Abstract
Parent sex ratio allocation has consequences for individual fitness, population dynamics, and conservation. Theory predicts that parents should adjust offspring sex ratio when the fitness returns of producing male or female offspring varies. Previous studies have assumed that only mothers are capable of biasing offspring sex ratios, but have neglected fathers, given the expectation of an equal proportion of X- and Y-chromosome-bearing (CBS) sperm in ejaculates due to sex chromosome segregation at meiosis. This assumption has been recently refuted and both paternal fertility and paternal genetic quality have been shown to bias sex ratios. Here, we simultaneously test the relative contribution of paternal, maternal, and individual genetic quality, as measured by inbreeding, on the probability of being born a son or a daughter, using pedigree and lifelong offspring sex ratio data for the eastern bongo (Tragelaphus eurycerus isaaci). Our models showed first, that surprisingly, as individual inbreeding decreases the probability of being born male increases, second, that paternal genetic effects on sex ratio were stronger than maternal genetic effects (which were absent). Furthermore, paternal effects were opposite in sign to those predicted; father inbreeding increases the probability of having sons. Previous paternal effects have been interpreted as adaptive due to sex-specific inbreeding depression for reproductive traits. We argue that in the eastern bongo, the opposite sign of the paternal effect on sex ratios results from a reversed sex-specific inbreeding depression pattern (present for female but not male reproductive traits). We anticipate that this research will help stimulate research on evolutionary constraints to sex ratios. Finally, the results open a new avenue of research to predict sex ratio allocation in an applied conservation context. Future models of sex ratio allocation should also include the predicted inbreeding level of the offspring and paternal inbreeding levels.
Keywords: sex ratio, Trivers and Willard hypothesis, genetic quality, male effects, sex allocation, Tragelaphus eurycerus isaaci
1. Introduction
Understanding the drivers of sex ratio variation has been a central question in evolutionary biology [1–3]. Theory poses that sex ratio allocation by parents should maximize parental fitness returns [4]. In species showing large variance in male reproductive success, such as polygynous ungulates, the direction of sex ratio bias is relatively consistent, with mothers in good condition biasing towards sons [5,6] (for a meta-analysis, see [7]), which is often the most costly sex [8,9]. Sons from mothers in good condition can advantageously compete for, and monopolize females, thus deriving fitness benefits for those mothers. By contrast, the relative contribution to sex ratio bias from fathers has remained largely unexplored, [2,10], except for a handful of recent papers [11–15]. This is because in mammalian males, sex chromosome segregation at meiosis is thought to preclude deviations from equal numbers of X-chromosome-bearing sperm (CBS) and Y-CBS. The first paper linking father phenotypic quality and sex ratio deviations in vertebrates, experimentally showed that highly fertile fathers bias towards sons [12]. Differences in X- and Y-CBS numbers can exist before ejaculation [11,16], and recent research on a wild mouse has shown that a non-additive fraction of genetic quality [17] affects offspring sex ratio at birth, with less paternal inbreeding leading to more sons, solely due to a precopulatory sperm trait effect [14]. We also showed, for the first time, the possibility that fathers adaptively control sex ratio bias, rather than being solely driven by a maternal effect over X- and Y-CBS. Thus, fathers, like mothers, can influence sex ratios independently. Here we go one step further, and test, simultaneously, the relative contribution of paternal, maternal, and individual genetic quality on the probability of being born a son or a daughter, using high-quality pedigree data and lifelong offspring sex ratio data from parents of the eastern bongo captive population.
The sex ratio is a key life-history trait that influences population viability through its effects on population dynamics and on the effective population size [18] (for details, see electronic supplementary material). Thus, from a conservation perspective, understanding what drives sex ratio variation is relevant for both in situ and ex situ population management [19,20]. On the one hand, a better assessment of the effects of paternal, maternal, and individual genetics on life-history traits such as sex ratios is fundamental to construct well-parametrized population models applicable to wild populations, and to increase our ability to manage them [21–23]. On the other, understanding the drivers of sex ratios in ungulates can help captive breeding programmes achieve desired ratios for particular breeding populations or reintroduction programmes.
The discovery that the effects of the father on offspring sex ratio can be independent from maternal effects [14] has opened up the question of whether fathers and mothers have divergent (antagonistic), coinciding, or neutral sex allocation interests [10]. This question has important implications for our understanding of the constraints to the evolution of sex ratios and sexual selection [16]. Here, we advance research in this direction by testing the contributions of father and mother quality simultaneously (and individual offspring quality).
The bongo is a species with strong sexual body size dimorphism, partly due to sexual selection [24], and in which males also sport large horns [25]. The species is not territorial [26] and has among the largest group sizes of the Tragelaphus genus [27]. These two features promote competition for mates and have been linked with the intensity of sexual selection [28]. Thus, the biology of the eastern bongo and its conservation status make it an excellent model to test these novel hypotheses with basic (ecological) and applied (conservation) implications.
There are two potential reasons why such a parental sex ratio bias driven by inbreeding can be adaptive. First, inbreeding can itself be heritable [29,30] as well as heterozygosity [31,32]. Secondly, inbreeding may affect other fitness-related traits such as growth, body size, competitive ability, or home range size [33], increasing the probability of inbred males of mating with kin, and of having more inbred offspring than outbred males, which can outcompete competitors, survive for longer to reproduce, and range farther to find females.
The bongo ex situ population offers advantages to test the paternal and maternal genetic quality effects on offspring sex ratio. First, it is a non-domestic ungulate where domestication has not depleted natural variation and hence is suitable to test sex ratio theory. This maximizes the applicability of our results to in situ and ex situ conservation contexts [34–36]. Second, using a population where pairings are controlled allows us to have certainty of paternity, and to account for maternal and paternal effects on sex ratio bias. Third, because variation in food supply between females is minimized, the relative contribution of maternal diet or body condition to sex ratio bias [37] is not expected. This also increases the statistical power to detect maternal and paternal genetic effects. Also, constant environmental conditions minimize the effects of seasonality, density, or social interactions on female sex ratio bias [38,39]. Thus, the probability of local resource competition (local mate competition) and local resource enhancement [40,41] of driving sex ratios [2] is minimized. Lastly, other variables influencing sex ratios, such as age of parents and total offspring number [39,42], were also recorded and accounted for in the statistical models conducted.
Here we tested whether paternal, maternal, and/or offspring genetic quality (a non-additive component [17], as measured by inbreeding [33]), predicted offspring sex ratio at birth. First, we predicted that fathers [12,14] and mothers [43] with higher genetic quality would both be interested in biasing offspring sex ratio towards sons as, according to theory [4], in a dimorphic ungulate, parents would both derive higher benefits. Second, we also predicted in line with previous results showing a paternal genetic effect [14] considering that mothers have phenotypic control over the sex of the offspring and fathers do not, that a father's genetic quality effect would be stronger than that of the mother. We did so accounting for father variation in lifespan, as this might lead to age effects introducing noise on sex ratios as individuals age. Third, because sex-specific post-weaning life trajectories fitness effects would be dependent on offspring quality (independent of maternal or paternal quality), we predicted that individual genetic variation would influence sex at birth. Specifically, low inbreeding would increase the probability of being born male, as higher variance in male reproductive success would make inbreeding impact a male's competitive ability and fitness, more than a female's.
2. Methods
(a). Study species
The eastern bongo, Tragelaphus eurycerus isaaci, is a highly social antelope forming large herds, with average group size in the wild of 13.5 individuals. It reaches sexual maturity approximately 2.5 years after birth. The litter size of this species is one, and the gestation length is nine months (Estes, [27]). Recorded maximum longevity is 19 years (PanTHERIA database) [44].
The International Union for Conservation of Nature (IUCN) lists the eastern bongo as Critically Endangered with a total of 75–140 individuals split between four wild isolated populations in Kenya: Aberdare Mountains, Mount Kenya, the Mau Escarpment, and Mount Eburu [45]. With greater numbers of individuals living in captivity than in the wild, WAZA (the World Association of Zoos and Aquariums) established an international studbook, and the North American AZA (Association of Zoos and Aquariums) and EAZA (European Association of Zoos and Aquaria) developed regionally coordinated captive breeding regions, for the eastern bongo. Currently, there are eight captive breeding regions for this species.
(b). Study populations and generations in captivity
Out of the eight captive breeding regions for the bongo, our team had historical responsibility for managing the European breeding program (EEP). Providing not only complete access to the dataset, but also a fully historical record of the programme management, which was not available for the other regions. We briefly compare below our European population with the other seven breeding regions. There was high variation in the total number of fathers with recorded offspring between regional breeding regions: Africa = 35, North America = 191, Europe = 117, Middle East = 5, East Asia = 6, southeast Asia = 3, Latin America = 3, Australasia = 2, see the electronic supplementary material, figure S1). We present below descriptive analyses for the three largest populations (Africa, Europe, and North America).
(c). Inbreeding and mean kinship
We calculated Wright's coefficient of inbreeding (f) from the pedigree and used it as an absolute measure of paternal and maternal genetic quality, and mean kinship (mk) as an indicator of genetic similarity and uniqueness (higher or lower mk, respectively). mk is defined as the average coefficient of kinship of an individual to every other living non-founder individual in the pedigree [46]. This measure summarizes genetic value [47,48] and breeding programmes use it to retain the highest possible gene diversity through minimizing the mean kinship of each male and female that are paired [48–51]. Interestingly, the EEP presented a paternal level of inbreeding that was closer to the range of values for which effects have been shown in a model mammal species; mice [14,34] (electronic supplementary material, figure S2).
(d). Life-history traits
For the first set of models, 367 fathers were initially considered, born from January to December. Each father sired an average of 7.23 offspring, over a range of 1–43 (see histogram of offspring sired by father in the electronic supplementary material, figure S3).
(i). Sex ratio
Secondary sex ratio (at birth) was calculated for fathers that sired at least 1 offspring (n = 367), of which 10.35% (n = 38) lost at least one offspring before weaning. The average number of dead offspring for those fathers that lost at least one offspring was 1.92 individuals (s.d. = 2.58).
(ii). Father longevity (years)
This is a fixed value per father, the total number of years from birth to death. It was included in the first model testing the drivers of total lifetime sex ratios and reproductive success. Fathers' lifespan was on average 9.03 years (s.d. = 3.084), with a range of 3–16.
(iii). Effects of breeding programme on variables of interest
There were no differences in mean sex ratio and number of offspring between European, North American, and African populations (ANOVA, F2,335 = 0.24, p = 0.77; F2,340 = 0.16, p = 0.84, respectively). There was, however, a nearly significant difference in longevity between regions (ANOVA, F2,338 = 2.78, p = 0.063; Africa, 10.03 ± 4.48, mean ± s.d., n = 33; North America, 8.48 ± 3.84, n = 191; Europe, 9.03 ± 3.07, n = 117). Pairwise tests showed longevity differs between Africa and North America (p = 0.039), but not between either of them and Europe (p > 0.14, in both cases).
(e). Data analysis
To test the novel hypotheses of this study, we used individuals from the EEP population. We first tested whether father f (coefficient of inbreeding) and mk (mean kinship) explained variation in the sex ratio of the total number of offspring fathers produced during their lifespan. The sex ratio was the response variable and included the father's f, the total number of offspring that the father sired throughout his lifetime, father longevity, and the following two-way interactions: number of offspring * father f, number of offspring * longevity, father f * longevity, in the model. In this analyses, each father that had produced offspring represented a single row in the analysis (n = 117). Thus, our model was unable to capture the effect of nuanced measures of paternal quality that vary throughout the lifetime of the individual, such as age or maternal contributions.
Second, we tested whether the probability that an individual would be born a male (1) or a female (0) (sex as response variable), was predicted by its own genetic quality (f), or by the f of the mother or the father (fixed for the whole lifetime of an individual), by the age at conception of each of the parents (we account for ontogeny variation on the quality of the parents throughout their reproductive lives), by the total number of offspring produced over their lifetimes and/or by generation. The correlation structure between the variables included in the analysis was also explored. This second model used all individuals born in the EEP that had captive-born parents as rows (n = 883) (note that in this case each individual born represents a row in the analysis, while in the first set of models only fathers that produced offspring represent a row).
To test whether the effect of male genetic quality varied throughout the reproductive life of an individual, we included father age at conception (how old the male was when he sired a given offspring) as well as its interaction with the father's f (father age at conception * f father). Likewise, we included mother age at conception and its interaction with the mother's f (mother age at conception * f mother). To ascertain whether the effect of individual, paternal, and maternal inbreeding on sex ratios was consistent across generations, the three interaction terms of generation with inbreeding level of the individual, the father and the mother (individual f*gen; father.f*gen; mother.f*gen) were included in the models. Generation was included in the models to control for sex ratio changes driven by a trend of inbreeding accumulation as generations in captivity increase. Means and standard deviations are used to describe the variables included in the models (table 1).
Table 1.
Descriptive statistics for the continuous variables included in the different models conducted for the second set of analyses (rows represent individual offspring) for the EEP. Father f and mother f stand for the coefficient of inbreeding of the father and the mother, respectively. Number of individuals = 883.
| f | father f | mother f | mk father | mk mother | father age conception (days) | mother age conception (days) | father number offspring | mother number offspring | generation | |
|---|---|---|---|---|---|---|---|---|---|---|
| min | 0 | 0 | 0 | 0 | 0 | 582 | 454 | 1 | 1 | 1 |
| max | 0.6694 | 0.4824 | 0.5977 | 0.0846 | 0.0805 | 6994 | 6826 | 30 | 15 | 7.2549 |
| range | 0.6694 | 0.4824 | 0.5977 | 0.0846 | 0.0805 | 6412 | 6372 | 29 | 14 | 6.2549 |
| median | 0.0739 | 0.0391 | 0.0352 | 0.0366 | 0.036 | 2478 | 2295 | 12 | 5 | 4.4375 |
| mean | 0.101 | 0.0760 | 0.0820 | 0.039 | 0.038 | 2653.940 | 2453.965 | 13.249 | 5.579 | 4.228 |
| SE.mean | 0.004 | 0.0033 | 0.0035 | 0.000 | 0.001 | 39.615 | 38.046 | 0.268 | 0.098 | 0.049 |
| CI.mean.0.95 | 0.007 | 0.0065 | 0.0069 | 0.001 | 0.001 | 77.750 | 74.672 | 0.526 | 0.193 | 0.096 |
| s.d. | 0.105 | 0.0986 | 0.1057 | 0.014 | 0.015 | 1177.169 | 1130.558 | 7.970 | 2.921 | 1.447 |
| coef.var | 1.035 | 1.2970 | 1.2897 | 0.352 | 0.393 | 0.444 | 0.461 | 0.602 | 0.524 | 0.342 |
(i). Model specification
We used a mixed effects model (glme, binomial model with logit link function) to include father ID and mother ID as random factors, and then used a corrected Akaike's Information Criterion to assess model support and to select the best model (electronic supplementary material; table S2). Before running the final models, variables were standardized rescaling the numeric parameters. The data were analysed using R software (version 3.3.2, http://www.R-project.org/) and STATISTICA (version 7.0, StatSoft Inc., Tulsa, OK, USA).
3. Results
We first analysed the effects of only lifelong paternal traits on sex ratio deviations. Overall, there were no significant differences in the mean sex ratios between all ex situ regions (electronic supplementary material, figure S1), and differences in the mean f of the offspring (a), father f (b), and mother f (c) (electronic supplementary material, figure S2, in all cases p < 0.0001) between the three regions with the largest sample sizes (Africa n = 35, North America n = 191, and Europe n = 115).
Using the EEP dataset, for which we had greatest historical detail, we then ran two different sets of analyses. This population also presented inbreeding levels closest to those previously observed as responsible for sex ratio deviations [14]. The first set of models, aimed at explaining paternal sex ratios, captured lifelong events and traits (electronic supplementary material; table S1) and used total sex ratio per father, for those that had produced offspring (n = 114), as the rows. Sex ratios (mean = 0.42, s.d. = 0.26) varied significantly across the global model, although there was little explanatory power (F3,111 = 2.98, p = 0.03, variance explained = 7.4%). Three variables were significant: father inbreeding (slope ± s.e. = 1.98 ± 0.76; β = 0.74 ± 0.29; t = 2.57, p = 0.01); father longevity (slope ± s.e. = 0.037 ± 0.013; β = 0.44 ± 0.16; t = 2.72, p = 0.007); and most importantly their interaction (slope ± s.e. = −0.183 ± 0.08; β = −0.664 ± 0.29; t = −2.21, p = 0.029). Revealing that the probability of having sons increased with inbreeding in younger fathers and reversed as fathers grew older, or that fathers with lower inbreeding had more sons as they grew older, while the opposite happened in more inbred fathers.
We then looked at the pairwise associations between paternal, maternal, and individual drivers of sex (figure 1). There were strong positive correlations between the mother and father mk, mother mk and generation, number of offspring of the father and mk father. A negative correlation appeared between number of offspring and generation. Interestingly, there was a negative relationship between f mother and number of offspring but not between father f and number of offspring suggesting that inbreeding depression on reproductive traits was only present in females, but not males.
Figure 1.
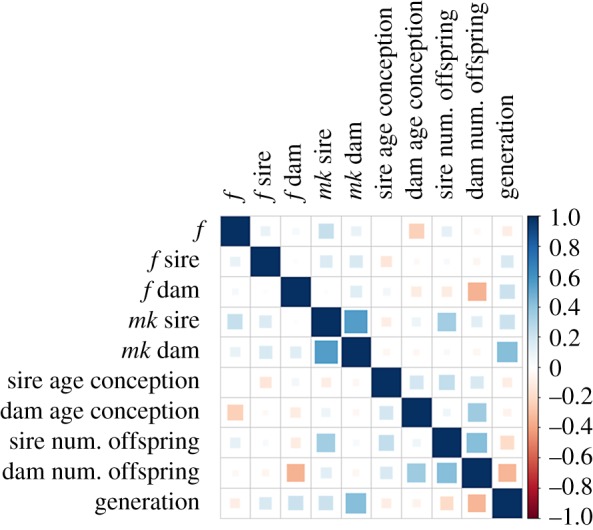
Correlations between genetic and life-history traits in the European breeding programme (EEP). Blue and red colours represent positive and negative correlations, respectively. Colours blue and red indicate the positive or negative sign of the relationship, respectively, colour intensity and the size of the coloured area within cells reflect the strength of the correlation.
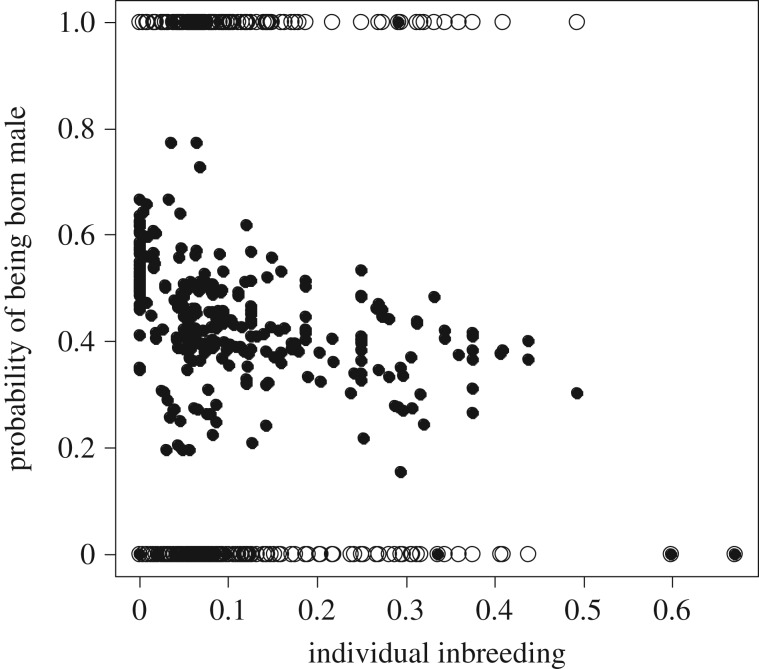
Interestingly, the linear mixed effect model, including father and mother ID as random factors, and scaling the variables, rendered a best-fit model (table 2) that showed a novel result. First, as the individual coefficient of inbreeding (f individual) increased, the probability of being a male decreased (slope ± s.e. = −0.191 ± 0.070, z = −2.707, p = 0.007, table 2 and figure 2). Second, as in the previous set of paternal-only models, father f was positively associated with the probability of producing sons (slope ± s.e. = 0.147 ± 0.069, z = 2.110, p = 0.034; table 2). Contradicting the paternal model, there was no interaction between father f and age at conception. No effect of female genetic quality explained variation in the probability of producing one sex or the other (z = −0.720, p = 0.471; table 2). Finally, as generations in captivity increased the probability of producing a male was also significantly reduced (slope ± s.e. = −0.192 ± 0.075, z = −2.546, p = 0.011; table 2). The same effects were observed when the terms were fitted independently.
Table 2.
Full and minimum adequate (final) general linear models after stepwise deletion of non-significant terms and scaling of numeric parameters, testing for drivers of individual sex ratios. Individual, paternal, and maternal factors are considered. Individual f offspring, father f, and mother f represent the coefficient of inbreeding of the individual, the father, and mother, respectively (n = 883). Father and mother age at conception is measured in days. The following two-way interactions (father.age.conception*father.f; mother.age.conception*mother.f; individual f*gen; father.f*gen; mother.f*gen) were included and tested for significance in the full and minimum adequate models, but none had a significant effect on sex at birth (p > 0.5 in all cases). Full model: null deviance: 1.213 on 882df. Residual deviance: 1191 on 870df. AIC: 1217. Final model: residual deviance: 1198 on 878df. AIC: 1207.
| estimate | s.e. | z | p | |
|---|---|---|---|---|
| full model term | ||||
| intercept | −0.250 | 0.069 | −3.587 | <0.001 |
| f individual | −0.1806 | 0.074 | −2.430 | 0.015 |
| f father | 0.138 | 0.071 | 1.938 | 0.052 |
| f mother | −0.054 | 0.074 | −0.720 | 0.471 |
| generation | −0.192 | 0.075 | −2.546 | 0.011 |
| father age at conception | −0.010 | 0.075 | −0.139 | 0.889 |
| mother age at conception | 0.076 | 0.078 | 0.969 | 0.332 |
| father number offspring | 0.041 | 0.079 | 0.517 | 0.605 |
| mother number offspring | −0.106 | 0.088 | −1.199 | 0.230 |
| final model term | ||||
| intercept | −0.224 | 0.068 | −3.284 | 0.001 |
| f individual | −0.191 | 0.070 | −2.707 | 0.007 |
| f father | 0.147 | 0.069 | 2.110 | 0.034 |
| generation | −0.183 | 0.070 | −2.623 | 0.009 |
Figure 2.
Effect of individual level of inbreeding on the probability of being born a male. A total of 883 bongos from the European breeding programme (EEP) were used for this analysis.
4. Discussion
Here we have tested the effects of paternal, maternal, and individual genetic quality on sex ratio bias in the eastern bongo, a species with strong sexual size dimorphism in which high-quality individuals are predicted to benefit more (increase their marginal fitness benefit) from being males as compared to being females. In contrast to the prevailing view in vertebrates (and specifically ungulates) that only females can bias offspring sex ratios [7], we did not detect maternal genetic effects on offspring sex ratios when testing both paternal and maternal genetic effects simultaneously. However, and in agreement with [14] we did detect a paternal genetic effect. We here also tested and detected for the first time, a relationship between individual quality and sex identity, as shown by the negative association between individual inbreeding and the probability of being born a male. As predicted, individuals with higher levels of inbreeding have a higher probability of being females than males. One possibility is that genetic variation determines sex, which is hard to envision. A second, more plausible explanation is that at some point between ejaculation and birth [10], Y-CBS sperm or male embryos of higher inbreeding have a lower probability of surviving up to birth than female embryos. Reduced food availability tends to negatively affect male embryo survival more than female survival [37], suggesting an increased susceptibility of male embryos. This susceptibility could also manifest differentially more in male embryos in the case of inbreeding.
The effect of fixed traits of parents on offspring should be larger in fathers as they mainly influence offspring genetically, while mothers can influence offspring phenotypically through gestation too. In agreement with this view, we did not find a significant effect of female inbreeding on sex ratios while we did detect a father effect. Previous results using lifelong measures of offspring sex ratio and genetic quality have allowed detection of paternal effects on sex ratios [14]. It has been recently argued that paternal inbreeding could lead to increased costs of producing Y-CBS (leading to sons) which could suffer a higher survival cost than X-CBS (leading to daughters). This could allow for adaptive manipulation of X versus Y sperm ratios. One possibility for this to happen is that genetic quality is heritable. In highly structured populations heritability of inbreeding [29,30] and heterozygosity [31,32] is expected, and we speculate this could be the case for wild bongo populations. However, under captive breeding programmes such as EEP or SSP, this structure might also dilute throughout time, leading to no expectation of heritability of inbreeding. Independently of the evidence of heritability of inbreeding other factors may also be at play. For instance, less inbred fathers have an overall higher probability of transmitting dominant advantageous alleles to their offspring than more inbred fathers. This is because the probability of having mildly deleterious alleles in homozygosis increases with inbreeding within and across loci [52]. Therefore, expectedly, there is a net fitness benefit for being less inbred (being more outbred, and having lower genetic loads), independently of the female that the son reproduces with on the next generation, and the father can be expected to contribute to the fitness of grand offspring in an adaptive manner.
Interestingly, in this study, after testing the genetic effect of the father, mother, and offspring on sex at birth we show that the strongest association detected occurred between the coefficient of inbreeding of the newborn and its sex, suggesting a new possibility hitherto not contemplated, i.e. that it is not only the independent qualities of the father or the mother that influence the sex ratio of the offspring, but the quality of the offspring (a property of the gene interactions between the parents) that influences the sex of the offspring. To our knowledge, this is the first report showing that the genetic quality of an individual significantly influences its own sex. In the eastern bongo, individuals that are conceived from matings of genetically dissimilar individuals, i.e. newborns with lower inbreeding levels, have a higher probability of being males, and more inbred individuals a higher probability of being females. This result suggests a parental (maternal or paternal) adjustment of the sex ratio of the offspring based, not only on their own genetic quality levels (on the expectation of consistent association between parental and offspring inbreeding levels), but on the predicted quality of the offspring. This adaptive assessment of future offspring quality by parents before mating is a condition for sex allocation strategies to evolve. The physiological and behavioural mechanisms that males use to avoid inbreeding have also been described [53–58]. Thus, given the likely fitness implications, mechanisms promoting outbred offspring through mating decisions, as well as offspring sex allocation mechanisms to future zygotes of differing genetic quality, should have evolved in mammalian parents. To anticipate heterosis or genetic dissimilarity (lower inbreeding) of future offspring, and adaptively bias offspring sex ratio, mates could use phenotypic clues to evaluate their dissimilarity, but there should exist a positive correlation between phenotypic and genetic dissimilarity [59].
It has been known for a long time that mothers can bias sex ratio [4,7,8,60]. Recent research and the present study have shown that fathers can also affect secondary sex ratios independently of maternal effects [11–14,61]. Two explanations are possible, either an ability of parents to make those strategic decisions (the adaptive explanation), or a higher susceptibility of male sperm or male embryos to inbreeding. Regarding susceptibility of male embryos to inbreeding there is evidence of preferential culling in utero of frail male fetuses in human populations [62,63].
As shown by the present study and another [14], the effect of the genetic quality of the father has a larger effect on sex ratio than that of the mother. It does seem reasonable that mothers, who can phenotypically influence the sex and the quality of the offspring [64] and are sensible to temporal variation in body condition [65] and environmental factors (benign versus stressful), would be less reliant on fixed characteristics, such as genetic quality, to make the sex allocation decisions to maximize its fitness than fathers. On the contrary, fathers of species that only provide sperm, and that only contribute to offspring quality genetically, but not phenotypically, should make their sex allocation decisions based on the assessment of their own quality. If this response is adaptive, the lack of a negative association (and presence of a positive one) between father f and sex ratio may be due to a lack of negative effect of paternal inbreeding on fertility traits [34,66,67], such as number of offspring produced during a lifetime (surrogate of fertility when there is no between-male variation in mating opportunities). This apparent absence of inbreeding depression for male reproductive traits in the eastern bongo would not provide a fitness advantage for those more outbred fathers biasing to sons, but to those shifting the sex ratio to daughters, in this case more sensitive reproductively to the negative effects of inbreeding. The possibility that purging of deleterious alleles with reproductive effects in males has occurred throughout the generations in captivity cannot be completely ruled out either.
Purging in captive breeding programmes [68,69], such as in Cuvier gazelles [36], has been shown to reverse the effects of inbreeding on viability traits such as juvenile survival. In our study, the evidence that inbreeding depression for reproductive traits is observed in fathers, might explain the absence of the negative effect of inbreeding on sex ratios.
Finally, two conservation considerations follow. First, male ungulates are generally more costly for captive breeding programmes than females because they are more aggressive, and do not produce offspring. Thus, in those species where available space in captivity is limited, managers are interested in predicting offspring sex at birth to reduce the production of males and reduce the need for ethical culling of males. Previous studies have not identified a male trait that could be used to predict the sign of sex ratio change. These results show that the coefficient of inbreeding of the expected offspring can be used to predict the probability of having a male. The magnitude of the effects observed, around 5–10% of the explained variance, seem strong enough to consider the use of inbreeding to predict sex ratio allocation.
Second, the results can also be relevant for small closed natural or managed populations. A small closed population (e.g. the bongo EEP) where mean inbreeding increases over time, is likely to become female biased. If this continues over generations, a handful of males may end up dominating breeding, thereby accelerating inbreeding accumulation. Over the generations, this may lead to inbreeding depression, loss of genetic diversity (but see [70]), and population extinction. This could be offset by bio-banking male gametes from the extant generation and using them to mitigate inbreeding depression (and sex ratio biases) in future generations, or in genetic exchange with other populations.
Supplementary Material
Acknowledgements
We thank the international studbook keeper, Lydia Frazier Bosley of Oregon Zoo, and the EEP coordinator, Nick Davis of the North of England Zoological Society, for their permission to use data from the international studbook database in this research and Paco Garcia-Gonzalez and Tim Woodfine for comments on a previous draft. A.F.M. thanks St Hilda's College, University of Oxford, for constant support.
Data accessibility
Data and code associated with this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.j64t6q9 [71].
Authors' contributions
A.F.M. conceived the project, analysed the data, and wrote the manuscript and the supplementary materials section with contributions from all authors. T.G. and P.R. contributed the data and insights into the dataset and the study system. All authors contributed valuable discussions and relevant edits to the manuscript.
Competing interests
We declare we have no competing interests.
Funding
Financial support for this work was provided by Marwell Wildlife. A.F.M. was also supported by the Ramon y Cajal program (RYC-2016-21114, MINECO).
References
- 1.Williams GC. 1966. Adaptation and natural selection: a critique of some current evolutionary thought. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.West SA. 2009. Sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 3.Charnov E.L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Trivers RL, Willard DE. 1973. Natural-selection of parental ability to vary sex-ratio of offspring. Science 179, 90–92. ( 10.1126/science.179.4068.90) [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock T, Albon SD, Guinness F. 1984. Maternal dominance, breeding success and birth sex ratios in red deer. Nature 308, 358–360. ( 10.1038/308358a0) [DOI] [Google Scholar]
- 6.Clutton-Brock TH, Albon SD, Guinness FE. 1986. Great expectations: dominance, breeding success and offspring sex ratios in red deer. Anim. Behav. 34, 460–471. ( 10.1016/S0003-3472(86)80115-4) [DOI] [Google Scholar]
- 7.Sheldon BC, West SA. 2004. Maternal dominance, maternal condition, and offspring sex ratio in ungulate mammals. Am. Nat. 163, 40–54. ( 10.1086/381003) [DOI] [PubMed] [Google Scholar]
- 8.Clutton-Brock TH, Iason GR. 1986. Sex ratio variation in mammals. Quart. Rev. Biol. 61, 339–374. ( 10.1086/415033) [DOI] [PubMed] [Google Scholar]
- 9.Lindstrom J, Coulson T, Kruuk L, Forchhammer MC, Coltman DW, Clutton-Brock T. 2002. Sex-ratio variation in Soay sheep. Behav. Ecol. Sociobiol. 53, 25–30. ( 10.1007/s00265-002-0545-4) [DOI] [Google Scholar]
- 10.Edwards AM, Cameron EZ. 2014. Forgotten fathers: paternal influences on mammalian sex allocation. Trends Ecol. Evol. 29, 158–164. ( 10.1016/j.tree.2013.12.003) [DOI] [PubMed] [Google Scholar]
- 11.Saragusty J, Hermes R, Hofer H, Bouts T, Goritz F, Hildebrandt TB. 2012. Male pygmy hippopotamus influence offspring sex ratio. Nat. Commun. 3, 697 ( 10.1038/ncomms1700). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomendio M, Malo AF, Soler AJ, Fernandez-Santos MR, Esteso MC, Garcia AJ, Roldan ERS, Garde J. 2006. Male fertility and sex ratio at birth in red deer. Science 314, 1445–1447. ( 10.1126/science.1133064) [DOI] [PubMed] [Google Scholar]
- 13.Douhard M, Festa-Bianchet M, Coltman DW, Pelletier F. 2016. Paternal reproductive success drives sex allocation in a wild mammal. Evolution 70, 358–368. ( 10.1111/evo.12860) [DOI] [PubMed] [Google Scholar]
- 14.Malo AF, Martinez-Pastor F, Garcia-Gonzalez F, Garde JJ, Ballou JD, Lacy RC. 2017. A father effect explains sex-ratio bias. Proc. R. Soc. B 284, 20171159 ( 10.1098/rspb.2017.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vanthournout B, Busck M, Bechsgaard J, Hendrickx F, Schramm A, Bilde T. 2018. Male spiders control offspring sex ratio through greater production of female determining sperm. Proc. R. Soc. B 285, 20172287 ( 10.1098/rspb.2017.2887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards AM, Cameron EZ, Pereira JC, Ferguson-Smith MA. 2016. Paternal sex allocation: how variable is the sperm sex ratio? J. Zool. 299, 37–41. ( 10.1111/jzo.12317) [DOI] [Google Scholar]
- 17.Neff BD, Pitcher TE. 2005. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 14, 19–38. ( 10.1111/j.1365-294X.2004.02395.x) [DOI] [PubMed] [Google Scholar]
- 18.Legendre S. 2004. Age structure, mating system, and population viability. In Evolutionary conservation biology (ed. Ferrière RDUCD.), pp. 41–58, Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Wedekind C. 2012. Managing population sex ratios in conservation practice: how and why? In Topics in conservation biology (ed. Povilitis T.). London, UK: InTechOpen. [Google Scholar]
- 20.Lambertucci SA, Carrete M, Speziale KL, Hiraldo F, Donazar JA. 2013. Population sex ratios: another consideration in the reintroduction - reinforcement debate? PLoS ONE 8, ARTN e7582 ( 10.1371/journal.pone.0075821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballou JD, Traylor-Holzer K, Turner A, Malo AF, Powell D, Maldonado J, Eggert L. 2008. Simulation model for contraceptive management of the Assateague Island feral horse population using individual-based data. Wildl. Res. 35, 502–512. ( 10.1071/WR07124) [DOI] [Google Scholar]
- 22.Eggert LS, Powell DM, Ballou JD, Malo AF, Turner A, Kumer J, Zimmerman C, Fleischer RC, Maldonado JE. 2010. Pedigrees and the study of the wild horse population of Assateague Island national seashore. J. Wildl. Manag. 74, 963–973. ( 10.2193/2009-231) [DOI] [Google Scholar]
- 23.Pozo R, Schindler S, Cusack JSC, Coulson T, Malo AF. 2016. Modelling the impact of selective harvesting on red deer. J. Wildl. Manag. 80, 978–989. ( 10.1002/jwmg.21089) [DOI] [Google Scholar]
- 24.Darwin C. 1871. Secondary sexual characters of mammals. In The descent of man. London, UK: Murray. [Google Scholar]
- 25.Emlen DJ. 2008. The evolution of animal weapons. Annu. Rev. Ecol. Evol. Syst. 39, 387–413. ( 10.1146/annurev.ecolsys.39.110707.173502) [DOI] [Google Scholar]
- 26.Kingdon J. 1988. East African mammals: an atlas of evolution in Africa. p. 404 Chicago, IL: University of Chicago Press. [Google Scholar]
- 27.Estes RD. 1991. The behavior guide to African mammals. South Africa: Russel Friedman Books. [Google Scholar]
- 28.Bro-Jorgensen J. 2007. The intensity of sexual selection predicts weapon size in male bovids. Evolution 61, 1316–1326. ( 10.1111/j.1558-5646.2007.00111.x) [DOI] [PubMed] [Google Scholar]
- 29.Reid JM, Arcese P, Keller LF. 2006. Intrinsic parent-offspring correlation in inbreeding level in a song sparrow (Melospiza melodia) population open to immigration. Am. Nat. 168, 1–13. ( 10.1086/504852) [DOI] [PubMed] [Google Scholar]
- 30.Nietlisbach P, Keller LF, Postma E. 2016. Genetic variance components and heritability of multiallelic heterozygosity under inbreeding. Heredity 116, 1–11. ( 10.1038/hdy.2015.59) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitton JB, Schuster WSF, Cothran EG, Defries JC. 1993. Correlation between the individual heterozygosity of parents and their offspring. Heredity 71, 59–63. ( 10.1038/hdy.1993.107) [DOI] [PubMed] [Google Scholar]
- 32.Thonhauser KE, Thoss M, Musolf K, Klaus T, Penn DJ. 2014. Multiple paternity in wild house mice (Mus musculus musculus): effects on offspring genetic diversity and body mass. Ecol. Evol. 4, 200–209. ( 10.1002/ece3.920) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. ( 10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 34.Malo AF, Martinez-Pastor F, Alaks G, Dubach J, Lacy RC. 2010. Effects of genetic captive-breeding protocols on sperm quality and fertility in the white-footed mouse. Biol. Reprod. 83, 540–548. ( 10.1095/biolreprod.110.085316) [DOI] [PubMed] [Google Scholar]
- 35.Cassinello J. 2005. Inbreeding depression on reproductive performance and survival in captive gazelles of great conservation value. Biol. Conserv. 122, 453–464. ( 10.1016/j.biocon.2004.09.006) [DOI] [Google Scholar]
- 36.Moreno E, Perez-Gonzalez J, Carranza J, Moya-Larano J. 2015. Better fitness in captive Cuvier's gazelle despite inbreeding increase: evidence of purging? PLoS ONE 10, ARTN e0145111 ( 10.1371/journal.pone.0145111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McClure PA. 1981. Sex-biased litter reduction in food-restricted wood rats (Neotoma floridana). Science 211, 1058–1060. ( 10.1126/science.211.4486.1058) [DOI] [PubMed] [Google Scholar]
- 38.Lambin X. 1994. Sex-ratio variation in relation to female philopatry in Townsend voles. J. Anim. Ecol. 63, 945–953. ( 10.2307/5271) [DOI] [Google Scholar]
- 39.Kruuk LEB, Clutton-Brock TH, Albon SD, Pemberton JM, Guinness FE. 1999. Population density affects sex ratio variation in red deer. Nature 399, 459–461. ( 10.1038/20917) [DOI] [PubMed] [Google Scholar]
- 40.Lambin X. 1994. Natal philopatry, competition for resources, and inbreeding avoidance in Townsend's voles (Microtus townsendii). Ecology 75, 224–235. ( 10.2307/1939396) [DOI] [Google Scholar]
- 41.Armitage KB. 1987. Do female yellow-bellied marmots adjust the sex-ratios of their offspring. Am. Nat. 129, 501–519. ( 10.1086/284654) [DOI] [Google Scholar]
- 42.Rosenfeld CS, Roberts RM. 2004. Maternal diet and other factors affecting offspring sex ratio: a review. Biol. Reprod. 71, 1063–1070. ( 10.1095/biolreprod.104.030890) [DOI] [PubMed] [Google Scholar]
- 43.Moreno E, Ibanez MB, Barbosa A. 2011. Mother traits and offpring sex in two threatened gazelle species in captivity. J. Nat. Conserv. 19, 148–153. ( 10.1016/j.jnc.2010.10.004) [DOI] [Google Scholar]
- 44.Jones KE, et al. 2009. PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90, 2648 ( 10.1890/08-1494.1) [DOI] [Google Scholar]
- 45.Group ISAS. 2008. Tragelaphus eurycerus ssp. isaaci. In The IUCN Red List of Threatened Species 2008: eT22057A9354511
- 46.Ballou JD. 1991. Management of genetic variation in captive populations. The Unity of Evolutionary Biology, Proceedings 4th International Congress of Systematic and Evolutionary Biology (1990 : University of Maryland). Portland, OR: Dioscorides Press. [Google Scholar]
- 47.Lacy RC. 1994. Managing genetic diversity in captive populations of animals. Restoration of Endangered Species (eds ML Bowles and CJ), pp. 63–89. Whelan Cambridge, UK: Cambridge University Press. [Google Scholar]
- 48.Ballou JD, Lacy RC. 1995. Identifying genetically important individuals for management of genetic variation in pedigreed populations. In Population management for survival and recovery: analytical methods and strategies in small population conservation (eds Ballou JD, Gilpin M, Foose TJ), pp. 76–111. New York, NY: Columbia University Press. [Google Scholar]
- 49.Fernandez J, Toro MA. 1999. The use of mathematical programming to control inbreeding in selection schemes. J. Anim. Breed. Genet. 116, 447–466. ( 10.1046/j.1439-0388.1999.00196.x) [DOI] [Google Scholar]
- 50.Fernandez J, Toro MA, Caballero A. 2004. Managing individuals' contributions to maximize the allelic diversity maintained in small, conserved populations. Conserv. Biol. 18, 1358–1367. ( 10.1111/j.1523-1739.2004.00341.x) [DOI] [Google Scholar]
- 51.Sonesson AK, Meuwissen THE. 2001. Minimization of rate of inbreeding for small populations with overlapping generations. Genet. Res. 77, 285–292. ( 10.1017/S0016672301005079) [DOI] [PubMed] [Google Scholar]
- 52.Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annu. Rev. Ecol. Syst. 18, 237–268. ( 10.1146/annurev.es.18.110187.001321) [DOI] [Google Scholar]
- 53.Isles AR, Baum MJ, Ma D, Szeto A, Keverne EB, Allen ND. 2002. A possible role for imprinted genes in inbreeding avoidance and dispersal from the natal area in mice. Proc. R. Soc. Lond. B 269, 665–670. ( 10.1098/rspb.2001.1911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keller L, Fournier D. 2002. Lack of inbreeding avoidance in the Argentine ant Linepithema humile. Behav. Ecol. 13, 28–31. ( 10.1093/beheco/13.1.28) [DOI] [Google Scholar]
- 55.Packer C. 1979. Inter-troop transfer and inbreeding avoidance in Papio anubis. Anim. Behav. 27(PART 1), 1–36. ( 10.1016/0003-3472(79)90126-X) [DOI] [Google Scholar]
- 56.Pusey A, Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. ( 10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 57.Sherborne AL, Thom MD, Paterson S, Jury F, Ollier WER, Stockley P, Beynon RJ, Hurst JL. 2007. The genetic basis of inbreeding avoidance in house mice. Curr. Biol. 17, 2061–2066. ( 10.1016/j.cub.2007.10.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolff JO, Lundy KI, Baccus R. 1988. Dispersal, inbreeding avoidance and reproductive success in white-footed mice. Anim. Behav. 36, 456–465. ( 10.1016/S0003-3472(88)80016-2) [DOI] [Google Scholar]
- 59.Ellers J, Rog S, Braam C, Berg MP. 2011. Genotypic richness and phenotypic dissimilarity enhance population performance. Ecology 92, 1605–1615. ( 10.1890/10-2082.1) [DOI] [PubMed] [Google Scholar]
- 60.Gomendio M, Clutton-Brock TH, Albon SD, Guinness FE, Simpson MJ. 1990. Mammalian sex ratios and variation in costs of rearing sons and daughters. Nature 343, 261–263. ( 10.1038/343261a0) [DOI] [PubMed] [Google Scholar]
- 61.Gomendio M, Malo AF, Soler AJ, Garde J, Roldan ERS. 2007. Testosterone and male fertility in red deer - Response. Science 316, 981–982. ( 10.1126/science.316.5827.981) [DOI] [PubMed] [Google Scholar]
- 62.Orzack SH, Stubblefield JW, Akmaev VR, Colls P, Munne S, Scholl T, Steinsaltz D, Zuckerman JE. 2015. The human sex ratio from conception to birth. Proc. Natl Acad. Sci. USA 112, E2102–E2111. ( 10.1073/pnas.1416546112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Catalano R, Bruckner T, Marks AR, Eskenazi B. 2006. Exogenous shocks to the human sex ratio: the case of September 11, 2001 in New York City. Hum. Reprod. 21, 3127–3131. ( 10.1093/humrep/del283) [DOI] [PubMed] [Google Scholar]
- 64.Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R. 1999. Experimental demonstration that offspring sex ratio varies with maternal condition. Proc. Natl Acad. Sci. USA 96, 570–573. ( 10.1073/pnas.96.2.570) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rubenstein DR. 2007. Temporal but not spatial environmental variation drives adaptive offspring sex allocation in a plural cooperative breeder. Am. Nat. 170, 155–165. ( 10.1086/518671) [DOI] [PubMed] [Google Scholar]
- 66.Saccheri IJ, Lloyd HD, Helyar SJ, Brakefield PM. 2005. Inbreeding uncovers fundamental differences in the genetic load affecting male and female fertility in a butterfly. Proc. R. Soc. B 272, 39–46. ( 10.1098/rspb.2004.2903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Losdat S, Chang SM, Reid JM. 2014. Inbreeding depression in male gametic performance. J. Evol. Biol. 27, 992–1011. ( 10.1111/jeb.12403) [DOI] [PubMed] [Google Scholar]
- 68.Boakes EH, Wang J, Amos W. 2007. An investigation of inbreeding depression and purging in captive pedigreed populations. Heredity 98, 172–182. ( 10.1038/sj.hdy.6800923) [DOI] [PubMed] [Google Scholar]
- 69.Crnokrak P, Barrett SCH. 2002. Perspective: purging the genetic load: a review of the experimental evidence. Evolution 56, 2347–2358. ( 10.1111/j.0014-3820.2002.tb00160.x) [DOI] [PubMed] [Google Scholar]
- 70.Lacy RC, Malo AF, Alaks G. 2018. Maintenance of genetic variation in quantitative traits of a woodland rodent during generations of captive breeding. Conserv. Genet. 19, 789–802. ( 10.1007/s10592-018-1054-y) [DOI] [Google Scholar]
- 71.Malo AF, Gilbert TC, Riordan P. 2019. Data from: Drivers of sex ratio bias in the eastern bongo: lower inbreeding increases the probability of being born male. Dryad Digital Repository. ( 10.5061/dryad.j64t6q9) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Malo AF, Gilbert TC, Riordan P. 2019. Data from: Drivers of sex ratio bias in the eastern bongo: lower inbreeding increases the probability of being born male. Dryad Digital Repository. ( 10.5061/dryad.j64t6q9) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data and code associated with this article are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.j64t6q9 [71].