Abstract
Many animals capable of deploying chemical defences are reluctant to use them, suggesting that synthesis of toxins imposes a substantial cost. Typically, such costs have been quantified by measuring the elevation in metabolic rate induced by toxin depletion (i.e. during replenishment of toxin stores). More generally, we might expect that toxin depletion will induce shifts in a broad suite of fitness-relevant traits. In cane toads (Rhinella marina), toxic compounds that protect against predators and pathogens are stored in large parotoid (shoulder) glands. We used correlational and experimental approaches in field and laboratory settings to investigate impacts of toxin depletion on growth rate and behaviour in cane toads. In free-ranging toads, larger toxin stores were associated with smaller gonads and livers, suggesting energetic trade-offs between toxin production and both reproduction and energy metabolism. Experimental removal of toxin (by manually squeezing parotoid glands) reduced rates of growth in body mass in both captive and free-ranging toads. Radio tracking demonstrated that de-toxined toads dispersed more slowly than did control toads. Given that toxin stores in cane toads take several months to fully replenish, deploying toxin to repel a predator may impose a substantial cost, explaining why toads use toxin only as a final line of defence.
Keywords: Bufo marinus, parotoid glands, radio telemetry, toxin production, toxin replenishment
1. Introduction
An organism's survival depends on its ability to evade predation, and a range of strategies have evolved to reduce the risk of detection and attack by a predator [1,2]. Chemical defences are common evolutionary adaptations for defence against predators as well as pathogens [2,3]. The production and maintenance of chemical defences, as well as the systems to deliver them, represent an energetic investment that occurs at the expense of allocation to other life-history traits [4–6]. The concept that venoms and toxins represent an expensive and limited resource in animals has led to formulation of ‘optimization hypotheses', which predict that individuals should gauge the use of their chemical defences and apply them judiciously [7–9]. Studies on a range of invertebrates and vertebrates provide broad support for optimization hypotheses [8,10].
Many studies documenting the costs of chemical defence in animals have focused on venomous rather than poisonous species [7,10–15]. The method of toxin delivery is the basis of the distinction between these two groups; venom is typically injected via fangs or spines, whereas poisons are secreted onto skin and delivered to a predator via contact or ingestion [3,16]. Importantly, venoms are often used to subdue prey as well as to deter predators. Thus, the selective forces acting on venom optimization (e.g. rapid replacement to avoid missed foraging opportunities) are likely to differ from those acting on defence optimization. The cutaneous glands of amphibians secrete toxic substances onto their skin, protecting against predators, parasites and microorganisms [1,17–19]. The cutaneous glands of amphibians are typically dispersed across all skin surfaces but sometimes are concentrated, as in the warts and parotoid glands of bufonids [17,20,21].
The parotoid glands of toads store large volumes of toxin, which is secreted onto the skin surface or expressed into a predator's mouth during an attack [21,22]. Parotoid toxin is a complex cocktail of peptides, alkaloids, steroids and biogenic amines [17,20]. The costs incurred in the production of toad toxins have not been measured, but several lines of evidence suggest that these costs are non-trivial. First, once toxin is released from the parotoid glands it takes several months to replenish [21]. Chen & Chen [23] expressed toxin from the glands of a cane toad (Rhinella marina) and 76 days later only 68% had been replenished. Thus, toads are incapable of repeatedly expending large volumes of toxin during predator encounters [24]. Second, if toxin is released through mechanical compression of glands (e.g. from biting or handling by a predator [25]), the replacement of collapsed alveoli may incur tissue repair and immune costs [21]. Finally, toads subject to attack rely on behavioural defences (such as crypsis, flight or threat) before they resort to excreting parotoid toxins [21,26–29]. This reluctance to deplete parotoid stores accords well with the optimization hypotheses [28], suggesting that toad toxin is expensive to produce and hence should not be expended except as a last resort.
Most studies investigating the cost of venom production have focused on energy, by comparing levels of oxygen consumption before and after depletion of venom stores [4,10,13–15,30]. However, increased oxygen consumption may only represent part of the cost of toxin regeneration. Other potential costs could include allocation of limited resources away from other functions [9,10,15,31]. Metabolic costs represent immediate proximate expenses, but other consequences for performance-related traits may be of greater ecological and evolutionary significance [15,32] and traits other than metabolism can affect energy budget dynamics during toxin replenishment.
Here, we investigated impacts of toxin depletion on growth rates and behaviour of cane toads. The study consisted of two parts. First, we dissected free-ranging toads to measure the caloric content of toxin and to assess morphological correlates of toxin volume. Second, we experimentally depleted parotoid toxin from toads (mimicking an encounter with a predator) to initiate the production of replacement toxin. We then compared subsequent growth rates, physical performance, and behaviour between de-toxined and control toads. This manipulative study was conducted in the wild as well as in a laboratory setting. If the production of toxin represents a major energetic expense, we predicted that replenishment of toxin stores would influence toad behaviour, physical performance and energy allocation.
2. Methods
(a). Study species
Cane toads are large bufonid anurans from Central and South America [33]. They were brought to Australia in 1935 as a biocontrol agent of insect pests of sugar cane. Since then, toads have spread thousands of kilometres westwards and southwards across the continent [33]. Like other bufonids, cane toads possess large parotoid glands located on their shoulders which contain defensive toxin (figure 1). Because this toxin is densely sequestered in discrete glands, it can be more easily removed as an experimental manipulation (figure 1) than can toxin in widely distributed skin glands.
Figure 1.
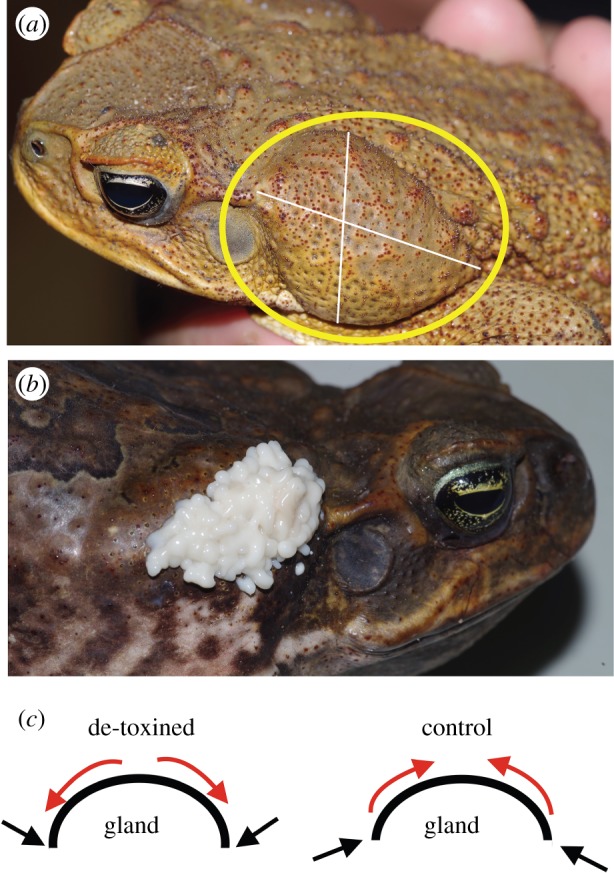
(a) Male cane toad with prominent parotoid gland (circled). White lines indicate approximate locations of length and width measurements. (b) Toxin expressed from the parotoid gland of a female cane toad. (c) Depiction of de-toxin versus control gland squeezing techniques. To squeeze toxin from glands, the fingers apply pressure (straight arrows) in a downward direction at the base of the gland. This outward pressure at the surface of the gland (curved arrows) forces toxin out. To squeeze a gland without removing toxin, pressure is applied in a more upward direction to compress the surface of the gland. (Online version in colour.)
(b). Study site
Our laboratory study took place at the University of Sydney's Tropical Ecology Research Facility at Fogg Dam (12.57°S, 131.30°E) located 60 km southeast of Darwin in Australia's Northern Territory. The area experiences wet–dry tropical seasonality, with 93% of the average annual rain (1400 mm) falling during a six-month wet season (November–April). Temperatures are high year-round, with average maximum temperatures during each month greater than 32°C [34]. Our study occurred during the months of October–November, which correspond to the harshest environmental conditions faced by resident toads [34]. During this hot dry time of year toads congregate at high density around dwindling sources of moisture. Leaning Tree Lagoon (12.71°S, 131.42°E) is one such area, and was the site chosen to collect toads and to conduct the field component of the study. The lagoon is a permanent waterbody and acts as a refuge for many organisms during the dry season. Cane toads arrived in the area in 2005 [34], and at the time of the present study in 2018, had been established for 14 years.
(c). Correlational study
Forty-three adult toads (22 females and 21 males) were collected by hand at night from Leaning Tree Lagoon and placed in damp cloth bags. They were returned to the University of Sydney's Tropical Ecology Research Facility at Fogg Dam and held overnight. The next day, the toads were humanely euthanized by an overdose of pentobarbitone sodium and measured for snout–urostyle length (SUL) and body mass. Sex was determined based on the presence of male secondary characteristics (release call, rugose skin, yellow coloration and nuptial pads). The length and width of each parotoid gland was measured (figure 1), and their toxins were extracted by manually compressing the gland between the thumb and forefinger several times until no more toxin could be extruded (figure 1). Squeezing glands in this manner collapses and empties the internal alveoli of stored toxin [21,35], although we did not examine glands histologically to quantify any toxin remaining after squeezing. Extracted toxin was scraped onto a glass microscope slide and weighed to 0.001 g. We determined the caloric content of toxin from a subsample of 13 toads (see below). Toads were dissected, and the liver, gonads, spleen and fat bodies were extracted, patted dry and weighed to 0.001 g.
(d). Experimental studies
(i). Free-ranging toads
Thirty-six toads were collected at night from the perimeter of Leaning Tree Lagoon, and the GPS coordinates of their locations recorded. They were placed in damp cloth bags and returned to the laboratory where they were kept overnight. The next day each toad was sexed and measured for SUL and mass. To be able to identify toads individually afterwards, we toe-clipped them (a procedure that causes minimal stress or after-effects [36]) and then randomly assigned toads to either control or de-toxined groups. Sixteen of the toads had their parotoid toxin manually expressed (as above). As a control group, 20 toads had their glands squeezed and manipulated for a similar duration, but without expressing any toxin. This control treatment was achieved by applying pressure to push the gland inwards (figure 1) and was performed for the same amount of time that it took to de-toxin a toad (approx. 90 s). Toads in the de-toxined group consisted of six females and 10 males and ranged in body size from 85.7 to 121 mm SUL. The control group contained eight females and 12 males, ranging in size from 83.7 to 120.6 mm. Each toad was then fitted with a bead chain belt bearing a 3 g radio-transmitter (model PD2; Holohil Systems, Ottawa, Canada). Body masses of the 36 radio-tracked toads ranged between 56 and 280 g, so the radio-transmitter and belt represented a burden of less than 6% of body mass. Toads were released back at their point of capture within 24 h. Radio-tagged toads were located daily over the subsequent 5 days and the GPS coordinates (accurate to 3 m) of their diurnal shelter sites were recorded. On each day, we calculated the distance each toad had moved from its original capture location and the distance moved from the previous day's location. After 5 days of radio-tracking, toads were recaptured and returned to the laboratory where they were euthanized, weighed and dissected. The liver, gonads, spleen and fat bodies were extracted, patted dry and weighed to 0.001 g.
Telemetry was conducted in two 5-day bouts, started one week apart. During each bout, 17–19 toads were tracked, consisting of approximately equal numbers of control and de-toxined individuals.
(ii). Captive toads
In early October 2018, 20 subadult toads (60–80 mm SUL) were captured at night from within 5 km of the Tropical Ecology Research Facility. They were individually toe-clipped for future identification and measured for SUL and mass. We then injected each toad subcutaneously with ivermectin at a dose of 0.02 mg/100 g to remove nematode lungworms (which are known to affect toad growth rates [37]). In addition, we orally dosed toads with metranidazole (10 mg per 100 g) to remove potentially pathogenic gastric protists [38]. Toads were then maintained in outdoor enclosures equipped with lights (to attract insects) and water for two weeks prior to the commencement of the experiment.
At the end of October, the 20 toads were collected from the outdoor enclosures, remeasured and randomly assigned to one of two toxin-gland-manipulation groups. Ten toads were de-toxined (as above) and 10 were treated as controls (as above). Toads in the de-toxined group consisted of six females and four males and ranged in body size from 61.2 to 84.9 mm SUL. The control group contained four females and six males, ranging in size from 62.2 to 85.4 mm. The toads were then housed in individual 12 l bins (40 × 30 × 20 cm), equipped with newspaper floor covering, a water dish and small shelter. Cages were held in a shaded building that experienced ambient levels of lighting, temperature and humidity. Each toad was fed five medium-sized, commercially sourced crickets daily for 20 days. After 20 days, we remeasured toads to assess growth and subjected them to physical and behavioural assays (below). We chose these assays to look for differences between de-toxined versus control toads because previous studies indicate they are effective tools to measure differences among groups of toads (e.g. as a function of infection status [37], invasion history [27,39] or immune response [40]).
(iii). Boldness and spontaneous activity
We used protocols described by Finnerty et al. [37] to measure aspects of boldness and spontaneous activity in toads. Toads were placed individually into 70 l arenas (64 × 42 × 30 cm) marked with 10 × 10 cm grid lines on the floor. Each arena held a black 1 l shelter box with a door that could be closed. Toads were placed into the closed shelters and after a 5-min acclimation period, the door was opened and the trial was filmed for 30 min. Four arenas were filmed simultaneously and arenas were sprayed and wiped with 10% ethanol between successive trials to remove scent cues. The following variables were scored from videotapes as measures of boldness and activity: (i) the time elapsed until the toad's head first emerged from the shelter, (ii) the time elapsed until its body fully emerged from the shelter, (iii) the number of grid lines crossed per minute, after emerging from the shelter and (iv) the time it escaped the arena (if applicable). These trials were conducted on the night of day 20 between the hours of 19.30–00.45 h and under ambient weather conditions (31–34.1°C, 52–60% relative humidity).
(iv). Locomotor performance
Running speeds of toads were measured on an indoor raceway (285 × 14.5 × 8.5 cm), the central 240 cm of which was demarked into four 60 cm sections. Toads were encouraged to run along the track by gently tapping their hindquarters. Each toad was raced up the 240 cm central section of the track and then back, and the time taken to traverse each of the eight 60 cm sections was recorded. The fastest time to cover any of the 60 cm sections was used as a measure of sprint speed and the cumulative time taken to traverse all eight 60 cm sections was used as a measure of endurance. If a toad was tapped more than 10 times in a row without response, the trial was terminated. Locomotor trials were conducted between 11.00 and 14.00 h on Day 21 under ambient conditions (31.4–34.5°C, 41–68% relative humidity).
(v). Foraging ability
Foraging success was measured by placing individual toads in 70 l arenas (64 × 42 × 30 cm) and then introducing 10 crickets. The number of crickets eaten within 5 min was used as a measure of foraging ability. These trials were conducted between 14.00 and 16.00 h on day 21 under ambient conditions (31.0–35.5°C, 37–56% relative humidity).
(vi). Dissection
On day 22, toads were euthanized and dissected. Liver, gonads, spleen and fat bodies were patted dry and weighed to 0.001 g.
(vii). Toxin calorimetry
We determined the energy content of toxin samples from 13 of the 43 toads collected for the correlational study (see above). Samples from the left- and right-hand glands of each toad were analysed separately. Briefly, samples were weighed and freeze-dried before duplicate combustion within a semi-micro oxygen vessel (PARR 1109A) and caloric determination in a bomb calorimeter (PARR 6200; John Morris Scientific Pty Limited, Australia). Energy equivalence of vessel and calorimeter (kJ per °C) was determined with benzoic acid (26.44 kJ g−1). We calculated the energy density of toxin samples (expressed as kJ g−1) by dividing the caloric content of each sample by its dry mass.
(e). Statistical analyses
We mainly used multiple regressions to investigate effects of toxin content (summed mass of toxin obtained from left and right glands in the correlational study) or toxin removal (in the experimental study) on morphological, and behavioural traits of toads. Because sex and body size influence many aspects of toad morphology, and behaviour (e.g. [27,39]), these two traits were included as independent variables in all multiple regressions. Residuals from regressions were inspected for violations of assumptions. Data on morphological traits (e.g. organ masses, SUL) were ln-transformed prior to analyses in order to achieve homogeneity of variances. Locomotor performance times (sprint and endurance) were ln-transformed in order to meet assumptions regarding normality.
Radio telemetry data consisted of multiple observations of distances moved for each individual, and the two radio telemetry bouts occurred during consecutive weeks that may have differed subtly in environmental conditions. To accommodate individual and temporal sources of variation into our analysis of telemetry data, we used mixed models, incorporating ID and telemetry bout (week 1 versus week 2) as random effects, and day number, toxin treatment, sex and body size as fixed effects.
We used a non-parametric Kruskal–Wallis test to determine whether toxin energy density varied among the 13 toads. To assess the effects of sex, body size and side (right versus left) on toxin energy density, we used a mixed model multiple regression with these morphological variables as fixed effects and with toad ID as a random effect. We used the Kenward–Rogers method to approximate degrees of freedom in all mixed models and rounded these values to the nearest whole number to present in tables. All analyses were conducted using JMP 13 software (SAS Institute, Cary NC) and significance was accepted at p < 0.05. The highest variance inflation factors from any of the multiple regression models used in our analyses was 1.81, indicating that multicollinearity was not a problem among our sets of independent variables.
3. Results
(a). Correlational study
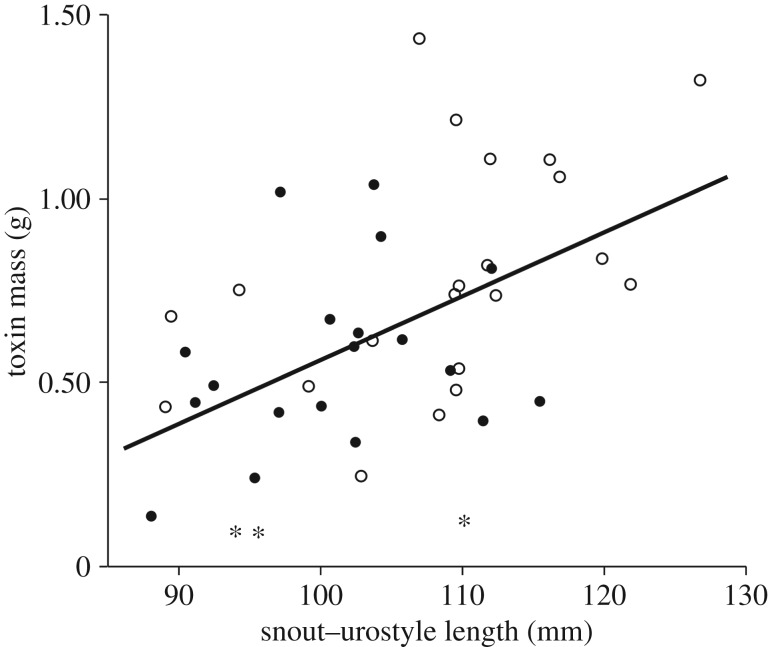
We measured the lengths and widths of 86 parotoid glands from 43 toads in the correlational study. The amount of toxin contained in each gland was positively correlated with both its length (r = 0.62, p < 0.0001) and its width (r = 0.67, p < 0.0001). Multiple regression indicated that larger toads held more toxin in their parotoid glands than did smaller toads (F1,39 = 7.19, p = 0.01; figure 2), but with no significant differences between males and females (F1,39 = 0.91, p = 0.35) and no interaction between sex and body size (F1,39 = 0.75, p = 0.39). Three toads (two males and one female) contained far less toxin than their body size would predict and were identified as outliers (based on Mahalanobis distances, p < 0.05; figure 2). These individuals might have expended toxin during a predator encounter in the recent past. We initially ran analyses on relationships between toxin content and organ masses including these three outliers and ran them again with the three individuals excluded.
Figure 2.
Positive relationship between body size and the mass of toxin contained in parotoid glands of 43 wild-caught cane toads used in the correlational study. Circles represent individual animals (open symbols for females, closed symbols for males), and the line of best fit is in black. Three outliers (asterisks) had unusually low levels of toxin for their size and were excluded from some analyses and from the line of best fit.
(i). Organ masses
Mass of livers, spleens and gonads increased significantly with toad body size, but fat body mass did not (table 1). Males had heavier livers than females and females had heavier gonads than males (table 1). Toads that contained more toxin had smaller gonads (table 1; electronic supplementary material, figure S1), and when three outliers were excluded toads with more toxin also had smaller livers (table 1; electronic supplementary material, figure S2).
Table 1.
Multiple regression results on the effects of sex, body size (SUL) and toxin content on organ masses of wild cane toads from the correlational study. Each regression analysis was carried first on the full dataset of 43 toads, and then re-run with three outliers excluded. Bold type indicates significance at p < 0.05. Shaded cells indicate results with outliers included in analyses.
| organ mass | effect | outliers included |
outliers excluded |
||||
|---|---|---|---|---|---|---|---|
| estimate | F1,39 | p-value | estimate | F1,36 | p-value | ||
| ln liver | intercept | −21.42 | −23.24 | ||||
| sex | −0.17 | 9.34 | 0.0040 | −0.14 | 6.72 | 0.0137 | |
| ln SUL | 4.82 | 54.98 | <0.0001 | 5.20 | 61.22 | <0.0001 | |
| ln toxin weight | −0.14 | 2.64 | 0.1124 | −0.28 | 5.23 | 0.0282 | |
| ln fat | intercept | −14.41 | −13.00 | ||||
| sex | −0.37 | 3.48 | 0.0696 | −0.35 | 2.99 | 0.0923 | |
| ln SUL | 2.63 | 1.24 | 0.2719 | 2.36 | 0.93 | 0.3412 | |
| ln toxin weight | −0.00 | 0.00 | 0.9978 | 0.36 | 0.66 | 0.4203 | |
| ln gonad | intercept | −58.73 | −60.02 | ||||
| sex | 1.28 | 52.41 | <0.0001 | 1.29 | 47.02 | <0.0001 | |
| ln SUL | 12.60 | 35.76 | <0.0001 | 12.87 | 32.08 | <0.0001 | |
| ln toxin weight | −0.69 | 6.37 | 0.0158 | −0.87 | 4.42 | 0.0426 | |
| ln spleen | intercept | −22.62 | −25.13 | ||||
| sex | −0.08 | 0.53 | 0.4697 | −0.05 | 0.17 | 0.6843 | |
| ln SUL | 4.22 | 10.18 | 0.0028 | 4.74 | 11.51 | 0.0017 | |
| ln toxin weight | 0.02 | 0.01 | 0.9253 | −0.17 | 0.46 | 0.5009 | |
(ii). Energy density of toxin
Average energy density of the 26 dried toxin samples from 13 toads was 22.29 kJ g−1 ± 0.18 s.e. (or 10.06 kJ g−1 wet weight). There was no significant difference in toxin energy density among the 13 individuals sampled (Kruskal–Wallis χ2 = 16.08, p = 0.19). Mixed model multiple regression indicated that caloric density of toxin was not significantly influenced by sex (F1,10 = 2.61, p = 0.14), body size (F1,10 = 0.02, p = 0.89), or whether the toxin was obtained from the left- or right-hand gland (F1,10 = 0.23, p = 0.64).
Because energy density of toxin did not differ significantly among the 13 sampled toads, we used the mean of these measurements (10.06 kJ g–1 wet weight) to estimate the total energy content of the wet toxin produced by the remaining 30 toads (whose toxin was weighed but not subjected to calorimetry). Based on the total mass of toxin collected from each individual, total caloric investment in parotoid stores ranged from 0.92 to 14.47 kJ, and increased with SUL (F1,41 = 13.68, p = 0.0006). Larger toads had a higher total caloric investment in toxin because they produced more toxin.
(b). Experimental studies
(i). Free-ranging toads
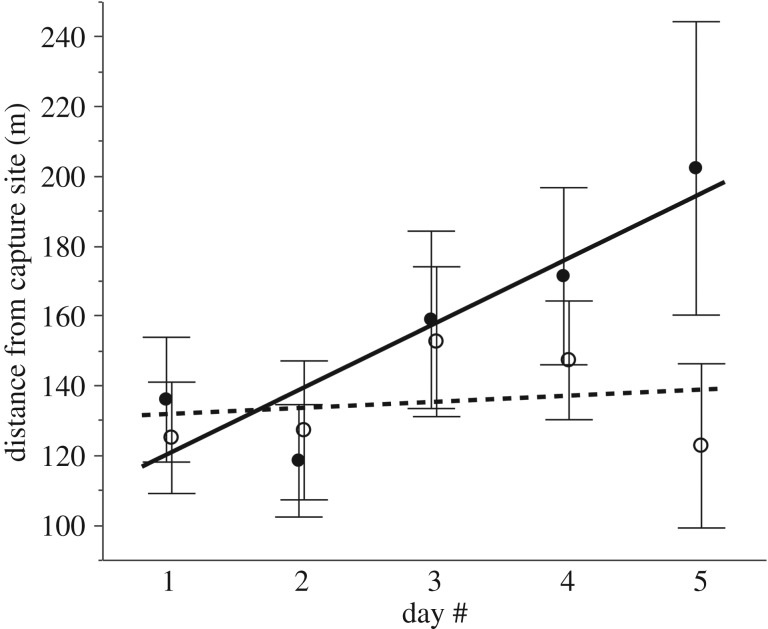
Movements. The distance between successive daytime refugia increased over the 5-day telemetry period but was not affected by toxin removal (table 2). However, toxin removal did affect the rate at which toads dispersed away from their point of capture, as indicated by a significant interaction between treatment and time (F1,142 = 4.21, p = 0.042, table 2). De-toxined toads remained at a relatively constant distance from their point of capture, whereas control toads moved increasingly further away over the 5 days. By the final day of telemetry, control toads had moved an average of 202 m ± 42 s.e. from their starting point, whereas de-toxined toads had only moved 122 m ± 24 s.e. (figure 3).
Table 2.
Mixed model analyses of the effects of toxin removal on movements of cane toads radio tracked over 5 days. Individual ID and telemetry bout were included as random effects in the model and the Kenward–Rogers method was used to approximate d.f. Bold type indicates significance at p < 0.05.
| trait | effect | d.f. | F | p-value |
|---|---|---|---|---|
| distance between diurnal refugia | sex | 1,32 | 0.26 | 0.6142 |
| SUL | 1,31 | 0.21 | 0.6536 | |
| toxin removal | 1,31 | 0.32 | 0.5768 | |
| day no. | 1,106 | 8.39 | 0.0046 | |
| toxin removal × day no. | 1,106 | 0.015 | 0.9039 | |
| distance from capture site | sex | 1,31 | 0.15 | 0.6985 |
| SUL | 1,32 | 0.19 | 0.6683 | |
| toxin removal | 1,31 | 1.22 | 0.2771 | |
| day no. | 1,142 | 7.08 | 0.0087 | |
| toxin removal × day no. | 1,142 | 4.21 | 0.0421 |
Figure 3.
Effects of toxin removal on rates of dispersal of 36 toads radio tracked for 5 days. De-toxined toads (dashed line, open circles) remained at a constant distance from their point of capture but control toads (solid line, filled circles) moved increasingly further away over the 5 days. Values at each time point represent means and bars denote associated standard errors. Lines depict best linear fits.
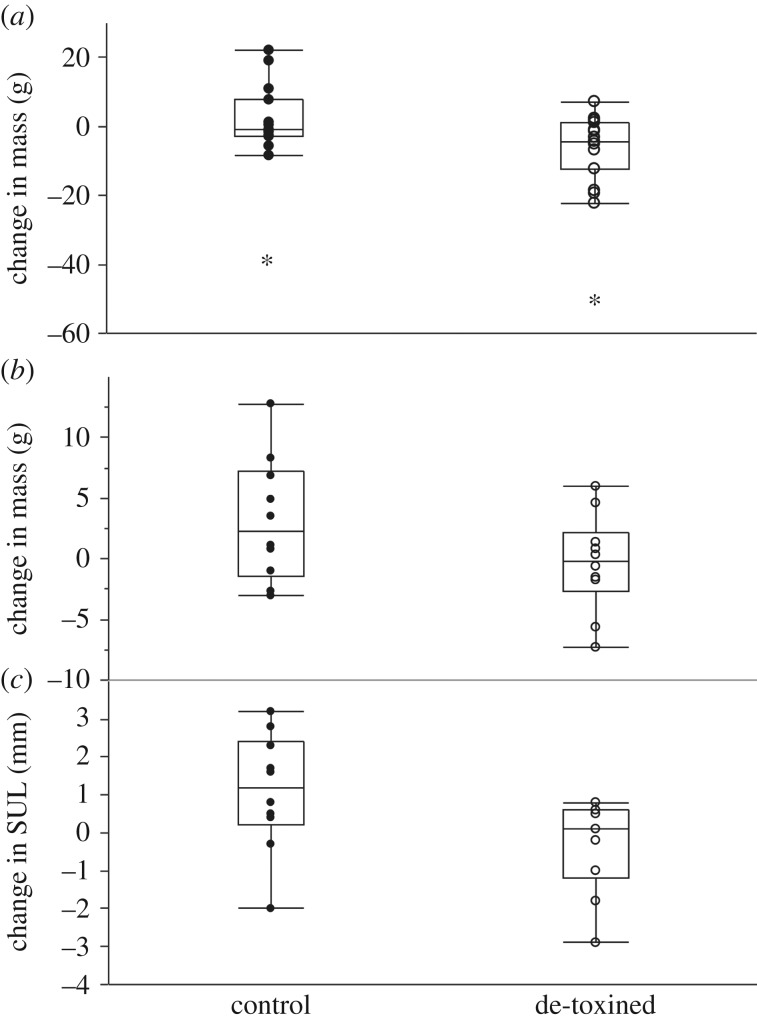
Change in mass. We identified two female toads (one de-toxined and one in the control group) that lost in excess of 35 g over the 5-day telemetry period. Such a rapid decrease in body mass suggests that these females had oviposited, so we excluded them from the analysis on mass change. With these outliers excluded, a multiple regression incorporating sex and SUL as covariates revealed a significant effect of toxin removal on mass change (table 3 and figure 4). On average, control toads gained 2.03 g ± 1.9 s.e. in mass over the 5-day telemetry period whereas de-toxined toads lost an average of 5.79 g ± 2.2 s.e. (excluding the mass of toxin removed). If the two females suspected of ovipositing are retained in the analysis, the significance of the effect of toxin removal on mass growth decreases to p = 0.058.
Table 3.
Multiple regression analyses on the effects of sex, body size and toxin removal on changes in body size of radio tracked and captive cane toads over 5 and 20 days, respectively. Bold type indicates significance at p < 0.05.
| study | trait | effect | d.f. | f | p-value |
|---|---|---|---|---|---|
| radio telemetry | change in mass | sex | 1,30 | 3.62 | 0.0668 |
| SUL | 1,30 | 0.48 | 0.4927 | ||
| toxin removal | 1,30 | 7.49 | 0.0103 | ||
| captive | change in mass | sex | 1,16 | 5.77 | 0.0288 |
| SUL | 1,16 | 0.39 | 0.5387 | ||
| toxin removal | 1,16 | 4.98 | 0.0404 | ||
| change in SUL | sex | 1,16 | 0.83 | 0.3770 | |
| SUL | 1,16 | 0.42 | 0.5275 | ||
| toxin removal | 1,16 | 5.04 | 0.0393 |
Figure 4.
Box plot of the effect of toxin removal on growth of cane toads. Circles represent individual animals. (a) Change in body mass over five days among radio-tracked cane toads. Two female toads (asterisks) that lost excessive amounts of mass (possibly through breeding) were excluded from analysis. (b) Change in mass over 20 days among toads maintained in captivity. (c) Change in SUL over 20 days among toads maintained in captivity.
Organ masses. Multiple regressions (incorporating sex and body size as covariates) on ln-transformed measures did not reveal any significant effects of toxin removal on organ masses of radio-tracked toads (electronic supplementary material, table S1). Predictably, all organ weights were positively related to body size, and females had heavier gonads than did males (electronic supplementary material, table S1). However, males had heavier fat bodies than did females (electronic supplementary material, table S1).
(c). Captive toads
(i). Growth
Toxin removal reduced growth over 20 days in both mass and SUL (table 3 and figure 4). On average, control toads increased mass by 3.2 g ± 1.6 s.e. and de-toxined toads lost 0.4 g ± 1.3 s.e. (excluding the mass of toxin removed). Control toads increased SUL by an average of 1.1 mm ± 0.5 s.e. and de-toxined toads decreased in SUL by 0.3 mm ± 0.4 s.e. The apparent loss of body length among de-toxined toads likely indicates measurement error around zero growth, rather than shrinkage.
(ii). Locomotor and feeding performance
Performance of the 20 captive toads in locomotor and feeding trials was independent of sex, body size and toxin removal (electronic supplementary material, table S2), although males tended (p = 0.05) to have lower sprint times (i.e. higher speeds) than did females.
(iii). Boldness trials
Males emerged both partially and completely from shelters more quickly than did females, were more active after emerging, and escaped from arenas more quickly than females (electronic supplementary material, table S2). Smaller toads completely emerged from shelters more quickly than did larger conspecifics (electronic supplementary material, table S2). De-toxined toads showed marginally (p = 0.07) higher levels of activity after emerging from shelters than did control toads (electronic supplementary material, table S2).
(iv). Organ masses
Among the 20 captive toads, larger individuals had heavier livers and gonads (electronic supplementary material, table S3), and females had larger gonads and spleens than did males (electronic supplementary material, table S3). There was no significant effect of toxin removal on mass of any organ (all p > 0.32; electronic supplementary material, table S3).
4. Discussion
Both our correlational and experimental studies suggest that toxin depletion in cane toads entails energetic trade-offs with liver stores, growth and reproduction. Our correlational study verified several a priori expectations, such as that larger toads carry more toxin than smaller toads, and that larger parotoid glands carry more toxin than smaller ones. We found negative correlations between the volume of toxin carried by toads and the size of their livers and gonads, suggesting energetic trade-offs. Toads that invest more in toxin production may do so at the expense of reproductive investment. The trade-off between toxin production and reproductive investment could arise through competing energy demands. Additionally, the bufodienolide component of toxin is synthesized from the same precursor molecules as are sex hormones [41]. Thus, competition for these precursors could also result in trade-offs between toxin production and reproduction. The liver is a major site of energy storage and biosynthesis in amphibians [42], suggesting that toxin production depletes energy or possibly other substances (e.g. soluble proteins) stored by the liver. Based on patterns of vascularization and density of organelles involved in biosynthesis, the parotoids themselves appear to be the main site of toxin production [20,43].
Concordantly, experimental removal of toxin generated growth deficits in toads, even over a relatively short duration (5–20 days). In the case of radio-tracked toads, the mass loss exhibited by de-toxined individuals could conceivably be due to elevated metabolic demands if synthesis of replacement toxin is initiated rapidly after toxin removal. It is also possible that toxin removal reduces general activity levels, including foraging. Toxin removal affected other measures of activity (i.e. dispersal) among radio-tracked toads. Alternatively, toads that have been stripped of their chemical defence may be more vulnerable to predation, and hence be reluctant to undertake potentially risky forays away from their home site. We did not detect any changes in activity among captive toads following toxin removal, perhaps because the artificiality of laboratory trials masked changes in behaviour that were expressed under natural conditions. In the case of captive toads, the reduced growth of de-toxined toads might be due to an elevation in metabolic rate (because all individuals were provided with similar amounts of food each day). In other taxa, toxin removal typically initiates an increase in metabolic rate as individuals upregulate the biochemical processes required for toxin synthesis [8,10,13–15].
Although toxin content was significantly related to SUL, there was considerable scatter around the line of best fit (figure 2). For instance, at SUL of 110 mm, individuals ranged more than 10-fold in the amount of toxin they possessed, from 0.12 to 1.4 g. These toads were collected from the wild and may have had differing predator-encounter experiences. Conceivably, some of these animals had partially depleted their toxin stores before capture and were in various stages of replenishment. Alternatively, differences in rates of predator encounter among toads could induce them to produce different amounts of toxin. Facultative adjustments of chemical defence in response to predation risk and other environmental cues have been reported in amphibians [9,12,41,44]. Because all toads used in the present study were collected from the same site, differences in external cues are unlikely to have impacted toxin levels.
Without data on the magnitude and duration of metabolic increase during toxin replenishment, it is difficult to place the cost into the context of a toad's energy budget. Using the energy content of an adult Tenebrio beetle as an example (5.8 kJ g−1 [45]), and a digestive conversion efficiency of 73% (for Bufo boreas [46]), it would take approximately 2.4 g (e.g. 24 beetles weighing 0.1 g each) to provide the calories present in 1 g of toxin (10.06 kJ; eight of the 43 toads in the correlational study contained greater than 1 g of toxin). Foraging cane toads have been described as ‘first-rate gluttons’ [47] and contingent upon prey availability, a large toad could likely ingest 24 beetles during a single foraging bout. Examination of cane toad stomach contents often shows very high levels of food intake. For instance, at a site in Panama, Zug & Zug [47] reported an average mass of stomach contents of cane toads of 8.9 g and a maximum of 35.4 g.
Although toads thus could rapidly replace the caloric value of expended toxin, the metabolic costs of toxin replenishment may be much more substantial than can be quantified by caloric units. Toxin replacement requires synthesis of complex compounds, as well as mobilization and catabolism of precursors and secretion of final products into extracellular spaces [10,30]. In addition, the process of toxin replenishment in toads is prolonged. Parotoid glands of R. icterica remain collapsed and empty for up to 105 days following manual compression to remove toxin [21]. Chen & Chen [23] reported expressing 0.71 g of toxin from a 254 g cane toad but 76 days later were only able to express 0.48 g from the same individual. At this replenishment rate (less than 0.89% of toxin volume per day), it would take greater than 112 days for a toad to replace the contents of its parotoid glands. If toxin replenishment necessitates an elevation in metabolic rate over this entire period, the cumulative costs could be substantial.
Our results suggest that toxin production by cane toads incurs costs that could impact fitness, favouring ‘toxin optimization’. That is, we would expect selection to favour frugal use of toxin. Many bufonids employ behavioural antipredator defences prior to releasing toxin, consistent with the idea that individuals avoid using toxin unnecessarily. In Australian cane toads, less than 9% of individuals responded to simulated standardized predator harassment by exuding toxin [27]. Toads were also more likely to exude toxin when temperatures were low, and the animals were thus less capable of rapid retreat [27]. Toads with relatively small parotoid glands were more likely to exude toxin than were toads with larger glands, suggesting that toads were more likely to expend toxin if they only had a small quantity to replace but were unable to control the amount released [27]. The extent to which toads are capable of metering their toxin remains to be determined.
Our experimental manipulations attempted to remove all the toxin from both glands, and this might not reflect a realistic toxin deployment by a threatened toad. Toads might secrete different amounts of toxin depending on the size or type of predator and the seriousness of the threat, similar to facultative adjustment of venom release by venomous animals [7,10]. Toads may even deploy toxin from one gland only, depending on which side the threat is on [22]. The level and circumstances of toads' ability to meter their toxin warrants future study.
A longer-term study to document the duration and consequences of the costs we document here would also be useful. For instance, it would be informative to determine whether different chemical components of the toxin are replenished at different rates [30]. Given that complete toxin replenishment by cane toads could take several months [23,24] the full costs could impact on direct determinants of fitness. For example, reduction in growth over several months would detrimentally affect all fitness components that were enhanced by large body size. Larger individuals have performance and fitness advantages in many amphibian species [48]. The small surface area : volume ratio of larger anurans also reduces their rates of desiccation [49], heating and cooling [50,51]. In cane toads specifically, larger size increases reproductive success of both males and females [52,53]. Although it would be difficult to document a reduction in lifetime reproductive success as a result of toxin depletion, a reduction in survivorship might be detectable and provide a useful indicator of the strength of selection acting on fine control of toxin expenditure.
Supplementary Material
Acknowledgements
We thank Student International Training and the Blennerhassett family. The Northern Territory Land Corporation provided research facilities. Two anonymous reviewers provided comments which improved the manuscript.
Ethics
The study was conducted under the approval of the University of Sydney Animal Care and Ethics Committee (permit numbers 2017/1195 and 2018/1441).
Data accessibility
Data available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.kp7r8b4 [54] and from Figshare at https://figshare.com/s/eb07628fd76b5ddc524d.
Authors' contributions
R.A.B. and G.P.B. collected data. K.B.-A. conducted calorimetry and G.P.B. analysed data. All authors contributed to writing.
Competing interests
We declare we have no competing interests.
Funding
Funding for this study was provided by the Australian Research Council.
References
- 1.Arbuckle K, Speed MP. 2015. Antipredator defenses predict diversification rates. Proc. Natl Acad. Sci. USA 112, 13 597–13 602. ( 10.1073/pnas.1509811112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, Cornell SJ, Speed MP. 2019. The evolution of variance in sequential defences. J. Theor. Biol. 462, 194–209. ( 10.1016/j.jtbi.2018.10.009) [DOI] [PubMed] [Google Scholar]
- 3.Brodie ED. 2009. Toxins and venoms. Curr. Biol. 19, R931–R935. ( 10.1016/j.cub.2009.08.011) [DOI] [PubMed] [Google Scholar]
- 4.Enzor L, Wilborn R, Bennett W. 2011. Toxicity and metabolic costs of the Atlantic stingray (Dasyatis sabina) venom delivery system in relation to its role in life history. J. Exp. Mar. Biol. Ecol. 409, 235–239. ( 10.1016/j.jembe.2011.08.026) [DOI] [Google Scholar]
- 5.Agrawal AA, Conner JK, Rasmann S. 2010. Tradeoffs and negative correlations in evolutionary ecology. In Evolution since Darwin: the first 150 years (eds MA Bell, DJ Futuyma, WF Eanes, JS Levinton), pp. 243–268. Sunderland, MA: Sinauer Associates.
- 6.Harris RJ, Jenner RA. 2019. Evolutionary ecology of fish venom: adaptations and consequences of evolving a venom system. Toxins 11, 60 ( 10.3390/toxins11020060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wigger E, Kuhn-Nentwig L, Nentwig W. 2002. The venom optimisation hypothesis: a spider injects large venom quantities only into difficult prey types. Toxicon 40, 749–752. ( 10.1016/S0041-0101(01)00277-X) [DOI] [PubMed] [Google Scholar]
- 8.Morgenstern D, King GF. 2013. The venom optimization hypothesis revisited. Toxicon 63, 120–128. ( 10.1016/j.toxicon.2012.11.022) [DOI] [PubMed] [Google Scholar]
- 9.Bókony V, et al. 2016. Variation in chemical defense among natural populations of common toad, Bufo bufo, tadpoles: the role of environmental factors. J. Chem. Ecol. 42, 329–338. ( 10.1007/s10886-016-0690-2) [DOI] [PubMed] [Google Scholar]
- 10.Hayes W. 2008. The snake venom-metering controversy: levels of analysis, assumptions, and evidence. In The biology of rattlesnakes (eds W Hayes, K Beaman, M Cardwell, S Bush), pp. 191–220. Loma Linda, CA: Loma Linda University Press.
- 11.Nazareth TM, Sudatti DB, Machado G. 2016. Chemical defense as a condition-dependent trait in harvestmen. J. Chem. Ecol. 42, 1047–1051. ( 10.1007/s10886-016-0749-0) [DOI] [PubMed] [Google Scholar]
- 12.Benard MF, Fordyce JA. 2003. Are induced defenses costly? Consequences of predator-induced defenses in western toads, Bufo boreas. Ecology 84, 68–78. ( 10.1890/0012-9658(2003)084[0068:AIDCCO]2.0.CO;2) [DOI] [Google Scholar]
- 13.McCue MD. 2006. Cost of producing venom in three North American pitviper species. Copeia 2006, 818–825. ( 10.1643/0045-8511(2006)6[818:COPVIT]2.0.CO;2) [DOI] [Google Scholar]
- 14.Pintor AF, Krockenberger AK, Seymour JE. 2010. Costs of venom production in the common death adder (Acanthophis antarcticus). Toxicon 56, 1035–1042. ( 10.1016/j.toxicon.2010.07.008) [DOI] [PubMed] [Google Scholar]
- 15.Smith MT, Ortega J, Beaupre SJ. 2014. Metabolic cost of venom replenishment by Prairie Rattlesnakes (Crotalus viridis viridis). Toxicon 86, 1–7. ( 10.1016/j.toxicon.2014.04.013) [DOI] [PubMed] [Google Scholar]
- 16.Nelsen DR, Nisani Z, Cooper AM, Fox GA, Gren EC, Corbit AG, Hayes WK. 2014. Poisons, toxungens, and venoms: redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 89, 450–465. ( 10.1111/brv.12062) [DOI] [PubMed] [Google Scholar]
- 17.Clarke BT. 1997. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. 72, 365–379. ( 10.1017/S0006323197005045) [DOI] [PubMed] [Google Scholar]
- 18.Apponyi MA, et al. 2004. Host-defence peptides of Australian anurans: structure, mechanism of action and evolutionary significance. Peptides 25, 1035–1054. ( 10.1016/j.peptides.2004.03.006) [DOI] [PubMed] [Google Scholar]
- 19.Rollins-Smith L, Woodhams D. 2011. Amphibian immunity: staying in tune with the environment. In Ecoimmunology (ed. Nelson GDAR.), pp. 92–143. New York, NY: Oxford University Press. [Google Scholar]
- 20.Mailho-Fontana PL, Antoniazzi MM, Sciani JM, Pimenta DC, Barbaro KC, Jared C. 2018. Morphological and biochemical characterization of the cutaneous poison glands in toads (Rhinella marina group) from different environments. Front. Zool. 15, 46 ( 10.1186/s12983-018-0294-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jared SG, Jared C, Egami MI, Mailho-Fontana PL, Rodrigues MT, Antoniazzi MM. 2014. Functional assessment of toad parotoid macroglands: a study based on poison replacement after mechanical compression. Toxicon 87, 92–103. ( 10.1016/j.toxicon.2014.05.020) [DOI] [PubMed] [Google Scholar]
- 22.Mailho-Fontana PL, Antoniazzi MM, Toledo LF, Verdade VK, Sciani JM, Barbaro KC, Pimenta DC, Rodrigues MT, Jared C. 2014. Passive and active defense in toads: the parotoid macroglands in Rhinella marina and Rhaebo guttatus. J. Exp. Zool A Ecol. Genet. Physiol. 321, 65–77. ( 10.1002/jez.1838) [DOI] [PubMed] [Google Scholar]
- 23.Chen K, Chen A. 1933. Notes on the poisonous secretions of twelve species of toads. J. Pharmacol. Exp. Ther. 47, 281–293. [Google Scholar]
- 24.Chen KK, Kovarikova A. 1967. Pharmacology and toxicology of toad venom. J. Pharm. Sci. 56, 1535–1541. ( 10.1002/jps.2600561202) [DOI] [PubMed] [Google Scholar]
- 25.Jared C, Antoniazzi MM, Jordao AE, Silva JRM, Greven H, Rodrigues MT. 2009. Parotoid macroglands in toad (Rhinella jimi): their structure and functioning in passive defence. Toxicon 54, 197–207. ( 10.1016/j.toxicon.2009.03.029) [DOI] [PubMed] [Google Scholar]
- 26.Hayes FE. 1989. Antipredator behavior of recently metamorphosed toads (Bufo a. americanus) during encounters with garter snakes (Thamnophis s. sirtalis). Copeia 1989, 1011–1015. ( 10.2307/1445987) [DOI] [Google Scholar]
- 27.Hudson CM, Brown GP, Shine R. 2017. Evolutionary shifts in anti-predator responses of invasive cane toads (Rhinella marina). Behav. Ecol. Sociobiol. 71, 134 ( 10.1007/s00265-017-2367-4) [DOI] [Google Scholar]
- 28.Kowalski K, Sawościanik O, Rychlik L. 2018. Do bufonids employ different anti-predator behaviors than ranids? Comparison among three European anurans. Copeia 106, 120–129. ( 10.1643/CE-16-567) [DOI] [Google Scholar]
- 29.Marchisin A, Anderson JD. 1978. Strategies employed by frogs and toads (Amphibia, Anura) to avoid predation by snakes (Reptilia, Serpentes). J. Herpetol. 12, 151–155. ( 10.2307/1563401) [DOI] [Google Scholar]
- 30.Nisani Z, Boskovic DS, Dunbar SG, Kelln W, Hayes WK. 2012. Investigating the chemical profile of regenerated scorpion (Parabuthus transvaalicus) venom in relation to metabolic cost and toxicity. Toxicon 60, 315–323. ( 10.1016/j.toxicon.2012.04.343) [DOI] [PubMed] [Google Scholar]
- 31.Kurali A, Pásztor K, Hettyey A, Tóth Z. 2016. Toxin depletion has no effect on antipredator responses in common toad (Bufo bufo) tadpoles. Biol. J. Linn. Soc. 119, 1000–1010. ( 10.1111/bij.12864) [DOI] [Google Scholar]
- 32.Hettyey A, Tóth Z, Van Buskirk J. 2014. Inducible chemical defences in animals. Oikos 123, 1025–1028. ( 10.1111/oik.01338) [DOI] [Google Scholar]
- 33.Lever C. 2001. The cane toad: the history and ecology of a successful colonist. Otley: Westbury Academic and Scientific Publishing. [Google Scholar]
- 34.Brown GP, Kelehear C, Shine R. 2011. Effects of seasonal aridity on the ecology and behaviour of invasive cane toads in the Australian wet–dry tropics. Funct. Ecol. 25, 1339–1347. ( 10.1111/j.1365-2435.2011.01888.x) [DOI] [Google Scholar]
- 35.Toledo R, Jared C, Brunner A Jr. 1992. Morphology of the large granular alveoli of the parotoid glands in toad (Bufo ictericus) before and after compression. Toxicon 30, 745–753. ( 10.1016/0041-0101(92)90008-S) [DOI] [PubMed] [Google Scholar]
- 36.Hudson CM, Brown GP, Shine R. 2017. Effects of toe-clipping on growth, body condition, and locomotion of cane toads (Rhinella marina). Copeia 105, 257–260. ( 10.1643/CE-16-564) [DOI] [Google Scholar]
- 37.Finnerty PB, Shine R, Brown GP. 2018. The costs of parasite infection: effects of removing lungworms on performance, growth and survival of free-ranging cane toads. Funct. Ecol. 32, 402–415. ( 10.1111/1365-2435.12992) [DOI] [Google Scholar]
- 38.Shilton CM, Šlapeta J, Shine R, Brown GP. 2018. Invasive colonic entamoebiasis in wild cane toads, Australia. Emerg. Infect. Dis. 24, 1541–1543. ( 10.3201/eid2408.180101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruber J, Whiting MJ, Brown G, Shine R. 2017. The loneliness of the long-distance toad: invasion history and social attraction in cane toads (Rhinella marina). Biol. Lett. 13, 20170445 ( 10.1098/rsbl.2017.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Llewellyn D, Brown G, Thompson M, Shine R. 2011. Behavioral responses to immune-system activation in an anuran (the cane toad, Bufo marinus): field and laboratory studies. Physiol. Biochem. Zool. 84, 77–86. ( 10.1086/657609) [DOI] [PubMed] [Google Scholar]
- 41.Bókony V, Üveges B, Verebélyi V, Ujhegyi N, Móricz ÁM. 2019. Toads phenotypically adjust their chemical defences to anthropogenic habitat change. Sci. Rep. 9, 3163 ( 10.1038/s41598-019-39587-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Withers P, Hillman S. 2001. Allometric and ecological relationships of ventricle and liver mass in anuran amphibians. Funct. Ecol. 15, 60–69. ( 10.1046/j.1365-2435.2001.00495.x) [DOI] [Google Scholar]
- 43.Hutchinson DA, Savitzky AH. 2004. Vasculature of the parotoid glands of four species of toads (Bufonidae: Bufo). J. Morphol. 260, 247–254. ( 10.1002/jmor.10219) [DOI] [PubMed] [Google Scholar]
- 44.Üveges B, Fera G, Móricz ÁM, Krüzselyi D, Bókony V, Hettyey A. 2017. Age- and environment-dependent changes in chemical defences of larval and post-metamorphic toads. BMC Evol. Biol. 17, 137 ( 10.1186/s12862-017-0956-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kouřimská L, Adámková A. 2016. Nutritional and sensory quality of edible insects. NFS J. 4, 22–26. ( 10.1016/j.nfs.2016.07.001) [DOI] [Google Scholar]
- 46.Lillywhite HB, Licht P, Chelgren P. 1973. The role of behavioral thermoregulation in the growth energetics of the toad, Bufo boreas. Ecology 54, 375–383. ( 10.2307/1934345) [DOI] [Google Scholar]
- 47.Zug GR, Zug PB. 1979. The marine toad, Bufo marinus: a natural history resume of native populations. Smithson Contrib. Zool. 284, 1–58. ( 10.5479/si.00810282.284) [DOI] [Google Scholar]
- 48.Wells KD. 2010. The ecology and behavior of amphibians. Chicago, IL: University of Chicago Press. [Google Scholar]
- 49.Reynolds SJ, Christian KA. 2009. Environmental moisture availability and body fluid osmolality in introduced toads, Rhinella marina, in monsoonal northern Australia. J. Herpetol. 43, 326–331. ( 10.1670/08-062R2.1) [DOI] [Google Scholar]
- 50.Olalla-Tárraga MÁ, Diniz-Filho JAF, Bastos RP, Rodríguez MÁ. 2009. Geographic body size gradients in tropical regions: water deficit and anuran body size in the Brazilian Cerrado. Ecography 32, 581–590. ( 10.1111/j.1600-0587.2008.05632.x) [DOI] [Google Scholar]
- 51.Carey C. 1978. Factors affecting body temperatures of toads. Oecologia 35, 197–219. ( 10.1007/BF00344732) [DOI] [PubMed] [Google Scholar]
- 52.Bowcock H, Brown GP, Shine R. 2013. Sexual selection in cane toads Rhinella marina: a male's body size affects his success and his tactics. Cur. Zool. 59, 747–753. ( 10.1093/czoolo/59.6.747) [DOI] [Google Scholar]
- 53.Alford RA, Cohen MP, Crossland MR, Hearnden MN, Schwarzkopf L. 1995. Population biology of Bufo marinus in northern Australia. In Supervisong scientist report 101: wetland research in the wet-dry tropics of Australia (ed. Finlayson CM.), pp. 173–181. Canberra, Australia: Commonwealth of Australia. [Google Scholar]
- 54.Blennerhassett RA, Bell-Anderson K, Shine R, Brown GP. 2019. Data from: The cost of chemical defence: the impact of toxin depletion on growth and behaviour of cane toads (Rhinella marina) Dryad Digital Repository. ( 10.5061/dryad.kp7r8b4) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Blennerhassett RA, Bell-Anderson K, Shine R, Brown GP. 2019. Data from: The cost of chemical defence: the impact of toxin depletion on growth and behaviour of cane toads (Rhinella marina) Dryad Digital Repository. ( 10.5061/dryad.kp7r8b4) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository at https://doi.org/10.5061/dryad.kp7r8b4 [54] and from Figshare at https://figshare.com/s/eb07628fd76b5ddc524d.