Despite its importance in the physiopathology of HSV-1 and -2 infections, the cell-to-cell spreading mechanism is still poorly understood. The data shown here suggest that infection-elicited neutralizing antibodies capable of inhibiting cell-to-cell virus spread can be underrepresented in most infected subjects. These observations can be of great help in better understanding the role of humoral immunity in controlling virus reactivation and in the perspective of developing novel therapeutic strategies, studying novel correlates of protection, and designing effective vaccines.
KEYWORDS: neutralizing activity, serum neutralizing antibodies, cell-to-cell virus spread, herpes simplex virus, human monoclonal antibodies, humoral immunity
ABSTRACT
Herpes simplex virus 1 (HSV-1) and HSV-2 can evade serum antibody-mediated neutralization through cell-to-cell transmission mechanisms, which represent one of the central steps in disease reactivation. To address the role of humoral immunity in controlling HSV-1 and HSV-2 replication, we analyzed serum samples from 44 HSV-1 and HSV-2 seropositive subjects by evaluating (i) their efficiency in binding both the purified viral particles and recombinant gD and gB viral glycoproteins, (ii) their neutralizing activity, and (iii) their capacity to inhibit the cell-to-cell virus passage in vitro. All of the sera were capable of binding gD, gB, and whole virions, and all sera significantly neutralized cell-free virus. However, neither whole sera nor purified serum IgG fraction was able to inhibit significantly cell-to-cell virus spreading in in vitro post-virus-entry infectious assays. Conversely, when spiked with an already described anti-gD human monoclonal neutralizing antibody capable of inhibiting HSV-1 and -2 cell-to-cell transmission, each serum boosted both its neutralizing and post-virus-entry inhibitory activity, with no interference exerted by serum antibody subpopulations.
IMPORTANCE Despite its importance in the physiopathology of HSV-1 and -2 infections, the cell-to-cell spreading mechanism is still poorly understood. The data shown here suggest that infection-elicited neutralizing antibodies capable of inhibiting cell-to-cell virus spread can be underrepresented in most infected subjects. These observations can be of great help in better understanding the role of humoral immunity in controlling virus reactivation and in the perspective of developing novel therapeutic strategies, studying novel correlates of protection, and designing effective vaccines.
INTRODUCTION
The importance of identifying a specific immunity directed against herpes simplex viruses (HSV) is well known. The significance of T-cell immunity in controlling HSV reactivation and shedding is also well described (1, 2). Less is known about the humoral branch of adaptive immune responses due to multifaceted clinical manifestations resulting from HSV-1 and -2 reactivations, which are often local and aviremic (3, 4). A number of unsuccessful vaccine clinical trials were performed based on the use of envelope virus glycoproteins to induce neutralizing humoral immunity despite the lack of information on molecular mechanisms present in the protection conferred by neutralizing serum antibodies targeting HSV (5–7). These trials demonstrated that vaccine candidates were able to elicit neutralizing serum antibodies. However, no long-lasting significant reduction in virus shedding or inhibition of recurrences were achieved (7). These observations contrast with the biological activity of certain monoclonal antibodies (MAbs) described as being able to reduce virus shedding when administered in animal models (8). Unfortunately, there are no animal models which properly mimic HSV reactivations or immunogens able to elicit in humans an antibody response featuring the anti-HSV activity of the monoclonal antibodies described until now (9). That is why the study of anti-HSV human humoral immunity should take into account antibodies of human origin, which should then be used to better understand and characterize natural responses to virus infection and the role of specific IgGs directed against the most immunogenic virus structures. Several correlations between the presence of specific antibodies tailoring HSV envelope glycoproteins and their putative anti-HSV activity were previously described, supporting the importance of gD in eliciting neutralizing antibodies (10). Moreover, glycoprotein B (gB) and gH/gL were also described as inducers of neutralizing antibodies (11, 12). From these studies it was evident that all those infected by HSV are able to produce such a neutralizing response, but to date it has not been possible to consider the presence of these antibodies as an effective correlate of protection from virus reactivation, a mechanism in which T-cell immunity is much more involved. In particular, it has already been described how low overall serum neutralizing antibody titers do not necessarily correlate with high virus shedding (13), suggesting that the evaluation of the neutralizing activity alone is not necessarily predictive of a definite virologic outcome. Great effort has been made to understand the functions of HSV glycoproteins by means of deleted recombinant viruses and their relative capability in inducing protective responses in animals, as well as the functional characterization of gD, gB, and gH/gL regions through the use of monoclonal antibodies which have been identified to date (14–17). Moreover, it has also been demonstrated how sera depleted by their anti-gD and -gB antibody fractions decreased their neutralizing capability (10).
Among the neutralizing monoclonal antibodies (MAbs) described so far, few are of human origin, and only one, IgG#33, previously characterized for its in vivo anti-HSV activity, is endowed with the capability of inhibiting in vitro virus infection of both HSV-1 and -2 after proper virus entry into target cells (18, 19). The focus of our attention is not on the fine dissection of antibody subpopulations from a quantitative point of view; instead, we give priority to the study of the biological effect of antibodies present within the sera. We therefore dissected the humoral response of 44 sera collected from HSV-1- or HSV-2-positive subjects by comparing their serum anti-HSV activity in terms of binding to recombinant gD, gB, and whole purified virions, neutralizing activity against both HSV types, and their capacity to inhibit, alone or in combination with the anti-gD MAb, the virus cell-to-cell transmission when added after virus entry.
RESULTS
Human serum preliminary stratification.
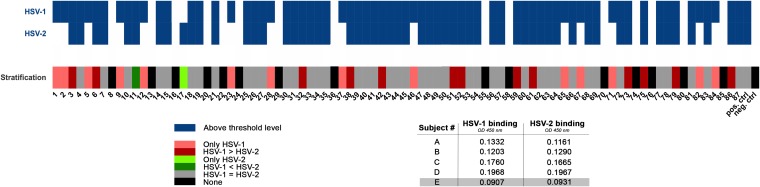
Eighty-seven sera were collected from patients who were positive in routine serological testing for the presence of anti-HSV-1/2 IgG. As this testing procedure is unable to discriminate between HSV-1 and HSV-2 antibodies, sera were further tested for their ability to bind HSV-1 HF and/or -2 MS purified virions coated on an enzyme-linked immunosorbent assay (ELISA) plate. A heatmap was generated according to pattern reactivity measured for all sera against the whole type 1 and 2 viruses (Fig. 1). An optical density at 450 nm (OD450) of 0.5 was set as the exclusion cutoff for discriminating strong serum reactivity against HSV-1 and/or HSV-2, and results showed that there are differences related to HSV-1 and -2 binding. In detail, there were sera able to bind efficiently to only one virus type, sera able to bind efficiently to both viruses but with different strengths, and a few sera that fell below our threshold, being a narrower exclusion cutoff than those of routine screening tests. On the basis of the binding patterns evaluated for all sera, they were further clustered into six categories, as depicted by different colors shown in Fig. 1. Since these binding categories are randomly represented among the 87 serum samples, only 44 sera representing all of the binding groups were selected for further evaluation, being a sufficient size for statistical correlations.
FIG 1.
Binding analysis of 87 human sera to HSV-1 and -2. A threshold OD450 of 0.5 was set to stratify binding activity of 87 human sera against HSV-1 HF and HSV-2 MS purified virions coated on an ELISA plate. In detail, a panel of 5 sera (A to E) from healthy uninfected subjects was tested for HSV-1 and -2 binding activity. The cutoff corresponding to an OD450 of 0.5 was set at 2.5 times the higher OD signal registered for the negatives in order to minimize the binding contribution of possible antibody cross-reactivity. Serum E represents the nonneutralizing (non-NT) control for the experiments. Blue boxes indicate positive sera, and white boxes indicate negative ones. Moreover, sera were stratified into six categories: sera able to bind only HSV-1 (light red) or only HSV-2 (light green), sera that bind both viruses equally (gray), sera that preferentially bind HSV-1 over HSV-2 (red) and vice versa (green), and negative sera (black). Positive and negative control sera were added as well.
Binding analysis against HSV whole virions and recombinant glycoproteins.
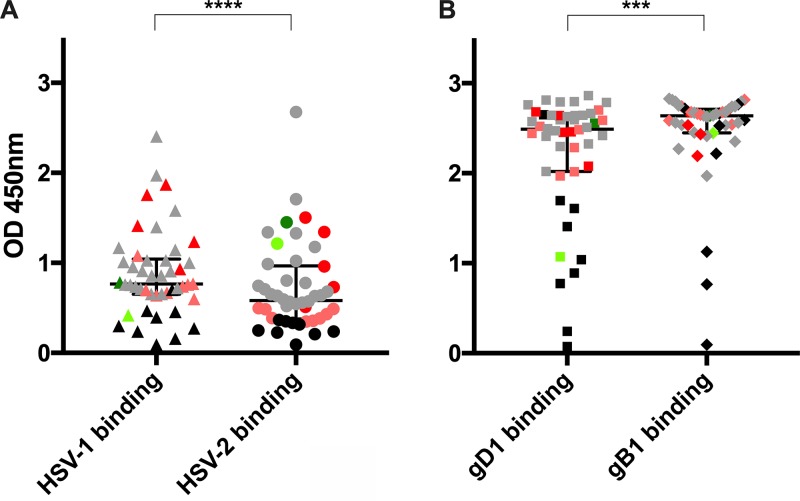
Selected sera were further characterized for their binding capability against both HSV-1 and -2 purified virions and soluble gB and gD of HSV-1 strain HF (gD1 and gB1) at a 1:200 dilution. Such a low dilution was used to better represent the physiological antibody concentration with respect to experimental limits; negative serum controls are included as well for monitoring possible nonspecific contributions resulting from this high concentration. Binding to soluble protein stocks was validated with the use of specific monoclonal antibodies (Fig. 2). A binding difference (P < 0.0001) was observed for the sera against the two virus types (median OD450, 0.765 for HSV-1 and 0.583 for HSV-2) (Fig. 3A and Table 1). On the other hand, all sera showed high reactivity when tested against recombinant soluble virus glycoproteins (OD450, 2.489 for gD1 and 2.639 for gB1) (Fig. 3B and Table 1). Moreover, gD1 seemed to be recognized more than gB1 (P < 0.001).
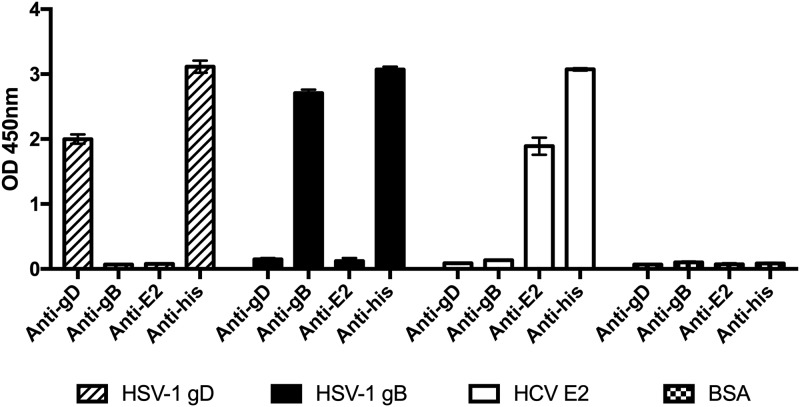
FIG 2.
Soluble protein purification. His-tagged soluble forms of HSV-1 glycoprotein D (gD1) and B (gB1) were affinity purified. Soluble HCV envelope protein E2 (HCV E2) was purified as a control. Protein stocks were validated using human monoclonal antibodies specific for the glycoproteins and a primary antibody specific for His-tagged proteins. Means ± standard deviations (SD) are reported.
FIG 3.
Characterization of binding to purified virions and soluble glycoproteins. Graphs show the results from binding analysis of 44 sera to HSV-1 (▲) and -2 virions (●) (A) and soluble glycoprotein D (■) and B (◆) of HSV-1 (B). Sera were used at a 1:200 dilution. The median optical density (OD450), with interquartile range, is reported for each binding analysis (***, P < 0.001; ****, P < 0.0001). Each data point is represented by its stratification color: sera able to bind only HSV-1 (light red) or only HSV-2 (light green), sera that bind both viruses equally (gray), sera that preferentially bind HSV-1 over HSV-2 (red) and vice versa (green), and negative sera (black).
TABLE 1.
Sera binding to purified virions and soluble glycoproteins and their activity against HSV-1 and -2 infectionsa
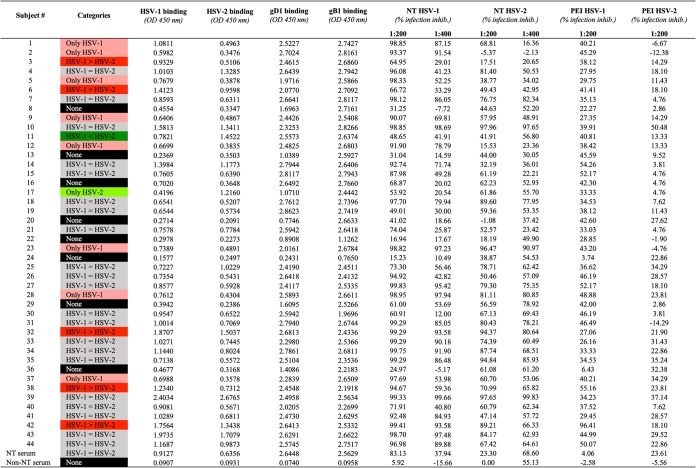 |
Results of neutralization (NT) experiments and postentry inhibition (PEI) assays are reported. Sera were tested at 1:200 and 1:400 dilutions.
On the basis of these binding experiments, no correlation was observed between the different categories in which sera have been previously clustered and their ability to recognize the two glycoproteins. As an example, the outlier sample of both HSV-1 and -2 binding data sets was serum 39 (Fig. 3A): its binding to HSV-1 had an OD450 of 2.40 and to HSV-2 had an OD450 of 2.677. However, when tested against the two soluble glycoproteins, results only slightly deviated from the medians for the two groups (gD1, OD450 of 2.496; gB1, OD450 of 2.563). Another example was represented by serum 11, which bound the HSV-2 purified virions above the median (OD450 of 1.452) and well recognizes HSV-1 (OD450 of 0.782). In this case, as expected, serum 11 also recognized well the two glycoproteins (gD1, OD450 of 2.557; gB1, OD450 of 2.637). Finally, a different behavior was also observed for serum 17, strongly recognizing only HSV-2 purified virus (OD450 of 1.216 versus 0.420 for HSV-1). It showed higher binding capability against gB1 than gD1 (OD450 of 2.444 for gB1 versus 1.071 for gD1), despite its higher binding capability against HSV type 2 whole virions, suggesting the contribution of cross-binding serum antibodies better recognizing the more conserved gB of HSV-1 HF and HSV-2 MS than the gD.
Therefore, as expected due to the high immunogenicity of both gB and gD, almost all sera recognized the major envelope glycoproteins when expressed and purified as recombinant soluble proteins. Conversely, the lower binding activity observed against whole viruses suggested an important contribution of IgG targets on the whole virus differing from the most immunogenic glycoproteins tested in these experiments.
NT of cell-free virus and postentry inhibition assays of serum samples.
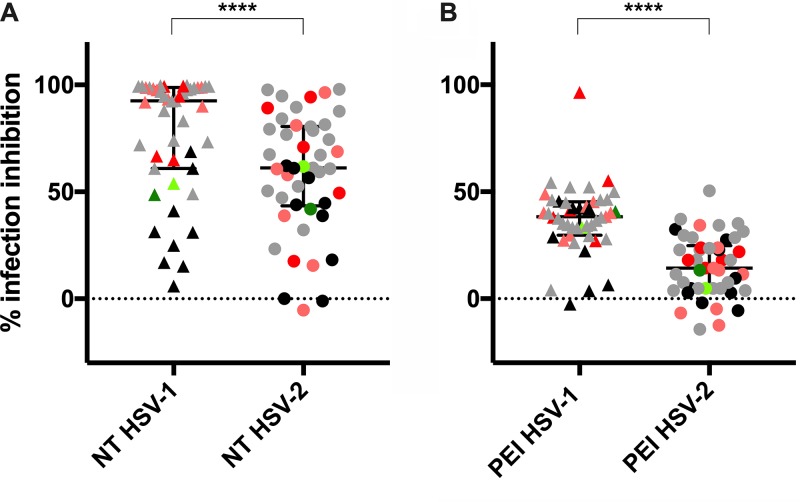
After the characterization of the binding ability of 44 selected sera, their ability to interfere with the replication of both virus types was tested. The median values (92.61% for HSV-1 and 61.13% for HSV-2) obtained with the neutralization (NT) assay indicated that the majority of samples contained antibodies able to block cell-free virus infection. Despite the wide variability observed, sera more efficiently neutralized HSV-1 HF than HSV-2 MS (P < 0.0001) (Fig. 4A and Table 1).
FIG 4.
Evaluation of serum activity against HSV-1 and -2 infections. (A) Neutralizing (NT) activity of 44 sera was assessed on Vero E6 cells, and results show that HSV-1 (▲) was more efficiently blocked than HSV-2 (●). (B) When tested in PEI assay on the same cells, no samples (except one) impaired virus replication efficiently, although higher activity again was observed against HSV-1 (▲) than HSV-2 (●). Sera were used at 1:200 dilution. Median values with interquartile ranges are reported for each experiment (****, P < 0.0001). Each data point is represented by its stratification color: sera able to bind only HSV-1 (light red) or only HSV-2 (light green), sera that bind both viruses equally (gray), sera that preferentially HSV-1 over HSV-2 (red) or vice versa (green), and negative sera (black).
Subsequently, postentry inhibition (PEI) assay was performed to evaluate the ability of sera to impair virus replication even after its entry into the target cells. Briefly, Vero cell monolayers were infected, and after washing cell-free virus particles, a layer of semisolid culture medium containing different dilutions of the sera was added. This method allowed us to appreciate virus spreading into adjacent cells via the cell-to-cell mechanism of infection (18, 20). Unexpectedly, and in contrast to the behavior of the same sera tested at the same dilutions for their neutralization capability, almost all sera failed to show consistent inhibition of the cell-to-cell transmission mechanism (Fig. 4B and Table 1). In detail, the majority of sera better constrained the HSV-1 cell-to-cell virus passage than HSV-2 (P < 0.0001). However, median values of inhibition obtained for HSV-1 and -2 were only 37.26% and 15.18%, respectively. Two outliers were identified: serum 42 was endowed with PEI activity against HSV-1 (96.41%) but not against HSV-2 (18.1%), and serum 10, which was the only serum inhibiting the HSV-2 tested isolate by 50.48% and showed partial inhibitory activity against HSV-1 (39.91%). PEI experiments highlighted how only a few tested sera were able to block HSV cell-to-cell spreading in vitro. However, as for the neutralizing activity, the postentry inhibition, when present at all, was preeminently type specific.
PEI activity of purified serum antibodies.
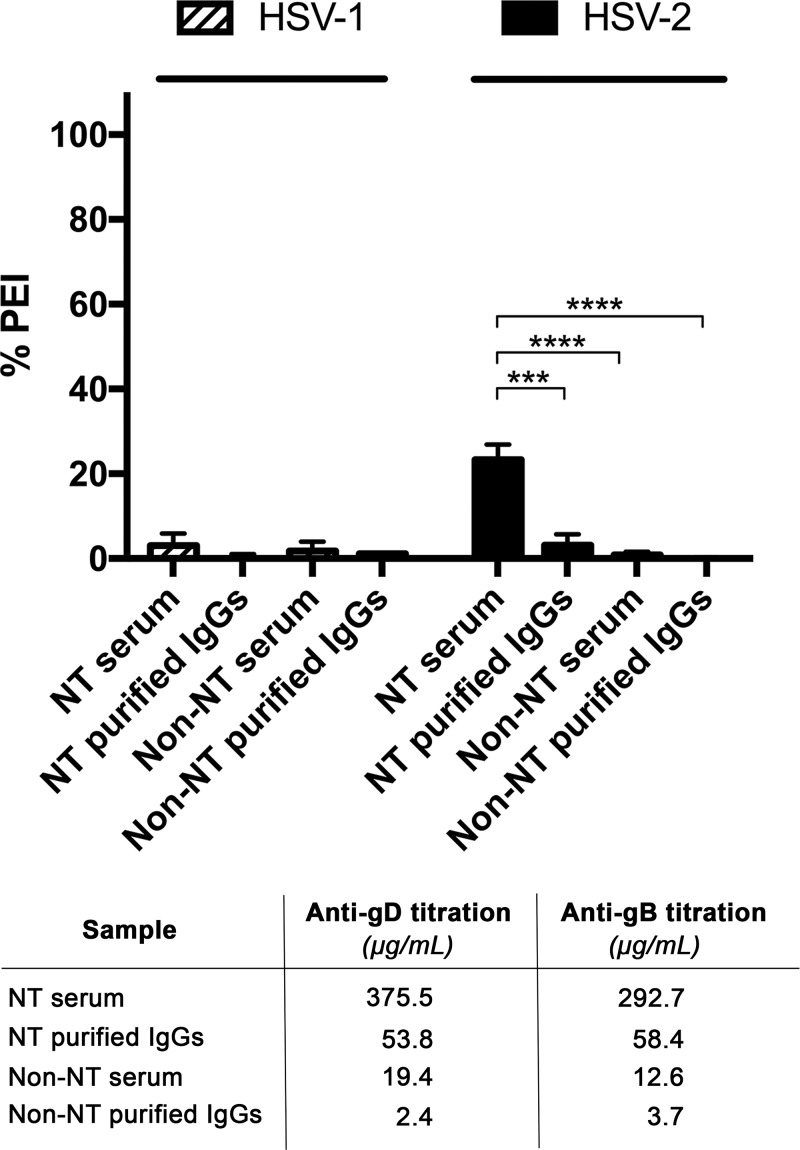
In order to push antibody concentration in PEI experiments over the physiological concentration of the total IgG fraction, serum IgGs were purified from a single positive neutralizing serum control as a proof of concept. Specifically, PEI assay was performed using both higher dilution of sera (1:100) and 100 μg/ml of purified IgGs, corresponding approximately to a 10-fold higher concentration than that of total IgGs in blood (21, 22) (Fig. 5). Results showed that even purified total IgGs at 100 μg/ml were only able to poorly inhibit postentry virus replication. Conversely, neutralizing serum lost its inhibitory activity against HSV-2 infection when only purified IgGs were used (P < 0.001). This behavior is probably related to other serum antibodies (including anti-gH/gL) not accounted for in this analysis and poor content of IgGs specific for HSV being able to recognize glycoproteins and epitopes crucial for the cell-to-cell mechanism of transmission still present.
FIG 5.
Evaluation of purified IgG activity against HSV-1 and -2 infections. PEI assay was performed on Vero E6 cells using lower dilutions of the neutralizing (NT) serum (1:100) control and purified total IgGs obtained from the same serum sample (100 μg/ml). Nonneutralizing (non-NT) serum and its purified total IgG fraction were used as a negative control. Mean values ± SD are reported for each experimental condition (***, P < 0.001; ****, P < 0.0001). Titration results for anti-gD and anti-gB present in NT and non-NT sera and their corresponding purified IgG fractions are indicated under the graph. Median values are reported.
Anti-gD and anti-gB IgG quantification.
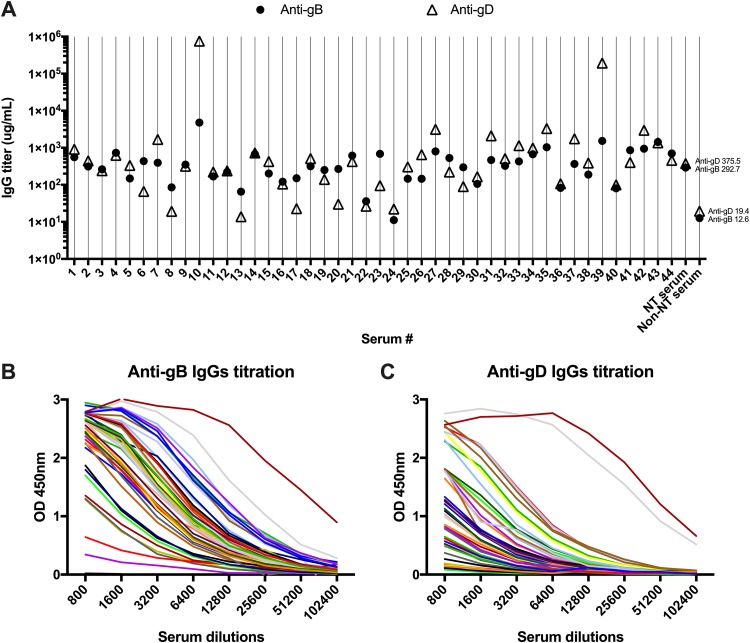
In order to evaluate the amount of serum IgG specific for gD or gB in all tested sera, both anti-gD and anti-gB antibodies were quantified (Fig. 6). Results indicate great variability among sera. Anti-gD antibody titers spanned across 3 logs (0.069 μg/ml to 3,765 μg/ml). The anti-gB humoral response was more homogenous, ranging from 0.056 μg/ml to 24 μg/ml. Only two outliers were identified for their anti-gD titer: sera 10 (3.8 mg/ml) and 39 (1 mg/ml). This assay allowed for an estimation of the amount of anti-gD and-gB antibodies used in the PEI experiment performed with a serum concentration of 1:200, 1.88 and 1.46 μg/ml for anti-gD and-gB, respectively, indicating overall low antibody titers specific to these proteins in naturally infected patients.
FIG 6.
Titration of specific serum IgGs. (A) IgG titers specific for gB (●) and gD (△) were obtained for 44 serum samples. Neutralizing (NT) and nonneutralizing (non-NT) serum controls are included as well. Median values are reported. Anti-gB (B) and anti-gD (C) antibodies were quantified in ELISA by performing titration curves on purified soluble glycoproteins (gB1 and gD1) in order to evaluate the amount of serum IgG specific for these glycoproteins in the 44 selected sera. Control sera (NT serum and non-NT serum) are included as well. Means are reported; SD are omitted for clarity.
From binding to biological function: a correlation analysis.
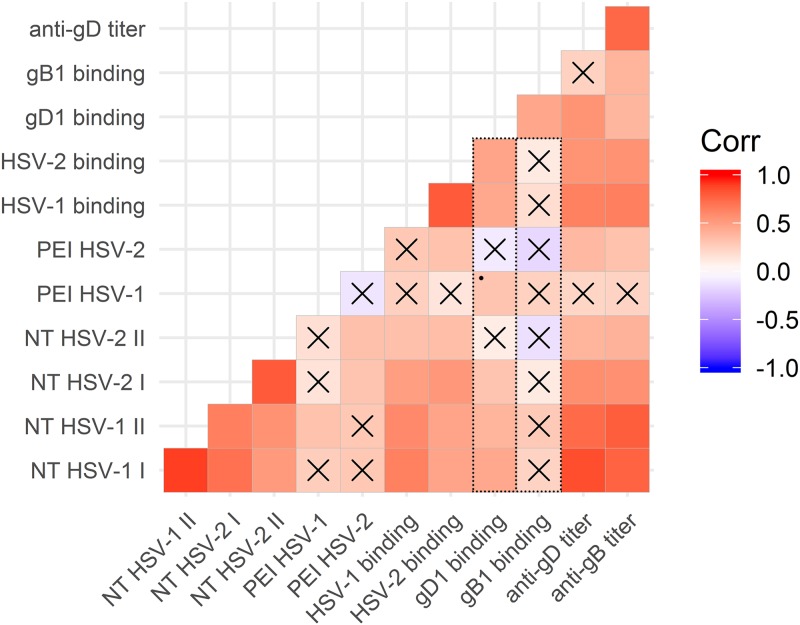
In light of the heterogeneous binding activity on the viral gD and the extremely variable anti-gD serum titer, possible correlations between binding activity and biological behavior of sera were inferred (Fig. 7). As expected, neutralizing activity was strongly related to whole virus binding (ρ = 0.643 and adjusted P value [Padj] of <0.001 for HSV-1; ρ = 0.533 and Padj < 0.001 for HSV-2), independent of the virus type. When considering recombinant glycoproteins, the ability of sera to recognize gD1 correlated with binding to the whole virus particle (ρ = 0.449, Padj < 0.01), NT (ρ = 0.450, Padj < 0.01), and, at the limit of statistical significance, PEI (ρ = 0.313, P < 0.05, Padj = 0.053). On the contrary, no significant correlation was observed between binding to gB1 and the ability of sera to block virus infection for both NT and PEI, although recognition of these two proteins has a similar trend but different binding capability (ρ = 0.464, Padj < 0.01). As depicted by boxes outlined with dots in Fig. 7, overall biological activities measured for the two virus types correlated more with the binding to gD1 than gB1.
FIG 7.
Graph of correlation matrix in R. Matrix heatmap plot corresponding to ρ correlation values between categories corresponding to parameters analyzed for the sera: binding to gD and gB, neutralizing activity, anti-gD and gB antibody titers, and postentry inhibition of the infection. Color scale indicates correlation values, with positive correlations assigned to red colors. X indicates Padj value of <0.05. *, Padj = 0.053. NT, neutralization assay. Roman numbers indicate 1:200 (I) and 1:400 (II) serum dilutions. Dotted boxes highlight how overall biological activities correlate with the binding to gD but not to gB.
The concentration of anti-gD antibodies and of those against gB might vary by orders of magnitude within the same patient, but together they fluctuated between serum samples (ρ = 0.749, Padj < 0.001). It is important to underline that correlation observed with titers does not necessarily reflect on the correlations observed for the binding activity due to their possible contribution in the binding of less represented high-affinity binders. High titers of anti-gD correlated with binding to HSV-1 (ρ = 0.642, Padj < 0.001) and HSV-2 whole virions (ρ = 0.549, Padj < 0.001), NT (ρ = 0.851, Padj < 0.001 for HSV-1; ρ = 0.578, Padj < 0.001 for HSV-2), and HSV-2 PEI (ρ = 0.369, Padj < 0.05 against HSV-2; ρ = 0.217, Padj = 0.18 against HSV-1). On the contrary, titer of anti-gB antibodies strongly correlated only with the NT (ρ = 0.769, Padj < 0.001 for HSV-1; ρ = 0.571, Padj < 0.001 for HSV-2) and only weakly with HSV-2 PEI (ρ = 0.321, Padj < 0.05) according to gD and gB titers fluctuating together.
Serum interference.
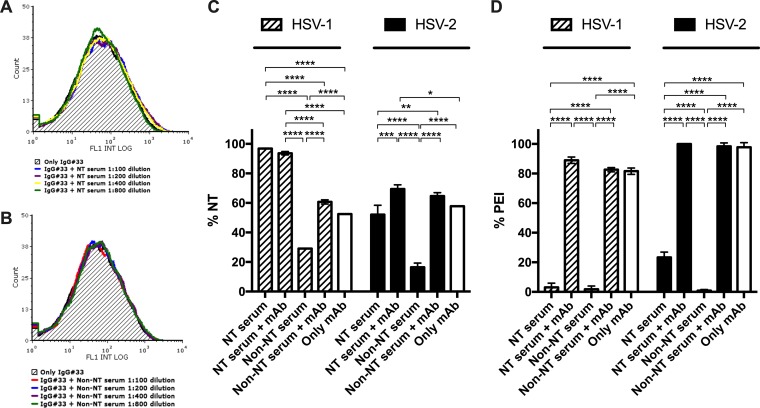
In order to assess if naturally elicited humoral response can impair activity of an already characterized neutralizing human monoclonal antibody able to strongly inhibit cell-to-cell transmission mechanisms and conferring systemic protection in vivo (18), competition analyses were performed. First, fluorescence-activated cell sorting (FACS) competition experiments evaluating the capability of serum IgGs to displace IgG#33 binding to gD1 transfected cells were carried out by combining a neutralizing and a nonneutralizing serum with the MAb (Fig. 8A and B). As a result, no decrease of IgG#33 binding was observed in the tested serum dilutions spanning from 1:100 to 1:800 for both neutralizing and nonneutralizing serum. Functional assays were then performed using the two sera supplemented with IgG#33. Accordingly, with FACS analysis, neither neutralizing nor nonneutralizing anti-gD serum antibodies hampered IgG#33 neutralizing and postentry inhibitory activity against both HSV-1 and -2. In detail, neutralizing serum retained its neutralizing activity against HSV-1 even at a very low dilution (1:100), while the MAb enhanced serum activity against HSV-2 at the same concentrations (P < 0.001) (Fig. 8C). On the other hand, a significant boost (P < 0.0001 and P < 0.05 against HSV-1 and -2, respectively) in the neutralizing activity was observed for the NT serum-MAb combination compared to that of the monoclonal alone, suggesting additive effect and excluding possible neutralization interference. Nonneutralizing serum acquired neutralizing capacity against both HSV-1 (P < 0.0001) and -2 (P < 0.0001) compared to serum alone. Interestingly, when supplemented with IgG#33, the two sera showed the same characteristics against HSV-2 infection.
FIG 8.
Competition evaluation. Dose-dependent binding competitions between sera and IgG#33 were performed. FACS analyses were performed using IgG#33 combined with serial dilutions (1:100 to 1:800) of both neutralizing (NT) serum (A) and nonneutralizing (non-NT) (B) serum to evaluate the possible competitive binding of antibodies to gD1-transfected cells. As shown by the graphs, no competition resulted even with the lower serum dilution tested. NT (C) and PEI (D) assays next were performed on Vero E6 cells with NT serum and a non-NT serum supplemented by IgG#33 against both HSV-1 and -2 infections. In NT assays, IgG#33 was used at its 50% inhibitory concentration (1.092 μg/ml against HSV-1 and 0.731 μg/ml against HSV-2 tested isolates), while in PEI assays it was used at 25 μg/ml and 100 μg/ml against HSV-1 and -2, respectively. Sera were used at 1:100 dilution, and the MAb alone control is indicated by white columns. Mean values ± SD are reported for each experimental condition (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Even more importantly, PEI experiments revealed that both neutralizing and nonneutralizing sera, unable to inhibit the virus replication when added after virus infection per se, acquired this feature when spiked with the monoclonal human IgG#33 (P < 0.0001) (Fig. 8D). Actually, PEI activity of sera mixed with the MAb depended only on MAb activity, as it was not statistically different from PEI activity of the MAb alone.
DISCUSSION
Overall, the presence of neutralizing antibodies is considered an important marker of protection against many viruses (23–25). However, it is also well known that the detection of only neutralizing antibodies can likewise be elusive for many viruses (26). Many anti-HSV vaccine attempts, trials, and projects, with the aim of eliciting neutralizing serum antibodies, such as those using whole virions or full-length viral immunogenic proteins, have been carried out in recent decades without much success in conferring long-lasting reduction of both clinical HSV recurrences and virus shedding rates (5–7). In this regard, several research groups have improved our knowledge of neutralizing epitopes present in HSV gD and gB (27–30). Moreover, it is also known that the presence of only neutralizing antibodies is not enough to confer protection from virus reactivations, it being a sufficient condition only for protection from de novo infection (1, 3). In order to further dissect the contribution of naturally elicited anti-HSV serum antibodies, we first analyzed sera according to their binding capability to both whole virions and recombinant glycoproteins. As expected, from this first round of screening we observed that almost all sera strongly recognized these two highly immunogenic HSV glycoproteins, the viral gD and gB. Conversely, the overall binding activity of the same sera to whole virions was orders of magnitude less strong, as highlighted by median values. This is probably due to the presence of serum antibodies hampering binding to the only recombinant glycoprotein for the sake of steric hindrance and/or to the relative amount of glycoproteins on the virion surface compared to recombinant protein amounts used in these assays. Due to these speculative assertions, our further analyses do not compare the binding magnitude of sera to virions versus that to glycoproteins. Despite this explanation, it is necessary to underline that serum IgG binding to recombinant gB is probably also heavily related to the presence of antibodies able to recognize the gB (also) in its postfusion conformation. This is why we should not exclude the possibility that these antibodies cannot bind the same protein in its metastable conformation present on the surface of the whole purified virions. Being unable to answer this question due to the lack of naturally elicited human antibodies well characterized so far for their specificity against the gB in its prefusion state, we further dissected another two macroscopic features of the selected sera: their neutralizing activity and, more relevantly, their ability to inhibit the cell-to-cell transmission mechanism when added to the cell monolayer after proper virus entry performed at 37°C. Importantly, it should be remembered that polyclonal antibodies in sera are a complex mixture; therefore, binding to discrete viral isolates might vary on the basis of antibody cross-isolate recognition. Gathering the overall results and keeping apart the single outlier sample deserving further attention, it is possible to state that almost all sera were endowed with appreciable neutralizing capability against both HSV-1 and -2 tested isolates. Interestingly, the global behavior of the same sera tested at the same dilution for their PEI activity was divergent to their global trend shown in neutralization experiments. This behavior has never been described before for naturally elicited humoral responses directed against HSV but has been described recently for human cytomegalovirus (31, 32). That is why, as a proof of concept, we also addressed the PEI experiment for purified serum IgGs from two different sera used as reference controls in our experiments. The results we gathered show how the purified IgG fractions were completely unable to inhibit virus cell passage even at a very high concentration, suggesting that their very low contribution in PEI activity is due to other antibody classes or underrepresented IgG not efficiently purified from the same serum, which, however, was unable to block efficiently the cell-to-cell transmission even at a lower dilution.
The next query to address was the evaluation of serum antibody titer specific for gD and gB within the different sera. This analysis evidenced that even using low serum dilutions, such as 1:100 and 1:200, the amount of these antibodies (<3.75 μg/ml) is sufficient for neutralizing virus infectivity but not enough to completely inhibit its replication once infection had occurred.
Once all of the data were gathered, a correlation analysis was performed to evidence a possible relationship between binding and anti-HSV activity. The most important information resulting from the correlation analysis of the data set was that gD binding ability can be considered the sole serum marker related to the PEI activity detectable in vitro. Moreover, higher neutralizing activity can be ascribed to gD binding capability as well as anti-gD-gB serum titers, further confirming what was already observed (10, 13). These observations suggest that anti-gD antibodies naturally elicited from infection or immunization with the whole gD format cannot abrogate the cell-to-cell transmission mechanism. This may be due to the presence of immunodominant gD regions biasing the antibody response (10), as suggested by PEI activity of sera tested at low dilution. We also observed the complete inability of highly concentrated purified serum total IgGs to inhibit virus replication after infection (0 and 3.2% inhibition versus HSV-1 and -2 at 100 μg/ml). However, for the sake of clarity, the amount of total Abs (containing anti-gD, gB, and gH/gL) recovered from serum contained within purified IgG samples were inevitably lowered due to purification procedures, and this mandatory attempt was also performed in order to push as much as possible the serum-only IgG concentration over the physiological amount. The observations on serum behavior suggest epitope-tailored vaccine approaches (33) properly eliciting PEI antibodies as well as for the setting up of novel immunotherapeutic regimens targeting HSV types that do not respond to currently available systemic drugs. Regarding the latter, we previously described a neutralizing human IgG that confers full protection from death in animals infected with HSV and that also inhibits the HSV cell-to-cell transmission mechanism (18, 19). This provides proof that even naturally elicited anti-gD antibodies are able to block this mechanism, but for some reason they are not particularly common and/or, when present, are probably underrepresented within the blood serum. Nonetheless, we should also consider that serum antibody interference hampers the activity of possibly present PEI serum IgGs (34). We inferred this possibility by spiking IgG#33 into human serum and checking for IgG#33 binding displacement on FACS experiments. As a result, we were able to assess how neither neutralizing nor nonneutralizing sera were able to displace IgG#33 from cells expressing virus gD as well as interfere with both neutralizing and PEI activity of the monoclonal antibody, suggesting that beyond the crucial role of protective epitopes on the gD discussed above, there is also a high affinity of antibodies for the viral target that is essential for avoiding interference.
These screening experiments further dissect several features of the humoral human response naturally elicited by HSV infection from a functional point of view. Our first findings in that direction seem to confirm that viral gD is naturally able to elicit a neutralizing response and also may be able to induce antibodies hampering the most important HSV escape strategy: cell to cell. Unfortunately, all classical therapeutic vaccine trials based on the whole virus or on the whole gD have failed to fulfill this requirement, suggesting the need for novel vaccine strategies and novel correlate-of-protection algorithms essential for evaluating all of the features of a truly protective immune response. Finally, further studies broadening the number of analyzed serum samples and including a fine dissection of sera collected from patients receiving vaccine candidates in clinical studies will be important for addressing this issue. These data elicit further investigations on the role played by cell-to-cell blocking antibodies in preventing clinical reactivations.
MATERIALS AND METHODS
Cells and viruses.
Vero E6 (Vero C1008, clone E6; ATCC CRL-1586) and HEK 293T (ATCC CRL-1586) cells were cultured in Dulbecco's modified Eagle medium (DMEM) containing 10% (vol/vol) fetal bovine serum (FBS). The laboratory strains HSV-1 HF (ATCC VR-260) and HSV-2 MS (ATCC VR-540) were used.
Recombinant proteins.
All recombinant proteins were cloned from the HSV-1 (strain HF) genome. Full-length (residues 1 to 394) glycoprotein D (gD1) was cloned into a mammalian expression vector (pcDNA 3.1 directional TOPO expression kit; Invitrogen). The full-length (residues 1 to 904) glycoprotein B (gB1) gene was synthesized and cloned into mammalian expression vector pcDNA3.1(+) by the Gene Synthesis Service of GenScript. The soluble form of glycoprotein D (residues 1 to 300) and of glycoprotein B (residues 1 to 733) were also cloned into pcDNA 3.1 vector. The soluble form of envelope protein E2 of hepatitis C virus (HCV) was cloned as already described (35) to be used as a purification control. HEK 293T cells were seeded in 6-well plates and were transfected the next day with the His-tagged protein expression vectors and Lipofectamine 2000 transfection reagent (ThermoFisher Scientific) in Opti-MEM reduced serum medium (Gibco, ThermoFisher Scientific) and serum-free DMEM without antibiotics. Six h later the medium was replaced with DMEM containing 2% FBS. After 48 h, cell supernatants were collected and His-tagged recombinant proteins were affinity purified over a nickel-nitrilotriacetic acid (Ni-NTA) agarose (Qiagen) column. Primers used were Fw.gBsoluble (5′-AAAAAGCTTACCATGCGACAGGGCGCACCTGCTCG), Rev.gBsoluble (5′-AACCGGTAGCGGCATTTGCGTCAGCATGAATCACG), Fw.gD (5′-GAAGGATCCATGATGGGGGGGGCTGCCGCCAG), Rev.gDfull (5′-CACCGGTATCCTCGGGGTCTTCCGGGGCG) and Rev.gDsoluble (5′-CACCGGTCATGTTGTTCGGGGTGGC)
Human sera.
All anonymized samples were obtained from 87 subjects consenting for both single blood sampling and specific serology for HSV-1 and -2 in 2017. Samples were selected according to their positivity for qualitative serological test for the determination of specific IgG antibodies to HSV-1 and -2 in human serum. In detail, an indirect chemiluminescence immunoassay (CLIA) was performed (LIAISON HSV-1/2 IgG assay; DiaSorin S.p.A., Saluggia, VC, Italy). A human serum recognizing both HSV-1 and -2 viruses and a human serum negative for anti-HSV antibodies were used as reference positive and negative controls, respectively. IgGs were purified from human neutralizing serum and a nonneutralizing serum control by a GammaBind Sepharose (GE Healthcare) column with goat anti-human IgG Fab (ThermoFisher Scientific) according to the manufacturer’s instructions.
Monoclonal antibodies.
Human IgG#33 has been previously described (18). It was selected using phage display technology from peripheral B cells of a donor showing strong serum IgG ELISA reactivity against both HSV-1 and -2 isolates. MAb#33 was selected against HSV-1- and -2-infected Vero E6 cells after a deselection step using uninfected cells. Anti-HSV gB human monoclonal antibody obtained by high-affinity selection by phage display was also used as a control for soluble HSV-1 gB purification and for estimating anti-gB IgG titer. Anti-HCV/E2 IgGe137 (36) was used in His-tagged purification control experiments.
ELISA.
HSV-1 HF and HSV-2 MS strains were expanded in confluent Vero E6 cells cultured in T-25 flasks (Corning CellBIND). The cells were infected with viruses in complete DMEM without FBS, and after 2 h the medium was replaced with fresh complete DMEM with 2% FBS and cultured for a further 2 to 3 days until cell lysis. Cells were scraped, and the suspensions were collected and, after clarification at 1,204 × g for 15 min at 4°C, heat inactivated for 30 min (HSV-1) or 10 min (HSV-2) at 56°C. After centrifugation at 48,384 × g for 30 min at 4°C, virus pellets were resuspended. Cell pellets were freeze-thawed three times and then sonicated three times. After centrifugation at 1,734 × g for 15 min at 4°C to pellet cell debris, the supernatant was centrifuged at 48,384 × g for 30 min at 4°C and resuspended together with virus pellets. Aliquots were stored at −80°C, and their titers were equal (2 × 106 PFU/ml). They were used for overnight coating of 96-well microplates (Corning Costar) at 4°C. His-tagged soluble recombinant envelope glycoproteins were coated by following the protocol described above, with 100 ng/well in phosphate-buffered saline (PBS). Coating the control with 1% bovine serum albumin (BSA; Sigma-Aldrich) solution in PBS was added as a nonspecific reactivity control. The following day, the plate was blocked with a 1% BSA solution in PBS to prevent nonspecific binding to wells, and 5 μg/ml of MAbs or 1:200 dilutions of sera were incubated with antigens for 1 h at 37°C. After washing with 0.1% Tween 20 (Sigma-Aldrich) in PBS solution, serum IgG was detected with goat anti-human IgG (Fab specific)-peroxidase antibody (A0293; Sigma-Aldrich), with His-tagged proteins with anti-His6-peroxidase antibody (11922416001; Sigma-Aldrich) as a coating control and using the Pierce TMB substrate kit (ThermoFisher Scientific). The OD450 was measured using Multiskan GO (ThermoFisher Scientific).
Antibody titrations.
His-tagged soluble recombinant gD1 and gB1 glycoproteins were used for overnight coating of 96-well microplates at 4°C with 100 ng/well in PBS. Coating control and blocking were performed as described above. Serial dilutions of sera (from 1:800 to 1:102,400) were incubated with antigens for 1 h at 37°C. Standard curves were added to each plate using known concentrations of human monoclonal antibodies directed against gD1 and gB1. After washing with 0.1% Tween 20 in PBS solution, serum IgGs were detected with goat anti-human IgG (Fab specific)-peroxidase antibody and a Pierce TMB substrate kit, measuring the OD450. Titers of specific IgGs were calculated by interpolating from the sigmoidal standard curves obtained with human monoclonal antibodies (37).
Flow cytometry (FACS).
HEK 293T cells were transfected with the gD1 expression vector as described for His-tagged soluble proteins, and transgene expression was tested after 48 h. After centrifugation at 2,000 × g for 5 min, cells were fixed with 4% paraformaldehyde (PFA)–PBS and incubated for 30 min at room temperature with serial dilutions of sera (1:100 to 1:800) and/or IgG#33-labeled Alexa Fluor488 at a concentration that resulted in gating 30% of cells, a percentage that facilitated interaction with human sera. Untransfected cells were used as a negative control. Afterwards, the cells were washed and analyzed by Beckman Coulter's Gallios flow cytometer. The FACS data were analyzed using FCS Express 6 Plus software (De Novo Software).
Microneutralization assay (NT).
Different complemented serum concentrations (1:200 and 1:400) were incubated with 200 50% tissue culture infectious doses of virus for 1 h at 37°C, and virus-serum mixture was applied to Vero E6 monolayers. In competition experiments, IgG#33 was also added at its 50% inhibitory concentration (1.092 μg/ml against HSV-1 and 0.731 μg/ml against HSV-2). After 2 h, medium was replaced with complete DMEM with 2% FBS and plates were incubated for 21 h. Cells were fixed and stained with anti-HSV1+HSV2 gD murine antibody 2C10 (ab6507; Abcam) and goat anti-mouse IgG-fluorescein isothiocyanate antibody (F0257; Sigma-Aldrich). Hoechst 33258 (Sigma-Aldrich) was used for nuclear staining. An IN Cell analyzer system allowed high-throughput acquisition, and analysis was performed using IN Cell Investigator software.
PEI assay.
The postentry assay was adapted from previous studies (20, 38) as previously described (18, 19). Confluent monolayers of Vero E6 cells were infected with 100 PFU of virus. After 20 min of adsorption at 37°C, the cell-free virus was removed. Cells were then incubated for 46 h in DMEM containing 2% FBS and 0.5% agarose in the presence of complemented serum (1:200 dilution). In competition experiments, 25 μg/ml and 100 μg/ml of IgG#33 was also added against HSV-1 and -2 infections, respectively. Cells were fixed and stained as described for the plaque reduction assay. Viral plaques were counted in silico using ImageJ 1.50c4 software (U.S. NIH, Bethesda, MD; http://imagej.nih.gov/ij/).
MST.
For performing experiments with the soluble glycoprotein D, a fluorescent label (NT-647) was attached to the protein (Tris-NTA/His tag coupling). In the microscale thermophoresis (MST) experiment, the concentration of NT-647-labeled gD was kept constant, while the concentration of the nonlabeled IgG#33 was varied between 1 μM and 0.06 nM. The assay was performed in PBS with 0.05% Tween 20. After a short incubation, the samples were loaded into MST NT.115 premium glass capillaries and the MST analysis was performed using a Monolith NT.115. Concentrations on the x axis are plotted in nanomolars. A Kd (dissociation constant) of ≤500 pM was determined for this interaction.
Statistical analysis.
Wilcoxon matched-pairs signed rank test was used for statistical analysis of medians of binding analysis data, neutralization assay, and PEI infection assay. t test followed by Welch's correction was used for statistical analysis of data generated by PEI assay performed with purified sera. Two-way analysis of variance and Tukey’s multiple-comparison test were performed for the evaluation of serum interfering activity with IgG#33 against HSV-1 and -2 infection in NT and PEI assays. The relationship between numerical variables was evaluated by means of Spearman’s correlation coefficient. Exact P values were computed by permutation methods to avoid any asymptotic approximation or distributional assumption. P values then were adjusted for false discovery rate.
ACKNOWLEDGMENTS
We are grateful to Dan McAuley for revising the English in the manuscript.
Experiments employing the IN Cell Analyzer 1000 system, Leica confocal SP2 microscope, and Zeiss Axio Observer.Z1 microscope with QImaging Exi-Blue were carried out in ALEMBIC, an advanced microscopy laboratory facility established by the San Raffaele Scientific Institute and the Vita-Salute San Raffaele University.
This work was partially supported by Polichem SA, Lugano, Switzerland. R.B. and M.C. are listed as inventors on a patent application of human monoclonal antibodies against HSV-1 and -2 for the treatment of HSV diseases under the name of Polichem SA. The remaining authors, including the corresponding author, have no competing interests to declare.
REFERENCES
- 1.Schiffer JT. 2013. Mucosal HSV-2 specific CD8+ T-cells represent containment of prior viral shedding rather than a correlate of future protection. Front Immunol 4:209. doi: 10.3389/fimmu.2013.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. 2009. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol 83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awasthi S, Belshe RB, Friedman HM. 2014. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J Infect Dis 210:571–575. doi: 10.1093/infdis/jiu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petro C, González PA, Cheshenko N, Jandl T, Khajoueinejad N, Bénard A, Sengupta M, Herold BC, Jacobs WR. 2015. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife 4:6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corey L, Langenberg AGM, Ashley R, Sekulovich RE, Izu AE, Douglas JM Jr, Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. 1999. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: two randomized controlled trials. JAMA 282:331–340. [DOI] [PubMed] [Google Scholar]
- 6.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapelière P, Dubin G, GlaxoSmithKline Herpes Vaccine Efficacy Study Group. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 7.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD, Herpevac Trial for Women. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clementi N, Criscuolo E, Cappelletti F, Burioni R, Clementi M, Mancini N. 2016. Novel therapeutic investigational strategies to treat severe and disseminated HSV infections suggested by a deeper understanding of in vitro virus entry processes. Drug Discov Today 21:682–691. doi: 10.1016/j.drudis.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Clementi N, Cappelletti F, Criscuolo E, Castelli M, Mancini N, Burioni R, Clementi M. 2017. Role and potential therapeutic use of antibodies against herpetic infections. Clin Microbiol Infect 23:1–19. doi: 10.1016/j.cmi.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 10.Cairns TM, Huang Z-Y, Gallagher JR, Lin Y, Lou H, Whitbeck JC, Wald A, Cohen GH, Eisenberg RJ. 2015. Patient-specific neutralizing antibody responses to herpes simplex virus are attributed to epitopes on gD, gB, or both and can be type specific. J Virol 89:9213–9231. doi: 10.1128/JVI.01213-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng T, Ponce de Leon M, Jiang H, Dubin G, Lubinski JM, Eisenberg RJ, Cohen GH. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol 72:65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krawczyk A, Krauss J, Eis-Hübinger AM, Daumer MP, Schwarzenbacher R, Dittmer U, Schneweis KE, Jager D, Roggendorf M, Arndt M. 2011. Impact of valency of a glycoprotein B-specific monoclonal antibody on neutralization of herpes simplex virus. J Virol 85:1793–1803. doi: 10.1128/JVI.01924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairns TM, Huang Z-Y, Whitbeck JC, Ponce-de-Leon M, Lou H, Wald A, Krummenacher C, Eisenberg RJ, Cohen GH. 2014. Dissection of the antibody response against herpes simplex virus glycoproteins in naturally infected humans. J Virol 88:12612–12622. doi: 10.1128/JVI.01930-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cairns TM, Whitbeck JC, Lou H, Heldwein EE, Chowdary TK, Eisenberg RJ, Cohen GH. 2011. Capturing the herpes simplex virus core fusion complex (gB-gH/gL) in an acidic environment. J Virol 85:6175–6184. doi: 10.1128/JVI.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whitbeck JC, Huang Z-Y, Cairns TM, Gallagher JR, Lou H, Ponce-de-Leon M, Belshe RB, Eisenberg RJ, Cohen GH. 2014. Repertoire of epitopes recognized by serum IgG from humans vaccinated with herpes simplex virus 2 glycoprotein D. J Virol 88:7786–7795. doi: 10.1128/JVI.00544-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicola AV, Ponce de Leon M, Xu R, Hou W, Whitbeck JC, Krummenacher C, Montgomery RI, Spear PG, Eisenberg RJ, Cohen GH. 1998. Monoclonal antibodies to distinct sites on herpes simplex virus (HSV) glycoprotein D block HSV binding to HVEM. J Virol 72:3595–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cairns TM, Fontana J, Huang Z-Y, Whitbeck JC, Atanasiu D, Rao S, Shelly SS, Lou H, Ponce-de-Leon M, Steven AC, Eisenberg RJ, Cohen GH. 2014. Mechanism of neutralization of herpes simplex virus by antibodies directed at the fusion domain of glycoprotein B. J Virol 88:2677–2689. doi: 10.1128/JVI.03200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clementi N, Criscuolo E, Cappelletti F, Quaranta P, Pistello M, Diotti RA, Sautto GA, Tarr AW, Mailland F, Concas D, Burioni R, Clementi M, Mancini N. 2017. Entry inhibition of HSV-1 and -2 protects mice from viral lethal challenge. Antiviral Res 143:48–61. doi: 10.1016/j.antiviral.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Criscuolo E, Clementi N, Mancini N, Burioni R, Miduri M, Castelli M, Clementi M. 2018. Synergy evaluation of anti-herpes simplex virus type 1 and 2 compounds acting on different steps of virus life cycle. Antiviral Res 151:71–77. doi: 10.1016/j.antiviral.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Krawczyk A, Arndt MAE, Grosse-Hovest L, Weichert W, Giebel B, Dittmer U, Hengel H, Jager D, Schneweis KE, Eis-Hubinger AM, Roggendorf M, Krauss J. 2013. Overcoming drug-resistant herpes simplex virus (HSV) infection by a humanized antibody. Proc Natl Acad Sci U S A 110:6760–6765. doi: 10.1073/pnas.1220019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. 2007. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol 151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah DK, Betts AM. 2013. Antibody biodistribution coefficients: inferring tissue concentrations of monoclonal antibodies based on the plasma concentrations in several preclinical species and human. MAbs 5:297–305. doi: 10.4161/mabs.23684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, Suscovich TJ, Alter G. 2016. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog 12:e1005315. doi: 10.1371/journal.ppat.1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itell HL, Nelson CS, Martinez DR, Permar SR. 2017. Maternal immune correlates of protection against placental transmission of cytomegalovirus. Placenta 60:S73–S79. doi: 10.1016/j.placenta.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. 2013. Correlates of vaccine-induced protection: methods and implications. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 26.Rolfes MA, Gross FL, Flannery B, Meyers AFA, Luo M, Bastien N, Fowler RA, Katz JM, Levine MZ, Kumar A, Uyeki TM, CSIS and ROSII Study Groups. 2018. Kinetics of serological responses in critically ill patients hospitalized with 2009 pandemic influenza A(H1N1) virus infection in Canada, 2009-2011. J Infect Dis 217:1078–1088. doi: 10.1093/infdis/jiy013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muggeridge MI. 2000. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J Gen Virol 81:2017–2027. doi: 10.1099/0022-1317-81-8-2017. [DOI] [PubMed] [Google Scholar]
- 28.Bender FC, Samanta M, Heldwein EE, de Leon MP, Bilman E, Lou H, Whitbeck JC, Eisenberg RJ, Cohen GH. 2007. Antigenic and mutational analyses of herpes simplex virus glycoprotein B reveal four functional regions. J Virol 81:3827–3841. doi: 10.1128/JVI.02710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazear E, Whitbeck JC, Ponce-de-Leon M, Cairns TM, Willis SH, Zuo Y, Krummenacher C, Cohen GH, Eisenberg RJ. 2012. Antibody-induced conformational changes in herpes simplex virus glycoprotein gD reveal new targets for virus neutralization. J Virol 86:1563–1576. doi: 10.1128/JVI.06480-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goade DE, Bell R, Yamada T, Mertz GJ, Jenison S. 1996. Locations of herpes simplex virus type 2 glycoprotein B epitopes recognized by human serum immunoglobulin G antibodies. J Virol 70:2950–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z, Sinzger C, Reichel JJ, Just M, Mertens T. 2015. Natural killer cells can inhibit the transmission of human cytomegalovirus in cell culture by using mechanisms from innate and adaptive immune responses. J Virol 89:2906–2917. doi: 10.1128/JVI.03489-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacob CL, Lamorte L, Sepulveda E, Lorenz IC, Gauthier A, Franti M. 2013. Neutralizing antibodies are unable to inhibit direct viral cell-to-cell spread of human cytomegalovirus. Virology 444:140–147. doi: 10.1016/j.virol.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Castelli M, Cappelletti F, Diotti RA, Sautto G, Criscuolo E, Dal Peraro M, Clementi N. 2013. Peptide-based vaccinology: experimental and computational approaches to target hypervariable viruses through the fine characterization of protective epitopes recognized by monoclonal antibodies and the identification of T-cell-activating peptides. Clin Dev Immunol 2013:1–12. doi: 10.1155/2013/521231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicasio M, Sautto G, Clementi N, Diotti RA, Criscuolo E, Castelli M, Solforosi L, Clementi M, Burioni R. 2012. Neutralization interfering antibodies: a “novel” example of humoral immune dysfunction facilitating viral escape? Viruses 4:1731–1752. doi: 10.3390/v4091731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sautto GA, Wisskirchen K, Clementi N, Castelli M, Diotti RA, Graf J, Clementi M, Burioni R, Protzer U, Mancini N. 2016. Chimeric antigen receptor (CAR)-engineered T cells redirected against hepatitis C virus (HCV) E2 glycoprotein. Gut 65:512–523. doi: 10.1136/gutjnl-2014-308316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perotti M, Mancini N, Diotti RA, Tarr AW, Ball JK, Owsianka A, Adair R, Patel AH, Clementi M, Burioni R. 2008. Identification of a broadly cross-reacting and neutralizing human monoclonal antibody directed against the hepatitis C virus E2 protein. J Virol 82:1047–1052. doi: 10.1128/JVI.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lilleri D, Zelini P, Fornara C, Zavaglio F, Rampino T, Perez L, Gabanti E, Gerna G. 2018. Human cytomegalovirus (HCMV)-specific T cell but not neutralizing or IgG binding antibody responses to glycoprotein complexes gB, gHgLgO, and pUL128L correlate with protection against high HCMV viral load reactivation in solid-organ transplant recipients. J Med Virol 90:1620–1628. doi: 10.1002/jmv.25225. [DOI] [PubMed] [Google Scholar]
- 38.De Logu A, Williamson RA, Rozenshteyn R, Ramiro-Ibañez F, Simpson CD, Burton DR, Sanna PP. 1998. Characterization of a type-common human recombinant monoclonal antibody to herpes simplex virus with high therapeutic potential. J Clin Microbiol 36:3198–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]