Abstract
Eosinophilic esophagitis (EoE) is an immune-mediated disease triggered by food antigens for which dietary elimination treatment can induce and sustain histologic remission. Our review aims to describe the state-of-the-art regarding dietary treatment of EoE, highlighting a number of areas of controversy related to dietary therapy in EoE, including novel modalities for determining food triggers, making the empiric dietary elimination process more efficient, issues of cross-contamination and “dosing” of how much food to avoid or add back, costs and effects on quality-of-life, long term efficacy, and the risk of developing immediate IgE-type reactions after initial dietary elimination. Elemental formulas, empiric elimination diets, and targeted allergy test-directed elimination diets are well-described treatments for EoE. Although elemental diets are most efficacious, their clinical use is limited by cost and the palatability of an exclusively liquid diet. While empiric elimination is less effective than elemental formula-based diets, they are more easily implemented and often sustainable. Since the comparative effectiveness of elimination diets with proton pump inhibitors and swallowed topical steroids remains unknown, there are multiple areas to address with future research.
Introduction
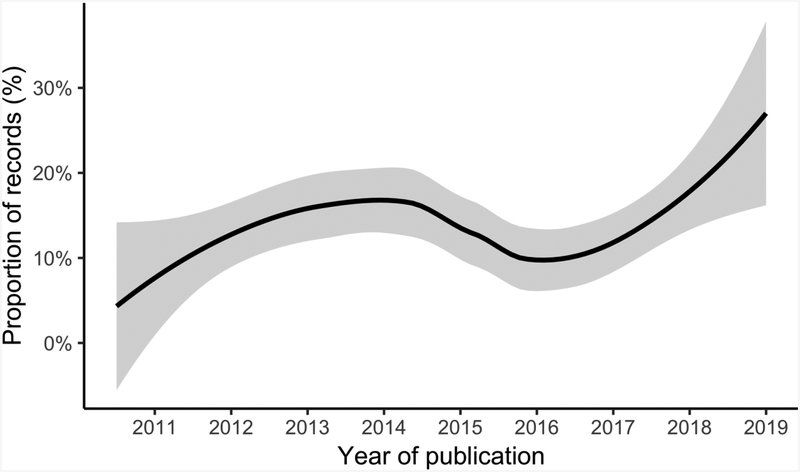
Eosinophilic esophagitis (EoE) is a chronic diseased characterized by infiltration of the esophageal mucosa with eosinophils and other inflammatory cells associated with characteristic endoscopic phenotypes and clinical symptoms. EoE has an incidence of one in 10,000 to 20,000 per person-year and a prevalence of one in 1,000 to 2,000 per person, with geographical heterogeneity and arising incidence and prevalence.1 The peak prevalence is in the fourth and fifth decades of life,2 with an inflammatory phenotype more common among younger patients and a fibrostenotic phenotype more common among older patients.3 While still a rare disease, EoE has become more common, with an outsized burden-of-disease, estimated at more than $1 billion annually in health care-related costs.4 Treatment of EoE involves targeting esophageal inflammation and, in patients with the fibrostenotic phenotype of EoE, addressing esophageal narrowing or strictures with dilation,5 with the goal of achieving clinical remission of symptoms, improvement of endoscopic findings, and histologic normalization.6–9 There are several pharmacologic treatment options for the esophageal mucosal inflammation, including the use of proton pump inhibitors, which restore mucosal integrity and block the expression of the cytokine eotaxin-3,10–13 swallowed topical steroids, ideally in an esophageal-specific preparation,14,15 and novel biologics that target proinflammatory mediators such as IL-5,16,17 IL-13,18,19 and IL-4,20 which have shown promise in initial investigations. The mainstay of non-pharmacologic therapy is dietary allergen elimination. Eliminations diets, one of the first treatments described for EoE21, helped support the hypothesized allergic pathogenesis of the disease.22 While studies of these diets were initially focused on children,23–26 newer studies have expanded the knowledge base in this age range, effectively extending the therapy to adults,27–30 as the number of studies continues to increase (Figure). The goal of this review is to evaluate the state of the art in dietary treatment of EoE while highlighting a number of areas of controversy and outlining directions for future research.
Figure.
The proportion of MEDLINE records with MESH term, “Eosinophilic Esophagitis,” that include diet therapy or elimination in their abstract as a LOESS-smoothed function of publication year.
The rationale for dietary elimination is directly related to EoE pathogenesis. EoE is thought to result from a cascade of cellular and humoral responses to a food allergen that produces a chronic inflammatory state of the esophageal epithelium,22,31 an hypothesis supported by a number of lines of evidence in both humans and murine models.32,22 Most importantly, it has been repeatedly demonstrated that removal of an offending food allergen or allergens results in clinical remission of EoE.26–28,30,33–39 There are three major approaches to dietary elimination in EoE: elemental formula, targeted elimination, and empiric elimination.40 The goal of elimination diets is not to indefinitely limit the diets of patients to highly-restricted regimens, but to induce clinicopathologic remission, after which foods can be added back sequentially in order to identify food triggers of esophageal eosinophilia and to establish a less restrictive, long-term, therapeutic diet for effective and least restrictive disease management.41
Elemental formulas consist only of free amino acids, corn syrup solids, and vegetable oils, components that are innately hypoallergenic.42,43 It was this therapy, in a landmark study by Kelly and colleagues of children with severe esophageal eosinophilia and GI symptoms despite high-dose PPI treatment, that provided the first clinical evidence that complete food allergen avoidance successfully treated EoE.21 Subsequent to this, additional studies showed that elemental formula-based diets were highly efficacious (85 – 95% response rates) for reducing inflammation and improving symptoms of EoE in patients of all ages.21,23,44–48 Despite this outstanding efficacy, there are some downsides to elemental formula including palatability, the need for administration via enteric feeding tube in some patients (either due to poor palatability or large volume required to meet nutritional needs), high costs (only a minority of states mandate insurance coverage of these formulas), and the extended and painstaking process of adding back potential food triggers after starting from a formula-only diet.
In contrast to removing all allergens, targeted elimination diets use allergy testing (skin prick testing, atopy patch testing, or serum-specific IgE) to attempt to identify foods for dietary elimination. Therefore, diets are constructed based on the allergy test results. While this approach makes intuitive sense, such diets are only modestly (35 – 55%) effective for histologic remission,33,39,49,50 since EoE is not an IgE-based disorder, which is what skin prick and serum-specific tests assess, and atopy patch testing has been reliable in only a few clinical settings.31,51 As such, there is poor agreement (13% or less) between these test results and food triggers determined clinically by dietary elimination and reintroduction.27–29 For these reasons, targeted elimination using currently clinically available allergy testing methods has fallen out of favor.
In this setting, empiric elimination diets are now commonly used. As more clinical experience with dietary elimination in EoE has accrued, the most common food triggers have been reported. This led to the development of the so-called six-food elimination diet (SFED), where dairy, wheat, egg, soy, nuts, and seafood are removed.27,28,30,33–36 This was first described as an alternative to elemental formula in children by Kagalwalla and colleagues, where rates of response were comparable to empiric elimination diets.26 Importantly, this diet was more acceptable to patients and caregivers than the elemental formula approach. Overall, SFED is effective at inducing and maintaining histologic remission of EoE in the majority of patients (66 – 78%).15,48 Once response is demonstrated with SFED, each food is sequentially added back, followed by a repeat endoscopy, approximately every 6 weeks, in order to identify the food triggers to avoid long-term.52 There are some data that suggest if patients can remain compliant long-term, these empiric elimination diets have a durable response.28,29,53
In practice, successful dietary therapy requires the patient to be engaged in the process, motivated to adhere to the elimination diet, understand how to avoid the eliminated foods and have the time and financial means to fully comply.40,54 This requires a multidisciplinary treatment approach, with gastroenterologists collaborating with allergists and registered dieticians or nutritionists. Substantial patient education is required related to label reading, avoiding cross-contact, and nutritionally adequate planning. Although it is beyond the scope of this review to discuss all of these details, a number of references are available for both providers and patients.40, 52
Controversies in dietary treatment
Despite its established contributions to the treatment of mucosal inflammation in EoE, a number of controversies exist with regard to the dietary treatment of EoE. These include the ways to identify food triggers, ways to streamline the empiric the dietary elimination process, issues of cross-contact and “dosing” of how much food allergen is required to trigger a disease flare, costs and patient burden, long-term efficacy, and risk of developing immediate IgE-type reactions after initial dietary elimination.
Determination of food triggers with allergy testing
As noted above, skin prick testing and serum-specific IgE generally have poor test characteristics, with sensitivity and positive predictive value both under 50%, with rates of agreement with food triggers of recurrent esophageal eosinophilia after food reintroduction have also been poor, 13% or less.27,28,33,55 These data suggest that if an allergy test is positive, the implicated food is most likely not causative, and if a food is causative, its allergy testing will most likely be negative. Additionally, while atopy patch testing has shown promise in some centers,39,56 it has not been extensively reproducible.33 While targeted elimination diets have been effective in some studies, the heterogeneity of results may be explained by variability in the allergy testing process, variability in the physician’s interpretation of test results, heterogeneity in the study populations, or by chance. Therefore, there is a pressing need for novel ways to simply identify allergy triggers, an area of active research.
One approach was component resolution diagnostics, which used purified or recombinant individual allergens instead of whole extracts to assess the presence of serum IgE, although this did not improve test characteristics for clinically significant food triggers in EoE.57–59 Moving away from IgE testing, the recent suggestion that IgG4 may be involved in EoE pathogenesis has been supported by the preliminary observations that food-specific IgG4 is elevated in some EoE patients and may correlate with known food triggers.51,60 This hypothesis has been recently investigated using an approach that combined in vitro measurement of lymphocyte proliferation in response to food allergens and esophageal food-specific IgG4, which was more accurate than ‘classical’ allergy tests with histologically proven food triggers used as the ‘gold standard’.61 Another novel approach is the esophageal prick test, where extracts of potential food triggers are endoscopically injected into the esophageal mucosa, either precipitating a blanching effect and functional obstruction or resulting in delayed formation of an esophageal ulcer.62 For both of these techniques, much work remains to be done before their clinical applicability is accepted in the identification of food triggers of EoE that in turn can inform highly-effective targeted elimination dietary regimens.
More efficient empiric elimination diets
While the SFED has become a preferred approach for dietary elimination, a natural area of controversy is the optimal process for elimination and reintroduction of foods. As such, there has been extensive interest in the efficacy of more efficient empiric diets. A four food elimination diet (4FED) of dairy products, wheat, egg, and either soy (in the U.S. studies) or legumes (in the Spanish studies) was effective in 54 – 64% of patients.63–65 Since elimination of four foods is less effective than elimination of six foods, but also less restrictive, further research is required to understand if this significantly lessens costs and quality-of-life burdens of therapy. In the “2–4–6” study, performed in Spain by investigators Molina-Infante and colleagues, a two food elimination diet of milk and wheat was effective in 40% of children and 44% of adults.66 Subjects who did not have histologic remission then attempted 4FED; subjects remaining without histologic remission after 4FED then attempted SFED. This “step-up” protocol was effective, with response rates to the 4FED and SFED comparable with what was previously reported. The benefit was that the process was more efficient, decreasing the endoscopic burden by 20–30%. Furthermore, they found that subjects responding only to SFED but not to 4FED had more than two identified triggers, which could make them poor candidates for adhering to an elimination diet. While a growing body of research describes the elimination of foods, little attention has been given to the process for reintroducing foods, a direction for future research.
Since dairy was the sole trigger identified in more than half of cases after a trial of SFED, a one-food elimination diet of dairy could thus be inferred to be effective in about one third of patients.27,47,67 Moreover, since randomized clinical trials are underway comparing dairy elimination only with the 4FED (in children) and with SFED (in adults), comparative efficacy should soon be known (NCT02610816 and NCT02778867, respectively). In light of these new data, it is possible that less restrictive empiric elimination diets can be used initially. If a response is not attained, additional foods could be eliminated. The possibilities for this were examined in a recent decision analysis, which found that an approach eliminating up to eight foods (beef and corn in addition to the traditional six foods) was optimal: first eliminating dairy (1FED), then also eliminating wheat and egg (3FED), and last eliminating the remaining five (8FED).68 A pooled analysis of previously reported triggers found that beef, corn, chicken, potato, pork, and rice were the most common histologically proven triggers after the six foods (dairy, wheat, egg, soy, nuts, and seafood).
Issues of cross-contamination and food trigger “dosing”.
How broadly to define wheat avoidance remains an area of difference in how elimination diets are administered, with European centers commonly eliminating wheat, barley, and rye, and with United States centers eliminating only wheat.69 Cross-reactivity to barley and rye can occur in food allergy to wheat, related to gluten avoidance in celiac disease, though it is unclear if this is generalizable to food triggers of EoE. Furthermore, wheat glutens contaminate most commercially-available cereal grains, leading some authors to espouse avoidance of all cereal grains, while the Work Group for Dietary Therapy and Nutrition Management of Eosinophilic Esophagitis recommends gluten-free oats due to gluten contamination of traditional oats, but allows other non-wheat grains.40 The amount of allergen needed to induce EoE remains unknown, and similarly the amount of cross-contamination should be avoided is also unknown.70 A related point is that the time for initial elimination, usually 6 weeks, may not hold for all patients, and a report has described a set of patients who did not have initial histologic response after 6 weeks, but who then responded after an additional 6–12 weeks of elimination.71
Costs and patient burden
The financial cost to patients of elimination diets as well as their effect on quality-of-life are important unanswered questions. Elimination diets increase the cost and complexity of grocery shopping for patients with EoE.54 Specifically, a SFED may increase the cost of groceries by up to $660 per year and require shopping at multiple stores to purchase the necessary items. Notably, these costs can be considered “hidden,” as they are not included in cost-effectiveness analyses that are conducted from the payer perspective72 and are not reimbursed by insurance. Elimination and reintroduction of foods also requires a number of confirmatory endoscopies. While this is resource-intensive and costly up-front, dietary elimination is cost-effective compared to long-term swallowed topical steroids, primarily due to the high costs of topical steroids for ongoing maintenance therapy.72 The costs and risks of elimination diets could nonetheless be reduced if less invasive monitoring of response could be achieved. One way to do this would be transnasal endoscopy, by which biopsies and phenotypic assessment can be safely performed without sedation in an office setting.73,74 The Cytosponge is a capsule with an attached string, which when swallowed opens into a spherical cytology sponge in the stomach, which is then removed through the esophagus and mouth to collect a histopathology specimen of esophageal epithelium.75 This method is an effective and accurate way to collect tissue for histologic analysis in EoE,76 having been successfully used in one small study in order to direct elimination diets.77 The one published study to our knowledge that has examined the effect of dietary therapy on quality-of-life reported a nonsignificant improvement with cow’s milk elimination diet in children.67 Remaining to be answered is how elimination diets effect quality-of-life in adults, how more burdensome elimination diets affect quality-of-life in all patients, how their effects on quality-of-life compare to other treatment modalities for EoE, and how to avoid nutritional deficiencies caused by the elimination of key foods.
Long-term effectiveness of dietary elimination.
While the immediate effectiveness of elimination diets is well described,27,28,30,33,33–36,39,49,50,63–65 relatively little is known about its long-term effectiveness. Moreover, little is known about whether patients are able to adhere to such diets in the long term. Several studies have examined long term outcomes of elimination diets, with up to six years follow-up, finding that long-term adherence to the diet did maintain clinical and histologic response, though long-term adherence was only maintained in about half of patients.28,29,53 Whether and how long-term adherence can be supported by clinicians remains unclear, although this issue is central for adequate long-term dietary maintenance therapy in EoE.
Possible risks of food reintroduction
The risks of elimination diets have thought primarily to be related to maintaining a balanced diet after eliminating trigger foods, for which collaboration with registered dietitians is key. There are several reports, however, that have described new IgE-mediated food allergies, including anaphylaxis, after re-exposure to previously eliminated foods.78–80 Food allergies are certainly common in EoE, but how common severe reaction after food reinduction is unknown, as are the steps that should be taken to minimize this risk. In general, it seems that the risk is small (mostly case-reportable), and may be limited to patients with prior sensitization. In cases where this is a potential concern, consultation with an allergist is key to ensuring safe food reintroduction.
Conclusions and future directions
Dietary therapies designed to eliminate trigger foods are a safe and effective treatment for EoE. Hypoallergenic elemental formulas are highly efficacious but not a long-term option for most patients. Since targeted elimination diets guided by skin prick testing or serum-specific IgE are much less effective, they generally should not be pursued. Empiric elimination diets are more effective, easier for patient adherence, eliminate common trigger foods, then serially reintroduce the foods with endoscopic assessment of disease activity. There are a number of controversies in the expanding literature describing dietary therapy of EoE: how to determine food triggers and avoid empiric elimination, how to optimally proceed through empiric elimination, how to broadly define food categories, how much exposure is needed to sustain disease, financial and quality-of-life costs to the patient, long-term effectiveness and adherence, and the risk of allergic reactions after food reintroduction.
Other important clinical questions regarding elimination diets for EoE remain. While elimination diets appear effective, there has never been a comparative study with topical steroids or PPIs. Furthermore, there have never been published randomized studies of dietary elimination compared to placebo, rendering novel techniques like network meta-analysis problematic for comparison. The comparative efficacy of dietary elimination with other therapies is therefore unknown. Furthermore, very little is known about why some patients fail to respond to elimination diets. Nonadherence is certainly one explanation for failure of empiric elimination diets, but likely not the sole reason. What proportion of treatment failures represent non-adherence, inadvertent contamination, gaps in education, atypical food triggers, or perhaps EoE that is not food-mediated is an important area for further research, with the goal of predicting which EoE therapy is most likely to succeed in each patient. As evidenced by the above discussion, there are multiple areas of research that should be explored to answer these questions (see Table). For now, the areas of debate include how many food groups to eliminate, whether small traces of trigger foods must be avoided, how best to histologically assess for response, and how to support long-term adherence to a diet after remission. Research comparing treatments for the mucosal inflammation in EoE, predicting individual response to dietary, steroid, or PPI treatment, and developing accurate simple testing methods to identify trigger foods has the potential to advance the care of the growing population of patients with EoE.
Table.
Directions for future research in dietary therapy for eosinophilic esophagitis by the clinical need they address.
| Clinical need | Approach in future research |
|---|---|
| Testing to determine triggers |
|
| Patient selection for dietary therapy |
|
| Less invasive assessment of response |
|
| Standardized approaches to elimination |
|
| Novel therapeutic targets |
|
Financial support:
This paper was supported by NIH R01 DK101856, NIH T32 DK007634, and CEGIR (U54 AI117804) which is part of the Rare Disease Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and is funded through collaboration between NIAID, NIDDK, and NCATS. CEGIR is also supported by patient advocacy groups including APFED CURED and EFC.
Footnotes
Disclosures: Dr. Dellon has received research funding from Adare, Allakos, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos, Regeneron, and Shire; has received consulting fees from Adare, Alivio, Allakos, AstraZeneca, Banner, Calypso, Enumeral, EsoCap, Celgene/Receptos, GSK, Regeneron, Robarts, Shire, and educational grants from Allakos, Banner, and Holoclara. The other authors have no potential conflicts of interest.
References
- 1.Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018. January;154(2):319–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD. Prevalence of Eosinophilic Esophagitis in the United States. Clinical Gastroenterology and Hepatology. 2014. April;12(4):589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointestinal Endoscopy. 2014. April;79(4):577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-Care Utilization, Costs and the Burden of Disease Related to Eosinophilic Esophagitis in the United States. The American Journal of Gastroenterology. 2015. May;110(5):626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dougherty M, Runge TM, Eluri S, Dellon ES. Esophageal dilation with either bougie or balloon technique as a treatment for eosinophilic esophagitis: a systematic review and meta-analysis. Gastrointestinal Endoscopy. 2017. October;86(4):581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Straumann A, Katzka DA. Diagnosis and Treatment of Eosinophilic Esophagitis. Gastroenterology. 2018. January;154(2):346–59. [DOI] [PubMed] [Google Scholar]
- 7.Dellon ES, Liacouras CA. Advances in Clinical Management of Eosinophilic Esophagitis. Gastroenterology. 2014. December;147(6):1238–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richter JE. Endoscopic Treatment of Eosinophilic Esophagitis. Gastrointestinal Endoscopy Clinics of North America. 2018. January;28(1):97–110. [DOI] [PubMed] [Google Scholar]
- 9.Reed CC, Dellon ES. Eosinophilic Esophagitis. Medical Clinics of North America. 2019. January;103(1):29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng E, Zhang X, Huo X, Yu C, Zhang Q, Wang DH, et al. Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 2013. June;62(6):824–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Rhijn BD, Weijenborg PW, Verheij J, van den Bergh Weerman MA, Verseijden C, van den Wijngaard RMJGJ, et al. Proton Pump Inhibitors Partially Restore Mucosal Integrity in Patients With Proton Pump Inhibitor–Responsive Esophageal Eosinophilia but Not Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2014. November;12(11):1815–23. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Cheng E, Huo X, Yu C, Zhang Q, Pham TH, et al. Omeprazole Blocks STAT6 Binding to the Eotaxin-3 Promoter in Eosinophilic Esophagitis Cells. Kalinichenko VV, editor. PLoS ONE [Internet]. 2012. November 21 [cited 2019 Feb 11];7(11). Available from: http://dx.plos.org/10.1371/journal.pone.0050037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucendo AJ, Arias Á, Molina-Infante J. Efficacy of Proton Pump Inhibitor Drugs for Inducing Clinical and Histologic Remission in Patients With Symptomatic Esophageal Eosinophilia: A Systematic Review and Meta-Analysis. Clinical Gastroenterology and Hepatology. 2016. January;14(1):13–22. [DOI] [PubMed] [Google Scholar]
- 14.Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Alimentary Pharmacology & Therapeutics. 2015. May;41(9):797–806. [DOI] [PubMed] [Google Scholar]
- 15.Cotton CC, Eluri S, Wolf WA, Dellon ES. Six-Food Elimination Diet and Topical Steroids are Effective for Eosinophilic Esophagitis: A Meta-Regression. Digestive Diseases and Sciences. 2017. September;62(9):2408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Straumann A, Conus S, Grzonka P, Kita H, Kephart G, Bussmann C, et al. Anti-interleukin-5 antibody treatment (mepolizumab) in active eosinophilic oesophagitis: a randomised, placebo-controlled, double-blind trial. Gut. 2010. January 1;59(01):21–30. [DOI] [PubMed] [Google Scholar]
- 17.Assa’ad AH, Gupta SK, Collins MH, Thomson M, Heath AT, Smith DA, et al. An Antibody Against IL-5 Reduces Numbers of Esophageal Intraepithelial Eosinophils in Children With Eosinophilic Esophagitis. Gastroenterology. 2011. November;141(5):1593–604. [DOI] [PubMed] [Google Scholar]
- 18.Rothenberg ME, Wen T, Greenberg A, Alpan O, Enav B, Hirano I, et al. Intravenous anti–IL-13 mAb QAX576 for the treatment of eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2015. February;135(2):500–7. [DOI] [PubMed] [Google Scholar]
- 19.Hirano I, Collins MH, Assouline-Dayan Y, Evans L, Gupta S, Schoepfer AM, et al. RPC4046, a Monoclonal Antibody Against IL13, Reduces Histologic and Endoscopic Activity in Patients With Eosinophilic Esophagitis. Gastroenterology. 2019. February;156(3):592–603. [DOI] [PubMed] [Google Scholar]
- 20.Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M, et al. Dupilumab efficacy and safety in adult patients with active eosinophilic oesophagitis: A randomised double-blind placebo-controlled phase 2 trial. United Eur Gastroenterol J. 2017;5(8):1146–7. [Google Scholar]
- 21.Kelly KJ, Lazenby AJ, Rowe PC, Yardley JH, Perman JA, Sampson HA. Eosinophilic esophagitis attributed to gastroesophageal reflux: improvement with an amino acid-based formula. Gastroenterology. 1995. November;109(5):1503–12. [DOI] [PubMed] [Google Scholar]
- 22.O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, et al. Pathophysiology of Eosinophilic Esophagitis. Gastroenterology. 2018. January;154(2):333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liacouras CA, Spergel JM, Ruchelli E, Verma R, Mascarenhas M, Semeao E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005. December;3(12):1198–206. [DOI] [PubMed] [Google Scholar]
- 24.Assa’ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: An 8-year follow-up. Journal of Allergy and Clinical Immunology. 2007. March;119(3):731–8. [DOI] [PubMed] [Google Scholar]
- 25.Markowitz JE, Spergel JM, Ruchelli E, Liacouras CA. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. The American Journal of Gastroenterology. 2003. April;98(4):777–82. [DOI] [PubMed] [Google Scholar]
- 26.Kagalwalla AF, Sentongo TA, Ritz S, Hess T, Nelson SP, Emerick KM, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2006. September;4(9):1097–102. [DOI] [PubMed] [Google Scholar]
- 27.Gonsalves N, Yang G, Doerfler B, Ritz S, Ditto AM, Hirano I. Elimination Diet Effectively Treats Eosinophilic Esophagitis in Adults; Food Reintroduction Identifies Causative Factors. Gastroenterology. 2012. June;142(7):1451–9. [DOI] [PubMed] [Google Scholar]
- 28.Lucendo AJ, Arias Á, González-Cervera J, Yagüe-Compadre JL, Guagnozzi D, Angueira T, et al. Empiric 6-food elimination diet induced and maintained prolonged remission in patients with adult eosinophilic esophagitis: A prospective study on the food cause of the disease. Journal of Allergy and Clinical Immunology. 2013. March;131(3):797–804. [DOI] [PubMed] [Google Scholar]
- 29.Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. A prospective open clinical trial of a proton pump inhibitor, elimination diet and/or budesonide for eosinophilic oesophagitis. Alimentary Pharmacology & Therapeutics. 2016. May;43(9):985–93. [DOI] [PubMed] [Google Scholar]
- 30.Wolf WA, Jerath MR, Sperry SLW, Shaheen NJ, Dellon ES. Dietary Elimination Therapy Is an Effective Option for Adults With Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2014. August;12(8):1272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon D, Cianferoni A, Spergel JM, Aceves S, Holbreich M, Venter C, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy. 2016. May;71(5):611–20. [DOI] [PubMed] [Google Scholar]
- 32.Mishra A, Hogan SP, Brandt EB, Rothenberg ME. An etiological role for aeroallergens and eosinophils in experimental esophagitis. Journal of Clinical Investigation. 2001. January 1;107(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson CJ, Abonia JP, King EC, Putnam PE, Collins MH, Franciosi JP, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2012. June;129(6):1570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colson D, Kalach N, Soulaines P, Vannerom Y, Campeotto F, Talbotec C, et al. The Impact of Dietary Therapy on Clinical and Biologic Parameters of Pediatric Patients with Eosinophilic Esophagitis. The Journal of Allergy and Clinical Immunology: In Practice. 2014. September;2(5):587–93. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Sánchez J, Gómez Torrijos E, López Viedma B, de la Santa Belda E, Martín Dávila F, García Rodríguez C, et al. Efficacy of IgE-targeted vs empiric six-food elimination diets for adult eosinophilic oesophagitis. Allergy. 2014. July;69(7):936–42. [DOI] [PubMed] [Google Scholar]
- 36.Arias Á, Lucendo AJ, Martínez-Fernández P, González-Castro AM, Fortea M, González-Cervera J, et al. Dietary treatment modulates mast cell phenotype, density, and activity in adult eosinophilic oesophagitis. Clinical & Experimental Allergy. 2016. January;46(1):78–91. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman JA, Morotti RA, Konstantinou GN, Yershov O, Chehade M. Dietary therapy can reverse esophageal subepithelial fibrosis in patients with eosinophilic esophagitis: a historical cohort. Allergy. 2012. October;67(10):1299–307. [DOI] [PubMed] [Google Scholar]
- 38.Leung J, Mehrzad R, Hundal NV, Alejos A, Hesterberg PE, Katz AJ, et al. Longitudinal Perspective on Managing Refractory Eosinophilic Esophagitis. The Journal of Allergy and Clinical Immunology: In Practice. 2015. November;3(6):951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spergel JM, Brown-Whitehorn TF, Cianferoni A, Shuker M, Wang M-L, Verma R, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. Journal of Allergy and Clinical Immunology. 2012. August;130(2):461–7. [DOI] [PubMed] [Google Scholar]
- 40.Groetch M, Venter C, Skypala I, Vlieg-Boerstra B, Grimshaw K, Durban R, et al. Dietary Therapy and Nutrition Management of Eosinophilic Esophagitis: A Work Group Report of the American Academy of Allergy, Asthma, and Immunology. The Journal of Allergy and Clinical Immunology: In Practice. 2017. March;5(2):312–24. [DOI] [PubMed] [Google Scholar]
- 41.Philpott H, Dellon ES. The role of maintenance therapy in eosinophilic esophagitis: who, why, and how? Journal of Gastroenterology. 2018. February;53(2):165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borschel MW, Antonson DL, Murray ND, Oliva-Hemker M, Mattis LE, Kerzner B, et al. Two single group, prospective, baseline-controlled feeding studies in infants and children with chronic diarrhea fed a hypoallergenic free amino acid-based formula. BMC Pediatrics [Internet]. 2014. December;14(136). Available from: http://bmcpediatr.biomedcentral.com/articles/10.1186/1471-2431-14-136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sicherer SH, Noone SA, Koerner CB, Christie L, Burks AW, Sampson HA. Hypoallergenicity and efficacy of an amino acid–based formula in children with cow’s milk and multiple food hypersensitivities. The Journal of Pediatrics. 2001. May;138(5):688–93. [DOI] [PubMed] [Google Scholar]
- 44.Warners MJ, Vlieg-Boerstra BJ, Verheij J, van Rhijn BD, Van Ampting MTJ, Harthoorn LF, et al. Elemental diet decreases inflammation and improves symptoms in adult eosinophilic oesophagitis patients. Alimentary Pharmacology & Therapeutics. 2017. March;45(6):777–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peterson KA, Byrne KR, Vinson LA, Ying J, Boynton KK, Fang JC, et al. Elemental Diet Induces Histologic Response in Adult Eosinophilic Esophagitis. The American Journal of Gastroenterology. 2013. May;108(5):759–66. [DOI] [PubMed] [Google Scholar]
- 46.Al-Hussaini A, Al-Idressi E, Al-Zahrani M. The role of allergy evaluation in children with eosinophilic esophagitis. Journal of Gastroenterology. 2013. November;48(11):1205–12. [DOI] [PubMed] [Google Scholar]
- 47.Kagalwalla AF, Amsden K, Shah A, Ritz S, Manuel-Rubio M, Dunne K, et al. Cowʼs Milk Elimination: A Novel Dietary Approach to Treat Eosinophilic Esophagitis. Journal of Pediatric Gastroenterology and Nutrition. 2012. December;55(6):711–6. [DOI] [PubMed] [Google Scholar]
- 48.Arias Á, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of Dietary Interventions for Inducing Histologic Remission in Patients With Eosinophilic Esophagitis: A Systematic Review and Meta-analysis. Gastroenterology. 2014. June;146(7):1639–48. [DOI] [PubMed] [Google Scholar]
- 49.Syrigou E, Angelakopoulou A, Zande M, Panagiotou I, Roma E, Pitsios C. Allergy-test-driven elimination diet is useful in children with eosinophilic esophagitis, regardless of the severity of symptoms. Pediatric Allergy and Immunology. 2015. June;26(4):323–9. [DOI] [PubMed] [Google Scholar]
- 50.Spergel JM, Andrews T, Brown-Whitehorn TF, Beausoleil JL, Liacouras CA. Treatment of eosinophilic esophagitis with specific food elimination diet directed by a combination of skin prick and patch tests. Annals of Allergy, Asthma & Immunology. 2005. October;95(4):336–43. [DOI] [PubMed] [Google Scholar]
- 51.Clayton F, Fang JC, Gleich GJ, Lucendo AJ, Olalla JM, Vinson LA, et al. Eosinophilic Esophagitis in Adults Is Associated With IgG4 and Not Mediated by IgE. Gastroenterology. 2014. September;147(3):602–9. [DOI] [PubMed] [Google Scholar]
- 52.Doerfler B, Bryce P, Hirano I, Gonsalves N. Practical approach to implementing dietary therapy in adults with eosinophilic esophagitis: the Chicago experience: Dietary therapy in adult EoE. Diseases of the Esophagus. 2015. January;28(1):42–58. [DOI] [PubMed] [Google Scholar]
- 53.Reed CC, Fan C, Koutlas NT, Shaheen NJ, Dellon ES. Food elimination diets are effective for long-term treatment of adults with eosinophilic oesophagitis. Alimentary Pharmacology & Therapeutics. 2017. November;46(9):836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asher Wolf W, Huang KZ, Durban R, Iqbal ZJ, Robey BS, Khalid FJ, et al. The Six-Food Elimination Diet for Eosinophilic Esophagitis Increases Grocery Shopping Cost and Complexity. Dysphagia. 2016. December;31(6):765–70. [DOI] [PubMed] [Google Scholar]
- 55.Philpott H, Nandurkar S, Royce SG, Thien F, Gibson PR. Allergy tests do not predict food triggers in adult patients with eosinophilic oesophagitis. A comprehensive prospective study using five modalities. Alimentary Pharmacology & Therapeutics. 2016. August;44(3):223–33. [DOI] [PubMed] [Google Scholar]
- 56.Spergel JM, Brown-Whitehorn T, Beausoleil JL, Shuker M, Liacouras CA. Predictive values for skin prick test and atopy patch test for eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2007. February;119(2):509–11. [DOI] [PubMed] [Google Scholar]
- 57.Armentia A, Martín-Armentia S, Martín-Armentia B, Santos-Fernández J, Álvarez R, Madrigal B, et al. Is eosinophilic esophagitis an equivalent of pollen allergic asthma? Analysis of biopsies and therapy guided by component resolved diagnosis. Allergologia et Immunopathologia. 2018. March;46(2):181–9. [DOI] [PubMed] [Google Scholar]
- 58.Armentia A, Martín S, Barrio J, Martín B, García JC, Vega JM, et al. Value of microarray allergen assay in the management of eosinophilic oesophagitis. Allergologia et Immunopathologia. 2015. January;43(1):73–80. [DOI] [PubMed] [Google Scholar]
- 59.Erwin EA, Tripathi A, Ogbogu PU, Commins SP, Slack MA, Cho CB, et al. IgE Antibody Detection and Component Analysis in Patients with Eosinophilic Esophagitis. The Journal of Allergy and Clinical Immunology: In Practice. 2015. November;3(6):896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wright BL, Kulis M, Guo R, Orgel KA, Wolf WA, Burks AW, et al. Food-specific IgG 4 is associated with eosinophilic esophagitis. Journal of Allergy and Clinical Immunology. 2016. October;138(4):1190–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dellon ES, Guo R, McGee SJ, Hamilton DK, Covington J, Moist SE, et al. An Allergen-Specific Immune Signature Identifies Food Triggers in Eosinophilic Esophagitis with High Accuracy. Gastroenterology. 2018. May;154(6):S260. [Google Scholar]
- 62.Warners MJ, Terreehorst I, van den Wijngaard RM, Akkerdaas J, van Esch BCAM, van Ree R, et al. Abnormal Responses to Local Esophageal Food Allergen Injections in Adult Patients With Eosinophilic Esophagitis. Gastroenterology. 2018. January;154(1):57–60. [DOI] [PubMed] [Google Scholar]
- 63.Molina-Infante J, Arias A, Barrio J, Rodríguez-Sánchez J, Sanchez-Cazalilla M, Lucendo AJ. Four-food group elimination diet for adult eosinophilic esophagitis: A prospective multicenter study. Journal of Allergy and Clinical Immunology. 2014. November;134(5):1093–9. [DOI] [PubMed] [Google Scholar]
- 64.Kagalwalla AF, Wechsler JB, Amsden K, Schwartz S, Makhija M, Olive A, et al. Efficacy of a 4-Food Elimination Diet for Children With Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2017. November;15(11):1698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gonsalves N, Doerfler B, Schwartz S, Yang G-Y, Zalewski A, Amsden K, et al. Prospective trial of four food elimination diet demonstrates comparable effectiveness in the treatment of adult and pediatric eosinophilic esophagitis. Gastroenterology. 2013;144(5):S154. [Google Scholar]
- 66.Molina-Infante J, Arias Á, Alcedo J, Garcia-Romero R, Casabona-Frances S, Prieto-Garcia A, et al. Step-up empiric elimination diet for pediatric and adult eosinophilic esophagitis: The 2–4–6 study. Journal of Allergy and Clinical Immunology. 2018. April;141(4):1365–72. [DOI] [PubMed] [Google Scholar]
- 67.Kruszewski PG, Russo JM, Franciosi JP, Varni JW, Platts-Mills TAE, Erwin EA. Prospective, comparative effectiveness trial of cow’s milk elimination and swallowed fluticasone for pediatric eosinophilic esophagitis: Pediatric eosinophilic esophagitis therapy. Diseases of the Esophagus. 2016. May;29(4):377–84. [DOI] [PubMed] [Google Scholar]
- 68.Zhan T, Ali A, Choi JG, Lee M, Leung J, Dellon ES, et al. Model to Determine the Optimal Dietary Elimination Strategy for Treatment of Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2018. November;16(11):1730–7. [DOI] [PubMed] [Google Scholar]
- 69.Kliewer KL, Venter C, Cassin AM, Abonia JP, Aceves SS, Bonis PA, et al. Should wheat, barley, rye, and/or gluten be avoided in a 6-food elimination diet? Journal of Allergy and Clinical Immunology. 2016. April;137(4):1011–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hernando A, Mujico JR, Mena MC, Lombardía M, Méndez E. Measurement of wheat gluten and barley hordeins in contaminated oats from Europe, the United States and Canada by Sandwich R5 ELISA: European Journal of Gastroenterology & Hepatology. 2008. June;20(6):545–54. [DOI] [PubMed] [Google Scholar]
- 71.Philpott H, Dellon E. Histologic improvement after 6 weeks of dietary elimination for eosinophilic esophagitis may be insufficient to determine efficacy. Asia Pac Allergy. 2018;8(2):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cotton CC, Erim D, Eluri S, Palmer SH, Green DJ, Wolf WA, et al. Cost Utility Analysis of Topical Steroids Compared With Dietary Elimination for Treatment of Eosinophilic Esophagitis. Clinical Gastroenterology and Hepatology. 2017. June;15(6):841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Friedlander JA, DeBoer EM, Soden JS, Furuta GT, Menard-Katcher CD, Atkins D, et al. Unsedated transnasal esophagoscopy for monitoring therapy in pediatric eosinophilic esophagitis. Gastrointestinal Endoscopy. 2016. February;83(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Philpott H, Nandurkar S, Royce SG, Gibson PR. Ultrathin unsedated transnasal gastroscopy in monitoring eosinophilic esophagitis: Transnasal gastroscopy for EoE. Journal of Gastroenterology and Hepatology. 2016. March;31(3):590–4. [DOI] [PubMed] [Google Scholar]
- 75.Januszewicz W, Tan WK, Lehovsky K, Debiram-Beecham I, Nuckcheddy T, Moist S, et al. Safety and Acceptability of Esophageal Cytosponge Cell Collection Device in a Pooled Analysis of Data From Individual Patients. Clinical Gastroenterology and Hepatology. 2019. March;17(4):647–656.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katzka DA, Smyrk TC, Alexander JA, Geno DM, Beitia RA, Chang AO, et al. Accuracy and Safety of the Cytosponge for Assessing Histologic Activity in Eosinophilic Esophagitis: A Two-Center Study. The American Journal of Gastroenterology. 2017. October;112(10):1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alexander JA, Katzka DA, Ravi K, Fitzgerald RC, Geno DM, Tholen C, et al. Efficacy of Cytosponge Directed Food Elimination Diet in Eosinophilic Esophagitis. a Pilot Trial. Gastroenterology. 2018. May;154(6):S76. [Google Scholar]
- 78.Gottlieb SJ, Markowitz JE, Dellon ES. New IgE Immediate Hypersensitivity Reactions Upon Reintroduction of Food Restricted for Treatment of Eosinophilic Esophagitis. Annals of Allergy, Asthma & Immunology. 2019. January;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Alsalamah M, Makhajia M, Somers G, Marcon M, Hummel D, Upton J. Anaphylaxis to Milk After Elimination Diet for Eosinophilic Gastrointestinal Disease. The American Journal of Gastroenterology. 2016. May;111(5):752–3. [DOI] [PubMed] [Google Scholar]
- 80.Hill DA, Shuker M, Cianferoni A, Wong T, Ruchelli E, Spergel JM, et al. The development of IgE-mediated immediate hypersensitivity after the diagnosis of eosinophilic esophagitis to the same food. The Journal of Allergy and Clinical Immunology: In Practice. 2015. January;3(1):123–4. [DOI] [PMC free article] [PubMed] [Google Scholar]