Abstract
Background
Diarrhoeal diseases are a leading cause of mortality and morbidity, especially among young children in low‐income countries, and are associated with exposure to human excreta.
Objectives
To assess the effectiveness of interventions to improve the disposal of human excreta for preventing diarrhoeal diseases.
Search methods
We searched the Cochrane Infectious Disease Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; the metaRegister of Controlled Trials (mRCT); and Chinese‐language databases available under the Wan Fang portal, and the China National Knowledge Infrastructure (CNKI‐CAJ). We also handsearched relevant conference proceedings, and contacted researchers and organizations working in the field, as well as checking references from identified studies.
Selection criteria
Randomized, quasi‐randomized, and non‐randomized controlled trials (RCTs) were selected, comparing interventions aimed at improving the disposal of human excreta to reduce direct or indirect human contact with no such intervention. Cluster (eg at the level of household or community) controlled trials were included.
Data collection and analysis
We determined study eligibility, extracted data, and assessed methodological quality in accordance with the methods prescribed by the protocol. We described the results and summarized the information in tables. Due to substantial heterogeneity among the studies in terms of study design and type of intervention, no pooled effects were calculated.
Main results
Thirteen studies from six countries covering over 33,400 children and adults in rural, urban, and school settings met the review's inclusion criteria. In all studies the intervention was allocated at the community level. While the studies reported a wide range of effects, 11 of the 13 studies found the intervention was protective against diarrhoea. Differences in study populations and settings, in baseline sanitation levels, water, and hygiene practices, in types of interventions, study methodologies, compliance and coverage levels, and in case definitions and outcome surveillance limit the comparability of results of the studies included in this review. The validity of most individual study results are further compromised by the non‐random allocation of the intervention among study clusters, an insufficient number of clusters, the lack of adjustment for clustering, unclear loss to follow‐up, potential for reporting bias and other methodological shortcomings.
Authors' conclusions
This review provides some evidence that interventions to improve excreta disposal are effective in preventing diarrhoeal disease. However, this conclusion is based primarily on the consistency of the evidence of beneficial effects. The quality of the evidence is generally poor and does not allow for quantification of any such effect. The wide range of estimates of the effects of the intervention may be due to clinical and methodological heterogeneity among the studies, as well as to other important differences, including exposure levels, types of interventions, and different degrees of observer and respondent bias. Rigorous studies in multiple settings are needed to clarify the potential effectiveness of excreta disposal on diarrhoea.
Keywords: Adult, Child, Humans, Feces, Diarrhea, Diarrhea/prevention & control, Sanitation, Sanitation/methods, Waste Management, Waste Management/methods
Interventions to improve disposal of human excreta for preventing diarrhoea
Diarrhoea is a major cause of death and disease, especially among young children in low‐income countries. Many of the microbial agents associated with diarrhoea are transmitted via the faecal‐oral route and are associated with exposure to human faeces. This review examined trials of interventions to improve the safe disposal of human faeces to prevent diarrhoea. In low‐income settings, among the estimated 2.6 billion people who lack basic sanitation, this mainly consists of introducing or expanding the number and use of latrines and other facilities to contain or dispose of faeces. We identified 13 studies of such interventions involving more than 33,400 people in six countries. These trials provide some evidence that excreta disposal interventions are effective in preventing diarrhoeal diseases. However, major differences among the studies, including the conditions in which they were conducted and the types of interventions deployed, as well as methodological deficiencies in the studies themselves, makes it impossible to estimate with precision the protective effective of sanitation against diarrhoea. Further research, including randomized controlled trials, is necessary to understand the full impact of these interventions.
Summary of findings
Summary of findings for the main comparison.
Measure of effect
| Reference | Outcome | Measure of effect | Estimate of effect* | Researchers' conclusion |
| Aziz 1990 | Incidence of diarrhoea | Risk ratio | 0.75 | Intervention beneficial |
| Garrett 2008 | Incidence of diarrhoea | Risk ratio | 0.71 (0.54‐0.92) | Intervention beneficial |
| Hu 1988 | Incidence of diarrhoea | Risk ratio | 0.56 | Intervention beneficial |
| Huttly 1990 | Incidence of diarrhoea | Risk ratio | 1.03 | No difference in effect |
| McCabe 1957 | Incidence of diarrhoea | Risk ratio | 0.53 | Intervention beneficial |
| Messou 1997 | Incidence of diarrhoea | Risk ratio | 0.64 | Intervention beneficial |
| Rubenstein 1965 | Incidence of diarrhoea | Risk ratio | 0.33 | Intervention beneficial |
| Wei 1998 | Incidence of diarrhoea | Risk ratio | 0.20 | Intervention beneficial |
| Xu 1990 | Incidence of diarrhoea | Risk ratio | 0.80 | Intervention beneficial |
| Xu 1994 | Incidence of diarrhoea | Risk ratio | 0.94 (0.54‐1.64) | No difference in effect |
| Yan 1986 | Incidence of diarrhoea | Risk ratio | 0.43 | Intervention beneficial |
| Zhang 2000 | Incidence of diarrhoea | Risk ratio | 0.37 | Intervention beneficial |
| Zhu 1997 | Incidence of diarrhoea | Risk ratio | 0.40 | Intervention beneficial |
| *Except for Garrett (2008) and Xu 1994, confidence intervals could not be calculated for these measures of effect due to an insufficient number of clusters. Refer to Methods. | ||||
Background
Introduction
An estimated 2.6 billion people or 39% of the world's population lack access to improved facilities for the disposal of human excreta, such as a basic pit latrine, a toilet connected to a septic tank or piped sewer system, or a composting toilet according to the World Health Organization (WHO) and the United Nations Children's Fund (UNICEF) (WHO/UNICEF 2010). In low‐income regions, where people are most vulnerable to infection and disease, only one in two people is covered by improved sanitation. More than one billion people still practice open defecation. In sub‐Saharan Africa and southern Asia coverage is just 31% and 33%, respectively. While the global population in 2006 is about equally divided between urban and rural dwellers, more than seven out of 10 people living without improved sanitation are rural inhabitants (WHO/UNICEF 2010).
The shortfall in sanitation coverage is not the result of a failure to recognize the need for it or declare goals to meet this need at the highest international level. The 1977 Mar del Plata Declaration by the United Nations expressed the goal of providing safe water and sanitation for all by 1990, launching the Water and Sanitation Decade (1981 to 1990). In 1990 the United Nations renewed the call and extended the deadline to the end of the 20th century. While sanitation was first omitted from the United Nations Millennium Development Goals (MDGs), it was added to the water target at the Johannesburg World Summit on Sustainable Development in 2002. Target 10 of Goal 7 is less ambitious than its predecessors , seeking only to reduce by half the portion of the population without access to 'basic' sanitation. Even so, the evidence suggests that current efforts will fall far short of even this scaleddown target. At the current rate, the world will miss the MDG sanitation target by 13 percentage points; in 2015, the number of people without basic sanitation will actually rise to 2.7 billion (WHO/UNICEF 2010). In sub‐Saharan Africa, where only 31% of people have access to improved sanitation, current efforts will actually result in an increase in the number who do not by 91 million (UNDP 2007; WHO/UNICEF 2005). Even if the MDG target could be met, it would still leave well more than 1.7 billion without such access.
Definitions of sanitation
In the broadest sense, sanitation deals not only with the collection, storage, treatment, disposal, reuse or recycling of human excreta (faeces and urine), but also the drainage, disposal, recycling, and re‐use of wastewater and storm water (sullage), and household, industrial, and hazardous solid waste. The MDG target, which is expressed in terms of 'basic sanitation', followed this broader approach and also included concepts of affordability, cultural acceptability, and environmental sustainability (United Nations 2002). The United Nations Millennium Task Force on Water and Sanitation attempted to consolidate these notions, defining basic sanitation as "the lowest‐cost option for securing sustainable access to safe, hygienic, and convenient facilities and services for excreta and sullage disposal that provide privacy and dignity, while at the same time ensuring a clean and healthful living environment both at home and in the neighbourhood of users" (Millennium Project 2005). The MDG definition is context specific. In dispersed, low‐income rural areas it may include a simple pit latrine, while in congested urban slums with a reliable water service, household‐based solutions would be deemed inadequate and low‐cost sewerage systems would be necessary to ensure the proper collection, treatment, and disposal or reuse of excreta and household wastewater (Millennium Project 2005).
The Joint Monitoring Programme for Water Supply and Sanitation (JMP) defines improved sanitation and unimproved sanitation in terms of the facilities for the disposal of human excreta (WHO 2002). Improved sanitation includes a private flush or pour‐flush toilet or latrine connected to a piped sewer system or septic system, a simple pit latrine with a slab, a ventilated improved pit (VIP) latrine or a composting toilet. Unimproved sanitation includes any other flush or pour‐flush latrine, an open pit latrine, bucket latrines, a hanging latrine, any public or shared facility or open defecation (WHO/UNICEF 2002).
Neither set of definitions is strictly health‐based. The MDG definition addresses not only safety and hygiene but also convenience, cost, privacy, and dignity. It emphasizes household and community impact, sustainability, and actual use by the population so protected. While the JMP classification is intended to reflect the health risk associated with safe excreta disposal, its distinction between improved and unimproved facilities is based mainly on observable criteria developed by the JMP to facilitate surveys of provision and promote the upgrading of facilities. The differences between these definitions are not merely academic; funding and other resources are largely directed at increasing levels of provision reported by the JMP and meeting MDG targets (UNDP 2007). At a minimum, however, both definitions agree that sanitation must include the safe disposal of human excreta, a criterion that is founded in health.
For this reason, this review focuses on sanitation interventions to introduce or expand the provision or use of facilities for excreta disposal. This includes steps to reduce open defecation by constructing basic sanitation in accordance with the MDG target. It also includes interventions to improve the disposal of child faeces, such as by promoting potties, when accompanied by the safe disposal of their contents (Traore 1994). This review does not extend, however, to interventions that are not principally aimed at the sanitary disposal of human faeces. Thus, it does not include efforts to use human waste in agricultural applications; an activity that may actually increase risks to health.
Diarrhoeal disease, disease agents, and pathways
Diarrhoeal diseases kill an estimated 1.8 million people each year (WHO 2005). Among infectious diseases, diarrhoea ranks as the third leading cause of both mortality and morbidity (after respiratory infections and HIV/AIDS). Young children are especially vulnerable, bearing 68% of the total burden of diarrhoeal disease (Bartram 2003). Among children younger than five years of age, diarrhoea accounts for 17% of all deaths (United Nations 2005).
The immediate threat from diarrhoea is dehydration, and a loss of fluids and electrolytes. Thus, the widespread promotion of oral rehydration therapy has significantly reduced the case‐fatality rate associated with the disease. Such improvements in case management, however, have not reduced morbidity, which is estimated at four billion cases annually (Kosek 2003). And since diarrhoeal diseases inhibit normal ingestion of foods and adsorption of nutrients, continued high morbidity is an important cause of malnutrition, leading to impaired physical growth and cognitive function (Guerrant 1999; Petri 20089), reduced resistance to infection (Baqui 1993), and potentially long‐term gastrointestinal disorders (Schneider 1978). With continued high attack rates, diarrhoeal disease is also an enormous economic burden, resulting in significant direct costs to the health sector and patients for treatment as well as in lost time at school, work, and in other productive activities (Mulligan 2005).
The infectious agents associated with diarrhoeal disease are transmitted chiefly through the faecal‐oral route (Byers 2001). A wide variety of bacterial, viral, and protozoan pathogens excreted in the faeces of humans and animals are known to cause diarrhoea. Among the most important of these are Escherichia coli, Salmonella spp., Shigella spp., Campylobacter jejuni, Vibrio cholerae, rotavirus, norovirus, Giardia lamblia, Cryptosporidium spp., and Entamoeba histolytica (Leclerc 2002). The importance of individual pathogens varies between settings, seasons, and conditions.
These pathogens may be transmitted through the ingestion of contaminated food, water or other beverages, by person‐to‐person contact, and by direct or indirect contact with infected faeces. Because of this variety of pathways, environmental interventions for the prevention of diarrhoeal disease typically include steps to improve the proper disposal of human faeces (sanitation), as well as improving water quality (Clasen 2006), water quantity and access, and promoting hand washing and other hygiene practices (Curtis 2003; Ejemot 2008).
In addition to diarrhoea, there are other important risks to health associated with poor sanitation. These include schistosomiasis, soil‐transmitted helminth infection (including ascariasis, trichuriasis, and hookworm infection), trachoma (Emerson 2004), and tropical enteropathy. Tropical enteropathy, a subclinical disorder of the small intestine caused by faecal bacteria ingested in large quantities by young children living in conditions of poor sanitation and hygiene, may be a substantial cause of under‐nutrition in young children that is entirely separate from diarrhoea (Humphrey 2009).
Excreta disposal and diarrhoea
While the biological association between diarrhoea and exposure to human faeces is well established, there is little rigorous epidemiological evidence of the effectiveness of sanitation interventions to prevent disease. Much of the evidence of the effectiveness and mechanisms of improved sanitation to prevent diarrhoea derives from observational studies (Barreto 2007; Genser 2008; Green 2009). There is little evidence of this from intervention studies. A previous Cochrane Review examined environmental sanitary interventions but it was limited to interventions to prevent active trachoma (Rabiu 2005).
There are three previous reviews of excreta disposal interventions (Esrey 1985; Esrey 1991; Fewtrell 2005; Waddington 2009 ). Esrey and colleagues identified 10 studies of improvements in excreta disposal with a median reduction in diarrhoea of 22% (ranging from 0% to 48%) (Esrey 1985). A subsequent review of 'sanitation' interventions reported a median reduction of 30% from 11 studies (36% from the five studies the investigators deemed to be rigorous) (Esrey 1991). Esrey and colleagues based their conclusions chiefly on observational studies. In addition to the confounding and bias inherent in such studies, Esrey and others have pointed out significant and widespread methodological problems in these studies (Blum 1983; Esrey 1986). Although these previous reviews were helpful in identifying the broad questions and suggesting answers, they did not employ the more rigorous methodologies and statistical methods of a systematic review (Egger 2001). In terms of coverage, for example, neither study (Blum 1983; Esrey 1986) involved a comprehensive search strategy. The reviews were also limited to studies in the English language. With respect to statistical methods, the simple use of the median fails to take into account the size of the study and the variance observed in the results (Deeks 2001). Moreover, they did not distinguish between the various case definitions (Moy 1991) and measures of diarrhoea morbidity (Morris 1996; Pickering 1987). Also, while Esrey attempted to incorporate qualitative criteria in the reviews, there was no independent assessment of the study quality or, for that matter, whether the studies identified met the inclusion criteria.
Fewtrell and colleagues conducted a more formal systematic review and meta‐analysis of environmental interventions against diarrhoea (Fewtrell 2005). They identified just four such studies of improved sanitation, only two of which provided data that they could use in a meta‐analysis. Fewtrell and colleagues reported that the interventions weree protective, with a pooled risk ratio (RR) of 0.68 (95% confidence interval (CI) 0.53 to 0.87) − a 32% reduction in diarrhoea that would appear to be consistent with Esrey's findings. In addition to being based on just two studies, however, there are other reasons to question the evidential weight of this estimate. Firstly, the review was limited to published studies and did not include a search of Chinese‐language databases in which a number of articles on sanitation interventions are believed to be indexed. Secondly, one of the two studies identified in the review (Azurin 1974) had cholera rather than general diarrhoea as its outcome. Cholera is usually a source of epidemic diarrhoea against which environmental interventions tend to be more effective than can be expected for general diarrhoea, leading to results that cannot be generalized to endemic diarrhoea (Gundry 2004). The other study (Daniels 1990) followed an observational design, and thus did not meet the eligibility criteria. Emerson and colleagues (Emerson 2004) have demonstrated that a health impact (trachoma) from latrines can be investigated using the more rigorous randomized, controlled trial design.
Waddington and colleagues undertook an update of the Fewtrell review (Waddington 2009). They identified six sanitation studies that met their inclusion criteria, yielding a pooled RR of 0.63 (95% CI 0.43 to 0.93). However, the pooled estimate of the three studies they regarded as being of 'high quality' was not statistically significant (0.64, 95% CI 0.37 to 1.10), and none of the six sanitation studies included in their review followed an intervention design.
Beyond the paucity of rigorous epidemiological evidence in support of sanitation interventions, there is relatively little evidence of the acceptability, scalability, and sustainability of steps to improve excreta disposal, especially in rural settings where provision is lowest (Jenkins 2005).
This review employs the rigorous methodology and other benefits of the Cochrane Collaboration to identify and summarize evidence on the effectiveness of sanitation interventions to prevent diarrhoeal diarrhoea. By highlighting such evidence or the paucity thereof, it seeks to remove a knowledge barrier that may be contributing to a comparatively slow progress in achieving the sanitation target of the MDGs.
Objectives
To assess the effectiveness of interventions to improve human excreta disposal for preventing diarrhoeal disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, quasi‐randomized, and non‐randomized controlled trials (RCTs). The unit of randomization may include individuals, families, households, villages, communities or other clusters.
Types of participants
Children and adults in any country or population.
Types of interventions
Interventions
Interventions aimed at introducing or expanding the coverage and use of facilities designed to reduce direct or indirect contact with human faeces. Such facilities including simple pit latrines, VIP latrines, bucket latrines, hanging toilets, water sealed pour‐flush toilets (whether or not connected to a vault, septic tank or sewer), and composting toilets. It also includes the promotion of apparatus to improve the safe disposal of child faeces, such as potties and scoops, when accompanied by improved disposal of their contents.
We included interventions that combine improvements in excreta disposal with other environmental interventions such as improvements in water quantity or access, and in water quality or in hygiene practices.
Control
Study participants who practice open defecation or who continue to follow their current practices with respect to excreta disposal rather than the prescribed intervention.
Types of outcome measures
Primary
Diarrhoea episodes among individuals, whether or not confirmed by microbiological examination.
The WHO's definition of diarrhoea is three or more loose or fluid stools (that take the shape of the container) in a 24‐hour period (WHO 1993). We defined diarrhoea and an episode in accordance with the case definitions used in each trial. We excluded trials that had no clinical outcomes; for example, trials that report only on microbiological pathogens in the stool. Where data were provided, we extracted and analysed data from the studies describing the method of diarrhoea surveillance and reporting, the severity of diarrhoea, hospital admission, and measures taken by individuals in response to diarrhoea.
Secondary
mortality
adverse events
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (whether published, unpublished, in press or ongoing).
Databases
We searched the following databases using the search terms detailed in Appendix 1: Cochrane Infectious Disease Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; and LILACS. We also searched Chinese‐language databases (Fung 2008) available under the China National Knowledge Infrastructure (CNKI‐CAJ) using comparable Chinese‐language search terms. We also searched the metaRegister of Controlled Trials (mRCT) using 'diarrhoea' and 'sanitation or latrine or toilet or privy or disposal' as search terms.
Conference proceedings
We searched the conference proceedings of the following organizations for relevant abstracts: International Water Association and the Water, Engineering and Development Centre, Loughborough University, UK.
Organizations and pharmaceutical companies
We contacted researchers and organizations including the Water, Sanitation and Health Programme of the WHO; World Bank Water and Sanitation Program; UNICEF Water, Environment and Sanitation; Environmental Health Project; IRC International Water and Sanitation Centre; Foodborne and Diarrheal Diseases Branch, Division of Bacterial and Mycotics Diseases, Centers for Disease Control and Prevention (CDC); US Agency for International Development (USAID); and the UK Department for International Development (DFID) for unpublished and ongoing trials.
Reference lists
We checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Except for Chinese‐language search results, Thomas Clasen (TC) and Kristof Boeston (KB) independently reviewed the titles and abstracts resulting from the searches and selected all studies that potentially met the inclusion criteria for the review. After obtaining full copies of all such studies, they independently determined if the trial met the inclusion criteria by completing an eligibility form. For Chinese‐language search results, Isaac Fung (IF) undertook the same process individually and summarized the article in English, and TC and Wolf‐Peter Schmidt (WS) reviewed the summaries to independently determine the eligibility of the study. Potentially relevant studies that we ultimately deemed did not meet the eligibility criteria for the study are nevertheless identified together with the reason for exclusion in the Characteristics of excluded studies section.
Data extraction and management
One author used a pre‐piloted form to extract and record the data described in Appendix 2, and attempted to contact authors to supply missing data. We recorded morbidity based on the measure used in the trial. We recorded whether the effect of the intervention on diarrhoea was expressed as a prevalence ratio (binary outcome), a cumulative RR (binary outcome) or an incidence rate ratio (count variable). We extracted the number of participants and events to calculate risk : prevalence ratios. We extracted the number of events and person‐time at risk for the calculation of incidence rate ratio. TC entered the extracted data into Review Manager 5.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias of each trial using an assessment form. While the protocol for the review contemplated assessing RCTs using the generation of allocation sequence, allocation concealment, blinding, and loss to follow‐up, no RCTs were actually identified that met the review's eligibility criteria. We assessed quasi‐randomized and non‐RCTs using the following criteria.
Comparability of characteristics between the intervention and control groups with respect to relevant baseline characteristics such as water quality, diarrhoeal morbidity, age, socioeconomic status, access to water, hygiene practices, and sanitation facilities. We classify this as adequate if no substantial differences are present; unclear if they are not reported or it is not known if substantial differences exist; or inadequate if one or more substantial difference exists.
Data collection for intervention and control groups at the same time. We classify this as adequate if the data were collected at similar points in time; unclear if this is not reported or not clear from the trial; or inadequate if data were not collected at similar points in time.
Loss to follow‐up. We classed the inclusion of randomized participants in the analysis as adequate if 90% or more of all participants enrolled at the outset of the trial were included in the analysis; unclear if it is not clear what portion of the participants who were enrolled at the outset of the trial were included in the analysis; or inadequate if less than 90% of all participants enrolled at the outset of the trial were included in the analysis.
Assessment of reporting biases
We plotted the RR against number of clusters in each study to explore the existence of publication bias. While this is not a classic funnel plot where the effect size is plotted against the standard error of the effect, this approach was the only option since most studies included too few clusters to calculate standard errors (Donner 2000).
Data synthesis
We compiled the data using Review Manager 5. Although trials of environmental intervention such as sanitation assess outcomes on an individual level, the unit of randomization is often not the individual but a household, a group of households, a school, a neighbourhood or a village. Some trials correct for this design effect by adjusting for the intra‐cluster correlation.
Where applicable, we calculated overall point estimates and 95% CI as the mean of the log cluster‐level data, following methods for paired and unpaired cluster randomized trials suggested by Bennett and colleagues (Bennett 2002). If cluster‐level disease rates were not reported, we relied on the point estimate and 95% CI given by the trial authors, provided that clustering had been accounted for in the analysis. In trials meeting the inclusion criteria but failing to adequately adjust for clustering (or to provide the data to allow adjustment), we extracted the unadjusted point estimates but rejected the 95% CI. We did not calculate the CI for trials with fewer than four clusters per study arm (Donner 2000). We pre‐specified that cluster randomized trials that did not adjust for clustering would not be combined with individual randomized trials in the meta‐analysis or tables. Due to substantial methodological heterogeneity among the studies included in the review and the absence of unreliable CI for most estimates of effect, we determined that a meta‐analysis was inappropriate.
Subgroup analysis and investigation of heterogeneity
Due to substantial clinical and methodological heterogeneity among the studies included in the review, we determined that subgroup analysis to investigate heterogeneity in outcomes was inappropriate. For the same reason, no sensitivity analysis was undertaken.
Results
Description of studies
Search results
Execution of the search strategy yielded 2028 titles and abstracts. These titles and abstracts were screened, and the full text articles of 38 studies were obtained for further assessment. Of these 38 studies, 13 met the review's inclusion criteria (see Characteristics of included studies), while 25 were excluded for other reasons (see Characteristics of excluded studies). Of the 13 included trials, all were published in journals. Seven of the studies were published in Chinese (Hu 1988, Wei 1998; Xu 1990; Xu 1994; Yan 1986; Zhang 2000; Zhu 1997), five in English (Aziz 1990; Garrett 2008; Huttly 1990; McCabe 1957; Rubenstein 1965), and one in French (Messou 1997). We worked with the original language version of each study.
Study characteristics
In all the trials included in the review, the intervention was allocated at a cluster (village/community, school, household) level and not at the individual level. Further details on the number and types of clusters is included in Characteristics of included studies.
The trials used a variety of methods for defining, assessing, and reporting outcomes. Eight trials used the WHO definition of diarrhoea (three or more loose stools in 24 hours) as the case definition of the disease (Aziz 1990; Garrett 2008; Hu 1988; Huttly 1990; Wei 1998; Xu 1994; Zhang 2000; Zhu 1997), two used hospital records (Rubenstein 1965; Xu 1990), and three reported no case definition (McCabe 1957; Messou 1997; Yan 1986). The episodes were either reported to the investigator without clinical confirmation (Aziz 1990;Garrett 2008; McCabe 1957; Messou 1997; Wei 1998; Xu 1994; Yan 1986), recorded by the participant or head of household (Huttly 1990; Zhu 1997) or based on hospital admission records (Rubenstein 1965; Xu 1990). Recall for diarrhoeal disease episode reporting was over periods of seven days (Aziz 1990; Garrett 2008), eight days (Huttly 1990), 15 days (Messou 1997), one month (Hu 1988; McCabe 1957; Xu 1994; Yan 1986) or a calendar season (Wei 1998). In one study, surveillance was through individuals reporting to clinics, with a four‐week follow‐up of non‐reporters (Zhang 2000). For each of the studies included in the review, the measure of diarrhoeal disease frequency was the cumulative incidence risk and the estimate of the effect of the intervention was a RR.
The follow‐up period for assessing the outcomes ranged from eight weeks (Garrett 2008) to 10 years (Zhang 2000), with a median of 15 months (Characteristics of included studies).
Only Garrett 2008 adjusted the data for clustering at the household level and for repeated observations of the same patients. As data collected in such cases are not independent observations, the failure to make such adjustments may result in overstating the precision of the measures of effect reported by such studies.
In addition to diarrhoea, other health outcomes included anthropometrics (Huttly 1990), outpatient visits and admissions for diarrhoea and all causes (Rubenstein 1965), the prevalence of positive shigella cultures from stool samples (McCabe 1957), and deaths related to diarrhoea (Messou 1997),
Study participants and settings
Details of the participants and setting for each trial appear in the Characteristics of included studies. Collectively, the 13 trials included in this review covered at least 33,417 participants, excluding two studies (Hu 1988; Wei 1998) that did not report the number of participants. The study populations ranged from 124 to 14,787 participants, with a mean of 3245 and a median of 1732.
Five studies reported outcomes for all ages (McCabe 1957; Xu 1990; Xu 1994; Yan 1986; Zhang 2000). The other studies limited participants to children under one year of age (Rubenstein 1965), under five years of age (Aziz 1990; Garrett 2008; Messou 1997), under six years of age (Hu 1988; Huttly 1990), and primary or secondary school children (Wei 1998; Zhu 1997). As the effect of sanitation on diarrhoea and certain infections may vary considerably with age, the comparability of results must be viewed in light of these significant differences in ages of the study populations.
All the included studies were conducted in low‐income or middle‐income settings. Seven were conducted in China (Hu 1988; Wei 1998; Xu 1990; Xu 1994; Yan 1986; Zhang 2000; Zhu 1997), two in rural USA (McCabe 1957; Rubenstein 1965), and one each in Bangladesh (Aziz 1990), the Ivory Coast (Messou 1997), Kenya (Garrett 2008), and Nigeria (Huttly 1990). Most studies were done in rural settings, though others were done in urban, suburban or school settings (Characteristics of included studies).
There was uncertainty, and considerable heterogeneity, in the pre‐intervention (control) settings in which the interventions were implemented (Table 2). Six of the included studies did not provide clear information on the pre‐intervention excreta disposal facilities of the study population even though this served as the control. Among the other seven studies that did provide this information, one reported open defecation (Aziz 1990) or a combination of open defecation or open pits (Garrett 2008; Huttly 1990). Others described the sanitation facilities of the controls as open pits (Zhang 2000), 'pit latrines' (Yan 1986), 'unsatisfactory facilities, including surface privies' (McCabe 1957) or 'shallow pit or single urns' (Hu 1988). Other pre‐intervention (control) conditions potentially relevant to the outcome of interest also varied or were not described in the studies. With respect to water supplies, five studies reported systems that would qualify as improved under WHO/UNICEF JMP definitions, two were unimproved and six studies provided insufficient information from which water supplies could be assessed (Table 2). None of the studies reported previous hygiene instruction in the settings prior to undertaking the intervention.
Table 1.
Pre‐intervention setting (used as control)
| Reference | Type of excreta disposal facility | Type of water supply* |
| Aziz 1990 | Open defecation | Improved |
| Garrett 2008 | 27% coverage of improved latrines | Unimproved |
| Hu 1988 | Shallow pit or single urn latrine | Improved |
| Huttly 1990 | Open defecation and open pit | Unimproved |
| McCabe 1957 | 52% unsatisfactory facilities, including surface privies; remainder had toilets connected to sewerage system with treatment | Unclear |
| Messou 1997 | Unclear | Unclear |
| Rubenstein 1965 | Unclear | Improved |
| Wei 1998 | Unclear | Unclear |
| Xu 1990 | Unclear ("normal toilet") | Improved |
| Xu 1994 | Unclear | Unclear |
| Yan 1986 | Pit latrine with no management of faeces removal | Unclear |
| Zhang 2000 | Open pit | Improved |
| Zhu 1997 | Unclear | Unclear |
| *Definition based on WHO/UNICEF 2002 | ||
Interventions
The studies included in this review involved a wide variety of interventions to improve excreta disposal facilities (Table 3). These consisted of the promotion or construction of VIP latrines (Huttly 1990), VIP latrines or sanitary platforms over pit latrines (Garrett 2008); borehole latrines with wooden superstructures and concrete slabs (McCabe 1957); household flush toilets connected to septic tanks or biogas reactors (Hu 1988); private latrines connected to a piped water system (Rubenstein 1965; Zhang 2000); private, multi‐compartment water‐sealed toilets (Aziz 1990; Yan 1986; Zhang 2000); school‐based latrines (Wei 1998; Zhu 1997), and public latrines (Messou 1997; Xu 1990).
Table 2.
Interventions
| Reference | Sanitation intervention | Sanitation coverage reached | Compliance with sanitation intervention | Other intervention components |
| Aziz 1990 | Double pit latrine | Unspecified | 88% use in 1987; 83% in use in 1993 | Improved water supply, hygiene promotion |
| Garrett 2008 | Sanitary platforms and ventilated improved pit (VIP) latrines | Baseline coverage was 39% in intervention households versus 27% for controls (P = 0.003). Coverage increased to 49% of intervention households versus 27% for controls (P < 0.001) | Unspecified | Improved water supply; household water treatment; hygiene promotion |
| Hu 1988 | Biogas latrine connected to fermentation reactor | Unspecified | Unspecified | None |
| Huttly 1990 | VIP latrines | 46% of households in intervention group had latrines at the end of the intervention | Unspecified | Improved water supply; hygiene promotion |
| McCabe 1957 | Bored hole privy | 100% | "Almost everyone" | None |
| Messou 1997 | Shared (public) double pit latrines | Unclear; designed to be shared by 10 people | Unspecified | Improved water supply; hygiene promotion; oral hydration therapy |
| Rubenstein 1965 | Water‐sealed pour‐flush latrines | Unspecified | Unspecified | Improved water supply |
| Wei 1998 | Unspecified | Unspecified | Unspecified | Improved handwashing facilities, hygiene promotion |
| Xiao 1997 | Relocate toilets away from water sources | Unspecified | Unspecified | Improved water supply; hygiene promotion |
| Xu 1990 | Public toilet connected to septic tank | Unspecified | Unspecified | None |
| Xu 1994 | Multi‐chambered toilets connected to septic tank or biogas reactor | 80% coverage at the end of the intervention period | Unspecified | None |
| Yan 1986 | Double urn funnel toilet | 85% coverage at the end of the intervention period | Unspecified | None |
| Zhang 2000 | Double urn funnel toilet | 91.12% coverage in 1986; 83.26% of toilets still in a good condition in 1996. | Unspecified | Improved water supply |
| Zhu 1997 | Flush toilets connected to septic tank; multi‐chamber latrines connected; open pit latrines | Unspecified | Unspecified | Improved hygiene facilities; health and hygiene promotion |
In two studies the intervention appeared to be mainly concerned with the capture and use of human faeces in biogas reactors (Hu 1988; Xu 1994 ). While this intervention meets the eligibility criteria for this review, it may have been driven by energy rather than health objectives.
As can be seen in Table 3, only five studies (Hu 1988; McCabe 1957;Xu 1990; Xu 1994; Yan 1986) included interventions that consisted solely of improvements to excreta disposal. In all but one of the other studies (Zhu 1997), the improvement in excreta disposal was, at the least, accompanied by improvements in water supplies, and in all but two studies (Rubenstein 1965; Zhang 2000), there was also a hygiene promotion component. In the other studies the excreta disposal and water supply activities were combined with other activities that could potentially impact on diarrhoea. Garrett 2008 evaluated a project involving not only improved latrines, water supplies (shallow wells and rainwater harvesting) and hygiene promotion but also point‐of‐use water treatment with chlorine. Wei 1998 also included the promotion of point‐of‐use water treatment (boiling water at school). The intervention reported in Messou 1997 included the provision of oral rehydration salts (ORS). Several studies included steps to improve the management of excreta disposal in addition to infrastructure enhancements (Wei 1998; Yan 1986; Zhu 1997). For these multiple‐component interventions it is not possible to isolate the effect of the improvement in excreta disposal or ascribe the difference in outcome solely to thesanitation component.
Despite the diversity in technologies and types of improvement in excreta disposal, it appears that all the interventions would result in 'improved' sanitation, as the term is used by the JMP (WHO/UNICEF 2002), except for those implemented in schools (Wei 1998; Zhu 1997 ) or other public settings (Messou 1997; Xu 1990), where the improvements would fail to meet the definition since they were shared facilities.
Coverage and use of the intervention
In most cases, details on the baseline coverage ‐ and in some cases, the end‐point coverage ‐ were not reported (Table 2; Table 3). Garrett 2008 reported that at baseline, 39% of intervention households had latrines compared with 28% of the control households; at the follow‐up point eight weeks after the launch of the intervention, coverage had increased among the intervention group, but to just 49%, compared to 27% for the controls (P < 0.001). McCabe 1957 observed that 52% of the intervention community had "unsatisfactory facilities" at the outset of the study, while the others were served by a community sewer treatment system; following implementation of the intervention, all households had satisfactory facilities. Two other studies reported that at the end of the intervention period, latrine coverage was 80% (Xu 1994) or 85% (Yan 1986): in neither of these cases, however, did the investigators report latrine coverage in the control group or at baseline. Studies evaluating public latrines reported coverage of one toilet for 10 inhabitants (Messou 1997). Other studies did not actually report on on the increase in coverage that was achieved as a result of the intervention.
Some studies reported on latrine use rather than intervention coverage. Aziz 1990, who reported that use of the latrine was constantly monitored, recorded that 88% of households used the latrines in 1987 against 83% in 1993. In 1993 a total of 64% of the latrines available in 1987 were still functioning properly. A subsequent evaluation reported that that most children aged 36‐59 months were said to use latrines, but few below this age did so (Hoque 1996). Huttly 1990 reported that 46% of households at the intervention were using a VIP latrine.They also observed that latrine use was high among adults but low (19%) among children. McCabe 1957 observed that "almost everyone" used the installed privies.
Risk of bias in included studies
In none of the studies was the allocation of the intervention among the participating clusters random. Rather, in each case, the intervention was allocated either by the researchers (Wei 1998,Zhu 1997) or the reasons were not specified in the reports or were provided subsequently (see Characteristics of included studies ). The allocation was not concealed and the intervention was not blinded.
The studies included in this review were assessed for risk of bias based only on the comparability of characteristics between the intervention and control groups and the contemporaneitys of the data collection. All studies met the definition of 'adequate' for the contemporaneity of the data collection, and 11 studies met the definitions of 'adequate' under both criteria (Table 4). Information on loss to follow‐up is presented in Table 4. Only three studies reported loss to follow‐up or provided information from which it could be calculated. Among these, only Messou 1997 and Xu 1990 had a less than 10% loss to follow‐up and were thus characterized as adequate on this criterion.
Table 3.
Risk of bias*
| Reference | Comparable study populations | Contemporary data collection | Study participant lost to follow‐up |
| Aziz 1990 | Adequate | Adequate | Unclear |
| Garrett 2008 | Unclear | Adequate | Inadequate |
| Hu 1988 | Adequate | Adequate | Unclear |
| Huttly 1990 | Adequate | Adequate | Unclear |
| McCabe 1957 | Adequate | Adequate | Unclear |
| Messou 1997 | Adequate | Adequate | Inadequate |
| Rubenstein 1965 | Inadequate | Adequate | Unclear |
| Wei 1998 | Adequate | Adequate | Unclear |
| Xu 1990 | Adequate | Adequate | Adequate |
| Xu 1994 | Adequate | Adequate | Unclear |
| Yan 1986 | Adequate | Adequate | Unclear |
| Zhang 2000 | Adequate | Adequate | Unclear |
| Zhu 1997 | Adequate | Adequate | Unclear |
| *Comparability of characteristics between groups, data collection for groups at the same time. Loss to follow up is adequate if <10%. Refer to Assessment of risk of bias in included studies | |||
Because of the lack of random allocation and the subjective reporting of diarrhoea as outcome measure, the risk of selection bias as well as of observer and responder bias in all trials must be considered high, even where the aforementioned criteria are satisfied.
Effects of interventions
See: Table 1
Diarrhoeal disease
The measure of effect for each of the 13 studies included in the review appears in Table 1.
Only two of the 13 studies included in this review reported no protective effect from the intervention (Huttly 1990; Xu 1994). Huttly and colleagues suggested that the intervention may not have been effective in preventing diarrhoea in the particular setting due to problems in implementation of the sanitation intervention that led to low levels of coverage and to low utilization by children.
Except for two studies (Garrett 2008; Xu 1994), CI could not be provided due to the insufficient number of clusters for which data was reported (Donner 2000). For the Garrett study, we relied on theCI provided by the authors. It is noted that the authors reported adjusting for clustering. For the Xu 1994 study we calculated the CI according to the methods proposed by Hayes and Moulton (Hayes 2008).
Due to the heterogeneity of studies and unavailability of reliable CI, no pooled effect was calculated. In general, however, the consistency of a protective effect against diarrhoea seen across the included studies indicates that these findings may be unlikely to be due to chance alone if there were no other factors that could explain the effect (ie baseline differences between communities, observer / responder bias).
Mortality
Only one trial reported on mortality as a study outcome. Messou 1997, which involved a combination of improved latrines with source water improvement, an oral rehydration intervention and hygiene instruction, reported an 85% reduction (from 27% to 4%) in the proportion of deaths related to diarrhoea in the villages with the intervention compared with no reduction in control villages. That trial also reported an 85% reduction (from 5.3% to 0.8%) in the mortality associated with diarrhoea among intervention villages with no correspondingly decline in control villages. We emphasize that the trial was primarily designed to investigate the impact of the intervention on death using a before and after study design, which is not optimal, especially since only two intervention and two control villages was included. Direct comparison between intervention and control villages resulted in a RR of 0.16 (mortality in intervention arm 2%, in control arm 11%). CIs could not be calculated due to an insufficient number of clusters.
Adverse events
None of the trials reported on adverse events from the intervention.
Publication bias
We were unable to create classic funnel plots that plot the effect size against the standard error of the effects, a method suitable to explore publication bias. However, the number of clusters can be regarded as a proxy for study power. Figure 1 shows the graph plotting the effect size against the number of clusters. The figure shows that studies with fewer clusters reported markedly larger effect sizes compared to studies that included more clusters. It is noted, however, that such asymmetry may also be due to clinical and methodological heterogeneity. Since we found substantial evidence of such heterogeneity, we cannot conclude that the funnel plot convincinlgy demonstrates evidence of publication bias in this case.
Figure 1.
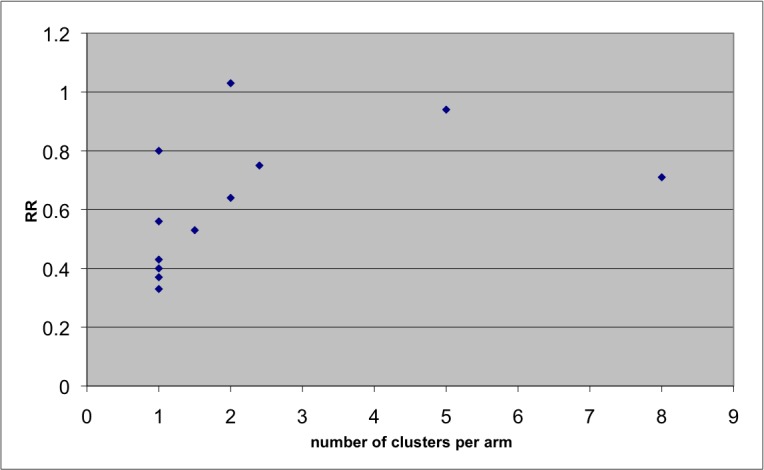
Effect size according to number of clusters per arm to explore publication bias
Discussion
Results suggest that interventions to improve excreta disposal are effective in preventing diarrhoeal disease. While the studies reported a range of effects, most found the intervention to be protective against diarrhoea. This is true not only for studies that combined the sanitation intervention with improvements in water or hygiene, but also for those that consisted solely of improvements in excreta disposal.
Nevertheless, this review provides only limited and preliminary evidence, and does not allow the quantification of such an effect. We were able to identify a significant number of studies that met the review's inclusion criteria, including five studies where the improvement in excreta disposal was not accompanied by other environmental interventions and thus could be investigated independently of activities that are also believed to prevent diarrhoea. Few if any of these studies were included in previous reviews of sanitation interventions (Esrey 1985; Esrey 1991; Fewtrell 2005; Waddington 2009). However, substantial heterogeneity among interventions, settings, and methodologies, and the absence of reliable CIs for most studies made it impossible to use meta‐analysis to calculate a pooled estimate of effect from the intervention. Accordingly, while we extracted and present information on pre‐intervention settings, type of intervention, coverage, compliance, and other components of the intervention, the analysis is only descriptive as no pooling of effects was possible. Despite most studies reporting a protective effect, it is not possible to actually derive a reliable estimate of the size of the effect.
Moreover, the strength of evidence must be qualified by certain methodlological issues presented by the studies included in the review. Firstly, while we were able to identify 13 studies that met the review's inclusion criteria, none randomized the intervention among the clusters comprising the study population. Most of these compared one or only a few intervention sites with a similar number of control sites. Many of the reasons for choosing one community for the intervention over the other may well be associated with disease risk (eg willingness to co‐operate, the presence of infrastructure, level of education, wealth), potentially introducing a systematic bias. Secondly, none of the studies included in the review assessed the effect of the sanitation intevention on intermediate outcomes, such as the quality of drinking water, microbial contamination of foods or presences of flies, which could suggest an objective impact of the intervention on common transmission pathways. Thus, there is no independent way to confirm that the intervention reduced exposure, much less disease.
A further shortcoming of most studies is the potential for observer and responder bias in assessing the disease outcomes. None of the studies was blinded, though this may be unavoidable with sanitation interventions. Most also relied on reported diarrhoea. The assessment of diarrhoeal diseases by active surveillance conducted by field workers is prone to cause bias both on the side of the study participants (responder bias) and the field worker (observer bias). These factors are known to potentially shift the effect measure towards a greater effect (Wood 2008). It may be possible to minimize such bias by including a passive surveillance component, where households are encouraged to seek treatment at a specified health centre, as was done in two of the included studies. However, the extent to which this outcome has increased validity has yet to be investigated.
In addition to the methodological limitations of all studies, differences in study populations, and settings, baseline sanitation levels, water, and hygiene practices, methodologies, case definitions, and outcome surveillance, and types of interventions limit the comparability of results from the studies included in this review. Failure to record or adjust for differences in the coverage and use of the excreta disposal intervention in many studies also raises issues about the conclusions that can be drawn from the findings. It may be expected, for example, that increasing coverage or use from 10% to 70% would yield different outcomes from those obtained by increasing coverage from 60% to 70%; however, this information is missing from most studies. Population density may also play an important role in the effectiveness of sanitation interventions but details on on density are absent from most studies.
Moreover, only five of the 13 included studies consisted solely of such improvements in excreta disposal. In all the other studies included in this review the sanitation intervention was accompanied at least by improvements in drinking water supply. And in some studies, there were hygiene promotion or other components to the intervention. Of the five sanitation‐only studies, four were concentrated geographically in a single country (China) and the other was conducted in a developed country (USA) more than 50 years ago.
The studies included in this review are suggestive of the wide variety of interventions being undertaken to improve excreta disposal in low‐income settings, both at the household and community level, and the extent to which they may be effective in minimizing human contact and pathogen exposure. They also suggest the considerable variations in quality, coverage, use, and sustainability of the interventions. Even uniform interventions implemented in a manner that is equally effective in containing excreta are nevertheless likely to yield different levels of effectiveness in reducing diarrhoea, depending on other exposure pathways. Thus, single, pooled estimates of the contribution that sanitation can make to preventing diarrhoeal disease are not only methodologically unsound but also misleading.
This review highlights some of the challenges in estimating the contribution of sanitation interventions to prevent diarrhoea and some of the shortcomings in the studies conducted to date. However, the more salient conclusion is the paucity of rigorous studies on the effectiveness of these sanitation interventions given the substantial burden of disease that is associated with direct or indirect contact with faeces. Research could help identify not only innovative solutions but also the key factors associated with the effectiveness of sanitation interventions and how they can best be targeted and delivered. This lack of research on the health impact of sanitation parallels, and may be partially responsible for, the lag in progress in extending sanitation coverage as reflected in the vast shortfall in achieving the MDG sanitation target.
Authors' conclusions
This review provides some evidence that excreta disposal interventions are effective in preventing diarrhoeal diseases. However, this conclusion is based largely on the consistency of some protective effect (11/13 studies). The quality of the evidence is generally poor and does not permit quantification of any such effect. The wide range of estimates of effect from the intervention may be due to clinical and methodological heterogeneity among the studies, including differences in study design, case definitions, outcomes, method, and length of follow‐up, and methods of estimating measures of effect. These and other differences could impact on levels of observer and responder bias. There were also important differences in the study populations, the levels of ambient exposure, the types of interventions, and the levels of coverage and use. The range in effect may also suggest that the contribution that excreta disposal interventions can make in preventing diarrhoea may depend on the local context and the exposure scenarios, and transmission dynamics that research to date cannot fully explain.
Perhaps the major finding of this review is the paucity of rigorous evidence demonstrating the effect of basic sanitation in preventing diarrhoea, a leading killer of young children. This is clearly not from a lack of attention to this sector by the public health community at the highest international level. Sanitation has attracted support (if not funding) at that level over the past 30 years. Neither is it because sanitation interventions cannot be assessed using experimental designs: Emerson 2004 demonstrated how a cluster‐based approach can be used to randomize excreta disposal interventions in a rigorous cluster RCT study design. While the MDG target for sanitation is intended to inspire the political will to advance the implementation of basic sanitation, it is possible that the pace of implementation is being retarded by this dearth of reliable evidence of the health outcomes that may be achieved thereby, and how they vary with exposure setting, type of improvement, and coverage achieved. Future research should address this need.
Rigorously conducted RCTs to assess interventions to improve basic sanitation will help clarify the potential contribution of such interventions. While conventional blinding may not be possible, it is important that such trials take steps to minimize an exaggerated effect from bias associated with open trials of non‐objective outcomes. If possible, such studies should assess the impact of sanitation on objective intermediate outcomes associated with exposure to excreta, such as contaminated food and water. Limiting exposure to child faeces, using potties or scoops, and properly disposing of their contents may be a particular priority, but no intervention studies have been conducted to date to determine or optimize the effect of such measures. Multiple trials in different settings will also help identify the circumstances in which improvements in excreta disposal should be targeted and given priority.
The effect of sanitation in the context of other environmental interventions to prevent diarrhoea should also be explored. Because sanitation is a primary barrier to faecal‐oral transmission it seems plausible under some circumstances to prevent most diarrhoea by implementing sanitation even without improvements in water supply or quality. It is also important to evaluate such interventions in rural versus urban settings where the challenges of implementation, transmission pathways, and exposure levels may vary. In high density settings it would also be useful to investigate whether and to what extent any benefit from increasing coverage and the use of latrines is conferred on non‐adopters, as with insecticide‐treated bednets, rendering the health impact of the intervention a "public good" in economic terms.
There is also a need for longer‐term effectiveness studies in programmatic (not‐research driven) settings. Rigorous observational studies and project evaluations can also contribute valuable evidence on the scalability and sustainability of sanitation interventions. Differences in programmatic approaches to optimize the adoption and long‐term utilisation of sanitation should also be investigated.
Finally, we note that this review does not address the potential contribution of improved excreta disposal to preventing other important health threats associated with inadequate sanitation, including schistosomiasis, other helminth infections, malnutrition, stunting and tropical enteropathy.
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | excreta disposal | excreta disposal | excreta disposal | excreta disposal | excreta disposal |
| 2 | sanitation | SANITATION | SANITATION | ENVIRONMENTAL SANITATION | sanitation |
| 3 | latrine OR toilet OR water closet OR privy | latrine OR toilet OR water closet OR privy | latrine OR toilet OR water closet OR privy | SANITATION | latrine OR toilet OR water closet OR privy |
| 4 | faeces OR defecation OR excrement OR waste | faeces OR defecation OR excrement OR waste | faeces OR defecation OR excrement OR waste | SOLID WASTE MANAGEMENT | faeces OR defecation OR excrement OR waste |
| 5 | 1 OR 2 OR 3 OR 4 | 1 OR 2 OR 3 OR 4 | 1 OR 2 OR 3 OR 4 | latrine OR toilet OR water closet OR privy | 1 OR 2 OR 3 OR 4 |
| 6 | diarrhea | DIARRHEA/EPIDEMIOLOGY OR DIARRHEA/MICROBIOLOGY OR DIARRHEA/PREVENTION AND CONTROL | DIARRHEA/EPIDEMIOLOGY OR DIARRHEA/MICROBIOLOGY OR DIARRHEA/PREVENTION AND CONTROL | faeces OR defecation OR excrement OR waste | diarrhea |
| 7 | waterborne | waterborne AND (infection* OR illness*) | waterborne AND (infection* OR illness*) | 1‐6/OR | waterborne |
| 8 | 6 OR 7 | cholera OR shigell* OR dysenter* OR cryptosporid* or giardia* OR Escherichia OR clostridium | cholera OR shigell* OR dysenter* OR cryptosporid* or giardia* OR Escherichia OR clostridium | DIARRHEA/EPIDEMIOLOGY OR DIARRHEA/DISEASE MANAGEMENT OR DIARRHEA/PREVENTION | 6 OR 7 |
| 9 | 5 AND 8 | ENTEROBACTERIACEAE | ENTEROBACTERIACEAE | waterborne AND (infection* OR illness*) | 5 AND 8 |
| 10 | — | 6 OR 7 OR 8 OR 9 | 6 OR 7 OR 8 OR 9 | cholera OR shigell* OR dysenter* OR cryptosporid* or giardia* OR Escherichia OR clostridium | — |
| 11 | — | 5 AND 10 | 5 AND 10 | ENTEROBACTERIACEAE | — |
| 12 | — | — | Limit 11 to Human | 8 OR 9 OR 10 OR 11 | — |
| 13 | — | — | — | 7 AND 12 | — |
| 14 | — | — | — | Limit 13 to Humans | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Data extracted from included studies
| Type of data | Fields |
| Trial data | Country and setting (urban, rural) |
| Number of participants/groups | |
| Unit of randomization and whether measurement of effect adjusts for clustering where randomization is other than individual | |
| Definition and practices of control group | |
| Type and details of excreta disposal intervention, including factors that may augment or diminish effectiveness (eg location, emptying practices, overflow protection) | |
| Other components of intervention (hygiene message, improved water supply, improved water quality, improved storage) | |
| Whether water is protected to point of use (ie by pipe, residual disinfection or safe storage) | |
| Case definition of diarrhoea | |
| Method for diarrhoea assessment (self‐reported, observedor clinically confirmed) | |
| Where self‐reported, recall period used | |
| Publication status | |
| Prescribed criteria of methodological quality | |
| Individual characteristics | Age group |
| Type of water source | |
| Level of faecal contamination of control water (low (< 100 thermotolerant coliforms (TTC)/100 mL), medium (100 to 1000 TTC/100 mL), and high (>1000 TTC/100 mL) | |
| Causative agents identified (yes or no) | |
| Water collection, storage, and drawing practices | |
| Distance to and other constraints regarding water supply | |
| Sanitation facilities (improved or unimproved) | |
| Hygiene practices | |
| Outcomes | Pre‐ and post‐intervention faecal contamination of drinking water, and method of assessment (including indicator used) |
| Diarrhoea morbidity and 95% confidence interval for each age group reported | |
| Manner of measuring diarrhoea morbidity | |
| Mortality attributed to diarrhoea | |
| Rate of utilisation of intervention and manner of assessing it |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 6, 2010
| Date | Event | Description |
|---|---|---|
| 8 May 2009 | Amended | Converted to new review format with minor editing. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cluster non‐randomized controlled trial among 5 clusters (2 intervention and 3 control villages). No information was provided on the method of allocation of intervention among clusters | |
| Participants | 1382 children < 5 years | |
| Interventions | Double pit, water‐sealed latrine, improved water supply, hygiene promotion | |
| Outcomes | Incidence of diarrhoea in children < 5 years, incidence of persistent diarrhoea, incidence of dysentery, longitudinal prevalence of diarrhoea | |
| Location | Bangladesh | |
| Lenth of follow up | 5 years | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 18 clusters (12 intervention and 6 control villages). Intervention was allocated by the researchers to villages based on qualifying water supply | |
| Participants | 960 children < 5 years old from 556 households | |
| Interventions | Cement sanitary platforms and VIP latrines, household water treatment with sodium hypochlorite, improved water storage vessel (clay pot with tap, narrow mouth and lid), improved water supplies (protection of shallow wells); hygiene promotion | |
| Outcomes | Incidence of diarrhoea in children < 5 years | |
| Location | Rural Kenya | |
| Lenth of follow up | 8 weeks | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 2 clusters (1 intervention and 1 control village). No information was provided on the method of allocation of the intervention between the clusters | |
| Participants | Unspecified number of individuals of all ages in 1 intervention village and 1 control village | |
| Interventions | Biogas latrine connected to fermentation reactor | |
| Outcomes | Incidence of diarrhoea | |
| Location | Rural China | |
| Lenth of follow up | 12 months | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 4 clusters (2 intervention villages and 2 control villages). No information was provided on the method of allocation of the intervention among the clusters | |
| Participants | Estimated 1405 children < 6 years old from 2 intervention and 2 control villages | |
| Interventions | VIP latrines, improved water supply | |
| Outcomes | Incidence of diarrhoea, anthropometrics | |
| Location | Rural Nigeria | |
| Lenth of follow up | 18 months | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 4 clusters (1 intervention and 3 control towns). Intervention was allocated to the clusters by the researchers based on intervention town's previous participation in a fly study | |
| Participants | 1332 individuals of all ages | |
| Interventions | Replaced "surface" and other "unsatisfactory privies" with new privy or rehabilitated old privy with 8‐ ft deep bored well, additional privies remodelled at schools, churches and commercial buildings | |
| Outcomes | Diarrhoea incidence, prevalence of Shigella spp. isolated from stools of children < 10 years of age, prevalence of flies breeding in privies | |
| Location | Rural USA | |
| Lenth of follow up | 18 months | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 4 clusters (2 intervention and 2 control villages). No information was provided on the method of allocation of the intervention among the clusters | |
| Participants | 1260 children < 5 years old | |
| Interventions | Double pit latrine, improved water supply, hygiene promotion, oral rehydration therapy | |
| Outcomes | Prevalence of diarrhoea in children < 5 years old, deaths related to diarrhoea | |
| Location | Rural Côte d'Ivoire | |
| Lenth of follow up | 4 years | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 2 clusters (1 intervention and 1 control village. Intervention was allocated by the researchers to the village that was the more receptive to it | |
| Participants | 124 children < 1 year old | |
| Interventions | Toilets connected to sewer, piped‐in water supplies | |
| Outcomes | Incidence of diarrhoea based on outpatient visits to clinc, outpatient visits for all causes, hospital admissions for all causes and for diarrhoea | |
| Location | Rural Native American population in the USA | |
| Lenth of follow up | 12 months | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 2 clusters (2 intervention and 2 control schools. No information was provided on method of allocation of the intervention among the clusters | |
| Participants | Unspecified number of primary school and secondary school students | |
| Interventions | Improve school toilets, maintain and improve school sanitary environment, hygiene promotion; improve water supply, point‐of‐use drinking water treatment (boiling), improve handwashing facilities | |
| Outcomes | Incidence of diarrhoea | |
| Location | Schools in rural China | |
| Lenth of follow up | 6 months | |
| Notes | No information provided on how the intervention was allocated to the clusters | |
| Methods | Cluster non‐randomized controlled trial among 2 clusters (1 intervention and 1 control neighbourhood) in a single community. No information provided on method of allocation of the intervention among the clusters | |
| Participants | 3599 individuals of all ages | |
| Interventions | Public latrines connected to septic tank | |
| Outcomes | Incidence of clinically confirmed diarrhoea cases extracted from hospital records | |
| Location | Urban China | |
| Lenth of follow up | 3 years | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 10 clusters (villages from 5 counties in the intervention group and villages from the same 5 countries in the control group). No information was provided on the method of allocation of the intervention among the clusters | |
| Participants | 14787 individuals of all ages | |
| Interventions | Toilet connected to septic tank or biogas reactor | |
| Outcomes | Incidence of diarrhoea | |
| Location | Rural China | |
| Lenth of follow up | 5 months | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 2 clusters (1 intervention and 1 control village. No information was provided on the method of allocation of the intervention among the clusters | |
| Participants | 2060 individuals of all ages | |
| Interventions | Construction of double urn funnel toilet plus faeces disposal management | |
| Outcomes | Incidence of diarrhoea | |
| Location | Rural China | |
| Lenth of follow up | 4 years | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 2 clusters (1 intervention and 1 control village). No information was provided on the method of allocation of the intervention among the clusters | |
| Participants | 3036 individuals of all ages | |
| Interventions | Double vault funnel toilet; improved water supply | |
| Outcomes | Prevalence of diarrhoea | |
| Location | Suburban China | |
| Lenth of follow up | 10 years | |
| Notes | ||
| Methods | Cluster non‐randomized controlled trial among 2 clusters (2 schools from 5 counties in the intervention group and 2 schools from the same 5 counties in the control group). No information was provided on the method of allocation of the intervention among the clusters | |
| Participants | 3472 primary school and secondary school students | |
| Interventions | Improved school‐based latrines (various types), with maintenance programme including non‐hazardous treatment of faeces; improved hygiene facilities; point‐of‐use water treatment; health and hygiene promotion | |
| Outcomes | Incidence of diarrhoea | |
| Location | Rural China | |
| Lenth of follow up | 5 months | |
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asaolu 2002 | Outcome was not diarrhoea |
| Azurin 1974 | Outcome was cholera, not endemic diarrhoea |
| Baltazar 2002 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Beck 1957 | Study design was not a controlled trial; outcome was not diarrhoea |
| Butz 1984 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Cao 2007 | Study design was not a controlled trial |
| Daniels 1990 | Study design was not a controlled trial |
| Gross 1989 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Guerrant 1983 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Gutierrez 1999 | Study design was not a controlled trial |
| Henry 1981 | Study design was not a controlled trial; outcome was not diarrhoea |
| Koopman 1978 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Kumar 1968 | No intervention to improve excreta disposal |
| Lou 1990 | Study design was not a controlled trial |
| Makoni 2004 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Meddings 2004 | Study design was not a controlled trial |
| Moore 1965 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Nanan 2003 | Study design was not a controlled trial. |
| Pickering 1985 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Pokhrel 2004 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Rego 2005 | Study design was not a controlled trial; no intervention to improve excreta disposal |
| Van Zil 1966 | Study design was not a controlled trial |
| Xiao 1995 | Study design was not a controlled trial |
| Xiao 1997 | Intervention was not improvement in excreta disposal |
| Zhou 1995 | Intervention was not improvement in excreta disposal |
Contributions of authors
Sandy Cairncross and Thomas Clasen conceived of the review. All the authors assisted in the design and development of the protocol, including the literature review used in the Background section. Thomas Clasen and Kristof Bostoen developed the search strategy. Wolf‐Peter Schmidt and Sophie Boisson provided additional epidemiological and statistical support. Isaac C‐H Fung assisted in developing the search strategy for the Chinese‐languagee databases. Marion Jenkins, Beth Scott, and Steven Sugden provided technical advice on sanitation interventions. All authors provided general advice for the review, commented on the protocol, and gave final approval on the version to be published.
Sources of support
Internal sources
No sources of support supplied
External sources
WaterAid, UK.
UNICEF, USA.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
- Aziz KMA, Hoque BA, Hasan KZ, Patwary MY, Huttly SRA, Rahman MM, et al. Reduction in diarrhoeal diseases in children in rural Bangladesh by environmental and behavioural modifications. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(3):433‐8. [DOI] [PubMed] [Google Scholar]; Hasan KZ, Briend A, Aziz KMA, Hoque BA, Patwary My, Huttly SRA. Lack of impact of a water and sanitation intervention on the nutritional status of children in rural Bangladesh. European Journal of Clinical Nutrition 1989;43(12):837‐43. [PubMed] [Google Scholar]; Hoque BA, Juncker T, Sack RB, Ali M, Aziz KMA. Sustainability of a water, sanitation and hygiene education project in rural Bangladesh: a 5‐year follow‐up. Bulletin World Health Organization 1995;74(4):431‐37. [PMC free article] [PubMed] [Google Scholar]
- Garrett V, Ogutu P, Mabonga P, Ombeki S, Mwaki A, Aluoch G, et al. Diarrhoea prevention in a high‐risk rural Kenyan population through point‐of‐use chlorination, safe water storage, sanitation, and rainwater harvesting. Epidemiology and Infection 2008;136(11):1463‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X‐R, Liu G, Liu S‐P, Yan‐Xin H, Zhang X‐M, Fan Y‐Y, et al. [Field evaluation of the effect of diarrhoea control of methanogenesis treatment of human and animal faeces and rubbish of cellulose nature in the rural areas of Xiang Cheng]. Henan Yu Fang Yi Xue Za Zhi [Henan Journal of Preventative Medicine] 1988;1988:11‐13. [Google Scholar]
- Blum D, Emeh R. Evaluation of the UNICEF‐assisted Imo State water supply and sanitation project: epidemiological and fieldwork methods. In: Briscoe J, Feachem RG, Rahaman MM editor(s). Evaluating Health Impacts: Water Supply, Sanitation and Hygiene Education. Toronto: International Development Research Centre Canada, 1986. [Google Scholar]; Huttly SR, Blum D, Kirkwood BR, Emeh RN, Feachem RG. The epidemiology of acute diarrhoea in a rural community in Imo State, Nigeria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(5):865‐70. [DOI] [PubMed] [Google Scholar]; Huttly SR, Blum D, Kirkwood BR, Emeh RN, Okeke N, Ajala M, et al. The Imo State (Nigeria) drinking water supply and sanitation project, 2: impact on dracunculiasis, diarrhoea and nutritional status. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84(2):316‐21. [DOI] [PubMed] [Google Scholar]; The Imo State Evaluatin Team. Evaluating water and sanitation projects: lessons from Imo State, Nigeria. Health Policy and Planning 1989;4(1):40‐9. [Google Scholar]
- McCabe LJ, Haines TW. Diarrheal disease control by improved human excreta disposal. Public Health Reports 1957;72(10):921‐8. [PMC free article] [PubMed] [Google Scholar]
- Messou E, Sangare SV, Josseran R, Corre C, Guelain J. [Effect of hygiene and water sanitation and oral rehydration on diarrhoea and mortality of children children less than five years old in the south of Ivory Coast]. Bulletin de la Société de Pathologie Exotique 1997;90(1):44‐7. [PubMed] [Google Scholar]; Messou E, Sangare SV, Josseran R, Corre C, Guelain J. [Effect of improvement in sanitary conditions and domestic hygiene on incidence of ascaridiasis and ankylostomiasis of children aged 2‐4 years in rural zones of Ivory Coaste]. Bulletin de la Société de Pathologie Exotique 1997;90(1):48‐50. [PubMed] [Google Scholar]
- Rubenstein A, Boyle J, Odoroff CL, Kunitz SJ. Effect of improved sanitary facilities on infant diarrhea in a Hopi village. Public Health Reports 1969;84(12):1093‐7. [PMC free article] [PubMed] [Google Scholar]
- Wei X‐N, Shao B‐C, Fang Z‐H, Gao M, Zhu X‐L. [Evaluation of the effect of prevention of diarrhoea and ascariasis among students of health intervetion measures in schools in rural areas. Zhongguo Gong Gong Wei Sheng [China Journal of Public Health] 1998;14:228. [Google Scholar]
- Xu J‐Z. [Observation on the efficacy of three squares septic tank lavatory for disease prevention]. Huan Jing Yu Jian Kang Za Zhi [Journal of Environment and Health] 1990;1990:250‐2. [Google Scholar]
- Xu G‐X, Zhu X‐L. [The assessment of the effects for prevention of diseases by non‐hazardous treatment of night soil at experimental spots in rural areas]. Wei Sheng Yan Jiu [Journal of Hygiene Research] 1994;23:23‐7. [Google Scholar]
- Yan Z‐S, Wang G‐F, Cui C, Yin L‐S, Su C‐H, Liu F, et al. [An observation of the effect on reducing the fly density and diarrhoea of the use of double urn funnel lavatory in faeces management]. Henan Yu Fang Yi Xue Za Zhi [Henan Journal of Preventive Medicine] 1986;1986(3):73‐6. [Google Scholar]
- Zhang W‐P, Liu M‐X, Yin W‐H, Zhang H‐J, Li G‐P, Liu L‐P, et al. [Evaluation of a long‐term effect on improving drinking water and lavatories in rural areas for prevention of diseases]. Ji Bing Kong Ji Za Zhi [Chinese Journal of Disease Control & Prevention] 2000;4(1):76‐8. [Google Scholar]
- Zhu X‐L, Xia Q‐Y, Meng Z‐Y, Wang X‐C, Shao B‐C, Yang S‐Y, et al. [Assessment of effects of disease prevention by intervention measures of school environmental hygiene in rural areas]. Wei Sheng Yan Jiu [Journal of Hygiene Research] 1997;26:378‐80. [Google Scholar]
References to studies excluded from this review
- Asaolu SO, Ofoezie IE, Odumuyiwa PA, Sowemimo OA, Oguniyi TA. Effect of water supply and sanitation on the prevalence and intensity of Ascaris lumbricodes among pre‐school‐age children in Ajebandele and Ifewara, Osun State, Nigeria. Tranactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(6):600‐4. [DOI] [PubMed] [Google Scholar]
- Azurin JC, Alvero M. Field evaluation of environmental sanitation measures against cholera. Bulletin of the World Health Organization 1974;51(1):19‐26. [PMC free article] [PubMed] [Google Scholar]
- Baltazar JC, Nadera DP, Victora CG. Evaluation of the National Control of Diarrhoeal Diseases Programme in the Philippines, 1980‐93. Bulletin of the World Health Organization 2002;80(6):637‐43. [PMC free article] [PubMed] [Google Scholar]
- Beck MD, Munoz JA, Scrimshaw NS. Studies on diarrheal diseases in Central America I: preliminary findings on cultural surveys of normal populations groups in Guatemala. The American Journal of Tropical Medicine and Hygiene 1957;6(1):62‐71. [DOI] [PubMed] [Google Scholar]
- Butz WP, Habicht J‐P, DaVanzo J. Environmental factors in the relationship between breast‐feeding and infant mortality: the role of sanitation and water in Malaysia. American Journal of Epidemiology 1984;119(4):516‐25. [DOI] [PubMed] [Google Scholar]
- Cao D‐Q, Zhang R. [A survey of the effect of the intervention measures of water and latrine improvement in rural areas on diarrhoea prevention]. Zhonngguo Nong Cun Wei Sheng Shi Ye Guan Li [Chinese Rural Health Service Administration] 2007;27:466. [Google Scholar]
- Daniels DL, Cousens SN, Makoae LN, Feachem RG. A case‐control study of the impact of improved sanitation on diarrhoea morbidity in Lesotho. Bulletin of the World Health Organization 1990;68(4):455‐63. [PMC free article] [PubMed] [Google Scholar]
- Gross R, Schell B, Bisi Molina MC, Cuelho Leao MA, Strack U. The impact of improvement of water supply and sanitation facilities on diarrhea and intestinal parasites: a Brazilian experience with children in two low‐income urban communities. Revista de saúde pública 1989;23(3):214‐20. [DOI] [PubMed] [Google Scholar]
- Guerrant RL, Kirchhoff LV, Shield DS, Natons MK, Leslie J, Sousa MA, et al. Prospective study of diarrheal illnesses in northeastern Brazil: patterns of disease, nutritional impact, etiologies, and risk factors. Journal of Infectious Diseases 1983;148(6):986‐99. [DOI] [PubMed] [Google Scholar]
- Guiterrez G, Reyes H, Fernandez S, Perez L, Perez‐Cuevas R, Guiscafre H. Impact of health care services, sanitation and education on mortality rates of children under five years of age [Impacto de los servicios de salud, el saneamiento y las alfabetizacion en la mortalidad de menores de cinco anos]. Salud Pública de México 1999;41(5):368‐75. [PubMed] [Google Scholar]
- Henry FJ. Environmental sanitation, infection and nutritional status of infants in rural St Lucia, West Indies. Transactions of the Royal Society of Tropical Medicine and Hygiene 1981;75(4):507‐13. [DOI] [PubMed] [Google Scholar]
- Koopman JS. Diarrhea and school toilet hygiene in Cali, Colombia. American Journal of Epidemiology 1978;107(5):412‐20. [DOI] [PubMed] [Google Scholar]
- Kumar P, Singh R, Sehgal BS. Fly control and diarrheal morbidity in a rural community in U.P. Indian Journal of Medical Sciences 1968:285‐91. [PubMed] [Google Scholar]
- Lou H, Liu M‐X, Song S‐M, Li Q‐M, Zhang W‐P, Xu G‐X, et al. [Effectiveness evaluation and cost‐effectiveness estimate for diarrhea control by environmental improvement in rural area]. Henan Yu Fang Yi Xue Za Zhi [Henan Journal of Preventive Medicine] 1989;1989:25‐9. [Google Scholar]
- Makoni FS, Ndamba J, Mbati PA, Manase G. Impact of waste disposal on health of a poor urban community in Zimbambwe. East African Medical Journal 2004;81(8):422‐6. [DOI] [PubMed] [Google Scholar]
- Meddings DR, Ronald LA, Marion S, Pinera JF, Oppliger A. Cost effectiveness of a latrine revision programme in Kabul, Afghanistan. Bulletin of the World Health Organization 2004;82(4):281‐8. [PMC free article] [PubMed] [Google Scholar]
- Moore HA, Cruz E, Vargas‐Mendez O. Diarreal disease studies in Costa Rica. IV. The influence of sanitation upon the prevalence of intestinal infection and diarrheal disease. American Journal of Epidemiology 1965;82(2):162‐184. [DOI] [PubMed] [Google Scholar]
- Nanan D, White F, Azam I, Afsar H, Hozhari S. Evaluation of a water, sanitation, and hygiene education intervention on diarrhoea in northern Pakistan. Bulletin of the World Health Organization 2003;81(3):160‐5. [PMC free article] [PubMed] [Google Scholar]
- Pickering H. Social and environmental factors associated with diarrhoea and growth in young children: child health in urban Africa. Social Science and Medicine 1985;21(2):121‐7. [DOI] [PubMed] [Google Scholar]
- Pokhrel D, Viraraghavan T. Diarrhoeal diseases in Nepal vis‐ à‐vis water supply and sanitation status. Journal of Water and Health 2004;2(2):71‐81. [PubMed] [Google Scholar]
- Rego RF, Moraes LRS, Dourado I. Diarrhoea and garbage disposal in Salvador, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005;99(1):48‐54. [DOI] [PubMed] [Google Scholar]
- Zijl WJ. Studies on diarrhoeal diseases in seven countries by the WHO Diarrhoeal Diseases Advisory Team. Bulletin of the World Health Organization 1966;35:249‐61. [PMC free article] [PubMed] [Google Scholar]
- Xiao S‐S, Lin L‐Y, Shi X‐Q, Lin C‐S, Chen K‐C. [A discussion on the control measures of diarrhoeal diseases in rural villages of eastern Fujian]. Zhongguo Gong Gong Wei Sheng [China Journal of Public Health] 1995;11:257. [Google Scholar]
- Xiao S, Lin C, Chen K. [Evaluation of effectiveness of comprehensive control for diarrhea diseases in rural areas of East Fujian and analysis of its cost‐benefit]. [Zhonghua Yu Fang Yi Xue Za Zhi] Chinese Journal of Preventive Medicine 1997;31(1):40‐1. [PubMed] [Google Scholar]
- Zhou TK, Wang CS, Gu BK, Tan JD, Su ZL, Fan PH. [Evaluation of effectiveness and cost‐effectiveness analysis of faeces management measures upon diarrhoea control]. Shanghai Yu Fang Yi Xue Za Zhi [Shanghai Journal of Preventive Medicine] 1995;7(9):388‐91. [Google Scholar]
Additional references
- Baqui AH, Black RE, Sack RB, Chowdhury HR, Yunus M, Siddique AK. Malnutrition, cell‐mediated immune deficiency, and diarrhea: a community‐based longitudinal study in rural Bangladeshi children. American Journal of Epidemiology 1993;137(3):355‐65. [DOI] [PubMed] [Google Scholar]
- Barreto ML, Genser B, Strina A, Teixeira MG, Assis AM, Rego RF, et al. Effect of city‐wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet 2007;370(9599):1622‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartram J. New water forum will repeat old message. Bulletin of the World Health Organization 2003;81(3):158. [PMC free article] [PubMed] [Google Scholar]
- Bennett S, Parpia T, Hayes R, Cousens S. Methods for the analysis of incidence rates in cluster randomized trials. International Journal of Epidemiology 2002;31(4):839‐46. [DOI] [PubMed] [Google Scholar]
- Blum D, Feachem RG. Measuring the impact of water supply and sanitation investments on diarrhoeal diseases: problems in methodology. International Journal of Epidemiology 1983;12(3):357‐65. [DOI] [PubMed] [Google Scholar]
- Byers KE, Guerrant RL, Farr BM. Fecal‐oral transmission. In: Thomas JC, Webber DJ editor(s). Epidemiologic Methods for the Study of Infectious Diseases. Oxford: Oxford University Press, 2001:228‐48. [Google Scholar]
- Clasen T, Roberts I, Rabie T, Schmidt W, Cairncross S. Interventions to improve water quality for preventing diarrhoea. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004794.pub2] [DOI] [PubMed] [Google Scholar]
- Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infectious Diseases 2003;3(5):275‐81. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care. 2nd Edition. London: BMJ Books, 2001:285‐312. [Google Scholar]
- Donner A, Klar N. Design and Analysis of Cluster Randomisation Trials in Health Research. London: Arnold, 2000. [Google Scholar]
- Egger M, Davey Smith G. Principles of and procedures for systematic reviews. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Books, 2001:23‐42. [Google Scholar]
- Ejemot RI, Ehiri JE, Meremikwu MM, Critchley JA. Hand washing for preventing diarrhoea. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004265.pub2] [DOI] [PubMed] [Google Scholar]
- Emerson PM, Lindsay SW, Alexander N, Bah M, Dibba SM, Faal HB. Role of flies and provision of latrines in trachoma control: cluster‐randomised controlled trial. Lancet 2004;363(9415):1093‐8. [DOI] [PubMed] [Google Scholar]
- Esrey SA, Feachem RG, Hughes JM. Interventions for control of diarrhoeal diseases among young children: improving water supplies and excreta disposal facilities. Bulletin of the World Health Organization 1985;63(4):757‐72. [PMC free article] [PubMed] [Google Scholar]
- Esrey SA, Habicht JP. Epidemiologic evidence for health benefits from improved water and sanitation in developing countries. Epidemiologic Reviews 1986;8:117‐28. [DOI] [PubMed] [Google Scholar]
- Esrey SA, Potash JB, Roberts L, Shiff C. Effects of improved water supply and sanitation on ascariasis, diarrhoea, dracunculiasis, hookworm infection, schistosomiasis, and trachoma. Bulletin of the World Health Organization 1991;69(5):609‐21. [PMC free article] [PubMed] [Google Scholar]
- Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta‐analysis. Lancet Infectious Diseases 2005;5(1):42‐52. [DOI] [PubMed] [Google Scholar]
- Fung ICH. Chinese journals: a guide for epidemiologists. Emerging Themes in Epidemiology 2008;5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genser B, Strina A, dos Santos LA, Teles CA, Prado MS, Cairncross S, et al. Impact of a city‐wide sanitationr intervention in a large urban centre on social, environmental and behavioural determinants of childhood diarrhoea: analysis of two cohort studies. International Journal of Epidemiology 2008;37(4):831‐40. [DOI] [PubMed] [Google Scholar]
- Green ST, Small MJ, Casman EA. Determinants of national diarrheal disease burden. Environmental Science & Technology 2009;43(4):993‐9. [DOI] [PubMed] [Google Scholar]
- Guerrant DI, Moore SR, Lima AA, Patrick PD, Schorling JB, Guerrant RL. Association of early childhood diarrhea and cryptosporidiosis with impaired physical fitness and cognitive function four‐seven years later in a poor urban community in northeast Brazil. American Journal of Tropical Medicine and Hygiene 1999;61(5):707‐13. [DOI] [PubMed] [Google Scholar]
- Gundry S, Wright J, Conroy R. A systematic review of the health outcomes related to household water quality in developing countries. Journal of Water and Health 2004;2(1):1‐13. [PubMed] [Google Scholar]
- Hayes RJ, Moulton LH. Cluster. Cluster Randomised Trials. London: Chapman & Hall/CRC Interdisciplinary Statistics Series, 2008. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 December 2007).
- Hoque BA, Juncker T, Sack RB, Ali M, Aziz KM. Sustainability of a water, sanitation and hygiene education project in rural Bangladesh: a 5‐year follow‐up. Bulletin of the World Health Organization 1996;74(4):431‐7. [PMC free article] [PubMed] [Google Scholar]
- Humphrey JH. Child undernutrition, tropical enteropathy, toilets, and handwashing. Lancet 2009;374(9694):1032‐35. [DOI] [PubMed] [Google Scholar]
- Jenkins MW, Curtis V. Achieving the 'good life': why some people want latrines in rural Benin. Social Science and Medicine 2005;61(11):2446‐59. [DOI] [PubMed] [Google Scholar]
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bulletin of the World Health Organization 2003;81(3):197‐204. [PMC free article] [PubMed] [Google Scholar]
- Leclerc H, Schwartzbrod L, Dei‐Cas E. Microbial agents associated with waterborne diseases. Critical Reviews in Microbiology 2002;28(4):371‐409. [DOI] [PubMed] [Google Scholar]
- McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American Journal of Epidemiology 2003;157(10):940‐3. [DOI] [PubMed] [Google Scholar]
- United Nations Millennium Project. UN Millennium Project Task Force on Water and Sanitation‐‐Health, dignity and development: what will it take?. London: Earthscan, 2005. [Google Scholar]
- Morris SS, Cousens SN, Kirkwood BR, Arthur P, Ross DA. Is prevalence of diarrhoea a better predictor of subsequent mortality and weight gain than diarrhea incidence?. American Journal of Epidemiology 1996;144(6):582‐8. [DOI] [PubMed] [Google Scholar]
- Moy RJ, Booth IW, McNeish AS, Choto RG. Definitions of diarrhoea. Journal of Diarrhoeal Diseases Research 1991;9(4):335. [PubMed] [Google Scholar]
- Mulligan J, Fox‐Rushby J, Adam T, Johns B, Mills A. Unit costs of health care inputs in low‐ and middle‐income regions. In: Jamison DT, Breman JG, Measham AR, Alleyn G, Claeson M, Evans DB, et al. editor(s). Disease Control Priorities in Developing Countries. Washington, DC: World Bank, 2006. [PubMed] [Google Scholar]
- Petri WA, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteri infections, diarrea, and their impact on function and development. Journal of Clinical Investigation 2008;118(4):1277‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering H, Hayes RG, Tomkins AM, Carson D, Dunn DT. Alternative measures of diarrhoeal morbidity and their association with social and environmental factors in urban children in The Gambia. Transactions of the Royal Society of Tropical Medicine and Hygiene 1987;81(5):853‐9. [DOI] [PubMed] [Google Scholar]
- Rabiu M, Alhassan M, Ejere H. Environmental sanitary interventions for preventing active trachoma. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004003.pub2] [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
- Schneider RE, Shiffman M, Faigenblum J. The potential effect of water on gastrointestinal infections prevalent in developing countries. American Journal of Clinical Nutrition 1978;31(11):2089‐99. [DOI] [PubMed] [Google Scholar]
- Traore E, Cousens S, Curtis V, Mertens T, Tall F, Traore A, Kanki B, Diallo I, Rochereau A, Chiron JP. Child defecation behaviour, stool disposal practices, and childhood diarrhoea in Burkina Faso: results from a case‐control study. J Epidemiol Community Health 1994;48(270‐275). [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Programme. Human Development Report 2007: Power, Poverty and the Global Water Crisis. New York: United Nations Development Programme, 2007. [Google Scholar]
- United Nations. Report of the World Summit on Sustainable Development: Johannesburg, South Africa, 26 August‐4 September 2002 [A/CONF.199/20*]. www.unctad.org/en/docs/aconf199d20&c1_en.pdf (accessed 3 October 2007).
- United Nations Statistics Division. Progress towards the Millennium Development Goals, 1990‐2005: Goal 4. Reduce child mortality. unstats/un.org/unsd/mi/goals_2005/goal_4.pdf (accessed 15 November 2005).
- United Nations. Resolution A/C.2/61/L.16/Rev.1 on the International Year of Sanitation 2008. United Nations General Assembly 4 December 2006.
- Waddinton H, Snilstveit B, White H, Fewtrell L. Water, sanitation and hygiene interventions to combat childhood diarrhoea in developing countries. Journal of Development Effectiveness2009; Vol. 1, issue 3:295‐335.
- World Health Organization. The management and prevention of diarrhoea: practical guidelines. 3rd Edition. Geneva: World Health Organization, 1993. [Google Scholar]
- World Health Organization. The World Health Report : 2002 : Reducing the Risks, PromotingHealthy Life [WHO/WHR/02.1]. Geneva: World Health Organization, 2002. [Google Scholar]
- World Health Organization. The World Health Report: 2005: Make Every Mother and Child Count. Geneva: World Health Organization, 2005. [Google Scholar]
- World Health Organization, United Nations Childrens' Fund. Global Water Supply and Sanitation Assessment. Geneva: World Health Organization, 2002. [Google Scholar]
- World Health Organization, United Nations Childrens' Fund. Progress towards the Millennium Development Goals, 1990‐2005. Geneva: World Health Organization, 2005. [Google Scholar]
- WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Meeting the MDG Drinking Water and Sanitation Target: The Urban and Rural Challenge of the Decade. Geneva and New York: World Health Organization and United Nations Children's Fund, 2006. [Google Scholar]
- WHO/UNICEF Joint Monitoring Programme for Water Supply and Sanitation. Progress on Drinking Water and Sanitation: 2010 Update. Geneva and New York: World Health Organization and United Nations Children's Fund, 2010. [Google Scholar]
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Emperical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998;280(19):1690‐1. [DOI] [PubMed] [Google Scholar]


