Abstract
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality in persons with types 1 or 2 diabetes (T1D, T2D). Although beneficial roles for strict control of hyperglycemia have been suggested, such a strategy is not without liabilities. Specifically, the risk of hypoglycemia and its consequences remain an omnipresent threat with such approaches. The advent of the Cardiovascular Outcomes Trials (CVOTs) for new anti-diabetes treatments has uncovered unexpected benefits of cardiovascular protection in some of the new classes of agents, such as the glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the sodium glucose cotransporter-2 (SGLT-2) inhibitors. Further, state-of-the-art approaches, such as antibodies to proprotein convertase subtilisin-kexin type 9 (PCKSK9); RNA therapeutics; agents targeting distinct components of the immune/inflammatory response; and novel small molecules that block the actions of receptor for advanced glycation endproducts (RAGE) signaling, also hold potential as new therapies for diabetes and CVD. Finally, interventions such as weight loss, such as through bariatric surgery, may hold promise for benefit in diabetes and CVD. In this Brief Review, some of the novel approaches and emerging targets for the treatment of diabetes and CVD are discussed. Ultimately, identification of the optimal timing and combinations of such interventions, especially in the context of personalized approaches, together with emerging disease-modifying agents, holds great promise to reduce the burden that diabetes poses to the cardiovascular system.
Introduction
Cardiovascular disease (CVD) remains a leading cause of morbidity and mortality in types 1 and 2 diabetes (T1D, T2D)1–3. Beyond the inherent increase in mortality in diabetic subjects, when diabetes is combined with manifestations of CVD, such as myocardial infarction or stroke, the mortality rate is nearly doubled, leading to an estimated reduction in life expectancy of approximately 12 years4. Notably, a recent study reporting on the Swedish National Diabetes Register included 271,174 patients with T2D and matched them with 1,355,870 control subjects; subjects were studied for median follow-up of 5.7 years. Five specific risk factors for CVD were included in the model, elevated level of glycated hemoglobin, elevated level of low density lipoprotein cholesterol level, albuminuria, smoking status and elevated blood pressure levels. The authors found that for the T2D subjects who had these five risk factor variables within the target range, there was no significant excess risk of death, myocardial infarction or stroke when compared to the control population. The authors did report, however, that in the T2D subjects, the risk for hospitalization for heart failure was higher than that observed in the control subjects. Importantly, elevation of the glycated hemoglobin outside the target range was the strongest predictor of stroke and acute myocardial infarction5. Yet, although strict control of hyperglycemia may afford some benefit in reduction of major macrovascular events in T1D and T2D patients, the increased risk of hypoglycemia and its associated consequences render such a therapeutic approach not necessarily applicable to all subjects6–8. Hence, there is an urgent need to identify new therapies for diabetes and its CVD consequences in order to enhance quality and duration of life in the ever-growing number of subjects affected by these disorders. This Brief Review highlights some of the recent therapeutic advances for diabetes and CVD and considers emerging pre-clinical approaches at various stages in the development pipeline.
Thiazolidinediones (TZDs), the advent of cardiovascular outcome trials (CVOT) and the effects of new diabetes medications on major adverse cardiovascular events (MACE)
The discovery that rosiglitazone was associated with a significant risk for myocardial infarction and possible increase risk of CVD death led the Food and Drug Administration (FDA) to issue a missive requiring that manufacturers of new diabetes drugs conduct “non-inferiority” trials to demonstrate that the emerging therapies would not result in increased CVD risk9. Recently, such CVOTs have led to the discovery of unexpected benefits of some of the newer classes of glucose-lowering agents on CVD.
Glucagon-Like Peptide-1 Receptor Agonists (GLP-1 RA) and Dipeptidyl peptidase-4 (DPP-4) Inhibitors: Targeting The GLP-1 Axis
GLP-1, a potent incretin hormone, is produced in the L-cells of the distal ileum and colon. It exerts distinct functions, depending on the specific site in the body. For example, in the periphery, GLP-1 functions to inhibit gastric acid secretion and inhibit glucagon secretion. Other actions are considered to be central, in the nervous system, in which GLP-1 induces satiety. At the level of the pancreas, GLP-1 enhances insulin secretion10. The receptor agonists, therefore, mimic the effect of endogenous GLP-1. Although not all members of the GLP-1 RA family of agents exerted benefit in MACE, a major trial known as LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) reported on findings in 9,340 T2D patients who were at high CVD risk. Subjects received either liraglutide or a placebo for a median follow-up period of 3.8 years. Significant cardiovascular benefits were observed in the liraglutide vs. placebo-treated subjects, as the rate of first occurrence of death from CV causes, nonfatal myocardial infarction or nonfatal stroke was lower in T2D patients treated with liraglutide vs. placebo11.
In other studies, testing a distinct GLP-1 RA, Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6), once weekly semaglutide was administered vs. placebo in T2D subjects with known high CVD risk. In that study, the rate of CVD death, nonfatal myocardial infarction or stroke was significantly lowered by semaglutide12. A recent report, however, indicated that semaglutide carries a safety warning, as its use was associated with mild to moderate gastrointestinal side effects and retinopathy vs. placebo treatment13. In the EXSCEL (Exenatide Study of Cardiovascular Event Lowering) study, once weekly treatment of T2D patients with or without previous CVD resulted in no significant difference in the incidence of any component of MACE between the two subject groups14. In the FREEDOM-CVO trial, subdermal implantation of exenatide is being tested for one year in patients with T2D; efficacy results regarding CVD and MACE are still pending15.
Other studies have focused on the testing of dipeptidyl peptidase-4 (DPP-4) inhibitors in T2D; DPP-4 inhibits GLP-1 degradation; therefore, agents that inhibit DPP-4 increase the availability of GLP-1. In a number of studies to date, SAVOR-TIMI (Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI)) (saxagliptin vs. placebo); EXAMINE (The Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care in patients with type 2 diabetes mellitus and acute coronary syndrome) (EXAMINE) (alogliptin vs. placebo); TECOS (Trial Evaluating Cardiovascular Outcomes with Sitagliptin) (sitagliptin vs. placebo) and Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus (CARMELINA) (linagliptin vs. placebo), no significant benefits of the DPP-4 inhibitor vs. placebo were observed with respect to any component of MACE. It remains uncertain whether this class of agents contributes to increased hospitalization for heart failure; further work will be required to settle that point16–19
Taken together, unlike the studies testing DPP-4 inhibitors, trials testing GLP-1-RAs revealed unexpected cardiovascular benefits. The reasons for the disparate effects of these two agents with distinct mechanisms of action, yet both targeting the GFP-1 RA pathway, on CVD, however, are not clear. Drucker and Nauck recently noted that although there do not appear to be substantial differences in the effects of either class of agents on the ability to lower HbA1c, there are notable distinctions. Whereas weight loss commonly accompanies use of the GLP-1 RAs, treatment with the DPP-4 inhibitors is associated with reduced gain of weight20. If and how body weight may be a surrogate for possible broader cardiometabolic benefits of the GLP-1 RAs has not been reported. Furthermore, if and how concerns regarding gastrointestinal side effects and retinopathy related to the GLP-1 RAs and if and how the possibility of increased hospitalizations for heart failure associated with the use of the DPP-4 inhibitors may affect their long-term usage remains to be determined.
Sodium-Glucose Cotransporter-2 (SGLT-2) Inhibitors
The SGLT-2 inhibitors, which decrease plasma glucose levels by prevention of renal glucose resorption, thereby causing glucosuria, also represent a new class of agents directly targeting hyperglycemia for which CVD benefits have been observed. In the first such study to report findings, Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial (EMPA-REG OUTCOME), empagliflozin was tested in subjects with T2D and established CVD and reported a decreased risk of MACE, as follows21. Although there were no significant between-group differences in the rates of myocardial infarction or stroke in the empagliflozin vs. placebo-treated subjects, empagliflozin was associated with significantly lower rates of death from cardiovascular causes, hospitalization for heart failure and death from any cause.
The Canagliflozin Cardiovascular Assessment Study (CANVAS) reported that treatment with canagliflozin vs. placebo significantly reduced the risk of CV death, non-fatal myocardial infarction or stroke compared to placebo in T2D subjects; there was no significant reduction in all-cause mortality22. In the Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58 (DECLARE–TIMI 58) trial, T2D patients were treated with dapagliflozin vs. vehicle. In patients who had or were at risk for atherosclerotic heart disease, treatment with dapagliflozin exerted no significant effect on MACE when compared with placebo; however, this treatment resulted in a lower rate of CVD death or hospitalization from heart failure vs. the placebo treatment23. In an additional trial, ertugliflozin is under study for CVD outcomes in patients with T2D24. The first three studies noted above have established the beneficial effects of SGLT-2 inhibitors in CVD but also showed that there were a number of notable adverse effects that require further consideration, including increased risk of genital mycotic infections, increased fracture risk, diabetic ketoacidosis and in patients with known peripheral arterial disease (PAD), an increased risk of amputation (canagliflozin) was observed. If and to what degree these possible complications affect long-term prospects for this class of agents remains to be determined.
Furthermore, at this time, it is not clear why certain of the SGLT-2 inhibitors, but not all, afford benefit for CVD outcomes. It is possible that there are yet-to-be-identified distinct effects of these various agents’ specific chemical structures on metabolism and CVD risk factors. It has been reported that among the various agents in this class, there are differences in selectivity for SGLT-1 vs. SGLT-2; along with differences in potency and pharmacokinetics25. If and how such factors may ultimately impact benefits in CVD, or not, will require further research to address such possibilities.
Finally, given the possible complications from the newer classes of anti-hyperglycemia agents discussed above, it will be important to outline specific indications and contra-indications for each of these newer agents that show cardiovascular benefit. In this context, Cosentino and colleagues recently published a report from the Cardiovascular Round Table (CRT) of the European Society of Cardiology (ESC) to consider potential guidelines for the implementation of these new agents that have shown CVD benefit26.
Novel Approaches to Lipid-Lowering in Diabetes: Targeting Proprotein Convertase subtilisin-kexin type 9 (PCSK9)
PCSK9 functions to promote the degradation of the low-density lipoprotein receptor (LDL-R), thereby reducing the clearance of LDL from the circulation. The development of novel antibodies that target PCSK9 has led to their testing in statin-treated subjects, including those with diabetes. In Efficacy and Safety of Alirocumab Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients with hypercholesterolemia (ODYSSEY COMBO-II) trial testing alirocumab vs. placebo, of the patients on maximally-tolerated statins and treated with alirocumab, 31% had diabetes. By 24 weeks of alirocumab therapy, in the diabetic subjects, LDL-C was reduced by 49.1% and in the non-diabetic subjects, LDL-C was reduced by 51.2%, that is, essentially comparable27. In the published reports on alirocumab in ODYSSEY OUTCOMES28, efficacy or not, in diabetic patients was not explicitly discussed, although it was noted that adverse events including “diabetes worsening or diabetic complication among patients with diabetes at baseline”, or “new-onset diabetes among patients without diabetes at baseline” did not differ between subjects treated with alirocumab vs. placebo28. However, in the case of the Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER) trial, evolocumab was tested in patients with and without diabetes who were on statins and had known atherosclerotic disease; the study showed that this treatment reduced CVD risk in both diabetic and non-diabetic subjects and, importantly, did not increase the risk of development of T2D29.
Collectively, these findings suggest that the PCSK9 inhibition appears safe in diabetic subjects and that patients with diabetes may benefit from this treatment. Certainly, however, more trials testing benefits of this class of agents will continue to provide the key data regarding the utility / safety of targeting this axis in diabetes.
Targeting the Immune/Inflammatory Response in Diabetes
Evidence suggests that diabetes is accompanied by a pro-inflammatory state, as there is upregulation of seminal factors that regulate and/or biomark inflammatory responses, such as high sensitivity C-reactive protein (hsCRP), toll-like receptors (TLRs), oxidative stress and Nuclear Factor-kB (NF-kB), and advanced glycation endproducts (AGEs) and their chief cell-surface receptor, RAGE (receptor for AGEs)30–35. Levels of hsCRP have been shown to predict CVD events in patients with diabetes36 and levels of hsCRP are associated with plasma soluble RAGE levels37, thus highlighting the interconnected nature of inflammatory sources and foci in both T1D and T2D. Burke and colleagues studied hearts from diabetic and non-diabetic subjects who died suddenly from CVD. They found that the atherosclerotic lesions from T2D vs. control subjects displayed higher levels of RAGE and one of its S100/calgranulin ligands (S100A12); larger necrotic cores; greater total and distal plaque load; and higher intimal staining for macrophages, T lymphocytes and HLA-DR expression compared to the non-diabetic subjects38. On account of these and other findings, therapeutic approaches have targeted the immune/inflammatory response for CVD outcomes and in such studies, diabetic subjects were included. Examples of such studies include the following, detailed below.
Targeting Interleukin-1 Beta
The Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), was a randomized double blind placebo study in which canakinumab vs. placebo was administered to subjects who had had recent vascular events and an hsCRP > 2.0 mg/L; the total number of subjects in the study was 10,061. Of these, 4,057 had T2D; 4,960 had “pre-diabetes” and the remainder had normal glucose levels. The patients were followed for a median period of 3.7 years. Overall, the trial was positive in considering all subjects; a significant reduction in death, non-fatal myocardial infarction or stroke was observed. In the subset analysis, the patients with diabetes benefitted as well and similarly to the subjects without diabetes39, 40. Interestingly, it was also found that canakinumab did not reduce the incidence of new-onset diabetes over the study duration, even in the face of significant reductions in levels of hsCRP and IL-6. In the first six to nine months of treatment, however, the patients treated with canakinumab did experience a reduction in HbA1c, the effects were not sustained over the duration40. Indeed, in an earlier Phase IIb study in which canakinumab was administered to 556 T2D patients at high vascular risk, the treatment resulted in reduced levels of hsCRP and IL-6, without major effect on atherogenic lipids41, thereby suggesting that the increased vascular risk in diabetes is not solely on account of lipid abnormalities, but, more broadly, due to unique effects on activation of inflammatory pathways specific to glucose and its direct and/or indirect consequences.
Of note, however, the treatment with canakinumab was associated with a higher incidence of fatal infection vs. placebo, although there were no significant differences in all-cause mortality40. If and how this finding affects usage in patients with diabetes remains to be seen.
Methotrexate
In a study targeting the immune/inflammatory response, the Cardiovascular Inflammation Reduction Trial (CIRT), low-dose methotrexate was administered to 4,786 patients with either T2D or metabolic syndrome who had previous myocardial infarction or who had evidence of multi-vessel coronary artery disease42. The trial’s primary endpoints were death from CVD or nonfatal myocardial infarction or stroke. The trial was stopped after a median of 2.3 years; it was found that the treatment with methotrexate did not lower levels of IL-1 beta, IL-6 or hsCRP when compared to placebo and that the primary outcome was not met.
Colchicine
A number of studies have addressed the effects of colchicine in CVD and immune/inflammatory responses. Nidorf and colleagues tested colchicine in 532 subjects with established coronary artery disease followed for a median of 3 years. The primary outcome (acute coronary syndrome, out of hospital cardiac arrest or noncardiometabolic ischemic stroke) was significantly reduced in the colchicine vs. placebo-treated subjects. Both diabetic and non-diabetic subjects were included in the trial; however, the authors did not specifically state if the benefits were equivalent in both groups43. In another study in which the effect of colchicine treatment on transcoronary gradients of levels of IL-1beta, IL-18 and IL-6 was probed, treatment with colchicine resulted in significant reductions in these levels. The authors noted that as there were significantly more diabetic patients in the acute coronary syndrome group treated with colchicine vs. the acute coronary syndrome group not treated with colchicine, they performed a sensitivity analysis with adjustment for diabetes and oral hypoglycemic agents. They reported that this adjustment yielded similar significant differences in cytokine levels between groups44. In addition, Deftereos and colleagues treated subjects (N=151) with acute ST-segment elevation myocardial infarction with colchicine vs. placebo (< 12 hours from the onset of pain) and found that subjects receiving colchicine demonstrated significant reductions in infarct size, as measured by area under the curve (AUC) for Creatine Kinase-MB fraction and MRI-determined infarct size. Both diabetic and non-diabetic subjects were included; however, the authors did not report treatment effects by diabetes status45.
In an imaging study, treatment of 80 subjects with recent acute coronary syndrome with colchicine plus optimal medical therapy vs. optimal medical therapy alone for approximately one year resulted in significant reductions in low attenuation plaque volume and hsCRP46. At this time, a number of new trials testing the effects of colchicine on CVD are under way.
Icosapent Ethyl
Icosapent ethyl is a stable eicosapentaenoic acid (EPA) that was recently tested in 8,179 subjects for prevention of cardiovascular events. In the Reduction of Cardiovascular Events Trial (REDUCE-IT), patients with hypertriglyceridemia despite the use of statins were followed for a median of 4.9 years and received either icosapent ethyl twice daily vs. placebo. The primary endpoint in the study was a composite of cardiovascular death, nonfatal myocardial infarction or stroke, coronary revascularization, or unstable angina. Compared to the placebo-treated group, the group receiving icosapent ethyl demonstrated significantly reduced primary endpoint47. It was noted in the study that the benefits of icosapent ethyl were observed in both diabetic and non-diabetic subjects. Side effects included higher hospitalization for atrial fibrillation or flutter in the icosapent ethyl group vs. the placebo with a trend toward more serious bleeding events in the former group as well. The authors of that work speculated that the mechanism of action of this agent might, in part, be explained through changes in inflammation, since the hsCRP levels were significantly reduced by this agent47.
In summary, the recent series of trials targeting the immune/inflammatory response have shown varied effects on CVD in general and, specifically, in patients with T2D and metabolic syndrome. These considerations underscore the premise that diabetes-associated inflammation may bear unique features, such that not all anti-immune/inflammatory response-targeted strategies will be effective. On the other hand, these studies may suggest that a key biomarker for utility, particularly in the diabetic / metabolic syndrome sub-group may be effectiveness on lowering hsCRP and other inflammatory markers.
Weight Loss – Medical & Surgical Approaches in Obesity and Diabetes
Obesity is an important risk factor for many disorders; among these are insulin resistance and T2D and, independently, CVD48–50. The broad benefits of healthy eating, for example, were suggested by a recently reported study called FINGER (Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability) in which cognition was improved in an elderly Finnish population practicing healthy eating, compared to a dietary counseling-only control51. In the context of metabolism, randomized clinical trials demonstrated that life style interventions to reduce body mass and to increase physical activity exerted a number of metabolic benefits, and, in persons with T2D, improved glycemic control52–54. Whether these results also imbued benefit in terms of reduced CVD was not clear. However, the Action for Health in Diabetes (Look AHEAD) Study, an intensive life style intervention study, demonstrated a number of benefits to health, however, the primary outcome was not met, as there were no significant reductions in CVD morbidity and mortality54–57.
In a follow-up subset analysis of Look AHEAD, the goal was to determine if the incidence of CVD varied on account of changes in weight or in fitness. The primary outcome of the study and the subset analysis was death from CV causes, non-fatal myocardial infarction or stroke or hospital admission for angina. The secondary outcomes included coronary artery bypass grafting, carotid endarterectomy, percutaneous coronary intervention, hospitalization for congestive heart failure, peripheral vascular disease, or total mortality58. The authors included as the reference group individuals who had received standard disease education and support intervention. In the aggregate, the interpretation of the data obtained by this analysis suggested that there was an association between the magnitude of the weight loss and the incidence of CVD in subjects with T2D58.
It was thus logical to determine if there were benefits of bariatric surgery on macrovascular disease outcomes in T2D. A retrospective observational matched cohort study of 20,235 T2D subjects with severe obesity compared cardiovascular outcomes in patients undergoing, or not, bariatric surgery59. In that study, 5,301 subjects had undergone bariatric surgery and 14,934 were considered the control subjects, without surgery. At the five years follow-up, the patients who had undergone bariatric surgery had a significantly lower risk of macrovascular events vs. the control group (2.1% vs. 4.3%; hazard ratio (HR)=0.60)59. It is important to note that bariatric surgery, compared to medical weight loss approaches, also may affect gut hormones and other factors that might exert distinct effects on risk factors related to CVD. Randomized controlled clinical trials will be required to address these key questions.
RNA Therapeutics
Emerging evidence links microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) to the pathogenesis of diabetes and its complications60–62. In the area of CVD and diabetes, experimental evidence has shown the utility of antagonizing these pathways in pre-clinical models. Impaired regression of diabetic atherosclerosis was overcome, in part, by administration of anti-miRNA33 oligonucleotides. Compared to control oligonucleotides, administration of oligonucleotides targeting miRNA33 resulted in decreased atherosclerotic plaque macrophage content and inflammation in diabetic Reversa mice after lipid lowering, which was traced to upregulation of the key cholesterol transporter, Abca163. In non-diabetic mice, targeting the lncRNA Chast (cardiac hypertrophy associated transcript) using GapmerR (single-stranded antisense oligonucleotides (ASOs) for silencing LncRNAs and mRNAs)-mediated silencing prevented and attenuated pathological cardiac remodeling in mice undergoing transverse aortic constriction (TAC)64. In non-diabetic mice, Malat1 (metastasis-associated lung adenocarcinoma transcript1) was shown to play important protective roles in ischemic stroke, as mice devoid of Malat1 displayed increased expression of pro-apoptotic and pro-inflammatory factors after ischemic stroke65. Further, Malat1 regulates angiogenesis after hind-limb ischemia via regulation of VEGFR266. In other studies, GapmeR-mediated silencing of the lncRNA maternally expressed gene 3 (Meg3) prevented the induction of MMP2 with diminished cardiac fibrosis and improved diastolic performance after TAC in mice67.
Therapies targeting RNA and DNA are emerging as opportunities for novel strategies against a variety of disorders68, 69. ASOs, Small interfering RNAs (siRNAs), miRNAs and aptamers are a few examples of such strategies. Examples of FDA-approved RNA therapeutics include fomiversen (ASO indicated for cytomegalovirus retinitis); pegaptanib targeting VEGF165, which was approved for use in age-related macular degeneration; eteplirsen, which restores the translational reading frame in the dystrophin gene, thereby serving as a therapy for Duchenne muscular dystrophy; and nusinersen, an ASO which is indicated for spinal muscular atrophy69.
Collectively, these examples of pre-clinical studies and FDA-approved therapies hold promise for novel targets for cardiovascular medicine and diabetes, particularly on account of the ability to target specific mutations in key genes related to cardiovascular homeostasis or injury. However, as diabetes is a chronic and long-term disease, the safety and tolerability of therapies targeting RNAs and DNAs will be critical to establish.
Novel Small Molecule Approaches and Diabetic Vascular Complications
A key consequence of the hyperglycemia associated with T1D and T2D is the generation of AGEs. AGEs result from nonenzymatic post-translational modification of proteins and lipids due to high glucose conditions; however, inflammatory and pro-oxidative mechanisms also result in the generation of AGEs. For example, a chief AGE found in vivo, carboxymethyl lysine (CML)-AGE may be produced by high glucose and via activation of the myeloperoxidase system, in a process that requires NADPH oxidases70, 71. AGEs may also be formed by hypoxia and ischemia/reperfusion, thereby highlighting further links to diabetes, in which tissue hypoxia and increased myocardial infarcts and strokes characterize the disease72. CML-AGE and other AGEs are detected in the plasma of diabetic patients73 and in atherosclerotic plaques74. Recent work has shown that CML-AGE is enriched in the adipose tissues of subjects with obesity, even in the absence of diabetes75. Further, in a mouse model of high fat feeding, feeding mice a diet of 60% kcal/fat resulted in increased accumulation of AGEs even before the animals were insulin resistant or diabetic76. Together, these two studies in human and animal models of obesity suggest that AGEs may increase even in the absence of hyperglycemia.
The discovery of RAGE as a chief signaling receptor for AGEs led to the finding that, beyond AGEs, RAGE was a multi-ligand receptor. In addition to AGEs, the immunoglobulin superfamily molecule RAGE also bound pro-inflammatory ligands such as S100/calgranulins, high mobility group box 1 (HMGB1) and lysophosphatidic acid (LPA)77–79. RAGE and its ligands are highly expressed in human diabetic atherosclerosis42, 80, 81. To test the role of RAGE in diabetic atherosclerosis and vascular diseases, numerous strategies have been employed in pre-clinical animal models, such as soluble RAGE (extracellular ligand-binding domains of RAGE that bind up RAGE ligands and block their activation of the cell surface receptor); anti-RAGE antibodies; and mice (or their bone marrow) devoid of Ager (the gene encoding RAGE)82–88. The discovery that the cytoplasmic RAGE domain bound the formin, DIAPH1, and that this interaction was important for RAGE signaling in vascular cells and monocytes/macrophages identified a novel platform for therapeutic intervention in disorders in which RAGE ligands accumulate and contribute to pathobiology, such as diabetic complications, obesity and CVD89–93. In contrast to the heterogeneous and multiple binding sites for RAGE ligands on the extracellular RAGE domains94–96, the binding site of DIAPH1 on the RAGE tail is suitable for small molecule binding. Solution NMR spectroscopy was used to identify interaction surfaces between the cytoplasmic domain of RAGE and DIAPH1 FH1 due to the exquisite sensitivity of chemical shifts to the chemical environment and showed residues 3–6 of the RAGE tail interact with FH1 and the KD is <10 μM. Mapping the observed chemical shift changes onto the molecular surface of the cytoplasmic domain of RAGE identified that interaction surface between the cytoplasmic domain of RAGE and DIAPH1 FH1 consists of a small positively charged patch formed by Q3, R4, R5, and Q6 with a total area less than 200 Å2 97. Recent work using super-resolution stochastic optical reconstruction microscopy (STORM) and single particle tracking (SPT) supported the strong connection between RAGE and DIAPH1, both spatially and with respect to their effects on the dynamics of the actin cytoskeleton98.
These findings prompted the screening of the ChemBridge CT488 library compounds at a single concentration, 10 μM, to test if such molecules would block the interaction of the cytoplasmic domain of RAGE with antibody-captured DIAPH1 from cultured cells. Thirteen compounds which specifically bound to the cytoplasmic domain of RAGE, not DIAPH1, were identified with nM dissociation constants. In vascular cells, they blocked RAGE ligand-induced inflammation and migration, suppressed ischemic injury in the diabetic isolated perfused heart and blocked upregulation of inflammatory mRNA transcripts in liver and kidney after infusion of RAGE ligand CML-AGE into wild-type mice99.
Taken together, these pre-clinical data support that the interaction of the cytoplasmic domain of RAGE with the formin, DIAPH1, may represent a novel platform for drug development for diabetes and its cardiovascular and other complications. Work is underway to advance the potency, safety, novelty and druggability of a primary and a “back-up” scaffold, all deduced from molecules identified through the initial screen from the ChemBridge library. Note that the RAGE/DIAPH1 axis is presented as one example of a signal transduction axis adversely affected by high levels of glucose and the generation of AGEs. Certainly, other targets in this area might include, but are not limited to, blocking AGE production itself and facilitating AGE clearance, all as efforts to quell AGE-associated augmentation of immune/inflammatory and pro-oxidative responses.
Perspectives – Looking Forward in Diabetes
Figure 1A summarizes the key endogenous and exogenous metabolic disturbances vis-à-vis glucose metabolism and their molecular consequences that contribute to increased CVD in diabetes. The advent of the CVOTs results and some of the surprising benefits on MACE uncovered in these trials underscores that the direct and/or indirect effects of higher than normal levels of glucose significantly perturb metabolic homeostasis, thereby exacerbating factors that increase CVD risk. Superimposed on these endogenous sources of aberrant glucose metabolism, exogenous factors, such as those in the diet and in the environment, may amplify AGEs exposure, thereby contributing to upregulation of pro-atherogenic processes. Although hosts of studies have uncovered adverse effects of glucose on extra- and intracellular properties, their long-term negative effects, such as on glycation (discussed above) and on epigenetic factors and “metabolic memory” 100, 101 also have been postulated to play key roles in CVD in diabetes. Further, the effects of diabetes on perturbation of lipid/lipoprotein metabolism, in addition to their unique and independent effects, also intersects with these glucose-driven mechanisms, as glycation of lipids and lipoproteins may change the function of these species and, further, through receptor/RAGE-dependent mechanism, may mediate and exacerbate cellular perturbation102, 103. Consequently, glucose-dependent immediate and long-term effects activate signaling pathways and alter gene expression programs that mediate vascular cell dysfunction. Coupled with immune cell perturbation, these factors may combine to increase vascular perturbation and, thereby, increase risk for CVD in these metabolic disorders.
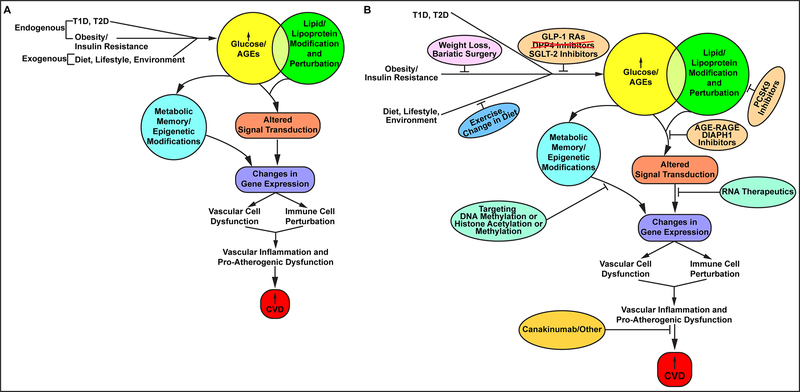
Figure 1. Pathobiological mechanisms and therapeutic targets for diabetes and CVD.
(A). Examples of proposed mechanisms of diabetes and CVD. Both endogenous and exogenous forces may converge to increase glucose levels, one consequence of which is the formation nonenzymatically glycated proteins or lipids, called AGEs, or advanced glycation endproducts – factors that have been linked mechanistically to the pathogenesis of CVD. Independently, lipid abnormalities in obesity and diabetes pose independent risk for CVD. Further, these two pathways may converge, as glycation of lipids and lipoproteins has been shown in multiple basic science experiments to regulate factors that aggravate CVD risk. Once ignited, the actions of glucose, AGEs and lipids modulate signaling pathways and factors that regulate gene expression, including microRNAs and lncRNAs. The consequences of these changes in gene expression may be vast, affecting the functions of both vascular cells and immune cells. Indeed, increased “vascular inflammation” occurs in diabetes and leads to the upregulation of factors that augment CVD risk. In addition, long-term epidemiologic studies have underscored that in both T1D and T2D, the effects of hyperglycemia may be long-lived, leading to epigenetic changes that may affect gene expression patterns and CVD risk for many years. Finally, fundamental changes in body mass and reductions in physical activity may portend increased obesity, insulin resistance, and if left unchecked, T2D. (B) Emerging therapeutic strategies in diabetes and metabolic dysfunction to combat CVD. Recent results from CVOTs demonstrated unexpected cardiovascular benefit from the use of newer classes of agents targeting hyperglycemia, namely, the GLP-1 RAs and the SGLT-2 inhibitors. However, the DPP4 inhibitors have not been shown to exert the same degree of benefits in CVD, but may be associated with higher rates of heart failure. Further, some of the members of these classes of agents have been associated with some risks, such as increased mycotic infections, increased retinopathy and risk of amputations, for unclear reasons. For maximally treated with statins subjects or in subjects with statin intolerance, studies have begun to show that the new series of antibodies targeting PCSK9 may exert equivalent benefit in CVD in non-diabetic and diabetic subjects. Recent discoveries on the roles of miRNAs and lncRNAs in diabetic complications in preclinical models may lead to broader testing and use of ASOs and GapmeRs for diabetes and CVD. The recent success of canakinumab, as illustrated in the CANTOS trial, solidified for the first time in a large clinical trial the benefits of targeting inflammation for CVD. In the CANTOS trial, it was shown that diabetic subjects and non-diabetic subjects benefitted from this approach. It is to be noted that there was an increased risk of serious infection in the canakinumab-treated group vs. the placebo. If and how this may affect overall utility for diabetic subjects remains to be determined. In the field of cancer, novel approaches to targeting DNA methylation and histone methylation and acetylation are gaining traction; given the evidence of “metabolic memory” in diabetic complications, such approaches may well soon be tested in diabetes and CVD. Lifestyle interventions, although tantalizing, have long proved to be difficult to achieve and sustain. Recent work has suggested that bariatric surgery may exert possible CVD benefits on account of weight loss, but, as well, to other to-be-elucidated factors (such as changes in gut hormones, as an example). Finally, novel approaches to disease modification, such as antagonism of the RAGE/DIAPH1 signaling pathway, have shown benefit in preclinical models of inflammation and diabetes. Work is ongoing to test these concepts. Note that this review was meant to illustrate but some of recently emerging targets for diabetes and CVD. Given the scope of the epidemics of obesity and diabetes, such efforts are both timely and, potentially, life-saving.
How, then, may emerging strategies be utilized to combat these risk-associated factors and, thereby, reduce the risks of CVD in diabetes and metabolic dysfunction? Figure 1B illustrates examples of the evolving agents and approaches for diabetes and CVD. Strategies to target the adverse effects of endogenous and exogenous glucose metabolism; to reduce pro-atherogenic low density lipoprotein levels in maximally-statin-treated subjects; to modulate RNA biology with novel therapeutics against miRNAs, lncRNAs or to silence mRNAs; to directly attack vascular inflammation and its consequences with agents against cytokines such as IL-1 beta; and novel, pre-clinical development targets such as RAGE/DIAPH1 (to block the adverse signaling of AGEs) all hold promise, perhaps in combination, to assuage the assault on the vascular and immune systems imparted by high levels of glucose and their direct/indirect consequences. Although not discussed in this review, efforts targeting epigenetic mechanisms, such as DNA methylation and histone methylation and acetylation are already in development, especially in cancer104–106. Given the emerging roles for epigenetics in the maladaptive “memories” imbued by high levels of glucose, it may be logical to test such agents in diabetes, as well.
Finally, it is very important to consider sex differences in CVD and associated mortality in diabetes. Yamagishi recently summarized results of multiple clinical studies that affirmed in a meta-analysis of 820,0000 people that the HR for death from CVD in diabetic persons was 2.32 compared to non-diabetic people, even after statistical measures to adjust for age, sex, smoking status and the BMI107, 108. That study, and others, reported that the excess relative risk for CVD of death was larger in women than in men with diabetes. Recognition of the importance of sex-based differences in CVD is already being strongly urged for the design of preclinical studies, as, recently, there is increased emphasis on the inclusion of male and female animals and their primary cells in pre-clinical studies for CVD-type pathologies109. Collectively, the dissection and consideration of sex-based differences in diabetic CVD in therapeutic approaches hold great promise for uncovering fundamental new insights into the pathogenesis of CVD as well as the development of new classes of personalized therapeutic strategies.
In conclusion, recent advances have paved the way for therapies that afford benefit to the cardiovascular system both by direct reduction of hyperglycemia and by mitigation of its long-term consequences. Given the vast epidemics of obesity and T2D110, such efforts to suppress CVD risk are essential to maintain long-term health and quality of life worldwide.
Supplementary Material
Highlights.
Cardiovascular disease remains a leading cause of morbidity and mortality in persons with types 1 or 2 diabetes.
The requirement for Cardiovascular Outcomes Trials (CVOTs) for new anti-diabetes treatments has uncovered unexpected cardioprotective benefits in some of the new classes of agents, such as the glucagon-like peptide 1 receptor agonists (GLP-1 RAs) and the sodium glucose cotransporter-2 (SGLT-2) inhibitors.
Recent studies testing antibodies to proprotein convertase subtilisin-kexin type 9 (PCKSK9) have shown cardiovascular benefit in subjects both without and with diabetes, thereby providing a new complementary therapy in diabetes and dyslipidemia.
Recent approaches to target inflammation have shown benefit in subjects with diabetes, such as antagonists of Interleukin-1 Beta. Other agents such as colchicine also show promise for cardiovascular protection in diabetes.
Novel and emerging targets for therapeutic intervention in diabetic cardiovascular disease, such as RNA therapeutics and targeting the Receptor for Advanced Glycation Endproducts (RAGE) axis also hold promise to reduce the burden that diabetes poses to the cardiovascular system
Acknowledgements
The author gratefully acknowledges the assistance of Ms. Latoya Woods in the preparation of this manuscript. This work is supported by grants from the United States Public Health Service and the American Heart Association. The author has no disclosures.
Abbreviations
- AGE
advanced glycation endproduct
- ASO
Antisense oligonucleotide
- CANTOS
Canakinumab Anti-Inflammatory Thrombosis Outcomes Study
- CANVAS
The Canagliflozin Cardiovascular Assessment Study
- CARMELINA
Cardiovascular and Renal Microvascular Outcome Study With Linagliptin in Patients With Type 2 Diabetes Mellitus
- Chast
Cardiac hypertrophy associated transcript
- CIRT
Cardiovascular Inflammation Reduction Trial
- CML-AGE
Carboxymethyl lysine advanced glycation endproduct
- CRT
Cardiovascular Round Table
- CVD
Cardiovascular disease
- CVOTs
Cardiovascular outcome trials
- DECLARE-TIMI 58
Dapagliflozin Effect on Cardiovascular Events–Thrombolysis in Myocardial Infarction 58
- DPP-4
Dipeptidyl peptidase-4
- EMPA-REG OUTCOME
Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes trial
- EPA
Eicosapentaenoic acid
- ESC
European Society of Cardiology
- EXAMINE
The Examination of Cardiovascular Outcomes With Alogliptin Versus Standard of Care in patients with type 2 diabetes mellitus and acute coronary syndrome
- EXSCEL
Exenatide Study of Cardiovascular Event Lowering
- FDA
Food and Drug Administration
- FINGER
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- FOURIER
Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk
- GLP-1 RAs
Glucagon-like peptide receptor agonists
- HMGB1
High mobility group box 1
- HR
Hazard Ratio
- HS-CRP
High Sensitivity C-reactive protein
- IL-1 beta
Interleukin-1 Beta
- LDL-R
Low density lipoprotein receptor
- LEADER
Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results
- Lnc RNA
Long noncoding RNA
- Look AHEAD
Action for Health in Diabetes
- LPA
Lysophosphatidic acid
- MACE
Major adverse cardiac events
- MALAT1
Metastasis-associated lung adenocarcinoma transcript 1
- MiRNA
Micro RNA
- NF-kB
Nuclear Factor-Kappa B
- ODYSSEY COMBO II
Efficacy and Safety of Alirocumab Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia
- PAD
Peripheral Arterial Disease
- PCSK9
Proprotein convertase subtilisin-kexin type 9
- RAGE
Receptor for advanced glycation endproducts
- REDUCE-IT
Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial
- SAVOR-TIMI
Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR)–Thrombolysis in Myocardial Infarction (TIMI))
- SGLT-2
Sodium glucose cotransporter-2
- SI RNA
Small interfering RNAs
- SPT
Single particle tracking
- STORM
Super-resolution stochastic optical reconstruction microscopy
- SUSTAIN-6
Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes
- T1D
Type 1 diabetes
- T2D
Type 2 diabetes
- TAC
Transverse aortic constriction
- TECOS
Trial Evaluating Cardiovascular Outcomes with Sitagliptin
- TLRs
Toll like receptors
- TZD
Thiazolidinedione
References
- 1.Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Livingstone SJ, Looker HC, Hothersall EJ, et al. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9:e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RG, Costacou T, Orchard TJ. Risk Factor Modeling for Cardiovascular Disease in Type I Diabetes in the Pittsburgh Epidemiology of Diabetes Complications (EDC) Study: A Comparison to the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study. Diabetes. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennells L, Kaptoge S, Wood A, et al. Equalization of four cardiovascular risk algorithms after systematic recalibration: individual-participant meta-analysis of 86 prospective studies. Eur Heart J. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rawshani A, Rawshani A, Franzen S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjornsdottir S. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633–644 [DOI] [PubMed] [Google Scholar]
- 6.Control Group, Turnbull FM, Abraira C, et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Lachin J, Cleary P, Orchard T, Brillon DJ, Backlund JY, O’Leary DH, Genuth S. Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med. 2003;348:2294–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guidance for Industry. Diabetes Mellitus-Evaluating Cardiovascular Risk in New Anti-diabetic Therapies to Treat Type 2 Diabetes. 2008. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf
- 10.MacDonald PE, El-Kholy W, Riedel MJ, Salapatek AM, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51 Suppl 3:S434–442 [DOI] [PubMed] [Google Scholar]
- 11.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marso SP, Bain SC, Consoli A, et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 13.Coon SA, Crannage EF, Kerwin LC, Guyton JE. Semaglutide once-weekly: improved efficacy with a new safety warning. Expert Rev Clin Pharmacol. 2018;11:1061–1072 [DOI] [PubMed] [Google Scholar]
- 14.Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377:1228–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittbrodt ET, Eudicone JM, Bell KF, Enhoffer DM, Latham K, Green JB. Generalizability of glucagon-like peptide-1 receptor agonist cardiovascular outcome trials enrollment criteria to the US type 2 diabetes population. Am J Manag Care. 2018;24:S146–s155 [PubMed] [Google Scholar]
- 16.Green JB, Bethel MA, Armstrong PW, et al. Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373:232–242 [DOI] [PubMed] [Google Scholar]
- 17.Rosenstock J, Perkovic V, Johansen OE, et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. Jama. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326 [DOI] [PubMed] [Google Scholar]
- 19.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335 [DOI] [PubMed] [Google Scholar]
- 20.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705 [DOI] [PubMed] [Google Scholar]
- 21.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 22.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 23.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2018 [DOI] [PubMed] [Google Scholar]
- 24.Scheen AJ. Cardiovascular Effects of New Oral Glucose-Lowering Agents: DPP-4 and SGLT-2 Inhibitors. Circ Res. 2018;122:1439–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaji M SGLT2 inhibitors: molecular design and potential differences in effect. Kidney Int Suppl. 2011:S14–19 [DOI] [PubMed] [Google Scholar]
- 26.Cosentino F, Ceriello A, Baeres FMM, et al. Addressing cardiovascular risk in type 2 diabetes mellitus: a report from the European Society of Cardiology Cardiovascular Roundtable. Eur Heart J. 2018 [DOI] [PubMed] [Google Scholar]
- 27.Leiter LA, Zamorano JL, Bujas-Bobanovic M, Louie MJ, Lecorps G, Cannon CP, Handelsman Y. Lipid-lowering efficacy and safety of alirocumab in patients with or without diabetes: A sub-analysis of ODYSSEY COMBO II. Diabetes Obes Metab. 2017;19:989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 2018;379:2097–2107 [DOI] [PubMed] [Google Scholar]
- 29.Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950 [DOI] [PubMed] [Google Scholar]
- 30.Dasu MR, Devaraj S, Park S, Jialal I. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622 [DOI] [PubMed] [Google Scholar]
- 32.Hanefeld M, Marx N, Pfutzner A, Baurecht W, Lubben G, Karagiannis E, Stier U, Forst T. Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT Study. J Am Coll Cardiol. 2007;49:290–297 [DOI] [PubMed] [Google Scholar]
- 33.Hayaishi-Okano R, Yamasaki Y, Katakami N, Ohtoshi K, Gorogawa S, Kuroda A, Matsuhisa M, Kosugi K, Nishikawa N, Kajimoto Y, Hori M. Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care. 2002;25:1432–1438 [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Diez R, Shekhtman A, Ramasamy R, Schmidt AM. Cellular mechanisms and consequences of glycation in atherosclerosis and obesity. Biochim Biophys Acta. 2016;1862:2244–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, Schmidt AM. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci. 2013;14:19891–19910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soinio M, Marniemi J, Laakso M, Lehto S, Ronnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care. 2006;29:329–333 [DOI] [PubMed] [Google Scholar]
- 37.An XF, Zhao Y, Yu JY, Liu JS, Gu WJ, Gao F. Plasma sRAGE is independently associated with high sensitivity C-reactive protein in type 2 diabetes without coronary artery disease. Diabetes Res Clin Pract. 2010;87:e19–22 [DOI] [PubMed] [Google Scholar]
- 38.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271 [DOI] [PubMed] [Google Scholar]
- 39.Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131 [DOI] [PubMed] [Google Scholar]
- 40.Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, Ridker PM. Anti-Inflammatory Therapy With Canakinumab for the Prevention and Management of Diabetes. J Am Coll Cardiol. 2018;71:2392–2401 [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, Thuren T. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126:2739–2748 [DOI] [PubMed] [Google Scholar]
- 42.Ridker PM, Everett BM, Pradhan A, et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N Engl J Med. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nidorf SM, Eikelboom JW, Budgeon CA, Thompson PL. Low-dose colchicine for secondary prevention of cardiovascular disease. J Am Coll Cardiol. 2013;61:404–410 [DOI] [PubMed] [Google Scholar]
- 44.Martinez GJ, Robertson S, Barraclough J, Xia Q, Mallat Z, Bursill C, Celermajer DS, Patel S. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J Am Heart Assoc. 2015;4:e002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deftereos S, Giannopoulos G, Angelidis C, Alexopoulos N, Filippatos G, Papoutsidakis N, Sianos G, Goudevenos J, Alexopoulos D, Pyrgakis V, Cleman MW, Manolis AS, Tousoulis D, Lekakis J. Anti-Inflammatory Treatment With Colchicine in Acute Myocardial Infarction: A Pilot Study. Circulation. 2015;132:1395–1403 [DOI] [PubMed] [Google Scholar]
- 46.Vaidya K, Arnott C, Martinez GJ, Ng B, McCormack S, Sullivan DR, Celermajer DS, Patel S. Colchicine Therapy and Plaque Stabilization in Patients With Acute Coronary Syndrome: A CT Coronary Angiography Study. JACC Cardiovasc Imaging. 2018;11:305–316 [DOI] [PubMed] [Google Scholar]
- 47.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC, Ballantyne CM. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22 [DOI] [PubMed] [Google Scholar]
- 48.Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167:1720–1728 [DOI] [PubMed] [Google Scholar]
- 49.Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med. 1995;122:481–486 [DOI] [PubMed] [Google Scholar]
- 50.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lehtisalo J, Levalahti E, Lindstrom J, Hanninen T, Paajanen T, Peltonen M, Antikainen R, Laatikainen T, Strandberg T, Soininen H, Tuomilehto J, Kivipelto M, Ngandu T. Dietary changes and cognition over 2 years within a multidomain intervention trial-The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER). Alzheimers Dement. 2018 [DOI] [PubMed] [Google Scholar]
- 52.Stevens VJ, Obarzanek E, Cook NR, et al. Long-term weight loss and changes in blood pressure: results of the Trials of Hypertension Prevention, phase II. Ann Intern Med. 2001;134:1–11 [DOI] [PubMed] [Google Scholar]
- 53.Aucott L, Gray D, Rothnie H, Thapa M, Waweru C. Effects of lifestyle interventions and long-term weight loss on lipid outcomes - a systematic review. Obes Rev. 2011;12:e412–425 [DOI] [PubMed] [Google Scholar]
- 54.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, Zhang Q. Lifestyle change and mobility in obese adults with type 2 diabetes. N Engl J Med. 2012;366:1209–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Look AHEAD Research Group. Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Look AHEAD Research Group, Gregg EW, Jakicic JM, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016;4:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher DP, Johnson E, Haneuse S, Arterburn D, Coleman KJ, O’Connor PJ, O’Brien R, Bogart A, Theis MK, Anau J, Schroeder EB, Sidney S. Association Between Bariatric Surgery and Macrovascular Disease Outcomes in Patients With Type 2 Diabetes and Severe Obesity. Jama. 2018;320:1570–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leung A, Natarajan R. Long Noncoding RNAs in Diabetes and Diabetic Complications. Antioxid Redox Signal. 2018;29:1064–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang HN, Xu QQ, Thakur A, Alfred MO, Chakraborty M, Ghosh A, Yu XB. Endothelial dysfunction in diabetes and hypertension: Role of microRNAs and long non-coding RNAs. Life Sci. 2018;213:258–268 [DOI] [PubMed] [Google Scholar]
- 62.Regazzi R MicroRNAs as therapeutic targets for the treatment of diabetes mellitus and its complications. Expert Opin Ther Targets. 2018;22:153–160 [DOI] [PubMed] [Google Scholar]
- 63.Distel E, Barrett TJ, Chung K, Girgis NM, Parathath S, Essau CC, Murphy AJ, Moore KJ, Fisher EA. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res. 2014;115:759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Viereck J, Kumarswamy R, Foinquinos A, et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8:326ra322. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long Noncoding RNA Malat1 Regulates Cerebrovascular Pathologies in Ischemic Stroke. J Neurosci. 2017;37:1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang X, Tang X, Hamblin MH, Yin KJ. Long Non-Coding RNA Malat1 Regulates Angiogenesis in Hindlimb Ischemia. Int J Mol Sci. 2018;19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piccoli MT, Gupta SK, Viereck J, Foinquinos A, Samolovac S, Kramer FL, Garg A, Remke J, Zimmer K, Batkai S, Thum T. Inhibition of the Cardiac Fibroblast-Enriched lncRNA Meg3 Prevents Cardiac Fibrosis and Diastolic Dysfunction. Circ Res. 2017;121:575–583 [DOI] [PubMed] [Google Scholar]
- 68.Kaczmarek JC, Kowalski PS, Anderson DG. Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017;9:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laina A, Gatsiou A, Georgiopoulos G, Stamatelopoulos K, Stellos K. RNA Therapeutics in Cardiovascular Precision Medicine. Front Physiol. 2018;9:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anderson MM, Requena JR, Crowley JR, Thorpe SR, Heinecke JW. The myeloperoxidase system of human phagocytes generates Nepsilon-(carboxymethyl)lysine on proteins: a mechanism for producing advanced glycation end products at sites of inflammation. J Clin Invest. 1999;104:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson MM, Heinecke JW. Production of N(epsilon)-(carboxymethyl)lysine is impaired in mice deficient in NADPH oxidase: a role for phagocyte-derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes. 2003;52:2137–2143 [DOI] [PubMed] [Google Scholar]
- 72.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102:905–913 [DOI] [PubMed] [Google Scholar]
- 73.Takeuchi M, Makita Z, Yanagisawa K, Kameda Y, Koike T. Detection of noncarboxymethyllysine and carboxymethyllysine advanced glycation end products (AGE) in serum of diabetic patients. Mol Med. 1999;5:393–405 [PMC free article] [PubMed] [Google Scholar]
- 74.Piarulli F, Lapolla A, Ragazzi E, Susana A, Sechi A, Nollino L, Cosma C, Fedele D, Sartore G. Role of endogenous secretory RAGE (esRAGE) in defending against plaque formation induced by oxidative stress in type 2 diabetic patients. Atherosclerosis. 2013;226:252–257 [DOI] [PubMed] [Google Scholar]
- 75.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, Rensen SS, Buurman WA, Greve JW, Blaak EE, van Zandvoort MA, Bierhaus A, Stehouwer CD, Schalkwijk CG. Nepsilon-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol. 2014;34:1199–1208 [DOI] [PubMed] [Google Scholar]
- 76.Song F, Hurtado del Pozo C, Rosario R, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63:1948–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901 [DOI] [PubMed] [Google Scholar]
- 78.Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem. 1995;270:25752–25761 [DOI] [PubMed] [Google Scholar]
- 79.Rai V, Toure F, Chitayat S, Pei R, Song F, Li Q, Zhang J, Rosario R, Ramasamy R, Chazin WJ, Schmidt AM. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med. 2012;209:2339–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cuccurullo C, Iezzi A, Fazia ML, De Cesare D, Di Francesco A, Muraro R, Bei R, Ucchino S, Spigonardo F, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A, Cipollone F. Suppression of RAGE as a basis of simvastatin-dependent plaque stabilization in type 2 diabetes. Arterioscler Thromb Vasc Biol. 2006;26:2716–2723 [DOI] [PubMed] [Google Scholar]
- 81.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, De Cesare D, De Blasis G, Muraro R, Bei R, Chiarelli F, Schmidt AM, Cuccurullo F, Mezzetti A. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108:1070–1077 [DOI] [PubMed] [Google Scholar]
- 82.Lopez-Diez R, Shen X, Daffu G, Khursheed M, Hu J, Song F, Rosario R, Xu Y, Li Q, Xi X, Zou YS, Li H, Schmidt AM, Yan SF. Ager Deletion Enhances Ischemic Muscle Inflammation, Angiogenesis, and Blood Flow Recovery in Diabetic Mice. Arterioscler Thromb Vasc Biol. 2017;37:1536–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Koulis C, Kanellakis P, Pickering RJ, Tsorotes D, Murphy AJ, Gray SP, Thomas MC, Jandeleit-Dahm KA, Cooper ME, Allen TJ. Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci (Lond). 2014;127:485–497 [DOI] [PubMed] [Google Scholar]
- 84.Bu DX, Rai V, Shen X, Rosario R, Lu Y, D’Agati V, Yan SF, Friedman RA, Nuglozeh E, Schmidt AM. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wendt T, Harja E, Bucciarelli L, Qu W, Lu Y, Rong LL, Jenkins DG, Stein G, Schmidt AM, Yan SF. RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis. 2006;185:70–77 [DOI] [PubMed] [Google Scholar]
- 86.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, Goova MT, Moser B, Kislinger T, Lee DC, Kashyap Y, Stern DM, Schmidt AM. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106:2827–2835 [DOI] [PubMed] [Google Scholar]
- 87.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr., Chow WS, Stern D, Schmidt AM. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031 [DOI] [PubMed] [Google Scholar]
- 88.Bucciarelli LG, Ananthakrishnan R, Hwang YC, Kaneko M, Song F, Sell DR, Strauch C, Monnier VM, Yan SF, Schmidt AM, Ramasamy R. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou X, Weng J, Xu J, Xu Q, Wang W, Zhang W, Huang Q, Guo X. Mdia1 is Crucial for Advanced Glycation End Product-Induced Endothelial Hyperpermeability. Cell Physiol Biochem. 2018;45:1717–1730 [DOI] [PubMed] [Google Scholar]
- 91.Toure F, Fritz G, Li Q, Rai V, Daffu G, Zou YS, Rosario R, Ramasamy R, Alberts AS, Yan SF, Schmidt AM. Formin mDia1 mediates vascular remodeling via integration of oxidative and signal transduction pathways. Circ Res. 2012;110:1279–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Y, Toure F, Qu W, Lin L, Song F, Shen X, Rosario R, Garcia J, Schmidt AM, Yan SF. Advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling and up-regulation of Egr-1 in hypoxic macrophages. J Biol Chem. 2010;285:23233–23240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Shea KM, Ananthakrishnan R, Li Q, Quadri N, Thiagarajan D, Sreejit G, Wang L, Zirpoli H, Aranda JF, Alberts AS, Schmidt AM, Ramasamy R. The Formin, DIAPH1, is a Key Modulator of Myocardial Ischemia/Reperfusion Injury. EBioMedicine. 2017;26:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie J, Burz DS, He W, Bronstein IB, Lednev I, Shekhtman A. Hexameric calgranulin C (S100A12) binds to the receptor for advanced glycated end products (RAGE) using symmetric hydrophobic target-binding patches. J Biol Chem. 2007;282:4218–4231 [DOI] [PubMed] [Google Scholar]
- 95.Xie J, Reverdatto S, Frolov A, Hoffmann R, Burz DS, Shekhtman A. Structural basis for pattern recognition by the receptor for advanced glycation end products (RAGE). J Biol Chem. 2008;283:27255–27269 [DOI] [PubMed] [Google Scholar]
- 96.Xue J, Rai V, Singer D, Chabierski S, Xie J, Reverdatto S, Burz DS, Schmidt AM, Hoffmann R, Shekhtman A. Advanced glycation end product recognition by the receptor for AGEs. Structure. 2011;19:722–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rai V, Maldonado AY, Burz DS, Reverdatto S, Yan SF, Schmidt AM, Shekhtman A. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem. 2012;287:5133–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Q, Smith EA. Diaphanous-1 affects the nanoscale clustering and lateral diffusion of receptor for advanced glycation endproducts (RAGE). Biochim Biophys Acta Biomembr. 2019;1861:43–49 [DOI] [PubMed] [Google Scholar]
- 99.Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci Rep. 2016;6:22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Costantino S, Ambrosini S, Paneni F. The epigenetic landscape in the cardiovascular complications of diabetes. J Endocrinol Invest. 2018 [DOI] [PubMed] [Google Scholar]
- 101.Reddy MA, Zhang E, Natarajan R. Epigenetic mechanisms in diabetic complications and metabolic memory. Diabetologia. 2015;58:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yamagishi SI, Matsui T. Role of Hyperglycemia-Induced Advanced Glycation End Product (AGE) Accumulation in Atherosclerosis. Ann Vasc Dis. 2018;11:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki K, Nakagawa K, Miyazawa T. Augmentation of blood lipid glycation and lipid oxidation in diabetic patients. Clin Chem Lab Med. 2014;52:47–52 [DOI] [PubMed] [Google Scholar]
- 104.Mazur PK, Herner A, Mello SS, et al. Combined inhibition of BET family proteins and histone deacetylases as a potential epigenetics-based therapy for pancreatic ductal adenocarcinoma. Nat Med. 2015;21:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song SH, Han SW, Bang YJ. Epigenetic-based therapies in cancer: progress to date. Drugs. 2011;71:2391–2403 [DOI] [PubMed] [Google Scholar]
- 106.Ferrari A, Longo R, Silva R, Mitro N, Caruso D, De Fabiani E, Crestani M. Epigenome modifiers and metabolic rewiring: New frontiers in therapeutics. Pharmacol Ther. 2019;193:178–193 [DOI] [PubMed] [Google Scholar]
- 107.Yamagishi SI. Sex disparity in cardiovascular mortality rates associated with diabetes. Diabetes Metab Res Rev. 2018;34:e3059. [DOI] [PubMed] [Google Scholar]
- 108.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, Norman PE, Sampson UK, Williams LJ, Mensah GA, Criqui MH. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340 [DOI] [PubMed] [Google Scholar]
- 109.Lu HS, Schmidt AM, Hegele RA, Mackman N, Rader DJ, Weber C, Daugherty A. Reporting Sex and Sex Differences in Preclinical Studies. Arterioscler Thromb Vasc Biol. 2018;38:e171–e184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chobot A, Gorowska-Kowolik K, Sokolowska M, Jarosz-Chobot P. Obesity and diabetes-Not only a simple link between two epidemics. Diabetes Metab Res Rev. 2018;34:e3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.