Abstract
Background
Paracetamol (acetaminophen) is widely used for treating fever in children. Like ibuprofen, aspirin, and physical methods (such as fanning), paracetamol aims to provide relief from symptoms and prevent febrile convulsions. Uncertainty exists about the benefits of using it to treat fever in children.
Objectives
To assess the effects of paracetamol for treating fever in children in relation to fever clearance time, febrile convulsions, and resolution of associated symptoms.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (May 2004), CENTRAL (The Cochrane Library Issue 2, 2004), MEDLINE (1966 to May 2004), EMBASE (1988 to May 2004), LILACS (May 2004), Science Citation Index (May 2004), and reference lists of articles. We also contacted researchers in the field.
Selection criteria
Randomized and quasi‐randomized trials of children with fever from infections comparing: (1) paracetamol with placebo or no treatment; and (2) paracetamol with physical cooling methods (eg, sponging, bathing, or fanning). The primary outcomes were fever clearance time and febrile convulsion.
Data collection and analysis
Two reviewers independently extracted data on methods, types of participants, interventions, and outcomes. The meta‐analysis was conducted using risk ratio with 95% confidence intervals for discrete variables, and mean differences for continuous outcomes.
Main results
12 trials (n = 1509 participants) met the inclusion criteria. Outcomes varied between trials. No data were available on the primary outcome. There is insufficient evidence to show whether paracetamol influenced the risk of febrile convulsions. In a meta‐analysis of two trials (n = 120), the proportion of children without fever by the second hour after treatment did not differ significantly between those given paracetamol and those sponged (risk ratio 1.84; confidence interval 0.94 to 3.61, random effects model). The statistical test showed significant heterogeneity between the groups receiving paracetamol or physical methods. No severe adverse events were reported. The number of children with mild adverse events did not differ significantly between paracetamol and placebo, or paracetamol and physical methods, but numbers were small.
Authors' conclusions
There are few trials that have directly compared the antipyretic properties of paracetamol against placebo or physical methods. Data on adverse events are limited. Establishing standard outcomes will help comparisons between studies and better meta‐analysis.
23 April 2019
No update planned
Other
This is currently not a priority for update
Plain language summary
Paracetamol for treating fever in children
Plain language summary pending.
Background
Fever is common within infections. It is caused by the body responding to disease or invasion by pathogens (Kluger 1992). Fever results in people feeling unwell, and occasionally in children, a rapidly rising temperature results in a convulsion (Behrmann 2000). Febrile convulsions are the most common type of convulsions in childhood, and are known to affect about two to five per cent of all children (Verity 1985). About 30 per cent of those who have had an episode of febrile convulsions have additional convulsions (Stuijvenberg 1998).
The physiological mechanism that results in fever is not clear. However, several immunological factors are known to interact in the process that leads to fever, notably the chemical factors called cytokines produced by white blood cells. Experts suggest that cytokines act on the centre in the brain that regulates temperature (thermoregulatory centre) to initiate the physiological responses that result in fever (Kwiatkowski 1995). Fever also increases the rate at which the body uses its energy reserve, especially when it occurs with chills and rigor (Mackowiak 1998).
The drugs most commonly used for treating fever are paracetamol, aspirin, and ibuprofen (Autret 1997). These drugs exert their effects by blocking different points in the chemical pathway that leads to fever. While aspirin and ibuprofen exert their effects on the central pathway (in the brain) as well as the peripheral (in other parts of the body), paracetamol is believed to act only on the central pathway (Mackowiak 1998). The action of these drugs on the temperature control pathway results in the peripheral blood vessels dilating to dissipate heat (Meyers 1980, Mackowiak 1998). People also use physical cooling methods, such as fanning and tepid sponging, which conduct heat from the skin (Agbolosu 1997).
Although the disease process that leads to fever is obviously harmful, some experts suggest that fever may have a beneficial effect of enhancing host resistance to infection (Kramer 1991, Kluger 1992, Roberts 1991). Some of these experts argue that interventions specifically targeted at resolution of fever may interfere with the beneficial role of fever during illness and adversely affect the outcome of the illness. One report suggests that treatment with antipyretic drugs could increase mortality in severe infections, prolong viral shedding, and impair antibody response to viral infection (Shann 1995). Another researcher has observed that giving paracetamol to African children with malaria fever prolonged the parasite clearance time (Brandts 1997). A Cochrane review has however shown that there is insufficient evidence to support the view that paracetamol prolongs parasite clearance in people with malaria (Meremikwu 2001).
In addition to the potentially harmful effects of reducing fever, there are harms associated with specific drugs. The ingestion of a high dose of paracetamol is known to cause liver damage (Meredith 1981, Plotz 1981). For instance, paracetamol toxicity from overdosing is the commonest cause of acute liver failure in the United Kingdom (Newsome 2001). Paracetamol overdose has also been reported to cause disorders of the kidneys, heart, blood cells, and metabolism (Jones 1997). Aspirin is reported to cause metabolic acidosis (which presents with rapid breathing), very low blood sugar (hypoglycaemia), lethargy, coma, and fits; symptoms which are common in severe malaria. Therefore its use in malaria patients may lead to diagnostic confusion, complications, and increase the risk of death (English 1996). Another major limitation to the use of aspirin in children is its association with Reye's syndrome, a rare adverse event that may lead to coma and death in children. The association of aspirin with Reye's syndrome has led to official ban on the use of aspirin for treating children in the United Kingdom and many other countries (Hall 1988, Porter 1990). Other reported adverse effects of antipyretic drugs (including ibuprofen) are gastrointestinal bleeding, heartburn, dyspepsia, nausea, and vomiting (Done 1983, Meyers 1980).
The common adverse effects of physical methods include shivering, crying, and discomfort. Sponging with cold water may cause peripheral cooling but the constriction of the blood vessels can actually cause heat conservation (Mackowiak 1998). The axillary temperature will fall and the rectal temperature will rise.
The uncertainties associated with antipyretic drugs and physical methods have led to a debate about the benefits and harms of methods of reducing fever (Choonara 1992, Done 1983). Most caregivers and many clinicians believe that treatment of fever will relieve symptoms and prevent harmful effects such as febrile convulsions (Stuijvenberg 1998). A recent study has shown that parental fear about presumed harmful effects of fever in children (also called "fever phobia") is still common, and in most cases, due to misconceptions (Crocetti 2001). Given that these drug and physical methods are widely recommended for treating children with a fever, we sought to examine reliable research evidence of the benefits and harms for each method through the following individual reviews.
1. Paracetamol for treating fever in children (this review). 2. Physical methods for treating fever in children (in preparation) 3. Ibuprofen for treating fever in children (in preparation). 4. Aspirin for treating fever in children (in preparation).
Comparisons between interventions in each review are structured in as follows.
1. PARACETAMOL Paracetamol compared to placebo. Paracetamol compared to physical methods.
2. PHYSICAL METHODS Physical methods compared to nothing or drug placebo. Physical methods added to any other drug intervention compared to the drug intervention alone.
3. IBUPROFEN Ibuprofen compared to placebo. Ibuprofen compared to physical methods. Ibuprofen compared to paracetamol.
4. ASPIRIN REVIEW Aspirin compared to placebo. Aspirin compared to physical methods. Aspirin compared to against paracetamol. Aspirin compared to non‐steroidal anti‐inflammatory drugs (NSAIDs).
Paracetamol for treating fever in children
Paracetamol (acetaminophen) is widely used for treating fever in children. This drug is reputed to be both effective and well tolerated (McIntyre 1996). On the other hand, some experts argue that the use of paracetamol may prolong the duration of certain infections (Brandts 1997). Some epidemiological reports suggest that the incidence of acute paracetamol poisoning may have increased in recent times owing probably to widespread use of the drug for treating fever (Meredith 1981). Children may develop paracetamol toxicity due to unintended inappropriate dosing, giving standard dose of the drug to children at increased risk of toxicity, or giving paracetamol concurrently with other drugs that are also capable of damaging the liver (AAP 2001).
There are obvious reasons for concern about the safety of this drug and uncertainties about its actual benefits in the treatment of childhood fever. We have addressed these questions in this systematic review with a view to providing reliable evidence for clinical practice and identifying areas for further research.
Objectives
To assess the effects of paracetamol for treating fever in children in relation to fever clearance time, incidence of febrile convulsions, and resolution of associated symptoms.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials.
Types of participants
Children aged 1 month to 15 years with fever of presumed infectious origin. Fever is defined as temperature of 37.5 ºC or more (axillary); or 38.0 ºC or more (core body temperature).
We excluded trials that studied only children who have had episodes of febrile convulsions in the past. A Cochrane review of prophylactic treatments for decreasing likelihood of future febrile convulsions is being prepared (Offringa 2001).
Types of interventions
Intervention
Paracetamol (acetaminophen).
Control
Placebo or physical methods (sponging, bathing, or fanning).
Types of outcome measures
Primary outcomes
1. Fever clearance time (time between onset of treatment and return of temperature to normal <37.5 ºC). 2. Children who have febrile convulsion after treatment started.
Secondary outcomes
1. Rate of temperature fall between 30 minutes and 6 hours of treatment, (expressed in ºC per hour). 2. Proportion without fever by first, second, and sixth hour of starting treatment. 3. Proportion in whom associated symptoms (discomfort, shivering, chills, anorexia, vomiting, irritability, headache, myalgia) resolved within six hours of starting treatment. 4. Adverse events. 5. Number of caregivers dissatisfied with treatment regimen.
Search methods for identification of studies
We selected the following search terms for searching all trial registers and databases for relevant trials: fever; anti‐pyretic drugs; non‐narcotic analgesic; paracetamol; acetaminophen; panadol; tylenol; tepid sponging; and fanning.
We searched the Cochrane Infectious Diseases Group (CIDG) Specialized Register for relevant trials (published, in press, or in progress) up to May 2004. Full details of the CIDG methods and the journals hand searched are published in The Cochrane Library in the section on Collaborative Review Groups.
We searched The Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 2, 2004). This contains mainly reference information to randomized controlled trials and controlled clinical trials in health care.
We searched the following electronic databases in combination with the search strategy developed by The Cochrane Collaboration and detailed in the Cochrane Reviewer's Handbook (Clarke 2000): MEDLINE (1966 to May 2004); LILACS (1982 to May 2004); EMBASE (1988 to May 2004); Science Citation Index (May 2004).
We checked the citations of all the trials identified by the above methods.
The external referees were asked to check the completeness of the search strategy, and to identify any additional unpublished, ongoing, or planned trials.
We contacted researchers who have done notable studies in infectious diseases and fever to check the completeness of the search strategy and supply information on any unpublished and ongoing trials not yet identified.
Data collection and analysis
Selection of studies
We independently applied the inclusion criteria for this review to the potentially relevant trials identified with the search strategy. Where there was any doubt, we consulted the Cochrane Infectious Diseases Group (CIDG) co‐ordinating editor. We included those trials that met the inclusion criteria.
Data extraction and management
We independently extracted data using a standard form. We wrote to the trial authors for additional data or clarification of analyses and outcomes where required using a standard form.
Assessment of risk of bias in included studies
We independently assessed the quality of included trials using the guidelines of the CIDG. The CIDG Co‐ordinating Editor was consulted where there were doubts. We planned to exclude any included trials subsequently shown to be of very poor methodological quality.
Data synthesis
We entered the data we extracted from the included trials into Review Manager 5 for the meta‐analysis. We prepared a narrative summary on groups of data or information considered inappropriate for meta‐analysis. We performed subgroup analysis of the incidence of febrile convulsion for children aged 6 months to 6 years (preschool children), since the risk of febrile convulsion is known to be particularly high in this age group (Behrmann 2000). We calculated risk ratio with 95% confidence intervals for comparisons of discrete variables, and calculated the mean difference for continuous data. We performed statistical tests to ascertain the homogeneity of the effects of compared interventions. Where we observed heterogeneity of effects, we attempted to explain this, including sensitivity analyses of indicators of study quality. We also considered publication bias.
Results
Description of studies
We identified 91 publications relevant to the review question and we formally applied the eligibility criteria to these. Twelve studies were included while 79 were excluded. The most frequent reasons for exclusion were non‐randomization of participants, failure to assess or give data on relevant outcome measures, failure to compare drug with placebo or other methods stipulated in the Protocol for this review, and inclusion of adult participants (see 'Characteristics of excluded studies').
The 12 trials that met the inclusion critieria had a total of 1509 children aged between 3 months and 15 years (see 'Characteristics of included studies'). Seven trials included only participants aged 6 years or less. Temperature was measured using digital electronic (six trials) and mercury thermometer (two trials); the method used was not mentioned in four trials. The sites at which temperature was measured were rectum (six trials), axilla (two trials), oral (two trials), or not mentioned (two trials).
Seven trials compared paracetamol with placebo (Brewer 1968, Steele 1970, Doran 1989, Walson 1989, Kramer 1991, Wilson 1991, Kauffman 1992a). Six trials compared paracetamol with physical methods (Steele 1970, Friedman 1990, Kinmonth 1992, Agbolosu 1997, Aksoylar 1997, Brandts 1997).
Both single doses (nine trials) and multiple doses (three trials) of paracetamol were evaluated. The dosage of paracetamol varied across the trials, ranging between 8 and 15 mg/kg per dose. One trial (Brandts 1997) administered paracetamol rectally, the others used the oral route.
Tepid sponging was the main physical method (five trials); one trial included warm sponging along with unwrapping the children (Kinmonth 1992). One trial (Brandts 1997) combined tepid sponging with using a wet blanket and fanning. Sponging was intermittent for 20 minutes or longer each time in all the trials except one in which the children were sponged continuously (Brandts 1997). The temperature of water used for warm sponging was described as being just below each participant's body temperature (32 to 41.7 ºC) while the duration of warm sponging varied (range of 1 to 82 minutes; median of 9 minutes). The water temperature ranged between 28 and 34 º C.
Outcome measures
The outcome measures varied widely between the included trials (see 'Characteristics of excluded studies'). The observation period also varied from 1 to 2 hours (3 trials); 3 to 6 hours (3 trials); 7 to 24 hours (3 trials); and 2 to 7 days (3 trials).
Some notable results unsuitable for meta‐analysis have been included in Appendix 1 and Appendix 2.
See the 'Characteristics of included studies' for further information on the included studies.
Risk of bias in included studies
The allocation sequence was adequately concealed only in 7 (Doran 1989, Kauffman 1992a, Kinmonth 1992, Kramer 1991, Steele 1970, Walson 1989, Wilson 1991) of the 12 included trials. 10 trials described satisfactory methods of generation of allocation sequence while 2 used a quasi‐randomization approach. Observers were blinded in six trials (Brewer 1968, Doran 1989, Kauffman 1992a, Kramer 1991, Walson 1989, Wilson 1991) partially blinded in one (Steele 1970), and unblinded in five trials. Dropout or exclusion rates were generally low in the trials (0 to 10.3%). The only trial with a relatively high dropout rate (Kramer 1991) performed an intention‐to‐treat analysis thereby minimizing the attrition bias associated with high losses to follow up. Since none of the other trials provided a detailed trial profile it is difficult to ascertain the actual magnitude of losses to follow up. All the trials stated the frequency of observation. We made no further exclusions on the basis of poor methodological quality.
Effects of interventions
1. Paracetamol compared to placebo
Seven trials compared paracetamol and placebo (Brewer 1968, Steele 1970, Doran 1989, Walson 1989, Kramer 1991, Kauffman 1992a, Wilson 1991).
Primary outcomes
Fever clearance time
Only one trial (Kramer 1991) involving 225 children reported this outcome over 2 to 6 days of observation. In the paracetamol group this was 34.7 hours (n = 123) and in the placebo group it was 36.1 hours (n = 102). The authors report that this was not statistically significantly different, using student t‐test and the Mann‐Whitney U test (standard deviation not given).
Febrile convulsion
Kramer 1991 reported that no febrile convulsion occurred in either the paracetamol or placebo group; participants were aged 6 months to 6 years. The other trials made no specific mention of the occurence of seizures.
Secondary outcomes
One trial (Steele 1970) reported on the proportion of children with fever by the second hour of observation while three trials (Brewer 1968, Steele 1970, Walson 1989) reported on adverse events. None of the trials reported on the rate of temperature fall or proportion in whom associated symptoms were resolved.
Without fever by two hours
One trial (Steele 1970) showed that significantly more children in the paracetamol group (17/25) than placebo (0/15) were without fever by the second hour of starting treatment (risk ratio (RR) 21.54; 95% confidence intervals (CI) 1.39 to 333.99; Analysis 1.1). No data were available for fever resolution at one and six hours.
1.1. Analysis.
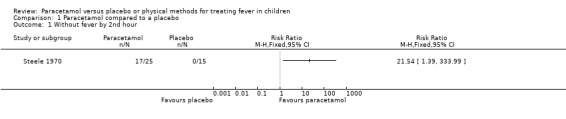
Comparison 1 Paracetamol compared to a placebo, Outcome 1 Without fever by 2nd hour.
Symptom resolution
Although none of the trials provided relevant data on this outcome, two trials showed that the mean time to resolution of symptoms or healing did not differ significantly between children who received paracetamol (multiple doses) and who were given placebo over two to six days of observation (see Appendix 1) (Doran 1989, Kramer 1991).
Adverse events
Meta‐analysis of data from three trials (Brewer 1968, Steele 1970, Walson 1989) involving a total of 254 participants showed that the incidence of adverse events in the paracetamol (9/130) and placebo (4/124) groups did not differ significantly (RR 1.84, 95% CI 0.65 to 5.18; Analysis 1.2). Adverse events were mild in all cases and included drowsiness and mild gastrointestinal symptoms.
1.2. Analysis.
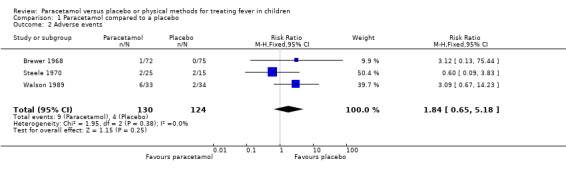
Comparison 1 Paracetamol compared to a placebo, Outcome 2 Adverse events.
2. Paracetamol compared to physical methods
Six trials (Steele 1970, Friedman 1990, Kinmonth 1992, Agbolosu 1997, Aksoylar 1997, Brandts 1997) compared paracetamol with physical methods (sponging with or without fanning or unwrapping). Data were adequate for meta‐analysis in two trials (Agbolosu 1997, Steele 1970).
Primary outcomes
Fever clearance time
Brandts 1997 showed no difference in fever clearance time following treatment with paracetamol or physical methods (sponging, fanning, and wet blanket) in a study of 50 children, but this could not confirmed by re‐analysis because the full data set was not available. This trial used multiple doses of paracetamol and followed the children (with respect to this outcome measure) for up to four days.
Febrile convulsion
One trial (Agbolosu 1997) reported one case of febrile convulsion among the sponging group (1/40) and none among the paracetamol group (0/40), but the difference was not statistically significant (RR 0.33; 95% CI 0.01 to 7.95; see Appendix 2). Five other trials (Steele 1970, Friedman 1990, Kinmonth 1992, Aksoylar 1997, Brandts 1997) made no specific reference to this outcome measure.
Secondary outcomes
Without fever by one and two hours
This outcome was assessed by two trials (Steele 1970, Agbolosu 1997). At one hour, there was no statistically significant difference in the number of children without fever in either the paracetamol or placebo group (fever free: paracetamol group 28/65; sponging group 18/55; RR 1.49; 95% CI 0.98 to 2.25; Analysis 2.1). At two hours, one trial (Agbolosu 1997) showed a statistically significant difference (RR 2.53; 95% CI 1.69 to 3.80), and the other trial (Steele 1970) did not (RR 1.27; 95% CI ‐0.74 to 2.20). The test for heterogeneity was statistically significant, and a combined analysis using a random effects model provides further uncertainty about the consistency of this effect (RR 1.84; 95% CI 0.94 to 3.61, random effects model).
2.1. Analysis.
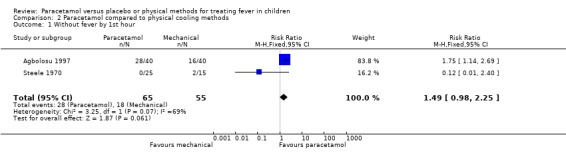
Comparison 2 Paracetamol compared to physical cooling methods, Outcome 1 Without fever by 1st hour.
Adverse events
The occurrence of adverse events as reported in the two trials (Steele 1970, Agbolosu 1997) was not statistically significantly different between the paracetamol (2/65) and sponging (6/55) groups (RR 0.26; 95% CI 0.07 to 1.01; Analysis 2.3). The other trials (Friedman 1990, Kinmonth 1992, Aksoylar 1997, Brandts 1997) did not report adverse events in either of the intervention groups. The adverse events reported were shivering, goose pimples, discomfort, and in one sponged participant, convulsion.
2.3. Analysis.
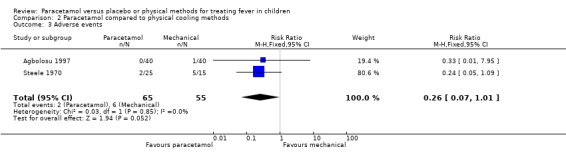
Comparison 2 Paracetamol compared to physical cooling methods, Outcome 3 Adverse events.
Other outcome measures
No data were available for meta‐analysis of other measures of antipyretic efficacy as stipulated in the Protocol for this review. Details of other additional outcomes are presented in Appendix 1 and Appendix 2. No trial assessed the attitude of caregivers to 'no intervention' compared with physical methods of treating fever.
Discussion
We found a small number of trials that tested paracetamol against placebo or physical methods for treating fever. Some of these studies were carefully conducted, while in others a lack of detail in the methods made it difficult to evaluate them.
Most studies were small, none identified primary outcomes in their research design, and the outcomes varied considerably between studies. In addition, data were often incomplete, with few providing standard deviation on mean measures or details of the statistical tests used by the authors.
We have not systematically assessed observational data of paracetamol efficacy, as we aimed to focus on more reliable comparisons between paracetamol and mechanical methods or placebo from randomized controlled trials. The comparative data were surprisingly sparse, and it is not clear whether paracetamol is effective when compared to placebo or physical methods in (1) reducing fever reduction and (2) reducing risk of febrile convulsions.
Authors' conclusions
Implications for practice.
This systematic review shows that inconsistent and weak evidence supports the use of paracetamol to reduce fever in children. This does not mean that paracetamol is ineffective, but simply that the number of reliable studies evaluating it against placebo or physical methods are too few to be sure it has a therapeutic effect.
In the absence of any obvious harms, a campaign to alter practice is not justifiable. Caregivers and doctors have faith in paracetamol, but its continued use needs to be justified in terms of benefit through future research or the outcome of the related Cochrane Reviews (see description in the 'Background') of the effects of other antipyretic drugs in the management of fever.
Implications for research.
We have not demonstrated any convincing direct evidence that paracetamol is effective in reducing fever or preventing febrile convulsions in children. This has the following research implications.
1. Further research in this area warrants larger studies measuring a few simple pragmatic outcomes, such as febrile convulsions; mean time to resolution of fever; and number of participants without fever by one hour.
2. A systematic review of the effects of paracetamol in adults could provide some evidence that clinicians may generalize for all age groups in the interim. Trials including only adults will however not provide any useful information on febrile convulsion since this is a childhood problem.
3. Further reviews of antipyretic drugs need to compare, in the first instance, the drug against placebo. Head to head comparisons against paracetamol presume that paracetamol is the standard treatment.
4. Monitoring of common adverse events and occasional rare events needs to be considered in all evaluations of antipyretic drugs in children.
What's new
| Date | Event | Description |
|---|---|---|
| 18 February 2009 | Amended | Title changed from Paracetamol for treating fever in children to Paracetamol versus placebo or physical methods for treating fever in children, in order to reflect the content of the review. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 1 October 2008 | Amended | Converted to new review format with minor editing (risk ratio used for all dichotomous outcomes). |
| 21 May 2004 | Amended | New studies found but not yet included or excluded. |
Appendices
Appendix 1. Paracetamol compared to placebo
| Trial | Outcome compared | Paracetamol (result) | Placebo (result) | Summary of effects | Source of data |
| Kramer 1991 | Fever clearance time (mean duration of fever) | 34.7 hours | 36.1 hours | No statistically significant difference. | Figure/Text |
| Kramer 1991 | Mean duration of other symptoms | 72.9 hours | 71.7 hours | No statistically significant difference. | Figure/Text |
| Walson 1989 | Mean percentage reduction in temperature at 1 hour, 2 hours, and 4 hours respectively | 34.2%, 57.5%, 59.0% respectively | 6.2%, 15.0%, 21.3% respectively | Statistically significant difference in all cases. | Tables/Text |
| Wilson 1991 | Mean (standard deviation) percentage efficacy at maximal effect | 76.80 (44.92%) | 14.88 (54.44%) | Statistically significantly different. | Table |
| Kauffman 1992a | Adverse events | None observed | None observed | No statistically significant difference. | Text |
| Kauffman 1992a | Mean temperature at 3 to 5 hours post‐treatment | Approximately 37.7 to 38.0 ºC | Approximately 39.1 to 39.3 ºC | Statistically significantly lower in paracetamol group. | Table/Text |
| Doran 1989 | Mean (standard deviation) time to total healing of children with chicken pox | 16.20 (5.80) days | 16.10 (5.60) days | No statistically significant difference. | Table |
| Doran 1989 | Mean (standard deviation) activity score on day 2 | 3.13 (0.23) | 2.82 (0.24) | Statistically significantly better in paracetamol group. | Text |
Appendix 2. Paracetamol compared to physical methods
| Trial | Outcome measure | Paracetamol (Result) | Mechanical (Result) | Summary of effects | Source of data |
| Aksoylar 1997 | Mean drop in temperature at 30 minutes | 0.3 ºC | 0.7 ºC | p<0.001 | Graph/Text |
| Aksoylar 1997 | Mean drop in temperature at 2 hours | 0.6 ºC | 1.3 ºC | p<0.001 | Graph/Text |
| Brandts 1997 | Fever clearance time | 32 hours | 43 hours | No statistically significant difference | Text |
| Friedman 1990 | Mean drop in temperature at 30 minutes | 0.9 ºF | 0.5 ºF | No statistically significant difference | Table |
| Friedman 1990 | Mean drop in temperature at 60 minutes | 1.7 ºF | 1.0 ºF | p=0.03 | Table |
| Kinmonth 1992 | Time below 37.2 ºC (period of antipyresis) | 129 minutes | 54 minutes | Paracetamol had longer period of antipyresis | Table |
| Kinmonth 1992 | Intervention well tolerated by child | 8 (n=26) | 12 (n=26) | Risk ratio 0.67; 95% confidence interval 0.33 to 1.36 | Table/Text (re‐analysis with Metaview) |
| Agbolosu 1997 | Number with febrile seizure | 0 (n=40) | 1 (n=40) | Risk ratio 0.33; 95% confidence interval 0.01 to 7.95; fixed effect model | Table (re‐analysis with Metaview) |
Data and analyses
Comparison 1. Paracetamol compared to a placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Without fever by 2nd hour | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Adverse events | 3 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.65, 5.18] |
Comparison 2. Paracetamol compared to physical cooling methods.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Without fever by 1st hour | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.98, 2.25] |
| 2 Without fever by 2nd hour | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.47, 2.80] |
| 3 Adverse events | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.07, 1.01] |
2.2. Analysis.
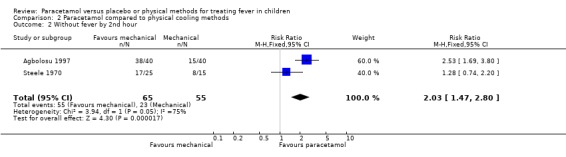
Comparison 2 Paracetamol compared to physical cooling methods, Outcome 2 Without fever by 2nd hour.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agbolosu 1997.
| Methods | Randomized (using block randomization), parallel, open trial Followed up for 2 hours |
|
| Participants | 80 children aged 6 to 60 months, axillary temperature 38.5 to 40 ºC with urinary tract infection and/or malaria Exclusion criteria: received antipyretic 2 hours prior to study entry; requiring admission; urgent investigation; or emergency treatment |
|
| Interventions | Paracetamol: 15mg/kg given as single dose Sponging: from head to toe (except scalp) until temperature is <38 ºC, using tepid water (temperature range 28 to 34 ºC) at ambient temperature 21 to 32 ºC |
|
| Outcomes | Mean temperature over time Mean fall in temperature by group Proportion of children whose temperature fell to <38.5 ºC at different time intervals |
|
| Notes | Study location: Malawi A thin layer of water left on the body of the children receiving tepid sponging until temperature fell below 38.5 ºCs C No losses to follow up or withdrawals recorded |
|
Aksoylar 1997.
| Methods | Randomized (method not specified), open parallel trial. Followed up for 3 hours | |
| Participants | 224 children aged 6 months to 5 years, rectal temperature 39 ºC, with viral and bacterial infections Exclusion criteria: received antipyretic 6 hours prior to study entry; allergy to study medications; renal, gastrointestinal, haematologic, cardiopulmonary, malignant, and central nervous system diseases; dehydration |
|
| Interventions | Paracetamol: 15mg/kg Ibuprofen: 8mg/kg Aspirin: 15mg/kg given as single dose Sponging: for 20 minutes with tepid water (feels neutral in temperature to a nurse's elbow) |
|
| Outcomes | Mean temperature over time Time of maximum fall in temperature Rate of fall of temperature Adverse events |
|
| Notes | Study location: Turkey 23 (10.3%) children withdrawn or lost to follow up |
|
Brandts 1997.
| Methods | Randomized, from random numbers table; no blinding; 3/50 (6%) children withdrawn; no intention‐to‐treat analysis; allocation concealment unclear | |
| Participants | 50 children with uncomplicated Plasmodium falciparum (parasite density: 25,000 to 200,000/uL); aged 2 to 7 years Exclusion criteria: complicated malaria; haemoglobin <8.0 g/dL (PCV<24%); glucose <2.8 mmol/L; lactate >3.5 mmol/L; schizontaemia >50/uL; platelets <50,000/uL; pigment containing neutrophils >2% |
|
| Interventions | 1. Mechanical antipyretic treatment (continuous electric fanning, tepid sponging, and cool blankets) plus paracetamol suppositories (50mg/kg/day at 10 to 15mg/kg 4 to 6 hourly); expelled suppositories replaced immediately
2. (Control): mechanical antipyretic therapy (as above) without paracetamol Similar antimalarials in both groups: intravenous quinine 15mg/kg 12 hourly x 4 days; then oral quinine 15mg/kg 12 hourly x 3 days |
|
| Outcomes | Fever clearance time Parasite clearance time Cure rate Tumour necrosis factor (TNF) PHA‐TNF Interleukin‐6 (IL‐6), (PHA‐IL6), and oxygen radicals |
|
| Notes | Study location: Gabon 3 (6.0%) children withdrawn or lost to follow up |
|
Brewer 1968.
| Methods | Randomized (using random code), parallel, placebo (double blind) controlled trial Followed up for 3 hours |
|
| Participants | 223 children aged <14 years, rectal temperature >38.3 ºC (101 ºF), with viral and bacterial infection Exclusion criteria: vomiting |
|
| Interventions | Paracetamol: 3mg/kg Indomethacin: 1mg/kg; placebo; given as single dose |
|
| Outcomes | Mean changes in temperature over time Proportion of children showing specified temperature reduction Proportion and mean temperature reduction in those with temperature >39.4 ºC (103 ºF) Adverse events |
|
| Notes | Study location: USA No losses to follow up or withdrawals recorded |
|
Doran 1989.
| Methods | Randomized (using table of random numbers), parallel, double blind, placebo controlled study Followed up for 6 days |
|
| Participants | 68 children aged 1 to 12 years with varicella exanthema Exclusion criteria: received any medication within 48 hours prior to study entry; history of seizure or other neurologic disorder; receiving long term medication; immunosuppressed |
|
| Interventions | Paracetamol: 10mg/kg Placebo Given as multiple dose at 4 hourly interval for 4 days |
|
| Outcomes | Time to total scabbing Itching on day 4 Activity score of the children on day 2 Time to last new vesicle Time to total healing |
|
| Notes | Study location: USA 6 (8.8%) children withdrawn or lost to follow up |
|
Friedman 1990.
| Methods | Randomized (using table of random numbers), parallel, open trial Followed up for 1 hour |
|
| Participants | 73 children aged 6 weeks to 4 years, rectal temperature 38.9 ºC (102 ºF), with viral and bacterial infection Exclusion criteria: received antipyretic 4 hours prior to study entry; and/or antibiotic in the past 72 hours; history of febrile convulsions; allergy to paracetamol |
|
| Interventions | Paracetamol: 10 to 15 mg/kg as single dose Sponging: with tepid water of about 37.8 ºC (100 ºF) for 20 minutes Paracetamol and tepid sponging |
|
| Outcomes | Mean temperature over time | |
| Notes | Study location: USA No losses to follow up or withdrawals recorded |
|
Kauffman 1992a.
| Methods | Randomized, double blind placebo controlled Follow up: antipyretic effect for 8 hours; adverse events for 24 hours |
|
| Participants | 37 children aged 2 to 12 years with intercurrent febrile illness, oral temperature at ≥ 38.3 ºC at least one hour before enrollment | |
| Interventions | Paracetamol: 10mg/kg Ibuprofen: 7.5 or 10mg/kg; placebo |
|
| Outcomes | Percentage decrease in temperature from baseline Area under the curve of percentage decrease in temperature against time Treatment failures and adverse events |
|
| Notes | Study location: USA Treatment failures were treated with 10mg/kg of paracetamol regardless of original treatment group |
|
Kinmonth 1992.
| Methods | Randomized (method not stated), parallel, open trial Followed up for 4 hours |
|
| Participants | 52 children aged 3 months to 5 years, axillary temperature 37.8 to 39.9 ºC, with mainly viral infection Exclusion criteria: received antipyretic 4 hours prior to study entry; temperature >40 ºC; serious concomitant disease; history of febrile convulsions; contraindication to paracetamol |
|
| Interventions | Paracetamol: 120mg for 1 year or less and 240mg for more than 1 year as single dose Unwrapping Sponging: with warm water (mean temperature 37.1 ºC) Paracetamol and warm sponging |
|
| Outcomes | Mean change in temperature over time Acceptability of treatment to child and parents Mean time of temperature below 37.2 ºC |
|
| Notes | Study location: UK No losses to follow up or withdrawals recorded |
|
Kramer 1991.
| Methods | Randomized (table of random numbers), parallel, double blind, placebo controlled Follow up: until fever free for at least 24 hours |
|
| Participants | 304 children aged 6 months to 6 years, rectal temperature ≥ 38 ºC with viral infection Exclusion criteria: onset >4 days; bacterial infection; history of convulsion; fever ≥ 41 ºC |
|
| Interventions | Paracetamol: 10 to 15mg/kg given 4 hourly versus placebo | |
| Outcomes | Mean duration of fever Mean duration of other symptoms Improvement in comfort/ behaviour |
|
| Notes | Study location: Canada 79 (26%) children randomized participants dropped |
|
Steele 1970.
| Methods | Quasi‐randomized (using serially numbered envelopes), parallel, placebo controlled, partially blinded (physical method arm not blinded) Followed up for 2 hours |
|
| Participants | 130 children aged 6 months to 5 years, rectal temperature 39.4 ºC or more, lasting more than 3 days, of viral and bacterial origin Exclusion criteria: received antipyretic 4 hours before study entry |
|
| Interventions | Paracetamol (alone or with sponging): 80mg, 160mg, 240mg, and 320mg for ages 6 to 18, 18 to 30, 30 to 48, and 48 to 60 months respectively Mechanical: sponging with tepid water (excluding face and head); 2 other mechanical interventions: sponging with ice water or alcohol plus water were also applied |
|
| Outcomes | Percentage with temperature ≤ 38.3 ºC at 1 and 2 hours; percentage with comfort rated as good, fair, or poor | |
| Notes | Study location: Hawaii No losses to follow up or withdrawals recorded |
|
Walson 1989.
| Methods | Randomized (using block randomization), parallel, double blind, placebo controlled trial Followed p for 8 hours |
|
| Participants | 127 children aged 2 to 11 years, oral temperature 38.3 to 40 ºC, (diagnosis not specified) Exclusion criteria: received antipyretic 8 hours before study entry; had prestudy salicylate or paracetamol of >50mg/L or >5mg/L respectively; hypersensitivity to medications; history of gastrointestinal tract, renal, liver, cardiopulmonary diseases; convulsive disorders; vomiting within 24 hours before study entry; dehydration |
|
| Interventions | Paracetamol:10mg/kg Ibuprofen: 5mg/kg and 10mg/kg; placebo; given as single dose. |
|
| Outcomes | Mean temperature over time Mean percent reduction in temperature Area under percent reduction‐time curve Adverse events |
|
| Notes | Study location: USA 9 (7.1%) children withdrawn or lost to follow up Antibiotics allowed to participants that required them |
|
Wilson 1991.
| Methods | Randomized (method not stated), parallel, modified double‐blind, placebo controlled trial Followed up for 6 to 12 hours |
|
| Participants | 178 children aged 3 months to 12 years, rectal temperature 38.3 to 40.5 ºC, with clinically stable condition Exclusion criteria: received antipyretic 2 hours before study entry; or antibiotics 12 hours before study entry; history of febrile seizures within 6 months; cancer; hypersensitivity to study or related drugs; severe illness |
|
| Interventions | Paracetamol: 12.5mg/kg Ibuprofen: 5 and 10mg/kg Placebo: 0.5ml/kg (single dose) |
|
| Outcomes | Rate of temperature fall Time to maximum antipyresis Mean change in temperature over time Area under the curve of antipyresis Adverse events |
|
| Notes | Study location: USA No losses to follow up or withdrawals recorded |
|
C: Centigrade; F: Fahrenheit; PCV: packed cell volume; PHA‐IL6: phytohaemagglutinin‐interleukin‐6; PHA‐TNF: phytohaemagglutinin‐tumor necrosis factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1994 | Not a clinical trial; review (not systematic). |
| Amdekar 1985 | Not compared with physical methods or placebo or no intervention. |
| Autret 1994 | Not compared with physical methods or placebo or no intervention. |
| Autret 1997 | Not compared with physical methods or placebo or no intervention. |
| Baker 1987 | Not compared with physical methods or placebo or no intervention. |
| Brown 1992 | No relevant outcome compared between paracetamol and placebo. |
| Carley 1999 | Review article; not randomized controlled trial. |
| Catti 1990 | No physical methods or placebo group. |
| Colgan 1957 | Not compared with physical methods or placebo or no intervention. |
| Cullen 1989 | Not compared with physical methods or placebo or no intervention. |
| D'Apuzzo 1992 | Not compared with physical methods or placebo or no intervention. |
| Duhamel 1993 | Not compared with physical methods or placebo or no intervention. |
| Eden 1967 | Not compared with physical methods or placebo or no intervention. |
| Eskerud 1991 | Not randomized controlled trial; a survey. |
| Fasan 1980 | Not compared with physical methods or placebo or no intervention; drug combined with chloroquine. |
| Fruthaler 1964 | Review article (not systematic); not a trial. |
| Fusi 1991 | Paracetamol in both arms of trial. |
| Gianiorio 1993 | Not compared with physical methods or placebo or no intervention. |
| Goyal 1998 | Not compared with physical methods or placebo or no intervention. |
| Houry 1999 | Not compared with physical methods or placebo or no intervention. |
| Ismail 1995 | Not compared with physical methods or placebo or no intervention; adults included. |
| Joshi 1990 | Not compared with physical methods or placebo or no intervention. |
| Keinanen 1977 | Not compared with physical methods or placebo or no intervention; not randomized. |
| Krishna 1995a | Not compared with physical methods or placebo or no intervention; adults included. |
| Krishna 1995b | Not compared with physical methods or placebo or no intervention; adults included. |
| Lal 2000 | Not compared with physical methods or placebo or no intervention. |
| Lesko 1995 | Not compared with physical methods or placebo or no intervention. |
| Lesko 1997 | Not compared with physical methods or placebo or no intervention. |
| Lewis 1988 | Participants not febrile at onset; prophylactic use. |
| Mahar 1994 | Not compared with placebo or no intervention; paracetamol in both arms of trial. |
| Maison 1998 | Not randomized controlled trial. |
| McIntyre 1996 | Not compared with physical methods or placebo or no intervention. |
| Moreno Martinez 1995 | Not compared with physical methods or placebo or no intervention. |
| Nahata 1984 | Not compared with physical methods or placebo or no intervention. |
| Newman 1985 | Participants in the same arm of study received either aspirin or paracetamol; not analysed in subgroups of types of drugs received. |
| Nwanyanwu 1999 | Not compared with physical methods or placebo or no intervention. |
| Pasquale 1993 | Not compared with physical methods or placebo or no intervention. |
| Polidori 1993 | Not compared with physical methods or placebo or no intervention. |
| Purssell 2000 | Review (not systematic); not a trial. |
| Schnaiderman 1993 | No placebo or physical methods arm; paracetamol in both arms. |
| Sharber 1997 | Paracetamol given to participants in both arms of trial. |
| Sheth 1980 | Not compared with physical methods or placebo or no intervention. |
| Sidler 1990 | Not compared with physical methods or placebo or no intervention. |
| Simila 1976 | Not compared with physical methods or placebo or no intervention. |
| Steele 1972 | Not compared with physical methods or placebo or no intervention. |
| Sugimura 1994 | Not randomized controlled trial, not compared with physical methods or placebo. |
| Ugazio 1993 | Not compared with physical methods or placebo or no intervention. |
| Uhari 1995 | Paracetamol combined with another drug or placebo. |
| Ulukol 1999 | Not compared with physical methods or placebo or no intervention. |
| Van Esch 1995 | Not compared with physical methods or placebo or no intervention. |
| Vauzelle‐Kervroedan | Not compared with physical methods or placebo or no intervention. |
| Vernon 1979 | Not compared with physical methods or placebo or no intervention. |
| Walker 1993 | Not compared with physical methods or placebo or no intervention. |
| Walson 1990 | Not compared with physical methods or placebo or no intervention. |
| Walson 1992 | Not compared with physical methods or placebo or no intervention. |
| Weippl 1985 | Not compared with physical methods or placebo or no intervention. |
| Wessie 1987 | Not compared with physical methods or placebo or no intervention. |
| Wilson 2000 | Paracetamol used in both arms of trial. |
| Yaffe 1981 | Review (not systematic); not a trial. |
Contributions of authors
Both reviewers prepared the protocol, selected trials, and extracted data. Martin Meremikwu performed data analysis and wrote the full review. Angela Oyo‐Ita checked it for accuracy and provided advice.
Sources of support
Internal sources
University of Calabar, Calabar, Nigeria.
External sources
Cochrane Child Health Field, Canada.
Effective Health Care Alliance Programme, funded by the Department for International Development (DFID), UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (eg, employment, consultancy, stock ownership, honoraria, expert testimony).
Unchanged
References
References to studies included in this review
Agbolosu 1997 {published data only}
- Agbolosu NB, Cuevas LE, Milligan P, Broadhead RL, Brewster D, Graham SM. Efficacy of tepid sponging versus paracetamol in reducing temperature in febrile children. Annals of Tropical Paediatrics 1997;17:283‐8. [DOI] [PubMed] [Google Scholar]
Aksoylar 1997 {published data only}
- Aksoylar S, Aksit S, Caglayan S, Yaprak I, Bakiler R, Cetin F. Evaluation of sponging and antipyretic medication to reduce body temperature in febrile children. Acta Paediatrica Japonica 1997;39:215‐7. [DOI] [PubMed] [Google Scholar]
Brandts 1997 {published data only}
- Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effects of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet 1997;350:704‐9. [DOI] [PubMed] [Google Scholar]
Brewer 1968 {published data only}
- Brewer EJ. A comparative evaluation of indomethacin, acetaminophen and placebo as antipyretic agents in children. Arthritis and Rheumatism 1968;11(5):645‐51. [DOI] [PubMed] [Google Scholar]
Doran 1989 {published data only}
- Doran TF, Angelis C, Baumgardner RA, David Mellits E. Acetaminophen: more harm than good for chickenpox?. Journal of Pediatrics 1989;114:1045‐8. [DOI] [PubMed] [Google Scholar]
Friedman 1990 {published data only}
- Friedman AD, Barton LL. Efficacy of sponging versus acetaminophen for reduction of fever. Pediatric Emergency Care 1990;6(1):6‐7. [DOI] [PubMed] [Google Scholar]
Kauffman 1992a {published data only}
- Kauffman RE, Sawyer LA, Scheinbaum ML. Antipyretic efficacy of ibuprofen vs acetaminophen. American Journal of Diseases of Children 1992;146:622‐5. [DOI] [PubMed] [Google Scholar]
Kinmonth 1992 {published data only}
- Kinmonth A, Fulton Y, Campbell MJ. Management of feverish children at home. British Medical Journal 1992;305:1134‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 1991 {published data only}
- Kramer MS, Naimark LE, Roberts‐Brauer R, McDougall A, Leduc DG. Risk and benefits of paracetamol antipyresis in young children with fever of presumed viral origin. Lancet 1991;337:591‐4. [DOI] [PubMed] [Google Scholar]
Steele 1970 {published data only}
- Steele RW, Tanaka PT, Lara RP, Bass JW. Evaluation of sponging and of oral antipyretic therapy to reduce fever. Journal of Pediatrics 1970;77(5):824‐9. [DOI] [PubMed] [Google Scholar]
Walson 1989 {published data only}
- Walson PD, Galletta G, Braden NJ, Alexander L. Ibuprofen, acetaminophen, and placebo treatment of febrile children. Clinical Pharmacology and Therapeutics 1989;46(1):9‐17. [DOI] [PubMed] [Google Scholar]
Wilson 1991 {published data only}
- Wilson JT, Brown RD, Kearns GL, Eichler VF, Johnson VA, Bertrand KM, et al. Single‐dose, placebo‐controlled comparative study of ibuprofen and acetaminophen antipyresis in children. Journal of Pediatrics 1991;119(5):803‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1994 {published data only}
- Adam D, Stankov G. Treatment of fever in childhood. European Journal of Pediatrics 1994;153:394‐402. [DOI] [PubMed] [Google Scholar]
Amdekar 1985 {published data only}
- Amdekar YK, Desai RZ. Antipyretic activity of ibuprofen and paracetamol in children with pyrexia. British Journal of Clinical Practice 1985;39(4):140‐3. [PubMed] [Google Scholar]
Autret 1994 {published data only}
- Autret E, Breart G, Jonville AP, Courcier S, Lassale C, Goehrs JM. Comparative efficacy and tolerance of ibuprofen syrup and acetaminophen syrup in children with pyrexia associated with infectious diseases and treated with antibiotics. European Journal of Clinical Pharmacology 1994;46:197‐201. [DOI] [PubMed] [Google Scholar]
Autret 1997 {published data only}
- Autret E, Reboul‐Marty J, Henry‐Launois B, Laborde C, Courcier S, Goehrs JM, et al. Evaluation of ibuprofen versus aspirin and paracetamol on efficacy and comfort in children with fever. European Journal of Clinical Pharmacology 1997;51:367‐71. [DOI] [PubMed] [Google Scholar]
Baker 1987 {published data only}
- Baker M, Fosarelli P, Carpenter R. Childhood fever: correlation of diagnosis with temperature response to acetaminophen. Pediatrics 1987;80(3):315‐8. [PubMed] [Google Scholar]
Brown 1992 {published data only}
- Brown DR, Wilson JT, Kearns GL, Eichler VF, Johnson VA, Bertrand KM. Single‐dose pharmacokinetics of ibuprofen and acetaminophen in febrile children. Journal of Clinical Pharmacology 1992;32:231‐41. [DOI] [PubMed] [Google Scholar]
Carley 1999 {published data only}
- Carley S, Thomas M. Paracetamol or ibuprofen in febrile children. Journal of Accident & Emergency Medicine 1999;16:137‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Catti 1990 {published data only}
- Catti A, Monti T. Treatment of infants with acute upper respiratory tract inflammation. A double‐blind comparison between nimesulide and paracetamol suppositories. Clinical Trials Journal 1990;27:327‐35. [Google Scholar]
Colgan 1957 {published data only}
- Colgan MT, Mintz AA. The comparative antipyretic effect of N‐acetyl‐P‐aminophenol and acetylsalicylic acid. Journal of Pediatrics 1957;50(5):552‐5. [DOI] [PubMed] [Google Scholar]
Cullen 1989 {published data only}
- Cullen S, Kenny D, Ward OC, Sabra K. Paracetamol suppositories: a comparative study. Archives of Disease in Childhood 1989;64:1504‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Apuzzo 1992 {published data only}
- D'Apuzzo V, Monti T. Pilot study of the antipyretic and analgesic activity of nimesulide pediatric suppositories. Drugs Under Experimental and Clinical Research 1992;18(2):63‐8. [PubMed] [Google Scholar]
Duhamel 1993 {published data only}
- Duhamel JF, Guillot M, Brouard J, Debosque S, Consten L, Dresco I, et al. Antipyretic efficacy of tiaprofenic acid versus acetaminophen in upper respiratory tract infections [Effet antipyretique de l'acide tiaprofenique chez l'enfant etude comparative au paracetamol]. Pediatrie 1993;48(9):655‐9. [PubMed] [Google Scholar]
Eden 1967 {published data only}
- Eden AN, Kaufman A. Clinical comparison of three antipyretic agents. American Journal of Diseases of Children 1967;114:284‐7. [DOI] [PubMed] [Google Scholar]
Eskerud 1991 {published data only}
- Eskerud JR, Hoftvedt BO, Laerum E. Fever: management and self‐medication. Results from a Norwegian population study. Family Practice 1991;8(2):148‐53. [DOI] [PubMed] [Google Scholar]
Fasan 1980 {published data only}
- Fasan P, Mabadeje A. A controlled trial of a combination of chloroquine with paracetamol in the treatment of acute malaria in a semi‐immune population. Journal of Tropical Medicine and Hygiene 1980;83:191‐3. [PubMed] [Google Scholar]
Fruthaler 1964 {published data only}
- Fruthaler GJ, Tilden T. Management of hyperpyrexia in children. Postgraduate Medicine 1964;35(6):643‐6. [DOI] [PubMed] [Google Scholar]
Fusi 1991 {published data only}
- Fusi G, Mezzopane A, Gramolini C, Careddu P. A double blind clinical trial of the effectiveness and safety of an association of paracetamol‐sobrerol vs paracetamol in pediatrics [Studio clinico in doppio cieco sulla efficacia e sulla tollerailita di una associazione di paracetamolo‐sobrelolo versus paracetamolo in et a pediatrica]. Giornale Italiano di Ricerche Cliniche e Terapeutiche 1991;12(1):17‐23. [Google Scholar]
Gianiorio 1993 {published data only}
- Gianiorio P, Zappa P, Sacco O, Fregonese B. Antipyretic and anti‐inflammatory efficay of nimesulide versus paracetamol in the symptomatic treatment of acute respiratory infections in children. Drugs 1993;46 Suppl 1:204‐7. [DOI] [PubMed] [Google Scholar]
Goyal 1998 {published data only}
- Goyal KP, Chandra J, Unnikrishnan G, Kumari S, Passah SM. Double blind randomized comparative evaluation of nimesulide and paracetamol as antipyretics. Indian Pediatrics 1998;35:519‐22. [PubMed] [Google Scholar]
Houry 1999 {published data only}
- Houry D, Ernst A, Weiss S, Ledbetter M. Ketorolac versus acetaminophen for treament of acute fever in the emergency department. Southern Medical Journal 1999;92(12):1171‐3. [DOI] [PubMed] [Google Scholar]
Ismail 1995 {published data only}
- Ismail S, Na Bangchang K, Karbwang J. Paracetamol deposition in Thai patients during and after treatment of falciparum malaria. European Journal of Clinical Pharmacology 1995;48:65‐9. [DOI] [PubMed] [Google Scholar]
Joshi 1990 {published data only}
- Joshi YM, Sovani VB, Joshi VV, Navrange JR, Benakappa DG, Shivananda P, et al. Comparative evaluation of the antipyretic efficacy of ibuprofen and paracetamol. Indian Pediatrics 1990;27:803‐6. [PubMed] [Google Scholar]
Keinanen 1977 {published data only}
- Keinanen S, Hietula M, Simila S, Kouvalainen K. Antipyretic therapy: comparison of rectal and oral paracetamol. European Journal of Clinical Pharmacology 1977;12:77‐80. [DOI] [PubMed] [Google Scholar]
Krishna 1995a {published data only}
- Krishna S, Pukrittayakamee S, Supanaranond W, ter Kuile F, Ruprah M, Sura T, et al. Fever in uncomplicated plasmodium falciparum malaria: randomized double‐ 'blind' comparison of ibuprofen and paracetamol treatment. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89:507‐9. [DOI] [PubMed] [Google Scholar]
Krishna 1995b {published data only}
- Krishna S, Supanaranond W, Pukrittayakamee S, ter Kulie F, Supputamangkol Y, Attatamsoonthorn K, et al. Fever in uncomplicated Plasmodium falciparum infection: effects of quinine and paracetamol. Transactions of the Royal Society of Tropical Medicine and Hygiene 1995;89:197‐9. [DOI] [PubMed] [Google Scholar]
Lal 2000 {published data only}
- Lal A, Gomber S, Talukdar B. Antipyretic effects of nimesulide, paracetamol and ibuprofen‐paracetamol. Indian Journal of Pediatrics 2000;67:865‐70. [DOI] [PubMed] [Google Scholar]
Lesko 1995 {published data only}
- Lesko SM, Mitchell AA. Assessment of the safety of pediatric ibuprofen: a practitioner‐based randomized clinical trial. Journal of the American Medical Association 1995;273(12):929‐33. [PubMed] [Google Scholar]
Lesko 1997 {published data only}
- Lesko SM, Mitchell AA. Renal function after short‐term ibuprofen use in infants and children. Pediatrics 1997;100(6):954‐7. [DOI] [PubMed] [Google Scholar]
Lewis 1988 {published data only}
- Lewis K, Cherry J, Sachs M, Woo D, Hamilton R, Tarle J, et al. The effect of prophylactic acetaminophen administration on reactions to DTP vaccination. American Journal of Diseases of Children 1988;142:62‐5. [DOI] [PubMed] [Google Scholar]
Mahar 1994 {published data only}
- Mahar AF, Allen SJ, Milligan P, Suthumnirund S, Chotpitayasunondh T, Sabchareon A, et al. Tepid sponging to reduce temperature in febrile children in a tropical climate. Clincal Pediatrics 1994;33(4):227‐31. [DOI] [PubMed] [Google Scholar]
Maison 1998 {published data only}
- Maison P, Guillemot D, Vauzelle‐Kervoedan F, Balkau B, Sermet C, Thibult N, et al. Trends in aspirin, paracetamol and non‐steroidal anti‐inflammatory drug use in children between 1981 and 1992 in France. European Journal of Clinical Pharmacology 1998;54:659‐64. [DOI] [PubMed] [Google Scholar]
McIntyre 1996 {published data only}
- McIntyre J, Hull D. Comparing efficacy and tolerability of ibuprofen and paracetamol in fever. Archives of Disease in Childhood 1996;74:164‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreno Martinez 1995 {published data only}
- Moreno Martinez JA, Sequeiros Loranca E, Medina Santillan R. A blind study to evaluate the efficacy and tolerance of sodium naproxen vs paracetamol in upper airway diseases [Estudio simple ciego para evaluar eficacia y tolerancia de naproxen sodico vs paracetamol en el trantamiento del sindrome febril en enfermedades de vias respiratorias atlas]. Investigacion Medica Internacional 1995;22(2):58‐61. [Google Scholar]
Nahata 1984 {published data only}
- Nahata MC, Powell DA, Durrell DE, Miller MA. Acetaminophen accumulation in pediatric patients after repeated therapeutic doses. European Journal of Clinical Pharmacology 1984;27:57‐9. [PubMed] [Google Scholar]
Newman 1985 {published data only}
- Newman J. Evaluation of sponging to reduce body temperature in febrile children. Canadian Medical Association Journal 1985;132:641‐2. [PMC free article] [PubMed] [Google Scholar]
Nwanyanwu 1999 {published data only}
- Nwanyanwu OC, Ziba C, Kazembe PN. Paracetamol and ibuprofen for treatment of fever in Malawian children aged less than five years. Transactions of the Royal Society of Tropical Medicine and Hygiene 1999;93:84. [DOI] [PubMed] [Google Scholar]
Pasquale 1993 {published data only}
- Pasquale G, Scaricabarozzi I, D'Agostino R, Taborelli G, Vallarino R. An assessment of the efficacy and tolerability of nimesulide versus paracetamol in children after adenotonsillectomy. Drugs 1993;46 Suppl 1:234‐7. [DOI] [PubMed] [Google Scholar]
Polidori 1993 {published data only}
- Polidori G, Titti G, Pieragostini P, Comito A, Scaricabarozzi I. A comparison of nimesulide and paracetamol in the treatment of fever due to inflammatory diseases of the upper respiratory tract in children. Drugs 1993;46 Suppl 1:231‐3. [DOI] [PubMed] [Google Scholar]
Purssell 2000 {published data only}
- Purssell E. Physical treatment of fever. Archives of Disease in Childhood 2000;82:238‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnaiderman 1993 {published data only}
- Schnaiderman D, Lahat E, Sheefer T, Aladjem. Antipyretic effectiveness of acetaminophen in febrile seizures: Ongoing prophylaxis versus sporadic usage. European Journal of Paediatrics 1993;152:747‐9. [DOI] [PubMed] [Google Scholar]
Sharber 1997 {published data only}
- Sharber 1997. The efficacy of tepid sponge bathing to reduce fever in young children. American Journal of Emergency Medicine 1997;15(2):188‐92. [DOI] [PubMed] [Google Scholar]
Sheth 1980 {published data only}
- Sheth UK, Gupta K, Paul T, Pispati PK. Measurement of antipyretic activity of ibuprofen and paracetamol in children. Journal of Clinical Pharmacology 1980;20(11‐12):672‐5. [DOI] [PubMed] [Google Scholar]
Sidler 1990 {published data only}
- Sidler J, Frey B, Baerlocher K. A double‐blind comparison of ibuprofen and paractamol in juvenile pyrexia. British Journal of Clinical Pharmacol 1990;70 Suppl:22‐5. [PubMed] [Google Scholar]
Simila 1976 {published data only}
- Simila S, Kouvalainen K, Keinanen S. Oral antipyretic therapy: Evaluation of Ibuprofen. Scandinavian Journal of Rheumatology 1976;5:81‐3. [DOI] [PubMed] [Google Scholar]
Steele 1972 {published data only}
- Steele RW, Young FSH, Bass JW, Shirkey HC. Oral antipyretic therapy. American Journal of Diseases of Children 1972;123:204‐6. [DOI] [PubMed] [Google Scholar]
Sugimura 1994 {published data only}
- Sugimura T, Fujimoto T, Motoyama H, Maruoka T, Korematu S, Asakuno Y, et al. Risk of antipyretics in young children with fever due to infectious disease. Acta Paediatrica Japonica 1994;36:375‐8. [DOI] [PubMed] [Google Scholar]
Ugazio 1993 {published data only}
- Ugazio AG, Guarnaccia S, Berardi M, Renzetti I. Clinical and pharmacokinetic study of nimesulide in children. Drugs 1993;46 Suppl 1:215‐8. [DOI] [PubMed] [Google Scholar]
Uhari 1995 {published data only}
- Uhari M, Rantala H, Vainiopaa L, Kurttila R. Effect of acetaminophen and low dose intermittent doses of diazepam on the prevention of recurrences of febrile seizures. Journal of Pediatrics 1995;126:991‐5. [DOI] [PubMed] [Google Scholar]
Ulukol 1999 {published data only}
- Ulukol B, Koksal Y, Cin S. Assessement of the efficacy and safety of paracetamol, ibuprofen and nimesulide in children with upper respiratory tract infections. European Journal of Clinical Pharmacology 1999;55:615‐8. [DOI] [PubMed] [Google Scholar]
Van Esch 1995 {published data only}
- Esch A, Steensel‐Moll HA, Steyerberg EW, Offringa M, Habbema JDF, Derksen‐Lubsen G. Antipyretic efficacy of ibuprofen and acetaminophen in children with febrile seizures. Archives of Pediatrics & Adolescent Medicine 1995;149:632‐7. [DOI] [PubMed] [Google Scholar]
Vauzelle‐Kervroedan {published data only}
- Vauzelle‐Kervroedan F, d'Athis P, Pariente‐Khayat A, Debregeas S, Olive G, Pons G. Equivalent antipyretic activity of ibuprofen and paracetamol in febrile children. Journal of Pediatrics 1997;131(5):683‐7. [DOI] [PubMed] [Google Scholar]
Vernon 1979 {published data only}
- Vernon S, Bacon C, Weightman D. Rectal paracetamol in small children with fever. Archives of Disease in Childhood 1979;54:469‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1993 {published data only}
- Walker O, Salako L, Sowunmi A, Olupitan S, Oyewo E. Parental piroxicam in the management of fever arthralgia and musculoskeletal conditions of acute malaria: an open randomised comparison with oral acetylsalicylic acid and paracetamol. Nigerian Medical Practitioner 1993;25(4):39‐42. [Google Scholar]
Walson 1990 {published data only}
- Walson PD. Ibuprofen versus paracetamol for the treatment of fever. British Journal of Clinical Practice 1990;70 Suppl:19‐21. [PubMed] [Google Scholar]
Walson 1992 {published data only}
- Walson PD, Galletta G, Chomilo F, Braden NJ, Sawyer LA, Scheinbaum ML. Comparison of multidose ibuprofen and acetaminophen therapy in febrile children. American Journal of Diseases of Children 1992;146:626‐32. [DOI] [PubMed] [Google Scholar]
Weippl 1985 {published data only}
- Weippl G, Michos N, Sundal EJ, Stocker H. Clinical experience and results of treatment with suprofen in pediatrics. Arzneimittel‐Forschung 1985;35(11):1724‐7. [PubMed] [Google Scholar]
Wessie 1987 {published data only}
- Wessie E, Miller G, Brien J. Fever response to acetaminophen in viral vs bacterial infections. Pediatric Infectious Disease Journal 1987;6:1091‐4. [PubMed] [Google Scholar]
Wilson 2000 {published data only}
- Wilson JT, Helms R, Pickering BD, Donahue L, Don Brown R. Acetaminophen controlled‐release sprinkles versus acetaminophen immediate‐release elixir in febrile children. Journal of Clinical Pharmacology 2000;40:360‐9. [DOI] [PubMed] [Google Scholar]
Yaffe 1981 {published data only}
- Yaffe SJ. Comparative efficacy of aspirin and acetaminophe in reduction of fever in children. Archives of Internal Medicine 1981;141:286‐92. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 2001
- American Academy of Pediatrics. Acetaminophen toxicity in children. Pediatrics 2001;108:1020‐4. [DOI] [PubMed] [Google Scholar]
Behrmann 2000
- Haslam RHA. Seizures in childhood. In: Behrmann M D, Kliegman R M, Jenson H B editor(s). Nelson textbook of paediatrics. 16th Edition. Philadelphia: W.B. Saunders Co., 2000:1813‐29. [Google Scholar]
Choonara 1992
- Choonara I, Nunn AJ, Barker C. Drugs for childhood fever (Letter). Lancet 1992;339:69. [DOI] [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD, editors. Optimal search strategy. Cochrane Reviewers' Handbook 4.1 [updated June 2000]; Appendix 5c. In: The Cochrane Library [database on disk and CDROM]. The Cochrane Collaboration. Oxford: Update Software; 2001, Issue 2.
Crocetti 2001
- Crocetti M, Moghbeli N, Serwint J. Fever phobia revisited: have parental misconceptions about fever changed in 20 years?. Pediatrics 2001;107(6):1241‐6. [DOI] [PubMed] [Google Scholar]
Done 1983
- Done AK. Treatment of fever in 1982: a review. American Journal of Medicine 1983;74(6A):27‐35. [DOI] [PubMed] [Google Scholar]
English 1996
- English M, Marsh V, Amukoya E, Lowe B, Murphy S, Marsh K. Chronic salicylate poisoning and severe malaria. Lancet 1996;347(9017):1736‐7. [DOI] [PubMed] [Google Scholar]
Hall 1988
- Hall SM, Plaster PA, Glasgow JFT, Hancock P. Preadmission antipyretics in Reye's syndrome. Archives of Disease in Childhood 1988;63:857‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1997
- Jones AL, Prescott LF. Unusual complications of paracetamol poisoning. Quarterly Journal of Medicine 1997;90:161‐8. [DOI] [PubMed] [Google Scholar]
Kluger 1992
- Kluger MJ. Drugs for childhood fever (Letter). Lancet 1992;339:70. [PubMed] [Google Scholar]
Kwiatkowski 1995
- Kwiatkowski D. The biology of malarial fever. Baillieres Clinical Infectious Diseases 1995;2(2):371‐88. [Google Scholar]
Mackowiak 1998
- Mackowiak PA, Plaisance KI. Benefits and risk of antipyretic therapy. Annals New York Academy of Sciences 1998;856:214‐23. [DOI] [PubMed] [Google Scholar]
Meredith 1981
- Meredith TJ, Vale JA, Goulding R. The epidemiology of acute acetaminophen poisoning in England and Wales. Archives of Internal Medicine 1981;141:397‐400. [DOI] [PubMed] [Google Scholar]
Meremikwu 2001
- Meremikwu M, Logan K, Garner P. Antipyretic measures for treating fever in malaria (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Meyers 1980
- Meyers FH, Jawetz E, Goldfien A. Review of medical pharmacology. 7th Edition. California: Lange Medical Publications, 1980. [Google Scholar]
Newsome 2001
- Newsome PN, Bathgate AJ, Henderson NC, MacGilchrist AJ, Plevris JN, Masterton G, et al. Referral pattern and social deprivation in paracetamol‐induced liver injury in Scotland. Lancet 2001;358:1612‐3. [DOI] [PubMed] [Google Scholar]
Offringa 2001
- Offringa M, Newton R. Prophylactic drug management for febrile convulsions in children (Cochrane Protocol). Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Plotz 1981
- Plotz PH, Kimberly RP. Acute effects of aspirin and acetaminophen on renal function. Archives of Internal Medicine 1981;141:343‐8. [DOI] [PubMed] [Google Scholar]
Porter 1990
- Porter JDH, Robinson PH, Glasgow JFT, Banks JH, Hall SM. Trends in the incidence of Reye's syndrome and the use of aspirin. Archives of Disease in Childhood 1990;65:826‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Roberts 1991
- Roberts NJ Jr. Impact of temperature elevation on immunologic defenses. Reviews of Infectious Diseases 1991;13:462‐72. [DOI] [PubMed] [Google Scholar]
Shann 1995
- Shann F. Antipyretics in severe sepsis (Comment). Lancet 1995;345(8946):338. [DOI] [PubMed] [Google Scholar]
Stuijvenberg 1998
- Stuijvenberg M, Derksen‐Lubsen G, Steyerberg EW, Habbema JDF, Moll HA. Randomized controlled trial of ibuprofen syrup administered during febrile illness to prevent febrile seizure recurrences. Pediatrics 1998;102(E51):1‐7. [DOI] [PubMed] [Google Scholar]
Verity 1985
- Verity CM, Butler NR, Golding J. Febrile convulsions in a national cohort followed up from birth. I. Prevalence and recurrence in the first year of life. British Medical Journal 1985;290:1307‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]


