Abstract
Background
Quinine and artemisinin drugs are used in severe malaria, but quinine resistance is increasing. Arteether is a recently developed artemisinin derivative that is oil soluble, has a long elimination half life, and is more stable than other derivatives.
Objectives
To compare intramuscular arteether with other antimalarial drugs to treat severe malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (October 2010), CENTRAL (The Cochrane Library Issue 3, 2010), MEDLINE (1966 to October 2010), EMBASE (1980 to October 2010), U.S. National Library of Medicine (NLM) Gateway (1953 to 1965), Web Science Citation (1981 to October 2010), LILACS (October 2010), Google search engine (October 2010), conference proceedings, and reference lists. We also contacted researchers, organizations, and pharmaceutical companies to help identify trials.
Selection criteria
Randomized and quasi‐randomized controlled trials of intramuscular arteether in adults and children with severe malaria.
Data collection and analysis
We independently assessed the risk of bias in the trials and extracted data, and analysed data using Review Manager 5.
Main results
Two small trials (n = 194) met the inclusion criteria. Both trials compared arteether with quinine in children with cerebral malaria and reported on similar outcomes. There was no statistically significant difference in the number of deaths (risk ratio 0.75, 95% confidence interval 0.43 to 1.30; n = 194, 2 trials), neurological complications (risk ratio 1.18, 95% confidence interval 0.31 to 4.46; n = 58, 1 trial), or other outcomes including time to regain consciousness, parasite clearance time, and fever clearance time. The meta‐analyses lack statistical power to detect important differences.
Authors' conclusions
More trials with a larger number of participants are needed before a firm conclusion about the efficacy and safety of arteether can be reached.
23 April 2019
No update planned
Other
This is not a current question.
Plain language summary
Intramuscular arteether to treat severe malaria
People with severe malaria are unconscious, have difficulty breathing, may convulse, and have low blood sugar. They need to be treated quickly, but because of their illness cannot take drugs by mouth. Arteether, an artemisinin derivative, is a possible alternative to the standard drug quinine. There are two types, artemotil and alpha/beta arteether, both are which are given as an intramuscular injection once a day. The authors of this review wanted to compare intramuscular arteether with other drugs used to treat severe malaria. They identified two small trials with 194 participants. Both trials compared arteether with quinine in children with cerebral malaria (an unrousable coma that cannot be attributed to any other cause other than malaria). There was no difference between the drugs in the number of deaths, people with neurological symptoms, or other outcomes, but there were probably too few participants to detect differences.
Background
Severe malaria, mostly due to Plasmodium falciparum infection, accounts for over one million deaths each year (WHO 2000). Most people with severe malaria require drugs by injections (intravenous or intramuscular) or given rectally. This is because people with severe malaria have a severe illness such as cerebral malaria (an unrousable coma that cannot be attributed to any other cause other than malaria) or repeated generalized convulsions, which may prevent them from taking oral medication, in addition to falciparum malaria parasites in their blood. Quinine has been the most widely used drug for severe malaria (WHO 2000), but it can cause low blood sugar (hypoglycaemia) (Okitolonda 1987), ringing of the ears (tinnitus), high‐tone hearing impairment, depressed mood (dysphoria), nausea, and vomiting (White 2000). Also, a decrease in quinine sensitivity has been recorded in areas of extensive use such as South‐East Asia (WHO 2001) and some parts of Africa (Mutanda 1999).
Artemisinin and its derivatives have been considered as alternatives to quinine because they are known to be effective against multiple‐drug‐resistant malaria parasites (White 1998), produce faster relief of symptoms and clearance of parasites than any other antimalarial drug (White 2000), and resistance towards them has not been documented. Artemether, artesunate, and artemisinin are used to treat severe malaria (White 2000). For non‐oral administration, artemether is available as intramuscular injection, artemisinin as rectal suppositories, and artesunate as rectal, intramuscular, and intravenous preparations (WHO 2001). Their elimination half lives vary from 45 minutes for intravenous artesunate, 4 to 11 hours for artemether, and 0 to 6 hours for rectal artemisinin (WHO 1993). Artemisinin is not very soluble in water or oil, artemether is oil soluble (lipophilic), and artesunate is water soluble (WHO 2001). The more lipophilic a drug is, the more likely it is to accumulate in brain tissue, which may be beneficial in cerebral malaria (Kain 1995; WHO 2002). However, a previous systematic review concluded that there was insufficient evidence to believe one of these derivatives was better than the rest (McIntosh 2003).
Arteether is an ethyl ether derivative of artemisinin that has recently been evaluated. It is oil soluble, has a long elimination half life (greater than 20 hours), and is more stable than the other artemisinin compounds (WHO 2001). Artemotil (previously known as beta arteether) and alpha/beta arteether are the two available formulations. Artemotil was registered in the Netherlands in May 2000 (Lugt 2002). Its use is restricted to children and adolescents under the age of 16 because of the possibility that it may have an effect on the heart, as some electrocardiogram abnormalities have been seen in some trials of artemotil (WHO 2002). Alpha/beta arteether was registered in India in January 1997 for use in both children and adults with severe malaria (WHO 2002). High doses of arteether have caused damage to tissues of the nervous system and heart in animal studies (Brewer 1994), but there is less evidence that these effects are seen in humans (de Vries 1996).
Both artemotil and alpha/beta arteether are given intramuscularly and can be given once daily because of their longer elimination half life (de Vries 1996). They have similar efficacy and clinical safety profiles (WHO 2002). The only differences in their formulations are that artemotil contains the beta form and is dissolved in sesame oil (Lugt 2002; WHO 2002), whereas alpha/beta arteether contains both alpha and beta forms and is dissolved in groundnut oil (Mohanty 1997).
Arteether is a potential substitute for quinine because of drug resistance and safety issues seen with quinine. It may also be more effective than other artemisinin derivatives because of its oil solubility, longer half life, and increased chemical stability. We have therefore summarized the available evidence on the two forms of arteether used to treat severe malaria.
Objectives
To compare intramuscular arteether with other antimalarial drugs for treating severe malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
Adults and children with severe malaria as defined by the World Health Organization (WHO 1990).
Types of interventions
Intervention
Intramuscular arteether (eg artemotil and alpha/beta arteether).
Control
Any other parenteral or rectally administered single antimalarial drug including quinine, artemether, artesunate, and artemisinin.
Types of outcome measures
Primary
Death.
Neurological complications at follow up.
Secondary
Time to regain consciousness.
Presence of parasites at days 7 and 28.
Parasite clearance time.
Fever clearance time.
Adverse events
Adverse events sufficient to cause withdrawal from treatment.
Life‐threatening clinical episodes, including hypoglycaemia.
Other adverse events, including tinnitus, hearing impairment, nausea, and vomiting.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Table 1: Cochrane Infectious Diseases Group Specialized Register (October 2010); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 3, 2010); MEDLINE (1966 to October 2010); EMBASE (1980 to October 2010); NLM Gateway (1953 to 1965); Web Science Citation (1981 to October 2010); and LILACS (1982 to October 2010).
1. Detailed search strategies.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | NLM Gatewayb | Web Science Citationb | LILACSb |
| 1 | artemotil | artemotil | artemotil | artemotil | artemotil | artemotil | artemotil |
| 2 | arteether | arteether | artemotil | arteether | arteether | arteether | arteether |
| 3 | beta arteether | beta arteether | artemotil | beta arteether | beta arteether | beta arteether | 1 or 2 |
| 4 | alpha arteether | alpha arteether | alpha arteether | alpha arteether | alpha arteether | alpha arteether | — |
| 5 | artecef | artecef | artecef | artecef | artecef | artecef | — |
| 6 | E‐MAL | 1 or 2 or 3 or 4 or 5 | E‐MAL | E‐MAL | E‐MAL | E‐MAL | — |
| 7 | malaria | malaria | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | 1 or 2 or 3 or 4 or 5 or 6 | — |
| 8 | — | 6 and 7 | Exp MALARIA | Exp MALARIA | Exp MALARIA | Malaria | — |
| 9 | — | — | 7 and 8 | 7 and 8 | 7 and 8 | 7 and 8 | — |
| 10 | — | — | Limit to human | Limit to human | — | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Internet search
We searched the Internet using the Google search engine (October 2010) with the search terms artemotil, arteether, ARTECEF, and E‐MAL.
Conference proceedings
We searched the following conference proceedings for relevant abstracts: the Third European Congress on Tropical Medicine and International Health, 8 to 12 September 2002, Lisbon, Portugal (www.kit.de/tropical2002/; accessed 16 July 2002); and the Third Multilateral Initiative on Malaria Pan‐African Conference, 18 to 22 November 2002, Arusha, Tanzania (mim.nih.gov/english/events/3rd_mim_conf/index.html; accessed 18 July 2002).
Researchers and pharmaceutical companies
After the search, we circulated a list of identified studies to individual researchers working in the field, to add any trials that may have been missed, unpublished or confidential reports, and information on ongoing trials. We also contacted the pharmaceutical companies Artecef BV (Netherlands) and Themis Medicare (India).
Reference lists
We checked the reference lists of existing reviews and of all trials identified by the above methods.
Data collection and analysis
Selection of studies
Bosede Afolabi (BA) selected potentially relevant trials from those identified by the search strategy and retrieved the full articles. BA and Christy Okoromah (CO) independently assessed each trial for inclusion in the review using the inclusion criteria. We stated the reason for excluding studies in the 'Characteristics of excluded studies'.
Data extraction and management
BA and CO independently extracted data from each included trial. BA entered the data into Review Manager 5. We calculated the percentage loss to follow up and reported it in the text of the review. For binary outcomes, we recorded the number of participants experiencing the event in each group of the trial; and for continuous outcomes, we extracted arithmetic means and standard deviations for each group.
Assessment of risk of bias in included studies
BA and CO independently assessed the risk of bias in the included trials using generation of allocation sequence, allocation concealment, blinding, and loss to follow up. We classed generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear according to Jüni 2001. We assessed blinding as open (all parties are aware of treatment), single (the participant or care provider/assessor know which treatment is given), or double (neither the participant or care provider/assessor know which treatment is given). For loss to follow up, we considered the inclusion of 90% of participants as adequate. We did not need to contact the trialists to clarify methodological issues.
Data synthesis
We carried out statistical analyses using Review Manager 5. We presented binary data as risk ratio (RR) and used the mean difference (MD) for continuous data. We used 95% confidence intervals (CI) and the fixed‐effect model for all analyses.
We assessed heterogeneity amongst trials by inspecting the forest plots and using the chi2 test for heterogeneity with a 10% level of statistical significance.
There was no need to explore the following potential sources of heterogeneity using subgroup analyses: (1) background resistance to either control or intervention drug; (2) age; and (3) different doses of control or intervention drug. This was because the first parameter was not reported and the two included trials were identical in the latter two parameters.
Results
Description of studies
We identified nine potentially eligible studies, seven of which did not meet our inclusion criteria for the reasons given in the 'Characteristics of excluded studies'. The two included studies, Thuma 2000 and Moyou 2001, are randomized controlled trials (see 'Characteristics of included studies').
Trial location
Both included trials were carried out in malaria‐endemic areas of sub‐Saharan Africa: one in Zambia (Thuma 2000) and the other in Cameroon (Moyou 2001). The study area in Cameroon was reported as characterized by perennial transmission of malaria with peaks in transmission at the beginning of the rainy seasons; the Zambia study did not report transmission patterns.
Participants
Both Thuma 2000 (n = 92) and Moyou 2001 (n = 102) included male and female children between 0 and 10 years old with cerebral malaria. Both trials included participants with asexual falciparum parasitaemia and a Blantyre coma score of two or less.
Intervention
Both trials used the same doses of intramuscular beta‐arteether. They also used the same dose and route of administration of quinine (intravenous initially, then oral when tolerated) as their control.
Outcomes
Both trials reported death, fever, and parasite clearance times, presence of parasites at day 7, and time to regain consciousness. Moyou 2001 also reported presence of parasites at day 28 and serious adverse events, and Thuma 2000 reported neurological complications and adverse events.
Risk of bias in included studies
Both trials used computer‐generated numbers (adequate method) to generate the allocation sequence and both used sealed, coded envelopes to conceal allocation (adequate method). Thuma 2000 reported using single blinding to assess parasite clearance time and the presence of parasites, and Moyou 2001 did not report any blinding. Both trials included more than 90% of the randomized participants in the analysis (adequate) of all outcomes. Two participants in the Cameroon trial were excluded from analysis because they had an unusually rapid recovery of consciousness (not previously been mentioned as an exclusion criterion), and two other participants were excluded because of sepsis. In the Zambian trial, three participants that died before beginning treatment were excluded from the analysis.
Effects of interventions
Death
There were 8/51 deaths in the arteether group and 14/51 in the quinine group in Moyou 2001 (RR 0.57, 95% CI 0.26 to 1.24), and 10/48 deaths in the arteether group and 9/44 deaths in the quinine group in Thuma 2000 (RR 1.02, 95% CI 0.46 to 2.27). The pooled meta‐analysis did not detect a statistically significant difference in the risk of death between arteether and quinine (RR 0.75, 95% CI 0.43 to 1.30; n = 194, Analysis 1.1).
1.1. Analysis.
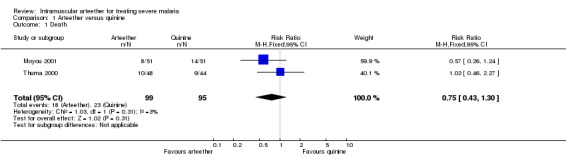
Comparison 1 Arteether versus quinine, Outcome 1 Death.
Neurological complications at follow up
Thuma 2000 reported residual neurological complications at 28 days in both the arteether (5/34) and quinine (3/24) groups (RR 1.18, 95% CI 0.31 to 4.46; n = 58, Analysis 1.2).
1.2. Analysis.
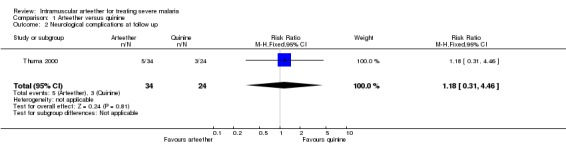
Comparison 1 Arteether versus quinine, Outcome 2 Neurological complications at follow up.
Time to regain consciousness, parasite clearance time, presence of parasites at day 7, and fever clearance time
We did not detect statistically significant differences in any of these outcomes between the arteether and quinine groups (seeAnalysis 1.3, Analysis 1.4, Analysis 1.5, and Analysis 1.7). In Thuma 2000, coma resolution time (which we interpreted as time to regain consciousness) could not be calculated for nine survivors (six in the arteether group and three in the quinine group) because they had persistent neurological complications lasting more than seven days, and the trialists recorded the coma resolution time as 168 hours (seven days) for these participants.
1.3. Analysis.
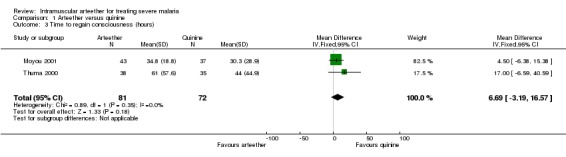
Comparison 1 Arteether versus quinine, Outcome 3 Time to regain consciousness (hours).
1.4. Analysis.
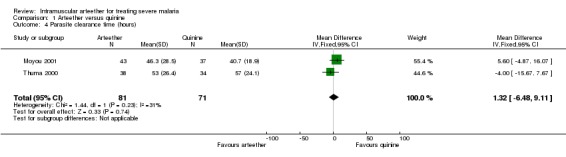
Comparison 1 Arteether versus quinine, Outcome 4 Parasite clearance time (hours).
1.5. Analysis.
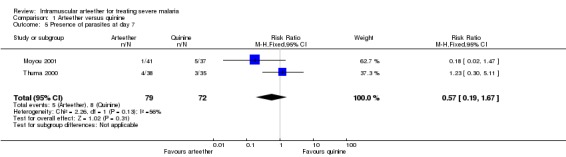
Comparison 1 Arteether versus quinine, Outcome 5 Presence of parasites at day 7.
1.7. Analysis.
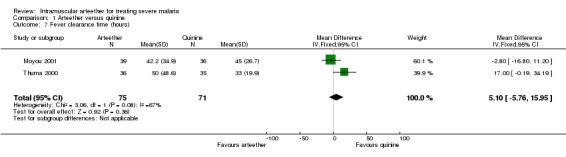
Comparison 1 Arteether versus quinine, Outcome 7 Fever clearance time (hours).
Presence of parasites at day 28
Moyou 2001 reported no statistically significant difference between the arteether and quinine groups (RR 0.76, 95% CI 0.39 to 1.49; n = 78, Analysis 1.6).
1.6. Analysis.
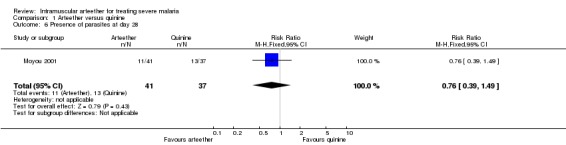
Comparison 1 Arteether versus quinine, Outcome 6 Presence of parasites at day 28.
Adverse events
Thuma 2000 reported people with one or more adverse events, and there was no statistically significant difference between the arteether and quinine groups (RR 0.97, CI 0.77 to 1.22; n = 92, Analysis 1.8). The types of adverse events included (arteether group, quinine group): weakness (2, 3), aphasia/speech disorder (8, 5), deafness (2, 1), fevers/rigors (2, 2), anorexia (6, 6), nausea/vomiting (2, 6), diarrhoea (5, 7), cough (7, 8), pneumonia (7, 6), conjunctivitis (2, 3), and other infections (5, 1).
1.8. Analysis.
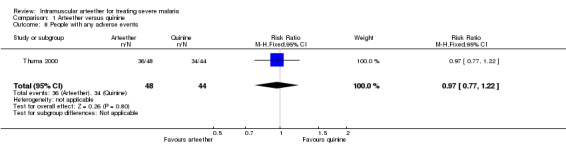
Comparison 1 Arteether versus quinine, Outcome 8 People with any adverse events.
Moyou 2001 reported one serious adverse event, fatal blackwater fever, in the quinine group (RR 0.33, 95% CI 0.01 to 8.00; n = 102, Analysis 1.9).
1.9. Analysis.
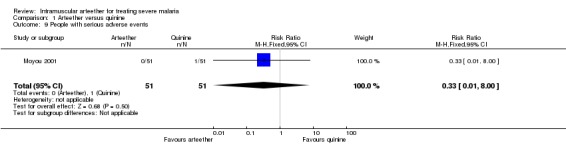
Comparison 1 Arteether versus quinine, Outcome 9 People with serious adverse events.
Discussion
This review was designed to assess the efficacy and safety of arteether for treating severe malaria. Two small trials in Africa involving 194 children with cerebral malaria met the inclusion criteria.
Both trials had similar inclusion criteria, used the same dose and route of administration of arteether and quinine (control), and reported on similar outcome measures. The total number of trials and participants were small, which means that the meta‐analyses lack power to detect any statistically significant differences between arteether and quinine. From the results, there was insufficient evidence to suggest that arteether reduces deaths, neurological complications, or any of the other measured outcomes compared to quinine.
Both trials adequately generated the allocation sequence and concealed allocation. The participants and the assessors did not appear to have been blinded in either study. As arteether was given intramuscularly and quinine was given intravenously, this may have been technically difficult. Also, with a primary outcome such as death, the anticipated gain in reduction of bias from blinding may not be worth the difficulty or cost (Schulz 1996). However blinding of other assessments is often advisable (Schulz 1996), but only Thuma 2000 used single blinding for measuring parasite clearance time and the presence of parasites. There have been a number of trials with other artemisinin derivatives, mainly artemether. Trials are expensive, and if further large‐scale trials are to be done with either artemotil or alpha/beta arteether, there needs to be a strong argument to justify the scientific rationale.
Authors' conclusions
Implications for practice.
The data on the comparative effects of arteether and quinine are too limited to determine the effects of arteether on death, neurological complications at follow up, time to regain consciousness, parasite clearance time, presence of parasites on days 7 and 28, fever clearance time, and adverse events. However, the evidence reviewed did not suggest that arteether is any worse than quinine for treating severe malaria in children.
Implications for research.
More trials that compare arteether with other antimalarial drugs in children and adults that have sufficient statistical power to detect clinically significant differences in the number of deaths and other important outcomes are needed. It is also important for subsequent trials to improve (for example, blinding of outcome assessments) and report all aspects of their methodological quality, and report all their outcomes, including adverse events and neurological complications, in greater detail.
What's new
| Date | Event | Description |
|---|---|---|
| 13 April 2011 | New search has been performed | A new search was conducted; no new studies have been identified |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 15 August 2008 | Amended | Converted to new review format and minor editing. |
| 28 April 2008 | New search has been performed | Three potentially relevant trials identified in a new search were excluded (Pareek 2006; Asimus 2007; Singh 2007). |
Acknowledgements
We initiated and developed the protocol for this review during the July 2002 Fellowship Programme, which is organized by the Cochrane Infectious Diseases Group, and completed the review during the October 2003 Fellowship Programme. The Department for International Development (UK) supports this Programme through the Effective Health Care Alliance Programme (EHCAP) at the Liverpool School of Tropical Medicine.
Data and analyses
Comparison 1. Arteether versus quinine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.43, 1.30] |
| 2 Neurological complications at follow up | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.31, 4.46] |
| 3 Time to regain consciousness (hours) | 2 | 153 | Mean Difference (IV, Fixed, 95% CI) | 6.69 [‐3.19, 16.57] |
| 4 Parasite clearance time (hours) | 2 | 152 | Mean Difference (IV, Fixed, 95% CI) | 1.32 [‐6.48, 9.11] |
| 5 Presence of parasites at day 7 | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.19, 1.67] |
| 6 Presence of parasites at day 28 | 1 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.39, 1.49] |
| 7 Fever clearance time (hours) | 2 | 146 | Mean Difference (IV, Fixed, 95% CI) | 5.10 [‐5.76, 15.95] |
| 8 People with any adverse events | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.22] |
| 9 People with serious adverse events | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.00] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Moyou 2001.
| Methods | Trial design: randomized controlled trial Generation of allocation sequence: computer‐generated randomization Allocation concealment: sealed, coded envelopes (information provided by author) Blinding: none as different routes of drug administration used Loss to follow up: 2 lost to follow up at day 28 in arteether group |
|
| Participants | 102 male and female children aged 0 to 10 years with cerebral malaria: 4/106 (4%) excluded; 102 evaluated (arteether (51), quinine (51)) Inclusion criteria: asexual falciparum parasitaemia; Blantyre coma score of 2 or less Exclusion criteria: children presenting with chronic illness, eg renal or liver diseases, frank acquired immune deficiency syndrome (AIDS), epilepsy, and cardiovascular accident/stroke; children taking cardioactive drugs; history of blackwater fever; positive blood culture for a pathogen or positive cerebrospinal fluid test result |
|
| Interventions | 1. Arteether for 5 days (3.2 mg/kg first day, then 1.6 mg/kg next 4 days) 2. Quinine for 7 days (20 mg/kg loading dose then 10 mg/kg every 8 h thereafter) | |
| Outcomes | 1. Mortality 2. Coma resolution time 3. Fever clearance time 4. Parasite clearance time 5. Serious adverse events | |
| Notes | Location: Cameroon | |
Thuma 2000.
| Methods | Trial design: randomized controlled trial Generation of allocation sequence: computer‐generated block randomization Allocation concealment: sealed, coded envelopes Blinding: none as different routes of drug administration used Loss to follow up: none |
|
| Participants | 92 male and female children aged 0 to 10 with cerebral malaria: 3/95 (3%) excluded; 92 evaluated (arteether (48), quinine (44)) Inclusion criteria: asexual falciparum parasitaemia; Blantyre coma score of 2 or less with no other cause for coma Exclusion criteria: history of chronic illness and blackwater fever |
|
| Interventions | 1. Arteether (Artemotil) for 5 days (3.2 mg/kg first day, then 1.6 mg/kg next 4 days) 2. Quinine for 7 days (20 mg/kg loading dose, then 10 mg/kg thereafter) | |
| Outcomes | 1. Mortality 2. Parasite clearance time 3. Fever clearance time 4. Coma resolution time 5. Residual neurological sequelae 6. Adverse events | |
| Notes | Location: Zambia | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asimus 2007 | Looked at the effects of arteether and other artemisinin derivatives on healthy people as opposed to those with malaria |
| Asthana 2001 | No control group |
| Mishra 1995 | No control group |
| Mohanty 1997 | No control group |
| Pareek 2006 | Compared arteether with arteether instead of with another drug |
| Shukla 2002 | No control group |
| Singh 2007 | Combined arteether with sulfadoxine‐pyrimethamine and does not appear to be randomized |
Contributions of authors
Bosede Afolabi developed and wrote the review, and designed the eligibility and extraction forms. Christy Okoromah commented on and helped revise the review. Both authors extracted the data.
Sources of support
Internal sources
College of Medicine, University of Lagos, Nigeria.
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Moyou 2001 {published and unpublished data}
- Moyou‐Somo R, Tietche F, Ondoa M, Kouemeni LE, Ekoe T, Mbonda E, et al. Clinical trial of beta‐arteether versus quinine for the treatment of cerebral malaria in children in Yaounde, Cameroon. American Journal of Tropical Medicine and Hygiene 2001;64(5‐6):229‐32. [DOI] [PubMed] [Google Scholar]
Thuma 2000 {published data only}
- Thuma PE, Bhat GJ, Mabeza GF, Osborne C, Biemba G, Shakankale GM, et al. A randomized controlled trial of artemotil (beta‐arteether) in Zambian children with cerebral malaria. American Journal of Tropical Medicine and Hygiene 2000;62(4):524‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Asimus 2007 {published data only}
- Asimus S, Elsherbiny D, Hai TN, Jansson B, Huong NV, Petzold MG, et al. Artemisinin antimalarials moderately affect cytochrome P450 enzyme activity in healthy subjects. Fundamental & Clinical Pharmacology 2007;21(3):307‐16. [DOI] [PubMed] [Google Scholar]
Asthana 2001 {published data only}
- Asthana OP, Srivastava JS, Pandey TK, Vishwanathan KA, Dev V, Mahapatra KM, et al. Multicentric clinical trials for safety an efficacy evaluation of alpha;beta arteether in complicated P. falciparum malaria. Journal of the Association of Physicians of India 2001;49:1155‐60. [PubMed] [Google Scholar]
Mishra 1995 {published data only}
- Mishra SK, Asthana OP, Mohanty S, Patnaik JK, Das BS, Srivastava JS, et al. Effectiveness of alpha,beta‐arteether in acute falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(3):299‐301. [DOI] [PubMed] [Google Scholar]
Mohanty 1997 {published data only}
- Mohanty S, Mishra SK, Satpathy SK, Dash S, Patnaik J. alpha, beta‐Arteether for the treatment of complicated falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(3):328‐30. [DOI] [PubMed] [Google Scholar]
Pareek 2006 {published data only}
- Pareek A, Nandy A, Kochar D, Patel KH, Mishra SK, Mathur PC. Efficacy and safety of beta‐arteether and alpha/beta‐arteether for treatment of acute Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 2006;75(1):139‐42. [PubMed] [Google Scholar]
Shukla 2002 {published data only}
- Shukla UK, Damle RK, Shukla MM, Singh N. Efficacy of alpha, beta ‐ Arteether in children with cerebral malaria in forested tribal belt. Indian Pediatrics 2002;39(6):565‐8. [PubMed] [Google Scholar]
Singh 2007 {published data only}
- Singh RK. Efficacy of combined treatment with alpha/beta‐arteether and sulfadoxine‐pyrimethamine, for cases of Plasmodium falciparum malaria in Jharkhand, India. Annals of Tropical Medicine and Parasitology 2007;101(3):271‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Brewer 1994
- Brewer TG, Grate SJ, Peggins JO, Weina PJ, Petras JM, Levine BS, et al. Fatal neurotoxicity of arteether and artemether. American Journal of Tropical Medicine and Hygiene 1994;51(3):251‐9. [DOI] [PubMed] [Google Scholar]
de Vries 1996
- Vries PJ, Dien TK. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs 1996;52(6):818‐36. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kain 1995
- Kain KC. Chemotherapy and prevention of drug‐resistant malaria. Wilderness and Environmental Medicine 1995;6(3):307‐24. [DOI] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Lugt 2002
- Lugt CB. Dutch registration for artemotil injections. www.who.int/tdr/publications/tdrnews/news63/artemotil.htm (accessed June 2002).
McIntosh 2003
- McIntosh HM, Olliaro P. Artemisinin derivatives for treating severe malaria. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD000527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mutanda 1999
- Mutanda LN. Assessment of drug resistance to the malaria parasite in residents of Kampala, Uganda. East African Medical Journal 1999;76(8):421‐4. [PubMed] [Google Scholar]
Okitolonda 1987
- Okitolonda W, Delacollete C, Malengreau M, Henquin JC. High incidence of hypoglycaemia in African patients treated with intravenous quinine for severe malaria. BMJ 1987;295(6600):716‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schulz 1996
- Schulz KF, Grimes DA, Altman DG, Hayes RJ. Blinding and exclusions after allocation in randomised controlled trials: survey of published parallel group trials in obstetrics and gynaecology. BMJ 1996;312(7033):742‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1998
- White NJ, Olliaro P. Artemisinin and derivatives in the treatment of uncomplicated malaria. Medecine Tropicale 1998;58 Suppl 3:54‐6. [PubMed] [Google Scholar]
White 2000
- White NJ. Malaria. In: Cook G editor(s). Manson's tropical diseases. 20th Edition. London: W.B. Saunders, 1996:1087‐164. [Google Scholar]
WHO 1990
- World Health Organization. Severe and complicated malaria. World Health Organization, Division of Control of Tropical Diseases. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84 Suppl 2:1‐65. [PubMed] [Google Scholar]
WHO 1993
- World Health Organization. The role of artemisinin and its derivatives in the current treatment of malaria (1994‐1995: report of an informal consultation, Geneva, 27‐29 September 1993). Geneva: World Health Organization, 1993. [Google Scholar]
WHO 2000
- World Health Organization. Severe falciparum malaria. World Health Organization, Communicable Diseases Cluster. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94 Suppl 1:1‐90. [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. The use of antimalarial drugs. Report of an informal consultation. World Health Organization, Geneva 2001.
WHO 2002
- World Health Organization. Review of application for inclusion of a drug in the WHO Essential Drugs List: Artemotil and alpha/beta arteether (CDS/RBM, 18 March 2002). www.who.int/medicines/organization/par/edl/arteether_review.doc (accessed 29 April 2003).


