Abstract
Chlorothalonil is a broad spectrum chloronitrile fungicide that has been identified as one of the most common pesticide contaminants found in managed honey bees (Hymenoptera: Apidae: Apis mellifera L.), their food stores, and the hive environment. While not acutely toxic to honey bees, several studies have identified potential sublethal effects, especially in larvae, but comprehensive information regarding the impact of chlorothalonil on adults is lacking. The goal of this study was to investigate the effects of exposure to a field relevant level of chlorothalonil on honey bee antiviral immunity and biochemical markers of general and social immunity, as well as macronutrient markers of nutrition and morphological markers of growth and development. Chlorothalonil exposure was found to have an effect on 1) honey bee resistance and/or tolerance to viral infection by decreasing the survival of bees following a viral challenge, 2) social immunity, by increasing the level of glucose oxidase activity, 3) nutrition, by decreasing levels of total carbohydrate and protein, and 4) development, by decreasing the total body weight, head width, and wing length of adult nurse and forager bees. Although more research is required to better understand how chlorothalonil interacts with bee physiology to increase mortality associated with viral infections, this study clearly illustrates the sublethal effects of chlorothalonil exposure on bee immunity, nutrition, and development.
Keywords: honey bee, chlorothalonil, immunity, nutrition, development
Honey bees are valued as both pollinators and honey producers, contributing to the estimated 35% of worldwide food production derived from crops that depend on pollinators (Klein et al. 2007). Globally, this contribution is valued at ca. $200 billion annually (Gallai et al. 2009), while in the United States, the annual value of pollination services is estimated at ca. $14 billion (Calderone 2012). A significant threat to the role that managed pollinators are expected to play in supporting global agriculture is the unsustainably high rate of annual colony loss reported by beekeepers in the United States and elsewhere throughout the last decade (vanEngelsdorp et al. 2011, vanEngelsdorp et al. 2012, Spleen et al. 2013, Steinhauer et al. 2014, Lee et al. 2015, Seitz et al. 2016, Kulhanek et al. 2017, Brodschneider et al. 2018). It is generally accepted that the cause of these losses is often complex and not driven by any single factor, but instead results from interactions between multiple stressors that can include parasites, pathogens, poor nutrition, and poor management practices (Goulson et al. 2015, Steinhauer et al. 2018). There is, however, a growing consensus that interactions between parasites such as the ectoparasitic mite Varroa destructor and pathogens such as deformed wing virus are the most important cause of unexplained colony loss (Genersch 2010). Parasitism by Varroa mites produces direct damage as a result of feeding (Rosenkranz et al. 2010), but also indirect damage in the form of altered physiological responses (Amdam et al. 2004) and potentially increased virulence of vectored viral pathogens (McMahon et al. 2016). With proper management of mite populations and adequate nutrition, colonies are typically able to tolerate the persistent, low-level infections that seem to be widespread in managed colonies (McMenamin and Genersch 2015, Locke et al. 2017). However, this delicate balance can be disrupted by exposure to environmental stressors such as pesticides, even when they are not acutely toxic, which may negatively impact the ability of bees to tolerate these infections (O’Neal et al. 2017b).
A growing body of literature documents the impact of pesticides on bee physiology that increase pathogen susceptibility (O’Neal et al. 2018) and alter behavior and development (Desneux et al. 2007, Wu et al. 2011). In addition to apicultural pesticides that are applied directly to the hive by beekeepers, bees can also be exposed to agricultural pesticides that are directly applied to flowering crop plants, as well as flowering noncrop plants that have received indirect pesticide contamination. Along with beekeeper-applied acaricides, fungicides are among the most common types of pesticides detected in bees and bee products (vanEngelsdorp et al. 2009b, Mullin et al. 2010), likely due to the application of fungicides during bloom when bees are present (Fell et al. 1983). The most commonly detected fungicide in bees and bee products is chlorothalonil (2,4,5,6-tetrachloro-1,3-benzenedicarbonitrile) (Mullin et al. 2010), which is a broad spectrum, nonsystemic, organochlorine fungicide in the chloronitrile family with multiple sites of action that is widely employed in a variety of agricultural and household settings (Van Scoy and Tjeerdema 2014). Although not acutely toxic to adult bees, chlorothalonil can significantly alter the gut microbial community of bees (Kakumanu et al. 2016), can increase susceptibility to the microsporidian gut pathogen Nosema ceranae (Pettis et al. 2013), and has also been detected at high levels in so-called ‘entombed’ pollen, which has been associated with increased risk of colony mortality (vanEngelsdorp et al. 2009a). Studies of bee larvae have suggested that larvae are more sensitive to chlorothalonil than adults (Zhu et al. 2014, Dai et al. 2018), and studies in both larvae and adults have demonstrated the potential of chlorothalonil to produce synergistic toxicity when administered in combination with other pesticides such as in-hive acaricides (Johnson et al. 2013, Zhu et al. 2014).
The overall goal of this study was to investigate the impact of chlorothalonil exposure at the colony level on the antiviral, general, and social immune responses of honey bees, as well as markers of adult bee nutrition and development. Until now, there have been no published studies that specifically investigate the effects of exposure to chlorothalonil, or any other fungicides, on the resistance and/or tolerance of honey bees to viral infections. One possible reason for this is the widespread prevalence of one or more viral pathogens in managed colonies (Chen et al. 2004, de Miranda et al. 2010, Runckel et al. 2011) that complicate studies intended to characterize viral infection dynamics. An even more significant challenge, however, is a general lack of readily available infectious clones of bee-specific viruses that could be used in controlled infection studies. Consequently, a recently-described model virus system (O’Neal et al. 2017a, b) was used to investigate the effects of chlorothalonil on the survival of bees challenged with a known quantity of virus. These findings were complemented by data showing the effects of chlorothalonil exposure on nurse and forager bees by examining changes in biochemical and morphological markers of 1) bee nutrition (total protein, carbohydrate, and lipid levels), 2) general immunity at the individual level (phenoloxidase [POX] activity), 3) immunity at the colony level (glucose oxidase [GOX] activity), and 4) development (body weight, head width, and wing length).
Materials and Methods
Chemicals
Anthrone, L-3,4-dihydroxyphenylalanine (L-DOPA), and vanillin reagents were purchased from Acros Organics (Fair Lawn, NJ). Bicinchoninic acid, chloroform, chlorothalonil, copper sulfate, sulfuric acid, Triton X-100, and glucose were purchased from Sigma Aldrich (St. Louis, MO). Chymotrypsin and o-dianisidine were purchased from MP Biomedicals (Solon, OH). Horseradish peroxidase was purchased from Novex Life Technologies (Grand Island, NY).
Impact of Chlorothalonil on Antiviral Immunity
Subjects
European honey bees (Apis mellifera) from three different colonies maintained by the Department of Entomology at the University of Nebraska were used for the survival study. Colonies were maintained according to standard beekeeping practices for commercial hives, except that they were not treated with pesticides or subjected to other in-hive interventions. In order to minimize age-related variability in survival experiments, frames of emerging worker brood from each colony were housed in a lab incubator at 32°C with a relative humidity of 50–80% and allowed to emerge for 24 h. Newly emerged bees from each colony were then collected and separately housed as age-matched cohorts in a lab incubator at 32°C with a relative humidity of 50–80% and ad libitum access to a 50% solution (w/v) of sucrose in water. The presence of an egg-laying queen was simulated by providing each cage with ¼ portions of a queen mandibular pheromone-impregnated strip purchased from Mann Lake Ltd. (Hackensack, MN) in order to reduce stress-related variability.
Infection with virus
Bees were individually infected with a known amount of gradient-purified (Marshall and Schneemann 2001) flock house virus (FHV), as previously described (O’Neal et al. 2017a,b). Briefly, each bee was cold anesthetized and received an intrathoracic injection of 50.6 nl of a 2 × 107 plaque-forming units (pfu)/µl viral suspension in 10 mM Tris–HCl, pH 7.5 using a Drummond Scientific Co. Nanoject II microinjection apparatus (Broomall, PA). The amount of virus that each bee received (1 × 106 pfu of FHV/bee) was determined based on the results of previous studies (O’Neal et al. 2017a,b) wherein the observed rate of mortality facilitated the detection of both increases and decreases in survival between treatment groups over time. As a vehicle control, groups were injected with 50.6 nl of 10 mM Tris–HCl, pH 7.5, which was not found to cause significantly greater mortality than a sham injection consisting of a thoracic puncture without fluid delivery. Neither vehicle injection nor sham injection were found to significantly increase mortality over a 10-d period when compared to age-matched, uninjected bees.
Survival study
The effect of chlorothalonil on the survival of virus-challenged bees was examined by orally exposing caged bees to either chlorothalonil (10 μg/L, or 10 parts per billion) in a 50% sucrose solution, or to untreated sucrose solution as a control, and then injecting each bee with either FHV or sterile vehicle as a control. The concentration used for the chlorothalonil treatment was calculated based on the median residue level of chlorothalonil detected in bees, as reported by Mullin et al. (Mullin et al. 2010), and the mean residue levels of chlorothalonil detected in stored pollen samples, as reported by Bernal et al. (Bernal et al. 2010). Each of the four treatment groups consisted of 6 replicates of 25 bees (150 bees per treatment), with 2 replicates from each colony. Bees were injected with either vehicle or virus following 24 h of exposure to chlorothalonil-supplemented or unsupplemented sucrose solution as the only source of food and water. Bees were provided access to the same sucrose solution, which was prepared fresh each day, for the duration of the test. Bees were monitored and survival was recorded daily for 10 d following injection.
Impact of Chlorothalonil on Nutrition, General Immunity, and Development
Experimental colonies
European honey bees (Apis mellifera) from colonies maintained by the Department of Entomology at Virginia Tech were used for the colony treatment studies. Starting in May, six experimental honey bee colonies were established at each of three apiaries (18 total colonies) and allowed to build colony strength through June. Each colony consisted of a single-story hive that was constructed using new frames and foundation in order to reduce exposure to pesticide residue. Sister queens were used in order to reduce genetic variability among the colonies. In order to reduce variability due to the age of the bees selected for analysis, age-matched adult bees were obtained by removing two frames of emerging worker brood from each colony. The frames were caged and maintained in a lab incubator at 32°C with a relative humidity of 50–80% for 8 h while adult bees emerged. At least 100 bees from each frame were collected and marked on the thorax after emergence using model paint (Testors, Vernon Hills, IL). Pine needle smoke was used to reduce paint odors before the marked bees were returned to their respective hives. This process was repeated several times over the course of successive weeks in order to ensure that marked groups of the appropriate age were available in all colonies for analysis.
Experimental treatments
At each apiary, three colonies were treated with chlorothalonil (10 μg/L, or parts per billion) in a 50% sucrose solution for 6 wk while the other three colonies were provided with untreated 50% sucrose solution for the same period. Samples of marked nurse bees were collected from brood frames and samples of marked forager bees were collected from the hive entrance at the end of the 6-wk treatment period. A minimum of 20 bees were collected from each age group in each colony in order to have 5 individuals for protein, carbohydrate, and lipid analysis, 5 individuals for POX and GOX activity measurement, and 10 individuals for morphometric analysis. This resulted in a total of 45 bees per treatment for protein, carbohydrate, and lipid analysis, 45 bees per treatment for POX and GOX activity, and 90 bees per treatment for morphometric analysis. Bee samples were immediately frozen in liquid nitrogen and stored at −80°C until needed for processing and analysis. All measurements were performed using a Molecular Devices SpectraMax M2 multimode microplate reader (Sunnyvale, CA).
Total proteins
Total protein concentration was measured as previously described (Reeves et al. 2018) using a modified version of the methods published by Smith et al. (Smith et al. 1985). Briefly, individual bees were homogenized in cold 0.1 M sodium phosphate buffer (pH 7.8) with 0.3% Triton X-100, centrifuged at 10,000 × g for 10 min at 4°C, and then the supernatant was loaded into a 96-well microplate along with 0.1 M sodium phosphate (pH 7.8) and bicinchoninic acid with 4% (v/v) copper sulfate. After incubating for 30 min at 37°C, samples were allowed to cool at room temperature for 5 min. The optical density of each protein sample was measured at 560 nm and compared to a protein standard curve generated using bovine serum albumin.
Total carbohydrates
Total carbohydrate concentration was measured as previously described (Reeves et al. 2018) using a modified version of the methods published by Van Handel and Day (Van Handel and Day 1988). Briefly, individual bees were homogenized in cold 0.1 M sodium phosphate buffer (pH 7.8) with 0.3% Triton X-100, centrifuged at 10,000 × g for 10 min at 4°C, and then the supernatant was transferred to a 5 ml glass centrifuge tube along with anthrone reagent. After incubating at 90°C for 15 min, samples were cooled at room temperature for 5 min and then loaded into a 96-well microplate. The optical density of each sample was measured at 625 nm and compared to a carbohydrate standard curve generated using glucose.
Total lipids
Total lipid concentration was measured as previously described (Reeves et al. 2018) using a modified version of the methods published by Van Handel and Day (Van Handel and Day 1988). Briefly, individual bees were homogenized in cold 0.1 M sodium phosphate buffer (pH 7.8) with 0.3% Triton X-100, washed with a chloroform:methanol solution, centrifuged at 10,000 × g for 10 min at 4°C, and then the supernatant was transferred to a 5 ml glass centrifuge tube along with sulfuric acid. After incubating at 90°C for 10 min, vanillin was added and samples were cooled at room temperature, then loaded into a 96-well microplate. The optical density of each sample was measured at 625 nm and compared to a lipid standard curve generated using vegetable oil.
POX activity
POX activity was measured as previously described (Reeves et al. 2018) using a modified version of the methods published by Laughton and Siva-Jothy (Laughton and Siva-Jothy 2011). Briefly, hemolymph was collected from individual bees and diluted in cold 0.1 M sodium phosphate buffer (pH 7.8) with 0.3% Triton X-100, then loaded into a 96-well microplate containing 0.1 M sodium phosphate buffer (pH 7.8) and deionized water. After adding chymotrypsin, samples were incubated for 5 min at 37°C, then L-DOPA was added. The change in optical density over time (ΔmOD) was measured at 490 nm in 15-s intervals for 60 min, then standardized using the total protein concentration of each hemolymph sample as determined using a bovine serum albumin standard curve.
GOX activity
GOX activity was measured as previously described (Reeves et al. 2018) using a modified version of the methods published by Alaux et al. (2010). Briefly, individual bee heads were homogenized in cold 0.1 M sodium phosphate buffer (pH 7.8) with 0.3% Triton X-100, then centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was loaded into a 96-well microplate containing 0.5 M potassium phosphate buffer (pH 7.0), 0.1 M glucose, and 2.5 U of horseradish peroxidase. After incubating for 10 min at 37°C, 3 mM o-dianisidine was added. The change in optical density over time (ΔmOD) was measured at 430 nm in 15-s intervals for 90 min, then standardized using the total protein concentration for each bee head as determined using a bovine serum albumin standard curve.
Body weight, head width, and wing length measurements
Measurements of each individual bee were performed as previously described (Reeves et al. 2018) using a modified version of the methods published by Wilson-Rich et al. (Wilson-Rich et al. 2008). Total body weight (wet weight) was recorded to the nearest milligram using a Mettler-Toledo AE 100 analytical balance (Columbus, OH). Head width (mm) and forewing length (mm) were measured using a Dinolite Pro AM413T/AD413T, produced by AnMo Electronics Corporation (New Taipei City, Sanchong District, Taiwan).
Statistical Analysis
Survival study results are reported as Kaplan–Meier survival curves with significant differences between curves determined by the log-rank (Mantel-Cox) test. Statistical analyses of differences in total proteins, carbohydrates, lipids, POX activity, GOX activity, and morphometric measurements based on treatment and age were conducted using a two-way analysis of variance and Dunnett’s multiple comparison test (Zar 2007). All calculations and statistical analyses were performed using GraphPad Prism 7 (La Jolla, CA) at a significance level (α) of 0.05.
Results
Impact of Chlorothalonil on Antiviral Immunity
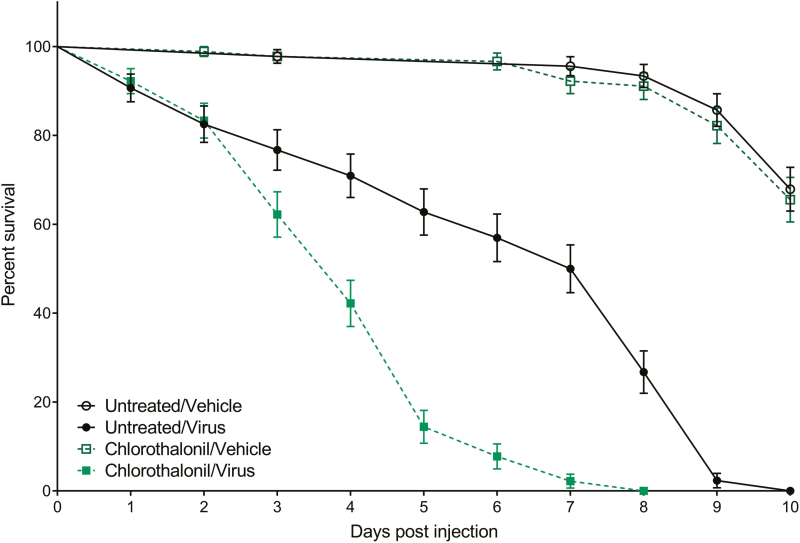
The difference in survival between chlorothalonil-treated and untreated bees following injection with either 1 × 106 pfu of FHV/bee or vehicle is shown in Fig. 1. Survival of bees in both uninfected control groups was greater than 95% at 5 d postinjection and approximately 65% when the study ended at 10 d postinjection. Survival of untreated bees infected with FHV was 62.79% at 5 d postinjection and did not reach 0% (100% mortality) until 10 d postinjection, whereas the survival of chlorothalonil-treated bees infected with FHV was only 14.44% at 5 d postinjection and reached 0% by 8 d postinjection. The survival of chlorothalonil-treated bees infected with FHV was significantly different from that of untreated bees infected with FHV (χ2 = 49.45; df = 1; P < 0.0001). Chlorothalonil-treated bees infected with FHV significantly differed from chlorothalonil-treated bees that were uninfected (χ2 = 183.5; df = 1; P < 0.0001), as well as untreated bees that were uninfected (χ2 = 190.1; df = 1; P < 0.0001). Untreated bees infected with FHV significantly differed from untreated bees that were uninfected (χ2 = 145.5; df = 1; P < 0.0001), as well as chlorothalonil-treated bees that were uninfected (χ2 = 132.5; df = 1; P < 0.0001). Uninfected control groups did not significantly differ from one another (χ2 = 0.1801; df = 1; P = 0.6713).
Fig. 1.
Effects of chlorothalonil exposure on honey bee survival following viral infection. Data analyzed as Kaplan–Meier survival curves where time points depict mean percent survival ± standard error for 150 adult bees (25 bees per replicate with 6 replicates per treatment). Survival of the Chlorothalonil/Virus group was significantly lower than the Untreated/Virus control group (Kaplan–Meier log-rank test; P < 0.0001). Both virus-infected groups experienced lower survival than the uninfected control groups (P < 0.0001), and uninfected control groups did not differ from one another (P = 0.6713).
Impact of Chlorothalonil on Nutrition, General Immunity, and Development
Total proteins
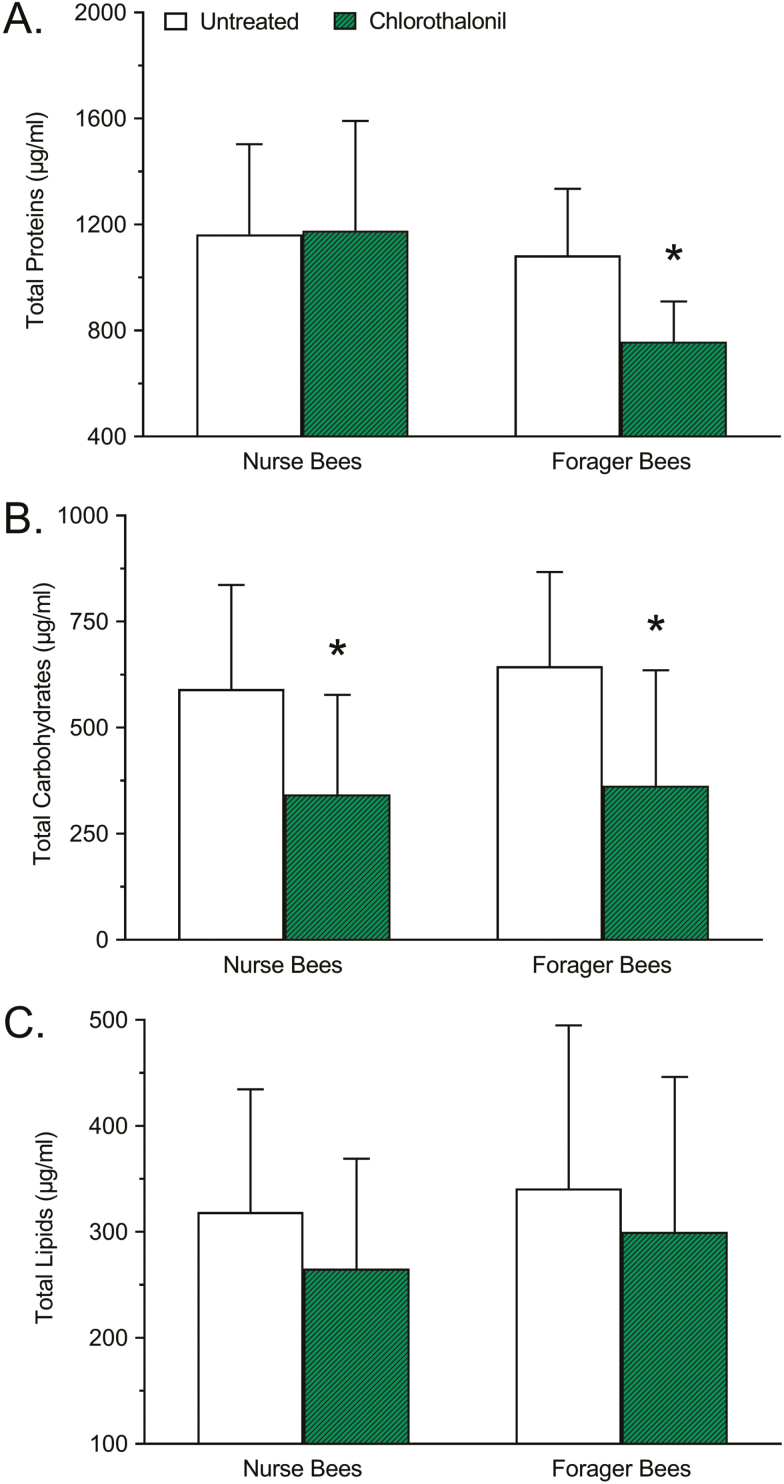
The difference in total protein concentration between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 2A. Significant differences due to age (F = 29.82; df = 1, 176; P < 0.0001) and treatment (F = 11.67; df = 1, 176; P = 0.0008) were detected. Relative to untreated controls, the total protein concentration of nurse bees treated with chlorothalonil did not significantly change (+1.203%; P = 0.9707), whereas the total protein concentration of forager bees treated with chlorothalonil decreased significantly (−30.03%; P < 0.0001).
Fig. 2.
Effects of chlorothalonil exposure on (A) total protein, (B) total carbohydrate, and (C) total lipid concentration of nurse and forager honey bees. Bars represent mean concentration (µg/ml) ± standard deviation (n = 45). Asterisks indicate a significant difference between the chlorothalonil treatment and the respective untreated control based on a two-way analysis of variance and Dunnett’s multiple comparison test where P < 0.05 was considered significant.
Total carbohydrates
The difference in total carbohydrate concentration between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 2B. No significant difference due to age was detected (F = 1.039; df = 1, 174; P = 0.3095), but there was a significant difference due to treatment (F = 52.34; df = 1, 174; P < 0.0001). Relative to untreated controls, the total carbohydrate concentration of nurse bees treated with chlorothalonil decreased significantly (−42.05%; P < 0.0001), as did the total carbohydrate concentration of forager bees treated with chlorothalonil (−43.66%; P < 0.0001).
Total lipids
The difference in total lipid concentration between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 2C. No significant difference due to age was detected (F = 2.092; df = 1, 176; P = 0.1498), but there was a significant difference due to treatment (F = 5.794; df = 1, 176; P = 0.0171). Relative to untreated controls, the total lipid concentration of nurse bees treated with chlorothalonil did not significantly change (−16.79%; P = 0.1082), nor did the total lipid concentration of forager bees treated with chlorothalonil (−12.06%; P = 0.2626).
POX activity
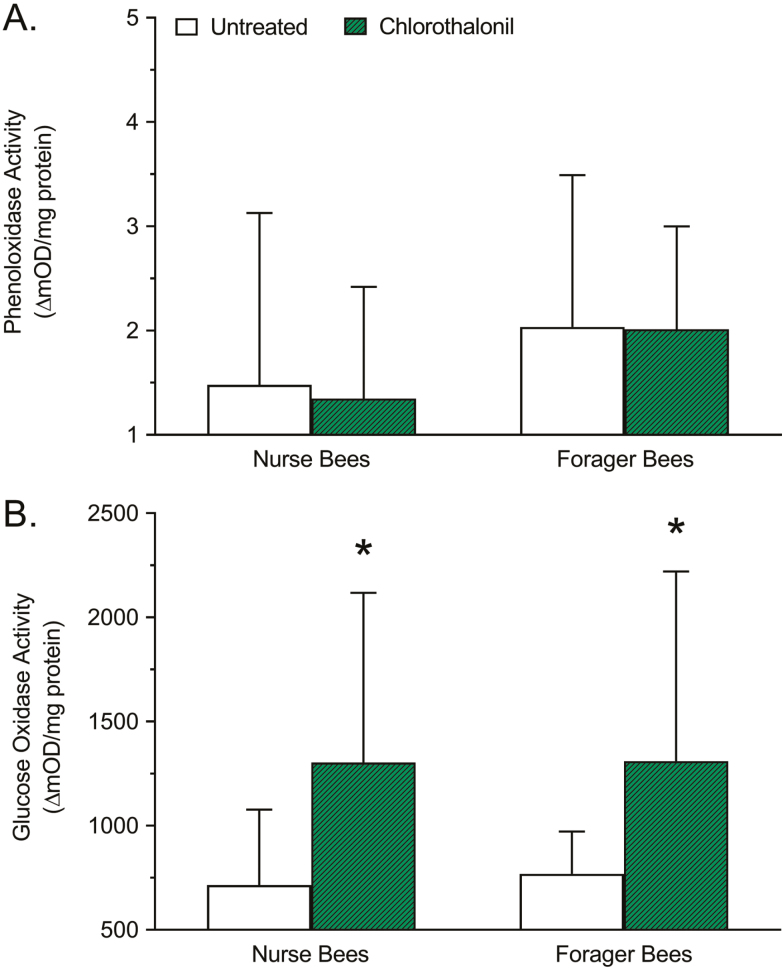
The difference in POX activity between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 3A. A significant interaction due to age was detected (F = 9.511; df = 1, 173; P = 0.0024), but there was no significant interaction due to treatment (F = 0.1523; df = 1, 173; P = 0.6968). Relative to untreated controls, the POX activity of nurse bees treated with chlorothalonil did not significantly change (−8.986%; P = 0.8642), nor did the POX activity of forager bees treated with chlorothalonil (−1.081%; P = 0.9964).
Fig. 3.
Effects of chlorothalonil exposure on (A) total phenoloxidase and (B) total glucose oxidase activity of nurse and forager honey bees. Bars represent mean activity level (ΔmOD/mg protein) ± standard deviation (n = 45). Asterisks indicate a significant difference between the chlorothalonil treatment and the respective untreated control based on a two-way analysis of variance and Dunnett’s multiple comparison test where P < 0.05 was considered significant.
GOX activity
The difference in GOX activity between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 3B. No significant interaction due to age was detected (F = 0.09719; df = 1, 176; P = 0.7556), but there was a significant interaction due to treatment (F = 34.43; df = 1, 176; P < 0.0001). Relative to untreated controls, the GOX activity of nurse bees treated with chlorothalonil increased significantly (+82.31%; P < 0.0001), as did the GOX activity of forager bees treated with chlorothalonil (+70.29%; P = 0.0002).
Body weight measurements
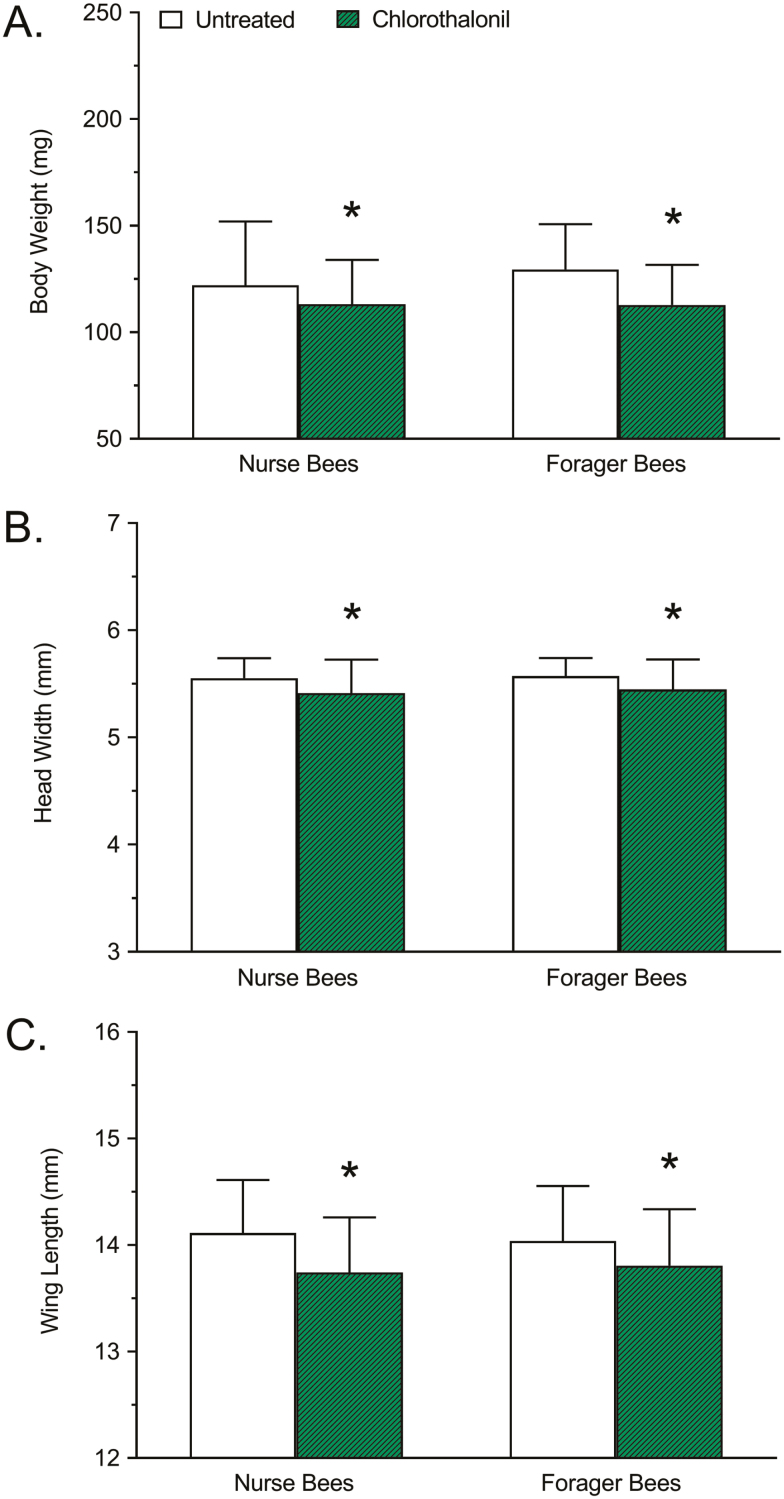
The difference in body weight between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 4A. No significant interaction due to age was detected (F = 2.096; df = 1, 356; P = 0.1486), but there was a significant interaction due to treatment (F = 27.42; df = 1, 356; P < 0.0001). Relative to untreated controls, the body weight of nurse bees treated with chlorothalonil decreased significantly (−7.213%; P = 0.0213), as did the body weight of forager bees treated with chlorothalonil (−12.83%; P < 0.0001).
Fig. 4.
Effects of chlorothalonil exposure on (A) body weight, (B) head width, and (C) wing length of nurse and forager honey bees. Bars represent mean body weight (mg), head width (mm), or wing length (mm) ± standard deviation (n = 90). Asterisks indicate a significant difference between the chlorothalonil treatment and the respective untreated control based on a two-way analysis of variance and Dunnett’s multiple comparison test where P < 0.05 was considered significant.
Head width measurements
The difference in head width between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 4B. No significant interaction due to age was detected (F = 1.155; df = 1, 356; P = 0.2832), but there was a significant interaction due to treatment (F = 26.43; df = 1, 356; P < 0.0001). Relative to untreated controls, the head width of nurse bees treated with chlorothalonil decreased significantly (−2.521%; P = 0.0003), as did the head width of forager bees treated with chlorothalonil (−2.243%; P = 0.0013).
Wing length measurements
The difference in wing length between chlorothalonil-treated and untreated nurse and forager honey bees is shown in Fig. 4C. No significant interaction due to age was detected (F = 0.008489; df = 1, 355; P = 0.9266), but there was a significant interaction due to treatment (F = 30.58; df = 1, 355; P < 0.0001). Relative to untreated controls, the wing length of nurse bees treated with chlorothalonil decreased significantly (−2.622%; P < 0.0001), as did the wing length of forager bees treated with chlorothalonil (−1.638%; P = 0.0056).
Discussion
The fungicide chlorothalonil is widely used for agricultural control of fungus and mildew. As it is not considered acutely toxic in adult bees, it is routinely applied around the time of bloom, resulting in a high potential for bee exposure. Indeed, chlorothalonil residues in pollen have been reported as high as 98.9 parts per million (Mullin et al. 2010) and studies have demonstrated detrimental health effects in both larvae (Zhu et al. 2014, Dai et al. 2018) and adults (vanEngelsdorp et al. 2009a, Pettis et al. 2013, Kakumanu et al. 2016). Furthermore, chlorothalonil is metabolized via oxidative dechlorination/hydrolysis to 4-hydroxy-2,5,6-trichloroisophthalonitrile, which is both more toxic and more likely to cause oxidative stress than the parent compound (Suzuki et al. 2004, Chaves et al. 2008). The work described here provides evidence that exposure to chlorothalonil at an environmentally relevant level (10 parts per billion) significantly decreased the survival of bees challenged with a viral infection. Furthermore, chlorothalonil exposure had a significant impact on 1) bee nutrition, evident through reduced protein and carbohydrate levels, 2) social immunity, evident through increased GOX activity, and 3) growth and development, evident through decreased body weight, head width, and wing length. This is the first study to report on the significant effect of chlorothalonil exposure on bee antiviral immunity, in addition to providing insight into the detrimental effects of dietary exposure to hive residue levels of chlorothalonil on the health and development of adult bees.
Chlorothalonil exposure was associated with a decrease in the levels of total carbohydrates in both nurse and forager bees, as well as a decrease in total protein levels in forager bees, but did not appear to alter lipid levels in nurse and forager bees, nor the protein levels of nurse bees. Nutrition is understood to play an important role in bee development and antiviral immunity (DeGrandi-Hoffman and Chen 2015), and low levels of macronutrients have been associated with reduced worker lifespan (Knox et al. 1971), reduced capacity for energy-intensive tasks such as flight, thermoregulation, comb building (Brodschneider and Crailsheim 2010), as well as reduced colony population growth (Zheng et al. 2014). It is worth noting that the decrease in total protein associated with chlorothalonil exposure was only observed in forager bees, which may be related to the high level of flight/foraging activity common at that time of year. Furthermore, previous work has shown a similar pattern of results when investigating the effects of exposure to the beekeeper-applied, in-hive acaricides coumaphos and tau-fluvalinate on bee macronutrient levels, markers of immunity, and morphometric markers of development (Reeves et al. 2018). Therefore, it is not surprising that bees with low macronutrient levels also display signs of impaired growth and development, as suggested by the decrease in body weight, head width, and wing length observed in both nurse and forager bees treated with chlorothalonil. These results, however, contradict previous reports showing that chlorothalonil exposure did not alter body weight, protein levels, or carbohydrate levels in worker bees (Feazel-Orr et al. 2016), or POX and GOX activity (Traver et al. 2018). The differing results between these previous reports and the study described here are likely due to differences in experimental methodology. One possible explanation is that differences between treatments were obscured by genetic variability, as neither of the previous studies attempted to control for this by establishing colonies using sister queens. Even more likely is that differences due to treatment were masked by age-related variability, as neither of the previous studies attempted to control for age by using age-matched bees, but instead randomly sampled bees from an area near the brood nest. These differences in observed results emphasize the importance of controlling for variables such as age, given that the nutritional needs of a bee shift with age and the corresponding role of that bee in the hive (Paoli et al. 2014).
Chlorothalonil exposure was associated with an increase in the levels of GOX activity, but not with a change in the levels of POX activity, in both nurse and forager bees. The observed increase in GOX activity suggests that exposure to chlorothalonil may be inducing a social immune response. Colony-level bacterial infection by American foulbrood (Paenibacillus larvae) was not found to increase GOX activity (López-Uribe et al. 2017), which was suggested by the authors to be the result of high levels of constitutive expression of GOX and other antimicrobial proteins. However, treatment with the organophosphate acaricide coumaphos and the pyrethroid acaricide tau-fluvalinate were previously shown to increase GOX levels in bees (Reeves et al. 2018), whereas the neonicotinoid pesticide imidacloprid had the opposite effect, decreasing GOX activity (Alaux et al. 2010). This difference may be the result of the different modes of action exhibited by these chemistries, or it could be explained by differences in experimental design between these studies. These findings also appear to support recent work that provides evidence for a trade-off between GOX activity and body mass (Jones et al. 2018), as bees treated with chlorothalonil display both increased GOX activity and decreased body weight. This suggests that the decrease in body weight may be the result of the physiologically taxing act of maintaining an enhanced social immune response. In contrast, however, chlorothalonil exposure did not appear to elicit a general immune response, as measured by POX activity, but this does not preclude the possibility of detrimental interactions with the bee immune response to a range of pathogens. Previous work has shown that bee larvae treated with chlorothalonil experience elevated transcript levels for prophenoloxidase-activating enzyme (Gregorc et al. 2012) and there is mounting evidence that chlorothalonil in combination with other pesticides yields increased toxicity (Johnson et al. 2013, Zhu et al. 2014) and pathogen susceptibility (Wu et al. 2012) in bees.
This study provides the first direct evidence of harmful interactions between chlorothalonil exposure and viral pathogens in bees, as is evident by the reduced ability of treated bees to resist and/or tolerate infection with a viral pathogen. As this portion of the study was, by necessity, conducted using laboratory assays rather than in the hive, more research is needed to determine the practical implications of this interaction at the colony level. This work also serves to emphasize the continuing need to develop better tools for answering questions related to honey bee antiviral immunity, which would provide significant insight into bee physiology and improve our collective understanding of bee antiviral immunity, disease tolerance that could be used to inform better management strategies and promote the future health and sustainability of global agriculture.
Acknowledgments
This study was partially funded by a grant from the Virginia Department of Agriculture and Consumer Services (MOA 2013-001; registration fees paid by pesticide companies).
Author Contributions
Conceptualization: S.T.O., A.M.R., R.D.F., C.C.B., and T.D.A. Investigation: S.T.O., A.M.R., and T.D.A. Formal analysis: S.T.O., C.C.B., and T.D.A. Original draft preparation: S.T.O. Review and editing: S.T.O., R.D.F., C.C.B., and T.D.A.
Author Competing Interests
The authors declare no competing financial interests.
References Cited
- Alaux C., Brunet J. L., Dussaubat C., Mondet F., Tchamitchan S., Cousin M., Brillard J., Baldy A., Belzunces L. P., and Le Conte Y.. 2010. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 12: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam G. V., K. Hartfelder K. Norberg A. Hagen, and Omholt S. W.. 2004. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering? J. Econ. Entomol. 97: 741–747. [DOI] [PubMed] [Google Scholar]
- Bernal J., E. Garrido-Bailón M. J. Del Nozal A. V. González-Porto R. Martín-Hernández J. C. Diego J. J. Jiménez J. L. Bernal, and Higes M.. 2010. Overview of pesticide residues in stored pollen and their potential effect on bee colony (Apis mellifera) losses in Spain. J. Econ. Entomol. 103: 1964–1971. [DOI] [PubMed] [Google Scholar]
- Brodschneider R., and Crailsheim K.. . 2010. Nutrition and health in honey bees. Apidologie. 41: 278–294. [Google Scholar]
- Brodschneider R., Gray A., Adjlane N., Ballis A., Brusbardis V., Charrière J.-D., Chlebo R., Coffey M. F., Dahle B., de Graaf D. C., . et al. 2018. Multi-country loss rates of honey bee colonies during winter 2016/2017 from the COLOSS survey. J Apicult Res. 57: 452–457. [Google Scholar]
- Calderone N. W. 2012. Insect pollinated crops, insect pollinators and US agriculture: trend analysis of aggregate data for the period 1992-2009. PLoS One 7: e37235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves A., D. Shea, and Danehower D.. 2008. Analysis of chlorothalonil and degradation products in soil and water by GC/MS and LC/MS. Chemosphere. 71: 629–638. [DOI] [PubMed] [Google Scholar]
- Chen Y., Y. Zhao J. Hammond H. T. Hsu J. Evans, and Feldlaufer M.. 2004. Multiple virus infections in the honey bee and genome divergence of honey bee viruses. J. Invertebr. Pathol. 87: 84–93. [DOI] [PubMed] [Google Scholar]
- Dai P., C. J. Jack A. N. Mortensen J. R. Bloomquist, and Ellis J. D.. 2018. The impacts of chlorothalonil and diflubenzuron on Apis mellifera L. larvae reared in vitro. Ecotoxicol. Environ. Saf. 164: 283–288. [DOI] [PubMed] [Google Scholar]
- DeGrandi-Hoffman G., and Chen Y.. . 2015. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 10: 170–176. [DOI] [PubMed] [Google Scholar]
- Desneux N., A. Decourtye, and Delpuech J. M.. 2007. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 52: 81–106. [DOI] [PubMed] [Google Scholar]
- Feazel-Orr H. K., Catalfamo K. M., Brewster C. C., Fell R. D., Anderson T. D., and Traver B. E.. . 2016. Effects of pesticide treatments on nutrient levels in worker honey bees (Apis mellifera). Insects. 7: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell R. D., Rajotte E. G., and Yoder K. S.. . 1983. Effects of fungicide sprays during apple bloom on pollen viability and honey bee foraging. Environ. Entomol. 12: 1572–1575. [Google Scholar]
- Gallai N., Salles J. M., Settele J., and Vaissiere B. E.. . 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ. 68: 810–821. [Google Scholar]
- Genersch E. 2010. Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 87: 87–97. [DOI] [PubMed] [Google Scholar]
- Goulson D., E. Nicholls C. Botías, and Rotheray E. L.. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science. 347: 1255957. [DOI] [PubMed] [Google Scholar]
- Gregorc A., J. D. Evans M. Scharf, and Ellis J. D.. 2012. Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). J. Insect Physiol. 58: 1042–1049. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Dahlgren L., Siegfried B. D., and Ellis M. D.. . 2013. Acaricide, fungicide and drug interactions in honey bees (Apis mellifera). PLoS One 8 (1): e54092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B., E. Shipley, and Arnold K. E.. 2018. Social immunity in honeybees-Density dependence, diet, and body mass trade-offs. Ecol. Evol. 8: 4852–4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakumanu M. L., Reeves A. M., Anderson T. D., Rodrigues R. R., and Williams M. A.. . 2016. Honey bee gut microbiome is altered by in-hive pesticide exposures. Front. Microbiol. 7: 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A. M., Vaissière B. E., Cane J. H., Steffan-Dewenter I., Cunningham S. A., Kremen C., and Tscharntke T.. . 2007. Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D. A., Shimanuki H., and Herbert E. W.. . 1971. Diet and the longevity of adult honey bees. J. Econ. Entomol. 64: 1415–1416. [Google Scholar]
- Kulhanek K., Steinhauer N., Rennich K., Caron D. M., Sagili R. R., Pettis J. S., Ellis J. D., Wilson M. E., Wilkes J. T., Tarpy D. R., . et al. 2017. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. J Apicult Res. 56: 328–340. [Google Scholar]
- Laughton A. M., and Siva-Jothy M. T.. . 2011. A standardised protocol for measuring phenoloxidase and prophenoloxidase in the honey bee, Apis mellifera. Apidologie. 42: 140–149. [Google Scholar]
- Lee K. V., Steinhauer N., Rennich K., Wilson M. E., Tarpy D. R., Caron D. M., Rose R., Delaplane K. S., Baylis K., Lengerich E. J., . et al. 2015. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie. 46: 292–305. [Google Scholar]
- Locke B., E. Semberg E. Forsgren, and de Miranda J. R.. 2017. Persistence of subclinical deformed wing virus infections in honeybees following Varroa mite removal and a bee population turnover. PLoS One 12: e0180910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Uribe M. M., A. Fitzgerald, and Simone-Finstrom M.. 2017. Inducible versus constitutive social immunity: examining effects of colony infection on glucose oxidase and defensin-1 production in honeybees. R. Soc. Open Sci. 4: 170224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D., and Schneemann A.. . 2001. Specific packaging of nodaviral RNA2 requires the N-terminus of the capsid protein. Virology. 285: 165–175. [DOI] [PubMed] [Google Scholar]
- McMahon D. P., Natsopoulou M. E., Doublet V., Fürst M., Weging S., Brown M. J. F., Gogol-Döring A., and Paxton R. J.. . 2016. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B: Biol. Sci. 283: 20160811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin A. J., and Genersch E.. . 2015. Honey bee colony losses and associated viruses. Curr. Opin. Insect Sci. 8: 121–129. [DOI] [PubMed] [Google Scholar]
- de Miranda J. R., G. Cordoni, and Budge G.. 2010. The Acute bee paralysis virus-Kashmir bee virus-Israeli acute paralysis virus complex. J. Invertebr. Pathol. 103(Suppl 1): S30–S47. [DOI] [PubMed] [Google Scholar]
- Mullin C. A., M. Frazier J. L. Frazier S. Ashcraft R. Simonds D. Vanengelsdorp, and Pettis J. S.. 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS One 5: e9754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal S. T., D. R. Swale, and Anderson T. D.. 2017a. ATP-sensitive inwardly rectifying potassium channel regulation of viral infections in honey bees. Sci. Rep. 7: 8668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neal S. T., C. C. Brewster J. R. Bloomquist, and Anderson T. D.. 2017b. Amitraz and its metabolite modulate honey bee cardiac function and tolerance to viral infection. J. Invertebr. Pathol. 149: 119–126. [DOI] [PubMed] [Google Scholar]
- O’Neal S. T., T. D. Anderson, and Wu-Smart J. Y.. 2018. Interactions between pesticides and pathogen susceptibility in honey bees. Curr. Opin. Insect Sci. 26: 57–62. [DOI] [PubMed] [Google Scholar]
- Paoli P. P., D. Donley D. Stabler A. Saseendranath S. W. Nicolson S. J. Simpson, and Wright G. A.. 2014. Nutritional balance of essential amino acids and carbohydrates of the adult worker honeybee depends on age. Amino Acids 46: 1449–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettis J. S., E. M. Lichtenberg M. Andree J. Stitzinger R. Rose, and Vanengelsdorp D.. 2013. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PLoS One 8: e70182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves A. M., O’Neal S. T., Fell R. D., Brewster C. C., and Anderson T. D.. . 2018. In-hive acaricides alter biochemical and morphological indicators of honey bee nutrition, immunity, and development. J. Insect Sci. 18: 8–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz P., P. Aumeier, and Ziegelmann B.. 2010. Biology and control of Varroa destructor. J. Invertebr. Pathol. 103(Suppl 1): S96–119. [DOI] [PubMed] [Google Scholar]
- Runckel C., M. L. Flenniken J. C. Engel J. G. Ruby D. Ganem R. Andino, and DeRisi J. L.. 2011. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, Nosema, and Crithidia. PLoS One 6: e20656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz N., Traynor K. S., Steinhauer N., Rennich K., Wilson M. E., Ellis J. D., Rose R., Tarpy D. R., Sagili R. R., Caron D. M., . et al. 2016. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J Apicult Res. 54: 292–304. [Google Scholar]
- Smith P. K., R. I. Krohn G. T. Hermanson A. K. Mallia F. H. Gartner M. D. Provenzano E. K. Fujimoto N. M. Goeke B. J. Olson, and Klenk D. C.. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. [DOI] [PubMed] [Google Scholar]
- Spleen A. M., Lengerich E. J., Rennich K., Caron D., Rose R., Pettis J. S., Henson M., Wilkes J. T., Wilson M., Stitzinger J., . et al. 2013. A national survey of managed honey bee 2011–12 winter colony losses in the United States: results from the Bee Informed Partnership. J Apicult Res. 52(2): 44–53. [Google Scholar]
- Steinhauer N. A., Rennich K., Wilson M. E., Caron D. M., Lengerich E. J., Pettis J. S., Rose R., Skinner J. A., Tarpy D. R., Wilkes J. T., . et al. 2014. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: results from the Bee Informed Partnership. J Apicult Res. 53: 1–18. [Google Scholar]
- Steinhauer N., K. Kulhanek K. Antúnez H. Human P. Chantawannakul M. P. Chauzat, and vanEngelsdorp D.. 2018. Drivers of colony losses. Curr. Opin. Insect Sci. 26: 142–148. [DOI] [PubMed] [Google Scholar]
- Suzuki T., H. Nojiri H. Isono, and Ochi T.. 2004. Oxidative damages in isolated rat hepatocytes treated with the organochlorine fungicides captan, dichlofluanid and chlorothalonil. Toxicology. 204: 97–107. [DOI] [PubMed] [Google Scholar]
- Traver B. E., H. K. Feazel-Orr K. M. Catalfamo C. C. Brewster, and Fell R. D.. 2018. Seasonal Effects and the Impact of In-Hive Pesticide Treatments on Parasite, Pathogens, and Health of Honey Bees. J. Econ. Entomol. 111: 517–527. [DOI] [PubMed] [Google Scholar]
- Van Handel E., and Day J. F.. . 1988. Assay of lipids, glycogen and sugars in individual mosquitoes: correlations with wing length in field-collected Aedes vexans. J. Am. Mosq. Control Assoc. 4: 549–550. [PubMed] [Google Scholar]
- Van Scoy A. R., and Tjeerdema R. S.. . 2014. Environmental fate and toxicology of chlorothalonil, pp. 89–105. In Whitacre D. M. (ed.), Reviews of environmental contamination and toxicology, Vol. 232 Springer International Publishing, Cham. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D., J. D. Evans L. Donovall C. Mullin M. Frazier J. Frazier D. R. Tarpy J. Hayes Jr, and Pettis J. S.. 2009a. “Entombed Pollen”: a new condition in honey bee colonies associated with increased risk of colony mortality. J. Invertebr. Pathol. 101: 147–149. [DOI] [PubMed] [Google Scholar]
- vanEngelsdorp D., J. D., Evans C., Saegerman C., Mullin E., Haubruge B. K., Nguyen M., Frazier J., Frazier D., Cox-Foster Y., Chen, et al. 2009b. Colony collapse disorder: a descriptive study. PLoS One 4: e6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanEngelsdorp D., Hayes J., Underwood R. M., Caron D., and Pettis J.. . 2011. A survey of managed honey bee colony losses in the USA, fall 2009 to winter 2010. J Apicult Res. 50: 1–10. [Google Scholar]
- vanEngelsdorp D., Caron D., Hayes J., Underwood R., Henson M., Rennich K., Spleen A., Andree M., Snyder R., Lee K., . et al. 2012. A national survey of managed honey bee 2010–11 winter colony losses in the USA: results from the Bee Informed Partnership. J Apicult Res. 51: 115–124. [Google Scholar]
- Wilson-Rich N., S. T. Dres, and Starks P. T.. 2008. The ontogeny of immunity: development of innate immune strength in the honey bee (Apis mellifera). J. Insect Physiol. 54: 1392–1399. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., C. M. Anelli, and Sheppard W. S.. 2011. Sub-lethal effects of pesticide residues in brood comb on worker honey bee (Apis mellifera) development and longevity. PLoS One 6: e14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., M. D. Smart C. M. Anelli, and Sheppard W. S.. 2012. Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. J. Invertebr. Pathol. 109: 326–329. [DOI] [PubMed] [Google Scholar]
- Zar J. H. 2007. Biostatistical analysis, 5th ed Pearson, Upper Saddle River, NJ. [Google Scholar]
- Zheng B., Z. Wu, and Xu B.. 2014. The effects of dietary protein levels on the population growth, performance, and physiology of honey bee workers during early spring. J. Insect Sci. 14: 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W., D. R. Schmehl C. A. Mullin, and Frazier J. L.. 2014. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS One 9: e77547. [DOI] [PMC free article] [PubMed] [Google Scholar]