Abstract
Background
Paracoccidioidomycosis is a fungal infection that occurs only in some particular places in Latin America. Treatment is long, the drugs have side effects, and patients can relapse. However, the disease is potentially fatal.
Objectives
To evaluate drugs for treating paracoccidioidomycosis.
Search methods
We searched the following databases: Cochrane Infectious Diseases Group Specialized Register (March 2011); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2011, Issue 1); PubMed (1966 to March 2011); EMBASE (1974 to March 2011); and LILACS (1982 to March 2011)
Selection criteria
Randomized controlled trials comparing drugs for treating people with paracoccidioidomycosis.
Data collection and analysis
Two authors independently assessed trial eligibility and risk of bias, and extracted data, including adverse events.
Main results
Two trials, one with 42 participants and another with 53 participants met the inclusion criteria. Risk of bias in the two trials was high, but most patients showed considerable clinical and mycological improvement. The first trial compared imidazoles (itraconazole and ketoconazole) with sulfadiazine (n=42). No difference was detected for cure (RR 0.77, 95% CI 0.52 to 1.16) or clinical improvement, or serological titres after 10 months of treatment, and there was no difference detected in adverse events. The second compared voriconazole with itraconazole (n=53) and did not demonstrate a difference in response. Two patients were withdrawn from voriconazole due to raised liver enzymes.
Authors' conclusions
The small number of participants and the short follow‐up period impede definitive conclusions on comparative effects.
8 May 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted in April 2019 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Plain language summary
Not enough good quality trials to assess the most effective drug treatment for paracoccidioidomycosis
Paracoccidioidomycosis is a fungal infection that causes ulcers, swelling, fever, and pain. If it also gets into the lungs, it can produce coughing, shortage of breath, chest pain, weight loss, and sometimes death. Without treatment, those suffering this disease may die in a few months or years. There are endemic areas between Mexico and southern Argentina. Drug treatments need to go on for many months and maybe years. There are various drugs that are used, but this review found only two small trials with too few data to say which drug was best, and the drugs all seem to have adverse effects. More research is needed.
Background
Paracoccidioidomycosis is a chronic illness that affects the lungs, lymph nodes, skin, and mucous membranes. People become infected by inhaling spores of the fungus Paracoccidioides brasiliensis.
Epidemiology
Paracoccidioidomycosis‐endemic areas stretch from Mexico to southern Argentina (Negroni 1993), particularly in regions with high humidity and rainfall (Calle 2001). Because of the long latency period, the disease can appear many years after the person has left an endemic area (Brummer 1993; Manns 1996). In areas where the disease is common the annual incidence is about one to three cases per 100,000 inhabitants (Rios‐Fabra 1994). One study in Brazil showed an annual mortality rate of one to 45 per million inhabitants (Coutinho 2002). The disease is more common in men, with an average male to female incidence of 13:1 (Brummer 1993), thought to be due to the protective effect of oestrogens inhibiting the transformation from mycelia to yeast (Restrepo 1984). The incidence in children is about 3% to 10% (Londero 1993). The overall incidence of P. brasiliensis infection is increasing, mainly among people with AIDS (Goldani 1995).
Clinical presentation
The fungus can remain inactive for a long period in healthy people, but immunodepression, including HIV infection and other systemic problems may turn the infection active (Brummer 1993). This illness mainly presents as pulmonary disease. In young people, the juvenile form involves mainly the lymphatic system with acute or subacute features, while the disease in adults tends to be chronic and affects mostly men older than 30 years (Franco 1987). This form can occur in one place in the body, usually the lungs, or in several sites. Without treatment, those suffering from the adult form die in a few years and those with the juvenile form die in a few months. The infection produces lesions that may spread throughout the body and symptoms are due to the local effects of these lesions, which can be on the skin, nose, mouth, or digestive tract, and which produce ulcers, swelling, pain, nausea, vomiting, headache, and fever. If the lesions are in the lungs then the symptoms include coughing, shortage of breath, chest pain, and weight loss. This can progress to respiratory failure and death.
Diagnosis
Diagnosis depends on where the lesion is located and uses microscopy, blood agar culture, serology, skin test, and chest radiography. Confirmation by histological examination is always necessary (Urib 1987; Del Negro 1991; Brummer 1993). The serological tests also have a prognostic value in which persistent higher values may predict a worse clinical response (Negroni 1974).
Treatment
Treatment usually lasts for one to two years and is often associated with complications and relapse. The definition of cure in this disease is very difficult because in many people with clinical and mycological cure, the level of the serological titres tends to remain raised even after a long period of treatment (Del Negro 2000).
The cure rate depends on the type of drug used, dosage, and timing of use. Various drugs are available for treating paracoccidioidomycosis, and, owing to the increase of the infection by the opportunistic fungus in people who are immunodepressed (eg with AIDS) or immunosuppressed (eg receiving cancer treatment), new antifungal agents have been developed for treating this and other mycoses (Maschmeyer 2002).
Sulphonamides
Sulphonamides, such as sulfadiazine, sulphamethoxypyridazine, and sulphamethoxazole, are among the original paracoccidioidomycosis treatments, and are given continuously for two to three years. They act by blocking folic acid formation and consequently fungal growth. The cure rate is around 70%, but it is associated with a 30% relapse rate (Del Negro 1974). These drugs, besides a great advantage of the low cost, can lead to death by bone marrow suppression, and toxicity through impaired hepatic and renal functions (Borelli 1986).
The combination of sulphamethoxazole and trimethoprim (an antimalarial derivative) also obstructs the synthesis of fungal DNA and has been recommended as an alternative for the use of sulphonamide alone. Although cheap, it is not exempt from toxicity and can lead to bone marrow suppression with thrombocytopenia (decrease of blood platelets) and leucopenia (decrease of blood leukocytes).
Amphotericin B
Amphotericin B was the drug of choice in most serious infections for many years. It stops the fungus multiplying by altering the cellular permeability of the fungal wall by binding to sterol (ergosterol) in the fungal membrane. Although 60% of people treated with amphotericin B are cured, its use is limited by the frequency of adverse effects and relapse rates as high as 20% to 30% (Restrepo 1994). It can provoke death from cardiac arrhythmia (abnormal heart rhythm), shock, and renal failure. Acute adverse drug reactions include fever, nausea, vomiting, headache, and tachypnoea (rapid breathing) (Sampaio 1962). Because of the adverse effects, this drug can be used only in hospitals. New formulations of amphotericin B, liposomal amphotericin B, amphotericin B lipid complex, and amphotericin B colloidal dispersion are less toxic but very expensive (Andrés 2001; Adler‐Moore 2002).
Azole derivatives
The azole derivatives, which kill the fungus by acting on the fungal cell membrane, appeared in the 1970s. The main azoles are ketoconazole, itraconazole, fluconazole, saperconazole, and voriconazole, with as many oral formulations as intravenous formulations. They are considered the drugs of choice for treating paracoccidioidomycosis, mainly because they are less toxic and have lower relapse rates (Restrepo 1983; Naranjo 1990). They are used for six or 12 months.
Objectives
To evaluate drugs used for treating paracoccidioidomycosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
People with clinical paracoccidioidomycosis (juvenile or adult form) and positive for P. brasiliensis identified by direct microscopy, histology, or culture from any biological sample.
Types of interventions
Comparisons between one or more drugs, including:
Amphotericins (eg amphotericin B, amphotericin B colloidal dispersion, amphotericin B lipid complex, and liposomal amphotericin B).
Azoles (eg ketoconazole, itraconazole, fluconazole, voriconazole and saperconazole).
Sulphonamides.
Co‐trimoxazole.
Types of outcome measures
Primary*
Cure, as defined by the authors (including combined clinical, mycological, immunological and radiological outcomes)
Secondary
All‐cause death.
Adverse events
Serious adverse events: life threatening or require hospitalization.
Adverse events requiring discontinuation of treatment.
Other adverse events of any description.
*Analysed at least six months after the beginning of therapy and as described by the trial authors.
Search methods for identification of studies
We have identified all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (March 2011); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2011, Issue 1); PubMed (1966 to March 2011); EMBASE (1974 to March 2011); and LILACS (1982 to March 2011).
Conference proceedings
We searched the following conference proceedings for relevant abstracts: II Congresso Brasileiro de Micologia, Brazil, 1998; VII Encontro Internacional sobre Paracoccidioidomicose, Brazil, 1999; III Congresso Latino‐Americano de Micologia, Venezuela, 1999; and the 9th International Congress on Infectious Diseases, Argentina, 2000.
Organizations and pharmaceutical companies
We contacted researchers in the following universities in Brazil for unpublished and ongoing trials: Universidade Federal de Mato Grosso; Universidade Federal de Goiás, Brazil; Universidade Federal do Amazonas; Universidade Federal do Rio de Janeiro; Universidade Federal de São Paulo; Universidade Federal de Minas Gerais; Universidade Estadual Paulista, Faculdade de Medicina de Botucatu; and Universidade de Sao Paulo SP, Faculdade de Medicina de Ribeirão Preto. We also contacted the two pharmaceutical companies that produce relevant medications, Janssen (Brazil) and Schering (Brazil).
Reference lists
We have also checked the reference lists of all trials identified by the above methods.
Data collection and analysis
Selection of studies
Two authors, Valfredo da Mota Menezes (VMM) and Bernardo Garcia Oliveira Soares (BGOS), independently assessed the titles and abstracts of all reports of trials identified by the search. We obtained full text hard copies of studies that fulfilled the inclusion criteria and for studies where there was some doubt. We then applied the inclusion criteria using an eligibility form and resolved any disagreements through discussion.
Data extraction and management
VMM and BGOS independently extracted data on trial characteristics including methods, participants, interventions, and outcomes. Any disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Two authors (VMM and BGOS) independently assessed the risk of bias in each trial in terms of generation of allocation sequence, allocation concealment, blinding, and inclusion of all randomized participants in the analysis (Schulz 1995). We classed generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear according to Jüni 2001, and considered the inclusion of all randomized participants in the final analysis to be adequate if more than 90%. We reported who was blinded in each trial, such as the participant, care provider, or outcome assessor. Any disagreements were resolved by discussion.
Data synthesis
VMM used Review Manager 5 for data analysis. For dichotomous outcomes, we calculated risk ratios (RR) with 95% confidence intervals (CI) using a fixed‐effect model (Mantel 1959). We analysed these data on an intention‐to‐treat basis.
In the event of additional trials, we intended to use (1) the following subgroup analysis to investigate potential source of heterogeneity: interventions lasting less than six months versus those lasting more than six months; and adults versus children; (2) after inclusion of each trial in the primary analysis, we will conduct, If necessary, sensitivity analyses for each of methodological quality factors; (3) to investigate whether publication bias might have adversely affected the results, we will use Egger's test of funnel plot asymmetry; (4) we will combine data from drugs of the same class; however, if there is statistically significant heterogeneity in the results, we will investigate it using a subgroup analysis by individual drugs.
Results
Description of studies
We found four studies through searching the electronic databases and cross‐checking the references. We excluded two retrospective studies (see 'Characteristics of excluded studies').
The remaining two trials was included in this review. 1. Shikanai‐Yasuda 2002: This trial evaluated 42 participants (37 male and 5 female; age range:14 to 75 years) with moderately severe paracoccidioidomycosis at the University Hospital in São Paulo, Brazil. After informed consent, they were randomized to receive itraconazole (14 participants), ketoconazole (14 participants), or sulfadiazine (14 participants) for a period of four to six months. The mean duration of treatment was 169.8 days for the itraconazole group, 154.1 days for the ketoconazole group, and 163.7 days for the sulfadiazine group. Further details, including the clinical and serological criteria used to evaluate this are described in the Characteristics of included studies 2.Queiroz‐Tellez 2007: This trial evaluated 53 patients (51 male and 2 female; age range:19 to 86 years) with acute or chronic paracoccidioidomycosis in three Brazilian tertiary care hospitals. Participants were randomized in a 2:1 ratio to receive voriconazole (35 participants) or itraconazole (18 participants) for a period minimum of six months and maximum of one year but "the actual duration of treatment was determined by the individual investigator". The median duration of therapy for the safety analysis was 169 days (range 5 to 353) for patients in the voriconazole group and 199.5 days (range, 32 to 363) in the itraconazole group. Actually, only 47 participants (30 in the voriconazole group and 17 in the itraconazole group) received six or more months of treatment. Other details, including eligibility, are described in the 'Characteristics of included studies'.
Risk of bias in included studies
1. Shikanai‐Yasuda 2002: The allocation sequence was generated using a randomization table. The trialists did not describe the methods used to conceal allocation concealment or report whether blinding was used. All randomized participants were included in the final analysis.
2. Queiroz‐Tellez 2007: The trialists indicate that randomization was performed according to a code generated by computer in a 2:1 ratio. However, because this is an open study, it would be important to show the baseline characteristics and also the distribution of the two forms of the disease in the two arms of studies. The authors did not perform a statistical calculation to define the sample size. All authors received financial support from the drug manufacturer. All randomized participants were included in the final analysis
Effects of interventions
1. Shikanai‐Yasuda 2002:The trial compared imidazoles (itraconazole and ketoconazole) with sulfadiazine in a sample of 42 participants with ages ranging from 14 to 75 years; we combined the imidazoles in the analysis.
For clinical status: The authors reported on three outcomes relevant to clinical status: cure, marked improvement, and moderate improvement (see definitions in the 'Characteristics of included studies'). These we have grouped into cure, cure plus marked clinical improvement, and cure plus any improvement. The majority of participants in all arms were reported as cured after six months of treatment (RR 0.77, 95% CI 0.52 to 1.16). No difference in clinically assessed 'marked improvement' and 'moderate and marked improvement' was demonstrated (RR 1.00, 95% CI 0.84 to 1.19) (Analysis 1.1, Analysis 1.2 and Analysis 1.3). One participant in the ketoconazole group showed treatment failure after 41 days of treatment.
1.1. Analysis.
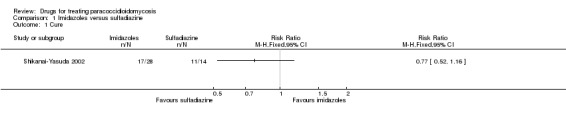
Comparison 1 Imidazoles versus sulfadiazine, Outcome 1 Cure.
1.2. Analysis.
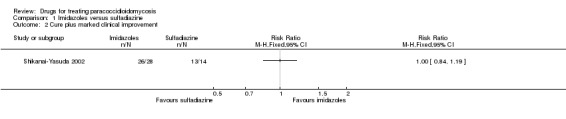
Comparison 1 Imidazoles versus sulfadiazine, Outcome 2 Cure plus marked clinical improvement.
1.3. Analysis.
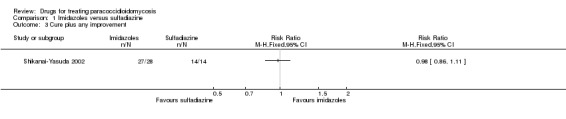
Comparison 1 Imidazoles versus sulfadiazine, Outcome 3 Cure plus any improvement.
For mycological status, 12 participants had mycological data before treatment and at follow up: two participants from the ketoconazole group, seven from the sulfadiazine group, and three from the itraconazole group. Each of these participants had repeated mycological examinations that became negative between six and 16 days after treatment.
For serological status, the antibody level had reduced significantly by 10 months of treatment for all three drugs. There was no statistically significant difference between the drugs. The trial authors presented the following P values: 0.0001 for itraconazole, 0.017 for ketoconazole, and 0.0012 for sulfadiazine.
For death, one participant in the ketoconazole group died after 90 days of treatment because of a complication with a tracheotomy.
For adverse events, the trial reported five adverse events assessed by blood examination and urinalysis during a period of one to three months after treatment. These were an increase in serum level of uric acid, cholesterol, triglycerides, alkaline phosphatase, and oliguria and haematuria; however, the trial authors did not report the baseline data. One participant in the sulfadiazine group had a renal biopsy that showed interstitial nephritis, but there is not information on the previous status of this participant. A statistical analysis does not show statistical significance between groups (Analysis 1.4).
1.4. Analysis.
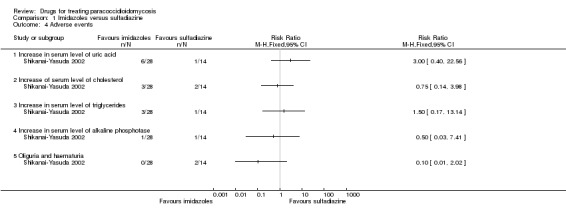
Comparison 1 Imidazoles versus sulfadiazine, Outcome 4 Adverse events.
2. Queiroz‐Tellez 2007: The trial compared voriconazole with itraconazole in a sample of 53 participants with ages ranging from 19 to 86 years. They reported "Global response" a composite score of clinical status, radiological status, mycological status and serological status. In comparison with baseline findings, the response was classified as complete, partial, stable disease, or failure. Complete or partial global response were classified as "satisfactory", satisfactory response was 88.6% in the voriconazole group and 94.4% in the itraconazole group (RR 0.94, CI 0.80 to 1.10) for an ITT analysis.(Analysis 2.2, Figure 1). The authors report that only 47 participants (30 in the voriconazole group and 17 in the itraconazole group) received continuous treatment for six months and for this group (treatment‐evaluable population) the rate of satisfactory response was 100% for both groups. Although the definition of global response included clinical, radiological, mycological and immunological status, the authors report that radiological data were accessed in only 39 participants and immunological data in only 32 participants.
2.2. Analysis.
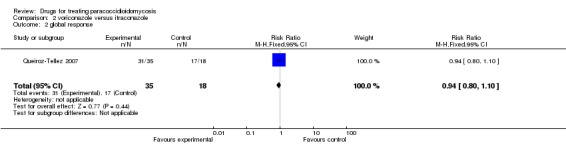
Comparison 2 voriconazole versus itraconazole, Outcome 2 global response.
1.
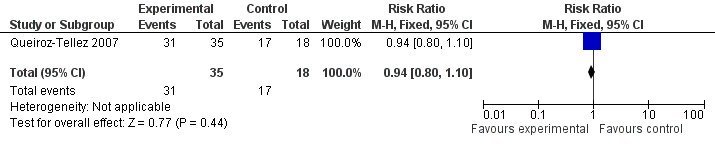
Forest plot of comparison: 2 voriconazole versus itraconazole, outcome: 2.2 global response.
For death, one patient died in the voricanazole group (of an aortic aneurysm).
For adverse events: Two patients of voriconazole group were withdrawn from treatment because of increased level of liver function tests.The majority of participants in both arms suffered some kind of adverse events (82.9% in the voriconazole group and 55.6% in the itraconazole group. RR 1.49, 95% CI 0.96 to 2.32). The most common adverse events in the voriconazole group were abnormal vision, chromatopsia, rash, and headache. In the itraconazole group the most common events were bradycardia and headache (Analysis 2.1, Figure 2). In four participants in the voriconazole group, there were 10 severe adverse events and there were three severe adverse events in two patients in the itraconazole group.
2.1. Analysis.
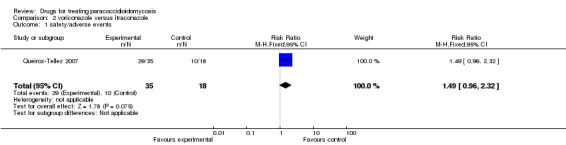
Comparison 2 voriconazole versus itraconazole, Outcome 1 safety/adverse events.
2.
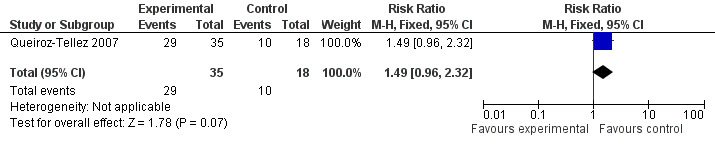
Forest plot of comparison: 2 voriconazole versus itraconazole, outcome: 2.1 safety/adverse events.
Discussion
One the included trials found that sulfadiazine and the imidazoles (itraconazole and ketoconazole) had similar effects for clinical and serological status. Open studies that analyse the use of sulfa derivatives, ketoconazole, and itraconazole have shown similar results; for example, Lopes 1966 and Passos Filho 1969, which analysed sulfadoxine, obtained cure rates varying between 59% and 90% depending on the dose. The same proportion of cure was demonstrated by Do Valle 1993, and Dillon 1986 demonstrated the substantial improvement of people treated with amphotericin B plus sulfa derivatives (sulfadimethoxine and sulfadoxine) when compared with those with amphotericin B alone.
Other studies have reported similar results in terms of cure rates and/or improvement varying from 65% to 100% with ketoconazole (Negroni 1980; Cucé 1981; Del Negro 1982; Marcondes 1984; Do Valle 1993). The same occurred with itraconazole, which achieved cure rates between 87.5% and 100% in three studies (Negroni 1987; Naranjo 1990; Marques 1998).
After more than half century of paracoccidioidomycosis therapeutic history, only two randomized trials have been conducted, one of them sponsored and edited by a pharmaceutical company, both small, with rather limited scope, including with all comparisons but one being between drugs in the same class. We remain uncertain about the ideal drug and dose needed to achieve complete cure and minimise adverse events. As things stand, there remain various therapeutic schemes to treat the same clinical form of the disease. Even in the same institution or service there are several therapeutic schemes (Do Valle 1993), which are used without supporting evidence.
Authors' conclusions
Implications for practice.
The small number of participants and the short follow‐up period in the two randomized controlled trials conducted on this topic impede definitive conclusions.
Implications for research.
Better designed, clinically relevant randomized controlled trials are needed with longer follow up. We know that the most widely used drug regimens are those with co‐trimoxazole (Paniago 2003; Pereira 2004) and those with itraconazole, which seems to have less toxicity (Naranjo 1990; Marques 1998), sometimes varying on the therapeutic doses or in the length of treatment. We believe that a randomized controlled trial that includes a comparison of these two drugs, with established doses, duration of treatment and follow‐up, and an analysis of the clinical, serological, radiological, mycological, and tolerability outcomes could assist the Health Ministries of the countries in Latin America to prepare a treatment policy.
What's new
| Date | Event | Description |
|---|---|---|
| 9 June 2011 | New search has been performed | One new study identified in March 2011. Review updated. |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 18 August 2008 | Amended | Converted to new review format with minor editing. |
Acknowledgements
Valfredo M Menezes developed the review while visiting the Cochrane Infectious Diseases Group at the Liverpool School of Tropical Medicine in February 2005. This Fellowship was provided by the Effective Health Care Research Alliance Programme, which is supported by the UK Department for International Development (DFID). The authors also acknowledge the support provided by the staff of the Brazilian Cochrane Centre and the Universidade Federal de Mato Grosso in Brazil.
This document is an output from a project funded by the DFID for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | PUBMEDb | EMBASEb | LILACSb |
| 1 | paracoccidioidomycosis | paracoccidioidomycosis | PARACOCCIDIOIDOMYCOSIS | paracoccidioidomycosis | paracoccidioidomycosis |
| 2 | paracoccidioides | paracoccidioides | paracoccidioidomycosis | PARACOCCIDIOIDES BRASILIENSIS | paracoccidioides |
| 3 | — | — | paracoccidioides | SOUTH AMERICAN BLASTOMYCOSIS | Blastomicose sul americana |
| 4 | — | — | Lutz's mycosis | Lutz$ mycosis | Blastomicose sud americana |
| 5 | — | — | South American Blastomycosis | 1 or 2 or 3 or 4 or 5 | Lutz micose |
| 6 | — | — | 1 OR 2 OR 3 OR 4 OR 5 | Limit 6 to human | 1 or 2 or 3 or 4 or 5 |
| 7 | — | — | Limit 6 to human | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term.
Data and analyses
Comparison 1. Imidazoles versus sulfadiazine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Cure plus marked clinical improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Cure plus any improvement | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Increase in serum level of uric acid | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Increase of serum level of cholesterol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Increase in serum level of triglycerides | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Increase in serum level of alkaline phosphotase | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Oliguria and haematuria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. voriconazole versus itraconazole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 safety/adverse events | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.96, 2.32] |
| 2 global response | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Queiroz‐Tellez 2007.
| Methods | Open, randomized controlled trial | |
| Participants | Number: 53; 51 male and 2 female Age: 19 to 86 years Inclusion criteria: age 18+ year; diagnosis by positive histopathologic examination or positive culture for P. brasiliensis; excluded: if intravenous therapy needed, prior treatment, or concomitant TB. liver disease, or renal disease | |
| Interventions | 1. Voriconazole: 400 mg twice in the first day, and after, 200 mg ‐ 2 per day for 6 months to 1 year; 2. Itraconazole: 100 mg twice day for 6 months to 1 year. |
|
| Outcomes | 1. Global response (clinical status, radiological status, mycological status and serological status). Classified as complete, partial, stable disease, or failure. Complete and partial were classified as satisfactory; stable and failure were classified as unsatisfactory 2. Safety (Adverse events):any, or discontinuation of treatment |
|
| Notes | Location: Brazil Date: 2007 Pharmaceutical company sponsored |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated pseudo‐random code |
| Allocation concealment (selection bias) | High risk | open study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | all patients reported on |
Shikanai‐Yasuda 2002.
| Methods | Randomized controlled trial Generation of allocation sequence: randomization table Allocation concealment: not described Blinding: not described Inclusion of all randomized participants in the final analysis: 100% for cure |
|
| Participants | ||
| Interventions | 1. Itraconazole: 50 to 100 mg/d for 4 to 6 months; mean duration 169.8 days 2. Ketoconazole: 200 to 400 mg/day for 4 to 6 months; mean duration 154.1 day 3. Sulfadiazine: 100 to 150 mg/kg/day for 4 to 6 months; mean duration 163.7 days | |
| Outcomes | 1. Cure, defined as total disappearance of clinico‐radiological lesion and of complaints due to their inflammatory activity 2. Marked clinical improvement, defined as reduction in lesion size and number of > 90% or persistence of residual fibrotic scars and disappearance of clinical complaints 3. Partial clinical improvement, defined as reduction in lesion size and number of < 90% and/or resolution of some lesion but persistence of others 4. Mycologic examination 5. Antibody level 6. Adverse events: increase of serum level of uric acid, cholesterol, triglycerides, alkaline phosphatase, and oliguria and haematuria | |
| Notes | Location: Brazil Date: 1988 to 1993 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | randomization table |
| Allocation concealment (selection bias) | Unclear risk | no detail given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no information given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | no information given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | no information given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | all patients reported on |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dillon 1986 | Retrospective and statistical study to compare amphotericin B with amphotericin B plus sulphamides |
| Marques 1985 | Retrospective study to compare ketoconazole with amphotericin B plus sulphamides |
Differences between protocol and review
2006, Issue 2 (first review version): quasi‐randomized controlled trials now excluded, as recommended by the Cochrane Infectious Diseases Group; to make the outcome measures easier to understand, we changed "clinical status: wound healing and clinical cure" to "clinical status: changes in the clinical signs and symptoms, as described by the trialists", "negative histology" to "mycological status, as described by the trialists", and "negative serology or fall of the specific antibody titres" to "serological status: changes in serological titres, as described by the trialists"; we added "radiological status: changes in radiological image, as described by the trialists" because of its importance in assessing clinical status; we changed "death" to "all‐cause death" and moved it from a primary to secondary outcome because it is a rare outcome; and removed the secondary outcome measure "loss to follow up and dropout rates" because it is considered as part of the risk of bias assessment.
Contributions of authors
Valfredo da Mota Menezes, Bernardo Garcia de Oliveira Soares, and Cor Jesus Fernandes Fontes all participated in writing the protocol and review.
Sources of support
Internal sources
Brazilian Cochrane Centre, Brazil.
Universidade Federal de Mato‐Grosso, Brazil.
External sources
Department for International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Queiroz‐Tellez 2007 {published data only}
- Queiroz‐Telles F, Goldani LZ, Schlamm HT, Goodrich JM, Espinel‐Ingroff A, Shikanai‐Yasuda MA. An open‐label comparative pilot study of oral voriconazole and itraconazole for long‐term treatment of paracoccidiodomycosis. Clinical Infectious Diseases 2007;45(11):1462‐9. [DOI] [PubMed] [Google Scholar]
Shikanai‐Yasuda 2002 {published data only}
- Shikanai‐Yasuda MA, Benardi G, Higaki Y, Negro GMB, Sun Hoo, Vaccari EH, et al. Randomized trial with itraconazole, ketoconazole and sulfadiazine in paracoccidioidomycosis. Medical Mycology 2002;40(4):411‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dillon 1986 {published data only}
- Dillon NL, Sampaio SAP, Haberman MC, Marques SA, Lastoria JC, Stolf HO, et al. Delayed results of treatment of paracoccidioidomycosis with amphotericin B plus sulfamides versus amphotericin B alone. Revista do Instituto de Medicina Tropical de São Paulo 1986;28(4):263‐6. [DOI] [PubMed] [Google Scholar]
Marques 1985 {published data only}
- Marques SA, Dillon NL, Franco MF, Habermann MC, Lastoria JC, Stolf HO, et al. Paracoccidioidomycosis: A comparative study of the evolutionary serologic, clinical and radiologic results for patients treated with ketoconazole or amphotericinB plus sulfonamides. Mycopathologia 1985;89(1):19‐23. [DOI] [PubMed] [Google Scholar]
Additional references
Adler‐Moore 2002
- Adler‐Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre‐clinical experience. Journal of Antimicrobial Chemotherapy 2002;49 Suppl 1:21‐30. [DOI] [PubMed] [Google Scholar]
Andrés 2001
- Andrés E, Tiphine M, Letscher‐Bru V, Herbrecht R. New lipid formulations of amphotericin B. Review of the literature [Nouvells formes lipidiques de l'amphotericine B. Revue de la literature]. Revue de Médicine Interne 2001;22(2):141‐50. [DOI] [PubMed] [Google Scholar]
Borelli 1986
- Borelli D. Mycosis: a therapeutic breviary. Medicina Cutanea Ibero‐Latino‐Americana 1986;14(3):147‐51. [PubMed] [Google Scholar]
Brummer 1993
- Brummer E, Castañeda E, Restrepo A. Paracoccidioidomycosis: an update. Clinical Microbiology Reviews 1993;6(2):89‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calle 2001
- Calle D, Rosero DS, Orozco LC, Camargo D, Castañeda E, Restrepo A. Paracoccidioidomycosis in Columbia: an ecological study. Epidemiology and Infection 2001;126(2):309‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coutinho 2002
- Coutinho ZF, Silva D, Lazéra M, Petri V, Oliveira RM, Sabroza PC, et al. Paracoccidioidomycosis mortality in Brazil (1980‐1995). Cadernos de Saude Publica 2002;18(5):1441‐54. [DOI] [PubMed] [Google Scholar]
Cucé 1981
- Cucé LC, Wrokalawski E, Sampaio SAP. Treatment of paracoccidioidomycosis with ketoconazole. Revista do Instituto de Medicina Tropical de São Paulo 1981;23(2):82‐5. [PubMed] [Google Scholar]
Del Negro 1974
- Negro GMB. Treatment of paracoccidioidomycosis [Tratamento da paracoccidioidomicose]. Revista da Associação Médica Brasileira 1974;20(6):231‐4. [PubMed] [Google Scholar]
Del Negro 1982
- Negro G. Ketoconazole in paracoccidioidomycosis. A long‐term therapy study with prolonged follow‐up. Revista do Instituto de Medicina Tropical de São Paulo 1982;24(1):27‐39. [PubMed] [Google Scholar]
Del Negro 1991
- Negro GMB, Garcia NM, Rodríguez EG, Cano MIN, Aguiar MSMV, Lirio VS, et al. The sensitivity, specificity and efficiency values of some serological test used in the diagnosis of paracoccidioidomycosis. Revista do Instituto de Medicina Tropical de São Paulo 1991;33(4):277‐80. [DOI] [PubMed] [Google Scholar]
Del Negro 2000
- Negro GMB, Pereira CN, Andrade HF, Palacios SA, Vidal MMS, Charbel CE, et al. Evaluation of tests for antibody response in the follow‐up of patients with acute and chronic forms of paracoccidioidomycosis. Journal of Medical Microbiology 2000;49(1):37‐46. [DOI] [PubMed] [Google Scholar]
Do Valle 1993
- Do Valle AC, Wanke B, Wank NCF, Lima NS, Perez M. Treatment of paracoccidioidomycosis: Retrospective study of 500 cases. Evaluation of therapeutic results with sulfonamides, amphotericin b, sulfamethoxazol plus trimethoprin, ketoconazole and miconazole [Tratamento da paracoccidioidomicose. Estudo retrospectivo de 500 casos. Avaliação dos resultados terapeuticos com sulfamidas, amfotericina b, sulfametoxazol com trimetoprim, cetoconazol e miconazol]. Anais Brasileiro de Dermatologia 1993;68(1):65‐70. [Google Scholar]
Franco 1987
- Franco M, Montenegro MR, Mendes RP, Marcos SA, Dillon NL, Mota NG. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Revista da Sociedade Brasileira de Medicina Tropical 1987;20(2):129‐32. [DOI] [PubMed] [Google Scholar]
Goldani 1995
- Goldani LZ, Sugar AM. Paracoccidioidomycosis and AIDS: an overview. Clinical Infectious Diseases 1995;21(5):1275‐81. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 updated May 2005; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 6 January 2006).
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Londero 1993
- Londero AT, Melo IS. Paracoccidioidomycosis in childhood: A critical review. Mycopathologia 1993;82(1):49‐55. [DOI] [PubMed] [Google Scholar]
Lopes 1966
- Lopes C, Furtado TA, Cisalpino EO, Hermeto A. Treatment of South American blastomycosis with sulfamide administered once per week. Follow‐up of 2 to 3 years [Tratamento da blastomicose sul‐americana por sulfamida administrada uma vez por semana‐ follow‐up de 2 a 3 anos]. O Hospital 1966;70(2):285‐97. [PubMed] [Google Scholar]
Manns 1996
- Manns B J, Baylis BW, Ubanski SJ, Gibb AP, Rabin HR. Paracoccidioidomycosis: case report and review. Clinical Infectious Diseases 1996;23(5):1026‐32. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. [PubMed] [Google Scholar]
Marcondes 1984
- Marcondes J, Meira DA, Mendes RP, Pereira PCM, Barraviera B, Mota NGS, et al. Evaluation of the treatment of paracoccidioidomycosis with ketoconazole [Avaliação do tratamento da paracoccidioidomicose com ketoconazol]. Revista do Instituto de Medicina Tropical de São Paulo 1984;26(2):113‐21. [DOI] [PubMed] [Google Scholar]
Marques 1998
- Marques SA. Paracoccidioidomycosis. Treatment with Itraconazole. Results achieved after long period follow‐up [thesis]. Faculdade de Medicina de Botucatú, Universidade Estadual Paulista, 1998. [Google Scholar]
Maschmeyer 2002
- Maschmeyer G. New antifungal agents‐treatment standards are beginning to grow old. Journal of Antimicrobial Chemotherapy 2002;49(2):239‐41. [DOI] [PubMed] [Google Scholar]
Naranjo 1990
- Naranjo MS, Trujillo M, Restrepo P, Gomez I, Restrepo A. Treatment of paracoccidioidomycosis with itraconazole. Journal of Medical and Veterinary Mycology 1990;28(1):67‐76. [DOI] [PubMed] [Google Scholar]
Negroni 1974
- Negroni R, Robles AM. Prognostic value of the skin test in paracoccidiodomycosis [El valor pronóstico de la prueba cutanea en paracoccidioido micosis]. Medicina Cutánea Ibero‐Latino‐Americana 1974;2(6):453‐7. [PubMed] [Google Scholar]
Negroni 1980
- Negroni R, Robles AM, Arechavala A, Tuculet MA, Galimbert R. Ketoconazole in the treatment of paracoccidioidomycosis and histoplasmosis. Reviews of Infectious Diseases 1980;2(4):643‐9. [DOI] [PubMed] [Google Scholar]
Negroni 1987
- Negroni R, Robles AM, Arachavala A, Niraboschi IN. Results of treatment with itraconazole by oral route in the paracoccidioidomycosis [Resultados del tratamiento con itraconazol por via oral en la paracoccidioidomicosis]. Revista Argentina de Micologia 1987;Suppl:27‐30. [Google Scholar]
Negroni 1993
- Negroni R. Paracoccidioidomycosis (south american blastomycosis, Lutz´s mycosis). International Journal of Dermatology 1993;32(12):847‐59. [DOI] [PubMed] [Google Scholar]
Paniago 2003
- Paniago AM, Aguiat JI, Aguiar ES, Cunha RV, Perira GR, Londero AT, et al. Paracoccidioidomycosis: a clinical and epidemiological study of 422 cases observed in Mato Grosso do Sul [Paracoccidioidomicoes: estudo clínico e epidemiológico de 422 casos observados em Mato Grosso do Sul]. Revista da Sociedade Brasileira de Medicina Tropical 2003;36(4):455‐9. [DOI] [PubMed] [Google Scholar]
Passos Filho 1969
- Passos Filho MC. Treatment of South American blastomycosis of pulmonary localization, with a sulfa administered in weekly doses [Tratamento da blastomicose sul‐americana de localização pulmonar com sulfa administravel em doses semanais]. O Hospital 1969;76(3):847‐56. [PubMed] [Google Scholar]
Pereira 2004
- Pereira RM, Bucharetchi F, Barison Ede M, Hessel G, Tresoldi AT. Paracoccidioidomycosis in children: clinical presentation, follow‐up and outcome. Revista do Instituto de Medicina Tropical de São Paulo 2004;46(3):127‐31. [DOI] [PubMed] [Google Scholar]
Restrepo 1983
- Restrepo A, Gomez I, Cano LE, Arango MD, Gutierrez F, Sanin A, et al. Treatment of paracoccidioidomycosis with ketoconazole: a three‐year experience. American Journal of Medicine 1983;74(1B):48‐52. [DOI] [PubMed] [Google Scholar]
Restrepo 1984
- Restrepo A, Salazar ME, Cano LE, Stover EP, Feldman D, Stevens DA. Estrogens inhibit mycelium‐to‐yeast transformation in the fungus Paracoccidioides brasiliensis: implications for resistance of females to paracoccidioidomycosis. Infection and Immunity 1984;46(2):346‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Restrepo 1994
- Restrepo A. Treatment of tropical mycoses. Journal of the American Academy of Dermatology 1994;31(3 Pt 2):91‐102. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Rios‐Fabra 1994
- Rios‐Fabra A, Moreno AR, Isturiz RE. Fungal infection in Latin American countries. Infectious Disease Clinics North America 1994;8(1):129‐54. [PubMed] [Google Scholar]
Sampaio 1962
- Sampaio SAP. Treatment of South American blastomycosis [Tratamento da blastomicose sul americana]. Jornal Brasileiro de Medicina 1962;6:516‐21. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Urib 1987
- Uribe F, Zuluaga AI, Leon W, Restrepo A. Histopathology of cutaneous and mucosal lesions in human paracoccidioidomycosis. Revista do Instituto de Medicina Tropical de São Paulo 1987;29(2):90‐6. [DOI] [PubMed] [Google Scholar]


