Abstract
Introduction:
Chlamydia trachomatis (CT) and human papillomavirus (HPV) are considered to be major sexually transmitted infections (STIs) and likely health hazard among women. In addition, HPV and CT are considered as potential cofactors in the development of cervical intraepithelial neoplasia (CIN).
Objectives:
The main objective of this study was to investigate the association of HPV and CT infection with the presence of abnormal cervical cytology.
Materials and Methods:
A cross-sectional study was carried out on 90 women with complaints of vaginal discharge attending STI clinic in a tertiary care hospital in New Delhi. Papanicolaou staining and polymerase chain reaction were done for the detection of HPV and CT. Statistical analyses were performed for comparison.
Results:
Abnormal cervical cytology was observed in 42.2% of the study participants (41.1% low-grade squamous intraepithelial neoplasia and 1.1% high-grade intraepithelial neoplasia). HPV and CT were positive in 21.1% and 31.5% of participants with abnormal cervical cytology, respectively. Coinfection with HPV and CT was observed in 84.2% of participants with cervical atypia. Further, genital herpes was diagnosed in 18.9% of the studied population and a significant association was observed between genital herpetic ulcers and abnormal cervical cytology (P = 0.016).
Conclusion:
CT was found to be a significant risk factor for cervical cytological abnormalities in our study. HPV and CT coinfection were also associated with a higher prevalence of cervical atypia. As chlamydial infection is easily treatable, we recommend screening and treatment of all women of the reproductive age group for Chlamydia to decrease the risk of cervical dysplasia.
Limitation:
This is a single-center STI clinic-based study. Multicenter and community-based studies with a larger cohort will confirm the association.
Key words: Cervical cytological abnormality, Chlamydia trachomatis, genital herpes, human papillomavirus
INTRODUCTION
Cervical cancer is the second most common malignancy worldwide.[1] As per GLOBOCON 2008 estimates, cervical cancers account for 9% of the total new cancer cases, with India contributing 20%–30% of the burden.[1,2] Further, India accounts for 77,100 of the total cervical cancer-related deaths.[2] Persistent human papillomavirus (HPV) infections have been incriminated in virtually all of the 500,000 cases of invasive cervical cancers (ICC) that occur per year worldwide.[3] Due to its central role in the etiology of cases of cervical cancer, HPV is correctly called a necessary, but not sufficient trigger of cervical cancer. Additional risk factors include HIV, immunosuppression, multiple sexual partners, and age of first intercourse, cigarette smoking, and the presence of other sexually transmitted infections (STIs) such as Chlamydia trachomatis (CT).[4]
CT is the most common bacterial sexually transmitted disease worldwide.[5] Various studies have reported a 5% prevalence of Chlamydia in the general population and 20%–40% in patients with STI.[6] HPV and CT play a central role in the etiology of cervical intraepithelial neoplasia (CIN) and subsequent development of cervical cancer. Chlamydia can lead to epithelial disruptions and microabrasions, thus increasing the susceptibility to HPV infection.[7] The prevalence of HPV/CT coinfection has been reported to be around 0.7%–23.5% in women with CIN and cervical cancer.[8] However, the association between HPV and CT infection still remains controversial as other studies failed to find an association between these infections and severity of cervical neoplasia.[7] Chlamydial infection is easily treatable, and therefore, may be an important therapeutic target for the prevention of cervical neoplasia. The aim of the present study was to ascertain the incidence of chlamydial infections in sexually active women, and the association of Chlamydia and HPV infection with cervical cytological abnormalities in women attending a STI clinic.
MATERIALS AND METHODS
Study subjects
A cross-sectional study applied to 90 women fulfilling the inclusion criteria was carried out at a STI clinic of a tertiary care hospital in North India, following approval from the Institutional Ethical Committee. The inclusion criteria included consecutive, sexually active women aged between 18 and 45 years presenting with complaints of vaginal discharge who consented for undergoing speculum examination and planned investigations. The exclusion criteria included pregnancy, women who had recently (within last 15 days) received antibiotics or were using intravaginal medications, menstruating or with the presence of active vaginal bleeding, visible cervical growth or ulceration, and history of prior treatment on the cervix.
A written and informed consent was obtained and each participant was asked a brief questionnaire regarding their personal details including socioeconomic status, medical history, sexual practices, smoking, and HIV status followed by a clinical examination as per the predesigned case record form. Laboratory investigations such as hemogram and Venereal Disease Research Laboratory were carried out. HIV sample was taken after informed and written consent with counseling done according to the National AIDS Control Organization guidelines.
Cervical samples were collected for conventional Pap smear. The Ayre's spatula and cytobrush used for cervical cytology were then immediately transferred to 15-ml plastic vials containing 5 ml of sterile phosphate-buffered saline and stored at − 70°C in a deep freezer until isolation of DNA for polymerase chain reaction (PCR) assay for HPV and Chlamydia.
A pathologist experienced in reading cervical cytology specimens and blinded from the clinical details participated in the study and reviewed the cytosmears. The diagnosis for cervical smears was classified according to Bethesda classification. PCR was done for detection of HPV and CT. Genomic DNA of the cervical scrapes from patients was extracted by a standard method described by Sambrook and Russell.[9] PCR assays utilized consensus sequence primers directed to a conserved L1 gene, and hence able to detect all mucosal HPV types. HPV typing was done using type-specific primers, that is, HPV-6, 11, 16, and 18. The PCR reaction for HPV detection consisted of an initial melting step of 95°C for 5 min; 35 cycles at 95°C for 30 s, at 55°C for 35 s and at 72°C for 30 s, and a final elongation step of 72°C for 10 min. PCR product was then visualized on ethidium bromide stained on 2% agarose gel.
Statistical analyses
For analysis, the study group was divided into two groups:
Group 1: Absence of cervical cytological atypia on Pap smear
Group 2: Cervical cytological atypia present on Pap smear.
All statistical analyses were carried out using SPSS software version 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp). Cytology was reported according to the Bethesda system. Association of outcome and exposure was tested with Fischer exact test and exposure to more than two categories with the Chi-square test. The significance of statistical Chi-square/Fisher's exact test, two-tailed P < 0.05 was considered as statistically significant.
RESULTS
Population characteristics
The mean age of the studied population was 28.8 ± 5.6 (20–43) years. Most of the patients belonged to the 18–29 years of age group (57.8%). Cervical cytological atypia was found to be the highest in the age group of 18–29 years (63.1%). The education level in the studied population was low with 53.3% of patients being uneducated. The mean income was INR 7880 ± 9710. About half of the patients with cervical cytological atypia (47.3%) belonged to the low-income group [Table 1].
Table 1.
Risk factors for cervical cytological abnormality in the studied population (number of participants with that variable present)
| Risk factors | Normal cytology (n=52) | Cervical cytological atypia (n=38) | P |
|---|---|---|---|
| Uneducated | 28 | 20 | 0.554 |
| Low SE status | 18 | 12 | 0.58 |
| Mean age at first sexual intercourse | 18.4 | 16.7 | 0.003 |
| Mean number of sexual exposures in the last 6 months | 16.3 | 27.5 | <0.0001 |
| Mean number of sexual exposures in the last 1 year | 28.8 | 54.4 | <0.0001 |
| >1 sexual partner | 4 | 10 | 0.047 |
| Contraceptive used | 27 | 16 | 0.085 |
| HIV positive | 2 | 1 | 1.000 |
| Herpetic ulcers | 5 | 12 | 0.016 |
SE: Socioeconomic
Risk factors associated with cervical cytological atypia
The mean age of first sexual intercourse of the study group was 17.7 ± 2.7 years. In the present study, early age of first sexual intercourse was found to be a significant risk factor for cervical cytological abnormalities (P = 0.003). The overall mean number of sexual intercourse in the past 6 months was 21.3 ± 14.4. The mean number of sexual intercourse in the past 6 months was found to be higher in patients with abnormal cytology and the association was found to be highly statistically significant (P = 0.0001). Sixteen patients (17.8%) reported contact with more than one sexual partner (range 2–4). However, none reported contact with more than one partner in the previous 6 months. A statistically significantly higher number of patients with abnormal cytology were found to have more than one sexual partner (P = 0.047). The number of nullipara women in the cohort with abnormal cytology (5.3%) was comparable with the study participants with normal cervical cytology (11.5; P = 0.307) and no association with parity was evident. Among the demographic factors, usage of contraceptive was reported by only 46.7% of the study cohort such that the contraceptive usage was higher in patients without unremarkable cytology versus those with abnormal cytology [P = 0.859; Table 1]. Condoms were the most common contraceptives used (76.2%) followed by intrauterine contraceptive device (12.0%) and oral contraceptives (9.5%). History of smoking was elicited in only four patients. None of the patients reported any history of chewing tobacco, alcohol, narcotics, or intravenous drug abuse. Overall only 3 (3.33%) study participants were found to be HIV positive.
Genital complaints
Genital discharge being an inclusion criterion was present in all the patients. Genital itching was the next most common genital complaint (65.6%), followed by abdominal/pelvic pain (20.0%), backache (14.4%), and burning micturition (2.2%). There was no significant difference in the frequency of these complaints between the two groups. None of the patients had complaints of postcoital bleeding or menstrual irregularities.
Prevalence of sexually transmitted infections
Genital herpes was diagnosed in 18.9% of the studied population. A significant association was observed between the presence of genital herpetic ulcers and abnormal cytology in our study (P = 0.016). Genital warts were present in 10 (11.1%) patients and a higher, though insignificant prevalence was seen in patients with abnormal cervical cytology (18.5%) as compared to those without atypia (5.7%; P = 0.085). Genital molluscum contagiosum was diagnosed in 4.4% of patients.
Cytology
Cervical abnormalities were detected in 44.4% of study participants with a reactive nuclear enlargement in 2.2% smears and dysplasia in 42.2% of study participants. Among the total study participants, 41.1% presented with low-grade squamous intraepithelial lesion neoplasia [LSIL; Figure 1] and 1.1% with high-grade squamous intraepithelial lesion neoplasia [HSIL; Figure 2].
Figure 1.
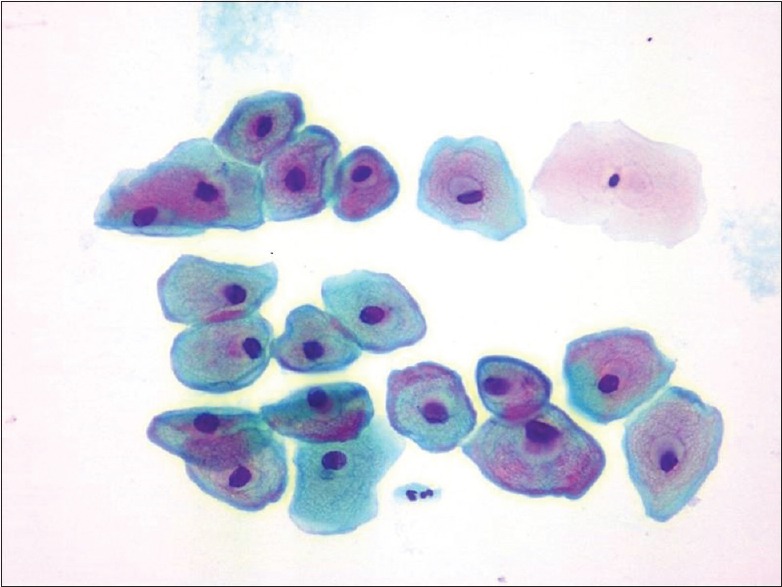
Cervical Pap staining (×40); cell showing low-grade squamous intraepithelial lesion
Figure 2.
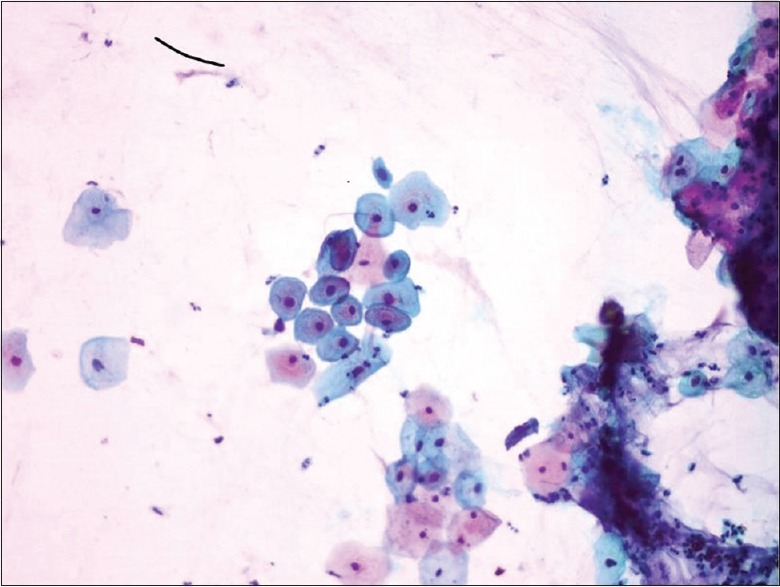
Cervical Pap staining (×40); cell showing high-grade squamous intraepithelial lesion
Abnormal flora was seen in 54 (60.0%) patients, columnar cells in 22 (24.4%) patients and inflammatory cells in 20 (22.2%) cytosmears, and multinucleate giant cells were detected in two smears from patients with a diagnosis of genital herpes.
Prevalence of HPV and CT in patients with cervical abnormal cytology: A total of 8 (8.9%) patients were found to be positive for cervical HPV and all were detected to have abnormal cytology on cervical cytology examination. Hence, a highly significant association was observed between cytological abnormality and HPV infection (P = 0.001). Moreover, the prevalence of HPV [Figure 3] in LSIL and HSIL was 18.9% and 100%, respectively. HPV16 [87.5%; Figure 4] was the most common type detected followed by HPV18 [12.5%; Figure 5].
Figure 3.
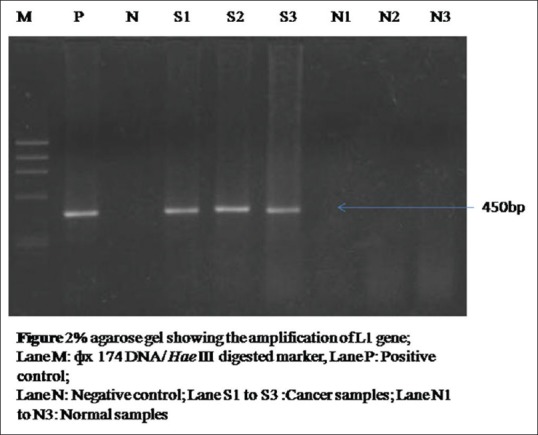
Two percent agarose gel showing the amplification of human papillomavirus L1 gene of 450 bp; Lane M: ϕ× 174 DNA/Hae III digested marker, Lane P: Positive control, Lane N: Negative control; Lane S1 to S3: Cancer samples; Lane N1 to N3: Normal samples
Figure 4.
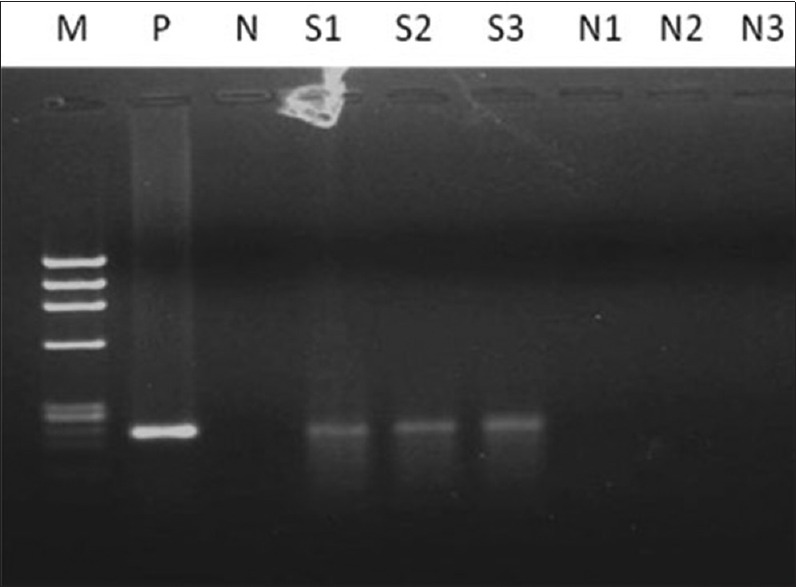
Two percent agarose gel showing the amplification of human papillomavirus 16 of 217 bp; Lane M: Ф×174 DNA/Hae III digested marker, Lane P: Positive control; Lane N: Negative control; Lane S1 to S3: Cancer samples; Lane N1 to N3: Normal samples
Figure 5.
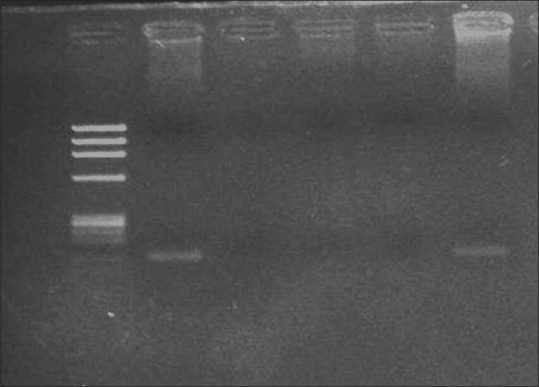
Two percent agarose gel showing the amplification of human papillomavirus-18 of 100 bp; Lane M: Ф×174 DNA/Hae III digested marker, Lane P: Positive control; Lane N: Negative control; Lane N1 control, S1 cancer sample
CT infection was detected in 16.7% smears by PCR method. [Figure 6]. Chlamydia isolation was significantly more in patients with abnormal cytology (31.5%) as compared to those without unremarkable cytology (5.8%; P = 0.004). Chlamydia was found to be positive in 27.7% of patients with LSIL and in the one patient with HSIL.
Figure 6.
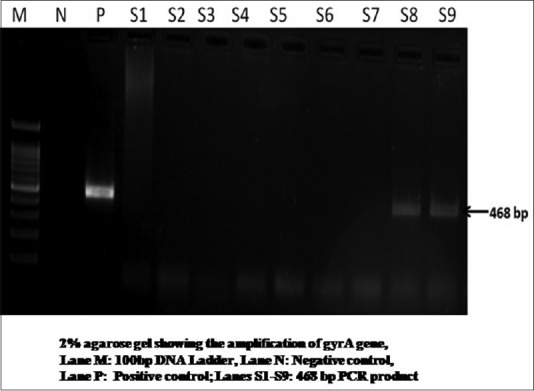
Two percent agarose gel showing the amplification of Chlamydia gyrA gene 468 bp, Lane M: 100 bp DNA Ladder, Lane N: Negative control, Lane P: Positive control; Lanes S1-S9: 468 bp polymerase chain reaction product
HPV and chlamydial coinfection were detected in 6.7% of our study population and all these patients were found to have abnormal cytology. A highly significant association was found between HPV/chlamydial coinfection with cervical abnormal cytology [P = 0.001, Table 2]. The coinfection was detected in the one patient with HSIL and in five patients with LSIL [Table 3].
Table 2.
Association of cervical cytological abnormality with human papillomavirus and Chlamydia trachomatis population (number of participants with that variable present)
| Normal cytology (n=52) | Cervical cytological abnormality (n=38) | P | |
|---|---|---|---|
| HPV positive | 0 | 8 | <0.0001 |
| CT positive | 3 | 12 | 0.004 |
| HPV and CT coinfection | 0 | 6 | <0.0001 |
CT: Chlamydia trachomatis; HPV: Human papillomavirus
Table 3.
Correlation of human papillomavirus and Chlamydia with grades of cervical atypia population (number of participants with that variable present)
| Infective agent detected | Atypia-absent (%) | Atypia-LSIL (n=37) (%) | Atypia-HSIL (n=1) (%) |
|---|---|---|---|
| HPV positive | 0 (0) | 7 (18.9) | 1 (100) |
| Chlamydia positive | 3 (6) | 11 (27.7) | 1 (100) |
HSIL: High-grade squamous intraepithelial lesion; LSIL: Low-grade squamous intraepithelial lesion; HPV: Human papillomavirus
DISCUSSION
Cervical cancer often arises from precancerous dysplastic lesions – CIN. The incidence of both cervical cancer and its precursor lesions has increased in the past decades.[2,10,11] HPV is a prerequisite for cervical cancer, but other cofactors have been implicated and these act by influencing HPV acquisition, its persistence, and development of neoplasia. STI such as herpes simplex-2 may act as one of the cofactors.[12,13,14] In our study, genital ulcers were present in 18.9% patients and all were diagnosed to be due to herpetic virus. A significantly higher number of patients with cervical abnormal cytology (31.5%) had the presence of genital herpes ulcers as compared to those with normal cytology (9.6%, P = 0.016). The increased cancer risk associated with these infections may be attributed to the inflammatory responses that are associated with the generation of free radicals and genetic instability. Castellsague et al. in their study found a two fold increased risk of cervical cancer in patients with HSV-2 infection.[13] Some other studies[12,13,14] have also reported HSV-2 as a cofactor, but the accumulated evidence is much less consistent and has not been confirmed in longitudinal studies. To confirm our findings, we recommend further studies on larger cohorts including patients with known abnormal cytology, with a focus on the role of genital herpes as a cofactor for cervical dysplasia.
The overall prevalence of HPV in our study was found to be 8.9%. Contrary to our findings, Bhatla et al.[15] in their study on 618 women attending their gynecology OPD reported a higher overall prevalence of high-risk HPV (18%). The prevalence of HPV in our patients with cervical abnormal cytology (21.1%) was found to be highly significant when compared to those without dysplasia (0%; P = 0.005). Basu et al.[16] and Yang et al.[17] reported a prevalence of 5.8% and 6.93%, respectively in their studies on healthy women of reproductive age group. In our patients, the prevalence of HPV in LSIL and HSIL was 18.9% and 100%, respectively. The prevalence of HPV in smears diagnosed as atypical squamous cells of undetermined significance (ASCUS), LSIL, and HSIL was reported to be 47.2%, 25%, and 83.3%, respectively by Bhatla et al. Ogembo et al.[10] in their meta-analysis reported HPV prevalence to be 16.4% in ASCUS, 24.5% in LSIL, 45.1% in HSIL, and 67.7% in ICC. Therefore, from data available so far, including findings of our study, we can conclude that HPV is a major risk factor for cervical dysplasia. Furthermore, the strength of this association between HPV and cervical dysplasia is found to be more evident in higher grades of dysplasia. The most common HPV found in our study was HPV16 (87.5%) followed by HPV18 (12.5%). This was similar to the results reported by Yang et al.[17] and Smith et al.,[18] where HPV16 was the most common HPV type detected.
Chlamydia has been widely studied as a potential HPV cofactor in cervical dysplasia due to possibility of concomitant infection, similar mode of transmission, asymptomatic nature, and persistence if untreated. CT can lead to epithelial damage allowing ease of HPV entry, enhancement of HPV viral load, inhibition of apoptosis, E6/E7 oncogenes overexpression, and cell transformation with genomic instability. Both Chlamydia and HPV infections increase the Ki-67 expression that is a cell proliferation marker of cervical epithelium.[19] Further, Chlamydia enhances HPV-16 protein infection in LSIL. Paba e t a l.[8] have also reported altered levels of mediators of the cell cycle and immune response in cases of synchronous coinfection. Gopalkrishna et al.[20] in their study in Indian women with known cervical dysplasia reported the prevalence of Chlamydia to be 12% in precancerous lesions and 22% in cancerous lesions. In contrast to the above studies, Madeleine et al. and Giuliano et al. found no significant association between Chlamydia infection and cervical dysplasia.[19] The methods used for Chlamydia detection in all the above studies were different. Some authors employed serological status for Chlamydia, whereas others used highly sensitive molecular techniques. Due to these differences, it is difficult to compare results from different studies.
In the present study, the prevalence of Chlamydia in patients with cervical cytological abnormalities (31.5%) was found to be significantly higher than those without atypia (5.8%, P = 0.004). As is evident, the prevalence of Chlamydi a in cervical abnormal cytology was found to be higher in our study when compared to the above studies. This could be attributed to the present study being based in a STI clinic at a tertiary care center. Further, the prevalence of Chlamydia in LSIL and HSIL was found to be 29.7% and 100%, respectively. Whereas, da Silva Barros et al.[21] found the prevalence to be 25% in LSIL and 29.3% in HSIL. Our study reports the comparable prevalence of Chlamydia in LSIL with the latter study. Therefore, to establish Chlamydia as a cofactor for cervical carcinogenesis, we require further larger cohort study that should include patients with known cervical dysplasia.
The specific question of the multistage process of HPV-related carcinogenesis which may be synergistically affected by Chlamydia has not been entirely elucidated. The inflammatory response caused by chlamydial infection may promote cell turn over that is needed for HPV replication. Persistent Chlamydia infections may induce an inflammatory milieu advantageous to HPV-related carcinogenesis by increasing the risk of DNA replication errors and active proliferation of genetically damaged cells.[19] The prevalence of HPV and Chlamydia coinfection in our study was found to be 6.7%. In the study done by Abreu et al.,[22] a comparable coinfection rate of 5.8% was reported. However, Bhatla et al.[23] in their study documented the coinfection rate to be only 0.7% and cervical dysplasia was present in 21.2% of the study population. Paba et al.[8] and da Silva Barros et al.[21] reported a much higher prevalence of 23.5% and 27.4%, respectively, in their study populations comprising patients with known cervical dysplasia, whereas the prevalence of coinfection in our study among patients with cervical dysplasia was 15%. It is pertinent to note that in our study HPV and Chlamydia coinfection was only found in patients with detection of cervical abnormal cytology as compared to those without any abnormality [P = 0.001, Table 2 and Table 3]. This indicates that Chlamydia and HPV may be acting synergistically in the pathway of cervical carcinogenesis. Luostarinen et al. in their cohort of Finnish women found that concomitant HPV18/45 and serologically detected CT infections were associated with very high CIN3 risk.[24] However, de Paula et al. failed to find any significant association between these coinfections and severity of cervical neoplasia.[25]
The limitations of the present study were that this was a cross-sectional, single-center study where the study participants were only recruited from the STI clinic. Further multicentric studies based on larger cohorts with longitudinal follow-up in relation to the CT acquisition and thorough evaluation of temporal relationships of concomitant infection with high-risk HPV types/Chlamydia and histologically proven cervical neoplasia are needed to demonstrate whether the chlamydial infection is a cofactor for cervical dysplasia. This clarification will enable early treatment of both CT and HPV infection and prevention of further morbidity.
Financial support and sponsorship
Intramural Research Grant of Rs 25,000 from University College of Medical Sciences, Delhi.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Chhabra S, Bhavani M, Mahajan N, Bawaskar R. Cervical cancer in Indian rural women: Trends over two decades. J Obstet Gynaecol. 2010;30:725–8. doi: 10.3109/01443615.2010.501412. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shew ML, Fortenberry JD, Tu W, Juliar BE, Batteiger BE, Qadadri B, et al. Association of condom use, sexual behaviors, and sexually transmitted infections with the duration of genital human papillomavirus infection among adolescent women. Arch Pediatr Adolesc Med. 2006;160:151–6. doi: 10.1001/archpedi.160.2.151. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J, Hook EW, Martin DH, Willis D, Fine P, Fuller D, et al. Confirming positive results of nucleic acid amplification tests (NAATs) for Chlamydia trachomatis: All NAATs are not created equal. J Clin Microbiol. 2005;43:1372–3. doi: 10.1128/JCM.43.3.1372-1373.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Judson FN. Assessing the number of genital chlamydial infections in the United States. J Reprod Med. 1985;30:269–72. [PubMed] [Google Scholar]
- 7.Swain GR, McDonald RA, Pfister JR, Gradus MS, Sedmak GV, Singh A, et al. Decision analysis: Point-of-care chlamydia testing vs. laboratory-based methods. Clin Med Res. 2004;2:29–35. doi: 10.3121/cmr.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paba P, Bonifacio D, Di Bonito L, Ombres D, Favalli C, Syrjänen K, et al. Co-expression of HSV2 and Chlamydia trachomatis in HPV-positive cervical cancer and cervical intraepithelial neoplasia lesions is associated with aberrations in key intracellular pathways. Intervirology. 2008;51:230–4. doi: 10.1159/000156481. [DOI] [PubMed] [Google Scholar]
- 9.Sambrook JF, Russell DW, editors. Molecular Cloning: A Laboratory Manual.3rd ed. 3rd ed. New York: Cold Spring Harbor Laboratory; 2001. [Google Scholar]
- 10.Ogembo RK, Gona PN, Seymour AJ, Park HS, Bain PA, Maranda L, et al. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: A systematic review and meta-analysis. PLoS One. 2015;10:e0122488. doi: 10.1371/journal.pone.0122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salit IE, Lytwyn A, Raboud J, Sano M, Chong S, Diong C, et al. The role of cytology (Pap tests) and human papillomavirus testing in anal cancer screening. AIDS. 2010;24:1307–13. doi: 10.1097/QAD.0b013e328339e592. [DOI] [PubMed] [Google Scholar]
- 12.Coker AL, Hulka BS, McCann MF, Walton LA. Barrier methods of contraception and cervical intraepithelial neoplasia. Contraception. 1992;45:1–10. doi: 10.1016/0010-7824(92)90136-h. [DOI] [PubMed] [Google Scholar]
- 13.Castellsagué X, Díaz M, de Sanjosé S, Muñoz N, Herrero R, Franceschi S, et al. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: Implications for screening and prevention. J Natl Cancer Inst. 2006 Mar 1;98:303–15. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 14.Schiffman MH. New epidemiology of human papillomavirus infection and cervical neoplasia. J Natl Cancer Inst. 1995;87:1345–7. doi: 10.1093/jnci/87.18.1345. [DOI] [PubMed] [Google Scholar]
- 15.Bhatla N, Dar L, Patro AR, Kriplani A, Gulati A, Verma K, et al. Human papillomavirus type distribution in cervical cancer in Delhi, India. Int J Gynecol Pathol. 2006;25:398–402. doi: 10.1097/01.pgp.0000209574.62081.e4. [DOI] [PubMed] [Google Scholar]
- 16.Basu P, Mittal S, Bhaumik S, Mandal SS, Samaddar A, Ray C, et al. Prevalence of high-risk human papillomavirus and cervical intraepithelial neoplasias in a previously unscreened population – A pooled analysis from three studies. Int J Cancer. 2013;132:1693–9. doi: 10.1002/ijc.27793. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, He Z, Huang XY, Liu HN, Tao JY. Prevalence of human papillomavirus and the correlation of HPV infection with cervical disease in Weihai, China. Eur J Gynaecol Oncol. 2015;36:73–7. [PubMed] [Google Scholar]
- 18.Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007;121:621–32. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 19.Silva J, Cerqueira F, Medeiros R. Chlamydia trachomatis infection: Implications for HPV status and cervical cancer. Arch Gynecol Obstet. 2014;289:715–23. doi: 10.1007/s00404-013-3122-3. [DOI] [PubMed] [Google Scholar]
- 20.Gopalkrishna V, Aggarwal N, Malhotra VL, Koranne RV, Mohan VP, Mittal A, et al. Chlamydia trachomatis and human papillomavirus infection in Indian women with sexually transmitted diseases and cervical precancerous and cancerous lesions. Clin Microbiol Infect. 2000;6:88–93. doi: 10.1046/j.1469-0691.2000.00024.x. [DOI] [PubMed] [Google Scholar]
- 21.da Silva Barros NK, Costa MC, Alves RR, Villa LL, Derchain SF, Zeferino LC, et al. Association of HPV infection and Chlamydia trachomatis seropositivity in cases of cervical neoplasia in Midwest Brazil. J Med Virol. 2012;84:1143–50. doi: 10.1002/jmv.23312. [DOI] [PubMed] [Google Scholar]
- 22.de Abreu AL, Nogara PR, Souza RP, da Silva MC, Uchimura NS, Zanko RL, et al. Molecular detection of HPV and Chlamydia trachomatis infections in Brazilian women with abnormal cervical cytology. Am J Trop Med Hyg. 2012;87:1149–51. doi: 10.4269/ajtmh.2012.12-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatla N, Puri K, Joseph E, Kriplani A, Iyer VK, Sreenivas V, et al. Association of Chlamydia trachomatis infection with human papillomavirus (HPV) and cervical intraepithelial neoplasia – A pilot study. Indian J Med Res. 2013;137:533–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Luostarinen T, Namujju PB, Merikukka M, Dillner J, Hakulinen T, Koskela P, et al. Order of HPV/Chlamydia infections and cervical high-grade precancer risk: A case-cohort study. Int J Cancer. 2013;133:1756–9. doi: 10.1002/ijc.28173. [DOI] [PubMed] [Google Scholar]
- 25.de Paula FD, Fernandes AP, Carmo BB, Vieira DC, Dutra MS, Santos CG, et al. Molecular detection of Chlamydia trachomatis and HPV infection infections in cervical samples with normal and abnormal cytopathological findings. Diagn Cytopathol. 2007;35:198–202. doi: 10.1002/dc.20629. [DOI] [PubMed] [Google Scholar]


