Abstract
Background
Women are more vulnerable to malaria during pregnancy, and malaria infection may have adverse consequences for the fetus. Identifying safe and effective treatments is important.
Objectives
To compare the effects of drug regimens for treating uncomplicated falciparum malaria in pregnant women.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (February 2008), CENTRAL (The Cochrane Library 2008, Issue 1), MEDLINE (1966 to February 2008), EMBASE (1974 to February 2008), LILACS (February 2008), mRCT (February 2008), reference lists, and conference abstracts. We also contacted researchers in the field, organizations, and pharmaceutical companies.
Selection criteria
Randomized and quasi‐randomized controlled trials of antimalarial drugs for treating uncomplicated malaria in pregnant women.
Data collection and analysis
Two authors assessed trial eligibility and risk of bias, and extracted data. We performed a quantitative analysis only where we could combine the data. We combined dichotomous data using the risk ratio (RR) and presented each result with a 95% confidence interval (CI).
Main results
Ten trials (1805 participants) met the inclusion criteria. Two were quasi‐randomized, seven did not describe allocation concealment, and all adjusted treatment failure to exclude new infections. One trial reported fewer treatment failures at day 63 with artesunate plus mefloquine compared with quinine (RR 0.09, 95% CI 0.02 to 0.38; 106 participants). One trial reported fewer treatment failures at day 63 with artesunate plus atovaquone‐proguanil compared with quinine (RR 0.14, 95% CI 0.03 to 0.57; 80 participants). One trial reported fewer treatment failures at day 28 when amodiaquine was compared with chloroquine (RR 0.20, 95% CI 0.08 to 0.46; 420 participants) and when amodiaquine plus sulfadoxine‐pyrimethamine was compared with chloroquine (RR 0.02, 95% CI 0.00 to 0.26; 418 participants). Compared with sulfadoxine‐pyrimethamine given alone, one trial reported fewer treatment failures at delivery (or day 40) with artesunate plus sulfadoxine‐pyrimethamine (RR 0.15, 95% CI 0.04 to 0.59; 79 participants) and azithromycin plus sulfadoxine‐pyrimethamine (RR 0.27, 95% CI 0.10 to 0.76; 82 participants).
Authors' conclusions
Data are scant. Some combination treatments appear to be effective at treating malaria in pregnancy; however, safety data are limited.
23 April 2019
No update planned
Other
Not a current question. The issue with ACTs in pregnancy is safety, and there is a comprehensive systematic review on this topic published by Dellicour 2017 https://doi.org/10.1371/journal.pmed.1002290 in PLoS Medicine
Plain language summary
Reliable research about the benefits and harms of treatments for malaria in pregnant women is scarce
Women are more vulnerable to malaria during pregnancy, and malaria may have harmful effects on the baby. Treatment options are becoming more limited because the malaria parasite is developing resistance to existing drugs and due to concerns about whether drugs may harm the baby. Evidence from randomized controlled trials is limited, with few drugs and drug combinations being evaluated.
Background
Susceptibility of pregnant women to malaria
Malaria is a parasitic disease spread by mosquitoes and endemic in parts of Africa, Asia, and South America. Pregnant women attract twice the number of mosquitoes as their non‐pregnant counterparts, which probably increases their exposure to infection (Lindsay 2000). Malaria contributes to illness and premature death in endemic areas, and pregnant women are particularly susceptible to the disease. It can cause severe maternal anaemia and malaria infection of the fetus (Hoffman 1992; Menendez 2000; Steketee 2001), and can contribute to an increased risk of maternal mortality (Stevens 2000; Brabin 2001; WHO 2004a) and low infant birthweight (WHO 2004a). As with any febrile illness, clinical disease also increases the risk of preterm birth, and preventing malaria infection in pregnant women with drugs appears to reduce perinatal mortality (Garner 2006). The burden of malaria infection during pregnancy is caused mainly by the Plasmodium falciparum parasite.
The specific manifestation of malaria during pregnancy depends, in part, on the mother's level of acquired clinical immunity. Women living in areas where malaria transmission is low and unstable, such as Asia, have little or no immunity. These women are at risk of developing severe clinical disease as well as severe anaemia, which can result in maternal and fetal mortality (WHO 2004a). In areas of low or unstable malaria transmission, the World Health Organization (WHO) estimates that the risk of severe malaria illness is at least doubled in pregnant women (WHO 2004a). Where there is a low incidence with less than one malaria infection per pregnancy some primigravidae will not become infected. This means that the higher risk of severe illness is likely to remain in subsequent pregnancies.
In areas where malaria transmission is high, such as parts of Africa, by the time women reach reproductive age they will normally have acquired partial immunity. This reduces the risk of illness and attenuates the clinical illness. The immunosuppression with pregnancy does, however, make women more susceptible and increase the risk of anaemia. Why this happens is debated: some suggest that the immune system may be down regulated in an attempt to protect the fetus (Duffy 2001). In these areas, pregnant women are most at risk during their first and second pregnancies, with less suppression in later pregnancies (WHO 2004a). Most women will not be aware that they are infected as they will experience no obvious clinical symptoms, but there is an increased risk of maternal anaemia and low birthweight (Brabin 2001).
Control of malaria in pregnant women
Regional leaders at the African Summit on Roll Back Malaria (April 2000) committed themselves to an intensive effort to halve the malaria mortality in Africa by 2010 (WHO 2003a). Progress towards this target has so far been limited by resource shortages in the most affected countries (WHO 2004b; RBM 2005a). In 2005, the WHO consolidated the strategy with the aim of covering 80% of pregnant women at risk of malaria with available control tools by 2010, and looking for equity and sustainability in the long‐term progress being made towards 2015 (RBM 2005b). To achieve this target the WHO promotes a strategic framework for malaria control during pregnancy. In Africa, where malaria transmission is high, the framework recommends a three‐pronged approach – intermittent preventive treatment, insecticide‐treated nets, and case management of malaria illness – to reduce the burden of malaria infection among pregnant women (WHO 2003b; WHO 2004a).
Cochrane Reviews have demonstrated the effects and safety of malaria prevention strategies for pregnant women, including intermittent preventive treatment (Garner 2006) and insecticide‐treated nets (Gamble 2006). In areas of low or unstable transmission (such as Asia), the focus is on the use of insecticide‐treated nets and prompt case management when women become ill. Preventive interventions are for all pregnant women, but there is little attention in policy documents to the treatment of women with moderate to severe anaemia or who have symptoms of malaria.
Treatment of malaria in pregnant women
Pregnant women with uncomplicated malaria infection should receive prompt treatment with effective antimalarial drugs to clear infection fast (Nosten 2001). Unlike severe malaria, where the aim is to save the pregnant woman's life, in the treatment of uncomplicated malaria a judgement must be made between the risks and benefits of a potentially toxic treatment and the consequences of infection. The options for treatment of pregnant women with malaria are few, and many endemic countries still lack an official policy (WHO 2007). Some data are available on the safety of drugs when used in prevention regimens, but pregnant women have been systematically excluded from most malaria treatment trials for fear of toxicity to the fetus (Guerin 2002). Thus the options for treatment are limited by potential teratogenicity (the ability to cause defects in the developing fetus) coupled with a paucity of safety data for the mother and fetus (Nosten 2001).
Some antimalarial drugs are not recommended in pregnancy because of recognized adverse effects. The tetracyclines and doxycycline are excluded because of adverse effects on bone growth (Nosten 2001; WHO 2004a). No data exist on the use of halofantrine in pregnant women, but the cardiotoxicity of the drug has compromised its role in the treatment of uncomplicated falciparum malaria (Nosten 1993a; Taylor 2004; WHO 2004a). Primaquine is not prescribed in pregnancy due to the risk of intravascular haemolysis in the mother and fetus (Clyde 1981; WHO 2004a). A study from Thailand examined stillbirth and exposure to mefloquine suggested that mefloquine use in pregnancy was associated with a small increased risk of stillbirth (Nosten 1999); conversely, a large randomized controlled trial showed no difference in the stillbirth rate between women who were treated with mefloquine or chloroquine before starting prophylaxis (Steketee 1996).
Treatment options are further reduced by the spread of drug‐resistant P. falciparum. In recognition of the widespread resistance and decreased efficacy of chloroquine and sulfadoxine‐pyrimethamine (particularly in Asia) (White 1999), the WHO now recommends the use of quinine plus clindamycin in all trimesters of pregnancy (WHO 2006). However, quinine sometimes causes hypoglycaemia (low blood sugar), even in uncomplicated infections (Nosten 2001; Taylor 2004). Artemisinin‐based combination therapies (ACTs) have been shown to be safe and effective, and increasing experience with the use of these treatments in over 1000 pregnancies has not reported any serious adverse effects (Adjuik 2004). The WHO now suggests that the ACTs being used in the region (or artesunate plus clindamycin) should be deployed, as an alternative to quinine plus clindamycin in the second and third trimester (RBM 2005b; WHO 2006). Current guidelines do not recommend artemisinin compounds for malaria in women in the first trimester of pregnancy unless treatment is believed to be life saving for the mother and other antimalarial drugs are considered unsuitable (RBM 2003). Furthermore, to slow the development of resistance, these drugs should only be used in combination. The selection of combination partner can be difficult, and the WHO gives no guidance on this. Further evidence of the safety of artemisinin compounds, and their partner treatments, in pregnancy will help inform decisions about their use as conventional therapies fail.
We have summarized the available evidence on the effects of various malaria treatments in pregnancy.
Objectives
To compare the effects of drug regimens for treating uncomplicated falciparum malaria in pregnant women.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
Pregnant women with uncomplicated falciparum malaria confirmed by a blood slide, irrespective of whether they are symptomatic or anaemic.
Trials may recruit both pregnant and non‐pregnant women so long as pregnant women form a subgroup in the analysis.
Types of interventions
Comparisons between drug regimens for treating uncomplicated falciparum malaria.
For asymptomatic infections, comparisons of treatments with placebo or no treatment are eligible.
Types of outcome measures
Maternal treatment response
Treatment failure, defined as parasitological or clinical evidence of treatment failure between the start of treatment and day 28 (for drugs with a half life of less than seven days) or day 42 (for drugs with a half life greater than seven days). This is equivalent to total failure defined in WHO 2003c. Data may be presented as treatment failure, including new infections, and as treatment failure adjusted to exclude new infections detected by a polymerase chain reaction (PCR) analysis.*
Fever clearance time.
Parasite clearance time.
Anaemia.
Maternal adverse events
Serious adverse events (fatal, life threatening, or require hospitalization).
Adverse events that result in the discontinuation of treatment.
Other adverse events.
Fetal outcomes
Low birthweight.*
Abortion; stillbirth; perinatal death.
Preterm delivery or gestational age.
Neonatal malaria.
Congenital anaemia or neonatal haemoglobin.
Congenital abnormality.
*Primary outcome measure.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Table 1: Cochrane Infectious Diseases Group Specialized Register (February 2008); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2008, Issue 1); MEDLINE (1966 to February 2008); EMBASE (1974 to February 2008); and LILACS (1982 to February 2008). We also searched the metaRegister of Controlled Trials (mRCT) using 'malaria' and 'pregnan' as search terms (February 2008).
1. Detailed search strategies.
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | malaria | malaria | malaria | malaria | malaria |
| 2 | pregnan* | pregnan* | Exp MALARIA | MALARIA | pregnan* |
| 3 | — | 1 and 2 | pregnan* | pregnan$ | 1 and 2 |
| 4 | — | — | PREGNANCY | PREGNANCY | — |
| 5 | — | — | 1 or 2 | 1 or 2 | — |
| 6 | — | — | 3 or 4 | 3 or 4 | — |
| 7 | — | — | 5 and 6 | 5 and 6 | — |
| 8 | — | — | Limit 7 to human | Limit 7 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Conference proceedings
We searched the following conference proceedings for relevant abstracts: Fourth MIM Pan‐African Malaria Conference, Yaoundé, Cameroon, November 2005; Third MIM Pan‐African Malaria Conference, Arusha, Tanzania, November 2002; Third European Congress on Tropical Medicine and International Health, Lisbon, Portugal, September 2002; Malaria: Current Status and Future Trends, Bangkok, Thailand, February, 2003; and the American Society of Tropical Medicine and Hygiene 52nd Annual Meeting, Philadelphia, USA, December 2003.
Researchers, organizations, and pharmaceutical companies
We contacted individual researchers working in the field, the WHO, PREMA‐EU, and GlaxoSmithKline, Novartis, and Merck (pharmaceutical companies) for unpublished and ongoing trials (contact made in 2005).
Reference lists
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
We assessed the results of the literature search for potentially relevant studies and obtained the full report for each.
The first author assessed the potentially relevant studies for inclusion using an eligibility form based on the inclusion criteria, and the second author checked the decisions. We scrutinized all trials for duplicate publication from the same data set. We resolved disagreements through discussion. We excluded all trials that did not meet the inclusion criteria and stated the reason in the 'Characteristics of excluded studies'.
Data extraction and management
The first author extracted data on the methods, types of participants, interventions, and outcomes from the included trials. The second author checked the extracted data against the trial reports to ensure accuracy and completeness. For dichotomous outcomes, we extracted the number of participants experiencing the event in each group. For continuous outcomes, we extracted a mean and standard deviation. We requested additional data from the trial authors.
Assessment of risk of bias in included studies
The first author assessed the risk of bias, and a second author checked the decisions. We classed generation of the allocation sequence and allocation concealment as adequate, inadequate, or unclear according to Jüni 2001. We recorded which people were blinded, such as the participants, care providers, or assessors. We considered the inclusion of all randomized participants in the analysis to be adequate if it was greater than or equal to 90% and inadequate if less than 90%. We displayed this information in a table and described it in the text.
Data synthesis
We interpreted the results qualitatively and performed a quantitative analysis only where we could combine the data. We stratified the results according to the drugs used in the trials.
The first author used Review Manager 5 to perform an intention‐to‐treat quantitative analysis where the data allowed. We combined dichotomous data using the risk ratio (RR) and continuous data using the mean difference (MD); we used the fixed‐effect model and reported the each result with a 95% confidence interval (CI).
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity by visually examining the forest plots and by using the chi‐squared test for heterogeneity with a 5% level of statistical significance.
We were unable to use some methods described in the published protocol because there were too few included trials. We will use these methods, including those for data analysis of continuous and time‐to‐event data, and criteria for exploring heterogeneity, should relevant data become available.
Results
Description of studies
We included 10 trials (see 'Characteristics of included studies') from 17 potentially relevant studies. Reasons for exclusion of the seven trials are documented (see 'Characteristics of excluded studies').
Trial design and location
Eight trials were randomized, and two were quasi‐randomized. Five were conducted in South‐East Asia: one in Thailand (Bounyasong 2001), and four in camps for displaced Karen people on the Thai‐Burma border (Nosten 1993a; McGready 2000; McGready 2001a; McGready 2005). Five trials were conducted in Africa – each in a different country: Burkina Faso (Coulibaly 2006); Democratic Republic of the Congo (DRC) (Mbanzulu 1993); Nigeria (Sowunmi 1998a); Ghana (Tagbor 2006); and Malawi (Kalilani 2007).
Local antimalarial resistance was not stated in the Malawi trial. The Ghana trial was conducted in an area with chloroquine resistance. The Burkina Faso trial in an area of increasing chloroquine and sulfadoxine‐pyrimethamine resistance. The DRC trial was conducted in an area with known resistance to a number of conventional treatments (including chloroquine, amodiaquine, and quinine). The other trials were conducted in areas with multiple‐drug‐resistant malaria.
Malaria transmission was stated for those trials carried out in the camps (low and seasonal), for the Burkina Faso trial (endemic and seasonal), and for the Malawi trial (perennial and seasonal).
Participants
This review includes 1805 women, 900 of these from the Ghana trial (Tagbor 2006). All women were recruited in their second or third trimester of pregnancy. Although some trials included a high proportion of primigravidae, all trials included women of a range of parities.
The Nigerian trial evaluated women with acute symptomatic malaria who had all previously failed treatment with chloroquine, sulfadoxine‐pyrimethamine, or chloroquine plus sulfadoxine‐pyrimethamine. The five trials from Thailand evaluated treatments in women who were usually asymptomatic with parasitaemia detected at regular screening. The Burkina Faso trial recruited symptomatic women only. It is unclear whether malaria was symptomatic in the other trials.
Interventions
Two trials compared artesunate plus mefloquine with quinine (McGready 2000; Bounyasong 2001); and two trials compared sulfadoxine‐pyrimethamine with chloroquine (Coulibaly 2006; Tagbor 2006). The remaining trials evaluated a wide variety of treatments, often in combination. All treatments were given at antenatal clinics. Women in the camps and in the Burkina Faso, Nigeria, and Malawi trials were supervised when taking the drugs. In the Ghana trial the first dose was supervised. It is not clear if treatment was supervised in the other trials. Kalilani 2007 gave two courses of treatment at least four weeks apart.
Outcomes
In most trials treatment failure was defined by parasite status only or the need for treatment. Clinical information was only reported in the Burkina Faso trial. Five trials excluded participants with new infections from the results. Seven of the 10 trials assessed a variety of pregnancy outcomes. Labour was monitored and newborns were generally followed up for up to 12 or 24 months. Five trials reported on low birthweight, and another two reported the mean birthweight only.
Risk of bias in included studies
The risk of bias assessment is summarized inTable 2.
2. Risk of bias of included trialsa.
| Trial | Generation of allocation sequence | Allocation concealment | Blinding | Inclusion of all randomized participants in the final analysisb |
| Bounyasong 2001 | Unclear | Unclear | None | Adequate |
| Coulibaly 2006 | Adequate | Unclear | None | Inadequate |
| Kalilani 2007 | Adequate | Adequate | Outcome assessor | Inadequate |
| Mbanzulu 1993 | Inadequate | Unclear | Outcome assessor | Adequate |
| McGready 2000 | Adequate | Unclear | None | Adequate |
| McGready 2001a | Unclear | Unclear | None | Inadequate |
| McGready 2005 | Adequate | Adequate | Outcome assessor | Adequate |
| Nosten 1993b | Inadequate | Unclear | None | Inadequate |
| Sowunmi 1998a | Unclear | Unclear | None | Adequate |
| Tagbor 2006 | Adequate | Adequate | Participant, treatment provider, outcome assessor, data analyst | Adequate |
aSee 'Assessment of risk of bias in included studies' for the assessment methods, and the 'Characteristics of included studies' for the methods used in each trial. bFor primary outcomes.
Generation of the allocation sequence
Seven trials were randomized, but only five described an adequate method of randomization (McGready 2000; McGready 2005; Coulibaly 2006; Tagbor 2006; Kalilani 2007). Two trials were quasi‐randomized: Nosten 1993a is described as a "paired restricted sequential trial", and Mbanzulu 1993 used alternate allocation.
Allocation concealment
Only three trials reported the method used to conceal allocation (envelopes: McGready 2005; Tagbor 2006; Kalilani 2007).
Blinding
In Tagbor 2006, the participants, treatment provider, outcome assessor and data analyst were all blinded. Kalilani 2007, Mbanzulu 1993, and McGready 2005 stated that the outcome assessor was blinded (but only for laboratory‐based outcomes in Kalilani 2007). The other trials did not report on blinding.
Inclusion of randomized participants in the analysis
In the analysis of the primary outcome measures – treatment failure and low birthweight – three trials included more than 90% of the randomized participants (Mbanzulu 1993; Sowunmi 1998a; McGready 2000), which we considered adequate, and four trials included less than 90% (Nosten 1993a; McGready 2001a; Coulibaly 2006; Kalilani 2007), which we considered inadequate. McGready 2005 and Tagbor 2006 included more than 90% of the randomized participants in the analysis of treatment failure but not birthweight. Although Bounyasong 2001 did not provide the number of participants analysed for any outcome, the trial authors stated that they considered follow up to be adequate.
Effects of interventions
1. Artesunate plus atovaquone‐proguanil versus quinine (80 participants, 1 trial)
Maternal treatment response
McGready 2005 reported fewer treatment failures at day 63 with artesunate plus atovaquone‐proguanil (RR 0.24, 95% CI 0.10 to 0.57; 80 participants; Analysis 1.1); also when new infections were excluded (RR 0.14, 95% CI 0.03 to 0.57; 80 participants; Figure 1, Analysis 1.2).
1.1. Analysis.
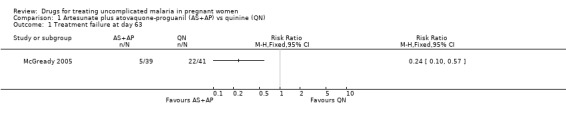
Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 1 Treatment failure at day 63.
1.
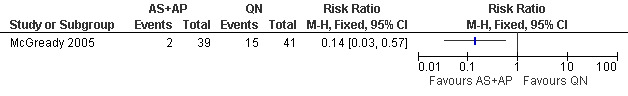
Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN): Treatment failure at day 63 (excludes new infections, detected by PCR)
1.2. Analysis.
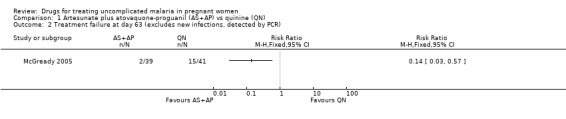
Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 2 Treatment failure at day 63 (excludes new infections, detected by PCR).
Median parasite clearance time was shorter in the artesunate plus atovaquone‐proguanil group than in the quinine group: two days (range 1 to 3) and four days (range 1 to 7), respectively, (trial authors' P < 0.0001; 79 participants).
Anaemia was not statistically significantly different between treatment groups (81 participants, Analysis 1.3).
1.3. Analysis.

Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 3 Anaemia.
Maternal adverse events
Fewer women in the artesuate plus atovaquone‐proguanil group reported tinnitus (RR 0.30, 95% CI 0.16 to 0.60; 58 participants; Analysis 1.4).
1.4. Analysis.

Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 4 Tinnitus.
Fetal outcomes
This small trial found no statistically significant difference in any of the fetal outcomes assessed: low birthweight (53 participants, Analysis 1.5); mean birthweight (53 participants, Analysis 1.6); preterm delivery (72 participants, Analysis 1.7); gestational age (72 participants, Analysis 1.8); intra‐uterine growth retardation (52 participants, Analysis 1.9); and congenital abnormality (72 participants, Analysis 1.10). Three deaths occurred in the quinine group (two premature infants and a term baby with complications related to birth asphyxia) and two in the artesunate plus atovaquone‐proguanil group (sepsis and unknown cause).
1.5. Analysis.
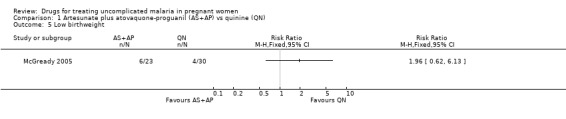
Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 5 Low birthweight.
1.6. Analysis.

Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 6 Mean birthweight.
1.7. Analysis.
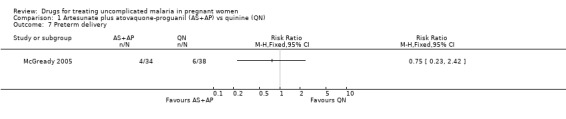
Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 7 Preterm delivery.
1.8. Analysis.

Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 8 Gestational age.
1.9. Analysis.

Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 9 Intra‐uterine growth retardation.
1.10. Analysis.

Comparison 1 Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN), Outcome 10 Congenital abnormality.
2. Artesunate plus mefloquine versus quinine (165 participants, 2 trials, different regimens)
Maternal treatment response
McGready 2000 reported fewer treatment failures (excludes new infections) at day 63 with artesunate plus mefloquine (RR 0.09, 95% CI 0.02 to 0.38; 106 participants; Figure 2, Analysis 2.1). There was also a trend towards better performance at day 28 in this trial (approximately 97% of the artesunate plus mefloquine group and 88% of the quinine group were without parasite recrudescence; data estimated from figure in original article). Bounyasong 2001 reported no treatment failures in either group (57 participants) at day 28.
2.
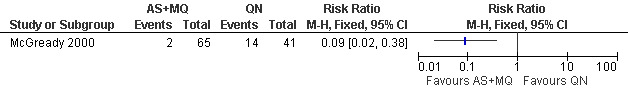
Artesunate plus mefloquine (AS+MQ) vs quinine (QN): Treatment failure at day 63 (excludes new infections, detected by PCR)
2.1. Analysis.
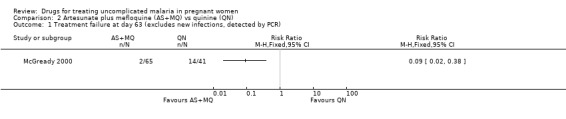
Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 1 Treatment failure at day 63 (excludes new infections, detected by PCR).
Bounyasong 2001 reported fever and parasite clearance times that were shorter with artesunate plus mefloquine (4.47 days and 3.46 days, respectively) than quinine (8.04 days and 7.03 days, respectively); the trial authors' P test was statistically significant.
Anaemia on admission was similar in both treatment groups in McGready 2000, but by day seven more women in the artesunate plus mefloquine group had anaemia (RR 1.57, 95% CI 1.01 to 2.45; 81 participants; Analysis 2.2). This did not persist at further time points (days 28, 42, and 63; Analysis 2.2), and by day 63 the number of women with anaemia in both treatment groups was about half that on admission. The trial authors also examined the number of women who developed anaemia during the 63 days of follow up (overall 35/53) and found no difference between the groups.
2.2. Analysis.

Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 2 Anaemia.
Bounyasong 2001 reported that mean haematocrit was not statistically significantly different between treatment groups on admission (artesunate plus mefloquine 34.29% versus quinine 35.17%). By the end of treatment haematocrit was slightly reduced in both treatment groups, but it was higher with artesunate plus mefloquine (33.2%) compared with quinine (28.4%); the trial authors' P test was statistically significant (Bounyasong 2001).
Maternal adverse events
Both trials reported on adverse events experienced by the women. Those affecting the nervous system, such as tinnitus, were generally more common with quinine (Analysis 2.3). Also, gastrointestinal adverse events (abdominal pain, anorexia, nausea, and vomiting) were more commonly reported with quinine (Analysis 2.4). The trials reported other adverse events − hypoglycaemia, muscle and joint pain, and palpitations − but there were no statistically significant differences between the groups (Analysis 2.5) except for hypoglycaemia, which was more common in the quinine group in Bounyasong 2001 (RR 0.15, 95% CI 0.05 to 0.44; 57 participants; Analysis 2.5). McGready 2000 did not report on hypoglycaemia. McGready 2000 reported one death, which was unrelated to malaria.
2.3. Analysis.

Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 3 Nervous system adverse events.
2.4. Analysis.

Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 4 Gastrointestinal adverse events.
2.5. Analysis.
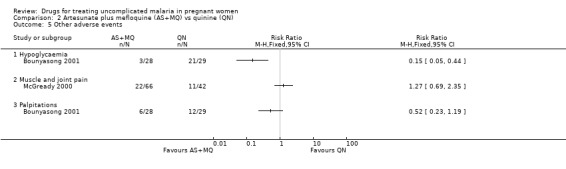
Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 5 Other adverse events.
Fetal outcomes
Both trials reported on mean birthweight. McGready 2000 reported no statistically significant difference in the proportion of infants with low birthweight (86 participants, Analysis 2.6) and no statistically significant difference in the mean birthweight (88 participants, Analysis 2.7). Bounyasong 2001 reported that infants born to mothers in the mefloquine group were 140 g heavier overall compared with quinine (trial authors' P = 0.041). McGready 2000 reported no stillbirths or congenital abnormalities in either group and no statistically significant difference in the number of abortions (108 participants, Analysis 2.8), but there were few participants. There were two neonatal deaths in the artesunate plus mefloquine group compared with one in the quinine group. Bounyasong 2001 did not demonstrate a statistically significant difference in gestational age (trial authors' data) or neonatal jaundice (57 participants, Analysis 2.9). Bounyasong 2001 did not report on congenital abnormalities.
2.6. Analysis.
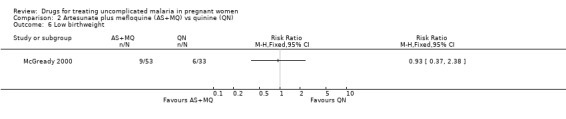
Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 6 Low birthweight.
2.7. Analysis.

Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 7 Mean birthweight.
2.8. Analysis.
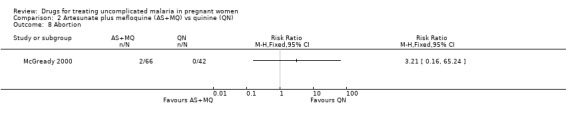
Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 8 Abortion.
2.9. Analysis.

Comparison 2 Artesunate plus mefloquine (AS+MQ) vs quinine (QN), Outcome 9 Neonatal jaundice.
3. Artesunate plus sulfadoxine‐pyrimethamine versus azithromycin plus sulfadoxine‐pyrimethamine (94 participants, 1 trial)
Participants in Kalilani 2007 received two courses of treatment.
Maternal treatment response
The proportion of treatment failures at delivery or day 40 was similar in both treatment groups (94 participants, Analysis 3.1); also when new infections were excluded (81 participants, Analysis 3.2). This was similar also for maternal anaemia (66 participants, Analysis 3.3).
3.1. Analysis.

Comparison 3 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP), Outcome 1 Treatment failure at delivery or day 40.
3.2. Analysis.
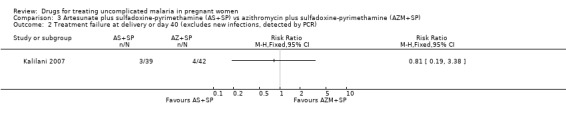
Comparison 3 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP), Outcome 2 Treatment failure at delivery or day 40 (excludes new infections, detected by PCR).
3.3. Analysis.
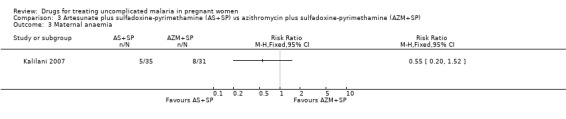
Comparison 3 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP), Outcome 3 Maternal anaemia.
Maternal adverse events
Adverse events could not be analysed as the data reported were insufficient.
Fetal outcomes
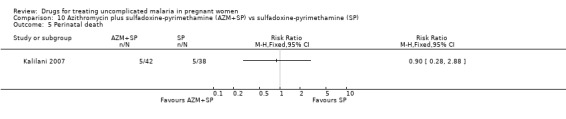
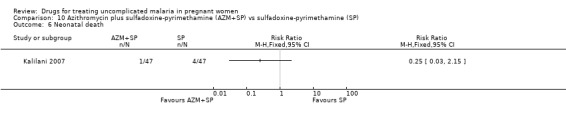
There was no statistically significant difference between treatment groups in low birthweight (70 participants, Analysis 3.4), perinatal death (80 participants, Analysis 3.5), or neonatal death (94 participants, Analysis 3.6).
3.4. Analysis.

Comparison 3 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP), Outcome 4 Low birthweight.
3.5. Analysis.
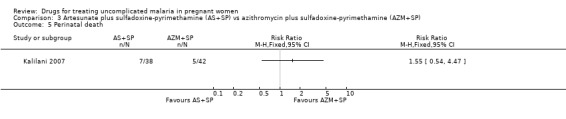
Comparison 3 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP), Outcome 5 Perinatal death.
3.6. Analysis.

Comparison 3 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP), Outcome 6 Neonatal death.
4. Artesunate plus sulfadoxine‐pyrimethamine versus sulfadoxine‐pyrimethamine (94 participants, 1 trial)
Participants in Kalilani 2007 received two courses of treatment.
Maternal treatment response
Treatment failure at delivery (or day 40) was statistically significantly reduced by adding artesunate to sulfadoxine‐pyrimethamine (RR 0.21, 95% CI 0.07 to 0.70; 94 participants; Analysis 4.1); also when new infections were excluded (RR 0.15, 95% CI 0.04 to 0.59; 79 participants; Figure 3, Analysis 4.2). Maternal anaemia was similar in both groups (68 participants, Analysis 4.3).
4.1. Analysis.

Comparison 4 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 1 Treatment failure at delivery or 40 days.
3.

Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP): Treatment failure at delivery or day 40 (excludes new infections, detected by PCR)
4.2. Analysis.

Comparison 4 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 2 Treatment failure at delivery or day 40 (excludes new infections, detected by PCR).
4.3. Analysis.
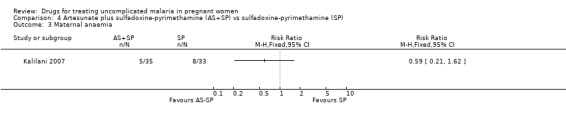
Comparison 4 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 3 Maternal anaemia.
Maternal adverse events
Adverse events could not be analysed as the data reported were insufficient.
Fetal outcomes
There was no statistically significant difference between treatment groups in low birthweight (70 participants, Analysis 4.4), perinatal death (76 participants, Analysis 4.5), or neonatal death (94 participants, Analysis 4.6).
4.4. Analysis.

Comparison 4 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 4 Low birthweight.
4.5. Analysis.

Comparison 4 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 5 Perinatal death.
4.6. Analysis.

Comparison 4 Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 6 Neonatal death.
5. Quinine plus spiramycin versus quinine (32 participants, 1 trial)
Maternal treatment response
Nosten 1993a reported that there was an equal distribution of treatment failures between the two groups. There was also no statistically significant difference for mean parasite clearance time in this small trial (32 participants, Analysis 5.1).
5.1. Analysis.

Comparison 5 Quinine plus spiramycin (QN+SPI) vs quinine (QN), Outcome 1 Mean parasite clearance time.
Fetal outcomes
This small trial reported one abortion in the quinine plus spiramycin group. There was no statistically significant difference in the mean gestational age at delivery (32 participants, Analysis 5.2).
5.2. Analysis.
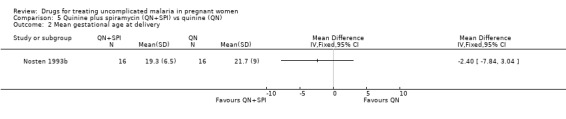
Comparison 5 Quinine plus spiramycin (QN+SPI) vs quinine (QN), Outcome 2 Mean gestational age at delivery.
6. Artesunate versus quinine plus clindamycin (129 participants, 1 trial)
Maternal treatment response
McGready 2001a reported more treatment failures (excluding new infections) at 48 hours in the quinine plus clindamycin group (RR 0.21, 95% CI 0.12 to 0.38; 129 participants, Analysis 6.1), but by day 42 all 129 women in both treatment groups were cured. The mean parasite clearance time was shorter in the artesunate group than the quinine plus clindamycin group (MD 0.60 days, 95% CI 0.23 to 0.97 days; 129 participants; Analysis 6.2).
6.1. Analysis.

Comparison 6 Artesunate (AS) vs quinine plus clindamycin (QN+CLD), Outcome 1 Treatment failure at 48 hours (excludes new infections, detected by PCR).
6.2. Analysis.

Comparison 6 Artesunate (AS) vs quinine plus clindamycin (QN+CLD), Outcome 2 Mean parasite clearance time.
Median haematocrit was similar in the two groups on admission (artesunate plus quinine: median 29.6, range 20.0 to 38.9, 64 participants; clindamycin: median 29.2, range 14.9 to 38.5, 65 participants). By day seven there were more participants with anaemia in the artesunate group (RR 1.57, 95% CI 1.01 to 2.45; 81 participants; Analysis 6.3), but this difference did not persist at later time points.
6.3. Analysis.

Comparison 6 Artesunate (AS) vs quinine plus clindamycin (QN+CLD), Outcome 3 Anaemia at day 7.
Maternal adverse events
Apart from tinnitus, the occurrence of adverse events experienced by the women was similar in both groups. When the trialists excluded from the analysis those with anaemia on admission there was no difference in the number of participants developing anaemia during follow up (39/59, trial authors' unpublished result).
Fetal outcomes
The proportion of infants were similar in the two groups for low birthweight (109 participants, Analysis 6.4), the mean birthweight (109 participants, Analysis 6.5), and the mean gestational age at delivery (115 participants, Analysis 6.5). There was one stillbirth in each treatment group. In the quinine plus clindamycin group there was one congenital abnormality (midline epidermoid cyst just superior to the bridge of the nose). One infant who had gastroschisis (infant abdominal hernia) did not survive.
6.4. Analysis.
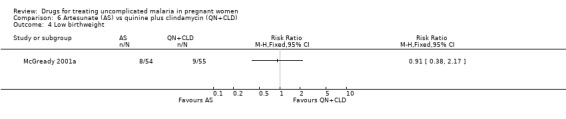
Comparison 6 Artesunate (AS) vs quinine plus clindamycin (QN+CLD), Outcome 4 Low birthweight.
6.5. Analysis.

Comparison 6 Artesunate (AS) vs quinine plus clindamycin (QN+CLD), Outcome 5 Mean birthweight.
7. Artemether plus mefloquine versus artemether (55 participants, 1 trial)
All participants in Sowunmi 1998a were recruited following failure with either chloroquine or sulfadoxine‐pyrimethamine treatment.
Maternal treatment response
All women treated with artemether plus mefloquine were aparasitaemic on day 28, and those treated with artemether were aparasitaemic on day 14 (after one treatment failure was treated again). The mean fever clearance time was similar in both groups (45 participants, Analysis 7.1) as was the mean parasite clearance time (45 participants, Analysis 7.2). Mean haematocrit did not change dramatically in either group between the first day of treatment (artemether plus mefloquine 29.0% (standard deviation 3.8); artemether 28.8% (4.3)) and day seven (artemether plus mefloquine 29.2% (3.1); artemether 29.1% (3.3)).
7.1. Analysis.

Comparison 7 Artemether plus mefloquine (ATM+MQ) vs artemether (ATM), Outcome 1 Mean fever clearance time.
7.2. Analysis.

Comparison 7 Artemether plus mefloquine (ATM+MQ) vs artemether (ATM), Outcome 2 Mean parasite clearance time.
Maternal adverse events
The trial authors described the adverse events as "minimal" and reported that they did not require treatment to be discontinued. Four events occurred in the artemether plus mefloquine group (two reports of abdominal discomfort and two of dizziness), but there was no statistically significant difference between the two treatment groups (Analysis 7.3).
7.3. Analysis.

Comparison 7 Artemether plus mefloquine (ATM+MQ) vs artemether (ATM), Outcome 3 Adverse events.
Fetal outcomes
Mean birthweight was similar in both groups (45 participants, Analysis 7.4), and none of the newborns were parasitaemic. There were no stillbirths or congenital abnormalities in either treatment group.
7.4. Analysis.

Comparison 7 Artemether plus mefloquine (ATM+MQ) vs artemether (ATM), Outcome 4 Mean birthweight.
8. Amodiaquine plus sulfadoxine‐pyrimethamine versus chloroquine (418 participants, 1 trial)
Maternal treatment response
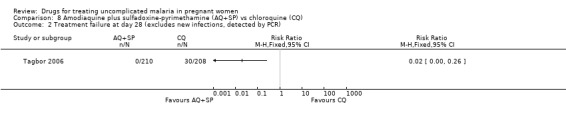
Tagbor 2006 reported fewer treatment failures at day 28 with amodiaquine plus sulfadoxine‐pyrimethamine (RR 0.08, 95% CI 0.03 to 0.19; 418 participants; Analysis 8.1); also when new infections were excluded (RR 0.02, 95% CI 0.00 to 0.26; 418 participants; Figure 4, Analysis 8.2).
8.1. Analysis.

Comparison 8 Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ), Outcome 1 Treatment failure at day 28.
4.
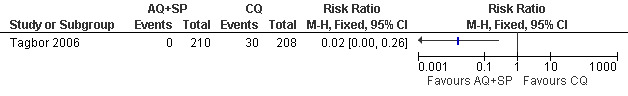
Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ): Treatment failure at day 28 (excludes new infections, detected by PCR)
8.2. Analysis.
Comparison 8 Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ), Outcome 2 Treatment failure at day 28 (excludes new infections, detected by PCR).
Maternal adverse events
This study reported on adverse events at day three and day seven; see Analysis 8.3. There were more "side effects" of any type reported with amodiaquine plus sulfadoxine‐pyrimethamine at both time points: day three (RR 1.19, 95% CI 1.09 to 1.30; 432 participants) and day seven (RR 1.30, 95% CI 1.01 to 1.68; 435 participants). General weakness was also experienced more with amodiaquine plus sulfadoxine‐pyrimethamine at both day three (RR 1.70, 95% CI 1.47 to 1.97; 432 participants) and day seven (RR 1.74, 95% CI 1.29 to 2.34; 435 participants).
8.3. Analysis.

Comparison 8 Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ), Outcome 3 Adverse events.
Other adverse events were more common with amodiaquine plus sulfadoxine‐pyrimethamine at day three, but the difference was not statistically significant at day seven: dizziness (RR 1.25, 95% CI 1.03 to 1.50; 432 participants); vomiting (RR 1.85, 95% CI 1.49 to 2.31; 432 participants); and nausea (RR 1.62, 95% CI 1.21 to 2.19; 432 participants).
Itching was more common with chloroquine at day three (RR 0.63, 95% CI 0.48 to 0.83; 432 participants), but the difference was not statistically significant at day seven.
Thirteen women in the chloroquine group and 16 in the amodiaquine plus sulfadoxine‐pyrimethamine group discontinued their treatment because of an adverse effect (statistical significance not calculable).
Fetal outcomes
No statistically significant difference was detected between the groups in terms of low birthweight (260 participants, Analysis 8.4) and preterm delivery (349 participants, Analysis 8.5). The trial authors also reported no statistically significant difference in abortions, stillbirths, and perinatal deaths. Congenital abnormalities (extra digits; external ear malformation) were reported in two infants in the chloroquine group and in one infant in the amodiaquine plus sulfadoxine‐pyrimethamine group.
8.4. Analysis.

Comparison 8 Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ), Outcome 4 Low birthweight.
8.5. Analysis.
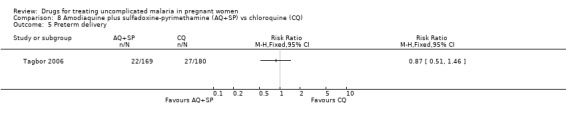
Comparison 8 Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ), Outcome 5 Preterm delivery.
9. Amodiaquine versus chloroquine (420 participants, 1 trial)
Maternal treatment response
Tagbor 2006 reported fewer treatment failures at day 28 with amodiaquine (RR 0.25, 95% CI 0.15 to 0.42; 420 participants; Analysis 9.1); also when new infections were excluded (RR 0.20, 95% CI 0.08 to 0.46; 420 participants; Figure 5, Analysis 9.2).
9.1. Analysis.

Comparison 9 Amodiaquine (AQ) vs chloroquine (CQ), Outcome 1 Treatment failure at day 28.
5.

Amodiaquine (AQ) vs chloroquine (CQ): Treatment failure at day 28 (excludes new infections, detected by PCR)
9.2. Analysis.

Comparison 9 Amodiaquine (AQ) vs chloroquine (CQ), Outcome 2 Treatment failure at day 28 (excludes new infections, detected by PCR).
Maternal adverse events
This trial reported on adverse events at day three and day seven; see Analysis 9.3. There were more "side effects" of any type reported with amodiaquine at day three (RR 1.13, 95% CI 1.03 to 1.24; 435 participants), but the difference was not statistically significant at day seven. General weakness was reported more among the amodiaquine group at both day three (RR 1.71, 1.47 to 1.98; 435 participants) and day seven (RR 1.48, 95% CI 1.08 to 2.01; 437 participants). At day three more women in the amodiaquine group had dizziness (RR 1.28, 95% CI 1.06 to 1.54; 435 participants) and vomiting (RR 1.41, 95% CI 1.11 to 1.79; 435 participants); the difference was not statistically significant at day seven. The difference in the incidence of itching and nausea was not statistically significant at day three or seven.
9.3. Analysis.
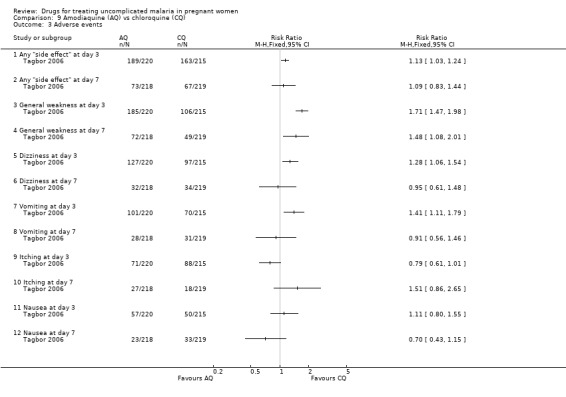
Comparison 9 Amodiaquine (AQ) vs chloroquine (CQ), Outcome 3 Adverse events.
Fetal outcomes
There was no statistically significant difference between the groups for low birthweight (262 participants, Analysis 9.4) and preterm delivery (356 participants, Analysis 9.5). The trial authors also reported no statistically significant difference in abortions, stillbirths, and perinatal deaths. Congenital malformations (extra digits) were reported in one baby in the chloroquine group and five in the amodiaquine group.
9.4. Analysis.

Comparison 9 Amodiaquine (AQ) vs chloroquine (CQ), Outcome 4 Low birthweight.
9.5. Analysis.

Comparison 9 Amodiaquine (AQ) vs chloroquine (CQ), Outcome 5 Preterm delivery.
10. Azithromycin plus sulfadoxine‐pyrimethamine versus sulfadoxine‐pyrimethamine (97 participants, 1 trial)
Participants in Kalilani 2007 received two courses of treatment.
Maternal treatment response
Treatment failure at delivery (or day 40) was statistically significantly reduced by adding azithromycin to sulfadoxine‐pyrimethamine (RR 0.29, 95% CI 0.10 to 0.80; 94 participants; Analysis 10.1); also when new infections were excluded (RR 0.27, 95% CI 0.10 to 0.76; 82 participants; Figure 6, Analysis 10.2). Maternal anaemia was similar in both groups (64 participants, Analysis 10.3).
10.1. Analysis.

Comparison 10 Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 1 Treatment failure at delivery or day 40.
6.

Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP): Treatment failure at delivery or day 40 (excludes new infections, detected by PCR)
10.2. Analysis.
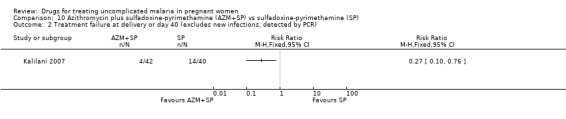
Comparison 10 Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 2 Treatment failure at delivery or day 40 (excludes new infections, detected by PCR).
10.3. Analysis.
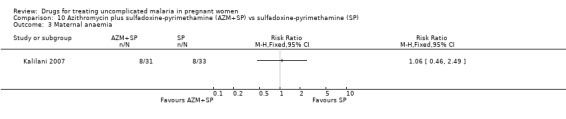
Comparison 10 Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 3 Maternal anaemia.
Maternal adverse events
Adverse events could not be analysed as the data reported were insufficient.
Fetal outcomes
There was no statistically significant difference between treatment groups in low birthweight (72 participants, Analysis 10.4), perinatal death (80 participants, Analysis 10.4), or neonatal death (94 participants, Analysis 10.4).
10.4. Analysis.

Comparison 10 Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 4 Low birthweight.
11. Sulfadoxine‐pyrimethamine versus chloroquine (538 participants, 2 trials)
Maternal treatment response
There was no statistically significant difference in treatment failure in either trial at day 14 (544 participants, Analysis 11.1). At day 28 there were fewer treatment failures with sulfadoxine‐pyrimethamine (RR 0.46, 95% CI 0.33 to 0.64; 538 participants; Analysis 11.2). In Tagbor 2006, when new infections were excluded, the difference was not statistically significant (416 participants, Analysis 11.3).
11.1. Analysis.

Comparison 11 Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ), Outcome 1 Treatment failure at day 14.
11.2. Analysis.

Comparison 11 Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ), Outcome 2 Treatment failure at day 28.
11.3. Analysis.

Comparison 11 Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ), Outcome 3 Treatment failure at day 28 (excludes new infections, detected by PCR).
Maternal adverse events
Coulibaly 2006 reported that there were no "adverse reactions attributable to treatment" during the trial. Tagbor 2006 reported on adverse events at day three and day seven; see Analysis 11.4. Vomiting was less frequent with sulfadoxine‐pyrimethamine at day three (RR 0.45, 95% CI 0.31 to 0.66; 432 participants) and day seven (RR 0.55, 95% CI 0.31 to 0.97; 437 participants). Other adverse events were less frequent with sulfadoxine‐pyrimethamine at day three, but there were no statistically significant differences at day seven: any "side effect" (RR 0.63, 95% CI 0.54 to 0.74; 432 participants); general weakness (RR 0.60, 95% CI 0.47 to 0.77; 432 participants); dizziness (RR 0.34, 95% CI 0.24 to 0.48; 432 participants); itching (RR 0.39, 95% CI 0.28 to 0.56; 432 participants); and nausea (RR 0.52, 95% CI 0.33 to 0.80; 432 participants).
11.4. Analysis.

Comparison 11 Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ), Outcome 4 Adverse events.
Fetal outcomes
Tagbor 2006 reported no statistically significant difference between the groups for low birthweight (271 participants, Analysis 11.5) and preterm delivery (260 participants, Analysis 11.6). The trial authors also reported no statistically significant difference in abortions, stillbirths, and perinatal deaths.
11.5. Analysis.

Comparison 11 Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ), Outcome 5 Low birthweight.
11.6. Analysis.
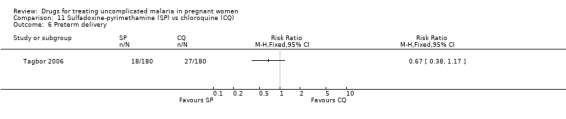
Comparison 11 Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ), Outcome 6 Preterm delivery.
12. Chloroquine plus clindamycin (for three or five days) versus chloroquine (132 participants, 1 trial)
Maternal treatment response
Mbanzulu 1993 compared two different regimens of chloroquine plus clindamycin (clindamycin given for three or five days) with chloroquine alone. At day 14 there were seven treatment failures in the chloroquine group and none in the chloroquine plus clindamycin groups, but this difference was not statistically significant (Analysis 12.1). All treatment failures were cured with chloroquine plus clindamycin.
12.1. Analysis.

Comparison 12 Chloroquine plus clindamycin (CQ+CLD) for 3 or 5 days vs chloroquine (CQ), Outcome 1 Treatment failure at day 14.
Maternal adverse events
No adverse events were reported for participants in the chloroquine plus clindamycin groups. Two participants in the chloroquine group developed itching (Analysis 12.2) and one developed diarrhoea (Analysis 12.3). Neither necessitated withdrawal from treatment.
12.2. Analysis.

Comparison 12 Chloroquine plus clindamycin (CQ+CLD) for 3 or 5 days vs chloroquine (CQ), Outcome 2 Adverse event: itching.
12.3. Analysis.

Comparison 12 Chloroquine plus clindamycin (CQ+CLD) for 3 or 5 days vs chloroquine (CQ), Outcome 3 Adverse event: diarrhoea.
Discussion
Ten trials assessing the safety and efficacy of treatments for uncomplicated malaria have involved 1805 pregnant women, 900 of which were involved in just one trial (Tagbor 2006). These 10 trials examined 12 different regimen comparisons, so statistical power to detect differences was generally low. As most trials were rather small and varied in the treatments evaluated, it is not surprising that this review was unable to demonstrate any clear direction for policy.
Data from two trials in Thailand have shown that artesunate plus mefloquine (given in two different regimens) appears to be better than quinine in terms of clearing parasites and fever (McGready 2000; Bounyasong 2001). Although one trial reported that hypoglycaemia occurred more frequently in the quinine group (Bounyasong 2001), no details of how hypoglycaemia was defined, how it was measured, and how frequently it was tested were given, so the clinical significance is difficult to determine. Furthermore, artesunate plus atovaquone‐proguanil appears to be more effective at clearing parasites than quinine (in Thailand); but these data are from one small trial (McGready 2005). Artesunate may be quicker at clearing parasites than quinine plus clindamycin (in Thailand; McGready 2001a), which is consistent with the known pharmacokinetic properties of artemisinin derivatives. When chloroquine was compared with amodiaquine and amodiaquine plus sulfadoxine‐pyrimethamine in one trial in Ghana (Tagbor 2006), the two newer regimens were found to be more effective at clearing parasites. There are early indications that adding artesunate or azithromycin to sulfadoxine‐pyrimethamine may improve parasite clearance (in Malawi; Kalilani 2007); however, there are no reliable data on adverse events with this regimen.
Apart from the one large trial that was well‐conducted and reported (Tagbor 2006), the methods used in the other trials were generally not clearly reported. Although it is not always easy to blind such trials, most trials were either quasi‐randomized or did not report the method of generation of the allocation sequence, and only three reported any method of concealing treatment allocation.
There remains a concern over a possible association between stillbirth and mefloquine. There were not enough data from trials to allow this review to evaluate this. However, one large trial of prevention in Malawi (which was not included in this review) evaluated mefloquine prophylaxis (Steketee 1996). Women received a treatment course of mefloquine on entry to the trial. The trial recruited over 4187 women and showed that those allocated to the mefloquine group had similar numbers of stillbirths to the control group taking chloroquine. This contrasts with a retrospective analysis of observational data from Thailand that suggested an association between mefloquine use and stillbirth, after making an adjustment for confounding (Nosten 1999). However, the possibility of residual confounding remains.
None of the trials included women in their first trimester. As many trials did not state if the women were symptomatic or how many previous pregnancies they had experienced, it is not possible to assess the impact of these factors on treatment outcomes. Most trials supervised treatment or followed up the participants intensively. Hence, trials may not reflect what effects the treatments would have had when used in standard conditions.
Generally, trials in non‐pregnant women are likely to inform treatment in pregnant women, and current treatments in Africa are moving towards artemisinin‐based combination treatments. Fetal safety is clearly important. In 2003, an expert panel reviewed evidence from a variety of sources and considered that the risks of not using artemisinin derivatives in pregnancy outweighed any putative adverse effects in relation to embryo‐lethality (WHO 2004a). The WHO's new treatment guidelines for malaria reflect this opinion (WHO 2006). However, there are still not enough safety data to recommend their use in the first trimester of pregnancy.
Clearly, more trials are needed evaluating the current best regimens for malaria. The trials must be well‐designed to reduce bias and methods must be clearly reported. Trialists should ensure that they investigate all relevant outcomes in the mother and fetus, including a systematic collection of data on adverse events and pregnancy outcomes.
Authors' conclusions
Implications for practice.
In South‐East Asia, artesunate plus mefloquine and artesunate plus atovaquone‐proguanil may be better than quinine at clearing parasites and the symptoms of uncomplicated malaria.
In West Africa, amodiaquine and amodiaquine plus sulfadoxine‐pyrimethamine may be more effective at clearing parasites than chloroquine.
In Southern Africa, the combination of artesunate or azithromycin with sulfadoxine‐pyrimethamine may be more effective than using sulfadoxine‐pyrimethamine alone in areas where there is resistance to sulfadoxine‐pyrimethamine.
Implications for research.
Well‐designed randomized controlled trials evaluating alternative treatment regimens for malaria in pregnancy are needed. These trials should assess both effectiveness and safety in the mother and fetus.
What's new
| Date | Event | Description |
|---|---|---|
| 4 May 2008 | New citation required but conclusions have not changed | New author: Aika Omari joined the author team, and Paul Garner stepped down. |
| 4 May 2008 | New search has been performed | New trials: Based on the updated search, we included four new trials (McGready 2005; Coulibaly 2006; Tagbor 2006; Kalilani 2007). |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 24 May 2006 | Amended | 2006, Issue 3: Nosten 1993a (additional reference) changed to Nosten 1993a, and Nosten 1993 (included study) changed to Nosten 1993b. |
| 4 May 2005 | Amended | New studies sought but none found. |
Acknowledgements
We thank Bernard Brabin and Francine Verhoeff for their comments during the development of the protocol for this review. We thank Paul Garner for his contributions as an author to the original version of the review (Orton 2005). The Liverpool School of Tropical Medicine provided support to Paul Garner.
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of developing countries.
Data and analyses
Comparison 1. Artesunate plus atovaquone‐proguanil (AS+AP) vs quinine (QN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at day 63 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at day 63 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Anaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Tinnitus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Mean birthweight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Preterm delivery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Gestational age | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Intra‐uterine growth retardation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Congenital abnormality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Artesunate plus mefloquine (AS+MQ) vs quinine (QN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at day 63 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Anaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 On admission | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Day 42 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Day 63 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Nervous system adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Abnormal neurology | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Blurring vision | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Tinnitus | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Vertigo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Gastrointestinal adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Abdominal pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Nausea | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Vomiting | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Other adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Hypoglycaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Muscle and joint pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Palpitations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Mean birthweight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Abortion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Neonatal jaundice | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at delivery or day 40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at delivery or day 40 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Maternal anaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Perinatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Neonatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Artesunate plus sulfadoxine‐pyrimethamine (AS+SP) vs sulfadoxine‐pyrimethamine (SP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at delivery or 40 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at delivery or day 40 (excludes new infections, detected by PCR) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Maternal anaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Perinatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Neonatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Quinine plus spiramycin (QN+SPI) vs quinine (QN).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean parasite clearance time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mean gestational age at delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Artesunate (AS) vs quinine plus clindamycin (QN+CLD).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 48 hours (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Mean parasite clearance time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Anaemia at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Mean birthweight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Mean gestational age at delivery | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
6.6. Analysis.

Comparison 6 Artesunate (AS) vs quinine plus clindamycin (QN+CLD), Outcome 6 Mean gestational age at delivery.
Comparison 7. Artemether plus mefloquine (ATM+MQ) vs artemether (ATM).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean fever clearance time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Mean parasite clearance time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Abdominal discomfort | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Dizziness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Mean birthweight | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. Amodiaquine plus sulfadoxine‐pyrimethamine (AQ+SP) vs chloroquine (CQ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at day 28 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Any "side effect" at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Any "side effect" at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 General weakness at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 General weakness at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Dizziness at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Dizziness at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Vomiting day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Vomiting at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Itching at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Itching at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Nausea at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Nausea at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Preterm delivery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Amodiaquine (AQ) vs chloroquine (CQ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at day 28 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Any "side effect" at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Any "side effect" at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 General weakness at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 General weakness at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Dizziness at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Dizziness at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Vomiting at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Vomiting at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Itching at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Itching at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 Nausea at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Nausea at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Preterm delivery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at delivery or day 40 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Treatment failure at delivery or day 40 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Maternal anaemia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Perinatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Neonatal death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
10.5. Analysis.
Comparison 10 Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 5 Perinatal death.
10.6. Analysis.
Comparison 10 Azithromycin plus sulfadoxine‐pyrimethamine (AZM+SP) vs sulfadoxine‐pyrimethamine (SP), Outcome 6 Neonatal death.
Comparison 11. Sulfadoxine‐pyrimethamine (SP) vs chloroquine (CQ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at day 14 | 2 | 544 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.29, 1.09] |
| 2 Treatment failure at day 28 | 2 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.64] |
| 3 Treatment failure at day 28 (excludes new infections, detected by PCR) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Any "side effect" at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Any "side effect" at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 General weakness at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 General weakness at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Dizziness at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Dizziness at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Vomiting at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Vomiting at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Itching at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Itching at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Nausea at day 3 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Nausea at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Low birthweight | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Preterm delivery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 12. Chloroquine plus clindamycin (CQ+CLD) for 3 or 5 days vs chloroquine (CQ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 3‐day clindamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 5‐day clindamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse event: itching | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 3‐day clindamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 5‐day clindamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse event: diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 3‐day clindamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 5‐day clindamycin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bounyasong 2001.
| Methods | Randomized controlled trial Generation of allocation sequence: no method reported Allocation concealment: no method reported Blinding: none Inclusion of all randomized participants: 95% (for treatment failure) |
|
| Participants | Number: 60 randomized, 57 analysed Inclusion criteria: pregnant women infected with P. falciparum; gestational age at least 28 weeks; not more than 4% parasitized red blood cells; could be followed up at Srisangwal Hospital; could take and tolerate oral form of the medicine and be admitted to the hospital for at least 7 days Exclusion criteria: former medication with quinine, artesunate (including its derivatives), or mefloquine within 28 days; history of quinine, artesunate, or mefloquine allergy; malaria with complications such as shock, renal failure, pulmonary oedema, or cerebral malaria; mixed malarial infection Age in years (mean): artesunate plus mefloquine group 27.207; quinine group 26.143 Parity (mean): artesunate plus mefloquine group 1.59; quinine group 1.36 Early/late pregnancy: second trimester Symptomatic/asymptomatic malaria: number not reported Anaemia on admission: number not reported |
|
| Interventions | 1. Artesunate plus mefloquine
Artesunate: 2 mg/kg loading dose and 1 mg/kg every 12 hours for at least 5 days (until parasites are absent and there is clinical improvement)
Mefloquine: 15 mg/kg on day 6 and 10 mg/kg 6 hours later 2. Quinine sulfate: 10 mg/kg every 8 hours for at least 7 days (until clinically recovered) |
|
| Outcomes | 1. Treatment failure at day 28
2. Fever clearance time
3. Parasite clearance time
4. Haematocrit
5. Birthweight
6. Gestational age at birth
7. Congenital abnormalities
8. Infant development
9. Adverse events Not included in review: 10. Treatment time of parasite presentation 11. Intra‐uterine growth retardation |
|
| Notes | Location: Mae Hong Son, Thailand Local malaria endemicity/transmission: not reported Local antimalarial drug resistance: multiple‐drug resistance Supervision of treatment: not reported |
|
Coulibaly 2006.
| Methods | Randomized controlled trial Generation of allocation sequence: computer‐generated, random‐number list Allocation concealment: no method reported Blinding: none Inclusion of all randomized participants: 83% (for treatment failure) |
|
| Participants | Number: 147 randomized, 122 analysed Inclusion criteria: primigravidae or secundigravidae; gestation 12 to 36 weeks (fundal height < 30 cm); axillary temperature ≥ 37.5 °C; recent history of fever (within 48 hours preceding enrolment); or presence of clinical malaria‐related symptoms; mono‐infection with P. falciparum density ≥ 2000 parasites/mm3 blood; absence of clinical signs of severe malaria; absence of other patent infections; absence of previous severe reaction to chloroquine or sulfadoxine‐pyrimethamine; staying in neighbouring district/village; able to come for follow up; consent Exclusion criteria: other febrile disease than malaria; use of interfering treatment during follow‐up period; high‐risk pregnancy Age in years (median (range)): 20 (15 to 29) Parity: primigravidae and secundigravidae Early/late pregnancy: second and third trimester Symptomatic/asymptomatic malaria: all women symptomatic Anaemia on admission: 81% (21% severe malaria) |
|
| Interventions | 1. Sulfadoxine‐pyrimethamine: 25 mg/kg sulfadoxine and 1.25 mg/kg pyrimethamine in 1 dose 2. Chloroquine: 10 mg/kg on days 0 and 1; 5 mg/kg on day 2 | |
| Outcomes | 1. Treatment failure at day 14
2. Treatment failure at day 28
3. Adverse reactions Not included in review: 4. Anaemia 5. Parasitaemia 6. Gametocytaemia 7. Early treatment failure 8. Late clinical failure 9. Late parasitological failure |
|
| Notes | Location: Ouagadougou, Burkina Faso Local malaria endemicity/transmission: endemic but seasonal Local antimalarial drug resistance: increasing resistance to chloroquine; and some resistance to sulfadoxine‐pyrimethamine Supervision of treatment: all treatments were supervised Data awaiting: none Additional notes: trial for monitoring of therapeutic efficacy; women who failed study treatment were given quinine |
|
Kalilani 2007.
| Methods | Randomized controlled trial Generation of allocation sequence: random‐number list; block randomized Allocation concealment: sealed envelopes Blinding: outcome assessor Inclusion of all randomized participants: 84% (for treatment failure) |
|
| Participants | Number: 141 randomized, 118 analysed Inclusion criteria: peripheral parasitaemia; P. falciparum; aged 15 to 49 years; estimated fetal gestational age 14 to 26 weeks; mother had felt fetal movement; available for follow up until delivery Exclusion criteria: multiple gestations; history of chronic disease such as tuberculosis or diabetes; mental health disorder; known allergies to drugs containing sulfonamides; macrolides or pyrimethamine; pregnancy complications; taken antimalarial drugs within 28 days before enrolment Age in years (median (interquartile range)): 20 (17 to 24) Parity: mostly primigravidae, some secundigravidae Early/late pregnancy: mainly third trimester Symptomatic/asymptomatic malaria: not stated Anaemia on admission: not stated |
|
| Interventions | 1. Sulfadoxine‐pyrimethamine: 1500 mg sulfadoxine, 75 mg pyrimethamine in 1 dose
2. Sulfadoxine‐pyrimethamine and azithromycin: 1500 mg sulfadoxine, 75 mg pyrimethamine in 1 dose, and 1 g/day azithromycin for 2 days
3. Sulfadoxine‐pyrimethamine and artesunate: 1500 mg sulfadoxine, 75 mg pyrimethamine in 1 dose, and artesunate 200 mg/day for 3 days All treatment courses given twice, at least 4 weeks apart All participants also given 200 mg ferrous sulfate and 0.25 mg folic acid for daily administration, and insecticide‐treated bed nets |
|
| Outcomes | 1. Treatment failure at delivery or 40 days
2. Treatment failure at delivery or 40 days (excludes new infections using PCR)
3. Maternal anaemia
4. Low birthweight
5. Perinatal death
6. Neonatal death Not included in review: 7. Placental parasite clearance |
|
| Notes | Location: Mpemba and Madziabango health centres, Blantyre District, Malawi Local malaria endemicity/transmission: perennial, peaks in rainy season Local antimalarial drug resistance: not reported Supervision of treatment: directly observed Additional notes: pilot for larger study of intermittent preventive treatment, therefore two treatment courses given; trial authors report that statistically significantly more women who received sulfadoxine‐pyrimethamine or azithromycin plus sulfadoxine‐pyrimethamine were diagnosed as HIV positive than those who received artesunate plus sulfadoxine‐pyrimethamine (but many women refused the routine testing) |
|
Mbanzulu 1993.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation Allocation concealment: no method reported Blinding: assessor blinded Inclusion of all randomized participants: 100% (for treatment failure) |
|
| Participants | Number: 132 randomized and analysed Inclusion criteria: at least 18 weeks pregnant; 1000 parasites per mm3; no prior antimalarial; not vomiting; consent Exclusion criteria: none reported Average age in years (mean (range)): not reported; most between 17 and 24 (15 to 40) Parity: 0 to 7; most 0 to 1 Early/late pregnancy: at least 18 weeks Symptomatic/asymptomatic malaria: number not reported Anaemia on admission: number not reported |
|
| Interventions | 1. Chloroquine plus clindamycin (for 3 days)
Chloroquine: 10 mg/kg on days 1 and 2; 5 mg/kg on day 3
Clindamycin: 10 mg/kg for 3 days 2. Chloroquine plus clindamycin (for 5 days) Chloroquine: 10 mg/kg on days 1 and 2; 5 mg/kg on day 3 Clindamycin: 10 mg/kg for 5 days 3. Chloroquine: 10 mg/kg on days 1 and 2; 5 mg/kg on day 3 |
|
| Outcomes | 1. Treatment failures at day 14 2. Adverse events | |
| Notes | Location: Kinshasa, Democratic Republic of the Congo Local malaria endemicity/transmission: not reported Local antimalarial drug resistance: some resistance to chloroquine, amodiaquine, and quinine Supervision of treatment: not reported Data awaiting: authors contacted 2005, awaiting additional trial data |
|
McGready 2000.
| Methods | Randomized controlled trial Generation of allocation sequence: block randomization Allocation concealment: no method reported Blinding: none Inclusion of all randomized participants: 92% (for treatment failure) and 75% (for low birthweight) |
|
| Participants | Number: 115 randomized, 108 analysed (86 and 108 for primary outcomes) Inclusion criteria: pregnant women in their second or third trimester seen at antenatal clinics of Shoklo and Maela camps; microscopy‐confirmed uncomplicated P. falciparum infection; fully informed verbal consent Exclusion criteria: severe complicated malaria; intercurrent infection requiring hospitalization; allergy to quinine or mefloquine; < 12 weeks gestation; history of mental disorder or mefloquine‐induced psychosis Age in years (median (range)): artesunate plus mefloquine group 24 (15 to 37); quinine group 23 (16 to 36) Parity: artesunate plus mefloquine group 18/66 primapara; quinine group 12/42 primapara Early/late pregnancy: second and third trimester Symptomatic/asymptomatic malaria: many women oligosymptomatic or asymptomatic Anaemia on admission: artesunate plus mefloquine group 33/66 anaemic; quinine group 22/42 anaemic |
|
| Interventions | 1. Artesunate plus mefloquine
Artesunate: 4 mg/kg on days 0, 1, and 2
Mefloquine: 15 mg/kg on day 1 and 10 mg/kg on day 2 2. Quinine sulfate: 10 mg/kg every 8 hours for 7 days |
|
| Outcomes | 1. Treatment failure at day 28 (excludes new infections using PCR)
2. Treatment failure at day 63 (excludes new infections using PCR)
3. Abortion
4. Stillbirth
5. Congenital abnormalities
6. Mean birthweight
7. Anaemia and haematocrit
8. Perinatal death
9. Adverse events Not included in review: 10. Treatment failure at 48 hours 11. Gametocyte positivity 12. Person‐gametocyte‐weeks 13. Placental weight 14. Estimated gestational age 15. Infant development |
|
| Notes | Location: Maela and Shoklo camps for displaced people of the Karen ethnic minority on the north‐west border of Thailand Local malaria endemicity/transmission: low and seasonal Local antimalarial drug resistance: multiple‐drug resistance Supervision of treatment: all treatments supervised Data awaiting: authors contacted, awaiting additional trial data Additional notes: Médecins Sans Frontières is the main provider of medicine; treatment failures (up to day 63) were given artesunate for a further 7 days; all mothers requested to deliver at clinic |
|
McGready 2001a.
| Methods | Randomized controlled trial Generation of allocation sequence: no method reported Allocation concealment: no method reported Blinding: none Inclusion of all randomized participants: 71% (for treatment failure) and 83% (for low birthweight) |
|
| Participants | Number: 131 randomized, 129 analysed (93 and 109 for primary outcomes) Inclusion criteria: pregnant women; second or third trimester; seen at antenatal clinics of Shoklo and Maela camps; microscopy‐confirmed uncomplicated P. falciparum infection; consent Exclusion criteria: severe or complicated malaria; intercurrent infections requiring hospitalization; allergy to quinine, artesunate, clindamycin; major liver or kidney disease; gestation < 12 weeks Age in years (median (range)): artesunate group 25 (15 to 41); quinine plus clindamycin group 24 (15 to 41) Parity: artesunate group 26.2% primipara; quinine plus clindamycin group 26.6% primipara Early/late pregnancy: second and third trimester Symptomatic/asymptomatic malaria: many women are oligosymptomatic or asymptomatic Anaemia on admission: number not reported |
|
| Interventions | 1. Artesunate: 2 mg/kg on days 0 to 4; 1 mg/kg on days 5 and 6 2. Quinine plus clindamycin Quinine: 10 mg/kg every 8 hours for 7 days Clindamycin: 5 mg/kg every 8 hours for 7 days | |
| Outcomes | 1. Treatment failure at day 42
2. Treatment failure (excludes new infections using PCR) at day 42
3. Congenital abnormalities
4. Stillbirth
5. Infant mortality
6. Low birthweight and mean birthweight
7. Anaemia
8. Haematocrit Not included in review: 9. Mean placental weight 10. Estimated gestational age at delivery |
|
| Notes | Location: Maela and Shoklo camps for displaced people of the Karen ethnic minority on the north‐west border of Thailand Local malaria endemicity/transmission: low and seasonal Local antimalarial drug resistance: multiple‐drug resistance Supervision of treatment: all treatments were supervised Data awaiting: authors contacted 2005, awaiting additional trial data Additional notes: Médecins Sans Frontières main provider of medicine; treatment given orally with a small amount of sugar and water; women asked to deliver at clinic (although usually deliver at home) |
|
McGready 2005.
| Methods | Randomized controlled trial Generation of allocation sequence: computer generated in blocks of 10 Allocation concealment: envelopes Blinding: outcome assessor Inclusion of all randomized participants: 99% (for treatment failure) |
|
| Participants | Number: 81 randomized, 80 analysed Inclusion criteria: healthy; first episode of (uncomplicated) falciparum or mixed malaria detected by weekly screening; haematocrit level ≥ 20%; second (> 13 weeks) or early third (< 32 weeks) trimester of pregnancy Exclusion criteria: known chronic disease; inability to follow antenatal clinic consultation; history of alcohol abuse; imminent delivery; inability to tolerate oral treatment Age in years (mean (standard deviation)): 26 (7) vs 26 (6) Parity: just under 1/3 primigravida Early/late pregnancy: second and third trimester Symptomatic/asymptomatic malaria: detected by screening so likely to be asymptomatic Anaemia on admission: not severe anaemia |
|
| Interventions | 1. Quinine sulfate: 10 mg/kg every 8 hours for 7 days 2. Atovaquone‐proguanil plus artesunate Atovaquone‐proguanil: fixed‐dose tablet (atovaquone 20 mg/kg and primaquine 8 mg/kg) once a day for 3 days Artesunate: 4 mg/kg once a day for 3 days | |
| Outcomes | 1. Treatment failure at day 63
2. Treatment failure at day 63 (excludes new infections using PCR)
3. Fever clearance time
4. Low birthweight and mean birthweight
5. Anaemia (and severe anaemia)
6. Prematurity and estimated gestational age at delivery
7. Intra‐uterine growth retardation
8. Congenital abnormality
9. Tinnitus Not included in review: 10. Stillbirth 11. Infant death in first 12 months (neonatal) 12. Total infant developmental score at 12 months 13. Vomiting drugs 14. Urticaria |
|
| Notes | Location: Maela and Shoklo camps for displaced people of the Karen ethnic minority on the north‐west border of Thailand Local malaria endemicity/transmission: low and seasonal Local antimalarial drug resistance: multiple‐drug resistance (only artemisinin therapies known to be effective) Supervision of treatment: all treatments supervised Additional notes: treatment given orally with sugar and water (quinine) or chocolate milk (atovaquone proguanil plus artesunate); any women with reappearance of parasites after the primary treatment were retreated with artesunate and clindamycin for 7 days |
|
Nosten 1993b.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: paired, restricted, sequential trial Allocation concealment: no method reported Blinding: none Inclusion of all randomized participants: 74.4% (for treatment failure) |
|
| Participants | Number: 43 randomized, 32 analysed Inclusion criteria: uncomplicated P. falciparum malaria; second or third trimester; fully informed consent Exclusion criteria: none reported Average age in years (mean (standard devation)): quinine plus spiramycin group 26 (6.2); quinine plus placebo group 28 (6.8) Parity (median): quinine plus spiramycin group 2; quinine plus placebo group 4 Early/late pregnancy in gestation weeks (median): second and third trimester Symptomatic/asymptomatic malaria: many women oligosymptomatic or asymptomatic Anaemia on admission: number not reported |
|
| Interventions | 1. Quinine plus spiramycin
Quinine sulfate salt: 30 mg/kg every day for 5 days
Spiramycin: 2 "Miu" 3 times a day for 5 days 2. Quinine plus placebo Quinine sulfate salt: 30 mg/kg every day for 5 days |
|
| Outcomes | 1. Treatment failure at day 28 2. Parasite clearance time 3. Abortion 4. Adverse events | |
| Notes | Location: Shoklo camp, Thai‐Burmese border Local malaria endemicity/transmission: not reported Local antimalarial drug resistance: multiple‐drug resistance (unsupervised 7‐day quinine treatment has a failure rate of 50% in pregnant women with uncomplicated malaria) Supervision of treatment: supervised Data awaiting: authors contacted, awaiting additional trial data Additional notes: despite high resistance, quinine is the only treatment available for pregnant women in the area; quinine was given as a supervised 5‐day course as this is the average actual intake when the standard 7‐day regimen is not supervised (usual practice) |
|
Sowunmi 1998a.
| Methods | Randomized controlled trial Generation of allocation sequence: no method reported Allocation concealment: no method reported Blinding: none Inclusion of all randomized participants: 100% (for treatment failure) |
|
| Participants | Number: 55 randomized, 55 analysed Inclusion criteria: non‐response to chloroquine or sulfadoxine‐pyrimethamine, or both, as shown by failure of complete clearance of parasitaemia in the 2 weeks following a course of treatment; oral fluid intolerance; no history of allergy to known antimalarial drugs; second or third trimester pregnancy; consent of patients Exclusion criteria: none reported Average age in years (mean (standard deviation; range)): artemether plus mefloquine group 29.9 (6.1; 21 to 41); artemether group 28.9 (5.1; 20 to 40) Parity: mostly primigravidae (29/45) Early/late pregnancy: second and third trimester Symptomatic/asymptomatic malaria: symptomatic Anaemia on admission: number not reported |
|
| Interventions | 1. Artemether plus mefloquine
Artemether: 3.2 mg/kg intramuscularly on day 0
Mefloquine: 7.5 mg/kg on days 1 and 2 2. Artemether: 3.2 mg/kg intramuscularly on day 0; 1.6 mg/kg daily for next 4 days |
|
| Outcomes | 1. Treatment failure at day 14 (for artemether plus mefloquine group) and day 28 (for artemether group)
2. Fever clearance time
3. Parasite clearance time
4. Stillbirth
5. Mean birthweight
6. Congenital (physical) abnormalities
7. Neonatal malaria
8. Haematocrit
9. Adverse events Not included in review: 10. Intra‐uterine growth retardation 11. Icterus in newborns 12. Infant development |
|
| Notes | Location: Nigeria Local malaria endemicity/transmission: not reported Local antimalarial drug resistance: multiple‐drug resistance (artemether and mefloquine resistance low, no artemisinin―mefloquine cross‐resistance) Supervision of treatment: supervised |
|
Tagbor 2006.
| Methods | Randomized controlled trial Generation of allocation sequence: randomized‐number list in blocks of 16 Allocation concealment: envelopes Blinding: participant, treatment provider, outcome assessor, and data analyst Inclusion of all randomized participants: 93% (for treatment failure) |
|
| Participants | Number: 900 randomized; 838 analysed Inclusion criteria: pregnant; gestational age ≥16 weeks; attended antenatal clinic between March 2003 and September 2004; with peripheral blood parasitaemia Exclusion criteria: multiple pregnancy; severe malaria; enrolled previously in the current study Age in years: roughly 23 Parity: all parities, about half primigravida Early/late pregnancy: 16 weeks plus, second and third trimester Symptomatic/asymptomatic malaria: detected by screening so likely to be asymptomatic Anaemia on admission: number not reported |
|
| Interventions | 1. Chloroquine: 600 mg on days 1 and 2; 300 mg on day 3 2. Amodiaquine: 600 mg on days 1 and 2; 300 mg on day 3 3. Sulfadoxine‐pyrimethamine Sulfadoxine: single dose 1500 mg Pyrimethamine: single dose 75 mg 4. Amodiaquine plus sulfadoxine‐pyrimethamine Amodiaquine: 600 mg on days 1 and 2; 300 mg on day 3 Sulfadoxine: single dose 1500 mg on day 1 Pyrimethamine: single dose 75 mg on day 1 |
|
| Outcomes | 1. Treatment failure at day 14
2. Treatment failure at day 28
3. Treatment failure at day 28 (excludes new infections using PCR)
4. Placental parasitaemia
5. Low birthweight
6. Preterm delivery
7. Adverse events Not included in review: 8. Placental parasite clearance 9. Birthweight 10. Abortion 11. Stillbirth 12. Perinatal death 13. Neonatal death 14. Congenital abnormality |
|
| Notes | Location: St Theresa's Hospital, Nkoranza, Ghana Local malaria endemicity/transmission: perennial (peaks in rainy season – July and August) Local antimalarial drug resistance: unclear Supervision of treatment: first dose supervised (given by study team) second and third doses taken at home |
|
HIV: human immunodeficiency virus; P. falciparum: Plasmodium falciparum; PCR: polymerase chain reaction.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Deen 2001 | Participants not diagnosed with malaria |
| Keuter 1990 | Nonrandomized study |
| McGready 2001b | Nonrandomized study |
| Naing 1998 | Nonrandomized study |
| Ndyomugyenyi 2000 | No control group |
| Sowunmi 1998b | No control group |
| Steketee 1996 | Some participants not parasitaemic |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN86353884.
| Trial name or title | "Co‐Artemether in pregnancy ‐ a pilot study (Thailand)" |
| Methods | Randomized controlled trial |
| Participants | Inclusion criteria: pregnant women with uncomplicated falciparum or mixed infection in second or third trimester who have failed after a course of quinine for 7 days; attend the Shoklo Malaria Research Unit AnteNatal Clinics regularly; agree to deliver at the Shoklo Malaria Research Unit Exclusion criteria: splenectomy; known chronic disease (cardiac, renal, hepatic); known haemoglobinopathy; known hepatic or renal impairment; inability to follow AnteNatal Clinics consultation; history of alcohol or narcotic abuse; inability to tolerate oral treatment; severe and complicated malaria; known hypersensitivity to artemisinin derivatives; taking any drug inhibiting the cytochrome enzyme CYP3A4 or drug that is metabolized by cytochrome enzyme CYPD or family; history of sudden death or of prolongation of QTc interval on electrocardiogram; cardiac arrytyhmia, congestive cardiac failure, or bradycardia accompanied by reduced left ventricular function; intake of drugs that prolong QTc interval |
| Interventions | 1. Artesunate: 50 mg tablets (2 mg/kg/day) for 7 days 2. Co‐artemether (20/120 mg artemether/lumefantrine): 4 tablets twice a day for 3 days with 200 mL chocolate milk at each dose |
| Outcomes | 1. PCR‐adjusted parasitological cure at day 42 or at delivery, depending on which occurs last 2. Gametocyte carriage 3. Pharmaokinetic parameters 4. Histopathology examination (presence of parasites, pigments, monocytes infiltrations, and other placental changes) of the placenta |
| Starting date | 6 February 2004 Anticipated end date: 1 January 2008 |
| Contact information | Dr Melba Gomes (gomesm@who.int), World Health Organization, Switzerland |
| Notes | Location: Thailand Registration number: ISRCTN86353884 Source of funding: NICEF/UNDP/World Bank/WHO – Special Programme for Research and Training in Tropical Diseases (TDR) |
PCR: polymerase chain reaction.
Differences between protocol and review
When we prepared the first version of the review (Orton 2005), we made some changes to the protocol. We specified the inclusion of quasi‐randomized controlled trials, split the outcome measures into "maternal treatment response", "maternal adverse events", and "fetal outcomes" for added clarity, and excluded placental infection as an outcome measure because it is not a measure of treatment response. We also updated the methods for assessing blinding, changed the phrase "loss to follow up" to "inclusion of all randomized participants in the analysis" to reflect changes within the Cochrane Infectious Diseases Group, and decided to use only the chi‐squared test for heterogeneity (and not the I2).
Contributions of authors
Lois Orton assessed eligibility, extracted data, and wrote the review. For the review update, Aika Omari assessed eligibility, extracted data, and helped write the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Department for International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Bounyasong 2001 {published data only}
- Bounyasong S. Randomized trial of artesunate and mefloquine in comparison with quinine sulphate to treat P. falciparum malaria pregnant women. Journal of the Medical Association of Thailand 2001;84(9):1289‐98. [PubMed] [Google Scholar]
Coulibaly 2006 {published data only}
- Coulibaly SO, Nezien D, Traore S, Kone B, Magnussen P. Therapeutic efficacy of sulphadoxine‐pyrimethamine and chloroquine for the treatment of uncomplicated malaria in pregnancy in Burkina Faso. Malaria Journal 2006;5:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalilani 2007 {published data only}
- Kalilani L, Mofolo I, Chaponda M, Rogerson SJ, Alker AP, Kwiek JJ, et al. A randomized controlled pilot trial of azithromycin or artesunate added to sulphadoxine‐pyrimethamine as treatment for malaria in pregnant women. PLos ONE 2007;11:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mbanzulu 1993 {published data only}
- Mbanzulu PN, Tona L, Nekwei W, Kobota V, Kisile M, Makengo M. Management of malaria during pregnancy in Kinshasa (Zaire) by chloroquine and clindamycin [Traitment du paludisme de la femme enceinte a Kinshasa (Republique democratique du Congo) par la chloroquine et la clinadmycine]. Revue Francaise de Gynecologie et d'Obstetrique 1998;93(6):433‐7. [Google Scholar]
McGready 2000 {published data only}
- McGready R, Brockman A, Cho T, Cho D, Vugt M, Luxemburger C, et al. Randomized comparison of mefloquine‐artesunate versus quinine in the treatment of multidrug‐resistant falciparum malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(6):689‐93. [DOI] [PubMed] [Google Scholar]
McGready 2001a {published data only}
- McGready R, Cho T, Samuel, Villegas L, Brockman A, Vugt M, et al. Randomized comparison of quinine‐clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(6):651‐6. [DOI] [PubMed] [Google Scholar]
McGready 2005 {published data only}
- McGready R, Ashley EA, Moo E, Cho T, Barends M, Hutagalung R, et al. A randomized comparison of artesunate‐atovaquone‐proguanil versus quinine in treatment for uncomplicated falciparum malaria during pregnancy. Journal of Infectious Diseases 2005;192(5):846‐53. [DOI] [PubMed] [Google Scholar]
Nosten 1993b {published data only}
- Nosten F, ter Kuile F, Thwai KL, Maelankirri L, White NJ. Spriamycin does not potentiate quinine treatment of falciparum malaria in pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 1993;87(3):305‐6. [DOI] [PubMed] [Google Scholar]
Sowunmi 1998a {published data only}
- Sowunmi A, Oduola AMJ, Ogundahunsi OAT, Fehintola FA, Ilesanmi OA, Akinyinka OO, et al. Randomised trial of artemether versus artemether and mefloquine for the treatment of chloroquine/sulphadoxine‐pyrimethamine‐resistant falciparum malaria during pregnancy. Journal of Obstetrics and Gynaecology 1998;18(4):322‐7. [DOI] [PubMed] [Google Scholar]
Tagbor 2006 {published data only}
- Tagbor H, Bruce J, Browne E, Randal A, Greenwood B, Chandramohan D. Efficacy, safety, and tolerability of amodiaquine plus sulphadoxine‐pyrimethamine used alone or in combination for malaria treatment in pregnancy: a randomised trial. Lancet 2006;368(9544):1349‐56. [DOI] [PubMed] [Google Scholar]
- Tagbor H, Bruce J, Ord R, Randall A, Browne E, Greenwood B, et al. Comparison of the therapeutic efficacy of chloroquine and sulphadoxine‐pyremethamine in children and pregnant women. Tropical Medicine and International Health 2007;12(11):1288‐97. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Deen 2001 {published data only}
- Deen JL, Seidlein L, Pinder M, Walraven GEL, Greenwood BM. The safety of the combination artesunate and pyrimethamine‐sulphadoxine given during pregnancy. Transactions of the Royal Society of Tropical Medicine and Hygiene 2001;95(4):424‐8. [DOI] [PubMed] [Google Scholar]
Keuter 1990 {published data only}
- Keuter M, Eijk A, Hoogstrate M, Raasveld M, Ree M, Ngwawe WA, et al. Comparison of chloroquine, pyrimethamine and sulfadoxine, and chloroproguanil and dapsone as treatment for falciparum malaria in pregnant and non‐pregnant women, Kakamega District, Kenya. BMJ 1990;301(6750):466‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGready 2001b {published data only}
- McGready R, Cho T, Keo NK, Thwai KL, Villegas L, Looaresuwan S, et al. Artemisinin antimalarials in pregnancy: a prospective treatment study of 539 episodes of multidrug‐resistant Plasmodium falciparum. Clinical Infectious Diseases 2001;33(12):2009‐16. [DOI] [PubMed] [Google Scholar]
Naing 1998 {published data only}
- Naing T, Win H, Nwe YY. Falciparum malaria and pregnancy: relationship and treatment response. Southeast Asian Journal of Tropical Medicine and Public Health 1998;19(2):253‐8. [PubMed] [Google Scholar]
Ndyomugyenyi 2000 {published data only}
- Ndyomugyenyi R, Magnussen P. Chloroquine prophylaxis, iron/folic‐acid supplementation or case management of malaria attacks in primigravidae in western Uganda: effects on congenital malaria and infant haemoglobin concentrations. Annals of Tropical Medicine and Parasitology 2000;94(8):759‐70. [DOI] [PubMed] [Google Scholar]
Sowunmi 1998b {published data only}
- Sowunmi A, Ilesanmi AO, Oduloa AMJ, Omitowoju GO, Ojengbede OA. Efficacy of mefloquine in uncomplicated chloroquine‐resistant falciparum malaria during pregnancy. Journal of Obstetrics and Gynaecology 1998;16(5):362‐3. [Google Scholar]
Steketee 1996 {published data only}
- Steketee RW, Wirima JJ, Hightower AW, Slutsker L, Heymann DL, Breman JG. The effect of malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:33‐41. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker L, Breman JG, Heymann DL. Comparability of treatment groups and risk factors for parasitemia at the first antenatal clinic visit in a study of malaria treatment and prevention in pregnancy in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:17‐23. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker L, Khoromana CO, Heymann DL, Breman JG. Malaria treatment and prevention in pregnancy: indications for use and adverse events associated with use of chloroquine and mefloquine. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:50‐6. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker L, Roberts JM, Khoromana O, Heymann DL. Malaria parasite infection during pregnancy and at delivery in mother, placenta, and newborn: efficacy of chloroquine and mefloquine in rural Malawi. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:24‐32. [DOI] [PubMed] [Google Scholar]
- Steketee RW, Wirima JJ, Slutsker WL, Khoromana CO, Breman JG, Heymann DL. Objectives and methodology in a study of malaria treatment and prevention in pregnancy in rural Malawi: The Mangochi Malaria Research Project. American Journal of Tropical Medicine and Hygiene 1996;55 Suppl 1:8‐16. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN86353884 {published data only}
- ISRCTN86353884. Co‐Artemether in pregnancy ‐ a pilot study (Thailand). www.controlled‐trials.com/ISRCTN86353884 (accessed February 2008).
Additional references
Adjuik 2004
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N, International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta‐analysis. Lancet 2004;363(9402):9‐17. [DOI] [PubMed] [Google Scholar]
Brabin 2001
- Brabin BJ, Rogerson SJ. The epidemiology and outcomes of maternal malaria. In: Duffy PE, Fried M editor(s). Malaria in pregnancy: deadly parasite, susceptible host. London and New York: Taylor & Francis, 2001:27‐52. [Google Scholar]
Clyde 1981
- Clyde DF. Clinical problems associated with the use of primaquine as a tissue schizontocidal and gametocytocidal drug. Bulletin of the World Health Organization 1981;59(3):391‐5. [PMC free article] [PubMed] [Google Scholar]
Duffy 2001
- Duffy PE. Immunity to malaria during pregnancy: different host, different parasite. In: Duffy PE, Fried M editor(s). Malaria in pregnancy: deadly parasite, susceptible host. London: Taylor & Francis, 2001:71‐127. [Google Scholar]
Gamble 2006
- Gamble C, Ekwaru JP, ter Kuile FO. Insecticide‐treated nets for preventing malaria in pregnancy. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003755.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garner 2006
- Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD000169.pub2] [DOI] [PubMed] [Google Scholar]
Guerin 2002
- Guerin PJ, Olliaro P, Nosten F, Druilhe P, Laxminarayan R, Binka F, et al. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infectious Diseases 2002;2(9):564‐73. [DOI] [PubMed] [Google Scholar]
Hoffman 1992
- Hoffman SL. Diagnosis, treatment, and prevention of malaria. Medical Clinics of North America 1992;76(6):1327‐55. [DOI] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Lindsay 2000
- Lindsay S, Ansell J, Selman C, Cox V, Hamilton K, Walraven G. Effect of pregnancy on exposure to malaria mosquitoes. Lancet 2000;355(9219):1972. [DOI] [PubMed] [Google Scholar]
Menendez 2000
- Menendez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, Kahigwa E, et al. The impact of placental malaria on gestational age and birth weight. Journal of Infectious Diseases 2000;181(5):1740‐5. [DOI] [PubMed] [Google Scholar]
Nosten 1993a
- Nosten F, ter Kuile FO, Luxemberger C, Woodrow C, Kyle DE, Chongsuphajaisiddhi T, et al. Cardiac effects of antimalarial treatment with halofantrine. Lancet 1993;341(8852):1054‐6. [DOI] [PubMed] [Google Scholar]
Nosten 1999
- Nosten F, Vincenti M, Simpson J, Yei P, Thwai KL, Vries A, et al. The effects of mefloquine treatment in pregnancy. Clinical Infectious Diseases 1999;28(4):808‐15. [DOI] [PubMed] [Google Scholar]
Nosten 2001
- Nosten F, McGready R. The treatment of malaria in pregnancy. In: Duffy PE, Fried M editor(s). Malaria in pregnancy: deadly parasite, susceptible host. London: Taylor & Francis, 2001:223‐41. [Google Scholar]
RBM 2003
- Global Partnership to Roll Back Malaria, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases. Assessment of the safety of artemisinin compounds in pregnancy: report of two informal consultations convened by WHO in 2002. Geneva: World Health Organization, 2003. [Google Scholar]
RBM 2005a
- Global Partnership to Roll Back Malaria. World malaria report: 2005. Geneva: World Health Organization, 2005. [Google Scholar]
RBM 2005b
- Roll Back Malaria (RBM) Partnership. Global Strategic Plan 2005‐2015. www.rollbackmalaria.org/forumV/docs/gsp_en.pdf November 2005 (accessed 1 May 2008).
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Steketee 2001
- Steketee RE, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria‐endemic areas. American Journal of Tropical Medicine and Hygiene 2001;64 Suppl 1‐2:28‐35. [DOI] [PubMed] [Google Scholar]
Stevens 2000
- Stevens RD. Anaemia ‐‐ the scourge of the Third World. Health Millions 2000;26(2):21‐3. [PubMed] [Google Scholar]
Taylor 2004
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Safety 2004;27(1):25‐61. [DOI] [PubMed] [Google Scholar]
White 1999
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Mrash K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
WHO 2003a
- World Health Organization, Global Partnership to Roll Back Malaria. The Abuja Declaration and the plan of action: an extract from the African Summit on Roll Back Marlaria, Abuja, 25 April 2000 [WHO/CDS/RBM/2000.17]. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2003b
- World Health Organization. Lives at risk: malaria in pregnancy. www.who.int/features/2003/04b/en (accessed December 2004).
WHO 2003c
- Global Partnership to Roll Back Malaria. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparium malaria. Geneva: World Health Organization, 2003. [Google Scholar]
WHO 2004a
- World Health Organization, Regional Office for Africa. A strategic framework for malaria prevention and control during pregnancy in the African region. Brazzaville: World Health Organization, 2004. [Google Scholar]
WHO 2004b
- World Health Organization, Regional Office for Africa. Malaria control in Africa: progress report on implementation of the plan of action of the Abuja Declaration. Brazzaville: WHO Regional Office for Africa, 2004. [Google Scholar]
WHO 2006
- World Health Organization Roll Back Malaria Department. Guidelines for the treatment of malaria [WHO/HTM/MAL/2006.1108]. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2007
- World Health Organization Global Malaria Programme. Anti‐malarial drug policies: AFRO, AMRO, EMRO, EURO, SEARO, WPRO. www.who.int/malaria/treatmentpolicies.html Updated April 2007 (accessed August 2007).
References to other published versions of this review
Orton 2005
- Orton L, Garner P. Drugs for treating uncomplicated malaria in pregnant women. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004912.pub2] [DOI] [PubMed] [Google Scholar]


