Abstract
Background
Childhood tuberculosis (TB) is a neglected global public health problem. Short treatment courses with rifampicin‐containing anti‐TB drugs given daily for six‐months cure over 90% of infected children, but poor adherence reduces treatment success. Intermittent, short‐course anti‐TB regimens, given two or three times a week under direct observation, are associated with higher adherence in observational studies; but how they compare with daily treatment in relation to cure is unclear. Current international and national recommendations differ on use of intermittent regimens to treat TB in children.
Objectives
To compare the efficacy and safety of intermittent, short‐course anti‐TB regimens (twice‐ or thrice‐weekly) with daily short‐course anti‐TB regimens in treating childhood TB.
Search methods
We searched the Cochrane Infectious Disease Group Specialized Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS, clinical trials registries, regional databases, conference proceedings, and references without language restrictions up to 30 May 2013; and contacted experts for relevant published, unpublished, and on‐going trials.
Selection criteria
Randomized controlled trials (RCTs) and quasi‐RCTs of children aged 15 years or younger, diagnosed with TB (according to the World Health Organization diagnostic categories 1, 2, or 3), who were treated with intermittent twice‐weekly or thrice‐weekly, short‐course anti‐TB regimens compared to daily short‐course anti‐TB treatment regimens. All regimens had to contain rifampicin for at least the first two months.
Data collection and analysis
The review authors independently screened and selected trials, assessed risk of bias, and extracted data. We sought clarifications from trial authors. We pooled relative risks with their 95% confidence intervals and used a random‐effects model where there was significant heterogeneity. We assessed overall evidence‐quality using the GRADE approach.
Main results
We included four trials published between 1996 to 2000 that randomized 563 children (465 evaluable) aged five months to 15 years to intermittent twice‐weekly versus daily anti‐TB treatment. Two trials were from India, one from South Africa, and one from Turkey. All trials used rifampicin and isoniazid, three trials used pyrazinamide, and one trial used streptomycin. The drug combination, and the duration of intermittent and daily treatments differed between trials, and no trials used drug combinations and schedules currently recommended for childhood TB. No trial reported if any child was HIV‐positive.
In comparisons of twice‐weekly versus daily anti‐TB treatment regimens, the trials did not detect differences in the number of patients cured, but trials were small, and the comparator regimens were not standard (four trials, 465 children; very low quality evidence). Trials were underpowered to provide estimates for death (two trials, 213 participants, very low quality evidence), relapse (one trial, 214 participants,very low quality evidence), and treatment limiting adverse events (four trials, 441 participants, very low quality evidence)
Reported adherence to treatment was similar (87% versus 84%; four trials, 458 children, very low quality evidence)
We did not find trials comparing the commonly used thrice‐weekly anti‐TB short‐course regimen with the daily treatment regimen.
Authors' conclusions
Trials conducted to date are insufficient to support or refute the use of intermittent twice‐ or thrice‐weekly, short‐course treatment regimens over daily short‐course treatment in children with TB. Further randomized trials conducted in high TB‐transmission settings will help inform policy and practice.
23 April 2019
No update planned
Research area no longer active
No longer an active research area.
Plain language summary
Twice‐ or thrice‐weekly doses versus daily doses of drugs to treat tuberculosis in children
About half a million children are diagnosed with tuberculosis (TB) every year, usually infecting the lungs, but also other organs of the body, and can cause meningitis. Infection in children is relatively common, and so establishing effective drug regimens that are easy to take and monitor is important.
TB drug regimens are standardised globally, and include a combination of drugs given daily for six months. More than 95% of children are cured with this treatment. Giving anti‐TB drugs twice‐ or thrice‐weekly is more convenient to supervise than daily treatment but may not be as effective as daily treatment in curing children of TB. The World Health Organization currently recommends only daily treatments, but some national governments recommend twice‐ or thrice‐weekly doses for children with TB.
In this Cochrane review, the review authors compared children given intermittent anti‐TB treatment to those given daily treatment. They examined the evidence up to 30 May 2013 and included four randomized trials that compared twice‐weekly treatment with daily doses of anti‐TB drugs, but none evaluated thrice‐weekly dosing. The four trials included 563 children aged five months to 15 years, not known to be resistant to TB drugs. The trials were published over 12 years ago and the regimens used are not those currently recommended.
The trials were small, and did not detect a difference between twice‐weekly or daily treatment in the number of children who were cured, died, relapsed, reported taking most or all of the drugs, or had adverse effects. Whether regimens of drugs two or three times a week are as good as regimens with daily doses remains unclear, as the evidence base to date is small, and the regimens tested are not the same as currently currently recommended drug combinations.
Summary of findings
Summary of findings for the main comparison. Intermittent short‐course anti‐TB regimens compared to daily anti‐TB regimens for treating TB in children.
| Intermittent short‐course anti‐TB regimens compared to daily anti‐TB regimens for treating TB in children with TB | ||||||
| Patient or population: Children with TB1 Intervention: Intermittent short‐course twice‐weekly anti‐TB regimens (six to nine months) Comparison: Daily anti‐TB regimens (six to 12 months) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Daily anti‐TB regimens | Intermittent short‐course anti‐TB regimens | |||||
|
Cure Follow‐up: 12 to 30 months |
836 per 1000 | 844 per 1000 (786 to 920) | RR 1.01 (0.94 to 1.1) | 465 (4 trials) | ⊝⊝⊝⊝ very low2,3,4,5 | |
| Death from any cause | 8 per 1000 | 13 per 1000 (2 to 75) | RR 1.52 (0.26 to 8.96) | 213 (2 trials)6 | ⊝⊝⊝⊝ very low,3,7,8,9 | |
|
Relapse Follow‐up: 12 to 30 months |
0 per 1000 | 0 per 1000 (0 to 0) | RR 3.68 (0.15 to 89.33) | 214 (1 trial)10 | ⊝⊝⊝⊝ very low11,12,13 | |
| Adherence to treatment | 840 per 1000 | 874 per 1000 (815 to 932) | RR 1.04 (0.97, 1.11) | 458 (4 trials) | ⊝⊝⊝⊝ very low3,4,14,15 | |
| Treatment‐limiting adverse events | 15 per 1000 | 6 per 1000 (1 to 39) | RR 0.4 (0.06 to 2.6) | 441 (4 trials) | ⊝⊝⊝⊝ very low2,3,4,16 | |
| *The basis for the assumed risk is the median control group risk across studies for pooled data and the control group risk for data from single studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The data in this table are from Kumar (India) 1990; Kansoy (Turkey) 1996; Ramachandran (India) 1998; and Te Water Naude (South Africa) 2000. 2 Downgraded by 1 for serious risk of bias: None of the trials were free of risk of bias. The trial that contributed the most weight (43%) to the pooled effect estimates (Te Water Naude (South Africa) 2000) was at high risk of selection bias. 3 No serious inconsistency: Statistical heterogeneity was low. 4 Downgraded by 1 for serious indirectness: Two of the trials (Kansoy (Turkey) 1996; Ramachandran (India) 1998) used a longer duration of treatment in the daily treatment arms than in the intermittent arms, and the intermittent arms in Kansoy (Turkey) 1996 and Ramachandran (India) 1998 used non‐standard regimens. None used drug combinations and schedules currently recommended for childhood TB. 5 Downgraded by 1 for serious imprecision: The 95% CI of the effect estimate indicated only non‐appreciable benefit with both interventions, but the sample size was smaller than the optimal information size for equivalence. 6 Data for deaths are from only two of the four trials as no deaths were reported in Kansoy (Turkey) 1996 and Te Water Naude (South Africa) 2000. 7 Downgraded by 1 for serious risk of bias: The trials were either unclear or at high risk of selection bias. 8 Downgraded by 1 for serious indirectness: Neither control group used drug combinations and schedules currently recommended for childhood TB. 9 Downgraded by 1 for serious imprecision: The number of deaths was very few, and the 95% CI for the risk difference are wide.
10 Data for relapse are from only one trial (Te Water Naude (South Africa) 2000) as no participant was reported to have had a confirmed relapse in Kansoy (Turkey) 1996; Kumar (India) 1990; and Ramachandran (India) 1998 over one to five years of follow‐up. 11 No serious study limitations: Only one relapse was reported with intermittent treatment in Te Water Naude (South Africa) 2000. This trial was at high risk of selection bias, but this is unlikely to have introduced bias in relapse estimates. 12 Downgraded by 1 for serious indirectness: The data for relapse comes from only one trial conducted in South Africa nearly 20 years ago and may not generalize to other settings today.
13Downgraded by 2 for serious imprecision: Only one child relapsed and the upper and lower limits of the 95% CI indicate appreciable benefits for both interventions with so significant differences. 14 Downgraded by 1 for serious risk of bias: The trials were open label in design and assessments of adherence were not done blind to treatment allocation The four trials used different methods to assess adherence and different definitions to define those adherent. 15 Downgraded by 1 for serious imprecision: The 95% CI of the pooled effect estimates indicated that the interventions did not appreciably increase adherence. 16 Downgraded by 1 for serious imprecision: Adverse events were infrequent and the 95% CI of the absolute risk difference indicated a non‐appreciable difference in the risk of adverse events requiring treatment interruptions with intermittent and with daily treatments.
Background
Description of the condition
Childhood tuberculosis (TB) is a major public health problem that was not considered a priority, (in public health, as authors we think the statement is clear as it is) due to difficulties in accurate diagnosis and a lack of reliable data on its prevalence (Walls 2004; Brent 2008). The World Health Organization (WHO) estimates that there were between 470,000 to 510,000 new cases of childhood TB worldwide in 2011, equivalent to approximately 6% of the global 8.3 to nine million new adult TB cases (WHO 2012). Also, there were 64,000 deaths among HIV‐negative children less than 15 years old (WHO 2012). Around 75% of the global incidence of childhood TB occurs in 22 high‐burden countries (Corbett 2003). India and China are the countries with the highest TB burden and together contribute nearly 40% of the global incidence of TB annually (WHO 2012).
Prevalence of childhood TB varies between countries. In industrialized countries it comprises a mere 5% of the total TB burden; in low‐income countries this proportion is as high as 40%; and in some of these countries childhood TB incidence is 50% of the adult TB incidence (Beyers 1996; Nelson 2004; IIPS 2007; Marais 2010). Even high‐income countries have been unable to match the decline in childhood TB incidence to that achieved in adult TB (CDC 1995). In fact, the incidence of childhood TB in both Europe and North America has recently increased, largely due to immigration from endemic areas (Magdorf 2008; Newton 2008).
Reports on the burden of TB generated by different countries rely on diagnosis by expectorated sputum stains that are positive for acid fast Mycobacterium tuberculosis bacilli (using special stains). However, smears are not routinely done in children in many countries with a high burden of TB; younger children rarely produce sputum, and childhood TB is largely sputum smear‐negative, resulting in an under‐estimation of prevalence (Shingadia 2003; Newton 2008; WHO 2012).
Diagnosis of childhood TB
Diagnosis of childhood TB is difficult because of the lack of specificity of diagnostic criteria. Unlike adult TB, where sputum‐positivity confirms the diagnosis, it is difficult to obtain sputum from children and many centres do not have facilities for bacterial culture after gastric aspiration (gastric lavage). Sputum microscopy is positive in under 10% to 15% of children with probable TB and under 30% to 40% of sputum cultures are positive (Marais 2007). While this could contribute to underestimation of childhood TB prevalence, the non‐specific nature of clinical diagnostic criteria might also result in an over‐diagnosis of TB, with the possibility that around 15% to 20% of the children diagnosed in high‐incidence communities may not have TB (Nelson 2004).
The WHO recommends that TB diagnosis is based on careful history, clinical examination, tuberculin skin testing, and, wherever possible, bacterial confirmation (Stop TB Partnership 2006a; WHO 2006b). The symptoms indicative of childhood TB are chronic cough for over 21 days, fever for more than 14 days (after excluding malaria or other causes of pneumonia), loss of weight, and failure to gain weight (WHO 2006b).
Description of the intervention
The recommended treatment for childhood TB is divided into two phases: an intensive phase and a continuation phase (Appendix 1). The intensive phase aims to help eliminate the majority of M. tuberculosis bacteria and prevent any emerging drug resistance; in the continuation phase the aim is to eradicate the dormant bacteria (Stop TB Partnership 2006b; WHO 2006b).
Short‐course treatments given daily or intermittently (twice‐ or thrice‐weekly) over six months to one year are commonly used in national TB control programs, and are based on treatment regimens in adults due to convenience in implementation (Graham 2004). Over 95% of childhood TB cases can be successfully treated with short‐course treatments, but rates of successful treatment are often much lower in low‐income countries, due to poorer compliance and treatment completion rates, delayed diagnosis, wrong diagnoses, co‐infection with HIV, poor absorption of anti‐TB drugs in malnourished or HIV‐infected children, and also due to drug resistance (Graham 2004). The potential for drug resistance with intermittent regimens is also a matter of concern in studies in adults. There is a lower risk of children with TB developing drug resistance as childhood TB is mostly paucibacillary (involves few bacilli) (Donald 2007a), and it is thought that children do not contribute to the transmission of drug‐resistant TB (Marais 2010). However, drug resistance in adult populations in some parts of the world is rising, and drug resistance in children reflects the community transmission of drug‐resistant strains from adults (Newton 2008; Schaaf 2009). Recent studies have demonstrated the potential for drug‐resistant TB to be transmitted within families by infected children (Seddon 2012).
Drugs used for childhood TB
Drugs used to treat children with TB are isoniazid, rifampicin, pyrazinamide, ethambutol, and streptomycin. Corticosteroids (prednisolone) are recommended for all children with TB meningitis. The WHO recommends that TB in children infected with HIV should be treated as in those without HIV infection: with a six‐month regimen (Stop TB Partnership 2006b; WHO 2006b). Rifampicin and isoniazid are bactericidal drugs, with isoniazid being the most potent drug, killing around 90% of the bacteria within 48 hours; rifampicin is half as potent as isoniazid (Donald 2008). Rifampicin and pyrazinamide are sterilizing agents that eliminate persisting, intermittently active or dormant bacilli, and without which any short‐course treatment should ideally not be given (Donald 2007b).
Diagnostic categories and treatment regimens
TB treatment regimens differ according to the diagnostic categories (see Appendix 1): Category 1 comprises new cases (new smear‐positive pulmonary TB; new smear‐negative pulmonary TB with extensive parenchymal lesions; severe forms of extrapulmonary TB; severe concomitant HIV disease). Category 2 comprises previously treated smear‐positive pulmonary TB cases. Category 3 is made up of new smear‐negative pulmonary TB cases other than those in category 1. Category 4 comprises chronic and multiple‐drug resistant TB (WHO 2006b; WHO 2010a).
Drug doses
Most TB drug regimens worldwide have been based on studies in adults and therefore for children the same mg/kg/bodyweight doses were given as in adults. However, this practice has been challenged (Schaaf 2005). Several studies have attempted to determine the effective doses of anti‐TB drugs in children, but the recommendations in many are discordant (Abernathy 1983; Varudkar 1985; Biddulph 1988; Snider 1988; Starke 1989a; Starke 1989b; Acocella 1990; Biddulph 1990; Kumar (India) 1990; Reis 1990; Starke 1990; Crofton 1992; Al‐Dossary 2002; Swaminathan 2005; WHO 2006b). Experts commissioned by the WHO conducted pharmacokinetic studies to determine the optimum doses of first line drugs used to treat childhood TB (rifampicin, isoniazid, and pyrazinamide); and the safety of these recommended doses of these drugs needs to be clarified in trials in children, especially those under 12 years of age (Hill 2008). Current recommended doses for TB treatment in children for the initial two months and the four months of continuation treatment (WHO 2010a) are higher than those used previously: isoniazid 10 mg/kg (range 10 to 15 mg/kg), maximum dose 300 mg/day; rifampicin 15 mg/kg (range 10 to 20 mg/kg), maximum dose 600 mg/day; pyrazinamide 35 mg/kg (30 to 40 mg/kg; maximum dose per day is 2000 mg; and ethambutol 20 mg/kg (15 to 25 mg/kg) dose dependant pharmacokinetic evidence and observational studies support the revised WHO recommendations (Donald 2011; Thee 2011; Kiser 2012).
Treatment adherence, cure, and relapse
One of the most important challenges for any anti‐TB programme is reducing the number of people who default, or discontinue treatment. The WHO defines a defaulter as a patient whose treatment was interrupted for two or more consecutive months (WHO 2006a; WHO 2006b). When a child is sputum‐negative in the last month of treatment for TB, and also on at least one previous occasion, he or she can be deemed cured (WHO 2006b), though this applies only to sputum‐positive pulmonary TB. In practice, resolution of clinical symptoms with significant weight gain, complete or partial resolution of radiological findings without the emergence of new radiological lesions, disappearance of lymph nodes in TB lymphadenitis, and regression in the size of any enlarged organs for disseminated TB are taken as equivalent to treatment response or cure. Some national guidelines for paediatric TB do not put a time limit after completion of treatment and the re‐appearance of disease (IAP 2010; RNTCP 2012). However re‐emergence of signs and symptoms of TB within 12 months after stopping treatment would appear to be a more appropriate cut‐off as re‐emergence of symptoms beyond this time would more likely be due to re‐infection in countries with a high‐TB burden, and not a relapse due to treatment failure (Mwandumba 2001).
How the intervention might work
Fully‐intermittent drug regimens for childhood TB require the child to take drugs just two or three times a week, preferably under direct observation and often preceded by daily treatment in the intensive phase (partially‐intermittent treatment). The rationale for intermittent dosing was the observation in vitro that following interruption of treatment with anti‐TB drugs, growth of M. tuberculosis takes some days to resume, thereby suggesting that short interruptions would be possible before resuming treatment; this interval is decided by the shortest time taken for resumption of bacterial activity after drug cessation that varies with anti‐TB drugs, and is shortest for rifampicin at two to three days (Dickinson 1966). In observational studies, this not only reduced the need for hospitalization for drug delivery but also increased compliance amongst children on anti‐TB drugs (Abernathy 1983; Biddulph 1988; Kiper 1998; Al‐Dossary 2002; Swaminathan 2005).
While treatment regimens for active TB that are intermittent, or use rifampin as part of combination treatment only during the initial phase, may offer practical advantages, their efficacy has often been in doubt. In a systematic review and meta‐analysis of studies in adults, TB treatment outcomes were significantly worse with shorter duration of rifampicin, or with initial drug resistance to isoniazid, or streptomycin, or both (Menzies 2009). Treatment outcomes were similar with all intermittent schedules evaluated, but there was insufficient evidence to support administration of treatment twice‐weekly throughout therapy (Menzies 2009).
Why it is important to do this review
The importance of treating childhood TB for global TB control
Most treatment strategies for childhood TB are based on the management of TB in adults (Mandalakas 2005), the rationale being that targeting adults with TB will automatically help reduce the incidence and prevalence of TB in children (Heymann 2000). Estimates from modelling studies suggest that even small increases in case detection and treatment of childhood TB would save millions more children's lives compared to the effect that treating increasing number of adults with TB would have on TB in children (Heymann 2000). Thus, a 5% increase in the number of children on anti‐TB treatment is estimated to lead to a 25% decline in the prevalence of childhood TB and also a 16% decline in mortality due to TB after 10 years, compared to no intervention (CDC 1995).
Untreated latent TB infection in children acts as a reservoir for future disease but since most active disease in children occurs in the first year after infection, the presence of childhood TB in a community is an indication of the ongoing disease transmission, including drug‐resistant strains, within it (Kant 2001; Marais 2004; Newton 2008).
The control of childhood TB now forms part of the 'Stop TB' strategy (Stop TB Partnership 2006a; Stop TB Partnership 2006b; Stop TB Partnership 2006c; Stop TB Partnership 2007) that builds on the WHO's DOTS (Directly Observed Treatment, Short‐course) programme (WHO 2006b), co‐developed by the International Union Against Tuberculosis and Lung Disease (IUTLD); and that aims to contribute to achieving goals four and six (to reduce child mortality and to improve maternal health) of the United Nations' Millennium Development Goals (http://www.undp.org/mdg/).
Differences in international and national recommendations
Inappropriate prescription of anti‐TB drugs for adults and children is common (Langendam 2012). There are also discrepancies in national and international recommendations for treatment schedules in high‐, low‐, and middle‐income countries and sometimes even within the same country. The WHO and the British Thoracic Society recommend either the daily or thrice‐weekly intermittent regimens, whereas the American Thoracic Society, along with the Centers for Disease Control and Prevention (CDC), recommend a daily or twice‐weekly regimen (Shingadia 2003). Current Indian guidelines recommend thrice‐weekly intermittent therapy given by DOTS in both the intensive phase and the continuation phase (IAP 2010; RNTCP 2012). All TB cases are to be reported to the Revised National Tuberculosis Control Programme (RNTCP), which supplies free drugs for treatment under the DOTS programme (Chauhan 2004).
Differences in recommendations have resulted in clinicians using a myriad of regimens. In India, the earlier Indian Academy of Paediatrics (IAP) recommendation of the daily regimen (IAP 1997) aimed primarily at paediatricians in the private sector (Amdekar 2009), led to the preferential use of daily dosing for their patients. The national programme, on the other hand, provides only intermittent therapy. Such a dichotomy resulted in a substantial proportion of patients not being registered in the National TB Control Programme statistics, since they did not access the drugs given under this programme. The more recent consensus statement (IAP 2010) recommends thrice‐weekly, intermittent DOTS in children at a higher dose; but fails to cite reliable evidence to support this change in recommendations.
Current evidence‐based guidance from the WHO on the treatment of paediatric TB (WHO 2010a; WHO 2010b) recommends against using intermittent regimens (twice‐ or thrice‐weekly) in children with suspected or confirmed pulmonary TB or tuberculous peripheral lymphadenitis living in settings with a high HIV prevalence (or with confirmed HIV infection). The guidance suggests that thrice‐weekly regimens can be considered only during the continuation phase of treatment for children known to be HIV‐uninfected and living in settings with well‐established DOTS; thus only partially‐intermittent regimens are recommenced, and fully‐intermittent schedules (without initial daily treatment for two months) are not currently recommended for children with TB (WHO 2010a).
A Cochrane Review of fully‐intermittent dosing with drugs for treating TB in adults produced inconclusive results (Mwandumba 2001). In this review, we systematically evaluated the effects of intermittent versus daily dosing with anti‐TB drugs for TB in children with, and without, HIV infection, in order to inform policy and clinical practice.
Objectives
To compare the efficacy and safety of intermittent, short‐course anti‐TB treatment regimens (twice‐ or thrice‐weekly) with daily short‐course anti‐TB treatment regimens in treating childhood TB.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and quasi‐RCTs.
Types of participants
Inclusion criteria
Children aged 15 years or younger, diagnosed as having TB in category 1, 2, or 3 according to the WHO diagnostic categories (Appendix 1).
Exclusion criteria
Children diagnosed under category 4 (multiple‐drug resistant TB) by the WHO (Appendix 1).
Types of interventions
Interventions*
Twice‐weekly short‐course anti‐TB chemotherapy.
Thrice‐weekly short‐course anti‐TB chemotherapy.
Control*
Daily short‐course anti‐TB chemotherapy.
* All regimens had to contain rifampicin for the intensive phase, which is the initial period when more intensive treatment is given. The total duration of treatment should ideally have been six months, but not have exceeded one year. We classified short course treatment regimens where daily dosing was used for the first two months as daily treatment, even if this was followed by intermittent treatment thereafter, since daily dosing in the intensive phase of treatment is considered critical for treatment success.
Types of outcome measures
Primary outcomes
Cure as defined by the trial authors and including the following:
negative sputum test (if appropriate);
weight gain;
-
resolution of symptoms and signs within one month after completion of treatment. These may include, but are not confined to:
fever or cough,
decrease in size of the lymph nodes, and
resolution of the chest X‐ray findings.
Secondary outcomes
Death from any cause during treatment or within one year after the completion of treatment.
Relapse: defined as re‐emergence of signs and symptoms of TB within 12 months after stopping treatment.
Adherence to treatment: defined as the proportion of children who are not defaulters (a defaulter being a child whose treatment was interrupted for two consecutive months or more).
Adverse effects
Serious adverse effects: as defined by the trial authors and based on clinical or laboratory findings, or both.
Treatment‐limiting adverse events: adverse effects during treatment requiring interruption or alteration of the treatment regimen.
Other adverse effects: including skin rash, nausea or vomiting, diarrhoea, epigastric pain, fatigue or malaise, dizziness, headache, fever or chills, arthralgia, peripheral neuropathy, anorexia or weight loss, insomnia, and pruritis.
Search methods for identification of studies
We attempted to identify all relevant trials, regardless of language or publication status (published, unpublished, or ongoing).
Electronic searches
Databases
Vittoria Lutje (VL), the Information Specialist of the Cochrane Infectious Diseases Group (CIDG) editorial base searched the following databases: the CIDG Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 5, 2013); MEDLINE; EMBASE; and LILACS, up to 30 May 2013 using the search terms detailed in Appendix 2.
In addition, on 28 May 2013 we searched the website of the Indian Medlars Center (IndMED; http://indmed.nic.in/) and the South Asian Database of Controlled Clinical Trials (http://www.cochrane‐sadcct.org/ ) using 'tuberculosis' and 'isoniazid' as search terms, to identify relevant trials from journals that may not be indexed in the databases named above.
Prospective clinical trials registries
On 30 May 2013, VL updated searches of the metaRegister of Controlled Trials (mRCT) and the search portal of the WHO International Clinical Trials Registry Platform (www.who.int/trialsearch) for ongoing trials.
Conference proceedings
In May 2013 we updated searches of proceedings from relevant conferences based on their availability (see Appendix 3).
Searching other resources
Researchers and organizations
We contacted researchers in the field to identify additional trials that might be eligible for inclusion. We also contacted relevant organizations, including the WHO, the Prevention of Tuberculosis Trials Consortium (TBTC), the International Union Against TB & Lung Disease, and the Global Partnership to Stop TB, for published, unpublished, and ongoing trials.
Reference lists
In addition we checked the reference lists of all trials identified by the above methods.
Data collection and analysis
Selection of studies
All review authors independently screened the citations and abstracts obtained by the searches to identify potentially eligible trials. We obtained the full articles for potentially eligible trials and independently evaluated them for inclusion. We excluded trials that recruited adults and children unless subgroup data for children were available from the trial report or from the trial authors. Disagreements were resolved by contacting the authors for clarification if needed, or by consensus. We documented the process of selection of trials in a flow diagram. The reasons for excluding studies are in the Characteristics of excluded studies section.
Data extraction and management
Using pre‐tested data extraction forms, all review authors independently extracted data from the included trials, including the number of patients randomized and the number for which outcome(s) were measured. We extracted the number of events and the number of patients in each treatment arm for dichotomous outcomes, and arithmetic means and standard deviations together with the number of patients in each group for continuous outcomes. For cluster‐RCTs, we extracted the number of clusters, average cluster size, unit of randomization, adjustment for clustering or other covariate in the statistical analysis, and estimates of the intra‐cluster correlation coefficient (ICC) for each outcome. Where results were adjusted for clustering, we extracted the point estimate with 95% confidence intervals (CIs); otherwise, we extracted the data as for individually randomized trials. We resolved any inadequacies or discrepancies by discussion, and if required by contacting the trial authors.
Assessment of risk of bias in included studies
All review authors independently assessed the risk of bias in the included trial reports. We assessed the six standard components: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other biases. For each of these components we assigned a judgment regarding the risk of bias as 'yes', 'no', or 'unclear' (Higgins 2011a). Where necessary, we attempted to contact the trial authors for clarifications if any components were unclear or not stated in the report. We recorded the results in the standard tables in Review Manager (RevMan) 5.1, and summarized the assessments in figures.
Measures of treatment effect
Soumik Kalita (SK) and Winsley Rose (WR) entered data into standard tables in Review Manager (RevMan) 5.1. Anuradha Bose (AB) and Prathap Tharyan (PT) independently checked all data. PT also performed additional data extraction and entry, which was checked by the other review authors. For dichotomous outcomes, we compared risk ratios and their 95% CIs.
Unit of analysis issues
For cluster‐RCTs, we combined the adjusted measures of effect with those from parallel group trials in meta‐analysis if the results were adjusted for clustering, using the generic inverse variance approach (Deeks 2011). If results were not adjusted for clustering, we attempted to adjust the results for clustering, by multiplying the standard errors of the estimates by the square root of the design effect where the design effect is calculated as DEff = 1 + (M ‐ 1) ICC, where M is the average cluster size and ICC is the intra‐cluster correlation. If the results were not adjusted for clustering, we extracted data as for parallel‐group RCTs and combined them in the meta‐analysis with data from other parallel group randomized trials; but, if possible, evaluated their inclusion in a sensitivity analysis.
Dealing with missing data
We attempted to obtain missing data from trial authors. We conducted an intention‐to‐treat analysis in trials with no loss to follow‐up and complete‐case analysis for trials with incomplete follow‐up, except for the primary outcome of cure, where we considered losses to follow‐up before symptom resolution not cured. We did not make any assumptions for the secondary outcomes or for adverse events, due to difficulties in making valid assumptions about those lost to follow‐up, apart from what was reported in the trials.
Assessment of heterogeneity
We assessed heterogeneity between trials by examining forest plots for inconsistency in the direction or magnitude of the effect estimates, with non‐overlapping CIs. We used the Chi2 test for heterogeneity with a 10% level of significance to detect inconsistency in trial results that exceeded chance, and the I2 statistic to denote the percentage of inconsistency in results due to inter‐trial variability that exceeded random‐error (Higgins 2003).
In general, we interpreted an I2 value of 50% or greater to denote significant heterogeneity (Higgins 2003), though we acknowledge that this cut‐off is arbitrary. Therefore we interpreted I2 values between 0% to 40% as possibly unimportant, 30% to 60% as possibly significant, 50% to 90% as possibly substantial, and 75% to 100% as possibly considerable; depending on whether the inconsistency in results was due to differences in the direction of effect estimates between trials, rather than due to differences in the magnitude of effect estimates favouring an intervention; as well as the strength of the evidence for heterogeneity from the P value for the Chi2 test for heterogeneity (Deeks 2011).
Assessment of reporting biases
If we had included at least 10 trials in a meta‐analysis, we would have considered assessing the likelihood of publication bias by examining the funnel plot for asymmetry due to small study effects.
Data synthesis
We stratified analyses by trial characteristics for the primary outcome (twice‐weekly and thrice‐weekly); or fully intermittent versus partially intermittent, and duration of interventions. We synthesized comparable data using the Mantel‐Haenszel method to derive pooled, weighted risk ratios in fixed‐effect meta‐analyses. We used the random‐effects model for data synthesis when we identified significant heterogeneity (see above) which could not be explained by subgroup analyses (see below). If I2 values revealed substantial inter‐trial variability in effect estimates in excess of chance that we thought were not due to variations in clinical or methodological attributes, we suggested caution in interpreting the pooled estimates. If substantial heterogeneity was unexplained, we presented the results of the trials in a forest plot, without summating their effect estimates.
Subgroup analysis and investigation of heterogeneity
The limited number of trials precluded subgroup analysis. In future editions with more trials, we would consider subgroups on the stratified analysis for fully intermittent chemotherapy (twice‐ or thrice‐weekly) and daily short‐course chemotherapy by age (younger or older than 4); HIV status; and previous TB treatment.
Sensitivity analysis
We attempted to conduct sensitivity analyses to investigate the robustness of the results to the various risk of bias components and the intention‐to‐treat sample versus completers, as well as to assumptions made in data analyses (such as when dealing with data from cluster‐RCTs).
Summarising and interpreting results
We used the GRADE approach to interpret findings (Schunemann 2008) and used GRADE Profiler (GRADE 2013) to import data from Review Manager (RevMan) 5.1 to create 'Summary of findings' tables for each comparison included in this review. These tables provide information concerning the overall quality of the evidence from the trials, the magnitude of effect of the interventions examined, and the sum of available data on the primary outcome and selected secondary outcomes.The GRADE approach integrates evaluations regarding study limitations; unexplained inconsistency in the results; indirectness (how representative of clinical practice the populations studied were; the deviations from accepted practice in the way interventions and comparisons were given; the choice of outcomes as representative of those considered important to clinical decision‐making; and the methods used in assessing these outcomes); imprecision in the estimates (in terms of statistical significance as well as clinical importance); and the likelihood that publication bias affected the estimates.
The outcomes we included in these tables, which were rated important or critically important to clinical decision‐making, were:
Cure
Death due to any cause
Relapse
Adherence
Treatment‐limiting adverse events.
We used this summary to guide our conclusions and recommendations.
The GRADE Working Group (Schunemann 2008) considershigh quality evidence to denote that the effect estimate is likely to reflect the effects obtained in clinical practice and that further research is very unlikely to change our confidence in the estimate of effect. Moderate quality evidence denotes that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality evidence indicates that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.Very low quality evidence denotes a serious lack of confidence in the effect estimates.
We standardized terminology to refect these grades of quality in this review;
High quality evidence is denoted by statements to this effect.
We used the term "probably" to denote the effects of treatment or control when evidence was of moderate quality evidence.
We used the word "may" to describe the likely effects when evidence was of low quality.
Very low overall quality assessments indicate considerable doubts of the internal or external validity of the effect estimate.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our search retrieved 82 records, of which we considered the abstracts of only 44 relevant. We scrutinised these abstracts, obtained and assessed 12 full text reports, and selected four trials for inclusion. See Figure 1 for a flow diagram of the selection process.
1.
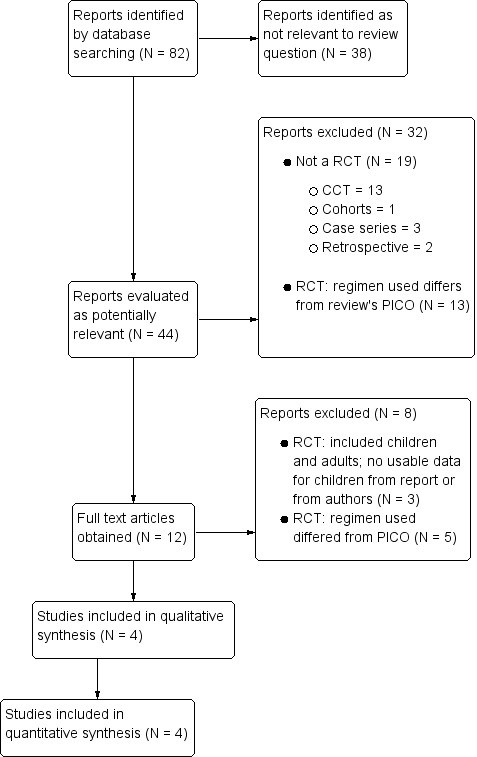
Flow diagram of the selection process.
No trials currently await classification and we are not aware of any ongoing trials.
Included studies
We included four RCTs published between 1996 and 2000 that compared various regimens of daily versus intermittent therapy of anti‐TB chemotherapy in 563 children (465 of whom were evaluable). One trial randomized children by households (Te Water Naude (South Africa) 2000) but did not report the proportions in each arm that were cluster‐randomized and individually randomized. In the other three trials, the unit of randomization was the individual. We have described the four trials in detail in Characteristics of included studies and have summarized them below.
Location
Two trials were conducted in India (Kumar (India) 1990; Ramachandran (India) 1998); one in South Africa (Te Water Naude (South Africa) 2000), and one in Turkey (Kansoy (Turkey) 1996).
Age
All four trials included children aged five months to 15 years. Kansoy (Turkey) 1996 recruited children aged five months to 13 years; Kumar (India) 1990 recruited children aged one to 15 years; Te Water Naude (South Africa) 2000 recruited children < 14 years of age; and Ramachandran (India) 1998 randomized children aged one to 12 years old.
Diagnosis of TB
All trials used a combination of clinical and radiological criteria (parenchymal or mediastinal lymph nodes in chest radiograph) to diagnose childhood TB; in addition Kansoy (Turkey) 1996 used epidemiological criteria (contact with adult TB) and immunological criteria (tuberculin skin test (TST) response). Kumar (India) 1990 used explicit criteria to diagnose children with TB lymphadenopathy, pulmonary TB, and disseminated TB; Ramachandran (India) 1998 reported detailed criteria used to diagnose 'most probable' and 'probable TB', with a course of antibiotics given to the latter and repeat chest X‐ray after two weeks of treatment, prior to inclusion and treatment of those with unresolved lung lesions. Te Water Naude (South Africa) 2000 used WHO criteria, current at the time of the trial, to diagnose suspected and confirmed cases of intra‐thoracic TB (see Characteristics of included studies).
Comparisons
The four included trials compared four different intermittent short‐course versus daily anti‐TB treatment regimens.
1. Partially‐intermittent (daily two weeks, intermittent 8.5 months) versus daily treatment (nine to 12 months) regimen
Kansoy (Turkey) 1996 used streptomycin in both treatment arms, and used different drugs and durations of treatment in the intermittent and daily treatment arms (Intermittent‐short course chemotherapy group: streptomycin, rifampicin, and isoniazid (INH) daily for two weeks, followed by INH and rifampicin twice‐weekly for eight and a half months versus conventional chemotherapy group: daily streptomycin for 40 days, rifampicin for nine months and INH for 12 months). While both arms had a daily treatment component, we classified this trial as a comparison of intermittent versus daily treatment, since the intermittent treatment arm used daily treatment only for two weeks of initial treatment.
2. Fully‐intermittent (six months) versus daily treatment plus intermittent treatment (daily two months, intermittent four months) regimen
Kumar (India) 1990 compared a fully‐intermittent twice‐weekly regimen (INH, rifampicin, and pyrazinamide given twice‐weekly for two months followed by INH and rifampicin twice‐weekly for four months versus a partially‐intermittent regimen with daily dosing in the intensive phase (INH, rifampicin, and pyrazinamide given daily for two months followed by INH and rifampicin twice‐weekly for four months). Though both arms used intermittent treatments, we considered this as a comparison of intermittent treatment versus daily treatment since daily treatment was used throughout the two‐month intensive treatment phase, This trial also permits an evaluation of intermittent treatment regimens with and without two‐months of daily treatment in the intensive treatment phase.
3. Fully intermittent (six months) versus daily treatment (nine months) regimen
Ramachandran (India) 1998 compared a fully‐intermittent regimen using thrice‐weekly and twice‐weekly dosing (INH, rifampicin, and pyrazinamide thrice‐weekly for two months, followed by INH and rifampicin twice‐weekly for four months) versus a longer nine month daily regimen (INH and rifampicin daily for nine months).
4. Fully intermittent (six months) versus daily treatment (six months) regimen
Te Water Naude (South Africa) 2000) compared a twice‐weekly fully intermittent six‐month regimen of INH, rifampicin, and pyrazinamide with a daily regimen of INH, rifampicin, and pyrazinamide (five days a week) for six months.
Drug doses
The doses used in the intermittent arms of the four trial were comparable with currently recommended dosing for intermittent regimens.
Outcomes
Childhood TB can affect several organs in the body, and response to therapy is defined according to the organ involved, in addition to general systemic signs and symptoms. The four trials used different definitions for the outcomes examined.
Primary outcome
Cure
Kansoy (Turkey) 1996 used clinical and radiological assessments and weight gain to monitor children for clinical improvement. Kumar (India) 1990 provided explicit criteria to define general improvement (afebrile, improved appetite, weight gain); and for marked, moderate, and poor improvement for pulmonary TB, with poor improvement defined as no significant general improvement, no radiological clearance, and increase in the size of pulmonary lesions or the appearance of new lesions. Ramachandran (India) 1998 monitored resolution of radiological lesions to evaluate treatment response and we used the proportions with complete resolution as well as those with general improvement but with residual lesions to define cure in this review.
Te Water Naude (South Africa) 2000 assessed response to treatment based on a composite score on four domains (parental assessment, clinical assessment, weight gain, and chest radiograph), with each scored from ‐1 (worse); 0 (no better); +1 (better); + 2 (much better). The combined scores ranged from ‐4 to +8. Trial authors considered any patient with a score of four or more as better or improved on all four domains; and those scoring eight as free of clinical and radiological evidence of TB. Assessments were at 3, 6, 12, and 18 to 30 months. For the 12‐month, and 18‐ to 30‐month assessments, parental ratings were omitted; therefore the composite scores ranged from ‐3 to +6. Thus, the trial authors considered anyone with a score of three or more as improved at 12 months or later. The outcomes in those assessed at the follow‐up time points were presented as median scores (with ranges), but the proportions in the treatment groups considered to be responders were not reported.
However, the authors reported that at six months, 163 of 206 randomized evaluable children were assessed for treatment response (77%); of whom 70/89 (79%) were in the arm allocated twice‐weekly intermittent treatment for six months and 93/117 (79%) were in the six month daily‐treatment arm (P = 0.40). The median (range) of combined outcome scores were 6 (5 to 7) in the intermittent arm and 6 (5 to 7) in the daily treatment arm at this time point (six months‐ the closest time point to our review’s time point of one month after completion of treatment). At 18‐ to 30‐month assessments, 71 (75%) in the intermittent treatment arm and 74 (63%) in the daily treatment arm were scored for treatment response (P = 0.73) and the median scores were 4 out of a possible 6 (range 3 to 5) in both arms (P = 0.949). Thus the six month outcomes were maintained at 18 to 30 month assessments. We interpreted the proportions 'cured' in our review as all those who scored four or more at six months. Since the lower limit of the score ranged from five to seven at six months, we interpreted this to mean that all children assessed fulfilled our description of 'cured'. The first author of Te Water Naude (South Africa) 2000 confirmed these interpretations in personal discussions with PT and in subsequent e‐mail communications.
Secondary outcomes
All trials reported relapse and adherence, and serious adverse events that were also defined and assessed in different ways (see Characteristics of included studies).
Two trials reported deaths (Kumar (India) 1990; Ramachandran (India) 1998), and we assumed that no deaths occurred in the other two included trials based on the number of children that completed follow‐up.
The included trials reported non‐serious adverse events poorly and it was unclear if they were systematically ascertained.
Excluded studies
We have described the 44 excluded trials in the Characteristics of excluded studies section, with the reasons for their exclusion. Thirteen were RCTs, of which 10 did not fulfil inclusion criteria. Three trials (TRC 1997; Jindani 2004; Jawahar 2005) included adults and children, but we were unable to obtain disaggregated data for children from the reports or the authors. We have discussed their exclusion under Potential biases in the review process.
Risk of bias in included studies
None of the included trials were free from risk of bias for many of the domains assessed (see Figure 2 and Figure 3).
2.
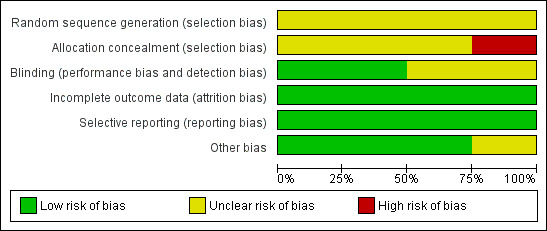
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included trials.
3.
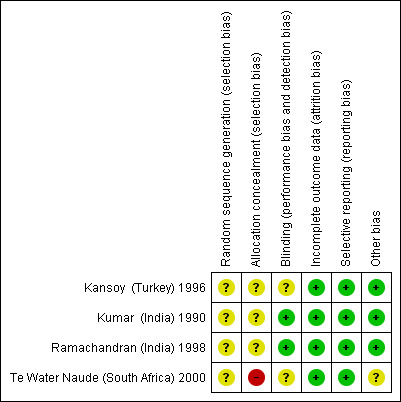
Methodological quality summary: review authors' judgements about each methodological quality item for each included trial.
Allocation
Te Water Naude (South Africa) 2000 was at unclear risk of bias for random sequence generation, but at high risk of bias due to inadequate allocation concealment arising from obtaining informed consent after randomization. This resulted in differential exclusion rates in the intervention arms. Of 153 children randomized to intermittent treatment, the trial only included 95 (62%) after exclusions; of 161 children randomized to daily treatment, the trial only included 118 (73%) after exclusions; and this difference was statistically significant (P < 0.05). The other trials were at unclear risk of selection bias due to lack of information in the trial reports on the methods.
Blinding
Kansoy (Turkey) 1996 and Te Water Naude (South Africa) 2000 were at unclear risk of detection bias due to the open‐label design of both trials, the lack of reporting of the use of independent outcome assessors in the former trial, and the difficulty in blinding the composite outcome used in the latter trial.
Incomplete outcome data
All trials were free of attrition risk of bias.
Selective reporting
None of the trials were prospectively registered or had a protocol that was available in the public domain. However, we did not detect any evidence of selective reporting.
Other potential sources of bias
It was unclear in Te Water Naude (South Africa) 2000 whether the lack of adjusting for clustering resulted in falsely imprecise effect estimates, as the trial authors did not report the proportions of children in each arm that were randomized by household, or as individuals. The first author indicated via correspondence and personal discussions that a low number of children were randomized by household, but the exact proportions in each intervention arm were not available.
Effects of interventions
See: Table 1
Cure
Intermittent and daily treatments cured similar proportions of children, in intention to treat analysis, where those lost to follow‐up before being judged treatment responders, and those who required extended treatment after the allocated treatment duration were considered not cured (RR 1.01, 95% CI 0.94 to 1.10, four trials, 465 children; Analysis 1.1; Figure 4).
1.1. Analysis.
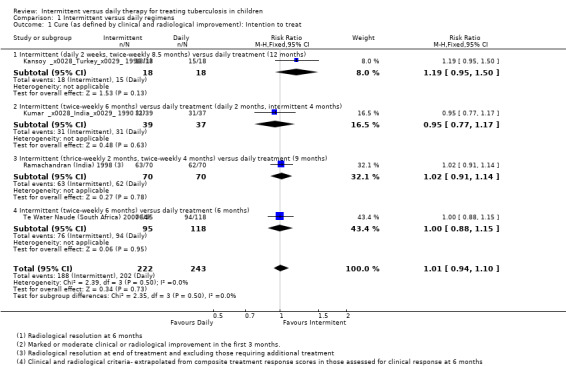
Comparison 1 Intermittent versus daily regimens, Outcome 1 Cure (as defined by clinical and radiological improvement): Intention to treat.
4.
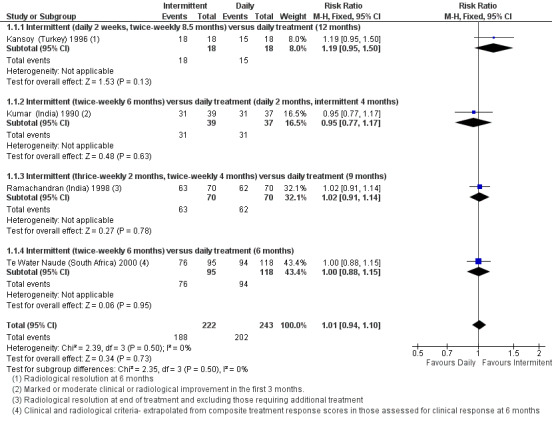
Forest plot: 1. Intermittent versus daily regimens, Outcome 1.1 Cure (as defined by clinical and radiological improvement): Intention to treat.
In per‐protocol sensitivity analysis of evaluable children judged cured (where drop‐outs were not included), the pooled estimate again did not significantly differ between daily treatment versus intermittent treatments (four trials, 441 children; Analysis 1.2). In additional sensitivity analysis, excluding Te Water Naude (South Africa) 2000 which was at high risk of selection bias, and also had data unadjusted for clustering, the pooled effect estimates continued to show no significant difference between intermittent and daily treatment regimens for children cured (RR 1.02; 95% CI 0.93 to 1.12; three trials, 352 children).
1.2. Analysis.
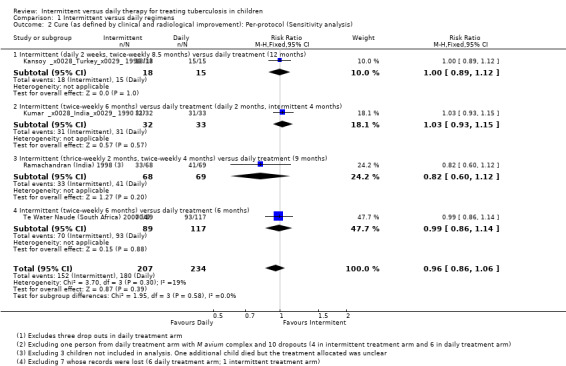
Comparison 1 Intermittent versus daily regimens, Outcome 2 Cure (as defined by clinical and radiological improvement): Per‐protocol (Sensitivity analysis).
Deaths due to any cause
Intermitttent treatments did not differ from daily treatments in the proportions who died due to any cause (four trials, 460 children; Analysis 1.3), though only two trials (Kumar (India) 1990; Ramachandran (India) 1998) reported any mortality due to TB. In the latter trial, one additional death that was not thought to be TB‐related was reported, but allocation to intermittent or daily treatment was not stated.
1.3. Analysis.
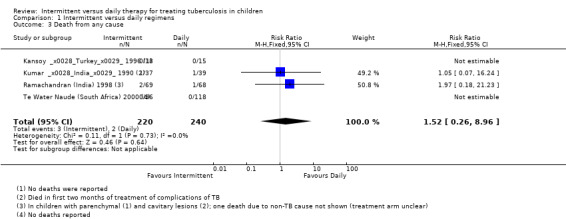
Comparison 1 Intermittent versus daily regimens, Outcome 3 Death from any cause.
Relapse
Only one trial reported one confirmed relapse among 449 evaluated children (with intermittent treatment) over 24 months of follow‐up (Te Water Naude (South Africa) 2000; Analysis 1.4).
1.4. Analysis.
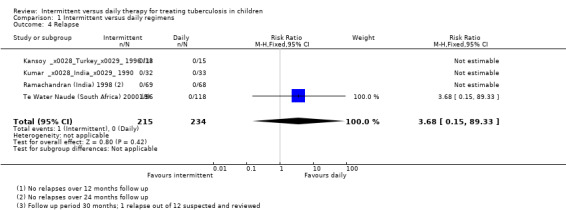
Comparison 1 Intermittent versus daily regimens, Outcome 4 Relapse.
Adherence to treatment
Adherence rates (defined and assessed in different ways) did not differ significantly with intermittent treatment or with daily treatment (four trials, 458 children; Analysis 1.5; Figure 5).
1.5. Analysis.
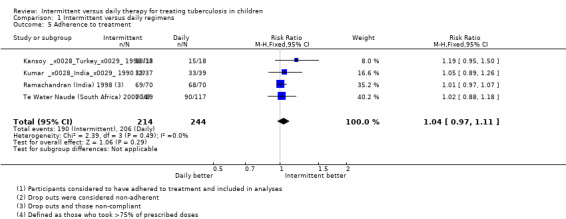
Comparison 1 Intermittent versus daily regimens, Outcome 5 Adherence to treatment.
5.
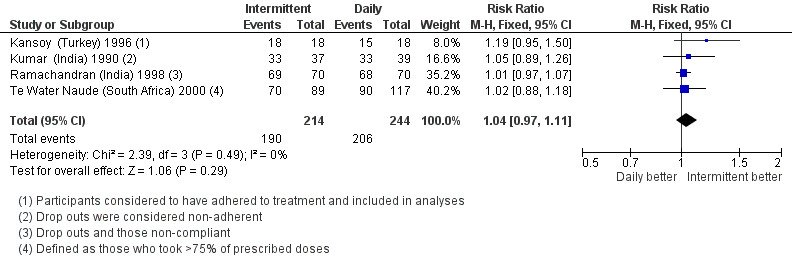
Forest plot: 1. Intermittent versus daily regimens, Outcome 1.5 Adherence to treatment.
Adverse events
None of the participants in the four trials were reported to have developed serious adverse events. Adverse events requiring interruption of treatment were not significantly different with both schedules (four trials, 441 children; Analysis 1.6). These events were transient hepatic toxicity in three children in two trials that required alterations in trial medication till resolution of symptoms and liver functions tests had normalized (Ramachandran (India) 1998 and Te Water Naude (South Africa) 2000). Among other adverse events, two trials reported transient episodes of vomiting and mild joint pains in the initial period.
1.6. Analysis.
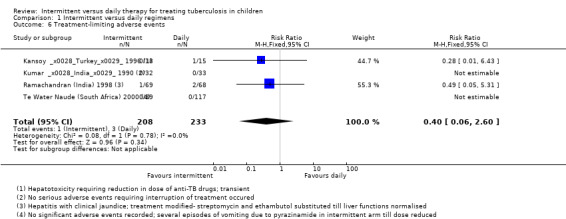
Comparison 1 Intermittent versus daily regimens, Outcome 6 Treatment‐limiting adverse events.
Discussion
Summary of main results
Four RCTs that were not free of study limitations compared different schedules of intermittent short‐course anti‐TB treatments versus daily treatments in 563 (465 evaluable) children aged five months to 15 years, who were not known to have drug resistance to anti‐TB drugs, The combination of drugs, and the duration of intermittent and daily treatments differed between trials, and none used drug combinations and schedules currently recommended for childhood TB.
Intermittent twice‐weekly, short‐course treatments and the daily treatment regimens cured 84% of the children treated with each regimen, but the risk of bias in the trials, the non‐standard treatments used, and imprecision in the estimate of cure due to the small number of children evaluated with these regimens limit our confidence in this estimate (Table 1).
Deaths due to any cause (1.3% versus 0.8%), and adherence to treatment were probably little different (87% versus 84%) with intermittent as with daily anti‐TB regimes, while relapses (only one in the four trials) may also be infrequent with both regimens.
Intermittent and daily treatments did not differ significantly in rates of adverse events requiring interruption of treatment (0.6% versus 1.5%). No child developed serious adverse events in the four trials, and other adverse events were infrequent and transient.
Overall completeness and applicability of evidence
Completeness of the evidence
We believe that we have identified all RCTs that fulfilled the inclusion and exclusion criteria used in this review. The fact that we found only four RCTs addressing the review question is evidence of the lack of importance given to research specific to children with TB, in spite of the importance of childhood TB to global TB control. Important gaps remain in the evidence base for treating childhood TB. Notably, we did not find any RCTs comparing the commonly used thrice‐weekly treatment regimens with daily treatment. None of the trials reported the HIV‐status of the children randomized.
These lacune in the body of evidence limit our ability to draw conclusions regarding the effects of intermittent dosing versus daily short‐course anti‐TB treatment, in HIV‐negative or HIV‐positive children with TB.
Applicability of the evidence
Although the included trials used different definitions for the diagnosis of childhood TB, they concur with current methods used in clinical practice to diagnose TB in children. All trials excluded children with primary complex and included the spectrum of childhood TB covered in the WHO Classification 1, 2, or 3, except Te Water Naude (South Africa) 2000 which excluded children with extrapulmonary TB. The trials were conducted in high TB‐burden low‐ and middle‐income counties, where most children with TB live, and recruited children as young as five months of age to 15 years of age. The doses of anti‐TB drugs used were comparable to currently recommended doses for anti‐TB drugs.The treatments in the trials were provided by DOTS, except Kansoy (Turkey) 1996 where supervision of treatment was unclear from the trial report.
However, the treatment regimens used in all of the trials were not similar to current recommendations for the treatment of childhood TB (WHO 2006b; WHO 2010a).
Kansoy (Turkey) 1996 used streptomycin in the intensive phase of the intermittent regimen and the daily regimen though this is recommended in current guidance only for children with multi‐drug resistant TB susceptible to streptomycin, and not as a first line treatment for children with pulmonary TB or TB lymphadenitis (WHO 2010a). In the intermittent‐treatment arm, the intensive phase was for only two weeks and the short‐course regimen in total lasted nine months, where INH and rifampicin were given. The daily treatment arm had streptomycin for 40 days, followed by INH and rifampicin for nine months and INH alone for another three months.
Kumar (India) 1990 included children with TB lymphadenitis, pulmonary TB and disseminated TB and used INH, rifampicin, and pyrazinamide in the two‐month intensive phase in the partially‐intermittent arm (daily treatment for the first two months) and the twice‐weekly fully‐intermittent arm. Current guidance (WHO 2006b; WHO 2010a) recommends the addition of ethambutol in the intensive phase for disseminated TB and severe forms of pulmonary TB.
Ramachandran (India) 1998 used a combination of thrice‐weekly and twice‐weekly regimens in the intermittent treatment arm. In the intensive treatment phase, INH, rifampicin, and pyrazinamide were given thrice‐weekly for two months, followed by INH and rifampicin given twice‐weekly for four months of continuation treatment. The daily treatment was for nine months and did not include pyrazinamide in the intensive treatment period.
Te Water Naude (South Africa) 2000 used pyrazinamide in the twice‐weekly and the daily treatment arms in the intensive as well as the continuation phases; while current guidance (WHO 2010a) recommends pyrazinamide only in the intensive treatment phase. Ethambutol did not form part of the treatment regimens.
Although not a stated outcome of this review, none of the included trials included estimates of resource costs and resource use or cost‐effectiveness of the intermittent versus daily regiments. Economic analytes derived from trials would have permitted better‐informed policies regarding the treatment of childhood TB.
Quality of the evidence
We assessed the overall quality of the evidence using the GRADE approach (Schunemann 2008), that considers ‘quality’ to be a judgment of the extent to which we can be confident that the estimates of effect are correct. Evidence from a RCT is initially graded as high and downgraded by one or two levels on each of five domains after full consideration of: any limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency and precision of the results, and the possibility of publication bias. A GRADE quality level of 'high' reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different. 'Low' and 'very low' quality evidence limit our confidence in the effect estimate (Balshem 2011).
The overall quality of the evidence for intermittent short‐course versus daily anti‐TB treatments from the four included trials was very low for all outcomes (Table 1).
Potential biases in the review process
We used standard methods described in the Cochrane handbook for systematic reviews of interventions (Higgins 2011b), and also ensured compliance with the Cochrane standards for the conduct of new reviews of interventions (MECIR 2011). Since the review protocol was developed and published before the GRADE approach was introduced into Cochrane reviews, the choice of critical outcomes selected for the Summary of Findings tables were done after the protocol was published; but we evolved these during discussions and before extracting data from the included trials.
We excluded three trials that met inclusion criteria for this review because data on children were not presented separately from that of adults, and we could not obtain data on children from the authors. TRC 1997 included participants aged > 12 years, and included some children aged 12 to 15, though the exact number in each intervention arm was unclear from the trial report, and was not available from the trial authors. The trial evaluated ethambutol‐containing versus non‐ethambutol containing regimens and supervised versus partially or completely unsupervised treatment; hence this trial is not strictly relevant to this review. Jindani 2004 recruited participants aged 15 years to 65 years and the number of children aged 15 years was not available. This trial had two daily treatment arms where four drugs (ethambutol, INH, rifampicin, and pyrazinamide) were given for two months of intensive treatment; continuation was with rifampicin and INH for four months in one arm and with ethambutol and INH in the other given for six months. A third arm studied thrice‐weekly administration of ethambutol, INH, rifampicin, and pyrazinamide for two months followed by daily ethambutol and INH for six months, and hence these comparisons are also not relevant to this review, or to current practice.
Jawahar 2005 randomly allocated 277 people in Madurai, South India (87 children < 12 years of age) with biopsy confirmed lymph node TB to receive either a daily self‐administered 6‐month regimen of rifampicin and INH, or a twice‐weekly, directly observed, 6‐month regimen of rifampicin and INH; with pyrazinamide given for the first two months. Patients were followed up for 36 months after completing treatment. At 36 months, 94% of those treated with the daily regimen and 96% of those treated with the twice‐weekly intermittent regimen had a successful outcome. Although the drug combinations used in this trial are not the currently recommended combinations, the result of this trial in children and adults is consistent with the results in this review.
Agreements and disagreements with other studies or reviews
The current evidence‐based guidance from the WHO on the treatment of paediatric TB (WHO 2010a; WHO 2010b) recommends only partially‐intermittent thrice‐weekly regimens (daily treatment for two months and intermittent treatment only in the continuation phase of four months) for children known to be HIV‐uninfected, living in settings with low HIV prevalence and with well‐established DOTS programmes. The revised guidelines of the Indian Academy of Pediatrics (IAP 2010) also recommends thrice‐weekly anti‐TB treatment. The National TB Control Programme of India (RNTCP 2012) recommends thrice‐weekly treatment in the intensive treatment and continuation phases. However, we did not find any RCTs of thrice‐weekly fully‐intermittent or partially‐intermittent treatment regimens versus daily or twice‐weekly, intermittent anti‐TB treatment regimens.
The WHO (WHO 2010b) recommends only partially‐intermittent thrice weekly regimens, and only in HIV‐negative children with TB from settings where effective DOTS can be assured; and does not recommend twice‐weekly intermittent regimens (WHO 2010a). This recommendation was influenced by a meta‐analysis (Menon 2010) that was published while this review was in progress. This meta‐analysis also included only the four trials included in our review. The results of the pooled estimates of effect in Menon 2010 indicated that children receiving twice‐weekly intermittent therapy were less likely to be cured than those receiving daily therapy (per protocol analysis: OR 0.27, 96% CI 0.15 to 0.51; four trials, 466 children), though the results were not statistically significant in intention‐to treat analysis (OR 0.66; 95% CI 0.23 to 1.84). Our review did not find any significant differences in intention to treat and per protocol analysis of cure rates between intermittent and daily therapy in the same trials. The reasons for the differences in the results of our review and that of Menon 2010 are:
Choice of effect measure: Menon 2010 used odds ratios as the effect measure while we chose risk ratios to express effects, since risks ratios are intuitively better understood by clinicians; and also because when event rates are common (risk is more than ˜ 20%), odds ratios and risk ratios differ in their magnitude and precision (with odds ratios often over‐estimating effects); this may alter the pooled effect estimates (Deeks 2011).
-
Interpretation of primary data: There were two important differences in the interpretation of primary data from the same trials used for meta‐analysis in our review and that of Menon 2010.
We defined cure as resolution of symptoms and signs within one month after completion of treatment and hence did not include those children in Ramachandran (India) 1998 who required extended treatment after the allocated treatment as among those cured in our intention‐to‐treat and in per‐protocol analyses. This is in conformity with internationally accepted definitions (WHO 2006b) where anyone requiring treatment at the end of therapy (if less than five months) or after at least five months of therapy, is considered a treatment failure. In the primary per‐protocol analysis in Menon 2010, the data for the nine children who required extended treatment were included among those cured. Menon 2010 also reported that when only those in Ramachandran (India) 1998 who did not need extended regimen were considered cured in sensitivity analysis, the pooled analysis did not reveal statistically significant differences between the two arms (OR 0.53, 95% CI 0.23 to 1.21).
Te Water Naude (South Africa) 2000 reported under treatment outcome in the results section that, "The number completing therapy on schedule was 199, with 85 (96%) in the intermittent group and 114 (97%) in the daily group (P = 0.59)". Menon 2010 interpreted this sentence to mean cure rates and were used in their data synthesis, while we believe that this sentence indicates treatment‐completion rates. The next two sentences in the trial report refer to the actual treatment outcome scores and state that "The treatment outcome scores for the two regimens scored at 3, 6, 12 and 18 to 30 months after starting treatment, and the numbers of children attending for evaluation of each of the time points are summarized in Table 4. No difference was found in the 2 groups." We used the data for the evaluation at six months in Table 4 of the report to derive the proportions cured, as described fully in the sub‐section on outcomes in the Results section under Included studies of this review. Our analysis of the data from this trial also demonstrates no significant difference between intervention arms, as does the trial report. However, in Menon 2010, the effect estimates for the proportions cured in this study indicated a benefit for daily treatment over twice‐weekly intermittent treatment (OR 0.30, 95% CI 0.09 to 0.98). This estimate is not in agreement with the conclusion in the trial report, or with our interpretation of the data for the proportions cured in the trial. The first author of this trial subsequently confirmed that the data used in Menon 2010 for cure rates were actually treatment completion rates, and also confirmed our interpretation of cure rates from the trial.
Discrepancies between intention to treat and per‐protocol analyses:Menon 2010 opted to present the statistically significant results of per‐protocol analyses over the statistically non‐significant results obtained with intention‐to‐treat analyses in framing conclusions. When sensitivity analyses show that the overall result and conclusions are not affected, then the results of a review can be regarded with a higher degree of certainty; otherwise the results must be interpreted with caution (Deeks 2011). The results of our intention‐to‐treat analysis and the per‐protocol sensitivity analysis did not differ, and confirmed our conclusions.
Linking overall quality of the evidence to the effect estimates in framing conclusions: We used the GRADE approach to assess the overall quality of evidence for all outcomes; this approach links the numerical effect estimates to the confidence we placed in these estimates in formulating conclusions. Menon 2010 used the Jadad scale (Jadad 1996) to assess study quality. There are problems reported in using summary scores from scales to assess methodological quality (Juni 1999). Moreover, Menon 2010 reported that "Out of four studies, three scored 2 on Jadad’s 5 point scale while one study scored 3 points suggesting that these studies were not of very good quality." However, this assessment of study limitations were not reflected in the review's conclusions that, "Twice‐weekly intermittent short course therapy is less likely to cure TB in children as compared to daily therapy." Our interpretation of the evidence from the same four trials included in our review and in Menon 2010 does not support this conclusion.
The two other studies included in the WHO rapid advice (WHO 2010a; WHO 2010b) evaluating intermittent versus daily treatments were a retrospective review of 130 children aged six months to 17 years (Göçmen 1993), and a prospective cohort of 185 children aged five months to 17 years (Al‐Dossary 2002) that evaluated partially intermittent treatments only without a daily treatment comparison arm.
We are not aware of any other systematic review evaluating the efficacy of intermittent versus daily treatment in children alone. Chang 2011 systematically reviewed the evidence on TB dosing schedules and efficacy and included nine systematic reviews, eight controlled studies, nine pharmacokinetic‐pharmacodynamic studies, and six animal studies. The clinical studies were mainly done on adults with TB and the review endorsed the use of daily dosing schedules, "especially during the initial phase in the presence of cavitation, INH resistance and advanced HIV co‐infection, to reduce the risk of treatment failure, recurrence and acquired drug resistance, including acquired rifamycin resistance" (Chang 2011). These caveats may well apply in childhood TB; but most cases of childhood pulmonary TB, particularly in younger children less than 10 years of age are not associated with cavitation (Marais 2004); and drug resistant TB, though increasingly a matter of concern, is not as widespread as in adults.
The evidence from our review indicates that the there is uncertainty regarding the comparative efficacy of intermittent short‐course versus daily short‐course anti‐TB treatment regimens; but no convincing evidence that daily short‐course anti‐TB treatment is superior to intermittent short‐course anti‐TB treatment in children with TB.
Authors' conclusions
Implications for practice.
There is insufficient evidence to support or refute the use of intermittent (twice‐weekly or thrice‐weekly) short‐course treatment regimens over daily short‐course treatment in children with TB. Intermittent and daily regimens may have similar effects in children with TB, but further research is required to confirm the observations in this review. However, the WHO 2010a strongly recommends against the use of intermittent anti‐TB treatment regimens in HIV‐positive children.
Implications for research.
Evidence from randomized controlled trials on these regimens is limited, and trials could help inform national and international public health policies for regimens in treating paediatric TB.
We propose that adequately powered, multicentre, multi‐country, randomized, non‐inferiority, pragmatic trials conducted in high TB‐transmission settings, in low and middle‐income countries that compare thrice‐weekly, and daily short‐course (six months) dosing, and use the currently recommended four drugs, in HIV‐negative children would help inform policy. Twice‐weekly regimens are not as commonly used as thrice‐weekly regimens and it would be important to establish the equivalence of thrice weekly regimens before considering trials of twice‐weekly anti‐TB regimens. Some national TB control programmes, such the one in India (RNTCP 2012), recommend only fully‐intermittent thrice‐weekly treatment under DOT, and thrice weekly fully intermittent treatment versus partially‐intermittent treatment (with initial daily dosing for the first two months) should also be evaluated in similar separate trials. Suggested design, conduct and reporting considerations for such trials are provided in Appendix 4.
In addition, national TB control programmes that treat children with TB routinely with intermittent regimens, should regularly report treatment success rates along with the criteria used to evaluate treatment success. They should also report adherence rates, relapse rates, and rates of adverse events, in order to build the evidence‐base for programmatic success with intermittent regimens. This is important since the WHO rapid evidence guidance based the recommendations for the continued use of thrice‐weekly, intermittent treatments in HIV‐negative children in the continuation phase of therapy, in spite of low quality evidence, on the consideration that children living in countries where intermittent treatment is given as part of national TB control programmes should not be excluded from the benefits of treatment (WHO 2010a). Such reports will then provide valuable updates of the benefits of intermittent treatment from programmatic evaluations to supplement data from future RCTs.
Acknowledgements
This document is an output of protocol‐development and review‐completion workshops organized by the South Asian Cochrane Network and Centre, hosted at the Prof BV Moses and ICMR Centre for Evidence‐Informed Health Policy at the Christian Medical College, Vellore that was partly funded by the Indian Council for Medical Research (ICMR) until August 2012. The views expressed in this review are not necessarily those of ICMR, the Liverpool School of Tropical Medicine, or the Christian Medical College, Vellore.
We are grateful to Jim Te Water Naude for meeting with PT in Cape Town in May 2013 for a detailed discussion of our interpretation of the data for treatment response from this trial (Te Water Naude (South Africa) 2000); and for confirming that the data we used in this review for the outcome of cure accurately represents the proportion of children in each treatment arm who had a treatment response that the authors of the trial considered synonymous with cure. We are also grateful to Jim for further correspondence providing unpublished details on the methods of the trial, and for permission to acknowledge his valuable contributions.
We thank the editorial base of the Cochrane Infectious Diseases Group (CIDG) for editorial support, in particular to Vittoria Lutje, information specialist, and Olalekan Uthman, the academic editor for this review. The editorial base for the CIDG is funded by the UK Department for International Development (DFID) for the benefit of developing countries. We thank the peer referees of this review (Prof Sushil K Kabra, All India Institute of Medical Sciences India; Andrea T. Cruz, Baylor University; and Laura Bonnett, Statistician, Institute of Translations Medicine, University of Liverpool) for helpful comments.
Appendices
Appendix 1. TB regimen according to categories recommended by the WHO*
| TB diagnostic category | TB cases | Regimen | |
| Intensive phase (daily or intermittent)** | Continuation phase (daily or intermittent)** | ||
| I | New smear‐positive pulmonary TB New smear‐negative pulmonary TB with extensive parenchymal involvement Severe forms of extrapulmonary TB (other than TB meningitis) Severe concomitant HIV disease |
2HRZE | 4HR or 6HE |
| I | TB meningitis | 2RHZS | 4RH |
| II | Previously treated smear‐positive pulmonary TB: ‐ relapse ‐ treatment after interruption ‐ treatment failure |
2HRZES+1HRZE | 5HRE |
| III | New smear‐negative pulmonary TB (other than in category I) Less severe forms of extrapulmonary TB |
2HRZ | 4HR or 6HE |
| IV | Chronic and multi‐drug resistant TB | Specially designed standardized or individualized regimens | ‐ |
| *Sources: Donald 2007b and WHO 2006b. **Recommended doses (WHO 2010a) isoniazid (H) – 10 mg/kg (range 10 to 15 mg/kg); maximum dose 300 mg/day; rifampicin (R) – 15 mg/kg (range 10 to 20 mg/kg); maximum dose 600 mg/day; pyrazinamide (Z) – 35 mg/kg (30 to 40 mg/kg); ethambutol (E) – 20 mg/kg (15 to 25 mg/kg). Additional recommendations (WHO 2010a):
| |||
Appendix 2. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis |
| 2 | isoniazid | ANTITUBERCULAR AGENTS/ADMINISTRATION AND DOSAGE | ANTITUBERCULAR AGENTS/ADMINISTRATION AND DOSAGE | TUBERCULOSIS/DRUG THERAPY | isoniazid |
| 3 | rifampicin | STREPTOMYCIN | STREPTOMYCIN | STREPTOMYCIN | rifampicin |
| 4 | rifampin | ISONIAZID | ISONIAZID | ISONIAZID | rifampin |
| 5 | pyrazinamide | RIFAMPIN | RIFAMPIN | RIFAMPICIN | pyrazinamide |
| 6 | ethambutol | rifampicin | rifampicin | rifampin | ethambutol |
| 7 | streptomycin | PYRAZINAMIDE | PYRAZINAMIDE | PYRAZINAMIDE | streptomycin |
| 8 | intermittent | ethambutol | ethambutol | ethambutol | intermittent |
| 9 | 2‐8/OR | intermittent | intermittent | intermittent | 2‐8/OR |
| 10 | 1 and 9 | 2‐9/OR | 2‐9/OR | 2‐9/OR | 1 and 9 |
| 11 | Child* | Child* | Child* | Child$ | Child$ |
| 12 | Pediatr* | Pediatr* | Pediatr* | Pediatr$ | Pediatr$ |
| 13 | Infant* | Infant* | Infant* | Infant$ | Infant$ |
| 14 | 11 or 12 or 13 | 11 or 12 or 13 | 11 or 12 or 13 | 11 or 12 or 13 | 11 or 12 or 13 |
| 15 | 10 and 14 | 1 and 10 and 14 | 1 and 10 and 14 | 1 and 10 and 14 | 10 and 14 |
| 16 | — | — | Limit 15 to Human | Limit 15 to humans | — |
Footnotes
aCochrane Infectious Diseases Group Specialized Register.
bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 3. Abstracts sought for additional trials
Conference proceedings of the American Thoracic Society (http://www.thoracic.org/journals/pats/index.php):
ATS International Conference, San Diego, May 2009
ATS International Conference, New Orleans, May 2010
ATS International Conference, Denver, Colorado, May 2011
ATS International Conference, San Francisco, May 2012
Conferences proceedings of the International Union against Tuberculosis and Lung Disease (http://www.theunion.org/index.php/en/conferences):
1st Conference of The Union South‐East Asia Region, New Delhi, India, September 2008
5th Conference of The Union Europe Region, Dubrovnik, Croatia, May 2009
13th Conference of The Union Latin American Region, San Salvador, El Salvador, March 2010
18th Union Conference for the African Region, Abuja, Nigeria, March 2011
3rd Conference of The Union Asia‐Pacific Region, Hong Kong, China, July 2011
42nd Union World Conference on Lung Health, Lille, France, October 2011
43rd Union World Conference on Lung Health, Kuala Lumpur, Malaysia, November 2012
Conference proceedings of the TB Education and Training Network (http://www.cdc.gov/tb/education/tbetn/pastconferences.htm ):
TB Education and Training Network's First Annual Meeting and Workshop: Culture, Language, and Literacy in TB Education and Training, Atlanta, Georgia, August 2001
2002 Conference ‐ TB Education and Training Network's Second Annual Meeting and Workshop: Reaching Key Audiences through Innovative TB Education and Training Methods, Atlanta, Georgia, August 2002
2003 Conference ‐ TB Education and Training Network's Third Annual Conference: Oh, the Places TB Education Can Go, Atlanta, Georgia, August, 2003
2004 Conference ‐ TB Education and Training Network's Fourth Annual Conference: TB Education and Training Survivor: Improving skills, building alliances, meeting challenges, Atlanta, Gerogia, August 2004
2005 Conference ‐ TB Education and Training Network's Fifth Annual Conference: Stepping Up Education and Training to Eliminate TB, Atlanta, Georgia, August 2005
2006 Conference ‐ TB Education and Training Network's Sixth Annual Conference: TB Education and Training Magic: Tricks of the Trade, Atlanta, Georgia, August, 2006
2007 Conference ‐ TB Education and Training Network's Seventh Annual Conference: The Amazing Race to Eliminate TB: Education and Training Skills to Succeed, Atlanta, Georgia, August 2007
2008 Conference ‐ The Eighth Annual TB ETN Conference: TB Education and Training: Going for the Gold, Atlanta, Georgia, August 2008
2009 Conference ‐ The Ninth Annual TB ETN Conference: TB Education and Training: Recipes for Success: Atlanta, Georgia, August 2009
2010 Conference ‐ The Tenth Annual TB ETN Conference: TB Education and Training: Fitting the Pieces Together, Atlanta, Georgia, August 2010
2011 Conference ‐ The 11th Annual TB ETN Conference: Waves of Change, Oceans of Opportunity, Atlanta, Georgia, September, 2011
2012 Conference‐ The 12th Annual TB ETN Conference: Lights, Camera, Action: Setting the Stage for TB Elimination, Atlanta, Georgia, September 2012
Appendix 4. Design, analysis, and reporting considerations for RCTs comparing intermittent versus daily treatment for children with TB
Population, setting, diagnoses, and comparators
Adequately powered, multicentre, multi‐country, randomized, allocation‐concealed, assessor‐blinded, non‐inferiority, pragmatic trials conducted in high TB‐transmission settings, in low and middle‐income countries, comparing thrice‐weekly, and daily short‐course (six months) treatment in HIV‐negative children should be considered a priority. Fully intermittent treatment versus partially‐intermittent treatment should be evaluated in similar separate trials, since there is no convincing evidence to support the current recommendations against the use of fully intermittent treatment, and some national programmes use fully intermittent treatment regimens.
Such trials should use standard methods to diagnose children with TB and recruit children with newly‐diagnosed TB using clinical, laboratory, radiological and immunological criteria, and stratify recruitment according to whether they had pulmonary TB, TB lymphadenitis, or disseminated TB. Since outcome trajectories differ in children who present to tertiary care and primary care settings, recruitment in such trials should ideally include children with TB identified from primary, secondary, and tertiary care settings. Children with TB meningitis, and bone and joint TB should be excluded from these trials since the recommended duration of treatment is longer than for other forms of TB in children; and they should be included in separate trials comparing intermittent versus daily treatment. Children should be tested for HIV‐infection and those testing positive should be excluded. Children less than one year of age born to HIV‐infected mothers and in whom the mother's HIV status is not known should be included only after HIV tests reliably reveal them to be HIV‐negative; or they should be treated for TB outside the trial. Children for whom the index case has drug‐resistant TB should also be excluded and treated appropriately outside the trial, unless cultures, if available, rule out drug‐resistant organisms.
Interventions, doses, and supervision
The doses of anti‐TB drugs should follow the currently recommended dose/kg/day stipulated in WHO 2010a; and the drug combinations and durations to be used in the intensive and continuation phases should follow the recommendations in WHO 2010a.
Treatment in the intermittent arms should be by DOT throughout and in the daily arm should be by DOT in the two‐month intensive phase at least.
Outcomes
The primary outcome should be treatment response using standard criteria that includes clinical, bacteriological, and radiological criteria for pulmonary and extra‐pulmonary TB. The first assessment for treatment response should be at three months after treatment‐initiation and monthly there‐after till six months. Those with no improvement or worsening in these criteria at six months or who require additional treatment should be considered treatment‐non‐responders. Adverse event monitoring should be actively undertaken in both intervention arms at regular periods using standard clinical and laboratory parameters throughout the six month period. Parents of children should be educated regarding the need for adherence and treatment completion and appropriately compensated for trial‐related expenses, to ensure continued participation. All recruited children should be assessed for relapse using the same criteria used for diagnosis at recruitment at nine, 12 and 18 months after treatment initiation; thereby providing relapse rates over one year of follow‐up after treatment. Further follow‐up may only provide re‐infection rates, rather than true relapse rates. Assessments for treatment‐response and relapse should be undertaken by independent assessors. Treatment failures should be evaluated for drug‐resistance, if not thought to be due to poor compliance. Consideration should be given to assessing pharmacokinetic data and evaluating the adequacy of drug levels, particularly in those considered treatment failures as well as in those with serious adverse events.
Evaluation of resource use and resource costs and full economic analyses should be conducted alongside the other evaluations and should be reported in full alongside the main results or in ancillary publications.
Analyses
If children are randomized by households, then the number of children randomized by households per intervention arm should be reported along with follow‐up information regarding the number of children randomized by households who completed the trial. Analysis should be by intention‐to‐treat where those lost to follow‐up before achieving treatment response should be considered treatment‐non‐responders. Results for those who completed treatment per protocol, and those who adhered to >75% of prescribed doses should also be reported for each intervention arm. Results should be adjusted for randomization by households (if appropriate) and any adjusted results, including the proportions considered treatment responders in each arm, should be reported, along with the intra‐cluster correlation coefficient (ICC). Quality of life assessments, if used, should use pre‐validated measures and should be reported for each assessment time point as means with standard deviations, in each group. If change scores are reported, then the standard deviation of the change score should also be reported.
Samples size estimates for non‐inferiority designs should pre‐set the treatment success rates at over 90% in each intervention arm in evaluating the numbers needed to be recruited; and should also pre‐state the confidence limits for an intention‐to‐treat and per‐protocol analyses to demonstrate non‐inferiority and for equivalence.
Reporting standards and prospective trials registration
Trials should follow CONSORT 2010 guidelines for reporting and the CONSORT extensions for cluster randomized and non‐inferiority trials, if these designs are used. They should ensure and report appropriate methods to avoid selection, performance, detection, attrition, and reporting biases. Trials should also be prospectively registered in a publicly accessible trials registry, and their protocols and supplementary analyses reported in full along with the trial publication.
Ethical issues
Ethical guidelines involving research in children should be followed since children represent a vulnerable population that require special precautions when clinical trials are performed (EU 2008).
Appendix 5. Optimal information size calculations (TB treatment outcomes)
| Outcome | Power | Significance level | Risk in control group | Hypothesis | Equivalence limit | RR deemed clinically significant (examples) | Sample size (Total) |
| Death | 80% | 5% | 5%1 | Equivalence | 5% | ‐ | 652 |
| 2% | ‐ | 4070 | |||||
| Superiority | ‐ | 0.5 | 2418 | ||||
| Cure | 80% | 5% | 80%2 | Equivalence | 10% | ‐ | 550 |
| 5% | ‐ | 2194 | |||||
| Superiority | ‐ | 1.06 | 1806 | ||||
| Relapse | 80% | 5% | 5% | Equivalence | 5% | ‐ | 652 |
| Superiority | ‐ | 0.5 | 2418 | ||||
| Adherence | 80% | 5% | 80% | Equivalence | 5% | ‐ | 2194 |
| Superiority | ‐ | 1.06 | 1806 |
Footnotes:
We performed all calculations using http://www.sealedenvelope.com
1Globally the risk of death in patients receiving treatment for TB is around 5%. 2The target cure rate for DOTS programs is 80%. The current global average is 86%.
Data and analyses
Comparison 1. Intermittent versus daily regimens.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure (as defined by clinical and radiological improvement): Intention to treat | 4 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.10] |
| 1.1 Intermittent (daily 2 weeks, twice‐weekly 8.5 months) versus daily treatment (12 months) | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.95, 1.50] |
| 1.2 Intermittent (twice‐weekly 6 months) versus daily treatment (daily 2 months, intermittent 4 months) | 1 | 76 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 1.3 Intermittent (thrice‐weekly 2 months, twice‐weekly 4 months) versus daily treatment (9 months) | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.91, 1.14] |
| 1.4 Intermittent (twice‐weekly 6 months) versus daily treatment (6 months) | 1 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.15] |
| 2 Cure (as defined by clinical and radiological improvement): Per‐protocol (Sensitivity analysis) | 4 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
| 2.1 Intermittent (daily 2 weeks, twice‐weekly 8.5 months) versus daily treatment (12 months) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.89, 1.12] |
| 2.2 Intermittent (twice‐weekly 6 months) versus daily treatment (daily 2 months, intermittent 4 months) | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.93, 1.15] |
| 2.3 Intermittent (thrice‐weekly 2 months, twice‐weekly 4 months) versus daily treatment (9 months) | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.12] |
| 2.4 Intermittent (twice‐weekly 6 months) versus daily treatment (6 months) | 1 | 206 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.86, 1.14] |
| 3 Death from any cause | 4 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 8.96] |
| 4 Relapse | 4 | 449 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.68 [0.15, 89.33] |
| 5 Adherence to treatment | 4 | 458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.11] |
| 6 Treatment‐limiting adverse events | 4 | 441 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.06, 2.60] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kansoy (Turkey) 1996.
| Methods |
Design: Randomized, parallel group, single‐centre, open‐labelled, controlled trial Trial duration: December 1988 to February 1992 (recruitment) |
|
| Participants |
Number randomized: 36 children Age: Five months to 13 years Gender: Both Inclusion criteria: Diagnosed cases of TB fulfilling the following criteria:
Exclusion criteria: Not reported |
|
| Interventions |
Intervention:
Control:
Doses used: Rifampicin‐ 15 mg/kg; maximum 600 mg/day; streptomycin‐ 20 mg/kg IM up to 1 g; INH 15 mg/kg up to 400 mg |
|
| Outcomes |
Outcomes used in this review
Outcomes reported and not used in this review
|
|
| Notes |
Country: Turkey Setting: SSK Tepecik Teaching Hospital, Izmir; outpatients Funding: Not mentioned Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Thirty six patients were randomly allocated either to the study group or conventional therapy group" Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method used to concealment allocation not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial was open labelled, and though outcomes are objective, some amount of detection bias is possible due to a degree of subjectivity in diagnosing TB in children. Unclear if outcome assessors were aware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The three drop outs after randomization during the follow‐up period in the control group due to poor family compliance are reported |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available but all relevant outcomes addressing the trial objectives were fully reported |
| Other bias | Low risk | The duration of INH in the control arm was for 12 months while INH was given for nine months in the intermittent treatment arm; the two arms are therefore not strictly comparable but this would affect external validity more than internal validity |
Kumar (India) 1990.
| Methods |
Trial Design: Randomized, parallel group, single‐centre, open‐labelled, controlled trial Trial duration: Not reported |
|
| Participants |
Number randomized: 76 children Age: One year to 15 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control:
Doses used: Intermittent regimen: INH 20 to 30 mg/kg body weight /dose; rifampicin 10 to 15 mg/kg body weight/dose; pyrazinamide 50 to 60 mg/kg body weight /dose Daily regimen: INH 10 to 15 mg/kg body weight/dose; rifampicin 10 to 15 mg/kg body weight/dose; pyrazinamide 20 to 30 mg/kg body weight/dose |
|
| Outcomes |
Outcomes used in this review
Criteria for improvement 1. General improvement : Disappearance of fever, improvement in appetite and weight gain 2. Pulmonary TB response
3. Tuberculous lymphadenitis response:
4. Disseminated TB response:
|
|
| Notes |
Country: India Setting: Post Graduate Institute of Medical Education and Research, Chandigarh Hospital; outpatients Funding: Indian Council of Medical Research Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "At enrolment any currency note available was taken and its number was noted. Even numbers were assigned to Regimen A and odd numbers to Regimen B". Quasi‐random assignment |
| Allocation concealment (selection bias) | Unclear risk | Quasi‐random allocation may lead to selection bias; however, groups were balanced at baseline on important prognostic indicators though the possibility of residual confounding cannot be ruled out |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial was open labelled, and though outcomes are objective, some amount of detection bias is possible due to a degree of subjectivity in diagnosing TB in children; however, the explicit pre‐stated criteria for defining response would have reduced the element of subjectivity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Ten withdrawals were reported; 4/37 in the intervention arm and 6/39 in the control arm which was not significantly different. The participants withdrawing from the trial were from families of migrant labourers. |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available but all relevant outcomes addressing the trial objectives were fully reported. |
| Other bias | Low risk | No other biases detected |
Ramachandran (India) 1998.
| Methods |
Trial Design: Randomized, parallel group, single‐centre, open‐label, stratified (by probability of TB) assessor blinded, controlled trial Trial duration: 1992 to 1997 |
|
| Participants |
Number randomized: 137 children Age: One year to 12 years Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control:
Doses used: INH 6 mg/kg (maximum 150 mg) for daily phase and 15 mg/kg (maximum 300 mg) for intermittent phases; rifampicin 12 mg/kg (maximum 300 mg); pyrazinamide 45 mg/kg (maximum 1 g) |
|
| Outcomes |
Outcomes used in this review
Outcomes reported but not used in this review
|
|
| Notes |
Country: India Settings: Tuberculosis Research Centre, Chennai; hospitalized for minimum two weeks or more; then outpatients Funding: Not stated
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated" Method of sequence generation is not stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "An important feature is that all the radiographs were read by a radiologist, an independent assessor, who was not aware of any of the details pertaining to the patients". Assessor blinding for the primary outcome and the use of objective secondary outcomes reduces the risk of detection bias; the open label design could introduce performance bias but this appears unlikely to have influenced outcome estimates |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All exclusions, deaths, and losses to follow‐up were reported. Treatment allocation for one death in the first month attributed to a non‐tuberculous cause was not reported; this does not affect relative estimates for deaths due to interventions. 11 children were lost to follow‐up for the final follow‐up at 60 months; the last available radiographs (all at or after 24 months) were considered for evaluation and included all 137 evaluable participants . |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available but all relevant outcomes addressing the trial objectives were fully reported. |
| Other bias | Low risk | The intermittent treatment arm used thrice‐weekly regimen for the first two months and a twice weekly regimen for the next four months that is a non‐standard regimen; Pyrazinamide was used for the first two months in the intermittent regimen and was not used in the 9‐month daily regimen. These would affect external validity rather than internal validity |
Te Water Naude (South Africa) 2000.
| Methods |
Trial design: Cluster‐randomized (by households), single‐centre, open labelled, controlled trial Trial duration: June 1991 to June 1995 |
|
| Participants |
Number randomized: 314 children Age: < 14 years; (range 13.4 months to 43.2 months) Gender: Both Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention:
Control:
|
|
| Outcomes |
Outcomes used in this review
Outcomes reported but not used in this review
|
|
| Notes |
Country: South Africa Setting: Idas Valley clinic, Stellenbosch district, Western Cape Province; outpatients Funding: South African Medical Council Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from report: "Children diagnosed as having tuberculosis were randomized by household unit to receive either intermittent treatment or daily treatment. Randomization was by random number tables." Quote from discussions and correspondence with first author: "The method of allocation: When a child fulfilled the eligibility criteria, the parent was asked if they would participate in the study. Households were assigned to one or the other group. A random number table was used: an odd number would assign the household to 2x weekly and an even number to 5x weekly". Comment: It is unclear if the use of odd and even numbers from a random number table would permit unpredictability in treatment allocation |
| Allocation concealment (selection bias) | High risk | Quote from report: "Informed consent was obtained after randomisation" Quote from correspondence with the first author,"Consent was taken after randomization, for purposes of informed choice or consent". Comment: As informed consent was taken after randomization, allocation was most likely not concealed. Of 153 randomized to intermittent treatment only 95 (62%) were included in the trial after exclusions; of 161 randomized to daily treatment, only 118 (73%) were included in the trial after exclusions (P < 0.05) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote from discussions and correspondence with the first author, "CXR readers were blinded to the regimen of treatment until after the reading was captured. Parental response was recorded by me yes. I saw all the cases clinically." Comment: The trial was open label, and though outcomes are mostly objective, some amount of detection bias is possible due to a degree of subjectivity in diagnosing TB in children using the composite of parent's report and clinical criteria, though radiological assessments were done blind to allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportions completing the trial after initiation of treatment in the intermittent treatment arm 89/95 (94%) and the daily arm 117/118 (99%) were not statistically significantly different. At six months, 163 of 206 randomized evaluable children were assessed for treatment response (77%); of which 70/89 (79%) were in the arm allocated twice weekly intermittent treatment and 93/117 (79%) were in the daily treatment arm (P = 0.40). |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available but all relevant outcomes addressing the trial objectives were fully reported. |
| Other bias | Unclear risk | Quote from trial report, "Households rather than patients were used in randomisation to avoid confusion in the event of more than one child from a particular household being enrolled". Quote from correspondence with author: "we randomised by household, but analysed by individual. There were few where there were more than one per household" Comment: Though the results were not adjusted for clustering; the proportions randomized by household are probably fewer than those individually randomized |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Dossary 2002 | Not a RCT: prospective cohort |
| Amrane 1989 | Not a RCT; controlled clinical trial |
| Anastasatu 1991 | Not a RCT; controlled clinical trial comparing two intermittent regimens |
| Anastasatu 1993 | Not a RCT; controlled clinical trial comparing two intermittent regimens |
| Balasubramanian 1990 | Not a RCT; controlled clinical trial comparing daily regimen with intermittent regimens |
| Bignall 1974 | Not a RCT, controlled clinical trial comparing five intermittent regimens |
| Carter 1954 | Not a RCT; study is on use of INH in treatment |
| Comstock 1969 | Not a RCT; controlled clinical trial comparing two doses of INH for prophylaxis of TB |
| Comstock 1970 | Not a RCT; controlled clinical trial aimed at finding the appropriate dosage of INH |
| Debre 1973 | Not a RCT; controlled clinical trial of INH chemoprophylaxis |
| Dingley 1974 | RCT; compared four daily regimens |
| Dingley 1982 | RCT; compared four daily regimens |
| Eule 1986 | RCT: compared four regimens but there was no daily six‐month regimen |
| Ganguin 1973 | Not a RCT; controlled clinical trial |
| Garciá López 1989 | Not a RCT; case series with historical controls |
| Göçmen 1993 | Not a RCT: retrospective study |
| HKCS‐BMRC 1991 | RCT: compared four intermittent regimens |
| HKTTS/BMRC 1975 | RCT: compared daily versus intermittent therapy but did not use rifampicin in the intensive phase. |
| Jawahar 1990 | Not a RCT: case series |
| Jawahar 2005 | RCT: compared intermittent versus daily regimens; included adults and children but separate data for 87 children not available from trial report or from authors |
| Jindani 2004 | RCT: adults and adolescents included; no disaggregated results |
| Karam‐Bechara 1994 | Not a RCT; follow‐up study of two cohorts |
| MRC 1993 | RCT: compared different daily regimens to treat spinal TB and there is no intermittent regimen. |
| No authors 1969 | RCT: compared four different regimens for re‐treatment of TB. |
| No authors 1974 | RCT: used only a once‐weekly regimen and was aimed at studying the side effects of rifampicin. |
| No authors 1975 | RCT: compared two daily followed by intermittent regimens and there was no comparison of daily versus intermittent regimens |
| No authors 1976 | RCT: compared three intermittent regimens |
| No authors 1977a | RCT: compared different intermittent regimens |
| No authors 1977b | RCT: compared three intermittent regimens |
| No authors 1981 | RCT: compared four different regimens of which one was daily and the other were intermittent. However there was no rifampicin in any of the regimens. |
| Rajeswari 1995 | RCT: initial phase of daily and intermittent arms was for three months |
| Ramachandran 1986 | Not a RCT: controlled clinical trial |
| Ramesh 1991 | Not a RCT; controlled clinical trial |
| Santha 1989 | RCT: compared non‐standard regimens |
| STS‐BMRC 1985 | RCT: compared three regimens of daily therapy for one or two months followed by intermittent therapy for a total treatment duration of six‐months. |
| Swaminathan 2005 | RCT: only daily treatment arm had rifampicin and INH |
| Tam 2002 | RCT: continuation phase had no daily therapy arm |
| Teo 2002 | RCT: compared two four‐month regimens |
| TRC 1970 | Not a RCT: controlled clinical trial |
| TRC 1997 | RCT: compared one daily regimen and two intermittent regimens. Included participants 12‐62 years of age. Data for children aged 12 to 15 years not available from report or from authors |
| WHO CCTC 1971 | RCT: compared daily treatment for three months followed by randomized intermittent treatment; participants were aged 15 to 70 years |
| WHO CCTC 1973 | RCT: compared daily treatment for three months followed by randomized intermittent treatment; participants were aged 15 to 70 years |
Differences between protocol and review
We updated the background section to incorporate changes in the recommended treatments for childhood TB that were published since the protocol was published. 'Risk of bias' tables and 'Summary of findings' tables were introduced as standard for Cochrane Reviews after this protocol was published. We generated 'Risk of bias' for the included trials in this review using the methods described in Higgins 2011a. We used GRADE profiler (GRADE 2013) and interpreted the evidence for each important and critically important outcome for the comparisons in the included trials using the GRADE approach (Schunemann 2008) to create 'Summary of findings' tables for each comparison. We selected outcomes to include in these tables though discussion before evaluating the search results.We described in our methods section, the GRADE approach to assessing overall study quality, the meaning of each grade of quality and the terminology we used to reflect these grades of quality.
We clarified in the review's methods our approach to dealing with Unit of analysis issues arising from cluster randomized trials, that were not described adequately in the protocol. These methods were based on advice provided in Higgins 2011b, that were updated after the protocol was published. In this section we had earlier stated that If the results of cluster‐randomized trials had not been adjusted for clustering, we would not have combined them in a meta‐analysis, but would have presented the results in an additional table. Te Water Naude (South Africa) 2000 partly randomized children by households but the author clarified that only a few children were thus randomized and the majority were individually randomized, and hence we extracted data as for individually randomized trials, and assessed the exclusion of the data from this trial in sensitivity analyses.
We also described in more detail in the methods section, our approach to assessing heterogeneity following guidance in Deeks 2011, that was also updated after our protocol was published.
In Types of interventions, we clarified that the duration of daily treatment could be up to one year. We added under Dealing with missing data that we conducted an intention‐to‐treat analysis in trials with no loss to follow‐up and completed‐case analysis for trials with incomplete follow‐up, except for the primary outcome of cure, where losses to follow‐up before meeting criteria treatment response were considered not cured. We also clarified that we did not make any assumptions for the secondary outcomes or for adverse events, due to difficulties in making valid assumptions about those lost to follow‐up for these outcomes, apart from what was reported in the trials.
Contributions of authors
AB conceived the idea for this review. All authors contributed to developing and writing the protocol. SK and WR extracted data and assessed risk of bias. AB, SK and WR drafted GRADE tables and the review. PT revised the GRADE tables and wrote the final version of the review. All authors helped to revise the review draft and approved the final review version.
Sources of support
Internal sources
-
Christian Medical College, Vellore, Tamil Nadu, India.
Employment for Anruradha Bose, Winsley Rose and Prathap Tharyan.
-
South Asian Cochrane Network & Centre, Vellore, Tamil Nadu, India.
Logistic support, protocol development and review completion workshops. Employment for Soumik Kalita during protocol development.
-
South Asian Network for Chronic Diseases, Public Health Foundation of India, India.
Employment for Soumik Kalita for a short period during review completion
External sources
-
UKAid: Department for International Development (DFID), UK.
Funding for the South Asian Cochrane Centre via the Effective Healthcare Research Consortium (Paul Garner, Liverpool School of Tropical Medicine, UK)
-
Indian Council of Medical Research, India.
Funding for Prof BV Moses and ICMR Centre for Evidence‐Informed Healthcare at CMC Vellore, that hosts the South Asian Cochrane Centre
Declarations of interest
AB, WR and PT have no financial or academic conflicts of interest.
Soumik Kalita is currently Head of Nutrition and Medical Affairs, GSK Consumer Healthcare‐Family Nutrition. In this position he looks after nutrition research for GSKCH Family Nutrition globally and this has no relationship with the work done by GSK Pharma which deals with drugs and vaccines against diseases. GSK Consumer Health is an entity that is separate from its GSK Pharma counterpart. Soumik was employed by the South Asian Cochrane Centre during the protocol phase of this review, and by the South Asian Network for Chronic Diseases, Public Health Foundation of India, during the initial phases of review completion. He joined GSKCH in September 2010. He declares no financial or academic conflicts of interest that would affect the conduct or conclusions of this review.
Unchanged
References
References to studies included in this review
Kansoy (Turkey) 1996 {published data only}
- Kansoy S, Kurtaş N, Akşít S, Aksoylar S, Yaprak I, Çağlayan S. Superiority of intermittent‐short course chemotherapy in childhood pulmonary tuberculosis. Turkish Journal of Medical Sciences 1996;26(1):41‐3. [Google Scholar]
Kumar (India) 1990 {published data only}
- Kumar L, Dhand R, Singhi PD, Rao KL, Katariya S. A randomized trial of fully intermittent vs. daily followed by intermittent short course chemotherapy for childhood tuberculosis. Pediatric Infectious Disease Journal 1990;9(11):802‐6. [DOI] [PubMed] [Google Scholar]
Ramachandran (India) 1998 {published data only}
- Ramachandran P, Kripasankar A S, Duraipandian M. Shortcourse chemotherapy for pulmonary tuberculosis in children. Indian Journal of Tuberculosis 1998;45:83–7. [Google Scholar]
Te Water Naude (South Africa) 2000 {published and unpublished data}
- Water Naude JM, Donald PR, Hussey GD, Kipel MA, Louw A, Perkins DR, et al. Twice weekly vs. daily chemotherapy for childhood tuberculosis. Pediatric Infectious Disease Journal 2000;19(5):405‐10. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Dossary 2002 {published data only}
- Al‐Dossary FS, Ong LT, Correa AG, Starke JR. Treatment of childhood tuberculosis with a six month directly observed regimen of only two weeks of daily therapy. Pediatric Infectious Disease Journal 2002;21(2):91‐7. [DOI] [PubMed] [Google Scholar]
Amrane 1989 {published data only}
- Amrane R, Mazouni L, Boulahbal F, Abtroun N, Zidane, C. A controlled survey comparing 2 short‐term therapeutic regimes in lymph node tuberculosis [Enquête contrôlée comparant deux régimes thérapeutiques de courte durée dans la tuberculose ganglionnaire]. Revue Des Maladies Respiratoires 1989;6(1):53‐7. [PubMed] [Google Scholar]
Anastasatu 1991 {published data only}
- Anastasatu C, Anastasatu O, Murgoci G, Dobre M, Bîscă N, Calciu M, et al. The late results of short‐term chemotherapy in benign forms of tuberculosis in children. Pneumoftiziologia 1991;40(4):7‐9. [PubMed] [Google Scholar]
Anastasatu 1993 {published data only}
- Anastasatu C, Anastasatu O, Murgoci G, Dobre M. The late results of intensive chemotherapy (9 months) in severe forms of tuberculosis in children [Rezultatele tardive ale chimioterapiei intensive (9 luni) în formele grave de tuberculoză la copil]. Pneumoftiziologia 1993;42(4):9‐12. [PubMed] [Google Scholar]
Balasubramanian 1990 {published data only}
- Balasubramanian R, Sivasubramanian S, Vijayan VK, Ramachandran R, Jawahar MS, Paramasivan CN, et al. Five year results of a 3‐month and two 5‐month regimens for the treatment of sputum‐positive pulmonary tuberculosis in south India. Tubercle 1990;71(4):253‐8. [DOI] [PubMed] [Google Scholar]
Bignall 1974 {published data only}
- Bignall JR. [Controlled therapeutic study of 5 intermittent chemotherapy regimens for pulmonary tuberculosis. 4th international study of the UICT]. Bulletin of the International Union against Tuberculosis 1974;49(1):422‐6. [PubMed] [Google Scholar]
Carter 1954 {published data only}
- Carter S. Primary tuberculosis in African children and the value of isoniazid in treatment. East African Medical Journal 1954;31(6):265‐76. [PubMed] [Google Scholar]
Comstock 1969 {published data only}
- Comstock GW, Hammes LM, Pio A. Isoniazid prophylaxis in Alaskan Boarding schools. A comparison of two doses. American Review of Respiratory Disease 1969;100(6):773‐9. [DOI] [PubMed] [Google Scholar]
Comstock 1970 {published data only}
- Comstock GW, Ferebee SH. How much isoniazid is needed for prophylaxis?. American Review of Respiratory Disease 1970;101(5):780‐2. [DOI] [PubMed] [Google Scholar]
Debre 1973 {published data only}
- Debre R, Perdrizet S, Lotte A, Naveau M, Lert F. Isoniazid chemoprophylaxis of latent primary tuberculosis: in five trial centres in France from 1959 to 1969. International Journal of Epidemiology 1973;2(2):153‐60. [DOI] [PubMed] [Google Scholar]
Dingley 1974 {published data only}
- Dingley HB, Sehgal KL. Treatment of pulmonary tuberculosis in children‐‐a controlled study. Indian Pediatrics 1974;11(4):289‐95. [PubMed] [Google Scholar]
Dingley 1982 {published data only}
- Dingley HB. Short term chemotherapy in children: Three years follow up. Indian Journal of Tuberculosis 1982;29(1):48‐54. [Google Scholar]
Eule 1986 {published data only}
- Eule H, Beck H, Evers H, Fischer P, Kwiatkowski H, Merkel S, et al. Daily ultrashort chemotherapy and intermittent short‐term chemotherapy with 4 drugs of communicable pulmonary tuberculosis treated for the first time. Results of a cooperative multicenter study. Zeitschrift für Erkrankungen der Atmungsorgane 1986;167(1‐2):29‐41. [PubMed] [Google Scholar]
Ganguin 1973 {published data only}
- Ganguin HG, Cramer G, Götz J, Holzhauer H, Niegsch G, Sieler H, et al. Proceedings: Results of a controlled trial of intermittent ambulatory therapy of infectious pulmonary tuberculosis with comparison of various maintenance regimens. Zeitschrift für Erkrankungen der Atmungsorgane mit Folia Bronchologica 1973;138(1):107‐15. [PubMed] [Google Scholar]
Garciá López 1989 {published data only}
- Garciá López JR, Vidal López ML, Castán Vidal ML, Borque Andrés C, José Gómez MI, García Hortelano J. Short term treatment of infantile pulmonary tuberculosis [Tratamiento de corta duración de la tuberculosis pulmonar infantil]. Anales Españoles De Pediatría 1989;31(2):110‐3. [PubMed] [Google Scholar]
Göçmen 1993 {published data only}
- Göçmen A, Özçelic U, Kiper M, Toppare M, Kaya S, Cengizlier R, et al. Short course intermittent chemotherapy in childhood tuberculosis. Infection 1993;21(5):324‐7. [DOI] [PubMed] [Google Scholar]
HKCS‐BMRC 1991 {published data only}
- Hong Kong Chest Service/British Medical Research Council. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6‐month, three‐times‐weekly regimens for smear‐positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide. Results at 30 months.. American Review of Respiratory Diseases 1991;143(4 Pt 1):700‐6. [DOI] [PubMed] [Google Scholar]
HKTTS/BMRC 1975 {published data only}
- Hong Kong Tuberculosis Treatment Services/British Medical Research Council. Controlled trial of 6‐ and 9‐month regimens of daily and intermittent streptomycin plus isoniazid plus pyrazinamide for pulmonary tuberculosis in Hong Kong. Tubercle 1975;56(2):81‐96. [DOI] [PubMed] [Google Scholar]
Jawahar 1990 {published data only}
- Jawahar MS, Sivasubramanian S, Vijayan VK, Ramakrishnan CV, Paramasivan CN, Selvakumar V, et al. Short course chemotherapy for tuberculous lymphadenitis in children. BMJ 1990;301(6748):359‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jawahar 2005 {published data only}
- Jawahar MS, Rajaram K, Sivasubramanian S, Paramasivan CN, Chandrasekar K, Kamaludeen MN, et al. Treatment of lymph node tuberculosis‐‐a randomized clinical trial of two 6‐month regimens. Tropical Medicine & International Health 2005;10(11):1090‐8. [PUBMED: 16262733] [DOI] [PubMed] [Google Scholar]
Jindani 2004 {published data only}
- Jindani A, Nunn AJ, Enarson DA. Two 8‐month regimens of chemotherapy for treatment of newly diagnosed pulmonary tuberculosis: international multicentre randomised trial. Lancet 2004;364(9441):1244‐51. [DOI] [PubMed] [Google Scholar]
Karam‐Bechara 1994 {published data only}
- Karam‐Bechara J, Naime‐Libien JE, Posada‐Maldonado EE, Aroch‐Calderón A, Olvera R. Treatment of tuberculosis in 100 children. A 5‐year follow‐up [Tratamiento de tuberculosis en 100 niños. Seguimiento durante cinco años]. Salud Pública De México 1994;36(1):30‐5. [PubMed] [Google Scholar]
MRC 1993 {published data only}
- Medical Research Council Working Party on Tuberculosis of the Spine. Controlled trial of short‐course regimens of chemotherapy in the ambulatory treatment of spinal tuberculosis. Results at three years of a study in Korea. Twelfth report of the Medical Research Council Working Party on Tuberculosis of the Spine. Journal of Bone and Joint Surgery. British Volume 1993;75(2):240‐8. [DOI] [PubMed] [Google Scholar]
No authors 1969 {published data only}
- No authors listed. A controlled comparison of four regimens of streptomycin plus pyrazinamide in the retreatment of pulmonary tuberculosis. Tubercle 1969;50(2):81‐114. [DOI] [PubMed] [Google Scholar]
No authors 1974 {published data only}
- No authors listed. A controlled clinical trial of small daily doses of rifampicin in the prevention of adverse reactions to the drug in a once‐weekly regimen of chemotherapy in Hong Kong: second report:‐the results at 12 months. Tubercle 1974;55(3):193‐210. [DOI] [PubMed] [Google Scholar]
No authors 1975 {published data only}
- No authors listed. A comparative study of daily followed by twice or once weekly regimens of ethambutol and rifampicin in retreatment of patients with pulmonary tuberculosis. The results at 1 year. A cooperative tuberculosis chemotherapy study in Poland. Tubercle 1975;56(1):1‐26. [DOI] [PubMed] [Google Scholar]
No authors 1976 {published data only}
- No authors listed. A study of two twice‐weekly and once‐weekly continuation regimen of tuberculosis chemotherapy, including a comparison of two durations of treatment. 1. First report: the results at 18 months. Tubercle 1976;57(4):235‐49. [DOI] [PubMed] [Google Scholar]
No authors 1977a {published data only}
- No authors. Controlled trial of intermittent regimens of rifampin plus isoniazid for pulmonary tuberculosis in Singapore. The results up to 30 months. American Review of Respiratory Diseases 1977;116(5):807‐20. [DOI] [PubMed] [Google Scholar]
No authors 1977b {published data only}
- No authors listed. A study of two twice‐weekly and a once‐weekly continuation regimen of tuberculosis chemotherapy, including a comparison of two durations of treatment. 2. Second report: the results at 36 months. Tubercle 1977;58(3):129‐36. [DOI] [PubMed] [Google Scholar]
No authors 1981 {published data only}
- No authors listed. Ethambutol plus isoniazid for the treatment of pulmonary tuberculosis‐‐a controlled trial of our regimens. Tubercle 1981;62(1):13‐29. [DOI] [PubMed] [Google Scholar]
Rajeswari 1995 {published data only}
- Rajeswari R, Sivasubramanian S, Balambal R, Parthasarathy R, Ranjani R, Santha T, et al. A controlled clinical trial of short‐course chemotherapy for tuberculoma of the brain. Tubercle and Lung Disease : the Official Journal of the International Union Against Tuberculosis and Lung Disease. 1995;76(4):311‐7. [DOI] [PubMed] [Google Scholar]
Ramachandran 1986 {published data only}
- Ramachandran P, Duraipandian M, Nagarajan M, Prabhakar R, Ramakrishnan CV, Tripathy SP. Three chemotherapy studies of tuberculous meningitis in children. Tubercle 1986;67(1):17‐29. [DOI] [PubMed] [Google Scholar]
Ramesh 1991 {published data only}
- Ramesh V, Misra RS, Saxena U, Mukherjee A. Comparative efficacy of drug regimens in skin tuberculosis. Clinical and Experimental Dermatology 1991;16(2):106‐9. [DOI] [PubMed] [Google Scholar]
Santha 1989 {published data only}
- Santha T, Nazareth O, Krishnamurthy MS, Balasubramanian R, Vijayan VK, Janardhanam B, et al. Treatment of pulmonary tuberculosis with short course chemotherapy in south India‐‐5‐year follow up. Tubercle 1989;70(4):229‐34. [DOI] [PubMed] [Google Scholar]
STS‐BMRC 1985 {published data only}
- Singapore Tuberculosis Service/British Medical Research Council. Clinical trial of three 6‐month regimens of chemotherapy given intermittently in the continuation phase in the treatment of pulmonary tuberculosis.. The American Review of Respiratory Diseases. 1985;132(2):374‐8. [DOI] [PubMed] [Google Scholar]
Swaminathan 2005 {published data only}
- Swaminathan S, Raghavan A, Duraipandian M, Kripasankar AS, Ramachandran P. Short‐course chemotherapy for paediatric respiratory tuberculosis: 5‐year report. International Journal of Tuberculosis and Lung Disease 2005;9(6):693‐6. [PubMed] [Google Scholar]
Tam 2002 {published data only}
- Tam CM, Chan SL, Kam KM, Goodall RL, Mitchison DA. Rifapentine and isoniazid in the continuation phase of a 6‐month regimen. Final report at 5 years: prognostic value of various measures. International Journal of Tuberculosis and Lung Disease 2002;6(1):3‐10. [PubMed] [Google Scholar]
Teo 2002 {published data only}
- Teo SK, Tan KK, Khoo TK. Four‐month chemotherapy in the treatment of smear‐negative pulmonary tuberculosis: results at 30 to 60 months. Annals of the Academy of Medicine, Singapore 2002;31(2):175‐81. [PubMed] [Google Scholar]
TRC 1970 {published data only}
- Tuberculosis Chemotherapy Centre, Madras. A controlled comparison of a twice‐weekly and three once‐weekly regimens in the initial treatment of pulmonary tuberculosis. Bulletin of the World Health Organization 1970;43(1):143‐206. [PMC free article] [PubMed] [Google Scholar]
TRC 1997 {published data only}
- Tuberculosis Research Centre. A controlled clinical trial of oral short‐course regimens in the treatment of sputum‐positive pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 1997;1(6):509‐17. [PubMed] [Google Scholar]
WHO CCTC 1971 {published data only}
- WHO Collaborating Centre for Tuberculosis Chemotherapy. A comparative study of daily and twice‐weekly continuation regimens of tuberculosis chemotherapy, including a comparison of two durations of sanatorium treatment. 1. First report: The results at 12 months. Bulletin of the World Health Organization 1971;45(5):573‐93. [PMC free article] [PubMed] [Google Scholar]
WHO CCTC 1973 {published data only}
- WHO Collaborating Centre For Tuberculosis Chemotherapy. A comparative study of daily and twice‐weekly continuation regimens of tuberculosis chemotherapy, including a comparison of two durations of sanatorium treatment. 2. Second report: the results from 12 to 24 months. Bulletin of the World Health Organization 1973;48(2):155‐65. [PMC free article] [PubMed] [Google Scholar]
Additional references
Abernathy 1983
- Abernathy RS, Dutt AK, Stead WW, MoersDJ. Short‐course chemotherapy for tuberculosis in children. Pediatrics 1983;72(6):801‐6. [PubMed] [Google Scholar]
Acocella 1990
- Acocella G. The use of fixed dose combinations in antituberculous chemotherapy. Rationale for their application in daily, intermittent and pediatric regimens. Bulletin of the International Union against Tuberculosis and Lung Disease 1990;65(2‐3):77‐83. [PubMed] [Google Scholar]
Amdekar 2009
- Amdekar Y. Changes in the management of tuberculosis. Indian Journal of Pediatrics 2009;76(7):739‐42. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Beyers 1996
- Beyers N, Gie RP, Zietsman HL, Kunneke M, Hauman J, Tatley M, et al. The use of a geographical information system (GIS) to evaluate the distribution of tuberculosis in a high‐incidence community. South African Medical Journal 1996;86(1):40‐4. [PubMed] [Google Scholar]
Biddulph 1988
- Biddulph J, Kokoha V, Sharma SS. Short course chemotherapy in childhood tuberculosis. Journal of Tropical Pediatrics 1988;34(1):20‐3. [DOI] [PubMed] [Google Scholar]
Biddulph 1990
- Biddulph J. Short course chemotherapy for childhood tuberculosis. Pediatric Infectious Disease Journal 1990;9(11):794‐801. [DOI] [PubMed] [Google Scholar]
Brent 2008
- Brent AJ, Anderson ST, Kampmann B. Childhood tuberculosis: out of sight, out of mind?. Transactions of the Royal Society of Tropical Medicine and Hygiene 2008;102(3):217‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 1995
- Centers for Disease Control and Prevention. Tuberculosis morbidity ‐ United States, 1994. MMWR. Morbidity and mortality weekly report 1995;44(20):387‐9, 395. [PubMed] [Google Scholar]
Chang 2011
- Chang KC, Leung CC, Grosset J, Yew WW. Treatment of tuberculosis and optimal dosing schedules. Thorax 2011;66(11):997‐1007. [DOI] [PubMed] [Google Scholar]
Chauhan 2004
- Chauhan LS, Arora VK. Formulation of guidelines for diagnosis and treatment of paediatric TB cases under RNTCP. Indian Journal of Tuberculosis 2004;51(1):102‐5. [Google Scholar]
Corbett 2003
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Archives of Internal Medicine 2003;163(9):1009‐21. [DOI] [PubMed] [Google Scholar]
Crofton 1992
- Crofton J, Horne N, Miller F. Clinical tuberculosis. 2nd Edition. London: Macmillan Education, 1999. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Chichester: John Wiley & Sons, Available from www.cochrane‐handbook.org.
Dickinson 1966
- Dickinson JM, Mitchison DA. In vitro studies on the choice of drugs for intermittent chemotherapy of tuberculosis. Tubercle 1966;47(4):370‐80. [Google Scholar]
Donald 2007a
- Donald PR, Schaaf HS. Old and new drugs for the treatment of tuberculosis in children. Paediatric Respiratory Reviews 2007;8(2):134–41. [DOI] [PubMed] [Google Scholar]
Donald 2007b
- Donald PR. The treatment of tuberculosis in childhood. South African Medical Journal 2007;97(10 Pt 2):992‐4. [PubMed] [Google Scholar]
Donald 2008
- Donald PR, Diacon AH. The early bactericidal activity of anti‐tuberculosis drugs: a literature review. Tuberculosis 2008;88(Suppl 1):S75‐83. [DOI] [PubMed] [Google Scholar]
Donald 2011
- Donald PR. Antituberculosis drug‐induced hepatotoxicity in children. Pediatric Reports 2011;3(2):e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
EU 2008
- European Union ad hoc group for the development of implementing guidelines for Directive 2001/20/EC . European Union. Ethical considerations for clinical trials on medicinal products conducted with the paediatric population. European Journal of Health Law 200;15(2):223‐50. [DOI] [PubMed] [Google Scholar]
GRADE 2013 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADE Profiler. Version 3.6 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2013.
Graham 2004
- Graham SM, Gie RM, Schaaf HS, Coulter JB, Espinal MA, Beyers N. Childhood tuberculosis: clinical research needs. International Journal of Tuberculosis and Lung Disease 2004;85(5):648‐57. [PubMed] [Google Scholar]
Heymann 2000
- Heymann SJ, Brewer TF, Wilson ME, Colditz GA, Fineberg HV. Pediatric tuberculosis: what needs to be done to decrease morbidity and mortality. Pediatrics 2000;106(1):e1. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available from www.cochrane‐handbook.org. [Google Scholar]
Hill 2008
- Hill S, Regondi L, Grzemska M, Matirua R. Children and tuberculosis medicines: bridging the research gap. Bulletin of the World Health Organization 2008;86(9):658. [DOI] [PMC free article] [PubMed] [Google Scholar]
IAP 1997
- Indian Academy of Pediatrics (IAP) Working Group. Treatment of childhood tuberculosis: consensus statement of IAP Working Group. Indian Pediatrics 1997;34(12):1093‐6. [PubMed] [Google Scholar]
IAP 2010
- Indian Academy of Pediatric (IAP), Working group on tuberculosis. Consensus statement on childhood tuberculosis. Indian Pediatrics 2010;47(1):41‐55. [DOI] [PubMed] [Google Scholar]
IIPS 2007
- International Institute for Population Sciences (IIPS) and Macro International. National Family Health Survey (NFHS‐3), 2005‐06: India. National Family Health Survey‐3 2007; Vol. 1:411.
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Juni 1999
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054‐60. [DOI] [PubMed] [Google Scholar]
Kant 2001
- Kant L. Childhood tuberculosis: increasing but neglected. Indian Journal of Tuberculosis 2001;48(1):1‐2. [Google Scholar]
Kiper 1998
- Kiper N, Göçmen A, Dilber E, Ozçelik U. Effectiveness of short‐course, intermittent chemotherapy for tuberculosis in children. Clinical Pediatrics 1998;37(7):433‐6. [DOI] [PubMed] [Google Scholar]
Kiser 2012
- Kiser JJ, Zhu R, D'Argenio DZ, Cotton MF, Bobat R, McSherry GD, et al. Isoniazid pharmacokinetics, pharmacodynamics, and dosing in South African infants. Therapeutic Drug Monitoring 2012;34(4):446‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langendam 2012
- Langendam MW, Werf MJ, Huitric E, Manissero D. Prevalence of inappropriate tuberculosis treatment regimens: a systematic review. European Respiratory Journal 2012;39(4):1012‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2008
- Lefebvre C, Eisinga A, McDonald S, Paul N. Enhancing access to reports of randomized trials published world‐wide ‐‐ the contribution of EMBASE records to the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library. Emerging Themes in Epidemiology 2008;5(13):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magdorf 2008
- Magdorf K, Detjen AK. Proposed management of childhood tuberculosis in low‐incidence countries. European Journal of Pediatrics 2008;167(8):927‐38. [DOI] [PubMed] [Google Scholar]
Mandalakas 2005
- Mandalakas AM, Starke JR. Current concepts of childhood tuberculosis. Seminars in Pediatric Infectious Diseases 2005;16(2):93‐104. [DOI] [PubMed] [Google Scholar]
Marais 2004
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, et al. The natural history of childhood intra‐thoracic tuberculosis: a critical review of literature from the pre‐chemotherapy era. International Journal of Tuberculosis and Lung Disease 2004;8(4):392‐402. [PubMed] [Google Scholar]
Marais 2007
- Marais BJ, Pai M. Recent advances in the diagnosis of childhood tuberculosis. Archives of Disease in Childhood 2007;92(5):446‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marais 2010
- Marais BJ, Schaaf HS. Childhood tuberculosis: An emerging and previously neglected problem. Infectious Diseases Clinics of North America 2010;24(3):729‐49. [DOI] [PubMed] [Google Scholar]
MECIR 2011
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.1, 8 December 2011 Available from http://www.editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_conduct_standards%202.1.pdf.
Menon 2010
- Menon PR, Lodha R, Sivanandan S, Kabra SK. Intermittent or daily short course chemotherapy for tuberculosis in children: meta‐analysis of randomized controlled trials. Indian Pediatrics 2010;47(1):67‐73. [DOI] [PubMed] [Google Scholar]
Menzies 2009
- Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, et al. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes:A systematic review and meta‐analysis. PLoS Medicine 2009;6(9):e1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mwandumba 2001
- Mwandumba HC, Squire SB. Fully intermittent dosing with drugs for treating tuberculosis in adults. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD000970] [DOI] [PubMed] [Google Scholar]
Nelson 2004
- Nelson LJ, Wells CD. Global epidemiology of childhood tuberculosis. International Journal of Tuberculosis and Lung Disease 2004;8(5):636‐47. [PubMed] [Google Scholar]
Newton 2008
- Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infectious Diseases 2008;8(8):498‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reis 1990
- Reis FJ, Bedran MB, Moura JA, Assis I, Rodrigues ME. Six‐month isoniazid‐rifampin treatment for pulmonary tuberculosis in children. American Review of Respiratory Disease 1990;142(5):996‐9. [DOI] [PubMed] [Google Scholar]
Review Manager (RevMan) 5.1 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
RNTCP 2012
- Revised National Tuberculosis Control Programme. National guidelines on diagnosis and treatment of pediatric tuberculosis. http://tbcindia.nic.in/documents.html. Directorate General of Health Services. Ministry of Health and Family Welfare, (accessed 1 November 2012).
Schaaf 2005
- Schaaf HS, Parkin DP, Seifart HI, Werely CJ, Hesseling PB, Helden PD, et al. Isoniazid pharmacokinetics in children treated for respiratory tuberculosis. Archives of Disease in Childhood 2005;90(6):614‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaaf 2009
- Schaaf HS, Marais BJ, Hesseling AC, Brittle W, Donald PR. Surveillance of antituberculosis drug resistance from the Western Cape province of South Africa‐ an upward trend. American Journal of Public Health 2009;99(8):1486‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schunemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Hanbook for Systematic Reviews of Intervention. Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org. The Cochrane Collaboration.
Seddon 2012
- Seddon JA, Warren RM, Enarson DA, Beyers N, Schaaf HS. Drug‐resistant tuberculosis transmission and resistance amplification within families. Emerging Infectious Diseases 2012;18(8):1342‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shingadia 2003
- Shingadia D, Novelli V. Diagnosis and treatment of tuberculosis in children. Lancet Infectious Diseases 2003;3(10):624‐32. [DOI] [PubMed] [Google Scholar]
Snider 1988
- Snider DE, Rieder HL, Combs D, Bloch AB, Hayden CH, Smith MH. Tuberculosis in children. Pediatric Infectious Disease Journal 1988;7(4):271‐8. [DOI] [PubMed] [Google Scholar]
Starke 1989a
- Starke JR, Taylor‐Watts KT. Tuberculosis in the pediatric population of Houston, Texas. Pediatrics 1989;84(1):28‐35. [PubMed] [Google Scholar]
Starke 1989b
- Starke JR, Taylor‐Watts KT. Six month chemotherapy of intrathoracic tuberculosis in children. American Reviews of Respiratory Diseases 1989;139(suppl):A314. [Google Scholar]
Starke 1990
- Starke JR. Multidrug therapy for tuberculosis in children. Pediatric Infectious Disease Journal 1990;9(11):785‐93. [DOI] [PubMed] [Google Scholar]
Stop TB Partnership 2006a
- Stop TB Partnership Childhood TB Subgroup: World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 1: Introduction and diagnosis of tuberculosis in children. International Journal of Tuberculosis and Lung Disease 2006;10(10):1091‐7. [PubMed] [Google Scholar]
Stop TB Partnership 2006b
- Stop TB Partnership Childhood TB Subgroup: World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 2: Anti‐tuberculosis treatment in children. International Journal of Tuberculosis and Lung Disease 2006;10(11):1205‐11. [PubMed] [Google Scholar]
Stop TB Partnership 2006c
- Stop TB Partnership Childhood TB Subgroup: World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 3: Management of TB in the HIV‐infected child. International Journal of Tuberculosis and Lung Disease 2006;10(12):1331‐6. [PubMed] [Google Scholar]
Stop TB Partnership 2007
- Stop TB Partnership Childhood TB Subgroup: World Health Organization. Guidance for National Tuberculosis Programmes on the management of tuberculosis in children. Chapter 4: Childhood contact screening and management. International Journal of Tuberculosis and Lung Diseases 2007;11(1):12‐5. [PubMed] [Google Scholar]
Thee 2011
- Thee S, Seddon JA, Donald PR, Seifart HI, Werely CJ, Hesseling AC, et al. Pharmacokinetics of isoniazid, rifampin, and pyrazinamide in children younger than two years of age with tuberculosis: evidence for implementation of revised World Health Organization recommendations. Antimicrobrobial Agents and Chemotherapy 2011;55(12):5560‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varudkar 1985
- Varudkar BL. Short‐course chemotherapy for tuberculosis in children. Indian Clinical Journal of Pediatrics 1985;52(419):593‐7. [DOI] [PubMed] [Google Scholar]
Walls 2004
- Walls T, Shingadia D. Global epidemiology of paediatric tuberculosis. Journal of Infection 2004;48(1):13‐22. [DOI] [PubMed] [Google Scholar]
WHO 2006a
- World Health Organization. Communicable diseases: tuberculosis, fact sheets on TB, TB and children. http://www.searo.who.int/EN/Section10/Section2097/Section2106_10681.htm (accessed 27 April 2009).
WHO 2006b
- World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. http://libdoc.who.int/hq/2006/WHO_HTM_TB_2006.371_eng.pdf (accessed 19 November 2008). [PubMed]
WHO 2010a
- World Health Organization. Rapid Advice. Treatment of tuberculosis in children. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
WHO 2010b
- World Health Organization. Annex 1 ‐ Evidence summary tables. Rapid Advice. Treatment of tuberculosis in children. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization, 2012. [Google Scholar]


