Abstract
Background
Rifamycins are an essential component of modern short‐course regimens for treating tuberculosis. Rifabutin has favourable pharmacokinetic and pharmacodynamic properties and is less prone to drug−drug interactions than rifampicin. It could contribute to shortening of therapy or simplify treatment in HIV‐positive people who also need antiretroviral drugs.
Objectives
To compare combination drug regimens containing rifabutin with those containing rifampicin for treating pulmonary tuberculosis
Search methods
We searched Cochrane Infectious Diseases Group Specialized Register (July 2009), CENTRAL (The Cochrane Library 2009, Issue 3), MEDLINE (1966 to July 2009), EMBASE (1974 to July 2009), and LILACS (1982 to July 2009). We also searched the Indian Journal of Tuberculosis (1983 to 2006), conference abstracts, reference lists, and unpublished data on file at Pfizer Inc.
Selection criteria
Randomized and quasi‐randomized trials including participants with sputum smear and/or culture‐confirmed tuberculosis that compared a rifabutin‐containing with an otherwise identical rifampicin‐containing regimen.
Data collection and analysis
Two authors independently assessed study eligibility and methodological quality, and extracted data. Dichotomous data were analysed and combined using relative risks (RR) with 95% confidence intervals (CI) using a fixed‐effect model. Subgroup analyses were carried out according to rifabutin dose.
Main results
Five trials with a total of 924 participants met the inclusion criteria; 5% of participants were HIV positive. Only one small trial was methodologically adequate. The two largest trials (818 participants) had unclear allocation concealment and included < 90% of randomized participants in the analysis. There was no statistically significant difference in between the regimens for cure (RR 1.00, 95% CI 0.96 to 1.04; 553 participants, 2 trials) or relapse (RR 1.23, 95% CI 0.45 to 3.35; 448 participants, 2 trials). The number of adverse events was not significantly different (RR 1.42, 95% CI 0.88 to 2.31; 714 participants, 3 trials), though the RR increased with rifabutin dose: 150 mg (RR 0.98, 95% CI 0.45 to 2.12; 264 participants, 2 trials); and 300 mg (RR 1.78, 95% CI 0.94 to 3.34; 450 participants, 2 trials). However, lack of dose adjustment by weight in the relevant trials complicates interpretation of this relationship.
Authors' conclusions
The replacement of rifampicin by rifabutin for first‐line treatment of tuberculosis is not supported by the current evidence. HIV‐positive people with tuberculosis, the group most likely to benefit from the rifabutin use, are under‐represented in trials to date, and further trials in this group would be useful.
Keywords: Humans; Antibiotics, Antitubercular; Antibiotics, Antitubercular/adverse effects; Antibiotics, Antitubercular/therapeutic use; Randomized Controlled Trials as Topic; Rifabutin; Rifabutin/adverse effects; Rifabutin/therapeutic use; Rifampin; Rifampin/therapeutic use; Tuberculosis, Pulmonary; Tuberculosis, Pulmonary/drug therapy; Uveitis; Uveitis/chemically induced
Rifabutin for treating pulmonary tuberculosis
Among current challenges in tuberculosis treatment are reducing the length of time that drugs must be taken to less than six months and finding ways to safely combine tuberculosis drugs with those used in the treatment of HIV infection. Rifabutin is a drug that has the potential to address these issues if substituted for rifampicin, a mainstay of current treatment. This review identified five trials involving 924 people, but none were of high quality. The review found no significant differences between rifabutin‐ and rifampicin‐containing treatment in curing tuberculosis and preventing relapse, but higher doses of rifabutin might be associated with more adverse effects and there was no evidence that it could shorten treatment. However, very few people with HIV and tuberculosis, who are most likely to benefit from use of rifabutin due to its lack of interaction with antiretroviral drugs, were included in the trials. Better quality clinical trials are needed to understand the place of rifabutin in the treatment of people with tuberculosis, particularly those who also have HIV.
Background
About a third of the world's population is infected with Mycobacterium tuberculosis. Tuberculosis remains one of the biggest killers among infectious diseases, with up to three million people dying from tuberculosis each year (Dye 1999). Diagnosis of tuberculosis generally relies on smear microscopy and culture of the sputum. The disease typically results in progressively destructive lung lesions but may affect almost any part of the body, usually with advanced wasting and death in more than half of cases in the absence of intervention. Despite the availability of increasingly effective treatment since the middle of the twentieth century the global burden of tuberculosis has continued to grow. This is partly because it is the commonest opportunistic infection in HIV‐infected individuals and partly due to the practicalities of organizing complicated and prolonged treatment, with drug resistance often the price of failure (Dye 2000).
The discovery of effective antituberculous agents such as streptomycin, isoniazid, and para‐aminosalicylic acid in the 1940s and early 1950s and their use in combination regimens to prevent drug resistance mutations arising in M. tuberculosis transformed the lives of tuberculosis sufferers. The introduction of the rifamycin class of antibiotics in the 1960s again revolutionized the treatment of tuberculosis and, as a component of a three‐ or four‐drug regimen also including isoniazid and pyrazinamide, it was a crucial factor in reducing the duration of treatment from up to 18 to six months, raising rates of cure at six months to more than 90%, and reducing relapse to less than 5% (Fox 2001). Rifampicin, the first clinically useful rifamycin, has remained a central component of therapy. Its principal action appears to be in the later 'sterilizing' phase of treatment, and its postulated activity against semi‐dormant organisms, which may form a significant component of the pool of persisting bacilli (Dickinson 1981), is probably the explanation for its efficacy (Mitchison 1992). The favourable pharmacokinetic and pharmacodynamic properties of rifamycins have also enabled treatment to be safely given as infrequently as twice a week rather than daily, helping to improve adherence to treatment in difficult situations. However, current regimens still require people to adhere to six months of treatment to reduce relapse rates to an acceptable level and new approaches to treatment clearly need to focus on improving the 'sterilizing' activity of the regimen (Gelband 2000).
New rifamycin derivatives with different properties have been synthesized, the first of which to reach clinical trials was rifabutin, a spiropiperidyl derivative of the parent compound rifamycin S (Marsili 1981). This drug was initially released on a compassionate basis in 1983 for the treatment of disseminated Mycobacterium avium intracellulare infection in patients with AIDS (O'Brien 1987). This initial experience suggested that it had good tolerability and safety, with the most prominent, and unusual, adverse effect being uveitis. At lower doses rifabutin also seemed to offer significant potential advantages over rifampicin for the treatment of M. tuberculosis (Kunin 1996). Several in vitro properties of rifabutin point to enhanced 'sterilizing' activity. Though the ratio of peak plasma concentration in humans to minimum inhibitory concentration (MIC) in the laboratory (Cmax/MIC) for rifabutin (7.5) is lower than that for rifampicin (67), it is less protein‐bound (71% vs 85%), and is more strongly accumulated by cells (ratio of intracellular to extracellular concentrations 9 vs 5). Rifabutin is much more fat soluble than rifampicin resulting in a much larger volume of distribution (9.3 L/kg vs 1 L/kg) and higher tissue/plasma concentration ratios. It also has several theoretical pharmacokinetic advantages that include minimal induction of CYP3A4/5, its principal metabolizing enzyme in the liver, and absorption that is typically unaffected by food (Burman 2001). The in vitro MIC of M. tuberculosis is lower for rifabutin (< 0.06 mg/mL) than for rifampicin (0.15 mg/mL) (Heifets 1988). Furthermore, rifabutin may retain its activity against isolates resistant to rifampicin in 10% to 30% of cases, possibly due to differences in affinity for mycobacterial RNA polymerase or additional inhibition of DNA biosynthesis (Ungheri 1984; Cavusoglu 2004). Studies of pharmacodynamics in a mouse model supported these pharmacokinetic and pharmacodynamic data and suggested that M. tuberculosis infection was eradicated approximately twice as quickly with rifabutin as with rifampicin (Ji 1993).
This evidence from animal models or studies of relevant pharmacokinetic and pharmacodynamic properties in humans suggests that rifabutin may have the capability of accelerating the sterilization phase of treatment that could result in shorter treatment regimens for tuberculosis. Are these potential advantages of rifabutin realized in clinical research? In the treatment of human tuberculosis the drug has been used in three clinical situations: previously untreated disease; multidrug‐resistant disease (Grassi 1996); and in HIV‐associated tuberculosis where drug interactions between non‐nucleoside reverse transcriptase inhibitors and particularly protease inhibitors and rifampicin have been a problem (CDC 2000). This review summarizes and evaluates the existing evidence from clinical trials comparing rifampicin‐ with rifabutin‐containing regimens in these three situations.
Objectives
To compare combination drug regimens containing rifabutin with those containing rifampicin for treating pulmonary tuberculosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials.
Types of participants
People being treated for pulmonary tuberculosis confirmed by sputum smear and/or culture.
Types of interventions
Intervention
Combination antituberculous drug regimens containing rifabutin given daily or two or three times weekly.
Control
Otherwise identical comparator regimen containing rifampicin.
Types of outcome measures
Primary
Cure (single negative M. tuberculosis culture at the completion of six months of therapy).
Secondary
Relapse (single positive M. tuberculosis culture up to two years after the completion of therapy).
Sputum smear and/or M. tuberculosis culture status two months after starting therapy.
Sputum smear and/or M. tuberculosis culture status three months after starting therapy (added to protocol post‐hoc).
Median time to sputum smear and/or M. tuberculosis culture conversion on therapy.
Hazard ratio of sputum smear and/or M. tuberculosis culture conversion on therapy.
Adverse events
Serious adverse events (death, leading to hospitalization or continuation of hospitalization, life threatening, or persistent or significant disability).
Adverse events leading to discontinuation of treatment.
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (July 2009); Cochrane Central Register of Controlled Clinical Trials (CENTRAL), published in The Cochrane Library (2009, Issue 3); MEDLINE (1966 to January 2007); EMBASE (1974 to January 2007); and LILACS (1982 to January 2007).
Conference proceedings
We searched the following conference proceedings for relevant abstracts from meetings held by the following organizations between 2000 and November 2006: International Union against Tuberculosis and Lung Disease; American Thoracic Society; British Thoracic Society; and the International Conference on Antimicrobial Agents and Chemotherapy.
Researchers, organizations, and pharmaceutical companies
We contacted Dr Andrew Vernon at the CDC Tuberculosis Clinical Trials consortium (January 2006), Drs Piero Olliaro and Phillip Onyebujoh at the Special Programme for Research and Training in Tropical Diseases (TDR) (September 2006), Dr Douglas Ross at Pfizer Inc (September 2006), Prof Dennis Mitchison (St George's Hospital Medical School), and Prof Andrew Nunn (MRC Clinical Trials Unit) regarding relevant unpublished or ongoing studies.
Reference lists and handsearching
We checked the reference lists of all the study reports retrieved by the above methods for any unidentified trials. We also handsearched the Indian Journal of Tuberculosis (1983 to July 2006; indexed on MEDLINE post‐2006).
Data collection and analysis
Selection of studies
G Davies (GD) scrutinized the abstracts identified through the searches for potential relevance and retrieved the full‐text versions of relevant abstracts. GD and S Cerri (SC) independently applied the inclusion criteria to the full‐text versions using an eligibility form. Initial agreement was only fair (kappa = 0.25) but disagreements, mostly caused by the use of monotherapy as the intervention in some studies, and definition of study endpoints, were easily resolved after further discussion between the three authors.
Data extraction and management
GD and SC independently extracted data onto data extraction forms; GD then imported the data into Review Manager 4.2. Discrepancies were resolved by further discussion between all three authors. For each outcome, the number of participants randomized and the number analysed in each treatment arm were extracted to allow assessment of loss to follow up. For dichotomous outcomes at fixed time points (sputum smear/culture status at two, three, and six months), we extracted the number of participants experiencing the event and the number assessed in each arm (negative/positive/lost to follow up). Relapse data were also expressed as proportions at the end of follow up since no other measures were made available in the trial reports. Time‐to‐event outcome measures were intended to be summarized only by extracting median times to or hazard ratios for smear or culture negativity where available. For most of the included trials these details were not reported, with only quoted P‐values from Cox modelling or the log‐rank test, and we were unable to obtain either the results of further unpublished analyses or individual patient data from the trial authors.
Assessment of risk of bias in included studies
GD and SC independently assessed the methodological quality of the retrieved trials using a methodological quality form. We classified the generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear (Juni 2001); and described who was blind to the interventions. We classified the inclusion of all randomized participants (proportion of participants included for which an efficacy endpoint was available) as adequate (if > 90%) or inadequate (if ≤ 90%). Disagreements were resolved by discussion between the two authors.
Data synthesis
GD analysed the data using Review Manager 4.2. The primary outcome and most of the secondary outcomes were analysed as a comparison of proportions using risk ratio (RR) as a measure of effect and presented with 95% confidence intervals (CI). Intention‐to‐treat analyses on an available‐case basis were possible and are presented for all of these outcomes. Best‐case and worst‐case analyses were also carried out for the relapse outcomes due to the differing quality of follow up between the two largest included trials; they are referred to only in the text. Heterogeneity amongst the included trials was sought by inspection of the forest plot and by formal testing using both the chi‐squared statistic with a significance level of 5% and the I2 statistic with a threshold of 50% representing a moderate level of heterogeneity. Funnel plots were constructed to look for evidence of publication bias. We combined the data using a fixed‐effect model; we would have used the random‐effects model had there been significant heterogeneity and it was still appropriate to combine trials. We carried out subgroup analyses according to the dose size of rifabutin used in the trials. One of the included trials, Gonzalez 1994, used an allocation ratio of 1:1:1, so for the purposes of the meta‐analysis we split the control arm in this trial into two equal groups, rounding up any non‐integers in the numerator and/or denominator.
Results
Description of studies
Trial selection
We identified eight trials with the search strategy. Five trials and a total of 924 participants were included in the review (see 'Characteristics of included studies'). We excluded three studies: two were monotherapy studies of early bactericidal activity and did not report on outcome measures relevant to the review (Chan 1992; Sirgel 1993); and one was a cohort study of rifabutin therapy in HIV‐positive patients on antiretroviral therapy that did not involve a rifampicin control arm (Burman 2006); see 'Characteristics of excluded studies'.
Trial characteristics
The included trials were conducted between 1992 and 1996. Two trials were moderately large with a total of 818 participants (Gonzalez 1994; McGregor 1996), while the three other trials were smaller with 106 participants in total (HKCS/BMRC 1992; Rowinska 1992; Schwander 1995). Two were conducted in Africa (one in each of Uganda and South Africa), one in Hong Kong, one in Poland, and one was a multicentre trial involving participants in Argentina, Brazil, and Thailand.
Participants
The trials involved diverse groups of participants: Rowinska 1992 involved Polish people with new or chronic disease; HKCS/BMRC 1992 was a paired study of Chinese people with multidrug‐resistant tuberculosis (and was designed to detect response to rifabutin in the presence of established rifampicin‐resistance); Gonzalez 1994 and McGregor 1996 included people with previously untreated tuberculosis in Africa, South‐East Asia, and South America; while Schwander 1995 was restricted to HIV‐positive Ugandan people with previously untreated disease. Overall, 90% of participants in the trials included were believed to be HIV negative, and none of the trials provided antiretroviral therapy.
Interventions
The trials employed regimens with three different doses of rifabutin (150 mg, 300 mg, or 600 mg) and also differed with respect to whether the dose was adjusted according to bodyweight, as summarized in Appendix 2.
Outcome measures
Gonzalez 1994 and McGregor 1996 were the only two trials to report on cure and relapse. Smear conversion only at two months was reported in three trials (HKCS/BMRC 1992; Rowinska 1992; Schwander 1995), and culture conversion at two months was reported only in McGregor 1996. McGregor 1996 and Gonzalez 1994 also reported on culture conversion at three months; since this outcome measure also appeared potentially informative, we included it in the review. Also, three trials carried out some form of time‐to‐event analysis using either smears (Schwander 1995) or cultures (Gonzalez 1994; McGregor 1996), though we could extract relevant time‐to‐event outcomes from only Gonzalez 1994. Adverse events were presented in four of the trials (HKCS/BMRC 1992; Rowinska 1992; Gonzalez 1994; McGregor 1996) as total numbers of adverse events, serious adverse events, and proportion of participants experiencing them during study follow up, rather than as rates. Only two trials, Gonzalez 1994 and McGregor 1996, specified whether adverse events resulted in discontinuation of treatment. The fifth trial, Schwander 1995, did not present data for adverse events, stating only that "no major differences between regimens were detectable"; no further information could be obtained.
Risk of bias in included studies
Generation of allocation sequence
One trial reported an adequate method of randomization (Schwander 1995); the method was unclear in the other trials.
Allocation concealment
Allocation concealment was adequate only in the three smaller trials and used either central randomization (HKCS/BMRC 1992; Rowinska 1992) or sealed envelopes (Schwander 1995). No details concerning allocation concealment were given in Gonzalez 1994 or McGregor 1996.
Blinding
Four of the included trials used blinding for the assessor only. Though Schwander 1995 reported blinding for the investigator and assessor, protection was weak since the drugs were formulated differently and no placebos were used. While the assessment of bacteriological outcomes in the laboratory appeared adequately blinded in all of the trials, this was not true for assessment of adverse event outcomes.
Inclusion of all randomized participants
The proportion of participants included for which an efficacy endpoint was available in the two trials that reported on cure and relapse, Gonzalez 1994 and McGregor 1996, was inadequate (≤ 90%). HKCS/BMRC 1992 was also assessed as inadequate, while Rowinska 1992 and Schwander 1995 both included more than 90% of all randomized participants and were assessed as adequate.
The reports of the two larger trials, Gonzalez 1994 and McGregor 1996, identified bacteriological conversion as the (composite) primary outcome measure. This was not predefined in the other trials, and no trial presented a power calculation.
Effects of interventions
Cure and relapse
Two trials reported these outcome measures (Gonzalez 1994; McGregor 1996). On the basis of available cases, both rifampicin‐ and rifabutin‐containing regimens achieved acceptable cure rates (≥ 95%). Relapse rates reported in McGregor 1996 (8% to 11%) were more than four‐fold higher than those in Gonzalez 1994 (0.8% to 1.8%). However, this heterogeneity was not statistically significant as assessed by the chi‐squared and I2 tests. Funnel plots were not very informative given that there were only two trials, but they did not suggest publication bias.
There was no statistically significant difference in cure (RR 1.00, 95% CI 0.96 to 1.04; 553 participants, 2 trials, Analysis 1.1) or relapse (RR 1.23, 95% CI 0.45 to 3.35; 448 participants, 2 trials, Analysis 1.2) despite there being numerically more relapses for the rifabutin‐based regimens in both trials. These results were unaffected by rifabutin dose (150 mg or 300 mg). Follow up to 24 months was only 38% in McGregor 1996, with 'best‐case' estimates of 3% and 4.1% relapse for the rifampicin‐ and rifabutin‐containing regimens respectively. While Gonzalez 1994 achieved 68% follow up, this included only 75% of the cohort at the time of the trial report with 'best‐case' estimates of 0.6% and 1.2% for the rifampicin‐ and rifabutin‐containing regimens.
Analysis 1.1.
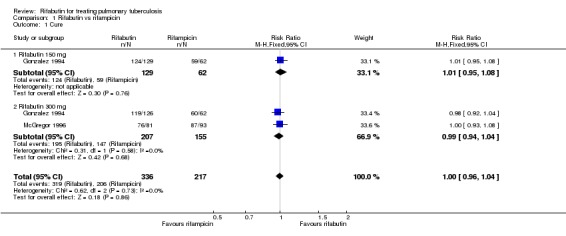
Comparison 1 Rifabutin vs rifampicin, Outcome 1 Cure.
Analysis 1.2.
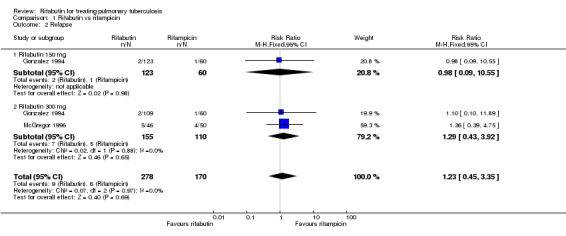
Comparison 1 Rifabutin vs rifampicin, Outcome 2 Relapse.
Smear and culture conversion
All of the included trials reported one or more of these outcome measures. There were no statistically significant differences in sputum culture conversion at two months (214 participants, 1 trial, Analysis 1.3) or at three months (654 participants, 2 trials,Analysis 1.4). Results of survival analysis and median conversion time based on culture were reported only in Gonzalez 1994. Only the outcome of the analysis was reported, and this did not support any statistical differences between the treatment groups (median time to negative culture 34 versus 37 days for the rifampicin‐ and rifabutin‐containing regimens, logrank test P = 0.59). McGregor 1996 reported only the mean time to culture conversion (99 versus 100 days for the rifampicin‐ and rifabutin‐containing regimens), so we could not combine these outcomes.
Analysis 1.3.

Comparison 1 Rifabutin vs rifampicin, Outcome 3 M. tuberculosis culture status 2 months after starting therapy.
Analysis 1.4.
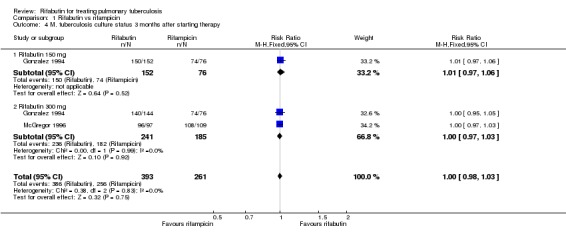
Comparison 1 Rifabutin vs rifampicin, Outcome 4 M. tuberculosis culture status 3 months after starting therapy.
The three small trials relied on outcomes based primarily on sputum smear conversion and used different and incompletely reported measures of effect. Of these, only Schwander 1995 reported the results of a time‐to‐event analysis, which included Cox regression. After an adjustment for radiological cavitation, an apparent advantage for the rifabutin‐containing regimen (P < 0.05) did not reach statistical significance (P = 0.1). Median times to smear conversion for the regimens were not reported.
Adverse events
Four trials reported information on adverse events (HKCS/BMRC 1992; Rowinska 1992; Gonzalez 1994; McGregor 1996). Overall reported proportions of participants experiencing any adverse event varied between trials, from 4% (McGregor 1996) to 70% (HKCS/BMRC 1992); the latter trial used higher doses of rifabutin in the context of different and more toxic companion drugs for multidrug‐resistant tuberculosis and hence was not directly comparable with the other three trials. Even within the three trials evaluating first‐line therapy, the incidence of adverse events varied widely; for example, 19% of participants receiving 300 mg rifabutin in Gonzalez 1994 experienced an adverse event compared to 4% in McGregor 1996, which used the same dose. Furthermore, dose adjustment by weight was not included in the protocol of either of these trials. On an available‐case basis, there was no significant difference in the risk of adverse events between rifampicin‐ and rifabutin‐containing regimens (RR 1.42, 95% CI 0.88 to 2.31; 714 participants, 3 trials, Analysis 1.5, though the RR increased from a dose of 150 mg rifabutin (RR 0.98, 95% CI 0.45 to 2.12; 264 participants, 2 trials, Analysis 1.5) to 300 mg (RR 1.78, 95% CI 0.94 to 3.34; 450 participants, 2 trials, Analysis 1.5). However, in no trial did there appear to be an increased incidence of specific relevant serious adverse events such as leucopoenia or hepatitis (common to all rifamycins) or of uveitis (specific to rifabutin), with no cases of the latter being reported in any of the included trials. In Gonzalez 1994, though it was claimed that adverse events tended to be classified as "severe" more often in the control arm, only 0.5% of controls discontinued whereas 3% of participants in the rifabutin 300 mg arm ultimately discontinued study medication. In McGregor 1996 the proportion discontinuing was 0.01% in both arms.
Analysis 1.5.
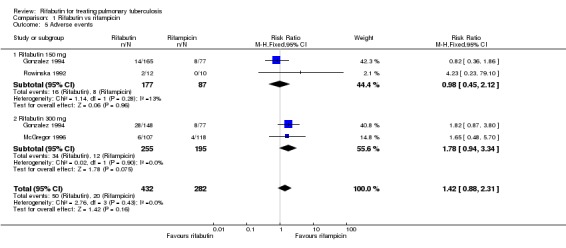
Comparison 1 Rifabutin vs rifampicin, Outcome 5 Adverse events.
Discussion
We identified five trials directly comparing regimens containing rifabutin with rifampicin for treating tuberculosis, all of which date from the period shortly after licensing of the drug. None of these trials was of high methodological quality, they were conducted in diverse patient populations, and the outcome measures chosen by the investigators varied. The included trials comprised less than 1000 participants and were dominated by two moderately sized multicentre trials, conducted on three continents, and in which few HIV‐positive individuals are likely to have been included. We assessed follow up in both of these trials as inadequate.
There was no evidence that rifabutin‐ and rifampicin‐containing regimens differed in terms of efficacy as assessed by sputum culture conversion at two, three, or six months on treatment. The proportion of participants who relapsed after treatment was different in the two major trials reporting this endpoint (8% to 11% in McGregor 1996 and 0.8% to 1.8% in Gonzalez 1994). While treatment was administered daily throughout the trial in Gonzalez 1994, it was given twice weekly in the continuation phase in McGregor 1996. In addition, this finding may reflect the more intense transmission environment in South Africa and the fact that in neither study were relapses distinguished from reinfections using molecular methods. Though relapses occurred more frequently on rifabutin‐containing regimens at either 150 mg or 300 mg, this was not a statistically significant finding, and the overall estimates of absolute relapse rates would be considered acceptable by current standards. In the context of multidrug‐resistant tuberculosis, the only published study did not support a role for rifabutin in the treatment of people harbouring rifampicin‐resistant organisms (HKCS/BMRC 1992). None of the trials stated whether demonstration of equivalence, non‐inferiority, or superiority was the purpose of the primary comparison. None of the secondary outcome measures defined in the review give any substantial support to superiority of rifabutin and were not designed to provide information relating specifically to reducing the duration of treatment. No power calculations were presented and the size of the trials is certainly too small to evaluate superiority. There does not therefore currently appear to be any case for replacing rifampicin with rifabutin in the first‐line regimen on the basis of efficacy alone, though it seems reasonable on the limited evidence available to assume that rifabutin‐containing regimens are likely to be similar in efficacy for practical purposes to those containing rifampicin. However, given that the CI for relapse currently includes a RR as high as three and the poor quality of follow up in the included trials to date, new, higher quality, equivalence trials would be needed to provide further reassurance on this point.
Participants taking rifabutin‐containing regimens were reported to have a similar number of adverse events as those taking rifampicin‐containing regimens in the three trials of first‐line treatment that reported adverse event data. Higher doses of rifabutin (300 mg) were associated with an increasing proportion of participants experiencing any adverse event, but at neither dose level did this proportion differ significantly from the standard dose of rifampicin (600 mg). In the largest trial, discontinuation rates in the rifabutin arm were as high as 3% on the higher dose. Notably, however, few of these adverse events were serious and did not include any of those of particular concern such as leucopoenia, hepatitis, or uveitis. Furthermore, the absence of any dose adjustment by weight in the two largest trials could have increased the frequency of adverse events in the rifampicin and rifabutin 300 mg arms. However, given that the review provides no evidence to prefer the higher dose of rifabutin in terms of efficacy, a case can be made for using lower doses in future trials or at the very least ensuring that adjustment of dose according to weight is used.
This review identified only one trial comprising a small number of HIV‐positive participants who were not receiving antiretroviral therapy (Schwander 1995). Though this trial was methodologically sound it did not report outcome measures that could easily be compared with those of the other trials. HIV‐positive people, who constitute the majority of tuberculosis patients in sub‐Saharan Africa, are therefore currently under‐represented in this review. Future trials in this group will be more complex to conduct than those included in the review to date, since their design must necessarily also include evaluation of the effect of the antiretroviral regimen selected. However, there is a clear need for more information about the use of rifabutin in these people, since it is precisely this group in whom the greatest practical benefits of substituting rifabutin for rifampicin can be envisaged.
Authors' conclusions
Rifabutin‐containing regimens perform as well as rifampicin‐containing regimens in achieving cure and preventing relapse, but higher doses of rifabutin may be associated with more adverse events and discontinuations. There is no evidence currently to support the replacement of rifampicin by rifabutin for the treatment of new cases of tuberculosis on the basis of efficacy. However, HIV‐positive people were under‐represented in the included trials and are the group most likely to benefit from substitution of rifampicin with rifabutin due to its lack of interaction with antiretroviral drugs.
Attempting to establish superiority of rifabutin alone in larger trials may not be a worthwhile goal, but well‐designed and executed equivalence trials with more precise confidence limits would be useful. Further trials evaluating the use of rifabutin and rifampicin in conjunction with antiretroviral therapy for people with HIV‐related tuberculosis must be a priority since no trials have yet evaluated this combined intervention, which has the potential to greatly simplify their care.
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of developing countries.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | tuberculosis | tuberculosis | tuberculosis | tuberculosis | tuberculosis |
| 2 | rifabutin | TUBERCULOSIS | TUBERCULOSIS | TUBERCULOSIS | rifabutin |
| 3 | rifampicin | 1 or 2 | 1 or 2 | 1 or 2 | rifampicin |
| 4 | 2 or 3 | rifabutin | rifabutin | rifabutin | rifampin |
| 5 | 1 and 4 | rifampicin | RIFABUTIN | RIFABUTIN | rifamycins |
| 6 | — | RIFAMYCINS | rifampicin | rifampicin | 2 or 3 or 4 or 5 |
| 7 | — | 4 or 5 or 6 | rifampin | rifampin | 1 and 6 |
| 8 | — | 3 and 7 | RIFAMPIN | RIFAMPICIN | — |
| 9 | — | — | RIFAMYCINS | RIFAMYCINS | — |
| 10 | — | — | 4 or 5 or 6 or 7 or 8 or 9 | 4 or 5 or 6 or 7 or 8 or 9 | — |
| 11 | — | — | 3 and 10 | 3 and 10 | — |
| 12 | — | — | Limit 11 to humans | Limit 11 to human | — |
aCIDG: Cochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Rifabutin dose adjustment according to weight
| Trial | Regimen | Dose |
| HKCS/BMRC 1992 | Matched individualized regimen for multidrug‐resistant disease | 600 or 450 mg of R or Rb according to weight |
| Rowinska 1992 | 9HRE vs 9HRbE | 150 mg Rb daily without dosage adjustment |
| Gonzalez 1994 | 2HRZE/4HR vs 2HRbZE/4HRb | 150 or 300 mg Rb daily without dosage adjustment |
| Schwander 1995 | 2HRZE/4HR vs 2HRbZE/4HRb | 150 or 300 mg Rb daily according to weight |
| McGregor 1996 | 2HRZE/4HR vs 2HRbZE/4HRb | 300 mg Rb daily/twice weekly without dosage adjustment |
E: ethambutol; H: isoniazid; R: rifampicin; Rb: rifabutin; Z: pyrazinamide.
Data and analyses
Comparison 1.
Rifabutin vs rifampicin
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure | 2 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.96, 1.04] |
| 1.1 Rifabutin 150 mg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.08] |
| 1.2 Rifabutin 300 mg | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.94, 1.04] |
| 2 Relapse | 2 | 448 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.45, 3.35] |
| 2.1 Rifabutin 150 mg | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.09, 10.55] |
| 2.2 Rifabutin 300 mg | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.43, 3.92] |
| 3 M. tuberculosis culture status 2 months after starting therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 M. tuberculosis culture status 3 months after starting therapy | 2 | 654 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.98, 1.03] |
| 4.1 Rifabutin 150 mg | 1 | 228 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.97, 1.06] |
| 4.2 Rifabutin 300 mg | 2 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] |
| 5 Adverse events | 3 | 714 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.88, 2.31] |
| 5.1 Rifabutin 150 mg | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.45, 2.12] |
| 5.2 Rifabutin 300 mg | 2 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.94, 3.34] |
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2009 | New search has been performed | New search conducted; no new studies found. |
| 9 November 2008 | Amended | Converted to new review format with minor editing. |
Differences between protocol and review
2007, Issue 4 (first review version): We included three‐month sputum culture conversion results as a secondary outcome measure since it also appeared potentially informative.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: assessor only Inclusion of all randomized participants: 88% (154/175) for rifampicin, 94.8% (165/174) for rifabutin 150 mg, and 86.5% (148/171) for rifabutin 300 mg at 6 months; 70.9% (124/175) for rifampicin, 74.1% (129/174) for rifabutin 150 mg, and 73.7% (126/171) for rifabutin 300 mg |
|
| Participants | Number: 520 enrolled; number screened for entry not reported Inclusion criteria: tuberculosis patients with previously untreated disease; HIV serology negative; Mycobacterial culture positive on 2 separate occasions |
|
| Interventions | 1. Rifabutin: 150 mg daily for 6 months 2. Rifabutin: 300 mg daily for 6 months 3. Rifampicin: 600 mg daily for 6 months Dose was not adjusted for weight Companion drugs: isoniazid (300 mg daily for 6 months); ethambutol (25 mg/kg daily for 2 months); and pyrazinamide (30 mg/kg daily for 2 months) |
|
| Outcomes | 1. "Conversion of sputum bacterial cultures" at weeks 12 and 24 (composite primary outcome measure) 2. Relapse (over 24 months) 3. Time to sputum culture conversion | |
| Notes | Location: multicentre study at 1 site in Argentina, 3 sites in Brazil, and 2 sites in Thailand Supervision: participants hospitalized for the first 2 months Follow up: sputum collected every 2 weeks during the initial phase of therapy, 89% follow up at 6 months, and 68% at 30 months; only 75% complete at the time of the available trial No power calculation was presented for any outcome measure |
|
| Methods | Randomized controlled trial; matched pairs design Generation of allocation sequence: unclear Allocation concealment: central randomization Blinding: assessor only Inclusion of all randomized participants: 64.7% (11/17) in both arms |
|
| Participants | Number: 34 enrolled Inclusion criteria: tuberculosis patients failing first‐line regimen and resistant to rifampicin, isoniazid, and streptomycin on susceptibility testing 34/88 screened were eligible: 18 were susceptible to 1 or more of above drugs; 13 culture negative; 13 died before entry; 6 declined to participate; and 4 were on ofloxacin |
|
| Interventions | 1. Rifabutin: 450 mg if < 50 kg and 600 mg if > 50 kg daily for 12 months 2. Rifampicin 450 mg if < 50 kg and 600 mg if > 50 kg daily for 12 months Companion drugs: 1 to 3 other drugs given daily chosen from: pyrazinamide (1.5/2.0 g); ethambutol (25 mg/kg for 2 months followed by 15 mg/kg thereafter); ethionamide/prothionamide (500 mg); kanamycin (750 mg); capreomycin (750 mg); and para‐aminosalicylic acid (10 g). Those who failed rifampicin could be switched to rifabutin, while those failing rifabutin could be switched to ofloxacin (800 mg daily) |
|
| Outcomes | 1. Cure/failure at 12 months 2. "Temporary response" (ie culture conversion of limited duration) Not included in this review: 3. Smear and culture status at 2 months |
|
| Notes | Location: Hong Kong Supervision: included a mixture of inpatients and outpatients; duration of hospitalization not stated Follow up: sputum collected every 2 weeks for first 2 months then monthly; follow up was 65% at 12 months; 3 participants were culture negative at baseline, and the rest were excluded due to protocol violations Other: "temporary" responses were observed in 18/22 and did not differ between the regimens No power calculation was presented for any outcome measure |
|
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: assessor only Inclusion of all randomized participants: 75.6% (118/156) for rifampicin and 75.4% (107/142) for rifabutin at 6 months; and 32.1% (50/156) for rifampicin and 32.4% (46/142) for rifabutin at 30 months |
|
| Participants | Number enrolled: 298; number screened for entry was not reported Inclusion criteria: tuberculosis patients with previously untreated disease; Mycobacterial culture positive |
|
| Interventions | 1. Rifabutin: 300 mg daily for 2 months then twice weekly for 4 months 2. Rifampicin: 600 mg daily for 2 months then twice weekly for 4 months Dose was not adjusted for weight Companion drugs: isoniazid (400 mg daily for 2 months then 600 mg twice weekly for 4 months); ethambutol (1200 mg daily for 2 months then 2400 mg twice weekly for 4 months); and pyrazinamide (2 g daily for 2 months) |
|
| Outcomes | 1. "Bacteriological conversion at weeks 8, 12 and 24" (composite primary outcome measure) 2. Relapse (over 24 months) 3. Time to sputum culture conversion | |
| Notes | Location: multicentre study at 8 sites in South Africa Supervision: ambulatory treatment from start was permitted, but most patients hospitalized were for 6 months Follow up: sputum collected every 2 weeks during initial phase of therapy; follow up was 75% at 6 months and 30% at 30 months HIV testing not carried out No power calculation was presented for any outcome measure |
|
| Methods | Randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: central randomization Blinding: assessor only Inclusion of all randomized participants: 100% (10/10 and 12/12 in the rifampicin and rifabutin arms respectively) |
|
| Participants | Number: 22 enrolled; number screened was not stated Inclusion criteria: tuberculosis patients with previously untreated disease; mycobacterial culture positive Exclusion criteria included alcoholism and psychological disturbance |
|
| Interventions | 1. Rifabutin: 150 mg daily for 9 months 2. Rifampicin: 600 mg daily for 9 months Companion drugs: isoniazid (300 mg daily for 9 months) and ethambutol (20 mg/kg for 9 months) |
|
| Outcomes | 1. Smear status at 2 months | |
| Notes | Location: multicentre trial at 2 sites in Poland (Warsaw and Lodz) Supervision: participants hospitalized for duration of their treatment Follow up: unclear how often sputum samples were collected Other: there was also an arm for participants who had previously received treatment, but this was uncontrolled HIV testing not carried out No power calculation was presented for the outcome measure |
|
| Methods | Randomized controlled trial Generation of allocation sequence: consecutive drawing of randomly generated treatment orders Allocation concealment: numbered opaque envelopes Blinding: investigator and assessor (though for the former blinding was weak, see notes) Inclusion of all randomized participants: 100% (25/25) and 96% (24/25) in the rifampicin and rifabutin arms |
|
| Participants | Number enrolled: 50 Inclusion criteria: HIV‐positive tuberculosis patients with previously untreated disease; sputum smear positive; suggestive chest x‐ray Exclusion criteria included alcoholism |
|
| Interventions | 1. Rifabutin: 150 mg if < 50 kg and 300 mg if > 50 kg daily for 6 months 2. Rifampicin: 450 mg if < 50 kg and 600 mg if > 50 kg daily for 6 months Companion drugs: isoniazid (300 mg daily for 6 months); pyrazinamide (1500 mg if < 50 kg and 2000 mg if > 50 kg daily for 2 months); and ethambutol (800 mg if < 50 kg and 1200 mg if > 50 kg daily for 2 months) |
|
| Outcomes | 1. Smear status at 2 months 2. Time to sputum smear conversion | |
| Notes | Location: Kampala, Uganda Supervision: ambulatory treatment in majority from an early stage, observed only once or twice a week Follow up: sputum samples collected every 2 weeks during the initial phase of therapy; cultures also done but only at the time of smear conversion Other: only 42/50 participants ultimately had culture confirmation of their positive smear; 7 participants who had no culture confirmation of Mycobacterium tuberculosis complex infection due to contamination were included in the analysis Note on allocation concealment: no placebos were used and the treatments differed in formulation so that both participants and carers could potentially determine allocation No power calculation was presented for any outcome measure |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Burman 2006 | Nonrandomized design with no rifampicin control arm |
| Chan 1992 | Monotherapy study in which no relevant outcome measures were reported |
| Sirgel 1993 | Monotherapy study in which no relevant outcome measures were reported |
Contributions of authors
G Davies formulated the review concept, wrote the protocol, extracted data, carried out the analysis, wrote the first draft, and co‐authored the final draft of the review. S Cerri assisted with protocol development, extracted data, and co‐authored the final draft of the review. L Richeldi assisted with protocol development, arbitrated during data extraction, and co‐authored the final draft of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
The Wellcome Trust, UK.
Declarations of interest
G Davies is a co‐applicant for research funding of a large randomized controlled trial evaluating the use of rifampicin and rifabutin in conjunction with antiretroviral therapy. None known for S Cerri and L Richeldi.
Unchanged
References
References to studies included in this review
- Gonzalez‐Montaner LJ, Natal S, Yongchaiyud P, Olliaro P. Rifabutin for the treatment of newly‐diagnosed pulmonary tuberculosis: a multinational, randomized, comparative study versus Rifampicin. Rifabutin Study Group. Tubercle and Lung Disease 1994;75(5):341‐7. [DOI] [PubMed] [Google Scholar]
- Hong Kong Chest Service/British Medical Research Council. A controlled study of rifabutin and an uncontrolled study of ofloxacin in the retreatment of patients with pulmonary tuberculosis resistant to isoniazid, streptomycin and rifampicin. Hong Kong Chest Service/British Medical Research Council. Tubercle and Lung Disease 1992;73(1):59‐67. [PubMed] [Google Scholar]
- McGregor MM, Olliaro P, Wolmarans L, Mabuza B, Bredell M, Felten MK, et al. Efficacy and safety of rifabutin in the treatment of patients with newly diagnosed pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 1996;154(5):1462‐7. [DOI] [PubMed] [Google Scholar]
- Rowinska‐Zakrzewska E, Slupek A, Graczyk J, Zwolska‐Kwiek Z, Augustynowicz‐Kopec E, Stambrowska A, et al. Preliminary results of rifabutine (ansamycine LM 427) treatment of patients with newly detected and chronic pulmonary tuberculosis and Mycobacterium infections [Wstepne wyniki leczenia ryfabutyna (ansamycyna LM 427) chorych na nowo wykryta i przewlekla gruzlice pluc oraz na mykobakteriozy]. Pneumologia i Alergologia Polska 1992;60(9‐10):81‐8. [PubMed] [Google Scholar]
- Schwander S, Rüsch‐Gerdes S, Mateega A, Lutalo T, Tugume S, Kityo C, et al. A pilot study of antituberculosis combinations comparing rifabutin with rifampicin in the treatment of HIV‐1 associated tuberculosis. Tubercle and Lung Disease 1995;76(3):210‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Burman W, Benator D, Vernon A, Khan A, Jones B, Silva C, et al. Tuberculosis Trials Consortium. Acquired rifamycin resistance with twice‐weekly treatment of HIV‐related tuberculosis. American Journal of Respiratory and Critical Care Medicine 2006;173(3):350‐6. [DOI] [PubMed] [Google Scholar]
- Chan SL, Yew WW, Ma WK, Girling DJ, Aber VR, Felmingham D, et al. The early bactericidal activity of rifabutin measured by sputum viable counts in Hong Kong patients with pulmonary tuberculosis. Tubercle and Lung Disease 1992;73(1):33‐8. [DOI] [PubMed] [Google Scholar]
- Sirgel FA, Botha FJ, Parkin DP, Wal BW, Donald PR, Clark PK, et al. The early bactericidal activity of rifabutin in patients with pulmonary tuberculosis measured by sputum viable counts: a new method of drug assessment. Journal of Antimicrobial Chemotherapy 1993;32(6):867‐75. [DOI] [PubMed] [Google Scholar]
Additional references
- Burman WJ, Gallicano K, Peloquin C. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clinical Pharmacokinetics 2001;40(5):327‐41. [DOI] [PubMed] [Google Scholar]
- Cavusoglu C, Karaca‐Derici Y, Bilgic A. In‐vitro activity of rifabutin against rifampicin‐resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Clinical Microbiology and Infection 2004;10(7):662‐5. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Updated guidelines for the use of rifabutin or rifampin for the treatment and prevention of tuberculosis among HIV‐infected patients taking protease inhibitors or non‐nucleoside reverse transcriptase inhibitors. Morbidity and Mortality Weekly Reports 2000;49(9):185‐9. [PubMed] [Google Scholar]
- Dickinson JM, Mitchison DA. Experimental models to explain the high sterilizing activity of rifampicin in the chemotherapy of tuberculosis. American Review of Respiratory Diseases 1981;123(4 Pt 1):367‐81. [DOI] [PubMed] [Google Scholar]
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 1999;282(7):677‐86. [DOI] [PubMed] [Google Scholar]
- Dye C, Williams BG. Criteria for the control of drug‐resistant tuberculosis. Proceedings of the National Academy of Sciences USA 2000;97(14):8180‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946‐1986, with relevant subsequent publications. International Journal of Tubercle and Lung Disease 2001;3(10 Suppl 2):231‐79. [PubMed] [Google Scholar]
- Gelband H. Regimens of less than six months for treating tuberculosis. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi C, Peona V. Use of rifabutin in the treatment of pulmonary tuberculosis. Clinical Infectious Diseases 1996;22 Suppl 1:50‐4. [DOI] [PubMed] [Google Scholar]
- Heifets LB, Lindholm‐Levy PJ, Iseman MD. Rifabutin: minimal inhibitory and bactericidal concentrations for Mycobacterium tuberculosis. American Review of Respiratory Diseases 1988;137(3):719‐21. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 January 2007).
- Ji B, Truffot‐Pernot C, Lacroix C. Effectiveness of rifampicin, rifabutin and rifapentine for preventive therapy of mouse tuberculosis in mice. American Review of Respiratory Disease 1993;148(6 Pt 1):1541‐6. [DOI] [PubMed] [Google Scholar]
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin CM. Antimicrobial activity of rifabutin. Clinical Infectious Diseases 1996;22 Suppl 1:3‐14. [DOI] [PubMed] [Google Scholar]
- Marsili L, Pasqualucci CR, Vigeyani A, Gioia B, Schioppacassi G, Oronzo G. New rifamycins modified at positions 3 and 4: synthesis, structure and biological evaluation. Journal of Antibiotics (Tokyo) 1981;34(8):1033‐8. [DOI] [PubMed] [Google Scholar]
- Mitchison DA. Understanding the chemotherapy of tuberculosis: current problems. Journal of Antimicrobial Chemotherapy 1992;29(5):477‐93. [DOI] [PubMed] [Google Scholar]
- O'Brien RJ, Lyle MA, Snider DE. Rifabutin (Ansamycin LM 427): A new rifamycin‐S derivative for the treatment of mycobacterial diseases. Reviews of Infectious Diseases 1987;9(3):519‐30. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
- Ungheri D, Della Bruna C, Sanfilippo A. Activity of the spiropiperidyl rifamycin LM 427 on rifampicin resistant Mycobacterium tuberculosis. Giornale Italiano di Chemioterapia 1984;31(3):211‐4. [PubMed] [Google Scholar]


