SUMMARY
Normal mitotic spindle assembly is a prerequisite for faithful chromosome segregation and unperturbed cell cycle progression. Precise functioning of the spindle machinery relies on conserved architectural features such as focused poles, chromosome alignment at the metaphase plate, and proper spindle length. These morphological requirements can be achieved only within a compositionally distinct cytoplasm that results from cell cycle-dependent regulation of specific protein levels and specific post-translational modifications. Here, we used cell-free extracts derived from Xenopus laevis eggs to recapitulate different phases of the cell cycle in vitro and to determine which components are required to render interphase cytoplasm spindle assembly competent in the absence of protein translation. We found that addition of a nondegradable form of the master cell cycle regulator cyclin B1 can indeed induce some biochemical and phenomenological characteristics of mitosis, but cyclin B1 alone is insufficient and actually deleterious at high levels for normal spindle assembly. In contrast, addition of a phosphomimetic form of the Greatwall-kinase effector Arpp19 with a specific concentration of nondegradable cyclin B1 rescued spindle bipolarity but resulted in larger-than-normal bipolar spindles with misalignment of chromosomes. Both were corrected by addition of exogenous Xkid (Xenopus homolog of human Kid/KIF22), indicating a role for this chromokinesin in regulating spindle length. These observations suggest that, of the many components degraded at mitotic exit and then replenished during the subsequent interphase, only a few are required to induce a cell cycle transition that produces a spindle assembly competent cytoplasm.
Graphical Abstract

eTOC Blurb
Addition of Cyclin B to interphase cytoplasm is sufficient to induce biochemical hallmarks of mitosis, but the resulting M-phase cannot produce normal spindles. Here Bisht et al. address the additional components required to make Cyclin B-induced M-phase cytoplasm spindle assembly competent and reveal a role for Xkid in spindle length regulation.
INTRODUCTION
The transition from mitosis to interphase depends on the proteolytic degradation of many proteins and subsequent dephosphorylation of other mitotic regulators [1-3]. Continuation of the cell cycle into the next mitosis and normal mitotic spindle assembly is thought to require the degraded proteins be translated and replenished again during the intervening interphase. Indeed, the APC/CCdc20 protein complex has been shown to mediate the ubiquitin-mediated proteolysis of cyclin A [4], Nek2A [5], HOXC10 [6], and E2F1 [7] during prometaphase, and cyclin B1 [8], cyclin B3 [8], Kif18A [9], and securin [10] during metaphase-anaphase transition. However, in Xenopus egg extracts specifically, only a subset of these proteins has been shown to be degraded by APC/CCdc20 including Nek2A [11], cyclin A [12], cyclin B1 [8], securin [10], and Xkid [13]. The actual list is probably longer, but we do not yet know how many additional proteins are targeted by APC/CCdc20 because a comprehensive list of proteins degraded during this cell cycle transition in extracts is currently unavailable. Regardless, precisely which proteins are indispensable for normal spindle assembly and what their specific contributions might be in defining the characteristic features of a “normal” mitotic spindle remain unanswered questions.
Cyclin B (here used as a generic term to describe any Cyclin B isoform or homolog) is arguably the most crucial protein for entry into mitosis [3, 14]. During interphase, it undergoes continual synthesis, accumulates up to a threshold concentration and concurrently binds Cdk1 kinase, thereby forming the inactive cyclin B–Cdk1 complex [15]. In turn, activation of cyclin B–Cdk1 is mediated by several positive-feedback mechanisms that constitute the Cdk1 autoamplification loop, which is primarily responsible for activation of Cdc25 phosphatase [16] and inactivation of Wee1 and Myt1 kinases [17]. A nondegradable form of cyclin B (e.g., cyclin B1–destruction mutant (dm) or truncated cyclin B) can induce biochemical and morphological hallmarks of mitosis [2, 18]. Many early studies did not specify which cyclin B isoform was being used, but it has since been established that the cyclin B1 isoform can activate Cdk1 in early embryogenesis and in Xenopus egg extracts [8, 19]. However, the cyclin B1 protein alone, either in its WT, dm, or nondegradable form, is insufficient to induce normal spindle assembly, as defined by a spindle with two focused poles, aligned chromosomes, and proper length [8, 20].
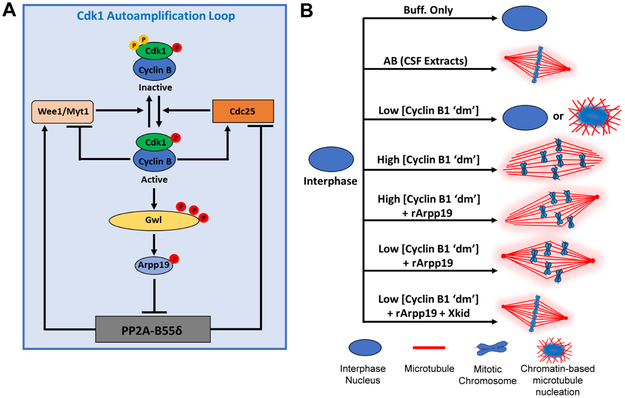
Greatwall (Gwl) kinase is also a critical regulatory component of the circuitry that regulates mitotic onset. Gwl is thought to contribute to the mitotic entry by phosphorylating two small homologous proteins, cAMP-regulated phosphoprotein (Arpp19) and α-Endosulfine, both of which are potent inhibitors of protein phosphatase PP2A-B55δ [21, 22]. Protein phosphatase PP2A-B55δ antagonizes the effects of cyclin B–Cdk1 by dephosphorylating the Cdk1 kinase substrates such as Cdc25 and Wee1/Myt1 [12]. Moreover, Gwl kinase is activated by phosphorylation downstream of cyclin B–Cdk1 and therefore participates in the Cdk1 autoamplification loop by inhibiting PP2A-B55δ [23]. As such, a small population of active cyclin B–Cdk1 complex is initially responsible for activation of the Gwl kinase pathway, which in turn further activates Cdc25 phosphatase and inhibits Wee1/Myt1 kinases, thereby promoting Gwl’s own activation (Refer to Figure 7A).
Xenopus egg extracts contain both Gwl substrates, Arpp19 and α-Endosulfine [21, 24]. Interestingly, depletion of Arpp19, but not of α-Endosulfine, completely inhibits mitotic entry and triggers mitotic exit, implying a role for endogenous Arpp19 in regulating both of these cell cycle transitions [21, 25]. Gwl-dependent phosphorylation of Arpp19 turns the effector into a potent inhibitor of PP2A-B55δ, thereby activating the Cdk1 autoamplification loop [21, 22]. Although Arpp19 is also phosphorylated by Cdk1 and PKA [22], these modifications seem to have relatively little to no effect on PP2A-B55δ activity [26]. Therefore, mitotic entry is mediated by inhibition of PP2A-B55δ through a cyclin B–Cdk1–Gwl–Arpp19/α-Endosulfine cascade, but how this pathway and its components contribute to normal spindle assembly remains poorly understood, due in part to the sheer number of candidate Cdk1 substrates that have been identified during mitosis (>500;[27]). Of these, over 40 are known to associate with the mitotic spindle or microtubule cytoskeleton [27].
In this study, we used Xenopus egg extract as a model system to study various aspects of the cell cycle, especially nuclear and mitotic spindle assembly [28]. The open nature of this system makes it tractable to molecular perturbations such that reagents can be either added or removed from the system with relative ease. These perturbations allow for exquisite control of progression through the cell cycle. Xenopus egg extracts are derived from eggs arrested in metaphase of meiosis II and remain arrested in meiosis because of cytostatic factor (CSF) activity [28]. Addition of calcium releases these CSF-arrested egg extracts from mitotic arrest, allowing them to proceed through the metaphase-to-anaphase transition and finally into interphase. In the presence of an exogenous chromatin source, typically demembranated sperm nuclei [28, 29], adding back an equal volume of fresh CSF-arrested extract induces mitosis and normal spindle assembly around each of the added nuclei [28].
Here we investigated which proteins could be added to interphase extract to induce an M-phase capable of normal spindle assembly in the absence of endogenous interphase protein synthesis. Our results demonstrate that high concentrations of a cyclin B1 destruction mutant (hereafter referred to as cyclin B1 ‘dm’) can indeed induce biochemical features of mitosis but are insufficient for normal spindle assembly. Addition of a phosphomimetic form of Arpp19 reduces the level of cyclin B1 ‘dm’ needed to induce mitosis and rescues spindle bipolarity. However, the bipolar spindles produced by this combination exhibited chromosome alignment defects and were longer than control spindles. These morphological defects were rescued by addition of exogenous chromokinesin Xkid, which is normally degraded during the metaphase-to-anaphase transition. Taken together, these data demonstrate that normal spindle assembly in our system requires initial activation of cyclin-dependent kinase at physiological levels of cyclin B1, a mitotically active state of the Gwl-effector Arpp19, and Xkid.
RESULTS
High levels of Xenopus cyclin B1 ‘dm’ induce mitosis but not normal spindle assembly following addition to interphase extracts.
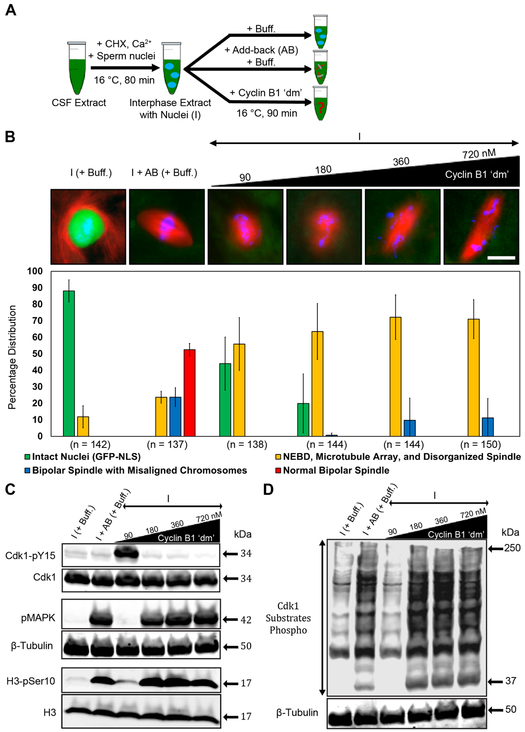
The ability of various forms of cyclin B to induce biochemical and morphological hallmarks of mitosis in Xenopus egg extracts is well documented in the literature [2, 15, 18]. However, it is not known whether cyclin B is sufficient to induce normal bipolar spindle assembly following protein degradation at the preceding metaphase-to-anaphase transition and in the absence of protein synthesis. As such, the question of which interphase-synthesized proteins are actually required for normal spindle assembly remains unanswered. To address this question, we made CSF-arrested egg extracts and released them into interphase via addition of calcium and demembranated Xenopus sperm nuclei. New protein synthesis was inhibited by addition of cycloheximide (CHX; hereafter, extracts treated this way will be referred to as either interphase or “I”; Figure 1A) [28, 30]. Upon completion of nuclear assembly (~80 min later), interphase egg extracts were supplemented with increasing concentrations of nondegradable Xenopus cyclin B1 ‘dm’. We chose a mutated form of the B1 isoform based on its essential role during early embryogenesis [19]. We observed that addition of cyclin B1 ‘dm’ at 90 nM concentration (the reported endogenous concentration of cyclin B1 during the first mitotic cycle is ~80 nM; [31]) promoted partial nuclear envelope breakdown (NEBD) and the formation of microtubule arrays around condensed chromatin clusters (Figure 1B). In contrast, these extracts exhibited many biochemical hallmarks of interphase, including increased inhibitory phosphorylation of Cdk1 on Tyr15 and the absence of phosphorylation of MAPK, of H3 on Ser10, and of Cdk1 substrates (Figures 1C and 1D).
Figure 1. Addition of cyclin B1 ‘dm’ to interphase extract induces mitosis but not normal spindle assembly.
A) Schematic diagram of the experimental protocol. The interphase and add back (AB) control conditions were diluted with 1× PBS buffer (Buff.) equivalent to the maximum volume of recombinant protein(s) added to the interphase extract. See also STAR Methods. B) Immunofluorescence images showing the most abundant structure assembled under the indicated conditions and labeled with Alexa Fluor 568–conjugated tubulin antibody to stain microtubules (red), Hoechst to stain chromosomes (blue), and GST-GFP-NLS to stain intact nuclei (green). Graph shows quantification of the percent of intact nuclei and/or chromatin associated microtubule-based structures assembled during interphase and mitosis for each of the conditions. Error bars represent standard deviation. n = number of nuclei and structures counted for each condition. C, D) Western blot analyses using antibodies against known mitotic markers and respective loading control markers. Labels on the left of blot images indicate the antibody epitopes. Data were taken from a minimum of three different extracts and blots shown are representative of three blots from independent extracts. See also Figure S5.
These results suggest that at this concentration of cyclin B1 ‘dm’ (90 nM; hereafter referred to as a “low concentration”), Cdk1 forms a complex with cyclin B1 ‘dm’ but remains in a partially active state, which is likely a result of activation of the Wee1/Myt1 kinases [32]. In contrast, addition of cyclin B1 ‘dm’ at concentrations greater than 90 nM, i.e., 180–720 nM (hereafter referred to as “higher concentrations”), resulted in the majority of nuclei undergoing NEBD, along with the formation of microtubule arrays around a larger proportion of chromatin clusters, some disorganized spindles, and even a small percentage of bipolar spindles with morphological aberrations including misaligned chromosomes and longer-than-normal pole-to-pole lengths (Figure 1B). These observations are consistent with spindle abnormalities seen in Xenopus following addition of nondegradable forms of cyclin B (e.g., cyclin B1 ‘dm’ or truncated cyclin B) [8, 20]. However, with higher concentrations of cyclin B1 ‘dm’, we observed several of the same biochemical hallmarks of mitosis induced by adding back an equal volume of cytostatic factor- (CSF) arrested extract (hereafter referred to as “AB”; Figures 1C and 1D). More specifically, we found that higher concentrations of cyclin B1 ‘dm’ resulted in reduced levels of inhibitory phosphorylation on Tyr15 of Cdk1 and increased immunoreactivity of phospho-specific antibodies against phosphorylated MAPK, phosphorylated Ser10 in histone H3, and phosphorylated Cdk1 substrates (Figures 1C and 1D). Collectively, these results indicate that although higher concentrations of cyclin B1 ‘dm’ can induce biochemical mitosis as well as NEBD, they are insufficient to support normal bipolar spindle assembly, suggesting that additional components might be necessary to recapitulate the process more accurately.
Excess cyclin B1 is deleterious for normal bipolar spindle assembly in Xenopus egg extracts.
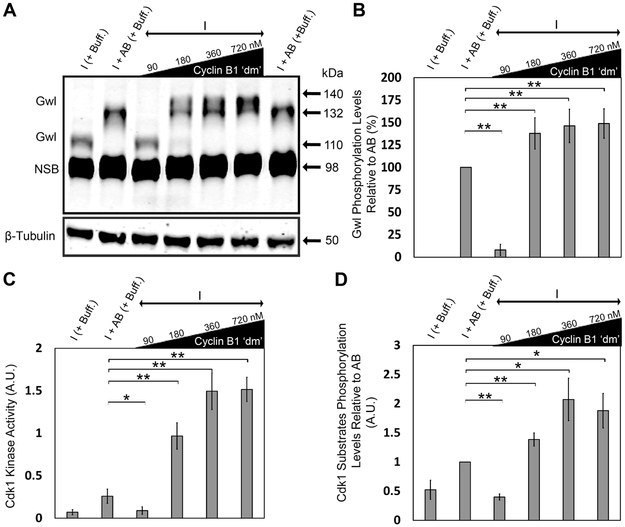
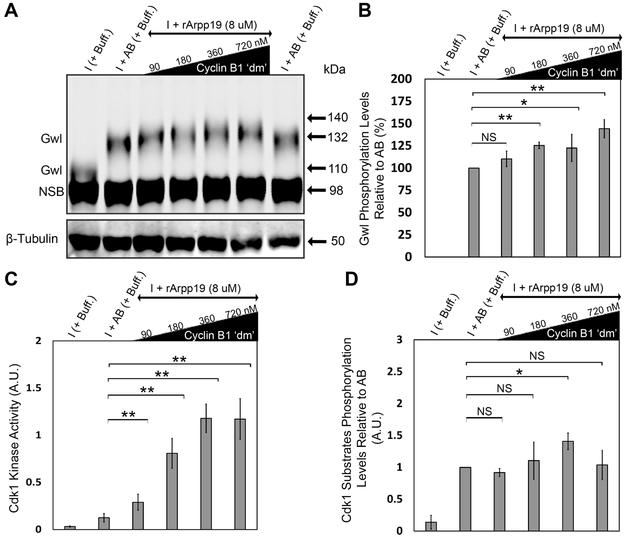
Proper progression through the cell cycle requires the activity of both Cdk1 and Gwl kinases [23]. Activation of Gwl during mitosis involves its phosphorylation at multiple sites by Cdk1 kinase and a concomitant electrophoretic mobility shift (Figure S1) [23]. To determine the phosphorylation state of Gwl in response to increasing concentrations of cyclin B1 ‘dm’, we added different concentrations of cyclin B1 ‘dm’ and looked for resulting changes in the electrophoretic mobility retardation of the kinase (hereafter referred to as “Gwl phosphorylation”). We observed that, at the low concentration of cyclin B1 ‘dm’, Gwl migrates with the same mobility as interphase Gwl, indicating that the kinase remains in its inactive state under these conditions. Surprisingly, at higher concentrations of cyclin B1 ‘dm’, we detected hyperphosphorylation of Gwl compared to the add-back control (Figures 2A and 2B). We also assessed Cdk1 kinase activity via its ability to phosphorylate a synthetic peptide as a substrate (see Experimental Procedures) in the presence of increasing concentrations of cyclin B1 ‘dm’. Higher concentrations of cyclin B1 ‘dm’ indeed increased the activity of Cdk1 kinase relative to the add-back control (Figure 2C), as shown previously [15, 33]. Similarly, following addition of higher concentrations of cyclin B1 ‘dm’ to interphase extracts, we observed an increasing trend in the Cdk1 substrates phosphorylation levels relative to the add-back control (Figures 1D and 2D). These results suggest that high concentrations of cyclin B1 ‘dm’ increase the kinase activity of Cdk1 relative to that achieved during a normal physiological mitosis, leading to hyperphosphorylation of Gwl and increased levels of Cdk1 substrate phosphorylation.
Figure 2. High levels of cyclin B1 ‘dm’ result in hyperphosphorylation of Gwl kinase and increases in Cdk1 kinase activity and Cdk1 substrates phosphorylation levels relative to add-back control.
A) Gwl phosphorylation levels under different conditions. B) Quantification of Gwl phosphorylation levels as a measure of the densitometric center of mass of the upper Gwl band (at ~135 kDa) along the vertical y-axis relative to that of the add-back control band (at ~132 kDa). C) Quantification of Cdk1 kinase activity as a measure of its ability to phosphorylate synthetic peptide substrate (using MESACUP Cdk1 Kinase Assay Kit) in different conditions. D) Quantification of the Cdk1 substrates phosphorylation levels in different conditions normalized to β-tubulin. Error bars represent standard deviation. Data were taken from a minimum of three different extracts and blots shown are representative of three blots from independent extracts. NSB, non-specific band. NS, not significant (p > 0.05), *p < 0.05, and **p < 0.005, as determined using the Student’s t-test. See also Figures S1 and S5.
In contrast to its low-concentration effect, increasing concentrations of cyclin B1 ‘dm’ resulted in a significant decrease in the percentage of normal bipolar spindles assembled in the extract as compared with normal add-back only controls (Figure 3A). We also noticed increased Gwl phosphorylation levels and a positive correlation between Cdk1 kinase activity and increasing concentrations of cyclin B1 ‘dm’ relative to the add-back control (Figures 3B, 3C, and 3D). These observations suggest that high, non-physiological levels of cyclin B1–mediated Cdk1 kinase activity are deleterious for normal spindle assembly, supporting the notion that some initial trigger or activation is required to induce mitosis and normal spindle assembly at a physiologically relevant concentration of cyclin B1 ‘dm’.
Figure 3. Excess cyclin B1 ‘dm’ is deleterious for normal spindle assembly in the add-back condition.
A) Immunofluorescence images showing the most abundant structure assembled under indicated conditions and labeled with Alexa Fluor 568–conjugated tubulin to stain microtubules (red) and Hoechst to stain chromosomes (blue). Graph shows quantification of the percent of chromatin associated microtubule-based structures assembled during mitosis for each of the conditions. Error bars represent standard deviation. Scale bar represents 20 μm. n = number of nuclei and structures counted for each condition. B) Extract samples from different conditions were subjected to western blotting using antibodies against Gwl. C) Quantification of Gwl phosphorylation levels as a measure of the densitometric center of mass of the upper Gwl band (at ~135 kDa) along the vertical axis relative to that of the add-back control band (at ~132 kDa). D) Quantification of Cdk1 kinase activity as a measure of its ability to phosphorylate synthetic peptide substrate (using MESACUP Cdk1 Kinase Assay Kit; see Experimental Procedures) under the indicated experimental conditions. Error bars represent standard deviation. Data were taken from a minimum of three different extracts and blots shown are representative of three blots from independent extracts. NSB, non-specific band. NS, not significant (p > 0.05), *p < 0.05, and **p < 0.005, as determined using the Student’s t-test. See also Figure S1.
A phosphomimetic form of the Gwl effector Arpp19 together with cyclin B1 ‘dm’ induces mitosis and bipolar spindle assembly.
To determine whether Gwl activity might be important for the induction of normal spindle assembly in extracts, we made a phosphomimetic “active” form of Arpp19 (hereafter referred to as rArpp19), one of only two known downstream effectors of the kinase [21, 22, 24], as a proxy for active Gwl kinase. Specifically, serines at positions 28 and 67 of wild-type Arpp19 were mutated to aspartates to mimic specific phosphorylation events by Cdk1 and Gwl, respectively [26, 34]. Additionally, the serine at position 109 was mutated to alanine to prevent a known inhibitory phosphorylation by PKA [35]. We did not consider creating a phosphomimetic mutant of Endosulfine, the other known Gwl kinase target, because α-Endosulfine does not seem to be involved in the regulation of mitotic entry and exit in Xenopus egg extracts [21].
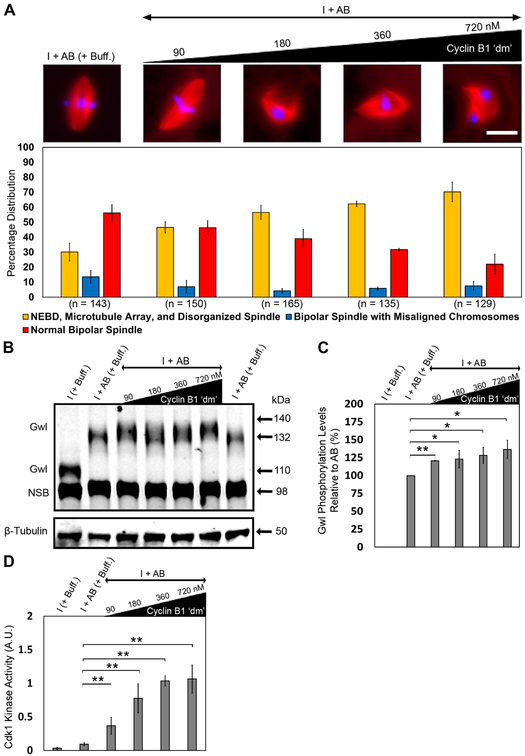
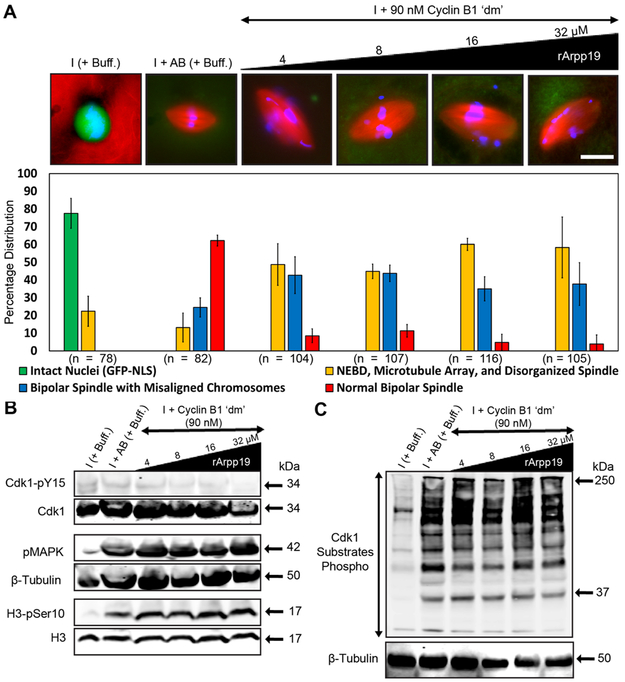
By itself, the low concentration of cyclin B1 ‘dm’ was unable to induce mitotic entry (Figure 1B); consistent with this observation, the same concentration promoted increased inhibitory phosphorylation on Tyr15 of Cdk1 (Figure 1C), corresponding to an inactive Cdk1 autoamplification loop. To determine whether rArrp19 together with the low concentration of cyclin B1 ‘dm’ could activate the Cdk1 autoamplification loop, we added increasing concentrations of rArpp19 (4–32 μM) to calcium-induced, CHX-treated interphase egg extracts while keeping the concentration of cyclin B1 ‘dm’ constant at 90 nM. The endogenous concentration of Arpp19 in Xenopus egg extracts has to our knowledge not been reported previously, however the Arpp19 concentration used in our study is in accord with a previously published research, where ~3.86 μM Arpp19 was used to induce mitosis in translationally active interphase extracts [21]. In contrast to extracts treated with rArpp19 alone (Figure S2), those that were treated with rArpp19 and cyclin B1 ‘dm’ resulted in the induction of mitosis as shown by the expected pattern of specific mitotic phosphorylation events (Figure 4B and 4C). Surprisingly, we observed a rescue of spindle bipolarity under these conditions, although other morphological spindle defects, namely chromosome misalignment and longer pole-to-pole lengths, were still present (Figure 4A). Quantification of these defects confirmed our initial qualitative assessment (Figure S3).
Figure 4. rArpp19 with a low cyclin B1 ‘dm’ concentration induces mitosis and generates bipolar spindles with defects in length and chromosome alignment.
A) Immunofluorescence images showing the most abundant structure assembled under the indicated conditions and labeled with Alexa Fluor 568–conjugated tubulin to stain microtubules (red), Hoechst to stain chromosomes (blue), and GST-GFP-NLS to stain intact nuclei (green). Graph shows quantification of the percent of intact nuclei and/or chromatin associated microtubule-based structures assembled during interphase and mitosis for each of the conditions. Error bars represent standard deviation. n = number of nuclei and structures counted for each condition. B, C) Extract samples from different conditions were subjected to western blotting using specific antibodies against mitotic markers and respective loading control markers. Labels on the left of blot images indicate the antibody epitopes. Data were taken from a minimum of three different extracts and blots shown are representative of three blots from independent extracts. See also Figures S2, S3, S4, and S6.
To determine whether these two kinds of spindle defects could be rescued by adjusting cyclin B1 ‘dm’ concentrations, we added an increasing concentration of cyclin B1 ‘dm’ (90–720 nM) while keeping the rArpp19 concentration constant at 8 μM. This concentration was chosen because of the higher percentage of bipolar spindles assembled at 8 μM rArpp19 together with 90 nM of cyclin B1 ‘dm’ (Figure 4A). Under these conditions, bipolarity was rescued; however, chromosome misalignment and defects in spindle length persisted (Figure S4A). We confirmed induction of mitosis by assessing the pattern of specific biochemical markers of mitosis (Figures S4B and S4C). Interestingly, increasing concentrations of cyclin B1 ‘dm’ resulted in a concomitant reduction in the percentage of bipolar spindles (but with phenotypic defects) and an increase in the percentage of microtubule arrays and disorganized spindles. This observation is consistent with our previous findings that high cyclin B1 ‘dm’ levels are deleterious for normal spindle assembly. It also demonstrates that the presence of rArpp19 reduces the threshold concentration of exogenous cyclin B1 ‘dm’ required for the induction of mitosis, as shown previously [36-38] and rescues bipolar spindle assembly.
In addition to the western blot analyses of mitosis-specific phosphorylation events, we also measured Gwl phosphorylation levels, Cdk1 kinase activity, and the Cdk1 substrates phosphorylation levels in the presence of an increasing concentration of cyclin B1 ‘dm’ (90–720 nM) and a constant rArpp19 concentration of 8 μM (Figure 5 and Figure S4C). Compared to cyclin B1 ‘dm’ alone at low concentration (i.e., Figure 2), we found increases in Gwl phosphorylation, Cdk1 kinase activity, and Cdk1 substrate phosphorylation, all at levels similar to those achieved in the add-back control. However, comparisons between higher concentrations of cyclin B1 ‘dm’ alone and higher concentrations of cyclin B1 ‘dm’ alone with 8 μM rArpp19 showed no significant differences. (Figures 5B, 5C, 5D, and Figure S4C). Comparative analysis of Figure 2B-D and Figure 5B-D is represented in Figure S5. These results indicate that rArpp19 together with the low concentration of cyclin B1 ‘dm’ reduces the hyperphosphorylation of Gwl kinase, Cdk1 kinase activity, and the Cdk1 substrate phosphorylation levels such that they are comparable to the normal spindle assembly–competent add-back control.
Figure 5. A low concentration of cyclin B1 ‘dm’ together with rArpp19 results in the reduction of Gwl kinase hyperphosphorylation and decreases in Cdk1 kinase activity and Cdk1 substrates phosphorylation levels.
A) Western blot analysis showing Gwl phosphorylation levels under the indicated experimental conditions. B) Quantification of Gwl phosphorylation levels as a measure of the densitometric center of mass of the upper Gwl band (at ~135 kDa) along the vertical axis relative to that of the add-back control band (at ~132 kDa). C) Quantification of Cdk1 kinase activity as a measure of its ability to phosphorylate synthetic peptide substrate (using MESACUP Cdk1 Kinase Assay Kit; see Experimental Procedures) under the indicated experimental conditions. D) Densitometric quantification of the Cdk1 substrates phosphorylation levels under different conditions normalized to β-tubulin loading controls. Error bars represent standard deviation. Data were taken from a minimum of three different extracts and blots shown are representative of three blots from independent extracts. NSB, non-specific band. NS, not significant (p > 0.05), *p < 0.05, and **p < 0.005, as determined using the Student’s t-test. See also Figures S1, S4, and S5.
Addition of exogenous Xkid is sufficient to rescue chromosome alignment and normal spindle length.
The observed chromosome alignment defect in our cyclin B1 ‘dm’ and rArpp19 spindles is consistent with reduced sister chromatid cohesion and/or diminished polar ejection forces (PEFs)[39]. Measurements of interkinetochore distances in spindles assembled under these conditions revealed no differences as compared with add-back controls (Figure S6), suggesting that issues with cohesion were not responsible for the aberrant chromosome spread. We then focused subsequent investigation on whether aberrant PEFs might be responsible. Previous studies in human cell lines and in vitro assays have shown that the chromokinesin Kid (KIF22) is responsible for generating PEFs [40]. In Xenopus egg extracts, Xkid is responsible for generating PEFs, and depletion of Xkid results in the formation of spindles with misaligned chromosomes [13]. Additionally, Xkid is the target of proteasomal degradation during the metaphase-to-anaphase transition in Xenopus egg extracts [13]. To confirm that Xkid is degraded following the progression of CSF-arrested extracts into interphase, we compared Xkid protein levels in CSF-arrested, interphase, and add-back extracts via western blotting. Xkid levels were indeed reduced in interphase extracts, suggesting that the protein is degraded at this transition (Figure S7). Importantly, the level of Xkid was also reduced in interphase extracts following the addition of cyclin B1 ‘dm’ and rArpp19 (Figure S7).
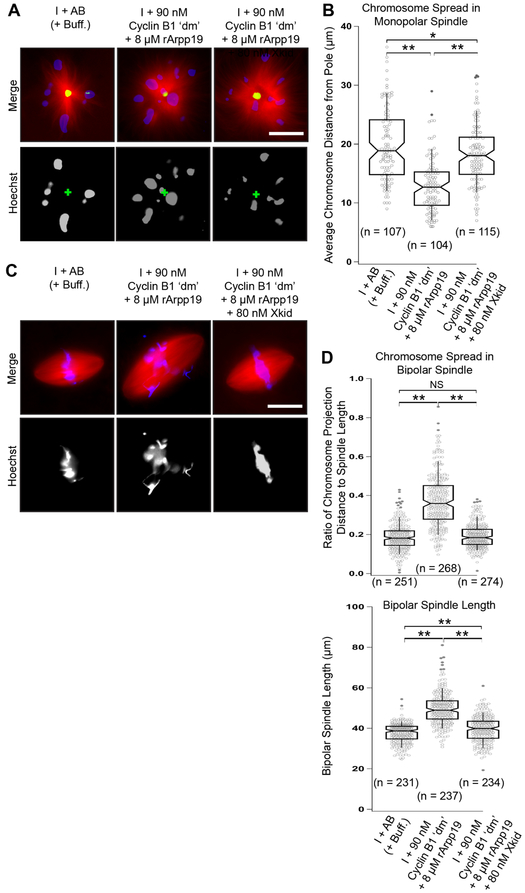
To determine whether reduced PEF levels were indeed responsible for the observed chromosome misalignment defect, we measured the pole-to-kinetochore distances in Eg5-inhibited monopolar spindles. In this well-established assay, the chromosomes are mono-oriented and typically positioned some distance away from the monopole center, with the distance being proportional to the magnitude of the PEFs acting on the chromosome [41]. We found that in the absence of supplemental exogenous Xkid, chromosomes were closer to the pole compared with chromosomes on monopoles assembled in the add-back control and in interphase extract containing cyclin B1, rArpp19, and supplemented exogenous Xkid at 80 Nm (Figures 6A and 6B). We note that the concentration of Xkid in Xenopus egg extracts has been reported to range from about 10 to 56 nM [13, 42].
Figure 6. Xkid is sufficient to rescue both chromosome misalignment and normal spindle length.
A) Immunofluorescence images showing representative monopolar spindles (induced by addition of the Eg5 inhibitor s-trytl-l-cysteine (STLC)) labeled with Alexa Fluor 488–conjugated anti-NuMA (green), Hoechst to stain chromosomes (blue), and Alexa Fluor 568–conjugated tubulin to stain microtubules (red). B) Quantification of the chromosome spread. Plots show measures of the distance between each monopolar spindle pole and chromosome center. For each boxplot, the top and bottom edges represent the 75th and 25th quartile boundaries, respectively. Whiskers extend to the 91st and 9th quartiles. Center lines represent median values, and notch widths correspond to 95% confidence intervals. n = the number of individual measurements of chromosome-to-pole distance made for each condition. C) Immunofluorescence images of assembled bipolar spindles labeled with Alexa Fluor 568–conjugated tubulin to stain microtubules (red) and Hoechst to stain chromosomes (blue). D) Quantification of the chromosome spread and spindle length of bipolar spindles under different conditions. Boxplot characteristics are as in B. n = the number of bipolar spindles examined. Data were gathered from a minimum of three different extracts. NS, not significant (p > 0.05), *p < 0.05, and **p < 0.005, as determined using the Student’s t-test. See also Figures S6 and S7.
The question remained as to whether exogenous Xkid could rescue the chromosome alignment defects in bipolar spindles assembled in mitotic extracts induced by low cyclin B1 ‘dm’ and rArpp19. Addition of exogenous Xkid at 80 nM was sufficient to rescue chromosome misalignment and led to normal metaphase spindle length as well (Figures 6C and 6D). In summary, these data support the conclusion that the chromosome alignment defects observed for spindles induced in the presence of cyclin B1 ‘dm’ and rArpp19 are the result of decreased Xkid levels and a reduction in chromokinesin-generated PEFs. Surprisingly, these findings also reveal a novel role for Xkid in metaphase spindle length regulation.
DISCUSSION
Cyclin B1 alone is insufficient to induce a spindle assembly competent M-phase
Our results indicate that in the absence of protein translation, addition of cyclin B1 ‘dm’ alone, at any of the concentrations tested, is not sufficient to generate a M-phase extract capable of normal spindle assembly despite possessing (particularly at higher concentrations) many of the characteristic biochemical hallmarks of proper M-phase. This result is in agreement with previous observations from several similar experiments. For example, spindles assembled in Xenopus oocytes following injection of mRNA encoding cyclin B1 ‘dm’ were longer than expected [8] and addition of cyclin BΔ90 (a non-degradable form of sea urchin cyclin B) to Xenopus egg extracts was found to produce spindles with misaligned chromosomes [20]. Additionally, in starfish oocytes, injection of tenfold excess of purified Cdk1 is required to induce the G2-M transition and NEBD, however, the large excess is unable to induce normal spindle assembly in this system [38]. It should be noted that cyclin B1 ‘dm’ treatment alone can indeed activate Gwl kinase activity, but the high, non-physiological concentrations of cyclin B1 ‘dm’ required to do so result in hyperphosphorylation Gwl and probably other Cdk1 substrates. In summary, these results suggest that components other than cyclin B1 are likely required to properly govern Cdk1-dependent Gwl phosphorylation levels and produce extracts capable of normal spindle assembly.
The relationship between Gwl activity and spindle bipolarity
The observation that the Gwl substrate rArpp19, in combination with cyclin B1 ‘dm’, rescues spindle bipolarity suggests an important role for Arpp19 in determining spindle morphology, but why does this combination rescue spindle bipolarity whereas addition of cyclin B1 ‘dm’ alone does not? Recent work has characterized the mitotic entry regulatory circuit as comprising two interdependent bistable switches – one regulating the auto-activation of Cdk1 and set to “on” to initiate and maintain a mitotic state, and the other regulating the antagonistic activity of PP2A-B55δ and set to “off” [37, 43]. In this context, we posit that high levels of cyclin B1 ‘dm’ might be sufficient to induce a partially mitotic state in the absence of protein translation, one in which the auto-activation switch is indeed set to “on” but one in which the PP2A-B55δ switch might erroneously occupy some other position besides “off”. Adding rArpp19 is expected to effectively inhibit PP2A-B55δ, thereby turning this switch “off” and inducing a fully mitotic state capable of near-normal spindle assembly. It is possible that the synthesis of other “triggering” proteins during interphase, such as cyclin A dependent activation of Cdc25 [12], might be required to turn the PP2A-B55δ circuit completely off and that addition of rArrp19 simply serves as an exogenous proxy to achieve the same functional end. It remains unclear to us as to why this state exists even when Gwl kinase is active, but perhaps it is because Gwl is hyperphosphorylated under these conditions.
The mechanistic link between rArpp19’s role in inducing spindle bipolarity, specifically pole-focusing, and the cell cycle regulatory circuitry is unclear. In Xenopus egg extracts, the generation of spindles with two focused spindle poles is known to be regulated by a number of proteins including the microtubule-based motors Eg5 and cytoplasmic dynein [44, 45], NuMA [46], TPX2 [47], Aurora A- and Aurora B-kinases [48, 49], MCAK [48, 49], Augmin [50], Kif4A [51], and others. Many of these proteins have also been shown to be regulated by Cdk1-dependent mitotic phosphorylations. That subset includes Eg5, NuMA, MCAK, Kif4a, Aurora-A and TPX2 [52-57], NuMA and TPX2 have also been shown to be substrates of PP2A-B55δ [58], PP2A [59], and other phosphatases. We posit the addition of cyclin B1 ‘dm’ alone is not sufficient to accurately recapitulate the level of mitotic phosphorylation of one (or more) of these proteins and that proper phosphorylation is only achieved when the cyclin is added in combination with rArpp19. Additional investigation is needed to determine whether this is indeed the case.
Although spindle bipolarity was rescued with the combination of rArrp19 and a low concentration of cyclin B1 ‘dm’, the assembled spindles were longer than normal and incapable of properly aligning their chromosomes into a tight metaphase plate. In apparent contradiction to this observation, experiments in starfish oocytes show that injection of cyclin B–Cdk1 and activated Gwl results in subjectively normal-appearing meiotic spindles with properly aligned chromosomes [38]. This discrepancy might be explained, at least in part, by the fact that the studies in starfish oocytes addressed spindle assembly during meiosis I, during which F-actin plays a predominant role in chromosome alignment [60]. In contrast, the Xenopus egg extracts used in our experiments were treated with cytochalasin B to inhibit F-actin assembly and cycled into interphase by addition of calcium to mimic fertilization. These discrepancies preclude direct comparison to a meiosis in vivo [28]. It should also be noted that normal protein synthesis was allowed to proceed in the starfish oocytes used by Hara and colleagues [38] but was inhibited in our experiments, i.e., our experiments relied exclusively on recombinant proteins to induce extracts to enter mitosis and ultimately generate spindles. RNAi-mediated knock-down of the Gwl substrate α-Endosulfine has been shown to result in longer spindles with misaligned chromosomes in drosophila S2 cells [61]. Xenopus egg extracts, however, contain both of the known Gwl kinase substrates, Arpp19 and α-Endosulfine [21, 22, 25], suggesting that the phenotypic defects we observed following cyclin B1 ‘dm’ and rArpp19 addition are not because the endogenous proteins are absent from Xenopus egg extracts. The persistence of these defects after rArpp19 addition also indicates that Gwl-mediated signaling is most likely not involved in their mechanistic underpinnings.
The roles of Xkid in regulating chromosome alignment and anastral spindle length
The observation of misaligned chromosomes in spindles assembled in the absence of replenished Xkid was to be expected based on the established function of the protein as a chromokinesin. The observation that spindles were longer under the same conditions, however, was not expected and suggests a role for Xkid in regulating the length of anastral spindles (though it should be noted that previous results hinted at this function, e.g. see [13]; Fig. 5). It is possible that this phenotype results from an increase in the effective area occupied by the chromosomes within the spindle. This idea is consistent with experiments involving spindles assembled around patterned islands of chromatin-coated beads bathed in extract, which showed that increasing the area occupied by chromatin results in a concomitant increase in spindle length [62]. Furthermore, recently proposed autocatalytic models of spindle length regulation predict that scattered and misaligned chromosomes would increase the effective area of chromosome-associated RanGTPase RCC1, thereby expanding the area occupied by the RanGTPase gradient [63] and increasing the distance over which microtubule nucleators remain active [64]. Thus, we would argue that the increase in spindle length we observed in the absence of replenished Xkid is actually a direct result of chromosome misalignment and not some separate and distinct function of the chromokinesin. We should acknowledge that there are some reported instances demonstrating that cells can assemble spindles with misaligned chromosomes and normal spindle lengths. For example, RPE1 cells lacking KIF18A function display this phenotype [65], so clearly more work is needed to validate this hypothesis.
That addition of Xkid could rescue the observed spindle length defect was surprising, in part, because several studies using human cell cultures have shown that RNAi of the human homolog of Xkid, KIF22, does not affect spindle length [66], whereas others have shown that reduced chromokinesin levels actually result in decreased spindle length [67]. These results, which seem to directly contradict our findings, might be explained by structural and functional differences between Xenopus Xkid and KIF22. KIF22 contains an additional microtubule-binding site within its stalk region that allows the protein to cross-link parallel microtubules [67, 68]. KIF22 is also phosphorylated by Cdk1 kinase, which reduces its affinity for microtubules [68, 69], and it remains unclear as to whether Xkid is regulated in the same way. Moreover, Xenopus Xkid undergoes degradation during the metaphase-anaphase transition by APC/CCdc20 protein complex, which is necessary for the poleward movement of the sister chromatids during anaphase [13], whereas KIF22 remains tightly associated with anaphase chromosomes and is degraded later during G1 phase by APC/CCdh1 protein complex [69, 70]. It is also possible that loss of chromokinesin function in anastral spindles found in Xenopus egg extracts has a more pronounced effect on chromosome alignment than in the cortically anchored astral spindles found in human cells.
Taken as a whole, this work advances our understanding of the core components required to render mitotic extracts competent to assemble normal spindles during mitosis (Figure 7B). Strikingly, just three components, cyclin B1 ‘dm’, rArpp19, and Xkid, are sufficient to induce an interphase extract into an M-phase that is capable of normal spindle assembly, suggesting that the other proteins degraded during the previous metaphase-to-anaphase transition and subsequently replenished via translation during the next interphase [6, 9, 71] are not necessary for spindle assembly (although we cannot rule out that some of them might be required for initial priming of cyclin B1–Cdk1 kinase). We acknowledge that our in vitro system is not mitotic. This distinction is important because, in addition to protein degradation mediated by APC/CCdc20 protein complex, progression through the somatic cell cycle also involves protein degradation via active APC/CCdh1 protein complex, which is responsible for ubiquitin-mediated proteasomal degradation of numerous other proteins [1, 71]. Therefore, it would be informative to determine whether this same minimal mixture of proteins can induce spindle assembly in normal somatic cells. If so, this minimal protein “cocktail” could be exploited for therapeutic benefit by forcing the induction of premature mitosis in dividing cells.
Figure 7. Cdk1 autoamplification loop and summary of spindle assembly phenotypes.
A) Schematic illustration of Cdk1 autoamplification loop. B) Cartoon summary describing reconstitution of spindle assembly under the experimental conditions indicated.
STAR*METHODS
CONTACT FOR REAGENT AND RESOURCES SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Jesse C. Gatlin (jgatlin@uwyo.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
CSF-arrested egg extract and sperm nuclei preparation
Cytostatic factor (CSF)-arrested extracts were prepared from freshly laid X. laevis eggs as described previously [28, 72]. Briefly, gravid female frogs (3-8 years of age) were primed by injection of 100U of pregnant mare serum gonadotropin 1-2 weeks prior to a 500U injection of human chorionic gonadotropin to induce laying. Eggs arrested in metaphase of meiosis-II were then collected, dejellied, packed, and fractionated via centrifugation. The cytoplasmic fraction was collected and supplemented with 10 μg/mL each of the protease inhibitors leupeptin, pepstatin, and chymostatin (LPC) and 10 μg/mL cytochalasin D (to prevent f-actin dependent gelation and contraction of the bulk extract). Extracts were kept on ice until induced to go into interphase. The demembranated sperm nuclei used in these studies was prepared as described in [29]. Briefly, excised testes were macerated and homogenized to release the sperm. The homogenate was then centrifuged to separate the testes tissue from the sperm. The isolated sperm cells were briefly treated with buffer containing lysolecithin to generate demembranated sperm nuclei.
All Xenopus laevis frogs used in these studies were purchased from Nasco which maintains a closed colony of frogs at least five generations removed from wild stock. The frogs were housed in filtered re-circulating aquaria rack systems (XenRack™; Aquatic Enterprises) and allowed at least 4 months to replenish oocytes between induced egg-laying cycles. All studies using Xenopus laevis followed the guidelines of the U.S. Department of Health and Human Service for the Care and Use of Laboratory Animals, and all experiments were performed in accordance with national regulatory standards and ethical rules.
METHOD DETAILS
Protein expression and purification
A pET-30a(+) vector encoding a cyclin B1 ‘dm’ expression construct was used to express and purify cyclin B1 ‘dm’ as follows. The expression construct was transformed into Rosetta 2(DE3) pLysS competent cells, which were then grown at 37°C to an OD600 of 0.6. The cyclin B1 ‘dm’ expression was induced for 14 hr at 16°C by adding IPTG (1 mM). The cells were then pelleted by centrifugation at 6,000 rpm for 20 min in a JA-10 rotor using a Beckman J2-21M centrifuge. The cell pellet was resuspended in lysis buffer (20 mM HEPES, 300 mM NaCl, and 20 mM imidazole at pH 8.0) containing 0.5% (v/v) Triton X-100 and 10 μg/ml each of leupeptin, pepstatin, and chymostatin; sonicated; and centrifuged at 14,000 rpm for 30 min in a JA-20 rotor using a Beckman J2-21M centrifuge. The supernatant was further incubated with HisPur Cobalt Resin for 60 min on a tube revolver rotator at 4°C. The resin was spun down at 2,000 rpm for 1 min in a Sorvall 75006445 rotor using a Sorvall Legend centrifuge (Thermo Scientific) and loaded onto an empty XK column (GE Healthcare); protein was purified using fast protein liquid chromatography (AKTA; GE Healthcare). Fractions were collected, dialyzed against 1× PBS buffer, and concentrated to ~0.45 mg/mL using Amicon Ultra-15 centrifugal filter devices (30,000 NMWL; Millipore).
The rArpp19 gene was synthesized in vitro and was cloned into the EcoRI and HindIII sites of the pET-30a(+) vector. The expression vector was transformed into BL21 (DE3) pLysS competent cells, which were then grown at 37°C to an OD600 of 0.7. rArpp19 expression was induced for 8 hr at 32°C by adding IPTG (1 mM). The cells were then pelleted and lysed, and protein was purified as described above. rArpp19 was concentrated to ~20 mg/mL using Amicon Ultra-15 centrifugal filter devices (10,000 NMWL; Millipore). Protein concentration was determined by running the protein samples and bovine serum albumin (BSA) standards on an SDS-polyacrylamide gel, which was then stained using Coomassie staining solution (0.1% [w/v] Coomassie Brilliant Blue, 50% [v/v] Methanol, 10% [v/v] Glacial acetic acid, and 40% H2O), imaged on a Li-Cor Odyssey Imager, and used to compare band intensities relative to BSA standards with ImageJ.
To express and purify recombinant 6xHis-Xkid from insect cells, the Xkid gene was cloned into pENTR-D-TOPO vector and transformed into XL-1 competent E. coli cells. Plasmid DNA was isolated from positive transformants, and the sequence was verified by DNA sequencing. The recombinant plasmid was further used to perform LR recombination reactions with the baculovirus genome using Gateway LR Clonase enzyme kit. Recombinant virus encoding Xkid was obtained, expressed in Sf9 cells, and purified as described [13, 42, 73] with the following modifications. The cells were pelleted at 6,000 rpm for 20 min in a JA-10 rotor using a Beckman J2-21M centrifuge, washed in cold 1× PBS buffer, and pelleted. The washed cell pellet was resuspended in Buffer A (50 mM NaPi, pH 8.0; 10 mM KCl; 1.5 mM MgCl2; 5 mM β-mercaptoethanol; and 10 μg/ml each of leupeptin, pepstatin, and chymostatin) and incubated on ice for 20 min. The suspension was homogenized with 40 strokes in a 15-ml Dounce homogenizer (#1315701; Wheaton) and centrifuged at 12,000 rpm for 10 min at 4 °C in a JA-20 rotor using a Beckman J2-21M centrifuge. The supernatant was saved, and the pellet was reextracted in Buffer B (50 mM NaPi, pH 8.0; 500 mM KCl; 5 mM MgCl2; 10% glycerol; 0.1% Tween 20; 5 mM β-mercaptoethanol; and 10 μg/ml each of leupeptin, pepstatin, and chymostatin) and incubated for 30 min on ice before being homogenized as described above and centrifuged for 10 min at 12,000 rpm in a JA-20 rotor using a Beckman J2-21M centrifuge. Both supernatants were mixed and incubated with HisPur Cobalt Resin on a tube revolver rotator at 4 °C for 60 min, and protein was purified as described above. The protein was concentrated to ~0.13 mg/mL using Amicon Ultra-15 centrifugal filter devices (50,000 NMWL; Millipore).
Nuclear and spindle assembly in Xenopus egg extract
The CSF-arrested extracts were released into interphase by the addition of calcium (to a final concentration of 0.4 mM) and demembranated Xenopus sperm nuclei (~200 sperm nuclei/μl) [29] and then incubated at 16°C for 80 min to allow for DNA synthesis and the formation of nuclei. To block reentry of interphase extracts into mitosis, cycloheximide (100 μg/mL) was added 10 min before calcium addition to inhibit endogenous protein synthesis in these extracts. After DNA replication and nuclear assembly, the interphase extracts were either treated with 1× CSF-XB buffer only or driven into mitosis and spindle assembly by addition of an equal volume of CSF-arrested extract (add-back) or partially purified recombinant protein(s) (e.g., cyclin B1 ‘dm’, rArpp19, and Xkid), followed by an 90 min incubation at 16°C (Figure 1A). During the incubation period, tubulin labeled with Alexa Fluor 488 (green) or Alexa Fluor 568 (red) [74] and GST-GFP-NLS (a chimeric protein consisting of glutathione S-transferase tag, green fluorescent protein, and SV40 nuclear localization signal) [75] were added to visualize microtubules and to assess the nuclear envelope integrity, respectively (Figures 1B, 3A, 4A, 6A, 6C, S2A, S4A, and S6A). For in vitro kinetochore labeling, Alexa Fluor 647–conjugated anti-CENP-C [76] was added to the extracts 15 min before the completion of the spindle assembly reaction (Figure S6A).
Immunoblotting
Egg extract samples were collected after completion of the indicated experiments and stored at −80°C until processed. For each condition, 10 μl of extract was diluted in 90 μl of 2× Laemmli sample buffer (Bio-Rad) [77] and boiled at 95°C for 5 min. Equal volumes of each sample were loaded into wells of either a 4–15% gradient or a 7.5% single-concentration SDS-polyacrylamide gel (Bio-Rad) along with protein standards (Bio-Rad) and separated on a protein gel electrophoresis apparatus (Bio-Rad). The proteins were then transferred from the gel to Bio-Rad nitrocellulose membrane with a wet electroblotting apparatus (Bio-Rad). The nitrocellulose membranes were then incubated in blocking buffer (1× PBS buffer [137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 2 mM KH2PO4 at pH 7.4] containing 5% [w/v] nonfat dry milk) for 1 hr at room temperature and further incubated with the indicated primary antibodies and secondary antibodies for western blot analysis. The following primary antibodies and dilutions were used: 1 μg/ml anti-Xkid, 1:500 anti-Gwl/MASTL, 2 μg/ml anti-phospho-H3 (Ser10), 1:1000 anti-Histone H3, 1:500 anti-phospho-MAPK, 1:200 anti-β-Tubulin, 1:200 anti-phospho-Cdk1 (Tyr15), 1:1000 anti-phospho-Cdk1 substrate motif, and 5 μg/ml anti-Cdk1. Secondary antibodies used were IRDye 680RD–conjugated anti–mouse IgG and IRDye 800CW–conjugated anti–rabbit IgG. Membranes were scanned using a Li-Cor Odyssey CLx Imager. Band intensities were normalized to loading controls (total H3 or β-Tubulin levels) and quantified in ImageJ.
Microscopy and imaging
Fixed samples were prepared for imaging by pipetting 6 μl of extract onto a microscopic slide and mixing it with an equal volume of spindle fix (48% [v/v] glycerol, 11% [v/v] formaldehyde from 37% [w/v] stock, and 1 μg/ml Hoechst 33258 in 1× MMR) before gently covering the mixture with an 18 × 18–mm coverslip. Coverslips were sealed using nail polish, and the slides were stored at 4°C until imaged. For in vitro kinetochore labeling, Alexa Fluor 647–conjugated anti-CENP-C was added to the extracts 15 min before the completion of the spindle assembly reaction. Images were acquired using an Olympus IX71 inverted epifluorescence microscope equipped with an automated shutter, stage, and CMOS digital camera (Orca Flash 2.8, Hamamatsu). All images were captured using Metamorph 7.7 software (Molecular Devices) and objectives of varying magnification: 40× oil (1.30 NA), 60× oil (1.35 NA), and 100× oil (1.40 NA). Images were analyzed and processed using ImageJ.
Protein phosphatase treatment and kinase assay
For Lambda Protein Phosphatase (LPP) treatment, 2 μl of extract from the indicated condition was resuspended in 48 μl of LPP buffer containing 2 mM MnCl2, 1× NEBuffer for Protein MetalloPhosphatases, and 200 U Lambda Protein Phosphatase and incubated at 30°C for 30 min. The reaction was terminated by heat inactivation at 65°C for 60 min in the presence of 20 μl 4× Laemmli sample buffer (Bio-Rad) (Figure S1).
Cdk1 kinase activity was assayed using MESACUP Cdk1/Cdk1 Kinase Assay Kit as described [78] with the following modifications (Figures 2C and 5C). A phosphorylation reaction mixture was made containing 6 μl of extract from the respective condition. The reaction was initiated by the addition of 2.5 μl ATP (2 mM) per 25 μl total reaction volume and incubated for 30 min at 30°C. The reaction was terminated by adding phosphorylation stop solution and centrifuging the sample at 13,000 rpm for 2 min using a Labnet Spectrafuge 24D microcentrifuge. The supernatant was then transferred to a microwell coated with anti-phospho-MV peptide monoclonal antibody (4A4) and incubated for 60 min at 25°C. The microwells were incubated with peroxidase (POD)-conjugated streptavidin. Biotinylated phospho-MV peptide bound to POD-conjugated streptavidin was detected by ABTS single solution and measured using a Synergy H4 microplate reader (BioTek) at 405 nm.
QUANTIFICATION AND STATISTICAL ANALYSIS
Spindle length and chromosome spread measurements
We determined the x and y coordinates for spindle poles and chromosomes for each spindle using ImageJ. Spindle length was determined by calculating the interpolar distance from the x,y coordinates of each spindle pole, whereas chromosome spread was measured as the ratio of chromosome projection distance (total distance between chromosomes on either side of the spindle equator) and spindle length using Excel (Microsoft) (Figure S3A).
Measurement of Gwl electrophoretic mobility retardation
To measure the Gwl kinase phosphorylation levels (as a measure of Gwl electrophoretic mobility retardation), densitometric analysis was used to determine the position of the center of mass of the upper-molecular-weight bands along the vertical y-axis (YM), which was compared to the YM value for the band in the add-back only condition (taken as an average of the bands in lanes 2 and 7; refer to Figure 2A).
Statistical analysis
Statistical analyses were performed using the two-tailed Student t-test in Excel (Microsoft) except where stated otherwise. All error bars represent standard deviation. N.S. refers to “not significant” or a p-value > 0.05, whereas significant differences are represented by one star if the p-value < 0.05 and two stars if the p-value < 0.005. Sample sizes are indicated in the figures and figure legends. All data were repeated using at least three independent extract preparations.
Supplementary Material
Highlights:
Cyclin B alone is insufficient to induce a spindle assembly competent M-phase.
Addition of Arpp19 with low Cyclin B levels rescues spindle bipolarity.
Addition of Xkid rescues remaining spindle length and chromosome alignment defects.
This three-part cocktail is sufficient to induce normal spindle assembly in extracts.
ACKNOWLEDGEMENTS
The authors would like to thank the funding agencies that supported this work: the National Institute of General Medical Sciences and grants R15GM101636 (to Dr. John Oakey; JC Gatlin, co-investigator), RO1GM102428 (to JC Gatlin), and R01GM113028 (to Dr. Daniel Levy; JC Gatlin, co-investigator). We would also like to express our thanks to the Marine Biological Laboratories Whitman center and its sponsors for several summer fellowships to JC Gatlin including the Laura and Arthur Colwin Endowed Summer Research Fellowship Fund and the Nikon Fellowship (both to JC Gatlin). Additional funding was provided by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant #2P20GM103432 and by the Pew Scholars Program in the Biomedical Sciences. We thank Dr. Don Jarvis and Missy Stuart for reagents, assistance, and training with the baculovirus protein expression system. We kindly thank Dr. Hironori Funabiki for the gift of the Xkid construct and antibody, Dr. Aaron Straight for Alexa 647–conjugated anti-CENP-C, Daniel Levy for GST-GFP-NLS protein and construct, and Dr. Grant Bowman for access to the Synergy H4 microplate reader. We thank Dr. Tim Hunt, Dr. Bela Novak, Daniel Levy, and Dr. David Fay for their insightful feedback and reviews of manuscript drafts and Dr. Amy Fluet for editing the document. The authors would like to thank Priscilla Phan for making all of the Xenopus egg extracts used for these experiments. We owe a tremendous amount of thanks to the referees for their critically insightful and constructive comments – the published version of our work represents a significant improvement over its previous incarnations. Lastly, we thank all the researchers whose work influenced our science, but who were not cited due to limits imposed by the publisher or our own unintended omission.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES LIST
- 1.Peters JM (2006). The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol 7, 644–656. [DOI] [PubMed] [Google Scholar]
- 2.Murray AW, Solomon MJ, and Kirschner MW (1989). The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature 339, 280–286. [DOI] [PubMed] [Google Scholar]
- 3.Nurse P (1990). Universal control mechanism regulating onset of M-phase. Nature 344, 503–508. [DOI] [PubMed] [Google Scholar]
- 4.Pines J, and Hunter T (1990). Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature 346, 760–763. [DOI] [PubMed] [Google Scholar]
- 5.Boekhout M, and Wolthuis R (2015). Nek2A destruction marks APC/C activation at the prophase-to-prometaphase transition by spindle-checkpoint-restricted Cdc20. J Cell Sci 128, 1639–1653. [DOI] [PubMed] [Google Scholar]
- 6.Gabellini D, Colaluca IN, Vodermaier HC, Biamonti G, Giacca M, Falaschi A, Riva S, and Peverali FA (2003). Early mitotic degradation of the homeoprotein HOXC10 is potentially linked to cell cycle progression. EMBO J 22, 3715–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peart MJ, Poyurovsky MV, Kass EM, Urist M, Verschuren EW, Summers MK, Jackson PK, and Prives C (2010). APC/C(Cdc20) targets E2F1 for degradation in prometaphase. Cell Cycle 9, 3956–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hochegger H, Klotzbucher A, Kirk J, Howell M, le Guellec K, Fletcher K, Duncan T, Sohail M, and Hunt T (2001). New B-type cyclin synthesis is required between meiosis I and II during Xenopus oocyte maturation. Development 128, 3795–3807. [DOI] [PubMed] [Google Scholar]
- 9.Sedgwick GG, Hayward DG, Di Fiore B, Pardo M, Yu L, Pines J, and Nilsson J (2013). Mechanisms controlling the temporal degradation of Nek2A and Kif18A by the APC/C-Cdc20 complex. EMBO J 32, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stemmann O, Zou H, Gerber SA, Gygi SP, and Kirschner MW (2001). Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726. [DOI] [PubMed] [Google Scholar]
- 11.Kimata Y, Baxter JE, Fry AM, and Yamano H (2008). A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell 32, 576–583. [DOI] [PubMed] [Google Scholar]
- 12.Lorca T, Bernis C, Vigneron S, Burgess A, Brioudes E, Labbe JC, and Castro A (2010). Constant regulation of both the MPF amplification loop and the Greatwall-PP2A pathway is required for metaphase II arrest and correct entry into the first embryonic cell cycle. J Cell Sci 123, 2281–2291. [DOI] [PubMed] [Google Scholar]
- 13.Funabiki H, and Murray AW (2000). The Xenopus chromokinesin Xkid is essential for metaphase chromosome alignment and must be degraded to allow anaphase chromosome movement. Cell 102, 411–424. [DOI] [PubMed] [Google Scholar]
- 14.Minshull J, Blow JJ, and Hunt T (1989). Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell 56, 947–956. [DOI] [PubMed] [Google Scholar]
- 15.Solomon MJ, Glotzer M, Lee TH, Philippe M, and Kirschner MW (1990). Cyclin activation of p34cdc2. Cell 63, 1013–1024. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann I, Clarke PR, Marcote MJ, Karsenti E, and Draetta G (1993). Phosphorylation and activation of human cdc25-C by cdc2--cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J 12, 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller PR, Coleman TR, and Dunphy WG (1995). Cell cycle regulation of a Xenopus Wee1-like kinase. Mol Biol Cell 6, 119–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glotzer M, Murray AW, and Kirschner MW (1991). Cyclin is degraded by the ubiquitin pathway. Nature 349, 132–138. [DOI] [PubMed] [Google Scholar]
- 19.Brandeis M, Rosewell I, Carrington M, Crompton T, Jacobs MA, Kirk J, Gannon J, and Hunt T (1998). Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc Natl Acad Sci U S A 95, 4344–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holloway SL, Glotzer M, King RW, and Murray AW (1993). Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell 73, 1393–1402. [DOI] [PubMed] [Google Scholar]
- 21.Gharbi-Ayachi A, Labbe JC, Burgess A, Vigneron S, Strub JM, Brioudes E, Van-Dorsselaer A, Castro A, and Lorca T (2010). The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677. [DOI] [PubMed] [Google Scholar]
- 22.Mochida S, Maslen SL, Skehel M, and Hunt T (2010). Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670–1673. [DOI] [PubMed] [Google Scholar]
- 23.Yu J, Zhao Y, Li Z, Galas S, and Goldberg ML (2006). Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol Cell 22, 83–91. [DOI] [PubMed] [Google Scholar]
- 24.Dupré A, and Jessus C (2017). ARPP19 Phosphorylations by PKA and Greatwall: The Yin and the Yang of the Cell Decision to Divide. Protein Phosphorylation, 3–29. [Google Scholar]
- 25.Haccard O, and Jessus C (2011). Greatwall kinase, ARPP-19 and protein phosphatase 2A: shifting the mitosis paradigm. Results Probl Cell Differ 53, 219–234. [DOI] [PubMed] [Google Scholar]
- 26.Dupre A, Buffin E, Roustan C, Nairn AC, Jessus C, and Haccard O (2013). The phosphorylation of ARPP19 by Greatwall renders the auto-amplification of MPF independently of PKA in Xenopus oocytes. J Cell Sci 126, 3916–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrone A, Adamo ME, Cheng C, and Kettenbach AN (2016). Identification of Candidate Cyclin-dependent kinase 1 (Cdk1) Substrates in Mitosis by Quantitative Phosphoproteomics. Mol Cell Proteomics 15, 2448–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai A, Murray AW, Mitchison T, and Walczak CE (1999). The Use of Xenopus Egg Extracts to Study Mitotic Spindle Assembly and Function In Methods in Cell Biology, Volume 61 (Academic Press; ), pp. 385–412. [DOI] [PubMed] [Google Scholar]
- 29.Hazel JW, and Gatlin JC (2018). Isolation and Demembranation of Xenopus Sperm Nuclei. Cold Spring Harb Protoc 2018, pdb prot099044. [DOI] [PubMed] [Google Scholar]
- 30.Field CM, Nguyen PA, Ishihara K, Groen AC, and Mitchison TJ (2014). Xenopus egg cytoplasm with intact actin. Methods Enzymol 540, 399–415. [DOI] [PubMed] [Google Scholar]
- 31.Tsai TY, Theriot JA, and Ferrell JE Jr. (2014). Changes in oscillatory dynamics in the cell cycle of early Xenopus laevis embryos. PLoS Biol 12, e1001788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller PR, Coleman TR, Kumagai A, and Dunphy WG (1995). Myt1: a membrane-associated inhibitory kinase that phosphorylates Cdc2 on both threonine-14 and tyrosine-15. Science 270, 86–90. [DOI] [PubMed] [Google Scholar]
- 33.Deibler RW, and Kirschner MW (2010). Quantitative reconstitution of mitotic CDK1 activation in somatic cell extracts. Mol Cell 37, 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okumura E, Morita A, Wakai M, Mochida S, Hara M, and Kishimoto T (2014). Cyclin B-Cdk1 inhibits protein phosphatase PP2A-B55 via a Greatwall kinase-independent mechanism. J Cell Biol 204, 881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupre A, Daldello EM, Nairn AC, Jessus C, and Haccard O (2014). Phosphorylation of ARPP19 by protein kinase A prevents meiosis resumption in Xenopus oocytes. Nat Commun 5, 3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domingo-Sananes MR, Kapuy O, Hunt T, and Novak B (2011). Switches and latches: a biochemical tug-of-war between the kinases and phosphatases that control mitosis. Philos Trans R Soc Lond B Biol Sci 366, 3584–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mochida S, Rata S, Hino H, Nagai T, and Novak B (2016). Two Bistable Switches Govern M Phase Entry. Curr Biol 26, 3361–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hara M, Abe Y, Tanaka T, Yamamoto T, Okumura E, and Kishimoto T (2012). Greatwall kinase and cyclin B-Cdk1 are both critical constituents of M-phase-promoting factor. Nat Commun 3, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieder CL, and Salmon ED (1994). Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol 124, 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brouhard GJ, and Hunt AJ (2005). Microtubule movements on the arms of mitotic chromosomes: polar ejection forces quantified in vitro. Proc Natl Acad Sci U S A 102, 13903–13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cassimeris L, Rieder CL, and Salmon ED (1994). Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J Cell Sci 107 (Pt 1), 285–297. [DOI] [PubMed] [Google Scholar]
- 42.Antonio C, Ferby I, Wilhelm H, Jones M, Karsenti E, Nebreda AR, and Vernos I (2000). Xkid, a chromokinesin required for chromosome alignment on the metaphase plate. Cell 102, 425–435. [DOI] [PubMed] [Google Scholar]
- 43.Rata S, Suarez Peredo Rodriguez MF, Joseph S, Peter N, Echegaray Iturra F, Yang F, Madzvamuse A, Ruppert JG, Samejima K, Platani M, et al. (2018). Two Interlinked Bistable Switches Govern Mitotic Control in Mammalian Cells. Curr Biol 28, 3824–3832 e3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawin KE, LeGuellec K, Philippe M, and Mitchison TJ (1992). Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359, 540–543. [DOI] [PubMed] [Google Scholar]
- 45.Heald R, Tournebize R, Blank T, Sandaltzopoulos R, Becker P, Hyman A, and Karsenti E (1996). Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425. [DOI] [PubMed] [Google Scholar]
- 46.Merdes A, Ramyar K, Vechio JD, and Cleveland DW (1996). A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87, 447–458. [DOI] [PubMed] [Google Scholar]
- 47.Wittmann T, Wilm M, Karsenti E, and Vernos I (2000). TPX2, A novel xenopus MAP involved in spindle pole organization. J Cell Biol 149, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohi R, Sapra T, Howard J, and Mitchison TJ (2004). Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell 15, 2895–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Ems-McClung SC, and Walczak CE (2008). Aurora A phosphorylates MCAK to control ran-dependent spindle bipolarity. Mol Biol Cell 19, 2752–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petry S, Pugieux C, Nedelec FJ, and Vale RD (2011). Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc Natl Acad Sci U S A 108, 14473–14478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walczak CE, Vernos I, Mitchison TJ, Karsenti E, and Heald R (1998). A model for the proposed roles of different microtubule-based motor proteins in establishing spindle bipolarity. Curr Biol 8, 903–913. [DOI] [PubMed] [Google Scholar]
- 52.Blangy A, Lane HA, d'Herin P, Harper M, Kress M, and Nigg EA (1995). Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83, 1159–1169. [DOI] [PubMed] [Google Scholar]
- 53.Van Horn RD, Chu S, Fan L, Yin T, Du J, Beckmann R, Mader M, Zhu G, Toth J, Blanchard K, et al. (2010). Cdk1 activity is required for mitotic activation of aurora A during G2/M transition of human cells. J Biol Chem 285, 21849–21857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cahu J, Olichon A, Hentrich C, Schek H, Drinjakovic J, Zhang C, Doherty-Kirby A, Lajoie G, and Surrey T (2008). Phosphorylation by Cdk1 increases the binding of Eg5 to microtubules in vitro and in Xenopus egg extract spindles. PLoS One 3, e3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotak S, Busso C, and Gonczy P (2013). NuMA phosphorylation by CDK1 couples mitotic progression with cortical dynein function. EMBO J 32, 2517–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanhaji M, Friel CT, Kreis NN, Kramer A, Martin C, Howard J, Strebhardt K, and Yuan J (2010). Functional and spatial regulation of mitotic centromere-associated kinesin by cyclin-dependent kinase 1. Mol Cell Biol 30, 2594–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dong Z, Zhu C, Zhan Q, and Jiang W (2018). Cdk phosphorylation licenses Kif4A chromosome localization required for early mitotic progression. J Mol Cell Biol 10, 358–370. [DOI] [PubMed] [Google Scholar]
- 58.Cundell MJ, Hutter LH, Nunes Bastos R, Poser E, Holder J, Mohammed S, Novak B, and Barr FA (2016). A PP2A-B55 recognition signal controls substrate dephosphorylation kinetics during mitotic exit. J Cell Biol 214, 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kang Q, Srividhya J, Ipe J, and Pomerening JR (2014). Evidence toward a dual phosphatase mechanism that restricts Aurora A (Thr-295) phosphorylation during the early embryonic cell cycle. J Biol Chem 289, 17480–17496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Burdyniuk M, Callegari A, Mori M, Nedelec F, and Lenart P (2018). F-Actin nucleated on chromosomes coordinates their capture by microtubules in oocyte meiosis. J Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Goshima G, Wollman R, Goodwin SS, Zhang N, Scholey JM, Vale RD, and Stuurman N (2007). Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 417–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dinarina A, Pugieux C, Corral MM, Loose M, Spatz J, Karsenti E, and Nedelec F (2009). Chromatin shapes the mitotic spindle. Cell 138, 502–513. [DOI] [PubMed] [Google Scholar]
- 63.Kalab P, Weis K, and Heald R (2002). Visualization of a Ran-GTP gradient in interphase and mitotic Xenopus egg extracts. Science 295, 2452–2456. [DOI] [PubMed] [Google Scholar]
- 64.Decker F, Oriola D, Dalton B, and Brugues J (2018). Autocatalytic microtubule nucleation determines the size and mass of Xenopus laevis egg extract spindles. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fonseca CL, Malaby HLH, Sepaniac LA, Martin W, Byers C, Czechanski A, Messinger D, Tang M, Ohi R, Reinholdt LG, et al. (2019). Mitotic chromosome alignment ensures mitotic fidelity by promoting interchromosomal compaction during anaphase. J Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stumpff J, Wagenbach M, Franck A, Asbury CL, and Wordeman L (2012). Kif18A and chromokinesins confine centromere movements via microtubule growth suppression and spatial control of kinetochore tension. Dev Cell 22, 1017–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tokai-Nishizumi N, Ohsugi M, Suzuki E, and Yamamoto T (2005). The chromokinesin Kid is required for maintenance of proper metaphase spindle size. Mol Biol Cell 16, 5455–5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiroguchi K, Ohsugi M, Edamatsu M, Yamamoto T, and Toyoshima YY (2003). The second microtubule-binding site of monomeric kid enhances the microtubule affinity. J Biol Chem 278, 22460–22465. [DOI] [PubMed] [Google Scholar]
- 69.Ohsugi M, Tokai-Nishizumi N, Shiroguchi K, Toyoshima YY, Inoue J, and Yamamoto T (2003). Cdc2-mediated phosphorylation of Kid controls its distribution to spindle and chromosomes. EMBO J 22, 2091–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feine O, Zur A, Mahbubani H, and Brandeis M (2007). Human Kid is degraded by the APC/C(Cdh1) but not by the APC/C(Cdc20). Cell Cycle 6, 2516–2523. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Z, He M, Shah AA, and Wan Y (2016). Insights into APC/C: from cellular function to diseases and therapeutics. Cell Div 11, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Maresca TJ, and Heald R (2006). Methods for studying spindle assembly and chromosome condensation in Xenopus egg extracts. Methods Mol Biol 322, 459–474. [DOI] [PubMed] [Google Scholar]
- 73.Harrison RL, and Jarvis DL (2016). Transforming Lepidopteran Insect Cells for Continuous Recombinant Protein Expression. Methods Mol Biol 1350, 329–348. [DOI] [PubMed] [Google Scholar]
- 74.Mooney P, Sulerud T, Pelletier JF, Dilsaver MR, Tomschik M, Geisler C, and Gatlin JC (2017). Tau-based fluorescent protein fusions to visualize microtubules. Cytoskeleton (Hoboken) 74, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levy DL, and Heald R (2010). Nuclear size is regulated by importin alpha and Ntf2 in Xenopus. Cell 143, 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milks KJ, Moree B, and Straight AF (2009). Dissection of CENP-C-directed centromere and kinetochore assembly. Mol Biol Cell 20, 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laemmli UK (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- 78.Ramos Gomes F, Romaniello V, Sanchez A, Weber C, Narayanan P, Psol M, and Pardo LA (2015). Alternatively Spliced Isoforms of KV10.1 Potassium Channels Modulate Channel Properties and Can Activate Cyclin-dependent Kinase in Xenopus Oocytes. J Biol Chem 290, 30351–30365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.