Abstract
Background
Malaria is an important cause of morbidity and mortality, in particular among children and pregnant women in sub‐Saharan Africa. Prompt access to diagnosis and treatment with effective antimalarial drugs is a central component of the World Health Organization's (WHO) strategy for malaria control. Home‐ or community‐based programmes for managing malaria are one strategy that has been proposed to overcome the geographical barrier to malaria treatment.
Objectives
To evaluate home‐ and community‐based management strategies for treating malaria.
Search methods
We searched the Cochrane Central Register of Controlled Trials published in The Cochrane Library; MEDLINE; EMBASE; Science Citation Index; PsycINFO/LIT; CINAHL; WHO clinical trial registry platform; and the metaRegister of Controlled Trials up to September 2012.
Selection criteria
Randomized controlled trials (RCTs) and non‐RCTs that evaluated the effects of a home‐ or community‐based programme for treating malaria in a malaria endemic setting.
Data collection and analysis
Two authors independently screened and selected studies, extracted data, and assessed the risk of bias. Where possible the effects of interventions are compared using risk ratios (RR), and presented with 95% confidence intervals (CI). The quality of the evidence was assessed using the GRADE approach.
Main results
We identified 10 trials that met the inclusion criteria. The interventions involved brief training of basic‐level health workers or mothers, and most provided the antimalarial for free or at a highly subsidized cost. In eight of the studies, fevers were treated presumptively without parasitological confirmation with microscopy or a rapid diagnostic test (RDT). Two studies trained community health workers to use RDTs as a component of community management of fever.
Home‐ or community‐based strategies probably increase the number of people with fever who receive an appropriate antimalarial within 24 hours (RR 2.27, 95% CI 1.79 to 2.88 in one trial; RR 9.79, 95% CI 6.87 to 13.95 in a second trial; 3099 participants, moderate quality evidence). They may also reduce all‐cause mortality, but to date this has only been demonstrated in rural Ethiopia (RR 0.58, 95% CI 0.44 to 0.77, one trial, 13,677 participants, moderate quality evidence).
Hospital admissions in children were reported in one small trial from urban Uganda, with no effect detected (437 participants, very low quality evidence). No studies reported on severe malaria. For parasitaemia prevalence, the study from urban Uganda demonstrated a reduction in community parasite prevalence (RR 0.22, 95% CI 0.08 to 0.64, 365 participants), but a second study in rural Burkina Faso did not (1006 participants). Home‐ or community‐based programmes may have little or no effect on the prevalence of anaemia (three trials, 3612 participants, low quality evidence). None of the included studies reported on adverse effects of using home‐ or community‐based programmes for treating malaria.
In two studies which trained community health workers to only prescribe antimalarials after a positive RDT, prescriptions of antimalarials were reduced compared to the control group where community health workers used clinical diagnosis (RR 0.39, 95% CI 0.18 to 0.84, two trials, 5944 participants, moderate quality evidence). In these two studies, mortality and hospitalizations remained very low in both groups despite the lower use of antimalarials (two trials, 5977 participants, low quality evidence).
Authors' conclusions
Home‐ or community‐based interventions which provide antimalarial drugs free of charge probably improve prompt access to antimalarials, and there is moderate quality evidence from rural Ethiopia that they may impact on childhood mortality when implemented in appropriate settings.
Programmes which treat all fevers presumptively with antimalarials lead to overuse antimalarials, and potentially undertreat other causes of fever such as pneumonia. Incorporating RDT diagnosis into home‐ or community‐based programmes for malaria may help to reduce this overuse of antimalarials, and has been shown to be safe under trial conditions.
Keywords: Child; Child, Preschool; Humans; Infant; Infant, Newborn; Antimalarials; Antimalarials/supply & distribution; Antimalarials/therapeutic use; Community Health Services; Community Health Services/methods; Community Health Services/organization & administration; Community Health Workers; Community Health Workers/education; Fever; Fever/drug therapy; Home Care Services; Home Care Services/organization & administration; Malaria; Malaria/drug therapy; Malaria/mortality; Mothers; Mothers/education; Randomized Controlled Trials as Topic
Home‐ or community‐based programmes for treating malaria
Malaria is an important cause of death especially in children and pregnant women living in sub‐Saharan Africa. In many rural areas, children are unable to access effective malaria treatment because health services are either too far away or antimalarial drugs are too expensive. Home‐ or community‐based programmes for managing malaria have been proposed as a key strategy to overcome these problems. In these programmes people living in rural settings, such as mothers, volunteers, or community health workers, are trained to recognise fever and provide antimalarial medicines at a low cost or for free. Malaria is not the only cause of fever and recently rapid diagnostic tests (RDTs) have become available. They are easy to use and enable trained workers to more accurately diagnose malaria and refer sick children without malaria for care elsewhere.
We examined the research published up to 12 September 2012 and we identified 10 studies for inclusion in this systematic review. In eight studies all people with fever were treated with antimalarial drugs by community health workers and in two studies community health workers were trained to confirm malaria in people using RDTs.
Home‐ or community‐based strategies probably increase the number of people with fever that receive an effective antimalarial within 24 hours (moderate quality evidence). They probably reduce the number of deaths in areas where malaria is common and there is poor access to health services (moderate quality evidence) but to date this has only been demonstrated in one study from a rural setting in Ethiopia. We do not know whether they reduce the number of people requiring admission to hospital (very low quality evidence), or the number of people with evidence of malaria infection in their blood (very low quality evidence). Home‐ or community‐based programmes may have little or no effect on the number of people with anaemia (low quality evidence). None of the included studies reported on adverse effects of using home‐ or community‐based programmes for treating malaria.
Use of RDTs instead of clinical diagnosis in home‐ or community‐based programmes for treating malaria probably reduces the overuse of antimalarials drugs (moderate quality evidence) and may have little or no difference upon the number of childhood deaths (low quality evidence), the number of children with evidence of malaria infection in their blood (low quality evidence), or the need for children to be admitted to hospital (low quality evidence) compared to use of clinical diagnosis.
Summary of findings
Summary of findings for the main comparison.
Home‐ or community‐based programmes versus standard care for treating malaria
| Home‐ or community‐based programmes for treating malaria versus facility based care | ||||||
|
Patient or population: Children with fever or malaria symptoms Settings: Malaria endemic areas Intervention: Home‐ or community‐based programmes Control: Standard care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard Care | Home‐ or community‐based programmes | |||||
| Prompt treatment with an effective antimalarial | 100 per 1000 |
469 per 1000 (100 to 1000) |
RR 4.69 (1.00 to 22.07) |
3099 (2 studies) | ⊕⊕⊕⊝ moderate1 |
Both studies found large statistically significant benefits. |
| All‐cause mortality | 50 per 1000 | 29 per 1000 (22 to 39) | RR 0.58 (0.44 to 0.77) | 13677 (1 study) | ⊕⊕⊕⊝ moderate2,3 |
|
| Hospitalizations | 230 per 1000 |
145 per 1000 (81 to 269) |
RR 0.63 (0.35 to 1.17) |
437 (1 study) | ⊕⊝⊝⊝ very low4,5 |
This single study was conducted in an urban setting. |
| Prevalence of parasitaemia | ‐ | ‐ | Not pooled | 1443 (2 studies) |
⊕⊝⊝⊝ very low6,7 |
Trials had mixed results. |
| Prevalence of anaemia | 44 per 1000 |
59 per 1000 (31 to 110) |
RR1.33 (0.70 to 2.51) |
3612 (3 studies) | ⊕⊕⊝⊝ low8,9 |
No statistically significant differences were seen. |
| The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for indirectness: All of these studies treated children with a clinical diagnosis of malaria, without parasitological confirmation. This approach is no longer recommended by the WHO and may lead to undertreatment of other illnesses which may require alternative treatments. 2 No serious risk of bias: Although the baseline characteristics of the intervention and control areas were not well described, deaths were well balanced at baseline between groups. 3 Downgraded by 1 for indirectness: The study was conducted in a setting where community‐based interventions such as this had been in operation for 20 years, and so the findings may not be easily generalised to other settings. 4 Downgraded by 2 for indirectness: This study was conducted in an urban setting, which is unusual for a home‐based programme. The findings may not be applicable elsewhere. 5 Downgraded by 1 for imprecision: The trend favours the intervention but the result is not statistically significant. 6 Downgraded by 1 for inconsistency: One trial from urban Uganda demonstrated a statistically significant difference in the prevalence of parasitaemia between the intervention and control groups, while one study from rural Burkina Faso did not. 7 Downgraded by 2 for imprecision: The data could not be pooled, and larger trials would be necessary to confidently prove or exclude a clinically important benefit on this outcome. 8 Downgraded by 1 for risk of bias: In all three of these trials, the prevalence of anaemia was significantly reduced in both the intervention and the control groups. The reasons for this are unclear, but include contamination or confounding. 9 Downgraded by 1 for imprecision: The confidence interval is very wide.
Summary of findings 2.
Home‐ or community‐based programmes using RDT diagnosis versus the same programmes using clinical diagnosis
| Home‐ or community‐based programmes using RDT diagnosis versus the same programme using clinical diagnosis | ||||||
|
Patient or population: Children with fever or malaria symptoms Settings: Malaria endemic areas Intervention: Home‐ or community‐based programmes using RDT diagnosis Control: Home‐ or community‐based programmes using clinical diagnosis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Clinical diagnosis | RDT diagnosis | |||||
| Treatment with an antimalarial | 980 per 1000 |
382 per 1000 (176 to 823) |
RR 0.39 (0.18, 0.84) |
5977 (2 studies) | ⊕⊕⊕⊝ moderate1 |
Absolute reductions in antimalarial use in these two trials were 72% and 43%. |
| All‐cause mortality | 1 per 1,000 |
2 per 1,000 (0 to 11) |
RR 3.51 (0.68 to 18.22) |
6055 (2 studies) | ⊕⊕⊝⊝ low2,3 |
Mortality was less than 2 per 1000 in both treatment groups. |
| Hospitalizations | 7 per 1000 |
2 per 1000 (0 to 11) |
RR 0.25 (0.04 to 1.50) |
3125 (1 study) | ⊕⊕⊝⊝ low2,3 |
|
| Treatment failure at day 7 | ‐ | ‐ | Not pooled | 5994 (2 studies) | ⊕⊕⊝⊝ low1, 4 |
Trials had mixed results. One study showed a statistically significant increase in treatment failure when RDTs were used while the other did not. |
| The assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 for serious indirectness: The introduction of RDTs was only tested in two settings. Compliance with the RDT protocol was high under trial conditions. Further effectiveness studies may be necessary to have full confidence in this results. 2 Downgraded by 1 for serious indirectness: The introduction of RDTs was only tested in two settings. It appeared safe under trial conditions without an increase in mortality or hospitalizations. Further effectiveness studies may be necessary to have full confidence in this. 3 Downgraded by 1 for serious imprecision: These two studies were not powered to look for effects on mortality of hospitalization. 4 Downgraded by 1 for serious inconsistency: One of the two studies found a statistically significant increase in patients reporting continued symptoms at day 7. The reasons for this are unclear.
Background
Malaria is an major cause of morbidity and mortality, especially among children and pregnant women in sub‐Saharan Africa. Prompt access to diagnosis and treatment with effective antimalarial drugs is a central component of the World Health Organization (WHO) strategy for malaria control (WHO 2006; WHO 2010).
Artemisinin‐based combination therapies (ACTs) are now the recommended first‐line therapy for malaria (WHO 2006; Sinclair 2009). However, access to these effective treatments continues to be a health policy challenge in many low resource settings. Results from household surveys conducted in 18 African countries between 2006 and 2007 showed that the mean proportion of children under five years of age with fever that were treated with an antimalarial drug was 38%, with only 3% of these receiving an ACT (WHO 2008). Similarly, in 2008, data from seven African countries revealed that only 16% of children who had fever received ACT (WHO 2009). In Kenya, about one year after the change in treatment policy to ACT, it was found that only about 10% of children received the recommended first line ACT (Gitonga 2008). Another study conducted in Burkina Faso found that less than 1% of households stocked effective combination therapy for malaria, and of those that did stock an antimalarial, 86% stocked chloroquine (CQ) (Tipke 2009).
Access to malaria treatment can be viewed as a multidimensional concept. The common dimensions of access include availability (sometimes referred to as physical or spatial access), affordability (sometimes referred to as financial access), acceptability (sometimes referred to as cultural access), accessibility, and adequacy (Andersen 1983; McIntyre 2007; Obrist 2007).
In most countries, the cost of ACTs is significantly greater than previously used antimalarial monotherapies, and represents a major barrier to care.
Other barriers to accessing effective treatment for malaria include the perceived quality of care, lack of knowledge, distance to health services, transport costs, treatment costs, and opportunity costs (Noor 2003; Whitty 2008). Therefore subsidies and reductions in the price of the ACTs alone will not automatically translate to improved access and other strategies will be needed.
Description of the intervention
Home‐ or community‐based programmes for managing malaria are one of the key strategies that have been proposed to overcome the geographical barrier to access to effective malaria treatment (WHO 2004). The WHO defines home‐based management of malaria as the presumptive treatment of febrile children at or near home with prepackaged antimalarial medicines distributed by trained community health workers (CHWs). However, different terminologies exist and are often used interchangeably in the literature which can become confusing: home‐based management of malaria (HBM), home management of malaria (HMM) and home‐based management of fever (HBMF). Staedke 2009a have argued that the term "home‐based management of malaria" should be used in cases with proven malaria and that "home‐based management of fever" should be reserved for the presumptive treatment of fevers at home without confirming a diagnosis of malaria.
In 2010, the WHO moved from presumptive malaria treatment to advocate parasitological confirmation prior to treatment of malaria in all patients. However this practice will not always be feasible, and the WHO recommendations do still allow for presumptive therapy when diagnostics are not available.
For the purpose of this review, we will explore the following home‐ or community‐based interventions:
Training mothers to presumptively treat fever with pre‐packaged antimalarials kept at home.
Training a basic health cadre (volunteers, CHWs, etc) to presumptively treat fever with pre‐packaged antimalarials supplied by the state or sold in pharmacies or shops.
Training a basic health cadre (volunteers, CHWs, etc) to diagnose malaria with rapid diagnostic tests (RDTs) and treat positive results with pre‐packaged antimalarials supplied by the state or sold in pharmacies or shops.
Training drug sellers to use a protocol involving positive RDT diagnosis prior to selling over the counter antimalarials.
How the intervention might work
Evidence from malaria endemic areas suggests that most episodes of fever are treated at home with over‐the‐counter medication bought from shops (McCombie 1996). In studies undertaken in Guatemala, Ethiopia, and Kenya over 60% of people self‐treated at home without seeking care from formal health facilities (Yeneneh 1993; Klein 1995; Snow 2005). In Ghana, Mali, Nigeria and Zambia, up to 90% of children with fever were treated at home (Salako 2001), and similarly in Sudan, people often started care at home, and then shifted to health workers if there was no improvement (Malik 2006).
Home‐ or community‐based programmes for the management of malaria therefore have the potential to reduce malaria related morbidity and mortality by: i) decreasing the time to treatment, and ii) improving the quality of treatments administered at home. This could also increase the proportion of people receiving appropriate treatment within 24 hours of the onset of fever or malaria which is one of the Roll Back Malaria (RBM) initiative indicators.
Conversely, in the context of the declining incidence of malaria, the proportion of fevers caused by other illnesses (such as pneumonia, measles, and diarrhoea) is increasing, and presumptively treating all fevers solely with antimalarials could adversely delay the diagnosis and treatment of other illnesses. Accurate diagnosis of malaria is therefore important and it has been suggested that RDTs to detect malaria could be incorporated into home‐ or community‐based programmes.
Why it is important to do this review
Home‐ or community‐based strategies for managing fever have been adopted by many countries in Africa, but there is limited and conflicting evidence on their effectiveness. A literature review of home‐based management strategies concluded that there was insufficient evidence to support its widespread implementation (Hopkins 2007). Moreover, the studies included in the review treated all fever cases presumptively with the older antimalarial CQ (Hopkins 2007). Following the adoption of ACTs as the first‐line antimalarial and the policy shift towards parasitological confirmation with RDTs, there is a need to re‐examine the effectiveness of home‐ and community‐based strategies at improving access to care and their impact on consequent childhood morbidity and mortality.
Objectives
To evaluate home‐based and community‐based management strategies for treating malaria or fever.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) for which the unit of randomization is the individual or cluster, and non‐RCTs including controlled before‐and‐after studies and interrupted‐time‐series studies.
Types of participants
People living in malaria endemic areas.
Types of interventions
Intervention
Any programme which trains mothers or caregivers, community‐based volunteers, community‐based health workers, or drug sellers to recognise and treat fevers with antimalarials presumptively or after a positive malaria RDT.
Control
Health facility‐based care; or an alternative home‐ or community‐based programme for recognizing and treating malaria or fevers.
Types of outcome measures
Primary outcomes
All‐cause mortality
Secondary outcomes
Malaria‐specific mortality
Hospitalizations
Severe malaria
Treatment with the recommended antimalarial within 24 hours
Treatment with any antimalarial
Parasitaemia
Anaemia
Adverse events (any adverse event as reported in the included studies)
Search methods for identification of studies
Electronic searches
We developed a highly sensitive search strategy to identify relevant studies. We searched the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; Science Citation Index; PsycINFO/LIT; and CINAHL using the search terms detailed in Appendix 1 up to 12 September 2012. We also searched the WHO clinical trial registry platform and the metaRegister of Controlled Trials (mRCT) for ongoing trials using the following search terms: malaria; child*; home‐based; community‐based; presumptive treatment.
Searching other resources
We handsearched conference proceedings, including recent MIM Pan‐African Malaria Conferences (2005 and 2009).
We contacted individual researchers working in this field for unpublished and ongoing trials.
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two authors (CO and SN) independently assessed titles and abstracts obtained from the searches to identify potentially eligible studies using a study selection form. We resolved any discrepancies through discussion. We obtained full text articles of all selected abstracts to formally assess eligibility using the pre‐specified eligibility criteria. We identified multiple publications of the same study using a reference manager and we have summarized the reasons for excluding studies in the ‘Characteristics of excluded studies’ section.
Data extraction and management
Individually randomized trials
For dichotomous outcomes in individually randomized trials, we extracted the number of patients with the event and the total number of patients in each group.
Cluster‐RCTs
Where a trial adjusted for clustering, we extracted the adjusted measure of effect and its 95% confidence interval (CI). However if the trial did not adjust for clustering, we extracted the same information as for individually randomized trials. We also aimed to extract the method used to adjust for clustering, the unit of randomization, the average cluster size, the number of clusters, and the intracluster correlation co‐efficient (ICC) for each outcome.
Non‐randomized trials
For dichotomous outcomes in controlled before‐and‐after studies, we aimed to extract event rates before and after the intervention for the intervention and control group. If measures of effect were presented that compared intervention versus control, we extracted the result and noted whether the measure of effect was adjusted for any confounders.
CO and SN independently extracted data from the studies using a detailed data extraction form. We resolved any differences in data extraction through discussion or, if necessary, by consulting the third author. We extracted data on:
Study details: citation, start and end dates, location, study design, and study details.
Participant details: study population eligibility (inclusion and exclusion) criteria, ages, population size, and attrition rate.
Details about the interventions: Nature of programme: Who was trained? How long were they trained for? What were they trained to do? How were they supervised? Who trained them?
Malaria treatment given.
Outcome details: Outcomes including malaria related morbidity, malaria related mortality, incidence of hospitalizations, all‐cause mortality, malaria parasitological prevalence, and adherence to recommended dosage.
Study site: Prevalence of malaria, available health services, and distance to health facilities.
Assessment of risk of bias in included studies
Two authors (CO and SN) performed the assessment independently. We resolved any differences through discussion or, if necessary, by consulting the third author, AM.
Individually randomized trials
We assessed the risk of bias of all RCTs using The Cochrane Collaboration’s tool for assessing the risk of bias. This approach assesses the risk of bias across six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential biases (Higgins 2008). For each domain we have assigned a judgment of ‘yes’ (low risk of bias), ‘no’ (high risk of bias), or ‘unclear’ (unclear risk of bias).
Cluster‐randomized trials
For cluster‐randomized trials, we assessed recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individual RCTs.
Non‐randomized trials
For non‐randomized trials, we used the Effective Practice and Organization of Care (EPOC) criteria for assessing the risk of bias (http://epoc.cochrane.org/epoc‐resources‐review‐authors).
Measures of treatment effect
We presented the measures of treatment effect as reported by the trial authors, with 95% CIs and tests of statistical significance where available. We summarized dichotomous outcomes using risk ratios with 95% CIs (or other measures of effect if risk ratios were not presented in the trial reports of non‐randomized or cluster‐RCTs).
Unit of analysis issues
Where cluster‐RCTs did not adjust for the cluster design, we contacted the authors to request estimates for the ICC values so that we could make appropriate adjustments in our analyses using the methods described in Section 16.3.4 and 16.3.5 of the Cochrane Handbook (Higgins 2008). None of the trial authors responded so we sought estimates of ICC values from similar trials in malaria. Unfortunately, we were unable to obtain any reliable estimates of the ICC, so we instead conducted a sensitivity analysis imputing three different ICC values of 0.01, 0.05, and 0.1 to assess the robustness of the results. Since all our outcomes were binary, we divided both the numerator and denominator by the design effect given by 1+(m‐1)*ICC, where m is the average cluster size (calculated by dividing the total number of participants by the total number of clusters in both intervention and control groups), and ICC is the intra‐cluster correlation co‐efficient.
As a guide to the value of this sensitivity analysis, we were able to obtain ICC values from a trial of intermittent preventive treatment of malaria in infants (Chandramohan 2005). This study was conducted in rural Ghana and randomized 96 clusters of 25 children. The estimates of ICC were 0.000 for mortality and hospital admission, 0.075 for clinical malaria, and 0.006 for severe anaemia (Meremikwu 2008).
For one cluster RCT (Kidane 2000) which did not adjust results for clustering but reported the mortality data for each matched pair of clusters, we conducted meta‐analysis across the 12 matched pairs of intervention and control groups, in order to estimate the treatment effect.
Dealing with missing data
We contacted authors where there was missing or unclear data. For one study which only presented baseline data as a rate, without giving specific numerators or denominators, we used the denominators from the outcome data for each cluster to calculate the number of participants and deaths in each cluster at baseline. We did not conduct any other imputation of results.
Assessment of heterogeneity
We assessed heterogeneity amongst trials by inspecting the forest plots (to detect overlapping CIs), the I² statistic with a level of 50% to denote moderate levels of heterogeneity, and applying the Chi2 test with a P value of 0.10 to indicate statistical significance.
Assessment of reporting biases
We identified an insufficient number of studies to enable an assessment of the likelihood of reporting bias.
Data synthesis
We analyzed the data using Review Manager (RevMan), and combined trial results in meta‐analysis where appropriate. We used the random‐effects model as we are looking for an 'average' effect rather than one true underlying effect. When a pooled meta‐analysis result was considered to be meaningless because of clinical or substantial statistical heterogeneity, we presented the results in a forest plot without a pooled estimate of effect. We presented results from cluster‐RCTs that did not adjust for clustering and non‐randomized studies in tables.
Quality of evidence
We assessed the quality of evidence across each outcome measure using the GRADE approach. The quality rating across studies has four levels: high, moderate, low, or very low. RCTs are initially categorized as high quality but can be downgraded after assessment of five criteria: risk of bias, consistency, directness, imprecision, and publication bias. Similarly, observational studies are initially categorized as low quality and can be downgraded by these same criteria. In exceptional circumstances they may be upgraded by three further criteria: large effect size, all plausible confounders would act to reduce the effect size, and evidence of a dose‐response effect (Guyatt 2008).
Subgroup analysis and investigation of heterogeneity
We planned to investigate statistical heterogeneity by conducting subgroup analysis with respect to age (< 5 years of age versus > 5 years of age), malaria endemicity, type of antimalarial used, form of training (leaflet, presentation, one‐to‐one); type of training (household versus community‐based); who was trained (eg family member versus drug seller); training area (eg recognizing fever/malaria versus treating individuals). However, we did not do so because of the limited number of studies identified for meta‐analyses.
Sensitivity analysis
We planned to carry out a sensitivity analysis by excluding studies with a high risk of bias from the meta‐analysis, but did not do so as there were so few trials in each comparison. However, post hoc we decided to carry out sensitivity analysis with respect to ICC values of 0.01, 0.05, and 0.1 in order to assess the effect of different ICC values on the significance of the treatment effect.
Results
Description of studies
Results of the search
The study flow diagram is shown in Figure 1. We identified 29 potentially eligible studies from 389 records. However, only 10 of these 29 studies met the inclusion criteria for this review.
Figure 1.
Study flow diagram.
On 1 May 2013 when this article was sent to press we noted several additional studies have been published since September 2012, the search date of this review. The editorial team briefly appraised these studies, and judged they are unlikely to overturn the conclusions of this review. They are being incorporated in the review update.
Included studies
We included 10 studies conducted in different African countries : Spencer 1987, Kenya; Delacollette 1996, Democratic Republic of Congo (DRC); Kidane 2000, Ethiopia; Nsungwa‐Sabiiti 2007, Uganda; Kouyate 2008, Burkina Faso; Staedke 2009, Uganda; Eriksen 2010, Tanzania; Yeboah‐Antwi 2010, Zambia; Kangwana 2011, Kenya; and Mubi 2011, Tanzania)).
Six studies were parallel cluster‐RCTs (Kidane 2000; Kouyate 2008; Staedke 2009; Eriksen 2010; Yeboah‐Antwi 2010; Kangwana 2011), one was a cross‐over cluster‐randomized trial (Mubi 2011), and three were controlled before‐and‐after studies (Spencer 1987; Delacollette 1996; Nsungwa‐Sabiiti 2007).
Only four of the seven cluster‐randomized studies made adjustments to their results to account for the cluster design (Staedke 2009; Yeboah‐Antwi 2010; Kangwana 2011; Mubi 2011), while three did not (Kidane 2000; Kouyate 2008; Eriksen 2010). One study was randomized by household (Staedke 2009; average cluster size: one child per cluster); four were randomized by village or clusters of villages (Kidane 2000; Kouyate 2008; Eriksen 2010; Kangwana 2011; average cluster sizes: 217, 42, 570, and 77 respectively), and two were randomized by CHW or health centre (Yeboah‐Antwi 2010; Mubi 2011; average cluster sizes: 133 and 101 respectively). In four studies, data collection was performed through proportional surveys pre and post intervention (Kouyate 2008; Eriksen 2010; Yeboah‐Antwi 2010; Kangwana 2011), and in three studies the CHWs or mothers providing the intervention collected the data (Kidane 2000; Staedke 2009; Mubi 2011).
All of the studies targeted children aged less than six years, except for three studies (Spencer 1987; Delacollette 1996; Mubi 2011) which treated all age groups.
The precise nature of the intervention varied between studies but all 10 studies involved the training of low‐level health workers or mothers to give antimalarials. In all 10 studies the antimalarial was provided free or at a highly subsidized cost. In eight studies the health workers or mothers treated all episodes of fever presumptively with an antimalarial and this was compared to standard (facility‐based) care (Spencer 1987; Delacollette 1996; Kidane 2000; Nsungwa‐Sabiiti 2007; Kouyate 2008; Staedke 2009; Eriksen 2010; Kangwana 2011). Two studies compared home‐ or community‐based programmes using RDTs to confirm malaria with programmes using presumptive treatment (Yeboah‐Antwi 2010; Mubi 2011). For further details see Table 5 and Table 6.
Table 1.
Summary of trials comparing home‐or community‐based interventions with facility‐ based care
| Study ID | Study Design |
Country (setting) |
Who was trained? | How long was the training? | What were they trained to do? | Were drugs given free? | How were they supervised? | Additional comments |
| Kangwana 2011 | Cluster‐RCT | Kenya (Rural) |
Retail outlet staff | 1 day | Treat clinical malaria with AL Referral criteria |
Subsidized | Retail staff kept records of prescription. Study staff visited after 3 months. |
Extensive social marketing of branded AL was conducted among the community. |
| Eriksen 2010 | Cluster‐RCT |
Tanzania (Unclear) |
Health workers | 7 days | Malaria case management | Yes | Health workers visited women leaders every 2 weeks. | Community awareness activities also took place. Women leaders were paid $20 per month. |
| Women leaders | 7 days | Treat clinical malaria with SP Referral criteria |
||||||
| Staedke 2009 | Cluster‐RCT | Uganda (Urban) |
Mothers | Unclear | Treat fever with AL | Yes | Study personnel visited every month. | |
| Kouyate 2008 | Cluster‐RCT | Burkina Faso (Rural) |
Nurses | 5 days | Malaria case management | Subsidized | Nurses visited women group leaders monthly. Study personnel visited monthly. |
Drugs supplied free to women group leaders who charged mothers a small fee. |
| Women group leaders | 2 days | Treat clinical malaria with CQ | ||||||
| Mothers | ½ day | Take children with fever to women leaders | ||||||
| Kidane 2000 | Cluster‐RCT | Ethiopia (Rural) |
Mother co‐ordinators | 2 months | Referral criteria To train mothers to treat clinical malaria |
Yes | Field supervisors visited the mother co‐ordinators 4 to 6 times per month and directly supervised a sample of mothers. | The mother co‐ordinators collected the data on death. |
| Mothers | Unclear | Treat clinical malaria with CQ | ||||||
| Nsungwa‐Sabiiti 2007 | CBA | Uganda (Rural) |
Community volunteers | 3 days | Treat fever with CQ+SP, and referral criteria | Yes | The district health team supervised the programme every 3 months. | Volunteers also educated mothers about care‐seeking at home visits and village meetings. |
| Delacollette 1996 | CBA | DRC (Rural) |
Literate volunteers | 2 weeks | Treat fever with CQ | Subsidized | Closely supervised by nurses from the health facility. | Volunteers received a small monetary incentive. |
| Spencer 1987 | CBA | Kenya (Rural) |
CHWs | Unclear | Treat fever with CQ | Yes | Unclear |
RCT = Randomized controlled trial, CBA = controlled before‐and‐after study, DRC = Democratic Republic of Congo, CHW = community health worker, SP = sulphadoxine‐pyrimethamine, CQ = chloroquine, AL = artemether‐lumefantrine, RDT = rapid diagnostic test
Table 2.
Summary of trials comparing home‐ or community‐based programmes using RDT diagnosis versus clinical diagnosis
| Study ID | Study Design |
Country (setting) |
Who was trained? | How long was the training? | What were they trained to do? | Were drugs given free? | How were they supervised? | Additional comments |
| Yeboah‐Antwi 2010 | Cluster‐RCT | Zambia (Rural) |
Community health Workers | 5 days | Treat fever + positive RDT with AL | Yes | Monthly supervision by the head nurse of each health centre | In the control arm, CHWs treated all fevers with AL. |
| Mubi 2011 | Cross‐over cluster‐RCT | Tanzania (Rural) |
Community health workers | 7 days | Treat fever + positive RDT with AL | Yes | Supervised throughout the study but no details provided | In the control arm, CHWs treated all fevers with AL. |
RCT = Randomized controlled trial, AL = artemether‐lumefantrine, RDT = rapid diagnostic test, CHW = community health workers
The mean duration of follow‐up of the 10 studies was 12 months; Delacollette 1996 (24 months), Kouyate 2008 (15 months), Mubi 2011 (5 months), Kidane 2000, Spencer 1987, Staedke 2009; and Yeboah‐Antwi 2010 (12 months each), Eriksen 2010 (9 months), Kangwana 2011 (6 months) and Nsungwa‐Sabiiti 2007 (18 months).
Excluded studies
We identified 19 studies as potentially relevant. However, these studies did not meet the review's inclusion criteria. We have listed the reasons for exclusion of these studies in the Characteristics of excluded studies section.
Risk of bias in included studies
For a summary of the risk of bias assessments, see Figure 2.
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six of the seven cluster‐RCTs described adequate random sequence generation. However, only two studies described an adequate method to conceal allocation and we considered them to be at low risk for selection bias (Staedke 2009; Mubi 2011). The risk of selection bias was unclear for the remaining five randomized trials (Kidane 2000; Kouyate 2008; Eriksen 2010; Yeboah‐Antwi 2010; Kangwana 2011), and high for the three controlled before‐and‐after studies (Spencer 1987; Delacollette 1996; Nsungwa‐Sabiiti 2007).
Baseline imbalance
Only two of the cluster‐RCTs provided adequate detail on baseline characteristics to be considered at low risk of bias (Staedke 2009; Kangwana 2011). Three studies provided only limited information and were judged to be at unclear risk (Kidane 2000; Eriksen 2010; Mubi 2011). Two studies had evidence of important differences between groups at baseline (Kouyate 2008; Yeboah‐Antwi 2010).
Of the three controlled before‐and‐after studies, Delacollette 1996 did not provide adequate information on the baseline characteristics of the two groups, and there was evidence of important baseline differences in both Nsungwa‐Sabiiti 2007 and Spencer 1987 which could have confounded the study findings.
Contamination
Of the 10 studies, there was high risk of contamination in one of the studies (Kouyate 2008), low risk of contamination in five studies (Delacollette 1996; Kidane 2000;Nsungwa‐Sabiiti 2007; Yeboah‐Antwi 2010; Mubi 2011) and unclear risk of contamination in four studies (Spencer 1987; Staedke 2009; Eriksen 2010; Kangwana 2011). See Characteristics of included studies for further details.
Blinding
Blinding of the participants in these types of studies would not be possible. However, blinding of the study statisticians during analysis would be possible and was not described for any of the included studies.
Incomplete outcome data
No loss of clusters was reported in any of the cluster‐RCTs, and six studies were judged to be at low risk of attrition bias (Kouyate 2008; Staedke 2009; Eriksen 2010; Yeboah‐Antwi 2010; Kangwana 2011;Mubi 2011).
Kidane 2000 was judged to be at high risk of bias for the outcome 'malaria specific mortality' as only one third of all deaths had undergone a verbal autopsy.
Selective reporting
We did not find evidence of selective outcome reporting.
Other potential sources of bias
Another potential source of bias was identified in Kouyate 2008, where all the outcomes were self‐reported.
Effects of interventions
Comparison 1: Home‐ or community‐based interventions versus facility‐based care
Treatment with the recommended antimalarial within 24 hours
Two cluster‐RCTs (Kangwana 2011; Staedke 2009) reported the proportion of fevers receiving prompt and effective treatment. Three cluster‐RCTs (Kangwana 2011; Staedke 2009; Kouyate 2008) and one controlled before‐and‐after study (Nsungwa‐Sabiiti 2007) reported the proportion of fevers receiving any antimalarial. In western Kenya, Kangwana 2011 trained private drug sellers and provided them with subsidized packs of artemether‐lumefantrine (AL). After six months, the proportion of children with fever receiving AL on the same day or the following day increased from 4.7% to 44.9% in the intervention groups, and from 5.3% to 19.9% in the controls (one trial, 2662 participants, P = 0.0001, authors own figures, see Table 7). In urban Uganda, Staedke 2009 reported that the proportion of participants with fevers receiving chloroquine (CQ) plus sulphadoxine‐pyrimethamine (SP), quinine, or an artemisinin within 24 hours of the onset of fever, was 51.5% in the intervention group compared to 5.2% in the controls (one trial, 437 participants, P < 0.0001, authors own figures, see Table 7). This result remained statistically significant with high ICC values (Analysis 1.4). All three cluster‐RCTs that reported the proportion of participants with fevers receiving any antimalarial demonstrated a larger increase in the intervention groups than in the controls (three trials, 4105 participants, see Table 7, Analysis 1.5). The sensitivity analysis adjusting these three trials for the cluster‐randomized design did not change the significance of the results (Analysis 1.5). In the controlled before‐and‐after study from rural Uganda (Nsungwa‐Sabiiti 2007), village volunteers were trained to recognise fever and treat with pre‐packaged antimalarials (CQ + SP). This study coincided with a change in national antimalarial policy from CQ to CQ + SP. The number of fevers which were treated with the correct dosage and duration of CQ at baseline was 7.4% in the intervention areas compared to 7.5% in control areas. The number of fevers treated with the correct dosage of CQ + SP post intervention was 13.5% in the intervention areas and 0.0% in control areas presumably because this combination was unavailable in the control areas.
Table 3.
Summary of results from cluster‐RCTs comparing home‐ or community‐based programmes with facility‐based care
|
Outcome |
Study ID |
Number of events/number of participants (percentage) |
Comment |
|||||
| Home‐ or community‐based programmes | Facility‐based care | |||||||
| Baseline | Follow‐up | Difference | Baseline | Follow‐up | Difference | |||
| All‐cause mortality | Kidane 2000 | ‐ | 190/6383 (29.8%) |
‐ | ‐ | 366/7294 (50.2%) |
‐ | Rate ratio 0.59, 95% CI 0.50 to 0.711 |
| Staedke 2009 | _ | 1/189 (0.5%) |
_ | _ | 1/176 (0.5%) |
_ | 2 | |
| Malaria‐specific mortality | Kidane 2000 | _ | 13/70 (18.6%) |
_ | _ | 68/120 (56.7%) |
_ | Determined by verbal autopsy3 |
| Hospitalization | Staedke 2009 | ‐ | 25/189 (13.2%) |
‐ | 40/176 (22.7%) |
Rate ratio 0.63, 95% CI 0.35 to 1.17 | ||
| Parasitaemia | Staedke 2009 | ‐ | 4/189 (1.8%) |
‐ | ‐ | 17/176 (9.7%) |
‐ | RR 0.21, 95% CI 0.07 to 0.64 |
| Kouyate 2008 | 455/542 (84%) |
379/496 (76%) |
‐8% | 438/541 (81%) |
366/510 (72%) |
‐9% | P = 0.05 | |
| Anaemia | Eriksen 2010 | 406/925 (43.9%) |
8/982 (0.8%) |
‐43.1% | 243/790 (30.8% |
2/1187 (0.2%) |
‐30.6% | |
| Staedke 2009 | 36/222 (16%) |
7/189 (4%) |
‐12% | 23/210 (11%) |
7/176 (4%) |
‐7% | ||
| Kouyate 2008 | 152/542 (28%) |
83/496 (17%) |
‐11% | 162/541 (30%) |
74/510 (15%) |
‐15% | P = 0.32 | |
| Fever episodes receiving prompt and effective treatment with antimalarials | Kangwana 20114 | N/R (4.7%) |
N/R (44.9%) |
+40.2% | N/R (5.3%) |
N/R (19.9%) |
+14.6% | P = 0.0001 |
| Staedke 20095 | ‐ | 444/862 (51.5%) |
‐ | ‐ | 30/570 (5.2%) |
‐ | P < 0.0001 | |
| Fever episodes treated with any antimalarial | Kangwana 2011 | N/R (45.5%) |
N/R (64.0%) |
+18.5% | N/R (38.9%) |
N/R (50.3%) |
11.4% | P = 0.0074 |
| Staedke 2009 | ‐ | 764/862 (88.7%) |
‐ | ‐ | 367/570 (64.4%) |
‐ | P < 0.0001 | |
| Kouyate 20086 | 66/179 (36.9%) |
208/241 (86.3%) |
‐ | 87/132 (65.9%) |
191/282 (67.7%) |
‐ | P = not reported | |
RR = risk ratio, N/R = not reported
1 This result was not adjusted for clustering so the 95% CI will be artificially narrow. 2Staedke 2009 was not adequately powered to look for an effect on mortality. 3 Verbal autopsy was only conducted on one third of all deaths. 4 In Kangwana 2011 'prompt and effective treatment' is defined as any brand of AL on the same day or following day. 5 In Staedke 2009 'prompt and effective treatment' is defined as CQ+SP, or quinine, or an artemisinin within 24 hours. 6 In Kouyate 2008 there is a large baseline imbalance in health seeking behaviour between the two groups.
Analysis 1.4.
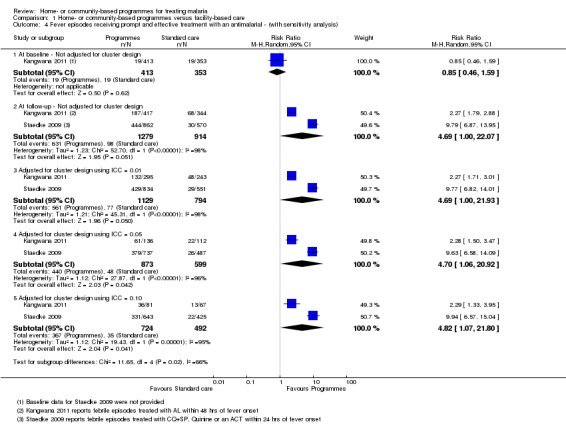
Comparison 1 Home‐ or community‐based programmes versus facility‐based care, Outcome 4 Fever episodes receiving prompt and effective treatment with an antimalarial ‐ (with sensitivity analysis).
Analysis 1.5.

Comparison 1 Home‐ or community‐based programmes versus facility‐based care, Outcome 5 Fever episodes receiving treatment with an antimalarial ‐ (with sensitivity analysis).
All‐cause mortality
Two randomized studies (Kidane 2000; Staedke 2009), and one controlled before‐and‐after study (Spencer 1987), reported on deaths occurring during follow‐up.
In rural villages in Ethiopia, mothers were trained to recognise and treat fever presumptively with CQ (Kidane 2000). The training was delivered by mother co‐ordinators who had undergone two months of training in malaria recognition and treatment, and deaths were recorded by these same mother co‐ordinators. Supervisors from the community‐based primary health care programme (which had been operating for over 20 years), visited the mother co‐ordinators and a small sample of mothers each month. During 12 months follow‐up, under‐5 mortality was significantly lower in the intervention areas than in the controls (one trial, 13,677 participants, RR 0.58, 95% CI 0.44 to 0.77, Analysis 1.1). We performed the meta‐analysis across the 12 matched pairs of intervention and control groups.
Analysis 1.1.
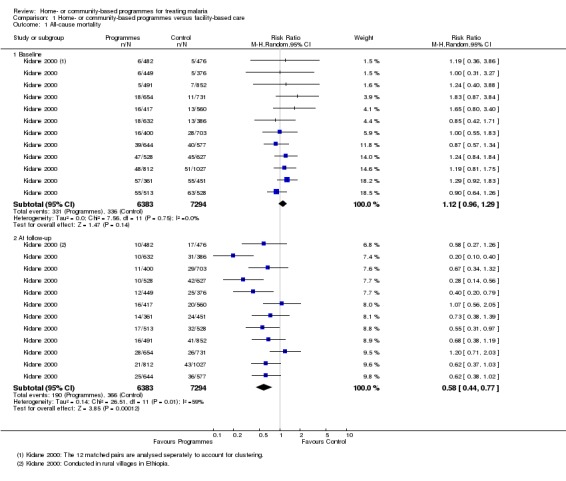
Comparison 1 Home‐ or community‐based programmes versus facility‐based care, Outcome 1 All‐cause mortality.
In urban Uganda, mothers were trained to recognise and treat fever presumptively with AL (Staedke 2009). The study was not powered to assess mortality and only two deaths occurred, one in each group (one trial, 437 participants, see Table 7).
In the controlled before‐and‐after study in a rural community in western Kenya, volunteer village health workers were trained to treat fever with CQ (Spencer 1987). The trial reported a reduction in all‐cause mortality in children under five years in the intervention areas. However, the authors reported that this was likely due to an increase in measles deaths in the intervention areas prior to the intervention.
Malaria‐specific mortality
Kidane 2000 used "verbal autopsy" to estimate the proportion of the observed deaths which might be due to malaria in rural Ethiopia. Deaths consistent with possible malaria were lower in the intervention group but only a third of all deaths were evaluated: 13/70 (19%) in the intervention group versus 68/120 (57%) in the controls (one trial, 13,677 participants, see Table 7).
In two controlled before‐and‐after studies from rural areas of DRC and Kenya respectively, volunteers were trained to treat fevers presumptively with CQ (Spencer 1987; Delacollette 1996). Tests for statistically significant differences between the two groups were not reported in either trial. In Delacollette 1996, the malaria‐specific mortality fluctuated in both the intervention and control groups over time, such that any effect of the intervention was impossible to determine. In Spencer 1987, the number of deaths attributable to malaria was low, but did not appear substantially different between groups (see Table 8).
Table 4.
Additional results from non‐randomized studies for malaria‐specific mortality
| Outcome | Study ID | Study design | Age group | Time period | Home‐ or community‐based programmes* | Control* | Comment |
| Malaria‐specific mortality | Delacollette 1996 | CBA | All | Aug 85 to Mar 86 (pre‐intervention) | 17 (102,410) | 27 (116,541) | Mortality per 10,000 patient months (number of patient months observed). |
| Apr 86 to Jul 86 (early intervention) | 21 (51,887) | 35 (59,490) | |||||
| Aug 86 to Mar 87 (full intervention) | 14 (103,704) | 27 (120,879) | |||||
| Apr 87 to Jul 87 (full intervention) | 32 (21,944) | 22 (36,530) | |||||
| Spencer 1987 | CBA | < 1 year | May 81 to April 82 (pre‐intervention) | 6.8 (8) | ‐ | Mortality per 1000 population (number of deaths). | |
| 1 to 4 years | Sept 82 to Aug 83 (during intervention) | 7.4 (10) | 4.4 (3) |
CBA = Controlled before‐and‐after study * Data as reported in these two papers. Tests of statistical significance were not reported.
Hospitalization
Only one RCT from urban Uganda reported on hospitalization (Staedke 2009). The rate of hospitalization was lower among households where mothers were trained to treat fevers with AL but this did not reach statistical significance (one trial, 437 participants, see Table 7).
Severe malaria
This outcome was not reported in any of the included studies.
Prevalence of parasitaemia
Two cluster‐RCTs (Kouyate 2008; Staedke 2009) and two controlled before‐and‐after studies (Spencer 1987; Delacollette 1996)) reported the prevalence of parasitaemia post‐intervention .
In urban Uganda, training mothers to treat fever with AL significantly reduced the prevalence of parasitaemia compared to attending standard care (RR 0.21, 95% CI 0.07 to 0.64, one trial, 437 participants, (authors own figures adjusted) see Table 7). However, in rural Burkina Faso, a complex intervention involving the training of mothers, mother co‐ordinators, and health workers to treat fevers with CQ found no statistically significant difference in parasitaemia between groups (one trial, 1006 participants, see Table 7). The sensitivity analysis adjusting these two trials for the cluster‐randomized design did not change the significance of either of these results (Analysis 1.2).
Analysis 1.2.
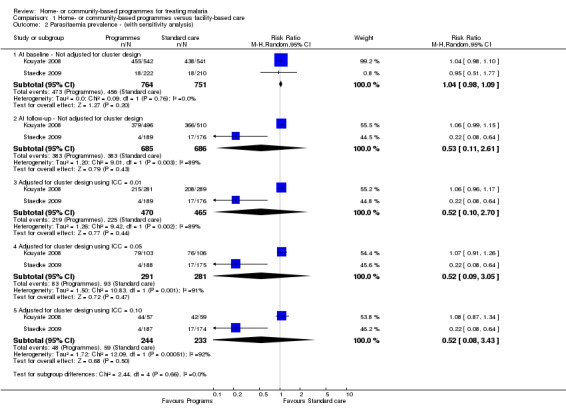
Comparison 1 Home‐ or community‐based programmes versus facility‐based care, Outcome 2 Parasitaemia prevalence ‐ (with sensitivity analysis).
Among the controlled before‐and‐after studies, Delacollette 1996 trained literate volunteers to treat fevers presumptively with CQ and found a five‐fold reduction in the prevalence of parasitaemia compared to only a two‐fold reduction in the control group (one trial, 446 participants). Spencer 1987 found no differences in the prevalence of parasitaemia between intervention and control groups in both the dry and rainy seasons (one trial, 1876 participants dry season, 520 participants rainy season, see Table 9).
Table 5.
Additional results from non‐randomized studies for prevalence of parasitaemia
| Outcome | Study ID | Study design | Age group | Detail | Number with parasitaemia/total number sampled (percentage) | Comment | |||||
| Home‐ or community‐based programmes | Control area | ||||||||||
| Baseline | Follow‐up | Difference | Baseline | Follow‐up | Difference | ||||||
| Parasitaemia | Delacollette 1996 | CBA | All ages | Any parasitaemia | 87/255 (34.1%) | 16/229 (7.0%) |
Rate Ratio1 4.9 (3‐8.1) | 96/254 (37.8) |
42/217 (19.3%) |
Rate Ratio 2.0 (1.4‐2.7) | Labelled as 'crude parasitological index' in paper. |
| High parasitaemia2 | 34/255 (13.3%) |
5/229 (2.2%) |
Rate Ratio 6.0 (2.4‐15.3) | 44/254 (17.3) |
20/217 (9.2%) |
Rate Ratio 1.9 (1.1‐3.1) | |||||
| Spencer 1987 | CBA | All ages | Dry season | 594/903 (65.7%) | 820/1291 (63.5%) | ‐2.2% | ‐ | 363/585 (62.1%) | ‐ | ||
| Rainy season | 516/586 (88.1%) | 273/361 (75.6%) | ‐12.5% | ‐ | 120/159 (75.5%) | ‐ | |||||
CBA = Controlled before‐and‐after study.
1The rate ratio was calculated as: the rate during Feb 1985/ rate during Feb 1987.
2 High parasitaemia defined as > 2,000 asexual forms of P. falciparum per mm3 of blood.
Prevalence of anaemia
Three cluster ‐RCTs reported the prevalence of anaemia before‐and‐after the intervention period (Kouyate 2008; Staedke 2009; Eriksen 2010). The prevalence of anaemia decreased in both the intervention and the control areas in all three trials without statistically significant differences between groups (three trials, 3612 participants, see Table 7). The sensitivity analysis using ICC values of 0.05, 0.01, and 0.1 did not change the significance of the results (Analysis 1.3).
Analysis 1.3.
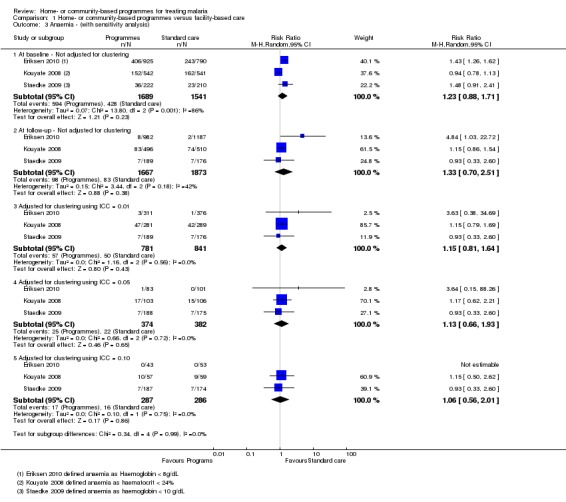
Comparison 1 Home‐ or community‐based programmes versus facility‐based care, Outcome 3 Anaemia ‐ (with sensitivity analysis).
It is likely that this observation was due to 'contamination' of the control areas (people living in the control areas also benefiting from the intervention), or confounding due to other health activities also being implemented in the study areas. Indeed, these phenomena were observed and commented on by Kouyate 2008 who noted that there were CQ and insecticide‐treated bednet (ITN) distribution activities by parallel programmes during the study period. Staedke 2009 and Eriksen 2010 did not comment on possible reasons for this observation.
Adverse events
None of the included studies reported on adverse events.
Comparison 2: Home‐ or community‐based programmes using RDTs versus using clinical algorithms
Two cluster‐RCTsevaluated the introduction of RDTs for malaria into home‐ or community‐based programmes (Yeboah‐Antwi 2010; Mubi 2011). In both studies CHWs were given one week of refresher training in fever case management. In the intervention areas, CHWs were trained to only treat people with fever with AL after a positive RDT, and in control areas all fevers were treated with AL. Mubi 2011 used a cross‐over design where the initial intervention and control CHWs swapped treatment arms half‐way through the study (see Table 6).
Treatment with an appropriate antimalarial
Neither Mubi 2011 nor Yeboah‐Antwi 2010 reported the proportion of children with fever receiving an antimalarial within 24 hours. However, in both studies compliance with the RDT protocol was high and antimalarial use was significantly lower in the intervention groups. In Yeboah‐Antwi 2010, the proportion of RDTs that were positive in the intervention arm was 271/975 (27.8%), and subsequently 265/963 (27.5%) were given antimalarials compared to 2066/2084 (99.1%) in controls. In Mubi 2011, the proportion of RDTs that were positive was 733/1457 (50.3%), and subsequently 775/1457 (53.2%) were given antimalarials compared to 1422/1473 (96.5%) of controls. The sensitivity analysis adjusting these two trials for the cluster‐randomized design did not change the significance of these results (see Analysis 2.5 and Table 10).
Table 6.
Summary of results for trials comparing RDT diagnosis versus clinical diagnosis
|
Outcome |
Study ID |
Study design | Number of episodes treated/Total number of episodes (%) |
Relative effect* (95% CI) |
Comment |
|
| RDT diagnosis | Clinical diagnosis | |||||
| Mortality | Yeboah‐Antwi 2010 | Cluster RCT | 2/1017 (0.2%) |
1/2108 (0.04%) |
‐ | The causes of death were not determined. |
| Mubi 2011 | Cluster RCT | 3/1457 (0.2%) |
1/1473 (0.06%) |
‐ | Malaria was confirmed as the cause of death in one patient from each group. | |
| Hospitalization | Yeboah‐Antwi 2010 | Cluster RCT | 4/1017 (0.4%) |
14/2108 (0.7%) |
RR 0.25 (0.04 to 1.50) |
|
| Referrals for further care | Mubi 2011 | Cluster RCT | 104/1457 (7.1%) |
49/1473 (3.3%) |
RR 1.89 (1.35 to 2.65) |
|
| Severe malaria | Yeboah‐Antwi 2010 | Cluster RCT | ‐ | ‐ | ‐ | Not reported. |
| Mubi 2011 | Cluster RCT | 1/1457 (0.06%) |
1/1473 (0.06%) |
‐ | Both of these children were given ACT and referred for further care. Both died. | |
| Treatment failure on day 7 | Yeboah‐Antwi 2010 | Cluster RCT | 95/1017 (9.3%) |
211/2108 (10.0%) |
RR 0.68 (0.39 to 1.19) |
Defined as continued symptoms, need for additional treatment, death or hospitalization. |
| Mubi 2011 | Cluster RCT | 94/1411 (6.7%) |
40/1458 (2.7%) |
RR 2.15 (1.50 to 3.09) |
Defined as incomplete recovery. | |
| Treatment with an appropriate antimalarial | Yeboah‐Antwi 2010 | Cluster RCT | 265/963 (27.5%) |
2066/2084 (99.1%) |
RR 0.23 (0.14 to 0.38) |
The proportion of positive RDT results was 271/975 (27.8%). |
| Mubi 2011 | Cluster RCT | 775/1457 (53.2%) |
1422/1473 (96.5%) |
RR 0.54 (0.46 to 0.62) |
The proportion of positive RDT results was 733/1457 (50.3%). | |
| Negative RDT tests given antimalarials | Yeboah‐Antwi 2010 | Cluster RCT | 3/704 (0.4%) |
‐ | ‐ | The parents of five additional children with negative RDT sought ACT elsewhere. |
| Mubi 2011 | Cluster RCT | 42/722 (5.8%) |
‐ | ‐ | ||
| Positive RDT tests not given antimalarials | Yeboah‐Antwi 2010 | Cluster RCT | ‐ | ‐ | ‐ | None reported. |
| Mubi 2011 | Cluster RCT | 2/733 (0.3%) |
‐ | ‐ | Both were fully recovered at day 7. | |
* Relative effects were adjusted for clustering by the study authors. Mubi 2011 presented results as cluster adjusted odds ratio (OR) which have been converted to risk ratio (RR) using the formula: RR=OR/(1‐ACR(1‐OR) where ACR = the Assumed Risk in the control group.
RCT = randomized controlled trial, RDT = rapid diagnostic test, ACT = artemisinin‐based combination therapy, CI = confidence interval
Analysis 2.5.
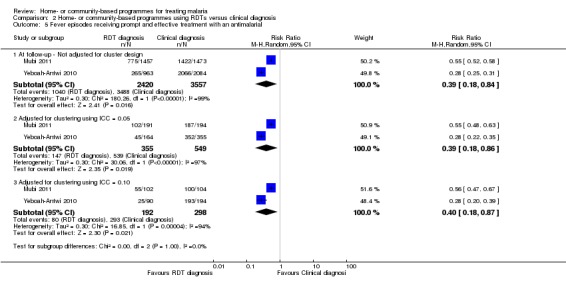
Comparison 2 Home‐ or community‐based programmes using RDTs versus clinical diagnosis, Outcome 5 Fever episodes receiving prompt and effective treatment with an antimalarial.
All‐cause mortality
The studies were not powered to detect an effect on mortality. In Yeboah‐Antwi 2010, three deaths occurred: 2/1017 (0.2%) in the intervention group versus 1/2082 (0.04%) in the control group. Both deaths in the intervention group occurred after a negative RDT. In Mubi 2011, four deaths occurred: 3/1457 (0.2%) in the intervention group versus 1/1473 (0.06%) in the control group. All four patients who died were treated with antimalarials and referred for further care. Malaria was confirmed as the cause of death in one patient in each group (see Table 10, Analysis 2.1).
Analysis 2.1.
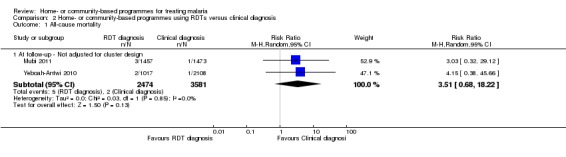
Comparison 2 Home‐ or community‐based programmes using RDTs versus clinical diagnosis, Outcome 1 All‐cause mortality.
Hospitalization
In Yeboah‐Antwi 2010, hospitalization was higher in the control group. However, this trial was not adequately powered to detect an effect and the result did not reach statistical significance: 4/1017 (0.4%) in the intervention group versus 14/2108 (0.7%) in the control group (RR 0.25, 95% CI 0.04 to 1.50, one trial, 3125 participants, ; authors' own figures adjusted for baseline fast breathing and fever, see Table 10, Analysis 2.2).
Analysis 2.2.
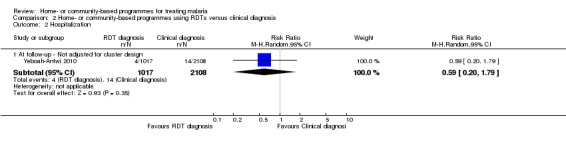
Comparison 2 Home‐ or community‐based programmes using RDTs versus clinical diagnosis, Outcome 2 Hospitalization.
In Mubi 2011 more patients in the intervention group were referred for further care: 104/1457 (7.1%) versus 49/1473 (3.3%) in the control group (RR 1.89, 95% CI 1.35 to 2.65, one trial, 2930 participants, Analysis 2.3). The potential reasons for this are not discussed by the study authors (see Table 10).
Analysis 2.3.

Comparison 2 Home‐ or community‐based programmes using RDTs versus clinical diagnosis, Outcome 3 Referrals for further care.
Treatment failure
Both studies reported treatment failure at Day‐7. In Yeboah‐Antwi 2010 there was no statistically significant difference detected in treatment failure (one trial, 3125 participants, Analysis 2.4), but Mubi 2011 reported that more than twice as many people reported symptoms at Day‐7 in the intervention group than in controls (RR 2.15, 95% CI 1.50 to 3.09, one trial, 2869 participants; Analysis 2.4, see Table 10).
Analysis 2.4.
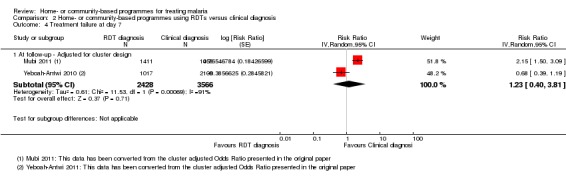
Comparison 2 Home‐ or community‐based programmes using RDTs versus clinical diagnosis, Outcome 4 Treatment failure at day 7.
Discussion
Summary of main results
We identified 10 trials that met the inclusion criteria. The interventions involved brief training of basic‐level health workers or mothers, and most provided the antimalarial for free or at a highly subsidized cost. In eight of the studies, fevers were treated presumptively without parasitological confirmation with microscopy or a rapid diagnostic test (RDT). Two studies trained community health workers to use RDTs as a component of community management of fever.
Home‐ or community‐based strategies probably increase the number of people with fever who receive an appropriate antimalarial within 24 hours (moderate quality evidence). They may also reduce all‐cause mortality, but to date this has only been demonstrated in rural Ethiopia (moderate quality evidence).
Hospital admissions in children were reported in one small trial from urban Uganda, with no effect detected (very low quality evidence). No studies reported on severe malaria. For parasitaemia prevalence, the study from urban Uganda demonstrated a reduction in community parasite prevalence, but a second study in rural Burkina Faso did not. Home‐ or community‐based programmes may have little or no effect on the prevalence of anaemia (low quality evidence). None of the included studies reported on adverse effects of using home‐ or community‐based programmes for treating malaria.
In two studies which trained community health workers to only prescribe antimalarials after a positive RDT, prescriptions of antimalarials were reduced compared to the control group where community health workers used clinical diagnosis (moderate quality evidence). In these two studies, mortality and hospitalizations remained very low in both groups despite the lower use of antimalarials (low quality evidence).
Overall completeness and applicability of evidence
The home‐ or community‐based programmes evaluated in the 10 included studies were all complex interventions combining several different elements, and often addressed more than one of the common barriers to accessing care. For example, home‐ and community‐based programmes were often discussed in the context of reducing the geographical barrier to care (the distance to the health facility), but the financial barrier was also reduced (by providing the antimalarial free or at a highly subsidized cost), and all studies addressed the educational barriers (through community awareness, social marketing, training of mothers or CHWs).
We are unable to determine which of these barriers was most important locally, or which of the elements were most responsible for the observed effects. Therefore, only broad conclusions can be drawn from these data, and local knowledge of the barriers to access will be of equal importance when designing and implementing new programmes.
Eight of the studies relied on the presumptive treatment of fevers without confirmation of malaria, and this strategy would undoubtedly result in significant overuse of antimalarials in most settings. To reduce this overtreatment and to refocus health providers on the alternative causes of fever, the WHO now recommends that all episodes of malaria are confirmed parasitologically prior to treatment (WHO 2010).
For basic health workers, RDTs are the most feasible option to achieve this. Yeboah‐Antwi 2010 and Mubi 2011 demonstrated that this can be done safely under trial conditions, but further monitoring of adherence to RDT protocols and safety under real‐life conditions is warranted. These studies demonstrated a significant reduction in use of antimalarials, but one study also demonstrated an increase in subsequent referrals to high levels of care. This increase is a potential benefit of programmes using RDTs if these children, who tested negative for malaria, now receive earlier management of their alternative diagnosis.
Nine of the 10 studies were conducted in rural areas of sub‐Saharan Africa countries where these programmes are currently promoted. However, Staedke 2009 demonstrated that these programmes could also be considered in urban settings where malaria is common and access to antimalarials is low.
Quality of the evidence
We assessed the quality of evidence provided by the randomized studies using the GRADE approach. We have presented these results in Table 1 and Table 2. The results of the non‐randomized studies were included as footnotes where appropriate. In general, the results of the non‐randomized studies were inconsistent, and did not contribute significantly to the overall body of evidence.
We judged the evidence that home‐ or community‐based strategies can increase access to, and use of, antimalarials to be of moderate quality, with consistent increases across all three trials. Also, we found the quality of evidence for the primary outcome (all‐cause mortality) to be moderate, which implies that we can have reasonable confidence in the result but further research may change the estimate of effect. The evidence from this single trial was downgraded due to concerns about generalizing this result to other settings. The reduction in mortality observed in this trial appeared large and important, but as the barriers to accessing care for malaria are likely to differ across settings, further studies from different settings are necessary to have full confidence that this result could be widely applied.
Potential biases in the review process
None were identified.
Agreements and disagreements with other studies or reviews
Three of the studies included in this review (Spencer 1987; Delacollette 1996; Kidane 2000) were also included in a review of home‐based management of malaria published in 2007 (Hopkins 2007). The authors of this review concluded that "Presumptive treatment of febrile children with pre‐packaged antimalarials in Home‐based Management of Malaria programmes is likely to increase delivery of effective drugs, and improve the timing, adherence, and dosing of treatment. Results from evaluations of community acceptability and feasibility are encouraging, but further study of health outcomes, including the impact on morbidity and mortality, will provide stronger evidence to support sustained implementation of community‐based interventions".
In this review, we excluded some of the observational studies included by Hopkins 2007, but we added several cluster‐RCTs that been published since the Hopkins 2007 review (Kouyate 2008; Staedke 2009, Yeboah‐Antwi 2010; Kangwana 2011; Mubi 2011). We also concluded that these interventions are likely to improve access to antimalarials, especially in rural or remote areas.
Authors' conclusions
Home‐ or community‐based interventions which provide antimalarial drugs free of charge probably improve prompt access to antimalarials. There is moderate quality evidence from rural Ethiopia that they may impact on childhood mortality when implemented in appropriate settings.
Programmes which treat all fevers presumptively with antimalarials are likely to overuse antimalarial drugs, and potentially undertreat other causes of fever such as pneumonia. Incorporating RDT diagnosis into home‐ or community‐based programmes for malaria may help to reduce this overuse of antimalarials, and has been shown to be safe under trial conditions.
Further well designed trials evaluating programmes which include parasitological confirmation with RDTs are needed to further guide practice.
The studies should report on adverse events, severe malaria and malaria‐specific mortality.
Acknowledgements
This project was funded by a grant from the Alliance for Health Policy and Systems Research (HSS/AHPSR), WHO, Geneva.
Matin Meremikwu is the Academic Editor of this review. The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. Search strategy
| Search set | Search terms to be used for all databases: |
| 1 | malaria |
| 2 | Child* |
| 3 | Infant* |
| 4 | Paediatr* |
| 5 | Pediatr* |
| 6 | Toddler* |
| 7 | 2 or 3 or 4 or 5 or 6 |
| 8 | Home‐base* |
| 9 | Homebase* |
| 10 | Community‐based |
| 11 | Presumptive treatment* |
| 12 | Self‐care |
| 13 | 8 or 9 or 10 or 11 or 12 |
| 14 | 1 and 7 and 13 |
Data and analyses
Comparison 1.
Home‐ or community‐based programmes versus facility‐based care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Baseline | 1 | 13677 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.96, 1.29] |
| 1.2 At follow‐up | 1 | 13677 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.44, 0.77] |
| 2 Parasitaemia prevalence ‐ (with sensitivity analysis) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 At baseline ‐ Not adjusted for cluster design | 2 | 1515 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.98, 1.09] |
| 2.2 At follow‐up ‐ Not adjusted for cluster design | 2 | 1371 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.61] |
| 2.3 Adjusted for cluster design using ICC = 0.01 | 2 | 935 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.10, 2.70] |
| 2.4 Adjusted for cluster design using ICC = 0.05 | 2 | 572 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.09, 3.05] |
| 2.5 Adjusted for cluster design using ICC = 0.10 | 2 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.08, 3.43] |
| 3 Anaemia ‐ (with sensitivity analysis) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 At baseline ‐ Not adjusted for clustering | 3 | 3230 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.71] |
| 3.2 At follow‐up ‐ Not adjusted for clustering | 3 | 3540 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.70, 2.51] |
| 3.3 Adjusted for clustering using ICC = 0.01 | 3 | 1622 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.81, 1.64] |
| 3.4 Adjusted for clustering using ICC = 0.05 | 3 | 756 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.66, 1.93] |
| 3.5 Adjusted for clustering using ICC = 0.10 | 3 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.56, 2.01] |
| 4 Fever episodes receiving prompt and effective treatment with an antimalarial ‐ (with sensitivity analysis) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 At baseline ‐ Not adjusted for cluster design | 1 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.46, 1.59] |
| 4.2 At follow‐up ‐ Not adjusted for cluster design | 2 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [1.00, 22.07] |
| 4.3 Adjusted for cluster design using ICC = 0.01 | 2 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 4.69 [1.00, 21.93] |
| 4.4 Adjusted for cluster design using ICC = 0.05 | 2 | 1472 | Risk Ratio (M‐H, Random, 95% CI) | 4.70 [1.06, 20.92] |
| 4.5 Adjusted for cluster design using ICC = 0.10 | 2 | 1216 | Risk Ratio (M‐H, Random, 95% CI) | 4.82 [1.07, 21.80] |
| 5 Fever episodes receiving treatment with an antimalarial ‐ (with sensitivity analysis) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At baseline ‐ Not adjusted for cluster design | 2 | 1077 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.45, 0.70] |
| 5.2 At follow‐up ‐ Not adjusted for cluster design | 3 | 2716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.26, 1.40] |
| 5.3 Adjusted for cluster design using ICC = 0.01 | 3 | 2299 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.27, 1.41] |
| 5.4 Adjusted for cluster design using ICC = 0.05 | 3 | 1648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.27, 1.44] |
| 5.5 Adjusted for cluster design using ICC = 0.10 | 3 | 1322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [1.26, 1.45] |
Comparison 2.
Home‐ or community‐based programmes using RDTs versus clinical diagnosis
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At follow‐up ‐ Not adjusted for cluster design | 2 | 6055 | Risk Ratio (M‐H, Random, 95% CI) | 3.51 [0.68, 18.22] |
| 2 Hospitalization | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 At follow‐up ‐ Not adjusted for cluster design | 1 | 3125 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.20, 1.79] |
| 3 Referrals for further care | 1 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 At follow‐up ‐ Adjusted for cluster design | 1 | 2930 | Risk Ratio (Random, 95% CI) | 1.89 [1.35, 2.65] |
| 4 Treatment failure at day 7 | 2 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 At follow‐up ‐ Adjusted for cluster design | 2 | 5994 | Risk Ratio (Random, 95% CI) | 1.23 [0.40, 3.81] |
| 5 Fever episodes receiving prompt and effective treatment with an antimalarial | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 At follow‐up ‐ Not adjusted for cluster design | 2 | 5977 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.84] |
| 5.2 Adjusted for clustering using ICC = 0.05 | 2 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.86] |
| 5.3 Adjusted for clustering using ICC = 0.10 | 2 | 490 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.18, 0.87] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: Controlled before‐and‐after study Study areas: ‘Area A’ Intervention: 12 villages, ‘Area B’ Control: Not described Data Collection: Four household surveys at six monthly intervals Length of follow‐up: 24 months |
|
| Participants | Target treatment group: All ages Sample size: Population of Area A approximately 13,000 Exclusions: None stated |
|
| Interventions | The intervention:
The control group: Facility‐based care only |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in this review:
|
|
| Notes | Country: Zaire, DRC Setting: Rural, Kotana health zone Malaria endemicity: Meso‐endemic, continuous transmission with seasonal fluctuations Study dates: 1985 to 1987 Study sponsor: UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases, and the Belgian Administration for Development Co‐operation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Controlled before‐and‐after study (no randomization) |
| Allocation concealment (selection bias) | High risk | Not applicable |
| Baseline imbalance | Unclear risk | Few details. 'The areas had the same malarial ecology, and malariometric indices' |
| Contamination | Low risk | Contamination is unlikely due to the distance between the study intervention and control sites |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data collection was through pre and post‐intervention cross‐sectional surveys. Each survey sampled between 200 to 300 participants from populations of around 14,000 |
| Loss of clusters | Low risk | Not applicable |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting was identified |
| Other bias | Low risk | No other potential sources of bias identified |
| Methods | Trial design: Cluster RCT Unit of randomization: Villages Number of clusters: 10 villages in total, five in intervention, five in control Data collection: Pre and post intervention survey Length of follow‐up: Nine months Authors did not adjust for clustering |
|
| Participants | Target treatment group: Children under the age of five Sample size: 1715 pre‐intervention survey and 2169 post‐intervention Exclusions: None described |
|
| Interventions | The intervention group:
The control group: Usual practice ‐ no details provided. |
|
| Outcomes | Included in this review:
Outcomes not included in this review:
|
|
| Notes | Country: Tanzania, Mkuranga District, Coast region of Tanzania Setting: Unclear Malaria endemicity: Holoendemic, peak transmission in January and June Study dates: April 2004 to May 2005 Study sponsors: EU INCO‐DEV funded collaboration between the Karolinska Institute (Sweden), Heidelberg University (Germany), Muhimbili University College of Health Sciences (Tanzania) and Centre de Recherce en Sante de Nouna (Burkina Faso) called the MAMOP project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "From the remaining 20 wards, 10 were randomly selected for the MAMOP project in a computer randomization (Excel)'. 'The intervention was implemented in 5 randomly chosen wards". |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not described. |
| Baseline imbalance | Unclear risk | Some baseline imbalance in education level's of mothers but statistical significance not reported. |
| Contamination | Unclear risk | The potential for contamination is not discussed by the study authors. However, the prevalence of anaemia substantially reduced in both treatment and control groups during the study period. The reasons for this are unclear but include contamination or confounding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | 'There was no blinding in the study design'. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data collection was through pre‐and post intervention surveys. One village from each ward was randomly selected for the survey. It is unclear what proportion of the total study population this represents. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting was identified. |
| Other bias | Low risk | No other bias identified. |
| Methods | Trial Design: Cluster RCT Unit of randomization: Sublocations (population 2,500 to 10,000) Number of clusters: 18, nine intervention and nine control sublocations Data collection: Pre and post‐intervention household surveys Length of follow‐up: Six months The authors adjusted for clustering |
|
| Participants | Target treatment group: Children aged three to 59 months Sample size: Estimated population: control 38,620 versus 44,538 intervention Exclusions: Urban and peri‐urban sublocations (due to risk of contamination) |
|
| Interventions | The intervention:
Control group: There was no intervention, but AL was available free at all government facilities. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Country: Kenya Setting: Three rural districts in Kenya's western province. Malaria endemicity: HIgh Study dates: August 2009 to May 2010 Study sponsors: KEMRI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers (Excel). Described as a 'modified randomization process' where if intervention and control sublocations were deemed to be too close and at risk of contamination, the list was reshuffled and the sublocation reselected. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Baseline imbalance | Low risk | The authors have presented the baseline characteristics for both in the intervention and control group at baseline and follow‐up. Although there are no direct statistical tests performed there appears to be low risk of imbalance of baseline characteristics. |
| Contamination | Unclear risk | 'In order to reduce the potential for contamination, a buffer zone was created around selected sublocations'. Despite the buffer zones, the proportion of children receiving antimalarials, and receiving AL increased substantially in the control groups. The authors comment that this may be due to reduced stock‐outs in the government facilities. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data collection was through pre‐and post intervention surveys. It is unclear what proportion of the total study population this represents. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Selective reporting (reporting bias) | Low risk | The authors have presented all outcomes which they intended to report and also provided further data. |
| Other bias | Low risk | No other forms of bias identified. |
| Methods | Trial design: Cluster RCT Unit of randomization: ‘tabias’ ‐ a cluster of villages Number of clusters: 24 tabias, 12 intervention and 12 control Data collection: The mother co‐ordinators kept records of births, deaths and migration. Malaria specific mortality was ascertained by verbal autopsy Length of follow‐up: 12 months The authors did not adjust for clustering |
|
| Participants | Target treatment group: Children under five Sample size: 13,677 children, control: 7924, intervention: 6383 Exclusions: None stated |
|
| Interventions | The intervention:
Control group: Facility‐based approach, mother coordinators were simply taught to record births, deaths and migration. |
|
| Outcomes | Outcomes included in this review:
Outcomes not included in this review: None |
|
| Notes | Country: Ethiopia Setting: Rural villages in an area where a community‐based primary health care programme had been operating the health system for over 20 years and the CHWs distributing the drugs had been frequently supervised Malaria endemicity: Seasonal hyperendemic Study dates: November 1996 to December 1997 Study sponsors: The UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Tabias were paired according to similar mortality rates. One tabia of each of the 12 pairs was allocated by random number to the intervention group and the other to control. It was not clear how this was generated, however it is likely to be at low risk. |
| Allocation concealment (selection bias) | Unclear risk | None described. |
| Baseline imbalance | Unclear risk | The baseline under‐five mortality rates of the 24 tabias varied from 8.4 per 1000 to 158.3 per 1000. The average baseline mortality in the control tabias was 47.6 per 1000, compared to 60.8 per 1000 in the intervention tabias, and this difference is not statistically significant. Other baseline characteristics were not presented. |
| Contamination | Low risk | No evidence of contamination was identified, and contamination would be likely to lead to an underestimation of any effect. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of children, parents and health workers was not be possible. As health workers collected the data some reporting bias is possible. No blinding is described at the analysis stage. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided on potential attrition. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting was identified. |
| Other bias | Low risk | Although only few verbal autopsies were performed this was assessed by two investigators and the second assessor was masked. |
| Methods | Trial design: Cluster RCT Unit of randomization: Villages Number of clusters: 13 villages, six intervention and seven control. Data collection: Pre and post‐intervention household surveys Length of follow‐up: 15 months The authors did not adjust for clustering. |
|
| Participants | Target treatment group: Children aged six to 59 months Sample size: 1083 children at baseline, 1006 at follow‐up Exclusions: None stated |
|
| Interventions | The intervention:
The control group: Village based health centres, no intervention. |
|
| Outcomes | Outcomes included in this review:
Outcomes not included in the review:
|
|
| Notes | Country: Burkina Faso Setting: Rural Malaria endemicity: Holoendemic but highly seasonal Study dates: July 2003 to October 2004 Study sponsor: EU INCO‐DEV |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Villages were selected by lottery until an approximate sample size of 1200 households per study arm was achieved. Assignment of clusters not clear. |
| Allocation concealment (selection bias) | High risk | None described. |
| Baseline imbalance | High risk | The baseline use of CQ was higher in the control compared to the intervention villages which shows that the that intervention and control area differed with regard to treatment behaviour. |
| Contamination | High risk | The prevalence of anaemia substantially reduced in both treatment and control groups during the study period. The authors note that there were CQ and ITN distribution activities in the control areas by parallel programmes during the study period. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding and all data is self reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data collection was through pre‐and post intervention surveys. It is unclear what proportion of the total study population this represents. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting was identified. |
| Other bias | High risk | No other forms of bias identified. |
| Methods | Trial design: cross‐over cluster RCT Unit of randomization: CHWs Number of clusters: 22 Data collection: CHWs collected data on new patients and at day 3 and 7. Length of follow‐up:Five months The authors adjusted data for clustering at the CHW level |
|
| Participants | Target treatment group: people aged three months and older Sample size: 3005 people with fever presented to CHWs during the study period Exclusions: Pregnancy, symptoms suggestive of severe disease and prior study inclusion within the previous 28 days |
|
| Interventions | The intervention:
The control group: CHWS diagnosed malaria using a clinical algorithm and treated with ACT. |
|
| Outcomes | Outcomes included in this review
Outcomes not included in this review
|
|
| Notes | Country: Tanzania Setting: Rural Malaria endemicity: Holoendemic. The study was conducted during the peak malaria transmission period. Study dates: March to August 2006 Study sponsor: Sida/SAREC |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Each of the 22 CHWs was assigned a unique number which was noted on a lottery ticket. 11 lottery tickets were then picked blindly by one researcher from a box after mixing.' |
| Allocation concealment (selection bias) | Low risk | See above. |
| Baseline imbalance | Low risk | No differences in age, sex or duration of fever are noted at baseline. |
| Contamination | Low risk | CHWs working within the same villages were randomized to intervention and control. No misuse of RDTs is reported during the cross‐over design and contamination is unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding is described but this is unlikely to have influenced the included outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For the primary outcome (proportion of fever cases prescribed ACT) no loss to follow‐up was reported. |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | No other forms of bias identified. |
| Methods | Trial design: Controlled before‐and‐after study Study areas: Two sub counties (Kyondo and Kitholhu) received the intervention and were compared to a control site comparable in population size (Kyarumba). Data collection: Cross sectional surveys at baseline and post intervention using cluster randomized sampling. Length of follow‐up: 17 to 22 months |
|
| Participants | Target group: Children under the age of five years. Sample size: At baseline 498 febrile children under five years were recruited into the study, and at post intervention 587 children were recruited. Exclusions: None stated |
|
| Interventions | The intervention:
Antimalarials were given as pre‐packaged CQ + SP labelled 'HOMAPAK'. Volunteers also educated mothers about care seeking at home visits and through village meetings. Control: In control areas antimalarials could be accessed over the counter at pharmacies or by attending health facilities. |
|
| Outcomes | Outcomes included in the review:
|
|
| Notes | Country: Uganda Setting: Rural, with 56% of the population living in absolute poverty Malaria endemicity: Hyperendemic Study dates: August 2002 to September 2004 Study sponsor: Department for Research Cooperation Makerere Univerity, Swedish Institute. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization. |
| Allocation concealment (selection bias) | High risk | Not applicable. |
| Baseline imbalance | High risk | Very few baseline data were presented. There were substantial differences in the use of antimalarials between intervention and control areas at base‐line which is likely to confound the results. |
| Contamination | Low risk | No evidence of contamination identified. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding is reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The data were collected via cross‐sectional surveys using cluster‐randomized sampling. The same households were not necessarily sampled pre‐ and post‐ intervention. |
| Loss of clusters | Low risk | Not applicable. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting was found. |
| Other bias | Low risk | We did not identify any other potential source of bias. |
| Methods | Trial design: Controlled before‐and‐after study Study areas: Community divided into three operational areas; (A & B = Intervention, C = Control) Data collection: Pre and post intervention household survey Length of follow‐up: 12 months |
|
| Participants | Target treatment group: Adults and children. Sample size: Unclear Exclusions: None stated |
|
| Interventions | The intervention:
The control group: Community zone C was designated as the control group. The volunteers were not supplied with CQ, but malaria treatment was available from the Saradidi community clinic, two Ministry of Health dispensaries in the areas and admission hospital. CQ could also be purchased from small shops. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Country: Kenya Setting: Saradidi near Lake Victoria, Rural Malaria endemicity: hyper to holoendemic area Study dates: 1981 to 1983 Study sponsor: Supported by WHO/UNDP and World Bank. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization process, sites selected on basis of development. |
| Allocation concealment (selection bias) | High risk | Not applicable. |
| Baseline imbalance | High risk | High level of presumptive treatment with CQ prior to intervention. Incomplete control group Data, Pre intervention data is not available due to overlap with the intervention and census dates. |
| Contamination | Unclear risk | The authors did not discuss any steps taken to reduce the risk of contamination. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data has been presented, however difficult to extrapolate actual values. |
| Loss of clusters | Low risk | Not applicable. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting was found. |
| Other bias | Low risk | We did not identify any other potential source of bias. |
| Methods | Trial design: Cluster RCT Unit of randomization: Household Number of clusters: 325 households. Data collection: Monthly diaries by mothers and questionnaires Length of follow‐up: 12 months |
|
| Participants | Target treatment group: Children aged 1 to 6 years Sample size: 437 children, 225 intervention versus 212 control group 1 Exclusions: Weight < 10 kg, serious chronic disease, intention to move out of study area, history of serious adverse reaction to study drug, severe malnutrition or anaemia. |
|
| Interventions | The intervention:
Control group 1: Caregivers advised to continue with their current approach to managing fevers. Control group 2: A non‐randomized comparison with a hospital based cohort was also reported. In this group children aged one to 10 received antimalarials only for microscopically‐confirmed malaria. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in the review:
|
|
| Notes | Country: Uganda Setting: Urban Malaria endemicity: mesoendemic, perennial Study dates: September 2005 to February 2007 Study sponsor: Gates Malaria Partnership |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "An independent project member, who had no further involvement in the rest of the trial, prepared a computer‐generated randomization list". |
| Allocation concealment (selection bias) | Low risk | "randomization numbers that correlated with the assigned study interventions were concealed in opaque envelopes". |
| Baseline imbalance | Low risk | The baseline characteristics between the intervention and standard care arm were similar. |
| Contamination | Unclear risk | The potential for contamination is not discussed by the study authors. However, the prevalence of anaemia substantially reduced in both treatment and control groups during the study period. The reasons for this are unclear but include contamination or confounding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding is described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No differential loss to follow‐up (28 participants out of 217 in the intervention group and 32 out of 208 participants in the control group were lost to follow‐up). |
| Loss of clusters | Low risk | No loss of clusters reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting was identified. |
| Other bias | Low risk | We did not identify any other potential source of bias. |
| Methods | Trial design: Cluster RCT Unit of randomization: Community health posts (CHPs) Number of clusters: 31 CHPs (15 in the intervention arm and 16 in the control arm) Data collection: Baseline and post intervention household survey Length of follow‐up: 12 months The authors adjusted for clustering |
|
| Participants | Target treatment group: Children aged six months to 5 years Sample size: 3,125 children (1,017 in the intervention arm and 2,108 in the control arm) Exclusions: None stated |
|
| Interventions | The intervention:
Control: CHWs in control areas underwent the same training except for the use of RDTs. All febrile children were treated with AL, and those with signs of pneumonia were referred to the health facility, as per Ministry of Health policy. |
|
| Outcomes | Outcomes included in the review:
Outcomes not included in this review:
|
|
| Notes | Country: Zambia Setting: Rural areas with poor road network Malaria endemicity: Hyperendemic Study dates: December 2007 to November 2008 Study sponsor: The United States Agency for International Development, President's Malaria Initiative |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Community health posts...were matched in pairs according to the distance from the health post. A random number generator was used to assign one post in the pair to the control arm, while the matched pair was assigned to the intervention arm." |
| Allocation concealment (selection bias) | High risk | Allocation concealment was not described. |
| Baseline imbalance | High risk | Baseline data were well presented. The proportion of children up‐to‐date with immunizations was significantly lower in the intervention areas which could indicate poorer access to health services. |
| Contamination | Low risk | No evidence of contamination identified. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding is described but this is unlikely to have affected the primary outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up was reported for the primary outcome (proportion of children presenting with fever prescribed ACT). |
| Loss of clusters | Low risk | No loss of clusters was reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective outcome reporting. |
| Other bias | Low risk | We did not identify any other potential source of bias. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abuya 2010 | No relevant outcomes reported |
| Ahorlu 2009 | This was an uncontrolled before‐and‐after study that evaluated the effects of intermittent preventive therapy in the context of home management of malaria |
| Ajayi 2008a | Assessment of accuracy of presumptive diagnoses |
| Ajayi 2008b | No relevant outcomes reported |
| Ajayi 2008c | No comparison between an intervention and a control. |
| Ansah 2010 | The study had no intervention or programme which trained people to recognize and treat fevers with antimalarials. |
| Bojang 2009 | This study was designed to test intermittent preventive therapy in the context of home management of malaria. |
| Chinbuah 2006 | No comparison between an intervention and a control. |
| Dunyo 2000 | The study tested the accuracy of malaria diagnosis at home versus health centre. |
| Elmardi 2008 | No comparison between an intervention and a control. |
| Greenwood 1988 | Not a home‐based or community‐based intervention trial. |
| Moir 1985 | No comparison between an intervention and a control. |
| Ngasala 2011 | Uncontrolled before‐and‐after study. This was a single arm study. |
| Pagnoni 1997 | No comparison between an intervention and a control. |
| Pence 2005 | The intervention was not restricted to malaria treatment. |
| Sesay 2011 | No comparison between an intervention and a control. |
| Sirima 2003 | No comparison between an intervention and a control. |
| Skarbinski 2009 | Not a home‐based or community‐based intervention trial. |
| Tiono 2008 | No outcomes relevant to this review. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Community volunteers as agents for improving early diagnosis and appropriate treatment of malaria in Bago Division, Myanmar |
| Methods | Cluster randomized controlled trial |
| Participants | Inclusion criteria: Subject older than six months with history of fever within one month, but not within 24 hours at the time of data collection in a malaria endemic village Exclusion criteria: Fever within 24 hours Age minimum: Six months Age maximum: No limit Gender: Both males and females |
| Interventions | Training of community volunteers on the use of malaria RDT for diagnosis of malaria and treatment with artemisinin based combination therapy (ACT) in remote villages where there is no health staff employed |
| Outcomes |
|
| Starting date | 21/05/2009 |
| Contact information | Dr Ohnmar Research Scientist Epidemiology Research Division Department of Medical Research (Lower Myanmar) No.5, Ziwaka Road, Dagon PO, Yangon, Myanmar |
| Notes | Sponsor: World Health Organization Regional Office for the South‐East Asia ‐ UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) Small Grants Programme (WHO/SEARO‐TDR Small Grants Programme) |
Contributions of authors
Charles Okwundu conceptualised and wrote the first draft of the review. Sukrti Nagpal and Dave Sinclair contributed to the background information, study selection and data extraction, and provided input to subsequent drafts of the review. Alfred Musekiwa commented on the draft review and contributed to the data extraction, synthesis, and analysis.
Sources of support
Internal sources
Centre for Evidence‐Based Health Care, Stellenbosch University, South Africa.
Wits Reproductive Health and HIV institute, South Africa.
External sources
The Alliance for Health Policy and Systems Research (HSS/AHPSR), WHO, Switzerland.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
- Delacollette C, Van der Stuyft P, Molima K. Using community health workers for malaria control: experience in Zaire. Bulletin of the World Health Organization 1996;74(4):423‐30. [PMC free article] [PubMed] [Google Scholar]
- Eriksen J, Mujinja P, Warsame M, Nsimba S, Kouyate B, Gustafsson LL, et al. Effectiveness of a community intervention on malaria in rural Tanzania ‐ a randomised controlled trial. African Health Sciences 2010;10(4):332‐40. [PMC free article] [PubMed] [Google Scholar]
- Kangwana BP, Kedenge SV, Noor AM, Alegana VA, Nyandigisi AJ, Pandit J, et al. The impact of retail‐sector delivery of artemether‐lumefantrine on malaria treatment of children under five in Kenya: a cluster randomized controlled trial. PLoSMedicine 2011;8(5):e1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidane G, Morrow RH. Teaching mothers to provide home treatment of malaria in Tigray, Ethiopia: a randomised trial. Lancet 2000;356(9229):550‐5. [DOI] [PubMed] [Google Scholar]
- Kouyaté B, Somé F, Jahn A, Coulibaly B, Eriksen J, Sauerborn R, et al. Process and effects of a community intervention on malaria in rural Burkina Faso: randomized controlled trial. Malaria Journal 2008;7(50):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mubi M, Janson A, Warsame M, Mårtensson A, Källander K, Petzold MG, et al. Malaria rapid testing by community health workers Is effective and safe for targeting malaria treatment: randomised cross‐over trial in Tanzania. PLoS ONE 2011;6(7):e19753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsungwa‐Sabiiti J, Peterson S, Pariyo G, Ogwal‐Okeng J, Petzold MG, Tomson G. Home‐based management of fever and malaria treatment practices in Uganda. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(12):1199‐207. [DOI] [PubMed] [Google Scholar]
- Spencer HC, Kaseje DC, Collins WE, Shehata MG, Turner A, Stanfill PS, et al. Community‐based malaria control in Saradidi, Kenya: description of the programme and impact on parasitaemia rates and antimalarial antibodies. Annals of Tropical Medicine and Parasitology April 1987;81(Suppl 1):13‐23. [DOI] [PubMed] [Google Scholar]
- Staedke SG, Mwebaza N, Kamya MR, Clark TD, Dorsey G, Rosenthal PJ, et al. Home management of malaria with artemether‐lumefantrine compared with standard care in urban Ugandan children: a randomised controlled trial. Lancet 2009;373(9675):1623‐31. [DOI] [PubMed] [Google Scholar]
- Yeboah‐Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever Due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Medicine 2010;7(9):e1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Abuya TO, Fegan G, Amin AA, Akhwale WS, Noor AM, Snow RW, et al. Evaluating different dimensions of programme effectiveness for private medicine retailer malaria control interventions in Kenya. PLoS One 2010;5(1):e8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahorlu CK, Koram KA, Seakey AK, Weiss MG. Effectiveness of combined intermittent preventive treatment for children and timely home treatment for malaria control. Malaria Journal 2009;8(292):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi IO, Falade CO, Bamgboye EA, Oduola AM, Kale OO. Assessment of a treatment guideline to improve home management of malaria in children in rural south‐west Nigeria. Malaria Journal 2008;7(24):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi IO, Falade CO, Kale OO. An assessment of accuracy of mothers' presumptive diagnosis of fever at home in southwest Nigeria: evidence for switch to parasite‐based diagnostic test. East African Journal of Public Health 2009;6(3):229‐34. [PubMed] [Google Scholar]
- Ajayi IO, Browne EN, Garshong B, Bateganya F, Yusuf B, Agyei‐Baffour P, et al. Feasibility and acceptability of artemisinin‐based combination therapy for the home management of malaria in four African sites. Malaria Journal 2008;7(6):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansah EK, Narh‐Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, et al. Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. BMJ 2010;340(c930):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojang KA, Sesay S, Sowe M, Conway D, Milligan P, Greenwood B. A study of intermittent preventive treatment and home based management of malaria in a rural area of The Gambia. American Journal of Tropical Medicine and Hygiene. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinbuah AM, Gyapong JO, Pagnoni F, Wellington EK, Gyapong M. Feasibility and acceptability of the use of artemether‐lumefantrine in the home management of uncomplicated malaria in children 6‐59 months old in Ghana. Tropical Medicine and International Health 2006;11(7):1003‐16. [DOI] [PubMed] [Google Scholar]
- Dunyo SK, Afari EA, Koram KA, Ahorlu CK, Abubakar I, Nkrumah FK. Health centre versus home presumptive diagnosis of malaria in southern Ghana: implications for home‐based care policy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(3):285‐8. [DOI] [PubMed] [Google Scholar]
- Elmardi KA, Malik EM, Abdelgadir T, Ali SH, Elsyed AH, Mudather MA, et al. Feasibility and acceptability of home‐based management of malaria strategy adapted to Sudan's conditions using artemisinin‐based combination therapy and rapid diagnostic test. Malaria Journal 2009;8(39):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BM, Greenwood AM, Bradley AK, Snow RW, Byass P, Hayes RJ, et al. Comparison of two strategies for control of malaria within a primary health care programme in the Gambia . Lancet 1988;1(8595):1121‐7. [DOI] [PubMed] [Google Scholar]
- Moir JS, Tulloch JL, Vrbova H, Jolley DJ, Heywood PF, Alpers MP. The role of voluntary village aides in the control of malaria by presumptive treatment of fever. 2. Impact on village health. Papua and New Guinea Medical Journal 1985;28(4):267‐278. [PubMed] [Google Scholar]
- Ngasala BE, Malmberg M, Carlsson AM, Ferreira PE, Petzold MG, Blessborn D, et al. Effectiveness of artemether‐lumefantrine provided by community health workers in under‐five children with uncomplicated malaria in rural Tanzania: an open label prospective study. Malaria Journal 2011;10(64):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoni F, Convelbo N, Tiendrebeogo J, Cousens S, Esposito F. A community‐based programme to provide prompt and adequate treatment of presumptive malaria in children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(5):512‐7. [DOI] [PubMed] [Google Scholar]
- Pence BW, Nyarko P, Phillips JF, Debpuur C. The effect of community nurses and health volunteers on child mortality: the Navrongo Community Health and Family Planning Project. Scandinavian Journal of Public Health 2005;35(6):599‐608. [DOI] [PubMed] [Google Scholar]
- Sesay S, Milligan P, Touray E, Sowe M, Webb EL, Greenwood BM, et al. A trial of intermittent preventive treatment and home‐based management of malaria in a rural area of The Gambia. Malaria Journal 2011;10(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirima SB, Konaté A, Tiono AB, Convelbo N, Cousens S, Pagnoni F. Early treatment of childhood fevers with pre‐packaged antimalarial drugs in the home reduces severe malaria morbidity in Burkina Faso. Tropical Medicine and International Health 2003;8(2):133‐9. [DOI] [PubMed] [Google Scholar]
- Skarbinski J, Ouma PO, Causer LM, Kariuki SK, Barnwell JW, Alaii JA, et al. Effect of malaria rapid diagnostic tests on the management of uncomplicated malaria with artemether‐lumefantrine in Kenya: a cluster randomized trial. The American Journal of Tropical Medicine and Hygiene 2009;80(6):919‐26. [PubMed] [Google Scholar]
- Tiono AB, Kaboré Y, Traoré A, Convelbo N, Pagnoni F, Sirima SB. Implementation of home based management of malaria in children reduces the work load for peripheral health facilities in a rural district of Burkina Faso. Malaria Journal 2008;7(201):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Ohnmar. Cluster randomized trial on the use of community volunteers to improve early diagnosis and appropriate treatment of malaria in Bago Division, Myanmar. Australian New Zealand Clinical Trials Registry Accessed 25 September 2012:ACTRN12610000706077.
Additional references
- Andersen RM, McCutcheon A, Aday LA, Chiu GY, Bell R. Exploring dimensions of access to medical care. Health Services Research 1983;18(1):49‐74. [PMC free article] [PubMed] [Google Scholar]
- Chandramohan D, Owusu‐Agyei S, Carneiro I, Awine T, Amponsa‐Achiano K, Mensah M, et al. Cluster randomised trial of intermittent preventive treatment for malaria in infants in area of high, seasonal transmission in Ghana. BMJ 2005;331(7519):727‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitonga CW, Amin AA, Ajanga A, Kangwana BB, Noor AM, Snow RW. The use of artemether‐lumefantrine by febrile children following national implementation of a revised drug policy in Kenya. Tropical Medicine and International Health 2008;13(4):487‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd., 2008. [Google Scholar]
- Hopkins H, Talisuna A, Whitty CJ, Staedke SG. Impact of home‐based management of malaria on health outcomes in Africa: a systematic review of the evidence. Malaria Journal 2007;6(134):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein RE, Weller SC, Zeissig R, Richards FO, Ruebush TK 2nd. Knowledge, beliefs, and practices in relation to malaria transmission and vector control in Guatemala. American Journal of Tropical Medicine and Hygiene 1995;52(5):383‐8. [DOI] [PubMed] [Google Scholar]
- Malik EM, Hanafi K, Ali SH, Ahmed ES, Mohamed KA. Treatment‐seeking behaviour for malaria in children under five years of age: implication for home management in rural areas with high seasonal transmission in Sudan. Malaria Journal 2006;5(60):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombie SC. Treatment seeking for malaria: a review of recent research. Social Science & Medicine 1996;43(6):933‐45. [DOI] [PubMed] [Google Scholar]
- McIntyre D, Mooney G (editors). The economics of health equity. Cambridge, UK: Cambridge University Press, 2007. [Google Scholar]
- Meremikwu MM, Donegan S, Esu E. Chemoprophylaxis and intermittent treatment for preventing malaria in children. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003756.pub3] [DOI] [PubMed] [Google Scholar]
- Noor AM, Zurovac D, Hay SI, Ochola SA, Snow RW. Defining equity in physical access to clinical services using geographical information systems as part of malaria planning and monitoring in Kenya. Tropical Medicine & International Health 2003;8(10):917‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist B, Iteba N, Lengeler C, Makemba A, Mshana C, Nathan R, et al. Access to health care in contexts of livelihood insecurity: a framework for analysis and action. PLoS Medicine2007; Vol. 4, issue 10:1584‐8. [DOI] [PMC free article] [PubMed]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Salako LA, Brieger WR, Afolabi BM, Umeh RE, Agomo PU, Asa S, et al. Treatment of childhood fevers and other illnesses in three rural Nigerian communities. Journal of Tropical Pediatrics 2001;47(4):230‐8. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005;434(7030):214‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedke SG, Mwebaza N, Kamya MR, Rosenthal PJ, Whitty CJM. Home management of malaria – Authors' reply. Lancet 2009;374(9686):289. 19632487 [Google Scholar]
- Tipke M, Louis VR, Yé M, De Allegri M, Beiersmann C, Sié A, et al. Access to malaria treatment in young children of rural Burkina Faso. Malaria Journal 2009;8(266):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitty CJM, Chandler C, Ansah E, Leslie T, Staedke SG. Deployment of ACT antimalarials for treatment of malaria: challenges and opportunities. Malaria Journal 2008;7(Suppl 1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Scaling up home‐based management of malaria: from research to implementation. http://apps.who.int/tdr/svc/publications/training‐guideline‐publications/scaling‐up‐home‐based‐management 2004 (accessed 12 April 2011).
- World Health Organization. WHO briefing on malaria treatment guidelines and artemisinin monotherapies. http://www.who.int/malaria/publications/atoz/meeting_briefing19april/en/index.html 2006 (accessed 12 April 2011).
- World Health Organization. World Malaria Report 2008. http://www.who.int/malaria/publications/atoz/9789241563697/en/index.html (accessed 12 April 2011).
- World Health Organization. World Malaria Report 2009. http://www.who.int/malaria/world_malaria_report_2009/en/index.html (accessed 12 April 2011).
- World Health Organization. Guidelines for the treatment of malaria. http://www.who.int/malaria/publications/atoz/9789241547925/en/index.html (accessed 12 April 2011).
- Yeneneh H, Gyorkos TW, Joseph L, Pickering J, Tedla S. Antimalarial drug utilization by women in Ethiopia: a knowledge‐attitudes‐practice study. Bulletin of the World Health Organization 1993;71(6):763‐72. [PMC free article] [PubMed] [Google Scholar]


