Abstract
Background
Bacillus Calmette‐Guerín (BCG) is a live attenuated vaccine to prevent tuberculosis, routinely administered at birth as part of the World Health Organization global expanded immunisation programme. Given intradermally, it can cause adverse reactions, including local, regional, distant and disseminated manifestations that may cause parental distress. Rarely, it can cause serious illness and even death. Among those patients with immunocompromised conditions, such as the human immunodeficiency virus (HIV) infection, the complication rate is even higher.
Objectives
To assess the effects of different interventions for treating BCG‐induced disease in children.
Search methods
The following databases were searched: the Cochrane Infectious Diseases Group Specialized Register and Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (The Cochrane Library 2012, Issue 4); MEDLINE (1966 to November 2012); EMBASE (1947 to November 2012); and LILACS (1980 to November 2012). The metaRegister of Controlled Trials (mRCT) and the WHO trials search portal. Conference proceedings for relevant abstracts and experts were also contacted to identify studies. No language restrictions were applied.
Selection criteria
Randomized controlled trials (RCTs) comparing any medical or surgical treatment modality for BCG‐induced disease in children.
Data collection and analysis
Two authors independently evaluated titles, applied inclusion criteria, and assessed the risk of bias of studies. The primary outcomes were the failure rate of therapies for all types of BCG vaccine‐induced complications and the time to resolution of illness measured in months. The secondary outcomes were death from BCG vaccine‐induced disease and the all‐cause mortality. Risk ratios (RRs) were used as measure of effect for dichotomous outcomes and mean differences for continuous outcomes.
Main results
Five RCTs analysing 341 children addressed the primary outcomes and were included. Four arms compared oral antibiotics to no intervention or placebo, one arm evaluated needle aspiration compared to no intervention, and another evaluated the use of locally instilled isoniazid versus oral erythromycin.
Two small studies evaluated oral isoniazid; we are uncertain of whether this intervention has an effect on clinical failure (RR 1.48; 95% Confidence Interval (CI) 0.79 to 2.78; 54 participants, two studies, very low quality evidence). Similarly, for oral erythromycin, we are uncertain if there is an effect (clinical failure RR 1.03; 95% CI 0.70 to 1.53; 148 participants, three studies, very low quality evidence), and for oral isoniazid plus rifampicin (clinical failure, RR 1.20; 95% CI 0.51 to 2.83; 35 participants, one study, very low quality evidence).
In patients with lymphadenitis abscess, needle aspiration may reduce clinically persistent BCG‐induced disease at 6 to 9 months of follow‐up (RR 0.13; 95% CI 0.03 to 0.55; 77 participants, one study, low quality evidence). In another study of patients with the same condition, aspiration plus local instillation of isoniazid reduces time to clinical cure compared to aspiration plus oral erythromycin (mean difference 1.49 months less; 95% CI 0.82 to 2.15 less; 27 participants, one study).
No RCTs of HIV‐infected infants with a BCG‐induced disease evaluated the use of antibiotics or other therapies for reducing the rate of clinical failure or the time to clinical resolution. No data on mortality secondary to the interventions for treating BCG‐induced disease were reported.
Authors' conclusions
It is unclear if oral antibiotics (isoniazid, erythromycin, or a combination of isoniazid plus rifampicin) are effective for the resolution of BCG‐induced disease. Most non‐suppurated lymphadenitis will resolve without treatment in 4 to 6 months. Patients with lymphadenitis abscess might benefit from needle aspiration and possibly local instillation of isoniazid could shorten recovery time. Included studies were generally small and could be better conducted. Further research should evaluate the use of needle aspiration and local instillation of isoniazid in fluctuant nodes. Therapeutic and preventive measures in HIV‐infected infants could be important given the higher risk of negative outcomes in this group.
23 April 2019
No update planned
Other
Although the CIDG conducted a new search up to 23 Jul, 2018 for potentially relevant studies, these studies have not yet been incorporated into this Cochrane Review as this review is not a current priority for update by CIDG.
Keywords: Child; Child, Preschool; Humans; Infant; Abscess; Abscess/microbiology; Abscess/therapy; Adjuvants, Immunologic; Adjuvants, Immunologic/adverse effects; Antibiotics, Antitubercular; Antibiotics, Antitubercular/therapeutic use; BCG Vaccine; BCG Vaccine/adverse effects; Erythromycin; Erythromycin/therapeutic use; Isoniazid; Isoniazid/therapeutic use; Lymphadenitis; Lymphadenitis/microbiology; Lymphadenitis/therapy; Mycobacterium bovis; Randomized Controlled Trials as Topic; Rifampin; Rifampin/therapeutic use; Suction; Suction/methods
Plain language summary
Therapies for BCG induced disease in children
Bacillus Calmette‐Guérin (BCG) is a widely used tuberculosis vaccine derived from a non‐infectious strain of the bovine tuberculosis bacillus (Mycobacterium bovis) and mainly given to young children. Usually, the only adverse reaction to the vaccine is an ulcer at the site of injection, which may leave a small scar.
Very occasionally, however, especially in children with weakened immune systems, the vaccine can cause more serious side effects. These can include local infections at the injection site, which may spread to the lymph nodes, causing lymphadenopathy, and the bones, and can even prove life‐threatening. These adverse reactions to the BCG vaccine are a particular risk for children infected with the Human Immunodeficiency Virus (HIV), where the condition is known as BCG immune reconstitution inflammatory syndrome (BCG‐IRIS).
In many cases, the infections resolve without any intervention, but treatments can include oral antibiotics, needle aspiration, draining abscesses, and surgically removing infected lymph nodes. This review was conducted to try to determine the effectiveness of these different treatments.
The review found no evidence of any benefit of using oral antibiotics to treat local or regional BCG‐induced disease. In patients with abscess‐forming lymphadenopathy, the only intervention with proven benefit was needle aspiration of the abscesses with or without local injection of the antibiotic isoniazid.
Based on these findings, the review authors recommend a 'wait and see' approach with follow‐up visits for minor reactions and lymphadenopathy without abscesses. For abscess‐forming lymphadenopathy, which can cause distress and discomfort, they advise needle aspiration. However, this review is based on only five studies, all of which were assessed as having a low or very low quality of evidence. As a consequence, the authors conclude there is an urgent need for more and better studies on ways to prevent and treat BCG‐induced disease, especially BCG‐IRIS.
Summary of findings
Summary of findings for the main comparison. Oral Isoniazid (INH) versus no intervention for BCG adverse reactions.
| Oral isoniazid (INH) versus no intervention for BCG adverse reactions | ||||||
| Patient or population: patients with BCG adverse reactions1 Settings: ambulatory setting Intervention: oral isoniazid (INH) Comparison: No intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Isoniazid (INH) | |||||
| Clinical failure clinical observation Follow‐up: 6 to 9 months2 | 36 per 100 | 53 per 100 (28 to 100) | RR 1.48 (0.79 to 2.78) | 54 (2 studies3) | ⊕⊝⊝⊝ very low4,5,6 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Includes regional ipsilateral subclavicular or axillary lymphadenitis >1.5 cm in diameter without fluctuation. 2 Follow up for 9 months and 6 months for Close 1985 and Caglayan 1987 respectively. 3Close 1985, Caglayan 1987. 4 Sequence generation, allocation concealment not described. Open randomized trials. 5Close 1985 is a small RCT with unknown full patient characteristics. Caglayan 1987 is an RCT with multiple comparisons other than INH. No heterogeneity could be detected. 6 Wide confidence intervals, which might include thresholds of clinical significance
Summary of findings 2. Oral erythromycin versus placebo or no intervention for BCG adverse reactions.
| Oral erythromycin compared to placebo or no intervention for BCG vaccine adverse reactions | ||||||
| Patient or population: patients with BCG adverse reactions Settings: ambulatory setting Intervention: erythromycin Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Erythromycin | |||||
| Clinical failure clinical observation Follow‐up: 1 to 6 months | 48 per 100 | 47 per 100 (36 to 62) | RR 1.03 (0.70 to 1.53) | 148 (3 studies1) | ⊕⊝⊝⊝ very low2,3,4 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Caglayan 1987, Noah 1993 and Kuyucu 1998. Include patients with regional ipsilateral subclavicular or axillary lymphadenitis >1.5 cm in diameter without fluctuation. 2 Sequence generation, allocation concealment not described. Only one study (Noah 1993) was blinded and used placebo in the control group. 3 Different doses (10, 40, and 50 mg/kg/day); different length of follow‐up. 4 Wide 95% CIs among different important clinical thresholds with moderate heterogeneity.
Summary of findings 3. Oral isoniazid plus rifampicin versus no intervention for BCG vaccine adverse reactions.
| Oral isoniazid plus rifampicin versus no intervention for BCG vaccine adverse reactions | ||||||
| Patient or population: infants with BCG vaccine adverse reactions1 Settings: ambulatory setting Intervention: isoniazid plus rifampicin Comparison: no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Isoniazid plus rifampicin | |||||
| Clinical failure clinical observation Follow‐up: mean 6 months | 33 per 100 | 43 per 100 (22 to 82) | RR 1.20 (0.51 to 2.83) | 35 (1 study2) | ⊕⊝⊝⊝ very low3,4 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Includes regional ipsilateral subclavicular or axillary lymphadenitis >1.5 cm in diameter without fluctuation.
2Caglayan 1987 3 Sequence generation and allocation concealment not described. Open randomized trial. 4 Wide 95% CI.
Summary of findings 4. Needle aspiration versus no intervention for BCG vaccine adverse reactions.
| Needle aspiration versus no intervention for BCG vaccine adverse reactions | ||||||
| Patient or population: infants with BCG vaccine adverse reactions1 Settings: ambulatory setting Intervention: needle aspiration Comparison: no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No intervention | Needle aspiration | |||||
| Clinical failure No regression of abscess by clinical evaluation2 Follow‐up: mean 6 months | 35 per 100 | 5 per 100 (1 to 19) | RR 0.13 (0.03 to 0.55) | 77 (1 study) | ⊕⊕⊝⊝ low3 | Only one study4 included patients with abscessed/fluctuant lymphadenitis. |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Regional adenitis with signs of fluctuation or abscess formation.
2 Regression of the lesion not adequately described by authors, possible measurement bias.
3 Sequence generation, allocation concealment not described.
Summary of findings 5. Local instillation of isoniazid versus erythromycin for BCG adverse reactions.
| Oral Erythromycin compared to locally instilled Isoniazid for BCG adverse reactions | ||||||
| Patient or population: patients with BCG adverse reactions1 Settings: ambulatory setting Intervention: oral erythromycin Comparison: locally‐instilled isoniazid | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oral erythromycin | Locally‐instilled isoniazid | |||||
| Time to resolution of illness Clinical observation Follow‐up: mean 6 months | The mean time to clinical resolution in the erythromycin group was 5.2 months. | The mean time to clinical resolution in the isoniazid group was 1.3 months less (95% CI 2.21 less to 0.39 less). | ‐ | 27 (1 study)2 | ⊕⊕⊝⊝ low3 | |
| *The basis for the assumed risk (eg the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Patients with axilar or subclavicular abscessed lymphadenitis; in all of them abscesses were previously aspirated.
3 Random sequence generation, allocation concealment not described.
Background
Description of the condition
Bacillus Calmette‐Guérin (BCG) is a live attenuated vaccine for preventing tuberculosis and is derived from Mycobacterium bovis. It has been used orally and intradermally since the 1920s (Sakula 1983), and is part of the immunization regime in many countries, as recommended by the World Health Organization (WHO) (Milstien 1993). Although it is considered to be safe, mild local reactions commonly occur. Usually, two or more weeks after its intradermal application, the BCG vaccine produces a papule that ultimately leads to an ulcer (10 weeks after vaccination) and a small scar (14 weeks after vaccination).
The BCG vaccine is routinely administered at birth in many countries as part of the global expanded programme of immunization recommended by the WHO (Milstien 1993). The most important risk factor for developing adverse reactions is an age of less than one month at time of application (Milstien 1990). Other risk factors are the method of application (subcutaneous instead of intradermic), number of viable bacilli, and changes in the strain used in current immunization regimes (Bolger 2006; Fine 1999).
In the era before the Human Immunodeficiency Virus (HIV), the frequency of complications seemed to be very low, even when taking into account that these complications were often under‐reported (Bonnlander 1993; FitzGerald 2000). Incidence of complications varies depending on the vaccine strain, the technique of administration, the age of the recipient, and the dose administered, with the rate ranging from 0.1 to 5 per 1000 vaccinated children (Lotte 1984; Lotte 1988). Complications arising from BCG vaccination have recently been classified as: a) local disease; b) regional disease; c) distant disease; and d) disseminated disease (Hesseling 2006). This classification also describes the BCG immune reconstitution inflammatory syndrome (BCG‐IRIS; see below).
Disseminated infection and osteitis in HIV‐uninfected children are extremely rare, but can result in serious morbidity and even death. The incidence rate for this event in immune competent children ranges from 0.19 to 1.56 per one million doses (Lotte 1984). However, a study in Canada estimated the occurrence at 205 cases per one million doses, much higher than had previously been reported (Deeks 2005).
Among HIV‐infected infants and children,the risk of BCG‐induced disease (both local and disseminated) is now considered a significant public health concern (Nutall 2011). Before the HIV pandemic, disseminated manifestations were restricted to patients with congenital or acquired severe immune deficiencies (Fine 1999; O'Brien 1995). Nowadays, the risk of disseminated BCG disease is several hundred fold higher in HIV‐infected children than in HIV‐uninfected children (Hesseling 2007). In countries where the BCG vaccine is routinely administered, children with undetected HIV infection may develop subclinical BCG infection, which may then be unmasked if given antiretroviral therapy (ART) (Rabie 2011). This important and recently described condition is called BCG‐IRIS and develops in up to 15% of children with HIV infection who have received the BCG vaccine. It usually presents within three months following initiation of ART as regional adenitis that suppurate and fistulate (French 2012; Nuttall 2008; Nutall 2011; Sharma 2011); risk factors for the development of BCG‐IRIS include a low CD4 count and high HIV‐1 RNA at initiation of the therapy (Rabie 2011).
Data on the protective effect of BCG vaccine in HIV‐exposed and infected children is lacking (Nutall 2011). Currently the WHO recommends that children who are known to be HIV‐infected, even if asymptomatic, should no longer be immunized with BCG vaccine (WHO 2007). Nonetheless, children with undiagnosed HIV may still be vaccinated, potentially leading to the development ofBCG‐induced disease. The timing of initiation of ART in HIV‐infected infants is a specific concern, as recent evidence suggests that early ART reduces the risk of BCG‐induced disease, including BCG‐IRIS (Rabie 2011).
Description of the intervention
Making a clear distinction between local/regional and distant/disseminated disease, the recommended medical therapies and surgical procedures are mainly aimed at the local/regional BCG‐induced diseases (Goraya 2001), with little attention paid to the distant/disseminated disease.
Medical therapies include anti‐tuberculosis drugs (isoniazid, rifampicin, ethambutol, ethionamide, ofloxacin, ciprofloxacin and erythromycin); topical corticosteroids; and antibiotics (Goraya 2002). Surgical options include needle aspiration, incision plus drainage of abscesses, and curettage or excision of involved nodes (Hengster 1997; Karpelowsky 2008). They are all meant to lessen the risk of complications, but their efficacy has not yet been adequately evaluated. In HIV‐infected children, the same therapeutic options are available, but again their efficacy is unclear. This potential lack of efficacy may be due to under‐reported cases, M. bovis resistance to anti‐tuberculosis drugs, and increased risk of BCG spread from regional to distant site, coupled with the documented poor outcome, which might warrant a more aggressive medical and/or surgical therapy (Hesseling 2006).
How the intervention might work
Anti‐tuberculosis drugs might effectively treat the BCG disease by directly attacking the attenuated M. bovis strain. However, chemotherapy is complicated by the inherent resistance of all M. bovis strains to pyrazinamide, and the emergence of additional resistance to other anti‐tuberculosis drugs as a result of inappropriate therapy (Hesseling 2004; Ritz 2009). The value of curettage, excision or therapeutic aspiration for minimizing the risk of potential hematogenous or lymphatic dissemination of BCG in HIV‐infected and uninfected children is unknown. Previous narrative and systematic reviews suggest needle aspiration can reduce the duration of suppuration, and surgery has been considered curative in cases of BCG suppurative lymphadenitis; however, both procedures are invasive and require sedation or general anaesthesia (Goraya 2002). There are reports that early ART (versus late ART) is associated with a significant reduction in BCG‐IRIS, probably by limiting the degree of CD4 cell depletion (Rabie 2008; Rabie 2011).
Why it is important to do this review
Whether local/regional or distant/disseminated, BCG vaccine‐induced disease can lead to parent anxiety, and increased infant morbidity and even death. In HIV‐infected children, BCG‐induced disease poses a higher risk for complications and dissemination (Hesseling 2006). Although the WHO guidelines recommend withholding the BCG vaccine from HIV‐infected children, a large number of unscreened children might go undetected and thus receive the vaccination.
A previous meta‐analysis concluded that oral erythromycin and other anti‐tuberculosis drugs do not reduce the risk of suppuration. However, this meta‐analysis does not describe extensively how it was conducted; in addition, the authors only searched one database and restricted the studies to those published in English (Goraya 2001). Beacuse of the variability of treatment modalities for BCG vaccine‐induced disease (ranging from no intervention to anti‐tuberculosis drugs and surgery) among different sub‐groups of patients (HIV‐infected and HIV‐uninfected) with a wide clinical spectrum of disease (from local to disseminated), there is an important lack of information on this topic and a systematic review is considered necessary to gather current available evidence and to direct future areas of research. Trials that incorporate patients treated with any medical or surgical therapies for BCG‐induced disease, including those with HIV infection, are included.
Objectives
To assess and summarize the evidence from randomized controlled trials on the effectiveness of medical and surgical interventions for treating BCG‐induced disease in children, distinguishing between local/regional disease, BCG‐IRIS and distant/disseminated disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Participants include all children (from 0 to 18 years of age) with BCG vaccine‐induced disease, with or without HIV‐infection. For HIV‐infected children, we included those with and without BCG‐IRIS.
Types of interventions
Medical therapies included any drug known or used for the treatment of BCG‐induced disease, distinguishing between local/regional disease, BCG‐IRIS and distant/disseminated disease. These interventions include oral anti‐tuberculous drugs (ie isoniazid, rifampicin, ethambutol, ethionamide, ofloxacin, ciprofloxacin, erythromycin), topical corticosteroids, and topical or locally‐instilled anti‐tuberculous drugs. Also included were surgical procedures such as needle aspiration with or without drug instillation, abscess open drainage, and node excision or curettage.
Types of outcome measures
Primary outcomes
-
Failure of therapy (medical and/or surgical), defined as any of the following:
progression from any enlarged node to suppuration;
progression of the BCG disease between stages (for example, from local BCG disease to disseminated disease) using any of the known classifications from Talbot 1997 or Hesseling 2006;
relapse or persistent enlarged nodes with or without suppuration despite treatment.
Time to resolution of illness. We used this outcome when the only information provided was the mean time (in months) measured from the period of study enrolment to the time the infant returned to baseline health status.
Secondary outcomes
Death from BCG vaccine‐induced disease.
Death from complications of the medical or surgical therapies.
All‐cause mortality.
For death from BCG vaccine induced‐disease and death from complications of the intervention, we planned to extract this data only if it was reported in the trial.
If death was directly related to the intervention (eg anaphylactic reaction from antibiotics), it would be classified accordingly. Otherwise, it would be annotated as a death from unknown causes and overall mortality.
Search methods for identification of studies
Collaborative Review Group search strategy. We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press and in progress).
Electronic searches
A systematic search was conducted in the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register and Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (The Cochrane Library 2012, Issue 4); MEDLINE (1966 to November 2012); EMBASE (Scopus) (1947 to November 2012); and LILACS (1980 to November 2012). We also searched the metaRegister of Controlled Trials (mRCT) and the WHO trials search portal using 'BCG vaccin*', 'complications' and 'adverse events' as search terms. Conference proceedings were consulted for relevant abstracts and experts were contacted to identify studies. No language restriction was applied.
Searching other resources
Conference proceedings
We searched the following conference proceedings for relevant abstracts:
The Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC)
The Infectious Diseases Society of America (IDSA) conferences
Each annual International Congress on Infectious Diseases (ICID) from the International Society for Infectious Diseases (ISID)
Each National Immunization Conference (NIC) from the Centers for Disease Control (CDC).
Researchers and organizations
We contacted researchers, authors of included trials, and other experts in the field of infectious diseases and epidemiology associated with the WHO's Immunization Safety Program, and also consulted vaccine safety web sites that met the credibility and content good information practices criteria of the WHO‐Vaccine Safety Net project.
Reference lists
We also checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Selection of studies
Two authors (CC and GP) independently screened the search results to select potentially relevant trials. Full articles for potentially relevant and relevant trials were retrieved. The authors independently applied the inclusion criteria using an eligibility form, and resolved any differences by discussing them with a third reviewer (CJ). The reports from each of the trials were scrutinized to ensure that multiple publications from the same trial were included only once. Excluded studies were listed together with the reasons for their exclusion in the Characteristics of excluded studies section. Finally, where we were still unsure if the study should be included because further information was necessary, we attempted to contact the study authors for clarification and allocated the study to the list of those awaiting assessment.
Data extraction and management
Two authors (CC and GP) independently extracted pre‐specified characteristics of each trial using a standardized, pre‐piloted, data extraction form. The two authors checked eligibility of candidate studies, while differences in data extraction were resolved by discussion with a third author (CJ). Study authors were contacted in cases of unclear or missing data. The following data was extracted:
the numbers of randomized and analysed participants in each treatment group for each outcome;
the number of participants with the event for each treatment group, and the mean and standard deviation for each treatment group for continuous outcomes.
Assessment of risk of bias in included studies
Two authors (CC and GP) assessed the risk of bias independently, and any disagreement was discussed and resolved by informal consensus with a third author (CJ). Study authors were contacted for unclear or unspecified information. We used the Cochrane Collaboration's tool for assessing risk of bias, which included the following domains (Higgins 2011).
Sequence generation: describe the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
Allocation concealment: describe the method used to conceal the allocation sequence in sufficient detail to determine whether intervention allocations could have been foreseen in advance of, or during, enrolment.
Blinding (masking) of participants, personnel and outcome assessors: describe all measures used, if any, to mask study participants and personnel from knowledge which intervention a participant received. Provide any information relating to whether the intended masking was effective.
Incomplete outcome data: describe the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. State whether attrition and exclusions were reported, the numbers in each intervention group (compared with total randomized participants), reasons for attrition or exclusions where reported, and any re‐inclusions in analyses performed by the review authors.
Selective outcome reporting: state how the possibility of selective outcome reporting was examined by the review authors, and what was found.
Other sources of bias: state any important concerns about bias not addressed in the other domains in the tool.
Judgements concerning the risk of bias for each component were assessed by the authors of the review using 'yes', 'no' or 'unclear', to indicate a low, high or unclear/unknown risk of bias respectively. The assessment of risk of bias is presented in a 'risk of bias graph' in addition to the 'risk of bias table'.
Measures of treatment effect
Dichotomous outcomes were evaluated using risk ratios (RR) as the measure of effect, with a 95% confidence interval (CI). For continuous outcomes, we used mean differences as the measure of effect, with a 95% CI.
Unit of analysis issues
When a particular multi‐arm study was included in the same meta‐analysis more than once, we split the control group to ensure that all participants in the meta‐analysis were independent.
Dealing with missing data
We did an intention‐to‐treat analysis (such that participants were kept in the intervention groups to which they were randomized, outcome data was available for all participants, and all randomized participants were included in the analysis). We contacted trial authors to request any missing data; if the authors did not respond within four to eight weeks, we conducted the review based only on the available information
Assessment of heterogeneity
We used forest plots to detect overlapping CIs, and applied the Chi2 test with a P value of 0.10 to indicate statistical significance. We also implemented the I2 statistic with a value of 30% to denote moderate levels of heterogeneity.
Assessment of reporting biases
We assessed reporting biases by examining asymmetry of funnel plots.
Data synthesis
One author (CC) performed the data analysis using Review Manager 5.1. Each individual therapy was treated separately in the analysis; we combined data using the fixed effects approach when there was no heterogeneity. If statistically significant heterogeneity was present (I2 statistic greater than 30% and/or the Chi2 test at a significant P value <0.1), we combined data using a random‐effects approach.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analyses for HIV‐infection status (infected versus not infected) when heterogeneity was present.
Sensitivity analysis
Sensitivity analyses regarding risk of bias were conducted to investigate the robustness of the results.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Studies awaiting classification / Ongoing studies.
Results of the search
The preliminary searches identified 30 potential citations. We read the titles and abstracts of these studies, selecting 19 articles as being eligible (Figure 1). Of these, five studies were included in the final analysis (Banani 1994; Caglayan 1987; Close 1985; Kuyucu 1998; Noah 1993).
1.

Study flow diagram.
Included studies
Close 1985 presents an investigation letter describing a study in which 18 patients were randomly allocated to receive either isoniazid (n = 8) at 10 mg/kg/day or no treatment (n = 10). Participants were followed up for nine months. There was only sufficient data to create one contingency table, and no additional information on patient baseline characteristics or other data on the methodology.
Caglayan 1987 took an original sample of 136 patients that had received the Pasteur vaccine intradermally. Six patients were excluded for not being available for adequate follow‐up and a further 10 were excluded because of 'untoward reactions', leaving 120 patients that were randomly allocated to no intervention (n = 42) or a medical therapy (n = 78). Within the medical therapy group, 21 patients were given oral isoniazid (10 mg/kg once a day for two months), 21 received isoniazid at the same dose plus rifampicin (10 mg/kg orally once a day for two months), and 36 received erythromycin stearate (30 mg/kg qid orally for one month). The decision to treat or not was 'at random' according to the authors. Failure of the medication was defined as an increase in the size of the lymph node, change in skin colour, and fluctuation progressing to drainage. Follow‐up was for six months. All patients had axillary or supraclavicular lymphadenitis (≧ 1.5 cm in diameter) without abscess formation.
Noah 1993 conducted a study on 96 patients with BCG adverse reactions, who were subdivided into two groups: those with lymphadenitis ≧ 2 cm in diameter but no fluctuation (lymphadenitis group, n = 69); and those with abscess formation at presentation (abscess group, n = 27).
After this categorization, patients in each group were randomized to intervention or control sub‐groups.
The abscess group had their collection aspirated with a gauge needle. They were then randomly allocated to receive either oral erythromycin succinate (50 mg/kg/day for one month) with a local instillation of placebo (normal saline) into the cavity, or local isoniazid instillation (50 mg) as a single dose into the cavity followed by oral placebo therapy qid for one month.
The lymphadenitis group was randomly assigned to receive either oral erythromycin succinate (50 mg/kg/day for one month) (n = 35) or oral placebo (n = 34).
Only for the lymphadenitis group was it possible to generate two‐by‐two tables for dichotomous outcomes (clinical resolution). The rest of the data were portrayed as continuous outcome, i.e. duration of resolution, defined as the time taken (in months) for the lymph nodes to become non‐palpable or less than 0.5 cm in size, without any evidence of residual ulceration, sinus formation and/or radiographic changes attributable to tuberculosis.
Banani 1994 randomly allocated 77 patients with regional BCG adverse reaction (fluctuant/suppurative adenitis) to needle aspiration (n = 43) or no intervention (n = 34). The main outcome measure consisted of the regression rate of lesions, with a maximum follow‐up of six months in all patients. Standard Pasteur vaccine was used in all patients. The authors excluded those with immunodeficiencies, generalized adenopathy and no signs of node pus collection, as well as those receiving an anti‐tuberculosis drug before referral. Aspiration was performed by needle subcutaneously. The authors do not report patients lost to follow‐up nor do they report who measured the main outcome and whether they were blinded to the group assignment. Spontaneous drainage of the lesion with development of sinus tract formation was less in the study group than in the control group at four months and six months. We determined the failure rate at six months as our main outcome.
Kuyucu 1998 conducted a study on infants two to six months of age with non‐suppurative, ipsilateral lymphadenitis (≧ 1.5 cm in diameter). Infants were vaccinated at birth or two months of age with BCG vaccine (Danish strain 1331). The authors randomized 45 patients into three groups: the first group received oral erythromycin stearate at 40 mg/kg/day for one month (n=15); the second group received a dose of streptomycin (20 mg/kg) injected directly into the node (n = 15); and the third group had no intervention (n = 15). Patients were evaluated every two weeks up to three months. Healing was defined as the regression of the lymph node to < 0.5 cm without evidence of residual ulceration and sinus formation, and no clinical or radiographic changes attributable to tuberculosis.
Excluded studies
Excluded studies and the reasons for their exclusion are detailed in the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias estimates are summarized in Figure 2 and Figure 3, and the reasons for these judgements are given in the Characteristics of included studies tables.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All five studies had an unclear risk of bias mainly because of an inadequate description or a failure to report the randomization process, sequence generation and allocation concealment.
Blinding
Only one study (Noah 1993) presented a low risk of bias: the outcome assessors were blinded to group assignment and a placebo was used in the control group. The study by Caglayan 1987 states that the outcome assessor was 'blind' to the group in which the child was enrolled, but the authors give no further explanation, so the risk of bias was deemed unclear. The studies by Close 1985, Banani 1994, and Kuyucu 1998 were deemed to have a high risk of bias: all three studies were open randomized trials without a blind assessment of the outcomes.
Incomplete outcome data
All studies had a low risk of bias because no patients were lost to follow‐up.
Selective reporting
There was insufficient information to permit judgement on reporting bias, hence the risk was deemed unclear.
Other potential sources of bias
Small sample size and poor reporting of the methodology was seen as a potential source of bias in all studies, but there was insufficient information to assess whether an important risk of bias existed. The risk of bias was classified as unclear.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
No trial reported any deaths or adverse events related to the treatment interventions for BCG‐induced disease.
For the main outcome of clinical resolution, we considered the following therapeutic strategies.
Oral isoniazid
Two open randomized trials, Close 1985 and Caglayan 1987, compared oral isoniazid versus no intervention. No significant difference in the risk of treatment failure (at six to nine months) for isoniazid and no intervention was detected (54 participants, two trials, Analysis 1.1; Figure 4). Using the GRADE approach, the quality of the evidence was assessed as very low (Table 1).
1.1. Analysis.
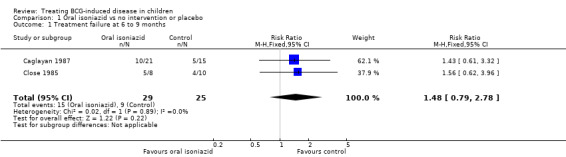
Comparison 1 Oral isoniazid vs no intervention or placebo, Outcome 1 Treatment failure at 6 to 9 months.
4.
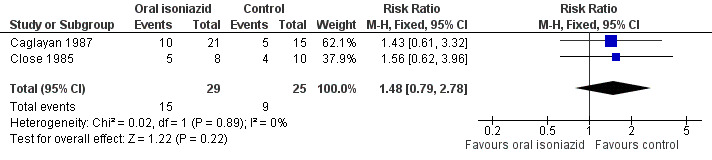
Forest plot of comparison: 1 Oral isoniazid vs no intervention or placebo, outcome: 1.1 Treatment failure at 6 to 9 months.
Oral erythromycin
Oral erythromycin was evaluated in three studies (Caglayan 1987; Kuyucu 1998; Noah 1993) and the effect was not statistically significant (148 participants, three trials, Analysis 2.1; Figure 5).
2.1. Analysis.

Comparison 2 Oral erythromycin vs no intervention or placebo, Outcome 1 Treatment failure at 6 to 9 months.
5.

Forest plot of comparison: 2 Oral erythromycin vs no intervention or placebo, outcome: 2.1 Treatment failure at 6 to 9 months.
Of these three studies, only the study by Noah 1993 used a placebo. Overall, the risk of bias was classified as either unclear or high, because the studies did not report the random sequence generation or concealment allocation. Also, there were mild concerns about the methodological heterogeneity, because the studies used different doses of erythromycin and adopted different lengths of follow‐up. Using the GRADE approach, the quality of the evidence was assessed as very low (Table 2).
Oral isoniazid plus rifampicin
Only the study by Caglayan 1987 compared this combined therapy against no intervention. The result was not significant (35 participants, one trial, Analysis 3.1; Figure 6) and the quality of the evidence (GRADE) was considered very low (Table 3).
3.1. Analysis.

Comparison 3 Oral isoniazid plus rifampicin vs no intervention or placebo, Outcome 1 Treatment failure at 6 to 9 months.
6.

Forest plot of comparison: 3 Oral isoniazid plus rifampicin vs no intervention or placebo, outcome: 3.1 Treatment failure at 6 to 9 months.
Needle aspiration compared to no intervention
Banani 1994 compared needle aspiration alone to no intervention and included patients with fluctuating lymph nodes (abscessed). The procedure was effective at reducing the persistence of the BCG‐induced lesions at follow‐up after six months (RR 0.13; 95% CI 0.03 to 0.55; 77 participants, one trial, Analysis 4.1; Figure 7). The quality of the evidence was considered low using the GRADE approach (Table 4).
4.1. Analysis.

Comparison 4 Needle aspiration vs no intervention, Outcome 1 Treatment failure at 6 months.
7.

Forest plot of comparison: 4 Needle aspiration vs no intervention, outcome: 4.1 Treatment failure at 6 months.
We did not find evidence for a direct comparison between needle aspiration and oral antibiotics.
Local instillation of isoniazid compared to oral erythromycin.
One study (Noah 1993) with unclear to low risk of bias compared the use of isoniazid locally instilled in a fluctuant node against oral erythromycin. The local instillation of isoniazid reduced the mean time to clinical resolution (mean difference 1.49 months less in the isoniazid group, 95% CI 0.82 to 2.68 less; 27 participants, one trial, Analysis 5.1; Figure 8). This evidence was classified as low quality (GRADE), mainly because random sequence generation and allocation concealment were not reported (Table 5).
5.1. Analysis.

Comparison 5 Local isoniazid versus oral erythromycin, Outcome 1 Time to resolution of illness (months).
8.

Forest plot of comparison: 5 Local isoniazid versus oral erythromycin, outcome: 5.1 Time to resolution of illness (months).
Discussion
Summary of main results
Oral antibiotics of any type were not effective at reducing the rate of clinical persistence of BCG‐induced lesions at six to nine months compared with no intervention or a placebo. This evidence comes from four clinical trials included in this review, with a total of 237 participants. Needle aspiration and local instillation of antibiotics were each evaluated in one study, while none of the studies evaluated HIV‐infected infants. None of the included studies reported any deaths or adverse events from the interventions.
Oral isoniazid
Two trials with 54 participants compared isoniazid against no intervention. Evidence from these studies was classified as having unclear to high risk of bias because the studies did not report the sequence generation or allocation concealment.
Oral erythromycin
Three randomized trials, assessed as having unclear to high risk of bias, recruited a total of 148 infants to compare oral erythromycin with a placebo or no intervention. Moderate clinical and methodological heterogeneity was observed, as the doses used in the studies ranged from 10 to 50 mg/kg/day and only one study (Noah 1993) was blinded.
Oral isoniazid plus rifampicin
One trial with 35 patients that had an overall unclear risk of bias compared isoniazid plus rifampin against no intervention, finding no major difference between them.
Needle aspiration
In one study (Banani 1994) involving 77 infants and children with suppurative lymphadenitis, needle aspiration reduced fluctuant adenopathy persistence at six months when compared with no intervention. The risk of bias was deemed unclear to high because the study did not report the randomization process or allocation concealment. In this case, however, the effect of the intervention was strong and the outcome could be evaluated more objectively (presence or absence of abscess). No deaths or adverse effects were reported with this procedure.
Local instillation of antibiotics
One trial (Noah 1993) with 27 participants compared oral erythromycin with local instillation of isoniazid in patients with fluctuant lymphadenitis. All participants had their collections previously aspirated. In this study, local instillation of isoniazid reduced the mean time for clinical resolution by 1.3 months. The risk of bias in this study was from unclear to low.
Overall completeness and applicability of evidence
The studies were from Turkey, Iran, and the Caribbean (Jamaica, Dominica). All trials included infants with local/regional lymphadenitis, while two of them also included infants with abscessed lymphadenitis. None of the studies included infants with HIV or other immunodeficiencies. We also did not find any studies including patients with distant/disseminated disease.
Three of the five studies specified the BCG strain administered to patients. Due to the small sample size, it is unclear whether different strains would have responded differently to the oral or locally‐instilled treatments. Isoniazid‐resistant strains in the BCG vaccine have been reported (Hesselberg 1972), which may explain the lack of response to oral isoniazid seen in two trials. Nevertheless, local instillation of isoniazid did reduce the mean time for clinical resolution in one study. Whether this was due to the local application of the antibiotic itself or due to another unknown mechanism is difficult to ascertain.
It's important to note that certain inherent differences among different populations and interventions might exist. For example, needle aspiration and/or locally instilled antibiotics would only be tested in individuals with fluctuant lymphadenopathy and not in individuals with non‐fluctuant lymphadenopathy, whereas antibiotics could be tested in individuals with all forms of BCG disease.
One study not included in our analysis but worth mentioning is Rabie 2011, which included infants with HIV infection that had been vaccinated with BCG. The treatment options considered for preventing the development of BCG‐induced disease (BCG‐IRIS) included early versus late ART. The study concluded that for patients with HIV infection and vaccinated with BCG an early ART, before immunological and/or clinical progression, substantially reduces the risk of BCG‐IRIS regional adenitis. In addition, it suggested that a low CD4 count and high HIV‐1 RNA at initiation of the ART are the strongest independent risk factors for BCG‐IRIS. Although the WHO specifies that BCG should not be administered to patients with HIV infection, this can still happen for HIV‐infected infants that haven't been formally diagnosed, who may then go on to develop these complications.
Quality of the evidence
This systematic review is limited, since the included trials were small and published between 1985 and 1998, and the reporting of the methodology was poor. Using the GRADE methodology, the quality of the evidence of included studies was assessed as being from very low to low. Although all five studies are classified as randomized, none reported sequence generation or allocation concealment adequately. Only one trial conducted blinding of participants.
Potential biases in the review process
Even though publication bias is an important threat to the validity of any systematic review, it is unlikely to have had an impact in this study. There was no evidence of funnel plot asymmetry on visual inspection.
Agreements and disagreements with other studies or reviews
A previous systematic review aimed at assessing the efficacy of various treatments for BCG‐induced disease concluded that neither oral erythromycin nor anti‐tuberculous drugs reduce the frequency of suppuration (Goraya 2001). Our results are consistent with this review. Despite conducting a more comprehensive search, with no language restrictions, we did not find any other trials that compared oral antibiotics against a control or placebo.
Authors' conclusions
Implications for practice.
BCG vaccine‐induced disease in children can produce discomfort and parental distress. The majority of reactions to the vaccine are localized and self‐limited, and in most cases observation for four to six months is usually sufficient. This systematic review found no evidence of any benefit of using oral antibiotics to treat local or regional BCG‐induced disease. There were no studies in distant or disseminated disease, including the newly described BCG‐IRIS. In patients with fluctuant/abscessed lymphadenopathy, the only intervention with proven benefit was needle aspiration with or without local instillation of isoniazid.
Treatment options should be considered and discussed with parents and caregivers, taking into account the different clinical situations. For minor reactions and non‐fluctuant lymphadenopathy, a 'wait and see' approach with close follow‐up visits seems most appropriate and should be recommended. For fluctuant lymphadenopathy, which causes distress, discomfort and potential for sinus development and dissemination, needle aspiration could be considered a viable option.
Implications for research.
Important uncertainty remains regarding the best therapy for BCG‐induced disease. For future trials to be adequately conducted, they should describe a better random sequence generation and allocation concealment than the studies considered in this review. In addition, they must be masked for both patients and researchers, and focus on clinically important outcomes such as time to heal, risk of suppuration, and risk of disseminated disease. Although oral antibiotics seem to be ineffective, it could be an area of further study if newly‐developed antibiotics show signs of efficacy. Future studies are also needed to investigate the benefit seen in the one small trial that evaluated the efficacy of needle aspiration with and without local instillation of isoniazid in patients with fluctuant nodes.
As BCG‐induced disease is a major public health concern in many countries with a high HIV prevalence, it is imperative to carry out more RCTs on ways to prevent or treat BCG‐induced disease in HIV‐infected infants. We only uncovered one RCT of HIV‐infected infants vaccinated with BCG, in which early ART therapy was found to prevent the development of BCG‐induced disease (Rabie 2011). Although this study did not meet our inclusion criteria for this systematic review, we suggest more studies are needed to replicate its findings.
Further RCTs must investigate therapeutic interventions for those HIV‐infected infants that have already developed BCG‐IRIS (ie antibiotics, corticosteroids, needle aspiration). Since the BCG vaccine is not recommended for HIV‐infected infants, these studies will probably have to involve infants that were only vaccinated because their HIV status was unknown at the time of vaccination.
A more effective tuberculosis vaccine is a major global health priority. Even though BCG vaccination protects against disseminated forms of childhood tuberculosis, it is not as effective against pulmonary tuberculosis. In recent years, efforts have been made to develop a new, safe and effective tuberculosis vaccine that could prevent all forms of tuberculosis, in all age groups and in HIV‐infected individuals. Human clinical trials evaluating at least 14 new tuberculosis vaccines are currently underway, including viral‐vectored vaccines, recombinant fusion proteins, recombinant BCG vaccines, and inactivated whole or fragmented mycobacteria (Hatherill 2011).
Acknowledgements
The editorial base of the Cochrane Infectious Diseases Group is funded by UKaid from the UK Government for the benefit of developing countries. Dr Olalekan Uthman was the academic editor for this review.
Appendices
Appendix 1. Search strategy
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | BCG vaccin* | BCG vaccin* | BCG vaccin* | BCG vaccin$ | BCG vaccin$ |
| 2 | Complication* | Complication* | Complication* | Complication$ | Complication$ |
| 3 | Adverse (effect* OR event* OR reaction*) | Adverse (effect* OR event* OR reaction*) | Adverse (effect* OR event* OR reaction*) | Adverse (effect$ OR event$ OR reaction$) | Adverse (effect$ OR event$ OR reaction$) |
| 4 | Lymph node swelling* | Lymph node swelling* | Lymph node swelling* | Lymph node swelling$ | Lymph node swelling$ |
| 5 | adenitis | adenitis | adenitis | adenitis | adenitis |
| 6 | 2 or 3 or 4 or 5 | LYMPHADENITIS | LYMPHADENITIS | LYMPHADENITIS | 2 or 3 or 4 or 5 |
| 7 | 1 and 6 | BCG VACCINE/ADVERSE EVENTS/ COMPLICATIONS/TOXICITY | BCG VACCINE/ADVERSE EVENTS/COMPLICATIONS/TOXICITY | BCG VACCINE/ADVERSE DRUG REACTION | 1 and 6 |
| 8 | 2 or 3 or 4 or 5 or 6 | 2 or 3 or 4 or 5 or 6 | 2 or 3 or 4 or 5 or 6 | ||
| 9 | 1 and 8 | 1 and 8 | 1 and 8 | ||
| 10 | 9 or 7 | 9 or 7 | 9 or 7 | ||
| 11 | Limit 10 to Humans | Limit 10 to Human | |||
|
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term. | |||||
Data and analyses
Comparison 1. Oral isoniazid vs no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 6 to 9 months | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.79, 2.78] |
Comparison 2. Oral erythromycin vs no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 6 to 9 months | 3 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.70, 1.53] |
Comparison 3. Oral isoniazid plus rifampicin vs no intervention or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 6 to 9 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Needle aspiration vs no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Local isoniazid versus oral erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to resolution of illness (months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by year of study]
Close 1985.
| Methods | Randomized controlled trial Duration: dates not described |
|
| Participants | Number: 18 enrolled infants Inclusion criteria: post‐BCG vaccine lymphadenitis Exclusion criteria: fluctuation of the adenitis |
|
| Interventions | Intervention group: isoniazid PO 10 mg/kg/day for a maximum of 9 months Control group: no intervention |
|
| Outcomes | Clinical failure (abscess formation/lymph node size increased) | |
| Notes | Location: Dominica, West Indies Setting: not described Source of funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Not described in the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not performed, open study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias exists |
Caglayan 1987.
| Methods | Randomized controlled trial Duration: 17 months, from January 1985 to June 1986 |
|
| Participants | Number: 120 infants enrolled (no age range specified) Inclusion criteria: post‐BCG vaccine lymphadenitis Exclusion criteria: patients with 'untoward' reactions (not adequately described) |
|
| Interventions | Group 1: isoniazid 10 mg/kg/day PO for two months Group 2: isoniazid plus rifampicin 10 mg/kg/day PO for two months Group 3: erythromycin 30 mg/kg/day PO for one month Group 4: no intervention |
|
| Outcomes | Clinical failure (abscess formation or lymph node size increased) | |
| Notes | Location: Turkey Setting: not described Source of funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Not described in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Describe the evaluation of the outcome as 'blind' with no further explanations |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The authors describe that they were 'blind' to where participants were allocated, although they do not specify how |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias exists |
Noah 1993.
| Methods | Randomized controlled trial Duration: 13 months, from November 1987 to December 1988 |
|
| Participants | Number: 96 infants enrolled: 69 with lymphadenitis; 27 with abscess formation Inclusion criteria: infants with lymphadenitis or abscess with a diameter of at least 2 cm within 6 months of BCG vaccination Exclusion criteria: not stated |
|
| Interventions | Group A (lymphadenitis group): received either 1) erythromycin PO (50 mg/kg/day for one month) or 2) oral placebo. Group B (abscessed group): all had their abscess aspirated, then randomized to 1) erythromycin PO (50 mg/kg/day for one month) plus a single local instillation of saline (placebo), or 2) oral placebo plus locally instilled isoniazid (50 mg single dose) |
|
| Outcomes | Clinical failure (abscess formation/lymph node size increased) | |
| Notes | Location: Jamaica Setting: emergency department community based‐trial Source of funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the study |
| Allocation concealment (selection bias) | Unclear risk | Not described in the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of placebo (coloured sugar water). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although subjective, the authors clearly define 'clinical resolution' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias exists |
Banani 1994.
| Methods | Randomized controlled trial Duration: 18 months, from September 1991 to March 1993 |
|
| Participants | Number: 77 infants enrolled Inclusion criteria: regional suppurative post‐BCG adenitis Exclusion criteria: a) no sonographic sign of node collection, b) generalised adenopathy, c) immunocompromised or d) if received an anti‐tuberculous drug before referral |
|
| Interventions | Group 1: needle aspiration Group 2: no intervention |
|
| Outcomes | Clinical failure (regression of the lesion not achieved) | |
| Notes | Location: Iran Setting: Not described Source of funding: Not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not adequate (every other week) |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not performed, open study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective (visual inspection and measurement) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias exists |
Kuyucu 1998.
| Methods | Randomized controlled trial Duration: 7 months, from June 1996 to January 1997 |
|
| Participants | Number: 45 enrolled infants (30 included in this review) Inclusion criteria: ipsilateral axillar or supraclavicular lymphadenitis (>1.5 cm) without suppuration Exclusion criteria: abscess formation |
|
| Interventions | Group 1: erythromycin (40 mg/kg/day PO for one month) Group 2: locally instilled streptomycin (20 mg/kg) ‐ group not included in this review Group 3: no intervention |
|
| Outcomes | Clinical failure (regression of the lesion not achieved) | |
| Notes | Location: Turkey Setting: community‐based trial Source of funding: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessment not blinded but measured in centimetres |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | There was insufficient information to assess whether an important risk of bias exists |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amato Neto 1973 | Wrong intervention: Oral BCG administration. Non‐randomized trial. |
| Au 1978 | Wrong intervention: BCG used as a therapy in adults for osteogenic sarcoma. |
| Balkan 1992 | Non‐randomized trial. |
| Bhandari 1980 | Observational non‐randomized trial. |
| Caglayan 1991 | Non‐randomized trial. |
| Cerda de Palou 2003 | Case report. |
| Chavganc 1969 | Case series. |
| De Britto 1997 | Wrong intervention: RCT of two BCG doses. Outcome not related to our review. |
| Easton 1984 | Case report. |
| Ferreira 1996 | Case series. |
| Goraya 2001 | Systematic review. |
| Guld 1955 | Case series. |
| Gur 2002 | Study evaluates tuberculin sensitivity, BCG scar and side effects after 0.1 and 0.05 ml of BCG at 3 months of age. No therapies evaluated. |
| Hanley 1985 | Non‐randomized trial. |
| Hengster 1997 | Non‐randomized trial. |
| Kendrick 1981 | Outcome not related to our review: cancer. |
| Koivisto 1974 | Comparative study of 3 BCG vaccines, not therapies for their complications. |
| Lachmann 1976 | Case series. |
| Mitinskaia 1994 | Case series. |
| Nazir 2005 | Case series. |
| Oguz 1992 | Non‐randomized trial. |
| Rabie 2011 | Important study about prevention of BCG‐IRIS. It is a randomized controlled trial evaluating the early initiation of antiretroviral therapy (ART) in HIV‐infected infants versus late ART for reducing the risk of BCG‐IRIS. Not included because it represents a different clinical question, where the condition of BCG‐induced disease (BCG‐IRIS) is the outcome to evaluate and not part of the problem/population. Ideally, the study would compare an intervention with a comparison for treating HIV‐infected infants with BCG‐IRIS. |
| Samileh 2006 | Case control study. |
| Teulieres 1991 | Wrong intervention: Compares two doses of BCG vaccine. Did not evaluate the treatment of BCG‐induced complications. |
| Thayyil‐Sudhan 1999 | Non‐randomized trial. Outcome not related to our review: Measurement of CD4 immunity in premature babies. |
| Yuan 2001 | Non‐randomized trial. |
Differences between protocol and review
The authors have changed since the published protocol. Out of five initial authors, three were able to work on the full review (CC, GP and CJ).
We added a new study in our references (Rabie 2011); although it was not included in our review, we considered it important to mention in the introduction and discussion sections.
In Data extraction and management, we originally planned to extract only dichotomous outcome data. However, in one study, the information was only expressed in number of months to reach clinical resolution of the illness, so we included the option of extracting continuous outcomes (ie months to reach clinical resolution), and extracted a mean and standard deviation for each treatment group.
Contributions of authors
Carlos Cuello and Giordano Pérez prepared the review and protocol. Carlos Jiménez checked the protocol, double checked the process and format of the review, and provided advice.
Sources of support
Internal sources
No sources of support supplied
External sources
Department for International Development, UK.
Declarations of interest
We certify that we have no affiliations with or involvement in any organization or entity with a direct financial interest in the subject matter of the review (eg employment, consultancy, stock ownership, honoraria, or expert testimony).
Unchanged
References
References to studies included in this review
Banani 1994 {published data only (unpublished sought but not used)}
- Banani SA, Alborzi A. Needle aspiration for suppurative post‐BCG adenitis. Archives of Disease in Childhood 1994;71(5):446‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caglayan 1987 {published data only (unpublished sought but not used)}
- Caglayan S, Yegin O, Kayran K, Timocin N, Kasirga E, Gun M. Is medical therapy effective for regional lymphadenitis following BCG vaccination?. American Journal of Diseases of Children 1987;141(11):1213‐4. [PubMed] [Google Scholar]
Close 1985 {published data only (unpublished sought but not used)}
- Close GC, Nasiiro R. Management of BCG adenitis in infancy. Journal of Tropical Pediatrics 1985;31(5):286. [DOI] [PubMed] [Google Scholar]
Kuyucu 1998 {published data only (unpublished sought but not used)}
- Kuyucu N, Kuyucu S, Ocal B, Tezic T. Comparison of oral erythromycin, local administration of streptomycin and placebo therapy for nonsuppurative Bacillus Calmette‐Guerin lymphadenitis. The Pediatric Infectious Disease Journal 1998;17(6):524‐5. [DOI] [PubMed] [Google Scholar]
Noah 1993 {published data only (unpublished sought but not used)}
- Noah PK, Pande D, Johnson B, Ashley D. Evaluation of oral erythromycin and local isoniazid instillation therapy in infants with Bacillus Calmette‐Guerin lymphadenitis and abscesses. The Pediatric Infectious Disease Journal 1993;12(2):136‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amato Neto 1973 {published data only}
- Amato Neto V, Oliveira e Silva YK, Finger H, Bianchi A, do Amaral JP, Cardozo BS, et al. Analysis of the adverse effects occurring after the administration of BCG by oral route in 5,579 healthy children [Análise das manifestações colaterais decorrentes da administração da vacina BCG, por via oral, a 5,579 crianças sadias]. Revista Paulista De Medicina 1973;82(3):135‐8. [PubMed] [Google Scholar]
Au 1978 {published data only}
- Au FC, Webber B, Rosenberg SA. Pulmonary granulomas induced by BCG. Cancer 1978;41(6):2209‐14. [DOI] [PubMed] [Google Scholar]
Balkan 1992 {published data only (unpublished sought but not used)}
- Balkan E, Sapan N, Unal M, Dogruyol H. Treatment of the suppurative BCG lymphadenitis [Süpüre BCG Lenfadenit Tedavisi]. Pediatrik Cerrahi Dergisi 1992;6:29‐32. [Google Scholar]
Bhandari 1980 {published data only (unpublished sought but not used)}
- Bhandari B, Khurana R, Mandowara SL. Management of post‐BCG lymphadenitis. Indian Journal of Pediatrics 1980;47(388):367‐70. [DOI] [PubMed] [Google Scholar]
Caglayan 1991 {published data only (unpublished sought but not used)}
- Caglayan S, Arikan A, Yaprak I, Aksoz K, Kansoy S. Management of suppuration in regional lymph nodes secondary to BCG vaccination. Acta Paediatrica Japonica 1991;33(6):699‐702. [DOI] [PubMed] [Google Scholar]
Cerda de Palou 2003 {published data only}
- Cerdá de Palou E, Santy HP. Lymphadenitis as a complication following vaccination with Bacillus Calmette‐Guérin (BCG). Nederlands Tijdschrift voor Geneeskunde 2003;147(12):569‐72. [PubMed] [Google Scholar]
Chavganc 1969 {published data only}
- Chavganc J, Hanák R, Dam HG. Epidemiological significance of the local reaction to direct BCG vaccination as assessed from a study in Mongolia. Bulletin of the World Health Organization 1969;41(1):45‐62. [PMC free article] [PubMed] [Google Scholar]
De Britto 1997 {published data only (unpublished sought but not used)}
- Britto RL, Ramanathan VD, Gupte MD. Regional lymphadenitis following antileprosy vaccine BCG with killed Mycobacterium leprae. International journal of leprosy and other mycobacterial diseases 1997;65(1):12‐9. [PubMed] [Google Scholar]
Easton 1984 {published data only (unpublished sought but not used)}
- Easton PA, Hershfield ES. Lymphadenitis as a late complication of BCG vaccination. Tubercle 1984;65(3):205‐8. [DOI] [PubMed] [Google Scholar]
Ferreira 1996 {published data only (unpublished sought but not used)}
- Ferreira AA, Bunn‐Moreno MM, Sant'Anna CC, Ferreira MF. BCG vaccination in low birth weight newborns: analysis of lymphocyte proliferation, IL‐2 generation and intradermal reaction to PPD. Tubercle and Lung Disease 1996;77(5):476‐81. [DOI] [PubMed] [Google Scholar]
Goraya 2001 {published and unpublished data}
- Goraya JS, Virdi VS. Treatment of Calmette‐Guérin bacillus adenitis: a metaanalysis. The Pediatric Infectious Disease Journal 2001;20(6):632‐4. [DOI] [PubMed] [Google Scholar]
Guld 1955 {published data only (unpublished sought but not used)}
- Guld J, Magnus K, Tolderlund K, Biering‐Sorensen K, Edwards PQ. Suppurative lymphadenitis following intradermal B.C.G. vaccination of the newborn; a preliminary report. British Medical Journal 1955;2(4947):1048‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gur 2002 {published data only (unpublished sought but not used)}
- Gür E, Arvas A. Dose of Calmette‐Guérin bacillus vaccination in infants. The Pediatric Infectious Disease Journal 2002;21(4):359‐60. [DOI] [PubMed] [Google Scholar]
Hanley 1985 {published data only (unpublished sought but not used)}
- Hanley SP, Gumb J, Macfarlane JT. Comparison of erythromycin and isoniazid in treatment of adverse reactions to BCG vaccination. British Medical Journal (Clinical research ed.) 1985;290(6473):970. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hengster 1997 {published data only (unpublished sought but not used)}
- Hengster P, Sölder B, Fille M, Menardi G. Surgical treatment of bacillus Calmette Guérin lymphadenitis. World Journal of Surgery 1997;21(5):520‐3. [DOI] [PubMed] [Google Scholar]
Kendrick 1981 {published data only (unpublished sought but not used)}
- Kendrick MA, Comstock GW. BCG vaccination and the subsequent development of cancer in humans. Journal of the National Cancer Institute 1981;66(3):431‐7. [PubMed] [Google Scholar]
Koivisto 1974 {published data only}
- Koivisto M, Brander E, Hakosalo J, Wasz‐Höckert O. Comparative study of 3 BCG vaccines [Kolmen BCG‐rokotteen vertaileva tutkimus]. Duodecim 1974;90(24):1717‐22. [PubMed] [Google Scholar]
Lachmann 1976 {published data only (unpublished sought but not used)}
- Lachmann D, Howanietz L. Clinical aspect and therapy of BCG‐lymphadenitis (author's transl). Pädiatrie und Pädologie 1976;11(1):283‐6. [PubMed] [Google Scholar]
Mitinskaia 1994 {published data only}
- Mitinskaia LA, Lukhimenko NV, Kamaeva VF. BCG vaccination and increasing the effectiveness of treatment of post‐vaccination complications by the use of rifampicin and dimexide. Problemy Tuberkuleza 1994;5:4‐7. [PubMed] [Google Scholar]
Nazir 2005 {published data only (unpublished sought but not used)}
- Nazir Z, Qazi SH. Bacillus Calmette‐Guerin (BCG) lymphadenitis‐changing trends and management. Journal of Ayub Medical College, Abbottabad: JAMC 2005;17(4):16‐8. [PubMed] [Google Scholar]
Oguz 1992 {published data only (unpublished sought but not used)}
- Oğuz F, Müjgan S, Alper G, Alev F, Neyzi O. Treatment of Bacillus Calmette‐Guérin‐associated lymphadenitis. The Pediatric Infectious Disease Journal 1992;11(10):887‐8. [PubMed] [Google Scholar]
Rabie 2011 {published data only}
- Rabie H, Violari A, Duong T, Madhi SA, Josipovic D, Innes S, et al. Early antiretroviral treatment reduces risk of bacille Calmette‐Guérin immune reconstitution adenitis. The International Journal of Tuberculosis and Lung Disease: the official journal of the International Union against Tuberculosis and Lung Disease 2011;15(9):1194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samileh 2006 {published data only (unpublished sought but not used)}
- Samileh N, Ahmad S, Farzaneh A, Shahnaz R, Lida F, Mohammad N. Immunity status in children with Bacille Calmette‐Guerin adenitis. A prospective study in Tehran, Iran. Saudi Medical Journal 2006;27(11):1719‐24. [PubMed] [Google Scholar]
Teulieres 1991 {published data only (unpublished sought but not used)}
- Teulieres L, Diouf MA, Chaud P, Saint‐Cyr A, Saliou P. Comparative trial of administration of half (0.05 mg) and quarter (0.025 mg) dose of intradermal Pasteur BCG on 291 infants from birth to 1 year in French Guyana. Vaccine 1991;9(7):521‐4. [DOI] [PubMed] [Google Scholar]
Thayyil‐Sudhan 1999 {published data only (unpublished sought but not used)}
- Thayyil‐Sudhan S, Kumar A, Singh M, Paul VK, Deorari AK. Safety and effectiveness of BCG vaccination in preterm babies. Archives of Disease in Childhood. Fetal and neonatal edition 1999;81(1):F64‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yuan 2001 {published data only (unpublished sought but not used)}
- Yuan Y, Wu M, Dai X, Ni Z, Su G. Study on different treatment methods of tuberculous lymphadenitis caused by BCG vaccination. Journal of Clinical Internal Medicine 2001;18(3):226. [Google Scholar]
Additional references
Bolger 2006
- Bolger T, O’Connell M, Menon A, Butler K. Complications associated with the bacille Calmette‐Guérin vaccination in Ireland. Archives of Disease in Childhood 2006;91(7):594‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonnlander 1993
- Bonnlander H, Rossignol AM. Complications of BCG vaccinations in rural Haiti. American Journal of Public Health 1993;83(4):583‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks SL, Clark M, Scheifele DW. Serious adverse events associated with Bacille Calmette‐Guérin vaccine in Canada. Pediatric Infectious Disease Journal 2005;24(6):538‐41. [DOI] [PubMed] [Google Scholar]
Fine 1999
- Fine P, Carneiro I, Milstein JB, Clements CJ. BCG immunization policies. Issues relating to the use of BCG in immunisation programmes. Geneva: World Health Organization (WHO/V&B/99.23) 1999.
FitzGerald 2000
- Fitzgerald JM. Management of adverse reactions to Bacille Calmette‐Guérin vaccine. Clinical Infectious Diseases 2000;31(Suppl 3):S75‐6. [DOI] [PubMed] [Google Scholar]
French 2012
- French MAH. Immune reconstitution inflammatory syndrome: immune restoration disease 20 years on. The Medical Journal of Australia 2012;196(5):318‐21. [DOI] [PubMed] [Google Scholar]
Goraya 2002
- Goraya JS, Virdi VS. Bacille Calmette‐Guérin lymphadenitis. Postgraduate Medical Journal 2002;78(920):327‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatherill 2011
- Hatherill M. Prospects for elimination of childhood tuberculosis: the role of new vaccines. Archives of Disease in Childhood 2011;96(9):851‐6. [DOI] [PubMed] [Google Scholar]
Hesselberg 1972
- Hesselberg I. Drug resistance in the Swedish/Norwegian BCG strain. Bulletin of the World Health Organization 1972;46(4):503‐7. [PMC free article] [PubMed] [Google Scholar]
Hesseling 2004
- Hesseling AC, Schaaf HS, Victor T, Beyers N, Marais BJ, Cotton MF, et al. Resistant Mycobacterium bovis Bacillus Calmette‐Guérin disease: implications for management of Bacillus Calmette‐Guérin Disease in human immunodeficiency virus‐infected children. Pediatric Infectious Disease Journal 2004;23(5):476‐9. [DOI] [PubMed] [Google Scholar]
Hesseling 2006
- Hesseling AC, Rabie H, Marais BJ, Manders M, Lips M, Schaaf HS, et al. Bacille Calmette‐Guérin Vaccine‐Induced Disease in HIV‐Infected and HIV‐Uninfected Children. Clinical Infectious Diseases 2006;42(4):548‐58. [DOI] [PubMed] [Google Scholar]
Hesseling 2007
- Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PE, Godfrey‐Faussett P, et al. The risk of disseminated Bacille Calmette‐Guerin (BCG) disease in HIV‐infected children. Vaccine 2007;25(1):14‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. 2011.
Karpelowsky 2008
- Karpelowsky JS, Alexander AG, Dix Peek S, Millar AJW, Rode H. Surgical complications of bacille Calmette‐Guérin (BCG) infection in HIV‐infected children: Time for a change in policy?. South African Medical Journal 2008;98(10):801‐4. [PubMed] [Google Scholar]
Lotte 1984
- Lotte A, Wasz‐Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E. BCG complications: estimates of risks among vaccinated subjects and statistical analysis of their main characteristics. Advances in Tuberculosis Research 1984;21:107‐93. [PubMed] [Google Scholar]
Lotte 1988
- Lotte A, Dam HG, Henderson R. Second IUATLD study on complications induced by intradermal BCG vaccination. Bulletin of the International Union Against Tuberculosis and Lung Disease 1988;63(2):47‐83. [PubMed] [Google Scholar]
Milstien 1990
- Milstien JB, Gibson JJ. Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bulletin of the World Health Organization 1990;68(1):93‐108. [PMC free article] [PubMed] [Google Scholar]
Milstien 1993
- Milstien JB. Immunological Basis for Immunization / Module 5: Tuberculosis. (WHO/EPI/GEN/93.15). Geneva: World Health Organization 1993.
Nutall 2011
- Nuttall JJC, Eley BS. BCG Vaccination in HIV‐Infected Children. Tuberculosis Research and Treatment 2011;2011:712736. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuttall 2008
- Nuttall JJ, Davies MA, Hussey GD, Eley BS. Bacillus Calmette‐Guérin (BCG) vaccine‐induced complications in children treated with highly active antiretroviral therapy. International Journal of Infectious Diseases 2008;12(6):e99‐105. [DOI] [PubMed] [Google Scholar]
O'Brien 1995
- O'Brien KL, Ruff AJ, Louis MA, Desormeaux J, Joseph DJ, McBrien M, et al. Bacillus Calmette‐Guérin complications in children born to HIV‐1‐infected women with a review of the literature. Pediatrics 1995;95(3):414‐8. [PubMed] [Google Scholar]
Rabie 2008
- Rabie H, Violari A, Madhi S, Gibb D, Steyn J, Niekerk R, et al. Complications of BCG Vaccination in HIV‐infected and uninfected Children: CHER Study. Proceedings of the 15th Conference on Retroviruses and Opportunistic Infections. Boston: Foundation for Retrovirology and Human Health, 2008.
Ritz 2009
- Ritz N, Tebruegge M, Connell TG, Sievers A, Robins‐Browne R, Curtis N. Susceptibility of Mycobacterium bovis BCG vaccine strains to antituberculous antibiotics. Antimicrobial Agents and Chemotherapy 2009;53(1):316‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakula 1983
- Sakula A. BCG: who were Calmette and Guérin?. Thorax 1983;38(11):806‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2011
- Sharma SK, Soneja M. HIV & immune reconstitution inflammatory syndrome (IRIS). The Indian Journal of Medical Research 2011;134(6):866‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talbot 1997
- Talbot EA, Perkins MD, Silva SF, Frothingham R. Disseminated bacille Calmette‐Guérin disease after vaccination: case report and review. Clinical Infectious Diseases 1997;24(6):1139‐46. [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. Revised BCG vaccination guidelines for infants at risk for HIV infection. The Weekly Epidemiological Record 2007;82(21):193‐6. [PubMed] [Google Scholar]


