Abstract
Background
Cerebral malaria is associated with swelling of the brain. Corticosteroid drugs could reduce the harmful effects of this swelling, but they could also suppress host immunity to infection.
Objectives
To assess the effects of corticosteroid drugs in patients with cerebral malaria on death, life‐threatening complications, and residual disability in survivors.
Search methods
In March 2008, we searched the Cochrane Infectious Disease Group Specialized Register, CENTRAL (The Cochrane Library 2008, Issue 1), MEDLINE, EMBASE, LILACS, and mRCT. We also checked reference lists.
Selection criteria
Randomized controlled trials comparing corticosteroids with no corticosteroids in addition to otherwise identical treatments for patients with cerebral malaria.
Data collection and analysis
Both authors independently assessed trial eligibility and risk of bias (methodological quality), and extracted data. Outcomes sought included death, death with life‐threatening complications, other complications, and disability.
Main results
Two trials with 143 participants met the inclusion criteria. There were 30 deaths in the two trials, distributed evenly between the corticosteroid and control groups (risk ratio 0.89; 95% confidence interval 0.48 to 1.68; 143 participants). Clinical complications were reported as the number of events in each trial arm and did not exclude complications occurring in fatalities. This made it difficult to interpret the reports of significantly more episodes of gastrointestinal bleeding and seizures in the corticosteroid group. Neither trial examined disability.
Authors' conclusions
There is currently no evidence of benefit from corticosteroids, but the small number of participants means it is difficult to exclude an effect on death in either direction. Data on clinical complications are difficult to assess.
23 April 2019
No update planned
Other
This is not a current question.
Plain language summary
Corticosteroids for treating cerebral malaria
Cerebral malaria is a severe form of the disease that can induce convulsions and coma; about 15% to 50% of patients with cerebral malaria will die, and 5% to 10% of survivors are left disabled as a result of brain damage.
In the past decades, health professionals often gave corticosteroids such as dexamethasone and hydrocortisone, as well as antimalarial drugs, to patients with cerebral malaria, with the aim of reducing the effects of swelling and inflammation in the brain.
This review assesses the effects of corticosteroid drugs given for cerebral malaria, on death, life‐threatening complications, and residual disability in survivors.
The authors included two trials with a total of 143 patients (both adults and children). There were no significant differences in the number of deaths between the corticosteroid and control groups, and data on clinical complications were difficult to assess. Neither trial examined disability.
Background
Many people continue to die from malaria, with deaths occurring in residents of malarious areas, as well as travellers visiting them. Cerebral malaria is a common severe form of the disease. These patients convulse, lapse into coma, and about 15% to 50% die (Warrell 1982; White 1982; Looareesuwan 1983; Philips 1985). An estimated 5% to 10% of survivors are left disabled as a result of brain damage caused by the infection (Valecha 1994; Warrell 1997). Interventions that improve outcomes in this condition are likely to have important health benefits.
From 1967 to 1982, health professionals often gave steroids (corticosteroids) such as dexamethasone and hydrocortisone as well as antimalarial drugs to patients with cerebral malaria (Daroff 1967; Oriscello 1968; Woodruff 1968; Shmitskamp 1971). The rationale for this was that corticosteroids would reduce the effects of oedema and inflammation in the brain, and consequently reduce mortality and long‐term disability.
Because of uncertainty about the effects of corticosteroids in cerebral malaria, we decided to systematically review the relevant trials testing corticosteroids in cerebral malaria.
Objectives
To assess the effects of corticosteroid drugs in patients with cerebral malaria on death, life‐threatening complications, and residual disability in survivors.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials. Trials of alternate allocation were excluded.
Types of participants
Children and adults with clinically diagnosed cerebral malaria, defined as a positive blood slide, disturbed mental status, with other causes excluded.
Types of interventions
Intervention
Corticosteroid drugs (hydrocortisone, dexamethasone, prednisolone) given in addition to specific malarial chemotherapy.
Control
Antimalarial chemotherapy identical to the intervention group.
Types of outcome measures
Main outcomes
Death.
Death or life‐threatening complication (such as pneumonia, septicaemia, pulmonary oedema, and seizures).
Death or any complication.
Death or disabling deficits (ie interfering with daily living) six months or more post‐randomization.
Other outcomes
Time to death.
Time to recover full consciousness.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published or unpublished, in press, or in progress).
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Disease Group Specialized Register (March 2008); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2008, Issue 1); MEDLINE (March 2008); EMBASE (March 2008); and LILACS (March 2008). We also searched the metaRegister of Controlled Trials (mRCT) using 'malaria' and 'steroid* or corticosteroid* or cortisone or hydrocortisone or prednisone or prednisolone' as search terms (March 2008).
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
The authors independently applied the inclusion criteria and resolved any disagreements by discussion. From each included trial, we extracted the method of treatment allocation, entry criteria, and outcome measures. We assessed each trial's risk of bias (methodological quality) using standard Cochrane Infectious Diseases Group methods. We also assessed completeness of follow up and sought to determine whether the trial authors had conducted an intention‐to‐treat analysis.
We analysed dichotomous data using the risk ratio (RR) and presented each result with a 95% confidence interval (CI).
Results
Description of studies
We identified four potentially relevant studies: we excluded two (Characteristics of excluded studies) and included two (Characteristics of included studies).
The two included trials had a total of 143 patients, both adults and children. One trial was conducted in Thailand (Warrell 1982) and the other in Indonesia (Hoffman 1988). We attempted to follow up an unpublished trial, which was conducted in Vietnam in the 1970s, but we have not been able to locate these data.
The trials appeared to take care to exclude other causes of disturbed consciousness in the patients. All patients had a blood slide showing asexual forms of the malaria parasite.
Both trials used the steroid dexamethasone (a corticosteroid). It was given in both trials as an intravenous injection, so there was no increased fluid load in the corticosteroid group. The doses given over the first 48 hours were different however; Warrell 1982 used an intended total dose of 2 mg/kg, while Hoffman 1988 used 11.4 mg/kg.
Both trials treated the corticosteroid and control groups with the same antimalarial regimen, which comprised of intravenous infusions of quinine given every eight hours. Hoffman 1988 used a loading dose of quinine.
The two trials appeared balanced, apart from coma. In Warrell 1982, 5/50 patients were unrousable in the corticosteroid group and 3/50 in the control group. In Hoffman 1988, 16/19 patients were comatose in the corticosteroid group compared to 12/19 in the control group. However, these differences are slight and not statistically significant.
Risk of bias in included studies
Warrell 1982 did not describe the quality of concealment well. The effectiveness of blinding of the clinicians assessing the patient outcomes was not described. Intention‐to‐treat analysis for death was possible in Hoffman 1988. For the analysis of complications, the authors withdrew five patients (two from the experimental group and three from the control group; all died within six hours after randomization) from their primary analysis, and data regarding complications in these patients were not provided. In these circumstances, we used the data available.
In both trials, the trial authors reported only the number of complications (not the number of patients with complications). As patients may have more than one complication, each complication has to be analysed independently. Thus no combined analysis of 'all complications' was possible with the data available. Also, we were careful in evaluating statistically significant differences between groups for these multiple associated outcomes as they could arise by chance alone.
Effects of interventions
Death
The meta‐analysis showed that deaths were distributed evenly between the corticosteroid and control groups in both trials (RR 0.89; 95% CI 0.48 to 1.68; 143 participants, 2 trials, Analysis 1.1).
1.1. Analysis.
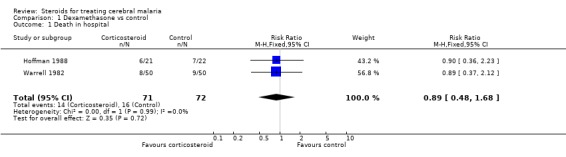
Comparison 1 Dexamethasone vs control, Outcome 1 Death in hospital.
Illness
The available data did not differentiate between survivors and fatalities, which meant it was not possible to identify the number of patients with life‐threatening morbidity who survived. The data also simply recorded the number of times the conditions occurred in each group; this means a particularly sick patient could have contributed to all six of the complication categories. The only analysis of complications by patient was in Warrell 1982, which reported "any complications" by corticosteroid and control group. This trial showed a significantly higher number of complications in the corticosteroid group (RR 1.59, 95% CI 1.00, 2.52; 100 participants, Analysis 1.2).
1.2. Analysis.
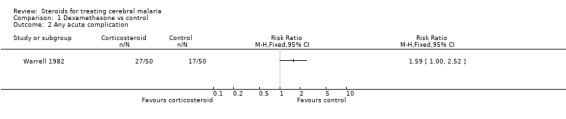
Comparison 1 Dexamethasone vs control, Outcome 2 Any acute complication.
As one patient can contribute to all outcomes, and these outcomes are closely associated, caution should be exercised in inferring any trends relating to different outcomes.
In relation to pneumonia, Warrell 1982 showed more morbid reports of pneumonia in the corticosteroid group (100 participants Analysis 1.3); however, there were fewer morbid reports of pulmonary oedema in the corticosteroid group (100 participants, Analysis 1.4). This contrasts with the smaller Hoffman 1988 trial, which recorded no cases of pneumonia in the corticosteroid group (43 participants, Analysis 1.3). Given this heterogeneity, it does not make sense to quote a RR; however, it is worth noting the confidence intervals around the estimate are wide and do not show any statistical significance.
1.3. Analysis.
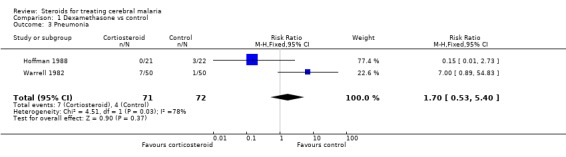
Comparison 1 Dexamethasone vs control, Outcome 3 Pneumonia.
1.4. Analysis.
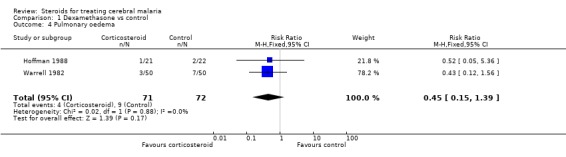
Comparison 1 Dexamethasone vs control, Outcome 4 Pulmonary oedema.
The meta‐analyses for pulmonary oedema (Analysis 1.4), urinary tract infection (Analysis 1.5), and bacteraemia/septicaemia (Analysis 1.6) do not show any significant difference; each meta‐analysis involved both trials and 143 participants.
1.5. Analysis.
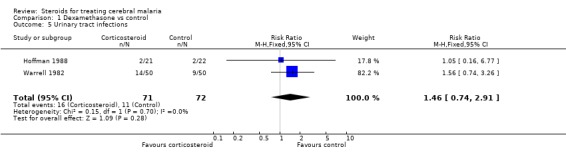
Comparison 1 Dexamethasone vs control, Outcome 5 Urinary tract infections.
1.6. Analysis.
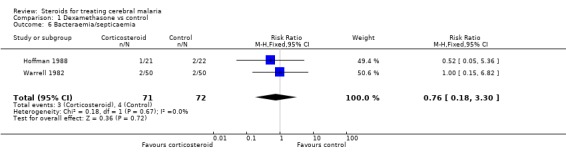
Comparison 1 Dexamethasone vs control, Outcome 6 Bacteraemia/septicaemia.
Gastrointestinal bleeding was more common in the corticosteroid group, and this was consistent in both trials (RR 8.17, 95% CI 1.05 to 63.6; 143 participants, Analysis 1.7).
1.7. Analysis.
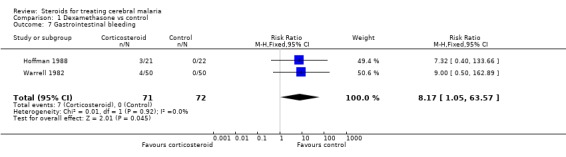
Comparison 1 Dexamethasone vs control, Outcome 7 Gastrointestinal bleeding.
Seizures were also more common in the corticosteroid group (RR 3.32, 95% CI 1.05 to 10.47; 143 participants, 2 trials, Analysis 1.8).
1.8. Analysis.
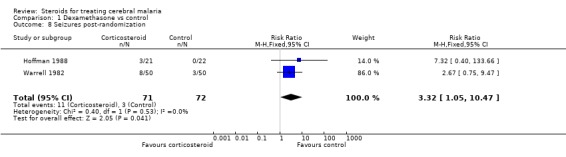
Comparison 1 Dexamethasone vs control, Outcome 8 Seizures post‐randomization.
Hypoglycaemia was reported only in Hoffman 1988, whereas bed sores and psychoses were reported only Warrell 1982; neither showed a significant difference between the two groups.
Survivors and fatalities: time to critical incident
In survivors, both trials measured mean or median time between the start of treatment and coma resolution. The mean time between start of treatment and coma resolution in the dexamethasone versus placebo group was 83.4 hours (standard deviation (SD) 49.3) versus 80.0 hours (SD 59.4) in Hoffman 1988 (not statistically significant). In Warrell 1982, the times were approximately 76 hours in the corticosteroid group and 57 hours in the control.
In fatalities, the mean time between start of treatment and death in the Hoffman 1988 was 47.7 hours (SD 43.9) in the corticosteroid group compared with 61.5 hours (SD 89.5) in the control group. In Warrell 1982, times were 53.3 hours (standard error (SE) 10.1) in the corticosteroid group compared with 25.1 hours (SE 3.6) in the control group.
Disability
Neither trial followed up beyond discharge from hospital, so we could not examine effects of corticosteroids on residual deficits at three to six months.
Discussion
We identified two trials that compared corticosteroids plus antimalarial treatment with antimalarial treatment alone (Warrell 1982; Hoffman 1988). Both were conducted in Asia, in Thailand (Warrell 1982) and Indonesia (Hoffman 1988)), and both enrolled small numbers of participants.
This review does not demonstrate an effect of corticosteroids on death from cerebral malaria, but the number of included participants is small. The review therefore cannot exclude potentially important effect of corticosteroids either increasing or decreasing the risk of death.
There were problems with interpreting the data on complications. Warrell 1982 reported an aggregate statistic of all complications, but this figure combined conditions irrespective of their severity; for example, urinary tract infection was combined with frank pulmonary oedema. In our protocol we intended to separate obviously life‐threatening complications from other complications, but this was not possible. The main difficulty was that morbidity was counted as episodes, so a single patient with multiple conditions could inflate morbidity counts across several categories, particularly the patients who died.
Each trial reported on seven to eight complications, all of which make clinical sense. Nevertheless, we would anticipate some statistically significant differences occurring by chance with this number of comparisons, so significance in one or two variables could occur by chance.
Pneumonia occurred more frequently in Warrell 1982, and the difference was statistically significant. The difference is also likely to convince clinicians (seven in the dexamethasone group, and one in the control group). However, Hoffman 1988 did not show a similar pattern. In fact, there were more cases of pneumonia in the control group, and, taken together, there was no significant difference in the occurrence of this condition. This therefore creates uncertainty about the extent to which the results from Warrell 1982 can be generalized in relation to pneumonia, and the cause of this heterogeneity between trials is not clear. It could be differences in diagnostic patterns between the two sites or true differences in pathology in the two settings.
There were more cases of gastrointestinal bleeding in the dexamethasone group, and no cases were reported in the control group, an effect known to be associated with corticosteroid administration. This result seems unlikely to have arisen by chance. It could be that there was differential reporting between the two groups in unblinded observations, as gastrointestinal bleeding is commonly associated with corticosteroids. It does seem unusual that in 72 severely ill people in the control group there was no gastrointestinal bleeding reported, which could suggest this complication is rare in severe malaria, or that there was differential reporting between the two trial arms. However, gastrointestinal bleeding was consistent between the trials, statistically significant, and thus the strongest evidence of a harmful effect.
The doses of corticosteroids varied between the trials, and this may have influenced the rate of complications. However, there was no striking association between the high‐dose regimen in Hoffman 1988 and the complication rate.
Outcomes measured were in the short term, and no data were available as to whether corticosteroids affect the chance of disability in survivors.
Time to critical events
We extracted data on time between the start of treatment and death. Specialists have argued that if corticosteroids prolong this interval, this provides evidence that the drug has potential to reduce the mortality rate. The reason for this is that a prolonged survival time gives specific antimalarial drugs longer time to kill the parasites, thereby enhancing survival. To examine this in more detail, the data need to be examined using survival analysis. However, the analysis will still only contribute to generating a hypothesis (that corticosteroids might be effective in preventing death) and not provide any evidence testing the hypothesis.
Another intermediate outcome that has caused controversy is the time between the start of treatment and coma recovery in survivors. A longer time may reflect more severe brain disease and a greater chance of long term damage. Warrell 1982 showed this to be prolonged in the corticosteroid group, and this is given as evidence of dexamethasone being harmful. Again, time‐to‐event calculations using a survival analysis may be helpful. Any further evidence would contribute to generating a hypothesis, not testing it. The latter requires follow‐up of patients at three months or more to seek residual deficits.
Any interpretation of these data on time to event between survivors and fatalities should be made with care as they are a subgroup analysis.
Authors' conclusions
Implications for practice.
There is insufficient evidence to support the use of corticosteroids in routine practice.
Implications for research.
The two included trials did not have sufficient power to exclude a clinically worthwhile benefit. The data on harm suggest that corticosteroids do more harm than good, but problems with the data make it difficult to be definite about this.
Some evidence of potential benefit of corticosteroids is needed to justify a trial. Without a good clinical rationale, it seems hard to justify a trial given the evidence of adverse effects to date, despite problems with potential bias in the data. Also, there are now more adjunctive treatments in severe malaria, which may limit the potential impact of corticosteroids. However, emerging evidence of raised intracranial pressure in children with cerebral malaria could support arguments of potential benefit.
What's new
| Date | Event | Description |
|---|---|---|
| 19 March 2008 | New search has been performed | Search updated, and no new studies were identified. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 11 November 2008 | Amended | Converted to new review format with minor editing. Summary statistics changed to risk ratios. |
Acknowledgements
Shell Petroleum, who provided a Research Fellowship for Dr Prasad.
This document is an output from a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Appendices
Appendix 1. Search methods: search strategies for databases
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | malaria | MALARIA, CEREBRAL | MALARIA, CEREBRAL | BRAIN MALARIA | malaria |
| 2 | (brain OR cerebr* OR mening*) | malaria | malaria | malaria | (brain OR cerebr$ OR mening$) |
| 3 | 1 AND 2 | (brain OR cerebr* OR mening*) | (brain OR cerebr* OR mening*) | (brain OR cerebr$ OR mening$) | 1 AND 2 |
| 4 | steroid* | 2 AND 3 | 2 AND 3 | 2 AND 3 | steroid$ |
| 5 | corticosteroid* | 1 OR 4 | 1 OR 4 | 1 OR 4 | corticosteroid$ |
| 6 | dexamethasone | steroid* | steroid* | steroid$ | hydrocortisone |
| 7 | hydrocortisone | corticosteroid* | STEROIDS | STEROIDS | dexamethasone |
| 8 | prednisolone | glucocorticoid* | corticosteroid* | corticosteroid$ | prednisolone |
| 9 | 4‐8/OR | hydrocortisone | glucocorticoid* | glucocorticoid$ | 4‐8/OR |
| 10 | 3 AND 9 | prednisolone | hydrocortisone | hydrocortisone | 3 AND 9 |
| 11 | — | dexamethasone | dexamethasone | dexamethasone | — |
| 12 | — | 6‐11/OR | prednisolone | prednisolone | — |
| 13 | — | 5 AND 12 | prednisone | methylprednisone | — |
| 14 | — | — | methylprednisone | 6‐13/OR | — |
| 15 | — | — | 6‐14/OR | 5 AND 14 | — |
| 16 | — | — | 5 AND 15 | Limit 15 to Humans | — |
| 17 | — | — | Limit 16 to Human | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Data and analyses
Comparison 1. Dexamethasone vs control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death in hospital | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.48, 1.68] |
| 2 Any acute complication | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pneumonia | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.53, 5.40] |
| 4 Pulmonary oedema | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.15, 1.39] |
| 5 Urinary tract infections | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.74, 2.91] |
| 6 Bacteraemia/septicaemia | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.18, 3.30] |
| 7 Gastrointestinal bleeding | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.17 [1.05, 63.57] |
| 8 Seizures post‐randomization | 2 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.32 [1.05, 10.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hoffman 1988.
| Methods | Randomized boxes, with allocation concealment | |
| Participants | Age: 1.5 to 42 years Gender: both sexes Excluded: patients with a history of seizure or receiving a central nervous system depressant drug Length of preceding illness: 6.2 days corticosteroid group; 4.8 days control group |
|
| Interventions | 1. Dexamethasone (3 mg doses 8 hourly for 48 hours) plus quinine (20 mg/kg loading, 10 mg/kg 8 hourly intravenous infusion) 2. Placebo plus quinine Both groups also received tracheal suction, cooling, aminopyrine, and seizure and hypoglycaemia management |
|
| Outcomes | 1. Death in hospital 2. Time to become rousable and time to regain a normal level of consciousness 3. Duration of fever 4. Infections, gastrointestinal bleeding, pulmonary oedema, and convulsions 5. No follow up after discharge | |
| Notes | Location: Indonesia over 3 years 1 patient withdrawn and not included in the analysis |
|
Warrell 1982.
| Methods | Randomization in sequential pairs, but allocation concealment unclear Double blind using placebo Intention‐to‐treat analysis |
|
| Participants | Age: 6 to 70 years Gender: both sexes; 7 patients were pregnant Inclusion criteria: coma with presence of asexual forms of Plasmodium falciparum in blood and with no other apparent cause of coma Exclusion criteria: coma following a generalized convulsion unless it lasted for > 6 hours; presence of a second disease (eg hepatitis B) that could not be regarded as a complication of cerebral malaria; history of recently received corticosteroid treatment Mean duration (days) of illness: 4 in each group |
|
| Interventions | 1. Quinine plus dexamethasone; 50 participants 2. Quinine plus placebo; 50 participants Quinine initially intravenous (oral as soon as patients could swallow), in 8 hourly dose of 12 mg/kg of the salt to children aged 6 to 14 years and 10 mg/kg to adults diluted in 250 to 500 mL of 5% dextrose infuses over 4 hours Dexamethasone intravenous for children 0.6 mg/kg at the start followed by 7 doses of 0.2 mg/kg at 6‐hour intervals; for adults 0.5 mg/kg at the start followed by 7 doses of 10 mg each (total duration of treatment 48 hours); total dose in 48 hours = 2 mg/kg for children |
|
| Outcomes | 1. Death in hospital 2. Neurological sequelae at discharge (between 10 to 15 days after admission; no follow up after discharge) 3. Time to become rousable and time to regain full consciousness 4. Duration of fever 5. Complications viz. infections, gastrointestinal bleeding, pulmonary oedema, psychosis and convulsions | |
| Notes | Location: Thailand | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Rampengan 1984 | Case series of 20 patients |
| Reid 1972 | Randomized comparison between dexamethasone (4 participants) with a defibrinating agent (Ancrod) (3 participants); 2 participants "received neither and acted as controls", implying they were not randomized |
Differences between protocol and review
1999, Issue 3: There were too few trials for some analyses. We intended to measure the number of participants developing single or multiple complications. As direct comparison of morbid episodes alone can be confounded by different death rates in the two groups, we intended to analyse death and life‐threatening conditions combined together. For time‐to‐event analysis (time to death or time to recover full consciousness), we intended to use survival analysis where possible. For analysis of time to recover consciousness, the participants who died were to be treated as 'unconscious' till the last time point in the survival curve.
Contributions of authors
Both authors contributed to the preparation of the review.
Sources of support
Internal sources
Neurosciences Centre and Clinical Epidemiology Unit, AIIMS, New Delhi, India.
External sources
Department for International Development, UK.
Shell Petroleum Ltd, UK.
European Commission (Development Directorate XII), Belgium.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Hoffman 1988 {published data only}
- Hoffman SL, Rustama D, Punjabi NH, Surampaet B, Sanjaya B, Dimpudus AJ, et al. High‐dose dexamethasone in quinine‐treated patients with cerebral malaria: a double‐blind, placebo‐controlled trial. Journal of Infectious Diseases 1988;158(2):325‐31. [DOI] [PubMed] [Google Scholar]
Warrell 1982 {published data only}
- Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, et al. Dexamethasone proves deleterious in cerebral malaria. A double‐blind trial in 100 comatose patients. New England Journal of Medicine 1982;306(6):31‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Rampengan 1984 {published data only}
- Rampengan TH, Salendu‐Warouw S, Wantania JM, Munir M. Corticosteroid in the treatment of cerebral malaria. Paediatrica Indonesia 1984;24(1‐2):11‐6. [PubMed] [Google Scholar]
Reid 1972 {published data only}
- Reid HA, Nkrumah FK. Fibrin‐degradation products in cerebral malaria. Lancet 1972;1(7744):218‐21. [DOI] [PubMed] [Google Scholar]
Additional references
Daroff 1967
- Daroff RB, Deller JJ Jr, Kastl AJ Jr, Blocker W Jr. Cerebral malaria. JAMA 1967;202(8):679‐82. [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Looareesuwan 1983
- Looareesuwan S, Warrell DA, White NJ, Sutharasamai P, Chanthavanich P, Sundaravej K, et al. Do patients with cerebral malaria have cerebral oedema? A computed tomography study. Lancet 1983;1(8322):434‐7. [DOI] [PubMed] [Google Scholar]
Oriscello 1968
- Oriscello RG. Cerebral malaria. British Medical Journal 1968;3(5618):617‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Philips 1985
- Philips RE, Warrell DA, White NJ, Looareesuwan S, Karbwang J. Intravenous quinidine for the treatment of severe falciparum malaria. Clinical and pharmacokinetic studies. New England Journal of Medicine 1985;312(20):1273‐8. [DOI] [PubMed] [Google Scholar]
Shmitskamp 1971
- Shmitskamp H, Wolthuis FH. New concepts in treatment of malignant tertian malaria and cerebral involvement. British Medical Journal 1971;1(5751):714‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valecha 1994
- Valecha N, Srivastava A, Sharma VP. Rational approach to the treatment of malaria. National Medical Journal of India 1994;7(6):281‐7. [PubMed] [Google Scholar]
Warrell 1997
- Warrell DA. Cerebral malaria: clinical features, pathophysiology and treatment. Annals of Tropical Medicine and Parasitology 1997;91(7):875‐84. [DOI] [PubMed] [Google Scholar]
White 1982
- White NJ, Looareesuwan S, Warrell DA, Warrell MJ, Bunnag D, Harinasuta T. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. American Journal of Medicine 1982;73(4):564‐72. [DOI] [PubMed] [Google Scholar]
Woodruff 1968
- Woodruff AW, Dickinson CJ. Use of dexamethasone in cerebral malaria. British Journal of Medicine 1968;3(5609):31‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]


