Abstract
Objectives
The exact underlying mechanism of rituximab treatment in patients with RA is poorly defined and knowledge about the effect of B cell depletion on immune cells in secondary lymphoid organs is lacking. We analysed lymphoid tissue responses to rituximab in RA patients.
Methods
Fourteen RA patients received 2 × 1000 mg rituximab intravenously, and lymph node (LN) biopsies were obtained before and 4 weeks after the first infusion. Tissues were examined by flow cytometry, immunohistochemistry and quantitative PCR. LN biopsies from five healthy individuals (HC) served as controls.
Results
LN biopsies of RA patients showed increased frequencies of CD21+CD23+IgDhighIgMvariable follicular B cells and CD3+CD25+CD69+ early activated, tissue resident T cells when compared with HCs. After treatment, there was incomplete depletion of LN B cells. There was a significant decrease in CD27−IgD+ naïve B cells, and CD27+IgD+ unswitched memory B cells including the CD27+IgD+IgM+ subset and follicular B cells. Strikingly, CD27+IgD− switched memory B cells persisted in LN biopsies after rituximab treatment. In the T cell compartment, a significant decrease was observed in the frequency of early activated, tissue resident T cells after rituximab treatment, but late activated T cells persisted. B cell proliferation inducing cytokine IL-21 was higher expressed in LN biopsies of RA patients compared with HC and expression was not affected by rituximab treatment.
Conclusion
Rituximab does not cure RA, possibly due to persistence of switched memory B cells in lymphoid tissues suggesting that factors promoting B cell survival and differentiation need to be additionally targeted.
Keywords: rheumatoid arthritis, lymphocytes, B cells, T cells, cytokines and inflammatory mediators
Rheumatology key messages
Rituximab treatment improves RA, in part because B cell depletion results in decreased T cell activation.
Rituximab does not cure RA, possibly due to persistent switched memory B cells in lymphoid tissues.
An RA cure may perhaps only be achieved if B cell survival and differentiation factors are also targeted.
Introduction
RA is an immune-mediated inflammatory disease affecting 1% of the population and is characterized by synovial inflammation leading to destruction of cartilage and bone. Autoreactive B cells are thought to be pivotal in the aetiology of RA because of its association with autoantibodies such as IgM-RF and/or ACPA, which can precede the clinical onset of the disease by many years [1, 2]. In addition, the inflamed synovial tissue contains expanded B cell clones [3] and ectopic germinal centre-like structures [4]. Also, the majority of RA patients show clinical benefit upon treatment with the B cell depleting anti-CD20 antibody rituximab [5–7]. However, rituximab treatment does not target antibody-producing plasma cells and does not cure RA as additional treatment is required upon B cell recovery.
Administration of rituximab results in a rapid, almost complete depletion of CD20 positive B cells in peripheral blood but only partial depletion in synovial tissue and bone marrow [8–13]. The relative resistance of B cells to the effects of rituximab in tissues may be related to sustained expression of protective factors such as B cell activating factor (BAFF, also called B lymphocyte stimulator), complement inhibitors CD55 (decay-accelerating factor) and CD59, or increased expression of receptors for B cell survival, such as BAFF receptor (BAFF-R) and transmembrane activator and CAML interactor (TACI).
The underlying mechanisms of anti-CD20 antibody therapy are still poorly defined, and it is not known why this treatment may result in long-lasting improvement but not in cure. We found that memory B cells infiltrate the inflamed synovial tissues of only a proportion of RA patients and that the extent of synovial B cell depletion varied between patients [11]. Presence or change in synovial B cells did not predict clinical response to treatment. We hypothesized that the variable clinical response and resistance to rituximab is caused by variable depletion and persistence of autoreactive memory B cells in secondary lymphoid tissue where autoantigen presentation and differentiation of these cells are likely to occur. Therefore, we investigated the effect of rituximab therapy on immune cells in lymph node (LN) biopsies of RA patients.
Methods
Patients
Fourteen RA patients were included in the study. All patients were diagnosed according to the ACR 1987 classification criteria for RA [14] and had active disease defined by a DAS evaluated in 28 joints (DAS28) >3.2. Stable treatment for at least 2 weeks with DMARDs, oral corticosteroids up to 10 mg daily and NSAIDs was permitted. Patients had previously failed treatment with at least one anti-TNF agent, defined as inadequate response or intolerance. Anti-TNF treatment was discontinued at least 4 weeks before start of treatment with rituximab. For comparison, five healthy individuals who were IgM-RF and ACPA negative were included. The study was approved by the Medical Ethics Committee of the AMC/University of Amsterdam and all patients gave written informed consent.
Study design
Patients were treated with intravenous infusions of 1000 mg of rituximab on day 1 and day 15 as previously described [11]. Premedication with methylprednisolone was omitted so as to be able to study the specific effects of rituximab. LN tissue was obtained as described previously [15] before and 4 weeks after the first infusion with rituximab. Immediately after collection biopsies were processed for flow cytometry analysis, snap-frozen en bloc in Tissue-Tek OCT compound (Miles, Elkhart, IN, USA) for immunohistochemistry analysis or snap frozen for RNA isolation. Clinical assessment was performed at day 0, and after 2, 4, 8, 12, 16, 20 and 24 weeks after start of treatment, including assessment of DAS28, CRP levels and ESR.
Flow cytometry analysis of peripheral blood
Peripheral blood was drawn before and 4 weeks after the start of rituximab treatment to determine ACPA and IgM-RF levels. B cell frequencies were determined by highly sensitive B cell analysis using flow cytometry [16]. B cells were defined as CD19+CD22+ double positive cells. Complete depletion of B cells was defined as <0.0001×109/L.
Flow cytometry analysis of lymph node tissue
LN tissue was put through a 70 μm (BD Falcon, San Jose, CA) cell strainer to obtain a single cell suspension. Cells were washed with PBS containing 0.01% NaN3 and 0.5% BSA and stained for 30 min at 4°C with directly labelled antibodies: CD45 PercP-Cy5.5, CD19 Alexa-700, CD27 APC-H7, IgD Pe-Cy7, CD20 PE, IgM FITC, CD69 PE, CD69 PerCP, CD21 APC, CD23 PE, CD25 APC, CD267 PE, BAFF-R FITC, CD16 Percp-Cy5.5, CD56 PE, CD55 PE, CD59 FITC (BD Biosciences, Breda, the Netherlands), HLA-DR Alexa-700 (eBioscience, Vienna, Austria), CD3 FITC (Sanquin, Amsterdam, the Netherlands). After incubation cells were washed and immediately analysed on a FACS CANTO II (BD Biosciences). To enable the measurement of different B cell subsets, a seven-colour FACS panel was set-up using antibodies against CD19, IgD, IgM, CD27, CD21, CD23 and CD45 (for normalization). Data were analysed using FlowJo software (Treestar, Ashland, OR, USA) and presented as frequencies, absolute numbers relative to 100 000 CD45+ lymphocytes or geometric mean fluorescence intensity (normalized on negative populations).
Immunohistochemical analysis of lymphoid tissue sections
Sections (5 μm each) were cut and mounted on StarFrost adhesive glass slides (Knittelgläser, Braunschweig, Germany). Sealed slides were stored at −80°C until further use. LN tissue sections were stained using mouse monoclonal antibodies against T cells (anti-CD3, clone SK7; Becton Dickinson, Breda, the Netherlands), B cells (anti-CD22, clone RFB4; Millipore, Amsterdam, the Netherlands) and plasma cells (anti-CD138, clone MI15; Dako, Heverlee, Belgium).
Staining was performed using a three-step immunoperoxidase method to detect bound anti-CD138 antibodies, as described previously [17]. For anti-CD3 and anti-CD22, we used a two-step immunoperoxidase method with a secondary polymer—horseradish peroxidase—conjugated anti-mouse antibody (EnVision+ System; Dako). As a negative control, irrelevant isotype-matched immunoglobulins, instead of the primary antibody, were applied to the sections. Staining was analysed by digital image analysis in a blinded fashion using a Syndia algorithm on a Qwin-based analysis system (Leica, Cambridge, UK) as described previously [18]. The number of positive cells was calculated for each section as the number of positive cells per square millimetre of tissue.
Quantitative real-time PCR
Total RNA was extracted from LN biopsies using the AllPrep DNA/RNA mini kit (Qiagen, Venlo, the Netherlands). RNA (500 ng) was reverse transcribed into cDNA using the Revertaid H-minus first strand cDNA synthesis kit (Thermo Fisher Scientific, Amsterdam, the Netherlands) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using a Step One Plus detection system [Thermo Fisher Scientific (Applied Biosystems)] using Taqman assays [Thermo Fisher Scientific (Applied Biosystems)] for 18S RNA (Hs99999901_s1) and IL-21 (Hs00222327_m1). Values for each gene were normalized to the expression level of 18S RNA. An arbitrary cDNA sample was used on each qPCR plate for normalization between different experimental runs.
Statistical analysis
Data not normally distributed are presented as medians with interquartile range. Differences were analysed with the two-tailed Mann–Whitney U test. The two-tailed Wilcoxon signed-rank test for paired data was used to analyse change after treatment. Correlations were calculated using a two-tailed Spearman’s rho. All statistical analyses were performed using Prism software (version 5, GraphPad Software, La Jolla, CA, USA).
Results
Clinical characteristics and clinical response to rituximab
Patient characteristics and clinical response to treatment according to the EULAR response criteria are summarized in Table 1. The mean age between the HCs and RA patients was significantly different (mean age HCs 36 years, mean age RA patients 53 years). However, none of the measured parameters correlated significantly with age. At baseline, patients had active disease (median DAS28 = 5.3), and after 24 weeks follow-up, three patients were classified as non-responders, seven patients were moderate responders and four patients were good responders according to EULAR response criteria.
Table 1.
Subjects characteristics and clinical responses in RA patients
| Healthy controls | RA | |
|---|---|---|
| (n=5) | (n=14) | |
| Sex, female, n (%) | 4 (80) | 12 (86) |
| Age, mean (s.d.), years | 36 (7.5) | 53 (13.0) |
| IgM-RF positive, n (%) | 0 (0) | 13 (93) |
| Anti-CCP positive, n (%) | 0 (0) | 11 (79) |
| IgM-RF and anti-CCP both positive, n (%) | 0 (0) | 10 (71) |
| IgM-RF and anti-CCP both negative, n (%) | 5 (100) | 1 (7) |
| ESR, median (IQR), mm/h | — | 21 (12, 32) |
| CRP, median (IQR), mg/L | 0.4 (0.3, 0.7) | 4.5 (2.9, 9.8) |
| DAS28, median (IQR) | 0 (0) | 5.3 (4.7, 6.0) |
| Clinical response at 24 weeks | ||
| Delta DAS28, median (IQR) | 1.6 (0.8, 2.1) | |
| EULAR good, n (%) | 4 (29) | |
| EULAR moderate, n (%) | 7 (50) | |
| EULAR non, n (%) | 3 (21) | |
Subjects characteristics and clinical responses in RA patients 24 weeks after the first rituximab infusion. Categorical variables: n (%); continuous variables (data not normally distributed): median (IQR). Anti-CCP: anti-citrullinated protein antibodies; DAS28: DAS in 28 joints; IQR: interquartile range.
Depletion of B cells in peripheral blood does not correlate with treatment response
In peripheral blood the frequencies of B cells, before rituximab treatment, did not correlate with ACPA levels, IgM-RF levels or DAS28 (Supplementary Fig. S1A, available at Rheumatology online). Four weeks after the first rituximab infusion there was complete depletion of peripheral blood B cells (cut off <0.0001×109/L) in 9 of the 13 patients measured. The extent of peripheral B cell depletion at this time point did not correlate with any of the baseline disease activity parameters or levels of CRP, ESR, IgM-RF or ACPA (Supplementary Fig. S1A, available at Rheumatology online).
Increased frequency of follicular B cells in lymph node biopsies of RA patients compared with healthy controls
In LN biopsies, the relative frequencies and absolute numbers of CD19+ B cells showed a trend towards an increase in RA patients (median 21% and 22 662 per 100 000 CD45+ cells) compared with HCs (median 11% and 11 597 per 100 000 CD45+ cells, P = 0.15 and P = 0.09, respectively) (Fig. 1A and Supplementary Fig. S2, available at Rheumatology online). Based on CD27 and IgD expression there was a numerical trend towards increase in the absolute numbers of unswitched memory (CD27+IgD+) B cells (median 4124 per 100 000 CD45+ cells) compared with HCs (median 1421 per 100 000 CD45+ cells, P = 0.13) (Supplementary Fig. S2, available at Rheumatology online). Otherwise, there was no difference in the relative or absolute frequencies of naïve (CD27−IgD+), unswitched memory (CD27+IgD+), switched memory (CD27+IgD−) or double negative (CD27−IgD−) B cells (Fig. 1A and B and Supplementary Fig. S2, available at Rheumatology online). The relative and absolute frequencies of CD21+CD23+IgDhighIgMvariable B cells, which represent a subpopulation of follicular B cells that has been associated with active arthritis in arthritis models and RA [19], were measured in a subset of patients (n = 7). These were increased in RA patients compared with HCs (P = 0.02 and P = 0.016) (Fig. 1C and Supplementary Fig. S2, available at Rheumatology online). Before as well as after rituximab treatment, the frequencies of total B cells in peripheral blood of RA patients did not correlate with the frequency of total B cells or any specific B cell subset in LN biopsies (data not shown).
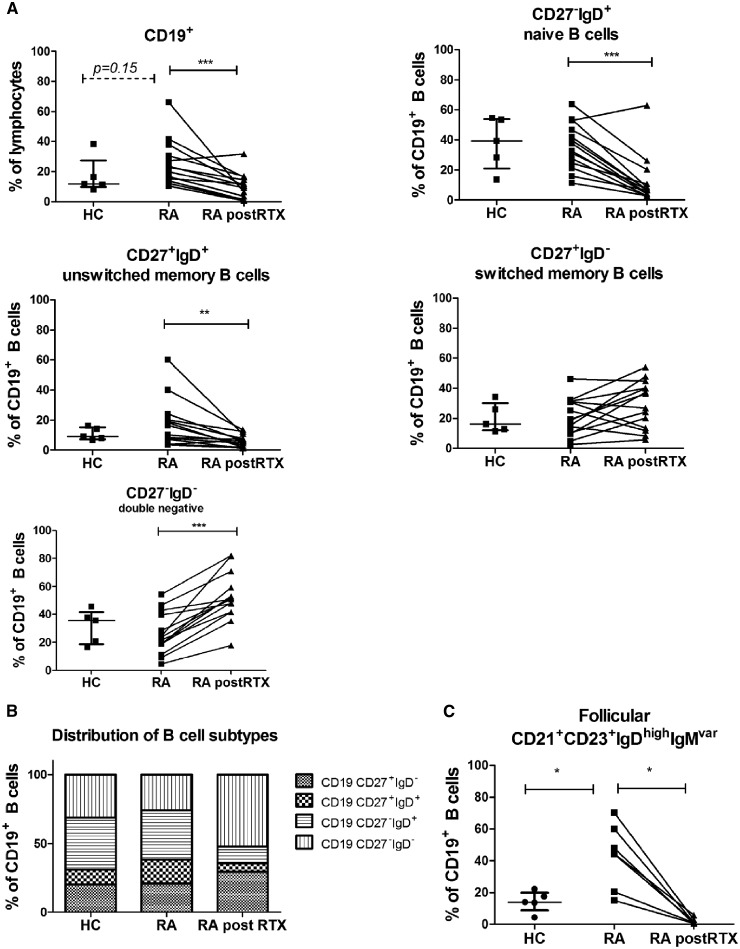
Fig. 1.
Lymphoid B cell subsets in healthy individuals and RA patients
LN single cells were analysed using flow cytometry. B cell subsets were analysed in healthy individuals (HC; n = 5), RA patients (RA; n = 14) and RA patients after two rituximab infusions (RA post-RTX; n = 14). (A) Frequencies of CD19+ B cells, unswitched memory (CD27+IgD+), switched memory (CD27+IgD−), naïve (CD27−IgD+) and double negative (CD27−IgD−) B cells were determined. (B) Relative distribution between these different B cell subsets present in healthy and RA patients (before and after RTX) is shown in a bar graph. (C) Frequencies of follicular (CD21+CD23+IgDhighIgMvariable) B cells were analysed in healthy individuals (HC; n = 5) and RA patients before and after two rituximab infusions (n = 7). Mann–Whitney test was used to analyse significant differences in cell subsets between healthy controls and RA patients (baseline). A Wilcoxon matched-pairs signed rank test was used to analyse significant changes before and after treatment. *P < 0.05, **P < 0.01, ***P < 0.001. HC: healthy controls; LN: lymph node; RTX: rituximab.
Rituximab treatment mainly depletes naïve and unswitched memory B cells in lymph node biopsies but switched memory B cells persist
Four weeks after rituximab treatment, the relative and absolute frequencies of total CD19+ B cells (P = 0.0004) was significantly decreased but there was still persistence of a proportion of B cells (median 10% of total lymphocytes compared with 21% before treatment) (Fig. 1A and Supplementary Fig. S2, available at Rheumatology online). With regard to B cell subsets, there was a pronounced decrease in the relative and absolute frequencies of naïve B cells (P = 0.0006 and P = 0.003) and unswitched memory B cells (P = 0.001 and P = 0.003). Also, CD21+CD23+IgDhighIgMvariable follicular B cells decreased (P = 0.02 and P = 0.03) (Fig. 1A and C and Supplementary Fig. S2, available at Rheumatology online). However, the relative and absolute frequencies of switched memory and double negative B cells were much less altered by rituximab treatment (switched memory: median 19% and 2359 per 100 000 CD45+ cells before compared with median 31% and 1290 per 100 000 CD45+ cells after treatment, P = 0.21 and P = 0.074, respectively; double negative B cells relatively increased: median 23% and 4476 per 100 000 CD45+ cells before compared with median 51% and 5654 per 100 000 CD45+ cells after treatment, P = 0.01 and P = 0.37, respectively) (Fig. 1A andSupplementary Fig. S2, available at Rheumatology online). In summary, the relative distribution of the different B cell subsets after two rituximab infusions was changed in favour of IgD− B cell populations (Fig. 1B). The changes in different B cell subsets are summarized in Table 2.
Table 2.
Frequencies of LN immune cells in healthy controls and RA patients
| Healthy | RA | RA post-RTX | P | |
|---|---|---|---|---|
| B cells | ||||
| CD19+ | 11.6 (9.7–27.3) | 21.3 (13.8–32.4) | 9.7 (1.3–14.9) | 0.0004 |
| CD27−IgD+ | 39.3 (21.0–54.1) | 35.2 (23.8–48.3) | 6.8 (3.8–13.1) | 0.0006 |
| CD27+IgD+ | 9.0 (7.4–15.2) | 13.4 (6.5–21.2) | 6.0 (1.9–8.5) | 0.0012 |
| CD27−IgD− | 35.5 (18.6–41.6) | 22.7 (16.8–40.4) | 50.8 (41.5–62.1) | 0.0001 |
| CD27+IgD− | 16.6 (12.1–30.1) | 19.1 (10.2–31.0) | 31.6 (13.1–41.2) | ns |
| CD21+CD23+IgDhighIgMvar | 14.0 (4.39–19.95) | 44.4 (15.1–60.0) | 1.2 (0.54–3.05) | 0.0156 |
| CD55+ | 99.8 (99.2–100) | 99.7 (99.1–99.7) | 99.1 (97.2–99.5) | ns |
| CD59+ | 84.4 (69.1–94.3) | 84.4 (67.4–95.7) | 81.7 (72.8–91.0) | ns |
| TACI+ | 59.9 (47.5–70.7) | 46.6 (33.2–61.1) | 53.9 (42.0–67.3) | ns |
| BAFF-R+ | 84.6 (77.2–94.1) | 92.9 (88.7–97.2) | 68.1 (46.8–94.3) | 0.03 |
| T cells | ||||
| CD3+ | 81.3 (63.9–87.4) | 75.8 (63.1–84.5) | 79.4 (62.7–91.6) | ns |
| CD3+CD69+ | 8.3 (5.7–12.7) | 24.0 (12.6–33.0) | 15.6 (4.1–33.2) | ns |
| CD3+CD25+ | 22.6 (18.5–24.4) | 28.1 (23.6–50.3) | 22.0 (15.5–40.6) | ns |
| CD3+CD25+CD69+ | 2.2 (1.1–3.1) | 8.4 (3.3–11.0) | 2.7 (0.8–5.0) | 0.03 |
| CD3+HLA-DR+ | 30.4 (25.5–32.6) | 21.3 (18.1–36.0) | 27.5 (11.4–37.7) | ns |
Data are frequencies of cells (%) presented as median (IQR). A Wilcoxon matched-pairs signed rank test was performed to analyse significant differences in subsets before vs after RTX treatment. BAFF-R: B cell activating factor receptor; IQR: interquartile range; LN: lymph node; ns: not significant; RTX: rituximab; TACI: transmembrane activator and CAML interactor.
No significant differences in the frequencies of B cell subsets between the clinical good, moderate and non-responders could be found at baseline or 4 weeks after treatment (data not shown). In addition, changes in frequencies of different B cell subsets in lymphoid tissue between baseline and 4 weeks after rituximab treatment did not correlate with the change in DAS28 at 24 weeks (data not shown). Of note, these results need to be interpreted with caution as there were only three non-responders in this exploratory study.
B cell follicular structures and CD138+ plasma cell numbers in lymph node tissue are unaffected by rituximab treatment
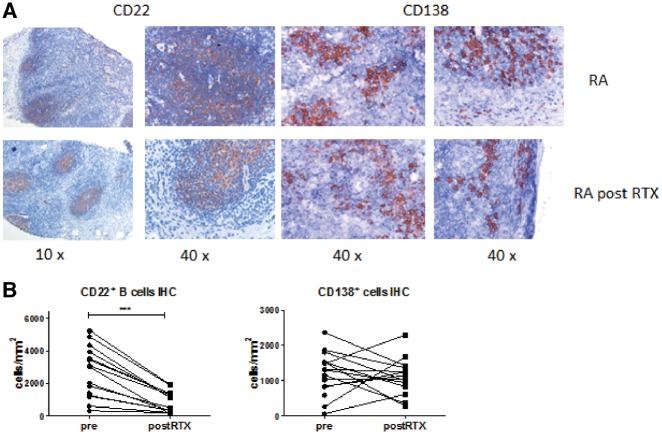
Since we found limited depletion of lymphoid switched memory B cells, we analysed if B cell follicles could still be discerned after treatment using immunohistochemical staining for CD22. In parallel we analysed the effect of rituximab on CD138+ plasma cells using immunohistochemistry since surface expression of CD138 to determine the frequency of plasma cells is challenging to measure by flow cytometry (Fig. 2A). In line with our flow cytometry data, CD22+ B cells could still be readily detected after rituximab treatment, and follicular structures were still present. Quantification of the staining confirmed that numbers of CD22+ cells/mm2 were decreased after rituximab treatment (P < 0.0001). The numbers of CD138+ cells/mm2 were unaltered after rituximab treatment (Fig. 2B).
Fig. 2.
Immunohistochemical analysis of CD22 and CD138 staining in lymphoid tissue of RA patients
(A) Representative images of CD22 (B cells) and CD138 (plasma cells) staining before and after two rituximab infusions. (B) The staining was quantified as positive cells per mm2. RTX: rituximab.
Taken together, histology data confirm that not all lymphoid B cells are depleted after rituximab treatment and that follicular structures and CD138+ plasma cells persist in LN tissue.
Complement inhibitor CD55 and BAFF-R are decreased on persisting B cells
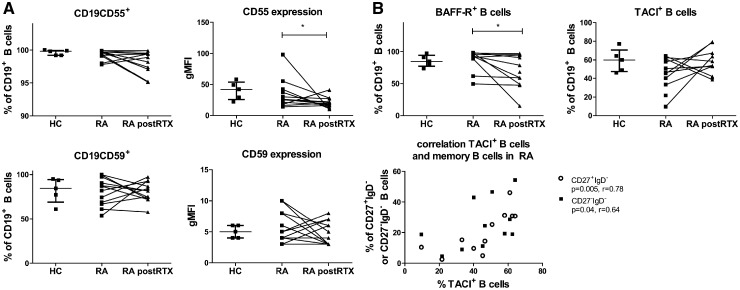
To explore a potential explanation behind the observed variable responsiveness of LN B cells to rituximab, we investigated the expression of membrane molecules on LN B cells potentially involved in resistance to rituximab. CD20, complement inhibitors CD55 and CD59 as well as the receptors for B cell survival factor BAFF, BAFF-R and TACI have all been reported to be associated with a decreased response to rituximab treatment in various haematological diseases [20–22]. In RA LN biopsies, expression level of CD20 was comparable between memory and naïve B cells indicating that there is no difference in susceptibility to CD20-mediated B cell depletion based on different CD20 levels (data not shown). CD20 was only measured at baseline, since rituximab may mask CD20. The expression levels of CD55 and CD59 (measured by geometric mean fluorescence intensity) on B cells were not different in RA patients compared with HCs (Fig. 3A). CD55 and CD59 expression levels were not correlated with B cell subset frequencies. After rituximab treatment the majority of persisting B cells expressed CD55 and CD59, although the expression levels of CD55 (geometric mean fluorescence intensity) were significantly lower on persisting lymphoid B cells in RA patients compared with baseline expression (P = 0.049; Fig. 3A). Expression levels of CD55 and CD59 on B cells were variable before and after rituximab treatment. There was no correlation between the expression levels of CD55 or CD59 at baseline or after treatment and the frequencies of B cell subsets after rituximab treatment or the extent of B cell depletion (data not shown).
Fig. 3.
Lymphoid B cell subsets expressing complement inhibitors and survival receptors
(A) Frequencies of CD19+ B cells positive for CD55 or CD59 and expression levels (gMFI) were analysed in healthy individuals (HC; n = 5), RA patients (RA; n = 12) and RA patients after two rituximab infusions (RA post-RTX; n = 12). (B) Frequencies of CD19BAFF-R+ B cells and CD19TACI+ B cells were analysed in the three study groups (HC; n = 5, RA; n = 10). For CD19TACI+ B cells the correlations with switched memory B cells (CD27+IgD−) and double negative B cells (CD27−IgD−) at baseline are shown (n = 11). BAFF-R: B cell activating factor receptor; gMFI: geometric mean fluorescence intensity; HC: healthy controls; RTX: rituximab; TACI: transmembrane activator and CAML interactor.
There were no significant differences in the frequency of BAFF-R+ or TACI+ lymphoid B cells between RA patients and HCs (Fig. 3B). BAFF-R is expressed on all B cell subsets. There was no correlation between the level of expression and B cell subset frequencies. Since TACI is mainly expressed on mature B cells and memory B cells, we investigated the possible relationship between the frequency of memory B cells and TACI+ B cells. Indeed, the frequency of TACI+ B cells in RA patients at baseline positively correlated with the frequency of unswitched memory B cells (P = 0.01, r = 0.75) switched memory (CD27+IgD−) B cells (P = 0.005, r = 0.78) and double negative (CD27−IgD−) B cells (P = 0.04, r = 0.64; Fig. 3B) but not with naïve B cells (Supplementary Fig. S1, available at Rheumatology online). After rituximab treatment the frequency of BAFF-R+ B cells significantly decreased (median decreased from 92.9–68.1% of total B cells; P = 0.03) while the frequency of TACI+ B cells within the total B cell population was unaltered, although the fraction of TACI+ B cells was numerically slightly higher [median 47% (interquartile range 33–61%) before to 54% (42–67%) after treatment P = 0.2; Fig. 3B].
Baseline expression of CD20, CD55, CD59, BAFF-R or TACI and changes in expression after rituximab treatment (ΔCD20, ΔCD55, ΔCD59, ΔBAFF-R or ΔTACI) did not correlate with ΔDAS28 at 24 weeks (data not shown). An important limitation of this exploratory part of the analysis was that there were only three non-responders in the whole study, and for this part of the analysis two of these non-responders could not be analysed because of low cell numbers.
Taken together, we found no indication of involvement of low CD20 expression, complement inhibitor expression levels or receptors for survival factors in resistance of LN B cells to rituximab. In addition, expression of these factors on the total LN B cell population was not related to clinical response, although these results need to be interpreted with caution.
Numbers of activated T cells are increased in lymphoid tissue of RA patients and early activation markers decrease after rituximab treatment
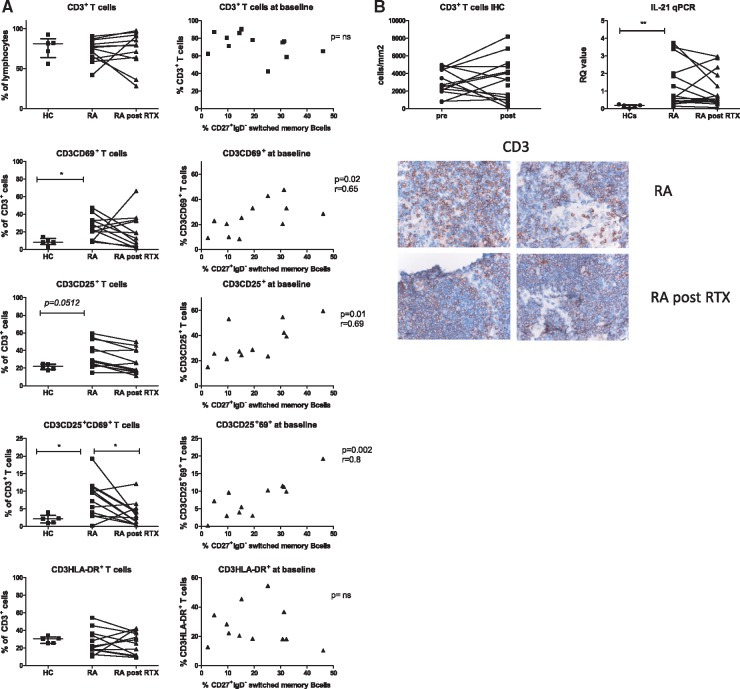
Therapeutic B cell depletion may diminish disease activity through various mechanisms [23]. Besides reducing the presence of excreted B cell products such as cytokines, autoantibodies and free light chains, T cell activation may be reduced, specifically at sites of antigen presentation [24]. Therefore, we analysed the activation status of T cells based on expression of CD69 (marker for very early activation and tissue residence [25]), CD25 (early activation) and HLA-DR (mid to late activation). Frequencies of tissue resident and early activated, tissue resident T cells, CD3+CD69+ and CD3+CD25+CD69+, were increased in RA patients compared with HCs (P = 0.01 and P = 0.02, respectively, Fig. 4A) while total CD3+ T cell frequencies were comparable. Furthermore, in contrast to HCs, the frequency of activated T cells in RA at baseline positively correlated with the frequency of switched memory B cells at baseline (CD3+CD69+; P = 0.02, r = 0.65: CD3+CD25+; P = 0.01, r = 0.69: CD3+CD25+CD69+ (P = 0.002, r = 0.80) but not with the frequency of naïve, unswitched or double negative B cells (Fig. 4A). After rituximab treatment the frequencies of total CD3+, CD3+CD69+, CD3+CD25+ and CD3+HLA-DR+ T cells were not altered. However, the frequency of activated CD3+CD25+CD69+ T cells significantly decreased (P = 0.03) (changes in different T cell subsets are summarized in Table 2). The baseline or post-treatment frequencies of activated T cells did not correlate to treatment response (data not shown). Since circulating T cells may carry CD20 at low expression levels on their surface, we analysed CD20 expression on LN CD3+ T cells at baseline and found that ∼99% of the CD3+ T cells expressing CD69 are negative for CD20 (data not shown) suggesting that the observed decrease in activated T cells is not a direct effect of rituximab on CD20+ T cells but rather an indirect consequence of B cell depletion. Based on histology, the distribution and number of CD3+ T cells in lymphoid tissue was unaltered after rituximab treatment (Fig. 4B). As an indication for follicular T cell activity, we analysed the relative quantity of IL-21 mRNA in whole LN biopsies from HCs and RA patients. IL-21 mRNA levels were higher in LN biopsies from RA patients compared with HCs (P = 0.004). After rituximab treatment, IL-21 mRNA levels remained stable.
Fig. 4.
Lymphoid T cell subsets; activation and relation with B cell subsets
(A) Frequencies of LN T cell subsets (CD3CD69+, CD3CD25+, CD3CD25+CD69+ and CD3HLA-DR+) were analysed in healthy individuals (HC; n = 5), RA patients (RA; n = 12) and RA patients after two rituximab infusions (RA post-RTX; n = 12). The correlation of different T cell subsets in LN of RA patients at baseline with the frequency of switched memory B cells are plotted on the right. (B) The amount of CD3+ T cells per mm2 of lymphoid tissue in RA patients before and after rituximab treatment was analysed with immunohistochemistry staining and digital image analysis. Representative images of CD3 staining are shown. Relative quantity of total IL-21 mRNA was analysed with qPCR in LN biopsies of healthy individuals (HC; n = 5), RA patients (RA; n = 15) and RA patients after two rituximab infusions (RA post-RTX; n = 15). HC: healthy controls; LN: lymph node; RTX: rituximab.
Taken together, these data show that in lymphoid tissue, T cell activation is increased in RA patients and related to switched memory B cell frequencies. The frequency of early activated, tissue resident T cells is decreased after rituximab treatment, but late activated T cells persist. In addition, total IL-21 mRNA levels in LN biopsies from RA patients remain high after rituximab treatment.
Discussion
The results presented here show for the first time that on group level the distribution of naïve and memory B cell subsets in lymphoid tissue of RA patients is comparable to the distribution observed in healthy individuals, while the frequencies of CD21+CD23+IgDhighIgMvariable follicular B cells that have been associated with active arthritis [19] are increased. Furthermore, the frequencies of early activated, tissue resident T cells are significantly increased in RA and related to the frequencies of lymphoid switched memory B cells. Rituximab treatment depletes IgD+ naïve, unswitched memory (a mixture of germinal centre derived and splenic marginal zone derived memory B cells) and CD21+CD23+IgDhighIgMvariable follicular B cells, while B cell follicular structures and switched memory B cells persist. Of interest, rituximab treatment is also associated with a significant decrease in the frequency of early activated, tissue resident CD3+CD25+CD69+ T cells, while markers of persistent T cell activation were unaffected. Rituximab treatment induces clinical improvement in the majority of autoantibody positive RA patients, but all patients eventually relapse [26]. We postulate that this may be explained by persistence of autoreactive switched memory B cells in secondary lymphoid tissues.
Previous studies have shown that rituximab causes almost complete depletion of B cells in peripheral blood, but only partial depletion in synovium [10, 11, 27] and bone marrow [9, 12]. Residual B cells in blood mainly consist of memory B cells with an activated phenotype [27]. Blood biomarker candidates like IgJhiFCRL5lo for CD20-negative plasmablasts and a higher ratio of memory B cells to transitional B cells at reconstitution have been described to predict the response to rituximab [28, 29]. However, understanding which B cell subsets persist in tissues may provide more insight into the mechanism of action of rituximab in peripheral tissues and B cell repopulation after treatment. Here we show that, similar to what has been observed in blood, switched memory B cells and B cell follicular structures persist in lymphoid tissue after rituximab treatment. This indicates that rituximab leads to varying levels of B cell depletion in different body compartments probably due to specific tissue microenvironment factors [30]. In addition, it has previously been shown that serum BAFF (B lymphocyte stimulator) levels rise following B cell depletion [26]. BAFF binding to BAFF-R may regulate B cell as well as plasma cell survival in the context of autoimmunity [31] and is likely to contribute to persistence of autoreactive B lineage cells. Of the BAFF targeting cells, we found that TACI+ B cells persist in lymphoid tissue while the frequency of BAFF-R+ B cells is decreased after rituximab treatment. TACI is mainly expressed on resting memory B cells while BAFF-R is expressed by all B cells including naïve B cells [32], which are the first B cells to repopulate in peripheral blood after rituximab treatment [33]. BAFF is constitutively produced by lymphoid stromal cells [34] and can drive B cell differentiation of the residual lymphoid naïve B cells expressing BAFF-R. BAFF signalling through the BAFF receptor and TACI receptor has been found especially important for naïve B cell survival. In this context, anti-BAFF treatment in patients with systemic lupus erythematosus has recently been associated with rapid and strong effects on naïve B cells and a delayed decrease in autoreactivity-associated B cells [35]. Co-operation of BAFF with other B cell survival factors may mediate memory B cell survival. Of interest, IL-21 has been shown to collaborate with BAFF in stimulating T cell-independent plasmablast formation from memory B cells in an experimental lupus model [36]. In another experimental lupus model, IL-15 stimulated memory B cell survival together with BAFF [37]. Taken together, the literature suggests that BAFF may be an important candidate for the B cell survival observed in the present study, as well as in the repopulation after rituximab treatment.
There is also a role for T cell-dependent activation of B cells in the pathogenesis of RA. Human synovium-SCID mouse chimeras [24] showed that T cell activation in RA synovium is B cell dependent and that T cells are involved in the formation of tertiary lymphoid structures in the inflamed tissue [38]. Consistent with this notion, we here show an increased frequency of activated LN T cells in RA patients correlating with the frequencies of switched memory B cells. In addition, we showed an increased frequency of a follicular B cell subset in RA patients, which is mainly involved in T cell-dependent immune responses and has been associated with active arthritis [19]. After B cell depleting treatment, a decreased frequency of markers of early T cell activation and a decrease in these follicular B cells was observed in LNs, but in the context of persistent late T cell activation. IL-21 mRNA levels were markedly increased in lymphoid tissue of RA patients but unaltered after rituximab treatment. IL-21 is mainly produced by follicular T helper cells and is crucial in providing signals for B cell proliferation and differentiation [39]. Persistent levels of IL-21, presumably produced by persistent follicular T helper cells, may provide positive signals for B cell repopulation in secondary lymphoid tissue. An effect of rituximab on activated T cells has been described in SLE where clinical remission was associated with decreased frequencies of activated T helper cells in peripheral blood [40]. A recent study analysed LN B cell persistence after CD20-mediated B cell depletion in experimental encephalomyelitis mouse models [41]. LN memory B cells persisted to some extent in naïve mice. In mice induced with a T–B cell-dependent form of experimental encephalomyelitis, an enhanced number of autoreactive memory B cells persisted in LNs. Clinical relapse was associated with enhanced expansions of these cells within an overall restricted B cell repertoire. This indicates that factors involved in LN T–B cell crosstalk are likely to be involved in both the clinical response to rituximab and clinical relapses after rituximab. In future research studies in LN biopsies, it will be of importance to investigate in more detail germinal centre B cells and Tfh cells, as well as factors produced by the LN microenvironment supporting this B–T cell crosstalk.
This is the first report describing the lymphoid tissue response to rituximab in RA patients; the results are consistent with and extend a previous report in four patients showing that a single dose of rituximab results in incomplete depletion of B cells in iliac LNs of patients receiving renal transplantation [42]. Similarly, there was persistence of B cells in LNs of a subset of cynomolgus monkeys treated with rituximab in a model of allotransplantation [43]. The results support the view that rituximab treatment improves RA in part because, next to B cell depletion, it results in decreased T cell activation. The observed persistence of switched memory B cells in lymphoid tissues after treatment may explain the observation that this therapy does not result in a cure for RA.
Supplementary Material
Acknowledgements
We thank the study participants in the study, the radiology department at the AMC for lymph node sampling, the flow cytometry facility at the Haematology department at AMC especially J. A. Dobber, the AMC KIR technicians for sample processing. Study conception and design: C.J.L., D.M.G., P.P.T., R.M.T., T.H.R. Acquisition of data: F.H.B., J.F.S., M.J.B., M.S., T.H.R. Analysis and interpretation of data: J.F.S., M.J.B., P.P.T., T.H.R. Drafting of manuscript: M.J.B., P.P.T., R.M.T., T.H.R. Critical revision: C.J.L., D.M.G., F.H.B., L.G.B., M.J.B., M.S., P.P.T., R.M.T., S.T.B., T.H.R. All authors read and approved the manuscript.
Funding: This study was supported by the Innovative Medicines Initiative (IMI) project BTCure (no. 115142-1), Euro-TEAM FP7 HEALTH programme under the grant agreement FP7-HEALTH-F2-2012-305549, Dutch Arthritis Foundation grant 11-1-308 and the Netherlands Organisation for Health Research and Development (ZonMw) Veni (project 916.12.109).
Disclosure statement: P.P.T. and D.M.G. are currently employees of GlaxoSmithKline. GSK was not involved in this study. The other authors have declared no conflicts of interest.
References
- 1. Rantapaa-Dahlqvist S, de Jong BA, Berglin E. et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 2. Nielen MM, van Schaardenburg D, Reesink HW. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum 2004;50:380–6. [DOI] [PubMed] [Google Scholar]
- 3. Doorenspleet ME, Klarenbeek PL, de Hair MJ. et al. Rheumatoid arthritis synovial tissue harbours dominant B-cell and plasma-cell clones associated with autoreactivity. Ann Rheum Dis 2014;73:756–62. [DOI] [PubMed] [Google Scholar]
- 4. Thurlings RM, Wijbrandts CA, Mebius RE. et al. Synovial lymphoid neogenesis does not define a specific clinical rheumatoid arthritis phenotype. Arthritis Rheum 2008;58:1582–9. [DOI] [PubMed] [Google Scholar]
- 5. Edwards JC, Szczepanski L, Szechinski J. et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. New Engl J Med 2004;350:2572–81. [DOI] [PubMed] [Google Scholar]
- 6. Emery P, Fleischmann R, Filipowicz-Sosnowska A. et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006;54:1390–400. [DOI] [PubMed] [Google Scholar]
- 7. Tak PP, Rigby WF, Rubbert-Roth A. et al. Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis 2011;70:39–46. [DOI] [PubMed] [Google Scholar]
- 8. Leandro MJ, Cooper N, Cambridge G, Ehrenstein MR, Edwards JC.. Bone marrow B-lineage cells in patients with rheumatoid arthritis following rituximab therapy. Rheumatology 2007;46:29–36. [DOI] [PubMed] [Google Scholar]
- 9. Teng YK, Levarht EW, Hashemi M. et al. Immunohistochemical analysis as a means to predict responsiveness to rituximab treatment. Arthritis Rheum 2007;56:3909–18. [DOI] [PubMed] [Google Scholar]
- 10. Kavanaugh A, Rosengren S, Lee SJ. et al. Assessment of rituximab’s immunomodulatory synovial effects (ARISE trial). 1: clinical and synovial biomarker results. Ann Rheum Dis 2007;67:402–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thurlings RM, Vos K, Wijbrandts CA. et al. Synovial tissue response to rituximab: mechanism of action and identification of biomarkers of response. Ann Rheum Dis 2008;67:917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakou M, Katsikas G, Sidiropoulos P. et al. Rituximab therapy reduces activated B cells in both the peripheral blood and bone marrow of patients with rheumatoid arthritis: depletion of memory B cells correlates with clinical response. Arthritis Res Ther 2009;11:R131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rehnberg M, Amu S, Tarkowski A, Bokarewa MI, Brisslert M.. Short- and long-term effects of anti-CD20 treatment on B cell ontogeny in bone marrow of patients with rheumatoid arthritis. Arthritis Res Ther 2009;11:R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnett FC, Edworthy SM, Bloch DA. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 15. de Hair MJ, Zijlstra IA, Boumans MJ. et al. Hunting for the pathogenesis of rheumatoid arthritis: core-needle biopsy of inguinal lymph nodes as a new research tool. Ann Rheum Dis 2012;71:1911–2. [DOI] [PubMed] [Google Scholar]
- 16. Dass S, Rawstron AC, Vital EM. et al. Highly sensitive B cell analysis predicts response to rituximab therapy in rheumatoid arthritis. Arthritis Rheum 2008;58:2993–9. [DOI] [PubMed] [Google Scholar]
- 17. Tak PP, van der Lubbe PA, Cauli A. et al. Reduction of synovial inflammation after anti-CD4 monoclonal antibody treatment in early rheumatoid arthritis. Arthritis Rheum 1995;38:1457–65. [DOI] [PubMed] [Google Scholar]
- 18. Haringman JJ, Vinkenoog M, Gerlag DM. et al. Reliability of computerized image analysis for the evaluation of serial synovial biopsies in randomized controlled trials in rheumatoid arthritis. Arthritis Res Ther 2005;7:R862–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuzin II, Kates SL, Ju Y. et al. Increased numbers of CD23(±) CD21(hi) Bin-like B cells in human reactive and rheumatoid arthritis lymph nodes. Eur J Immunol 2016;46:1752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prevodnik VK, Lavrencak J, Horvat M, Novakovic BJ.. The predictive significance of CD20 expression in B-cell lymphomas. Diagnostic Pathol 2011;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rezvani AR, Maloney DG.. Rituximab resistance. Best Pract Res Clin Haematol 2011;24:203–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vallerskog T, Heimburger M, Gunnarsson I. et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 2006;8:R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorner T, Kinnman N, Tak PP.. Targeting B cells in immune-mediated inflammatory disease: a comprehensive review of mechanisms of action and identification of biomarkers. Pharmacol Ther 2010;125:464–75. [DOI] [PubMed] [Google Scholar]
- 24. Takemura S, Klimiuk PA, Braun A, Goronzy JJ, Weyand CM.. T cell activation in rheumatoid synovium is B cell dependent. J Immunol 2001;167:4710–8. [DOI] [PubMed] [Google Scholar]
- 25. Sathaliyawala T, Kubota M, Yudanin N. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013;38:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cambridge G, Perry HC, Nogueira L. et al. The effect of B-cell depletion therapy on serological evidence of B-cell and plasmablast activation in patients with rheumatoid arthritis over multiple cycles of rituximab treatment. J Autoimmun 2014;50:67–76. [DOI] [PubMed] [Google Scholar]
- 27. Teng YK, Levarht EW, Toes RE, Huizinga TW, van Laar JM.. Residual inflammation after rituximab treatment is associated with sustained synovial plasma cell infiltration and enhanced B cell repopulation. Ann Rheum Dis 2009;68:1011–6. [DOI] [PubMed] [Google Scholar]
- 28. Adlowitz DG, Barnard J, Biear JN. et al. Expansion of activated peripheral blood memory B cells in rheumatoid arthritis, impact of B cell depletion therapy, and biomarkers of response. PLoS One 2015;10:e0128269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Owczarczyk K, Lal P, Abbas AR. et al. A plasmablast biomarker for nonresponse to antibody therapy to CD20 in rheumatoid arthritis. Sci Transl Med 2011;3:101ra192. [DOI] [PubMed] [Google Scholar]
- 30. Boumans MJ, Thurlings RM, Gerlag DM, Vos K, Tak PP.. Response to rituximab in patients with rheumatoid arthritis in different compartments of the immune system. Arthritis Rheum 2011;63:3187–94. [DOI] [PubMed] [Google Scholar]
- 31. Avery DT, Kalled SL, Ellyard JI. et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest 2003;112:286–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mackay F, Schneider P.. Cracking the BAFF code. Nat Rev Immunol 2009;9:491–502. [DOI] [PubMed] [Google Scholar]
- 33. Leandro MJ, Cambridge G, Ehrenstein MR, Edwards JC.. Reconstitution of peripheral blood B cells after depletion with rituximab in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:613–20. [DOI] [PubMed] [Google Scholar]
- 34. Gorelik L, Gilbride K, Dobles M. et al. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med 2003;198:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang W, Quach TD, Dascalu C. et al. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. J Clin Invest Insight 2018;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakayama Y, Kosek J, Capone L. et al. Aiolos overexpression in systemic lupus erythematosus B cell subtypes and BAFF-induced memory B cell differentiation are reduced by CC-220 modulation of cereblon activity. J Immunol 2017;199:2388–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma N, Xiao H, Marrero B. et al. Combination of TACI-IgG and anti-IL-15 treats murine lupus by reducing mature and memory B cells. Cell Immunol 2014;289:140–4. [DOI] [PubMed] [Google Scholar]
- 38. Takemura S, Braun A, Crowson C. et al. Lymphoid neogenesis in rheumatoid synovitis. J Immunol 2001;167:1072–80. [DOI] [PubMed] [Google Scholar]
- 39. Ozaki K, Spolski R, Ettinger R. et al. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol 2004;173:5361–71. [DOI] [PubMed] [Google Scholar]
- 40. Sfikakis PP, Boletis JN, Lionaki S. et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum 2005;52:501–13. [DOI] [PubMed] [Google Scholar]
- 41. Häusler D, Häusser-Kinzel S, Feldmann L. et al. Functional characterization of reappearing B cells after anti-CD20 treatment of CNS autoimmune disease. Proc Natl Acad Sci U S A 2018;115:9773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamburova EG, Koenen HJ, Borgman KJ. et al. A single dose of rituximab does not deplete B cells in secondary lymphoid organs but alters phenotype and function. Am J Transplant 2013;13:1503–11. [DOI] [PubMed] [Google Scholar]
- 43. Schroder C, Azimzadeh AM, Wu G. et al. Anti-CD20 treatment depletes B-cells in blood and lymphatic tissue of cynomolgus monkeys. Transplant Immunol 2003;12:19–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.