Abstract
Hydrolysis of phosphatidylcholine to choline was found to be catalysed by phospholipase D enzymes from diverse members of the gut microbiota, revealing a mechanism by which commensals obtain choline for subsequent production of disease-associated trimethylamine.
It is increasingly apparent that metabolites generated by commensal microorganisms in the gut have profound impacts on human health. However, a key challenge remains in our functional understanding of the microbiome, which is to achieve a detailed mechanistic insight into how individual microorganisms contribute to specific biochemical conversions and the subsequent impact on host physiology. In this issue of Nature Microbiology, Chittim et al.1 reveal a mechanism by which microorganisms in the human gut hydrolyse phosphatidylcholine (PC) to release the essential nutrient choline for downstream metabolism. Previous work has shown that gut microorganisms can anaerobically convert choline to trimethylamine (TMA), which is further metabolized by the host to trimethylamine N-oxide (TMAO). TMAO has been implicated in a number of adverse host pathologies, such as cardiovascular disease2. In the current study, the authors report that several microorganisms have phospholipase D (PLD) enzymes that produce choline from PC. Interestingly, they also demonstrate that other microorganisms can influence TMA production through PLD activity, even though they themselves cannot convert choline to TMA. This has several important implications for elucidating and translating the genetic potential of choline metabolism to actual biochemical activity. For example, definition of the individual roles of microorganisms for PC and choline metabolism, the interplay between microorganisms in metabolite transformations, and finally the differences between PLDs from pathogenic and commensal bacteria, which is suggestive of a therapeutic opportunity for treating TMA-induced pathologies via the inhibition of commensal PLDs.
PC is an abundant phospholipid and the primary form of choline in the human body and diet. Previous research by Tang et al.3 revealed an association between elevated levels of PC and cardiovascular risk due to TMAO production. Whether host or gut microbiota enzymes were involved in PC conversion to choline was unclear until the new study by Chittim et al.1 (Fig. 1). The authors initially evaluated a panel of 15 gut microorganisms for choline formation from diverse phospholipids, including PC. They found that the initial release of choline, followed by TMA production, occurs with microorganisms that have the cut gene cluster responsible for anaerobic TMA formation from choline4. This result showed that diverse phospholipids could supply choline to the enzymes in the cut gene cluster, but importantly, was suggestive that an unanticipated ability to convert PC was at play. A bioinformatic analysis using two characterized PLDs with only 12% sequence identity revealed a putative PLD-encoding gene in all 9 bacterial strains that were capable of transforming PC to TMA; no such gene was found in those microorganisms unable to convert PC. To confirm their computational analysis, a pld knockout construct was prepared in the human gut isolate, Escherichia coli MS 200-1. The knockout strain was incapable of PC conversion and subsequent TMA formation, but when the pld gene was placed back into the knockout strain, metabolic activity and TMA formation was restored. Although this set of experiments may be considered somewhat routine for single organism or human cell metabolism studies at face value, this ‘dissection’ of a critical metabolic pathway has only occurred minimally in gut microbiome studies, yet it affords so much power in increasing our mechanistic understanding and identifying viable opportunities for therapeutic intervention in the gut.
Fig. 1|. Gut commensal organisms play an important role in the conversion of choline to disease-associated TMA.
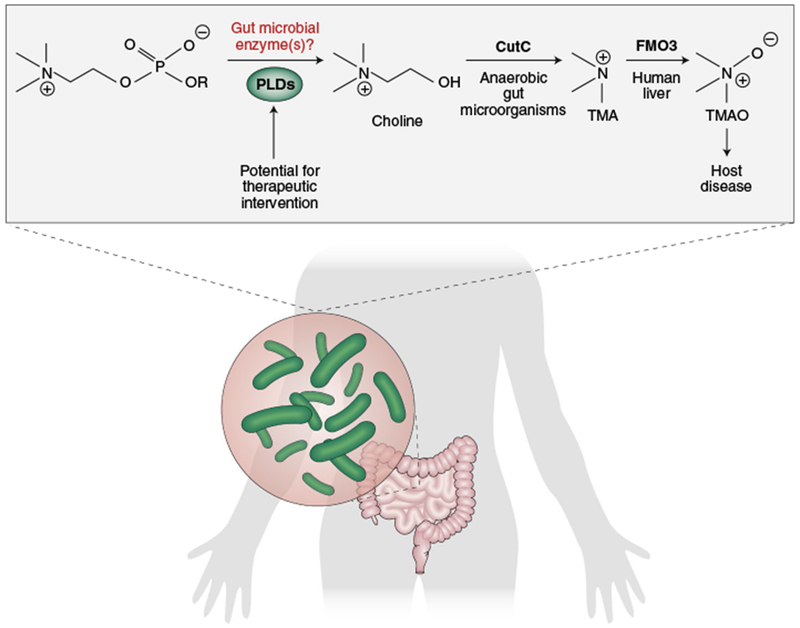
By narrowing in on the biochemical activity of individual gut microorganisms, it was revealed that commensals can convert PC to choline using PLD enzymes. This process occurs in microorganisms that can (that is, those harbouring the CutC gene) and cannot further convert choline to TMA, which is then converted to TMAO by the host via FMO3 (flavin-containing monooxygenase 3). Gut commensal PLD enzymes differ from pathogenic PLDs and host PLDs, and thus may prove to be a viable therapeutic target.
To identify opportunities for therapeutic intervention when evaluating microbial enzyme targets, one must determine the catalytic features of the enzyme responsible for activity and the relationship of the enzyme to other bacterial and human enzymes, and in the case of the gut microbiome, how broad their distribution is throughout diverse microbiota. Chittim et al. found a conserved amino acid residue in PLD-containing bacteria that probably plays a role in interacting with PC and influencing substrate specificity5. When they tested human PLD inhibitors against the E. coli PLD, there was no inhibition; this is exciting because it suggests a real therapeutic opportunity to target commensal PLDs without impacting host PLDs. Finally, PC-metabolizing PLDs were found widely throughout gut microbiota, and are distinct from pathogen PLDs. All of these components taken together suggest that much more research is needed to understand the role of TMA production in the human gut, how it impacts host disease, and how gut microorganism PLDs may be targeted as a potential therapeutic treatment.
As gut microbiome research has rapidly increased over the last several years, there is a desperate need to move from genome-inspired inference of biochemical function and mechanisms, to direct characterization and quantification6. Genomes and transcriptomes provide an initial view into the metabolic potential of gut microbiota, but they fail to reveal how biochemical activity is influenced by various routes of inhibition, protein modifications, host–microorganism interactions and localization, and interactions between the microorganisms themselves, among other events7. Chittim et al. reveal the importance of microbial interactions in TMA production. They unexpectedly found PLDs in organisms that cannot metabolize choline to TMA, suggesting that these microorganisms are providing choline to choline-utilizers within the community. Undoubtedly, future research will continue to show that factors beyond expression are critical to the biochemistry of the gut microbiome.
To enable a functional understanding of these gut commensals in host physiology and disease, novel tools, technologies and approaches capable of measuring molecular-scale gut microbial function are required, together with a coupling between the identification of the responsible microorganisms and enzymes8,9. This is an excellent time for researchers from diverse backgrounds to bring mechanisms in the gut microbiome to light. The Chittim et al. study excellently illuminates the value of biochemical studies in the gut microbiome, and will probably propel PLDs in the gut microbiome forward as an avenue for therapeutic intervention of host diseases associated with TMA production.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Chittim LC, Martinez del Campo A & Balskus EP Nat. Microbiol 10.1038/s41564-018-0294-4 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Wang Z et al. Nature 472, 57–63 (2012). [Google Scholar]
- 3.Tang WHW et al. N. Engl. J. Med 368, 1575–1584 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romano KA, Vivas EI, Amador-Noguez D & Rey FE mBio 6, e02481–14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bodea S, Funk MA, Balskus EP & Drennan CL Cell Chem. Biol. 23, 1206–1216 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koppel N & Balskus EP Cell Chem. Biol. 23, 18–30 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Tropini C, Earle KA, Huang KC & Sonnenburg JL Cell Host Microbe 21, 433–442 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bitteen JS et al. ACS Nano 10, 6–37 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Whidbey C & Wright AT Curr. Top. Microbiol. Immunol. http://doi.org/cw6n (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


