The ability to protect vulnerable populations such as pregnant women and children from Zika virus and other arbovirus infections is essential to preventing the devastating complications induced by these viruses. One class of antiviral therapies may lie in known pregnancy-acceptable drugs that have the potential to mitigate arbovirus infections and disease, yet this has not been explored in detail. In this study, we show that the common antiparasitic drug atovaquone inhibits arbovirus replication through intracellular nucleotide depletion and can impair ZIKV infection in an ex vivo human placental explant model. Our study provides a novel function for atovaquone and highlights that the rediscovery of pregnancy-acceptable drugs with potential antiviral effects can be the key to better addressing the immediate need for treating viral infections and preventing potential birth complications and future disease.
KEYWORDS: Zika, antiviral, arbovirus, atovaquone, pregnancy
ABSTRACT
Arthropod-borne viruses represent a significant public health threat worldwide, yet there are few antiviral therapies or prophylaxes targeting these pathogens. In particular, the development of novel antivirals for high-risk populations such as pregnant women is essential to prevent devastating disease such as that which was experienced with the recent outbreak of Zika virus (ZIKV) in the Americas. One potential avenue to identify new and pregnancy-acceptable antiviral compounds is to repurpose well-known and widely used FDA-approved drugs. In this study, we addressed the antiviral role of atovaquone, an FDA Pregnancy Category C drug and pyrimidine biosynthesis inhibitor used for the prevention and treatment of parasitic infections. We found that atovaquone was able to inhibit ZIKV and chikungunya virus virion production in human cells and that this antiviral effect occurred early during infection at the initial steps of viral RNA replication. Moreover, we were able to complement viral replication and virion production with the addition of exogenous pyrimidine nucleosides, indicating that atovaquone functions through the inhibition of the pyrimidine biosynthesis pathway to inhibit viral replication. Finally, using an ex vivo human placental tissue model, we found that atovaquone could limit ZIKV infection in a dose-dependent manner, providing evidence that atovaquone may function as an antiviral in humans. Taken together, these studies suggest that atovaquone could be a broad-spectrum antiviral drug and a potential attractive candidate for the prophylaxis or treatment of arbovirus infection in vulnerable populations, such as pregnant women and children.
IMPORTANCE The ability to protect vulnerable populations such as pregnant women and children from Zika virus and other arbovirus infections is essential to preventing the devastating complications induced by these viruses. One class of antiviral therapies may lie in known pregnancy-acceptable drugs that have the potential to mitigate arbovirus infections and disease, yet this has not been explored in detail. In this study, we show that the common antiparasitic drug atovaquone inhibits arbovirus replication through intracellular nucleotide depletion and can impair ZIKV infection in an ex vivo human placental explant model. Our study provides a novel function for atovaquone and highlights that the rediscovery of pregnancy-acceptable drugs with potential antiviral effects can be the key to better addressing the immediate need for treating viral infections and preventing potential birth complications and future disease.
INTRODUCTION
Recent outbreaks of significant human vector-borne pathogens have left us with the uncertainty of potential future devastating epidemics (1, 2). In particular, Zika virus (ZIKV), a flavivirus and close relative of dengue virus (DENV), has led to an overwhelming spectrum of diseases, including Guillain-Barré syndrome, microcephaly, ocular damage, and even meningitis, encephalitis, thrombocytopenia, sperm alterations, and multiorgan failure (3–9). This, coupled with the widespread and invasive Aedes species of mosquito, makes it easy to envision another epidemic when environmental, ecological, and human factors meet (10). Unfortunately, there are no antiviral treatments or prophylaxes targeting these viruses, and thus efforts to mitigate the impact of and ultimately prevent the disease are urgent and need to be addressed.
Pregnant women carry a particularly high risk for complications caused by ZIKV and other prevalent arbovirus such as chikungunya virus (CHIKV) and DENV (11–17). Importantly, the capacity of the virus to infect trophoblasts, Hofbauer macrophages, and endothelial cells (1, 18), thus allowing it to infect the fetus at any stage of growth, challenges the protective function of the placenta in the maternal-fetal interface (19, 20). Despite the significant morbidity observed in newborns (21), there are no antivirals available to treat this population, in part due to safety concerns during pregnancy, lack of biosafety studies, and nonexistent clinical trials. With this in mind, and given the urgency of this need, we propose to repurpose existing drugs with an acceptable profile in pregnancy.
Nucleotide biosynthesis inhibitors such as ribavirin, brequinar, and mycophenolic acid (MPA) have been shown extensively to inhibit a wide array of viral infections both in vitro and in vivo (22–28). In addition, a number of small compounds that possess antiviral function through the depletion of intracellular nucleotide pools have been identified, suggesting that this cellular pathway may be a prime target for antiviral development (29–33). Unfortunately, many of these compounds have numerous side effects and are not approved for use in high-risk populations such as pregnant women or children; thus, safe and pregnancy-acceptable nucleotide biosynthesis inhibitors would be ideal candidates as antivirals.
In these studies, we address the antiviral role of atovaquone, an FDA Pregnancy Category C and well-known antimalarial and antiparasitic drug that has been used repeatedly in the clinical setting for nearly 2 decades (34–37). Atovaquone is a ubiquinone (coenzyme Q) analogue that functions through the inhibition of the mitochondrial cytochrome complex III (38, 39). However, it has also been shown to inhibit dihydroorotate dehydrogenase (DHODH), an enzyme required for de novo pyrimidine synthesis, leading to specific depletion of intracellular nucleotide pools (38, 40–42). Given these capacities, we hypothesized that atovaquone may function similarly to other known nucleotide biosynthesis inhibitors and may inhibit RNA virus replication.
Here, we show that atovaquone is able to inhibit ZIKV and chikungunya virus (CHIKV) replication and virion production in human cells, similar to what has been shown for other pyrimidine biosynthesis inhibitors. Moreover, we found this effect to occur early in infection, during the initial steps of viral RNA synthesis, and that viral inhibition can be rescued with the addition of exogenous pyrimidines, indicating that this drug functions through the blocking of DHODH and depletion of intracellular nucleotides. Finally, we show that atovaquone can inhibit ZIKV infection in an ex vivo human placental tissue model. Taken together, these studies identify atovaquone as an antiviral compound with potential pregnancy-acceptable benefits. More importantly, they highlight the potential to repurpose available drugs in the hopes to one day translate these findings to novel and safe approaches, preventing arbovirus-related outcomes in vulnerable populations.
(This article was submitted to an online preprint archive [43].)
RESULTS
Atovaquone inhibits arbovirus replication in vitro.
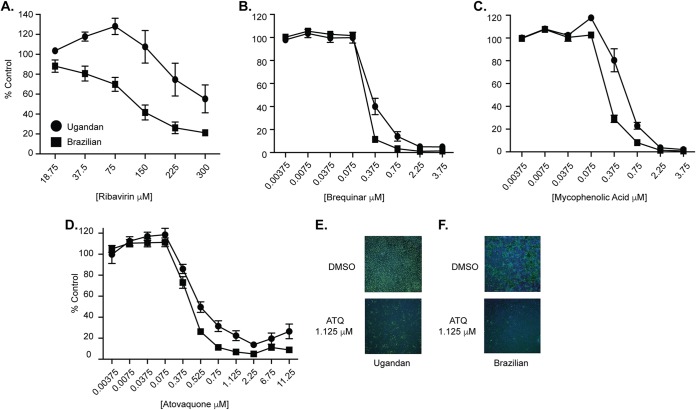
Nucleotide biosynthesis inhibitors have been shown to have antiviral activity toward a wide range of RNA viruses both in vitro and in vivo (22, 26, 31, 44–46), suggesting that manipulating this pathway is a potential avenue for antiviral development. However, many of these compounds are not approved for use in high-risk populations such as pregnant women and children. Atovaquone is a well-tolerated antiparasitic drug that has been used extensively for the treatment and prevention of Pneumocystis jirovecii pneumonia (PCP), toxoplasmosis, babesiosis, and malaria (35–37, 39), yet the antiviral role of atovaquone has not been addressed. To examine the antiviral activity of atovaquone, we pretreated Vero cells with increasing concentrations of atovaquone as well as ribavirin, MPA, and brequinar, known nucleotide biosynthesis inhibitors that have been shown to have antiviral function (Fig. 1). The cells were subsequently infected with either the Ugandan or Brazilian strain of Zika virus (ZIKV), and viral inhibition was assessed at 72 h postinfection by immunostaining for the ZIKV envelope (E) protein. We found that nucleotide biosynthesis inhibitors were able to inhibit ZIKV replication of both strains (Fig. 1A to C) and that atovaquone exhibited similar antiviral activity over the concentrations tested (Fig. 1D to F). Taken together, these studies show that atovaquone inhibits ZIKV infection and spread, potentially through the depletion of intracellular nucleotide pools.
FIG 1.
Nucleotide biosynthesis inhibitors impair ZIKV replication. (A to D) Vero cells were pretreated with ribavirin (A), brequinar (B), mycophenolic acid (C), atovaquone (D), or carrier controls for 2 h and subsequently infected with either the Ugandan (MR766) or Brazilian (Paraiba_01/2015) strain of ZIKV at an MOI of 0.1. Cells were fixed at 72 h postinfection and stained with anti-flavivirus E antibody, and infected cells were quantified on a CellInsight CX7 high-content microscope. Data are represented as percent ZIKV-positive cells compared to the carrier control. (E and F) Representative images of Ugandan strain (E) and Brazilian strain (F) atovaquone (ATQ) inhibition at 1.125 μM. Data represent three independent experiments, each with internal duplicates.
Atovaquone impairs ZIKV virion production in mammalian cells.
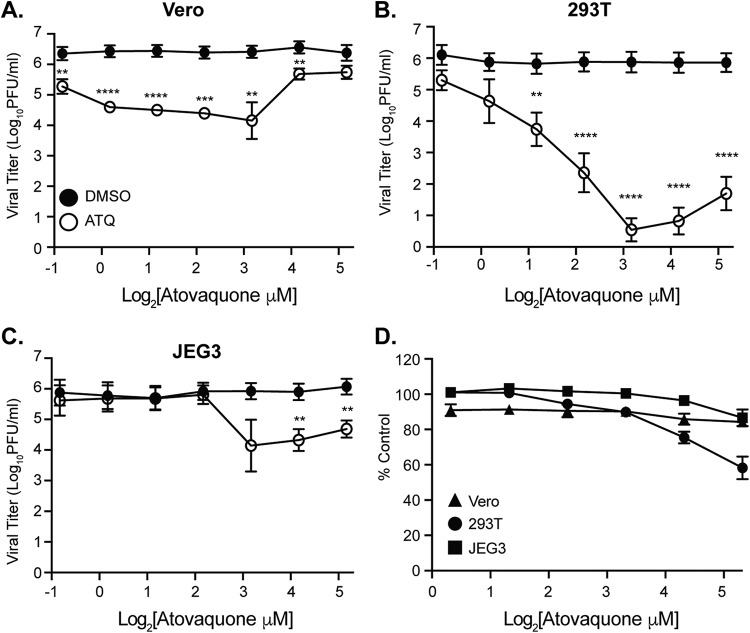
Given the inhibition of viral replication in Vero cells, we next addressed whether atovaquone was able to reduce the production of infectious ZIKV particles in mammalian cell types, including human cells. For these and all subsequent studies, we chose to use the Ugandan strain of ZIKV due to its robust replication in vitro (Fig. 1). We infected Vero, human 293T, and human placental JEG3 cells with the Ugandan strain of ZIKV in the presence of atovaquone or a dimethyl sulfoxide (DMSO) control and quantified infectious virion production by plaque assay. We found that atovaquone significantly impaired virion production in all cell types, tested although the peak of inhibition varied between cell type (Fig. 2A to C).
FIG 2.
Atovaquone inhibits ZIKV infectious virion production in mammalian cells. (A to C) Vero (A), 293T (B), and JEG3 (C) cells were infected with ZIKV MR766 at an MOI of 0.1 in the presence of atovaquone (open symbols) or DMSO control (closed symbols). Virus-containing supernatants were collected at 36 h postinfection, and infectious virus was quantified by plaque assay on Vero cells. The mean and standard error of the mean (SEM) are shown. For Vero and JEG3 cells, data represent three independent experiments, each with internal triplicates; for 293T cells, data represent four independent experiments, each with internal technical triplicates (**, P < 0.01; ***, P < 0.005; ****, P < 0.0001 [Student’s t test]). (D) Cells were incubated with increasing concentrations of atovaquone or DMSO control for 36 h and subsequently stained with Sytox green reagent to visual dye-permeable dead cells. Dead (Sytox-positive) cells were quantified by microscopy on a CellInsight CX7 high-throughput platform. Data are represented as percentage of alive cells (Sytox negative) compared to those in the DMSO control. The mean and SEM are shown. Data represent three independent experiments, each with internal technical triplicates.
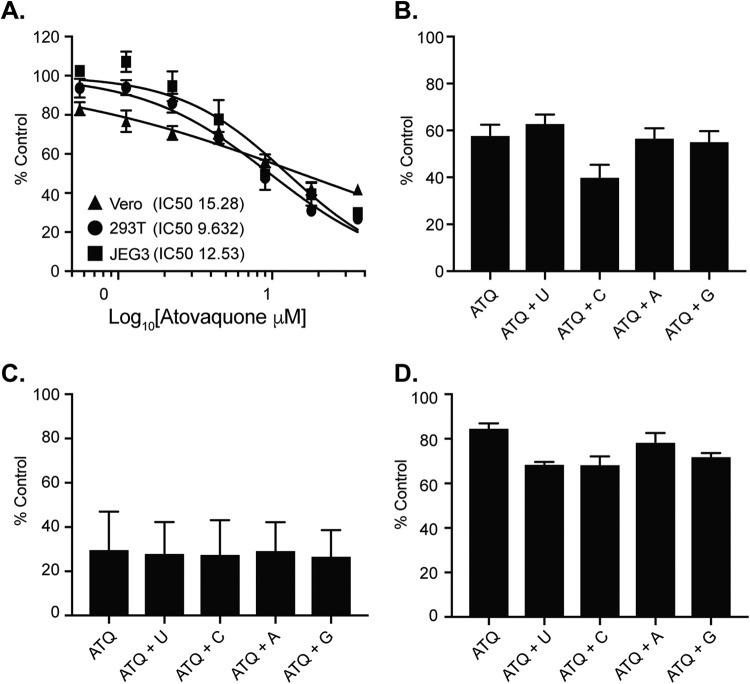
One potential explanation for these results could be that atovaquone is toxic and this leads to reduced virus production. To address this, we measured cell viability with Sytox green, a dye that binds nucleic acids when both the plasma and the nuclear membrane are permeabilized and thus represents dying cells (Fig. 2D). Using this assay, we found that high concentrations of atovaquone, particularly in 293T cells, did lead to more cell death than the DMSO control; however, lower concentrations had minimal effects on cell viability. As a control to make sure that atovaquone was working in these cells, we used the fact that atovaquone is a mitochondrial cytochrome complex III inhibitor (38, 41, 47) and measured its activity by a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) cell proliferation assay. As expected, we found that atovaquone caused a dose-dependent reduction in mitochondrial activity compared to the DMSO control (Fig. 3A), indicating that it is functional yet does not lead to significant cell death. Moreover, these results were confirmed in our data as although we observed a reduction in mitochondrial function by MTT assay, we found that in Vero and 293T cells, virus production increased at higher concentrations of atovaquone, suggesting that the cells are still competent for virus production under these conditions. Taken together, these results show that atovaquone is able to inhibit ZIKV virion production in mammalian cell types.
FIG 3.
Atovaquone inhibits mitochondrial function and is unable to be rescued by exogenous nucleosides. (A) Mitochondrial function and cell proliferation were measured by MTT assay. Each cell type was incubated with increasing concentrations of atovaquone or DMSO control, and MTT assay was performed at 36 h postincubation. Data are represented at a percentage of the value for the DMSO control. The mean and SEM are shown. Data represent three independent experiments, each with internal technical triplicates. (B to D) Vero (B), JEG3 (C), and 293T (D) cells were incubated with 4.5 μM, 18 μM, and 4.5 μM atovaquone, respectively (concentrations where we found maximum inhibition of virion production) in the presence or absence of 100 μM exogenous nucleosides for 36 h at 37°C. Following incubation, cell proliferation was analyzed by MTT assay. Data are represented as a percentage of the value for the DMSO control. The mean and SEM are shown. Data represent three independent experiments, each with internal technical triplicates.
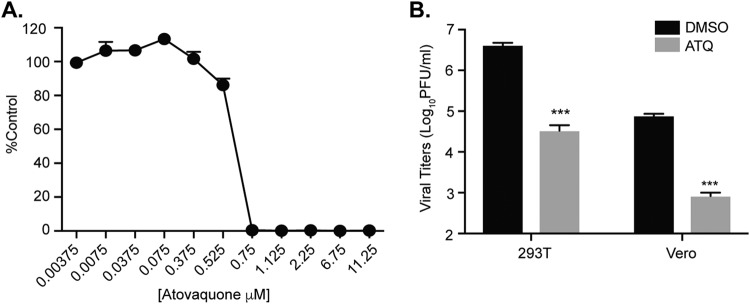
To expand on these findings, we addressed whether atovaquone could inhibit CHIKV, an arbovirus transmitted by Aedes mosquitoes and capable of causing severe human disease and significant outbreaks (48–50). Importantly, CHIKV has been shown to be inhibited by nucleotide biosynthesis inhibitors (27, 51, 52), and thus we hypothesized that it would be inhibited by atovaquone. We infected Vero cells with a CHIKV expressing a ZsGreen reporter in the presence of increasing concentrations of atovaquone and quantified the number of ZsGreen-positive cells after treatment by microscopy. Similar to the case for ZIKV, CHIKV replication was inhibited by atovaquone in a dose-dependent manner (Fig. 4A). This inhibition in replication was further confirmed in Vero and 293T cells, where we observed reductions in infectious CHIKV virions after treatment with atovaquone (Fig. 4B). These results suggest that atovaquone can inhibit multiple arboviruses and has the potential to be used as a well-tolerated antiviral therapy.
FIG 4.
Atovaquone inhibits chikungunya virus replication. (A) Vero cells were pretreated with DMSO or atovaquone (ATQ) for 2 h and subsequently infected with CHIKV (IOL) expressing ZsGreen at an MOI of 0.1. After infection, virus was removed, cells were washed, and medium containing atovaquone was added and left for 24 h. Cells were then fixed, and ZsGreen-positive cells were quantified with a CellInsight CX7 high-content microscope. Data are represented as percent ZsGreen-positive cells compared to the DMSO control. Data represent three independent experiments. (B) 293T and Vero cells were infected with CHIKV-ZsGreen at an MOI of 0.1 in the presence of 4.5 μM atovaquone. Unabsorbed virus was washed off, and medium containing DMSO or 4.5 μM atovaquone was added. Infectious virus was quantified at 24 h postinfection by plaque assay. The mean and SEM are shown. Data represent three independent experiments. ***, P < 0.005 (Student’s t test).
Atovaquone inhibits ZIKV at early stages of infection.
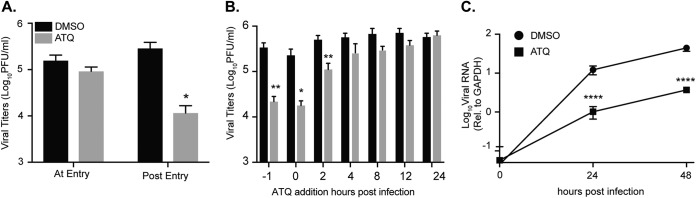
To explore which stage of the viral life cycle atovaquone targets, we first addressed viral entry by treating cells with atovaquone or DMSO during virus entry, washing the cells extensively, and adding back complete medium (Fig. 5A, at entry). As a control, we added atovaquone during entry and added back medium containing atovaquone after infection (Fig. 5A, post entry). We found that when cells were treated during viral entry, there was no change in production of viral particles compared to that with DMSO, yet when added after entry, atovaquone was able to inhibit ZIKV replication, suggesting that atovaquone functions postentry. To investigate this further, we performed time-of-addition experiments to test which part of the intracellular viral life cycle is targeted by atovaquone. We infected 293T cells with ZIKV, added atovaquone or a DMSO control at different time points during infection (Fig. 5B), and quantified viral titers by plaque assay at 36 h postinfection. We found that ZIKV virion production is most inhibited at early stages of infection (up to 4 h postinfection) and that this effect diminishes as the infection progresses, suggesting that atovaquone acts early during infection, potentially inhibiting RNA replication. Finally, to investigate the impact of atovaquone on ZIKV RNA replication, we analyzed ZIKV intracellular RNA at multiple time points postinfection (Fig. 5C). We found that whereas viral RNA levels were equal after infection (time zero), again confirming that there is no effect of atovaquone on viral entry, there was a significant difference in viral RNA at 24 and 48 h postinfection, indicating that atovaquone acts to inhibit ZIKV RNA replication early during infection.
FIG 5.
Atovaquone acts early during ZIKV infection and inhibits viral RNA synthesis. (A) 293T cells were infected with ZIKV MR766 at an MOI of 0.5 in the presence of 4.5 μM atovaquone. After absorption, cells were washed extensively, and medium was added without (at entry) or with (post entry) 4.5 μM atovaquone. Infectious virus was quantified by plaque assay at 36 h postinfection. The mean and SEM are shown. Data represent four independent experiments, each with internal technical triplicates. *, P < 0.05 (Student’s t test). (B) 293T cells were treated with 4.5 μM atovaquone 1 h prior, during, or postinfection with ZIKV MR766 at an MOI of 1. After infection, virus was removed and medium containing 4.5 μM atovaquone was added and left for 36 h. Infectious virus was quantified by plaque assay at 36 h postinfection. The mean and SEM are shown. Data represent three independent experiments, each with internal technical triplicates. *, P < 0.05; **, P < 0.01 (Student’s t test). (C) Vero cells were infected with ZIKV MR766 at an MOI of 10 in the presence of 4.5 μM atovaquone. After infection, virus was removed, cells were washed extensively, and medium containing 4.5 μM atovaquone was added. At time zero (after infection) and at 24 and 48 h postinfection, medium was removed, cells were washed, and intracellular RNA was extracted with TRIzol. Viral RNA was quantified by SYBR green in comparison to GAPDH. The mean and SEM are shown. Data represent three independent experiments, each with internal technical triplicates ****, P < 0.0001 (Student’s t test).
ZIKV RNA replication and virion production are rescued by the addition of exogenous nucleosides.
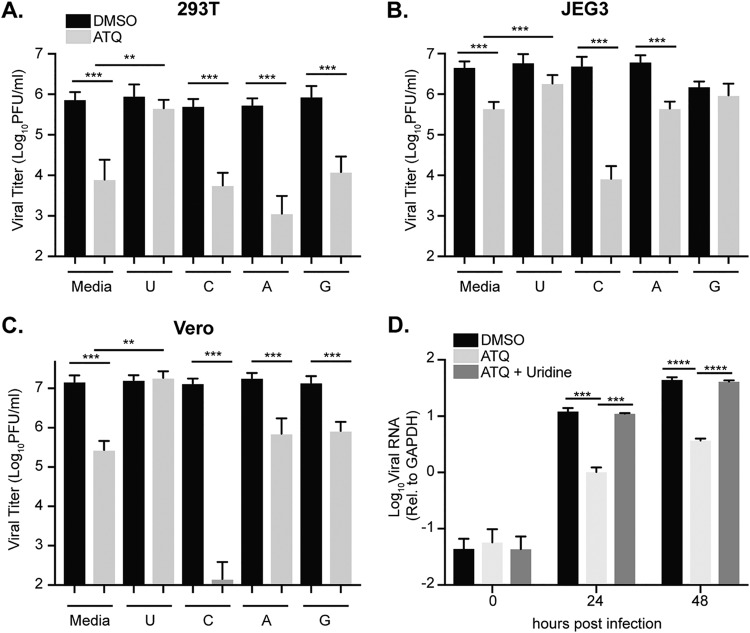
Given the role of atovaquone in the inhibition of DHODH and viral inhibition curves to those for brequinar, another inhibitor of DHODH and pyrimidine biosynthesis (Fig. 1B), we hypothesized that atovaquone may function through a similar pathway. To address this, we performed a rescue experiment where we infected cells with ZIKV in the presence of atovaquone followed by medium with atovaquone supplemented with 100 μM uridine, cytidine, adenosine, or guanosine. As a control, we addressed the impact of nucleosides on DMSO-treated cells. We found that nucleoside addition led to no significant increases in virus production, yet the addition of guanosine to JEG3 cells seemed to decrease virus production. Nonetheless, in atovaquone-treated cells, we found that in all cells types, ZIKV infectious particle production was rescued only when uridine was added to the medium (Fig. 6A to C). Given these results and the dual function of atovaquone in blocking both mitochondrial function and DHODH, it is possible that the addition of exogenous nucleosides could simply have rescued the MTT phenotype (mitochondrial function) that we see for atovaquone and thus ZIKV replication. However, we found that when cells were incubated in the presence of atovaquone and nucleoside, there was no change in MTT cell proliferation (Fig. 3B to D), indicating that the ZIKV inhibition and rescue we observed are not through mitochondrial inhibition but rather through the inhibition of DHODH. Furthermore, we addressed the ability of uridine to complement ZIKV RNA synthesis and found that indeed the addition of exogenous uridine rescued this phenotype to levels similar to those for the DMSO control (Fig. 6D). Taken together, these results show that the inhibition of ZIKV RNA replication and virion production by atovaquone can be rescued by the addition of exogenous uridine.
FIG 6.
Exogenous uridine rescues ZIKV virion production and RNA synthesis. (A to C) 293T (A), JEG3 (B), and Vero (C) cells were infected with ZIKV MR766 at an MOI of 0.1 in the presence of DMSO or atovaquone (293T and Vero, 4.5 μM atovaquone; JEG,318 μM atovaquone) with complete medium (Media) or 100 μM uridine (U), cytidine (C), adenosine (A), or guanosine (G). After infection, virus was removed, cells were washed, and medium containing DMSO or atovaquone with complete medium or 100 μM nucleoside was added. Infectious virus was quantified at 36 h postinfection by plaque assay. The mean and SEM are shown. Data represent three independent experiments, each with internal technical duplicates **, P < 0.05; ***, P < 0.005; ****, P < 0.0001 (Mann-Whitney test). (D). Vero cells were infected with ZIKV MR766 at an MOI of 10 in the presence of DMSO, 4.5 μM atovaquone, or 4.5 μM atovaquone with 100 μM uridine. Intracellular viral RNA was extracted with TRIzol at 0, 24, and 48 h postinfection, and viral RNA relative to GAPDH was quantified with SYBR green. The mean and SEM are shown. Data represent three independent experiments, each with internal technical duplicates ****, P < 0.0001 (two-way ANOVA).
Atovaquone inhibits ZIKV infection in an ex vivo human placental tissue model.
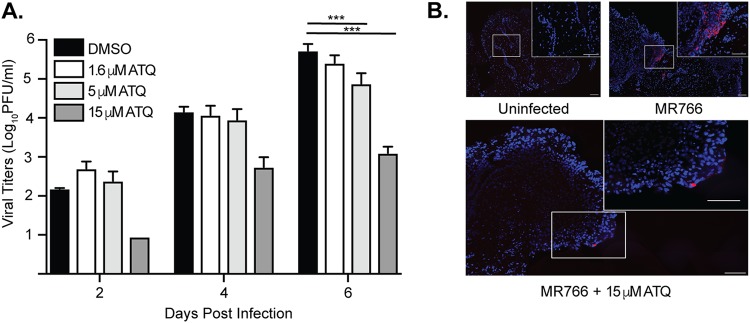
We found that atovaquone significantly inhibited ZIKV in human placental JEG3 cells in vitro, and thus we were interested in determining the extent to which this compound could inhibit ZIKV infection in an ex vivo human placental tissue model. To investigate this, we infected human placental chorionic villus explants with the Ugandan strain of ZIKV in the presence of increasing doses of atovaquone. Similar to data in cell lines, we found that ZIKV infection and virion production were inhibited in a dose-dependent manner in the human placental tissue (Fig. 7A). These results were confirmed by fluorescence in situ hybridization probing for ZIKV RNA in ZIKV-infected tissue where atovaquone treatment reduced ZIKV spread (Fig. 7B), showing a dose-dependent decrease in ZIKV-infected cells in the presence of increasing amounts of atovaquone. To rule out any adverse effects of atovaquone on these tissues, we performed terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining on uninfected and infected tissue at all atovaquone concentrations and, similar to the in vitro results, found no significant differences in cell death (data not shown). These data highlight that atovaquone may provide protection in the human maternal-fetal interface during ZIKV infection.
FIG 7.
Atovaquone inhibits ZIKV infection and spread in an ex vivo human placental tissue model. Human placental chorionic villus explants were infected with 105 PFU ZIKA (MR766) in the presence of increasing concentrations of atovaquone or a DMSO carrier control. Supernatants were collected at days 2, 4, and 6 postinfection, and viral titers were quantified by plaque assay. (A) Total virus accumulation over the course of infection. The mean and SEM are shown. Data represent three independent experiments. ***, P < 0.005 (two-way ANOVA). (B) Fluorescence in situ hybridization of ZIKV RNA (red)-infected human tissues counterstained with DAPI at 6 days postinfection.
DISCUSSION
It remains unknown when the next outbreak of ZIKV will occur, yet we know through past devastating epidemics, in which millions of individuals, including women and children, were affected by this virus that we still have an urgent need for effective therapies against ZIKV infection (16, 53, 54). Despite current potential protection due to herd and self-immunity, environmental factors, and host-vector-virus interactions that keep ZIKV at low incidence (1, 55, 56), preventing ZIKV and other arbovirus infections should be a priority. In recent years, many compounds have been proposed as potential anti-ZIKV agents following in vitro results (29, 30, 57–66). Some of these drugs have an extensive background in the medical field and offer attractive options, either alone or in combination, for treatment and perhaps prophylaxis of ZIKV infections; however, most of them remain inadequate to be used during pregnancy. Only chloroquine has been demonstrated in animal models of pregnancy to be effective against ZIKV (64, 65), and none of them have been tested in humans in the context of ZIKV infection. Here, we propose a pregnancy-acceptable drug candidate for the treatment of ZIKV and other potential viral infections, highlighting the repurposing of FDA-approved drugs as a possible avenue for antiviral development.
Atovaquone, a ubiquinone analogue approved in humans since 1999 for the treatment of Pneumocystis jirovecii pneumonia (PCP) (37, 39) and prevention of malaria (36), has no antiviral activity described in the literature to date. In this work, we addressed the antiviral role of atovaquone in ZIKV infections in vitro and in an ex vivo human placental tissue model and also explored the antiviral effect on CHIKV in vitro. We found that atovaquone, similar to the known antiviral compounds ribavirin, MPA, and brequinar, was able to inhibit ZIKV replication and infectious particle production in multiple mammalian cell types, including human placental cells. Interestingly, we found that in Vero and 293T cells, higher concentrations of drug had the least impact on virion production. One explanation for this could be that the virus has evolved to be resistant to atovaquone. However, we find this unlikely given the short time of infection and that drug resistance typically takes multiple passages. Additional explanations could be that at high concentrations, the inhibition of de novo pyrimidine biosynthesis leads to an increased utilization in the nucleotide salvage pathways, thus restoring intracellular nucleotide levels and viral replication, or that atovaquone is simply interfering with other cellular pathways that impede its antiviral effects. This may be of particular importance for the use of atovaquone as an antiviral in humans. In the in vitro human cell culture system, the 50% inhibitory concentration (IC50) of atovaquone for mitochondrial inhibition was roughly 10 μM, which contrasts with historical trials of atovaquone taken orally at a doses of 750 mg every 6 h for the treatment of toxoplasmosis, which reported steady serum concentrations in humans of roughly 50 μM without associated toxicity (41, 67, 68). One possible explanation for this difference is that in vitro studies do not entirely represent all the biological interactions that take place in the human body. In addition, it is possible that at this high concentration, atovaquone is not antiviral and thus would need to be optimized at lower concentrations for its antiviral function in humans.
To address the stage in the viral life cycle where atovaquone acts, we first performed viral entry assays and found that adding atovaquone at the time of infection had no effect on virion production, whereas adding atovaquone after infection was able to reduce ZIKV replication, indicating that atovaquone acts postentry. We then performed time-of-addition assays and found that only early during infection, within the first 2 to 4 h postinfection, was atovaquone able to inhibit viral replication. These data are similar to what has been seen for the antiviral effects of brequinar on DENV (23, 69), suggesting that atovaquone may function by a similar mechanism. Given these results, we hypothesized that atovaquone functions during the initial steps of ZIKV RNA replication and blocks virion production through the inhibition of RNA replication. When we quantified ZIKV RNA levels over time, we found that indeed atovaquone treatment significantly reduced viral RNA synthesis.
Atovaquone is thought to function primarily through the inhibition of mitochondrial cytochrome complex III and thus inhibition of mitochondrial function in the parasite (39, 41, 42). Using an MTT assay of cell proliferation measured through mitochondrial function we also saw that atovaquone was able to reduce mitochondrial function in all cell lines we analyzed, yet these cells were shown to be viable by Sytox green staining. Atovaquone also functions through inhibiting dihydroorotate dehydrogenase (DHODH) (38), an enzyme involved in pyrimidine biosynthesis and in particular the synthesis of UMP. Given the striking similarities to brequinar, another pyrimidine biosynthesis inhibitor, and that atovaquone inhibited ZIKV RNA synthesis, we hypothesized that this inhibition was through intracellular nucleotide depletion. To address this, we added exogenous nucleosides in the presence of atovaquone and indeed found that the addition of uridine was able to rescue ZIKV infection in all cell types. Moreover, we found that the addition of uridine was able to specifically rescue ZIKV RNA synthesis, suggesting that atovaquone functions through the depletion of intracellular nucleotide pools, and the addition of exogenous uridine can rescue ZIKV replication via the pyrimidine salvage pathway, bypassing the inhibition of atovaquone at critical steps in the de novo pyrimidine synthesis inhibition. We found it interesting that human 293T and JEG3 cells were unable to be completely rescued with the addition of uridine. However, it has been shown that nucleotide depletion will induce an antiviral innate immune response (31, 32, 46), and we hypothesize that a similar mechanism may be induced in these cells, allowing them to retain antiviral function in the presence of exogenous uridine. One striking finding was that atovaquone in the presence of cytidine in Vero and JEG3 cells, and to a lesser extent adenosine in 293T cells, had an additive antiviral effect. Moreover, we found that the addition of guanosine alone with DMSO was able to reduce viral titers, suggesting that multiple cell-specific metabolic pathways are at play, including key regulatory mechanisms controlling pyrimidine levels within individual cell types. Future studies exploring the nucleotide metabolism of specific cell types, including placental cells, could shed light on novel pathways needed for viral replication. Finally, we show that atovaquone also works to inhibit ZIKV infection in human first- and second-trimester placental explants. Many studies have shown that placental cytotrophoblasts, trophoblasts, Hofbauer cells, and fibroblasts are susceptible to ZIKV (18, 20, 70–74); thus, recreating the dynamics of this infection and host-virus interaction at the placental level makes this study relevant to the most vulnerable target of ZIKV, pregnant women.
Taking our results together, we found that atovaquone, a pregnancy-acceptable and common antiparasitic drug, has antiviral activity against ZIKV and CHIKV which can be translated in the clinical setting into an attractive candidate for the treatment and prevention of arbovirus infections in vulnerable populations as well as in individuals who live in or travel to areas of endemicity. Furthermore, patients with AIDS, chronic steroid users, and posttransplant patients who take atovaquone daily for the prevention of PCP remain at high risk of acquiring multiple viral infections due to their impaired immune system. Therefore, it could be valuable to estimate the effect of atovaquone in viruses relevant to these patients, such as human cytomegalovirus (75), herpes simplex virus (76), JC virus (77), and respiratory syncytial virus (78). Atovaquone (in combination with proguanil hydrochloride) is already commercially available and broadly prescribed for malaria prophylaxis, yet so far there have not been proposed any clinical trials that address the relationship of atovaquone and ZIKV or CHIKV infections. This raises the questions of whether (i) individuals who are/were taking atovaquone-proguanil (Malarone) are protected from viral threats as well and (ii) the broad administration of drugs which may possess unknown antiviral functions impact the evolution of viral infections. Future studies addressing these questions will be essential to understanding the antiviral effect of atovaquone on viral evolution and disease. Nonetheless, these results contribute to the urgent need of finding effective ZIKV treatments, especially for pregnant women, as these treatments should be readily accessible in order to ameliorate the teratogenic consequences of ZIKV across all trimesters of pregnancy. The studies completed here identified a potential candidate for these at-risk populations, yet more work is needed to define the complete antiviral role of atovaquone in vivo. Moreover, these studies have highlighted that repurposing drugs may provide fast avenues to the development of novel antiviral therapies and that we can potentially exploit FDA-approved drugs to fight emerging viral threats.
MATERIALS AND METHODS
Cells and viruses.
Vero cells (ATCC CCL-81) were cultured in Dulbecco modified Eagle medium (DMEM) (Corning) supplemented with 10% new born calf serum (NBCS) (Gibco) and 100 μg/ml penicillin-streptomycin (P/S) (Corning) at 37°C with 5% CO2. BHK-21 (ATCC CCL-10), 293T (ATCC CRL-3216), and JEG3 (provided by Carolyn Coyne, University of Pittsburgh) cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), 1% nonessential amino acids (NEAA) (Corning), and 1% P/S at 37°C with 5% CO2. All cell lines were confirmed to be mycoplasma free.
The Ugandan (MR766) (79) and Brazilian (Paraiba_01/2015) (80) strains of ZIKV were generated from infectious clones provided by Matthew Evans (Icahn School of Medicine at Mount Sinai) and Alexander Pletnev (National Institutes of Health), respectively. To generate initial viral stocks, each plasmid was transfected via Lipofectamine 2000 reagent (Invitrogen) into 293T cells, and virus-containing supernatant was harvested at 48 h posttransfection. A working viral stock was then generated by passaging the initial viral stock over Vero cells. Viral titers were quantified by plaque assay. In brief, 10-fold serial dilutions of each virus in DMEM were added to a confluent monolayer of Vero cells and left for 1 h at 37°C. Following incubation, cells were overlaid with 0.8% agarose in DMEM and 2% NBCS and incubated at 37°C for 5 days. The cells were fixed with 4% formalin, agarose plugs were removed, and plaques were visualized with crystal violet.
Wild-type CHIKV was generated from the La Reunion 06-049 infectious clone as previously described (51, 81). A CHIKV La Reunion infectious clone expressing ZsGreen was constructed by standard molecular biology techniques. First, an AvrII restriction enzyme site was inserted 5′ of the subgenomic promoter by site-directed mutagenesis using the primers 5′-CACTAATCAGCTACACCTAGGATGGAGTTCATCCC-3′ (forward) and 5′-GGGATGAACTCCATCCTAGGTGTAGCTGATTAGTG-3′ (reverse). The CHIKV subgenomic promoter was then amplified by PCR (forward primer, 5′-CCTAGGCCATGGCCACCTTTGCAAG-3′; reverse primer, 5′-ACTAGTTGTAGCTGATTAGTGTTTAG-3′) and subcloned into the AvrII site to generate a CHIKV infectious clone containing two subgenomic promoters. Finally, the ZsGreen cassette was amplified by PCR (forward primer, 5′-GTGTACCTAGGATGGCCCAGTCCAAGCAC-3′; reverse primer, 5′-GCTATCCTAGGTTAACTAGTGGGCAAGGC-3′) from a CHIKV infectious clone obtained from Andres Merits (University of Tartu) and subcloned into the AvrII restriction enzyme site. The complete cassette and subgenomic regions were sequenced to ensure that there were no second-site mutations. To generate infectious virus, each plasmid was linearized overnight with NotI, phenol-chloroform extracted, ethanol precipitated, and used for in vitro transcription using the SP6 mMessage mMachine kit (Ambion). In vitro-transcribed RNAs were phenol-chloroform extracted, ethanol precipitated, aliquoted at 1 μg/μl, and stored at −80°C. Ten micrograms of each RNA was electroporated into BHK-21 cells (28), and virus was harvested at 48 h postelectroporation. Working virus stocks were generated by passaging virus over BHK-21 cells, and viral titers were quantified by plaque assay as described above.
Ethics statement.
For these experimental studies, first- and second-trimester human placental tissue was obtained within 2 h of surgery from donors undergoing elective termination under an institutional review board (IRB) protocol approved by the Institutional Review Board for the Icahn School of Medicine at Mt. Sinai (HS 12-00145). All subjects provided informed written surgical consent for the use of deidentified waste materials for educational research. Tissue specimens are considered to be nonhuman subjects since they are deidentified. Following the surgery, tissue specimens are delivered to Mount Sinai's Institutional Biorepository and Molecular Pathology Shared Resource Facility (SRF) in the Department of Pathology. The biorepository operates under a Mount Sinai Institutional Review Board (IRB)-approved protocol and follows guidelines set by HIPAA. All samples are linked, with appropriate IRB approval and consent, to clinical and pathological data and are open to all investigators of the institution, as well as for specific third-party collaborative efforts with investigators from other institutions.
Ex vivo infection of human placental tissue.
First- and second-trimester human placental tissue was obtained as described above. Chorionic villi adjacent to the fetal chorionic plate were placed into prewarmed DMEM containing 25% F-12 medium, 10% FBS, 5 mM HEPES, 2 mM glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B (Fungizone), and 300 ng/ml Timentin as previously described (82). After removal of the amnion and decidua, the chorionic villi were cut into 0.2-cm3 blocks; nine blocks were plated per well of a six-well plate onto collagen gel foams (Cardinal Health) in 3 ml medium, and 3 wells were used per condition (27 tissue blocks). Following an overnight incubation at 37°C, tissue blocks were individually infected with 1 × 105 PFU ZIKVMR766 in a volume of 5 μl that was preincubated with 15 μM, 5 μM, or 1.6 μM atovaquone for 1 h. Atovaquone was maintained in the culture medium at the same concentrations throughout 6 days of culture. Supernatants were collected and medium changed every other day.
ZIKV PFU assay.
ZIKV PFU were quantified on Vero cell monolayers, whereby 250 μl of tissue culture supernatant was adsorbed for 2 h at 37°C in 12-well plates and cells were overlaid with 1.5 ml DMEM (Invitrogen) supplemented with 0.8% methyl cellulose, 2% FBS, and 50 μg/ml gentamicin sulfate. Cells were incubated for 5 days at 37°C, fixed with 4% paraformaldehyde, and stained with crystal violet for plaque visualization.
ZIKV RNA detection by in situ hybridization.
Placental tissues from day 6 postinfection were fixed in 10% neutral buffered formalin for 24 h and placed back into phosphate-buffered saline (PBS) until paraffin embedding. In situ hybridization using RNAscope was performed on 5-μm paraffin-embedded sections. Deparaffinization and target retrieval were performed using RNAscope universal pretreatment reagents (ACD 322380) following the manufacturer’s protocol, and fluorescence in situ hybridization was subsequently performed according to the manufacturer’s protocol (ACD 323110) with RNAscope probe V-ZIKVsph2015 (ACD 467871) as previously described (65). Following in situ hybridization, slides were mounted with Vectashield hard-set mounting medium with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories) and analyzed using an AxioImager Z2 microscope (Zeiss) and Zen 2012 software (Zeiss).
Apoptosis detection assay.
Formalin-fixed paraffin-embedded placental tissues were cut into 8-μm sections and stained using a TACS 2 TdT fluorescein in situ apoptosis detection kit (Trevigen, catalog number 4812-30-K). Slides were then mounted with Vectashield hard-set mounting medium containing DAPI (Vector Laboratories) and analyzed using an AxioImager Z2 microscope (Zeiss) and Zen 2012 software (Zeiss).
Drug sensitivity assays.
Vero cells (10,000 cells/well in a 96-well plate) were pretreated with medium containing a carrier control or drug (ribavirin, mycophenolic acid, brequinar [Sigma], or atovaquone (ABCAM) for 2 h at 37°C. Following preincubation, cells were incubated with ZIKV or CHIKV-ZsGreen at a multiplicity of infection (MOI) of 0.1 in the presence of each drug or carrier control for 1 h at 37°C. Cells were then washed extensively, and medium containing drug or carrier was added to each well. After incubation at 37°C for 48 h, cells were fixed with 4% paraformaldehyde and subject to immunostaining or visualized directly in the case of CHIKV-ZsGreen. In brief, following fixation, the cells were washed with Perm-Wash buffer (BD Bioscience), permeabilized with 0.25% Triton X-100 in phosphate-buffered saline (PBS) (Gibco), and blocked with 0.2% bovine serum albumin (BSA) and 0.05% saponin in PBS for 1 h at room temperature (RT). Cells were then incubated with a monoclonal mouse antibody to the Flavivirus envelope protein (4G2) (Millipore) for 1 h at RT. Following primary antibody incubation, cells were washed with Perm-Wash buffer and incubated with a secondary anti-mouse IgG antibody conjugated to Alexa488 for 1 h at RT. Cells were then washed, and infected cells were quantified on a CellInsight CX7 high-content microscope and screening platform using uninfected cells as a negative control and a cutoff for three standard deviations from negative to be scored as an infected cell.
To address the effect of atovaquone on infectious virion production, cells were seeded as described above and infected with ZIKV at an MOI of 0.1 in the presence of increasing concentrations of atovaquone for 1 h at 37°C. Cells were washed with PBS and incubated in medium containing atovaquone or DMSO as a control for 36 h at 37°C. Virus-containing supernatants were collected, and viral titers were quantified by plaque assay.
Cell viability assays.
Cell viability was assayed using Sytox green (Invitrogen) following the manufacturer’s instructions. In brief, Vero, 293T, and JEG3 cells (10,000 cells/well in a 96-well plate) were treated with increasing concentrations of atovaquone and incubated for 36 h at 37°C. Following the incubation, cells were washed with phosphate-free buffer, treated with a 1:20,000 dilution of the Sytox green stain for 30 min, and washed again to remove unbound stain. Sytox green positive-cells were quantified on the CellInsight CX7 high-content microscope as described above. Mitochondrial function and cell proliferation were measured using the CellTiter 96 nonradioactive cell proliferation assay (Promega), according to the manufacturer’s protocol. Vero, 293T, and JEG3 cells (10,000 cells/well in a 96-well plate) were treated with increasing concentrations of atovaquone and incubated for 36 h at 37°C. Following the incubation, 15 μl of dye solution was added to each well and incubated for 4 h at 37°C with 5% CO2. The reaction was stopped by the addition of 100 μl of solubilization/stop solution, and the absorbance was measured at 570 nm in an EnVision microplate reader. The 50% cytotoxic concentration (CC50) was calculated by a nonlinear regression analysis of the dose-response curves.
RNA extractions and reverse transcription-quantitative PCR.
Intracellular viral RNA was extracted with TRIzol reagent (Invitrogen) following the manufacturer’s instructions and used directly for cDNA synthesis with the Maxima H minus-strand kit (Thermo). Relative viral RNA levels were quantified using Power SYBR green (Applied Biosystems) with the following primers (83): GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 5′-GAAGGTCGGAGTCAACGGATTT-3′ and 5′-GAATTTGCCATGGGTGGAAT-3′, and ZIKV, 5′-AGATGACTGCGTTGTGAAGC-3′ and 5′-GAGCAGAACGGGACTTCTTC-3′.
Virus entry and time-of-addition assays.
To assay for the role of atovaquone in viral entry, 293T cells were infected with ZIKV at an MOI of 0.1 in the presence of 4.5 μM atovaquone for 1 h at 37°C. Cells were washed extensively, complete medium with or without 4.5 μM atovaquone was added back to the cells, and virus-containing supernatants were collected at 36 h postinfection. Viral titers were quantified by plaque assay. As a control, medium containing atovaquone was added after infection.
For time-of-addition studies, 293T cells, pretreated with 4.5 μM atovaquone for 1 h or left untreated, were infected with ZIKV at an MOI of 1 in the presence or absence of atovaquone for 1 h at 37°C. Following incubation, cells were washed to remove unabsorbed virus, and medium with or without atovaquone was added. At different time points postinfection, medium was removed and medium containing 4.5 μM atovaquone was added to the infected cells. Culture medium was collected at 36 h postinfection, and viral titers were quantified by plaque assay. Medium containing DMSO was used as a control for all time points.
Rescue assay.
Vero, 293T, and JEG3 cells were seeded in a 96-well plate as described above. Cells were infected with ZIKV diluted to an MOI of 0.1 in DMEM containing atovaquone for 1 h at 37°C. Following incubation, cells were washed three times with PBS to remove unabsorbed virus. After washing, cells were incubated with medium containing atovaquone with either DMSO or 100 μM uridine, cytidine, adenosine, or guanosine for 36 h. Culture medium was collected at 36 h postinfection and viral titers quantified by plaque assay.
Data analysis and statistics.
GraphPad Prism 7.0 software was used for all analyses. The equations to fit the best curve were generated based on r2 values of ≥0.9 inhibitory concentration versus normalized response). Two-way analysis of variance (ANOVA) and Student t tests were also used, with P values of <0.05 considered statistically significant. All experiments were completed at least three independent times.
ACKNOWLEDGMENTS
We thank all members of the Stapleford lab for support and helpful discussions during the course of the study. We thank Aaron Briley and Meike Dittmann for technical assistance with the CX7 CellInsight high-content microscopy and helpful discussions. Finally, we thank the labs of Carolyn Coyne, Matthew Evans, and Alexander Pletnev for essential cells and reagents.
REFERENCES
- 1.Pierson TC, Diamond MS. 2018. The emergence of Zika virus and its new clinical syndromes. Nature 560:573–581. doi: 10.1038/s41586-018-0446-y. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MR, Chen TH, Hancock WT, Powers AM, Kool JL, Lanciotti RS, Pretrick M, Marfel M, Holzbauer S, Dubray C, Guillaumot L, Griggs A, Bel M, Lambert AJ, Laven J, Kosoy O, Panella A, Biggerstaff BJ, Fischer M, Hayes EB. 2009. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 360:2536–2543. doi: 10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Joguet G, Mansuy JM, Matusali G, Hamdi S, Walschaerts M, Pavili L, Guyomard S, Prisant N, Lamarre P, Dejucq-Rainsford N, Pasquier C, Bujan L. 2017. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 17:1200–1208. doi: 10.1016/S1473-3099(17)30444-9. [DOI] [PubMed] [Google Scholar]
- 4.de Oliveira Dias JR, Ventura CV, de Paula Freitas B, Prazeres J, Ventura LO, Bravo-Filho V, Aleman T, Ko AI, Zin A, Belfort R Jr, Maia M, Zika Virus Study Group. 2018. Zika and the eye: pieces of a puzzle. Prog Retin Eye Res 66:85–106. doi: 10.1016/j.preteyeres.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Carteaux G, Maquart M, Bedet A, Contou D, Brugieres P, Fourati S, Cleret de Langavant L, de Broucker T, Brun-Buisson C, Leparc-Goffart I, Mekontso Dessap A. 2016. Zika virus associated with meningoencephalitis. N Engl J Med 374:1595–1596. doi: 10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- 6.Pradhan F, Burns JD, Agameya A, Patel A, Alfaqih M, Small JE, Ooi W. 2017. Case report: Zika virus meningoencephalitis and myelitis and associated magnetic resonance imaging findings. Am J Trop Med Hyg 97:340–343. doi: 10.4269/ajtmh.16-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karimi O, Goorhuis A, Schinkel J, Codrington J, Vreden SGS, Vermaat JS, Stijnis C, Grobusch MP. 2016. Thrombocytopenia and subcutaneous bleedings in a patient with Zika virus infection. Lancet 387:939–940. doi: 10.1016/S0140-6736(16)00502-X. [DOI] [PubMed] [Google Scholar]
- 8.Swaminathan S, Schlaberg R, Lewis J, Hanson KE, Couturier MR. 2016. Fatal Zika virus infection with secondary nonsexual transmission. N Engl J Med 375:1907–1909. doi: 10.1056/NEJMc1610613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dirlikov E, Torres JV, Martines RB, Reagan-Steiner S, Perez GV, Rivera A, Major C, Matos D, Munoz-Jordan J, Shieh WJ, Zaki SR, Sharp TM. 2018. Postmortem findings in patient with Guillain-Barre syndrome and Zika virus infection. Emerg Infect Dis 24:114–117. doi: 10.3201/eid2401.171331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, Moore CG, Carvalho RG, Coelho GE, Van Bortel W, Hendrickx G, Schaffner F, Elyazar IR, Teng HJ, Brady OJ, Messina JP, Pigott DM, Scott TW, Smith DL, Wint GR, Golding N, Hay SI. 2015. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 4:e08347. doi: 10.7554/eLife.08347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos R, Viana R, Brainer-Lima A, FloreAncio T, Carvalho MD, van der Linden V, Amorim A, Rocha MA, Medeiros F. 2018. Perinatal chikungunya virus-associated encephalitis leading to postnatal-onset microcephaly and optic atrophy. Pediatr Infect Dis J 37:94–95. doi: 10.1097/INF.0000000000001690. [DOI] [PubMed] [Google Scholar]
- 12.Basurko C, Everhard S, Matheus S, Restrepo M, Hilderal H, Lambert V, Boukhari R, Duvernois JP, Favre A, Valmy L, Nacher M, Carles G. 2018. A prospective matched study on symptomatic dengue in pregnancy. PLoS One 13:e0202005. doi: 10.1371/journal.pone.0202005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira R, Barreto FKA, Maia A, Gomes IP, Simiao AR, Barbosa RB, Rodrigues ASR, Lopes KW, Araujo FMC, do Vale RLS, Cavalcante JW, Cavalcanti L. 2018. Maternal and infant death after probable vertical transmission of chikungunya virus in Brazil—case report. BMC Infect Dis 18:333. doi: 10.1186/s12879-018-3243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charlier C, Beaudoin MC, Couderc T, Lortholary O, Lecuit M. 2017. Arboviruses and pregnancy: maternal, fetal, and neonatal effects. Lancet Child Adolesc Health 1:134–146. doi: 10.1016/S2352-4642(17)30021-4. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. 2016. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg?. Ultrasound Obstet Gynecol 47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 16.Honein MA. 2018. Recognizing the global impact of Zika virus infection during pregnancy. N Engl J Med 378:1055–1056. doi: 10.1056/NEJMe1801398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marinho PS, Cunha AJ, Amim Junior J, Prata-Barbosa A. 2017. A review of selected arboviruses during pregnancy. Matern Health Neonatol Perinatol 3:17. doi: 10.1186/s40748-017-0054-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Noronha L, Zanluca C, Burger M, Suzukawa AA, Azevedo M, Rebutini PZ, Novadzki IM, Tanabe LS, Presibella MM, Duarte Dos Santos CN. 2018. Zika virus infection at different pregnancy stages: anatomopathological findings, target cells and viral persistence in placental tissues. Front Microbiol 9:2266. doi: 10.3389/fmicb.2018.02266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheridan MA, Yunusov D, Balaraman V, Alexenko AP, Yabe S, Verjovski-Almeida S, Schust DJ, Franz AW, Sadovsky Y, Ezashi T, Roberts RM. 2017. Vulnerability of primitive human placental trophoblast to Zika virus. Proc Natl Acad Sci U S A 114:E1587–E1596. doi: 10.1073/pnas.1616097114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quicke KM, Bowen JR, Johnson EL, McDonald CE, Ma H, O’Neal JT, Rajakumar A, Wrammert J, Rimawi BH, Pulendran B, Schinazi RF, Chakraborty R, Suthar MS. 2016. Zika virus infects human placental macrophages. Cell Host Microbe 20:83–90. doi: 10.1016/j.chom.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hussain A, Ali F, Latiwesh OB, Hussain S. 2018. A comprehensive review of the manifestations and pathogenesis of Zika virus in neonates and adults. Cureus 10:e3290. doi: 10.7759/cureus.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goebel S, Snyder B, Sellati T, Saeed M, Ptak R, Murray M, Bostwick R, Rayner J, Koide F, Kalkeri R. 2016. A sensitive virus yield assay for evaluation of antivirals against Zika Virus. J Virol Methods 238:13–20. doi: 10.1016/j.jviromet.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Qing M, Zou G, Wang QY, Xu HY, Dong H, Yuan Z, Shi PY. 2010. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob Agents Chemother 54:3686–3695. doi: 10.1128/AAC.00561-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kamiyama N, Soma R, Hidano S, Watanabe K, Umekita H, Fukuda C, Noguchi K, Gendo Y, Ozaki T, Sonoda A, Sachi N, Runtuwene LR, Miura Y, Matsubara E, Tajima S, Takasaki T, Eshita Y, Kobayashi T. 2017. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antiviral Res 146:1–11. doi: 10.1016/j.antiviral.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hinton TM, Zuwala K, Deffrasnes C, Todd S, Shi S, Marsh GA, Dearnley M, Wohl BM, Tolstrup M, Zelikin AN. 2016. Polyanionic macromolecular prodrugs of ribavirin: antiviral agents with a broad spectrum of activity. Adv Healthc Mater 5:534–540. doi: 10.1002/adhm.201500841. [DOI] [PubMed] [Google Scholar]
- 26.Snell NJ. 2001. Ribavirin—current status of a broad spectrum antiviral agent. Expert Opin Pharmacother 2:1317–1324. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- 27.Coffey LL, Beeharry Y, Borderia AV, Blanc H, Vignuzzi M. 2011. Arbovirus high fidelity variant loses fitness in mosquitoes and mice. Proc Natl Acad Sci U S A 108:16038–16043. doi: 10.1073/pnas.1111650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozen-Gagnon K, Stapleford KA, Mongelli V, Blanc H, Failloux AB, Saleh MC, Vignuzzi M. 2014. Alphavirus mutator variants present host-specific defects and attenuation in mammalian and insect models. PLoS Pathog 10:e1003877. doi: 10.1371/journal.ppat.1003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.da Silva S, Oliveira Silva Martins D, Jardim A. 2018. A review of the ongoing research on Zika virus treatment. Viruses 10:E255. doi: 10.3390/v10050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saiz JC, Oya NJ, Blazquez AB, Escribano-Romero E, Martin-Acebes MA. 2018. Host-directed antivirals: a realistic alternative to fight Zika virus. Viruses 10:E453. doi: 10.3390/v10090453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucas-Hourani M, Dauzonne D, Jorda P, Cousin G, Lupan A, Helynck O, Caignard G, Janvier G, Andre-Leroux G, Khiar S, Escriou N, Despres P, Jacob Y, Munier-Lehmann H, Tangy F, Vidalain PO. 2013. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog 9:e1003678. doi: 10.1371/journal.ppat.1003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luthra P, Naidoo J, Pietzsch CA, De S, Khadka S, Anantpadma M, Williams CG, Edwards MR, Davey RA, Bukreyev A, Ready JM, Basler CF. 2018. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antiviral Res 158:288–302. doi: 10.1016/j.antiviral.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raveh A, Delekta PC, Dobry CJ, Peng W, Schultz PJ, Blakely PK, Tai AW, Matainaho T, Irani DN, Sherman DH, Miller DJ. 2013. Discovery of potent broad spectrum antivirals derived from marine actinobacteria. PLoS One 8:e82318. doi: 10.1371/journal.pone.0082318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan KR, Fairley JK, Wang M, Gutman JR. 2018. A survey on outcomes of accidental atovaquone-proguanil exposure in pregnancy. Malar J 17:198. doi: 10.1186/s12936-018-2352-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krause PJ, Lepore T, Sikand VK, Gadbaw J Jr, Burke G, Telford SR 3rd, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. 2000. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 343:1454–1458. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 36.Ling J, Baird JK, Fryauff DJ, Sismadi P, Bangs MJ, Lacy M, Barcus MJ, Gramzinski R, Maguire JD, Kumusumangsih M, Miller GB, Jones TR, Chulay JD, Hoffman SL, Naval Medical Research Unit 2 Clinical Trial Team. 2002. Randomized, placebo-controlled trial of atovaquone/proguanil for the prevention of Plasmodium falciparum or Plasmodium vivax malaria among migrants to Papua, Indonesia. Clin Infect Dis 35:825–833. doi: 10.1086/342578. [DOI] [PubMed] [Google Scholar]
- 37.Kovacs JA. 1992. Efficacy of atovaquone in treatment of toxoplasmosis in patients with AIDS. The NIAID-Clinical Center Intramural AIDS Program. Lancet 340:637–638. doi: 10.1016/0140-6736(92)92172-C. [DOI] [PubMed] [Google Scholar]
- 38.Ashton TM, Fokas E, Kunz-Schughart LA, Folkes LK, Anbalagan S, Huether M, Kelly CJ, Pirovano G, Buffa FM, Hammond EM, Stratford M, Muschel RJ, Higgins GS, McKenna WG. 2016. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat Commun 7:12308. doi: 10.1038/ncomms12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Artymowicz RJ, James VE. 1993. Atovaquone: a new antipneumocystis agent. Clin Pharm 12:563–570. [PubMed] [Google Scholar]
- 40.Yeo AE, Seymour KK, Rieckmann KH, Christopherson RI. 1997. Effects of dual combinations of antifolates with atovaquone or dapsone on nucleotide levels in Plasmodium falciparum. Biochem Pharmacol 53:943–950. doi: 10.1016/S0006-2952(96)00835-0. [DOI] [PubMed] [Google Scholar]
- 41.Baggish AL, Hill DR. 2002. Antiparasitic agent atovaquone. Antimicrob Agents Chemother 46:1163–1173. doi: 10.1128/AAC.46.5.1163-1173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cushion MT, Collins M, Hazra B, Kaneshiro ES. 2000. Effects of atovaquone and diospyrin-based drugs on the cellular ATP of Pneumocystis carinii f. sp. carinii. Antimicrob Agents Chemother 44:713–719. doi: 10.1128/AAC.44.3.713-719.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kottkamp AC, Di Jesus E, Grande R, Brown JK, Jacobs AR, Lim JK, Stapleford K. 2018. The antiparasitic drug atovaquone inhibits arbovirus replication through the depletion of intracellular nucleotides. bioRxiv doi: 10.1101/507152. [DOI] [PMC free article] [PubMed]
- 44.Adcock RS, Chu YK, Golden JE, Chung DH. 2017. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antiviral Res 138:47–56. doi: 10.1016/j.antiviral.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Tong X, Smith J, Bukreyeva N, Koma T, Manning JT, Kalkeri R, Kwong AD, Paessler S. 2018. Merimepodib, an IMPDH inhibitor, suppresses replication of Zika virus and other emerging viral pathogens. Antiviral Res 149:34–40. doi: 10.1016/j.antiviral.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Chung DH, Golden JE, Adcock RS, Schroeder CE, Chu YK, Sotsky JB, Cramer DE, Chilton PM, Song C, Anantpadma M, Davey RA, Prodhan AI, Yin X, Zhang X. 2016. Discovery of a broad-spectrum antiviral compound that inhibits pyrimidine biosynthesis and establishes a type 1 interferon-independent antiviral state. Antimicrob Agents Chemother 60:4552–4562. doi: 10.1128/AAC.00282-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siregar JE, Kurisu G, Kobayashi T, Matsuzaki M, Sakamoto K, Mi-Ichi F, Watanabe Y, Hirai M, Matsuoka H, Syafruddin D, Marzuki S, Kita K. 2015. Direct evidence for the atovaquone action on the Plasmodium cytochrome bc1 complex. Parasitol Int 64:295–300. doi: 10.1016/j.parint.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Hua C, Combe B. 2017. Chikungunya virus-associated disease. Curr Rheumatol Rep 19:69. doi: 10.1007/s11926-017-0694-0. [DOI] [PubMed] [Google Scholar]
- 49.Burt FJ, Chen W, Miner JJ, Lenschow DJ, Merits A, Schnettler E, Kohl A, Rudd PA, Taylor A, Herrero LJ, Zaid A, Ng LFP, Mahalingam S. 2017. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis 17:e107–e117. doi: 10.1016/S1473-3099(16)30385-1. [DOI] [PubMed] [Google Scholar]
- 50.Vanlandingham DL, Higgs S, Huang YJ. 2016. Aedes albopictus (Diptera: Culicidae) and mosquito-borne viruses in the United States. J Med Entomol 53:1024–1028. doi: 10.1093/jme/tjw025. [DOI] [PubMed] [Google Scholar]
- 51.Stapleford KA, Rozen-Gagnon K, Das PK, Saul S, Poirier EZ, Blanc H, Vidalain PO, Merits A, Vignuzzi M. 2015. Viral polymerase-helicase complexes regulate replication fidelity to overcome intracellular nucleotide depletion. J Virol 89:11233–11244. doi: 10.1128/JVI.01553-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gallegos KM, Drusano GL, D Argenio DZ, Brown AN. 2016. Chikungunya virus: in vitro response to combination therapy with ribavirin and interferon alfa 2a. J Infect Dis 214:1192–1197. doi: 10.1093/infdis/jiw358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos B, Coelho FC, Armstrong M, Saraceni V, Lemos C. 2018. Zika: an ongoing threat to women and infants. Cad Saude Publica 34:e00038218. doi: 10.1590/0102-311X00038218. [DOI] [PubMed] [Google Scholar]
- 54.Fauci AS, Morens DM. 2016. Zika virus in the Americas—yet another arbovirus threat. N Engl J Med 374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y, Liu J, Du S, Shan C, Nie K, Zhang R, Li XF, Zhang R, Wang T, Qin CF, Wang P, Shi PY, Cheng G. 2017. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 545:482–486. doi: 10.1038/nature22365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sassetti M, Zé-Zé L, Franco J, Cunha JD, Gomes A, Tomé A, Alves M-J. 2018. First case of confirmed congenital Zika syndrome in continental Africa. Trans R Soc Trop Med Hyg 112:458–462. doi: 10.1093/trstmh/try074. [DOI] [PubMed] [Google Scholar]
- 57.Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming GL, Zheng W, Song H, Tang H. 2016. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med 22:1101–1107. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z, Brecher M, Deng YQ, Zhang J, Sakamuru S, Liu B, Huang R, Koetzner CA, Allen CA, Jones SA, Chen H, Zhang NN, Tian M, Gao F, Lin Q, Banavali N, Zhou J, Boles N, Xia M, Kramer LD, Qin CF, Li H. 2017. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res 27:1046–1064. doi: 10.1038/cr.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao RY, Xu YF, Zhang TH, Yang JJ, Yuan Y, Hao P, Shi Y, Zhong J, Zhong W. 2017. Pediatric drug nitazoxanide: a potential choice for control of Zika. Open Forum Infect Dis 4:ofx009. doi: 10.1093/ofid/ofx009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y, Mesplede T, Xu H, Quan Y, Wainberg MA. 2018. The antimalarial drug amodiaquine possesses anti-ZIKA virus activities. J Med Virol 90:796–802. doi: 10.1002/jmv.25031. [DOI] [PubMed] [Google Scholar]
- 61.Sacramento CQ, de Melo GR, de Freitas CS, Rocha N, Hoelz LVB, Miranda M, Fintelman-Rodrigues N, Marttorelli A, Ferreira AC, Barbosa-Lima G, Abrantes JL, Vieira YR, Bastos MM, de Mello Volotão E, Nunes EP, Tschoeke DA, Leomil L, Loiola EC, Trindade P, Rehen SK, Bozza FA, Bozza PT, Boechat N, Thompson FL, de Filippis AMB, Brüning K, Souza TML. 2017. The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci Rep 7:40920. doi: 10.1038/srep40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Retallack H, Di Lullo E, Arias C, Knopp KA, Laurie MT, Sandoval-Espinosa C, Mancia Leon WR, Krencik R, Ullian EM, Spatazza J, Pollen AA, Mandel-Brehm C, Nowakowski TJ, Kriegstein AR, DeRisi JL. 2016. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc Natl Acad Sci U S A 113:14408–14413. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang S, Xu M, Lee EM, Gorshkov K, Shiryaev SA, He S, Sun W, Cheng YS, Hu X, Tharappel AM, Lu B, Pinto A, Farhy C, Huang CT, Zhang Z, Zhu W, Wu Y, Zhou Y, Song G, Zhu H, Shamim K, Martinez-Romero C, Garcia-Sastre A, Preston RA, Jayaweera DT, Huang R, Huang W, Xia M, Simeonov A, Ming G, Qiu X, Terskikh AV, Tang H, Song H, Zheng W. 2018. Emetine inhibits Zika and Ebola virus infections through two molecular mechanisms: inhibiting viral replication and decreasing viral entry. Cell Discov 4:31. doi: 10.1038/s41421-018-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shiryaev SA, Mesci P, Pinto A, Fernandes I, Sheets N, Shresta S, Farhy C, Huang CT, Strongin AY, Muotri AR, Terskikh AV. 2017. Repurposing of the anti-malaria drug chloroquine for Zika virus treatment and prophylaxis. Sci Rep 7:15771. doi: 10.1038/s41598-017-15467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao B, Parnell LA, Diamond MS, Mysorekar IU. 2017. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J Exp Med 214:2303–2313. doi: 10.1084/jem.20170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chan JF, Chik KK, Yuan S, Yip CC, Zhu Z, Tee KM, Tsang JO, Chan CC, Poon VK, Lu G, Zhang AJ, Lai KK, Chan KH, Kao RY, Yuen KY. 2017. Novel antiviral activity and mechanism of bromocriptine as a Zika virus NS2B-NS3 protease inhibitor. Antiviral Res 141:29–37. doi: 10.1016/j.antiviral.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Basco LK, Ramiliarisoa O, Le Bras J. 1995. In vitro activity of atovaquone against the African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg 53:388–391. doi: 10.4269/ajtmh.1995.53.388. [DOI] [PubMed] [Google Scholar]
- 68.Shanks GD, Gordon DM, Klotz FW, Aleman GM, Oloo AJ, Sadie D, Scott TR. 1998. Efficacy and safety of atovaquone/proguanil as suppressive prophylaxis for Plasmodium falciparum malaria. Clin Infect Dis 27:494–499. doi: 10.1086/514710. [DOI] [PubMed] [Google Scholar]
- 69.Yeo KL, Chen YL, Xu HY, Dong H, Wang QY, Yokokawa F, Shi PY. 2015. Synergistic suppression of dengue virus replication using a combination of nucleoside analogs and nucleoside synthesis inhibitors. Antimicrob Agents Chemother 59:2086–2093. doi: 10.1128/AAC.04779-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vermillion MS, Lei J, Shabi Y, Baxter VK, Crilly NP, McLane M, Griffin DE, Pekosz A, Klein SL, Burd I. 2017. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun 8:14575. doi: 10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ribeiro MR, Moreli JB, Marques RE, Papa MP, Meuren LM, Rahal P, de Arruda LB, Oliani AH, Oliani D, Oliani SM, Narayanan A, Nogueira ML. 2018. Zika-virus-infected human full-term placental explants display pro-inflammatory responses and undergo apoptosis. Arch Virol. doi: 10.1007/s00705-018-3911-x. [DOI] [PubMed] [Google Scholar]
- 72.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. 2016. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szaba FM, Tighe M, Kummer LW, Lanzer KG, Ward JM, Lanthier P, Kim IJ, Kuki A, Blackman MA, Thomas SJ, Lin JS. 2018. Zika virus infection in immunocompetent pregnant mice causes fetal damage and placental pathology in the absence of fetal infection. PLoS Pathog 14:e1006994. doi: 10.1371/journal.ppat.1006994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tabata T, Petitt M, Puerta-Guardo H, Michlmayr D, Wang C, Fang-Hoover J, Harris E, Pereira L. 2016. Zika virus targets different primary human placental cells, suggesting two routes for vertical transmission. Cell Host Microbe 20:155–166. doi: 10.1016/j.chom.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de la Camara R. 2016. CMV in hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis 8:2016031. doi: 10.4084/mjhid.2016.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Munawwar A, Singh S. 2016. Human herpesviruses as copathogens of HIV infection, their role in HIV transmission, and disease progression. J Lab Physicians 8:5–18. doi: 10.4103/0974-2727.176228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beltrami S, Gordon J. 2014. Immune surveillance and response to JC virus infection and PML. J Neurovirol 20:137–149. doi: 10.1007/s13365-013-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waghmare A, Campbell AP, Xie H, Seo S, Kuypers J, Leisenring W, Jerome KR, Englund JA, Boeckh M. 2013. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin Infect Dis 57:1731–1741. doi: 10.1093/cid/cit639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schwarz MC, Sourisseau M, Espino MM, Gray ES, Chambers MT, Tortorella D, Evans MJ. 2016. Rescue of the 1947 Zika virus prototype strain with a cytomegalovirus promoter-driven cDNA clone. mSphere 1:e00246-16. doi: 10.1128/mSphere.00246-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsetsarkin KA, Kenney H, Chen R, Liu G, Manukyan H, Whitehead SS, Laassri M, Chumakov K, Pletnev AG. 2016. A full-length infectious cDNA clone of Zika virus from the 2015 epidemic in Brazil as a genetic platform for studies of virus-host interactions and vaccine development. mBio 7:e01114-16. doi: 10.1128/mBio.01114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Coffey LL, Vignuzzi M. 2011. Host alternation of chikungunya virus increases fitness while restricting population diversity and adaptability to novel selective pressures. J Virol 85:1025–1035. doi: 10.1128/JVI.01918-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weisblum Y, Oiknine-Djian E, Vorontsov OM, Haimov-Kochman R, Zakay-Rones Z, Meir K, Shveiky D, Elgavish S, Nevo Y, Roseman M, Bronstein M, Stockheim D, From I, Eisenberg I, Lewkowicz AA, Yagel S, Panet A, Wolf DG. 2017. Zika virus infects early- and midgestation human maternal decidual tissues, inducing distinct innate tissue responses in the maternal-fetal interface. J Virol 91:e01905-16. doi: 10.1128/JVI.01905-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bayer A, Lennemann NJ, Ouyang Y, Bramley JC, Morosky S, Marques ET Jr, Cherry S, Sadovsky Y, Coyne CB. 2016. Type III interferons produced by human placental trophoblasts confer protection against Zika virus infection. Cell Host Microbe 19:705–712. doi: 10.1016/j.chom.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]