Abstract
Background
This study aimed to use gas chromatography-mass spectrometry (GC-MS) to identify the chemical constituents of volatile oil extracted by steam distillation from Cichorium glandulosum Boiss et Huet (CG), a traditional Uyghur medicine, and to investigate its effects on carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats.
Material/Methods
Sprague-Dawley rats (n=60) included six groups: the control group (n=10), untreated model group (n=10), the volatile oil of CG high-dose group (0.15 ml/kg) (n=10), the volatile oil of CG medium-dose group (0.10 ml/kg) (N=10), the volatile oil of CG low-dose group (0.05 ml/kg) (n=10), and the silybin-treated group (0.20 ml/kg) (n=10). Rats given the essential oil extract of CG by intragastric administration, and then subcutaneously injected with a solution of CCl4 in olive oil to create the rat model of hepatic fibrosis. Serum samples were analyzed for markers of liver function, including aspartate transaminase (AST), alanine transaminase (ALT), malondialdehyde (MDA), hydroxyproline (Hyp), γ-glutamyl transpeptidase (γ-GT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and albumin (Alb). Histology and immunohistochemistry were performed on rat liver tissue.
Results
Thirty-eight compounds were identified from the volatile oil of CG (total, 98.058%), with terpenoids, including citronellol, being the most abundant. In the animal model of liver fibrosis, all doses of volatile oil of CG significantly reduced the serum levels of AST, ALT, MDA, Hyp, γ-GT, LDH, ALP, and Alb.
Conclusions
GC-MS identified the components of the volatile oil of CG, which included citronellol. Treatment with volatile oil of CG reduced liver fibrosis in a rat model.
MeSH Keywords: Gas Chromatography-Mass Spectrometry; Medicine, East Asian Traditional; Vacuum Extraction, Obstetrical
Background
Cichorium glandulosum Boiss et Huet (CG) is a traditional Chinese herbal medicine derived from chicory roots and seeds, which has been used to treat liver and gallbladder disease [1]. Currently, CG is widely used in Xinjiang Uygur Autonomous Region in China, as well as in the Caucasus [1]. CG is a Chinese Uygur medicinal herb with a bitter taste and has the functions of clearing the liver and gallbladder, improving digestion, and acts as a diuretic. CG has been reported to be used to treat jaundice, stomach ache, to treat obesity, edema, hepatitis, nephritis, enteritis, tracheitis, and other diseases [1].
Recently, the chemical constituents of CG and its clinical toxicological and pharmacological effects have been studied. It has been shown that CG has protective effects on liver fibrosis, but the mechanism of its pharmacological effects remains unknown. Recent studies have shown that CG contains flavone, sesquiterpene, coumarin, carbohydrates, and other active ingredients, which have strong biological activity and medicinal value [2–4]. Current studies on CG have mainly focused on the separation and purification of sesquiterpenes [5–7] and the extraction of flavonoids [8,9]. Studies on the extraction and chemical composition of volatile oil from the whole plant have not previously been undertaken, and a comprehensive study of its pharmacological effects has not been reported.
Therefore, this study aimed to use gas chromatography-mass spectrometry (GC-MS) to isolate and identify the chemical constituents of volatile oil extracted from CG by steam distillation and to compare its effects with silybin, the active component of silymarin extracted from Silybum marianum (milk thistle), on carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats. The liver and spleen coefficients, serum levels of aspartate transaminase (AST), alanine transaminase (ALT), malondialdehyde (MDA), hydroxyproline (Hyp), γ-glutamyl transpeptidase (γ-GT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and albumin (Alb) were measured, and the histopathological and immunohistochemical changes of the liver were observed to investigate the hepatoprotective and anti-fibrotic effects of volatile oil extracted from CG.
Material and Methods
Equipment used in this study
The following equipment was used in this study. D-200 small Chinese medicine crusher (Changhong Pharmaceutical Machinery and Equipment Factory of Changsha City, Hunan Province, China); KQ2200DE numerical control ultrasonic cleaner (Zhangjiagang Haina Ultrasonic Electric Co., Ltd., China); CL-2 constant temperature heating magnetic stirrer (Shanghai Yingyu Scientific Research Instrument Equipment Co., Ltd., China); BSA822 electronic balance (Xi Jie Balance Instrument Co., Ltd., Beijing, China); a DHG-9030A electric thermostat blast dryer (Beijing Hengtai Fengke Test Equipment Co., Ltd., China); Agilent 7890A/5975C Gas Chromatography-Mass Spectrometer (Agilent, Santa Clara, CA, USA); volatile oil extractor, reflux condenser, circular bottom Flask (1000 ml), disposable injector, and TDL-50 desktop centrifuge (Shanghai Anting Science Instrument Factory, China); TS-8 vortex mixer (Jiangsu Haimen Qilin Medical Instrument Factory, China); FA210B electronic balance (Shanghai Precision Science Instrument Co., Ltd., China); electrothermal constant temperature water bath pot (Beijing Chang’an Science Instrument Factory, China); Optima MAX-XP ultra-speed refrigerated centrifuge (Beckman Coulter, Brea, CA, USA); 722N visible spectrophotometer (Shanghai Precision Scientific Instrument Co., Ltd., China); electric thermostat water bath (Beijing Chang’an Scientific Instrument Factory, China), 1ml, 5ml syringes (Shanghai Kangdelai Enterprise Development Group Co., Ltd., China); −80°C refrigerator and DB-09 paraffin embedding machine (Hubei Technology Co., Ltd., Hubei, China).
Reagents used in this study
The following reagents were used in this study. Carbon tetrachloride (CCl4) (analytical purity) (Tianjin Zhiyuan Chemical Reagent, China); olive oil (Shanghai Jiali Food Industry Co., Ltd, China); glacial acetic acid (analytical purity) (Tianjin Guangfu Technology Development Co., Ltd., China); normal saline (Xi’an Jingxi Shuanghe Pharmaceutical Co., Ltd., China); chloral hydrate (Shanghai Chemical Reagent Purchasing and Supply Station, Xinhua Chemical Factory, China); liquid nitrogen (Shihezi Animal Epidemic Prevention Center); 10% formalin (Shihezi University Medical College Laboratory, China); a kit was used to analyze serum alkaline phosphatase (ALP), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), hydroxyproline (Hyp), malondialdehyde (MDA), albumin, γ-glutamyl transpeptidase (γ-GT) (Nanjing Institute of Bioengineering, China). Ether, formaldehyde, and other reagents were analytically pure grade.
Volatile oil of Cichorium glandulosum Boiss et Huet (CG)
Volatile oil of Cichorium glandulosum Boiss et Huet (CG) was extracted by steam distillation and was obtained from Professor Mao Juju, Professor of Chinese Medicine at Jing Shi He Zi College of Pharmacy. Following extraction, the volatile oil of CG was suspended in distilled water, with a ratio of 1: 10, and shaken vigorously 20 times to form an emulsion before gastric administration to the rats in this study.
Experimental animals
Sixty specific pathogen-free (SPF) Sprague-Dawley (SD) rats, 30 male rats, and 50 female rats, with a mean weight of 200±30 g, were purchased from the Experimental Animal Research Center of Xinjiang Medical University (Animal License No. SCXK 2011-0004). Standard food and drink were given, and the study began after two days of feeding. The study was approved by the Ethics Committee of Shihezi University (Approval No. 2015-030-01).
Treatment of medicinal materials
The partially dried chicory samples were crushed, and the whole dried grass and crushed residue (2–5 cm). Powdered medicinal materials were divided into 100 g samples, placed in flasks, and 600 ml of normal saline and magnetite were added. After shaking and mixing, the powder was soaked for 12 hours and treated with ultrasonic dispersion for 30 minutes.
Extraction of volatile oil of Cichorium glandulosum Boiss et Huet (CG)
The volatile oil extractor was connected to a reflux condenser. Water was added from the upper end of the condenser tube to fill the volatile oil extractor and the gently heated to boiling point and boiling was continued for 5 hours. When the oil volume was reduced to 2.0 ml in the extractor, heating stopped, the water was drained, and the oil was collected.
Gas chromatography-mass spectrometry (GC-MS) to identify the chemical constituents of volatile oil of (CG)
GC-MS was performed using a quartz capillary column. The heating program used an initial temperature of 40°C, increasing to 280°C at a rate of 5°C/min, with this temperature being maintained for 60 minutes until the analysis was completed. The carrier gas was helium, the inlet temperature was 240°C, the split ratio was 40: 1, and the injection volume was 10 L. The mass spectrometry conditions included a standard electron ionization (IE) source (70eV), an ion source temperature of 180°C, and an interface temperature of 240°C. The quadrupole mass analyzer had a scan range of 20–700 amu and a scan speed of 4.0 scans/sec).
The rat liver fibrosis model
In rats in the five model groups (n=50), 40% carbon tetrachloride (CCl4) in olive oil was injected in the back to develop the rat model of liver fibrosis. The first dose of CCl4 was 1.0 ml/kg, and then 0.5 ml/kg was injected twice weekly for 13 weeks. In the rat control group (n=10), rats were injected subcutaneously with an equivalent volume of normal saline, twice weekly for 13 weeks.
Treatment groups in the rat model of liver fibrosis
In the five model groups, there was one untreated model group (n=10), and four treated rat model groups included the volatile oil of CG high-dose group (0.15 ml/kg) (n=10), the volatile oil of CG medium-dose group (0.10 ml/kg) (N=10), the volatile oil of CG low-dose group (0.05 ml/kg) (n=10), and the silybin-treated group (0.20 ml/kg) (n=10). Treatment began on the fifth week following the development of the rat model of liver fibrosis and continued for 13. After 13 weeks, the rats fasted for 24 hours and then euthanized. Blood was collected from the abdominal aorta. Blood samples were centrifuged to obtain the serum, which was cryopreserved at −20°C. The liver and spleen were removed from each rat and weighed, and part of the liver was fixed in 10% formaldehyde at −80°C. Histological examination and immunohistochemistry were performed on the rat liver tissue sections.
Liver and spleen coefficients
The rats were injected with 10% chloral hydrate into the abdominal cavity. The liver and spleen were removed from the abdomen after they were euthanized. The rats were washed with normal saline at 4°C, and the excess water was removed using filter paper. After weighing, the coefficient (index) of the liver and spleen were calculated as follows: organ index=organ quality (g)/individual quality (g) ×100%.
Measurement of serum biochemical indicators of liver function
Blood from the rat abdominal aorta was incubated in a water bath at 37°C for 1 h and centrifuged for 15 min at 3,000 rpm. Serum was used to measure the levels of aspartate transaminase (AST), alanine aminotransferase (ALT), malondialdehyde (MDA), albumin (Alb), γ-glutamyl transpeptidase (γ-GT), hydroxyproline (Hyp), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP). All serum biochemical markers were were tested according to the instructions from the manufacturers of the kit. The ratio of AST to serum glutamic-oxaloacetic transaminase (AST/GOT) was determined by Kim’s method. The ratio of ALT to glutamate-pyruvate transaminase (GPT) (ALT/GPT) was determined by Rye’s method. Serum Hyp was measured using an enzyme digestion method, and serum Alb was measured by bromocresol green colorimetry. Serum MDA was measured using the thiobarbituric acid (TBA) method.
Rat liver histology
From each rat, part of the right lobe of the liver was fixed in 10% formalin. Tissues were processed and embedded in paraffin wax and tissue sections were cut onto glass slides. Sections of rat liver were routinely stained with hematoxylin and eosin (H&E) for light microscopy. Liver fibrosis was assessed histologically using the histochemical stain, elastic van Giesen (EVG). Immunohistochemistry was performed with primary antibodies to Smad7, Smad3, Toll-like receptor 4 (TLR4), alpha smooth muscle actin (ASMA), and transforming growth factor-beta 1 (TGF-β1) and immunostaining was evaluated by light microscopy.
Statistical analysis
Data were analyzed using SPSS version 17.0 software (IBM, Chicago, IL, USA). The measurement data were expressed as the mean ± standard deviation (SD). Analysis of variance (ANOVA) was used for comparison between groups. The test level α was 0.05/10. A P-value <0.05 was considered to be statistically significant.
Results
Gas chromatography-mass spectrometry (GC-MS) analysis of the volatile oil of Cichorium glandulosum Boiss et Huet (CG)
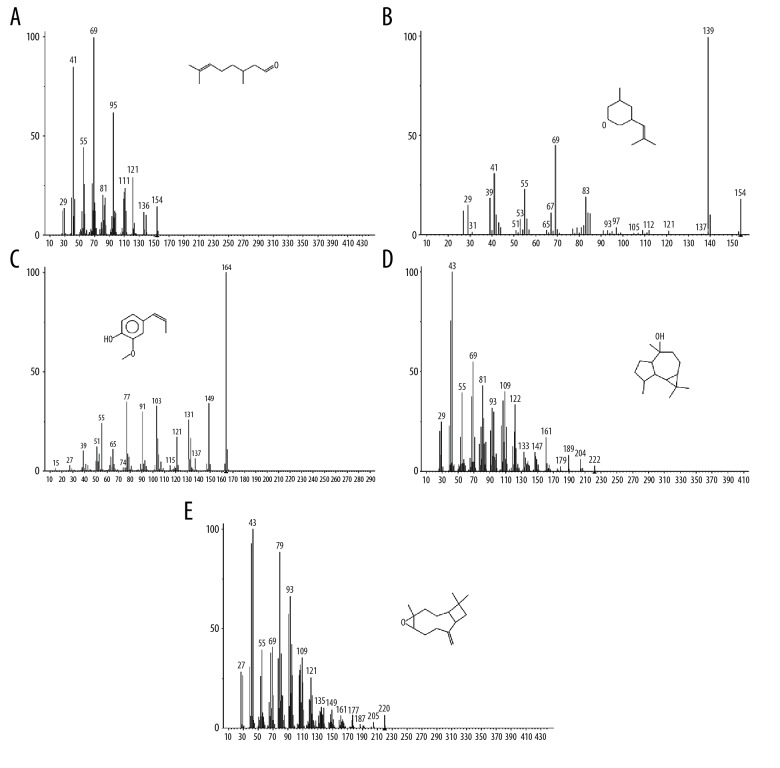
A total of 38 compounds were identified by gas chromatography-mass spectrometry (GC-MS) analysis of the volatile oil of Cichorium glandulosum Boiss et Huet (CG) (Table 1). The detected compounds accounted for 98.058% of the total amount. The total ion chromatogram obtained is shown in Figure 1. Terpenoids were the most abundant compounds. The relative content of citronellol was 74.060%, which peaked at 37.039 min. The structure and mass spectrogram of citronellol are shown in Figure 2A. Citronellol, or dihydrogeraniol, derived from lemongrass can be used as an antibacterial agent.
Table 1.
Chemical composition and content of GC-MS analysis of volatile oil from Cichorium glundulosum Boiss et Hout.
| No. | Retention time (min) | Compounds | Molecule |mass | Relative contents (%) |
|---|---|---|---|---|
| 1 | 20.892 | Benzaldehyde | C7H6O | 0.40% |
| 2 | 29.657 | 1,6-Octadien-3-ol, 3,7-dimethyl- 3,7-dimethylocta-1,6-dien-3-ol | C10H18O | 0.39% |
| 3 | 30.365 | 2H-Pyran,tetrahydro-4-methyl-2-(2-methyl-1-propenyl)- | C10H18O | 5.34% |
| 4 | 31.334 | Tetrahydro-4-methyl-2-(2-methyl-1-propenyl)-2H-pyran | C10H18O | 2.77% |
| 5 | 32.597 | 6-Octenal, 3,7-dimethyl- | C10H18O | 0.27% |
| 6 | 37.039 | 6-Octen-1-ol, 3,7-dimethyl-, (R)- | C10H20O | 74.06% |
| 7 | 37.708 | 6-Octen-1-ol, 3,7-dimethyl- | C10H20O | 0.46% |
| 8 | 38.346 | 2,6-Octadien-1-ol, 3,7-dimethyl- | C10H18O | 0.28% |
| 9 | 38.662 | 2-Isopropenyl-5-methylhex-4-enal | C10H16O | 0.73% |
| 10 | 41.316 | Cyclohexene, 2-ethenyl-1,3,3-trimethyl- | C11H18 | 0.39% |
| 11 | 43.838 | Phenol, 2-methoxy-4-(1-propenyl)-, (Z)- | C10H12O2 | 3.17% |
| 12 | 44.931 | 2,6-Octadien-1-ol, 3,7-dimethyl-, acetate | C12H20O2 | 0.27% |
| 13 | 46.411 | Benzene, 1,2-dimethoxy-4-(2-propenyl)- | C11H14O2 | 0.58% |
| 14 | 47.785 | Caryophyllene | C15H24 | 0.44% |
| 15 | 49.246 | α-Caryophyllene | C15H24 | 0.93% |
| 16 | 49.861 | 1H-Benzocycloheptene,2,4a,5,6,7,8-hexahydro-3,5,5,9-tetramethyl-, (R)- | C15H24 | 0.14% |
| 17 | 50.217 | 1H-Cyclopenta[1,3]cyclopropa[1,2]benzene,octahydro-7-methyl-3-methylene-4-(1-methylethyl)-,[3aS-(3a.alpha.,3b.beta.,4.beta.,7.alpha.,7aS*)]- | C15H24 | 0.35% |
| 18 | 50.545 | 1,3-Benzodioxole, 4-methoxy-6-(2-propenyl)- | C11H12O3 | 0.15% |
| 19 | 50.754 | Cyclohexane,1-ethenyl-1-methyl-2-(1-methylethenyl)-4-(1-methylethylidene)- | C15H24 | 0.14% |
| 20 | 50.943 | 1H-3a,7-Methanoazulene,2,3,4,7,8,8a-hexahydro-3,6,8,8-tetramethyl-,[3R-(3.alpha.,3a.beta.,7.beta.,8a.alpha.)]- | C15H24 | 0.22% |
| 21 | 51.382 | 1,3-Benzodioxole, 4-methoxy-6-(2-propenyl)- | C11H12O3 | 0.06% |
| 22 | 52.065 | Ledol | C15H26O | 1.49% |
| 23 | 52.391 | Caryophyllene oxide | C15H24O | 1.21% |
| 24 | 52.985 | 1-Hydroxy-6-(3-isopropenyl-cycloprop-1-enyl)-6-methyl-heptan-2-one | C14H22O2 | 0.12% |
| 25 | 53.16 | Epiglobulol | C15H26O | 0.47% |
| 26 | 53.279 | (−)-Spathulenol | C15H24O | 0.55% |
| 27 | 53.76 | Cedren-13-ol, 8- | C15H24O | 0.33% |
| 28 | 54.067 | 12-Oxabicyclo[9.1.0]dodeca-3,7-diene, 1,5,5,8-tetramethyl-, [1R-(1R*,3E,7E,11R*)]- | C15H24O | 0.70% |
| 29 | 54.683 | tau.-Cadinol | C15H26O | 0.53% |
| 30 | 55.177 | 4aH-Cycloprop[e]azulen-4a-ol,decahydro-1,1,4,7-tetramethyl,[1aR(1a.alpha.,4.beta.,4a.beta.,7.alpha.,7a.beta.,7b.alpha.)]- | C15H26O | 0.36% |
| 31 | 57.842 | Phenanthrene | C14H10 | 0.35% |
| 32 | 58.155 | 2-Pentadecanone,6,10,14-trimethyl- | C18H36O | 0.70% |
| 33 | 58.775 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | C16H22O4 | 0.27% |
| 34 | 59.901 | 1-Heptatriacotanol | C37H76O | 0.11% |
| 35 | 60.511 | Dibutyl phthalate | C16H22O4 | 0.24% |
| 36 | 61.778 | Linoleic acid ethyl ester | C20H36O2 | 0.11% |
| 37 | 76.285 | Pentacosane | C25H52 | 0.17% |
| 38 | 84.419 | Heptacosane | C27H56 | 0.18% |
Figure 1.
Gas chromatography-mass spectrometry (GC-MS) total ion chromatogram for the volatile oil of Cichorium glandulosum Boiss et Huet (CG).
Figure 2.
Gas chromatography-mass spectrometry (GC-MS) full scan mass spectrometric analysis of components (A–E) in the volatile oil of Cichorium glandulosum Boiss et Huet (CG) (A) 6-Octen-1-ol, 3,7-dimethyl-. (B) 2H-pyran, tetrahydro-4-methyl-2-(2-methyl-1-propenyl)-. (C) Phenol, 2-methoxy-4-(1-propenyl)-. (D) Ledol. (E) (Z)-caryophyllene oxide.
The relative content of rose oxide was 5.342%, which peaked at 30.365 min (Figure 2B). The relative content of isoeugenol at 41.838 min was 3.167% (Figure 2C). The relative content of the bicyclic sesquiterpene, caryophyllin, was 1.489% (Figure 2D) and 1.210% (Figure 2E) at 52.365 min and 52.391 min, respectively. The volatile oil of CG also contained relatively low levels of alcohols (37 alcohols at 59.901 min), aldehydes (including, 3,7-dimethyl-6-octenaldehyde), ethers (including 1,3-benzodioxole, 4-methoxy-6-(2-propenyl), ketones (including, 6,10,14-trimethyl-2-pentadecane at 58.155 min), and lipids (including, ethyl linoleate at 61.778 min), long-chain alkanes, including twenty-five alkane at 76.285 min twenty-five alkane.
The protective effect of CG on hepatic fibrosis in the rat model: The liver and spleen coefficient
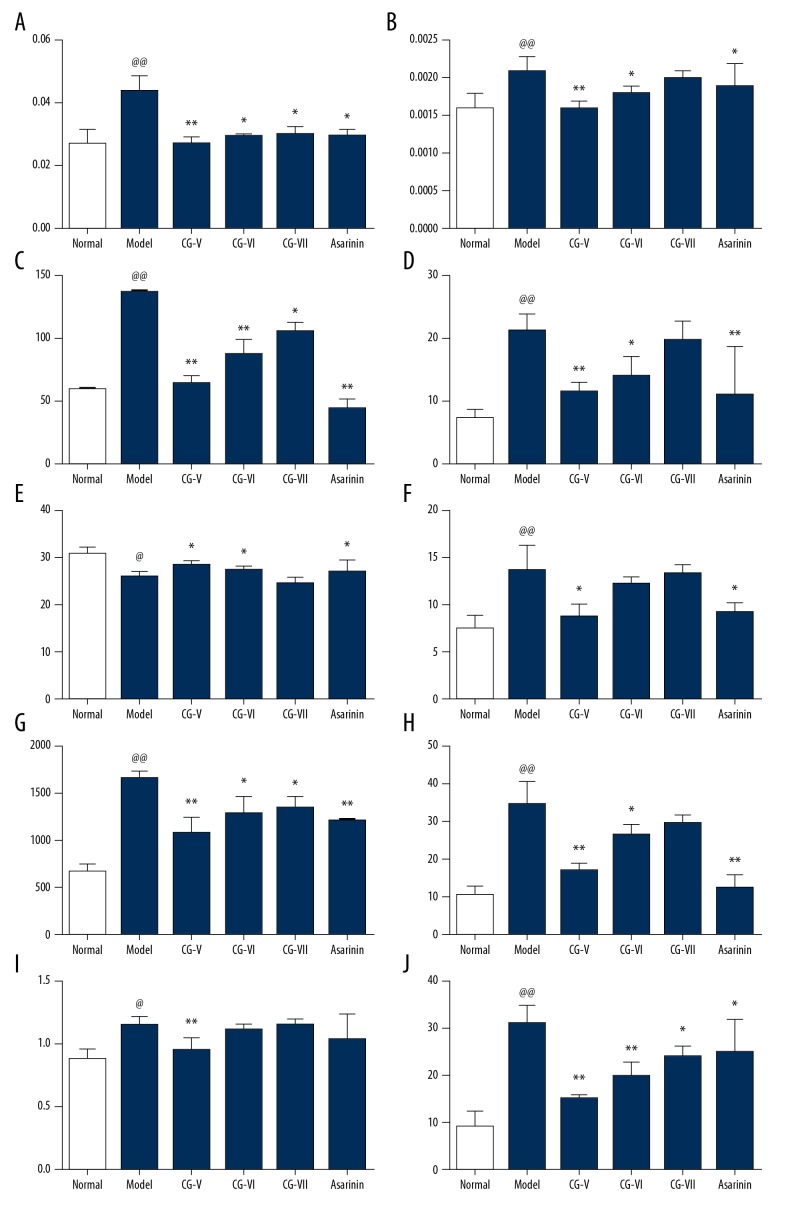
Compared with the normal control rat group, the liver and spleen coefficients of the rat model groups were significantly increased (P<0.01), indicating that the model was successful. Compared with the model group, the liver coefficient and spleen coefficient of the volatile oil of CG high-dose treatment group was significantly reduced (P<0.01). Compared with the model group, the liver coefficient and spleen coefficient of the volatile oil of CG medium-dose treatment group was significantly reduced (P<0.01). Also, the liver coefficient of the volatile oil of CG low-dose treatment group was significantly reduced (P<0.05), while the spleen coefficient of the volatile oil of CG low-dose treatment group was reduced, but this did not reach statistical significance (P>0.05). Compared with the model group, the liver and spleen coefficient of the silybin-treated group was significantly reduced. The results are shown in Figure 3A, 3B.
Figure 3.
The protective effect of volatile oil of Cichorium glandulosum Boiss et Huet (CG) on hepatic fibrosis in the rat model of carbon tetrachloride (CCl4)-induced hepatic fibrosis. (A, B) Effects of volatile oil of Cichorium glandulosum Boiss et Huet (CG) on rat liver and spleen indices. (B–J) Effects of CG on serum aspartate transaminase (AST), alanine transaminase (ALT), albumin (Alb), malondialdehyde (MDA), lactate dehydrogenase (LDH), γ-glutamyl transpeptidase (γ-GT), hydroxyproline (Hyp), and alkaline phosphatase (ALP) activities. (n=10) (mean ±SD). Compared with the normal group, @@ p<0.01, @ p<0.05. Compared with the model group, ** p<0.01, * p<0.05.
Serum levels of aspartate transaminase (AST) and alanine transaminase (ALT)
Compared with the normal control rat group, the serum levels of AST and ALT in the model group were significantly increased, which indicated that the model was successful. Compared with the model group, the levels of AST and ALT in the serum of rats in the volatile oil of CG high-dose group were significantly reduced (P<0.01). The serum levels of AST in the rats in the volatile oil of CG medium-dose group were significantly reduced (P<0.01) and the levels of ALT were significantly reduced (P<0.05). The levels of AST in the serum of rats in the volatile oil of CG low-dose group were significantly reduced (P<0.05). The serum levels of ALT were reduced but this did not reach significance (P>0.05). Serum AST and ALT levels in the silybin-treated group were significantly reduced when compared with the model group (P<0.01). The results are shown in Figure 3C, 3D.
Serum albumin (Alb) and malondialdehyde (MDA) levels
Compared with the normal control rat group, serum albumin levels in the model group were significantly reduced, and the concentration of MDA were significantly increased, indicating that the model was successful. Compared with the model group, the serum Alb levels of rats in the volatile oil of CG high-dose group were significantly increased, and the MDA concentration was significantly reduced (P<0.05). Compared with the model group, the serum Alb levels of rats in the volatile oil of CG medium-dose group were significantly increased (P<0.05), and the MDA levels were reduced, but this finding did not reach statistical significance (P>0.05). Compared with the model group, the serum Alb levels of rats in the volatile oil of CG medium-dose group were significantly increased (P<0.05). There was no significant difference between serum Alb levels and MDA levels (P>0.05). The serum albumin levels and MDA levels in the silybin-treated group were also reduced. The result are shown in Figure 3E, 3F.
Serum lactate dehydrogenase (LDH) and γ-glutamyl transpeptidase (γ-GT) levels
Compared with the normal control rat group, the levels of LDH and γ-GT in the model group were significantly increased (P<0.01), indicating that the model was successful. Compared with the model group, the serum levels of LDH and γ-GT in the volatile oil of CG high-dose group were significantly reduced (P<0.01). The serum levels of LDH and γ-GT in the volatile oil of CG medium-dose group and low-dose group were significantly reduced (P<0.05). The serum LDH and γ-GT levels in the silybin-treated group were significantly decreased (P<0.01). The results are shown in Figure 3G, 3H.
Serum hydroxyproline (Hyp) and alkaline phosphatase (ALP) levels
Compared with the normal control rat group, the serum levels Hyp (P<0.05) and ALP (P<0.01) in the model group were significantly increased, indicating that the rats had developed hepatic fibrosis and the model was successful. Compared with the model group, the levels of Hyp and ALP in the serum of the volatile oil of CG high-dose group were significantly reduced (P<0.01). There was no significant difference in serum Hyp levels between the model group and the volatile oil of CG medium-dose group (P>0.05), but there was a significant difference in ALP levels between the model group and the volatile oil of CG medium dose group (P<0.01). Serum Hyp levels of the volatile oil of CG low-dose group were significantly increased (P>0.05). Serum levels of ALP were significantly reduced when compared with the model group (P<0.05). The serum levels of Hyp in the silybin-treated group were reduced when compared with the model group, but this difference did not reach statistical significance (P>0.05), but the serum levels of ALP were significantly reduced (P<0.05). The results are shown in Table 2 and Figure 3I, 3J.
Table 2.
Effect of VOCGB extract on serum hydroxyproline activity in rats with hepatic fibrosis(χ̄±S, n=10).
| Group | Dose (mg/kg.d) | Hyp |
|---|---|---|
| Normal group | – | 0.877±0.075 |
| Model group | – | 1.146±0.069* |
| VOCGB high-dose group | 0.15 ml·kg−1 | 0.956±0.085# |
| VOCGB medium dose group | 0.10 ml·kg−1 | 1.111±0.046 |
| VOCGB low concentration group | 0.05 ml·kg−1 | 1.156±0.038 |
| Silybin group | 0.20 mg·kg−1 | 1.035±0.196 |
Comparing with normal group
P<0.05;
Comparing with model group
P<0.01.
VOCGB – volatile oil of Cichorium glundulosum Boiss et Hout.
Histology of the rat liver in the normal control, untreated and treated rat model groups
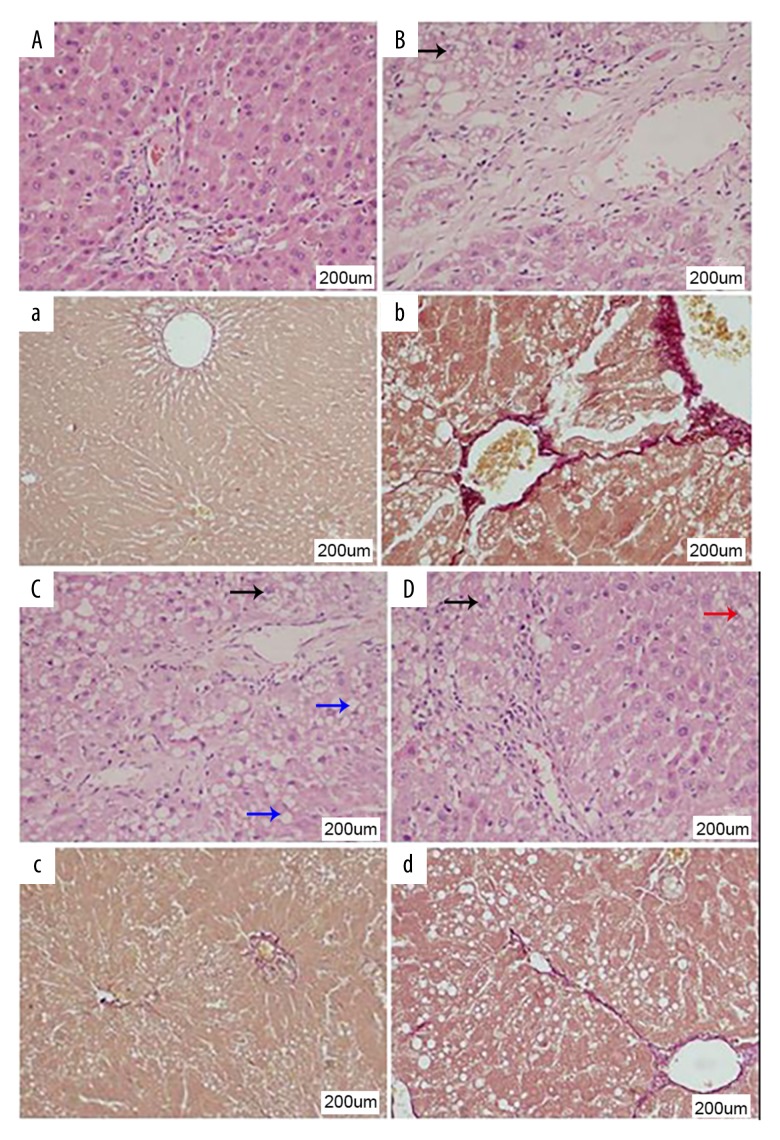
In the normal control rat group, the histology of the liver was normal. In particular, the central vein could be identified and was associated with normal liver architecture and normal hepatocytes, without necrosis, inflammation, steatosis, or fibrosis (Figure 4A). In the untreated rat model group, histology confirmed the presence of liver fibrosis with lymphocyte infiltrates. The normal structure of the hepatic lobules was disorganized, with loss of the normal arrangement of the liver trabeculae, loss of liver sinusoids, and edema of the liver tissue. The hepatocytes were swollen and steatosis was present with intracellular fat droplets that varied in size. Some hepatocytes showed vesicular degeneration and necrosis (Figure 4B).
Figure 4.
Histology of the liver tissues for hepatic fibrosis in the rat model of carbon tetrachloride (CCl4)-induced hepatic fibrosis. (A–D) Photomicrographs of the histology of the rat liver tissues show fibrosis. Hematoxylin and eosin (H&E) and elastic van Giesen (EVG). Magnification ×200. (A) Liver histology in the normal rat group. (B) Liver histology in the untreated rat model group. (C) Liver histology in the rat model group treated with volatile oil of Cichorium glandulosum Boiss et Huet (CG). (D) Liver histology in the rat model group treated with silybin. The blue arrow in C shows hepatocyte steatosis; the black arrow in B, C, and D shows vesicular hepatocyte degeneration; the red arrow in D shows cell necrosis and loss of cell nuclei.
Compared with the untreated model group, in the rats treated with high-dose volatile oil of CG, there was reduced liver fibrosis and the normal liver architecture was preserved. There was some steatosis, and cell vacuolation, but edema, inflammation, and liver cell necrosis were reduced. Therefore, treatment with volatile oil of CG appeared to improved the morphological changes of liver fibrosis induced by carbon tetrachloride (CCl4) in the rat model. The effects of treatment with high-dose volatile oil of CG were more apparent when compared with the medium-dose and low-dose groups. These results indicated that the volatile oil of CG had a protective effect on hepatic fibrosis induced by carbon tetrachloride (CCl4) in rats (Figure 4C). The rat liver histology in the silybin-treated rat model group showed mild hepatic fibrosis with retained liver architecture that included discernible sinusoids, homogeneous cytoplasm of hepatocytes, a marked reduction of cell necrosis, and some vesicular degeneration (Figure 4D).
Histology of liver fibrosis using elastic van Giesen (EVG) staining in the normal control, untreated and treated rat model groups
In the normal control rat group, the structure of central venous area was clear, with only a few collagen fibers, stained red with EVG, found around the blood vessels, and the liver architecture was normal (Figure 4A). In the untreated rat model group, EVG staining confirmed extensive hepatic fibrosis was observed, with collagen fibers extending outward from the central vein or portal area, which connected to form septal pseudolobules that divided the liver parenchyma. There was hepatocyte degeneration associated with hepatic fibrosis (Figure 4B).
Compared with the untreated model group, the degree of liver fibrosis in the high-dose group treated with volatile oil of CG was significantly reduced, and a small amount of collagen was present. There was a small amount of fibrous septum formation, but the degree of cell degeneration reduced, and there was no pseudolobule formation. The effects on reduction of liver fibrosis in the volatile oil of CG high-dose treatment group were significantly greater when compared with the low-dose and medium-dose volatile oil of CG-treated groups. These findings indicated that volatile oil of CG had a protective effect on the hepatic fibrosis induced by carbon tetrachloride (CCl4) in the rat model of liver fibrosis (Figure 4C). Compared with the untreated model group, the silybin-treated group showed no pseudolobule formation, mild degeneration of hepatocytes, and only a small amount of hepatic collagen (Figure 4D).
Immunohistochemistry for the expression of transforming growth factor-beta 1 (TGF-β1) in the rat liver
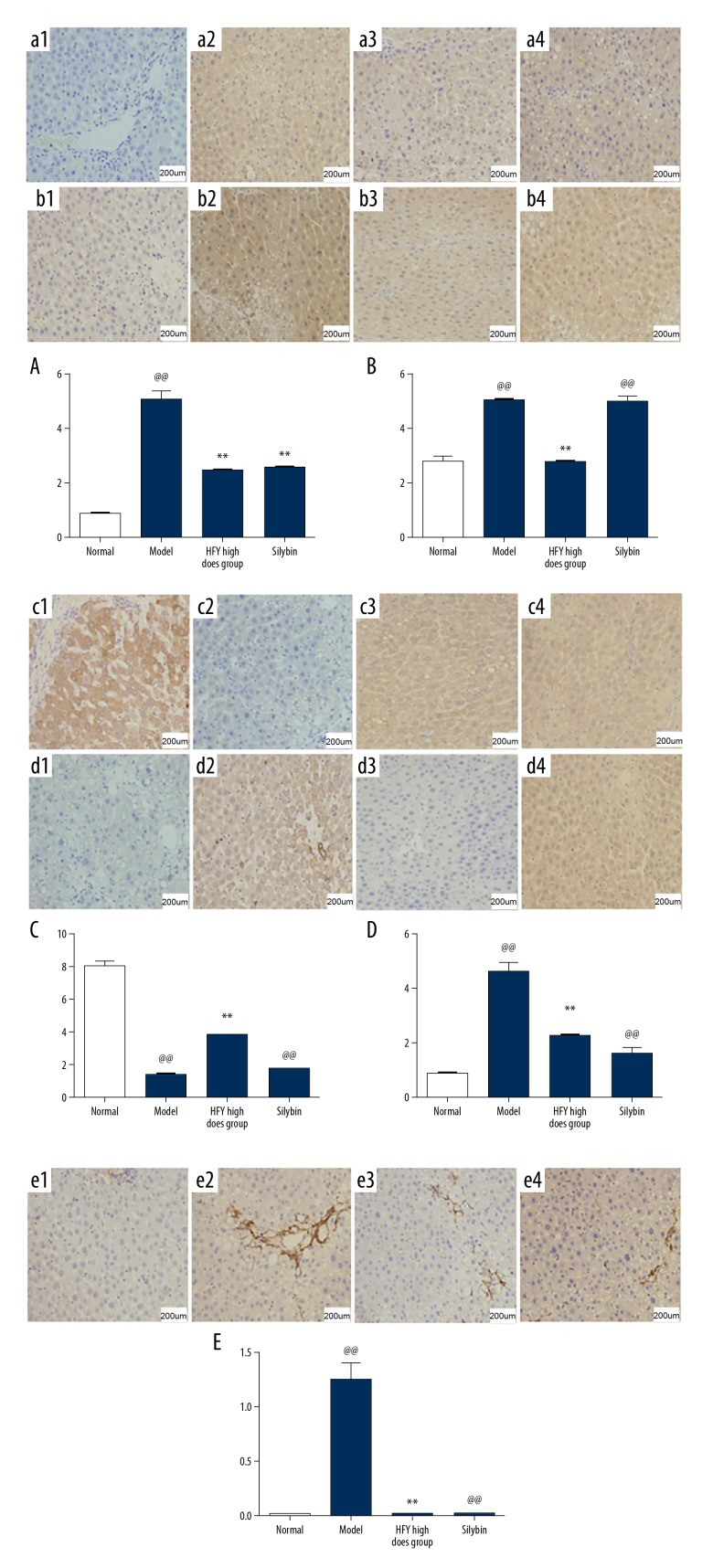
The liver tissue from rats in the normal control group showed low expression of transforming growth factor-beta 1 (TGF-β1) (Figure 5A1). The untreated model group showed the highest levels of expression of TGF-β1 in the liver tissue, mainly in the cytoplasm of hepatocytes, and some in the cells around the central lobular vein and the fibrous septae (intensity score, ++) (Figure 5A2). In rat liver tissue from the model group treated with volatile oil of CG, the degree of immunostaining for TGF-β1 was reduced (intensity score, +) when compared with that found in the untreated model group (Figure 5A3). The expression intensity of the silybin-treated group was weaker than that of the model group, and was weakly positive (intensity score, +) (Figure 5A4). The results of semi-quantitative analysis of the immunostaining of liver tissue for the expression of TGF-β1 showed that the rats in the model group treated with volatile oil of CG had significantly reduced expression when compared with the untreated model group (p<0.05, p<0.01) (Figure 5A).
Figure 5.
Immunohistochemistry of rat liver tissues for the expression of transforming growth factor-beta 1 (TGF-β1), Smad3, Smad7, Toll-like receptor 4 (TLR4), and alpha smooth muscle actin (ASMA) in the rat model of carbon tetrachloride (CCl4)-induced hepatic fibrosis. 1 – the normal rat group; 2 – the untreated rat model group; 3 – the rat model group treated with volatile oil of Cichorium glandulosum Boiss et Huet (CG); 4 – the rat model group treated with silybin. (A) Immunohistochemistry shows the expression of transforming growth factor-beta 1 (TGF-β1) in the rat liver tissue. (B) Immunohistochemistry shows the expression of Smad3 in the rat liver tissue. (C) Immunohistochemistry shows the expression of Smad7 in the rat liver tissue. (D) Immunohistochemistry shows the expression of Toll-like receptor 4 (TLR4) in the rat liver tissue. (E) Immunohistochemistry shows the expression alpha smooth muscle actin (ASMA) in the rat liver tissue. (n=10) (Mean ± SD). Compared with the normal group, @@ p<0.01; compared with the model group, ** p<0.01, * p<0.05.
Immunohistochemistry for the expression of Smad3 in the rat liver
The liver tissue from rats in the normal control group showed weak expression of Smad3 (intensity score, ±) (Figure 5B1). The strongest expression was found in the untreated rat model group, with positive immunostaining in the area around the central vein. In the hepatocytes, there was expression of Smad3 in the nucleus and cytoplasm. There was strongly positive expression of Smad 3in the untreated rat model group (intensity score, +++) (Figure 5B2). Immunostaining for Smad3 in the volatile oil of CG-treated rat model group was weaker than that of model group (intensity score, ++) (Figure 5B3). The expression of Smad3 in the silybin-treated group was significantly lower than that of the model group (intensity score, ++) (Figure 5B4). The results of semi-quantitative analysis of the immunostaining of liver tissue for the expression of Smad3 showed that the rats in the model group treated with volatile oil of CG had significantly reduced expression when compared with the untreated model group (p<0.01) (Figure 5B).
Immunohistochemistry for the expression of Smad7 in the rat liver
The liver tissue from rats in the normal control group showed strong positive immunostaining for Smad7 (intensity score, +++) (Figure 5C1). Immunostaining for Smad7 was localized to the cytoplasm of normal hepatocytes, and there was no immunostaining of sinusoidal and interstitial cells. In the untreated rat model group, there was minimal positive immunostaining for Smad7 (intensity score, ±) (Figure 5C2). In the rat livers from the rat model group treated with volatile oil of CG showed increased immunostaining for Smad7 (intensity score, ++) when compared with the untreated rat model group, and weaker immunostaining when compared with the normal control group (Figure 5C3). The degree of positive immunostaining for Smad7 in the silybin-treated group (intensity score, +) was increased when compared with the untreated model group, and was weaker than that of the normal group (Figure 5C4). The results of semi-quantitative analysis of the immunostaining of liver tissue for the expression of Smad 7 showed that the rats in the model group treated with volatile oil of CG had significantly increased expression when compared with the untreated model group (p<0.01) (Figure 5C).
Immunohistochemistry for the expression of Toll-like receptor 4 (TLR4) in the rat liver
Normal liver tissues from the normal control rat group showed very low expression of TLR4 (intensity score, ±) (Figure 5D1). The expression of TLR4 in the untreated model group was strong, mainly in the cytoplasm of hepatocytes. In the model group, TLR4 immunostaining was moderately positive (intensity score, ++) (Figure 5D2). The expression of TLR4 in the volatile oil of CG-treated group was significantly weaker than that of the untreated model group, and the immunostaining was weakly positive (intensity score, +) (Figure 5D3). The expression intensity of the silybin-treated group was weaker than that of the model group, and was weakly positive (intensity score, +) (Figure 5D4). The results of semi-quantitative analysis of the immunostaining of liver tissue for the expression of TLR4 showed that the rats in the model group treated with volatile oil of CG had significantly reduced expression when compared with the untreated model group (p<0.01) (Figure 5D).
Immunohistochemistry for the expression of alpha smooth muscle actin (ASMA) in the rat liver
Normal liver tissues from the normal control rat group showed weak positive expression of alpha smooth muscle actin (ASMA) in vascular smooth muscle cells and cells in the periportal area of liver tissues (intensity score, +) (Figure 5E1). The rat liver tissue from the untreated model showed increased expression of ASMA, mainly in fibrous stromal cells of liver tissue (intensity score, +++) (Figure 5E2). The expression levels of ASMA in the rat model group treated with volatile oil of CG were significantly lower compared with the untreated model group (intensity score, +) (Figure 5E3). The intensity of immunostaining for ASMA in the silybin-treated group was significantly weaker when compared with the untreated model group (intensity score, +) (Figure 5E4). The results of semi-quantitative analysis of the immunostaining of liver tissue for the expression of ASMA showed that the rats in the model group treated with volatile oil of CG had significantly reduced expression when compared with the untreated model group (p<0.01) (Figure 5E).
Discussion
In this study, gas chromatography-mass spectrometry (GC-MS) isolated and identified the chemical constituents of volatile oil extracted by steam distillation from Cichorium glandulosum Boiss et Huet (CG), a traditional Uyghur medicine, and to study its effects on carbon tetrachloride (CCl4)-induced hepatic fibrosis in rats. Thirty-eight compounds were identified from the volatile oil of CG (total, 98.058%), with terpenoids being the most abundant. In the animal model of liver fibrosis, all doses of volatile oil of CG significantly reduced the serum levels of aspartate transaminase (AST), alanine transaminase (ALT), malondialdehyde (MDA), hydroxyproline (Hyp), γ-glutamyl transpeptidase (γ-GT), lactate dehydrogenase (LDH), alkaline phosphatase (ALP), and albumin (Alb).
GC-MS showed that two materials, the polyvinyl chloride (PVC) plasticizers, diisobutyl phthalate and dibutyl phthalate, were found at the peak time of 58.775 min and 60.511 min. Extracts from these two materials could be filtered with plastic appliances before injection into the rat experimental model. According to recent reports [10,11], the electron ionization (IE) scanning sensitivity of the gas chromatograph used in the experiment included a 1L standard sample of octafluoronaphthalene at a concentration of 1 pg/L, which was injected and scanned in the mass range of 50–300 u. The anion at m/z 272, corresponded to deprotonated gamma-glutamyl-dehydroalanyl-glycine (EdAG). For the m/z 272 ion, the signal-to-noise ratio of turbine pump and diffusion pump was 400: 1 and 200: 1 respectively, which means that the possibility of detection of these two substances exists.
Wu et al. [12] reported that most of the volatile oils in the seeds of CG were long-chain alkanes, but there were no seeds in the whole grass of CG used in this experiment. Terpenoids were the most commonly detected compounds, which showed that the volatile oils in the whole grass and seeds of CG were quite different. In addition to two interfering substances, the other 36 substances were identified to comprise almost 100% of the sample, and the relative content of all substances was 98.058%. On GC-MS, the main components were citronellol, rose ether, isoeugenol, alcohol, and caryophyllin. Therefore, the findings of this study could be used for the quality control of the aerial parts of the Cichorium family of herbal medicines.
By GC-MS analysis, citronella was the highest in volatile oil of CG, which was as high as 74.060%. Citronellol is a terpenoid compound, and terpenoids have been reported to have bactericidal, anti-inflammatory, and anti-tumor physiological activities [13]. However, terpenoids are the main constituents in Cichorium tomentosum L. Our previous study found that the effective fractions with high terpenoids in CG, had significant hepatoprotective and anti-hepatic fibrosis effects [14,15]. Previously published studies have shown that terpenoids from CG also have an antibacterial effect, including for infections of the gastrointestinal tract [16,17]. The transmission of gastrointestinal microbial factors may also promote the development and progression of liver disease, including alcohol-induced liver diseases [18,19], and non-alcoholic steatohepatitis (NASH) [20,21]. A previously published study also found that Lactobacillus acidophilus could disseminate to multiple organs and was associated with the development of autoimmunity, and inflammation of the liver [22]. The antibacterial and hepatoprotective mechanisms of the terpenoids in CG may have synergistic effects.
The findings of the present study showed that the volatile oil of CG had a protective effect on liver fibrosis in a rat model. The effects of the volatile oil of CG might be due to the terpenoid, citronellol, which was found to be the main component on GC-MS. Jiang et al. reported that citronella microemulsion gel had an antimicrobial effect in the treatment of Staphylococcus aureus and Candida albicans [23]. Shi et al. reported that citronellol significantly inhibited the growth and toxin production of Aspergillus flavus [24].
Conclusions
In this study, gas chromatography-mass spectrometry (GC-MS) identified the components of the volatile oil of Cichorium glandulosum Boiss et Huet (CG), a traditional Uyghur medicine, and identified a major component, citronellol. Treatment with volatile oil of CG reduced liver fibrosis in a rat model. Although citronellol has been reported to be a major bacteriostatic agent, its hepatoprotective effect has not previously been reported. The mechanism of the hepatoprotective effect of citronellol may occur by regulating the immune response in the liver through the intestinal route. The preliminary findings from this study in a rat model of liver fibrosis require further investigation using in vitro and in vivo studies.
Footnotes
Source of support: This study was supported by the Natural Science Foundation of China (no. 81560680)
Conflict of interest
None.
References
- 1.Zhang HY, Zhang B, Liu XQ, et al. Assay of herba cichorii polysaccharide content. Chin Trad Pat Med. 2011;33(1):114–17. [Google Scholar]
- 2.Heimler D, Isolani L, Vignolini P, et al. Polyphenol content and antiradical activity of Chechorium intybus L. From biodynamic and conventional farming. Food Chem. 2009;114(3):765–70. [Google Scholar]
- 3.Wu HK, Su Z, Yi Li A, et al. [Components of Cichorium glandulosum seeds]. Chem Nat Compd. 2007;43(1):109. [in Chinese] [Google Scholar]
- 4.Michalska K, Kisiel W. The first guaian-12-oic acid glucopyranosyl ester and other constituents from Picris rhagadioloides (L.) Desf. (Asteraceae) Molecules. 2008;13(2):444–51. doi: 10.3390/molecules13020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu H, Su Z, Yang Y, et al. Isolation of three sesquiterpene lactones from the roots of Cichorium glandulosum Boiss et Huet. by high-speed counter-current chromatography. J Chromatogr A. 2007;1176:217–22. doi: 10.1016/j.chroma.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 6.He MQ, Miao D, Gao F, Ju J. [Chemical constituents of the roots of Cichorium tomentosum]. Pharmaceutical and Clinical Studies. 2014;22(3):234–36. [in Chinese] [Google Scholar]
- 7.Hu J, Dilibel M, Li Y, et al. [Simultaneous determination of 5 chemical constituents in chicory and chicory by UPLC]. Chinese Journal of Experimental Traditional Medical Formulae. 2014;17:65–68. [in Chinese] [Google Scholar]
- 8.El Lakany AM, Ghani MMA. Chemical constituents and biological activities of Cichorium intybus L. Nat Prod Sci. 2004;10(2):69–73. [Google Scholar]
- 9.Pan L, Jia X, Shi M, et al. [Studies on the chemical constituents of Chicory]. Journal of Xinjiang Medical University. 2015;9:1088–90. [in Chinese] [Google Scholar]
- 10.Zeng P. [How to choose test level in statistics]. Chinese Journal of Cardiovascular Medicine. 2011;1:25. [in Chinese] [Google Scholar]
- 11.Agilent 5975C series MSD Operation Manual. Available at: https://www.agilent.com/cs/library/usermanuals/public/G3170-90036.pdf.
- 12.Wu H, Fan Y, Liao BL, Aijiaikebeier A. [Chemical constituents of essential oils from chicory seeds were analyzed by GC-MS]. Spectroscopic Laboratory. 2005;22:7. [in Chinese] [Google Scholar]
- 13.Mou Y, Yan H, Gong L. [General situation of research on terpenoids]. Chemical Enterprise Management. 2018;11:12–13. [in Chinese] [Google Scholar]
- 14.Qin D, Wen Z, Nie Y, Yao G. Effect of Cichorium glandulosum extracts on CCl4-induced hepatic fibrosis. Iran Red Crescent Med J. 2013;15(12):1–8. doi: 10.5812/ircmj.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin D, Nie Y, Wen Z. Protection of rats from thioacetamide-induced hepatic fibrosis by the extracts of a traditional Uighur medicine Cichorium glandulosum. Iran J Basic Med Sci. 2014;17(11):880–85. [PMC free article] [PubMed] [Google Scholar]
- 16.Kamada N, Seo SU, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 17.Nakamoto N, Amiya T, Aoki R, et al. Commensal Lactobacillus controls immune tolerance during acute liver injury in mice. Cell Rep. 2017;21:1215–26. doi: 10.1016/j.celrep.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Betrapally NS, Gillevet PM, Bajaj JS. Changes in the intestinal microbiome and alcoholic and nonalcoholic liver diseases: Causes or effects? Gastroenterology. 2016;150:1745–55.e3. doi: 10.1053/j.gastro.2016.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012;302(9):G966–78. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–75. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: A connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 22.Manfredo Vieira S, Hiltensperger M, Kumar V, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–61. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang F, Wang Y, Yan J, et al. [Preparation of citronella microemulsion gel and its antibacterial test in vitro]. Chinese Journal of Experimental Traditional Medical Formulae. 2017;20:8–13. [in Chinese] [Google Scholar]
- 24.Shi C, Zhang C, Du Y, et al. Effects of different plant inhibitors on the growth and toxicity of Aspergillus flavus. J Food Saf Qual. 2017;8:2892–97. [Google Scholar]