Abstract
Background
The World Health Organization recommends uncomplicated P. falciparum malaria is treated using Artemisinin‐based Combination Therapy (ACT). This review aims to assist the decision making of malaria control programmes by providing an overview of the relative benefits and harms of the available options.
Objectives
To compare the effects of ACTs with other available ACT and non‐ACT combinations for treating uncomplicated P. falciparum malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE; EMBASE; LILACS, and the metaRegister of Controlled Trials (mRCT) to March 2009.
Selection criteria
Randomized head to head trials of ACTs in uncomplicated P. falciparum malaria.
This review is limited to: dihydroartemisinin‐piperaquine; artesunate plus mefloquine; artemether‐lumefantrine (six doses); artesunate plus amodiaquine; artesunate plus sulfadoxine‐pyrimethamine and amodiaquine plus sulfadoxine‐pyrimethamine.
Data collection and analysis
Two authors independently assessed trials for eligibility and risk of bias, and extracted data. We analysed primary outcomes in line with the WHO 'Protocol for assessing and monitoring antimalarial drug efficacy' and compared drugs using risk ratios (RR) and 95% confidence intervals (CI). Secondary outcomes were effects on P. vivax, gametocytes, haemoglobin, and adverse events.
Main results
Fifty studies met the inclusion criteria. All five ACTs achieved PCR adjusted failure rates of < 10%, in line with WHO recommendations, at most study sites.
Dihydroartemisinin‐piperaquine performed well compared to the ACTs in current use (PCR adjusted treatment failure versus artesunate plus mefloquine in Asia; RR 0.39, 95% CI 0.19 to 0.79; three trials, 1062 participants; versus artemether‐lumefantrine in Africa; RR 0.39, 95% CI 0.24 to 0.64; three trials, 1136 participants).
ACTs were superior to amodiaquine plus sulfadoxine‐pyrimethamine in East Africa (PCR adjusted treatment failure versus artemether‐lumefantrine; RR 0.12, 95% CI 0.06 to 0.24; two trials, 618 participants; versus AS+AQ; RR 0.44, 95% CI 0.22 to 0.89; three trials, 1515 participants).
Dihydroartemisinin‐piperaquine (RR 0.32, 95% CI 0.24 to 0.43; four trials, 1442 participants) and artesunate plus mefloquine (RR 0.30, 95% CI 0.21 to 0.41; four trials, 1003 participants) were more effective than artemether‐lumefantrine at reducing the incidence of P.vivax over 42 days follow up.
Authors' conclusions
Dihydroartemisinin‐piperaquine is another effective first‐line treatment for P. falciparum malaria.
The performance of the non‐ACT (amodiaquine plus sulfadoxine‐pyrimethamine) falls below WHO recommendations for first‐line therapy in parts of Africa.
In areas where primaquine is not being used for radical cure of P. vivax, ACTs with long half‐lives may provide some benefit.
23 April 2019
No update planned
Review superseded
Please refer to the Cochrane Special Collection: Sinclair 2014 https://doi.org/10.1002/14651858.SC000007/full
Plain language summary
Artemisinin‐based combination treatments for uncomplicated malaria
Malaria is a major cause of illness and death in many of the world's poorest countries. It is spread from person to person by the bite of mosquitoes infected with a microorganism called Plasmodium. The Plasmodium species P. falciparum is the most common cause of malaria worldwide and causes the majority of deaths. Uncomplicated malaria is the mild form of the disease which, if left untreated, can progress rapidly to become life threatening. The drugs traditionally used to treat uncomplicated malaria have become ineffective in many parts of the world due to the development of drug resistance.
The World Health Organization now recommends Artemisinin‐based Combination Therapy (ACTs) for treating uncomplicated malaria. The ACTs combine an artemisinin‐derivative (a relatively new group of drugs which are very effective) with another longer‐lasting drug to try and reduce the risk of further resistance developing.
This review summarizes the relative benefits and harms of the four ACTs in common use, one relatively new ACT (dihydroartemisinin plus piperaquine), and one combination which does not contain an artemisinin derivative but remains in use in some African countries (amodiaquine plus sulfadoxine‐pyrimethamine).
All five ACTs were shown to be highly effective at treating P. falciparum in most places where they have been studied. However, there were several trials where ACTs had high levels of treatment failure, which emphasises the need to continue to monitor their performance.
The new ACT, dihydroartemisinin plus piperaquine, was shown to be at least as effective as the ACTs currently in widespread use in Asia and Africa, and represents another option for malaria treatment.
ACTs were shown to be more effective than amodiaquine plus sulfadoxine‐pyrimethamine in countries from East Africa which probably represents high levels of resistance, to both drugs in this combination, in this region.
The second most common form of malaria, P. vivax, can also be treated with ACTs but requires additional treatment to cure the patient completely. This is because the P. vivax parasite can lie dormant in the liver for months or years before becoming active again. ACTs where the partner drug has a long duration of action may help to delay these relapses.
The ACTs seem to be relatively safe with few serious side effects. Minor side effects are more common but can be difficult to distinguish from the symptoms of malaria itself. Fifty trials were included in this review but did not include the most vulnerable populations; pregnant women and young infants (age < six months).
Background
Malaria is a disease of global public health importance. Its social and economic burden is a major obstacle to human development in many of the world's poorest countries. In heavily affected countries, malaria alone accounts for as much as 40% of public health expenditure, 30% to 50% of hospital admissions, and up to 60% of outpatient visits (WHO 2007). It has an annual incidence of approximately 250 million episodes and is the cause of more than a million deaths, most of them in infants, young children, and pregnant women (WHO 2008b).
Malaria is transmitted from person to person by the bite of mosquitoes infected with the protozoan parasite Plasmodium. Four Plasmodium species are capable of causing malaria in humans: P. falciparum, P. vivax, P. malariae, and P. ovale. Of these P. falciparum is responsible for over 90% of cases and almost all of the malaria deaths worldwide (WHO 2008b). P. vivax is also common and often presents as a co‐infection with P. falciparum in a single illness (Mayxay 2004). Uncomplicated malaria is the mild form of the disease which presents as a febrile illness with headache, tiredness, muscle pains, abdominal pains, rigors (severe shivering), and nausea and vomiting. If left untreated P. falciparum malaria can rapidly develop into severe malaria with anaemia (low haemoglobin in the blood), hypoglycaemia (low blood sugar), renal failure (kidney failure), pulmonary oedema (fluid in the lungs), convulsions (fitting), coma, and eventually death (WHO 2006). A clinical diagnosis of malaria can be confirmed by detection of the malaria parasite in the patient's blood. This has traditionally been done by light microscopy but increasingly rapid diagnostic tests are being used.
Resistance of P. falciparum to the traditional antimalarial drugs (such as chloroquine, sulfadoxine‐pyrimethamine, amodiaquine, and mefloquine) is a growing problem and is thought to have contributed to increased malaria mortality in recent years (WHO 2006). Chloroquine resistance has now been documented in all regions except Central America and the Caribbean. There is high‐level resistance to sulfadoxine‐pyrimethamine throughout South East Asia and increasingly in Africa. Mefloquine resistance is common in the border areas of Cambodia, Myanmar, and Thailand, but uncommon elsewhere. Resistance of P. vivax to sulfadoxine‐pyrimethamine is also increasing, and chloroquine resistance has been reported in some parts of Asia and Oceania (WHO 2006).
Artemisinin‐based antimalarials
Artemisinin and its derivatives (such as artesunate, artemether, and dihydroartemisinin) are antimalarial drugs with a unique structure and mode of action. The first published report of clinical trials appeared in the Chinese Medical Journal in 1979 (Qinghaosu 1979). Until recently there had been no reported resistance to the artemisinin derivatives; however the possibility of emerging resistance, on the Thai‐Cambodian border, is currently being investigated (WHO 2008a).
Artemisinin derivatives have been shown to produce faster relief of clinical symptoms and faster clearance of parasites from the blood than other antimalarial drugs (McIntosh 1999; Adjuik 2004; WHO 2006). When used as monotherapy, the short half‐life of the artemisinin derivatives (and rapid elimination from the blood) means that patients must take the drug for at least seven days (Meshnick 1996; Adjuik 2004). Failure to complete the course, due to the rapid improvement in clinical symptoms, can lead to high levels of treatment failure even in the absence of drug resistance. Artemisinin derivatives are therefore usually given with another longer‐acting drug, with a different mode of action, in a combination known as artemisinin‐based combination therapy or ACT. These combinations can then be taken for shorter durations than artemisinin alone (White 1999; WHO 2006).
The artemisinin derivatives also reduce the development of gametocytes (the sexual form of the malaria parasite that is capable of infecting mosquitoes) and consequently the carriage of gametocytes in the peripheral blood (Price 1996; Targett 2001). This reduction in infectivity has the potential to reduce the post‐treatment transmission of malaria (particularly in areas of low or seasonal transmission), which may have significant public health benefits (WHO 2006).
Artemisinin and its derivatives are generally reported as being safe and well tolerated, and the safety profile of ACTs may be largely determined by the partner drug (WHO 2006; Nosten 2007). Studies of artemisinin derivatives in animals have reported significant neurotoxicity (brain damage), but this has not been seen in human studies (Price 1999). Animal studies have also shown adverse effects on the early development of the fetus, but the artemisinin derivatives have not been fully evaluated during early pregnancy in humans (Nosten 2007). Other reported adverse events include gastrointestinal (GI) disturbance (stomach upset), dizziness, tinnitus (ringing in the ears), neutropenia (low levels of white blood cells), elevated liver enzymes (a marker for liver damage), and electrocardiographic (ECG) abnormalities (changes in cardiac conduction). Most studies however, have found no evidence of ECG changes, and only non‐significant changes in liver enzymes (WHO 2006; Nosten 2007). The incidence of type 1 hypersensitivity (allergic) reactions is reported to be approximately 1 in 3000 patients (Nosten 2007).
Assessing antimalarial efficacy
The World Health Organization (WHO) recommends that first‐line antimalarials should have a treatment failure rate of less than 10%, and failure rates higher than this should trigger a change in treatment policy (WHO 2006). Treatment failure can be classified as:
Early treatment failure:
the development of danger signs or severe malaria on days one, two, three in the presence of parasitaemia;
parasitaemia on day two higher than on day 0;
parasitaemia and axillary temperature > 37.5 °C on day three;
parasitaemia on day three > 20% of count on day 0.
or late treatment failure:
development of danger signs, or severe malaria, after day three with parasitaemia;
presence of P. falciparum parasitaemia and axillary temperature > 37.5 °C on or after day four;
presence of P. falciparum parasitaemia after day seven.
The late reappearance of P. falciparum parasites in the blood can be due to failure of the drug to completely clear the original parasite (a recrudescence) or due to a new infection, which is especially common in areas of high transmission. A molecular genotyping technique called polymerase chain reaction (PCR) can be used in clinical trials to distinguish between recrudescence and new infection, giving a clearer picture of the efficacy of the drug and its post‐treatment prophylactic effect (White 2002; Cattamanchi 2003).
The WHO recommends a minimum follow‐up period of 28 days for antimalarial efficacy trials, but longer periods of follow up may be required for antimalarials with long elimination half‐lives (White 2002; WHO 2003). This is because treatment failure due to true recrudescence of malaria parasites may be delayed until the drug concentration falls below the minimum concentration required to inhibit parasite multiplication, which may be beyond 28 days. The WHO recommends 42 days follow up for trials involving lumefantrine and 63 days for trials of mefloquine (WHO 2003).
P. vivax malaria
P. vivax differs from P. falciparum in generally producing a milder illness and in having a liver stage known as a hypnozoite. These hypnozoites can lie dormant in the liver following an acute infection and cause spontaneous relapses at later dates.
As P. vivax often co‐exists with P. falciparum in a single illness, it is important to assess the effect of ACTs on the P. vivax parasite (Mayxay 2004; WHO 2006). ACTs have been shown to clear P. vivax from the peripheral blood, but they do not have a substantial effect on the liver stage of the parasite (Pukrittayakamee 2000). Although ACTs cannot provide a radical cure for P. vivax, their ability to delay the eventual relapse of P. vivax and provide a prolonged malaria free period may produce significant public health benefits.
It is important to note that when P. vivax parasitaemia occurs following initial treatment, PCR is unable to distinguish a recrudescence of the original infection (due to failure to clear the parasite from the peripheral blood) from a spontaneous relapse (due to failure to clear the liver stage) (WHO 2006).
Choice of combination treatment
The WHO now recommends that P. falciparum malaria is always treated using a combination of two drugs that act at different biochemical sites within the parasite (WHO 2006). If a parasite mutation producing resistance arises spontaneously during treatment, the parasite should then be killed by the partner drug, thereby reducing or delaying the development of resistance to the artemisinin derivatives, and increasing the useful lifetime of the individual drugs (White 1996; White 1999; WHO 2006). This policy emerged at the time when ACTs were primarily being considered, but other possibilities such as amodiaquine combined with sulfadoxine‐pyrimethamine (non‐ACTs) are also available.
The decision of which ACT to adopt into national malaria control programmes has been based on a combination of research and expert opinion. Systematic reviews can contribute to this decision by providing evidence on the:
relative effects on cure between combinations;
absolute cure levels achieved by a drug in a particular region;
safety and risk of adverse effects of the combination;
impact on gametocytes;
impact on haemoglobin levels; and
relative effects on P. vivax.
Other information that is also important to decision‐making include:
the appropriateness of the partner drug within a locality, based on informed judgements related to regional and national overviews of drug resistance and the intensity of malaria transmission;
the simplicity of the treatment regimen (co‐formulated products are generally preferred as they reduce the availability and use of monotherapy, which may in turn reduce the development of resistance);
the cost (since the ACT is likely to represent a large percentage of the annual health expenditure in highly endemic countries); and
other concerns such as fetal toxicity and teratogenicity.
To contribute to informed decision‐making, we have examined the comparative effects of ACTs for which co‐formulated products are currently available or shortly to be made available. We have included trials that have used co‐packaged or loose preparations of these same ACTs to provide information on relative effects of the different treatment options. While recent Cochrane Reviews have synthesized the evidence around individual ACT comparisons (Bukirwa 2005; Omari 2005; Bukirwa 2006; Omari 2006), this review broadens the inclusion criteria and pools the data into a single Cochrane Review. A comprehensive list of the available drugs and the treatment comparisons that have been assessed is shown in Appendix 1. The data are presented in answer to four questions:
How does dihydroartemisinin‐piperaquine (DHA‐P) perform?
How does artesunate‐mefloquine (AS+MQ) perform?
How does artemether‐lumefantrine (AL6) perform?
How does artesunate plus amodiaquine (AS+AQ) perform?
The comparison drugs were any of the above plus artesunate plus sulfadoxine‐pyrimethamine (AS+SP) and amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP).
Objectives
To compare the effects of ACTs with other available ACT and non‐ACT combinations for treating uncomplicated P. falciparum malaria.
A secondary objective was to explore the effects of the combinations on P. vivax infection.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials. Quasi‐randomized studies were excluded.
Types of participants
Adults and children (including pregnant women and infants) with symptomatic, microscopically confirmed, uncomplicated P. falciparum malaria.
Trials that included participants with P. vivax co‐infection and mono‐infection were also eligible.
Types of interventions
Intervention
Three‐day course of an ACT (fixed dosed, co‐blistered, or individually packaged (loose)).
Control
Three‐day course of an alternative ACT or non‐artemisinin combination treatment (amodiaquine plus sulfadoxine‐pyrimethamine).
The specific ACTs included are: dihydroartemisinin‐piperaquine; artesunate plus mefloquine; artemether‐lumefantrine (six doses); artesunate plus amodiaquine and artesunate plus sulfadoxine‐pyrimethamine (Appendix 1).
Types of outcome measures
Primary outcomes
Total failure at days 28, 42, and 63; PCR‐adjusted and PCR‐unadjusted.
Secondary outcomes
P. vivax parasitaemia at day 28, 42, or 63 (all participants).
P. vivax parasitaemia at day 28, 42, or 63 (only participants with P. vivax at baseline).
Gametocyte carriage at day 7 or 14 (preference for day 14 in data analysis).
Gametocyte development (negative at baseline, and positive at follow up).
Change in haemoglobin from baseline (minimum 28 day follow up).
Adverse events
Deaths occurring during follow up.
Serious adverse events (life threatening, causing admission to hospital, or discontinuation of treatment).
Haematological and biochemical adverse effects (e.g. neutropenia, liver toxicity).
Early vomiting.
Other adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases using the search terms detailed in Appendix 2: Cochrane Infectious Diseases Group Specialized Register (March 2009); Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library (2009, issue 1); MEDLINE (1966 to March 2009); EMBASE (1974 to March 2009); and LILACS (1982 to March 2009). We also searched the metaRegister of Controlled Trials (mRCT) using 'malaria' and 'arte* OR dihydroarte*' as search terms (March 2009).
Searching other resources
We contacted individual researchers working in the field, organizations including the World Health Organization, and pharmaceutical companies (Atlantic, Guilin, Holleykin, HolleyPharm, Mepha, Novartis, Parke‐Davis, Pfizer, Sanofi‐Aventis, Roche) for information on unpublished trials (August 2008).
We also checked the reference lists of all trials identified by the methods described above.
Data collection and analysis
Selection of studies
David Sinclair (DS) and Babalwa Zani (BZ) reviewed the results of the literature search and obtained full‐text copies of all potentially relevant trials. DS scrutinized each trial report for evidence of multiple publications from the same data set. DS and BZ then independently assessed each trial for inclusion in this review using an eligibility form based on the inclusion criteria. We resolved any disagreements through discussion or, where necessary, by consultation with Paul Garner (PG). If clarification was necessary we attempted to contact the trial authors for further information. We have listed the trials that were deemed ineligible and the reasons for their exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
DS and BZ independently extracted data using a pre‐tested data extraction form. We extracted data on trial characteristics including methods, participants, interventions, and outcomes as well as data on dose and drug ratios of the combinations.
We extracted the number randomized and the number analysed in each treatment group for each outcome. We calculated and reported the loss to follow up in each group.
For dichotomous outcomes, we recorded the number of participants experiencing the event and the number of participants in each treatment group. For continuous outcomes, we extracted the arithmetic means and standard deviations for each treatment group together with the numbers of participants in each group. If the data were reported using geometric means, we recorded this information and extracted standard deviations on the log scale. If medians were extracted we also extracted ranges.
Primary outcome
The primary analysis drew on the WHO's protocol for assessing and monitoring antimalarial drug efficacy (WHO 2003). This protocol has been used to guide most efficacy trials since its publication in 2003, even though it was designed to assess the level of antimalarial resistance in the study area rather than for comparative trials. As a consequence a high number of randomized participants are excluded from the final efficacy outcome as losses to follow up or voluntary or involuntary withdrawals. For this reason we conducted a sensitivity analysis which aimed to restore the integrity of the randomization process (as is usual in trial analysis) and test the robustness of the results to this methodology. (For a summary of the methodology and sensitivity analysis see Appendix 3)
PCR‐unadjusted total failure
PCR‐unadjusted total failure (P. falciparum) was calculated as the sum of early treatment failures and late treatment failures (without PCR adjustment). The denominator excludes participants for whom an outcome was not available (e.g. those who were lost to follow up, withdrew consent, took other antimalarials, or failed to complete treatment) and those participants who were found not to fulfil the inclusion criteria after randomization.
PCR‐adjusted total failure
PCR‐adjusted total failure (P. falciparum) was calculated as the sum of early treatment failures, and late treatment failures due to PCR‐confirmed recrudescence. Participants with indeterminate PCR results, missing PCR results, or PCR‐confirmed new infections were treated as involuntary withdrawals and excluded from the calculation. Late treatment failures that occurred between days 4 and 14 were assumed to be recrudescences of the original parasite without the need for PCR genotyping (unless genotyped in the trial). The denominator excludes participants for whom an outcome was not available (e.g. those who were lost to follow up, withdrew consent, took other antimalarials, or failed to complete treatment) and those participants who were found not to fulfil the inclusion criteria after randomization.
These primary outcomes relate solely to failure due to P. falciparum. For both PCR‐unadjusted and PCR‐adjusted total failure, participants who experienced P. vivax during follow up were retained in the calculation if they were treated with chloroquine and continued in follow up. As long as they did not go on to develop P. falciparum parasitaemia they were classified as treatment successes. We excluded from the calculation those participants who experienced P. vivax and were removed from the trial's follow up at the time of P. vivax parasitaemia.
It was not always possible to guarantee that individual trials used the standard WHO definitions. We have accepted the trial authors' data unless we had specific reason to reclassify an individual participant or reject the data. Where this has been done we have stated clearly the reasons for doing so.
Secondary outcomes and adverse events
In a secondary analysis we examined the effects of ACTs on P. vivax. We have reported the incidence of P. vivax parasitaemia during follow up at days 28, 42, and 63. Where possible, we have stratified this analysis into participants who had P. vivax co‐infection at baseline and those negative for P. vivax at baseline.
Extracting data on gametocyte carriage was difficult due to the variety of ways that these data are presented in individual papers. In order to try to present useful data we contacted the lead author of all trials that reported on gametocytes for additional information which fitted our specified outcomes.
Haematological outcomes were also presented in a multitude of ways which prevented meta‐analysis. We have therefore presented these data as a narrative summary with forest plots where possible.
Other secondary outcomes have been presented using forest plots, tables, or narrative summaries as appropriate.
We extracted the number of serious adverse events and deaths and have presented these data in a forest plot. We have only included those trials that specifically report serious adverse events.
Data on early vomiting were extracted as a measure of tolerability of these combinations, and are presented as a forest plot. Other adverse events are presented in tables with a narrative summary.
Assessment of risk of bias in included studies
DS and BZ independently assessed the risk of bias for each trial using 'The Cochrane Collaboration's tool for assessing the risk of bias' (Higgins 2008). Differences of opinion were discussed with PG. We followed the guidance to assess whether adequate steps had been taken to reduce the risk of bias across six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias. We have categorized these judgments as 'yes' (low risk of bias), 'no' (high risk of bias), or 'unclear'. Where our judgement is unclear we attempted to contact the trial authors for clarification.
This information was used to guide the interpretation of the data that are presented.
Measures of treatment effect
We analysed the data using Review Manager 5. Dichotomous data are presented and combined using risk ratios. For continuous data summarized by arithmetic means and standard deviations, data have been combined using mean differences. Risk ratios and mean differences are accompanied by 95% confidence intervals. Medians and ranges are only reported in tables.
Dealing with missing data
If data from the trial reports were insufficient, unclear, or missing, we attempted to contact the trial authors for additional information. If we judged the missing data to render the result uninterpretable we excluded the data from the meta‐analysis and clearly stated the reason. The potential effects of missing data have been explored through a series of sensitivity analyses (Appendix 3).
Assessment of heterogeneity
We assessed for heterogeneity amongst trials by inspecting the forest plots, applying the Chi² test with a 10% level of statistical significance, and also using the I² statistic with a value of 50% used to denote moderate levels of heterogeneity.
Data synthesis
The included trials have been given identity codes which include the first author, the year the study was conducted (not the year it was published) and the three‐letter international country code. Studies in forest plots are also listed in chronological order (by the final date of enrolment). We hope this will aid with interpretation of the review and forest plots.
Treatments have been compared directly using pair‐wise comparisons. For outcomes that are measured at different time points we have stratified the analysis by the time point. The primary outcome analysis is also stratified by geographical region as a crude marker for differences in transmission and resistance patterns.
Meta‐analysis has been performed within geographic regions where appropriate after assessment and investigation of heterogeneity. A random‐effects model was used where the Chi² test P value was less than 0.1 or the I² statistic was greater than 50%.
In addition, Olliaro‐Vaillant plots have been used to simultaneously display the absolute and relative benefits of individual ACTs at day 28.
Subgroup analysis and investigation of heterogeneity
We investigated potential sources of heterogeneity through the following subgroup analyses: geographical region, intensity of malaria transmission (low to moderate versus high malaria transmission), known parasite resistance, allocation concealment, participant age, and drug dose (comparing regimens where there are significant variations in drug dose).
Sensitivity analysis
We conducted a series of sensitivity analyses to investigate the robustness of the methodology used in the primary analysis. Our aim was to restore the integrity of the randomization process by adding excluded groups back into the analysis in a stepwise fashion (see Appendix 3 for details). Where these analyses altered the direction or significance of the measure of effect the revised results are presented and discussed.
Results
Description of studies
Results of the search
The search was conducted on 12 August 2008 and repeated on 26 March 2009. In total 517 trials were identified. Full text copies were obtained for 85 trials. Fifty trials are included in this review and 35 were excluded. A further four trials (Bousema 2004 KEN; Koram 2003 GHA; Martensson 2003 TZA; Van den Broek 2004 ZAR) were excluded from the primary analysis due to baseline differences between groups which had the potential to severely bias the result. These trials were retained for their data on adverse events.
Included studies
Forty‐six of the fifty included trials were conducted between 2003 and 2009.
Thirty‐one trials were conducted in Africa, 17 in Asia, one in South America (DHA‐P versus AS+MQ) and one in Oceania (DHA‐P versus AL6 versus AS+SP). There is obvious regional variability in which drugs are being studied. Trials from Asia mainly involve AS+MQ, AL6 and DHA‐P (plus one trial from Indonesia with AS+AQ). Only two studies from Africa have evaluated AS+MQ.
Pregnant and lactating women were excluded from all trials. The study population in Asian trials is older, with exclusion of children aged less than one year. African studies concentrated more on children and included those as young as six months.
Three trials (Hasugian 2005 IDN; Karunajeewa 2007 PNG; Ratcliff 2005 IDN) included participants with P. vivax mono‐infection at baseline. For our primary analysis we obtained data from the authors for only those participants who had P. falciparum or mixed infection (falciparum andvivax) at baseline.
One trial (Dorsey 2006 UGA) had an unusual study design where participants were followed up for more than one episode of malaria. For our primary analysis we obtained data from the authors for first episodes of malaria only.
The characteristics of the included studies are given in the 'Characteristics of included studies' table.
Excluded studies
The reasons for exclusion are given in the 'Characteristics of excluded studies' table.
The four additional studies excluded from the primary analysis had different inclusion criteria for different arms of the trial. Children aged less than one year were excluded from the AL6 treatment arm and reassigned to either AS+AQ or AS+SP. In these studies this led to significant baseline differences in age and weight, factors known to be associated with the outcomes. We explored the effects of including these trials in the largest meta‐analysis (AL6 versus AS+AQ, Analysis 9.9; Analysis 9.10). Inclusion of the trials with this bias shifted the results from no difference detected to favouring AL6. In the light of this we decided to exclude all trials that had systematically reallocated patients after randomization.
9.9. Analysis.
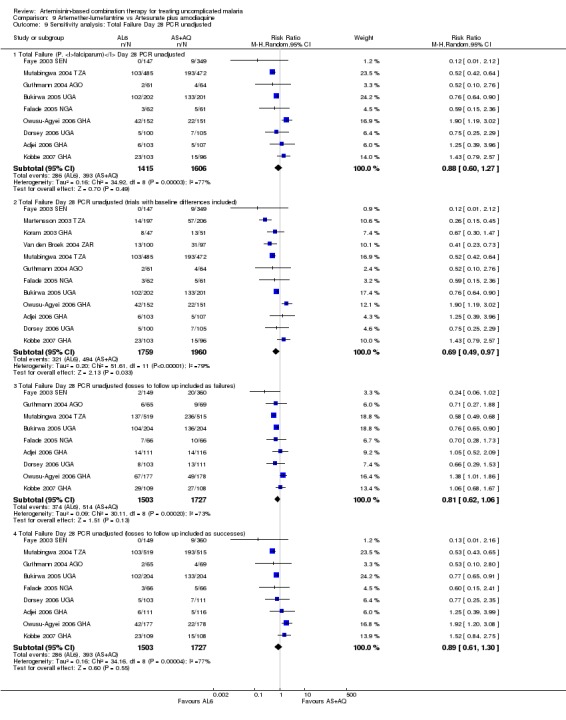
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 9 Sensitivity analysis: Total Failure Day 28 PCR unadjusted.
9.10. Analysis.
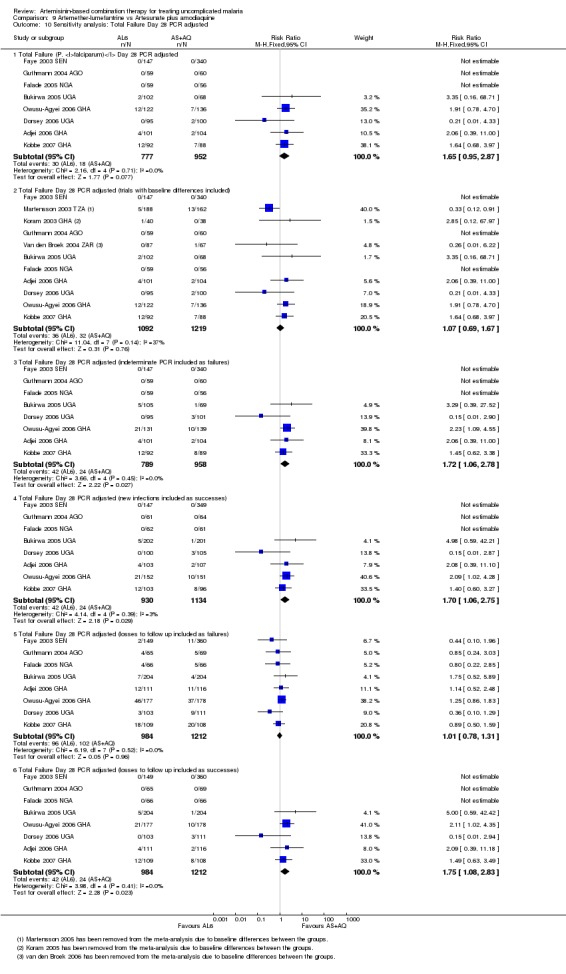
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 10 Sensitivity analysis: Total Failure Day 28 PCR adjusted.
Risk of bias in included studies
For a summary of the 'Risk of bias' assessments please see Figure 1 and Figure 2.
1.
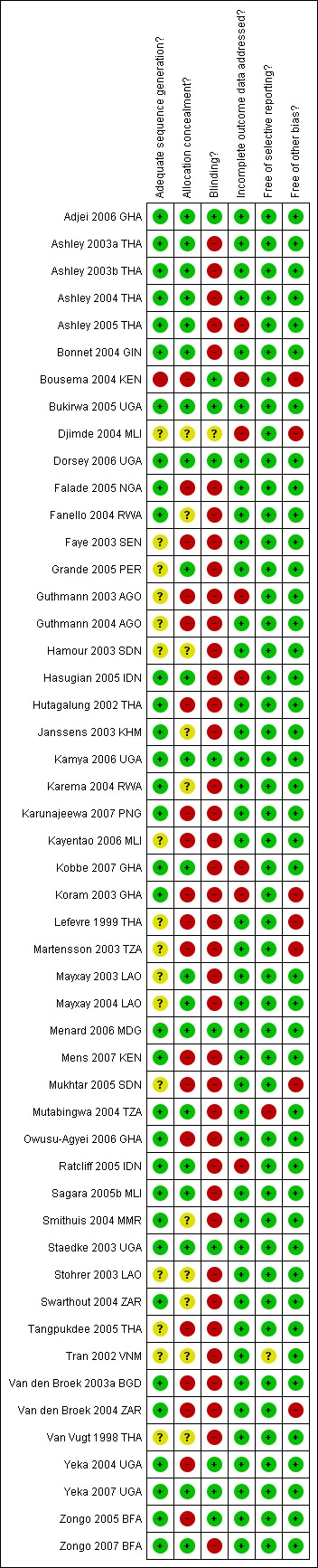
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
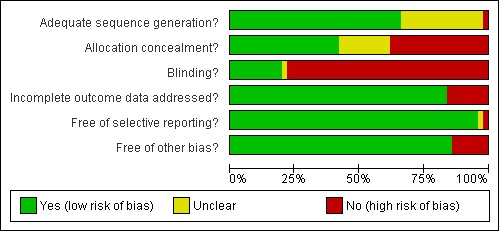
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Generation of the randomized sequence was judged to be at low risk of bias for 33 trials, high risk of bias for 1 trial, and 16 trials were unclear regarding randomization methods.
Allocation concealment was judged to be at low risk of bias in 21 studies, high risk of bias in 19 studies and unclear in 10 studies. Descriptions which included the following details were accepted as adequate for concealment: opaque sealed envelopes; sealed sequentially numbered envelopes; or third party allocation. For primary outcomes we conducted a sensitivity analysis including only the trials with adequate allocation concealment.
Blinding
Of the included trials only 10 were judged to be at low risk of bias due to adequate blinding. Blinding or quality control of laboratory staff was conducted in 34 studies. Although this may be reassuring with regard to parasitological outcomes, secondary outcomes and particularly adverse event reporting will remain at high risk of bias.
Incomplete outcome data
We have reported the proportion of participants in each treatment arm for whom an outcome was not available and conducted sensitivity analyses to test the possible effect of these losses. Eight trials were judged to be at high risk of bias due to either moderate drop‐out (> 15%), differential drop‐out between groups that had the potential to alter the result, or participants missing from the primary analysis who could not be accounted for.
Selective reporting
Due to the varying half‐lives of drugs, the choice of which day to measure outcomes can influence the comparative effects of the drugs. If a drug with a long half‐life (DHA‐P or AS+MQ) is compared to a drug with a short half‐life (AS+AQ or AS+SP), day 28 outcomes may underestimate PCR adjusted failure with the long half‐life drug. At later time points (day 42 and 63) drugs with long half‐lives are likely to appear superior in preventing new infections (PCR unadjusted failure) which represents a prophylactic effect. We have kept this in mind when interpreting the data but did not judge the trials to be at high risk of bias.
Other potential sources of bias
Pharmaceutical companies provided financial support or study drugs in 15 trials. Further involvement of the pharmaceutical company in trial design or reporting is only described in one study (Djimde 2004 MLI).
Effects of interventions
In April 2009 we conducted the sensitivity analysis as described in Table 3 to test the robustness of our methodology. In general these analyses did not substantially change the direction, magnitude, or confidence intervals of the estimate of effect. Examples are shown in Analysis 1.12 and Analysis 1.13. Only sensitivity analyses of interest remain linked in this review.
1.12. Analysis.
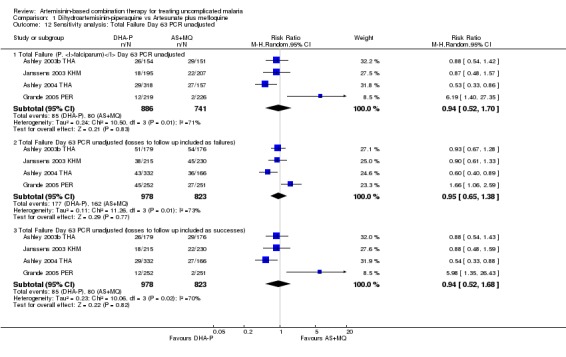
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 12 Sensitivity analysis: Total Failure Day 63 PCR unadjusted.
1.13. Analysis.
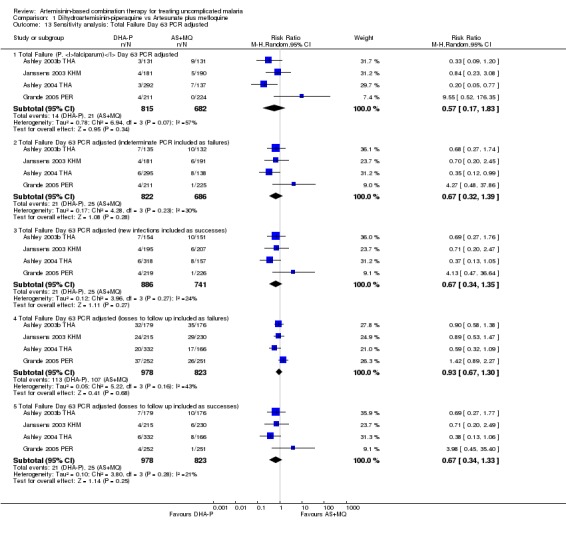
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 13 Sensitivity analysis: Total Failure Day 63 PCR adjusted.
Question 1. How does dihydroartemisinin‐piperaquine (DHA‐P) perform?
Dosing concerns
Two dosing regimens have been commonly used in clinical trials of DHA‐P. These two regimens give the same total dose, but divided into three or four doses, given over three days. One trial (Ashley 2004 THA) directly compared the three‐dose regimen with the four‐dose regimen and found no difference at any time point (one trial, 318 participants, Analysis 14.1, Analysis 14.2).
14.1. Analysis.
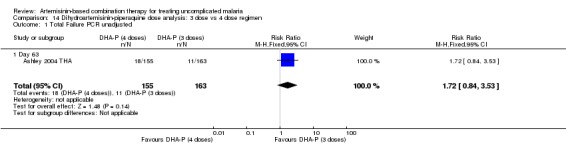
Comparison 14 Dihydroartemisinin‐piperaquine dose analysis: 3 dose vs 4 dose regimen, Outcome 1 Total Failure PCR unadjusted.
14.2. Analysis.
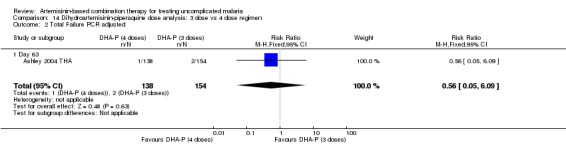
Comparison 14 Dihydroartemisinin‐piperaquine dose analysis: 3 dose vs 4 dose regimen, Outcome 2 Total Failure PCR adjusted.
In comparisons comparing DHA‐P to AS+MQ, four trials used the three‐dose regimen, three trials used the four‐dose regimen and one trial used both. Stratifying the analysis by dosing regimen did not reveal any significant differences in efficacy between the two regimens (Analysis 15.1; Analysis 15.2; Analysis 15.3; Analysis 15.4; Analysis 15.5; Analysis 15.6).
15.1. Analysis.
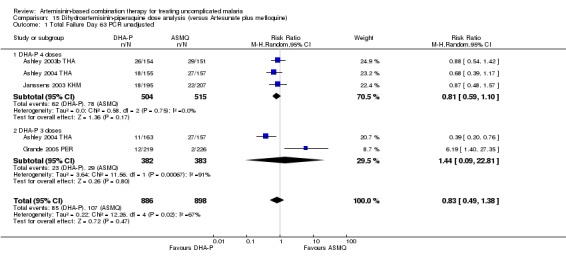
Comparison 15 Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine), Outcome 1 Total Failure Day 63 PCR unadjusted.
15.2. Analysis.
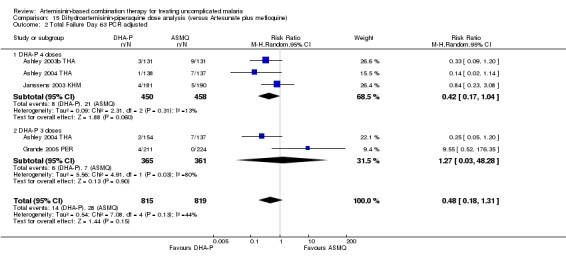
Comparison 15 Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine), Outcome 2 Total Failure Day 63 PCR adjusted.
15.3. Analysis.
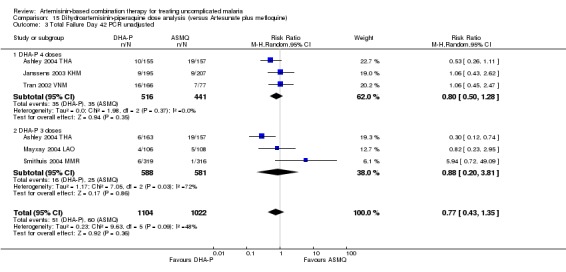
Comparison 15 Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine), Outcome 3 Total Failure Day 42 PCR unadjusted.
15.4. Analysis.
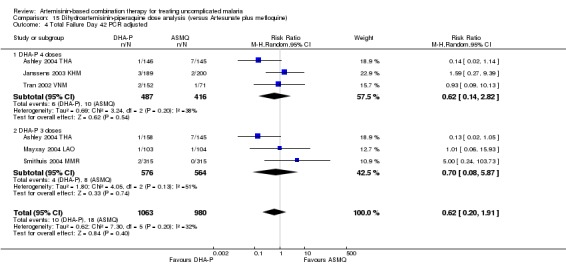
Comparison 15 Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine), Outcome 4 Total Failure Day 42 PCR adjusted.
15.5. Analysis.
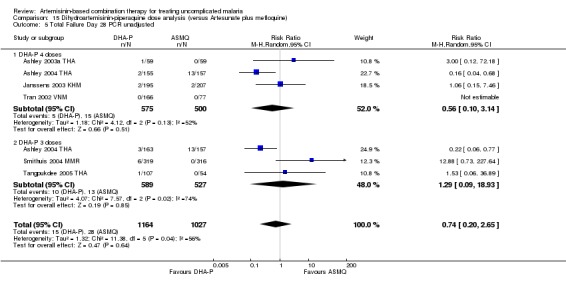
Comparison 15 Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine), Outcome 5 Total Failure Day 28 PCR unadjusted.
15.6. Analysis.
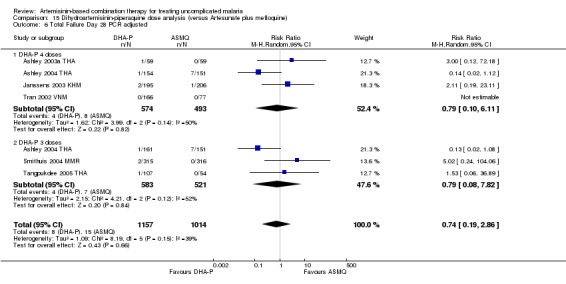
Comparison 15 Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine), Outcome 6 Total Failure Day 28 PCR adjusted.
Comparison 1. DHA‐P versus artesunate plus mefloquine
We found nine trials which assessed this comparison (eight in Asia and one in South America). Allocation concealment was assessed as 'low risk of bias' in five trials (Ashley 2003a THA; Ashley 2003b THA; Ashley 2004 THA; Grande 2005 PER; Mayxay 2004 LAO). Laboratory staff (outcome assessors) were blinded to treatment allocation in three trials (Ashley 2003a THA; Ashley 2003b THA; Ashley 2005 THA), and no other blinding is described.
Total failure
PCR adjusted treatment failure with DHA‐P was below 5% in all nine studies, and with AS+MQ in seven out of nine studies.
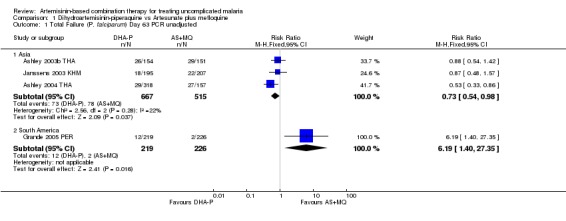
At day 63 comparative results were mixed. Trials from Asia favoured DHA‐P (Day 63, three trials, 1182 participants: PCR unadjusted RR 0.73, 95% CI 0.54 to 0.98, Analysis 1.1; PCR adjusted RR 0.39, 95% CI 0.19 to 0.79, Analysis 1.2) and the one trial from South America favoured AS+MQ (one trial, 445 participants: PCR unadjusted RR 6.19, 95% CI 1.40 to 27.35, Analysis 1.1; PCR adjusted no significant difference, Analysis 1.2). This difference may reflect the level of mefloquine resistance at the study sites. The performance of DHA‐P in the study in South America is similar to that in Asia, but the performance of AS+MQ was much improved with no PCR confirmed recrudescences.
1.1. Analysis.
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 1 Total Failure (P. falciparum) Day 63 PCR unadjusted.
1.2. Analysis.
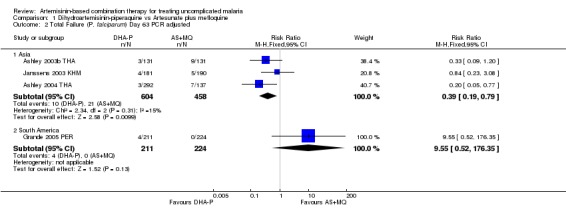
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 2 Total Failure (P. falciparum) Day 63 PCR adjusted.
No significant differences were shown at other time points (Day 42, five trials, 1969 participants, Analysis 1.3, Analysis 1.4; Day 28, six trials, 2034 participants, Analysis 1.5, Analysis 1.6).
1.3. Analysis.
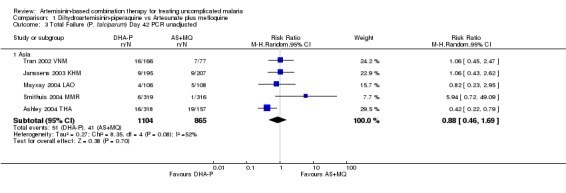
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 3 Total Failure (P. falciparum) Day 42 PCR unadjusted.
1.4. Analysis.
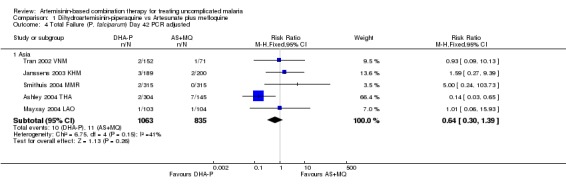
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 4 Total Failure (P. falciparum) Day 42 PCR adjusted.
1.5. Analysis.
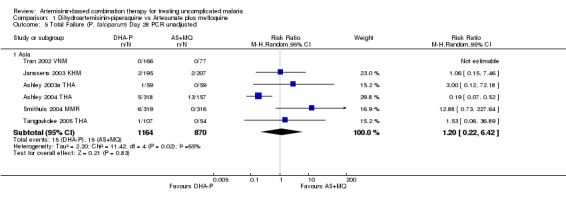
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 5 Total Failure (P. falciparum) Day 28 PCR unadjusted.
1.6. Analysis.
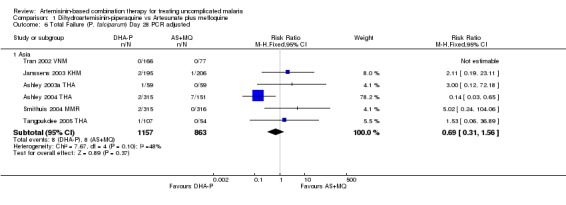
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 6 Total Failure (P. falciparum) Day 28 PCR adjusted.
P. vivax
No significant difference was shown in the incidence of P. vivax parasitaemia at any time point (Day 63, four trials, 1661 participants; Day 42, three trials, 1251 participants; Day 28, one trial, 402 participants; Analysis 1.7). There were no significant differences in the incidence of P. vivax between groups with or without P. vivax at baseline.
1.7. Analysis.
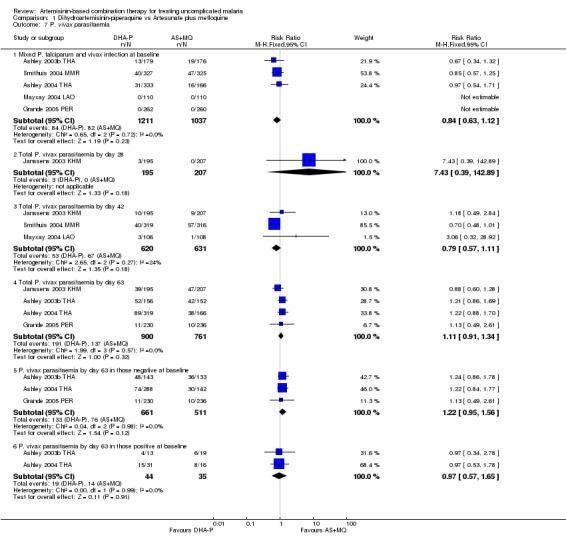
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 7 P. vivax parasitaemia.
Gametocytes
The number of participants who developed detectable gametocytes (after being negative at baseline) was low in both groups, but significantly lower with AS+MQ (three trials, 1234 participants: RR 3.06, 95% CI 1.13 to 8.33, Analysis 1.8). AS+MQ may also clear gametocytes quicker than DHA‐P but the analysis is confounded by differences in gametocyte carriage at baseline (two trials, 1174 participants, Analysis 1.9).
1.8. Analysis.
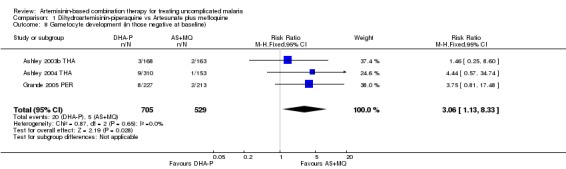
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 8 Gametocyte development (in those negative at baseline).
1.9. Analysis.
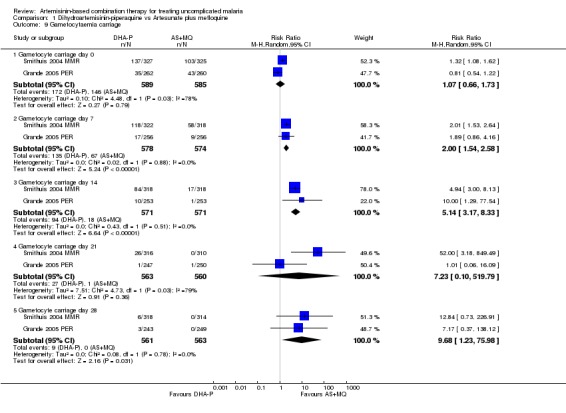
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 9 Gametocytaemia carriage.
Anaemia
Five trials report on haematological changes. Individual studies did not show significant differences between groups (see Appendix 5). Two trials (Ashley 2003b THA; Ashley 2004 THA) report a decrease in haematocrit over the first seven days followed by recovery in both groups (figures not reported).
Adverse events
No difference has been shown in the frequency of serious adverse events (seven trials, 2374 participants, Analysis 1.10).
1.10. Analysis.
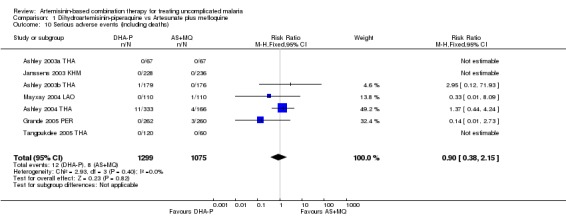
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 10 Serious adverse events (including deaths).
There is some evidence that DHA‐P is better tolerated than AS+MQ. Cental nervous system (CNS) related adverse events (at least one of sleep disturbance, dizziness, or anxiety) were reported as more common with AS+MQ in five out of the nine trials. Five trials also report significantly more nausea and vomiting with AS+MQ and two trials report more palpitations and dyspnoea. Abdominal pain and diarrhoea were reported as significantly more common with DHA‐P in one trial each. For a summary of adverse event findings see Appendix 4.
Early vomiting
Seven trials report some measure of early vomiting (vomiting related to drug administration) and no difference was shown in any trial (seven trials, 2473 participants, Analysis 1.11).
1.11. Analysis.
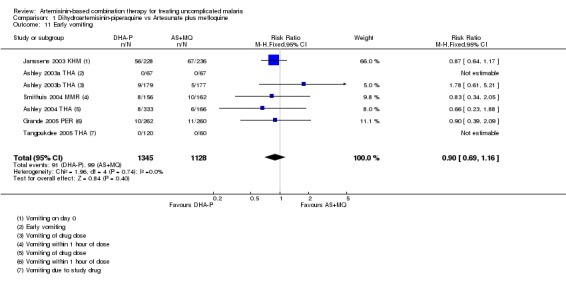
Comparison 1 Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine, Outcome 11 Early vomiting.
Comparison 2. DHA‐P versus artemether‐lumefantrine (six doses)
We found six trials (four in Africa, one in Asia and one in Oceania) which assessed this comparison. Allocation concealment was assessed as low risk of bias in four trials (Kamya 2006 UGA; Ratcliff 2005 IDN; Yeka 2007 UGA; Zongo 2007 BFA). Laboratory staff were blinded to treatment allocation in five out of six trials.
Total failure
PCR adjusted treatment failure with DHA‐P was below 5% in four out of six studies and with AL6 in two out of six studies. Of note, one trial from Africa (Kamya 2006 UGA) found PCR adjusted failure to be > 10% with both combinations.
In trials from Africa DHA‐P performed significantly better than AL6 at day 42 (three trials, 1136 participants: PCR unadjusted Heterogeneity: Chi² P < 0.0001, I² = 91%, Analysis 2.1; PCR adjusted RR 0.39, 95% CI 0.24 to 0.64, Analysis 2.2). Although there is substantial heterogeneity among PCR unadjusted results the direction of effect is consistently in favour of DHA‐P.
2.1. Analysis.
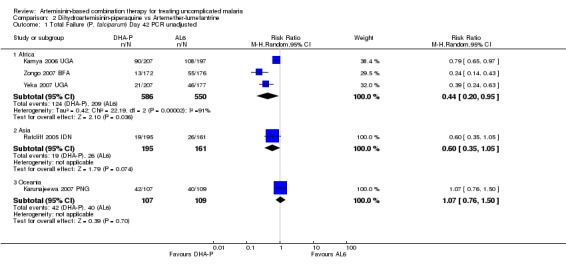
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 1 Total Failure (P. falciparum) Day 42 PCR unadjusted.
2.2. Analysis.
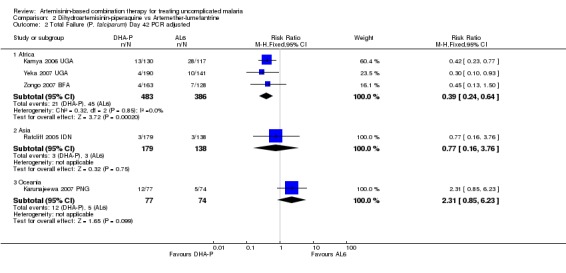
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 2 Total Failure (P. falciparum) Day 42 PCR adjusted.
In the one trial from Asia both drugs performed well with a non significant trend towards reduced re‐infections with DHA‐P (one trial, 356 participants, Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.3. Analysis.
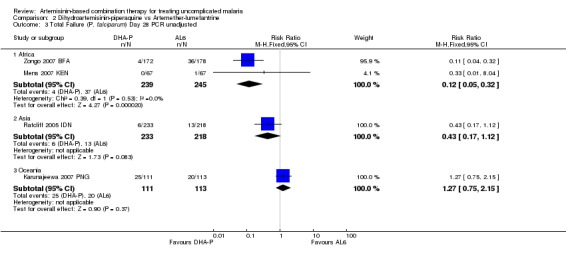
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 3 Total Failure (P. falciparum) Day 28 PCR unadjusted.
2.4. Analysis.
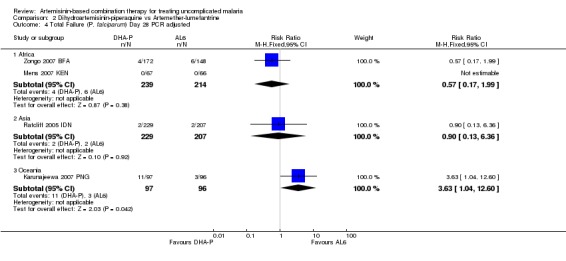
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 4 Total Failure (P. falciparum) Day 28 PCR adjusted.
In Oceania Karunajeewa 2007 PNG showed a reduction in PCR adjusted treatment failure at day 28 with AL6 but this effect was no longer significant at day 42 (one trial, 356 participants, Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4).
P. vivax
Participants treated with DHA‐P had significantly fewer episodes of P. vivax parasitaemia during 42 days follow up (four trials, 1442 participants: RR 0.32, 95% CI 0.24 to 0.43, Analysis 2.5). Of these four trials only one (Ratcliff 2005 IDN) included participants with P. vivax co‐infection at baseline.
2.5. Analysis.
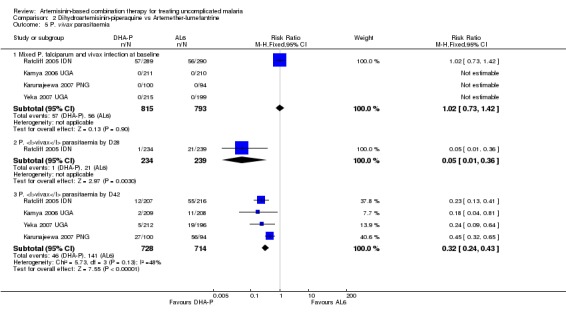
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 5 P. vivax parasitaemia.
Gametocytes
Four trials reported the development of gametocytes in those negative at baseline and the results were highly heterogenous and could not be pooled (four trials, 1203 participants, heterogeneity: Chi² P = 0.006, I² = 76%, Analysis 2.6). This heterogeneity is consistent with the performance of the two drugs for total failure. In the two trials from Uganda (Kamya 2006 UGA and Yeka 2007 UGA) DHA‐P had significantly fewer treatment failures and was also significantly better at reducing gametocyte development. In trials with no difference for treatment failure (Zongo 2007 BFA and Mens 2007 KEN) there was also no difference in gametocyte development. Karunajeewa 2007 PNG and Ratcliff 2005 IDN report no differences in gametocyte carriage between groups but did not give figures.
2.6. Analysis.
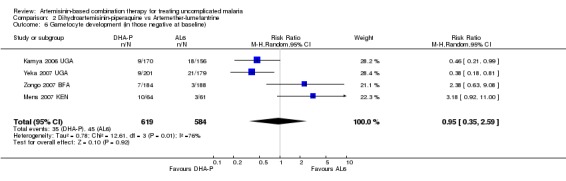
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 6 Gametocyte development (in those negative at baseline).
Anaemia
Four trials report changes in haemoglobin from baseline to the last day of follow up (day 28 or 42). There is a non significant trend towards a benefit with DHA‐P but this is unlikely to be of clinical significance (four trials, 1356 participants, Analysis 2.7). In addition Karunajeewa 2007 PNG reports that haemoglobin remained similar in all groups (no figures given).
2.7. Analysis.
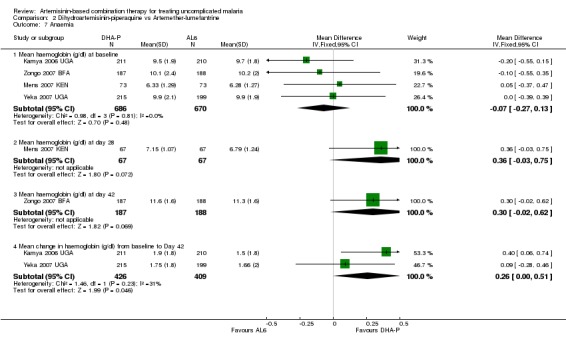
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 7 Anaemia.
Adverse events
No significant difference has been shown in the frequency of serious adverse events (five trials, 2110 participants, Analysis 2.8).
2.8. Analysis.
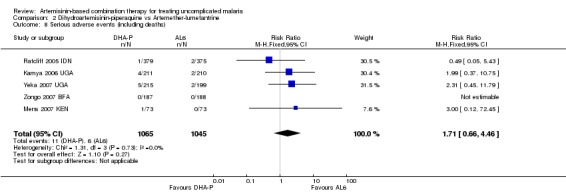
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 8 Serious adverse events (including deaths).
Kamya 2006 UGA and Karunajeewa 2007 PNG report no differences between groups (two trials, 671 participants). Ratcliff 2005 IDN reports more diarrhoea (P = 0.003) with DHA‐P (774 participants). Mens 2007 KEN reports more weakness (P = 0.035) with AL6 (146 participants). Yeka 2007 UGA reports more abdominal pain (P = 0.05) with AL6 (414 participants). Zongo 2007 BFA reports more abdominal pain (P < 0.05) and headache (P < 0.05) with AL6 (375 participants). For a summary of adverse event findings see Appendix 4.
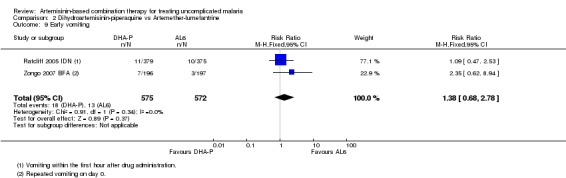
Early vomiting
No difference has been shown in the frequency of drug related vomiting (two trials,1147 participants, Analysis 2.9).
2.9. Analysis.
Comparison 2 Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine, Outcome 9 Early vomiting.
Comparison 3. DHA‐P versus artesunate plus amodiaquine
We found two trials (one in Africa and one in Asia) which assessed this comparison. Allocation concealment was assessed as low risk of bias in one trial (Hasugian 2005 IDN) and unclear in the other. In both trials laboratory staff were blinded to treatment allocation, but other staff and participants were unblinded.
Total failure
PCR adjusted treatment failure with DHA‐P was below 5% in both trials, and below 10% with AS+AQ.
DHA‐P performed significantly better than AS+AQ at day 28 (two trials, 679 participants: PCR unadjusted RR 0.53, 95% CI 0.35 to 0.81, Analysis 3.1; PCR adjusted RR 0.47, 95% CI 0.23 to 0.94, Analysis 3.2). The one trial that reports outcomes at day 42 (Hasugian 2005 IDN) had high losses to follow up (> 20%) at this time point (Analysis 3.3; Analysis 3.4).
3.1. Analysis.
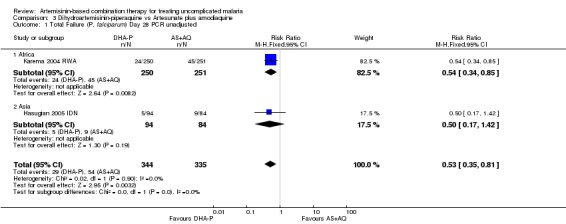
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
3.2. Analysis.
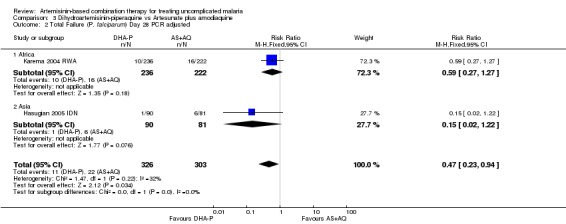
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
3.3. Analysis.
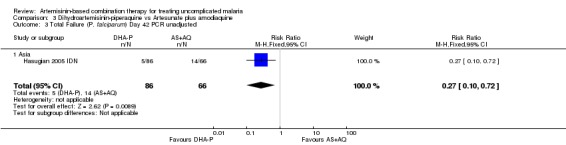
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 3 Total Failure (P. falciparum) Day 42 PCR unadjusted.
3.4. Analysis.
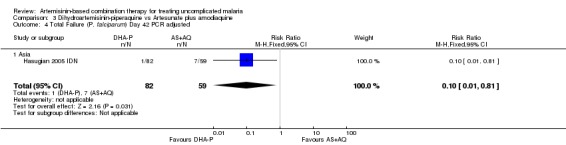
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 4 Total Failure (P. falciparum) Day 42 PCR adjusted.
P. vivax
Hasugian 2005 IDN reports significantly fewer episodes of P. vivax parasitaemia with DHA‐P by day 42 (one trial, 170 participants: RR 0.25, 95% CI 0.09 to 0.74, Analysis 3.5).
3.5. Analysis.
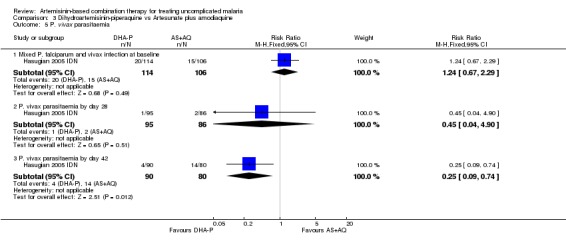
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 5 P. vivax parasitaemia.
Gametocytes
Both trials report no significant differences in gametocyte carriage during follow up (figures not reported).
Anaemia
Hasugian 2005 IDN found that the prevalence of anaemia at day seven (P = 0.02) and 28 (P = 0.006) was significantly higher with AS+AQ (authors own figures); in this trial recurrence of parasitaemia with both P. falciparum and P. vivax was higher in the AS+AQ group. Karema 2004 RWA found no significant difference in PCV between groups at days 0 or 14.
Adverse events
Hasugian 2005 IDN reports three serious adverse events with AS+AQ (two patients with recurrent vomiting on day three, one patient with bilateral cerebellar signs) (one trial, 334 participants, Analysis 3.6). Karema 2004 RWA does not comment on serious adverse events.
3.6. Analysis.
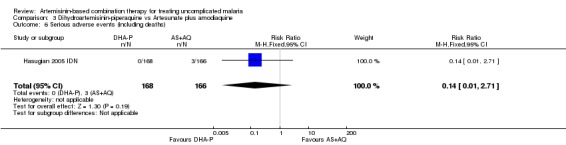
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 6 Serious adverse events (including deaths).
Hasugian 2005 IDN reports more nausea (P = 0.004), vomiting (P = 0.02), and anorexia (P = 0.007) with AS+AQ (334 participants). Karema 2004 RWA reports more vomiting (P = 0.007), anorexia (P = 0.005) and fatigue (P = 0.001) with AS+AQ (504 participants). For a summary of adverse event findings see Appendix 4.
Early vomiting
Hasugian 2005 IDN found no significant difference in the number of participants who vomited at least one dose of medication (one trial, 334 participants, Analysis 3.7).
3.7. Analysis.
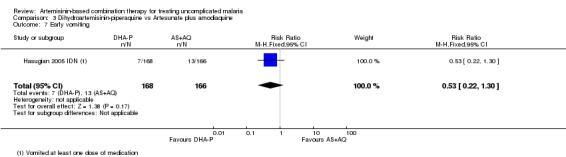
Comparison 3 Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine, Outcome 7 Early vomiting.
Comparison 4. DHA‐P versus artesunate plus sulfadoxine‐pyrimethamine
We found one trial (from Oceania) which assessed this comparison. No attempt to conceal allocation was described. Laboratory staff were blinded to treatment allocation.
Total failure
At day 42 PCR adjusted treatment failure was > 10% in both groups.
There were no significant differences in treatment failure between the two arms (one trial, 215 participants, Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4)
4.1. Analysis.
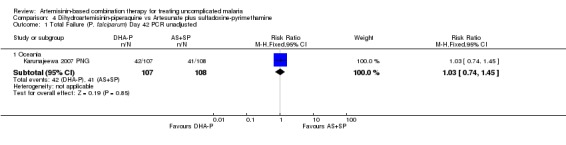
Comparison 4 Dihydroartemisinin‐piperaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 42 PCR unadjusted.
4.2. Analysis.
Comparison 4 Dihydroartemisinin‐piperaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 42 PCR adjusted.
4.3. Analysis.
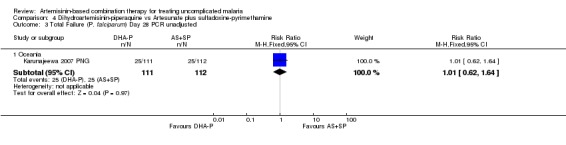
Comparison 4 Dihydroartemisinin‐piperaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 3 Total Failure (P. falciparum) Day 28 PCR unadjusted.
4.4. Analysis.
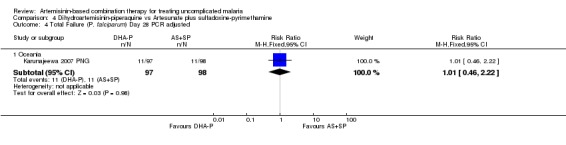
Comparison 4 Dihydroartemisinin‐piperaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 4 Total Failure (P. falciparum) Day 28 PCR adjusted.
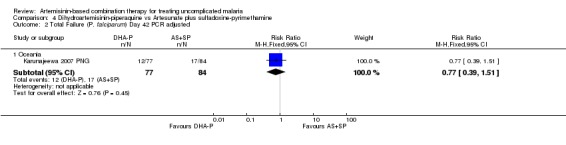
P. vivax
Compared to AS+SP, DHA‐P significantly reduced the incidence of P. vivax parasitaemia by day 42 in participants treated for P. falciparum mono‐infection at baseline (one trial, 194 participants: RR 0.45, 95% CI 0.32 to 0.65, Analysis 4.5), or P. vivax ± P. falciparum at baseline (one trial, 75 participants: RR 0.46, 95% CI 0.27 to 0.79, Analysis 4.5).
4.5. Analysis.
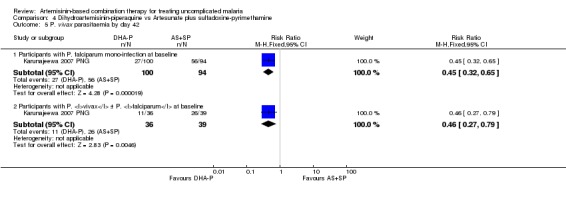
Comparison 4 Dihydroartemisinin‐piperaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 5 P. vivax parasitaemia by day 42.
Gametocytes
No significant differences in gametocyte carriage during follow up are reported (figures not reported).
Anaemia
Haemoglobin levels were reported to remain similar in both groups throughout follow up (figures not reported).
Adverse events
Monitoring for adverse events was undertaken but no differences between the groups were reported (see Appendix 4).
Early vomiting
Not reported.
Comparison 5. DHA‐P versus amodiaquine plus sulfadoxine‐pyrimethamine
We found two trials (both in Africa) which assessed this comparison. Allocation concealment was assessed as low risk of bias in one trial (Zongo 2007 BFA) and unclear in the other. Karema 2004 RWA blinded laboratory staff to treatment allocation. No other blinding is described.
Total failure
PCR adjusted treatment failure with DHA‐P was below 5% in both trials. In Rwanda, PCR adjusted treatment failure with AQ+SP was above 10%.
DHA‐P performed significantly better than AQ+SP at 28 days (two trials, 848 participants: PCR unadjusted RR 0.37, 95% CI 0.25 to 0.55, Analysis 5.1; PCR adjusted RR 0.30, 95% CI 0.17 to 0.54, Analysis 5.2). Zongo 2007 BFA did not show a difference at day 42 with both drugs performing well at this site (one trial, 341 participants, Analysis 5.3; Analysis 5.4).
5.1. Analysis.
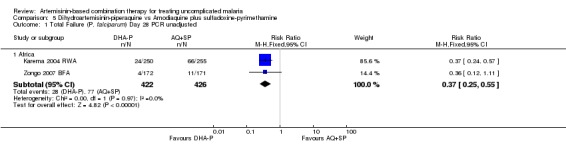
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
5.2. Analysis.
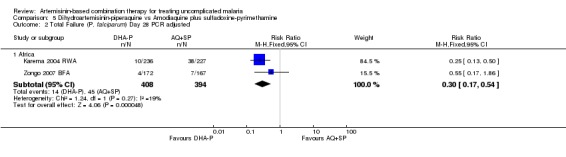
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
5.3. Analysis.
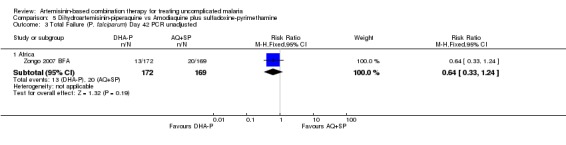
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 3 Total Failure (P. falciparum) Day 42 PCR unadjusted.
5.4. Analysis.
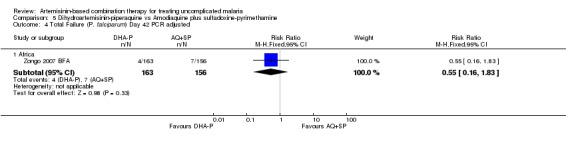
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 4 Total Failure (P. falciparum) Day 42 PCR adjusted.
P. vivax
Not reported.
Gametocytes
Zongo 2007 BFA found no difference in the development of gametocytaemia in participants who did not have detectable gametocytes at baseline (one trial, 367 participants, Analysis 5.5). Karema 2004 RWA reported no significant difference in gametocyte carriage during follow up but figures were not reported (one trial, 510 participants).
5.5. Analysis.
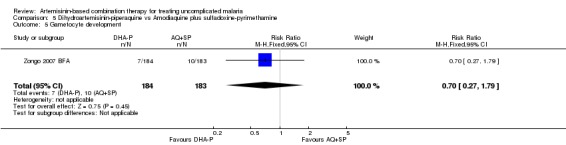
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 5 Gametocyte development.
Anaemia
Zongo 2007 BFA found no significant difference in haemoglobin at baseline or at day 42 (1 trial, 371 participants, Analysis 5.6). Karema 2004 RWA found that the packed cell volume (PCV) increased from baseline to day 14 in both groups, but at day 14 it was significantly lower with DHA‐P (one trial, 510 participants: MD ‐1.10, 95% CI ‐1.73 to ‐0.47, Analysis 5.6). This difference is unlikely to be of clinical significance.
5.6. Analysis.
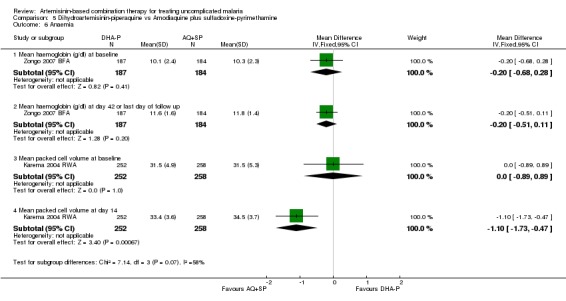
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 6 Anaemia.
Adverse events
Zongo 2007 BFA reports that there were no serious adverse events (one trial, 371 participants). Karema 2004 RWA does not comment on serious adverse events.
Zongo 2007 BFA reports more abdominal pain (P < 0.05) and pruritis (P < 0.05) with AQ+SP (371 participants). Karema 2004 RWA reports more vomiting (P = 0.007), anorexia (P = 0.005), and fatigue (P = 0.001) with AQ+SP (510 participants). For a summary of adverse event findings see Appendix 4.
Early vomiting
Zongo 2007 BFA reports on vomiting medication on day 0 (as an exclusion criteria not an outcome) and there was no difference between groups (one trial, 383 participants, Analysis 5.7).
5.7. Analysis.
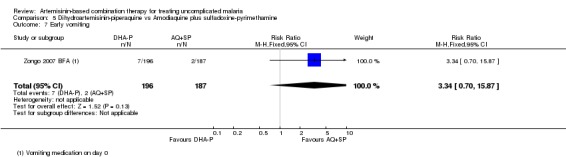
Comparison 5 Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 7 Early vomiting.
Question 2. How does artesunate mefloquine (AS+MQ) perform?
Dosing concerns
AS+MQ has traditionally been administered using 15 mg/kg mefloquine on day one and 10 mg/kg on day two. A new fixed‐dose combination of AS+MQ is now available where mefloquine is given as a once daily dose of 8 mg/kg. One trial (Ashley 2005 THA) has directly compared these two regimens and found no significant difference (one trial, 423 participants, Analysis 16.1; Analysis 16.2). In addition five trials used loose tablets to deliver a once daily dose of mefloquine of 8 mg/kg in combination with artesunate. In all of these trials the proportion of treatment failures with the new regimen was below 10% and in three trials below 5% (Analysis 17.1; Analysis 17.2)
16.1. Analysis.
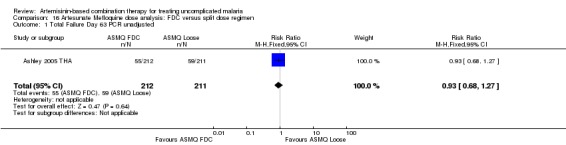
Comparison 16 Artesunate Mefloquine dose analysis: FDC versus split dose regimen, Outcome 1 Total Failure Day 63 PCR unadjusted.
16.2. Analysis.
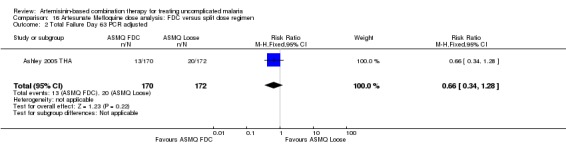
Comparison 16 Artesunate Mefloquine dose analysis: FDC versus split dose regimen, Outcome 2 Total Failure Day 63 PCR adjusted.
17.1. Analysis.
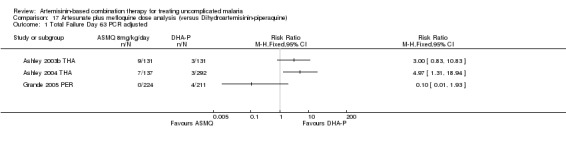
Comparison 17 Artesunate plus mefloquine dose analysis (versus Dihydroartemisinin‐piperaquine), Outcome 1 Total Failure Day 63 PCR adjusted.
17.2. Analysis.
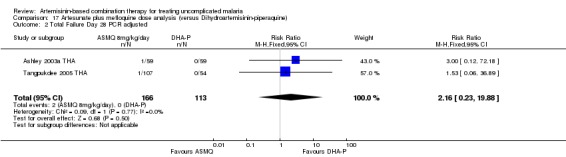
Comparison 17 Artesunate plus mefloquine dose analysis (versus Dihydroartemisinin‐piperaquine), Outcome 2 Total Failure Day 28 PCR adjusted.
Comparison 6. AS+MQ versus artemether‐lumefantrine (six doses)
We found eight trials (six in Asia and two in Africa) which assessed this comparison. Allocation concealment was assessed as low risk of bias in two trials (Mayxay 2003 LAO; Sagara 2005b MLI). Only one trial blinded microscopists to treatment allocation.
Total failure
In all eight trials both combinations performed well with PCR adjusted treatment failures below 5%.
In Asia, AS+MQ reduced overall treatment failure by day 42 compared to AL6 (four trials, 1000 participants: PCR unadjusted RR 0.53, 95% CI 0.29 to 0.94, Analysis 6.1). For PCR adjusted treatment failure there was substantial heterogeneity (four trials, 904 participants: heterogeneity Chi² P = 0.04, I² = 64%, Analysis 6.2), which related to one trial (Hutagalung 2002 THA). This trial was unusual in that P. vivax was very common during follow up and significantly more common following treatment with AL6. P. vivax was treated with chloroquine and participants continued in follow up. Therefore significantly more participants in the AL6 group received additional antimalarials which may have affected the result. Sensitivity analysis removing this trial shifts the result significantly in favour of AS+MQ.
6.1. Analysis.
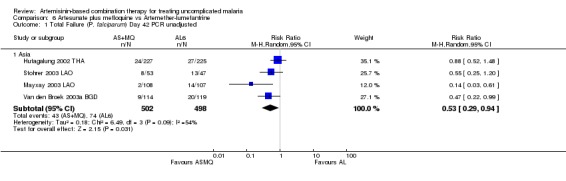
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 1 Total Failure (P. falciparum) Day 42 PCR unadjusted.
6.2. Analysis.
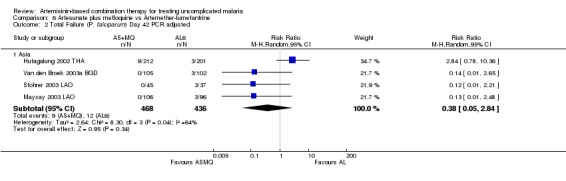
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 2 Total Failure (P. falciparum) Day 42 PCR adjusted.
There were no significant differences in PCR adjusted treatment failure at day 28 (five trials, 1479 participants, Analysis 6.4). One trial from Africa (Sagara 2005b MLI) did find a significant reduction in re‐infections with AS+MQ but this was not repeated elsewhere (Analysis 6.3).
6.4. Analysis.
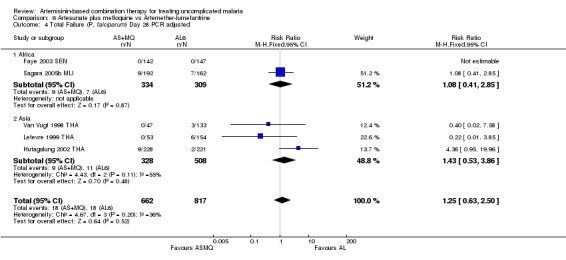
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 4 Total Failure (P. falciparum) Day 28 PCR adjusted.
6.3. Analysis.
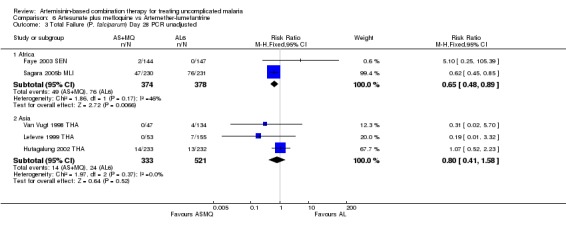
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 3 Total Failure (P. falciparum) Day 28 PCR unadjusted.
P. vivax
AS+MQ performed significantly better than AL6 at reducing the incidence of P. vivax during 42 days of follow up (four trials, 1003 participants: RR 0.30, 95% CI 0.21 to 0.41, Analysis 6.5).
6.5. Analysis.
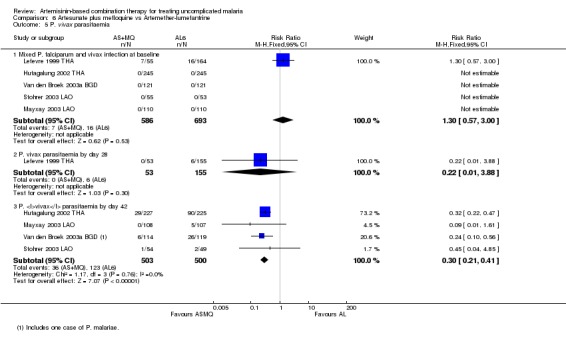
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 5 P. vivax parasitaemia.
Gametocytes
There is no evidence of an advantage with either drug at reducing gametocytaemia. There was no significant difference in gametocyte development in those negative at baseline (three trials, 883 participants, Analysis 6.6). Gametocyte carriage was generally low in the three trials which report it, with a statistically significant reduction in gametocyte carriage with AS+MQ on day seven, but not day three or 14 (three trials, 636 participants: Gametocyte carriage day seven RR 0.35, 95% CI 0.14 to 0.85, Analysis 6.7). Sagara 2005b MLI reports no differences between groups (no figures given).
6.6. Analysis.
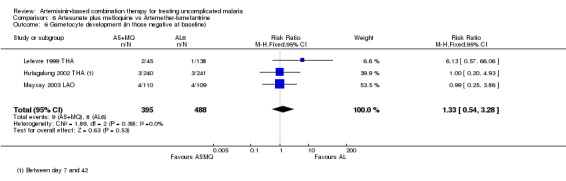
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 6 Gametocyte development (in those negative at baseline).
6.7. Analysis.
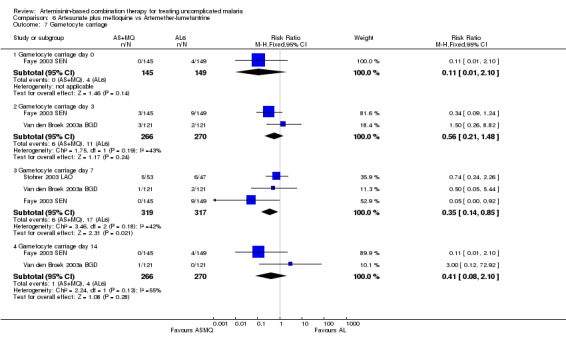
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 7 Gametocyte carriage.
Anaemia
Six trials report some measure of haematological recovery. Hutagalung 2002 THA found a greater decrease in haematocrit at day seven with AS+MQ (9.3% AS+MQ versus 6.7% AL6, P = 0.02; authors own figures). None of the remaining five trials report a significant difference (see Appendix 5).
Adverse events
No difference has been shown in the frequency of serious adverse events (seven trials, 1773 participants, Analysis 6.8).
6.8. Analysis.
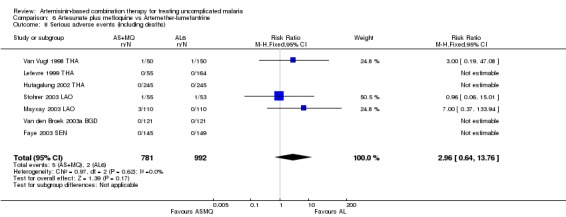
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 8 Serious adverse events (including deaths).
Three trials report significantly more CNS symptoms with AS+MQ (dizziness, headache, confusion, or sleep disturbance) and one reports more with AL6. Gastrointestinal (GI) symptoms (nausea, vomiting, abdominal pain, or anorexia) were significantly more common with AS+MQ in four trials. For a summary of adverse events see Appendix 4.
Early vomiting
No difference has been shown in the frequency of early vomiting (six trials, 1479 participants, Analysis 6.9).
6.9. Analysis.
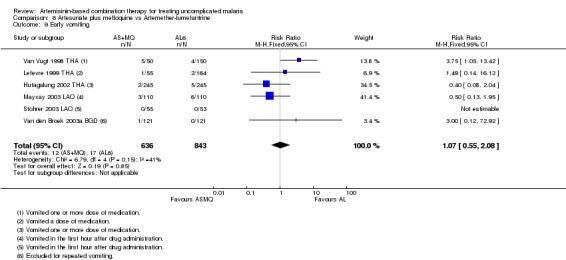
Comparison 6 Artesunate plus mefloquine vs Artemether‐lumefantrine, Outcome 9 Early vomiting.
Comparison 7. AS+MQ versus artesunate plus amodiaquine
We only found one trial in Africa (Faye 2003 SEN) which assessed this comparison. Allocation concealment and blinding were not described.
Total failure
In the 28 days of this trial, treatment failure was very low in both groups. It is therefore not possible to draw conclusions on the benefits of either drug. There were no significant differences in PCR unadjusted failure (one trial, 493 participants, Analysis 7.1) and no episodes of PCR confirmed recrudescence.
7.1. Analysis.
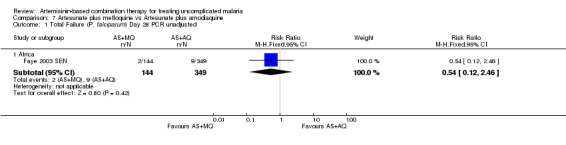
Comparison 7 Artesunate plus mefloquine vs Artesunate plus amodiaquine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
P. vivax
Not reported.
Gametocytes
Gametocyte carriage was very low in both groups. Gametocytes were only detectable in three participants in the AS+MQ group on day three. At baseline, day seven and day 14 gametocytes were undetectable in all participants.
Anaemia
Twenty‐five percent of participants had haemoglobin measured on days 0 and 14 and no significant differences are reported.
Adverse events
In this trial there were no serious adverse events (one trial, 505 participants) and no differences between groups reported (see Appendix 4).
Early vomiting
Not reported.
Comparison n/a. AS+MQ versus artesunate plus sulfadoxine‐pyrimethamine
We did not find any trials which assessed this comparison.
Comparison 8. AS+MQ versus amodiaquine plus sulfadoxine‐pyrimethamine
We only found one trial in Africa (Faye 2003 SEN) which assessed this comparison. Allocation concealment and blinding were not described.
Total failure
In the 28 days of this trial, treatment failure was very low in both groups. It is therefore not possible to draw conclusions on the benefits of either drug. There were no differences in PCR unadjusted failure (one trial, 300 participants, Analysis 8.1) and there were no episodes of PCR confirmed recrudescence.
8.1. Analysis.
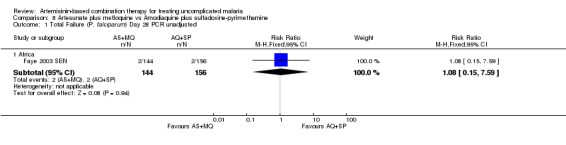
Comparison 8 Artesunate plus mefloquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
P. vivax
Not reported.
Gametocytes
Detectable gametocytaemia was significantly less common with AS+MQ at days three and seven (Gametocyte carriage day three: RR 0.21, 95% CI 0.06 to 0.70; Gametocyte carriage day seven: RR 0.03, 95% CI 0.00 to 0.47, Analysis 8.3). At day 14 gametocytes were undetectable in all participants.
8.3. Analysis.
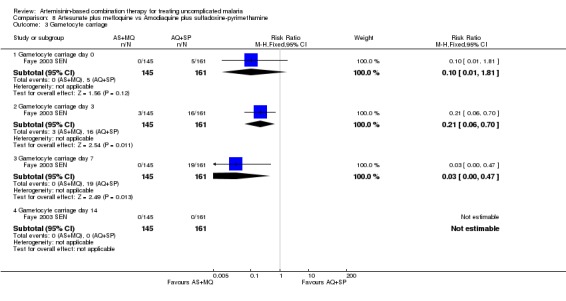
Comparison 8 Artesunate plus mefloquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 3 Gametocyte carriage.
Anaemia
Twenty five percent of participants had haemoglobin measured on days 0 and 14 and no significant differences were reported.
Adverse events
In this trial there were no serious adverse events in either group (one trial, 306 participants) and no differences between groups reported (see Appendix 4).
Early vomiting
Not reported.
Question 3. How does artemether‐lumefantrine (6 doses) perform?
Dosing concerns
The six‐dose regimen of AL6 has been shown to be superior to the four‐dose regimen (Vugt 1999; Omari 2006). In this review we have only included the six‐dose regimen.
Comparison 9. AL6 versus artesunate plus amodiaquine
We found twelve trials (all in Africa) which assessed this comparison. Three of these trials were excluded after sensitivity analysis due to baseline differences which had the potential to bias the result in favour of AL6 (Analysis 9.9; Analysis 9.10). Of the remaining nine trials allocation concealment was assessed as low risk of bias in five trials (Adjei 2006 GHA; Bukirwa 2005 UGA; Dorsey 2006 UGA; Kobbe 2007 GHA; Mutabingwa 2004 TZA) and laboratory staff were blinded to treatment allocation in four trials.
Total failure
PCR adjusted treatment failure was below 5% for both AL6 and AS+AQ in six out of eight trials. In two more recent trials (both from Ghana), PCR adjusted treatment failure for both arms was above 5% and for AL6 above 10% (Analysis 9.2).
9.2. Analysis.
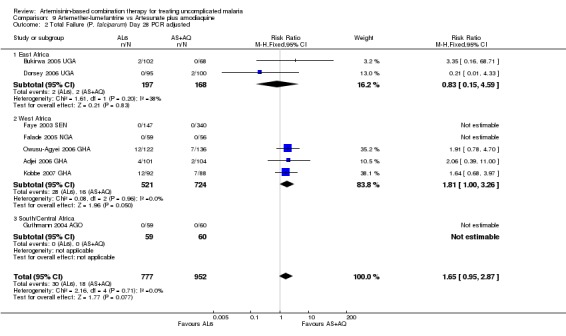
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
No difference has been shown in PCR adjusted total failure at day 28, either within individual trials or after pooling (eight trials, 1729 participants, Analysis 9.2). There is substantial heterogeneity in PCR unadjusted failure (nine trials, 3021 participants: heterogeneity Chi² P < 0.0001, I² = 76%, Analysis 9.1). Subgroup analysis seems to suggest regional differences, with studies from East Africa showing benefit with AL6 and recent studies from West Africa favouring AS+AQ (Analysis 9.1). However, substantial heterogeneity remains, and further subgroup analysis by trial characteristics and transmission intensity did not expand the interpretation of this heterogeneity.
9.1. Analysis.
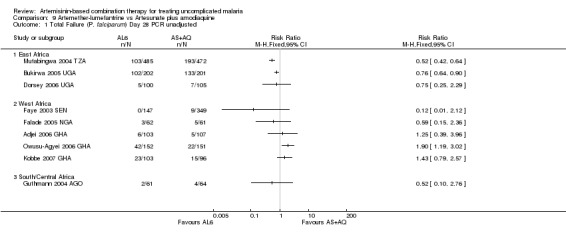
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
P. vivax
One trial (Dorsey 2006 UGA) reported on P. vivax but there were too few patients to draw a conclusion (AL6: 8/202 at baseline and 3/202 during follow up, AS+AQ: No vivax at any time point).
Gametocytes
Bukirwa 2006 found that AL6 significantly reduced the development of gametocytaemia in patients who did not have detectable gametocytes at baseline (one trial, 305 participants: RR 0.34, 95% CI 0.15 to 0.74, Analysis 9.3). Three trials reporting gametocyte carriage over 14 days of follow up do not show a clear advantage with either combination (three trials, 1078 participants, Analysis 9.4).
9.3. Analysis.
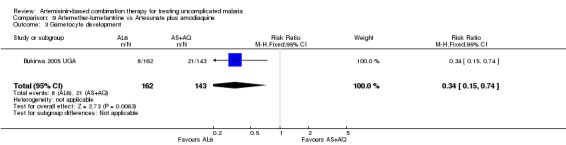
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 3 Gametocyte development.
9.4. Analysis.
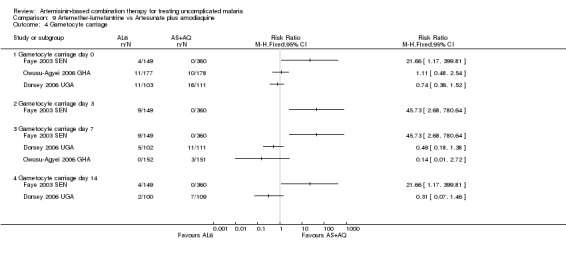
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 4 Gametocyte carriage.
Anaemia
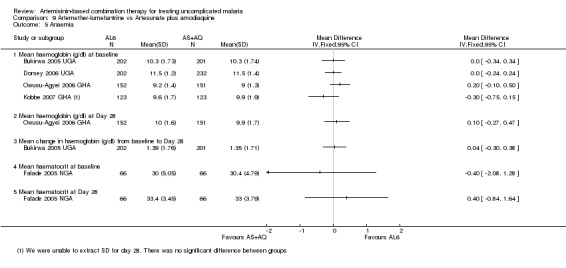
Four studies reported some measure of haematological recovery from baseline to day 28 and did not show a difference between the two combinations (four trials, 2356 participants, Analysis 9.5). Guthmann 2004 AGO reported the proportion of participants who were anaemic (Hb < 11 g/dl) at day 0 and 28 and did not show a difference (one trial, 123 participants, Analysis 9.6). Three trials (Dorsey 2006 UGA; Faye 2003 SEN; Mutabingwa 2004 TZA) also reported measures of anaemia at day 14 and did not show a difference.
9.5. Analysis.
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 5 Anaemia.
9.6. Analysis.
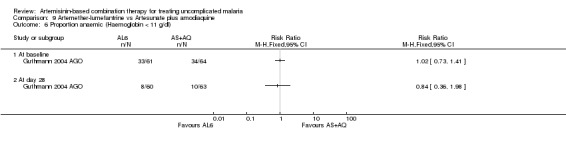
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 6 Proportion anaemic (Haemoglobin < 11 g/dl).
Adverse events
No difference has been shown in the frequency of serious adverse events (six trials, 2749 participants, Analysis 9.7).
9.7. Analysis.
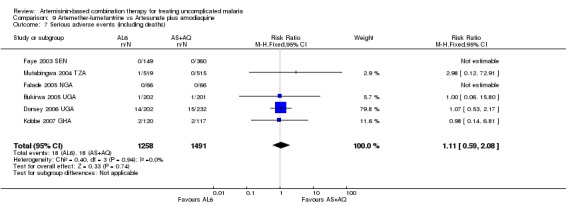
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 7 Serious adverse events (including deaths).
No important differences in adverse events were reported between groups. For a summary of adverse events see Appendix 4.
Early vomiting
No difference has been shown in the frequency of early vomiting (five trials, 1097 participants, Analysis 9.8).
9.8. Analysis.
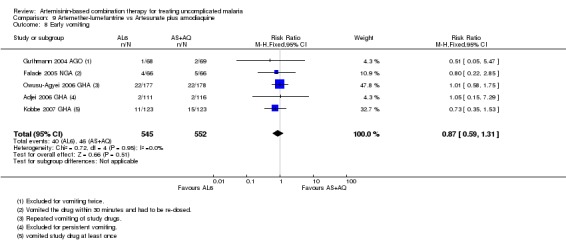
Comparison 9 Artemether‐lumefantrine vs Artesunate plus amodiaquine, Outcome 8 Early vomiting.
Comparison 10. AL6 versus artesunate plus sulfadoxine‐pyrimethamine
We found four trials (three from Africa and one from Oceania) which assessed this comparison. Two of these trials were excluded from the primary analysis due to baseline differences between the groups (Analysis 10.6; Analysis 10.7). Allocation concealment was judged to be at high risk of bias in the two remaining trials. Laboratory staff were blinded to treatment allocation in one trial.
10.6. Analysis.
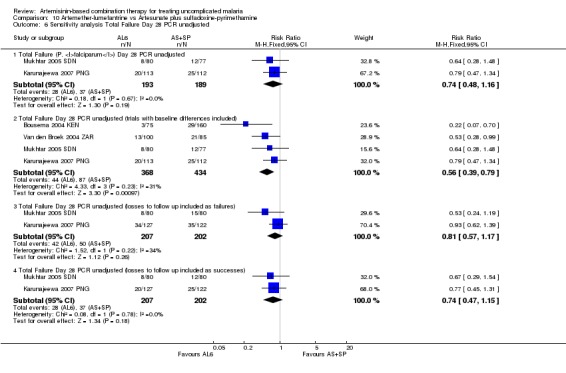
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 6 Sensitivity analysis Total Failure Day 28 PCR unadjusted.
10.7. Analysis.
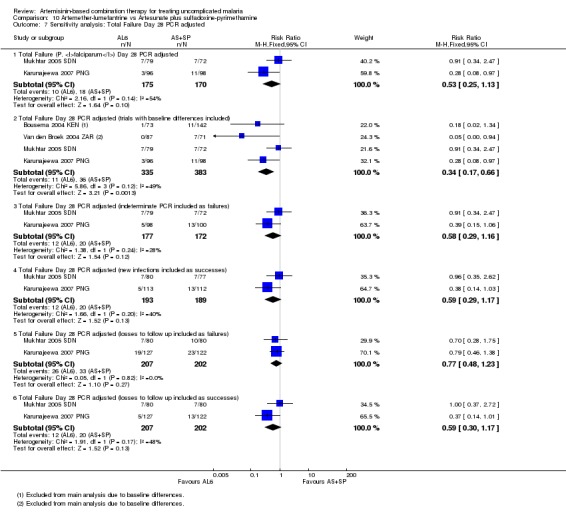
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 7 Sensitivity analysis: Total Failure Day 28 PCR adjusted.
Total failure
In Oceania, Karunajeewa 2007 PNG found no difference in PCR unadjusted failure (one trial, 217 participants, Analysis 10.1; Analysis 10.3), but did show a significant reduction in PCR adjusted treatment failure with AL6 at both day 28 and day 42 (one trial, 217 participants: Day 42 RR 0.33, 95% CI 0.13 to 0.86, Analysis 10.2; Day 28 RR 0.28, 95% CI 0.08 to 0.97, Analysis 10.4). PCR adjusted treatment failure with AS+SP was > 20% at day 42.
10.1. Analysis.
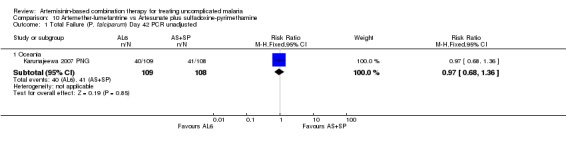
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 42 PCR unadjusted.
10.3. Analysis.
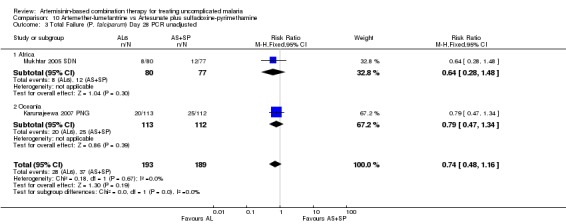
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 3 Total Failure (P. falciparum) Day 28 PCR unadjusted.
10.2. Analysis.
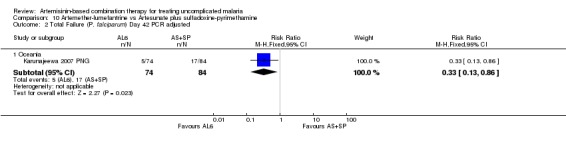
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 42 PCR adjusted.
10.4. Analysis.
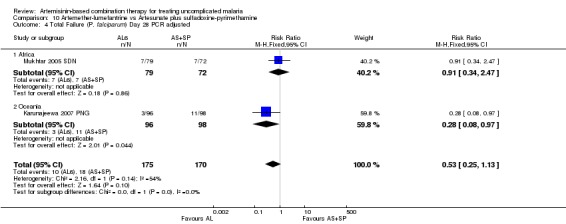
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 4 Total Failure (P. falciparum) Day 28 PCR adjusted.
In Africa, Mukhtar 2005 SDN found no difference between the two groups (one trial, 157 participants, Analysis 10.3, Analysis 10.4).
P. vivax
Karunajeewa 2007 PNG found no differences in the incidence of P. vivax parasitaemia by day 42 in participants treated for P. falciparum mono‐infection at baseline (one trial, 196 participants), or those treated for P. vivax at baseline (one trial, 72 participants, Analysis 10.5)
10.5. Analysis.
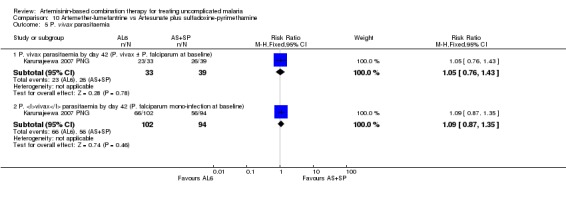
Comparison 10 Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 5 P. vivax parasitaemia.
Gametocytes
Karunajeewa 2007 PNG reports no differences in gametocyte carriage between the two groups during follow up (figures not reported).
Anaemia
Karunajeewa 2007 PNG reports no differences in mean haemoglobin during follow up (figures not reported).
Adverse events
Two trials report on adverse events and no differences are noted between the two groups (Karunajeewa 2007 PNG; Van den Broek 2004 ZAR). For a summary of adverse events see Appendix 4.
Early vomiting
Not reported.
Comparison 11. AL6 versus amodiaquine plus sulfadoxine‐pyrimethamine
We found seven trials (all in Africa) which assessed this comparison. One trial was excluded from the primary analysis due to baseline differences between groups. Of the remaining trials allocation concealment was assessed as low risk of bias in two trials (Dorsey 2006 UGA; Zongo 2007 BFA) and laboratory staff were blinded to treatment allocation in four trials.
Total failure
PCR adjusted treatment failure with AL6 was below 5% in all six trials. The performance of AQ+SP was much more variable.
In East Africa, where treatment failure with AQ+SP was high, AL6 performed markedly better at day 28 (three trials, 1646 participants: PCR unadjusted RR 0.35, 95% CI 0.30 to 0.41, Analysis 11.1; PCR adjusted RR 0.12, 95% CI 0.06 to 0.24, Analysis 11.2).
11.1. Analysis.
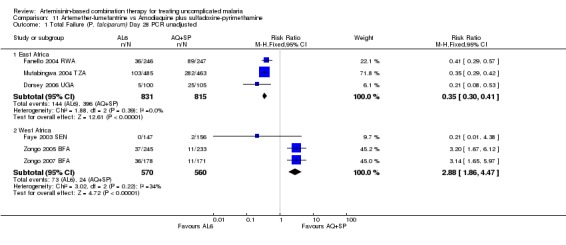
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
11.2. Analysis.
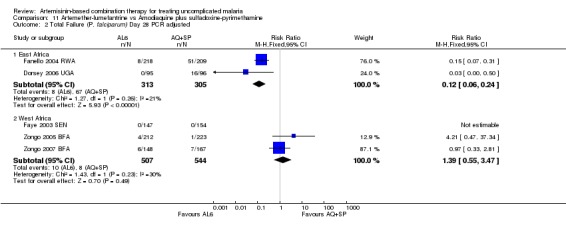
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
In contrast, in West Africa, where AQ+SP performed much better, there were fewer PCR unadjusted treatment failures with AQ+SP at both day 28 (three trials, 1130 participants: PCR unadjusted RR 2.88, 95% CI 1.86 to 4.47, Analysis 11.1) and day 42 (one trial, 345 participants: PCR unadjusted RR 2.64, 95% CI 1.66 to 4.21, Analysis 11.3). There were no significant differences between the two combinations after PCR adjustment (Analysis 11.2; Analysis 11.4).
11.3. Analysis.
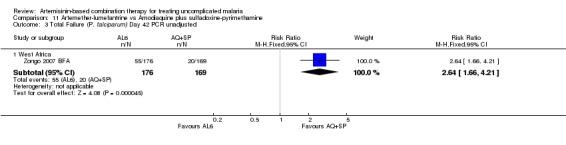
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 3 Total Failure (P. falciparum) Day 42 PCR unadjusted.
11.4. Analysis.
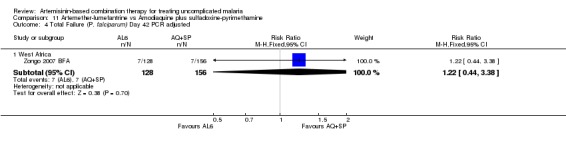
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 4 Total Failure (P. falciparum) Day 42 PCR adjusted.
P. vivax
Only one trial (Dorsey 2006 UGA) reported on P. vivax and there were too few patients to draw a conclusion (AL6 8/202 at baseline and 3/202 during follow up, AQ+SP 4/253 at baseline and 0 during follow up).
Gametocytes
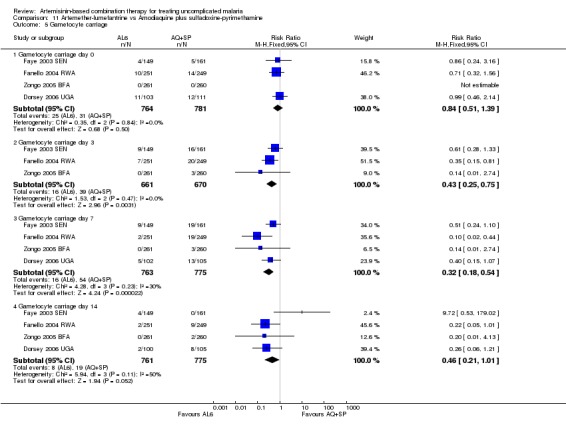
The prevalence of gametocyte carriage was significantly lower with AL6 at day three (three trials, 1331 participants: RR 0.43, 95% CI 0.25 to 0.75, Analysis 11.5) and day seven (four trials,1538 participants: RR 0.32, 95% CI 0.18 to 0.54, Analysis 11.5). Zongo 2007 BFA found no significant difference in the development of gametocytaemia in participants without detectable gametocytes at baseline (one trial, 371 participants, Analysis 11.6).
11.5. Analysis.
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 5 Gametocyte carriage.
11.6. Analysis.
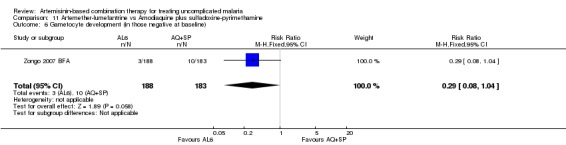
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 6 Gametocyte development (in those negative at baseline).
Anaemia
Zongo 2005 BFA reports change in haemoglobin from baseline to day 28; Zongo 2007 BFA reports mean haemoglobin at baseline and day 42. Neither of these trials showed a clinically significant difference (two trials, 893 participants, Analysis 11.7). Four other trials assessed haematological recovery at shorter time points and did not detect a difference (Dorsey 2006 UGA; Fanello 2004 RWA; Faye 2003 SEN; Mutabingwa 2004 TZA).
11.7. Analysis.
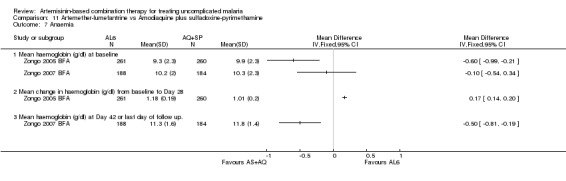
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 7 Anaemia.
Adverse events
No difference has been shown in the frequency of serious adverse events (five trials, 2684 participants, Analysis 11.8).
11.8. Analysis.
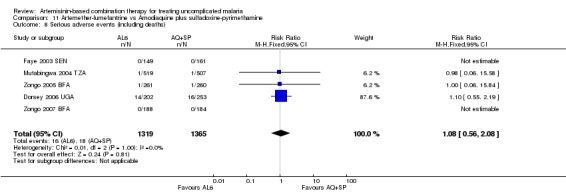
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 8 Serious adverse events (including deaths).
Dorsey 2006 UGA reports more anorexia (P < 0.05) and weakness (P < 0.05) with AQ+SP (455 participants). Two trials report a significant increase in pruritis (P < 0.05, P < 0.0001) with AQ+SP. No further differences are noted. For a summary of adverse events see Appendix 4.
Early vomiting
Two trials report on the number of participants excluded for persistent vomiting on day 0. There were no differences between groups (two trials, 893 participants, Analysis 11.9).
11.9. Analysis.
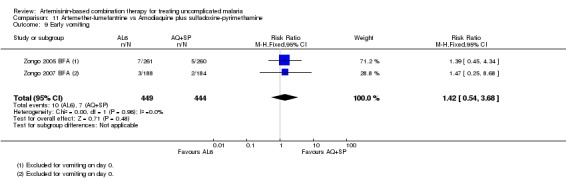
Comparison 11 Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 9 Early vomiting.
Question 4. How does artesunate plus amodiaquine perform?
Comparison 12. AS+AQ versus artesunate plus sulfadoxine‐pyrimethamine
We found seven trials (all in Africa) which assessed this comparison. Allocation concealment was judged as low risk of bias in only one trial (Bonnet 2004 GIN) and unclear in four. Laboratory staff were blinded to treatment allocation in two trials.
Total failure
PCR adjusted treatment failures with AS+AQ were < 10% in all seven trials, and with AS+SP in six out of seven trials.
Overall the number of PCR adjusted failures was low with no significant difference between groups (seven trials, 1419 participants, Analysis 12.2). There was substantial heterogeneity in PCR unadjusted failure rates between trials (seven trials, 1419 participants: heterogeneity: Chi² P < 0.00001, I² = 88%, Analysis 12.1). We attempted to investigate this heterogeneity with subgroup analysis on geographical region, allocation concealment, drug dose, stated resistance pattern, and age of participants, with no clear findings.
12.2. Analysis.
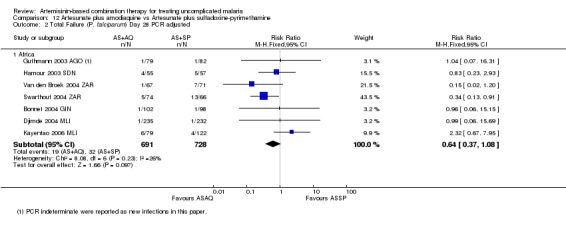
Comparison 12 Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
12.1. Analysis.
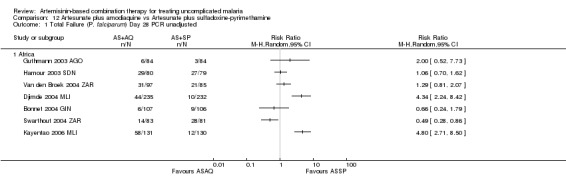
Comparison 12 Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
P. vivax
Not reported.
Gametocytes
We were able to combine the results of three trials reporting gametocyte carriage on days three, seven and 14 and no difference was shown at any time point (three trials, 532 participants, Analysis 12.3). The remaining four trials report that there were no differences in carriage between groups but do not give figures.
12.3. Analysis.
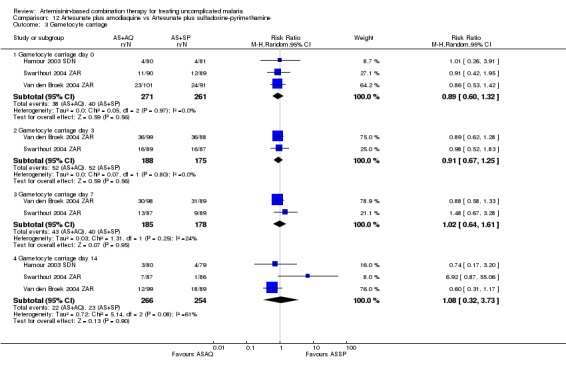
Comparison 12 Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 3 Gametocyte carriage.
Anaemia
Five trials report that levels of anaemia improved following treatment in both groups. Three of these trials did not give figures (Djimde 2004 MLI; Swarthout 2004 ZAR; Van den Broek 2004 ZAR). Two trials report the proportion of patients with anaemia at baseline and day 28. The proportion improved in both groups with no significant differences between the two treatments (two trials, 452 participants, Analysis 12.4).
12.4. Analysis.
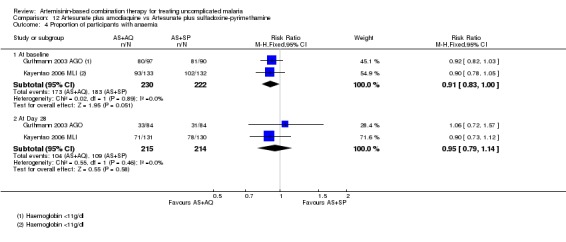
Comparison 12 Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 4 Proportion of participants with anaemia.
Adverse events
No difference has been shown in the frequency of serious adverse events (four trials, 1108 participants, Analysis 12.5).
12.5. Analysis.
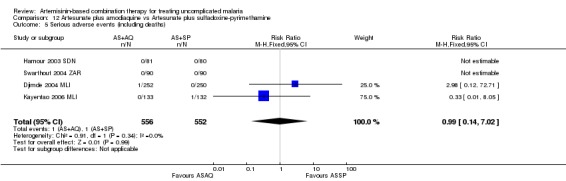
Comparison 12 Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine, Outcome 5 Serious adverse events (including deaths).
Five trials reported on adverse events and no significant differences between treatments were noted. One trial (Djimde 2004 MLI) performed haematological and biochemical tests on days 7, 14, and 28 and no significant abnormalities were noted. For a summary of adverse events see Appendix 4.
Early vomiting
Not reported.
Comparison 13. AS+AQ versus amodiaquine plus sulfadoxine‐pyrimethamine
We found eight trials which assessed this comparison (all in Africa). Allocation concealment was assessed as low risk of bias in four trials (Dorsey 2006 UGA; Menard 2006 MDG; Mutabingwa 2004 TZA; Staedke 2003 UGA) and unclear in two. Laboratory staff were unaware of treatment allocation in seven trials.
Total failure
The efficacy of both drugs in these trials was highly variable.
A subgroup analysis demonstrates that it is in East Africa that AQ+SP is failing as a first‐line therapy. Heterogeneity is high, limiting meaningful pooling of data, but trials from East Africa tend to favour AS+AQ (five trials, 3317 participants, PCR unadjusted heterogeneity: Chi² P < 0.0001, I² = 91%, Analysis 13.1; three trials, 1515 participants, PCR adjusted heterogeneity: Chi² P = 0.03, I² = 73%, Analysis 13.2). AQ+SP performed well in Senegal in 2003, Mali in 2006 and Madagascar in 2006. We further investigated this heterogeneity with subgroup analysis on allocation concealment, drug dose, stated resistance pattern, and age of participants, with no clear findings.
13.1. Analysis.
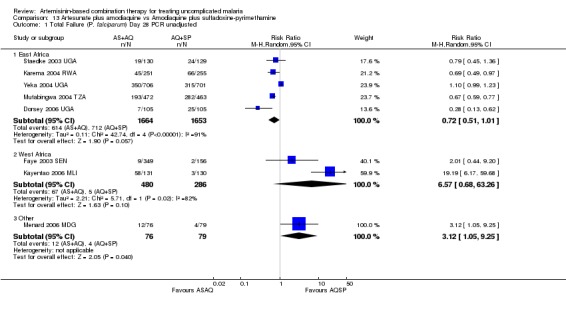
Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 1 Total Failure (P. falciparum) Day 28 PCR unadjusted.
13.2. Analysis.
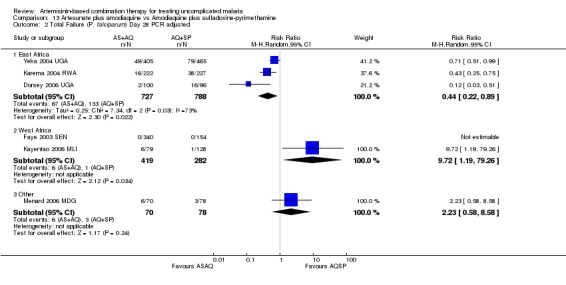
Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
P. vivax
Not reported.
Gametocytes
AS+AQ significantly reduced the development of gametocytes in those negative at baseline (two trials, 1354 participants: RR 0.67, 95% CI 0.54 to 0.82, Analysis 13.3). Six trials measured gametocyte carriage during follow up. Three of these reported that there were no differences but did not give figures. Of the three trials which gave figures, only one (Faye 2003 SEN) found that AS+AQ significantly reduced carriage rates at days three and seven (Analysis 13.4).
13.3. Analysis.
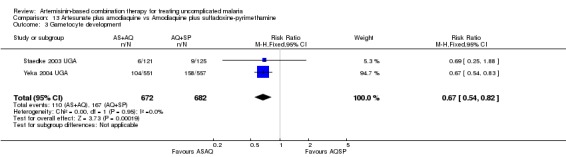
Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 3 Gametocyte development.
13.4. Analysis.
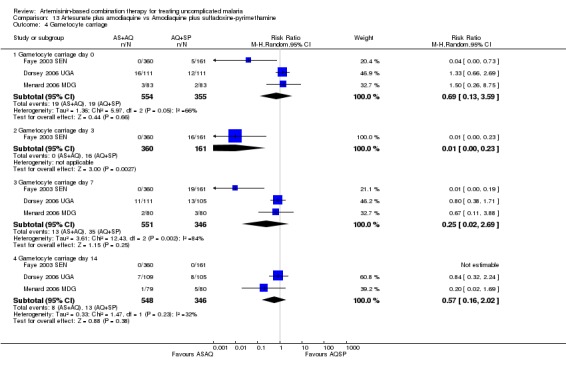
Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 4 Gametocyte carriage.
Anaemia
All eight trials reported some measure of haematological recovery. No individual trial has reported a clinically important difference at day 14 or 28 (see Appendix 5).
Adverse events
No difference has been shown in the frequency of serious adverse events (seven trials, 4200 participants, Analysis 13.6).
13.6. Analysis.
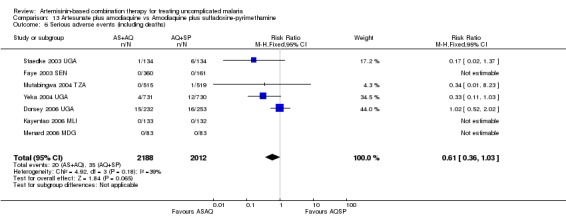
Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 6 Serious adverse events (including deaths).
Dorsey 2006 UGA reports more anorexia (P < 0.05) and weakness (P < 0.05) with AQ+SP (485 participants). No differences are noted in any other trial. Four trials also undertook some biochemical monitoring and no important differences are noted. For a summary of adverse events see Appendix 4.
Early vomiting
Not reported.
Discussion
Summary of main results
Efficacy (as measured by total failure)
The WHO has set two standards for antimalarial drugs:
that a total failure rate (adjusted for new infections) of > 10% should trigger a change of first‐line drug policy; and
that a new drug being adopted as policy should have total failure rates (adjusted for new infections) of < 5%.
This review has demonstrated that:
In head to head trials the newest ACT, dihydroartemisinin‐piperaquine, achieved the standard of < 5% total failure in 15 out of the 17 studies it was involved in. DHA‐P appears to be at least as effective as AS+MQ in Asia (eight trials) providing a valuable alternative to current therapy. In clinical trials in Africa, DHA‐P may be more effective than the current widely used options AL6 (four trials) and AS+AQ (one trial), although these two drugs continue to perform well in many areas (Figure 3; Figure 4).
AS+MQ has performed well in trials from Asia and South America, with failure rates consistently low, but has been little studied in the African context (Figure 5; Figure 6).
AL6 and AS+AQ performed well in almost all studies they were involved in but Kamya 2006 UGA found failure rates in excess of 10% with AL6 and Yeka 2004 UGA reported > 10% failure with AS+AQ (Figure 7; Figure 8; Figure 9; Figure 10).
There is very little good quality evidence available comparing AS+SP to DHA‐P, AS+MQ or AL6 but it has performed well in head to head trials with AS+AQ.
The performance of the non‐ACT AQ+SP (which is only recommended as an interim measure by the WHO), was inadequate for first‐line use in several countries from East Africa. It was, however, still performing well in Senegal in 2003 (Faye 2003 SEN), Madagascar in 2006 (Menard 2006 MDG), and Burkina Faso in 2005 (Zongo 2005 BFA).
3.
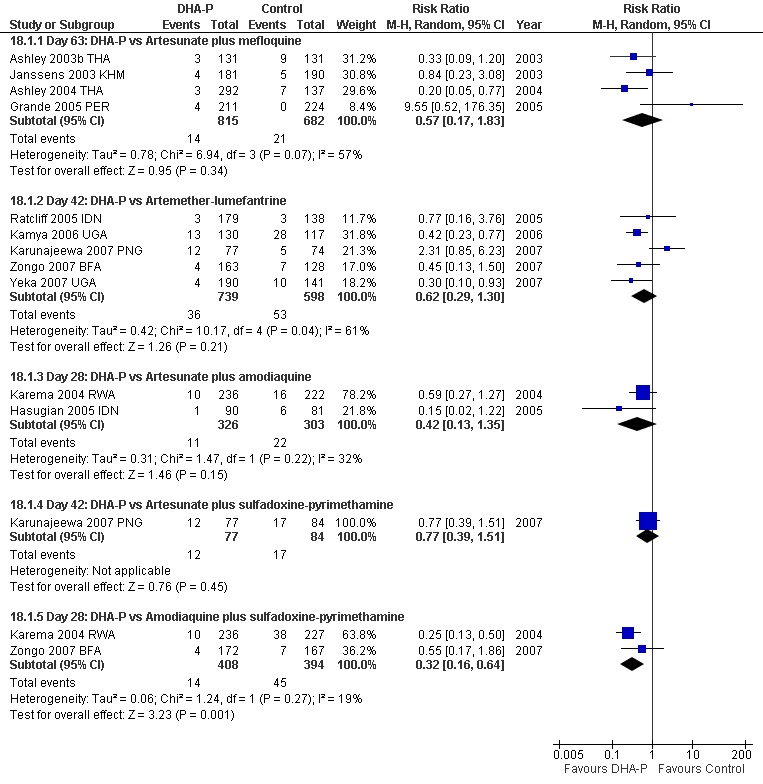
How does Dihydroartemisinin‐piperaquine perform? Summary of primary outcome: Effectiveness: Total Failure (P. falciparum) PCR adjusted.
4.
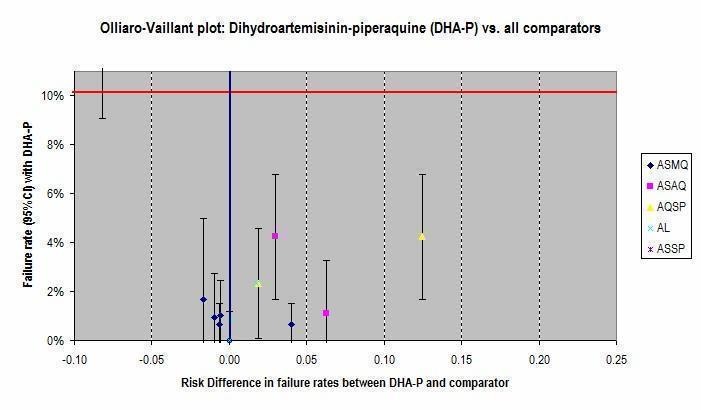
Olliaro‐Vaillant plot. Day 28 PCR adjusted treatment failure data for trials of DHA‐P against all comparators are presented in this plot.
The horizontal red line represents the WHO standard of 10% treatment failure (PCR corrected). Plots below this line represent trials where DHA‐P performed to this standard.
The vertical blue line represents no difference between the two drugs. Plots to the right of this line represent trials where DHA‐P performed better than the comparator drug, and plots to the left represent trials where the comparator drug performed better than DHA‐P.
5.
How does Artesunate plus mefloquine perform? Summary of primary outcome: Effectiveness: Total Failure (P. falciparum) PCR adjusted.
6.
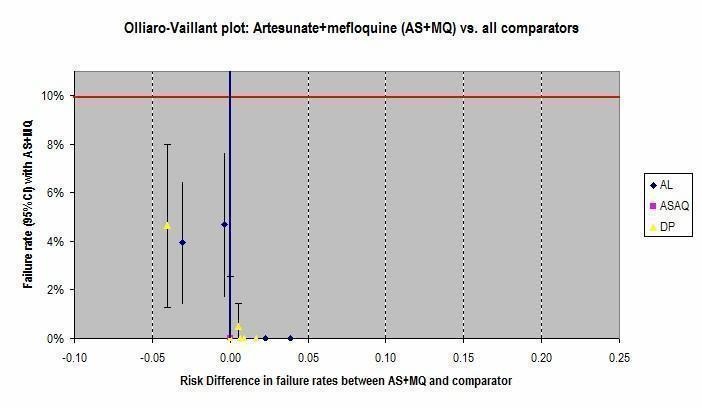
Olliaro‐Vaillant plot. Day 28 PCR adjusted treatment failure data for trials of AS+MQ against all comparators are presented in this plot.
The horizontal red line represents the WHO standard of 10% treatment failure (PCR corrected). Plots below this line represent trials where AS+MQ performed to this standard.
The vertical blue line represents no difference between the two drugs. Plots to the right of this line represent trials where AS+MQ performed better than the comparator drug, and plots to the left represent trials where the comparator drug performed better than AS+MQ.
7.
How does Artemether‐lumefantrine perform? Summary of primary outcome: Effectiveness: Total Failure (P. falciparum) Day PCR adjusted.
8.
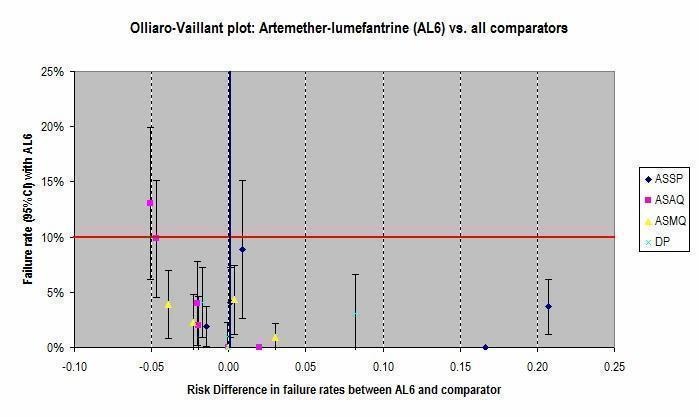
Olliaro‐Vaillant plot. Day 28 PCR adjusted treatment failure data for trials of AL6 against all comparators are presented in this plot.
The horizontal red line represents the WHO standard of 10% treatment failure (PCR corrected). Plots below this line represent trials where AL6 performed to this standard.
The vertical blue line represents no difference between the two drugs. Plots to the right of this line represent trials where AL6 performed better than the comparator drug, and plots to the left represent trials where the comparator drug performed better than AL6.
9.
How does Artesunate plus amodiaquine perform? Summary of primary outcome: Effectiveness: Total Failure (P. falciparum) PCR adjusted.
10.
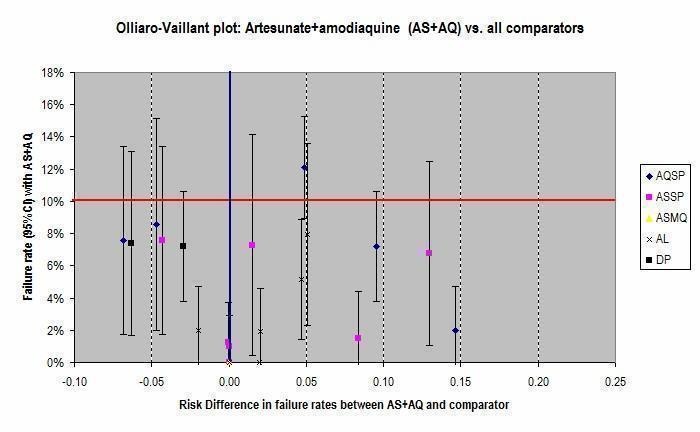
Olliaro‐Vaillant plot. Day 28 PCR adjusted treatment failure data for trials of AS+AQ against all comparators are presented in this plot.
The horizontal red line represents the WHO standard of 10% treatment failure (PCR corrected). Plots below this line represent trials where AS+AQ performed to this standard.
The vertical blue line represents no difference between the two drugs. Plots to the right of this line represent trials where AS+AQ performed better than the comparator drug, and plots to the left represent trials where the comparator drug performed better than AS+AQ.
Efficacy (P. vivax)
The two drugs with long half‐lives (DHA‐P and AS+MQ) have been shown to be superior to AL6 in reducing the incidence of P. vivax following treatment (for either P. falciparum or P. falciparum/P. vivax co‐infections). DHA‐P has also been shown to reduce the incidence of P. vivax compared to AS+AQ. Five trials have compared DHA‐P and AS+MQ and shown no difference.
There could be some public health benefits to using drugs with long half‐lives in this way, to prolong the malaria free period. One trial (Hasugian 2005 IDN) demonstrated a reduced risk of anaemia after treatment with DHA‐P. This is likely to be due to the lower incidence of both P. falciparum re‐infections and P. vivax in this group. As ACTs are ineffective at treating the liver stages of P. vivax, this effect may be lost as follow up continues as the majority of P. vivax will eventually relapse.
Prevention of transmission (as measured by gametocytes)
ACTs may be superior to AQ+SP (the only combination not containing an artemisinin derivative) in their effect on gametocytes. Gametocyte carriage at days three and seven was higher with AQ+SP compared to AS+MQ (one trial, 306 participants, Analysis 8.3) and AL6 (four trials, 1538 participants, Analysis 11.5). Gametocyte development in those negative at baseline was also higher with AQ+SP compared to AS+AQ (two trials, 1354 participants, Analysis 13.3). No difference was shown between AQ+SP and DHA‐P.
Artesunate plus mefloquine seems to be superior to DHA‐P in reducing the carriage of gametocytes and preventing gametocyte development. This effect may be a result of the relatively low artemisinin content of this combination. Pharmokinetic data suggest that dihydroartemisinin and artesunate are broadly bioequivalent (Newton 2002) but at current dosing the total dose of dihydroartemisinin over three days (6 mg/kg) is only half the total dose of artesunate (12 mg/kg).
DHA‐P did perform well against other combinations, and there is currently no evidence that it is inferior to AL6, AS+AQ or AQ+SP in its effect on gametocytes.
It should be noted that there is evidence that even submicroscopic levels of gametocytes (which are present in a significant number of patients after treatment) are capable of transmission (Bousema 2004 KEN).
Haematological recovery
Anaemia is a common complication of malaria. Following successful treatment of the parasite, the level of anaemia should improve gradually over time, provided there is no further re‐infection. This process can be hastened by supplementation with oral iron therapy.
In this review, where measures of haematological recovery were reported, there is no evidence of clinically important differences between the different ACTs.
Harms (as measured by adverse events)
The general lack of standardization in recording and reporting of adverse events unfortunately precludes the use of meta‐analysis to analyse safety data. In addition, very few of the included trials involved adequate blinding to prevent bias in adverse event reporting. Although serious adverse events seem to be uncommon, very few trials undertook the biochemical or haematological monitoring necessary to detect neutropenia or hepatotoxicity which have been previously reported.
DHA‐P seems to have a favourable profile in comparison to the other drugs. In the 17 trials involving DHA‐P, results are inconsistent, but individual trials have shown reduced incidence of vomiting, anorexia, abdominal pain, fatigue, and pruritis compared to AQ+SP, vomiting, anorexia, and fatigue compared to AS+AQ, abdominal pain and headache compared to AL6 and sleep disturbance, dizziness, anxiety, nausea and vomiting compared to AS+MQ.
AS+MQ seems to cause more sleep disturbance and dizziness than DHA‐P and AL6. Overall there are also probably more gastrointestinal symptoms with AS+MQ.
Combinations including amodiaquine do seem to cause more gastrointestinal upset when compared to DHA‐P but there is no convincing evidence of increased vomiting compared to AL6.
No clinically severe alterations in biochemical tests were noted in any of these trials.
AS+MQ tolerability in African children
There has been concern regarding the tolerability of AS+MQ in African children (WHO 2006). This concern was raised by Slutsker 1990 in a trial of mefloquine monotherapy in children aged three months to five years. They found vomiting rates of 16/56 (29%) with a single dose of 25 mg/kg and 26/65 (40%) with15 mg/kg; 13% and 8% were unable to tolerate a second dose respectively. Three important details from this trial should be noted: i) there was no comparison with an alternative therapy, ii) the one‐off dose was higher than in current regimens, and iii) the mean age of children was 13 months which is considerably younger than most trials of mefloquine in Asia.
In this review, we found two head to head trials of AS+MQ in Africa. Both of these studies excluded children aged < one year but vomiting was noted to be more common with AS+MQ in one of these trials (Sagara 2005b MLI). There are, in addition, several published single‐arm or excluded trials of AS+MQ use in Africa (Massougbodji 2002; Agomo 2008; Sagara 2008), but again these do not include the very young children as included in Slutsker 1990. It is therefore not possible with current evidence to say whether this poor tolerance is a consistent finding, whether it is substantially different from other available ACTs or whether the new regime of mefloquine 8 mg/kg/day is better tolerated.
Overall completeness and applicability of evidence
Due to the changing patterns of resistance, summary statistics should be interpreted with caution as the effectiveness of these combinations is likely to vary from place to place, and to change with time.
Evidence is generally lacking on the safety and efficacy of these combinations in very young children (< six months) and in pregnant and lactating women who were excluded from all of the included trials.
In addition to the ACTs presented here, two further combinations (dihydroartemisinin plus naphthoquine and artesunate plus sulfamethoxypyrazine‐pyrimethamine) are beginning to appear in the published literature and the market place, and these will be added to future updates of this review.
Quality of the evidence
The quality of the evidence has been assessed using the GRADE process (Guyatt 2008) and the results presented in the 'Summary of findings tables'. For these tables we asked the following questions:
1) Is dihydroartemsinin‐piperaquine a suitable alternative to the currently recommended ACTs?
There is high quality evidence that DHA‐P is at least as effective (at reducing PCR corrected treatment failure) as AS+MQ in Asia, and AL6 in Africa, and moderate quality evidence that DHA‐P is at least as effective as AS+AQ (Appendix 6).
2) Does amodiaquine plus sulfadoxine‐pyrimethamine remain a valid alternative to ACTs?
The performance of AQ+SP is highly variable and so it is difficult to make general statements on relative effects. There is moderate quality evidence that AQ+SP is inferior to DHA‐P and AL6 in East Africa and very low quality evidence that it is also inferior to AS+AQ (Appendix 6).
3) Does artesunate plus sulfadoxine‐pyrimethamine remain a valid alternative to other ACTs?
There is no good quality evidence comparing AS+SP to DHA‐P, AS+MQ or AL6. In trials comparing AS+SP to AS+AQ both drugs performed well and no clear difference was shown (Appendix 6).
4) Is artesunate plus mefloquine a valid alternative to the currently used ACTs in Africa?
AS+MQ generally performed well in trials in Asia against DHA‐P and AL6 (Appendix 6). The direct evidence from Africa versus AS+AQ and AQ+SP is of low quality (Summary of findings table 7; Summary of findings table 8). The high performance of AS+MQ is likely to be maintained in Africa where resistance to mefloquine is low.
For the comparison artemether‐lumefantrine versus artesunate plus amodiaquine see Appendix 6.
Potential biases in the review process
Data extraction was unblinded. All included trials are published; we were unable to obtain further unpublished data from pharmaceutical companies.
Authors' conclusions
Implications for practice.
All five ACTs performed adequately, to be used as first‐line therapies, in most sites where they were studied, however there are examples of failure rates above 10% with all combinations, emphasizing the need for continued monitoring and evaluation.
There is now a growing weight of evidence available to justify the use of dihydroartemisinin‐piperaquine as a first‐line treatment option for P. falciparum malaria.
There is evidence that the non‐artemisinin combination AQ+SP is failing in parts of East Africa where DHA‐P, AL6, and AS+AQ have been shown to be superior. There is also evidence that ACTs have a superior effect on gametocytes that may be of public health benefit particularly in low transmission settings.
The ACTs appear to be effective in treating the blood stage of P. vivax. There may also be some benefit in using drugs with long half‐lives to delay spontaneous relapses. This prophylactic effect needs to be balanced with the theoretical risk of promoting the development of drug resistance. Additionally, in areas where primaquine is being used to provide a radical cure this effect may not be be of clinical significance.
Evidence of the safety of artemisinins is accumulating. Serious adverse events with these drugs appear to be rare. However, these trials are not powered to detect rare but clinically important events and so it is imperative that active monitoring continues.
Implications for research.
There are several new ACT combinations in development which are likely to become commercially available in the next few years. Policy makers therefore have a greater range of potential products. In these circumstances, improved information on comparative efficacy, adverse events, and tolerability is invaluable for informed decision making.
Many trials are using relatively standardized primary outcomes. A move towards standardized approaches to measuring and reporting secondary outcomes, and adverse events, would greatly improve comparability between trials and meta‐analysis.
In the absence of mefloquine resistance, AS+MQ is likely to be highly effective in African countries but concerns regarding poor tolerability in young infants have restricted its use in this setting. There is in fact little evidence on the use of any of the ACTs in this age group, and head to head randomized trials are necessary to clarify or refute the specific concerns regarding AS+MQ and to provide more general guidance on the choice and use of ACTs in infants.
Further research is needed to clarify the role of specific ACTs in the treatment of P. vivax. It remains unclear as to whether a long acting ACT offers individual or public health benefits compared to standard treatments for radical cure.
The most vulnerable populations (pregnant women and very young infants) were excluded from all trials, and represent a critical gap in current knowledge.
What's new
| Date | Event | Description |
|---|---|---|
| 12 August 2009 | Amended | Tables for treatment comparisons, search strategy, primary outcome measures, adverse events, anaemia, and summary of findings moved to appendices. |
Acknowledgements
This document is an output from a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. We thank Hasifa Bukirwa for assistance with data extraction.
Appendices
Appendix 1. Treatment comparisons eligible for review
| Question | Analysis | Comparisons |
| 1. How does dihydroartemisinin‐piperaquine perform? |
1 | vs artesunate plus mefloquine |
| 2 | vs artemether‐lumefantrine (6 doses) | |
| 3 | vs artesunate plus amodiaquine | |
| 4 | vs artesunate plus sulfadoxine‐pyrimethamine | |
| 5 | vs amodiaquine plus sulfadoxine‐pyrimethamine | |
| 2. How does artesunate plus mefloquine perform? |
1 | vs dihydroartemisinin‐piperaquine |
| 6 | vs artemether‐lumefantrine (6 doses) | |
| 7 | vs artesunate plus amodiaquine | |
| ‐ | vs artesunate plus sulfadoxine‐pyrimethamine | |
| 8 | vs amodiaquine plus sulfadoxine‐pyrimethamine | |
| 3. How does artemether‐lumefantrine (6 doses) perform? |
2 | vs dihydroartemisinin‐piperaquine |
| 6 | vs artesunate plus mefloquine | |
| 9 | vs artesunate plus amodiaquine | |
| 10 | vs artesunate plus sulfadoxine‐pyrimethamine | |
| 11 | vs amodiaquine plus sulfadoxine‐pyrimethamine | |
| 4. How does artesunate plus amodiaquine perform? |
3 | vs dihydroartemisinin‐piperaquine |
| 7 | vs artesunate plus mefloquine | |
| 9 | vs artemether‐lumefantrine (6 doses) | |
| 12 | vs artesunate plus sulfadoxine‐pyrimethamine | |
| 13 | vs amodiaquine plus sulfadoxine‐pyrimethamine |
Footnotes
aTo contribute to informed decision‐making, the review is limited to artemisinin combination therapies (ACTs) for which co‐formulated products are currently available or shortly to be made available (trials using co‐packaged or loose preparations of these same ACTs are included).
Appendix 2. Detailed search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | malaria | malaria | malaria | malaria | malaria |
| 2 | arte* | arte* | arte* | arte* | arte* |
| 3 | dihydroarte* | dihydroarte* | dihydroarte* | dihydroarte* | dihydroarte* |
| 4 | amodiaq* | amodiaq* | amodiaq* | amodiaq$ | amodiaq$ |
| 5 | lumefantrine | lumefantrine | lumefantrine | lumefantrine | lumefantrine |
| 6 | Coartem* | Coartem* | Coartem* | Coartem$ | Coartem$ |
| 7 | mefloquine | mefloquine | mefloquine | mefloquine | mefloquine |
| 8 | 2 or 3 | 2 or 3 | 2 or 3 | 2 or 3 | 2 or 3 |
| 9 | 4 or 5 or 6 or 7 | 4 or 5 or 6 or 7 | 4 or 5 or 6 or 7 | 4 or 5 or 6 or 7 | 4 or 5 or 6 or 7 |
| 10 | 1 and 8 and 9 | 1 and 8 and 9 | 1 and 8 and 9 | 1 and 8 and 9 | 1 and 8 and 9 |
| 11 | — | — | Limit 10 to humans | Limit 10 to human | — |
Footnotes
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 3. Primary outcome measure (Total Failure) and sensitivity analyses
| Analysis | Participants | PCRb‐unadjusted | PCR‐adjusted | ||
| Numerator | Denominator | Numerator | Denominator | ||
| Primary analysis | Exclusions after enrolment | Excludedc | Excluded | Excluded | Excluded |
| Missing or indeterminate PCR | Included as failures | Included | Excluded | Excluded | |
| New infections | Included as failures | Included | Excluded | Excluded | |
| Sensitivity analysis 1d | As 'Primary analysis' except: missing or indeterminate PCR | — | — | Included as failures | Included |
| Sensitivity analysis 2e | As 'Sensitivity analysis 1' except: new infections | — | — | Included as successes | Included |
| Sensitivity analysis 3f | As 'Sensitivity analysis 2' except: exclusions after enrolment | Included as failures | Included | Included as failures | Included |
| Sensitivity analysis 4g | As 'Sensitivity analysis 2' except: exclusions after enrolment | Included as successes | Included | Included as successes | Included |
Footnotes
aNote: participants who were found to not satisfy the inclusion criteria after randomization are removed from all calculations. bPCR: polymerase chain reaction. c'Excluded' means removed from the calculation. dTo re‐classify all indeterminate or missing PCR results as treatment failures in the PCR‐adjusted analysis. eTo re‐classify all PCR‐confirmed new infections as treatment successes in the PCR‐adjusted analysis. (This analysis may overestimate efficacy as PCR is not wholly reliable and some recrudescences may be falsely classified as new infections. Also some participants may have gone on to develop a recrudescence after the new infection.) fTo re‐classify all exclusions after enrolment (losses to follow up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment failures. For PCR‐unadjusted total failure this represents a true worse‐case scenario. gTo re‐classify all exclusions after enrolment (losses to follow up, withdrawn consent, other antimalarial use, or failure to complete treatment) as treatment successes.
Appendix 4. Adverse event tables
| Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine | |||
| Study ID | Adverse event monitoring | Blinding | Summary of adverse event findings |
|
Ashley 2003a THA (134 participants) |
Inpatient monitoring until day 28 FBC, U&E, LFT on days 0 and 7 |
Open label | SAE: No serious adverse events observed Biochemical: No evidence of toxicity observed Other: No differences between the groups reported |
|
Ashley 2003b THA (356 participants) |
Daily review until parasites cleared then weekly until day 63 A subset of patients in the DHA‐P group had FBC, U&E and LFT on days 0 and 7 and ECG monitoring before and after treatment |
Open label | SAE: No serious adverse events observed GI: More abdominal pain reported with DHA‐P (P = 0.025) Nausea, vomiting, and diarrhoea not significantly different CNS: More sleep disturbance with AS+MQ (P = 0.008) Dizziness not significantly different Biochemical: Some minor fluctuations in LFTs CVS: No comment |
|
Ashley 2004 THA (499 participants) |
Clinical examination, symptom enquiry, and haematocrit daily until parasites cleared then weekly until day 63 |
Open label | SAE: 4 serious events with AS+MQ (death, severe anaemia, febrile convulsion, coagulopathy) and 11 with DHA‐P (2 deaths, bacterial sepsis, febrile convulsion, leptospirosis, haematemesis, nephritic syndrome, severe anaemia, respiratory infection, epigastric pain and vomiting). All except the one case of severe vomiting were judged to be unrelated or unlikely to be due to the study treatment GI: More diarrhoea with DHA‐P (P = 0.026); nausea, vomiting, and abdominal pain not significantly different CNS: No significant difference in dizziness or sleep disturbance Other: Urticaria occurred in 1 patient with DHA‐P but none with AS+MQ |
|
Grande 2005 PER (522 participants) |
Clinical assessment daily until day 3 then weekly until day 63 FBC, U&E, LFT, and PCV days 0 and 7, PCV days 14 and 63 |
Open label | SAE: 3 serious drug related events with AS+MQ requiring stopping treatment (encephalopathy, anxiety and arrhythmia, palpitations, and chest pain) GI: More nausea and vomiting with AS+MQ in adults (P = 0.02) but not significantly different in children. Abdominal pain and anorexia not significantly different CNS: More insomnia, dizziness and anxiety with ASMQ in adults (P = < 0.001) and more insomnia and anxiety with AS+MQ in children (P = < 0.001, 0.02). More somnolence with DHA‐P (P = 0.02) Biochemical: No clinically significant abnormal renal or liver test results |
|
Janssens 2003 KHM (464 participants) |
Clinical examination and symptom questionnaire days 0, 1, 2, 3. Only adverse events occurring in these 3 days are reported. |
Open label | SAE: No serious adverse events observed GI: More nausea, vomiting, and anorexia with AS+MQ, only vomiting was significant (P = 0.03) CNS: More dizziness and sleep disturbance with AS+MQ (P = 0.002, 0.03) CVS: More palpitations with AS+MQ (P = 0.04) |
|
Mayxay 2004 LAO (220 participants) |
Daily review until parasites cleared then weekly until day 42 | Open label | SAE: One neuropsychiatric reaction in AS+MQ group GI: More nausea and vomiting with AS+MQ (P = < 0.001, 0.02), abdominal pain and diarrhoea not significantly different CNS: More dizziness, sleep disturbance, nightmares, headache and weakness with AS+MQ (P = < 0.001, 0.02, 0.003, 0.001, 0.009) CVS/RS: More palpitations and dyspnoea with AS+MQ (P = 0.002, 0.04) |
|
Smithuis 2004 MMR (652 participants) |
Symptom questionnaire at days 0, 1, 2, 3 and 7. Only adverse events occurring in the first 7 days are reported. |
Open label | SAE: No serious adverse events reported in the first 7 days GI: More nausea with AS+MQ but only significant in the group having supervised treatment (P = 0.05), diarrhoea, vomiting, and abdominal pain were not significantly different CNS: More dizziness with AS+MQ but only significant in the group having unsupervised treatment (P = 0.03), no other symptoms reported |
|
Tangpukdee 2005 THA (180 participants) |
Inpatient monitoring until day 28. Assessed using non‐suggestive questioning. | Open label | SAE: No serious adverse events observed Other: Reported as minor. No differences between groups reported |
|
Tran 2002 VNM (243 participants) |
Review at days 0, 2 and 7 LFTs on days 3, 7 and 28. Further follow‐up is unclear. |
Open label | SAE: 12 events (10 vomiting, 2 dizziness) described as significant in AS+MQ group and none with DHA‐P (P = 0.002) Biochemical: No significant differences Other: All other adverse events described as minor with no differences between groups reported |
| Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Kamya 2006 UGA (421 participants) |
Assessed daily until day 3 then weekly until day 42. A standardized history, physical exam, including neurological assessment at each visit. Haemoglobin was checked at baseline and last day of follow up. |
Double‐blind | SAE: four with DHA‐P, 2 with AL, all judged to be unrelated to study meds (3 febrile convulsions, otitis media, asthma attack, pyomyositis) GI: No difference in vomiting, diarrhoea, abdominal pain, or anorexia CNS: No differences presented CVS/RS: No difference in cough |
|
Karunajeewa 2007 PNG (250 participants) |
Standardized follow up on days 0, 1, 2, 3, 7, 14, 28, and 42. Adverse event monitoring not described. | Open label | Overall comment: No treatment withdrawals were attributable to adverse events related to a study drug No other significant differences are noted between treatments |
|
Mens 2007 KEN (146 participants) |
Adverse events were recorded at each visit in the case record form (days 0, 1, 2, 3, 7, 14, and 28). An adverse event defined as any unfavourable and unintended sign. | Open label | SAE: 1 patient treated with DHA‐P died on day 14. Assessed as unrelated to treatment. GI: No difference in anorexia, abdominal pain, diarrhoea, or vomiting CVS/RS: No difference in cough CNS: Weakness more common with AL6 (P = 0.035). No difference in headache. Derm: No difference in pruritis |
|
Ratcliff 2005 IDN (774 participants) |
Assessed daily until fever and parasites cleared then weekly until day 42. A symptom questionnaire and physical exam at each visit. Haemoglobin was checked at each visit. |
Open label | SAE: 1 death 60 days after treatment. Cause not known GI: Diarrhoea was more common with DHA‐P (P = 0.003). Nausea, vomiting, abdominal pain, and anorexia not different CNS: Headache and dizziness not significantly different CVS/RS: Palpitations and cough not different Other: No difference in rash or myalgia |
|
Yeka 2007 UGA (414 participants) |
Standardized history, physical exam, and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42 and any other day they were unwell Assessed at each visit including neurological examination. Adverse events described as any untoward medical occurrence. |
Single Blind | SAE: 2 with AL, 5 with DHA‐P, all judged unrelated to study meds (2 convulsions, 2 pyomyositis, vomiting, severe anaemia, dehydration) GI: Abdominal pain more common with AL (P = 0.05). No difference in anorexia, vomiting or diarrhoea. RS/CVS: No difference in cough or coryza CNS: No difference in malaise/weakness Derm: No difference in pruritis Overall comment: Most AE were of mild to moderate severity and consistent with symptoms of malaria |
|
Zongo 2007 BFA (375 participants) |
Assessed daily until day 3 then weekly until day 42. A standardized history and physical exam at each visit. Haemoglobin was checked at baseline and last day of follow up. | Open label | SAE: None observed GI: Less abdominal pain with DHA‐P (P < 0.05), vomiting, diarrhoea, and anorexia not different CNS: Less headache with DHA‐P (P < 0.05), no difference in weakness CVS/RS: No difference in cough |
| Dihydroartemisinin‐piperaquine vs Artemether plus amodiaquine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Hasugian 2005 IDN (334 participants) |
Assessed at each follow‐up visit (daily until afebrile and clear of parasites, then weekly to day 42) An adverse event defined as a symptom that developed after starting treatment |
Open label | SAE: 3 with AS+AQ (2 vomiting, 1 ataxia), none with DHA‐P GI: On days 1 and 2 more nausea (P = 0.004), vomiting (P = 0.02), anorexia (P = 0.007) with AS+AQ No further comment |
|
Karema 2004 RWA (504 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, 21, and 28) An adverse event defined as any unfavourable and unintended sign associated temporally with the use of the drug administered Differential WBC count (and liver function tests at one site only) assessed at days 0 and 14 |
Open label | SAE: Not reported (one seizure with AS+AQ) GI: More vomiting (P = 0.007) and anorexia (P = 0.005) with AS+AQ. No difference in abdominal pain, diarrhoea, nausea CNS: More fatigue with AS+AQ (P = 0.001). No difference in seizures, headache, dizziness, drowsiness CVS/RS: No difference in cough, angina, oedema Biochemical: No differences in mean PCV or mean WBC. No hepatotoxicity observed (one site only) Other: No difference in rash |
| Dihydroartemisinin‐piperaquine vs artesunate plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Karunajeewa 2007 PNG (245 participants) |
Adverse event monitoring not described | Open label | Overall comment: No treatment withdrawals were attributable to adverse events related to a study drug No other significant differences are noted between treatments |
| Dihydroartemisinin‐piperaquine vs amodiaquine plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Karema 2004 RWA (510 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, 21, and 28) An adverse event defined as any unfavourable and unintended sign associated temporally with the use of the drug administered Differential WBC count (and liver function tests at 1 site only) assessed at days 0 and 14 |
Open label | SAE: Not reported (1 seizure with AQ+SP) GI: More vomiting (P = 0.007) and anorexia (P = 0.005) with AQ+SP. No difference in abdominal pain, diarrhoea, nausea CNS: More fatigue with AQSP (P = 0.001). No difference in seizures, headache, dizziness, drowsiness CVS/RS: No difference in cough, angina, oedema Biochemical: No differences in mean PCV or mean WBC. No hepatotoxicity observed (one site only) Other: No difference in rash |
|
Zongo 2007 BFA (371 participants) |
A standardized history and examination on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42 Adverse events defined as untoward medical occurrences Haemoglobin measured on days 0 and 42 or day of clinical failure |
Open label | SAE: No serious adverse events were observed GI: Abdominal pain was more common with AQ+SP (P < 0.05). No difference in vomiting, diarrhoea, or anorexia. CNS: No difference in headache or weakness CVS/RS: No difference in cough Other: Pruritis more common with AQ+SP (P < 0.05) |
| Artesunate plus mefloquine vs Artemether‐lumefantrine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Faye 2003 SEN (294 participants) |
All side effects were monitored actively (days 0, 1, 2, 7, 14, 21, and 28) and passively during the study 25% were randomly selected for blood counts, liver and renal function tests at days 0, 14, and 28 |
Open label | SAE: No serious adverse events Overall comment: The side effects observed with each treatment combination were minor, mainly gastralgia, dizziness, pruritis, asthenia, and vomiting Biochemical: No severe alterations in renal or hepatic function were observed |
|
Hutagalung 2002 THA (490 participants) |
Routine follow up daily until fever and parasites cleared then weekly to day 42 or any other day they became unwell At each visit a questionnaire on adverse events was completed An adverse event defined as symptoms or signs that were not present on admission and that developed after the start of treatment |
Open label |
SAE: None reported Overall comment: Both treatment regimens were well tolerated |
|
Lefevre 1999 THA (219 participants) |
Routine follow up at days 1, 2, 3, 7, 14, 21, and 28. Adverse events assessed at each visit. ECG monitoring and laboratory tests (including FBC liver and renal function tests) at baseline and each day of follow‐up. |
Open label | SAE: No comment. GI: Abdominal pain, nausea, vomiting, diarrhoea, anorexia, constipation 18.3% AL vs 21.8% AS+MQ CNS: Headache, dizziness, and sleep disorder‐ 27.4% AL vs 16.4% AS+MQ CVS/RS: ECG 2% of each group showed QT prolongation of potential relevance with no cardiac complication Haematological: Slight worsening of anaemia after 3 days in both groups Biochemical: Liver function tests slightly abnormal at baseline. All baseline parameters normalized over the course of treatment. Renal function, electrolytes, glucose. Protein, urine tests showed no relevant changes after baseline in either group. Other: Skin reactions 8 AL vs 2 AS+MQ |
|
Mayxay 2003 LAO (220 participants) |
Routine follow up daily until fever and parasites cleared then weekly until day 42 or anytime they felt unwell Potential side effects were recorded at each visit |
Open label |
SAE: 3 serious neuropsychiatric events in AS+MQ group GI: Nausea and vomiting, abdominal pain, and diarrhoea more common with AS+MQ (P < 0.05) CNS: Weakness, dizziness, headache, confusion, and irritable/angry all more common with AS+MQ (P < 0.05). No difference in nightmares and tinnitus. CVS/RS: No difference in palpitations or dyspnoea Other: No difference in urticaria, herpes or blurred vision |
|
Sagara 2005b MLI (270 participants) |
Routine follow up on days 1, 2, 3, 7, 14, 21, and 28 Complete blood count, ALT and creatinine on 20% of participants on days 0 and 14 A serious adverse event was defined according to the International Conference on Harmonisation |
Open label | SAE: Not mentioned GI: Vomiting more common with AS+MQ (P = 0.04). No significant difference in abdominal pain or diarrhoea. CNS: No significant difference in headache, weakness, dizziness (P = 0.06) or malaise Dermatological: No significant difference in pruritis or rash Biochemical: States 'both treatments were similar for laboratory adverse events' |
|
Stohrer 2003 LAO (108 participants) |
Treatment emergent symptoms and signs were recorded on days 0 to 3 |
Open label |
SAE: 1 AL: severe diarrhoea, 1 ASMQ heavy sleep disorder and dizziness GI: None of the patients in either arm vomited within 1 hour of drug intake. No differences in abdominal pain, nausea, vomiting, diarrhoea, anorexia. CNS: Headache, dizziness, weakness, sleep disorder: 14 AL vs 22 ASMQ no significant difference |
|
Van den Broek 2003a BGD (242 participants) |
Routine follow up on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42 and any other day when feeling ill Possible side effects assessed at each visit |
Open label |
SAE: None observed During the first 3 days headache, vomiting, nausea, and dizziness were significantly more common with AS+MQ (P < 0.05) Other complaints were: sleeplessness, pruritis/rash, epigastric pain, sweating with AS+MQ; blurred vision and anorexia with AL |
|
Van Vugt 1998 THA (200 participants) |
Routine follow up daily until fever and parasites cleared then weekly to day 28 A questionnaire for adverse effects was completed at each visit. Full neurological examination on days 0, 3, 7, and 28. Complete haematology and biochemistry (at one centre) on days 0, 3, 7, and 28. |
Open label |
SAE: 1 with AL: coma lasting 4 days 12 days after treatment, 1 with AS+MQ; generalized urticaria on day 1 Vomiting of medication: 4/150 AL vs 5/50 ASMQ (P = 0.045) GI: Anorexia, vomiting, nausea, abdominal pain, hepatomegaly less common with AL (12.7% AL vs 26% AS+MQ, P = 0.043) CVS: No electrocardiographic changes CNS: CNS symptoms (dizziness, sleep disorder, headache) less common with AL (6% AL vs 34% AS+MQ, P < 0.0001). One case of tremor and 2 cases of numbness with AL. Overall: Possible drug related adverse events less common with AL (33/150 AL vs 23/50 ASMQ, P = 0.002) |
| Artesunate plus mefloquine vs Artesunate plus amodiaquine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Faye 2003 SEN (505 participants) |
All side effects were monitored actively (days 0, 1, 2, 7, 14, 21, and 28) and passively during the study 25% were randomly selected for blood counts, liver, and renal function tests at days 0, 14, and 28 |
Open label | SAE: No serious adverse events Overall comment: The side effects observed with each treatment combination were minor; mainly gastralgia, dizziness, pruritis, asthenia, and vomiting Biochemical: No severe alterations in renal or hepatic function were observed |
| Artesunate plus mefloquine vs Amodiquine plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Faye 2003 SEN (306 participants) |
All side effects were monitored actively (days 0, 1, 2, 7, 14, 21, and 28) and passively during the study 25% were randomly selected for blood counts, liver, and renal function tests at days 0, 14, and 28 |
Open label | SAE: No serious adverse events Overall comment: The side effects observed with each treatment combination were minor, mainly gastralgia, dizziness, pruritis, asthenia, and vomiting Biochemical: No severe alterations in renal or hepatic function were observed |
| Artemether‐lumefantrine vs Artesunate plus amodiaquine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Adjei 2006 GHA (227 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, and 28), including neurological assessment Audiological assessment on days 0, 3, 7, and 28 Total and differential WBC counts and liver enzymes on days 0, 3, 7, 14, and 28 |
Single blind (outcome assessors) | SAE: 1 patient treated with AS+AQ had severe anaemia on day 14 GI: No significant difference in nausea and vomiting between groups CNS: No significant difference in dizziness, fatigue, or excessive sleepiness between groups. Nystagmus was observed in 1 patient in each group, both cases had potential explanations from the past medical history. A positive Romberg's test was observed in 1 child treated with AL, again with a possible alternative diagnosis. Audiology: Hearing thresholds were significantly elevated in treated subjects as days 0, 3, 7, and 28 but no differences between participants and controls after 9 months Haematological: The mean neutrophil count was lower than baseline in both groups throughout follow up but there was no significant difference between groups. There was no significant difference in the incidence of neutropenia between groups (14/111 AL vs 13/116) Biochemical: No difference in liver enzymes were observed between groups. Liver enzymes were not observed to increase in response to treatment. |
|
Bukirwa 2005 UGA (408 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, and 28), including neurological assessment An adverse event defined as any untoward medical occurrence |
Single blind (outcome assessors) | SAE: One serious adverse event in each group (AL6 convulsion; AS+AQ pneumonia) both judged unlikely to be related to study meds CNS: No abnormalities in hearing or fine finger dexterity Overall comment: Adverse events of at least moderate severity: 125/202 AL vs 136/201 ASAQ (P = 0.25) |
|
Dorsey 2006 UGA (434 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, and 28) An adverse event defined as any untoward medical occurrence Complete blood count and liver enzymes on days 0 and 14 |
Single blind (outcome assessors) | SAE: 29 serious adverse events (14/202 AL vs 15/232 ASAQ). Majority were seizures associated with fever. None considered probably or definitely related to study meds GI: Anorexia more common with ASAQ (P < 0.05). No significant difference in abdominal pain, vomiting or diarrhoea CVS/RS: No significant difference in cough CNS: No other significant differences in weakness Biochemical: Elevated liver enzymes occurred in 7 patients, all were attributed to other causes (6 viral hepatitis and 1 Salmonella bacteraemia) Other: No significant difference in pruritis |
|
Falade 2005 NGA (132 participants) |
Assessed at each visit (days 0 to 7, 14, 21, and 28) FBC, WBC and liver enzymes on days 0, 7 and 28 An adverse event defined as not present at enrolment but occurring during follow ‐up |
Open label | SAE: There were no serious adverse events GI: No significant difference in abdominal pain or vomiting CVS/RS: No significant difference in cough or palpitations Haem: A significant transient decline in neutrophil counts between days 0 and 7 with AL which recovered by day 28 Biochemical: No statistically significant disturbance in blood chemistry. The study drugs did not adversely affect liver enzymes |
|
Faye 2003 SEN (509 participants) |
All side effects were monitored actively (days 0, 1, 2, 7, 14, 21, and 28) and passively during the study 25% were randomly selected for blood counts, liver and renal function tests at days 0, 14, and 28 |
Open label | SAE: No serious adverse events Overall comment: The side effects observed with each treatment combination were minor, mainly gastralgia, dizziness, pruritis, asthenia, and vomiting Biochemical: No severe alterations in renal or hepatic function were observed |
|
Guthmann 2004 AGO (134 participants) |
Adverse event monitoring not described | Unclear | AE not reported (2 patients excluded from AS+AQ group for vomiting and 1 from AL) |
|
Kobbe 2007 GHA (237 participants) |
'The comparative tolerability was assessed by the risk of occurrence of an adverse event' For each adverse event causality was assessed as recommended by the WHO |
Open label | SAE: 2 SAE in each group, all classified as unlikely to be related to the treatment (asthma attack, febrile convulsion, enteritic bacterial infection, and severe anaemia) GI: No difference in GI symptoms including vomiting CVS/RS: No difference in respiratory symptoms Derm: No difference in dermatological symptoms |
|
Koram 2003 GHA (105 participants) |
Adverse event monitoring not described | Open label | AE not reported (3 patients with AS+AQ and 1 with AL were withdrawn for excessive vomiting) |
|
Martensson 2003 TZA (407 participants) |
Possible adverse events recorded at each visit (days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42) Differential white cell counts at days 0, 3, 7, 14, 21, and 28 An adverse event was defined as any undesirable medical occurrence regardless of whether it was related to the treatments |
Unclear | SAE: 9 severe adverse events (2/200 AL vs 7/208 AS+AQ) all associated with clinically suspected severe malaria and not attributed to study drugs Haematological: No significant differences in mean WBC or neutrophil count between groups Overall comment: Both regimens generally well tolerated |
|
Mutabingwa 2004 TZA (1034 participants) |
Parents or guardians were asked to report on side effects, tolerability and usefulness of the treatment (days 0, 14, and 28) | Unclear | SAE: 1 death in the group treated with AL No other reporting of AE |
|
Van den Broek 2004 ZAR (207 participants) |
Possible side effects as passively reported to the examiner were recorded at each visit (days 0, 1, 2, 3, 7, 14, 21, and 28) | Open label | SAE: No severe adverse events judged to be related to the treatment given Overall comment: Common complaints were vomiting, diarrhoea, abdominal pain, and anorexia The frequency of potential adverse events was low (around 10%) and did not differ between groups. 1 case of urticaria occurred with AS+AQ |
|
Owusu‐Agyei 2006 GHA (355 participants) |
Field workers visited their homes to solicit adverse events on days 0, 2, 3, 7, 14, and 28 | Open label | SAE: Not reported GI: No significant difference in diarrhoea, vomiting, nausea, anorexia, abdominal pain CNS: No significant difference in difficulty sleeping CVS/RS: No significant difference in cough, dyspnoea, palpitation Other: Body pain more common with AS+AQ. No difference in fever, runny nose, itching, joint pain, ulcers, yellow eyes |
| Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Bousema 2004 KEN (249 participants) |
Adverse event monitoring not described | Single blind (outcome assessors) | AE not reported |
|
Karunajeewa 2007 PNG (249 participants) |
Adverse event monitoring not described | Open label | Overall comment: No treatment withdrawals were attributable to adverse events related to a study drug No other significant differences are noted between treatments |
|
Mukhtar 2005 SDN (160 participants) |
Adverse event monitoring not described | Unclear | AE not reported |
|
Van den Broek 2004 ZAR (197 participants) |
Possible side effects as passively reported to the examiner were recorded at each visit (days 0, 1, 2, 3, 7, 14, 21, and 28) | Open label | SAE: No severe adverse events judged to be related to the treatment given Overall comment: Common complaints were vomiting, diarrhoea, abdominal pain and anorexia The frequency of potential adverse events was low (around 10%) and did not differ between groups. 1 case of urticaria occurred with AS+SP |
| Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Dorsey 2006 UGA (455 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, and 28) An adverse event defined as any untoward medical occurrence Complete blood count and liver enzymes on days 0 and 14 |
Single blind (outcome assessors) | SAE: 30 serious adverse events (14/202 AL vs 16 AQ+SP). Majority were seizures associated with fever. None considered probably or definitely related to study meds. GI: Anorexia more common with AQ+SP (P < 0.05). No significant difference in abdominal pain, vomiting, or diarrhoea. CVS/RS: No significant difference in cough CNS: Weakness more common with AQ+SP (P < 0.05). No other significant differences. Biochemical: Elevated liver enzymes occurred in 7 patients, all were attributed to other causes (6 viral hepatitis and 1 Salmonella bacteraemia) Other: No significant difference in pruritis |
|
Fanello 2004 RWA (500 participants) |
All adverse events were recorded on the clinical record form (days 7, 14, 21, and 28) and a causality assessment was made PCV and WBC days 0 and 14 |
Open label | SAE: No comment on serious AE Overall comment: 251 patients reported one AE concomitant with administration of the drug with no differences between groups. AE possibly or probably related to the study drugs 22/251 AL, 35/249 AQ+SP P = 0.06 Haem: Mean WBC count at day 14 was similar in both groups (data not shown). |
|
Faye 2003 SEN (310 participants) |
All side effects were monitored actively (days 0, 1, 2, 7, 14, 21, and 28) and passively during the study 25% were randomly selected for blood counts, liver and renal function tests at days 0, 14, and 28 |
Open label | SAE: No serious adverse events Overall comment: The side effects observed with each treatment combination were minor, mainly gastralgia, dizziness, pruritis, asthenia, and vomiting Biochemical: No severe alterations in renal or hepatic function were observed |
|
Mutabingwa 2004 TZA (1026 participants) |
Parents or guardians were asked to report on side effects, tolerability, and usefulness of the treatment (days 0, 14, and 28) | Unclear | SAE: 1 death in each group No other reporting of AE |
|
Zongo 2007 BFA (372 participants) |
Assessed at each visit (days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42) Adverse events defined as any untoward medical occurrence |
Open label | SAE: No serious adverse events GI: No significant difference in abdominal pain, vomiting, diarrhoea, or anorexia CVS/RS: No significant difference in cough CNS: No significant difference in headache or weakness. Other: Pruritis more common with AQ+SP (P < 0.05) |
|
Zongo 2005 BFA (521 participants) |
Assessed at each visit (days 0, 1, 2, 3, 7, 14, 21, and 28) Adverse events defined as any untoward medical occurrence |
Double blind | SAE: 1 serious AE in each group (severe anaemia) GI: No significant difference in abdominal pain, vomiting, diarrhoea, or anorexia CVS/RS: No significant difference in cough or coryza CNS: No significant difference in headache or weakness Other: Pruritis more common with AQ+SP (P < 0.0001) |
| Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Bonnet 2004 GIN (220 participants) |
Adverse event monitoring not described | Open label | AE not reported |
|
Djimde 2004 MLI ( participants) |
Haemoglobin, glucose, complete blood count, liver enzymes, and creatinine were measured on days 0, 7, 14, and 28 | Single blind (details not given) | SAE: One with AS+AQ. Overall comment: Adverse event distribution was unremarkable. Haematological: All treatment decreased the prevalence of abnormal values of leucocytes and platelets (figures not given) Biochemical: At day 14 the prevalence of grade 1 ALT toxicity was 9.7% AS+AQ vs 2.5% AS+SP (figures not given). These changes not thought to be clinically significant. |
|
Guthmann 2003 AGO (187 participants) |
Adverse event monitoring not described | Open label | AE not reported |
|
Hamour 2003 SDN (161 participants) |
Adverse event monitoring not described | Open label | SAE: No significant adverse events Overall comment: No significant adverse events were reported |
|
Kayentao 2006 MLI (265 participants) |
Adverse event monitoring not described | Single blind | One death occurred at day 7 after treatment with AS+SP. The parasitaemia was reported as cleared and cause of death unknown. Other AE not reported |
|
Swarthout 2004 ZAR (180 participants) |
Parents and guardians were asked about tolerability and potential side effects of the drugs (days 0, 1, 2, 3, 7, 14, 21, and 28) | Open label | SAE: None reported Overall comment: There were no adverse side effects reported by parents and both regimens were well tolerated |
|
Van den Broek 2004 ZAR (192 participants) |
Possible side effects as passively reported to the examiner were recorded at each visit (days 0, 1, 2, 3, 7, 14, 21, and 28) | Open label | SAE: No severe adverse events judged to be related to the treatment given Overall comment: Common complaints were vomiting, diarrhoea, abdominal pain and anorexia The frequency of potential adverse events was low (around 10%) and did not differ between groups. 1 case of urticaria occurred with AS+SP. |
| Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine | |||
| Study ID | Adverse event monitoring | Blinding | Adverse events |
|
Dorsey 2006 UGA (485 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, and 28) An adverse event defined as any untoward medical occurrence Complete blood count and liver enzymes on days 0 and 14 |
Single blind (outcome assessors) | SAE: 31 serious adverse events (15/232 AS+AQ vs 16/253 AQSP). Majority were seizures associated with fever. None considered probably or definitely related to study meds. GI: Anorexia more common with AQ+SP (P < 0.05). No significant difference in abdominal pain, vomiting or diarrhoea. CVS/RS: No significant difference in cough CNS: Weakness more common with AQ+SP (P < 0.05). No other significant differences Biochemical: Elevated liver enzymes occurred in 7 patients, all were attributed to other causes (6 viral hepatitis and 1 Salmonella bacteraemia) Other: No significant difference in pruritis |
|
Faye 2003 SEN (521 participants) |
All side effects were monitored actively (days 0, 1, 2, 7, 14, 21, and 28) and passively during the study 25% were randomly selected for blood counts, liver and renal function tests at days 0, 14, and 28 |
Open label | SAE: No serious adverse events Overall comment: The side effects observed with each treatment combination were minor, mainly gastralgia, dizziness, pruritis, asthenia and vomiting Biochemical: No severe alterations in renal or hepatic function were observed |
|
Karema 2004 RWA (510 participants) |
Assessed at each follow‐up visit (days 0, 1, 2, 3, 7, 14, 21, and 28) An adverse event defined as any unfavourable and unintended sign associated temporally with the use of the drug administered Differential WBC count (and liver function tests at one site only) assessed at days 0 and 14 |
Open label | SAE: Not reported (one seizure with AS+AQ, one with AQ+SP) GI: No differences in nausea, vomiting, diarrhoea, abdominal pain, or anorexia CVS/RS: No difference in cough, angina, oedema CNS: No difference in seizures, headache, dizziness, drowsiness, or fatigue Biochemical: No differences in mean PCV or mean WBC. No hepatotoxicity observed (1 site only) Other: No difference in rash |
|
Kayentao 2006 MLI (265 participants) |
Adverse event monitoring not described | Single blind | AE not reported |
|
Menard 2006 MDG (166 participants) |
Adverse event monitoring not described | Single blind (outcome assessors) | SAE: ‘No severe side effects attributable to the study medication’ No other reporting of AE |
|
Mutabingwa 2004 TZA (1022 participants) |
Parents or guardians were asked to report on side effects, tolerability, and usefulness of the treatment (days 0, 14, and 28) | Unclear | SAE: 1 death in the AQ+SP group died on the day of randomization No other reporting of AE |
|
Staedke 2003 UGA (268 participants) |
Assessed at each visit with a standardized history and examination. Neurological assessment on days 0, 7, 14, and 28. CBC, creatine and alanine transferase on days 0, 7, and 28. |
Single blind (outcome assessors) | SAE: 16 serious adverse events (1/134 AS+AQ vs 6/134 AQ+SP CNS: ‘No important neurological events were seen’ Biochem: 1 severe anaemia with AS+AQ, 1 severe neutropenia with AQ+SP, 1 elevated alanine transaminase with AQ+SP No other comment on adverse events |
|
Yeka 2004 UGA (1461 participants) |
Adverse event monitoring not described | Single blind (outcome assessors) | SAE: 4/731 AS+AQ vs 10/730 AQ+SP. 2 additional patients died in the AQ+SP group No other reporting of AE |
Footnotes
AE = adverse event DHA‐P = dihydroartemisinin‐piperaquine AS = artesunate MQ = mefloquine AL = artemether‐lumefantrine AQ = amodiaquine SP = sulfadoxine‐pyrimethamine SAE = serious adverse event GI = gastrointestinal system CVS = cardiovascular system RS = respiratory system CNS = central nervous system ECG = electrocardiogram QT = interval between the Q and T waves of an ECG U&E = urea and electrolytes FBC = full blood count LFT = liver function tests PCR = polymerase chain reaction PCV = packed cell volume WBC = white blood cells
Appendix 5. Anaemia tables
| Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine | ||
| Study ID | Outcome measure and result | Significance test |
| Ashley 2003b THA | Median decrease in haematocrit by day 7: DHA‐P 6.3% (0% to 13.6%) vs AS+MQ 9.4% (2.6% to 14.3%) Mean haematocrit weekly from day 0 to 63: Presented graphically |
P = 0.21 |
| Ashley 2004 THA | Median change in haematocrit in each group, each week, from day 0 to 63: 'a decrease in haematocrit in both groups between days 0 and 7 followed by recovery in both groups'. Figures presented graphically. | Not reported |
| Janssens 2003 KHM | Mean haematocrit at day 63: DHA‐P 40.0% vs AS+MQ 40.2% (No differences at baseline) |
Not reported |
| Mayxay 2004 LAO | Mean haematocrit following treatment (days 7 to 42): 'did not significantly differ between groups'. Figures not given. | Not significant P > 0.05 |
| Smithuis 2004 MMR | Mean haemoglobin at day 28 (supervized treatment): DHA‐P 10.4g/dl vs AS+MQ 10.5g/dl Proportion anaemic (Hb < 10g/dl) on day 28 (superviZed treatment): DHA‐P 56/152 vs AS+MQ 59/156 (no differences at baseline) |
P = 0.65 P = 0.85 |
| Artesunate plus mefloquine vs Artemether‐lumefantrine | ||
| Study ID | Outcome measure and result | Significance test between groups |
| Faye 2003 SEN | Proportion with anaemia (Hb < 12) on day 0: AS+MQ 15/24 (62.5%) vs AL6 24/35 (68.6%) Proportion with anaemia (Hb < 12) on day 14: AS+MQ 17/24 (70.8%) vs AL6 24/35 (68.6%) (On 25% randomly selected participants) |
Not reported |
| Hutagalung 2002 THA | Mean decrease in haematocrit by day 7: AS+MQ 9.3% (SD 11.5%, 95% CI 7.7% to 10.9%) vs AL6 6.7% (SD 11.4%, 95% CI 5.1% to 8.3%) | P = 0.023 |
| Lefevre 1999 THA | Mean haemoglobin on day 0: AS+MQ 11.5 g/dl vs AL6 11.6 g/dl Mean haemoglobin on day 29: AS+MQ 12.2 g/dl vs AL6 12.4 g/dl |
Not reported |
| Mayxay 2003 LAO | Mean haematocrit after treatment (day 7 to 42): Data presented graphically | P > 0.05 |
| Van Vugt 1998 THA | Proportion with anaemia (haematocrit < 30%) on day 0: AS+MQ 10% vs AL6 6% Proportion with anaemia (haematocrit < 30%) on day 28: AS+MQ 2.4% vs AL6 2.3% |
Not reported |
| Sagara 2005b MLI | Proportion with anaemia (Hb < 10g/dl) on day 0: AS+MQ 24/213 (11.3%) vs AL6 27/193 (14.0%) Proportion with anaemia (Hb < 10g/dl) on day 28: AS+MQ 10/213 (4.7%) vs AL6 10/193 (5.2%) |
Not reported |
| Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine | ||
| Study ID | Outcome measure and result | Significance test between groups |
| Dorsey 2006 UGA | Mean (SD) change in haemoglobin from baseline to Day 14: AS+AQ ‐0.03 (1.10) g/dl vs AQ+SP 0.16 (1.03) g/dl | Not reported |
| Faye 2003 SEN | Proportion with anaemia (Hb < 12g/dl) on day 0: AS+AQ 35/52 (68.6%) vs AQ+SP 19/27 (70.3%) Proportion with anaemia (Hb < 12g/dl) on day 14: AS+AQ 40/51 (80.4%) vs AQ+SP 21/27 (77.7%) In random 25% or study population |
Not reported |
| Karema 2004 RWA | Mean (SD) PCV at day 14: AS+AQ 34.0% (3.7) vs AQ+SP 34.5 (3.7) | Not significant P not given |
| Kayentao 2006 MLI | Mean (SD) haemoglobin day 14: AS+AQ 10.17 (1.5) g/dl vs AQ+SP 10.43 (1.49) g/dl Mean (SD) haemoglobin day 28: AS+AQ 10.78 (1.49) g/dl vs AQ+SP 11.05 (1.52) g/dl |
Not significant (P value not given) Not significant (P value not given) |
| Menard 2006 MDG | Median (IQR) of individual increases in Hb from baseline to day 28 (95% CI): AS+AQ 1.1 g/dl (‐2.6 to 5.2) vs AQ+SP 0.5 g/dl (‐4.4 to 5.8) | Not significant P not given |
| Mutabingwa 2004 TZA | Mean (SD) change in haemoglobin from baseline to Day 14: AS+AQ 0.58 (1.4) g/dl vs AQ+SP 0.54 (1.4) g/dl | Not reported |
| Staedke 2003 UGA | Median (SD not reported) change in haemoglobin from baseline to day 28: AS+AQ 1.9 g/dl vs AQ+SP 1.3 g/dl | P = 0.004 |
| Yeka 2004 UGA | Mean increase in haemoglobin by Day 28: Jinja site: AS+AQ 0.95 (1.91) g/dl vs AQ+SP 1.15 (1.93) g/dl Arua site: AS+AQ 1.44 (1.67) g/dl vs AQ+SP 1.44 (1.60) g/dl Tororo site: AS+AQ 1.14 (1.48) g/dl vs AQ+SP 1.58 (1.55) g/dl Apac site: AS+AQ 1.76 (1.55) g/dl vs AQ+SP 1.77 (1.79) g/dl |
P > 0.05 P > 0.05 P < 0.05 P > 0.05 |
Footnotes
DHA‐P = dihydroartemisinin‐piperaquine AS = artesunate MQ = mefloquine AL6 = artemether‐lumefantrine AQ = amodiaquine SP = sulfadoxine‐pyrimethamine Hb = haemoglobin IQR = interquartile range PCV = packed call volume SD = standard deviation
Appendix 6. Summary of findings tables
| Is Dihydroartemisinin‐piperaquine as effective as Artesunate plus mefloquine for uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Dihydroartemisinin‐piperaquine Comparison: Artesunate plus mefloquine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate plus mefloquine | Dihydroartemisinin‐piperaquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 63 PCR adjusted ‐ Asia | 46 per 1000 | 18 per 1000 (9 to 36) | RR 0.39 (0.19 to 0.79) | 1062 (3) | ⊕⊕⊕⊕ high1,2,3,4,5,6 |
| Efficacy: Total Failure (P. falciparum) Day 63 PCR unadjusted ‐ Asia | 151 per 1000 | 110 per 1000 (82 to 148) | RR 0.73 (0.54 to 0.98) | 1182 (3) | ⊕⊕⊕⊕ high1,2,3,4,5,6 |
| Efficacy: Total Failure (P. falciparum) Day 63 PCR adjusted ‐ South America | 0 per 1000 | Not estimable | RR 9.55 (0.52 to 176.35) | 435 (1) | ⊕ very low7,8,9,10 |
| Efficacy: Total Failure (P. falciparum) Day 63 PCR unadjusted ‐ South America | 9 per 1000 | 56 per 1000 (13 to 246) | RR 6.19 (1.4 to 27.35) | 445 (1) | ⊕⊕⊕ moderate7,8,9,11 |
| Vivax efficacy: P. vivax parasitaemia by day 63 | 180 per 1000 | 200 per 1000 (164 to 241) | RR 1.11 (0.91 to 1.34) | 1661 (4) | ⊕⊕⊕ moderate4,12,13,14,15 |
| Transmission potential: Gametocyte development (in those negative at baseline) | 9 per 1000 | 28 per 1000 (10 to 79) | RR 3.06 (1.13 to 8.83) | 1234 (3) | ⊕⊕⊕⊕ high4,11,13,16 |
| Harms: Serious adverse events (including deaths) | 7 per 1000 | 6 per 1000 (3 to 15) | RR 0.9 (0.38 to 2.15) | 2617 (8) | ⊕⊕ low4,10,13,17 |
| Harms: Early vomiting | 88 per 1000 | 79 per 1000 (61 to 102) | RR 0.90 (0.69 to 1.16) | 2473 (7) | ⊕⊕ low4,13,18,19 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Data on treatment failure at days 42 and 28 were also available and no differences between the two drugs were shown. 2Ashley 2003b THA, Ashley 2004 THA and Janssens 2003 KHM. 3No serious limitations: Allocation concealment was judged to be at 'low risk of bias' in two trials and 'unclear' in one. Sensitivity analysis only including trials with adequate concealment did not substantially change the result. Laboratory staff were blinded in two of the trials. 4No serious inconsistency: Heterogeneity was low. 5No serious indirectness: Trials were conducted in Asia (Thailand and Cambodia) in areas of low and unstable transmission. Children age < one year and pregnant or lactating women were excluded. 6 No serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with DHA‐P over AS+MQ and no appreciable benefit. 7Grande 2005 PER. 8No serious limitations: Allocation concealment was assessed as 'low risk of bias'. No blinding was described in this trial. 9Serious indirectness: Only one trial conducted in Peru in a low transmission setting. Children age < 5 years and pregnant and lactating women were excluded. 10Very serious imprecision: The 95% CI of the pooled estimate is wide including appreciable benefit or harm with each drug over the other. Both drugs performed very well and there were too few events to detect a difference between the two drugs. 11No serious imprecision: Both limits of the 95% CI suggest appreciable benefit with AS+MQ. 12Overall five trials assessed P. vivax response. No differences were shown in occurrence of vivax parasitaemia at any time point or between those with or without vivax co‐infection at baseline. 13No serious indirectness: Trials conducted in Asia and South America in low and unstable transmission areas. 14No serious limitations: Allocation concealment was assessed as 'low risk of bias' in three out of four trials. 15Serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with AS+MQ over DHA‐P and crosses the line of no effect. 16No serious limitations: Allocation concealment was assessed as 'low risk of bias' in all four trials. 17No serious limitations: Allocation concealment was judged to be at 'low risk of bias' in five out of eight trials. 18Serious limitations: All trials were open label and judged to be at 'high risk of bias' for blinding. 19Serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with DHA‐P and crosses the line of no effect.
| Is Dihydroartemisinin‐piperaquine as effective as Artemether‐lumefantrine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Dihydroartemisinin‐piperaquine Comparison: Artemether‐lumefantrine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artemether‐ lumefantrine | Dihydroartemisinin‐ piperaquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 42 PCR adjusted ‐ Africa | 117 per 1000 | 46 per 1000 (28 to 75) | RR 0.39 (0.24 to 0.64) | 869 (3) | ⊕⊕⊕⊕ high1,2,3,4,5,6,7 |
| Efficacy: Total Failure (P. falciparum) Day 42 PCR unadjusted ‐ Africa | 380 per 1000 | 167 per 1000 (76 to 361) | RR 0.44 (0.20 to 0.95) | 1136 (3) | ⊕⊕⊕ moderate2,3,4,6,8,9 |
| Efficacy: Total Failure (P. falciparum) Day 42 PCR adjusted ‐ Asia | 22 per 1000 | 17 per 1000 (4 to 83) | RR 0.77 (0.16 to 3.76) | 317 (1) | ⊕ very low1,10,11,12,13 |
| Efficacy: Total Failure (P. falciparum) Day 42 PCR unadjusted ‐ Asia | 161 per 1000 | 97 per 1000 (56 to 169) | RR 0.60 (0.35 to 1.05) | 356 (1) | ⊕⊕ low10,11,12,14 |
| Vivax efficacy: P. vivax parasitaemia by D42 | 197 per 1000 | 63 per 1000 (47 to 85) | RR 0.32 (0.24 to 0.43) | 1442 (4) | ⊕⊕⊕⊕ high2,5,7,15,16 |
| Transmission potential: Gametocyte development (in those negative at baseline) | — | — | — | 1203 (4) | ⊕ very low17,18,19 |
| Harms: Serious adverse events (including deaths) | 6 per 1000 | 10 per 1000 (4 to 27) | RR 1.71 (0.66 to 4.46) | 2110 (5) | ⊕⊕ low5,20,21 |
| Harms: Early vomiting | 23 per 1000 | 32 per 1000 (16 to 64) | RR 1.38 (0.68 to 2.78) | 1147 (2) | ⊕ very low5,21,22 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its longer half‐life, PCR adjusted treatment failure with DHA‐P may be underestimated at this time point. 2Data are also available for treatment failure at day 28 but provide no further useful information. 3Kamya 2006 UGA, Yeka 2007 UGA and Zongo 2007 BFA. 4No serious limitations: Allocation concealment was assessed as 'low risk of bias' in all trials. Laboratory staff were blinded in two trials. 5No serious inconsistency: Heterogeneity was low. 6No serious indirectness: Trials were conducted in Africa (Uganda and Burkina Faso) in areas of high and moderate transmission. Children aged < six months and pregnant or lactating women were excluded. 7No serious imprecision: Both limits of the 95% CI of the pooled estimate imply appreciable benefit with DHA‐P. 8Serious inconsistency: Heterogeneity was high (I2 = 91%) reflecting differences in the magnitude of effect but not the direction. 9No serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and non‐appreciable benefit with DHA‐P over AL6 but does not cross the line of no effect. 10Ratcliff 2005 IDN. 11Serious limitations: Allocation concealment was assessed as 'low risk of bias' in this trial. At day 42 loss to follow‐up was high: > 20% in both groups. 12Serious indirectness: Only one trial from Asia. 13Serious imprecision: The 95% CI is very wide including appreciable benefit or harm with each drug over the other. 14No serious imprecision: The 95% CI includes appreciable benefit with DHA‐P and crosses the line of no effect but does not include appreciable benefit with AS+AQ. 15Allocation concealment was assessed as 'low risk of bias' in three out of four trials. Laboratory staff were blinded in 4 trials. 16No serious indirectness: Although the strongest data are from Asia (Ratcliff 2005 IDN and Karunajeewa 2007 PNG) these are consistent with the data from Africa. 17No serious limitations: Allocation concealment was assessed as 'low risk of bias' in two out of four trials. Laboratory staff were blinded in three trials. 18Very serious inconsistency: Heterogeneity was high (I2 = 76%) with two trials (Kamya 2006 UGA;Yeka 2007 UGA) favouring DHA‐P and two (Mens 2007 KEN;Zongo 2007 BFA) favouring AL6. 19Very serious imprecision: Data not pooled. 20No serious limitations: Allocation concealment was assessed as 'low risk of bias' in four trials. 21Very serious imprecision: The 95% CI of the pooled estimate is wide including appreciable benefit and harm with each drug over the other. 22Serious limitations: Allocation concealment was assessed as 'low risk of bias' in both trials. Both trials were unblinded.
| Is Dihydroartemisinin‐piperaquine as effective as Artesunate plus amodiaquine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Dihydroartemisinin‐piperaquine Comparison: Artesunate plus amodiaquine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate plus amodiaquine | Dihydroartemisinin‐piperaquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | 73 per 1000 | 34 per 1000 (17 to 69) | RR 0.47 (0.23 to 0.94) | 629 (2) | ⊕⊕⊕ moderate1,2,3,4,5,6,7,8 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted | 161 per 1000 | 85 per 1000 (56 to 130) | RR 0.53 (0.35 to 0.81) | 679 (2) | ⊕⊕⊕ moderate2,3,4,5,6,7,8 |
| Vivax efficacy: P. vivax parasitaemia by day 42 | 175 per 1000 | 44 per 1000 (16 to 130) | RR 0.25 (0.09 to 0.74) | 170 (1) | ⊕⊕⊕ moderate9,10,11 |
| Transmission potential: Gametocyte carriage | — | — | — | 881 (2) |
—12 |
| Harms: Serious adverse events (including deaths) | 18 per 1000 | 3 per 1000 (0 to 49) | RR 0.14 (0.01 to 2.71) | 334 (1) | ⊕ very low9,10,13 |
| Harms: Early vomiting | 78 per 1000 | 41 per 1000 (17 to 101) | RR 0.53 (0.22 to 1.3) | 334 (1) | ⊕ very low10,13,14 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its longer half‐life, PCR adjusted treatment failure with DHA‐P may be underestimated at this time point. 2One trial (Hasugian 2005 IDN) also reported outcomes at day 42 but losses to follow up were very high (> 20%) at this time point. 3Hasugian 2005 IDN and Karema 2004 RWA. 4No serious limitations: Allocation concealment was assessed as 'low risk of bias' in one trial and 'unclear' in one trial. Laboratory staff were blinded in both trials. 5No serious inconsistency: Heterogeneity was low. 6One trial was conducted in Africa (Rwanda, transmission intensity not reported) and one in Asia (Indonesia, unstable transmission). Children aged < one year and pregnant or lactating women were excluded. 7Serious indirectness: Due to variable resistance rates to amodiaquine extrapolation to other areas is likely to be unreliable. 8No serious imprecision: The 95% CI of the pooled estimate includes appreciable and non‐appreciable benefit with DHA‐P over AS+AQ but does not cross the line of no effect. 9No serious limitations: Allocation concealment was assessed as 'low risk of bias' in this trial (Hasugian 2005 IDN). 10Serious indirectness: Only one trial (Hasugian 2005 IDN) assessed this outcome. 11No serious imprecision: Both limits of the 95% CI imply appreciable benefit with DHA‐P over AS+AQ. 12Both trials report no differences in gametocyte carriage but figures were not given. 13Very serious imprecision: The 95% CI includes appreciable benefit or harm with each drugs over the other. 14Serious limitations: This trial was open label.
| Is Dihydroartemisinin‐piperaquine superior to Artesunate plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas excluding Southeast Asia Intervention: Dihydroartemisinin‐piperaquine Comparison: Artesunate plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate plus sulfadoxine‐pyrimethamine | Dihydroartemisinin ‐piperaquine | ||||
| Efficacy: Total Failure Day 42 PCR adjusted | 202 per 1000 | 156 per 1000 (79 to 305) | RR 0.77 (0.39 to 1.51) | 161 (1) | ⊕ very low1,2,3,4 |
| Efficacy: Total Failure Day 42 PCR unadjusted | 380 per 1000 | 391 per 1000 (281 to 551) | RR 1.03 (0.74 to 1.45) | 215 (1) | ⊕ very low1,2,3,4 |
| Vivax efficacy: P. vivax parasitaemia Day 42 | 596 per 1000 | 268 per 1000 (191 to 387) | RR 0.45 (0.32 to 0.65) | 194 (1) | ⊕⊕ low1,2,3,5 |
| Transmission potential: Gametocyte carriage | — | — | — | 215 (1) | —6 |
| Harms: Serious adverse events (including deaths) | — | — | — | — | Not reported |
| Harms: Early vomiting | — | — | — | — | Not reported |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Karunajeewa 2007 PNG. 2Serious limitations: No allocation concealment was described. Laboratory staff were blinded to treatment allocation. 3Serious indirectness: Data only available from one country. 4Very serious imprecision: The 95% CI includes appreciable benefit and harm of one drug over the other. 5No serious imprecision: Both limits of the 95% CI suggest appreciable benefit with DHA‐P. 6Karunajeewa 2007 PNG reports that there were no differences in gametocyte carriage but no figures were given.
| Is Dihydroartemisinin‐piperaquine superior to Amodiaquine plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Africa Intervention: Dihydroartemisinin‐piperaquine Comparison: Amodiaquine plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Amodiaquine plus sulfadoxine‐pyrimethamine | Dihydroartemisinin‐ piperaquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | 114 per 1000 | 34 per 1000 (19 to 62) | RR 0.3 (0.17 to 0.54) | 802 (2) | ⊕⊕⊕ moderate1,2,3,4,5,6,7 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted | 181 per 1000 | 67 per 1000 (45 to 100) | RR 0.37 (0.25 to 0.55) | 848 (2) | ⊕⊕⊕ moderate1,2,3,4,5,6,7 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported |
| Transmission potential: Gametocyte development (in those negative at baseline) | 55 per 1000 | 38 per 1000 (15 to 98) | RR 0.7 (0.27 to 1.79) | 367 (1) | ⊕ very low5,8,9 |
| Harms: Serious adverse events (including deaths) | — | — | — | 374 (1) |
⊕ very low8,10,11 |
| Harms: Early vomiting | — | — | — | — | Not reported12 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its longer half‐life treatment failure due to DHA‐P may be underestimated at this time point. One trial (Zongo 2007 BFA) also reported treatment failure at day 42 and did not show a difference. 2Karema 2004 RWA and Zongo 2007 BFA. 3No serious limitations: Allocation concealment was judged to be at 'low risk of bias' in one trial and 'unclear' in the other. Laboratory staff were blinded to treatment allocation in one trial. 4No serious inconsistency: Heterogeneity was low. 5Serious indirectness: Due to variable resistance rates to AQ and SP, extrapolation of results to other areas is likely to be unreliable. 6Trials conducted in Rwanda (transmission not stated) and Burkina Faso (holoendemic). Children aged < 6 months and pregnant or lactating women were excluded. 7No serious imprecision: Both limits of the 95% CI of the pooled estimate imply appreciable benefit with DHA‐P over AQ+SP. 8No serious limitations: Allocation concealment was judged to be 'low risk of bias' in this trial (Zongo 2007 BFA). This trial was unblinded. 9Very serious imprecision: The 95% CI of the pooled estimate is wide including appreciable benefit or harm with each drug over the other. 10Serious indirectness. Only one trial (Zongo 2007 BFA) reported this outcome. 11Very serious imprecision: No serious adverse events were recorded. It is unlikely that a trial of this size would detect rare but important adverse events. 12One trial (Zongo 2007 BFA) reports vomiting medication on day 0 (as an exclusion criteria not an outcome) and found no difference.
| Is Artesunate plus mefloquine superior to Artemether‐lumefantrine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Artesunate plus mefloquine Comparison: Artemether‐lumefantrine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artemether‐ lumefantrine | Artesunate plus mefloquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 42 PCR adjusted | 28 per 1000 | 11 per 1000 (1 to 80) | RR 0.38 (0.05 to 2.84) | 904 (4) | ⊕ very low1,2,3,4,5,6 |
| Efficacy: Total Failure (P. falciparum) Day 42 PCR unadjusted | 149 per 1000 | 79 per 1000 (43 to 140) | RR 0.53 (0.29 to 0.94) | 1000 (4) | ⊕⊕ low1,2,3,4,5,7 |
| Vivax efficacy: P. vivax parasitaemia by day 42 | 246 per 1000 | 74 per 1000 (52 to 101) | RR 0.3 (0.21 to 0.41) | 1003 (4) | ⊕⊕⊕⊕ high2,5,8,9 |
| Transmission potential: Gametocyte carriage day 14 | 15 per 1000 | 6 per 1000 (1 to 31) | RR 0.41 (0.08 to 2.1) | 536 (2) | ⊕⊕ low8,10,11 |
| Harms: Serious adverse events (including deaths) | 2 per 1000 | 6 per 1000 (1 to 28) | RR 2.96 (0.64 to 13.76) | 1773 (7) | ⊕⊕ low8,11,12 |
| Harms: Early vomiting | 20 per 1000 | 21 per 1000 (11 to 42) | RR 1.07 (0.55 to 2.08) | 1479 (6) | ⊕ very low8,11,12,13 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Data were also available for treatment failure at day 28 but these did not add any further information. 2Hutagalung 2002 THA, Mayxay 2003 LAO, Stohrer 2003 LAO, and Van den Broek 2003a BGD. 3Serious limitations: Allocation concealment was assessed as 'low risk of bias' in 1 trial and 'unclear in 1. Sensitivity analysis removing the trials with inadequate concealment substantially alters the result. In one trial (Hutagalung 2002 THA) a disproportionate number of participants in the AL6 arm received additional antimalarials. Trials were unblinded. 4Serious inconsistency: There was moderate heterogeneity (PCR adjusted I2 = 64%, PCR unadjusted I2 = 54%) relating to one trial (Hutagalung 2002 THA). Removal of this trial shifted the result significantly in favour of AS+MQ. 5No serious indirectness: Trials were conducted in Asia (Thailand, Laos, and Bangladesh) in areas of low and high transmission. Children aged < one year and pregnant or lactating women were excluded. 6Very serious imprecision: The 95% CI of the pooled estimate is wide including appreciable benefit and harm with each drug over the other. Both drugs performed very well in all four trials. 7No serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with AS+MQ but does not cross the line of no effect. 8No serious inconsistency: Heterogeneity was low. 9No serious imprecision: Both limits of the 95% CI of the pooled estimate imply appreciable benefit with AS+MQ. 10Allocation concealment was assessed as at 'high risk of bias' in both trials (Faye 2003 SEN, van den Broek2003a BGD). The number of gametocyte carriers was generally low in both groups. One trial showed a statistical difference at day seven but not day three or 14. 11Very serious imprecision: The 95% CI of the pooled estimate are very wide including appreciable benefit or harm with both drugs. 12Allocation concealment was assessed as 'high risk of bias' in three out of seven trials. Sensitivity analysis removing the trials without adequate allocation concealment did not substantially alter the result. 13Serious limitations: All trials were open label.
| Is Artesunate plus mefloquine superior to Artesunate plus amodiaquine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Artesunate plus mefloquine Comparison: Artesunate plus amodiaquine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate plus amodiaquine | Artesunate plus mefloquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | — | — | — | 482 (1) | ⊕ very low1,2,3,4,5,6 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted | 26 per 1000 | 14 per 1000 (3 to 64) | RR 0.54 (0.12 to 2.46) | 493 (1) | ⊕ very low2,3,4,5,7 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported |
| Transmission potential: Gametocyte carriage day 14 | — | — | — | 505 (1) | ⊕ very low2,3,4,5,7 |
| Harms: Serious adverse events (including deaths) | ‐ | ‐ | ‐ | 505 (1) | ⊕ very low2,3,4,5,9 |
| Harms: Early vomiting | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its longer half‐life treatment failure with AS+MQ may be underestimated at this time‐point. 2Faye 2003 SEN. 3Serious limitations: Allocation concealment was assessed as 'high risk of bias' and no blinding is described. 4Serious indirectness: Only one trial from Senegal reported this outcome. Extrapolation of this result to other countries is likely to be unreliable. 5Children aged < one year and pregnant or lactating women were excluded. 6Very serious imprecision: There were no PCR adjusted treatment failures in either group. 7Very serious imprecision: The 95% CI is wide including appreciable benefit and harm with each drug over the other. 8Very serious imprecision: There were no participants with detectable gametocytes in either arm. There were no significant differences in gametocyte carriage at days three or seven. 9Very serious imprecision: No serious adverse events were recorded in this trial. A trial of this size would be unlikely to detect rare but important adverse events.
| Is Artesunate plus mefloquine superior to Amodiaquine plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Africa Intervention: Artesunate plus mefloquine Comparison: Amodiaquine plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Amodiaquine plus sulfadoxine‐pyrimethamine | Artesunate plus mefloquine | ||||
| Efficacy: Total Failure Day 28 PCR adjusted | — | — | — | 296 (1) |
⊕ very low1,2,3,4,5,6 |
| Efficacy: Total Failure Day 28 PCR unadjusted | 13 per 1000 | 14 per 1000 (2 to 99) | RR 1.08 (0.15 to 7.59) | 300 (1) | ⊕ very low2,3,4,5,7 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported |
| Transmission potential: Gametocyte carriage day 7 | 118 per 1000 | 4 per 1000 (0 to 55) | RR 0.03 (0 to 0.47) | 306 (1) | ⊕⊕ low2,3,4,5,8 |
| Harms: Serious adverse events (including deaths) | — | — | — | 306 (1) | ⊕ very low2,3,4,5,9 |
| Harms: Early vomiting | — | — | — | — | Not reported |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its longer half‐life, treatment failure with AS+MQ may be underestimated at this timepoint. 2Faye 2003 SEN. 3Serious limitations: Allocation concealment was assessed as 'high risk of bias' and no blinding is described. 4Serious indirectness: Only one trial from Senegal reported this outcome. Extrapolation of this result to other countries is likely to be unreliable. 5Children aged < 1 year and pregnant or lactating women were excluded. 6Very serious imprecision: No PCR adjusted treatment failures were recorded in either treatment group. 7Very serious imprecision: The 95% CI is wide including appreciable benefit and harm with each drug over the other. 8No serious imprecision: Both limits of the 95% CI imply appreciable benefit with AS+MQ. At day 14 there were no participants with detectable gametocytes in either group. 9Very serious imprecision: No serious adverse events were recorded in this trial. A trial of this size would be unlikely to detect rare but important adverse events.
| Is Artemether‐lumefantrine superior to Artesunate plus amodiaquine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Africa Intervention: Artemether‐lumefantrine Comparison: Artesunate plus amodiaquine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | Artesunate plus amodiaquine | Artemether‐lumefantrine | |||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | 19 per 1000 | 31 per 1000 (18 to 55) | RR 1.65 (0.95 to 2.87) | 1729 (8) | ⊕⊕⊕ moderate1,2,3,4,5,6 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted | — | — | — | 2617 (5) | ⊕ very low2,5,7,8,9 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported10 |
| Transmission potential: Gametocyte carriage day 14 | — | — | — | 718 (2) | ⊕ very low11,12,13,14 |
| Harms: Serious adverse events (including deaths) | 13 per 1000 | 14 per 1000 (8 to 27) | RR 1.11 (0.59 to 2.08) | 2617 (5) | ⊕⊕ low3,4,5,15 |
| Harms: Early vomiting | 83 per 1000 | 72 per 1000 (49 to 109) | RR 0.87 (0.59 to 1.31) | 1097 (5) | ⊕ very low4,15,16 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its long half‐life PCR adjusted treatment failure with AL6 may be underestimated at this time point. 2Adjei 2006 GHA, Bukirwa 2005 UGA, Dorsey 2006 UGA, Falade 2005 NGA, Faye 2003 SEN, Guthmann 2004 AGO, Kobbe 2007 GHA and Owusu‐Agyei 2006 GHA (and Mutabingwa 2004 TZA for PCR unadjusted only). 3No serious limitations: Allocation concealment was assessed as 'low risk of bias' in four trials. Sensitivity analysis removing the trials with inadequate allocation concealment did not substantially alter the result. 4No serious inconsistency: Heterogeneity was low. 5No serious indirectness: Trials were conducted in a variety of African countries with variable transmission and resistance patterns. Children aged < four months and pregnant or lactating women were excluded. 6Serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with ASAQ over AL6 and crosses the line of no effect. 7No serious limitations: Allocation concealment was assessed as 'low risk of bias' in five trials. Sensitivity analysis removing the trials with inadequate allocation concealment did not substantially alter the result. 8Very serious inconsistency: Heterogeneity was high so data were not pooled. This heterogeneity seemed to be related to region (with trials from East Africa favouring AL6 and trials from West Africa favouring ASAQ) and transmission intensity (with two trials experiencing very high rates of new infections). 9Very serious imprecision: Data were not pooled due to heterogeneity. The effect estimate is likely to vary between settings. 10Only one trial reported P. vivax and there were too few events to draw a conclusion. 11Dorsey 2006 UGA had adequate allocation concealment and blinding. In Faye 2003 SEN no allocation concealment or blinding was described. 12Very serious inconsistency: Heterogeneity was high so data were not pooled. 13Trials were conducted in Senegal (moderate transmission) and Uganda (mesoendemic). 14Very serious imprecision: The two trials reporting this outcome had very different results. 15Very serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit and harm with each drug over the other. 16Serious limitations: Four out of five trials were unblinded.
| Is Artemether‐lumefantrine superior to Artesunate plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Artemether‐lumefantrine Comparison: Artesunate plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate plus sulfadoxine‐pyrimethamine | Artemether‐lumefantrine | ||||
| Efficacy: Total Failure (P. falciparum) Day 42 PCR adjusted | 202 per 1000 | 67 per 1000 (26 to 174) | RR 0.33 (0.13 to 0.86) | 158 (1) | ⊕ very low1,2,3,4,5 |
| Efficacy: Total Failure (P. falciparum) Day 42 PCR unadjusted | 380 per 1000 | 369 per 1000 (258 to 517) | RR 0.97 (0.68 to 1.36) | 217 (1) | ⊕ very low2,3,4,6 |
| Vivax efficacy: P. vivax parasitaemia by Day 42 | 667 per 1000 | 700 per 1000 (507 to 954) | RR 1.05 (0.76 to 1.43) | 72 (1) | ⊕ very low2,3,7,8 |
| Transmission potential: Gametocyte carriage | — | — | — | 158 (1) | —9 |
| Harms: Serious adverse events (including deaths) | — | — | — | 197 (1) |
⊕ very low10,11 |
| Harms: Early vomiting | — | — | — | — | Not reported |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note that due to its longer half‐life, PCR adjusted treatment failure with AL6 may be underestimated at this time point. 2Karunajeewa 2007 PNG. 3Serious limitations: Allocation concealment was assessed as 'high risk of bias' in this trial. Only microscopists were blinded to treatment allocation. 4Very serious indirectness: Data are only available from one country (Papua New Guinea). One other trial from Sudan with high risk of bias (Mukhtar 2005 SDN) reports data for day 28 and did not find a difference. 5No serious imprecision: The 95% CI includes appreciable and non‐appreciable benefit with AL6 over AS+SP but does not cross the line of no effect. 6Very serious imprecision: The 95% CI is very wide including appreciable benefit and harm with each drug over the other. 7Serious indirectness: Data are only available from one country (Papua New Guinea). This outcome is for participants with P. vivax ± P. falciparum at baseline. 8Serious imprecision: The 95% CI includes appreciable benefit with AS+SP and crosses the line of no effect. 9Karunajeewa 2007 PNG reports no differences in gametocyte carriage between the two groups during follow up (figures not given). 10Very serious limitations: The only trial which reports this outcome (Van den Broek 2004 ZAR) was excluded from the primary outcome due to baseline differences between groups. 11Very serious imprecision: There were no serious adverse events in this trial. Trials of this size would be unlikely to detect rare but clinically important adverse events.
| Is Artemether‐lumefantrine superior to Amodiaquine plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Africa Intervention: Artemether‐lumefantrine Comparison: Amodiaquine plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Amodiaquine plus sulfadoxine‐pyrimethamine | Artemether‐lumefantrine | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted ‐ East Africa | 220 per 1000 | 26 per 1000 (13 to 53) | RR 0.12 (0.06 to 0.24) | 618 (2) | ⊕⊕⊕ moderate1,2,3,4,5,6,7,8 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted ‐ East Africa | 486 per 1000 | 170 per 1000 (146 to 199) | RR 0.35 (0.3 to 0.41) | 1646 (3) | ⊕⊕⊕ moderate2,10,4,5,6,7,8,9 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted ‐ West Africa | 15 per 1000 | 21 per 1000 (8 to 52) | RR 1.39 (0.55 to 3.47) | 1051 (3) | ⊕ very low1,3,4,5,6,11,12,13 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted ‐ West Africa | 43 per 1000 | 124 per 1000 (80 to 192) | RR 2.88 (1.86 to 4.47) | 1130 (3) | ⊕⊕⊕ moderate3,5,6,11,12,14 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported15 |
| Transmission potential: Gametocyte carriage day 14 | 25 per 1000 | 11 per 1000 (5 to 25) | RR 0.46 (0.21 to 1.01) | 1536 (4) | ⊕⊕ low16,17,18 |
| Harms: Serious adverse events (including deaths) | 13 per 1000 | 14 per 1000 (7 to 27) | RR 1.08 (0.56 to 2.08) | 2684 (5) | ⊕⊕ low5,13,19 |
| Harms: Early vomiting | — | — | — | — | Not reported20 |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Please note due to its longer half‐life, treatment failure with AL6 may be underestimated at this time point. 2Dorsey 2006 UGA, Fanello 2004 RWA. 3No serious limitations: Allocation concealment was assessed as 'low risk of bias' in one of the trials. Sensitivity analysis removing the trials without adequate concealment did not substantially change the result. 4Only one trial had adequate blinding. 5No serious inconsistency: Heterogeneity was low. 6Serious indirectness: There is considerable variability in the efficacy of AQSP which makes extrapolation of results to other settings unreliable. 7Trials were conducted in Uganda (mesoendemic), Rwanda (transmission not reported). Children aged < six months and pregnant or lactating women were excluded. 8No serious imprecision: Both limits of the 95% CI of the pooled estimate imply appreciable benefit with AL6 over AQ+SP. 9No serious limitations: Allocation concealment was assessed as 'low risk of bias' in two of the three trials. Sensitivity analysis removing the trial with unclear concealment did not substantially change the result. 10and Mutabingwa 2004 TZA, Tanzania, very high transmission. 11Zongo 2005 BFA, Zongo 2007 BFA and Faye 2003 SEN. 12Trials conducted in Burkina Faso (holoendemic) and Senegal (moderate transmission). Children aged < six months and pregnant or lactating women were excluded. 13Very serious imprecision: The 95% CI of the pooled estimate is wide including appreciable benefit and harm with each drug over the other. 14No serious imprecision: Both limits of the 95% CI of the pooled estimate imply appreciable benefit with AQSP over AL6. 15Only one trial reported on P. vivax and there were too few events to draw a conclusion. 16Data were also available for day seven where gametocyte carriage was significantly lower with AL6. 17Serious limitations: Only one of the four trials had adequate allocation concealment. 18Serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with AL6 and crosses the line of no effect. 19No serious limitations: Allocation concealment was assessed as 'low risk of bias' in three trials. 20Two trials reported vomiting of medication on day 0 (as an exclusion criteria not an outcome) and found no difference.
| Is Artesunate plus amodiaquine superior to Artesunate plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Endemic areas worldwide Intervention: Artesunate plus amodiaquine Comparison: Artesunate plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Artesunate plus sulfadoxine‐pyrimethamine | Artesunate plus amodiaquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | 44 per 1000 | 28 per 1000 (16 to 48) | RR 0.64 (0.37 to 1.08) | 1419 (7) | ⊕⊕ low1,2,3,4,5 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR unadjusted | — | — | — | 1614 (7) | ⊕ very low1,2,4,6,7 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported |
| Transmission potential: Gametocyte carriage day 14 | 91 per 1000 | 81 per 1000 (46 to 140) | RR 0.89 (0.51 to 1.54) | 520 (3) | ⊕ very low8,9,10,11 |
| Harms: Serious adverse events (including deaths) | 2 per 1000 | 2 per 1000 (0 to 14) | RR 0.99 (0.14 to 7.02) | 1108 (4) | ⊕ very low9,10,11 |
| Harms: Early vomiting | — | — | — | — | Not reported |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Bonnet 2004 GIN;Djimde 2004 MLI;Guthmann 2003 AGO;Hamour 2003 SDN;Kayentao 2006 MLI;Swarthout 2004 ZAR;Van den Broek 2004 ZAR. 2Serious limitations: Allocation concealment was assessed as 'low risk of bias' in only one trial. Only one trial had adequate blinding of laboratory staff. 3No serious inconsistency: Heterogeneity was low. 4Trials were conducted in a variety of African countries (Guinea, Mali, Angola, DRC) and transmission intensities in children aged 6 to 59 months. 5Serious imprecision: The 95% CI of the pooled estimate includes appreciable benefit with ASAQ and crosses the line of no effect. 6Very serious inconsistency: Heterogeneity was high (I2 = 88%) with some trials showing benefit with AS+AQ and some with AS+SP. 7Very serious imprecision: Data were not pooled due to high heterogeneity. 8No difference was shown in gametocyte carriage at day three or seven. 9Serious limitations: No trial adequately described an allocation concealment procedure. 10No serious inconsistency: Heterogeneity was low. 11Very serious imprecision: The 95% CI of the pooled estimate is wide including appreciable benefit or harm of each drug over the other.
| Is Artesunate plus amodiaquine superior to Amodiaquine plus sulfadoxine‐pyrimethamine for treating uncomplicated malaria? | |||||
| Patient or population: Patients with uncomplicated malaria Settings: Africa Intervention: Artesunate plus amodiaquine Comparison: Amodiaquine plus sulfadoxine‐pyrimethamine | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Amodiaquine plus sulfadoxine‐pyrimethamine | Artesunate plus amodiaquine | ||||
| Efficacy: Total Failure (P. falciparum) Day 28 PCR adjusted | — | — | — | 2346 (6) | ⊕ very low1,2,3,4,5 |
| Efficacy: Total Failure (P. falciparum) Day 28 PCR un adjusted‐ | — | — | — | 4220 (8) | ⊕ very low1,4,5,6,7,8 |
| Vivax efficacy: P. vivax parasitaemia | — | — | — | — | Not reported |
| Transmission potential: Gametocyte carriage day 14 | 38 per 1000 | 22 per 1000 (6 to 77) | RR 0.57 (0.16 to 2.02) | 894 (3) | ⊕ very low4,9,10,11,12 |
| Harms: Serious adverse events (including deaths) | 17 per 1000 | 1 per 1000 (6 to 18) | RR 0.61 (0.36 to 1.03) | 4200 (7) | ⊕⊕⊕ moderate13,14,15 |
| Harms: Early vomiting | — | — | — | — | Not reported |
| *The assumed risk is the mean risk from the studies included in this review, calculated as the number of patients in the control groups with the event divided by the total number of patients in control groups. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
Footnotes
1Dorsey 2006 UGA; Faye 2003 SEN; Karema 2004 RWA; Kayentao 2006 MLI; Menard 2006 MDG; Yeka 2004 UGA. 2No serious limitations: Allocation concealment was assessed as 'low risk of bias' in two trials. Laboratory staff were blinded in 3 trials. 3Serious inconsistency: Substantial heterogeneity (I2 = 77%). In the three trials from east Africa AS+AQ tended to perform better that AQ+SP, but AQ+SP still performed well elsewhere. 4Serious indirectness: Due to variability in resistance rates generalization of results is likely to be unreliable. 5Very serious imprecision: Data not pooled due to high heterogeneity. The magnitude of effect is likely to vary between settings. 6and Mutabingwa 2004 TZA and Staedke 2003 UGA. 7No serious limitations: Allocation concealment was assessed as 'low risk of bias' in four trials. Laboratory staff were blinded in four trials. 8Serious inconsistency: Substantial heterogeneity (I2 = 91%). In the five trials from east Africa AS+AQ tended to perform better than AQ+SP, but AQ+SP still performed well elsewhere. 9Dorsey 2006 UGA; Faye 2003 SEN; Menard 2006 MDG. 10No serious limitations: Allocation concealment was assessed as 'low risk of bias' in two trials. 11 Very serious imprecision: The 95% CI is very wide including appreciable benefit and harm or each drug over the other. 12Faye 2003 SEN found a significant reduction in gametocytaemia at day three with AS+AQ. Staedke 2003 UGA found a significant reduction in gametocyte development with AS+AQ. 13No serious limitations. 14No serious inconsistency: Heterogeneity is low. 15Serious imprecision: The 95%CI of the pooled estimate includes appreciable benefit with AS+AQ over AQ+SP and crosses the line of no effect.
Data and analyses
Comparison 1. Dihydroartemisinin‐piperaquine vs Artesunate plus mefloquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 63 PCR unadjusted | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Asia | 3 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.54, 0.98] |
| 1.2 South America | 1 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.19 [1.40, 27.35] |
| 2 Total Failure (P. falciparum) Day 63 PCR adjusted | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Asia | 3 | 1062 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.19, 0.79] |
| 2.2 South America | 1 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.55 [0.52, 176.35] |
| 3 Total Failure (P. falciparum) Day 42 PCR unadjusted | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Asia | 5 | 1969 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.46, 1.69] |
| 4 Total Failure (P. falciparum) Day 42 PCR adjusted | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Asia | 5 | 1898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.30, 1.39] |
| 5 Total Failure (P. falciparum) Day 28 PCR unadjusted | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Asia | 6 | 2034 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.22, 6.42] |
| 6 Total Failure (P. falciparum) Day 28 PCR adjusted | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Asia | 6 | 2020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.56] |
| 7 P. vivax parasitaemia | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Mixed P. falciparum and vivax infection at baseline | 5 | 2248 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.63, 1.12] |
| 7.2 Total P. vivax parasitaemia by day 28 | 1 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.43 [0.39, 142.89] |
| 7.3 Total P. vivax parasitaemia by day 42 | 3 | 1251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.11] |
| 7.4 Total P. vivax parasitaemia by day 63 | 4 | 1661 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.91, 1.34] |
| 7.5 P. vivax parasitaemia by day 63 in those negative at baseline | 3 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.95, 1.56] |
| 7.6 P. vivax parasitaemia by day 63 in those positive at baseline | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.65] |
| 8 Gametocyte development (in those negative at baseline) | 3 | 1234 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [1.13, 8.33] |
| 9 Gametocytaemia carriage | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Gametocyte carriage day 0 | 2 | 1174 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.66, 1.73] |
| 9.2 Gametocyte carriage day 7 | 2 | 1152 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.54, 2.58] |
| 9.3 Gametocyte carriage day 14 | 2 | 1142 | Risk Ratio (M‐H, Random, 95% CI) | 5.14 [3.17, 8.33] |
| 9.4 Gametocyte carriage day 21 | 2 | 1123 | Risk Ratio (M‐H, Random, 95% CI) | 7.23 [0.10, 519.79] |
| 9.5 Gametocyte carriage day 28 | 2 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 9.68 [1.23, 75.98] |
| 10 Serious adverse events (including deaths) | 7 | 2374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.38, 2.15] |
| 11 Early vomiting | 7 | 2473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.69, 1.16] |
| 12 Sensitivity analysis: Total Failure Day 63 PCR unadjusted | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 Total Failure (P. falciparum) Day 63 PCR unadjusted | 4 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.52, 1.70] |
| 12.2 Total Failure Day 63 PCR unadjusted (losses to follow up included as failures) | 4 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.65, 1.38] |
| 12.3 Total Failure Day 63 PCR unadjusted (losses to follow up included as successes) | 4 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.52, 1.68] |
| 13 Sensitivity analysis: Total Failure Day 63 PCR adjusted | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Total Failure (P. falciparum) Day 63 PCR adjusted | 4 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.83] |
| 13.2 Total Failure Day 63 PCR adjusted (indeterminate PCR included as failures) | 4 | 1508 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.32, 1.39] |
| 13.3 Total Failure Day 63 PCR adjusted (new infections included as successes) | 4 | 1627 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.35] |
| 13.4 Total Failure Day 63 PCR adjusted (losses to follow up included as failures) | 4 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.67, 1.30] |
| 13.5 Total Failure Day 63 PCR adjusted (losses to follow up included as successes) | 4 | 1801 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.33] |
Comparison 2. Dihydroartemisinin‐piperaquine vs Artemether‐lumefantrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 42 PCR unadjusted | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Africa | 3 | 1136 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.20, 0.95] |
| 1.2 Asia | 1 | 356 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.35, 1.05] |
| 1.3 Oceania | 1 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.76, 1.50] |
| 2 Total Failure (P. falciparum) Day 42 PCR adjusted | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Africa | 3 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.24, 0.64] |
| 2.2 Asia | 1 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.16, 3.76] |
| 2.3 Oceania | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.85, 6.23] |
| 3 Total Failure (P. falciparum) Day 28 PCR unadjusted | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Africa | 2 | 484 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.32] |
| 3.2 Asia | 1 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.17, 1.12] |
| 3.3 Oceania | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.75, 2.15] |
| 4 Total Failure (P. falciparum) Day 28 PCR adjusted | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Africa | 2 | 453 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.17, 1.99] |
| 4.2 Asia | 1 | 436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.13, 6.36] |
| 4.3 Oceania | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.04, 12.60] |
| 5 P. vivax parasitaemia | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Mixed P. falciparum and vivax infection at baseline | 4 | 1608 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.42] |
| 5.2 P. vivax parasitaemia by D28 | 1 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.36] |
| 5.3 P. vivax parasitaemia by D42 | 4 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.24, 0.43] |
| 6 Gametocyte development (in those negative at baseline) | 4 | 1203 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.35, 2.59] |
| 7 Anaemia | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Mean haemoglobin (g/dl) at baseline | 4 | 1356 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.27, 0.13] |
| 7.2 Mean haemoglobin (g/dl) at day 28 | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 0.36 [‐0.03, 0.75] |
| 7.3 Mean haemoglobin (g/dl) at day 42 | 1 | 375 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.02, 0.62] |
| 7.4 Mean change in haemoglobin (g/dl) from baseline to Day 42 | 2 | 835 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.00, 0.51] |
| 8 Serious adverse events (including deaths) | 5 | 2110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.66, 4.46] |
| 9 Early vomiting | 2 | 1147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.68, 2.78] |
Comparison 3. Dihydroartemisinin‐piperaquine vs Artesunate plus amodiaquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.35, 0.81] |
| 1.1 Africa | 1 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.34, 0.85] |
| 1.2 Asia | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.17, 1.42] |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 2 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 0.94] |
| 2.1 Africa | 1 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.27, 1.27] |
| 2.2 Asia | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.22] |
| 3 Total Failure (P. falciparum) Day 42 PCR unadjusted | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.72] |
| 3.1 Asia | 1 | 152 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.10, 0.72] |
| 4 Total Failure (P. falciparum) Day 42 PCR adjusted | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 4.1 Asia | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 0.81] |
| 5 P. vivax parasitaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Mixed P. falciparum and vivax infection at baseline | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.67, 2.29] |
| 5.2 P. vivax parasitaemia by day 28 | 1 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.04, 4.90] |
| 5.3 P. vivax parasitaemia by day 42 | 1 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.09, 0.74] |
| 6 Serious adverse events (including deaths) | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.71] |
| 7 Early vomiting | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.22, 1.30] |
Comparison 4. Dihydroartemisinin‐piperaquine vs Artesunate plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 42 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oceania | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.74, 1.45] |
| 2 Total Failure (P. falciparum) Day 42 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oceania | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.39, 1.51] |
| 3 Total Failure (P. falciparum) Day 28 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oceania | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.62, 1.64] |
| 4 Total Failure (P. falciparum) Day 28 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oceania | 1 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 5 P. vivax parasitaemia by day 42 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Participants with P. falciparum mono‐infection at baseline | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.32, 0.65] |
| 5.2 Participants with P. vivax ± P. falciparum at baseline | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.27, 0.79] |
Comparison 5. Dihydroartemisinin‐piperaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Africa | 2 | 848 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.25, 0.55] |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Africa | 2 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.17, 0.54] |
| 3 Total Failure (P. falciparum) Day 42 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Africa | 1 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.33, 1.24] |
| 4 Total Failure (P. falciparum) Day 42 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Africa | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.16, 1.83] |
| 5 Gametocyte development | 1 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.79] |
| 6 Anaemia | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Mean haemoglobin (g/dl) at baseline | 1 | 371 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.68, 0.28] |
| 6.2 Mean haemoglobin (g/dl) at day 42 or last day of follow up | 1 | 371 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.51, 0.11] |
| 6.3 Mean packed cell volume at baseline | 1 | 510 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.89, 0.89] |
| 6.4 Mean packed cell volume at day 14 | 1 | 510 | Mean Difference (IV, Fixed, 95% CI) | ‐1.10 [‐1.73, ‐0.47] |
| 7 Early vomiting | 1 | 383 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.34 [0.70, 15.87] |
Comparison 6. Artesunate plus mefloquine vs Artemether‐lumefantrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 42 PCR unadjusted | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Asia | 4 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.29, 0.94] |
| 2 Total Failure (P. falciparum) Day 42 PCR adjusted | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Asia | 4 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.05, 2.84] |
| 3 Total Failure (P. falciparum) Day 28 PCR unadjusted | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Africa | 2 | 752 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.48, 0.89] |
| 3.2 Asia | 3 | 854 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.41, 1.58] |
| 4 Total Failure (P. falciparum) Day 28 PCR adjusted | 5 | 1479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.63, 2.50] |
| 4.1 Africa | 2 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.41, 2.85] |
| 4.2 Asia | 3 | 836 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.53, 3.86] |
| 5 P. vivax parasitaemia | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Mixed P. falciparum and vivax infection at baseline | 5 | 1279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.57, 3.00] |
| 5.2 P. vivax parasitaemia by day 28 | 1 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 3.88] |
| 5.3 P. vivax parasitaemia by day 42 | 4 | 1003 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.21, 0.41] |
| 6 Gametocyte development (in those negative at baseline) | 3 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.54, 3.28] |
| 7 Gametocyte carriage | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Gametocyte carriage day 0 | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.10] |
| 7.2 Gametocyte carriage day 3 | 2 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.48] |
| 7.3 Gametocyte carriage day 7 | 3 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.14, 0.85] |
| 7.4 Gametocyte carriage day 14 | 2 | 536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.10] |
| 8 Serious adverse events (including deaths) | 7 | 1773 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.96 [0.64, 13.76] |
| 9 Early vomiting | 6 | 1479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.55, 2.08] |
Comparison 7. Artesunate plus mefloquine vs Artesunate plus amodiaquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Africa | 1 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.12, 2.46] |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Africa | 1 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Gametocyte carriage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Gametocyte carriage day 0 | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Gametocyte carriage day 3 | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.31 [0.90, 332.99] |
| 3.3 Gametocyte carriage day 7 | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Gametocyte carriage day 14 | 1 | 505 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.2. Analysis.
Comparison 7 Artesunate plus mefloquine vs Artesunate plus amodiaquine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
7.3. Analysis.
Comparison 7 Artesunate plus mefloquine vs Artesunate plus amodiaquine, Outcome 3 Gametocyte carriage.
Comparison 8. Artesunate plus mefloquine vs Amodiaquine plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Africa | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.15, 7.59] |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Africa | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Gametocyte carriage | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Gametocyte carriage day 0 | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.01, 1.81] |
| 3.2 Gametocyte carriage day 3 | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.70] |
| 3.3 Gametocyte carriage day 7 | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.47] |
| 3.4 Gametocyte carriage day 14 | 1 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
8.2. Analysis.
Comparison 8 Artesunate plus mefloquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 2 Total Failure (P. falciparum) Day 28 PCR adjusted.
Comparison 9. Artemether‐lumefantrine vs Artesunate plus amodiaquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 East Africa | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 West Africa | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 South/Central Africa | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 8 | 1729 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.95, 2.87] |
| 2.1 East Africa | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.15, 4.59] |
| 2.2 West Africa | 5 | 1245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.00, 3.26] |
| 2.3 South/Central Africa | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Gametocyte development | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.74] |
| 4 Gametocyte carriage | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Gametocyte carriage day 0 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Gametocyte carriage day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Gametocyte carriage day 7 | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Gametocyte carriage day 14 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Anaemia | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Mean haemoglobin (g/dl) at baseline | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mean haemoglobin (g/dl) at Day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Mean change in haemoglobin (g/dl) from baseline to Day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Mean haematocrit at baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Mean haematocrit at Day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Proportion anaemic (Haemoglobin < 11 g/dl) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 At baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Serious adverse events (including deaths) | 6 | 2749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.59, 2.08] |
| 8 Early vomiting | 5 | 1097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.31] |
| 9 Sensitivity analysis: Total Failure Day 28 PCR unadjusted | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 9 | 3021 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.60, 1.27] |
| 9.2 Total Failure Day 28 PCR unadjusted (trials with baseline differences included) | 12 | 3719 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.49, 0.97] |
| 9.3 Total Failure Day 28 PCR unadjusted (losses to follow up included as failures) | 9 | 3230 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 9.4 Total Failure Day 28 PCR unadjusted (losses to follow up included as successes) | 9 | 3230 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.61, 1.30] |
| 10 Sensitivity analysis: Total Failure Day 28 PCR adjusted | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Total Failure (P. falciparum) Day 28 PCR adjusted | 8 | 1729 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.95, 2.87] |
| 10.2 Total Failure Day 28 PCR adjusted (trials with baseline differences included) | 11 | 2311 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.67] |
| 10.3 Total Failure Day 28 PCR adjusted (indeterminate PCR included as failures) | 8 | 1747 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.06, 2.78] |
| 10.4 Total Failure Day 28 PCR adjusted (new infections included as successes) | 8 | 2064 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [1.06, 2.75] |
| 10.5 Total Failure Day 28 PCR adjusted (losses to follow up included as failures) | 8 | 2196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.78, 1.31] |
| 10.6 Total Failure Day 28 PCR adjusted (losses to follow up included as successes) | 8 | 2196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.08, 2.83] |
Comparison 10. Artemether‐lumefantrine vs Artesunate plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 42 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oceania | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.68, 1.36] |
| 2 Total Failure (P. falciparum) Day 42 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oceania | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.13, 0.86] |
| 3 Total Failure (P. falciparum) Day 28 PCR unadjusted | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 3.1 Africa | 1 | 157 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.28, 1.48] |
| 3.2 Oceania | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 4 Total Failure (P. falciparum) Day 28 PCR adjusted | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.13] |
| 4.1 Africa | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.34, 2.47] |
| 4.2 Oceania | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.08, 0.97] |
| 5 P. vivax parasitaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 P. vivax parasitaemia by day 42 (P. vivax ± P. falciparum at baseline) | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.43] |
| 5.2 P. vivax parasitaemia by day 42 (P. falciparum mono‐infection at baseline) | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.87, 1.35] |
| 6 Sensitivity analysis Total Failure Day 28 PCR unadjusted | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.16] |
| 6.2 Total Failure Day 28 PCR unadjusted (trials with baseline differences included) | 4 | 802 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.39, 0.79] |
| 6.3 Total Failure Day 28 PCR unadjusted (losses to follow up included as failures) | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.17] |
| 6.4 Total Failure Day 28 PCR unadjusted (losses to follow up included as successes) | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.47, 1.15] |
| 7 Sensitivity analysis: Total Failure Day 28 PCR adjusted | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Total Failure (P. falciparum) Day 28 PCR adjusted | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.25, 1.13] |
| 7.2 Total Failure Day 28 PCR adjusted (trials with baseline differences included) | 4 | 718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.17, 0.66] |
| 7.3 Total Failure Day 28 PCR adjusted (indeterminate PCR included as failures) | 2 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.29, 1.16] |
| 7.4 Total Failure Day 28 PCR adjusted (new infections included as successes) | 2 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.29, 1.17] |
| 7.5 Total Failure Day 28 PCR adjusted (losses to follow up included as failures) | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.48, 1.23] |
| 7.6 Total Failure Day 28 PCR adjusted (losses to follow up included as successes) | 2 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.30, 1.17] |
Comparison 11. Artemether‐lumefantrine vs Amodiaquine plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 East Africa | 3 | 1646 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.30, 0.41] |
| 1.2 West Africa | 3 | 1130 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.88 [1.86, 4.47] |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 East Africa | 2 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.06, 0.24] |
| 2.2 West Africa | 3 | 1051 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.55, 3.47] |
| 3 Total Failure (P. falciparum) Day 42 PCR unadjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 West Africa | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.66, 4.21] |
| 4 Total Failure (P. falciparum) Day 42 PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 West Africa | 1 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.44, 3.38] |
| 5 Gametocyte carriage | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Gametocyte carriage day 0 | 4 | 1545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.51, 1.39] |
| 5.2 Gametocyte carriage day 3 | 3 | 1331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.25, 0.75] |
| 5.3 Gametocyte carriage day 7 | 4 | 1538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.18, 0.54] |
| 5.4 Gametocyte carriage day 14 | 4 | 1536 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.21, 1.01] |
| 6 Gametocyte development (in those negative at baseline) | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.04] |
| 7 Anaemia | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Mean haemoglobin (g/dl) at baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Mean change in haemoglobin (g/dl) from baseline to Day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Mean haemoglobin (g/dl) at Day 42 or last day of follow up. | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Serious adverse events (including deaths) | 5 | 2684 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.56, 2.08] |
| 9 Early vomiting | 2 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.54, 3.68] |
Comparison 12. Artesunate plus amodiaquine vs Artesunate plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 7 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Africa | 7 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Africa | 7 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.37, 1.08] |
| 3 Gametocyte carriage | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Gametocyte carriage day 0 | 3 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.60, 1.32] |
| 3.2 Gametocyte carriage day 3 | 2 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.67, 1.25] |
| 3.3 Gametocyte carriage day 7 | 2 | 363 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.64, 1.61] |
| 3.4 Gametocyte carriage day 14 | 3 | 520 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.32, 3.73] |
| 4 Proportion of participants with anaemia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At baseline | 2 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 4.2 At Day 28 | 2 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 5 Serious adverse events (including deaths) | 4 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.02] |
Comparison 13. Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure (P. falciparum) Day 28 PCR unadjusted | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 East Africa | 5 | 3317 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.01] |
| 1.2 West Africa | 2 | 766 | Risk Ratio (M‐H, Random, 95% CI) | 6.57 [0.68, 63.26] |
| 1.3 Other | 1 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 3.12 [1.05, 9.25] |
| 2 Total Failure (P. falciparum) Day 28 PCR adjusted | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 East Africa | 3 | 1515 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.89] |
| 2.2 West Africa | 2 | 701 | Risk Ratio (M‐H, Random, 95% CI) | 9.72 [1.19, 79.26] |
| 2.3 Other | 1 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 2.23 [0.58, 8.58] |
| 3 Gametocyte development | 2 | 1354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.82] |
| 4 Gametocyte carriage | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Gametocyte carriage day 0 | 3 | 909 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.13, 3.59] |
| 4.2 Gametocyte carriage day 3 | 1 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.23] |
| 4.3 Gametocyte carriage day 7 | 3 | 897 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.02, 2.69] |
| 4.4 Gametocyte carriage day 14 | 3 | 894 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.16, 2.02] |
| 5 Anaemia | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Mean haemoglobin (g/dl) at baseline | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mean change in haemoglobin (g/dl) from baseline to day 14 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Mean change in haemoglobin (g/dl) from baseline to Day 28 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Mean haemoglobin (g/dl) at Day 28 | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Serious adverse events (including deaths) | 7 | 4200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.36, 1.03] |
13.5. Analysis.
Comparison 13 Artesunate plus amodiaquine vs Amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 5 Anaemia.
Comparison 14. Dihydroartemisinin‐piperaquine dose analysis: 3 dose vs 4 dose regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure PCR unadjusted | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.84, 3.53] |
| 1.1 Day 63 | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.84, 3.53] |
| 2 Total Failure PCR adjusted | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 6.09] |
| 2.1 Day 63 | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.05, 6.09] |
Comparison 15. Dihydroartemisinin‐piperaquine dose analysis (versus Artesunate plus mefloquine).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure Day 63 PCR unadjusted | 4 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.49, 1.38] |
| 1.1 DHA‐P 4 doses | 3 | 1019 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.59, 1.10] |
| 1.2 DHA‐P 3 doses | 2 | 765 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.09, 22.81] |
| 2 Total Failure Day 63 PCR adjusted | 4 | 1634 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.31] |
| 2.1 DHA‐P 4 doses | 3 | 908 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.04] |
| 2.2 DHA‐P 3 doses | 2 | 726 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.03, 48.28] |
| 3 Total Failure Day 42 PCR unadjusted | 5 | 2126 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.43, 1.35] |
| 3.1 DHA‐P 4 doses | 3 | 957 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.50, 1.28] |
| 3.2 DHA‐P 3 doses | 3 | 1169 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.20, 3.81] |
| 4 Total Failure Day 42 PCR adjusted | 5 | 2043 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.20, 1.91] |
| 4.1 DHA‐P 4 doses | 3 | 903 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.14, 2.82] |
| 4.2 DHA‐P 3 doses | 3 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.08, 5.87] |
| 5 Total Failure Day 28 PCR unadjusted | 6 | 2191 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.20, 2.65] |
| 5.1 DHA‐P 4 doses | 4 | 1075 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.10, 3.14] |
| 5.2 DHA‐P 3 doses | 3 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.09, 18.93] |
| 6 Total Failure Day 28 PCR adjusted | 6 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.19, 2.86] |
| 6.1 DHA‐P 4 doses | 4 | 1067 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.10, 6.11] |
| 6.2 DHA‐P 3 doses | 3 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.08, 7.82] |
Comparison 16. Artesunate Mefloquine dose analysis: FDC versus split dose regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure Day 63 PCR unadjusted | 1 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.27] |
| 2 Total Failure Day 63 PCR adjusted | 1 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.34, 1.28] |
Comparison 17. Artesunate plus mefloquine dose analysis (versus Dihydroartemisinin‐piperaquine).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Failure Day 63 PCR adjusted | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Total Failure Day 28 PCR adjusted | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.23, 19.88] |
Comparison 18. How does Dihydroartemisinin‐piperaquine perform?
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness: Total Failure (P. falciparum) PCR adjusted | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 63: DHA‐P vs Artesunate plus mefloquine | 4 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.17, 1.83] |
| 1.2 Day 42: DHA‐P vs Artemether‐lumefantrine | 5 | 1337 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.29, 1.30] |
| 1.3 Day 28: DHA‐P vs Artesunate plus amodiaquine | 2 | 629 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.13, 1.35] |
| 1.4 Day 42: DHA‐P vs Artesunate plus sulfadoxine‐pyrimethamine | 1 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.39, 1.51] |
| 1.5 Day 28: DHA‐P vs Amodiaquine plus sulfadoxine‐pyrimethamine | 2 | 802 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.64] |
18.1. Analysis.
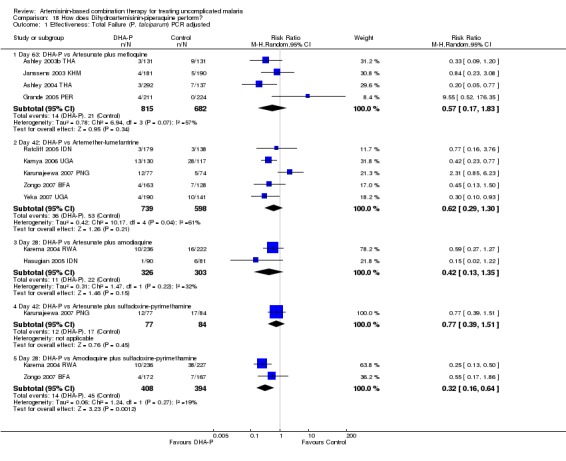
Comparison 18 How does Dihydroartemisinin‐piperaquine perform?, Outcome 1 Effectiveness: Total Failure (P. falciparum) PCR adjusted.
Comparison 19. How does Artesunate plus mefloquine perform?
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness: Total Failure (P. falciparum) PCR adjusted | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 63: AS+MQ vs Dihydroartemisinin‐piperaquine | 4 | 1497 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.55, 5.72] |
| 1.2 Day 42: AS+MQ vs Artemether‐lumefantrine | 4 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.05, 2.84] |
| 1.3 Day 28: AS+MQ vs Artesunate plus amodiaquine | 1 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Day 28: AS+MQ vs Artesunate plus sulfadoxine‐pyrimethamine | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Day 28: AS+MQ vs Amodiaquine plus sulfadoxine‐pyrimethamine | 1 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
19.1. Analysis.
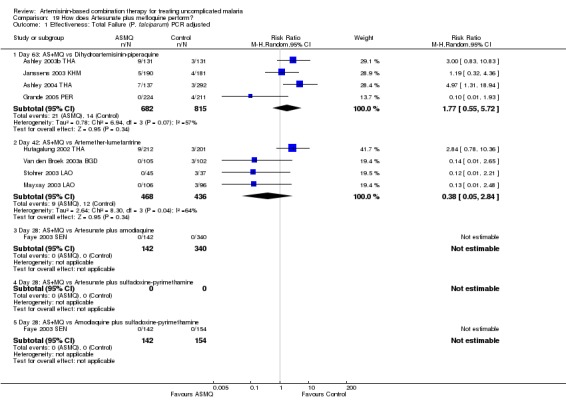
Comparison 19 How does Artesunate plus mefloquine perform?, Outcome 1 Effectiveness: Total Failure (P. falciparum) PCR adjusted.
Comparison 20. How does Artemether‐lumefantrine perform?
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness: Total Failure (P. falciparum) Day PCR adjusted | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 42: AL vs Dihydroartemisinin‐piperaquine | 5 | 1337 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.77, 3.39] |
| 1.2 Day 42: AL vs Artesunate plus mefloquine | 4 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 2.66 [0.35, 20.09] |
| 1.3 Day 28: AL vs Artesunate plus amodiaquine | 8 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.97, 3.02] |
| 1.4 Day 42: AL vs Artesunate plus sulfadoxine‐pyrimethamine | 1 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.13, 0.86] |
| 1.5 Day 28: AL vs Amodiaquine plus sulfadoxine‐pyrimethamine | 5 | 1669 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.11] |
20.1. Analysis.
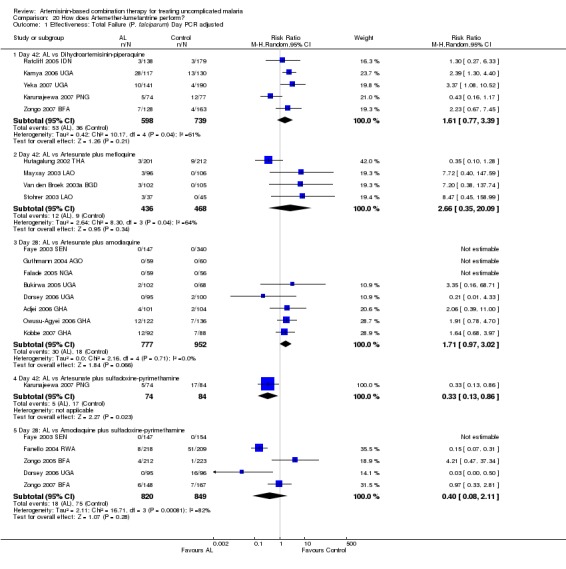
Comparison 20 How does Artemether‐lumefantrine perform?, Outcome 1 Effectiveness: Total Failure (P. falciparum) Day PCR adjusted.
Comparison 21. How does Artesunate plus amodiaquine perform?
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness: Total Failure (P. falciparum) PCR adjusted | 19 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 28: AS+AQ vs Dihydroartemisinin‐piperaquine | 2 | 629 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.74, 7.54] |
| 1.2 Day 28: AS+AQ vs Artesunate plus mefloquine | 1 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Day 28: AS+AQ vs Artemether‐lumefantrine | 8 | 1729 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.33, 1.03] |
| 1.4 Day 28: AS+AQ vs Artesunate plus sulfadoxine‐pyrimethamine | 7 | 1419 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.34, 1.45] |
| 1.5 Day 28: AS+AQ vs Amodiaquine plus sulfadoxine‐pyrimethamine | 6 | 2364 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.33, 1.63] |
21.1. Analysis.
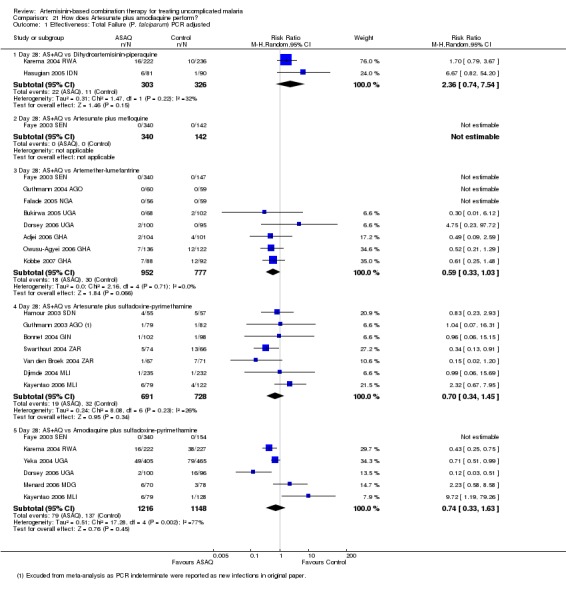
Comparison 21 How does Artesunate plus amodiaquine perform?, Outcome 1 Effectiveness: Total Failure (P. falciparum) PCR adjusted.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adjei 2006 GHA.
| Methods | Trial design: A single blind randomized controlled trial Follow up: Clinical and laboratory assessment on days 0, 1, 2, 3, 7, 14, 28 and then monthly for 1 year Adverse event monitoring: Assessed at each visit up to 1 year using open questions about side effects, behavioural and developmental concerns. Neurological examination at each visit. Audiometry assessment on days 0, 3, 7, 28, and 1 year. WBC, aminotransferase and total bilirubin at days 0, 3, 7, 14, and 28. |
|
| Participants | Number: 227 randomized Inclusion criteria: Age 6 months to 14 yrs, axillary temp > 37.5 ºC, signs and symptoms of uncomplicated malaria, P. falciparum mono‐infection 2000 to 200,000/µl, willingness to comply with the follow up, informed consent Exclusion criteria: Signs or symptoms of severe malaria, chronic malnutrition or other severe disease, known intolerance or allergy to study meds, reported treatment with any of the study drugs during preceding month |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination (Plasmotrim: Mepha, Camoquine: Pfizer)
Only the first dose each day was supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Ghana Setting: Urban primary health facilities Transmission: Not described Resistance: AQ Dates: Oct 2004 to Dec 2006 Funding: Danish Council for Development Research, Global Fund for AIDS, TB and Malaria through the National Malaria Control Programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A computer generated randomisation scheme was prepared in advance' |
| Allocation concealment? | Low risk | 'Allocated treatments were kept in sealed opaque envelopes' |
| Blinding? All outcomes | Low risk | 'All study personnel (except project nurses) were unaware of the assigned treatments' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (7.2% AL6 vs 7.8% AS+AQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate treatment failure with AL6. |
| Free of other bias? | Low risk | No other sources of bias identified |
Ashley 2003a THA.
| Methods | Trial design: A 3‐arm randomized controlled trial Follow up: All patients admitted to hospital for 28 days, oral temperature taken every 6 hours, parasite counts 12‐hourly until negative then daily for 28 days Adverse event monitoring: Adverse events defined as signs or symptoms that occurred or became more severe after treatment started. All patients had full blood counts, urea, electrolytes, creatinine, and liver function tests at days 0 and 7. |
|
| Participants | Number: 134 randomized into included treatment arms Inclusion criteria: Age > 14 yrs, weight > 40 kg, symptoms of malaria, P. falciparum parasitaemia, informed consent Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% of red blood cells parasitized, contraindication to mefloquine, treatment with mefloquine in the previous 60 days, sulphonamides or 4‐aminoquinolones present in urine on admission |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilin, Mequin: Atlantic)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Thailand Setting: Bangkok Hospital for Tropical Diseases Transmission: Low transmission Resistance: Multiple‐drug resistance Dates: Jul 2002 to Apr 2003 Funding: Mahidol University, Tak Malaria Initiative Project, supported by Bill and Melinda Gates Foundation, Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'The randomisation was computer generated (STATA; version 7; Statacorp)'. Randomized in blocks of 6 |
| Allocation concealment? | Low risk | 'The treatment allocation was concealed in sealed envelopes labelled with the study code' |
| Blinding? All outcomes | High risk | 'Laboratory staff reading the blood smears had no knowledge of the treatment received'. No other blinding described |
| Incomplete outcome data addressed? All outcomes | Low risk | Similar loss to follow up in all groups (10.6% DHA‐P vs 11.9% AS+MQ) |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 28 outcomes are likely to underestimate treatment failure with AS+MQ and DHA‐P. |
| Free of other bias? | Low risk | No other sources of bias identified |
Ashley 2003b THA.
| Methods | Trial design: A randomized controlled trial Follow up: Temperature and blood smears daily until clearance of fever and parasites, then weekly attendance until day 63 Adverse event monitoring: Adverse events defined as signs or symptoms that occurred or became more severe after treatment started. A subset of 55 patients in the DHA‐P group had full blood counts, urea, electrolyte, creatinine and liver function tests at days 0 and 7. 32 patients from the DHA‐P group also had ECG monitoring before and after treatment. |
|
| Participants | Number: 355 randomized into included treatment arms Inclusion criteria: Age 1 to 65 yrs, symptomatic P. falciparum parasitaemia, informed consent Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% of red blood cells parasitized, contraindication to mefloquine, treatment with mefloquine in the previous 60 days |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilin, Mequin: Atlantic)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Thailand Setting: 4 clinics on the Thai‐Myanmar border Transmission: Unstable low and seasonal transmission Resistance: Multiple‐drug resistance Dates: Jul 2002 to Apr 2003 Funding: Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'The randomisation was computer generated (STATA; version 7; Statacorp)'. Randomized in blocks of 9. |
| Allocation concealment? | Low risk | 'The treatment allocation was concealed in sealed envelopes labelled with the study code' |
| Blinding? All outcomes | High risk | 'Laboratory staff reading the blood smears had no knowledge of the treatment received'. No other blinding described. |
| Incomplete outcome data addressed? All outcomes | Low risk | Similar losses to follow up in all groups (12.8% DHA‐P vs 13.6% AS+MQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Ashley 2004 THA.
| Methods | Trial design: A 3‐arm randomized controlled trial Follow up: Temperature and blood smears daily until clearance of fever and parasites, then weekly attendance for examination, symptom enquiry, malaria smear and haematocrit until day 63 Adverse event monitoring: Adverse events defined as signs or symptoms that occurred or became more severe after treatment started. Symptoms were screened at each visit |
|
| Participants | Number: 499 randomized Inclusion criteria: Age 1 to 65 yrs, symptomatic P. falciparum mono‐infection or mixed infections, informed consent Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% of red blood cells parasitized, treatment with mefloquine in the previous 60 days |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination (Artekin: Holleykin)
2. Dihydroartemisinin‐piperaquine, fixed dose combination (Artekin: Holleykin)
3. Artesunate plus mefloquine, loose combination (Artesunate: Guilin, Mequin: Atlantic)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Thailand Setting: 4 clinics on the Thai‐Myanmar border Transmission: Unstable low and seasonal transmission Resistance: Multiple‐drug resistance Dates: Apr 2003 to Apr 2004 Funding: Medicines for Malaria Venture, Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'The randomisation list was generated using STATA; version 7 (Stata)'. Randomized in blocks of 9. |
| Allocation concealment? | Low risk | 'The treatment allocation was concealed in sealed envelopes labelled with the study code' |
| Blinding? All outcomes | High risk | 'Laboratory staff reading the blood smears had no knowledge of the treatment received'. No other blinding described. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were low in all groups (4.2% DHA‐P vs 4.8% AS+MQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. 2 patients were considered to be early treatment failures by the reviewers and reclassified as such. This was not clearly stated in the paper. |
| Free of other bias? | Low risk | No other sources of bias identified |
Ashley 2005 THA.
| Methods | Trial design: An open label randomized controlled trial Follow up: Temperature and blood smears daily until clearance of fever and parasites, then weekly attendance for clinical examination, symptom enquiry, malaria smear, and haematocrit until day 63 Adverse event monitoring: Adverse events were actively screened at each visit. Adverse events were defined as signs or symptoms that occurred or became more severe after treatment started. |
|
| Participants | Number: 500 randomized Inclusion criteria: Age 6 months to 65 yrs, weight > 5 kg, symptomatic P. falciparum mono‐infection or mixed infections, informed consent Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% of red blood cells parasitized, treatment with mefloquine in the previous 60 days, contraindication to mefloquine |
|
| Interventions | 1. Artesunate plus mefloquine, fixed‐dose combination, adult tablets 100 mg/220 mg, paediatric tablets 25 mg/55 mg (Far‐Manguinhos)
2. Artesunate plus mefloquine, loose combination, (Arsumax: Sanofi‐Synthelabo, Lariam: Roche)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Thailand Setting: 6 clinics on the Thai‐Myanmar border Transmission: Unstable low and seasonal transmission Resistance: Multiple‐drug resistance Dates: Nov 2004 to Jun 2005 Funding: DNDi, European Union International Co‐operation programme, Médecins sans Frontières, WHO/TDR, Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomised in blocks of 10 by a statistician using a computer‐generated randomisation' |
| Allocation concealment? | Low risk | 'The treatment allocation was concealed in numbered, sealed envelopes...opened only after enrolment in the study' |
| Blinding? All outcomes | High risk | An open label study. '50% of enrolment slides, 10% of follow up slides and all slides reported as showing recrudescence were subjected to a second blind reading' |
| Incomplete outcome data addressed? All outcomes | High risk | Losses to follow‐up are moderate (15.5% FDC vs 15.3% loose). Reasons are not clearly stated and some losses may represent early treatment failures. |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Bonnet 2004 GIN.
| Methods | Trial design: A randomized controlled trial Follow up: Clinical and parasitological assessment on days 0, 1, 2, 3, 7, 14, 21 and 28. Gametocyte carriage measured at day 0 and 28. PCR genotyping on all reappearances after day 9. Adverse event monitoring: None described |
|
| Participants | Number: 220 randomized Inclusion criteria: Age 6 to 59 months, axillary temp > 37.5 ºC, P. falciparum mono‐infection 2000 to 200,000/µl Exclusion criteria: Signs of severity or severe malaria, severe anaemia (Hb < 5 g/dl), severe malnutrition, concomitant febrile condition with the potential to confound study outcome, history of allergic reaction to the study drugs |
|
| Interventions | 1. Artesunate plus amodiaquine, loose combination (Arsumax: Guilin, Camoquin: Parke‐Davis)
2. Artesunate plus sulfadoxine‐pyrimethamine, loose combination, (Arsumax: Guilin, Fansidar: Roche)
All doses supervized |
|
| Outcomes |
|
|
| Notes | Country: Guinea Setting: Outpatient department Transmission: Perennial seasonal malaria with increased transmission between June and October Resistance: CQ, AQ and SP resistance Dates: Jun 2004 to Sept 2004 Funding: Médecins sans Frontières |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A randomization list with a block size of 20 was electronically generated by the methodological center (Epicentre, Paris)' |
| Allocation concealment? | Low risk | 'Sealed opaque envelopes corresponding to each inclusion number, and containing the name of the allocated treatment regimen, were prepared before the study started.' (Additional information from authors) |
| Blinding? All outcomes | High risk | No comment on blinding. A random sample of 92 slides were cross‐checked by an independent technician. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss to follow up in both groups (2.7% AS+AQ vs 3.6% AS+SP) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Bousema 2004 KEN.
| Methods | Trial design: A 3‐arm, single blind (outcome assessors) randomized controlled trial Follow up: Days 0, 1, 2, 3, 7, 14, and 28 or any other day they became ill Adverse event monitoring: None described |
|
| Participants | Number: 376 randomized to included treatment arms Inclusion criteria: Age 6 months to 10 yrs, temp > 37.5 ºC or history of fever, P. falciparum mono‐infection > 500/µl. Additionally for AL group: weight > 10 kg and living < 5 km from the clinic. Exclusion criteria: Signs of severe malaria, inability to take meds orally, evidence of chronic disease or an acute infection other than malaria, known hypersensitivity to any of the study drugs, reported treatment with antimalarials in the previous 2 weeks, resident outside of study area |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus sulfadoxine‐pyrimethamine, loose combination (Arsumax: Sanofi‐Aventis, Fansidar: Roche)
3. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination (Camoquine: Pfizer, Fansidar: Roche)
All doses supervized and given with a fatty meal |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Kenya Setting: Rural clinic Transmission: High and perennial Resistance: Not reported Dates: Oct to Dec in 2003 and 2004 Funding: Foundation for the Advancement of Tropical Research, Netherlands Organization for Scientif Research, Ter Meulen Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Children were divided in age strata and randomized to different treatment regimens using Excel generated randomization tables. Serious flaws in randomization. |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | Low risk | 'Other than those administering the medication, all staff engaged in the trial were blinded to allocation' |
| Incomplete outcome data addressed? All outcomes | High risk | Losses to follow up were different between groups with no losses in the AL group (0% AL6 vs 8.0% AS+SP vs 9.4% AQ+SP). This is likely to be related to the different inclusion criteria for AL6. |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may underestimate treatment failure with AL6. |
| Free of other bias? | High risk | Due to differing inclusion criteria for the 3 arms children in the AL6 group were older, heavier and had higher Hb levels at baseline. This may improve outcome in this group and consequently the AL6 arm was excluded from this review. |
Bukirwa 2005 UGA.
| Methods | Trial design: A single blind randomized controlled trial Follow up: Days 0, 1, 2, 3, 7, 14, and 28 or any other day they became ill, for a standardized history, examination and malaria film. Haemoglobin measurement day 0, 28 or day of failure. Participants with Hb < 10 g/dl given ferrous sulphate and antihelminthic treatment. Adverse event monitoring: Assessed at each follow‐up visit, an adverse event defined as any untoward medical occurrence |
|
| Participants | Number: 419 randomized Inclusion criteria: Age 1 to 10 yrs, axillary temp > 37.5 ºC or history of fever in previous 24 hrs, P. falciparum mono‐infection 2000 to 200,000/µl, informed consent Exclusion criteria: Danger signs or evidence of severe malaria, evidence of a concomitant febrile illness, repeated vomiting of first dose of medication, history of serious side effects to study drugs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi‐Aventis, Camoquin: Parke‐Davis)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Uganda Setting: Rural health centre Transmission: High transmission, holoendemic with peaks following 2 rainy seasons Resistance: CQ and SP resistance Dates: Dec 2004 to July 2005. Funding: Centers for Disease Control and Prevention, Association of Schools of Public Health, DfID |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'An off‐site investigator prepared computer‐generated age‐stratified randomisation codes' |
| Allocation concealment? | Low risk | 'The randomisation list was secured in a locked cabinet accessible only by the study nurse. Participants were enrolled by study physicians and treatments were assigned by the study nurse' |
| Blinding? All outcomes | Low risk | 'Only the study nurse was aware of treatment assignments. All other study personnel including study physicians and laboratory personnel involved in assessing outcomes were blinded' |
| Incomplete outcome data addressed? All outcomes | Low risk | Participants were excluded before enrolment only by predefined criteria. Losses to follow up after enrolment were low (1% AL6 vs 1.5% AS+AQ) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate treatment failure with AL6. |
| Free of other bias? | Low risk | No other sources of bias identified |
Djimde 2004 MLI.
| Methods | Trial design: A single blind (outcome assessors) randomized controlled trial Follow up: Days 0, 1, 2, 3, 7, 14, 21, and 28 or any other day they became ill, for a clinical assessment and malaria film Adverse event monitoring: Haemoglobin, glucose, complete blood count, liver enzymes, and creatinine were measured on days 0, 7, 14, and 28 |
|
| Participants | Number: 502 randomized to included treatment arms Inclusion criteria: Age > 6 months, weight > 5 kg, axillary temp > 37.5 ºC, uncomplicated malaria of any species 2000 to 200,000/µl, able to tolerate oral treatment, resident of study area for entire period of follow up, informed consent Exclusion criteria: Pregnancy, symptoms of severe malaria, allergy to a study drug, documented consumption of 1 of the study drugs in the previous 7 days |
|
| Interventions | 1. Artesunate plus amodiaquine, fixed dose combination, 50/153 mg tablets (Arsucam: Sanofi‐Aventis)
2. Artesunate plus sulfadoxine‐pyrimethamine, loose combination (Arsumax: Sanofi‐Aventis, Fansidar: Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Mali Setting: A village Transmission: Hyperendemic with seasonal peaks Resistance: CQ and SP resistance Dates: Dec 2002 to Oct 2004 Funding: Access to Medicines, Sanofi‐Aventis and the International Atomic Energy Agency |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Enrolled patients were randomly assigned to treatment groups'. No further details. |
| Allocation concealment? | Unclear risk | 'The randomisation list was concealed to clinicians'. No further details. |
| Blinding? All outcomes | Unclear risk | Described as single blind, although details not given |
| Incomplete outcome data addressed? All outcomes | High risk | In the day 28 efficacy analysis 13 patients in the AS+AQ group and 9 in the AS+SP group are unaccounted for |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | High risk | 'The study sponsor was involved in the protocol development and reporting of severe adverse events' |
Dorsey 2006 UGA.
| Methods | Trial design: A 3‐arm, single blind (outcome assessors) randomized controlled trial. An unusual design where participants were randomized to a treatment and followed up through however many episodes of malaria happened to occur during the time period. Follow up: Days 0, 1, 2, 3, 7, 14, and 28 or any other day they became ill, for a standardized history, examination and malaria film. Anthelminthics, iron sulphate, and vitamin A were prescribed as per IMCI guidelines. Participants with P. vivax during follow up were censored on day of occurrence Adverse event monitoring: Assessed at each follow‐up visit, an adverse event defined as any untoward medical occurrence. Complete blood count and alanine aminotransferase on day 0 and 14. |
|
| Participants | Number: 329 children randomized to a treatment group Inclusion criteria: Age 1 to 10 yrs, weight >10 kg, agreement to remain in Kampala, agreement to attend the study clinic for any febrile illness, agreement to avoid medications outside of the study, informed consent Exclusion criteria: Known adverse reactions to study meds, severe malnutrition, known serious chronic disease, life threatening lab results on screening |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets
2. Artesunate plus amodiaquine, loose combination
3. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination
Only the first dose was supervized each day |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Uganda Setting: Urban clinic Transmission: Mesoendemic with peaks during the 2 rainy seasons Resistance: CQ, AQ and SP resistance Dates: Nov 2004 to June 2006 Funding: National Institutes of Health, Doris Duke Charitable Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A randomisation list was computer generated with variable blocks of 3, 6, and 9 by an off‐site investigator' |
| Allocation concealment? | Low risk | 'Sequentially numbered, sealed envelopes containing the treatment group assignments were prepared from the randomisation list' |
| Blinding? All outcomes | Low risk | 'All study personnel involved in outcome assessment were blinded to treatment allocation' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in all groups and reasons given (2.9% AL6 vs 5.4% AS+AQ vs 5.4% AQ+SP) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow‐up in studies of AL6. Day 28 outcomes may underestimate the failure rate with AL6. |
| Free of other bias? | Low risk | No other sources of bias identified |
Falade 2005 NGA.
| Methods | Trial design: An open‐label randomized controlled trial Follow up: Examination and malaria film on days 0 to 7, 14, 21, and 28. Participants were admitted to hospital for the first 3 days then seen at days 7, 14, 21, and 28. Adverse event monitoring: Assessed at each visit by examination and questioning about the progress of presenting symptoms and new symptoms. FBC, WBC, and liver enzymes on days 0, 7, and 28. An adverse event defined as not present at enrolment but occurring during follow up. |
|
| Participants | Number: 132 participants randomized Inclusion criteria: Age 6 months to 10 yrs, axillary temp > 37.5 ºC, signs and symptoms of malaria, P. falciparum mono‐infection 2000 to 200,000/µl, willingness to comply with the protocol, informed consent Exclusion criteria: Signs of severe and complicated malaria or other febrile illness, severe malnutrition, history of hypersensitivity to any of the study drugs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi‐Synthelabo, Camoquine: Pfizer)
All doses supervized and given with food, fruit drink, or dissolved in water |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Nigeria Setting: General Outpatient Department of University College Hospital Transmission: Intense and occurs all year round Resistance: CQ and SP Dates: Aug 2004 to Aug 2005 Funding: Study meds were supplied by Novartis, Sanofi‐Sycitilabo and Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A pregenerated randomisation table' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of lab staff |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (7.5% AL6 vs 6.0% AS+AQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate treatment failure with AL6. |
| Free of other bias? | Low risk | No other sources of bias identified |
Fanello 2004 RWA.
| Methods | Trial design: An open‐label randomized controlled trial Follow up: Participants were admitted to hospital for the first 3 days then seen at days 7, 14, 21, and 28. At each visit history, clinical signs and symptoms, temperature and malaria film. PCV and WBC were recorded on days 0 and 14. Adverse event monitoring: All adverse events were recorded on the clinical record form and a causality assessment was made |
|
| Participants | Number: 500 randomized Inclusion criteria: Age 12 to 59 months, weight >10 kg, axillary temp > 37.5 ºC or history of fever in the previous 24 hrs, P. falciparum mono‐infection 2000 to 200,000/µl, informed consent Exclusion criteria: Severe malaria, concomitant illness or underlying disease, known allergy to the study drugs, a clear history of adequate antimalarial treatment in the previous 72 hrs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets
2. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Rwanda Setting: Rural health clinics Transmission: Variable Resistance: Not described Dates: July 2004 to Dec 2004 Funding: Belgian Development Co‐operation (DGIS) and the Prince Leopold Institute of Tropical Medicine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomly allocated in blocks of 20...according to a randomization list prepared in Belgium' |
| Allocation concealment? | Unclear risk | 'Allocation of treatment was concealed from both the doctor and the patient, until final recruitment of the patient'. Method not described. |
| Blinding? All outcomes | High risk | An open‐label trial. 'Laboratory technicians reading malaria slides did not know the treatment received by individual patients' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up (2% AL6 vs 0.8% AQ+SP) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may overestimate the efficacy of AL6. |
| Free of other bias? | Low risk | No other sources of bias identified |
Faye 2003 SEN.
| Methods | Trial design: A 5‐arm, open‐label randomized controlled trial Follow up: Days 0, 1, 2, 7, 14, 21, and 28 for a clinical examination and malaria film Adverse event monitoring: All side effects were monitored actively and passively during the study. 25% randomly selected for blood counts, liver, and renal function tests at days 0, 14, and 28. |
|
| Participants | Number: 815 randomized into included treatment arms Inclusion criteria: 'as per WHO 2002 protocol' Exclusion criteria: 'as per WHO 2002 protocol' |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, co‐blistered (Artequine: Mepha)
3. Artesunate plus amodiaquine, co‐blistered (Arsucam: Sanofi‐Aventis)
4. Amodiaquine plus sufadoxine‐pyrimethamine (Pharmacie Nationale d'Approvisionnement d Senegal)
All doses supervized |
|
| Outcomes |
|
|
| Notes | Country: Senegal Setting: Healthcare centres Transmission: Moderate with a peak in the rainy season Resistance: High levels of chloroquine resistance Dates: The transmission periods of 2002 and 2003 Funding: Study drugs supplied by Sanofi‐Aventis, Mepha, and Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. Only described as 'randomized' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of lab staff |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were not reported in the original paper and figures were only given as percentages. Unpublished data reveal loss to follow up as low in all groups (3.1% AS+AQ, 0.7% AS+MQ, 1.3% AL6, 3.1% AQ+SP). |
| Free of selective reporting? | Low risk | The WHO recommend 42 days follow up for studies involving AL6 and 63 days for AS+MQ. Day 28 outcomes may underestimate treatment failure with AL6 and AS+MQ. |
| Free of other bias? | Low risk | No other sources of bias identified |
Grande 2005 PER.
| Methods | Trial design: An open‐label randomized controlled trial Follow up: Days 0, 1, 2, 3, 7, 14, 21, 28, 35, 42, 49, 56, and 63 or any other day they became ill, for a clinical assessment and malaria film. PCV measurement day 0, 7, 14 and 63. P. vivax treated with CQ. Adverse event monitoring: Assessed at each follow‐up visit, an adverse event defined as any unfavourable and unintended sign, symptom or disease temporally associated with the drug administered. Complete blood count, liver, and renal function tests at days 0 and 7. |
|
| Participants | Number: 522 randomized Inclusion criteria: Age 5 to 60 yrs, fever > 37.5 ºC or history of fever in the previous 24 hours, P. falciparum mono‐infection 1000 to 200,000/µl Exclusion criteria: Pregnancy or lactation, severe malaria, any concomitant illness or underlying disease, contraindication to any of the trial drugs, history of treatment with mefloquine in the previous 60 days or chloroquine, primaquine or quinine in previous 14 days |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilin, Lariam: Hoffman La‐Roche)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Peru Setting: 9 rural health posts Transmission: Low malaria transmission Resistance: High CQ and SP resistance Dates: July 2003 to July 2005 Funding: Directorate‐General for Development and Cooperation of the Belgian Government |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized in blocks of 10'. No further details given. |
| Allocation concealment? | Low risk | 'Sealed opaque envelopes were opened only after the final decision to recruit the patient had been made' |
| Blinding? All outcomes | High risk | An open‐label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Similar loss to follow up in both groups (8.7% DHA‐P vs 5.9% AS+MQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Guthmann 2003 AGO.
| Methods | Trial design: An open label randomized controlled trial Follow up: Reassessed clinically and parasitologically on days 0, 3, 7, 14, 21, and 28. Gametocytes were measured at each visit. Haemoglobin was measured at days 0 and 28. Adverse event monitoring: None described |
|
| Participants | Number: 187 randomized into included treatment arms Inclusion criteria: Age 6 to 59 months, weight > 5 kg, axillary temp > 37.5 ºC or history of fever in the previous 24 hours, P. falciparum mono‐infection 2000 to 100,000/µl, living within 1 hours walk of the clinic, informed consent Exclusion criteria: Signs of severity or severe malaria, severe anaemia (Hb < 5 g/dl), severe malnutrition, any concomitant febrile condition with the potential to confound the study outcome, history of allergic reaction to the study drug, reported intake of a full course of antimalarials in the previous 7 days |
|
| Interventions | 1. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi‐Aventis, Camoquin: Parke‐Davis)
2. Artesunate plus sulfadoxine‐pyrimethamine, loose combination (Arsumax: Sanofi‐Aventis, Fansidar: Roche)
All doses supervized |
|
| Outcomes |
|
|
| Notes | Country: Angola Setting: Hospital outpatient dept., health centre, 3 health posts and 1 maternal and child health centre Transmission: Mesoendemic with stable and seasonal transmission with a peak from September to April Resistance: CQ and SP resistance Dates: March 2003 to July 2003 Funding: Médecins sans Frontières |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly allocated in blocks of 20'. Due to technical problems randomization only started after the first 30 patients had been enrolled. |
| Allocation concealment? | High risk | 'Without a concealment procedure' |
| Blinding? All outcomes | High risk | No comment on blinding. External quality control on a random sample of malaria films was conducted. |
| Incomplete outcome data addressed? All outcomes | High risk | 3 times as many withdrawals in AS+AQ group vs AS+SP (12% vs 4%). Reasons for this disparity are not given. |
| Free of selective reporting? | Low risk | Only PCR adjusted results given, PCR unadjusted is unpublished data |
| Free of other bias? | Low risk | No other sources of bias identified |
Guthmann 2004 AGO.
| Methods | Trial design: A randomized controlled trial Follow up: Days 0, 1, 2, 3, 7, 14, 21, and 28, for a clinical assessment and malaria film. Haemoglobin and gametocyte measurement on days 0 and 28. Adverse event monitoring: Not described |
|
| Participants | Number: 137 randomized Inclusion criteria: Age 6 to 59 months, confirmed clinical P. falciparum malaria, informed consent Exclusion criteria: As per WHO 2003 protocol |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi‐Aventis, Camoquin: Parke‐Davis)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Angola Setting: Health centre Transmission: High transmission, mesoendemic Resistance: CQ and SP resistance Dates: Apr 2004 to Jul 2004 Funding: Médecins sans Frontières, The American Society of Tropical Medicine and Hygiene (ASTMH) and the American Committee on Clinical Tropical Medicine and Travelers’ Health (ACCTMTH) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as 'randomized' but no other details |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | Blinding not mentioned. 100 malaria films were checked by an independent laboratory |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up low in both groups (6.2% AL6 vs 7.2% AS+AQ) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate treatment failure with AL6. |
| Free of other bias? | Low risk | No other sources of bias identified |
Hamour 2003 SDN.
| Methods | Trial design: An open label randomized controlled trial Follow up: Reassessed clinically and parasitologically on days 0, 1, 2, 3, 7, 14, 21, and 28 Adverse event monitoring: Not described |
|
| Participants | Number: 161 randomized Inclusion criteria: Age 6 to 59 months, weight > 5 kg, axillary temp > 37.5 ºC, P. falciparum mono‐infection 2000 to 200,000/µml, informed consent Exclusion criteria: Signs of severe malaria, concomitant febrile conditions except mild viral upper respiratory tract infections, hypersensitivity to study drugs |
|
| Interventions | 1. Artesunate plus sulphadoxine‐pyrimethamine, loose combination (Arsumax: Sanofi‐Aventis, Fansidar: Roche)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi‐Aventis, Camoquin: Parke‐Davis)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Sudan Setting: Rural health care centre Transmission: Markedly seasonal Resistance: CQ resistance Dates: Sept 2003 to Nov 2003 Funding: Médecins sans Frontières |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized by sealed envelopes'. No further details given. |
| Allocation concealment? | Unclear risk | 'Sealed envelopes'. No further details. |
| Blinding? All outcomes | High risk | An open‐label trial. No comment on blinding of laboratory staff to allocation, but slides read independently with external quality control. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (2.5% AS+SP vs 0% AS+AQ). A large number of PCR samples were indeterminate but equally distributed across groups. |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Hasugian 2005 IDN.
| Methods | Trial design: An open label randomized controlled trial Follow up: Daily until fever and parasites cleared then weekly until day 42, for a physical examination, a symptom questionnaire and malaria film. Haemoglobin measured on days 0, 7, and 28. Adverse event monitoring: Assessed at each follow‐up visit |
|
| Participants | Number: 340 randomized Inclusion criteria: Age > 1 yr, weight > 5 kg, slide confirmed malaria (P. falciparum, P. vivax or both), fever or history of fever in the preceding 48 hours Exclusion criteria: Pregnancy or lactation, danger signs or signs of severe malaria, > 4% red blood cells parasitized, concomitant disease that required hospital admission |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination (Artekin: Holley)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Guilin, Flavoquine: Aventis)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Indonesia Setting: Rural clinics Transmission: Unstable Resistance: Chloroquine and SP resistance Dates: Jul 2005 to Dec 2005 Funding: Wellcome Trust ‐ National Health and Medical Research Council |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A randomisation list was generated in blocks of 20 by an independent statistician' |
| Allocation concealment? | Low risk | 'Treatment allocation concealed in an opaque, sealed envelope that was opened once the patient had been enrolled' |
| Blinding? All outcomes | High risk | An open‐label trial. 'All slides were read by a certified microscopist who was blinded to treatment allocation'. |
| Incomplete outcome data addressed? All outcomes | High risk | The primary outcome data are unpublished data including only participants with P. falciparum mono or co‐infection at baseline. High losses to follow up in both groups at day 42 (21% DHA‐P vs 24.5 % AL6), moderate at day 28 (16.6% DHA‐P vs 18.8 % AL6). |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 42 outcomes may underestimate failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
Hutagalung 2002 THA.
| Methods | Trial design: An open‐label randomized controlled trial Follow up: Examination and malaria film daily until fever and parasites cleared then weekly to day 42 or any other day they became unwell P. vivax during follow up was treated with CQ and continued in follow up Adverse event monitoring: At each visit a questionnaire on adverse events was completed |
|
| Participants | Number: 490 randomized Inclusion criteria: Weight > 10 kg, slide confirmed P. falciparum, informed consent Exclusion criteria: Pregnancy, clinical or laboratory signs of severe illness and/or severe and complicated malaria severe malaria, treatment with mefloquine in previous 63 days |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilan, Lariam: Hoffman‐La Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Thailand Setting: Malaria clinics of the Shoklo Malaria Research Unit Transmission: Low and unstable Resistance: Multiple‐drug resistance Dates: July 2001 to June 2002 Funding: Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computerized randomisation was in blocks of ten' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up balanced and low in both groups (8% AL6 vs 7% AS+MQ) |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 42 outcomes may under estimate treatment failure with AS+MQ. |
| Free of other bias? | Low risk | No other sources of bias identified |
Janssens 2003 KHM.
| Methods | Trial design: An open label randomized controlled trial Follow up: Monitored daily until fever and parasites cleared then weekly to day 63. Temperature, symptom questionnaire, malaria film, and haematocrit at each visit. Adverse event monitoring: An adverse event defined as any new sign or symptom appearing after treatment started. At each visit a symptom questionnaire was completed. |
|
| Participants | Number: 464 randomized Inclusion criteria: Age > 1 yr, axillary temp > 37.5 ºC or history of fever, signs and symptoms of uncomplicated malaria, P. falciparum mono or mixed infections, written informed consent Exclusion criteria: Pregnancy or lactation, signs or symptoms of severe malaria, > 4% red blood cells parasitized, a history of convulsions or neuropsychiatric disorder, treatment with mefloquine in the past 60 days |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilin, Mefloquine: Mepha)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Cambodia Setting: Rural health centres and outreach malaria clinics Transmission: Low and seasonal Resistance: Multiple‐drug resistance Dates: Oct 2002 to March 2003 Funding: Médecins sans Frontières |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer generated randomisation (STATA version 8, Statacorp)' |
| Allocation concealment? | Unclear risk | 'Treatment allocations were concealed in sealed envelopes'. No further details. |
| Blinding? All outcomes | High risk | An open‐label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up balanced and low in both groups (9.3% DHA‐P vs 10% AS+MQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Kamya 2006 UGA.
| Methods | Trial design: A single blind (outcome assessors) randomized controlled trial Follow up: Standardized history and examination and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, 35, 42 and any other day they felt unwell. Haemoglobin measured at day 0 and day 42 or day of failure. Anaemia was treated with ferrous sulphate and anthelminthics according to IMCI guidelines. Adverse event monitoring: Assessed for any new or worsening event at each visit. An adverse event defined as any untoward medical occurrence, irrespective of its suspected relationship to the study medications. |
|
| Participants | Number: 509 randomized Inclusion criteria: Age 6 m to 10 yrs, weight > 5 kg, axillary temp > 37.5 ºC or history of fever in the past 24 hours, P. falciparum mono‐infection 2000 to 200,000/µl, informed consent Exclusion criteria: Danger signs or signs of severe malaria, evidence of concomitant febrile illness, history of serious side effects to study medication |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Duocotexin: HolleyPharm)
All doses supervized. All participants received a glass of milk after each dose |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Uganda Setting: Rural health centre Transmission: Perennial holoendemic malaria with very high transmission intensity Resistance: Not reported Dates: Mar 2006 to July 2006 Funding: US Centres for Disease Control, Malaria Consortium Drugman, DFID, DHA‐P supplied by HolleyPharm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A randomisation list was computer generated by an off‐site investigator' |
| Allocation concealment? | Low risk | 'Sequentially numbered, sealed envelopes containing the treatment group assignments were prepared from the randomisation list' |
| Blinding? All outcomes | Low risk | 'Study physicians and laboratory personnel involved in assessing outcomes were blinded to treatment assignments' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (0.9% AL6 vs 0.9% DHA‐P). A large number of participants were excluded after randomization for failing to meet the entry criteria. |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 42 outcomes may underestimate failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
Karema 2004 RWA.
| Methods | Trial design: A 3‐arm open label randomized controlled trial Follow up: History, clinical signs and symptoms, and malaria film on days 0, 1, 2, 3, 7, 14, 21, and 28 and any other day they felt unwell. PCV measured at days 0 and 14. Adverse event monitoring: An adverse event defined as any unfavourable and unintended sign associated temporally with the use of the drug administered. Differential WBC count (and liver function tests at 1 site only) assessed at days 0 and 14. |
|
| Participants | Number: 762 randomized Inclusion criteria: Age 12 to 59 months, weight > 10 kg, axillary temp > 37.5 ºC or history of fever in the preceding 24 hrs, P. falciparum mono‐infection 2000 to 200,000/µl Exclusion criteria: Severe malaria, any other concomitant illness or underlying disease, known allergy to study drugs, clear history of adequate antimalarial treatment in the previous 72 hours, PCV < 15% |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleypharm)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi)
3. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination.
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Rwanda Setting: Peri‐urban and rural health centres Transmission: Not reported Resistance: Not reported Dates: Oct 2003 to Apr 2004 Funding: Belgian Development Co‐operation in collaboration with the Prince Leopold Institute of Tropical Medicine. DHA‐P provided by Holleypharm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomly allocated in blocks of 15', computer generated sequence (information from author) |
| Allocation concealment? | Unclear risk | 'Allocation of treatment was concealed until final recruitment'. No further details |
| Blinding? All outcomes | High risk | An open‐label trial. 'Laboratory technicians reading malaria slides did not know the treatment received' |
| Incomplete outcome data addressed? All outcomes | Low risk | Very low losses to follow up in all groups (0.8% DHA‐P vs 0.4% AS+AQ vs 1.2% AQ+SP) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 28 outcomes may underestimate failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
Karunajeewa 2007 PNG.
| Methods | Trial design: A 4‐arm open label randomized controlled trial Follow up: Standardized follow up including temperature and malaria film on days 0, 1, 2, 3, 7, 14, 28, and 42. Drug levels assayed on day 7. Adverse event monitoring: None described |
|
| Participants | Number: 372 randomized to included treatment arms Inclusion criteria: Age 0.5 to 5 years, axillary temp > 37.5 ºC or history of fever in the preceding 24 hrs, > 1000/µl asexual P. falciparum or > 250/µl asexual P. vivax, P. ovale or P. malariae, informed consent Exclusion criteria: Features of severe malaria, evidence of another infection or coexisting condition including malnutrition, intake of study drug in previous 14 days |
|
| Interventions | 1. Artesunate plus sulfadoxine‐pyrimethamine, loose combination (Sanofi‐Aventis, Roche)
2. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Beijing Holley‐Cotec)
3. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Novartis), given with milk
All doses supervized except the evening dose of AL6 |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Papua New Guinea Setting: Health centres Transmission: Holoendemic Resistance: CQ and SP Dates: Apr 2005 to Jul 2007 Funding: WHO Western Pacific Region, Rotary against Malaria in Papua New Guinea, National Health and Medical Research Council of Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer‐generated randomised assignment with blocks of 24 for each site' |
| Allocation concealment? | High risk | Not described |
| Blinding? All outcomes | High risk | An open label trial. Microscopists were unaware of treatment assignments. |
| Incomplete outcome data addressed? All outcomes | Low risk | Moderate losses to follow up in all groups (11.5% AS+SP vs 13.0% DHA‐P vs 14.2% AL6) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 42 outcomes may underestimate failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
Kayentao 2006 MLI.
| Methods | Trial design: An open label 3‐arm randomized controlled trial Follow up: Assessment and malaria film on days 0, 1, 2, 7, 14, and 28. Haemoglobin on days 0, 14, 28 or day of failure. Adverse event monitoring: None described |
|
| Participants | Number: 397 randomized Inclusion criteria: Age 6 to 59 months, axillary temp > 37.5 ºC, P. falciparum mono‐infection of 2000 to 200,000/µl, informed consent Exclusion criteria: Danger signs, evidence of another febrile illness, haemoglobin < 5 g/dl |
|
| Interventions | 1. Artesunate plus amodiaquine, loose combination
2. Artesunate plus sulfadoxine‐pyrimethamine, loose combination
3. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Mali Setting: Rural health centre Transmission: Seasonal with peak in October Resistance: CQ Dates: Jul 2005 to Jan 2006 Funding: US Centers for Disease Control and Prevention, Malaria and Research Training Center, University of Bamako |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Block randomisation (block size of 20)'. No further details. |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | Described as 'open‐label'. Patients were not informed of the drug received but no placebos were used. Microscopists were unaware of treatment allocation. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in all groups (1.5% AS+AQ vs 1.5% AS+SP vs 1.5% AQ+SP) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Kobbe 2007 GHA.
| Methods | Trial design: An open label randomized controlled trial Follow up: Standardized history and examination, malaria film and haemoglobin on days 0, 3, 7, 14, and 28 and any other day they felt unwell Adverse event monitoring: 'The comparative tolerability was assessed by the risk of occurrence of an adverse event'. For each adverse event causality was assessed as recommended by the WHO. |
|
| Participants | Number: 246 randomized Inclusion criteria: Age 6 to 59 months, axillary temp > 37.5 ºC or history of fever in the preceding 24 hrs, P. falciparum mono‐infection 2000 to 200,000/µl, informed consent Exclusion criteria: Danger signs or signs of severe malaria, any other severe underlying disease, severe malnutrition, antibiotics or adequate antimalarials in the previous 7 days, a history of hypersensitivity to study drugs, unable to tolerate oral treatment |
|
| Interventions | 1. Artesunate plus amodiaquine, co‐blister combination 50 mg AS/153 mg AQ, (Arsucam: Sanofi‐Aventis)
2. Artemether‐lumefantrine, fixed dose combination 20/120 mg (Coartem: Novartis)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Ghana Setting: District Hospital Transmission: Holoendemic with seasonal peaks Resistance: CQ Dates: Oct 2006 to Sept 2007 Funding: Vereinigung der Freunde des Tropeninstituts Hamburg E.V., German Academic Exchange Service. Drugs supplied free of charge by Novartis and Sanofi‐Aventis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer generated list with randomisation in blocks of ten' |
| Allocation concealment? | Low risk | 'Children received the first dose of the individually allocated treatment (in sealed, numbered, opaque envelopes)' |
| Blinding? All outcomes | High risk | An open label trial. 10% of malaria slides were cross‐checked by a blinded microscopist. |
| Incomplete outcome data addressed? All outcomes | High risk | Moderate losses to follow up in both groups (14% AL6 vs 16% AS+AQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Koram 2003 GHA.
| Methods | Trial design: A 4‐arm, open‐label randomized controlled trial Follow up: Examination, symptoms recorded, temperature and pulse and malaria film on days 0, 1, 2, 3, 7, 14, 21 and 28 and any other day they felt unwell. Full blood count and haemoglobin measured at days 14 and 28. Adverse event monitoring: None |
|
| Participants | Number: 105 randomized into included treatment arms Inclusion criteria: Age 6 to 59 months, signs and symptoms of uncomplicated malaria including axillary temp > 37.5 ºC, P. falciparum mono‐infection of 2000 to 200,000/µl, informed consent Exclusion criteria: Signs and symptoms of severe malaria, other diseases requiring drugs with antimalarial or antihistaminic activities, Hb < 5 g/dl |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Ghana Setting: Hohoe District Hospital and Navrongo War Memorial Hospital Transmission: High transmission and markedly seasonal Resistance: CQ and SP resistance Dates: June 2003 to Aug 2003 Funding: Multilateral Initiative on Malaria, UNICEF/UNDP/World Bank/WHO Special Program for Research & Training in Tropical Diseases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer generated random list based on a simple random selection procedure' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open‐label trial. No comment on blinding of laboratory staff |
| Incomplete outcome data addressed? All outcomes | High risk | 'Patients who showed signs/symptoms of severe malaria, had serious adverse events or required blood transfusion were withdrawn from the study'. These events after enrolment would represent treatment failure and should not be withdrawn. |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate treatment failure with AL6. |
| Free of other bias? | High risk | Participants in the AL6 group were significantly older and had a higher Hb at baseline. This is due to differing inclusion criteria for the 2 groups and is likely to affect the result. |
Lefevre 1999 THA.
| Methods | Trial design: An open‐label clinical and pharmacokinetic randomized controlled trial Follow up: Monitored 3 times daily until parasites and fever cleared. Then follow up at days 1, 2, 3, 7, 14, 21, and 28 for temp and malaria film. P. vivax during follow up was treated with CQ and primaquine and continued in follow up Adverse event monitoring: Assessed at each visit. ECG monitoring and laboratory tests (including FBC liver and renal function tests) at baseline and each day of follow up. |
|
| Participants | Number: 219 randomized Inclusion criteria: Age > 12 yrs, weight > 35 kg, microscopically confirmed P. falciparum, informed consent Exclusion criteria: Signs or symptoms of severe malaria, heart disease or significant ECG abnormalities, psychiatric disorders, severe renal or hepatic impairment, history of drug hypersensitivity or allergy |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilan, Lariam: Hoffman‐La Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Thailand Setting: Bangkok Hospital for Tropical Diseases Transmission: Low transmission Resistance: Multiple‐drug resistance Dates: Sept 1998 to Jan 1999 Funding: Novartis Pharma AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized in a ratio of 3:1'. No further details given. |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open‐label trial. No comment on blinding of laboratory staff |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were low and proportional in the 2 groups (5.4% AL6 vs 3.6% AS+MQ) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6 and 63 days with AS+MQ. Day 28 outcomes may overestimate the efficacy of AL6 and AS+MQ. |
| Free of other bias? | High risk | It is stated that participants whose condition deteriorated were to be excluded from the trial. There is no flow chart so it is unclear how many participants this represented, and whether these should have been classified as early treatment failures. |
Martensson 2003 TZA.
| Methods | Trial design: A randomized controlled trial Follow up: Clinical assessment, malaria film, and haemoglobin measurement on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42 Adverse event monitoring: Possible adverse events recorded at each visit. Differential white cell counts at days 0, 3, 7, 14, 21, and 28. An adverse event was defined as any undesirable medical occurrence regardless of wether it was related to the treatments. |
|
| Participants | Number: 408 randomized Inclusion criteria: Age 6 to 59 months and weight > 6 kg for AS+AQ group, 9 to 59 months and > 9 kg for AL6 group, axillary temp > 37.5 ºC or history of fever in previous 24 hrs, P. falciparum parasitaemia 2000 to 200,000/µl Exclusion criteria: Symptoms and signs of severe malaria, any danger sign, serious underlying disease, Hb < 5 g/dl, known allergy to study drugs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination (Plasmotrim: Mepha, Flavoquin: Roussel)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Zanzibar, Tanzania Setting: Outpatient departments in densely populated rural areas Transmission: Holoendemic Resistance: Not reported Dates: Nov 2002 to Feb 2003 Funding: UNDP/World Bank/WHO Special Program for Research & Training in Tropical Diseases, Swedish Development Co‐operation Agency Department for Research Cooperation, European 5th Framework Project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as 'randomized' but no details given |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | No blinding is described. 10% of malaria films were cross‐checked by an independaent examiner in a central laboratory |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up (1.5% AL6 vs 1% AS+AQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | High risk | Due to different inclusion criteria for the 2 groups, participants in the AL6 group were, on average, older and heavier at baseline |
Mayxay 2003 LAO.
| Methods | Trial design: A 3‐arm, open label randomized controlled trial Follow up: Temperature was measured every 6 hours and patient reviewed daily until fever and parasites cleared then weekly until day 42 or any time they felt unwell. At each visit a malaria film and haematocrit measurement was taken. Adverse event monitoring: Potential side effects were recorded at each visit |
|
| Participants | Number: 220 randomized into included treatment arms Inclusion criteria: Age > 1 yr, axillary temp > 37.5 ºC or history of fever in previous 3 days, P. falciparum parasitaemia 5000 to 200,000/µl, likely to stay in hospital until fever cleared and complete 42 days follow up, informed consent Exclusion criteria: Pregnancy or lactation, signs of severe malaria, history of allergy or contraindication to the study drugs, a full course of antimalarials in the previous 3 days |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, loose combination (artesunate: Guilan, Lariam: Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Lao People's Democratic Republic Setting: District clinic Transmission: Not stated Resistance: CQ and SP resistance Dates: June to Oct in 2002 and 2003 Funding: Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized in blocks of 15'. No further details given. |
| Allocation concealment? | Low risk | 'The treatment choice was kept in a sealed opaque envelope that was opened only after the decision to recruit had been made' |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (2.7% AL6 vs 1.8% AS+MQ) |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 42 outcomes may underestimate treatment failure with AS+MQ. |
| Free of other bias? | Low risk | No other sources of bias identified |
Mayxay 2004 LAO.
| Methods | Trial design: An open label randomized controlled trial Follow up: Temperature was measured every 6 hours and patient reviewed daily until fever and parasites cleared then weekly until day 42 or anytime they felt unwell. At each visit a malaria film and haematocrit measurement was taken. Adverse event monitoring: Potential adverse events were recorded at each visit |
|
| Participants | Number: 220 randomized Inclusion criteria: Age > 1 year, axillary temp > 37.5 ºC or history of fever in the previous 3 days, P. falciparum mono‐infection 1000 to 200,00/µl, were likely to stay in hospital until parasite clearance and complete 42 days follow up, informed consent Exclusion criteria: Pregnancy or lactation, signs of severe malaria, antimalarials in the previous 3 days, contraindications to the study drugs |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination (Artesunate: Guilin, Lariam: Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Lao People's Democratic Republic (Laos) Setting: District clinic Transmission: Not reported Resistance: Not reported Dates: May 2004 to Sept 2004 Funding: Western Pacific Regional office of WHO, Wellcome Trust of Great Britain, Artekin provided by Holleykin Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomized in blocks of 10'. No further details given. |
| Allocation concealment? | Low risk | 'The treatment choice was kept in a sealed opaque envelope, which was opened only after the decision to recruit' |
| Blinding? All outcomes | High risk | An open‐label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (3.6% DHA‐P vs 1.8% AS+MQ) |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 42 outcomes are likely to overestimate the efficacy of the 2 drugs. |
| Free of other bias? | Low risk | No other sources of bias identified |
Menard 2006 MDG.
| Methods | Trial design: A 5‐arm single blind (outcome assessors) randomized controlled trial Follow up: Patients returned for malaria films on days 0, 1, 2, 3, 7, 14, 21, 28, and any other day they felt ill. Haemoglobin was assessed on days 0 and 28. Adverse event monitoring: Not described |
|
| Participants | Number: 166 randomized to included treatment arms Inclusion criteria: Age 6 months to 15 yrs, weight > 5 kg, axillary temp > 37.5 ºC, P. falciparum mono‐infection 1000 to 200,000/µl, informed consent Exclusion criteria: Danger signs, severe or complicated malaria, febrile conditions other than malaria, severe malnutrition, severe anaemia (Hb < 5 g/dl), development of concomitant disease which could interfere with study outcome, known hypersensitivity to the study drugs, repeated vomiting of the first dose |
|
| Interventions | 1. Artesunate plus amodiaquine
2. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Madagascar Setting: Primary health centres Transmission: Low and predominantly seasonal Resistance: CQ resistance Dates: Feb 2006 to June 2006 Funding: Natixis, the Global Fund to Fight AIDS, Tuberculosis and Malaria, and the IAEA project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomization was in blocks of 5'. Drawing numbered papers from a box (additional detail from author). |
| Allocation concealment? | Low risk | 'Treatment regimens were allocated by an independent individual not involved in the analysis of the study' |
| Blinding? All outcomes | Low risk | 'All other study personnel were blinded to the treatment assignments, and patients not informed of their treatment regimen' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (8.4% AS+AQ vs 4.8% AQ+SP) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Mens 2007 KEN.
| Methods | Trial design: An open label randomized controlled trial Follow up: Malaria film and haemoglobin level on days 0, 1, 2, 3, 7, 14, and 28, plus QT‐NASBA for detection of sub‐microscopic gametocytaemia Adverse event monitoring: Adverse events were recorded at each visit in the case record form. An adverse event defined as any unfavourable and unintended sign. |
|
| Participants | Number: 146 randomized Inclusion criteria: Age 6 months to 12 years, axillary temp > 37.5 ºC or history of fever, P. falciparum mono‐infection 1000 to 200,000/µl, informed consent Exclusion criteria: Severe malaria, any other underlying illness |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 20 mg/160 mg tablets (Sigma‐Tau)
2. Artemether‐lumefantrine, fixed dose combination, 20/120 mg tablets (Novartis)
All doses supervized and given with a glass of milk |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Kenya Setting: Health centre Transmission: High transmission Resistance: Not reported Dates: Apr 2007 to July 2007 Funding: The Knowledge and Innovation Fund, Koninklijk Instituut voor de Tropen/Royal Tropical Institute. DHA‐P provided free of charge by Sigma‐Tau. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A computer generated randomisation list' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | Microscopists were blinded to treatment allocation. No other blinding described. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (8.2% DHA‐P vs 8.2% AL6) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may underestimate treatment failure with AL6 and DHA‐P. |
| Free of other bias? | Low risk | No other sources of bias identified |
Mukhtar 2005 SDN.
| Methods | Trial design: A randomized controlled trial Follow up: On days 0, 1, 2, 3, 7, 14, 21, and 28. A malaria film taken at each visit Adverse event monitoring: None described |
|
| Participants | Number: 160 randomized Inclusion criteria: All age groups, as per WHO protocol 2003 Exclusion criteria: As per WHO protocol 2003 |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus sulfadoxine‐pyrimethamine, loose combination
Only first dose of each day was supervized |
|
| Outcomes |
|
|
| Notes | Country: Sudan Setting: 3 villages in eastern Sudan Transmission: Low endemicity Resistance: CQ and SP resistance Dates: Oct to Dec in 2004 and 2005 Funding: National Centre for Research, drugs provided by Novartis, Amipharma and the national Malaria Control Programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'A simple random technique of a hat draw' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | No details of blinding given. Malaria films were read by 2 independent microscopists. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (0% AL6 vs 3.8% AS+SP) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate the failure rate of AL6. |
| Free of other bias? | High risk | In general details of the trial were limited. Very few baseline data given and no detail on drug regimens. |
Mutabingwa 2004 TZA.
| Methods | Trial design: A 4‐arm, randomized controlled trial Follow up: Participants were assessed clinically and by malaria film on days 0, 14, and 28 or any other day they were unwell Adverse event monitoring: Parents or guardians were asked to report on side effects, tolerability, and usefulness of the treatment |
|
| Participants | Number: 1541 randomized into included treatment arms Inclusion criteria: Age 4 to 59 months, symptoms suggestive of malaria, P. falciparum > 2000/µl, able to take oral meds, able to attend clinic for follow up, informed consent Exclusion criteria: Mixed infections, severe or complicated malaria, concomitant disease masking assessment of the response to treatment, intake of antimalarials other than CQ within the past 7 days, known hypersensitivity to any of the study drugs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, co‐blistered/loose (Sanofi)
3. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination (Sanofi, Roche)
All doses unsupervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Tanzania Setting: Maternal and child health clinic Transmission: Very high Resistance: High level CQ and SP resistance Dates: Sept 2002 to Oct 2004 Funding: Gates Malaria Partnership. AS+AQ donated by Sanofi. AL6 donated by WHO |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomization was done by computer (Stata Version 6), with blocks of variable sizes' |
| Allocation concealment? | Low risk | 'Treatment allocations were put into opaque, sealed and countersigned, sequentially numbered envelopes' |
| Blinding? All outcomes | High risk | Malaria films were read by 2 different laboratories unaware of treatment allocation. No other blinding is reported. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were low in all groups (6.5% AL6 vs 8.3% AS+AQ vs 8.7% AQ+SP) |
| Free of selective reporting? | High risk | No baseline data is given on gametocytes. PCR data is only given for 1 year of the trial. It is not possible to calculate attrition for this period. |
| Free of other bias? | Low risk | No other sources of bias identified |
Owusu‐Agyei 2006 GHA.
| Methods | Trial design: A 3‐arm, randomized controlled trial Follow up: Participants were assessed for adverse events and by malaria film on days 0, 2, 3, 7, 14, and 28 or any other day they were unwell. Haemoglobin measured on days 1, 2, 3, 7, and 28. Anaemia was treated with iron according to national guidelines Adverse event monitoring: Field workers visited their homes to solicit adverse events on days 0, 2, 3, 7, 14, and 28 |
|
| Participants | Number: 355 randomized into included treatment arms Inclusion criteria: Age 6 months to 10 yrs, weight > 5 kg, axillary temp > 37.5 ºC or history of fever, parasitaemia 2000 to 200,000/µl, informed consent Exclusion criteria: Danger signs, signs of severe malaria, concomitant febrile illness, Hb < 7 g/dl |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, co‐blistered (Arsucam: Sanofi‐Aventis)
All doses supervized for 3 days |
|
| Outcomes |
|
|
| Notes | Country: Ghana Setting: District hospital Transmission: Perennial, high with a peak July to August Resistance: Not stated Dates: June 2005 to May 2006 Funding: Gates Malaria Partnership of the London School of Hygiene and Tropical Medicine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomization was done using Microsoft Excel 2003 randomisation generator' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of lab staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Moderate losses to follow up but similar in both groups (14% AL6 vs 15% AS+AQ) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Biochemical monitoring is stated although this outcome is not reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Ratcliff 2005 IDN.
| Methods | Trial design: An open‐label randomized controlled trial Follow up: A symptom questionnaire, physical examination, malaria film and haemoglobin measurement daily until fever and parasites cleared then weekly to day 42 Adverse event monitoring: A symptom questionnaire at each visit |
|
| Participants | Number: 774 randomized Inclusion criteria: Weight >10 kg, fever or a history of fever in the preceding 48 hours, slide confirmed malaria (P. falciparum, P. vivax or mixed infections) Exclusion criteria: Pregnancy or lactation, danger signs or signs of severity, parasitaemia > 4%, concomitant disease requiring hospital admission |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
2. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
Only the first dose of each day was supervized. All participants advised to take each dose with a biscuit or milk. |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Indonesia Setting: Rural outpatient clinics Transmission: Unstable Resistance: Multiple‐drug resistance Dates: Jul 2004 to Jun 2005 Funding: Wellcome Trust UK and National Health and Medical Research Council Australia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A randomisation list was generated in blocks of 20 patients by an independent statistician' |
| Allocation concealment? | Low risk | 'With each treatment allocation concealed in an opaque sealed envelope'. No further details given. |
| Blinding? All outcomes | High risk | An open label trial. The microscopists were blinded to treatment allocation. |
| Incomplete outcome data addressed? All outcomes | High risk | The primary outcome data are unpublished data including only participants with P. falciparum mono or co‐infection at baseline. Losses to follow up were high in both groups at day 42 (28.4 % DHA‐P vs 25.6 % AL6) and moderate at day 28 (19% DHA‐P vs 17.6% AL6). |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 42 outcomes may underestimate failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
Sagara 2005b MLI.
| Methods | Trial design: An open label randomized controlled trial Follow up: Examination and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, and any day they felt unwell. Haemoglobin on days 0, 14, and 28. Adverse event monitoring: CBC, ALT, and creatinine on 20% of participants on days 0 and 14 |
|
| Participants | Number: 470 randomized Inclusion criteria: Age > 1 yr, weight >10 kg, axillary temperature > 37.5 ºC, P.falciparum mono‐infection 2000 to 200,000, resident at study site, able to take oral medication, informed consent Exclusion criteria: Pregnancy, severe malaria, a serious underlying disease, an allergy to 1 or more study drugs, use of study drugs within 28 days |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, co‐blistered (Artequin: Mepha)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Mali Setting: Peri‐urban Transmission: Hyperendemic with highly seasonal transmission Resistance: Not stated Dates: Aug 2004 to Feb 2005 Funding: Pharmatech Inc (also donated AS+MQ), and Mepha Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A bloc randomisation code with treatment arm was computer generated by the study statistician' |
| Allocation concealment? | Low risk | 'Study codes were sealed in individual opaque and sequentially numbered envelopes' |
| Blinding? All outcomes | High risk | An open label trial. Microscopists were blinded to the treatment arm. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were low in both groups (2.1% AS+MQ vs 1.7% AL6) |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6 and 63 days with AS+MQ. Day 28 outcomes may overestimate the efficacy of AL6 and AS+MQ. |
| Free of other bias? | Low risk | No other sources of bias identified |
Smithuis 2004 MMR.
| Methods | Trial design: A 4‐arm open‐label randomized controlled trial Follow up: A symptom questionnaire, malaria film, and gametocyte count on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42. Haemoglobin was measured on days 0 and 28. Adverse event monitoring: A symptom questionnaire at each visit |
|
| Participants | Number: 652 randomized Inclusion criteria: Age > 1 year, axillary temperature > 37.5 ºC or history of fever in the previous 48 hrs, P. falciparum mono‐infection 500 to 100,000 parasites/µl or co‐infection with P. vivax, informed consent Exclusion criteria: Pregnancy, signs of severe malaria, signs or symptoms of other diseases, history of taking mefloquine in the previous 2 months or any other antimalarial in the previous 48 hrs, history of psychiatric disease |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
2. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
3. Artesunate plus mefloquine, loose combination (artesunate: Guilin, Lariam: Hoffman‐La Roche)
4. Artesunate plus mefloquine, loose combination (artesunate: Guilin, Lariam: Hoffman‐La Roche)
|
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Myanmar Setting: Rural village tracts Transmission: Seasonal with peaks in the monsoon season Nov to Jan and sometimes in the early monsoon, May to June Resistance: Very high rates of CQ and SP resistance Dates: Nov 2003 to Feb 2004 Funding: Médecins sans Frontières (Holland) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Unmarked and sealed envelopes, containing the treatment allocation were drawn from a box |
| Allocation concealment? | Unclear risk | 'Unmarked and sealed envelopes'. No further details given. |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Very low losses to follow up in both groups |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 42 outcomes are likely to overestimate the efficacy of the 2 drugs. |
| Free of other bias? | Low risk | No other sources of bias identified |
Staedke 2003 UGA.
| Methods | Trial design: An open label randomized controlled trial Follow up: A standardized history and examination and malaria film on days 1, 2, 3, 7, 14, 21, and 28 or other times if they were unwell. Haemoglobin was measured on days 0, 7, and 28. Adverse event monitoring: Assessed at each visit. Neurological assessment on days 0, 7, 14, and 28. Complete blood count, creatinine, and alanine transferase on days 0, 7, and 28. |
|
| Participants | Number: 278 randomized into included treatment arms Inclusion criteria: Age 6 months to 10 yrs, tympanic temp > 38.0 ºC or febrile symptoms in previous 48 hrs, P. falciparum mono‐infection 500 to 200,000/µl, willingness to participate in 28 day follow up, informed consent Exclusion criteria: Danger signs, severe malaria, alternative diagnosis for febrile illness, antifolate use in the previous 4 weeks, history of serious side effects to any of the study drugs, severe anaemia (Hb < 5 g/dl) |
|
| Interventions | 1. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination
2. Artesunate plus amodiaquine
All doses supervized. Meds crushed and mixed with chocolate to mask the colour and taste. |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Uganda Setting: Urban hospital Transmission: Mesoendemic with peaks in the 2 rainy seasons Resistance: CQ and SP resistance Dates: Aug 2002 to July 2003 Funding: NIH and the Fogarty International Centre/NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'An off‐site investigator generated randomization codes with a computer for two age groups using variable blocking' |
| Allocation concealment? | Low risk | 'Sequentially numbered sealed envelopes containing the treatment group assignments were prepared from the randomization lists' |
| Blinding? All outcomes | Low risk | 'All study personnel (excluding study nurse), including the doctors, were unaware of the treatment assignments' |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were low in both groups (3% AS+AQ vs 3.7% AQ+SP) |
| Free of selective reporting? | Low risk | We were unable to use PCR adjusted data as PCR was only performed on late clinical failures, not on late parasitological failures |
| Free of other bias? | Low risk | No other sources of bias identified |
Stohrer 2003 LAO.
| Methods | Trial design: An open label randomized controlled trial Follow up: A history, axillary temperature and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, and 42 or other times if they were unwell. Haemoglobin was measured on days 0 and 28 Participants experiencing P. vivax during follow up were withdrawn Adverse event monitoring: Treatment emergent symptoms and signs were recorded on days 0 to 3 |
|
| Participants | Number: 108 randomized Inclusion criteria: Weight > 10 kg, axillary temperature > 37.5 ºC, P. falciparum mono‐infection 1000 to 100,000/µl, ability to attend follow up, informed consent Exclusion criteria: Pregnancy or lactation, signs of severe or complicated malaria, severe malnutrition, febrile diseases other than malaria, history of hypersensitivity reaction to any of the study drugs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2, Artesunate plus mefloquine, loose combination (Plasmotrim: Mepha, Mephaquine: Mepha)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Lao People's Democratic Republic Setting: Hospital and community based Transmission: Perennial with peaks during the rainy season May to Oct Resistance: CQ and SP resistance Dates: Oct to Dec 2003 Funding: USAID, mefloquine and artesunate donated by Mepha, Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Envelope randomisation' in blocks of various sizes, no further details given |
| Allocation concealment? | Unclear risk | 'A sealed envelope was opened which assigned patients to one of the two treatment arms'. No further details given. |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff, quality control was conducted by rechecking malaria films by expert microscopists. |
| Incomplete outcome data addressed? All outcomes | Low risk | Disproportionate losses to follow up (11.3% AL6 vs 3.6% AS+MQ) but unlikely to have affected the overall result |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 42 outcomes may overestimate the efficacy of AS+MQ. |
| Free of other bias? | Low risk | No other sources of bias identified |
Swarthout 2004 ZAR.
| Methods | Trial design: An open label randomized controlled trial Follow up: Examination and malaria film on days 0, 1, 2, 3, 7, 14, 21, and 28, or other times if they were unwell Adverse event monitoring: Parents and guardians were asked about tolerability and potential side effects of the drugs |
|
| Participants | Number: 180 randomized Inclusion criteria: Age 6 to 59 months, symptoms suggestive of malaria, P. falciparum mono‐infection 2000 to 200,000/µl, able to take the study drugs orally, able to attend follow up, informed consent Exclusion criteria: Severe or complicated malaria, concomitant disease that could mask response to antimalarial treatment, known hypersensitivity to any of the study drugs |
|
| Interventions | 1. Artesunate plus amodiaquine
2. Artesunate plus sulfadoxine‐pyrimethamine
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Democratic Republic of Congo Setting: Small town health centre Transmission: Highly endemic and seasonal with peaks in the rainy seasons; March to May and September to November Resistance: CQ and SP resistance Dates:April 2004 to May 2004 Funding: Médecins sans Frontières (Holland) and ECHO |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomization in blocks of 12 was performed by computer before the study started' |
| Allocation concealment? | Unclear risk | 'A sealed envelope containing the treatment allocation...was opened only after informed consent had been obtained' |
| Blinding? All outcomes | High risk | 'Neither patients nor clinicians were blinded to the treatment given, microscopists unaware of treatment allocation read all slides' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (7.8% AS+AQ vs 10% AS+SP) |
| Free of selective reporting? | Low risk | All WHO outcomes reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Tangpukdee 2005 THA.
| Methods | Trial design: An open label randomized controlled trial Follow up: The patients were admitted to hospital for 28 days. Clinical evaluation and parasite counts were performed 12‐hourly until parasites cleared then daily for 28 days. Adverse event monitoring: Assessed daily using non‐suggestive questioning. Side effects were defined as signs and symptoms which occurred or became more severe after treatment started. Routine haematology, biochemistry, and urinalysis were conducted and baseline and weekly during follow up. |
|
| Participants | Number: 180 randomized Inclusion criteria: Age >14 years, weight > 40 kg, P. falciparum on blood smear, ability to take oral medicines, agree to stay in hospital for 28 days, informed consent Exclusion criteria: Pregnancy or lactation, severe malaria, severe vomiting, concomitant systemic diseases, other antimalarials in the previous 14 days or the presence of sulphonamides or 4‐aminoquinolones in the urine |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Thailand Setting: Bangkok Hospital for Tropical Diseases Transmission: Low Resistance: Multiple‐drug resistance Dates: Not given Funding: Mahidol University Research Grant, Artekin supplied by Holleykin Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Randomly treated at a ratio of 1:2'. No further details given. |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow up were low and similar between groups (10.8% DHA‐P vs 10% AS+MQ) |
| Free of selective reporting? | Low risk | Day 28 outcomes may overestimate the efficacy of drugs with long half‐lives such as AS+MQ and DHA‐P |
| Free of other bias? | Low risk | No other sources of bias identified |
Tran 2002 VNM.
| Methods | Trial design: An open label randomized controlled trial Follow up: Malaria film on days 0, 2, and 7. Participants followed up to day 56 but further details not described Adverse event monitoring: Not described |
|
| Participants | Number: 243 randomized to included treatment arms Inclusion criteria: Age > 2 yrs, microscopically confirmed uncomplicated P. falciparum malaria Exclusion criteria: Pregnancy, evidence of organ dysfunction, unable to tolerate oral medication, unable to return for follow up, resident in Dac O for > 2 years |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Artekin: Holleykin)
2. Artesunate plus mefloquine, loose combination (artesunate: Guilin, Lariam: Hoffman‐La Roche)
|
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Vietnam Setting: Health station Transmission: Low and seasonal Resistance: Multiple‐drug resistance Dates: Nov 2001 to Mar 2002 Funding: Wellcome Trust of Great Britain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Patients were randomly allocated one of three treatments in a ratio of 2:2:1'. No further details given. |
| Allocation concealment? | Unclear risk | 'Drugs were kept in identically numbered opaque envelopes'. No further details. |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff. |
| Incomplete outcome data addressed? All outcomes | Low risk | 'There were no losses to follow‐up' |
| Free of selective reporting? | Unclear risk | It is unclear from the paper whether it is only clinical failure that is being reported |
| Free of other bias? | Low risk | No other sources of bias identified |
Van den Broek 2003a BGD.
| Methods | Trial design: A 3‐arm, open label randomized controlled trial Follow up: Clinical assessment and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42 and any other day when feeling ill P. vivax or P. malariae during follow up were treated with CQ and continued in follow up Adverse event monitoring: Possible side effects assessed at each visit |
|
| Participants | Number: 242 randomized to included treatment arms Inclusion criteria: Age > 1 yr, history of fever, P. falciparum mono‐infection 1000 to 100,000/µl, informed consent Exclusion criteria: Pregnancy, signs of severe malaria, signs of another febrile illness or severe illness requiring treatment, Hb < 6 g/dl |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, loose combination
All doses supervized |
|
| Outcomes |
|
|
| Notes | Country: Bangladesh Setting: Outpatient clinics Transmission: High endemicity with a clear seasonal pattern Resistance: Multiple‐drug resistance Dates: May 2003 to Sept 2003 Funding: Médecins sans Frontières (Holland) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomisation was done in blocks of 30 by drawing a card from a box' |
| Allocation concealment? | High risk | 'Treatment allocation was done by drawing a card from a box containing three types of cards coding for treatments' |
| Blinding? All outcomes | High risk | An open label trial. No comment on blinding of laboratory staff. 10% of slides were cross‐checked. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up (1.6% AL6 vs 5.8% AS+MQ) |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ. Day 42 outcomes may underestimate treatment failure with AS+MQ. |
| Free of other bias? | Low risk | No other sources of bias identified |
Van den Broek 2004 ZAR.
| Methods | Trial design: A 3‐arm, open label randomized controlled trial Follow up: Clinical assessment and malaria film on days 0, 1, 2, 3, 7, 14, 21, and 28. Haemoglobin measured at days 0, 14, and 28 Adverse event monitoring: Possible side effects as passively reported to the examiner were recorded at each visit |
|
| Participants | Number: 298 randomized Inclusion criteria: Age 6 to 59 months, weight > 5 kg for AS+AQ and AS+SP groups and > 10 kg for AL6, fever > 37.5 ºC or history of fever in the previous 24 hrs, P. falciparum mono‐infection 2000 to 200,000/µl, lives within 2 hours walking distance, informed consent Exclusion criteria: Signs of severe or complicated malaria, any danger sign, a serious concomitant illness, malnutrition, known hypersensitivity to the study drugs |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus amodiaquine, loose combination (Arsumax: Sanofi‐Aventis, Camoquin: Parke‐Davis)
3. Artesunate plus sulphadoxine‐pyrimethamine, loose combination (Arsumax: Sanofi‐Aventis, Fansidar: La Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Republic of Congo Setting: Health centre Transmission: Holoendemic with a peak in the rainy seasons Resistance: CQ, SP, and AQ resistance Dates: May 2004 to Oct 2004 Funding: Médecins sans Frontières (Holland) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomized to the three treatments by a random number list' (information from author) |
| Allocation concealment? | High risk | Allocation was not concealed (information from author) |
| Blinding? All outcomes | High risk | An open label trial. 10% of malaria films were cross‐checked by external laboratories. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in all groups (5.7% AL6 vs 4% AS+AQ vs 6.6% AS+SP). A significant number of PCR samples were indeterminate or missing which may affect the result. |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may underestimate the failure rate with AL6. |
| Free of other bias? | High risk | Due to differing inclusion criteria for the 3 arms children in the AL6 group were older, heavier and had higher Hb levels at baseline. This may improve outcome in this group and consequently the AL6 arm was excluded from this review. |
Van Vugt 1998 THA.
| Methods | Trial design: An open‐label randomized controlled trial Follow up: Examination and malaria film daily until fever and parasites cleared then weekly to day 28 Adverse event monitoring: A questionnaire for adverse effects was completed at each visit. Full neurological examination on days 0, 3, 7, and 28. Complete haematology and biochemistry (at 1 centre) on days 0, 3, 7, and 28. |
|
| Participants | Number: 200 randomized Inclusion criteria: Age > 2 yrs, P. falciparum parasitaemia > 500/µl, informed consent Exclusion criteria: Pregnancy or lactation, severe or complicated malaria |
|
| Interventions | 1. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Artesunate plus mefloquine, loose combination (artesunate: Guilan, Lariam: Hoffman‐La Roche)
All doses supervized |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Thailand Setting: Bangkok Hospital for Tropical Diseases and an outpatient clinic Transmission: Not reported Resistance: Multiple‐drug resistance Dates: Nov 1997 to Mar 1998 Funding: Wellcome Trust of Great Britain, Novartis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Using a 3:1 randomization scheme'. No further details given. |
| Allocation concealment? | Unclear risk | 'The allocation was in sealed envelopes'. No further details given. |
| Blinding? All outcomes | High risk | An open label trial. No other comment on blinding. |
| Incomplete outcome data addressed? All outcomes | Low risk | Different losses to follow up in each group (11% AL6 vs 6% AS+MQ) but unlikely to affect the overall result |
| Free of selective reporting? | Low risk | The WHO recommends 63 days follow up in studies of AS+MQ, and 42 days with AL6. Day 28 outcomes may underestimate treatment failure with both drugs. |
| Free of other bias? | Low risk | No other sources of bias identified |
Yeka 2004 UGA.
| Methods | Trial design: A 3‐arm single blind randomized controlled trial Follow up: Malaria film on days 0, 1, 2, 3, 7, 14, 21, 28 and any other day they were unwell. Haemoglobin on days 0 and 28 or the day of failure. Adverse event monitoring: Not described |
|
| Participants | Number: 1537 randomized to included treatment arms Inclusion criteria: Age > 6 months, axillary temp > 37.5 ºC or history of fever in the previous 24 hours, P. falciparum mono‐infection 2000 to 200,000/µl, informed consent Exclusion criteria: Pregnancy, danger signs, signs of severe malaria, concomitant febrile illness, history of treatment with an antifolate or amodiaquine during the previous week, history of serious side effects to the study meds |
|
| Interventions | 1. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination
2. Artesunate plus amodiaquine
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Uganda Setting: District health centres Transmission: 4 sites with medium‐high to high endemicity Resistance: CQ and SP resistance Dates: Nov 2002 to May 2004 Funding: CDC/Association of Schools of Public Health co‐operative agreement, Malaria Surveillance and Control in Uganda, DfID |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomisation codes were computer generated' |
| Allocation concealment? | High risk | Not described |
| Blinding? All outcomes | Low risk | 'All other study personnel (except study nurse) were blinded to the treatment assignments and participants were not informed of their treatment regimen' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (3.4% AS+AQ vs 4.0% AQ+SP). High transmission with very high reinfection rates results in very high exclusions from primary analysis. |
| Free of selective reporting? | Low risk | Outcomes only presented as percentages. Additional data gained from authors. |
| Free of other bias? | Low risk | No other sources of bias identified |
Yeka 2007 UGA.
| Methods | Trial design: A single blind randomized controlled trial Follow up: Standardized history, physical exam, and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42 and any other day they were unwell. Haemoglobin on days 0 and 42 or the day of failure. Anaemia was treated with ferrous sulphate and antihelminthics according to IMCI guidelines. Adverse event monitoring: Assessed at each visit including neurological examination. Adverse events described as any untoward medical occurrence. |
|
| Participants | Number: 461 randomized Inclusion criteria: Age 6 months to 10 yrs, weight > 5 kg, axillary temp > 37.5 ºC or history of fever in the previous 24 hours, P. falciparum mono‐infection 2000 to 200,000/µl, informed consent Exclusion criteria: Danger signs or evidence of severe malaria, concomitant febrile illness, history of serious side effects to the study meds |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Duocotexin: HolleyPharm)
2. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
All doses supervized and given with a glass of milk |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Uganda Setting: Health centre Transmission: Moderate transmission Resistance: Not stated Dates: Aug 2006 to Apr 2007 Funding: CDC, DfID, DHA‐P supplied by Holleypharm, AL6 supplied by Uganda Ministry of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'A randomisation list was computer generated by an off‐site investigator' |
| Allocation concealment? | Low risk | 'Sealed opaque envelopes containing the study number and assigned treatment were secured in a locked cabinet' |
| Blinding? All outcomes | Low risk | 'Only the study nurse was aware of assignments. All other study personnel were blinded. Patients were not informed of their treatment regimen'. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in both groups (1.4% DHA‐P vs 1.5% AL6) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 42 outcomes may underestimate treatment failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
Zongo 2005 BFA.
| Methods | Trial design: A randomized controlled trial Follow up: A standardized history, examination, and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, or any other day they felt unwell. Haemoglobin measured on days 0 and 28 or day of clinical failure. Children with Hb < 10 g/dl were treated with ferrous sulphate and antihelminthic treatment. Adverse event monitoring: Assessed at each visit |
|
| Participants | Number: 580 randomized Inclusion criteria: Age > 6 months, weight > 5 kg, axillary temp > 37.5 ºC or history of fever in the last 24 hours, P. falciparum mono‐infection 2000‐200,000/µl, the ability to participate in 28 days follow up, informed consent Exclusion criteria: Danger signs or signs of severe malaria, history of serious adverse effects related to study meds, evidence of concomitant febrile illness, antimalarial use other than chloroquine in previous 2 weeks, haemoglobin < 5 g/dl |
|
| Interventions | 1. Artemether‐Lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
2. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination (Amodiaquine: Aventis, Fansidar: Roche)
Placebos were used to simulate equal numbers of pills. All doses supervized. |
|
| Outcomes |
Not included in the review:
|
|
| Notes | Country: Burkina Faso Setting: Urban health centres Transmission: Holoendemic with transmission peaks during the rainy season Resistance: Not stated Dates: Aug 2005 to Dec 2005 Funding: Fogarty International Centre of the National Institutes of Health, International Atomic Energy Agency, National Budget of the Institut de Recherche en Sciences de la Sante |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Computer‐generated randomisation lists' |
| Allocation concealment? | High risk | None described |
| Blinding? All outcomes | Low risk | 'Investigators responsible for classification of treatment outcomes were unaware of treatment assignment'. Placebos were used and participants not informed of allocation. |
| Incomplete outcome data addressed? All outcomes | Low risk | Mildly disparate losses to follow up (6.1% AL6 vs 10.4% AQ+SP), unlikely to have affected overall result |
| Free of selective reporting? | Low risk | The WHO recommends 42 days follow up in studies of AL6. Day 28 outcomes may under estimate treatment failure with AL6 and DHA‐P. |
| Free of other bias? | Low risk | No other sources of bias identified |
Zongo 2007 BFA.
| Methods | Trial design: A 3‐arm randomized controlled trial Follow up: A standardized history, examination, and malaria film on days 0, 1, 2, 3, 7, 14, 21, 28, 35, and 42. Haemoglobin measured on days 0 and 42 or day of clinical failure. Children with Hb < 10 g/dl were treated with ferrous sulphate and antihelminthic treatment. Adverse event monitoring: Assessed at each visit. Adverse events defined as untoward medical occurrences. |
|
| Participants | Number: 580 randomized Inclusion criteria: Age > 6 months, weight > 5 kg, axillary temp > 37.5 ºC or history of fever in the last 24 hours, P. falciparum mono‐infection 2000 to 200,000/µl, the ability to participate in 42 days follow up, informed consent Exclusion criteria: Danger signs or signs of severe malaria, history of serious adverse effects related to study meds, evidence of concomitant febrile illness, antimalarial use other than chloroquine in previous 2 weeks, haemoglobin < 5 g/dl |
|
| Interventions | 1. Dihydroartemisinin‐piperaquine, fixed dose combination, 40 mg/320 mg tablets (Duocotexin: HolleyPharm)
2. Artemether‐lumefantrine, fixed dose combination, 20 mg/120 mg tablets (Coartem: Novartis)
3. Amodiaquine plus sulfadoxine‐pyrimethamine, loose combination (Flavoquine: Aventis, Fansidar: Roche)
All doses supervized |
|
| Outcomes |
Not included in this review:
|
|
| Notes | Country: Burkino Faso Setting: Health dispensaries Transmission: Holoendemic, transmission principally in the rainy season May to Oct Resistance: Not reported Dates: Not reported Funding: Doris Duke Charitable Foundation, Holley Cotec Pharmaceuticals, International Atomic Energy Agency, National Budget of the Institut de Recherche en Sciences de la Sante |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Randomly assigned on the basis of a computer‐generated code provided by an off‐site investigator' |
| Allocation concealment? | Low risk | 'Referred for treatment allocation by a study nurse not involved in enrolment or assessment of treatment outcomes' |
| Blinding? All outcomes | High risk | 'The study was not blinded' |
| Incomplete outcome data addressed? All outcomes | Low risk | Low losses to follow up in all groups (8% DHA‐P vs 6.4% AL6 vs 8.2% AQ+SP) |
| Free of selective reporting? | Low risk | All WHO outcomes reported. Day 42 outcomes may underestimate treatment failure with DHA‐P due to its long half‐life. |
| Free of other bias? | Low risk | No other sources of bias identified |
A = artemether ACPR = adequate clinical and parasitological response AL = artemether‐lumefantrine AL6 = artemether‐lumefantrine (six doses) AQ = amodiaquine AS = artesunate CQ = chloroquine DFID = Department for International Development (UK) DHA‐P = dihydroartemisinin‐piperaquine FBC = full blood count HCT = haematocrit L = lumefantrine m = months MQ = mefloquine PCR = polymerase chain reaction PCV = packed cell volume SP = sulfadoxine‐pyrimethamine vs = versus WBC = white blood cell yrs = years
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abacassamo 2002 MOZ | Only 21 days follow up |
| Abuaku 2005 | Conference presentation of Koram 2003 GHA |
| Adjei 2005 | Conference presentation of Adjei 2006 GHA |
| Bell 2008 | Comparison not relevant to this review: artesunate plus sulfadoxine‐pyrimethamine vs amodiaquine plus sulfadoxine‐pyrimethamine |
| Blair 2006 | Duration of follow up in the group given amodiaquine plus sulfadoxine‐pyrimethamine was only 21 days. The randomization procedure is also unclear. |
| Denis 2006 | Not randomized |
| Dorsey 2002 | Comparison not relevant to this review: artesunate plus sulfadoxine‐pyrimethamine vs amodiaquine plus sulfadoxine‐pyrimethamine |
| Dorsey‐G 2003 | A paper based on the trial reported in Dorsey 2002. Contains no new efficacy data. |
| Fofana 2005 | Conference presentation of Djimde 2004 MLI |
| Ibrahium 2007 | Quasi‐randomized |
| Jiao 1997 | Comparison not relevant to this review: benflumetol vs artesunate plus benflumetol |
| Kabanywanyi 2007 | Not randomized. Participants were randomized to monotherapy or artemether‐lumefantrine at 1 site and monotherapy or artesunate plus amodiaquine at a second site. This does not allow a proper randomized comparison of AL6 vs AS+AQ. |
| Massougbodji 2005 | Comparison not relevant to this review: trial of 2 different regimens of artesunate plus mefloquine |
| Meremikwu 2004 NGA | Only 14 days follow up |
| Mockenhaupt 2005 | Comparison not relevant to this review: artesunate plus sulfadoxine‐pyrimethamine vs amodiaquine plus sulfadoxine‐pyrimethamine |
| Mohamed 2006 | Not randomized. Participants at 1 centre received artemether‐lumafantrine, participants at a second centre received artesunate plus sulfadoxine‐pyrimethamine. |
| Mulenga 2006 | Comparison not relevant to this review: artemether‐lumefantrine vs sulfadoxine‐pyrimethamine |
| Ndayiragije 2004 | Follow up only 14 days. Differences between groups at baseline. Not randomized. |
| Ndiaye 2005 | Conference presentation of Faye 2003 SEN |
| Obonyo 2007 | A meta‐analysis of trials included in this review |
| Okell 2008 | A meta‐analysis of 6 trials. All trials relevant to this review are included. |
| Piola 2005 | Comparison not relevant to this review: artemether‐lumefantrine supervized vs unsupervized |
| Rwagacondo 2003 | Comparison not relevant to this review: artesunate plus sulfadoxine‐pyrimethamine vs amodiaquine plus sulfadoxine‐pyrimethamine |
| Sagara 2006 | Comparison not relevant to this review: artesunate plus sulphamethoxypyrazine‐pyrimethamine vs artemether lumefantrine |
| Sowunmi 2007a | Reports the same trial as Sowunmi 2007b. No new efficacy data. |
| Sowunmi 2007b | Comparison not relevant to this review: artemether‐lumefantrine vs amodiaquine‐sulphalene‐pyrimethamine |
| Tall 2005 | A conference presentation of Tall 2007 |
| Tall 2007 | Quasi‐randomized |
| Thapa 2007 | Quasi‐randomized. Comparison not relevant to this review: artemether‐lumefantrine vs sulfadoxine‐pyrimethamine. |
| Tranh 2009 | Quasi‐randomized |
| van den Broek 2005b | Quasi‐randomized |
| van Vugt 1998 | Comparison not relevant to this review: artemether‐lumefantrine (4 doses) vs artesunate plus mefloquine |
| Vugt 1999 | Comparison not relevant to this review: artemether‐lumefantrine (4 doses) vs 2 different 6‐dose regimens of artemether‐lumefantrine |
| Wilairatana 2002 | Comparison not relevant to this review: Artecom (dihydroartemisinin‐piperaquine ‐trimethoprim) vs artesunate mefloquine |
| Wiseman 2006 | A cost‐effectiveness analysis based on the findings of Mutabingwa 2005. Contains no new efficacy data. |
AL6 = artemether‐lumefantrine (six doses) AQ = amodiaquine AS = artesunate
Differences between protocol and review
Gametocyte clearance has been removed as a secondary outcome as the effect of ACTs on gametocytes is adequately assessed using the remaining two outcomes.
The multiple treatment comparison methodology as described under 'data synthesis' in the protocol was not used and this description has been removed.
The clinical questions posed under 'quality of evidence' were not stated in the protocol. These were added as currently relevant questions regarding the use of ACTs.
We did not use funnel plots to assess for publication bias as there were too few trials under each comparison for meaningful analysis.
Contributions of authors
All authors were involved in the conception and design of the protocol. Data extraction and assessment of risk of bias was performed by David Sinclair and Babalwa Zani. David Sinclair, Piero Olliaro, and Paul Garner worked on the analysis of secondary outcomes. Data input and analysis was conducted by David Sinclair with input from Piero Olliaro and Paul Garner and statistical advice from Sarah Donegan. The text was drafted by David Sinclair with input from all other authors.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Adjei 2006 GHA {published data only}
- Adjei GO, Kurtzhals JAL, Rodrigues OP, Alifrangis M, Hoegberg LCG, Kitcher ED, et al. Amodiaquine‐artesunate vs artemether‐lumefantrine for uncomplicated malaria in Ghanaian children: a randomized efficacy and safety trial with one year follow‐up. Malaria Journal 2008;7(127):DOI: 10.1186/1475‐2875‐7‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ashley 2003a THA {published and unpublished data}
- Ashley EA, Krudsood S, Phaiphun L, Srivilairit S, McGready R, Leowattana W, et al. Randomized, controlled dose‐optimization studies of dihydroartemisinin‐piperaquine for the treatment of uncomplicated multidrug‐resistant falciparum malaria in Thailand. Journal of Infectious Diseases 2004; Vol. 190, issue 10:1773‐82. [DOI] [PubMed]
Ashley 2003b THA {published and unpublished data}
- Ashley EA, Krudsood S, Phaiphun L, Srivilairit S, McGready R, Leowattana W, et al. Randomized, controlled dose‐optimization studies of dihydroartemisinin‐piperaquine for the treatment of uncomplicated multidrug‐resistant falciparum malaria in Thailand. Journal of Infectious Diseases 2004;190(10):1773‐82. [DOI] [PubMed] [Google Scholar]
Ashley 2004 THA {published and unpublished data}
- Ashley EA, McGready R, Hutagalung R, Phaiphun L, Slight T, Proux S, et al. A randomized, controlled study of a simple, once‐daily regimen of dihydroartemisinin‐piperaquine for the treatment of uncomplicated, multidrug‐resistant falciparum malaria. Clinical Infectious Diseases 2005; Vol. 41, issue 4:425‐32. [DOI] [PubMed]
Ashley 2005 THA {published and unpublished data}
- Ashley EA, Lwin K, McGready R, Simon WH, Phaiphun L, Proux S, et al. An open label randomized comparison of mefloquine–artesunate as separate tablets vs. a new co‐formulatedcombination for the treatment of uncomplicated multidrug‐resistant falciparum malaria in Thailand. Tropical Medicine and International Health 2006;11(11):1653‐60. [DOI] [PubMed] [Google Scholar]
Bonnet 2004 GIN {published and unpublished data}
- Bonnet M, Roper C, Felix M, Coulibaly L, Kankolongo GM, Guthmann JP. Efficacy of antimalarial treatment in Guinea: in vivo study of two artemisinin combination therapies in Dabola and molecular markers of resistance to sulphadoxine‐pyrimethamine in N'Zerekore. Malaria Journal 2007; Vol. 6:54. [DOI] [PMC free article] [PubMed]
Bousema 2004 KEN {published and unpublished data}
Bukirwa 2005 UGA {published data only}
- Bukirwa H, Yeka A, Kamya MR, Talisuna A, Banek K, Bakyaita N, et al. Artemisinin combination therapies for treatment of uncomplicated malaria in Uganda. PLoS Clinical Trials 2006; Vol. 1, issue 1:e7. [DOI] [PMC free article] [PubMed]
Djimde 2004 MLI {published and unpublished data}
- Djimde AA, Fofana B, Sagara I, Sidibe B, Toure S, Dembele D, et al. Efficacy, Safety, and Selection of Molecular Markers of Drug Resistance by Two ACTs in Mali. American Journal of Tropical Medicine and Hygiene 2008; Vol. 78, issue 3:455‐61. [PubMed]
Dorsey 2006 UGA {published and unpublished data}
Falade 2005 NGA {published data only}
- Falade CO, Ogundele AO, Yusuf BO, Ademowo OG, Ladipo SM. High efficacy of two artemisinin‐based combinations (artemether‐lumefantrine and artesunate plus amodiaquine) for acute uncomplicated malaria in Ibadan, Nigeria. Tropical Medicine and International Health 2008;13(5):635‐643. [DOI] [PubMed] [Google Scholar]
Fanello 2004 RWA {published data only}
- Fanello CI, Karema C, Doren W, Overmeir C, Ngamije D, D'Alessandro U. A randomised trial to assess the safety and efficacy of artemether‐lumefantrine (Coartem) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwanda. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007; Vol. 101, issue 4:344‐50. [DOI] [PubMed]
Faye 2003 SEN {published and unpublished data}
- Faye B, Ndiaye JL, Ndiaye D, Dieng Y, Faye O, Gaye O. Efficacy and tolerability of four antimalarial combinations in the treatment of uncomplicated Plasmodium falciparum malaria in Senegal. Malaria Journal 2007; Vol. 6, issue 1:80. [DOI] [PMC free article] [PubMed]
Grande 2005 PER {published data only}
- Grande T, Bernasconi A, Erhart A, Gamboa D, Casapia M, Delgado C, et al. A randomised controlled trial to assess the efficacy of dihydroartemisinin‐piperaquine for the treatment of uncomplicated falciparum malaria in Peru. PLoS ONE 2007; Vol. 2, issue 10:e1101. [DOI] [PMC free article] [PubMed]
Guthmann 2003 AGO {published and unpublished data}
- Guthmann JP, Ampuero J, Fortes F, Overmeir C, Gaboulaud V, Tobback S, et al. Antimalarial efficacy of chloroquine, amodiaquine, sulfadoxine‐pyrimethamine, and the combinations of amodiaquine + artesunate and sulfadoxine‐pyrimethamine + artesunate in Huambo and Bie provinces, central Angola. Trans R Soc Trop Med Hyg 2005; Vol. 99, issue 7:485‐92. [DOI] [PubMed]
Guthmann 2004 AGO {published data only}
- Guthmann JP, Cohuet S, Rigutto C, Fortes F, Saraiva N, Kiguli J, et al. High efficacy of two artemisinin‐based combinations (artesunate + amodiaquine and artemether + lumefantrine) in Caala, Central Angola. American Journal of Tropical Medicine and Hygiene 2006; Vol. 75, issue 1:143‐5. [PubMed]
Hamour 2003 SDN {published data only}
- Hamour S, Melaku Y, Keus K, Wambugu J, Atkin S, Montgomery J, et al. Malaria in the Nuba Mountains of Sudan: baseline genotypic resistance and efficacy of the artesunate plus sulfadoxine‐pyrimethamine and artesunate plus amodiaquine combinations. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005; Vol. 99, issue 7:548‐54. [DOI] [PubMed]
Hasugian 2005 IDN {published and unpublished data}
- Hasugian AR, Purba HL, Kenangalem E, Wuwung RM, Ebsworth EP, Maristela R, et al. Dihydroartemisinin‐piperaquine versus artesunate‐amodiaquine: superior efficacy and posttreatment prophylaxis against multidrug‐resistant Plasmodium falciparum and Plasmodium vivax malaria. Clinical Infectious Diseases 2007; Vol. 44, issue 8:1067‐74. [DOI] [PMC free article] [PubMed]
Hutagalung 2002 THA {published data only}
- Hutagalung R, Paiphun L, Ashley EA, McGready R, Brockman A, Thwai KL, et al. A randomized trial of artemether‐lumefantrine versus mefloquine‐artesunate for the treatment of uncomplicated multi‐drug resistant Plasmodium falciparum on the western border of Thailand. Malaria Journal 2005; Vol. 4:46. [DOI] [PMC free article] [PubMed]
Janssens 2003 KHM {published data only}
- Janssens B, Herp M, Goubert L, Chan S, Uong S, Nong S, et al. A randomized open study to assess the efficacy and tolerability of dihydroartemisinin‐piperaquine for the treatment of uncomplicated falciparum malaria in Cambodia. Tropical Medicine and International Health 2007; Vol. 12, issue 2:251‐9. [DOI] [PubMed]
Kamya 2006 UGA {published data only}
- Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, et al. Artemether‐lumefantrine versus dihydroartemisinin‐piperaquine for treatment of malaria: a randomized trial. PLoS Clinical Trials 2007; Vol. 2, issue 5:e20. [DOI] [PMC free article] [PubMed]
Karema 2004 RWA {published and unpublished data}
- Karema C, Fanello CI, Overmeir C, Geertruyden JP, Doren W, Ngamije D, et al. Safety and efficacy of dihydroartemisinin/piperaquine (Artekin) for the treatment of uncomplicated Plasmodium falciparum malaria in Rwandan children. Transactions of the Royal Society of Tropical Medicine and Hygiene 2006; Vol. 100, issue 12:1105‐11. [DOI] [PubMed]
Karunajeewa 2007 PNG {published data only}
- Karunajeewa HA, Mueller I, Senn M, Lin E, Law I, Gomorrai PS, et al. A trial of combination antimalarial therapies in children from Papua New Guinea. New England Journal of Medicine 2008;359(24):2545‐57. [DOI] [PubMed] [Google Scholar]
Kayentao 2006 MLI {published data only}
- Kayentao K, Maiga H, Newman RD, McMorrow ML, Hoppe A, Yattara O, et al. Artemisinin‐based combinations versus amodiaquine plus sulphadoxine‐pyrimethamine for the treatment of uncomplicated malaria in Faladje, Mali. Malaria Journal 2009;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobbe 2007 GHA {published data only}
- Kobbe R, Klein P, Adjei S, Amemasor S, Thompson WN, Heidemann H, et al. A randomized trial on effectiveness of artemether‐lumefantrine versus artesunate plus amodiaquine for unsupervised treatment of uncomplicated Plasmodium falciparum malaria in Ghanaian children. Malaria Journal 2008;7:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koram 2003 GHA {published data only}
Lefevre 1999 THA {published data only}
- Lefevre G, Looareesuwan S, Treeprasertsuk S, Krudsood S, Silachamroon U, Gathmann I, et al. A clinical and pharmacokinetic trial of six doses of artemether‐ lumefantrine for multidrug‐resistant Plasmodium falciparum malaria in Thailand. American Journal of Tropical Medicine and Hygiene 2001; Vol. 64, issue 5‐6:247‐56. [DOI] [PubMed]
Martensson 2003 TZA {published data only}
- Martensson A, Stromberg J, Sisowath C, Msellem MI, Gil JP, Montgomery SM, et al. Efficacy of artesunate plus amodiaquine versus that of artemether‐lumefantrine for the treatment of uncomplicated childhood Plasmodium falciparum malaria in Zanzibar, Tanzania. Clinical Infectious Diseases 2005; Vol. 41, issue 8:1079‐86. [DOI] [PubMed]
Mayxay 2003 LAO {published and unpublished data}
- Mayxay M, Khanthavong M, Lindegardh N, Keola S, Barends M, Pongvongsa T, et al. Randomized comparison of chloroquine plus sulfadoxine‐pyrimethamine versus artesunate plus mefloquine versus artemether‐lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People's Democratic Republic. Clinical Infectious Diseases 2004; Vol. 39, issue 8:1139‐47. [DOI] [PubMed]
Mayxay 2004 LAO {published and unpublished data}
- Mayxay M, Thongpraseuth V, Khanthavong M, Lindegardh N, Barends M, Keola S, et al. An open, randomized comparison of artesunate plus mefloquine vs. dihydroartemisinin‐piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in the Lao People's Democratic Republic (Laos). Tropical Medicine and International Health 2006; Vol. 11, issue 8:1157‐65. [DOI] [PubMed]
Menard 2006 MDG {published data only}
- Menard D, Andrianina NN, Ramiandrasoa Z, Randriamanantena A, Rasoarilalao N, Jahevitra M, et al. Randomized clinical trial of artemisinin versus non‐artemisinin combination therapy for uncomplicated falciparum malaria in Madagascar. Malaria Journal 2007; Vol. 6:65. [DOI] [PMC free article] [PubMed]
Mens 2007 KEN {published data only}
- Mens PF, Sawa P, Amsterdam SM, Versteeg I, Omar SA, Schallig HD, et al. A randomized trial to monitor the efficacy and effectiveness by QT‐NASBA of artemether‐lumefantrine versus dihydroartemisinin‐piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malaria Journal 2008;7(237):Doi:10.1186/1475‐2875‐7‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mukhtar 2005 SDN {published data only}
- Mukhtar EA, Gadalla NB, El‐Zaki SE, Mukhtar I, Mansour FA, Babiker A, et al. A comparative study on the efficacy of artesunate plus sulphadoxine/pyrimethamine versus artemether‐lumefantrine in eastern Sudan. Malaria Journal 2007; Vol. 6:92. [DOI] [PMC free article] [PubMed]
Mutabingwa 2004 TZA {published data only}
- Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, et al. Amodiaquine alone, amodiaquine+sulfadoxine‐pyrimethamine, amodiaquine+artesunate, and artemether‐lumefantrine for outpatient treatment of malaria in Tanzanian children: a four‐arm randomised effectiveness trial. Lancet 2005; Vol. 365, issue 9469:1474‐80. [DOI] [PubMed]
Owusu‐Agyei 2006 GHA {published data only}
- Owusu‐Agyei S, Asante KP, Owusu R, Adjuik M, Amenga‐Etego S, Dosoo DK, et al. An open label, randomised trial of artesunate+amodiaquine, artesunate+chlorproguanil‐dapsone and artemether‐lumefantrine for the treatment of uncomplicated malaria. PLoS ONE 2008;3(6):e2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ratcliff 2005 IDN {published and unpublished data}
- Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung RM, Laihad F, et al. Two fixed‐dose artemisinin combinations for drug‐resistant falciparum and vivax malaria in Papua, Indonesia: an open‐label randomised comparison. Lancet 2007; Vol. 369, issue 9563:757‐65. [DOI] [PMC free article] [PubMed]
Sagara 2005b MLI {published and unpublished data}
- Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, et al. A randomized trial of artesunate‐mefloquine versus artemether‐lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. American Journal of Tropical Medicine and Hygiene 2008;79(5):655‐61. [PubMed] [Google Scholar]
Smithuis 2004 MMR {published data only}
Staedke 2003 UGA {published and unpublished data}
Stohrer 2003 LAO {published data only}
- Stohrer JM, Dittrich S, Thongpaseuth V, Vanisaveth V, Phetsouvanh R, Phompida S, et al. Therapeutic efficacy of artemether‐lumefantrine and artesunate‐mefloquine for treatment of uncomplicated Plasmodium falciparum malaria in Luang Namtha Province, Lao People's Democratic Republic. Tropical Medicine and International Health 2004; Vol. 9, issue 11:1175‐83. [DOI] [PubMed]
Swarthout 2004 ZAR {published data only}
- Swarthout TD, Broek IV, Kayembe G, Montgomery J, Pota H, Roper C. Artesunate + amodiaquine and artesunate + sulphadoxine‐pyrimethamine for treatment of uncomplicated malaria in Democratic Republic of Congo: a clinical trial with determination of sulphadoxine and pyrimethamine‐resistant haplotypes. Tropical Medicine and International Health 2006; Vol. 11, issue 10:1503‐11. [DOI] [PubMed]
Tangpukdee 2005 THA {published data only}
- Tangpukdee N, Krudsood S, Thanachartwet W, Chalermrut K, Pengruksa C, Srivilairit S, et al. An open randomized clinical trial of Artekin vs artesunate‐mefloquine in the treatment of acute uncomplicated falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 2005; Vol. 36, issue 5:1085‐91. [PubMed]
Tran 2002 VNM {published data only}
Van den Broek 2003a BGD {published and unpublished data}
- Broek IV, Maung UA, Peters A, Liem L, Kamal M, Rahman M, et al. Efficacy of chloroquine + sulfadoxine‐‐pyrimethamine, mefloquine + artesunate and artemether + lumefantrine combination therapies to treat Plasmodium falciparum malaria in the Chittagong Hill Tracts, Bangladesh. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005; Vol. 99, issue 10:727‐35. [DOI] [PubMed]
Van den Broek 2004 ZAR {published and unpublished data}
- Broek I, Kitz C, Al Attas S, Libama F, Balasegaram M, Guthmann JP. Efficacy of three artemisinin combination therapies for the treatment of uncomplicated Plasmodium falciparum malaria in the Republic of Congo. Malaria Journal 2006; Vol. 5:113. [DOI] [PMC free article] [PubMed]
Van Vugt 1998 THA {published data only}
Yeka 2004 UGA {published and unpublished data}
- Yeka A, Banek K, Bakyaita N, Staedke SG, Kamya MR, Talisuna A, et al. Artemisinin versus nonartemisinin combination therapy for uncomplicated malaria: randomized clinical trials from four sites in Uganda. PLoS Medicine 2005; Vol. 2, issue 7:e190. [DOI] [PMC free article] [PubMed]
Yeka 2007 UGA {published data only}
- Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, et al. Artemether‐lumefantrine versus dihydroartemisinin‐piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS ONE 2008;3(6):e2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zongo 2005 BFA {published and unpublished data}
Zongo 2007 BFA {published and unpublished data}
- Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Sere Y, Rosenthal PJ, et al. Randomized comparison of amodiaquine plus sulfadoxine‐pyrimethamine, artemether‐lumefantrine, and dihydroartemisinin‐piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clinical Infectious Diseases 2007; Vol. 45, issue 11:1453‐61. [DOI] [PubMed]
References to studies excluded from this review
Abacassamo 2002 MOZ {published data only}
- Abacassamo F, Enosse S, Aponte JJ, Gomez‐Olive FX, Quinto L, Mabunda S, et al. Efficacy of chloroquine, amodiaquine, sulphadoxine‐pyrimethamine and combination therapy with artesunate in Mozambican children with non‐complicated malaria. Tropical Medicine and International Health 2004; Vol. 9, issue 2:200‐8. [DOI] [PubMed]
Abuaku 2005 {published data only}
- Abuaku B, Koram K, Quashie N, Duah N. Efficacy of chloroquine, sulfadoxine‐pyrimethamine, amodiaquine + artesunate and artemether + lumefantrine in treating uncomplicated malaria in Ghana. Acta Tropica 2005;95 Suppl 1 [Abstracts from the 4th MIM Pan‐African Conference]:335. [Google Scholar]
Adjei 2005 {published data only}
- Adjei G, Goka B, Rodrigues O, Kitcher E, Badoe E, Alifrangis M, et al. Amodiaquine‐artesunate versus artemether‐lumefantrine: Efficacy and safety for single or repeat episodes of uncomplicated malaria in Ghanaian children. Acta Tropica 2005;95 Suppl 1 [Abstracts from the 4th MIM Pan‐African Conference]:38. [Google Scholar]
Bell 2008 {published data only}
- Bell DJ, Nyirongo SK, Mukaka M, Zijlstra EE, Plowe CV, Molyneux ME. Sulfadoxine‐pyrimethamine‐based combinations for malaria: a randomised blinded trial to compare efficacy, safety and selection of resistance in Malawi. PLoS ONE 2008;3(2):e1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blair 2006 {published data only}
- Blair S, Carmona‐Fonseca J, Pineros JG, Rios A, Alvarez T, Alvarez G, et al. Therapeutic efficacy test in malaria falciparum in Antioquia, Colombia. Malaria Journal 2006; Vol. 5:14. [DOI] [PMC free article] [PubMed]
Denis 2006 {published data only}
Dorsey 2002 {published data only}
Dorsey‐G 2003 {published data only}
Fofana 2005 {published data only}
- Fofana B, Sidibe B, Dembele D, Toure S, Maiga H, Sagara I, et al. Comparison of the efficacy, safety and tolerability of three treatment regimens for uncomplicated P. falciparum malaria in Mali: artesunate+amodiaquine (3 days) versus artesunate (3 days) + sulfadoxine‐pyrimethamine (1 day) versus artesunate (5 days). Acta Tropica 2005;95 Suppl 1 [Abstracts from the 4th MIM Pan‐African Conference]:345‐6. [Google Scholar]
Ibrahium 2007 {published data only}
- Ibrahium AM, Kheir MM, Osman ME, Khalil IF, Alifrangis M, Elmardi KA, et al. Efficacies of artesunate plus either sulfadoxine‐pyrimethamine or amodiaquine, for the treatment of uncomplicated, Plasmodium falciparum malaria in eastern Sudan. Annals of Tropical Medicine and Parasitology 2007; Vol. 101, issue 1:15‐21. [DOI] [PubMed]
Jiao 1997 {published data only}
- Jiao XQ, Liu EY, Shan CQ, Shan CQ, Dal P, Gathmann I. A double‐blind comparative trial of benflumetol, a novel antimalarial, and CGP 56697, a combination of benflumetol and artemether, in the treatment of acute P. falciparum malaria in adults in China. Fifth International Conference on Travel Medicine Program and Abstracts. Geneva, 1997:Abstract 108.
Kabanywanyi 2007 {published data only}
- Kabanywanyi AM, Mwita A, Sumari D, Mandike R, Mugittu K, Abdulla S. Efficacy and safety of artemisinin‐based antimalarial in the treatment of uncomplicated malaria in children in southern Tanzania. Malaria Journal 2007; Vol. 6, issue 1:146. [DOI] [PMC free article] [PubMed]
Massougbodji 2005 {published data only}
- Massougbodji A, Agbo K, Faye O, Guiguemde R, Kone M, Heidecker J, et al. Efficacy and safety of a combination of artsesunate/mefloquine, Artequin (TM), in African children and adults with uncomplicated P. falciparum malaria. Acta Tropica 2005;95 Suppl 1 [Abstracts from the 4th MIM Pan‐African Conference]:234‐5. [Google Scholar]
Meremikwu 2004 NGA {published data only}
- Meremikwu M, Alaribe A, Ejemot R, Oyo‐Ita A, Ekenjoku J, Nwachukwu C, et al. Artemether‐lumefantrine versus artesunate plus amodiaquine for treating uncomplicated childhood malaria in Nigeria: randomized controlled trial. Malaria Journal 2006; Vol. 5:43. [DOI] [PMC free article] [PubMed]
Mockenhaupt 2005 {published data only}
- Mockenhaupt FP, Ehrhardt S, Dzisi SY, Teun Bousema J, Wassilew N, Schreiber J, et al. A randomized, placebo‐controlled, double‐blind trial on sulfadoxine‐pyrimethamine alone or combined with artesunate or amodiaquine in uncomplicated malaria. Tropical Medicine and International Health 2005; Vol. 10, issue 6:512‐20. [DOI] [PubMed]
Mohamed 2006 {published data only}
- Mohamed AO, Eltaib EH, Ahmed OA, Elamin SB, Malik EM. The efficacies of artesunate‐sulfadoxine‐pyrimethamine and artemether‐lumefantrine in the treatment of uncomplicated, Plasmodium falciparum malaria, in an area of low transmission in central Sudan. Annals of Tropical Medicine and Parasitology 2006; Vol. 100, issue 1:5‐10. [DOI] [PubMed]
Mulenga 2006 {published data only}
- Mulenga M, Geertruyden JP, Mwananyanda L, Chalwe V, Moerman F, Chilengi R, et al. Safety and efficacy of lumefantrine‐artemether (Coartem(R)) for the treatment of uncomplicated Plasmodium falciparum malaria in Zambian adults. Malaria Journal 2006; Vol. 5, issue 1:73. [DOI] [PMC free article] [PubMed]
Ndayiragije 2004 {published data only}
- Ndayiragije A, Niyungeko D, Karenzo J, Niyungeko E, Barutwanayo M, Ciza A, et al. Efficacy of therapeutic combinations with artemisinin derivatives in the treatment of non complicated malaria in Burundi.[Efficacite de combinaisons therapeutiques avec des derives de l'artemisinine dans le traitement de l'acces palustre non‐complique au Burundi]. Tropical Medicine and International Health 2004; Vol. 9, issue 6:673‐9. [DOI] [PubMed]
Ndiaye 2005 {published data only}
- Ndiaye P, Faye B, Ndiaye J, Ndiaye D, Diallo I, Seck P, et al. Efficacite et tolerance de l'association Artesunate plus Amodiaquine (Amonate®) versus Arthemether plus lumefantrine (Coartem®) six doses dans le traitement des acces palustres simples a Plasmodium falciparum au Senegal. Acta Tropica 2005;95 Suppl 1 [Abstracts from the 4th MIM Pan‐African Conference]:254‐5. [Google Scholar]
Obonyo 2007 {published data only}
- Obonyo CO, Juma EA, Ogutu BR, Vulule JM, Lau J. Amodiaquine combined with sulfadoxine/pyrimethamine versus artemisinin‐based combinations for the treatment of uncomplicated falciparum malaria in Africa: a meta‐analysis. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(2):117‐26. [DOI] [PubMed] [Google Scholar]
Okell 2008 {published data only}
- Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: A pooled analysis of six randomized trials. Malaria Journal 2008;7:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piola 2005 {published data only}
- Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, Ruzagira E, et al. Supervised versus unsupervised intake of six‐dose artemether‐lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet 2005; Vol. 365, issue 9469:1467‐73. [DOI] [PubMed]
Rwagacondo 2003 {published data only}
- Rwagacondo CE, Niyitegeka F, Sarushi J, Karema C, Mugisha V, Dujardin JC, et al. Efficacy of amodiaquine alone and combined with sulfadoxine‐pyrimethamine and of sulfadoxine pyrimethamine combined with artesunate. American Journal of Tropical Medicine and Hygiene 2003; Vol. 68, issue 6:743‐7. [PubMed]
Sagara 2006 {published data only}
- Sagara I, Dicko A, Djimde A, Guindo O, Kone M, Tolo Y, et al. A randomized trial of artesunate‐sulfamethoxypyrazine‐pyrimethamine versus artemether‐lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Mali. American Journal of Tropical Medicine and Hygiene 2006; Vol. 75:630‐636. [PubMed]
Sowunmi 2007a {published data only}
Sowunmi 2007b {published data only}
- Sowunmi A, Gbotosho GO, Happi CT, Adedeji AA, Fehintola FA, Folarin OA, et al. Therapeutic efficacy and effects of artemether‐lumefantrine and amodiaquine‐sulfalene‐pyrimethamine on gametocyte carriage in children with uncomplicated Plasmodium falciparum malaria in Southwestern Nigeria. American Journal of Tropical Medicine and Hygiene 2007; Vol. 77, issue 2:235‐41. [PubMed]
Tall 2005 {published data only}
- Tall A, Rabarijaona L, Bedja S, Rahamatou S, Ahmed O, Ratsimbasoa A, et al. Efficacy of artesunate + amodiaquine, artesunate + sulfadoxine‐pyrimethamine, chloroquine + sulfadoxine‐pyrimethamine in P.falciparum malaria in Comoros. Acta Tropica 2005;95 Suppl 1 [Abstracts from the 4th MIM Pan‐African Conference]:223‐4. [Google Scholar]
Tall 2007 {published data only}
- Tall A, Rabarijaona LP, Robert V, Bedja SA, Ariey F, Randrianarivelojosia M. Efficacy of artesunate plus amodiaquine, artesunate plus sulfadoxine‐pyrimethamine, and chloroquine plus sulfadoxine‐pyrimethamine in patients with uncomplicated Plasmodium falciparum in the Comoros Union. Acta Tropica 2007. [DOI] [PubMed]
Thapa 2007 {published data only}
- Thapa S, Hollander J, Linehan M, Cox‐Singh J, Bista MB, Thakur GD, et al. Comparison of artemether‐lumefantrine with sulfadoxine‐pyrimethamine for the treatment of uncomplicated Falciparum malaria in Eastern Nepal. American Journal of Tropical Medicine and Hygiene 2007; Vol. 77, issue 3:423‐30. [PubMed]
Tranh 2009 {published data only}
- Thanh NX, Trung TN, Phong NC, Thien NX, Dai B, Dennis Shanks G, et al. Open label randomized comparison of dihydroartemisinin‐piperaquine and artesunate‐amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in central Vietnam. Tropical Medicine and International Health 2009;14(5):1‐8. [DOI] [PubMed] [Google Scholar]
van den Broek 2005b {published data only}
- Broek I, Amsalu R, Balasegaram M, Hepple P, Alemu E, Hussein el B, et al. Efficacy of two artemisinin combination therapies for uncomplicated falciparum malaria in children under 5 years, Malakal, Upper Nile, Sudan. Malaria Journal 2005; Vol. 4, issue 1:14. [DOI] [PMC free article] [PubMed]
van Vugt 1998 {published data only}
- Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, et al. Randomized comparison of artemether‐benflumetol and artesunate‐mefloquine in treatment of multidrug‐resistant falciparum malaria. Antimicrobial Agents Chemotherapy 1998; Vol. 42, issue 1:135‐9. [DOI] [PMC free article] [PubMed]
Vugt 1999 {published data only}
Wilairatana 2002 {published data only}
- Wilairatana P, Krudsood S, Chalermrut K, Pengruksa C, Srivilairit S, Silachamroon U, et al. An open randomized clinical trial of Artecom vs artesunate‐mefloquine in the treatment of acute uncomplicated falciparum malaria in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2002; Vol. 33, issue 3:519‐24. [PubMed]
Wiseman 2006 {published data only}
- Wiseman V, Kim M, Mutabingwa TK, Whitty CJ. Cost‐effectiveness study of three antimalarial drug combinations in Tanzania. PLoS Medicine 2006; Vol. 3, issue 10. [DOI] [PMC free article] [PubMed]
Additional references
Adjuik 2004
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N, International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta‐analysis. The Lancet 2004;363(9402):9‐17. [DOI] [PubMed] [Google Scholar]
Agomo 2008
- Agomo PU, Meremikwu MM, Watila IM, Omalu IJ, Odey FA, Oguche S, et al. Efficacy, safety and tolerability of artesunate‐mefloquine in the treatment of uncomplicated Plasmodium falciparum malaria in four geographic zones of Nigeria. Malaria Journal 2008;7:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bukirwa 2005
- Bukirwa H, Orton L. Artesunate plus mefloquine versus mefloquine for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD004531.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bukirwa 2006
- Bukirwa H, Critchley J. Sulfadoxine‐pyrimethamine plus artesunate versus sulfadoxine‐pyrimethamine plus amodiaquine for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004966.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cattamanchi 2003
- Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp‐1, msp‐2, and glurp. American Journal of Tropical Medicine and Hygiene 2003;68(2):133‐9. [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. GRADE Working Group.Rating quality of evidence and strength of recommendations: What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors), Cochrane Handbook of Systematic Reviews of Intervention. Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. Chichester: John Wiley & Sons Ltd.
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Massougbodji 2002
- Massousgbodji A, Kone Kinde Gazard D, same‐Ekobo A, Cambon N, Mueller EA. A randomized double‐blind study on the efficacy and safety of a practical three‐day regimen with artesunate and mefloquine for the treatment of uncomplicated Plasmodium falciparum malaria in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 2002;96(6):655‐659. [DOI] [PubMed] [Google Scholar]
Mayxay 2004
- Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed‐species malaria infections in humans. Trends in Parasitology 2004;20(5):233‐40. [DOI] [PubMed] [Google Scholar]
McIntosh 1999
- McIntosh HM, Olliaro P. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000256] [DOI] [PMC free article] [PubMed] [Google Scholar]
Meshnick 1996
- Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiological Reviews 1996;60(2):301‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Newton 2002
- Newton PN, Vugt M, Teja‐Isavadharm P, Siriyanonda D, Rasameesoroj M, Teerapong P, et al. Comparison of oral artesunate and dihydroartemisinin antimalarial bioavailabilities in acute Falciparum malaria. Antimicrobial Agents Chemotherapy 2002;46(4):1125‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nosten 2007
- Nosten F, White NJ. Artemisinin‐based combination treatment of falciparum malaria. American Journal of Tropical Medicine and Hygiene 2007;77 (6 Suppl):181‐92. [PubMed] [Google Scholar]
Omari 2005
- Omari AAA, Gamble C, Garner P. Artemether‐lumefantrine (six‐dose regimen) for treating uncomplicated falciparum malaria. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD005564] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omari 2006
- Omari AAA, Gamble C, Garner P. Artemether‐lumefantrine (four‐dose regimen) for treating uncomplicated falciparum malaria. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 1996
- Price RN, Nosten F, Luxemburger C, ter Kuile FO, Paiphun L, Chongsuphajaisiddhi T, et al. Effects of artemisinin derivatives on malaria transmissibility. The Lancet 1996;347(9016):1654‐8. [DOI] [PubMed] [Google Scholar]
Price 1999
- Price R, Vugt M, Phaipun L, Luxemburger C, Simpson J, McGready R, et al. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. American Journal of Tropical Medicine and Hygiene 1999;60(4):547‐55. [DOI] [PubMed] [Google Scholar]
Pukrittayakamee 2000
- Pukrittayakamee S, Chantra A, Simpson JA, Vanijanonta S, Clemens R, Looareesuwan S, et al. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrobial Agents and Chemotherapy 2000;44(6):1680‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qinghaosu 1979
- Qinghaosu Antimalarial Coordinating Research Group. Antimalarial studies on qinghaosu. Chinese Medical Journal 1979;92:811‐6. [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Sagara 2008
- Sagara I, Diallo A, Kone M, Coulibaly M, Diawara SI, Guindo O, et al. A Randomized Trial of Artesunate‐Mefloquine versus Artemether‐Lumefantrine for Treatment of Uncomplicated Plasmodium falciparum Malaria in Mali. American Journal of Tropical Medicine and Hygiene 2008;79(5):655‐661. [PubMed] [Google Scholar]
Slutsker 1990
- Slutsker LM, Khoromana CO, Payne D, Allen CR, Wirima JJ, Heymann DL, et al. Mefloquine therapy for Plasmodium falciparum malaria in children under5 years of age in Malawi: In vivo/in vitro efficacy and correlation of drug concentration with parasitological outcome. Bulletin of the World Health Organisation 1990;68(1):53‐59. [PMC free article] [PubMed] [Google Scholar]
Targett 2001
- Targett G, Drakeley C, Jawara M, Seidlein L, Coleman R, Deen J, et al. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. Journal of Infectious Diseases 2001;183(8):1254‐9. [DOI] [PubMed] [Google Scholar]
Vugt 1999
- Vugt MV, Wilairatana P, Gemperli B, Gathmann I, Phaipun L, Brockman A, et al. Efficacy of six doses of artemether‐lumefantrine (benflumetol) in multidrug‐resistant Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 1999;60(6):936‐42. [DOI] [PubMed] [Google Scholar]
White 1996
- White NJ, Olliaro PL. Strategies for the prevention of antimalarial drug resistance: rationale for combination chemotherapy for malaria. Parasitology Today 1996;12(10):399‐401. [DOI] [PubMed] [Google Scholar]
White 1999
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. The Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
White 2002
- White NJ. The assessment of antimalarial drug efficacy. Trends in Parasitology 2002;18(10):458‐64. [DOI] [PubMed] [Google Scholar]
WHO 2003
- Bloland PB. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria [WHO/HTM/RBM/2003.50]. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2006
- World Health Organization. Roll Back Malaria Dept. Guidelines for the treatment of malaria [WHO/HTM/MAL/2006.1108]. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2007
- World Health Organization. Malaria [Fact sheet no. 94]. www.who.int/mediacentre/factsheets/fs094/en/index.html May 2007 (accessed 1 July 2008).
WHO 2008a
- World Health Organization. Global malaria control and elimination: report of a meeting on containment of artemisinin tolerance, 19 January 2008, Geneva, Switzerland. Geneva: World Health Organization, 2008. [Google Scholar]
WHO 2008b
- WHO Global Malaria Programme. World Malaria Report: 2008. Geneva: World Health Organization, 2008. [Google Scholar]


