Abstract
Objectives
To evaluate the relationship between radiographic progression and disease activity in subjects with PsA treated with adalimumab (ADA) or placebo (PBO) and the impact of concomitant MTX.
Methods
This was a post hoc analysis of the randomized, double-blind, PBO-controlled ADEPT trial. Subjects were categorized according to time-averaged (TA) disease activity (remission, low, moderate or high) based on Disease Activity Score of 28 joints with CRP [DAS28(CRP)], Disease Activity Index for Psoriatic Arthritis (DAPSA) or Psoriatic Arthritis Disease Activity Score (PASDAS), and achievement of minimal disease activity (MDA) at week 24. Radiographic progression was assessed as change in modified total Sharp score (ΔmTSS) from baseline to week 24. The analyses included interaction terms between disease activity and treatment on radiographic progression, comparison of radiographic progression in subjects categorized by disease activity and treatment, and correlation between disease activity and radiographic progression by treatment.
Results
The interaction terms for TA disease activity and treatment on ΔmTSS were significant (P = 0.002–0.008). Irrespective of concomitant MTX, ΔmTSS was lower with ADA vs PBO in all disease activity categories. Importantly, even in subjects having moderate or high disease activity or not achieving MDA, ΔmTSS was significantly lower on ADA than PBO (P = 0.05–0.001 for TA-DAPSA, TA-PASDAS and MDA). Correlations between TA disease activity scores and ΔmTSS were moderately positive and significant (P < 0.001) with PBO but non-significant with ADA.
Conclusion
Among subjects with PsA treated with ADA, there was evidence of a ‘disconnect’ between disease activity and radiographic progression: inhibition of radiographic progression was greater than expected based on control of clinical disease activity alone. MTX had no added effect.
Trial registration
ClinicalTrials.gov, http://clinicaltrials.gov, NCT00646386.
Keywords: psoriatic arthritis, adalimumab, ADEPT, radiographic progression, disconnect, MTX
Rheumatology key messages
Evidence is provided for a disconnect between disease activity and radiographic progression in PsA patients receiving adalimumab.
Disease activity correlated with radiographic progression in patients receiving placebo, but not patients receiving adalimumab.
Concomitant methotrexate treatment appeared to have little influence on radiographic progression.
Introduction
PsA, an inflammatory peripheral joint disease associated with psoriasis, is often characterized by structural damage as a result of cartilage degradation, bone resorption and osteoproliferation [1, 2]. Therapies for PsA, which include conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) and biologic agents (bDMARDs), aim to reduce the underlying inflammation, thereby also preventing structural damage. bDMARDs, such as TNF inhibitors as well as IL-12/23 and IL-17 inhibitors, are effective in controlling the signs and symptoms of PsA and have been shown to inhibit radiographic progression [3–6], whereas csDMARDs have not shown effective inhibition of structural damage [3, 4, 7–9].
In RA, inhibition of radiographic progression under TNF inhibitor therapy has been shown to be greater than expected based on the control of clinical disease activity. This ‘disconnect’ phenomenon, an uncoupling between inflammation and structural progression, has been observed in subjects with RA treated with infliximab in combination with MTX in the ATTRACT and ASPIRE trials [10, 11], as well as after treatment with etanercept in the TEMPO trial [12]. However, this phenomenon has yet to be explored in PsA.
In the ADEPT trial of subjects with PsA, adalimumab (ADA) significantly inhibited structural progression over 24 weeks compared with placebo (PBO), and the inhibitory effect was maintained in a 2-year open-label extension period [13]. Preliminary results showed that a disconnect phenomenon, similar to that observed in subjects with RA, may also be seen in subjects with PsA following ADA therapy [14]. The objective of this analysis was to further examine the relationship between inhibition of structural progression and control of clinical disease activity in subjects with PsA treated with ADA within the ADEPT trial, and to assess the impact of concomitant MTX usage.
Methods
Subjects and study design
ADEPT was a double-blind, randomized, PBO-controlled, parallel-group, 24-week trial comparing the efficacy and safety of ADA therapy with PBO in subjects with active PsA. Details of subjects, methods and the primary results of the ADEPT study have been published previously [15].
In brief, subjects included in ADEPT were ⩾18 years old and had moderately to severely active PsA (⩾3 swollen joints and ⩾3 tender or painful joints) with either active psoriatic skin lesions or a documented history of psoriasis. Subjects had a history of inadequate response or intolerance to non-steroidal anti-inflammatory drug therapy and were TNF inhibitor naive. MTX use was allowed prior to and during the study if it had been taken for ⩾3 months with stable dosage for ⩾4 weeks prior to the baseline visit. Use of csDMARDs other than MTX was not permitted. Subjects were grouped according to MTX use and degree of psoriasis at baseline [⩾3% or ⩽3% of body surface area (BSA)], and were randomized 1:1 to receive ADA or PBO 40 mg subcutaneously every other week. This post hoc analysis included subjects who had evaluable radiographs at baseline and week 24.
All research was conducted in compliance with the Declaration of Helsinki, and the protocol was approved by the institutional review boards of the participating centres. Investigators at each site enrolled patients into the trial, and all patients gave their written informed consent before participating in the study.
Study assessments
The following composite measures of PsA disease activity were used: Disease Activity Score of 28 joints with CRP [DAS28(CRP)] [16]; Disease Activity Index for Psoriatic Arthritis (DAPSA) [17, 18]; and Psoriatic Arthritis Disease Activity Score (PASDAS) [19, 20]. Disease activity was categorized as follows: DAS28(CRP) remission (<2.6), low (⩾2.6–<3.2), moderate (⩾3.2–⩽5.1) and high (>5.1) disease activity; DAPSA remission (⩽4), low (>4–⩽14), moderate (>14–⩽28) and high (>28) disease activity [21]; and PASDAS low (⩽3.2), moderate (>3.2–<5.4) and high (⩾5.4) disease activity [22]. Subjects were also categorized by achieving or not achieving minimal disease activity (MDA) [23].
Radiographic progression was measured by the modified total Sharp score (mTSS) on the radiographs of hands and feet taken at baseline and week 24.
Statistical analyses
The analyses were performed using as-observed data.
Disease activity by continuous measures (DAS28, DAPSA and PASDAS) was averaged over the period from baseline to week 24: time-averaged (TA) data were calculated as area under the curve for each disease activity measure and standardized by the length of the observation (24 weeks). The achievement of MDA, which is a binary measure (yes/no), was considered as a point assessment at week 24.
The interaction between treatment (ADA or PBO) and disease activity on ΔmTSS was evaluated using an analysis of covariance model adjusting for baseline mTSS, with disease activity, treatment and their interaction included in the model. TA-DAS28(CRP), TA-DAPSA and TA-PASDAS were modelled as continuous variables and MDA as a categorical variable. The different disease activity measures were introduced in separate models.
Changes in mTSS from baseline to week 24 in subjects categorized by TA disease activity or MDA status were compared between the treatments received (ADA or PBO).
The correlation between disease activity and ΔmTSS was assessed by Pearson [TA-DAS28(CRP), TA-DAPSA or TA-PASDAS as continuous variable] or biserial (MDA status as binary variable) correlation coefficients.
To assess the impact of MTX on the relationship between disease activity and structural progression, the same analyses were performed taking into account the presence or absence of concomitant MTX therapy in subjects treated with either ADA or PBO.
The α level of all statistical analyses was 0.05.
Results
Baseline demographics and disease characteristics
Of the 315 subjects randomized to receive treatment in ADEPT, 313 had evaluable radiographs at baseline and week 24. Of these, 151 received ADA and 162 PBO; approximately half of the subjects in both the ADA and PBO groups received concomitant MTX. Subjects’ baseline demographic and disease characteristics were comparable between the treatment groups. Baseline characteristics based on MTX use (Table 1) were also comparable across subgroups; only the frequency of baseline psoriasis BSA ⩾3% was lower in subjects receiving MTX.
Table 1.
Baseline demographics and disease characteristics for subjects receiving ADA or PBO with or without concomitant MTX
| Characteristica | Treatment group | |||||
|---|---|---|---|---|---|---|
| ADA (n = 151) | PBO (n = 162) | |||||
| +MTX (n = 77) | No MTX (n = 74) | Overall (n = 151) | +MTX (n = 81) | No MTX (n = 81) | Overall (n = 162) | |
| Age, mean (s.d.) (range), years | 48.0 (12.1) (20.0–76.0) | 49.3 (13.0) (24.0–88.0) | 48.6 (12.5) (20.0–88.0) | 50.9 (11.0) (24.0–73.0) | 47.6 (11.1) (21.0–79.0) | 49.2 (11.1) (21.0–79.0) |
| Sex male, n (%) | 42 (54.5) | 43 (58.1) | 85 (56.3) | 44 (54.3) | 45 (55.6) | 89 (54.9) |
| Baseline psoriasis BSA ≥3%, n (%) | 29 (37.7) | 41 (55.4) | 70 (46.4) | 28 (34.6) | 42 (51.9) | 70 (43.2) |
| TJC78 | 22.2 (16.8) | 25.7 (17.8) | 23.9 (17.3) | 25.7 (17.1) | 25.9 (19.0) | 25.8 (18.0) |
| SJC76 | 12.4 (9.7) | 16.3 (14.1) | 14.3 (12.2) | 12.9 (9.7) | 15.8 (12.3) | 14.3 (11.1) |
| Oligoarthritis, n (%)b | 2 (2.6) | 3 (4.1) | 5 (3.3) | 1 (1.2) | 1 (1.2) | 2 (1.2) |
| Polyarthritis, n (%)c | 75 (97.4) | 71 (95.9) | 146 (96.7) | 80 (98.8) | 80 (98.8) | 160 (98.8) |
| PhGA (VAS 0–100) | 53.8 (16.7) | 53.8 (14.7)** | 53.8 (15.7)** | 54.0 (15.0) | 53.0 (16.5)* | 53.5 (15.7)* |
| PtGA (VAS 0–100) | 44.9 (22.9) | 49.3 (23.4) | 47.1 (23.2) | 48.3 (19.4)* | 47.9 (22.9) | 48.1 (21.2)* |
| Pain (VAS 0–100) | 50.6 (22.7) | 51.5 (20.1) | 51.1 (21.4) | 47.4 (20.4)* | 50.1 (23.0) | 48.8 (21.7)* |
| CRP, mg/l | 1.5 (2.2) | 1.2 (2.0) | 1.4 (2.1) | 1.3 (1.6) | 1.5 (1.8) | 1.4 (1.7) |
| HAQ-DI | 1.0 (0.6) | 1.0 (0.6) | 1.0 (0.6) | 1.0 (0.6) | 1.0 (0.7) | 1.0 (0.7) |
| SF-36 PCS | 32.7 (10.3)* | 33.8 (9.5)** | 33.2 (9.9)*** | 32.5 (9.1) | 34.1 (10.4) | 33.3 (9.8) |
| Dactylitis | 1.9 (4.1)* | 2.7 (5.3)* | 2.3 (4.7)** | 2.1 (6.8)* | 2.0 (4.4) | 2.1 (5.7)* |
| Enthesitis | 0.9 (1.3)* | 0.6 (1.1)* | 0.8 (1.2)** | 1.0 (1.3)* | 0.8 (1.3)* | 0.9 (1.3)** |
Values are mean (s.d.) unless otherwise stated.
≤4 involved joints.
≥5 involved joints. Data missing for: *1 subject, **2 subjects, ***3 subjects. ADA: adalimumab; BSA: body surface area; HAQ-DI: HAQ-disability index; PCS: physical component summary; PhGA: physician global assessment; PBO: placebo; PtGA: patient global assessment; SF-36: Medical Outcomes Study Short-Form (36-item) Health Survey; SJC: swollen joint count; TJC: tender joint count; VAS: visual analogue scale.
Interaction between treatment and disease activity on radiographic progression
We first assessed the interaction between treatment (ADA or PBO) and disease activity on the change in mTSS at week 24. The interaction term was significant for TA-DAS28(CRP), TA-DAPSA and TA-PASDAS (P = 0.002, P = 0.008 and P = 0.006, respectively), indicating that the relationship between disease activity and radiographic progression did indeed differ between the subjects treated with ADA or PBO. No significant interaction between treatment and disease activity on structural progression could be observed by using MDA status at week 24 rather than TA measures of disease activity. This led us to further investigate the changes in radiographic progression across different levels of disease activity in patients receiving ADA or PBO.
Radiographic progression in subjects categorized by disease activity and treatment
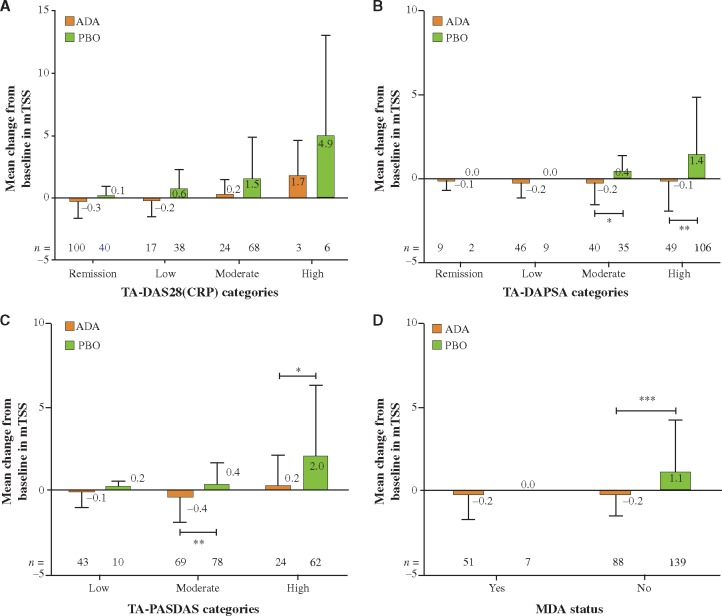
At week 24, the mean change in mTSS was low in subjects with remission or low disease activity, and increased in those with moderate or high disease activity (Fig. 1A–D). The subjects treated with ADA experienced lower mean changes in mTSS in all disease activity categories and by all disease activity measures compared with PBO (Fig. 1A–D). Remarkably, there was significantly less progression on ADA than PBO in subjects with moderate or high disease activity based on TA-DAPSA and -PASDAS or achievement of MDA at week 24. Although the numerical differences between radiographic progression on ADA and PBO were the largest with TA-DAS28, statistical significance was not reached, which could be due to low patient numbers in the categories of moderate and high disease activity (Fig. 1A–D). Similar results were seen with changes in both erosion and joint space narrowing scores across the TA-DAS28(CRP) disease activity categories (Supplementary Fig. 1, available at Rheumatology online).
Fig. 1.
Change in mTSS from baseline to week 24 in subjects grouped by disease activity and treatment
(A) TA-DAS28(CRP); (B) TA-DAPSA; (C) TA-PASDAS; (D) MDA status at week 24. *P < 0.05, **P < 0.01, ***P < 0.001 for ADA vs PBO. Error bars indicate s.d. ADA: adalimumab; DAS28: Disease Activity Score of 28 joints; MDA: minimal disease activity; mTSS: modified total Sharp score; DAPSA, Disease Activity Index for Psoriatic Arthritis; PASDAS: Psoriatic Arthritis Disease Activity Score; PBO: placebo; TA: time averaged.
Correlation between disease activity and radiographic progression
In subjects receiving PBO, there was a medium sized and statistically significant positive correlation between TA disease activity expressed by all disease activity measures and change in mTSS at week 24 (Table 2). No such relationship was observed in subjects treated with ADA. There was also no correlation between disease activity and structural progression for subjects treated with either ADA or PBO using MDA status at week 24 as a binary PsA disease activity measure, possibly due to the low number of patients achieving MDA in the PBO group.
Table 2.
Correlation between TA disease activity or MDA status and change in mTSS at week 24
| Disease activity measurea | Treatment | n | Correlation (95% CI) | P-value |
|---|---|---|---|---|
| TA-DAS28(CRP) | PBO | 152 | 0.351 (0.202, 0.482) | <0.001 |
| ADA | 144 | 0.123 (−0.042, 0.280) | 0.144 | |
| TA-DAPSA | PBO | 152 | 0.338 (0.188, 0.471) | <0.001 |
| ADA | 144 | 0.141 (−0.023, 0.298) | 0.091 | |
| TA-PASDAS | PBO | 150 | 0.300 (0.146, 0.438) | <0.001 |
| ADA | 136 | 0.076 (−0.094, 0.241) | 0.381 | |
| MDA | PBO | 146 | −0.078 (−0.237, 0.086) | 0.353 |
| ADA | 139 | −0.020 (−0.186, 0.147) | 0.815 |
TA-DAS28(CRP), TA-DAPSA and TA-PASDAS assessed using Pearson correlation; MDA assessed using biserial correlation. ADA: adalimumab; DAS28: Disease Activity Score of 28 joints; MDA: minimal disease activity; mTSS: modified total Sharp score; DAPSA, Disease Activity Index for Psoriatic Arthritis; PASDAS: Psoriatic Arthritis Disease Activity Score; PBO: placebo; TA: time averaged.
Impact of MTX on the relationship between disease activity and radiographic progression
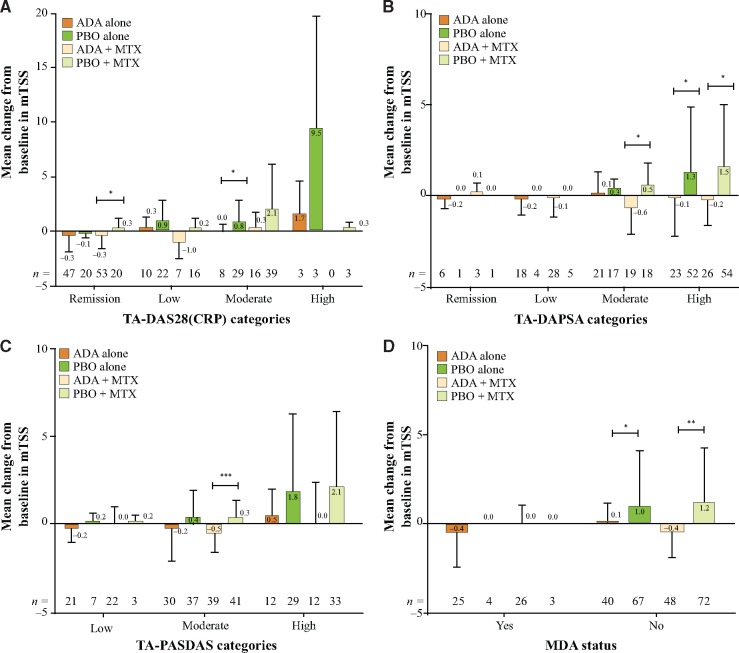
Irrespective of the presence or absence of MTX, the subjects treated with ADA experienced numerically less radiographic progression than those treated with PBO within all disease activity categories and by all disease activity measures (Fig. 2A–D). Further, concomitant MTX did not appear to markedly influence structural progression in subjects treated with ADA. Although not formally tested, there was also little difference between structural progression on PBO+MTX compared with PBO alone (Fig. 2A–D).
Fig. 2.
Change in mTSS from baseline to week 24 in subjects grouped by disease activity and treatment
(A) TA-DAS28(CRP); (B) TA-DAPSA; (C) TA-PASDAS; (D) MDA status at week 24 *P < 0.05, **P < 0.01, ***P < 0.001 for ADA vs PBO. Error bars indicate s.d. ADA: adalimumab; DAPSA: Disease Activity Index for Psoriatic Arthritis; DAS28: Disease Activity Score of 28 joints; MDA: minimal disease activity; mTSS: modified total Sharp score; PASDAS: Psoriatic Arthritis Disease Activity Score; PBO: placebo; TA: time averaged.
Discussion
The present analyses confirmed that treatment with ADA inhibited radiographic progression to a larger extent than might be expected based on the control of clinical disease activity alone, and that this was irrespective of concurrent MTX use. We first showed that the interaction terms for TA disease activity and treatment on radiographic progression were significant, implying that the relationship between disease activity and radiographic progression differed between ADA and PBO. Further investigation found that, as expected, radiographic progression was low in subjects in remission or low disease activity irrespective of treatment (although it was numerically lower in subjects treated with ADA). Radiographic progression increased in subjects with moderate or high disease activity, reflecting the fact that disease activity drives radiographic progression. However, the increase in radiographic progression in subjects with moderate or high disease activity was mainly seen in those receiving PBO, suggesting a disconnect between disease activity and radiographic progression in subjects treated with ADA. In support of this disconnect, radiographic progression only correlated positively with disease activity in subjects receiving PBO (the higher the disease activity the greater progression), but not in those receiving ADA.
Evidence for a disconnect between disease activity and radiographic progression has been observed previously in subjects with RA. In the ATTRACT study, infliximab appeared to inhibit radiographic progression independently of its effect on disease activity [10]. However, the statistical interaction between disease activity and radiographic progression as a result of treatment group was not formally assessed, so false correlations could be inferred when separating data into treatment subgroups [24]. Additionally, these analyses compared radiographic progression data solely with the achievement of 20% improvement in the American College of Rheumatology (ACR20) criteria rather than with more robust measures of disease activity. The ASPIRE study later confirmed the inhibitory effect of infliximab plus MTX (but not MTX alone) on radiographic progression in subjects with RA across all disease activity levels classified by the Simplified Disease Activity Index [11]. The disconnect phenomenon in RA has been further demonstrated in subsequent exploratory analyses of the TEMPO study of etanercept, using TA-CRP and TA-DAS to measure disease activity over time [12]. Similar to the present data in PsA, the authors found a significant interaction between treatment and disease activity with respect to radiographic progression, suggesting that the relationship between disease activity and radiographic progression differed by treatment. Higher disease activity was related to increased radiographic progression in subjects treated with MTX only; this relationship became less clear upon treatment with etanercept and was absent in subjects receiving etanercept in combination with MTX.
Importantly, in our PsA analyses MTX appeared to have little influence on radiographic progression and the relationship between disease activity and radiographic progression. Subject numbers in the treatment groups receiving ADA or PBO with or without MTX were probably too low to allow for a more definite conclusion. Our observation is nevertheless in line with previous findings that MTX in combination with a TNF inhibitor does not add a clear benefit in terms of either clinical or structural outcomes in PsA [15, 25–27]. The reasons for this remain largely unclear. In contrast, findings from studies in RA, such as TEMPO and PREMIER, have shown that MTX enhances inhibition of structural progression in subjects treated with a TNF inhibitor [28, 29]. Landewé and colleagues [12] suggested that etanercept and MTX may block different pathways of joint damage in RA, but these treatments may have different effects in PsA due to differing pathogenetic disease mechanisms.
One may argue that the conventional radiography used to measure structural changes in our study is not sensitive enough and therefore more subtle structural changes may have been missed. Furthermore, osteoproliferative changes are not captured by the mTSS scoring method. An imaging study using more sensitive techniques, such as magnetic resonance imaging and computed tomography, in addition to conventional radiography showed no significant progression of erosive or proliferative structural lesions either, despite persistence of certain levels of inflammation [30]. The subjects with PsA in that study otherwise demonstrated a clinical response to ADA therapy after 48 weeks. The authors speculated that TNF antagonists may inhibit the development of structural changes through an additional pathway and not only by suppressing inflammation [30]. Interestingly, evidence for uncoupling between disease activity and structural progression has been seen in our study for both erosions and joint space narrowing (Supplementary Fig. 1, available at Rheumatology online). This indicates that the disconnect phenomenon in PsA may be due to ADA inhibiting a common pathogenetic pathway for erosions and joint space narrowing, but certainly more research would be needed for a better understanding.
The limited duration of follow-up in the imaging study [30] was one drawback, and this may have also been a limitation of our analyses, which included ADEPT data through the initial 24-week PBO-controlled period [15]. The long-term open-label extension period of ADEPT showed that ADA maintained inhibition of radiographic progression in most subjects [13]. Additional radiographs taken at weeks 48 and 144 as part of this long-term extension could be analysed for correlation with disease activity in future work.
The vast majority of PsA subjects included in the ADEPT study and our analyses had polyarthritis. The existence of the disconnect phenomenon under ADA therapy remains to be investigated in the oligo- and mono-articular phenotypes of PsA, although there is little reason to believe that it would be different. It would also be of interest to investigate the relationship between radiographic progression and disease activity after treatment with other TNF inhibitors as well as other bDMARDs in subjects with PsA. In RA, the disconnect phenomenon has also been demonstrated with the IL-6 inhibitor tocilizumab and the B cell directed agent rituximab, both in combination with MTX [31, 32]. As these two bDMARDs are generally not efficacious in PsA, the ability of IL-17 and IL-23 inhibitors to retard radiographic progression beyond the control of clinical disease activity, and the dependency of this uncoupling on the use of concomitant MTX, would be of major interest.
In summary, this is the first in-depth analysis of the relationship between radiographic progression and disease activity under treatment with a TNF inhibitor in subjects with PsA. Treatment with ADA resulted in the inhibition of radiographic progression that was greater than expected based on clinical disease activity. The data suggest that even if a treatment target of remission or low disease activity is not achieved, radiographic progression may be inhibited in subjects with PsA receiving ADA. This may be particularly relevant in PsA subjects who are at high risk of comorbidities and may find it difficult to tolerate intensified treatment. Further analyses are needed to explore the long-term effect of this disconnect phenomenon and to better understand its potential thresholds and underlying mechanisms.
Supplementary Material
Acknowledgements
Medical writing support was provided by John Ewbank, PhD, of 2 the Nth, funded by AbbVie.
Funding: AbbVie funded the study (NCT00646386), contributed to its design, participated in data collection, analysis and interpretation of the data, and in writing, review, and approval of the publication.
Disclosure statement: R.L. has served as a consultant/participated in advisory boards for AbbVie, BMS, Janssen (formerly Centocor), Galapagos, Merck, Novartis, Pfizer and UCB; is Director of Rheumatology Consultancy BV; and has received research grants and speaker grants from AbbVie, Novartis, Pfizer and UCB. C.T.R. has received research grants from AbbVie, Amgen, Janssen and UCB, and consulting fees from AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer and UCB. L.C.C. has received honoraria and/or research funding from AbbVie, Amgen, BMS, Celgene, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer, Prothena, Sun Pharma and UCB. D.A. has received research grants, consulting fees and/or speaker’s fees from AbbVie, BMS, Lilly, Pfizer, Merck, Medac, UCB, Mitsubishi/Tanabe, Janssen and Roche. Y.Z., F.G. and M.H. are employees of AbbVie and may own stock/options.
Data sharing
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. Access is provided to anonymized, patient and trial-level data (analysis data sets), as well as other information (e.g. protocols and clinical study reports) from AbbVie-sponsored Phase II–IV global interventional clinical trials conducted in patients (completed as of May 2004, for products and indications approved in either the United States or the European Union), as long as the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications.
Access to this clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research, and will be provided following review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time and the data will be accessible for 12 months, with possible extensions considered. For more information on the process, or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
References
- 1. Kane D, Stafford L, Bresnihan B, FitzGerald O.. A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology (Oxford) 2003;42:1460–8. [DOI] [PubMed] [Google Scholar]
- 2. Ritchlin CT, Colbert RA, Gladman DD.. Psoriatic arthritis. N Engl J Med 2017;376:2095–6. [DOI] [PubMed] [Google Scholar]
- 3. Eder L, Thavaneswaran A, Chandran V, Gladman DD.. Tumour necrosis factor alpha blockers are more effective than methotrexate in the inhibition of radiographic joint damage progression among patients with psoriatic arthritis. Ann Rheum Dis 2014;73:1007–11. [DOI] [PubMed] [Google Scholar]
- 4. Gossec L, Smolen JS, Ramiro S. et al. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis 2016;75:499–510. [DOI] [PubMed] [Google Scholar]
- 5. Goulabchand R, Mouterde G, Barnetche T. et al. Effect of tumour necrosis factor blockers on radiographic progression of psoriatic arthritis: a systematic review and meta-analysis of randomised controlled trials. Ann Rheum Dis 2014;73:414–9. [DOI] [PubMed] [Google Scholar]
- 6. Ornbjerg LM, Ostergaard M, Boyesen P. et al. Impact of tumour necrosis factor inhibitor treatment on radiographic progression in rheumatoid arthritis patients in clinical practice: results from the nationwide Danish DANBIO registry. Ann Rheum Dis 2013;72:57–63. [DOI] [PubMed] [Google Scholar]
- 7. Acosta Felquer ML, Coates LC, Soriano ER. et al. Drug therapies for peripheral joint disease in psoriatic arthritis: a systematic review. J Rheumatol 2014;41:2277–85. [DOI] [PubMed] [Google Scholar]
- 8. Jones G, Crotty M, Brooks P.. Interventions for psoriatic arthritis. Cochrane Database Syst Rev 2000;(2):CD000212. [DOI] [PubMed] [Google Scholar]
- 9. Kaltwasser JP, Nash P, Gladman D. et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 2004;50:1939–50. [DOI] [PubMed] [Google Scholar]
- 10. Smolen JS, Han C, Bala M. et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum 2005;52:1020–30. [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Han C, van der Heijde DM. et al. Radiographic changes in rheumatoid arthritis patients attaining different disease activity states with methotrexate monotherapy and infliximab plus methotrexate: the impacts of remission and tumour necrosis factor blockade. Ann Rheum Dis 2009;68:823–7. [DOI] [PubMed] [Google Scholar]
- 12. Landewé R, van der Heijde D, Klareskog L, van Vollenhoven R, Fatenejad S.. Disconnect between inflammation and joint destruction after treatment with etanercept plus methotrexate: results from the trial of etanercept and methotrexate with radiographic and patient outcomes. Arthritis Rheum 2006;54:3119–25. [DOI] [PubMed] [Google Scholar]
- 13. Mease PJ, Ory P, Sharp JT. et al. Adalimumab for long-term treatment of psoriatic arthritis: 2-year data from the adalimumab effectiveness in psoriatic arthritis trial (ADEPT). Ann Rheum Dis 2009;68:702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mease P. Disconnect between radiographic progression and clinical outcomes in psoriatic arthritis patients treated with adalimumab. Arthritis Rheum 2010;62: abstract 1935. [Google Scholar]
- 15. Mease PJ, Gladman DD, Ritchlin CT. et al. Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial. Arthritis Rheum 2005;52:3279–89. [DOI] [PubMed] [Google Scholar]
- 16. Fransen J, Stucki G, van Riel PLCM.. Rheumatoid arthritis measures: disease activity score (DAS), disease activity score-28 (DAS28), rapid assessment of disease activity in rheumatology (RADAR), and rheumatoid arthritis disease activity index (RADAI). Arthritis Rheum 2003;49:S214–24. [Google Scholar]
- 17. Schoels M, Aletaha D, Funovits J. et al. Application of the DAREA/DAPSA score for assessment of disease activity in psoriatic arthritis. Ann Rheum Dis 2010;69:1441–7. [DOI] [PubMed] [Google Scholar]
- 18. Smolen JS, Schoels M, Aletaha D.. Disease activity and response assessment in psoriatic arthritis using the disease activity index for psoriatic arthritis (DAPSA). A brief review. Clin Exp Rheumatol 2015;33:S48–50. [PubMed] [Google Scholar]
- 19. Coates LC, FitzGerald O, Mease PJ. et al. Development of a disease activity and responder index for psoriatic arthritis—report of the psoriatic arthritis module at OMERACT 11. J Rheumatol 2014;41:782–91. [DOI] [PubMed] [Google Scholar]
- 20. Helliwell PS, FitzGerald O, Fransen J. et al. The development of candidate composite disease activity and responder indices for psoriatic arthritis (GRACE project). Ann Rheum Dis 2013;72:986–91. [DOI] [PubMed] [Google Scholar]
- 21. Schoels MM, Aletaha D, Alasti F, Smolen JS.. Disease activity in psoriatic arthritis (PsA): defining remission and treatment success using the DAPSA score. Ann Rheum Dis 2016;75:811–8. [DOI] [PubMed] [Google Scholar]
- 22. Helliwell PS, FitzGerald O, Fransen J.. Composite disease activity and responder indices for psoriatic arthritis: a report from the GRAPPA 2013 meeting on development of cutoffs for both disease activity states and response. J Rheumatol 2014;41:1212–7. [DOI] [PubMed] [Google Scholar]
- 23. Coates LC, Fransen J, Helliwell PS.. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. [DOI] [PubMed] [Google Scholar]
- 24. Altman DG, Bland JM.. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Antoni C, Krueger GG, de Vlam K. et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT-2 trial. Ann Rheum Dis 2005;64:1150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Behrens F, Koehm M, Arndt U. et al. Does concomitant methotrexate with adalimumab influence treatment outcomes in patients with psoriatic arthritis? Data from a large observational study. J Rheumatol 2016;43:632–9. [DOI] [PubMed] [Google Scholar]
- 27. Mease PJ, Kivitz AJ, Burch FX. et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004;50:2264–72. [DOI] [PubMed] [Google Scholar]
- 28. Breedveld FC, Weisman MH, Kavanaugh AF. et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 29. Klareskog L, van der Heijde D, de Jager JP. et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 2004;363:675–81. [DOI] [PubMed] [Google Scholar]
- 30. Poggenborg RP, Wiell C, Boyesen P. et al. No overall damage progression despite persistent inflammation in adalimumab-treated psoriatic arthritis patients: results from an investigator-initiated 48-week comparative magnetic resonance imaging, computed tomography and radiography trial. Rheumatology (Oxford) 2014;53:746–56. [DOI] [PubMed] [Google Scholar]
- 31. Aletaha D, Alasti F, Smolen JS.. Rituximab dissociates the tight link between disease activity and joint damage in rheumatoid arthritis patients. Ann Rheum Dis 2013;72:7–12. [DOI] [PubMed] [Google Scholar]
- 32. Smolen JS, Avila JC, Aletaha D.. Tocilizumab inhibits progression of joint damage in rheumatoid arthritis irrespective of its anti-inflammatory effects: disassociation of the link between inflammation and destruction. Ann Rheum Dis 2012;71:687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.