Abstract
Background
Using a pilot system we have categorised this review as: “Historical question – no update intended: monotherapy no longer recommended" (see published notes).
Multiple‐drug‐resistant malaria is widespread, and in South‐East Asia resistance is high against nearly all single therapy antimalarial drugs. Here, and in other areas with low malaria transmission, the combination of artesunate and mefloquine may provide an effective alternative.
Objectives
To compare artesunate plus mefloquine with mefloquine alone for treating uncomplicated Plasmodium falciparum malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (May 2005), CENTRAL (The Cochrane Library Issue 2, 2005), MEDLINE (1966 to May 2005), EMBASE (1988 to May 2005), LILACS (May 2005), BIOSIS (1985 to June 2005), conference proceedings, and reference lists. We also contacted researchers, organizations, and pharmaceutical companies.
Selection criteria
Randomized and quasi‐randomized controlled trials comparing artesunate plus mefloquine with mefloquine alone for treating uncomplicated malaria.
Data collection and analysis
Two authors independently applied the inclusion criteria, extracted data, and assessed methodological quality. The primary outcome was treatment failure by day 28, defined as evidence of parasitaemia with or without clinical failure between days zero (start of treatment) and 28. For dichotomous data we calculated risk ratios (RR) and 95% confidence intervals (CI).
Main results
Eight trials involving 1996 participants met the inclusion criteria. All were conducted in areas with low malaria transmission, seven in South‐East Asia and one in the Peruvian Amazon. The doses and dosing regimens of artesunate and mefloquine varied across trials. The trials using a total dose of 25 mg/kg mefloquine and 10 mg artesunate reported fewer treatment failures with the combination at all time points: day 28 (RR 0.17, 95% CI 0.06 to 0.47; 824 participants, 4 trials), day 42 (RR 0.23, 95% CI 0.14 to 0.39; 298 participants, 1 trial), and day 63 (RR 0.26, 95% CI 0.09 to 0.77; 501 participants, 2 trials). The results for parasitaemia showed a similar trend. Trials using a lower dose of artesunate tended to favour the artesunate plus mefloquine combination. Overall, adverse events were similar across treatment arms.
Authors' conclusions
Artesunate plus mefloquine performs better than mefloquine alone for treating uncomplicated falciparum malaria in areas with low malaria transmission. A total dose of 25 mg/kg mefloquine and at least 10 mg artesunate leads to higher cure rates. Better reporting of methods and standardisation of outcomes would help the interpretation of future trials.
2008: As monotherapy is no longer recommended by the World Health Organization for malaria treatment, the authors do not intend to update this review.
23 April 2019
No update planned
Review superseded
Please refer to the Cochrane Special Collection: Sinclair 2014 https://doi.org/10.1002/14651858.SC000007/full
Keywords: Humans; Antimalarials; Antimalarials/therapeutic use; Artemisinins; Artemisinins/therapeutic use; Artesunate; Drug Therapy, Combination; Malaria, Falciparum; Malaria, Falciparum/drug therapy; Mefloquine; Mefloquine/therapeutic use; Sesquiterpenes; Sesquiterpenes/therapeutic use
Plain language summary
Artesunate plus mefloquine in areas with low malaria transmission performed better than mefloquine alone for uncomplicated P. falciparum malaria
Using a pilot system we have categorised this review as: “Historical question – no update intended: monotherapy no longer recommended" (see published notes).
Malaria is a parasitic disease spread by mosquitoes that kills thousands of people worldwide. Multiple‐drug‐resistant malaria is widespread, and in South‐East Asia resistance is high against nearly all single therapy antimalarial drugs. Here, and in other areas with low malaria transmission, the combination of artesunate and mefloquine may provide an effective alternative. The review includes eight trials, mainly from South‐East Asia, that compared artesunate plus mefloquine with mefloquine alone for treating uncomplicated malaria. Artesunate plus mefloquine performed better at destroying blood parasites and reducing fever. Adverse events were similar with both treatments.
Background
Plasmodium falciparum malaria continues to be a major cause of ill health and death in many areas, especially in the tropics (WHO/UNICEF 2003). Uncomplicated falciparum malaria is the commonest form of the disease and accounts for the greatest proportion of morbidity (Olliaro 1996).
Antimalarial combination therapy is currently regarded as a major strategy to combat drug‐resistant malaria (RBM 2001a). This is because malaria parasites can rapidly develop resistance to antimalarial drugs when they are used alone (single therapy). Combination therapy involves the simultaneous use of two or more blood schizonticides (drugs acting on the blood stage of the malaria parasites) that have independent modes of action and different biochemical targets in the parasite (White 1999; RBM 2001b). The combination may be more effective than single therapy, if the components exert a synergistic or additive antimalarial effect and there is decreased effectiveness due to resistance to at least one of the component drugs. The combination of artemisinin derivatives with other antimalarial drugs is widely advocated because it produces rapid clinical and parasitological response, may delay the development of resistance, and may reduce malaria transmission (RBM 2001a).
Artesunate is a water‐soluble derivative of dihydroartemisinin and the most widely used member of the artemisinin derivatives. It is effective against gametocytes (Price 1996), the sexual forms of the parasites responsible for the transmission of malaria, and is one of the most rapidly acting antimalarial agents available. There is no documented resistance against artesunate, but because it has a very short half life (less than two hours), long treatment courses of five to seven days are necessary to prevent recurrence of the parasites (Bethell 1997; White 1999).
Mefloquine is a 4‐quinoline methanol antimalarial drug. It has been used as single therapy in areas of low malaria transmission and where chloroquine resistance is common (White 1996). Because its terminal half life is about 20 days, it is eliminated slowly from the body (Looareesuwan 1992b). This means that the malaria parasites are exposed to sub‐therapeutic levels of the drug over long periods of time, which encourages the development of resistant strains (White 1997). Reports from Thailand and neighbouring countries show that parasites are rapidly developing resistance to mefloquine (Wongsrichanalai 2002), and its use as a single antimalarial treatment is no longer recommended in these areas (Looareesuwan 1998).
In South‐East Asia, mefloquine is often combined with artesunate to treat multiple‐drug‐resistant uncomplicated malaria (Angus 2001). The rationale is to provide improved early clearance of parasites in order to reduce the probability of any remaining parasites surviving the residual effect of mefloquine. By preventing the exposure of the initial high load of parasites to mefloquine alone the spread of parasite resistance to mefloquine may be delayed. The gametocidal action of artesunate may also reduce the transmission of mefloquine‐resistant malaria strains. The addition of mefloquine to artesunate allows the use of a practical short course of artesunate, that is, three days compared with five or seven days for artesunate alone. The combination may also reduce recrudescence rates; for example, recrudescence rates as high as 37% have been observed with a five‐day course of artesunate compared with 7% when artesunate is given together with mefloquine (Price 1998; Trung 2001).
Results from clinical trials comparing artesunate plus mefloquine with mefloquine alone suggest that the combination is superior. These trials have however used varying regimens of the combination with mefloquine given either during or after the artesunate course (Hoshen 2000). In addition, current dosage schedules are based on available clinical data and not from well‐designed dose‐finding trials (RBM 2001c). Therefore, it is still unclear which regimen and dosing schedule are most suitable.
The use of artesunate and mefloquine either alone or in combination may be associated with unwanted effects. Mefloquine is associated with neuropsychiatric disorders, including psychosis and convulsions, which have been observed both during treatment and prophylaxis (Weinke 1991). Some artemisinin derivatives have been associated with fatal neurotoxicity in animals, and a few people taking artemisinin drugs have been reported with transient first‐degree heart block, but artesunate itself seems to be well tolerated (Brewer 1994; Kain 1995; Gilles 2000). Reports from trials assessing the effects of the combination have reported moderate tolerability with no increase in adverse events over those related to the individual drugs (WHO 2002).
McIntosh and Olliaro prepared a Cochrane review of all artemisinin drugs for treating uncomplicated malaria (McIntosh 1999). Although they reviewed the effects of combining artemisinins with mefloquine, no two trials used the same treatment regimens and thus the findings were inconclusive. However, the review authors do not intend to update their review because their objectives were achieved. Our review attempts to answer the still outstanding questions regarding the combination of artesunate plus mefloquine.
Objectives
To compare artesunate plus mefloquine with mefloquine alone for treating uncomplicated Plasmodium falciparum malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
Adults and children with uncomplicated P. falciparum malaria, as confirmed by microscopy.
Types of interventions
Intervention
Artesunate plus mefloquine.
Control
Mefloquine.
Mefloquine dose should be the same in both the intervention and control groups.
Types of outcome measures
Primary
Treatment failure (parasitological) by day 28, defined as evidence of parasitaemia with or without clinical failure between day zero (start of treatment) and day 28.
Secondary
Treatment failure (parasitological) by days 28, 42, and 63, excluding new infections where possible.
Parasitaemia on thick blood film on days seven and 14.
Resolution of fever: fever clearance time (time for temperature to return to normal as defined by the trial authors; and fever clearance within 48 hours of starting treatment).
Parasite clearance: parasite clearance time; and rate of 50% and 90% parasite clearance (PC 50, PC 90) as reported.
Number of participants developing severe malaria, as defined by the World Health Organization (WHO 2001), during follow up.
Adherence (number of participants completing treatment).
Adverse events
Number of adverse events.
Serious adverse events: adverse events that lead to death, require hospitalization or prolongation of existing hospitalization, are life threatening, or result in persistent or significant disability or incapacity.
Adverse events that require the discontinuation of treatment.
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Table 1: Cochrane Infectious Diseases Group Specialized Register (May 2005); Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library (Issue 2, 2005); MEDLINE (1966 to May 2005); EMBASE (1974 to May 2005); LILACS (1982 to May 2005); and BIOSIS (1985 to June 2005).
1. Detailed search strategies.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb | BIOSIS |
| 1 | artesunate | artesunate | ARTESUNATE | ARTESUNATE | artesunate | artesunate |
| 2 | mefloquine | mefloquine | artesunate | artesunate | mefloquine | mefloquine |
| 3 | Lariam | Lariam | arsumax | arsumax | malaria | Lariam |
| 4 | — | 2 or 3 | 1 or 2 or 3 | 1 or 2 or 3 | 1 and 2 and 3 | — |
| 5 | — | 1 and 4 | MEFLOQUINE | mefloquine | — | — |
| 6 | — | — | mefloquine | MEFLOQUINE | — | — |
| 7 | — | — | Lariam | mephaquim | — | — |
| 8 | — | — | 5 or 6 or 7 | Lariam | — | — |
| 9 | — | — | 4 and 8 | 5 or 6 or 7 or 8 | — | — |
| 10 | — | — | exp MALARIA | 4 and 9 | — | — |
| 11 | — | — | malaria | malaria | — | — |
| 12 | — | — | exp PLASMODIUM | MALARIA | — | — |
| 13 | — | — | plasmodium | PLASMODIUM‐FALCIPARUM | — | — |
| 14 | — | — | 10 or 11 or 12 or 13 | 11 or 12 or 13 | — | — |
| 15 | — | — | 9 and 14 | 10 and 14 | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration Higgins 2005; upper case: MeSH or EMTREE heading; lower case: free text term.
Conference proceedings
We searched the following conference proceedings for relevant abstracts: Third European Congress on Tropical Medicine and International Health, Lisbon, Portugal, 8 to 11 September 2002; and The Third Multilateral Initiative on Malaria Pan‐African Conference, Arusha, Tanzania, 18 to 22 November 2002.
Researchers, organizations, and pharmaceutical companies
We circulated a list of the trials identified using this search strategy to organizations and individual researchers working in the field to help identify any additional trials. We also sought unpublished or confidential reports and information on ongoing trials. We contacted the following pharmaceutical companies: Arenco (France); Mepha (Switzerland); Rhone‐Poulenc Rorer (France); Propharma (Scotland); Novartis (Switzerland); Sanofi‐Winthrop (France); Guilin Pharmaceutical Company (China); Kunning Pharmaceutical Corporation (Malaysia); Thua Thien Pharmaceutical Company (Viet Nam); and the National Pharmaceutical Plant Company (Viet Nam).
Reference lists
We checked the reference lists of existing reviews (McIntosh 1999; McIntosh 2000) and of all trials identified by the above methods.
Data collection and analysis
Selection of studies
The first author scanned the results of the search strategy for all potentially relevant trials before retrieving the full articles and scrutinizing them for possible multiple publications. Both authors independently assessed each potentially relevant trial for inclusion in the review using an eligibility form based on the inclusion criteria. There were no disagreements. We give the reason for excluding trials in the 'Characteristics of excluded studies'.
Data extraction and management
Both authors independently extracted data using a data extraction form; there were no disagreements. The first author entered the data into Review Manager 5. For trials where some outcomes were not reported or were not clear, we wrote to the trial authors for more information.
We approximated intention‐to‐treat analysis wherever possible but generally the reports had insufficient information. We calculated the percentage loss to follow up and reported this information in the 'Characteristics of included studies'. For dichotomous outcomes, we recorded the number of participants experiencing the event in each group, and, for continuous outcomes, we extracted the arithmetic means and standard deviations.
Assessment of risk of bias in included studies
Both authors independently assessed the methodological quality of each included trial. We classed generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear according to Jüni 2001. We described who was blinded, such as the participants, care provider, or outcome assessors, and classed the inclusion of all randomized participants in the final analysis as adequate if 90% or more were included.
Data synthesis
We analysed the data with Review Manager 5 using risk ratio (RR) for dichotomous data, mean difference(MD) for continuous data, and 95% confidence intervals (CI). Because the dose and regimen of both artesunate and mefloquine varied, we categorized trials based on total dose of artesunate and mefloquine used. We categorized artesunate as at least 10 mg/kg (multiple doses) and less than 10 mg/kg (single dose) and mefloquine as either 25 mg/kg or 15 mg/kg.
We assessed heterogeneity amongst trials by inspecting the forest plots and by using the chi‐squared test for heterogeneity with a 10% level of statistical significance. We used the random‐effects model where there was statistically significant heterogeneity and it was still appropriate to combine the trials.
Future updates
We were unable to use some methods in the protocol because there were too few trials, but we intend to use these methods, described in Table 2, in future updates.
2. Methods for future updates.
| Method | Details |
| Recrudesced and new infections | In areas of intense malaria transmission, blood smears positive for malaria parasites after day 14 may be a result of new infections or a recrudescence of the original infection. Polymerase chain reaction (PCR) is a method that can be used to differentiate between new and old infections. We will use the results of PCR analyses, if they become available, to differentiate between recrudesced and new infections |
| Continuous data reported with geometric means | We will extract the standard deviations on the log scale, and extract minimum and maximum values for medians. We will combine the findings on a log scale and report on the original scale; we will report medians and ranges in tables |
| Exploring potential sources of heterogeneity using subgroup analyses | 1. Intervention: simultaneous versus sequential regimens; mefloquine dose; and artesunate dose 2. Trial setting: level of background mefloquine resistance; high malaria transmission (an area of hyperendemicity or holoendemicity) versus low transmission (an area of hypoendemicity or mesoendemicity) 3. Pre‐treatment malaria parasite density: < 250,000/µL or 5% of total red blood cells and at least 250,000/µL or 5% of total red blood cells 4. Age: ≤ 5 years and > 5 years |
| Sensitivity analyses | We will conduct sensitivity analyses for each of the components of methodological quality |
| Funnel plots | We will examine funnel plots for asymmetry, keeping in mind that the asymmetry could be caused by publication bias, differences in methodological quality, or heterogeneity |
Results
Description of studies
See 'Characteristics of included studies' for individual trial details.
Eight of 12 potentially relevant trials met our inclusion criteria. One trial was reported on in two separate publications (Marquino 2003), and two trials were reported in a single publication (Nosten 1994a; Nosten 1994b).
Source of funding
The trials were funded by various sources, including nongovernmental organizations (eg Médecins Sans Frontières), governmental organizations (eg US Agency for International Development and the Government of Peru), international organizations (eg World Health Organization), and pharmaceutical companies. The trial reports did not state the role of the funding agencies in the trial process.
Trial design and location
All trials were described as randomized, but most trial authors provided no information on the method of randomization. The trials were conducted in low malaria transmission areas. Seven trials were conducted in South‐East Asia − Thai‐Burmese border (two), Thai‐Cambodia border (one), Thai‐Myanmar border (one), most northern state of the Union of Myanmar (one), and the interior of Thailand (two). One trial was conducted in the Peruvian Amazon Basin in South America.
Participants
The eight trials included 1155 participants. Five trials included children and adults, and three trials included adults with the lowest cut‐off age of 15 years.
Interventions
All trials compared artesunate plus mefloquine with mefloquine alone. Four trials had additional arms that were not considered in this review. As shown in Table 3, the total dose of artesunate and mefloquine varied across the trials; some trials used single doses and others divided the doses.
3. Total dose and regimens: artesunate and mefloquine.
| Trial | Artesunate | Mefloquine | ||
| Total dose (mg/kg) | Regimen | Total dose (mg/kg) | Regimen | |
| Karbwang 1994 | 3.33a | 1 dose | 21b | 2 doses over 1 day |
| Looareesuwan 1992a | 10 | 11 doses over 5 days | 21b | 2 doses over 1 day |
| Marquino 2003 | 12 | 3 doses over 3 days | 15 | 1 dose |
| Nosten 1994a | 4 | 1 dose | 25 | 1 dose |
| Nosten 1994b | 10 | 4 doses over 3 days | 25 | 1 dose |
| Price 1995 | 12 | 3 doses over 3 days | 25 | 1 dose |
| Smithius 2004 | 4 | 1 dose | 15 | 1 dose |
| Thimasarn 1997 | 10a | 2 doses over 2 days | 21b | 2 doses over 3 days |
aAssuming 60 kg person (actual dose 200 mg). bAssuming 60 kg person (actual dose 1250 mg).
Drug resistance
The seven trials conducted in South‐East Asia were in areas described as having multiple‐drug‐resistance to antimalarial drugs; nearly all mentioned high resistance to mefloquine. Marquino 2003 did not mention resistance to mefloquine, but it was likely to be low.
Outcome measures
All trials reported the presence of parasites at various time points in addition to other review outcomes. None reported on adherence to the treatment schedule or the progression to severe malaria. All trials except Smithius 2004 mentioned adverse events, although some did not report them in detail.
Risk of bias in included studies
SeeTable 4and the 'Characteristics of included studies' for details.
4. Risk of bias assessment.
| Trial | Generation of allocation sequencec | Allocation concealmentd | Blindinge | Inclusion of all randomized participants in final analysisb |
| Karbwang 1994 | Unclear | Unclear | Unclear | Adequate |
| Looareesuwan 1992a | Unclear | Unclear | Unclear | Inadequate |
| Marquino 2003 | Adequate | Unclear | Unclear | Inadequate |
| Nosten 1994a | Unclear | Unclear | Unclear | Inadequate |
| Nosten 1994b | Unclear | Unclear | Unclear | Inadequate |
| Smithius 2004 | Unclear | Unclear | Unclear | Inadequate |
| Thimasarn 1997 | Unclear | Unclear | Unclear | Adequate |
| Price 1998 | Unclear | Unclear | Unclear | Inadequate |
aSee the 'Assessment of risk of bias in included studies' for the assessment methods, and the Characteristics of included studies' for the methods used in each trial. bFor primary outcomes. cUnclear: reported as random but method not revealed. dUnclear: not mentioned. eUnclear: unlikely to be blinded.
All trials reported using random methods to generate the allocation sequence, but only Marquino 2003 mentioned the method, a table of random numbers. None of the trials referred to allocation concealment. None of the trials mentioned blinding, but they were unlikely to have used blinding based on the nature of the treatment regimens. Losses to follow up were high and no trial included all the enrolled participants in the final analysis. Karbwang 1994 and Thimasarn 1997 included more than 90% of the participants in the final analysis (adequate). It was not possible to calculate the losses to follow up for Smithius 2004, and all the other trials included less than 90% of the enrolled participants in the final analysis (inadequate).
Effects of interventions
We have stratified the trials based on the total dose of mefloquine, either 25 mg/kg or 15 mg/kg. Within these stratifications we have grouped the trials according to the total dose of artesunate. The total doses in milligrams per kilogram are in Table 3; for some trials, we converted the doses into milligrams per kilogram from a total dose in milligrams.
1. Mefloquine total dose: 25 mg/kg
a. Artesunate total dose: ≥ 10 mg/kg
Four trials: Looareesuwan 1992b, Nosten 1994b, Price 1995, and Thimasarn 1997
Treatment failure
There were statistically significantly fewer treatment failures for participants taking artesunate plus mefloquine at all time points: day 28 (RR 0.17, 95% CI 0.06 to 0.47; 824 participants, 4 trials), day 42 (RR 0.23, 95% CI 0.14 to 0.39; 298 participants, 1 trial), and day 63 (RR 0.26, 95% CI 0.09 to 0.77; 501 participants, 2 trials) (Analysis 1.1)
1.1. Analysis.
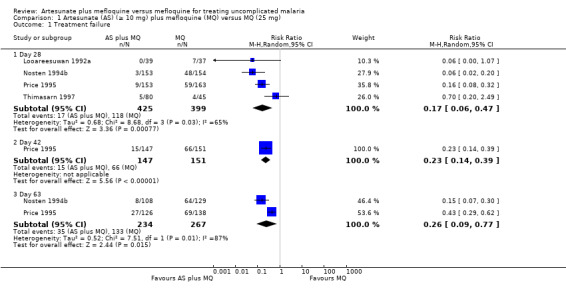
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 1 Treatment failure.
Parasitaemia
Artesunate plus mefloquine was associated with a statistically significant lower risk of parasitaemia at day three (RR 0.04, 95% CI 0.02 to 0.11; 716 participants, 2 trials), day seven (RR 0.02, 95% CI 0.00 to 0.11; 700 participants, 2 trials), and day 14 (RR 0.09, 95% CI 0.03 to 0.28; 339 participants, 1 trial) (Analysis 2.2)
2.2. Analysis.
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 2 Parasitaemia.
Resolution of fever
Nosten 1994b reported fewer participants still with fever in the artesunate plus mefloquine group at day two (RR 0.22, 95% CI 0.11 to 0.45; 348 participants) and day three (RR 0.02, 95% CI 0.00 to 0.38; 348 participants). Looareesuwan 1992b reported shorter fever clearance times with artesunate plus mefloquine (MD ‐32.20, 95% CI ‐46.54 to ‐17.86; 76 participants) (Analysis 1.1 and Analysis 1.4)
1.4. Analysis.
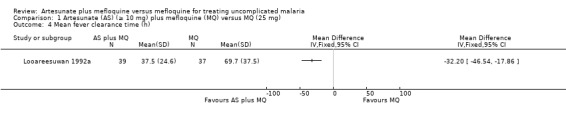
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 4 Mean fever clearance time (h).
Parasite clearance
Looareesuwan 1992b reported shorter parasite clearance times with artesunate plus mefloquine (MD ‐26.00, 95% CI ‐34.81 to ‐17.19; 76 participants) (Analysis 1.5)
1.5. Analysis.
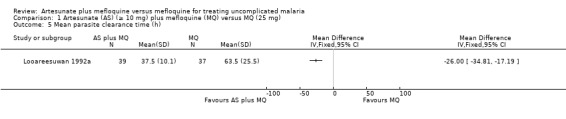
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 5 Mean parasite clearance time (h).
Adverse events
The results for Nosten 1994a and Nosten 1994b, for psychosis and vomiting, are combined under Nosten 1994a because the single publication containing these two trials did not provide a breakdown by trial.
Generally, the adverse events were similar for the two treatment groups although headache and nausea were statistically significantly more frequent in the mefloquine only group. One participant in the mefloquine group had a serious adverse event (frank psychosis requiring hospitalization; 1/183 versus 0/185) and another had treatment discontinued due to severe vomiting (1/183 versus 0/185) (Analysis 1.6)
1.6. Analysis.
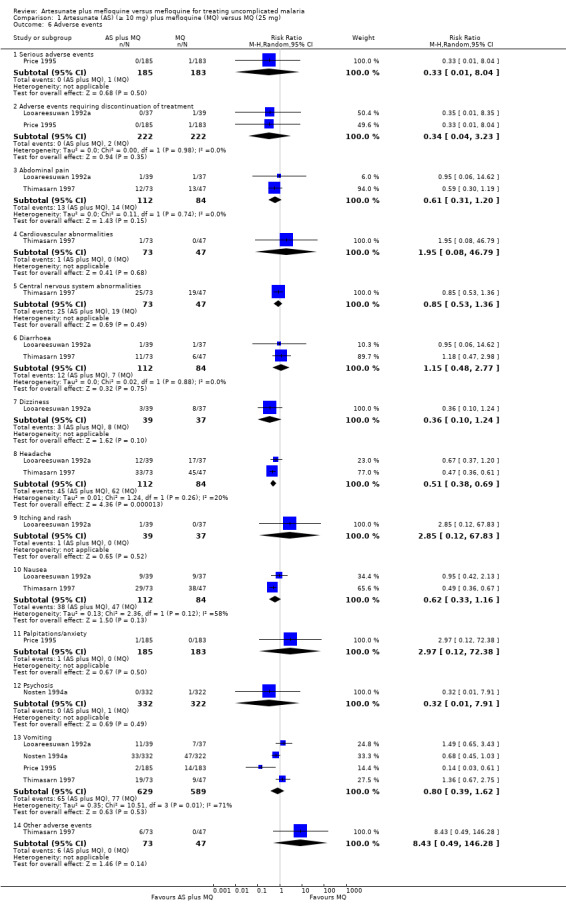
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 6 Adverse events.
b. Artesunate total dose: 4 mg/kg
Two trials: Nosten 1994a and Karbwang 1994
Treatment failure
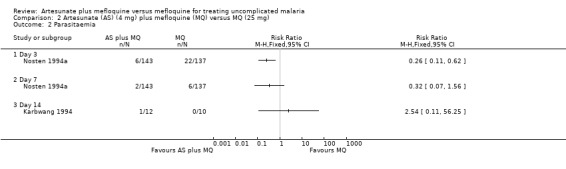
Artesunate plus mefloquine tended to perform better than mefloquine alone, but the effect was not statistically significant (RR 0.83, 95% CI 0.50 to 1.38; 259 participants, 2 trials). Although the point estimates for the individual trials are in opposite directions, the confidence intervals overlap. Karbwang 1994 also reported on treatment failure at day 42: four of 12 participants in the artesunate plus mefloquine group failed treatment compared with one of eight in the mefloquine group. This very small trial has low statistical power to detect differences, and thus results should be interpreted with caution (Analysis 2.1)
2.1. Analysis.
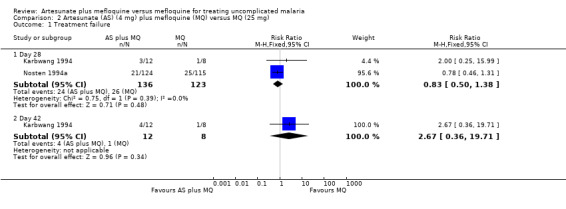
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 1 Treatment failure.
Parasitaemia
Artesunate plus mefloquine tended to reduce the risk of parasitaemia at days three and seven in Nosten 1994a, but the effect was only statistically significant at day three (RR 0.26, 95% CI 0.11 to 0.62; 280 participants). Karbwang 1994 reported on parasitaemia at day 14, but the results are difficult to interpret because of the very small numbers (Analysis 2.2) .
Resolution of fever
Fever resolved statistically significantly faster in participants in the artesunate plus mefloquine group at day two (RR 0.37, 95% CI 0.19 to 0.74; 239 participants) and day three (RR 0.33, 95% CI 0.12 to 0.89; 239 participants) in Nosten 1994a. These results were statistically significant unlike the shorter mean fever clearance times reported for the artesunate plus mefloquine group in Karbwang 1994 (Analysis 2.3 and Analysis 2.4)
2.3. Analysis.
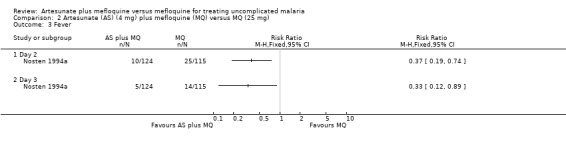
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 3 Fever.
2.4. Analysis.
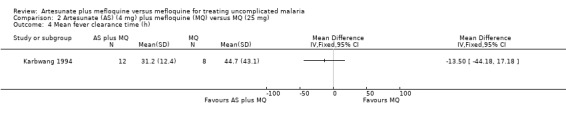
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 4 Mean fever clearance time (h).
Parasite clearance
Karbwang 1994 reported shorter mean parasite clearance times for the artesunate plus mefloquine group, but the difference was not statistically significant (Analysis 2.5)
2.5. Analysis.
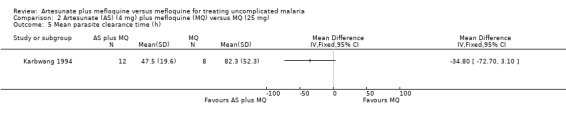
Comparison 2 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 5 Mean parasite clearance time (h).
Adverse events
Karbwang 1994 did not give details of adverse events but reported that nausea, vomiting, diarrhoea, dizziness, and bradycardia were similar for the two treatment groups. The single publication reporting on Nosten 1994a and Nosten 1994b did not give a breakdown of adverse events by trial; they are reported under Nosten 1994a in the meta‐analysis.
2. Mefloquine total dose: 15 mg/kg
a. Artesunate total dose: 12 mg/kg
One trial: Marquino 2003
Parasitaemia
Fewer participants had parasitaemia in the artesunate plus mefloquine group on day three (RR 0.80, 95% CI 0.01 to 0.57; 98 participants) (Analysis 3.1)
3.1. Analysis.
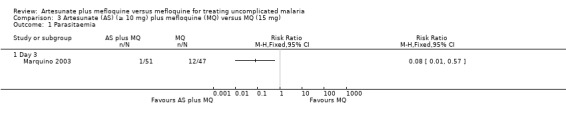
Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 1 Parasitaemia.
Resolution of fever
There was no difference in resolution of fever between the two treatment groups, but the participant numbers were very small and confidence intervals wide (Analysis 3.2).
3.2. Analysis.
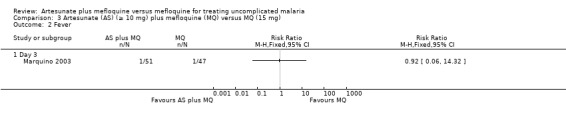
Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 2 Fever.
Adverse events
Insomnia was reported in one participant in the mefloquine group (Analysis 3.3).
3.3. Analysis.
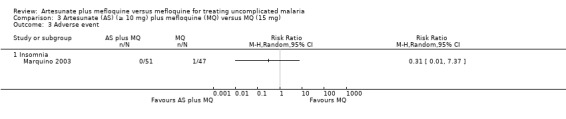
Comparison 3 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 3 Adverse event.
b. Artesunate total dose: 4 mg/kg
One trial: Smithius 2004
Treatment failure
Levels of treatment failure were similar across the two treatment groups at days 14, 28, and 42 (Analysis 4.1).
4.1. Analysis.
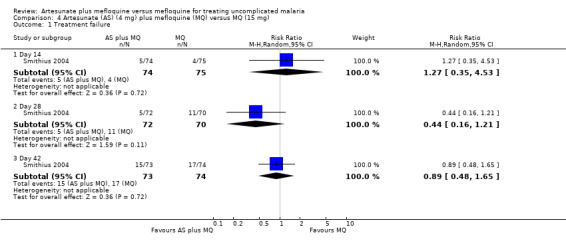
Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 1 Treatment failure.
Parasitaemia
There were similar results for parasitaemia at day three although more participants in the mefloquine group had positive blood slides (10/80 versus 4/76 for mefloquine and artesunate plus mefloquine, respectively) (Analysis 4.2).
4.2. Analysis.
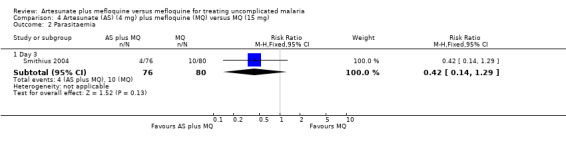
Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 2 Parasitaemia.
Resolution of fever
Nine of 76 participants on artesunate plus mefloquine compared with seven of 78 participants on mefloquine still had documented fever at day three (Analysis 4.3)
4.3. Analysis.
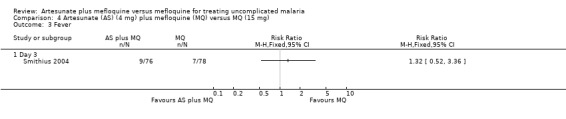
Comparison 4 Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg), Outcome 3 Fever.
Adverse events
This trial did not comment on adverse events.
Discussion
This review includes 1155 participants from eight trials. The data are mainly from South‐East Asia where malaria transmission is low and multiple‐drug resistance is prevalent.
Few trials reported the number of participants screened and eligible, the numbers excluded or withdrawn, or the numbers lost to follow up in each treatment group at each target visit. Where possible we used the randomized participants to form our denominator (approximated intention‐to‐treat analyses), but where this information was unavailable we used the numbers as given in the trial report. It was not clear from the trial reports if any of the trials that concealed allocation of treatment also used blinding, but based on the description of the regimens this was unlikely. This might affect the quality of trial results by introducing bias and therefore limiting the conclusions that can be made.
Although some trials included both children and adults, we were unable to analyse outcomes by age group because the trial reports did not provide details.
Few participants contributed to each given outcome because of a lack of uniformity in the way the trials measured and reported on outcomes, and in the drug doses and regimens. This creates uncertainty with the observed result and some comparisons did not have much statistical power to detect differences.
The combination of artesunate and mefloquine generally results in higher cure rate and better fever control than mefloquine alone. A total dose of 25 mg/kg mefloquine and at least 10 mg/kg artesunate gave the best results. The effects were more varied with a lower dose of artesunate (4 mg/kg), but the combination was still better than mefloquine alone. There were insufficient data to determine the effect of using at least 10 mg/kg artesunate and 15 mg/kg mefloquine. The combination of 4 mg/kg artesunate and 15 mg/kg mefloquine did not perform better than mefloquine alone.
One serious adverse event (psychosis) was observed in the mefloquine alone group. Other adverse events were similar across treatment groups, sometimes leading to discontinuation of treatment. Because the number of participants is still small to enable the risk of rare but important adverse events to be determined, surveillance of artesunate plus mefloquine is needed.
Authors' conclusions
Implications for practice.
Artesunate plus mefloquine is better than mefloquine alone for clearing malaria parasites and resolving fever in people with uncomplicated P. falciparum malaria in areas with low malaria transmission, although the trials reviewed were of variable methodological quality. Adding artesunate to mefloquine does not lead to any more adverse events than when mefloquine is used alone. A total dose of 25 mg/kg mefloquine and at least 10 mg artesunate lead to faster symptom relief and better cure. There are insufficient data to determine the effect of using lower doses of mefloquine.
Implications for research.
Randomized controlled trials are needed from areas outside of South‐East Asia, as are trials in children. These trials need to determine the optimal regimen of artesunate plus mefloquine and should use uniform outcome definitions and standardized reporting to allow for comparability of results. Trials should also include outcomes on symptom resolution as these are of particular interest to malaria patients. Trialists should use rigorous methodology, particularly for allocation concealment and blinding, and thorough reporting.
2008: As monotherapy is no longer recommended by the World Health Organization for malaria treatment (WHO 2006), the authors do not intend to update this review.
What's new
| Date | Event | Description |
|---|---|---|
| 14 March 2012 | Amended | The CIDG is piloting a new classification system for reviews. The classification for this review has now been added; description included in "Published notes" section of review |
| 13 August 2008 | Review declared as stable | As monotherapy is no longer recommended by the World Health Organization for malaria treatment (WHO 2006), the authors do not intend to update this review. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format and minor editing. |
Notes
2012, Issue 4: Status: Historical question – no update intended: monotherapy no longer recommended
As of August 2008, this Cochrane Review is no longer being updated. The question addressed by this Cochrane Review is no longer considered to be relevant to decision making, as monotherapy has been replaced by Artemisinin‐based combination therapy, and is no longer used. For the most up‐to‐date information regarding malaria treatments, please see: Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD007483. DOI: 10.1002/14651858.CD007483.pub2
The review status is a pilot system used by the Cochrane Infectious Diseases Group to help the reader understand whether the review is concerns a current question, and is up to date.
We report on:
1. The question the review addresses. Is it a:
Historical question, where the intervention or policy has been superseded by new medical developments (such as a new drug); or a
Current question, which is still relevant to current policy or practice.
2. Whether the review is up to date. Is the review:
Up to date;
Update pending; or
No update intended.
We then provide comment on the review status, to help explain the categories selected.
Acknowledgements
The Protocol for this Cochrane Review was developed during the July 2002 Fellowship Programme organized by the Cochrane Infectious Diseases Group through the Effective Health Care Alliance Programme (EHCAP) at the Liverpool School of Tropical Medicine.
This document is an output from a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Data and analyses
Comparison 1. Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 28 | 4 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.06, 0.47] |
| 1.2 Day 42 | 1 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.14, 0.39] |
| 1.3 Day 63 | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.09, 0.77] |
| 2 Parasitaemia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 3 | 2 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.11] |
| 2.2 Day 7 | 2 | 700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.11] |
| 2.3 Day 14 | 1 | 339 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.03, 0.28] |
| 3 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean fever clearance time (h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean parasite clearance time (h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Serious adverse events | 1 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 8.04] |
| 6.2 Adverse events requiring discontinuation of treatment | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.04, 3.23] |
| 6.3 Abdominal pain | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.20] |
| 6.4 Cardiovascular abnormalities | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.08, 46.79] |
| 6.5 Central nervous system abnormalities | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.53, 1.36] |
| 6.6 Diarrhoea | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.48, 2.77] |
| 6.7 Dizziness | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.10, 1.24] |
| 6.8 Headache | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.38, 0.69] |
| 6.9 Itching and rash | 1 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 2.85 [0.12, 67.83] |
| 6.10 Nausea | 2 | 196 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.33, 1.16] |
| 6.11 Palpitations/anxiety | 1 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 72.38] |
| 6.12 Psychosis | 1 | 654 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.91] |
| 6.13 Vomiting | 4 | 1218 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.62] |
| 6.14 Other adverse events | 1 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 8.43 [0.49, 146.28] |
1.2. Analysis.
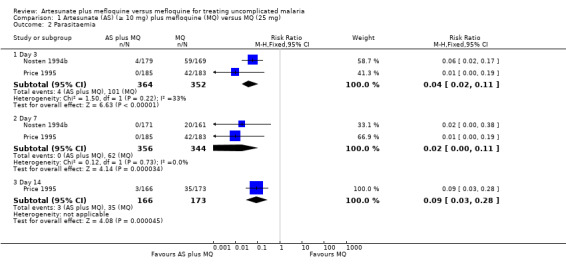
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 2 Parasitaemia.
1.3. Analysis.
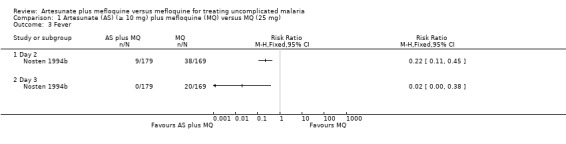
Comparison 1 Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (25 mg), Outcome 3 Fever.
Comparison 2. Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (25 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Day 28 | 2 | 259 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.38] |
| 1.2 Day 42 | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.36, 19.71] |
| 2 Parasitaemia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean fever clearance time (h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean parasite clearance time (h) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Artesunate (AS) (≥ 10 mg) plus mefloquine (MQ) versus MQ (15 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Parasitaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse event | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Insomnia | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Artesunate (AS) (4 mg) plus mefloquine (MQ) versus MQ (15 mg).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Day 14 | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.35, 4.53] |
| 1.2 Day 28 | 1 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.16, 1.21] |
| 1.3 Day 42 | 1 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.48, 1.65] |
| 2 Parasitaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day 3 | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.14, 1.29] |
| 3 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Karbwang 1994.
| Methods | Randomized controlled trial Length of follow up: 42 d Generation of allocation sequence: no details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 9.1% (2/22) excluded after randomization due to vomiting of trial drugs |
|
| Participants | Number enrolled: 22 participants aged 17 to 48 years Inclusion criteria: adult male patients; aged 17 to 48 years; 45 to 70 kg bodyweight; uncomplicated malaria; asexual parasites < 5% Exclusion criteria: history of liver or kidney disease |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 200 mg Mefloquine: 750 mg followed by 500 mg 6 h later |
|
| Outcomes | 1. Mean fever clearance time 2. Mean parasite clearance time 3. Adverse events 4. Parasite counts at various time points reportedly measured but not reported | |
| Notes | Location: Bangkok, Thailand Date: not given Source of funding: UNDP/World Bank/WHO Special Programme for Training in Tropical Diseases, Atlantic Co. Ltd (Thailand), and Hoffmann‐La Roche (Thailand) |
|
Looareesuwan 1992a.
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: no details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 9.4% (8/85) excluded at day 28 from analysis due to severe vomiting of study drugs and concomitant illness but other losses to follow up were not accounted for |
|
| Participants | Number enrolled: 127 participants between 16 and 56 years old Inclusion criteria: acute uncomplicated falciparum malaria; 100 to 200,000 parasites/µL blood; 16 to 60 years old; 45 to 60 kg bodyweight who agreed to stay in hospital for 28 days Exclusion criteria: pregnancy; severe malaria; and history of antimalarial drug treatment in preceding 1 week |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 100 mg followed by 50 mg/12 h for 5 d Mefloquine: 750 mg followed by 500 mg after 6 h Not included in this review: 3. Artesunate |
|
| Outcomes | 1. Number cured at 28 d 2. Mean parasite clearance time 3. Mean fever clearance time 4. Adverse events | |
| Notes | Location: Bangkok, Thailand Date: January to May 1991 Source of funding: Mahidol University research grant and Roche Research Foundation of Hong Kong; Atlantic Pharmaceutical Co. Ltd supplied artesunate tablets |
|
Marquino 2003.
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: table of random numbers Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 14.8% (17/115) were excluded from analysis at day 28 because of loss to follow up and taking antimalarial medication outside of the trial |
|
| Participants | Number enrolled: 115 children and adults aged 5 to 50 years Inclusion criteria: falciparum mono infection with 500 to 30,000 parasites/µl of blood; axillary temperature at least 37.5 °C and/or history of fever in previous 48 h Exclusion criteria: severe malaria; presence of other causes of fever; pregnancy; and history of allergy to trial medication |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 4 mg/kg/d for 3 d, single dose Mefloquine: 15 mg/kg, single dose |
|
| Outcomes | 1. Fever on day 3 2. Parasite carriage at days 3, 7, 14, 21, and 28 3. Adverse events | |
| Notes | Location: Peruvian Amazon Date: June to September 2000 Source of funding: US Agency for International Development, the US Naval Medical Research Command, and the Government of Peru |
|
Nosten 1994a.
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: randomization in pairs, no further details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 21.4% (65/304) excluded at day 28 due to vomiting trial medication and loss to follow up |
|
| Participants | Number enrolled: 304 adults and children Inclusion criteria: slide‐confirmed malaria; weight > 5 kg; no use of other antimalarial in previous month; no signs of severe disease; and not pregnant |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 4 mg/kg, single dose Mefloquine: 25 mg/kg, single dose |
|
| Outcomes | 1. Fever clearance time 2. Parasite clearance time 3. Parasite carriage at days 1, 2, 3, 4, 5, 7, 14, and 28 4. Adverse events | |
| Notes | Location: Thai‐Burmese boarder near Mae Sot Date: January to July 1992 Source of funding: not reported Nosten 1994a and Nosten 1994b are published in the same article |
|
Nosten 1994b.
| Methods | Randomized controlled trial Length of follow up: 63 d Generation of allocation sequence: no details Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 11.8% excluded at day 28 and 31.9% excluded at day 63 due to vomiting trial medication and attrition |
|
| Participants | Number enrolled: 348 adults and children Inclusion criteria: slide‐confirmed malaria; weight > 5 kg; no use of other antimalarial in previous month; no signs of severe disease; and not pregnant |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 4 mg/kg followed by 2 mg daily for 3 d Mefloquine: 25 mg/kg, single dose |
|
| Outcomes | 1. Fever clearance time 2. Parasite clearance time 3. Parasite carriage at days 1, 2, 3, 4, 5, 7, 14, and 28 4. Adverse events | |
| Notes | Location: Thai‐Burmese boarder near Mae Sot Date: October 1992 to June 1993 Source of funding: not reported Nosten 1994a and Nosten 1994b are published in the same article |
|
Price 1995.
| Methods | Randomized controlled trial Length of follow up: 63 d Generation of allocation sequence: randomized in blocks of three, no further details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 14.1% (46/362) lost to follow up at day 28, and 28.3% (98/362) lost to follow up at day 63; participants excluded because of vomiting and for reasons reportedly unrelated to the trial |
|
| Participants | Number enrolled: 550 Inclusion criteria: weight at least 5 kg; slide‐confirmed falciparum malaria; and no antimalarial treatment in preceding 63 d Exclusion criteria: pregnancy; signs of severe malaria or concomitant illness requiring hospitalization; and history of neuropsychiatric illness |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 4 mg/kg/d for 3 d Mefloquine: 25 mg/kg, single dose on day 2 Not included in this review: 3. Artemether plus mefloquine |
|
| Outcomes | 1. Parasite clearance time 2. Fever clearance time 3. Symptom clearance time 4. Adjusted cumulative failure rates at days 7, 28, 42, and 63 5. Adverse events | |
| Notes | Location: Thai‐Myanmar border Date: June 1993 to May 1994 Source of funding: Wellcome Trust of Great Britain |
|
Smithius 2004.
| Methods | Randomized controlled trial Length of follow up: 42 d Generation of allocation sequence: not mentioned Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: not possible to calculate losses to follow up because numbers were not clear |
|
| Participants | Number enrolled: 317, but only 156 given interventions relevant to this review Inclusion criteria: axillary temperature at least 37.5 °C or history of fever in previous 2 d; Plasmodium falciparum parasites of at least 1000/mm3 blood Exclusion criteria: body weight < 5 kg; pregnant women; signs and symptoms of complicated malaria or suggestive of another cause of fever; patients with a recent history of mefloquine use; patients with a parasite count > 250,000/mm3 and mixed infections |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine Artesunate: 4 mg/kg, single dose Mefloquine: 15 mg/kg, single dose Not included in this review: 3. Chloroquine 4. Sulfadoxine‐pyrimethamine |
|
| Outcomes | 1. Treatment failure at days 14, 28, and 42 2. Parasitaemia at day 3 3. Fever at day 3 | |
| Notes | Location: Kachin State, north Myanmar Date: July to August 2004 Source of funding: Médecins Sans Frontières (Holland) |
|
Thimasarn 1997.
| Methods | Randomized controlled trial Length of follow up: 28 d Generation of allocation sequence: no details available Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: 3% (12/394) excluded post‐randomization for taking wrong regimen and vomiting of trial drugs but distribution across groups not given |
|
| Participants | Number enrolled: 394 participants Inclusion criteria: symptomatic adults > 15 years old with malaria contracted within 50 km radius of the trial clinics; asexual parasitaemia 500 to 400,000 parasites/µL blood; no signs of complications; no history of antimalarial drug intake in previous 2 weeks; and consent to stay in malaria free area for the period of follow up Exclusion criteria: pregnancy |
|
| Interventions | 1. Artesunate plus mefloquine
2. Mefloquine
Artesunate: 300 mg/d for 2 d
Mefloquine: 750 mg on day 1 and 500 mg on day 2 Not included in this review: 3. Artesunate 4. Artemether 5. Quinine 6. Artemether‐mefloquine 7. Artesunate plus mefloquine with a different mefloquine dose |
|
| Outcomes | 1. Cure rate at day 28
2. Adverse events Not included in this review: 3. Parasite malaria drug sensitivity 4. Parasite malaria drug resistance |
|
| Notes | Location: Thai‐Cambodia border Date: July 1993 to December 1994 Source of funding: World Health Organization country budget for Thailand (THA/DPC/001) |
|
Allocation concealment: B = unclear, see 'Methods of the review' for details and a summary of the quality assessment in Table 04
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 2005 | Used different mefloquine doses for combination and mefloquine alone study arms |
| Cardoso 1996 | Used different mefloquine doses for combination and mefloquine alone study arms |
| Looareesuwan 1993 | Used different mefloquine doses for combination and mefloquine alone study arms |
| Luxemburger 1991 | Used different mefloquine doses for combination and mefloquine alone study arms |
| Price 1998 | Compared artesunate plus mefloquine with artesunate rather than mefloquine |
Differences between protocol and review
We changed the title of the review from 'Artesunate plus mefloquine for treating malaria' to the current title to reflect the review's inclusion criteria. We include only one primary outcome measure, treatment failure, following recent developments in knowledge around the accuracy of some malaria treatment outcomes. In line with changes in the guidance from the Cochrane Infectious Diseases Group, we updated the methods for assessing blinding and changed the wording of "loss to follow up" to "inclusion of all randomized participants in the final analysis".
Contributions of authors
Hasifa Bukirwa extracted and analysed data, and drafted the review. Lois Orton extracted data, and edited and advised on the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Karbwang 1994 {published data only}
- Karbwang J, Na Bangchang K, Thanavibul A, Back DJ, Bunnag D, Harinasuta T. Pharmacokinetics of mefloquine alone or in combination with artesunate. Bulletin of the World Health Organization 1994;72(1):83‐7. [PMC free article] [PubMed] [Google Scholar]
Looareesuwan 1992a {published data only}
- Looareesuwan S, Viravan C, Vanijanonta S, Wilairatana P, Suntharasamai P, Charoenlarp P, et al. Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria. Lancet 1992;339(8797):821‐4. [DOI] [PubMed] [Google Scholar]
Marquino 2003 {published data only}
- Marquino W, Huilca M, Calampa C, Falconi E, Cabezas C, Naupay R, et al. Efficacy of mefloquine and mefloquine‐artesunate combination therapy for the treatment of uncomplicated Plasmodium falciparum malaria in the Amazon Basin of Peru. American Journal of Tropical Medicine and Hygiene 2003;68(5):608‐12. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Hijar G, Montoya Y, Marouino W, Ruebush TK 2nd, Wongsrichanalai C, et al. Lack of prediction of mefloquine and mefloquine‐artesunate treatment outcome by mutations in the Plasmodium falciparum multidrug resistance 1 (pfmdr1) gene for P. falciparum malaria in Peru. American Journal of Tropical Medicine and Hygiene 2003;68(1):107‐10. [PubMed] [Google Scholar]
Nosten 1994a {published data only}
- Nosten F, Luxemburger C, ter Kuile FO, Woodrow C, Pah Eh J, Chongsuphajaisiddhi T, et al. Treatment of multidrug‐resistant Plasmodium falciparum malaria with 3‐day artesunate‐mefloquine combination. Journal of Infectious Diseases 1994;170(4):971‐7. [DOI] [PubMed] [Google Scholar]
Nosten 1994b {published data only}
- Nosten F, Luxemburger C, ter Kuile FO, Woodrow C, Pah Eh J, Chongsuphajaisiddhi T, et al. Treatment of multidrug‐resistant Plasmodium falciparum malaria with 3‐day artesunate‐mefloquine combination. Journal of Infectious Diseases 1994;170(4):971‐7. [DOI] [PubMed] [Google Scholar]
Price 1995 {published data only}
- Price RN, Nosten F, Luxemburger C, Kham A, Brockman A, Chongsuphajaisiddhi T, et al. Artesunate versus artemether in combination with mefloquine for the treatment of multidrug‐resistant falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89(5):523‐7. [DOI] [PubMed] [Google Scholar]
Smithius 2004 {published data only}
- Smithuis F, Shahmanesh M, Kyaw MK, Savran O, Lwin S, White NJ. Comparison of chloroquine, sulfadoxine/pyrimethamine, mefloquine and mefloquine‐artesunate for the treatment of falciparum malaria in Kachin State, North Myanmar. Tropical Medicine and International Health 2004;9(11):1184‐90. [DOI] [PubMed] [Google Scholar]
Thimasarn 1997 {published data only}
- Thimasarn K, Sirichaisinthop J, Chanyakhun P, Palananth C, Rooney W. A comparative study of artesunate and artemether in combination with mefloquine on multidrug resistant falciparum malaria in Eastern Thailand. SouthEast Asian Journal of Tropical Medicine and Public Health 1997;28(3):465‐71. [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 2005 {published data only}
- Adam I, A‐Elbasit IE, Elbashir MI. Efficacies of mefloquine alone and of artesunate followed by mefloquine, for the treatment of uncomplicated, Plasmodium falciparum malaria in eastern Sudan. Annals of Tropical Medicine and Parasitology 2005;99(2):111‐7. [DOI] [PubMed] [Google Scholar]
Cardoso 1996 {published data only}
- Cardoso Bda S, Dourado HV, Pinheiro Mda C, Crescente JA, Amoras WW, Baena J, et al. An efficacy and tolerance study of oral artesunate alone and in combination with mefloquine in the treatment of umcomplicated falciparum malaria in an endemic area of Para, Brazil [Estudo da eficacia e tolerancia do artesunato oral isolado e em associacao com mefloquina, no tratamanto da malaria falciparum nao complicada em area endemica do Para, Brasil]. Revista da Sociedade Brasileira de Medicina Tropical 1996;29(3):251‐7. [DOI] [PubMed] [Google Scholar]
Looareesuwan 1993 {published data only}
- Looareesuwan S, Vanijanonta S, Viravan C, Wilairatana P, Charoenlarp P, Andrial M. Randomized trial of mefloquine alone and artesunate followed by mefloquine for the treatment of acute uncomplicated falciparum malaria. Annals of Tropical Medicine and Parasitology 1994;88(2):131‐6. [DOI] [PubMed] [Google Scholar]
Luxemburger 1991 {published data only}
- Luxemburger C, ter Kuile FO, Nosten F, Dolan G, Bradol JH, Phaipun L, et al. Single day mefloquine‐artesunate combination in the treatment of multi‐drug resistant falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88(2):213‐7. [DOI] [PubMed] [Google Scholar]
Price 1998 {published data only}
- Price R, Luxemburger C, Vugt M, Nosten F, Kham A, Simpson J, et al. Artesunate and mefloquine in the treatment of uncomplicated multidrug‐resistant hyperparasitaemic falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1998;92(2):207‐11. [DOI] [PubMed] [Google Scholar]
Additional references
Angus 2001
- Angus BJ, Thaiaporn I, Chanthapadith K Suputtamongkol Y, White NJ. Oral artesunate dose‐response relationship in acute falciparum malaria. Journal of the Medical Association of Thailand 2001;84(9):1289‐99. [Google Scholar]
Bethell 1997
- Bethell DB, Teja‐Isavadharm P, Cao XT, Pham TT, Ta TT, Tran TN, et al. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1997;91(2):195‐8. [DOI] [PubMed] [Google Scholar]
Brewer 1994
- Brewer GT, Peggins OJ, Grate JS, Petras JM, Levine BS, Weina PJ, et al. Neurotoxicity in animals due to arteether and artemether. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88 Suppl 1:33‐6. [DOI] [PubMed] [Google Scholar]
Gilles 2000
- Gilles HM. Management of severe malaria: a practical handbook. 2nd Edition. Geneva: World Health Organization, 2000. [Google Scholar]
Higgins 2005
- Higgins J, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions [updated March 2005]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 18 April 2005).
Hoshen 2000
- Hoshen MB, Na‐Bangchang K, Stein WD, Ginsburg H. Mathematical modelling of the chemotherapy of Plasmodium falciparum malaria with artesunate: Postulation of "dormancy" a partial cystostatic effect of the drug, and its implication of treatment regimens. Parasitology 2000;121(3):237‐46. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in healthcare: Assessing the quality of controlled trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kain 1995
- Kain KC. Chemotherapy and prevention of drug‐resistant malaria. Wilderness & Environmental Medicine 1995;6(3):307‐24. [DOI] [PubMed] [Google Scholar]
Looareesuwan 1992b
- Looareesuwan S, Harinasuta T, Chongsuphajaisiddhi T. Drug resistant malaria, with special reference to Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 1992;23(4):621‐34. [PubMed] [Google Scholar]
Looareesuwan 1998
- Looareesuwan S, Wilairatana P, Chokejindachai W, Viriyavejakul P, Krudsood S, Singhasivanon P. Research on new antimalarial drugs and the use of drugs in combination at the Bangkok Hospital for Tropical Diseases. Southeast Asian Journal of Tropical Medicine and Public Health 1998;29(2):344‐54. [PubMed] [Google Scholar]
McIntosh 1999
- McIntosh HM, Olliaro P. Artemisinin derivatives for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD000256] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 2000
- McIntosh HM, Olliaro P. Artemisinin derivatives for treating severe malaria. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Olliaro 1996
- Olliaro O, Cattani J, Wirth D. Malaria, the submerged disease. JAMA 1996;275(3):230‐3. [PubMed] [Google Scholar]
Price 1996
- Price RN, Nosten F, Luxemburger C, ter Kuile FO, Paiphun L, Chongsuphajaisiddhi T, et al. Effects of artemisinin derivatives on malaria transmissibility. Lancet 1996;347(9016):1654‐8. [DOI] [PubMed] [Google Scholar]
RBM 2001a
- Global Partnership to Roll Back Malaria. Combination therapy with antimalarial drugs. Antimalarial drug combination therapy: report of a WHO technical consultation, 4‐5 April 2001. Geneva: World Health Organization, 2001:7‐8. [Google Scholar]
RBM 2001b
- Global Partnership to Roll Back Malaria. 2.2 Rationale for the use of combination therapy & 2.3 Artemisinin‐based combination therapy. The use of antimalarial drugs: report of a WHO informal consultation, 13‐17 November 2000. Geneva: World Health Organization, 2001:17‐9. [Google Scholar]
RBM 2001c
- Global Partnership to Roll Back Malaria. Artemisinin‐based combinations. The use of antimalarial drugs: Report of a WHO informal consultation, 13‐17 November 2000. Geneva: World Health Organization, 2001:12‐5. [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Trung 2001
- Trung TN, Davis TM, Hewitt S, Thuan LK, Quang HH, Anh CV, et al. Treatment of falciparum malaria in Vietnamese children: the need for combination therapy and optimized dosage regimens. Annals of Tropical Paediatrics 2001;21(4):307‐12. [DOI] [PubMed] [Google Scholar]
Weinke 1991
- Weinke T, Trautmann M, Helh T, Weber G, Eichenlaub D, Fleischer K, et al. Neuropsychiatric side effects after the use of mefloquine. American Journal of Tropical Medicine and Hygiene 1991;45(1):86‐91. [DOI] [PubMed] [Google Scholar]
White 1996
- White NJ. Can amodiaquine be resurrected?. Lancet 1996;348(9036):1184‐5. [DOI] [PubMed] [Google Scholar]
White 1997
- White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrobial agents and chemotherapy 1997;41(7):1413‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1999
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization/AFRO. Malaria. www.afro.who.int/malaria/bulletins/2001 (accessed 12 March 2003).
WHO 2002
- World Health Organization. Regional Office for Western Pacific. Report: meeting on antimalarial drug development / convened by: World Health Organization, Regional Office for the Western Pacific, Shanghai, China 16‐17 November 2001. Manila: WHO Regional Office for the Western Pacific, 2002. [Google Scholar]
WHO 2006
- World Health Organization, Roll Back Malaria Dept. Guidelines for the treatment of malaria [WHO/HTM/MAL/2006.1108]. Geneva: World Health Organization, 2006. [Google Scholar]
WHO/UNICEF 2003
- World Health Organization. Malaria Control Unit, UNICEF. The Africa Malaria Report 2003. Geneva: World Health Organization, 2003. [Google Scholar]
Wongsrichanalai 2002
- Wongsrichanalai C, Picjard AL, Wersdorfer WH, Meshnick SR. Epidemiology of drug resistant malaria. Lancet Infectious Diseases 2002;2(4):209‐18. [DOI] [PubMed] [Google Scholar]


