Abstract
Background
Acute diarrhoea is one of the principal causes of morbidity and mortality among children in low‐income countries. The cornerstone of treatment is oral rehydration therapy and dietary management. However, there is a lack of data and studies on both the timing and type of feeding that should be adopted during the course of the illness.
Objectives
To compare the efficacy and safety of early and late reintroduction of feeding in children with acute diarrhoea.
Search methods
In May 2011, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2011, Issue 1), MEDLINE, EMBASE, LILACS, and mRCT. We also contacted researchers and organizations, and searched reference lists.
Selection criteria
Randomized controlled trials of early versus late refeeding among children less than 10 years old with acute diarrhoea. Early refeeding was defined as within 12 hours of start of rehydration and late refeeding was defined as more than 12 hours after start of rehydration.
Data collection and analysis
Two authors independently assessed the search results and the risk of bias, and extracted data. We present risk ratios for dichotomous outcomes and mean differences for continuous outcomes. We combined the results of the trials using meta‐analysis when heterogeneity was not substantial.
Main results
Twelve trials involving 1283 participants wereincluded; 1226 participants were used in the analysis (724 in the early refeeding group and 502 in the late refeeding group). Nine trials described their allocation sequence, but only two used concealed allocation. One trial reported single‐blinding but did not clearly identify the person who was blinded. Early refeeding meant intake during or immediately after start of rehydration, while late refeeding meant intake only 20 hours to 48 hours after start of rehydration. Significant heterogeneity was noted in the data for the duration of diarrhoea. There was no significant difference between the two refeeding groups in the number of participants who needed unscheduled intravenous fluids (six trials with 813 participants), who experienced episodes of vomiting (five trials with 466 participants), and who developed persistent diarrhoea (four trials with 522 participants). The mean length of hospital stay was also similar (two trials with 246 participants).
Authors' conclusions
There was no evidence that early refeeding increases the risk of unscheduled intravenous fluid use, episodes of vomiting, and development of persistent diarrhoea. No conclusion could be made regarding the duration of diarrhoea.
23 March 2018
No update planned
Research area no longer active
This research area is no longer active.
Keywords: Child; Child, Preschool; Humans; Infant; Eating; Fluid Therapy; Acute Disease; ; Diarrhea; Diarrhea/diet therapy; Diarrhea/therapy; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Reintroducing a normal diet following acute diarrhoea
Many children in developing countries die from acute diarrhoea. Although it is usually caused by infectious viruses or bacteria, the exact organism is rarely known, as it is impractical to test for the organism. Treating the diarrhoea is thus standard therapy, with the recommended policy of using oral rehydration therapy and dietary supplements. Because the gut can be damaged by the infection, many doctors recommend a period of fasting followed by gradual reintroduction of food, although the evidence for when exactly a “normal” diet should be reintroduced is lacking. The authors here looked at children who received ‘early’ refeeding (within 12 hours of the start of rehydration) or ‘late’ refeeding (after 12 hours from the start of rehydration). The authors identified 12 trials that met their inclusion criteria, with a total of 1283 children under 5 years; of these, 1226 were used in the analysis (724 given early refeeding; 502 given late refeeding). There was no significant difference between the two refeeding groups in the number of participants who needed unscheduled intravenous fluids (813 participants, 6 trials), who experienced episodes of vomiting (466 participants, 5 trials), and who developed persistent diarrhoea i.e. greater than 14 days in duration (522 participants, 4 trials). The mean length of hospital stay was also similar (246 participants, 2 trials).There is therefore no evidence to suggest that early refeeding increases the risk of complications after acute diarrhoea such as the need for IV fluids, or increases the risk of developing persistent diarrhoea. Further studies are needed to fully examine other parameters such as duration of diarrhoea, and effect on weight gain.
Background
Acute diarrhoea remains one of the major causes of morbidity and preventable deaths among children, especially in the developing world (WHO/UNICEF 2004). A review of 27 prospective studies from 20 countries published between 1990 and 2000 estimated the incidence of diarrhoea at 3.2 episodes per child per year for children under five years of age (Kosek 2003). Although there was a declining trend, it was still estimated that diarrhoea claimed 1.4 million to 2.5 million lives in 2000 (Kosek 2003). Acute diarrhoea is conventionally defined as increased frequency of defecation (three or more times in 24 hours) (WHO 1995) and faeces that are sufficiently liquid to take the shape of the container in which they are placed (Keusch 2006). Persistent diarrhoea is defined as diarrhoea lasting for 14 days or longer (WHO 1995).
The most common causes of acute diarrhoea in children are infectious agents (viruses, bacteria, and parasites) (Podewils 2004). Most cases of acute diarrhoea in infants and young children have viral causes and are usually short‐lived; antibiotics are not routinely prescribed for viral diarrhoea. Transmission may occur through faecal‐oral routes (transmitted from the stool of one individual to the mouth of another), respiratory secretions, or fomites (inanimate objects such as kitchen utensils) (Keusch 2006). Aetiologic diagnosis of acute diarrhoea is more important epidemiologically and from a public health perspective than for clinical management. Standard diagnostic tests, such as microbiological culture and microscopy, are not cost‐effective or practical for managing individual cases (Keusch 2006). Most cases of acute diarrhoea are managed clinically, especially in developing countries where resources are limited.
Acute diarrhoea may be accompanied by nausea, vomiting, abdominal cramping, clinically significant systemic symptoms or malnutrition. Acute watery diarrhoea is rapidly dehydrating and can be life‐threatening unless fluid therapy is instigated , especially for the very young. Prevention of complications among affected children depends on an accurate assessment of the hydration status and timely instigation of appropriate oral rehydration therapy (ORT), and dietary and zinc supplements (WHO 1995; WHO/UNICEF 2004).
Children used to be starved during and after the diarrhoeic episode for fear of exacerbating the symptoms and worsening the course of the illness (WHO 1999). Although oral hydration therapy has been studied and recommended for the past 30 years, there is a lack of data and studies on the timing and type of feeding that should be adopted during the course of diarrhoea. Early refeeding has been recommended as part of the management of acute diarrhoea (Walker‐Smith 1997). Non‐clinical studies have shown that early refeeding may induce digestive enzymes, improve absorption of nutrients, enhance enterocyte regeneration, and promote recovery of the brush border disaccharidase (Hageman 1977; Hirshhorn 1980; Isolauri 1989; Williamson 1978).
Early studies showed that early refeeding has a significant nutritional advantage, especially among malnourished children (Brown 1988). Some cohort and non‐blinded studies have shown that early refeeding has the potential to reduce stool frequency and volume, and hasten recovery (Chung 1948; Nanulescu 1995; Sarker 1983). Controlled non‐randomized studies assessing the reintroduction of milk formula at different times and concentrations showed no significant difference in the duration of hospitalization (Rees 1979) or the duration of diarrhoea between the different feeding regimens (Santosham 1991; Soeprapto 1979), although vomiting was more frequent among those who had higher concentrations of milk formula (Rees 1979).
A meta‐analysis of 29 randomized clinical trials compared the effects of continuous feeding with lactose‐containing versus lactose‐free diets to young children suffering from acute diarrhoea (Brown 1994). There was a significantly higher treatment failure rate among those receiving lactose‐containing diets: this was noted among patients with initial severe dehydration and in studies conducted before 1985. The authors thus concluded that routine dilution of milk and use of lactose‐free milk formulas are unnecessary for mildly dehydrated cases, especially when ORT and early feeding (in addition to milk) are already part of the routine management. In a controlled clinical trial, a formula containing soy fiber improved the consistency of stools during diarrhoea, with no effect on stool volume (Brown 1993). Adding soy to formula also decreased the duration of antibiotic‐associated diarrhoea among older children (Burks 2001). Continued breastfeeding during diarrhoea was also found to cause a significant decrease in the volume and number of stools (Khin‐Maung‐U 1985). But despite these studies and the quoted clinical recommendations, nutritional management during diarrhoea still varies among health practitioners (Bezerra 1992; Chongban 2005; Santosham 1997).
Since there is still uncertainty and incomplete information on the use of early refeeding in the management of acute diarrhoea in children, this review clarifies and reviewes the clinical evidence to support early refeeding. We define early refeeding as within 12 hours from start of rehydration and define late refeeding as more than12 hours after start of rehydration. The division between early and late refeeding was arbitrary but was guided by the authors' knowledge of the subject. This review only covers children, as the aetiologic distribution, clinical course and approach to management is different for adults. The main outcomeswere clinically‐relevant endpoints such as duration of diarrhoea, total stool output, percentage weight gain at resolution of illness, unscheduled use of intravenous fluids, and episodes of vomiting. These outcomes addressed the common concerns and doubts harbored by most health practitioners, mothers and caregivers with regard to early refeedings.
Objectives
To compare the efficacy and safety of early and late reintroduction of feeding in children with acute diarrhoea.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Children less than 10 years old with acute diarrhoea, including both breastfed and non‐breastfed. Acute diarrhoea was defined as increased frequency of defecation (three or more times in 24 hours) and faeces that are sufficiently liquid to take the shape of the container in which they are placed, with a duration of 14 days or less at the time of presentation.
Types of interventions
Intervention: Early refeeding group
Feeding was reintroduced within 12 hours from start of rehydration; continuous breastfeeding during rehydration was included in this group.
Control: Late refeeding group
Feeding was reintroduced more than 12 hours after start of rehydration.
Types of outcome measures
Primary
Duration of diarrhoea (hours) from admission until cessation of diarrhoea.
Secondary
Total stool output (ml/kg) during the first 24 hours and 48 hours after start of rehydration.
Percentage weight gain 24 hours after start of rehydration and at resolution of diarrhoea.
Unscheduled intravenous (IV) fluid therapy.
Cases of vomiting.
Adverse events
All adverse events, including hyponatraemia (low sodium; serum sodium level ≤130 mmol/L), hypokalaemia (low potassium; serum potassium level ≤3 mol/L) (), and development of persistent diarrhoea.
Search methods for identification of studies
All relevant trials regardless of language or publication status (published, unpublished, in press, and ongoing).
Databases
We searched the following databases using the search terms and strategy described in Table 1: Cochrane Infectious Diseases Group Specialized Register (May 2011); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (2011, Issue 1); MEDLINE (1966 to May 2011; EMBASE (1974 to May 2011; and LILACS (1982 to May 2011). We also searched themetaRegister of Controlled Trials (mRCT) using 'diarrhoea', 'refeeding', 'breastfeeding' and 'feeding' as search terms.
1. Detailed Search Strategies.
| Search Set | CIDG SR | CENTRAL | MEDLINE | EMBASE | LILACS |
| 1 | diarrhoea | diarrhoea | diarrhoea | diarrhoea | diarrhoea |
| 2 | diarrhoea | gastroenteritis | gastroenteritis | gastroenteritis | diarrhoea |
| 3 | gastroenteritis | 1 or 2 | 1 or 2 | 1 or 2 | gastroenteritis |
| 4 | 1 or 2 or 3 | Feed* | Feed* | Feed$ | 1 or 2 or 3 |
| 5 | Feed* | Refeed* | Refeed* | Refeed$ | Feeding |
| 6 | Refeed* | Re‐feed* | Re‐feed* | Re‐feed$ | Refeeding |
| 7 | Re‐feed* | Nutrition* | Nutrition* | Nutrition$ | Re‐feeding |
| 8 | 5 or 6 or 7 | 4 or 5 or 6 or 7 | NUTRITIONAL SUPPORT | DIET RESTRICTION | 5 or 6 or 7 |
| 9 | 4 and 8 | 3 AND 8 | TIME FACTORS | FEEDING BEHAVIOUR | 4 AND 8 |
| 10 | 4‐9/OR | 4‐9/OR | |||
| 11 | 3 AND 10 | 3 AND 10 | |||
| 12 | Limit 12 to Human | Limit 12 to Humans | |||
| 13 | |||||
| 14 | |||||
| 15 | |||||
| Cochrane Infectious Diseases Group Specialized register | Search terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins); Upper case: MeSH or EMTREE heading; Lower Case: free text term | ||||
Researchers and organizations
To help identify unpublished and ongoing trials, we searched (May 2007 to Dec 2009) the web sites of the following organizations: World Health Organization (www.who.int); Child Health and Nutrition Research Initiative (CHNRI) (www.chnri.org); International Clinical Epidemiology Network (INCLEN) (www.inclen.org); USAID (www.usaid.gov); Asian Development Bank (www.adb.org); World Bank (www.worldbank.org); and the International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B) (www.icddrb.org). We also questioned individual researchers working in the field of general paediatrics and gastroenterology, and the members of local and international societies for paediatric gastroenterology, hepatology and nutrition (such as the Philippine Society of Pediatric Gastroenterology and Nutrition and the Asia Pacific Society of Pediatric Gastroenterology, Hepatology and Nutrition), about whether they had conducted trials relating to feeding in acute diarrhoea.
We checked the reference lists of all studies identified by the above methods.
Data collection and analysis
Trial selection
The first two authors ( GV Gregorio and LF Dans) independently assessed the results of the literature search to determine the eligibility of the trials. We then retrieved the full reports of all trials considered by one or both authors to be potentially relevant, as well as trials with unclear treatment allocation. We used a standard eligibility form based on the inclusion criteria to independently assess the trials. We resolved disagreements through discussions or by consulting the third author (MAA Silvestre). If eligibility was unclear due to inadequate data, or if a multiple publication of the same trial was observed, we attempted to contact the trial authors for clarification. We appraised each of the trials to ensure that multiple publication was not an issue. We listed the excluded studies and the reasons for the exclusion.
Assessment of risk of bias
The first two authors ( GV Gregorio and LF Dans) independently assessed the risk of bias of each trial using six components: sequence generation; allocation concealment; blinding; incomplete outcome data; selective outcome reporting; and other biases. Using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions, a judgment of yes, no or unclear to indicate a low, high, or unclear/unknown risk of bias, respectively, was used to describe each component. Although trial participants or care providers might be impossible to blind, we noted if other study personnel were blinded. The percentage of missing outcome data was reported in the risk of bias tables.
We classified the trials as having a high or low risk of bias and included studies with a low risk of bias in a sensitivity analysis. A high risk was defined as trials with unclear sequence generation or allocation concealment, and trials where less than 90% of randomized participants completed the trial. Disagreements over the risk of bias assessment were resolved by a third author. A ‘risk of bias summary’ and ‘risk of bias graph; in addition to the risk of bias tables, were completed. .
Data extraction and management
The first two authors ( GV Gregorio and LF Dans) independently extracted data from the trials using pre‐tested data extraction forms. We extracted the number of participants who were randomized and the number who were analyzed for all outcomes for each treatment arm in each trial to determine loss to follow‐up, whether loss was comparable across treatments, and the type of analysis used. For continuous outcomes, we extracted arithmetic means and standard deviations for each treatment group and noted the number of participants in each group. In trials with multiple interventions (where two or more types of feeding were used as treatment groups), we pooled the means and standard deviations of the different feeding groups across the treatment arms. We used as our unit of analysis the mean duration of diarrhoea in hours and the total stool output as ml/kg.
For data that reported the outcome as a median, we extracted the ranges and presented the data in a separate table.
For dichotomous outcomes, we recorded the number of participants experiencing the event and the number of participants in each treatment group. For trials with multiple treatment arms, we combined the numbers experiencing the outcome in two or more experimental interventions and also combined the total number of participants in the combined treatment arms, whenever appropriate. We then compared collectively with the identified control group.
We resolved any disagreements over data extraction by referring to the trial report and through discussion, or, if that failed, by consulting with another author. Where data were insufficient or missing, we attempted to contact the trial authors. LF Dans entered the data into Review Manager 5.
Data analysis
The first two authors (GV Gregorio and LF Dans) analyzed the data using Review Manager 5 and all results were presented with a 95% confidence interval (CI). We combined trials that compared early versus late feeding using meta‐analysis. We analyzed data using an available case approach (i.e. all patients for whom an outcome was measured and reported are included in the analysis). We aimed to include all the originally randomized patients in the analysis, including protocol‐violators
We compared dichotomous data using risk ratio. The mean difference was used to combine continuous data summarized by arithmetic means and standard deviation.
We checked for features of a normal distribution by calculating the ratio of the mean and standard deviation. If the ratio (mean/SD) was less than two, then it was likely that the data were skewed. We considered the skewed data in the primary analysis but excluded it in the sensitivity analysis.
For continuous outcomes reported in medians and ranges, the results were reported in a table. Similarly, when the outcome was reported using a different unit (e.g. ml/kg/patient or ml/patient rather than ml/kg/day), the results were tabulated.
Some of the data reported by the trials could not be used in the meta‐analysis. One trial reported the duration of diarrhoea as a median instead of a mean (Hoghton 1996; Table 2); another compared the data of those who did not (considered a success) and did (considered a failure) require IV fluids (Shaikh 1991; Table 3).
2. Additional data provided by the studies used in the review.
| Outcome and unit of analysis | Early refeeding | Late refeeding | ||
| Hoghton 1996 | Median (range) of duration of diarrhoea (hours) | 66.5 (11‐192) | 56 (24‐216) | |
| Khin‐Maung‐U 1985 | Mean (SE) of total stool output in the first 24 hours, ml/kg/patient | 89.2 (10) | 115.8 (14.5) | |
| Khin‐Maung‐U 1986 | Mean (SE) of total stool output in the first 24 hours, ml/patient | 1447.5 (214.4) | 870.6 (152.3) |
3. Mean (SD) of stool output at different periods of observation (gm/kg/day).
| Early refeeding | Late refeeding | |||
| Shaikh 1991 | Successfully treated | Clinical failure | Successfully treated | Clinical failure |
| Day 1 | 195 (116) | 300 (111) | 256 (157) | 354 (156) |
| Day 2 | 192 (153) | 436 (102) | 202 (223) | 412 (183) |
| Day 3 | 166 (132) | 420 (109) | 177 (201) | 336 (164) |
Subgroup analysis and investigation of heterogeneity
We evaluated the presence of statistical heterogeneity among the interventions by inspecting the forest plot and by performing a Chi2 test for heterogeneity using a P value of 0.10 to determine statistical significance. Also, we used a I2 value of >50% as an indication of moderate heterogeneity. If there was statistically‐significant heterogeneity, we used the random‐effects model (DerSimonian and Laird method) to combine data, otherwise we applied a fixed‐effect model.
We used subgroup analysis to investigate the effect of age , nutritional status (normal and mild malnutrition versus moderate and severe malnutrition), breastfeeding (breastfed and non‐breastfed infants) and type of food reintroduced (diluted versus full‐strength milk formula, lactose‐free versus lactose‐containing). When there was substantial statistical heterogeneity, we did not combine the trials in the meta‐analysis.
Sensitivity analysis
We planned to conduct a sensitivity analyses to assess the robustness of the meta‐analysis by excluding trials with high risk of bias. The number of studies judged as having a high/low/unclear risk of bias were reported. Trials with skewed data were excluded from the analysis.
Assessment of reporting bias
A funnel plot was constructed to look for evidence of reporting bias
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
We assessed the abstracts of 98 references uncovered in the primary search up until 27 May 2011 and retrieved the full papers for 22 potentially relevant trials. A total of 12 trials met the inclusion criteria (see Characteristics of included studies). Ten trials were excluded: five studies did not satisfy the definition of early or late refeeding used in this review (Armistead 1989; Chew 1993; Fox 1990; Haffejee 1990; Soeprapto 1979); three trials were unclear about when the refeeding started (Haque 1983; Hjelt 1989; Ransome 1984); and two were not randomized controlled trials (Parker 1981; Nanulescu 1995).
No additional or unpublished trials were identified from searching organization web sites or from questioning individual researchers.
Included studies
The 12 included trials enrolled a total of 1283 participants (757 for early refeeding and 526 for late refeeding), all of whom were children. However, only 1226 were used in the final analysis (724 for early refeeding and 502 for late refeeding) as some of the randomized patients were withdrawn or were considered as drop‐outs. All available data up to the time of withdrawal were included. All trial reports were in English. All trials were published between 1979 and 1997, and there were no multiple publications.
Location
The 12 trials were conducted in 16 different countries (see details in Characteristics of included studies). There was one multicenter study involving 11 European countries (Sandhu 1997), including hospitals in the United Kingdom, Italy, Finland, the Netherlands, Croatia, Slovenia, Czechoslovakia, Belgium, Portugal, Poland, and Israel. Two trials each were conducted in the United Kingdom (Conway 1989; Rees 1979); the United States (Santosham 1985; Santosham 1991); Burma (Khin‐Maung‐U 1985, Khin‐Maung‐U 1986); and Israel (Gazala 1988; Rees 1979); and one trial each in Egypt (Santosham 1990), Pakistan (Shaikh 1991), and Peru (Brown 1988).
Ten trials were conducted in a hospital setting, while two studies (Gazala 1988; Santosham 1991) enrolled patients from an out‐patient clinic.
Source of Funding
Three trials were funded by milk companies (Santosham 1985; Santosham 1990; Santosham 1991); two trials were partially supported by the Department of Medical Research in Burma (Khin‐Maung‐U 1985; Khin‐Maung‐U 1986); two trials were supported by a grant from the US Agency for International Development (Brown 1988; Shaikh 1991), but one had additional support from the Diarrheal Disease Control Programme of the World Health Organization and the Nestle Milk Company (Brown 1988). Five trials did not state the source of funding (Conway 1989; Gazala 1988; Hoghton 1996; Rees 1979; Sandhu 1997), including the multicenter study (Sandhu 1997), which was conducted on behalf of the European Society of Paediatric Gastroenterology and Nutrition.
Participants
Diagnosis of diarrhoea
All trials included children with acute diarrhoea of 14 days or less in duration. Six trials included children with diarrhoea of between five and seven days duration (Gazala 1988; Rees 1979; Hoghton 1996; Santosham 1985; Santosham 1990; Santosham 1991). Another four trials included children with diarrhoea of less than 72 hours in duration (Brown 1988; Khin‐Maung‐U 1985; Shaikh 1991).
Age
All trials included children less than five years old. In six trials, the children were less than two years old (Gazala 1988; Khin‐Maung‐U 1985; Sandhu 1997; Santosham 1985; Santosham 1990; Santosham 1991); in two trials, they were between three months and three years old(Brown 1988; Hoghton 1996); in three trials, they were between 1.5 months and four years old(Conway 1989; Rees 1979; Shaikh 1991); and in one trial they were between one and five years old (Khin‐Maung‐U 1986). Only two trials considered the nutritional status of the participants (Brown 1988; Shaikh 1991).
Type and timing of feeding
For those in the early refeeding group, the feeding consisted of either half‐ or full‐strength cow's milk formula (four trials) (Brown 1988; Rees 1979; Santosham 1985; Santosham 1991); boiled rice or the child's usual diet (three trials) (Hoghton 1996; Khin‐Maung‐U 1986; Sandhu 1997); soy‐based milk formula (two trials) (Santosham 1985; Santosham 1991); or breast milk or cow's milk formula (one trial) (Gazala 1988). One trial randomized the patients into either a soy‐ or rice‐based formula or pre‐cooked rice (Santosham 1990). Another trial allocated patients to receive either oral rehydration solution and breastfeeding during the rehydration phase or oral rehydration alone for 24 hours (Khin‐Maung‐U 1986).
For those in the late refeeding group, feeding after start of rehydration was allowed either after 24 hours (seven trials) (Conway 1989; Gazala 1988; Khin‐Maung‐U 1985; Khin‐Maung‐U 1986; Santosham 1990; Santosham 1991; Shaikh 1991); 48 hours (two trials) (Brown 1988; Santosham 1985); 20 hours (one trial) (Sandhu 1997); or between 24 and 48 hours (one trial) (Hoghton 1996). One trial allowed feeding only after the diarrhoea had stopped (Rees 1979).
Duration of follow‐up
The patients were monitored either until resolution of diarrhoea (six trials) (Conway 1989; Hoghton 1996; Khin‐Maung‐U 1985, Khin‐Maung‐U 1986; Santosham 1990; Shaikh 1991); two weeks after hospital discharge (five trials) (Brown 1988; Gazala 1988; Sandhu 1997; Santosham 1985; Santosham 1991); or once full strength milk formula could be tolerated (one trial) (Rees 1979).
Outcomes reported
Most of the trials reported the overall mean duration of diarrhoea from admission to resolution (seven trials) (Conway 1989; Hoghton 1996; Khin‐Maung‐U 1985; Khin‐Maung‐U 1986; Santosham 1990; Shaikh 1991) and the number who required unscheduled use of IV fluids (six trials) (Conway 1989; Hoghton 1996; Santosham 1985; Santosham 1990; Santosham 1991; Shaikh 1991). A few trials also reported the total stool output in the first 24 hours (three trials) (Brown 1988; Santosham 1985; Santosham 1991); oral intake in the form of ORS, formula or rice between 24 and 48 hours (six trials) (Khin‐Maung‐U 1985; Khin‐Maung‐U 1986; Santosham 1985; Santosham 1990; Santosham 1991; Shaikh 1991); mean percentage weight gain at the 24th hour after start of rehydration (three trials) (Santosham 1985; Santosham 1991; Shaikh 1991) and at the resolution of diarrhoea (three trials) (Santosham 1985; Santosham 1990; Santosham 1991); the number of participants with vomiting (four trials) (Conway 1989; Hoghton 1996; Rees 1979; Santosham 1985; ); the development of persistent diarrhoea (four trials) (Conway 1989; Santosham 1985; Santosham 1990; Santosham 1991); and the length of hospital stay (two trials) (Conway 1989; Rees 1979; ). Three trials monitored patients for development of hyponatraemia or hypokalaemia.
Some of the data reported by the trials could not be used in the meta‐analysis. For the duration of diarrhoea, one trial reported it as a median instead of a mean (Hoghton 1996; Table 2). For the mean total stool output in the first 24 hours, two trials reported it as either ml/kg/patient (Khin‐Maung‐U 1985) or ml/patient (Khin‐Maung‐U 1986) rather than ml/kg. Another compared data for the mean stool output of those who did (considered a failure) and did not (considered a success) require IV fluids (Table 3; Shaikh 1991).
Risk of bias in included studies
No trial reported appropriate procedures for all the components used to assess risk of bias (allocation concealment, generation of the allocation sequence, blinding of either the care providers, participants or outcome assessors, inclusion of all randomized participants, selective outcome reporting and other biases). No trial was identified as having selective outcome reporting or other biases.
Allocation
Nine trials had adequate allocation sequence, with either the use of random‐number tables (eight trials) or coin toss (one trial) (Gazala 1988). Three trials were randomized but did not describe the allocation method (Conway 1989; Hoghton 1996; Rees 1979).
Of the 12 included trials, ten had unclear allocation concealment. One trial used sealed envelopes (Brown 1988), while another assigned groups to treatment allocations by flipping a coin (Gazala 1988).
Blinding
Only one trial reported single‐blinding, but it is unclear who was blinded (Hoghton 1996). The participants of all other trials were not blinded and it is unclear if the caregivers or outcome assessors were blinded.
Incomplete outcome data
The number of participants followed up was complete for five trials (Conway 1989; Khin‐Maung‐U 1985; Khin‐Maung‐U 1986; Rees 1979; Shaikh 1991); adequate (90% to 99%)for at least one outcome in five trials (Brown 1988; Hoghton 1996; Sandhu 1997; Santosham 1985; Santosham 1991); and 89% for two trials (Gazala 1988; Santosham 1990).
Effects of interventions
Duration of diarrhoea (hours) from admission until cessation of diarrhoea
A shorter duration of diarrhoea was observed with early refeeding in two trials (Santosham 1985; Santosham 1991) and with late refeeding in one trial (Khin‐Maung‐U 1986), while for four trials the outcome was similar in both groups (Conway 1989; Gazala 1988; Khin‐Maung‐U 1985; Santosham 1990). Overall, the late refeeding group showed longer duration compared with the early refeeding group, although the mean difference was not significant (MD ‐6.90 hrs, 95% CI ‐18.70 to 4.91; 685 participants, seven trials, Analysis 1.1). Considerable heterogeneity among the limited number of trials was observed (Chi2 test, P=0.11, I2 = 82%). There were only two trials where the data were not skewed (Santosham 1990; Santosham 1991), but similar results were seen (Chi² test, P = 0.04; I2 = 77%). Subgroup analysis could not be done because of the limited number of trials.
1.1. Analysis.
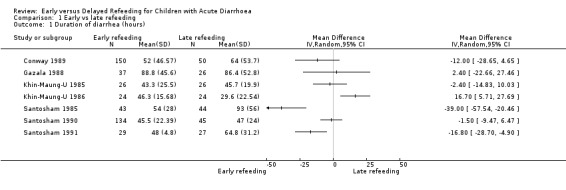
Comparison 1 Early vs late refeeding, Outcome 1 Duration of diarrhea (hours).
Total stool output (ml/kg) during the first 24 and 48 hours after start of rehydration
Three trials each reported the total stool output in the first 24 hours (Brown 1988; Santosham 1985; Santosham 1990) and 48 hours (Khin‐Maung‐U 1985; Santosham 1985; Santosham 1990) after start of rehydration. One trial favoured early refeeding (Santosham 1985) and another favoured late refeeding at both periods of observation (Santosham 1990). Less stool output was also shown on the 24th hour and the 48th hour with early and late refeeding, respectively (Khin‐Maung‐U 1985). All but one study (Khin‐Maung‐U 1985) showed skewed results.
We used an I2 value of >50% as an indication of moderate heterogeneity. Overall, the comparison of the mean total stool in the first 24 hours and 48 hours (Analysis 1.2 and Analysis 1.3) after start of rehydration showed significant heterogeneity: I2 of 85% and 87%, respectively.
1.2. Analysis.
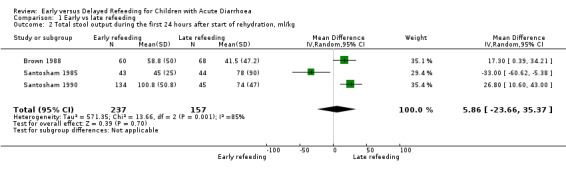
Comparison 1 Early vs late refeeding, Outcome 2 Total stool output during the first 24 hours after start of rehydration, ml/kg.
1.3. Analysis.
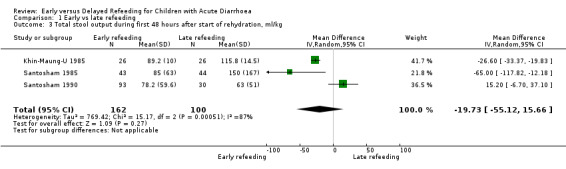
Comparison 1 Early vs late refeeding, Outcome 3 Total stool output during first 48 hours after start of rehydration, ml/kg.
Percentage weight gain at the 24th hour after start of rehydration and at resolution of diarrhoea
No difference was observed in the mean percentage weight gain at the 24th hour after start of rehydration (Analysis 1.4) and at resolution of illness (Analysis 1.5) . Skewed data were observed for the results of the mean percentage weight gain at the 24th hour (Santosham 1985; Santosham 1991; Shaikh 1991) and at cessation of diarrhoea (Santosham 1985; Santosham 1990; Santosham 1991; Shaikh 1991).
1.4. Analysis.
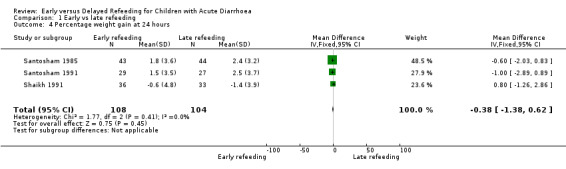
Comparison 1 Early vs late refeeding, Outcome 4 Percentage weight gain at 24 hours.
1.5. Analysis.
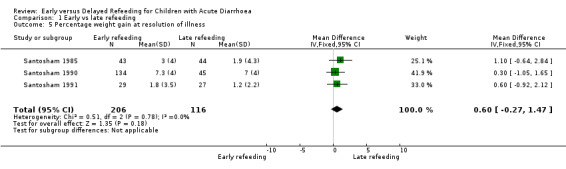
Comparison 1 Early vs late refeeding, Outcome 5 Percentage weight gain at resolution of illness.
Unscheduled intravenous fluid therapy
There was no significant difference in both groups in the number of participants who needed IV fluids (RR 0.87, 95% CI 0.48 to ‐1.59; 813 participants, six trials, Analysis 1.6, Figure 1).
1.6. Analysis.
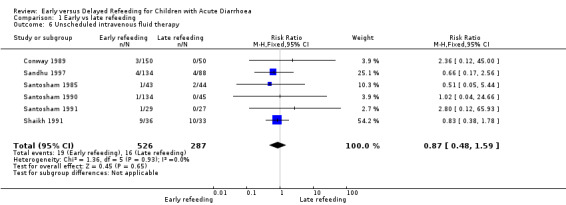
Comparison 1 Early vs late refeeding, Outcome 6 Unscheduled intravenous fluid therapy.
1.
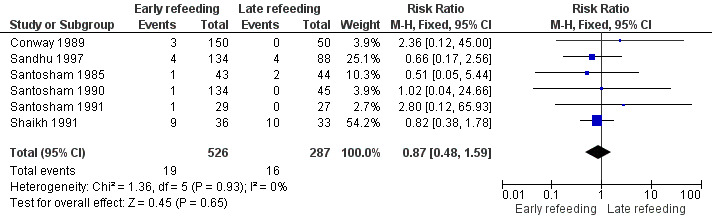
Figure 1. Forest plot of early versus late refeeding in the outcome of unscheduled use of intravenous fluids
Cases of vomiting
There was no significant difference between the two groups in the number of patients with episodes of vomiting (RR 1.16, 95% CI 0.72 to 1.86; 456 participants, five trials, Analysis 1.7).
1.7. Analysis.
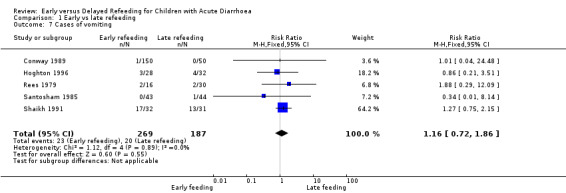
Comparison 1 Early vs late refeeding, Outcome 7 Cases of vomiting.
Adverse events: development of persistent diarrhoea
There was no significant difference in the number of patients who developed persistent diarrhoea (RR 0.57, 95% CI 0.18 to 1.85; 522 participants, four trials, Analysis 1.8).
1.8. Analysis.
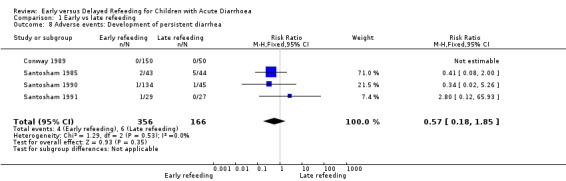
Comparison 1 Early vs late refeeding, Outcome 8 Adverse events: Development of persistent diarrhea.
Adverse events: development of hyponatraemia
Three trials monitored sodium and potassium concentrations on admission and at different intervals during the illness (Brown 1988; Santosham 1985; Santosham 1990). No patient was reported to have developed hypokalaemia. Hyponatraemia (Analysis 1.9) was reported in four patients: two in the early refeeding group (Santosham 1985) and one in the late refeeding group (Santosham 1990), while another trial (Brown 1988) reported one patient who developed hyponatraemia but did not specify to which group they belonged.
1.9. Analysis.
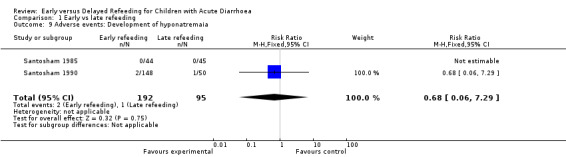
Comparison 1 Early vs late refeeding, Outcome 9 Adverse events: Development of hyponatremaia.
Publication bias
We observed significant heterogeneity in the primary outcomes among the limited trials and therefore we decided to use a funnel plot for the secondary outcome, where the data were homogenous. We constructed a funnel plot of six trials to compare early and late refeeding and measure the outcome of unscheduled use of IV fluid (Figure 2). The funnel plot is symmetrical but the number of studies is limited and so we cannot conclude whether the results are free from publication biases for unscheduled IV fluids.
2.
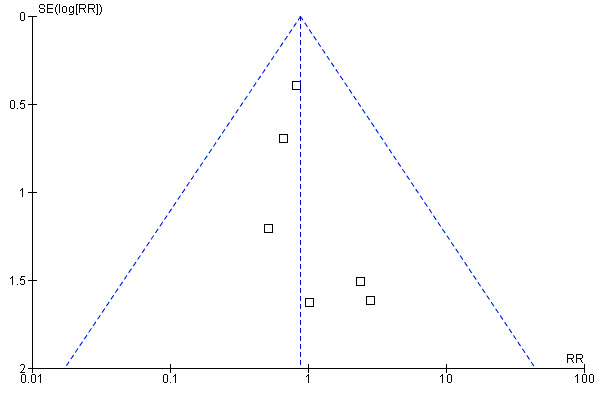
Figure 2. Funnel plot of early vs late refeeding on the number of unscheduled use of intravenous fluids
Discussion
The present meta‐analysis did not provide evidence that early refeeding increases unscheduled use of IV fluids, episodes of vomiting, and development of persistent diarrhoea. The results support existing practice of early refeeding during or after start of rehydration of patients.
Up to the present time, some physicians still recommend variable periods of fasting during acute diarrhoea to allow 'bowel rest' followed by gradual reintroduction of food. The proponents of this practice contend that early refeeding may increase the stool output and lead to more complications, such as unscheduled use of IV fluids, episodes of vomiting, and persistent diarrhoea.
Our results suggest that the number of patients who develop these complications are similar whether early or late refeeding is practiced. However, early refeeding is advocated in order to counteract the transient malabsorption of nutrients that can occur during an episode of acute diarrhoea. This may have a negative influence on growth and contribute to malnutrition, which may, in turn, predispose to persistent diarrhoea. It is for this reason that relevant outcomes in a study of this nature should include the duration of diarrhoeal disease, total stool output, and percentage weight gain at resolution of illness. However, these important outcomes could not be assessed in the present study because of skewed data or heterogeneity among the limited number of trials that reported this information. Heterogeneity in the treatment effect may have been influenced by the way the outcomes were measured (methodological diversity). It was unclear in most of the trials whether the duration of diarrhoea was measured from the initial onset of the disease, before admission to the study, or only from admission up to the time of discharge. Ideally, measurement of stool output should be made by taking the difference in the weight of the diaper before and after use. In some studies where both males and females were included, the urine output may have been inadvertently mixed with the stool, giving an erroneously high stool output. The difference in the type of milk (cow‐ or soy‐based milk) or food that was used to re‐feed the patient might also have contributed to the heterogeneity of the different trials.
Since most of the studies were conducted more than 20 years ago, reporting of the methodology of the trials was incomplete. In the majority of the studies, it's unclear how the random allocation of patients to groups was concealed. It was also unclear if blinding was observed, although this was difficult to implement because of the nature of the interventions. Overall, therefore, the quality of these studies was relatively difficult to assess because of incomplete reporting.
Whilst previous clinical practice guidelines imply that early refeeding is acceptable (Bezerra 1992; Chongban 2005; MMWR 2003; Murphy 1998; WHO/UNICEF 2004), this is the first systematic review conducted on this topic to synthesize the available evidence.
Authors' conclusions
Implications for practice.
The results of this systematic review summarize the available evidence on the timing of feeding during cases of acute diarrhoea in children. It reveals that there is little additional risk of unscheduled use of IV fluids, persistent diarrhoea, vomiting or longer hospital stays for children who were re‐fed early.
Implications for research.
Further studies are needed into whether the timing of refeeding has any effect on the duration of diarrhoea, the total stool output, and weight gain in childhood acute diarrhoea.
History
Protocol first published: Issue 3, 2008 Review first published: Issue 7, 2011
| Date | Event | Description |
|---|---|---|
| 1 April 2008 | New citation required and major changes | Substantive amendment |
Acknowledgements
This document is an output of a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Data and analyses
Comparison 1. Early vs late refeeding.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration of diarrhea (hours) | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Total stool output during the first 24 hours after start of rehydration, ml/kg | 3 | 394 | Mean Difference (IV, Random, 95% CI) | 5.86 [‐23.66, 35.37] |
| 3 Total stool output during first 48 hours after start of rehydration, ml/kg | 3 | 262 | Mean Difference (IV, Random, 95% CI) | ‐19.73 [‐55.12, 15.66] |
| 4 Percentage weight gain at 24 hours | 3 | 212 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.38, 0.62] |
| 5 Percentage weight gain at resolution of illness | 3 | 322 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐0.27, 1.47] |
| 6 Unscheduled intravenous fluid therapy | 6 | 813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.48, 1.59] |
| 7 Cases of vomiting | 5 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.72, 1.86] |
| 8 Adverse events: Development of persistent diarrhea | 4 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.18, 1.85] |
| 9 Adverse events: Development of hyponatremaia | 2 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.06, 7.29] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 1988.
| Methods | Randomized controlled trial | |
| Participants | Number enrolled: 138 participants (128 were analyzed) Inclusion criteria: 3 to 36 months, male, non‐malnourished, diarrhoea for less than 60 hours Exclusion criteria: female, received more than one dose of antibiotics for the diarrhoea, more than one episode of breastfeeding per day, diarrhoea within the last 3 weeks, weight for length <2 SD, presence of edema or serum albumin <2.5 g/dL |
|
| Interventions | 1. Formula diet composed of casein, sucrose, dextrin with maltose (Dextri‐Maltose), and vegetable oil to provide 110 kcal/kg body weight/d (CSO‐110): 34 participants 2. CSO to provide 55 kcal/kg/d (CSO‐55) for 2 days and then CSO‐140: 29 participants 3. Oral glucose‐electrolyte solution (GES) for 2 days, CSO‐55 for the next 2 days, and then CSO‐110: 34 participants 4. Intravenous GES was used for the first 2 days, CSO‐55 for the next 2 days, and then CSO‐110 : 34 participants |
|
| Outcomes | Duration of diarrhoea; apparent absorption of macronutrients and retention of nitrogen; changes in anthropometric indicators of nutritional status monitored at intervals during and after illness. | |
| Setting | Hospital based trial Location: Lima, Peru |
|
| Notes | Interventions 1 and 2 were considered early refeeding while 3 and 4 were late refeeding groups The mean duration of the diarrhoea in each group was divided among the treatment success and treatment failures Two patients had prolonged severe diarrhoea on the 8th hospital day but their group assignments were unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to one of four dietary groups by means of a block randomization procedure |
| Allocation concealment (selection bias) | Low risk | In each sub‐stratum, 12 envelopes were filled randomly (three envelopes each) with the numbers of the four dietary groups and sealed. Once all envelopes of a substratum were exhausted, 12 new envelopes were prepared. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who were randomized were included in the final analysis |
| Selective reporting (reporting bias) | High risk | Two patients with severe diarrhoea were excluded |
| Other bias | Unclear risk | No details given in trial report |
Conway 1989.
| Methods | Randomized controlled trial | |
| Participants | Number: 200 enrolled Inclusion criteria: 6 weeks to 12 months, fed on formula feeds, with acute onset of watery or extremely loose stools for less than 14 days, no systemic illness Exclusion criteria: None |
|
| Interventions | 1. Oral rehydration solution (Dextrolyte, Cow and Gate) for 24 hours, followed by 24 hours of half strength and 24 hours of three‐quarter strength SMA Gold Cap (Wyeth) before continued feeding with the full strength formula milk: 50 participants 2. HN25 formula (Milupa) for two days after the stools returned to normal followed, on successive days, by replacement of one, three, and then all HN25 feeds by full strength SMA Gold Cap: 50 participants 3. Continued feeding with full strength SMA Gold Cap from the time of admission: 50 participants 4. Continued feeding with Formula S (Cow and Gate) from the time of admission. 50 participants |
|
| Outcomes | Duration of diarrhoea after admission, percentage weight change noted on days 2 and 5 and on discharge | |
| Setting | Hospital based trial Location: Seacroft Hospital admissions, Leeds, UK |
|
| Notes | Data from Group 2, 3 and 4 were combined in the early refeeding group Data on weight change was only presented in a graph format Only one patient in Group 2 had persistent diarrhoea but recorded only until day 10 One patient in group 1 had continuous vomiting but it was not clear how long it lasted Location: Seacroft Hospital admissions in Leeds |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given in trial report |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who were randomized were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Gazala 1988.
| Methods | Randomized controlled trial | |
| Participants | Number: 90 enrolled Inclusion criteria: 1 to 12 months, acute (<4 days duration) watery (at least 4 watery stools per day) diarrhoea, mild dehydration |
|
| Interventions | 1. Refeeding was started after 6 hours of oral rehydration with ORS 2. Refeeding was started after 24 hours of rehydration. |
|
| Outcomes | Percentage weight gain, duration of diarrhoea, number of infants admitted to the hospital | |
| Setting | Private out‐patient trial Location: Primary care clinic in Rahat, Israel |
|
| Notes | Clinical features were assessed at 24 hours and 2 weeks following the initial visit We assumed that the reported number of infants admitted to the hospital are interval numbers between the 2 follow‐up evaluations Thirty percent of the infants were lost to follow‐up during the 2‐week period Percentage weight change was reported but not its SD |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study started on Sunday and infants were randomly assigned, starting on the last day of the week (Friday), to either group by flipping a coin, then alternated everyday. The daily change was to minimize mothers belonging to one group from influencing other mothers in a different group. |
| Allocation concealment (selection bias) | High risk | Those assigning will be able to decipher the next treatment allocation for the subsequent days after the initial flipping of the coin |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and caregivers were not blinded, but it was not mentioned if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 16 out of 53 and 11 out of 37 in the early and late refeeding group, respectively, were not reported in the assessment of outcome 2 weeks following initial visit |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | High risk | Thirty percent of patients lost to follow up |
Hoghton 1996.
| Methods | Single‐blind randomized controlled trial | |
| Participants | Number: 62 enrolled Inclusion criteria: children <3 years of age who had acute gastroenteritis of less than 7 days duration, with liquid stools and increased frequency of defecation but with no other associated illness Exclusion criteria: presence of severe vomiting or in those with >5% dehydration |
|
| Interventions | 1. Oral rehydration therapy (ORT) with glucose electrolyte solution alone for 24 to 48 hours without food: 33 participants 2. ORT with a modified diet: 29 participants |
|
| Outcomes | Duration of diarrhoea (reported as median, range); percentage weight gain; consistency of stool output; vomiting episodes; incidence of lactose intolerance | |
| Setting | Hospital based trial Location: Casualty department of Bristol Children's Hospital, Bristol, UK |
|
| Notes | Who was blinded was unclear. There were 62 infants recruited in the study: Group 1 had 33 participants while Group 2 had 29. One in each group was withdrawn by their parents. For the outcome of vomiting, we considered 32 participants for Group 1 and 28 participants for Group 2. We included a participant supposedly withdrawn from Group 2 for severe vomiting who had to be hospitalized. Duration of diarrhoea and percentage change in weight gain were reported as median and therefore were not included in the meta‐analysis. Length of follow up was 5 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given in trial report |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The study indicated that the patients were randomly allocated into two groups in a single‐blinded fashion, although it was unclear who was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27 out of 29 and 32 out of 33 patients in the early and late refeeding group, respectively, were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Khin‐Maung‐U 1985.
| Methods | Randomized controlled trial | |
| Participants | Number: 52 enrolled Inclusion criteria: 6 to 24 months, with acute watery diarrhoea <48 hours duration, with moderate or severe dehydration, breastfed Exclusion criteria: presence of systemic illness, clinically evident malnutrition, bottle fed and children who had received antibiotics |
|
| Interventions | 1. Oral rehydration solution alone: 26 participants 2. Breast feeding plus oral rehydration solution: 26 participants |
|
| Outcomes | Total input (oral and intravenous) and total output (stool, urine, and vomitus) every hour and body weights | |
| Setting | Hospital based trial Location: Pediatric wards in Infectious Disease Hospital in Rangoon, Burma |
|
| Notes | We converted the SE to SD for the duration of diarrhoea. Follow‐up period was only for 48 hours and all the participants had resolution of diarrhoea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each child entered into the study was allocated by random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding is not possible if patients were monitored every hour |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who were randomized were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Khin‐Maung‐U 1986.
| Methods | Randomized controlled trial | |
| Participants | Number: 48 enrolled Inclusion criteria: 2 to 5 years with watery diarrhoea <48 hours duration, with moderate to severe dehydration Exclusion criteria: presence of systemic illness, clinically evident malnutrition, children who had received antibiotics |
|
| Interventions | 1. Oral rehydration solution (ORS) alone during the first 24 hours of admission: 24 participants 2. ORS with boiled rice feeding: 24 participants |
|
| Outcomes | Duration of diarrhoea (hrs), total stool output and volume of vomitus (ml/patient) | |
| Setting | Hospital based trial Location: Pediatric wards in Infectious Disease Hospital in Rangoon, Burma |
|
| Notes | We converted SE to SD for the duration of diarrhoea Follow‐up period was only for 48 hours and all the participants had resolution of diarrhoea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each child allocated by random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who were randomized were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Rees 1979.
| Methods | Randomized controlled trial | |
| Participants | Number: 46 enrolled Inclusion criteria: children between 6 weeks to 4 years, with diarrhoea with or without vomiting of less than 5 days duration, <5% dehydrated; gastroenteritis was the only disease |
|
| Interventions | 1. Full‐strength milk: 16 participants 2. 0.18% sodium chloride and 4% dextrose in water (clear fluids) until the diarrhoea settled, when full‐strength milk was reintroduced: 16 participants 3. Clear fluids until the diarrhoea settled when milk was reintroduced in increasing concentrations, by a quarter strength every 8 hours until full strength was reached, unless the diarrhoea recurred: 14 participants |
|
| Outcomes | Daily records of weight, stool and vomitus; length of hospital stay | |
| Setting | Hospital based trial Location: Primary care clinic in Rahat, Israel |
|
| Notes | The control group was the combined 2nd and 3rd group, which had delayed refeeding of full‐strength milk and increasing concentrations of milk No vomiting in any group after day 3 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given in trial report |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients who were randomized were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Sandhu 1997.
| Methods | Multicenter randomized controlled trial in twelve European hospitals | |
| Participants | Number: 230 enrolled Inclusion criteria: <3 years old, weaned children, with acute diarrhoea (≥4 watery stools per day for >1 but <5 days) Exclusion criteria: previous intake of oral rehydration solution or on intravenous fluid; previous treatment with antidiarrhoeal drugs; children with short gut syndrome, chronic inflammatory bowel disease, ileus, associated hepatic or renal disease |
|
| Interventions | 1. Reguar diet: 134 participants 2. Oral rehydration solution (ORS) for 20 hours and then fed with child's regular diet: 96 participants If a child was breastfed, breast‐feeding was to continue throughout, and in addition the child was given ORS (10 ml/kg/watery stool) and regular diet as appropriate |
|
| Outcomes | Weight gain (gms) during hospitalization, and on day 5 and 14 of hospitalization; stool frequency and the type of stool (watery, loose, or formed); number of patients with vomiting | |
| Setting | Hospital based trial Location: Multicentre study based in 13 European hospitals in 11 countries: Royal Hospital for Sick Children, Bristol, UK; Ospedale Maria Nuova, Reggio Emilia, Italy; Department of Clinical Sciences, University of Tampere, Finland; Departimento Universita Di Napoli, Napoli, Italy; The Children's AMC, Amsterdam, The Netherlands; Groot Ziekengasthius, `s‐Hertogenbosch, The Netherlands; Children's Hospital Zagreb, Republic of Croatia; Maribor Teaching Hospital, Ljubljanska, Slovenia; Department of Paediatrics, Charles University, Czech Republic; Antwerp Children's Hospital, Belgium; Hospital de S. Joao, Porto, Portugal; Katedra Pediatrii Akademii Medycznej, Warszawa, Dzialdowska, Poland; and Soroka Medical Centre, Beer Sheva, Israel |
|
| Notes | The figures showed that there were still participants who had diarrhoea and vomiting at Day 5 , although it was unclear how many were the actual counts. However, it was mentioned that none of the participants still had persistent diarrhoea and vomiting by Day 14. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were allocated to the two groups according to random numbers by the 12 centres included in the study |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants and caregivers were not blinded, but it was not mentioned if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 134 out of 134 and 88 out of 96 patients in the early and late refeeding group, respectively, were included in the final analysis |
| Selective reporting (reporting bias) | High risk | There were still participants who had diarrhoea and vomiting at Day 5, although it was unclear how many were the actual counts |
| Other bias | Unclear risk | No details given in trial report |
Santosham 1985.
| Methods | Randomized controlled trial | |
| Participants | Number: 89 enrolled Inclusion criteria: 0 to 12 months with acute watery diarrhoea (<7 days duration, at least 5 watery stools per day) |
|
| Interventions | 1. Soy‐based lactose‐free formula 4 hrs after hospitalization: 43 participants 2. Food was withheld for the first 48 hours of hospitalization: 44 participants |
|
| Outcomes | Stool output in the first 24 and 48 hours and during illness (ml/kg); percentage weight gain on the following: 24th and 48th hour, resolution of illness and two weeks after discharge; duration of diarrhoea (hrs); serum sodium and potassium on admission, during and at resolution of illness | |
| Setting | Hospital based trial Location: Indian Health Service Hospital, Whiteriver, Arizona, USA |
|
| Notes | One person in each group was excluded because food other than that allowed for the study was taken Persistent vomiting was defined as more than 3 times in an 8 hour interval necessitating intravenous therapy Persistent diarrhoea defined as more than 7 days of diarrhoea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned using block randomization of groups of four |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 43 out of 44 and 44 out of 45 patients in the early and late refeeding group, respectively, were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Santosham 1990.
| Methods | Randomly assigned | |
| Participants | Number: 200 enrolled Inclusion criteria: children 3 to 18 months with acute watery diarrhoea (<7 days duration, at least 5 watery stools per day) Exclusion criteria: exclusively breastfed, with illness requiring intravenous fluids or antibiotic therapy, <5% dehydration, clinical signs of kwashiorkor |
|
| Interventions | 1. Glucose oral rehydration solution (G‐ORS) for 4 hours followed by soy‐based formula (SF): 50 participants 2. G‐ORS for 4 hours followed by rice‐based formula: 50 participants 3. Rice‐based ORS for 24 hours followed by SF: 50 participants 4. G‐ORS for 4 hours followed by pre‐cooked rice: 50 participants |
|
| Outcomes | Stool output during the first 24 hours and during illness (ml/kg); percentage weight gain during illness; duration of diarrhoea (hrs); serum sodium and potassium on admission, during and at resolution of illness | |
| Setting | Hospital based trial Location: Abu El‐Reeche Hospital, Cairo, Egypt |
|
| Notes | The third group (R‐ORS § SF), which continued to receive R‐ORS for the first 24 hours of the maintenance period followed by a soy‐based lactose‐free formula, was considered late refeeding or the control group in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled infants were randomly assigned in blocks of eight to one of four groups. |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 148 out of 150 and 49 out of 50 patients in the early and late refeeding group, respectively, were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | High risk | Eleven percent were lost to follow‐up |
Santosham 1991.
| Methods | Randomized controlled trial | |
| Participants | Number: 59 enrolled Inclusion criteria: 2 to 12 months with acute watery diarrhoea (<7 days duration, at least 5 watery stools per day), <7% dehydration |
|
| Interventions | 1. Soy‐based lactose‐free formula and oral electrolyte solution (Resol) on the first 24 hours: 29 participants 2. Oral electrolyte solution (Resol) alternating with water in the first 24 hours; day 2, half‐strength soy‐based formula alternating with ORS; day 3, full strength soy based formula: 30 participants |
|
| Outcomes | Duration of diarrhoea (days); percentage weight gain at 24 hours after entry, at resolution of illness and at 2 weeks after therapy. CBC, serum electrolytes, total proteins and glucose were only monitored on admission | |
| Setting | Out‐patient trial Locations: US Public Health Service Hospital in Whiteriver, Arizona; and private and city health clinics in Baltimore, Maryland |
|
| Notes | 3 dropped out within 24 hours because of non‐compliance with the study regimen. No data were obtained for these 3 participants. Persistent diarrhoea lasting for more than 7 days after start of therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomized to groups of four using the table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded, but it was unclear if the caregivers or the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 56 out of 59 patients who were randomized in the trial were included in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Shaikh 1991.
| Methods | Randomized controlled trial | |
| Participants | Number: 69 enrolled Inclusion criteria: children 9 to 48 months, acute watery diarrhoea <72 hours, moderate to severe dehydration, no previous antibiotic treatment, no complications other those related to dehydration, weaned from mother's milk |
|
| Interventions | 1. 'Khitchri' and half‐strength cow's milk formula in addition to WHO ORS after 4 to 6 hours rehydration: 36 participants 2. WHO ORS with no food in the first 24 hours, then traditional legume‐based weaning diet 'khitchri' and half‐strength cow's milk formula freely: 33 participants |
|
| Outcomes | Stool output (g/kg/day), percentage weight gain after start of rehydration and at 24 hours and 72 hours post‐rehydration | |
| Setting | Hospital based trial Location: Civil Hospital, Karachi, Pakistan. |
|
| Notes | 6 children were withdrawn and did not complete the study due to intercurrent infections and for non‐medical reasons (2 from Group A, 4 from Group B) Treatment failures were defined as a need to restart administeringintravenous fluids Data were separated from those who were treatment successes and failures; we were not able to extract the data for appropriate meta analysis Vomiting was monitored only until day 3 11 children in Group A and 15 in Group B had high stool rates on day 3 but it was unclear if this persisted for more than 7 days. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to one of the two treatment groups using a random number table |
| Allocation concealment (selection bias) | Unclear risk | No details given in trial report |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 32 out of 36 and 31 out of 33 in the early and late refeeding group, respectively, were reported in the final analysis |
| Selective reporting (reporting bias) | Unclear risk | No details given in trial report |
| Other bias | Unclear risk | No details given in trial report |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armistead 1989 | This is a randomized controlled trial of 68 hospitalized babies for acute diarrhoea to compare full‐strength versus quarter‐strength formula. However, both groups were given feedings only after 24 hours of oral rehydration with glucose electrolyte mixture. |
| Chew 1993 | The study assessed the effects on clinical course of two feeding regimens in 159 Guatemalan and Brazilian infant boys aged 2 weeks to 6 months who had acute diarrhoea. They were assigned randomly to one of two feeding regimens: full‐strength milk formula (group A), or progressive reintroduction of full‐strength milk formula (half‐strength for the first 24 hours, two‐thirds strength for the second 24 hours, and full‐strength thereafter) (group B). Both groups received refeeding after 4 to 6 hours of oral rehydration. |
| Fox 1990 | Sixty‐two babies under the age of 6 months who were admitted with gastroenteritis were randomly allocated to gradual refeeding or abrupt refeeding. In both groups, refeeding was started after a period of 12 hours rehydration. |
| Haffejee 1990 | A therapeutic trial of hospitalized children with acute diarrhoea randomized to receive either lactose‐free soya formula or their original cow's milk‐based formula. However, both comparison groups were re‐fed at time of admission. |
| Haque 1983 | One hundred and fifty children with acute enteritis were randomly allocated to three feeding regimens: a. clear fluids (glucose electrolyte solution) then quarter‐strength formula; b. clear fluids then full‐strength formula; c. continuing full‐strength milk. In the first two groups, the initial fluids were given within 6 to 24 hours and therefore it was unclear whether there were children who received refeeding early. |
| Hjelt 1989 | Fifty‐two children aged 6 to 46 months who were hospitalized for acute gastroenteritis were randomized after start of rehydration to receive either traditional gradual or rapid refeeding. The gradual refeeding group was allowed to take only syrupy water gruel and apple porridge for at least one day and therefore it was unclear how many in this group would have been re‐fed early. |
| Nanulescu 1995 | A quasi‐randomized trial compared early versus late re‐feeding in the management of acute diarrhoea in the first year of life. |
| Parker 1981 | The participants were 9 infants with protracted diarrhoea and malnutrition and 2 infants with surgically created short bowel. Patients were not selected randomly for allocation to the treatment groups. |
| Ransome 1984 | A blind randomized controlled trial was performed to compare full strength versus graduated milk formula given on the first day of hospital admission for the treatment of acute infantile gastroenteritis. |
| Soeprapto 1979 | The study included babies aged 4 to 24 months with diarrhoea, but it was not clear whether they were suffering from acute watery diarrhoea. Both comparison groups were re‐fed after start of rehydration, but the the interval until the participants were given full feeding differed between the groups. |
Differences between protocol and review
Authorship: GV Gregorio was designated as the principal investigator for the review.
Data extraction: We originally planned to extract data on the number of patients requiring hospitalization. However, most of the trials were conducted in a hospital setting, and the outcome reported by two trials was the mean length of hospital stay. We also intended to obtain count data by determining the total number of episodes in each group (if the episode was rare) or the number of person years in each group for each treatment arm (if the episode was common). However, during our assessment of the trials, we realised that the trials reported the number of participants with vomiting, and thus it was considered to be a dichotomous outcome rather than a count outcome. There were also no studies that reported the mean caloric intake, deaths and all‐cause mortality.
Data analysis: In multiple treatment arms with two or more types of feeding as treatment groups, the outcomes were combined as appropriate and compared collectively with the control group.
Subgroup analyses: This could not be done because of the limited number of trials in each outcome.
Contributions of authors
All the authors wrote the protocol. The first two authors carried out the risk of bias (methodological quality) assessment, data extraction, data analysis, and wrote the final manuscript.
Sources of support
Internal sources
Effective Health Care Research Programme Consortium, UK, Not specified.
External sources
Department of International Development, UK.
Declarations of interest
None known
Unchanged
References
References to studies included in this review
Brown 1988 {published data only}
- Brown KH, Gastanaduy AS, Saavedra JM, Lembcke J, Rivas D, Robertson AD, et al. Effect of continued oral feeding on clinical and nutritional outcomes of acute diarrhoea in children. The Journal of Pediatrics 1988;112:191‐200. [DOI] [PubMed] [Google Scholar]
Conway 1989 {published data only}
- Conway SP, Ireson A. Acute gastroenteritis in well nourished infants: comparison of four feeding regimens. Archives of Diseases of Childhood 1989;64:87‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gazala 1988 {published data only}
- Gazala E, Weitzman S, Weitzman Z, Gross J, Bearman J, Gorodischer R. Early vs late refeeding in acute infantile diarrhea. Israel Journal of Medical Science 1988;24:174‐9. [PubMed] [Google Scholar]
Hoghton 1996 {published data only}
- Hoghton MAR, Mittal NK, Sandhu BK, Mahdi G. Effects of immediate modified feeding on infantile gastroenteritis. British Journal of General Practice 1996;46:173‐5. [PMC free article] [PubMed] [Google Scholar]
Khin‐Maung‐U 1985 {published data only}
- Khin MU, Nyunt‐Nyunt‐Wai, Myo‐Khin, Mu‐Mu‐Khin, Tin U, Thane‐Toe. Effect on clinical outcome of breast feeding during acute diarrhoea. British Medical Journal 1985;290:587‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khin‐Maung‐U 1986 {published data only}
- Khin‐maung‐u, Nyunt‐nyunt‐wai, Myo‐khin, Mo‐mo‐khin, Tin‐u, Thane‐toe. Effect of boiled‐rice feeding in childhood cholera on clinical outcome. Human Nutrition: Clinical Nutrition 1986;40C:249‐54. [PubMed] [Google Scholar]
Rees 1979 {published data only}
- Rees L, Brook CGD. Gradual reintroduction of full‐strength milk after acute gastroenteritis in children. Lancet 1979;1:770‐1. [DOI] [PubMed] [Google Scholar]
Sandhu 1997 {published data only}
- Sandhu BK, Isolauri E, Walker‐Smith JA, Banchini G, VanCaillie‐Bertrand M, Dias J, et al. A multicentre study on behalf of the European Society of Paediatric Gastroenterology and Nutrition Working Group on acute diarrhoea: early feeding in childhood gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition 1997;24:522‐7. [DOI] [PubMed] [Google Scholar]
Santosham 1985 {published data only}
- Santosham M, Foster S, Reid R, Bertrando R, Yolken R, Burns B, et al. Role of soy‐based, lactose‐free formula during treatment of acute diarrhea. Pediatrics 1985;76:292‐8. [PubMed] [Google Scholar]
Santosham 1990 {published data only}
- Santosham M, Fayad IM, Hashem M, Goepp JG, Refat M, Sack RB. A comparison of rice‐based oral rehydration solution and "early feeding" for the treatment of acute diarrhea in infants. Pediatrics 1990;116:868‐75. [DOI] [PubMed] [Google Scholar]
Santosham 1991 {published data only}
- Santosham M, Greenough WB III. Oral rehydration therapy: a global perspective. Journal of Pediatrics 1991;llS(4):S44‐S51. [DOI] [PubMed] [Google Scholar]
Shaikh 1991 {published data only}
- Shaikh S, Molla AM, Islam A, Billoo AG, Hendricks K, Stryder J. A traditional diet as part of oral rehydration therapy in severe acute diarrhoea in young children. Journal of Diarrhoeal Disease Research 1991;3:258‐63. [PubMed] [Google Scholar]
References to studies excluded from this review
Armistead 1989 {published data only}
- Armistead J, Kelly D, Walker‐Smith J. Evaluation of infant feeding in acute gastroenteritis. Journal of Pediatric Gastroenterology and Nutrition 1989;8:240‐4. [DOI] [PubMed] [Google Scholar]
Chew 1993 {published data only}
- Chew F, Penna FJ, Peret Filho LA, Quan C, Lopes MC, Mota JAC, et al. Is dilution of cows’ milk formula necessary for dietary management of acute diarrhoea in infants age less than 6 months?. Lancet 1993;341:194‐7. [DOI] [PubMed] [Google Scholar]
Fox 1990 {published data only}
- Fox R, Leen CLS, Dunbar EM, Ellis ME, Mandal BK. Acute gastroenteritis in infants under 6 months old. Archives of Disease in Childhood 1990;65:936‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haffejee 1990 {published data only}
- Haffejee IE. Cow's milk‐based formula, human milk, and soya feeds in acute infantila diarrhea: a therapeutic trial. Journal of Pediatric Gastroenterology and Nutrition 1990;10:193‐8. [DOI] [PubMed] [Google Scholar]
Haque 1983 {published data only}
- Haque KN, Al‐Frayh A, El‐Rifai R. Is it necessary to regraduate milk after acute gastroenteritis in children?. Tropical and Geographical Medicine 1983;35:369‐73. [PubMed] [Google Scholar]
Hjelt 1989 {published data only}
- Hjelt K, Paerregaard A, Petersen W, Christiansen L, Krasilnikoff PA. Rapid versus gradual refeeding in acute gastroenteritis in childhood: energy intake and weight gain. Journal of Pediatric Gastroenterology and Nutrition 1989;8:75‐80. [DOI] [PubMed] [Google Scholar]
Nanulescu 1995 {published data only}
- Nanulescu M, Condor M, Popa M, Muregan M, Panfa P, Ionac S, et al. Early re‐feeding in the management of acute diarrhoea in infants of 0‐1 year of age. Acta Paediatrica 1995;84:1002‐6. [DOI] [PubMed] [Google Scholar]
Parker 1981 {published data only}
- Parker P, Stroop S, Greene H. A controlled comparison of continuous versus intermittent feeding in the treatment of infants with intestinal disease. Journal of Pediatrics 1991;99:360‐4. [DOI] [PubMed] [Google Scholar]
Ransome 1984 {published data only}
- Ransome OJ, Roode H. Early introduction of milk feeds in acute infantile gastro‐enteritis. South African Medical Journal 1984;65:127‐8. [PubMed] [Google Scholar]
Soeprapto 1979 {published data only}
- Soeprapto, Soenarto Y, Nelwan, Moenginah PA, Ismangoen. Feeding Children with Diarrhea. Journal of Tropical Pediatrics 1979;25:97‐100. [DOI] [PubMed] [Google Scholar]
Additional references
Bezerra 1992
- Bezerra JA, Stathos TH, Duncan B, Gaines JA, Udall JN Jr. Treatment of infants with acute diarrhea: what's recommended and what's practiced. Pediatrics 1992;90 (1 Pt 1):1‐4. [PubMed] [Google Scholar]
Brown 1993
- Brown KH, Perez F, Peerson JM, Fadel J, Brunsgaard, Ostrom KM, et al. Effect of dietary fiber (soy polysaccharide) on the severity, duration, and nutritional outcome of acute, watery diarrhea in children. Pediatrics 1993;92:241–7. [PubMed] [Google Scholar]
Brown 1994
- Brown KH, Peerson JM, Fontaine O. Use of nonhuman milks in the dietary management of young children with acute diarrhea: a meta‐analysis of cllnical trials. Pediatrics 1994;93:17‐27. [PubMed] [Google Scholar]
Burks 2001
- Burks AW, Vanderhoof JA, Mehra S, Ostrom KM, Baggs G. Randomized clinical trial of soy formula with and without added fiber in antibiotic‐induced diarrhea. Journal of Pediatrics 2001;139:578–82. [DOI] [PubMed] [Google Scholar]
Chongban 2005
- Chongbanyatcharoen P. Acute Diarrhea’s Recommendations on Oral Rehydration Therapy and Feeding. Journal of the Medical Association of Thailand 2005;88 (Suppl 1):S30‐4. [PubMed] [Google Scholar]
Chung 1948
- Chung AW, Viscorova B. The effect of oral feeding versus early oral starvation on the course of infantile diarrhea. Journal of Pediatrics 1948;33:14‐22. [DOI] [PubMed] [Google Scholar]
Hageman 1977
- Hageman RF, Strogand JJ. Fasting and refeeding: cell kinetic response of jejunum, ileum and colon. Cell Tissue Kinetics 1977;10:3‐14. [DOI] [PubMed] [Google Scholar]
Hirshhorn 1980
- Hirshhorn N. The treatment of acute diarrhea in children. An historical and physiological perspective. American Journal of Clinical Nutrition 1980;33:637‐63. [DOI] [PubMed] [Google Scholar]
Isolauri 1989
- Isolauri E, Juntunen M, Wiren S, Vuorinen P, Koivula T. Intestinal permeability changes in acute gastroenteritis: effects of clinical factors and nutritional management. Journal of Pediatric Gastroenterology and Nutrition 1989;8:466–73. [DOI] [PubMed] [Google Scholar]
Keusch 2006
- Keusch GT, Fontaine O, Bhargava A, Boschi‐Pinto C, Bhutta ZA, Gotuzzo E, et al. Chapter 16. Diarrheal Diseases. Disease Control Priorities in Developing Countries (2nd Edition).. Oxford University Press , World Bank ISBN: 0‐8213‐6179‐1, 2006. [Google Scholar]
Kosek 2003
- Kosek M, Bern C, Guerrant RL. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bulletin of the World Health Organization 2003;81:197‐204. [PMC free article] [PubMed] [Google Scholar]
MMWR 2003
- King CK, Glass R, Bresee JS, Duggan C. Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: oralrehydration, maintenance, and nutritional therapy. MMWR 2003;52:9. [PubMed] [Google Scholar]
Murphy 1998
- Murphy MS. Guidelines for managing acute gastroenteritis based on a systematic review of published research. Archives of Disease in Childhood 1998;79:279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Podewils 2004
- Podewils LJ, Mintz ED, Nataro JP, Parashar UD. Acute, Infectious Diarrhea Among Children in Developing Countries. Seminars in Pediatric Infectious Diseases 2004;15:155‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Santosham 1997
- Santosham M, Keenan EM, Tulloch J, Broun D, Glass R. Oral Rehydration Therapy for Diarrhea: An Example of Reverse Transfer of Technology. Pediatrics 1997;100:e10. [DOI] [PubMed] [Google Scholar]
Sarker 1983
- Sarker SA, Molla AM, Rahaman MM. Impact of supplementary food on intake of breast milk in diarrhoea. Lancet 1983;2(8363):1349‐51. [DOI] [PubMed] [Google Scholar]
Walker‐Smith 1997
- Walker‐Smith JA, Sandhu BK, Isolauri E, Banchini G, Caillie‐Bertrand M, Dias JA, et al. Guidelines prepared by the ESPGAN Working Group on Acute Diarrhoea. Recommendations for feeding in childhood gastroenteritis. European Society of Pediatric Gastroenterology and Nutrition. Journal of Pediatric Gastroenterology and Nutrition 1997;24(5):619‐20. [DOI] [PubMed] [Google Scholar]
WHO 1995
- World Health Organization. The Treatment of Diarrhoea. A Manual for Physicians and Other Senior Health Workers. Geneva: World Health Organization, 1995. [Google Scholar]
WHO 1999
- Management of severe malnutrition: a manual for physicians and other senior health workers. WHO Geneva 1999; Vol. Available at: www.who.int/nutrition/publications/malnutrition/en/index.html (Accessed on 20 February 2008).
WHO/UNICEF 2004
- WHO UNICEF Joint Statement. Clinical Management of Acute Diarrhoea, WHO/FCH/CAH/04.7 or UNICEF/PD/Diarrhoea/01. The United Nations Children’s Fund/World Health Organization, May 2004.
Williamson 1978
- Williamson RCN. Intestinal adaptation: I. Structural, Functional and cytokinetic changes. New England Journal of Medicine 1978;298:1393‐402. [DOI] [PubMed] [Google Scholar]


