Abstract
Purpose
The concentration of protons in the aqueous humor (AH) of the vertebrate eye is maintained close to blood pH; however, pathologic conditions and surgery may shift it by orders of magnitude. We investigated whether and how changes in extra- and intracellular pH affect the physiology and function of trabecular meshwork (TM) cells that regulate AH outflow.
Methods
Electrophysiology, in conjunction with pharmacology, gene knockdown, and optical recording, was used to track the pH dependence of transmembrane currents and mechanotransduction in primary and immortalized human TM cells.
Results
Extracellular acidification depolarized the resting membrane potential by inhibiting an outward K+-mediated current, whereas alkalinization hyperpolarized the cells and augmented the outward conductance. Intracellular acidification with sodium bicarbonate hyperpolarized TM cells, whereas removal of intracellular protons with ammonium chloride depolarized the membrane potential. The effects of extra- and intracellular acid and alkaline loading were abolished by quinine, a pan-selective inhibitor of two-pore domain potassium (K2P) channels, and suppressed by shRNA-mediated downregulation of the mechanosensitive K2P channel TREK-1. Extracellular acidosis suppressed, whereas alkalosis facilitated, the amplitude of the pressure-evoked TREK-1–mediated outward current.
Conclusions
These results demonstrate that TM mechanotransduction mediated by TREK-1 channels is profoundly sensitive to extra- and intracellular pH shifts. Intracellular acidification might modulate aqueous outflow and IOP by stimulating TREK-1 channels.
Keywords: trabecular meshwork, mechanotransduction, K2p channels, TREK-1
The pH of the aqueous humor (pHAH) reflects the activity of regulatory mechanisms and buffers in the anterior eye.1–3 Intracellular protons are generated by respiration, anaerobic glycolysis, and calcium clearance, sequestered by buffering mechanisms, and extruded via Na+/H+ exchange, Na+-driven Cl−/HCO3− transport, lactate export, glutamine import, and other processes.4–6 In addition to biological mechanisms, clinical interventions such as cataract surgery, antiglaucoma drugs, and contact lens insertion may lower pHAH from its average level of ∼7.4 to below 6.0.7–9 Although the (patho)physiologic significance of such pHAH shifts has not been studied at the cellular/molecular levels, impetus for such investigations comes from early clinical investigations that showed that metabolic acidosis, diabetic acidosis, respiratory alkalosis, and exercise markedly lower IOP,10–13 and from studies showing that pH affects the delivery and biodegradation of IOP lowering drugs.14 pH shifts may modulate HCO3− and AH production/transport in the ciliary body3 and permeability of the blood–ciliary barrier,15 however, recent studies, which localized TREK-1, a mechanosensitive ion channel with exquisite sensitivity for pH16 in mouse and human trabecular meshwork cells,17,18 raise the question whether the pH dependence of IOP observed in clinical studies10–13 might include the effects of pH on TM mechanotransduction itself.
The 426-residue TREK-1 (TWIK-related potassium; OMIM 603219) channel subunit encoded by the human KCNK2 gene contains two pore-forming P domains and four transmembrane segments. Its expression covers the brain, heart, kidneys, ovaries, and eye, as well as smooth muscle cells and mechanosensitive neurons that innervate the colon and bladder.19–22 TREK-1 gating is inhibited by extracellular protons but somewhat uniquely among ion channels, the channel activity can be potentiated by intracellular protons.23 Studies in recombinant systems identified its cytosolic C-terminal domain as the integrator of the modulatory effects of heat, mechanical force, and pH on TREK-1 currents24–26: substitution of the proton-binding E306 residue locks the channel in an open configuration,27 whereas external proton sensors include H126 in the first extracellular loop and W275 in the fourth transmembrane domain.16,28,29 Despite the importance of proton binding for the gating of recombinant TREK-1 channels, the physiologic significance of this process and its relevance for multimodal integration remain unknown.
We recently identified TREK-1 as a principal regulator of the membrane potential, calcium homeostasis, and pressure sensitivity in trabecular meshwork (TM) cells18: mechanosensitive, smooth muscle-like cells in the iridocorneal angle that control the aqueous fluid outflow in the mammalian eye30 and play a central role in the etiology of glaucoma.31 The ability of TM cells to sense mechanical stress32 and match mechanotransduction to fluctuations in the local physicochemical environment allows them to maintain IOP within an acceptable physiologic range33; however, TM function is adversely impacted by glaucoma and may involve aberrant TREK-1 signaling.17,34 Given the significance of protonation for TREK-1 activity,27,28,35 we wondered how acidic and alkaline pH shifts encountered under physiologic and pathologic conditions might influence signals across the TM membrane and whether they affect TREK-1–dependent mechanosensitivity. We report that the background membrane conductance and sensitivity to pressure in these cells can be modulated by external and internal protons and that pH shifts act almost exclusively via TREK-1 channels. These findings implicate activity-dependent, metabolic, and pathologic pH shifts in the regulation of TREK-1–dependent pressure sensing, multimodal transduction, and control of the conventional outflow pathway in the primate eye.
Methods
Cell Culture and Transfection
Human trabecular meshwork (hTM cells), isolated from the juxtacanalicular and corneoscleral regions of the human eye (ScienCell Research Laboratories, Carlsbad, CA, USA), were grown in Trabecular Meshwork Cell Medium (ScienCell, Catalog#6591) at 37°C and 5% CO2. Confluent cells showed the flattened phenotype that is typical of cultured hTMs and expressed TM marker genes, including Aqp1, Timp3, Myoc, Cryab1, and Acta2 (Supplementary Fig. S1). The phenotype of the hTM cell line was further confirmed by testing for steroid-induced upregulation of the myocilin gene. As shown in Supplementary Figure S2, 120-hour incubation with dexamethasone (DEX; 500 nM) potentiated Myoc expression. These data are consistent with our previous characterizations of the same cell line.18,36 A subset of experiments was conducted with primary TM (pTM) cells isolated from corneal rims from three donors (aged between 35 and 60 years) and dissected out of the eye of two additional donors with no history of eye disease. The tissues were acquired and used in concordance with the tenets of the WMA Declaration of Helsinki and the Department of Health and Human Services Belmont Report.
Cells were transiently transfected with TREK-1 shRNA (Catalog No: TL312003; OriGene Technologies, Inc., Rockville, MD, USA) or scrambled shRNA-mCherry using Lipofectamine 3000 reagent by manufacturer's instructions. The efficiency of knockdown estimated by RT-PCR analysis was 30%; however, construct-expressing cells were identified for patch-clamp experiments by green fluorescent protein (GFP) reporter fluorescence. Transfected cells were studied at the third or fourth day after transfection.
Electrophysiology
Whole-cell patch-clamp data were collected as described,18,37 using pClamp 10.7 acquisition software, a Multiclamp 700B amplifier, and DigiData 1550 (Molecular Devices, San Jose, CA, USA). Recordings were sampled at 5 kHz and filtered at 2 kHz. Pipettes, fabricated from borosilicate glass capillaries (WPI; outer diameter 1.5 mm, inner diameter 0.84 mm) had a resistance of 6 to 9 MOhm when filled with the K-gluconate–based pipette solution. Whole-cell current was elicited by −100- to 100-mV ramps from the holding potential of −40 mV. RAMP pulses were of 1-second duration and applied at 0.1 Hz. Membrane potential was recorded in the current-clamp mode in which no holding current was applied. The standard extracellular recording solution contained the following (mM): 140 NaCl, 2.5 KCl, 1.5 MgCl2, 1.8 CaCl2, 10 HEPES, and 5.5 d-glucose. The pipette solution contained the following (mM): 120 K-gluconate, 10 KCl, 10 HEPES, 1 MgCl2, 4 Mg-ATP, 0.6 Na-GTP, and 0.5 EGTA; pH was adjusted to 7.3 with KOH. All experiments were performed at room temperature (22–23°C). In experiments using NH3Cl, 10 mM NaCl was replaced with an equimolar amount of NH3Cl, and in experiments using NaHCO3, 90 mM NaCl was replaced with an equimolar amount of NaHCO3. The pipette solution in this experiment contained the following (mM): 135 K-gluconate, 10 KCl, 2 HEPES, 1 MgCl2, 4 Mg-ATP, 0.6 Na-GTP, and 0.5 EGTA; pH was adjusted to 7.3 with KOH.
High-Speed Pressure Clamp
Membrane stretch was induced with the HSPC-1 device from ALA Scientific Instruments (Farmingdale, NY, USA). Positive pressure steps (15 mm Hg, 3-second duration) were delivered through the recording electrode. Acquisition of the pressure-induced current was controlled via pClamp 10.7 (Molecular Devices). Before pressure applications, 100 μM 4,4′-Diisothiocyano-2,2′-stillbenedisulfonic acid (DIDS) was added to the extracellular solution to inhibit volume-regulated anion conductance.
Fluorescent Imaging
pHi levels were tracked with BCECF AM (5 μM; 2′,7′-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein, acetoxymethyl ester), as described.36,38,39 Emitted epifluorescence was visualized on an upright E600 FN microscope (Nikon Instruments, Tokyo, Japan) with 40× (0.8 N.A.) water objectives following sequential excitation at 480 nm. The acquisition was controlled, backgrounds were subtracted, and the ratios were computed by Nikon Elements software. The saline perfusate contained the following (mM): 140 NaCl, 2.5 KCl, 1.5 MgCl2, 1.8 CaCl2, 10 HEPES, and 5.5 d-glucose, with 10 mM NaCl in some experiments replaced with equimolar NH4Cl. Intracellular acidification was achieved by application of external solution contained 90 mM NaHCO3. BCECF fluorescence was normalized to averaged baseline values measured 60 to 0 seconds before application of NaHCO3 or NH4Cl. Results are plotted as average values (three to four slides, each containing 5–10 cells) from at least two separate experiments.
PCR
Total RNA was isolated from hTM cells using RNAeasy mini kit (Qiagen, Hilden, Germany). The complement DNA was synthesized using the QuantiTect Reverse Transcription Kit (Qiagen). RT-PCR was used to detect the mRNA expression of TM markers with the primers listed in Supplementary Figure S1. The PCR amplification was done using T100 Thermal Cycler (Biorad, Hercules, CA, USA) with the program (95°C for 3 minutes; 95°C for 20 seconds, 60°C for 30 seconds, 72°C for 30 seconds, 45 cycles; 75°C for 5 minutes, 4°C for ∞. For quantitative PCR, hTM cells were treated with or without 500 nM dexamethasone for 5 days and then harvested for RNA isolation. The expression of the myocilin mRNA was normalized to an internal control (β-tubulin). The relative mRNA value was calculated from Ct value by using the 2−ΔΔCT method for relative gene expression analysis.
Reagents
The reagents used for extracellular and intracellular (pipette) solutions were obtained from Sigma-Aldrich (St. Louis, MO, USA) or Calbiochem (San Diego, CA, USA). BCECF AM was from Molecular Probes (Eugene, OR, USA).
Data Analysis
Clampfit 10.7 and OriginPro8 were used for patch-clamp data analysis. Imaging data were analyzed using Nikon NIS-Elements software. Unless specified, the two-sample t-test was used to compare two mean values. The difference between the two mean values was considered significant if P < 0.05.
Results
pHo Modulates TM Pressure Sensing
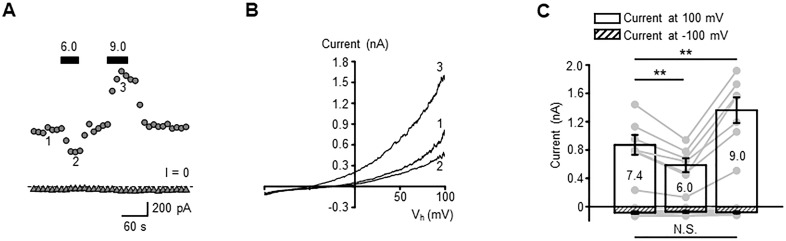
We first determined how external pH affects the resting current in whole-cell recordings from unstimulated hTM cells that were voltage clamped at ±100 mV. Acidification decreased the current amplitude from 872.2 ± 140.6 (control) to 585.2 ± 99.0 pA, whereas alkalinization increased it to 1361.8 ± 181.2 pA (Figs. 1A–1C; P < 0.01). The amplitude of the inward current was not significantly modulated by acidic and alkaline pHo shifts (Fig. 1C). Hence, changes in the extracellular proton concentration mainly affect the outward current that subserves the resting TM conductance.
Figure 1.
External pH modulates the transmembrane current and the plasma membrane potential of hTM cells. (A) A representative trace of the whole-cell current illustrating inhibition and potentiation of current by extracellular acidification and alkalinization. The cells were held at +100 (circles) and −100 mV (triangles); solid bars indicate the application time of the external solution with specified pH. (B) Current–voltage relationship of current recorded at time points shown in A. (C) Summary of experiments shown in A and B, with the average ± SEM of current amplitudes recorded at +100 (open bars) and −100 mV (patterned bars). **P < 0.01; Pair-sample t-test, n = 8 cells.
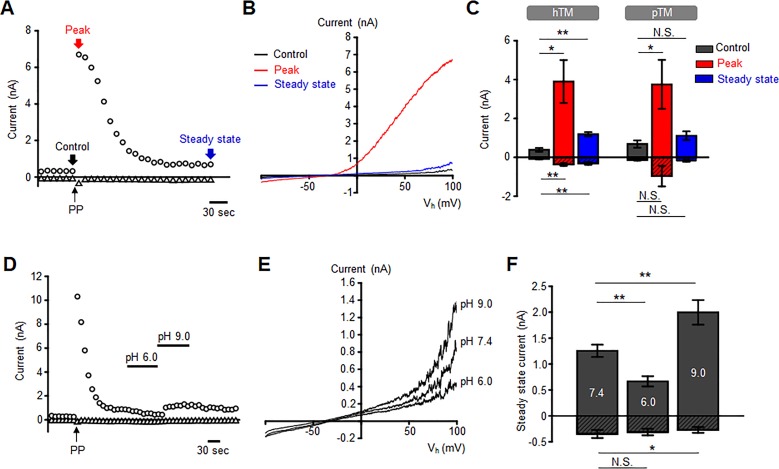
We next studied whether pHo affects mechanosensitive TM currents by stretching the plasma membrane with steps of positive pressure delivered with high-speed pressure clamp.40 The I-V relationship of the pressure-induced whole-cell current showed weak outward rectification, presumably due to the presence of extracellular magnesium.41 When the membrane potential was held at +100 mV, 15 mm Hg steps elicited a robust outward current that peaked at 3898.8 ± 1104.8 pA (n = 8 cells; P < 0.01). At −100 mV, the pressure-activated inward current reached a maximum of −368.3 ± 67.5 pA (Figs. 2A–2C). Following the outward and inward peaks, the current relaxed to a steady-state plateau at 1186.7 ± 108.8 pA (n = 8 cells; P < 0.05) and −314 ± 68.3 pA (n = 8 cells; P < 0.01), respectively (Figs. 2A–2C). Primary TM cells isolated from human donor eyes responded similarly to extracellular pH shifts (Fig. 2C), with inward and outward peaks at −965.18 ± 530 and 3752.3 ± 1260 pA and steady-state plateaus at −182.6 ± 43.3 and 1110.7 ± 225.1 pA (n = 7 cells, N = 2 donors), respectively. Acidic conditions (superfusion with pH 6.0 saline) suppressed the outward plateau current from 1254.9 ± 118.9 (control) to 666.4 ± 99.5 pA, a 47% decrease (n = 8 cells; P < 0.01), whereas alkaline (pH 9.0) conditions increased the current to 1996 ± 238 pA, which is a 59% increase (n = 8 cells; P < 0.01; Figs. 2D–2F).
Figure 2.
External pH modulates mechanogated current in hTM cells. (A) A representative time course of pressure-induced current in hTM cells induced by a pressure pulse (PPs) (arrow) in the presence of 100 μM DIDS; the holding potentials were −100 (triangles) and +100 mV (circles). (B) Current–voltage relationship of control, peak, and steady-state current recorded in time-points shown in A. (C) Summary of peak and steady-state components of the pressure-induced current (−100 mV, patterned bars and +100 mV, open bars) for hTM (n = 8 cells) and pTM cells (n = 8 cells, N = 2 donors). Shown are the mean ± SEM. Not Significant (N.S.)P > 0.05; *P < 0.05. (D) The steady-state component of pressure-induced current is sensitive to pHo. (E) The current–voltage relationships from the experiment is shown in D. (F) Summary of the experiments in D and E. Patterned and open bars represent the currents recorded at −100 and +100 mV, respectively, and plotted as means ± SEM. Pair-sample t-test. n = 8 cells. N.S.P > 0.05; *P < 0.05; **P < 0.01.
TM K2P Channels Function as pHo Sensors
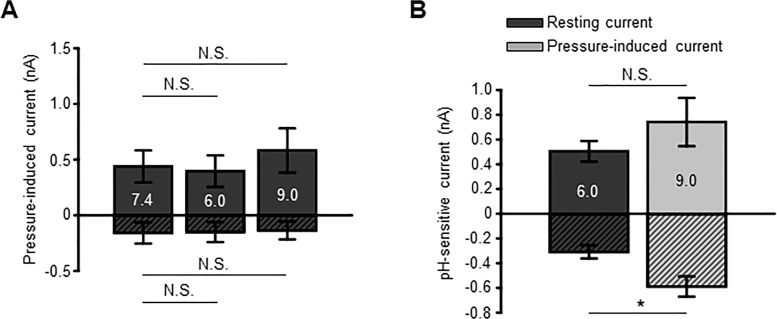
The principal fraction of resting and pressure-evoked outward currents in human TM cells is mediated by K2P channels.18 Consistent with this, the pan-K2P inhibitor quinine (100 μM) reduced the peak outward current to 950.6 ± 153 pA (n = 8 cells; P < 0.05) without affecting inward current significantly (−507.4 ± 127.9 pA; n = 8 cells; P > 0.05), whereas the plateau responses were reduced to 393 ± 142.9 (n = 8 cells; P < 0.001) and −154 ± 99.4 pA (n = 8 cells; P > 0.05), respectively. Under these conditions, TM cells lost their responsiveness to acid and alkaline shifts (Fig. 3A). Hence, the pH sensitivity of the resting current is mediated through steady-state activation of TREK-1. Figure 3B shows that the effect of pH on the membrane current (and thus TREK-1 activation) is augmented in cells with active mechanotransduction (i.e experiencing pressure/stretch). This finding predicts that cells experiencing elevated IOP levels will be subject to stronger TREK-1 modulation by protons.
Figure 3.
The sensitivity of hTM cell transmembrane current to pHo is mediated by K2P channels. (A) Quinine abolishes modulation of the transmembrane current by pHo. Patterned and open bars represent the currents recorded at −100 and 100 mV, respectively. Mean ± SEM. N.S.P > 0.05. Pair-sample t-test. n = 9 cells. P > 0.05. (B) Quinine affects the acid sensitivity of the steady-state component of pressure-induced current. The pH-sensitive current was calculated by subtracting the current at pH 7.4 from the minimum and maximum currents recorded at pH 6.0 and 9.0, respectively. Patterned and open bars represent the currents inhibited by pH 6.0 and potentiated by pH 9.0, respectively. *P < 0.05; N.S.P > 0.05; Two-sample t-test, n = 7 cells and n = 9 cells for background (resting) and pressure-induced currents, respectively. Quinine (100 μM) was applied before pressure stimulation.
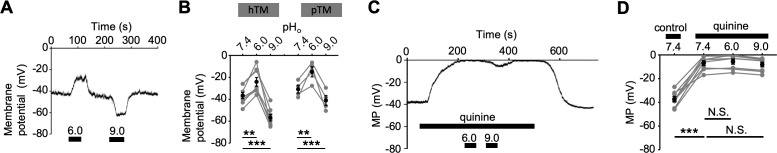
We investigated how the pHo dependence of transmembrane current translates to changes in the cells' resting potential (Vrest) by exposing them to extracellular acid and alkaline shifts under current-clamp conditions. Acidification from pH 7.4 to pH 6.0 depolarized the cells from −36.6 ± 2.8 (control) to −24.1 ± 4.0 mV (n = 8 cells; P < 0.01; Figs. 4A, 4B), whereas alkalinization to pH 9.0 evinced a significant (P < 0.001) hyperpolarization (to −56.9 ± 2.9 mV; n = 8 cells). Vrest in pTM cells was similarly sensitive to pHo (Fig. 4B). Quinine abolished the pHo dependence of the TM membrane potential, depolarizing Vrest to −7.0 ± 2.0 mV (n = 8 cells, P < 0.001) and occluding the effects of acidification and alkalinization (n = 8 cells, P > 0.05; Figs. 4C, 4D).
Figure 4.
Inhibition of K2p channels abolishes pHo-dependent modulation of the membrane potential. (A) Representative trace of the membrane potential: pHo shifts profoundly modulate Vrest. (B) Summary of results illustrated in A. Means (black symbols) ± SEM and individual values (gray symbols). **P < 0.01; ***P < 0.001. Pair-sample t-test, n = 8 cells. pTM stands for primary TM cells; hTM for immortalized TM cells. (C) Quinine depolarizes hTM cells and suppresses their sensitivity to pHo. (D) Summary of the data presented in C, with the mean ± SEM of the average membrane potential values (black symbols) and individual values (gray symbols). N.S.P > 0.05; ***P < 0.001. Pair-sample t-test, n = 8 cells.
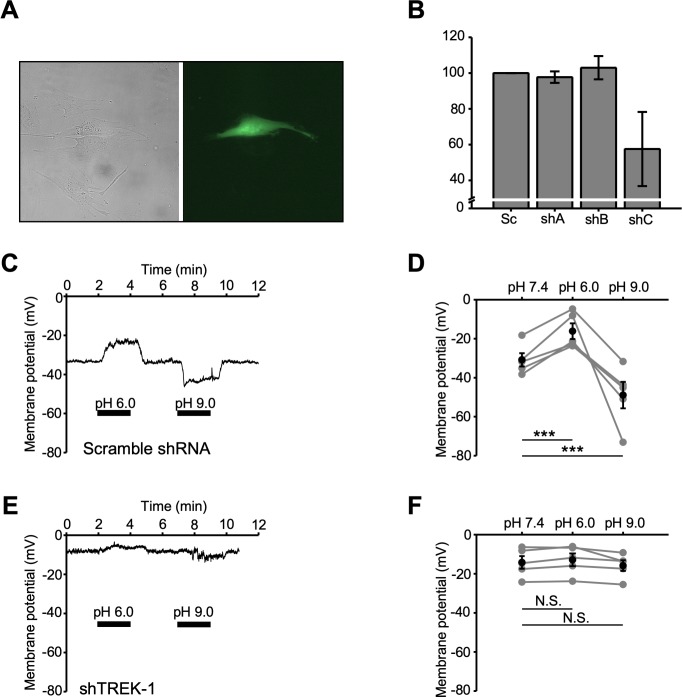
To establish whether the pHo dependence of the resting conductance reflects modulation of TREK-1 channels, cells were transfected with scrambled (control) (Sc-shRNA) and TREK-1-specific short hairpin RNAs (TREK1-shRNA). Transfection efficiency of the construct was evaluated with RT-PCR, and transfected cells were identified by a reporter (GFP) fluorescence (Figs. 5A, 5B). At pH 7.4, Sc-shRNA–treated TM cells had a Vrest of −30.9 ± 3.4 mV and showed pHo dependence that mirrored wild-type cells. Thus, acidification induced a ∼48% depolarization (to −16.7 ± 4.0 mV; n = 5 cells; P < 0.001), whereas alkalinization hyperpolarized Sc-shRNA–treated cells to −48.9 ± 6.8 mV (n = 5 cells; P < 0.001) (Figs. 5C, 5D). Consistent with TREK-1 channel's role in maintaining the TM background conductance,18 its knockdown depolarized the cells to −14.2 ± 3.2 mV and largely obliterated pHo-dependent modulation of Vrest. TREK-1 shRNA-treated cells responded to external acidification with a small depolarization (∼2 mV; n = 5 cells), whereas alkalinization evinced a hyperpolarization (∼2 mV), neither of which were statistically significant (P > 0.05; Figs. 5E, 5F). These results identify TREK-1 channels as the primary effectors of Vrest modulation by external pH.
Figure 5.
TREK-1 knockdown abolishes the effects of external pH on the plasma membrane potential. (A) Representative bright-field (left) and fluorescent (right) images of TM cells. The fluorescent cell expresses TREK-1 shRNA constract. (B) Bar graphs summarizing knockdown eficiency of shRNA constructs. (C, E) pHo-dependent modulation of Vrest is abolished in TREK-1 shRNA (shTREK-1) but not scrambled shRNA (Sc-shRNA)–overexpressing hTM cells. Shown are representative traces. (D, F) Summary of data shown in C and E. Mean ± SEM (black symbols) and individual membrane potential values (gray symbols). P > 0.05; ***P < 0.001. Pair-sample t-test, n = 5 cells for shTREK-1 and Sc-shRNA cohorts.
TREK-1 Functions as a Transducer of Trabecular pHi
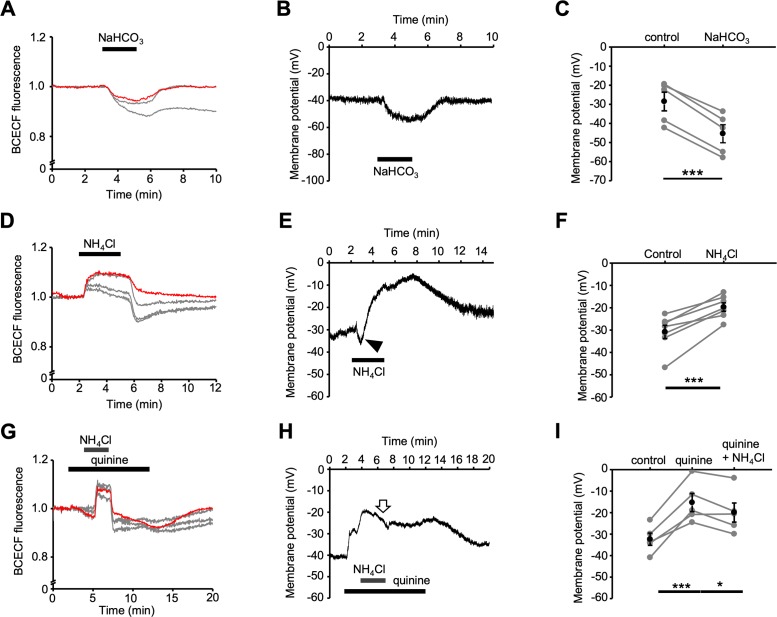
Uniquely among pH-sensitive K2P channels, the TREK family can be modulated by both external and internal pH.23,27 Because human TM cells do not express TREK-2,18 pHi dependence of TREK gating is expected to involve the TREK-1 isoform. Two complementary approaches, consisting of intracellular acidification through exposure to external HCO3−, and alkalinization delivered via the “ammonium prepulse,” were used to modulate TREK-1 via pHi. Intracellular acidification, imposed by application of 90 mM NaHCO3−, was tracked by the proton indicator dye BCECF AM. Figure 6A shows representative traces of simultaneous recordings from “nonpatched” cells (gray traces) and a current-clamped cell (red trace), all of which showed an increase in the proton loading caused by the diffusion of dissolved CO2. Intracellular bicarbonate loading and acidosis resulted in sustained and reversible changes in the TM membrane potential. Consistent with TREK-1 activation, HCO3− loading hyperpolarized the membrane by −16.9 ± 1.2 mV (n = 5 cells; P < 0.001; Figs. 6B, 6C).
Figure 6.
The hTM membrane potential is pHi dependent. (A) Bicarbonate loading. NaHCO3 acidifies pHi recorded from intact (gray traces) and patched (red trace) cells loaded with BCECF-AM. (B) The patched cell (red trace in A) hyperpolarizes during the acid load. (C) Summary of the data in B, with the mean ± SEM (black symbols) and individual Vrest values (gray symbols). ***P < 0.001. Pair-sample t-test, n = 7 cells. (D) Effects of NH4Cl on pHi in intact (gray traces) and patched (red trace) cells. (E) Current-clamp. Intracellular alkalinization induces a transient hyperpolarization (arrowhead) followed by sustained depolarization. (F) Summary for D and E, with the mean ± SEM (black symbols) and individual values (gray symbols). ***P < 0.001. Pair-sample t-test, n = 7 cells. (G) The NH4Cl prepulse alkalinizes, then acidifies the pHi in intact (gray traces) and patched (red trace) hTM cells. Quinine does not affect ΔpHi. (H) Representative current-clamp recording. Quinine inhibits NH4Cl-induced depolarization. (I) Summary of data illustrated in G and H; mean ± SEM (black symbols) and individual values (gray symbols). *P < 0.05, ***P < 0.001. Pair-sample t-test, n = 5 cells.
The ammonium prepulse is based on the dissolution of ammonium chloride NH4Cl in the external saline; as the ammonia gas (NH3) crosses into the cell interior, it removes protons by ionizing into NH4+, effecting an intracellular alkalinization that is visualized by BCECF.38 This is shown in Figure 6D: NH4Cl (10 mM) alkalinized three intact cells (gray traces), whereas NH4Cl removal resulted in transient acidification. A simultaneously recorded cell was current-clamped (red trace). In this cell, alkalinization was associated with a significant (P < 0.001) and reversible depolarization from the resting potential of −30.8 ± 3.0 to −19.7 ± 1.9 mV (n = 7 cells; Figs. 6E, 6F). Quinine did not affect NH4Cl-induced changes in pHi (Fig. 6G), whereas NH4Cl had no additive depolarizing effects in quinine-treated cells (n = 5 cells; P > 0.05; Figs. 6H, 6I). These data support the conclusion that the pHi dependence of Vrest is mediated largely via the internal proton binding site(s) of TREK-1. Occasionally, cytosolic alkalinization under control conditions evoked a small hyperpolarization (arrow in Fig. 6E) that appeared to be missing in quinine-treated cells but was not examined further. Altogether, these results suggest that modulation of intracellular pH (by metabolism, cell activity, external modulators) may regulate the TM plasma membrane via the proton dependence of TREK-1.
Discussion
This study demonstrates that pH changes control trabecular mechanotransduction by modulating TM-intrinsic mechanosensitive K+ channels. Extracellular acidification depolarized the resting potential and suppressed pressure sensitivity by inhibiting an outwardly rectifying current, whereas intracellular acidification promoted TREK-1 activation. These effects were abolished through pharmacology and by shRNA-mediated inhibition of TREK-1 channels. Our findings, therefore, suggest that acid/alkaline loading of the aqueous humor and TM cytosol could profoundly influence TREK-1–dependent physiologic states such as IOP.17
TM function in the healthy eye requires the ability to sense pressure stimuli and adjust fluid outflow to match an increase in pressure.32,42 In a predominantly glycolytic tissue such as TM,43 pHi and pHo changes that might modulate the pressure dependence and physiology of TM-resident ion channels could arise from multiple sources that include metabolic activity, proton clearance, buffering, and lactate release. The relative involvement of carbonic anhydrases and concentration of the AH HCO3− seem to vary across species, with pHAH ranging between slightly alkaline (∼7.6) in rabbits to acidic (∼7.18) in pigs and neutral in nonhuman primates (∼7.44) and humans (∼7.38).8,9,14,44 Although its functions in bicarbonate production/transport point at the ciliary body as a critical regulator of pHAH,45,46 our results are, to the best of our knowledge, the first to identify TM mechanotransduction as a potential target of pH shifts in the anterior eye.
Our voltage- and current-clamp data show that altering the pHi or pHo to match the conditions that might be expected during drug/laser exposure can substantially affect the resting potential and pressure sensitivity of TM cells. pHo lowering to 6.0 depolarized the cells by ∼10 mV, with comparable hyperpolarizations seen in response to extracellular alkalinization. Intracellular acid loads (mediated by HCO3−) and alkalinization (mediated via ammonium prepulse), respectively, hyperpolarized and depolarized, Vrest. We identified the principal target of pHo/pHi as the mechanosensitive TREK-1 channel. The sensitivity to arachidonic acid, spadin, insensitivity to “classical” K+ channel blockers and absence of TREK-2 and TRAAK expression18 established that Vrest and pressure-activated K+-mediated current in TM cells are subserved by TREK channels rather than four- and six-pore K+ channels (e.g., Kv, Kir, KATP, and KCa channels). Consistent with this, pan-K2P blockers and TREK-1–specific shRNA suppressed the depolarization and inhibition of the outward current induced by external protons together with blocking the hyperpolarization and current facilitation mediated by external alkaline shifts.
Further identifying TREK-1 as the central target of proton modulation was the abrogation of acid/alkaline loading effects on the membrane potential and current by TREK-1 inhibition/deletion. The pH dependence of the TREK-1 current in TM cells largely mirrors the properties of recombinant channels and smooth muscle cells,20,47 with the relatively weak outward rectification seen at positive potentials presumably reflecting intracellular Mg2+ modulation at physiologic (asymmetrical) K+ concentrations.41,48 Although TASK-1 is expressed in hTM cells and sensitive to pH,49 its minimal contribution to Vrest and absence of mechanosensitivity18 argue against its involvement in the pH dependence of TM mechanotransduction. Mild internal acidosis resulting from metabolic activity might, therefore, be expected to facilitate constitutive TREK-1 opening by shifting the pressure–activation curve toward positive pressures and converting the channel from the voltage-dependent into a “leak” mode.23,24 The opposite would be the case during respiratory acidosis when IOP elevation13 would result from suppression of TREK-1.
Pharmacologic or surgical interventions could affect TM-dependent regulation of fluid outflow by modulating the TREK-1 selectivity filters at conserved sites (e.g., W275 within TM4) that govern multimodal transduction of heat, pressure, and protons,28 PIP2-interacting domains that are responsible for acidic activation (e.g., E306 within the C terminus)16,27 and residues that mediate external pH sensing (e.g., H126).29 For example, antiglaucoma medications were reported to lower pHAH to ∼5.3 to 5.8,8 levels at which we observe substantial modulation of TREK-1. Although it remains to be seen whether protons regulate (conventional and uveoscleral) outflow, TM mechanotransduction is modulated by acidifications resulting from inhibition of carbonic anhydrases (Trusopt), stimulation of PGF2α receptors (Xalatan), and a block of β-adrenergic receptors (Timoptol).8 Another clinical intervention that may transiently affect pHAH and TM signaling is the use of femtosecond laser during cataract surgery, reported to lower pH to <6.4.9
pH-dependent generation and sensing of forces by TM cells almost certainly involve additional extra-/intracellular inputs that modulate TREK-1 channels (Fig. 7) together with other pH-sensitive ion channels, cytoskeletal elements, polyunsaturated fatty acids, and phagocytosis/secretion processes. TRPV4, a nonselective TM-resident cation channel activated by stretch and pressure36 that may counter TREK-1 activation by depolarizing the TM membrane,18 appears to be activated by acidosis at ∼pHo 6.0 with peak activation at ∼pH 4.0.50 Intracellular acid loading could modulate contractility by displacing Ca2+ ions from internal buffers,38 depressing the effectiveness of actin–myosin interactions,51 and modulate the actomyosin contractility.52 Signaling pathways downstream from stretch sensors might themselves contribute to cytosol acidification by producing lactate.53 By analogy with tissues from TREK-1–null mice that are more sensitive to a variety of external stressors,54 we propose that TREK-1 activation (by intracellular acidosis and extracellular alkalosis) is protective because it counters the overactivation of mechanosensitive TRP channel isoforms.36 Our finding that TREK-1 channels integrate the TM response to pHo, pHi, and pressure represents only a fraction of its overall polymodal potential given that TREK-1 in vivo is likely to combine mechano- and pH-transduction with temperature sensing (TREK-1 might sense the temperature gradient across the anterior chamber that controls the velocity of the AH55–57) and phospholipid modulation21 (Fig. 7). It also must be noted that many studies of trabecular outflow have been conducted in enucleated eyes, which are likely to show profound lowering of pHAH14; it is thus possible that trabecular mechanosensitivity and potentially outflow facility might be affected by postmortem changes in proton/bicarbonate activities. Taken together, the results presented in this study suggest that IOP sensing can only be understood by taking into account the dynamic properties of the intra- and extracellular milieu that include the production and removal of protons. Given the widespread expression of TREK-121,58 and ubiquitous nature of proton homeostasis, the significance of pH-dependent mechanotransduction transcends the eye. Relevant examples can be found in cancer and cardiovascular biology, where pathology remodels tissue mechanics and architecture. High lactic acid production and carbonic anhydrase activity in the hypertrophic heart and tumors may promote reactive oxygen generation, atrial fibrillation, cancer progression, and metastasis59,60 due to interstitial acidification and pH dependence of mechanotransduction.
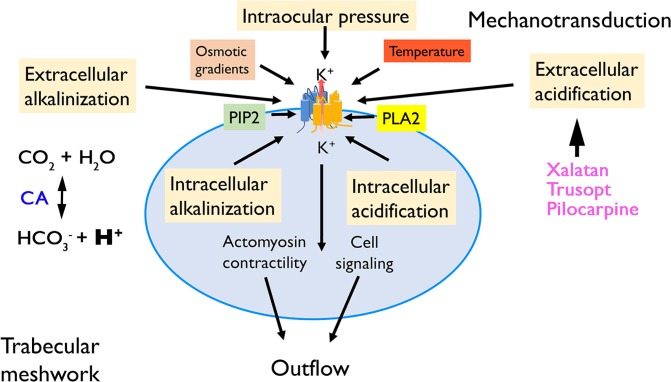
Figure 7.
Hypothetical model of pH dependence of trabecular mechanotransduction. The mechanosensitivity of TM cells is modulated by the effects of intra- and extracellular pH shifts on TREK-1 gating and pressure transduction. The process is influenced by the polymodality of TREK-1 activation, which is sensitive to temperature, membrane content of phosphatidylinositol bisphosphate (PIP2), phospholipase A2 (PLA2) activation, cell swelling, fluid shear, and potentially antiglaucoma drugs. A key player in local pH regulation is the carbonic anhydrase (CA), which regulates the local availability of bicarbonate ions and protons. Through their effects on the pressure dependence of TREK-1, the mechanisms that produce, buffer, and remove protons modulate the drainage of the aqueous fluid from the anterior eye.
Supplementary Material
Acknowledgments
Supported by the National Institutes of Health Grants R01EY022076, R01EY027920, and P30EY014800; The Willard L. Eccles Foundation; Glaucoma Research Foundation; University of Utah Technology Acceleration Grant; and an Unrestricted Grant from Research to Prevent Blindness to the Department of Ophthalmology at the University of Utah.
Disclosure: O. Yarishkin, None; T.T.T. Phuong, None; D. Križaj, None
References
- 1.Helbig H, Korbmacher C, Kuhner D, Berweck S, Wiederholt M. Characterization of Cl-/HCO3- exchange in cultured bovine pigmented ciliary epithelium. Exp Eye Res. 1988;47:515–523. doi: 10.1016/0014-4835(88)90091-7. [DOI] [PubMed] [Google Scholar]
- 2.Hou Y, Delamere NA. Studies on H(+)-ATPase in cultured rabbit nonpigmented ciliary epithelium. J Membr Biol. 2000;173:67–72. doi: 10.1007/s002320001008. [DOI] [PubMed] [Google Scholar]
- 3.Shahidullah M, To CH, Pelis RM, Delamere NA. Studies on bicarbonate transporters and carbonic anhydrase in porcine nonpigmented ciliary epithelium. Invest Ophthalmol Vis Sci. 2009;50:1791–1800. doi: 10.1167/iovs.08-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry FA, Schmitz D, Reimer RJ, et al. Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giasson C, Bonanno JA. Corneal epithelial and aqueous humor acidification during in vivo contact lens wear in rabbits. Invest Ophthalmol Vis Sci. 1994;35:851–861. [PubMed] [Google Scholar]
- 8.Veselovsky J, Olah Z, Vesela A, Gressnerova S. The free amino acids and the aqueous humor pH after antiglaucomatics in vitro. Bratisl Lek Listy. 2003;104:14–18. [PubMed] [Google Scholar]
- 9.Rossi M, Di Censo F, Di Censo M, Oum MA. Changes in aqueous humor pH after femtosecond laser-assisted cataract surgery. J Refract Surg. 2015;31:462–465. doi: 10.3928/1081597X-20150623-04. [DOI] [PubMed] [Google Scholar]
- 10.Marcus DF, Krupin T, Podos SM, Becker B. The effect of exercise on intraocular pressure. I. Human beings. Invest Ophthalmol. 1970;9:749–752. [PubMed] [Google Scholar]
- 11.Kielar RA, Teraslinna P, Kearney JT, Barker D. Effect of changes in Pco2 on intraocular tension. Invest Ophthalmol Vis Sci. 1977;16:534–537. [PubMed] [Google Scholar]
- 12.Krupin T, Weiss A, Becker B, Holmberg N, Fritz C. Increased intraocular pressure following topical azide or nitroprusside. Invest Ophthalmol Vis Sci. 1977;16:1002–1007. [PubMed] [Google Scholar]
- 13.Petounis AD, Chondreli S, Vadaluka-Sekioti A. Effect of hypercapnea and hyperventilation on human intraocular pressure general anaesthesia following acetazolamide administration. Br J Ophthalmol. 1980;64:422–425. doi: 10.1136/bjo.64.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorget F, Parenteau A, Carrier M, et al. Characterization of the pH and temperature in the rabbit, pig, and monkey eye: key parameters for the development of long-acting delivery ocular strategies. Mol Pharm. 2016;13:2891–2896. doi: 10.1021/acs.molpharmaceut.5b00731. [DOI] [PubMed] [Google Scholar]
- 15.Cole DF. Aqueous humour formation. Documenta Ophthalmol. 1966;21:116–238. [Google Scholar]
- 16.Sandoz G, Douguet D, Chatelain F, Lazdunski M, Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc Natl Acad Sci U S A. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carreon TA, Castellanos A, Gasull X, Bhattacharya SK. Interaction of cochlin and mechanosensitive channel TREK-1 in trabecular meshwork cells influences the regulation of intraocular pressure. Sci Rep. 2017;7:452. doi: 10.1038/s41598-017-00430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarishkin O, Phuong TTT, Bretz CA, et al. TREK-1 channels regulate pressure sensitivity and calcium signaling in trabecular meshwork cells. J Gen Physiol. 2018;150:1660–1675. doi: 10.1085/jgp.201812179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink M, Duprat F, Lesage F, et al. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- 20.Koh SD, Monaghan K, Sergeant GP, et al. TREK-1 regulation by nitric oxide and cGMP-dependent protein kinase. An essential role in smooth muscle inhibitory neurotransmission. J Biol Chem. 2001;276:44338–44346. doi: 10.1074/jbc.M108125200. [DOI] [PubMed] [Google Scholar]
- 21.Enyedi P, Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- 22.Hughes S, Foster RG, Peirson SN, Hankins MW. Expression and localisation of two-pore domain (K2P) background leak potassium ion channels in the mouse retina. Sci Rep. 2017;7:46085. doi: 10.1038/srep46085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 24.Chemin J, Patel AJ, Duprat F, Lauritzen I, Lazdunski M, Honore E. A phospholipid sensor controls mechanogating of the K+ channel TREK-1. EMBO J. 2005;24:44–53. doi: 10.1038/sj.emboj.7600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brohawn SG, Su Z, MacKinnon R. Mechanosensitivity is mediated directly by the lipid membrane in TRAAK and TREK1 K+ channels. Proc Natl Acad Sci U S A. 2014;111:3614–3619. doi: 10.1073/pnas.1320768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagriantsev SN, Peyronnet R, Clark KA, Honore E, Minor DL., Jr Multiple modalities converge on a common gate to control K2P channel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen A, Ben-Abu Y, Hen S, Zilberberg N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J Biol Chem. 2008;283:19448–19455. doi: 10.1074/jbc.M801273200. [DOI] [PubMed] [Google Scholar]
- 30.Freddo TF, Gong H. Etiology of IOP elevation in primary open angle glaucoma. Optom Glaucoma Soc E J. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3432647/ Accessed May 13, 2019. [PMC free article] [PubMed]
- 31.Tektas OY, Lutjen-Drecoll E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp Eye Res. 2009;88:769–775. doi: 10.1016/j.exer.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Battista SA, Lu Z, Hofmann S, Freddo T, Overby DR, Gong H. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49:5346–5352. doi: 10.1167/iovs.08-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Acott TS, Kelley MJ, Keller KE, et al. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014;30:94–101. doi: 10.1089/jop.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goel M, Sienkiewicz AE, Picciani R, Lee RK, Bhattacharya SK. Cochlin induced TREK-1 co-expression and annexin A2 secretion: role in trabecular meshwork cell elongation and motility. PLoS One. 2011;6:e23070. doi: 10.1371/journal.pone.0023070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma L, Zhang X, Zhou M, Chen H. Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J Biol Chem. 2012;287:37145–37153. doi: 10.1074/jbc.M112.398164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryskamp DA, Frye AM, Phuong TT, et al. TRPV4 regulates calcium homeostasis, cytoskeletal remodeling, conventional outflow and intraocular pressure in the mammalian eye. Sci Rep. 2016;6:30583. doi: 10.1038/srep30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo AO, Lakk M, Frye AM, et al. Differential volume regulation and calcium signaling in two ciliary body cell types is subserved by TRPV4 channels. Proc Natl Acad Sci U S A. 2016;113:3885–3890. doi: 10.1073/pnas.1515895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krizaj D, Mercer AJ, Thoreson WB, Barabas P. Intracellular pH modulates inner segment calcium homeostasis in vertebrate photoreceptors. Am J Physiol Cell Physiol. 2011;300:C187–C197. doi: 10.1152/ajpcell.00264.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szikra T, Barabas P, Bartoletti TM, et al. Calcium homeostasis and cone signaling are regulated by interactions between calcium stores and plasma membrane ion channels. PLoS One. 2009;4:e6723. doi: 10.1371/journal.pone.0006723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besch SR, Suchyna T, Sachs F. High-speed pressure clamp. Pflugers Arch. 2002;445:161–166. doi: 10.1007/s00424-002-0903-0. [DOI] [PubMed] [Google Scholar]
- 41.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 42.Bradley JM, Kelley MJ, Zhu X, Anderssohn AM, Alexander JP, Acott TS. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42:1505–1513. [PubMed] [Google Scholar]
- 43.Anderson PJ, Wang J, Epstein DL. Metabolism of calf trabecular (reticular) meshwork. Invest Ophthalmol Vis Sci. 1980;19:13–20. [PubMed] [Google Scholar]
- 44.Maren TH. Chemistry of the renal reabsorption of bicarbonate. Can J Physiol Pharmacol. 1974;52:1041–1050. doi: 10.1139/y74-138. [DOI] [PubMed] [Google Scholar]
- 45.Maren TH, Brechue WF, Bar-Ilan A. Relations among IOP reduction, ocular disposition and pharmacology of the carbonic anhydrase inhibitor ethoxzolamide. Exp Eye Res. 1992;55:73–79. doi: 10.1016/0014-4835(92)90094-9. [DOI] [PubMed] [Google Scholar]
- 46.Madshus IH, Olsnes S. Selective inhibition of sodium-linked and sodium-independent bicarbonate/chloride antiport in Vero cells. J Biol Chem. 1987;262:7486–7491. [PubMed] [Google Scholar]
- 47.Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–F801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 48.Renigunta V, Schlichthorl G, Daut J. Much more than a leak: structure and function of K(2)p-channels. Pflugers Arch. 2015;467:867–894. doi: 10.1007/s00424-015-1703-7. [DOI] [PubMed] [Google Scholar]
- 49.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 51.Metzger JM, Moss RL. Effects of tension and stiffness due to reduced pH in mammalian fast- and slow-twitch skinned skeletal muscle fibres. J Physiol. 1990;428:737–750. doi: 10.1113/jphysiol.1990.sp018238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kohler S, Schmoller KM, Crevenna AH, Bausch AR. Regulating contractility of the actomyosin cytoskeleton by pH. Cell Rep. 2012;2:433–439. doi: 10.1016/j.celrep.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pugin J, Dunn-Siegrist I, Dufour J, Tissieres P, Charles PE, Comte R. Cyclic stretch of human lung cells induces an acidification and promotes bacterial growth. Am J Respir Cell Mol Biol. 2008;38:362–370. doi: 10.1165/rcmb.2007-0114OC. [DOI] [PubMed] [Google Scholar]
- 54.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–2695. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumar S, Acharya S, Beuerman R, Palkama A. Numerical solution of ocular fluid dynamics in a rabbit eye: parametric effects. Ann Biomed Eng. 2006;34:530–544. doi: 10.1007/s10439-005-9048-6. [DOI] [PubMed] [Google Scholar]
- 56.Maingret F, Lauritzen I, Patel AJ, et al. TREK-1 is a heat-activated background K(+) channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider ER, Anderson EO, Gracheva EO, Bagriantsev SN. Temperature sensitivity of two-pore (K2P) potassium channels. Curr Top Membr. 2014;74:113–133. doi: 10.1016/B978-0-12-800181-3.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feliciangeli S, Chatelain FC, Bichet D, Lesage F. The family of K2P channels: salient structural and functional properties. J Physiol. 2015;593:2587–2603. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halestrap AP, Wang X, Poole RC, Jackson VN, Price NT. Lactate transport in heart in relation to myocardial ischemia. Am J Cardiol. 1997;80:17A–25A. doi: 10.1016/s0002-9149(97)00454-2. [DOI] [PubMed] [Google Scholar]
- 60.Glitsch M. Protons and Ca2+: ionic allies in tumor progression? Physiology (Bethesda) 2011;26:252–265. doi: 10.1152/physiol.00005.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.