Abstract
Background
Blood transfusion is used in patients with severe malarial anaemia, but risks adverse reactions, transmission of disease, and is complicated to organize in developing countries.
Objectives
This review evaluates the effects of routine blood transfusion for severe anaemia on death and adverse outcomes in malarious areas.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (April 2010), CENTRAL (The Cochrane Library Issue 2, 2010), MEDLINE (1966 to April 2010), EMBASE (1980 to April 2010), LILACS (April 2010), and reference lists of relevant articles. We contacted researchers and organizations working in the field.
Selection criteria
Randomized and quasi‐randomized trials of blood transfusion compared with conservative management in malaria‐associated severe anaemia.
Data collection and analysis
Trials were identified and data extracted by a single reviewer (MM) and checked by a second (HS). Inclusion criteria were applied and data were extracted independently by both reviewers.
Main results
Two randomized trials of 230 children were included. In the transfusion group, there was a non‐significant tendency towards fewer deaths (RR 0.41, 95% CI 0.06 to 2.70), but a trend towards more severe adverse events (RR 8.60, 95% CI 1.11 to 66.43). In one trial by Bojang (1997a) respiratory distress was less common and hospital stay was shorter in the transfusion group (MD 1.88 days, 95% CI 2.41 to 1.35). Subsequent need for urgent blood transfusion was less common in the transfusion group (RR 0.12, 95% CI 0.02 to 0.68). Day 28 packed cell volume was less in the transfusion group (MD ‐1.34, 95% CI ‐2.57 to ‐0.11). There was no information on HIV or Hepatitis B virus transmission.
Authors' conclusions
There is insufficient data to be sure whether routinely giving blood to clinically stable children with severe anaemia in endemic malarious areas reduces death, or results in higher haematocrit measured at one month.
23 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Plain language summary
Blood transfusion for treating malarial anaemia
Malaria may cause anaemia. In areas where malaria is common and transmission is intense, many children are infected with the parasite, and severe anaemia can commonly cause death. Blood transfusions can be important for preventing deaths in very ill patients, although there are also some risks involved. This review was designed to assess the benefits and harms of giving a blood transfusion to all children with severe malarial anaemia but otherwise not in distress or severely unwell.
Two studies met our inclusion criteria, with a total of 230 children included in them; no studies looked at adults. There were fewer deaths in children who had blood transfusion compared with those who did not, but the numbers were small and not statistically significant. There was a trend towards more severe adverse events in the transfusion group, but a trend toward shorter hospital stay. The proportion of blood volume made up of red blood cells (haematocrit) was lower in transfused versus non‐transfused children at day 28.
In conclusion, for a clinician faced with a child with severe anaemia who is otherwise stable and not distressed, there is insufficient evidence to know whether the risks of routine blood transfusion outweigh the benefit.
Background
Severe anaemia and cerebral malaria complicate Plasmodium falciparum infection, and cause most of the 1‐2 million deaths caused by malaria each year (WHO 1990). In areas with stable and intense transmission of malaria, anaemia may cause more deaths than cerebral malaria (Slutsker 1994, Snow 1994).
Blood transfusion is often given to patients with severe malarial anaemia, and can be important in preventing death in very ill patients (English 1996). In other patients who are clinically stable, blood may be given simply on the basis of low haemoglobin, to prevent clinical deterioration. The other potential benefits are that it shortens recovery from anaemia, shortens hospital stay, reduces the length of follow up, and reduces the period in which patients are vulnerable as a result of their anaemic state (Bojang 1997a).
There are risks in blood transfusion: it can cause circulatory overload, transfusion reactions, and occasionally, death (Ness 1990, Garratty 1997, Goodnough 1999). There is also the risk of infection with HIV, hepatitis B or other pathogens (Greenberg 1988, Sazama 1994). Some experts recommend that patients with severe malarial anaemia who are otherwise clinically stable should be managed conservatively as haemoglobin has been shown to rise rapidly following antimalarial chemotherapy in a remarkable proportion of such cases (Pape 1989, Holzer 1993).
Conservative management corresponds with consensus guidelines for severe anaemia of any cause (ACP 1992, WHO 1989). The World Health Organization recommends that red cell transfusion is necessary only if the anaemia is associated with incipient or established cardiac failure (WHO 1989). Similarly, the American College of Physicians has recommended that blood transfusion in acute or chronic anaemia should only be given in symptomatic patients who failed to respond to other suitable resuscitative measures (ACP 1992). However some practice audit reports have shown that inappropriate blood transfusion is still common (Mozes 1989, Jager 1990, Ghali 1994).
In developing countries, blood transfusion may be further complicated by inadequate supply of donor blood, trained personnel and other basic requirements for good quality care. The potentially harmful effects of blood may be increased in these resource‐poor settings since inadequate supervision of laboratory and clinical staff may increase the risk of incorrect cross matching, inadequate screening, poorly given transfusions and badly managed reactions.
The aim of this systematic review is to assess the benefits and harms of blood transfusion in severe malarial anaemia from randomised controlled trials. Since the balance of effects could vary depending on age and severity of illness, we intend to stratify the analysis by severity, and to consider sub‐group analysis of children, adults and pregnant women.
Objectives
To evaluate the effect of routine blood transfusion in patients with malaria and severe anaemia on death and severe adverse events.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials of routine blood transfusion.
Types of participants
Children or adults (irrespective of any inherited red blood cell disorders) with severe anaemia and confirmed malaria parasitaemia. Diagnosis of malaria was applied as a criterion for inclusion regardless of the species of malaria parasites.
Definition of severe anaemia: Although the most widely cited definition of severe anaemia is haematocrit < 15%, the definition of severe anaemia in reviewed literature varied from haematocrit of 13% or less (Fullerton 1962) to < 20% (Hedberg 1993). Haematocrit < 20% has been adopted as the definition for this review in order to avoid exclusion of many good trials on the basis of haematocrit cut‐off points.
Inclusion of participants with inherited red cell disorders: Indications other than severe anaemia are well recognised in patients with inherited red cell disorders, notably sickle cell anaemia. Patients with those specific indicators would be expected to be excluded from trials strictly designed to determine the effects of blood transfusion in severe malarial anaemia patients who are otherwise stable. It is also possible that some good trials may not consider these disorders as criteria for exclusion. This explanation justifies the inclusion of this category of participants in the review.
Types of interventions
Intervention
Blood transfusion.
Control
Conservative management.
Types of outcome measures
Primary outcome
Death within two months.
Other outcomes
Severe adverse events.
Duration of stay in hospital.
Re‐admissions.
Respiratory distress in the 1st week.
Need for additional transfusion.
Increase in haematocrit during follow‐up.
HIV and Hepatitis B status.
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
We used the following search terms for all trial registers and databases: blood transfusion, haemotherapy, anaemia and malaria. The search strategy was further enlarged to include two inherited red cell disorders (sickle cell anaemia and thalassaemia) which are commonly associated with severe anaemia in many malarious areas.
We searched the Cochrane Infectious Diseases Group Specialized Register for relevant trials up to April 2010. Full details of the Cochrane Infectious Diseases Group methods and the journals hand searched are published in The Cochrane Library in the section on Collaborative Review Groups.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 2, 2010). This contains mainly reference information to randomized controlled trials and controlled clinical trials in health care.
We searched the following electronic databases using the search terms in combination with the search strategy developed by the Cochrane Collaboration and detailed in Chapter 6 of the Cochrane Handbook (Higgins 2008): MEDLINE (1966 to April 2010); African Index Medicus (1998); EMBASE (1980 to April 2010); and LILACS (La Literatura Latinoamericana y del Caribe de Informacion en Ciencias de Salud; www.bireme.br, accessed April 2010).
Organizations and individuals working in the field were contacted, and these included: Medical Research Council, The Gambia; Ifakara Centre (National Institute of Medical Research,Tanzania); Wellcome Groups (Thailand and Vietnam); World Health Organization (Child Health/TDR) and Departments of Paediatrics in malarious areas of Africa, Asia and Pacific.
The reviewers also drew on existing reviews of this topic, and checked the citations of all the trials identified by the above methods.
The review panel and external referees, were asked to check the completeness of the search strategy, and to identify any additional unpublished, ongoing and planned trials.
Data collection and analysis
Selection of studies
The inclusion criteria were independently applied to all identified studies by the two reviewers, and when in doubt the opinion of the editor was sought.
Data extraction and management
Data were extracted from the selected trials using a standard form. Data retrieved included methods, types of participants, interventions and outcome. Unpublished data, and additional data for published studies, requested from individuals or organizations, were obtained on a standard form.
Assessment of risk of bias in included studies
Study quality was assessed using the standard methods of the Cochrane Infectious Diseases Group.
Data synthesis
Anaemia severity was defined as "very severe" for haematocrit (PCV) < 12%, and "moderately severe" for haematocrit = 12‐19%. Presence of signs of cardiac failure in severe anaemia was stratified as "very severe" regardless of PCV. This definition is a consensus view based on published studies and personal communication with over twenty experts.
Comparison was between transfused participants and the non‐transfused controls. Estimates of effect were assessed using Risk Ratio (RR) or Mean Difference (MD) with 95% Confidence Intervals. A fixed effects model was used for meta‐analysis. Where analysis showed evidence of heterogeneity of effectiveness between studies, sub‐group analysis was used to explore plausible explanations, including severity of anaemia, age, co‐existing morbidity (especially sickle cell anaemia) and variations in the type, speed and mode of blood transfusion.
Results
Description of studies
Eligibility
A total of 42 studies were identified but only two met the inclusion criteria (see 'Characteristics of included studies'). The main reasons for exclusion were non‐randomisation of blood transfusion, non‐inclusion of severe anaemia as a selection criterion and location outside malarious areas (see 'Characteristics of excluded studies').
Location
The included studies were conducted in The Gambia and Tanzania. A total of 230 children aged two months to nine years with severe anaemia were included in the two trials.The detail of the characteristics of each study is shown in the Table of included studies.
Study details
Participants – both studies excluded children with PCV < 12%, haemorrhage or features of congestive cardiac failure (eg respiratory distress and gallop rhythm). One study (Holzer 1993) also excluded patients with temperature > 38 °C while the other (Bojang 1997a) excluded those with sickle cell disease or severe malnutrition. The PCV range of participants was 12‐17% (Holzer 1993) and 12% to <15% (Bojang 1997a) respectively. This means that both trials exlcuded the "very severe" cases. Sickle cell anaemia patients were excluded from one trial (Bojang 1997a) while the other trial was unclear regarding the inclusion or exclusion of this category of patients.
Intervention – A total number of 118 received blood transfusion while 112 did not. Both trials used whole blood for transfusion but the volume transfused was 15ml/kg in one (Bojang 1997a) and 20ml/kg in the other trial. Non‐transfused participants received oral iron supplements in one study (Bojang 1997a) and none in the other trial.
In Holzer 1993, all patients received treatment for malaria with chloroquine (25 mg/kg) followed by prophylaxis (5 mg/kg/week). In Bojang 1997a, a combination of chloroquine and sulfadoxine‐pyrimethamine was used, followed by chemoprophylaxis (weekly Maloprim – dapsone+pyrimethamine) by the 28th day of follow‐up in randomly selected sub‐groups of transfused (19) and non‐transfused (16) participants.
Risk of bias in included studies
Both trials were randomised but the method of generation of allocation sequence was adequate in one (Holzer 1993) and unclear in the other. The adequacy of allocation concealment could not be determined in both trials. Investigators were not blinded probably due to the nature of the intervention. Studies were not analysed according to the intention‐to‐treat principle.
Both trials recorded high percentage of loss to follow‐up. In Holzer 1993, 16 (13.8%) participants were lost to follow‐up by the 8th week (10 transfused and 6 non‐transfused) but mortality data was ascertained in five of them (2 transfused and 3 non‐transfused). In Bojang 1997a, 26 (22.8%) participants were lost to follow‐up by the 8th week (11 transfused and 15 non‐transfused). Five children in the transfusion group were not transfused becaused their PCV increased while they were waiting for blood to be provided. Both studies did not give clear indication of the sources of the blood used in the trials.
Effects of interventions
Primary outcomes
Death
Combining the two trials, there were fewer deaths among transfused participants (1/118) than non‐transfused patients (3/112) but the numbers were small, and the result not statistically significant (Analysis 1.1).
1.1. Analysis.
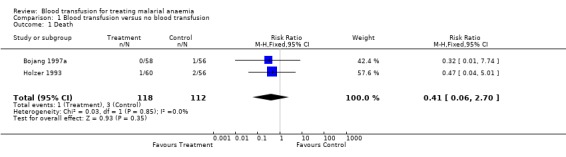
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 1 Death.
Severe adverse events
Bojang 1997a reported convulsions (4 patients) and coma (3 patients) following transfusion but none in the non‐transfused. Holzer 1993 reported chills and fever in one transfused participant who later died.
Seven out of eight adverse events occured in one trial (Bojang 1997a), with only one event in the other. Results were not combined since the risk effect between the studies is so different (Analysis 1.2).
1.2. Analysis.
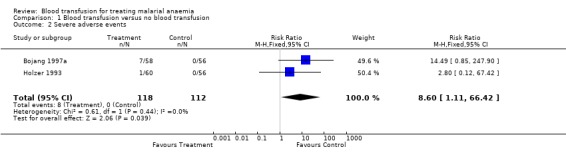
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 2 Severe adverse events.
Number of deaths and severe adverse events were pooled. One participant (Holzer 1993), who experienced an adverse event then later died, was counted only once in this analysis. The meta‐analysis showed no significant difference in risk of either death or an adverse event between transfused and non‐transfused participants (RR 2.54 95% CI 0.69 to 9.34, Analysis 1.3).
1.3. Analysis.
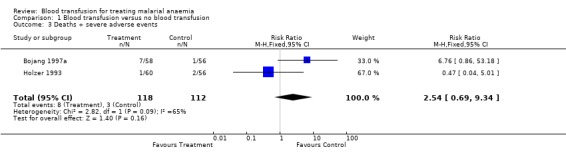
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 3 Deaths + severe adverse events.
Other outcomes
Duration of stay in hospital
Bojang 1997a reported duration of stay in hospital, and this was significantly longer in the non‐transfused group of participants (MD 1.88, 95% CI 2.41 to 1.35, Analysis 1.4). Holzer 1993 reported a higher rate of re‐admission among the non‐transfused (RR 0.23, 95% CI 0.03 to 2.03, Analysis 1.5) but did not report length of stay.
1.4. Analysis.
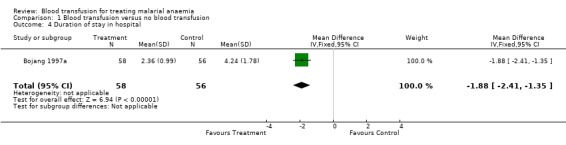
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 4 Duration of stay in hospital.
1.5. Analysis.
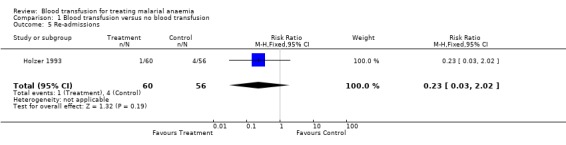
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 5 Re‐admissions.
Respiratory distress in the first week
The risk of developing severe respiratory distress in the follow‐up period was shown by one trial (Bojang 1997a) to be significantly higher among non‐transfused participants (RR 0.04, 95% CI 0.00 to 0.70, Analysis 1.6). The other trial did not report this event but identified one case of bilateral pneumonia among the non‐transfused.
1.6. Analysis.
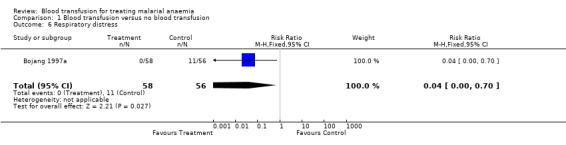
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 6 Respiratory distress.
Need for additional transfusion
See Analysis 1.7. One trial showed that more participants in the non‐transfused group (10/56) than the transfused group (0/58) needed blood transfusion in addition to their primary allocation of the intervention. The criterion for additional transfusion was 'clinical assessment' in Holzer 1993, but not stated by Bojang 1997a. In the Bojang trial, 10 children from the non‐transfused arm required additional transfusion because they developed respiratory distress, and in Holzer 1993 one child from the non‐transfused arm was treated with quinine and blood was transfused, but the child died soon after transfusion.
1.7. Analysis.
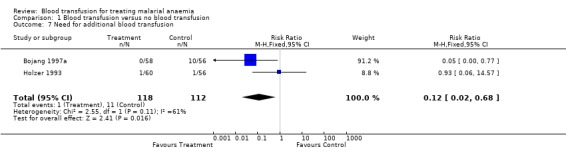
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 7 Need for additional blood transfusion.
Increase in haematocrit during follow‐up
In both studies, mean haematocrit at baseline in the transfusion and non‐transfusion groups were similar.
The haematocrit by day 7 of follow‐up reported by Bojang 1997a was significantly higher in transfused participants (MD 3.39, 95% CI 1.5 to 5.3, Analysis 1.8).
1.8. Analysis.
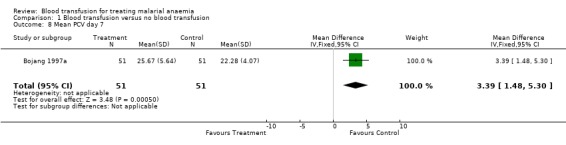
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 8 Mean PCV day 7.
By day 28, haematocrit was significantly higher in the non‐transfused participants in one study (Bojang 1997a; MD 2.10, 95% CI 3.52 to 0.68, Analysis 1.9) and was not significantly different in the other trial (Holzer 1993; MD 0.90, 95% CI 1.55 to 3.35, Analysis 1.9).
1.9. Analysis.
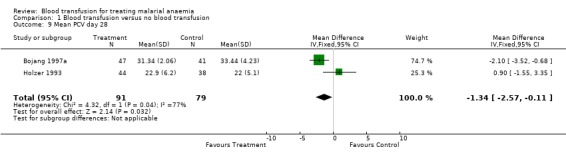
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 9 Mean PCV day 28.
Day 56 haematocrit (Holzer 1993) showed no significant difference between transfused and non‐transfused participants (MD 0.70, 95% CI ‐2.98 to 1.59, Analysis 1.10). The other trial did not report data on haematocrit by day 56.
1.10. Analysis.
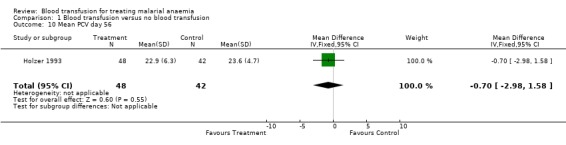
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 10 Mean PCV day 56.
Effect on Plasmodium parasitaemia
In the trial that compared blood transfusion with iron supplements (Bojang 1997a), there was no significant difference in the proportion of participants with parasitaemia on days 7 and 28 of follow‐up (RR 0.97, 95% CI 0.06 to 15.06; same for both days, Analysis 1.11 and Analysis 1.12). This outcome was not pre‐specified.
1.11. Analysis.
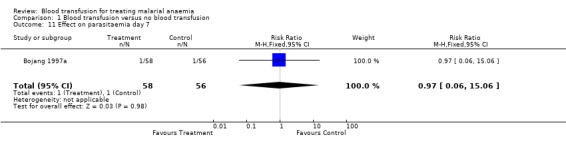
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 11 Effect on parasitaemia day 7.
1.12. Analysis.
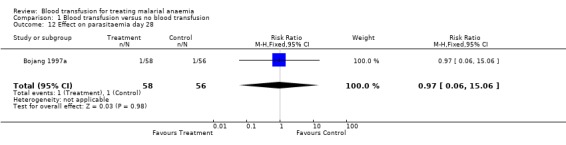
Comparison 1 Blood transfusion versus no blood transfusion, Outcome 12 Effect on parasitaemia day 28.
HIV and hepatitis B transmission
None of the trials reported the evaluation of these outcomes.
Other adverse events
No additional severe adverse events attributable to blood or the other drugs was recorded.
Discussion
These studies were small, and neither study appeared to conceal allocation. The small size means we cannot be sure whether early transfusion improves survival in children with moderate malaria associated with anaemia. Loss to follow up was >10%, which could also bias the results.
The trials did not evaluate the risk of HIV or hepatitis B virus transmission which would require longer term follow up. Clearly in centres where screening is routine this is less of a risk, but these are often fatal infections.
There was also lack of information on patients with sickle cell anaemia since these were excluded from one trial (Bojang 1997a) and inadequately reported in the other.
There is evidence from one trial that non‐transfused children stayed longer in hospital than the transfused. Whether this is a result of arbitrary policies about haemoglobin level at discharge or some other clinical decision is not clear.
The mean value in haematocrit during follow‐up was higher in transfused children during the first week but this did not persist. The non‐transfused children had clearly higher mean haematocrit in one trial (Bojang 1997a) but lower mean haematocrit in the other trial by day 28. One of the trials (Bojang 1997a) gave iron supplements to the non‐transfused while the other (Holzer 1993) gave none and this may explain the more rapid increase in the haematocrit in the former.
Blood transfusion is given for several other reasons than severe anaemia in clinical practice (Hebert 1997b, Goodnough 1999). Many of these other indications have been the subject of good clinical trials (Koshy 1988, Lorente 1993, Vichinsky 1995, Adams 1998, Yu 1998, Hebert 1999). However, these important trials do not provide an answer to the question of what the effective and safe standards should be for the use of blood transfusion for severe anaemia in malarious areas. The geographical and epidemiological relevance of this research question lies in the fact that malaria which is the leading cause of severe anaemia in endemic communities can be cured in most cases and recovery from malarial anaemia is known to be rapid following successful chemotherapy (van den Hombergh 1996, Pape 1989, WHO 1989). The data were too limited to explore potential factors modifying effects. However, both studies were conducted in stable endemic malarious areas: the effects of malaria may be more severe in people with little immunity, so transfusion in these groups could potentially be of greater benefit.
We found no information was available on adults. Evidence in these groups are important, especially pregnant women who are also exposed to risk of significantly higher morbidity and mortality from malarial anaemia.
Authors' conclusions
Implications for practice.
In children living in malarious areas with severe anaemia and no respiratory distress, there is insufficient reliable information to know whether routine administration of blood does more good than harm.
Arguments that blood transfusion helps haematocrit recovery over the subsequent weeks is not supported by evidence from one trial.
Implications for research.
Larger randomized trials of blood transfusion with death as the primary outcome would be useful. The trials could also evaluate as secondary outcomes: respiratory distress and subsequent need for blood (within the first week); adverse events; increase in haematocrit (up to the 2nd month); duration of hospital stay; re‐admission rate; HIV and Hepatitis B virus status (within 3‐6 months). A carefully conducted large trial is likely to under‐estimate the risk of HIV/HBV transmission, since greater care might be taken to avoid these infections in a trial, compared with routine practice.
Some clinicians believe that it is unethical to conduct studies on children with PCV between 12% and 17%, and believe their clinical judgement is better. We maintain that the best way of assessing this policy is to conduct a randomized controlled trial.
The reviewed trials excluded children with PCV < 12% who did not have signs of heart failure thereby losing information on this category of patients. Subsequent trials should include such patients and make room for sub‐group analysis if necessary. A multicentre trial, contributing data to an individual patient data analysis, would help to explore the various factors that might modify effects. Potential sources of heterogeneity are clinical status on admission, age, pregnancy, nutritional status, presence of sickle cell trait or various forms of thalassaemia, and endemicity of malaria.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2010 | New search has been performed | New search conducted; no new trials found. |
History
Protocol first published: Issue 2, 1999 Review first published: Issue 4, 1999
| Date | Event | Description |
|---|---|---|
| 29 July 2008 | Amended | Converted to new review format. |
| 15 March 2007 | New search has been performed | New studies sought but none found. |
Acknowledgements
This review was supported by a Fellowship co‐funded by the Department for International Development (UK) and the European Union DG XII. Authors are grateful to Professor B. M. Greenwood, Professor Kevin Marsh, Dr Brian Coulter and Dr Elizabeth Topley for advice and support.
Data and analyses
Comparison 1. Blood transfusion versus no blood transfusion.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.06, 2.70] |
| 2 Severe adverse events | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.60 [1.11, 66.42] |
| 3 Deaths + severe adverse events | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.69, 9.34] |
| 4 Duration of stay in hospital | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.88 [‐2.41, ‐1.35] |
| 5 Re‐admissions | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 2.02] |
| 6 Respiratory distress | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.70] |
| 7 Need for additional blood transfusion | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.68] |
| 8 Mean PCV day 7 | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | 3.39 [1.48, 5.30] |
| 9 Mean PCV day 28 | 2 | 170 | Mean Difference (IV, Fixed, 95% CI) | ‐1.34 [‐2.57, ‐0.11] |
| 10 Mean PCV day 56 | 1 | 90 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐2.98, 1.58] |
| 11 Effect on parasitaemia day 7 | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.06] |
| 12 Effect on parasitaemia day 28 | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 15.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bojang 1997a.
| Methods | Randomised controlled trial, method of randomisation not specified, allocation concealment unclear | |
| Participants | 114 children aged 6 months to 9 years with malaria and severe anaemia (PCV < 15%) Exclusion criteria: PCV <12%, respiratory distress, gallop rhythm, haemorrhage, severe malnutrition or sickle cell disease |
|
| Interventions | Blood transfusion versus no blood transfusion (with oral iron) All particpicants treated with chloroquine (25 mg/kg) plus sulfadoxine‐pyrimethamine (SP) |
|
| Outcomes | 1. Death 2. Increase in PCV 3. Duration of stay in hospital 4. Additional need for transfusion 5. Severe adverse events 6. Respiratory distress 7. Effect on parasitaemia | |
| Notes | Malaria microscopically confirmed by asexual parasitaemia or malaria pigments in leukocytes Excluded patients with PCV<12% or respiratory distress or other signs of cardiac failure had immediate transfusion (not randomised) |
|
Holzer 1993.
| Methods | Randomised controlled trial; allocation sequence generated from table of random numbers, allocation concealment unclear | |
| Participants | 116 children aged 2 months to 6 years PVC 12‐17% Temperature < 38 °C Exclusion criteria: pneumonia, signs of cardiac failure or haemorrhage |
|
| Interventions | Blood transfusion versus no blood transfusion All participants treated for malaria with chloroquine (CQ = 25 mg/kg) and mebendazole All had chemoprophylaxis with CQ (5 mg/kg/week) |
|
| Outcomes | 1. Death 2. Increase in PCV 3. Need for additional transfusion, re‐admission 4. Adverse events | |
| Notes | Microscopic confirmation of malaria not specified | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Addo‐Yobo 1991 | Not randomised clinical trial |
| Bojang 1997b | Allocation of participants to blood transfusion not randomised |
| Camacho 1998 | Did not involve randomised trial of blood transfusion |
| Carson 1988 | Not randomised clinical trial |
| Corwin 1995 | Not randomised clinical trial |
| Craighead 1993 | Not randomised clinical trial |
| Delzanno 1996 | Not in malarious area |
| Dorward 1989 | Not a randomised clinical trial |
| Elechi 1995 | Not randomised clinical trial |
| English 1996 | Participants not randomised for blood transfusion |
| Fullerton 1962 | Not randomised clinical trial |
| Ghali 1994 | Not randomised clinical trial |
| Gumodoka 1993 | Not randomised clinical trial |
| Harrison 1971 | Blood transfusion not randomised |
| Hebert 1997a | Not randomised clinical trial |
| Hebert 1999 | Participants were critically ill but not all were severely anaemic; location of study is not a malarious area |
| Hedberg 1993 | Not randomised clinical trial of blood transfusion |
| Jackson 1982 | Not clinical trial of blood transfusion |
| Jager 1990 | Not randomised clinical trial |
| Koshy 1988 | Severe anaemia not inclusion criterion; not in malarious area |
| Lackritz 1992 | Not randomised clinical trial |
| Lackritz 1993 | Not randomised clinical trial |
| Mozes 1989 | Not randomised clinical trial |
| Pape 1989 | Not randomised clinical trial |
| Poets 1997 | Not randomised clinical trial |
| Poletes 1994 | Not randomised clinical trial |
| Saxena 1993 | Not randomised clinical trial |
| Schmutzhard 1988 | Not randomised clinical trial |
| Slutsker 1994 | Not a clinical trial |
| Srichaikul 1993 | Not randomised clinical trial |
| van den Hombergh 1996 | Allocation randomised for iron therapy but not for blood transfusion |
| Vichinsky 1995 | Severe anaemia was not an inclusion criterion; not in malarious area |
| Vos 1994 | Not randomised clinical trial |
| Yu 1998 | Severe anaemia was not an inclusion criterion, not in malarious area |
| Zucker 1994 | Not randomised clinical trial |
Sources of support
Internal sources
University of Calabar, Nigeria.
External sources
Department for International Development, UK.
European Commission (Directorate General XII), Belgium.
Liverpool School of Tropical Medicine, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of the review (e.g. employment, consultancy, stock ownership, honoraria, expert testimony).
Unchanged
References
References to studies included in this review
Bojang 1997a {published data only}
- Bojang KA, Palmer A, Boele van hensbroek M, Banya WA, Greenwood BM. Management of severe malarial anaemia in Gambian children. Trans R Soc Trop Med Hyg 1997;91:557‐561. [DOI] [PubMed] [Google Scholar]
Holzer 1993 {published data only}
- Holzer BR, Egger M, Teuscher T, Koch S, Mboya DM, Smith GD. Childhood anemia in Africa: to transfuse or not transfuse?. Acta Tropica 1993;55:47‐51. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Addo‐Yobo 1991 {published data only}
- Addo‐Yobo EO, Lovel H. How well are hospitals preventing iatrogenic HIV?: a study of the appropriateness of blood transfusion in three hospitals in Ashanti Region, Ghana. Tropical Doctor 1991;21:162‐164. [DOI] [PubMed] [Google Scholar]
Bojang 1997b {published data only}
- Bojang KA, Boele van Hensbroek M, Palmer A, Banya WA, Jaffar S, Greenwood BM. Predictors of mortality in Gambian children with severe malarial anaemia. Ann Trop Paediatr 1997;17:355‐359. [DOI] [PubMed] [Google Scholar]
Camacho 1998 {published data only}
- Camacho LH, Gorduek VR, Wilairatana P, Pootrakul P, Brittenham GM, Looareesuwan S. The course of anaemia after treatment of acute, falciparum malaria. Ann Trop Med Parasitol 1998;92:525‐537. [DOI] [PubMed] [Google Scholar]
Carson 1988 {published data only}
- Carson JL, Poses RM, Spence RK, Bonavita G. Severity of anaemia and operative mortality and morbidity. Lancet 1988;i:727‐729. [DOI] [PubMed] [Google Scholar]
Corwin 1995 {published data only}
- Corwin HL, Parsonnet KC, Gettinger A. RBC transfusion in the ICU is there a reason?. Chest 1995;108:767‐771. [DOI] [PubMed] [Google Scholar]
Craighead 1993 {published data only}
- Craighead IB, Knowles JK. Prevention of transfusion‐associated HIV transmission with use of a transfusion protocol in under 5s. Tropical Doctor 1993;23:59‐61. [DOI] [PubMed] [Google Scholar]
Delzanno 1996 {published data only}
- Delzanno G, Falcone M, Gaudiano L, Bertinetti G, Demarchi A, Paotetti R. Human recombinant erythropoietin in the treatment of anaemia in obstetric‐gynaecologic patients. Evaluation of such treatment as an alternative to blood transfusion (Italian). Minerva Ginecol 1996;48:115‐118. [PubMed] [Google Scholar]
Dorward 1989 {published data only}
- Dorward JA, Knowles JK, Dorward IM. Treatment of severe anaemia in children in a rural hospital. Tropical Doctor 1989;19:155‐158. [DOI] [PubMed] [Google Scholar]
Elechi 1995 {published data only}
- Elechi EN, Elechi GN. Surgical management of patients with severe anaemia due to acute blood loss: a case for withholding perioperative blood transfusion. East Afr Med J 1995;72:343‐344. [PubMed] [Google Scholar]
English 1996 {published data only}
- English M, Waruiru C, Marsh K. Transfusion for respiratory distress in life‐threatening childhood malaria. Am J Trop Med Hyg 1996;55:525‐530. [DOI] [PubMed] [Google Scholar]
Fullerton 1962 {published data only}
- Fullerton WT, Turner AG. Exchange transfusion in treatment of severe anaemia in pregnancy. Lancet 1962;i:75‐78. [DOI] [PubMed] [Google Scholar]
Ghali 1994 {published data only}
- Ghali WA, Palepu A, Paterson WG. Evaluation of red blood cell transfusion practices with use of preset criteria. Can Med Assoc J 1994;150:1449‐1454. [PMC free article] [PubMed] [Google Scholar]
Gumodoka 1993 {published data only}
- Gumodoka B, Vos J, Kigadye FC, Asten H, Dolmans WV, Borgdorff MW. Blood transfusion practices in Mwanza Region, Tanzania. AIDS 1993;7:387‐392. [DOI] [PubMed] [Google Scholar]
Harrison 1971 {published data only}
- Harrison KA, Ajabor LN, Lawson JB. Ethacrynic acid and packed‐blood‐cell transfusion in treatment of severe anaemia in pregnancy. Lancet 1971;1:11‐14. [DOI] [PubMed] [Google Scholar]
Hebert 1997a {published data only}
- Hebert PC, Wells G, Tweedale M, et al. Does transfusion practice affect mortality in critically ill patients?. Am J Respir Crit Care Med 1997;155:1618‐1623. [DOI] [PubMed] [Google Scholar]
Hebert 1999 {published data only}
- Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999;340:409‐417. [DOI] [PubMed] [Google Scholar]
Hedberg 1993 {published data only}
- Hedberg K, Shaffer N, Davachi F, et al. Plasmodium falciparum‐associated anemia in children at a large urban hospital in Zaire. Am J Trop Med Hyg 1993;48:365‐371. [DOI] [PubMed] [Google Scholar]
Jackson 1982 {published data only}
- Jackson RT, Latham MC. Anemia of pregnancy in Liberia, West Africa: a therapeutic trial. Am J Clin Nutr 1982;35:710‐714. [DOI] [PubMed] [Google Scholar]
Jager 1990 {published data only}
- Jager H, N'Galy B, Perriens J, et al. Prevention of transfusion‐associated HIV transmission in Kinshasa, Zaire: HIV screening is not enough. AIDS 1990;4:571‐574. [DOI] [PubMed] [Google Scholar]
Koshy 1988 {published data only}
- Koshy M, Burd L, Wallace D, Moawad A, Baron J. Prophylactic red‐cell transfusions in pregnant patients with sickle cell disease. A randomized cooperative study. N Engl J Med 1988;319:144‐152. [DOI] [PubMed] [Google Scholar]
Lackritz 1992 {published data only}
- Lackritz EM, Campbell CC, Ruebush TK, et al. Effect of blood transfusion on survival among children in a Kenyan hospital. Lancet 1992;340:524‐528. [DOI] [PubMed] [Google Scholar]
Lackritz 1993 {published data only}
- Lackritz EM, Ruebush TK, Zucker JR, Adungosi JE, Were JB, Campbell CC. Blood transfusion practices and blood‐banking services in a Kenyan hospital. AIDS 1993;7:995‐999. [DOI] [PubMed] [Google Scholar]
Mozes 1989 {published data only}
- Mozes B, Epstein M, Ben‐Bassat I, Modan B, Halkin H. Evaluation of the appropriateness of blood and blood product transfusion using preset criteria. Transfusion 1989;29:473‐476. [DOI] [PubMed] [Google Scholar]
Pape 1989 {published data only}
- Pape GL. Response to drug treatment in children with chronic anaemia. Tropical Doctor 1989;1:46. [PubMed] [Google Scholar]
Poets 1997 {published data only}
- Poets CF, Pauls U, Bohnhorst B. Effect of blood transfusion on apnoea, bradycardia and hypoxaemia in preterm infants. Eur J Pediatr 1997;156:311‐316. [DOI] [PubMed] [Google Scholar]
Poletes 1994 {published data only}
- Poletes GP, Miller SF, Finley RK, Lincks J. Blood use in the burn unit: a possible role for erythropoietin. J Burn Care Rehabil 1994;15:37‐41. [DOI] [PubMed] [Google Scholar]
Saxena 1993 {published data only}
- Saxena S, Weiner JM, Rabinowitz A, Fridey J, Shulman IA, Carmel R. Transfusion practice in medical patients. Arch Intern Med 1993;153:2575‐2580. [PubMed] [Google Scholar]
Schmutzhard 1988 {published data only}
- Schmutzhard E, Rainer J, Rwechungura RI. Treatment of severe malarial anaemia in East Africa's underfives ‐ an unsolvable problem since the advent of AIDS. Trans R Soc Trop Med Hyg 1988;82:220. [DOI] [PubMed] [Google Scholar]
Slutsker 1994 {published data only}
- Slutsker L, Taylor TE, Wirima JJ, Sketeke RW. In‐hospital morbidity and mortality due to malaria‐associated severe anaemia in two areas of Malawi with different patterns of malarial infection. Trans R Soc Trop Med Hyg 1994;88:548‐551. [DOI] [PubMed] [Google Scholar]
Srichaikul 1993 {published data only}
- Srichaikul T, Leelasiri A, Polvicha P, et al. Exchange transfusion therapy in severe complicated malaria. Southeast Asian J Trop Med Public Health 1993;24(Suppl 1):100‐105. [PubMed] [Google Scholar]
van den Hombergh 1996 {published data only}
- Hombergh J, Dalderop E, Smit Y. Does iron therapy benefit children with severe malaria‐associated anaemia? A clinical trial with 12 weeks supplementation of oral iron in young children from the Turiani Division, Tanzania. J Trop Pediatr 1996;42:220‐227. [DOI] [PubMed] [Google Scholar]
Vichinsky 1995 {published data only}
- Vichinsky EP, Haberkem CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group. N Engl J Med 1995;333:206‐213. [DOI] [PubMed] [Google Scholar]
Vos 1994 {published data only}
- Vos J, Gumodoka B, Asten HA, Berege ZA, Dolmans WM, Borgdorff MW. Changes in blood transfusion practices after introduction of consensus guidelines in Mwanza region, Tanzania. AIDS 1994;8:1135‐1140. [DOI] [PubMed] [Google Scholar]
Yu 1998 {published data only}
- Yu M, Burchell S, Hasaniya NW, Takanishi DM, Myers SA, Takiguchi SA. Relationship of mortality to increasing oxygen delivery in patients > or = 50 years of age: a prospective, randomized trial. Crit Care Med 1998;26:981‐983. [DOI] [PubMed] [Google Scholar]
Zucker 1994 {published data only}
- Zucker JR, Lackritz EM, Ruebush TK, et al. Anaemia, blood transfusion practices, HIV and mortality among women of reproductive age in western Kenya. Trans R Soc Trop Med Hyg 1994;88:173‐176. [DOI] [PubMed] [Google Scholar]
Additional references
ACP 1992
- American College of Physicians. Practice strategies for elective red blood transfusion. Ann Intern Med 1992;116:403‐406. [DOI] [PubMed] [Google Scholar]
Garratty 1997
- Garratty G. Severe reactions associated with transfusion of patients with sickle cell disease. Transfusion 1997;37:357‐361. [DOI] [PubMed] [Google Scholar]
Goodnough 1999
- Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion Medicine. Blood Transfusion. N Engl J Med 1999;340:438‐447. [DOI] [PubMed] [Google Scholar]
Greenberg 1988
- Greenberg AE, Nguyen‐Dinhh P, Mann JM, et al. The association between malaria, blood transfusion and HIV seropositivity in a pediatric population in Kinshasa, Zaire. J Am Med Assoc 1988;259:245‐249. [PubMed] [Google Scholar]
Hebert 1997b
- Hebert PC, Schweitzer I, Calder L, Blajchman M, Guilivi A. Review of the clinical practice literature on allogeneic red blood cell transfusion. Can Med Assoc J 1997;156(11 suppl):S9‐S26. [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.0.0. The Cochrane Collaboration, 2008. [Google Scholar]
Ness 1990
- Ness PM, Shirey RS, Thoman SK, Buck SA. The differentiation of delayed serologic and delayed hemolytic transfusion reactions: incidence, long‐term serologic findings, and clinical significance. Transfusion 1990;30:688‐693. [DOI] [PubMed] [Google Scholar]
Sazama 1994
- Sazama K. Bacteria in blood for transfusion A review. Arch Pathol Lab Med 1994;18:350‐365. [PubMed] [Google Scholar]
Snow 1994
- Snow RW, Bastos de Azevedo I, Lowe BS, et al. Severe childhood malaria in two areas of markedly different falciparum transmission in East Africa. Acta Tropica 1994;57:289‐300. [DOI] [PubMed] [Google Scholar]
WHO 1989
- World Health Organization. Global Blood Safety Initiative. Guidelines for the appropriate use of blood. Geneva: WHO, 1989. [Google Scholar]
WHO 1990
- World Health Organization, Division of Control of Tropical Diseases. Severe and complicated malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 1990;84 Suppl 2:1‐65. [PubMed] [Google Scholar]


