Abstract
Background
Using a pilot system we have categorised this review as: Current question ‐ update pending (See "Published notes" section for an explanation).
Traveller's diarrhoea is a syndrome frequently encountered in persons crossing an international boundary. Diarrhoea can lead to significant discomfort and interference with travel plans. Bacterial pathogens are a frequent cause of this syndrome. Several antibiotics have been tested for efficacy in reducing the duration and severity of the illness.
Objectives
The aims of this review were to assess the effects of antibiotics on traveller's diarrhoea in relation to duration of illness, severity of illness, and adverse effects of medications.
Search methods
The Cochrane Collaboration Trials Register, MEDLINE, and EMBASE were searched. Additional trials were identified by hand searching. Content experts were contacted.
Selection criteria
All trials in any language in which travellers older than 5 years were randomly allocated to treatment for acute non‐bloody diarrhoea with antibiotics and where the causative organism is not known at allocation.
Data collection and analysis
Two reviewers assessed trial quality and extracted data.
Main results
Twenty published studies met inclusion and quality criteria for inclusion. Twelve studies were placebo‐controlled.
A meta‐analysis for the primary outcome was not feasible. All of the 10 trials reported a significant reduction in duration of diarrhoea in participants treated with antibiotics compared with placebo.
Data from two trials demonstrated a small reduction for antibiotic treated patients in the number of unformed stools passed per each 24 hour period from randomisation up to 72 hours.
Data from six trials demonstrated a greater number of participants being cured of diarrhoea by 72 hours (odds ratio 5.90, 95% confidence interval 4.06 to 8.57).
Data regarding side effects were available from five trials. There was wide variation in the prevalence of side effects reported in different trials. Persons taking antibiotics experienced more side effects than those taking placebo (odds ratio 2.37, 95% confidence interval 1.50 to 3.75).
Authors' conclusions
Antibiotic treatment is associated with shorter duration of diarrhoea but higher incidence of side‐effects. Trials generally do not report duration of post‐treatment diarrhoea using time‐to‐event analyses, and should do.
8 May 2019
No update planned
Other
Many trials of antibiotics in travellers' diarrhoea have been conducted and demonstrate shorter illness following antibiotic treatment. Travellers' diarrhoea is a self‐limiting illness. Therefore an update is not a current priority for the CIDG.
Keywords: Adult; Child; Humans; Travel; Acute Disease; Administration, Oral; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Diarrhea; Diarrhea/drug therapy
Plain language summary
Antibiotic treatment reduces duration and severity of travellers' diarrhoea.
Using a pilot system we have categorised this review as: Current question ‐ update pending. (See "Published notes" section for an explanation).
Diarrhoea is a common problem for travellers, particularly travellers going from developed to less developed nations. The illness is frequently caused by a bacterial infection. Although the illness is unlikely to result in death, diarrhoea can disrupt travel plans and lead to severe or incapacitating symptoms. The review showed that antibiotic treatment shortens the duration and severity of diarrhoea. Persons taking antibiotics reported more side effects, but most side effects were minor, or resolved on stopping the antibiotic.
Background
Travellers' diarrhoea is an acute syndrome occurring in people who have crossed a national boundary and who experience watery or unformed bowel motions which are passed more frequently than usual, sometimes accompanied by nausea, vomiting, abdominal pain or cramping. Recovery within five days is normal, although in about 10% of cases symptoms will persist beyond seven days (DuPont 1993, Farthing 1992). The syndrome is most commonly caused by intestinal bacteria known as enterotoxigenic Escherichia coli (ETEC), although other bacteria including Campylobacter, Shigella, and Salmonella species have all been frequently incriminated (Black 1986, Gorbach 1975, Taylor 1991). Travellers' diarrhoea lasting for 14 days or more is sometimes termed "persistent diarrhoea" and is considered separately as the causes and management differ from the usual travellers' illness. Some 10% of travellers with diarrhoea develop dysentery, defined as diarrhoea mixed with visible blood, indicating a potentially serious condition which differs from travellers' diarrhoea and warrants separate investigation and management. Mortality is rare in simple travellers' diarrhoea although the condition is potentially serious in the very young and the elderly, who may not tolerate fluid loss, as well as in children and younger adults. Those with an impaired immune response, reduced gastric acidity, and some forms of heart disease may also be considered at risk of more severe effects from travellers' diarrhoea (BSSI 1996).
The magnitude of the problem is considerable. With the advent of modern air travel, between 1950 and 1990 there was an increase from approximately 25 million to 425 million persons travelling worldwide (Hundt 1996). Travellers' diarrhoea has been commonly reported to occur in 30‐60% of travellers to developing countries, in contrast to reported incidence rates of 5% or less in travellers to Europe and North America (Cartwright 1997).
The incidence of the syndrome appears to vary according to the itinerary, to the season in which travel occurs, and to factors surrounding eating behaviour and style of travel, with the younger adult more at risk than the middle‐aged (Steffen 1983, Mattila 1993). Nevertheless, diarrhoea still occurs in the well‐informed and prepared traveller (McIntosh 1997), including those who stay exclusively in "five star" accommodation.
As the international traveller is often on a restricted schedule because of pre‐planned tour itineraries or business demands, even a day lost to illness can be unexpectedly disruptive, creating a demand for prompt relief. Medical treatment may not always be easily accessible to the traveller because of factors such as an unfamiliar health system, language barriers, remoteness, and time constraints, so it is an attractive option for the traveller to carry self‐treatment for some common conditions.
The mainstay of treatment for diarrhoea of all degrees of severity is adequate replacement of fluids and electrolytes. Moreover, the use of antibiotics to treat diarrhoea has been tested in a number of clinical trials (Katelaris 1995, NIH‐CDCS 1985).
This review was designed to examine the effectiveness of antibiotic treatment for acute diarrhoea. We also considered adverse effects reported in trials, as the aim of treatment is termination of diarrhoea and relief of symptoms, rather than reduction in a more serious clinical endpoint. We did not examine several other important issues, such as cost of treatment, which may be better evaluated in other ways.
Several related reviews are planned, including assessment of the effectiveness of non‐antibiotic treatments, and of combination treatments for diarrhoea.
Objectives
To assess the effects of antibiotics on diarrhoea in relation to: (a) duration of illness; (b) severity of illness; and (c) adverse effects.
Methods
Criteria for considering studies for this review
Types of studies
All trials in any language in which travellers are randomly allocated to a treatment for non‐bloody diarrhoea lasting not more than 14 days and where the causative organism is not known at allocation; to include placebo controlled trials and comparison trials.
Types of participants
Adults and children 5 years or older who develop acute non‐bloody diarrhoea lasting not more than 14 days (i.e. to exclude dysentery and persistent diarrhoea at randomisation).
Travellers are defined as people who are travelling outside their usual country of residence, and are resident in the country of study for less than six months.
Types of interventions
1. Any antibiotic therapy versus placebo. 2. Any antibiotic versus another antibiotic.
Treatments were self‐administered or issued by a physician according to a study protocol with no prior knowledge of bacterial culture results.
Other treatments were acceptable within intervention and control groups, so long as both groups remain comparable in this respect; examples include fluid replacement, dietary advice, and malaria prophylaxis.
Types of outcome measures
Duration
Duration of diarrhoea, as assessed by time to last unformed stool.
Severity
Number of loose stools passed per 24 hour period: 0‐24 hours; 25‐48 hours; 49‐72 hours; > 72 hours.
Tolerability
Number of subjects who report any side effect of treatment.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press or in progress).
The topic search terms used for searching trial registers and databases were: "travel$" for traveler, traveller, "diarr$" for "diarrhea", "diarrhoea", "diarrea", "reisediarrhoe", and "turista".
The Cochrane Infectious Diseases Group (CIDG) Specialized Register was searched for relevant trials up to September 2003. Details of the CIDG methods and the journals hand‐searched are published in The Cochrane Library in the section on Collaborative Review Groups.
The Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library (Issue 3, 2004) was searched.
The following databases were searched using the topic search terms using the topic search terms in combination with the Cochrane highly sensitive search strategy for identifying trials as outlined in Appendix 5c of the Cochrane Handbook: MEDLINE (1966 to September 2003); and EMBASE (1988 to September 2003).
Additional trials were identified through hand searching of abstracts from the biennial International Society for Travel Medicine Conferences and other selected meetings including the annual meetings of the American Gastroenterological Society.
We contacted content experts in the field who have conducted and published randomised controlled trials on travellers' diarrhoea, aiming to identify any remaining published or unpublished trials. We also attempted to get original data from North American trialists, but these data could not be retrieved. Finally, the reference lists in all identified studies were scanned in an effort to identify missing trials.
Data collection and analysis
A full text version of each article identified through the various search strategies was independently assessed by two reviewers in terms of its quality in the following categories (for details see the Policy Statement of the Cochrane Infectious Diseases Group): allocation concealment; generation of allocation sequence; inclusion of all randomised participants.
Data extraction was performed for outcome variables.
Calculation of treatment effects was performed using Cochrane statistical software, RevMan version 4.04. Results of dichotomous variables are expressed as Mantel Haenszel odds ratio (OR) and 95% confidence interval (95% CI). Continuous outcomes are expressed as mean difference (MD) and 95% CI.
Results
Description of studies
Thirty‐three potentially eligible trials of antibiotic treatment for diarrhoea were identified. Twenty five studies were identified by searching MEDLINE, 11 by searching EMBASE (two unique), and four by hand searching of abstracts. An additional two unpublished articles were found through consultation with a content expert.
Six studies are awaiting further evaluation (See 'Characteristics of studies awaiting classification'). This includes three trials published in German language journals. Two studies are unpublished trials about which the principal investigator provided methodological details, but requested that the data not be included. One trial was available only in abstract form, and further data are awaited. Two studies were excluded for not meeting inclusion criteria and three trials did not meet predefined quality assessments (see 'Characteristics of excluded studies'). Of the remaining studies, two were duplicate publications of prior reports, and were considered together with the original study. This left a total of 20 studies which were included in the present analysis (see 'Characteristics of included studies').
Seven trials (Christensen 1988, DuPont 1992, Ericsson 1983, Mattila 1993, Salam 1994, Wistrom 1989, Wistrom 1992) were studies of a single antibiotic agent against a placebo agent. Five trials (DuPont 1982, DuPont 1992b, Ericsson 1987, Ericsson 1990, Steffen 1993) were comparisons of multiple agents, or multiple regimens of a single agent, and included a placebo group. Five studies (DuPont 1984, Ericsson 1992, Ericsson 1997, Kuschner 1995, Thornton 1992) were direct comparisons of one antibiotic against another antibiotic, or multiple regimens of different durations using the same antibiotic, without use of a placebo group. Three studies (Ericsson 1986, Petrucelli 1992, Taylor 1991) compared an antibiotic against an antibiotic‐antimotility agent combination.
Eleven studies were conducted in Mexico, of which 10 were among US adult students attending summer classes in Guadalajara, Mexico. Two studies were based in Thailand, and one each in Belize, Egypt, The Gambia, India, and Morocco. Two studies included people travelling to various destinations in developing nations.
The travellers in these studies were students (10 studies), military personnel (5 studies), "package" tourists (2 studies), resort guests (1 study), independent travellers (1 study), or volunteers recruited for the purpose of the study (1 study).
Inclusion criteria were fairly uniform across the studies, with all of the studies but three (Christensen 1988, Salam 1994, Steffen 1993) requiring participants to have a minimum of three loose stools per 24 hours before presentation or a smaller number of stools in a shorter period, such as two stools in 8 hours, and have evidence of an enteric illness, such as abdominal cramps, nausea, vomiting, and low grade fever. Inclusion criteria were not explicitly stated in one study (Christensen 1988), and a single loose stool was sufficient for inclusion in two studies (Salam 1994, Steffen 1993).
The agents evaluated in these trials included an oral flouroquinolone (ciprofloxacin, norfloxacin, or ofloxacin) in 12 studies (DuPont 1992b, Ericsson 1987, Ericsson 1997, Kuschner 1995, Mattila 1993, Petrucelli 1992, Salam 1994, Steffen 1993, Wistrom 1989, Wistrom 1992), trimethoprim/ sulfamethoxazole in six studies (DuPont 1982, Ericsson 1986, Ericsson 1987, Ericsson 1990, Ericsson 1992, Thornton 1992), and in one study each for the following agents: ampicillin (DuPont 1984), azithromycin (Kuschner 1995), aztreonam (DuPont 1992), bicozamycin (Ericsson 1983), furazolidone (DuPont 1984), pivmecillinam (Christensen 1988), and trimethoprim alone (DuPont 1982).
Risk of bias in included studies
Allocation
All of the studies were randomised studies. Only one study reported on the method of randomisation used (DuPont 1982).
Blinding
Eighteen studies were double‐blinded, with one being single blinded (Ericsson 1997), and one study (Christensen 1988) not reporting whether blinding was used.
Inclusion of all randomised patients
Included trials had losses to follow up or excluded patients from efficacy analysis that were less than 10% of randomised participants in all cases. Few studies reported an intention to treat analysis (Ericsson 1990, Ericsson 1992, Ericsson 1997).
Effects of interventions
Data from studies comparing antibiotic treatment with placebo are presented in the summary analysis and in Table 1. A total of 1474 patients were included in placebo‐controlled studies.
1. Duration of post‐treatment diarrhoea (hours).
| Study | Treatment | Control | Pooled SD | ||||
| N | Mean | SD | N | Mean | SD | ||
| DuPont 1992b (Ofloxacin 3‐days) | 81 | 28 | 30 | 79 | 56 | 48 | — |
| DuPont 1992b (Ofloxacin 5‐days) | 66 | 39 | 36 | 79 | 56 | 48 | — |
| Ericsson 1983 | 72 | 28.2 | 27.3 | 68 | 63.7 | 46.1 | — |
| Salam 1994 | 45 | 24.8 | 25.49 | 38 | 53.5 | 27.12 | — |
| Wistrom 1992 | 8 | 26 | — | 9 | 60 | — | 27.989 |
A meta‐analysis of the primary outcome, duration of diarrhoea after initiation of antibiotic treatment, or time to last unformed stool, was not performed. Of 12 placebo‐controlled studies identified, 10 studies (DuPont 1982, DuPont 1992, DuPont 1992b, Ericsson 1983, Ericsson 1987, Ericsson 1990, Mattila 1993, Salam 1994, Steffen 1993, Wistrom 1989) reported data for this outcome. However, only three of these (DuPont 1992b, Ericsson 1983, Salam 1994) reported mean and standard deviation (SD) time to last unformed stool. One other study reported mean and a P value for a statistical test from which an imputed pooled SD could be derived (Wistrom 1992). The remaining six studies reported statistical comparisons that used non‐parametric tests. In five cases, trials used the Wilcoxon test and one used the Mann‐Whitney test.
In all 10 trials, a statistically significant reduction in time to last unformed stool was reported for participants receiving antibiotic treatment when compared with placebo, with the exception of the five day ofloxacin arm in DuPont 1992b. The data from the trials from which mean and SD could be obtained or imputed are presented in Table 1.
For the secondary efficacy endpoint of number of patients cured by 72 hours after randomisation, data from six trials (DuPont 1982, Ericsson 1983, Mattila 1993, Salam 1994, Steffen 1993, Wistrom 1989) were available. Treatment with antibiotics resulted in a greater number of participants being cured of diarrhoea by 72 hours (OR 5.90, 95% CI 4.06 to 8.57, Analysis 1.1).
1.1. Analysis.
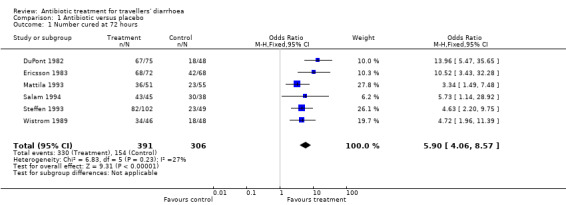
Comparison 1 Antibiotic versus placebo, Outcome 1 Number cured at 72 hours.
For the secondary endpoint of change in severity (number of unformed stools per 24 hour time interval), two trials (Ericsson 1983,Salam 1994) demonstrated a small reduction for antibiotic treated patients in the number of unformed stools passed per each 24 hour period from randomisation up to 72 hours (see Analysis 1.2). For the first 24 hours after randomisation, the difference was ‐1.59 stools (95% CI ‐2.66 to 0.52). For the period 25‐48 hours, the difference was ‐2.10 stools (95% CI ‐2.78 to ‐1.42). For the third post‐randomisation day, the difference was ‐1.38 stools (95% CI ‐1.94 to ‐0.82).
1.2. Analysis.
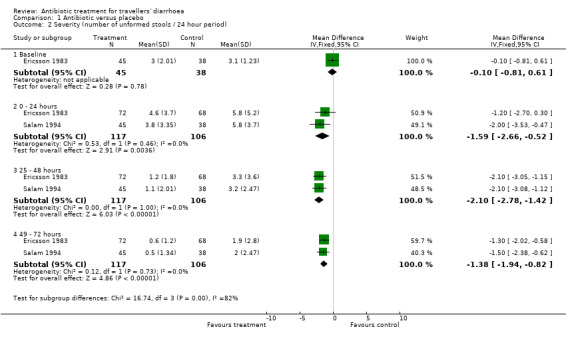
Comparison 1 Antibiotic versus placebo, Outcome 2 Severity (number of unformed stools / 24 hour period).
For the secondary endpoint of prevalence of any reported side effect of treatment, data from five trials (DuPont 1982, DuPont 1992, DuPont 1992b, Ericsson 1983, Steffen 1993) were available. There were more side effects reported by persons taking antibiotics than those taking placebo (OR 2.37, 95% CI 1.50 to 3.75, Analysis 1.3). There was significant variation in the incidence of side effects reported in different trials. Elimination of trials with the largest weight did not affect the overall result. Side effects reported were all noted to be not clinically serious, or resolved on withdrawal of the drug. Studies did not report examination for Clostridium difficile exotoxin in participants with recurrent diarrhoea. No studies reported fatalities or participants needing to be hospitalised after randomisation.
1.3. Analysis.
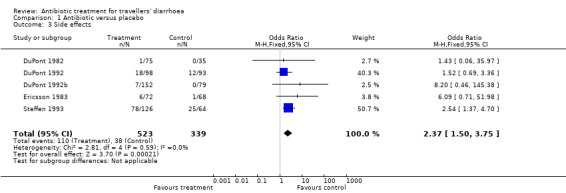
Comparison 1 Antibiotic versus placebo, Outcome 3 Side effects.
Discussion
We examined the use of oral antibiotics for acute episodes of diarrhoea in travellers. The principal finding of this review was that a meta‐analysis of the primary outcome was not feasible. The pooled results of the secondary endpoints demonstrate a significant beneficial effect of antibiotic treatment in terms of the proportion of persons taking antibiotics who were cured of diarrhoea by 72 hours of treatment, and in reducing the severity of illness. There were significantly more adverse effects reported in those taking antibiotics than those taking placebo. However, most of these side effects were minor, and resolved either spontaneously or on stopping the antibiotic. The planned analyses of data for comparative trials of antibiotics and combination treatment were not carried out, principally because of concerns that the skewed nature of published data would imply a revision of the review methods.
Ten eligible trials reported summary measures based on mean, but only three reported both mean and SD. The ratios of mean and SD in these three trials also suggested that data for the primary outcome might be skewed; mean < 1.6 SD. In one trial, a pooled SD could be calculated. The other six trials used non‐parametric tests to compare the means from treatment and control groups. From this we infer that this was also because the data were not normally distributed, yet it was not stated clearly in any trials. For these reasons meta‐analysis for the primary outcome data was not feasible.
One major problem with the analyses presented in these trials was that the primary outcome was not treated as a time‐to‐event outcome. The proper analysis of such data would require time to event analysis, such as Kaplan‐Meier life table methods, comparing groups using the log rank test. If such an analysis can be undertaken, it would strengthen the reliability of the conclusions.
The trials examined in this systematic review used similar entry criteria, with three exceptions (Christensen 1988, Salam 1994, Steffen 1993). Participants could be regarded as having had "classic travellers' diarrhoea" (Steffen 1999), or moderate to severe diarrhoeal illness to have been included in these studies. Part of the reason for this uniformity is that many of the studies were performed in the same geographic location, using similar protocols, in consistent participant populations.
Authors' conclusions
Implications for practice.
Available trial data demonstrate that antibiotic treatment is effective in reducing the duration of post‐treatment diarrhoea, and severity of diarrhoea. However, this is at the price of an increased chance of side effects from antibiotic treatment.
Implications for research.
The quality of information included in reports of therapeutic trials must meet certain minimum criteria. It is no longer accepted practice for authors to omit confidence interval data or measures of variation of the mean from reports of statistical testing. It is also crucial that data be appropriately analysed. The primary outcome for this review is ideally represented in a time‐to‐event analysis. Thorough statistical review of papers before publication should prevent such errors.
What's new
| Date | Event | Description |
|---|---|---|
| 5 January 2012 | Amended | The CIDG is piloting a new classification system for reviews. The classification for this has now been added; description included in "Published notes" section of review |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format with minor editing. |
| 8 September 2003 | New search has been performed | New studies found but not yet included or excluded. |
Notes
2012, Issue 2: Review status: Current question ‐ update pending. New studies have been identified; a new author team is required for this review.
The Cochrane Infectious Diseases Group assesses Review Status using a pilot system to help the reader understand whether the review concerns a current question, and is up to date. We report on:
1. The question the review addresses. Is it a:
Historical question, where the intervention or policy has been superseded by new medical developments (such as a new drug); or a
Current question, which is still relevant to current policy or practice.
2. Whether the review is up to date. Is the review:
Up to date;
Update pending; or
No update intended.
We then provide comment of the review status, to help explain the categories selected.
Acknowledgements
Dr Paul Garner for general guidance, and Dr Paula Williamson for statistical advice. Dr HL DuPont provided access to unpublished trials, which are awaiting further analysis.
Data and analyses
Comparison 1. Antibiotic versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number cured at 72 hours | 6 | 697 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.90 [4.06, 8.57] |
| 2 Severity (number of unformed stools / 24 hour period) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Baseline | 1 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.81, 0.61] |
| 2.2 0 ‐ 24 hours | 2 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐1.59 [‐2.66, ‐0.52] |
| 2.3 25 ‐ 48 hours | 2 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐2.10 [‐2.78, ‐1.42] |
| 2.4 49 ‐ 72 hours | 2 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐1.38 [‐1.94, ‐0.82] |
| 3 Side effects | 5 | 862 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.37 [1.50, 3.75] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Christensen 1988.
| Methods | Randomised, placebo‐controlled trial | |
| Participants | 65 Danish tourists on a 2 week trip to India | |
| Interventions | Treatment: Pivmecillinam 400 mg orally three times daily for 3 days Comparison: Matching placebo |
|
| Outcomes | Duration of post‐treatment diarrhoea | |
| Notes | Stool cultures were not obtained by investigators | |
DuPont 1982.
| Methods | Randomised, double‐blind, placebo‐controlled trial Patients sequentially allocated to one of three preparations, which had been randomly ordered using procedure PLAN of the Statistical Analysis System Patients kept daily diary of symptoms and medication doses They were seen daily in the study clinic |
|
| Participants | 110 US students attending summer school in Guadalajara, Mexico during the summer of 1981 Entry criteria: four or more unformed stools per 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 48 hours' duration; at least one of: abdominal pain, cramps, nausea, vomiting, or dysentery |
|
| Interventions | Treatment:
1. Trimethoprim/ sulfamethoxazole 160/800 mg twice daily for 5 days
2. Trimethoprim 200 mg twice daily for 5 days Comparison: 3. Placebo twice daily for 5 days |
|
| Outcomes | 1. Number of unformed stools passed 2. Number of hours to last unformed stool 3. Failure: Diarrhoea persisting longer than the 5 day treatment period or with clinical evidence of worsening illness while on treatment | |
| Notes | 83 participants (75%) had positive stool cultures Major pathogenic organisms: ETEC ‐ (58%), Shigella ‐ 23 (21%), Salmonella ‐ 5 (5%) No pre‐ or post‐treatment antibiotic sensitivities reported |
|
DuPont 1984.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses |
|
| Participants | 100 US students attending summer school in Guadalajara, Mexico Entry criteria: four or more unformed stools pers 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 60 hours' duration; at least one of: abdominal pain, cramps, nausea, vomiting, fever |
|
| Interventions | Treatment: Furazolidone 100 mg orally four times daily for 5 days Comparison: Ampicillin 500 mg orally four times daily for 5 days |
|
| Outcomes | Time from initiation of treatment to passage of last unformed stool | |
| Notes | ||
DuPont 1992.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses |
|
| Participants | 191 US students attending summer school in Guadalajara, Mexico during the summers of 1989 and 1990 Entry criteria: four or more unformed stools pers 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 72 hours' duration; at least one of: abdominal pain, cramps, nausea, vomiting, fever |
|
| Interventions | Treatment: Aztreonam 100 mg three times a day for 5 days Placebo: Matching placebo |
|
| Outcomes | 1. Clinical recovery: duration of post‐treatment diarrhoea, proportion of each group with clinical recovery by time interval post initiation of treatment 2. Treatment failures 3. Adverse events | |
| Notes | 81 participants (42%) had positive stool cultures Major pathogenic organisms: ETEC ‐ 61 (32%), Shigella ‐ 7 (4%). Others: Aeromonas, Edwardsiella, Plesiomonas, Salmonella 100% of pretreatment stools sensitive to aztreonam and norfloxacin. Reduced sensitivity to TMP/SMX: ETEC 91%, Shigella 91%, Salmonella 60% |
|
DuPont 1992b.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Two patients were assigned ofloxacin for every patient assigned placebo Patients kept daily diary of symptoms and medication doses Patients were seen daily and questioned by investigators |
|
| Participants | 232 US students attending summer school in Guadalajara, Mexico during the period from June 1986 and December 1988 Entry criteria: four or more unformed stools pers 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 60 hours' duration; other abdominal complaints, such as abdominal discomfort, urgency; or fever |
|
| Interventions | Treatment groups:
1. Ofloxacin 300 mg orally twice daily for three days
2. Ofloxacin 300 mg orally twice daily for five days Placebo groups: 3. Placebo orally twice daily for three days 4. Placebo orally twice daily for five days |
|
| Outcomes | 1. Clinical response 2. Cure: complete resolution of illness 3. Duration of post treatment diarrhoea 4. Failure: Deterioration of clinical status during treatment or diarrhoea persisting 5 days or longer after treatment was begun | |
| Notes | 103 participants (44%) had 124 pathogens isolated Major pathogenic organisms: ETEC ‐ 66 (28%), Shigella ‐ 24 (10%), Plesiomonas ‐14 (6%) Others: Aeromonas, Campylobacter, ETEC, Salmonella Pre‐ and post‐treatment antibiotic sensitivities not reported |
|
Ericsson 1983.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses |
|
| Participants | 148 US students attending summer school in Guadalajara, Mexico during the summer of 1980 Entry criteria: four or more unformed stools pers 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 48 hours' duration; at least one of: abdominal pain, cramps, nausea, vomiting, fever |
|
| Interventions | Treatment: Bicozamycin 500 mg orally 4 times daily for 3 days Comparison: Placebo orally 4 times daily for 3 days |
|
| Outcomes | 1. Time from initiation of treatment to passage of last unformed stool 2. Number of unformed stools passed per 24 hour interval from initiation of treatment 3. Failed: Moderate to severe illness with more than 6 unformed stools per day and moderate cramps, nausea or vomiting | |
| Notes | 97 participants (66%) had positive stool cultures Major pathogenic organisms: ETEC ‐ 49 (33%), Shigella ‐ 25 (17%), Salmonella ‐ 6 (4%), Rotavirus ‐ 5 (3%) Data on pre‐ and post‐treatment antibiotic sensitivities not reported |
|
Ericsson 1986.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses, and were observed daily at the clinic site |
|
| Participants | 134 US students attending summer school in Guadalajara, Mexico during the summer of 1983 Entry criteria: four or more unformed stools per 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 48 hours' duration; at least one of: abdominal pain, cramps, nausea, vomiting, fever Participants with a temperature above 38.9 centigrade or with frank blood in the stools were excluded |
|
| Interventions | Groups and initial doses:
1. BW942 C 20 mg orally and placebo TMP/SMX
2. TMP/SMX 160/800 mg orally plus placebo BW942C
3. Both active formulations as above
4. Both placebo as above Subsequent doses: 1. BW924C 10 mg orally five times daily and one placebo morning and night for 3 days 2. TMP/SMX 160/800 mg orally twice daily and placebo BW942C five times daily for 3 days 3. Both active formulations as above 4. Both placebo formulations as above All groups: no dietary restrictions. Other antidiarrhoeal agents were prohibited |
|
| Outcomes | 1. Time to last unformed stool 2. Passage of half or less than half the number of unformed stools in a given interval compared with the numbers passed in a corresponding pre‐treatment interval 3. Relapse: recurrence of symptoms at least 24 hours after what was thought to be the last unformed stool 4. Failure: worsening clinically within or after the 3‐day treatment period or diarrhoea persisting beyond day 5 | |
| Notes | ||
Ericsson 1987.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses |
|
| Participants | 191 US students attending summer school in Guadalajara, Mexico during the summer of 1984 Entry criteria: four or more unformed stools pers 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 72 hours' duration; at least one of: abdominal pain, cramps, nausea, vomiting, fever Passage of frank blood or fever above 38.9 centigrade were exclusion criteria |
|
| Interventions | Treatment:
1. Ciprofloxacin 500 mg orally twice daily for 5 days
2. TMP/SMX 160/800 mg orally twice daily for 5 days Comparison: 3. Placebo twice daily for 5 days All groups: no specific dietary restrictions. Use of other antidiarrhoeal medication was prohibited |
|
| Outcomes | 1. Time to last unformed stool 2. Well: Total diarrhoeal index score (defined in text) less than 1 or 0 3. Improvement: Passage of half or less than half the number of unformed stools in a given interval compared with the number in a corresponding pretreatment interval; Total diarrhoeal index half or less than half the enrollment index 4. Relapse: rise in patients' diarrhoeal index 24 or more hours after passage of what appeared to be the last unformed stool 5. Failed: Diarrhoea worsened clinically within the 5 day treatment period; diarrhoea persisting beyond day 5 of the study | |
| Notes | Results of pretreatment stool cultures not presented Pretreatment antibiotic sensitivity patterns not disclosed |
|
Ericsson 1990.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses. Intention to treat analysis performed |
|
| Participants | 227 US students attending summer school in Guadalajara, Mexico Entry criteria: three or more unformed stools pers 24 hours; diarrhoea of no more than 14 days' duration; at least one of: abdominal cramps, nausea, or vomiting Patients with frankly bloody stools or temperature above 39 centigrade were excluded |
|
| Interventions | 1. Single oral dose sulfamethoxazole 1600 mg/trimethoprim 320 mg
2. SMX/TMP 800/160 twice daily for 3 days
3. Loperamide hydrochloride orally, 4 mg given as a loading dose, and 2 mg after each loose bowel movement (daily dose not to exceed 16 mg/ day)
4. Combination SMX/TMP and loperamide as in 2. and 4. above
5. Placebo The medications were reformulated into two different colour capsules. White capsules contained 80 mg Trimethoprim/ 400 mg sulfamethoxazole or placebo. Yellow capsules contained 2 mg of loperamide or placebo. All subjects received a loading dose of 4 white and 2 yellow capsules All groups received advice about fluid replacement and dietary adjustments |
|
| Outcomes | 1. Duration of diarrhoea: number of hours from enrollment to passage of last unformed stool 2. Treatment failure: Passing more than half the number of stools in a 24‐hour period that qualified them for enrollment, so long as that number exceeded three | |
| Notes | 90 participants (40%) had positive stool cultures Major pathogenic organisms: ETEC ‐ 46 (20%), Shigella ‐ 18 (8%), Aeromonas ‐ 8 (4%), Campylobacter ‐ 7 (3%), Plesiomonas ‐ 7 (3%), Salmonella ‐ 2 (1%) No pre‐ or post‐treatment antibiotic sensitivities reported |
|
Ericsson 1992.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses Patients were seen daily at the clinic site. Intention to treat analysis used |
|
| Participants | 190 US students attending summer school in Guadalajara, Mexico during the summers of 1988 and 1989 Entry criteria: three or more unformed stools per 24 hour period; diarrhoea of no more than 14 days' duration; at least one of: abdominal cramps, nausea, vomiting, or fever Patients with frankly bloody stools or temperature above 39 centigrade were excluded |
|
| Interventions | 1. TMP/SMX 160/800 twice daily for 6 doses
2. TMP/SMX 320/1600 single dose with remaining doses of placebo
3. TMP/SMX 320/1600 single dose then 160/800 for 5 doses All groups: Open label loperamide 4 mg loading dose and 2 mg following each loose stool (not exceeding 16 mg /day) Information regarding fluid replacement and dietary adjustments |
|
| Outcomes | Duration of diarrhoea: number of hours from enrollment to passage of the last unformed stool, followed by at least 24 hours free of all symptoms | |
| Notes | ||
Ericsson 1997.
| Methods | Randomised, single‐blind, placebo‐controlled trial Randomisation was performed using a table of random numbers Patients kept daily diary of symptoms and medication doses The primary outcome parameter was determined by a blinded evaluator Intention to treat analysis used |
|
| Participants | 166 US students attending summer school in Guadalajara, Mexico during the summers of 1992‐1994 Entry criteria: three or more unformed stools per 24 hours and at least one of: abdominal cramps, nausea, vomiting, or fever |
|
| Interventions | 1. Ofloxacin 400 mg orally single dose 2. Ofloxacin 200 mg orally twice daily for 3 days 3. Ofloxacin 400 mg orally single dose plus loperamide for up to 3 days (4 mg loading dose, followed by 2 mg after each loose stool to a maximum of 16 mg/24 hour period) | |
| Outcomes | Time from initiation of treatment to passage of last unformed stool | |
| Notes | ||
Kuschner 1995.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept a daily diary of symptoms and timing of medication doses Patients were seen daily for at least 3 days and then on day 10 |
|
| Participants | 79 US military personnel participating in a military exercise in Thailand during May 1993 Entry criteria: three or more liquid bowel movements per 24 hour period; or two liquid stools plus cramps, nausea, vomiting, or fever; diarrhoea of no more than 48 hours' duration |
|
| Interventions | Treatment: Azithromycin 500 mg orally daily for three days Comparison: Ciprofloxacin 500 mg orally daily for three days Both groups: Doxycycline daily as malaria prophylaxis |
|
| Outcomes | 1. Clinical cure: resolution of diarrhoea and all symptoms within 72 hours after taking first dose of study medication 2. Treatment failure: persistency of diarrhoea or any symptom for longer than 72 hours after treatment was initiated | |
| Notes | ||
Mattila 1993.
| Methods | Randomised, double‐blind, placebo‐controlled study Efficacy analysis based on assigned treatment, i.e. not per intention to treat |
|
| Participants | 106 Finnish tourists visiting Agadir, Morocco in 1989 Entry criteria: four or more unformed stools per 24 hours or three unformed stools per 8 hours; at least one of: abdominal pain or spasms, nausea, vomiting, fever |
|
| Interventions | Treatment: Norfloxacin 400 mg by mouth twice daily for three days Control: Identical‐looking placebo twice daily for three days Both groups: No specific dietary restrictions, use of other antidiarrhoeal medication was prohibited, oral rehydration solution provided if required |
|
| Outcomes | 1. Duration of diarrhoea: time to the passage of last unformed stool 2. Full recovery: cessation of all symptoms and signs by the fourth day after beginning treatment 3. Treatment failure: passage of at least one unformed stool during the day after the treatment period or deterioration on treatment 4. Relapse: diarrhea recurring at least 24 hours after the 3‐day treatment period and after apparent recovery 5. Adverse effects | |
| Notes | This study spanned two seasons, namely Jan ‐ February (winter) and October ‐ November (fall) 67 participants (63%) had positive stool cultures, 21 cases with more than one pathogen Major pathogenic organisms: ETEC ‐ 27 (25%), Salmonella ‐ 23 (22%), Campylobacter ‐ 22 (21%), Aeromonas ‐ 9 (8%), EPEC ‐ 6 (6%), Rotavirus ‐ 3 (3%), Shigella ‐ 2 (2%) 100% of pretreatment stools sensitive to norfloxacin |
|
Petrucelli 1992.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses |
|
| Participants | 142 US military personnel participating in a military exercise in Thailand during May 1990 Entry criteria: three or more liquid bowel movements per 24 hour period; or two liquid stools plus cramps, nausea, or vomiting; diarrhoea of no more than 4 days duration Patients were excluded if they had grossly bloody stools or high fever (38.3 centigrade) |
|
| Interventions | 1. Ciprofloxacin 750 mg orally plus placebo
2. Ciprofloxacin 750 mg plus loperamide 4 mg followed by 2 mg after each liquid bowel movement (maximum 16 mg/day)
3. Ciprofloxacin 500 mg twice daily for three days plus loperamide 4 mg followed by 2 mg after each liquid bowel movement (maximum 16 mg/day) All groups: Daily doxycycline for malaria prophylaxis |
|
| Outcomes | 1. Duration of illness: Last unformed stool Last liquid stool 2. Complete recovery from all symptoms 3. Severity: Cumulative number of liquid bowel movements at 24, 48, and 72 hours after enrollment 4. Number of loperamide caplets taken after the loading dose | |
| Notes | ||
Salam 1994.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept daily diary of symptoms and medication doses |
|
| Participants | 88 British marines on military exercises in Belize and within 8 weeks of arrival Inclusion criteria: one or more unformed stools Participants with blood in their stools, fever > 38.5 centigrade, or volume depletion requiring intravenous fluid therapy were excluded |
|
| Interventions | Treatment: Ciprofloxacin 500 mg orally as a single dose Comparison: Identical placebo orally as a single dose Both groups: Chloroquine weekly and proguanil daily for malaria prophylaxis |
|
| Outcomes | 1. Duration of illness:
Time to last liquid stool
Time to last unformed stool (semi‐solid or soft) 2. Severity: Cumulative numbers of liquid and unformed stools at 24, 48, and 72 hours after enrolment |
|
| Notes | Stool cultures were not obtained by investigators | |
Steffen 1993.
| Methods | Randomised, double‐blind, placebo‐controlled trial Randomisation by computer‐generated list Patients were seen daily by a physician who also administered the dose of medication Patients kept a daily diary of symptoms |
|
| Participants | 195 hotel guests at a resort in The Gambia Diarrhoea of less than 6 days duration defined as at least one watery stool; one soft stool with abdominal cramps, vomiting, or nausea |
|
| Interventions | Treatment: 1. Fleroxacin 400 mg orally single dose day one, placebo day 2 2. Fleroxacin 400 mg orally for two days Placebo: 3. Placebo orally for two days | |
| Outcomes | Cure: Passage of a formed stool unless followed thereafter by unformed stool; no stools passed within 24 hours | |
| Notes | 44 participants excluded from efficacy analysis, but 190 analysed for safety 79 participants (52%) had positive stool cultures Major pathogenic organisms: ETEC ‐ 44 (29%), Salmonella ‐ 12 (8%), Plesiomonas ‐ 4 (3%), Aeromonas ‐ 2 (1%) Pre‐ and post‐treatment antibiotic sensitivities not reported |
|
Taylor 1991.
| Methods | Randomised, double‐blind trial Method of randomisation not stated Patients kept daily diary of symptoms, time and form of stool, and medication doses |
|
| Participants | 162 military personnel participating in field training exercises in Egypt from October to November 1989 Inclusion criteria were three or more liquid stools in 24 hours; or one or two liquid stools accompanied by abdominal cramps, or vomiting Participants with fever >38.3 degrees centigrade, grossly bloody diarrhoea, or diarrhoea for longer than 60 hours were excluded |
|
| Interventions | Intervention: Ciprofloxacin 500 mg by mouth twice daily for three days plus loperamide, 4 mg initial dose followed by 2 mg with each unformed stool (to a maximum of 16 mg per 24 hours) Comparison: ciprofloxacin 500 mg by mouth twice daily for three days plus placebo, taken in the same manner as loperamide |
|
| Outcomes | 1. Improvement: 50% decrease in daily number of stools compared to the previous 24 hours 2. Recovery: Total relief from all symptoms 3. Length of time lost from duty | |
| Notes | First dose observed, subsequent doses self‐ administered | |
Thornton 1992.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients were seen daily in clinic and at follow‐up visits at one and four weeks following onset of illness |
|
| Participants | 142 male military personnel participating in 1986 and 1987 UNITAS/WATC shipboard exercises or 1986 Blue Horizon land‐based exercise Entry criteria: four or more unformed stools per 24 hours or three unformed stools per 8 hours; diarrhoea of no more than 48 hours' duration; at least one other sign of gastrointestinal illness |
|
| Interventions | Treatment: Norfloxacin 400 mg orally twice daily for 5 days Comparison: TMP/SMX 160/800 orally twice daily for 5 days |
|
| Outcomes | 1. Duration of post‐treatment diarrhoea 2. Cure: resolution of diarrhoea after 5 days of treatment 3. Failure: continued diarrhoea after treatment 4. Non‐evaluable | |
| Notes | ||
Wistrom 1989.
| Methods | Randomised, double‐blind, placebo‐controlled multi‐centre trial Method of randomisation not stated Patients kept daily diary of symptoms, signs, and medication doses |
|
| Participants | 447 Swedish travellers on short duration trips (1‐6 weeks) to developing countries who visited three participating travel clinics for pre‐travel immunisations between December 1986 and April 1988 Entry criteria: four or more loose stools per 24 hours; two loose stools/24 hours plus any of the following: abdominal cramps, nausea, vomiting, or fever (> 38 centigrade rectal temperature) |
|
| Interventions | Self treatment Treatment: Norfloxacin 400 mg twice daily orally for three days Comparison: Identical placebo twice daily orally for three days Both: Written and verbal instructions regarding the conduct of the trial. Sealed envelopes containing the randomisation code to be opened in the event of a medical emergency and returned to the investigators if not opened |
|
| Outcomes | 1. Cure: one or fewer loose stools per 24 hours without additional symptoms Improving: two or three loose stools/24 hours without additional symptoms; one loose stool/24 hours with accompanying symptoms 2. Recurrence: return to inclusion criteria within 7 days after the last treatment dose 3. Failed | |
| Notes | 24 participants excluded initially 106 travellers developed diarrhoea meeting entry criteria 12 pathogens isolated on post‐travel stool cultures |
|
Wistrom 1992.
| Methods | Randomised, double‐blind, placebo‐controlled trial Method of randomisation not stated Patients kept a daily diary of symptoms and timing of medication doses Patients were seen daily for at least 3 days and then on day 10 |
|
| Participants | 42 US volunteers taken to a hotel in Puerto Vallarta, Mexico for 11 days Entry criteria: four or more unformed stools in a 24 hour period; two loose stools in a 24 hour period plus any of: abdominal cramps, vomiting, fever > 38 centigrade oral temperature |
|
| Interventions | Treatment: Ciprofloxacin 250 mg twice daily orally for three days Comparison: Placebo twice daily for three days |
|
| Outcomes | 1. Cure: one or fewer loose stools per 24 hours without additional symptoms Improving: two or three loose stools/24 hours without additional symptoms; one loose stool/24 hours with accompanying symptoms 2. Failed | |
| Notes | 17 participants developed diarrhoea Major pathogenic organism: ETEC ‐ 7 (41%) 100% of pretreatment stools sensitive to ciprofloxacin ETEC found with in vitro resistance on pretreatment stools: Ampicillin ‐ 63%, Doxycycline ‐ 47%, chloramphenicol ‐ 32%, SMX/TMP 17% |
|
ETEC, enterotoxigenic Escherichia coli; TMP, trimethoprim; SMX, sulfamethoxazole.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bouree 1987 | This was a study of prophylaxis not treatment of travellers' diarrhoea. In this double‐blind, placebo‐controlled study, 75 French adults participating in a car and motorcycle rally were randomised to nifuroxazide 400 mg per day or placebo as prophylaxis against travellers' diarrhoea |
| Chagnon 1996 | This was a review article and not a clinical trial |
| Hill 1961 | This unblinded trial allocated in rotation patients with acute onset of diarrhoea to one of four treatment groups:
1. "Empiric treatment" with magnesium sulphate and chlorodyne
2. Oxytetracycline 500 mg loading dose followed by 250 mg 6‐hourly for 5 days
3. Sulfonamide (phthalylsulfathiazole) 4 g five times daily plus oral streptomycin 1 g four times daily for 6 days
4. Sulfonamide alone ‐ phthalylsulfathiazole 4 g five times daily for 6 days No inclusion criteria for frequency of stools, stool consistency, or other symptoms were defined in the report. Allocation was done before obtaining culture results. Results for patients with dysentery were considered separately to those with no identified pathogens |
| Richards 1970 | This study was a single‐blind, non‐randomised, placebo‐controlled trial of prophylaxis with cloquinol 250 mg by mouth three or four times daily. The participants were British footballers playing abroad in the off season |
| Steffen 1988 | This was a randomised, double‐blind, placebo‐controlled study of self‐treatment of travellers' diarrhoea. The 2580 participants were Swiss residents visiting the Zurich University Vaccination Centre before travel to developing countries between July 1984 and December 1986. They were randomised, with stratification by destination, to one of seven regimens:
1. Bismuth subsalicylate 262.5 mg, 2 every 30 mins (total 8 doses per 24 hours)
2. Doxycycline 100 mg twice daily
3. Loperamide 4 mg loading dose and 2 mg after each unformed stool (max 16 mg per 24 hours)
4. Mecillinam 400 mg three times daily
5. Streptococcus faecium 75 x 10E6 SF68 three times daily
6. Trimethoprim/sulfamethoxazole 160/800 mg twice daily
7. Placebo Participants were instructed to commence self‐treatment with the development of any of the following symptoms: at least one watery stool; a pasty stool accompanied by cramps, nausea, vomiting, or fever In this study, 482 participants were considered drop outs (18.9%) prior to travel. Of the 2098 who travelled, 798 developed diarrhoea, and 654 took the study medication (82% of eligible participants), but a further 115 were excluded for protocol violations, 35 for taking concurrent medications, and 17 for unspecified reasons. A total of 530 participants were analysed for drug efficacy (66% of eligible participants). Because of the high drop out rate the trial was excluded |
Characteristics of studies awaiting assessment [ordered by study ID]
Anonymous 1995.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Bruns 1995.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
DuPont 1994.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
DuPont 1994b.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
DuPont 1997.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Ziegenhagen 1992.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
Contributions of authors
Not stated.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Unchanged
References
References to studies included in this review
Christensen 1988 {published data only}
- Christensen OE, Tuxen KK, Menday P. Treatment of travellers' diarrhoea with pivmecillinam [letter]. J Antimicrob Chemother 1988;22:570‐1. [DOI] [PubMed] [Google Scholar]
DuPont 1982 {published data only}
- DuPont HL, Ericsson CD, Reves RR, Galindo E. Antimicrobial therapy for travelers' diarrhea. Rev Infect Dis 1986;8(Suppl 2):S217‐22. [DOI] [PubMed] [Google Scholar]
- DuPont HL, Reves RR, Galindo E, Sullivan PS, Wood LV, Mendiola JG. Treatment of travelers' diarrhea with trimethoprim / sulfamethoxazole and with trimethoprim alone. N Engl J Med 1982;307:841‐4. [DOI] [PubMed] [Google Scholar]
- Dupont HL, Ericsson CD, Galindo E, Dupont MW, Mendiola Gomez J. Antimicrobial therapy of travellers' diarrhoea. Scand J Gastroenterol Suppl 1983;84:99‐105. [PubMed] [Google Scholar]
DuPont 1984 {published data only}
- DuPont HL, Ericsson CD, Galindo E, Wood LV, Morgan D, Bitsura JA, Mendiola JG. Furazolidone versus ampicillin in the treatment of traveler's diarrhea. Antimicrob Agents Chemother 1984;26:160‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPont HL, Ericsson CD, Reves RR, Galindo E. Antimicrobial therapy for travelers' diarrhea. Rev Infect Dis 1986;8(Suppl 2):S217‐22. [DOI] [PubMed] [Google Scholar]
DuPont 1992 {published data only}
- DuPont HL, Ericsson CD, Mathewson JJ, Cabada FJ, Conrad DA. Oral aztreonam, a poorly absorbed yet effective therapy for bacterial diarrhea in US travelers to Mexico. JAMA 1992;267:1932‐1935. [PubMed] [Google Scholar]
DuPont 1992b {published data only}
- DuPont HL, Ericsson CS, Mathewson JJ, DuPont MW. Five versus three days of ofloxacin therapy for traveler's diarrhea: a placebo ‐ controlled study. Antimicrob Ag Chemo 1992;36:87‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ericsson 1983 {published data only}
- DuPont HL, Ericsson CD, Reves RR, Galindo E. Antimicrobial therapy for travelers' diarrhea. Rev Infect Dis 1986;8(Suppl 2):S217‐22. [DOI] [PubMed] [Google Scholar]
- Dupont HL, Ericsson CD, Galindo E, Dupont MW, Mendiola Gomez J. Antimicrobial therapy of travellers' diarrhoea. Scand J Gastroenterol Suppl 1983;84:99‐105. [PubMed] [Google Scholar]
- Ericsson CD, DuPont HL, Sullivan P, Galindo E, Evans DG, Evans DJ Jr. Bicozamycin, a poorly absorbable antibiotic, effectively treats travelers' diarrhea. Ann Intern Med 1983;98:20‐5. [DOI] [PubMed] [Google Scholar]
Ericsson 1986 {published data only}
- Ericsson CD, Johnson PC, DuPont HL, Morgan DR. Role of a novel antidiarrheal agent, BW942C, alone or in combination with trimethoprim‐sulfamethoxazole in the treatment of traveler's diarrhea. Antimicrob Agents Chemother 1986;29:1040‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ericsson 1987 {published data only}
- Ericsson CD, Johnson PC, Dupont HL, Morgan DR, Bitsura JA, Cabada FJ. Ciprofloxacin or trimethoprim‐sulfamethoxazole as initial therapy for travelers' diarrhea, A placebo‐controlled, randomized trial. Ann Intern Med 1987;106:216‐20. [DOI] [PubMed] [Google Scholar]
Ericsson 1990 {published data only}
- Ericsson CD, DuPont HL, Mathewson JJ, West MS, Johnson PC, Bitsura JA. Treatment of traveler's diarrhea with sulfamethoxazole and trimethoprim and loperamide. JAMA 1990;263:257‐61. [PubMed] [Google Scholar]
Ericsson 1992 {published data only}
- Ericsson CD, Nicholls‐Vasquez I, DuPont HL, Mathewson JJ. Optimal dosing of trimethoprim‐sulfamethoxazole when used with loperamide to treat traveler's diarrhea. Antimicrob Agents Chemother 1992;36:2821‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ericsson 1997 {published data only}
- Ericsson CD, DuPont HL, Mathewson JJ. Single dose ofloxacin plus loperamide compared with single dose or three days of ofloxacin in the treatment of traveler's diarrhea. J Travel Med 1997;4:3‐7. [DOI] [PubMed] [Google Scholar]
Kuschner 1995 {published data only}
- Kuschner RA, Trofa AF, Thomas RJ, et al. Use of azithromycin for the treatment of campylobacter enteritis in travelers to Thailand, an area where ciprofloxacin resistance is prevalent. Clin Infect Dis 1995;21:536‐41. [DOI] [PubMed] [Google Scholar]
Mattila 1993 {published data only}
- Mattila L, Peltola H, Siitonen A, Kyronseppa H, Simula I, Kataja M. Short term treatment of traveler's diarrhea with norfloxacin: a double‐blind, placebo‐controlled study during two seasons. Clin Infect Dis 1993;17:779‐82. [DOI] [PubMed] [Google Scholar]
Petrucelli 1992 {published data only}
- Petruccelli BP, Murphy GS, Sanchez JL, et al. Treatment of traveler's diarrhea with ciprofloxacin and loperamide. J Infect Dis 1992;165:557‐60. [DOI] [PubMed] [Google Scholar]
Salam 1994 {published data only}
- Salam I, Katelaris P, Leigh‐Smith S, Farthing MJ. Randomised trial of single‐dose ciprofloxacin for travellers' diarrhoea. Lancet 1994;344:1537‐9. [DOI] [PubMed] [Google Scholar]
Steffen 1993 {published data only}
- Steffen R, Jori R, DuPont HL, Mathewson JJ, Sturchler D. Efficacy and toxicity of fleroxacin in the treatment of traveler's diarrhea. Am J Med 1993;Vol 94(suppl 3A):S182‐186. [PubMed] [Google Scholar]
- Steffen R, Jori R, DuPont HL, Mathewson JJ, Sturchler D. Treatment of travellers' diarrhoea with fleroxacin: a case study. J Antimicrob Chemo 1993;31:767‐776. [DOI] [PubMed] [Google Scholar]
Taylor 1991 {published data only}
- Taylor DN, Sanchez JL, Candler W, Thornton S, McQueen C, Echeverria P. Treatment of travelers' diarrhea: ciprofloxacin plus loperamide compared with ciprofloxacin alone. A placebo‐controlled, randomized trial. Ann Intern Med 1991;114:731‐4. [DOI] [PubMed] [Google Scholar]
Thornton 1992 {published data only}
- Thornton SA, Wignall SF, Kilpatrick ME, Bourgeois AL, Gardiner C, Batchelor RA, Burr DH, Oprandy JJ, Garst P, Hyams KC. Norfloxacin compared to trimethoprim / sulfamethoxazole for the treatment of travelers' diarrhea among U.S. military personnel deployed to South America and West Africa. Mil Med 1992;157:55‐8. [PubMed] [Google Scholar]
Wistrom 1989 {published data only}
- Wistrom J, Jertborn M, Hedstrom SA, Alestig K, Englund G, Jellheden B, Norrby SR. Short‐term self‐treatment of travellers' diarrhoea with norfloxacin: a placebo‐controlled study. J Antimicrob Chemother 1989;23:905‐13. [DOI] [PubMed] [Google Scholar]
Wistrom 1992 {published data only}
- Wistrom J, Gentry LO, Palmgren AC, Price M, Nord CE, Ljungh A, Norrby SR. Ecological effects of short‐term ciprofloxacin treatment of travellers' diarrhoea. J Antimicrob Chemother 1992;30:693‐706. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bouree 1987 {published data only}
- Bouree P, Kouchner G, Ponti M. Double‐blind study of traveller's diarrhoea using Nifuroxazide. Trans R Soc Trop Med Hyg 1987;81:859. [DOI] [PubMed] [Google Scholar]
Chagnon 1996 {published data only}
- Chagnon A. Travelers' diarrhea. Revue du Praticien 1996;46(2):189‐95. [PubMed] [Google Scholar]
Hill 1961 {published data only}
- Hill JA. Diarrhoea in Aden ‐ a therapeutic trial. Trans R Soc Trop Med Hyg 1961;55:355‐360. [DOI] [PubMed] [Google Scholar]
Richards 1970 {published data only}
- Richards DA. A controlled trial in travelers' diarrhoea. Practitioner 1970;204:822‐824. [PubMed] [Google Scholar]
Steffen 1988 {published data only}
- Steffen R, Heusser T, Schopp A, DuPont H. Efficacy and side‐effects of six agents in the self‐treatment of traveler's diarrhea. Travel Medicine International 1988;6:153‐157. [Google Scholar]
References to studies awaiting assessment
Anonymous 1995 {published data only}
- No authors listed. Single‐dose ciprofloxacin in the treatment of traveller's diarrhoea [Ciprofloxacin‐einmaldosis zur behandlung der reisediarrhoe]. Deutsche Medizinische Wochenschrift 1995;120(25‐6):943. [PubMed] [Google Scholar]
Bruns 1995 {published data only}
- Bruns R, Raedsch R. Therapy of traveller's diarrhea [Therapie der reisediarrhoe]. Medizinische Welt 1995;46(12):591‐6. [Google Scholar]
DuPont 1994 {published data only (unpublished sought but not used)}
- Otsuka Pharmaceuticals, study no 106‐92‐212.
DuPont 1994b {published data only (unpublished sought but not used)}
- Otsuka Pharmaceuticals study no 106‐92‐209.
DuPont 1997 {published data only}
- DuPont HL, Ericsson CD, Mathewson JJ, Palazzini E, DuPont MW, DeLa Cabada FJ. Rifaximin ‐ a nonabsorbed antimicrobial in the therapy of travelers' diarrhea in Guadalajara, Mexico. The Fifth International Conference on Travel Medicine. March 1997.
Ziegenhagen 1992 {published data only}
- Ziegenhagen DJ, Raedsch R, Kruis W. Traveler's diarrhea in Turkey. Prospective randomized therapeutic comparison of charcoal versus tannin albuminate/ethacridine lactate [Reisediarrho in der Turkei. Prospektiv randomisierter therapievergleich kohle versus tanninalbuminat/ethacridinlactat]. Medizinische Klinik 1992;87(12):637‐9. [PubMed] [Google Scholar]
Additional references
Black 1986
- Black RE. Pathogens that cause traveler's diarrhea in Latin America and Africa. Rev Infect Dis 1986;8(Suppl 2):S131‐S135. [DOI] [PMC free article] [PubMed] [Google Scholar]
BSSI 1996
- Farthing M, Feldman R, Finch R Fox R, Leen C, Mandal B Moss P, Nathwani D, Nye F, Ritchie L, Todd W, Wood M. The management of infective gastroenteritis in adults. A consensus statement by an expert panel convened by the British Society for the Study of Infection. J Inf 1996;33:143‐152. [DOI] [PubMed] [Google Scholar]
Cartwright 1997
- Cartwright RY, Chahed M. Foodborne diseases in travellers. World Health Statistics Quarterly 1997;50:102‐110. [PubMed] [Google Scholar]
DuPont 1993
- DuPont HL, Ericsson CD. Prevention and treatment of traveler's diarrhea. N Engl J Med 1993;328:1821‐1827. [DOI] [PubMed] [Google Scholar]
Farthing 1992
- Farthing M, et al. Treatment and prevention of traveller's diarrhoea. Gastroenterology International 1992;5:162‐175. [Google Scholar]
Gorbach 1975
- Gorbach SL, Kean BH, Evans DG, Evans D, Bessudo D. Traveler's diarrhea and toxigenic Escherichia coli. N Engl J Med 1975;292:933‐936. [DOI] [PubMed] [Google Scholar]
Hundt 1996
- Hundt A. Impact of tourism development on the economy and health of Third World Nations. J Travel Med 1996;3:107‐112. [DOI] [PubMed] [Google Scholar]
Katelaris 1995
- Katelaris PH, Farthing MJ. Traveler's diarrhea: clinical presentation and prognosis. Chemotherapy 1995;41 Suppl 1:40‐7. [DOI] [PubMed] [Google Scholar]
McIntosh 1997
- McIntosh IB, Reed JM, Power KG. Traveller's diarrhoea and the effect of pre‐travel health advice in general practice. Br J Gen Pract 1997;47:71‐75. [PMC free article] [PubMed] [Google Scholar]
NIH‐CDCS 1985
- [No authors listed]. Travelers' diarrhea. NIH Consensus Development Conference. JAMA 1985;253(18):2700‐4. [PubMed] [Google Scholar]
Steffen 1983
- Steffen R, Linde F, Gyr K, Schar M. Epidemiology of Diarrhea in Travelers. JAMA 1983;249:1176‐1180. [PubMed] [Google Scholar]
Steffen 1999
- Steffen R, Collard F, Tornieporth N, et al. Epidemiology, etiology, and impact of traveler's diarrhea in Jamaica. JAMA 1999;281:811‐17. [DOI] [PubMed] [Google Scholar]


