Abstract
No vaccine exists against visceral leishmaniasis. To develop effective vaccines, we have previously reported protective role of live attenuated centrin gene–deleted Leishmania donovani (LdCen−/−) parasites through induction of Th1 type immune response in mice, hamsters, and dogs. In this study, we specifically explored the role of Th17 cells in LdCen−/−-induced host protection in mice. Our results showed that compared with wild-type L. donovani infection, LdCen−/− parasites induce significantly higher expression of Th17 differentiation cytokines in splenic dendritic cells. There was also induction of IL-17 and its promoting cytokines in total splenocytes and in both CD4 and CD8 T cells following immunization with LdCen−/−. Upon challenge with wild-type parasites, IL-17 and its differentiating cytokines were significantly higher in LdCen−/−-immunized mice compared with nonimmunized mice that resulted in parasite control. Alongside IL-17 induction, we observed induction of IFN-γ-producing Th1 cells as reported earlier. However, Th17 cells are generated before Th1 cells. Neutralization of either IL-17 or IFN-γ abrogated LdCen−/−-induced host protection further confirming the essential role of Th17 along with Th1 cytokines in host protection. Treatment with recombinant IL-23, which is required for stabilization and maintenance of IL-17, heightened Th17, and Tc17 responses in immunized mice splenocytes. In contrast, Th17 response was absent in immunized IL-23R−/− mice that failed to induce protection upon virulent Leishmania challenge suggesting that IL-23 plays an essential role in IL-17–mediated protection by LdCen−/− parasites. This study unveiled the role of IL-23–dependent IL-17 induction in LdCen−/− parasite-induced immunity and subsequent protection against visceral leishmaniasis.
Leishmaniasis is a spectrum of diseases caused by the protozoan intracellular Leishmania parasites. Among them, visceral leishmaniasis (VL), caused by Leishmania donovani and L. infantum, is the fatal form of the leishmaniasis disease complex (1). The treatment of leishmaniasis relies primarily on chemotherapeutic drugs that have undesired side effects and the emergence of drug-resistant parasites makes treatment of leishmaniasis challenging (2, 3). To date there is no licensed vaccine available against any form of leishmaniasis.
Several approaches have been taken to develop a vaccine for leishmaniasis, among them live attenuated vaccines are very promising (4, 5). The advantage of a live attenuated vaccine is that it provides unbiased and complete array of immune potent Ags to induce host protective immune response. In our laboratory we have developed a genetically modified live attenuated L. donovani parasite (centrin gene–deleted L. donovani [LdCen−/−]) and tested it as a vaccine against VL (6). Our previous studies have shown LdCen−/− parasites are capable of protecting mice, hamsters, and dogs against different forms of leishmaniasis via induction of a robust protective response (6–9). As the immune response in VL is orchestrated by various cell types, a thorough understanding of vaccine-induced immunity will further reveal the important mediators of protective response induced by a vaccine. It has been demonstrated that both CD4 and CD8 T cells are important for host protection in VL (10). Innate immune cells like macrophages and dendritic cells (DCs) priming the T cells toward a Th1 type of immune response, particularly via generation of IFN-γ, is known to control Leishmania parasite growth in a proinflammatory cytokine dominant milieu (11, 12). Apart from Th1 cytokines, several studies have also demonstrated the role of Th17-mediated immune response in generation of vaccine-induced protective cellular immunity against various pathogens such as Mycobacterium tuberculosis, Helicobacter pylori, Pseudomonas aeruginosa, amongst others. (13–15).
IL-17 is characterized as a proinflammatory cytokine capable of mediating killing of several intra- and extracellular pathogens (16, 17). Th17 cells represent a unique helper T cell subset characterized by their ability to produce IL-17 and RORγt transcription factor (18). Th17 cells differentiate from naive CD4 T cells in response to TGF-β and IL-6 (19), amplified by IL-1β and stabilized and maintained by IL-23 (19). In addition to CD4 Th17 cells, CD8 T cells (Tc17), γδ T cells, neutrophils, and innate lymphoid cells also secrete IL-17 (18). Recent studies have found a protective role for IL-17A, the major cytokine produced by CD4 Th17 cells against the intracellular pathogen Trypanosoma cruzi (16, 20). Likewise in case of Leishmania infection, several studies have demonstrated that, apart from Th1 cytokines, IL-17 plays a crucial role in host protection against both human and canine VL caused by L. donovani and L infantum respectively (21, 22). Successful therapy of VL with different immune modulatory molecules has been shown to induce Th1 cytokines, along with IL-17, IL-22, and IL-23 (23–25). A recent report demonstrated that leishmanial Ag-stimulated DCs exhibit a potent anti-leishmanial role by the induction of proinflammatory cytokines and generation of Th17 cells during experimental VL infection (26). In contrast, in cutaneous leishmaniasis caused by L. major it has been shown that Th17 cells influence disease progression via regulation of tissue-destructive neutrophil recruitment at the lesion site (27).
Besides CD4 T cells, CD8 T cell responses play an important role in controlling intracellular pathogens including Leishmania by mechanisms that are mainly dependent on IFN-γ, granzyme, and perforin (28). Recently it has been established that a distinct subset of IL-17–producing CD8 T cells, termed as Tc17 cells, also play a critical role in host defense against several infections. Tc17 cells are protective against vaccinia and influenza virus infections (29, 30) and are indispensable for vaccine immunity against fungal pneumonia (17). Similar to CD4 Th17 cells, Tc17 cells can also be induced from naive CD8 T cells in presence of TGF-β and IL-6 or IL-21 (31, 32). In addition to IL-17, Tc17 cells secrete IL-17F and IL-22, and express IL-23R together with Th17 lineage-specific transcription factors RORγ and RORα. Like CD4 Th17 cells, differentiated Tc17 cells also require IL-23 for their maintenance (33, 34). IL-22 is another Th17 cytokine often secreted from IL-17 producing cells, and has been shown to be involved with the protection against VL in humans (21).
Although several studies demonstrated live attenuated Leishmania parasites inducing robust and durable protection against reinfection, none of them explored the role of IL-17/IL-23 immune axis in protective immune response. Hence, in the current study we studied the role of Th17 cells in LdCen−/− immunization-induced protective immunity. We showed that LdCen−/−-infected DCs produce IL-1β, IL-6, and TGF-β to establish the Th17 lineage, and upon challenge both CD4 and CD8 T cells produce IL-17 resulting in the protection against wild-type L. donovani (LdWT) infection. The direct role of LdCen−/−-induced IL-17 in protection was demonstrated either by using anti-IL-17 Abs to neutralize the IL-17 effect or by using IL-23 receptor gene-deleted mice. Further, we demonstrate that both Th17 and Th1 arms of protective immunity are induced by the LdCen−/− parasite immunization and importantly CD8 T cells have a major role in both arms of protective immunity.
Materials and Methods
Animals and parasites
Five- to six-week-old female C57BL/6 mice were obtained from the National Cancer Institute, National Institutes of Health, Bethesda, MD. IL-23R−/− mouse was a gift from Dr. V. Kuchroo (Harvard Medical School, Boston). An IL-23R−/− mouse breeding pair was housed in a conventional, pathogen-free facility and maintained by in-house breeding program at the Center for Biologics Evaluation and Research, Food and Drug Administration. We have used 6–8-wk-old female mice for all our experiments. All mice were maintained at the center’s American Association for the Accreditation of Laboratory Animal Care’s accredited facility under standard environmental conditions for this species. Both LdWT (MHOM/SD/62/1S) parasites and LdCen−/− line of L. donovani (Ld1S2D) used in our experiments were maintained in golden Syrian hamsters to maintain the infectivity. The parasites were cultured according to the procedure previously described (6, 7). Red fluorescent protein (RFP)-expressing LdWT parasites were developed using the pA2RFPhyg plasmid for integration of a RFP/Hygromycin B resistance gene expression cassette into the parasite 18S rRNA gene locus as described previously (35). mCherry expressing LdCen−/− parasites were generated using the pLEXSY-cherry-sat2 plasmid as per the company’s protocols (Jena Bioscience). The parasites were cultured according to the procedure previously described (36).
Ethics statement
The animal protocol for this study has been approved by the Institutional Animal Care and Use Committee at the Center for Biologics Evaluation and Research, Food and Drug Administration (ASP 1995#26). Further, the animal protocol is in full accordance with the “Guide for the Care and Use of Laboratory Animals” as described in the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals 2015 (http://grants.nih.gov/grants/olaw/references/phspolicylabanimals.pdf).
Infection of mice and isolation of parasitized splenic DCs
The mice were infected through tail vein with 3 × 106 stationary phase red fluorescent LdWT-RFP or LdCen−/− mCherry promastigotes. In each study, at least six mice were used per group. Age-matched naive mice were used as a control. At 1 and 2 wk postinfection, mice were sacrificed and infected splenic DCs (Cd11−Cd11c+) from different groups of mice were sort selected by high-speed FACS cell sorter system (BD FACS Aria-IITM). Single-cell suspensions were prepared from spleens, and RBCs were lysed using ammonium-chloride-potassium lysing buffer. Splenocytes were then labeled with APC-tagged anti-TCR-β, anti-NK1.1, anti-Cd19, anti-Ly6G, and anti-Cd11b Abs using anti-APC magnetic beads, and passed through LS columns to select out these cell types. Flow through enriched DC population was collected and stained with Cd11c-FITC Ab and further sort selected. In some experiments, we have isolated conventional Cd11b+Cd11c+ DCs. Two weeks postimmunization mice spleens were collected and digested with collagenase (1 mg/ml) and DNase I (20 μg/ml) to make single-cell suspension (37). Splenocytes were labeled with APC-tagged anti-TCR-β, anti-NK1.1, anti-Cd19, and anti-Ly6G Abs. Anti-APC magnetic beads were used and passed through the LS columns to select out specific cell types. Flow through enriched DC population was collected and stained with Cd11b+, F4/80, and Cd11c+-FITC Ab and further sort selected.
Immunization and challenge studies
The mice were immunized via tail vein with 3 × 106 stationary phase LdCen−/− promastigotes; 2 or 5 wk postimmunization mice were then challenged via tail vein with 105 virulent L. donovani (LdWT) metacyclic parasites. Infective-stage metacyclic promastigotes of L. donovani were isolated from stationary cultures by density gradient centrifugation as described previously (38). In each study, at least four mice were used per group. Age-matched naive mice used as controls were also similarly challenged with 105 virulent L. donovani metacyclic parasites. At 2, 6, and 12 wk of postchallenge period, parasite load was measured from spleens of challenged mice by culturing the separated host cell preparations by limiting dilutions as previously described (6). In a separate experiment, IL-23R−/− mice were immunized with LdCen−/− and then challenged with LdWT parasites as described above. At 10 wk of postchallenge, parasite load was measured from spleens of challenged mice by serial dilution.
Ex vivo treatment with recombinant IL-23
Mice were immunized via tail vein with 3 × 106 stationary phase LdCen−/− promastigotes. At 12 wk postimmunization mice were sacrificed and splenocytes were isolated and cultured in the presence of exogenously added recombinant IL-23 (20 ng/ml) for 24 h. Flow cytometry analysis of IL-17 producing CD4 and CD8 effector memory cells were performed.
In vivo IL-17 and IFN-γ neutralization
Lyophilized rat anti-mouse IL-17 (Amgen) and IFN-γ mAbs (eBioscience) and control rat IgG (R&D Systems) were resuspended in PBS and injected i.p. (200 μg per mouse) at 0, 3, 6, 9, 12, 19, 26, and 33 d after LdCen−/− immunization. Proinflammatory cytokines were measured from the Leishmania Ag–stimulated splenocyte culture supernatants 5 wk postimmunization. Immunized mice (after IL-17/IFN-γ neutralization) were challenged via tail vein with 105 virulent L. donovani (LdWT) metacyclic parasites. Mice were then euthanized at 10 wk postchallenge and spleens were collected for assessing the parasite burden.
RT-PCR for cytokines
Total RNA was extracted from the parasitized splenic DCs by using an RNAqueous-Micro kit (AM1931; Ambion) and RNA was extracted from total mouse splenocytes using PureLink RNA Mini kit (Ambion). Aliquots (400 ng) of total RNA were reverse transcribed into cDNA by using random hexamers from a high-capacity cDNA reverse transcription kit (Applied Biosystems). Cytokine gene expression levels were determined using the TaqMan gene expression master mix and premade TaqMan gene expression assays (Applied Biosystems) using a CFX96 Touch Real-Time System (Bio-Rad, Hercules, CA). The data were analyzed with CFX Manager Software. Expression of the following genes was determined using TaqMan gene expression assays (Applied Biosystems) in the CFX96 Touch Real-Time System: TGF-β1 (Mm01178819_m1); IL-10 (Mm00439614_m1); IL-6 (Mm00446190_m1); IL-1β (Mm00434228_m1); IFN-γ (Mm01168134_m1); IL-23 (Mm00518984_m1); IL-27 (Mm00461164_m1); GAPDH (Mm99999915_g1). Expression values were determined by the 2−ΔΔCt method; samples were normalized to GAPDH expression and determined relative to expression values from naive mice.
Multiplex cytokine ELISA
Splenocytes from different groups of mice were plated in 24-well culture plates and stimulated with L. donovani freeze-thaw Ag (FTAg) (80 μg/ml) in complete RPMI 1640 medium and cells were incubated at 37°C in 5% CO2, with 95% humidity. After 72 h of culture, cell supernatants were collected and stored at −80°C until cytokines were analyzed using two multiplex custom-built kits: IL-17A, IL-1β, IL-6, IL-10, IFN-γ (Bio-Plex EXP Mo Cyto Grp1 5-plex, Cat No. Y6000007KB); and IL-17F, IL-21, IL-22 and IL-23p19 (Mouse Cytokine Th17 Group III, 4-plex, Cat No. YJ0000000U). The plate was read in a Luminex-100 (Luminex) system using Bio-Plex Manager software 5.0. The cytokine analysis procedure has been performed according to the manufacturer’s instructions, and the level of cytokine concentration was measured using a standard curve of each specific cytokine.
Cytokine ELISA
Splenocytes obtained from different groups of treated mice were cultured in complete RPMI 1640 medium in the presence of FTAg (80 μg/ml) for 24 h at 37°C and were assayed for mouse cytokines (IL-27, IL-17A, IL-17F, IL-6, IFN-γ) with use of the sandwich ELISA kit (eBioscience). The assay was performed according to the manufacturer’s instructions.
Intracellular staining and flow cytometry
Splenocytes isolated from different groups of mice were cultured in 24-well plates in complete RPMI 1640 medium at 37°C and stimulated with Leishmania FTAgs isolated from purified metacyclics (80 μg/ml). After 24 h cells were treated with protein transport inhibitor (BD GolgiStop; BD Pharmingen) and the plate was incubated at 37°C for 6 h. In some experiments after 24 h stimulation with FTAg, cells were restimulated with PMA (10 ng/ml) and ionomycin (1 μg/ml) for 4 h along with protein transport inhibitor (BD GolgiStop; BD Pharmingen). Cells were then blocked at 4°C with rat anti-mouse CD16/32 (5 μg/ml) from BD Pharmingen for 20 min. For surface staining, cells were then stained with anti-mouse CD3 APC-Cy7, anti-mouse CD4 eFluor-450 and anti-mouse CD8 eFluor-605NC or anti-mouse CD3 Alexa Fluor-700, anti-mouse CD4 FITC, anti-mouse CD25 PerCP Cy5.5 or anti-mouse CD3 APC-eFluor@780, anti-mouse CD4 eFluor@450, anti-mouse CD8a eFluor@605NC, anti-mouse CD44 FITC, and anti-mouse CD62L PE-Cy5 for 30 min (each with 1/300 dilution; 4°C). The cells were then stained with LIVE/DEAD fixable aqua (Invitrogen/Molecular Probes) to stain dead cells. Cells were washed with wash buffer and fixed with the Cytofix/Cytoperm Kit (BD Biosciences) for 20 min (room temperature). Intracellular staining was done with anti-mouse IL-17A PE, anti-mouse IFN-γ FITC, anti-mouse IL-10 APC, and anti-mouse Foxp3 PE for 30 min (each with 1:300 dilution; 4°C). In some experiments isotype controls for Abs used under similar conditions indicated specific binding of the test Abs. Cells were acquired on an LSRII (BD Biosciences) equipped with 405, 488, 532, and 638 laser lines using DIVA 6.1.2 software. Data were analyzed with FlowJo software version 9.1.5 (Tree Star). For analysis, first doublets were removed using the width parameter; dead cells were excluded based on staining with the LIVE/DEAD aqua dye. Lymphocytes were identified according to their light-scattering properties. CD4 and CD8 T cells were identified as CD3+ lymphocytes uniquely expressing either CD4 or CD8. Upon further gating, intracellular cytokines were measured in CD4 and CD8 cells. Fluorescence minus one controls were used for proper gating of positive events for designated cytokines.
Statistical analysis
Statistical analysis of differences between means of groups was determined by unpaired two-tailed Student t test, using GraphPad Prism 5.0 software. A p value < 0.05 was considered significant, and a p value < 0.005 was considered highly significant.
Results
LdCen−/− immunization induced the expression of cytokines promoting Th17 cells and inhibits Th2 cytokines in splenic DCs isolated from infected C57BL/6 mice
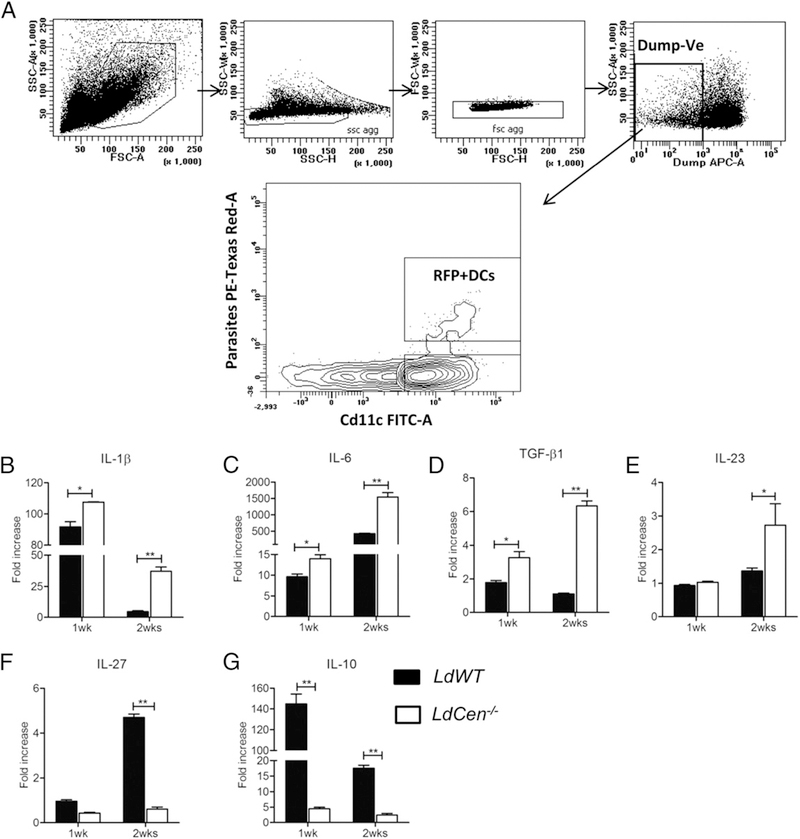
We have previously shown that bone marrow-derived macrophage cells infected with live attenuated LdCen−/− parasites were capable of inducing a strong proinflammatory response in vitro and in vivo leading to the proliferation of Th1 cells (39). In this study, we investigated whether LdCen−/− parasites induced DC-mediated Th17 polarization in spleen in vivo. We infected mice intravenously with red fluorescent LdWT-RFP, or LdCen−/− mCherry parasites. One and two weeks postinfection, we enriched parasitized DCs from spleen by gating live single cells for (lineage [T cells, B cells, NK cells, and macrophages]− RFP/mCherry+Cd11c+) populations (Fig. 1A) and assessed the expression of Th17 cell differentiation cytokines (IL-1β, IL-6, and TGF-β1), stabilizing cytokine (IL-23) and suppressing cytokines (IL-27 and IL-10) in these cells. RT-PCR analysis showed that three major Th17-inducing cytokines, IL-1β, IL-6, and TGF-β1, were significantly elevated in parasitized splenic DCs isolated from LdCen−/−-immunized mice compared with LdWT-infected mice, both at 1 and 2 wk postimmunization (Fig. 1B–D). Although, a similar level of IL-23 was observed in LdWT- and LdCen−/−-infected DCs at 1 wk postinfection, IL-23 mRNA expression was significantly increased in LdCen−/−-infected DCs compared with LdWT-infected DCs at 2 wk postinfection (Fig. 1E). In contrast, IL-27 and IL-10, the major Th17-suppressing and anti-inflammatory cytokines respectively, were significantly reduced in parasitized splenic DCs isolated from LdCen−/−-immunized mice compared with LdWT-infected mice (Fig. 1F, 1G). Of note, conventional Cd11chi DCs in the spleen are mostly Cd11b+ (80–90%). Hence, we have further characterized the splenic DCs which are not only Cd11c+ but also Cd11b+ (Supplemental Fig. 1A). Two weeks postimmunization, we enriched parasitized DCs from the spleen by gating live single cells for (lineage [T cell, B cell, NK cell, Ly6G]− Cd11b+F4/80−Cd11c+ RFP/mCherry+) and assessed the expression of Th17 cell differentiation cytokines. RT-PCR analysis showed that Th17-inducing cytokines IL-1β and IL-6 were significantly elevated along with significant abrogation of anti-inflammatory cytokine IL-10 in parasitized splenic DCs isolated from LdCen−/−-infected mice compared with LdWT-infected mice at 2 wk postimmunization (Supplemental Fig. 1B–D). Thus, from the in vivo gene expression profiling it is evident that LdCen−/− parasite immunization induced a strong splenic DC-mediated Th17 polarization.
FIGURE 1.
LdCen−/− immunization induce the expression of Th17 cells promoting cytokines and inhibit Th2 cytokines in splenic DCs isolated from infected C57BL/6 mice. (A) Infected cells were sorted from the spleen of different groups of mice after indicated period of postinfection with either LdWT-RFP or LdCen−/−-mCherry by gating live single cells for (lineage [T cells, B cells, NK cells, and macrophages]− and RFP/mCherry+ Cd11c+) cells. (B–G) mRNA expression levels of IL-1β, IL-6, TGF-β1, IL-23, IL-27, and IL-10 were measured from infected DCs, and expressed as fold increase over naive DCs. The data represent the mean values + SEM of results from two independent experiments; in each experiment more than eight mice splenocytes were pooled to produce enough infected DCs. *p < 0.05, **p < 0.005.
LdCen−/− immunization induced Th17 cytokines concomitant with the inhibition of anti-inflammatory cytokines compared with LdWT infection in splenocytes
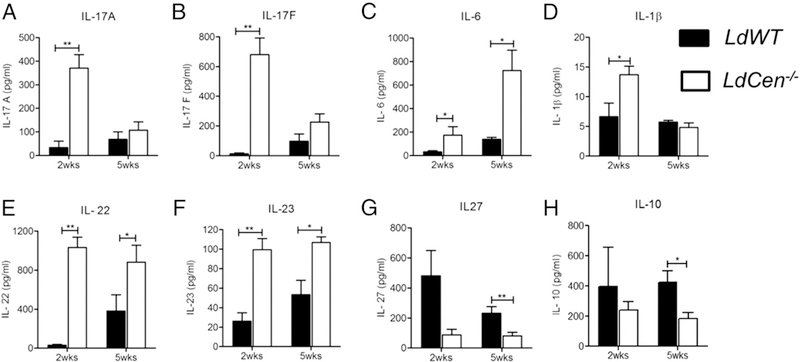
Because significant induction of Th17-stimulating cytokine expression like IL-1β, IL-6, and TGF-β1 was observed in splenic DCs isolated from LdCen−/−-immunized mice, we evaluated the cytokine production in splenocytes of LdCen−/−-immunized or LdWT-infected mice restimulated with L. donovani Ag. Multiplex cytokine ELISA analysis showed LdCen−/− immunization caused significant induction of IL-17 (IL-17A and IL-17F) as early as at 2 wk with a less pronounced increase at 5 wk compared with LdWT-infected mice (Fig. 2A, 2B). Interestingly, LdCen−/−-immunized mice showed significant increase in the Th17-inducing cytokine level such as IL-6 at 2 and 5 wk as well as IL-1β at 2 wk compared with LdWT-infected mice (Fig. 2C, 2D). Even though we could detect significant TGF-β expression at RNA level (Fig. 1D), we were not able to detect appreciable production of TGF-β in the splenocyte cultures (data not shown). Apart from IL-17, the levels of IL-22 and IL-23 were significantly high along with a concomitant suppression of IL-27 and anti-inflammatory cytokine IL-10 in LdCen−/−-immunized mice compared with LdWT-infected mice at both time points (Fig. 2E–H). Thus, LdCen−/− immunization significantly induced Th17 cytokine secretion along with concomitant suppression of anti-inflammatory cytokines in the splenocytes compared with LdWT infection.
FIGURE 2.
LdCen−/− immunization induced Th17 along with the inhibition of anti-inflammatory cytokines compared with LdWT infection in splenocytes. LdCen−/−-immunized or LdWT-infected mice were sacrificed at 2 and 5 wk postinfection. Splenocytes were collected and cultured in presence of Leishmania Ag. Culture supernatants were collected and concentrations of cytokines IL-17A, IL-17F, IL-6, IL-1β, IL-22, IL-23, IL-27, IL-10 (A–H) were measured by the multiplex or single plex (IL-27) mouse cytokine ELISA kit as described in the Materials and Methods section. The data represent the mean values + SEM of results from two independent experiments. *p < 0.05, **p < 0.005.
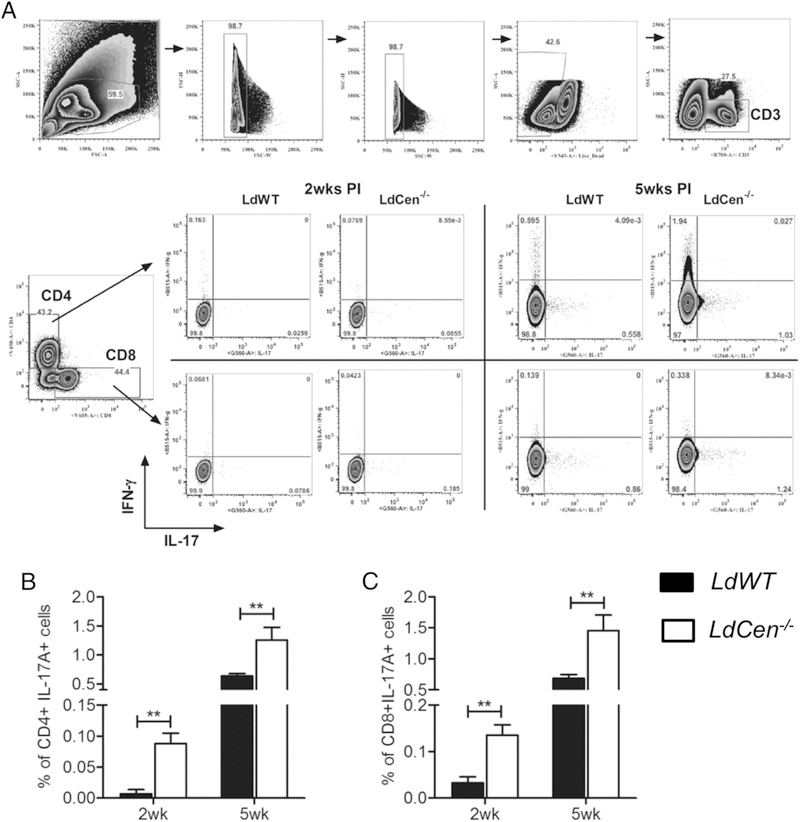
LdCen−/− immunization induced secretion of IL-17 and from both CD4 and CD8 T cells
T cell–mediated immune response plays an important role in VL (10). To further characterize the cellular sources of IL-17, we determined the frequency of IL-17–producing CD4 and CD8 T cells in Ag–stimulated splenocytes by flow cytometry. CD4 and CD8 T cells were gated and further separated into distinct subpopulation based on their production of IL-17 (Fig. 3A). The results showed at both 2 and 5 wk postimmunization a significantly higher frequency of IL-17–producing CD4 and CD8 T cells in LdCen−/−-immunized mice compared with LdWT-infected mice (Fig. 3B, 3C). We have also observed that the overall expression of IL-17 in CD8 T cells is less compared with IL-17 expression in CD4 T cells, presumably due to MHC I activation-induced endocytosis of CD8 receptors in activated cells (40).
FIGURE 3.
LdCen−/− immunization induced IL-17 secretion from both CD4 and CD8 T cells compared with LdWT infection. LdCen−/−-immunized or LdWT-infected mice were sacrificed at 2 and 5 wk postinfection. Flow cytometry analysis of Leishmania Ag-stimulated splenocytes. (A) Gating strategy for flow cytometry analysis and representative flow plots. (B and C) Bar diagrams represent IL-17 secreting CD4 and CD8 T cells. The data represent the mean values + SEM of results from three independent experiments. **p < 0.005. PI, postimmunization.
Because our results showed that LdCen−/− immunization significantly inhibited the IL-10 induction compared with LdWT infection in mice (Fig. 2H) and as it has been reported earlier that IL-10 acts as a negative regulator of Th17 cells (41), we further characterized frequency of IL-10–producing CD4 T cells. We observed a significantly lower frequency of IL-10–producing CD4 T cells in LdCen−/−-immunized mice compared with LdWT-infected mice (Supplemental Fig. 2A, 2B). A recent study indicates a crucial role of IL-10–producing Th1 cells in mediating disease progression during VL (42). Hence, we also looked at IL-10+ CD4 T cells that coproduce IFN-γ cytokine after immunization with LdCen−/− parasites. The results showed a significantly higher percentage of CD4+ T cells positive for both IFN-γ and IL-10 in LdWT-infected mice compared with LdCen−/−-immunized mice (Supplemental Fig. 2C). We further delineate the phenotype and frequency of IL-10–producing CD4 T cells based on their expression of CD25 and Foxp3 (Supplemental Fig. 2D). IL-10–producing CD4+CD25+Foxp3+ cells are classified as naturally occurring regulatory T (nTreg) cells and CD4+CD25−Foxp3− as Tr1 cells (43). Although we did observe expansion of IL-10–producing CD4+ T cells in LdWT-infected mice (Supplemental Fig. 2B), the frequency of nTreg cells significantly decreased in LdWT-infected and LdCen−/−-immunized mice compared with naive mice (Supplemental Fig. 2E), which is in accordance with previous reports (42, 44). Additionally, we observed an appreciably lower frequency of IL-10–producing nTreg and Tr1 cells in LdCen−/−-immunized mice compared with LdWT-infected mice (Supplemental Fig. 2F, 2G) at 5 wk postimmunization. Further, we did not observe any marked difference in the frequency of IFN-γ, IL-10 double producing nTreg cells between LdWT- and LdCen−/−-immunized mice (Supplemental Fig. 2H); however, the frequency of IFN-γ+ IL-10+ Tr1 cells are comparatively lower although not statistically significant in LdCen−/−-immunized mice compared with LdWT-immunized mice (Supplemental Fig. 2I).
LdCen−/− immunization induced Th17 cytokines following virulent Leishmania challenge
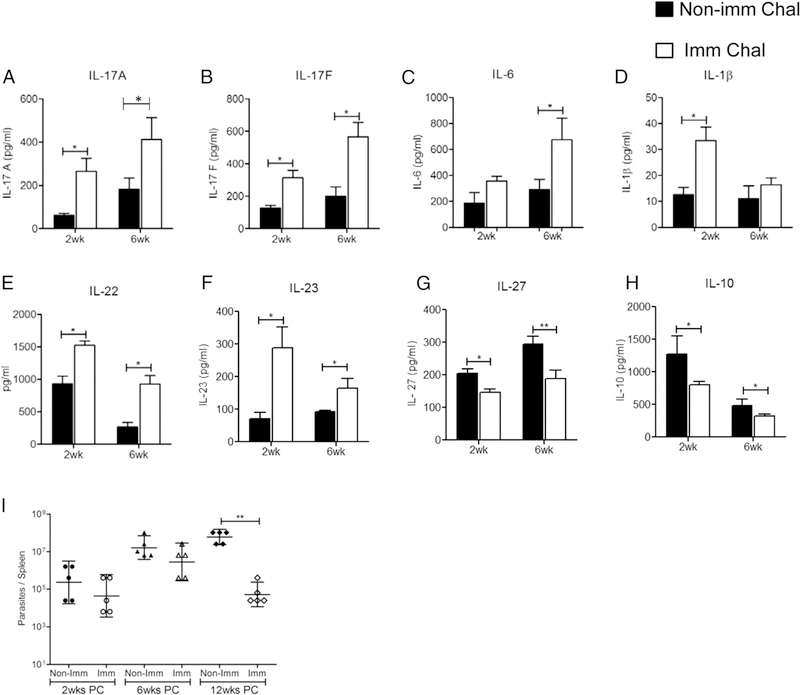
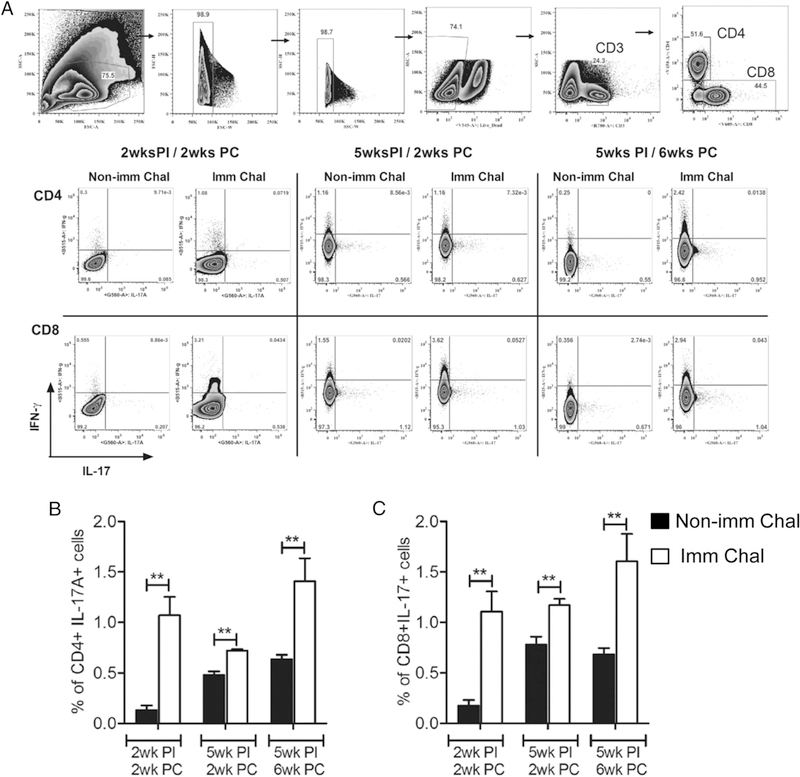
Having established that LdCen−/− immunization induced a cytokine response characterized by IL-17–producing CD4 and CD8 T cells, we characterized the immune response in mice upon virulent challenge. At 5 wk postimmunization, mice were challenged with LdWT parasites, and Ag-specific cytokine secretion from splenocytes was analyzed at 2 and 6 wk postchallenge by multiplex cytokine ELISA. The results showed that LdCen−/−-immunized-challenged mice had a significantly higher level of IL-17 (IL-17A and IL-17F) compared with naive-challenged mice at both time points (Fig. 4A, 4B). Additionally, immunized mice showed an appreciable increase in IL-6 at 6 wk postchallenge along with IL-1β at 2 wk postchallenge compared with nonimmunized-challenged mice (Fig. 4C, 4D). Furthermore, following challenge with LdWT parasites, immunized mice showed a substantially enhanced secretion of IL-22 and IL-23 (Fig. 4E, 4F) along with an appreciable decrease in IL-27 and IL-10 levels compared with naive-challenged mice at both time points (Fig. 4G, 4H). Finally, the effect of IL-17 induction upon immunization on parasite control was analyzed in animals at various times after L. donovani infection. The result showed that immunized mice tended to control the parasite burden as early as 2 wk postchallenge that finally resulted in >2 log-fold reduction in spleen compared with nonimmunized-challenged mice at 12 wk postchallenge (Fig. 4I). We further investigated whether LdCen−/− immunization at earlier (2 wk) and later (5 wk) time points could induce both CD4 and CD8 T cells to secrete IL-17 following challenge with virulent LdWT parasites (Fig. 5A). Flow cytometry analysis showed that 2-wk-postimmunized mice at 2 wk postchallenge had a significantly higher frequency of IL-17–producing CD4 (Th17) and CD8 T (Tc17) cells compared with naive-challenged mice. Thus, induction of IL-17 ensued as early as 2 wk postimmunization. Additionally, 5-wk-postimmunized mice at 2 and 6 wk postchallenge had a considerably higher frequency of IL-17–producing CD4 and CD8 T cells compared with naive-challenged mice (Fig. 5B, 5C).
FIGURE 4.
LdCen−/− immunization confers host protection by induction of Th17 cytokines. Five weeks postimmunized mice were challenged with virulent L. donovani parasites. At 2 and 6 wk postchallenge both immunized and nonimmunized mice were euthanized, and splenocytes were cultured and stimulated with Leishmania Ag. Culture supernatants were collected and concentrations of cytokines IL-17A, IL-17F, IL-6, IL-1p, IL-−/−, IL-23p19, IL-27, IL-10 (A–H) were measured by the multiplex or single plex (IL-27) mouse cytokine ELISA kit as described in the Materials and Methods section. (I) At 2, 6, and 12 wk postchallenge (PC) parasite burden was measured in the spleen of immunized-challenged and nonimmunized-challenged animals by serial dilution. The data represent the mean values + SEM of results from two independent experiments. *p < 0.05, **p < 0.005.
FIGURE 5.
LdCen−/−-immunized mice induce IL-17 secretion from both CD4 and CD8 T cells upon challenge with virulent L. donovani infection. Two and five weeks postimmunized mice were challenged with LdWT parasites as described in Fig. 4 legend. (A) Gating strategy for flow cytometry analysis and representative flow plots. (B and C) Bar diagrams represent IL–17-secreting CD4 and CD8 T cells in nonimmunized-challenged and immunized-challenged mice. The data represent the mean values + SEM of results from three independent experiments. **p < 0.005. PC, postchallenge; PI, postimmunization.
LdCen−/− immunization induced synergism between Th17 and Th1 axis
We have previously shown that immunization with LdCen−/− in animal models induces an IFN-γ-predominant Th1 response (6–9). To test whether LdCen−/− immunization resulted in synergism between Th1 and Th17 axis, we further analyzed Th1 response in this study along with Th17. We specifically evaluated the signature Th1 cytokine, IFN-γ production in splenocytes of immunized mice before and after challenge. We found that LdCen−/−-immunized mice showed significant induction of Th1 cytokine IFN-γ in the splenocytes at 2 and 5 wk compared with LdWT-infected mice (Supplemental Fig. 3A). Furthermore, immunized mice showed an increased level of IFN-γ in the splenocytes at 2 and 6 wk postchallenge compared with naive-challenged mice (Supplemental Fig. 3B).
We also determined the frequency of IFN-γ–producing CD4 and CD8 T cells in Ag-stimulated splenocytes by flow cytometry. CD4 and CD8 T cells were gated, further separated into distinct subpopulations based on their production of IFN-γ or IL-17 and generated a comparative plot (Supplemental Fig. 3C, 3D). We found that although the frequency of IFN-γ–producing CD4 and CD8 T cells increased significantly in LdCen−/−-immunized mice compared with LdWT-infected mice at 5 wk postimmunization, IL-17-producing CD4 and CD8 T cells generated as early as 2 wk postimmunization with LdCen−/− (Supplemental Fig. 3C, 3D).
Moreover, although IL-17–secreting CD4/CD8 T cells were significantly high in 2-wk-immunized/2-wk-postchallenged mice and 5-wk-immunized/2-wk-postchallenged mice, only IFN-γ–secreting CD8 T cells and not IFN-γ–secreting CD4 T cells were significantly high compared with nonimmune-challenged mice (Supplemental Fig. 3E, 3F). Thus IFN-γ–producing CD8 T cells preceded IFN-γ–producing CD4 T cells in immunized mice, which is in accordance with a previous report (45). However, the percent of IFN-γ–producing CD4 and CD8 T cells were simultaneously significantly increased along with IL-17–secreting T cells in 5-wk-immunized/6-wk-postchallenged mice (Supplemental Fig. 3E, 3F). Noteworthy, we did not observe IL-17 and IFN-γ double-producing T cells in any experimental groups.
Neutralization of IL-17 or IFN-γ abrogates the LdCen−/−-induced host protective immunity
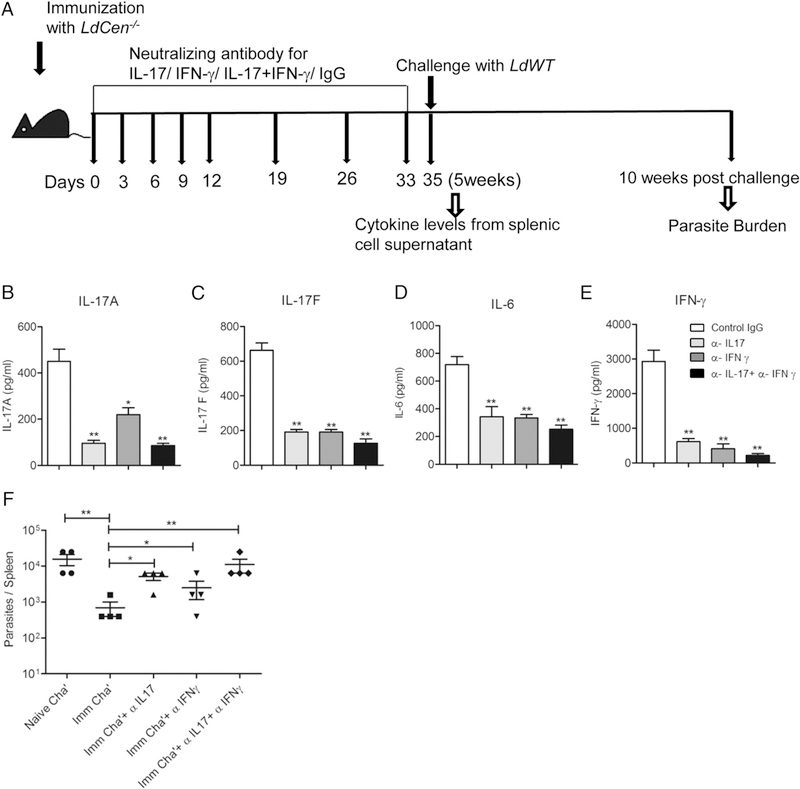
To ascertain the specific roles of Th17 along with Th1 cytokines in LdCen−/−-mediated protection in vivo, mice were immunized with LdCen−/− plus 200 μg of either anti–IL-17 or anti–IFN-γ mAb alone, or 400 μg total combination of anti–IL-17 and anti–IFN-γ mAbs. One set of animals received control IgG (Fig. 6A) at regular intervals. We specifically determined the level of cytokines produced by Leishmania Ag–restimulated splenocytes derived from the LdCen−/−-immunized mice after treatment with the Abs at 5 wk postimmunization. Anti–IL-17 and anti–IFN-γ mAb treatment either alone or in combination significantly reduced IL-17A and IL-17F in LdCen−/−-immunized mice splenocytes compared with control IgG-treated immunized mice (Fig. 6B, 6C). Additionally, IL-6, which is involved in the differentiation of Th17 cells from naive T cells, and the signature proinflammatory Th1 cytokine IFN-γ were also significantly reduced by both treatments (Fig. 6D, 6E). Importantly, anti–IL-17 and anti–IFN-γ mAb treatment either alone or in combination significantly reduced LdCen−/−-mediated protection as indicated by significantly increased spleen parasite burden at 10 wk postchallenge (Fig. 6F). These results suggest that similar to IFN-γ, IL-17 also plays an important role in LdCen−/−-induced protective immunity.
FIGURE 6.
IL-17 or IFN-γ neutralization abrogates the LdCen−/−-induced host protective immunity. Mice were immunized with LdCen−/−, and different groups of mice were treated either with anti–IL-17 or anti–IFN-γ mAb, or anti–IL-17 and anti–IFN-γ mAb, or control IgG as described in Materials and Methods. (A) Schematic diagram showing the treatment regimen. After 5 wk postimmunization mice were euthanized and splenocytes were cultured in presence of Leishmania Ag. (B–E) IL-17A, IL-17F, IL-6, and IFN-γ were measured from the culture supernatants by sandwich ELISA. The data represent the mean values + SEM of results from two independent experiments. *p < 0.05, **p < 0.005 compared with IgG treated-immunized mice. (F) Mice from each group were challenged with L. donovani parasites and splenic parasite burden was measured after 10 wk postchallenge. The data represent the mean values + SEM of results from two independent experiments. *p < 0.05, **p < 0.005.
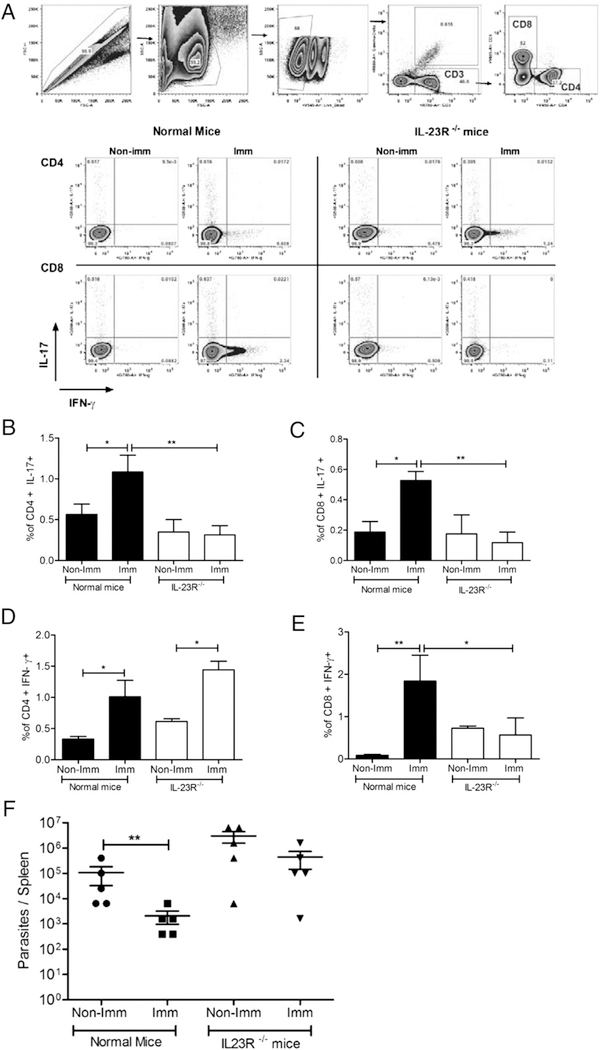
LdCen−/−-immunized IL-23R−/− mice failed to induce IL-17–mediated host protection upon virulent challenge with L. donovani parasites
Because IL-23 plays an essential role in the stabilization and maintenance of the Th17 response (18) and from our observation that there is a significant stimulation of IL-23 in LdCen−/−-immunized (Fig. 2F) and in immunized-challenged mice (Fig. 4F), we wanted to determine whether IL-23 plays a direct role in LdCen−/−-induced host protection via IL-17. We specifically determined the frequency of IL-17 and IFN-γ–producing CD4 and CD8 T cells in normal and IL-23R−/− mice upon immunization by flow cytometry (Fig. 7A). The results showed that at 5 wk postimmunization, the frequency of IL-17–producing CD4 and CD8 T cells was significantly reduced in IL-23R−/− mice compared with normal mice (Fig. 7B, 7C). LdCen−/−-immunized IL-23R−/− mice also failed to induce IFN-γ production from CD8 T cells. On the contrary, immunized IL-23R−/− mice specifically exhibited a significantly increased frequency of IFN-γ-producing CD4 T cells (Fig. 7D, 7E). These data suggest that IL-23 has a differential impact on IFN-γ-producing CD4 Th17 and Th1 cells in response to LdCen−/− immunization after challenge.
FIGURE 7.
LdCen−/−-immunized IL-23R−/− mice failed to induce host protection upon virulent challenge with L. donovani parasites. Normal mice and IL-23R−/− mice were immunized with LdCen−/− and at 5 wk postimmunization mice were sacrificed. Cell specific cytokine secretions were measured by flow cytometry. (A) Gating strategy and representative flow plots; (B and C) bar diagrams represent IL-17–producing CD4 and CD8 T cells; and (D and E) IFN-γ-producing CD4 and CD8 T cells. (F) Spleen parasite burden was measured by serial dilution after 10 wk postchallenge with LdWT (MHOM/SD/62/1S) parasites. The data represent the mean values + SEM of results from three independent experiments. *p < 0.05, **p < 0.005.
Previously, it was reported that IL-23 was required for enhanced recall response of Th17 cells (46). Therefore, to test whether IL-23 plays a role in LdCen−/−-mediated activation of effector memory Th17 and Tc17 cells, we performed an ex vivo study in which recombinant IL-23 (rIL-23) was added exogenously to the splenocytes isolated from 12-wk-postimmunized mice. Results showed that the percentage of IL-17-producing CD4 and CD8 effector memory cells (CD4+CD44hiCD62L−IL-17+/Th17 and CD8+CD44hiCD62L−IL-17+/Tc17) was significantly higher in the LdCen−/−-immunized group upon treatment with rIL-23 (Supplemental Fig. 4).
To demonstrate the impact of IL-23–mediated IL-17 induction in the protective response, both normal mice and IL-23R−/− mice were immunized with LdCen−/− parasites. Five weeks post-immunization, mice were challenged with virulent LdWT parasites. Spleens were analyzed for parasite burden 10 wk postchallenge. The results showed that LdCen−/−-immunized-challenged IL-23R−/− mice failed to control parasitemia compared with the immunized-challenged normal mice (Fig. 7F). These results suggest that an intact IL-17 response is necessary for protection and that IL-23 plays a significant role in sustaining the IL-17-mediated protection by LdCen−/− parasites.
Discussion
The role of Th1 cells in mediating protective immunity against protozoans, intracellular bacteria, and viruses is well documented (47) and is achieved due to the ability to secrete IFN-γ and consequent initiation of the microbicidal action in macrophages (47, 48). Several studies suggested that apart from Th1 immune response, Th17 cells have evolved to confer protective immunity against various infectious diseases (20, 21, 24, 49–52). Indeed, blocking of the IL-17 pathway led to increased susceptibility to intracellular Francisella tularensis and Candida muridarum infection due to impaired Th1 response suggesting that both Th1 and Th17 arms could contribute to protection (53, 54). Studies using the intracellular pathogen Salmonella have also shown that absence of IL-23, IL-17 stabilizing and proliferating cytokine, and IL-17R signaling resulted in increased dissemination of the bacteria to the lymph nodes due to reduced neutrophil recruitment (55). Additionally, both IL-23−/− mice and IL-17R−/− mice were more susceptible to Listeria monocytogenes infection and had reduced neutrophil recruitment to the liver (56). Overall, these studies indicate the critical role of IL-17 in controlling pathogen via induction of both innate and adaptive immune response.
Effective clearance of Leishmania parasites from host cells requires Th1 cells to secrete a substantial amount of IFN-γ. DC-derived IL-12 skews naive CD4 T cells into IFN-γ-producing Th1 cells that can mediate the induction of protective Th1 immune response during VL (57, 58). However, the current understanding of the role of Th17 cells during leishmaniasis is rapidly evolving. A recent study has reported an increased amount of IL-17 in the serum of asymptomatic individuals with a delayed-type hypersensitivity reaction to L. donovani compared with that in symptomatic patients (21). Additionally, heightened Th17 type responses were also observed in post-kala-azar dermal leishmaniasis (59). In both studies it has been demonstrated that PBMCs from healed VL patients, upon stimulation with Leishmania Ags, strongly induce IL-17 and IL-23, suggesting that IL-17 might have a protective role in parasite clearance (21, 59). Furthermore, infection with L. infantum, the causative agent of canine VL, caused a heightened IL-17A production that was shown to control parasite replication in conjunction with Th1 response (22). Interestingly, it has been demonstrated that the successful therapy of VL with several immunomodulators involves Th17 cytokines. For example, treatment of L. donovani-infected mice with curdlan, a naturally occurring p-glucan immunomodulatory molecule, was shown to exert an anti-leishmanial effect via induction of IL-17 (23). Additionally, another immunomodulator, astrakurkurone (a fungal extract), restricted L. donovani infection in experimental VL by potentiating the induction of IL-17 along with IFN-γ from CD4 T cells (25). Together, these results strongly suggested that IL-17 plays a complementary role in protection against VL along with Th1 cells. These results also highlight the importance of assessing any putative role of IL-17 while evaluating anti-Leishmania vaccines (60). However, the role of IL-17 in modulating immune responses in the context of live attenuated Leishmania vaccine-induced immunity has not been ascertained. Previously our laboratory has shown LdCen−/− parasites can be used as a vaccine-induced protection against virulent L. donovani infection in several animal models via induction of Th1 type immune response (6–9). In the current study we show, for the first time, to our knowledge, that LdCen−/− parasites significantly elicit a Th17 type protective immune response against virulent L. donovani infection and such an induction does not impede the development of Th1 immune response. Further, both Th17 and Th1 responses are simultaneously induced by LdCen−/− parasites in the murine model, which in turn render protection against virulent challenge.
The DC-derived cytokines are crucial in initiating and shaping T cell responses (57). Activation of DCs transforms them into fully functional APCs capable of priming naive T cell (Th0) differentiation toward Th1/Th2/Treg/Th17 phenotype (18). Specifically, DC-derived cytokines IL-6, TGF-β1, IL-1β, and IL-23 play important roles in the differentiation, proliferation, and maintenance of Th17 cells (18, 61), whereas IL-27 and IL-10 are key mediators in suppressing Th17 cells (62, 63). In the current study we found that live attenuated LdCen−/− parasites stimulated splenic DCs to produce proinflammatory cytokines like IL-6, IL-1β, TGF-β1, and IL-23 and reduced expression of IL-27 and IL-10. The cytokine expression profile in LdCen−/−-immunized mice splenic DCs clearly suggests a cytokine milieu conducive to generation of Th17 cells in immunized mice. This was further confirmed by a significant induction of IL-17, IL-6, IL-1β, IL-23, and IL-22 in the total splenocytes culture supernatant from LdCen−/−-immunized mice.
T cell-mediated immune response plays an important role in VL. Both CD4 and CD8 T cells have been shown to be involved in immune regulation during VL (64). In healed VL patients, CD4 T cells producing IL-17 have been shown to be strongly associated with protection against kala azar (21). Similarly, in a recent study, we showed an increase in IL-17-secreting CD4 T cells in the PBMCs isolated from healed VL individuals after stimulation with LdCen−/− parasites ex vivo (65). In another study using Leishmania Ag-stimulated DCs as a vaccine, induction of IL-6, IL-23, and TGF-β expression was observed, which in turn resulted in IL-17-producing CD4 T cells leading to host protection against experimental VL (26). Thus, both experimental animal studies and observations from clinical studies in endemic areas support a strong role for Th17 in protection against VL. In the current study we demonstrated that LdCen−/− immunization in mice resulted in significant induction of IL-17 production from both CD4 and CD8 T cells before and after challenge, which correlate with protective response.
Apart from CD4+ IL-17 (Th17), a growing body of evidence implicates the presence of IL-17-producing CD8+ T (Tc17) cells in various diseases such as infections, cancer, and autoimmunity (31). Tc17 cells are found in mice responding to influenza A infection (66). Moreover, both CD4 and CD8 T cells producing IL-17 might have roles in controlling parasite proliferation and invasion in human toxoplasmosis (67, 68). Importantly, our data show that CD8 T cells are also involved in the production of IL-17 in LdCen−/−-immunized mice before and after challenge, further showing the importance of this novel T cell subset in protection against L. donovani infection.
IL-10 and IL-27 negatively regulate Th17 differentiation (62, 69). Regulatory T cells are the most abundant IL-10-producing T cells during VL and play a crucial role in pathogenesis. In human VL it has been shown that splenic CD4+CD25−Foxp3− (Tr1 cells) cells are the major producers of IL-10 (43). It has also been shown both CD4+CD25+Foxp3+ (nTreg) and CD4+CD25−Foxp3− (Tr1) cells produce IL-10 in an Ag-dependent manner (70). Moreover, in IL-17Rα−/− mice both CD4+IL-10+ cells and Foxp3 expressions were significantly enhanced upon L. infantum infection (22). Similarly, it has been reported that IL-27 induction mediates susceptibility to VL by suppressing the IL-17-neutrophil response (71). In the current study we observed LdCen−/− immunization suppresses IL-10 from Tr1 and nTreg, as well as suppressing IL-27 generation. Therefore, suppression of IL-10 and IL-27 further confirms heightened Th17 differentiation in LdCen−/−-immunized mice. In addition to the aforementioned roles for IL-17, Th17 cells also produce IL-22, which promotes the growth of epithelial cells thereby enabling tissue repair and liver protection against chronic infection (72, 73). It has been shown that IL-22 is also associated with the protection in human VL (21). We observed heightened expression of IL-22 in LdCen−/−-immunized mice further confirming the critical roles of Th17 cytokines in host protection. All these studies suggest that there is strong induction of Th17-related cytokines upon LdCen−/− immunization. Further, immunized mice upon challenge with virulent L. donovani showed sustained induction of IL-17 and its promoting cytokines (IL-6, IL-1β, and TGF-β1), stabilizing cytokines (IL-23), and concomitant decrease in both IL-10 and IL-27 cytokines in total splenocytes as well as heightened IL-17-producing CD4 and CD8 T cell population. Taken together our studies using LdCen−/− immunization suggest that IL-17 induction and concomitant IL-27 suppression have a role in protection against Leishmania infection as was observed recently (71).
To further elucidate the direct involvement of IL-17 in LdCen−/−-induced immunity, we administered IL-17-specific neutralizing Ab in postimmunized mice. Of note, we used the neutralization Ab approach to inhibit the functional activity of IL-17 rather than using IL-17−/− mouse model because it is well documented that IL-17 gene deletion is associated with several other congenital defects and impaired delayed-type hypersensitivity response (74). Further, neutralization at the time of T cell priming stage early in the immunization alone would not alter the overall homeostatic functions mediated by IL-17. Treatment with neutralization Abs to IL-17 resulted in inhibition of proinflammatory cytokine (IL-17, IL-6, IFN-γ) generation in immunized mice leading to the abrogation of LdCen−/− immunization-induced host protection. Lack of Th17 response induction due to the IL-17 neutralizing Abs immediately after the immunization resulted in lack of parasite control after challenge. These results further affirm the direct protective role of IL-17 in immunized mice against L. donovani infection.
Of interest, in contrast to our present observation, one recent report demonstrated that IL-17 helps in pathogen establishment during VL at early phase of infection (75). Notably, the authors reported that IL-17-deficient mice had decreased accumulation of neutrophils and monocytes leading to reduced L. donovani infection in the visceral organs. However, there is overwhelming evidence both in human and animal studies, including our current study, that IL-17 plays a protective role in VL (21–23). The cause of the difference in outcomes is unknown at this time and may need further analysis.
IL-23 has been proposed to induce the proliferation and stabilization of IL-17-secreting cells (67, 76). Furthermore, IL-23 is also required for activation of the recall Th17 memory response (46) and IL-23 receptor (IL-23R) is required for effector Th17 cell responses in vivo (77). Indeed, there is growing evidence suggesting that Th17 lineage may be critical for vaccine-induced memory immune responses against bacterial, fungal, and parasitic infection (78). Importantly, in the absence of IL-23R, activated Th17 cells retain low IL-7Rα expression, known to be important for the survival of memory CD8 T cells (79). Our ex vivo study showed that treatment with recombinant IL-23 enhanced the percentage of IL-17-producing CD4 and CD8 effector memory cells in the LdCen−/−-immunized group in comparison with the untreated immunized group, indicating that IL-23 plays an important role in the vaccine-induced immunity. This was further substantiated through our in vivo finding where immunized IL-23R−/− mice failed to induce IL-17-producing CD4 and CD8 T cells and consequently were unable to induce host protective immunity against virulent L. donovani infection. Our observation is in agreement with the previous report describing an essential role for IL-23R in the terminal differentiation of Th17 effector cells (77). We also found LdCen−/−-immunized IL-23R−/− mice failed to induce IFN-γ production from CD8 T cells. On the contrary, immunized IL-23R−/− mice specifically exhibited a significant increased frequency of IFN-γ-producing CD4 T cells. The cause of difference in IFN-γ response in CD8 and CD4 T cells when IL-23 pathway is disrupted is not known at this time and needs further study.
The role of IL-17 in regulating the IFN-γ response has been demonstrated in several infectious diseases. Specifically, immunization against H. pylori infection rapidly accumulates IL-17-producing CD4 T cells prior to IFN-γ-producing cells, suggesting that IL-17-secreting cells can help to recruit Th1 cells (14). Several reports have shown that IL-17 might contribute to the recruitment of IFN-γ-producing cells via induction of chemokines such as CXCL9, CXCL10, and CXCL11 (13, 80, 81). Additionally, IL-17 is shown to play a role in the induction of IL-12 and IFN-γ by macrophages (53). It has been proposed that vaccination against M. tuberculosis resulted in the generation of IFN-γ- or IL-17-producing T cells. Importantly, IL-17 recruits IFN-γ-producing T cells in the affected organs, which in turn ameliorate the bacterial growth (13). During L. infantum infection it has been shown that IL-17 in association with IFN-γ plays a crucial role in host protection (22). Previously we had shown that immunization with LdCen−/− in animal models induces Th1 response as indicated by IFN-γ-producing CD4 and CD8 T cells in BALB/c mice (6–9). To test whether both Th1 and Th17 responses can simultaneously be induced due to LdCen−/− immunization, in the current study we analyzed Th1 response along with Th17 in C57BL/6 mice. Interestingly, we observed that LdCen−/− immunization induces IL-17 secretion from both CD4 and CD8 T cells as early as 2 wk postimmunization even before induction of IFN-γ, confirming previous studies with H. pylori infection (14). We found that there was no difference in the quality and magnitude of IFN-γ response either in the presence of IL-17 induction or due to difference in the strain of mice used. In addition, treatment with Abs to IFN-γ resulted in the abrogation of both IL-17 and IFN-γ production and lack of protection against L. donovani infection suggesting that IFN-γ and IL-17 may work synergistically in LdCen−/−-mediated protection. It is important to note that we did not observe IL-17 and IFN-γ double-producing T cells upon immunization with LdCen−/− parasites.
In summary, we have systematically demonstrated that vaccination with LdCen−/− parasite strongly induces both innate and adaptive immunity to produce Th17 cytokines from both CD4 and CD8 cells along with other Th1 cytokines. The ultimate effector function to kill L. donovani parasite involves both Th17 and Th1 cells. Further, IL-23 plays an important role in IL-17-mediated immune protection induced by LdCen−/−. Hence, Th17-IL-23 immune pathway should be explored further as an important target for vaccine development against VL.
Supplementary Material
Acknowledgments
We thank Amgen for providing the anti-IL-17 mAb. We thank Prof. Vijay Kuchroo for helpful discussion throughout the study and providing IL-23R−/− mice used in this study. Critical review of the manuscript by Dr. Sreenivas Gannavaram is highly appreciated.
This work was supported by intramural funds and the Critical Path Initiative of the Center for Biologics Evaluation and Research, U.S. Food and Drug Administration. A.B. received a fellowship from the University Grants Commission, Government of India as a Raman Postdoctoral Fellow under the Indo-U.S. 21st Century Knowledge Initiative program for postdoctoral studies in the United States.
The findings of this study are an informal communication and represent the authors’ own best judgments. These comments do not bind or obligate the Food and Drug Administration.
Abbreviations used in this article
- DC
dendritic cell
- FTAg
freeze-thaw Ag
- LdCen−/−
centrin gene-deleted Leishmania donovani
- LdWT
wild-type Leishmania donovani
- nTreg
naturally occurring regulatory T
- RFP
red fluorescent protein
- VL
visceral leishmaniasis
Footnotes
Disclosures
The authors have no financial conflicts of interest.
The online version of this article contains supplemental material.
References
- 1.Marinho DS, Casas CN, Pereira CC, and Leite IC 2015. Health economic evaluations of visceral leishmaniasis treatments: a systematic review. PLoS Negl. Trop. Dis 9: e0003527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh OP, Singh B, Chakravarty J, and Sundar S 2016. Current challenges in treatment options for visceral leishmaniasis in India: a public health perspective. Infect. Dis. Poverty 5: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.No JH 2016. Visceral leishmaniasis: revisiting current treatments and approaches for future discoveries. Acta Trop. 155: 113–123. [DOI] [PubMed] [Google Scholar]
- 4.Saljoughian N, Taheri T, and Rafati S 2014. Live vaccination tactics: possible approaches for controlling visceral leishmaniasis. Front. Immunol 5: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khamesipour A, Rafati S, Davoudi N, Maboudi F, and Modabber F 2006. Leishmaniasis vaccine candidates for development: a global overview. Indian J. Med. Res 123: 423–38. [PubMed] [Google Scholar]
- 6.Selvapandiyan A, Dey R, Nylen S, Duncan R, Sacks D, and Nakhasi HL 2009. Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis. J. Immunol 183: 1813–1820. [DOI] [PubMed] [Google Scholar]
- 7.Dey R, Natarajan G, Bhattacharya P, Cummings H, Dagur PK, Terrazas C, Selvapandiyan A, McCoy JP Jr., Duncan R, Satoskar AR, and Nakhasi HL 2014. Characterization of cross-protection by genetically modified live-attenuated Leishmania donovani parasites against Leishmania mexicana. J. Immunol 193: 3513–3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiuza JA, Gannavaram S, Santiago HC, Selvapandiyan A, Souza DM, Passos LS, de Mendonca LZ, Lemos-Giunchetti DS, Ricci ND, Bartholomeu DC, et al. 2015. Vaccination using live attenuated Leishmania donovani centrin deleted parasites induces protection in dogs against Leishmania infantum. Vaccine 33: 280–288. [DOI] [PubMed] [Google Scholar]
- 9.Fiuza JA, Dey R, Davenport D, Abdeladhim M, Meneses C, Oliveira F, Kamhawi S, Valenzuela JG, Gannavaram S, and Nakhasi HL 2016. Intradermal immunization of Leishmania donovani centrin knock-out parasites in combination with salivary protein LJM19 from sand fly vector induces a durable protective immune response in hamsters. PLoS Negl. Trop. Dis 10: e0004322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaye PM, and Aebischer T 2011. Visceral leishmaniasis: immunology and prospects for a vaccine. Clin. Microbiol. Infect 17: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 11.Kaye PM, and Beattie L 2016. Lessons from other diseases: granulomatous inflammation in leishmaniasis. Semin. Immunopathol 38: 249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanley AC, and Engwerda CR 2007. Balancing immunity and pathology in visceral leishmaniasis. Immunol. Cell Biol 85: 138–147. [DOI] [PubMed] [Google Scholar]
- 13.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol 8: 369–377. [DOI] [PubMed] [Google Scholar]
- 14.DeLyria ES, Redline RW, and Blanchard TG 2009. Vaccination of mice against H. pylori induces a strong Th-17 response and immunity that is neutrophil-dependent. Gastroenterology 136: 247–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, and Pier GB 2008. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J. Immunol 181: 4965–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai CW, Blase JR, Zhang X, Eickhoff CS, and Hoft DF 2016. Th17 cells are more protective than Th1 cells against the intracellular parasite Trypanosoma cruzi. PLoS Pathog. 12: e1005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nanjappa SG, Hernandez-Santos N, Galles K, Wuthrich M, Suresh M, and Klein BS 2015. Intrinsic MyD88-Akt1-mTOR signaling coordinates disparate Tc17 and Tc1 responses during vaccine immunity against fungal pneumonia. PLoS Pathog. 11: e1005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korn T, Bettelli E, Oukka M, and Kuchroo VK 2009. IL-17 and Th17 cells. Annu. Rev. Immunol 27: 485–517. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Korn T, Oukka M, and Kuchroo VK 2008. Induction and effector functions of T(H)17 cells. Nature 453: 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki Y, Hamano S, Wang S, Shimanoe Y, Iwakura Y, and Yoshida H 2010. IL-17 is necessary for host protection against acute-phase Trypanosoma cruzi infection. J. Immunol 185: 1150–1157. [DOI] [PubMed] [Google Scholar]
- 21.Pitta MG, Romano A, Cabantous S, Henri S, Hammad A, Kouriba B, Argiro L, el Kheir M, Bucheton B, Mary C, et al. 2009. IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J. Clin. Invest 119: 2379–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nascimento MS, Carregaro V, Lima-Junior DS, Costa DL, Ryffel B, Duthie MS, de Jesus A, de Almeida RP, and da Silva JS 2015. Interleukin 17A acts synergistically with interferon γ to promote protection against Leishmania infantum infection. J. Infect. Dis 211: 1015–1026. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh K, Sharma G, Saha A, Kar S, Das PK, and Ukil A 2013. Successful therapy of visceral leishmaniasis with curdlan involves T-helper 17 cytokines. J. Infect. Dis 207: 1016–1025. [DOI] [PubMed] [Google Scholar]
- 24.Sacramento LA, Cunha FQ, de Almeida RP, da Silva JS, and Carregaro V 2014. Protective role of 5-lipoxigenase during Leishmania infantum infection is associated with Th17 subset. BioMed Res. Int 2014: 264270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallick S, Dutta A, Chaudhuri A, Mukherjee D, Dey S, Halder S, Ghosh J, Mukherjee D, Sultana SS, Biswas G, et al. 2016. Successful therapy of murine visceral leishmaniasis with astrakurkurone, a triterpene isolated from the mushroom astraeus hygrometricus, involves the induction of protective cell-mediated immunity and TLR9. Antimicrob. Agents Chemother 60: 2696–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jawed JJ, Majumder S, Bandyopadhyay S, Biswas S, Parveen S, and Majumdar S 2016. SLA-PGN-primed dendritic cell-based vaccination induces Th17-mediated protective immunity against experimental visceral leishmaniasis: a crucial role of PKCβ. Pathog. Dis 74: ftw041. [DOI] [PubMed] [Google Scholar]
- 27.Lopez Kostka S, Dinges S, Griewank K, Iwakura Y, Udey MC, and von Stebut E 2009. IL-17 promotes progression of cutaneous leishmaniasis in susceptible mice. J. Immunol 182: 3039–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stäger S, and Rafati S 2012. CD8(+) T cells in leishmania infections: friends or foes? Front. Immunol 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh N, Glosson NL, Wang N, Guindon L, McKinley C, Hamada H, Li Q, Dutton RW, Shrikant P, Zhou B, et al. 2010. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J. Immunol 185: 2089–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamada H, Bassity E, Flies A, Strutt TM, Garcia-Hernandez ML, McKinstry KK, Zou T, Swain SL, and Dutton RW 2013. Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J. Immunol 190: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srenathan U, Steel K, and Taams LS 2016. IL-17+ CD8+ T cells: differentiation, phenotype and role in inflammatory disease. Immunol. Lett 178: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen HR, Harris TJ, Wada S, Grosso JF, Getnet D, Goldberg MV, Liang KL, Bruno TC, Pyle KJ, Chan SL, et al. 2009. Tc17 CD8 T cells: functional plasticity and subset diversity. J. Immunol 183: 7161–7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stritesky GL, Yeh N, and Kaplan MH 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol 181: 5948–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glosson-Byers NL, Sehra S, and Kaplan MH 2014. STAT4 is required for IL-23 responsiveness in Th17 memory cells and NKT cells. JAK-STAT 3: e955393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chagas AC, Oliveira F, Debrabant A, Valenzuela JG, Ribeiro JM, and Calvo E 2014. Lundep, a sand fly salivary endonuclease increases Leishmania parasite survival in neutrophils and inhibits XIIa contact activation in human plasma. PLoS Pathog. 10: e1003923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvapandiyan A, Duncan R, Debrabant A, Bertholet S, Sreenivas G, Negi NS, Salotra P, and Nakhasi HL 2001. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J. Biol. Chem 276: 43253–43261. [DOI] [PubMed] [Google Scholar]
- 37.Arora P, and Porcelli SA 2016. An efficient and high yield method for isolation of mouse dendritic cell subsets. J. Vis. Exp 110: e53824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Späth GF, and Beverley SM 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol 99: 97–103. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharya P, Dey R, Dagur PK, Kruhlak M, Ismail N, Debrabant A, Joshi AB, Akue A, Kukuruga M, Takeda K, et al. 2015. Genetically modified live attenuated Leishmania donovani parasites induce innate immunity through classical activation of macrophages that direct the Th1 response in mice. Infect. Immun 83: 3800–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blum JS, Wearsch PA, and Cresswell P 2013. Pathways of antigen processing. Annu. Rev. Immunol 31: 443–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo B 2016. IL-10 modulates Th17 pathogenicity during autoimmune diseases. J. Clin. Cell. Immunol 7: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owens BM, Beattie L, Moore JW, Brown N, Mann JL, Dalton JE, Maroof A, and Kaye PM 2012. IL-10-producing Th1 cells and disease progression are regulated by distinct CD11c+ cell populations during visceral leishmaniasis. PLoS Pathog. 8: e1002827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nylén S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, and Sacks D 2007. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25 + (Foxp3) regulatory T cells in human visceral leishmaniasis. J. Exp. Med 204: 805–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maurya R, Kumar R, Prajapati VK, Manandhar KD, Sacks D, Sundar S, and Nylen S 2010. Human visceral leishmaniasis is not associated with expansion or accumulation of Foxp3+ CD4 cells in blood or spleen. Parasite Immunol. 32: 479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colmenares M, Kima PE, Samoff E, Soong L, and McMahon-Pratt D 2003. Perforin and gamma interferon are critical CD8+ T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun 71: 3172–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, Porth K, Boniface K, Mattson J, Basham B, et al. 2013. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Reports 3: 1378–1388. [DOI] [PubMed] [Google Scholar]
- 47.Szabo SJ, Sullivan BM, Peng SL, and Glimcher LH 2003. Molecular mechanisms regulating Th1 immune responses. Annu. Rev. Immunol 21: 713–758. [DOI] [PubMed] [Google Scholar]
- 48.Arango Duque G, and Descoteaux A 2014. Macrophage cytokines: involvement in immunity and infectious diseases. Front. Immunol 5: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilke CM, Bishop K, Fox D, and Zou W 2011. Deciphering the role of Th17 cells in human disease. Trends Immunol. 32: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, et al. 2008. IL-17A produced by gammadelta T cells plays a critical role in innate immunity against listeria monocytogenes infection in the liver. J. Immunol 181: 3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin W, and Dong C 2013. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect 2: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khader SA, and Gopal R 2010. IL-17 in protective immunity to intracellular pathogens. Virulence 1: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, Guglani L, Alcorn JF, Strawbridge H, Park SM, et al. 2009. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31: 799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai H, Cheng J, Gao X, Joyee AG, Fan Y, Wang S, Jiao L, Yao Z, and Yang X 2009. IL-17/Th17 promotes type 1 T cell immunity against pulmonary intracellular bacterial infection through modulating dendritic cell function. J. Immunol 183: 5886–5895. [DOI] [PubMed] [Google Scholar]
- 55.Godinez I, Raffatellu M, Chu H, Paixao TA, Haneda T, Santos RL, Bevins CL, Tsolis RM, and Baumler AJ 2009. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect. Immun 77: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meeks KD, Sieve AN, Kolls JK, Ghilardi N, and Berg RE 2009. IL-23 is required for protection against systemic infection with Listeria monocytogenes. J. Immunol 183: 8026–8034. [DOI] [PubMed] [Google Scholar]
- 57.Gorak PM, Engwerda CR, and Kaye PM 1998. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur. J. Immunol 28: 687–695. [DOI] [PubMed] [Google Scholar]
- 58.León B, Lopez-Bravo M, and Ardavin C 2007. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity 26: 519–531. [DOI] [PubMed] [Google Scholar]
- 59.Katara GK, Ansari NA, Singh A, Ramesh V, and Salotra P 2012. Evidence for involvement of Th17 type responses in post kala azar dermal leishmaniasis (PKDL). PLoS Negl. Trop. Dis 6: e1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banerjee A, Bhattacharya P, Joshi AB, Ismail N, Dey R, and Nakhasi HL 2016. Role of pro-inflammatory cytokine IL-17 in Leishmania pathogenesis and in protective immunity by Leishmania vaccines. Cell. Immunol 309: 37–41. [DOI] [PubMed] [Google Scholar]
- 61.Mills KH 2008. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol 38: 2636–2649. [DOI] [PubMed] [Google Scholar]
- 62.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, and Weiner HL 2009. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol 183: 2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. 2006. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat. Immunol 7: 937–945. [DOI] [PubMed] [Google Scholar]
- 64.Faleiro RJ, Kumar R, Hafner LM, and Engwerda CR 2014. Immune regulation during chronic visceral leishmaniasis. PLoS Negl. Trop. Dis 8: e2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Avishek K, Kaushal H, Gannavaram S, Dey R, Selvapandiyan A, Ramesh V, Negi NS, Dubey US, Nakhasi HL, and Salotra P 2016. Gene deleted live attenuated Leishmania vaccine candidates against visceral leishmaniasis elicit pro-inflammatory cytokines response in human PBMCs. Sci. Rep 6: 33059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamada H, Garcia-Hernandez ML, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, and Dutton RW 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol 182: 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, and Gurney AL 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem 278: 1910–1914. [DOI] [PubMed] [Google Scholar]
- 68.Silva JL, Rezende-Oliveira K, da Silva MV, Gómez-Hernández C, Peghini BC, Silva NM, Mineo JR, and Rodrigues Júnior V 2014. IL-17-expressing CD4+ and CD8+ T lymphocytes in human toxoplasmosis. Mediators Inflamm. 2014: 573825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ansari NA, Kumar R, Gautam S, Nylén S, Singh OP, Sundar S, and Sacks D 2011. IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J. Immunol 186: 3977–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rai AK, Thakur CP, Singh A, Seth T, Srivastava SK, Singh P, and Mitra DK 2012. Regulatory T cells suppress T cell activation at the pathologic site of human visceral leishmaniasis. PLoS One 7: e31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quirino GF, Nascimento MS, Davoli-Ferreira M, Sacramento LA, Lima MH, Almeida RP, Carregaro V, and Silva JS 2016. Interleukin-27 (IL-27) mediates susceptibility to visceral leishmaniasis by suppressing the IL-17-neutrophil response. Infect. Immun 84: 2289–2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boniface K, Bernard FX, Garcia M, Gurney AL, Lecron JC, and Morel F 2005. IL-22 inhibits epidermal differentiation and induces proinflammatory gene expression and migration of human keratinocytes. J. Immunol 174: 3695–3702. [DOI] [PubMed] [Google Scholar]
- 73.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, and Flavell RA 2007. Interleukin-22 butnot interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity 27: 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, and Iwakura Y 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17: 375–387. [DOI] [PubMed] [Google Scholar]
- 75.Terrazas C, Varikuti S, Kimble J, Moretti E, Boyaka PN, and Satoskar AR 2016. IL-17A promotes susceptibility during experimental visceral leishmaniasis caused by Leishmania donovani. FASEB J. 30: 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Khader SA, and Cooper AM 2008. IL-23 and IL-17 in tuberculosis. Cytokine 41: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, and Cua DJ 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol 10: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin Y, Slight SR, and Khader SA 2010. Th17 cytokines and vaccine-induced immunity. Semin. Immunopathol 32: 79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, and Ahmed R 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol 4: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 80.Zrioual S, Toh ML, Tournadre A, Zhou Y, Cazalis MA, Pachot A, Miossec V, and Miossec P 2008. IL-17RA and IL-17RC receptors are essential for IL-17A-induced ELR+ CXC chemokine expression in synoviocytes and are overexpressed in rheumatoid blood. J. Immunol 180: 655–663. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Y, Chen L, Gao W, Hou X, Gu Y, Gui L, Huang D, Liu M, Ren C, Wang S, and Shen J 2012. IL-17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum infected mice. Eur. J. Immunol 42: 1523–1535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.