Induction of type I IFNs and inflammatory cytokines plays pivotal roles in host antiviral innate immune responses. Viruses have evolved various mechanisms to interfere with these processes. HCMV causes severe ailments in immunodeficient populations and is a major cause of birth defects. It has been shown that HCMV antagonizes host innate immune defenses, which is important for establishing immune evasion and latent infection. In this study, we identified the HCMV DNA polymerase subunit UL44 as a suppressor of antiviral innate immune responses. Overexpression of UL44 impaired HCMV-triggered induction of type I IFNs and other antiviral genes and thus potentiated viral replication, whereas UL44 deficiency showed opposite effects. Mechanistic studies indicated that UL44 acts by inhibiting the binding of IRF3 and NF-κB to the promoters of downstream antiviral genes. These findings defined an important mechanism of HCMV immune evasion at the transcriptional level, which may provide a therapeutic target for the treatment of HCMV infection.
KEYWORDS: HCMV, IRF3, NF-κB, UL44, immune evasion, innate immunity, type I interferons
ABSTRACT
Innate immunity is the first line of host defense against viral invasion. The induction of type I interferons (IFNs) and inflammatory cytokines is essential to host antiviral immune responses, which are also key targets of viral immune evasion. Human cytomegalovirus (HCMV) can establish long-term latent infections, in which immune evasion is a pivotal step. In this study, we identified HCMV protein UL44, a DNA polymerase processivity factor, as an inhibitor of the interferon regulatory factor 3 (IRF3)- and NF-κB-dependent antiviral response. Ectopic expression of UL44 inhibited HCMV-triggered induction of downstream effector genes and enhanced viral replication. Conversely, knockdown of UL44 potentiated HCMV-triggered induction of downstream antiviral genes. UL44 interacted with IRF3 and p65, and it inhibited the binding of IRF3 and NF-κB to the promoters of their downstream antiviral genes. These findings reveal an important mechanism of immune evasion by HCMV at the transcriptional level.
IMPORTANCE Induction of type I IFNs and inflammatory cytokines plays pivotal roles in host antiviral innate immune responses. Viruses have evolved various mechanisms to interfere with these processes. HCMV causes severe ailments in immunodeficient populations and is a major cause of birth defects. It has been shown that HCMV antagonizes host innate immune defenses, which is important for establishing immune evasion and latent infection. In this study, we identified the HCMV DNA polymerase subunit UL44 as a suppressor of antiviral innate immune responses. Overexpression of UL44 impaired HCMV-triggered induction of type I IFNs and other antiviral genes and thus potentiated viral replication, whereas UL44 deficiency showed opposite effects. Mechanistic studies indicated that UL44 acts by inhibiting the binding of IRF3 and NF-κB to the promoters of downstream antiviral genes. These findings defined an important mechanism of HCMV immune evasion at the transcriptional level, which may provide a therapeutic target for the treatment of HCMV infection.
INTRODUCTION
Human cytomegalovirus (HCMV), a member of the human herpesvirus family, is a typical double-stranded DNA (dsDNA) virus that encodes >200 proteins (1, 2). HCMV is a common opportunistic virus causing severe ailments and deaths in people with immature or compromised immune systems, such as organ transplant recipients and HIV-infected individuals (3). HCMV infection increases the expression of type I interferons (IFNs), proinflammatory cytokines, and chemokines in early stages (4, 5). Conversely, HCMV proteins can suppress cellular and organismal defenses, a process that is pivotal for establishing immune evasion and latent infection (6). Therefore, HCMV has become an ideal model for investigating the mechanism of viral immune evasion due to its multiple strategies for modulating host innate and adaptive responses (7).
Upon infection of a permissive cell, the genes of HCMV are expressed in a regulated cascade: immediate early genes are expressed first, followed by early and then late genes. Various HCMV proteins target many steps of innate and adaptive immune responses. For example, UL82 (8), UL31 (9), US9 (10), UL23 (11), UL38 (12), UL83 (13, 14), TRS1 (15), and UL26 (16) modulate innate and adaptive immune responses through distinct mechanisms.
Innate immunity is the first line of host defense against viral infection. Type I IFNs and inflammatory cytokines are two vital arms of the innate immune system, which play pivotal roles in eliminating viruses at early stages of infection (17–20). Upon viral infection, germ line-encoded pattern recognition receptors (PRRs) recognize viral nucleic acids, such as viral DNA and RNA (21), to initiate the transcription of type I IFNs and other antiviral genes. In recent years, several viral DNA sensors have been identified, among which cyclic GMP-AMP (cGAMP) synthase (cGAS) is well established as a cytosolic DNA sensor that induces type I IFNs in most cell types (22). Upon its binding to viral DNA, the enzymatic activity of cGAS is activated, leading to the synthesis of cGAMP, which functions as a second messenger and binds to the endoplasmic reticulum (ER)-associated adaptor protein MITA (also called STING) (23, 24). MITA then recruits TBK1 (TANK-binding kinase 1) and the transcription factor IRF3 (interferon regulatory factor 3), as well as the IKK (IκB kinase) complex, leading to the phosphorylation of IRF3 or IκBα by TBK1 or IKKβ, respectively, and the induction of type I IFNs and proinflammatory cytokines (25, 26).
IRF3 is a key transcription factor in the induction of type I IFNs (27). Active IRF3 must go through sequential posttranslational modifications before associating with the IFN-β promoter (28). So far, some viruses have been reported to induce the degradation of activated IRF3. Only a few viruses have been shown to interfere with the binding of activated IRF3 to the IFNB promoter; such interference, in turn, suppresses the transcription of type I IFNs (29, 30).
Another transcription factor that plays an essential role in the induction of type I IFNs is NF-κB. The mammalian NF-κB family consists of five members: p65/RelA, RelB, p50/NF-κB1, p52/NF-κB2, and c-Rel (31). The N termini of these proteins share a conserved structure known as the Rel homology domain (RHD). The RHD is responsible for binding to DNA, forming homo- or heterodimers with the family members, interacting with IκBα (an inhibitor of NF-κB), and translocating into the nucleus (32). In addition, Rel proteins (p65/RelA, RelB, c-Rel) contain a C-terminal transactivation domain, which is missing in p50 and p52. Although p50, p52, and Rel proteins could form multiple kinds of homo- and heterodimers, as reported previously, the predominant form of NF-κB is a heterodimer of p65 and p50 (33).
Despite the association of HCMV with various human health problems, our knowledge of its immune evasion strategies is still limited. Previously, we screened for HCMV proteins that inhibit DNA-triggered transcription of antiviral genes (8). In this study, we identified HCMV DNA polymerase processivity factor UL44 as an inhibitor of virus-triggered induction of antiviral genes. Overexpression of UL44 inhibited antiviral immune responses. Conversely, knockdown of UL44 potentiated HCMV-triggered transcription of IFNB1 and other antiviral genes. UL44 enhanced virus replication by inhibiting host antiviral responses. Mechanistic studies indicated that UL44 inhibited the binding of IRF3 and NF-κB to promoters of antiviral genes. Our findings suggest that UL44-mediated inhibition of the induction of downstream antiviral genes at the transcriptional level provides a mechanism for HCMV immune evasion.
RESULTS
HCMV UL44 antagonizes signaling triggered by viruses and TNF-α.
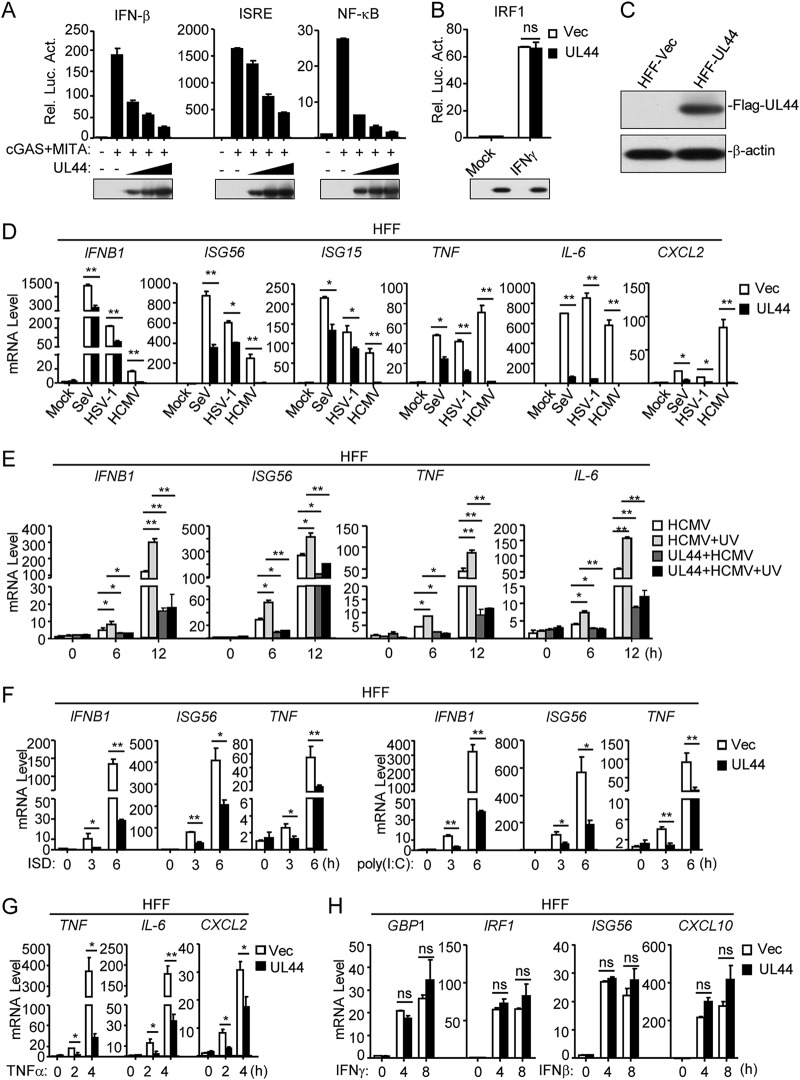
Previously, we screened a total of 64 HCMV proteins and identified UL82 as a negative regulator of cGAS-MITA-triggered signaling (8). In these screens, we also found that UL44 substantially inhibited cGAS-MITA-induced activation of the interferon-sensitive response element (ISRE) reporter in HEK293 cells (8). In reporter assays, ectopic expression of UL44 inhibited cGAS-MITA-induced activation of the IFN-β promoter, ISRE, and NF-κB in a dose-dependent manner in HEK293 cells (Fig. 1A). In reporter assays, UL44 did not affect IFN-γ-induced activation of the IRF1 reporter (Fig. 1B). Previously, it has been shown that human primary foreskin fibroblasts (HFFs) can express downstream antiviral genes in response to DNA virus infection (34, 35). We established HFF lines stably expressing UL44 (Fig. 1C). Quantitative PCR (qPCR) analysis indicated that ectopic expression of UL44 inhibited HCMV-induced transcription of the IFNB1, ISG56, ISG15, TNF, IL-6, and CXCL2 genes in HFFs (Fig. 1D). Interestingly, UL44 also inhibited the induction of downstream antiviral genes by herpes simplex virus 1 (HSV-1) (a DNA virus) and Sendai virus (SeV) (an RNA virus) (Fig. 1D), suggesting that UL44 inhibits shared components in the antiviral signaling pathways in response to both DNA and RNA viruses. UV-inactivated HCMV, which does not undergo viral transcription and translation after infection, induced higher levels of mRNAs of antiviral genes (IFNB1, ISG56, TNF, and IL-6) than untreated HCMV, which was also inhibited by UL44 (Fig. 1E). These results suggest that UL44 directly affects HCMV-induced transcription of downstream antiviral genes. In addition, UL44 also inhibited transcription of the IFNB1, ISG56, and TNF genes induced by transfected DNA mimic ISD (interferon-stimulatory DNA) and RNA mimic poly(I:C) in HFFs (Fig. 1F). UL44 also inhibited tumor necrosis factor alpha (TNF-α)-induced transcription of the TNF, IL-6, IL-8, and CXCL2 genes in HFFs (Fig. 1G). However, UL44 did not affect IFN-γ-induced transcription of the GBP1 and IRF1 genes or IFN-β-induced transcription of the ISG56 and CXCL10 genes (Fig. 1H). Taken together, these results suggest that UL44 inhibits shared components in virus- and TNF-α-triggered signaling pathways.
FIG 1.
Inhibition of innate antiviral signaling by HCMV UL44. (A) UL44 inhibits cGAS-MITA-induced activation of the IFN-β promoter, ISRE, and NF-κB in a dose-dependent manner. HEK293 cells (1 × 105) were transfected with an IFN-β promoter (0.05 μg), ISRE (0.03 μg), or NF-κB (0.005 μg) luciferase reporter plasmid, as well as with expression plasmids for cGAS (0.01 μg) and MITA (0.01 μg) and increased amounts of the UL44 plasmid, for 20 h before luciferase assays. Rel. Luc. Act., relative luciferase activity. The lower blots show the expression levels of the transfected UL44 protein. (B) Effects of UL44 on IFN-γ-induced IRF1 promoter activation. HEK293 cells (1 × 105) were transfected with IRF1 promoter reporter (0.05 μg) and UL44 expression (0.05 μg) plasmids for 20 h. The cells were then either left untreated or treated with IFN-γ for 12 h before luciferase assays. Vec, vector; ns, not significant. The lower blots show the expression levels of the transfected UL44 plasmids. (C) Expression of UL44 in a stable HFF-UL44 cell line. Control HFFs (2 × 105) (HFF-Vec) or HFFs stably expressing UL44 (2 × 105) were collected. Immunoblot analyses were performed with the indicated antibodies. (D) UL44 inhibits HCMV-, HSV-1-, and SeV-induced transcription of antiviral genes in HFFs. HFFs stably expressing UL44 (5 × 105) were either left uninfected or infected with HCMV, HSV-1, or SeV (each at an MOI of 1) for 12 h before qPCR analysis. (E) Inhibition by UL44 of IFNB1, ISG56, TNF, and IL-6 transcription induced by UV-inactivated HCMV. Control cells or cells stably expressing UL44 (5 × 105) were infected with wild-type or UV-inactivated HCMV for the indicated times before qPCR analysis. (F) UL44 inhibits dsDNA- and dsRNA-induced transcription of antiviral genes in HFFs. HFFs stably expressing UL44 (5 × 105) were transfected with ISD (2 μg) or poly(I:C) (2 μg) for the indicated times before qPCR analysis. (G) Effects of U44 on TNF-α-induced transcription of the TNF, IL-6, and CXCL2 genes in HFFs. HFFs stably expressing UL44 (5 × 105) were treated with TNF-α for the indicated times before qPCR analysis. (H) Effects of UL44 on IFN-γ-induced transcription of the GBP1 and IRF1 genes or IFN-β-induced transcription of the ISG56 and CXCL10 genes in HFFs. HFFs stably expressing UL44 (5 × 105) were either left untreated or treated with IFN-γ or IFN-β for the indicated times before immunoblot analyses were performed. Graphs show means ± SD (n = 3). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) by the unpaired t test.
Knockdown of UL44 enhances the innate antiviral response to HCMV.
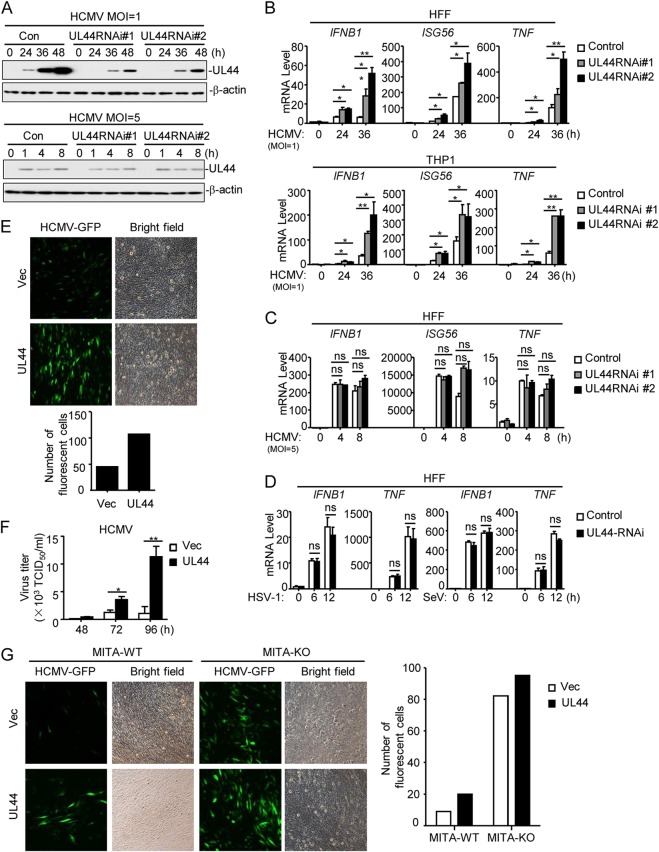
We next examined the role of endogenous UL44 in the innate antiviral response. We used the RNA interference (RNAi) knockdown strategy that has been successfully used in previous studies (8, 9, 36). We constructed two UL44-RNAi plasmids that could inhibit the expression of UL44 protein (Fig. 2A, top). qPCR analysis indicated that knockdown of UL44 promoted HCMV-induced transcription of the IFNB1, ISG56, and TNF genes in both HFFs and THP1 cells (Fig. 2B). Since the UL44 gene is an essential HCMV gene, in order to exclude the possibility that the enhanced signaling in UL44 knockdown cells resulted from indirect reductions in the levels of other viral proteins rather than in the level of UL44 itself, we examined the effects of UL44 knockdown at early times with a higher multiplicity of infection (MOI) of 5. Since UL44 is a tegument protein, as reported previously (37–39), UL44 derived from viral particles was detected at early times postinfection. However, since the UL44 gene is a late gene, its transcription had not yet started at these time points; therefore, transfection of UL44-RNAi plasmids showed little effect on UL44 expression at early times postinfection (MOI, 5) (Fig. 2A, bottom). In agreement with these results, UL44-RNAi barely affected the transcription of antiviral genes at early times at a high MOI (Fig. 2C). Notably, at 24 h postinfection, when UL44 was initially detected, UL44-RNAi markedly inhibited the expression of UL44 and promoted the transcription of antiviral genes (Fig. 2A and B), suggesting that UL44 itself is responsible for the regulation of HCMV-triggered antiviral signaling. We further examined the effects of UL44-RNAi on HSV-1- and SeV-induced signaling and found that UL44 knockdown did not affect HSV-1- or SeV-induced transcription of IFNB1 and TNF (Fig. 2D).
FIG 2.
UL44 deficiency increases HCMV-induced expression of downstream antiviral genes. (A) Effects of UL44-RNAi on the expression of HCMV UL44. HFFs stably expressing UL44-RNAi (2 × 106) were infected with HCMV (MOI, 1 or 5) for the indicated times before immunoblot analyses were performed. (B) Effects of UL44-RNAi plasmids on HCMV-induced transcription of antiviral genes. HFFs or THP1 cells stably expressing UL44-RNAi (5 × 105) were infected with HCMV (MOI, 1) for the indicated times before qPCR analysis. (C) Effects of UL44-RNAi on HCMV-induced transcription of antiviral genes. HFFs stably expressing UL44-RNAi (5 × 105) were infected with HCMV (MOI, 5) for the indicated times before qPCR analysis. (D) Effects of UL44-RNAi plasmids on HSV-1- or SeV-induced transcription of antiviral genes. HFFs stably expressing UL44-RNAi (5 × 105) were infected with HSV-1 or SeV (each at an MOI of 1) for the indicated times before qPCR analysis. (E) UL44 promotes HCMV production. Control HFFs or HFFs stably expressing UL44 (2 × 105) were infected with HCMV-GFP (MOI, 1), and the supernatants were harvested at 96 h postinfection before fluorescence microscopy. (F) UL44 enhances virus replication. Control HFFs or HFFs stably expressing UL44 (2 × 105) were infected with HCMV (MOI, 1), and the supernatants were harvested at the indicated times postinfection for measurement of viral titers by standard TCID50 assays. (G) UL44 enhances HCMV replication by inhibiting cellular antiviral responses in HFFs. MITA-deficient (knockout [KO]) HFFs were generated with CRISPR-Cas9 technology. MITA-KO and control (wild-type [WT]) HFFs (2 × 105) were infected with HCMV-GFP (MOI, 1), and the supernatants were harvested at 96 h postinfection before florescent microscopy. Graphs show means ± SD (n = 3). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) by the unpaired t test; ns, not significant.
We next investigated the roles of UL44 in cellular antiviral responses. For these experiments, we used green fluorescent protein (GFP)-tagged HCMV, in which a GFP-coding sequence was inserted downstream of the US28 gene without deletion of any viral genome (40). Overexpression of UL44 markedly enhanced the replication of HCMV-GFP in HFFs (Fig. 2E). Viral 50% tissue culture infective dose (TCID50) assays indicated that UL44 markedly increased the progenies after HCMV infection at 48, 72, and 96 h in HFFs (Fig. 2F). These results suggest that UL44 plays important roles in HCMV immune evasion. Previous reports indicated that MITA is an adaptor protein for the viral-DNA-induced innate antiviral response (24, 41). Overexpression of UL44 markedly enhanced the replication of HCMV in wild-type but not MITA-deficient HFFs (Fig. 2G). These results suggest that UL44 promotes the replication of HCMV by inhibiting MITA-mediated innate antiviral responses.
UL44 inhibits IRF3- and NF-κB-induced transcription of antiviral genes.
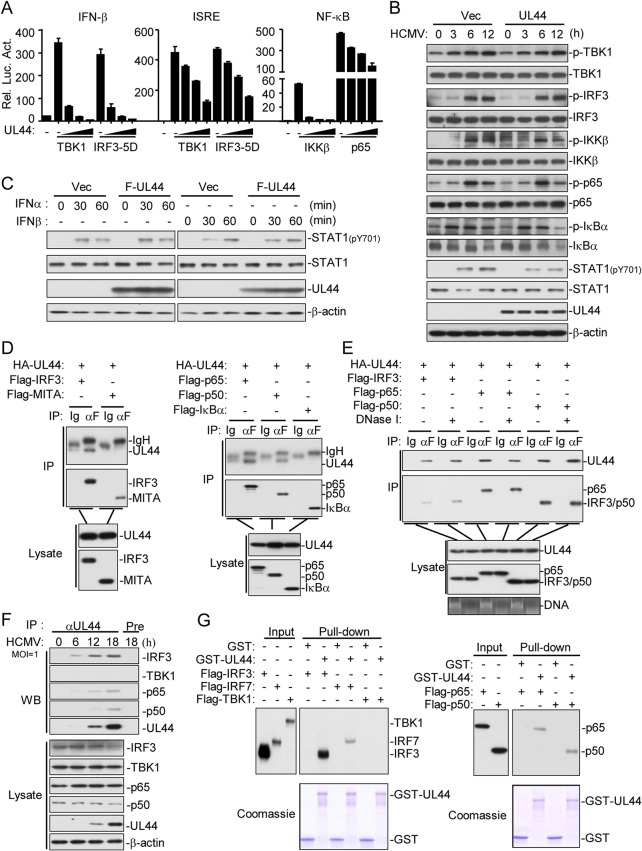
We next determined the molecular mechanisms by which UL44 functions. As shown in Fig. 3A, UL44 inhibited the activation of the IFN-β promoter and ISRE induced by overexpression of TBK1 and a constitutively active mutant of IRF3 (IRF3-5D). UL44 also inhibited the activation of NF-κB induced by overexpression of IKKβ and p65 (Fig. 3A). UL44 did not inhibit HCMV-induced phosphorylation of TBK1, IRF3, IKKβ, p65, and IκBα but did inhibit HCMV-induced phosphorylation of STAT1 (at Y701), which is a downstream component of type I IFNs (Fig. 3B). Furthermore, UL44 did not affect IFN-α- and IFN-β-induced phosphorylation of STAT1 at Y701 in HFFs (Fig. 3C). These data suggest that UL44 functions to inhibit IRF3- and NF-κB-mediated transcription.
FIG 3.
UL44 inhibits IRF3- and NF-κB-mediated transcription of antiviral genes. (A) Effects of UL44 on the activation of the IFN-β promoter, ISRE, and NF-κB mediated by various components. HEK293 cells (1 × 105) were transfected with the IFN-β promoter, ISRE, and the NF-κB reporter, UL44, and the indicated expression plasmids for 20 h before luciferase assays. (B) Effects of UL44 on HCMV-induced phosphorylation of downstream components. HFFs stably expressing UL44 (2 × 106) were either left untreated or infected with HCMV (MOI, 1) for the indicated times before immunoblot analysis. (C) Effects of UL44 on IFN-α- and IFN-β-induced phosphorylation of STAT1. HFFs stably expressing UL44 (2 × 106) were either left untreated or treated with IFN-α or IFN-β for the indicated times before immunoblot analysis. F, Flag. (D) Association of UL44 with IRF3, p65, and p50. HEK293T cells (5 × 106) were transfected with the indicated plasmids for 20 h before coimmunoprecipitation and immunoblot analysis were performed with the indicated antibodies. IP, immunoprecipitation. αF, anti-Flag. (E) The association of UL44 with IRF3, p65, and p50 is DNA independent. HEK293T cells (5 × 106) were transfected with the indicated plasmids for 20 h. Cells were either left untreated or treated with DNase I (2 μg/ml) for 2 h before coimmunoprecipitation and immunoblot analysis were performed with the indicated antibodies. (F) Association of endogenous UL44 with IRF3, p65, and p50 in HFFs. HFFs (1 × 107) either were left untreated or were infected with HCMV for the indicated times before coimmunoprecipitation and immunoblot analysis with the indicated antibodies. Pre, preimmune sera. (G) UL44 binds to IRF3, IRF7, p65, and p50 in vitro. Purified GST and GST-UL44 were used to pull down transiently expressed Flag-TBK1, Flag-IRF3, Flag-IRF7, Flag-p65, and Flag-p50 as indicated.
Coimmunoprecipitation experiments indicated that UL44 was associated with IRF3, p65, and p50, but not with MITA or IκBα, in an overexpression system (Fig. 3D) and that the interactions were independent of DNA (Fig. 3E). Endogenous coimmunoprecipitation experiments indicated that UL44 was associated with IRF3, p65, and p50, but not with TBK1, following HCMV infection (Fig. 3F). In vitro glutathione S-transferase (GST) pulldown assays further confirmed that UL44 interacted with IRF3, IRF7, p65, and p50 but not with TBK1 (Fig. 3G). These results suggest that UL44 is associated with IRF3 and NF-κB after HCMV infection.
UL44 suppresses the binding of IRF3 and NF-κB to the promoters of antiviral genes.
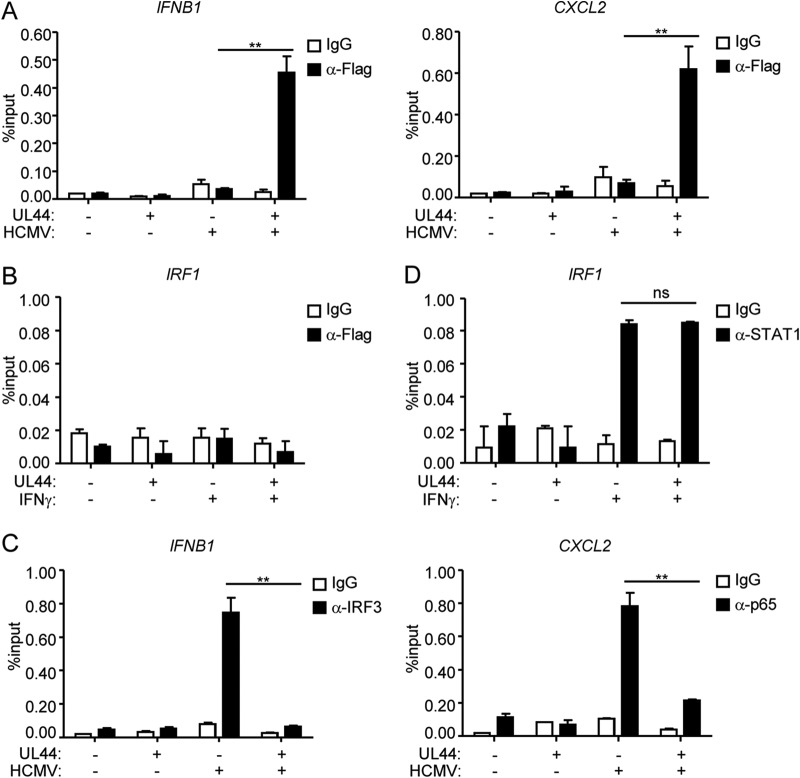
Previously, it has been shown that UL44 is localized to the nucleus (42–45). We found that UL44 was bound to the promoters of two downstream antiviral genes, IFNB1 and CXCL2, following HCMV infection (Fig. 4A) but was not bound to the promoter of IRF1 following IFN-γ treatment (Fig. 4B). In addition, UL44 inhibited the binding of IRF3 or p65 to the promoter of IFNB1 or CXCL2, respectively, after HCMV infection (Fig. 4C) but did not inhibit the binding of STAT1 to the promoter of IRF1 after IFN-γ treatment (Fig. 4D).
FIG 4.
UL44 suppresses the binding of IRF3 and NF-κB to the promoters of antiviral genes. (A) UL44 binds to the promoters of the IFNB and CXCL2 genes. Control HFFs or HFFs stably expressing UL44 (2 × 107) were either left uninfected or infected with HCMV for 4 h. ChIP assays were performed with the indicated antibodies. The binding of UL44 to the IFNB and CXCL2 promoters was determined by qPCR. (B) UL44 does not bind to the promoter of IRF1. Control HFFs or HFFs stably expressing UL44 (2 × 107) were either left untreated or treated with IFN-γ for 2 h. ChIP assays were performed with the indicated antibodies. The binding of UL44 to the IRF1 promoter was determined by qPCR. (C) UL44 diminishes the binding of IRF3 and p65 to the promoters of IFNB1 and CXCL2, respectively. Control HFFs or HFFs stably expressing UL44 (2 × 107) were either left untreated or infected with HCMV for 4 h. ChIP assays were performed with the indicated antibodies. The binding of IRF3 and p65 to the indicated promoters was determined by qPCR. (D) Effects of UL44 on IFN-γ-induced binding of STAT1 to the IRF1 promoter. Control HFFs or HFFs stably expressing UL44 (2 × 107) were either left untreated or treated with IFN-γ for 2 h. ChIP assays were performed with the indicated antibodies. The binding of STAT1 to the IRF1 promoter was determined by qPCR. Graphs show means ± SD (n = 3). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) by the unpaired t test; ns, not significant.
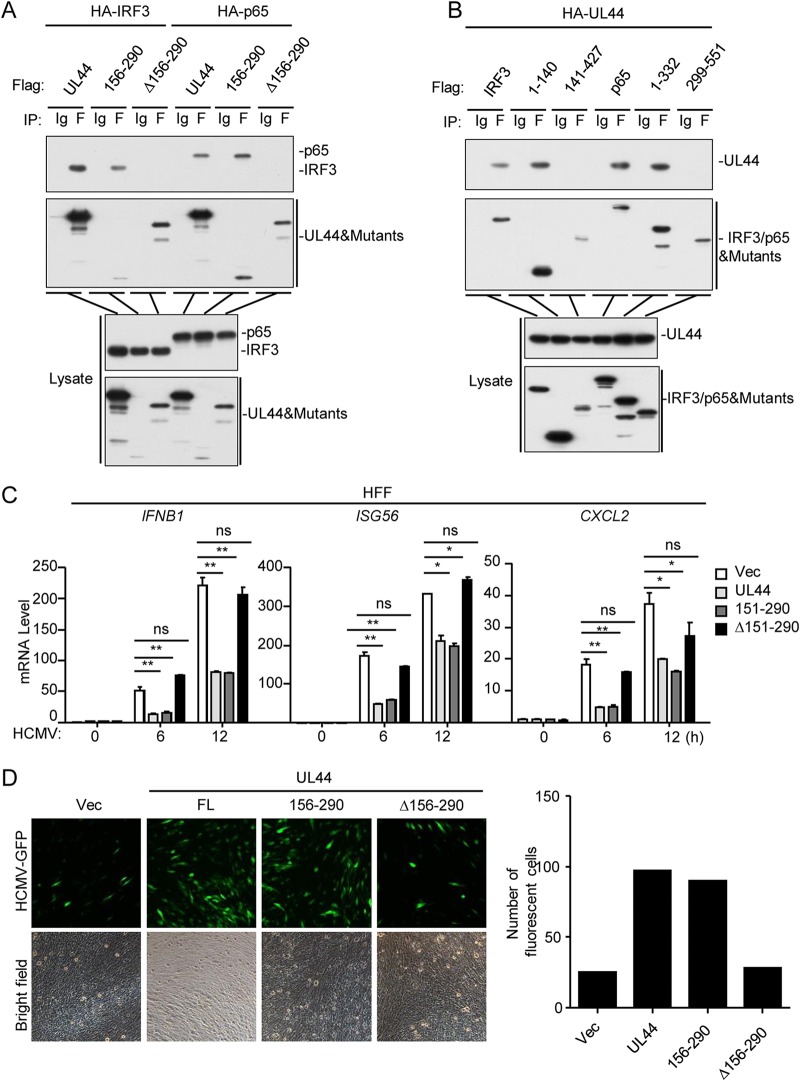
The DNA-binding domain is required for UL44 to inhibit innate antiviral responses.
Previous studies have demonstrated that amino acids (aa) 156 to 290 of UL44 constitute a DNA-binding domain (46). Domain-mapping experiments indicated that the DNA-binding domain of UL44 and the N termini of IRF3 (aa 1 to 140) and p65 (aa 1 to 332) were sufficient for their interactions (Fig. 5A and B). As shown in Fig. 5C, UL44(156–290), but not UL44(Δ156–290), inhibited HCMV-induced transcription of the IFNB1, ISG56, and CXCL2 genes. In agreement with these findings, the level of HCMV replication in HFFs stably expressing wild-type UL44 or UL44(156–290) was higher than that in control or UL44(Δ156–290)-expressing cells (Fig. 5D). These results suggest that the DNA-binding domain of UL44 is essential for its inhibitory role in the innate antiviral response.
FIG 5.
The DNA-binding domain of UL44 inhibits the host antiviral immune response. (A and B) Domain mapping of the UL44–IRF3 and UL44–p65 interactions. HEK293T cells (5 × 106) were transfected with the indicated plasmids for 20 h before coimmunoprecipitation and immunoblot analysis were performed with the indicated antibodies. (C) Effects of UL44 mutants on HCMV-induced transcription of antiviral genes in HFFs. HFFs (5 × 105) stably expressing wild-type UL44 or one of the indicated UL44 mutants were either left uninfected or infected with HCMV (MOI, 1) for the indicated times before qPCR analysis. (D) Effects of UL44 mutants on cellular antiviral responses. HFFs (2 × 105) stably expressing wild-type UL44 or one of the indicated UL44 mutants were infected with HCMV-GFP (MOI, 0.1) for 96 h before fluorescence microscopy. Graphs show means ± SD (n = 3). Asterisks indicate significant differences (*, P < 0.05; **, P < 0.01) by the unpaired t test; ns, not significant.
DISCUSSION
The transcription factors IRF3 and NF-κB are critically involved in virus-triggered induction of downstream genes (47). The induction of downstream genes by IRF3 and NF-κB promotes an antiviral state that limits viral replication. Thus, many distinct types of viruses attenuate IRF3 and NF-κB activation as part of their infectious program (48). In this study, we identified HCMV UL44 as a negative regulator of IRF3- and NF-κB-mediated transcription of downstream antiviral genes and of the innate immune response.
HCMV UL44 was identified as a DNA polymerase processivity subunit, which is important for HCMV replication. UL44 is a 433-aa protein that contains an N-terminal dimerization domain (aa 2 to 155), a middle DNA-binding domain (aa 156 to 290), and a C-terminal phosphorylation domain (aa 291 to 433). A recent study showed that UL44 could inhibit the transcriptional activity of p53 and contributed to the canceration process (49). Several lines of evidence suggest that UL44 plays an important role in the innate immune response to HCMV. Overexpression of UL44 inhibited cGAS-MITA-induced activation of ISRE, IFN-β, and NF-κB in HEK293 cells. In agreement with these results, UL44 inhibited HCMV-, HSV-1-, SeV-, and cytosolic dsDNA (ISD)- or dsRNA [poly(I:C)]-induced transcription of downstream effector genes, whereas knockdown of UL44 accelerated the HCMV-triggered production of type I IFNs and downstream antiviral genes. In addition, we found that ectopic expression of UL44 increased HCMV replication and that these effects are dependent on IRF3- and p65-mediated signaling pathways.
Mechanistic studies suggest that UL44 inhibits the binding of IRF3 and p65 to the promoters of downstream antiviral genes. Overexpression of UL44 inhibited IRF3- and p65-induced activation of ISRE and NF-κB, respectively, but did not affect HCMV-induced phosphorylation of IRF3 and p65. UL44 interacted with IRF3 and p65, and their endogenous associations were increased following HCMV infection. Chromatin immunoprecipitation (ChIP) assays showed that in the absence of HCMV infection, UL44 did not bind to the promoters of IFNB and CXCL2. However, following HCMV infection, UL44 was bound to the promoters of the IFNB and CXCL2 genes and inhibited the binding of IRF3 and p65 to the promoters of these genes. Based on these results, two possible scenarios for the mechanisms of UL44 function exist. In the first model, upon HCMV infection, activated IRF3 and p65 enter the nucleus, where UL44 binds to them and is brought into proximity to the promoters of their target genes. Since the interactions of UL44 with IRF3 and p65 are mediated by their DNA-binding domains, it is possible that such interactions somehow dampen the DNA-binding activities of IRF3 and p65 while enhancing the DNA-binding activity of UL44, resulting in the binding of UL44 to the promoters and the subsequent dissociation of UL44 from IRF3 or p65. In the second model, HCMV infection activates signaling cascades and leads to the activation of IRF3 and p65 as well as chromatin remodeling, which enhances the accessibility of these transcription factors to the promoters of their target genes (50, 51). In the nucleus, a fraction of UL44 binds to IRF3 and p65 to sequester and/or dissociate them from the promoters of their target genes. Simultaneously, an excess of UL44 binds to and occupies the promoters of IRF3/p65-downstream antiviral genes to prevent their induction. Taken together, our results suggest that UL44 acts by inhibiting the binding of IRF3 and NF-κB to the promoters of downstream antiviral genes, thus defining an important mechanism for HCMV immune evasion.
MATERIALS AND METHODS
Reagents and antibodies.
Lipofectamine 2000 (Invitrogen), Polybrene (Millipore), puromycin and RNase inhibitor (Thermo), the Dual-Specific Luciferase Assay kit (Promega), SYBR green (Bio-Rad), digitonin (Sigma), and mouse antibodies against Flag and β-actin (Sigma), hemagglutinin (HA) (Covance), phospho-NF-κB p65, phospho-Stat1 (Tyr 701) (9167), and phospho-IRF-3 (4947S) (Cell Signaling Technology), phospho-TBK1 (ab109272) and TBK1 (ab40676) (Abcam), IRF3 (sc-9082), and STAT1 (sc-346) (Santa Cruz Biotechnology) were purchased from their respective manufacturers. Antisera against UL44 and UL82 were generated by immunizing rabbits or mice with purified recombinant UL44 or UL82.
Cells.
HEK293 cells were obtained from the ATCC. HFFs were provided by Min-Hua Luo (Wuhan Institute of Virology, Chinese Academy of Sciences [CAS]). These cells were cultured in Dulbecco’s modified Eagle medium (DMEM; HyClone) supplemented with 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin (Thermo Fisher Scientific) at 37°C under 5% CO2. All cells were negative for mycoplasma.
Viruses.
HCMV (strain AD169) and HSV-1–GFP were provided by Min-Hua Luo (Wuhan Institute of Virology, CAS) and Chun-Fu Zheng (Suzhou University) (52), respectively. HCMV-GFP was generated with a bacterial artificial chromosome (BAC) carrying GFP-tagged HCMV genome, provided by Dong Yu (Washington University School of Medicine). HSV-1 (strain KOS) was from the China Center for Type Culture Collection, Wuhan, China. SeV has been described previously (53). HCMV and HCMV-GFP stocks were prepared on HFFs, and virus titers were determined by standard TCID50 assays.
Constructs.
Expression plasmids for HA-tagged, FLAG-tagged UL44 and its truncation mutants were constructed by standard molecular biology techniques. Expression plasmids for FLAG-tagged IRF3, TBK1, p65, p50, IRF7, MITA, and IκBα and the ISRE, IFN-β, and NF-κB promoter reporter plasmids have been described previously (8, 54–56).
DNA oligonucleotide.
The ISD oligonucleotide (5′-TACAGATCTACTAGTGATCTATGACTGATCTGTACATGATCTACA-3′) was used to stimulate cells.
Transfection and reporter assays.
Transfection and reporter assays were performed as described previously (57). HEK293 cells were transfected by the standard calcium phosphate precipitation method. HFFs were transfected with Lipofectamine 2000. To ensure that each transfection supplied the same amount of total DNA, the empty control plasmid was added in each transfection. To normalize for transfection efficiency, the pRL-TK (Renilla luciferase) reporter plasmid (0.01 μg) was added in each transfection. Luciferase assays were performed using a Dual-Specific Luciferase Assay kit. Firefly luciferase activities were normalized on the basis of Renilla luciferase activities.
RNAi.
Double-stranded oligonucleotides corresponding to the target sequences were cloned into the pSuper.retro RNAi plasmid (Oligoengine). The following sequences were targeted for UL44 mRNA: sequence 1 (5′-GCCGTCGCTTATCTTGCAAAC-3′) and sequence 3 (5′-GCTGGAATTCACAGCCAATAA-3′). The sequence targeted by the control RNAi plasmid is 5′-GGAAGATGTATGGAGACATGG-3′.
RNAi-transduced stable HFFs.
The HEK293T cells were transfected with two packaging plasmids (pGAG-Pol and pVSV-G) together with a control UL44-RNAi retroviral plasmid. Twenty-four hours later, cells were incubated with a new medium without antibiotics for another 24 h. The recombinant-virus-containing medium was first filtered and then added to HFFs in the presence of Polybrene (4 μg/ml). The infected cells were selected with puromycin (0.5 μg/ml) for 10 days before additional experiments.
Preparation of MITA-KO cells by CRISPR-Cas9 technology.
Double-stranded oligonucleotides corresponding to the target sequences were cloned into the lenti-CRISPR (clustered regularly interspaced short palindromic repeat)-V2 vector, which was cotransfected with packaging plasmids into HEK293T cells. Two days after transfection, the viruses were harvested, ultrafiltered (0.22-mm filter; Millipore), and used to infect HFFs in the presence of Polybrene (4 μg/ml). The infected cells were selected with puromycin (0.5 μg/ml) for at least 5 days.
Coimmunoprecipitation and immunoblot analysis.
HEK293 cells (1 × 107), THP1 cells, or HFFs (3 × 107) were lysed in l ml NP-40 lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). Coimmunoprecipitation and immunoblot analysis were performed as described previously (53, 58).
GST pulldown assay.
GST-UL44-bound glutathione agarose beads were incubated for 3 h with lysates from HEK293T cells transiently expressing a Flag-IRF3, IRF7, TBK1, p65, or p50 plasmid. The beads were first washed three times with lysis buffer (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride), then mixed with an equal volume of 2× SDS loading buffer, and boiled for 10 min. The input/elutes were resolved by SDS-PAGE and analyzed by Coomassie staining and/or immunoblot analysis (59).
qPCR.
Total RNA was isolated for qPCR analysis to measure the mRNA levels of various genes. The data shown in the figures are the relative abundances of the indicated mRNAs normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primer sequences for IFNB1, ISG56, ISG54, CXCL10, IL-6, GBP1, IRF1, and GAPDH have been described previously (52, 60).
ChIP assay.
Chromatin immunoprecipitation (ChIP) assays were performed as described previously (33, 61). Immunoprecipitation was performed with 2 μg of an IRF3, p65, or STAT1 antibody, 2 μg of a Flag antibody, or 5 μg of a UL44 antibody. The immune complexes were absorbed with protein A beads blocked with bovine serum albumin and salmon sperm DNA (Millipore). Gene-specific primer sequences were as follows: for IFNB1, 5′-CTGAAAGGGAGAAGTGAAAGTGG-3′ (forward) and 5′-TCGAAAGGTTGCAGTTAGAATGTC-3′ (reverse); for CXCL2, 5′-CTCGCAGGCGGTTATCTCGGTATC-3′ (forward) and 5′-GGGGGTCGGGGCACTCACG-3′ (reverse); for IRF1, 5′-GAGGAGGTGAAAGACCAGAGCA-3′ (forward) and 5′-TAGCATCTCGGCTGGACTTCGA-3′ (reverse).
Statistical analysis.
All statistical tests were performed using GraphPad Prism, version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). All experiments were repeated at least twice. The data are presented as means and standard deviations (SD) from one representative experiment. For comparisons consisting of two groups, means were compared using two-tailed Student t tests; for comparisons consisting of three groups, means were compared using one-way or two-way analysis of variance (ANOVA) with Bonferroni’s posttest. Differences were considered statistically significant at P values of <0.05.
ACKNOWLEDGMENTS
This study was supported by the National Science Fund for Distinguished Young Scholars (grant 31425010), the Pilot Project of Chinese Academy of Sciences (CAS) (grant XDB29010302), the National Natural Science Foundation of China (grants 31621061 and 31800732), the Ministry of Science and Technology of China (grant 2015CB554302), the Key Research Programs of Frontier Sciences (funded by the Chinese Academy of Sciences), the National Postdoctoral Program for Innovative Talents (grant BX201700277), and the China Postdoctoral Science Foundation (grant 2018M630894).
REFERENCES
- 1.Murphy E, Rigoutsos I, Shibuya T, Shenk TE. 2003. Reevaluation of human cytomegalovirus coding potential. Proc Natl Acad Sci U S A 100:13585–13590. doi: 10.1073/pnas.1735466100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy E, Yu D, Grimwood J, Schmutz J, Dickson M, Jarvis MA, Hahn G, Nelson JA, Myers RM, Shenk TE. 2003. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci U S A 100:14976–14981. doi: 10.1073/pnas.2136652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hommes DW, Sterringa G, van Deventer SJ, Tytgat GN, Weel J. 2004. The pathogenicity of cytomegalovirus in inflammatory bowel disease: a systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis 10:245–250. doi: 10.1097/00054725-200405000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Boyle KA, Pietropaolo RL, Compton T. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol 19:3607–3613. doi: 10.1128/MCB.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol 77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amsler L, Verweij M, DeFilippis VR. 2013. The tiers and dimensions of evasion of the type I interferon response by human cytomegalovirus. J Mol Biol 425:4857–4871. doi: 10.1016/j.jmb.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson SE, Mason GM, Wills MR. 2011. Human cytomegalovirus immunity and immune evasion. Virus Res 157:151–160. doi: 10.1016/j.virusres.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Fu YZ, Su S, Gao YQ, Wang PP, Huang ZF, Hu MM, Luo WW, Li S, Luo MH, Wang YY, Shu HB. 2017. Human cytomegalovirus tegument protein UL82 inhibits STING-mediated signaling to evade antiviral immunity. Cell Host Microbe 21:231–243. doi: 10.1016/j.chom.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Huang ZF, Zou HM, Liao BW, Zhang HY, Yang Y, Fu YZ, Wang SY, Luo MH, Wang YY. 2018. Human cytomegalovirus protein UL31 inhibits DNA sensing of cGAS to mediate immune evasion. Cell Host Microbe 24:69–80.e4. doi: 10.1016/j.chom.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Choi HJ, Park A, Kang S, Lee E, Lee TA, Ra EA, Lee J, Lee S, Park B. 2018. Human cytomegalovirus-encoded US9 targets MAVS and STING signaling to evade type I interferon immune responses. Nat Commun 9:125. doi: 10.1038/s41467-017-02624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng L, Sheng J, Vu GP, Liu Y, Foo C, Wu S, Trang P, Paliza-Carre M, Ran Y, Yang X, Sun X, Deng Z, Zhou T, Lu S, Li H, Liu F. 2018. Human cytomegalovirus UL23 inhibits transcription of interferon-gamma stimulated genes and blocks antiviral interferon-gamma responses by interacting with human N-myc interactor protein. PLoS Pathog 14:e1006867. doi: 10.1371/journal.ppat.1006867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moorman NJ, Cristea IM, Terhune SS, Rout MP, Chait BT, Shenk T. 2008. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 3:253–262. doi: 10.1016/j.chom.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li T, Chen J, Cristea IM. 2013. Human cytomegalovirus tegument protein pUL83 inhibits IFI16-mediated DNA sensing for immune evasion. Cell Host Microbe 14:591–599. doi: 10.1016/j.chom.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biolatti M, Dell’Oste V, Pautasso S, Gugliesi F, von Einem J, Krapp C, Jakobsen MR, Borgogna C, Gariglio M, De Andrea M, Landolfo S. 2018. Human cytomegalovirus tegument protein pp65 (pUL83) dampens type I interferon production by inactivating the DNA sensor cGAS without affecting STING. J Virol 92:e01774-17. doi: 10.1128/JVI.01774-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent HA, Ziehr B, Moorman NJ. 2017. Mechanism of protein kinase R inhibition by human cytomegalovirus pTRS1. J Virol 91:e01574-16. doi: 10.1128/JVI.01574-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathers C, Schafer X, Martinez-Sobrido L, Munger J. 2014. The human cytomegalovirus UL26 protein antagonizes NF-κB activation. J Virol 88:14289–14300. doi: 10.1128/JVI.02552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Medzhitov R. 2007. Recognition of microorganisms and activation of the immune response. Nature 449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 19.Paludan SR, Bowie AG. 2013. Immune sensing of DNA. Immunity 38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter S, Ricci EP, Mercier BC, Moore MJ, Fitzgerald KA. 2014. Post-transcriptional regulation of gene expression in innate immunity. Nat Rev Immunol 14:361–376. doi: 10.1038/nri3682. [DOI] [PubMed] [Google Scholar]
- 21.Hu MM, Shu HB. 2018. Cytoplasmic mechanisms of recognition and defense of microbial nucleic acids. Annu Rev Cell Dev Biol 34:357–379. doi: 10.1146/annurev-cellbio-100617-062903. [DOI] [PubMed] [Google Scholar]
- 22.Sun LJ, Wu JX, Du FH, Chen X, Chen Z. 2013. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa H, Barber GN. 2008. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455:674–678. (Erratum, 456:274.) doi: 10.1038/nature07432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao FC, Lei CQ, He X, Zhang L, Tien P, Shu HB. 2008. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity 29:538–550. doi: 10.1016/j.immuni.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Abe T, Barber GN. 2014. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol 88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shu HB, Wang YY. 2014. Adding to the STING. Immunity 41:871–873. doi: 10.1016/j.immuni.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Cai X, Chiu YH, Chen ZJ. 2014. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell 54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, Liu Y, Wang P, Guan X, He S, Luo S, Li C, Hu K, Jin W, Du T, Yan Y, Zhang Z, Zheng Z, Wang H, Hu Q. 2015. HSV-2 immediate-early protein US1 inhibits IFN-β production by suppressing association of IRF-3 with IFN-β promoter. J Immunol 194:3102–3115. doi: 10.4049/jimmunol.1401538. [DOI] [PubMed] [Google Scholar]
- 30.Xing J, Ni L, Wang S, Wang K, Lin R, Zheng C. 2013. Herpes simplex virus 1-encoded tegument protein VP16 abrogates the production of beta interferon (IFN) by inhibiting NF-κB activation and blocking IFN regulatory factor 3 to recruit its coactivator CBP. J Virol 87:9788–9801. doi: 10.1128/JVI.01440-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vallabhapurapu S, Karin M. 2009. Regulation and function of NF-κB transcription factors in the immune system. Annu Rev Immunol 27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S, May MJ, Kopp EB. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Lei CQ, Hu YH, Xia T, Li M, Zhong B, Shu HB. 2014. Kruppel-like factor 6 is a co-activator of NF-κB that mediates p65-dependent transcription of selected downstream genes. J Biol Chem 289:12876–12885. doi: 10.1074/jbc.M113.535831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konno H, Konno K, Barber GN. 2013. Cyclic dinucleotides trigger ULK1 (ATG1) phosphorylation of STING to prevent sustained innate immune signaling. Cell 155:688–698. doi: 10.1016/j.cell.2013.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gray EE, Winship D, Snyder JM, Child SJ, Geballe AP, Stetson DB. 2016. The AIM2-like receptors are dispensable for the interferon response to intracellular DNA. Immunity 45:255–266. doi: 10.1016/j.immuni.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau L, Gray EE, Brunette RL, Stetson DB. 2015. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 350:568–571. doi: 10.1126/science.aab3291. [DOI] [PubMed] [Google Scholar]
- 37.Smith RM, Kosuri S, Kerry JA. 2014. Role of human cytomegalovirus tegument proteins in virion assembly. Viruses 6:582–605. doi: 10.3390/v6020582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn W, Chou C, Li H, Hai R, Patterson D, Stolc V, Zhu H, Liu F. 2003. Functional profiling of a human cytomegalovirus genome. Proc Natl Acad Sci U S A 100:14223–14228. doi: 10.1073/pnas.2334032100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalejta RF. 2008. Tegument proteins of human cytomegalovirus. Microbiol Mol Biol Rev 72:249–265. doi: 10.1128/MMBR.00040-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D, Smith GA, Enquist LW, Shenk T. 2002. Construction of a self-excisable bacterial artificial chromosome containing the human cytomegalovirus genome and mutagenesis of the diploid TRL/IRL13 gene. J Virol 76:2316–2328. doi: 10.1128/jvi.76.5.2316-2328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. 2005. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol Cell 19:727–740. doi: 10.1016/j.molcel.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Alvisi G, Roth DM, Camozzi D, Pari GS, Loregian A, Ripalti A, Jans DA. 2009. The flexible loop of the human cytomegalovirus DNA polymerase processivity factor ppUL44 is required for efficient DNA binding and replication in cells. J Virol 83:9567–9576. doi: 10.1128/JVI.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvisi G, Jans DA, Ripalti A. 2006. Human cytomegalovirus (HCMV) DNA polymerase processivity factor ppUL44 dimerizes in the cytosol before translocation to the nucleus. Biochemistry 45:6866–6872. doi: 10.1021/bi060086u. [DOI] [PubMed] [Google Scholar]
- 44.Strang BL, Boulant S, Coen DM. 2010. Nucleolin associates with the human cytomegalovirus DNA polymerase accessory subunit UL44 and is necessary for efficient viral replication. J Virol 84:1771–1784. doi: 10.1128/JVI.01510-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strang BL, Boulant S, Chang L, Knipe DM, Kirchhausen T, Coen DM. 2012. Human cytomegalovirus UL44 concentrates at the periphery of replication compartments, the site of viral DNA synthesis. J Virol 86:2089–2095. doi: 10.1128/JVI.06720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva LA, Loregian A, Pari GS, Strang BL, Coen DM. 2010. The carboxy-terminal segment of the human cytomegalovirus DNA polymerase accessory subunit UL44 is crucial for viral replication. J Virol 84:11563–11568. doi: 10.1128/JVI.01033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu B, Ren Y, Sun X, Han C, Wang H, Chen Y, Peng Q, Cheng Y, Cheng X, Zhu Q, Li W, Li HL, Du HN, Zhong B, Huang Z. 2017. Induction of INKIT by viral infection negatively regulates antiviral responses through inhibiting phosphorylation of p65 and IRF3. Cell Host Microbe 22:86–98.e4. doi: 10.1016/j.chom.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 48.Ma Z, Damania B. 2016. The cGAS-STING defense pathway and its counteraction by viruses. Cell Host Microbe 19:150–158. doi: 10.1016/j.chom.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon Y, Kim MN, Young Choi E, Heon Kim J, Hwang ES, Cha CY. 2012. Inhibition of p53 transcriptional activity by human cytomegalovirus UL44. Microbiol Immunol 56:324–331. doi: 10.1111/j.1348-0421.2012.00446.x. [DOI] [PubMed] [Google Scholar]
- 50.Honda K, Takaoka A, Taniguchi T. 2006. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Agalioti T, Lomvardas S, Parekh B, Yie J, Maniatis T, Thanos D. 2000. Ordered recruitment of chromatin modifying and general transcription factors to the IFN-β promoter. Cell 103:667–678. doi: 10.1016/S0092-8674(00)00169-0. [DOI] [PubMed] [Google Scholar]
- 52.Li Y, Chen R, Zhou Q, Xu Z, Li C, Wang S, Mao A, Zhang X, He W, Shu HB. 2012. LSm14A is a processing body-associated sensor of viral nucleic acids that initiates cellular antiviral response in the early phase of viral infection. Proc Natl Acad Sci U S A 109:11770–11775. doi: 10.1073/pnas.1203405109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ran Y, Zhang J, Liu LL, Pan ZY, Nie Y, Zhang HY, Wang YY. 2016. Autoubiquitination of TRIM26 links TBK1 to NEMO in RLR-mediated innate antiviral immune response. J Mol Cell Biol 8:31–43. doi: 10.1093/jmcb/mjv068. [DOI] [PubMed] [Google Scholar]
- 54.Yang Q, Liu TT, Lin H, Zhang M, Wei J, Luo WW, Hu YH, Zhong B, Hu MM, Shu HB. 2017. TRIM32-TAX1BP1-dependent selective autophagic degradation of TRIF negatively regulates TLR3/4-mediated innate immune responses. PLoS Pathog 13:e1006600. doi: 10.1371/journal.ppat.1006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo WW, Li S, Li C, Lian H, Yang Q, Zhong B, Shu HB. 2016. iRhom2 is essential for innate immunity to DNA viruses by mediating trafficking and stability of the adaptor STING. Nat Immunol 17:1057–1066. doi: 10.1038/ni.3510. [DOI] [PubMed] [Google Scholar]
- 56.Hu MM, Yang Q, Xie XQ, Liao CY, Lin H, Liu TT, Yin L, Shu HB. 2016. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity 45:555–569. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 57.Zhou Q, Lin H, Wang SY, Wang S, Ran Y, Liu Y, Ye W, Xiong XZ, Zhong B, Shu HB, Wang YY. 2014. The ER-associated protein ZDHHC1 is a positive regulator of DNA virus-triggered, MITA/STING-dependent innate immune signaling. Cell Host Microbe 16:450–461. doi: 10.1016/j.chom.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 58.Yang Y, Wang SY, Huang ZF, Zou HM, Yan BR, Luo WW, Wang YY. 2016. The RNA-binding protein Mex3B is a coreceptor of Toll-like receptor 3 in innate antiviral response. Cell Res 26:288–303. doi: 10.1038/cr.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu JJ, Li W, Shao Y, Avey D, Fu B, Gillen J, Hand T, Ma S, Liu X, Miley W, Konrad A, Neipel F, Sturzl M, Whitby D, Li H, Zhu F. 2015. Inhibition of cGAS DNA sensing by a herpesvirus virion protein. Cell Host Microbe 18:333–344. doi: 10.1016/j.chom.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei J, Guo W, Lian H, Yang Q, Lin H, Li S, Shu HB. 2017. SNX8 mediates IFNγ-triggered noncanonical signaling pathway and host defense against Listeria monocytogenes. Proc Natl Acad Sci U S A 114:13000–13005. doi: 10.1073/pnas.1713462114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo WW, Lian H, Zhong B, Shu HB, Li S. 2016. Kruppel-like factor 4 negatively regulates cellular antiviral immune response. Cell Mol Immunol 13:65–72. doi: 10.1038/cmi.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]