Abstract
Background
Around 120 million women worldwide suffer fromTrichomonas vaginalis vaginitis every year. The infection is sexually transmitted and is believed to facilitate HIV transmission.
Objectives
To assess the effects of various treatment strategies for trichomoniasis in women.
Search methods
We searched the Cochrane Central Register of Controlled Trials , MEDLINE, and EMBASE. Trials were also identified from reference lists of reviews, through pharmaceutical companies, and by informal discovery. Only published data were used in this review. Date of the most recent search: November, 2002.
Selection criteria
Randomised or quasi‐randomised trials of different treatment strategies in women with trichomoniasis. Different antitrichomonal drugs or doses were eligible, as were comparisons of treatment with no treatment or placebo.
Data collection and analysis
Trial quality was assessed and data extracted by two reviewers independently using standard criteria.
Main results
Fifty‐four trials were included. Nitroimidazole drugs seem to be effective in achieving parasitological cure in short term follow up. Partner treatment can be effective in decreasing longer term reinfection rates.
Authors' conclusions
Parasitological cure can be achieved by single oral dose of nitroimidazole. Further research should focus on developing effective partner treatment strategies to prevent reinfections and reduce trichomoniasis prevalence.
Keywords: Female, Humans, Antitrichomonal Agents, Antitrichomonal Agents/therapeutic use, Clinical Trials as Topic, Metronidazole, Metronidazole/therapeutic use, Nitroimidazoles, Nitroimidazoles/therapeutic use, Trichomonas Vaginitis, Trichomonas Vaginitis/drug therapy
Nitroimidazole drugs are effective in the treatment of trichomoniasis in women.
Trichomoniasis is a sexually transmitted infection that affects about 120 million women worldwide every year. This review examines the effectiveness of various treatments and found that oral nitroimidazole drugs are effective in treating trichomoniasis in women.
Background
Trichomonas vaginalis vaginitis is one of the most common sexually transmitted diseases, with around 120 million women worldwide estimated to suffer from trichomoniasis every year (WHO 1994). It has been shown to be a common infection in some communities in developing countries (WHO 1994).
Trichomoniasis infection is characterised by green‐yellow frothy vaginal discharge, pain on sexual intercourse, vulvovaginal soreness and itching, and pain on urination. However, many women with trichomoniasis have no symptoms (asymptomatic). Clinical diagnosis is not specific and laboratory confirmation is necessary. The parasite can be found in vaginal secretions, glands (both Bartholin's gland and Skene's gland), and the urethra. The wet mount smear is a cheap and quick diagnostic method but its sensitivity depends on the experience of the examiner and the amount of parasites in the vagina. Standard culture, transport/culture kits, enzyme immunoassay, nucleic acid amplification, and immunofluorescence methods are also available.
T. vaginalis vaginitis requires prompt and effective treatment. Metronidazole and other nitroimidazole drugs (such as ornidazole, tinidazole, nimorazole, and carnidazole) have been used as antitrichomonal agents for more than 30 years. Despite their widespread use, resistance has been relatively rare and generally managed by either higher doses or other nitroimidazoles. Clotrimazole, povidone‐iodine, and nonoxynol‐9 have been used as local intravaginal applications. Although oral medication is generally preferred, because of the presence of infection in the vaginal glands and urethra, therapeutic blood concentrations are also achieved with local (vaginal or rectal) application of metronidazole. The usual dose of metronidazole is a 2 g single dose or 250 mg three times daily for seven days. However, there are many variations of dose and duration of treatment with metronidazole and other nitroimidazoles. Repeat testing at 5‐7 days and at around 30 days may be done to evaluate the immediate success of the treatment and the short term recurrence rate respectively. In clinical practice, however, repeat testing is rarely done unless treatment failure is suspected.
Treatment strategies aim to treat infected women and ensure that sexual partners are also treated. Recurrence of infection is thought to be mainly caused by reinfection from a partner or failure to complete the treatment course. In addition to the morbidity caused by the infection, a main concern about the high prevalence of trichomoniasis is the possibility that reproductive tract infections (ulcerative or non‐ulcerative) increase the transmission rate of HIV. If this is so with trichomoniasis, high prevalence rates of the infection in some populations means the infection may be important in facilitating HIV transmission.
This review attempts to evaluate the evidence with regard to the most effective treatment strategy for T. vaginalis vaginitis in women.
Objectives
To compare the effectiveness of various treatment strategies for trichomoniasis in women who are not pregnant.
Methods
Criteria for considering studies for this review
Types of studies
Any trial where an attempt is made to allocate different forms of trichomoniasis treatment by a random or quasi‐random method was considered for inclusion.
Types of participants
Symptomatic or asymptomatic women, including adolescents, with confirmed (by a laboratory technique) Trichomonas vaginalis vaginitis. The setting where participants were enrolled (such as gynaecology outpatient, sexually transmitted disease or family planning clinic) was noted, as were other indicators of risk status (e.g. commercial sex workers).
Exclusion criteria: trials during pregnancy; trials in men; prophylactic interventions; interventions aimed at symptomatic relief only.
Types of interventions
Any treatment versus no treatment
Short (single dose, repeat doses up to 1‐2 days) versus longer (5‐10 day) treatment
Systemic versus local treatment
Partner treatment versus no treatment
Different partner treatment strategies (giving the medication to the women or seeing and counselling partners individually)
Comparison of two different agents
Comparison of different doses of the same agent
Types of outcome measures
Parasitological cure
Clinical cure (clearance of discharge, soreness, itching)
Side effects and complications of treatment
Search methods for identification of studies
Electronic literature search of MEDLINE and EMBASE. Standard three‐stage Cochrane search strategy was used for the MEDLINE search from 1966 to 2002 with the following disease terms: Trichomonas vaginalis (explode/all subheadings); Trichomonas vaginitis (explode/all subheadings); Trichomonas infections (explode/all subheadings); Trichomon*, Trichomon*, in title or abstract.
EMBASE was searched from 1980 to 2002 using "trichomonas" and "treatment" as search terms. African Index Medicus was searched to 1996. The specialised register of the Cochrane Infectious Diseases Group and the reference lists of identified trials and current reviews were searched. Manufacturers of metronidazole and tinidazole in the UK were contacted.
Letters were sent to authors of reviews on trichomoniasis treatment and staff at the Centers for Disease Control and Prevention (Sexually Transmitted Disease Control Programme) were contacted.
The Cochrane Central Register of Controlled Trials was searched in each successive issue of The Cochrane Library using the search term trichomon* . The latest search was in November 2002.
No study was excluded on the basis of the language in which it was written.
Data collection and analysis
All trials identified were considered for inclusion and are referenced in this review. Trials with objectives other than treatment of trichomoniasis in women where no indication of any kind of random allocation could be found were excluded without further evaluation. Authors were contacted if there were doubts about randomisation or the data were not in a suitable form for analysis. The risk of bias in eligible trials was evaluated in terms of allocation concealment, generation of the allocation sequence, blinding, and inclusion of all randomised participants according to the Cochrane Infectious Diseases Group guidelines.
In addition to prespecified outcomes, the following characteristics of trials were extracted:
Country
Characteristics of the study population
Exclusion criteria
Partner treatment measures
Loss to follow up
Women excluded from analyses
Diagnostic procedures used
Data were extracted independently from a random sample of trials that met the inclusion criteria (n = 4). This was done by an editor in the Infectious Diseases Group and compared with the data entered by the reviewer as part of routine quality monitoring. In the most recent update of this review duplicate data extraction has been done by the two reviewers for the newly included trials.
Treatment with a single dose, or two doses 12 to 48 hours apart, were regarded as "short" treatment regimens. Others ranging from 5 to 10 days were regarded as "long" treatment regimens.
In trials with multiple arms comparing different dosages of the same drug, these arms have been reduced to two for analysis purposes (e.g. metronidazole 1 g or less versus metronidazole greater than 1 g).
Results
Description of studies
Fifty‐four studies are included in this review. Ten of these were conducted in developing countries in Asia and Africa. Details are given in the table of 'Characteristics of included studies'. This review excluded treatment during pregnancy. There were few studies which combined pregnant and non‐pregnant women; when it was not possible to obtain separate data these studies were excluded (see 'Characteristics of excluded studies').
Of the trials included in this review the majority (40/54) compared different antitrichomonal drugs and/or different doses. Other treatment strategies included short versus long treatment (four trials), vaginal versus oral plus vaginal treatment (one trial), oral versus oral plus vaginal treatment (four trials), oral versus vaginal treatment (two trials), partner treatment versus no treatment (one trial), and comparison with a no treatment group (six trials). Some trials had more than one arm with different treatments versus control (i.e. no treatment). In all short versus long treatment trials metronidazole was used in the long treatment arm, compared to short treatment with metronidazole in two (Hager 1980, Thin 1979), ornidazole in one (Nygaard 1977). and tinidazole in one (Aimakhu 1975).
Study populations were heterogeneous. Women attending emergency departments, venereal disease clinics, gynaecology outpatient clinics, cancer screening clinics, prisons, and private practices were recruited in different trials.
All trials used at least one laboratory method for diagnosis.
Partner treatment measures are generally mentioned briefly. One trial (Lyng 1981) randomised partners of women with trichomoniasis to treatment or placebo. Others, in general, did not focus on partner treatment as part of the intervention although most attempted to treat the partners if they were available.
Some trials used extensive exclusion criteria such as the presence of other sexually transmitted diseases, poor general health, any other illnesses, and presumed promiscuity. This created rather atypical study populations. These were generally dose comparison trials possibly motivated by pharmaceutical companies. On the other hand, trials which looked at treating women who came for care (Hager 1980, Chunge 1992, Tidwell 1994) as well as others, had either high losses to follow up or exclusions which resulted in small proportions of enrolled women remaining for analysis. These women were also more likely to have concurrent sexually transmitted diseases. High losses to follow up, exclusions, and concurrent infections and treatments raise questions about the comparability of study groups within trials and the possibility of bias should be kept in mind when interpreting the results.
Risk of bias in included studies
Of 54 trials, 16 were classified as category A for allocation concealment, 30 as category B, and 8 as category C. Trials were given a quality score of A if they used secure concealment methods such as central randomisation, or sealed, opaque, envelopes. Inadequately concealed trials, such as those with open randomisation methods, received a score of C. Trials in category B described allocation as randomised but gave no further details on how allocation concealment was done. Some of these trials were reported as double blind. Since no further details were provided these trials were classified as randomised, allocation concealment unclear. Trials were generally small and only two that met the inclusion criteria had comparator arms containing more than 100 patients (Forster 1963, Saeed 1976).
The small dose/drug comparison trials had very few losses to follow up, while trials which studied higher risk women (venereal disease, emergency clinics) had higher loss to follow up rates (11‐62%). In the Hager trial, where short and long treatment regimens were compared, only 38% of the 468 women attended the follow up visit.
Only 13 trials (13/54) specifically reported that outcome assessment was blinded. It was not possible to ascertain whether outcome assessment was blinded in the others.
Effects of interventions
Treatment versus no treatment
Forster 1963 conducted a placebo controlled trial where the intervention groups were given oral metronidazole alone, or oral combined with vaginal metronidazole, over 10 days. At six weeks follow up there was a parasitological failure rate of 6% (18/287) in the treatment group. Trichomonas was still detected in 78% (111/142) of women in the placebo group.
Two trials (Gorlero 1992, Gorlero 1994) compared two different doses of fenticonazole vaginal ovules with a placebo group. Fenticonazole was given as a single dose in the first trial and as two doses on consecutive days in the second trial. Follow up parasitological examination was between days 4 and 7. The two different dose arms in each trial were grouped together for this comparison. Treatment resulted in a significantly higher number of women with parasitological cure (53/105 v 8/51). Of note is the relatively low overall cure rate with treatment (50%).
One trial (Csonka 1963) compared metronidazole with no treatment, two other trials (Rees 1974, Mati 1974) compared tinidazole with no treatment, while another trial (Lean 1972) compared metronidazole, tinidazole, and placebo. All these studies showed evidence of effective parasitological cure by nitroimidazoles.
Short versus longer duration of treatment
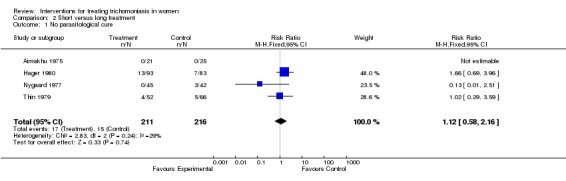
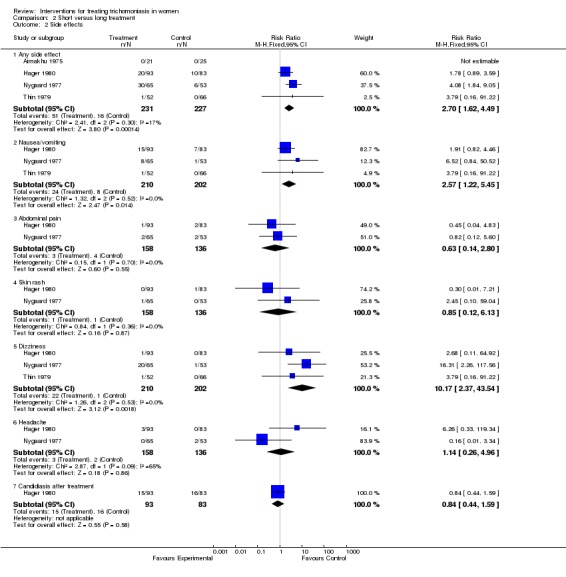
Two trials (Thin 1979, Hager 1980) compared a single 2 g oral dose of metronidazole with 5‐7 day course of metronidazole. Parasitological cure was achieved in 88% and 92% of women with short and long treatments, respectively. Side effects were mainly gastrointestinal (nausea, vomiting) and more frequent with the single dose (15% vs 7%). Although the Hager trial was relatively large (468 women enrolled), only 38% attended the follow up visit.
Hager 1980 also compared failure rates in subgroups of women who said that their partners took the treatment versus those whose partners did not, and those who admitted to sexual intercourse (they were advised against it) versus those who did not. No difference was found in either comparison (7‐21 days after treatment). However, high loss to follow up raises concern about the comparability of the two groups.
Two studies (Aimakhu 1975, Nygaard 1977) compared a standard one week course of metronidazole with short course tinidazole and ornidazole, respectively.
Overall, short treatment was comparable to long treatment in terms of no parasitological cure (RR 1.12, 95% CI 0.58 to 2.16). Side effects however, especially nausea/vomiting and dizziness, were significantly more frequent with short treatment.
Oral versus intravaginal treatment
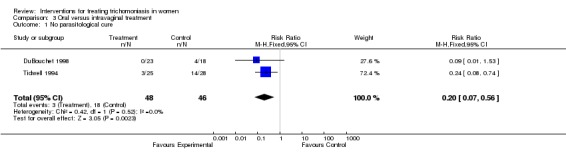
Two trials (Tidwell 1994, DuBouchet 1998) compared oral versus intravaginal treatment. Tidwell enrolled only culture‐positive women which (as they had to be called back) resulted in the loss of one third of eligible women. DuBouchet also lost 27% of participants by the first follow up visit, and 44% by the second follow up visit. Data from the first follow up visit were used. Our analysis showed that oral treatment was more successful in achieving parasitological cure.
One trial (Forster 1963) compared oral metronidazole treatment with oral and intravaginal treatment together (both long term) and found the combined regimen more effective.
Oral versus oral plus intravaginal treatment
Four trials (Chung 1978, Diwald 1971, Forster 1963, Gummerus 1983) compared oral versus oral plus intravaginal treatment. All of the trials except one (Diwald 1971) found combined oral and intravaginal treatment more effective.
Partner treatment versus no partner treatment
One trial (Lyng 1981) randomised partners of women with trichomoniasis (who received standard treatment with tinidazole 2 g) to treatment (tinidazole 2 g single dose) or placebo. Although there was no difference in parasitological results at the first follow up, significantly more women (3/59 vs 14/59) whose partners received placebo were Trichomonas positive by the second follow up at around two months compared with women whose partners were treated. History of intercourse did not affect the results.
No trials were identified that examined other different partner treatment strategies such as seeing or counselling partners individually.
Drug/dose comparisons
A number of small trials compared the effectiveness of different doses of the same drug, or compared various nitroimidazole drugs with each other.
Metronidazole was compared with tinidazole in eight studies (Anjaneyulu 1977, Begum 1980, Gabriel 1982, Garud 1978, Lean 1972, O‐Prasertsawat 1992, Rao 1978, Sandvei 1979). Except for one study (Lean 1972), all compared short regimens of each drug. There were no parasitological failures in two of the trials; however, our meta‐analysis results indicate a statistically significant higher treatment failure rate (RR 3.24, 95% CI 1.66 to 6.32), higher clinical failure rate (RR 3.81, 95% CI 1.83 to 7.90), and higher side effect rate (RR 1.65, 95% CI 1.35 to 2.02) with metronidazole. These results should be interpreted with caution as blind assessment of outcomes was reported in only one of the eight trials (Gabriel 1982). There was no statistical difference in parasitological or clinical outcomes in this trial.
Six trials compared tinidazole with ornidazole (Hillstroem 1977, Chaisilwattana 1980, Serup 1978, Chunge 1992, Sesti 1990, Sandvei 1979). These trials showed no difference in parasitological cure, and the one trial that reported on clinical cure showed no difference. The ornidazole group had a higher incidence of side effects, most notably fatigue (RR 0.18, 95% CI 0.05 to 0.58).
In most trials single dose treatment with any nitroimidazole drug resulted in parasitological cure rates above 90%. Although rarely severe, side effects seem to be relatively common and dose related.
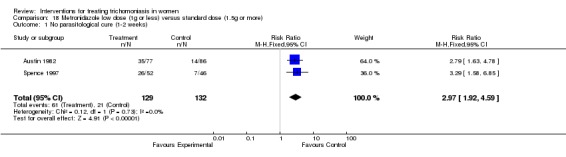
Two trials (Spence 1997, Austin 1982) compared different doses of short treatment metronidazole. One trial (Austin 1982) compared 1 g with 2 g, whereas the other (Spence 1997) compared 0.5 g, 1 g, 1.5 g, and 2 g. For the purpose of this review meta‐analysis was conducted comparing high (1.5 g or more) with low (1 g or less) dose treatment regimens. Lower dose treatment was found to be inferior to high dose (or rather standard dose) metronidazole in terms of failure to achieve parasitological cure (RR 2.97, 95% CI 1.92 to 4.59) with similar rates of side effects. Laboratory assessments were blinded in both trials.
Discussion
Strength of the evidence
This review includes multiple small trials that examine parasitological outcomes in comparisons of effectiveness between different nitroimidazole drugs, different doses, and different regimens. Such variation among the trials lend significant heterogeneity to the meta‐analysis, and results should be cautiously interpreted.
Only two trials had comparison groups of more than 100 women. Only one trial examined broader aspects of effective care in trichomoniasis by evaluating the effectiveness of partner treatment in reducing persistent infection.
Treatment effectiveness
The included trials showed that almost any nitroimidazole drug given as a single dose or over a longer period results in parasitological cure in 90% of cases. Oral single dose treatment with any nitroimidazole seems to be effective in achieving short term parasitological cure, but is associated with more frequent side effects than either longer oral or intravaginal treatment. Intravaginal treatment showed parasitological cure rates around 50% which is unacceptably low. The data on symptomatic relief from intravaginal treatment compared with oral treatment are not consistent. However, the trial by Bremond (Bremond 1987), which was excluded from the review because the focus was on symptomatic relief rather than parasitological cure, showed significant benefit from locally applied anti‐inflammatory treatment.
It is not possible to conclude that tinidazole is superior to metronidazole from the evidence reviewed. Outcome assessments were blinded in only one study that showed no difference between the two drugs. One argument in favour of tinidazole has been its longer half‐life in the body, hence possibly longer duration of effect when compared to metronidazole.
Partner treatment
Although effective clinical treatments exist, Trichomonas vaginalis vaginitis is still one of the most common sexually transmitted diseases. Reinfection by partners appears to be a major problem, and the one trial that examined partner treatment showed that this intervention reduced reinfection significantly. One other trial, which examined differential cure rates by a history of whether the partner had been treated, showed no difference. The latter data, however, are based on reported treatment history, are not from randomised comparisons, and have been gathered from short term follow up studies.
Drug resistance
Another problem that may not be as widespread as reinfection is drug resistance. Metronidazole is probably the most widely used nitroimidazole for trichomoniasis, and several case reports of resistance have been published although the true extent of this is not known.
Applicability
Effective treatment of trichomoniasis is important internationally, but particularly important in some developing countries where HIV transmission is high and resources for health care are scarce. The results suggest that reinfection is common if partners are not treated. Since many women in developing countries attend clinics alone it is not clear whether giving the medication to the woman to take to her partner is effective or not. Partner notification, on the other hand, can be time consuming, expensive, and difficult to achieve. These problems are similar for all sexually transmitted diseases, but the mild symptoms and non‐specific symptoms of Trichomonas infection in men means that compliance with partner treatment is less likely. Another problem that confronts all sexually transmitted disease control strategies is that those groups at greatest risk of these diseases (including commercial sex workers, users of commercial sex workers, and people with multiple concurrent sexual partners) are often difficult to follow up.
By looking at the number of trials published in recent years, enthusiasm for research in the treatment of trichomoniasis seems to be low. This is most probably because of the successful short term parasitological cure that is achieved by drug treatment. However, T. vaginitis remains one of the most common sexually transmitted diseases, and the possibility of an increase in HIV transmission because of vaginitis makes it even more important to investigate effective strategies to decrease the prevalence of the disease.
Authors' conclusions
Nitroimidazoles seem to be effective in achieving short term parasitological cure. Single dose treatment is adequate but patients should be warned about the side effects; compliant patients may be offered a longer treatment regimen with less risk of side effects. Women with severe symptoms may benefit from intravaginal nitroimidazoles or anti‐inflammatory agents in addition to oral treatment. Every effort should be made to treat partners.
From the limited evidence reviewed here there seems to be little difference between drugs.
Strategies to ensure effective partner treatment for the reduction of disease prevalence should be investigated. Future trials should be designed appropriately for this purpose. It is difficult and probably unrealistic to expect women and their partners to attend long term follow up, which makes immediate partner treatment strategies more important. Such trials need to be conducted in settings where infected women are most likely to be seen, such as sexually transmitted disease clinics or gynaecology outpatient clinics. The challenge is to test strategies to increase partner treatment, with longer follow up to evaluate the success of treatment in those settings. Trials could usefully investigate giving partner treatment to women compared with tracing partners by letter, telephone or through extension workers. Using financial incentives to encourage follow up, such as paying for transport and health service fee exemption, could also be researched.
Although it is not a priority for health services research, a carefully conducted metronidazole versus tinidazole comparison with blind assessment of outcomes in settings where both drugs are used and available may be worthwhile. The sample size in such a trial should take into account that both drugs are likely to achieve high rates of parasitological cure and the difference between them would be rather small.
Acknowledgements
Thanks to Mrs Barbara Aaronson, Dr Michel Boulvain, Dr Ellen Ingham, Dr Regina Kulier, Dr Helena von Hertzen, and Dr Stanislaw Orzeszyna for assistance with translation; to Rhone‐Poulenc Rorer (UK) and Pfizer (UK) for providing articles from their database; to the authors for providing additional information, and to Nicola Dollimore (late) for her constructive criticisms and comments in earlier versions of this review. Thanks also to Dr George Schmid for his critical review and comments on the latest version of the review.
Data and analyses
Comparison 1.
Treatment versus no treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (day 4 to 2 weeks) | 6 | 672 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.15, 0.23] |
| 2 No parasitological cure (6 weeks) | 1 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.05, 0.13] |
| 3 No parasitological cure (3 months) | 2 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.13, 0.22] |
| 4 Side effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Any side effect | 3 | 524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.96, 1.78] |
| 4.2 Unpleasant taste | 1 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [1.09, 8.66] |
| 4.3 Nausea | 3 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.14, 5.66] |
| 4.4 Vomiting | 3 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.45, 2.83] |
| 4.5 Headache | 3 | 503 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.46, 1.93] |
| 4.6 Dizziness | 2 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.41, 1.12] |
Analysis 1.1.
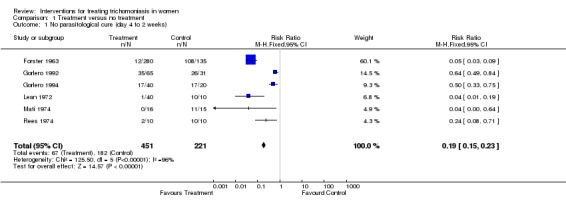
Comparison 1 Treatment versus no treatment, Outcome 1 No parasitological cure (day 4 to 2 weeks).
Analysis 1.2.
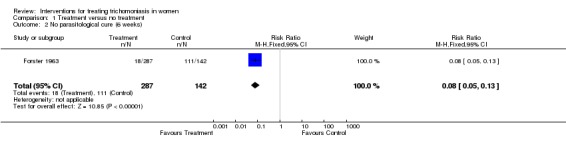
Comparison 1 Treatment versus no treatment, Outcome 2 No parasitological cure (6 weeks).
Analysis 1.3.
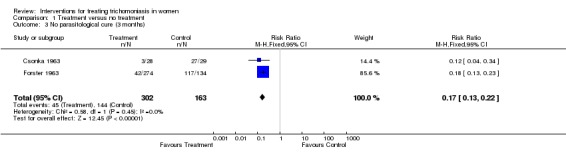
Comparison 1 Treatment versus no treatment, Outcome 3 No parasitological cure (3 months).
Analysis 1.4.
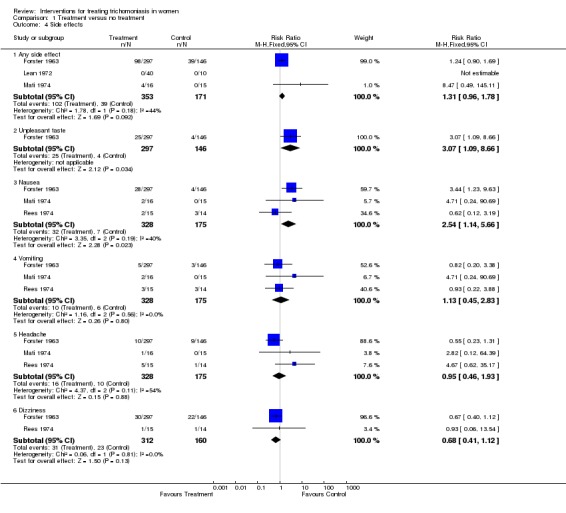
Comparison 1 Treatment versus no treatment, Outcome 4 Side effects.
Comparison 2.
Short versus long treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 4 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.58, 2.16] |
| 2 Side effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any side effect | 4 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.62, 4.49] |
| 2.2 Nausea/vomiting | 3 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [1.22, 5.45] |
| 2.3 Abdominal pain | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.14, 2.80] |
| 2.4 Skin rash | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.12, 6.13] |
| 2.5 Dizziness | 3 | 412 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.17 [2.37, 43.54] |
| 2.6 Headache | 2 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.26, 4.96] |
| 2.7 Candidiasis after treatment | 1 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.44, 1.59] |
Analysis 2.1.
Comparison 2 Short versus long treatment, Outcome 1 No parasitological cure.
Analysis 2.2.
Comparison 2 Short versus long treatment, Outcome 2 Side effects.
Comparison 3.
Oral versus intravaginal treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 2 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.07, 0.56] |
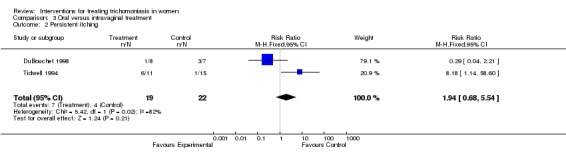
| 2 Persistent itching | 2 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [0.68, 5.54] |
| 3 Persistent discharge | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.50, 3.61] |
| 4 Persistent dysuria | 2 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.38 [0.60, 146.87] |
Analysis 3.1.
Comparison 3 Oral versus intravaginal treatment, Outcome 1 No parasitological cure.
Analysis 3.2.
Comparison 3 Oral versus intravaginal treatment, Outcome 2 Persistent itching.
Analysis 3.3.
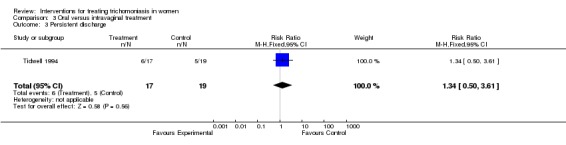
Comparison 3 Oral versus intravaginal treatment, Outcome 3 Persistent discharge.
Analysis 3.4.
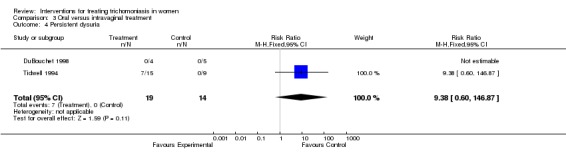
Comparison 3 Oral versus intravaginal treatment, Outcome 4 Persistent dysuria.
Comparison 4.
Oral versus oral plus intravaginal
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (2 weeks) | 4 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [1.10, 8.16] |
| 2 No parasitological cure (6 weeks) | 1 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.55 [0.93, 6.96] |
| 3 No parasitological cure (3 months) | 1 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.97, 3.14] |
Analysis 4.1.
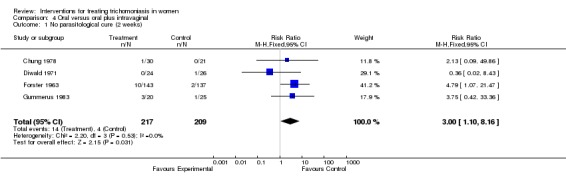
Comparison 4 Oral versus oral plus intravaginal, Outcome 1 No parasitological cure (2 weeks).
Analysis 4.2.
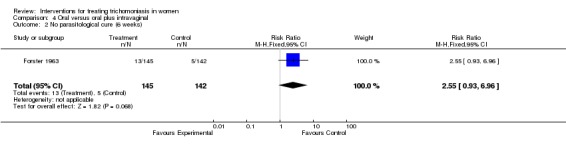
Comparison 4 Oral versus oral plus intravaginal, Outcome 2 No parasitological cure (6 weeks).
Analysis 4.3.
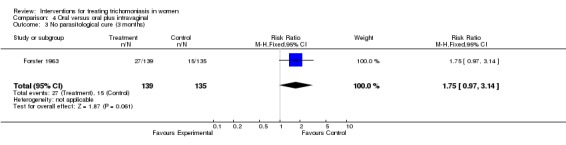
Comparison 4 Oral versus oral plus intravaginal, Outcome 3 No parasitological cure (3 months).
Comparison 5.
Partner treatment versus no partner treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (at average 10 days) | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.92] |
| 2 No parasitological cure (at average 2 months) | 1 | 118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.06, 0.71] |
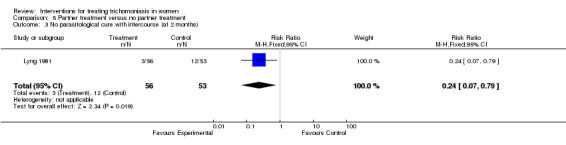
| 3 No parasitological cure with intercourse (at 2 months) | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.07, 0.79] |
Analysis 5.1.
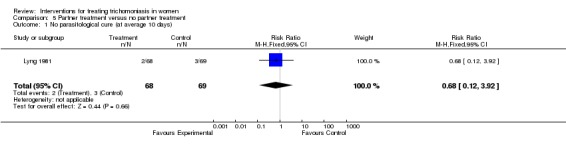
Comparison 5 Partner treatment versus no partner treatment, Outcome 1 No parasitological cure (at average 10 days).
Analysis 5.2.
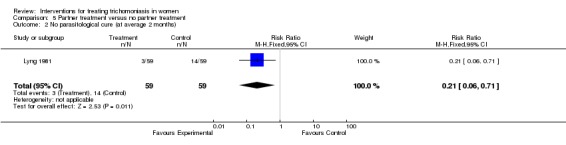
Comparison 5 Partner treatment versus no partner treatment, Outcome 2 No parasitological cure (at average 2 months).
Analysis 5.3.
Comparison 5 Partner treatment versus no partner treatment, Outcome 3 No parasitological cure with intercourse (at 2 months).
Comparison 6.
Metronidazole versus ornidazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (day 3) | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.42 [0.27, 107.20] |
| 2 No parasitological cure (4 weeks) | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.21, 21.32] |
| 3 Side effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Any side effect | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.14, 0.71] |
| 3.2 Nausea/vomiting | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.18] |
| 3.3 Fatigue | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.11] |
| 3.4 Dizziness | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.00, 1.13] |
Analysis 6.1.
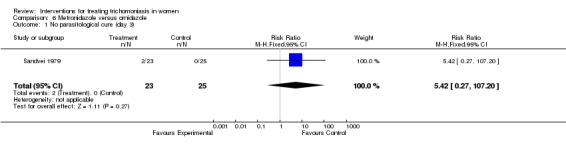
Comparison 6 Metronidazole versus ornidazole, Outcome 1 No parasitological cure (day 3).
Analysis 6.2.
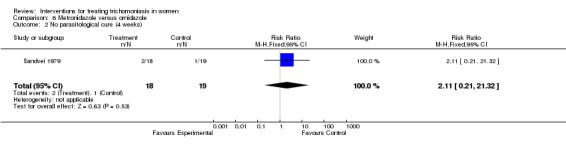
Comparison 6 Metronidazole versus ornidazole, Outcome 2 No parasitological cure (4 weeks).
Analysis 6.3.
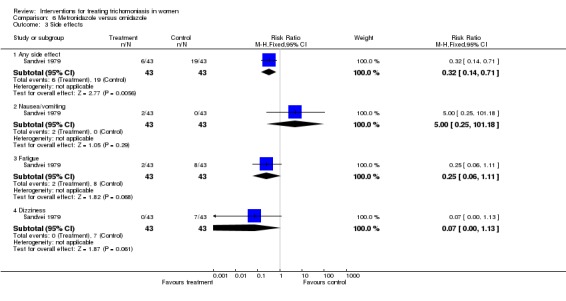
Comparison 6 Metronidazole versus ornidazole, Outcome 3 Side effects.
Comparison 7.
Metronidazole versus nimorazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 8 | 1005 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 1.01] |
| 2 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea/vomiting | 2 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.22, 12.19] |
| 2.2 Dizziness | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.98] |
Analysis 7.1.
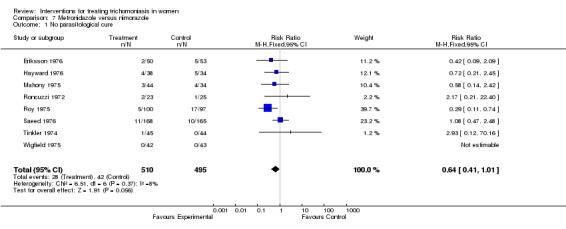
Comparison 7 Metronidazole versus nimorazole, Outcome 1 No parasitological cure.
Analysis 7.2.
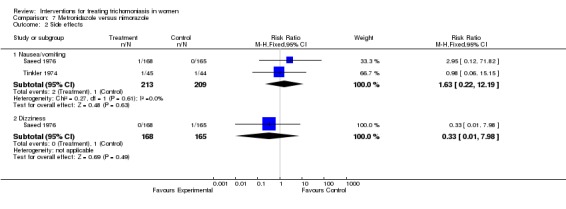
Comparison 7 Metronidazole versus nimorazole, Outcome 2 Side effects.
Comparison 8.
Metronidazole versus nifuratel
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.30, 1.05] |
| 2 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any side effect | 2 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.31, 4.87] |
| 2.2 Allergic symptoms | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.69 [0.23, 95.00] |
| 2.3 Nausea | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [0.12, 67.27] |
Analysis 8.1.
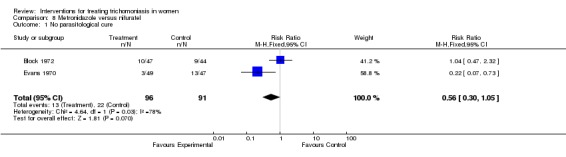
Comparison 8 Metronidazole versus nifuratel, Outcome 1 No parasitological cure.
Analysis 8.2.
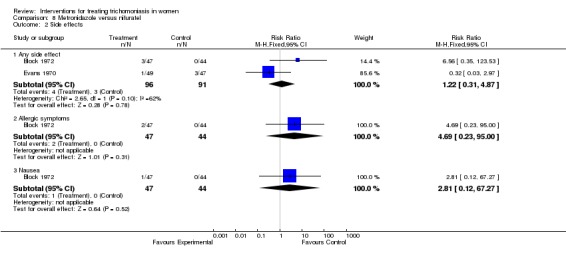
Comparison 8 Metronidazole versus nifuratel, Outcome 2 Side effects.
Comparison 9.
Metronidazole versus tinidazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (3‐21 days) | 8 | 595 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [1.66, 6.32] |
| 2 No parasitological cure (4 weeks) | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.49] |
| 3 No clinical improvement | 5 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.81 [1.83, 7.90] |
| 4 Side effects | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Any side effect | 6 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.35, 2.02] |
| 4.2 Side effect requiring treatment | 2 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.56 [0.79, 26.34] |
| 4.3 Bitter taste | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.51] |
| 4.4 Anorexia | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.84, 3.22] |
| 4.5 Nausea | 4 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.03, 2.17] |
| 4.6 Vomiting | 4 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [1.42, 8.16] |
| 4.7 Ataxia | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.49, 12.06] |
| 4.8 Dizziness | 4 | 342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.59, 2.72] |
| 4.9 Abdominal discomfort | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.13, 4.08] |
Analysis 9.1.
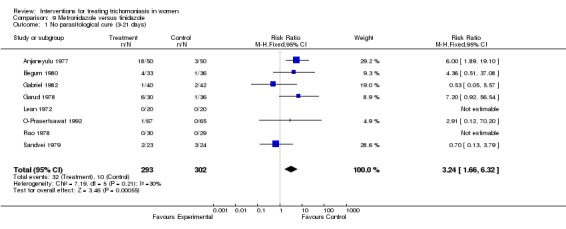
Comparison 9 Metronidazole versus tinidazole, Outcome 1 No parasitological cure (3‐21 days).
Analysis 9.2.
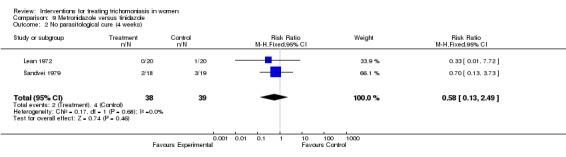
Comparison 9 Metronidazole versus tinidazole, Outcome 2 No parasitological cure (4 weeks).
Analysis 9.3.
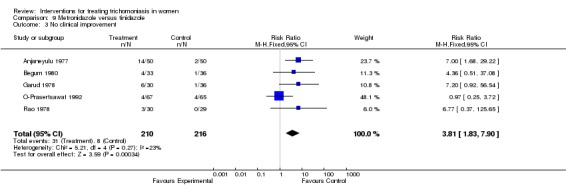
Comparison 9 Metronidazole versus tinidazole, Outcome 3 No clinical improvement.
Analysis 9.4.
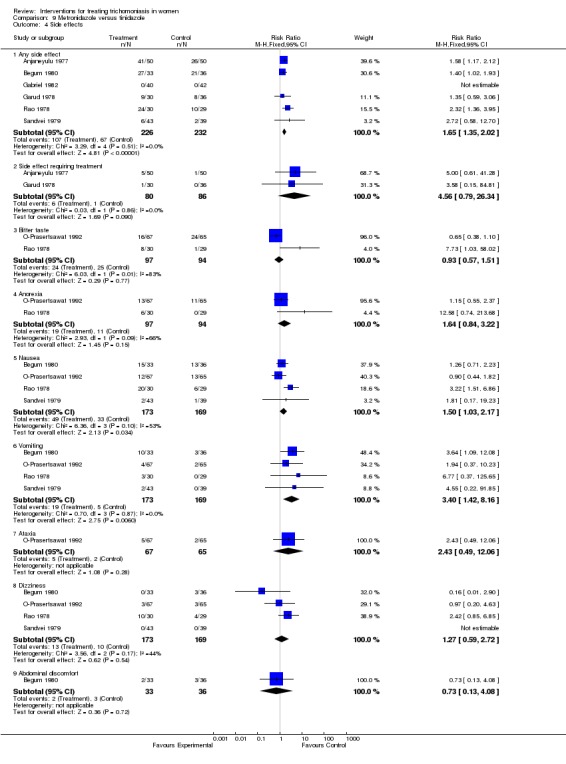
Comparison 9 Metronidazole versus tinidazole, Outcome 4 Side effects.
Comparison 10.
Metronidazole versus nitrimidazine
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.09] |
| 2 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any side effect | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.33, 8.72] |
| 2.2 Nausea | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.23, 8.03] |
Analysis 10.1.
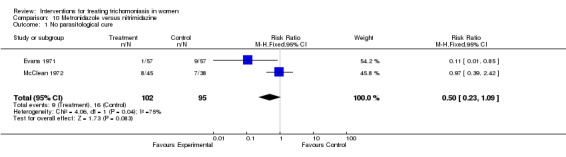
Comparison 10 Metronidazole versus nitrimidazine, Outcome 1 No parasitological cure.
Analysis 10.2.
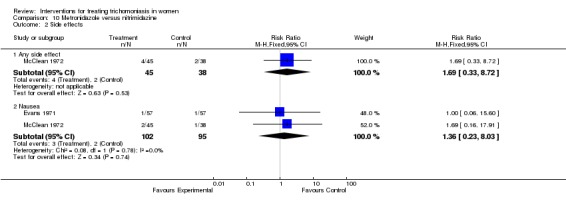
Comparison 10 Metronidazole versus nitrimidazine, Outcome 2 Side effects.
Comparison 11.
Intravaginal: metronidazole versus methyl patricin
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.6 [0.89, 2.86] |
Analysis 11.1.
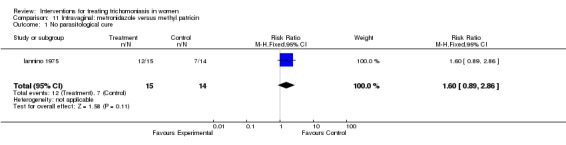
Comparison 11 Intravaginal: metronidazole versus methyl patricin, Outcome 1 No parasitological cure.
Comparison 12.
Intravaginal: metronidazole versus lotrimazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (2 weeks) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Wetmount | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.22, 0.84] |
| 1.2 Culture | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.08] |
Analysis 12.1.
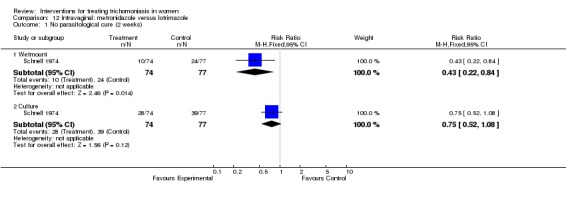
Comparison 12 Intravaginal: metronidazole versus lotrimazole, Outcome 1 No parasitological cure (2 weeks).
Comparison 13.
Intravaginal: clotrimazole versus AVC suppositories
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (2‐3 weeks) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.47] |
| 2 No parasitological cure (4‐6 weeks) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.30] |
| 3 Any side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Analysis 13.1.
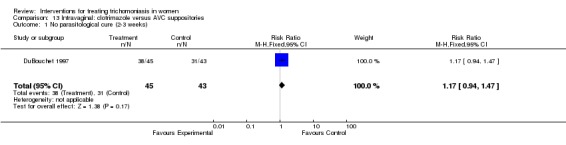
Comparison 13 Intravaginal: clotrimazole versus AVC suppositories, Outcome 1 No parasitological cure (2‐3 weeks).
Analysis 13.2.
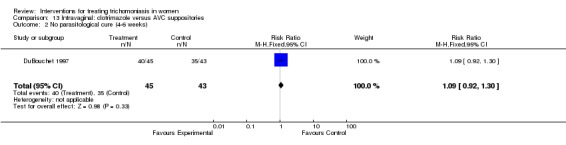
Comparison 13 Intravaginal: clotrimazole versus AVC suppositories, Outcome 2 No parasitological cure (4‐6 weeks).
Analysis 13.3.
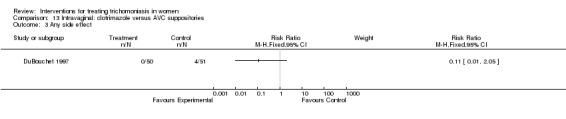
Comparison 13 Intravaginal: clotrimazole versus AVC suppositories, Outcome 3 Any side effect.
Comparison 14.
Tinidazole versus ornidazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (days 3‐14) | 6 | 347 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.61, 2.01] |
| 2 No parasitological cure (4 weeks) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.65, 14.53] |
| 3 No clinical cure | 1 | 27 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.15, 5.67] |
| 4 Side effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Nausea/vomiting | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.49, 4.23] |
| 4.2 Fatigue | 4 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.05, 0.58] |
| 4.3 Dizziness | 3 | 277 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.04, 0.87] |
Analysis 14.1.
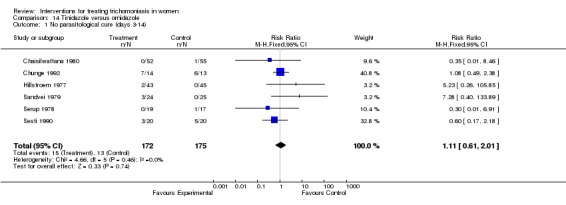
Comparison 14 Tinidazole versus ornidazole, Outcome 1 No parasitological cure (days 3‐14).
Analysis 14.2.
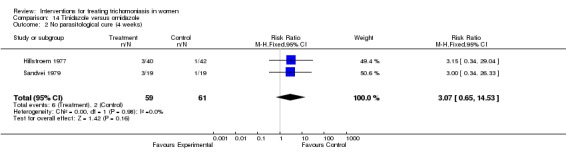
Comparison 14 Tinidazole versus ornidazole, Outcome 2 No parasitological cure (4 weeks).
Analysis 14.3.
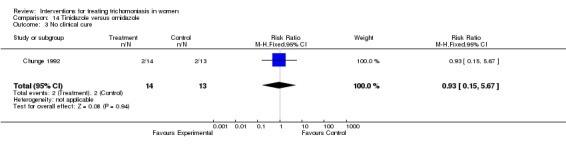
Comparison 14 Tinidazole versus ornidazole, Outcome 3 No clinical cure.
Analysis 14.4.
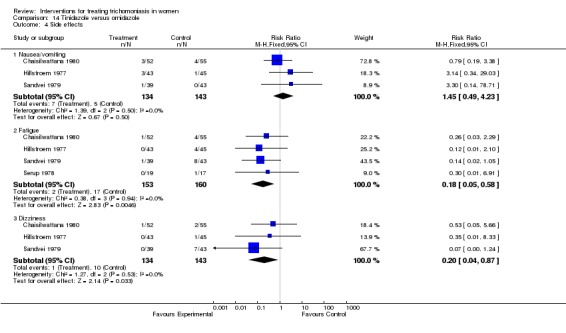
Comparison 14 Tinidazole versus ornidazole, Outcome 4 Side effects.
Comparison 15.
Tinidazole versus nimorazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [1.51, 9.32] |
| 2 No clinical cure | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.40, 11.56] |
Analysis 15.1.
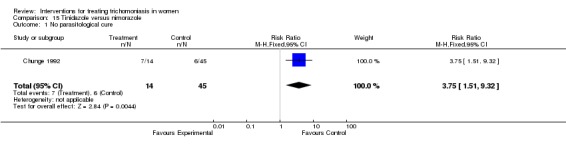
Comparison 15 Tinidazole versus nimorazole, Outcome 1 No parasitological cure.
Analysis 15.2.
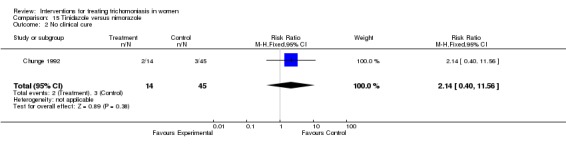
Comparison 15 Tinidazole versus nimorazole, Outcome 2 No clinical cure.
Comparison 16.
Tinidazole versus carnidazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (1 week) | 1 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.57 [0.28, 112.12] |
| 2 No parasitological cure (2 weeks) | 1 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.13 [0.25, 103.43] |
| 3 Any side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Analysis 16.1.
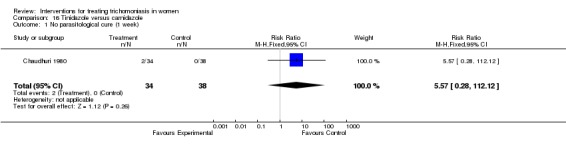
Comparison 16 Tinidazole versus carnidazole, Outcome 1 No parasitological cure (1 week).
Analysis 16.2.
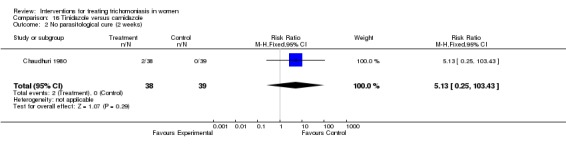
Comparison 16 Tinidazole versus carnidazole, Outcome 2 No parasitological cure (2 weeks).
Analysis 16.3.
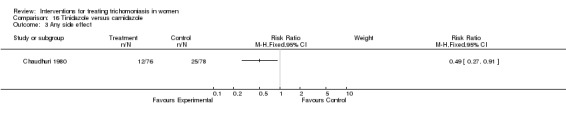
Comparison 16 Tinidazole versus carnidazole, Outcome 3 Any side effect.
Comparison 17.
Ornidazole versus nimorazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.46 [1.34, 8.94] |
| 2 No clinical cure | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [0.43, 12.37] |
Analysis 17.1.
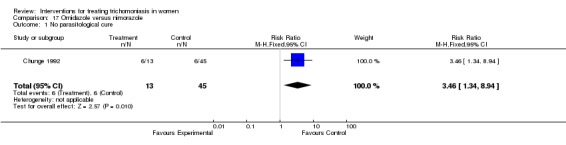
Comparison 17 Ornidazole versus nimorazole, Outcome 1 No parasitological cure.
Analysis 17.2.
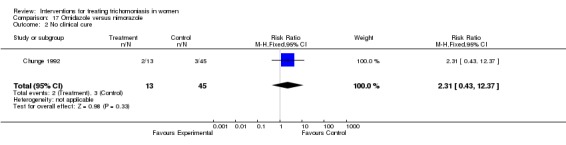
Comparison 17 Ornidazole versus nimorazole, Outcome 2 No clinical cure.
Comparison 18.
Metronidazole low dose (1g or less) versus standard dose (1.5g or more)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (1‐2 weeks) | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.97 [1.92, 4.59] |
| 2 Side effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Any side effect | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.24, 2.04] |
| 2.2 Nausea/vomiting | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.29, 9.90] |
| 2.3 Bitter taste | 1 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.99] |
Analysis 18.1.
Comparison 18 Metronidazole low dose (1g or less) versus standard dose (1.5g or more), Outcome 1 No parasitological cure (1‐2 weeks).
Analysis 18.2.
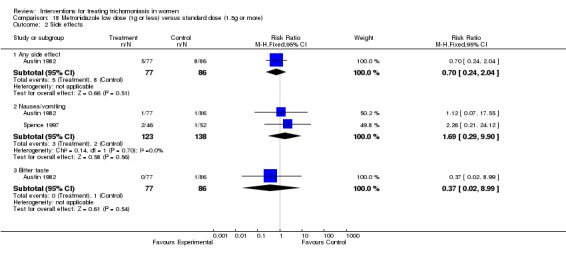
Comparison 18 Metronidazole low dose (1g or less) versus standard dose (1.5g or more), Outcome 2 Side effects.
Comparison 19.
Ornidazole 0.5‐1g versus 1.5‐2g
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 3 | 214 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.38, 4.33] |
| 2 No clinical cure | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.12 [0.95, 240.11] |
| 3 Any side effect | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Analysis 19.1.
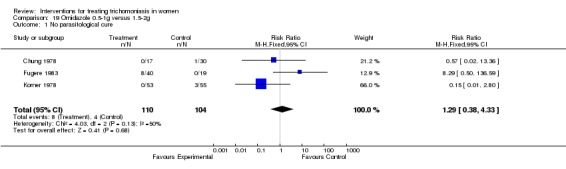
Comparison 19 Ornidazole 0.5‐1g versus 1.5‐2g, Outcome 1 No parasitological cure.
Analysis 19.2.
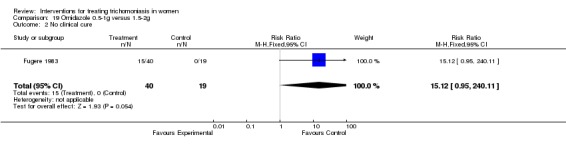
Comparison 19 Ornidazole 0.5‐1g versus 1.5‐2g, Outcome 2 No clinical cure.
Analysis 19.3.
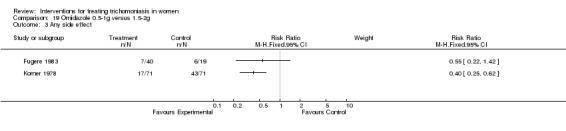
Comparison 19 Ornidazole 0.5‐1g versus 1.5‐2g, Outcome 3 Any side effect.
Comparison 20.
Nimorazole dose comparisons
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 750mg versus 1000mg | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.04, 4.10] |
| 1.2 2g versus 3‐4g | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.0 [1.28, 78.12] |
| 2 No clinical cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 2g versus 3‐4g | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.56 [0.75, 246.76] |
Analysis 20.1.
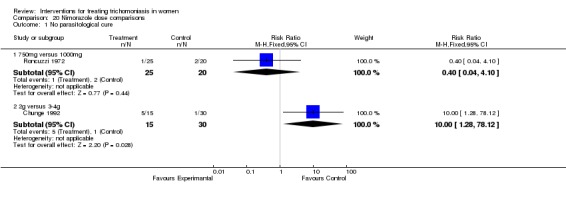
Comparison 20 Nimorazole dose comparisons, Outcome 1 No parasitological cure.
Analysis 20.2.
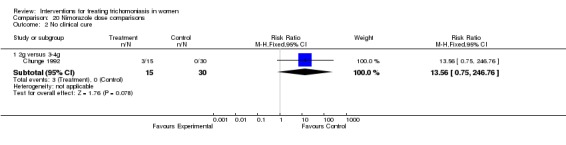
Comparison 20 Nimorazole dose comparisons, Outcome 2 No clinical cure.
Comparison 21.
Carnidazole 1.5g versus 2g
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (1‐3 weeks) | 1 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.16, 5.36] |
Analysis 21.1.
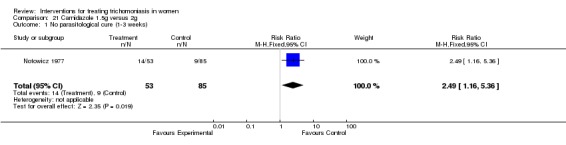
Comparison 21 Carnidazole 1.5g versus 2g, Outcome 1 No parasitological cure (1‐3 weeks).
Comparison 22.
Intravaginal: fenticonazole 600 mg versus 1000 mg
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 3 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.98, 2.23] |
Analysis 22.1.
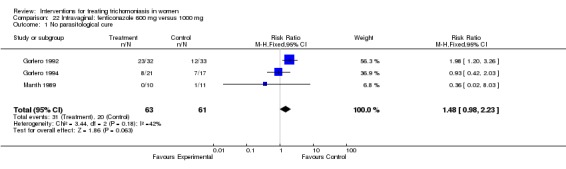
Comparison 22 Intravaginal: fenticonazole 600 mg versus 1000 mg, Outcome 1 No parasitological cure.
Comparison 23.
Nifuratel 7 days versus 10 days
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.57, 1.94] |
Analysis 23.1.
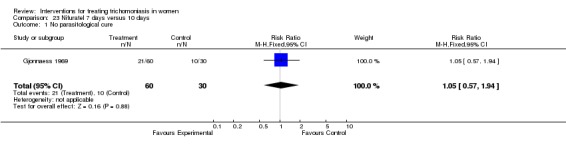
Comparison 23 Nifuratel 7 days versus 10 days, Outcome 1 No parasitological cure.
Comparison 24.
Oral metronidazole versus intravaginal clotrimazole
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (2‐3 weeks) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.04, 0.27] |
| 2 No parasitological cure (4‐6 weeks) | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.12, 0.41] |
| 3 Any side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Analysis 24.1.
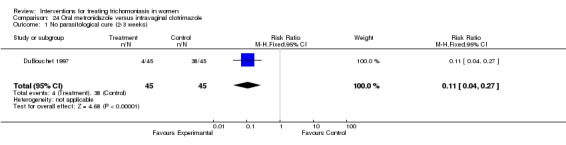
Comparison 24 Oral metronidazole versus intravaginal clotrimazole, Outcome 1 No parasitological cure (2‐3 weeks).
Analysis 24.2.
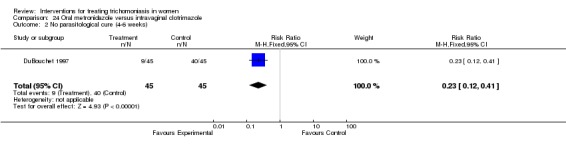
Comparison 24 Oral metronidazole versus intravaginal clotrimazole, Outcome 2 No parasitological cure (4‐6 weeks).
Analysis 24.3.
Comparison 24 Oral metronidazole versus intravaginal clotrimazole, Outcome 3 Any side effect.
Comparison 25.
Oral metronidazole versus intravaginal AVC suppositories
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure (2‐3 weeks) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.05, 0.32] |
| 2 No parasitological cure (4‐6 weeks) | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.13, 0.45] |
| 3 Any side effect | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Analysis 25.1.
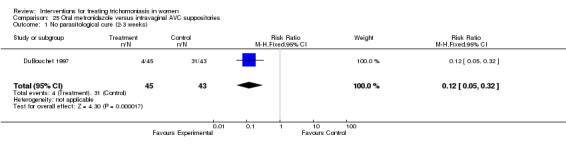
Comparison 25 Oral metronidazole versus intravaginal AVC suppositories, Outcome 1 No parasitological cure (2‐3 weeks).
Analysis 25.2.
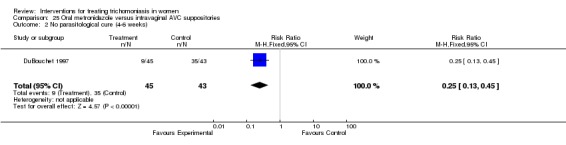
Comparison 25 Oral metronidazole versus intravaginal AVC suppositories, Outcome 2 No parasitological cure (4‐6 weeks).
Analysis 25.3.
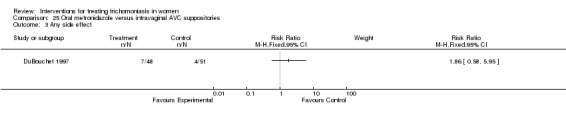
Comparison 25 Oral metronidazole versus intravaginal AVC suppositories, Outcome 3 Any side effect.
Comparison 26.
Oral plus intravaginal versus intravaginal
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oral Aminitrozole plus intravaginal Acigel versus intravaginal Acigel | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.38, 0.94] |
Analysis 26.1.
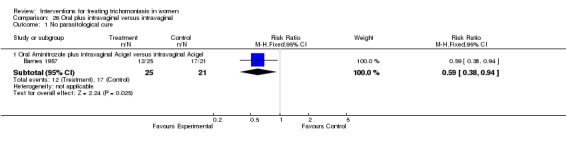
Comparison 26 Oral plus intravaginal versus intravaginal, Outcome 1 No parasitological cure.
Comparison 27.
Oral metronidazole versus intravaginal nonoxynol 9
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 No parasitological cure | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.57] |
Analysis 27.1.
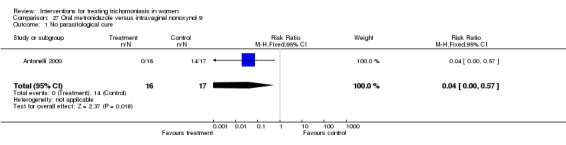
Comparison 27 Oral metronidazole versus intravaginal nonoxynol 9, Outcome 1 No parasitological cure.
What's new
| Date | Event | Description |
|---|---|---|
| 18 August 2008 | Amended | Converted to new review format with minor editing. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 13 January 2003 | New citation required and conclusions have changed | Included one new trial comparing oral metronidazole to intravaginal nonoxynol‐9 (Antonelli 2000), and excluded one trial for lack of randomisation (Wladeck 1981). |
| 24 November 2002 | New search has been performed | New studies found and included or excluded. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Cards labelled with either drug in sealed envelopes were picked up by nurses from a basket. No placebos were used. No mention of blind outcome assessment. |
|
| Participants | Pregnant (4) and non‐pregnant (53) women with vaginal discharge in Ibadan, Nigeria were included in this study. However, only the data for non‐pregnant women have been extracted. Partners were treated if available. Abstinence from sexual intercourse was advised. |
|
| Interventions | Metronidazole 200 mg 3 times daily by mouth for 7 days versus tinidazole 5 x 400 mg capsules taken as single dose in clinic. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by smear and confirmation by culture. Follow up on days 3, 5, and 15. Excluded 1 tinidazole and 3 metronidazole group women because wetmount diagnosis was not confirmed and 2 tinidazole and 1 metronidazole participants were lost to follow up. |
|
| Methods | Consecutive women allocated in a balanced randomisation schedule. No mention of blind outcome assessment. |
|
| Participants | 100 women with vaginal discharge in Poona, India were included. Most partners were treated. Those receiving antitrichomonal treatment in the past 4 weeks were ineligible. |
|
| Interventions | Metronidazole 2 g (5 x 400 mg tabs) by mouth single dose versus tinidazole 2 g (4 x 500 mg tabs) by mouth single dose. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount. Follow up on days 4, 8, and 12. No exclusions or loss to follow up reported. |
|
| Methods | Sequentially numbered opaque envelopes containing medications. No placebo use. Outcome assessment was blinded. |
|
| Participants | 46 women with motile trichopmonads on wet mount at gynecology clinic in North Carolina, USA. | |
| Interventions | Metronidazole 2 g (5 x 400 mg tabs) versus Nonoxynol‐9 450 mg per vagina (3 x 150mg suppositories) suppositories per vagina. | |
| Outcomes | Parasitological cure. | |
| Notes | Diagnosis by wet mount. Follow up 6‐22 days after treatment. Study terminated after results of first 46 patients because of poor clinical response with nonoxynol 9 suppositories. 26% (12 patients) loss to follow up. Data from 1 patient missing. |
|
| Methods | Series of random numbers prepared by the pharmacy. Laboratory assessments were blinded. Method of allocation concealment not stated. |
|
| Participants | 186 women with vaginal discharge attending sexually transmitted disease (STD) clinics and family planning clinics in Ontario, Canada. Partners were treated if seen. Abstinence from intercourse was advised. |
|
| Interventions | Metronidazole 1g versus 2 g, both given as single, oral dose. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount confirmed by culture. Follow up weekly. Loss to follow up 23/186 (12.4%). No post‐ randomisation exclusions reported. |
|
| Methods | Alternate allocation. | |
| Participants | Women with trichomoniasis in England. | |
| Interventions | Aminitrozole, 100 mg three times daily by mouth for 10 days + aci‐gel versus aci‐gel only. Aci‐gel is a buffered vaginal jelly with a pH of 4.0 given twice daily. |
|
| Outcomes | Parasitological cure. | |
| Notes | Diagnosis by wetmount and culture. Loss to follow up rate was 23% (14/60). |
|
| Methods | Randomisation table. No mention of blinding. |
|
| Participants | 69 women with trichomoniasis in Dacca and Sylhet, Bangladesh. Most partners were treated. Exclusion criteria: pregnancy, overt hepatic dysfunction. |
|
| Interventions | Tinidazole 2 g by mouth single dose versus metronidazole 1 g by mouth twice a day for 2 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by smear and symptomology. Follow up on post‐treatment days 7, 14, and 21. No mention of dropouts. |
|
| Methods | Randomisation done, description not given. Double blind trial. Preparations in neutral packaging. |
|
| Participants | 100 women with trichomoniasis in Sweden. Most partners were treated. Exclusion criteria: concurrent infection with other pathogen found. |
|
| Interventions | Nifuratel 200 mg by mouth 3 times a day for 7 days plus 250 mg intravaginally daily for 10 days, versus metronidazole 200 mg by mouth 3 times a day for 7 days plus 500 mg intravaginally daily for 10 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by smear and symptomology. Follow up 14 days after treatment (patients found positive receive another treatment with the same regimen and are examined again after 14 days). 12% loss to follow up in nifuratel group, 3% in metronidazole group. |
|
| Methods | Randomised, double blind. Sealed pre‐numbered treatment packs were provided by the drug company. The method of randomisation is not stated. Code was broken after all trial procedures were finished implying blinded outcome assessment. |
|
| Participants | Women at reproductive age with trichomoniasis attending a venereal disease clinic in Bangkok, Thailand. | |
| Interventions | Tinidazole 2 g versus ornidazole 1.5 g, both given as a single oral dose. The male partners also received similarly coded corresponding medication. |
|
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount. Loss to follow up rate was 11% (13/120). More than half of patients had chronic or recurrent vaginitis. |
|
| Methods | Random allocation, double blind, coded treatment packs were used. No mention of blind outcome assessment. |
|
| Participants | Women with trichomoniasis in Rotterdam, Holland. | |
| Interventions | Carnidazole versus tinidazole, both 2 g, single oral dose. Partners received same treatment and an instruction leaflet was given to all patients recommending condom use for 2 weeks and alcohol abstinence. |
|
| Outcomes | Parasitological cure after 1 and 2 weeks. Side effects. | |
| Notes | Diagnosis by wetmount. Side effects reported for all (patients and partners). Five patients did not attend the first follow up, all were seen at 2 weeks, no exclusions reported. |
|
| Methods | Patients allocated at random to 3 groups. No further details provided. |
|
| Participants | 109 patients with trichomoniasis seen at gynaecology outpatient clinics in two hospitals in Seoul and Hong Kong. No partner treatment was employed although instructions for using a condom were given. |
|
| Interventions | Ornidazole 2 g single dose by mouth versus ornidazole 1 g by mouth plus 0.5 g vaginal tablet short course (24 h) versus ornidazole 1 g by mouth, short course (24 h). | |
| Outcomes | Clinical and parasitological cure. Side effects. | |
| Notes | Diagnosis by wetmount. Follow up on day 3 and after the first menstruation after treatment. Loss to follow up 37.7% after 3 days and 88.1% after menses. |
|
| Methods | Patients were randomised into 5 groups. No details of the procedure given, placebos were not used. |
|
| Participants | Symptomatic women attending a family planning clinic and a female outpatient clinic in Kenya who agreed to take the medications in the clinic and to come back for follow up were enrolled. Women with obvious clinical problems were excluded. |
|
| Interventions | The women were divided into 5 groups: Group 1: Nimorazole 2 g, oral single dose. Group 2: Nimorazole 3 g, oral single dose. Group 3: Nimorazole 4 g, oral in two doses 24 hours apart Group 4: Tinidazole 2 g, oral single dose Group 5: Ornidazole 1.5 g, oral single dose. Advice on coital abstinence was withheld on purpose to find out those who had sex between the treatment and follow up visit. |
|
| Outcomes | Parasitological cure on day 3. Clinical cure (defined as absence of any 3 of the initial symptoms that were present). Side effects. | |
| Notes | Diagnosis by wetmount smear. 121 out of 153 women came for follow up on day 3 (21% loss to follow up). A further 49 women were excluded from analysis because they had sexual intercourse during the study period. Results reported for 47% of all women enrolled. |
|
| Methods | Randomly allocated tablets put in identical, numbered packets, with key to code kept by hospital pharmacy. Double blind, placebo used. |
|
| Participants | 67 women with trichomoniasis in England. Exclusion criteria: prostitutes and women thought to be promiscuous. |
|
| Interventions | Metronidazole 200 mg three times a day for 10 days versus placebo. | |
| Outcomes | Parasitological cure. | |
| Notes | Diagnosis by smear. Follow up to three months after treatment. Loss to follow up ‐ 10 women: 7 in placebo group, 3 in metronidazole group. |
|
| Methods | Randomised following a list of random numbers. Double blind. Unclear whether lab assessments were blinded. |
|
| Participants | 50 women with trichomoniasis, in Steyr, Austria. Partners received active treatment in both groups. |
|
| Interventions | Tinidazole 150 mg by mouth twice daily + tinidazole 150 mg vaginal tablet once daily, for 5 days versus tinidazole 150 mg by mouth twice daily + placebo vaginal tablets for 5 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount and culture (in 37/50 cases). Follow up on day 7. No loss to follow up reported. |
|
| Methods | Random allocation in order of study entry, based on table of random numbers. Open label study. No placebos were used. |
|
| Participants | 168 women with trichomoniasis from Brooklyn, Charlottesville, and Baltimore, USA. Patients were advised to use condoms to prevent reinfection and to refer partners for treatment. Exclusion criteria: pregnancy or suspected pregnancy, hypersensitivity to imidazoles, central nervous system (CNS) disease, severe hepatic disease, blood dyscrasias, using oral anticoagulants, treatment for trichomoniasis in preceding 4 weeks, inability to complete treatment between menstrual periods. |
|
| Interventions | Metronidazole 2 g by mouth single dose versus two 100 mg clotrimazole vaginal tablets once a day for 7 days versus AVC vaginal suppository (containing 1.05 g sulfanilamide, 14 mg aminacrine hydrochloride, and 140 mg allantoin) twice a day for 7 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by smear, culture, and symptomatic score. Follow up 2‐3 and 4‐6 weeks after treatment. Patients dropped from the study were replaced with patients who received the same treatment. Exclusion from analysis (35 women): failure to return for first follow up visit (19), invalid spacing of visits (5), discontinuance of drug because of adverse reactions (2), other protocol violations (9). 168 women enrolled, 133 (79%) evaluable for efficacy. |
|
| Methods | Randomised, open label pilot study. Randomised based on table of random numbers. |
|
| Participants | 56 women with trichomoniasis in USA. Patients were urged to abstain from intercourse during treatment and their partners were to use condoms until completion of the study. Male partners were offered treatment. Exclusion criteria: women under 18, pregnant women, women with candidiasis at diagnosis, lactation, intrauterine device (IUD) in situ, menstruation at time of diagnosis. |
|
| Interventions | 0.75% metronidazole gel, 5 g intravaginally, two times a day for 7 days, versus 250 mg oral metronidazole three times a day for 7 days. | |
| Outcomes | Parasitological cure. | |
| Notes | Diagnosis by smear and culture Follow up 5‐7 days and 21‐28 days after completion of treatment. Exclusion from analysis: 8 patients dropped for not meeting entry criteria, 2 lost to follow up, 3 did not return for follow up within the allotted time period. |
|
| Methods | Randomised in groups of 10. The investigator did not know the group allocation but no further details reported. No mention of blind outcome assessment. |
|
| Participants | Women attending a venereal disease clinic in Stockholm, Sweden. Pregnant women were excluded. Partner treatment was usually not possible. |
|
| Interventions | Metronidazole 200 mg 3 times a day for 7 days versus nimorazole 300 mg twice daily for 7 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis was by culture and wetmount in the first half and by culture only in the second half of the study. Loss to follow up was 3/53 (5.6%) in the metronidazole group and 6/59 (10.2%) in the nimorazole group. No post‐randomisation exclusions reported. |
|
| Methods | Randomly allocated consecutive women in a double blind way. No placebos were used and because one treatment was oral and intravaginal and the other oral only, blinding was until the time of allocation. |
|
| Participants | Women attending sexually transmitted disease clinic in London, England. No mention of any partner treatment attempt. |
|
| Interventions | Metronidazole 200 mg by mouth three times daily for 7 days versus nifuratel 200 mg by mouth three times daily for 7 days + one 250 mg vaginal pessary for 10 nights. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount in most cases and with culture and smear in some. Loss to follow up 17% (19/115). |
|
| Methods | Consecutive women randomly allocated to two treatment groups in a double blind way. No mention of blind outcome assessment. |
|
| Participants | Women attending a sexually transmitted disease clinic in London, England. | |
| Interventions | Metronidazole 200 mg by mouth three times daily for 7 days versus nitrimidazine 250 mg tablets by mouth, twice daily for 6 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount Loss to follow up 26/142 (18%) and an additional 2 women were excluded because the diagnosis was based on cervical smears rather than wetmount technique. |
|
| Methods | Randomisation from a table of random numbers, drugs numbered consecutively and placed in sealed envelopes. Placebos were used for similar route schedules (i.e. oral and intravaginal) separately. Combined oral and vaginal treatment and placebos had two envelopes, containing each treatment stapled together. Outcome assessments were blinded. |
|
| Participants | Women attending a cancer detection clinic in Puerto Rico who had Trichomonas identified in their cervical and vaginal smears were eligible. Mean age of women was 40, and 70% were between 30‐49. Eligible women were sent reminders and responders were included if they were wetmount positive. |
|
| Interventions | There were 4 study groups. All groups received metronidazole or placebo according to the following regimes: Oral drug 250 mg three times daily for 10 days. Vaginal drug 500 mg once daily for 10 days in addition to oral drug as above. Oral placebo. Oral and vaginal placebo. |
|
| Outcomes | Parasitological cure after 2 weeks, 6 weeks, and 3 months. Clinical cure. Side effects. | |
| Notes | Diagnosis and enrolment by wetmount smear. Loss to follow up was 35/450 at 2 weeks, 21/450 at 6 weeks, and 42/450 at 3 months. Three in the drug and 4 in the placebo group had no follow up. |
|
| Methods | Randomised from a table of random numbers. Placebos were not used but the trial was conducted in a double blind fashion. No mention of blind outcome assessment. |
|
| Participants | Symptomatic and asymptomatic women with trichomoniasis in Montreal, Canada. Women with serious medical disorders, gonorrhoea or syphilis or who had been treated with metronidazole in the last 3 months were excluded. |
|
| Interventions | Ornidazole was given as a single, oral dose of 0.5 g or 1.0 g or 1.5 g to three groups, respectively. Women were given information about the nature of the disease, advised to either refrain from coitus or use a condom and were given a bottle of metronidazole tablets with instructions for the partner. They were also advised to abstain from using douches, creams, and vaginal jellies. |
|
| Outcomes | Presence of infection at days 7‐12 and around day 30. Side effects. | |
| Notes | Trial enrolment was according to wetmount smear results but samples were also collected for culture, urine microscopy, and Papanicolaou smears. | |
| Methods | Random allocation to two groups. Placebos were not used, however investigators were blind to the group allocation during the course of the study and for outcome assessments. Follow up at 2 weeks. |
|
| Participants | Non‐pregnant women with vaginal discharge who could attend a follow up visit after 2 weeks were eligible. Trial conducted in a venereal disease clinic in London, England. |
|
| Interventions | Metronidazole 2 g (400 g tabs x 5) versus tinidazole 2 g (500g x 4) as single dose by mouth. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount and culture. Loss to follow up 6/46 and 7/49 in the metronidazole and tinidazole groups, respectively. |
|
| Methods | Women randomly allocated to two groups. No further details given. No mention of blind outcome assessment. |
|
| Participants | Women with vaginal discharge attending gynaecological outpatient departments in Bombay, India. Partners treated in most cases. Abstinence from sexual intercourse was advised. |
|
| Interventions | Metronidazole 2 g by mouth single dose versus tinidazole 2 g by mouth single dose. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount and culture. Follow up on days 6, 14 and 21. Loss to follow up 24/90 (27%) in whole group. No exclusions reported. |
|
| Methods | Random division into groups. No mention of blinding. Method of allocation concealment not stated. |
|
| Participants | 95 women with trichomoniasis in Norway. Most partners were treated. |
|
| Interventions | Nifuratel 200 mg by mouth three times a day for 7 days plus a 250 mg vaginal tablet once a day for 10 days versus nifuratel 200 mg by mouth three times a day for 10 days plus a 250 mg vaginal tablet once a day for 10 days. | |
| Outcomes | Parasitological cure during follow up and during first 4 months following therapy. Side effects. | |
| Notes | Diagnosis by smear. Patients were re‐treated (with 200 mg nifuratel three times a day for 10 days plus a 250 mg vaginal tablet twice daily for 10 days) if trichomonads were found during follow up. Follow up 10 days after end of therapy. 5 patients lost to follow up, no breakdown into treatment groups. |
|
| Methods | Random allocation to three groups. No details of randomisation procedure given. Placebos were used for the no‐treatment group. No mention of blind outcome assessment. |
|
| Participants | Women with trichomoniasis, >18 years, in Italy. Exclusion criteria: patients with serious metabolic or systemic disorders and women who were treated for vaginal infections in the last 4 weeks. |
|
| Interventions | Three groups: Fenticonazole, 600 mg single intravaginal ovule versus fenticonazole 1000 mg single intravaginal ovule versus placebo. No mention of any partner treatment or notification. |
|
| Outcomes | Presence of infection on day 4. Clinical cure was assessed by the women (w) and physicians (p) separately using a semi‐quantitative scale (symptoms (w)/vaginal erythema and oedema (p): none, mild, moderate, severe). Side effects. |
|
| Notes | Diagnosis was made by wetmount smear. All women were asked to come for a second follow up visit on day 8; those who were Trichomonas positive on day 4 were given an additional 600 mg ovule regardless of their allocated group. No mention of exclusions or loss to follow up. |
|
| Methods | Randomised, double blind, ran parallel in two centres. Placebos were used. No mention of blind outcome assessment. |
|
| Participants | Women with trichomoniasis, aged 18‐70 years, in Italy. Women with serious systemic disorders and who received vaginitis treatment in the last 2 weeks were excluded. Presence of another infectious agent was also a reason for exclusion. |
|
| Interventions | Three groups: Group 1: Fenticonazole 600 mg intravaginal ovule on days 1 & 2 followed by placebo on days 3 & 4. Group 2: Fenticonazole 1000 mg intravaginal ovule on days 1 & 2 followed by placebo on days 3 & 4. Group 3: Placebo ovules for four days. No mention of any partner treatment or notification measures. |
|
| Outcomes | Presence of infection on day 7. Clinical cure. Side effects. | |
| Notes | Diagnosis was made by wetmount smear. Of the 61 women enrolled, 2 withdrew and 1 stopped because of side effects resulting in 3 exclusions. |
|
| Methods | Random allocation of participants. No further information is given. |
|
| Participants | 45 women with trichomoniasis. All husbands were treated. |
|
| Interventions | Tinidazole 2 g orally as a single dose versus tinidazole 2 g single dose + 500 mg vaginally every night for 7 days. | |
| Outcomes | Parasitological cure on day 14. Side effects. | |
| Notes | Diagnosis made by culture and wet smear. Follow up 2 weeks after treatment. No mention of losses to follow up. |
|
| Methods | Method of randomisation not specified. Placebos were used, the trial was conducted in a double blind fashion. No mention of blind outcome assessment. |
|
| Participants | Symptomatic women attending a venereal disease clinic in Georgia, USA. Women younger than 18 years, who had missed a period and who were treated for trichomoniasis in the last month were excluded. |
|
| Interventions | Single dose versus 7 day regimen. Single dose regimen: Metronidazole 2 g (8 x 250 mg tabs) followed by one placebo tablet three times daily for 7 days. Seven day regimen: Eight placebo tablets followed by one metronidazole tablet three times daily for 7 days. Each women was given 2 g metronidazole for partner treatment and were asked to abstain from coitus and alcohol during the treatment period. |
|
| Outcomes | Parasitological cure 7‐21 days after completing therapy. Side effects. | |
| Notes | Diagnosis was based on wetmount and culture. Participants were given standard treatments for any other sexually transmitted disease identified. Neisseria gonorrhoea was identified in 14% and 18% of single dose and 7 day groups respectively. Overall loss to follow up was 62% (292/468). |
|
| Methods | Alternate allocation. | |
| Participants | 105 women attending a venereal disease clinic in Bournemouth, England. Abstinence from sexual intercourse advised. Partners treated when they were available. |
|
| Interventions | Metronidazole 400 mg immediately followed by 4, 12 hourly doses of 400 mg (2 g) versus nimorazole 750 mg stat followed by 3, 12 hourly doses of 750 mg (3 g), oral. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount confirmed by culture. Follow up at days 7, 14, and 28. Loss to follow up and exclusion because of not taking drugs appropriately (1 in each group) accounted for 15/53 (28%) and 18/52 (35%) with metronidazole and nimorazole respectively. Excluded: those with less than 3 visits or attended follow ups for less than 28 days. |
|
| Methods | Random numbers used to generate two groups. Each treatment was separately packed, numbered and women were enrolled consecutively. Placebos were used and outcome assessments were blinded. |
|
| Participants | Women with trichomoniasis attending two gynaecology outpatient and one venereal disease clinic in Sweden. Women who had recently been treated for other sexually transmitted diseases (STDs) were also included. Age range was 14‐71 years. |
|
| Interventions | Ornidazole 1.5 g versus tinidazole 2 g, both given as a single oral dose. Of the sexual partners, 27/45 and 25/43 received treatment. |
|
| Outcomes | Parasitological cure on day 7 and 30. Side effects. | |
| Notes | Enrolment was by wetmount smear but all women also had cultures. No exclusions or loss to follow up reported. |
|
| Methods | No description of how groups were randomised. No mention of blinding. |
|
| Participants | 29 women with trichomoniasis. | |
| Interventions | Metronidazole 500 mg, intravaginally, 1 tablet per day versus Methyl‐Patricin 25,000 units, intravaginally, 1 tablet per day. | |
| Outcomes | Parasitological cure. | |
| Notes | Diagnosis by smear. Follow up on post‐treatment day 2 to 4. |
|
| Methods | Randomisation from a list of random numbers. Double blind, placebos were used. Outcome assessments were blinded. |
|
| Participants | Women with trichomoniasis, attending general practice clinics in Copenhagen, Denmark. Women with liver, blood diseases, who could not attend follow up visits and who were known to be promiscuous were excluded. |
|
| Interventions | Ornidazole, 1 g (500 mg tabs x 2) + 2 placebo tabs versus ornidazole 2 g (500 mg tabs x 4). Women were given an extra pack of tablets to take to their partners with written instructions. |
|
| Outcomes | Parasitological cure on day 7. Side effects. | |
| Notes | Diagnosis was made by wetmount and culture. Culture negative patients (18 and 16 in the 1 g and 2 g groups, respectively) were excluded from further analysis except for side effects. Loss to follow up was 6 and 1 in the 1 g and 2g groups. |
|
| Methods | Randomised, double blind. Identical capsules were used for the two treatment groups and the placebo group. Outcome assessments were blinded. |
|
| Participants | 50 non‐pregnant women with vaginal discharge in Singapore were included. Women with neurological diseases were excluded. |
|
| Interventions | Metronidazole versus tinidazole versus placebo all administered 200 mg 3 times daily for 10 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by dark ground microscopy. Follow up examinations on day 5, day 10, 3rd week and 4th week. No losses to follow up. 45/50 remained in hospital for the first 5 days, the remainder were hospitalised for the whole course. |
|
| Methods | Allocation on a random basis. Active or placebo treatments were given in coded boxes. No mention of blind outcome assessment. |
|
| Participants | Partners of women who had trichomoniasis were randomised to treatment and control groups. The trial was conducted in Copenhagen, Denmark. |
|
| Interventions | To the partners: Tinidazole 2 g oral, single dose versus placebo. All women received standard treatment. |
|
| Outcomes | Parasitological cure on days 7‐14 and around day 30. Side effects. | |
| Notes | Diagnosis was made by culture. Loss to follow up rate was 8% (12/149) in the first and 11% (14/132) in the second follow up. |
|
| Methods | Containers only identifiable by numbers were given to women and their partners (separately marked) where possible. Neither the nurse administering the treatment nor the women could identify the treatment arms. Outcome assessments were blinded. |
|
| Participants | Patients attending "special clinics" (venereal disease) in south east England. | |
| Interventions | Single dose, 2 g metronidazole versus nimorazole. Partners offered concomitantly coded treatment if they were available. |
|
| Outcomes | Parasitological cure. Side effects. | |
| Notes | Diagnosis by wetmount. 12/93 (13%) lost to follow up. |
|
| Methods | Patients were randomly assigned to two groups, method of randomisation not mentioned, double blind trial. Placebos not used. No mention of blind outcome assessment. |
|
| Participants | Symptomatic women with trichomoniasis. Patients with serious medical disorders, poor general health, immunodeficient, and those considered unsuitable or unreliable were excluded. Trial conducted in Europe. |
|
| Interventions | Fenticonazole intravaginal ovule 600 mg versus 1000 mg. No mention of partner treatment, notification, and other precautions. |
|
| Outcomes | Parasitological cure on day 1or 2. Clinical cure was assessed semi‐ quantitatively (symptoms cleared, improved, unchanged, worsened). Side effects. | |
| Notes | Diagnosis was made by wetmount smear. No loss to follow up. |
|
| Methods | Randomised double blind. Paired (for partner treatment) numbered envelopes containing either active or placebo were given to the women. No mention of blind outcome assessment. |
|
| Participants | 31 non‐pregnant women attending gynaecology outpatient clinics in Nairobi, Kenya. No advice on sexual intercourse was given during the course of the study. |
|
| Interventions | Tinidazole 2 g single dose (4 tablets) by mouth versus 4 yeast tablets as single dose. | |
| Outcomes | Clinical and parasitological cure. Side effects. | |
| Notes | Diagnosis by wetmount. Follow up on day 7. No report of losses to follow up or post‐randomisation exclusions. |
|
| Methods | Random, both the patient and prescriber blind to allocation. No further details given. No mention of blind outcome assessment. Follow up intended until 3 months. |
|
| Participants | Women with trichomoniasis in London, England. | |
| Interventions | Metronidazole 400 mg twice daily for 5 days versus nitrimidazine 250 mg twice daily for 6 days. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount smear. Loss to follow up was 16% (5 v 11 in metronidazole and nitrimidazine groups, respectively) and one patient who did not complete the treatment was excluded. |
|
| Methods | Alternate allocation. | |
| Participants | Women with trichomoniasis, attending a venereal disease clinic in Rotterdam, Holland. Thirty women (20%) were commercial sex workers. |
|
| Interventions | Carnidazole 2 g versus 1.5 g, oral single dose. | |
| Outcomes | Parasitological cure after 1‐3 weeks. | |
| Notes | Diagnosis by wetmount and culture. Concomitant treatment was given to 49 women with gonorrhoea and one woman with syphilis. Loss to follow up was 6.6% and 13.1% in the 2 g and 1.5 g groups, respectively. |
|
| Methods | Random allocation to two groups. Blinding could not be maintained after allocation, however no details are given about how allocation concealment was done. |
|
| Participants | 119 Women attending general practices in Copenhagen, Denmark, who required a gynaecological examination were recruited. Pregnancy, presumed promiscuity, and systemic disorders were reasons for exclusion. |
|
| Interventions | Metronidazole 200 mg 3 times daily for 7 days versus ornidazole 500 mg 4 tablets by mouth as single dose. Partner treatments were given to the women to take to their partners. |
|
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount and culture. Only those verified by culture results were analysed. Excluded wetmount positive culture negatives (10/53 in the metronidazole and 12/66 in the ornidazole groups). Only 1 woman in the ornidazole group was lost to follow up at 1 week, 8 in the ornidazole, and 3 in the metronidazole group were lost at 5 weeks. |
|
| Methods | Double blind randomised controlled trial. Patients were asked to choose a box that contained one of the drugs. Outcome assessments were blinded. |
|
| Participants | Symptomatic women with trichomoniasis attending a gynaecology outpatient clinic in Bangkok, Thailand. | |
| Interventions | Metronidazole 1.6 g split into two doses versus tinidazole 2 g as a single oral dose. Same treatments were given to the partners. They were also asked to refrain from coitus or to use condoms. |
|
| Outcomes | Parasitological cure between 6‐16 days after treatment. Clinical improvement (self‐assessed by the women). Side effects. | |
| Notes | Both wetmount smear and culture were used for diagnosis. Dropout rate was 16.3% (13/67) in the metronidazole and 18.8% (15/65) in the tinidazole groups. |
|
| Methods | Allocation according to a randomisation schedule. No further details reported. No mention of blind outcome assessment. |
|
| Participants | 60 non‐pregnant women with vaginal discharge, without gonorrhoea or candidiasis, and no history of antitrichomonal treatment in the past 2 weeks. Sexual intercourse abstinence was advised. Partners were treated when possible. Trial conducted in Mangalore, India. |
|
| Interventions | Metronidazole 2 g (400 mg x 5 tabs) versus tinidazole 2 g (500 mg x 4 tabs) both given as single oral dose. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount. Follow up on days 4, 8, and 12. One patient in tinidazole group was lost to follow up. |
|
| Methods | Random allocation to one of two treatment groups. Outcome assessments were blinded. |
|
| Participants | 29 prison women in Nairobi, Kenya. Pregnancy, coexistent disease, and unwillingness to participate were reasons for exclusion. |
|
| Interventions | Tinidazole 2 g (5 x 400 mg tablets) single dose by mouth versus placebo (ascorbic acid 5 x 25 mg tablets) single dose by mouth. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount. Follow up at days 4, 5, 7, and at 2 months. Excluded 9/29 because transfer to another prison, discharge or reluctance to carry on with examinations. Only 6 women in each group were examined at 2 months. |
|
| Methods | Simple randomisation using numbered sealed envelopes containing the instructions for therapy. No mention of blinding. Follow up less than one month and greater than one month after treatment. |
|
| Participants | 68 women with trichomoniasis in Italy. Partners were not treated. Exclusion criteria: women who were pregnant or suspected to be pregnant. |
|
| Interventions | Metronidazole 600 mg every 24 hours for 7 days versus nimorazole 750 mg every 12 hours for 4 days versus nimorazole 1000 mg every 12 hours for 3 days. | |
| Outcomes | Parasitological cure | |
| Notes | Diagnosis by smear. Loss to follow up at less than 1 month after treatment: none; greater than one month after treatment: 11 in metronidazole group, 9 in nimorazole 750 mg group, 7 in nimorazole 1000 mg group. |
|
| Methods | Alternate allocation. | |
| Participants | 218 consecutive women attending a venereal disease clinic in Bournemouth, England. Abstinence from sexual intercourse was advised. |
|
| Interventions | Metronidazole 200 mg 3 times daily by mouth for 7 days versus nimorazole 250 mg 12 hourly for 6 days. Contact slips were issued for male partners of all women requesting them to attend the clinic within 24 hours and they were given the same treatment if they attended. |
|
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount and culture. Follow up on days 7, 14, 28, and 42. Loss to follow up 21/218 (9.6%) in total with 9/109 in metronidazole and 12/109 in nimorazole groups. |
|
| Methods | Consecutive women attending the clinic were enrolled by alternate allocation. | |
| Participants | Women attending veneral disease clinic in Bournemouth, England. | |
| Interventions | Metronidazole 400 mg tablets 12 hourly for 48 hours versus nimorazole 250 mg tablets 12 hourly for 48 hours. Women were advised against sexual intercourse and asked to bring their partners to the clinic the next day. Additional female contacts of male partners were sent contact slips. Reminders and social worker contact were attempted for women who did not attend follow up visits. |
|
| Outcomes | Parasitological cure around days 7‐9, 14‐21, and 21‐35. Side effects. | |
| Notes | Diagnosis was made by wetmount and/or culture. Diagnostic tests for candida, syphilis, and gonorrhoea were also carried out. Of the 333 cases followed up, 208 had other sexually transmitted diseases as well. Loss to follow up rate was 11.4% (43/376). |
|
| Methods | Randomised by a table of random numbers. Placebos were not used but it was a double blind trial. No mention of blind outcome assessment. Method of allocation concealment not stated. |
|
| Participants | Symptomatic women with trichomoniasis, between 13‐57 years, in Norway. Those living too far to attend follow up were excluded. |
|
| Interventions | Metronidazole 2 g versus tinidazole 2 g versus ornidazole 2 g. All were given oral as a single dose. Same medication with written instructions for the partner. |
|
| Outcomes | Parasitological cure on day 3 and around 4 weeks. Side effects. | |
| Notes | Diagnosis by wetmount and culture. Cultures for candida and gonorrhoea were also taken. Side effects were reported in all persons who took the drug (including the partners). Loss to follow up was 2/25, 1/25, and 0/25 in the metronidazole, tinidazole, and ornidazole groups, respectively on day 3 and 7/25, 6/25, and 6/25 after 4 weeks. |
|
| Methods | Randomised, double blind. No other details given. No mention of blind outcome assessment. |
|
| Participants | Women with trichomoniasis in Germany. | |
| Interventions | Intravaginal, metronidazole (250 mg) versus clotrimazole (100 mg), both for 6 days to be inserted in the evenings. Partners were treated with metronidazole. |
|
| Outcomes | Parasitological cure after 2 and 4 weeks. Clinical cure. | |
| Notes | Both wetmount and culture were used for initial diagnosis and follow ups. Loss to follow up was 8% v 4% (metronidazole and clotrimazole) at 2 weeks, and 23% and 36% after 4 weeks. Treatment failures after 2 weeks were given oral and vaginal metronidazole. It is not clear whether they were included in 4 weeks follow up results. |
|
| Methods | Randomised, double blind, random number tables used, no placebos. No mention of blind outcome assessment. |
|
| Participants | Women with trichomoniasis, most (33/47) were admitted to the hospital for various gynaecological problems. The trial took place in Copenhagen, Denmark. Patients with known liver disorders, neurological and haematological disorders were excluded. |
|
| Interventions | Single, oral 2 g dose of tinidazole versus ornidazole. Partners were given corresponding treatments. |
|
| Outcomes | Parasitological cure. Side effects. | |
| Notes | Both wetmount and culture methods were used. Of the 47 patients enrolled in the trial, 7 were excluded because of negative culture results and 4 were lost to follow up. |
|
| Methods | Patients randomly divided into two treatment groups, no further details given. No mention of blind outcome assessment. |
|
| Participants | Women with trichomoniasis in Rome, Italy. | |
| Interventions | Tinidazole 2 g oral single dose + tinidazole 150 mg and nystatine 100.000 IU ovule for 7 days versus ornidazole 2 g oral single dose + ornidazole 500 mg ovule single dose. Corresponding oral treatments given to the partners. |
|
| Outcomes | No parasitological cure (2 weeks). Side effects. | |
| Notes | Diagnosis by wetmount. No mention of exclusions or loss to follow up. |
|
| Methods | Randomised by table of random numbers, identical packs numbered consecutively. Double blind, placebos were used. Outcome assessments were blinded. |
|
| Participants | 167 women attending an inner city sexually transmitted disease (STD) clinic in Philadelphia, USA. | |
| Interventions | Metronidazole 0.5 g v 1 g v 1.5 g v 2 g, all administered as single, oral dose. All women received 4 tablets (active and placebo according to the dose). |
|
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by wetmount. Follow up 7‐10 days after treatment. Loss to follow up was 18/41 (44%), 15/44 (34%), 15/42 (36%), and 18/37 (49%) in the 0.5, 1, 1.5, and 2 g groups,respectively (overall 66/164, 40%). Women lost to follow up were similar in baseline demographic characteristics to those attending follow up examinations. Excluded 3 women (2 with 2 g and 1 with 0.5 g dose) who vomited after ingestion of the medication. |
|
| Methods | Randomised, double blind, placebos used. Individual, numbered treatment packs were randomly arranged to be given to patients. Outcome assessments were blinded. |
|
| Participants | Women with trichomoniasis who could attend follow up visits after 2 weeks in London, England. | |
| Interventions | Single dose versus five day regimen: Single dose regimen: Metronidazole 2 g (5 x 400 mg tabs) followed by placebo for 5 days (1 tablet twice daily). Five day regimen: 5 placebo tablets as a single dose followed by metronidazole 400 mg tabs twice daily for 5 days. No partner treatment or notification measures are mentioned. It is not clear whether the women were advised against coitus during the treatment period or not. |
|
| Outcomes | Parasitological cure on day 7 and day 14. Side effects. | |
| Notes | Both wetmount smear and culture were used for diagnosis. Loss to follow up on day 14 was 46% and 31% in the single dose and 5 day groups, respectively. |
|
| Methods | Random number table used to prepare consecutively numbered, sealed envelopes containing the medication. Placebos were used. Outcome assessments were blinded. |
|
| Participants | Women, 15‐65 years old, seen in the emergency department (Jackson, USA) with a diagnosis of trichomoniasis. | |
| Interventions | Metronidazole 2 g vaginal cream + oral placebo versus metronidazole 2 g oral + vaginal placebo. Partners were offered treatment with a single 2 g dose of metronidazole. Women were asked to abstain from coitus and alcohol until follow up. |
|
| Outcomes | Parasitological cure on day 3‐5. Clinical cure. Side effects. | |
| Notes | Both wetmount smear and culture were done but patients were enrolled according to culture results. All patients had cultures for N. gonorrhea, 20% and 14% were positive in the oral and vaginal groups, respectively. Of the 305 patients tested for possible enrolment, 302 could be evaluated. 94 were positive and 61 of these could be enrolled (they needed to be called back). Loss to follow up was 7/61 (11.4%) and one was excluded because of bacterial overgrowth in culture. |
|
| Methods | Randomised, double blind but no placebos used. No mention of blind outcome assessment. |
|
| Participants | 100 women attending Bristol Royal Infirmary, England. Exclusion criteria: women with more than one partner, who were thought insufficiently intelligent to understand the study, not reliable to take medications as prescribed, and those with partners unwilling to take concurrent medication. Abstinence from sexual intercourse was advised. |
|
| Interventions | Metronidazole 200 mg 3 times daily for 7 days versus nimorazole 250 mg twice daily for 6 days, both by mouth. | |
| Outcomes | Parasitological and clinical cure. Side effects. | |
| Notes | Diagnosis by dark field microscopy and culture. Follow up on days 8 and 14. Loss to follow up 4/100 and 7 others excluded for not taking drugs as prescribed. |
|
| Methods | Alternate allocation. No mention of blinding. |
|
| Participants | 199 women with trichomoniasis in England. Some partners were treated. Patients were advised to avoid sexual intercourse. Exclusion criteria: pregnancy or suspected pregnancy. |
|
| Interventions | Nimorazole 3 g (two 500 mg tablets 12 hourly for three doses) versus metronidazole 4.2 g (one 200 mg tablet three times daily for 7 days). | |
| Outcomes | Parasitological cure. | |
| Notes | Diagnosis by smear. Follow up on completion of treatment and 3 more times at weekly intervals. Loss to follow up: 23 in metronidazole group and 20 in nimorazole group. |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akinla 1975 | No randomised or quasi‐randomised comparisons were made. |
| Ali 1975 | No randomised or quasi‐randomised comparisons were made. |
| Andersen 1975 | No randomised or quasi‐randomised comparisons were made. |
| Apte 1978 | No randomised or quasi‐randomised comparisons were made. |
| Arnold 1974 | No randomised or quasi‐randomised comparisons were made. |
| Arya 1974 | Women with both trichomoniasis and candidiasis were included in this trial. |
| Aubert 1982 | Women attending family planning clinics were offered either a single, 2 g or 250 mg three times daily for 7 days metronidazole treatment. There was no randomised comparison between the two groups. |
| Aure 1974 | Double blind trial comparing metronidazole with tinidazol. The trial is excluded because the data were reported per couple rather than individuals. |
| Azevedo 1985 | No randomised or quasi‐randomised comparisons were made. |
| Bedoya 1974 | No randomised or quasi‐randomised comparisons were made. |
| Bertini de Oliveira | No randomised or quasi‐randomised comparisons were made. |
| Bloch 1985 | This trial was excluded because the data were not in a suitable form for analysis. Correspondence with the author revealed that the data were destroyed during a move. Women with trichomoniasis were randomly allocated to metronidazole 2 g oral tablets, benzoyl metronidazole suspension (2 g) or tinidazole (2 g) oral treatment. On day 7, 1.7%, 4.5%, and 1.7 % were Trichomonas positive respectively, on day 14, none of the metronidazole and 5.1 % of tinidazole groups were positive. The side effects were highest with metronidazole tablets (22.4 %) and lowest with metronidazole suspension (7.5 %). |
| Botero 1977 | This clinical trial was excluded as there was no randomised or quasi‐randomised comparison between women treated with metronidazole or panidazole for trichomoniasis. |
| Bremond 1987 | The objective of this randomised trial was symptomatic treatment of vaginitis. Women with trichomoniasis and candidiasis were randomised to benzydamine versus placebo in addition to standard treatment with tinidazole 2 g for Trichomonas and econazole for candida. Self assessment by the women showed that benzydamine + specific treatment was more effective than specific treatment alone in providing symptomatic relief. |
| Burkett 1975 | No randomised or quasi‐randomised comparisons were made. |
| Catterall 1957 | There were no randomised comparisons between groups treated with trichomycin and acetarsol. |
| Cohen 1975 | No randomised or quasi‐randomised comparisons were made. |
| Csonka 1971 | Fifty‐four women attending sexually transmitted disease clinics were treated with a single 2 g dose of metronidazole. Subsequently, a group of 58 women treated with the 7 day regime were taken as a control group. There was no randomised comparison between the two groups. |
| Davidson 1973 | There was no randomised comparison group. |
| Dellenbach 1972 | The study was conducted in two parts. In the first part, all women received tinidazole; in the second, women were randomly allocated to tinidazole oral or oral + vaginal suppository. The results, however, are reported together and it is not possible to separate the randomised part from the first part. |
| Dellenbach 1974 | No randomised or quasi‐randomised comparisons were made. |
| Djahangiri 1976 | No randomised or quasi‐randomised comparisons were made. |
| Dubois 1970 | No randomised or quasi‐randomised comparisons were made. |
| Dunlop 1958 | The data in this study were not available in a suitable form to be extracted. Eight patients were treated with 2‐acetylamino‐5‐nitrothiazole and 5 control patients were given calcium lactate tablets. Outcomes were reported as number of vaginal smears positive or negative for trichomonads, and not as number of patients cured. |
| Dykers 1978 | No randomised or quasi‐randomised comparisons were made. |
| Erb 1975 | No randomised or quasi‐randomised comparisons were made. |
| Feo 1961 | There was no comparison group in this study. |
| Figueroa 1997 | The data from this study was not in a suitable form for analysis. |
| Ganju 1966 | There was no indication that any randomised or quasi‐randomised comparison were made between study groups. Women with trichomoniasis (pregnant and non‐pregnant) were divided into four groups (metronidazole, hamycin, stovarsol vaginal compound, and placebo) |
| Giordano 1974 | No randomised or quasi‐randomised comparisons were made. |
| Goldman 1974 | No randomised or quasi‐randomised comparisons were made. |
| Goncalves 1987 | This RCT was excluded because outcome of interest was symptomatic relief from Trichomonas and candida infections. Vinegar was compared to salicylic acid/boric acid/ammonium alum combination in hip bath in addition to specific therapy. |
| Goodwin 1972 | Randomised crossover study to investigate the disulfiram‐like effects of flunidazole, a nitroimidazole structurally similar to metronidazole but lacking the metallic taste effect. The study was excluded because the objective was not the treatment of trichomoniasis. However, investigators could not substantiate the reported disulfiram‐like effects of nitroimidazoles in this small study. |
| Gyorik 1971 | No randomised or quasi‐randomised comparisons were made. |
| Heiss 1971 | No randomised or quasi‐randomised comparisons were made. |
| Helmy 1974 | Allocation was not randomised in this study. Initially, consecutive women were given tinidazole and the third given metronidazole and later this was changed to alternation. |
| Henderson 1975 | No randomised or quasi‐randomised comparisons were made. |
| Jones 1977 | No randomised or quasi‐randomised comparisons were made. |
| Karagiosov 1979 | No randomised or quasi‐randomised comparisons were made. |
| Kawamura 1978 | No randomised or quasi‐randomised comparisons were made. In the first recruitment period patients (male Trichomonas positive) were treated with tinidazole, in the second, with metronidazole. |
| Kholodovskaya 1979 | This study examined the pharmacokinetics of tinidazole versus metronidazole. |
| Kim 1993 | This study involved treatment of nongonococcal urethritis in males, and included diseases other than trichomoniasis. |
| Kulseng‐Hanssen 1975 | No randomised or quasi‐randomised comparisons were made. |
| Lanceley 1953 | There were no randomised comparisons. Cultured Trichomonas vaginalis were inoculated in the urethra of 5 and culture media only to 5 other men. |
| Liechti 1971 | It was not possible to clarify whether the study was a true randomised controlled trial or not. The study involves several groups of patients with trichomoniasis treated with different doses of tinidazole, metronidazole, and vaginal and oral route combinations. For groups concerning tinidazole oral versus oral + vaginal with two different doses (4 groups compared altogether) it is stated that the study was double blind. However there is no mention of randomisation and one group has significantly fewer number of participants compared to other three. |
| Lotvin 1973 | No randomised or quasi‐randomised comparisons were made. |
| Lyon 1963 | Women with trichomoniasis were given metronidazole in three different regimes (oral or intravaginal alone and in combination) but there were no randomised comparisons between the three groups. |
| Magnier 1976 | No randomised or quasi‐randomised comparisons were made. |
| Marianowski 1976 | No randomised or quasi‐randomised comparisons were made. |
| Massa 1976 | No randomised or quasi‐randomised comparisons were made. |
| McCann 1972 | Pregnant women were included in this trial and it was not possible to look at them separately. The trial was a randomised comparison of metronidazole and nitrimidazine, both given by mouth as "long'"treatments. |
| Milek 1974 | No randomised or quasi‐randomised comparisons were made. Tinidazole, single, oral dose was tested in doses of 1.5 g, 1.6 g, 1.8 g and 2.0 g. |
| Miller 1969 | No randomised or quasi‐randomised comparisons were made. |
| Mischer 1976 | No randomised or quasi‐randomised comparisons were made. |
| Moore 1979 | There was no comparison group in this study. |
| Mukherjee 1979 | There was no randomised comparison group. |
| Naguib 1975 | No randomised or quasi‐randomised comparisons were made. |
| Obel 1975 | No randomised or quasi‐randomised comparisons were made. |
| Ongom 1974 | No randomised or quasi‐randomised comparisons were made. |
| Ozbilgin 1974 | No randomised or quasi‐randomised comparisons were made. |
| Peeters 1974 | The main reasons for excluding this trial was inclusion of pregnant women (n=1), and the aim was to investigate the effect of miconazole on the incidence of yeast infections occurring after trichomoniasis treatment. It was a randomised controlled trial in which the experiment group received metronidazole 250 mg tablets twice daily for 10 days + miconazole vaginal cream for once in the evening for 15 days while the control group received only metronidazole in the same way. In the metronidazole + miconazole group the number of + yeast cultures were 6 and 0, before and after the treatment, and in the metronidazole only group, all 3 of the yeast positive women remained positive. The parasitological cure rates were 100% in the combined treatment group and 90% (18/20) in the metronidazole only group. |
| Pereyra 1972 | This trial was excluded because three pregnant women were included in the study. The three pregnant women were treated with flunidazole, but their treatment outcomes were not noted. |
| Phillips 1976 | No randomised or quasi‐randomised comparisons were made. |
| Plotho 1971 | No randomised or quasi‐randomised comparisons were made. |
| Porapakkham 1967 | Although the allocation was reported as "random", this study was excluded because there was a large unexplained imbalance in the numbers of women allocated to metronidazole oral + vaginal and metronidazole oral only. Also pregnant women were included in the trial and data for them were not reported separately. |
| Psaroudakis 1977 | No randomised or quasi‐randomised comparisons were made. |
| Quartararo 1974 | No randomised or quasi‐randomised comparisons were made. |
| Rainer 1974 | No randomised or quasi‐randomised comparisons were made. |
| Robinson 1965 | This trial was excluded because it was not clear whether the comparisons had been made between randomised groups. The authors extended a series of metronidazole treated pregnant and non‐pregnant women to include a group of women randomly allocated to a treatment and placebo. Consequently, there is an imbalance in the sample sizes of two groups and it is not possible to identify randomised groups in the tables presented. |
| Ross 1973a | This study was excluded because it was not possible to separate pregnant from non‐pregnant women. Alternate women with trichomoniasis were given 2 g metronidazole or 2 g nimorazole as single dose. Eleven of 75 in the metronidazole group and 6 of 73 were regarded as treatment failures. |
| Ross 1973b | No randomised or quasi‐randomised comparisons were made. |
| Rzempoluch 1976 | This trial examined the treatment of mixed infections. |
| Rösemann 197 | No randomised or quasi‐randomised comparisons were made. |
| Salinas 1971 | No randomised or quasi‐randomised comparisons were made. |
| Sandront‐Degee 1975 | No randomised or quasi‐randomised comparisons were made. |
| Schellen 1974 | No randomised or quasi‐randomised comparisons were made. |
| Schmoer 1974 | No randomised or quasi‐randomised comparisons were made. |
| Shutsky 1978 | No randomised or quasi‐randomised comparisons were made. |
| Sternadel 1974 | No randomised or quasi‐randomised comparisons were made. |
| Sternadel 1975 | No randomised or quasi‐randomised comparisons were made. |
| Swarz 1974a | No randomised or quasi‐randomised comparisons were made. |
| Swarz 1974b | No randomised or quasi‐randomised comparisons were made. |
| Thavabalan 1974 | No randomised or quasi‐randomised comparisons were made. |
| Tran Dinh De 1963 | There was no randomised comparison between groups. |
| Turanova 1977 | No randomised or quasi‐randomised comparisons were made. |
| Underhill 1974 | No randomised or quasi‐randomised comparisons were made. |
| Vartiainen 1966 | No randomised or quasi‐randomised comparisons were made. |
| Wacha 1976 | No randomised or quasi‐randomised comparisons were made. |
| Wallin 1974 | No randomised or quasi‐randomised comparisons were made. |
| Ward 1976 | No randomised or quasi‐randomised comparisons were made. |
| Watt 1960 | There was no comparison group in this study. |
| Weidenbach 1974 | No randomised or quasi‐randomised comparisons were made. |
| Weitgasser 1976 | This trial is reported as double blind, but no mention of random allocation is made. |
| Wladeck 1981 | No randomised or quasi‐randomised comparisons were made. |
| Woodcock 1972 | Metronidazole was given 400 mg/day for 2 days when the researcher was on duty, 200 mg/day for 7 days when the researcher was off duty. There was thus no randomised comparison between the two groups. |
| Zaremba 1980 | No randomised or quasi‐randomised comparisons were made. |
| Zwierz 1969 | No randomised or quasi‐randomised comparisons were made. |
Contributions of authors
Metin Gülmezoglu wrote the initial version of the review. Fatu Forna is currently the responsible author for the review. She contributed to updating the review by extracting data from studies not included before and by revising the text of the review.
Sources of support
Internal sources
National Perinatal Epidemiology Unit, Oxford, UK, Not specified.
HRP ‐ UNDP/UNFPA/WHO/World Bank Special Programme in Human Reproduction, Geneva, Switzerland.
Duke University School of Medicine, North Carolina, USA.
Department of Maternal & Child Health, University of North Carolina‐Chapel Hill, USA.
External sources
Department for International Development, UK.
Nuffield Provincial Hospitals Trust, UK.
European Commission (Directorate General XII), Belgium.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Unchanged
References
References to studies included in this review
- Aimakhu VE. Vaginal trichomoniasis: One stat dose of tinidazole compared with a seven‐day course of metronidazole. West Afr Med J, 1975;23:97‐100. [Google Scholar]
- Anjaneyulu R, Gupte SA, Desai DB. Single‐dose treatment of trichomonal vaginitis: a comparison of tinidazole and metronidazole. J Int Med Res, 1977;5:438‐441. [DOI] [PubMed] [Google Scholar]
- Antonelli NM, Diehl SJ, Wright JW. A randomized trial of intravaginal nonoxynol 9 versus oral metronidazole in the treatment of vaginal trichomoniasis. Am J Obstet Gynecol 2000;182:1008‐10. [DOI] [PubMed] [Google Scholar]
- Austin TW, Smith EA, Darwish R, Ralph ED, Pattison FLM. Metronidazole in a single dose for the treatment of trichomoniasis. Failure of a 1‐g single dose. Br J Vener Dis, 1982;58:121‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Ralph ED, Darwish R, Austin TW, Smith EA, Pattison FLM. Susceptibility of Trichomonas vaginalis strains to metronidazole: response to treatment. Sex Trans Dis, 1983;10:119‐122. [DOI] [PubMed] [Google Scholar]
- Barnes J, Boutwood A, Haines E, Levington W, Lister E, Haram BJ. Oral treatment of trichomonas vaginitis with aminitrozole. Br Med J, 1957;1:1160‐1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum SF, Ali SE, Ali S. Short course nitroimidazole in the treatment of vaginal trichomoniasis. Curr Ther Res, 1980;28:922‐926. [Google Scholar]
- Block E. The effect of nifuratel and metronidazole in the treatment of trichomoniasis. Lakartidningen, 1972;69:5210‐5212. [PubMed] [Google Scholar]
- Chaisilwattana P, Bhiraleus P, Patanaprnich P, Bhadrakom C. Double blind comparative study of tinidazole and ornidazole as a single dose treatment of vaginal trichomoniasis. J Med Assoc Thailand, 1980;63:448‐452. [PubMed] [Google Scholar]
- Chaudhuri P, Drogendijk AC. A double‐blind controlled clinical trial of carnidazole and tinidazole in the treatment of vaginal trichomoniasis. Eur J Obstet Gynecol Reprod Biol, 1980;10:325‐328. [DOI] [PubMed] [Google Scholar]
- Chung SO, Yoo BI, Park YD, Lasserre R. Single‐day treatment of trichomonas vaginitis with low dose of ornidazole. Southeast Asian J Trop Med Pub Health, 1978;9:74‐78. [PubMed] [Google Scholar]
- Chunge CN, Kangethe S, Pamba HO, Owate J. Treatment of symptomatic trichomoniasis among adult women using oral nitroimidazoles. East Afr Med J, 1992;69:398‐401. [PubMed] [Google Scholar]
- Csonka GW. Long‐term aspect of treatment with metronidazole (flagyl) in trichomonal vaginitis. Brit J Vener Dis, 1963;39:258‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divald VJ. Trichomoniasis ‐ behandlung der frau mit tinidazol. Wien Med Wochen, 1971;24:492‐494. [PubMed] [Google Scholar]
- duBouchet L, Spence MR, Rein MF, Danzig MR, McCormack WM. Multicenter comparison of clotrimazole vaginal tablets, oral metronidazole, and vaginal suppositories containing sulfanilamide, aminacrine hydrochloride, and allantoin in the treatment of symptomatic trichomoniasis. Sex Trans Dis, 1997;24:156‐160. [DOI] [PubMed] [Google Scholar]
- duBouchet L, McGregor JA, Ismail M, McCormack WM. A pilot study of metronidazole vaginal gel versus oral metronidazole for the treatment of Trichomonas vaginalis vaginitis. Sex Trans Dis, 1998;25:176‐179. [DOI] [PubMed] [Google Scholar]
- Eriksson G, Wanger L. Treatment of trichomoniasis in females with and without gonorrhoea. Br J Vener Dis, 1976;52:276‐278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Catterall RD. Nifuratel compared with metronidazole in the treatment of trichomonal vaginitis. Br Med J, 1970;1:335‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans BA, Catterall RD. Nitrimidazine compared with metronidazole in the treatment of vaginal trichomoniasis. Br Med J, 1971;4:146‐147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster SA, Ramirez OG, Rapoport AH. Metronidazole and trichomonal vaginitis. Am J Obstet Gynecol, 1963;87:1013‐1023. [PubMed] [Google Scholar]
- Fugere P, Verschelden G, Caron M. Single dose of ornidazole in women with vaginal trichomoniasis. Obstet Gynecol, 1983;62:502‐505. [PubMed] [Google Scholar]
- Gabriel G, Robertson E, Thin RN. Single dose treatment of trichomoniasis. J Int Med Res, 1982;10:129‐130. [DOI] [PubMed] [Google Scholar]
- Garud M, Lulla M, Saraiya U, Vaidya S. Oral single dose therapy of trichomonal vaginitis: comparison of tinidazole and metronidazole. J Obstet Gynaecol India, 1978;28:347‐350. [Google Scholar]
- Gjonnaess H, Aure JC. Treatment of trichomonas vaginitis with nifuratel. Acta Obstet Gynecol Scand, 1969;48:85‐94. [DOI] [PubMed] [Google Scholar]
- Gorlero F, Bosco P, Barbieri M, Bertulessi C, Pulici L, Polvani F, Cecco L. Fenticonazole ovules in the treatment of vaginal trichomonas infections. A double‐blind randomized pilot clinical trial. Curr Ther Res, 1992;51:367‐376. [Google Scholar]
- Gorlero F, Macchiavello S, Pellegatta L, Airoldi ML, Gaffuri B, Pulici L, Cecco L. Evaluation of the efficacy and tolerability of two different dosages of fenticonazole vaginal ovules (600 mg and 1000 mg) in patients with vaginal trichomoniasis: A controlled, double‐blind, randomized clinical trial versus placebo. Curr Ther Res, 1994;55:510‐518. [Google Scholar]
- Gummerus M. Tinidazole in vaginal trichomoniasis [Trikomonaskolpiitin hoito tinidatsolilla]. Suomen Laakarilehti 1983;38(13):1216‐1217. [Google Scholar]
- Hager WD, Brown ST, Kraus SJ, Kleris GS, Perkins GJ, Henderson M. Metronidazole for vaginal trichomoniasis. JAMA, 1980;244:1219‐1220. [PubMed] [Google Scholar]
- Hayward MJ, Roy RB. Two‐day treatment of trichomoniasis in the female. Comparison of metronidazole and nimorazole. Br J Vener Dis, 1976;52:63‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillstroem L, Pettersson L, Palsson E, Sandstroem SO. Comparison of ornidazole and tinidazole in single‐dose treatment of trichomoniasis in women. Br J Ven Dis, 1977;53:193‐194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannino A, Testa P. [Sul trattamento locale delle vaginiti da T. vaginalis. Richerche comparative con metil‐patricina e metronidazolo]. Minerva Ginecol 1975;27:929‐32. [PubMed] [Google Scholar]
- Korner B, Nygaard B, Jensen RH. A controlled trial of single‐dose treatment with ornidazol (Tiberal) for vaginal trichomoniasis in general practice [Engangsbehandling med ornidazol (Tiberal) ved vaginal trichomoniasis]. Ugeskr Laeg, 1978;140:1485‐1487. [PubMed] [Google Scholar]
- Lean TH, Vengadasalam D. Treatment of vaginal trichomoniasis with a new anti‐protozal compound (oc‐chloromethyl‐2‐methyl‐ 5‐nitro‐1‐imidazole‐ethanol). Br J Vener Dis, 1973;49:69‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng J, Christensen J. A double‐blind study of the value of treatment with a single dose tinidazole of partners to females with trichomoniasis. Acta Obstet Gynecol Scand, 1981;60:199‐201. [PubMed] [Google Scholar]
- Mahony JDH, Harris JRW, Farrer CJ. Nimorazole and metronidazole in the treatment of trichomonal vaginitis. Br J Clin Pract, 1975;29:71‐72. [PubMed] [Google Scholar]
- Manth SM, Rindt W, Schnitker J, Weigerding A, Colli E, Scatigna M. Fenticonazole in the treatment of vaginal trichomonas infections. Curr Ther Res, 1989;45:1060‐1066. [Google Scholar]
- Mati JKG, Wallace RJ. The treatment of trichomonal vaginitis using a single dose of tinidazole by mouth. East Afr Med J, 1974;51:883‐888. [PubMed] [Google Scholar]
- McClean AN. Nitrimidazine (Naxogin) compared with metronidazole (Flagyl) in the treatment of trichomonal vaginitis. Br J Vener Dis, 1972;48:69‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notowicz A, Stolz E, Koning GA. First experiences with single‐dose treatment of vaginal trichomoniasis with carnidazole (R 25831). Br J Ven Dis, 1977;53:129‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard B, Kjaersgaard H, Korner B, Hammer Jensen R. Single‐dose treatment with ornidazole (Tiberal(R)) and seven‐day treatment with metronidazole (Flagyl(R)) in vaginal trichomoniasis. Ugeskr Laeg, 1977;139:524‐526. [PubMed] [Google Scholar]
- O‐Prasertsawat P, Jetsawangsri T. Split‐dose metronidazole or single‐dose tinidazole for the treatment of vaginal trichomoniasis. Sex Trans Dis, 1992;19:295‐297. [DOI] [PubMed] [Google Scholar]
- Rao HTM, Shenoy DR. Single‐dose oral treatment of vaginal trichomoniasis with tinidazole and metronidazole. J Int Med Res, 1978;6:46‐49. [DOI] [PubMed] [Google Scholar]
- Rees PH, McGlashan HE, Mwega V. Single‐dose treatment of vaginal trichomoniasis with tinidazole. East Afr Med J, 1974;51:782‐785. [PubMed] [Google Scholar]
- Roncuzzi R, Fortuna A. [Ricerca controllata sul trattamento della tricomoniasi vaginale con nimorazolo (nitrimidazina) o metronidazolo]. Giornale Italiano Di Chemioterapia, 1972;19:69‐71. [PubMed] [Google Scholar]
- Roy RB, Laird SM, Heasman L. Treatment of trichomoniasis in the female. A comparison of metronidazole and nimorazole. Br J Vener Dis, 1975;51:281‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed A, Roy RB, Huq MA. Two‐day treatment of trichomoniasis in the female ‐ A comparison of metronidazole and nimorazole. Br J Clin Pract, 1976:41‐44. [PubMed] [Google Scholar]
- Sandvei R, Hernborg K. [Behandling av trichomonas vaginalis]. Tidsskr Nor Loegeforen 1979;99:316‐317. [PubMed] [Google Scholar]
- Schnell JD. The incidence of vaginal candida and trichomonas infections and treatment of trichomonas vaginitis with clotrimazole. Postgrad Med J 1974;July Suppl:79‐81. [PubMed] [Google Scholar]; Schnell JD, Ruether C. [Therapie kombinierter vaginal infektionen von trichomonas vaginalis und sprosspilzen]. Geburtsh Frauenheilk 1974;34:551‐7. [PubMed] [Google Scholar]
- Serup J, Jensen RH. Treatment of trichomoniasis vaginalis with single oral dose of ornidazole (Tiberal) and tinidazole (Fasigyn). A controlled investigation. Ugeskr Laeg, 1978;140:1483‐1484. [PubMed] [Google Scholar]
- Sesti F, Valli E, Troisi C, Ciancio F, Piccione E. Comparative clinical study of the therapeutic efficacy of tinidazole and ornidazole in the treatment of vaginal trichomoniasis [Studio clinico comparativo sull'efficacia terapeutica di tinidazolo vs. ornidazolo nel trattamento della trichomoniasi vaginale]. Giorn It Ost Gin, 1990;12:83‐84. [Google Scholar]
- Spence MR, Harwell TS, Davies MC, Smith JL. The minimum single oral metronidazole dose for treating trichomoniasis: a randomized blinded study. Obstet Gynecol, 1997;89:699‐703. [DOI] [PubMed] [Google Scholar]
- Thin RN, Symonds MAE, Booker R, Cook S, Langlet F. Double‐blind comparison of a single dose and a five‐day course of metronidazole in the treatment of trichomoniasis. Br J Ven Dis, 1979;55:354‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell BH, Lushbaugh WB, Laughlin M, Cleary JD, Finley RW. A double‐blind placebo‐controlled trial of single‐dose intravaginal versus single‐dose oral metronidazole in the treatment of trichomonal vaginitis. J Infect Dis, 1994;170:242‐246. [DOI] [PubMed] [Google Scholar]
- Tinkler AE. Nimorazole compared with metronidazole in the treatment of vaginal trichomoniasis. Practitioner, 1974;212:115‐119. [PubMed] [Google Scholar]
- Wigfield AS. Trichomonal vaginitis. A 24‐hr regimen of nimorazole compared with a 7‐day regimen of metronidazole. Br J Vener Dis, 1975;51:54‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Akinla O, Ogunbi O. Treatment of trichomonal vaginitis with single dose tinidazole (Fasigyn). West Afr J Pharmac Drug Res, 1975;2:31‐37. [PubMed] [Google Scholar]
- Ali SE. Clinical evaluation of a single dose of tinidazole in trichomoniasis. Curr Ther Res, 1975;18:669‐672. [PubMed] [Google Scholar]
- Andersen HJ. [Behandling af trichomoniasis urogenitalis med en engagngsdosis af tinidazolum]. Ugeskr Laeg, 1975;137:676‐678. [PubMed] [Google Scholar]
- Apte VV, Packard RS. Tinidazole in the treatment of trichomoniasis, giardiasis and amoebiasis. Drugs, 1978;15(Suppl. 1):43‐48. [DOI] [PubMed] [Google Scholar]
- Arnold M. [Vergleich von Nifuratel und Tinidazol bei Trichomonadenvaginitis]. Ther Umsch, 1974;31:202‐204. [PubMed] [Google Scholar]
- Arya OP, Alergant CD. Comparison of magmilor with flagyl compaq in women with trichomoniasis and candidiasis. Unpublished1974.
- Aubert JM, Sesta HJ. Treatment of vaginal trichomoniasis: Single, 2‐gram dose of metronidazole as compared with a seven‐day course. J Reprod Med, 1982;27:743‐745. [PubMed] [Google Scholar]
- Aure MT. [Estudio comparativo entre el tinidazole y el metronidazol en el tratamiento de la tricomoniasis vaginal]. Rev Obstet Gynecol Venezuela, 1974;34:437‐440. [Google Scholar]
- Azevedo EMM, Fonseca AM, Souza AZ, Bagnoli VR, Salvatore CA. Vaginal trichomoniasis treatment by immunization with lactobacillus acidophilus vaccine. Archives of Gynecology 1985;237:65. [Google Scholar]
- Bedoya JM. Short treatment for human urogenital trichomoniasis with tinidazole: a preliminary report. Curr Med Res Opin, 1974;2:165‐168. [DOI] [PubMed] [Google Scholar]
- Bertini de Oliveira AM, Delascio D, Oliveira N. Treatment of vaginitis due to trichomonas. Results with a new imidazole derivative: Nitrimidazine. Minerva Ginecol, 1972;7:342‐353. [PubMed] [Google Scholar]
- Bloch B, Smyth E. The treatment of Trichomonas vaginalis vaginitis. An open controlled prospective study comparing a single dose of metronidazole tablets, benzoyl metronidazole suspension and tinidazole tablets. S Afr Med J, 1985;67:455‐457. [PubMed] [Google Scholar]
- Botero D, Perez A. Treatment of intestinal amoebiasis and vaginal trichomoniasis with panidazole and its comparison with metronidazole. Trans R Soc Trop Med Hyg, 1977;71:508‐511. [DOI] [PubMed] [Google Scholar]
- Bremond A. Clinical evaluation of benzydamine for treatment of vaginitis: Results of a randomized study with emphasis on functional symptoms. Int J Tiss Reac, 1987;9:147‐149. [PubMed] [Google Scholar]
- Burkett G, Noel B. Single dose treatment of trichomonas vaginalis with tinidazole (Fasigyn). W I Med J, 1975;24:179‐181. [PubMed] [Google Scholar]
- Catterall RD, Nicol CS. Systemic treatment of trichomonal infections. Br Med J, 1957;2:29‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Merger R. [Un traitement‐minute de la trichomonase vaginale]. Gynecologie, 1975;26:27‐28. [Google Scholar]
- Csonka GW. Trichomonal vaginitis treated with one dose of metronidazole. Br J Vener Dis, 1971;47:456‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson F. Short‐term high‐dose metronidazole for vaginal trichomoniasis. J Obstet Gynecol, 1973;80:368‐370. [DOI] [PubMed] [Google Scholar]
- Dellenbach P, Muller P. [Traitement des trichomonases urogenitales par le Fasigyn]. J Gynecol Obstet Biol Reprod, 1972;1(Suppl. 2):369‐371. [PubMed] [Google Scholar]
- Dellenbach P, Muller P. Single dose therapy of urogenital trichomoniasis with 2 grams tinidazole. Curr Med Res Opin, 1974;2:142‐146. [DOI] [PubMed] [Google Scholar]
- Djahangiri MH. [Eindosis‐Therapie der Trichomonaden ‐Kolpitis mit tinidazol]. Muench Med Wschr, 1976;118:47‐48. [PubMed] [Google Scholar]
- Dubois P, Lambotte R, Plomteux G. [Essais therapeutiques cliniques avec le fasigyn dans la trichomoniase vaginale]. Rev Med Liege, 1970;25:586‐590. [PubMed] [Google Scholar]
- Dunlop EMC, Philipp E, Watt JD. Oral treatment of trichomonal vaginitis with 2‐Acetylamino‐5‐Nitrothiazole. Br J Vener Dis, 1958;34:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykers JR. Single‐dose metronidazole for trichomonal vaginitis: patient and consort. Am J Obstet Gynecol, 1978;132:579‐580. [DOI] [PubMed] [Google Scholar]
- Erb H. [Eindosis‐therapie bei trichomoniasis]. Geburtsh Frauenheilk, 1975;35:44‐47. [PubMed] [Google Scholar]
- Feo LG. Flagyl in the oral treatment of trichomonal vaginitis. Br J Vener Dis, 1961;37:219‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa RBG, Lopez FGB, Rechy GO, Romero‐Cabello R. [Estudio Comparativo para evaluar la eficacia y seguridad de metronidazol y secnidazol en presentacion de ovulos, para el tratamiento de tricomoniases vaginal]. Ginecologia y Obstet De Mexico, 1997;65:487‐491. [PubMed] [Google Scholar]
- Ganju D, Anjaneyulu R. Leucorrhoea due to Trichomonas vaginalis ‐ Clinical trial with Hamycin. Hindustan Antibiotics Bulletin, 1966;9:69‐75. [PubMed] [Google Scholar]
- Giordano C, Casanova R, Montagnoli JR, Sousa MJ, Martinez A, dos Santos JF, Silva SMM. [Tratamento da tricomoniase vaginal com dose unica de tinidazol]. Ginecologia, 1974;68:557‐565. [Google Scholar]
- Goldman JA. A comparative study of nitrimidazine and metronidazole in the treatment of trichomonas infections. Harefuah, 1974;86:463‐465. [PubMed] [Google Scholar]
- Goncalves NMC. Clinical evaluation of a salicylic acid/boric acid/ammonium alum combination as coadjuvants in the treatment of Trichomonas vaginalis and Candida albicans vulvovaginitis. J Bras Ginecol, 1987;97:359‐362. [Google Scholar]
- Goodwin DW, Reinhard J. Disulfiramlike effects of trichomonocidal drugs. A review and double‐blind study. Quart J Stud Alc, 1972;33:734‐740. [PubMed] [Google Scholar]
- Gyorik W, Wenner R. [Therapie der Trichomonadeninfektion mit Tinidazol im Vergleich zu Metronidazol]. Schweiz Rundsch Med Prax, 1971;60:1612‐1614. [Google Scholar]
- Heiss VH. [Klinische auswertung aktiver Arzneimittel gegen Trichomoniasis vaginalis im Doppel‐blindversuch]. Wien Med Wochen, 1971;46:832‐834. [PubMed] [Google Scholar]
- Helmy NI. A new anti‐trichomonal agent. Br J Clin Pract, 1974;28:411‐412. [PubMed] [Google Scholar]
- Henderson JN, Tait IB. The use of povidone‐iodine ('Betadine') pessaries in the treatment of candidal and trichomonal vaginitis. Curr Med Res Opin, 1975;3:157‐162. [DOI] [PubMed] [Google Scholar]
- Jones R, Enders P. An evaluation of tinidazole as single‐dose therapy for the treatment of Trichomonas vaginalis. Med J Aust, 1977;2:679‐680. [DOI] [PubMed] [Google Scholar]
- Karagiosov I, Stoilov L, Trayanova E. One day treatment of genital trichomoniasis with Fasigyn. Akush Ginekol, 1979;18:98‐104. [PubMed] [Google Scholar]
- Kawamura N. Metronidazole and tinidazole in a single large dose for treating urogenital infections with trichomonas vaginalis in men. Br J Vener Dis, 1978;54:81‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodovskaya IV, Minasova GS, Khokhlov AP. Clinicolaboratory evaluation of tinidazole (Facizhine) "Polfa" in treatment of vaginal trichomoniasis. Vestn Dermatol Venerol, 1979;4:58‐60. [PubMed] [Google Scholar]
- Kim HJ, Kim JH, Kim YT. Tetracycline versus combination of tetracycline and metronidazole in the treatment of male patients with nongonococcal urethritis. Korean J Dermatol, 1993;31:937‐943. [Google Scholar]
- Kulseng‐Hanssen S, Ladehaug B. [Vaginal trichomoniasis behandlet med tinidazol (Fasigyn) i engangsdose]. Tidsskrift for Den Norske Laegeforening, 1975;95:1003‐1004. [PubMed] [Google Scholar]
- Lanceley F, McEntegart MG. Trichomonas vaginalis in the male. The experimental infection of a few volunteers. Lancet, 1953;1:668‐669 (April 4). [DOI] [PubMed] [Google Scholar]
- Liechti R. [Perorale behandlung der Trichomonadenkolpitis mit Fasigyn]. Schweiz Z Gynaekol Geburtsh, 1971;2:229‐235. [Google Scholar]
- Lotvin BR, Moreno JAR, Larios NM, Arista RB. [Estudio doble ciego de un nuevo tricomonicida de uso oral exclusivamente]. Ginec Obstet Mex, 1973;34:397‐400. [PubMed] [Google Scholar]
- Lyon FA, Sinykin MB, Barr MM. Trichomonal vaginitis treated with metronidazole. Am J Obstet Gynecol, 1963;85:955‐958. [DOI] [PubMed] [Google Scholar]
- Magnier PA, Cohen A. [La trichomonase uro‐genitale et son traitement minute par la tinidazole 500]. Lyon Med, 1976;236:279‐284. [Google Scholar]
- Marianowski J. [Leczenie rzesistkowego zapalenia pochwy jednorazowa dawka tinidazolu]. Gynecol Pol, 1976;47:317‐319. [PubMed] [Google Scholar]
- Massa M, Arias B, Subiabre V, Rojo M. Therapeutic trial of Trichomonas vaginalis in male by using a single dose of tinidazole [Ensayo terapéutico de la infeccion por Trichomonas vaginalis en el hombre mediante una dosis unica de tinidazol]. Bol Chile Parasit, 1976;31:46‐47. [PubMed] [Google Scholar]
- McCann JS, Mahony JDH, Harris JRW. Comparison of nitrimidazine and metronidazole in the treatment of trichomonal vaginitis. Br J Vener Dis, 1972;48:387‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milek E, Nedelkova E. Single‐dose therapy with tinidazole in trichomoniasis. Curr Med Res Opin, 1974;2:169‐177. [DOI] [PubMed] [Google Scholar]
- Miller MW, Howes HL, English AR. Tinidazole, a potent new antiprotozoal agent. Antimicrob Agents and Chemother, 1970;9:261‐266. [PubMed] [Google Scholar]
- Mischer P, Soeltz‐Szoets J, Thurner J. [Kurzzeitbehandlung der trichomoniasis]. Z Hautkr, 1976;51:465‐468. [PubMed] [Google Scholar]
- Moore JR. One‐gram, single‐dose metronidazole (flagyl) for trichomonal vaginitis. J Am Coll Health Assoc, 1979;28:128. [DOI] [PubMed] [Google Scholar]
- Mukherjee K. Clinical experience with Flagyl suspension in vaginal trichomoniasis. Indian Practitioner, 1979;32:291‐294. [Google Scholar]
- Naguib YA, Nagui A, Bassaly M, Gaber A. Treatment of trichomonas vaginitis with a single dose of tinidazole. J Egypt Med Assoc, 1975;58:633‐644. [PubMed] [Google Scholar]
- Obel E. [Behandling af Trichomonas vaginalis med "enkeltdosis" metronidazol (Flagyl, Trichomol), tinidazol (Fasigyn)]. Ugeskr Laeg, 1975;137:693‐694. [PubMed] [Google Scholar]
- Ongom VL, Wamboka JW, Odong EAI, Mafigiri J. A single dose treatment of Trichomonas infection with tinidazole (Fasigyn) in Uganda. East Afr Med J, 1974;51:878‐882. [PubMed] [Google Scholar]
- Ozbilgin A, Ozbel Y, Alkan MZ, Guruz Y, Atambay M, Tasci S, Ozcel MA. Trichomoniasis in non‐gonococcic urethritis among male patients. J Egypt Soc Parasitol, 1994;24:621‐625. [PubMed] [Google Scholar]
- Peeters R, Snauwaert R, Cutsem JV, Amery W. A controlled trial with miconazole (R 14889) in the prevention of yeast infections after treatment of vaginal trichomoniasis. Eur J Obstet Gynecol Reprod Biol, 1974;4:95‐99. [Google Scholar]
- Pereyra AJ, Nelson RM, Ludders DJ. Flunidazole ‐ A new drug for systemic treatment of urogenital trichomoniasis. Am J Obstet Gynecol, 1972;112:963‐966. [DOI] [PubMed] [Google Scholar]
- Phillips C, Shrivastava M. Single‐dose therapy of trichomonal vaginitis with tinidazole. J Obstet Gynaecol India, 1976;26:893‐895. [Google Scholar]
- Plotho B, Koelbl H. [Die behandlung der trichomoniasis urogenitalis mit tinidazol*) einer neuen, trichomonaziden substanz]. Wien Med Wochen, 1971;40:707‐709. [PubMed] [Google Scholar]
- Porapakkham S. Metronidazole treatment of vaginal trichomoniasis II. Oral vs Vaginal Therapy. J Obstet Gynecol, 1967;29:213‐216. [PubMed] [Google Scholar]
- Psaroudakis A, Kalogeropoulos G, Michalopoulos A, Antoniu K, Tsatsiadis K, Golemati E, Kaskarelis D. Treatment of vaginal trichomoniasis with a single oral dose of tinidazole. Curr Ther Res, 1977;21:473‐478. [Google Scholar]
- Quartararo P, Florino S. Treatment of vaginal trichomoniasis with a single dose of tinidazole. Curr Med Res Opin, 1974;2:153‐157. [DOI] [PubMed] [Google Scholar]
- Rainer VA. [Uber die behandlung der trichomoniasis urogenitalis beider geschiechter mit tinidazol (Fasigyn)]. Wien Med Wochen, 1974;42‐43:625‐626. [PubMed] [Google Scholar]
- Robinson SC, Gopi M. Trichomonas vaginalis. V. Further observations on metronidazole (Flagyl) (including infant follow‐up). Am J Obstet Gynecol, 1965;93:502‐505. [PubMed] [Google Scholar]
- Ross SM. Vaginal and oral nitrimidazine in the treatment of vaginal trichomoniasis. Br J Vener Dis, 1973;49:310‐313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SM. Single and triple dose treatment of trichomonas infection of the vagina. Br J Vener Dis, 1973;49:475‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzempoluch E, Cwik F, Szymanska A, Lubelska K. [Porownanie skutecznosci leczenia rzesistkowicy metronidazolem, fasigynem i pimafucina]. Polski Tygodnik Lekarski, 1976;31:2021‐2023. [PubMed] [Google Scholar]
- Rösemann GWE, Vaughan J. Treatment of trichomoniasis in the female with a single dose of tinidazole. S Afr Med J, 1973;47:1222‐1224. [PubMed] [Google Scholar]
- Salinas PL. [Tinidazol (CP, 12‐574) en el tratamiento de la tricomoniasis vaginal]. Rev Clin Espanol, 1971;123:173‐176. [PubMed] [Google Scholar]
- Sandront‐Degee M, Werbrouck‐Navette J, Lambotte R. [Acquisitions nouvelles dans la therapeutique des vaginites a trichomonas vaginalis]. Rev Med Liege, 1975;30:560‐562. [PubMed] [Google Scholar]
- Schellen TMCM, Meinhardt G. Treatment of trichomoniasis with a single oral dose of tinidazole. Curr Med Res Opin, 1974;2:158‐164. [DOI] [PubMed] [Google Scholar]
- Schmoer J. Single‐dose treatment with tinidazole: progress in the therapy of trichomoniasis. Curr Med Res Opin, 1974;2:138‐141. [DOI] [PubMed] [Google Scholar]
- Shutsky IV, Bratus FF, Melnichenko AI, Borosvky AV, Arabadzhi TM, Kunina EM. Comparative evaluation of some methods for treatment of trichomonosis in women. Vestn Dermatol Venerol, 1978;9:71‐74. [PubMed] [Google Scholar]; Szczucki IV, Bratus FF, Melitczenko AI, Borowski AV, Vinogradova MP, Elenieva LV, Kunina EM. Results of treatment with metronidazole according to various schedules in trichomonadosis alone or coexistent with gonorrhoea, syphilis and bacterial infections. Przegl Dermatol, 1987;2:133‐138. [PubMed] [Google Scholar]
- Sternadel Z, Peksa A, Karwan‐Plonska. Treatment of trichomonas vaginitis with tinidasol [Leczenie rzesistkowego zapalenia pochwy tinidazolem]. Gin Pol, 1974;9:110‐1111. [PubMed] [Google Scholar]
- Sternadel Z, Karwan‐Plonska A, Peksa A. [Ocena skutecznosci leczniczej jednorazowej dawki doustnej tinidazolu (2000 mg) w leczeniu rzesistkowego zapalenia pochwy]. Gynecol Pol, 1975;46:787‐789. [PubMed] [Google Scholar]
- Swarz H, Lahon HFJ. Introduction. Curr Med Res Opinion, 1974;2:127‐129. [DOI] [PubMed] [Google Scholar]
- Swarz H. International experience with a new single 2 gram dose of tinidazole ('Fasigyn'). Curr Med Res Opinion, 1974;2:181‐187. [DOI] [PubMed] [Google Scholar]
- Thavabalan PB, Oriel JD. Single‐dose treatment of vaginal trichomoniasis with tinidazole ('Fasigyn'). Curr Med Res Opinion, 1974;2:178‐180. [DOI] [PubMed] [Google Scholar]
- Tran Dinh De, Nguyen Van Tu. Clinical and statistical study of trichomonad infestation in Vietnamese women with special reference to treatment by a new imidazole derivative. Am J Obstet Gynecol, 1963;87:92‐104. [DOI] [PubMed] [Google Scholar]
- Turanova EN, Delektorsky VV, Nyunikova OI, Yashkova GN, Voskresenskaya GA. Fasigyn in the treatment of Trichomonas vaginitis. Akush Ginekol, 1977;4:49‐51. [PubMed] [Google Scholar]
- Underhill RA, Peck JE. Causes of therapeutic failure after treatment of trichomonal vaginitis with metronidazole: Comparison of single‐dose treatment with a standard regimen. Br J Clin Pract, 1974;28:134‐136. [PubMed] [Google Scholar]
- Vartiainen E, Widholm O. Treatment of trichomonas colpitis with thyadione [Uusi Isske trikomonaskolpiitin hoitoon]. Duodecim 1966;82:755‐757. [PubMed] [Google Scholar]
- Wacha DSO, Zeigler O. The treatment of trichomonal vaginitis using single dose tinidazole (fasign‐pfizer). Med J Zambia, 1976;10:157‐159. [PubMed] [Google Scholar]
- Wallin J, Forsgren A. Tinidazole‐a new preparation for T.vaginalis infections. Br J Vener Dis, 1974;50:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JP. Tinidazole (Fasigyn)‐single dose therapy for Trichomonas vaginalis. Med J Aust, 1976;2:651‐652. [PubMed] [Google Scholar]
- Watt L, Jennison RF. Clinical evaluation of metronidazole. A new systemic trichomonacid. Br Med J, 1960;2:902‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidenbach A, Leix H. Treatment of trichomonal vaginitis with a single dose of tinidazole. Curr Med Res Opin, 1974;2:147‐151. [DOI] [PubMed] [Google Scholar]
- Weitgasser H. Treatment of vaginal trichomoniasis with Tiberal Roche under particular consideration of single day therapy [Die behandlung der trichomoniasis vaginalis mit Tiberal Roche unter besonderer berucksichtigung der ein‐tages‐therapie]. Wien Med Wochenschr 1976;126(11‐12):162‐5. [PubMed] [Google Scholar]
- Wladeck WG. A comparative study with Tinidazole and Nimorazole in the treatment of Trichomoniasis [Estudo comparativo entre o Tinidazol e o Nimorazol no Tratamento da Tricomoniase]. Rev Bras Med 1981;38(7):439‐442. [Google Scholar]
- Woodcock KR. Treatment of trichomonal vaginitis with a single oral dose of metronidazole. Br J Vener Dis, 1972;48:65‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]; Woodcock KR. Two‐day treatment with metronidazole in vaginal trichomoniasis. Br J Vener Dis, 1972;48:383‐386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba A, Szarmach H, Trybula J. [Badania porownawcze nad skutecznoscia leczenia rzesistkowicy jednorazowa dawka metronidazolu i fasyginu]. Przeg Derm, 1980;67:229‐231. [PubMed] [Google Scholar]
- Zwierz C, Weryk‐Wojciechowicz Z, Spiralska I. Clinical evaluation of metronidazol "polfa" in the treatment of trichomonas vaginalis. Wiad Parazytol, 1969;15:387‐388. [PubMed] [Google Scholar]
Additional references
- Khanna J, Look PFA, Griffin PD. Challenges in reproductive health research. Biennial Report 1992‐1993, WHO. Geneva: WHO, 1994. [Google Scholar]
References to other published versions of this review
- Gülmezoglu AM, Garner P. Trichomoniasis treatment in women: a systematic review. Tropical Medicine and International Health 1998;3(7):553‐558. [DOI] [PubMed] [Google Scholar]


