Abstract
Objectives
Although knee osteoarthritis (KOA) is a leading cause of impaired functioning among older adults globally, little is still known about the complex mechanisms of disability accumulation in these patients. The aim of the study was to analyze the clinical parameters of patients with KOA in a Bulgarian population and to determine which of these clinical characteristics define disability to the greatest extent.
Material and methods
Patients aged 40–80 years with symptomatic KOA were included. The assessment tools for pain, clinical disease severity, and disability were the pain visual analogue scale (VAS) and disease-specific questionnaires: Algofunctional Index of Lequesne, Western Ontario and McMaster Universities OA Index (WOMAC), and the Health Assessment Questionnaire-Disability Index (HAQ-DI), respectively. Radiographs of the knees were obtained and graded according to the Kellgren-Lawrence (KL) system.
Results
One hundred and thirty-two patients (81% women) participated in the study. The median values of VAS (mm), WOMAC, Lequesne, and HAQ-DI scores were 52, 37.5, 11, 0.88, respectively. Men had milder disease, resulting in lower VAS, WOMAC, Lequesne, and HAQ-DI scores and less structural damage compared to women (p < 0.05). WOMAC index correlated positively with age of the patients but not with duration of the complaints. Patients with severe and very severe pain did not differ in their HAQ-DI, disease severity and KL grading. WOMAC physical function score and Lequesne index were independent predictors for the HAQ-DI in patients with KOA.
Conclusions
Bulgarian patients with KOA had moderate disability which showed a strong relationship with physical function of WOMAC and disease severity. Multiple layers of causality coexist to determine the knee pain in Bulgarian patients with KOA.
Keywords: quality of life, disability, self-esteem, knee osteoarthritis
Introduction
Osteoarthritis (OA) is the most common cause of joint pain associated with varying degrees of functional deficiency, decreased quality of life and life expectancy [1–3]. Knee OA (KOA), as the most common peripheral localization of OA [4], affects people of all ages with different levels of physical activity [5]. According to data from the global burden of disease study for 2016, osteoarthritis was the ninth most common cause of disability in Bulgaria [6]. The complex clinical evaluation of patients with KOA includes assessment of pain, stiffness and function, as well as the measurement of various aspects of health [7, 8]. Among the various assessment tools for measurement of OA clinical severity, the Algofunctional Index of Lequesne [9] and the Western Ontario and McMaster Universities OA Index (WOMAC) [10] are the most commonly used. These two disease-specific evaluation tools have similar overall statistical effectiveness, although WOMAC appears to be slightly more effective in pre-screening patients with KOA detecting changes in pain and physical function with internal consistency and reliability [11].
In addition to disease-specific questionnaires, the use of generic (non-disease-specific) questionnaires may be beneficial in evaluation of overall health and quality of life in patients with chronic illnesses. The Health Assessment Questionnaire-Disability Index (HAQ-DI) may be utilized to assess functional disability in patients with KOA. According to the Arthritis, Rheumatism and Aging Medical Information System (ARAMIS), by 2003 the HAQ questionnaire was used more than 200,000 times in routine practice and research. The mean scores reported in patients with OA and rheumatoid arthritis were 0.8 and 1.2, respectively. In the total population this value was 0.49 [12].
Although KOA is a leading cause of disability among older adults globally [6], little is still known about the complex mechanisms of disability accumulation in these patients. Furthermore, KOA has a variable clinical course among different patients depending on genetic and environmental factors [13]. Given the fact that most of the published studies have been conducted in North American and Western European populations, it is entirely possible that their results may not be entirely reproducible for the Central and Eastern European population [14]. Studying the complex mechanisms of disability accumulation in patients with KOA will allow us to identify rational mechanism-based treatment targets.
In this study we analyzed the clinical and demographic parameters of patients with KOA in the Bulgarian population and determined which of these clinical characteristics define the disability in KOA to the greatest extent.
Material and methods
This was a cross-sectional study carried out for a period of 2 years and 5 months from October 2014 to February 2017 from a single team at the largest inpatient rheumatology center in Bulgaria – the Rheumatology Clinic of University Hospital “St. Ivan Rilski”, Sofia, Bulgaria – with an annual patient load figure between 4,000 and 5,000 patients with rheumatic diseases. Patients with KOA who met the American College of Rheumatology (ACR) criteria for KOA [15] and were symptomatic with either unilateral or bilateral KOA in the medial tibio-femoral compartment, with a pain duration more than 6 months, were included. The radiological severity of KOA was classified according to the Kellgren-Lawrence (KL) scale. For this purpose, radiographs of both knees in an upright weight-bearing position were obtained.
We excluded patients who met one or more of the following criteria: KL grade IV, comorbidity with another rheumatic disease, a pre-existing intra-articular fracture or documented high-energy trauma of the lower limb, decompensated metabolic or cardiovascular disease, treatment with systemic glucocorticoids (doses > 7.5 mg) in the previous 3 months as well as intraarticular hyaluronan, glucocorticoids or orthobiotics in the past 6 months, treatment with symptomatic slow acting drugs for OA (glucosamine, chondroitin, avocado/soybean unsaponifiables) in the previous 6 months.
The intensity of knee pain was evaluated with a 100 mm visual analogue scale (VAS). According to the intensity of the pain, the patients were divided into four groups: mild (< 40 mm), moderate (40–60 mm), severe (60–80 mm) and very severe (> 80 mm) pain. To achieve an adequate assessment of pain and physical function, patients were asked not to take pain-relieving drugs (including analgesics and non-steroidal anti-inflammatory drugs) within 48 hours before the study.
Assessment of clinical disease severity (pain status and function) was done by the algofunctional index of Lequesne and by Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC). The value of Lequesne’s index ranges from 0 to 24 points. Sections of the index include pain or discomfort, maximum walking distance, and activities of daily living [9]. The severity of the disease in the studied group was classified as follows: “mild” (1–4 points), “moderate” (5–7 points), “severe” (8–10 points), “very severe” (11–13 points) and “extremely severe” (≥ 14 points). WOMAC is an OA disease-specific questionnaire that is self-reported by the patient and provides information about disease activity evaluating the underlying disease symptoms. The WOMAC score ranges from 0 to 96 points and the questionnaire is divided into three main sections: pain (total: 20 points), stiffness (total: 8 points), and functional impairment (total: 68 points). Higher values of the index are associated with more severe symptoms and impaired joint function [10].
Assessment of disability was done by the Health Assessment Questionnaire-Disability Index (HAQ-DI). Responses for evaluation of disability were reported by the patients on a 4-point Likert-type scale ranging from 0 (without any difficulty) to 3 (unable to do it). Higher values of the HAQ-DI index are associated with a higher degree of disability.
All participants gave informed consent and the study was approved by the local ethics committee. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration, revised in 2008.
Statistical analysis
The statistical analysis was performed using the SPSS 21 software. Descriptive statistics, parametric and nonparametric tests and linear regression analysis were used. The distribution of the data was calculated by the Shapiro-Wilk test. Fisher’s exact test was used to analyze the presence of a linear relationship between categories. Spearman rank analysis was used to investigate correlations in data with non-normal distribution. Nonparametric Mann-Whitney U-test and parametric Student’s t-test were used to compare the variables.
When continuous data were non-normally distributed, the assumption of homogeneity of variance was assessed by Levene’s test for equality of variances. If the distributions were identically shaped, the hypotheses were stated in terms of a difference between medians (min and max). Multivariate linear regression analysis was performed to predict the main clinical and demographic parameters influencing the values of HAQ-DI.
Results
One hundred and thirty-two patients (mean age 63.45 ±8.71, range 40–80 years) participated in the study, of whom 81% were women. Their BMI was 28.93 ±3.88 kg/m2. The median duration of knee pain (by history) was 3 years (range 0.5 to 17 years). Disease activity, pain intensity, disease severity, and disability and quality of life as evaluated using WOMAC, VAS, Lequesne’s index, and HAQ-DI, respectively, as well as other clinical and imaging parameters of the KOA patients, are presented in Table I.
Table I.
Demographic, clinical and radiographic characteristics of the study group
| Variables | Patients with KOA (n = 132) | Clinical groups | Radiographic groups | |||||
|---|---|---|---|---|---|---|---|---|
| Patients with isolated KOA (n = 65) | Patients with generalized OA (n = 67) | p | KL I patients (n = 39) | KL II patients (n = 65) | KL III patients (n = 28) | p | ||
| Demographic variables | ||||||||
| Age (years)* | 63.45 ±8.71 | 61.74 ±8.48 | 65.12 ±8.68 | 0.025 | 58.92 ±9.1 | 65.74 ±7.61 | 64.46 ±8.5 | > 0.001 |
| Gender (% women) | 81 | 75 | 87 | NS | 67 | 86 | 88 | 0.024 |
| BMI (kg/m2)* | 28.93 ±3.88 | 28.26 ±4.08 | 29.58 ±3.59 | NS | 28.60 ±3.81 | 28.91 ±3.92 | 29.45 ±3.98 | NS |
| Clinical variables | ||||||||
| Isolated KOA (%) | 49.2 (65/132) | – | – | – | 59 (23/39) | 43.1 (28/65) | 50 (14/28) | NS |
| Generalized OA (%) | 50.8 (67/132) | – | – | – | 41 (16/39) | 56.9 (37/65) | 50 (14/28) | NS |
| Pain duration (years)** | 3.5 (0.5; 17) | 3 (0.5; 15) | 4 0.5; 17) | NS | 2 (0.5; 15) | 5 (0.5; 17) | 7 (0.5; 15) | 0.003 |
| Morning stiffness (minutes)** | 12.5 (5; 60) | 12.5 (5; 60) | 15 (5; 60) | NS | 10 (5; 45) | 15 (5; 60) | 15 (5; 60) | NS |
| Pain (VAS mm)** | 52 (22; 95) | 52 (22; 95) | 53 (26; 95) | NS | 46 (22; 95) | 51 (32; 95) | 70 (29; 95) | 0.001 |
| HAQ-DI** | 0.88 (0; 2.25) | 0.75 (0; 2.25) | 0.88 (0.10; 2.25) | NS | 0.50 (0; 2.0) | 0.75 (0.10; 2.25) | 1.00 (0.13; 2.00) | 0.001 |
| WOMAC index** | 37.5 (6; 92) | 46 (13; 92) | 31 (6; 91) | NS | 32 (6; 92) | 42 (11; 81) | 50 (13; 91) | 0.004 |
| Lequesne index** | 11 (4; 23) | 12 (4.5; 23) | 11 (4; 23) | NS | 9.50 (4; 23) | 12 (5; 21.5) | 14.25 (4.5; 23) | 0.010 |
| Radiographic stage (KL) | ||||||||
| KL I (%) | 29.5 (39/132) | 35.3 (23/65) | 23.9 (16/67) | NS | – | – | – | – |
| KL II (%) | 49.3 (65/132) | 43 (28/65) | 55.2 (37/67) | NS | – | – | – | – |
| KL III (%) | 21.2 (28/132) | 21.5 (14/65) | 20.9 (14/67) | NS | – | – | – | – |
BMI – body mass index; HAQ-DI – Health Assessment Questionnaire Disability Index; KL – Kellgren-Lawrence; KOA – knee osteoarthritis; NS – not significant; OA – osteoarthritis; WOMAC – Western Ontario and McMaster Universities index; VAS – visual analogue scale;
normally distributed data are presented as mean (±SD)
non-normally distributed data are presented as median (min; max).
The median value of VAS was 52 mm (range: 22–95 mm). It correlated positively with age and duration of the pain (rs = 0.173; p = 0.047 and rs = 0.395; p < 0.001, respectively), but not with BMI. The older the patients were or the longer they suffered from pain, the higher the VAS level was. Women and men had significantly different pain values on VAS: 57 (22; 95) mm and 45 (26; 87) mm, respectively, Mann-Whitney U = 882.5; p = 0.008. The same correlations were observed for the pain measured by the Likert scale included in the WOMAC questionnaire. After categorization of patients according to their pain levels, no significant difference was observed between the two groups with the highest intensity of pain (“severe” and “very severe”) in terms of health-related quality of life, disease activity, disease severity and KL grading.
Clinical severity of knee osteoarthritis by WOMAC
The median total WOMAC score was 37.5 (range: 22–92 mm). Total WOMAC values correlated with age (rs = 0.257; p = 0.003) but not with pain duration (rs = 0.102; p = 0.242) or BMI (rs = 0.158; p = 0.070). Women and men had significantly different disease severity scores: 45 (6; 92) and 33 (7; 64), respectively, Mann-Whitney U = 742.5; p = 0.001.
When the specific sections of the WOMAC index were separately analyzed, we observed that stiffness was associated with pain duration (rs = 0.24, p = 0.029) but not with the age of the patients. Stiffness also did not differ significantly between men and women, unlike the physical function, which showed a significant gender difference (p = 0.027). Physical function correlated positively with age (rs = 0.31, p = 0.004).
Clinical disease severity by Lequesne index
The median Lequesne index value in the studied group was 11 (range: 4–23). The values of the Lequesne index correlated with age (rs = 0.250; p = 0.004), pain VAS (rs = 0.191; p = 0.029) and BMI (rs = 0.199; p = 0.022). Women and men had a significantly different disease burden according to the Lequesne index: 12.5 (4; 23) and 9 (5; 15), respectively, Mann-Whitney U = 719.5; p < 0.001.
Functional disability
The median HAQ-DI was 0.88 (range: 0–2.25). HAQ-DI values correlated with pain duration (rs = 0.187; p = 0.032), but not with age (rs = 0.128; p = 0.143) or BMI (rs = 0.068; p = 0.441). Women and men had significantly different values of HAQ-DI: 1 (0, 2.25) and 0.5 (0; 2.25), respectively, Mann-Whitney U = 786.5; p = 0.001. All the self-reported clinical measures, including VAS, both WOMAC physical function and stiffness, Lequesne, and HAQ-DI, correlated strongly or very strongly (rs > 0.6) with each other and are presented in Table II.
Table II.
Correlations among clinical variables
| Variable | HAQ-DI | VAS | Total WOMAC | Lequesne | |
|---|---|---|---|---|---|
| HAQ-DI | Correlation coefficient | – | 0.692** | 0.674** | 0.625** |
| Sig. (2-tailed) | – | 0.000¥ | 0.000¥ | 0.000¥ | |
| Pain intensity (VAS) | Correlation coefficient | 0.692 | – | 0.815* | 0.748* |
| Sig. (2-tailed) | 0.000¥ | – | 0.000¥ | 0.000¥ | |
| Physical function (WOMAC) | Correlation coefficient | 0.742* | 0.654* | – | 0.736* |
| Sig. (2-tailed) | 0.000¥ | 0.000¥ | – | 0.000¥ | |
| Stiffness (WOMAC) | Correlation coefficient | 0.612 | 0.654 | – | 0.686 |
| Sig. (2-tailed) | 0.000¥ | 0.000¥ | – | 0.000¥ | |
| Disease severity (Lequesne index) | Correlation coefficient | 0.625** | 0.748* | 0.724* | – |
| Sig. (2-tailed) | 0.000¥ | 0.000¥ | 0.000¥ | – |
0.7 < rs < 0.9 – very strong correlation
0.5 < rs < 0.7 – strong correlation
p < 0.001
HAQ-DI – Health Assessment Questionnaire Disability Index; QoL – quality of life; WOMAC – Western Ontario and McMaster Universities index; VAS – visual analogue scale.
Based on univariate analyses of clinical and demographic variables, individuals who had longer pain duration, more severe stiffness, worse physical function, higher disease severity, higher pain levels, and women scored higher on the HAQ-DI. These six variables were entered into the multivariate regression model, wherein the variables most strongly associated with higher HAQ-DI were physical function (p = 0.001) and disease severity (p = 0.001).
A multivariate linear regression analysis was calculated to predict HAQ-DI from the WOMAC physical function score and total Lequesne score. A significant regression equation (F [2, 130] = 69.99; p < 0.001) was found, with adjusted R2 = 0.624. In this case, the predictive HAQ-DI values were determined using the following analytical dependence:
HAQ – DI = –0.255 + 0.02 * (WOMACPhys) + 0.053 * (LEQ),
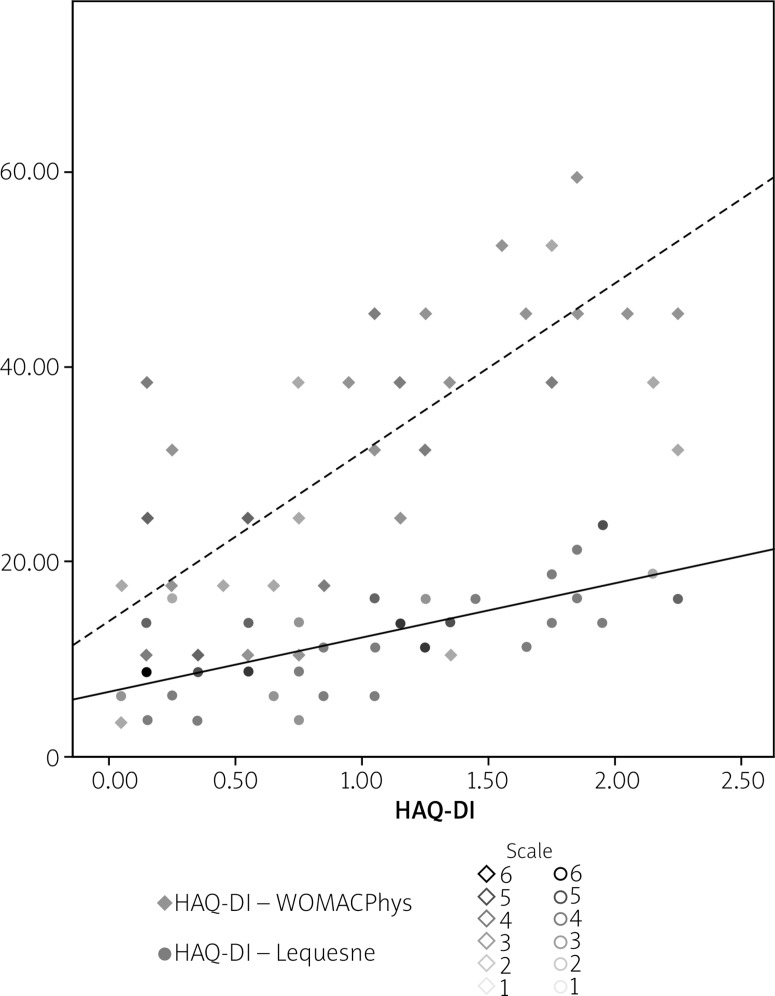
where WOMACPhys and LEQ are the scores of the WOMAC physical function section and Lequesne indices, respectively. According to the formula, participants’ average HAQ-DI is increased by 0.02 and 0.053 for each added point of the WOMAC physical function and Lequesne indices, respectively, which were independent predictors for the HAQ-DI score in patients with KOA. Figure 1 shows the resulting scatter plot characterizing the relationship between HAQ-DI (X) and Lequesne index (Y) and WOMAC physical function score (Y).
Fig. 1.
Scatter plot showing the relationship between HAQ-DI (X) and disease-specific indices – WOMAC physical function (Y) and Lequesne (Y).
Discussion
In this cohort from Bulgaria we found that pain correlated positively with age and the duration of OA. Pain is the dominant symptom in OA, which usually causes the patient to visit the rheumatologist’s office. Correlations between pain and age and between pain and its duration may be partly attributed to the advanced structural changes, but also to the central mechanisms of sensitization inherent in OA patients [16] which could be merely a matter of the time characteristic of the longer history and more persistent symptoms. “Sensitization” to painful stimuli is achieved by development of sensitizing central nociceptive circuits that amplify pain sensation, accompanied by highly variable degrees of peripheral tissue damage [17].
In our cohort of Bulgarian KOA patients, men as a whole had milder disease, resulting in lower pain levels and structural damage. Nevertheless, gender may be an independent risk factor for sensitization, as recent studies have suggested [18]. Some authors suggest that gender differences may be attributed to a lower pain threshold combined with greater sensitivity of women to pain than to advanced structural changes [19, 20]. Besides age and gender, ethnicity alone may both influence the link between radiographic features and pain, and vary among different populations [21]. Surprisingly, pain levels in our cohort did not correlate with BMI, although obesity is a well-known risk factor for KOA. Our results, nevertheless, are in consistence with another study of Caucasian patients with KOA [22]. In both studies patients were not categorized according to their BMI.
In the analysis of our data, the WOMAC-derived Likert pain score did not show any marked differences from the VAS, which suggests the interchangeability of the two scales. These data are consistent with findings in other studies of patients with KOA [23]. However, the Likert scale has some advantages over the VAS, namely the possibility of enhanced data processing using categorization in data analysis and better perception by patients. The main disadvantage is the lack of freedom in response, as opposed to a 100-mm VAS [24, 25].
A significant difference was not found between the two groups reporting the most severe pain (pain VAS 60–80 mm versus VAS ≥ 80 mm) in terms of disease activity (WOMAC), HAQ-DI, and structural changes assessed by imaging. The discordance between pain levels and radiographic severity was previously observed in a well-controlled study [26] and suggests the multifactorial mechanisms that play a role in determining the perception of pain in KOA patients. This demonstrates once more that central mechanisms, similar to those in fibromyalgic syndrome, may play a pivotal role in pain genesis. Obviously, as reported by Bedson et al., the above-mentioned findings can coexist at the same time, making up multiple layers of causality of knee pain in patients with KOA [21].
The other important finding of our study was that the WOMAC index, which assessed the main symptoms of the disease, namely pain, physical function and stiffness, correlated positively with age of the patient but did not correlate with pain duration. Since OA is an excellent example of an age-related illness [27], it is no surprise that its clinical severity correlates with age. Being not related to duration of pain, the composite nature of the WOMAC index may be accompanied by lack of sensitization over time.
Originally developed to assess disability in rheumatoid arthritis, the HAQ-DI questionnaire is used in a wide range of diseases, including KOA. The median we reported for HAQ-DI (0.88) is similar to the reported values in epidemiological studies of larger cohorts of patients with OA [12].
The performed multivariate regression analysis showed that HAQ-DI values can be predicted when the WOMAC physical function score and total Lequesne score are known. Considering also the determinant coefficient of the presented model, both scores account for approximately 62% of variations in HAQ-DI values, despite the putative polymorbidity of the study population. Interestingly, the WOMAC physical function score and total Lequesne score independently contribute to the HAQ-DI, thus reflecting different aspects of disability among patients with KOA. These results suggest the lack of interchangeability between WOMAC and Lequesne indices, although they both aim at addressing physical function in patients with KOA and highly correlate with one another. Surprisingly, according to our model, pain was not a determinant of disability, in contrast to the conclusions made by McAlindon et al. [28].
It should be noted however that in their study physical function in knees was assessed by quadriceps femoris isometric strength. Nevertheless, to reduce disability of patients with KOA, the treatment should not only target palliative pharmacological pain relief, but also other factors contributing to impaired physical function such as joint misalignment, obesity and muscle weakness. Despite the confirmatory nature of our data, little is still known about the complex mechanisms of disability accumulation in patients with OA to enable rational mechanism-based management of the disease.
To the best of our knowledge, this is the first study that predicts disability level based on disease-specific indices. Even though the clinical assessment of patients with KOA should include both disease-specific and generic instruments for more general insight into the patient’s health, the predicted value of the HAQ-DI may serve as a starting point for determining disability/health related quality of life in patients with KOA.
The first and the most significant limitation of the study is the relatively small sample size, especially for the male group (n = 25). Because symptomatic KOA is more prevalent and severe in women [29] on the one hand and regional gender differences in patients’ attitude towards the Bulgarian healthcare system may exist on the other, a larger than expected proportion of female patients have sought medical attention in the inpatient research center and agreed to participate in the survey. Secondly, patients were recruited from an inpatient setting and thus the study group may not be representative of an outpatient KOA population. Based on patient-reported questionnaires, our data may have some limitations with regard to accuracy and recall bias. Nevertheless, self-reported questionnaires provide an indispensable perspective of the quality of OA care and reflect the care as perceived by the patient [30]. Mixing patients with isolated KOA and KOA in the context of generalized OA may have an effect on the non-knee-specific measurements but in this way we aimed at studying the full clinical spectrum of the disease.
Conclusions
In conclusion, multiple layers of causality coexist to determine the knee pain in patients with KOA. Patients with KOA in the study group were characterized by moderate disability which showed a strong relationship with physical function and disease severity. Understanding the complex mechanisms of disability accumulation will allow rational management of patients with KOA.
Footnotes
The author declares no conflict of interest.
References
- 1.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Knee pain and disability in the community. Br J Rheumatol. 1992;31:189–192. doi: 10.1093/rheumatology/31.3.189. [DOI] [PubMed] [Google Scholar]
- 2.Nüesch E, Dieppe P, Reichenbach S, et al. All cause and disease specific mortality in patients with knee or hip osteoarthritis: population based cohort study. BMJ. 2011;342:d1165. doi: 10.1136/bmj.d1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 4.Oliveria SA, Felson DT, Reed JI, et al. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 5.Weiss JM, Noble PC, Conditt MA, et al. What functional activities are important to patients with knee replacements? Clin Orthop Relat Res. 2002;404:172–188. doi: 10.1097/00003086-200211000-00030. [DOI] [PubMed] [Google Scholar]
- 6.Vos T, Abajobir AA, Abbafati C, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theiler R, Brooks P, Ghosh P. Clinical, biochemical and imaging methods of assessing osteoarthritis and clinical trials with agents claiming chondromodulating activity. Osteoarthritis Cartilage. 1994;2:1–23. doi: 10.1016/s1063-4584(05)80002-0. [DOI] [PubMed] [Google Scholar]
- 8.Georgiev T, Ivanova M, Stoilov R. Clinical methods to evaluate patients with knee osteoarthritis. Revmatologiia (Bulgaria) 2018;26:3–10. [Google Scholar]
- 9.Lequesne M, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Scand J Rheumatol. 1987;65(Suppl):85–89. doi: 10.3109/03009748709102182. [DOI] [PubMed] [Google Scholar]
- 10.Bellamy N, Buchanan WW. A preliminary evaluation of the dimensionality and clinical importance of pain and disability in osteoarthritis of the hip and knee. Clin Rheumatol. 1986;5:231–241. doi: 10.1007/BF02032362. [DOI] [PubMed] [Google Scholar]
- 11.Altman R, Brandt K, Hochberg M, et al. Design and conduct of clinical trials in patients with osteoarthritis: Recommendations from a task force of the Osteoarthritis Research Society: Results from a workshop. Osteoarthritis Cartilage. 1996;4:217–243. doi: 10.1016/s1063-4584(05)80101-3. [DOI] [PubMed] [Google Scholar]
- 12.Bonnie B, Fries JF. The Stanford Health Assessment Questionnaire: a review of its history, issues, progress, and documentation. J Rheumatol. 2003;30:167–178. [PubMed] [Google Scholar]
- 13.Gardiner BS, Woodhouse FG, Besier TF, et al. Predicting knee osteoarthritis. Ann Biomed Eng. 2016;44:222–233. doi: 10.1007/s10439-015-1393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgiev T, Stoilov R. Bulgarian rheumatology: science and practice in a cost-constrained environment. Rheumatol Int. 2019;39:417–429. doi: 10.1007/s00296-018-4202-2. [DOI] [PubMed] [Google Scholar]
- 15.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 16.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Finan PH, Buenaver LF, Bounds SC, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. 2013;65:363–372. doi: 10.1002/art.34646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartley EJ, King CD, Sibille KT, et al. Enhanced pain sensitivity among individuals with symptomatic knee osteoarthritis: potential sex differences in central sensitization. Arthritis Care Res. 2016;68:472–480. doi: 10.1002/acr.22712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perrot S, Poiraudeau S, Kabir-Ahmadi M, Rannou F. Correlates of pain intensity in men and women with hip and knee osteoarthritis. Results of a national survey: The French ARTHRIX study. Clin J Pain. 2009;25:767–772. doi: 10.1097/AJP.0b013e3181b43d4f. [DOI] [PubMed] [Google Scholar]
- 20.Paradowski PT, Bergman S, Sundén-Lundius A, et al. Knee complaints vary with age and gender in the adult population. Population-based reference data for the Knee Injury and Osteoarthritis Outcome Score (KOOS) BMC Musculoskelet Disord. 2006;7:38. doi: 10.1186/1471-2474-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lethbridge-Çejku M, Scott WW, Jr, Reichle R, et al. Association of radiographic features of osteoarthritis of the knee with knee paIn: data from the Baltimore Longitudinal Study of Aging. Arthritis Rheum. 1995;8:182–188. doi: 10.1002/art.1790080311. [DOI] [PubMed] [Google Scholar]
- 23.Bolognese JA, Schnitzer TJ, Ehrich EW. Response relationship of VAS and Likert scales in osteoarthritis efficacy measurement. Osteoarthritis Cartilage. 2003;11:499–507. doi: 10.1016/s1063-4584(03)00082-7. [DOI] [PubMed] [Google Scholar]
- 24.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult paIn: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(Suppl 11):240–252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Townsend M, Berman LB, Keller JL. A comparison of Likert and visual analogue scales for measuring change in function. J Chronic Dis. 1987;40:1129–1133. doi: 10.1016/0021-9681(87)90080-4. [DOI] [PubMed] [Google Scholar]
- 26.Arendt-Nielsen L, Egsgaard LL, Petersen KK, et al. A mechanism – based pain sensitivity index to characterize knee osteoarthritis patients with different disease stages and pain levels. Eur J Pain. 2015;19:1406–1417. doi: 10.1002/ejp.651. [DOI] [PubMed] [Google Scholar]
- 27.Anderson, Shane A, Richard FL. Why is osteoarthritis an agerelated disease? Best Pract Res Clin Rheumatol. 2010;24:15–26. doi: 10.1016/j.berh.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srikanth VK, Fryer JL, Zhai G, et al. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Østerås N, Jordan KP, Clausen B, et al. Self-reported quality care for knee osteoarthritis: comparisons across Denmark, Norway, Portugal and the UK. RMD open. 2015;1:e000136. doi: 10.1136/rmdopen-2015-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]