Abstract
Background
Brucellosis is the most common zoonotic infection in the world. Several antibiotics, separately or in combination, have been tried for treatment of human brucellosis. The inconsistencies between different treatment regimens warrants the need for a systematic review to inform clinical practice and future research.
Objectives
To evaluate the effects of various antibiotic regimens, monotherapy or in combination with other antibiotics, for treating human brucellosis.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, and LILACS until May 2012. We browsed the abstract books of several international infectious diseases conferences. We also checked the reference lists of all studies identified
Selection criteria
We included the randomized controlled trials on the pharmaceutical interventions in treatment of acute, chronic, non‐complicated, and complicated human brucellosis. The outcomes of interest were relapse, persistence of symptoms at the end of treatment, and adverse drug effects.
Data collection and analysis
Two authors independently assessed the studies for inclusion, risk of bias, and extracted relevant data using pre‐designed extraction forms. The findings of homogenous studies were pooled using fixed‐effect meta‐analysis.
Main results
In total we included 25 studies comparing various antibiotic regimens. Methods of allocation and concealment were inadequately described in half the studies, and only three were blinded. In comparisons of doxycycline plus rifampicin versus doxycycline plus streptomycin we found eight studies with 694 participants. For treatment failure, the doxycycline plus rifampicin regimen was less effective (risk ratio (RR) 1.91, 95% confidence interval (CI) 1.07 to 3.42, seven studies, 567 participants), relapse (RR 2.39, 95% CI 1.17 to 4.86), and minor adverse drug reactions (RR 1.38, 95% CI 0.99 to 1.92). In comparisons of doxycycline plus rifampicin against quinolone (ciprofloxacin or ofloxacin) plus rifampicin we found five studies of 336 participants. The pooled analysis did not demonstrate any significant difference between two regimens in terms of relapse and symptom persistence, but showed a non‐significant higher risk of minor adverse reactions in doxycycline plus rifampicin (RR 1.80, 95% CI 0.78 to 4.18). Other comparisons were reported in a few heterogenous studies, and the pooled analyses, where applied, did not show any significant difference.
Authors' conclusions
Doxycycline (six weeks) plus streptomycin (two or three weeks) regimen is more effective regimen than doxycycline plus rifampicin (six weeks) regimen. Since it needs daily intramuscular (IM) injection, access to care and cost are important factors in deciding between two choices. Quinolone plus rifampicin (six weeks) regimen is slightly better tolerated than doxycycline plus rifampicin, and low quality evidence did not show any difference in overall effectiveness.
22 March 2019
Update pending
Authors currently updating
The update is due to be published in 2019.
Keywords: Humans, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Brucellosis, Brucellosis/drug therapy, Ciprofloxacin, Ciprofloxacin/therapeutic use, Doxycycline, Doxycycline/therapeutic use, Ofloxacin, Ofloxacin/therapeutic use, Randomized Controlled Trials as Topic, Rifampin, Rifampin/therapeutic use, Streptomycin, Streptomycin/therapeutic use
Plain language summary
Antibiotics for treating human brucellosis
Brucellosis is a common infection caused by Brucella bacteria species and can infect both people and animals. It is spread by eating infected food products and through direct contact with infected animals. The bacterial infection can affect different tissues and organs and is treated using antibiotics. Current recommended treatment regimens involve the use of two or more antibiotics in order to avoid relapses occurring and to prevent prolonged use of these drugs, which may lead to problems of drug resistance arising. Drug resistance is a particularly important issue as most people infected with brucellosis live in low socioeconomic areas of developing countries, where tuberculosis is also an endemic health problem. Thus there are concerns over the potential increase in resistance to tuberculosis drugs due to their prolonged use in treating brucellosis.
This review evaluates different drug regimens for treatment of brucellosis in terms of treatment failure and side effects: doxycycline plus rifampicin, doxycycline plus streptomycin, quinolones plus rifampicin or doxycycline plus gentamycin.
Based on currently available evidence, there is probably a lower incidence of total drug treatment failure in people that take doxycycline plus streptomycin instead of doxycycline plus rifampicin to treat brucellosis. However, we are uncertain whether either one of these two treatment regimens results in people having fewer adverse drug reactions.
There may not be any difference between the two drug regimens, doxycycline plus rifampicin versus quinolones plus rifampicin, with respect to total treatment failure. Notably, use of doxycycline plus rifampicin instead of quinolones plus rifampicin may result in more people suffering adverse drug reactions.
Giving doxycycline plus gentamycin to people with brucellosis may reduce the incidence of total treatment failure compared to administration of doxycycline plus streptomycin. However, comparing these two drug regimens, there may not be any difference in the number of people that have drug reactions.
Importantly studies included in this review were limited to adult patients with brucellosis, and the findings of this review are not applicable to children, pregnant women, and patients with complications like spondylitis and neurobrucellosis. Some studies did not perform any explicit assessment of minor adverse reactions, so the findings regarding adverse drug reactions should be interpreted with caution.
Summary of findings
Summary of findings for the main comparison. Doxycycline+Rifampicin compared to Doxycycline+Streptomycin for human Brucellosis.
| Doxycycline+Rifampicin compared to Doxycycline+Streptomycin for human Brucellosis | ||||||
| Patient or population: patients with human Brucellosis Settings: inpatient or ambulatory Intervention: Doxycycline+Rifampicin Comparison: Doxycycline+Streptomycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline+Streptomycin | Doxycycline+Rifampicin | |||||
| total treatment failure Follow‐up: mean 1 years | 5 per 100 | 10 per 100 (6 to 18) | RR 1.91 (1.07 to 3.42) | 567 (7 studies) | ⊕⊕⊕⊝ moderate1 | |
| Minor adverse drug reactions Follow‐up: mean 3 months | 18 per 100 | 25 per 100 (18 to 35) | RR 1.38 (0.99 to 1.92) | 473 (5 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 on risk of bias. All but one study had unclear allocation concealment and were not blinded. Effect not statistically significant if only trials that were adequately concealed were included. 2 Downgraded by 1 on publication bias. Since, the minor adverse drug reactions were possibly under‐reported in the studies.
Summary of findings 2. Doxycycline+Rifampicin compared to Quinolones+Rifampicin for human Brucellosis.
| Doxycycline+Rifampicin compared to Quinolones+Rifampicin for human Brucellosis | ||||||
| Patient or population: patients with human Brucellosis Settings: inpatient or ambulatory Intervention: Doxycycline+Rifampicin Comparison: Quinolones+Rifampicin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Quinolones+Rifampicin | Doxycycline+Rifampicin | |||||
| total treatment failure Follow‐up: mean 1 years | 135 per 1000 | 128 per 1000 (66 to 236) | OR 0.94 (0.45 to 1.98) | 181 (3 studies) | ⊕⊕⊕⊝ moderate1 | |
| Minor adverse drug reactions Follow‐up: mean 3 months | 17 per 100 | 30 per 100 (13 to 71) | RR 1.80 (0.78 to 4.18) | 296 (4 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 on risk of bias. Concealment unknown in Agalar and Ersoy and not concealed in Akova; none blinded. 2 Downgraded by 1 on publication bias. Since, the minor adverse drug reactions were possibly under‐reported in the studies
Summary of findings 3. Doxycycline+Streptomycin compared to Doxycycline+Gentamycin for human Brucellosis.
| Doxycycline+Streptomycin compared to Doxycycline+Gentamycin for human Brucellosis | ||||||
| Patient or population: patients with human Brucellosis Settings: inpatient or ambulatory Intervention: Doxycycline+Streptomycin Comparison: Doxycycline+Gentamycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Doxycycline+Gentamycin | Doxycycline+Streptomycin | |||||
| total treatment failure Follow‐up: mean 1 years | 51 per 1000 | 91 per 1000 (43 to 180) | OR 1.85 (0.84 to 4.08) | 345 (2 studies) | ⊕⊕⊝⊝ low1,2 | |
| Minor adverse drug reactions Follow‐up: mean 3 months | 284 per 1000 | 241 per 1000 (170 to 347) | RR 0.85 (0.60 to 1.22) | 345 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 on indirectness. Two studies by the same author at the same location.
2 Downgraded by 1 on imprecision. Wide confidence intervals that mean the estimate could mean one combination is superior, inferior or no different to the other.
3 Downgraded by 1 on publication bias. Since, the minor adverse drug reactions were possibly under‐reported in the studies
Background
Brucellosis is the most common zoonotic disease worldwide (Pappas 2005a) and an important health problem in the Middle East, Central Asia, Africa, and Latin America (Hall 1990; Solera 1997a; Hassanjani Roushan 2004). It has been imported to countries in other geographical areas through migration, travel, and infected food products (Pappas 2005a). Brucellosis is caused by intracellular bacteria from the genus Brucella. Several Brucella species have been identified; Brucella melitensis is the most common cause of human brucellosis (Corbel 1997), while B. abortus is the most common cause of infection in animals. Human infection with B. abortus is often subclinical and less severe than the disease caused by B. melitensis (WHO 2006). Human reaction to different species ranges from acute inflammation to chronic granulomatous infection. Transmission generally occurs through eating unpasteurized or raw milk, or other dairy products. Other possible routes of transmission are direct contact with infected animal parts, inhalation of infected particles, and accidental inoculation. In developed countries, it is mostly an occupational disease in veterinarians, microbiologic laboratories staff, dairy industry professionals, and abattoir workers.
Clinical presentation
People with brucellosis usually present with non‐specific symptoms like fever, sweating, fatigue, loss of appetite, and weight loss (WHO 2006). Diagnosis is made by isolation of Brucella bacteria or positive tests for anti‐Brucella antibodies plus compatible clinical findings (fever, sweats, arthralgias, hepatomegaly, splenomegaly, or signs of focal disease) (Sauret 2002). Depending on the time and also the adequacy of received treatment, complications can occur. Musculoskeletal complications are the most common, particularly osteoarticular disease, monoarthritis (one‐joint inflammation), low back pain, osteomyelitis (bone infection), and septic arthritis (joint infection) (Corbel 2005). The reproductive system is the second common site of focal brucellosis. Neurological symptoms like depression and lethargy are frequently seen (Corbel 2005). There is no universal definition for chronic brucellosis. Some experts suggest the term 'chronic brucellosis' in patients whose clinical symptoms persist for 12 months or more from the time of the diagnosis (WHO 2006). The mortality rate due to Brucella infection is low, but morbidity is high because this systemic infection involves many organs and tissues, often causing diminished activity.
Treatment
Brucellosis is treated with antibiotics, although the most effective antibiotic regimens and treatment durations are unclear (Cisneros 1990; Karabay 2004). There are some limitations in choosing the best regimen: the choice of antibiotics that act intracellularly (eg doxycycline) are limited (Agalar 1999); and prolonged treatment needed to prevent relapse may increase the occurrence of adverse reactions (including gastric discomfort, hepatotoxicity (liver toxicity), nephrotoxicity (kidney toxicity), and allergic reactions), and may reduce adherence to the treatment. Regimens that combine two or more antibiotics are now recommended by most experts due to high relapse rates with monotherapy (Agalar 1999; Pappas 2005b). In addition, since the majority of brucellosis cases are in low socioeconomic areas of developing countries, where tuberculosis is also an endemic health problem, the overlap between treatment regimens of two diseases have raised concerns over the potential increase in resistance to tuberculosis drugs due to their prolonged use in brucellosis treatment (Ariza 2007). The significant health consequences of multiple drug resistant tuberculosis has resulted in calls for new drug regimens and shorter brucellosis drug treatment regimens.
In 1971, the World Health Organization (WHO) suggested a 21‐day regimen of tetracycline plus streptomycin as the treatment of choice for human brucellosis (WHO 1971). Although this regimen was successful in reducing the early symptoms, it failed to treat the disease completely, and immediate relapse was observed in some patients (Ariza 1985; Cisneros 1990). Accordingly, in 1986 the Joint Food and Agriculture Organization of the United Nations (FAO)/ WHO Expert Committee on Brucellosis proposed two new regimens: rifampicin (600 to 900 mg/day orally) plus doxycycline (200 mg/day orally) for six weeks; and doxycycline (200 mg/day orally) for 45 days plus streptomycin (1 g/day intramuscularly) for two to three weeks (WHO 1986). However, later studies showed a treatable but high rate of relapse for the mentioned regimens. The rifampicin plus doxycycline regimen is the most popular treatment, and favourable to the more effective regimen of streptomycin plus doxycycline, possibly because its lower price and ease of administration (Pappas 2005b; Pappas 2007); while streptomycin requires parenteral administration in a hospital setting or in an appropriately set‐up primary care network, both of which are restricted in lower income countries (Pappas 2005a). The above‐mentioned treatment regimens were replicated in the 2006 WHO recommendations (WHO 2006). Streptomycin plus doxycycline regimen was stated as the gold standard for the treatment of brucellosis by the recommendations developed through an international consensus meeting of experts in Ioannina, Greece (Ariza 2007). Streptomycin plus doxycycline was considered superior to the doxycycline plus rifampicin regimen by the Ioannina expert panel, because of its higher effectiveness, some supporting evidence of pharmacokinetics, and its lack of overlap with tuberculosis treatments. However, the expert panel suggested the need for further studies on the potential side effects of aminoglycoside‐containing regimens.
Other antibiotic formulations, including quinolones (eg ciprofloxacin and ofloxacin) and trimethoprim/sulphamethoxazole (co‐trimoxazole), have been used (Pappas 2005b; WHO 2006). However, questions remain about their effectiveness (Pappas 2006; WHO 2006). In addition, monotherapy with tetracyclines has been considered another less costly and easier to administer by some authors; while the evidence on its comparative effectiveness with combination therapies is scarce and controversial (Solera 2010).
Treatment protocols may differ in children aged less than eight years and pregnant women, because of adverse reactions of some medications, including inhibition of bone growth due to tetracycline treatment in children and teratogenic potential of some drugs, such as streptomycin (WHO 2006).
Rationale for the review
Despite years of research on the treatment of human brucellosis, the choice of antibiotics and the optimal duration of treatment have remained controversial (Ariza 2007). Skalsky 2008 conducted a systematic review on the effectiveness of antibiotic regimens in treating human brucellosis. Despite the comprehensiveness of the search and rigor of quality assessment, the study had some limitations. The authors pooled studies based on drug classes (eg comparing all quinolone and non‐quinolone based regimens, no matter with what other antibiotic they were administered), and did not take the within class heterogeneity into account. In addition, they pooled spondylitis cases with non‐complicated brucellosis, despite differences in the treatment duration in many studies. In addition, while they stated that they used a modified intention‐to‐treat analysis, in which all drop outs were counted as failure, it seems that they did not apply that rule consistently to all included studies. For instance, 16 patients who were withdrawn from the Ariza 1992 trial were not considered as failure in the meta‐analysis. Moreover, they limited the review to a few comparisons among different combinations of treatment regimens. Solis Garcia del Pozo 2012 conducted a more recent systematic review on the same issue, trying to address the limitations of the Skalsky 2008 study. However, we believe that the authors misclassified several studies. For example, even though they stated that the meta‐analyses were done only on randomized controlled trials, several quasi and non‐randomized studies were also included (eg Ariza 1985b; Saltoglu 2002; Solera 1991; Solera 1995). In addition, despite stating that they excluded studies with less than six months follow‐up, Karabay 2004 with three months follow‐up was still used in the analysis. Moreover, they combined the results of Keramat 2009 study (with 38% of included patients suffering from spondylitis) with the studies on non‐complicated brucellosis.
The inconsistencies among different treatment regimens and lack of high quality reviews comparing various treatment regimens (especially comparisons other than doxycycline plus streptomycin versus doxycycline plus rifampicin) demonstrate the need for a more comprehensive systematic review. We aimed to collate the evidence on the effectiveness of the frequently used regimens for treating human brucellosis, to inform practice and research priorities.
Objectives
To evaluate the effects of various antibiotic regimens, monotherapy or in combination with other antibiotics, for treating human brucellosis in terms of treatment failure and side effects.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials. Non‐randomized and quasi‐randomized trials were excluded.
Types of participants
People with brucellosis diagnosed by the presence of compatible clinical findings (fever, sweats, arthralgias, hepatomegaly, splenomegaly, or signs of focal disease) with:
isolation of Brucella species from blood, other tissues, or fluids.
or standard tube agglutination titres of 1/160, or more for anti‐Brucella antibodies.
Patients with brucellosis spondylitis were excluded, because of the considerable differences in the treatment principles of spinal complications.
Types of interventions
Interventions
Combination of doxycycline and rifampicin, doxycycline and streptomycin, quinolones and rifampicin, doxycycline and gentamycin, and other combinations, with any dosage for any period of treatment.
Control
Placebo, or other antibiotics given alone or in combination.
Types of outcome measures
Primary
Total treatment failure, defined as:
the relapse of symptoms and signs of the disease accompanied by increasing serological tests and/or a positive culture after the end of treatment or
persistence of disease symptoms or signs after the end of treatment
Secondary
Time to defervescence, defined as the number of days after the beginning of treatment until the fever ends.
Adverse drug reactions
Serious adverse reactions, including those that result in death, hospitalisation, prolongation of hospital stay, discontinuation of treatment, or persistent or significant long‐term disability.
Minor adverse reactions, including gastrointestinal upset, central nervous system events, hepatotoxicity, nephrotoxicity, ototoxicity, or dermatitis.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Table 4: Cochrane Infectious Diseases Group Specialized Register, Cochrane Central Register of Controlled Trials (CENTRAL) issue, published in The Cochrane Library (2012, issue 2), MEDLINE (9 May 2012), EMBASE (9 May 2012), and LILACS (9 May 2012).
1. Detailed search strategies.
| Search set | CIDG SR^ | CENTRAL | MEDLINE^^ | EMBASE^^ | LILACS^^ |
| 1 | brucell* | brucell* | brucell* | brucell$ | brucellosis |
| 2 | Malta fever | Malta fever | Malta fever | Malta fever | Malta fever |
| 3 | undulant fever | undulant fever | undulant fever | undulant fever | undulant fever |
| 4 | 1 or 2 or 3 | BRUCELLOSIS/DRUG THERAPY/PREVENTION AND CONTROL THERAPY | BRUCELLOSIS/DRUG THERAPY/PREVENTION AND CONTROL THERAPY BRUCELLOSIS/THERAPY | BRUCELLOSIS | 1 or 2 or 3 |
| 5 | ‐ | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | 1 or 2 or 3 or 4 | ‐ |
| 6 | ‐ | ‐ | Limit 5 to Human | Limit 5 to Human | ‐ |
| ^Cochrane Infectious Diseases Group Specialized Register | ^^Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006); upper case: MeSH or EMTREE heading; lower case: free text term |
The electronic search was updated by the Cochrane Infectious Diseases Group on 9 May 2012.
We also searched the International Clinical Trials Registry Platform Search Portal (http://www.who.int/trialsearch/) for ongoing and unpublished trials until 9 May 2012, using 'brucellosis' as the search term.
Conference proceedings
We searched the following conference proceedings for relevant abstracts from year 2000 to 2008: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); Annual meetings of the Infectious Diseases Society of America (IDSA); and European Congress of Clinical Microbiology and Infectious Diseases (ECCMID).
Researchers and pharmaceutical companies
We contacted individual researchers working in the field and pharmaceutical companies including Bayer, Aventis, Novartis, and CollaGenex for unpublished and ongoing trials.
Reference lists
We checked the reference lists of all studies identified by the above methods, looking for cited relevant studies.
Data collection and analysis
Selection of studies
Two authors independently checked the results of the literature search for potentially relevant trials following the inclusion criteria, and determined the eligibility for obtaining full articles. If it was obvious from the abstract that the study did not meet the selection criteria, we excluded the study. If it was unclear from the abstract whether the study met the selection criteria, or it was included by each author, then we retrieved the full text article. Two authors independently assessed the full‐text articles for inclusion using the study eligibility criteria. We resolved disagreements in a consensus meeting, or by seeking the opinion of a third author. We scrutinized each trial report to find potential duplicates. We have listed the excluded studies along with the reason for exclusion in the Characteristics of excluded studies. If eligibility was unclear, we attempted to contact the trial authors for further information, and a final decision made to include or exclude.
Data extraction and management
Two authors independently extracted data using a pre‐designed data extraction form. We resolved disagreements by consensus or by consulting a third author.
We used an available case analysis, ie all patients with a measured outcome were included in the analysis. For studies in which patients with spondylitis were also included, we extracted the data for the subgroup without spondylitis and, if available, we used this data in the analysis.
We extracted data for dichotomous outcomes, such as relapse rate and therapeutic failure, by recording the total number of participants randomized, those that experienced outcomes, and the number analysed. For continuous outcomes, such as time to defervescence, we extracted the total number of participants analysed, arithmetic means, and the standard deviations. If the number of participants randomized to each group was not identical to the number analysed for a given outcome, we calculated the percentage of participants lost to follow‐up. We attempted to contact trial authors where published data were unclear or missing.
Assessment of risk of bias in included studies
Two authors independently assessed the risk of bias using the Cochrane Collaboration Risk of Bias form. We resolved disagreements through consensus or by consulting a third author. We assessed six components of risk of bias for each study: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other biases. For each component, the judgment of "yes", "no", or "unclear" was made, which represented either a high, low, or unclear risk of bias.
Assessment of reporting biases
We intended to explore the presence of reporting biases using funnel plots but we were unable to do so due to the nature of the data and the limited number of included studies.
Data synthesis
We used Review Manager (RevMan) to analyse data. We used risk ratio (RR) as a measure of effect for dichotomous data, and the weighted mean difference for continuous data. The results were presented with 95% CI. If we suspected the data were skewed and inappropriately summarized as means and standard deviations, we did not combine the data in a meta‐analysis.
For each outcome, we stratified the analyses by comparison (ie we did not combine trials with different control groups). For total treatment failure and relapse, we also stratified the analyses by follow‐up time (less than six months versus greater than six months). For adverse drug reactions, we stratified the analyses by severity, classified as serious, minor (mild and moderate), and all adverse reactions. We combined trials within the same strata using meta‐analysis.
For dichotomous outcomes, where data available, the number of patients randomized was used as the denominator, and all missing data were considered as
To check for heterogeneity, we visually examined the forest plots, used the Chi2 test with 10% level of statistical significance and performed the I2 statistic (50% represents moderate level of heterogeneity). Where the number of trials permitted, we planned to explore the following potential sources of heterogeneity in subgroup analyses: Brucella species (B. melitensis or B. abortus); antibiotic dose; treatment duration; follow‐up duration (less than six months; six months or more); participant age (less than eight years versus older); pregnancy; and local organ involvements (central nervous system involvement, endocarditis).
Sensitivity analysis
We intended to perform a sensitivity analysis to assess the role of adequate allocation concealment and other quality indicators, but we were unable to do so due to the limited number of included studies.
Results
Description of studies
Results of the search
Of the potentially relevant studies, we excluded 32 (Characteristics of excluded studies), and included 24 citations for 25 studies (Characteristics of included studies). Of the included studies, one citation consisted of two separate studies (Montejo 1993a; Montejo 1993b). A preliminary report of one study was reported in an earlier citation in the form of excerpt or conference proceeding (Rodriguez Zapata 1987). Our efforts in finding the full text of one potentially included study have been unsuccessful to date (Studies awaiting classification).
Included studies
We included 25 randomized controlled trials. All of the studies used a combination of clinical assessment, culture, and serology to diagnose brucellosis. Fifteen studies diagnosed the brucellosis by a positive culture or a combination of clinical features (including fever, sweats, arthralgia, hepatomegaly, splenomegaly) and a standard tube agglutination titre (STAT) of 1/160 or more. Acocella 1989 used a Standard tube agglutination test above 125 International Units (IU), and Alavi 2007 a STAT titre of 1/80; which are in both studies a more sensitive and less specific serologic cut‐point. Conversely, Hassanjani Roushan 2006, and Hassanjani Roushan 2010 used a titer of 1/320 or more for STAT, as diagnostic cut‐point. The Lang 1990, Lang 1992, and Rodriguez Zapata 1987 studies used the Rose‐Bengal and a four‐fold increase in hemagglutination titres as serologic criteria. The Agalar 1999, Buzon 1982, and Sarmadian 2009 did not specify the serologic criteria . The percentage of positive culture for brucellosis ranged between 60 to 75% of participants in included studies, apart from Agalar 1999 which excluded all patients with negative culture. In most of the studies B. melitensis was the only detected Brucella species in cultures. Patients with neurobrucellosis or endocarditis were excluded from most of the studies, excluding Colmenero 1994, Karabay 2004, and Rodriguez Zapata 1987. Agalar 1999 reported no occurrence of endocarditis. All studies were restricted to adult patients, but the minimum age varied from six years (Lang 1992) to 18 years (Lang 1990;Solera 2004). Pregnant women were excluded. Details about each included trial are given in the table of Characteristics of included studies.
Doxycycline plus rifampicin regimen was compared with doxycycline plus streptomycin in eight studies (Acocella 1989;Ariza 1992;Colmenero 1989;Colmenero 1994;Ersoy 2005;Hashemi 2012; Montejo 1993b;Rodriguez Zapata 1987 ). It was compared with a combination of a quinolone (ciprofloxacin or ofloxacin) with rifampicin in six studies (Agalar 1999;Akova 1993;Ersoy 2005;Hashemi 2012; Karabay 2004;Sarmadian 2009). Some studies compared it with other antibiotic combinations: with tetracycline plus streptomycin (Acocella 1989), with doxycycline plus co‐trimoxazole (Alavi 2007), with doxycycline plus ciprofloxacin (Kalo 1996;Sarmadian 2009), with ciprofloxacin (Lang 1990), with co‐trimoxazole (Montejo 1993a), and with doxycycline plus rifampicin plus amikacin (Ranjbar 2007).
Only nine studies defined the relapse based on the presence of both clinical and serologic conditions (Agalar 1999; Alavi 2007; Ersoy 2005; Hashemi 2012; Hassanjani Roushan 2004; Hassanjani Roushan 2006; Hassanjani Roushan 2010; Karabay 2004; Lang 1990). The rest of the studies defined it based on reappearance of clinical signs and symptoms, or the presence of either clinical, serologic, or bacteriologic criteria.
Risk of bias in included studies
Details of the risk of bias of included studies are provided in Figure 1 and Figure 2.
1.
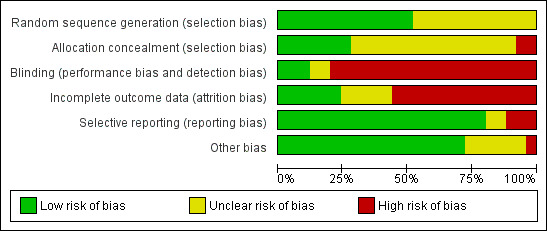
Risk of Bias graph: review authors' judgements about each Risk of Bias item presented as percentages across all included studies.
2.
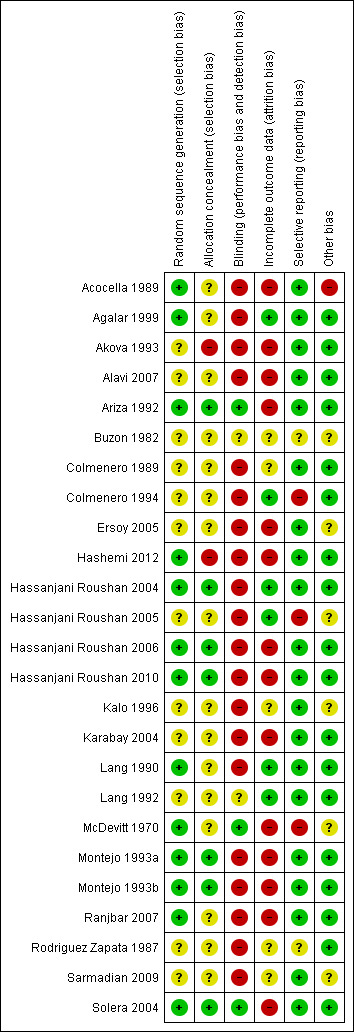
Risk of Bias summary: review authors' judgements about each Risk of Bias item for each included study.
Allocation
All studies were randomized controlled trials, but the method of sequence generation was adequately described in 12 of the studies (Characteristics of included studies). The allocation of patients was sufficiently concealed in seven studies (Ariza 1992;Hassanjani Roushan 2004;Hassanjani Roushan 2006;Hassanjani Roushan 2010;Montejo 1993a;Montejo 1993b;Solera 2004).
Blinding
Most studies were open, and the patients, care providers, and outcome assessors were not blinded; except in Ariza 1992, McDevitt 1970, and Solera 2004 in which patients and investigators were kept blinded to treatments.
Incomplete outcome data
Generally, studies reported the number of patients missed during the study period. However, because of the low proportion of the primary outcome (total treatment failure), the percentage of missing outcomes compared with observed event risk was high enough to induce bias in intervention effect estimate in most of the studies. The percentage of patients lost to follow‐up was considerable among the studies, and some mistakenly defined the non‐compliant patients or who developed complications as excluded. Most of the studies did not explicitly report the number of patients missed at each follow‐up session.
Selective reporting
Relapse and persistence of symptoms at the end of treatment was reported in most studies. Three studies did not report the frequency of adverse drug events (Alavi 2007; Colmenero 1994;Hassanjani Roushan 2005).
Other potential sources of bias
The provided information in most of the studies was not sufficient to assess these sources of bias. Acocella 1989 had a considerable baseline imbalance between study groups.
Effects of interventions
See: Table 1; Table 2; Table 3
1. Doxycycline plus rifampicin versus doxycycline plus streptomycin
Six studies used 1 g/day of streptomycin for 21 days, administered intramuscularly (Acocella 1989;Colmenero 1989;Colmenero 1994;Ersoy 2005;Hashemi 2012; Rodriguez Zapata 1987). Ariza 1992, and Montejo 1993b applied it for two weeks. In the doxycycline plus streptomycin arm, most studies administered 200 mg/day of doxycyline for six weeks, except for Colmenero 1989 which used it for 30 days, and Ersoy 2005 who used a dosage of 100 mg/day of doxycycline for six weeks.
1.1 Total treatment failure
The pooled analysis of six trials (n = 490, Figure 3) did not show any significant difference in total treatment failure between doxycycline plus rifampicin and doxycycline plus streptomycin in short‐term follow‐up (Acocella 1989, Ariza 1992, Colmenero 1994, Ersoy 2005, Hashemi 2012, Rodriguez Zapata 1987). But in follow‐ups of longer than six months in duration, pooling the results of seven studies (n = 567) showed that the doxycycline plus rifampicin regimen resulted in significantly more treatment failures with a fixed‐effect Mantel‐Hanszel RR of 1.91 (95% CI 1.07 to 3.42, Analysis 1.1: subgroup 2) (Acocella 1989, Ariza 1992, Colmenero 1989, Colmenero 1994, Ersoy 2005, Montejo 1993b, Rodriguez Zapata 1987). The point estimates were all placed on one side of the no‐effect line, and were not significantly heterogeneous.
3.
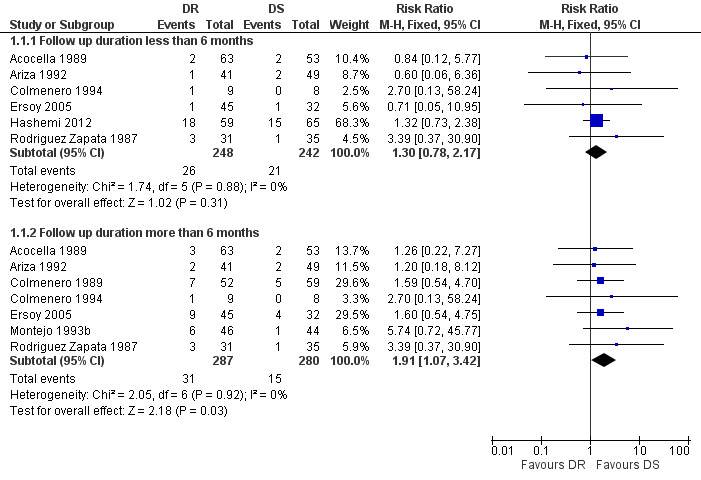
Forest plot of comparison: 1 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Streptomycin(DS), outcome: 1.1 Total treatment failure.
1.1. Analysis.
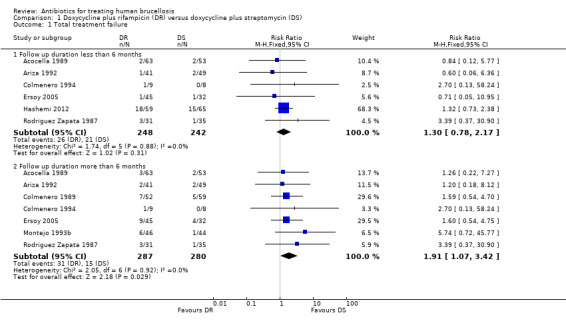
Comparison 1 Doxycycline plus rifampicin (DR) versus doxycycline plus streptomycin (DS), Outcome 1 Total treatment failure.
Following visual inspection of the forest plot, the point estimates of RR for two studies with different doxycycline plus streptomycin regimens (Colmenero 1989, Ersoy 2005), or with different durations of streptomycin administration (Ariza 1992; Montejo 1993b) did not differ from the other studies. Ariza 1992, Colmenero 1994, and Rodriguez Zapata 1987 also included seven, two, and two patients with spondylitis respectively. That subgroup was removed from the analysis, since the information regarding the relapse and treatment failure was available for it.
1.2 Relapse
The pooled analysis of relapse in seven studies (n = 567, Figure 4) showed that doxycycline plus rifampicin regimen resulted in more relapses than doxycycline plus streptomycin during follow‐up periods of more than six months, with the fixed‐effect Mantel‐Hanszel RR of 2.39 (95% CI 1.17 to 4.86, Analysis 1.2: subgroup 2) (Acocella 1989, Ariza 1992, Colmenero 1989, Colmenero 1994, Ersoy 2005, Montejo 1993b, Rodriguez Zapata 1987). The pooled analysis of relapse rate during follow‐ups shorter than six months in five studies (n = 413) did not show any significant difference.
4.
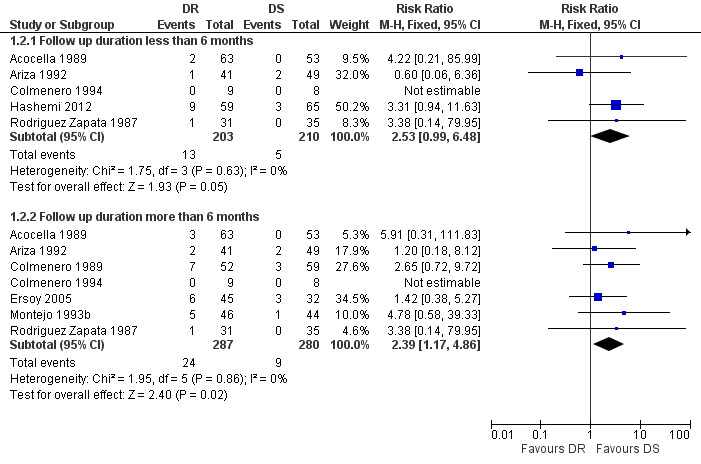
Forest plot of comparison: 1 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Streptomycin(DS), outcome: 1.2 Relapse.
1.2. Analysis.
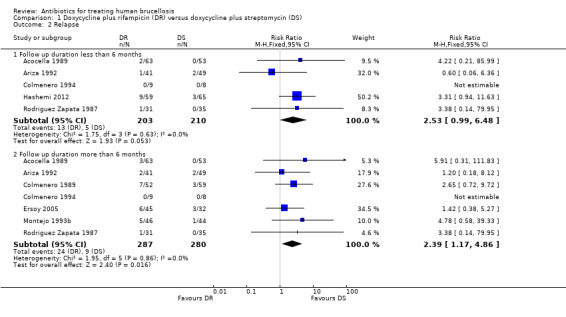
Comparison 1 Doxycycline plus rifampicin (DR) versus doxycycline plus streptomycin (DS), Outcome 2 Relapse.
1.3 Persistence of symptoms
The pooled analysis of persistence of symptoms in eight studies (n = 696, Figure 5) did not show any significant difference between two regimens, with the fixed‐effect Mantel‐Hanszel RR of 1.69 (95% CI 0.79 to 3.59, Analysis 1.3) (Acocella 1989;Ariza 1992;Colmenero 1989;Colmenero 1994;Ersoy 2005;Hashemi 2012; Montejo 1993b;Rodriguez Zapata 1987). The variation among studies was not significantly greater than by chance. Montejo 1993b defined the persistence of symptoms as if the symptoms did not resolve two weeks after treatment had begun, which was slightly different from other studies (persistence after the end of treatment).
5.
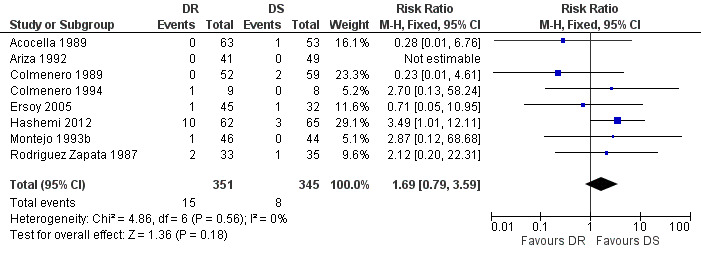
Forest plot of comparison: 1 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Streptomycin(DS), outcome: 1.3 Persistence of symptoms.
1.3. Analysis.
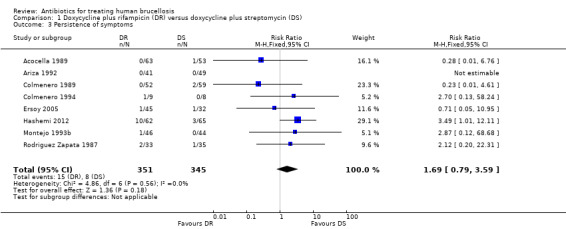
Comparison 1 Doxycycline plus rifampicin (DR) versus doxycycline plus streptomycin (DS), Outcome 3 Persistence of symptoms.
1.4 Adverse drug reactions
We divided adverse drug effects into two subgroups of serious adverse reactions, including those that resulted in death, hospitalisation, prolongation of hospital stay, discontinuation of treatment, or persistent or significant long‐term disability, and minor adverse reactions. Only two studies reported cases of serious adverse reactions, resulted in the change of treatment regimen (Acocella 1989;Ersoy 2005), which did not differ in two study groups. Five studies observed minor adverse drug effects, mainly gastro‐intestinal complaints (Ariza 1992;Colmenero 1989;Ersoy 2005;Hashemi 2012; Rodriguez Zapata 1987). Pooled analysis of minor adverse drug reactions of five studies (n = 473, Figure 6) did not show a significantly higher risk of minor drug reactions in doxycycline plus rifampicin than doxycycline plus streptomycin, with fixed‐effect Mantel‐Hanszel RR of 1.38 (95% CI 0.99 to 1.92, Analysis 1.4: subgroup 2).
6.
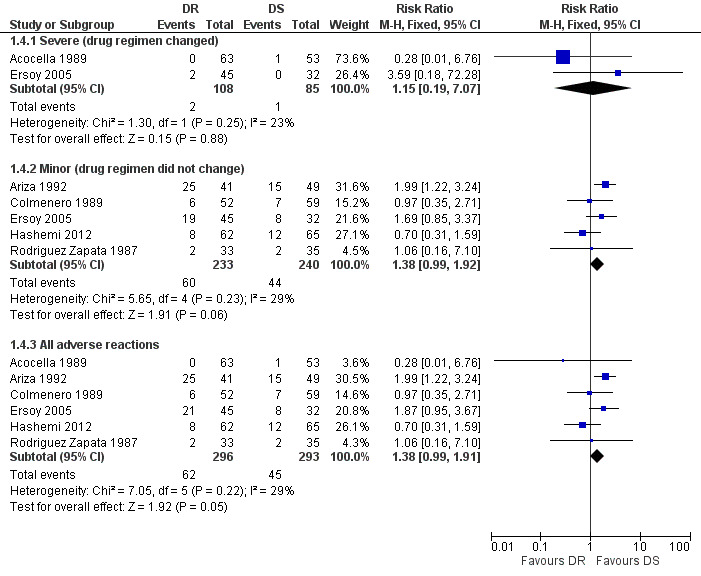
Forest plot of comparison: 1 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Streptomycin(DS), outcome: 1.4 Adverse drug reactions.
1.4. Analysis.
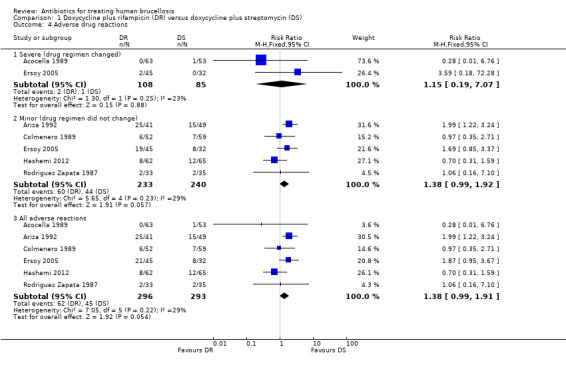
Comparison 1 Doxycycline plus rifampicin (DR) versus doxycycline plus streptomycin (DS), Outcome 4 Adverse drug reactions.
1.5 Time to defervescence
Two studies reported the average time to defervescence (Ariza 1992;Colmenero 1989). None of them reported the standard deviation of measure, so meta‐analysis was not possible. The average time to defervescence in doxycycline plus rifampicin and doxycycline plus streptomycin groups was 4.2 and 3.2 days in Ariza 1992, and 3.5 and 3.5 days in Colmenero 1989 respectively.
2. Doxycycline plus rifampicin versus quinolone plus rifampicin
Five studies compared doxycycline plus rifampicin with a combination of a quinolone (ciprofloxacin or ofloxacin) with rifampicin (Agalar 1999;Akova 1993;Ersoy 2005;Hashemi 2012; Karabay 2004). Three studies used the combination of ofloxacin 400 mg/day with rifampicin 600 mg/day for six weeks (Akova 1993;Ersoy 2005;Karabay 2004). Hashemi 2012 administered ofloxacin 800 mg/day for six weeks, combined with 15 mg/kg/day of rifampicin. Agalar 1999 administered ciprofloxacin 1 g/day and rifampicin 600 mg/day for 30 days. Akova 1993 included six patients with spondylitis who were excluded from the analysis.
2.1 Total treatment failure
The pooled analysis of total treatment failure of five studies (n = 333, Figure 7) for short term follow‐up did not show any significant difference between two regimens, with a fixed‐effect Mantel‐Hanszel RR of 1.41(95% CI 0.87 to 2.28, Analysis 2.1: subgroup 1). Furthermore, pooling of the results for the follow‐up durations longer than six months did not show any significant difference (RR 0.94, 95% CI 0.45 to 1.98, 181 participants, three trials, Analysis 2.1: subgroup 2). In short‐term follow‐up, Hashemi 2012, which used a doubled daily dose of ofloxacin, showed a considerably better point estimates for the ofloxacin plus rifampicin treatment, in comparison to the other four studies. Agalar 1999, which used ciprofloxacin, did not show any considerable difference in comparison to other studies using ofloxacin.
7.
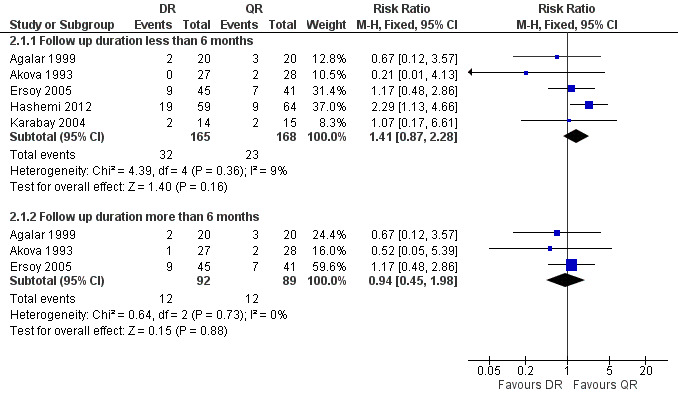
Forest plot of comparison: 2 Doxycycline plus Rifampicin(DR) versus Quinolones plus Rifampicin(QR), outcome: 2.1 Total treatment failure.
2.1. Analysis.
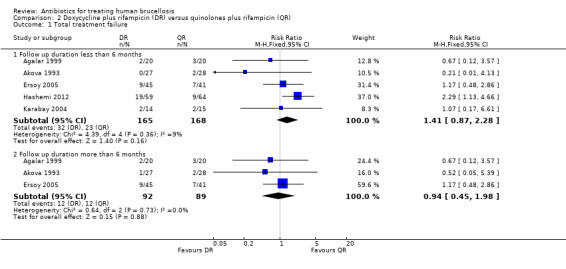
Comparison 2 Doxycycline plus rifampicin (DR) versus quinolones plus rifampicin (QR), Outcome 1 Total treatment failure.
2.2 Relapse
The pooled analysis of short‐term and long‐term follow‐ups of risk of relapse (Figure 8) did not show any significant difference between two regimens, with a fixed‐effect Mantel‐Hanszel RR of 0.95 (95% CI 0.40 to 2.27, 181 participants, three trials, Analysis 2.2: subgroup 2) for long‐term follow‐up. In short‐term follow‐up, Hashemi 2012, showed a considerably better point estimates in favour of ofloxacin plus rifampicin treatment, in comparison to the other three studies.
8.
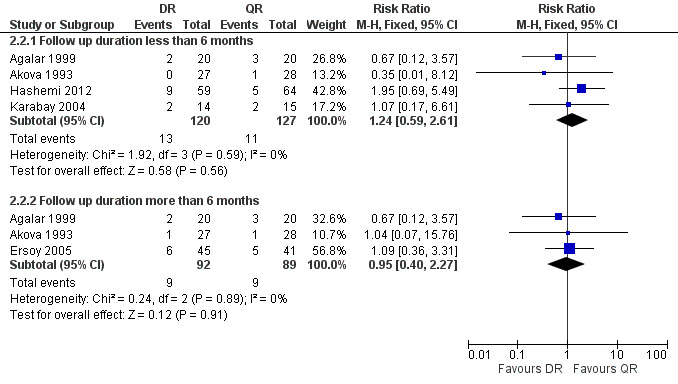
Forest plot of comparison: 2 Doxycycline plus Rifampicin(DR) versus Quinolones plus Rifampicin(QR), outcome: 2.2 Relapse.
2.2. Analysis.
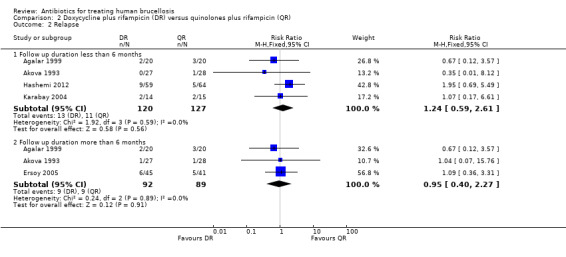
Comparison 2 Doxycycline plus rifampicin (DR) versus quinolones plus rifampicin (QR), Outcome 2 Relapse.
2.3 Persistence of symptoms
The pooled estimate of the risk of symptom persistence at the end of treatment in three studies (n = 267, Figure 9) was not statistically significant, with a fixed‐effect Mantel‐Hanszel RR of 1.80 (95% CI 0.72 to 4.53, Analysis 2.3). Again, the findings of Hashemi 2012 study was in favour of ofloxacin plus rifampicin treatment, considerably different from the other two studies.
9.
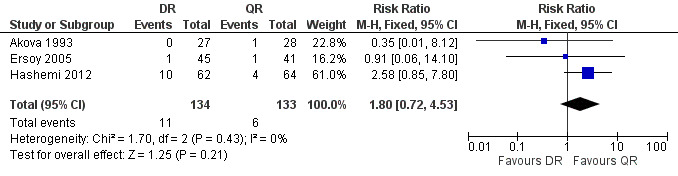
Forest plot of comparison: 2 Doxycycline plus Rifampicin(DR) versus Quinolones plus Rifampicin(QR), outcome: 2.3 Persistence of symptoms.
2.3. Analysis.
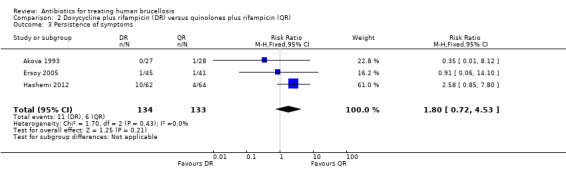
Comparison 2 Doxycycline plus rifampicin (DR) versus quinolones plus rifampicin (QR), Outcome 3 Persistence of symptoms.
2.4 Adverse drug reactions
Serious drug effects, leading to the change of treatment regimen, was only reported in Ersoy 2005 (three events). The pooled estimate of the random effect analysis of minor adverse reactions in four studies (n = 296, Figure 10) was not significant, with a Mantel‐Hanszel RR of 1.80 (95% CI 0.78 to 4.18, Analysis 2.4: subgroup 2). Hashemi 2012 was the only study reporting a higher proportion of minor adverse drug reactions in ofloxacin plus rifampicin group, compared to the doxycycline plus rifampicin. The observed adverse reactions were mainly gastrointestinal upset.
10.
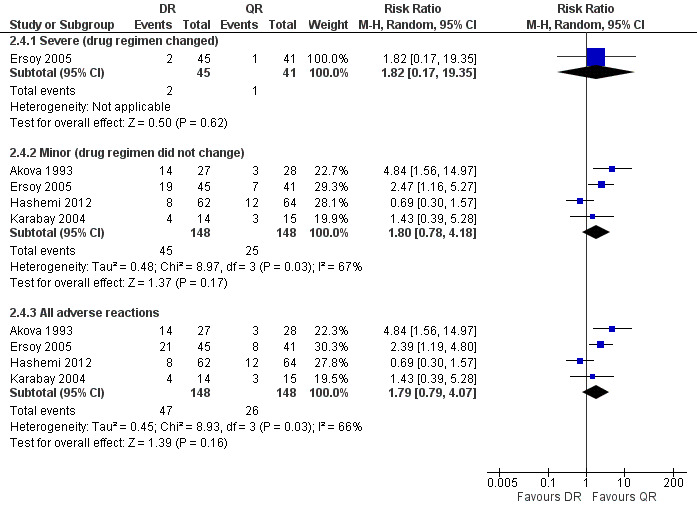
Forest plot of comparison: 2 Doxycycline plus Rifampicin(DR) versus Quinolones plus Rifampicin(QR), outcome: 2.4 Adverse drug reactions.
2.4. Analysis.
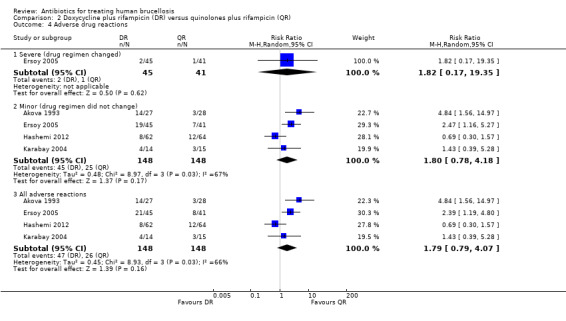
Comparison 2 Doxycycline plus rifampicin (DR) versus quinolones plus rifampicin (QR), Outcome 4 Adverse drug reactions.
2.5 Time to defervescence
Three studies reported the average time to defervescence. Akova 1993, which only reported the means, showed 5.1 and 6.3 days for defervescence in doxycycline plus rifampicin and quinolone plus rifampicin groups. The meta‐analysis on two studies (n = 69, Figure 11) showed a significantly shorter fever duration in quinolone plus rifampicin group, with a fixed‐effect WMD of 1.2 days (95% CI 0.55 to 1.85, Analysis 2.5) (Agalar 1999;Karabay 2004).
11.
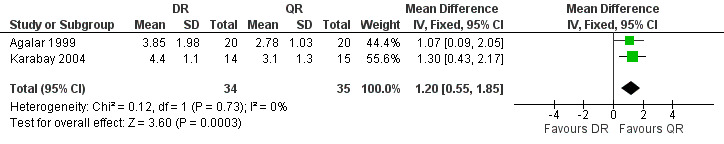
Forest plot of comparison: 2 Doxycycline plus Rifampicin(DR) versus Quinolones plus Rifampicin(QR), outcome: 2.5 Time to defervescence (days).
2.5. Analysis.
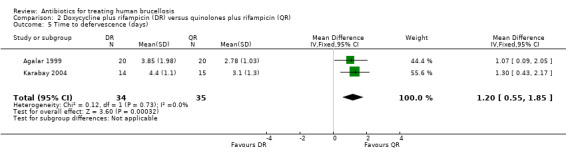
Comparison 2 Doxycycline plus rifampicin (DR) versus quinolones plus rifampicin (QR), Outcome 5 Time to defervescence (days).
3. Doxycycline plus streptomycin versus quinolone plus rifampicin
Ersoy 2005 compared doxycycline plus streptomycin for 45 days with a combination of ofloxacin 400 mg/day with rifampicin 600mg/day for 45 days. Hashemi 2012 compared a six‐week treatment with doxycycline plus streptomycin with a combination of ofloxacin 800 mg daily plus rifampicin 15 mg/kg daily for six weeks.
3.1 Total treatment failure
The pooled analysis of short‐term follow‐up in two studies (n = 202, Figure 12) did not show any significant difference between two treatment regimens, with a fixed‐effect Mantel‐Hanszel RR of 0.69 (95% CI: 0.33 to 1.44). Ersoy 2005 did not show a significant difference in long term follow‐ups between two regimens, with a RR of 0.73 (95% CI 0.23 to 2.29, Analysis 3.1).
12.
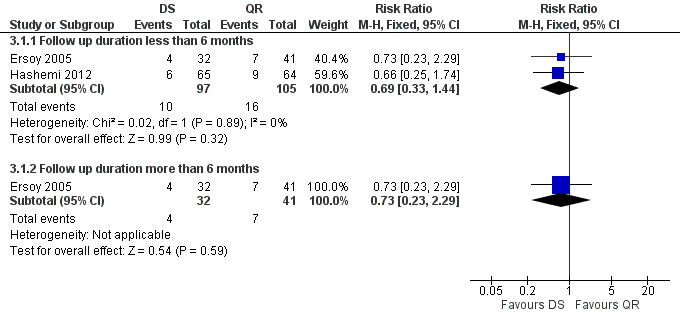
Forest plot of comparison: 3 Doxycycline plus Streptomycin(DS) versus Quinolones plus Rifampicin(QR), outcome: 3.1 Total treatment failure.
3.1. Analysis.
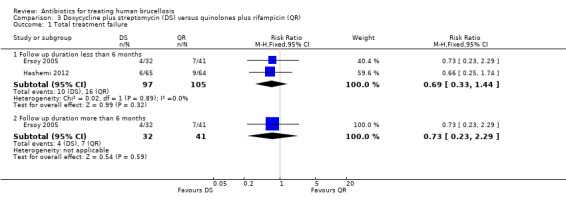
Comparison 3 Doxycycline plus streptomycin (DS) versus quinolones plus rifampicin (QR), Outcome 1 Total treatment failure.
3.2 Relapse
Hashemi 2012 reported the relapse rate for the short‐term follow‐up (Figure 13), which was not significantly different in two treatment regimens (RR 0.59, 95% CI 0.15 to 1.37). Ersoy 2005 only reported the relapse rate at long‐term follow‐up, with no significant difference (RR 0.77, 95% CI 0.20 to 2.98, Analysis 3.2: subgroup 2).
13.
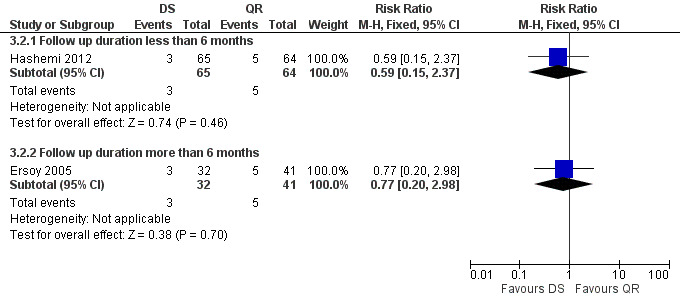
Forest plot of comparison: 3 Doxycycline plus Streptomycin(DS) versus Quinolones plus Rifampicin(QR), outcome: 3.2 Relapse.
3.2. Analysis.
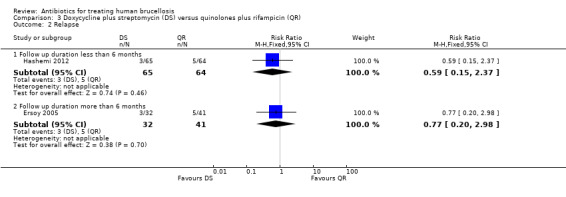
Comparison 3 Doxycycline plus streptomycin (DS) versus quinolones plus rifampicin (QR), Outcome 2 Relapse.
3.3 Persistence of symptoms
The pooled analysis of the persistence of symptoms at the end of treatment was not significant (Figure 14), with a fixed‐effect Mantel‐Hanszel RR of 0.74 (95% CI 0.17 to 3.17, Analysis 3.3).
14.
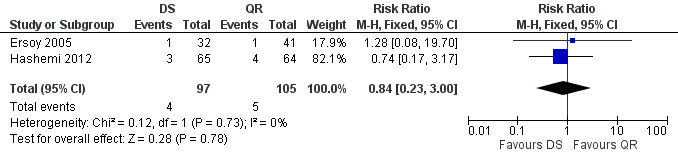
Forest plot of comparison: 3 Doxycycline plus Streptomycin(DS) versus Quinolones plus Rifampicin(QR), outcome: 3.3 Persistence of symptoms.
3.3. Analysis.
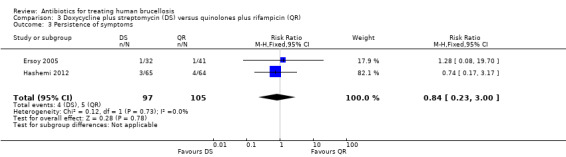
Comparison 3 Doxycycline plus streptomycin (DS) versus quinolones plus rifampicin (QR), Outcome 3 Persistence of symptoms.
3.4 Adverse drug reactions
Serious drug reactions were reported in Ersoy 2005 (one event in quinolone plus rifampicin group). The pooled analysis of minor adverse reactions in two studies (n = 202) did not show any significant difference in two treatment regimens, with a fixed‐effect Mantel‐Hanszel RR of 1.15 (95% CI 0.65 to 2.01). The side effects were mainly gastrointestinal upset.
3.5 Time to defervescence
Time to defervescence was not reported in any of the studies.
4. Doxycycline plus rifampicin versus doxycycline plus quinolone
Two studies compared doxycycline plus rifampicin with a combination of a quinolone (ciprofloxacin or ofloxacin) with doxycyline (Kalo 1996;Sarmadian 2009). Both used ciprofloxacin 1g/day as the quinolone, in combination with rifampicin 600 mg/day. The duration of treatment was six weeks in Kalo 1996, and eight weeks in Sarmadian 2009.
4.1 Total treatment failure
Sarmadian 2009 reported the total treatment failure for short‐term follow‐up, and did not show any significant difference (RR 0.78, 95% CI 0.32 to 1.88, Analysis 4.1, Figure 15).
4.1. Analysis.
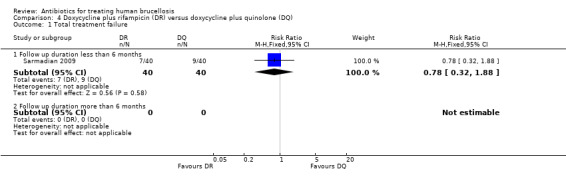
Comparison 4 Doxycycline plus rifampicin (DR) versus doxycycline plus quinolone (DQ), Outcome 1 Total treatment failure.
15.
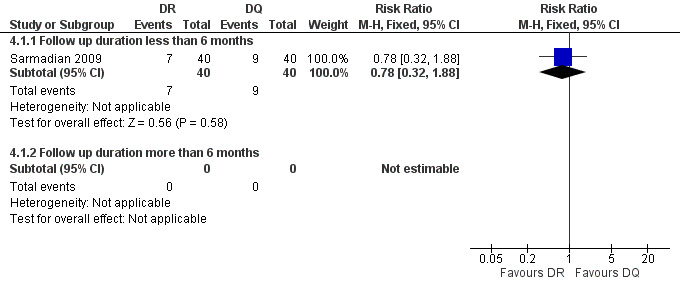
Forest plot of comparison: 4 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Quinolone(DQ), outcome: 4.1 Total treatment failure.
4.2 Relapse
The pooled analysis of short‐term follow‐ups of risk of relapse in two studies (Figure 16) did not show any significant difference between two regimens, with a fixed‐effect Mantel‐Hanszel RR of 1.5 (95% CI 0.26 to 8.55, 104 participants, two trials, Analysis 4.2: subgroup 1) for short‐term follow‐up.
16.
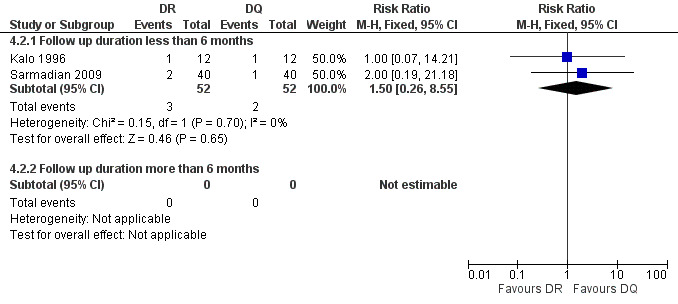
Forest plot of comparison: 4 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Quinolone(DQ), outcome: 4.2 Relapse.
4.2. Analysis.
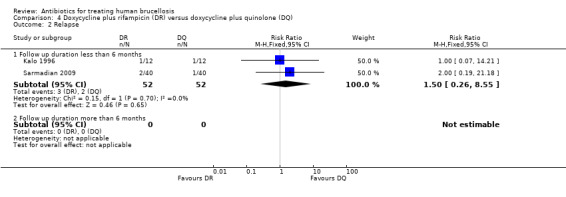
Comparison 4 Doxycycline plus rifampicin (DR) versus doxycycline plus quinolone (DQ), Outcome 2 Relapse.
4.3 Persistence of symptoms
Sarmadian 2009 did not show any significant difference for the symptom persistence at the end of treatment (RR 0.6, 95% CI 0.22 to 1.75, Analysis 4.3, Figure 17).
4.3. Analysis.
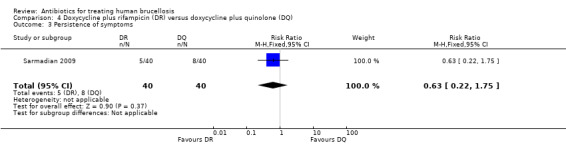
Comparison 4 Doxycycline plus rifampicin (DR) versus doxycycline plus quinolone (DQ), Outcome 3 Persistence of symptoms.
17.
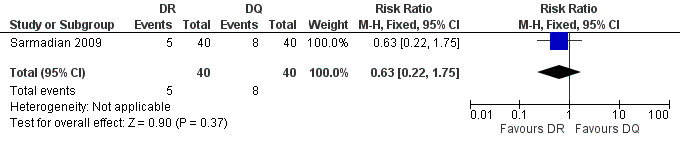
Forest plot of comparison: 4 Doxycycline plus Rifampicin(DR) versus Doxycycline plus Quinolone(DQ), outcome: 4.3 Persistence of symptoms.
4.4 Adverse drug reactions
None of the studies reported the occurrence of adverse reactions.
4.5‐time to defervescence
Only Kalo 1996 reported the mean time to defervescence. The average febrile duration was four or five days in doxycycline plus rifampicin and quinolone plus doxycycline groups respectively. The study did not report the standard deviation values.
5 Doxycycline plus streptomycin versus doxycycline plus gentamycin
Two studies compared doxycycline plus streptomycin and doxycycline plus gentamycin regimens. Hassanjani Roushan 2006 (n = 191) compared a doxycycline plus streptomycin (45 and 14 days) regimen with a doxycycline plus gentamicin (45 and 7 days) regimen. In another study, Hassanjani Roushan 2010 (n = 154) compared a doxycycline plus streptomycin (45 and 14 days) regimen with a doxycycline plus gentamicin (56 and 5 days) regimen.
Solera 2004 (n = 146) compared two durations of doxycycline therapy (30 days versus 45 days) as a part of doxycycline plus gentamycin regimen with each other.
5.1 Total treatment failure
The pooled analysis of total treatment failure of two studies (n = 345, Figure 18) for short term and long term follow‐ups did not show any significant difference between two regimens, with a fixed‐effect Mantel‐Hanszel RR of 1.96 (95% CI 0.85 to 4.49, Analysis 5.1: subgroup 1) and 1.85 (95% CI 0.84 to 4.08, Analysis 5.1: subgroup 2) respectively. The point estimate of total treatment failure was in favour of doxycycline plus gentamycin regimen.
18.
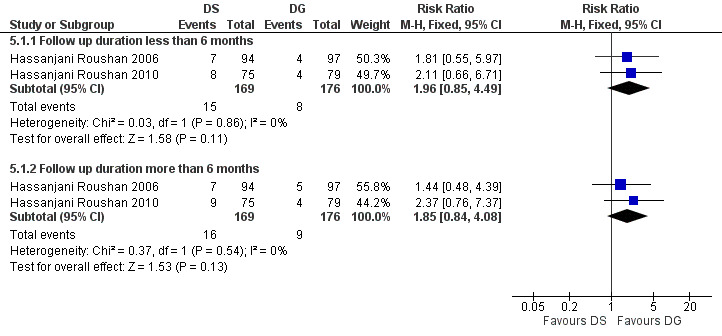
Forest plot of comparison: 5 Doxycycline plus Streptomycin(DS) versus Doxycyline plus gentamycin(DG), outcome: 5.1 Total treatment failure.
5.1. Analysis.
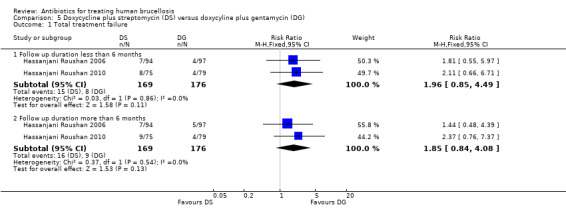
Comparison 5 Doxycycline plus streptomycin (DS) versus doxycyline plus gentamycin (DG), Outcome 1 Total treatment failure.
5.2 Relapse
The pooled analysis of short‐term and long‐term follow‐ups of risk of relapse in two studies (Figure 19) did not show any significant difference between two regimens, with a fixed‐effect Mantel‐Hanszel RR of 1.83 (95% CI 0.54 to 6.12, 345 participants, two trials, Analysis 5.2: subgroup 1) and 1.67 (95% CI 0.56 to 5.0, 345 participants, two trials, Analysis 5.2: subgroup 2) respectively, in favour of doxycycline plus gentamycin regimen.
19.
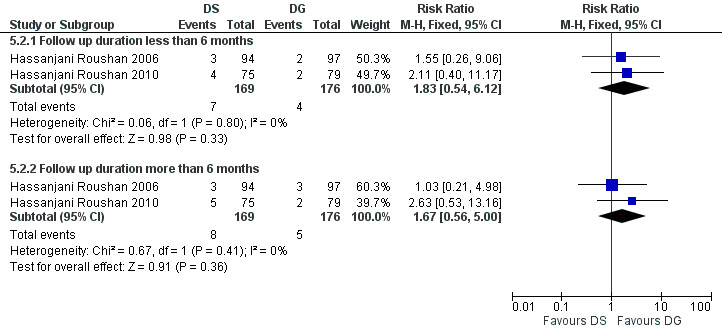
Forest plot of comparison: 5 Doxycycline plus Streptomycin(DS) versus Doxycyline plus gentamycin(DG), outcome: 5.2 Relapse.
5.2. Analysis.
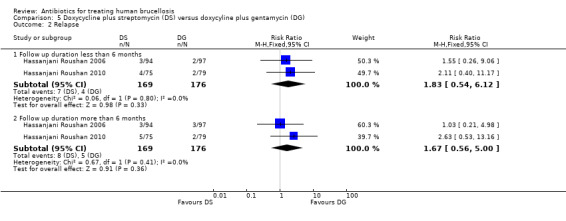
Comparison 5 Doxycycline plus streptomycin (DS) versus doxycyline plus gentamycin (DG), Outcome 2 Relapse.
Comparing two doxycycline plus gentamycin regimens, Solera 2004 reported 15 cases of relapse in the group treated with doxycycline for 30 days, versus 9 cases in the group treated for 45 days, at one year follow‐up.
5.3 Persistence of symptoms
The pooled estimate of the risk of symptom persistence at the end of treatment in two studies (n = 345, Figure 20) did not significantly differ, with a fixed‐effect Mantel‐Hanszel RR of 2.09 (95% CI 0.64 to 6.79, Analysis 5.3).
20.
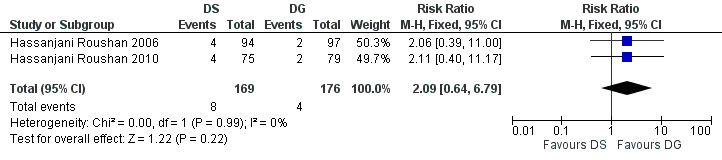
Forest plot of comparison: 5 Doxycycline plus Streptomycin(DS) versus Doxycyline plus gentamycin(DG), outcome: 5.3 Persistence of symptoms.
5.3. Analysis.
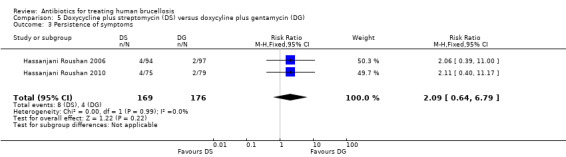
Comparison 5 Doxycycline plus streptomycin (DS) versus doxycyline plus gentamycin (DG), Outcome 3 Persistence of symptoms.
5.4 Adverse drug reactions
Only minor adverse effects occurred in both studies, which did not warrant change in treatment regimen. The pooled estimate of the risk of minor adverse reactions (Figure 21) was 0.85 (95% CI 0.60 to 1.22, Analysis 5.4: subgroup 2). The most frequent adverse reactions in both studies were photosensitivity and abdominal discomfort. Hassanjani Roushan 2006 reported two cases of ototoxicity in doxycycline plus streptomycin group.
21.
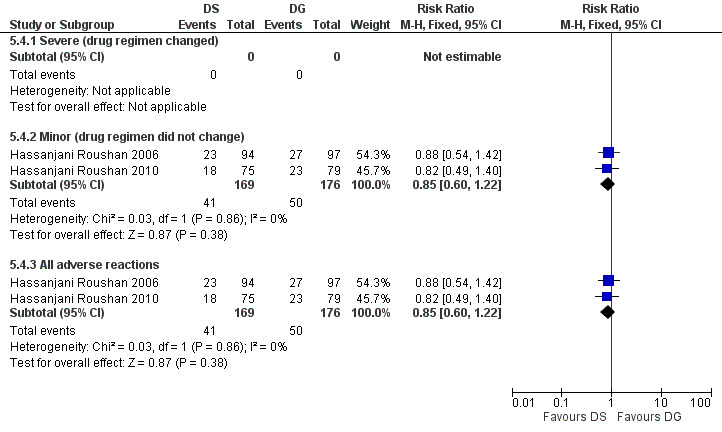
Forest plot of comparison: 5 Doxycycline plus streptomycin(DS) versus doxycyline plus gentamycin (DG), outcome: 5.4 Adverse drug reactions.
5.4. Analysis.
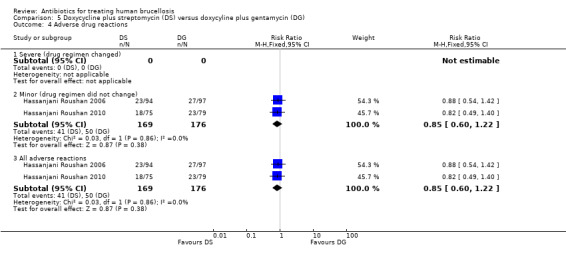
Comparison 5 Doxycycline plus streptomycin (DS) versus doxycyline plus gentamycin (DG), Outcome 4 Adverse drug reactions.
Comparing two doxycycline plus gentamycin regimens, Solera 2004 did not report any serious adverse reactions in neither groups. They detected 29 minor adverse reactions in 30‐day doxycycline treatment and 28 in 45‐day treatment (mainly photosensitivity and epigastric pain).
6 Doxycycline plus rifampicin versus other regimens
Ranjbar 2007 (n = 220) reported the comparison between doxycycline plus rifampicin (eight weeks) and doxycycline plus rifampicin plus amikacin (amikacin 20 mg/day IM for seven days added to the regimen). Lang 1990 (n = 10) compared doxycycline plus rifampicin regimen with two dosages of ciprofloxacin monotherapy.
6.1‐Total treatment failure
In Ranjbar 2007, at six‐month follow‐up, doxycycline plus rifampicin regimen showed a higher risk of total treatment failure compared to the amikacin added regimen, with a Mantel‐Hanszel RR of 1.48 (95% CI 1.02 to 2.15).
In Lang 1990, no failure was observed in doxycycline plus rifampicin regimen. While five out of six patients in ciprofloxacin monotherapy groups relapsed a few weeks after treatment.
6.2 Relapse
In Ranjbar 2007, at six‐month follow‐up, the relapse was seen in nine patients in doxycycline plus rifampicin group and six patients in the amikacin added group, with a Mantel‐Hanszel RR of 1.5 (95% CI 0.55 to 4.07).
Lang 1990 reported no relapse in doxycycline plus rifampicin group in one year follow‐up. But three patients in group 1gr bd ciprofloxacin and two patients in 750 mg bd ciprofloxacin groups showed the signs of relapse during one month after treatment, resulting in the termination of the study.
6.3 Persistence of symptoms
In Ranjbar 2007, persistence of symptoms at the eighth week after beginning of treatments was seen in 37 patients in doxycycline plus rifampicin group and in 25 patients in amikacin added group, with a Mantel‐Hanszel RR of 1.48 (95% CI 0.96 to 2.28).
6.4 Adverse drug reactions
Ranjbar 2007 did not report any serious adverse reactions, but they reported minor reactions in four cases of doxycycline plus rifampicin, and six cases of amikacin added group. Two patients in doxycycline plus rifampicin had mild gastric complaints, one patient had vomiting, and one had genital candidiasis. In the amikacin added group, four patients had mild gastric complaints and two had phototoxicity.
Lang 1990 reported no serious adverse reactions in any groups. They reported a transient increase in hepatocellular enzymes in all treatment groups.
7 Tetracycline plus streptomycin versus other regimens
Acocella 1989 (n = 146) reported the comparison between a regimen consisting of tetracycline 2 g/day for 21 days, plus streptomycin 1g/day for the first two weeks with doxycycline plus rifampicin (45 days) and doxycycline plus streptomycin (45 and 21 days). The sample size in tetracycline plus streptomycin group was considerably smaller than other two groups.
7.1 Total treatment failure
During the one‐year follow‐up, total treatment failure was seen in 11 (41%) patients in the tetracycline plus streptomycin group, which was higher than the doxycycline plus rifampicin and the doxycycline plus streptomycin groups, with 3 (5%) and 2 (4%) cases respectively.
7.2 Relapse
During the six‐month follow‐up, relapse was seen in six (22%) patients in the tetracycline plus streptomycin group, which was higher than the doxycycline plus rifampicin and doxycycline plus streptomycin groups with 2 (4%) and 0 cases respectively. The relapse rates at 1 year follow‐up were 22%, 6%, and 0% in the three groups respectively.
7.3 Persistence of symptoms
Persistence of symptoms at the end of treatment was seen in 4, 0, and 1 patient in the tetracycline plus streptomycin, doxycycline plus rifampicin, and doxycycline plus streptomycin groups respectively.
7.4 Adverse drug reactions
Serious drug reactions leading in treatment regimen change were seen in one patient in doxycycline plus streptomycin (vertigo related to streptomycin), and one patient in tetracycline plus streptomycin group (lupus‐like rash on the face and erythema related to tetracycline).
8 Doxycycline plus streptomycin versus other regimens
Lang 1992 (n = 18) compared doxycycline plus streptomycin regimen (28 and 14 days) with a monotherapy with ceftriaxone maximum 2 g daily for at least two weeks. The initial goal was to include 66 patients but the study was discontinued early after evaluation of the first 18, because six out of eight patients in the ceftriaxone group showed treatment failure, and were switched to the other group after one week. Out of two who responded initially in ceftriaxone group, one developed relapse three weeks after completion of therapy. In contrast, all 10 patients in the doxycycline plus streptomycin group responded well to the therapy and did not show any signs of relapse or therapeutic failure during the six month follow‐up. No adverse reaction was seen in either group.
9 Co‐trimoxazole versus other regimens
Alavi 2007 (n = 102) compared doxycycline plus rifampicin regimen with doxycycline plus co‐trimoxazole. The follow‐up period was about three months in this study.
Montejo 1993a (n = 200) reported the comparison between doxycycline plus rifampicin regimen administered for 28 days with co‐trimoxazole (six months) and doxycycline (six weeks) monotherapy.
Buzon 1982 (n = 84) reported the comparison of a regimen consisting of tetracycline plus rifampicin for four weeks with a six month treatment with co‐trimoxazole.
Hassanjani Roushan 2004 (n = 280) compared co‐trimoxazole plus doxycycline for eight weeks with co‐trimoxazole plus rifampicin for eight weeks.
Hassanjani Roushan 2005 (n = 79) compared two regimens of co‐trimoxazole plus rifampicin administered for six and eight weeks.
9.1 Total treatment failure
In Alavi 2007, the risk of total treatment failure was higher in doxycycline plus rifampicin group compared to doxycycline plus co‐trimoxazole, with a Mantel‐Hanszel RR of 2.8 (95% CI 0.95 to 8.24).
In Montejo 1993a, at one year follow‐up, total treatment failure was higher among doxycycline plus rifampicin (30 days) than doxycycline alone (6 weeks) group, with a Mantel‐Hanszel RR of 1.64 (95% CI 0.79 to 3.39). The risk of total treatment failure was also higher in the doxycycline plus rifampicin (30 days) group than co‐trimoxazole alone (six months) group, with a M‐H RR of 2.46 (95% CI 1.02 to 5.94).
In Buzon 1982, at six‐month follow‐up, total treatment failure was seen in 17 (45%) patients in co‐trimoxazole group and 8 (17%) patients in tetracycline plus rifampicin group.
In Hassanjani Roushan 2004, at one year follow‐up, total treatment failure was seen in 22 cases of co‐trimoxazole plus doxycycline, and 37 cases of co‐trimoxazole plus rifampicin group, with a Mantel‐Hanszel RR of 0.59 (95% CI 0.39 to 0.9).
At one‐year follow‐up, Hassanjani Roushan 2005 reported six cases of total treatment failure in the six‐week group, and one in the eight‐week group, with a Mantel‐Hanszel RR of 5.56 (95% CI 0.7 to 44.09).
9.2 Relapse
At the six month follow‐up, Alavi 2007 reported six cases of relapse in the doxycycline plus rifampicin group, and three in the doxycycline plus co‐trimoxazole group.
In Montejo 1993a, at one year follow‐up, relapse was reported in 14 cases of doxycycline plus rifampicin (30 days) group, two cases of co‐trimoxazole alone (six months), and 10 cases of doxycycline alone.
Buzon 1982 did not report the frequency of relapse in the study groups.
At 12 month follow‐up, Hassanjani Roushan 2004 reported 10 cases of symptom persistence in co‐trimoxazole plus doxycycline group, and 14 cases in co‐trimoxazole plus rifampicin group, with a Mantel‐Hanszel RR of 0.71 (95% CI 0.34 to 1.5).
At one year follow‐up, Hassanjani Roushan 2005 reported three cases of relapse in the six week group, and only one case of relapse in the eight week group.
9.3 Persistence of symptoms
Alavi 2007 reported five cases of symptom persistence in doxycycline plus rifampicin group, and one in the doxycycline plus co‐trimoxazole group.
Montejo 1993a reported symptom persistence in one patient of doxycycline plus rifampicin (30 days) group, four patients in co‐trimoxazole alone (six months), and no one in the doxycycline alone group.
Buzon 1982 did not report the frequency of symptom persistence in the study groups.
Hassanjani Roushan 2004 reported 12 cases of relapse in co‐trimoxazole plus doxycycline group, and 23 cases in co‐trimoxazole plus rifampicin group, with a Mantel‐Hanszel RR of 0.52 (95% CI 0.28 to 0.96).
Hassanjani Roushan 2005 reported three cases of persistence of symptoms in the six week group, and nobody in the eight week group.
9.4 Adverse drug reactions
Alavi 2007 did not address the assessment of adverse reactions.
Montejo 1993a did not report the rate of adverse reactions in each group separately.
In Buzon 1982, serious adverse reactions warranting the change in regimen happened in eight cases in co‐trimoxazole group and no one in the tetracycline plus rifampicin group.
Hassanjani Roushan 2004 reported two cases of adverse reaction in cotrimoxazole plus doxycycline group (erythema multiform in one case, vomiting in one case), and seven cases in co‐trimoxazole plus rifampin group (three cases had skin rash and four experienced vomiting).
Hassanjani Roushan 2005 did not address the assessment of adverse reactions.
10 Ampicillin versus other regimens
McDevitt 1970 (n = 54) conducted a double‐blind study to compare ampicillin 1 g four times a day orally for 28 days with placebo. They did not report the relapse and persistence of symptoms as study outcomes. At one month follow‐up, a total of six patients in the ampicillin group and seven patients in the placebo group subjectively felt better.
Discussion
Summary of main results
The meta‐analysis showed that, in adult patients suffering from brucellosis, doxycycline plus rifampicin regimen administered for six weeks results in slightly more treatment failure (relapse and persistence of symptoms) than the regimen consisting of doxycycline for six weeks and streptomycin for two or three weeks, with a Number Needed to Treat (NNT) of 18, as well as a non‐significant more minor adverse reactions with a Number Needed to Harm (NNH) of 13. These findings are based on pooling of the findings from seven and five low quality, randomized controlled trials respectively.
In addition, the meta‐analysis of three small studies showed that doxycycline plus rifampicin regimen for six weeks results in a slightly more minor adverse reactions (NNH = 7) and fairly longer fever duration (approximately one day) than the regimen consisting of a quinolone (ofloxacin or ciprofloxacin) and rifampicin administered for six weeks. Pooling of the results of three small studies did not show any significant difference in terms of total treatment failure between two regimens. The quality of evidence was low.
Adding another antibiotic, eg amikacin (Ranjbar 2007), to the doxycycline plus rifampicin regimen may have some beneficial effects on the relapse and treatment failure rates. However, this finding is only based on a single low quality randomized controlled trial.
Current evidence is not enough to compare various dosages and treatment durations in doxycycline plus rifampicin regimens. Only one study (Montejo 1993a) showed that, the regimen lasted for 30 days is significantly less effective than the doxycycline monotherapy for six weeks.
The evidence is not also sufficient to compare the effectiveness of quinolone plus rifampicin with doxycyline plus streptomycin. Based on the current available evidence, it is not possible to draw firm conclusions on the effectiveness of other antibiotic combinations, including doxycycline plus quinolone, tetracycline plus streptomycin, co‐trimoxazole plus rifampicin, and co‐trimoxazole plus doxycycline.
The meta‐analysis of two studies on comparing doxycyline plus gentamycin with doxycycline plus streptomycin showed a slight non‐significant superiority of doxycycline plus gentamycin in terms of treatment failure (NNT = 23). However, doxycycline plus gentamycin showed a modestly higher risk of minor adverse reactions (NNH = 24).
Overall completeness and applicability of evidence
All included studies were limited to adult patients with brucellosis, and current findings are not applicable to children, pregnant women, and patients with complications like spondylitis and neurobrucellosis. The dosages and treatment durations were quite homogenous among studies assessing the effect of doxycycline plus rifampicin and doxycycline plus streptomycin. In terms of outcomes, some studies did not perform any explicit assessment of minor adverse reactions, so the findings regarding adverse drug reactions should be interpreted with caution. We excluded all studies which included only the patients with spondylitis, or the studies in which separate information were not provided for the patients without spondylitis. The reason is that, spinal and neurological complications of brucellosis do not respond well to conventional treatment regimens, and generally require longer treatment durations, which is an important source of heterogeneity.
Quality of the evidence
All studies were randomized controlled trials. However, more than half of studies did not describe the methods of allocation and concealment sufficiently. In addition, only three studies were blinded, and patients and care providers were not aware of group allocation. The definition of relapse and treatment failure in most of studies was based on objective bacteriological or serological measures, which were not very sensitive to masking of group allocation.
Some studies did not explicitly describe missing patients at each follow‐up session. Since the effect of treatment is assessed by the frequency of relapse and persistence of symptoms, detailed reporting of missing data is of special importance. Lack of sufficient reporting of missing data in some studies affects the overall quality of evidence.
Potential biases in the review process
Our search strategy was comprehensive, and was not limited to language and publication status. We covered the major international and local bibliographic databases, specialty journals, grey literature, and conferences in the field. We used a clear inclusion criteria and thorough quality assessment methodology to appraise the studies. Some information were missing in a few studies which were mainly published in the form of abstracts. This was reflected in the risk of bias tables. Two independent reviewers screened and appraised the studies, and extracted data, using pre‐designed assessment and data extraction forms. However, since brucellosis is the disease of the developing world, publication bias seems likely.
Agreements and disagreements with other studies or reviews
We showed that, doxycycline plus streptomycin regimen is superior to doxycycline plus rifampicin and other alternatives. This finding is consistent with the recommendations of Ioannina expert panel (Ariza 2007). WHO guidelines on treatment of human brucellosis recommended both regimens, doxycycline plus rifampicin and doxycycline plus streptomycin, as equally effective in uncomplicated brucellosis (WHO 2006). This recommendation is based on expert opinions and contradicts with the findings of current systematic review. Doxycycline plus streptomycin results in a significantly lower rate of relapse, symptom persistence, and adverse drug reactions in comparison to doxycycline plus rifampicin regimen. Skalsky 2008 and Solis Garcia del Pozo 2012 in a systematic review on treatment of human brucellosis similarly showed the superiority of doxycycline plus streptomycin regimen. Despite superiority in effectiveness, it has been shown that, practitioners prefer the doxycycline plus rifampicin regimen over doxycycline plus streptomycin because of the ease of administration and lower costs (Pappas 2007). Shorter treatment durations for streptomycin administration may improve the adherence. We found that the administration of streptomycin for two or three weeks yielded fairly similar results, which was consistent with the findings of Solis Garcia del Pozo 2012. On the other hand, the non‐significant superiority of five or seven days of gentamycin administration as an alternative to streptomycin is worth more attention.These findings should be investigated in future studies, in order to find a regimen which is both effective and is tolerated well.
We did not find any significant difference in total treatment failure between doxycycline plus rifampicin and quinolone plus rifampicin treatments, and showed non‐significant favourable results for quinolone plus rifampicin in terms of side effects, and a significant shorter time to defervescence. Based on these findings, there is not sufficient evidence for inferiority of quinolone‐containing regimens compared to other drug treatments. Ioannina expert panel did not support wide use of quinolone‐containing regimens, due to the lack of convincing evidence of efficacy, higher cost, and the risk of resistance to quinolones in the community (Ariza 2007). Skalsky 2008 pooled all the studies with the regimens containing quinolones and compared them with non‐quinolone regimens. They showed a significant difference in favour of non‐quinolone regimens in terms of total treatment failure. Based on this comparison they did not recommend quinolones as a treatment option for treating human brucellosis. However, quinolone and non‐quinolone groups were obviously heterogeneous regarding treatment duration and other administered drugs. While Solis Garcia del Pozo 2012 did not show any difference between doxycycline plus rifampicin and quinolone plus rifampicin treatments.
Authors' conclusions
Implications for practice.
Where applicable, doxycycline (six weeks) plus streptomycin (two or three weeks) regimen is more effective and more tolerable regimen than doxycycline plus rifampicin (six weeks). Since daily IM injection is required, access to care and cost are important factors in deciding between two choices.
It seems that the doxycycline plus rifampicin regimen should be administered long enough to be effective, and one month is not an adequate treatment duration.
Based on low quality evidence, quinolone plus rifampicin (six weeks) regimen did not have any difference with doxycycline plus rifampicin, in terms of overall effectiveness, and was slightly better tolerated.
Monotherapy is not recommended in treatment of brucellosis.
Implications for research.
Most of the trials in the field of brucellosis have several weaknesses in terms of design and reporting. The details of random allocation and concealment were not reported in many studies. In addition, since the outcomes of interest ie symptom persistence, relapse, and adverse drug effects, does not occur frequently, even a small number of missing values may affect the dependability of findings. We suggest conducting large, well‐designed, randomized controlled trials with more precise definitions of the disease, study population, treatment regimens, outcomes, and long enough follow‐ups,especially on the effectiveness of shorter durations of streptomycin treatment (eg two weeks), short‐term administration of gentamycin as an alternative to streptomycin, and newer generations of quinolone containing regimens. More detailed reporting of the reasons for patient drop‐outs is also recommended.
We recommend a cost‐effectiveness analysis to formally compare doxycycline plus rifampicin and doxycycline plus streptomycin regimens, since the latter is more effective and also more costly.
Given that a noticeable percentage of relapse happened later than six months after beginning of treatment, we suggest brucellosis trials to follow patients for at least one year. Six month follow‐up is insufficient in assessing the overall effectiveness of treatment regimens.
We also recommend high quality randomized controlled trials on the treatment of childhood and complicated brucellosis (e.g. spondylitis), since there is no convincing evidence on the effectiveness of pharmaceutical treatments on these sub‐populations.
History
Protocol first published: Issue 2, 2008 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 2 November 2010 | New search has been performed | The contact reviewer changed. Two authors of the protocol acknowledged. |
| 22 February 2009 | Amended | Converted to new review format. |
Acknowledgements
We thank Dr Mahtab Rabbani‐Anari for her contributions in developing the protocol and assessing some studies. We thank Dr Arash Rashidian (Center for Academic and Health Policy, Tehran University of Medical Sciences) and Dr Sirus Jafari (Infectious Diseases Department, Imam Khomeini hospital) for commenting on the draft protocol. The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of developing countries.
Data and analyses
Comparison 1. Doxycycline plus rifampicin (DR) versus doxycycline plus streptomycin (DS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total treatment failure | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up duration less than 6 months | 6 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.78, 2.17] |
| 1.2 Follow up duration more than 6 months | 7 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.07, 3.42] |
| 2 Relapse | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow up duration less than 6 months | 5 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [0.99, 6.48] |
| 2.2 Follow up duration more than 6 months | 7 | 567 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.17, 4.86] |
| 3 Persistence of symptoms | 8 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.79, 3.59] |
| 4 Adverse drug reactions | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Severe (drug regimen changed) | 2 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.19, 7.07] |
| 4.2 Minor (drug regimen did not change) | 5 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.99, 1.92] |
| 4.3 All adverse reactions | 6 | 589 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.99, 1.91] |
Comparison 2. Doxycycline plus rifampicin (DR) versus quinolones plus rifampicin (QR).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total treatment failure | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up duration less than 6 months | 5 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.87, 2.28] |
| 1.2 Follow up duration more than 6 months | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.98] |
| 2 Relapse | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow up duration less than 6 months | 4 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.59, 2.61] |
| 2.2 Follow up duration more than 6 months | 3 | 181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.40, 2.27] |
| 3 Persistence of symptoms | 3 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.72, 4.53] |
| 4 Adverse drug reactions | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Severe (drug regimen changed) | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.17, 19.35] |
| 4.2 Minor (drug regimen did not change) | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.78, 4.18] |
| 4.3 All adverse reactions | 4 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [0.79, 4.07] |
| 5 Time to defervescence (days) | 2 | 69 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [0.55, 1.85] |
Comparison 3. Doxycycline plus streptomycin (DS) versus quinolones plus rifampicin (QR).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total treatment failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up duration less than 6 months | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.44] |
| 1.2 Follow up duration more than 6 months | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.23, 2.29] |
| 2 Relapse | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow up duration less than 6 months | 1 | 129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.37] |
| 2.2 Follow up duration more than 6 months | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.20, 2.98] |
| 3 Persistence of symptoms | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.23, 3.00] |
| 4 Adverse drug reactions | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Severe (drug regimen changed) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 10.08] |
| 4.2 Minor (drug regimen did not change) | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.65, 2.01] |
| 4.3 All adverse reactions | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.63, 1.90] |
3.4. Analysis.
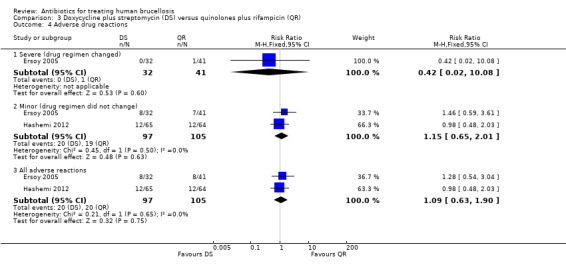
Comparison 3 Doxycycline plus streptomycin (DS) versus quinolones plus rifampicin (QR), Outcome 4 Adverse drug reactions.
Comparison 4. Doxycycline plus rifampicin (DR) versus doxycycline plus quinolone (DQ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total treatment failure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up duration less than 6 months | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.32, 1.88] |
| 1.2 Follow up duration more than 6 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Relapse | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow up duration less than 6 months | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.55] |
| 2.2 Follow up duration more than 6 months | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Persistence of symptoms | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.22, 1.75] |
Comparison 5. Doxycycline plus streptomycin (DS) versus doxycyline plus gentamycin (DG).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total treatment failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Follow up duration less than 6 months | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [0.85, 4.49] |
| 1.2 Follow up duration more than 6 months | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.84, 4.08] |
| 2 Relapse | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Follow up duration less than 6 months | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.54, 6.12] |
| 2.2 Follow up duration more than 6 months | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.56, 5.00] |
| 3 Persistence of symptoms | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.09 [0.64, 6.79] |
| 4 Adverse drug reactions | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Severe (drug regimen changed) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Minor (drug regimen did not change) | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.22] |
| 4.3 All adverse reactions | 2 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acocella 1989.
| Methods | Randomized controlled trial Generation of allocation sequence: randomization tables Allocation concealment: not described Blinding: not used Inclusion of all randomized participants in the final analysis: not reported Duration: 3 years, from May 1981 to July 1984 |
|
| Participants | Number: 146 adults enrolled Inclusion criteria: positive blood culture for Brucella or (i) clinical picture compatible with acute brucellosis and (ii) Standard Tube agglutination Test above 125 IU, or complement fixation positive at a dilution of 1/8 or more. Exclusion criteria: severe concomitant diseases, requiring treatment with corticosteroids, barbiturates or other antibiotics, pregnancy, younger than 10, or older than 70 years, allergic to any of the four antibiotics to be used. |
|
| Interventions | A:rifampicin 900 mg per day in a single administration for 45 days, plus doxycycline 200 mg per day in a single administration for 45 days B:doxycycline 200 mg per day in a single administration for 45 days, plus streptomycin sulphate 1 g per day by IM route for 21 days C:tetracycline 2 g per day (0‐5 g four times daily) for 21 days by oral route, plus streptomycin sulphate 1 g per day by IM route for 14 days |
|
| Outcomes | 1‐relapse 2‐therapeutic failure due to persistent symptoms 3‐theraputic failure due to medication side effects |
|
| Notes | Location: Six centres: three in France, one in Greece and two in Spain Setting: hospitalised and ambulatory patients Source of funding: a grant from Merrell‐Dow‐Lepetit drug company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation of patients to the regimen was carried out through the use of randomization tables. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study design |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 63, 53, and 27 patients were randomized to the regimens A, B, and C respectively. The reasons for the missingness of 8 patients, who completed the treatment, but did not appear in follow‐up assessment was not described. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported. |
| Other bias | High risk | Considerable baseline imbalance in study groups. Financially supported by a drug company. |
Agalar 1999.
| Methods | Randomized controlled trial Duration: October 1995 and January 1998 |
|
| Participants | Number: 40 patients enrolled Inclusion criteria:Patients suspected to have Brucella infection based on clinical and laboratory findings and positive culture. Exclusion criteria: age under 15 years, history of seizures, recent antibiotic use, allergy to the study antibiotics, and pregnancy. Patients with negative culture were excluded. |
|
| Interventions | 1‐doxycycline 100 mg b.i.d. and rifampicin 600 mg once daily P.O. for 45 days 2‐ciprofloxacin 500 mg b.i.d. and rifampicin 600 mg once daily P.O. for 30 days |
|
| Outcomes | 1‐clinical relapse 2‐bacteriological relapse 3‐advers drug reactions 4‐time to defervescence |
|
| Notes | Location: Social Security Institution Educational Hospital in Ankara, Turkey Setting: hospitalised patients Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out using a randomization table. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20 were randomized to doxycycline plus rifampicin, 20 to ciprofloxacin plus rifampicin. No missing outcome data was reported. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported. |
| Other bias | Low risk | |
Akova 1993.
| Methods | Randomized controlled trial Duration: March 1989 and March 1992 |
|
| Participants | Number: 61 patients enrolled [3 in DR group and 3 in OR group were excluded from this review due to spondylitis] Inclusion criteria:a standard tube agglutination titer of 1/160 or more for anti‐Brucella antibodies in the presence of compatible clinical findings (fever, night sweats, arthralgia, hepatomegaly, splenomegaly, and lymphadenopathy) or isolation of a Brucella sp. from blood or bone marrow cultures. Exclusion criteria: endocarditis or neurobrucellosis. Individuals who received antimicrobial therapy prior to the study, pregnant women, and patients allergic to any of the drugs employed in the regimens |
|
| Interventions | 1‐ 200 mg of doxycycline plus 600 mg of rifampicin per day for a total of 6 weeks. All medications were administered once daily. 2‐ 400 mg of ofloxacin plus 600 mg of rifampicin per day for a total of 6 weeks. All medications were administered once daily. |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐advers drug reactions 4‐time to defervescence |
|
| Notes | Location: Hacettepe University hospital, Turkey Setting: hospitalised patients Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | High risk | The patients were then randomized in a non blinded fashion. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 randomized to doxycycline plus rifampicin, 31 to ofloxacin plus rifampicin. No information was provided about the numbers at follow‐up. Not all patients followed for one year. Mean follow up (SD) in DR and DS groups were 14.2(5.6) and 15.1(5.9) months respectively. The reasons were not described. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Alavi 2007.
| Methods | Randomized controlled trial Duration: April 2004 to January 2006 |
|
| Participants | Number: 105 patients enrolled Inclusion criteria: the finding of >1/80 standard tube agglutination titer (STAT) of antibodies to Brucella (Wright) with a 2 mercaptoethanol (2 ME) >1/40, in association with compatible clinical findings such as back pain, sweating and fever Exclusion criteria: age less than 15 years, pregnancy, spondylitis, endocarditis, meningoencephalitis, previous history of brucellosis, and antimicrobial therapy for more than seven days before enrolment |
|
| Interventions | 1‐ doxycycline (100mg twice a day), and rifampicin (300mg every 8 hour) for 8 weeks 2‐ doxycycline (100mg twice a day), and co‐trimoxazole (2 adult tablets, 960mg twice a day) for 8 weeks |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐laboratory improvement |
|
| Notes | Location: The private clinic and infectious disease ward of Razi hospital affiliated to Joundishapour University, Ahwaz, Iran Setting: hospitalised and ambulatory patients Source of funding: supported by Joundishapoor Infectious and Tropical Diseases Research Center |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52 patients were randomized to doxycycline plus rifampicin, and 53 to doxycycline plus co‐trimoxazole. At 3 month follow‐up, 51 were remained in each group.The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Ariza 1992.
| Methods | Randomized controlled trial Duration: 1986‐89 |
|
| Participants | Number: 111 patients enrolled [3 in DR group and 4 in DS group were excluded from this study due to spondylitis] Inclusion criteria: a standard tube agglutination titer of 1/160 or more for anti‐Brucella antibodies in the presence of compatible clinical findings (fever, sweat, arthralgias, hepatomegaly, splenomegaly, and lymphadenopathy) or isolation of a Brucella sp. from blood cultures. Exclusion criteria: Endocarditis, neurobrucellosis |
|
| Interventions | 1‐ Doxycycline 100 mg twice a day orally for 45 days plus Rifampicin 15mg/kg body weight per day in a single morning dose for 45 days plus 14ml of saline solution IM as placebo for 15 days 2‐ Doxycycline 100 mg twice a day orally for 45 days plus Streptomycin 1g/d IM for 15 days (750 mg/d for patients more than 60 yrs) plus oral placebo in a single morning dose for 45 days |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐advers drug reactions 4‐time to defervescence |
|
| Notes | Location: Bellvitge hospital, Barcelona, Spain Setting: hospitalised patients Source of funding: Rifampicin was supplied by Merrill Dow S.A., Barcelona Spain |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using the table of random numbers |
| Allocation concealment (selection bias) | Low risk | All drugs were coded numerically. The code list was kept in the pharmacy and was open only after the study completed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of patients and care providers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 53 were randomized to doxycycline plus rifampicin, 58 to doxycycline plus streptomycin. Sixteen patients (9 in DR, 7 in DS) were withdrawn. Nine did not adhere to the treatment, 7 lost to follow up. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Buzon 1982.
| Methods | Randomized controlled trial Duration: not described |
|
| Participants | Number: 84 patients Inclusion criteria: Patients with acute brucellosis diagnosed through isolation ofBrucella and/or seroconversion with a compatible clinical setting. Exclusion criteria: not described |
|
| Interventions | 1‐tetracycline 500mg PO ,QID, plus rifampicin 1.2 g PO daily during the first week and 600 mg PO daily for the following three weeks for a 4 weeks period 2‐ TXP/SMZ 480/2400 for 10 days, 320/1600 for 20 days and 160/400 until the end of treatment during 6 months. |
|
| Outcomes | 1‐therapeutic failure 2‐side effects |
|
| Notes | Location: not described Setting: not described Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The random allocation was carried out on the 92 courses of treatment, rather than 84 patients. No information about the completeness of follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Not mentioned |
| Other bias | Unclear risk | Insufficient information |
Colmenero 1989.
| Methods | Randomized controlled trial Duration: 1985‐1986 |
|
| Participants | Number: 111 patients analysed Inclusion criteria: 1‐isolation of Brucella from blood or any other body fluid and/or 2‐clinical picture compatible with the disease together with (i) Wright's seroagglutination at titers equal to or higher than 1/160; or (ii) indirect immunofluorescence with titers equal to or higher than 1/100 of the IgS or IgG conjugates and equal to or higher than 1/50 for IgM or IgA conjugates; or (iii) seroconversion of four or more times the initial titers in two separate serum samples taken within a minimum interval of 3 weeks between them. Exclusion criteria: Patients with neuromeningeal complications or those treated within the preceding 96 h with either tetracycline, streptomycin, rifampicin or co‐trimoxazole |
|
| Interventions | 1‐100mg Doxycycline/12h orally for 30 days plus single IM dose of 1g streptomycin sulphate for the first 21 days. In patients with osteoarticular or visceral complications treatment with doxycycline extended to 60 days 2‐Single morning dose of 15mg/kg rifampicin for 45 days plus 100mg/12 doxycycline for 45 days. In patients with osteoarticular or visceral complications treatment with doxycycline extended to 60 days |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐advers drug reactions 4‐time to defervescence |
|
| Notes | Location: Hospital regional "Carlos Haya", Malaga, Spain Setting: hospitalised patients Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only analysed patients were reported |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Colmenero 1994.
| Methods | Randomized controlled trial Duration: not mentioned |
|
| Participants | Number: 19 patients enrolled [one patient in each group was excluded from this review due to spondylitis] Inclusion criteria: A diagnosis of brucellosis was made by the isolation of the organism or the presence of compatible clinical features together with the demonstration of specific antibodies at significant titers. Significant titers were considered to be.1/160 for seroagglutination and .1/320 by the anti‐Brucella Coombs test. Exclusion criteria: abnormal hepatic and renal functions, and receiving any drugs or taking any alcohol during the treatment period. |
|
| Interventions | 1‐Doxycycline at 100 mg orally every 12 h for 6 weeks plus streptomycin (sulphate) at 1 g intramuscularly daily for the first 3 weeks. 2‐Doxycycline at 100 mg orally every 12 h for 6 weeks plus rifampicin: 600 mg/day orally for those who weighed between 50 and 60 kg, 900 mg/day for those who weighed between 61 and 80 kg, and 1,200 mg/day for those who weighed more than 80kg. The single doses were given in the morning for 6 weeks. |
|
| Outcomes | 1‐relapse 2‐therapeutic failure |
|
| Notes | Location: Unit of Infectious Diseases of Malaga Regional Hospital, Spain Setting: not described Source of funding: The study was supported in part by grants CYTIT‐SAF92‐0333, FISS 89/0305, and FISS 93/0632. This work has been carried out within the European Collaborative Group of the Center for Cooperation in the Field of Scientific and Technical Research of the European Communities, project Bl‐phase II. The doxycycline and rifampicin standards were provided by Pfizer Corporation (Brussels, Belgium) and Marion Merrell Dow (Madrid, Spain). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9 patients were randomized to DR group, and 10 to DS group. No missing outcome data |
| Selective reporting (reporting bias) | High risk | Assessment of side effects and time to defervescence are not reported |
| Other bias | Low risk | |
Ersoy 2005.
| Methods | Randomized controlled trial Duration: May 1997 and December 2002 |
|
| Participants | Number: 128 patients enrolled Inclusion criteria: Patients older than 16 years diagnosed as uncomplicated brucellosis. The diagnostic criteria were: (i) isolation of Brucella spp. from blood or other fluids, or (ii) the finding of ≥ 1/160 titre or four‐fold rise over 2‐3 weeks in titre of antibodies to Brucella by a standard‐tube agglutination test, in association with characteristic clinical findings, and history of consuming unpasteurized or raw milk. Exclusion criteria: pregnancy or nursing, known or suspected hypersensitivity or any contraindication to rifampicin, tetracyclines or amino glycosides, severe concomitant disease and effective antimicrobial therapy within 10 days before starting the study. Patients with central nervous system involvement, spondylitis, endocarditis or children under16 years of age were also excluded from the study. |
|
| Interventions | 1‐ofloxacin 400 mg/day plus rifampicin 600 mg/day for 6 weeks 2‐doxycycline 200 mg/day plus rifampicin 600 mg/day for 6 weeks 3‐doxycycline 100 mg/day for 6 weeks plus streptomycin 1 g, intramuscularly for 3 weeks |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐adverse drug reactions |
|
| Notes | Location: Turkey Setting: hospitalised and ambulatory patients Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 128 patients were enrolled. Ten patients did not appear in follow ups. The reasons were not described. The data of 41 patients in OR, 45 in DR, and 32 in DS was analysed. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Unclear risk | The number of patients in DS group were considerably smaller than two others, implying the larger proportion of non‐responders |
Hashemi 2012.
| Methods | Randomized controlled trial Duration: April 2008 to March 2010 |
|
| Participants | Number: 219 patients enrolled Inclusion criteria: Brucellosis was diagnosed based on clinical presentation compatible with brucellosis in the presence of significant titers of specific antibodies (standard tube agglutination 1/160, Coombs test 1/160, 2‐mercaptoetanol (2‐ME) 1‐80, or Brucella IgG‐ELISA >12) and/or a positive blood culture. Exclusion criteria: age under 17 years, endocarditis, neurobrucellosis, spondylitis, renal failure, hepatic failure, or a history of treatment for brucellosis in the last 6 months. |
|
| Interventions | 1‐doxycycline 200 mg daily for 6 weeks plus streptomycin 1000 mg daily for the first 3 weeks 2‐doxycycline 200 mg daily plus rifampin 15 mg/kg daily for 6 weeks 3‐ofloxacin 800 mg daily plus rifampin 15 mg/kg daily for 6 weeks. |
|
| Outcomes | 1‐Clinical response: clinical improvement of primary signs (objective) and symptoms (subjective) of disease at the end of treatment 2‐Therapeutic failure: persistence of symptoms and signs at the end of 6 weeks of therapy 3‐Relapse: reappearance of symptoms and signs accompanied by a 2‐ME titer 1/80 during the follow‐up period. 4‐Adverse drug reactions |
|
| Notes | Location: Sina referral hospital, Hamedan province, Iran Setting: outpatient and in‐patient Source of funding: supported in part by the Vice‐Chancellor of Research and Technology, Hamedan University of Medical Sciences, Hamedan, Iran |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Open randomization |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 219 patients were enrolled, out of whom 28 were excluded, since they did not attend the first follow‐up.Outcome for therapeutic failure was available for 65 patients in doxycycline‐streptomycin group, 62 in doxycycline‐rifampicin group, and 64 in ofloxacin plus rifampicin group. Outcome for relapse at 6 months was available for 65 patients in doxycycline plus streptomycin group, 59 in doxycycline plus rifampicin group, and 64 in ofloxacin plus rifampicin group. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hassanjani Roushan 2004.
| Methods | Randomized controlled trial Duration: April 1999 to January 2002 |
|
| Participants | Number: 280 patients enrolled Inclusion criteria: the finding of >=1/320 standard tube agglutination titer (STAT) of antibodies to Brucella with a 2 mercaptoethanol (2 ME) >=1/160, in association with compatible clinical findings. For all of these cases, confirmatory tests were also performed using Elisa with significant titers of IgM and IgG specific Brucella antibodies. Exclusion criteria: age less than 10 years, pregnancy, spondylitis, endocarditis, meningoencephalitis, previous history of brucellosis, and antimicrobial therapy for more than 7 days before enrolment |
|
| Interventions | 1‐Co‐trimoxazole 8 mg/kg/day of the trimethoprim component divided into 3 doses plus doxycycline 100mg twice daily for eight weeks 2‐Co‐trimoxazole 8 mg/kg/day of the trimethoprim component divided into 3 doses plus rifampicin 15 mg/kg once a day for eight weeks |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐adverse drug reactions |
|
| Notes | Location: Cities of Babol and Amol and surrounding villages in Northern Iran Setting: not mentioned Source of funding: the research centre of Babol Medical University. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 140 patients were randomized to each group. Cases that refused to follow‐up were considered as treatment failure (3 patients in each group) |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hassanjani Roushan 2005.
| Methods | Randomized controlled trial Duration: March 1998 to July 2003 |
|
| Participants | Number: 79 patients enrolled Inclusion criteria: not described Exclusion criteria: not described |
|
| Interventions | 1‐cotrimoxasole plus rifampicin for six weeks 2‐cotrimoxasole plus rifampicin for eight weeks |
|
| Outcomes | 1‐relapse 2‐treatment failure |
|
| Notes | Location: Babol province, Iran Setting: not described Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Patients were not blinded. Other details were not provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 41 and 38 patients were randomized to six and eight weeks of treatment. It is stated that "all patients were followed‐up for one year after completion of therapy." |
| Selective reporting (reporting bias) | High risk | |
| Other bias | Unclear risk | Insufficient information |
Hassanjani Roushan 2006.
| Methods | Randomized controlled trial Duration: October 2003 through June 2005 |
|
| Participants | Number: 200 patients enrolled Inclusion criteria: a Standard Tube Agglutination Test (STAT) titer >=1:320 and 2‐Mercaptoethanol (2ME) titer >=1:80 in people who had clinical findings compatible with the diagnosis. Exclusion criteria: age of <10 years, spondylitis, neurobrucellosis, pregnancy, and the history of >1 week of antibiotic treatment before enrolment. |
|
| Interventions | 1‐doxycycline 100 mg orally twice daily for 45 days, plus streptomycin 1g intramuscularly daily for 14 days 2‐doxycycline 100 mg orally twice daily for 45 days, plus gentamicin 5 mg/kg/day intramuscularly for 7 days |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐adverse drug reactions |
|
| Notes | Location: Babol, Northern Iran Setting: hospitalised patients Source of funding: Razi Medical Research Center of Babol and the Iranian Society for Support of Patients with Infectious Diseases. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 100 patients were randomized to each group. Three refused follow‐up in DG, and 5 refused follow‐up in DS. One patient in DS died in a car accident. The analysis was carried out on 97 and 94 patients in DG and DS groups. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Hassanjani Roushan 2010.
| Methods | Randomized controlled trial Duration: April 2005 to September 2008 |
|
| Participants | Number: 164 patients enrolled [2 patients in DS and 1 in DG groups excluded due to spondylitis] Inclusion criteria: Patients > 10 yrs. The diagnosis of brucellosis was made by using standard tube agglutination (STA) titre >= 1:320 and 2‐mercaptoethanol (2ME) titre >= 1:160, together with compatible clinical findings (fever, sweating, arthralgias, peripheral arthritis, sacroiliitis, and epididymo‐orchitis). Exclusion criteria: age of <10 years, spondylitis, endocarditis, neurobrucellosis, pregnancy, and the history of >2 days of antibiotic treatment before enrolment. |
|
| Interventions | 1‐doxycycline 100 mg orally twice daily for 45 days, plus streptomycin 1g intramuscularly daily for 14 days 2‐doxycycline 100 mg orally twice daily for 8 weeks, plus gentamicin 5 mg/kg/day intramuscularly for 5 days |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐adverse drug reactions |
|
| Notes | Location: Babol, Northern Iran Setting: inpatient and outpatient Source of funding: the Infectious Diseases Research Center and Tropical Diseases of Babol Medical University. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | "For each patient, a record was drawn and the therapy regimen, which was noted on it, was administered. We could not predict the therapy regimen for any patient." |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 82 patients were randomized to each group. Three were excluded due to the diagnosis of spondylitis. Five patients in DS and 2 in DG lost to follow‐ups. The proportion of missing outcomes compared with observed events was considerable. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Kalo 1996.
| Methods | Randomized controlled trial Duration: January 1992 to December 1994 |
|
| Participants | Number: 24 patients enrolled Inclusion criteria: positive serology and/or isolation of a Brucella sp. from blood in the presence of compatible epidemiological and clinical findings. The serological criteria were the following : Wright seroagglutination assay titers equal to or higher than 1/160 and/or indirect immunofluorescence assay titers higher than 1/100. Exclusion criteria: Individuals who received antimicrobial therapy prior to the study, patients allergic to the drugs employed, children, under 15 years of age, pregnant women, patients with complicated brucellosis, such as central nervous system involvement, endocarditis or spondylitis. |
|
| Interventions | 1‐ciprofloxacin (1 g/d, P.O) plus doxycycline (200 mg/d) for 6 weeks 2‐rifampicin (900 mg/d P.O) plus doxycycline (200 mg/d) for 6 weeks |
|
| Outcomes | 1‐relapse 2‐time to defervescence |
|
| Notes | Location: University Hospital Centre of Tirana, Albania Setting: not described Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. There is a quote in the study in which "the first 12 patients" were assigned to the first regimen, and "the second twelve" to the other. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Twelve patients were randomized to each group. No details of follow‐up were provided. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | No baseline comparison |
Karabay 2004.
| Methods | Randomized controlled trial Duration: December 1999 and December 2001 |
|
| Participants | Number: 34 patients enrolled Inclusion criteria: the presence of signs and symptoms compatible with brucellosis including a positive agglutination titre (≥1/160) and/or a positive culture. Exclusion criteria: history of seizure, pregnancy, and age under 15 years. |
|
| Interventions | 1‐doxycycline 100 mg two times daily and rifampicin 600 mg once daily for 45 days 2‐ofloxacin 400 mg once daily and rifampicin 600 mg once daily for 30 days |
|
| Outcomes | 1‐clinical relapse 2‐adverse drug reactions 3‐time to defervescence |
|
| Notes | Location: the Social Security Duzce Hospitaland Abant Izzet Baysal University, Duzce Medical School, Turkey. Setting: hospitalised patients Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18 patients were randomized to DR, and 16 to OR. Four patients in DR and 1 in OR did not appear in follow‐ups. Four left because they felt well, and one refused to use the drugs. However, the proportion of missing outcomes compared with observed events was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Lang 1990.
| Methods | Randomized controlled trial Duration: not mentioned |
|
| Participants | Number: 11 patients enrolled Inclusion criteria: patients over 18 years old, with a four‐fold rise in quantitative hemagglutination titres, with or without positive cultures for B. melitensis from blood, bone marrow or other body fluids. Exclusion criteria: ? |
|
| Interventions | 1‐doxycycline 100 mg bd plus rifampicin 300 mg bd for 6 weeks 2‐ciprofloxacin 1 g bd for 6 weeks 3‐ciprofloxacin 750 mg bd for 6 weeks |
|
| Outcomes | 1‐clinical relapse 2‐bacteriological relapse 3‐time to defervescence |
|
| Notes | Location: Israel Setting: not described Source of funding: the study was supported, in part, by Bayer AG, D‐5600 Wuppertal, Germany. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were assigned by a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients were randomized to the regimen 1, 4 to the regimen 2, and 2 to the regimen 3. One patient missed because of non‐compliance. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Lang 1992.
| Methods | Randomized controlled trial Duration: 1989‐6 month follow‐up |
|
| Participants | Number: 18 patients Inclusion criteria: Patients 6 years of age and older were enrolled if they presented with clinical signs suggestive of brucellosis (a temperature >38C for at least 72 hours, arthralgia, back pain, rigors, and night sweats). The diagnosis of brucellosis was confirmed by a fourfold increase in titers of Brucella antibody between collection of acute‐phase and convalescent‐phase sera and/or positive cultures of blood, bone marrow, or other sterile fluids. Exclusion criteria: not described |
|
| Interventions | 1‐ ceftriaxone maximum 2 gr single daily IM injection for at least 2 weeks 2‐ doxycycline 100 mg P.O. daily for 4 weeks, plus streptomycin 20 mg/kg (maximum 1gr) I.M. once daily for 14 days |
|
| Outcomes | 1‐therapeutic failure | |
| Notes | Location: Israel Setting: inpatient Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 patients were randomized to the ceftriaxone and 8 to DS groups. No missing data were reported. |
| Selective reporting (reporting bias) | Low risk | No adverse reactions related to the treatment were observed in any of the patients in either group during one week of study. |
| Other bias | Low risk | Initial goal was to include 66 patients but study discontinued early after evaluation of the first 18. The reason was initial failure of 6/8 patients in ceftriaxone group who were switched to the other group after one week. |
McDevitt 1970.
| Methods | Randomized controlled trial Duration: not mentioned |
|
| Participants | Number: 68 Inclusion criteria: A positive result was one with a Coombs titre of 1/80 or greater, irrespective of complement‐fixation titre, or a Coombs titre of 1/40 for B. abortus in the presence of a complement fixation titre of 1/16 or greater. Exclusion criteria: not described |
|
| Interventions | 1‐ampicillin 1 gr four times a day orally for 28 days 2‐placebo, in identical capsules, for the same period.( four times a day orally for 28 days) |
|
| Outcomes | 1‐side effects 2‐Subjective improvement |
|
| Notes | Location: Ireland Setting: outpatients Source of funding: the research funds of the Queen's University of Belfast and the Northern Ireland Hospitals Authority. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number with pre selection for some characteristics |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both patients and observers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 patients were randomized to ampicillin and 38 to placebo. In the placebo group, one patient was withdrawn due to the progression of the disease, three failed to complete the treatment, and four did not complete the follow‐up. In the ampicillin group, one did not complete the treatment, and five did not attend the follow‐up assessment. |
| Selective reporting (reporting bias) | High risk | Neither failure nor relapse were assessed |
| Other bias | Unclear risk | Insufficient information |
Montejo 1993a.
| Methods | Randomized controlled trial Duration: 1980‐83 |
|
| Participants | Number: about 200 patients enrolled Inclusion criteria: ≥ 14 years of age, a clinical picture consistent with a diagnosis of brucellosis, isolation of germs from clinical specimens, and/ or titers of the standard tube agglutination test (STAT) of 1/160. Exclusion criteria: antecedents of brucellosis in the previous year, the presence of serious associated illness, pregnancy, a reported allergy to one or more of the antimicrobial agents used in this study, and a diagnosis of endocarditis, spondylitis, or CNS affected by Brucella. |
|
| Interventions | 1‐1200 mg of rifampicin once a day for 7 days and then 600 mg/d for 21 days together with 200 mg of doxycycline/d for 28 days 2‐160 mg of trimethoprim plus 800 mg of sulfa‐methoxazole every 8 hours for 10 days, then the same dose every 12 hours until the end of the 4‐week period, and finally 80 mg of trimethoprim and 400 mg of sulphamethoxazole every 12 hours until the end of the 6‐month treatment period 3‐200 mg of doxycycline/d for 6 weeks |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐drug regimen change due to non‐compliance |
|
| Notes | Location: Hospital de Cruces, Baracaldo, Vizcaya, Spain Setting: not described Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a table of random numbers |
| Allocation concealment (selection bias) | Low risk | The results of this random distribution were placed in sealed, numbered envelopes, which were opened once it was established that the patient fulfilled the criteria for inclusion in the study. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 5 patients were excluded due to the allergy to medications, 5 because of receiving treatment in the previous year, 4 due to brucellosis complications, and 13 due to loss to follow‐up. At one‐year follow‐up, 65 patients were analysed to the regimen 1, 64 to the regimen 2, and 71 to the regimen 3. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Montejo 1993b.
| Methods | Randomized controlled trial Duration: 1984‐87 |
|
| Participants | Number: about 130 patients enrolled Inclusion criteria: ≥ 14 years of age, a clinical picture consistent with a diagnosis of brucellosis, isolation of germs from clinical specimens, and/ or titers of the standard tube agglutination test (STAT) of 1/160. Exclusion criteria: antecedents of brucellosis in the previous year, the presence of serious associated illness, pregnancy, a reported allergy to one or more of the antimicrobial agents used in this study, and a diagnosis of endocarditis, spondylitis, or affection of the CNS by Brucella. |
|
| Interventions | 1‐ 1 g of streptomycin/d (intramuscularly) for 3 weeks together with 200 mg of doxycycline/d (orally) for 6 weeks 2‐900 mg of rifampicin/d plus 200 mg of doxycycline/d (orally) for 6 weeks 3‐1 g of streptomycin/d (intramuscularly) for 2 weeks plus 200 mg of doxycycline/d (orally) for 6 weeks. |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐drug regimen change due to non‐compliance |
|
| Notes | Location: Hospital de Cruces, Baracaldo, Vizcaya, Spain Setting: not described Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a table of random numbers |
| Allocation concealment (selection bias) | Low risk | The results of this random distribution were placed in sealed, numbered envelopes, which were opened once it was established that the patient fulfilled the criteria for inclusion in the study. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 5 patients were excluded due to the allergy to medications, 5 because of receiving treatment in the previous year, 4 due to brucellosis complications, and 13 due to loss to follow‐up. At one‐year follow‐up, 44 patients were analysed to the regimen 1, 46 to the regimen 2, and 40 to the regimen 3. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes are reported |
| Other bias | Low risk | |
Ranjbar 2007.
| Methods | Randomized controlled trial Duration: 1999‐2001 |
|
| Participants | Number: 228 patients enrolled Inclusion criteria: (1) brucellosis clinical features including fever, sweats, arthralgia, hepatomegaly, splenomegaly, and/or signs of focal disease with a ≥1/160 standard tube agglutination titer of antibodies to Brucella; or (2) a tissue sample or blood culture positive for Brucella bacteria; or (3) a four‐fold increase in Wright titer in a two‐week interval with compatible clinical findings Exclusion criteria: Pregnant women, children under eight years of age, and patients with endocarditis and neurobrucellosis |
|
| Interventions | 1‐ doxycycline 100 mg twice a day plus rifampicin 10 mg/kg body weight/day every morning, both taken orally for eight weeks 2‐doxycycline 100 mg twice a day and rifampicin 10 mg/kg body weight/day every morning, both taken orally for eight weeks, plus 7.5 mg/kg amikacin intramuscularly twice a day for seven days. |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐symptom relief 4‐negative 2ME titer 5‐adverse drug reactions |
|
| Notes | Location: Sina Hospital, Hamedan, Iran Setting: hospitalised and ambulatory patients Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 114 patients were randomized to each group. In DR group, 3 did not take the drugs correctly, and 1 lost to follow up. In DRA group, 2 did not complete the treatment, and 2 lost to follow up. The proportion of missing outcomes compared with observed event was considerable. |
| Selective reporting (reporting bias) | Low risk | Important outcomes were reported |
| Other bias | Low risk | |
Rodriguez Zapata 1987.
| Methods | Randomized controlled trial Duration: not stated |
|
| Participants | Number: 72 patients enrolled. [two patients were excluded from this review due to spondylitis] Inclusion criteria:1)presence of a clinical picture compatible with acute brucellosis.2) Rose Bengal test positive and/or positive blood culture for Brucella. Exclusion criteria: patients with severe concomitant diseases, patients requiring treatments with corticosteroids, barbiturates or other antibiotics, pregnant women, patients younger than 13 or older than 70 years, patients for whom any of the four antibiotics to be used were contraindicated. |
|
| Interventions | 1‐ doxycycline 200 mg once daily, plus rifampicin 900 mg orally once daily plus for 45 days 2‐ doxycycline 200 mg orally once daily plus streptomycin 1 g intramuscularly, daily for 21 days |
|
| Outcomes | 1‐relapse 2‐therapeutic failure 3‐adverse drug reactions |
|
| Notes | Location: Spain Setting: hospitalised patients (?) Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 36 patients were randomized to each group. In DR group, 2 patients did not complete the treatment, and 2 were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Time to defervescence was measured, but not reported. |
| Other bias | Low risk | |
Sarmadian 2009.
| Methods | Randomized controlled trial Duration: 2006‐2008 |
|
| Participants | Number: 80 patients enrolled Inclusion criteria:Patient over 13yrs age, with brucellosis Exclusion criteria: not described |
|
| Interventions | 1‐ Doxycycline 100 mg BID, plus Rifampicin 10 mg/kg/day for 8 weeks 2‐ Doxycycline 100 mg BID, plus Ciprofloxacin 500 mg BID for 8 weeks |
|
| Outcomes | 1‐relapse 2‐symptom relief 3‐adverse drug reactions |
|
| Notes | Location: Arak, Iran Setting: outpatient Source of funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided regarding missing values |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Only abstract was available |
Solera 2004.
| Methods | Randomized controlled trial Duration: July 1995 and January 1999 |
|
| Participants | Number: 167 patients randomized Inclusion criteria: "Eligible patients were >=18 years of age with brucellosis diagnosed on the basis of at least 1 of the following criteria: isolation of Brucella species from samples of blood or other fluids or tissues; detection of antibodies to Brucella species at a titer of >=1:160 by use of a standard tube agglutination method; or a >= 4‐fold increase in the Brucella antibody titer to >= 1:80, revealed by standard tube agglutination, between serum specimens obtained >=2 weeks apart and studied in the same laboratory. Additionally, at least 2 of the following compatible clinical findings must have been present: fever, arthralgias, weight loss, hepatosplenomegaly, or signs of focal disease." Exclusion criteria: "known or suspected hypersensitivity (or another contraindication) to tetracyclines or aminoglycosides,severe concomitant disease, body weight of <50 kg, or receipt of effective antimicrobial therapy for brucellosis <= 30 days before entering the study. We also excluded patients with endocarditis, spondylitis, or neurobrucellosis." |
|
| Interventions | 1‐Doxycycline 100 mg twice daily for 30 days, plus placebo for an additional 15 days, plus gentamicin 240 mg daily during the first 7 days 2‐ Doxycycline 100 mg twice daily for 45 days, plus gentamicin 240 mg daily during the first 7 days |
|
| Outcomes | 1‐relapse 2‐adverse drug reactions |
|
| Notes | Location: 5 general hospitals in central Spain Setting: hospitalised patients Source of funding: Fondo de Investigacion Sanitaria, Spanish Ministry of Health (grant FIS 96/0293). Laboratorios Wasserman donated study drug and matching placebo. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random treatment assignments |
| Allocation concealment (selection bias) | Low risk | The master list of random assignments was stored at the Pharmacy Department at the General Hospital in Albacete, Spain |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patients and care providers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 167 patients were randomized (84 in group 1 and 83 in group 2). Seven patients in group 1 and 5 in group 2 were excluded due to the diagnosis of spondylitis or no brucellosis. Four patients in group 1 and 5 in group 2 were lost to follow up. The analysis was carried out on 73 patients in each group. The proportion of missing outcomes compared with observed event risk were enough to induce clinically relevant bias in intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abramson 1997 | Non‐randomized |
| Alfaro 2007 | Non‐randomized |
| Alp 2006 | Non‐randomized |
| Ariza 1985a | Included some patients with spondylitis in both groups who received an additional treatment |
| Ariza 1985b | Non‐randomized. Allocation was based on odd and even number of age. |
| Bayindir 2003 | Brucellosis spondylitis |
| Bertrand 1979 | Non‐randomized |
| Colmenero 1986 | Non‐randomized |
| De Mello Vieira 1984 | Non‐randomized |
| De Rosa 1970 | Non‐randomized |
| Doğanay 1992 | Non‐randomized |
| El Miedany 2003 | Non‐randomized |
| Feiz 1973 | Quazi‐randomized. Patients were assigned to groups, according to the order of appearance in the clinic. The sequence generation was not randomized. |
| Fiaccadori 1972 | Non‐randomized |
| Hassanjani Roushan 2007 | Non‐randomized |
| Irmak 2003 | The study is about the effects of Levamizole, which is not considered an antibiotic. |
| Keramat 2009 | Totally, 38% of cases had the signs of spondylitis, and the results were not reported separately for that subgroup. In addition, there was a loss to follow up of 57% |
| Kurmanova 1995 | Non‐randomized |
| Llorens‐Terol 1980 | Non‐randomized |
| Lubani 1989 | No effective randomization of treatment regimens. Patients were randomized into three groups according to the duration of treatment. In each group, several treatment regimens were administered in a non‐random fashion. |
| Lulu 1988 | Non‐randomized |
| Magill 1953 | Non‐randomized |
| Mukovozova 1987 | Non‐randomized |
| Rizzo‐Naudi 1967 | Non‐randomized |
| Saltoglu 2002 | Non‐randomized |
| Solera 1991 | Non‐randomized |
| Solera 1993 | Out of 204 randomized patients, the outcomes were only reported in 115 non‐complicated subgroup, out of whom 8 had spondylitis. |
| Solera 1995 | No effective randomization. Sequence generated by odd or even age |
| Solera 1997 | Non‐randomized |
| Sánchez 1991 | Non‐randomized |
| Sánchez 1994 | Non‐randomized |
| Vargas 1980 | Non‐randomized |
Characteristics of studies awaiting assessment [ordered by study ID]
Feng 1994.
| Methods | Randomized controlled trial Generation of allocation sequence: not known Allocation concealment: not known Blinding: not known Inclusion of all randomized participants in the final analysis: not known Duration: not known |
| Participants | Number: not known Inclusion criteria: not known Exclusion criteria: not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | Location: not known Setting: not known Source of funding: not known |
Differences between protocol and review
The contact reviewer changed. Two authors of the protocol were acknowledged for their contributions.
Contributions of authors
All authors participated in the preparation of the protocol, assessing the quality of studies, and data extraction. RYN performed all the analyses. All authors commented and endorsed the final review contents.
Sources of support
Internal sources
Tehran University of Medical Sciences, Iran.
External sources
No sources of support supplied
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Acocella 1989 {published data only}
- Acocella G, Bertrand A, Beytout J, Durrande JB, Garcia Rodriguez JA, Kosmidis J, et al. Comparison of three different regimens in the treatment of acute brucellosis: a multicenter multinational study. Journal of Antimicrobial Chemotherapy 1989;23:433‐9. [DOI] [PubMed] [Google Scholar]
Agalar 1999 {published data only}
- Agalar C, Usubutun S, Turkyilmaz R. Ciprofloxacin and rifampicin versus doxycycline and rifampicin in the treatment of brucellosis. European Journal of Clinical Microbiology & Infectious Diseases 1999;18:535‐8. [DOI] [PubMed] [Google Scholar]
Akova 1993 {published data only}
- Akova M, Uzun O, Akalin HE, Hayran M, Unal S, Gür D. Quinolones in treatment of human brucellosis: comparative trial of ofloxacin‐rifampin versus doxycycline‐rifampin. Antimicrobial Agents and Chemotherapy 1993;37:1831‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alavi 2007 {published data only}
- Alavi SM, Rajabzadeh AR. Comparison of two chemotherapy regimen: Doxycycline‐rifampicin and doxycycline cotrimoxazol in the brucellosis patients Ahvaz, Iran,2004‐2006. Pakistan Journal of Medical Sciences 2007;23:889‐92. [Google Scholar]
Ariza 1992 {published data only}
- Ariza J, Gudiol F, Pallarés R, Rufí G, Fernández‐Viladrich P. Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double‐blind study. Annals of Internal Medicine 1992;117:25‐30. [DOI] [PubMed] [Google Scholar]
Buzon 1982 {published data only}
- Buzon L, Bouza E, Rodriguez M. Treatment of brucellosis with rifampicin+tetracycline vs TMP/SMZ. A prospective and randomized study. Chemioterapia 1982;1(4 Suppl):221. [Google Scholar]
Colmenero 1989 {published data only}
- Colmenero Castillo JD, Hernandez Marquez S, Reguera Iglesias JM, Cabrera Franquelo F, Rius Diaz F, Alonso A. Comparative trial of doxycycline plus streptomycin versus doxycycline plus rifampin for the therapy of human brucellosis. Chemotherapy 1989;35:146‐52. [DOI] [PubMed] [Google Scholar]
Colmenero 1994 {published data only}
- Colmenero JD, Fernández‐Gallardo LC, Agúndez JA, Sedeño J, Benítez J, Valverde E. Possible implications of doxycycline‐rifampin interaction for treatment of brucellosis. Antimicrobial Agents and Chemotherapy 1994;38:2798‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ersoy 2005 {published data only}
- Ersoy Y, Sonmez E, Tevfik MR, But AD. Comparison of three different combination therapies in the treatment of human brucellosis. Tropical Doctor 2005;35:210‐2. [DOI] [PubMed] [Google Scholar]
Hashemi 2012 {published data only}
- Hashemi SH, Gachkar L, Keramat F, Mamani M, Hajilooi M, Janbakhsh A, et al. Comparison of doxycycline–streptomycin, doxycycline–rifampin,and ofloxacin–rifampin in the treatment of brucellosis:a randomized clinical trial. International Journal of Infectious Diseases 2012;16:e247–e251. [DOI] [PubMed] [Google Scholar]
Hassanjani Roushan 2004 {published data only}
- Hasanjani Roushan MR, Gangi SM, Ahmadi SA. Comparison of the efficacy of two months of treatment with co‐trimoxazole plus doxycycline vs. co‐trimoxazole plus rifampin in brucellosis. Swiss Medical Weekly 2004;134:564‐8. [DOI] [PubMed] [Google Scholar]
Hassanjani Roushan 2005 {published data only}
- Hasanjani Roushan MR, Smailnejad Gangi SM, Janmohammadi N, Soleimani Amiri MJ. Efficacy of cotrimoxasol plus rifampin for six and eight weeks of therapy in childhood brucellosis. European Congress of Clinical Microbiology and Infectious Diseases. 2005.
Hassanjani Roushan 2006 {published data only}
- Hasanjani Roushan MR, Mohraz M, Hajiahmadi M, Ramzani A, Valayati AA. Efficacy of gentamicin plus doxycycline versus streptomycin plus doxycycline in the treatment of brucellosis in humans. Clinical Infectious Diseases 2006;42:1075‐80. [DOI] [PubMed] [Google Scholar]
Hassanjani Roushan 2010 {published data only}
- Roushan MR, Amiri MJ, Janmohammadi N, Hadad MS, Javanian M, Baiani M, et al. Comparison of the efficacy of gentamicin for 5 days plus doxycycline for 8 weeks versus streptomycin for 2 weeks plus doxycycline for 45 days in the treatment of human brucellosis: a randomized clinical trial. The Journal of Antimicrobial Chemotherapy 2010;65(5):1028‐35. [DOI] [PubMed] [Google Scholar]
Kalo 1996 {published data only}
- Kalo T, Novi S, Nushi A, Dedja S. Ciprofloxacin plus doxycycline versus rifampicin plus doxycycline in the treatment of acute brucellosis. Medecine Et Maladies Infectieuses 1996;26:587‐9. [Google Scholar]
Karabay 2004 {published data only}
- Karabay O, Sencan I, Kayas D, Sahin I. Ofloxacin plus rifampicin versus doxycycline plus rifampicin in the treatment of brucellosis: a randomized clinical trial. BMC Infectious Diseases. 2004;4:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lang 1990 {published data only}
- Lang R, Raz R, Sacks T, Shapiro M. Failure of prolonged treatment with ciprofloxacin in acute infections due to Brucella melitensis. The Journal of Antimicrobial Chemotherapy 1990;26:841‐6. [DOI] [PubMed] [Google Scholar]
Lang 1992 {published data only}
- Lang R, Dagan R, Potasman I, Einhorn M, Raz R. Failure of ceftriaxone in the treatment of acute brucellosis. Clinical Infectious Diseases 1992;14:506‐9. [DOI] [PubMed] [Google Scholar]
McDevitt 1970 {published data only}
- McDevitt DG. Ampicillin in the treatment of brucellosis. A controlled therapeutic trial. British Journal of Industrial Medicine 1970;27:67‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montejo 1993a {published data only}
- Montejo JM, Alberola I, Glez‐Zarate P, Alvarez A, Alonso J, Canovas A, et al. Open, randomized therapeutic trial of six antimicrobial regimens in the treatment of human brucellosis. Clinical Infectious Diseases 1993;16:671‐6. [DOI] [PubMed] [Google Scholar]
Montejo 1993b {published data only}
- Montejo JM, Alberola I, Glez‐Zarate P, Alvarez A, Alonso J, Canovas A, et al. Open, randomized therapeutic trial of six antimicrobial regimens in the treatment of human brucellosis. Clinical Infectious Diseases 1993;16:671‐6. [DOI] [PubMed] [Google Scholar]
Ranjbar 2007 {published data only}
- Ranjbar M, Keramat F, Mamani M, Kia AR, Khalilian FO, Hashemi SH, et al. Comparison between doxycycline‐rifampin‐amikacin and doxycycline‐rifampin regimens in the treatment of brucellosis. International Journal of Infectious Diseases 2007;11:152‐6. [DOI] [PubMed] [Google Scholar]
Rodriguez Zapata 1987 {published data only}
- Rodriguez Zapata M, Gamo Herranz A, Morena Fernández J. Comparative study of two regimens in the treatment of brucellosis. International Journal of the Mediterranean Society of Chemotherapy 1987;6(2 Suppl):360‐2. [PubMed] [Google Scholar]
- Rodríguez Zapata M, Gamo A, Morena J. Evaluation of several regimens for the treatment of brucellosis: rifampicin‐doxycycline vs. streptomycin‐doxycycline. SEMER 1986;80:32‐3. [Google Scholar]
Sarmadian 2009 {published data only}
- Sarmadian H, Didgar F, Sufian M, Zarinfar N, Salehi F. Comparison Between Efficacy of Cipofoxacin Doxycycline and Rifampin ‐ Doxycycline Regimens in Treatment and Relapse of Brucellosis. Tropical Medicine & International Health 2009;14(suppl 2):209. [Google Scholar]
Solera 2004 {published data only}
- Solera J, Geijo P, Largo J, Rodriguez‐Zapata M, Gijón J, Martinez‐Alfaro E, et al. A randomized, double‐blind study to assess the optimal duration of doxycycline treatment for human brucellosis. Clinical Infectious Diseases 2004;39:1776‐82. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abramson 1997 {published data only}
- Abramson O, Abu‐Rashid M, Gorodischer R, Yagupsky P. Failure of short antimicrobial treatments for human brucellosis. Antimicrobial Agents and Chemotherapy 1997;41:1621‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfaro 2007 {published data only}
- Alfaro ME, Geijo P, Marcos F, Mateos F, Blanch J, Tárraga I, et al. Doxycycline and streptomycin versus doxycycline alone in the treatment of human brucellosis. 17th European Congress of Clinical Microbiology and Infectious Diseases, Munich, Germany. 2007.
Alp 2006 {published data only}
- Alp E, Koc RK, Durak AC, Yildiz O, Aygen B, Sumerkan B, et al. Doxycycline plus streptomycin versus ciprofloxacin plus rifampicin in spinal brucellosis. BMC Infectious Diseases 2006;6:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ariza 1985a {published data only}
- Ariza J, Gudiol F, Pallarés R, Rufí G, Fernández‐Viladrich P. Comparative trial of co‐trimoxazole versus tetracycline‐streptomycin in treating human brucellosis. The Journal of Infectious Diseases 1985;152:1358‐9. [DOI] [PubMed] [Google Scholar]
Ariza 1985b {published data only}
- Ariza J, Gudiol F, Pallarés R, Rufí G, Fernández‐Viladrich P. Comparative trial of rifampin‐doxycycline versus tetracycline‐streptomycin in the therapy of human brucellosis. Antimicrobial Agents and Chemotherapy 1985;28:548‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bayindir 2003 {published data only}
- Bayindir Y, Sonmez E, Aladag A, Buyukberber N. Comparison of five antimicrobial regimens for the treatment of brucellar spondylitis: a prospective, randomized study. Journal of Chemotherapy 2003;15:466‐71. [DOI] [PubMed] [Google Scholar]
Bertrand 1979 {published data only}
- Bertrand A, Roux J, Janbon F, Jourdan J, Jonquet O. The treatment of brucellosis using rifampicine. La Nouvelle Presse Medicale 1979;8(44):3635‐9. [PubMed] [Google Scholar]
Colmenero 1986 {published data only}
- Colmenero JD, Porras JJ, Valdivielso P, Porras JA, Ramon E, Cause M, et al. Brucellosis: a prospective study of 100 cases. Medecina Clinica (Barc) 1986;86(2):43‐8. [PubMed] [Google Scholar]
De Mello Vieira 1984 {published data only}
- Mello Vieira J. Treatment of brucellosis with doxycycline and rifampicin: a study of 50 cases. Current Therapeutic Research 1984;35:944. [Google Scholar]
De Rosa 1970 {published data only}
- Rosa F, Fabiani F. Therapy of human brucellosis with cephalexin. La Clinica Terapeutica 1970;53(2):141‐4. [PubMed] [Google Scholar]
Doğanay 1992 {published data only}
- Doganay M, Aygen B. Use of ciprofloxacin in the treatment of brucellosis. European Journal of Clinical Microbiology and Infectious Diseases 1992;11(1):74‐5. [DOI] [PubMed] [Google Scholar]
El Miedany 2003 {published data only}
- Miedany YM, Gaafary M, Baddour M, Ahmed I. Human brucellosis: do we need to revise our therapeutic policy?. The Journal of Rheumatology 2003;30:2666‐72. [PubMed] [Google Scholar]
Feiz 1973 {published data only}
- Feiz JM, Sabbaghian H, Sohrabi FA. comparative study of therapeutic agents used for treatment of acute brucellosis. The British Journal of Clinical Practice 1973;27:410‐3. [PubMed] [Google Scholar]
Fiaccadori 1972 {published data only}
- Fiaccadori F, Camilloni R, Ghinelli F, Pizzigoni G. Comparative study of the use of tetracycline in low dosage in the treatment of brucellosis]. Giornale Di Clinica Medica 1972;53:290‐9. [PubMed] [Google Scholar]
Hassanjani Roushan 2007 {published data only}
- Hasanjani Roushan MR, Soleimani Amiri J, Janmohammadi N, Soleimani Amiri S. Efficacy of gentamicin for 5 days plus doxycycline for eight weeks versus streptomycin for 2 weeks plus doxycycline for 45 days in the treatment of human brucellosis. European Congress of Clinical Microbiology and Infectious Diseases. 2007.
Irmak 2003 {published data only}
- Irmak H, Buzgan T, Karahocagil MK, Evirgen O, Akdeniz H, Demiröz AP. The effect of levamisole combined with the classical treatment in chronic brucellosis. The Tohoku Journal of Experimental Medicine 2003;201:223‐8. [DOI] [PubMed] [Google Scholar]
Keramat 2009 {published data only}
- Keramat F, Ranjbar M, Mamani M, Hashemi SH, Zeraati F. A comparative trial of three therapeutic regimens: ciprofloxacin‐rifampin, ciprofloxacin‐doxycycline and doxycycline‐rifampin in the treatment of brucellosis. Tropical Doctor 2009;39(4):207‐10. [DOI] [PubMed] [Google Scholar]
Kurmanova 1995 {published data only}
- Kurmanova KB, Kkhamfyang S, Iudin SM. Effectiveness of pefloxacin in brucellosis. Antibiot Khimioter 1995;40:39‐42. [PubMed] [Google Scholar]
Llorens‐Terol 1980 {published data only}
- Llorens‐Terol J, Busquets RM. Brucellosis treated with rifampicin. Archives of Disease in Childhood 1980;55(6):486‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lubani 1989 {published data only}
- Lubani MM, Dudin KI, Sharda DC, Ndhar DS, Araj GF, Hafez HA, et al. A multicenter therapeutic study of 1100 children with brucellosis. The Pediatric Infectious Disease Journal 1989;8:75‐8. [PubMed] [Google Scholar]
Lulu 1988 {published data only}
- Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, Fenech FF. Human brucellosis in Kuwait: a prospective study of 400 cases. The Quarterly Journal of Medecine 1988;66(249):39‐54. [PubMed] [Google Scholar]
Magill 1953 {published data only}
- Magill GB, Killough JH. Oxytetracycline‐streptomycin therapy in brucellosis due to Brucella melitensis. Archives of Internal Medicine 1953;91:204‐11. [DOI] [PubMed] [Google Scholar]
Mukovozova 1987 {published data only}
- Mukovozova LA. Efficacy of various methods of therapy of chronic brucellosis. Terapevticheskii Arkhiv 1987;59(11):93‐96. [PubMed] [Google Scholar]
Rizzo‐Naudi 1967 {published data only}
- Rizzo‐Naudi J, Griscti‐Soler N, Ganado W. Human brucellosis: An evaluation of antibiotics in the treatment of brucellosis. Postgraduate Medical Journal 1967;43(502):520‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saltoglu 2002 {published data only}
- Saltoglu N, Tasova Y, Inal AS, Seki T, Aksu HS. Efficacy of rifampicin plus doxycycline versus rifampicin plus quinolone in the treatment of brucellosis. Saudi Medical Journal 2002;23:921‐4. [PubMed] [Google Scholar]
Sánchez 1991 {published data only}
- Sánchez H, Luis M, Guillén O. Tetracycline and rifampin association in the treatment of brucellosis. Boletin de la Sociedad Peruana Medicina Interna 1991;4:6‐8. [Google Scholar]
Sánchez 1994 {published data only}
- Sánchez H, Luis M, Guillén O, Quispe M, Salcedo E, Castillo G. Doxycicline and Rifampin in the treatment of Brucellosis. Boletin de la Sociedad Peruana Medicina Interna 1994;7:66‐7. [Google Scholar]
Solera 1991 {published data only}
- Solera J, Medrano F, Rodríguez Zapata M, Geijo P, Paulino J. A comparative therapeutic and multicenter trial of rifampicin and doxycycline versus streptomycin and doxycycline in human brucellosis]. Medicina Clínica 1991;96:649‐53. [PubMed] [Google Scholar]
Solera 1993 {published data only}
- Solera J, Paulino J, Rodrígeuz Zapata M, Geijó P, Medrano F, Jiménez‐Zorzo F, et al. Multicentre comparative therapeutic trial of rifampicin and doxycycline versus streptomycin and doxycycline in human brucellosis. Primary evaluation results. Revista Española De Reumatología 1992;19:219. [Google Scholar]
- Solera J, Paulino J, Zapata M, Geijo P, Largo J, Grupo GECMEI. Brucellosis with osteoarticular involvement: Must it be treated in a diffrent way?. Revista Española De Reumatología 1993;20(1 Suppl):153. [Google Scholar]
Solera 1995 {published data only}
- Solera J, Rodríguez‐Zapata M, Geijo P, Largo J, Paulino J, Sáez L, et al. Doxycycline‐rifampin versus doxycycline‐streptomycin in treatment of human brucellosis due to Brucella melitensis. Antimicrobial Agents and Chemotherapy 1995;39:2061‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solera 1997 {published data only}
- Solera J, Espinosa A, Martínez‐Alfaro E, Sánchez L, Geijo P, Navarro E, et al. Treatment of human brucellosis with doxycycline and gentamicin. Antimicrobial Agents and Chemotherapy 1997;41:80‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vargas 1980 {published data only}
- Vargas V, Pedreira JD, Clotet B, Juste C, Guardia J, Bacardi R. Treatment of acute brucellosis with cotrimoxazole, doxicyclin and streptomycin. A comparative study. Medicina Clinica (Barc) 1980;75:418‐20. [PubMed] [Google Scholar]
References to studies awaiting assessment
Feng 1994 {published data only}
- Feng YM. Randomized controlled trial of six antibacterial methods in brucellosis. Endemic Diseases Collections 1994;15:66. [Google Scholar]
Additional references
Ariza 1985
- Ariza J, Gudiol F, Pallarés R, Rufi G, Fernández‐Viladrich P. Comparative trial of rifampin‐doxycycline versus tetracycline‐streptomycin in the therapy of human brucellosis. Antimicrobial Agents and Chemotherapy 1985;28(4):548‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ariza 2007
- Ariza J, Bosilkovski M, Cascio A, Colmenero JD, Corbel MJ, Falagas ME, et al. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med 2007;4:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cisneros 1990
- Cisneros JM, Viciana P, Colmenero J, Pachón J, Martinez C, Alarcón A. Multicenter prospective study of treatment of Brucella melitensis brucellosis with doxycycline for 6 weeks plus streptomycin for 2 weeks. Antimicrobial Agents and Chemotherapy 1990;34(5):881‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corbel 1997
- Corbel MJ. Brucellosis: an overview. Emerging Infectious Diseases 1997;3(2):213‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corbel 2005
- Corbel MJ, Beeching NJ. Brucellosis. Harrison's principles of internal medicine. 16th Edition. Vol. 1, McGraw‐Hill, 2005:914‐7. [Google Scholar]
Hall 1990
- Hall W. Modern chemotherapy for brucellosis in humans. Reviews of Infectious Diseases 1990;12(6):1060‐99. [DOI] [PubMed] [Google Scholar]
Pappas 2005a
- Pappas G, Solera J, Akritidis N, Tsianos E. New approaches to the antibiotic treatment of brucellosis. International Journal of Antimicrobial Agents 2005;26(2):101‐5. [DOI] [PubMed] [Google Scholar]
Pappas 2005b
- Pappas G, Akritidis N, Bosilkovski M, Tsianos E. Brucellosis. New England Journal of Medicine 2005;352(22):2325‐36. [DOI] [PubMed] [Google Scholar]
Pappas 2006
- Pappas G, Christou L, Akritidis N, Tsianos EV. Quinolones for brucellosis: treating old diseases with new drugs. Clinical Microbiology & Infection 2006;12(9):823‐5. [DOI] [PubMed] [Google Scholar]
Pappas 2007
- Pappas G, Siozopoulou V, Akritidis N, Falagas ME. Doxycycline‐rifampicin: physicians' inferior choice in brucellosis or how convenience reigns over science. The Journal of Infection 2007;54(5):459‐62. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagan: The Nordic Cochrane Centre, The Cochrane Collaboration, 2010.
Sauret 2002
- Sauret JM, Vilissova N. Human brucellosis. Journal of the American Board of Family Practice 2002;15(5):401‐6. [PubMed] [Google Scholar]
Skalsky 2008
- Skalsky K, Yahav D, Bishara J, Pitlik S, Leibovici L, Paul M. Treatment of human brucellosis: systematic review and meta‐analysis of randomised controlled trials. BMJ 2008;336(7646):701‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solera 1997a
- Solera J, Martínez‐Alfaro E, Espinosa A. Recognition and optimum treatment of brucellosis. Drugs 1997;53:245‐56. [DOI] [PubMed] [Google Scholar]
Solera 2010
- Solera J. Update on brucellosis: therapeutic challenges. International Journal of Antimicrobial Agents 2010;36:s18‐s20. [DOI] [PubMed] [Google Scholar]
Solis Garcia del Pozo 2012
- Solis Garcia del Pozo J, Solera J. Systematic review and meta‐analysis of randomized clinical trials in the treatment of human brucellosis. PLoS ONE 2012;7:e32090. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 1971
- Joint FAO/WHO Expert Committee on Brucellosis. Joint FAO/WHO Expert Committee on Brucellosis [meeting held in Geneva, 29 June ‐ 6 July 1970]: fifth report. WHO Technical Report Series No. 464/FAO Agricultural Series No. 85. Geneva: World Health Organization, 1971:41‐3. [PubMed] [Google Scholar]
WHO 1986
- Joint FAO/WHO Expert Committee on Brucellosis. Joint FAO/WHO Expert Committee on Brucellosis [meeting held in Geneva from 12 to 19 November 1985]: sixth report. WHO Technical Report Series No. 740. Geneva: World Health Organization, 1986:56‐7. [Google Scholar]
WHO 2006
- Corbel MJ. Brucellosis in humans and animals [WHO/CDS/EPR/2006.7]. Geneva: World Health Organization, 2006:36‐41. [Google Scholar]


