Abstract
HIV is a chronic well manageable disease. Highly active antiretroviral therapy improves the quality of life of people living with HIV, but the treatment has to be continued lifelong, as the total cure has not been established. Cost of treatment, drug toxicities, interaction with other drugs and persistence of inflammation and acceleration of the aging process, all put together, warrant an urgent need for a total cure. Even though one case had been proved to be cured, still a practical cure is far beyond the reach. Numerous approaches and strategies had been put forth to achieve a cure; still they are to be proved with human studies. This article reviews the major approaches, recent advances in the venture of HIV cure, and the safety concerns involved.
Key words: Early treatment, genetic manipulation, immunology, kill and kick strategy, sterilizing cure, strategies for cure
INTRODUCTION
As of now, HIV management needs life long treatment. A total cure is the need of the hour to restore a normal life expectancy and the immune function, to cut short the cost effect for the individual, family and to his country, to reduce morbidity, mortality, to avoid exposing to drug toxicity, to avoid subsequent loss of functions of vital organs such as kidney, liver, heart, bone marrow, and nervous system, to avoid unnecessary drug interaction, and to avoid acceleration of aging.[1]
Since the advent of highly active antiretroviral therapy from 1996 onward there came a breakthrough in the management of HIV disease to the extent of a “functional” cure. However, at any time, these individuals would be likely to go for a viremia when these individuals either stop the treatment or attain resistance to antiretroviral therapy (ART).[2,3,4,5,6] The main barrier to a sterilizing cure is the presence of a “latent reservoir;” a population of HIV-infected cells those persist for the lifetime of the individual despite ART- and the HIV-specific immune response.[3,4] Eliminating the viral reservoir, make the latent virus susceptible for ART or make the reservoir inactive forever would prevent the virus from reestablishing the infection after the discontinuation of the treatment. Hence, the identification of various sources of viral reservoirs such as latent CD4 cells, central nervous system – microglial cells, macrophages, monocytes - (peripheral blood mononuclear cells), reproductive system, lymph nodes, spleen, and gut tissues get the prime importance in the research.[5]
Allogeneic hematopoietic stem cell transplantation from a donor homozygous for HIV-resistant CCR5Δ32 mutation,[7] alteration of the host genome using genetic engineering techniques, Kick and kill (also known as “shock and kill”), altering the immune system for better clearance of the HIV-infected targets, “Hit early and Hit hard” (early treatment) are some of the strategies to be discussed.[1]
GENETIC STRATEGIES
Co-Receptor CCR5 (or CRCX4) is essential for the entry of HIV into the CD4-bearing cell to get established. About 1% of the Caucasian population[8] and 1.44% of the Western African population[9] have the mutation, known as Delta 32, inherited from both parents which prevents the protein CCR5 from appearing on the cell surface. Since HIV enters the cell through CCR5 molecules, when they are absent HIV cannot penetrate. They are immune to HIV infection. The prevalence of Delta 32 gene defect (absence of CCR5) in other communities has to be estimated in the future.[6] Although a previous study has shown a 2% heterozygous CCR5Δ32 deletion in 300 studied women, these 6 women belonged to the Muslim community, as was reported among the Muslims of northern India.[10]
Stem cell transplantation
Stem cells are undifferentiated biological cells that can differentiate into specialized cells and can divide (through mitosis) to produce more stem cells. Two broad types of stem cells namely embryonic stem cells and adult stem cells. Stem cells can also be taken from umbilical cord blood just after birth. Stem cells and progenitor cells act as a repair system for the body, such as blood, skin, or intestinal tissues. Adult stem cells are frequently used in medical therapies, for example in bone marrow transplantation. Bone marrow is a rich source of adult stem cells.[7] Long-term control of HIV is possible with CCR5 Delta32/Delta32 Stem-Cell Transplantation [Figure 1].
Figure 1.
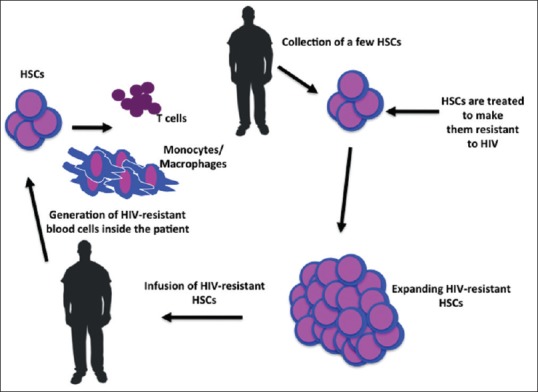
Stem Cell Transplantation
With this theory, one patient had been cured completely.[11]
Berlin patient
Timothy Ray Brown, an HIV-positive American living in Germany, also had leukemia, received a transplant of stem cells from a donor carrying a rare, inherited gene mutation (CCR5Δ32) that seems to make carriers virtually immune to HIV infection during February 2007. Timothy had stem cell transplant twice as there was not adequate expected response through the first one. The transplant appeared to wipe out both diseases, giving hope to doctors. However, this transplant process is not only expensive, it is incredibly painful and complicated, and requires the patient to start a whole new regimen of drugs. Only after a search of 70 donors only, Timothy Brown got his transplant. The patient remains off ART, with a normal CD4 cell count. The HIV RNA levels were lower than those typically seen in virologically suppressed patients on ART. HIV DNA was detected in a rectal biopsy sample by one laboratory. No HIV has been detected in CSF or peripheral blood mononuclear cells. No replication-competent HIV has been isolated.[7] However, the two “Boston patients”—nicknamed for the Massachusetts city where they were treated and received bone-marrow transplants with cells that were not resistant to HIV.[7] Researchers reported that the virus has rebounded in both of the Boston patients.[12]
Other genetic modulations
With the help of zinc finger nucleases, DNA editing enzymes, eliminating the CCR5 expression over CD4 cells seems to be safe and have modified cells have the half-life of 48 weeks, but was expensive and difficult to scale upward.[13]
Using the CRISPR/Cas gene-editing technology, the Cowan and Rossi teams knocked the CCR5 receptor out of blood stem cells that they showed could give rise to differentiated blood cells that did not have CCR5. In theory, such gene-edited stem cells could be introduced into HIV patients through bone marrow transplantation, the procedure used to transplant blood stem cells into leukemia patients, to give rise to HIV-resistant immune systems.[14] Researchers engineered a molecule known as a chimeric antigen receptor (CAR) and inserted a gene for that molecule into blood-forming stem cells, which they transplanted into mice genetically engineered to have human immune systems. It significantly reduced HIV levels in mice with a genetic therapy that induces immune cells to fight better against the virus. The CAR is a two-part receptor that recognizes an antigen (such as HIV) and in this case instructs immune cells to locate and kill HIV-infected cells. The transplant of the CAR-carrying blood stem cells gave rise to functional immune cells that could kill HIV in the mice. Consequently, the mice experienced an 80%–95% drop in viral load. The researchers concluded that such genetic therapy might be feasible in HIV-positive humans.[15]
KICK AND KILL (SHOCK AND KILL) METHOD
At present, the reason for which we are not able to achieve a complete cure with the help of ART, in spite our achievement of undetectable viral load, is said to be the presence of a dormant virus or HIV latency. In shock and kill, immune stimulants shock the latent virus from hidden reservoirs and then attempt to kill reactivated HIV.
Now an enzyme had been identified which is called as histone deacetylase (HDAC) which is responsible to keep up latency. Several companies are looking into HDAC-inhibitors. Some in vitro H-DAC studies seemed to be promising but yet to be confirmed by clinical studies. Flushing these latent CD4 HIV-infected cells with drugs such as Vorinostat and Panobinostat (HDAC inhibitors) into the circulation, make them susceptible for ART after expressed out of these reservoirs.[16]
Histone deacetylase inhibitors have a broad spectrum of epigenetic activities. Vorinostat is marketed under the name Zolinza for the treatment of cutaneous T-cell lymphoma (CTCL).[17] This was approved by the U. S. Food and Drug Administration for the treatment of CTCL on October 6, 2006. They flush the virus from the reservoir to the circulation. The dose is 400 mg statum.[18]
GS-9620 – TLR7 agonist[19] and Protein kinase C agonist bryostatin-1[20] are some other drugs on the pipeline. Studies with primates were successful.
Romidepsin (Celgene), another HDAC inhibitor drug, as intravenous injection 5 mg/m2 once weekly for 3 weeks while maintaining the ART, safely induced HIV-1 transcription resulting in plasma HIV-1 RNA that was readily detected with standard commercial assays demonstrating that significant reversal of HIV-1 latency in vivo is possible without blunting T-cell-mediated immune responses. These findings have major implications for future trials aiming to eradicate the HIV-1 reservoir.[21] Keeping the latency to remain inactive forever:
A natural compound called as Cortistatin A has been found to significantly reduce the rate of reactivation of immune cells latently infected with HIV. Cortistatin A was isolated from a marine sponge known as Corticium simplex. It has been shown to inhibit Tat, a viral protein that is instrumental in prompting the virus to replicate. This study showed that the compound reduced viral reactivation by an average of 92.3%.[22]
IMMUNOLOGICAL APPROACH
Administration of a Rhesus CMV vaccine vector containing SIV Gag, Rev/Tat/Nef, Env and Pol inserts led to viral control of pathogenic SIV infection in a subset of Primates. After initial viremia, viral loads and interestingly SIV DNA became undetectable after 30 weeks of infection. Data also confirm that levels of replication-competent virus in the reservoir decreased over time. The results indicate vaccine contributed to viral clearance.[23]
SAV001-H is the first preventive HIV vaccine using a killed or “dead version” of HIV-1 virus. Sumagen's SAV 001 is unique and different from other vaccine candidates, as it uses genetically re-engineered whole virus genome and thereby eliminates its pathogenicity and then inactivates its virulence through chemical treatment and irradiation methods, finally arriving at the first “whole-killed virus” based HIV vaccine [Figure 2].
Figure 2.
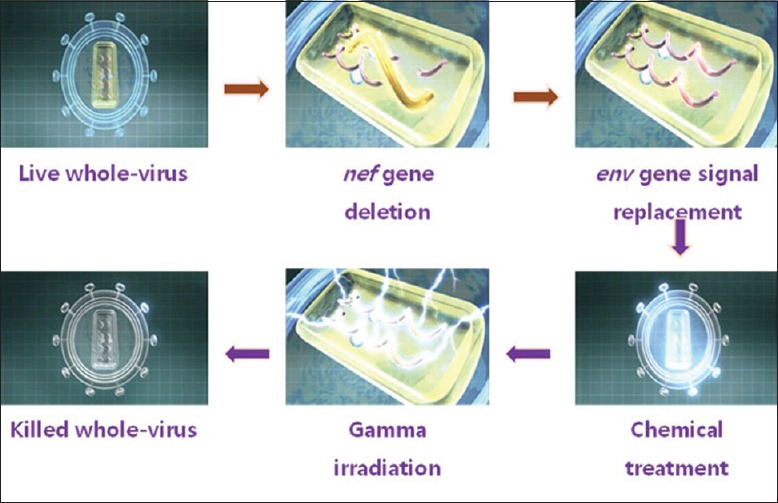
Principles of Vaccination
Researchers observed no serious adverse events and also found a surprising boost in antibody production. The antibody against p24 capsid antigen increased as much as 64-fold in some vaccines while the antibody against gp120 surface antigen increased up to eight-fold. p24 is a structural protein that makes up most of the HIV viral core also known as the “capsid.” High levels of p24 are present in the blood serum of newly infected individuals during the short period between infection and seroconversion, making p24 antigen assays useful in diagnosing primary HIV infection. A glycoprotein, gp120, is necessary for attachment to cell surface receptors and also allows for the HIV to enter cells. The increased antibody titers were maintained during the 52-week study period.
Another one promising vaccine is Kang's vaccine. Here also, the use of a killed-whole HIV-1, which is similar to the vaccines developed for polio, influenza, and rabies. HIV-1 is also genetically engineered; this raises its safety profile and the possibility of it being produced in large quantities.[24]
The scientists tested an immunogen called eOD-GT8 60mer, a protein nanoparticle designed to mimic a critical part of the HIV envelope protein and to bind and activate B cells to produce antibodies needed to fight HIV. The eOD-GT8 60mer was developed in the Schief laboratory and tested in mouse models engineered by the Nemazee laboratory to produce human-like antibodies. The researchers showed that immunization with eOD-GT8 60mer produced antibody “precursors” with some of the traits necessary to recognize and block HIV infection, suggesting that eOD-GT8 60mer could be a good first step in a series of immunizations against HIV. “The vaccine appears to work well in mouse model to “prime” the antibody response.” “The immunogen proved capable of launching the mouse immune systems in the right direction.” The researchers are now investigating other immunogens that could work in conjunction with eOD-GT8 60mer.[25]
EARLY TREATMENT
“Hit Early and Hit and Hard” – That is, treatment should be started as early as possible during Primary HIV Infection (Acute infection). That is to hit hard with ART before the latent reservoir formed inside the body.
Mississipi baby
Mother of the baby was not identified as HIV positive until labor started. She was not on ART. After confirmation of the presence of HIV-DNA particles with the baby, ART started within 30 h after birth. By 18 months baby missed from follow-up. ART stopped for more than a year and once again the baby came to follow-up after 2 years. Surprisingly, viral load was undetectable with the baby. ART withheld and the baby was under continuous vigilance. However, viral relapse happened after 3 years.[26]
CONCLUSION
There are many other trials that are also going on throughout the world. Enumerating all is beyond the purview of this article. Newer reports coming daily have encouraging outcome. Some of these trials are nearing the winning post. We could expect a sterilizing cure for HIV disease within another 5–10 years.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Pace M, Frater J. A cure for HIV: Is it in sight? Expert Rev Anti Infect Ther. 2014;12:783–91. doi: 10.1586/14787210.2014.910112. [DOI] [PubMed] [Google Scholar]
- 2.Chun TW, Nickle DC, Justement JS, Large D, Semerjian A, Curlin ME, et al. HIV-infected individuals receiving effective antiviral therapy for extended periods of time continually replenish their viral reservoir. J Clin Invest. 2005;115:3250–5. doi: 10.1172/JCI26197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF, et al. In vivo fate of HIV-1-infected T cells: Quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–90. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- 4.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 5.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chun TW, Justement JS, Murray D, Hallahan CW, Maenza J, Collier AC, et al. Rebound of plasma viremia following cessation of antiretroviral therapy despite profoundly low levels of HIV reservoir: Implications for eradication. AIDS. 2010;24:2803–8. doi: 10.1097/QAD.0b013e328340a239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 8.Kaslow RA, McNicholl JM. Genetic determinants of HIV-1 infection and its manifestations. Proc Assoc Am Physicians. 1999;111:299–307. doi: 10.1046/j.1525-1381.1999.99238.x. [DOI] [PubMed] [Google Scholar]
- 9.Kokkotou E, Philippon V, Gueye Ndiaye A, Mboup S, Wang WK, Essex M, et al. Role of the CCR5 delta 32 allele in resistence to HIV 1 infection in West Africa. J Hum Virol. 1996;1(7):469–74. [PubMed] [Google Scholar]
- 10.Chaudhari DV, Kerkar SC, Chavan V, Mehta PR, Mania-Pramanik J. Chemokine receptors CCR5 and CCR2 genes in HIV positive, HIV exposed seronegative and in HIV unexposed individuals: A study from Mumbai. Indian J Dermatol Venereol Leprol. 2015;81:548. doi: 10.4103/0378-6323.158642. [DOI] [PubMed] [Google Scholar]
- 11. [Last accessed on 2019 Feb 11]. Available from: https://en.wikipedia.org/wiki/Hematopoietic_stem_cell_transplantation .
- 12.Hayden EC. Stem-cell transplants may purge HIV. Nature. 2013 13297 ISSN 0028-0836 EISSN 1476-4687. [Google Scholar]
- 13.Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in anologus CD4 T cells of persons infected with HIV. N Eng J Med. 2014;28:839–47. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ebina H, Miswa N, Kanemura Y, Koyanagi Y. Harnessing the CRISPR/Cas9 system to disrupt latent HIV-1 provirus. Sci Rep. 2013;3:2510. doi: 10.1038/srep02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Patel B, Ghanem MH, Bundoc V, Zheng Z, Morgan RA, et al. Novel CD4-based bispecific chimeric antigen receptor designed for enhanced anti-HIV potency and absence of HIV entry receptor activity. J Virol. 2015;89:6685–94. doi: 10.1128/JVI.00474-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirakawa K, Chavez L, Hakre S, Calvanese V, Verdin E. Reactivation of latent HIV by histone deacetylase inhibitors. Trends Microbiol. 2013;21:277–85. doi: 10.1016/j.tim.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. [Last accessed on 2019 Feb 11]. Available from: https://www.en.wikipedia.org/wiki/Vorinostat .
- 18.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. [Last accessed on 2019 Feb 11];Nature. 2012 487:482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sloan D, Irrinki A, Tsai A, Kaur J, Lalezari J, Murry J, et al. TLR7 Agonist GS-9620 Activates HIV-1 in PBMCs from HIV-Infected Patients on cART. [Last accessed on 2019 Feb 11]. Available from: http://www.croiconference.org/sites/default/files/posters-2015/417.pdf .
- 20.Mehla R, Bivalkar-Mehla S, Zhang R, Handy I, Albrecht H, Giri S, et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. [Last accessed on 2019 Feb 11];PLoS One. 2010 5:e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Søgaard OS, Graversen ME, Leth S, Olesen R, Brinkmann CR, Nissen SK, et al. The depsipeptide romidepsin reverses HIV-1 latency in vivo. PLoS Pathog. 2015;11:e1005142. doi: 10.1371/journal.ppat.1005142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mediouni S, Jablonski J, Paris JJ, Clementz MA, Thenin-Houssier S, McLaughlin JP, et al. Didehydro-cortistatin A inhibits HIV-1 tat mediated neuroinflammation and prevents potentiation of cocaine reward in tat transgenic mice. Curr HIV Res. 2015;13:64–79. doi: 10.2174/1570162x13666150121111548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen SG, Piatak M, Jr, Ventura AB, Hughes CM, Gilbride RM, Ford JC, et al. Immune clearance of highly pathogenic SIV infection. Nature. 2013;502:100–4. doi: 10.1038/nature12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. [Last accessed on 2019 Feb 11]. Available from: http://www.sumagen.com/english/business/aids_vaccine.htm .
- 25.Immunogen eOD-GT8 60mer can Stimulate Immune System to Block HIV/AIDS Infection in Mice: Study. [Last accessed on 2019 Feb 11]. Available from: http://www.eurekalert.org/pub_releases/2015-06/sri-ssa061715.php .
- 26. [Last accessed on 2019 Feb 11]. Available from: https://en.wikipedia.org/wiki/Mississippi_baby .


