Abstract
Background
Fever is common in malaria, and drugs and sponging are widely used for symptomatic relief. Some researchers have suggested that fever reduction may prolong malaria illness.
Objectives
We aimed to assess whether treatments to reduce fever in malaria influence the course of the illness.
Search methods
We searched the Cochrane Infectious Diseases Group Trial Register (June 2012), the Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 6, 2012), MEDLINE (1966 to June 2012); EMBASE (1980 to June 2012) and LILACS (June 2012). We contacted researchers and organisations working in the field to enable us identify other unpublished or ongoing trials.
Selection criteria
Randomized controlled trials of fever reduction measures in adults or children with confirmed malaria.
Data collection and analysis
Inclusion criteria were independently applied by two authors. We extracted data from trials that met our pre‐specified criteria using a standard data extraction form. Mean differences with 95% confidence intervals (CI) were calculated for continuous data. GRADE was used to evaluate and summarize the quality of the evidence.
Main results
Ten randomized controlled trials with 990 participants including both adults and children met our inclusion criteria. All were small scale trials with methodological limitations and were conducted in a variety of patients. Some trials detected an impact of antipyretic drugs on fever clearance time, while others did not. Regarding parasite clearance,no clear influence of anti‐pyresis was demonstrated (six trials, 423 participants, very low quality of evidence). No difference in the number or severity of adverse events between antipyretic drugs and control was detected.
Authors' conclusions
We do not know whether antipyretics alter parasite clearance time. Whether further trials are worthwhile to investigate this or not would require a judgement of whether this was an important question to resolve using interventional trials.
23 April 2019
No update planned
Research area no longer active
Research area no longer active: the specific question with malaria is no longer being pursued
Keywords: Adult; Child; Humans; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/therapeutic use; Antimalarials; Antimalarials/therapeutic use; Antipyretics; Antipyretics/adverse effects; Antipyretics/therapeutic use; Cryotherapy; Cryotherapy/methods; Fever; Fever/parasitology; Fever/therapy; Malaria; Malaria/complications; Malaria/drug therapy; Malaria/parasitology; Parasitemia; Parasitemia/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Antipyretic measures for treating fever in malaria
Fever is a common symptom of malaria. Antipyretic drugs (fever‐relieving medicines) and physical measures (such as tepid sponging) are widely used by caregivers and health care workers to treat fever in adults and children with malaria. Some researchers have questioned the belief that treating fever with antipyretic drugs is beneficial. They suggest that it may actually prolong the time taken for the malaria parasite to be cleared from the blood system. This review looked for evidence from appropriate types of research that addressed these issues. We found only a few small trials and could not obtain sufficient information from these trials to reach a conclusion on whether the antipyretic drugs actually help to resolve malaria symptoms or prolong the illness.
Summary of findings
Summary of findings for the main comparison. Antipyretic drugs compared to no antipyretic drug or placebo for treating fever in malaria.
| Antipyretic drugs compared to no antipyretic drug or placebo for treating fever in malaria | ||||||
| Patient or population: patients with fever in malaria Settings: malaria endemic areas Intervention: antipyretic drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antipyretic drugs | |||||
| FCT | See comment | See comment | Not estimable | 59 (2 studies) | ⊕⊝⊝⊝ very low1,2,3,4,5 | |
| PCT | See comment | See comment | Not estimable | 59 (2 studies) | ⊕⊝⊝⊝ very low1,6 | |
| Adverse Events | none reported | |||||
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Random sequence generation was not specified. Allocation concealment was unclear. Trial was an open trial and blinding of outcome assessors was not done. 2 Trials did not show any consistent pattern of effect on this outcome. 3 Trials were in malaria endemic areas. One of the trials (Matsegui 2008) included only children while the other (Krishna 1995a) included only adults. 4 Studies were not pooled but the confidence intervals were wide and none demonstrated a statistically significant effect. 5 No publication bias detected. 6 No explanation was provided
Background
Description of the condition
Malaria is a common public health problem in the developing countries of Central and South America, Hispaniola (Haiti and the Dominican Republic), sub‐Saharan Africa, the Middle East, the Indian subcontinent, Southeast Asia and Oceania. An estimated 250 million cases of malaria occur annually, with more than 80% of the cases found in endemic regions of Africa and Asia (WHO 2008). The disease accounts for about one million deaths annually, of which more than 90% are in Africa and 85% in African children below the age of five years (WHO 2008).
The common symptoms of malaria include fever, poor appetite, vomiting, malaise and convulsions. Fever is the most common of the symptoms of malaria. The fear of fever‐induced convulsions (febrile convulsions) is a common reason why caregivers of young children use various forms of fever remedies. Simple febrile convulsions are generally known not to cause serious morbidity or fatality. However, some other forms of malaria‐induced convulsions arise from potentially fatal complications, notably cerebral malaria, malaria induced hypoglycemia and metabolic acidosis (Strengell 2009).
Fever in malaria is believed to be associated with release of toxins and antigenic substances which induce the release of cytokines by white blood cells. The cytokines, such as tumour necrosis factor‐alpha and interleukin‐6, in turn activate the cyclooxygenase pathway leading to the production of prostaglandin E2. In the thermoregulatory centre of the brain, prostaglandin E2 acts on thermosensitive cells to induce fever. Fever is postulated to be a host response to curb infections and achieve recovery but its role in defence against malaria is unclear. It has been suggested that fever and associated cytokines may have beneficial roles in malaria (Dodoo 2002; Mordmüller 1997). Thus more harm than good may be done by blunting the febrile host response by administering antipyretic drugs to people with fever due to malaria.
Description of the intervention
The primary goal of treating malaria is to eliminate the malaria parasites with effective anti‐malarial agents. However, other treatments are often required to ameliorate the symptoms of malaria and its complications. Fever, aches, convulsions and dehydration are some of the symptoms and signs of malaria that often require treatment. Common treatment modalities for fever include anti‐pyretic drugs (such as paracetamol, ibuprofen and other nonsteroidal anti‐inflammmatory drugs (NSAIDs)) and physical methods (including sponging with tepid water, fanning and use of cooling blankets) (WHO 2006). Measures to treat fever are thought to make the patients feel better and prevent febrile convulsions in children. Physical methods especially tepid sponging, are sometimes used in combination with anti‐pyretic drugs to treat fever in malaria.
Paracetamol, also known as acetaminophen, is an antipyretic drug commonly used in children (Prescott 2000) and for treating fever in malaria (WHO 2008). It also has analgesic effects. It is derived from the parent compound, N‐acetyl‐p aminophenol, 4‐hydroxyacetanilide, which is an active metabolite of phenacetin. It is administered via the enteral and parenteral routes. Its side effects include vomiting, diarrhoea and abdominal pain. In overdose, paracetamol causes hepatotoxicity which can rapidly progress to hepatic failure if appropriate treatment is not commenced early (Prescott 2000).
NSAIDs are another group of antipyretic drugs used in treating fever due to malaria (Matsegui 2008). They are weak organic acids with wide chemical diversity and include aspirin, ibuprofen, metamizol, naproxen etc. Adverse effects associated with the use of NSAIDs include gastric irritation and anti‐platelet effects, which can cause prolonged bleeding and renal damage. Reye's syndrome is associated with aspirin use in paediatric age groups.
How the intervention might work
There is evidence from previous systematic reviews that both physical methods and common antipyretic drugs (notably paracetamol and ibuprofen) lead to short‐lived reduction in body temperature in feverish conditions caused by infections (Meremikwu 2002; Meremikwu 2003). Physical antipyretic measures such as tepid sponging, fanning and cooling blankets induce reduction in body temperature by conduction, convection or by evaporation. Antipyretic effects of physical methods tend to be more short‐lived necessitating frequent application, which tends to increase discomforting effects such as shivering with goose pimples (Meremikwu 2003).
Paracetamol is the most commonly used antipyretic agent. The World Health Organization (WHO) malaria treatment guideline recommends paracetamol, ibuprofen and tepid sponging as measures for treating fever in malaria (WHO 2006). The mode of action of paracetamol is poorly understood, but it is thought to achieve this effect by blocking the effects of endogenous pyrogens on the hypothalamus (Prescott 2000). NSAIDs exert their antipyretic effect by deactivating the cycloxygenase pathway with subsequent inhibition of prostaglandin production (Lell 2001).
In recent times, the role of antipyretic measures in treatment of infections has been called into question (Warwick 2008;Kramer 1991). This is particularly important in malaria cases as there is evidence that febrile temperatures inhibit in vitro growth of Plasmodium falciparum (Long 2001). A study of people infected with malaria showed that paracetamol prolonged parasitaemia (Brandts 1997). Recent studies have shown that although NSAIDs such as ibuprofen, metamizol and naproxen reduce fever peaks, they do not play a significant role in reducing fever clearance time in malaria (Matsegui 2008; Lell 2001).
The prevention of febrile convulsions is one of the reasons practitioners use antipyretic measures, but there is no evidence to show that antipyretic drugs prevent febrile convulsions (Strengell 2009). A study of African children with malaria showed that 54% of convulsions occurred at rectal temperatures below 38°C, indicating that factors other than high grade fever account for the majority of convulsions in children with malaria (Waruiru 1996). It has been postulated that the convulsions associated with fever in malaria may be due to systemic pathophysiological changes caused by these infections (Waruiru 2004).
Why it is important to do this review
Paracetamol and ibuprofen are widely used in fever management, and have been recommended by the WHO malaria treatment guideline group (WHO 2006). Paracetamol is generally known to be safe and well tolerated when used in the recommended doses but the controversies regarding observations that antipyresis may prolong malaria parasitaemia remain unresolved (Hayward 1999). The first version of this systematic review aimed to test the null hypothesis that paracetamol and other fever control measures do not prolong malaria illness (Meremikwu 2000). This review found insufficient data to confirm or refute an impact of antipyretic measures on parasitaemia or malarial illness (Meremikwu 2000) and did not make a head to head comparison of the effects of paracetamol and NSAIDs. Since the completion of that review, more studies that addressed this question have been conducted. The WHO malaria treatment guideline recommends ibuprofen along with paracetamol for treating fever in malaria but acknowledges the fact that experience with use of ibuprofen is limited (WHO 2006). Considering that available evidence of any advantage of one antipyretic measure over the other is inconclusive, we have decided in the update of this systematic review to also study the comparative effectiveness of the various antipyretic measures on the treatment of malaria.
Objectives
To assess the effects of drugs and non‐pharmacological methods used for treating fever in malaria.
Hypotheses
Antipyretic measures do not influence the complete and sustained resolution of fever in malaria.
Antipyretic measures do not affect the time to clear parasitaemia during an episode of malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Children or adults with fever due to malarial illness confirmed by microscopic examination of blood slides or antigen detection techniques.
Types of interventions
1. Antipyretic drugs: paracetamol, Ibuprofen and other NSAIDs. 2. Physical methods such as tepid sponging, fanning, cooling blankets.
Types of outcome measures
Primary outcomes
Fever clearance time (FCT) (the time between onset of treatment and sustained resolution of fever i.e. return of temperature to normal (< 37.5 °C) without recurrence during same illness).
Parasite clearance time (PCT) (time between onset of treatment and clearance of malaria parasites from peripheral blood film).
Secondary outcomes
-
Resolution of fever:
Proportion of participants without fever within six and twelve hours of starting treatment. Fever is defined as a temperature above 37.2 °C for adults and 37.5 °C for children.
Mean drop in temperature within the first six hours of starting treatment.
Fever time (duration in hours that an individual's temperature was above an indicated fever threshold).
Absence of parasitaemia by 3rd, 7th and 14th days after treatment started.
Length of hospital stay.
Adverse events
Convulsions, vomiting or gastrointestinal bleeding.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press and in progress).
We used the following search terms for all trial registers and databases: pyrexia, fever, antipyretic and malaria.
Electronic searches
We searched the following electronic databases using the search terms in combination with the search strategy developed by the Cochrane Collaboration and detailed in the latest version of the Cochrane Reviewers' Handbook (Higgins 2008): the Cochrane Infectious Diseases Group specialized trials register in the Cochrane Library (up to June 2012);the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 6, 2012); MEDLINE (1966 to June 2012); African Index Medicus (1998); EMBASE (1980 to June 2012); LILACS (La Literatura Latinoamericana y del Caribe de Informacion en Ciencias de Salud) www.bireme.br; accessed June 2012; and Science Citation Index (1981 to June 2012).
Searching other resources
We also checked the reference lists of all trials identified by the above methods.
We contacted researchers and organisations working in the field for information on unpublished and ongoing trials.
Data collection and analysis
Two reviewers (BN,EU) applied the inclusion criteria to all potential trials. Where there was any doubt, we consulted the third reviewer (CO).
We extracted data using a standard data extraction form.
We assessed the study quality using the standard methods of the Cochrane Collaboration as stipulated in the latest edition of Cochrane Reviewers' Handbook. We planned to explore potential sources of heterogeneity such as type, dosage, route and method of administration of antipyretic measures, age of participants (adults versus children) and site of temperature measurement (core or axillary).
Selection of studies
Two authors (BN and EU) independently applied the inclusion criteria to all identified studies and made a decision on which studies to include.We retrieved the full papers and we applied the eligibility criteria to all potentially relevant papers. When disagreement occured, we consulted a third author (CO).There were no language restrictions in the search or the selection of articles.
Data extraction and management
Data extraction was performed by two authors (BN and CO) using a standard data extraction form. The data we extracted from studies that qualified for inclusion included:
Methods: generation of allocation sequence, method of allocation concealment, blinding, study duration.
Participants: study setting (including country and hospital), age, gender and other socio‐demographic characteristics of participants.
Intervention: nature of intervention delivered to the treatment and control groups, route of administration, dosage and duration.
Outcome measures and results: differences between intervention and control groups in terms of parasite clearance time, fever clearance time,
proportion without fever six and twelve hours after treatment started, mean drop in temperature within the first six hours of treatment, vomiting episodes after treatment, length of hospital stay, fever time, occurence of convulsions and other adverse effects.
Missing data: we extracted information on missing data arising from participant attrition or missing statistical information.
Assessment of risk of bias in included studies
Risk of bias was assessed independently by three review authors (MM, BN and CO) according to the specifications of the Cochrane Handbook (Higgins 2008). We judged the risk of bias within each included study in relation to six domains; sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other sources of bias using ratings of 'Yes' (low risk of bias), 'No' (high risk of bias) and 'Unclear' (unknown risk of bias).
Measures of treatment effect
Continuous data
We extracted and analysed continuous data if mean and standard deviation values were available and there was no clear evidence of skewed distribution. If the mean difference values had been provided, we would have extracted and utilized this information for the analysis irrespective of provision of mean and standard deviation. We were interested in post‐intervention values. We re‐calculated the standard deviation in instances where the standard error was reported.
Binary data
We analysed binary outcomes by calculating the risk ratio with 95% CI.
Unit of analysis issues
Four studies had multiple groups (Hemmer 1991; Lell 2001; Walker 1993; Hugosson 2003). We combined the relevant intervention groups into a single group for extraction of data and reporting in an additional table (Table 2). The same was done for the relevant control groups. In Lell 2001, we divided the shared intervention groups approximately evenly among comparison groups. We were unable to combine data from these studies for meta‐analysis as they differed significantly in the baseline antimalarial treatment and scale of measurement of outcome measures.
1. Outcomes from included studies.
| FEVER CLEARANCE | |||||
| Study ID | Treatment group | FCT in treatment group | Control group | FCT in control group | Summary of effects |
| Brandts 1997 | Paracetamol = 24 | 32 hours | Mechanical antipyretic = 23 | 43 hours | The difference of 11 hours was not significant (95% CI ‐2 to 24, P = 0.176). |
| Hemmer 1991 | Aspirin = 31 | No data | No adjunct = 33 | No data | No significant difference in defervescence time in the two groups |
| Hugosson 2003 | Sulphadoxine/pyremethamine +paracetamol = 38 | No data | Sulphadoxine/pyremethamine only = 41 | No data | There was no significant difference between the group in terms of temperature after the 72 hours of treatment The paracetamol group showed the best early reduction in fever from 0 to 6 hours and this was significant between the two groups (P < 0.01). |
| Krishna 1995b | Ibuprofen = 8 | No data | Paracetamol=8 | No data | The reduction in fever by ibuprofen was significantly greater than that in paracetamol. |
| Lell 2001 | Naproxen = 30 Metamizol = 30 |
At a fever threshold of 38°C FCT was: Naproxen = 24hours Metamizol = 39 hours |
Mechanical antipyretic = 30 | 39 hours | This difference is significant. |
| Walker 1993 | Piroxicam = 39 Aspirin = 36 |
No data | Paracetamol = 40 | No data | No difference in mean FCT between the patients treated with paracetamol, piroxicam or acetylsalicylic acid. |
| Krudsood 2010 | Ibuprofen = 30 | Time for the mean temperature to fall below 37.0°C = 3 hours |
Placebo = 30 | Time for the mean temperature to fall below 37.0°C = 20 hours | This difference is significant. |
| PARASITE CLEARANCE | |||||
| Study ID | Treatment group | PCT in treatment group | Control group | PCT in control group | Summary of effects |
| Brandts 1997 | Paracetamol = 24 | 75 hours | Mechanical antipyretic = 23 | 59 hours | Parasite clearance time was significantly longer in the paracetamol teatment group.The mean difference in PCT was 16 hours (95% CI 8‐24 hours). |
| Hemmer 1991 | Aspirin = 31 | no data | No adjunct = 33 | no data | No significant difference in parasite clearance in the two groups. |
| Hugosson 2003 | Sulphadoxine/pyremethamine + paracetamol = 38 | 11% of patients were positive for P. falciparum after 72 hours of treatment | Sulphadoxine/pyremethamine only = 41 | 12% of patients were positive for P. falciparum after 72 hours of treatment | No significant difference in parasitaemia reduction in the two groups. However parasite reduction between was greater in the paracetamol treatment group (p < 0.005). |
| Lell 2001 | Naproxen = 30 Metamizol = 30 |
Naproxen = 66 hours Metamizol = 63 hours |
Mechanical antipyretic = 30 | 60 hours | No significant difference in three groups. |
| Krishna 1995b | Ibuprofen = 8 | No data | Paracetamol = 8 | No data | No comments were made on parasite clearance. |
| Walker 1993 | Piroxicam = 39 Aspirin = 36 |
No data | Paracetamol = 40 | No data | The mean PCT was similar in the three groups. |
| Krudsood 2010 | Ibuprofen = 30 | Range = 14.0‐50.0 hours Median = 37.3 hours |
Placebo = 30 | Range = 13.5‐50.0 hours Median = 23.7 hours |
PCT was longer in the ibuprofen treated group compared to the placebo group. |
| FEVER TIME | |||||
| Study ID | Summary of effect | ||||
| Lell 2001 | Fever time was significantly lower in the naproxen group than for those in metamizol and mechanical antipyretic groups at wide range of fever tresholds (4.5 hours,6.8 hours,10.4 hours respectively at a fever treshold of 38.5°C; P=0.009). | ||||
| LENGTH OF HOSPITAL STAY | |||||
| Hemmer 1991 | Length of hospital stay was similar: 8 (4‐27) days and 8 (4‐31) in aspirin and control groups respectively. | ||||
| ADVERSE EFFECTS | |||||
| Matsegui 2008 | Occurence of adverse effects was similar in the ibuprofen and placebo group (7 versus 9) respectively. | ||||
| Krishna 1995b | No difference in adverse events (one in the ibuprofen group and one in the paracetamol group). | ||||
| Walker 1993 | No statistically significant difference in the three treatment arms in the number of reported adverse effects. More than 50% of the adverse effects reported were abdominal discomfort. | ||||
| Krudsood 2010 | No significant difference in incidence of adverse events were noted between the ibuprofen and placebo groups. A total of 31 adverse events were reported in 26 patients with 16 of the adverse events reported by 14 patients who received ibuprofen and 15 reported by 12 patients who received placebo. GI disorders particularly abdominal pain was the commonest. No GI hemorrhage was noted. No deaths and no Serious Adverse Events(SAEs). | ||||
Dealing with missing data
Missing data may arise as a result of participant attrition or missing values.
If a study reported outcomes only for participants who completed the trial or only for participants who followed the protocol, we contacted authors and we asked them to provide additional information to facilitate an intention‐to‐treat analysis. Where the information was unavailable due to data loss or non‐response, we reported the available results as stated in the trial report in (Table 2).
Assessment of heterogeneity
We planned to assess data sets for heterogeneity by visual assessment of forest plots and Chi2 test for heterogeneity with a 10% level of statistical significance, and applied the I2 test statistic with a value of 50% or higher denoting a significant level of heterogeneity.
Assessment of reporting biases
Since asymmetry of funnel plots may result from publication bias, heterogeneity or poor methodological quality, we planned to examine funnel plots using Review Manager (RevMan). However, we found an insufficient number of trials to do this.
We planned to assess selective outcome reporting by checking the protocols of included trials if possible (within trial registries, conference proceedings, etc) but found no protocols for any of the included trials. We found no internal evidence of selective outcome reporting within published trials.
Data synthesis
We used Review Manager (RevMan) to perform statistical analyses.
Where the studies were similar enough and results were presented in similar statistical methods, we performed a meta‐analysis. Fixed‐effect model (FEM) was used for data synthesis. We used random‐effects model where we detected significant heterogeneity (I2 >50%) but thought it appropriate to perform meta‐analysis. We assesed the quality of the evidence and constructed a summary of findings table using the GRADE method.
Subgroup analysis and investigation of heterogeneity
We had planned to perform a subgroup analysis. However, no subgroup analysis was done due to insufficient trials.
Sensitivity analysis
We had planned to perform a sensitivity analysis to investigate the impact of adequate allocation concealment and other indicators of high methodological quality on the results. However, this was not possible as the number of trials that used adequate allocation concealment was insufficient to allow for sensitivity analysis to assess the possible influence of high risk of bias in trials that did not apply allocation concealment.
Results
Description of studies
We identified 22 clinical trials, of which ten met the criteria for inclusion (Characteristics of included studies), 12 were excluded (Characteristics of excluded studies).
Included studies
There were a total of 990 participants in the 10 trials that met the inclusion criteria, but only 854 patients were relevant to the research question of the review. We excluded 33, 96 and seven participants (n = 136) from Hemmer 1991; Hugosson 2003, and Krishna 1995a, respectively.
Brandts 1997 compared a combination of physical methods (fanning and sponging) and rectal paracetamol with physical methods alone in 50 slide‐confirmed cases of uncomplicated P. falciparum malaria (aged 2 to 7 years) in Gabon. Three randomized patients were excluded from the analysis of intervention efficacy.
Hemmer 1991 in Germany, studied three groups with malaria who either received a) intravenous acetylsalicylic acid (aspirin; n = 31); b) intravenous heparin (n = 33) or c) neither of these drugs (n = 33). All had confirmed P. falciparum malaria (19 were complicated), and were aged over 14 years. The heparin group was excluded from this review leaving a total of 64 participants in the aspirin (n = 31) and control (n = 33) groups.
Krishna 1995a in Thailand studied a total of 21 adult cases of uncomplicatedP. falciparum malaria in three groups of seven participants each, but only two groups were analysed for this review. The group which received quinine followed by paracetamol after two hours was excluded from the review, while the group that received paracetamol followed by quinine and the control group (which had no antipyretic) were included. The group that received delayed paracetamol was excluded from data synthesis because many participants (3/7: 42.9%) were withdrawn.
Matsegui 2008 in Gabon compared the effect of ibuprofen versus placebo on fever in 50 children (aged 2 to 7 years) with uncomplicated malaria. Three were withdrawn.The data on FCT and PCT presented were suitable for meta‐analysis.
Hugosson 2003 in Tanzania studied a total of 175 children (aged between 12 to 59 months) with uncomplicated malaria in groups who either received chloroquine alone, sulphadoxine/pyrimethamine alone, chloroquine and sulphadoxine/pyrimethamine, or sulphadoxine/pyrimethamine and paracetamol. The groups included 56, 41, 40 and 38 participants respectively. However, only the two groups that received sulphadoxine/pyrimethamine alone (n = 41) and sulphadoxine/pyrimethamine and paracetamol (38) were included in the review. Thus, only 79 participants were included out of the total 175.
Krishna 1995b in Thailand studied a total of 16 patients with uncomplicated malaria in two groups who received either ibuprofen or paracetamol. The data on temperature changes could not be included in meta‐analysis.
Lell 2001 in Gabon studied 90 children with uncomplicated malaria in three groups who received metamizol, naproxen or physical methods treatment alone. Data was not presented in a form that could be included in meta‐analysis.
Walker 1993 in Nigeria studied 118 patients with malaria and compared piroxicam to paracetamol and aspirin. The antimalarial drug used in this study was sulphadoxine/pyrimethamine.
Krudsood 2010 in Thailand studied 60 patients with uncomplicated malaria in two groups who received intravenous ibuprofen and placebo intravenous infusion. Data on PCT and FCT was not presented in a form that will be suitable for inclusion in meta‐analysis.
Kofoed 2011 in Guinea Bissau randomized 338 children with uncomplicated malaria to either paracetamol or placebo. The authors did not report any data with regard to FCT and PCT.
The data provided by Krishna 1995a which were suitable for meta‐analysis were temperature changes within first six hours, FCT and PCT. The data on FCT and PCT presented by Matsegui 2008 were suitable for meta‐analysis. Other trials did not fully report their data in a form that could be used in meta‐analysis, with no statistical measure of variance for FCT or PCT provided in either Brandts 1997, or Hugosson 2003. Where information for inclusion in meta‐analysis was not available, the results have been summarised in "other data" table.
Types of patients
Five trials (Brandts 1997; Hugosson 2003; Lell 2001; Matsegui 2008; Kofoed 2011) included children (aged between one and seven years) and the other five included adults. Walker 1993 included patients with ages ranging from 11 to 67 years. Only one trial included patients who had severe malaria (Hemmer 1991). Three trials (Hemmer 1991; Krishna 1995a; Krudsood 2010) that involved adults excluded pregnant patients. All the trials excluded patients who had underlying diseases, had taken previous effective antimalarial or antipyretic treatment close to the time of entry to the study, or had a history of allergy to a drug in the same class as the study drug. All trials that included children included those of an age susceptible to febrile convulsion, mainly between the ages of six months and six years. Kofoed 2011 included children up to 15 years of age.
Types of intervention
Comparisons included:
Paracetamol plus physical methods versus physical methods alone (Brandts 1997).
Ibuprofen plus physical methods versus placebo plus physical methods (Matsegui 2008).
Paracetamol plus quinine versus quinine alone (Krishna 1995a).
Paracetamol plus sulphadoxine/pyrimethamine versus sulphadoxine/pyrimethamine alone (Hugosson 2003).
Ibuprofen versus paracetamol (Krishna 1995b).
Aspirin versus no treatment (Hemmer 1991).
Metamizol plus physical methods versus naproxen plus physical methods versus physical methods alone (Lell 2001).
Piroxicam versus aspirin versus paracetamol (Walker 1993).
Ibuprofen versus placebo (Krudsood 2010).
Paracetamol versus placebo (Kofoed 2011).
For purposes of meta analysis and reporting of data in an additional tables (Table 2), we subdivided the trials into three groups:
a) Antipyretic drug (paracetamol and NSAIDs) versus no antipyretic drug or placebo (1, 2, 3, 4, 6,7, 9, and 10 above).
b) Antipyretic drug versus physical methods.
c) Paracetamol versus NSAIDs (5 and 8 above).
We did not find any trials that directly compared an antipyretic drug to physical methods for the group of patients of interest to the review.
Outcome assessment
Our primary outcomes on FCT and PCT were defined and assessed in such a way that we could extract data on them from seven trials but only two trials (Krishna 1995a; Matsegui 2008) assessed the outcomes and reported the data in a way that permitted a meta analysis. Lell 2001; Brandts 1997 and Krishna 1995b assessed these outcomes but did not report the respective standard deviations to permit meta analysis. One trial (Hemmer 1991) reported the FCT as the median with the range. Krudsood 2010 reported PCT in a similar manner with median and range. Hugosson 2003 assessed the proportion of patients who had experienced parasite clearance at 72 hours post treatment. However this outcome must be interpreted in light of the fact that this trial included the administration of an effective antimalarial agent which was taken by the patients in both groups that are of relevance to this review. Kofoed 2011 did not report FCT and PCT.
Two trials measured axillary temperatures (Hugosson 2003; Kofoed 2011 ), three trials measured oral temperatures (Krishna 1995a; Krishna 1995b; Krudsood 2010) while three trials measured rectal temperatures (Brandts 1997; Lell 2001; Matsegui 2008).The site of temperature measurement was not reported in two trials (Hemmer 1991, Walker 1993).
Differences between the previous version of this review and the present version
The present review has been revised to allow for the inclusion of trials that performed a head‐to‐head comparison of paracetamol and NSAIDs. This has permitted the inclusion of Krishna 1995b and Walker 1993 in this review as these trials were excluded from the previous version of this review.
Excluded studies
The main reasons for exclusion (see Characteristics of excluded studies) were inclusion of non‐malarial cases and administration of the same antipyretic drug on both arms.
Risk of bias in included studies
All the trials were described as randomized. See Figure 1 and Figure 2.
1.
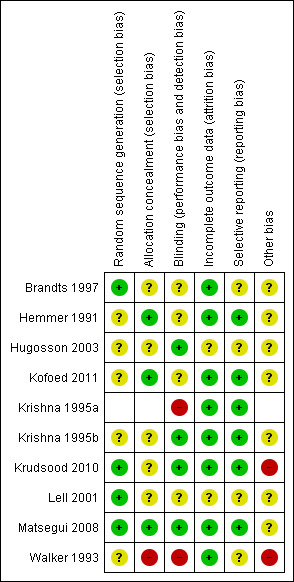
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
2.
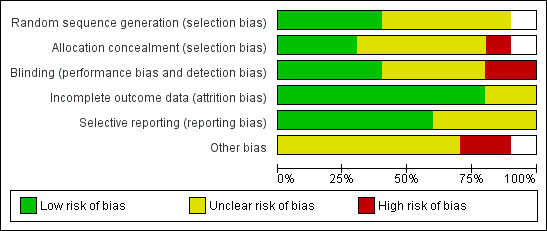
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
Allocation
Generation of allocation sequence: four trials stated the method of generation of allocation sequence; Brandts 1997; Krudsood 2010; Lell 2001 used a table of random numbers while Matsegui 2008 used computer generated random numbers. Generation of allocation sequence was unclear in the other studies although they were described as randomized.
Allocation concealment: this was adequate in three trials (Hemmer 1991;Matsegui 2008; Kofoed 2011) in which sealed envelopes were used or randomization numbers kept separately and unclear in the other trials. Krishna 1995a and Walker 1993 were open trials.
Blinding
Three trials (Krishna 1995b; Matsegui 2008; Krudsood 2010 ) were double blinded trials where the participants and study personnel were blinded. In Hugosson 2003, outcome assessors only were blinded. Kofoed 2011 did not explicitly state who was blinded but described the administration of numbered boxes that had paracetamol or indistinguishable placebo tablets. Blinding was not feasible in the other trials as they included dissimilar comparisons of antipyretics versus mechanical methods. Krishna 1995a and Walker 1993 were open trials.
Incomplete outcome data
Attrition rates ranged from 0% in three trials (Hemmer 1991; Krishna 1995b; Lell 2001),1.6% in Krudsood 2010, 3% in three trials (Brandts 1997; Hugosson 2003; Matsegui 2008), 10% in Kofoed 2011 and 14% in Krishna 1995a.
Data on all 50 patients was available for intention‐to‐treat analysis in Matsegui 2008. Kofoed 2011 performed both an intention‐to‐treat analysis and a per protocol analysis.
No intention‐to‐treat analysis was done in the other trials.
Selective reporting
A range of primary outcomes was reported by the trials. However, they reported primary and secondary outcomes specified in the methods section of the trials. Both significant and non‐significant results were reported.
Other potential sources of bias
It was not clear if the trials had other sources of bias such as early stopping, influence of funders and deviation from trial protocol. With regard to funding, most of the trials were funded by university research project grants although the trialists did not explicitly state that the funders had no role in the design, analysis and interpretation of the trial findings. Three trials (Walker 1993Krudsood 2010; Kofoed 2011) were funded by the pharmaceutical companies who manufactured the drugs used for the trial. In Krudsood 2010, the funders played a role in trial design, data collection and analysis as well as approval of the published report although the authors claim autonomy to the contents of the report. We have judged this as high risk in these two trials. However in Kofoed 2011 the authors explicitly stated that the funders had no role in the design, analysis and interpretation of trial data.
Effects of interventions
See: Table 1
Interventions versus placebo or no drug
We assessed eight trials under this comparison group (Brandts 1997; Hemmer 1991; Hugosson 2003; Lell 2001; Krishna 1995a; Matsegui 2008; Krudsood 2010; Kofoed 2011)
Fever clearance
This was measured in a variety of ways: FCT (the time between onset of treatment and sustained resolution of fever without recurrence during same illness) and fever time (FT) (duration in hours that a patient's temperature was above an indicated fever threshold).
Fever clearance time and fever time
In the seven studies measuring this, some reported demonstrating an effect, others did not.
Krishna 1995a did not detect a difference (60 versus 44 hours; mean difference (MD) 16.0, 95% CI ‐24.10 to 56.10); although the mean maximum fall in temperature was more rapid in the paracetamol plus quinine treated group 2.1°C (SD 0.79) compared to the group that received quinine alone 0.9°C (SD 1.1). Hemmer 1991 and Brandts 1997 reported no difference detected although they provided no measures of variance.
Matsegui 2008 reported a difference (ibuprofen 41.0 hours ± 21; placebo group (52.0 hours ± 24) in FCT, and the total time the patient was febrile 18.3 (15.5) hours and 28.0 (16.0) hours. However, this difference was not statistically significant. Lell 2001 reported that at fever threshold of 38°C, naproxen significantly reduced FCT compared to physical methods only (24 versus 39 hours respectively), and the temperature fell below the threshold quicker. We are unable to include this in a meta analysis because the trialists did not report standard deviation or standard error. Krudsood 2010 reported that time from onset of treatment to fall of the mean temperature below 37.0°C was three hours in the ibuprofen treatment group and 20 hours in the placebo group.
Hugosson 2003 also reported that mean axillary temperature six hours after treatment in the sulphadoxine/pyrimethamine only group was higher than that in sulphadoxine/pyrimethamine and paracetamol group. The temperature values were 38.1°C and 36.9°C respectively six hours after treatment and this different was statistically significant. However, these trials did not specifically define FCT. These improvements were mainly short‐lived. Kofoed 2011 did not report FCT.
Parasite clearance
Parasite clearance time
Six studies reported on PCT (Krishna 1995a; Brandts 1997; Matsegui 2008; Krudsood 2010; Hemmer 1991; Hugosson 2003). Results were mixed, with three studies showing longer PCT, and three shorter PCT.
Studies suggesting longer PCT:
Krishna 1995a, PCT was longer in the paracetamol group than the controls (72 versus 53 hours; MD 19.00, 95% CI 1.38 to 36.62).
Brandts 1997 reported a longer PCT in the paracetamol plus mechanical antipyresis group than the mechanical antipyresis alone group (75 hours versus 59 hours); they reported this as significant, but the method of analysis was not given (difference = 16 hours, 95% CI 8 to 24); as data on variance in the two arms were not given, we could not repeat this analysis and we could not include this in the meta analysis.
Krudsood 2010 PCT was also delayed in the antipyretic arm. In the ibuprofen treatment group, it was in the range of 14.0 to 50.0 hours with a median of 37.3 hours (P = 0.0024 Kruskall‐Wallis test). While in the placebo treatment group, it was in the range of 13.5 to 50.0 hours and a median of 23.7 hours(p= 0.0024 Kruskall Wallis test).
Studies reporting shorter PCT or no difference detected:
In Matsegui 2008, PCT was shorter in the ibuprofen group (47.0 hr (± 23) than in the placebo group (66.0 hours (± 15 hours) but was reported as not significant. When we combined the results from these two trials, the weighted mean difference was ‐0.63 hours 95% CI ‐37.85 to 36.59; random‐effects model). We used random‐effects model due to the high level of statistical heterogeneity (I2=92%) in the results of the two trials. In view of the high statistical hetrogeneity and clinical heterogeneity , we opted to report the meta analysis graph without the pooled estimate of effects Analysis 1.2.
Hemmer 1991 provided no data but reported that PCT did not differ between the aspirin group and controls.
Hugosson 2003 found no significant difference in parasite density reduction between the groups receiving sulphadoxine/pyremetahamine only and sulphadoxine/pyrimethamine with paracetamol after 72 hours of treatment. However the parasite reduction between 0‐24 hours was greater in the group that received sulphadoxine/pyrimethamine with paracetamol.
1.2. Analysis.
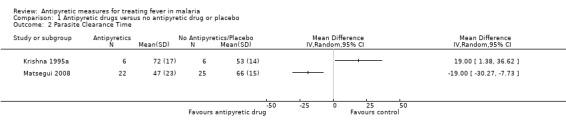
Comparison 1 Antipyretic drugs versus no antipyretic drug or placebo, Outcome 2 Parasite Clearance Time.
Presence of parasites after treatment
At three days after treatment had started, Hugosson 2003 showed that five (12%) of the 41 patients treated with sulphadoxine/pyrimethamine only and four (11%) of the 38 patients who received sulphadoxine/pyrimethamine and paracetamol were positive for P. falciparum after 72 hrs (three days) of treatment. There was no significant difference in the two groups.
Kofoed 2011 reported that 19 out of 167 patients who were randomized to paracetamol against 21 out of 171 patients randomized to placebo still had parasites three days after treatment (early treatment failure). 25 paracetamol patients and 16 placebo patients had parasites seven days after treatment (late parasitological failure). These results were not statistically significant.
Parasitaemia at later time points was not reported by any studies.
Length of hospital stay
Hemmer 1991 and Matsegui 2008 assessd this outcome and reported no significant difference in length of hospital stay between the treatment groups. Patients were hospitalized for a mean (± SD) of 80.0 hours (± 10.0) versus 79.0 hours (± 11.0) for ibuprofen and placebo, respectively in the Matsegui 2008 trial.
Alleviation of symptoms and signs
Headache: One trial reported that paracetamol alleviated headache but gave no data or comparative analysis (Krishna 1995a). Nausea, vomiting and myalgia which were also monitored half‐hourly by Krishna 1995a and did not differ between the study groups.
Convulsions: One trial reported the occurrence of convulsions in one participant (Matsegui 2008); this was seen as an adverse event and led to the exclusion of the participant from the trial. Kofoed 2011 reported that two paracetamol patients as against six placebo patients had convulsions following treatment. However this difference was not statistically significant.
Vomiting after treatment: no trials reported this.
Cure rate
In Kofoed 2011 no difference in malaria cure by day 28 was reported: 108/113 patients randomized to paracetamol showed an adequate clinical and parasitological response on day 28 post treatment (adjusted by PCR to exclude new infections), as against 111 out of 116 patients randomized to placebo.
Adverse events
Paracetamol
Krishna 1995a reported adverse effects such as urticarial rash, abdominal pain and diarrhoea.
Ibuprofen
Seven of the 25 patients who received ibuprofen in the Matsegui 2008 trial had adverse events. The events that were reported included vomiting, headache, abdominal pain, fatigue, diarrhoea, cough, fever and conjunctivitis. One child in the ibuprofen group experienced convulsions. The trialists noted that the incidence of adverse events was not statistically different between the ibuprofen and placebo groups.
The number of patients who experienced an adverse effect, such as gastrointestinal disorders (abdominal pain) and respiratory disorders (nasal congestion), on receiving Ibuprofen was similar to those who received placebo in Krudsood 2010. None of the patients in both treatment arms reported a gastrointestinal haemorrhage.
Aspirin
There was no report on adverse events in the Hemmer 1991 trial, which included aspirin.
Mechanical antipyresis
The studies reported no adverse effects.
Paracetamol versus NSAIDs
We assessed two trials (Krishna 1995b and Walker 1993) under this comparison group.
Fever clearance
The trialists in Krishna 1995b assessed mean temperature response for which they reported that the mean temperature nadir was significantly lower in the ibuprofen group compared to the paracetamol group.
Walker 1993 reported that there was no difference in mean FCT between the patients treated with paracetamol, piroxicam or acetylsalicylic acid, but did not give any data.
Parasite clearance time
Walker 1993 reported no difference between the three groups in the trial, but no data was given.
Alleviation of other symptoms
Krishna 1995b reported that alleviation of headache by ibuprofen was sustained over a longer period of time than paracetamol. All patients were headache‐free for 1.5 hours with ibuprofen. Paracetamol did not abolish headache in all patients and the incidence of headaches increased after one hour of symptomatic improvement.
Walker 1993 reported the effect of the study medications on mean ache scores for generalized joint aches. Aspirin appeared better initially with a 43% fall versus piroxicam (30%) and paracetamol (13%). However, this trend did not persist as aspirin had only produced a 54% decrease by Day 4 as against 100% for piroxicam and paracetamol respectively. Aspirin achieved 100% reduction by Day 7. A similar trend was also observed for mean headache scores except that there was still residual headache by Day 4 in all the groups, which was cleared by Day 7. The mean headache score was however less severe in the paracetamol‐treated group from the onset.
Adverse Events
Krishna 1995b reported adverse effects such as urticarial rash, abdominal pain and diarrhoea.
Walker 1993 reported adverse events like diarrhoea in one patient who received piroxicam, abdominal discomfort, dizziness and itching. The trialists reported that there were no statistically significant differences between drug treatments in the incidence of the reported adverse events.
Discussion
Summary of main results
The major objective of this review was to ascertain from reliable research whether antipyretic measures impact on the treatment of malaria. Biological theory led researchers to ask if fever control could prolong parasitaemia and do harm in patients with malaria. We set out to confirm or refute our apriori hypotheses.
We were unable to demonstrate a clinically meaningful or statistically significant effect of the antipyretic drugs under review on FCT and PCT. This was mainly due to the small size of the studies, and the level of heterogeneity between them.
For FCT (ie time to sustained resolution of fever during an episode of malaria), most trials did not detect an impact of antipyretic drugs on this outcome. Antipyretic drugs induced short‐lived relief of fever with each dose of treatment but did not contribute significantly to sustained resolution of fever in malaria.
As regards our hypothesis that antipyretic measures do not prolong malaria parasitemia, we are unable to make any meaningful conclusion as we found an inconsistent pattern of the effects of antipyretics on this outcome. Some of the trials reported no significant difference in PCT while others found a significant difference due to the fact that antipyretics prolonged PCT. Also statistical and clinical heterogeneity made it inappropriate to pool data from the included studies.
Many clinicians treat febrile children with antipyretics because of the risk of febrile convulsions (Strengell 2009), and we had hoped to inform this practice by seeking data that would either support or discourage this practice. However, trials were too small to examine the incidence of febrile convulsions and most of the included trials did not report this outcome. In Kofoed 2011, more patients in the placebo arm had convulsions but this was not statistically significant. Only one participant in the Matsegui 2008 trial had a convulsion.
We found insufficient data to show whether the antipyretic effects of ibuprofen and paracetamol differed significantly when used to treat fever in patients with malaria. Only one trial with few participants reported this comparison; there is a need for this to be replicated in larger, better powered trials.
Overall completeness and applicability of evidence
The studies we included in this review were mostly for patients with uncomplicatedP. falciparum malaria. The studies did not assess the effect of antipyretics in severe malaria, as severe illness was an exclusion criteria in almost all of the studies. We therefore cannot answer the question as to the benefit or otherwise of antipyretic intervention in this group of patients.
We also did not find any trial that performed a head‐to‐head comparison of antipyretic drugs versus physical methods. Therefore, we are unable to comment on the superiority or otherwise of antipyretic drugs over physical methods or vice versa. Some of the trials used physical methods in both the treatment and control arms as the baseline standard of care.
Quality of the evidence
This review included data from randomized controlled trials but about half did not report the method of randomization. Only a few (3/10) explicitly reported allocation concealment indicating a rather high risk of bias.The quality of the evidence for fever clearance time and parasite clearance time was categorised as "very low".
Authors' conclusions
Implications for practice.
Fever management in malaria with antipyretic drugs remains a common practice in both mild and severe disease. There is currently insufficient evidence to recommend a change in practice.
Implications for research.
This review shows that evidence on potential harms of using antipyretic drugs in the treatment of malaria is scanty. It highlights a significant gap in knowledge and lack of reliable data to inform a common clinical practice for a disease that affects several millions of people every year. Additional research evidence will be needed to draw reliable conclusions on the questions addressed in this review. There is also a need for primary research to assess the effects of antipyretic measures on the treatment of fever in severe malaria. The absence of data to make a direct head‐to‐head comparison of antipyretic drugs and physical methods calls for the design of randomised control trials to assess these treatment options. An important outcome to incoporate into these trials would be the patients' views with regard to antipyretics leading to faster resolution of symptoms.
Additional information could also be obtained from trials designed to examine fever control in all patients (especially children) with fever living in a malarious area, regardless of the aetiology, and explore malaria infection as a dominant sub‐group.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2012 | New search has been performed | New search and seven new studies added. Author team changed. GRADE has been used to grade the evidence. Review methods slightly changed to allow inclusion of trials with head to head comparison of antipyretic drugs. Data was re‐extracted but overall conclusions not changed. |
| 29 May 2012 | New citation required but conclusions have not changed | New studies added. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 29 July 2008 | Amended | Converted to new review format with minor editing. |
| 30 October 2004 | Amended | New studies found but not yet included or excluded. |
| 11 December 2002 | Amended | Abstract and search strategy edited. |
Acknowledgements
The Institute of Tropical Diseases Research and Prevention, University of Calabar Teaching Hospital (represented by Martin Meremikwu) receives support from UK Department for International Development (DFID) Effective Health Care Research Programme Consortium, and collaborates with the Liverpool School of Tropical Medicine UK and the South African Cochrane Centre in the implementation of this grant programme. The editorial base for the Cochrane Infectious Diseases Group is funded by DFID for the benefit of developing countries.The funders take no responsibility for the data presented or the views expressed.
Data and analyses
Comparison 1. Antipyretic drugs versus no antipyretic drug or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fever Clearance Time | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Parasite Clearance Time | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Mean drop in temperature within first 6 hrs of starting treatment | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.12, 2.28] |
| 4 Length of Hospital stay | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐5.00, 7.00] |
1.1. Analysis.
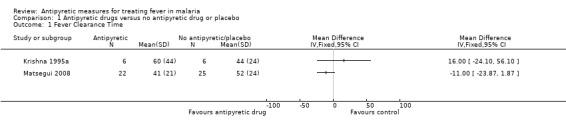
Comparison 1 Antipyretic drugs versus no antipyretic drug or placebo, Outcome 1 Fever Clearance Time.
1.3. Analysis.
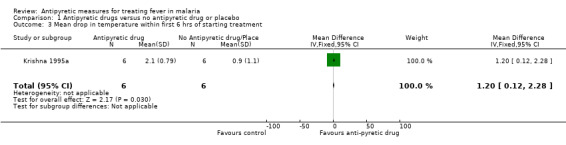
Comparison 1 Antipyretic drugs versus no antipyretic drug or placebo, Outcome 3 Mean drop in temperature within first 6 hrs of starting treatment.
1.4. Analysis.
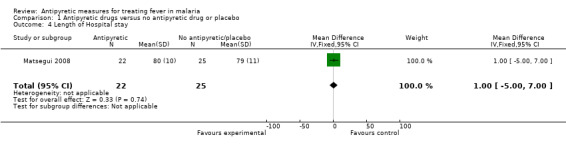
Comparison 1 Antipyretic drugs versus no antipyretic drug or placebo, Outcome 4 Length of Hospital stay.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brandts 1997.
| Methods | Randomized from random numbers table. Allocation concealment unclear, unblinded. 3/50 (6%) withdrawn from the analysis after randomization. No intention‐to‐treat analysis. Ethical approval was given by the Ethics Committee of the International Foundation of the Albert Schweitzer Hospital in Lambaréné, Gabon. Informed consent was sought from patients. The study was funded by the Fortune Medical Faculty, University of Tubingen, Germany and the Osterrieichesche Gessellschaft fur Chemotherapie Austria and the AUPELF‐UREF/ARC, France. |
|
| Participants | 50 children with uncomplicated P. falciparum malaria. Study was conducted in Gabon, West Africa. Patients were included in the trial if they had initial asexual parasitemia between 25,000 and 200,000 parasites/μL blood, aged 2 to 7 years, had temperatures above 38 °C on admission or fever in the preceeding 24 hours, had not taken antipyretic treatment in the preceeding 8 hours and not received effective antimalarial treatment for the acute infection. Exclusion criteria: complicated malaria, Hb < 8.0 g/dL (PCV < 24%), glucose < 2.8 mmol/L, lactate > 3.5 mmol/L, schizontaemia > 50/µL, platelets < 50,000/µL, pigment‐containing neutrophils > 2%. |
|
| Interventions | A: mechanical antipyretic treatment (continuous electric fanning, tepid sponging and cool blankets) plus paracetamol suppositories (50 mg/kg/day at 10 to 15 mg/kg, 4 to 6 hourly); expelled suppositories replaced immediately.
B (control): mechanical antipyretic therapy (as above) without paracetamol. Similar antimalarials in both groups: intravenous quinine 15 mg/kg, 12 hourly for 4 days; then oral quinine at 15 mg/kg, 12 hourly, for 3 days. |
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants reported as withdrawn from the treatment group and reasons for withdrawal given; one from the paracetamol(treatment) group and two from the mechanical antipyretics (control) group. However data on 47 patients were available for per protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | All pre‐specified primary and secondary outcomes were reported |
| Other bias | Unclear risk | No information |
Hemmer 1991.
| Methods | Randomized, method of randomization not specified, separate randomization for complicated and uncomplicated. Allocation adequately concealed in sealed envelopes by another department Blinding was unclear. None lost to follow‐up. No intention‐to‐treat analysis. The study was ethically approved by the Ethics Committee of the Hamburg Medical Association. The study was supported by grants from Deutsche Forschungsgemeinschaft, Mildred‐Scheel Foundation, the German Association of Cancer Research and the State of Baden‐Wurttenberg Research Focuses on Inflammation and Transport Mechanisms. |
|
| Participants | 64 patients were included in the meta‐analysis out of 97 patients, aged > 14 years. Heparin group excluded. All were treated in Hamburg, Germany (18 African, 79 European). All had history of fever (1 to 30 days). 78 were uncomplicated. 19 complicated (impaired cerebral function = 14; clotting disorder = 4; impaired renal function = 4; others = 3). |
|
| Interventions | Antipyretics: Control: No antipyretic. Intervention 1: Intravenous (IV) acetylsalicylic acid (ASA: 500 mg; days 0, 2, 4). Intervention 2: subcutaneous heparin 70 units/kg, 3/day for 5 days, with no ASA. Anti‐malarials: Uncomplicated: randomized to oral quinine (20‐25 mg/kg/day) plus doxycycline (100mg/day) or mefloquine 20mg/kg in 3 doses, 6 hours apart Complicated: standard therapy with intravenous quinine |
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes were reported |
| Other bias | Unclear risk | No information |
Hugosson 2003.
| Methods | Randomized, unblinded, method of randomization not stated. Concealment of allocation unclear. Attrition not clearly stated. Ethical clearance was obtained from the Ministry of Health of Tanzania and the Clinical Research Committee of the Karolinska Institutet (Stockholm, Sweden). The study was funded by the Swedish International Development Cooperation Agency (Stockholm, Sweden). |
|
| Participants | 175 children aged 12‐59 months participated in the study but 79 only were included for meta‐analysis. All had uncomplicated malaria with fever. Patients were included in the trial if they had uncomplicated mono‐infection with P. falciparum malaria, parasite density of 2,000‐250,000 parasites per μL, an axillary temperature of 37.5 to 40 °C, no history of drug intake in the last two weeks and no history of hypersensitivity drug reactions. Patients were excluded if they had any of the signs of severe malaria eg. hyperparasitemia, and severe anemia. They were also excluded if they had signs of co‐existing diseases. |
|
| Interventions | Group A: Chloroquine given at a dose of 25 mg base/kg of body weight over a period of three days. Group B: Single dose of 1.25 mg of pyrimethamine and 25 mg of sulfadoxine/kg of body weight Group C: Chloroquine and SP Group D: SP and paracetamol, 15 mg/kg of body weight every eight hours for 72 hours. |
|
| Outcomes |
Primary; Temperature at 48 hours Secondary;
|
|
| Notes | For purposes of this review, we were interested in extracting data for the patients in the sulphadoxine pyrimethamine alone group versus the sulphadoxine pyrimethamine plus paracetamol group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double(participants and study personell) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3% attrition observed but reasons not stated in the report |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Kofoed 2011.
| Methods | Randomized controlled trial | |
| Participants | Children aged between 3 and 190 months, weighing at least 7.5kg, with positive thick film for malaria, monoinfection with P. falciparum and 20 or more parasites per 2000 leukocytes. Patients were excluded if they had convulsions, severe vomitting, severe anemia, severe concurrent infection or needed hospital care for any other reason. | |
| Interventions | Paracetamol at 50 mg/kg body weight per day for three days. | |
| Outcomes | Early treatment failure, late parasitological failure, adequate clinical and parasitologial response (PCR adjusted), incidence of convulsions. | |
| Notes | Ethical approval was obtained from the Direccao de Higiene e Epidemiologia, Ministerio da Saude Publica in Guinea Bissau. Study was funded by the Department of Infectious Diseases, Malarsjukhuset, Eskildstuna. Also the drugs were donated by GSK (paracetamol/placebo) and Recip (Chloroquine). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated explicitly |
| Allocation concealment (selection bias) | Low risk | Randomization numbers were kept separately at the Department of Pediatrics in Kolding, Denmark |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated explicitly who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | An intention‐to‐treat analysis was performed and also authors attempted to account for all missing data by employing a best case, worst case scenario |
| Selective reporting (reporting bias) | Low risk | Although we do not have the protocol, we have no reason to believe the authors selectively reported the study outcomes. |
| Other bias | Unclear risk | No information provided on this. |
Krishna 1995a.
| Methods | Randomized, unblinded, method of randomization not stated. Concealment of allocation unclear. Losses to follow‐up not clearly stated but calculated to be 5 (23.8%), since final report of FCT and PCT indicated that n=16 out of 21. No intention‐to‐treat analysis. The study was approved by the ethical review subcommittee of the Ministry of Health, Tanzania. The study was a component of Wellcome‐ Mahidol University‐ Oxford Tropical Medicine Research Programme. |
|
| Participants | 14 patients were included out of 21 adults (7 per group). All had uncomplicated P. falciparum malaria. Kanchanaburi, Thailand Oral temperature > 38 ºC Range of parasitaemia: 1130 to 24,9600/µL Exclusion criteria: age < 14 years, pregnancy, paracetamol taken < 6 hours before |
|
| Interventions | Group A: Quinine (10 mg/kg, oral) followed 2 hours later by paracetamol (15 mg/kg, oral)
Group B: Paracetamol (15 mg/kg) followed 2 hours later by quinine (10 mg/kg)
Group C: Quinine (10 mg/kg) with no paracetamol Group A was excluded from review |
|
| Outcomes |
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Low risk | |
Krishna 1995b.
| Methods | Randomized, double blind trial. Method of randomization not stated. Allocation concealment was unclear. Ethical clearance obtained from the Faculty of Medicine, Ramathipodi Hospital, Bangkok, Thailand. Funding from the Oxford‐Welcome‐Mahidol Research Project. |
|
| Participants | 16 patients with uncomplicated P. falciparum malaria. Patients enrolled into the study had uncomplicatedP. falciparum malaria and an oral temperature > 38.5 °C, and gave informed consent. Patients were excluded if they had contraindications to the use of paracetamol or ibuprofen (specifically a history of asthma, dyspeptic symptoms, gastro‐intestinal bleeding or allergy to ibuprofen), or gave a history of antipyretic or antimalarial drug use within 6 hours of presentation. |
|
| Interventions | Group A received oral quinine sulphate 10 mg/kg and paracetamol elixir 15mg/kg in a 50 mg/mL suspension. Group B received oral quinine sulphate 10 mg/kg and ibuprofen suspension 10 mg/kg in a 20 mg/mL suspension. |
|
| Outcomes | Clinical features, which included temperature responses to ibuprofen and paracetamol Parasite clearance Adverse events |
|
| Notes | Outcome assessments were not reported in a way that would allow for a meaningful analysis of its results in the context of this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding (participants and personnel) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No attrition reported |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes were reported. |
| Other bias | Unclear risk | No information |
Krudsood 2010.
| Methods | Prospective, double‐blind, placebo controlled trial. Randomization was done using table of random numbers. Allocation concealment was not stated. Ethical clearance obtained from the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. This study and its report were supported by Cumberland Pharmaceuticals Inc. (Nashville, TN). Informed consent was obtained before enrolment into the trial. |
|
| Participants | 60 patients with uncomplicated malaria. Patients were included if they had microscopically proven P. falciparum malaria, > 17 years of age, had oral temperature of > 38.0°C and had been febrile for at least 12 hours. Patients were excluded if weighed < 40kg, received antipyretic treatment 8 hours or less before dosing, had history of adverse effects to any NSAID, were pregnant or nursing, had features of severe malaria, and any additional medical problems. |
|
| Interventions | Group A received oral artesunate (4 mg/kg/day) and mefloquine (8 mg/kg/day for 72 hours) plus IV‐ibuprofen 400 mg ((400 mg ibuprofen (4 mL) diluted in 100 mL of isotonic saline) infused every 6 hours for 72 hours, followed by doses every 6 hours as needed for a further 2 days to treat fever > 38.0°C. Group B received oral artesunate (4 mg/kg/day) and mefloquine (8 mg/kg/day for 72 hours) plus placebo (intravenous infusion of 100 mL isotonic saline) infused every 6 hours for 72 hours, followed by doses every 6 hours as needed for a further 2 days to treat fever > 38.0°C. |
|
| Outcomes | Primary Endpoints
within the first 24 hours of treatment. Secondary Endpoints
Tolerability Endpoints
Safety Endpoints
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers was used for allocation sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not stated in the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinding (patients, investigators, nursing staff and study monitoring staff, including microscopists, were all blinded to study treatments) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only one patient withdrew from the study due to social reasons |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes were reported |
| Other bias | High risk | Funders played a role in study design, data collection and analysis. They also approved the study for publishing although the authors claim autonomy for contents of the report. |
Lell 2001.
| Methods | Randomized controlled trial. Generation of allocation sequence was done using a table of random numbers. Allocation concealment was unclear. Ethical approval was obtained from the the Ethics Committee, International Foundation of Albert Schweitzer Hospital (Lambarene, Gabon). Study was funded by a Fortune grant from the Medical Faculty, University of Tubingen, Tubingen, Germany and the Gessellschaft fur Chemotherapie, Vienna, Austria. |
|
| Participants | 90 children with uncomplicated P. falciparum (parasite density: 20,000 to 200,000/µL) Aged 2 to 7 years Gabon, West Africa Exclusion criteria: Antipyretic use within 8 hours of presentation, haemoglobin less than 8g/dL, PCV less than 24%, white cell count > 12 x 109/L, platelet count < 50 x 109/L, schizontaemia > 50/uL; glucose < 50 mg/dL; lactate level > 3.5mM, severe malaria |
|
| Interventions | Group A (metamizol group): infusion of 5% glucose with 12 mg/kg of quinine dihydrochloride every 12 hours + single dose of SP at discharge + mechanical antipyretic (continous fanning and tepid sponging) + oral metamizol at 10mg/kg every 6 hours. Group B (naproxen group): infusion of 5% glucose with 12 mg/kg of quinine dihydrochloride every 12 hours + single dose of SP at discharge + mechanical antipyretic (continous fanning and tepid sponging)+ rectal suppositories of naproxen at 7.5mg/kg every 12 hours. Group C (mechanical antipyretics only): infusion of 5% glucose with 12 mg/kg of quinine dihydrochloride every 12 hours + single dose of SP at discharge + mechanical antipyretic (continous fanning and tepid sponging). |
|
| Outcomes |
|
|
| Notes | This study investigated metamizole (dipyrone) which has been banned by the National regulating bodies of many countries because of its risk of agranulocytosis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No attrition was reported as the children were seen to have all recovered completely |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Matsegui 2008.
| Methods | Randomized double blind placebo trial. Method of randomization was computer generated. Allocation was concealed using sealed envelopes. Per protocol analysis was done for primary outcome while intention to treat analysis was done for adverse effects. Data on all patients was available for intention‐to‐treat analysis. Three participants were withdrawn (one had a convulsion after randomization and two violated the protocol). The study was ethically approved by the Ethics Committee, International Foundation of Albert Schweitzer Hospital (Lambare´ne´, Gabon). Some form of support was obtained from the University of Tubingen, Germany. |
|
| Participants | 50 children with uncomplicated P. falciparum (parasite density: 20,000 to 200,000/µL). Aged 2 to 7 years. Gabon, West Africa. Exclusion criteria: complicated malaria, Hb < 7.0 g/dL (PCV < 20%), schizontaemia > 50/µL, platelets < 50,000/µL, white cell count > 16,000/µL, platelet count< 40,000/µL. |
|
| Interventions | Group A (treatment): infusion of 250 ml of 5% glucose with 12 mg/kg of quinine dihydrochloride every 12 hours for 72 hours + single dose of SP + mechanical treatment (continous fanning and cooling blanket) + ibuprofen syrup (nurofen 7 mg/kg every eight hours) until fever and parasite clearance achieved. Group B (placebo): infusion of 250 ml of 5% glucose with 12mg/kg of quinine dihydrochloride every 12 hours for 72 hours + single dose of SP + mechanical treatment (continous fanning and cooling blanket). |
|
| Outcomes |
Primary outcomes: Fever clearance time in hours at a threshold of 37.5 °C Secondary outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double (participants and study personel) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants were withdrawn and the reasons were stated. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified primary and secondary outcomes were reported. |
| Other bias | Unclear risk | No information |
Walker 1993.
| Methods | Open randomized controlled trial. Method of sequence generation unclear, no allocation concealment Study was conducted in Nigeria. Ethical approval was from the College of Medicine and University College Hospital, University of Ibadan. Study was funded by Pfizer. |
|
| Participants | 118 participants were randomized. Participants were aged between 11 and 67 years, and all had parasitologically proven P. falciparum malaria. Three patients were subsequently withdrawn because of associated sickle cell anemia and parasitological failure of antimalarial drug, leaving 115 for analysis. Exclusion criteria for this trial included: history of allergy or hypersensitivity to analgesics, peptic ulceration or significant gastrointestinal disease, renal or hepatic failure, history of alcoholism or drug abuse, concurrent medication with anticoagulants or lithium, presence of any febrile illness in addition to malaria or chronic musculoskeletal disease or sickle cell disease. |
|
| Interventions | Group A ‐ 39 patients received piroxicam(40 mg daily by the Intramuscular route on Day 0 and 1 and 20 mg orally on days 2, 3, 4). Group B‐ 40 patients received Paracetamol (1 g every eight hours for 4 days via the oral route). Group C‐ 36 patients received acetylsalicylic acid (600 mg every eight hours from Day 0 to Day 4). |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Open trial |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients were excluded from the study and reason for exclusion was stated and they were not randomized into any of the groups. |
| Selective reporting (reporting bias) | Unclear risk | We could not judge if the report was free from selective reporting as the outcome measures to be assessed in this trial were not pre‐specified in the methods section of the report |
| Other bias | High risk | Study was funded by Pfizer Pharmaceuticals |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agbolosu 1997 | Randomized trial of paracetamol versus tepid sponging, but 56.2% of participants did not have malaria. |
| Aksoylar 1997 | Randomized trial of tepid sponging versus antipyretic drugs but participants did not have malaria. |
| Fasan 1980 | Clinical trial of a combination of paracetamol and chloroquine; included no placebo or mechanical antipyretic group. |
| Ismail 1995 | Randomized trial of paracetamol in regimens of artemisinin derivatives; did not compare paracetamol with placebo or mechanical antipyretic group. |
| Kramer 1991 | Randomized, placebo‐controlled trial of febrile children but participants did not have malaria. |
| Mahar 1994 | Open randomized comparison of paracetamol and tepid sponging; participants had fever of presumed viral origin and not malaria. |
| Newman 1985 | Not confirmed malaria patients. |
| Nwanyanwu 1999 | Mechanical measures or non‐treated controls not included. |
| Sharber 1997 | Randomized trial but both arms received paracetamol alone or with tepid sponging. |
| Steele 1970 | Not confirmed malaria patients. |
| Tarimo 2002 | Compared sulphadoxine and pyremethamine with chloroquine. |
| Thomas 2009 | Randomized trial but had paracetamol in both arms. Compared paracetamol only with paracetamol and tepid sponging. |
Contributions of authors
Martin Meremikwu, Karl Logan and Paul Garner wrote the protocol and the first edition of the review. In this update, Chibuzo Odigwe, Bridget Nwagbara and Ekong Udoh selected studies for inclusion. Bridget Nwagbara and Chibuzo Odigwe performed data extraction. Martin Meremikwu and Chibuzo Odigwe performed data analysis. All the authors contributed to the preparation of the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
-
University of Calabar, Nigeria.
Provides salary cost for Prof Martin Meremikwu and office space, electricity and other utilities for the four authors based in Nigeria.
External sources
Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, expert testimony).
Unchanged
References
References to studies included in this review
Brandts 1997 {published data only}
- Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effects of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet 1997;350(9079):704‐9. [DOI] [PubMed] [Google Scholar]
Hemmer 1991 {published data only}
- Hemmer CJ, Kern P, Holst FG, Nawroth PP, Dietrich M. Neither heparin nor acetylsalicylic acid influence the clinical course in human Plasmodium falciparum malaria: a prospective randomised study. American Journal of Tropical Medicine and Hygiene 1991;45(5):608‐12. [DOI] [PubMed] [Google Scholar]
Hugosson 2003 {published data only}
- Hugosson E, Tarimo D, Troye‐Blomberg M, Montgomery SM, Premji Z, Bjorkman A. Antipyretic, parasitologic, and immunologic effects of combining sulfadoxine/pyrimethamine with chloroquine or paracetamol for treating uncomplicated Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 2003;69(4):366‐71. [PubMed] [Google Scholar]
Kofoed 2011 {published data only}
- Kofoed PE, Ursing J, Rodrigues A, Rombo L. Paracetamol versus placebo in the treatment of non severe malaria in children in Guinea Bissau: a randomized controlled trial. Malaria Journal 2011;10:148‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishna 1995a {published data only}
- Krishna S, Supanaranond W, Pukrittayakamee S, ter Kuile F, Supputamangkol Y, Attatamsoonthorn K, et al. Fever in uncomplicated Plasmodium falciparum infection: effects of quinine and paracetamol. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(2):197‐9. [DOI] [PubMed] [Google Scholar]
Krishna 1995b {published data only}
- Krishna S, Pukrittayakamee S, Supanaranond W, ter Kuile F, Ruprah M, Sura T, et al. Fever in uncomplicated Plasmodium falciparum malaria: randomized double‐blind comparison of ibuprofen and paracetamol treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(5):507‐9. [DOI] [PubMed] [Google Scholar]
Krudsood 2010 {published data only (unpublished sought but not used)}
- Krudsood S, Tangpukdee N, Wilairatana P, Pothipak N, Duangdee C, Warrell DA, et al. Intravenous ibuprofen (IV‐ibuprofen) controls fever effectively in adults with acute uncomplicated Plasmodium falciparum malaria but prolongs parasitemia. American Journal of Tropical Medicine and Hygiene 2010;83(1):51‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lell 2001 {published data only}
- Lell B, Sovric M, Schmid D, Luckner D, Herbich K, Long HY, et al. Effect of antipyretic drugs in children with malaria. Clinical Infectious Diseases 2001;32(5):838‐41. [DOI] [PubMed] [Google Scholar]
Matsegui 2008 {published data only}
- Matsiegui PB, Missinou MA, Necek M, Mavoungou E, Issifou S, Lell B, et al. Antipyretic effect of ibuprofen in Gabonese children with uncomplicated falciparum malaria: a randomized, double‐blind, placebo‐controlled trial. Malaria Journal 2008;7:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1993 {published data only}
- Walker O, Salako LA, Sowumi A, Olupitan SB, Oyewo EA. Parenteral piroxicam in the management of fever, arthralgia and musculoskeletal conditions of acute malaria: an open randomised comparison with oral acetysalicylic acid and paracetamol. Nigerian Medical Practioner 1993;25(4):39‐42. [Google Scholar]
References to studies excluded from this review
Agbolosu 1997 {published data only}
- Agbolosu NB, Cuevas LE, Milligan P, Broadhead RL, Brewster D, Graham SM. Efficacy of tepid sponging versus paracetamol in reducing temperature in febrile children. Annals of Tropical Paediatrics 1997;17(3):283‐8. [DOI] [PubMed] [Google Scholar]
Aksoylar 1997 {published data only}
- Aksoylar S, Aksit S, Caglayan S, Yaprak I, Bakiler R, Cetin F. Evaluation of sponging and antipyretic medication to reduce body temperature in febrile children. Acta Paediatrica Japonica 1997;39(2):215‐7. [DOI] [PubMed] [Google Scholar]
Fasan 1980 {published data only}
- Fasan PO, Mabadeje AFB. A controlled trial of a combination of chloroquine with paracetamol in the treatment of acute malaria in a semi‐immune population. Journal of Tropical Medicine and Hygiene 1980;83(5):191‐3. [PubMed] [Google Scholar]
Ismail 1995 {published data only}
- Ismail S, Na Bangchang K, Karbwang J, Back DJ, Edwards G. Paracetamol disposition in Thai patients during and after treatment of falciparum malaria. European Journal of Clinical Pharmacology 1995;48(1):65‐9. [DOI] [PubMed] [Google Scholar]
Kramer 1991 {published data only}
- Kramer MS, Naimark LE, Roberts‐Brauer R, McDougall A, Leduc DG. Risks and benefits of paracetamol antipyresis in young children with fever of presumed viral origin. Lancet 1991;337(8741):591‐4. [DOI] [PubMed] [Google Scholar]
Mahar 1994 {published data only}
- Mahar AF, Allen SJ, Milligan P, Suthumnirund S, Chotpitayasunondh T, Sabchareon A, et al. Tepid sponging to reduce temperature in febrile children in a tropical climate. Clinical Pediatrics 1994;33(4):227‐31. [DOI] [PubMed] [Google Scholar]
Newman 1985 {published data only}
- Newman J. Evaluation of sponging to reduce body temperature in febrile children. Canadian Medical Association Journal 1985;132(6):641‐2. [PMC free article] [PubMed] [Google Scholar]
Nwanyanwu 1999 {published data only}
- Nwanyanwu OC, Ziba C, Kazembe PN. Paracetamol and ibuprofen for treatment of fever in Malawian children aged less than five years. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93(1):84. [DOI] [PubMed] [Google Scholar]
Sharber 1997 {published data only}
- Sharber J. The efficacy of tepid sponge bathing to reduce fever in young children. American Journal of Emergency Medicine 1997;15(2):188‐92. [DOI] [PubMed] [Google Scholar]
Steele 1970 {published data only}
- Steele RW, Tanaka PT, Lara RP, Bass JW. Evaluation of sponging and oral antipyretic therapy to reduce fever. Journal of Pediatrics 1970;77(5):824‐9. [DOI] [PubMed] [Google Scholar]
Tarimo 2002 {published data only}
- Tarimo DS, Minjas JN, Bygbjerg IC. Sulfadoxine‐pyeimethamine monotherapy in Tanzanian children gives rapid parasite clearance but slower fever clearance that is improved by chloroquine in combination therapy. Tropcal Medicine & International Health 2002;7(7):592‐8. [DOI] [PubMed] [Google Scholar]
Thomas 2009 {published data only}
- Thomas S, Vijaykumar C, Naik R, Moses PD, Antonisamy B. Comparative effectiveness of tepid sponging and antipyretic drug versus only antipyretic drug in the management of fever among children: a randomized controlled trial. Indian Pediatrics 2009;46(2):133‐6. [PubMed] [Google Scholar]
Additional references
Dodoo 2002
- Dodoo D, Omer FM, Todd J, Akanmori BD, Koram KA, Riley EM. Absolute levels and ratios of proinflammatory and anti‐inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. The Journal of Infectious Disease 2002;185(7):971‐9. [DOI] [PubMed] [Google Scholar]
Hayward 1999
- Hayward J, Veale B. Paracetamol for fever in children: high time for systematic reviews of the evidence. Australian Family Physician 1999;28(2):105. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. [Google Scholar]
Long 2001
- Long HY, Lell B, Dietz K, Kremsner PG. Plasmodium falciparum: in vitro growth inhibition by febrile temperatures. Parasitology Research 2001;87(7):553‐5. [DOI] [PubMed] [Google Scholar]
Meremikwu 2002
- Meremikwu M, Oyo‐Ita AE. Paracetamol versus placebo or physical methods for treating fever in children. Cochrane Database of Systematic Reviews 2002, (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
Meremikwu 2003
- Meremikwu MM, Oyo‐Ita A. Physical methods versus drug placebo or no treatment for managing fever in children. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mordmüller 1997
- Mordmüller BG, Metzger WG, Juillard P, Brinkman BM, Verweij CL, Grau GE, et al. Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. European Cytokine Network 1997;8(1):29‐35. [PubMed] [Google Scholar]
Prescott 2000
- Prescott LF. Paracetamol: past, present, and future. American Journal of Therapeutics 2000;7(2):143‐7. [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Strengell 2009
- Strengell T, Uhari M, Tarka R, Uuisimaa J, Alen R, Lautala P, et al. Antipyretic agents for preventing recurrences of febrile seizures: randomized controlled trial. Archives of Pediatrics and Adolescent Medicine 2009;163(9):799‐804. [DOI] [PubMed] [Google Scholar]
Waruiru 1996
- Waruiru CM, Newton CR, Foster D, New L, Winstanley P, Mwangi I, et al. Epileptic seizures and malaria in Kenyan children. Transactions of the Royal Society of Tropical Medicine and Hygiene 1996;90(2):152‐5. [DOI] [PubMed] [Google Scholar]
Waruiru 2004
- Waruiru C, Appleton R. Febrile seizures: an update. Archives of Disease in Childhood 2004;89(8):751‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Warwick 2008
- Warwick C. Paracetamol and fever management. Journal of the Royal Society for the Promotion of Health 2008;128(6):320‐3. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Use of antipyretics. In: Guidelines for the treatment of malaria. Geneva: World Health Organization 2006:27‐8.
WHO 2008
- World Health Organization. Estimated burden of malaria in 2006. In: World Malaria Report 2008. Geneva:World Health Organization 2008:9‐15.
References to other published versions of this review
Meremikwu 2000
- Meremikwu MM, Logan K, Garner P. Antipyretic measures for treating fever in malaria. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI] [PubMed] [Google Scholar]


