Abstract
Background
To prevent the development of drug resistance, the World Health Organization (WHO) recommends treating malaria with combination therapy. Azithromycin, an antibiotic with antimalarial properties, may be a useful additional option for antimalarial therapy.
Objectives
To compare the use of azithromycin alone or in combination with other antimalarial drugs with the use of alternative antimalarial drugs for treating uncomplicated malaria caused by Plasmodium falciparum or Plasmodium vivax.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (August 2010); CENTRAL (The Cochrane Library Issue 3, 2010); MEDLINE (1966 to August 2010); EMBASE (1974 to August 2010); LILACS (August 2010); the metaRegister of Controlled Trials (mRCT, August 2010); conference proceedings; and reference lists. We also contacted researchers and a pharmaceutical company.
Selection criteria
Randomized controlled trials comparing azithromycin, either alone or combined with another antimalarial drug, with another antimalarial drug used alone or combined with another antimalarial drug, or with azithromycin combined with another antimalarial drug if different combinations or doses of azithromycin were used. The primary outcome was treatment failure by day 28, defined as parasitological or clinical evidence of treatment failure between the start of treatment and day 28. Secondary outcomes included treatment failure by day 28 corrected for new infections confirmed by polymerase chain reaction (PCR), fever and parasite clearance time, and adverse events.
Data collection and analysis
Two people independently applied the inclusion criteria, extracted data and assessed methodological quality. We used risk ratio (RR) and 95% confidence intervals (CI).
Main results
Fifteen trials met the inclusion criteria (2284 participants, 69% males, 16% children). They were conducted in disparate malaria endemic areas, with the earlier studies conducted in Thailand (five) and India (two), and the more recent studies (eight) spread across three continents (South America, Africa, Asia). The 15 studies involved 41 treatment arms, 12 different drugs, and 28 different treatment regimens. Two studies examined P. vivax.
Three‐day azithromycin (AZ) monotherapy did not perform well for P. vivax or P. falciparum (Thailand: P. vivax failure rate 0.5 g daily, 56%, 95% CI 31 to 78. India: P. vivax failure rate 1 g daily,12%, 95% CI 7 to 21; P. falciparum failure rate 1 g daily, 64%, 95% CI 36 to 86.) A 1 g azithromycin and 0.6 g chloroquine combination daily for three days for uncomplicated P. falciparum infections was associated with increased treatment failure in India and Indonesia compared with the combination of sulphadoxine‐pyrimethamine and chloroquine (pooled RR 2.66, 95% CI 1.25 to 5.67), and compared with the combination atovaquone‐proguanil in a multicentre trial in Columbia and Surinam (RR 24.72, 95% CI 6.16 to 99.20). No increased risk of treatment failure was seen in two studies in Africa with mefloquine as the comparator drug (pooled RR 2.02, 95% CI 0.51 to 7.96, P = 0.3); the pooled RR for PCR‐corrected data for the combination versus mefloquine was 1.01, 95% CI 0.18 to 5.84 (P = 1.0). An increased treatment failure risk was seen when comparing azithromycin in a dose of 1.2 to 1.5 mg in combination with artesunate (200 mg per day for three days) with artemether‐lumefantrine (pooled RR 3.08, 95% CI 2.09 to 4.55; PCR‐corrected pooled RR 3.63, 95% CI 2.02 to 6.52).
Serious adverse events and treatment discontinuation were similar across treatment arms. More adverse events were reported when comparing the 1 g azithromycin/ 0.6 g chloroquine combination with mefloquine (pooled RR 1.20, 95% CI 1.06 to 1.36) or atovaquone‐proguanil (RR 1.41, 95% CI 1.09 to1.83).
Authors' conclusions
Currently, there is no evidence for the superiority or equivalence of azithromycin monotherapy or combination therapy for the treatment of P. falciparum or P. vivax compared with other antimalarials or with the current first‐line antimalarial combinations. The available evidence suggests that azithromycin is a weak antimalarial with some appealing safety characteristics. Unless the ongoing dose, formulation and product optimisation process results in a universally efficacious product, or a specific niche application is identified that is complementary to the current scala of more efficacious antimalarial combinations, azithromycin's future for the treatment of malaria does not look promising.
Keywords: Female; Humans; Male; Antimalarials; Antimalarials/therapeutic use; Artemether, Lumefantrine Drug Combination; Artemisinins; Artemisinins/therapeutic use; Artesunate; Atovaquone; Atovaquone/therapeutic use; Azithromycin; Azithromycin/therapeutic use; Chloroquine; Chloroquine/therapeutic use; Drug Combinations; Drug Therapy, Combination; Ethanolamines; Ethanolamines/therapeutic use; Fluorenes; Fluorenes/therapeutic use; Malaria, Falciparum; Malaria, Falciparum/drug therapy; Malaria, Vivax; Malaria, Vivax/drug therapy; Mefloquine; Mefloquine/therapeutic use; Proguanil; Proguanil/therapeutic use; Pyrimethamine; Pyrimethamine/therapeutic use; Randomized Controlled Trials as Topic; Sulfadoxine; Sulfadoxine/therapeutic use; Treatment Failure
Azithromycin is not useful as monotherapy for uncomplicated malaria. In combinations with other antimalarials, it may need to be used at high doses, potentially affecting tolerability.
To help prevent the malaria parasite from developing resistance to antimalarial medicines, the WHO recommends the use of combination therapy, where malaria infections are treated with more than one drug simultaneously. As azithromycin is an antibiotic that also has an effect on the malaria parasite, we assessed its efficacy and tolerability as an antimalarial when used alone or as part of combination therapy with other antimalarials. Our review of studies conducted over the past 14 years suggests that azithromycin is a relatively weak antimalarial whose efficacy depends on the drug dose and the partner drug in the combination therapy. The data suggest that, among adults, the higher doses needed to achieve an acceptable level of treatment success with malaria may be less well tolerated. Unless the ongoing product and dose optimisation process results in a universally efficacious product or identifies a specific niche application that is complementary to the current scala of more efficacious antimalarial combinations, azithromycin's future as an antimalarial does not look promising.
Background
The World Health Organization (WHO) treatment guidelines for uncomplicated malaria recommend combination therapy to reduce the development of drug resistance and to improve therapeutic efficacy. Over the past decade highly efficacious artemisinin‐based combination therapies (ACTs) have been implemented across the world (WHO/RBM 2006). Despite this substantial progress, there is a clear need for further antimalarial combinations, involving both candidates for the next generation of first‐line antimalarials and those that address specific niches in malaria control, such as options that are safe for use during early pregnancy or that can be used for syndromic approaches that target multiple diseases.
Three antibiotic groups have been shown to have antimalarial activity in studies using experimental malaria models: tetracyclines, lincosamides, and macrolides (Pradines 2001). Antibiotics have also been used in clinical practice to enhance the effect of available antimalarials. For example, tetracycline and doxycycline (tetracyclines) and clindamycin (a lincosamide) are recommended in combination with quinine as a second‐line treatment option for uncomplicated malaria following a treatment failure (WHO/RBM 2006). However, because they can disturb bone and teeth development, the tetracyclines are contraindicated among pregnant women and children, frequently the most vulnerable groups for malaria (Nosten 2006).
Azithromycin is a potential alternative treatment option. This relatively new macrolide antibiotic has a longer half‐life (approximately 60 hours) and better pharmacokinetic properties (adult treatment dose for bacterial infections 500 mg orally once daily for three to seven days) compared to the macrolide erythromycin, which is widely available in developing countries (standard adult treatment dose for bacterial infections is 250 to 500 mg orally every six hours or 0.5 to 1 g every 12 hours for seven days or more). Azithromycin can treat a broad spectrum of bacterial infections; it has an attractive safety profile and could be an option for use in pregnancy, where the currently available arsenal of effective and safe antimalarials is limited (Nosten 2006). Several trials have used azithromycin to treat sexually‐transmitted diseases and genital infections during pregnancy with no reports of adverse neonatal outcomes (Adair 1998; Gray 2001; Ogasawara 1999).
Studies of the efficacy of azithromycin in the treatment of uncomplicated malaria in experimental and animal models have shown that azithromycin is a relatively weak antimalarial with a slow parasite clearance rate. The treatment dose, duration of therapy, and antimalarial partner drug are reported to be important determinants of efficacy (Andersen 1995; Biswas 2001; Gingras 1992; Gingras 1993; Neerja 2004; Noedl 2001; Ohrt 2002; Puri 2000; Yeo 1995;). Results from experimental clinical studies using azithromycin (in combination or alone) for treating symptomatic malaria in Asia were inconsistent in methodology, dose used, and outcome measures (Dunne 2005a; Dunne 2005b; Krudsood 2000; Krudsood 2002; Miller 2006; Na‐Bangchang 1996). In this review, we aim to summarize the available data from randomized controlled trials to assess the usefulness of azithromycin for the treatment of uncomplicated malaria and to identify its potential niche among the available antimalarial drugs.
Objectives
To compare the efficacy and safety of azithromycin, alone or in combination with other antimalarial drugs, with alternative antimalarial drugs for treating uncomplicated malaria caused by Plasmodium falciparum or Plasmodium vivax.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Children and adults presenting with uncomplicated malaria (P. falciparum or P. vivax) confirmed by microscopy.
Excluded: studies in participants with signs of complicated malaria as defined by the authors (including cerebral malaria, convulsions, circulatory collapse, abnormal breathing, jaundice, macroscopic haemoglobinuria, prostration, renal impairment, severe anaemia, and hypoglycaemia) (WHO/AFRO 2001), and studies in which the participants are exclusively pregnant women.
Types of interventions
Interventions Azithromycin alone, combined with another antimalarial drug, or combined with a placebo. Control
Other antimalarial drug used alone, combined with other antimalarial drugs, or combined with a placebo.
Azithromycin alone if the intervention is azithromycin combined with another antimalarial drug.
Azithromycin combined with another antimalarial drug if different combinations or doses of azithromycin have been used.
Types of outcome measures
Primary Treatment failure by day 28 (unadjusted for new infections), defined as parasitological or clinical evidence of treatment failure between the start of treatment and day 28. This is equivalent to total failure defined in the current WHO guidelines (Bloland 2003) and includes new infections. Parasitological treatment failure means failure of the malaria parasite to clear from the blood during the follow‐up time, or returning of malaria parasites in the blood within 28 days after initial clearance. Clinical evidence of treatment failure means parasitaemia in the presence of danger signs (vomiting, history of convulsions, inability to sit, inability to drink, lethargy); parasitaemia in the presence of symptoms of complicated malaria as described above (WHO/AFRO 2001); parasitaemia in the presence of documented fever (37.5 °C when measured axillary or 38.0 °C or more when measured rectally); or clinical treatment failure as defined by the trial authors for trials conducted before 2003. Secondary
Treatment failure by day 28 corrected for new infections confirmed by polymerase chain reaction (PCR), defined as parasitological or clinical evidence of treatment failure between the start of treatment and day 28.
Fever clearance time.
Parasite clearance time.
Presence of gametocytes on day 7, 14 or 28.
Morbidity other than clinical malaria (eg diarrhoea, respiratory tract infections) up to 28 days.
Adverse events
Serious adverse events defined as fatal, life threatening, or those that require hospitalisation.
Adverse events that may lead to discontinuation of the drug.
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases We searched the following databases using the search terms and strategy described in Table 1: Cochrane Infectious Diseases Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; LILACS; and the metaRegister of Controlled Trials (mRCT). Conference proceedings We searched the conference proceedings of the Multilateral Initiative on Malaria Pan‐African Conferences and the Annual meetings of the American Society of Tropical Medicine and Hygiene (ASTMH) for relevant abstracts. Researchers, organizations, and pharmaceutical companies We contacted individual researchers working in the field, organizations including the WHO, and pharmaceutical companies (Pfizer, Sandoz) for unpublished and ongoing trials.
Reference lists We checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Trial selection The first author screened the results of the search strategy for potentially relevant trials and retrieved the full articles of these trials. Each trial was scrutinized for multiple publications from the same study. Using an eligibility criteria form based on the inclusion criteria, two authors independently assessed the trials for inclusion in the review. Discrepancies between assessments were resolved through discussion or referred to an editor of the Cochrane Infectious Diseases Group when needed. We excluded studies that did not meet the inclusion criteria for the efficacy assessment and stated the reason for exclusion; however, we included these studies for adverse events.
Data extraction We piloted the data extraction form before using it for independent data extraction. We attempted to contact the corresponding author if the available data was unclear, missing, or reported in a format that was different to the format that we required.
We extracted the number of participants analysed and the number randomized for each treatment arm and for each outcome. We calculated the percentage loss to follow‐up and reported this information in the characteristics table. For continuous outcomes, we extracted the arithmetic means and standard deviations for each treatment group, together with the numbers of participants in each group. If medians were extracted, we also extracted ranges. For dichotomous outcome measures (eg treatment failure, presence of gametocytes, adverse events), we recorded the number of participants experiencing the event and the number analysed in each group. We entered the data in Review Manager 5.0.
Methodological quality assessment We used 'The Cochrane Collaboration's tool for assessing the risk of bias' (Higgins 2008). We followed the guidance to assess whether adequate steps had been taken to reduce the risk of bias across six domains: sequence generation; allocation concealment; blinding (of participants, personnel, and outcome assessors); incomplete outcome data; selective outcome reporting; and other sources of bias (Juni 2001; Schulz 2002). We have categorized these judgments as 'yes' (low risk of bias), 'no' (high risk of bias), or 'unclear'. Where our judgement was unclear, we attempted to contact the trial authors for clarification.
Data analysis We analysed the data using Review Manager 5.0 and presented all results with 95% CI. If no participants were lost to follow‐up, we used an intention‐to‐treat analysis. For trials with loss to follow‐up, we undertook an available case analysis, in which trial participants were analysed by the arm they were randomised into and participants for whom the outcome was not collected were excluded.
We compared dichotomous data using relative risks. Where data were presented using medians and ranges, we presented the data in tables only. We aimed to conduct sensitivity analyses based on allocation concealment and loss to follow‐up. We did not intend to combine trials of different comparator drugs.
We assessed heterogeneity by inspecting the forest plots and assessing the heterogeneity statistics (Chi2 test < 0.1, I2 statistic > 50%). If we detected heterogeneity and considered it clinically meaningful to combine the trials, we used a random‐effects model. We intended to explore the following potential sources of heterogeneity using subgroup analyses: participants (children versus adults, as defined by the included trials); trial setting (high versus low malaria transmission ); drug dose (total dose and division of doses over days); and presence of drug resistance to comparator drug. However, the small number of studies per comparison did not allow these analyses. They also precluded meaningful use of funnel plots and sensitivity analyses.
Results
Description of studies
We identified 19 potential studies for inclusion. Two studies were excluded because their results were not yet available: one was still ongoing at the time of the search (Pfizer 677833), and one study was completed but the analysis was not yet finished (NIAID 379821). Two other studies were excluded from the efficacy analysis because they were not randomized (Krudsood 2002; Pfizer 282919); however, these studies were included in the evaluation of adverse events. In the remaining 15 studies, 2284 participants were enrolled and 2083 (91.2%) were available for the analysis of the primary endpoint. Participants were children and adults: the lowest age for inclusion was six months (Sykes 2009). However, most studies included adolescents and adults only (13 out of the 15 studies); we estimate that 374 of the enrolled participants (16.4%) were children < 60 months and/or with a weight < 35 kg. Sixty‐nine percent of participants were male and two studies included men only (Na‐Bangchang 1996; Pukrittayakamee 2001); all studies excluded pregnant women. The earliest study started in 1995 and the most recent study finished in 2009. The majority of studies were funded by Pfizer alone (nine) or in combination with others (two). Six trial synopses were retrieved from the web from studies sponsored by Pfizer; the role of the funding agency was not stated in these reports.
Two studies examined P. vivax (Dunne 2005a; Pukrittayakamee 2001), whereas P. falciparum was the species of primary interest in all other studies. The early studies were conducted in Thailand (five) and India (two), whereas the more recent studies (eight) were conducted across three continents (South America, Africa, Asia). Thailand is known for its multi‐resistant P. falciparum, with described resistance to chloroquine, sulphadoxine‐pyrimethamine, mefloquine and quinine; malaria transmission is generally low, unstable, and seasonal. There is no malaria transmission in Bangkok, the site of three studies. Malaria transmission within the area investigated in the early Indian study was reported as "stable throughout the year" (Dunne 2005b). One of the Pfizer studies was conducted in an area of intense malaria transmission in Kenya (Pfizer 82563), but the type of malaria transmission in the areas of the other Pfizer studies was not reported. Malaria transmission was high in the study area in Tanzania (Sykes 2009), but moderate‐to‐low in the study area in Bangladesh (Thriemer 2010). All studies followed participants up to day 28, with seven of them reporting PCR‐adjusted failure rates and seven studies continuing follow‐up until day 42. However, only three studies reported day 42 results (Pfizer 84227, Sykes 2009, Thriemer 2010).
There was a wide variety in the drug combinations, doses of azithromycin and comparator drugs, and regimens used, with 41 treatment arms of the 15 studies involving 12 different antimalarials, and 28 different treatment regimens (see Characteristics of included studies or Table 2). The most common azithromycin combination was 1 g azithromycin with 0.6 g chloroquine daily for three days (1 g azithromycin/chloroquine), used in six arms. The characteristics of the included studies are given in the Characteristics of included studies table.
All studies except one (Pfizer 367653) reported excluding subjects with a recent history of intake of an antimalarial, although the period of intake could vary between the last 48 hours to 42 days prior to enrolment. In Tanzania, only subjects with a history of intake of an effective antimalarial were excluded, because local drug resistance to commonly used antimalarials such as sulfadoxine‐pyrimethamine, amodiaquine and chloroquine was reported to be > 70% (Sykes 2009). Two studies used drug screening tests to confirm the absence of antimalarial metabolites (Na‐Bangchang 1996; Pukrittayakamee 2001).
Risk of bias in included studies
The available information did not allow an appropriate evaluation of the quality of the data. The six trial synopses from Pfizer that were publicly available contained limited information on the methods, and did not specify randomization and allocation concealment. Baseline characteristics were broadly comparable between arms except for the baseline parasite density in the combination arm of a study in India, which was lower than the other arms (Table 4, Dunne 2005b). Four trials conducted by Pfizer included an arm with 0.5 g azithromycin and 600 mg chloroquine for three days; when one of these trials in India showed low efficacy in a second interim analysis, this arm was stopped in this and all other trials where this arm was used (Pfizer 74841, Pfizer 82576, Pfizer 84227, Pfizer 84240). Results were presented for the 59 participants in the arm for the trial in India (Pfizer 74841), but not for this arm in any of the other trials in which nine, 14, and seven participants were enrolled, respectively (Pfizer 82576, Pfizer 84227, Pfizer 84240). Two studies were reportedly terminated because of a slow rate of recruitment (Pfizer 84240, Pfizer 82563) and two arms in one trial were terminated prematurely because of a lack of efficacy (Dunne 2005b); results of the enrolled participants in these studies were included in our analyses (except for the 0.5 g azithromycin/600 mg chloroquine arm in Pfizer 84240). The comparator drugs for the studies by Pfizer in Africa, Indonesia, and South America were not the first‐line drug in the respective countries.
A summary of the 'Risk of bias' assessments is presented in Figure 1 and Figure 2. Of the 15 trials included in the assessment of efficacy, the sequence generation and allocation concealment was adequate and judged to be at low risk of bias in three studies, and unknown in the remaining trials. Only five studies were judged to be at low risk of bias due to adequate blinding. Blinding of laboratory staff was conducted in five open label studies, reducing the bias for the efficacy outcome; however, adverse event reporting will remain at risk of bias in these studies.Two trials were considered to be at high risk of bias due to a moderate loss of participants (> 15%), and/or unequal distribution of loss of participants between treatment arms. We considered almost all trials at risk of selective reporting for one or more of the following reasons: the outcome assessment seemed subjective; there was no baseline data provided by treatment arm; adverse events were not reported by treatment arm; prespecified outcomes were not reported; treatment arms were included that were not randomized; only men were included; interim analyses and subsequent halting of treatment arms; and halting of complete studies before the sample size had been reached. Only one study reported that the sponsor had no role in data analysis and writing of the report (Thriemer 2010).
Figure 1.
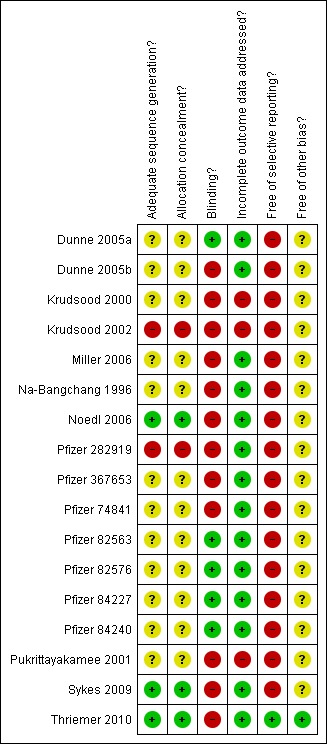
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 2.
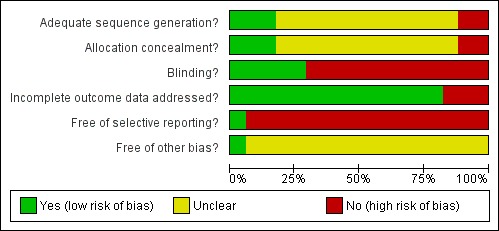
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Details of how we derived estimates of total failure from the data in each trial report can be found in Table 2. Six treatment arms used the same treatment combination and dose for azithromycin (1 g of azithromycin for three days in combination with 0.6 g of chloroquine for three days); however, only three comparison regimens were the same. An overview of the RR for comparing an azithromycin‐containing regimen with an alternative regimen can be seen in Table 3 (P. falciparum and P. vivax) and Analysis 1.1 (P. falciparum). A risk ratio (RR) greater than one indicates that the azithromycin regimen is more likely to result in treatment failure compared to the alternative regimen; a RR less than one indicates the opposite. The attained efficacy and 95% confidence interval for each arm, stratified by dose, is presented in Figure 3 and Figure 4, with PCR‐adjusted results used for P. falciparum where available. To allow comparison and for completeness, we also show the information from the excluded trials. A meta‐analysis could be conducted for the comparisons between azithromycin/chloroquine and sulphadoxine‐pyrimethamine/chloroquine, azithromycin/chloroquine and mefloquine, azithromycin/chloroquine and chloroquine, and azithromycin/artesunate and artemether/lumefantrine. For the comparison between azithromycin/chloroquine and chloroquine, the chloroquine doses used differed (0.3 g on day three versus 0.6 g on day three), and the sample sizes of the second study were very small (Dunne 2005b; Pfizer 82563); for this reason, the results of the first study and not the meta‐analysis have been presented where necessary (Dunne 2005b).
Analysis 1.1.

Comparison 1 Overview treatment failure for AZ or AZ combination vs. control for P. falciparum, Outcome 1 Treatment failure on day 28, not corrected by PCR.
Figure 3.
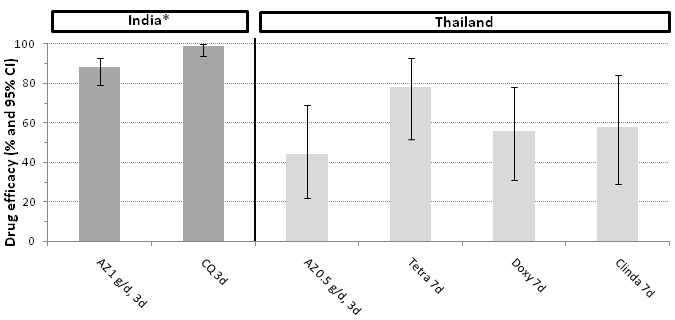
Efficacy of azithromycin and comparison drugs for P. vivax (28 days follow up)
Abbreviations: AZ: azithromycin; CQ: chloroquine; Tetra: tetracycline; Doxy: doxycycline; Clinda: clindamycin; CI: confidence interval
*Note that in the study in India, participants were treated with primaquine from day 7 until day 20 in both arms
Figure 4.

Efficacy of azithromycin containing treatment regimens for P. falciparum (28 days follow‐up)
Abbreviations: AZ: azithromycin; CQ: chloroquine; Artm: artemether; Art: artesunate; dihydroart: dihydroartemisinin; Q: quinine; CI: confidence interval; d: days
Symbols: *: PCR‐corrected; **: partially PCR‐corrected; §: study conducted in an area without malaria transmission (Bangkok). The AZ dose in the combination with artemisinin 300 mg was 500 mg at start, followed by 250 mg after 24 hours and 48 hours.
An interrupted line has been drawn at the 90% efficacy level, the minimum level for the 95% confidence interval for a potentially useful drug regimen as recommended by WHO (WHO/RBM 2006).
P. vivax
There was no difference in total treatment failure rates for a three‐day course of azithromycin monotherapy (azithromycin 0.5 g per day) compared with seven‐day monotherapy courses of tetracycline, doxycycline or clindamycin in Thailand (Figure 3). In India, a three‐day course of azithromycin (1 g per day) was significantly less efficacious than the standard first‐line chloroquine course. In the latter study, all participants were treated with primaquine from day 7 until day 20, which may partly explain the overall higher efficacy at day 28. Compared to the other antibiotics, the time to P. vivax parasite clearance was longer with azithromycin monotherapy (Table 5).
P. falciparum
The main meta‐analyses used a fixed‐effect model (except for the comparison between azithromycin/chloroquine and chloroquine) and data for participants who could be evaluated at follow‐up. Because of the limited number of comparisons that could be made, no exploration of the effect of trial methodology on the estimate of treatment effect was conducted.
Total failure by day 28
Azithromycin combination therapy at a dose of 0.5 g per day in combination with chloroquine at 0.6 g per day for three days resulted in a high level of day 28 treatment failures in a study in India (Pfizer 74841) compared with a sulphadoxine‐pyrimethamine/chloroquine combination (RR 6.10, 95% CI 2.21 to 16.87), and a combination of chloroquine and 1 g of azithromycin per day (RR 2.96, 95% CI 1.00 to 8.75). This led to the premature termination of the 0.5 g azithromycin/chloroquine arm in all Pfizer sponsored trials (Pfizer 82576, Pfizer 84227, Pfizer 84240) at that time. The results of the 0.5 g azithromycin/chloroquine arm from the other studies before termination were not available.
The combination of an azithromycin dose of ≤0.5 g per day for three days in combination with artesunate or artemether did not perform well. Krudsood et al (Krudsood 2000) report an increased risk of day 28 treatment failures when the combination of 0.5 g azithromycin and artesunate for three days is compared with mefloquine/artesunate (RR 24.00, 95% CI 3.36 to 171.27), but not when compared with three days of artesunate monotherapy (RR 0.78, 95% CI 0.54 to 1.14). Na‐Bangchang et al report an increased risk of treatment failure when comparing the combination of azithromycin at 0.5 g for three days and artemether at 300 mg at start with doxycycline for five days and artemether in a higher dose (300 mg at start and 100 mg after 12 hours) (RR 1.83, 95% CI 1.21 to 2.76) (Na‐Bangchang 1996).
Cure rates using an azithromycin dose of 0.5 mg ranged from 15% (95% CI 5% to 35%, in combination with artemether; Na‐Bangchang 1996) to 66% (95% CI 53% to 78%, in combination with chloroquine; Pfizer 74841). A higher cure rate of 86% (95% CI 42% to 99%, in combination with chloroquine) was reported among seven adult participants in Africa (Pfizer 82576).
When using azithromycin doses of 1 g per day or higher, no difference was seen in treatment failures when comparing azithromycin/quinine combinations (different dose regimens for three or five days) with a doxycycline/quinine combination for seven days (Miller 2006) or azithromycin/artesunate combinations for three days (different dose regimens) (Noedl 2006) in Thailand, but sample sizes were small (< 30 participants).
Azithromycin doses of > 1 g per day for three days (1.5 g per day in Bangladesh, and 20 mg/kg/day among children in Tanzania) in combination with artesunate were associated with increased treatment failure compared to artemether‐lumefantrine (pooled RR 3.08, 95% CI 2.09 to 4.55; Analysis 1.1) (Sykes 2009, Thriemer 2010).
Dunne et al in India showed reduced treatment failure for the combination of 1 g azithromycin daily for three days and chloroquine (the standard dose of 0.6 g on day 1 and 2, and 0.3 g on day 3) versus chloroquine alone (RR 0.04, 95% CI 0.01 to 0.18) or azithromycin alone (1 g daily for three days: RR 0.05, 95% CI 0.01 to 0.20) (Dunne 2005b). A 1 g azithromycin/chloroquine combination (1 g azithromycin and 0.6 g chloroquine daily for three days) was associated with increased treatment failure in India and Indonesia compared with the combination sulphadoxine‐pyrimethamine/chloroquine (pooled RR 2.66, 95% CI 1.25 to 5.67; Analysis 1.1) (Pfizer 74841, Pfizer 84240) and compared with the combination atovaquone‐proguanil in a multi‐site trial in Columbia and Surinam (RR 48.43, 95% CI 6.80 to 344.84) (Pfizer 84227). No increased risk on treatment failure was seen in two multi‐country studies in Africa with mefloquine (750 mg at start, followed by 500 mg after six to 10 hours) as comparator drug (pooled RR 2.02, 95% CI 0.51 to 7.98, P = 0.3; Analysis 1.1) (Pfizer 82576, Pfizer 367653).
Of the seven studies that followed participants until day 42, three studies reported their day 42 results. For one study, this was not for the evaluable study population (Pfizer 84240); the results of the other two studies are presented in Analysis 1.3. The pooled RR for the combination of azithromycin and artesunate versus artemether‐lumefantrine on day 42 was 1.90 (95% CI 1.44 to 2.49, Analysis 1.3).
Analysis 1.3.

Comparison 1 Overview treatment failure for AZ or AZ combination vs. control for P. falciparum, Outcome 3 Treatment failure on day 42, not corrected by PCR.
PCR‐adjusted failure rates by day 28
PCR‐adjusted (partially) failure rates were available for eight studies. For the two studies in Africa comparing the 1 g azithromycin/chloroquine combination with mefloquine monotherapy, there was sufficient information to repeat the meta‐analysis with PCR‐adjusted data. The pooled RR was 1.01 (95% CI 0.18 to 5.87), suggesting there was no difference in treatment failure among the two treatment regimens (Analysis 1.2; Pfizer 82576, Pfizer 367653). However, the fact that the RR decreased from 2.02 to 1.01 may indicate there were more new infections in the azithromycin/chloroquine group and that post‐treatment prophylaxis is shorter with azithromycin/chloroquine than with mefloquine. For the two studies comparing azithromycin/artesunate with artemether‐lumefantrine, the PCR‐adjusted pooled RR on day 28 was 3.63 (95% CI 2.02 to 6.52, Analysis 1.2); on day 42 it was 2.47 (95% CI 1.53 to 3.99, Analysis 1.4).
Analysis 1.2.
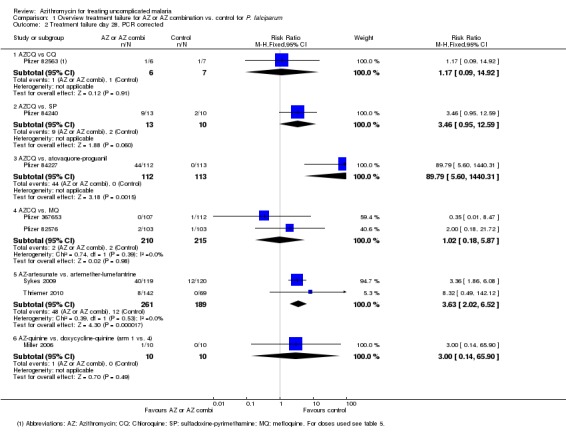
Comparison 1 Overview treatment failure for AZ or AZ combination vs. control for P. falciparum, Outcome 2 Treatment failure day 28, PCR corrected.
Analysis 1.4.
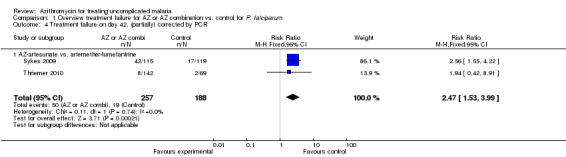
Comparison 1 Overview treatment failure for AZ or AZ combination vs. control for P. falciparum, Outcome 4 Treatment failure on day 42, (partially) corrected by PCR.
Figure 4 shows the treatment efficacy and 95% CI for each drug regimen in order of increasing azithromycin dose, using PCR‐adjusted data where available. Depending on location and combination drug, there is a tendency for improved efficacy with increasing azithromycin dose. Overall, the highest efficacy level was observed in studies in Africa with a 1.0 g azithromycin/chloroquine combination among adults. However, the only study in African children < five years so far, which used an equivalent or higher azithromycin dose in combination with artesunate, showed insufficient efficacy.
Fever and parasite clearance
Six and eight studies reported data on fever and parasite clearance, respectively, although 12 and 13 studies, respectively, mentioned that this information would be collected. The available data was insufficient for meta‐analysis and is presented in Table 4 and Table 5. Drug combinations with artesunate tended to have shorter fever and parasite clearance times compared with other combinations. Asexual parasite clearance was reported to be significantly shorter for atovaquone‐proguanil compared with the 1 g azithromycin/chloroquine combination but the underlying data was not provided (Pfizer 84227).
Gametocytes
Six studies reported gametocyte outcomes; information from one study could not be interpreted (Pfizer 84240). In India, azithromycin monotherapy for P. vivax resulted in more frequent gametocytaemia at day 7 (17 out of 95) compared with chloroquine (0 out of 99, P < 0.001, Dunne 2005a). In Thailand, Noedl et al did not see a difference in the mean gametocyte clearance time among the regimens used (azithromycin/artesunate combinations and azithromycin/quinine combinations, four arms) (Noedl 2006). Gametocyte clearance was similar between a 1 g azithromycin/chloroquine combination and mefloquine monotherapy until day 28 in a study in Africa. On and beyond day 28, mefloquine showed a slightly higher gametocyte clearance rate than 1 g azithromycin/chloroquine (Pfizer 82576); unfortunately, the underlying data was not provided. No significant difference was seen in gametocyte clearance between 1 g azithromycin/chloroquine and atovaquone‐proguanil in South America, although lower gametocytaemia clearance was reported with atovaquone‐proguanil from week 3 onwards (Pfizer 84227). In the study among children and adults in Bangladesh, no difference in median gametocyte clearance was found between the azithromycin‐artesunate and artemether‐lumefantrine regimens (46.1 hours versus 97.2 hours, among 128 and 65 participants respectively; no interquartile range available) (Thriemer 2010).
Morbidity other than clinical malaria (eg diarrhoea, respiratory tract infections) up to 28 days
There was not enough systematic information in the trials to assess this secondary outcome. One trial reported a higher occurrence of upper respiratory tract infections among participants in the control group using artemether‐lumefantrine compared to the azithromycin‐artesunate arm (9.5% among 65 participants versus 2.3% among 128 participants respectively, P = 0.06, Thriemer 2010). Diarrhoea is discussed under specific adverse events.
Adverse events (All studies)
Details of the safety reporting methods used and the inclusion and exclusion criteria are presented in the Characteristics of included studies table. Entry criteria were rigorous for some studies, limiting the amount of adverse effects that can be expected. Ten studies excluded subjects with an allergy to study drugs, ten studies excluded persons with abnormal laboratory tests, and seven studies excluded persons with severe vomiting. As reported before, pregnant women were excluded in all studies. Three studies did not provide any baseline information. For the other studies, the presented baseline information was comparable between arms, except for the open label arm of 1 g azithromycin/chloroquine in the early study in India (Dunne 2005b), where a lower baseline parasite density was reported. Four studies did not report the procedures used to monitor adverse events. In four studies, the denominator of the study population for the evaluation of adverse events was not reported. Six studies used placebos.
Adverse events overall
The number of persons with any adverse events are presented in Table 6, and reported in terms of 'treatment related' (TR) and 'any cause' (AC) adverse events where this information was provided. TR adverse events are events which in the opinion of the investigators (of the trial) are likely, probably, or possibly related to the intervention the participant received. A participant can have more than one adverse event, and the last columns indicate the total number of adverse events which were reported by arm; the denominator here is the number of subjects, with the average number of adverse events per person in brackets.
No clear difference in frequency of adverse events was detected in studies involving P. vivax (Table 6); in one study the frequency of adverse events tended to be lower in the azithromycin arm (Dunne 2005a), whereas in the other study they tended to be higher (Pukrittayakamee 2001).
The open label arm of the 1 g azithromycin/chloroquine combination in the early study in India reported less adverse events compared to the (blinded) chloroquine (RR 0.37, 95% CI 0.19 to 0.70) or azithromycin (RR 0.41, 95% CI 0.21 to 0.82) monotherapy arms (Dunne 2005b). The azithromycin/artesunate combinations had less TR adverse events compared to the azithromycin/quinine combinations in Thailand (Noedl 2006); the azithromycin/artemether combination had less AC adverse events compared to doxycycline/artemether in the same country (Na‐Bangchang 1996). No difference was seen in adverse events when the 1 g azithromycin/ chloroquine combination was compared with sulphadoxine‐pyrimethamine/chloroquine; however, the information on adverse events in studies using sulphadoxine‐pyrimethamine/chloroquine was very limited (Pfizer 74841, Pfizer 84240). More adverse events were reported in the 1 g azithromycin/chloroquine combination arm compared with mefloquine (RR 1.26, 95% CI 1.06 to 1.50; Pfizer 82576) or atovaquone‐proguanil (RR 1.41, 95% CI 1.09 to 1.83; Pfizer 84227) in placebo‐controlled studies. This was not seen in the open‐label study comparing 1 g azithromycin/chloroquine to mefloquine (RR 1.14, 95% CI 0.95 to 1.37; Pfizer 367653). The pooled RR for TR adverse events when comparing the 1 g azithromycin/chloroquine combination with mefloquine was 1.20, 95% CI 1.06 to 1.36 (Analysis 2.1, Pfizer 82576, Pfizer 367653). No difference in adverse events was detected in the studies comparing azithromycin/artesunate versus artemether‐lumefantrine.
Serious adverse events and discontinuations
Serious adverse events and discontinuations are presented in Table 7, with a distinction between TR adverse events and AC adverse events, where this information was provided. We also included any other adverse events reported for laboratory tests or physical examination. Types of serious adverse events are presented in the last column. There were no deaths, and no significant differences between regimens for serious adverse events and discontinuations. Some laboratory abnormalities were reported, but these all seemed transient.
Specific adverse events
Where this information was available, we summarized findings for specific adverse events in Table 8. Because different doses of azithromycin were used over the different trials, we assessed the occurrence of a dose‐response relationship for azithromycin. We were able to explore this for nausea (Figure 5), vomiting (Figure 6), diarrhoea (Figure 7), and pruritis (Figure 8). A higher prevalence of nausea (33.0% or 33/100) was reported among participants using 2 g azithromycin/chloroquine (open label study in Columbia/India) compared to participants using 1 g azithromycin/chloroquine (9.6% or 11/114 in placebo controlled trial, RR 3.1, 95% CI 1.7 to 5.8, and 11.5% or 13/113 in open label trial, RR 2.6, 95% CI 1.5 to 4.7, comparing 2 g versus 1 g azithromycin dose). No consistent difference was detected for the other adverse events examined. Although Figure 5 suggested there might be an association between azithromycin dose and nausea, this did not seem to translate into vomiting (Figure 6). No dose‐response relationship was apparent for diarrhoea (Figure 7), and pruritis was mainly reported in studies in Africa and South America (Figure 8).
Figure 5.

Adverse event of nausea in study arms with a sample size of 50 or more.
Abbreviations: AZ: azithromycin; CQ: chloroquine; S Am: South America; Col: Colombia; MQ: mefloquine; SP: sulphadoxine‐pyrimethamine; AT/PG: atovaquone/proguanil; Art: artesunate
No information available on nausea in study using 0.5 g AZ in India, in one of the 1 g AZ studies in India, and in the 1 g AZ study in South America, or in the study using SP/CQ in India, and AT/PG in South America.
* P < 0.05 comparing the AZ 2 g study with the AZ 1 g studies
Figure 6.
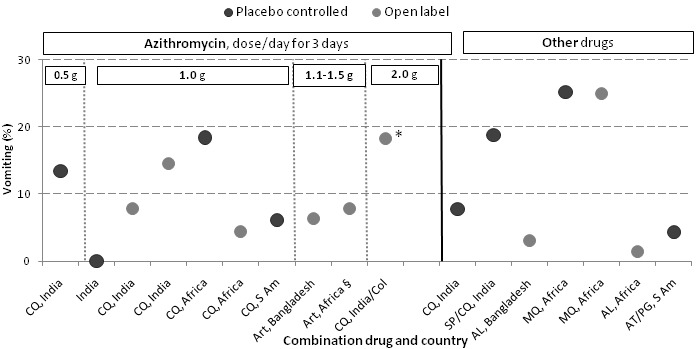
Adverse event of vomiting in study arms with a sample size of 50 or more.
Abbreviations: AZ: azithromycin; CQ: chloroquine; S Am: South America; Col: Colombia; MQ: mefloquine; SP: sulphadoxine‐pyrimethamine; AT/PG: atovaquone/proguanil; Art: artesunate
* P < 0.05 compared to AZ 1 g placebo‐controlled study in South America and AZ 1 gram open‐label study in Africa
Figure 7.
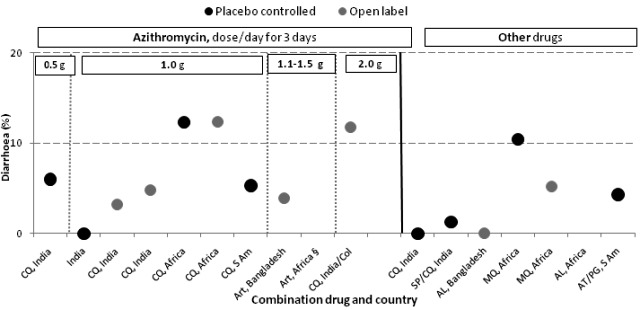
Adverse event of diarrhoea in study arms with a sample size of 50 or more.
Abbreviations: AZ: azithromycin; CQ: chloroquine; S Am: South America; Col: Colombia; MQ: mefloquine; SP: sulphadoxine‐pyrimethamine; AT/PG: atovaquone/proguanil; Art: artesunate
Figure 8.

Adverse event of pruritis in study arms with a sample size of 50 or more.
Abbreviations: AZ: azithromycin; CQ: chloroquine; S Am: South America; Col: Colombia; MQ: mefloquine; SP: sulphadoxine‐pyrimethamine; AT/PG: atovaquone/proguanil; Art: artesunate
* P < 0.05 compared to all other studies
Discussion
This review covers azithromycin efficacy and safety data for uncomplicated malaria among participants from controlled trials across malaria endemic regions in Africa, South America, and Asia conducted over a 14‐year period. Overall, the reviewed studies show the evidence is scattered, variable in quality, and with a considerable risk of bias; additionally, there are substantial gaps in knowledge.
For P. vivax there is simply insufficient data, with only two studies available. From the evidence provided, azithromycin is inadequate as a monotherapy for P. vivax using a dose of 0.5 to1 g per day for three days, and, like other antimalarial antibiotics, has no apparent effect on the P. vivax hypnozoites.
For uncomplicated P. falciparum malaria, the evidence covered a wide variety of dose regimens, partner drugs, and comparators, although the varied reporting quality and low adherence to the widely recommended CONSORT guidelines for the reporting of clinical trials complicated assessment of the evidence (Hopewell 2008). The available data suggest that azithromycin has weak dose‐dependent antimalarial activity and is not efficacious as a monotherapy. The current evidence does not support firm conclusions about the use of azithromycin as part of combination therapy for treating uncomplicated P. falciparum. The wide variety of involved treatment arms limited meta‐analyses, but individual trial results showed that the efficacy of a combination of 1 g azithromycin/chloroquine for three days was significantly lower when compared with sulphadoxine‐pyrimethamine/chloroquine in Asian adults or atovaquone‐proguanil in South American adults, and equivalent when compared to mefloquine in African adults. Compared with artemether‐lumefantrine, one of the WHO recommended ACTs (WHO/RBM 2006), a higher dose of azithromycin (1.1 to 1.5 g/day for three days) in combination with artesunate was inadequate among African children and inferior among adults in Bangladesh. Only studies of the azithromycin/chloroquine combination among adults in Africa (Pfizer 367653) and one study in India/Colombia had an efficacy level > 95% and a lower 95% CI limit of 90% or above, which is the recommended cure rate for a new antimalarial medicine to be considered for policy (Figure 3, WHO/RBM 2006). For the azithromycin/chloroquine combination, 2 g azithromycin per day for three days may be needed to reach universal cure rates in excess of 90%, but this may not be as well tolerated as 1 g/day.
There were no differences in serious adverse events among the regimens used. There was no difference in adverse events overall when comparing azithromycin/chloroquine with sulphadoxine‐pyrimethamine/chloroquine (Asia), but there were significantly more adverse events when comparing azithromycin/chloroquine with mefloquine (Africa) or atovaquone/proguanil (South America). Higher doses of azithromycin may result in nausea, but not necessarily vomiting (Figure 5 and Figure 6). However, a note of caution should be mentioned, because different studies have different systems and rigor regarding the detection of adverse events and so comparing adverse events between studies is not as reliable as comparing adverse events between different arms of the same study.
Besides the standard challenges to meta‐analysis in Cochrane reviews related to variations in definitions, endpoints and study design between reviewed studies, two issues deserve specific mention. Over the past few years the assessment of efficacy of antimalarials with longer half‐lives has moved towards the use of day 42 PCR‐adjusted findings as the primary endpoint of interest (Stepniewska 2004). PCR‐adjustment is particularly important in areas of high malaria transmission, where on the one hand a high rate of re‐infection may mask successful clearance of the original infection, while on the other a higher background immunity of older children or adult participants may complement the drug treatment and may mask a low‐grade level of drug resistance (White 2002). Of the 13 randomized trials for P. falciparum, three were conducted in an area without malaria transmission (Bangkok, Thailand), while (partial) PCR adjustment for day 28 was available for eight trials, including trials in Africa that were presumably conducted in areas of higher transmission. Although seven studies reportedly followed patients until day 42, those outcomes were not presented in the publications for five of these studies.The half life of azithromycin (approximately 60 hours or 2.5 days) suggests that treatment failures are likely to occur by day 28, but the day 28 PCR‐adjusted rates may underestimate the true failure rates. The RR for the azithromycin‐combination compared with a comparator drug may also depend on the pharmacokinetic qualities of the comparator drug, eg if this is a short‐acting or a long‐acting drug, or a drug with a post‐treatment prophylactic effect.
This review is also defined by the fact that almost half of the reviewed trial information originated from publicly‐available trial synopses prepared by the manufacturer Pfizer. The company has made a commendable effort to provide public access to their unpublished research findings on azithromycin, allowing us to include the majority of relevant azithromycin treatment trials in this review. Although these synopses are limited in the amount of detail and did not go through the standard peer review process, they make up a substantial proportion of the available 'azithromycin for malaria' literature. The reported methodology sections in the manuscripts and trial synopses frequently lacked the required level of detail to assess the quality of the trials. Pfizer and authors of non‐Pfizer‐related articles were contacted for additions, clarification and verification of the quality, methods and data where needed, which was partially obtained.
One study, conducted between 1998 and 2001, needs specific mention from a methodological perspective. The promising results of this study for the 1 g/day azithromycin dose in combination with chloroquine (Dunne 2005b) likely guided Pfizer’s subsequent efforts to assess the antimalarial properties of azithromycin, but we had serious quality concerns: the azithromycin/chloroquine combination arm was not blind and was not conducted concurrently with the other arms. This suggests that randomization may not have occurred for this last arm, and technically this arm could be excluded from this review. Results may indeed have been somewhat biased, as the enrolment parasitaemia of the last arm was lower compared to the other arms and enrolment parasite density is a well‐known determinant of treatment outcome (Stepniewska 2004). In addition, the outcome assessment appeared subjective ("Any participant who, in the opinion of the investigator, had not sufficiently improved received additional antimalarial therapy"). This may have contributed to the higher efficacy level observed in the initial study in India compared to the later study in the same country.
The variation in assessed azithromycin doses and treatment arms for the azithromycin‐chloroquine combinations that followed the initial Indian study in part depicts the product development and dose‐optimisation process Pfizer undertook to adapt a widely used antimicrobial for an antimalarial application. Unfortunately, although there are guidelines for assessing drug efficacy, there are no clear guidelines for the development and field assessment of optimal antimalarial doses and dose regimens for programmatic conditions, taking into account efficacy and safety as well as costs, user‐friendliness, and long‐term prospects, particularly the potential for drug resistance (Barnes 2008). It is increasingly being recognized that new antimalarial candidates may not achieve their full potential in terms of impact and useful life‐span because of a lack of attention to dose‐optimization, and a tendency to select, register and market doses at the lower end of the therapeutic range, which before long fuel the development of resistance.
The assessed azithromycin doses, observed dose‐dependency, and regional variation in efficacy illustrate this issue (Figure 2). Sub‐optimal results were obtained among adults in India, Colombia, Surinam, and Indonesia when a 1 g azithromycin/chloroquine combination was used; however, this same dose and combination performed well in Africa, where a higher level of pre‐existing antimalarial immunity in adult study participants may have contributed to high clearance rates. A relatively high dose of 1.2 g azithromycin per day in combination with artesunate was insufficient for African children, confirming the likely role of pre‐existing immunity in the observed difference in parasite clearance. The level of background chloroquine resistance in the study area will also have contributed to the difference in the azithromycin/chloroquine combination; in areas with a high level of chloroquine resistance, the combination is effectively reduced to an azithromycin monotherapy. In addition, the effect of dose may depend on a patient's weight, with higher treatment failure rates reported in heavier subjects in two studies (Pfizer 74841, Pfizer 82576). A recent single arm study using a high dose of azithromycin (2 g/day for three days) in combination with chloroquine did show a higher efficacy in a multi‐site study in adults in India and Columbia (Pfizer 282919), suggesting that a 2 g azithromycin dose may be needed to achieve universal high efficacy. This higher azithromycin dose seemed less well tolerated, however, and was associated with an increased risk of nausea, but not vomiting or diarrhoea (Figures 3 to 5). The reported incidence of nausea and vomiting with 2 g azithromycin/chloroquine (30% and 18%, respectively) was higher than in a study using a single 2 g dose of azithromycin for community‐based pneumonia or acute sinusitis among adults (4% and 1%, respectively) (Pfizer 2008). Apart from differences in monitoring, it is possible that the duration of therapy and the malarial illness may have contributed to the higher occurrence of these adverse events.
A cumulative dose of 3 to 6 g of azithromycin is relatively high compared with other applications of azithromycin. Lower doses are used for bacterial infections (eg 500 mg per day for three days) and single treatments of azithromycin with 1 to 2 g have been used successfully for treating otitis media among children (a dose of 30 mg/kg once up to a maximum of 2 g), community‐acquired pneumonia among adults (one single dose of 2 g), acute bacterial sinusitis among adults (one single dose of 2 g), and sexually‐transmitted diseases (one single dose of 1 g) (Pfizer 2008). In bacteria, macrolides such as azithromycin and erythromycin bind to the 50S ribosomal subunit and inhibit protein synthesis, causing premature detachment of incomplete peptide chains and subsequent cell death (Retsema 2001). In bacterial infections, azithromycin works effectively because it concentrates in tissues and white blood cells; neutrophils can become vehicles for drug delivery at a site of infection and a higher dose can enhance drug efficacy (Blumer 2005; Retsema 2001). For malaria, higher and longer treatment doses are needed because of a different mechanism of action: azithromycin affects the 'house keeping' function of the apicoplast in the progeny, causing 'delayed death' (Dahl 2008). The apicoplast is an organelle in the plasmodium; its function is currently unclear , but it is thought to have an origin in prokaryotes. Although malaria parasites directly exposed to azithromycin do not seem to be functionally affected, and can go on to invade an erythrocyte and develop into a schizont, these schizonts are unable to form functional merozoites and will subsequently die (Dahl 2008). A higher efficacy of azithromycin can be expected when at least two asexual cycles (approximately 96 hours) have occurred (Biswas 2001), which might translate into a four‐day course of azithromycin. Given the relatively slow and weak antimalarial action of azithromycin, the use of this drug for case‐management would need to rely heavily on combination with a fast‐acting efficacious antimalarial such as a member of the artemisinin group (Noedl 2001).
In vitro studies have produced conflicting results for interactions of azithromycin combinations: Edgie‐Mark et al (2009) reported indifference between azithromycin and chloroquine, whereas others indicated that there may be an additive or synergistic effect of the combination (Edgie‐Mark 2009; Nakornchai 2006; Noedl 2007; Ohrt 2002 ). But the level of azithromycin‐chloroquine interaction may depend on the background resistance level of the P. falciparum strain to chloroquine (Ohrt 2002). For the azithromycin‐artesunate combination, antagonism has been reported (Nakornchai 2006), whereas an additive to synergistic interaction was reported for azithromycin‐dihydroartemisinin by Noedl et al (2007) and an additive with a trend for antagonism was reported by Ohrt et al (2002) (Noedl 2007; Ohrt 2002). An additive effect has been reported for azithromycin‐mefloquine (Nakornchai 2006), and an additive to synergistic effect for azithromycin‐quinine, azithromycin‐pyroniradine, azithromycin‐tafenoquine, and azithromycin‐primaquine (Nakornchai 2006; Noedl 2007; Ohrt 2002). Thus far, azithromycin has mainly been combined with chloroquine. A few studies in Thailand assessed other azithromycin combinations for P. falciparum, but, except for the study by Krudsood and colleagues, these had relatively small sample sizes (30 participants or less per arm) (Krudsood 2000; Miller 2006; Na‐Bangchang 1996; Noedl 2006 ). The studies where azithromycin was combined with artesunate did not show promising results compared with artemether‐lumefantrine.
Given the weak antimalarial properties and low prospects as a competitor drug for the current range of ACTs, the remaining interest in an azithromycin combination seems mainly based on its appealing safety profile. This would allow two potential niche applications among pregnant women and children: intermittent preventive treatment (IPT) and use as a potential syndromic treatment, eg for malaria and respiratory tract infections in children and malaria and sexually‐transmitted diseases in pregnant women (Chico 2008). Findings in this review were mainly based on adult males and care should be taken when extrapolating efficacy and tolerability findings from adults to a paediatric or pregnant population, whose different pharmacokinetic profiles may require different doses. However, the two studies which included children and compared an azithromycin/artesunate combination with an azithromycin dose of > 1 g per day with artemether‐lumefantrine showed that the azithromycin/artesunate combination was three times more likely to result in failure (Analysis 1.1). Two more paediatric studies are in analysis or ongoing in African countries (Pfizer 677833: Burkina Faso, Cote d'Ivoire, Ghana, Kenya, Mali, Zambia, a comparison of fixed‐dose azithromycin/chloroquine versus artemether‐lumefantrine; NIAID 379821: Malawi, comparison of azithromycin/chloroquine versus chloroquine alone, chloroquine and artesunate, and chloroquine and malarone), assessing azithromycin doses ranging from 20 to 30 mg/kg/day for three days. This is equivalent to 1.2 to 1.8 g in a person of 60 kg. Findings from these studies will be included in a future update of this review.
A small study in Malawi evaluated the usefulness of sulphadoxine‐pyrimethamine in combination with 1 g azithromycin per day for two days for treating uncomplicated malaria in pregnancy (Kalilani 2007). This combination resulted in fewer recrudescent episodes when compared to sulphadoxine‐pyrimethamine monotherapy, but the authors reported one abortion that was possibly related to treatment, four days after exposure (Kalilani 2007). Azithromycin monotherapy has been evaluated for malaria prophylaxis among healthy, non‐pregnant, semi‐immune adults in doses ranging from 250 mg daily to 1000 mg weekly. The reported prophylactic efficacy ranged from 98% to99% for P. vivax in two studies with a total of 327 participants (Heppner 2005; Taylor 1999), and 64% to 83% for P. falciparum in three studies with a total of 444 participants (Andersen 1998; Heppner 2005; Taylor 1999). For P. vivax, the results were better than the treatment results of azithromycin monotherapy for clinical malaria. When considering the drug for IPT in pregnancy, azithromycin is not optimal given the need for a longer duration of treatment. This may cause substantial challenges for adherence, driving resistance. In addition, the combination of azithromycin with chloroquine is not ideal for IPT, given the efforts by countries to remove chloroquine from the national shelves in favour of ACTs (Sipilanyambe 2008; WHO 2008), and the problems with adherence and adverse events such as pruritis, which are well‐described in the African region (Taylor 2004). As resistance to sulphadoxine‐pyrimethamine is increasing and the effective lifespan of IPT with sulphadoxine‐pyrimethamine may be coming to an end (Ter Kuile 2007), plans are underway to test a 1 g azithromycin/chloroquine combination as a fixed‐dose option for IPTp (IPT for pregnant women) in Africa (Dr. Chandra, personal communication, Chico 2008). Given the above dose‐dependent data in non‐pregnant adults, and the challenges to successful implementation, this may not be a potential niche product just yet.
Authors' conclusions
The available evidence suggests that azithromycin has relatively weak antimalarial activity.
Given the availability of highly efficacious and cheaper first and second line antimalarials, there does not seem to be a place for wide‐scale programmatic use of the assessed azithromycin‐chloroquine combinations and the azithromycin‐artesunate combination for case‐management of uncomplicated P. falciparum malaria (WHO/RBM 2006); there is insufficient data available on the use of azithromycin with other partner drugs.
The available evidence is very limited. The main reasons for this are the small number of studies using the same treatment combination, azithromycin dose and comparison group; uncertainty over trial methodological quality; the sub‐optimal results reported so far, mainly with chloroquine; and the availability of superior alternatives in the form of ACTs. In addition, the current cost of azithromycin would be a barrier as well: a treatment course of 1 g azithromycin/0.6 g chloroquine for three days was estimated at $7 (Chico 2008).
While the current evidence is insufficient for policy makers, the fact that azithromycin has a different mechanism of action to other antimalarials, together with its safety profile, may offer treatment options for pregnant women and children; for example, there may be opportunities to use it for a syndromic approach that could be explored further.
We also understand that Pfizer is working on optimizing the drug formulation in order to overcome some of the described challenges. Unless those efforts are fruitful, however, the current move towards a ‘low’ 1 g dose azithromycin/chloroquine fixed‐dose product focusing on IPTp may be somewhat impetuous. Furthermore, the need for a treatment course lasting several days (and the potential for pruritis when combined with chloroquine) poses a considerable challenge for wide‐scale use as IPTp.
Given the limited data, and the potential further improvements in drug formulation, it remains important to explore further the use of azithromycin in combination with fast‐acting drugs with different mechanisms of action for case management, or in combination with other medium/long‐acting drugs for IPT, or at higher daily doses. For case management, exploration of a twice daily versus once daily regimen may be worthwhile to assess if such a regimen would reduce nausea when used in combination with other antimalarials.
In conclusion, azithromycin is a weak antimalarial with some appealing characteristics. Unless it overcomes some of the highlighted challenges and finds a specific niche that is complementary to the current scala of more efficacious antimalarial combinations, its future as an antimalarial does not look promising.
Acknowledgements
A M van Eijk was awarded a Reviews for Africa Programme Fellowship (www.mrc.ac.za/cochrane/rap.htm), funded by a grant from The Nuffield Commonwealth Programme, through The Nuffield Foundation. D J Terlouw received financial support from the Centers for Disease Control. The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development for the benefit of developing countries.
We are grateful to Dr R Chandra from Pfizer for additional information on Pfizer‐led trials, and to Dr Noedl and Dr Thriemer for their kind assistance in providing additional information. We thank Dr Gertrude Kalanda for her help in verifying trial information, and Professor F ter Kuile for his review of the manuscript.
Appendices
Appendix 1. Detailed search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | mRCT |
| 1 | azithromycin | azithromycin | azithromycin | azithromycin | azithromycin | azithromycin |
| 2 | azithromycin | azithromycin | azithromycin | ERYTHROMYCIN | azithromycin | malaria |
| 3 | 1 or 2 | ERYTHROMYCIN/ANALOGS AND DERIVATIVES | ERYTHROMYCIN/ANALOGS AND DERIVATIVES | 1 or 2 | 1 or 2 | 1 and 2 |
| 4 | malaria | 1 or 2 or 3 | 1 or 2 or 3 | malaria | malaria | ‐ |
| 5 | 3 and 4 | malaria | malaria | 3 and 4 | 3 and 4 | ‐ |
| 6 | ‐ | 4 and 5 | 4 and 5 | Limit 5 to human | ‐ | ‐ |
Footnotes
a Cochrane Infectious Diseases Group Specialized Register
b Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006); upper case: MeSH or EMTREE heading; lower case: free text term
Appendix 2. Total failure (TF): method used to derive TF values from reported data
| Trial Country | Treatment regimen | Randomized | TF | Data to derive TF | Definitions of outcomes used in studies |
| P. vivax | |||||
| Dunne 2005a India |
Azithromycin 1 g/day for 3 days Primaquine from day 7 to 20 |
98 | 12/97 | 85/97 parasitological success (clinical response 86/97) | No systematic evaluation of outcome. Parasitological failure defined as RI (recrudescence after clearance), RII (reduction in parasitaemia by > 75% of baseline without clearance, RIII (failure to reduce parasitaemia to < 25% of baseline) but no clear clinical consequence to parasitological failure (eg RII failure at day 7). “Patients who the investigator felt were not responding to therapy were given alternative therapies at their discretion.” Clinical response: Resolution of fever, without relapse at day 28. |
| Chloroquine 0.6 g on day 0 to 1, 0.3 g on day 2 Primaquine from day 7 to 20 |
102 | 1/102 | 101 parasitological success (clinical response 101/102) | ||
| Pukrittayakamee 2001 Thailand |
Azithromycin 0.5 g/day for 3 days | 20 | 10/18 |
P. vivax cure: 8/18 Note: P. falciparum 6/18 |
Treatment failure: persistence of fever and parasitaemia for > 7 days or persistence of parasitaemia in the absence of fever for > 2 weeks. Reappearance of infection after clearance within 28 days. |
| Tetracycline 250 mg 4x/day for 7 days | 27 | 4/18 | P. vivax cure: 14/18 | ||
| Doxycycline 200 mg/day for 7 days | 25 | 8/18 | P. vivax cure: 10/18 | ||
| Clindamycin 300 mg 4x/day for 7 days | 20 | 5/12 | P. vivax cure: 7/12 | ||
| P. falciparum | |||||
| Dunne 2005b India |
Azithromycin 1 g/day for 3 days | 16 | 9/14 | 9 had RI (3), RII (3) or RIII (3) failures (5/15 clinical cure) | No systematic evaluation of outcome. Treatment failure defined in terms of RI (recrudescence after clearance of parasitaemia), RII (reduction in parasitaemia to < 25% of baseline levels without clearance), and RIII (failure to reduce parasitaemia to < 25% of baseline levels), but time of evaluation and clinical consequences for type of resistance level not reported. Clinical response not defined (but one of the main outcomes). Term 'Eradication' used in table, not defined. “Any participant who, in the opinion of the investigator, had not sufficiently improved received additional antimalarial therapy.” |
| Chloroquine 0.6 g on day 0 to 1, 0.3 g on day 2 | 16 | 11/15 | 11 had RI (9), RII (1) or RIII (1) failures (clinical cure 4/15) | ||
| Azithromycin 1 g/day for 3 days, Chloroquine 0.6 g on day 0 to 1, 0.3 g on day 2 | 64 | 2/63 | 2 had RII (1) or RIII (1) failures (clinical cure 61/63) | ||
| Krudsood 2000 Thailand |
Azithromycin 0.5 g/day, Artesunate 200 mg/day for 3 days | 67 | 24/55 | 24 RI response, 31 cure | 28‐day cure rate used. Sensitive: no reappearance of asexual forms of P. falciparum within 28 day follow‐up. Resistance: Resistance at level I (reappearance after day 7), II (parasitaemia at day 7), and III (failure to reduce parasitaemia by > 75% of initial count or clinical deterioration after 48 hours). |
| Artesunate 200 mg/day for 3 days and mefloquine 10 mg/kg on day 0 to 1, 5 mg/kg on day 2 | 67 | 1/55 | 1 RI response, 54 cure | ||
| Artesunate 200 mg/day for 3 days | 68 | 34/61 | 34 RI response, 27 cure | ||
| Miller 2006 Thailand |
Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 3 days | 10 | 1/10 | 1 R1 response, 9 cure | Cure: clearance of P. falciparum parasitaemia without recrudescence of malaria in a 28‐day period. |
| Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 5 days | 20 | 0/20 | All cure | ||
| Azithromycin 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | 20 | 0/20 | All cure. Note: this arm was not randomized | ||
| Doxycycline 100 mg 2x/day for 7 days and quinine 10 mg/kg 3x/day for 7 days | 10 | 0/10 | All cure | ||
| Na‐Bangchang 1996 Thailand |
Azithromycin 0.5 g at start, 0.25 g at 24 and 48 hours, and artemether 300 mg at start | 30 | 23/27 | 4/27 were sensitive, 23 RI response | Rate of treatment failure (RI‐RIII, WHO 1973). |
| Doxycycline 100 mg 2x/day for 5 days and artemether 300 mg at start and 100 mg after 12 hrs | 30 | 14/30 | 16/30 sensitive, 14 RI response | ||
| Noedl 2006 Thailand |
Azithromycin 0.75 g 2x/day and artesunate 100 mg 2x/day for 3 days | 27 | 2/25 | 23/25 cure. 2 RI | 28 day cure. Treatment failure divided in RI: clearance of asexual parasites within 7 days after start treatment, followed by recrudescence; RII: decrease in parasite count < 25% of baseline value within 48 hours but failure to clear parasites by day 7; RIII: failure of the parasite count to decrease to < 25% of the baseline value within 48 hours (WHO 1973). |
| Azithromycin 1 g/day and artesunate 200 mg/day for 3 days | 27 | 3/27 | 24/27 cure, 3 RI | ||
| Azithromycin 0.75 g 2x/day and quinine 10 mg/kg 2x/day for 3 days | 16 | 4/15 | 11/15 cure, 1 RI and 3 RIII | ||
| Azithromycin 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | 27 | 2/25 | 23/25 cure, 2 RI | ||
| Pfizer 74841 India |
Azithromycin 1 g/day and chloroquine 600 mg/day for 3 days | 83 | 12/73 | 61 eradicated | Outcome: Asexual parasite clearance rate at day 28. No further definition given. Term 'eradication' used in table, not defined. |
| Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | 67 | 20/59 | 39 eradicated | ||
| Sulfadoxine‐pyrimethamine 1500mg/75 mg at start, chloroquine 0.6 g on day 0 to 1, 0.3 g on day 2 | 79 | 4/72 | 68 eradicated | ||
| Pfizer 82563 Kenya |
Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | 7 | 1/5 | 1 RIII failure which cleared by day 7 (Eradication: 5/5) |
Eradication: Clearance of asexual parasitaemia within 7 days of initiation of treatment, without subsequent recurrence through day 28. Failure: RI: clearance of asexual parasitaemia before day 7, followed by recurrence on or after day 7. RII: marked reduction (< 25% of baseline) of asexual parasitaemia but no clearance prior to and up to day 7. RIII: no marked reduction ( > 25% of baseline) of parasitaemia within 48 hours. If a subject remained parasitaemic on day 7, curative treatment was provided. Terms of eradication and failure not mutually exclusive for RIII resistance: we used failure (RI‐RIII) for consistency with other studies. |
| Chloroquine 0.6 g/day for 3 days | 7 | 2/7 | 1 RIII failure which cleared by day 7, and one RI failure (the other RI failure mentioned was the untreated RIII failure) (Eradication: 5/7) PCR adjusted: 1/7 |
||
| Pfizer 82576 Ghana, Kenya, Mali, Uganda, Zambia |
Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | 114 | 5/103 | 5 late treatment failures (4.85%) PCR adjusted: 2/103 |
Primary outcome table presumably PCR‐adjusted; data for PCR‐unadjusted results on day 28 not clearly presented. Percent early and late treatment failure used (assuming the investigators follow WHO 2003) but this may not be reliable because the trial ended at day 42. Two RIII failures in 1 g azithromycin/chloroquine arm reported, but not labelled as early treatment failures. |
| Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | 9 | NR | |||
| Mefloquine 750 mg at start, 500 mg after 6 hours | 115 | 2/103 | 1 early treatment failure and 1 late treatment failure (1.94%) PCR adjusted: 1/103 |
||
| Pfizer 84227 Columbia/Surinam |
Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | 114 | 48/112 | 64 eradicated PCR partially adjusted: 68/112 (35 analysed) |
Primary endpoint asexual parasite clearance rate at day 28, not further defined. “Treatment when persistent or recurrent parasitaemia”, but time point of evaluation for treatment not defined. Used 'eradicated': asexual parasite clearance rate on day 28 among evaluable subjects. |
| Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | 14 | NR | |||
| Atovaquone 1000 mg/day and proguanil 400 mg/day for 3 days | 116 | 1/113 | 112 eradicated PCR adjusted: 113/113 |
||
| Pfizer 84240 Indonesia |
Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | 13 | 9/13 | 4 eradicated PCR partially adjusted 4/13 eradicated (5 analysed) |
Eradication: Clearance of asexual Pf parasitaemia within 7 days of initiation of treatment, without subsequent recurrence through day 28. Failure: failure to achieve clearance of Pf asexual parasitaemia within 7 days of initiation of treatment or subsequent recurrence through day 28 after achieving clearance. Subjects with persistent or recurrent parasitaemia during the follow up period were treated with antimalarial drugs and were withdrawn from further participation in the study. |
| Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | 7 | NR | |||
| Sulfadoxine‐Pyrimethamine 1500mg/75 mg at start, chloroquine 0.6 g on day 0 to 1, 0.3 g on day 2 | 12 | 3/10 | 7 eradicated PCR adjusted: 8/10 |
||
| Pfizer 367653 Burkina Faso, Ghana, Kenya, Mali, Senegal, Zambia |
Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | 113 | 1/107 | As per correspondence with Pfizer PCR adjusted: 0/107 |
Eradicated: clearance of asexual P. falciparum parasitaemia within 7 days of initiation of treatment, without subsequent recrudescence through day 28. Failure: Failure to achieve clearance of asexual P. falciparum parasitaemia within 7 days of initiation of treatment or subsequent recrudescence through day 28 after achieving initial clearance. |
| Mefloquine 750 mg at start, 500 mg after 6 to 10 hours | 116 | 1/111 | As per correspondence with Pfizer PCR adjusted: 1/111 |
||
| Sykes 2009 Tanzania |
Azithromycine 20 mg/kg/day and artesunate 4 mg/kg/day for 3 days | 129 | 69/119 | Day 28 partially PCR‐adjusted: 40/119 (6 not tested) Day 42:TF 76/115, partially PCR‐adjusted: 42/115 (5 not tested) |
Parasitological failure: the presence of asexual malaria parasites after treatment (after day 3) on or before day 28, irrespective of symptoms. |
| Artemether 20 mg and lumefantrine 120 mg 2x/day for < 15 kg and 40 mg/240 mg 2x/day for ≥15 kg for 3 days | 132 | 24/120 | Day 28 partially PCR‐adjusted: 12/120 (3 not tested) Day 42: TF 41/119, partially PCR adjusted: 17/119 (4 not tested) |
||
| Thriemer 2010 Bangladesh |
Azithromycin 1.5 g/day and artesunate 200 mg/day for 3 days. Children < 35 kg: azithromycin 30 mg/kg/day, and artesunate 4 mg/kg/day for 3 days | 152 | 13/142 | Day 28: PCR‐adjusted: 8/142 Day 42: TF 22/142, PCR‐adjusted: 8/142 |
Primary endpoint was cure: adequate clinical and parasitological response as defined by WHO criteria by day 42. Day 28 data kindly provided by the authors. |
| Artemether 80 mg and lumefantrine 480 mg 2x/day for 3 days. Children < 35 kg: artemether 4 mg/kg and lumefantrine 24 mg/kg 2x/day for 3 days | 76 | 1/69 | Day 28: PCR‐adjusted: 0/69 Day 42: TF 6/69, PCR‐adjusted: 2/69 |
||
| Excluded studies | |||||
| Krudsood 2002 Thailand |
Azithromycin 0.5 g/day and dihydroartemisinin 80 mg/day for 3 days | 82 | 20/66 | 46 cure | 28 day cure rate: the proportion of patients who cleared asexual parasitaemia within 7 days of initiation of treatment without subsequent recrudescence within 28 days. |
| Mefloquine 10 mg/kg on day 1,2; 5 mg/kg on day 3; dihydroartemisinin 80 mg/day for 3 days | 88 | 68/68 | All cure | ||
| Pfizer 282919 India/Columbia |
Azithromycin 2 g/day and chloroquine 0.6 g/day for 3 days | 110 | 5/107 | As per presentation ASTMH December 08, New Orleans PCR‐adjusted: 3/107 |
Parasitological clearance day 28 (PCR unadjusted), and eradication not defined. |
Footnotes
Appendix 3. Results: Treatment failure by day 28
|
Trial country |
Treatment regimen |
Blind ing |
Total failure | Comparison |
Risk ratio (95% CI) (or P‐value if 0 failures) |
Cure day 28 (95% CI) |
| P. vivax | ||||||
| Dunne 2005A India | 1) Azithromycin 1 g/day for 3 days | P | 12/97 | 1 vs. 2 | 12.62 (1.67‐95.22) | 88 (79‐93) |
| 2) Chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 1/102 | 99 (94‐100) | |||
| Pukrittayakamee 2001 Thailand |
1) Azithromycin 0.5 g/day for 3 days | O | 10/18 | 44 (22‐69)§ | ||
| 2) Tetracycline 250 mg 4x/day for 7 days | O | 4/18 | 1 vs. 2 | 2.50 (0.96‐6.52) | 78 (52‐93)§ | |
| 3) Doxycycline 200 mg/day for 7 days | O | 8/18 | 1 vs. 3 | 1.25 (0.65‐2.42) | 56 (31‐78)§ | |
| 4) Clindamycin 300 mg 4x/day for 7 days | O | 5/12 | 1 vs. 4 | 1.33 (0.61‐2.93) | 58 (29‐84)§ | |
| P. falciparum | ||||||
| Dunne 2005B India |
1) Azithromycin 1 g/day for 3 days | P | 9/14 | 1 vs. 2 | 0.88 (0.53‐1.44) | 36 (14‐64) |
| 2) Chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 11/15 | 3 vs. 2 ¶ | 0.04 (0.01‐0.18) | 27 (9‐55) | |
| 3) Azithromycin 1 g/day for 3 days, chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | O | 2/63 | 3 vs. 1 ¶ | 0.05 (0.01‐0.20) | 97 (88‐99) | |
| Krudsood 2000 Thailand |
1) Azithromycin 0.5 g/day, artesunate 200 mg/day for 3 days | O | 24/55 | 56 (42‐69)§ | ||
| 2) Artesunate 200 mg/day for 3 days, mefloquine 10 mg/kg on day 1 to 2, 5 mg/kg on day 3 | O | 1/55 | 1 vs. 2 | 24.00 (3.36‐171.27) | 98 (89‐100)§ | |
| 3) Artesunate 200 mg/day for 3 days | O | 34/61 | 1 vs. 3 | 0.78 (0.54‐1.14) | 44 (32‐57)§ | |
| Miller 2006 Thailand |
1) Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 3 days | O | 1/10 | 1 vs. 2 1 vs. 3 ¶ 1 vs. 4 |
P=0.33 (Fisher) P=0.33 (Fisher) P=1.0 (Fisher) |
90 (54‐99)* |
| 2) Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 5 days | O | 0/20 | 2 vs. 1 2 vs. 3 ¶ 2 vs. 4 |
P=0.33 (Fisher) P=1.0 (Fisher) P=1.0 (Fisher) |
100 (80‐100) | |
| 3) Azithromycin 0.5 g 3x/days and quinine 10 mg/kg 3x/day for 3 days | O | 0/20 | 3 vs. 1 ¶ 3 vs. 2 ¶ 3 vs. 4 ¶ |
P=0.33 (Fisher) P=1.0 (Fisher) P=1.0 (Fisher) |
100 (80‐100) | |
| 4) Doxycycline 100 mg 2x/day and quinine 10 mg/kg 3x/day for 7 days | O | 0/10 | 100 (66‐100) | |||
| Na‐Bangchang 1996 Thailand |
1) Azithromycin 0.5 g at start, 0.25 g at 24 and 48 hrs, and artemether 300 mg at start | O | 23/27 | 1 vs. 2 | 1.83 (1.21‐2.76) | 15 (5‐35) |
| 2) Doxycycline 100 mg 2x/day for 5 days and artemether 300 mg at start and 100 mg after 12 hours | O | 14/30 | 53 (35‐71) | |||
| Noedl 2006 Thailand |
1) Azithromycin 0.75 g 2x/day and artesunate 100 mg 2x/day for 3 days | O | 2/25 | 1 vs. 2 1 vs. 3 1 vs. 4 |
0.72 (0.13‐3.96) 0.30 (0.06‐1.44) 1.00 (0.15‐6.55) |
92 (73‐99)§ |
| 2) Azithromycin 1 g/day and artesunate 200 mg/day for 3 days | O | 3/27 | 2 vs. 1 2 vs. 3 2 vs. 4 |
1.39 (0.25‐7.64) 0.42 (0.11‐1.62) 1.39 (0.25‐7.64) |
89 (70‐97)§ | |
| 3) Azithromycin 0.75 g 2x/day and quinine 10 mg/kg 2x/day for 3 days | O | 4/15 | 3 vs. 1 3 vs. 2 3 vs. 4 |
3.33 (0.69‐16.06) 2.40 (0.62‐9.33) 3.33 (0.69‐16.06) |
73 (45‐91)§ | |
| 4) Azithromycin 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | O | 2/25 | 4 vs. 1 4 vs. 2 4 vs. 3 |
1.00 (0.15‐6.55) 0.72 (0.13‐3.96) 0.30 (0.06‐1.44) |
92 (73‐99)§ | |
| Pfizer74841 India |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P/O | 12/73 | 1 vs. 2 1 vs. 3 |
0.48 (0.26‐0.91) 2.96 (1.00‐8.75) |
84 (73‐91) |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | 20/59 | 2 vs. 1 2 vs. 3 |
2.06 (1.10‐3.86) 6.10 (2.21‐16.87) |
66 (53‐78) | |
| 3) Sulfadoxine‐pyrimethamine 1500 mg/75 mg at start, chloroquine 600 mg on day 1 to 2, 300 mg on day 3 | O | 4/72 | 94 (86‐98) | |||
| Pfizer 82563 Kenya |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 1/5 | 1 vs. 2 | 0.70 (0.09‐5.76) | 80 (30‐99) |
| 2) Chloroquine 0.6 g/day for 3 days | P | 2/7 | 86 (42‐99)* | |||
| Pfizer 82576 Ghana, Kenya, Mali, Uganda, Zambia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 5/103 | 1 vs. 3 | 2.50 (0.50‐12.59) | 98 (92‐100)* |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | NR | ||||
| 3) Mefloquine 750 mg at start, 500 mg after 6 hours | P | 2/103 | 99 (94‐100)* | |||
| Pfizer 84227 Colombia/Surinam |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 48/112 | 1 vs. 3 | 48.43 (6.80‐344.84) | 61 (51‐70)** |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | NR | ||||
| 3) Atovaquone 1000 mg/day and proguanil 400 mg/day for 3 days | P | 1/113 | 100 (96‐100)* | |||
| Pfizer 84240 Indonesia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 9/13 | 1 vs. 3 | 2.31 (0.84‐6.36) | 31 (10‐61)** |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | NR | ||||
| 3) Sulfadoxine‐pyrimethamine 1500 mg/75 mg at start, chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 3/10 | 80 (44‐96)* | |||
| Pfizer 367653 Burkina Faso, Ghana, Kenya, Mali, Senegal, Zambia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | O | 1/107 | 1 vs. 2 | 1.05 (0.07‐16.52) | 100 (96‐100)* |
| 2) Mefloquine 750 mg at start, 500 mg after 6to 10 hours | O | 1/112 | 99 (94‐100)* | |||
| Sykes 2009 Tanzania |
1) Azithromycine 20 mg/kg/day and artesunate 4 mg/kg/day for 3 days | O | 69/119 | 1 vs. 2 | 2.90 (1.96‐4.28) | 66 (57‐75)** |
| 2) Artemether 20 mg and lumefantrine 120 mg 2x/day for < 15 kg and 40 mg/240 mg 2x/day for ≥15 kg for 3 days | O | 24/120 | 90 (93‐95)** | |||
| Thriemer 2010 Bangladesh |
1) Azithromycin 1.5 g/day and artesunate 200 mg/day for 3 days. Children < 35 kg: azithromycin 30 mg/kg/day, and artesunate 4 mg/kg/day for 3 days. | O | 13/142 | 1 vs. 2 | 6.32 (0.84‐47.31) | 94 (89‐97)* |
| 2) Artemether 80 mg and lumefantrine 480 mg 2x/day for 3 days. Children < 35 kg: artemether 4 mg/kg and lumefantrine 24 mg/kg 2x/day for 3 days | O | 1/69 | 100 (93‐100)* | |||
| Excluded studies (P. falciparum) | ||||||
| Krudsood 2002 Thailand |
1) Azithromycin 0.5 g/day and dihydroartemisinin 80 mg/day for 3 days | O | 46/66 | 1 vs. 2 | P < 0.001 (Fisher) | 70 (57‐80)§ |
| 2) Mefloquine 10 mg/kg on day 1 and 2, and 5 mg/kg on day 3, and dihydroartemisinin 80 mg/day for 3 days | O | 0/68 | 100 (93‐100)§ | |||
| Pfizer 282918 India, Colombia |
1) Azithromycin 2 g/day and chloroquine 600 mg/day for 3 days | O | 5/107 | 97 (91‐99)* |
Footnotes
Abbreviations:CI: confidence interval; NR: not reported; O: open Label; P: use of placebo. Significant risk ratios printed in bold
Calculations of risk ratios and cure with 95% confidence intervals using Epicalc 2000 version 1.02 (Gilman & Myatt: http://www.brixtonhealth.com/epicalc.html)
* PCR‐adjusted
** Partially PCR‐adjusted. Pfizer 84227: 35/49 infections analysed, 4 reinfection. Pfizer 84240: 5/9 treatment failures analysed, all recrudescent.
§ Study follow‐up conducted in area without malaria transmission
¶ Treatment arms not conducted at the same time period
Appendix 4. Results: Fever clearance time
| Study | Definition | Treatment | N | Median, hrs | Mean (SD), hrs | Range, hrs |
| P. vivax | ||||||
| Pukrittayakamee 2001 | Time taken for body temperature to fall below 37.5 °C and remain below this value for > 48 hours | AZ | 20 | 61 | 4‐156 | |
| TET | 27 | 57 | 4‐125 | |||
| DX | 25 | 68 | 6‐128 | |||
| CL | 20 | 43 | 4‐110 | |||
| P. falciparum | ||||||
| Krudsood 2000 | Time from the start of treatment until the oral temperature fell to ≤37.5 ºC and remained below that level for the next 48 hours | AZ+AT | 67 | 33.6 (27.2) | 0‐120 | |
| AT+MQ | 67 | 38.8 (32.1) | 0‐152 | |||
| AT | 68 | 37.9 (29.0) | 0‐152 | |||
| Miller 2006 | Time (in hours) until temperature was ≤37.4 °C and remained there for at least an additional 48 hours | AZ3+Q | 10 | 52.5 (42.0) | ‐ | |
| AZ5+Q | 20 | 41.5 (30.9) | ‐ | |||
| AZ4.5+Q | 20 | 49.8 (44.0) | ‐ | |||
| Q+DX | 10 | 52.8 (42.0) | ‐ | |||
| Na‐Bangchang 1996 | The time taken for the temperature to return to normal i.e. below 37.3 °C and remain at that value for at least 24 hours | ART+AZ | 27 | 20 | 8‐35 | |
| ART+DX | 30 | 26.5 | 3‐63 | |||
| Noedl 2006 | Time from initiation of treatment until oral temperature decreased to < 37.5°C and remained at less than this temperature for the next 48 hours | AZ4.5+AT | 25 | 23.4 (22.5) | 0.0‐76.2 | |
| AZ3+AT | 27 | 16.7 (16.2) | 0.0‐61.3 | |||
| AZ4.5+60Q | 15 | 51.4 (32.8) | 0.0‐114.0 | |||
| AZ4.5+90Q | 25 | 38.1 (38.3) | 0.0‐140.0 | |||
| Thriemer 2010 | Interval from start of treatment until the axillary temperature decreased to < 37.5°C and remained below this temperature for the next 48 hours | AZ4.5+AT | 128 | 6.3 (19.3)* |
IQR: 0.0‐19.6 (16.2‐21.5)* |
|
| AL | 65 | 3.4 (18.5)* |
0.0‐19.1 (6.5‐28.2)* |
Footnotes
Abbreviations: AZ: azithromycin; TET: tetracycline; DX: doxycycline; CL: clindamycin; AT: artesunate; Q: quinine; MQ: mefloquine; ART: artemether; AL: artemether‐lumefantrine; SD: Standard deviation; hrs: hours, IQR: inter‐quartal range. The numbers in the treatment column indicate the full treatment course dose in g for azithromycin and mg/day for quinine.
*Only patients included who were febrile at enrolment
Appendix 5. Results: Parasite clearance time
| Study | Definition | Treatment | N | Median, hrs | Mean (SD), hrs | Range, hrs |
| P. vivax | ||||||
| Dunne 2005a | Time to clearance of parasitaemia | AZ | 97 | 55 | NR | |
| CQ | 102 | 20 | NR | |||
| Pukrittayakamee 2001 | Time to fall below detectable levels in a peripheral blood smear. | AZ | 20 | 146 (65) | ||
| TET | 27 | 131 (35) | ||||
| DX | 25 | 145 (45) | ||||
| CL | 20 | 110 (42) | ||||
| P. falciparum | ||||||
| Dunne 2005b | Time at resolution of parasitaemia | AZ | 16 | 96 | NR | |
| CQ | 16 | 57 | NR | |||
| AZ/CQ | 63 | 36 | NR | |||
| Krudsood 2000 | Time from the start of treatment until the first negative blood film, which remained negative for the next 24 hours | AZ+AT | 67 | 43.5 (17.3) | 5‐103 | |
| AT+MQ | 67 | 45.3 (14.7) | 11‐97 | |||
| AT | 68 | 43.7 (13.6) | 21‐80 | |||
| Miller 2006 | Time from the start of treatment until the first negative blood smear for asexual stages, which remained negative for an additional 24 hours | AZ3+Q | 10 | 78.9 (25.8) | ‐ | |
| AZ5+Q | 20 | 77.7 (21.4) | ‐ | |||
| AZ4.5+Q | 20 | 77.7 (23.1) | ‐ | |||
| Q+DX | 10 | 68.8 (23.1) | ‐ | |||
| Na‐Bangchang 1996 | Time taken for the parasite count to fall below the level of microscopic detection | ART+AZ | 27 | 28 | 18‐48 | |
| ART+DX | 30 | 31 | 8‐54 | |||
| Noedl 2006 | Time from initiation of treatment until the first time that blood films were negative for asexual parasites of P. falciparum and remained negative for the next 48 hours | AZ4.5+AT | 25 | 33.1 (15.3) | 8.8‐79.1 | |
| AZ3+AT | 27 | 35.7 (10.5) | 18.2‐55.6 | |||
| AZ4.5+60Q | 15 | 93.1 (32.0) | 37.0‐149.1 | |||
| AZ4.5+90Q | 25 | 62.9 (26.1) | 14.5‐115.5 | |||
| Thriemer 2010 | The interval from the start of the treatment until the first time a blood smear was negative for asexual P. falciparum and two consecutive smears were negative. | AZ4.5+AT | 128 | 25.1 | IQR 18.5‐30.3 |
|
| AL | 65 | 27.2 | 18.3‐30.2 |
Footnotes
Abbreviations: AZ: azithromycin; TET: tetracycline; DX: doxycycline; CL: clindamycin; AT: artesunate; Q: quinine; MQ: mefloquine; ART: artemether; AL: artemether‐lumefantrine, SD: Standard deviation; hrs: hours; NR: not reported, IQR: inter‐quartal range. The numbers in the treatment column indicate the full treatment course dose in g for azithromycin and mg/day for quinine.
Appendix 6. Results: Adverse events, treatment related and all cause
|
Trial country |
Treatment regimen | Blinding |
AE treatment related (TR) (%) |
Risk ratio AE* (95% CI) | AE any cause (AC) (%) | Number AE | |
|
TR (average AE/person) |
AC (average AE/person) |
||||||
| P. vivax | |||||||
| Dunne 2005A India | 1) Azithromycin 1 g/day for 3 days | P | 13/97 (13.4) | 0.57 (0.31‐1.05) | 15/97 (0.2) | ||
| 2) Chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 24/102 (23.5) | Reference | 35/102 (0.3) | |||
| Pukrittayakamee 2001 Thailand |
1) Azithromycin 0.5 g/day for 3 days | O | 6/18 (33.3)^ | P = 0.06 (Fisher) | |||
| 2) Tetracycline 250 mg 4x/day for 7 days | O | 0/18 (0.0) | |||||
| 3) Doxycycline 200 mg/day for 7 days | O | 0/18 (0.0) | |||||
| 4) Clindamycin 300 mg 4x/day for 7 days | O | 0/12 (0.0) | Reference | ||||
| P. falciparum | |||||||
| Dunne 2005B India |
1) Azithromycin 1 g/day for 3 days | P | 8/16 (50.0) | 0.78 (0.42‐1.46) | 8/16 (0.5) | ||
| 2) Chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 9/16 (56.3) | Reference | 10/16 (0.6) | |||
| 3) Azithromycin 1 g/day for 3 days, chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | O | 13/63 (20.6) | 0.37 (0.19‐0.70) | 18/63 (0.3) | |||
| Krudsood 2000 Thailand |
1) Azithromycine 0.5 g/day, Artesunate 200 mg/day for 3 days | O | NR | "Most adverse events were mild to moderate severity, difficult to distinguish from complaints of malaria, and disappeared within a week after the drugs were administered." | |||
| 2) Artesunate 200 mg/day for 3 days, mefloquine 10 mg/kg on day 1 to 2, 5 mg/kg on day 3 | O | NR | |||||
| 3) Artesunate 200 mg/day for 3 days | O | NR | |||||
| Miller 2006 Thailand |
1) Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 3 days | O | 10/10 (100) |
16/10 (1.6) | 32/10 (3.2) | ||
| 2) Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 5 days | O | 20/20 (100) | 28/20 (1.4) | 45/20 (2.3) | |||
| 3) Azithromycin 0.5 mg 3x/day and quinine 10 mg/kg 3x/day for 3 days | O | 21/21 (100) | 32/21 (1.5) | 55/21 (2.6) | |||
| 4) Doxycycline 100 mg 2x/day and quinine 10 mg/kg 3x/day for 7 days | O | 10/10 (100) | 20/10 (2.0) | 31/10 (3.1) | |||
| Na‐Bangchang 1996 Thailand |
1) Azithromycin 0.5 g at start, 0.25 g at 24 hours and 48 hours, and artemether 300 mg at start | O | NR | 0.28 (0.09‐0.88) | 3/27 (11.1) | ||
| 2) Doxycycline 100 mg 2x/day for 5 days and artemether 300 mg at start and 100 mg after 12 hours | O | NR | Reference | 12/30 (40.0) | |||
| Noedl 2006 Thailand |
1) Azithromycin 0.75 g 2x/day and artesunate 100 mg 2x/day for 3 days | O | 1/27 (3.7) | 0.05 (0.01‐0.37) | 26/27 (96.3) | 113/27(4.2) | |
| 2) Azithromycin 1 g/day and artesunate 200 mg/day for 3 days | O | 3/27 (11.1) | 0.16 (0.05‐0.47) | 27/27 (100) | 154/27 (5.7) | ||
| 3) Azithromycin 0.75 g 2x/day and quinine 10 mg/kg 2x/day for 3 days | O | 8/16 (50.0) | 0.71 (0.41‐1.23) | 16/16 (100) | 102/16 (6.4) | ||
| 4) Azithromycin 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | O | 19/27 (70.4) | Reference | 27/27 (100) | 169/27 (6.3) | ||
| Pfizer74841^^ India |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P/O | NR | 7/83 (0.1) | 24/83 (0.3) | ||
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | NR | 2/67 (0.03) | 20/67 (0.3) | |||
| 3) Sulfadoxine‐pyrimethamine 1500 mg/75 mg at start, chloroquine 600 mg on day 1 to 2, 300 mg on day 3 | O | NR | 3/80 (0.04) | 22/80 (0.3) | |||
| Pfizer 82563 Kenya |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 7/7 (100) | 7/7 (1.0) | |||
| 2) Chloroquine 0.6 g/day for 3 days | P | 7/7 (100) | 18/7 (2.6) | ||||
| Pfizer 82576 Ghana, Kenya, Mali, Uganda, Zambia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 89/114 (78.1) | 1.26 (1.06‐1.50) | 106/114 (93.0) | 175/114 (1.5) | 327/114 (2.9) |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | 4/9 (44.4) | 0.72 (0.34‐1.52) | 6/9 (66.7) | 7/9 (0.8) | 11/9 (1.2) | |
| 3) Mefloquine 750 mg at start, 500 mg after 6 hours | P | 71/115 (61.7) | Reference | 107/115 (93.0) | 150/115 (1.3) | 337/115 (2.9) | |
| Pfizer 84227^^ Colombia/Surinam |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | NR | 1.41 (1.09‐1.83) | 68/114 (59.6) | ||
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | NR | 1.52 (0.98‐7.80) | 9/14 (64.3) | |||
| 3) Atovaquone 1000 mg/day and proguanil 400 mg/day for 3 days | P | NR | Reference | 49/116 (42.2) | |||
| Pfizer 84240 Indonesia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 8/13 (61.5) | 1.48 (0.67‐3.27) | 10/13 (76.9) | 22/13 (1.7) | 46/13 (3.5) |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | 6/7 (85.7) | 2.06 (0.99‐4.29) | 7/7 (100) | 12/7 (1.7) | 26/7 (3.7) | |
| 3) Sulfadoxine‐Pyrimethamine 1500mg /75 mg at start, chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 5/12 (41.7) | Reference | 7/12 (58.3) | 8/12 (0.7) | 18/12 (1.5) | |
| Pfizer 367653 Burkina Faso, Ghana, Kenya, Mali, Senegal, Zambia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | O | 80/113 (70.8) | 1.14 (0.95‐1.37) | 95/113 (84.1) | 173/113 (1.5) | 264/113 (2.3) |
| 2) Mefloquine 750 mg at start, 500 mg after six to10 hours | O | 72/116 (62.1) | Reference | 92/116 (79.3) | 175/116 (1.5) | 281/116 (2.4) | |
| Sykes 2009 Tanzania |
1) Azithromycine 20 mg/kg/day and artesunate 4 mg/kg/day for 3 days | O | 1.21 (0.81‐1.80) | 39/129 (30.2) | 44/129 (0.3) | ||
| 2) Artemether 20 mg and lumefantrine 120 mg 2x/day for < 15 kg and 40 mg/240 mg 2x/day for ≥15 kg for 3 days | O | Reference | 33/132 (25.0) | 38/132 (0.3) | |||
| Thriemer 2010 Bangadesh |
1) Azithromycin 1.5 g/day and artesunate 200 mg/day for 3 days. Children <35 kg: azithromycin 30 mg/kg/day, and artesunate 4 mg/kg/day for 3 days. | O | 27/128 (0.2) | ||||
| 2) Artemether 80 mg and lumefantrine 480 mg 2x/day for 3 days. Children <35 kg: artemether 4 mg/kg and lumefantrine 24 mg/kg 2x/day for 3 days | O | 33/65 (0.5) | |||||
| Excluded studies (P. falciparum) | |||||||
| Krudsood 2002 Thailand |
1) Azithromycin 0.5 g/day and dihydroartemisinin 80 mg/day for 3 days | O | NR | "Regarding safety, it was difficult to distinguish between symptoms of acute malaria and drug‐related effects. All signs and symptoms and parasitaemia simultaneously disappeared within few days." | |||
| 2) Mefloquine 10 mg/kg on day 1 and 2, and 5 mg/kg on day 3, dihydroartemisinin 80 mg/day for 3 days | |||||||
| Pfizer 282918 India, Colombia |
1) Azithromycin 2 g/day and chloroquine 0.6 g/day for 3 days | O | 48/110 (43.6) | NR | 78/110 (0.7) | ||
Footnotes
Abbreviations: AE: adverse events; TR: treatment related; AC: any cause; CI: confidence interval; NR: not reported; O: open label; P: use of placebo
Total number of adverse events reported as number of adverse events/number of participants.
Calculations of risk ratios and cure with 95% confidence intervals using Epicalc 2000 version 1.02 (Gilman & Myatt: http://www.brixtonhealth.com/epicalc.html)
*Risk ratio for treatment‐related adverse events if data is available, otherwise risk ratio for adverse events of 'any cause'
^Only delayed appearance of P. falciparum reported as adverse events
^^Only frequent adverse events (eg 2% to 3% or more) reported
Appendix 7. Results: Serious adverse events (SAE), discontinuations and other adverse events (laboratory, ECG, physical examination
|
Trial country |
Treatment regimen | Blinding | SAE | Discontinuation | Lab and other | Notes | ||
| TR | AC | TR | AC | |||||
| P. vivax | ||||||||
| Dunne 2005a India | 1) Azithromycin 1 g/day for 3 days | P | 0 | 0 | 0 | NR | NR | 2 discontinued in arm 2 because of pruritis (1) and maculopapular rash (1) |
| 2) Chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 0 | 0 | 2 | NR | NR | ||
| Pukrittayakamee 2001 Thailand |
1) Azithromycin 0.5 g/day for 3 days | O | 0 | 0 | NR | NR | "None of the patients developed serious adverse effects as monitored by clinical symptoms and laboratory data." | |
| 2) Tetracycline 250 mg 4x/day for 7 days | O | 0 | 0 | NR | NR | |||
| 3) Doxycycline 200 mg/day for 7 days | O | 0 | 0 | NR | NR | |||
| 4) Clindamycin 300 mg 4x/day for 7 days | O | 0 | 0 | NR | NR | |||
| P. falciparum | ||||||||
| Dunne 2005b India |
1) Azithromycin 1 g/day for 3 days | P | 0 | 0 | 0 | 0 | NR | |
| 2) Chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 0 | 0 | 0 | 0 | NR | ||
| 3) Azithromycin 1 g/day for 3 days, chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | O | 0 | 0 | 0 | 0 | NR | ||
| Krudsood 2000 Thailand |
1) Azithromycin 0.5 g/day, artesunate 200 mg/day for 3 days | O | 0 | 0 | NR | NR | "Several patients had abnormal haematology and biochemistry at the beginning, but all of these improved a few weeks after treatment." | |
| 2) Artesunate 200 mg/day for 3 days, mefloquine 10 mg/kg on day 1 to 2, 5 mg/kg on day 3 | O | 0 | 0 | NR | NR | |||
| 3) Artesunate 200 mg/day for 3 days | O | 0 | 0 | NR | NR | |||
| Miller 2006 Thailand |
1) Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 3 days | O | 0 | 0 | NR | NR | Elevated ALT level (1 in each group). Resolved within 10 days of completion of therapy. | Most side effects related to quinine (tinnitus, prolonged QTc) |
| 2) Azithromycin 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 5 days | O | 0 | 0 | NR | NR | |||
| 3) Azithromycin 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | O | 0 | 0 | NR | NR | |||
| 4) Doxycycline 100 mg 2x/day and quinine 10 mg/kg 3x/day for 7 days | O | 0 | 0 | NR | NR | |||
| Na‐Bangchang 1996 Thailand |
1) Azithromycin 0.5 g at start, 0.25 g at 24 hours and 48 hours, and artemether 300 mg at start | O | 0 | 0 | NR | NR | NR | "Transient mild nausea, abdominal discomfort and loss of appetite were common adverse effects." |
| 2) Doxycycline 100 mg 2x/day for 5 days and artemether 300 mg at start and 100 mg after 12 hours | O | 0 | 0 | NR | NR | NR | ||
| Noedl 2006 Thailand |
1) Azithromycin 0.75 g 2x/day and artesunate 100 mg 2x/day for 3 days | O | 0 | 0 | 0 | 0 | NR | One participant with food poisoning withdrawn in arm 4. |
| 2) Azithromycin 1 g/day and artesunate 200 mg/day for 3 days | O | 0 | 0 | 0 | 0 | NR | ||
| 3) Azithromycin 0.75 g 2x/day and quinine 10 mg/kg 2x/day for 3 days | O | 0 | 0 | 0 | 0 | ECG: quinine groups significant prolongation of QT interval. | ||
| 4) Azithromycin 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | O | 0 | 1 | 0 | 0 | |||
| Pfizer74841 India |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P/O | 0/83 | 5/83 (6.0%) | 0 | 3/83 (3.6%) | “No clinical significant laboratory findings or changes in vital signs." | SEA any cause: arm 1: cerebral malaria (1), fever (4); arm 2: fever (3), convulsion (1), pneumonia (1); arm 3: fever (2), abnormal behaviour (1, TR). |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | 0/67 | 5/67 (7.5%) | 0 | 0/67 | Discontinuations: 1 cerebral malaria and 2 malarial fever in arm 1, and 1 malarial fever in arm 3. | ||
| 3) Sulfadoxine‐pyrimethamine 1500mg /75 mg at start, chloroquine 0.6 g on day 1 to 2, 0.3 mg on day 3 | O | 1/80 (1.3%) | 3/80 (3.8%) | 0 | 1/80 (1.3%) | |||
| Pfizer 82563 Kenya |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 0 | 1/6 (16.7%) | 0 | 0 | Similar incidence of lab abnormalities in both groups. "None were unexpected for this population." Vital signs: larger mean decrease in BP and larger mean increase in heart rate from baseline in group 2. |
One SEA in group 1 (seizures) in a person with a history of seizures. |
| 2) Chloroquine 0.6 g/day for 3 days | P | 0 | 0 | 0 | 0 | |||
| Pfizer 82576 Ghana, Kenya, Mali, Uganda, Zambia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 0 | 1/114 (0.9%) | 2/114 (1.8%) | 3/114 (2.6%) | "The incidence of laboratory abnormalities was similar in arm 1 and arm 3." | SEA: Dyspnoea and confusional state in arm 1, mental disorder and nephritic syndrome in arm 3 (TR) |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | 0 | 0 | 0 | 0 | Discontinuations: arm 1: confusion 1, vomiting 2 (TR), arm 3: vomiting 2, (1 TR), jaundice 1, fever 1 (TR) | ||
| 3) Mefloquine 750 mg at start, 500 mg after 6 hours | P | 2/115 (1.7%) | 2/115 (1.7%) | 2/115 (1.7%) | 4/115 (3.5%) | |||
| Pfizer 84227 Colombia/Surinam |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | NR | 3/114 (2.6%) | NR | 6/114 (5.3%) | NR | SEA: Arm 1: complicated malaria, progressive renal failure, sepsis; arm 2: enterocolitis; arm 3: diabetic neuropathy, progressive severe anaemia. |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | NR | 1/14 (7.1%) | NR | NR | NR | Discontinuations: Arm 1: vomiting 2, infection 2, sepsis 1, electrolyte imbalance 1; arm 3: infection 1. |
|
| 3) Atovaquone 1000 mg/day and proguanil 400 mg/day for 3 days | P | NR | 2/116 (1.7%) | NR | 1/116 (0.9%) | NR | ||
| Pfizer 84240 Indonesia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | P | 0 | 1/13 (7.7%) | 0 | 0 | "All treatment groups showed a similar incidence of laboratory abnormalities (1 subject in each group) with regard to normal baseline. None of the laboratory abnormalities was unexpected for this disease population." | SEA arm 1: recurrent malaria; arm 2: appendicitis |
| 2) Azithromycin 0.5 g/day and chloroquine 0.6 g/day for 3 days | P | 0 | 1/7 (14.3%) | 0 | 0 | |||
| 3) Sulfadoxine‐Pyrimethamine 1500 mg/75 mg at start, chloroquine 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 0 | 0/12 | 0 | 0 | |||
| Pfizer 367653 Burkina Faso, Ghana, Kenya, Mali, Senegal, Zambia |
1) Azithromycin 1 g/day and chloroquine 0.6 g/day for 3 days | O | 0/113 | 0/113 | NR | 1/113 (0.9%) | "No subjects were discontinued from the study due to abnormal laboratory test results." | SEA: dizziness and intentional self injury |
| 2) Mefloquine 750 mg at start, 500 mg after 6 to 10 hours | O | 1/116 (0.9%) | 2/116 (1.7%) | NR | 0/116 | Discontinuation in arm 1 because of pruritis | ||
| Sykes 2009 Tanzania |
1) Azithromycine 20 mg/kg/day and artesunate 4 mg/kg/day for 3 days | O | 1/120 | Lab‐abnormalities on day 14: 18/120 |
||||
| 2) Artemether 20 mg and lumefantrine 120 mg 2x/day for <15 kg and 40 mg/240 mg 2x/day for ≥15 kg for 3 days | O | 9/129 | Lab‐abnormalities on day 14: 22/121 |
|||||
| Thriemer 2010 Bangladesh |
1) Azithromycin 1.5 g/day and artesunate 200 mg/day for 3 days. Children < 35 kg: azithromycin 30 mg/kg/day, and artesunate 4 mg/kg/day for 3 days. | O | 0 | 8 | NR | Discontinuations: Development of P. vivax ≥ day 21 | ||
| 2) Artemether 80 mg and lumefantrine 480 mg 2x/day for 3 days. Children < 35 kg: artemether 4 mg/kg and lumefantrine 24 mg/kg 2x/day for 3 days | O | 0 | 4 | NR | ||||
| Excluded studies (P. falciparum) | ||||||||
| Krudsood 2002 Thailand |
1) Azithromycin 0.5 g/day and dihydroartemisinin 80 mg/day for 3 days | O | 0 | 0 | 0 | 0 | "Some baseline laboratory parameters were affected by disease status. However, they all returned to normal within 1‐2 weeks." | |
| 2) Mefloquine 10 mg/kg on day 1 and 2, and 5 mg/kg on day 3, dihydroartemisinin 80 mg/day for 3 days | 0 | 0 | 0 | 0 | ||||
| Pfizer 282919 India, Colombia |
1) Azithromycin 2 g/day and chloroquine 0.6 g/day for 3 days | O | 0 | NR | 0 | NR | ||
Footnotes
Abbreviations: SEA: serious adverse event; AE: adverse event; TR: treatment related; AC: any cause; NR: not reported; O: open label; P: use of placebo
There were no significant differences for SEA and discontinuations among treatment arms
Appendix 8. Common side effects among studies with detailed reporting of adverse events
| Study | Treatment regimes | Blinding | Nausea | Vomiting | Diarrhoea | Pruritis | Dizziness | |||||
| % | n/N | % | n/N | % | n/N | % | n/N | % | n/N | |||
| Dunne 2005a India |
1) AZ 1 g/day for 3 days | P | 0.0 | 0/97 | 0.0 | 0/97 | 0.0 | 0/97 | 1.0 | 1/97 | NR | |
| 2) CQ 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 4.9 | 5/102 | 7.8 | 8/102 | 0.0 | 0/102 | 7.8 | 8/102 | NR | ||
| Dunne 2005b India |
1) AZ 1 g/day for 3 days | P | 0.0 | 0/16 | 0.0 | 0/16 | 12.5 | 2/16 | 6.3 | 1/16 | 6.3 | 1/16 |
| 2) CQ 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 0.0 | 0/16 | 0.0 | 0/16 | 6.3 | 1/16 | 18.8 | 3/16 | 18.8 | 3/16 | |
| 3) AZ 1 g/day for 3 days, CQ 0.6 mg on day 1 to 2, 0.3 g on day 3 | O | 6.3 | 4/63 | 7.9 | 5/63 | 3.2 | 2/63 | 1.6 | 1/63 | 0.0 | 0/63 | |
| Miller 2006 Thailand |
1) AZ 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 3 days | O | NR | 10.0 | 1/10 | 20.0 | 2/10 | NR | 100.0 | 10/10 | ||
| 2) AZ 0.5 g 2x/day and quinine 10 mg/kg 3x/day for 5 days | O | NR | 20.0 | 2/20 | 15.0 | 3/20 | NR | 100.0 | 20/20 | |||
| 3) AZ 0.5 g 3x/day and quinine 10 mg/kg 3x/day for 3 days | O | NR | 19.0 | 4/21 | 9.5 | 2/21 | NR | 90.5 | 19/21 | |||
| 4) Doxycycline 100 mg 2x/day and quinine 10 mg/kg 3x/day for 7 days | O | NR | 40.0 | 4/10 | 10.0 | 1/10 | NR | 100.0 | 10/10 | |||
| Pfizer74841§ India |
1) AZ 1 g/day and CQ 0.6 g/day for 3 days | P/O | NR | 14.5 | 12/83 | 4.8 | 4/83 | 2.4 | 2/83 | NR | ||
| 2) AZ 0.5 g/day and CQ 0.6 g/day for 3 days | P | NR | 13.4 | 9/67 | 6.0 | 4/67 | 3.0 | 2/67 | NR | |||
| 3) SP 1500 mg/75 mg at start, CQ 0.6 g on day 1 to 2, 0.3 g on day 3 | O | NR | 18.8 | 15/80 | 1.3 | 1/80 | 0.0 | 0/80 | NR | |||
| Pfizer 82576 Ghana, Kenya, Mali, Uganda, Zambia |
1) AZ 1 g/day and CQ 0.6 g/day for 3 days | P | 9.6 | 11/114 | 18.4 | 21/114 | 12.3 | 14/114 | 51.8 | 59/114 | 16.7 | 19/114 |
| 2) AZ 0.5 g/day and CQ 0.6 g/day for 3 days | P | 0.0 | 0/9 | 22.2 | 2/9 | 0.0 | 0/9 | 22.2 | 2/9 | 0.0 | 0/9 | |
| 3) Mefloquine 750 mg at start, 500 mg after 6 hours | P | 17.4 | 20/115 | 25.2 | 29/115 | 10.4 | 12/115 | 9.6 | 11/115 | 33.9 | 39/115 | |
| Pfizer 84227 Colombia/Surinam |
1) AZ 1 g/day and CQ 0.6 g/day for 3 days | P | NR | 6.1 | 7/114 | 5.3 | 6/114 | 25.4 | 29/114 | NR | ||
| 2) AZ 0.5 g/day and CQ 0.6 g/day for 3 days | P | NR | 14.3 | 2/14 | 0.0 | 0/14 | 28.6 | 4/14 | NR | |||
| 3) Atovaquone 1000/proguanil 400 mg/day for 3 days | P | NR | 4.3 | 5/116 | 4.3 | 5/116 | 2.6 | 3/116 | NR | |||
| Pfizer 84240 Indonesia |
1) AZ 1 g/day and CQ 0.6 g/day for 3 days | P | 53.8 | 7/13 | 30.8 | 4/13 | 7.7 | 1/13 | NR | 15.4 | 2/13 | |
| 2) AZ 0.5 g/day and CQ 0.6 g/day for 3 days | P | 28.6 | 2/7 | 42.9 | 3/7 | 14.3 | 1/7 | NR | 28.6 | 2/7 | ||
| 3) SP 1500 mg/75 mg at start, CQ 0.6 g on day 1 to 2, 0.3 g on day 3 | P | 16.7 | 2/12 | 16.7 | 2/12 | 16.7 | 2/12 | NR | 8.3 | 1/12 | ||
| Pfizer 367653 Burkina Faso, Ghana, Kenya, Mali, Senegal, Zambia |
1) AZ 1 g/day and CQ 0.6 g/day for 3 days | O | 11.5 | 13/113 | 4.4 | 5/113 | 12.4 | 14/113 | 28.3 | 32/113 | 19.5 | 22/113 |
| 2) Mefloquine 750 mg at start, 500 mg after 6 to 10 hours | O | 15.5 | 18/116 | 25.0 | 29/116 | 5.2 | 6/116 | 1.7 | 2/116 | 23.3 | 27/116 | |
| Sykes 2009 Tanzania |
1) Azithromycine 20 mg/kg/day and artesunate 4 mg/kg/day for 3 days | O | NR | 7.8 | 10/129 | NR | 5.4 | 7/129 | 1.6 | 2/129 | ||
| 2) Artemether 20 mg and lumefantrine 120 mg 2x/day for < 15 kg and 40 mg/240 mg 2x/day for ≥15 kg for 3 days | O | NR | 1.5 | 2/132 | NR | 3.0 | 4/132 | 0.8 | 1/132 | |||
| Thriemer 2010* Bangladesh |
1) Azithromycin 1.5 g/day and artesunate 200 mg/day for 3 days. Children < 35 kg: azithromycin 30 mg/kg/day, and artesunate 4 mg/kg/day for 3 days. | O | 7.8 | 10/128 | 6.3 | 8/128 | 3.9 | 5/128 | NR | NR | ||
| 2) Artemether 80 mg and lumefantrine 480 mg 2x/day for 3 days. Children < 35 kg: artemether 4 mg/kg and lumefantrine 24 mg/kg 2x/day for 3 days | O | 18.5 | 12/65 | 3.1 | 2/65 | 0 | 0/65 | NR | NR | |||
| Excluded studies | ||||||||||||
| Pfizer 282919 India, Colombia |
1) AZ 2 g/day and CQ 0.6 g/day for 3 days | O | 30.0 | 33/110 | 18.2 | 20/110 | 11.8 | 13/110 | 3.6 | 4/110 | NR | |
Footnotes
Abbreviations: AZ: azithromycin; CQ: chloroquine; NR: not reported. If both 'All cause' and 'treatment related' adverse events were reported, we included 'All cause' adverse events.
*In the study by Thriemer et al, nausea defined as abdominal pain and discomfort, and diarrhoea defined as loose stools.
Azithromycin arms: Nausea: Pfizer 282919 versus 82576: RR 3.11, 95% CI 1.66 to 5.84; Pfizer 282919 versus 367653: RR 2.61, 95% CI 1.45 to 4.68; Pfizer 282919 versus Dunne 2005b: RR 4.72, 95% CI 1.75 to 12.72;
Azithromycin arms: Vomiting: Pfizer 282919 versus 367653: RR 4.11, 95% CI 1.6 to 10.6; Pfizer 282919 versus 84227: RR 2.96, 95% CI 1.30 to 6.72
Azithromycin arms: Diarrhoea: No significant differences
Azithromycin arms: Pruritis: Pfizer 82576 versus 84227: RR 2.03, 95% CI 1.42 to 2.92 (Pruritis in AZ/CQ arm in Pfizer 82576 also significant higher versus AZ regimens in other studies which reported on pruritis)
Data and analyses
Comparison 1.
Overview treatment failure for AZ or AZ combination vs. control for P. falciparum
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure on day 28, not corrected by PCR | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 AZ vs. CQ | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.53, 1.44] |
| 1.2 AZCQ vs. CQ (not randomized) | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.01, 2.35] |
| 1.3 AZCQ vs. AZ monotherapy (not randomized) | 1 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.20] |
| 1.4 AZCQ vs. SPCQ | 2 | 168 | Risk Ratio (M‐H, Random, 95% CI) | 2.59 [1.24, 5.43] |
| 1.5 AZCQ vs. atovaquone‐proguanil | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 48.43 [6.80, 344.83] |
| 1.6 AZCQ vs. MQ | 2 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.50, 8.07] |
| 1.7 AZ‐artesunate vs. artesunate | 1 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.54, 1.14] |
| 1.8 AZ‐artesunate vs. MQ‐artesunate | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 24.00 [3.36, 171.26] |
| 1.9 AZ‐artesunate vs. AZ‐quinine (arm 1 vs. 4) | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.15, 6.55] |
| 1.10 AZ‐artesunate vs. artemether‐lumefantrine | 2 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 2.98 [2.03, 4.37] |
| 1.11 AZ‐artemether vs. doxycycline‐artemether | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.21, 2.76] |
| 1.12 AZ‐quinine vs. doxycycline‐quinine (arm 1 vs. 4) | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.14, 65.90] |
| 2 Treatment failure day 28, PCR corrected | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 AZCQ vs CQ | 1 | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.09, 14.92] |
| 2.2 AZCQ vs. SP | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [0.95, 12.59] |
| 2.3 AZCQ vs. atovaquone‐proguanil | 1 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 89.79 [5.60, 1440.31] |
| 2.4 AZCQ vs. MQ | 2 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.18, 5.87] |
| 2.5 AZ‐artesunate vs. artemether‐lumefantrine | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [2.02, 6.52] |
| 2.6 AZ‐quinine vs. doxycycline‐quinine (arm 1 vs. 4) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.14, 65.90] |
| 3 Treatment failure on day 42, not corrected by PCR | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.44, 2.49] |
| 3.1 AZ‐artesunate vs. artemether‐lumefantrine | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.44, 2.49] |
| 4 Treatment failure on day 42, (partially) corrected by PCR | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.53, 3.99] |
| 4.1 AZ‐artesunate vs. artemether‐lumefantrine | 2 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.53, 3.99] |
Comparison 2.
Overview adverse events for AZ or AZ combinations vs. control for P. falciparum
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment related adverse events | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 AZ vs. CQ | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.71] |
| 1.2 AZCQ vs. CQ | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.19, 0.70] |
| 1.3 AZCQ vs. AZ monotherapy | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.21, 0.82] |
| 1.4 AZCQ vs. SPCQ | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.67, 3.27] |
| 1.5 AZCQ vs. MQ | 2 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.06, 1.36] |
| 1.6 AZ‐artesunate vs. AZ‐quinine (arm 1 vs. 4) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.01, 0.37] |
| 2 All adverse events (any cause) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 AZCQ vs. SPCQ | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.75, 2.32] |
| 2.2 AZCQ vs. atovaquone‐proguanil | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.09, 1.83] |
| 2.3 AZCQ vs. MQ | 2 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.10] |
| 2.4 AZ‐artemether vs. doxycycline‐artemether | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.09, 0.88] |
| 2.5 AZ‐artesunate vs. artemether‐lumefantrine | 1 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.81, 1.80] |
Analysis 2.1.
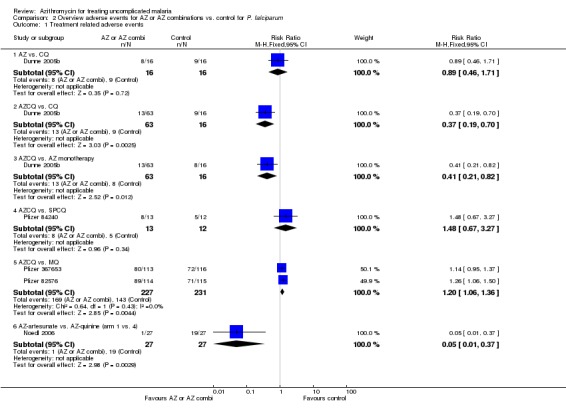
Comparison 2 Overview adverse events for AZ or AZ combinations vs. control for P. falciparum, Outcome 1 Treatment related adverse events.
Analysis 2.2.
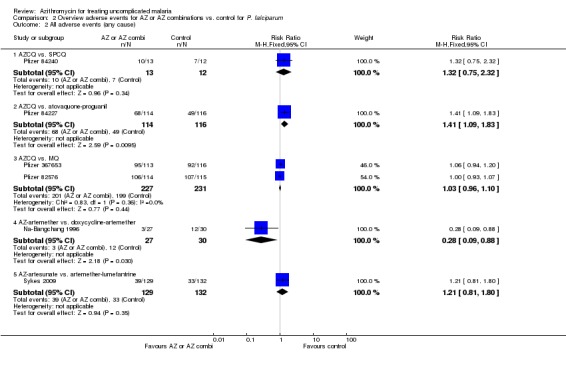
Comparison 2 Overview adverse events for AZ or AZ combinations vs. control for P. falciparum, Outcome 2 All adverse events (any cause).
What's new
| Date | Event | Description |
|---|---|---|
| 14 March 2011 | Amended | Additional tables moved to appendices to aid readability |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 2, 2011
| Date | Event | Description |
|---|---|---|
| 18 July 2008 | Amended | Converted to new review format. |
Differences between protocol and review
Although we initially intended to assess the possibility of subgroup analysis by species of malaria, it proved irrelevant to compare studies for P. vivax directly with studies for P. falciparum given the different efficacy of the drugs used for these species. We decided instead to keep all data stratified by malaria species. The trials excluded for meta‐analyses were included in the assessment of adverse events and drug efficacy, because they provided valuable information for these outcomes. We believe that excluding these trials would deprive the reader of a complete overview of the topic. This review was conducted over several years and because of the adoption of new methods by the Cochrane group for assessing risk of bias, we changed our assessment accordingly using the revised guidelines in 2008.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods |
Trial design: Double blind, randomized, controlled trial with two arms. Follow‐up: Hospitalized, eight‐hourly blood smears. Medical history and exam at baseline. Discharged when two consecutive blood smears negative. Follow up and blood smears on day 7, 14, and 28. Adverse event monitoring: Not reported. G6PD test at baseline. Adverse event data for those analysed. |
|
| Participants |
Number enrolled: 200 Number available for analysis: 199 Inclusion criteria: 18 to 65 years, Plasmodium vivax infection with parasite density < 100,000 parasites/µl in blood smear, Parasight F test negative for Plasmodium falciparum, a history of fever in the previous 48 hours, written informed consent. Exclusion criteria: Impaired consciousness, jaundice, respiratory distress; haematuria (self‐report); treated with an antimalarial or antibiotic with antimalarial activity in the previous 15 days; laboratory evidence or history of significant cardiovascular, liver or renal functional abnormality that in the opinion of the investigator would place them at increased risk; a serum glucose level less than the lower limit of normal; a history of allergy to or hypersensitivity to study drugs; a blood transfusion in the previous 28 days; any situation that would prevent follow up visits. Age range: Not reported. Mean age (SD): 31.7 (11.6) years in arm 1, 30.0 (11.8) in arm 2. Proportion male: 159/200 (79.5%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 1 gram per day for three days plus chloroquine placebo arm 2) Chloroquine 600 mg per day for two days, 300 mg on day three plus azithromycin placebo Supervision of intake: Not reported, but participants initially hospitalised. Other medication: Primaquine provided from day 7 to day 20. No report on symptomatic treatment. |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (secondary endpoint for this study) Parasite clearance time Presence of gametocytes on day 7 Adverse events (Fever clearance time mentioned as an outcome, but results not reported) |
|
| Notes |
Location: India, six sites (New Delhi, Baroda, Berhampur, Karamsad, Jabalpur and Guwhati) Date: July 1998 to October 2001 Malaria endemicity: Stable throughout the year, increased incidence in rainy season (Dunne 2005b) Drug resistance: Not reported Method of determining parasite density: Giemsa stained thin and thick smear, otherwise no details. Baseline data: Baseline data for gender, age, days of illness, mean P. vivax parasite density, reported for those randomized. Look similar. Drug source: Not reported Source of funding: Pfizer Global Research and Development, Inc. Other notes: RII and RIII failures on day three were not a routine reason to treat with alternative therapies. The investigators report: “Patients who the investigator felt were not responding to therapy were given alternative therapies at their discretion, including quinine and mefloquine.” Slower resolution of parasitaemia and fever in azithromycin group may have biased findings to earlier labelling of treatment failure. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | "Study drug was provided to sites in blocks of four." |
| Allocation concealment? | Unclear risk | "Sealed envelopes were available at each site to be opened only in case of emergencies. The integrity of these envelopes was monitored periodically at each site." |
| Blinding? All outcomes | Low risk | All parties remained blinded to treatment assignment |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss to follow up: 98 and 102 randomised in respective groups, 97 (99.0%) and 102 (100%) results at day 28. Not clear why one participant was excluded. |
| Free of selective reporting? | High risk | Definition of treatment failure potentially subjective. Slower resolution of parasitaemia and fever in azithromycin group may have biased findings to earlier labelling of treatment failure. Fever clearance time results not reported. No follow‐up visit at day 21. |
| Free of other bias? | Unclear risk | Not clear how much study sponsor was involved in reporting of results and adverse events |
| Methods |
Trial design: A placebo‐controlled, randomized trial for two arms, and open label for one arm. Follow‐up: Hospitalized and eight‐hourly blood smears until two consecutive smears negative. Medical history, physical examination and blood smear once a day until day 7, and on day 14, and 28. Adverse event monitoring: Not reported. G6PD at baseline, blood count, bilirubin and aspartate aminotransferase at baseline and day 3. Adverse event data for those treated. |
|
| Participants |
Number enrolled: 32 in trial and 64 in open‐label study Number available for analysis: 29 in trial and 63 in open‐label study Inclusion criteria: 18 to 65 years, Plasmodium falciparum infection with parasite density < 100,000 parasites/µl in blood smear, Parasight F test or ICT positive for Plasmodium falciparum, a history of fever > 38°C in the previous 48 hours, and written informed consent. For women in addition: negative pregnancy test, and willing to use contraception during and three months after the study. Exclusion criteria: Impaired consciousness, jaundice and/or respiratory distress; haematuria (self‐report); a serum glucose level less than the lower limit of normal; pregnant or lactating; treated with any antimalarial drug in the previous 15 days; a blood transfusion in the previous 28 days; laboratory or clinical evidence of significant abnormality of cardiovascular, liver or renal function. Age range: not reported; mean of 30.2 years in arm 1, 31.8 in arm 2 and 31.7 in arm 3 Proportion male: 29/32 (90.6%) in trial and 53/64 (82.8%) in open‐label arm Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 1 gram per day for three days plus chloroquine placebo arm 2) Chloroquine 600 mg per day for two days, 300 mg on day three plus azithromycin placebo arm 3) Azithromycin 1 gram per day for three days plus chloroquine 600 mg per day for two days, 300 mg on last day Supervision of intake: Yes Other (symptomatic) medication: Not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (secondary endpoint for this study) Parasite clearance time Adverse events |
|
| Notes |
Location: India, six sites (New Delhi, Baroda, Berhampur, Karamsad, Jabalpur and Guwhati; Dunne 2005a) Date: July 1998 to October 2001 Malaria endemicity: Stable throughout the year, increased incidence in rainy season. Drug resistance: Not reported Method of determining parasite density: Giemsa‐stained thick and thin smears, count of asexual parasites per 200 white blood cells (WBCs) in the thick blood smear, using the WBC count from the coincident hematologic evaluation at baseline. Negative if no parasites in 50 high‐power fields. Baseline data: Baseline date for gender, age, days of illness, mean P. falciparum parasite density, and hematocrit by treatment arm. Open‐label study: lower baseline parasite density compared to participants in randomized blind trial. Other variables are similar. Drug source: Pfizer Global Research and Development Source of funding: Pfizer Global Research and Development Other notes: No clear criteria for failure; RI‐III criteria not reported for specific time point. The investigators report: “Any participant who, in the opinion of the investigator, had not sufficiently improved received additional antimalarial therapy.” Data presented for “evaluable participants” who had baseline falciparum parasitaemia < 100,000 parasites/µl, had completed three days of therapy, and had an assessment of fever (considered to be the highest temperature recorded that day), all occurring within the appropriate time frame. Open‐label arm not recruiting at the same time as other arms (historical comparison). Lower baseline parasitaemia in open label arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | None described. "Participants were randomly assigned to one of the following groups.." |
| Allocation concealment? | Unclear risk | None described |
| Blinding? All outcomes | High risk | Incomplete blinding, placebo used in two arms but not in third arm |
| Incomplete outcome data addressed? All outcomes | Low risk | Moderate loss to follow‐up. Blinded part of trial: 16 and 16 randomised in respective groups, 14 (87.5%) and 15 (93.8%) results at day 28. Two lost to follow‐up, 1 not evaluable. Open‐label study: 64 randomized, 63 (98.4%) results available at day 28. One lost before taking drug. |
| Free of selective reporting? | High risk | Definition of treatment failure potentially subjective. Third arm not at the same time as other arms (historical comparison). No follow‐up visit at day 21. A planned Interim analysis was conducted, leading to the abandonment of arm 1 and 2, and the introduction of arm 3. |
| Free of other bias? | Unclear risk | Not clear how much study sponsor was involved in reporting of results and adverse events. |
| Methods |
Trial design: An open‐label, randomized, controlled trial. Follow‐up: Participants were hospitalised. The oral temperature, pulse and respiratory rate were obtained every four hours. Blood pressure was measured once daily. Twelve‐hourly blood smears until negative, then once daily for 28 days in hospitalised participants or weekly after discharge. Adverse event monitoring: All participants were admitted for 28 days in order to observe adverse events; daily examinations for the first seven days and weekly thereafter. Laboratory tests at baseline: blood count, urinalysis, liver function, blood urea nitrogen, creatinine and serum electrolytes. Repeated weekly until day 28. Number of participants assessed for adverse events not explicit. |
|
| Participants |
Number enrolled: 202 Number available for analysis: 171 Inclusion criteria: At least 14 years, body weight at least 40 kg, asexual forms of Plasmodium falciparum in blood smear, informed consent. Exclusion criteria: Signs of severe or complicated malaria (WHO criteria 1990); inability to tolerate oral medications; pregnant or lactating; allergy or sensitivity to drugs or a history of serious allergy to any medication; consumption of any antimalarial drug therapy within two weeks prior to admission. Age range: 12 to 61 years; mean (SD) 24.8 (8.7) in arm 1, 24.5 years (8.8) in arm 2, 25.7 (8.4) in arm 3 Proportion male: 135/202 (66.8%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 500 mg plus artesunate 200 mg once a day for three days. arm 2) Artesunate 200 mg once a day for three days plus mefloquine 10 mg/kg on the first twp days and 5 mg/kg on last day. arm 3) Artesunate 200 mg once a day for three days. Supervision of intake: Yes Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint) Parasite and fever clearance time (mean) (primary endpoint) Adverse events |
|
| Notes |
Location: Bangkok, Thailand Date: December 1998 to May 1999 Malaria endemicity: No transmission in Bangkok Drug resistance: Not reported Method of determining parasite density: Giemsa stained thin and thick blood smears, count of asexual parasites per 200 WBCs in thick film or against 1000 red blood cells in thin films. Negative if no parasites in 200 high power fields in thick film. Baseline data: Gender, age, height, weight, duration of fever, first attack, P. falciparum density, hepatomegaly, splenomegaly, laboratory data (haemoglobin, WBC counts, kidney and liver functions) reported by treatment for those randomized. Look similar. Drug source: Not reported Source of funding: Not reported Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No description provided |
| Allocation concealment? | Unclear risk | "Each patient was randomly assigned to one of three treatment regimens..." |
| Blinding? All outcomes | High risk | Open label study |
| Incomplete outcome data addressed? All outcomes | High risk | Moderate loss to follow up: 67, 67, and 68 randomised in respective arms, and 55 (82.1%), 55 (82.1%) and 61 (89.7%) results at day 28 respectively. Loss to follow up for “reasons unrelated to drug treatment or side effects”. Those lost to follow up were asymptomatic and blood smear negative before dropping out. |
| Free of selective reporting? | High risk | Adverse events not reported by treatment arms |
| Free of other bias? | Unclear risk | Insufficient information |
| Methods |
Trial design: A sequential, open‐label trial. Follow up: Participants were hospitalised for 28 days or agreed to come for follow‐up weekly and stay in non‐endemic malaria area. Medical history, physical exam at baseline. Clinical signs and symptoms daily until day seven, and afterwards weekly. The oral temperature, pulse and respiratory rate were obtained every four hours. Blood pressure was measured once daily. Twelve‐hourly blood smears until negative, then once daily for 28 days in hospitalised participants or weekly after discharge. Adverse event monitoring: All participants were admitted for 28 days in order to observe adverse events; daily examinations for the first seven days and weekly thereafter. Routine haematology and biochemistry at baseline, repeated weekly until day 28. Number of participants assessed for adverse events not explicit. |
|
| Participants |
Number enrolled: 170 Number available for analysis: 134 Inclusion criteria: At least 15 years, body weight at least 40 kg, asexual forms of Plasmodium falciparum in blood smear, informed consent. Exclusion criteria: Signs of severe or complicated malaria (WHO criteria 2000); inability to tolerate oral medications; pregnant or lactating; allergy or sensitivity to drugs; consumption of any antimalarial drug therapy within two weeks prior to admission. Age range: 15 to 72 years; mean (SD) 27.0 (11.9) in arm 1, 25.2 years (11.1) in arm 2 Proportion male: 122/170 (71.8%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 500 mg plus dihydroartemisinin 80 mg once a day for three days. arm 2) Dihydroartemisinin 80 mg once a day for three days plus mefloquine 10 mg/kg on the first two days and 5 mg/kg on last day. Supervision of intake: Yes Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes: Total failure by day 28 (primary endpoint) (not used) Parasite and fever clearance time (primary endpoint) (not used) Adverse events (used) |
|
| Notes |
Location: Bangkok Hospital for Tropical Diseases, Bangkok, Thailand Date: Not reported Malaria endemicity: No transmission in Bangkok Drug resistance: Not reported Method of determining parasite density: Giemsa stained thin and thick blood smears, count of asexual parasites per 200 WBCs in thick film or against 1000 red blood cells in thin films. Negative if no parasites in 200 high power fields in thick film. Baseline data: Gender, age, duration of fever, first attack, P. falciparum density, hepatomegaly, splenomegaly, laboratory data (haemoglobin, WBC counts, kidney and liver functions) reported by treatment for those randomized. Look similar. Drug source: dihydroartemisinin: Cotexin, Beijing Cotec New Technology Corp. Beijing Wan Hui pharmaceutical group, China, 20 mg/tablet. Mefloquine: not reported Source of funding: Mahidol University Research Grant Other notes: Study included for adverse events, but not for efficacy analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Sequential assignment to one of two regimens |
| Allocation concealment? | High risk | Sequential assignment to one of two regimens |
| Blinding? All outcomes | High risk | Open‐label study |
| Incomplete outcome data addressed? All outcomes | High risk | Moderate loss to follow‐up, similar across arms: 82 and 88 enrolled in respective arms, 66 (80.5%) and 68 (77.3%) results at day 28 respectively. All persons lost were blood smear negative at discharge from the hospital. |
| Free of selective reporting? | High risk | Adverse events not reported by treatment arm |
| Free of other bias? | Unclear risk | Insufficient information; no date for study reported |
| Methods |
Trial design: Open‐label, randomized, controlled trial with three arms, followed by an open‐label study with one arm. Follow‐up: Hospitalized, medical history and examination on enrolment. Daily history of signs, symptoms and medication. Routine physical exam on "a periodic schedule". Blood smears twice daily until malaria cleared, then daily for a week, then weekly or as clinically warranted until day 28. Adverse event monitoring: All patients were evaluated daily during treatment for adverse events that were new in onset or aggravated in severity or frequency after administration of the study drugs. An adverse event was considered drug related if its relationship to treatment was rated definite or probably by a study clinician. Complete blood count, urinalysis, blood urea nitrogen, creatinine, glucose, ALT, gamma‐glutamyl transferase, urine pregnancy test (women only), and ECG at enrolment. Not clear when laboratory tests were repeated ("on a periodic schedule"). Adverse events reported for those treated. |
|
| Participants |
Number enrolled: 61 Number available for analysis: 60 Inclusion criteria: age ≥20 years; males and non‐pregnant females, visiting the OPD with fever; Plasmodium falciparum infection; written informed consent. Exclusion criteria: Known history of chronic illness; signs or symptoms of severe or complicated P. falciparum malaria (WHO 2000) or hyperparasitaemia (> 5% of red blood cells infected), or sustained hyperpyrexia (fever > 40 °C); significant liver dysfunction (ALT > 300IU/l); known pregnancy or positive urine test result for betha‐hCG; mixed malaria infection by Giemsa smear; drug therapy for P. falciparum in the previous 42 days; history of allergy to study medicines or concurrent use of drugs with known interactions with study drugs. Age range: 20 to 60 years, mean 36 (arm 1), 31 (arm 2), 35 (arm 3), 35 (arm 4) Proportion male: 44/60 (73.3%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 500 mg two times per day for three days plus quinine 10 mg/kg three times per day for three days. arm 2) Azithromycin 500 mg two times per day for five days plus quinine 10 mg/kg three times per day for five days. arm 3) Doxycycline 100 mg two times per day for seven days plus quinine 10 mg/kg three times per day for seven days. arm 4) Azithromycin 500 mg three times per day for three days plus quinine 10 mg/kg three times per day for three days: This arm was evaluated a rainy season after the other arms without comparator drug (so non‐randomized) Supervision of intake: Yes Other (symptomatic) treatment: Acetaminophen and dimenhydrate provided as needed for symptoms by participant. Other medications or intravenous fluids as prescribed by a physician |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint), PCR‐adjusted failure rate by day 28 Parasite and fever clearance time (secondary outcomes) Adverse events |
|
| Notes |
Location: Armed Forces Research Institute of Medical Sciences inpatient clinical trials centre, Kwai River Christian Hospital, Sanghlaburi district, Kanchanaburi Province, Thailand (western border with Myanmar). Date: July 1998 to October 2001 Malaria endemicity: Low seasonal transmission Drug resistance: Resistance in study area reported to chloroquine, sulphadoxine‐pyrimethamine and mefloquine Method of determining parasite density: Giemsa stained thick and thin smears; count of asexual and sexual parasites per 200 WBCs; if less than 10 parasites, counted against 500 WBCs. Negative if no parasites in 200 high‐power fields. Smears read on site, and re‐read by off‐site blinded microscopist. Parasite density was the mean of non‐discrepant values. Discrepant results decided by third microscopist. Baseline data: Gender, age, P. falciparum parasite density, temperature, baseline laboratory data (white blood cell count, red blood cell counts, platelets, kidney and liver functions), reported by treatment arm for those analysed. Look similar. Drug source: Quinine sulphate: Zenith Goldline Pharmaceuticals, Miami, Fl, provided by Walter Reed Army Medical Center (Washington DC); Doxycycline hyclate: Qualitest Parmaceuticals, Huntsville, AL, provided by Walter Reed Army Medical Center (Washington DC); Azithromycin: produced and provided by Pfizer Inc, New York, NY. Source of funding: Pfizer Inc. and U.S. Army Medical Research and Materiel Command. Other notes: Enrolment in high dose azithromycin arm in later (different) malaria season and without comparator arm (historical comparison), so might not have been randomized. Facility had screens on windows, so low risk on re‐infection inside the facility. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | A block randomization table of 10 QAZ to 2 QD subjects was generated. To improve power of the study, enrolment to a well‐tolerated QAZ arm was increased with 10 (year 1). In the subsequent year's malaria season (year 2), an additional regimen with a higher daily azithromycin regimen was assessed without comparator. Thirty women were enrolled in year 1 in an azithromycin regimen, but there were 10 in the QD arm, whereas there should have been six. Volunteers withdrawing before trial completion were replaced in the randomization schedule (applicable to one participant in an azithromycin arm). |
| Allocation concealment? | Unclear risk | Not reported if and how allocation was concealed |
| Blinding? All outcomes | High risk | Microscopists were blinded to treatment regimen (reported by authors). Otherwise open label |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss to follow‐up: one participant elected to withdraw on day two and was replaced |
| Free of selective reporting? | High risk | One arm was not at the same time as the other three regimens (not randomized) |
| Free of other bias? | Unclear risk | Not clear how much study sponsor was involved in reporting of results and adverse events |
| Methods |
Trial design: Open‐label, randomized, controlled trial with two arms. Follow up: Physical exam at baseline. Hospitalized until day 28, blood smear every six hours until negative, then daily until day 28. Adverse event monitoring: Monitoring of adverse events was performed daily until day 7, then once weekly until day 28. Baseline date for age, parasite density (P. falciparum), and red blood cell count, among those randomized. Look similar. Adverse events reported among participants who completed the study. |
|
| Participants |
Number enrolled: 60 Number available for analysis: 57 Inclusion criteria: 15 to 59 years, uncomplicated Plasmodium falciparum infection with density < 100,000/µl, willing to provide informed consent Exclusion criteria: History of mefloquine or quinine treatment over the last four weeks (confirmed by drug levels determined in blood using HPLC) Age range: 15 to 59 years, median 23 in group 1, 25 in group 2. Proportion male: 60/60 (100%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 500 mg at start, 250 mg after 24 and 48 hrs, plus artemether 300 mg once at start. arm 2) Doxycycline 100 mg every 12 hrs for five days, plus artemether 300 mg once at the start and 100 mg after 12 hours. Supervision of intake: Yes Other (symptomatic) medication: Not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 Parasite and fever clearance time Adverse events |
|
| Notes |
Location: Mae Sot, Tak Province, Thailand (Thai‐Myanmar border) Date: June to August 1995 (rainy season) Malaria endemicity: Not reported Drug resistance: Resistance in study area reported to chloroquine, sulphadoxine‐pyrimethamine, and mefloquine. Method of determining parasite density: Giemsa stained thick and thin smear, parasite count per 1000 red blood cells or per 200 WBC. No definition of negative smear. Drug source: artemether: Artenam, Arenco nv, Belgium; doxycycline: Vibramycin, Pfizer; azithromycin: Zithromax, Pfizer. Source of funding: Japan Association of Tropical Medicine Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No information |
| Allocation concealment? | Unclear risk | No information |
| Blinding? All outcomes | High risk | Not blinded for participants or investigators, not clear for microscopist. |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss to follow‐up, similar across arms: 30 and 30 randomized in respective groups, 27 (90%) and 30 (100%) results at day 28. Three were lost to follow‐up; reason not specified, but reportedly not related to malaria or adverse effects at the time of study departure. |
| Free of selective reporting? | High risk | Only males included |
| Free of other bias? | Unclear risk | Insufficient information |
| Methods |
Trial design: Open‐label, randomized clinical trial with four arms. Follow up: Hospitalized until day 28. Physical exam, vital signs, clinical signs and symptoms and medication history daily until day 7, and on day 14, 21, 28 or when needed. Blood smears twice daily until negative on at least two successive smears, then weekly on day 7, 14, 21 and 28 or when signs or symptoms of malaria. Adverse event monitoring: Adverse events recorded daily until day 7, and then on day 14, 21, 28 or whenever adverse event occurred. Laboratory tests (blood cell counts and chemistry) on day 0, 3, 7, 14; on day 21 and 28 if abnormal. ECG on day 0, 1 and 3. Urinalysis on day 0, 3, (28 if abnormalities). All randomized subjects were analyzed for safety. |
|
| Participants |
Number enrolled: 97 Number available for analysis: 92 Inclusion criteria: 20 to 65 years; men and non‐pregnant women; acute uncomplicated falciparum malaria with density of asexual parasites of 100/µl to 100,000/µl; febrile (37.5 °C during the current illness or history of fever within the last 48 hours). Exclusion criteria: Pregnant or nursing; signs and symptoms of severe malaria; mixed malaria infection at the time of admission to the hospital; received malaria drug therapy or blood transfusions in the preceding 30 days; laboratory evidence or history of significant cardiovascular, liver, or renal functional abnormality; severe vomiting; report of alcohol or drug abuse; any clinically significant illness; likelihood that patient would require treatment with drugs not permitted by the protocol. Age range: Not reported, mean (SD) 28.0 (7.1) in arm 1, 28.8 (6.9) in arm 2, 26.4 (6.0) in arm 3, 27.7 (7.6) in arm 4. Proportion male: 86/97 (88.7%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 750 mg twice a day plus artesunate 100 mg twice a day for three days arm 2) Azithromycin 1 g once a day plus artesunate 200 mg once a day for three days arm 3) Azithromycin 750 mg twice a day plus quinine 10 mg/kg twice a day for three days arm 4) Azithromycin 500 mg three times a day plus quinine 10 mg/kg three times a day for three days Supervision of intake: Yes, interventions taken with meals or a snack Other (symptomatic) treatment: Acetaminophen and dimenhydrate provided as needed by participant. Other medications or intravenous fluids as prescribed by physician. |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint) Parasite and fever clearance time (secondary endpoints) Adverse events |
|
| Notes |
Location: Bangkok, Thailand (Hospital for Tropical Diseases, Mahidol University) Date: 23 May 2004 to 2 May 2005 Malaria endemicity: Intermittent and unstable Drug resistance in area: Chloroquine, sulfadoxine‐pyrimethamine, mefloquine, quinine Method of determining parasite density: Staining not reported. Parasite count and gametocyte count per 200 WBC in thick film or per 1000 red blood cells on thin film if too numerous. Negative if no parasite in 200 oil‐immersion fields in thick smear. Smears read on site, and re‐read by off‐site blinded microscopist. Discrepant results re‐read by third reader for final result. Microscopists blinded to treatment arms. Baseline data: Gender, age, weight, height, enrolment P. falciparum density by treatment regimen for those randomized. Look similar. Drug source: Quinine: provided by Walter Reed Army Medical Center; artesunate: Plasmotrim‐50 Lactab, Mepha, purchased locally; azithromycin: Zithromax, produced and provided by Pfizer. Source of funding: Global Pharmaceuticals Pfizer and National Institutes of Allergy and Infectious Diseases, National Institute of Health. Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Patients were randomly assigned to 1 of 4 treatment groups according to a statistical series based on random sampling numbers that had been drawn up for each patient by the study staff." |
| Allocation concealment? | Low risk | "The details of the series were unknown to any of the investigators or to the coordinator and were contained in a set of sealed envelopes." |
| Blinding? All outcomes | High risk | Open‐label but microscopists were blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss to follow‐up, similar across arms: 27, 27, 16, and 27 randomised in respective arms, 25 (92.6%), 27 (100%), 15 (93.8%) and 25 (92.6%) results at day 28. Four lost because of non‐compliance with follow‐up; 1 discontinued because of severe adverse event (food poisoning unrelated to study drugs). Enrolment in arm 3 halted after three early treatment failures. |
| Free of selective reporting? | High risk | Arm 3 halted prematurely because of treatment failures |
| Free of other bias? | Unclear risk | Insufficient information |
| Methods |
Trial design: Multi‐centre, non‐randomized, open‐label clinical trial with one treatment regimen. Follow up: Hospitalized for at least three days or until three consecutive peripheral negative smears. Follow up visits on day 7, 14, 21, 28, 35 and 42 Adverse event monitoring: All treated subjects analyzed for adverse events. No other information available. |
|
| Participants |
Number enrolled: 110 Number available for analysis: 107 Inclusion criteria: Males and females ≥18 years with uncomplicated, symptomatic malaria as indicated by the presence of blood smears positive for P. falciparum asexual parasitemia between 1000 and 100 000 parasites/µl and documented fever ≥38.5 ºC/101.3 F rectal or ≥38 ºC/100.4 F oral or history of fever as reported by subject within the prior 24 hours, positive rapid diagnostic test, and written informed consent Exclusion criteria: Subjects with severe or complicated malaria. Pregnant or nursing women. Age range: 18 to 77, mean 31.0 years, no SD reported Proportion male: 85/110 (77.3%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 2 g plus chloroquine 600 mg once a day for three days Supervision of intake: Yes Other (symptomatic) treatment: Information not available |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint) Parasite and fever clearance time (secondary endpoints, not used) Adverse events (used in this review) |
|
| Notes |
Location: Tumaco, Colombia, and Goa, India Date: March 2006 to February 2008 Malaria endemicity: Information not available Drug resistance in area: Information not available Method of determining parasite density: Information not available Baseline data: Age, race, weight Drug source: Not reported Source of funding: Pfizer Other notes: Information from the ASTMH presentation in December 2008, as kindly provided by Dr. Chandra, and the entry in clinicaltrials.gov |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | Single arm efficacy study |
| Allocation concealment? | High risk | Single arm efficacy study |
| Blinding? All outcomes | High risk | Open label |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss to follow‐up. 110 participants enrolled, results for 107 (97%) on day 28. No explanation provided for loss of three participants. |
| Free of selective reporting? | High risk | Not all of the study’s pre‐specified outcomes have been reported, scanty information on protocol |
| Free of other bias? | Unclear risk | Not clear if and how sponsor was involved in analysis and reporting |
| Methods |
Trial design: Multi‐centre, open‐label, randomized, controlled trial with two treatment regimens Follow up: Hospitalized, eight‐hourly blood smear until three consecutive smears were negative for P. falciparum and the investigators deemed discharge from the hospital appropriate. Follow‐up visits and blood smears were conducted on day 3, 7, 14, 21, 28, 35 and 42, or when needed. Adverse event monitoring: Safety evaluations included adverse event assessments, haematology and serum chemistry laboratory tests, physical exams and vital sign measurements. Adverse events were reported using preferred terms (MedDRA: Medical Dictionary for Regulatory Activities). All treated subjects were analyzed for adverse events. |
|
| Participants |
Number enrolled: 229 Number available for analysis: 219 Inclusion criteria: Females and males ≥18 years of age with uncomplicated, symptomatic monoinfection with P. falciparum malaria as indicated by the presence of both of the following: a) blood smears positive for Plasmodium falciparum asexual parasitaemia between 1000 and100,000 parasites; b) documented fever (38.5 ºC/101.3 F rectal or tympanic; 37.5 ºC/99.5 F axillary or 38 ºC/100.4 F oral) or history of fever (as reported by subject) within the previous 24 hours. Exclusion criteria: Severe or complicated malaria; pregnant or breast‐feeding women. Age range: 17 to 71 years, mean age (SD): 30.2 (11.0) years in arm 1; 31.2 (12.4) years in arm 2. Proportion male: 128/229 (55.9%) Pregnant women: Excluded |
|
| Interventions | Arm 1) Azithromycin 1 g and chloroquine 600 mg once a day for three days Arm 2) Mefloquine 750 mg at start and 500 mg six to 10 hours later Supervision of intake: Yes, intake with a minimum of 240 ml water Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28, PCR‐adjusted failure rate by day 28 (primary endpoint) Adverse events (secondary endpoint) |
|
| Notes |
Location: Seven centres in Africa: Nouna and Ouagadougou in Burkina Faso, Navrongo in Ghana, Kisumu in Kenya, Bamako in Mali, Senegal, Ndola in Zambia. Date: 6 November 2006 to 19 September 2007 Malaria endemicity: Not reported Drug resistance in area: Not reported Method of determining parasite density: Giemsa stain, otherwise no information Other Laboratory tests: Haematology and serum chemistry, not clear how often repeated Baseline data: age, gender, weight, height, parasite density, and participants with a weight < 35 kg. Look similar Drug source: Azithromycin: Zithromax, Pfizer Inc. Source of funding: Pfizer Inc. Notes: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described. Only described as 'randomized in a 1:1 ratio' |
| Allocation concealment? | Unclear risk | None described |
| Blinding? All outcomes | High risk | Open‐label study. Microscopists were blinded. |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow‐up low and similar in both groups: 113 and 116 randomised in respective arms (1 and2, see below), 107 (94.7%) and 112 (96.6%) completed, respectively, reason for study departure of 10 participants is not clear. |
| Free of selective reporting? | High risk | Not all outcomes described in methods section reported |
| Free of other bias? | Unclear risk | Not clear if and how the sponsor was involved in analysis and writing of report |
| Methods |
Trial design: Multi‐centre, randomized, controlled trial with three treatment regimens, blinded for the azithromycin dose. Follow‐up: Subjects were hospitalised for a minimum of three days with eight‐hourly blood smears until three consecutive blood smears were negative for asexual parasitaemia and the investigator deemed discharge from hospital inappropriate. Post‐therapy evaluations and blood smears at day 3, 14, 21, 28, 35 and 42. Adverse event monitoring: "Safety evaluations included adverse events throughout the study, hematology and serum chemistry laboratory evaluations at baseline and day 3, and a complete (day 0) and focused (day 1 to 3, 7, 28) physical exam." Use of COSTART (Coding Symbol Thesaurus of Adverse Reaction Terms) terms for description of adverse events. Adverse events reported for all treated subjects who received at least one dose of study medication. |
|
| Participants |
Number enrolled: 230 Number available for analysis: 204 Inclusion criteria: Females and males ≥18 years of age with uncomplicated, symptomatic malaria as indicated by the presence of both of the following: a) blood smear positive for Plasmodium falciparum asexual parasitaemia between 1000 and100,000 parasites/µL; b.) fever or history of fever (38.5 ºC/101.2F rectal or tympanic; ≥37.5 ºC/99.5 F axillary or ≥38 ºC/100.4F oral) within the prior 24 hours; serum glucose ≥ 60 mg/dL (by fingerstick or peripheral blood collection); positive rapid diagnostic test (Binax NOW ICT) positive for P. falciparum; women of childbearing potential had a negative urine gonadotropin prior to entry into the study and agreed to use adequate contraception during the entire study. Exclusion criteria: Severe or complicated malaria, including subjects with any of the following: a) impaired consciousness (eg obtundation, unarousable coma), seizures or abnormal neurologic exam suggestive of severe or complicated malaria; b) haemoglobinuria; c) jaundice; d) respiratory distress (respiratory rate >= 30/min); e) persistent vomiting; f) haematuria, as reported by the patient; pregnant or breast‐feeding women; history of allergy to or hypersensitivity to azithromycin or any macrolide, sulphonamides, pyrimethamine, or chloroquine; known or suspected folate deficiency; known history of blood dyscrasias (eg, megaloblastic anaemia, agranulocytosis, aplastic anaemia, thrombocytopenia, leukopenia, neutropenia, haemolytic anaemia); known G‐6PD deficiency; history of epilepsy or psoriasis; history of treatment with any antimalarial drug (chloroquine, quinine, mefloquine, Malarone, sulphadoxine‐pyrimethamine, artemisinin compounds) or antibacterial with known antimalarial activity (macrolides, doxycycline, clindamycin) within two weeks prior to enrolment into the study; known or suspected cardiovascular, hepatic or renal abnormality that in the opinion of the investigator would place the subject at increased risk if they participated in the study. The following findings were specific exclusions: a) serum creatinine > 2.0 x ULN; b) ALT and/or AST > 3 x ULN; inability to swallow oral medication in tablet form; treatment with other investigational drugs within 30 days prior to enrolment into the study; alcohol and/or any other drug abuse; requirement to use medication during the study that might interfere with the evaluation of the study drug; specific systemic diseases or other medical conditions that would interfere with the evaluation of the therapeutic response or safety of the study drug; inability to comprehend and/or unwillingness to follow the study protocol; prior participation in this study. Age range: 18 to 75 years, mean age (SD) 31.7 (12.9) years in arm 1; 29.5 (9.6) years in arm 2; 31.7 (11.1) years in arm 3 Proportion male: 199/230 (86.5%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 1 gram plus chloroquine 600 mg once a day for three days arm 2) Azithromycin 500 mg plus azithromycin placebo plus chloroquine 600 mg once a day for three days arm 3) Sulfadoxine‐pyrimethamine (500 mg/25mg), three tablets at start plus chloroquine 600 mg once a day for two days and 300 mg for one day. Arm 2 removed based on second interim analysis when 190 subjects were enrolled (156 in database) on request of DSMB. Supervision of intake: Yes Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint) Adverse events |
|
| Notes |
Location: India: Indore, Dispur Guwahati (Assam), Bambolim (Gao), Parel Mumbai (Maharashtra), Nagpur (Maharashtra), Rourkela (Orissa), Vellore (Tamil Nadu). Date: 01 September 2003 to 31 January 2005 Malaria endemicity: Not reported Drug resistance in area: Not reported Method of determining parasite density: Not reported Baseline data: Age, duration of infection by treatment arm. Look similar Drug source: Azithromycin: Zithromax, Pfizer Inc. Not reported for chloroquine or sulfadoxine‐pyrimethamine Source of funding: Pfizer Inc. Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | None described |
| Blinding? All outcomes | High risk | Blinded for azithromycin dose in arm 1 and 2, until arm 2 was removed. Then open label. Not clear if microscopists were blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Losses to follow‐up similar in all groups: 83, 67 and 80 randomized in respective arms; 73 (88.0%), 59 (88.1%) and 72 (90.0%) results at day 28; 17 subjects lost to follow up (7, 7, and 3 respectively) and reasons for loss of other nine not clear. |
| Free of selective reporting? | High risk | Two interim analyses conducted: one planned and one at the request of the DSMB. One arm discontinued. |
| Free of other bias? | Unclear risk | Not clear if and how the sponsor was involved in analysis and writing of report |
| Methods |
Trial design: Single‐centre, randomized, placebo‐controlled trial with two treatment regimens. Follow‐up: Not clear if hospitalised. Daily blood smears between day 0 and 6 until three consecutive smears were negative. Then weekly on day 7, 14, 21 and 28. Evaluations included assessment of vital signs (blood pressure, heart rate, respirations, temperature), clinical signs and symptoms, and a physical exam. Focused physical exams were performed for the first three days, and as needed based on clinical presentation. Adverse event monitoring: Adverse events evaluated daily at first three days, and as needed. Laboratory tests on day 7, if abnormal repeated on day 14. Type of tests not specified. All randomized participants were evaluated for adverse events. |
|
| Participants |
Number enrolled: 14 Number available for analysis: 13 Inclusion criteria: 18 to 60 years; healthy male and female subjects; asymptomatic P. falciparum mono‐infection with a parasite density of 1000 to 30,000/µl; willingness to sign and ability to understand consent form; willingness and ability to return for scheduled follow up visits. Exclusion criteria: Mixed malaria infection by Giemsa smear; history of allergy to or hypersensitivity to chloroquine, azithromycin or other macrolides (eg erythromycin, clarithromycin); any of the following: a) antimalarial therapy administered in the past four weeks, including quinine therapy or an artemisinin derivative; or b) an antibacterial with known antimalarial activity (including erythromycin, doxycycline, clindamycin, cotrimoxazole) within one week prior to enrolment into the study; fever, history of fever in past 48 hours, or signs/symptoms of malaria (including acute or subacute headache, nausea, or vomiting); inability to swallow oral medication; laboratory evidence or history of significant cardiovascular, liver, haematologic or renal functional abnormality; any situation that could prevent the patient from returning for follow‐up visits; pregnancy or breast feeding; any other concurrent illness that may confound the result; any other condition or circumstance that in the opinion of the investigator may pose a threat to the study participant or study. Age range: 19 to 53 years, mean 39.3 year in arm 1 and 28.7 years in arm 2. Proportion male: 7/14 (50%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 1 g plus chloroquine 600 mg once a day for three days arm 2) Azithromycin placebo plus chloroquine 600 mg once a day for three days Supervision of intake: yes Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (secondary endpoint) Adverse events |
|
| Notes |
Location: One centre in western Kenya Date: 02 August 2004 to 09 November 2004 Malaria endemicity: Intense malaria transmission Drug resistance in area: Not reported Method of determining parasite density: Not reported Baseline data: Age, gender, weight, BMI, of randomised participants by treatment regimen. Younger age in chloroquine‐only group. Drug source: Azithromycin: Zithromax, Pfizer Inc. Not reported for chloroquine. Source of funding: Pfizer Inc. Other notes: Trial terminated prematurely due to inability to recruit the planned number of subjects (487 screened resulted in 14 enrolled). There were no safety or efficacy concerns regarding the study in the decision to terminate the trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | None reported |
| Blinding? All outcomes | Low risk | Placebo controlled |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss to follow‐up higher in one group; however, groups were small: 7 and 7 randomised in respective arms, 5 and 7 results at day 28. One participant was withdrawn because the person was treated with CoArtem on day 8 by an outside health care facility, one participant was withdrawn because of lack of smears on follow up dates. |
| Free of selective reporting? | High risk | Study was closed prematurely due to a lack of subjects meeting protocol criteria. |
| Free of other bias? | Unclear risk | Not clear if and how the sponsor was involved in analysis and writing of report |
| Methods |
Trial design: Multi‐centre, randomized, placebo‐controlled trial with three treatment regimens. Follow up: Subjects were hospitalised for at least three days until three consecutive blood smears were negative for asexual parasitaemia and the investigator deemed discharge appropriate. Post‐therapy evaluation visits and blood smears at day 3, 7, 14, 21, 28, 35 and 42. Adverse event monitoring: Safety evaluations included adverse event and vital sign monitoring throughout the study, haematology and serum chemistry laboratory evaluation (baseline and day 3), and a complete (day 0) and focused (day 1 to 3, 7, 28) physical exam. Use of COSTART terms for reporting adverse events. Adverse events reported for all treated subjects. |
|
| Participants |
Number enrolled: 238 Number available for analysis: 213 Inclusion criteria: Written informed consent; males and females ≥18 years with symptomatic, uncomplicated malaria as indicated by the presence of the following: a) blood smears with mono infection with P. falciparum with asexual parasitaemia between 1000 and 100,000 parasites/µl; b) fever or a history of fever (≥38.5 °C rectal or tympanic; ≥37.5 °C axillary or ≥38 °C oral) within the prior 24 hours; serum glucose ≥60 mg/dl or 3.3 mmol/l, and rapid diagnostic test (Binax , now ICT) positive forP. falciparum; subjects must be willing to be treated in the inpatient setting for a minimum of three days; women of childbearing potential had a negative urine gonadotropin prior to entry into the study and agreed to use adequate contraception during the entire study. Exclusion criteria: Severe or complicated malaria including subjects with any of the following: a) impaired consciousness (eg obtundation, unarousable coma), seizures (any seizure within 24 hours prior to enrolment) or abnormal neurologic exam suggestive of severe or complicated malaria; b) haemoglobinuria; c) jaundice; d) respiratory distress (respiratory rate ≥30/min); e) persistent vomiting; f) haematuria, as reported by the patient; presence of non‐falciparum species on microscopy; pregnant or breast‐feeding women; history of allergy to or hypersensitivity to azithromycin or any macrolide, mefloquine or related compounds (eg quinine and quinidine), or chloroquine; known or suspected folate deficiency; known history of blood dyscrasias (eg megaloblastic anaemia, agranulocytosis, aplastic anaemia, thrombocytopenia, leukopenia, neutropenia, haemolytic anaemia); known G‐6PD deficiency; history of epilepsy or psoriasis; history of treatment with any antimalarial drug (chloroquine, quinine, mefloquine, Malarone, sulphadoxine‐pyrimethamine, artemisinin compounds) or antibacterial with known antimalarial activity (macrolides, doxycycline, clindamycin) within two weeks prior to enrolment into the study; known or suspected cardiovascular, hepatic or renal abnormality that in the opinion of the investigator would place the subject at increased risk if they participated in the study. The following findings are specific exclusions: a) serum creatinine > 2.0 x ULN b) ALT and/or AST > 3 x ULN; active depression or a recent history of depression, generalized anxiety disorder, psychosis, schizophrenia or other major psychiatric disorders; inability to swallow oral medication in tablet form; treatment with other investigational drugs within 30 days prior to enrolment into the study; alcohol and/or any other drug abuse; requirement to use medication during the study that might interfere with the evaluation of the study drug; specific systemic diseases or other medical conditions that would interfere with the evaluation of the therapeutic response or safety of the study drug; inability to comprehend and/or unwillingness to follow the study protocol; prior participation in this study. Age range: 18 to 68 years, mean age not reported Proportion male: 134/238 (56.3%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 1 g plus chloroquine 600 mg once a day for three days plus matching placebo for mefloquine on day 0 arm 2) Azithromycin 500 mg and azithromycin placebo and chloroquine 600 mg once a day for three days plus matching placebo for mefloquine on day 0 arm 3) Mefloquine 750 mg at start followed by 500 mg after six hours, plus matching placebos for azithromycin and chloroquine once daily for three days After four months, arm 2 removed based on interim review of the entire program including studies in South America and India by the DSMB (November 2004). Supervision of intake: Yes, intake with at least 240 ml of water Other (symptomatic) medication: Not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (secondary endpoint), PCR‐adjusted failure rates by day 28 (primary endpoint, available for two of three arms) Adverse events |
|
| Notes |
Location: Five centres in Africa: Navrongo, Ghana; Nairobi, Kenya; Bamako, Mali; Jinja and Kampala, Uganda; Ndola, Zambia. Date: 28 June 2004 to 01 May 2006 Malaria endemicity: Not reported Drug resistance in area: Not reported Method of determining parasite density: Not reported Baseline data: No baseline data by treatment regimen provided Drug source: Azithromycin: Zithromax, Pfizer Inc. Source of funding: Pfizer Inc. Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | None reported |
| Blinding? All outcomes | Low risk | Placebo controlled |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss to follow‐up low and similar in group 1 and 3; higher in group 2, but this was small sample size: 114, 9 and 115 randomised in respective arms, 103 (90.4%), 7 (77.8%) and 103 (89.6%) results at day 28. Not clear how many defaulted and how many had adverse events by day 28. |
| Free of selective reporting? | High risk | One study regimen prematurely terminated. Outcomes were not reported by treatment regimens (fever clearance time, parasite clearance time, gametocyte clearance rate). Asexual P. falciparum clearance rate by day 28 not clearly reported. No baseline information by treatment arm. |
| Free of other bias? | Unclear risk | Not clear if and how the sponsor was involved in analysis and writing of report |
| Methods |
Trial design: Multi‐centre, randomized, placebo‐controlled trial with three treatment regimens. Follow‐up: Hospitalized with eight‐hourly blood smears until three consecutive blood smears were negative for asexual parasitaemia and the investigator deemed discharge from the hospital appropriate. Insecticide treated net at discharge. Post‐therapy evaluation visits and blood smears at day 7, 14, 21, 28, 35 and 42. Adverse event monitoring: Safety evaluations included adverse event and vital sign monitoring throughout the study, haematology and serum chemistry evaluations (baseline and day 3), and a complete (day 0) and focused (day 1 to 3, 7, 28) physical exam. Use of COSTART terms for the presentation of adverse events. Evaluated for adverse events all subjects who received at least one dose of study medication. |
|
| Participants |
Number enrolled: 244 Number available for analysis: 225 (group 1 and 3) Inclusion criteria: Written informed consent; male and female subjects ≥18 years with symptomatic, uncomplicated mono‐infection with P. falciparum as indicated by the following: a) blood smear with asexual parasitaemia between 1000 and 40,000 parasites/µl; b) fever or a history of fever (≥38.5 °C rectal or tympanic; ≥37.5 °C axillary or ≥38 °C oral) within the prior 24 hours; serum glucose ≥60 mg/dl or 3.3 mmol/l; rapid diagnostic test (Binax NOW ICT) positive forP. falciparum; willing to be treated in inpatient setting for a minimum of three days or more until parasitaemia has cleared and the investigator deems the subject fit for discharge; negative urine gonadotropin urine test among women of child‐bearing potential; willingness in the last group to use adequate contraception during the study and one month after the last visit. Exclusion criteria: Severe or complicated malaria including subjects with any of the following: a) impaired consciousness (eg obtundation, unarousable coma, delirium, stupor), seizures (any seizure within a 24 hour prior to enrolment) or abnormal neurologic exam suggestive of severe or complicated malaria; b) haemoglobinuria; c) jaundice; d) respiratory distress (respiratory rate of 30 breaths/minute or more); e) persistent vomiting; f) haematuria, as reported by the patient; pregnant or breast‐feeding women; history of allergy to or hypersensitivity to azithromycin or any macrolide, atovaquone, proguanil or chloroquine; concomitant administration of rifampin or rifabutin and metoclopramide; history of epilepsy or psoriasis; history of treatment with any antimalarial drug (chloroquine, quinine, mefloquine, atovaquone/proguanil, sulphadoxine‐pyrimethamine, artemisinin compounds) or antibacterial with known antimalarial activity (macrolides, doxycycline, clindamycin) within two weeks prior to enrolment into the study; known or suspected cardiovascular, hepatic or renal abnormality that in the opinion of the investigator would place the subject at increased risk if they participated in the study. The following findings are specific exclusions: a) known or suspected creatinine clearance < 30 mL/min; b) ALT and/or AST > 3 x upper limit of normal; inability to swallow oral medication in tablet or capsule form; treatment with other investigational drugs within 30 days prior to enrolment in the study; alcohol and/or any other drug abuse; requirement to use medication during the study that might interfere with the evaluation of the study drug; specific systemic diseases or other medical conditions that would interfere with the evaluation of the therapeutic response or safety of the study drug; inability to comprehend and/or unwillingness to follow the study protocol; prior participation in this study. Age range: 18 to 86 years, mean age not provided Proportion male: 139/244 (57.0%) Pregnant women: Excluded |
|
| Interventions | arm1) Azithromycin 1 g plus chloroquine 600 mg once a day for three days plus matching placebo for atovaquone‐proguanil arm 2) Azithromycin 500 mg plus azithromycin placebo and chloroquine 600 mg once a day for three days plus matching placebo for atovaquone‐proguanil arm 3) Four capsules of atovaquone 250 mg‐proguanil 100 mg once a day for three days plus matching placebos for azithromycin and chloroquine once daily for three days. After four months, arm 2 was removed based on interim review of the entire programme including studies in India and additional treatment failures in South America by the DSMB in November 2004. In May 2005, recruitment in Surinam halted because of poor blinded efficacy. Supervision of intake: Yes, intake of interventions with food or milky drink Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint), PCR‐adjusted treatment failure by day 28 Gametocyte on day 28 Adverse events |
|
| Notes |
Location: Three centres in Colombia and one in Suriname Date: 05 July 2004 to 05 July 2005 Malaria endemicity: Not reported Drug resistance in area: Not reported Method of determining parasite density: Not reported Baseline: No information on characteristics by treatment arm Drug source: Azithromycin: Zithromax, Pfizer Inc. Not reported for chloroquine or atovaquone‐proguanil. Source of funding: Pfizer Inc. Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | None described |
| Blinding? All outcomes | Low risk | Placebo controlled |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss of participants low and similar among arms: 114, 14 and 116 randomised in respective arms; 112 (98.2%) and 113 (97.4%) results at day 28 in arms 1 and 3 respectively. Five participants defaulted. Results for arm 2 not presented. |
| Free of selective reporting? | High risk | No clear definition of treatment failure at day 28. One treatment arm prematurely terminated; results not reported. Another study arm in one site prematurely terminated because of poor efficacy of blinded arm. No baseline information by treatment arm. Fever clearance time and parasite clearance time not reported by treatment arm. |
| Free of other bias? | Unclear risk | Not clear if and how the sponsor was involved in analysis and writing of report |
| Methods |
Trial design: Multi‐centre, randomized, placebo‐controlled trial with three treatment regimens. Follow up: Hospitalised with eight‐hourly blood smears until three consecutive blood smears (thick and thin) were negative for asexual parasitaemia and investigator deemed discharge appropriate. Post‐therapy evaluation visits and blood smears at day 3 to 7, 14, 21, 28, 35 and 42. Adverse event monitoring: Adverse events were monitored throughout the study. Physical examinations and laboratory evaluations (haematology and serum chemistry) were done at protocol‐specified times. Reporting of adverse events for all randomized and treated subjects. |
|
| Participants |
Number enrolled: 32 Number available for analysis: 23 (arm 1 and 3) Inclusion criteria: Females and males > 18 years with uncomplicated symptomatic malaria as indicated by the presence of both of the following: a) mono‐infection with P. falciparum on blood smear, asexual parasitaemia between 1000 and 100,000 parasites/µl; b) fever or a history of fever (≥38.5 °C rectal or tympanic; ≥37.5 °C axillary or ≥38 °C oral) within the prior 24 hours; serum glucose ≥60 mg/dL (by fingerstick or peripheral blood collection); rapid diagnostic test (Binax NOWTM ICT) positive for P. falciparum; women of childbearing potential had a negative urine gonadotropin test prior to entry into the study and agreed to use adequate contraception during the entire study. Exclusion criteria: Severe or complicated malaria including subjects with any of the following: a) impaired consciousness, seizures or abnormal neurologic exam; b) jaundice; c) respiratory distress; d) persistent vomiting; e) haematuria, as reported by the patient; f) parasite density > 100,000 parasites/mL; g) presence of non‐falciparum species on microscopy; pregnant or breast‐feeding women; history of allergy to or hypersensitivity to azithromycin or any macrolide, sulphonamides, pyrimethamine, or chloroquine; known history of blood dyscrasias (eg megaloblastic anaemia, agranulocytosis, aplastic anaemia, thrombocytopenia, leukopenia, neutropenia, haemolytic anaemia); history of epilepsy or psoriasis; history of treatment with any antimalarial drug (chloroquine, quinine, mefloquine, malarone, sulphadoxine‐pyrimethamine, artemisinin compounds) or antibacterial with known antimalarial activity (macrolides, doxycycline, clindamycin) within two weeks prior to enrolment into the study; known or suspected cardiovascular, hepatic or renal abnormality that in the opinion of the investigator would place the subject at increased risk if they participated in the study. Other specific exclusion criteria were: a) serum creatinine > 2.0 x ULN; b) ALT and/or AST > 3 x ULN; inability to swallow oral medication in tablet form; treatment with other investigational drugs within 30 days prior to enrolment into the study; alcohol and/or any other drug abuse; requirement to use medication during the study that might interfere with the evaluation of the study drug (nelfinavir, digoxin, ergot alkaloids, terfenadine, cyclosporine, hexobarbital and phenytoin); specific systemic diseases or other medical conditions that would interfere with the evaluation of the therapeutic response or safety of the study drug; inability to comprehend and/or unwillingness to follow the study protocol; intentions to leave the vicinity of the trial site in the next 42 days; previous participation in this study. Age range: 19 to 58 years, mean 31.1 years in arm 1, 27.1 years in arm 2 and 27.2 years in arm 3. Proportion male: 27/32 (84.4%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 1 g plus chloroquine 600 mg once a day for three days plus matching placebo for sulphadoxine‐pyrimethamine on first day arm 2) Azithromycin 500 mg plus azithromycin placebo plus chloroquine 600 mg once a day for three days plus matching placebo for sulphadoxine‐pyrimethamine on first day arm 3) Three tablets of sulphadoxine‐pyrimethamine (1500mg/75 mg in total respectively) once plus matching placebos for azithromycin and chloroquine once daily for three days. After 19 randomizations, arm 2 was stopped based on inadequate efficacy seen with this arm during interim analysis of another protocol in this program. Supervision of intake: Yes Other (symptomatic) medication: not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary endpoint), PCR‐adjusted failure rate by day 28 Adverse events |
|
| Notes |
Location: Two centres in Indonesia Date: 31 March 2004 to 19 April 2005 Malaria endemicity: Not reported Drug resistance in area: Not reported Method of determining parasite density: Not reported Baseline data: No information on characteristics by treatment arm except for age Drug source: Azithromycin: Zithromax, Pfizer Inc. Source of funding: Pfizer Inc. Other notes: The trial was terminated prematurely, reportedly due to inability to recruit the planned number of subjects (and the tsunami). The Pfizer trial synopsis states: "There were no safety concerns regarding the study in the decision to terminate the trial." However, failure rate among 13 persons enrolled in arm 1 was high (9/13) and this may have influenced the decision. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not described |
| Allocation concealment? | Unclear risk | None reported |
| Blinding? All outcomes | Low risk | Placebo controlled |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss of participants low and not different by arm: 13, 7 and 12 randomised in respective groups; 13 (100%) and 10 (83.3%) results at day 28 in arm 1 and 3 respectively. One participant in group 3 had mixed infection with P. vivax at day 1, and one received chloroquine for P. vivax on day 21. Arm 2 was stopped early; results not reported. |
| Free of selective reporting? | High risk | One arm prematurely terminated, before study was completely terminated due to a lack of subjects meeting protocol criteria and the devastating tsunami of December 2004. Result for terminated arm not presented. Fever clearance time, parasite clearance time and gametocyte clearance rate not presented by treatment arm. |
| Free of other bias? | Unclear risk | Not clear if and how the sponsor was involved in analysis and writing of report |
| Methods |
Trial design: Single‐centre, open‐label, randomized, controlled trial with four treatment regimens. Follow up: Hospitalized until clearance of parasitemia. Blood smear every six hours in thin film until only detectable in thick film and then every 12 hours in thick film until clearance, then daily for 28 days. Vital signs recorded four‐hourly until resolution of fever, and thereafter six‐ to12‐hourly. Adverse event monitoring: Routine biochemical and haematological test performed on admission and repeated weekly. Only adverse event of delayed appearance of P. falciparum reported for 66 participants who completed the study. |
|
| Participants |
Number enrolled: 92 Number available for analysis: 66 Inclusion criteria: Adult male patients with acute symptomatic P. vivax malaria; informed consent. Exclusion criteria: Mixed infections; a history of drug hypersensitivity; intake of any antimalarial drug within the previous 48 hours; urine positive in screening tests for sulphonamides (lignin test) or 4‐aminoquinolones (Wilson‐Edeson test). Age range: 14 to 51 years. Mean age: 24 (SD 8) years Proportion male: 92/92 (100%) Pregnant women: Excluded |
|
| Interventions | arm 1) Azithromycin 500 mg/day for three days arm 2) Tetracycline 250 mg four times a day for seven days arm 3) Doxycycline 200 mg/day for seven days arm 4) Clindamycin 300 mg four times a day for seven days Supervision of intake: yes Other (symptomatic) medication: Paracetamol (0.5 to 1 g four‐hourly) was given for fever ≥38 °C. Note: Only after P. vivax treatment failure was established, participants were treated with primaquine for 14 days and chloroquine |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 Parasite and fever clearance time Adverse events |
|
| Notes |
Location: Bangkok Hospital for Tropical Diseases, Bangkok, Thailand Date: 1995 to 1998 Malaria endemicity: No transmission in Bangkok, unstable/seasonal in parts of country Drug resistance: P. vivax: pyrimethamine reported in the study area Method of determining parasite density: Giemsa or field‐stained thick and thin smears; parasite density counted per 1000 red blood cells in a thin smear or per 200 WBC in a thick smear. No definition for negative smear. Baseline data: Age, number of previous malaria attacks, haematocrit, WBC, creatinine, total bilirubin and SGOT of randomised participants. Look similar. Drug source: Azithromycin: donated by Pfizer International Corp, Thailand; tetracycline: Thai Government Pharmaceutical Organization; doxycycline: Siam Pharmaceutical Co.; clindamycin: Pharmacia & Upjohn Pharmaceuticals, donated by R.X. Company Limited, Thailand. Source of funding: Wellcome Trust, UK Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Not reported |
| Allocation concealment? | Unclear risk | None described |
| Blinding? All outcomes | High risk | Open label, not clear if microscopist is blinded |
| Incomplete outcome data addressed? All outcomes | High risk | Moderate to high loss to follow‐up; not equally distributed across arms: 20, 27, 25, and 20 randomised in respective groups, 18 (90.0%), 18 (66.7%), 18 (72.0%) and 12 (60.0%) results at day 28. Not reported why no results for 26 participants. |
| Free of selective reporting? | High risk | All male participants. Only adverse event of P. falciparum infection during follow‐up reported by treatment arm; other adverse events not reported by treatment regimen. |
| Free of other bias? | Unclear risk | Insufficient information |
| Methods |
Trial design: Single‐centre, open‐label, randomized, controlled trial with two treatment regimens. Follow up: Evaluation visits and blood smears, filter paper sample and haemoglobin tests on day 0 to 2, 7, 14, 28 and 42, or on any day if the child was unwell. Adverse event monitoring: Mothers or guardians were asked about possible adverse effects, which were classified according to WHO criteria. Follow‐up visits on day 0 to 2, 7,14, 28, and 42, or if child unwell. Blood count and liver function tests on day 0 and 14. Number available for adverse events not reported. |
|
| Participants |
Number enrolled: 261 Number available for analysis: 236 Inclusion criteria: Children aged six months to 59 months with fever (axillary temperature > 37.5 ºC or a history of fever within the previous 48 hours); positive rapid diagnosis test (Parahit); Plasmodium falciparum asexual parasite density on blood smear of > 2000 and < 200,000 parasites/µl of blood; haemoglobin > 7 g/dl; living in the catchment area; willingness to attend study follow‐up visits and to provide informed consent. Exclusion criteria: Children with symptoms suggestive of severe febrile disease (based on the modified WHO criteria for severe malaria); unable to tolerate drugs orally; inability to feed; an obvious alternative cause of fever; use of an effective antimalarial drug in the previous seven days; mixed plasmodial infection; known hypersensitivity to a study drug. Age range: Not reported. Median age: 30 months (interquartal range 18 to 42) in arm 1, and 27 months (interquartal range 16 to 42) in arm 2 Proportion male: 124/261 (47.5%) (estimated from percentages in table 1 and enrolment in figure 1) Pregnant women: Not applicable |
|
| Interventions | arm 1) Azithromycin 20 mg/kg body weight, and artesunate 4 mg/kg once a day for three days arm 2) Artemether‐lumefantrine (fixed dose combination tablets 20 mg artemether and 120 mg lumefantrine), one tablet two times per day for those with body weight < 15 kg and 2 tablets two times per day for a body weight > 15 kg for three days Supervision of intake: Complete observed intake for arm 1, only observed intake for morning dose for arm 2 Other (symptomatic) medication: Not reported |
|
| Outcomes | Outcomes used in this review: Total failure by day 28 (primary outcome). PCR‐adjusted failure. Adverse events |
|
| Notes |
Location: Muheza Designated District Hospital, Muheza, Tanzania Date: June 2008 to February 2009 Malaria endemicity: Perennial, stable, hyper‐holoendemic Drug resistance: Reported to be > 70% for sulfadoxine‐pyrimethamine, chloroquine, and amodiaquine in the district Method of determining parasite density: Giemsa‐stained thick and thin smears. Parasite density calculated from thick smears, assuming a white blood cell count of 8000 cells/µl. No definition for negative smear. Read by two readers, discordant results solved by third reader. Drug source: Not reported Source of funding: The Gates Malaria Partnership Other notes: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | "Randomization occurred in blocks of random sizes using Stata software" |
| Allocation concealment? | Low risk | "Slips with study allocation were placed in sealed opaque envelopes and were opened in front of parents or guardians." |
| Blinding? All outcomes | High risk | Open‐label study; microscopists were blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Low loss of participants and equally distributed across arms: 129 and 132 randomised in respective groups, 119 (92.2%) and 120 (90.9%) results at day 28. Of the 25 participants who did not complete, 16 withdrew (seven in arm 1 and nine in arm 2), and nine were lost to follow‐up (six in arm 1 and three in arm 2) |
| Free of selective reporting? | High risk | Study stopped after planned interim analysis of DSMB. Drug intake in arm 1 completely observed; only half of drug intake in arm 2 observed. However, results are in the opposite direction of the potential effect of this bias. No follow‐up visit on day 21. |
| Free of other bias? | Unclear risk | Not sufficient information |
| Methods |
Trial design: Single‐centre, open‐label, randomized, controlled trial with two treatment regimens. Follow up: Patients were admitted to the hospital for the duration of study drug administration or until complete fever and parasite clearance, whichever took longer. Blood smears were conducted twice daily until two slides were negative. Findings of the physical examination, clinical signs and symptoms, and medication history were recorded daily until discharge and at follow‐up visit. Follow‐up visits on day 7, 14, 21, 28, 35, and 42, and whenever signs and symptoms consistent with malaria were reported. At enrolment, participants received an insecticide‐treated net. Adverse event monitoring: Mothers or guardians were asked about possible adverse effects, which were classified according to WHO criteria. Findings of adverse events were recorded daily until discharge and at follow‐up visit. Blood cell count and chemistry analyses on day 0 and day 3, or whenever clinically warranted. Adverse events reported for evaluable population (128 in arm 1 and 65 in arm 2). |
|
| Participants |
Number enrolled: 228 Number available for analysis: 211 Inclusion criteria: Persons between 8 and 65 years of age with acute symptomatic (fever or a reported history of fever within the last 48 h) Plasmodium falciparum monoinfection, with a parasite density of 100–100,000 asexual parasites per microliter of blood, as determined from the screening blood smear. Written informed consent. Females 12 years of age or older were required to have a negative human chorionic gonadotropin pregnancy test (hCG One Step, ACON International) and to use an acceptable method of contraception throughout the study. Exclusion criteria: Known history of intolerance or hypersensitivity to the study drugs, a history of receiving antimalarial therapy in the past 4 weeks, signs and symptoms of severe malaria according to WHO criteria, laboratory evidence of significant liver or renal function abnormalities (creatinin and/or bilirubin levels of 13 mg/dL). Age range: 8‐67 years. Mean age: 22 years (SD 13.4) in arm 1, and 21 years (SD 13.5) in arm 2 Proportion male: 162/228 (71.1%) Pregnant women: Not applicable |
|
| Interventions | arm 1) Azithromycin 1.5 g and 200 mg artesunate once a day for three days. Children < 35 kg: azithromycin 30 mg/kg body weight and artesunate 4 mg/kg once a day for three days. arm 2) Artemether‐lumefantrine (fixed dose combination tablets 20 mg artemether and 120 mg lumefantrine). Adults four tablets two times per day for three days; children < 35 kg: 4 mg/kg artemether and 24 mg/kg lumefantrine two times per day for three days. Supervision of intake: Complete observed intake for both arms (admitted to hospital). Milk provided in artemether‐lumefantrin group with medication. Other (symptomatic) medication: Paracetamol, domperidon, and/or metoclopramid provided as needed for fever, headache, myalgias, nausea and/or dizziness. Four patients received omeprazole. |
|
| Outcomes | Outcomes used in this review: Total failure and PCR adjusted failure by day 28. Fever and parasite clearance time Adverse events |
|
| Notes |
Location: Malaria Research Initiative Bandarban (MARIB) field site, Bandarban Sadar Hospital, Southeastern Bangladesh Date: Enrolment between August 2006 and August 2007 Malaria endemicity: Low‐to‐moderate transmission Drug resistance: Not reported for the region Method of determining parasite density: Thick and thin smears, type of staining not reported. Asexual parasite counted against 200 WBCs on thick film or per 2000 red blood cells on thin film. Negative smear if no asexual parasite in 200 oil‐immersion fields. All positive smears after initial clearance read by two readers, discordant results solved by third reader. Baseline data: Age, gender, weight, height, parasite density, and participants with a weight < 35 kg. Look similar. Drug source: Azithromycin: Zithromax 500 mg tablets provided by Pfizer. Artesunate: Artesunet 50 mg tablets from Aexim Pharmaceuticals, purchased locally. Coartem: Novartis, purchased locally. Source of funding: Investigator Initiated Research grant (IIR grant GA9001DK) from Pfizer Inc. New York Other notes: Participants received an insecticide‐treated net at enrolment. Of all participants, 113/228 (50%) were children defined as persons with a weight < 35 kg. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Patients were randomly assigned in a 2:1 ratio to treatment groups 1 and 2 by reference to a statistical series based on random sampling numbers drawn up for each patient by study staff. |
| Allocation concealment? | Low risk | The details of the series were unknown to any of the investigators or the coordinator, and were contained in a set of sealed envelopes. |
| Blinding? All outcomes | High risk | Open‐label, but microscopists were blinded |
| Incomplete outcome data addressed? All outcomes | Low risk | Loss to follow‐up low and similar across arms: 152 and 76 randomised in respective groups, 142 (93.4%) and 69 (90.8%) completed. Eight developed P. vivax and two were lost to follow‐up in arm 1; four developed P. vivax and three were lost to follow up in arm 2. |
| Free of selective reporting? | Low risk | All outcomes reported by treatment arm, no interim analysis |
| Free of other bias? | Low risk | Sponsor had no role in data analysis and report writing |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Chloroquine alone or in combination for malaria in children in Malawi |
| Methods |
Trial design: Open‐label, randomized, controlled trial with four treatment arms. Follow‐up: No details available. Weekly follow‐up until day 28 for episode of uncomplicated malaria. Participants can take part with more than one episode of malaria during the course of one year. They will be treated with the same regimen throughout the year. Adverse event evaluation: No details available. |
| Participants |
Inclusion criteria: Subjects aged greater than or equal to six months to five years presenting to Ndirande Health Centre with signs or symptoms consistent with malaria including, but not limited to, one or more of the following: fever at the time of evaluation (axillary temperature greater than or equal to 37.5 ºC by digital thermometer); report of fever within the last two days; clinically‐profound anaemia (conjunctival or palmar pallor); headache; body aches; abdominal pain; decreased intake of food or fluids; weakness. Weight greater than or equal to 5kg. Positive malaria smear for P. falciparum mono‐infection with parasite density between 2,000/mm3 and 200,000/mm3. Planning to remain in the study area for one year. Willingness to return for four‐weekly routine visits, as well as unscheduled sick visits. Parental/guardian consent for each participant. Exclusion criteria: Signs of severe malaria; one or more of the following: haemoglobin less than or equal to 5 g/dL; prostration; respiratory distress; bleeding; recent seizures; coma or obtundation (Blantyre coma score < 5); inability to drink; persistent vomiting. Known allergy or history of adverse reaction to chloroquine, artesunate, azithromycin, erythromycin or atovaquone‐proguanil. Chronic medication with any antibiotic or antimalarial medication. Previous enrolment in this study. Alanine aminotransferase more than five times the upper limit of normal or creatinine greater than three times the upper limit of normal. Evidence of chronic disease or physical stigmata of severe malnutrition (i.e. loss of muscle mass or subcutaneous tissue, edema, or skin or hair findings consistent with severe malnutrition). |
| Interventions | arm 1) Azithromycin 30 mg/kg once a day for three days (200 mg/5cc suspension), and chloroquine 10 mg/kg on day 0 and 1, 5 mg/kg on day 2 (100 mg tablets) arm 2) Artesunate 4 mg/kg once a day for three days (50 mg tablets), and chloroquine 10 mg/kg on day 0 and 1, 5 mg/kg on day 2 (100 mg tablets) arm 3) chloroquine 10 mg/kg on day 0 and 1, 5 mg/kg on day 2 (100 mg tablets) arm 4) Atovaquone‐proguanil once a day for three days (paediatric tablet 62.5 mg/25 mg, full strength tablet 250 mg/100 mg) and chloroquine 10 mg/kg on day 0 and 1, 5 mg/kg on day 2 (100 mg tablets) |
| Outcomes | Outcomes which will be used in the review: Total failure by day 28 after first drug administration (secondary endpoint) Adverse events (safety of all study regimens) |
| Starting date | February 2007 |
| Contact information | Professor Plowe, Dr. Laufer |
| Notes |
Location: Ndirande Health Centre in Blantyre, Malawi Sponsor: National Institute of Allergy and Infectious Diseases |
| Trial name or title | Azithromycin plus chloroquine versus artemether‐lumefantrine for the treatment of uncomplicated P. falciparum malaria in children in Africa |
| Methods |
Trial design: Open‐label, randomized, controlled trial with two treatment arms. Follow up: No details available. Weekly follow up until day 42. Adverse event evaluation: No details available. |
| Participants |
Inclusion criteria: Age five to 12 years (cohort 1), and six to 59 months (cohort 2) with uncomplicated, symptomatic malaria as indicated by the presence of the following: blood smears positive for mono‐infection with P. falciparum and asexual parasitaemia between 1000 and 100,000 parasites/µl, documented fever (≥38.0 ºC rectal; 37.2 ºC axillary or 37.5 ºC oral) or a reported history of fever in the last 24 hours. Appropriate for outpatient treatment, blood glucose ≥ 60mg/dl, haemoglobin ≥ 6 g/dl or haematocrit ≥18% without signs of anaemia‐induced congestive heart failure. Females ≥ 10 years: negative urine pregnancy test. Exclusion criteria: Peripheral blood smear positive for mixed infection with multiple Plasmodium spp; severe or complicated malaria, including subjects with any of the following: Impaired consciousness (eg obtundation, unarousable coma); seizures or abnormal neurologic exam suggestive of severe or complicated malaria; known haemoglobinuria; jaundice; respiratory distress; persistent vomiting; gross haematuria, as reported by the subject's legally‐acceptable representative; inability to drink or breast‐feed; unable to sit or stand as appropriate for age; recent history of convulsions; known pregnancy or breast‐feeding or positive urine pregnancy test (females ≥10 years of age and of child bearing potential); history of allergy to or hypersensitivity to azithromycin, any macrolide, chloroquine, artemether, any artemisinin derivative, lumefantrine; any contraindication to any study drug including azithromycin, chloroquine and artemether‐lumefantrine; history of treatment with any antimalarial drug (such as halofantrine, chloroquine, quinine, mefloquine, malarone, sulphadoxine‐pyrimethamine, artemisinin compounds) or antibacterial with known antimalarial activity (macrolides, doxycycline, clindamycin) within two weeks prior to enrolment of a subject (and/or of the mother of a subject who is being breast‐fed) into the study; known or suspected cardiovascular, hepatic or renal abnormality that in the opinion of the investigator would place the subject at increased risk if they participated in the study. |
| Interventions | arm 1) Azithromyin 30 mg/kg and chloroquine 10 mg/kg once a day for three days arm 2) Artemether‐lumefantrine (fixed dose combination tablets 20 mg artemether and 120 mg lumefantrine) based on weight, once daily for three days |
| Outcomes | Outcomes which will be used in the review: Total failure by day 28 (primary endpoint) Asexual parasite clearance time, fever clearance time, P. falciparum gametocytes absence rate Adverse events (safety of all study regimen) |
| Starting date | June 2008 |
| Contact information | Pfizer CT.gov Call Center 1‐800‐718‐1021 |
| Notes |
Location: Nouna and Ouagadougou in Burkina Faso, Abidjan in Cote d'Ivoire, Kisumu in Kenya, Bamako and Sikasso in Mali Sponsor: Pfizer |
Contributions of authors
AM van Eijk wrote the protocol and review. DJ Terlouw edited the protocol and review, and assisted with the gathering of data.
Sources of support
Internal sources
No sources of support supplied
External sources
Nuffield Commonwealth Foundation, UK.
Department for International Development (DFID), UK.
Liverpool School of Tropical Medicine, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
- Dunne MW, Singh N, Shukla M, Valecha N, Bhattacharyya PC, Patel K, et al. A double‐blind, randomized study of azithromycin compared to chloroquine for the treatment of Plasmodium vivax malaria in India. American Journal of Tropical Medicine and Hygiene 2005;73(6):1108‐11. [PubMed] [Google Scholar]
- Dunne MW, Singh N, Shukla M, Valecha N, Bhattacharyya PC, Dev V, et al. A multicenter study of azithromycin, alone and in combination with chloroquine, for the treatment of acute uncomplicated Plasmodium falciparum malaria in India. Journal of Infectious Diseases 2005;191(10):1582‐8. [DOI] [PubMed] [Google Scholar]
- Krudsood S, Silachamroon U, Wilairatana P, Singhasivanon P, Phumratanaprapin W, Chalermrut K, et al. A randomized clinical trial of combinations of artesunate and azithromycin for treatment of uncomplicated Plasmodium falciparum malaria in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 2000;31(4):801‐7. [PubMed] [Google Scholar]
- Krudsood S, Buchachart K, Chalermut K, Charusabha C, Treepasertsuk S, Haoharn O, et al. A comparative clinical trial of combinations of dihydroartemisinin plus azithromycin and dihydroartemisinin plus mefloquine for treatment of multidrug resistant falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 2002;33(3):525‐31. [PubMed] [Google Scholar]
- Miller RS, Wongsrichanalai C, Buathong N, McDaniel P, Walsh DS, Knirsch C, et al. Effective treatment of uncomplicated Plasmodium falciparum malaria with azithromycin‐quinine combinations: a randomized, dose ranging study. American Journal of Tropical Medicine and Hygiene 2006;74(3):401‐6. [PubMed] [Google Scholar]
- Na‐Bangchang K, Kanda T, Tipawangso P, Thanavibul A, Suprakob K, Ibrahim M, et al. Activity of artemether‐azithromycin versus artemether‐doxycycline in the treatment of multiple drug resistant falciparum malaria. Southeast Asian Journal of Tropical Medicine and Public Health 1996;27(3):522‐5. [PubMed] [Google Scholar]
- Noedl H, Krudsood S, Chalermratana K, Silachamroon U, Leowattana W, Tangpukdee N, et al. Azithromycin combination therapy with artesunate or quinine for the treatment of uncomplicated Plasmodium falciparum malaria in adults: A randomized, phase 2 clinical trial in Thailand. Clinical Infectious Diseases 2006;43:1264‐71. [DOI] [PubMed] [Google Scholar]
- Pfizer Inc. A three day trial of azithromycin plus chloroquine for the treatment of uncomplicated Plasmodium falciparum malaria. http://www.clinicaltrials.gov/ct/show/NCT00282919 2008. [Google Scholar]
- Pfizer Inc. A phase‐3, randomized, open‐label, comparative trial of azithromycin plus chloroquine versus mefloquine for the treatment of uncomplicated Plasmodium falciparum malaria in Africa. http://pdf.clinicalstudyresults.org/documents/company‐study_4619_0.pdf2008.
- Pfizer Inc. A phase 2/3 randomized, comparative trial of azithromycin plus chloroquine for the treatment of uncomplicated Plasmodium falciparum malaria in India. http://pdf.clinicalstudyresults.org/documents/company‐study_1101_0.pdf2007.
- Pfizer Inc. A phase 2, double blind, randomized, comparative trial of azithromycin in combination with chloroquine versus chloroquine in the eradication of asymptomatic Plasmodium falciparum infection in semi‐immune adults. http://pdf.clinicalstudyresults.org/documents/company‐study_1764_0.pdf 2007. [Google Scholar]
- Pfizer Inc. A phase 2/3, randomized, double‐blind, comparative trial of azithromycin plus chloroquine versus mefloquine for the treatment of uncomplicated Plasmodium falciparum malaria in Africa. http://pdf.clinicalstudyresults.org/documents/company‐study_2362_0.pdf2007.
- Pfizer Inc. A phase 2/3, randomized, double blind, comparative trial of azithromycin plus chloroquine versus atovaquone‐proguanil for the treatment of uncomplicated Plasmodium falciparum malaria in South America. http://pdf.clinicalstudyresults.org/documents/company‐study_1770_0.pdf2007.
- Pfizer Inc. A phase 2/3, randomized, comparative, double blind trial of azithromycin plus chloroquine versus sulfadoxine‐pyrimethamine plus chloroquine for the treatment of uncomplicated, symptomatic falciparum malaria in Southeast Asia. http://pdf.clinicalstudyresults.org/documents/company‐study_1765_0.pdf2007.
- Pukrittayakamee A, Clemens R, Chantra A, Nontprasert A, Luknam T, Looareesuwan S, et al. Therapeutic responses to antibacterial drugs in vivax malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95:524‐8. [DOI] [PubMed] [Google Scholar]
- Sykes A, Hendriksen I, Mtove G, Mandea V, Mrema H, Rutta B, et al. Azithromycin plus artesunate versus artemether‐lumefantrine for treatment of uncomplications malaria in Tanzanian children: a randomized, controlled trial. Clinical Infectious Diseases 2009;49:1195‐1201. [DOI] [PubMed] [Google Scholar]
- Thriemer K, Starzengruber P, Khan WA, Haque R, Marma ASP, Ley B, et al. Azithromycin combination therapy for the treatment of uncomplicated falciparum malaria in Bangladesh: an open‐label randomized controlled clinical trial. Journal of Infectious Diseases 2010;202(3). [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- National Institute of Allergy and Infectious Diseases. Chloroquine alone or in combination for malaria in children in Malawi. http://www.clinicaltrials.gov/ct/show/NCT00379821.2006.
- Pfizer Inc. Azithromycin plus chloroquine versus artemether‐lumefantrine for the treatment of uncomplicated P. falciparum malaria in children in Africa. http://www.clinicaltrials.gov/ct/show/NCT00677833.2008. [DOI] [PMC free article] [PubMed]
Additional references
- Adair CD, Gunter M, Stovall TG, McElroy G, Veille JC, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstetrics and Gynecology 1998;91(2):165‐8. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Ager A, McGreevy P, Schuster BG, Wesche D, Kuschner R, et al. Activity of azithromycin as a blood schizonticide against rodent and human plasmodium in vivo. American Journal of Tropical Medicine and Hygiene 1995;52(2):159‐61. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Oloo AJ, Gordon DM, Ragama OB, Aleman GM, Bermand JD, et al. Successful double‐blinded, randomized, placebo‐controlled field trial of azithromycin and doxycycline as prophylaxis for malaria in Western Kenya. Clinical Infectious Diseases 1998;26:146‐50. [DOI] [PubMed] [Google Scholar]
- Barnes KI, Watkins WM, White NJ. Antimalarial dosing regimens and drug resistance. Trends in Parasitology 2008;24(3):127‐134. [DOI] [PubMed] [Google Scholar]
- Biswas S. In‐vitro antimalarial activity of azithromycin against chloroquine sensitive and chloroquine resistant Plasmodium falciparum. Journal of Postgraduate Medicine 2001;47(4):240‐3. [PubMed] [Google Scholar]
- Bloland PB, Ringwald P, Snow RW. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria [WHO/HTM/RBM/2003.50]. Geneva: World Health Organization, 2003. [Google Scholar]
- Blumer JL. Evolution of a new drug formulation: the rationale for high‐dose, short‐course therapy with azithromycin. International Journal of Antimicrobial Agents 2005;26 Suppl 3:S143‐7. [DOI] [PubMed] [Google Scholar]
- Chico RM, Pittro R, Greenwood B, Chandramohan D. Azithromycin‐chloroquine and the intermittent preventive treatment of malaria in pregnancy. Malaria Journal 2008;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl EL, Rosenthal PJ. Apicoplast translation, transcription and genome replication: targets for antimalarials antibiotics. Trends in Parasitology 2008;24(6):279‐84. [DOI] [PubMed] [Google Scholar]
- Edgie‐Mark A, Dennull RA, Reinbold DD, Waters NC, Johnson JD. Assessment of malaria in vitro drug combination screening and mixed‐strain infections using the malaria Sybr Green I‐based fluorescence assay. Antimicrobial Agents and Chemotherapy 2009;53(6):2557‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras BA, Jensen JB. Activity of azithromycin (CP‐62,993) and erythromycin against chloroquine‐sensitive and chloroquine resistant strains of Plasmodium falciparum in vitro. American Journal of Tropical Medicine and Hygiene 1992;47(3):378‐82. [DOI] [PubMed] [Google Scholar]
- Gingras BA, Jensen JB. Antimalarial activity of azithromycin and erythromycin against Plasmodium berghei. American Journal of Tropical Medicine and Hygiene 1993;49(1):101‐5. [DOI] [PubMed] [Google Scholar]
- Gray RH, Wabwire‐Mangen F, Kigozi G, Sewankambo NK, Serwadda D, Moulton LH, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. American Journal of Obstetrics and Gynecology 2001;185(5):1209‐17. [DOI] [PubMed] [Google Scholar]
- Heppner DG Jr, Walsh DS, Uthaimongkol N, Tang DB, Tulyayon S, Permpanich B, et al. Randomized, controlled, double‐blind trial of daily oral azithromycin in adults for the prophylaxis of Plasmodium vivax malaria in western Thailand. American Journal of Tropical Medicine and Hygiene 2005;73(5):842‐9. [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, Version 5.0.2 (updated September 2009). [Google Scholar]
- Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG, et al. CONSORT for reporting randomized controlled trials in journal and conference abstracts: explanation and elaboration. PLoS Medicine 2008;5(1):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. British Medical Journal 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Alker AP, Kwiek JJ, et al. A randomized controlled pilot trial of azithromycin or artesunate added to sulfadoxine‐pyrimethamine as treatment for malaria in pregnant women. PLoS ONE 2007;2(11):e1 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakornchai S, Konthiang P. Activity of azithromycin or erythromycin in combination with antimalarial drugs against multidrug‐resistant Plasmodium falciparum in vitro. Acta Tropica 2006;100:185‐91. [DOI] [PubMed] [Google Scholar]
- Neerja J, Puri SK. Plasmodium yoelii: activity of azithromycin in combination with pyrimethamine or sulfadoxine against blood and sporozoite induced infections in Swiss mice. Experimental Parasitology 2004;107(3‐4):120‐4. [DOI] [PubMed] [Google Scholar]
- Noedl H, Wernsdorfer WH, Krudsood S, Wilairatana P, Kollaritsch H, Wiedermann G, et al. Antimalarial activity of azithromycin, artemisinin and dihydroartemisinin in fresh isolates of Plasmodium falciparum in Thailand. Acta Tropica 2001;80(1):39‐44. [DOI] [PubMed] [Google Scholar]
- Noedl H, Krudsood S, Leowattana W, Tangpukdee N, Thanachartwet W, Looareesuwan S, et al. In vitro antimalarial activity of azithromycin, artesunate, and quinine in combination and correlation with clinical outcome. Antimicrobial Agents and Chemotherapy 2007;51(2):651‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosten F, McGready R, d'Alessandro U, Bonell A, Verhoeff F, Menendez C, et al. Antimalarial drugs in pregnancy: a review. Current Drug Safety 2006;1(1):1‐15. [DOI] [PubMed] [Google Scholar]
- Ogasawara KK, Goodwin TM. Efficacy of azithromycin in reducing lower genital Ureaplasma urealyticum colonization in women at risk for preterm delivery. Journal of Maternal‐Fetal Medicine 1999;8(1):12‐6. [DOI] [PubMed] [Google Scholar]
- Ohrt C, Willingmyre GD, Lee P, Knirsch C, Milhous W. Assessment of azithromycin in combination with other antimalarial drugs against Plasmodium falciparum in vitro. Antimicrobial Agents and Chemotherapy 2002;46(8):2518‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizer Labs. Zmax U.S. Physician Prescribing Information. http://media.pfizer.com/files/products/uspi_zmax.pdf.2008.
- Pradines B, Rogier C, Fusai T, Mosnier J, Daries W, Barret E, et al. In vitro activities of antibiotics against Plasmodium falciparum are inhibited by iron. Antimicrobial Agents and Chemotherapy 2001;45(6):1746‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri SK, Singh N. Azithromycin: antimalarial profile against blood‐ and sporozoite‐induced infections in mice and monkeys. Experimental Parasitology 2000;94(1):8‐14. [DOI] [PubMed] [Google Scholar]
- Retsema J, Fu W. Macrolides: structures and microbial target. International Journal of Antimicrobial Agents 2001;18:S3‐10. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
- Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet 2002;359:614‐8. [DOI] [PubMed] [Google Scholar]
- Sipilanyambe N, Simon JL, Chanda P, Olumese P, Snow RW, Hamer DH. From chloroquine to artemether‐lumefantrine: the process of drug policy change in Zambia. Malaria Journal 2008;7(25):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska K, Taylor WRJ, Mayxay M, Price R, Smithuis F, Guthmann J, et al. In vivo assessment of drug efficacy against Plasmodium falciparum malaria: duration of follow up. Antimicrobial Agents and Chemotherapy 2004;48(11):4271‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor WRJ, Richie RL, Fryauff DJ, Picarima H, Ohrt C, Tang D, et al. Malaria prophylaxis using azithromycin: a double‐blind, placebo‐controlled trial in Irian Jaya, Indonesia. Clinical Infectious Diseases 1999;28:74‐81. [DOI] [PubMed] [Google Scholar]
- Taylor WRJ, White NJ. Antimalarial drug toxicity. Drug Safety 2004;27(1):25‐61. [DOI] [PubMed] [Google Scholar]
- Ter Kuile FO, Eijk AM, Filler SJ. Effect of sulfadoxine‐pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. Journal of the American Medical Association 2007;297(23):2603‐16. [DOI] [PubMed] [Google Scholar]
- White NJ. The assessment of antimalarial drug efficacy. Trends in Parasitology 2002;18(10):458‐64. [DOI] [PubMed] [Google Scholar]
- WHO. Chemotherapy of malaria and resistance to antimalarials. WHO Technical Report Series1973. [PubMed]
- WHO Global Malaria Programme. Global antimalarial drug policy database. http://www.who.int/malaria/amdp/amdp_afro.html. http://www.who.int/malaria/amdp/amdp_searo.html.2008.
- World Health Organization/AFRO. Severe malaria in the African region: results of a multicentre study. Liaison Bulletin of the Malaria Programme WHO/AFRO2001; Vol. 4, issue 2:1‐3.
- World Health Organization, Roll Back Malaria Department. Guidelines for the treatment of malaria [WHO/HTM/MAL/2006.1108]. Geneva: World Health Organization, 2006. [Google Scholar]
- Yeo AE, Rieckmann KH. Increased antimalarial activity of azithromycin during prolonged exposure of Plasmodium falciparum in vitro. International Journal for Parasitology 1995;25(4):531‐2. [DOI] [PubMed] [Google Scholar]


