Abstract
Background:
Self-report does not provide complete information about tobacco smoke exposure among users and is not relevant for secondhand exposure detection. Biochemical screening for primary metabolite of nicotine would be useful to validate the smoking status and exposure to secondhand smoke.
Aims and Objectives:
This study was designed to evaluate the performance of a sensitive and rapid method to verify smoking status among smokers, passive smokers, and nonsmokers by quantification of cotinine in saliva and urine using liquid chromatography and mass spectrometry.
Materials and Methods:
Cotinine (urine and saliva) levels were measured in 98 participants out of which active users (smoked tobacco users; n = 56) and persons exposed to tobacco smoke (passive smokers; n = 15). Values obtained were compared with nonusers (nonsmokers; n = 27). A simple, rapid, and sensitive method was developed and validated for this purpose. With minimal sample preparation, the current analytical procedure showed a wide detection range (1.1–1000 ng/mL) which made it suitable for analyzing various biological matrices.
Results:
The mean cotinine levels of urine for smokers, passive smokers, and nonsmokers were 1043.7, 36.63, and 13.6 ng/ml, respectively, while in saliva, it was 327.39, 18.31, and 9.53 ng/ml, respectively.
Conclusion:
Analysis of variance showed that cotinine levels (urine and saliva) of smokers were significantly higher levels than passive smokers and nonsmokers (P < 0.01). Similarly, passive smokers also had significantly higher cotinine levels (urine and saliva) than nonsmokers (P < 0.001).
Keywords: Cotinine, Fagerstrom Test for Nicotine Dependence, liquid chromatography and mass spectrometry, passive smokers
INTRODUCTION
The World Health Organization estimates that tobacco use causes nearly 6 million deaths worldwide.[1] Southeast Asia and India are one of the largest tobacco users in the world. Tobacco use is a major concern in this region as both smoked and smokeless forms are prevalent.[2] Cigarette smoking is a public health problem and is preventable cause of many noncommunicable diseases. Tobacco use has been identified as a risk factor for cardiovascular diseases, lung and other cancers, chronic respiratory diseases, stroke, and complications of pregnancy.[3]
Reducing tobacco use and dependence has been identified as a key strategy in reducing the significant long-term health effects and associated economic costs of tobacco use. Clinical practice guidelines recommend consistent identification and documentation of tobacco users along with determination of smoking status as the first step in clinical interventions to counsel and treat tobacco users.[4]
Due to various highly adverse effects of smoking, there is a need to monitor the extent of exposure among users and nonusers. Evaluation of smoking status among users and persons exposed (friends or family members) by self-report questionnaire is inconclusive most of the times. Data suggest that a percentage of patients with respiratory disease (18%), cancer patients (20%), and pregnant women (35%) will self-report inaccurately due to a variety of factors (such as misunderstanding, intentional deception, embarrassment, denial, and shame).[5]
Passive or secondhand smokers (SHSs) are not smoked tobacco users but exposed to smoked tobacco. Attention needs to be paid on the ill effect of SHS on nonsmokers. Around 50,000 people die each year because of exposure to SHS and it is a known risk factor for asthma, bronchitis, and coronary artery disease.[6] Understanding SHS exposure is important in measuring and preventing exposure to this widespread environmental contaminant.
The most commonly used biomarker of tobacco exposure is cotinine. Measurement of cotinine, a primary metabolite of nicotine having a half-life of 16–18 h, provides a reliable means of determining smoking status and other tobacco product uses or exposures.[5] Cotinine concentration in various biological fluids such as urine, saliva, or serum is directly proportional to the degree of nicotine exposure.[7,8] The advantage of using cotinine as a biomarker of tobacco smoke and environmental tobacco smoke (ETS) is the fact that 72% of nicotine is converted to cotinine, and it has a longer half-life (17 h) in comparison to nicotine (3 h).[9,10]
Confirmation by a laboratory analysis may provide correct information about smoking status and smoke exposure. Various analytical techniques are available to measure cotinine levels in biological matrices including colorimetric assays, immunoassays, NicAlert™ saliva tests, gas chromatography (GC), and high-performance liquid chromatography (HPLC). Quantification by mass spectrometry techniques (GC-MS or liquid chromatography and mass spectrometry [LC-MS] is considered more and reliable than colorimetric assays and immunoassays.[11] While screening techniques can be semi-quantitative, GC-MS and LC-MS provide accurate quantitative results. However, disadvantages of these techniques are higher cost and time consumption. Cotinine measurement has not been routinely used in clinical settings mainly due to the time involved, the methods required to collect the sample, and the cost involved.
The combination of LCMS/MS is recognized as offering the best sensitivity and specificity for the detection and measurement of drugs and their metabolites in biological specimens.[12] The primary aim was to validate a method for cotinine quantification in urine and saliva in a broad range of concentrations. This analytical technique will help in accurate determination of degree of exposure among smokers, passive smokers, and nonsmokers. This may have implication in India where smoking in public is common.
MATERIALS AND METHODS
Population
Data were collected from the participants attending the outpatient department of our institute over a period of 1 year (August 2015–July 2016). Smoked tobacco users, passive smokers, and nonsmokers were selected by simple random sampling, and the study design was cross sectional. To all the smokers (actively using cigarette, bidi, and smoked tobacco products form the last 1 year), the Fagerstrom Test for Nicotine Dependence (FTND) was administered as a screening tool and participants having FTND score ≥4.0 were considered as tobacco users with low-to-high dependence.[2]
We screened 134 participants out of which 104 had FTND score ≥4.0. Of them, 29 participants were having other complications and were not fit for the study. Out of 75 which meet the criterion, only 56 agreed to be a part of the study. Hence, finally, 56 participants were considered as smokers. Passive smokers were eligible family members of the smokers exposed to tobacco smoke at home. They were identified by self-report, and demographic information (age, sex, and last exposure) was also obtained. In most of the cases, eligible smoked tobacco users either were not accompanied by the family members or they refrained from smoking in the presence of family members. We got 22 eligible passive smokers and 15 agreed to be a part of the study. Nonsmokers claimed that they were not exposed to tobacco smoke[13] and were randomly selected from nearby community. The Alcohol Use Disorders Identification Test, FTND, and Drug Abuse Screening Test were administered to rule out any exposure to alcohol, drugs, and tobacco. Out of 35 eligible nonsmokers, only 27 consented to be a part of the study.
The sample size was calculated using G*Power 3 (Heinrich Heine University Düsseldorf, Germany). for analysis of variance (ANOVA) fixed effects, one-way by assuming effect size for α = 0.05 and 1− β = 0.8. In the current research, number groups were three and total sample size was 98. For smokers, sufficient sample size (56) was available, but for passive smokers, it was difficult as patient were not always accompanied by the family members or they weren’t ready to be a part of the study. Hence, we were not able to maintain the equal sample size in all the groups. Sighting variation in the number of participants in all the three groups’ power of the test for T-distribution of comparing pairs was examined. It ranged from 93% to 98% showing adequate sample size.
Saliva and urine samples of all the three groups were collected. Consent along with requisite ethics permission was obtained. All samples were labeled and stored in −80°C till analysis. Urine and saliva were collected as follows: smokers (urine: n = 56 and saliva: n = 49), passive smokers (urine and saliva: n = 15), and nonsmokers (urine: n = 27 and saliva: n = 26).
Standards and reagents
Cotinine (98%), internal standard (IS) acetanilide, and formic acid were procured from Sigma-Aldrich. Acetonitrile and methanol ultra-gradient (LC-MS grade) were supplied by Sigma-Aldrich. Ultrapure water was obtained from Milli-Q Plus, Ultrapure Water System (Millipore USA). All working standards were prepared in methanol and stored at −20°C. For centrifugation, Spinwin 2 microcentrifuge was used. Control urine lyophilized samples were purchased from Medichem, Germany.
Sample collection
About 2–5 ml of urine sample and 1–2 ml of saliva were collected for analysis. Participants were asked to avoid taking edible ½ h prior to sample collection. Saliva was collected by passive drooling, and samples were kept 2°C–8°C immediately and later stored at −80°C till analysis.
Sample preparation
Samples (urine and saliva) were centrifuged at 10,000 rpm in cooling centrifuge at 5°C for 5 min. For 100 μl of urine, an equal volume of acetone was added, and for 200 μl of saliva, 100 μl of acetone was added. After proper vortex mixing, the final volume was made up to 1 ml with 0.1% formic acid in methanol. Samples were kept at 5°C for 5 min to avoid the acetone evaporation at room temperature. Finally, the samples were centrifuged for 5 min at 10,000 rpm and passed through Millex-HV, 4 mm, 0.45 μm, and were ready for LC-MS-MS analysis.
Instrumentation
Agilent 1200 series liquid chromatograph (with degasser, column oven that included column switcher) with binary pump and thermostatic autosampler was used for sample introduction. Mass Spectrometer (MS-MS) 6460 Triple Quadrupole system was operated in electrospray ionization (ESI) in positive mode.
Liquid chromatography and mass spectrometry conditions
Samples were placed in temperature controlled autosampler tray (4°C). Samples (2.0 μl) were injected into Polaris 3C-18 silica-based column (150 mm × 3.0 × particle size 5 μm) from Agilent Technologies. The column was mounted on thermostatic column compartment at room temperature. The flow rate was 0.30 ml/min. The mobile phase consisted of Milli-Q water and acetonitrile (80:20) in isocratic mode.
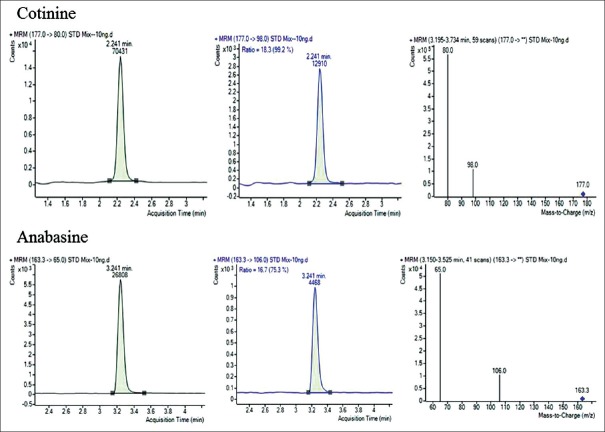
The ESI operated in positive ion mode with collision energy 25-V collision gas temperature was 300°C with gas flow of 11.0 L/min. Nebulizer operated at 40 psi and capillary voltage was 4000 V. Cotinine was analyzed independently by injecting each sample in duplicate. Q1 (parent mass) and Q3 (product ion) were cotinine 177 → 98, 80. Acetanilide was IS, and Q1 and Q3 for acetanilide were 163 → 64.9, 106. Acquisition setting was optimized using Mass Hunter Software Agilent technologies, Germany.
Statistical analysis
ANOVA test was used for group comparisons. FTND associated with the cotinine values in urine and saliva was also compared. For all statistical procedures, significance was assessed at P < 0.05.
RESULTS
Optimization of Liquid chromatography and mass spectrometry conditions
Introduction of stable liquid chromatography systems coupled with tandem mass spectrometers frequently referred as LC-MS/MS has done major advances in analytical technology. The mass spectrometer is electronically tuned to focus the molecular ion of interest using quadrupole. Triple-quadrupole mass spectrometer consists of three compartments; selected parent ions (177 for cotinine) pass through first compartment (q1) and reach to next compartment that is known as collision cell. In collision chamber (q2), it reacts with an inert gas to fragment into daughter ions (98, 80). The daughter ions are then directed to the third compartment (q3), which separates the daughter ions and focuses them on detector. Based on abundance of ions, concentration of analyte can be measured [Figure 1]. HPLC-MS/MS method parameters were optimized by monitoring cotinine and acetanilide ion pairs for quantification in multiple reaction monitoring (MRM) mode (cotinine 177 → 98, 80, acetanilide 136.1 → 94.1, 77.0). The retention time was 2.24 min for cotinine and for acetanilide 3.20 min. A total run time was 5 min. The MRM spectra were illustrated in Figure 1. For quantification, quantifier ion was 80 and qualifier was 98 depending on their abundance.
Figure 1.
Good chromatographic separation along with m/z was achieved on Polaris 3C-18 silica-based column for cotinine and acetanilide
Method validation
The current analytical procedure is rapid, accurate, and reproducible for simultaneous extraction and quantification of cotinine in saliva and urine samples. The method was validated as per the United Nations Office on Drugs and Crime guidelines.
Linearity
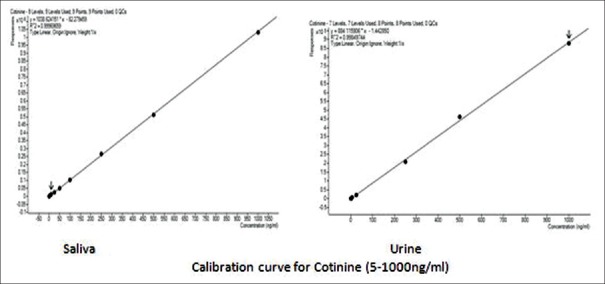
Calibration standards were prepared by spiking the intended matrix. Linear regression analysis calculated regression constants to document linearity, as shown in Figure 2. Calibration curve was in the range of 1.1 ng/ml–1000.0 ng/ml. Coefficient of correlation (r) was >0.9996 and 0.9998 for saliva and urine, respectively.
Figure 2.
Calibration curve showing good linear responses for cotinine in urine and saliva
Sensitivity
Limit of detection (LOD) was 0.50 ng/ml, while limit of quantification (LOQ) was 1.1 ng/ml.
Precision
Six replicates for each concentration (3.3, 500, and 750 ng/ml) were analyzed as per guidelines. Intra- and inter-day precision was observed within range [Table 1]. Matrix effect in urine and serum was investigated using six lots of blank matrix from individual donors was ≤10% while test carry over was negligible.
Table 1.
Accuracy, precision, and recovery data of method validation
| Matrix | LOD (ng/ml) | LOQ (ng/ml) | Recovery | Linearity range (ng/ml) | Correlation coefficient (r2) | RSD (%) | Ruggedness accuracy (%) | |
|---|---|---|---|---|---|---|---|---|
| Intraday within run | Inter day between run | |||||||
| Urine | 0.50 | 1.1 | 100.26 | 5.0-1000 ng/ml | >0.99 | 9.24 | 9.5 | 90 |
| Saliva | 0.50 | 1.1 | 99.59 | 5.0-1000 ng/ml | >0.99 | 9.24 | 9.5 | 90 |
LOD – Limit of detection, LOQ – Limit of quantification, RSD – Relative standard deviation
Recovery
Mean recovery was 100.26% in urine, while in saliva, cotinine recovery was 99.59%.
Robustness
Robustness of the method was evaluated by introducing small changes in matrix, mobile phase, and analyst. We report accuracy of 90% which is well within the range (80%–120%).
Sample stability
Ten individual samples of urine and saliva from tobacco users were prepared to access analyte stability, and we report 24–48 h sample stability at 4°C–8°C, while standard was stable up to 72 h at the same conditions.
Method applicability
Out of 98 smokers, samples selected for this study were males aged 21–60 years. Among passive smokers, seven males (17–55 years old) and eight females (25–60 years old) participated. Nonsmokers group had 24 males (25–71 years old) and 8 females (24–40 years old). The mean age among groups was comparable, and effect of age on cotinine excretion was not noticed.
We analyzed 56 urine and 49 saliva samples of smoked tobacco users, quality or quantity of saliva sometimes makes it unsuitable for analysis. Passive smokers counted less (n = 15) as tobacco users were not always accompanied by their family members and some users reported that they did smoke in the presence of family or friends.
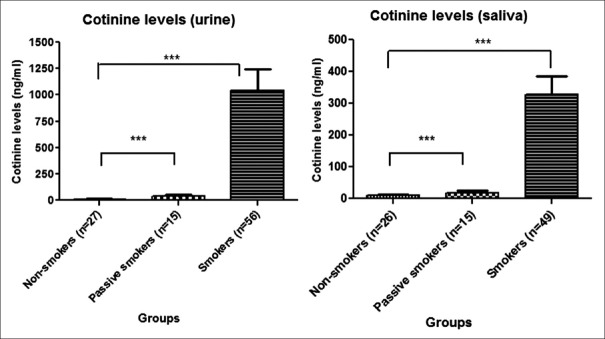
The mean urine cotinine levels for nonsmokers (n = 27) was 13.6 ng/ml, for passive smokers (n = 15) was 36.63 ng/ml, and for smokers (n = 56) was 1043.7 ng/ml. We report that the mean urine cotinine level of passive smokers was almost three times higher than that of nonsmokers. For smokers and nonsmokers, the cotinine ratio was 1:76.
A total of 90 saliva samples were analyzed for cotinine. The mean cotinine levels in saliva for nonsmokers (n = 26) was 9.53 ng/ml, for passive smokers (n = 15) was 18.31 ng/ml, and for smokers (n = 49) was 327.39 ng/ml. The mean saliva cotinine level of passive smokers was almost twice that of nonsmokers.
Urine cotinine levels among smokers, passive smokers, and nonsmokers were examined as shown in Figure 3. ANOVA results were significant, indicating that the mean levels of cotinine in smokers, passive smokers, and nonsmokers were not the same, i.e., it was significantly different (P < 0.001). A similar trend was observed upon examining the saliva cotinine levels from smokers, passive smokers, and nonsmokers. The ANOVA results of saliva cotinine showed that the means of the three groups differed significantly (P < 0.001).
Figure 3.
***P<0.001. Comparison of mean urine and saliva cotinine levels among nonsmokers, passive smokers, and smokers
Post hoc analysis was carried out after ANOVA for both the urine and saliva, and a significant difference was observed (P < 0.001). All possible three comparisons were significant (P < 0.001) for both urine and saliva, [Table 2] indicating that highest mean value for smokers (1043.60 and 327.39 ng/ml) followed by passive smoker (36.63 and 18.30 ng/ml) and the least value was among nonsmokers (13.60 and 9.52 ng/ml). We report that the significant difference was observed due to the lowest value of urine and saliva cotinine in nonsmokers. On comparing urine and saliva cotinine, we observed ratio of 4.98.
Table 2.
Comparison of cotinine (urine and saliva) among smokers, passive smokers, and nonsmokers
| n | Mean±SD | F | Significant | |
|---|---|---|---|---|
| Urine | ||||
| Nonsmokers | 27 | 13.60 (12.72) | 9.439 | 0.000 |
| Passive smokers | 15 | 36.63 (64.57) | ||
| Smokers | 56 | 1043.60 (1514.01) | ||
| Saliva | ||||
| Nonsmokers | 26 | 9.52 (11.86) | 11.839 | 0.000 |
| Passive smokers | 15 | 18.30 (28.88) | ||
| Smokers | 49 | 327.39 (411.06) |
SD – Standard deviation
The FTND score for smokers ranged from 4 to 10. ANOVA was carried on urine and saliva cotinine levels to assess their correlation with FTND score. We found that there was no correlation between both urine and saliva cotinine levels and self-report through FTND score.
DISCUSSION
Rapid method with simplified sample preparation for cotinine quantification (urine and saliva) is described in this study. Biological matrices such as urine and saliva were used as they are noninvasive, and the sample collection and preservation are easy. Various analytical techniques have been applied for cotinine determination in urine and saliva, for example, immune sorbent assay (ELISA, LOD = 1.3 ng/ml). Moreover, cross-reactivity is a major concern, especially with nicotine metabolites such as 3-hydroxycotinine.[14] GC either with flame ionization or mass detector can be applied for cotinine quantification, and higher LOQ is restricting factor for accessing ETS exposure.[11] For the current method (LCMS-MS), LOQ is 1.1 ng/ml and can easily distinguish among smokers, passive smokers, and nonsmokers. To evaluate such low concentrations by tandem mass spectrometer, minimization of ion suppression is required. ISs estimate loss of analyte in sample preparation. Structurally similar compounds may help to decrease ion suppressions.[12] Anticipating issues in purchasing d-3 cotinine import, we opted for acetanilide as IS.
The present method using LCMS-MS-ESI (positive mode) offers better sensitivity and specificity approach for assessment of cotinine in urine and saliva among active smokers, passive smokers, and nonsmokers. Despite variation in cotinine levels in three different groups and different biological matrices, the current technique can effectively measure cotinine levels. It has advantage of simplified sample preparation as well and good sensitivity and specificity with low LOQ like 1.1 ng/ml for saliva and urine which makes the current application useful for all the three groups.
Cotinine levels have earlier been used to validate the smoking status of an individual. The current work on cotinine can distinguish between nonsmokers, passive smokers, and smokers based on tobacco smoke exposure. Not much research has been focused on defining a cutoff value for passive smokers and nonsmokers in India and Southeast Asian region.
Behera reported urinary cotinine values having a mean of 2736.20 ± 983.29 ng/ml. The cotinine values for passive smokers were 285.75 ± 86.30 ng/ml which was significantly higher than that of nonsmokers (7.30 ± 2.47 ng/ml).[13] We observed that the smokers had a mean urinary cotinine level of 1043.69 ± 1514.01 ng/ml. The passive smokers had a mean of 36.63 ± 64.57 ng/ml and the nonsmokers had a mean of 13.6 ± 12.73 ng/ml.
Etzel reported that salivary cotinine was highest in smokers with an average of 318.0 ng/ml and significantly higher in passive smokers as compared to nonsmokers.[15] We observed that the smokers had a mean salivary cotinine level of 327.39 ± 411.06 ng/ml. The passive smokers had a mean value of 18.30 ± 28.88 ng/ml, and the nonsmokers had a mean value of 9.52 ± 11.86 ng/ml.
On comparing cotinine levels (urine: saliva), we found that the levels of urinary cotinine were 4.98 times higher than that of salivary cotinine levels. Other studies have shown that the ratio of urine cotinine to saliva cotinine falls within the range of 4–6.[16] Our value of 4.98 falls within the accepted range.
The most common tools to measure self-reported nicotine dependence are FTND and the biochemical assessment through the measurement of cotinine level in biological matrices. Cotinine, a major metabolite of nicotine, is considered the most appropriate parameter to evaluate tobacco exposure and smoking status due to its higher stability and half-life compared to nicotine. Both measurements such as FTND and cotinine were taken and studied. We found a significant difference (P < 0.001) for cotinine levels (urine and saliva) among smokers, passive smokers, and nonsmokers. We further investigated the correlation between self-report by FTND score with cotinine levels (urine and saliva) using Pearson correlation test. We did not find any correlation between FTND scores and cotinine levels. Self-report through FTND could be a good screening tool but could not detect the magnitude of smoking. For detection of magnitude of smoking, cotinine quantification is essential.
ROC curve analysis was used to identify the optimal cutoff for cotinine levels for distinguishing T-smokers from nonsmokers. Cotinine (urine) cutoff level for smokers from nonsmokers was 44.5 ng/mL, with 98.7% sensitivity, 96.1% specificity, 95.7% positive predictive value, and 94.1% negative predictive value.
Salivary cotinine concentrations >10 ng/ml were found in nonexposed nonsmokers, 10–100 ng/ml in passive smokers, and ≥100 ng/ml in smokers.[15] We report salivary cotinine concentrations >20.0 ng/ml among nonsmokers, 40–80 ng/ml for passive smokers, and ≥300 ng/ml for smokers.
CONCLUSION
We report an ESI-LC-MS/MS method to identify smoked tobacco exposure (active or passive) in saliva and urine. Results were in correlation with last use or exposure. LOD and LOQ were 0.50 and 1.1 ng/ml, respectively, and recovery was 99.56% showing the sensitivity of the method. We conclude that the current analytical procedure can identify and validate the smoking status and exposure in our population.
The current work establishes the potency of cotinine in the context of smoking and exposure. Further research relating to smoking and secondhand exposure with other health issues can be planned. We conclude that the current validated application was efficient enough to quantify the magnitude of exposure of smoked tobacco among users and passive smokers.
Comparison of the current technique was not made against an established gold technique along with small, and variable sample size was our limitation.
Limitation
Unequal sample size was the limitation of the study.
Financial support and sponsorship
This work was supported by the Center for Addiction Medicine and all experiments were carried out at Drug Toxicology Laboratory at National Institute of Mental Health and Neurosciences, Bangalore, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
This work was supported by the Center for Addiction Medicine and all experiments were carried out at Drug Toxicology Laboratory at National Institute of Mental Health and Neurosciences, Bangalore, India. Authors acknowledge contribution of Mr. Chowdaiah M and Shrvanthi Daphne Anand for technical help and standardization of experimental technique.
REFERENCES
- 1.WHO Report on the Global Tobacco Epidemic. Geneva: World Health Organization; 2011. World Health Organization. [Google Scholar]
- 2.Sharma MK, Sharma P. Need for validation of Fagerstrom test for nicotine dependence in Indian context: Implications for nicotine replacement therapy. Indian J Psychol Med. 2016;38:105–8. doi: 10.4103/0253-7176.178768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P, Murthy P, Shivhare P. Nicotine quantity and packaging disclosure in smoked and smokeless tobacco products in India. Indian J Pharmacol. 2015;47:440–3. doi: 10.4103/0253-7613.161273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miranda JJ, Kinra S, Casas JP, Davey Smith G, Ebrahim S. Non-communicable diseases in low- and middle-income countries: Context, determinants and health policy. Trop Med Int Health. 2008;13:1225–34. doi: 10.1111/j.1365-3156.2008.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montalto NJ, Wells WO. Validation of self-reported smoking status using saliva cotinine: A rapid semiquantitative dipstick method. Cancer Epidemiol Biomarkers Prev. 2007;16:1858–62. doi: 10.1158/1055-9965.EPI-07-0189. [DOI] [PubMed] [Google Scholar]
- 6.Slowik N, Ma S, He J, Lin YY, Soldin OP, Robbins RA, et al. The effect of second hand smoke exposure on markers of elastin degradation. Chest. 2011;140:946–53. doi: 10.1378/chest.10-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benowitz NL. Biomarkers of environmental tobacco smoke exposure. Environ Health Perspect. 1999;107(Suppl 2):349–55. doi: 10.1289/ehp.99107s2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 9.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56:483–93. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18:188–204. doi: 10.1093/oxfordjournals.epirev.a017925. [DOI] [PubMed] [Google Scholar]
- 11.Toraño JS, van Kan HJ. Simultaneous determination of the tobacco smoke uptake parameters nicotine, cotinine and thiocyanate in urine, saliva and hair, using gas chromatography-mass spectrometry for characterisation of smoking status of recently exposed subjects. Analyst. 2003;128:838–43. doi: 10.1039/b304051h. [DOI] [PubMed] [Google Scholar]
- 12.Apinan R, Choemung A, Na-Bangchang K. A sensitive HPLC-ESI-MS-MS method for the determination of cotinine in urine. J Chromatogr Sci. 2010;48:460–5. doi: 10.1093/chromsci/48.6.460. [DOI] [PubMed] [Google Scholar]
- 13.Behera D, Uppal R, Majumdar S. Urinary levels of nicotine & cotinine in tobacco users. Indian J Med Res. 2003;118:129–33. [PubMed] [Google Scholar]
- 14.Matsumoto A, Ino T, Ohta M, Otani T, Hanada S, Sakuraoka A, et al. Enzyme-linked immunosorbent assay of nicotine metabolites. Environ Health Prev Med. 2010;15:211–6. doi: 10.1007/s12199-009-0129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etzel RA. A review of the use of saliva cotinine as a marker of tobacco smoke exposure. Prev Med. 1990;19:190–7. doi: 10.1016/0091-7435(90)90020-k. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P, 3rd, et al. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11:954–60. doi: 10.1093/ntr/ntp092. [DOI] [PMC free article] [PubMed] [Google Scholar]