Abstract
BACKGROUND AND OBJECTIVES:
Hypertension in pregnancy is one of the most important unsolved problems in midwifery, and since it is the main cause of maternal death, preventive intervention measures are essential to control this serious complication. This study aimed to determine the effect of walking on gestational hypertension disorders in women prone to hypertension.
MATERIALS AND METHODS:
This randomized clinical trial was conducted on 72 pregnant women susceptible to gestational hypertension who were randomly (through a random number table) assigned into two groups of 36. The pregnant women in the experimental group had walking program for 20–30 min from weeks 14–34, four times a week. Data were analyzed, via independent t-test, Fisher's exact test, and Chi-square test.
RESULTS:
The results indicated that in the experimental group, 2 cases with transient gestational hypertension and 1 case of preeclampsia existed, and in the control group, 9 pregnant women were with gestational hypertension and 4 pregnant women were with preeclampsia. Therefore, the incidence of these two complications in the experimental group was significantly lower than the control group (P < 0.05). Moreover, the mean systolic and diastolic blood pressures in the experimental group were significantly lower than the control group (P < 0.05).
CONCLUSION:
Based on the results, the moderate walking, as an easy physical activity, is recommended for pregnant women susceptible to pregnancy hypertension.
Keywords: Hypertension in pregnancy, physical activity, preeclampsia, walking
Introduction
Despite extensive research in recent decades, how creating hypertension by pregnancy remains unanswered, while these blood pressure disorders are among the most important unsolved problems in the midwifery. The initial classification describing the four types of hypertension includes pregnancy-induced hypertension, preeclampsia and eclampsia syndrome, chronic hypertension for any reason, and preeclampsia superimposed on chronic hypertension. Among these disorders, preeclampsia syndrome alone or along with chronic hypertension is considered the most dangerous mode.[1]
Preeclampsia affects 3.9% of all pregnancies and 20% of the first pregnancy, and about 5000 mothers’ deaths are associated to preeclampsia and its complications.[2] In a study conducted by Ramírez-Vélez et al.[3] in 2009, it was indicated that the preeclampsia occurrence in developing countries is twenty times more than that in the developed countries and it is one of the common causes of mortality and maternal and perinatal morbidity throughout the world.[4,5,6] Janani et al. in Iran showed that the prevalence of blood pressure disorders is 3.8%.[7]
Considering the relatively high prevalence of hypertension in pregnancy and its role in the maternal and neonatal mortality and being affected, it is necessary to provide the necessary knowledge to the mothers and prevent this complication during pregnancy through complete care.[5] Therefore, compliance with standard sports programs can have beneficial effects, such as reducing the risk factors for hypertension, in this regard.[8]
Today, the sport is important in the world, and all societies try to incorporate physical activities into the daily agenda of humans. Currently, exercise at the reproductive age, especially during pregnancy, is emphasized by medical science;[9] however, despite the variety in physical activities during pregnancy, there is still insufficient scientific evidence of the superiority of an exercise method to another.[10]
The American College of Obstetricians and Gynecologists in 2002 stated that if the pregnant women do not have medical and obstetric disorders, they can have moderate physical activity at most days of the week for about 30 min, provided that this activity does not have any effect on the embryo and does not directly damage the womb. The association emphasizes the training and encouragement of pregnant mothers to physical activity by their caregivers since most of these activities are inexpensive and affordable and one can be protected from imposing a heavy economic burden caused by the pregnancy complications, in addition to benefiting from health benefits during pregnancy.[4] Today, evidence suggests that despite increased maternal health literacy, physical activity is not done effectively during pregnancy. In a study conducted in Shahid Beheshti University of Medical Sciences, on 386 pregnant women with normal pregnancies, it was stated that even in active women who continued to exercise during pregnancy, the activity was reduced compared to the prepregnancy conditions,[11] which indicates nonconsidering the benefits of physical activity in pregnancy.
Among the physical activities that can be done during pregnancy, it can refer to swim, mild aerobic exercise, such as walking, yoga, and stretching exercises, of which walking is likely the mild, easy, and inexpensive exercise. These mild physical activities stimulate the main muscle groups and improve vascular perfusion;[12] therefore, they can be effective in reducing pregnancy complications if done correctly and continuously.
Physical exercise and activities are currently associated with reduced risk and the necessity of using drugs to treat hypertension in associated nonpregnancy. They are also recommended in nonrisky or the low-risk pregnancies. Furthermore, in some studies, exercise was recommended in pregnant women with hypertension or preeclampsia risks aimed to reduce the incidence of hypertension including the reduced preeclampsia incidence,[13,14] which further studies are required in this regard.
Therefore, considering the high prevalence of gestational hypertension disorders in the recent years and the possibility of more mothers affected by these disorders risk, as well as the high contribution of these disorders to the maternal and neonatal mortalities and complications, and the lack of studies on the effects of various exercises and physical activities on prevention of these disorders in susceptible pregnant women, it is essential to recognize the pregnant women at pregnancy-related hypertensive disorders risk so that with recommendations about having physical activity, their pregnancies end with the least complication and the best outcome. This study aimed to determine the effect of walking on hypertensive disorders in pregnancy in women susceptible to hypertension referring to midwifery service centers.
Materials and Methods
This prospective randomized clinical trial was conducted with a clinical trial code of 30997. The research sample including 72 pregnant women referring to the comprehensive health service centers in Rafsanjan was randomly divided into two groups of 36. The randomization process was performed through a random number table. The researcher chose the table numbers from the top and considered the even numbers for the experimental group and the odd numbers for the control group. Furthermore, the process of this sequence was in hidden form.
The sample size was determined using the following formula:
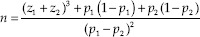
All the pregnant women in this study were susceptible to gestational hypertension and had a history of transient gestational hypertension, history of preeclampsia or eclampsia, chronic hypertension, autoimmune disease, history of transient hypertension or preeclampsia in a sister or mother, or obvious diabetes. The participants were included in case they had no restriction on physical activity and psychological illness such as depression and anxiety and had a singleton pregnancy.
The participants were excluded if they suffered from pregnancy complications such as bleeding during pregnancy or preterm labor or if the participants did not go on walking or declared lack of walking within one or more weeks.
Both groups received routine prenatal care at weeks 16–20, 24–30, 31–34, 35–37, 38, 39, and 40, based on the country plan. The mothers in the control group received just the same routine pregnancy care, and the mothers in the experimental group had the walking program.
These women were asked to have an average moderate walk for 20–30 min each week from 14 to 34 weeks of the pregnancy, four times a week so that they can talk when walking. Before walking, the pregnant women were recommended to use adequate fluids, walk with comfortable clothes, and do not walk with the full or empty stomach. The pregnant women could do walking at home or in a free environment cautiously. The mothers were asked to refer to their physician and inform the researcher in cases they had signs of danger such as shortness of breath, palpitations, difficulty, and imbalance while walking.
A 1-day follow-up was conducted through the phone by the researcher from week 14 to the end of week 34 to examine the experimental group in terms of walking. Moreover, from week 20 of the gestation until the end of week 34, to examine the control and experimental groups in terms of the development of hypertension, the researcher contacted to the relevant midwifery service providers and the hospital high-risk care ward to identify the type of hypertension such as pregnancy-associated transient hypertension, preeclampsia, eclampsia, and preeclampsia superimposed on chronic hypertension. In the case of maternal affection, the mother's name was included into the list of mothers with hypertension, and the mothers of the experimental group were excluded from the walking program. Ultimately, the chart of the blood pressure changes from the beginning of pregnancy to the end of the study was recorded in the checklist.
Data were collected using the checklist for fertility and hypertension characteristics, and the data were analyzed by SPSS (SPSS V.23 Inc., Chicago, IL, USA) using paired and independent t-test, Fisher's exact test, and Chi-square test.
This study was conducted with an ethics code of 396,533. At the beginning of the study, written consents were obtained from all the participants participating in the project. Participants at each stage of the study could leave the study, and the implementation of the plan did not endanger the participants. The information obtained in this plan was confidential.
Results
Of the 74 participants in this project, 38 were assigned in the experimental group and 36 were in the control group, of which 2 were excluded from the experimental group.

After examining the demographic and fertility characteristics of the two groups, the mean demographic variables of age and body mass index (BMI) were not significantly different between the two groups (P > 0.05). The age range of pregnant women in the experimental group was 19–40 years and it was 19–41 years in the control group. The mean of fertility variables including gestational age, number of deliveries, and the number of abortions in the two groups was not significantly different (P > 0.05) [Table 1].
Table 1.
Comparison of the average of the different quantitative variables between the two groups
| Variable | Experimental | Control | Independent t-test | |||
|---|---|---|---|---|---|---|
| Average | SD | Average | SD | t | P | |
| Age (year) | 31.91 | 4.62 | 31 | 5.29 | 0.76 | 0.45 |
| Gestational age at the start of the study according to LMP (week) | 14.02 | 0.30 | 14.94 | 1.40 | 1.57 | 0.11 |
| Pregnancy number | 2.78 | 1.17 | 2.58 | 1.25 | 0.68 | 0.50 |
| Number of deliveries | 1.64 | 1.06 | 1.22 | 1.05 | 1.67 | 0.10 |
| Number of abortions | 0.39 | 0.77 | 0.50 | 0.77 | 0.61 | 0.54 |
| BMI | 27.36 | 3.64 | 34.97 | 43.77 | 1.04 | 0.30 |
SD=Standard deviation, BMI=Body mass index, LMP=Last menstrual period
There was no significant difference between the two groups in terms of the history of hypertension during the previous pregnancy, obvious diabetes, autoimmune diseases, and chronic hypertension (P > 0.05) [Table 2].
Table 2.
The frequency distribution of hypertension records in pregnancy in two groups
| Records | Experimental, n (%) | Group, n (%) | χ2 | P |
|---|---|---|---|---|
| Previous gestational hypertension | 9 (25) | 9 (25) | - | 1 |
| Previous preeclampsia | 11 (30.6) | 12 (33.3) | 0.06 | 0.80 |
| Chronic hypertension | 7 (19.4) | 5 (13.9) | 0.40 | 0.53 |
| Previous preeclampsia superimposed on chronic hypertension | 0 | 0 | - | 1 |
| Previous eclampsia | 0 | 0 | - | 1 |
| Autoimmune disease | 3 (8.3) | 6 (16.7) | - | 0.24 |
| Obvious diabetes | 6 (16.7) | 5 (13.9) | 0.11 | 0.74 |
| The history of preeclampsia in first-degree relatives (sister and mother) | 1 (2.8) | 3 (3.8) | - | 0.31 |
After analyzing the data, it was determined that among 36 pregnant women in the experimental group, 2 pregnant women had transient gestational hypertension and 1 pregnant woman had preeclampsia. In the control group, 9 pregnant women were with transient gestational hypertension and 4 pregnant women with preeclampsia. These findings indicated that transient hypertension and preeclampsia in the experimental group were significantly lower than the control group (P < 0.05) [Table 3].
Table 3.
The frequency distribution of blood pressure disorders in the current pregnancy in the two groups
| Variable | Experimental, n (%) | Control, n (%) | χ2 | P | ||
|---|---|---|---|---|---|---|
| Gestational hypertension | 2 | 5.6 | 9 | 25 | 5.26 | 0.02 |
| Preeclampsia | 1 | 2.8 | 4 | 11.1 | - | 0.042 |
| Eclampsia | 0 | 0 | 0 | 0 | - | 1 |
| Preeclampsia superimposed on chronic hypertension | 0 | 0 | 0 | 0 | - | 1 |
Other findings showed that no preeclampsia superimposed on chronic hypertension, and no cases of eclampsia were observed in both the groups (P > 0.05) [Table 3].
Furthermore, the average changes in systolic and diastolic blood pressures after walking compared to before walking indicated that this average in the experimental group was significantly lower than the control group (P < 0.05) [Table 4].
Table 4.
Comparison of mean systolic and diastolic blood pressure changes after walking compared to prewalk between the two groups
| Changes | Experimental | Control | Independent t-test | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | P | |
| Systolic blood pressure | 1.81 | 2.40 | 9.86 | 2.87 | 2.15 | 0.03 |
| Diastolic blood pressure | −0.28 | 1.57 | 7.78 | 1.96 | 3.21 | 0.002 |
SD=Standard deviation
Discussion
In the present study, the demographic and fertility characteristics such as age and BMI were not significantly different between the two groups (P > 0.05). The age range of women in the experimental group was 19–40 years and it was 19–41 years in the control group indicating the proper distribution of pregnant women in terms of these two variables in two groups. In the study of Hu et al., no significant difference was found between the two groups after analyzing the demographic information.[15] Moreover, in the study of Sorensen et al.,[16] Saftlas et al. ( 2004),[17] and Rudra et al.,[18] there was no significant difference between the mean age and BMI between the two groups.
In the present study, it was found that the mean of fertility variables including gestational age, number of deliveries, and the number of abortions was not significantly different in the two groups (P > 0.05). The mean of these variables was also not significant in the studies conducted by Hu et al.,[15] Sorensen et al.,[16] and Saftlas et al. ( 2004).[17]
The demographic and fertility characteristics were considered in this study, and the above studies are based on the references,[19] related to the development of various hypertension disorders types. Therefore, the significance of these variables is important in the two groups.
There was no significant difference between the two groups in terms of the history of hypertension during the previous pregnancy, obvious diabetes, autoimmune diseases, and chronic hypertension (P > 0.05), which could be effective in comparing the two groups in terms of the approximately similar samples regarding the gestational blood pressure disorders.
In the study of Kasawara et al., no significant difference was indicated in gestational hypertension in both case and control groups,[20] which is consistent with our study.
Based on the findings, it was found that walking for 20–30 min, four times a week, from 14 to 34 weeks of pregnancy can prevent pregnancy transient hypertension and preeclampsia in women at risk of gestational abnormal blood pressures. Since after analyzing the data, it was found that among 36 pregnant women in the experimental group, 2 pregnant women were with transient gestational hypertension and 1 pregnant woman was with preeclampsia; however, in the control group, 9 pregnant women were with transient gestational hypertension and 4 pregnant women were with preeclampsia. These findings showed that the transient and preeclampsia in the test group were significantly lower than the control group (P < 0.05).
Exercise and walking can improve endothelial function of the arteries and help to reduce endothelin-1 levels and vasodilatation-inducing factors by stimulating the release of nitric oxide, which is the most important mechanism of walking to control gestational blood pressure abnormalities.[15]
Hu et al. also stated that the aerobic exercise three times a week from 16 to 31 weeks of the pregnancy for 50 min increases nitric oxide. Considering the similarity of the method of Hu et al. study with the present study, it can be concluded that due to the vascular changes, the frequency of transient gestational hypertension and preeclampsia in the experimental group significantly decreased in both works.[15]
In this regard, the study of Sorensen et al., a case–control study, also indicated that doing any physical activity and exercise during the first 20 weeks of pregnancy can reduce the risk of preeclampsia in pregnancy. In this work, the risk of preeclampsia in pregnant women who had physical activity during the first 20 weeks of pregnancy was 34% lower than the inactive pregnant women.[16] According to the results of this study, it can be concluded that in case of beginning the physical activity in the first half of pregnancy, it is possible to control pregnancy-related hyperbaric abnormalities.
In the present study, comparing the two control and postintervention groups, it was found that the frequency of transient hypertension compared to preeclampsia was higher in the control group. Moreover, it was found that the ratio of reduction of preeclampsia in the experimental group to the control group was one-quarter and this ratio for transient hypertension was less than one-quarter, indicating the effectiveness of walking on the control of transient gestational hypertension. In the study of Magro-Malosso et al., it was showed that doing aerobic exercise 2–7 times/week for 30–60 min from 23 weeks of gestation in uncomplicated pregnant women could reduce the incidence of transient pregnancy hypertension; however, it had no significant effect on preeclampsia prevalence.[21]
In another review of the findings, it was found that the mean changes in systolic and diastolic blood pressures after walking were significantly less in the experimental group compared to the control group before walking (P < 0.05). The study of Mohazab et al. ( 2015) on 14 nonpregnant women with blood pressure disorders within the fertility age range, who performed 4 weeks of handgrip isometric exercise program three times a week, determined significant changes in vascular dilatation and systolic and diastolic blood pressures.[22] The findings of this study regarding the reduction of systolic and diastolic blood pressures are consistent with the present study.
Other findings showed that there were no eclampsia and preeclampsia superimposed on chronic hypertension in the two groups.
In the study of Kasawara et al., aimed to determine the relationship between the controlled physical activity in pregnant women with chronic hypertension or with a history of preeclampsia, no significant difference was found between the two groups in terms of severe maternal morbidity, intensive care unit admission, and neonatal morbidity.[19]
Lack of these two complications in this study and Kasawara study can be associated with the timely and useful care of pregnant women with chronic hypertension and preeclampsia that prevent the preeclampsia superimposed on chronic hypertension as well as eclampsia.
In Iran, according to the Ministry of Health's instruction to report the at-risk pregnant women urgently and to record these cases in the mother and baby system, as well as the formation of a therapeutic team for pregnant women morbidity for these mothers, the women with severe preeclampsia are under special care and counseling with the groups of doctors such as internal medicine and hematology physicians as well as the direct supervision the vice-chancellor for treatment of the university, preventing the deterioration of mother's disease. On the other hand, identifying the at-risk pregnant women in the health centers of Rafsanjan and introducing them to the health deputy and sending their names to the health centers and hospitals can also be effective to early diagnose these pregnant women and begin timely treatment and to form a committee of morbidity as soon as possible.
The study participants were pregnant women susceptible to hypertensive disorders of pregnancy. Therefore, they repeatedly and unpredictably referred to comprehensive health centers for measuring and recording blood pressure. For this reason, measuring and recording the blood pressure of these women were given to the mother-care providers in these centers that were the limit of research.
Conclusion
Considering the fact that gestational hypertension is one of the health problems in Iran and is one of the main causes of morbidity and mortality of pregnant women, it is recommended to perform moderate walking, which is an easy and accessible physical activity and effective to reduce the incidence of these disorders and consequently the complications of pregnancy and delivery in these pregnant women. Lack of the preeclampsia superimposed on chronic hypertension in this study can also indicate a positive effect of walking on chronic hypertension management in the affected mothers. Presenting the finding of this study to deputies of health and treatment in medical sciences universities of the country, in order to inform the gynecologists and midwives about the effect of this type of physical activity on hypertensive disorders during pregnancy, can improve pregnant women's health, especially those who are susceptible to this disorder.
Financial support and sponsorship
This article was financially supported by Isfahan and Rafsanjan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The present research is based on the results of the research project with the code IR. MUI. RES. 396533 approved by Isfahan University of Medical Sciences. For this purpose, we thank all from Rafsanjan Comprehensive Health Centers and the research deputy of Isfahan and Rafsanjan University of Medical Sciences.
References
- 1.Dodange A. Ahvaz, Iran: Jondi Shapoor University of Medical Sciences; 2016. Comparison of Pregnancy Outcomes in Women with Early and Late Hypertensive Disorders of Pregnancy. [Google Scholar]
- 2.Kahnamouei-aghdam F, Amani F, Hamidimoghaddam S. Prevalence of pre-eclampsia and eclampsia risk factors among pregnant women 2013-2011. Int J Adv Med. 2015;2:132–28. [Google Scholar]
- 3.Ramírez-Vélez R, Aguilar AC, Mosquera M, Garcia RG, Reyes LM, López-Jaramillo P, et al. Clinical trial to assess the effect of physical exercise on endothelial function and insulin resistance in pregnant women. Trials. 2009;10:104. doi: 10.1186/1745-6215-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehchenari S, BorujeniJ The effects of active and inactive lifestyles with controlled diet on insulin resistance of AST and ALT serum levels of pregnant women's liver enzymes. Iran J Obstet Gynecol Infertil. 2016;19:9–16. [Google Scholar]
- 5.Irani E. Ardabil, Iran: Ardabil University of Medical Sciences; 2001. The Prevalence of Epidemiologic Factors and Complications of Preeclampsia in Patients Admitted to Alavi Hospital in Ardabil, 2001. [Google Scholar]
- 6.Dildy GA, 3rd, Belfort MA, Smulian JC. Preeclampsia recurrence and prevention. Semin Perinatol. 2007;31:135–41. doi: 10.1053/j.semperi.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Janani F, Changaee F. seasonal variation in the prevalence of the preeclampsia. J family Med prim care. 2017;6:766–9. doi: 10.4103/jfmpc.jfmpc_132_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esmat N, Mahshid NS, Nozar N. Assessing the knowledge, attitude and practice of puerpera admitted to maternity hospitals in Kerman in terms of exercise in pregnancy. Iranian Journal of Nursing. 2010;66:64–72. [Google Scholar]
- 9.Samira R, Elaheh SR. Investigating the awareness and practice of pregnant women toward exercise during pregnancy. Iran J Nurs. 2004;40:6–10. [Google Scholar]
- 10.Soleyman Z, Ashraf Z. Evaluating the affect of simple exercise and the correct condition of daily work on the outcome of pregnancy. Iran J Obstet Gynecol Infertil. 2009;12:51–7. [Google Scholar]
- 11.Zadeh I, Taavoni S, Ahmadi Z, HaqqaniH Comparison of pre-pregnancy sports activities to the first trimester after delivery. Iran J Nurs. 2008;54:135–41. [Google Scholar]
- 12.Masoume SN. Tehran: Golban; 2016. Exercise During Pregnancy and Postpartum; pp. 9–10. [Google Scholar]
- 13.Burgos CS, Kasawara KT, Costa ML, Pinto E Silva JL. PP041. The effect of exercise in pregnant women with chronic hypertension and/or previous preeclampsia on blood pressure and heart rate variability. Pregnancy Hypertens. 2012;2:263–4. doi: 10.1016/j.preghy.2012.04.152. [DOI] [PubMed] [Google Scholar]
- 14.Gavard JA, Artal R. Effect of exercise on pregnancy outcome. Clin Obstet Gynecol. 2008;51:467–80. doi: 10.1097/GRF.0b013e31816feb1d. [DOI] [PubMed] [Google Scholar]
- 15.Hu LH, Xie P, Chen DH. Effect of medical nutrition combined with exercise intervention on the placental ischemic hypoxic injury and serum angiogenesis factors in patients with gestational hypertantion. J Hainan Med Univ. 2017;23:112–5. [Google Scholar]
- 16.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA, et al. Recreational physical activity during pregnancy and risk of preeclampsia. Hypertension. 2003;41:1273–80. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 17.Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. Am J Epidemiol. 2004;160:758–65. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- 18.Rudra CB, Williams MA, Lee IM, Miller RS, Sorensen TK. Perceived exertion during prepregnancy physical activity and preeclampsia risk. Med Sci Sports Exerc. 2005;37:1836–41. doi: 10.1249/01.mss.0000175862.41620.41. [DOI] [PubMed] [Google Scholar]
- 19.Gary Cunningham F, leveno C, Bloom S, Spong C, Dashe J, Hoffman B, et al. 24th ed. Tehran: Golban; 2014. Williams Obstetrics; pp. 728–31. [Google Scholar]
- 20.Kasawara KT, Burgos CS, do Nascimento SL, Ferreira NO, Surita FG, Pinto E Silva JL, et al. Maternal and perinatal outcomes of exercise in pregnant women with chronic hypertension and/or previous preeclampsia: A randomized controlled trial. ISRN Obstet Gynecol. 2013;2013:857047. doi: 10.1155/2013/857047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magro-Malosso ER, Saccone G, Di Tommaso M, Roman A, Berghella V. Exercise during pregnancy and risk of gestational hypertensive disorders: A systematic review and meta-analysis. Acta Obstet Gynecol Scand. 2017;96:921–31. doi: 10.1111/aogs.13151. [DOI] [PubMed] [Google Scholar]
- 22.Mohazab M, Daryanoosh F, Baba Beigi MA, Rasekhi A, Jahromi MK, Tehrani NH. Evaluating the effect of four weeks of hand grip isometric exercise on vascular changes associated with blood flow and blood pressure in women with hypertension. J Shahid Sadoughi Univ Med Sci Yazd. 2015;631:7–639. [Google Scholar]


