Abstract
Background:
It is well known that depression improves faster with electroconvulsive treatment (ECT) than with antidepressant medications. N-methyl-D-aspartate-receptor antagonists (ketamine) have been shown to have rapid antidepressant effects when given as an intravenous infusion. Faster recovery with ECT is likely when used with ketamine as anesthetic.
Aim:
The aim of the study is to compare the outcome of modified electroconvulsive therapy (MECT) in major depressive disorder patients undergoing MECT with ketamine versus thiopentone anesthesia.
Materials and Methods:
Sixty hospitalized patients (age: 18–45 years) with major depressive disorder (Diagnostic and Statistical Manual of Mental Disorders-IV Text Revision) were randomly allocated to either of the two MECT groups (30 patients each) receiving ketamine or thiopentone as anesthetic agent. The participants were assessed on a weekly basis on Hamilton Rating Scale for Depression (HAM-D) and Beck Depression Inventory (BDI).
Results:
Ketamine group required significantly lesser number of MECT sessions for achieving remission and had rapid improvement in HAM-D and BDI scores compared to the thiopentone group. Furthermore, the stimulus intensity required to elicit seizures was significantly less and seizure duration was longer in ketamine group compared to the thiopentone group.
Conclusion:
The use of ketamine for anesthesia led to rapid recovery from depressive symptoms and seems to be a better option for depressive patients, especially when a rapid response is desired.
Keywords: Depression, electroconvulsive therapy, ketamine, thiopentone
INTRODUCTION
The World Health Organization has ranked depression fourth in the list of the most urgent health problems worldwide. It is associated with significant morbidity and mortality, including impaired social and physical functioning and increased risk for suicide.[1]
The latency of the onset of action and limited efficacy of currently available antidepressants can result in considerable morbidity, including increased suicide risk.[2] Recent evidence suggests that N-methyl-D-aspartate (NMDA) receptor antagonists (e.g., ketamine) are associated with antidepressant effects in several animal models of depression, and clinical studies show that they induce a rapid-onset antidepressant response (within 2 h) in individuals with major depressive disorder when given parenterally.[3,4,5]
It is well known that depression improves faster with electroconvulsive treatment (ECT) than with antidepressant medications. Recovery of depression with ECT may be hastened using ketamine as the anesthetic agent.[6] Some empirical studies also favor this hypothesis. Superior efficacy of modified electroconvulsive therapy (MECT) with ketamine (NMDA antagonist) as anesthetic agent has been demonstrated in case reports,[7] open-label studies,[8] and chart review studies.[9] Few double-blind ECT studies have demonstrated its antidepressant effects.[10,11,12] Limitations of previous studies include small sample size,[10,11,12] lack of randomization,[8] and retrospective designs.[9] Besides these limitations, some other randomized ECT studies did not confirm the antidepressant effect of ketamine.[13,14,15,16] Different meta-analyses of such randomized controlled trials have also reported conflicting findings.[6,17,18] These inconsistencies could be attributed to differences in study design,[10,14] sites of application of ECT,[9,11,13] type,[11,12,13,16] and dose[11,14] of anesthetic agent used, or the termination criteria used in these studies.[10,11,14]
Existing evidence obviously does not make a strong case in favor of antidepressant effects of ketamine when used as an ECT anesthetic agent. Hence, this study was planned to compare the outcome of electroconvulsive therapy in major depressive disorder patients undergoing ECT with thiopentone versus ketamine anesthesia.
MATERIALS AND METHODS
The study was carried out in the department of psychiatry at a tertiary care hospital in North India. As this study was planned as a part of postgraduate training of the first author (AJ), the complete proposal of study was presented before the Postgraduate Board of Education for ethical and methodological clearance.
The sample
Study sample consisted of 60 hospitalized patients with Diagnostic and Statistical Manual of Mental Disorders- IV Text Revision diagnosis of major depressive disorder, of either sex, of age 18–45 years, having a score of 15 or more on 17-item Hamilton Depression Rating Scale (HAM-D),[19] and Status 3 or less on Preoperative Risk Criteria of the American Society of Anesthesiologists.[20] Bipolar depressed patients were not included in the study. The diagnosis of major depressive disorder was made by a semistructured clinical interview. All patients were selected after a decision to start ECT was taken by the treatment team based on the common indications such as suicidality or no response to two adequate trials of antidepressant medications.
The patients were excluded if they had any comorbid medical/surgical disease, history of substance abuse or dependence except nicotine-containing substances, history of serious adverse effects related to anesthetics (e.g., allergy), any implant in the body, such as a pacemaker, intracranial electrode, and clip, pregnancy, and concomitant presence of a mental disorder other than major depression. The patients requiring any change in the anesthetic drug during the course of study were also excluded from the analysis.
After taking informed consent, detailed physical examination and investigations were carried out to rule out physical comorbidities. Detailed examination and investigations to know patients’ fitness for anesthesia was also carried out. Fitness for anesthesia was decided by an anesthetist (NM).
Each patient was allotted randomly to either Group “A” or “B” by the nursing assistant, who was not involved in the study, for deciding the choice of anesthetic drug, i.e., whether to use thiopentone or ketamine for MECT. Both drugs are routinely used in this hospital for anesthetic procedure. For this, chit-pull method was used. Thirty chits marked with “A” and 30 chits marked with “B” were kept in a container. The patient's group was decided by picking up a chit randomly from the container by the nurse on ECT duty. This chit was then discarded. In this manner, two equal groups of 30 patients each were created for the purpose of study. The researchers (AJ and HK) and the patients were blind to the choice anesthetic drug done by anesthetist (NM). The choice of anesthetic drug was revealed only after the data analysis was complete. The choice of oral antidepressant medications in individual study participants in both groups was random, as decided by the treating psychiatrists (who were not concerned with the study) on the basis of clinical characteristics and patient's choice.
Electroconvulsive treatment procedure
MECT was given thrice a week on alternate days using MEDICAID BPE-2000 instrument to deliver brief-pulse wave stimulation for bilateral ECT. The stimulus intensity was decided by empirical titration. Seizure threshold in all patients was titrated during the first treatment, and energy was subsequently increased if patients showed insufficient seizures during the ECT course (i.e., electroencephalography [EEG] seizure activity <30 s). Duration of seizures during ECT was monitored by EEG recording. Pulse width, pulse frequency, and pulse amplitude of brief-pulse stimulation were kept constant at 1.0 ms, 70 hertz, and 800 milliampere, respectively. The dose of ECT was titrated by changing the duration of stimulation. The total electrical dose was calculated as milli-Coulomb of electrical charge at each session of ECT. The anesthetic consisted of intravenous atropine sulfate (0.25 mg) with either ketamine (1 mg/kg) or thiopentone (2.5 mg/kg). Succinylcholine (0.5 mg/kg) was given intravenously as muscle relaxant after induction of anesthesia. All these drugs are available free of cost in hospital supply. The stimulation intensity and the seizure duration on the EEG were recorded for each ECT session. Individuals requiring more than the prescribed doses of anesthetic drugs were excluded from the study.
Assessment
Baseline clinical assessment was carried out by the researcher (AJ) with 17-item HAM-D[19] and Beck Depression Inventory (BDI)[21] a day before starting MECT. The sociodemographic details and clinical history were also recorded at the same time. Thereafter, the assessment on these tools was carried out weekly (day after ECT) by the researcher (AJ) till MECT was stopped. The patients were assessed again at 1 week and 6 weeks after the discontinuation of ECT on all the above-mentioned tools. Side effects of anesthetic procedure, if any, were recorded separately after each MECT. Patients were also assessed in the evening (6 h after ECT) for cognitive side effects using Mini-Mental State Examination (MMSE).[22]
The MECT was stopped if (a) the patient achieved score of ≤4 on 17-item HAM-D; (b) if the patient did not show progressive decrease in HAM-D score >3 in three successive ECTs; (c) the patient developed any complication; and (d) the patient or caregiver withdrew consent.
Statistical analysis
The Statistical Package for the Social Sciences version 16.0 (SPSS Inc., Chicago, IL) for Windows was used for statistical analysis. Frequencies with percentages were calculated for categorical variables, and mean and standard deviation were calculated for continuous variables. Categorical data were analyzed using Chi-square test; continuous data were analyzed using unpaired t-test. Mann–Whitney U-test was used to compare mean duration of current depressive episode as the data were not normally distributed. Mixed model design ANOVA, with duration of current depressive episode as the covariate, was used to assess changes in BDI and HAM-D scores over time in the two treatment groups. All statistics were two tailed, carried out with α set at 0.05. The anesthetist (NM) was not involved in the statistical analysis.
RESULTS
A total of 60 patients were assessed – 30 in thiopentone group and 30 in ketamine group. None of the patients withdrew from the study [Figure 1]. There were no significant differences in the sociodemographic profile of the two groups [Table 1].
Figure 1.
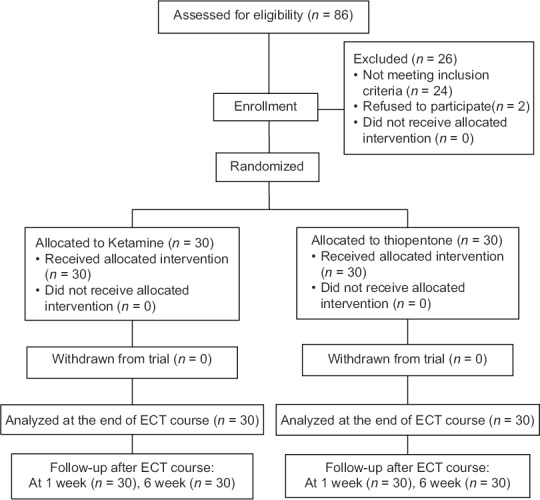
Consort diagram showing recruitment and progress of study participants
Table 1.
Baseline variables of the study groups
| Thiopentone group (n=30) | Ketamine group (n=30) | P | |
|---|---|---|---|
| Age (mean±SD) | 34.97±8.17 | 35.37±8.97 | 0.86c |
| Gender, n (%) | |||
| Male | 17 (56.7) | 13 (43.3) | 0.30a |
| Female | 13 (43.3) | 17 (56.7) | |
| Locality, n (%) | |||
| Rural | 20 (66.7) | 20 (66.7) | 1.0a |
| Urban | 10 (33.3) | 10 (33.3) | |
| Income group (Rs./month), n (%) | |||
| <7000 | 17 (56.7) | 16 (53.3) | 0.79a |
| 7000-28000 | 13 (43.3) | 14 (46.7) | |
| >28000 | 0 | 0 | |
| Mean±SD (median) duration of current depressive episode (months) | 6.83±6.81 (4) | 3.55±5.17 (1.25) | 0.002b |
| Baseline HAM-D score | 32.0±7.60 | 29.53±4.56 | 0.13c |
| Baseline BDI score | 52.17±3.55 | 51.37±2.41 | 0.31c |
| Baseline MMSE score | 27.83±1.39 | 27.70±1.62 | 0.73c |
aChi-square test; bMann-Whitney U-test; cUnpaired t-test. HAM-D – Hamilton Rating Scale for Depression; BDI – Beck Depression Inventory; MMSE – Mini-Mental Status Examination; SD – Standard deviation
Baseline clinical assessment revealed that thiopentone group had significantly longer duration of illness, but no significant differences were found in HAM-D, BDI, and MMSE scores [Table 1].
On comparing the ECT-induced seizure pattern in the two groups [Table 2], the ketamine group required significantly lesser number of ECT sessions (t = 10.53; P < 0.001) and had significantly longer seizures as recorded by EEG (t = 4.94; P < 0.001). However, none of the patients had prolonged seizures in this group. Furthermore, the mean stimulus intensity required to elicit seizures was significantly less in ketamine group compared to the thiopentone group (t = 6.46; P < 0.001).
Table 2.
Electroconvulsive therapy-related variables
| Thiopentone group (n=30) | Ketamine group (n=30) | t | P | |
|---|---|---|---|---|
| Number of ECT sessions, mean±SD | 8.33±1.24 | 5.57±0.73 | 10.53 | <0.001 |
| EEG seizure duration (s) | 42.63±5.82 | 50.33±6.25 | 4.94 | <0.001 |
| Stimulus intensity (mC) | 92.93±9.03 | 80.97±4.61 | 6.46 | <0.001 |
ECT – Electroconvulsive therapy; EEG – Electroencephalogram; mC – milli-Coulomb; SD – Standard deviation
Table 3 shows weekly changes in HAM-D and BDI scores over the course of ECT treatment in the two groups. Mixed model design ANOVA with duration of current depressive episode as the covariate showed significant time × group interaction (within-subject effects) after applying Greenhouse–Geisser correction for BDI (F [1.86, 106] =23.82, P < 0.001, partial η2 = 0.29) as well as HAM-D scores (F [5, 95.38] =19.38, P < 0.001, partial η2 = 0.25). Test of between-individual effects was also significant (P < 0.001). Further analysis of the data by unpaired t-test was done to determine the time points where the two ECT groups differed significantly. The HAM-D and BDI scores were significantly lower in the ketamine group at the end of 1st and 2nd week of ECT. At the end of ECT sessions, mean BDI scores were similar in the two treatment groups, but mean HAM-D scores were significantly lower in the ketamine group. There were no differences in the HAM-D or BDI scores in the study groups during subsequent follow-ups [Table 3].
Table 3.
Depression ratings over the course of electroconvulsive therapy
| Week 1 | Week 2 | At completion of ECT sessions | 1 week follow up | 6 weeks follow up | |
|---|---|---|---|---|---|
| HAM-D scores (mean±SD) | |||||
| Thiopentone (n=30) | 24.33±6.75 | 13.43±5.91 | 3.43±0.94 | 1.60±2.34 | 0.70±1.51 |
| Ketamine (n=30) | 17.37±4.29 | 4.17±3.40 | 2.83±1.12 | 1.0±1.11 | 0.27±0.58 |
| P | <0.001 | <0.001 | 0.03 | 0.21 | 0.15 |
| BDI scores (mean±SD) | |||||
| Thiopentone (n=30) | 41.63±9.51 | 22.67±11.01 | 6.97±3.20 | 3.07±3.47 | 1.17±2.69 |
| Ketamine (n=30) | 30.30±11.69 | 7.77±4.45 | 6.03±1.56 | 2.87±1.63 | 0.73±1.26 |
| P | <0.001 | <0.001 | 0.16 | 0.78 | 0.43 |
HAM-D – Hamilton Rating Scale for Depression; BDI – Beck Depression Inventory; SD – Standard deviation; ECT – Electroconvulsive therapy
The common side effects that were reported in each treatment group immediately after the ECT sessions are shown in Table 4. Nausea/vomiting, increased secretions (requiring suctioning more than once), emergence reactions, delirium, and rise in blood pressure (MAP >160 mm of mercury) and heart rate (>120 beats/min) were reported more often in the ketamine group.
Table 4.
Side effects reported immediately after electroconvulsive therapy sessions
| Thiopentone group (n=30), n (%) | Ketamine group (n=30), n (%) | |
|---|---|---|
| Emergence reactions | 0 (0) | 2 (6.6) |
| Increased secretions | 3 (10.0) | 9 (30.0) |
| Nausea/vomiting | 0 (0) | 4 (13.3) |
| Headache | 12 (40.0) | 10 (33.3) |
| Delirium | 0 (0) | 2 (6.6) |
| BP rise | 2 (6.6) | 6 (20.0) |
| HR rise | 1 (3.3) | 4 (13.3) |
BP – Blood pressure; HR – Heart rate
There were no significant differences in the mean MMSE scores of the two groups (thiopentone: 26.07 ± 1.92 and ketamine: 27.75 ± 0.95; P = 0.10) at the completion of ECT sessions.
DISCUSSION
There are few case reports and planned studies depicting rapid antidepressant effects of intravenous ketamine infusion.[3,5] The antidepressant effect of ketamine as an ECT anesthetic in depressive patients has been assessed in very few studies.[7,8,9,10,11,12,13,14,15,16]
The sociodemographic and baseline clinical details in both the treatment groups of our study were similar except for the mean duration of current depressive episode [6.83 ± 6.81 in thiopentone and 3.55 ± 5.17 in ketamine group; P = 0.002, Table 1]. Exactly similar domiciliary distribution of patients and an inversely equal gender distribution in the two treatment groups was a chance finding.
In contrast to other studies,[10,11,16] we kept upper cutoff for age at 45 years to make the sample homogeneous.
Efficacy
We found that fewer number of ECT treatments were required in the ketamine group (5.57 ± 0.73) than in the thiopentone group (8.33 ± 1.24) until completion [Table 2]. This shows superiority of ketamine anesthesia over thiopentone for ECT in depressive episodes. The total number of sessions can be used as surrogate parameter representing the clinical response to the treatment.[23] We also found that depression ratings (HAM-D and BDI scores) were significantly lower in the ketamine group than the thiopentone group at the end of 1st as well as 2nd week after commencing ECT [Table 3], which indicates that there was a rapid onset of response and earlier improvement in depression (lesser number of ECT sessions required) in the group receiving ECT with ketamine as the anesthetic agent. This finding is consistent with previous reports of the rapid onset of antidepressant effects with ketamine infusion.[3,5]
Very similar outcomes were reported by Okamoto et al.[8] in a nonrandomized study of ketamine versus propofol anesthesia in 31 treatment-resistant depressed inpatients referred for ECT. They found that the speed of response and magnitude of response (decrease in mean HAM-D scores) were both greater with ketamine anesthesia at the end of a course of eight ECTs administered across 4 weeks. Similarly, Wang et al.,[10] in a randomized controlled trial on 48 patients, reported greater speed and magnitude of response in patients receiving ketamine (0.8 mg/kg) alone or ketamine (0.8 mg/kg) + propofol (1.5 mg/kg) than those receiving only propofol (1.5 mg/kg) as the ECT anesthetic agent.
Yoosefi et al.[11] in a randomized, double-blind clinical trial compared the effects of ketamine (N = 15) and thiopental (n = 14) administration during electroconvulsive therapy in patients with major depressive disorder. They found that at the end of the study (after 6 ECT sessions), depression improved significantly in both groups. However, a significant difference in depression improvement was noted only before the second ECT with ketamine compared with thiopental.
Gamble et al.[12] in a randomized clinical trial compared ECT with ketamine-based anesthesia versus propofol-based anesthesia (12 patients in each arm) and found that ketamine-based ECT provided response and remission after fewer ECT sessions.
Our study suggests rapid improvement with ketamine, but we did not find a higher magnitude of response in ketamine group [Table 3] which could be because our study was different with respect to study sample chosen, the termination criteria for MECT, and different anesthetic agents used.
Better outcome in the ketamine group can be explained by the synergistic antidepressant effects of ECT and the ketamine. This finding corroborates reports about inherent antidepressant properties of ketamine.[3,5,7]
In contrast, some other randomized ECT studies did not confirm the antidepressant effect of ketamine.[13,14,15,16] This might be due to the anticonvulsant effects of thiopentone or propofol which were administered along with ketamine during the ECT in some studies[13,14,15] or due to different doses of the anesthetic agents used.[16]
Seizure expression
We found that patients receiving ECT with ketamine as an anesthetic agent needed significantly lower stimulation intensity for eliciting seizures and significantly longer seizures were recorded by EEG [Table 2]. These findings are in line with findings of Krystal et al.[24] who reported that seizure parameters in the ketamine group were overall more favorable with respect to seizure duration. Kranaster et al.,[9] in a retrospective chart review, examined the effects of ketamine as ECT anesthetic in 42 patients suffering from treatment-resistant depression who received ECT treatment with either ketamine (n = 16) or thiopental (n = 26). They found that the duration of motor seizures as well as the duration of EEG activity did not differ between groups; however, concordance as a parameter of seizure quality was significantly higher in the ketamine group. These findings can be explained by the anticonvulsant property of barbiturate anesthetics which is not found in ketamine.[25] However, we have reservations in attributing long duration of seizure to poor control of seizures with ketamine because all patients had therapeutic duration of seizures and none of the patients in this group exhibited prolonged seizure. This observation also reflects the dissonance between the statistical and therapeutic significance of seizures.
Side effects
As depicted in Table 4, we found that patients in both the treatment groups had very few side effects immediately after the treatment sessions. Headache was the most commonly reported side effect in both the treatment groups. This is due to the fact that headache is a common side effect of ECT procedure itself.[26] Side effects such as emergence reactions, increased secretions, nausea/vomiting, delirium, increased BP, and increased HR were reported more in ketamine group compared to thiopentone group. This could be due to inherent property of ketamine to produce such side effects. The central nervous system (CNS) side effects of ketamine such as emergence reactions are due to the NMDA blocking actions of ketamine. These receptors play a major role in the transmission of sensory information and are thought to mediate the excitation of neurons in the CNS secondary to interactions with excitatory amino acid neurotransmitters.[27] The cardiovascular side effects of ketamine can be explained by its inherent property of causing a systemic release of catecholamines and inhibition of norepinephrine reuptake at peripheral nerves and nonneuronal tissues such as the myocardium.[28]
There has been interest in using ketamine in ECT anesthesia as a way of possibly blocking ECT-induced cognitive dysfunction. Some retrospective chart reviews have reported better cognitive outcomes with ketamine as the anesthetic agent.[9,24] However, we did not find any cognitive benefit of using ketamine as MMSE scores were statistically similar in both groups at the completion of ECT sessions. Loo et al.,[15] similarly, found no cognitive advantage of adding ketamine to thiopental anesthesia for ECT.
In brief, the findings appear to suggest that ketamine anesthetic had led to rapid recovery with MECT. The patients in this group had rapid decrease in HAM-D and BDI scores.
The exact mechanism of antidepressant action of ketamine is not known. Initially, NMDA receptor antagonism was postulated as the possible mechanism of antidepressant action of ketamine,[29] but other NMDA antagonists do not seem to produce the same antidepressant effects as ketamine, suggesting that its antidepressant properties may not be mediated through NMDA pathways. Furthermore, it has been postulated that ketamine's antidepressant effect involves activation of alpha amino-3-hydroxy-5-methyl-4-isoxzole propionic acid receptors either directly or through metabolism to (2R, 6S)-hydroxynorketamine which ultimately increases brain-derived neurotrophic factor, thereby increasing synaptogenesis between neurons and leading to recovery from depression.[30] Other postulated mechanisms for the antidepressant effect of ketamine include activity at sigma receptors[31] and effects within the dopaminergic[32] or serotonergic systems.[33]
Limitations
We could not control the bias arising because of the antidepressant medication. However, the variance due to the choice of antidepressant medication was equal and random between the two groups and cannot account for the observed difference. Furthermore, we did not estimate the sample size required for our study and chose a sample size of 30 in each treatment group based on our convenience.
CONCLUSION
The study highlights the beneficial role of using ketamine as an anesthetic in MECT for patients with depressive disorder compared to conventional barbiturates. As the use of ketamine for anesthesia led to rapid recovery from depressive symptoms, ketamine assisted MECT seems to be a better option for depressive patients, especially when a rapid response is desired, for example, severely depressed patients with active suicidal ideation with strong suicidal intent.[34] In such patients, it helps in rapidly resolving suicidal ideation secondary to its rapid antidepressant property.[34,35]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rihmer Z, Merikangas KR. Mood disorders: Epidemiology. In: Sadock VA, Sadock BJ, Ruiz P, editors. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 10th ed. Vol. 1. Philadelphia, PA: Williams & Wilkins; 2017. p. 1614. [Google Scholar]
- 2.Jick H, Kaye JA, Jick SS. Antidepressants and the risk of suicidal behaviors. JAMA. 2004;292:338–43. doi: 10.1001/jama.292.3.338. [DOI] [PubMed] [Google Scholar]
- 3.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–4. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 4.Harihar C, Dasari P, Srinivas JS. Intramuscular ketamine in acute depression: A report on two cases. Indian J Psychiatry. 2013;55:186–8. doi: 10.4103/0019-5545.111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 6.Ren L, Deng J, Min S, Peng L, Chen Q. Ketamine in electroconvulsive therapy for depressive disorder: A systematic review and meta-analysis. J Psychiatr Res. 2018;104:144–56. doi: 10.1016/j.jpsychires.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Ostroff R, Gonzales M, Sanacora G. Antidepressant effect of ketamine during ECT. Am J Psychiatry. 2005;162:1385–6. doi: 10.1176/appi.ajp.162.7.1385. [DOI] [PubMed] [Google Scholar]
- 8.Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T, et al. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: Comparing ketamine and propofol anesthesia. J ECT. 2010;26:223–7. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- 9.Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: A retrospective study. Eur Arch Psychiatry Clin Neurosci. 2011;261:575–82. doi: 10.1007/s00406-011-0205-7. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C, et al. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT. 2012;28:128–32. doi: 10.1097/YCT.0b013e31824d1d02. [DOI] [PubMed] [Google Scholar]
- 11.Yoosefi A, Sepehri AS, Kargar M, Akhondzadeh S, Sadeghi M, Rafei A, et al. Comparing effects of ketamine and thiopental administration during electroconvulsive therapy in patients with major depressive disorder: A randomized, double-blind study. J ECT. 2014;30:15–21. doi: 10.1097/YCT.0b013e3182a4b4c6. [DOI] [PubMed] [Google Scholar]
- 12.Gamble JJ, Bi H, Bowen R, Weisgerber G, Sanjanwala R, Prasad R, et al. Ketamine-based anesthesia improves electroconvulsive therapy outcomes: A randomized-controlled study. Can J Anaesth. 2018;65:636–46. doi: 10.1007/s12630-018-1088-0. [DOI] [PubMed] [Google Scholar]
- 13.Abdallah CG, Fasula M, Kelmendi B, Sanacora G, Ostroff R. Rapid antidepressant effect of ketamine in the electroconvulsive therapy setting. J ECT. 2012;28:157–61. doi: 10.1097/YCT.0b013e31824f8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Järventausta K, Chrapek W, Kampman O, Tuohimaa K, Björkqvist M, Häkkinen H, et al. Effects of S-ketamine as an anesthetic adjuvant to propofol on treatment response to electroconvulsive therapy in treatment-resistant depression: A randomized pilot study. J ECT. 2013;29:158–61. doi: 10.1097/YCT.0b013e318283b7e9. [DOI] [PubMed] [Google Scholar]
- 15.Loo CK, Katalinic N, Garfield JB, Sainsbury K, Hadzi-Pavlovic D, Mac-Pherson R, et al. Neuropsychological and mood effects of ketamine in electroconvulsive therapy: A randomised controlled trial. J Affect Disord. 2012;142:233–40. doi: 10.1016/j.jad.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Rasmussen KG, Kung S, Lapid MI, Oesterle TS, Geske JR, Nuttall GA, et al. A randomized comparison of ketamine versus methohexital anesthesia in electroconvulsive therapy. Psychiatry Res. 2014;215:362–5. doi: 10.1016/j.psychres.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 17.McGirr A, Berlim MT, Bond DJ, Chan PY, Yatham LN, Lam RW, et al. Adjunctive ketamine in electroconvulsive therapy: Updated systematic review and meta-analysis. Br J Psychiatry. 2017;210:403–7. doi: 10.1192/bjp.bp.116.195826. [DOI] [PubMed] [Google Scholar]
- 18.Li DJ, Wang FC, Chu CS, Chen TY, Tang CH, Yang WC, et al. Significant treatment effect of add-on ketamine anesthesia in electroconvulsive therapy in depressive patients: A meta-analysis. Eur Neuropsychopharmacol. 2017;27:29–41. doi: 10.1016/j.euroneuro.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960 Feb;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dripps RD. New classification of physical status. Anaesthesiology. 1963;24:111. [Google Scholar]
- 21.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Sartorius A, Muñoz-Canales EM, Krumm B, Krier A, Andres FJ, Bender HJ, et al. ECT anesthesia: The lighter the better? Pharmacopsychiatry. 2006;39:201–4. doi: 10.1055/s-2006-950395. [DOI] [PubMed] [Google Scholar]
- 24.Krystal AD, Weiner RD, Dean MD, Lindahl VH, Tramontozzi LA, 3rd, Falcone G, et al. Comparison of seizure duration, ictal EEG, and cognitive effects of ketamine and methohexital anesthesia with ECT. J Neuropsychiatry Clin Neurosci. 2003;15:27–34. doi: 10.1176/jnp.15.1.27. [DOI] [PubMed] [Google Scholar]
- 25.Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94:1351–64. doi: 10.1097/00000539-200205000-00057. [DOI] [PubMed] [Google Scholar]
- 26.Prudic J, Duan Y. Electroconvulsive therapy. In: Sadock VA, Sadock BJ, Ruiz P, editors. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 10th ed. Vol. 2. Philadelphia, PA: Williams and Wilkins; 2017. p. 3298. [Google Scholar]
- 27.Haas DA, Harper DG. Ketamine: A review of its pharmacologic properties and use in ambulatory anesthesia. Anesth Prog. 1992;39:61–8. [PMC free article] [PubMed] [Google Scholar]
- 28.Timm C, Linstedt U, Weiss T, Zenz M, Maier C. Sympathomimetic effects of low-dose S(+)-ketamine. Effect of propofol dosage. Anaesthesist. 2008;57:338–46. doi: 10.1007/s00101-008-1331-0. [DOI] [PubMed] [Google Scholar]
- 29.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–5. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–6. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robson MJ, Elliott M, Seminerio MJ, Matsumoto RR. Evaluation of sigma (σ) receptors in the antidepressant-like effects of ketamine in vitro and in vivo . Eur Neuropsychopharmacol. 2012;22:308–17. doi: 10.1016/j.euroneuro.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Belujon P, Grace AA. Restoring mood balance in depression: Ketamine reverses deficit in dopamine-dependent synaptic plasticity. Biol Psychiatry. 2014;76:927–36. doi: 10.1016/j.biopsych.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto S, Ohba H, Nishiyama S, Harada N, Kakiuchi T, Tsukada H, et al. Subanesthetic doses of ketamine transiently decrease serotonin transporter activity: A PET study in conscious monkeys. Neuropsychopharmacology. 2013;38:2666–74. doi: 10.1038/npp.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andrade C. Ketamine for depression, 2: Diagnostic and contextual indications. J Clin Psychiatry. 2017;78:e555–8. doi: 10.4088/JCP.17f11629. [DOI] [PubMed] [Google Scholar]
- 35.Sathyanarayana Rao TS, Andrade C. A possible role for ketamine in suicide prevention in emergency and mainstream psychiatry. Indian J Psychiatry. 2017;59:259–61. doi: 10.4103/psychiatry.IndianJPsychiatry_345_17. [DOI] [PMC free article] [PubMed] [Google Scholar]


