Abstract
Critical thermal limits (CTLs) show much variation associated with the experimental rate of temperature change used in their estimation. Understanding the full range of variation in rate effects on CTLs and their underlying basis is thus essential if methodological noise is not to overwhelm or bias the ecological signal. We consider the effects of rate variation from multiple intraspecific assessments and provide a comprehensive empirical analysis of the rate effects on both the critical thermal maximum (CTmax) and critical thermal minimum (CTmin) for 47 species of ectotherms, exploring which of the available theoretical models best explains this variation. We find substantial interspecific variation in rate effects, which takes four different forms (increase, decline, no change, mixed), with phylogenetic signal in effects on CTmax, but not CTmin. Exponential and zero exponential failure rate models best explain the rate effects on CTmax. The majority of the empirical rate variation in CTmin could not be explained by the failure rate models. Our work demonstrates that rate effects cannot be ignored in comparative analyses, and suggests that incorporation of the failure rate models into such analyses is a useful further avenue for exploration of the fundamental basis and implications of such variation.
Keywords: critical thermal limits, critical thermal maxima, critical thermal minima, rate of temperature change, thermal tolerance, heat stress
1. Introduction
Thermal performance curves describe the effects of temperature on physiological processes. The endpoints of a typical thermal performance curve (TPC) are the upper and lower critical thermal (CT) limits (CTLs), where performance declines to zero [1–4]. Practicably, these endpoints are often defined behaviourally as the temperatures that result in loss of the righting response, coordination, or equilibrium, or the onset of stereotypical behaviour or thermal spasms [5–7]. CTLs are usually measured using a dynamic (ramping) method where temperature is gradually increased or lowered at a constant rate of temperature change until an endpoint is observed. Typically, CTLs of ectotherms increase with faster rates of temperature change, but the converse has also been found [8–10]. Irrespective, the effects of varying rates of temperature change can be large [8,9], and can also interact unpredictably with acclimation effects [11]. Given the use of CTLs to explore fundamental ecological questions, such as the functional basis of community structure [12] and the influence of trait variation on niche modelling [13–15], and to estimate important environmental change impacts [16–18], understanding the full range of variation in rate effects on CTLs is essential.
Several proposals have now been made for the ways in which rate effects (which are related to changes in exposure time to stressful temperature [2,19,20]) influence CTLs and their estimation. The most comprehensive of these is the idea of thermal tolerance landscapes [2]. The approach is based on the premise that a single underlying relationship exists between temperature, exposure time, and mortality (see also [21]), which follows a typical dose–response curve. Here, critical thermal maximum (CTmax) is defined as a knockdown (death) temperature at 1 min of exposure [2] (not 0 min of exposure [20]). A similar argument is applied to critical thermal minimum (CTmin). In both cases, the theoretical expectation is that CTLs should improve (higher CTmax, lower CTmin) with increasing rates of experimental temperature change (or ramping rate). Several studies have now considered the thermal tolerance landscape approach, the most comprehensive of which is a recent analysis of 11 Drosophila species focusing especially on knockdown time [22].
A related approach models CTmax based on the assumption that failure rate increases with rising temperature [23]. The statistical failure rate model predicts that CTmax should increase, typically in a nonlinear fashion, with both experimental ramping rate and experimental starting temperature, and is capable of explaining more than half of the variation in CTmax values associated with changing ramping rates and starting temperatures. This model is thought to be likewise applicable to CTmin estimates [23]. Notably, the failure rate model is considered a first order model to distinguish statistical effects, which are expected to lead to the positive relationship between an increasing ramping rate and CTLs, from biological effects that might lead either to no effect or to a negative relationship. Here, statistical effects represent variance in CTLs explained by the failure rate model showing an improvement in CTLs (higher CTmax, lower CTmin), where failure rate (M = 1/time) is expected to be higher at the higher (or at the lower for CTmin) temperature of failure. Residual variance not explained by the failure rate model is attributed to the biological effects.
A recent work, particularly focused on predicting the thermal acclimation capacity of ectotherms across many different species, body sizes, latitudes, traits, and habitats, also includes the effect of ramping rate as one of the variables, but only at the interspecific level and at the high temperature end [24]. However, the study uses an interspecific approach, which confounds intraspecific with interspecific variation, assuming, in contrast to existing empirical data [8–11], that rate effects at the two levels have similar magnitudes and take the same form, and leaving the effect of ramping rate on the extent of variation of both CTmax and CTmin at the intraspecific level unexplored.
Given the growing significance of CTL estimates in both basic and applied ecological research [16–18,25,26], methodological variation in their estimates, and the existence of various theoretical frameworks for the expected nature of its impacts, here, we examine CTL variation with time of exposure to stressful temperatures within species, considering also how exposure time effects on CTLs vary among species. To investigate what the form of the intraspecific response is, across multiple taxa, we quantify how much overall variance can be explained by time alone, how much by species identity, and how much by higher level phylogenetic effects. In doing so we consider closely Rezende et al.'s [2] variables CTmax and z (CTmin and z′). The variable z is defined as a constant that characterizes the sensitivity to temperature change, and is a key component of the thermal tolerance landscape models [2]. We do so to determine whether z (z′) conform to the original theoretical estimates, and whether CTmax and CTmin estimated using this approach provide values in keeping with what has been measured. Next, we examine the failure rate model approach [23], which provides more flexibility than the log-linear model of the thermal tolerance landscape approach [2], to determine whether each species follows the response expected by the set of failure rate models originally proposed. We provide the best fitting model to the data at the species level and determine the variation attributed to the statistical effects of failure rate and to residual biological variation [23]. Furthermore, we extend the set of failure rate models to analyse CTmin responses.
2. Methods
(a). Data collection
We used a systematic review approach [27] to find studies that measure upper and/or lower CTLs of ectotherms using different rates of temperature change. Search databases included Web of Science, Google Scholar, and Research Gate using the keywords (‘critical thermal limits’ OR ‘thermal tolerance’) AND (‘rate of temperature change’ OR ‘ramping rate’ OR ‘heating rate’ OR ‘cooling rate’) AND (‘critical thermal maxima’ OR ‘CTmax’ OR ‘CTmin’ OR ‘critical thermal minima’). In addition, we examined studies listed in the references of the articles found through the database search. Upper and/or lower CTLs were extracted from tables, figures and the main text. Data extraction from figures was undertaken using Plot Digitizer software [28].
Because the effect of ramping rate (i.e. time of exposure) on CTLs of ectotherms at the intraspecific level is of central interest, inclusion criteria for studies comprised: (i) at least two different rates of temperature change per species to establish a time-temperature pattern of response, (ii) the same life stage, and (iii) a consistent acclimation temperature within single species across multiple ramping rates. If the study contained more than one acclimation temperature, while simultaneously testing for the effect of ramping rate, we chose the acclimation temperature that was the closest to the recorded environmental temperature at the time of the species collection reported in the study. Based on these criteria the CTmax and CTmin datasets included 41 (184 data points) and 23 (77 data points) species of ectotherms, respectively. Each data point represents an arithmetic mean of a CTL corresponding to a particular rate of temperature change. Thus, these datasets differ from those used in a recent synthesis which focuses on thermal tolerance and acclimation capacities of ectotherms at different acclimation temperatures that use a single ramping rate per species in all of the 254 cases investigated [24].
(b). Analytical approach
Time of exposure for each CTL with its corresponding rate of temperature change was calculated according to:
| 2.1 |
| 2.2 |
where ST is the starting experimental temperature, r is the rate of temperature change, t is the time of exposure (t′ is the equivalent of t for decreasing temperatures), CTmax is the upper critical limit, and CTmin is the lower critical limit. To examine the CTL variation with time of exposure to stressful temperature and quantify how much overall variance can be explained by time alone, time and species, and by phylogenetic signal, we undertook two sets of analyses. First, we used a linear model on a semi-logarithmic scale to determine how much of the overall variance in CTLs (i.e. separately for CTmax and CTmin) is explained by the time of exposure across the entire dataset. In essence, the analyses suggest how much variance might be accounted for by a simple failure rate type model [23]. Then, we included species as an additional term to determine how much variance is attributable to species-specific responses. Because we found a strong species-specific response of the pattern variation in the CTLs to the time of exposure, we also undertook a phylogenetic analysis in each case using the phylogenetic generalized least-squares (PGLS) method with the ‘caper’ package in R [29]. Phylogenetic trees were generated using the ‘rotl’ package [30] from the comprehensive tree of life [31]. Tree branch lengths were estimated using Grafen's arbitrary branch lengths transformation where branch length is set to a length equal to the number of descendant tips minus one [32]. All analyses were performed in R v.3.5.1 [33].
Following the approach recommended by Rezende et al. [2], we tested the expectation that CTLs should improve (higher CTmax, lower CTmin) with increasing rates of experimental temperature change following the proposed log-linear model [2]. We used a linear model of CTLs against a logarithm of time for each species to calculate the intercept and slope of these lines (electronic supplementary material, tables S1 and S2), which, according to the thermal tolerance landscape framework, correspond to parameters CTmax and z (and CTmin and z′ for low temperatures), respectively [2]. We also calculated goodness-of-fit of the line for each species (as the coefficient of determination). In a few instances where we had only two ramping rates per species, we used the CTL data only to establish the time-temperature pattern of response, but did not include the goodness-of-fit. Two species, Cryptolestes ferrugineus and Glossina pallidipes, along with Thaumatotibia leucotreta (larval stage only), were removed from examination of the association between CTmin and z′ parameters because the CTmin estimates were major outliers. We tested whether parameters z (z′) and CTmax (CTmin) were as strongly correlated as proposed in the thermal tolerance landscape framework [2] and also performed a phylogenetic analysis on these traits.
To test the failure rate model approach and determine whether each species follows the response expected by the set of proposed failure rate models (that assume that the relationship between the failure rate and temperature can follow different parametric forms, such as exponential and power-law forms), we modified and expanded the R code provided by the authors [23]. Because the authors provide the full code for the best fitting model to their species' dataset only, we expanded the code to provide the probability density function (pdf), expected time to failure, and CTL estimates for the remaining seven models (https://doi.org/10.26180/5c467981f3158). We selected the best fitting failure rate model for each species in our datasets using the corrected Akaike information criterion (AICc) [34] because mean CTL values were used within species, and we reported the variance attributed to the statistical effects of failure rate previously described above (electronic supplementary material, table S3). Five species showing a converse response from the one expected by the set of the failure rate models (i.e. a CTmax decreasing with increasing rate) were excluded from the CTmax analysis, because the statistical effects described by the failure rate models cannot account for a converse response [23]. Nevertheless, we applied these models to species exhibiting no effect (i.e. no change in CTLs) or a mixed response (i.e. an increase in CTL followed by a decrease at faster ramping rates) to evaluate if statistical effects account, at least partially, for any CTL variation of these types of responses. Likewise, we extend this code to analyse the response patterns of CTmin and exclude nine species owing to their converse response not described by the failure rate models (electronic supplementary material, table S4 and https://doi.org/10.26180/5c467981f3158). Both of these scripts can be easily applied to any species dataset investigating the effect of ramping rate and starting temperature on either heat or cold tolerance at the level of species. It is important to highlight that we tested these models using the reported mean values of CTLs for each ramping rate and a single experimental starting temperature per species, with the analyses focusing on the effect of ramping rate at the intraspecific level. All analyses were performed in R v.3.5.1 [32] using ‘bbmle’ package v.1.0.20 applying the Nelder–Mead algorithm within function mle2 [35].
3. Results
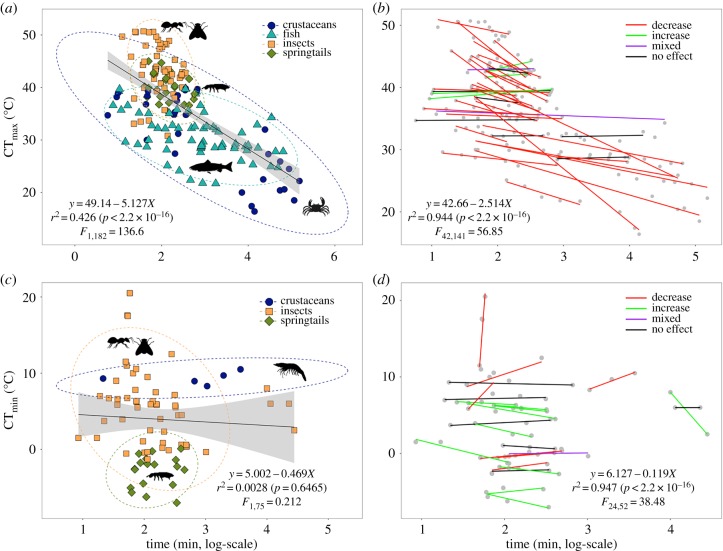
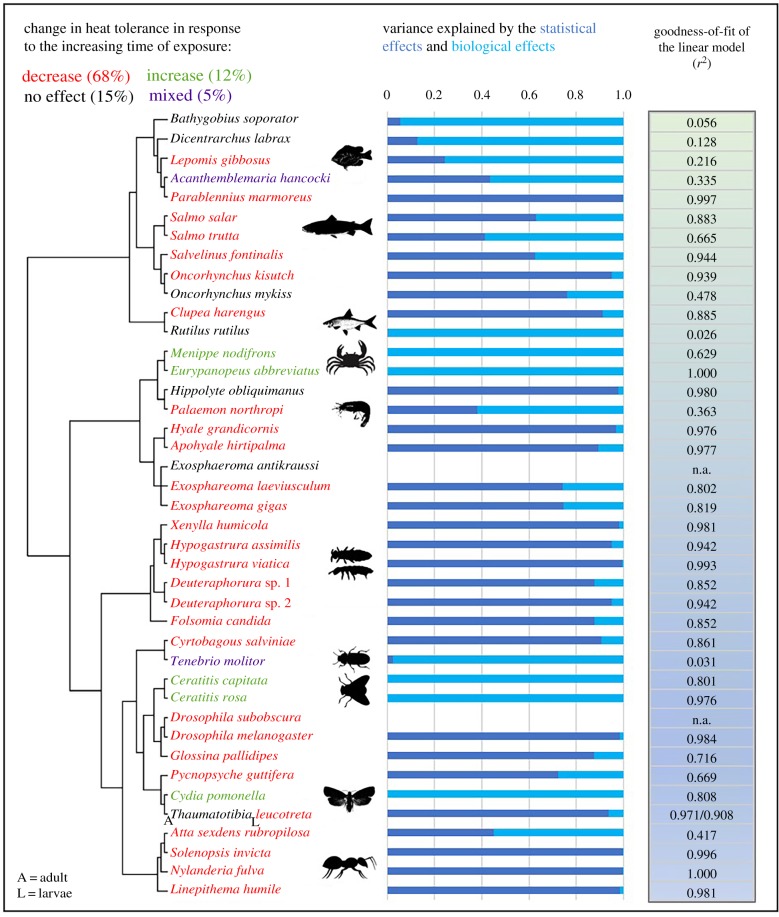
In the overall time-temperature relationship, 43% of the overall variance in CTmax can be explained by the time of exposure to heat stress (r2 = 0.426, p < 0.0001; figure 1a). If the analyses are undertaken using rate data only (i.e. excluding starting times) the outcomes are similar (electronic supplementary material, figure S4). On the other hand, there was no relationship between CTmin and time of exposure to cold stress (r2 = 0.0028, p > 0.60; figure 1c). Adding species identity as an additional factor revealed a strong species-specific response incorporating four different time-temperature patterns of response. Most (94.4%) of variance in CTmax can be explained by the time of exposure to heat stress and species identity (r2 = 0.944, p < 0.0001; figure 1b). Similarly, 94.7% of variance in CTmin is explained by the time of exposure to cold stress and species identity (r2 = 0.947, p < 0.0001; figure 1d). Variation among species in the relationships was not a consequence of body size variation among them as no interaction between a body size measure and rate was found when body size was included in an examination of the relationship between time and CTL (electronic supplementary material, figure S3 and tables S6 and S7). Of the 40 species examined in the CTmax analysis (T. leucotreta was excluded from percentage analysis because adults and larvae have different responses for both CTmax and CTmin), more than half showed a decline in CTmax with exposure time (figure 2). Fifteen per cent of species showed no effect, 12% an increase in thermal tolerance with exposure time, and 5% had a mixed response (figure 2). In the case of CTmin, response patterns were more evenly distributed among categories (electronic supplementary material, figure S1). Forty-one per cent of the 22 species showed an increase in CTmin with exposure, which is the contrary of what is typically predicted [2], 27% showed a decrease, 5% had a mixed response, and cold tolerance of 27% of the species remained unchanged regardless of the time of exposure (i.e. ramping rate) (electronic supplementary material, figure S1).
Figure 1.
Time-temperature relationship for upper and lower critical thermal limits of ectotherms. (a) General relationship between time of exposure and CTmax of 41 species of crustaceans, fishes, insects and springtails. (b) The association between time of exposure and CTmax at the intraspecific level reveals four different patterns of species-specific responses to the heat stress for 41 species of ectotherms. (c) General relationship between time of exposure and CTmin of 23 species of crustaceans, insects, and springtails. (d) The association between time of exposure and CTmin at the intraspecific level reveals four different patterns of species-specific responses to the cold stress for 23 species of ectotherms. (Online version in colour.)
Figure 2.
Phylogeny of 41 species of ectotherms used in the CTmax analysis and their response patterns describing the change in heat tolerance with the increasing exposure time (i.e. slower ramping rates). Variance explained by the statistical (dark blue) and biological (light blue) effects using a failure rate model [23] and goodness-of-fit of the thermal landscape model [2]. (Online version in colour.)
Following the thermal tolerance landscape approach [2], we calculated the intercept and slope of the lines for log10-transformed time of exposure on CTmax values for each species that correspond to the CTmax and z. We also calculated variance explained by the time of exposure for each species excluding species that had only two ramping rates. Regressing log10-transformed time of exposure on CTmax values of 39 species resulted in relationships with lower goodness-of-fit (median r2 = 0.872, 95% confidence interval (CI) between 0.656 and 0.976) (figure 2 and electronic supplementary material, table S1) than those derived from upper lethal limit approaches reported in the original study seeking to integrate the two approaches (median r2 = 0.985, 95% CI between 0.876 and 0.999) [2]. Analyses show that z accounts for 70% of the variation in CTmax as estimated by the coefficient of determination (slope = 2.90, p < 0.0001, r2 = 0.702; table 1a and electronic supplementary material, figure S2a). Within insects and crustaceans, the variance explained by this model corresponds to 76% and 67%, respectively (slopes = 2.52 and 1.88, p < 0.0001 and p = 0.0069), while within fishes and springtails this model cannot explain the variance in CTmax (slopes = −0.12 and 4.21, p = 0.9145 and p = 0.0694). Parameters CTmax and z vary across species, with CTmax ranging between 27.8°C and 64.9°C and z ranging between 0.04 and 8.9. Likewise, we performed the same analysis on cold tolerance (electronic supplementary material, figure S1 and table S2). CTmin curves of 21 species of ectotherms resulted in relationships with lower goodness-of-fit (median r2 = 0.858, 95% CI between 0.694 and 0.956) (electronic supplementary material, figure S1) than those reported using lower lethal limit values in the original study seeking to integrate the two approaches (median r2 = 0.955, 95% CI between 0.876 and 0.993) [2]. After excluding three outliers from the analysis, we found that the association between parameters CTmin and z′ was not significant (slope = −0.68, p = 0.5167, r2 = 0.022; table 1b and electronic supplementary material, figure S2b), suggesting that z′ does not explain the variation in CTmin in 21 species of ectotherms, which is in contrast with the high correlation found in studies using lower lethal limits and the general thermal tolerance landscape expectation (slope = −4.99, p < 0.0001, r2 = 0.965 [2]). The range of CTmin variation was lower than for CTmax, with CTmin ranging between −7.30°C and 9.61°C, and z′ between 0 and 4.03, which is not in keeping with the results of the thermal landscape approach [2].
Table 1.
Outcome of the linear models examining the relationship between parameters CTmax and z and CTmin and z′ among 41 and 23 species of ectotherms, respectively.
| (a) CTmax | estimate | s.e. | t | p |
|---|---|---|---|---|
| CTmax (intercept) | 34.696 | 1.147 | 30.240 | <0.0001 |
| z | 2.905 | 0.299 | 9.703 | <0.0001 |
| F1,40 = 94.14, p < 0.0001, r2 = 0.7018 | ||||
| (b) CTmin | estimate | s.e. | t | p |
| CTmin (intercept) | 3.413 | 1.780 | 1.918 | 0.0703 |
| z′ | −0.681 | 1.031 | −0.661 | 0.5167 |
| F1,19 = 0.44, p = 0.5167, r2 = 0.022 | ||||
Estimates of a phylogenetic signal in z, a constant characterizing the sensitivity to temperature change, were moderate. Pagel's λ in z alone was 0.43, while lower phylogenetic signals were detected in the effects of response (λ = 0.26), habitat (λ = 0.05), and climate (λ = 0.40) on z. On the other hand, no phylogenetic signal was detected in z′ alone, nor in the effects of response, habitat, and climate on z′ (electronic supplementary material, table S5).
The most common failure rate models across species in both CTmax and CTmin analyses were exponential and zero exponential models, followed by a zero-power threshold, zero exponential threshold and exponential threshold models. Five species had a converse trend to which we could not apply statistical effects described by the failure rate models as recognized by the authors, thus, we assigned the response to the biological effects only (figure 2 and electronic supplementary material, table S3). Outcomes of the CTmin analysis showed that the most common cold tolerance response was a converse response, which could not be accounted for by the statistical effects of the failure rate models. Therefore, we similarly assigned the variance to the biological effects (electronic supplementary material, table S4 and figure S1). The zero-power threshold model was the most common best-fit model closely followed by the exponential and zero exponential models, including exponential threshold and zero exponential threshold models. Using mean CTmax values for each ramping rate we found that the best-fitting model for G. pallidipes are exponential and zero exponential models, giving a different outcome from the zero-power threshold model, which was the best-fitting model for the full G. pallidipes dataset containing CTmax values for each individual tested.
4. Discussion
Our results demonstrate that the commonly expected direction of response characterized by an improvement of CTLs (higher CTmax, lower CTmin) with the increasing rate of temperature change is not a universally observed pattern among the ectotherms. While time of exposure generally has a negative effect on CTmax, such a generalization cannot be made for CTmin. Similarly, ontogeny, body size, and sex, along with nutritional status and the extent of desiccation stress have varying effects on heat and cold tolerance [36–40].
A strong species-specific response for both CTmax and CTmin, independent of body size (unlike the situation found in interspecific analyses [24]), reveals four different thermal tolerance response patterns to the increasing exposure time (i.e. slower ramping rate). The proposal that static and dynamic experimental methods share similar relationships with exposure time in a thermal tolerance landscape framework [2] thus appears to be an oversimplification of empirical observations. Species showing an increase in thermal tolerance with the exposure time might fit the log-linear time-temperature trend [2] well, but in the opposite direction from the one expected. This model generally produces a poor fit for species with no effect and mixed trends in their CTLs. Indeed, assessment of the method used by Rezende et al. [2] revealed that the parameters z (z′), a constant characterizing the sensitivity to the temperature change, and CTmax (CTmin), redefined as a knockdown temperature at 1 min of exposure, are not always highly correlated as originally proposed. These outcomes are in agreement with a recent comprehensive assessment of the thermal landscapes approach, which demonstrated that heat tolerance parameters, z and CTmax at 1 min of exposure in 11 Drosophila species are not correlated [22]. The lower correlation of z and CTmax than the one found in the study by Rezende and colleagues [2] may have several explanations. One of these may be variation of species' CTmax response patterns to the ramping rate, as two thirds of the species show the decline in thermal tolerance with the longer exposure time (i.e. slower ramping rate), while the rest of the species yield different responses. The other explanation, supported by evidence from modelling analyses, is that experimental noise (or small sample size), autocorrelation and unwarranted extrapolation, are responsible for the initial finding of a strong relationship between z and CTmax [22]. Perhaps unsurprisingly, therefore, we also found no correlation between z′ and CTmin, a result while different to the original thermal landscapes idea [2], is in keeping with the growing body of literature testing it [22]. The variation of CTmin with the dynamic temperature change is much more pronounced, where the most dominant pattern among the species tested is an increase in cold tolerance with the increasing exposure time. Parameter z also appears to be more phylogenetically constrained (λ = 0.43) than parameter z′ where no phylogenetic signal was detected (λ = 0), which is in agreement with the previous studies detecting a phylogenetic constraint in upper thermal limits [41,42].
In addition, significant disparities exist when comparing CTmin estimates from the empirical studies using a dynamic method and CTmin values predicted by the thermal tolerance landscape. For example, Jian et al. [43] found that the overall limits to activity (CTmin) for three species of beetles in the adult life stage, Cryptolestes ferrugineus, Tribolium castaneum, and Sitophilus oryzae, were 2.0°C, 6.0°C, and 6.5°C respectively [43]. Empirical results from this study differ substantially from CTmin values predicted by Rezende et al. [2], where CTmin limits for C. ferrugineus, T. castaneum, and S. oryzae were estimated to be −100.96°C, −85.17°C, and −38.98°C respectively [2]. This discrepancy arises from redefining CTmin as death or knockdown temperature at 1 min of exposure [2] as opposed to the lowest limit to activity, as used across a multitude of cold tolerance studies [44–46]. The lowest ever reported CTmin of −16°C for Diamesa Meigen, a Himalayan glacier species, remains much higher than these three estimates [47]. Because the thermal tolerance landscape framework seems to overestimate empirical CTmin values, the approach requires more exploration.
The set of failure rate models proposed by Kingsolver & Umbanhowar [23], where failure rate is higher at the higher (or at the lower for CTmin) temperature of failure, generally corresponds to a decline in thermal tolerance with exposure time, a similar expectation to the thermal tolerance landscape framework [2]. However, failure rate models are more flexible than the log-linear models in a sense that they allow curves to take different shapes and include the presence or absence of a threshold temperature. In addition, other patterns of thermal tolerance response to the increasing ramping rate, which cannot be attributed to the statistical effects of failure rate, can also be incorporated generally [23]. Contribution of the statistical effects to the mixed and no effect responses is generally small, probably because the variation is owing to the biological effects. The models are not applicable to the converse response currently including 12% (for CTmax) and 41% (for CTmin) of species, which can be also attributed to the biological effects. It is important to note that we obtained a different best-fitting model for G. pallidipes than the authors [23] because we used mean CTmax values per ramping rate as opposed to CTmax values for each individual tested, which demonstrates how the outcome of the analysis may change when an individual variation is incorporated into or excluded from the intraspecific study. The most common best-fit models in CTmax analysis suggest that failure rate increases exponentially from the experimental starting temperature until CTmax is reached. Results of other species support the presence of a temperature threshold after which failure rate follows an exponential or a power increase before reaching CTmax. CTmin analysis supports the presence of a temperature threshold, but there are also species showing an exponential increase in failure rate from the experimental starting temperature until CTmin is reached.
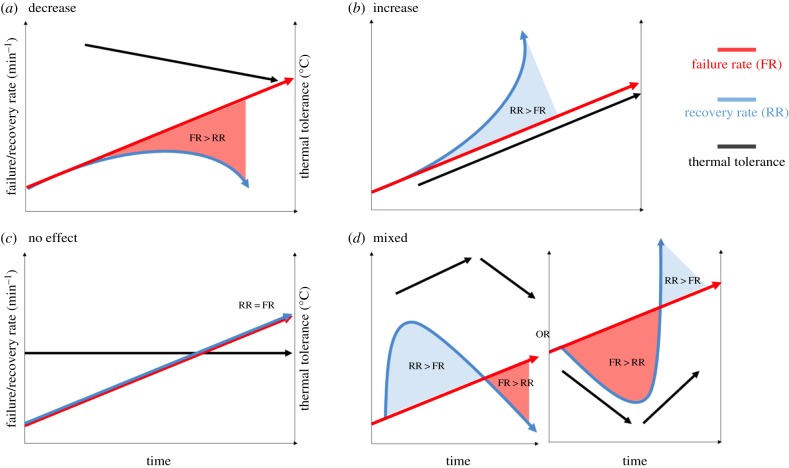
Based on the four response patterns found, we propose a set of hypothetical relationships between failure rate [23], recovery rate, and CTLs that may explain the variation in species' responses (figure 3). If recovery rate cannot catch up with the failure rate during prolonged time of exposure (figure 3a), thermal tolerance declines with exposure time. On the other hand, if recovery rate improves with time owing to a rapid physiological response, thermal tolerance increases with exposure time (figure 3b). In the case of the no effect response, time of exposure (i.e. ramping rate) might have no effect on thermal tolerance (figure 3c). Alternatively, failure rate and recovery rate could be matched because of some form of beneficial physiological response by an organism. Finally, a mixed response could reveal an inflection point [8], with a pattern of declining thermal tolerance from an intermediate ramping rate towards faster and slower rates (figure 3d). This response potentially reveals an optimal ramping rate at which maximum tolerance gain is achieved owing to acclimation to the rapid change in temperature, while an organism simultaneously becomes exposed to the deleterious effects of temperature extremes.
Figure 3.
Hypothetical relationship between failure rate and recovery rate and their effect on thermal tolerance. (a) Thermal tolerance declines with exposure time if recovery rate cannot catch up with the failure rate. (b) Thermal tolerance increases with exposure time if recovery rate improves over time and overcomes failure rate. (c) Thermal tolerance is not affected by exposure time when failure rate and recovery rate are closely matched. (d) Thermal tolerance initially shows an increase (or a decrease) in thermal tolerance, reaches a peak high (or low) temperature at a certain ramping rate (i.e. time of exposure), followed by a decrease (or an increase) in thermal tolerance. This response potentially reveals an inflection point or ramping rate, which either decreases or improves recovery rate relative to the failure rate. (Online version in colour.)
What these four relationships provide is a framework for further exploration of the way in which differing damage accumulation rates and organismal-level physiological and biochemical response rates interact to determine thermal tolerance. Clearly, time of exposure (given different ramping rates) is an important component thereof, especially given the high Q10 of the processes leading to heat stress-related physiological failure [22]. However, as an early study showed [9], so too is the starting temperature of the process, because this may determine the extent to which an organism is already outside the zone of tolerance [48], which precedes the onset of damage. Just what the effect is of starting temperature on experimental outcomes is not yet well resolved. The proposed framework suggests that future work should focus on three main areas. First, determining whether starting temperature has an as large effect as ramping rate on outcomes, as a single study suggests it might [9] and whether a threshold effect, indicating that differences in starting temperatures inside or outside the organism's zone of tolerance (i.e. on either side of the incipient lethal temperature [48]) are important. Second, further considering the outcomes of the failure rate approach in the context of the framework proposed here to determine the extent to which simple failure rate models may afford the null expectation for thermal limits in the absence of biological responses [23]. The failure rate models provide good fits to the available data and are readily interpretable both in a statistical and physiological context. Finally, investigation of whether differential rates of damage and repair really are responsible for variation in the rate-thermal limit response. Very high rates of Q10 for thermal limits [22] suggest that, at least at the highest temperatures, any form of repair will be rapidly overwhelmed given generally lower thermal sensitivities of routine physiological functions including, for example, protein synthesis [49]. Of course, even in the absence of investigation of these questions, it is clear that rate variation cannot be ignored in compiled comparative studies, either of CTLs or of their implications for environmental change.
Supplementary Material
Acknowledgements
We thank Craig R. White for helpful discussions and valuable advice on phylogenetic modelling and analysis, and two anonymous referees for their helpful comments on an earlier draft of the manuscript.
Data accessibility
All data and R code for analyses shown in figures, tables and results, including R code for failure rate models are deposited in Monash Figshare repository at: https://doi.org/10.26180/5c467981f3158.
Authors' contributions
A.K. and S.L.C. designed the study. A.K. and G.L. conducted the analyses. A.K. and S.L.C. wrote the original draft. All authors edited and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
This project was funded by the Holsworth Wildlife Research Endowment – Equity Trustees Charitable Foundation & the Ecological Society of Australia, and by Australian Research Council Discovery Project DP170101046.
References
- 1.Angilletta MJ., Jr 2009. Thermal adaptation: a theoretical and empirical synthesis. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Rezende EL, Castañeda LE, Santos M. 2014. Tolerance landscapes in thermal ecology. Funct. Ecol. 28, 799–809. ( 10.1111/1365-2435.12268) [DOI] [Google Scholar]
- 3.Deutsch CA, Tewksbury JJ, Huey RB, Sheldon KS, Ghalambor CK, Haak DC, Martin PR. 2008. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sinclair BJ, et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19, 1372–1385. ( 10.1111/ele.12686) [DOI] [PubMed] [Google Scholar]
- 5.Lutterschmidt WI, Hutchison VH. 1997. The critical thermal maximum: history and critique. Can. J. Zool. 75, 1561–1574. ( 10.1139/z97-783) [DOI] [Google Scholar]
- 6.Chown SL, Nicolson SW. 2004. Insect physiological ecology mechanisms and patterns. Oxford, UK: Oxford University Press. [Google Scholar]
- 7.Owen KJ, Dillon ME. 2018. Critical thermal limits of bumblebees (Bombus impatiens) are marked by stereotypical behaviors and are unchanged by acclimation, age or feeding status. J. Exp. Biol. 221, 165589 ( 10.1242/jeb.165589) [DOI] [PubMed] [Google Scholar]
- 8.Mora C, Maya MF. 2006. Effect of the rate of temperature increase of the dynamic method on the heat tolerance of fishes. J. Therm. Biol. 31, 337–341. ( 10.1016/j.jtherbio.2006.01.005) [DOI] [Google Scholar]
- 9.Terblanche JS, Deere JA, Clusella-Trullas S, Janion C, Chown SL. 2007. Critical thermal limits depend on methodological context. Proc. R. Soc. B 274, 2935–2943 ( 10.1098/rspb.2007.0985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinagre C, Leal I, Mendonça V, Flores AAV. 2015. Effect of warming rate on the critical thermal maxima of crabs, shrimp and fish. J. Therm. Biol. 47, 19–25. ( 10.1016/j.jtherbio.2014.10.012) [DOI] [PubMed] [Google Scholar]
- 11.Allen JL, Chown SL, Janion-Scheepers C, Clusella-Trullas S. 2016. Interactions between rates of temperature change and acclimation affect latitudinal patterns of warming tolerance. Conserv. Physiol. 4, cow053 ( 10.1093/conphys/cow053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamblin AL, Youngsteadt E, López-Uribe MM, Frank SD. 2017. Physiological thermal limits predict differential responses of bees to urban heat-island effects. Biol. Lett. 13, 20170125 ( 10.1098/rsbl.2017.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valladares F, et al. 2014. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17, 1351–1364 ( 10.1111/ele.12348) [DOI] [PubMed] [Google Scholar]
- 14.Kearney MR, Isaac AP, Porter WP. 2014. microclim: global estimates of hourly microclimate based on long-term monthly climate averages. Sci. Data 1, 140006 ( 10.1038/sdata.2014.6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrando-Pérez S, et al. 2018. Intraspecific variation in lizard heat tolerance alters estimates of climate impact. J. Anim. Ecol. 88, 247–257 ( 10.1111/1365-2656.12914) [DOI] [PubMed] [Google Scholar]
- 16.Diamond SE, Sorger DM, Hulcr J, Pelini SL, Toro ID, Hirsch C, Oberg E, Dunn RR. 2011. Who likes it hot? A global analysis of the climatic, ecological, and evolutionary determinants of warming tolerance in ants. Glob. Change Biol. 18, 448–456. ( 10.1111/j.1365-2486.2011.02542.x) [DOI] [Google Scholar]
- 17.Overgaard J, Kearney MR, Hoffmann AA. 2014. Sensitivity to thermal extremes in Australian Drosophila implies similar impacts of climate change on the distribution of widespread and tropical species. Glob. Change Biol. 20, 1738–1750. ( 10.1111/gcb.12521) [DOI] [PubMed] [Google Scholar]
- 18.García-Robledo C, Kuprewicz EK, Staines CL, Erwin TL, Kress WJ. 2016. Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proc. Natl Acad. Sci. USA 113, 680–685. ( 10.1073/pnas.1507681113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilgour DM, McCauley RW. 1986. Reconciling the two methods of measuring upper lethal temperatures in fishes. Environ. Biol. Fishes 17, 281–290. ( 10.1007/bf00001494) [DOI] [Google Scholar]
- 20.Cooper BS, Williams BH, Angilletta MJ Jr. 2008. Unifying indices of heat tolerance in ectotherms. J. Therm. Biol. 33, 320–323. ( 10.1016/j.jtherbio.2008.04.001) [DOI] [Google Scholar]
- 21.Tang J, Mitcham E, Wang S, Lurie S. 2007. Heat treatments for postharvest pest control: theory and practice. Wallingford, UK: CABI. [Google Scholar]
- 22.Jørgensen LB, Malte H, Overgaard J. 2019. How to assess Drosophila heat tolerance: unifying static and dynamic tolerance assays to predict heat distribution limits. Funct. Ecol. 33, 629–642. ( 10.1111/1365-2435.13279) [DOI] [Google Scholar]
- 23.Kingsolver JG, Umbanhowar J. 2018. The analysis and interpretation of critical temperatures. J. Exp. Biol. 221, 167858 ( 10.1242/jeb.167858) [DOI] [PubMed] [Google Scholar]
- 24.Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI. 2018. The complex drivers of thermal acclimation and breadth in ectotherms. Ecol. Lett. 21, 1425–1439. ( 10.1111/ele.13107) [DOI] [PubMed] [Google Scholar]
- 25.Bennett JM, et al. 2018. GlobTherm, a global database on thermal tolerances for aquatic and terrestrial organisms. Sci. Data 5, 180022 ( 10.1038/sdata.2018.22) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araújo MB, Ferri-Yáñez F, Bozinovic F, Marquet PA, Valladares F, Chown SL. 2013. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219. ( 10.1111/ele.12155) [DOI] [PubMed] [Google Scholar]
- 27.Pullin AS, Stewart GB. 2006. Guidelines for systematic review in conservation and environmental management. Conserv. Biol. 20, 1647–1656. ( 10.1111/j.1523-1739.2006.00485.x) [DOI] [PubMed] [Google Scholar]
- 28.Huwaldt JA, Steinhorst S. 2015. Plot Digitizer (Version 2.6.8) [Software]. Retrieved from http://plotdigitizer.sourceforge.net/.
- 29.Orme D. 2013. The caper package: comparative analysis of phylogenetics and evolution in R. R package version 5.2 See http://cran.r-project.org/web/packages/caper/vignettes/caper.pdf.
- 30.Michonneau F, Brown JW, Winter DJ. 2016. rotl: an R package to interact with the Open Tree of Life data. Methods Ecol. Evol. 7, 1476–1481. ( 10.1111/2041-210x.12593) [DOI] [Google Scholar]
- 31.Hinchliff CE, et al. 2015. Synthesis of phylogeny and taxonomy into a comprehensive tree of life. Proc. Natl Acad. Sci. USA 112, 12 764–12 769. ( 10.1073/pnas.1423041112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grafen A. 1989. The phylogenetic regression. Phil. Trans. R. Soc. Lond. B 326, 119–157. ( 10.1098/rstb.1989.0106) [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 34.Burnham KP, Anderson DR (eds). 2004. Model selection and multimodel inference. New York, NY: Springer. [Google Scholar]
- 35.Bolker B, R Development Core Team. 2011. bbmle: tools for general maximum likelihood estimation. R package version 1.0.3 See http://CRAN.R.project.org/package=bbmle.
- 36.Madeira D, Narciso L, Cabral HN, Diniz MS, Vinagre C. 2012. Thermal tolerance of the crab Pachygrapsus marmoratus: intraspecific differences at a physiological (CTMax) and molecular level (Hsp70). Cell Stress Chaperones 17, 707–716. ( 10.1007/s12192-012-0345-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klockmann M, Günter F, Fischer K. 2016. Heat resistance throughout ontogeny: body size constrains thermal tolerance. Glob. Change Biol. 23, 686–696. ( 10.1111/gcb.13407) [DOI] [PubMed] [Google Scholar]
- 38.Terblanche JS, Mitchell KA, Uys W, Short C, Boardman L. 2017. Thermal limits to survival and activity in two life stages of false codling moth Thaumatotibia leucotreta (Lepidoptera, Tortricidae). Physiol. Entomol. 42, 379–388. ( 10.1111/phen.12210) [DOI] [Google Scholar]
- 39.Overgaard J, Kristensen TN, Sørensen JG. 2012. Validity of thermal ramping assays used to assess thermal tolerance in arthropods. PLoS ONE 7, e32758 ( 10.1371/journal.pone.0032758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terblanche JS, Hoffmann AA, Mitchell KA, Rako L, le Roux PC, Chown SL. 2011. Ecologically relevant measures of tolerance to potentially lethal temperatures. J. Exp. Biol. 214, 3713–3725. ( 10.1242/jeb.061283) [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann AA, Chown SL, Clusella-Trullas S. 2012. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934–949. ( 10.1111/j.1365-2435.2012.02036.x) [DOI] [Google Scholar]
- 42.Kellermann V, Overgaard J, Hoffmann AA, Flojgaard C, Svenning J-C, Loeschcke V. 2012. Upper thermal limits of Drosophila are linked to species distributions and strongly constrained phylogenetically. Proc. Natl Acad. Sci. USA 109, 16 228–16 233. ( 10.1073/pnas.1207553109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jian F, Fields PG, Hargreaves K, Jayas DS, White NDG. 2015. Chill-coma and minimum movement temperatures of stored-product beetles in stored wheat. J. Econ. Entomol. 108, 2471–2478. ( 10.1093/jee/tov196) [DOI] [PubMed] [Google Scholar]
- 44.Huey RB, Crill WD, Kingsolver JG, Weber KE. 1992. A method for rapid measurement of heat or cold resistance of small insects. Funct. Ecol. 6, 489 ( 10.2307/2389288) [DOI] [Google Scholar]
- 45.Kelty JD, Lee RE Jr. 1999. Induction of rapid cold hardening by cooling at ecologically relevant rates in Drosophila melanogaster. J. Insect Physiol. 45, 719–726. ( 10.1016/s0022-1910(99)00040-2) [DOI] [PubMed] [Google Scholar]
- 46.Powell SJ, Bale JS. 2006. Effect of long-term and rapid cold hardening on the cold torpor temperature of an aphid. Physiol. Entomol. 31, 348–352. ( 10.1111/j.1365-3032.2006.00527.x) [DOI] [Google Scholar]
- 47.Kohshima S. 1984. A novel cold-tolerant insect found in a Himalayan glacier. Nature 310, 225–227. ( 10.1038/310225a0) [DOI] [Google Scholar]
- 48.Cossins AR, Bowler K. 1987. Temperature biology of animals. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 49.Elias M, Wieczorek G, Rosenne S, Tawfik DS. 2014. The universality of enzymatic rate–temperature dependency. Trends Biochem. Sci. 39, 1–7. ( 10.1016/j.tibs.2013.11.001) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and R code for analyses shown in figures, tables and results, including R code for failure rate models are deposited in Monash Figshare repository at: https://doi.org/10.26180/5c467981f3158.