Abstract
Objectives
To evaluate the efficacy of denosumab for progressive bone erosion in risk factor subgroups of Japanese RA patients.
Methods
This study included 340 RA patients on MTX from the dose-response study of Denosumab in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE study-a 12-month, multicentre, randomized, double-blind, placebo-controlled, phase II study). The patients were randomized to receive placebo or denosumab 60 mg every 6 months, 3 months or 2 months. Subgroup analyses involved baseline RF, ACPA, swollen joint count, CRP level, RA duration, ESR and glucocorticoid use.
Results
Patients with risk factor positivity generally showed consistent results for the primary endpoint of the change in the modified Sharp erosion score at 12 months from baseline. In the placebo, every 6 months, every 3 months and every 2 months groups, the mean changes in the erosion score, according to the RF status (RF-positive vs -negative subgroups), were 1.18 vs 0.59, 0.25 (P = 0.0601 vs placebo) vs 0.31 (P = 0.0827), 0.21 (P = 0.0422) vs −0.02 (P = 0.0631) and 0.15 (P = 0.0010) vs −0.05 (P = 0.0332), respectively, while the mean changes in the erosion score, according to the ACPA status (ACPA-positive vs -negative subgroups), were 1.30 vs 0.07, 0.26 (P = 0.0142) vs 0.33 (P = 0.2748), 0.16 (P = 0.0058) vs 0.08 (P = 0.7166) and 0.09 (P < 0.0001) vs 0.08 (P = 0.8939), respectively.
Conclusion
Denosumab is a potentially useful treatment option for RA patients who are positive for RF, ACPA and other possible risk factors.
Trial registration
JAPIC Clinical Trials Information, http://www.clinicaltrials.jp/user/cteSearch_e.jsp, JapicCTI-101263.
Keywords: rheumatoid arthritis, denosumab, bone erosion, subgroup analysis, rheumatoid factor, anti-cyclic citrullinated peptide antibody
Rheumatology key messages
RF and ACPA are prognostic factors that predict progressive bone erosion in RA patients.
Denosumab effectively prevents bone erosion progression in patients receiving MTX with these risk factors.
Denosumab is expected to show reliable efficacy in patients with extensive erosion progression.
Introduction
RA is a chronic disease characterized by persistent synovitis, systemic inflammation and joint destruction [1]. One of the causes of bone erosion and bone loss is increased osteoclast activity [2]. M-CSF and receptor activator of nuclear factor kB (RANK) ligand (RANKL) are considered to be essential for osteoclast differentiation, activation and survival [3–7]. Denosumab is a fully human mAb that binds specifically to human RANKL and inhibits bone resorption [8]; thus, it is expected to prevent the progression of bone erosion and bone loss.
We previously reported the efficacy of denosumab with regard to the inhibition of bone erosion among Japanese RA patients in the dose-response study of Denosumab in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE study), which was a 12-month, multicentre, randomized, double-blind, placebo-controlled, phase II study of denosumab administered subcutaneously at a dosage of 60 mg every 6 months (Q6M), 3 months (Q3M) or 2 months (Q2M) with MTX [9]. The results indicated that denosumab inhibited the progression of bone erosion. However, differences in the treatment response according to subgroups based on the bone erosion progression risk factor status have not been reported. Previous studies have reported that poor prognostic factors, such as RF and ACPA, are risk factors for the progression of radiographic erosion in RA patients [10, 11]. In the present study, we focused on the results of subgroups that had poor prognostic factors in the DRIVE study. We grouped patients according to the swollen joint count (SJC) and CRP level, in addition to RF and ACPA, because these have been reported as risk factors for bone destruction [12–14]. The primary objective of this subgroup analysis was to evaluate the effect of denosumab on bone erosion progression among RA patients in risk factor subgroups.
Methods
Patients and study design
The eligibility criteria and design of this study have been described in detail in our previous report [9]. It is important to note that, at registration, we excluded patients who were orally administered Bisphosphonates (BPs) for a total of 2 years (104 weeks) or more in the past, as well as patients who were intravenously administered BPs in the past.
Patients were randomly registered under a double-blind design to subcutaneously receive either placebo or denosumab 60 mg Q6M, Q3M or Q2M. Randomization was stratified according to glucocorticoid use and RF status at baseline. All patients basically continued MTX treatment (6–16 mg/week) with vitamin D ⩾400 IU and calcium ⩾600 mg/day. The investigators were allowed to prescribe bucillamine, salazosulfapyridine, glucocorticoid and/or NSAIDs at any time throughout the study. Patient eligibility depended on a diagnosis of RA by the investigator and fulfilment of the 1987 American College of Rheumatology (ACR) criteria [15], with RA disease duration of 6 months to <5 years and at least 6 swollen joints among a total of 58 joints assessed by the investigator at screening. For enrolment, patients also had to demonstrate radiographic bone erosion as assessed by the investigator or meet the following criteria at screening: CRP level ⩾1.0 mg/dl or ESR ⩾28 mm/h, and ACPA positivity or RF level >20 IU/ml. The main exclusion criterion was previous or current use of biologics for the treatment of RA. The use of bisphosphonates and oral glucocorticoids at a dose of >10 mg/day (prednisolone equivalent) was prohibited throughout the study.
This study was approved by the institutional review board of each participating institution and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Radiographic assessment
Radiographs of the hands, wrists and feet, obtained at baseline and at 6 and 12 months, were assessed according to the van der Heijde modified Sharp method [16]. Two well-trained readers who were blinded to the treatment groups, patient characteristics and time order evaluated the images. The average scores of the two readers were used for analyses. The changes in the modified Sharp erosion score from baseline were calculated at 6 and 12 months.
Statistical analysis
Changes in the erosion score at 12 months were analysed according to subgroups based on the RF status (positive or negative), ACPA status (positive or negative), baseline SJC (<10 or ⩾10), baseline CRP level (<1.0 or ⩾1.0 mg/dl), RA duration (<3 or ⩾3 years, this cut-off indicates early RA or not), glucocorticoid use (absence or presence) and baseline ESR (<28 or ⩾28 mm/h). Two-sided van Elteren stratified rank tests, adjusting for randomized strata (four strata according to the combination of baseline glucocorticoid use and baseline RF), were performed for comparisons between the denosumab groups and placebo group with regard to changes in the radiographic score. In post hoc analysis, the proportion of patients without radiographic progression (change in the erosion score from baseline ⩽0.5) and the proportion of patients with rapid erosion progression (change in the erosion score from baseline ⩾3.0 or ⩾5.0) among patients in risk factor subgroups were analysed using Fisher’s exact test.
Efficacy endpoints were analysed using the full analysis set that included all randomized patients who received at least one dose of denosumab or placebo and had a baseline radiographic score, as well as at least one post-baseline radiographic score. Missing values for the radiographic scores were imputed using linear extrapolation/interpolation. All statistical analyses were performed using SAS software (release 9.2; SAS Institute, Inc., Cary, NC, USA). The significance level was set at 0.05.
Results
Baseline characteristics and patient disposition
The study included 340 RA patients. Baseline demographics and characteristics were generally comparable between the treatment groups [9].
The mean (s.d.) patient ages were 57.0 (10.57) years in the placebo group, 54.4 (10.57) years in the Q6M group, 52.0 (11.65) years in the Q3M group and 54.6 (10.51) years in the Q2M group. However, the proportion of female patients was slightly higher in the placebo group (86.4%) than in the other groups (76.5% in the Q6M group, 72.0% in the Q3M group and 77.6% in the Q2M group).
Radiographic progression
Denosumab significantly inhibited the progression of bone erosion at 12 months when compared with the progression on placebo treatment in the overall population of the DRIVE study. The mean changes in the modified Sharp erosion score at 12 months from baseline in the placebo, Q6M, Q3M and Q2M groups were 0.99, 0.27 (P = 0.0082 vs placebo), 0.14 (P = 0.0036) and 0.09 (P < 0.0001), respectively [9].
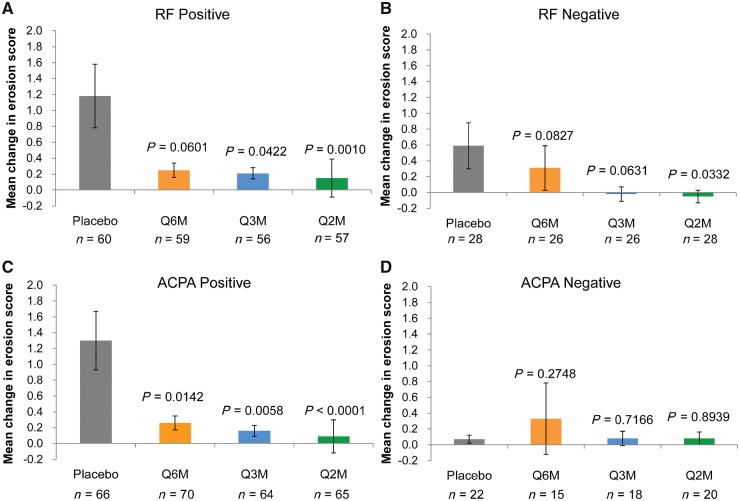
The score change was greater among RF-positive patients than among RF-negative patients in the placebo group. On the other hand, the difference in the score change between RF-positive and RF-negative patients was less pronounced in the denosumab groups (Fig. 1A and B). A similar tendency was noted for ACPA (Fig. 1C and D).
Fig. 1.
Mean change in modified Sharp erosion score from baseline to 12 months in the subgroups
(A) RF-positive subgroup, (B) RF-negative subgroup, (C) ACPA-positive subgroup and (D) ACPA-negative subgroup. The means ± s.e.m. are presented. P-values were calculated using the two-sided van Elteren stratified rank test adjusted for randomized strata (four strata according to the combination of baseline glucocorticoid use and baseline RF) vs placebo. Q2M: denosumab 60 mg every 2 months; Q3M: denosumab 60 mg every 3 months; Q6M: denosumab 60 mg every 6 months.
Changes in the modified Sharp erosion score were numerically smaller among RF-positive patients in all denosumab groups than in the placebo group (Fig. 1A and Table 1). The difference between the placebo and Q3M or Q2M groups was statistically significant; however, the difference between the placebo and Q6M groups was not significant. Similarly, the changes were numerically and significantly smaller among ACPA-positive patients in all denosumab groups than in the placebo group (Fig. 1C and Table 1). Changes in the bone erosion score among denosumab-treated patients decreased dose-dependently in the RF- and ACPA-positive subgroups. These results are consistent with the primary endpoint in the overall population [the mean changes in the bone erosion score in the placebo, Q6M, Q3M and Q2M groups were 0.99 (95% CI 0.42, 1.56), 0.27 (0.06, 0.48, P = 0.0082 vs placebo), 0.14 (0.02, 0.26, P = 0.0036) and 0.09 (−0.24, 0.41, P < 0.0001), respectively].
Table 1.
Changes in erosion score from baseline to 12 months, according to RF and ACPA status
| Denosumab | ||||
|---|---|---|---|---|
| Placebo (N = 88) | 60 mg Q6M (N = 85) | 60 mg Q3M (N = 82) | 60 mg Q2M (N = 85) | |
| RF status | ||||
| Positive | ||||
| n | 60 | 59 | 56 | 57 |
| Mean (s.d.) | 1.18 (3.08) | 0.25 (0.73) | 0.21 (0.55) | 0.15 (1.83) |
| Median (Q1, Q3) | 0.00 (0.00, 1.00) | 0.000 (0.00, 0.50) | 0.000 (0.00, 0.00) | 0.000 (0.00, 0.00) |
| P-value | – | 0.0601 | 0.0422 | 0.0010 |
| Negative | ||||
| n | 28 | 26 | 26 | 28 |
| Mean (s.d.) | 0.59 (1.53) | 0.31 (1.41) | −0.02 (0.46) | −0.05 (0.44) |
| Median (Q1, Q3) | 0.00 (0.00, 0.50) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.0827 | 0.0631 | 0.0332 |
| ACPA status | ||||
| Positive | ||||
| n | 66 | 70 | 64 | 65 |
| Mean (s.d.) | 1.30 (3.04) | 0.26 (0.75) | 0.16 (0.57) | 0.09 (1.73) |
| Median (Q1, Q3) | 0.00 (0.00, 1.50) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.0142 | 0.0058 | <0.0001 |
| Negative | ||||
| n | 22 | 15 | 18 | 20 |
| Mean (s.d.) | 0.07 (0.23) | 0.33 (1.73) | 0.08 (0.39) | 0.08 (0.37) |
| Median (Q1, Q3) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.2748 | 0.7166 | 0.8939 |
The erosion score was assessed using the van der Heijde modified Sharp method. P-values were calculated using the two-sided van Elteren stratified rank test adjusted for randomized strata (four strata according to the combination of baseline glucocorticoid use and baseline RF) vs placebo. Q2M: denosumab 60 mg every 2 months; Q3M: denosumab 60 mg every 3 months; Q6M: denosumab 60 mg every 6 months; N: number of patients who received ≥1 dose of the investigational product and had baseline measurement and at least one post-baseline measurement of the radiographic score; n: number of patients in the subgroup; Q1, Q3: quartile 1, 3.
On the other hand, the difference in the score between the placebo group and the Q6M, Q3M or Q2M group was numerically lower among RF-negative patients than among RF-positive patients, while a significant difference was observed for only the Q2M group (Fig. 1B and Table 1). Additionally, among ACPA-negative patients, no numerical or significant differences were observed between the placebo and denosumab groups (Fig. 1D and Table 1).
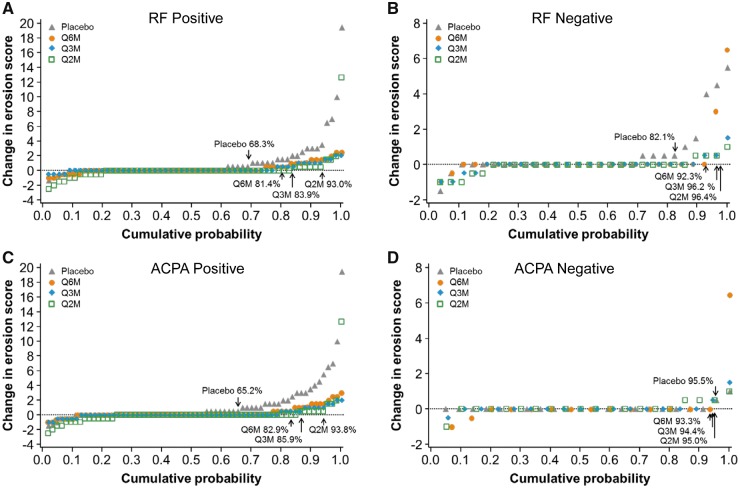
The proportion of patients with rapid erosion progression (change in erosion score ⩾3 or ⩾5) was larger among RF-positive patients than among RF-negative patients in the placebo group (Table 2). The proportion decreased in the denosumab groups, regardless of the baseline RF status; however, statistical significance was observed only for erosion score ⩾3 in the RF-positive subgroup. With regard to the subgroups according to the baseline ACPA status, only ACPA-positive patients in the placebo group showed rapid erosion progression. The proportion of patients with a change in erosion score ⩾3 was significantly lower in the denosumab groups than in the placebo group (Table 2). The cumulative probability plots of changes in the erosion score showed that the proportion of patients without radiographic progression was quantitatively larger in all denosumab groups than in the placebo group among the RF-positive, RF-negative and ACPA-positive subgroups, but not the ACPA-negative subgroup (Fig. 2).
Table 2.
Proportion of patients with rapid erosion progression within the subgroups (RF and ACPA status)
| Change in erosion score ≥3 | Change in erosion score ≥5 | |||
|---|---|---|---|---|
| n (%) | P-value | n (%) | P-value | |
| RF status | ||||
| Positive | ||||
| Placebo (N = 60) | 8 (13.3) | – | 4 (6.7) | – |
| 60 mg Q6M (N = 59) | 0 (0.0) | 0.0061 | 0 (0.0) | 0.1187 |
| 60 mg Q3M (N = 56) | 0 (0.0) | 0.0063 | 0 (0.0) | 0.1194 |
| 60 mg Q2M (N = 57) | 1 (1.8) | 0.0325 | 1 (1.8) | 0.3649 |
| Negative | ||||
| Placebo (N = 28) | 3 (10.7) | – | 1 (3.6) | – |
| 60 mg Q6M (N = 26) | 2 (7.7) | 1.0000 | 1 (3.8) | 1.0000 |
| 60 mg Q3M (N = 26) | 0 (0.0) | 0.2369 | 0 (0.0) | 1.0000 |
| 60 mg Q2M (N = 28) | 0 (0.0) | 0.2364 | 0 (0.0) | 1.0000 |
| ACPA status | ||||
| Positive | ||||
| Placebo (N = 66) | 11 (16.7) | – | 5 (7.6) | – |
| 60 mg Q6M (N = 70) | 1 (1.4) | 0.0018 | 0 (0.0) | 0.0248 |
| 60 mg Q3M (N = 64) | 0 (0.0) | 0.0006 | 0 (0.0) | 0.0579 |
| 60 mg Q2M (N = 65) | 1 (1.5) | 0.0043 | 1 (1.5) | 0.2079 |
| Negative | ||||
| Placebo (N = 22) | 0 (0.0) | – | 0 (0.0) | – |
| 60 mg Q6M (N = 15) | 1 (6.7) | 0.4054 | 1 (6.7) | 0.4054 |
| 60 mg Q3M (N = 18) | 0 (0.0) | – | 0 (0.0) | – |
| 60 mg Q2M (N = 20) | 0 (0.0) | – | 0 (0.0) | – |
P-values were calculated using Fisher’s exact test. N: number of patients who received ≥1 dose of the investigational product and had baseline measurement and at least one post-baseline measurement of the radiographic score in the subgroup; n: number of patients with an erosion score ≥3 or ≥5.
Fig. 2.
Cumulative probability plot of the change from baseline to 12 months in the subgroups
(A) RF-positive subgroup, (B) RF-negative subgroup, (C) ACPA-positive subgroup and (D) ACPA-negative subgroup. The figures in the plots are the proportions of patients without erosion progression (Δ erosion score ≤0.5). Q2M: denosumab 60 mg every 2 months; Q3M: denosumab 60 mg every 3 months; Q6M: denosumab 60 mg every 6 months.
Furthermore, we performed subgroup analysis involving other potential prognostic factors. Changes in the bone erosion score were smaller in the denosumab groups than in the placebo group among all subgroups that were based on factors such as baseline SJC, baseline CRP level, RA duration and baseline ESR status (Table 3 and supplementary Figs S1–S4, available at Rheumatology online). A numerically great change in the erosion scores of patients in the placebo group was observed in the patients with a baseline SJC ⩾10, baseline CRP level ⩾1.0 mg/dl, RA duration ⩾3 years or baseline ESR ⩾28 mm/h. However, significant differences were only observed in the Q3M and Q2M groups, among patients with a baseline SJC ⩾10 and in the Q2M group among those with a baseline ESR ⩾28 mm/h. With regard to the counterpart subgroups (i.e. baseline SJC <10, baseline CRP level <1.0 mg/dl, RA duration <3 years and baseline ESR <28 mm/h) that showed a small change (around 0.5) in the bone erosion score in the placebo group, significant decreases in the score were observed in all denosumab groups, excluding the Q6M group, among patients with an RA duration <3 years.
Table 3.
Changes in the erosion score from baseline to 12 months according to other risk factors
| Denosumab | ||||
|---|---|---|---|---|
| Placebo (N = 88) | 60 mg Q6M (N = 85) | 60 mg Q3M (N = 82) | 60 mg Q2M (N = 85) | |
| Baseline SJC | ||||
| ≥10 | ||||
| n | 39 | 27 | 42 | 40 |
| Mean (s.d.) | 1.50 (3.61) | 0.71 (1.40) | 0.25 (0.64) | 0.03 (0.53) |
| Median (Q1, Q3) | 0.00 (0.00, 1.00) | 0.00 (0.00, 1.50) | 0.00 (0.00, 0.50) | 0.00 (0.00, 0.00) |
| P-value | – | 0.4747 | 0.038 | 0.0009 |
| <10 | ||||
| n | 49 | 58 | 40 | 45 |
| Mean (s.d.) | 0.59 (1.55) | 0.07 (0.63) | 0.03 (0.37) | 0.14 (2.04) |
| Median (Q1, Q3) | 0.00 (0.00, 0.50) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.0228 | 0.0479 | 0.0186 |
| Baseline CRP | ||||
| ≥1.0 mg/dl | ||||
| n | 21 | 13 | 13 | 11 |
| Mean (s.d.) | 1.69 (4.35) | 0.89 (1.89) | 0.42 (0.61) | 0.32 (1.03) |
| Median (Q1, Q3) | 0.00 (0.00, 2.00) | 0.00 (0.00, 1.50) | 0.00 (0.00, 1.00) | 0.00 (−0.50, 1.50) |
| P-value | – | 0.6656 | 0.6310 | 0.3966 |
| <1.0 mg/dl | ||||
| n | 67 | 72 | 69 | 74 |
| Mean (s.d.) | 0.77 (1.90) | 0.16 (0.67) | 0.09 (0.51) | 0.05 (1.58) |
| Median (Q1, Q3) | 0.00 (0.00, 1.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.0213 | 0.0078 | 0.0002 |
| Duration of RA | ||||
| ≥3 years | ||||
| n | 28 | 26 | 24 | 25 |
| Mean (s.d.) | 1.65 (4.20) | 0.03 (0.64) | 0.04 (0.55) | 0.59 (2.63) |
| Median (Q1, Q3) | 0.00 (0.00, 1.75) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.1549 | 0.1746 | 0.1702 |
| <3 years | ||||
| n | 60 | 59 | 58 | 60 |
| Mean (s.d.) | 0.68 (1.51) | 0.37 (1.09) | 0.18 (0.53) | −0.13 (0.57) |
| Median (Q1, Q3) | 0.00 (0.00, 0.75) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.1036 | 0.0298 | <0.0001 |
| Baseline ESR | ||||
| ≥28 mm/h | ||||
| n | 33 | 23 | 26 | 29 |
| Mean (s.d.) | 1.88 (3.97) | 0.81 (1.60) | 0.42 (0.64) | 0.03 (0.77) |
| Median (Q1, Q3) | 0.00 (0.00, 2.00) | 0.00 (0.00, 1.50) | 0.00 (0.00, 1.00) | 0.00 (0.00, 0.50) |
| P-value | – | 0.4237 | 0.2920 | 0.0144 |
| <28 mm/h | ||||
| n | 55 | 62 | 56 | 56 |
| Mean (s.d.) | 0.46 (1.23) | 0.07 (0.50) | 0.01 (0.42) | 0.11 (1.80) |
| Median (Q1, Q3) | 0.00 (0.00, 0.50) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.0173 | 0.0081 | 0.0034 |
| Glucocorticoid use | ||||
| Present | ||||
| n | 37 | 36 | 37 | 37 |
| Mean (s.d.) | 1.37 (3.74) | 0.33 (1.24) | 0.23 (0.57) | −0.07 (0.73) |
| Median (Q1, Q3) | 0.00 (0.00, 0.50) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.50) | 0.00 (0.00, 0.00) |
| P-value | – | 0.1885 | 0.5262 | 0.0072 |
| Absent | ||||
| n | 51 | 49 | 45 | 48 |
| Mean (s.d.) | 0.72 (1.51) | 0.22 (0.74) | 0.07 (0.50) | 0.20 (1.92) |
| Median (Q1, Q3) | 0.00 (0.00, 1.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.00) |
| P-value | – | 0.0307 | 0.0023 | 0.0041 |
The erosion score was assessed using the van der Heijde modified Sharp method. P-values were calculated using the two-sided van Elteren stratified rank test adjusted for randomized strata (four strata according to the combination of baseline glucocorticoid use and baseline RF) vs placebo. Q2M: denosumab 60 mg every 2 months; Q3M: denosumab 60 mg every 3 months; Q6M: denosumab 60 mg every 6 months; N: number of patients who received ≥1 dose of the investigational product and had baseline measurement and at least one post-baseline measurement of the radiographic score; n: number of patients in the subgroup; SJC: swollen joint count; Q1, Q3: quartile 1, 3.
In addition, subgroup analysis involving glucocorticoid use (one of the stratification factors) was performed. In the placebo group, bone erosion progression was greater among patients with basal glucocorticoid use than among those without glucocorticoid use (Table 3 and supplementary Fig. S5, available at Rheumatology online). Denosumab effectively reduced the mean change in the erosion score in both subgroups. Among patients with glucocorticoid use, a significant reduction in the erosion score was observed only in the Q2M group, while among patients in all denosumab groups without glucocorticoid use, a significant reduction was observed.
Discussion
Bone erosion is a central clinical feature of RA and is associated with enhanced osteoclast differentiation stimulated by two essential mediators (M-CSF and RANKL) [3]. Denosumab, an anti-RANKL antibody, was found to be effective for bone erosion; however, it likely has no effect on disease activity [9, 17]. In this study, we evaluated the efficacy of denosumab with regard to the progression of bone erosion in patients receiving MTX among subgroups positive for the risk factors of progression, such as RF, ACPA status, baseline SJC and CRP level. The results were generally consistent for the primary endpoint, with regard to the overall population of the DRIVE study. Additionally, the quantitative trend of dose-dependent inhibition in the risk factor subgroups was consistent with the result in the overall population. However, there were differences among the subgroups according to the presence/absence of the risk factors of progression in, not only the placebo group, but also the denosumab groups.
Previous studies have shown that positive RF and ACPA findings are risk factors for bone erosion progression in patients with RA [11, 12]. Our results are consistent with these findings. Radiographic progression at 12 months was much greater among RF-positive and ACPA-positive patients than among negative patients, especially in the placebo group. Denosumab showed a potent inhibitory effect on bone erosion in a dose-dependent manner among RF-positive and ACPA-positive patients. Thus, denosumab is considered as a treatment option for RA patients with bone erosion progression, despite a positive RF and/or ACPA status.
The present study also evaluated the efficacy of denosumab in RF-negative and ACPA-negative patients, who may experience only limited progression of joint destruction. Denosumab did not show a clear effect in these patients. Accordingly, these patients do not appear to benefit from denosumab treatment. However, the assessment of negative subgroups involved small sample sizes; thus, the findings should be interpreted with caution. Moreover, the disease activity of patients in this study was lower than that of patients in clinical studies involving other biologics [18–20]. It is likely that the investigators controlled the disease activity well in this study, as they could adjust the dosage of MTX within approved limits and prescribe salazosulfapyridine and/or bucillamine as needed throughout the study period. Nevertheless, 17.9 and 4.5% of patients experienced ongoing bone erosion (score >0.5) in the RF-negative and ACPA-negative subgroups of the placebo group, respectively. However, denosumab was shown to reduce this proportion. Previous studies reported that erosion progresses in patients who have not achieved remission, despite control of disease activity with conventional synthetic DMARDs [21, 22]. In addition, a substantial proportion of patients treated with MTX monotherapy show rapid radiographic progression despite RF and/or ACPA negativity [13]. Thus, the current prognostic risk factors are not sufficient to identify progressive patients, and it remains unclear whether denosumab is beneficial for RA patients with a low risk for progressive bone erosion.
Denosumab was significantly effective at all assessed doses among patients with a baseline SJC ⩾10, baseline CRP <1.0 mg/dl, RA duration <3 years, baseline ESR <28 mm/h and no glucocorticoid use. However, in contrast to the subgroups with the RF and ACPA status, the changes in bone destruction were smaller among these subgroups in the placebo group. In this study, only RF and glucocorticoid use were stratification factors, while the treatment assigned within these subgroups was balanced for known and unknown prognostic factors. The other subgroups were created post hoc, so there may exist an imbalance in the other prognostic factors. Additionally, interactions between the subgroups were not assessed because of the small sample size. Therefore, the statistical results of this study should be interpreted carefully.
The present study had some limitations. First, the treatment duration was relatively short (12 months). Second, the sample size was small, resulting in seemingly underpowered significance analyses in some subgroups. Finally, only a partial post hoc analysis was performed (proportions of patients without radiographic progression and rapid radiographic progression in the DRIVE study).
In conclusion, RF and ACPA are poor prognostic factors and denosumab is a potentially useful treatment option for RA patients receiving MTX with these factors. Although denosumab was found to reduce the proportion of progressive patients in the risk factor-negative subgroups, a further clinical trial with an adequate sample size is required to confirm the findings.
Supplementary Material
Acknowledgements
The authors would like to thank the patients and the investigators who were involved in this study. We also acknowledge the assistance of Amgen Inc. for editorial assistance on the manuscript. We thank WysiWyg Co., Ltd for manuscript editing.
Funding: This work was supported by Daiichi-Sankyo Co., Ltd. The sponsor was involved in the study conception, design, conduct, data collection and data analyses.
Disclosure statement: T.O. and N.O. are employees of Daiichi Sankyo. N.I. has received research grants from Abbott, Astellas, Bristol-Myers-Squibb, Chugai and Daiichi Sankyo and has served on speakers’ bureaus for Hisamitsu, Janssen, Kaken, Mitsubishi Tanabe, Taisho Toyama and UCB. Y.T. has received research grants from AbbVie, Astellas, Chugai, Bristol-Myers Squibb, Daiichi Sankyo, Eisai, Kyowa Hakko Kirin, Mitsubishi Tanabe, MSD, Ono, Pfizer and Takeda, and has received personal fees from Astellas, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eli Lilly, Janssen, Mitsubishi Tanabe, Pfizer, Sanofi, UCB and YL Biologics. H.Y. has received research grants from AbbVie, Astellas, AYUMI, BMS, Chugai, Daiichi Sankyo, Eisai, Kaken, Mitsubishi Tanabe, MSD, Nippon Shinyaku Ono, Pfizer, Takeda, Teijin, Torii and UCB, and has received consulting fees from Astellas, BMS, Chugai, Daiichi Sankyo, Mitsubishi Tanabe, Nippon Kayaku, Pfizer, Takeda, Teijin and YL Biologics. T.Y. has received Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT #17H04377) and has received consulting fees from Daiichi Sankyo. H.K.G. has received consulting fees from Amgen, Agnovos, Bioclinica, Biomarin, Clementia, Daiichi Sanyo, Eli Lilly, Janssen, Medimmune, Merck, Novartis, Pfizer, Regeneron, Servier and Takeda. D.v.d.H. has received consulting fees from AbbVie, Amgen, Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Galapagos, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB, and is the director of Imaging Rheumatology BV. T.T. has received research grants from AbbVie, Asahi Kasei, Astellas, AYUMI, Chugai, Daiichi Sankyo, Eisai, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Pfizer and Takeda, and has received personal fees from AbbVie, Astellas, Astra Zeneca, Bristol-Myers Squibb, Chugai, Daiichi Sankyo, Eisai, Eli Lilly, GlaxoSmithKline, Janssen, Mitsubishi Tanabe, Nippon Kayaku, Novartis, Pfizer, Sanofi, Taiho, Taisho Toyama, Takeda, Teijin and UCB.
References
- 1. Scott DL, Wolfe F, Huizinga TW.. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- 2. Schett G, Hayer S, Zwerina J, Redlich K, Smolen JS.. Mechanisms of disease: the link between RANKL and arthritic bone disease. Nat Clin Pract Rheumatol 2005;1:47–54. [DOI] [PubMed] [Google Scholar]
- 3. Schett G, Gravallese E.. Bone erosion in rheumatoid arthritis: mechanism, diagnosis and treatment. Nat Rev Rheumatol 2012;8:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fuller K, Wong B, Fox S, Choi Y, Chambers TJ.. TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 1998;188:997–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lacey DL, Tan HL, Lu J. et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol 2000;157:435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lacey DL, Timms E, Tan HL. et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998;93:165–76. [DOI] [PubMed] [Google Scholar]
- 7. Yasuda H, Shima N, Nakagawa N. et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA 1998;95:3597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kostenuik PJ, Nguyen HQ, McCabe J. et al. Denosumab, a fully human monoclonal antibody to RANKL, inhibits bone resorption and increases BMD in knock-in mice that express chimeric (murine/human) RANKL. J Bone Miner Res 2009;24:182–95. [DOI] [PubMed] [Google Scholar]
- 9. Takeuchi T, Tanaka Y, Ishiguro N. et al. Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose–response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)—a 12-month, multicentre, randomised, double-blind, placebo-controlled, phase II clinical trial. Ann Rheum Dis 2016;75:983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aletaha D, Alasti F, Smolen JS.. Rheumatoid factor determines structural progression of rheumatoid arthritis dependent and independent of disease activity. Ann Rheum Dis 2013;72:875–80. [DOI] [PubMed] [Google Scholar]
- 11. Katchamart W, Koolvisoot A, Aromdee E, Chiowchanwesawakit P, Muengchan C.. Associations of rheumatoid factor and anti-citrullinated peptide antibody with disease progression and treatment outcomes in patients with rheumatoid arthritis. Rheumatol Int 2015;35:1693–9. [DOI] [PubMed] [Google Scholar]
- 12. Dixey J, Solymossy C, Young A.. Is it possible to predict radiological damage in early rheumatoid arthritis (RA)? A report on the occurrence, progression, and prognostic factors of radiological erosions over the first 3 years in 866 patients from the Early RA Study (ERAS). J Rheumatol Suppl 2004;69:48–54. [PubMed] [Google Scholar]
- 13. Visser K, Goekoop-Ruiterman YP, de Vries-Bouwstra JK. et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 2010;69:1333–7. [DOI] [PubMed] [Google Scholar]
- 14. Vastesaeger N, Xu S, Aletaha D, St Clair EW, Smolen JS.. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1114–21. [DOI] [PubMed] [Google Scholar]
- 15. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 16. van der Heijde D. How to read radiographs according to the Sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 17. Cohen SB, Dore RK, Lane NE. et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum 2008;58:1299–309. [DOI] [PubMed] [Google Scholar]
- 18. Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF. et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. [DOI] [PubMed] [Google Scholar]
- 19. Genovese MC, Bathon JM, Martin RW. et al. Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum 2002;46:1443–50. [DOI] [PubMed] [Google Scholar]
- 20. Takeuchi T, Tanaka Y, Kaneko Y. et al. Effectiveness and safety of adalimumab in Japanese patients with rheumatoid arthritis: retrospective analyses of data collected during the first year of adalimumab treatment in routine clinical practice (HARMONY study). Mod Rheumatol 2012;22:327–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Molenaar ET, Voskuyl AE, Dinant HJ. et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004;50:36–42. [DOI] [PubMed] [Google Scholar]
- 22. Mulherin D, Fitzgerald O, Bresnihan B.. Clinical improvement and radiological deterioration in rheumatoid arthritis: evidence that the pathogenesis of synovial inflammation and articular erosion may differ. Br J Rheumatol 1996;35:1263–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.