Abstract
Background
Artemisinin‐based combination treatments are strongly advocated, but supplies are limited. Sulfadoxine combined with amodiaquine is an alternative non‐artemisinin combination.
Objectives
To compare sulfadoxine‐pyrimethamine plus amodiaquine (SP plus AQ) with sulfadoxine‐pyrimethamine plus artesunate (SP plus AS) for treating uncomplicated Plasmodium falciparum malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (October 2005), CENTRAL (The Cochrane Library 2005, Issue 4), MEDLINE (1966 to October 2005), EMBASE (1988 to October 2005), LILACS (October 2005), and reference lists. We also contacted researchers and organizations working in this field.
Selection criteria
Randomized controlled trials comparing SP plus AS with SP plus AQ for treating uncomplicated P. falciparum malaria.
Data collection and analysis
Two authors independently applied the inclusion criteria, extracted data, and assessed methodological quality. The primary outcome measure was treatment failure (parasitological or clinical evidence of treatment failure between start of treatment and day 28). We calculated the risk ratio (RR) with 95% confidence intervals (CI) for dichotomous data.
Main results
Four trials (775 participants) met the inclusion criteria. All were from areas of high and seasonal malaria transmission in Africa. Fewer participants using SP plus AQ failed treatment by day 28 (RR 0.59, 95% CI 0.42 to 0.83; 652 participants, 3 trials). Even excluding new infections, SP plus AQ performed better (RR 0.62, 95% CI 0.40 to 0.96; 649 participants, 3 trials). There was no statistically significant difference between the two treatments for treatment failure at day 14 (RR 1.14, 95% CI 0.47 to 2.78; 775 participants, 4 trials). SP plus AS was more effective at reducing gametocyte carriage at day seven (RR 2.31, 95% CI 1.36 to 3.92; 220 participants, 1 trial). One trial reported that one person − in the SP plus AQ group − developed severe malaria. Adverse events were poorly reported, but did not seem to differ in type and number between the two treatment combinations.
Authors' conclusions
SP plus AQ performed better at controlling treatment failure at day 28, but was not as good as SP plus AS at reducing gametocyte carriage at day seven. Careful consideration of local resistance patterns is required because resistance to sulfadoxine‐pyrimethamine and amodiaquine are high in many areas. In order to delay development of resistance to artesunate, the combination with sulfadoxine‐pyrimethamine should only be considered where both drugs are known to be effective. Data on adverse events are still lacking.
8 May 2019
No update planned
Intervention not in general use or been superseded
Historical question. The WHO does not recommend non‐artemisinin combinations. No update intended.
Plain language summary
Sulfadoxine‐pyrimethamine plus artesunate versus sulfadoxine‐pyrimethamine plus amodiaquine for treating uncomplicated malaria
Sulfadoxine‐pyrimethamine plus amodiaquine (SP plus AQ) performed better than sulfadoxine‐pyrimethamine plus artesunate (SP plus AS) when treating uncomplicated malaria
Malaria is a parasitic disease spread by mosquitoes that kills thousands of people worldwide. Artemisinin‐based combination treatments are strongly advocated, but uncertainty about their availability (and cost) remains a major concern. The review includes four small randomized controlled trials, all from Africa, comparing SP plus AS with SP plus AQ for treating uncomplicated malaria. SP plus AQ performed better at destroying blood parasites at 28 days, although resistance to the drugs may have increased since the trials were performed. Adverse events were poorly reported.
Background
Malaria is a mosquito‐borne disease that is the leading cause of morbidity and mortality in sub‐Saharan Africa, affecting 300 to 500 million people every year (WHO 2000a). Of the four malaria parasite species that infect humans, Plasmodium falciparum is most common and can cause severe malaria. Uncomplicated malaria is the more frequent presentation but can progress to severe malaria if not adequately treated. The level of immunity to malaria varies with transmission intensity of the setting and age. Residents of high malaria transmission areas usually develop a level of immunity to malaria that increases with age up to about five years. This immunity may protect an individual from developing severe malaria. In areas of low and seasonal malaria transmission, people do not develop immunity and are therefore more susceptible to development of severe malaria.
Effective malaria treatment is important to minimize the effects of malaria and is a major control strategy (WHO 2000a). Parasite resistance to many of the available antimalarial drugs continues to be a problem and makes effective treatment of malaria episodes difficult. Chloroquine had been first‐line treatment until the 1990s when parasite resistance led to its replacement with other regimens (White 1998). Parasites have developed resistance to sulfadoxine‐pyrimethamine rapidly because its long half life encourages the selection of the resistant parasites; some fear that it will become completely ineffective (WHO 1988; Warsame 2002). As a consequence, the search for effective antimalarial regimens continues; new antimalarial drugs are not being developed fast enough to meet the demand, and new ways of using already available drugs are being explored.
Combination therapy, when two or more antimalarial drugs with different modes of action are used together, is a widely recommended strategy for increasing the effectiveness of the component drugs and delaying the development of resistance to them (White 1999). In particular, artemisinin‐based combination treatments are recommended because the artemisinin drug produces rapid clinical and parasitological response, may delay the development of resistance, and may reduce malaria transmission by killing gametocytes (Adjuik 2004).
Several countries in Africa have either changed or are considering changing their treatment policies to artesunate (an artemisinin drug) combined with amodiaquine, or other artemisinin‐containing regimens. Although artemisinin‐based combinations have been shown to be effective, implementing any policy involving their use could be faced with several problems, namely poor availability since artemisinin drugs are not yet widely available in Africa and may not be for some time because of low production, comparatively high cost, dosing complexity, and the lack of clinical experience with artemisinin‐based combinations (Bloland 2003).
SP and amodiaquine are both widely available and inexpensive. There has been interest in combining these two drugs, and recent studies in Africa have explored this possibility. The studies have shown that SP combined with amodiaquine (SP plus amodiaquine) may be comparable to SP combined with artesunate (SP plus artesunate) for treating malaria (Bloland 2003). The World Health Organization's Roll Back Malaria programme recommends using SP plus amodiaquine in areas where efficacy of both drugs is high (RBM 2003a).
The use of these combinations may be associated with unwanted adverse effects, possibly not observed when either drug is used singly. In particular there have been concerns about the safety profile of amodiaquine (Olliaro 2004), though a recent systematic review found no evidence of increased risks of serious adverse events (MacLehose 2003). Sulfadoxine‐pyrimethamine is rarely associated with severe adverse reactions in standard treatment (Sturchler 1993). Some artemisinin derivatives have been associated with fatal neurotoxicity in animals, and transient first‐degree heart block has been reported in a few people taking artemisinin drugs, but artesunate itself seems to be well tolerated (Brewer 1994; Kain 1995; WHO 2000b). It is however still important to look out for any adverse events that may occur. We therefore compared SP plus amodiaquine with SP plus artesunate for treating uncomplicated P. falciparum malaria.
We have examined pragmatic outcomes, which include clinical endpoints. Treatment failure by day 28 is our primary outcome because this captures the majority of failures with these drugs, and recent evidence has shown that measures before this time underestimate the true failure rate (RBM 2003b; Stepniewska 2004). Parasitaemia after day 14 could be the result of a new infection and some studies detect these new infections using polymerase chain reaction (PCR) analysis, a method that can be used to differentiate between new and old infections; we also examined the outcome of treatment failure by day 28 with new infections excluded.
Objectives
To compare sulfadoxine‐pyrimethamine plus artesunate (SP plus AS) with sulfadoxine‐pyrimethamine plus amodiaquine (SP plus AQ) for treating uncomplicated P. falciparum malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Adults and children with microscopically confirmed P. falciparum malaria. We only included trials where participants had uncomplicated P. falciparum malaria as defined by the World Health Organization (WHO 2001).
Types of interventions
SP plus AS versus SP plus AQ.
Types of outcome measures
Primary
Treatment failure by day 28, defined as parasitological or clinical evidence of treatment failure between start of treatment and day 28; this includes new infections.
Secondary
Treatment failure, defined as parasitological or clinical evidence of treatment failure between start of treatment and day 28; excludes new infections detected by PCR where reported.
Time to parasite clearance, defined as the first negative blood slide.
Time to fever clearance, defined as the time between the start of treatment and the temperature returning to normal and remaining so for at least 48 hours.
Presence of gametocytes at day 28.
Adverse events
Serious adverse events, defined as a sign, symptom, or intercurrent illness that is fatal, life‐threatening, or requires hospital admission.
Adverse events leading to discontinuation of treatment.
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (October 2005); Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2005, Issue 4); MEDLINE (1966 to October 2005); EMBASE (1980 to October 2005); and LILACS (1982 to October 2005).
Researchers and pharmaceutical companies
For unpublished and ongoing trials, we contacted individual researchers working in the field and the following pharmaceutical companies: Arenco (France), Mepha (Switzerland), Rhone‐Poulenc Rorer (France); Propharma (Scotland), Novartis (Switzerland), Sanofi‐Winthrop (France), Dafra (Belgium), Guilin Pharmaceutical Company (China), Kunning Pharmaceutical Corporation (Malaysia), Thua Thien Pharmaceutical Company (Viet Nam), and the National Pharmaceutical Plant Company (Viet Nam).
Reference lists
We also checked the reference lists of all trials identified by the above methods.
Data collection and analysis
Selection of studies
Hasifa Bukirwa (HB) scanned the results of the literature search, retrieved potentially relevant trials, and checked the eligibility with Julia Critchley (JC) using a standard form. We resolved ambiguity by discussion.
Data extraction and management
HB and JC independently extracted trial characteristics and data. Where the number randomized and the numbers analysed were inconsistent, we calculated the percentage loss to follow up and reported this in 'Characteristics of included studies. We had intended to primarily consider trials that used an intention‐to‐treat analysis, but only one trial approximated such an analysis, two used a per‐protocol analysis, and the fourth did not specify the method although it was clearly not an intention‐to‐treat analysis.
For dichotomous outcomes, we recorded the number of participants experiencing the event in each group of the trial. For continuous outcomes, we extracted the arithmetic means and standard deviations for each group.
Assessment of risk of bias in included studies
HB and JC independently assessed the methodological quality of the included trials. We classed the generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear according to Jüni 2001. We considered the inclusion of all randomized participants in the analysis to be adequate (acceptable) if it was 90% or more. We described who was blinded, such as the participants and care providers or assessors.
Data synthesis
We analysed data using Review Manager 4.2. We calculated the risk ratio (RR) with 95% confidence intervals (CI) for dichotomous data. We assessed heterogeneity among included trials by visually inspecting forest plots, carrying out a chi‐squared test for heterogeneity (statistical significance at 10% level) and calculating I2 statistic. We used the fixed‐effect model to pool data because we did not detect heterogeneity.
We planned to carry out subgroup analyses based on the following subgroups but this was not possible. We could not subgroup by participant age (less than five years versus greater or equal to five years) because all the identified trials were done in one age group. There were too few trials allow any meaningful subgroup analyses for trial setting, namely high (hyperendemicity or holoendemicity) versus low endemicity (hypoendemicity or mesoendemicity) and level of resistance to the comparator drug. Subgroup analyses may be possible in future updates of this review.
Results
Description of studies
Four trials (775 participants) met our inclusion criteria (see 'Characteristics of included studies'). They were carried out in areas of high and seasonal malaria transmission in Africa. All trials were single centre trials, except for Rwagacondo 2003, which was conducted at three sites within Rwanda. We have used the published analysis, which combined data from the three sites.
Source of funding
The four trials were supported by international and bilateral organizations such as the World Health Organization, and one, Mockenhaupt 2005, also had contributions in the form of the study drugs from pharmaceutical companies.
Location
All trials were conducted in Africa: Mockenhaupt 2005 in a hyperendemic area of Ghana; Abacassamo 2004 in an area of seasonal transmission in Mozambique; Rwagacondo 2003 described transmission as stable with seasonal peaks at the trial site in Rwanda; and Dorsey 2002 was conducted in a mesoendemic area of Uganda.
Participants
All the four trials were in children aged between six months and five years.
Interventions
All trials compared SP plus AQ with SP plus AS. All trials also had additional arms that were not pertinent to this review: Mockenhaupt 2005 and Dorsey 2002 included a sulfadoxine‐pyrimethamine alone arm; Abacassamo 2004 had an amodiaquine plus artesunate arm; and Rwagacondo 2003 had an amodiaquine alone arm.
Dose and regimen
The trials used similar dose and regimens for SP plus AS and SP plus AQ.
Length of follow up
Follow up was 28 days for Mockenhaupt 2005 and Rwagacondo 2003. Abacassamo 2004 followed participants for 21 days and Uganda had total follow up per participant of one year, although individual episodes were followed up to 28 days.
Drug resistance
Resistance to sulfadoxine‐pyrimethamine was reported in two trials. Rwagacondo 2003 reported resistance to sulfadoxine‐pyrimethamine between 11% and 45% and Dorsey 2002 reported it as common: a study done around the same period as the Ugandan trial reported parasitological failure levels of about 61% with sulfadoxine‐pyrimethamine (Talisuna 2004).
Outcomes
Treatment failure was reported by all the trials either at day 14 or 28 or both. One trial, Dorsey 2002, reported on progression to severe malaria. Mockenhaupt 2005 reported on gametocyte carriage at day seven and Abacassamo 2004 at day 14. Three trials mentioned adverse events, but none of them in sufficient detail.
Risk of bias in included studies
Generation of allocation sequence was adequate in three trials; it was unclear in one trial that did not mention the method of generation (Rwagacondo 2003). For allocation concealment, one trial used adequate methods (Abacassamo 2004) and the other three did not provide this information). Two trial reports mentioned blinding: Dorsey 2002 used a placebo in the sulfadoxine‐only arm to blind the participants and study investigators; and Mockenhaupt 2005 used a double dummy placebo technique to blind participants from the intervention, but it was not clear who else was blinded. Three trials did not include all the enrolled participants in the final analysis: Mockenhaupt 2005 and Abacassamo 2004 included adequate numbers of the enrolled participants in the final analysis (90% and 91% respectively). Dorsey 2002 had a one‐year follow up and included 89%, which we classed as inadequate. The fourth trial, Rwagacondo 2003, did not provide this information.
Effects of interventions
Treatment failure by day 28
Three trials reported results for this outcome (Dorsey 2002; Rwagacondo 2003; Mockenhaupt 2005). Fewer participants failed treatment with SP plus AQ (RR 0.59, 95% CI 0.42 to 0.83; 652 participants, 3 trials; comparison 01‐01), with a consistent effect across all trials and no evidence of heterogeneity (I2 = 0%). When new infections were excluded, SP plus AQ still performed better (RR 0.62, 95% CI 0.40 to 0.96; 649 participants, 3 trials; comparison 01‐02).
Treatment failure by day 14
Fewer people failed treatment with SP plus AS in three of the four trials, but the overall effect was not statistically significant (RR 1.14, 95% CI 0.47 to 2.78; 775 participants, 4 trials; comparison 01‐03).
Treatment failure: progression to severe malaria
Dorsey 2002 reported one participant with severe malaria − in the SP plus AQ group (comparison 01‐04).
Gametocyte carriage
Mockenhaupt 2005 reported that fewer participants in the SP plus AS group had gametocytes at day seven (RR 2.31, 95% CI 1.36 to 3.92; 220 participants; comparison 01‐05). Abacassamo 2004 reported a similar trend at day 14, but the difference was not statistically significant (113 participants, comparison 01‐06).
Adverse events
Most trials did not clearly describe the methods for reporting adverse events or the precise numbers of events. Abacassamo 2004 reported "no severe adverse reactions attributable to treatment", Rwagacondo 2003 reported "no major drug related adverse effects", and Dorsey 2002 reported "no severe adverse reactions to trial drugs" and that "mild adverse reactions did not differ between the three treatment groups". Mockenhaupt 2005 did not mention adverse events.
Discussion
Four trials are included in this review. The data come from mainly high malaria transmission areas in Africa, but the level of background resistance to the component drugs of the combinations is not clear. All trials enrolled children aged six to 59 months.
The methodological quality of the included trials could not be conclusively determined as a consequence of poor reporting but is believed to be generally good. Three trials had adequate generation of allocation sequence, but only one had adequate allocation concealment and only two used blinding. None of the trials included all the enrolled participants in the final analysis, although losses to follow up were within acceptable limits and the reasons for exclusion and loss to follow up were always given.
All trials compared SP plus AQ with SP plus AS, and all used the same dose and regimens and in a similar population. This has made comparison of reported outcomes possible.
Reported outcomes were treatment failure, progression to severe malaria, and gametocyte carriage. Three trials mentioned adverse events, but none of them in sufficient detail and none described the procedure to assess them, which create uncertainty over the completeness of these data. Some of the trials reported on symptom resolution (parasite clearance and fever clearance), but this was not reported in a format that could be used in this review and therefore it has not been possible to report them here.
The combination of SP plus AQ resulted in better cure of malaria when assessed at day 28. At day 14 the result was not conclusive and it is not possible to determine which, if any, treatment is better than the other at this point. These observed effects may be because the longer acting amodiaquine still offers protection beyond day 14 compared to artesunate, which is quickly eliminated from the body and whose effect in the combination lasts a short time. If artesunate has disappeared from the patient's system and there is resistance to sulfadoxine‐pyrimethamine then day 28 cure rates are likely to be very low unless the patient has some level of immunity to malaria. SP plus AS was better at controlling gametocytes than SP plus AQ. This result, as may be expected, is because the artesunate component (like the other artemisinin drugs) is effective in destroying gametocytes.
There are presently insufficient data to determine the effects of the two treatments on outcomes such as symptom resolution and adverse events.
Although there was little statistical heterogeneity in results across trials, some caution is still required to generalize from four small trials carried out at different times, in separate parts of Africa, and with different malaria transmission intensities. All these trials took place at a time of rapid spread of sulfadoxine‐pyrimethamine resistance and the emergence of amodiaquine resistance. Resistance patterns may have worsened since the trials were carried out, particularly for the SP plus AQ combination. Resistance to sulfadoxine‐pyrimethamine has spread rapidly across Africa and manifests initially as recrudescence of the same parasite genotype between 14 and 28 days or longer. Combination therapy with sulfadoxine‐pyrimethamine is therefore likely to be a 'stop‐gap' measure until artemisinin‐based combination therapies are widely available, or limited to areas with documented low sulfadoxine‐pyrimethamine resistance. It is possible that sulfadoxine‐pyrimethamine may not be recommended at all for first‐line treatment of any malaria in Africa in the future. The SP plus AQ combination should therefore only be considered where both sulfadoxine‐pyrimethamine and amodiaquine resistance (measured at 28 days) are known to be low. It is particularly important that effective drugs (such as artesunate, with no documented resistance) are protected and not exposed to the possibility of developing resistance by partnering them with ineffective drugs. This also implies that SP plus AS combination therapy should only be considered where both drugs are known to be effective.
Authors' conclusions
Implications for practice.
In these trials, SP plus AQ was better than SP plus AS at controlling treatment failure at day 28, but there are insufficient data to determine performance at day 14. Careful consideration of local resistance patterns is however required, as resistance to both sulfadoxine‐pyrimethamine and amodiaquine are high in many areas and may be worsening. In order to delay development of resistance to artesunate, the SP plus AS combination should only be considered in areas where both drugs are known to be effective. Data on adverse events are still lacking.
Implications for research.
Randomized controlled trials that include outcomes on symptom resolution and adverse effects are needed. These are not only important to help further assess effectiveness and safety but are also of particular interest to people with malaria. Trial methodology needs to be reported more clearly. Research in other locations (such as Central Asia) where sulfadoxine‐pyrimethamine is more effective might be useful.
What's new
| Date | Event | Description |
|---|---|---|
| 4 November 2008 | Amended | Converted to new review format. |
Acknowledgements
This review was developed by Hasifa Bukirwa during a one‐year fellowship organized by the Effective Health Care Alliance Programme at the Liverpool School of Tropical Medicine. The UK Department for International Development (DFID) supports this Programme. This document is an output from a project funded by the DFID for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | sulfadoxine‐pyrimethamine | sulfadoxine‐pyrimethamine | sulfadoxine‐pyrimethamine | sulfadoxine ADJ pyrimethamine | sulfadoxine‐pyrimethamine |
| 2 | fansidar | fansidar | fansidar | fansidar | fansidar |
| 3 | amodiaquine | amodiaquine | amodiaquine | amodiaquine | amodiaquine |
| 4 | artesunate | artesunate | AMODIAQUINE | AMODIAQUINE | artesunate |
| 5 | — | 1 or 2 | ARTESUNATE | ARTESUNATE | 1 or 2 |
| 6 | — | 3 and 5 | artesunate | artesunate | 3 and 5 |
| 7 | — | 4 and 5 | 1 or 2 | 1 or 2 | 4 and 5 |
| 8 | — | — | 3 or 4 | 3 or 4 | 6 and 7 |
| 9 | — | — | 5 or 6 | 5 or 6 | — |
| 10 | — | — | 7 and 8 | 7 and 8 | — |
| 11 | — | — | 7 and 9 | 7 and 9 | — |
| 12 | — | — | 10 and 11 | 10 and 11 | — |
| 13 | — | — | MALARIA | malaria | — |
| 14 | — | — | malaria | MALARIA | — |
| 15 | — | — | 13 or 14 | PLASMODIUM‐FALCIPARUM | — |
| 16 | — | — | 12 and 15 | 13 or 14 or 15 | — |
| 17 | — | — | — | 12 and 16 | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term.
Data and analyses
Comparison 1. Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure by day 28 | 3 | 652 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.42, 0.83] |
| 2 Treatment failure by day 28 (excludes new infections) | 3 | 649 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.40, 0.96] |
| 3 Treatment failure by day 14 | 4 | 775 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.47, 2.78] |
| 4 Treatment failure: progress to severe malaria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Gametocyte carriage at day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Gametocyte carriage at day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
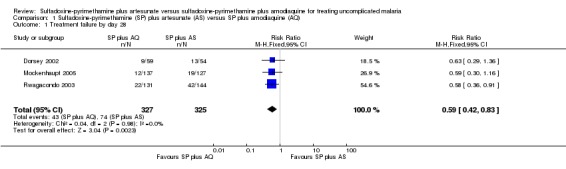
Comparison 1 Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ), Outcome 1 Treatment failure by day 28.
1.2. Analysis.
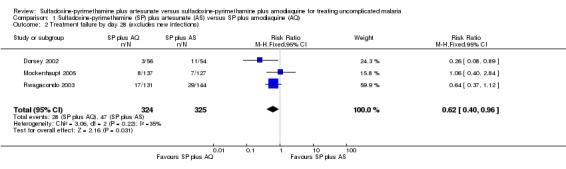
Comparison 1 Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ), Outcome 2 Treatment failure by day 28 (excludes new infections).
1.3. Analysis.
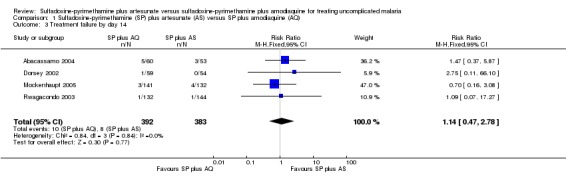
Comparison 1 Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ), Outcome 3 Treatment failure by day 14.
1.4. Analysis.
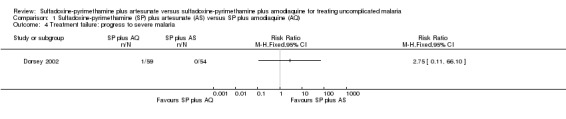
Comparison 1 Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ), Outcome 4 Treatment failure: progress to severe malaria.
1.5. Analysis.
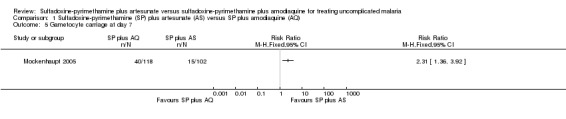
Comparison 1 Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ), Outcome 5 Gametocyte carriage at day 7.
1.6. Analysis.
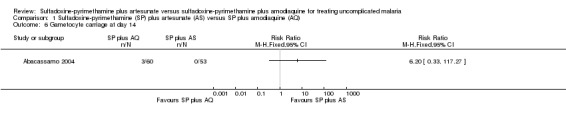
Comparison 1 Sulfadoxine‐pyrimethamine (SP) plus artesunate (AS) versus SP plus amodiaquine (AQ), Outcome 6 Gametocyte carriage at day 14.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abacassamo 2004.
| Methods | Randomized open trial Generation of allocation sequence: random permuted block Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: no (113/124) Length of follow up: 21 days |
|
| Participants | Number: 124 enrolled, 113 analysed Inclusion criteria: children 6 to 59 months living within the study area; axillary temperature 37.5 ºC to 40 ºC; acute non‐complicated Plasmodium falciparum; parasitaemia 2000 to 100,000 asexual parasites/µL of blood; parent or guardian written informed consent Exclusion criteria: danger signs (not able to drink, eat or breastfeed; severe vomiting – > 2 times in 24 hours; unconscious and unable to sit or stand; signs of severe malaria – cerebral malaria defined as inability to localize pain; severe anaemia – haemoglobin < 5 g/dL or a packed cell volume less < 15% or with spontaneous bleeding and repeated generalized convulsions |
|
| Interventions | 1. Sulfadoxine‐pyrimethamine (SP) plus amodiaquine (AQ)
2. SP plus artesunate (AS) AQ: 10 mg/kg/day for 3 days AS: 4 mg/kg/day for 3 days SP: 25 mg/kg sulfadoxine and 1.25 mg/kg pyrimethamine Third arm not relevant to review: AQ plus AS |
|
| Outcomes | 1. Therapeutic response, according World Health Organization (WHO) 1996 definitions (reference in Abacassamo 2004) 2. Presence of fever 3. Gametocyte carriage | |
| Notes | Location: Mozambique Date: February to June 2002 Funding: DBL/INS joint research programme and the WHO/Special Programme for Research and Training in Tropical Diseases (TDR) |
|
Dorsey 2002.
| Methods | Randomized placebo controlled trial Generation of allocation sequence: computer‐generated randomization list Allocation concealment: not mentioned Blinding: participants and study investigators blinded Inclusion of all randomized participants: no (93/122) Length of follow up: 14 days |
|
| Participants | Number: 211 children enrolled Inclusion criteria: children 6 months to 5 years old; no history of treatment for malaria in previous 2 weeks or fever in last 48 h; no history of adverse reactions to any of the study drugs; no history of sickle cell disease; haemoglobin 50 g/L or more; willingness to remain in the city of Kampala and follow the study protocol for the next 12 months; parent or guardian written informed consent |
|
| Interventions | 1. Sulfadoxine‐pyrimethamine (SP) plus amodiaquine (AQ)
2. SP plus artesunate (AS) AQ: 10 mg/kg/day for 2 days and 5 mg/kg for 1 day AS: 4 mg/kg/day for 3 days SP: 25 mg/kg sulfadoxine and 1.25 mg/kg pyrimethamine; single dose Third arm not relevant to review: SP plus vitamin C placebo |
|
| Outcomes | 1. Treatment failure at day 14 and 28; at day 28 both adjusted and unadjusted for new infections 2. Mentions adverse events but did not report any 3. Parasite carriage reported for episodes not patients 3. Presence of fever 4. Mentioned adverse events but did not give details by treatment arm | |
| Notes | Location: Mulago Hospital, Kampala, Uganda Date: July 2000 to August 2001 Follow up was 14 days for each episode of malaria over 1‐year period Funding: Fogarty International Center/National Institutes of Health and the UNDP/World Bank/World Health Organization (WHO) Special Programme for Research and Training in Tropical Diseases (TDR) |
|
Mockenhaupt 2005.
| Methods | Randomized placebo controlled trial Generation of allocation sequence: computer‐generated block randomization Allocation concealment: not mentioned Blinding: participants, not clear whom else Inclusion of all randomized participants: no (264/293) Length of follow up: 28 days |
|
| Participants | Number: 293 enrolled, 273 analysed at day 14, and 264 analysed at day 28 Inclusion criteria: children 6 to 59 months attending Bulpeila Health Centre; mono‐infection with Plasmodium falciparum; parasitaemia 2000 to 100,000 asexual parasites/µL of blood; axillary temperature at least 37.5 ºC; body weight > 5 kg; absence of severe malnutrition; no other causes of febrile illness; no danger signs and no severe and complicated malaria; haemoglobin at least 5 g/dL; and parent or guardian written informed consent Exclusion criteria: known hypersensitivity to study drugs; detection during follow up of mixed malarial infections; and development of concomitant disease which would interfere with classification of treatment outcome |
|
| Interventions | 1. Sulfadoxine‐pyrimethamine (SP) plus amodiaquine (AQ)
2. SP plus artesunate (AS) AQ: 10 mg/kg/day for 3 days plus AS placebo AS: 4 mg/kg/day for 3 days plus AQ placebo SP: 25 mg/kg sulfadoxine and 1.25 mg/kg pyrimethamine; single dose Third arm not relevant to review: SP alone (plus AQ and AS placebos once a day for 3 days) |
|
| Outcomes | 1. Treatment failure according to the World Health Organization (WHO) classification; at day 28 both adjusted and unadjusted for new infections 2. Presence of malaria parasites at various time points up to day 28 3. Presence of fever at various time points up to day 28 4. Gametocyte prevalence at day 7 | |
| Notes | Location: Tamale, Northern Region, Ghana Date: August to December 2002 Funding: World Health Organization; Dafra Pharma (Belgium) and Park‐Davis (Senegal) provided study medication |
|
Rwagacondo 2003.
| Methods | Randomized open trial Generation of allocation sequence: not mentioned Allocation concealment: not mentioned Blinding: not mentioned Inclusion of all randomized participants: no (275/276) Length of follow up: 28 days |
|
| Participants | Number: 276 children enrolled Inclusion criteria: children 6 to 59 months old; fever, temperature at least 37.5 ºC; weight at least 5 kg; parasitaemia 1000 to 100,000 asexual parasites/µL of blood; Plasmodium falciparum pure infection; parent or guardian written informed consent Exclusion criteria: danger signs (not able to drink or breastfeed; vomiting > 2 times in 24 hours; recent history of convulsions; unconscious state or unable to sit or stand; signs of severe malaria; a packed cell volume < 15%; a clear history of adequate malaria treatment in the preceding 72 h; or evidence of chronic disease |
|
| Interventions | 1. Sulfadoxine‐pyrimethamine (SP) plus amodiaquine (AQ)
2. SP plus artesunate (AS) AQ: 10 mg/kg/day for 3 days AS: 4 mg/kg/day for 3 days SP: 25 mg/kg sulfadoxine and 1.25 mg/kg pyrimethamine Third arm not relevant to review: AQ alone |
|
| Outcomes | 1. Treatment failure at day 28 both adjusted and unadjusted for new infections 2. Mentions adverse events but did not report any | |
| Notes | Location: Rwanda in 3 sites of Kicukiro, Rukara and Mashesha health centres Date: May to August 2001 Funding: Belgian Development Co‐operation (DGIS) in collaboration with the Prince Leopold Institute of Tropical Medicine (Antwerp, Belgium) |
|
Contributions of authors
Hasifa Bukirwa extracted and analysed data, and drafted the review. Julia Critchley extracted data and edited the review.
Sources of support
Internal sources
Makerere University Malaria Project, Uganda.
External sources
Department for International Development, UK.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Abacassamo 2004 {published data only}
- Abacassamo F, Enosse S, Aponte JJ, Gomez‐Olive FX, Quinto L, Mabunda S, et al. Efficacy of chloroquine, amodiaquine, sulphadoxine‐pyrimethamine and combination therapy with artesunate in Mozambican children with non‐complicated malaria. Tropical Medicine and International Health 2004;9(2):200‐8. [DOI] [PubMed] [Google Scholar]
Dorsey 2002 {published data only}
- Dorsey G, Njama D, Kamya MR, Cattamanchi A, Kyabayinze D, Staedke SG, et al. Sulfadoxine/pyrimethamine alone or with amodiaquine or artesunate for treatment of uncomplicated malaria: a longitudinal randomised trial. Lancet 2002;360(9350):2031‐8. [DOI] [PubMed] [Google Scholar]
Mockenhaupt 2005 {published data only}
- Mockenhaupt FP, Ehrhardt S, Dzisi SY, Teun Bousema J, Wassilew N, Schreiber J, et al. A randomized, placebo‐controlled, double‐blind trial on sulfadoxine‐pyrimethamine alone or combined with artesunate or amodiaquine in uncomplicated malaria. Tropical Medicine and International Health 2005;10(6):512‐20. [DOI] [PubMed] [Google Scholar]
Rwagacondo 2003 {published data only}
- Rwagacondo CE, Niyitegeka F, Sarushi J, Karema C, Mugisha V, Dujardin JC, et al. Efficacy of amodiaquine alone and combined with sulfadoxine‐pyrimethamine and sulfadoxine‐pyrimethamine combined with artesunate. American Journal of Tropical Medicine and Hygiene 2003;68(6):743‐7. [PubMed] [Google Scholar]
Additional references
Adjuik 2004
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N, International Artemisinin Study Group. Artesunate combinations for treatment of malaria: meta‐analysis. Lancet 2004;363(9402):9‐17. [DOI] [PubMed] [Google Scholar]
Bloland 2003
- Bloland PB. A contrarian view of malaria therapy policy in Africa. American Journal of Tropical Medicine and Hygiene 2003; Vol. 68, issue 2:125‐6. [PubMed]
Brewer 1994
- Brewer GT, Peggins OJ, Grate JS, Petras JM, Levine BS, Weina PJ, et al. Neurotoxicity in animals due to arteether and artemether. Transactions of the Royal Society of Tropical Medicine and Hygiene 1994;88 Suppl 1:33‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 October 2005).
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in healthcare: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kain 1995
- Kain KC. Chemotherapy and prevention of drug‐resistant malaria. Wilderness & Environmental Medicine 1995;6(3):307‐24. [DOI] [PubMed] [Google Scholar]
MacLehose 2003
- MacLehose HG, Klaes D, Garner P. Amodiaquine: A systematic review of adverse events [Draft]. www.who.int/medicines/organization/par/edl/expcom13/expcom03add.shtml 2003.
Olliaro 2004
- Olliaro P, Mussano P. Amodiaquine for treating malaria. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
RBM 2003a
- Roll Back Malaria. Position of WHO's Roll Back Malaria Department on malarai treatment policy. www.emro.who.int/rbm/WHOPositionStatement.pdf (accessed 28 July 2004).
RBM 2003b
- Global Partnership to Roll Back Malaria. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: World Health Organization, 2003. [Google Scholar]
Review Manager 4.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Stepniewska 2004
- Stepniewska K, Taylor WR, Mayxay M, Price R, Smithuis F, Guthmann JP, et al. In vivo assessment of drug efficacy against Plasmodium falciparum malaria: duration of follow‐up. Antimicrobial Agents and Chemotherapy 2004;48(11):4271‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturchler 1993
- Sturchler D, Mittelholzer ML, Kerr L. How frequent are notified severe cutaneous adverse reactions to Fansidar?. Drug Safety 1993;8(2):160‐8. [DOI] [PubMed] [Google Scholar]
Talisuna 2004
- Talisuna AO, Nalunkuma‐Kazibwe A, Bakyaita N, Langi P, Mutabingwa TK, Watkins WW, et al. Efficacy of sulphadoxine‐pyrimethamine alone or combined with amodiaquine or chloroquine for the treatment of uncomplicated falciparum malaria in Ugandan children. Tropical Medicine and International Health 2004;9(2):222‐9. [DOI] [PubMed] [Google Scholar]
Warsame 2002
- Warsame M, Abdillahi A, Nur Duale A, Nur Ismail A, Hassan AM, Mohamed A, et al. Therapeutic efficacy of chloroquine and sulfadoxine/pyrimethamine agaisnt Plasmodium falciparum infection in Somalia. Bulletin of the World Health Organization 2002;80(9):704‐8. [PMC free article] [PubMed] [Google Scholar]
White 1998
- White NJ. Drug resistance in malaria. British Medical Bulletin 1998;54(3):703‐15. [DOI] [PubMed] [Google Scholar]
White 1999
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
WHO 1988
- World Health Organization. Development of recommendations for the protection of short‐stay travellers to malaria endemic areas: Memorandum from two WHO Meetings. Bulletin of the World Health Organization 1988;66(2):177‐96. [PMC free article] [PubMed] [Google Scholar]
WHO 2000a
- WHO Expert Committee on Malaria (1998: Geneva, Switzerland), World Health Organization. WHO Expert Committee on Malaria: twentieth report. Vol. WHO Technical Report Series; 892, Geneva: World Health Organization, 2000. [PubMed] [Google Scholar]
WHO 2000b
- World Health Organization. Management of severe malaria: a practical handbook. 2nd Edition. Geneva: World Health Organization, 2000. [Google Scholar]
WHO 2001
- World Health Organization. Severe malaria in the African region: results of a multicentre study. Malaria Liason Bulletin of the Malaria Programme WHO/AFRO 2001; Vol. 4, issue 2:1‐3.


