HSV-2 is a ubiquitous important human pathogen that causes recurrent infections for the life of its host. We hypothesized that the autonomic ganglia have important roles in viral reactivation, and this study sought to determine whether this is correct in the clinically relevant guinea pig vaginal infection model. Our findings indicate that sympathetic ganglia are sources of reactivating virus, helping explain how the virus causes lifelong recurrent disease.
KEYWORDS: latency, neuron, parasympathetic, reactivation, sympathetic
ABSTRACT
Herpes simplex virus 2 (HSV-2) can be transmitted in the presence or absence of lesions, allowing efficient spread among the general population. Recurrent HSV genital lesions are thought to arise from reactivated latent virus in sensory cell bodies of the dorsal root ganglia (DRG). However, HSV-2 has also been found latent in autonomic ganglia. Spontaneous reactivation or a low level of chronic infection could theoretically also occur in these peripheral nervous tissues, contributing to the presence of infectious virus in the periphery and to viral transmission. Use of a recently described, optimized virus with a monomeric mNeonGreen protein fused to viral capsid protein 26 (VP26) permitted detection of reactivating virus in explanted ganglia and cryosections of DRG and the sacral sympathetic ganglia (SSG) from latently infected guinea pigs. Immediate early, early, and late gene expression were quantified by droplet digital reverse transcription-PCR (ddRT-PCR), providing further evidence of viral reactivation not only in the expected DRG but also in the sympathetic SSG. These findings indicate that viral reactivation from autonomic ganglia is a feature of latent viral infection and that these reactivations likely contribute to viral pathogenesis.
IMPORTANCE HSV-2 is a ubiquitous important human pathogen that causes recurrent infections for the life of its host. We hypothesized that the autonomic ganglia have important roles in viral reactivation, and this study sought to determine whether this is correct in the clinically relevant guinea pig vaginal infection model. Our findings indicate that sympathetic ganglia are sources of reactivating virus, helping explain how the virus causes lifelong recurrent disease.
INTRODUCTION
Herpes simplex virus 2 (HSV-2) is globally pervasive, with approximately 417 million people aged 15 to 49 years old infected—an estimated 11.3% global prevalence (1) with a reported U.S. prevalence of 15.7% (2). Episode severity ranges from brief and asymptomatic, the most prevalent means of HSV-2 transmission, to painful genital lesions (3, 4). Severe acute genital herpes may be associated with sensory deficits, urinary retention, constipation, and erectile dysfunction (5, 6). Up to 15% of women report urinary retention during HSV-2 infection, but the mechanism remains unclear (7). While primary infections are more often symptomatic, recurrences are frequently asymptomatic, facilitating transmission of virus even when no lesions are present (3, 4). In addition, HSV-2 infection during pregnancy can have devastating effects, including spontaneous abortion, low birth weight, premature delivery, and infection of the neonate. In neonates, localized infection, dissemination, and encephalitis can result in severe disability or death. Approximately 70% of mothers are asymptomatic at the time of birth, when neonatal herpes is typically transmitted (8, 9).
After the initial infection of epidermal and mucosal keratinocytes within the reproductive tract, the virus travels retrograde from the axon terminal to the soma of the sensory neuron. The virus forms an episome within the neuronal cell body’s nucleus, persisting for the lifetime of the host in latency, evading detection and immunity, with episodic reactivation to cause lesions or asymptomatic shedding. Upon reactivation, the virus travels anterograde, exiting the axons to replicate in the periphery. There is ample evidence that the virus also infects and establishes latency in peripheral autonomic ganglia, potentially giving rise to autonomic symptoms during acute infection (10–16), and HSV-1 can be induced to reactivate from autonomic ganglia by neurectomy (17), but spontaneous reactivation via autonomic pathways has not yet been demonstrated.
It is known that HSV-2 enters the axon termini of sensory neurons whose somata congregate in the coccygeal and sacral plexus of the dorsal root ganglion (DRG). These neurons innervate a specific dermatome of the genital skin and buttocks associated with sites of viral reactivation-induced lesions, but sensory neurons are not the only neurons that innervate the reproductive tract or this specific dermatome. Autonomic sympathetic and parasympathetic termini represent alternative neuronal pathways for the virus. The parasympathetic preganglionic neurons that innervate the genitourinary tract arise from the sacral region of the spinal cord (SC) (S2-S4), the same spinal level as the DRGs in which HSV-2 establishes latency during genital infection. Similarly, the sympathetic ganglia have highly branched axons that innervate the genitourinary tract, as well as the dermis of the skin, providing potential routes for anterograde and retrograde transport of virus to and from the peripheral tissues.
After infection of rabbit eyes with HSV-1, reactivating HSV was detected both in tears and in saliva, suggesting more loci of reactivation than could be explained by reactivation within a single division of the sensory trigeminal ganglion (18). Recent human studies suggest that asymptomatic reactivation, contributing to viral shedding in the reproductive tract, is not rare. Analyses of human biopsy specimens revealed multiple sites of reactivation, widely distributed over the genital tract and deep within the dermis in the absence of obvious peripheral lesions (19). The large number of concurrent noncontiguous reactivation sites suggests that these recurrences may be derived from a neuron that innervates the deep dermis at multiple peripheral locations, such as those located in peripheral autonomic ganglia (20). Thus, reactivation within peripheral autonomic ganglia could explain some important aspects of viral pathogenesis.
While humans are the only natural host for HSV-2, mice, guinea pigs, and rabbits can be infected and used for study of reactivation in vivo. In the murine vaginal infection model, HSV-2 can spread not only to the dorsal root sensory ganglia (DRG), but to the autonomic parasympathetic neurons of the major pelvic ganglia (MPG) and the sympathetic chain (12). HSV-1 has also been reported in enteric neurons following vaginal infection of mice, resulting in “toxic megacolon,” a neutrophil-mediated inflammatory response that led to mortality. Homogenates of parasympathetic enteric neurons and sensory DRG, but not of the sympathetic chain, contained infectious virus in these studies (11). However, the role of the sympathetic chain could not be established in these experiments, since HSV does not spontaneously reactivate to cause lesions in latently infected mice. In contrast, HSV-2 does reactivate spontaneously to cause lesions in vaginally infected female guinea pigs, more closely modeling human disease and allowing study of both acute infection and viral recurrence. During acute infection of guinea pigs, typical human autonomic symptoms, such as urinary retention, may also be observed. Rapid but independent spread of acutely infecting virus both to DRG and spinal cord (SC) suggests that the virus may spread to the SC via nonsensory (e.g., autonomic) neuronal pathways (15). In guinea pigs with latent HSV-2 ocular infection, immediate early and early genes were detected in sympathetic superior cervical ganglia, while only immediate early genes were found in parasympathetic ciliary ganglia. Furthermore, HSV-2 reactivated from in vitro-infected, cultured primary adult sympathetic superior cervical and parasympathetic ciliary neurons as well as from sensory trigeminal neurons (21), indicating that HSV-2 is capable of reactivating in autonomic ganglia.
Using the guinea pig as a spontaneous reactivation model with a recently described and optimized fluorescing virus, we investigated the ability of HSV-2 to reactivate from autonomic ganglia after genital infection. We used viral fluorescence in neurons during latency to detect likely viral reactivation, which we confirmed by also detecting viral glycoproteins via immunostaining. We also quantified expression of viral genes in autonomic and sensory ganglia to assess potential viral reactivation from these locations.
RESULTS
Autonomic and sensory ganglia harbor reactivation-competent HSV-2.
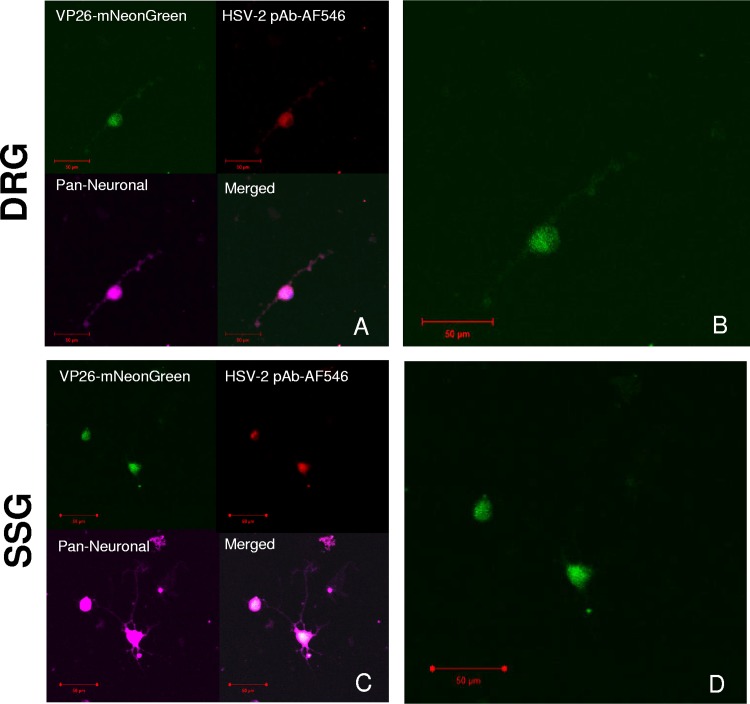
To detect reactivation-competent latent virus in the dorsal root ganglia (DRG) and the sacral sympathetic ganglion (SSG), we infected guinea pigs intravaginally with “Nedel,” a fluorescent mNeonGreen minor capsid fusion (VP26) HSV-2 recombinant virus that expresses the VP26-mNeonGreen fusion protein only during productive infection (22). Nedel replicates with wild-type kinetics and reactivates from latency in vivo. We then explanted ganglia from the animals 36 days later, during the latent phase of infection. Ganglia were enzymatically and mechanically dissociated for plating into chamber slides. In this model, axotomy during dissection stimulates reactivation, while the nutrient-rich medium allows for the regrowth of axons (23, 24). Fluorescence became visible at 48 h with maximum intensity materializing 72 h after plating. Fluorescence emerged from the expected sensory DRG (Fig. 1A and B) and from the sympathetic SSG (Fig. 1C and D). The explanted ganglia at 72 h were fragile, and many did not remain adherent to the slide following triple labeling and intermittent washes, preventing quantification of reactivation in these experiments. The fluorescent green capsid protein (VP26-mNeonGreen) colocalized with the red fluorescent HSV-2 polyclonal antibody (pAb) (AF546) within magenta-labeled neurons (pan-neuronal stain) from the DRG and SSG. Thus, autonomic ganglia that innervate the genital tract harbor reactivation-competent latent HSV, as do the DRGs.
FIG 1.
HSV-2 reactivates from both sensory DRG and sympathetic SSG. (A) Representative images of in vivo Nedel-infected, dissociated, and explanted sensory dorsal root ganglia (DRG). (Top left) VP26-mNeonGreen expressed from Nedel; (top right) HSV-2 pAb AF546 against HSV-2 gH/gL; (bottom left) pan-neuronal stain; (bottom right) merge. (B) Expanded image from panel A showing mNeonGreen expressed from Nedel in DRG neuron. (C) Sacral sympathetic ganglia (SSG). (Top left) VP26-mNeonGreen expressed from Nedel; (top right) HSV2 pAb AF546 against HSV-2 gH/gL; (bottom left) pan-neuronal stain; (bottom right) merge. (D) Expanded image from panel C showing mNeonGreen expressed from Nedel in SSG neurons.
HSV-2 reactivation occurs in vivo in both sensory and autonomic neurons.
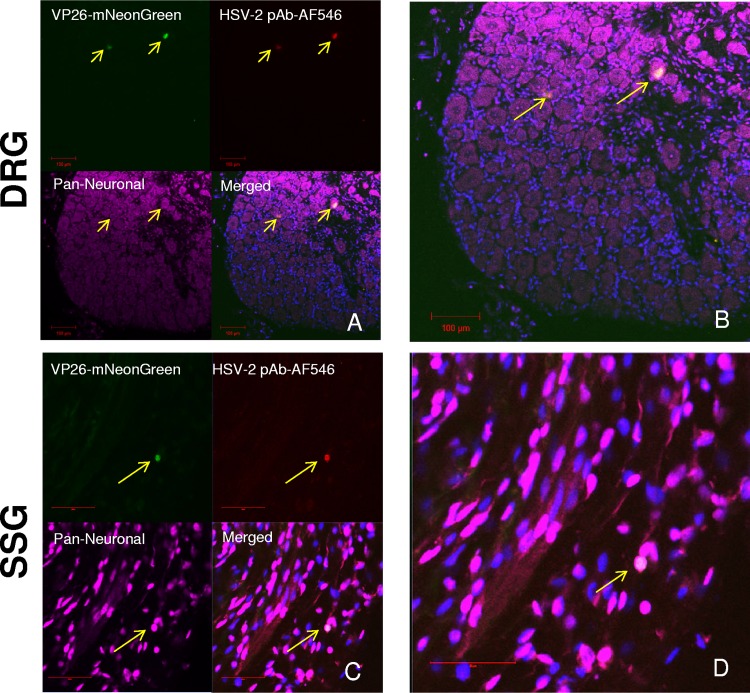
To determine whether virus in autonomic ganglia is reactivation competent in vivo, latently infected guinea pigs were deeply anaesthetized and underwent cardiac perfusion with paraformaldehyde 36 days postinfection, followed by fluorescent immunolabeling of cryosections from the DRG and SSG. The cardiac perfusion ensured that euthanasia would not stimulate reactivation postmortem and that samples obtained would accurately reflect what was occurring in vivo. Green fluorescence was observed in neurons in the DRG (Fig. 2A and B) and SSG (Fig. 2C and D), indicating the presence of fluorescent capsid protein expressed from Nedel. Since this productive infection occurred in animals 36 days after infection and Nedel expresses mNeonGreen only during productive infection, the observed green fluorescence is presumed to be due to reactivation of the latent virus. The colocalization of capsid fluorescence and HSV-2 antigen detected by immunostaining was similar throughout the DRG. In some small neurons of the SSG, the capsid’s fluorescent signal colocalized with anti-HSV-2 glycoprotein, confirming the presence of virus. As expected, in vivo reactivation was too rare to draw statistically evaluable conclusions. Nonetheless, with 3 reactivating neurons found per 1,200 counted neurons in both the DRG and SSG, this observation was consistent with similar rates of in vivo viral reactivation in sensory ganglia versus autonomic ganglia.
FIG 2.
In vivo reactivation. Confocal images of immunolabeled, Nedel-infected frozen tissue sections. (A) DRG. (Top left) Detection of mNeonGreen fluorescence; (top right) immunofluorescence using pAb HSV-2-AF546; (bottom left) pan-neuronal stain; (bottom right) merged. Yellow arrows point to cells exhibiting fluorescent capsid and anti-HSV-2 pAb. (B) Expanded merged DRG from panel A. (C) SSG. (D) Expanded merged SSG from panel C. Bar (in panels C and D) = 50 μm.
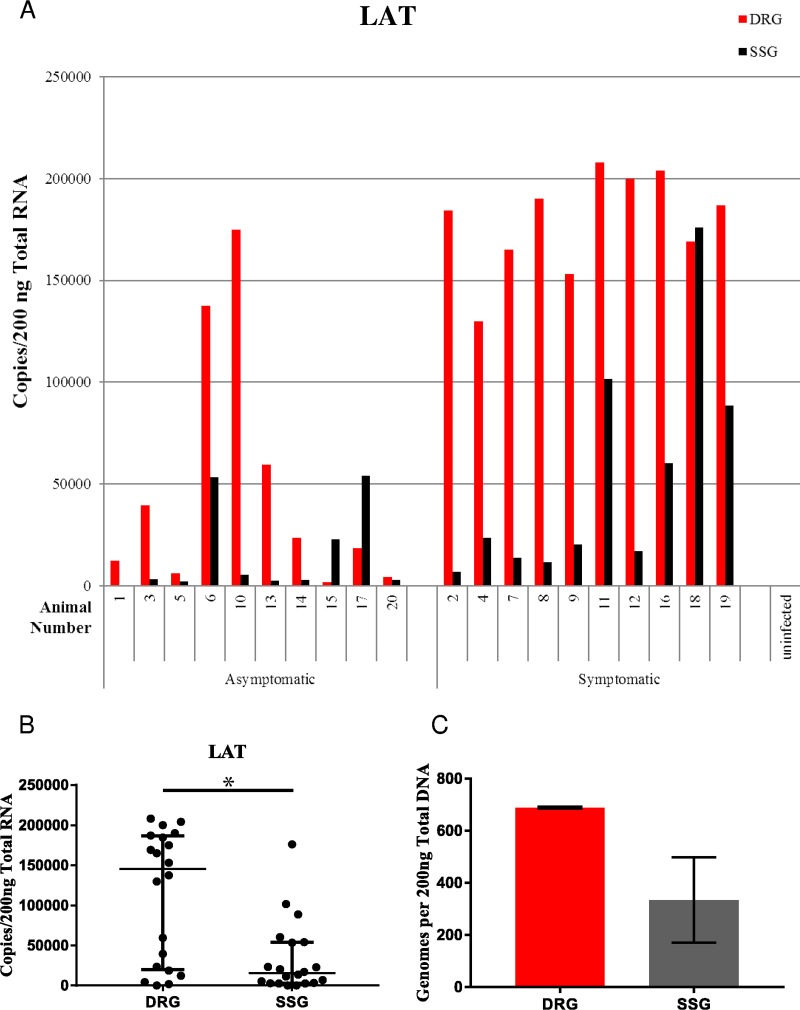
To further evaluate our observations of capsid protein and viral glycoprotein production in the labeled cryosections, HSV-2 viral RNA was quantified in 20 additional latently infected guinea pigs by droplet digital reverse transcription-PCR (ddRT-PCR). We evaluated the expression of the latency-associated transcript (LAT), which is the only transcript abundantly expressed during latency. LAT was detected in all 20 infected animals in both the DRG and SSG, providing further evidence that latency can be established and maintained in the autonomic ganglia. The finding of more LAT in symptomatic versus asymptomatic animals in both the DRG and SSG (Fig. 3A) may be because animals harboring more latent virus (as suggested by more LAT) also had a greater likelihood of symptomatic reactivation. Significantly more LAT expression was detected in the DRG than in the SSG, suggesting the presence of more gene expression-competent latent virus in the DRG (Fig. 3A and B, Mann-Whitney P = 0.0032). Consistent with this interpretation, in a separate experiment, greater concentrations of viral DNA were also observed in the DRG versus the SSG (Fig. 3C) (689 and 335 genomes, respectively; P = 0.163; t test; n = 6 each group). These experiments do not rule out a contribution to LAT expression of reactivating virus, though the relative infrequency of reactivation relative to latency in ganglia suggests that most LAT in ganglia taken from animals undergoing spontaneous reactivation is expressed from latent virus.
FIG 3.
LAT gene expression per 200 ng total RNA from the DRG and SSG per individual animal (A) and aggregate LAT gene expression from the DRG and SSG (B). In panel B, median values with interquartile ranges (error bars) (Mann-Whitney, P = 0.0032, n = 20 per group) are shown. (C) Genomes per 200 ng of total DNA. Column heights represent the mean values for six animals (689 and 335, respectively). Error bars represent the standard errors of the means.
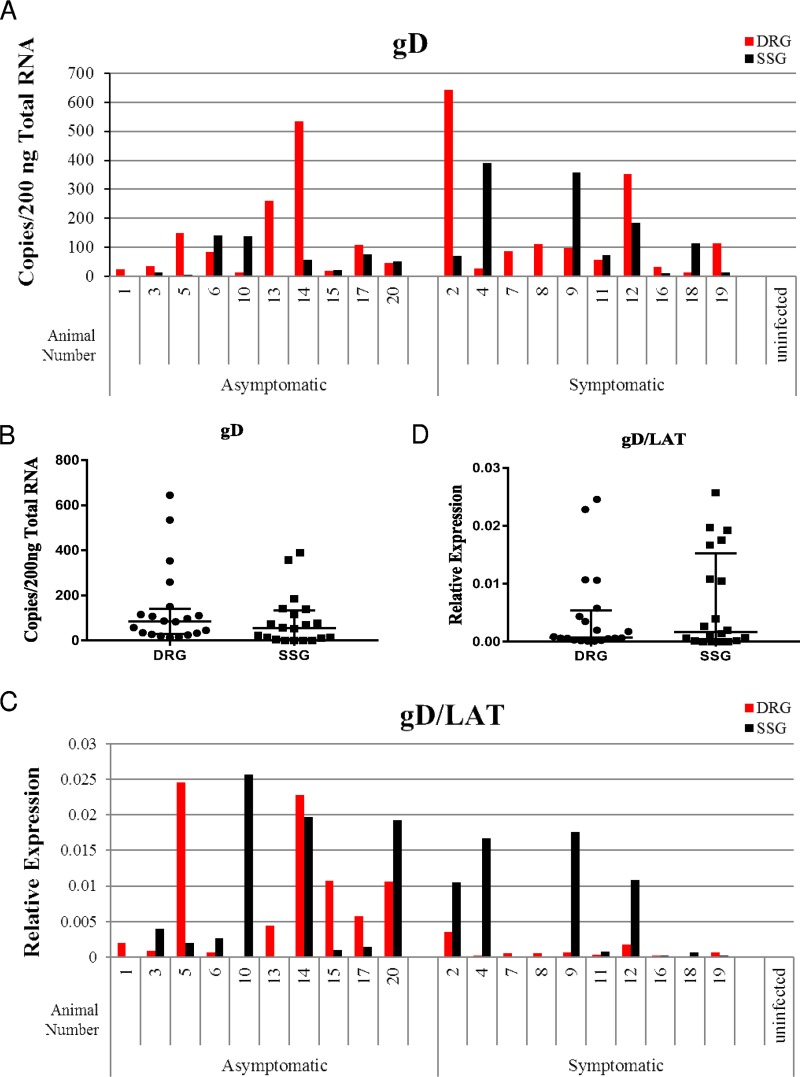
Expression of glycoprotein D, a late gene, is likely to represent ongoing or recent reactivation. While gD levels were not correlated with the presence of symptomatic recurrence (suggesting that gD expression in ganglia might sometimes either lead or trail peripheral observations), relatively high levels of gD were observed both in the DRG (in 20/20 animals) and SSG (in 16/20 animals) (Fig. 4A). Greater quantities of gD expression were detected in the DRG compared to the SSG in 12/20 animals. However, 7/20 animals exhibited greater quantities of gD transcripts in the SSG than in the DRG. When examined across all animals, transcript levels of gD were not statistically different between the DRG and SSG (Fig. 4B, P = 0.1222). Identification of gD RNA in both of these locations is consistent with the qualitative findings in fluorescently labeled cryosections, which also indicate that in vivo reactivations can occur in both of these ganglia. Normalization of gD transcript levels to LAT expression levels (Fig. 4C and D) revealed that on a LAT-adjusted basis, gD levels were higher in the SSG of 10 animals and in the DRG of 8 animals, suggesting that if LAT expression levels are proportional to the quantity of latent virus, there is a similar propensity for latent virus to reactivate in each location, consistent with the microscopic observations (Mann-Whitney test, P = 0.7533).
FIG 4.
(A and B) Late gene gD expression per individual animal (A) and aggregate expression from the DRG and SSG (B). In panel B, median values with interquartile ranges (error bars) (n = 20, Mann-Whitney, P = 0.1222) are shown. (C and D) Relative gD gene expression normalized to LAT per individual animal (C) and shown as aggregate expression from the DRG and SSG (D) (n = 20, Mann-Whitney, P = 0.7533).
DISCUSSION
We used three different approaches to investigate the ability of HSV-2 to reactivate from autonomic ganglia, and we consistently found that the virus is latent and reactivation competent in both DRG and SSG. Given previous experiments that revealed LAT and viral DNA in the autonomic ganglia, viral reactivation in ganglia after an axotomy-induced neural stress signal is not unexpected (4, 15, 22). However, this is the first description of HSV-2 reactivation from latently infected autonomic ganglia in a clinically relevant vaginal model of infection, in which virus reactivates spontaneously to cause recurrent disease.
Use of the guinea pig model, in which spontaneous reactivation gives rise to peripheral recurrent lesions, is a strength of our study. Using measures of capsid expression sufficient to give rise to substantial fluorescence, combined with expression of viral glycoproteins in the same cell, along with expression of gD RNA, we did not observe substantial differences in the propensity of virus to reactivate in the autonomic SSG, compared with the DRG, which is a known source of reactivating virus.
The ability of HSV-2 to reactivate from autonomic ganglia has important implications for our understanding of viral pathogenesis. Autonomic reactivation leading to shed virus in the reproductive tract may explain both the relatively high frequency and the relatively diffuse locality of subclinical reactivations observed in otherwise asymptomatic humans, thus contributing to HSV-2’s spread among the general population (25). Viral shedding from autonomic ganglia, which may lead to recruitment of deep, dermal, resident T cells that are susceptible to HIV infection (26) also could help explain the association of HSV-2 infection with the increased risk of HIV infection. If, as has been hypothesized (26), widespread autonomic reactivation recruits deep, dermal, resident T cells that are susceptible to HIV infection, this might further explain how HSV predisposes to HIV acquisition.
The presence of HSV latency in distinct arms (somatic versus autonomic) of the peripheral nervous system suggests the possibility that stimuli for reactivation from different types of neurons may vary. Clinical interventions specifically targeted to subtypes of neurons might thus influence reactivation rates. Animal models that use pain, heat, or UV light to stimulate reactivation most likely target sensory neurons that respond to these stimuli. Therefore, those models may not reflect reactivation from autonomic neurons, which are more sensitive to hormonal fluctuation than sensory neurons. Increased hormone levels, which have also been found to impact HSV reactivation (27), seem more likely to specifically target autonomic neurons, as is also likely the case for iontophoresis of epinephrine, which has been used as a reactivation stimulus in in vivo ocular models. Ex vivo and in vitro models that are focused on sympathetic neurons likely have specific relevance to clinically important reactivation from autonomic neurons (27, 28), since different stimuli induce reactivation in sensory neurons (29).
The knowledge that HSV-2 is latent and reactivation competent in autonomic ganglia also has practical implications for preclinical study of vaccines and interventions against recurrent genital herpes. To the extent that viral latency is evaluated only in dissected dorsal root ganglia, it is possible that important sites of latency could be missed. For example, the emergence of the fairly diffuse autonomic ganglia as an important reservoir of reactivating virus suggests that gene therapy directed only at the DRG is unlikely to be successful in eradicating all latent or reactivating virus (30). Similarly, antivirals designed to specifically target reactivation mechanisms in sensory neurons may not be effective in autonomic neurons.
Previous reports of autonomic neuron involvement following HSV infections drew various conclusions. Differences in tissue dissections, animal models, and identification of neuronal tissue could potentially account for those discrepancies (11, 12, 21). In the acute murine vaginal model, HSV-2 was found in sympathetic neurons (12), while in a different experiment in the same model, HSV-1 gene expression and infectious virus from homogenate was detected in only parasympathetic neurons (11). In the guinea pig model, both HSV-1 and HSV-2 DNA and viral gene expression were detected in sympathetic and parasympathetic ganglia during latency after ocular infection (21). These differences in observations could be explained by differences in the animal models, the tropism between HSV-1 and HSV-2, the quantity of virus used to infect, or the method’s sensitivity for detection of reactivating virus. Since the sacral sympathetic ganglia innervate the skin and reproductive tract in small-animal models, this is the autonomic site that is most likely infected during genital infection (31, 32). However, our study is the first to specifically assess latent and recurrent HSV-2 infection in these ganglia. The ability of HSV-2 to reactivate from the SSG suggests that other autonomic ganglia may also be sources of viral reactivation. Future studies could help to define the relative roles of sensory, parasympathetic, and sympathetic reactivation in recurrent human disease and the relative importance of their roles in other aspects of HSV pathogenesis.
MATERIALS AND METHODS
Viral strains and stock production.
HSV-2 strain 333 was originally obtained from Gary Hayward (Johns Hopkins University, Baltimore, MD). mNeonGreen was obtained from Allele Biotech (San Diego, CA), and the recombinant fluorescent virus Nedel has recently been described (22). Nedel expresses mNeonGreen as a fusion protein with VP26 during productive infection. The recombinant and parental strain virus were propagated in Vero cells (ATCC CCL-81).
Animals.
Female Hartley guinea pigs (5 used for explant, 5 used for cryosections, 20 used for ddRT-PCR; 125 to 150 g; Charles River Breeding Laboratories, Wilmington, MA) were intravaginally inoculated with 1 × 106 PFU HSV-2 strain 333 or recombinant virus Nedel, as previously described (33).
Ex vivo explant reactivation.
Dorsal root ganglia and sacral sympathetic ganglia were harvested and cultured as previously described (34). Ganglia were enzymatically dissociated in collagenase, papain, and dispase (Worthington) before mechanically triturating and plating on Matrigel-coated eight-well Lab-Tek II chamber slides (Thermo Fisher Scientific). Cultures were then fixed for 5 min in 4% paraformaldehyde, gently rinsed in phosphate-buffered saline (PBS) and immunolabeled. Immunolabeled neuronal cultures were evaluated by using a Zeiss LSM 710 upright confocal laser scanning microscope.
In vivo. (i) Cryosectioning.
Under anesthesia, animals were cardiac perfused with PBS followed by cardiac perfusion with 4% paraformaldehyde. Dorsal root ganglia and the sacral sympathetic chains were dissected, rinsed in PBS, and sucrose protected overnight. Tissues were embedded in OCT (TissueTek), and 10-μm sections were made with a cryostat (Leica). Sections were permeabilized, blocked, and immunolabeled before viewing with a Zeiss LSM 710 upright confocal laser scanning microscope.
(ii) Immunolabeling of explanted ganglia and cryosections.
The antibodies used included the following: NeuroTrace 640/660 deep-red or 594 red fluorescent Nissl stain (catalog no. N21483; Thermo Fisher Scientific), mouse anti-HSV-2 gH/gL (catalog no. H2A269-100; Virusys, Taneytown, MD), mouse anti-HSV gB (catalog no. HA056-100; Virusys), mouse anti-HSV gD (HA025-100; Virusys), sheep anti-rat choline acetyltransferase 1:500 (catalog no. OSC00041W; Invitrogen), goat anti-mouse IgG (H+L) highly cross-adsorbed secondary antibody, Alexa Fluor 546 (catalog no. A-11030; Thermo Fisher Scientific), donkey anti-sheep IgG H&L (Alexa Fluor 594) (ab150180; Thermo Fisher Scientific). After permeabilization and blocking in blocking/permeabilizing buffer (5% serum [goat or donkey], 0.1% bovine serum albumin [BSA], 0.01% Triton X-100) for 1 h at room temperature (RT), samples were labeled with the primary antibody for 24 h at 4°C, secondary antibody for 24 h at 4°C, labeled with Nissl stain for 2 h at RT before 4′,6′-diamidino-2-phenylindole (DAPI) staining. Cryosections also were treated with TrueBlack for 30 s.
Droplet digital RT-PCR of HSV-2 genes in ganglia.
Tissue was harvested from CO2-euthanized animals during the latent phase of infection and homogenized using the Precellys 24 homogenizer and CKMix beads (ceramic zirconium oxide mix beads of 1.4 mm and 2.8 mm in 2-ml standard tubes). All animals were studied at least 21 days after vaginal infection. Animals denoted as having symptomatic recurrent lesions were euthanized on the first day of symptoms (after at least a 3-day symptom-free period), while asymptomatic animals were lesion-free for at least 3 days prior to euthanasia. RNA was extracted in TRISure per the manufacturer’s instructions (Bioline, London, UK). Previously described primers and probes were used (35) for gD and LAT. Bio-Rad’s QX100 system was used to generate and read droplets. ddRT-PCR conditions were as follows, all single cycle except as otherwise noted: 60 min at 50°C; 10 min at 95.5°C; 40 cycles with each cycle consisting of 30 s at 95°C and 1 min at 60°C; and 10 min at 98°C. Statistics were performed using GraphPad Prism version 7.0 for Windows (GraphPad Software, La Jolla, CA).
qPCR of HSV-2 genomes in ganglia.
Tissue was harvested from six CO2-euthanized animals during the latent phase of infection (77 days postinfection) and homogenized using the Precellys 24 homogenizer and CKMix beads (ceramic zirconium oxide mix beads of 1.4 mm and 2.8 mm in 2-ml standard tubes) in RLT lysis buffer (Qiagen). DNA was isolated manually with the AllPrep DNA/RNA minikit (Qiagen). Previously described primers and probes were used (35) for LAT using the following thermal conditions: 2 min at 50°C; 10 min at 95°C; 40 cycles with each cycle consisting of 15 s at 95°C and 1 min at 60°C. Genomes were quantified based on standards created by serial dilutions of known quantities of HSV-2 DNA. Results reflect the means from two independent quantitative PCRs (qPCRs).
ACKNOWLEDGMENTS
This work would not have been possible without the assistance of the veterinarians and veterinary technicians at the FDA White Oak Vivarium, especially Jill Ascher, Jessica Dewar, Xiaohong Li, and Moya Getrouw. We also thank Kaz Takeda for confocal microscopy guidance, Steve Rubin and Derek Ireland for instruction and use of the cryostats, and Allen C. Myers for in-lab explanations of ganglia anatomy.
J.R.P. and P.R.K. were funded by the FDA intramural research program. A.S.B. was funded by NIH grants K22 AI097299 and R01 NS104351.
Philip Krause and Julianna Pieknik conceived and designed the experiments. Julianna Pieknik and Andrea S. Bertke performed the ganglia explantation jointly. Julianna Pieknik performed the dissections, cryosectioning, immunohistochemical staining, microscopy, ddRT-PCR, and analyzed the data.
REFERENCES
- 1.Looker KJ, Magaret AS, Turner KME, Vickerman P, Gottlieb SL, Newman LM. 2015. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One 10:e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley H, Markowitz LE, Gibson T, McQuillan GM. 2014. Seroprevalence of herpes simplex virus types 1 and 2–United States, 1999-2010. J Infect Dis 209:325–333. doi: 10.1093/infdis/jit458. [DOI] [PubMed] [Google Scholar]
- 3.Mark KE, Wald A, Magaret AS, Selke S, Olin L, Huang M-L, Corey L. 2008. Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 198:1141–1149. doi: 10.1086/591913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiffer JT, Corey L. 2013. Rapid host immune response and viral dynamics in herpes simplex virus-2 infection. Nat Med 19:280–290. doi: 10.1038/nm.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caplan LR, Kleeman FJ, Berg S. 1977. Urinary retention probably secondary to herpes genitalis. N Engl J Med 297:920–921. doi: 10.1056/NEJM197710272971708. [DOI] [PubMed] [Google Scholar]
- 6.Goodell SE, Quinn TC, Mkrtichian E, Schuffler MD, Holmes KK, Corey L. 1983. Herpes simplex virus proctitis in homosexual men. N Engl J Med 308:868–871. doi: 10.1056/NEJM198304143081503. [DOI] [PubMed] [Google Scholar]
- 7.Whitley RJ, Kimberlin DW, Roizman B. 1998. Herpes simplex viruses. Clin Infect Dis 26:541–555. doi: 10.1086/514600. [DOI] [PubMed] [Google Scholar]
- 8.Kesson AM. 2001. Management of neonatal herpes simplex virus infection. Paediatr Drugs 3:81–90. doi: 10.2165/00128072-200103020-00001. [DOI] [PubMed] [Google Scholar]
- 9.Berger JR, Houff S. 2008. Neurological complications of herpes simplex virus type 2 infection. Arch Neurol 65:596–600. doi: 10.1001/archneur.65.5.596. [DOI] [PubMed] [Google Scholar]
- 10.Rodahl E, Stevens JG. 1992. Differential accumulation of herpes simplex virus type 1 latency-associated transcripts in sensory and autonomic ganglia. Virology 189:385–388. doi: 10.1016/0042-6822(92)90721-Z. [DOI] [PubMed] [Google Scholar]
- 11.Khoury-Hanold W, Yordy B, Kong P, Kong Y, Ge W, Szigeti-Buck K, Ralevski A, Horvath TL, Iwasaki A. 2016. Viral spread to enteric neurons links genital HSV-1 infection to toxic megacolon and lethality. Cell Host Microbe 19:788–799. doi: 10.1016/j.chom.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parr MB, Parr EL. 2003. Intravaginal administration of herpes simplex virus type 2 to mice leads to infection of several neural and extraneural sites. J Neurovirol 9:594–602. doi: 10.1080/jnv.9.6.594.602. [DOI] [PubMed] [Google Scholar]
- 13.Price RW. 1977. Viral infections of the autonomic nervous system and its target organs: pathogenetic mechanisms. Med Hypotheses 3:33–36. doi: 10.1016/0306-9877(77)90049-4. [DOI] [PubMed] [Google Scholar]
- 14.Sanjuan NA, Lascano EF. 1986. Autonomic nervous system involvement in experimental genital infection by herpes simplex virus type 2. Arch Virol 91:329–339. doi: 10.1007/BF01314291. [DOI] [PubMed] [Google Scholar]
- 15.Ohashi M, Bertke AS, Patel A, Krause PR. 2011. Spread of herpes simplex virus to the spinal cord is independent of spread to dorsal root ganglia. J Virol 85:3030–3032. doi: 10.1128/JVI.02426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labetoulle M, Maillet S, Efstathiou S, Dezelee S, Frau E, Lafay F. 2003. HSV1 latency sites after inoculation in the lip: assessment of their localization and connections to the eye. Invest Ophthalmol Vis Sci 44:217–225. doi: 10.1167/iovs.02-0464. [DOI] [PubMed] [Google Scholar]
- 17.Price RW, Schmitz J. 1978. Reactivation of latent herpes simplex virus infection of the autonomic nervous system by postganglionic neurectomy. Infect Immun 19:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill JM, Nolan NM, McFerrin HE, Clement C, Foster TP, Halford WP, Kousoulas KG, Lukiw WJ, Thompson HW, Stern EM, Bhattacharjee PS. 2012. HSV-1 latent rabbits shed viral DNA into their saliva. Virol J 9:221. doi: 10.1186/1743-422X-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnston C, Zhu J, Jing L, Laing KJ, McClurkan CM, Klock A, Diem K, Jin L, Stanaway J, Tronstein E, Kwok WW, Huang ML, Selke S, Fong Y, Magaret A, Koelle DM, Wald A, Corey L. 2014. Virologic and immunologic evidence of multifocal genital herpes simplex virus 2 infection. J Virol 88:4921–4931. doi: 10.1128/JVI.03285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulais N, Misery L. 2008. The epidermis: a sensory tissue. Eur J Dermatol 18:119–127. doi: 10.1684/ejd.2008.0348. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Ives AM, Bertke AS. 2015. Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J Virol 89:8383–8391. doi: 10.1128/JVI.00468-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pieknik JR, Bertke AS, Tang S, Krause PR. 2018. A VP26-mNeonGreen capsid fusion HSV-2 mutant reactivates from viral latency in the guinea pig genital model with normal kinetics. Viruses 10:E246. doi: 10.3390/v10050246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawtell NM, Thompson RL. 2004. Comparison of herpes simplex virus reactivation in ganglia in vivo and in explants demonstrates quantitative and qualitative differences. J Virol 78:7784–7794. doi: 10.1128/JVI.78.14.7784-7794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiner I, Spivack JG, Deshmane SL, Ace CI, Preston CM, Fraser NW. 1990. A herpes simplex virus type 1 mutant containing a nontransinducing Vmw65 protein establishes latent infection in vivo in the absence of viral replication and reactivates efficiently from explanted trigeminal ganglia. J Virol 64:1630–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tronstein E, Johnston C, Huang ML, Selke S, Magaret A, Warren T, Corey L, Wald A. 2011. Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 305:1441–1449. doi: 10.1001/jama.2011.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. 2009. Persistence of HIV-1 receptor-positive cells after HSV-2 reactivation is a potential mechanism for increased HIV-1 acquisition. Nat Med 15:886–892. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ives AM, Bertke AS. 2017. Stress hormones epinephrine and corticosterone selectively modulate herpes simplex virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J Virol 91:e00582-17. doi: 10.1128/JVI.00582-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertke AS, Ma A, Margolis MS, Margolis TP. 2013. Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons. J Virol 87:6512–6516. doi: 10.1128/JVI.00383-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanez A, Harrell T, Sriranganathan H, Ives A, Bertke A. 2017. Neurotrophic factors NGF, GDNF and NTN selectively modulate HSV1 and HSV2 lytic infection and reactivation in primary adult sensory and autonomic neurons. Pathogens 6:5. doi: 10.3390/pathogens6010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stone D, Niyonzima N, Jerome KR. 2016. Genome editing and the next generation of antiviral therapy. Hum Genet 135:1071–1082. doi: 10.1007/s00439-016-1686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindh B, Lundberg JM, Hokfelt T. 1989. NPY-, galanin-, VIP/PHI-, CGRP- and substance P-immunoreactive neuronal subpopulations in cat autonomic and sensory ganglia and their projections. Cell Tissue Res 256:259–273. [DOI] [PubMed] [Google Scholar]
- 32.Kozlowska A, Mikolajczyk A, Majewski M. 2017. Detailed characterization of sympathetic chain ganglia (SChG) neurons supplying the skin of the porcine hindlimb. Int J Mol Sci 18:E1463. doi: 10.3390/ijms1807146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanberry LR, Kern ER, Richards JT, Abbott TM, Overall JC Jr. 1982. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent disease. J Infect Dis 146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 34.Stanberry LR, Kern ER, Richards JT, Overall JC Jr. 1985. Recurrent genital herpes simplex virus infection in guinea pigs. Intervirology 24:226–231. doi: 10.1159/000149647. [DOI] [PubMed] [Google Scholar]
- 35.Tang S, Bertke AS, Patel A, Wang K, Cohen JI, Krause PR. 2008. An acutely and latently expressed herpes simplex virus 2 viral microRNA inhibits expression of ICP34.5, a viral neurovirulence factor. Proc Natl Acad Sci U S A 105:10931–10936. doi: 10.1073/pnas.0801845105. [DOI] [PMC free article] [PubMed] [Google Scholar]