Abstract
Human kidney organoids are complex structures resembling nephron arrays, which can be derived in a variety of ways. Whether all of these differentiation protocols produce qualitatively similar organoid cell types is not yet clear. A comparative analysis of two organoid differentiation protocols is recently reported in Cell Stem Cell, using single cell RNA sequencing (scRNA-seq) as an analytical tool. This demonstrates that the two protocols have much in common, and that neither produces kidney cells in a pure or comprehensive manner. Ureteric lineages appear to be absent, and organoids are contaminated with non-kidney cell types, including neurons and muscle cells. Based on the scRNA-seq datasets, a new differentiation protocol is devised to reduce non-kidney cell types, without adversely affecting organoid epithelial cells. Together with published analyses of a third differentiation protocol, these findings suggest more commonalities than differences between kidney organoid platforms, and identify critical strategies for functional improvement of these cellular structures.
An organoid is a multicellular unit in vitro that resembles a tissue or organ of the body. Human kidney organoids contain complex, nephron-like structures with utility for both disease modeling and regenerative medicine. As these organoids are a relatively recent invention, it is not yet crystal clear what the composition of these cell cultures actually is. A variety of protocols have been published for organoid differentiation derived from pluripotent stem cells. These studies differ substantially, with one reporting generation of a collecting duct network alongside tubules [1], another claiming 90 % efficiency in producing nephron progenitor cells [2], and yet a third observing both kidney and non-kidney cell types in organoid cultures [3]. Recent studies have further begun to catalog the different types of cells present in organoids with transcriptomic analysis of single cells (single cell RNA sequencing, or scRNA-seq), to gain a more comprehensive view [4–7]. In this ‘second opinion’, we will take a careful look at one such study, recently published in Cell Stem Cell [5], and attempt to place its findings within the greater context of the literature.
It would be premature to conclude, as a recent review has done [8], that any of the purportedly ‘unique’ features of the published differentiation protocols are real or substantial. On the contrary, all of these protocols clearly share certain key commonalities, such as the formation of three nephron segments along a proximal-to-distal axis, and induction of nephrogenesis with the kinase inhibitor CHIR99021 [1–3,9]. As each of these protocols has been developed by a different group, any perceived differences may merely be in the eye of the beholder. This is the entry point for the Wu et al. study, in which two protocols are examined side-by-side by third party ‘objective observers’ that were not involved in either of the original studies they seek to reproduce. Although the authors claim to “provide the first direct comparison of separate differentiation protocols”, this isn’t really true – such comparisons have been published previously [4,10]. Nevertheless, this paper does do a good job of comparing two of these differentiation protocols using scRNA-seq.
In order to properly understand this particular study, it is important to clearly grasp the experimental design. The authors perform the two selected differentiation protocols essentially as described [1,2] to produce organoids in two different batches and from two different pluripotent stem cell lines (one embryonic and one induced), although the concentration of CHIR99021 needs to be reduced to successfully differentiate one of these. Organoids are generated, and immunofluorescence snapshots demonstrate the presence of nephron-like segments within these, although the extent of this microscopy characterization is rather limited. The organoids are dissociated into individual cells, whose messenger RNA molecules are bar coded in tiny droplets prior to reverse transcription, PCR-based amplification, and deep sequencing [11,12]. Although this technique can produce transcriptomic data for thousands of cells, it can detect only ~ 20 % of the genes expressed in any given cell, with only snippets of any given mRNA being actually sequenced. The results can therefore depend greatly upon the analytical approach.
In Wu et al., the raw sequence data from all of the experiments (different protocols, cell lines, and batches) is normalized and pooled together into a single large dataset. This is then analyzed computationally to identify populations of related cells (“clusters”). By manually scanning the gene lists in each cluster for known marker genes, specific clusters are associated with known cell types. The contribution of the individual protocols, cell lines, batches etc. to each of these clusters is then de-coded for individual cells to identify their samples of origin. Altogether, the pooled dataset encompasses over 70,000 cells, which unsupervised clustering suggests represented 23 different clusters.
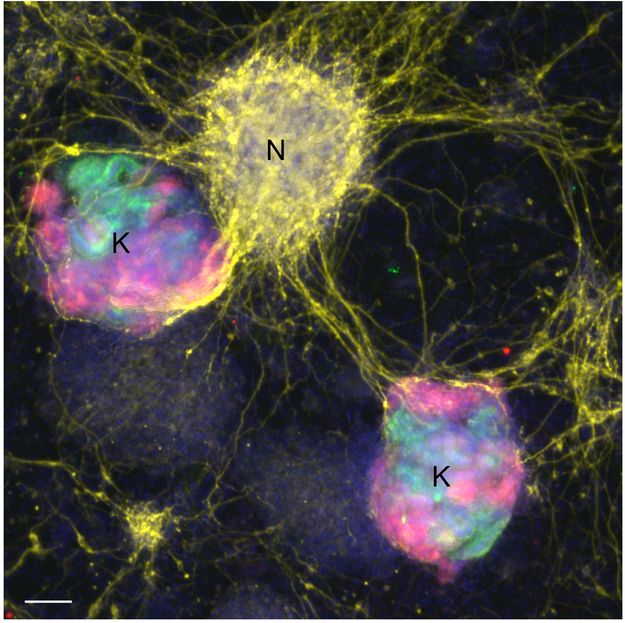
One prominent finding is that, regardless of differentiation protocol, many of these clusters are not specific to the kidney. There is a substantial population of neurons and muscle (~ 20 % of all cells), an even larger ‘mesenchymal’ population which may or may not be kidney stroma (~ 30 %), and a smattering of endothelial cells. This is not the first time non-kidney cells have been noticed in organoids. Indeed, neuronal contaminants were described in one of the earliest reports of these structures (Figure 1) [3]. Other ‘off target’ cell types including myogenic lineages have also previously been detected in organoids using standard immunofluorescence staining [1,3,10]. Thus, the discovery of ‘off-target’ cell types in these organoids is not wholly unexpected, but it is highlighted here in an interesting way, and establishes this phenomenon as a common event amongst the various protocols. As neurons derive from ectoderm while kidneys derive from mesoderm, even the very early differentiation stages of these two protocols are unlikely to be germ-layer specific. This is difficult to square with previous claims of 90 % efficiency of differentiation into SIX2+ nephron progenitor cells [2], which typically generate kidney epithelia [13].
Figure 1. Neurons are present in kidney organoid cultures.
Image reproduced from reference 3, showing neuronal contaminants (yellow, neuron-specific class III β-tubulin) in cultures of kidney organoids (green, Lotus tetragonolobus lectin; red, synaptopodin). Scale bar, 100 μm. A third-party re-analysis of two other protocols, references 1 and 2, finds neurons in these organoid cultures, as well.
The kidney-specific cell types are also somewhat interesting, not so much for what is present, as for what is absent. Specifically, no clusters can be unambiguously defined as the ureteric bud lineage or its descendants, the collecting ducts. This contrasts with the conclusion of a previous Nature paper, whose protocol was tested here, albeit with a different iPS cell line [1]. The absence of collecting ducts is in line with the previous observations of several others in the field, who have eschewed classifying CDH1hi cells in organoids as ureteric [2,3,9]. Although the consensus seems to be leaning in this direction, the gene expression signature of these CDH1hi cells still shows some overlap with collecting ducts, and cannot be definitively identified by scRNA-seq. To truly rule out the identity of these cells as ureteric bud or collecting ducts, it will be essential to actually generate bona fide collecting ducts in human kidney organoids, which has not yet been done convincingly by any group.
One limitation of these organoid cultures, also supported by the findings in Wu et al., is that none of the kidney cell types appears to be fully mature, particularly when compared to embryonic kidney tissue in vivo [4,14]. This underscores the need for exercising caution when describing structures such as ‘foot processes’ in the absence of tertiary interdigitations, or ‘glomeruli’ in the absence of a functional vasculature. It is also notable that several prominent cell types associated with kidney nephrons, such as parietal cells, pericytes, and mesangial cells, cannot clearly be identified in these organoids with scRNA-seq. Whether this indicates absence or immaturity of these cell types in organoids, or reflects a more fundamental inadequacy of scRNA-seq technology to detect them, is not yet clear.
Overall, the findings of Wu et al. match up very nicely with a recent scRNA-seq analysis of a third differentiation protocol, published earlier this year [4]. That study, using a combination of scRNA-seq and immunofluorescence, also reveals the presence of non-kidney cells, absence of collecting ducts, and general immaturity of the organoid cell types. It is striking that three different protocols have now been shown to contain similar repertoires of both kidney-specific and contaminating cell types. The likelihood that any of these protocols is qualitatively unique or advantageous in terms of differentiation capacity seems to be diminishing.
There are some weaknesses in this comparative analysis. The study relies very heavily on scRNA-seq, which is a highly processed, inherently descriptive, low coverage method. While normalizing and pooling the data from distinct conditions enables the authors to identify many clusters, it also carries a risk of producing false associations (analogous to pooling lysates from different conditions in a single Western blot). For instance, the basal media for the two differentiation protocols used are very different – one is a relatively rich media (APEL2), and the other is practically a starvation condition (Advanced RPMI). This would be expected to produce some pronounced differences in many cell types over the ~ 2 week incubation prior to the scRNA-seq harvest. It also would have been helpful to complement the pooled analysis with a more detailed, separate analysis of the two protocols, to test for this. It would also have been enlightening to include additional controls for the pooling approach, such as directed differentiations of these cell lines into non-kidney lineages. Interpretations of scRNA-seq datasets can be highly subjective and need to be considered carefully, particularly when the data are presented in graphical ‘plot’ forms with little to no raw data to inspect.
Perhaps illustrating some of these limitations, in one piece of data, pseudotemporal trajectory analysis is performed, which uses a computational algorithm to attempt to discern temporal relationships between different cell types in the culture (analagous to how evolutionary trees are built based on genetic similarity) [15]. This produces an unexpected bifurcation between podocytes, neurons, and stromal cells on one branch of the tree, and tubular lineages on the other. From a developmental standpoint, this trajectory is highly unlikely to be correct - it is well established that podocytes and proximal tubules derive from the same SIX2+ nephron progenitor cell population, which is distinct from stromal cells and far removed from neurons (which derive from a distinct germ layer) [13]. Indeed, in organoids, proximal tubules are typically juxtaposed to podocytes, suggesting a close developmental relationship. Thus, the trajectory analysis suggests that scRNA-seq is inadequate to predict temporal relationships in this system, even between closely related cell types. Why this should be so is unclear, but it may relate to the general immaturity of organoid cells and the lack of sufficient definition among the cell types themselves in the culture, compared to in vivo. Alternatively, it may reflect bias of scRNA-seq for highly expressed genes, such as cytoskeletal components present in both podocytes and neurons, that overshadow more subtle developmental characteristics of these individual cell types.
The final experiment brings greater depth to the story, in that the authors perform a functional experiment to improve the organoids based on their scRNA-seq dataset. By examining cognate ligand-receptor pairs in the non-tubular lineages, the authors identify brain-derived neurotrophic factor (BDNF) as possibly involved in neuronal differentiation. Addition of a BDNF inhibitor to the differentiating organoids dramatic decreases the levels of ‘off-target’ neuronal cell differentiation, which is confirmed in a qualitative way by immunofluorescence. Although chemical depletion of neurons could probably have been conceived and accomplished successfully without the need for scRNA-seq, this is nevertheless a solid demonstration that ‘omics’ methodologies can be used to identify interesting pairs of ligands and receptors that are functional in the organoid cell types.
This experiment is also interesting conceptually. When neurons were originally identified in human kidney organoids, it was speculated that they might serve as a substitute for the ureteric bud in epithelial cell differentiation, analagous to embryonic spinal cord in metanephric organ culture [3]. The experiments here suggest that ureteric bud is not present, but that neurons are also dispensable for organoid differentiation. Thus, these off-target cells may be disposed of without concern for the health of the organoids, likely improving any functional utility they may have down the road. This constitutes the flip side to another recent study, in which the number of vascular endothelial cells were dramatically increased in human kidney organoid cultures by adding in vascular endothelial growth factor at a specific time point in the differentiation protocol [4]. That study also utilized scRNA-seq to analyze this improvement, and collectively the two reports suggest this may be a generally useful tool for improving organoid differentiations.
In conclusion, the manuscript by Wu et al. provides a multi-faceted analysis of scRNA-seq data obtained from thousands of cells and two different flavors of kidney organoids. The conclusions of this ‘third party’ analysis are that the organoids generated from these two protocols are, in fact, very similar to one another, and neither is pristine. Significant discrepancies with the published reports are observed regarding the efficiency of one protocol, and the range of cell types produced by the other. The results match very well with what has been observed with a third organoid differentiation protocol using scRNA-seq and side by side comparisons [4]. A novel aspect of the Wu et al. study lies in describing a modified protocol, based in part on the scRNA-seq resource, that successfully eliminates the vast majority of neuronal cells, without adversely affecting the desirable tubular and podocyte lineages. More generally, the paper serves as a useful demonstration of how third party analyses can clarify and synthesize reproducible features of discoveries from different groups. This ‘technology transfer’ approach has also recently been used to test the reproducibility of kidney-on-a-chip microphysiological devices with cells from different sources [16]. Although such detailed comparative analyses remain relatively rare, they are hopefully gaining traction, and undoubtedly play a valuable role for the field.
ACKNOWLEDGEMENTS
The Freedman laboratory is supported by NIH Awards K01DK102826, R01DK117914, UG3TR002158, an Allen Institute Translational Science Grant, a Regular Award from the United States – Israel Binational Science Foundation, an Institute for Stem Cell and Regenerative Medicine Innovation Pilot Award, a Brotman-Baty Institute Seattle Single Cell Initiative award, a gift from the Northwest Kidney Centers to the Kidney Research Institute, and start-up funds from the University of Washington.
REFERENCES
- 1.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH: Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 2015;526:564–568. [DOI] [PubMed] [Google Scholar]
- 2.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 2015;33:1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV: Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 2015;6:8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czerniecki SM, Cruz NM, Harder JL, Menon R, Annis J, Otto EA, Gulieva RE, Islas LV, Kim YK, Tran LM, Martins TJ, Pippin JW, Fu H, Kretzler M, Shankland SJ, Himmelfarb J, Moon RT, Paragas N, Freedman BS: High-Throughput Screening Enhances Kidney Organoid Differentiation from Human Pluripotent Stem Cells and Enables Automated Multidimensional Phenotyping. Cell Stem Cell 2018;22:929–940 e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD: Comparative Analysis and Refinement of Human PSC-Derived Kidney Organoid Differentiation with Single-Cell Transcriptomics. Cell Stem Cell 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borestrom C, Jonebring A, Guo J, Palmgren H, Cederblad L, Forslow A, Svensson A, Soderberg M, Reznichenko A, Nystrom J, Patrakka J, Hicks R, Maresca M, Valastro B, Collen A: A CRISP(e)R view on kidney organoids allows generation of an induced pluripotent stem cell-derived kidney model for drug discovery. Kidney Int 2018;94:1099–1110. [DOI] [PubMed] [Google Scholar]
- 7.Harder JL, Menon R, Otto EA, Zhou J, Eddy S, Wys NL, Nair V, Cebrian C, Spence J, Troyanskaya OG, Hodgin J, Wiggins RC, Freedman BS, Kretzler M: Organoid single-cell profiling identifies a transcriptional signature of glomerular disease. JCI Insight, in press 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morizane R, Bonventre JV: Kidney Organoids: A Translational Journey. Trends Mol Med 2017;23:246–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, Nishinakamura R: Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 2014;14:53–67. [DOI] [PubMed] [Google Scholar]
- 10.Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchil l A.J., Kim YK, Winston K, Diaz MA, Fu H, Finn LS, Pei Y, Himmelfarb J, Freedman BS: Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nature Materials 2017;doi: 10.1038/NMAT4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA: Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015;161:1202–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, Peshkin L, Weitz DA, Kirschner MW: Droplet barcoding for single-cell transcriptomics applied to embryonic stem cells. Cell 2015;161:1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 2008;3:169–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, Cruz NM, Liu Y, Churchill AJ, Wang Y, Fu H, Pippin JW, Lin LY, Shankland SJ, Vogl AW, McNagny KM, Freedman BS: Gene-edited Human Kidney Organoids Reveal Mechanisms of Disease in Podocyte Development. Stem Cells 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, Trapnell C: Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 2017;14:979–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakolish C, Weber EJ, Kelly EJ, Himmelfarb J, Mouneimne R, Grimm FA, House JS, Wade T, Han A, Chiu WA, Rusyn I: Technology Transfer of the Microphysiological Systems: A Case Study of the Human Proximal Tubule Tissue Chip. Sci Rep 2018;8:14882. [DOI] [PMC free article] [PubMed] [Google Scholar]