Abstract
Purpose
Overall survival (OS) is the definitive and best-established primary efficacy end point to evaluate diffuse large B-cell lymphoma (DLBCL) therapies, but it requires prolonged follow-up. An earlier end point assessed post-treatment would expedite clinical trial conduct and accelerate patient access to effective new therapies. Our objective was to formally evaluate progression-free survival (PFS) and PFS at 24 months (PFS24) as surrogate end points for OS in first-line DLBCL.
Patients and Methods
Individual patient data were analyzed from 7,507 patients from 13 multicenter randomized controlled trials of active treatment in previously untreated DLBCL, published after 2002, with sufficient PFS data to predict treatment effects on OS. Trial-level surrogacy examining the correlation of treatment effect estimates of PFS/PFS24 and OS was evaluated using both linear regression (R2WLS) and Copula bivariable (R2Copula) models. Prespecified criteria for surrogacy required either R2WLS or R2Copula ≥ 0.80 and neither < 0.7, with lower-bound 95% CI > 0.60.
Results
Trial-level surrogacy for PFS was strong (R2WLS = 0.83; R2Copula = 0.85) and met the predefined criteria for surrogacy. At the patient level, PFS strongly correlated with OS. The surrogate threshold effect had a hazard ratio of 0.89. Surrogacy was consistent across comparisons with or without rituximab and with rituximab maintenance trials. Trial-level surrogacy for PFS24 was relatively strong (R2WLS = 0.77; R2Copula = 0.78) but did not meet prespecified criteria. At the patient level, PFS24 significantly correlated with OS. The surrogate threshold effect had an odds ratio of 1.51.
Conclusion
This large pooled analysis of individual patient data supports PFS as a surrogate end point for OS in future randomized controlled trials evaluating chemoimmunotherapy in DLBCL. Use of this end point may expedite therapeutic development with the intent of bringing novel therapies to this patient population years before OS results are mature.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) accounts for approximately 32% of non-Hodgkin lymphoma (NHL) diagnoses.1 The addition of rituximab to cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as initial therapy for DLBCL was one of the key contributors to improved 5-year standardized cancer-specific survival from 52% in 1999 to 66% in 20052 and led to R-CHOP becoming the most common first-line treatment. Relative 10-year survival for patients with DLBCL is approximately 50%,1 illustrating the persisting need for improved therapies. However, no regimen has demonstrated a survival benefit compared with R-CHOP in a randomized trial.3-5 Approximately 10% to 15% of patients with DLBCL have disease that is refractory to initial therapy, and another 20% to 25% who experience relapse after an initial response to treatment will experience worse outcomes.6,7 Ten-year DLBCL-specific survival is considerably dependent on the extent of disease and prognostic score and has been shown to be 80% for patients with low-risk, 60% for those with intermediate-risk, and 36% for those with high-risk disease.8 Genomic tools for risk stratification and molecular surveillance of DLBCL have been presented,9,10 but neither these tools nor novel agents have improved patient outcomes.
Overall survival (OS) is the gold-standard primary efficacy end point for clinical trials of antilymphoma treatment. On the basis of studies evaluated here, it may take > 8 years to achieve median OS for first-line DLBCL and, depending on accrual time, approximately 10 years to complete phase III trials using OS as an end point. For example, a randomized controlled trial (RCT) comparing R-CHOP with an alternative dose-intensive regimen (CALGB [Cancer and Leukemia Group B]/Alliance 50303) opened in 2005 and reported initial findings in December 2016.5 Results from previous studies in DLBCL and other aggressive types of NHL suggest that progression-free survival (PFS) and short-term outcomes, including event-free survival and PFS at 0.5, 2, or 3 years, should be explored as candidates for an earlier surrogate end point for OS.11-14 However, these studies were based on suboptimal data and/or validation methods.
We established the Surrogate Endpoints for Aggressive Lymphoma (SEAL) international collaboration to construct a large database integrating individual patient data (IPD) from completed, published, multicenter RCTs in DLBCL to evaluate potential surrogate end points for OS in DLBCL trials and support continuous translational research (eg, prognostic analyses, risk classifications, subgroup analyses).15 Several studies have been successful in using metadatabases to provide a strong foundation and methodology for establishing statistical criteria that assesses clinically meaningful relationships between end points and surrogates. These studies combined IPD from multiple studies to identify earlier surrogate end points for adjuvant treatment in colorectal cancer (ACCENT [Adjuvant Colon Cancer End Points]),16 metastatic colorectal cancer (ARCAD [Analysis and Research in Cancers of the Digestive System]),17 and follicular lymphoma (FLASH [Follicular Lymphoma Analysis of Surrogate Hypothesis]).18 The specific objectives of the SEAL study are to determine trial-level (primary) and patient-level (secondary) correlations between two surrogate end point candidates, PFS and PFS at 24 months (PFS24), with OS after first-line treatment of DLBCL using well-established statistical methods on IPD. Here we report results of an analysis of data from 13 studies in a total of 7,507 patients.
PATIENTS AND METHODS
Trial Selection and Comparison Definition
On June 30, 2015, we used the search terms DLBCL, NHL, and aggressive or advanced or diffuse histiocytic in the title and keywords up-front or first-line or untreated or newly diagnosed or initial/primary/treatment/therapy or no prior therapy or naïve to conduct a comprehensive search of published DLBCL clinical studies in the Medline database maintained by the US National Library of Medicine. To be included, studies were required to be multicenter RCTs published in English from 2002 to October 13, 2015, and designed to evaluate active treatment in ≥ 100 adult (no pediatric) patients with previously untreated DLBCL or aggressive NHL defined by WHO/Revised European American Lymphoma classification. Studies were excluded if they were reviews; if they enrolled patients with early-stage (I or II) disease only, low-grade or indolent or HIV-related lymphoma, or relapsed or refractory disease; or if the intervention focused on salvage treatment or supportive care, such as growth factors, palliative care, quality of life, or health economics.
The sponsors of all identified studies meeting the prespecified inclusion criteria were contacted to determine their interest in confidentially sharing IPD. Studies unable to transfer IPD before December 2016 were excluded for this analysis. All exclusions were based only on data quality and availability and were determined before any statistical analysis of the end points.
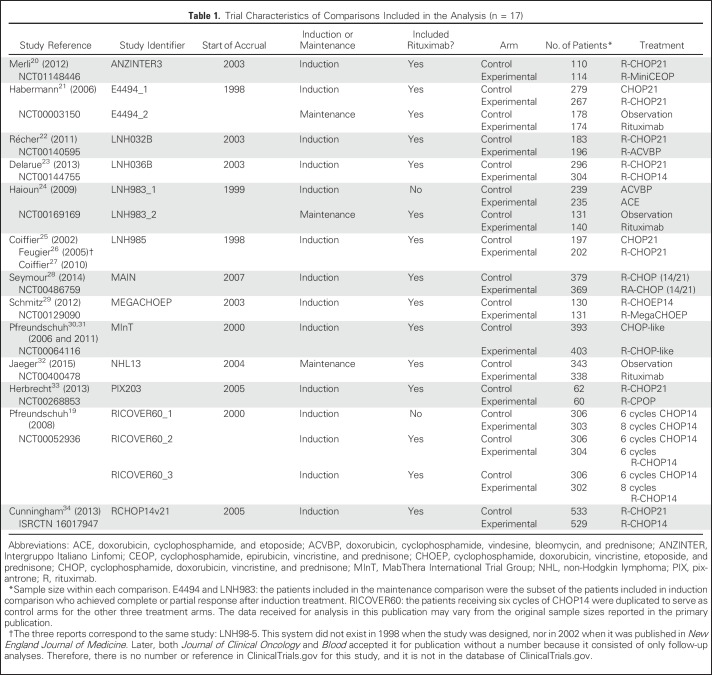
As of August 2016, 13 of 14 studies collected in the SEAL database met all of the inclusion criteria. Among those studies, there were 10 studies comparing induction treatments only, two studies comparing both induction and maintenance treatments, and one study comparing maintenance treatments only. One study19 (RICOVER60 [Rituximab With CHOP Over Age 60 Years]) included three experimental arms compared with one control arm. The meta-analytic unit for surrogacy estimation was predefined as the comparison between two arms (experimental v control) nested within trials. The induction and maintenance comparisons within the same study were treated as two comparison units. As such, a total of 17 comparison units were predefined (Table 1).
Table 1.
Trial Characteristics of Comparisons Included in the Analysis (n = 17)
Surrogate End Point Candidates
Potential surrogate candidates for OS included PFS and PFS24. PFS was a time-to-event end point defined as the time from initiation of induction treatment to the earliest occurrence of progressive disease, relapse, or death resulting from any cause. Living patients without documented disease progression were censored on the date of their last disease evaluation. PFS24 was a binary end point where patients who were alive and in disease-free status up to 24 months after initiation of induction treatment were considered a success. The primary end point and surrogate candidates were derived according to consistent calculation rules across studies.
Statistical Methods
True end point.
The primary clinical end point was OS, defined as time from initiation of induction treatment to death resulting from any cause. Living patients were censored on the date when they were last documented as alive.
General statistical methods.
Within-trial treatment effects for time-to-event end points OS and PFS were quantified using hazard ratios (HRs). Within-trial treatment effects for the binary end point PFS24 were quantified using odds ratios (ORs), and 95% CIs were calculated for each. Analyses were performed by using SAS software (version 9.4; SAS Institute, Cary, NC) and R software (version 2.14; R Foundation for Statistical Computing, Vienna, Austria).
Surrogacy evaluation.
The primary surrogacy evaluation method was trial-level surrogacy, which measured how precisely treatment effect on the true end point may be predicted based on observed treatment effects on the surrogate end point. At the trial level, two commonly used trial-level surrogacy measures were considered: Copula bivariable (R2Copula)35,36 and linear regression based on weighted least squares regression method (R2WLS),16,37 where R2Copula takes into account patient-level correlation between the two end points and R2WLS does not. After publication of surrogacy qualification criteria established in the follicular lymphoma setting,18 the predefined rule for declaring trial-level surrogacy required either R2WLS or R2Copula ≥ 0.80, with the lower boundary of a 95% CI of > 0.6, and neither estimate < 0.7.
Supplemental trial-level surrogacy measures included the surrogate threshold effect,38 the minimum treatment effect on the surrogate required to confidently predict a significant treatment effect on OS in a future trial. For patient-level surrogacy, the correlations between the candidate surrogate end point and OS were quantified through rank correlation coefficient (ρ) for PFS via the bivariable Clayton Copula model36; for PFS24, the global OR for comparing the odds of remaining alive beyond a particular time point between patients with different PFS24 status was estimated through the bivariable Plackett Copula model35 over the entire duration of OS. Patient-level correlation was considered a supportive but not sufficient condition for surrogacy validation. ρ closer to 1.0 indicated a stronger correlation. For the global OR, a 95% CI excluding 1 indicated significant individual-level correlation.
Sensitivity and Subpopulation Analysis
Leave-one-out cross-validation, which compares the predicted log (HR) values with the observed values on OS based on the estimated trial-level model, leaving one trial out at a time, assessed the predictive performance of the regression model. Leave-one-out estimation, which re-estimated R2 when one trial was excluded at a time, was performed to identify potentially influential trials. Surrogacy was further examined within subpopulations defined by treatment type (ie, comparisons with rituximab, induction comparisons only).
RESULTS
Patient Characteristics
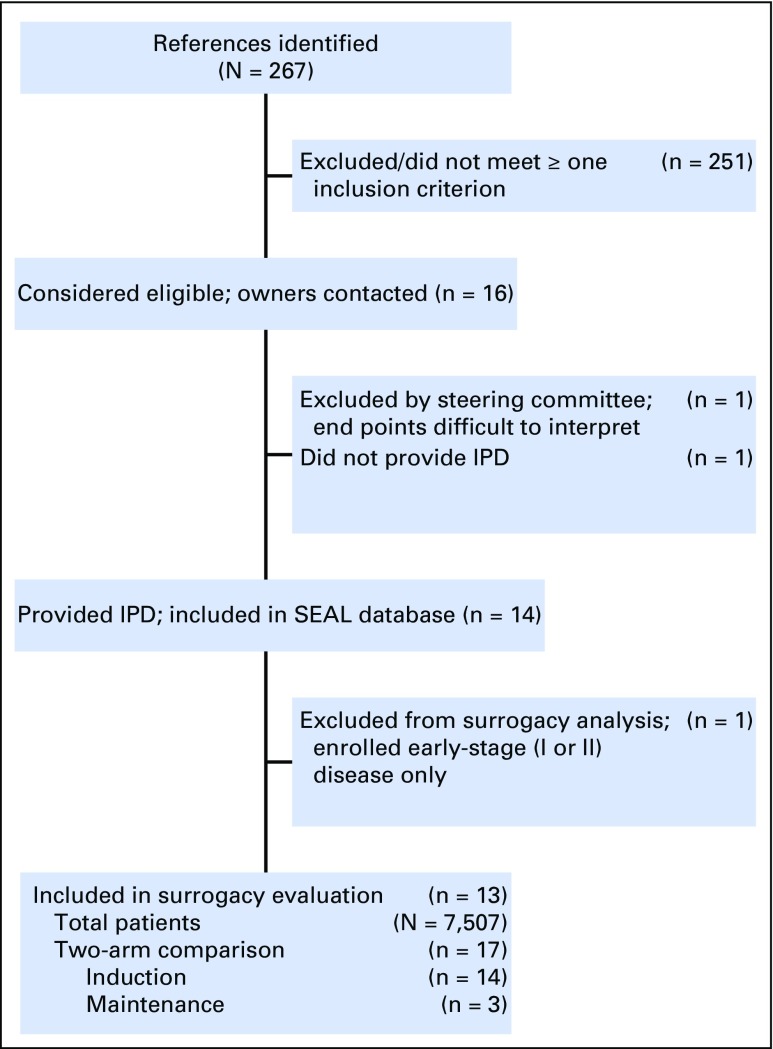
Of a total of 267 references individually examined, 14 studies were included in the SEAL database and 13 studies met all inclusion criteria (Fig 1). Table 1 summarizes trial-level characteristics of the 13 studies included,19,20-34 involving IPD from 7,507 patients and 17 two-arm comparisons, of which 14 compared induction regimens and three compared maintenance regimens. Fifteen comparisons involved rituximab.
Fig 1.
Flowchart of study selection. Abbreviation: IPD, individual patient data.
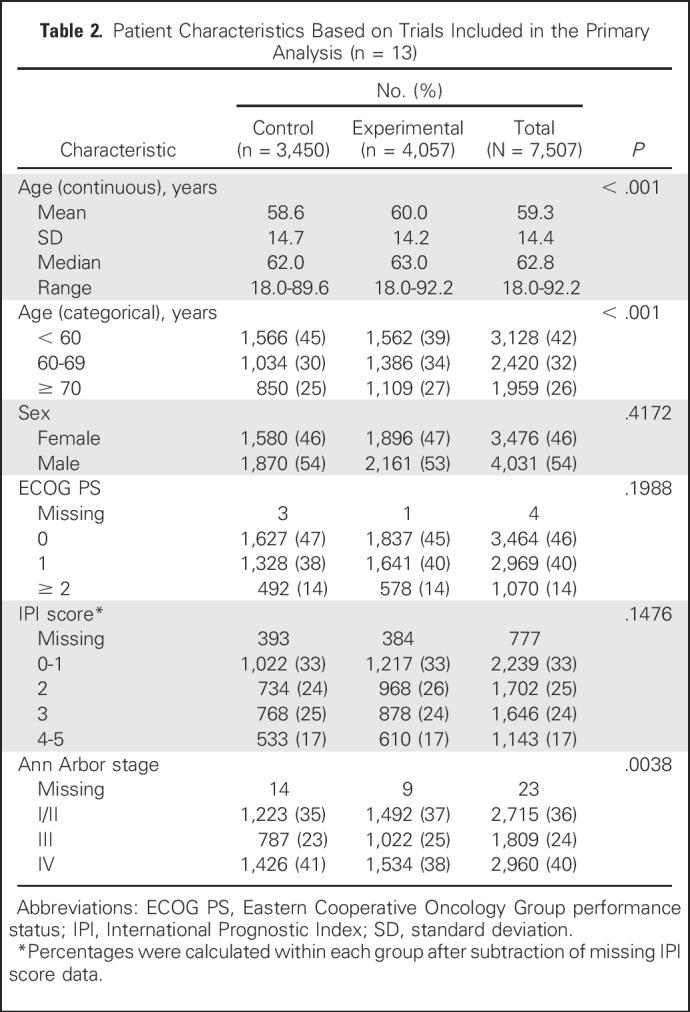
Baseline characteristics were well balanced between arms (Table 2), with a median age of 62.8 years; 42% of patients were age < 60 years, 32% were age 60 to 69 years, and 26% were ≥ 70 years; 54% were men, and 14% had Eastern Cooperative Oncology Group performance status ≥ 2. One third of patients (33%) had International Prognostic Index (IPI) scores of 0 to 1; 25%, IPI 2; 24%, IPI 3; and 17%, IPI 4 to 5. Ann Arbor stage was I/II in 36% of patients, III in 24%, and IV in 40%. Overall, the median follow-up time for OS was 52 months. The distributions of PFS and OS in overall population pooling all patients across studies are shown in Appendix Figures A1A and A1B (online only).
Table 2.
Patient Characteristics Based on Trials Included in the Primary Analysis (n = 13)
PFS: Trial- and Patient-Level Surrogacy Measures
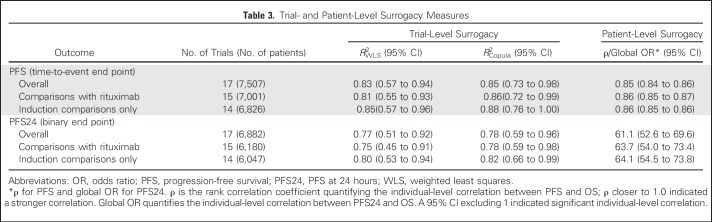
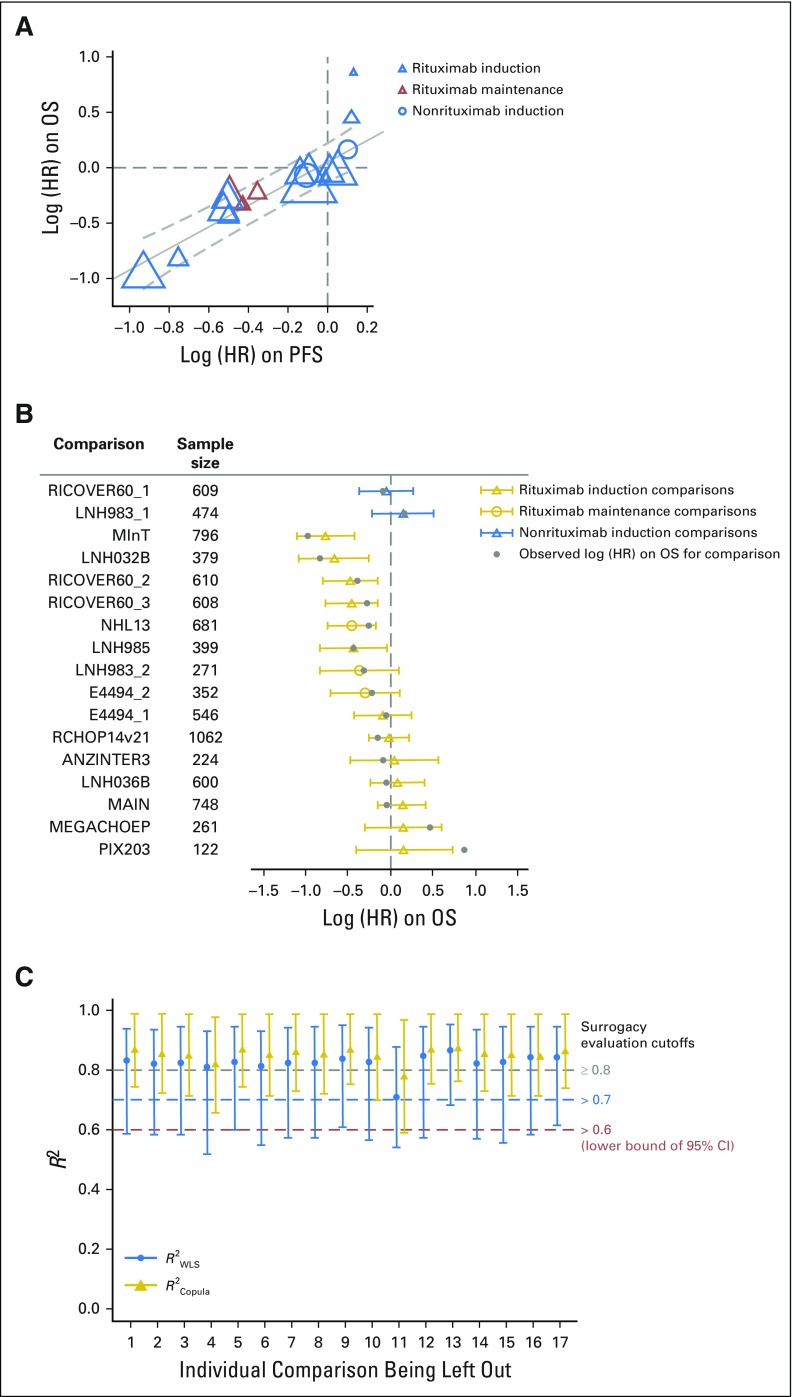
As summarized in Table 3 and Figure 2A, trial-level surrogacy for PFS was strong (R2WLS = 0.83; 95% CI, 0.57 to 0.94; R2Copula = 0.85; 95% CI, 0.73 to 0.98) and met the predefined criteria for surrogacy. This implies a strong prediction of treatment effect on OS based on observed treatment effect on PFS. The patient-level correlation between PFS and OS considering the entire duration of follow-up was strong, with a rank correlation coefficient ρ of 0.85 (95% CI, 0.84 to 0.86; Table 3). The surrogate threshold effect had an HR of 0.89, which indicated that an observed HR ≤ 0.89 for PFS would predict a significant treatment effect on OS in a future trial. Surrogacy was consistent across comparisons with and without rituximab induction and with rituximab maintenance.
Table 3.
Trial- and Patient-Level Surrogacy Measures
Fig 2.
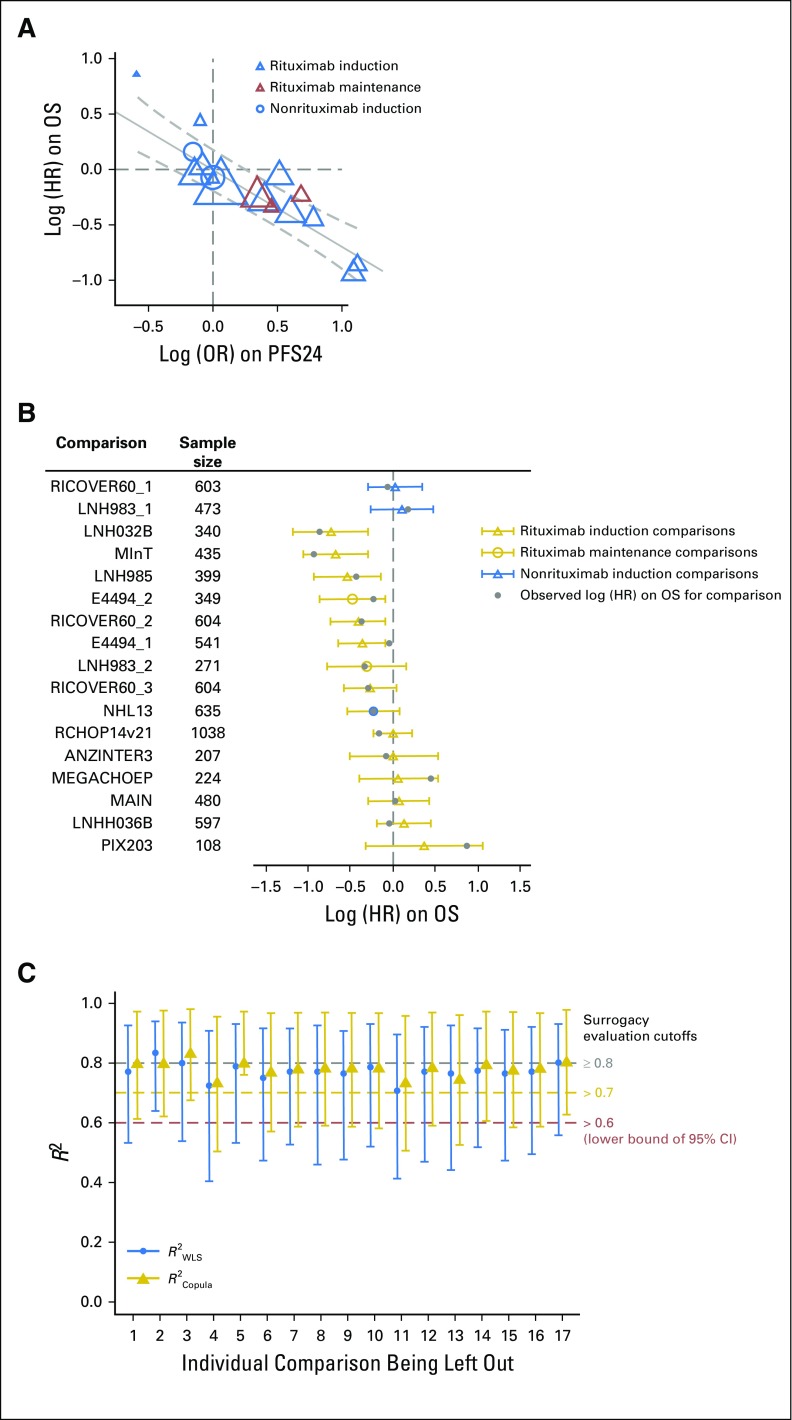
(A) Trial-level treatment effect correlation between progression-free survival (PFS) and overall survival (OS). Circle/triangle size is proportional to sample size. Solid line indicates fitted weighted least squares (WLS) regression line; gray dashed lines indicate 95% prediction limits. (B) PFS surrogacy sensitivity analysis: leave-one-out cross-validation. For each comparison, the open circle/triangle is the predicted log (hazard ratio [HR]) on OS based on the estimated WLS regression line at trial level, removing the comparison listed; the horizontal bars indicate 95% prediction interval. (C) PFS surrogacy sensitivity analysis: leave-one-out re-estimation. For each labeled comparison, R2WLS and R2Copula were estimated by excluding the labeled comparison; vertical bars indicate 95% CI. ANZINTER, Intergruppo Italiano Linfomi; MegaCHOEP, cyclophosphamide, doxorubicin, vincristine, and prednisone plus etoposide; MInT, MabThera International Trial Group; NHL, non-Hodgkin lymphoma; PIX, pixantrone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
Leave-one-out cross-validation demonstrated consistency between observed and predicted OS treatment effects for each comparison unit on the basis of PFS (Fig 2B), except when leaving one comparison (PIX203) out. This comparison was also shown to be a high influence outlier when evaluating the re-estimated R2 when one comparison at a time was excluded (Fig 2C). Exclusion of this comparison yielded an R2WLS of 0.87 (95% CI, 0.70 to 0.95) and R2Copula of 0.88 (95% CI, 0.76 to 0.99).
PFS24: Trial- and Patient-Level Surrogacy Measures
As summarized in Table 3 and Figure 3A, trial-level surrogacy for PFS24 (R2WLS = 0.77; 95% CI, 0.51 to 0.92; R2Copula = 0.78; 95% CI, 0.59 to 0.96) was slightly less robust than PFS and did not meet the prespecified surrogacy qualification criteria. At the patient level, the global OR was 61.1 (95% CI, 52.6 to 69.6), which indicates substantially higher odds of remaining alive beyond a particular time point for patients who were alive and disease free at 24 months after initiation of induction treatment (Table 3). The surrogate threshold effect had an OR of 1.51, which indicated that an observed OR ≥ 1.51 for PFS24 would predict a significant treatment effect on OS in a future trial. Surrogacy performance improved when restricted to induction comparisons. Sensitivity analyses showed similar results (Figs 3B and 3C).
Fig 3.
(A) Trial-level treatment effect correlation between progression-free survival at 24 months (PFS24) and overall survival (OS). Circle/triangle size is proportional to sample size. Solid line indicates fitted weighted least squares (WLS) regression line; gray dashed lines indicate 95% prediction limits. (B) PFS24 surrogacy sensitivity analysis: leave-one-out cross-validation. For each comparison, the open circle/triangle is the predicted log (hazard ratio [HR]) on OS based on the estimated WLS regression line at trial level, removing the comparison listed; horizontal bars indicate 95% prediction interval. (C) PFS24 surrogacy sensitivity analysis: leave-one-out re-estimation. For each labeled comparison, R2WLS and R2Copula were estimated by excluding the labeled comparison; vertical bars indicate 95% CI. ANZINTER, Intergruppo Italiano Linfomi; MegaCHOEP, cyclophosphamide, doxorubicin, vincristine, and prednisone plus etoposide; MInT, MabThera International Trial Group; NHL, non-Hodgkin lymphoma; PIX, pixantrone; R-CHOP, rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone.
DISCUSSION
This pooled analysis compiled IPD from 13 DLBCL RCTs published from 2002 to 2015. The acquisition and integration of IPD from these trials were conducted by an independent data center at Mayo Clinic according to predefined end point derivations and statistical plans. The SEAL group, which conducted these analyses, included involvement from international lymphoma experts and biostatisticians who had previously developed these statistical approaches in consultation with regulatory agencies from the United States and Europe. To our knowledge, this is the first analysis based on integrated IPD from RCTs involving patients with DLBCL. Unlike literature-based meta-analyses, use of IPD ensured the consistent determination of end points and the consistent interpretation of within-trial treatment effects across all studies. Our analysis demonstrates that treatment effect on PFS is a strong predictor of treatment effect on OS. These results were consistent across different surrogacy estimation methods and sensitivity analyses. The strong association between PFS and OS was maintained irrespective of the inclusion of rituximab in the induction and/or maintenance regimen. Together, these results indicate that PFS serves as a strong surrogate for OS in trials evaluating first-line therapy for DLBCL.
DLBCL is the most prevalent aggressive form of NHL and a heterogeneous disease with subtypes distinguishable by molecular, immunophenotypic, morphologic, and clinical characteristics.39 In the prerituximab era, the chemotherapy combination CHOP was established as standard first-line treatment of DLBCL based on results from a randomized study comparing CHOP with three other more aggressive combination chemotherapy regimens.40 Integration of the monoclonal anti-CD20 antibody rituximab into chemotherapy regimens was one of the most important advances in the treatment of DLBCL based on data first published in 200220 and confirmed in subsequent trials.23,24 Despite promising data from early-phase clinical trials, since 2002, no regimen has demonstrated significant clinical benefit over R-CHOP in a randomized controlled clinical trial. Surrogate end points such as PFS are needed to reduce the evaluation time for new agents and regimens so that effective first-line regimens and strategies can be delivered in clinical practice sooner, and ineffective regimens can be abandoned without prolonged evaluation.
Current approaches for improving outcomes in DLBCL focus on addressing therapeutic targets present in nearly all patients with DLBCL, such as CD19 and CD79b, and characterizing the genetic mutations and pathways involved in DLBCL along with their functional roles to define risk stratification models and identify new therapeutic targets. Clinically, patients with DLBCL who achieve event-free survival at 24 months after initiation of therapy experience survival similar to that of matched controls, whereas those who do not have poor expected survival.12 Targeting poor-risk patients identified by clinical or genetic factors has become a priority for defining patient groups, where improving upon outcomes with R-CHOP is needed and feasible in a suitable timeframe for drug development. Recognition and continued investigation into the complexity and heterogeneity of DLBCL led to classifications based on immunohistochemistry and gene expression profiling that distinguish two major subtypes of DLBCL: germinal center B cell–like DLBCL and activated B cell–like DLBCL, with the latter type being associated with worse prognosis in some41-45 but not all studies.46 Fluorescence in situ hybridization and immunohistochemistry also differentiate patients with DLBCL who are positive for MYC and BCL2 and/or BCL6; these patients experience worse outcomes when treated with R-CHOP.47,48 Precision medicine approaches that perform DLBCL subtyping in all patients and then add a novel agent to R-CHOP therapy for a poor-risk subgroup have been projected to provide benefits that outweigh their costs,49 but at present, such strategies have failed to demonstrate clinical benefit. Three RCTs demonstrated that substituting bortezomib for vincristine or adding bortezomib to R-CHOP did not significantly improve outcomes.3,50,51 A randomized trial substituting obinutuzumab for rituximab demonstrated that this regimen did not significantly improve investigator-assessed PFS compared with R-CHOP.4 Likewise, altering the administration of R-CHOP to every 14 days instead of every 21 days increased toxicity without providing benefits.30,31 Failure of these trials has been attributed to selection bias, leading to prospectively enrolled patients with DLBCL who had more favorable survival than was expected with R-CHOP based on historical controls, and limited additive benefits of these approaches. These trials also reinforce the use of PFS in the clinical research community as an acceptable surrogate for OS. Novel agents and approaches are emerging from early phase I/II clinical trials that may improve survival for all patients with DLBCL, with particular attention to poor-risk groups that may benefit more from a selected therapy.52-54 Randomized studies are ongoing to demonstrate the potential benefit of these approaches over R-CHOP.55,56 These trials and future studies should examine design strategies to reduce selection bias and include surrogate end points (such as PFS) to improve the likelihood of success, along with reducing the time for evaluating the clinical benefit of interventions.
Future research examining PFS24 and new candidates for surrogacy should be carried out as the SEAL collaboration continues to actively pursue additional trials and data. For emerging novel agents substituted in or combined with R-CHOP, PFS can be an appropriate surrogate end point when the expected mechanism for improving OS is long-term disease control. For agents expected to improve OS in settings where DLBCL progression is expected to occur on treatment surrogacy, re-evaluation may be required. A majority of clinical trials included in our analysis focused on the induction setting. As a result, a subgroup analysis of PFS surrogacy in the maintenance setting was not possible. As more maintenance studies (eg, REMARC [Study of Lenalidomide Maintenance Versus Placebo in Responding Elderly Patients With DLBCL and Treated With R-CHOP] NCT01122472) become available to SEAL, the surrogacy of PFS and PFS24 could be specifically tested in the maintenance setting. Additional surrogate end point candidates in DLBCL, such as response-based end points and event-free survival, should be evaluated as additional data from current SEAL studies and new studies are added.
In the first-line setting, DLBCL treatment is administered with the intent to achieve long-term disease control and ultimately a cure. Although R-CHOP provides significant clinical benefits to patients, these advantages challenge the conduct of future trials of newer therapeutic options. More efficient approaches are needed to promote the development of novel therapies in a timely manner, provide patients earlier access to more effective therapeutic options, and more rapidly evaluate and discard approaches that do not have a clinically significant impact. Establishing surrogate RCT end points that are measured earlier than current approaches can facilitate future drug development. The results of the SEAL analyses have demonstrated that treatment effects on PFS strongly predicts for treatment effects on OS. Although PFS24 was significantly correlated with longer OS at the patient-level analysis, it did not meet the trial-level prespecified surrogacy qualification criteria. Future analyses with additional trials should re-evaluate the role of PFS24 as a surrogate end point, focusing on the trial-level correlation with OS. In conclusion, this pooled analysis of IPD from randomized clinical trials of first-line treatments for patients with DLBCL demonstrates that the primary surrogate candidate, PFS, met the qualification criteria to be a robust surrogate end point for OS. These results support the use of PFS as an appropriate primary end point in future studies evaluating chemoimmunotherapy in patients with DLBCL.
ACKNOWLEDGMENT
We thank and dedicate this manuscript to Dr Daniel Sargent, who was a principal leader in integrating his expertise in biostatistics and oncology with vision and foresight for establishing the importance of identifying early surrogate markers of prolonged survival outcomes. Dan was vital in bringing global investigators together to build data-sharing programs with large metadatabases across academic and industry teams for purposes of answering advanced research questions across multiple diverse studies, and with the continuation of these studies, his legacy continues to live on. We thank all the patients, families, and caregivers who participated in each of these studies, as well as all of the investigators, contributors, and study groups included in the analysis and for providing study data. We also thank all the SEAL group coinvestigators and study groups who contributed data for this analysis and the Mayo Clinic. Editorial support was provided by Julie Kern and Monica Nicosia with Bio Connections and funded by Celgene.
Appendix
The following investigators, contributors, and study groups were included in the analysis: Francesco Merli and the Intergruppo Italiano Linfomi (ANZINTER), Thomas M. Habermann and the US Intergroup (ECOG 4494), Groupe d’Etude des Lymphomes de l’Adulte (GELA; now LYSARC), Amgen, and Roche, along with Christian Récher (LNH03-2B), Richard Delarue (LNH03-6B), Corrinne Haioun (LNH 98-3), Bertrand Coiffier and Pierre Feugier (LNH98-5), John F. Seymour and F. Hoffman-La Roche (MAIN), Norbert Schmitz and the German High-Grade Lymphoma Study Group (DSHNHL 2002-1), Michael Pfreundschuh and the MabThera International Trial Group (MInT) and Roche, Ulrich Jaeger and the Arbeitsgemeinschaft Medikamentöse Tumortherapie (AGMT; NHL13), Raoul Herbrecht and Cell Therapeutics (PIX203), Michael Pfreundschuh and Deutsche Krebshilfe (RICOVER-60), and David Cunningham and Cancer Research UK (UCL).
Surrogate Endpoints for Aggressive Lymphoma (SEAL) group membership: The SEAL group (in alphabetic order) consists of: Bertrand Coiffier, David Cunningham, Jocelyne Flament, Christopher R. Flowers, Tommy Fu, Hervé Ghesquieres, Thomas M. Habermann, Corinne Haioun, Raoul Herbrecht, Ulrich Jaeger, Matthew J. Maurer, Francesco Merli, Tina Nielsen, Fang-Shu Ou, Michael Pfreundschuh, Daniel J. Sargent, Norbert Schmitz, John F. Seymour, Qian Shi, Hervé Tilly, Lixia Wang, and Marita Ziepert.
Fig A1.
Distribution of (A) progression-free survival in overall population pooling of all patients across studies and (B) overall survival in overall population pooling of all patients across studies at a median follow-up time of 52 months.
Footnotes
Supported by grants from Celgene.
Presented in part at the 58th Annual Meeting of the American Society of Hematology, San Diego, CA, December 3-6, 2016.
Written on behalf of the Surrogate Endpoints for Aggressive Lymphoma group.
Clinical trial information: NCT01148446.
Listen to the podcast by Dr Abramson at ascopubs.org/jco/podcasts
AUTHOR CONTRIBUTIONS
Conception and design: Qian Shi, Norbert Schmitz, Michael Pfreundschuh, Ulrich Jaeger, Thomas M. Habermann, Corinne Haioun, Raoul Herbrecht, Jocelyne Flament, Tommy Fu, Bertrand Coiffier, Christopher R. Flowers
Provision of study materials or patients: Norbert Schmitz, John F. Seymour, Francesco Merli, Raoul Herbrecht, Bertrand Coiffier
Collection and assembly of data: Qian Shi, Fang-Shu Ou, Jesse G. Dixon, John F. Seymour, Ulrich Jaeger, Hervé Tilly, Hervé Ghesquieres, Francesco Merli, Marita Ziepert, Raoul Herbrecht, Jocelyne Flament, Bertrand Coiffier
Data analysis and interpretation: Qian Shi, Jesse G. Dixon, David Cunningham, John F. Seymour, Ulrich Jaeger, Thomas M. Habermann, Corinne Haioun, Christopher R. Flowers
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Progression-Free Survival as a Surrogate End Point for Overall Survival in First-Line Diffuse Large B-Cell Lymphoma: An Individual Patient–Level Analysis of Multiple Randomized Trials (SEAL)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Qian Shi
No relationship to disclose
Norbert Schmitz
Stock or Other Ownership: Celgene
Honoraria: Riemser, Takeda Pharmaceuticals, Novartis, Kite Pharma, Janssen Oncology, Hexal
Consulting or Advisory Role: Hexal, Kite Pharma, Riemser
Research Funding: Janssen Oncology
Travel, Accommodations, Expenses: Roche, Riemser, Novartis, Kite Pharma, Janssen Oncology
Fang-Shu Ou
No relationship to disclose
Jesse G. Dixon
No relationship to disclose
David Cunningham
Research Funding: Amgen (Inst), AstraZeneca (Inst), Bayer HealthCare Pharmaceuticals (Inst), Celgene (Inst), Merrimack (Inst), MedImmune (Inst), Merck Serono (Inst), Sanofi (Inst)
Michael Pfreundschuh
Honoraria: Roche, Takeda Pharmaceuticals
Consulting or Advisory Role: Amgen, Boehringer Ingelheim, Celgene, Roche, Spectrum Pharmaceuticals, Novartis, Bayer HealthCare Pharmaceuticals, Bristol-Myers Squibb, Janssen, Sandoz, Mundipharma, MorphoSys, Seattle Genetics, Merck Sharp & Dohme, Kite Pharma
Research Funding: Amgen, Roche, Spectrum Pharmaceuticals
Travel, Accommodations, Expenses: Amgen, Roche, Janssen
John F. Seymour
Honoraria: AbbVie, Celgene, Janssen, Roche
Consulting or Advisory Role: AbbVie, Celgene, Janssen, Roche
Speakers’ Bureau: AbbVie, Roche
Research Funding: AbbVie, Janssen, Roche
Travel, Accommodations, Expenses: Roche, AbbVie, Celgene
Ulrich Jaeger
Honoraria: Amgen, Celgene, AbbVie, Roche, Novartis, Gilead Sciences
Consulting or Advisory Role: Amgen, Celgene, AbbVie, Roche, Novartis, Gilead Sciences
Research Funding: Novartis (Inst), Gilead Sciences (Inst), Roche (Inst),
Thomas M. Habermann
No relationship to disclose
Corinne Haioun
Consulting or Advisory Role: Roche, Celgene, Janssen-Cilag, Gilead Sciences, Amgen, Novartis, Takeda Pharmaceuticals
Travel, Accommodations, Expenses: Roche, Amgen,
Hervé Tilly
Honoraria: Celgene, Roche/Genentech, Servier
Consulting or Advisory Role: Roche, AstraZeneca, Karyopharm Therapeutics, Bristol-Myers Squibb
Research Funding: Celgene (Inst)
Travel, Accommodations, Expenses: Roche
Hervé Ghesquieres
Honoraria: Gilead Sciences, Sanofi
Consulting or Advisory Role: Celgene
Francesco Merli
Honoraria: Roche
Consulting or Advisory Role: Roche, Celgene
Research Funding: Roche
Travel, Accommodations, Expenses: Roche
Marita Ziepert
Research Funding: Asklepios Kliniken Hamburg (Inst)
Raoul Herbrecht
Consulting or Advisory Role: Astellas Pharma, Basilea, Gilead Sciences, Merck Sharp & Dohme, Novartis, Pfizer
Speakers’ Bureau: Pfizer, Gilead Sciences, Basilea
Research Funding: Cell Therapeutics, Pfizer
Travel, Accommodations, Expenses: Basilea, Celgene, Gilead Sciences, Merck Sharp & Dohme, Pfizer
Jocelyne Flament
Employment: Celgene
Stock or Other Ownership: Celgene
Tommy Fu
Employment: Celgene
Stock or Other Ownership: Celgene
Bertrand Coiffier
Consulting or Advisory Role: Celgene, Celltrion, Erytech Pharma, MorphoSys, Mundipharma, Novartis, Pfizer
Research Funding: Celgene (Inst)
Expert Testimony: Gilead Sciences
Other Relationship: Celltrion, Celgene
Christopher R. Flowers
Consulting or Advisory Role: OptumRx, Seattle Genetics, Bayer HealthCare Pharmaceuticals, Gilead Sciences, Spectrum Pharmaceuticals, AbbVie, AstraZeneca, BeiGene, Denovo Biopharma, Karyopharm Therapeutics, Pharmacyclics/Janssen
Research Funding: Acerta Pharma (Inst), Infinity Pharmaceuticals (Inst), Onyx Pharmaceuticals (Inst), Janssen Oncology (Inst), Gilead Sciences (Inst), Celgene (Inst), TG Therapeutics (Inst), Genentech/Roche (Inst), Pharmacyclics (Inst), AbbVie (Inst), Immune Design (Inst), BeiGene (Inst)
Travel, Accommodations, Expenses: Celgene, Genentech/Roche
REFERENCES
- 1.Teras LR, DeSantis CE, Cerhan JR, et al. : 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 66:443-459, 2016 [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Noone A, Krapcho M, et al: SEER cancer statistics review, 1975-2012. Bethesda, MD, National Cancer Institute, 2015.
- 3.Leonard JP, Kolibaba KS, Reeves JA, et al. : Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell–like diffuse large B-cell lymphoma. J Clin Oncol 35:3538-3546, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Vitolo U, Trněný M, Belada D, et al. : Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vncristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 35:3529-3537, 2017 [DOI] [PubMed] [Google Scholar]
- 5. Wilson WH, Sin-Ho J, Pitcher BN, et al: Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood 128, 2016 (abstr 469) [Google Scholar]
- 6.Sehn LH, Gascoyne RD: Diffuse large B-cell lymphoma: Optimizing outcome in the context of clinical and biologic heterogeneity. Blood 125:22-32, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Crump M, Neelapu SS, Farooq U, et al. : Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 130:1800-1808, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howlader N, Mariotto AB, Besson C, et al. : Cancer-specific mortality, cure fraction, and noncancer causes of death among diffuse large B-cell lymphoma patients in the immunochemotherapy era. Cancer 123:3326-3334, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Kurtz DM, Green MR, Bratman SV, et al. : Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 125:3679-3687, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddy A, Zhang J, Davis NS, et al: Genetic and functional drivers of diffuse large B cell lymphoma. Cell 171:481-494.e15, 2017. [DOI] [PMC free article] [PubMed]
- 11.Lee L, Wang L, Crump M: Identification of potential surrogate end points in randomized clinical trials of aggressive and indolent non-Hodgkin’s lymphoma: Correlation of complete response, time-to-event and overall survival end points. Ann Oncol 22:1392-1403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maurer MJ, Ghesquières H, Jais JP, et al. : Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol 32:1066-1073, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maurer MJ, Habermann TM, Shi Q, et al: Utility of progression-free survival at 24 months (PFS24) to predict subsequent outcome for patients with diffuse large B-cell lymphoma (DLBCL) enrolled on randomized clinical trials: Findings from a Surrogate Endpoint in Aggressive Lymphoma (SEAL) analysis of individual patient data from 5853 patients. Blood 128, 2016 (abstr 3027) [Google Scholar]
- 14.Zhu R, Lu D, Chu YW, et al. : Assessment of correlation between early and late efficacy endpoints to identify potential surrogacy relationships in non-Hodgkin lymphoma: A literature-based meta-analysis of 108 phase II and phase III studies. AAPS J 19:669-681, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Sargent DJ, Shi Q, Flowers CR, et al. : The search for surrogate endpoints in trials in diffuse large B-cell lymphoma: The Surrogate Endpoints for Aggressive Lymphoma project. Oncologist 22:1415-1418, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargent DJ, Wieand HS, Haller DG, et al. : Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 23:8664-8670, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Sargent DJ, Köhne CH, Sanoff HK, et al. : Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol 27:1948-1955, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi Q, Flowers CR, Hiddemann W, et al. : Thirty-month complete response as a surrogate end point in first-line follicular lymphoma therapy: An individual patient-level analysis of multiple randomized trials. J Clin Oncol 35:552-560, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Pfreundschuh M, Schubert J, Ziepert M, et al. : Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: A randomised controlled trial (RICOVER-60). Lancet Oncol 9:105-116, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Merli F, Luminari S, Rossi G, et al. Cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab versus epirubicin, cyclophosphamide, vinblastine, prednisone and rituximab for the initial treatment of elderly “fit” patients with diffuse large B-cell lymphoma: Results from the ANZINTER3 trial of the Intergruppo Italiano Linfomi. Leuk Lymphoma. 2012;53:581–588. doi: 10.3109/10428194.2011.621565. [DOI] [PubMed] [Google Scholar]
- 21.Habermann TM, Weller EA, Morrison VA, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 22.Récher C, Coiffier B, Haioun C, et al. Intensified chemotherapy with ACVBP plus rituximab versus standard CHOP plus rituximab for the treatment of diffuse large B-cell lymphoma (LNH03-2B): An open-label randomised phase 3 trial. Lancet. 2011;378:1858–1867. doi: 10.1016/S0140-6736(11)61040-4. [DOI] [PubMed] [Google Scholar]
- 23.Delarue R, Tilly H, Mounier N, et al. Dosedense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): A randomized phase 3 trial. Lancet Oncol. 2013;14:525–533. doi: 10.1016/S1470-2045(13)70122-0. [DOI] [PubMed] [Google Scholar]
- 24.Haioun C, Mounier N, Emile JF, et al. Rituximab versus observation after high-dose consolidative first-line chemotherapy with autologous stem-cell transplantation in patients with poor-risk diffuse large B-cell lymphoma. Ann Oncol. 2009;20:1985–1992. doi: 10.1093/annonc/mdp237. [DOI] [PubMed] [Google Scholar]
- 25.Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 26.Feugier P, Van Hoof A, Sebban C, et al. Longterm results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: A study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 27.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seymour JF, Pfreundschuh M, Trnĕný M, et al. R-CHOP with or without bevacizumab in patients with previously untreated diffuse large B-cell lymphoma: Final MAIN study outcomes. Haematologica. 2014;99:1343–1349. doi: 10.3324/haematol.2013.100818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz N, Nickelsen M, Ziepert M, et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: An open-label, randomised, phase 3 trial (DSHNHL 2002-1) Lancet Oncol. 2012;13:1250–1259. doi: 10.1016/S1470-2045(12)70481-3. [DOI] [PubMed] [Google Scholar]
- 30.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 31.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-Bcell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 32.Jaeger U, Trneny M, Melzer H, et al. Rituximab maintenance for patients with aggressive B-cell lymphoma in first remission: Results of the randomized NHL13 trial. Haematologica. 2015;100:955–963. doi: 10.3324/haematol.2015.125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbrecht R, Cernohous P, Engert A, et al. Comparison of pixantrone-based regimen (CPOP-R) with doxorubicin-based therapy (CHOP-R) for treatment of diffuse large B-cell lymphoma. Ann Oncol. 2013;24:2618–2623. doi: 10.1093/annonc/mdt289. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: A phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381:1817–1826. doi: 10.1016/S0140-6736(13)60313-X. [DOI] [PubMed] [Google Scholar]
- 35.Burzykowski T, Molenberghs G, Buyse M: The validation of surrogate end points by using data from randomized clinical trials: A case-study in advanced colorectal cancer. J R Stat Soc Ser A Stat Soc 167:103-124, 2004 [Google Scholar]
- 36.Burzykowski T, Molenberghs G, Buyse M, et al. : Validation of surrogate end points in multiple randomized clinical trials with failure time end points. Appl Stat 50:405-422, 2001 [Google Scholar]
- 37.Sargent DJ, Patiyil S, Yothers G, et al. : End points for colon cancer adjuvant trials: Observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol 25:4569-4574, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Burzykowski T, Buyse M: Surrogate threshold effect: An alternative measure for meta-analytic surrogate endpoint validation. Pharm Stat 5:173-186, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Armitage JO, Weisenburger DD: New approach to classifying non-Hodgkin’s lymphomas: Clinical features of the major histologic subtypes—Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 16:2780-2795, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Fisher RI, Gaynor ER, Dahlberg S, et al. : Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 328:1002-1006, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Alizadeh AA, Eisen MB, Davis RE, et al. : Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511, 2000 [DOI] [PubMed] [Google Scholar]
- 42.Rosenwald A, Wright G, Chan WC, et al. : The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 346:1937-1947, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Hans CP, Weisenburger DD, Greiner TC, et al. : Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 103:275-282, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Meyer PN, Weisenburger DD, Choi WL, et al. : LMO2 expression and the Hans algorithm in predicting germinal center phenotype and survival in diffuse large B-cell lymphoma treated with rituximab. Lab Invest 89:1258, 2009 [Google Scholar]
- 45.Scott DW, Wright GW, Williams PM, et al. : Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood 123:1214-1217, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Staiger AM, Ziepert M, Horn H, et al. : Clinical impact of the cell-of-origin classification and the MYC/ BCL2 dual expresser status in diffuse large B-cell lymphoma treated within prospective clinical trials of the German High-Grade Non-Hodgkin’s Lymphoma study group. J Clin Oncol 35:2515-2526, 2017 [DOI] [PubMed] [Google Scholar]
- 47.Petrich AM, Gandhi M, Jovanovic B, et al. : Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: A multicenter retrospective analysis. Blood 124:2354-2361, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Swerdlow SH, Campo E, Pileri SA, et al. : The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127:2375-2390, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Q, Staton AD, Ayer T, et al. : Exploring the potential cost-effectiveness of precision medicine treatment strategies for diffuse large B-cell lymphoma. Leuk Lymphoma 25:1-10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Davies AJ, Caddy J, Maishman T, et al: A prospective randomised trial of targeted therapy for diffuse large B-cell lymphoma (DLBCL) based upon real-time gene expression profiling: The Remodl-B study of the UK NCRI and SAKK lymphoma groups. Blood 126, 2015 (abstr 812) [Google Scholar]
- 51.Offner F, Samoilova O, Osmanov E, et al. : Frontline rituximab, cyclophosphamide, doxorubicin, and prednisone with bortezomib (VR-CAP) or vincristine (R-CHOP) for non-GCB DLBCL. Blood 126:1893-1901, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nowakowski GS, LaPlant B, Macon WR, et al. : Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: A phase II study. J Clin Oncol 33:251-257, 2015 [DOI] [PubMed] [Google Scholar]
- 53. Tilly H, Morschhauser F, Bartlett NL, et al: Polatuzumab vedotin combined with rituximab, cyclophosphamide, doxorubicin, and prednisone (R-CHP) for patients with previously untreated diffuse large B-cell lymphoma (DLBCL): updated results of a phase Ib/II study. Blood 128, 2016 (abstr 1853) [Google Scholar]
- 54.Younes A, Thieblemont C, Morschhauser F, et al. : Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: A non-randomised, phase 1b study. Lancet Oncol 15:1019-1026, 2014 [DOI] [PubMed] [Google Scholar]
- 55. A study of the Bruton’s tyrosine kinase inhibitor, PCI-32765 (ibrutinib), in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone in patients with newly diagnosed non-germinal center B-cell subtype of diffuse large B-cell lymphoma ( NCT02073097). https://clinicaltrials.gov/ct2/show/NCT01855750.
- 56.Nowakowski GS, Chiappella A, Witzig TE, et al. : ROBUST: Lenalidomide-R-CHOP versus placebo-R-CHOP in previously untreated ABC-type diffuse large B-cell lymphoma. Future Oncol 12:1553-1563, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]