Dietary fats take a circuitous route from the intestine to the bloodstream, where they serve as nutrients or are stored in adipose tissue. Fats processed in the digestive tract are packaged by the intestinal epithelium into tiny lipid-protein particles called chylomicrons. Chylomicrons are taken up by lymphatic channels (lacteals) within villi that line the small intestine and are transported by lymphatic vessels to the blood circulation. How chylomicrons enter lacteals has long been a mystery, as the particles are too large to cross the endothelial cells that line lymphatics. Moreover, scattered openings observed in lacteals seemed too sparse to explain chylomicron entry, and evidence for vesicular transport was limited (1, 2). The discovery of specialized, discontinuous button-like junctions between lacteal endothelial cells raised another possibility (3). Button junctions have open regions and closed regions and are strikingly different from zipper junctions that tightly seal endothelial cells in blood vessels and collecting lymphatics. On page 599 of this issue, Zhang et al. (4) show that chylomicron entry is dependent on button junctions. Importantly, experimental manipulations that led to transformation of button junctions into zippers prevented chylomicron uptake and protected mice from developing obesity while on a high-fat diet.
Intestinal lymphatic vessels not only transport chylomicrons but are also routes for the recirculation of fluid into blood. According to the conventional view, fluid flows into lacteals along hydrostatic pressure gradients when their endothelial cells are pulled apart by interstitial forces (5). The extent of separation of the cells at openings is governed by forces within villi transmitted to the lacteal wall by anchoring filaments. Intake of dietary fat promotes chylomicron production and water absorption by the intestinal epithelium that lead to higher interstitial pressure, lymph flow, and chylomicron transport through lacteals (5). Lacteal contractility, which is driven by longitudinal smooth muscle cells in intestinal villi, can propel fluid and cells in lymph at velocities up to 150 µm/s into downstream lymphatics en route to the bloodstream (6).
Zhang et al. sought to determine the contributions of the endothelial cell receptors, vascular endothelial growth factor receptor–1 (VEGFR1) and neuropilin-1 (NRP1), to the regulation of fat transport. By deleting the corresponding genes, Vegfr1 (also known as Flt1) and Nrp1, in endothelial cells of mice, they unexpectedly found that button junctions in lacteals transformed into zipper junctions and that this conversion was accompanied by resistance to obesity when mice were given a high-fat diet (see the figure).
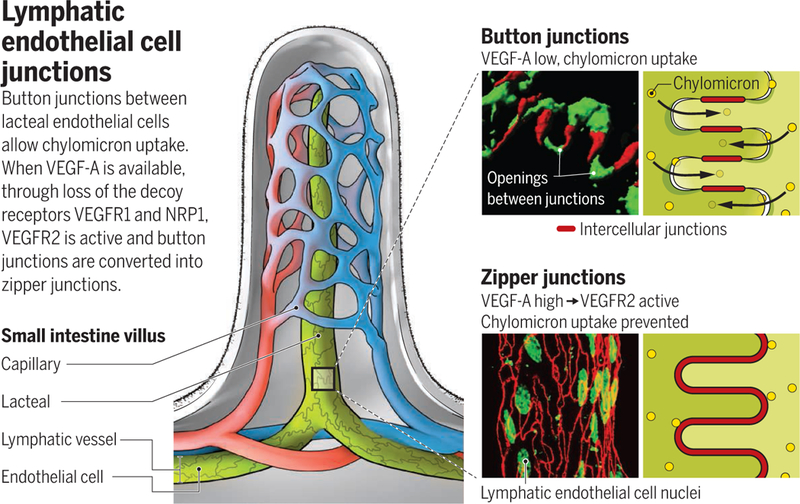
This work was initiated to resolve a controversy over the involvement of VEGFR1 and NRP1 in fat transport in obesity and type 2 diabetes. According to one concept, VEGF-B, a ligand of VEGFR1 and NRP1, increases expression of fatty acid transporters in blood vessels through binding and activating VEGFR1 and NRP1, which consequently promote fat transport into tissues (7). A contrasting interpretation is that VEGF-B improves vascular and metabolic health by displacing another ligand, VEGF-A, which can then activate VEGFR2 signaling (8). VEGFR2 signaling is essential for maintaining vascular function, which is compromised in metabolic syndrome and type 2 diabetes. In the absence of VEGF-B, VEGFR1 and NRP1 can act as decoy receptors that compete for VEGF-A binding to VEGFR2. In support of this idea, Zhang et al. provide evidence that VEGFR1 and NRP1 bind VEGF-A and prevent excessive VEGFR2 signaling that would otherwise promote button-to-zipper transformation and thereby reduce chylomicron uptake into lacteals.
Zhang et al. add to the evidence that endothelial cell junctions are dynamic structures. Button junction plasticity is evident during development: Initial lymphatics have zipper junctions until late gestation, when they change into button junctions through a process that requires the growth factor angiopoietin-2 (ANGPT2) (9, 10). Alternatively, button junctions change into zippers in growing lymphatics in inflamed tissues (9). This transformation is reversible by treatment with the corticosteroid dexamethasone (9). Further evidence of plasticity is provided by the dependence of lacteal function on VEGF-C, a ligand for VEGFR3, and on delta-like ligand 4 (DLL4), a NOTCH ligand. Mice lacking Vegfc or Dll4 have reduced dietary lipid absorption and are resistant to obesity (11, 12). Genetic inactivation of Dll4 in lymphatic endothelial cells results in transformation of button junctions into zippers (12). In addition to the gatekeeper function of cellular junctions, chylomicron entry into lacteals is controlled by the expression of the transcription factor pleomorphic adenoma gene-like 2 (PLAGL2) in intestinal epithelial cells. Plagl2-deficient mice have impaired lacteal uptake of chylomicrons and die postnatally from lack of fat absorption (13).
The findings by Zhang et al. raise the question of how VEGF-A can have opposite effects on lymphatics, where it reduces permeability by promoting zipper junctions, and on blood vessels, where it increases leakage by opening normally closed zipper junctions in endothelial cells. Although the basis of this dichotomy is unclear, mechanistic insights come from studies of Rho-associated protein kinases (ROCKs). Pharmacologic inhibition of ROCKs promote zippering of endothelial cell junctions and suppress chylomicron uptake into lacteals. ROCK inhibitors also reduce leakage in blood vessels (14). In both cases, ROCK inhibitors increase the barrier function of endothelial cells by stabilizing proteins at intercellular junctions.
These advances highlight the importance of button junctions in intestinal lacteals for the uptake of dietary fat. Although button junctions were identified in lymphatics more than 10 years ago (3), the finding that the junctions are essential for chylomicron uptake by lacteals and can be genetically and pharmacologically regulated point to new strategies for management of obesity. However, understanding the mechanism and consequences of the transformation of button junctions into zippers is at an early stage. Zippering junctions in lymphatics could impair uptake of essential nutrients in chylomicrons or have adverse effects by compromising fluid drainage and immune cell trafficking. Nonetheless, the discovery that the uptake of intestinal fat is determined by the structure of junctions in lymphatic endothelial cells opens new avenues for investigating the regulation of body weight.
REFERENCES
- 1.Casley-Smith JR, J. Cell Biol 15, 259 (1962). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobbins WO 3rd, Rollins EL, J. Ultrastruct. Res 33, 29 (1970). [DOI] [PubMed] [Google Scholar]
- 3.Baluk P et al. , J. Exp. Med 204, 2349 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang F et al. , Science 361, 599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kvietys PR, Granger DN, Ann. N. Y. Acad. Sci 1207 (suppl. 1), E29 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choe K et al. , J. Clin. Invest 125, 4042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagberg C et al. , Physiology 28, 125 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robciuc MR et al. , Cell Metab 23, 712 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao L-C et al. , Am. J. Pathol 180, 2561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng W et al. , Genes Dev 28, 1592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nurmi H et al. , EMBO Mol. Med 7, 1418 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier-Latmani J et al. , J. Clin. Invest 125, 4572 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dyck F et al. , Cell Metab 6, 406 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Mikelis CM et al. , Nat. Commun 6, 6725 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]


