Abstract
Background
Scabies is an intensely itchy parasitic infection of the skin caused by the Sarcoptes scabiei mite. It is a common public health problem with an estimated global prevalence of 300 million cases. Serious adverse effects have been reported for some drugs used to treat scabies.
Objectives
To evaluate topical and systemic drugs for treating scabies.
Search methods
In June 2010, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2010, Issue 2), MEDLINE, EMBASE, LILACS, and INDMED. In August 2010, we also searched the grey literature and sources for registered trials. We also checked the reference lists of retrieved studies.
Selection criteria
Randomized controlled trials of drug treatments for scabies.
Data collection and analysis
Two authors independently assessed trial quality and extracted data. Results were presented as risk ratios with 95% confidence intervals and data combined where appropriate.
Main results
Twenty‐two small trials involving 2676 people were included. One trial was placebo controlled, 18 compared two or more drug treatments, three compared treatment regimens, and one compared different drug vehicles.
Fewer treatment failures occurred by day seven with oral ivermectin compared with placebo in one small trial (55 participants). Topical permethrin appeared more effective than oral ivermectin (140 participants, 2 trials), topical crotamiton (194 participants, 2 trials), and topical lindane (753 participants, 5 trials). Permethrin also appeared more effective in reducing itch persistence than either crotamiton (94 participants, 1 trial) or lindane (490 participants, 2 trials). No difference was detected between permethrin (a synthetic pyrethroid) and a natural pyrethrin‐based topical treatment (40 participants, 1 trial), and between permethrin and benzyl benzoate (53 participants, 1 trial), however both these trials were small.
No significant difference was detected in the number of treatment failures between crotamiton and lindane (100 participants, 1 trial), lindane and sulfur (68 participants, 1 trial), benzyl benzoate and sulfur (158 participants, 1 trial), and benzyl benzoate and natural synergized pyrethrins (240 participants, 1 trial); all were topical treatments. No trials of malathion were identified.
No serious adverse events were reported. A number of trials reported skin reactions in participants randomized to topical treatments. There were occasional reports of headache, abdominal pain, diarrhoea, vomiting, and hypotension.
Authors' conclusions
Topical permethrin appears to be the most effective treatment for scabies. Ivermectin appears to be an effective oral treatment. More research is needed on the effectiveness of malathion, particularly when compared to permethrin, and on the management of scabies in an institutional setting and at a community level.
23 April 2019
No update planned
Review superseded
This review includes an evaluation of crotamiton, lindane, sulfur, and benzyl benzoate. However, these are not active areas of research and are not widely used for treatment. A new assessment of ivermectin and permethrin alone is justified and thus this Cochrane Review has been superseded by Rosumeck 2018 https://doi.org/10.1002/14651858.CD012994
Plain language summary
Interventions for treating scabies
Scabies is a parasitic infection of the skin. It occurs throughout the world, but is particularly problematic in areas of poor sanitation, overcrowding, and social disruption, and is endemic in many resource‐poor countries. The global prevalence of scabies is estimated at 300 million cases, but the level of infection varies between countries and communities. The female mite burrows into the skin to lay eggs which then hatch out and multiply. The infection can spread from person to person via direct skin contact, including sexual contact. It causes intense itching with eruptions on the skin. Various drugs have been developed to treat scabies, and herbal and traditional medicines are also used. The review of trials attempted to cover all these. The authors identified 22 small trials involving 2676 people, with 19 of the trials taking place in resource‐poor countries. Permethrin appeared to be the most effective topical treatment for scabies, and ivermectin appeared to be an effective oral treatment. However, ivermectin is unlicensed for this indication in many countries. Adverse events such as rash, vomiting, and abdominal pain were reported, but the trials were too small to properly assess serious but rare potential adverse effects. No trials of herbal or traditional medicines were identified for inclusion.
Background
What is scabies?
Scabies is an intensely itchy parasitic infection of the skin that is caused by the Sarcoptes scabiei mite. It occurs throughout the world, but is particularly problematic in areas of poor sanitation, overcrowding, and social disruption. The global prevalence of scabies is estimated at 300 million cases (Alexander 1984), with large variations between countries. In the UK, no up‐to‐date robust prevalence data exist, but general practitioners recorded approximately 1200 new cases per year in the 1990s (Downs 1999). In resource‐rich communities, scabies tends to occur in cyclical epidemics, particularly within institutional‐living situations such as nursing homes (Scheinfeld 2004) or the army (Mimouni 1998; Mimouni 2003). There is some seasonal variation with incidence being greater in the winter than the summer, perhaps related to the tendency for more indoor overcrowding in colder weather (Downs 1999). In resource‐poor communities, the occurrence pattern is quite different with the disease being endemic in many areas (Chosidow 2000). For example, the prevalence of scabies among the remote Aboriginal communities of Northern Australia is around 50% in children and 25% in adults (Wong 2002). The prevalence of infection in a community is potentially influenced by changes in social attitudes, population movements, wars, misdiagnosis, inadequate treatment, and changes in the immune status of the population. Scabies infestation represents a considerable burden of ill health in many communities, and although the disease is rarely life threatening, it causes widespread debilitation and misery (Green 1989).
The life cycle of S.scabiei begins with the pregnant female laying two to three eggs a day in burrows several millimetres to several centimetres in length in the stratum corneum (outermost layer) of the skin. After about 50 to 72 hours, larvae emerge and make new burrows. They mature, mate, and repeat this 10‐ to 17‐day cycle. Mites usually live for 30 to 60 days (Green 1989).
Humans are the main reservoir for S.scabiei var. hominis (variety of the mite named to reflect the main host species). Scabies is usually spread person to person via direct skin contact, including sexual contact, though transfer via inanimate objects such as clothing or furnishings is also possible (Hay 2004). The mite can burrow beneath the skin within 2.5 minutes, though around 20 minutes is more usual (Alexander 1984). The level of infectiousness of an individual depends in part on the number of mites harboured, which can vary from just a single mite to millions (Chosidow 2000). Humans can also be transiently infected by the genetically distinct animal varieties of S. scabiei (eg var. canis), though cross infectivity is low (Fain 1978; Walton 2004).
Clinical infection with the scabies mite causes discomfort and often intense itching of the skin, particularly at night, with irritating papular or vesicular eruptions. While infestation with the scabies mite is not life threatening, the severe, persistent itch debilitates and depresses people (Green 1989). The classical sites of infestation are between the fingers, the wrists, axillary areas, female breasts (particularly the skin of the nipples), peri‐umbilical area, penis, scrotum, and buttocks. Infants are usually affected on the face, scalp, palms, and soles. Much of the itching associated with scabies is as a result of the host immune reaction, and symptoms can take several weeks to appear after initial infection in a person exposed to scabies for the first time. Symptoms appear after a much shorter interval (one to two days) after reinfestation (Arlian 1989).
A more severe or 'crusted' presentation of infestation is associated with extreme incapacity and with disorders of the immune system, such as HIV infection. Clinically this atypical form of scabies presents with a hyperkeratotic dermatosis resembling psoriasis. Lymphadenopathy and eosinophilia can be present, but itching may be unexpectedly mild. Patients with crusted scabies may harbour millions of mites and are highly infectious (Meinking 1995a). The dermatological distribution of mites in such patients is often atypical (eg including the head), and treatment in hospital is often advised (Chosidow 2000).
Complications are few although secondary bacterial infection of the skin lesions by group A Streptococcus pyogenes or Staphylococcus aureus, or both, can occur following repeated scratching, particularly in warmer climates (Meinking 1995a). Secondary infection with group A Streptococcus can lead to acute glomerulonephritis, outbreaks of which have been associated with scabies (Green 1989).
Diagnosis, treatment, and prevention
Diagnosis on clinical grounds is usually made on a history of itching (particularly if contacts are also affected) and the finding of lesions in the classical sites. The diagnosis can in most cases be confirmed by microscopically identifying a mite, egg, or mite faeces in a skin scraping, or by extracting a mite from a burrow (Chosidow 2000).
Various treatments are available for scabies. These include sulfur compounds, which have been used for centuries; benzyl benzoate (first used in 1931); crotamiton (used since the late 1970s); hexachlorocyclohexane, which is also known as gamma benzene hexachloride or the commercial purified form lindane ('lindane' is used in this review) (available since 1948); malathion (used since the mid 1970s); permethrin (first licensed in 1985 by the US Food and Drug Administration); and oral ivermectin (first used in humans in the 1980s). A number of herbal remedies have also been proposed (Oladimeji 2000; Alebiosu 2003; Oladimeji 2005).
Serious adverse effects have been associated with the use of some antiscabietic treatments. Convulsions and aplastic anaemia have been reported with the use of lindane (Rauch 1990; Elgart 1996), and an increased risk of death amongst elderly patients has been reported with the use of ivermectin (Barkwell 1997).
Evidence of cure ideally requires follow up for about one month. This allows time for lesions to heal and for any eggs and mites to reach maturity if treatment fails (ie beyond the longest incubation interval). Patients should be warned that itching may persist for one to two weeks after treatment, even if the mite is successfully eradicated (Buffet 2003). Because of this delay in symptom relief it may sometimes be difficult to distinguish reinfestation from primary treatment failure.
Contacts of cases are usually advised to treat themselves at the same time as the case in order to reduce the risk of reinfestation (Orkin 1976). Prevention is based on principles common to most infectious diseases, that is, limitation of contact with the mite.
Using data from randomized controlled trials, this review examines the existing evidence of effectiveness of treatments for scabies.
Objectives
To evaluate topical and systemic drugs for treating scabies.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Children or adults with a clinical or parasitological diagnosis of scabies.
Types of interventions
Intervention
Drug treatment (systemic or local).
Herbal or traditional medicine treatment.
Any combination of above.
Treatment of contacts in addition to cases.
Control
Placebo or no intervention.
A different drug intervention, drug intervention vehicle, intervention regimen, or combination of interventions.
Different or no treatment of contacts.
Types of outcome measures
Primary
Treatment failure in a clinically diagnosed case.
Treatment failure in a parasitologically confirmed case.
Treatment failure is defined in both the above cases as the persistence of original lesions, the appearance of new lesions, or confirmation of a live mite.
Secondary
Persistence of patient‐reported itch.
Adverse events
Serious adverse event that leads to death, is life threatening, results in persistent or significant disability or incapacity, or requires hospitalization.
Adverse event that requires discontinuation of treatment.
Other adverse event.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (June 2010); Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 2); MEDLINE (1966 to June 2010); EMBASE (1974 to June 2010); LILACS (1982 to June 2010); and INDMED (June 2010).
Grey literature
In August 2010, we searched the following sources for published and unpublished trials using the term 'scabies': British Library Index of Conference Proceedings (catalogue.bl.uk/); British Library for Development Studies (blds.ids.ac.uk/); BRIDGE (www.bridge.ids.ac.uk/); Social Care Online (www.scie‐socialcareonline.org.uk/); EconLit; ERIC; Institute for Development Studies (www.ids.ac.uk/ids/particip/information/readrm.html); IIED (www.iied.org/). We searched Science.gov (www.science.gov/) using the terms 'scabies' AND ('trial' OR 'study').
Registered trials
In August 2010, we searched the following sources for registered trials using the term 'scabies': Current Controlled Trials (www.controlled‐trials.com/); Thompson CenterWatch Clinical Trials Listing Service (www.centerwatch.com/); US National Institutes of Health ClinicalTrials.gov (www.clinicaltrials.gov/); TrialsCentral (www.trialscentral.org/); and the UK Department of Health National Research Register (www.nihr.ac.uk/).
Reference lists
We checked the reference lists of all retrieved trials.
Data collection and analysis
Selection of studies
All identified trials were entered into a trials register. MS and PJ independently applied the inclusion criteria to the potentially relevant trials. If a trial's eligibility was unclear, we attempted to contact the trial authors for further information. MS reassessed all included and excluded trials cited in the previous review version (Walker 2000). Where the review authors disagreed, the Co‐ordinating Editor of the Cochrane Infectious Diseases Group was consulted, and a consensus reached among the three parties; this process was also used for assessing the risk of bias in trials, and extracting data. The trial reports were scrutinized to ensure that multiple publications from the same trial were included only once. We listed the reasons for excluding studies in the 'Characteristics of excluded studies'.
Data extraction and management
We independently extracted data from the newly included trials. Where important data were missing, we attempted to contact the trial authors for further information. MS entered the data into Review Manager 5.0. We extracted the number of patients randomized and the number analysed for each group for each trial. For each dichotomous outcome, we recorded the number of participants experiencing the event in each arm of the trial.
Assessment of risk of bias in included studies
Both authors independently assessed the quality of the newly included trials. We assessed the generation of allocation sequence and allocation concealment as adequate, inadequate, or unclear (Juni 2001). We assessed the inclusion of randomized participants in the analysis to be adequate if greater than 80%. We recorded who was blinded (eg participants or investigators) rather than using potentially ambiguous terms such as double blind or single blind. MS reassessed the included trials from the previous review version (Walker 2000).
Data synthesis
MS analysed the data using Review Manager 5.0. Analyses were stratified by comparison. We undertook an available case analysis, that is, participants were analysed in the group to which they were randomized regardless of treatment received, but only where an outcome was recorded (Higgins 2005).
Results were presented as risk ratios (RRs) with 95% confidence intervals (CIs) around these estimates. RRs less than one were taken to demonstrate a favourable outcome of the intervention of interest, and these are presented to the left of the line of no effect.
For those comparisons in which there were data from more than one trial we assessed heterogeneity by visually inspecting forest plots, calculating an I2 statistic, and carrying out a chi square test for heterogeneity. If heterogeneity was detected we undertook a subgroup analysis, grouping trials according to drug regimen and follow up time (1 week vs 2 weeks vs 3 weeks vs 4 weeks) in order to explore causes of heterogeneity. If heterogeneity was not detected we pooled results from trials in a fixed‐effect meta‐analysis.
Results
Description of studies
Trial selection
Of the 79 trials identified and included in our trials register, 57 were excluded (see 'Characteristics of excluded studies') and 22 met the inclusion criteria (see 'Characteristics of included studies'). All trials were identified from published literature. One ongoing study, Naeyaert ongoing has also been identified.
Trial location
Nineteen of the 22 included studies were conducted in resource‐poor countries, although one, Schultz 1990, was a large multicentre trial involving eight centres (four sexually transmitted disease clinics, two dermatology clinics, and two family practice clinics), with one of the family practice centres in Mexico and the others in the USA. Of the other three trials, one was carried out in the USA (Hansen 1986) and two in Italy (Amerio 2003; Biele 2006).
Participants
Three trials included only adults (Chouela 1999; Amerio 2003; Biele 2006), six included only children (Maggi 1986; Schenone 1986; Taplin 1990; Avila‐Romay 1991; Brooks 2002; Singalavanija 2003); and the other 13 included both adults and children. The total number of participants randomized in the 22 trials was 2676; all had a clinical diagnosis of scabies, with a subset of 903 identified as having their diagnosis confirmed parasitologically.
Interventions
The effectiveness of the following drugs was tested: topical benzyl benzoate; crotamiton; decamethrin; lindane; permethrin; synergized natural pyrethrins; sulfur; and oral ivermectin. Eighteen trials compared one drug with at least one other drug, one trial compared ivermectin against placebo, three trials compared different drug treatment regimens, and one trial compared two different vehicles for the same drug. No randomized controlled trials investigating malathion were identified.
Clinicians and drug companies recommended treatment of family members and close contacts at the same time as cases, to improve cure rates and reduce reinfection (Taplin 1986). None of the trials tested this hypothesis. Close and family contacts in both intervention and control groups were treated, however, in all but six trials (Hansen 1986; Maggi 1986; Amer 1992; Macotela‐Ruiz 1993; Amerio 2003; Biele 2006).
Five trials stipulated that a bath or shower should be taken before treatment (Gulati 1978; Schenone 1986; Taplin 1990; Avila‐Romay 1991; Bachewar 2009); and ten trials stipulated that participants should change and wash their linen after treatment (Avila‐Romay 1991; Glaziou 1993; Chouela 1999; Usha 2000; Madan 2001; Nnoruka 2001; Brooks 2002; Singalavanija 2003; Zargari 2006; Ly 2009).
Dosing and regimen
Benzyl benzoate
The strength of the topical benzyl benzoate solution varied with three trials using 10% (Glaziou 1993; Brooks 2002; Biele 2006), one trial using 12.5% (Ly 2009) and three trials using 25% (Gulati 1978; Nnoruka 2001; Bachewar 2009). The treatment regimen was different in each trial: it was applied once and left overnight in Brooks 2002; applied twice, 12 hours apart in Glaziou 1993; applied twice and left overnight on two consecutive nights in Bachewar 2009; applied three times, 12 hours apart in Gulati 1978; applied on five consecutive days in Biele 2006; and a single application was left for 72 hours in Nnoruka 2001. Ly 2009 included two benzyl benzoate intervention groups, one in which the drug was applied once and left for 24 hours, and another in which the drug was applied twice, 24 hours apart, left in each case for 24 hours.
Crotamiton
A 10% topical preparation was used in two trials (Taplin 1990; Amer 1992). It was applied overnight on two consecutive nights in Amer 1992, and was applied once overnight in Taplin 1990.
Decamethrin
Schenone 1986 compared 0.02% decamethrin lotion applied daily for two days repeated on two more days a week later with 0.02% decamethrin lotion applied daily for four consecutive days.
Lindane
Each lindane trial used a 1% topical preparation, except for Singalavanija 2003, which used a 0.3% preparation. The number of applications ranged from one (Hansen 1986; Maggi 1986; Taplin 1986; Schultz 1990; Chouela 1999) to two (Amer 1992; Zargari 2006) to seven (Singalavanija 2003). Maggi 1986 compared a single application of lindane left on for four days, washed off and then repeated after a week with a single one‐hour application of lindane, repeated after a week.
Permethrin
A 5% topical preparation was used in each permethrin trial. The number of applications ranged from one (Schultz 1990; Taplin 1990; Usha 2000; Bachewar 2009) to two (Amer 1992; Zargari 2006) to two consecutive overnight applications repeated after 14 days (Amerio 2003).
Synergized natural pyrethrins
A 0.16% topical preparation of natural pyrethrins synergized with pyperonil butoxide was used in Amerio 2003, applied on two successive nights and repeated 14 days later. In Biele 2006, a 0.165% preparation was applied on three consecutive days.
Sulfur
Two of the three sulfur trials used a 10% topical preparation (Avila‐Romay 1991; Singalavanija 2003). In the third trial, Gulati 1978, the strength of the preparation was not stated. Avila‐Romay 1991 compared sulfur in cold cream with sulfur in pork fat; both medications were applied nightly for three nights and then once three nights later. Singalavanija 2003 applied the sulfur on seven consecutive nights. Gulati 1978 applied sulfur once in the morning, once in the evening, and once again the next morning; treatment was repeated after 10 days if lesions persisted.
Ivermectin
The oral dose of ivermectin varied from a 100 µg/kg bodyweight (Glaziou 1993) to 200 µg/kg bodyweight (Macotela‐Ruiz 1993; Usha 2000; Madan 2001; Nnoruka 2001; Brooks 2002; Bachewar 2009). The Chouela 1999 and Ly 2009 trials used an ivermectin dose between 150 µg/kg and 200 µg/kg bodyweight. Each trial gave a single dose.
Length of follow up
Follow up ranged from seven days to one month. In 11 trials it was possible to extract outcome data at 28 to 31 days after treatment (Hansen 1986; Taplin 1986; Schultz 1990; Taplin 1990; Amer 1992; Glaziou 1993; Madan 2001; Nnoruka 2001; Amerio 2003; Singalavanija 2003; Biele 2006). Follow up was at 21 days in two trials (Schenone 1986; Brooks 2002); 14 to 15 days in six trials (Gulati 1978; Maggi 1986; Chouela 1999; Usha 2000; Zargari 2006; Ly 2009); and seven to 10 days in the remaining three trials (Avila‐Romay 1991; Macotela‐Ruiz 1993; Bachewar 2009).
Outcome measures
The review's primary outcome measure (treatment failure) was reported in 21 of the 22 trials. Six of these 21 trials reported treatment failure in both clinically diagnosed cases and in microscopically confirmed cases (Schultz 1990; Taplin 1990; Amer 1992; Amerio 2003; Singalavanija 2003; Biele 2006); the other 13 trials reported treatment failure in clinically diagnosed cases who may or may not have been confirmed microscopically. Seven trials reported the secondary outcome measure (itch persistence) in addition to treatment failure (Hansen 1986; Schultz 1990; Taplin 1990; Brooks 2002; Amerio 2003; Singalavanija 2003; Biele 2006). Itch persistence alone was reported by Maggi 1986. Adverse events were reported as an outcome in all trials except Gulati 1978 and Maggi 1986. The seven trials that reported on itch varied in their methods to assess this outcome:
Hansen 1986: did not report on the method used.
Maggi 1986: participants reported on itch using a three‐point scale ("absent", "moderate", and "intense") before and after treatment; numbers in each category were reported.
Schultz 1990: participants reported the presence or absence of itch before and after treatment; numbers in each category were reported.
Taplin 1990: participants were reported as either having presence or absence of itch; no further details of assessment were given.
Brooks 2002: participants described itch severity on a visual analogue scale before and after treatment; mean scores were reported along with the number of participants with absence of night‐time itch.
Amerio 2003 and Biele 2006: participants reported on itch using a five‐point scale (from 0 = "no itch" to 4 = "severe itch") before and after treatment; mean scores were reported along with the number of participants with complete relief from itching.
Singalavanija 2003: participants were divided into those who reported a decrease or absence of itch, and those who reported no improvement.
Sources of support
Seven trials stated that funding or support had been provided by drug companies (Taplin 1986; Schultz 1990; Taplin 1990; Glaziou 1993; Usha 2000; Amerio 2003; Zargari 2006).
Background prevalence
Fifteen trial reports did not state the background prevalence of scabies. In the four trials where prevalence was stated, it ranged from 9% in India (Gulati 1978) to 14% among children in a boarding school in Chile (Schenone 1986) to 36% in French Polynesia (Glaziou 1993) to 67% in Panama (Taplin 1990).
Risk of bias in included studies
See Table 16 for a summary assessment and the 'Characteristics of included studies' for details.
1. Quality assessment.
| Trial | Allocation sequence generation | Allocation concealment | Blinding | Inclusiona |
| Amer 1992 | Unclear | Unclear | Unclear | Adequate |
| Amerio 2003 | Adequate | Adequate | Investigators | Adequate |
| Avila‐Romay 1991 | Unclear | Unclear | Unclear | Adequate |
| Bachewar 2009 | Adequate | Unclear | None | Inadequate |
| Biele 2006 | Adequate | Unclear | Investigators | Adequate |
| Brooks 2002 | Adequate | Unclear | Investigators | Inadequate |
| Chouela 1999 | Unclear | Unclear | Described as "double blind"; participants blinded | Adequate |
| Glaziou 1993 | Unclear | Unclear | Outcomes assessor | Adequate |
| Gulati 1978 | Unclear | Unclear | Unclear | Adequate |
| Hansen 1986 | Unclear | Unclear | "Single blind", unclear who was blinded | Adequate |
| Ly 2009 | Adequate | Unclear | None | Adequate |
| Macotela‐Ruiz 1993 | Unclear | Unclear | Participant and outcomes assessor | Adequate |
| Madan 2001 | Unclear | Unclear | Outcomes assessor | Inadequate |
| Maggi 1986 | Unclear | Unclear | Unclear | Adequate |
| Nnoruka 2001 | Adequate | Unclear | Unclear | Adequate |
| Schenone 1986 | Unclear | Unclear | Unclear | Adequate |
| Schultz 1990 | Unclear | Adequate | Outcomes assessor | Adequate |
| Singalavanija 2003 | Adequate | Unclear | Unclear | Inadequate |
| Taplin 1986 | Unclear | Adequate | Investigators | Adequate |
| Taplin 1990 | Unclear | Adequate | Investigators | Adequate |
| Usha 2000 | Adequate | Adequate | None | Adequate |
| Zargari 2006 | Unclear | Adequate | Investigators and participants | Adequate |
aInclusion of randomized participants in analysis.
Generation of allocation sequence
Eight trials described an adequate method of generating a random allocation sequence: by computer in Usha 2000, Brooks 2002, Amerio 2003, Biele 2006 and Bachewar 2009; and by random‐number table in Nnoruka 2001, Singalavanija 2003 and Ly 2009. The method was unclear in the other trials.
Allocation concealment
Six trials reported adequate allocation concealment: by phone call to third party‐based procedure in Amerio 2003; by use of identical coded medication containers in Taplin 1986, Schultz 1990, Taplin 1990, and Zargari 2006; and the author of Usha 2000 confirmed that the allocation was by a third party, not the investigator. The remaining trials had methods of concealment that were either unclear or not reported.
Blinding
Twelve trials reported blinding. In two of these trials both the investigators or outcome assessors and the participants were described as blinded (Macotela‐Ruiz 1993; Zargari 2006), and in eight trials the investigators or outcome assessors alone were described as blinded (Taplin 1986; Schultz 1990; Taplin 1990; Glaziou 1993; Madan 2001; Brooks 2002; Amerio 2003; Biele 2006). Chouela 1999 described the participants as blinded but also reported the trial as "double blind". Hansen 1986 described the trial as "single blind", but it is unclear who was blinded.
Inclusion of randomized participants in the analysis
Ten trials included all randomized participants in the analysis with no mention of losses to follow up. The completeness of follow up was greater than 80% (ie adequate) in eight trials (Hansen 1986; Taplin 1986; Schultz 1990; Taplin 1990; Macotela‐Ruiz 1993; Chouela 1999; Zargari 2006; Ly 2009). The remaining four trials reported completeness of follow up less than 80% (Brooks 2002 − 27% lost to follow up, Madan 2001 − 25% lost to follow up, Singalavanija 2003 − 32% lost to follow up; Bachewar 2009 ‐ 22% lost to follow up).
Effects of interventions
1. Ivermectin
Only one trial assessed the effectiveness against placebo, while eight trials compared it with another drug.
1.1. Versus placebo (55 participants, 1 trial)
Macotela‐Ruiz 1993 compared 200 µg/kg bodyweight oral ivermectin with placebo.
Treatment failure in clinically diagnosed cases
Macotela‐Ruiz 1993 reported fewer treatment failures in the ivermectin group at seven days (RR 0.24, 95% CI 0.12 to 0.51; 55 participants, Analysis 1.1). Figure 1.
1.1. Analysis.
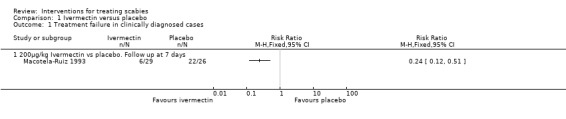
Comparison 1 Ivermectin versus placebo, Outcome 1 Treatment failure in clinically diagnosed cases.
1.

Forest plot of comparison: 1 Ivermectin versus placebo, outcome: 1.1 Treatment failure in clinically diagnosed cases.
Adverse events.
None were reported.
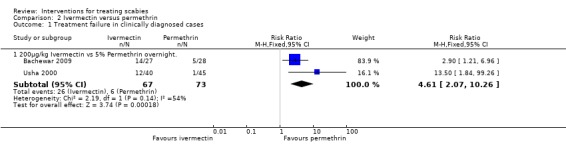
1.2. Versus permethrin (153 participants, 2 trials)
Usha 2000 and Bachewar 2009 both compared 200 µg/kg bodyweight oral ivermectin with 5% topical permethrin cream.
Treatment failure in clinically diagnosed cases
Usha 2000 reported more treatment failures in the ivermectin group at two weeks (RR 13.50, 95% CI 1.84 to 99.26; 85 participants), as did Bachewar 2009 at one week follow up (RR 2.90, 95% CI 1.21 to 6.96; 55 participants, Analysis 2.1). Significant heterogeneity was not detected and the trials' combined estimate showed more treatment failures with ivermectin (RR 4.61, 95% CI 2.07 to 10.26, fixed‐effect model; 140 participants). Figure 2.
2.
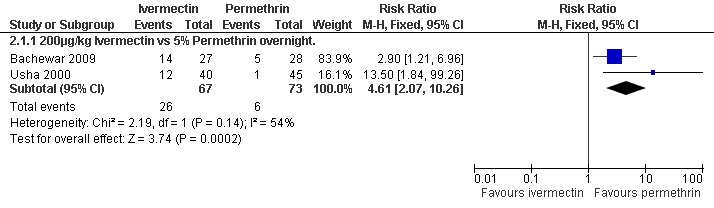
Forest plot of comparison: 2 Ivermectin versus permethrin, outcome: 2.1 Treatment failure in clinically diagnosed cases.
Adverse events
Three of 43 participants in the ivermectin group in the Usha 2000 trial reported aggravation of symptoms. No adverse events were reported in Bachewar 2009 (see Table 17).
2. Adverse events.
| Comparison | Trial | Adverse event | Intervention | n/Na | Intervention | n/Na |
| Ivermectin vs placebo | Macotela‐Ruiz 1993 | None recorded | Ivermectin | — | Placebo | — |
| Ivermectin vs permethrin | Usha 2000 | Aggravation of symptoms | Ivermectin | 3/43 | Permethrin | 0/45 |
| Bachewar 2009 | None recorded | Ivermectin | — | Permethrin | — | |
| Ivermectin vs lindane | Chouela 1999 | Headache | Ivermectin | 1/26 | Lindane | 6/27 |
| Headache | Ivermectin | 1/26 | Lindane | 0/27 | ||
| Hypotension | Ivermectin | 1/26 | Lindane | 0/27 | ||
| Abdominal pain | Ivermectin | 1/26 | Lindane | 0/27 | ||
| Vomiting | Ivermectin | 1/26 | Lindane | 0/27 | ||
| Madan 2001 | Severe headache | Ivermectin | 1/100 | Lindane | 0/100 | |
| Ivermectin vs benzyl benzoate | Glaziou 1993 | Mild increase in pruritus | Ivermectin | 0/23 | Benzyl benzoate | 5/21 |
| Nnoruka 2001 | Pruritus and irritation | Ivermectin | 0/29 | Benzyl benzoate | 7/29 | |
| Brooks 2002 | Pustular rash | Ivermectin | 3/43 | Benzyl benzoate | 0/37 | |
| Cellulitis | Ivermectin | 1/43 | Benzyl benzoate | 0/37 | ||
| Burning or stinging | Ivermectin | 0/43 | Benzyl benzoate | 6/37 | ||
| Dermatitis | Ivermectin | 0/43 | Benzyl benzoate | 6/37 | ||
| Bachewar 2009 | None recorded | Ivermectin | — | Benzyl benzoate | — | |
| Ly 2009 | Abdominal pain | Ivermectin | 5/65 | Benzyl benzoate | 0/116 | |
| Mild diarrhoea | Ivermectin | 2/65 | Benzyl benzoate | 0/116 | ||
| Irritant dermatitis | Ivermectin | 0/65 | Benzyl benzoate | 30/116 | ||
| Permethrin vs crotamiton | Taplin 1990 | Worsening of symptoms | Permethrin | 0/48 | Crotamiton | 10/48 |
| Amer 1992 | None recorded | Permethrin | — | Crotamiton | — | |
| Permethrin vs lindane | Hansen 1986 | Mild burning, stinging, or itching | Permethrin | 5/49 | Lindane | 5/50 |
| Taplin 1986 | None recorded | Permethrin | — | Lindane | — | |
| Schultz 1990 | Burning/stinging | Permethrin | 23/234 | Lindane | 12/233 | |
| Pruritus | Permethrin | 15/234 | Lindane | 17/233 | ||
| Erythema | Permethrin | 5/234 | Lindane | 3/233 | ||
| Tingling | Permethrin | 4/234 | Lindane | 5/233 | ||
| Rash | Permethrin | 2/234 | Lindane | 2/233 | ||
| Diarrhoea | Permethrin | 1/234 | Lindane | 1/233 | ||
| Persistent excoriation | Permethrin | 1/234 | Lindane | 0/233 | ||
| Contact dermatitis | Permethrin | 0/234 | Lindane | 1/233 | ||
| Phemphigus | Permethrin | 0/234 | Lindane | 1/233 | ||
| Papular rash | Permethrin | 0/234 | Lindane | 1/233 | ||
| Amer 1992 | None recorded | Permethrin | — | Lindane | — | |
| Zargari 2006 | Skin irritation | Permethrin | 2/59 | Lindane | 1/58 | |
| Permethrin versus benzyl benzoate | Bachewar 2009 | None recorded | Permethrin | — | Benzyl benzoate | — |
| Permethrin vs synergized natural pyrethrins | Amerio 2003 | Secondary skin infection | Permethrin | 10/20 | Synergized pyrethrins | 2/20 |
| Crotamiton vs lindane | Amer 1992 | None recorded | Lindane | — | Crotamiton | — |
| Lindane vs sulfur | Singalavanija 2003 | Foul odour | Lindane | 3/50 | Sulfur | 10/50 |
| Burning | Lindane | 6/50 | Sulfur | 2/50 | ||
| Erythema | Lindane | 5/50 | Sulfur | 2/50 | ||
| Benzyl benzoate vs sulfur | Gulati 1978 | None recorded | Benzyl benzoate | — | Sulfur | — |
| Benzyl benzoate vs synergized natural pyrethrins | Biele 2006 | Skin irritation and burning sensations | Benzyl benzoate | 22/120 | Synergized pyrethrins | 3/120 |
| Lindane: short vs long application | Maggi 1986 | None recorded | Lindane (short course) | — | Lindane (long course) | — |
| Decamethrin: 2‐day + 2‐day vs 4‐day application | Schenone 1986 | Moderate skin hotness | Decamethrin (both regimens) | 15/127 | ||
| Sulfur: pork fat vehicle vs cold cream vehicle | Avila‐Romay 1991 | Pruritus | Sulfur/salicylic acid in pork fat | 32/53 | Sulfur in cold cream | 18/58 |
| Xerosis | Sulfur/salicylic acid in pork fat | 18/53 | Sulfur in cold cream | 14/58 | ||
| Burning sensations | Sulfur/salicylic acid in pork fat | 9/53 | Sulfur in cold cream | 6/58 | ||
| Keratosis pilaris | Sulfur/salicylic acid in pork fat | 8/53 | Sulfur in cold cream | 0/58 | ||
| Erythema | Sulfur/salicylic acid in pork fat | 1/53 | Sulfur in cold cream | 6/58 | ||
| Keratosis follicularis | 0/53 | Sulfur in cold cream | 1/58 | |||
aNo. participants reporting event/total no. participants.
1.3. Versus lindane (253 participants, 2 trials)
Chouela 1999 compared 150 µg/kg bodyweight oral ivermectin with 1% topical lindane, while Madan 2001 compared 200 µg/kg ivermectin with 1% lindane.
Treatment failure in clinically diagnosed cases
Chouela 1999 found no significant difference between the groups at 15 days (43 participants), while at four weeks Madan 2001 found that treatment failures were reduced in the ivermectin group (RR 0.31, 95% CI 0.18 to 0.54; 150 participants, Analysis 3.1). Heterogeneity was not detected and the trials' combined estimate showed a benefit of ivermectin over lindane (RR 0.36, 95% CI 0.23 to 0.58, fixed‐effect model; 193 participants). Figure 3.
3.1. Analysis.
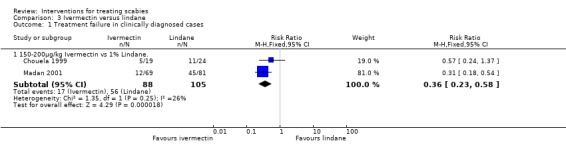
Comparison 3 Ivermectin versus lindane, Outcome 1 Treatment failure in clinically diagnosed cases.
3.
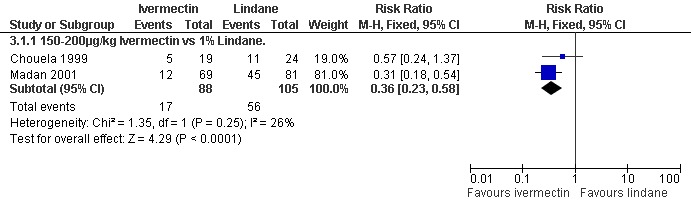
Forest plot of comparison: 3 Ivermectin versus lindane, outcome: 3.1 Treatment failure in clinically diagnosed cases.
Adverse events
See Table 17. Chouela 1999 reported adverse events in 4/26 participants in the ivermectin group (headache, hypotension, abdominal pain, and vomiting) and in 6/37 participants in the lindane group (headache). Madan 2001 reported an adverse event in 1/100 participants in the ivermectin group (severe headache); there were none in the lindane group.
1.4. Versus benzyl benzoate (462 participants, 5 trials)
Brooks 2002 compared 200 µg/kg bodyweight oral ivermectin with 10% topical benzyl benzoate. Glaziou 1993 compared 100 µg/kg bodyweight ivermectin with 10% benzyl benzoate. Nnoruka 2001 and Bachewar 2009 compared 200 µg/kg bodyweight ivermectin with 25% benzyl benzoate. Ly 2009 compared 150 to 200 µg/kg bodyweight ivermectin with 12.5% benzyl benzoate.
Treatment failure in clinically diagnosed cases
See Analysis 4.1. No significant difference between the two groups was found in Bachewar 2009 at one week follow up (52 participants). After 14 days Ly 2009 found a significant difference in favour of benzyl benzoate compared with ivermectin (RR 2.00, 95% CI 1.47 to 2.72; 162 participants). No significant difference between the two groups was found in Brooks 2002 at three weeks (80 participants) or by Glaziou 1993 at 30 days (44 participants). At 30 days Nnoruka 2001 found a significant difference in favour of ivermectin (RR 0.13, 95% CI 0.03 to 0.53; 58 participants). Heterogenity was detected between the trials (Chi2 = 27.97, df = 4, P < 0.0001; I2 = 86%; see Figure 4 for forest plot). The differences in drug regimen and length of follow up that exist between the five trials may explain this heterogeneity.
4.1. Analysis.
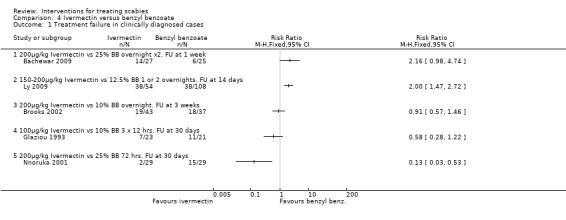
Comparison 4 Ivermectin versus benzyl benzoate, Outcome 1 Treatment failure in clinically diagnosed cases.
4.
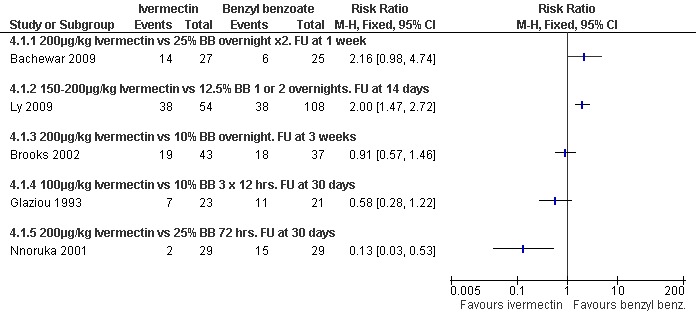
Forest plot of comparison: 4 Ivermectin versus benzyl benzoate, outcome: 4.1 Treatment failure in clinically diagnosed cases.
Itch persistence
See Analysis 4.2. Brooks 2002 found no significant difference in the number of participants who reported night‐time itch at three weeks (58 participants). Figure 5.
4.2. Analysis.
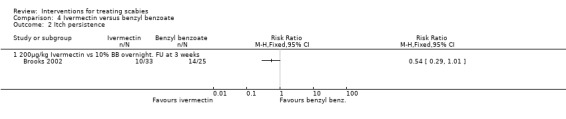
Comparison 4 Ivermectin versus benzyl benzoate, Outcome 2 Itch persistence.
5.

Forest plot of comparison: 4 Ivermectin versus benzyl benzoate, outcome: 4.2 Itch persistence.
Adverse events
See Table 17. Brooks 2002 reported adverse events in 4/43 participants in the ivermectin group (pustular rash, cellulitis) and in 12/37 participants in the benzyl benzoate group (burning or stinging, dermatitis). Glaziou 1993 and Nnoruka 2001 reported adverse events only in the benzyl benzoate group: 5/21 participants (mild increase in pruritus) in Glaziou 1993; and 7/29 participants (pruritus and irritation) in Nnoruka 2001. Ly 2009 reported adverse events in 7/65 participants in the ivermectin group (abdominal pain, diarrhoea) and in 30/116 participants in the benzyl benzoate groups. Bachewar 2009 reported no adverse events.
2. Permethrin
2.1. Versus crotamiton (196 participants, 2 trials)
Two trials compared 5% permethrin with 10% crotamiton (Taplin 1990; Amer 1992). In Taplin 1990 the drugs were applied for 8 to 10 hours, whereas in Amer 1992 the drugs were applied overnight on two consecutive days.
Treatment failure
See Analysis 5.1 and Analysis 5.2. Participants in both trials had their scabies clinically diagnosed and microscopically confirmed. The comparative treatment failure rates described for clinically diagnosed cases therefore apply equally to microscopically diagnosed cases in these trials. Taplin 1990 found that treatment failure was reduced in the permethrin group after 28 days (RR 0.26, 95% CI 0.11 to 0.65; 94 participants, Analysis 5.1.3). Amer 1992 found no significant difference in outcome between the groups after 28 days (100 participants). Heterogeneity was not detected and a combined estimate showed a benefit of permethrin over crotamiton (RR 0.24, 95% CI 0.10 to 0.55, fixed‐effect analysis; 194 participants). Figure 6 and Figure 7.
5.1. Analysis.
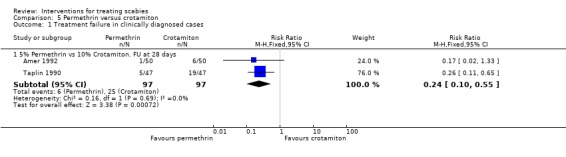
Comparison 5 Permethrin versus crotamiton, Outcome 1 Treatment failure in clinically diagnosed cases.
5.2. Analysis.
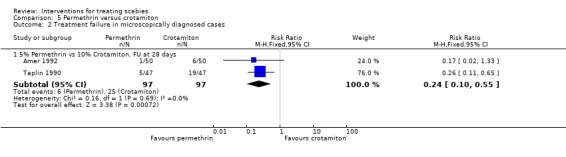
Comparison 5 Permethrin versus crotamiton, Outcome 2 Treatment failure in microscopically diagnosed cases.
6.
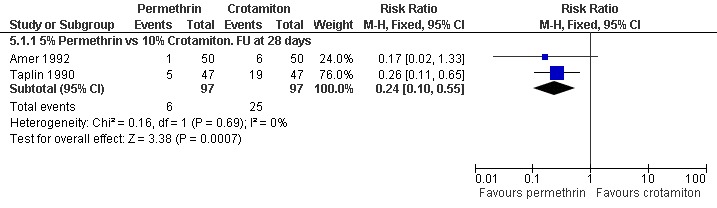
Forest plot of comparison: 5 Permethrin versus crotamiton, outcome: 5.1 Treatment failure in clinically diagnosed cases.
7.
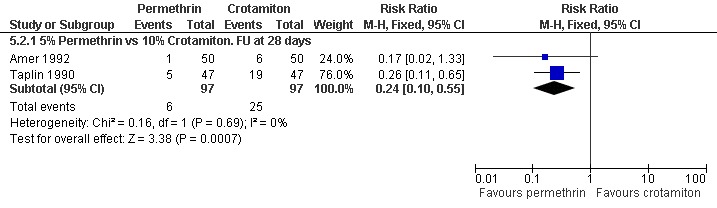
Forest plot of comparison: 5 Permethrin versus crotamiton, outcome: 5.2 Treatment failure in microscopically diagnosed cases.
Itch persistence
See Analysis 5.3. Permethrin reduced the number of participants with itch persistence in Taplin 1990 (RR 0.26, 95% CI 0.11 to 0.65; 94 participants). Figure 8.
5.3. Analysis.
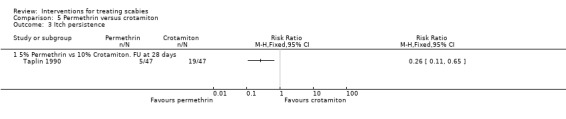
Comparison 5 Permethrin versus crotamiton, Outcome 3 Itch persistence.
8.

Forest plot of comparison: 5 Permethrin versus crotamiton, outcome: 5.3 Itch persistence.
Adverse events
See Table 17. Taplin 1990 reported no adverse events in the permethrin group, but did report adverse events in 10/47 participants in the crotamiton group (worsening of symptoms). Amer 1992 reported no adverse events.
2.2. Versus lindane (835 participants, 5 trials)
Five trials compared 5% topical permethrin with 1% topical lindane (Hansen 1986; Taplin 1986; Schultz 1990; Amer 1992; Zargari 2006).
Treatment failure in clinically diagnosed cases
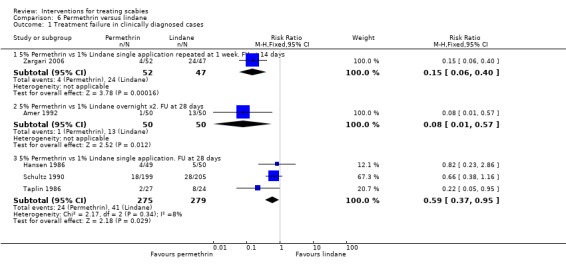
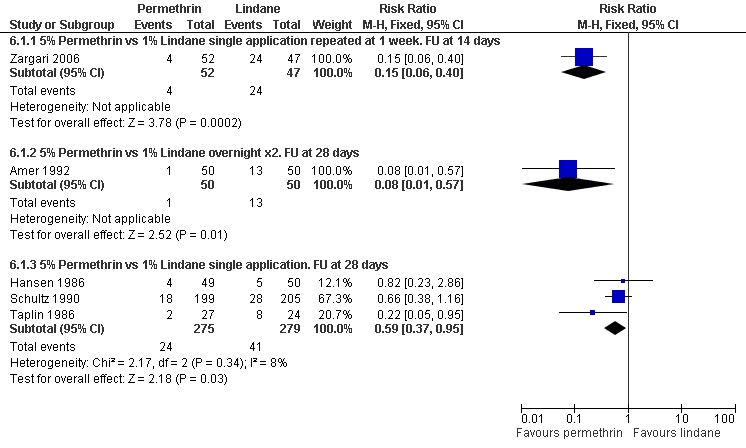
See Analysis 6.1. Zargari 2006 reported fewer treatment failures in the permethrin group after 14 days (RR 0.15, 95% CI 0.06 to 0.40; 99 participants). At 28 days Amer 1992 found two consecutive overnight applications of permethrin to be superior to two consecutive overnight applications of lindane (RR 0.08, 95% CI 0.01 to 0.57; 100 participants). Three trials compared a single application of permethrin with a single application of lindane, with follow up at 28 days (Hansen 1986, Schultz 1990 and Taplin 1986). No benefit was found for either treatment by Hansen 1986 (28 days, 99 participants) or Schultz 1990 (28 +/‐ 7 days, 404 participants), whereas Taplin 1986 found permethrin to be superior (RR 0.22, 95% CI 0.05 to 0.95; 51 participants).
6.1. Analysis.
Comparison 6 Permethrin versus lindane, Outcome 1 Treatment failure in clinically diagnosed cases.
Heterogenity was detected between the results of the five studies (Chi2 = 11.83, df = 4, P = 0.02; I2 = 66%; see Figure 9 for forest plot) so the trials were grouped by drug regimen and length of follow up in order to explore causes of heterogeneity. The pooled effect for the three trials sharing the same drug regimen (single application) and length of follow up (four weeks) showed a significant effect in favour of permethrin (RR 0.59, 95% CI 0.37 to 0.95, fixed‐effect model; 554 participants). Statistical heterogeneity was not detected in this group of three trials.
9.
Forest plot of comparison: 6 Permethrin versus lindane, outcome: 6.1 Treatment failure in clinically diagnosed cases.
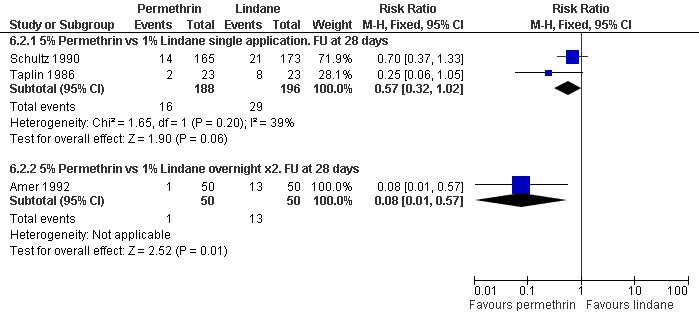
Treatment failure in microscopically confirmed cases
See Analysis 6.2. Two consecutive overnight applications of permethrin was found to be superior to two consecutive overnight applications of lindane after 28 days in Amer 1992 (RR 0.08, 95% CI 0.01 to 0.57; 100 participants). Taplin 1986 (46 participants) and Schultz 1990 (338 participants) both compared single applications of permethrin and lindane with follow up at 28 days. Neither trial showed a significant difference between the interventions. Figure 10.
6.2. Analysis.
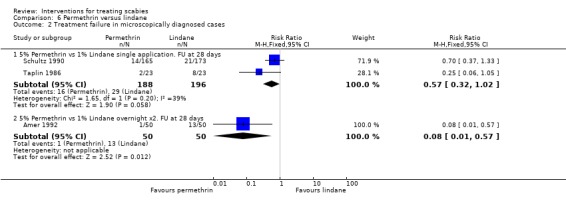
Comparison 6 Permethrin versus lindane, Outcome 2 Treatment failure in microscopically diagnosed cases.
10.
Forest plot of comparison: 6 Permethrin versus lindane, outcome: 6.2 Treatment failure in microscopically diagnosed cases.
Itch persistence
See Analysis 6.3. The two trials that reported on itch persistence found different effects: Hansen 1986 found no significant difference between the two interventions after 28 days (99 participants), whereas Schultz 1990 found permethrin to be superior after 28 +/‐ 7 days (RR 0.56, 95% CI 0.37 to 0.86; 391 participants). Heterogeneity was not detected and a combined estimate showed permethrin to be superior (RR 0.61, 95% CI 0.44 to 0.86, fixed effects model; 490 participants). Figure 11.
6.3. Analysis.
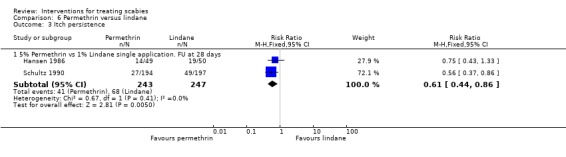
Comparison 6 Permethrin versus lindane, Outcome 3 Itch persistence.
11.
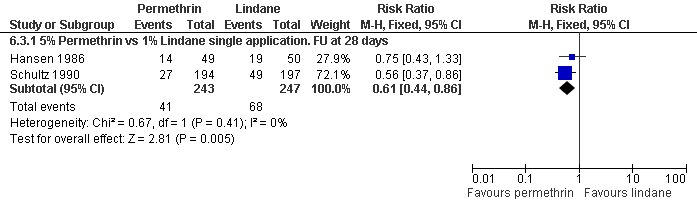
Forest plot of comparison: 6 Permethrin versus lindane, outcome: 6.3 Itch persistence.
Adverse events
See Table 17. Hansen 1986 recorded mild burning, stinging, or itching in both groups (5/49 participants in the permethrin group, 5/50 participants in the lindane group). Schultz 1990 reported adverse events in 51/234 participants in the permethrin group (burning/stinging, pruritus, erythema, tingling, rash, diarrhoea, persistent excoriation) and in 43/233 participants in the lindane group (burning/stinging, pruritus, tingling, erythema, rash, papular rash, diarrhoea, contact dermatitis, phemphigus). Zargari 2006 reported skin irritation in both groups (2/59 participants in the permethrin group, 1/58 participant in the lindane group). Amer 1992 and Taplin 1986 both reported no adverse events.
2.3 Versus benzyl benzoate (69 participants, 1 trial)
Treatment failure in clinically diagnosed cases
See Analysis 7.1. In Bachewar 2009 there was no significant difference in treatment failure between the two groups after one week (RR 0.74, 95% CI 0.26 to 2.14; 53 participants). Figure 12.
7.1. Analysis.
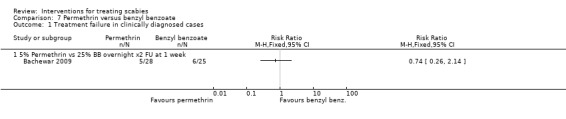
Comparison 7 Permethrin versus benzyl benzoate, Outcome 1 Treatment failure in clinically diagnosed cases.
12.

Forest plot of comparison: 7 Permethrin versus benzyl benzoate, outcome: 7.1 Treatment failure in clinically diagnosed cases.
Adverse events
No adverse events were reported in Bachewar 2009.
2.4. Versus synergized natural pyrethrins (40 participants, 1 trial)
Amerio 2003 compared 5% topical permethrin with topical 0.16% natural pyrethrins synergized with 1.65% pyperonil butoxide.
Treatment failure
All participants had their scabies both clinically diagnosed and microscopically confirmed. There were no treatment failures in either group after 28 days (40 participants).
Itch persistence
See Analysis 8.1.There was no significant difference in itch persistence between the two groups after 28 days (40 participants). Figure 13.
8.1. Analysis.
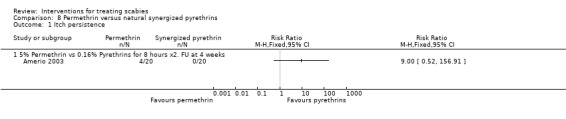
Comparison 8 Permethrin versus natural synergized pyrethrins, Outcome 1 Itch persistence.
13.

Forest plot of comparison: 8 Permethrin versus natural synergized pyrethrins, outcome: 8.1 Itch persistence.
Adverse events
See Table 17. Ten of the 20 participants in the permethrin group and two of the 20 participants in the synergized pyrethrin group were reported as having secondary skin infections requiring antibiotic treatment. It was not clear from the trial report whether this was considered an adverse event or rather a baseline characteristic.
3. Other drug comparisons
3.1. Crotamiton versus lindane (100 participants, 1 trial)
Amer 1992 compared 10% topical crotamiton with 1% topical lindane.
Treatment failure
See Analysis 9.1 and Analysis 9.2. All participants in Amer 1992 had their scabies both clinically diagnosed and microscopically confirmed. There was no significant difference in treatment failure between the two groups after 28 days (100 participants). Figure 14 and Figure 15.
9.1. Analysis.
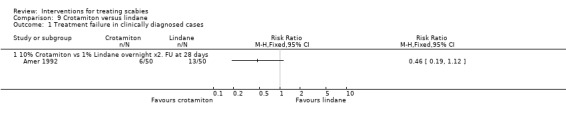
Comparison 9 Crotamiton versus lindane, Outcome 1 Treatment failure in clinically diagnosed cases.
9.2. Analysis.
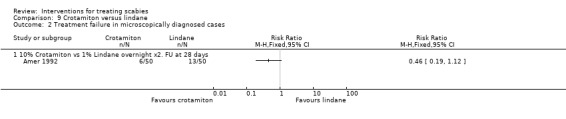
Comparison 9 Crotamiton versus lindane, Outcome 2 Treatment failure in microscopically diagnosed cases.
14.

Forest plot of comparison: 9 Crotamiton versus lindane, outcome: 9.1 Treatment failure in clinically diagnosed cases.
15.

Forest plot of comparison: 9 Crotamiton versus lindane, outcome: 9.2 Treatment failure in microscopically diagnosed cases.
Adverse events
None were reported.
3.2. Lindane versus sulfur (100 participants, 1 trial)
Singalavanija 2003 compared 0.3% topical lindane with 10% topical sulfur.
Treatment failure in clinically diagnosed cases
See Analysis 10.1. There was no significant difference between the two groups after 28 days in Singalavanija 2003 (68 participants). Figure 16.
10.1. Analysis.
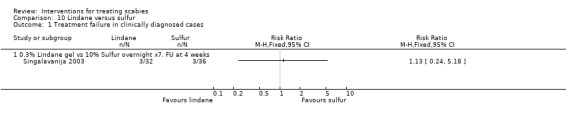
Comparison 10 Lindane versus sulfur, Outcome 1 Treatment failure in clinically diagnosed cases.
16.

Forest plot of comparison: 10 Lindane versus sulfur, outcome: 10.1 Treatment failure in clinically diagnosed cases.
Itch persistence
See Analysis 10.2. There was no significant difference between the groups in the number of participants in whom itch persisted at 28 days (68 participants). Figure 17.
10.2. Analysis.
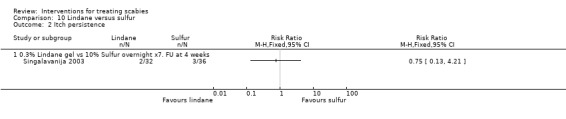
Comparison 10 Lindane versus sulfur, Outcome 2 Itch persistence.
17.

Forest plot of comparison: 10 Lindane versus sulfur, outcome: 10.2 Itch persistence.
Adverse events
See Table 17. The reported adverse events (foul odour, burning, erythema) occurred in the sulfur group (14/50 participants) and the lindane group (14/50 participants).
3.3. Benzyl benzoate versus sulfur (158 participants, 1 trial)
Gulati 1978 compared 25% topical benzyl benzoate with topical sulfur ointment.
Treatment failure in clinically diagnosed cases
See Analysis 11.1. There was no significant difference between the two groups after 15 days in Gulati 1978 (158 participants). Figure 18.
11.1. Analysis.
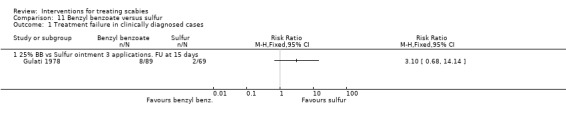
Comparison 11 Benzyl benzoate versus sulfur, Outcome 1 Treatment failure in clinically diagnosed cases.
18.

Forest plot of comparison: 11 Benzyl benzoate versus sulfur, outcome: 11.1 Treatment failure in clinically diagnosed cases.
Adverse events
None were reported.
3.4. Benzyl benzoate versus synergized natural pyrethrins (240 participants, 1 trial)
Biele 2006 compared 10% topical benzyl benzoate with topical 0.165% natural pyrethrins synergized with 1.65% pyperonil butoxide.
Treatment failure
See Analysis 12.1 and Analysis 12.2. All participants had their scabies both clinically diagnosed and microscopically confirmed. There was no significant difference in treatment failure between the two groups after four weeks in Biele 2006 (240 participants). Figure 19 and Figure 20.
12.1. Analysis.
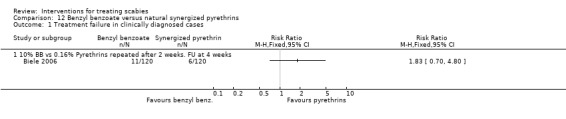
Comparison 12 Benzyl benzoate versus natural synergized pyrethrins, Outcome 1 Treatment failure in clinically diagnosed cases.
12.2. Analysis.
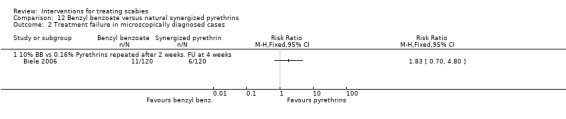
Comparison 12 Benzyl benzoate versus natural synergized pyrethrins, Outcome 2 Treatment failure in microscopically diagnosed cases.
19.

Forest plot of comparison: 12 Benzyl benzoate versus natural synergized pyrethrins, outcome: 12.1 Treatment failure in clinically diagnosed cases.
20.

Forest plot of comparison: 12 Benzyl benzoate versus natural synergized pyrethrins, outcome: 12.2 Treatment failure in microscopically diagnosed cases.
Itch persistence
See Analysis 12.3. There was no significant difference in itch persistence between the two groups after four weeks (240 participants). Figure 21.
12.3. Analysis.
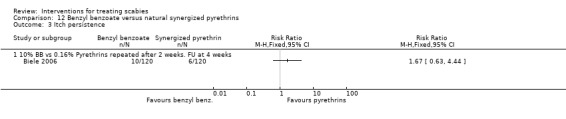
Comparison 12 Benzyl benzoate versus natural synergized pyrethrins, Outcome 3 Itch persistence.
21.

Forest plot of comparison: 12 Benzyl benzoate versus natural synergized pyrethrins, outcome: 12.3 Itch persistence.
Adverse events
Twenty‐two of the 120 participants in the benzyl benzoate group and three of the 120 participants in the synergized natural pyrethrins group experienced skin irritation and burning sensations after drug application (see Table 17).
4. Length of treatment comparisons
4.1. Benzyl benzoate: one overnight application versus two overnight applications (116 participants, 1 trial)
Treatment failure in clinically diagnosed cases.
There was no significant difference in treatment failure between the two groups after 14 days in Ly 2009 (108 participants, Analysis 13.1). Figure 22.
13.1. Analysis.
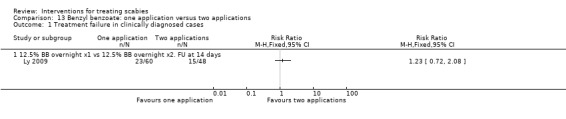
Comparison 13 Benzyl benzoate: one application versus two applications, Outcome 1 Treatment failure in clinically diagnosed cases.
22.

Forest plot of comparison: 13 Benzyl benzoate: one application versus two applications, outcome: 13.1 Treatment failure in clinically diagnosed cases.
Adverse events
Irritant dermatitis was reported in 30 out of 116 participants (see Table 17).
4.2. Lindane: short application versus long application (87 participants, 1 trial)
Treatment failure
Maggi 1986 did not assess this outcome measure.
Itch persistence
A short application of lindane reduced itch persistence at 14 days (RR 0.34, 95% CI 0.12 to 0.98; 87 participants, Analysis 14.1). However, the trial authors did suggest that the pruritus experienced by the participants could have been due to a lindane‐associated contact dermatitis. Figure 23.
14.1. Analysis.
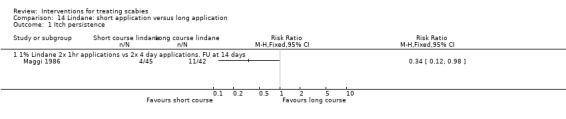
Comparison 14 Lindane: short application versus long application, Outcome 1 Itch persistence.
23.
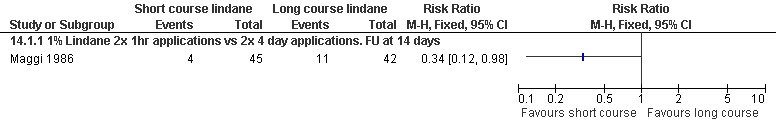
Forest plot of comparison: 14 Lindane: short application versus long application, outcome: 14.1 Itch persistence.
Adverse events
None other than pruritus (see above) were reported.
4.3. Decamethrin: two‐day plus two‐day application versus four‐day application (127 participants, 1 trial)
Treatment failure in clinically diagnosed cases.
There were no treatment failures in either group in Schenone 1986 after 21 days: 0/53 treatment failures in the two‐plus‐two‐day group; and 0/74 treatment failures in the four‐day group. Five participants in each group received a second treatment at seven days due to the presence of active lesions. This second treatment consisted of two applications of 0.02% decamethrin on consecutive days.
Adverse events
Fifteen of 127 participants experienced "moderate skin hotness" after application of decamethrin (see Table 17).
5. Drug vehicle comparisons
5.1. Sulfur: pork fat vehicle versus cold cream vehicle (51 participants, 1 trial)
Treatment failure in clinically diagnosed cases
See Analysis 15.1. There was no significant difference in the number of treatment failures between the two groups after 10 days in Avila‐Romay 1991 (51 participants). Figure 24.
15.1. Analysis.
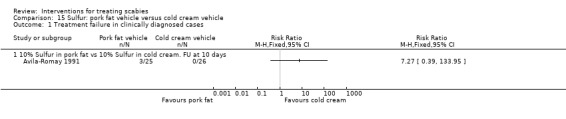
Comparison 15 Sulfur: pork fat vehicle versus cold cream vehicle, Outcome 1 Treatment failure in clinically diagnosed cases.
24.

Forest plot of comparison: 15 Sulfur: pork fat vehicle versus cold cream vehicle, outcome: 15.1 Treatment failure in clinically diagnosed cases.
Adverse events
See Table 17. Pruritus, xerosis, burning sensation, and erythema were reported for cases and contacts in both groups. There were adverse events in 68/53 participants in the pork fat vehicle group, including keratosis pilaris. There were adverse events in 45/58 participants in the cold cream vehicle group, including keratosis follicularis.
Discussion
The review's objective was to evaluate the effectiveness of current treatments for scabies in order to inform practice and guide future research. The previous version of this review noted that clinicians faced considerable uncertainty when choosing the best treatment for scabies (Walker 2000). Ten years later the picture is a little clearer, but there are still considerable gaps in our knowledge.
Trial quality
All 22 included trials were designed to test the effectiveness of one or more treatments for scabies. Methodological quality varied between trials. Only two trials described both adequate randomization sequence generation and adequate allocation concealment, and the majority of the reports described neither adequately. The blinding was absent, or the degree of blinding was unclear in ten of the 22 identified trials, and losses to follow up were greater than 20% of the enrolled participants in four of the trials.
Effectiveness
The results of this review suggest that, of the topical treatments for scabies, permethrin is most effective. Permethrin has been tested against topical crotamiton, topical lindane, and oral ivermectin in randomized controlled trials, and it appears to be superior to all three in terms of minimizing treatment failure in participants with a clinical diagnosis of scabies. In the one trial that tested permethrin against topical benzyl benzoate no difference in cure rate was detected, however this trial was small (53 participants) and the data used in the review related only to one week follow up.
In the subgroup of participants with microscopically confirmed scabies, permethrin was again superior to crotamiton, but there is uncertainty as to whether permethrin is superior to lindane. Permethrin also appears to be better at relieving itch than either crotamiton or lindane (itch was not reported as a separate outcome in the ivermectin versus permethrin trial). Unfortunately no trials comparing permethrin with either topical sulfur or topical malathion were identified; permethrin's relative effectiveness against these treatments therefore remains unknown.
In some countries natural pyrethrin‐based topical treatments are available as an alternative to permethrin cream (Biele 2006). Pyrethrins are naturally occurring insecticidal compounds found in the Compositae family of plants (Wagner 2000), whereas permethrin is a synthetic pyrethroid analogue. Results from the two Italian trials included in this review suggest that pyrethrin is equivalent in effectiveness to both permethrin and benzyl benzoate.
Trials comparing crotamiton with lindane, lindane with sulfur, and sulfur with benzyl benzoate have all produced equivocal results, suggesting that there is no single most effective treatment out of these four topical options. In most countries the choice is in any case restricted, either due to lack of availability, or the lack of a licence for scabies.
Ivermectin is currently the only oral treatment for scabies that is in routine use. It appears to be more effective than both placebo and lindane, but less effective than permethrin. There was significant heterogeneity in the results of the five trials that compared ivermectin and benzyl benzoate, which may be explained by differences in drug regimen and length of follow up between the trials. After stratifying by drug regimen and length of follow up the relative effectiveness of ivermectin appeared to increase with increasing length of follow up. This may mean that ivermectin is slower in achieving cure than topical benzyl benzoate, however, this conclusion is rather speculative given these data.
An advantage of an oral antiscabietic treatment over a topical one is ease of use, particularly in hot humid climates, when engaging in mass treatment, or when treating children. However, ivermectin is not presently licensed for the treatment of scabies in most countries. Ivermectin's effectiveness, cost effectiveness, and safety in mass treatment in areas of high endemicity (preferably as a sustainable public health intervention) need to be further evaluated in larger trials of sufficient power.
Topical ivermectin has also been suggested to be effective after success in uncontrolled studies (Yeruham 1998; Victoria 2001). At present there is no commercially available topical ivermectin preparation available for the treatment of scabies, and randomized controlled trials are needed to evaluate this potential new treatment option.
There are still no published reports of randomized controlled trials that test the effectiveness of malathion against either placebo or another drug, despite over 30 years passing by since a non‐controlled study first suggested that the drug was effective (Hanna 1978). The 2010 British National Formulary recommends malathion as the treatment of choice if permethrin is inappropriate (BNF 2010), despite the lack of evidence from randomized controlled trials. Such a trial comparing malathion with permethrin is needed to test their relative effectiveness.
We found trials of the herbal remedies toto soap (Alebiosu 2003) and lippia oil (Oladimeji 2000; Oladimeji 2005), but these trials did not meet the review's inclusion criteria. Both treatments look promising, but randomized controlled trials making direct comparisons with the existing best treatments are needed to assess their true relative effectiveness.
Treatment regimen was assessed in two trials. Maggi 1986 found that a one‐hour application of lindane reduced itch compared with a much longer four‐day application; the authors suggested that the itch may, at least in part, have been due to a dermatitis caused by the lindane treatment itself. Schenone 1986 compared two different regimens using decamethrin, a pyrethroid insecticide in the same class as permethrin. All participants were cured in both groups. Decamethrin is not commercially available for the treatment of scabies and we found no trials that tested its effectiveness against other treatments. Decamethrin (as deltamethrin) is usually used as an agricultural insecticide and its safety as an antiscabietic medication has not been established (WHO 1990).
The formulation of a topically applied product may influence its efficacy. For example, a 1% permethrin formulation marketed for the treatment of head lice appears to be less effective than the conventional antiscabietic 5% preparation, according to case reports (Cox 2000). None of the trials included in this review directly compared different strength formulations of the same treatment.
One trial compared different vehicles for the same drug (Avila‐Romay 1991). Cold cream as a treatment vehicle for sulfur may be more effective than pork fat, with fewer adverse events. For resource‐poor countries this could be a cheap and safe option, which in some circumstances might also be more culturally acceptable.
This review did not seek to assess the relative cost effectiveness of the various treatments for scabies; however, large cost differences are apparent. In the UK, costs are: permethrin £5.51 per 30 g of cream, benzyl benzoate £0.50 per 100 mL, crotamiton £2.99 per 100 mL, and malathion £2.96 per 100 mL (BNF 2010). When lindane was marketed in the UK it was a fifth the cost of permethrin per treatment (BNF 1997).
We did not specifically attempt to assess the effectiveness of treatments for crusted scabies, and none of the included trials selected participants with this diagnosis. Caution should therefore be exercised in generalizing the results of this review to the treatment of patients with atypical severe scabies infection. This is an important area where more research is needed.
Caution should also be exercised in generalizing these results, which were obtained from trials that recruited individual participants (mostly in the outpatient setting), to the management of outbreaks in institutions. Given the burden of disease caused by scabies within institutions, such as long‐term healthcare facilities, the inclusion of such patients in randomized controlled trials of effectiveness would be beneficial.
Mass treatment of a community in order to eradicate scabies has been tested in two studies (Dunne 1991; Bockarie 2000), both of which used oral ivermectin. Unfortunately neither of these studies met the review's inclusion criteria (Bockarie 2000 was an uncontrolled trial, and Dunne 1991 recruited participants on the basis of a diagnosis of onchocerciasis). Further research is needed to test the effectiveness, safety, and practicality of this approach to the management of scabies, particularly in areas of high prevalence.
Safety
Serious adverse events leading to death or permanent disability were not reported in any of the included or excluded trials. This review did not seek to systematically review the literature on the safety of antiscabietic treatments, but a number of notable reports of serious adverse events that have been published elsewhere are discussed below.
Convulsions and aplastic anaemia have both been reported with the use of lindane (Rauch 1990; Elgart 1996); in some cases this being thought to be due to the application of the drug to non‐intact skin. Lindane was withdrawn by the manufacturer from the UK market in 1996, but this was for commercial and not toxicological reasons. In 1995, the US Food and Drug Administration designated lindane as a second‐line treatment due to its potential toxicity; only to be used in those who have failed to respond to, or who are intolerant of, other antiscabietic treatments (WHO 2003).
Ivermectin has been very widely used in the treatment of onchocerciasis (predominantly in adults) and even with repeated doses serious adverse effects have been rare (DeSole 1989; Pacque 1990). However, an increased risk of death among a group of elderly patients with scabies in a long‐term care facility has been reported (Barkwell 1997). Whether this was due to ivermectin or to interactions with other scabicides, including lindane and permethrin, or other treatments such as psychoactive drugs was not clear and there was considerable discussion of the validity of this report (Bredal 1997; Coyne 1997; Diazgranados 1997; Reintjes 1997).
Rare adverse reactions have been reported with the use of both permethrin (dystonia, Coleman 2005) and natural pyrethrin (fatal asthma, Wagner 2000).
The relative purity of the active ingredients of certain topical treatments and their isomeric ratios may also affect drug toxicity. In particular, very little is known about the effects of exposure to different isomeric grades of permethrin. Clinical grade material is 25:75 cis isomer:trans isomer and agricultural grade is 40:60. The cis isomer has 10 times the acute toxicity and there could be dangers in people in resource‐poor countries using agricultural‐grade permethrin for treating human infestations (personal communication from Ian Burgess, Medical Entomology Centre, Cambridge). Similar problems have been reported with the inappropriate use of agricultural grade malathion for treating human infestations (Petros 1990).
A search of the WHO Adverse Drug Reaction Database in 1998 for a previous version of this review found reports of serious adverse drug reactions for convulsions (benzyl benzoate 4, crotamiton 1, lindane 38, malathion 2, permethrin 6) and death (benzyl benzoate 0, crotamiton 1, lindane 1, malathion 0, permethrin 5) (Walker 2000). A search for this update of the review of the UK Medicines and Healthcare Products Regulatory Agency database of suspected drug reactions found reports for convulsions (benzyl benzoate 1, crotamiton 0, lindane 3, malathion 0, permethrin 0, sulfur 0, ivermectin 1) and death (benzyl benzoate 0, crotamiton 0, lindane 1, malathion 0, permethrin 1 (intra uterine death), sulfur 0, ivermectin 3) (MHRA 2006). Extreme caution must be shown in interpreting these reports, as they are clearly influenced by the extent to which the products are used and by the quality of the reporting. Neither can a causal link be assumed for any of the reported events.
Authors' conclusions
Implications for practice.
On the basis of the available evidence from randomized controlled trials, topical permethrin appears to be the most effective treatment for scabies. Ivermectin appears to be an effective oral treatment, but in many countries it is not licensed for this indication.
Implications for research.
Trials are needed to evaluate the relative effectiveness of malathion against permethrin, and the relative effectiveness of herbal treatments against existing treatments. The effectiveness of topical ivermectin also needs to be explored. The most appropriate treatment for the severe crusted form of scabies has not yet been established in randomized controlled trials.
Researchers should ensure that toxicity and safety outcomes are systematically collected in future trials as well as being notified through routine monitoring of adverse events in clinical practice.
Approaches to the control of outbreaks in institutions and public health programmes to control scabies in populations with high prevalence require evaluation.
What's new
| Date | Event | Description |
|---|---|---|
| 11 August 2010 | New search has been performed | Two new trials Bachewar 2009 and Ly 2009 added. Trials now stratified according to drug regimen and length of follow up if heterogeneity detected. Removal of meta‐analyses where heterogeneity detected. Minor changes to conclusion regarding effectiveness of ivermectin compared with benzyl benzoate. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 4, 1997
| Date | Event | Description |
|---|---|---|
| 18 August 2008 | Amended | Converted to new review format with minor editing. |
| 30 April 2007 | New citation required and conclusions have changed | 2007, Issue 3: A substantive update with new authors. We included nine new trials and excluded two studies (Dunne 1991 and Macotela‐Ruiz 1996) included in Walker 2000 after re‐evaluation, as noted in the 'Characteristics of excluded studies'. The review has been rewritten and reformatted throughout, and the conclusions of the review have been updated to reflect the new trial evidence. We used more precise definitions in the 'Types of interventions' and separated the 'Types of outcome measures' into primary, secondary, and adverse events. Treatment failure in those clinically diagnosed and treatment failure in those microscopically confirmed are considered as separate outcome measures, while parasitological cure is no longer an outcome measure. We reformatted the search strategy section, but did not attempt to systematically search literature for adverse events. For data analysis, we used relative risks rather than odds ratios, and used a random‐effects model for meta‐analysis if significant heterogeneity was present. We used available‐case analyses rather than intention‐to‐treat analyses using imputed data. |
| 1 February 2006 | New search has been performed | New studies found and included or excluded. |
| 1 January 2000 | New search has been performed | 2000, Issue 3: Revised, synopsis added, and updated with new studies (Walker 2000). |
| 1 January 1999 | New search has been performed | 1999, Issue 3: Revised and updated with new studies (Walker 1999b). |
| 1 January 1999 | New search has been performed | Revised with new title 'Interventions for treating scabies' (Walker 1999a). |
Acknowledgements
We are heavily indebted to Dr Godfrey Walker who, with Paul Johnstone, co‐authored the previous version (Walker 2000). The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINE/EMBASEb | LILACSb | INDMED |
| 1 | scabies | scabies | scabies | scabies | scabies |
| 2 | — | Sarcoptes scabiei | SCABIES | treatment | Sarcoptes scabiei |
| 3 | — | 1 or 2 | 1 or 2 | 1 and 2 | 1 or 2 |
| 4 | — | — | treatment | — | — |
| 5 | — | — | benzyl benzoate | — | — |
| 6 | — | — | crotamiton | — | — |
| 7 | — | — | lindane | — | — |
| 8 | — | — | malathion | — | — |
| 9 | — | — | permethrin | — | — |
| 10 | — | — | ivermectin | — | — |
| 11 | — | — | sulphur | — | — |
| 12 | — | — | hexachlorocyclohexane | — | — |
| 13 | — | — | gamma benzene hexachloride | — | — |
| 14 | — | — | 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 | — | — |
| 15 | — | — | 3 and 14 | — | — |
| 16 | — | — | Limit 15 to human | — | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2006); upper case: MeSH or EMTREE heading; lower case: free text term.
Data and analyses
Comparison 1. Ivermectin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 200μg/kg Ivermectin vs placebo. Follow up at 7 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Ivermectin versus permethrin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 200μg/kg Ivermectin vs 5% Permethrin overnight. | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.61 [2.07, 10.26] |
2.1. Analysis.
Comparison 2 Ivermectin versus permethrin, Outcome 1 Treatment failure in clinically diagnosed cases.
Comparison 3. Ivermectin versus lindane.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 150‐200μg/kg Ivermectin vs 1% Lindane. | 2 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.23, 0.58] |
Comparison 4. Ivermectin versus benzyl benzoate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 200μg/kg Ivermectin vs 25% BB overnight x2. FU at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 150‐200μg/kg Ivermectin vs 12.5% BB 1 or 2 overnights. FU at 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 200μg/kg Ivermectin vs 10% BB overnight. FU at 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 100μg/kg Ivermectin vs 10% BB 3 x 12 hrs. FU at 30 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 200μg/kg Ivermectin vs 25% BB 72 hrs. FU at 30 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Itch persistence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 200μg/kg Ivermectin vs 10% BB overnight. FU at 3 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Permethrin versus crotamiton.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 5% Permethrin vs 10% Crotamiton. FU at 28 days | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 2 Treatment failure in microscopically diagnosed cases | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 5% Permethrin vs 10% Crotamiton. FU at 28 days | 2 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.10, 0.55] |
| 3 Itch persistence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 5% Permethrin vs 10% Crotamiton. FU at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Permethrin versus lindane.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 5% Permethrin vs 1% Lindane single application repeated at 1 week. FU at 14 days | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.06, 0.40] |
| 1.2 5% Permethrin vs 1% Lindane overnight x2. FU at 28 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.57] |
| 1.3 5% Permethrin vs 1% Lindane single application. FU at 28 days | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.37, 0.95] |
| 2 Treatment failure in microscopically diagnosed cases | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 5% Permethrin vs 1% Lindane single application. FU at 28 days | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.32, 1.02] |
| 2.2 5% Permethrin vs 1% Lindane overnight x2. FU at 28 days | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.57] |
| 3 Itch persistence | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 5% Permethrin vs 1% Lindane single application. FU at 28 days | 2 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.44, 0.86] |
Comparison 7. Permethrin versus benzyl benzoate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 5% Permethrin vs 25% BB overnight x2 FU at 1 week | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Permethrin versus natural synergized pyrethrins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Itch persistence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 5% Permethrin vs 0.16% Pyrethrins for 8 hours x2. FU at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. Crotamiton versus lindane.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 10% Crotamiton vs 1% Lindane overnight x2. FU at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment failure in microscopically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 10% Crotamiton vs 1% Lindane overnight x2. FU at 28 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Lindane versus sulfur.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 0.3% Lindane gel vs 10% Sulfur overnight x7. FU at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Itch persistence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 0.3% Lindane gel vs 10% Sulfur overnight x7. FU at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Benzyl benzoate versus sulfur.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 25% BB vs Sulfur ointment 3 applications. FU at 15 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. Benzyl benzoate versus natural synergized pyrethrins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 10% BB vs 0.16% Pyrethrins repeated after 2 weeks. FU at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment failure in microscopically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 10% BB vs 0.16% Pyrethrins repeated after 2 weeks. FU at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Itch persistence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 10% BB vs 0.16% Pyrethrins repeated after 2 weeks. FU at 4 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. Benzyl benzoate: one application versus two applications.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 12.5% BB overnight x1 vs 12.5% BB overnight x2. FU at 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Lindane: short application versus long application.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Itch persistence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 1% Lindane 2x 1hr applications vs 2x 4 day applications. FU at 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Sulfur: pork fat vehicle versus cold cream vehicle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure in clinically diagnosed cases | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 10% Sulfur in pork fat vs 10% Sulfur in cold cream. FU at 10 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amer 1992.
| Methods | Design: randomized controlled trial Generation of allocation sequence: "according to code" Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 150 enrolled (all ages; sex not stated) Inclusion criteria: clinically diagnosed and microbiologically confirmed scabies Exclusion criteria: significant impetiginization |
|
| Interventions | 1. 5% permethrin (50 participants)
2. 10% crotamiton (50 participants)
3. 1% lindane (50 participants) Each medication applied "neck to toe" on 2 successive nights |
|
| Outcomes | 1. Number of participants clinically cured (no new lesions and all original lesions healed) at 28 days | |
| Notes | Location: Egypt Date: not stated Colour photographs used for comparison before and after treatment |
|
Amerio 2003.
| Methods | Design: randomized controlled trial Generation of allocation sequence: computer generated Allocation concealment: phone call‐based procedure Blinding: investigators only Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 40 enrolled (mean age 44, standard deviation 17; 19 males, 21 females) Inclusion criteria: immunocompetent; aged 18 to 75; microscopically confirmed uncomplicated scabies Exclusion criteria: HIV positive; severe renal failure; liver insufficiency; acute or chronic leukaemia; lymphoma; use of antiscabietic preparations in previous 30 days; pregnancy; breastfeeding |
|
| Interventions | 1. 5% permethrin cream (20 participants)
2. 0.16% natural pyrethrins synergized with pyperonil butoxide (1.65%) in thermolabile foam ("Milice", Mipharm, Italy) (20 participants) Both medications applied to entire body surface except head for 8 h overnight on 2 consecutive days, and then same treatment repeated after 14 days |
|
| Outcomes | 1. Number of participants with clearance of lesions at 4 weeks
2. Number of participants with complete relief of itching at 4 weeks Not included in this review: 3. Number of participants with clearance of lesions at 2 weeks 4. Number of participants with complete relief of itching at 2 weeks 5. Clinical grading score (semi‐quantitative measure of numbers of lesions) at 2 and 4 weeks 6. Itching score at 2 and 4 weeks 7. Numbers of days taking antihistamine drugs 8. Numbers of participants with secondary skin infection |
|
| Notes | Location: Italy Date: March 2001 to October 2001 Trial supported by unrestricted grant from Mipharm SpA |
|
Avila‐Romay 1991.
| Methods | Design: randomized controlled trial Generation of allocation sequence: "randomly assigned" Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 51 cases and 60 contacts enrolled (children 6 to 17 years old; sex not stated) Inclusion criteria: clinically compatible lesions associated with itching Exclusion criteria: secondary infection |
|
| Interventions | 1. 10% sulfur in pork fat with 1% salicylic acid as preservative (25 cases and 28 contacts)
2. 10% sulfur in cold cream (26 cases and 32 contacts) Both medications applied nightly for 3 nights then once 3 nights later, average dose 7 g Both medications applied by the patients from shoulders to feet for about 5 minutes, under supervision of a physician |
|
| Outcomes | 1. Number of participants clinically cured at 10 days (defined as absence of cutaneous lesions and itching)
2. Secondary cutaneous reactions in cases and contacts Not included in this review: 3. Patient preference (not further defined) |
|
| Notes | Location: Mexico; participants from a house for orphan children Date: not stated 60 contacts also randomly assigned to treatment with sulfur in either pork fat or cold cream |
|
Bachewar 2009.
| Methods | Design: randomized controlled trial Generation of allocation sequence: computer generated Allocation concealment: unclear Blinding: none Inclusion of randomized participants in the analysis: 78% (23/103 lost to follow up) |
|
| Participants | Number: 103 enrolled (aged over 12; 63 males, 40 females) Inclusion criteria: clinically diagnosed scabies Exclusion criteria: pregnancy; lactation; women of child bearing age; abnormal liver or kidney function; thyroid disease; cardiac disorders; nervous system disorders; psychiatric illness; diabetes mellitus; hypertension; chronic infectious disease; any concurrent medication; consuming tobacco, alcohol, or any substance of abuse; any other associated skin disease which could alter the picture of scabies; known/suspected immunocompromised individuals; having scabies with atypical presentations including crusted scabies and scabies incognito; any antiscabetic treatment in the preceding week; noncompliant participants. |
|
| Interventions | 1. 25% benzyl benzoate lotion applied to whole body below neck and left overnight, on 2 consecutive nights (35 participants) 2. 5% permethrin cream applied to whole body below neck and left overnight (34 participants) 3. Oral ivermectin 200 µg/kg bodyweight single dose (34 participants) Not included in this review: 4. Second topical application of 25% benzyl benzoate lotion at 1 week for treatment failures in intervention group 1 (benzyl benzoate) 5. Second topical application of 5% permethrin cream at 1 week for treatment failures in intervention group 2 (permethrin) 6. Second dose of oral ivermectin at 1 week for treatment failures in intervention group 3 (ivermectin) |
|
| Outcomes | 1. Number of participants cured at 1 week (defined as absence of new papules, vesicles or classical burrows) 2. Adverse events Not included in this review: 3. Number of participants cured at 2 weeks (defined as absence of new papules, vesicles or classical burrows) 4. Itching recorded on visual analogue scale |
|
| Notes | Location: Nagpur, India Date: March to July 2007 All family members and close contacts treated at same time as the participant with 25% benzyl benzoate lotion |
|
Biele 2006.
| Methods | Design: randomized controlled trial Generation of allocation sequence: computer generated Allocation concealment: unclear Blinding: investigators Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 240 enrolled (aged 18 to 75 years, mean age 31 years (pyrethrin group) and 30 years (benzyl benzoate); males only)
Inclusion criteria: clinically diagnosed and microscopically confirmed scabies Exclusion criteria: treatment for scabies within previous 15 days; renal failure (plasma creatinine > 2.5 mg/dL); liver insufficiency (alanine aminotransferase or aspartate aminotransferase > 3 upper normal limit); acute or chronic leukaemia or lymphoma |
|
| Interventions | 1. 10% benzyl benzoate lotion ("SCAB", PentaMedical, Milan, Italy), topical application on 5 consecutive days (120 participants)
2. 0.165% natural pyrethrins synergized with pyperonil butoxide (1.65%) in thermolabile foam ("Milice", Mipharm, Italy), topical application on 3 consecutive days (120 participants) Both treatments were applied to all skin surfaces from scalp to soles of feet Treatment was repeated after 2 weeks if participant was not considered clinically cured |
|
| Outcomes | 1. Number of participants clinically cured at 4 weeks
2. Number of participants with relief of itching at 4 weeks
3. Adverse events Not included in this review: 4. Number of participants with clearance of lesions at 2 weeks 5. Clinical grading score (semi‐quantitative measure of numbers of lesions) at 4 weeks 6. Itching score at 4 weeks |
|
| Notes | Location: Italy Date: October 2003 to July 2004 |
|
Brooks 2002.
| Methods | Design: randomized controlled trial Generation of allocation sequence: computer generated Allocation concealment: unclear Blinding: investigators Inclusion of randomized participants in the analysis: 73% (30/110 lost to follow up) |
|
| Participants | Number: 110 enrolled (children 6 months to 14 years old; sex not stated) Inclusion criteria: clinically diagnosed scabies Exclusion criteria: treatment for scabies within previous 2 months; major intercurrent illness; history of meningitis or neurological illness |
|
| Interventions | 1. Oral ivermectin 200 µg/kg bodyweight single dose (55 participants) 2. 10% benzyl benzoate applied neck to toe overnight (55 participants) | |
| Outcomes | 1. Number of participants clinically cured at 3 weeks (defined as absence of skin lesions)
2. Number of participants with persistence of night‐time itch at 3 weeks
3. Adverse events Not included in this review 4. Itch severity 5. Numbers of lesions |
|
| Notes | Location: Vanuatu Date: January to April 2001 Family contacts treated with same drug as the participant Author confirmed equal numbers of participants randomized to each intervention |
|
Chouela 1999.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: participants (study described as double blind) Inclusion of randomized participants in the analysis: 81% (10/53 participants lost to follow up or withdrew) |
|
| Participants | Number: 53 enrolled (aged over 18 years with a mean age of 40.8 years; 19 males, 34 females) Inclusion criteria: clinical or parasitological signs compatible with scabies Exclusion criteria: pregnancy; breastfeeding; treatment for scabies within previous 4 weeks; renal dysfunction; hepatic dysfunction; concomitant antidepressant; anxiolytic or antipruritic drug use; severe immunodeficiency; HIV infection; clinically high risk for HIV; neoplasia affecting immunity; immunosuppressive treatment; gastrointestinal dysfunction; history of convulsions |
|
| Interventions | 1. Single dose of oral ivermectin, 150 to 200 µg/kg in 6 mg tablets plus single topical application of 60 mL placebo solution (26 participants)
2. Single topical application of 60 mL 1% lindane topical solution plus placebo tablets (27 participants) Both placebo and 1% lindane solutions applied neck to toe and kept on for 8 h Not included in this review: 3. Second dose of oral ivermectin, 150 to 200 µg/kg in 6 mg tablets plus single topical application of 60 mL placebo solution at 15 days for treatment failures in intervention group 1 (ivermectin) 4. Second topical application of 60 mL 1% lindane topical solution plus placebo tablets at 15 days for treatment failures in intervention group 2 (lindane) |
|
| Outcomes | 1. Number of participants cured at 15 days (defined as absence of pruritus and clinical lesions or a reduction of signs and symptoms to a score of 1 (mild pruritus and mild lesions))
2. Adverse events Not included in this review 3. Number of participants receiving second dose at 15 days who were cured at 29 days |
|
| Notes | Location: Argentina Date: April 1996 to February 1997 Members of the same household who were infested but could not be included in the study treated with 1% lindane (adults) or 6% sulfur cream (infants) |
|
Glaziou 1993.
| Methods | Design: randomized controlled trial Generation of allocation sequence: "randomly allocated" Allocation concealment: unclear Blinding: outcomes assessor Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 44 enrolled (aged 5 to 56 years, mean 17.5 years; 23 males, 21 females) Inclusion criteria: clinically diagnosed scabies defined as the association of pruritus with at least 1 classical burrow Exclusion criteria: other disease; pregnancy; abnormal physical examination (except for cutaneous lesions); abnormal laboratory screen; refused consent |
|
| Interventions | 1. Oral ivermectin 100 µg/kg bodyweight single dose (23 participants) 2. 10% benzyl benzoate applied to entire body except head on 3 occasions 12 h apart (21 participants) | |
| Outcomes | 1. Number of participants clinically cured at 30 days (defined as complete disappearance of initial lesions and pruritus)
2. Adverse events Not included in this review: 3. Number of participants clinically cured at 7 days 4. Number of participants clinically cured at 14 days 5. Mean clinical score (based on number and activity of lesions) |
|
| Notes | Location: French Polynesia Date: 1992 All household contacts treated at same time as the participant with 10% benzyl benzoate Merck Sharp and Dohme supplied the ivermectin tablets at no cost |
|
Gulati 1978.
| Methods | Design: randomized controlled trial Generation of allocation sequence: "cases ... divided at random" Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 158 enrolled (mean age 16.6 years; 75 males, 83 females) Inclusion criteria: clinical diagnosis of scabies Exclusion criteria: none stated |
|
| Interventions | 1. 25% benzyl benzoate emulsion (89 participants)
2. Sulfur ointment (69 participants) Both medications "applied all over the body after a thorough scrub bath with soap and water once in the morning, then again at night and again the next morning" Treatments were repeated in those whose lesions persisted after the 10th day |
|
| Outcomes | 1. Number of participants with clinically assessed "clearance of lesions" at 15 days Not included in this review: 2. Numbers of participants with clearance of lesions at 3 to 5, 6 to 8, 9 to 11, and 12 to 14 days 3. Number of days until clearance of lesions |
|
| Notes | Location: India Date: not stated Family contacts treated concurrently with same drug as the participant 33% of participants had secondarily infected lesions Prevalence of scabies in this study was 158/1727 (9.1%) |
|
Hansen 1986.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear, "single blind" Inclusion of randomized participants in the analysis: 95% (5/104 lost to follow up) |
|
| Participants | Number: 104 enrolled (aged 2 to 71 years) Inclusion criteria: clinical and/or microscopic diagnosis of scabies Exclusion criteria: none stated |
|
| Interventions | 1. 1% lindane lotion (50 participants)
2. 5% permethrin lotion (49 participants) Both medications applied as a single application |
|
| Outcomes | 1. Number of participants with absence of lesions at 28 days
2. Number of participants with persistence of pruritus at 28 days
3. Adverse events Not included in this review: 4. Number of participants with absence of lesions at 14 days |
|
| Notes | Location: not stated Date: not stated Data taken from a conference abstract |
|
Ly 2009.
| Methods | Design: randomized controlled trial Generation of allocation sequence: random number table Allocation concealment: unclear Blinding: none Inclusion of randomized participants in the analysis: 90% (19/181 lost to follow up) |
|
| Participants | Number: 181 enrolled (mean age 16.5 years; 116 males, 65 females) Inclusion criteria: clinical diagnosis of scabies Exclusion criteria: pruritus due to insect bites; chickenpox in participant or member of participant's family; treatment for scabies within previous month; under 5 years or over 65 years of age; weight less than 15 kg; pregnancy; breastfeeding; use of bleaching products for cosmetic purposes; crusted scabies; diabetes; hypertension; cardiovascular disease; neurological disease; living outside of Dakar district |
|
| Interventions | 1. Oral ivermectin 150‐200 µg/kg bodyweight single dose (65 participants) 2. 12.5% benzyl benzoate to whole body except head (single application left on for 24 hours, 68 participants; two consecutive 24 hour applications, 48 participants) Not included in this review 4. Second dose of oral ivermectin at 14 days for treatment failures in intervention group 1 (ivermectin) 5. Second single application of 12.5% benzyl benzoate at 14 days for treatment failures in intervention group 2 (benzyl benzoate single application) 6. Second double application of 12.5% benzyl benzoate at 14 days for treatment failures in intervention group 3 (benzyl benzoate double application) |
|
| Outcomes | 1. Number of participants cured at 14 days (defined as complete disappearance of visible lesions and itching) 2. Adverse events Not included in this review 3. Number of participants cured at 28 days 4. Number of participants with bacterial superinfection 5. Compliance with medication regimen |
|
| Notes | Location: Dakar, Senegal Date: July 2003 to September 2004 |
|
Macotela‐Ruiz 1993.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: participants and outcomes assessor Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 55 enrolled (aged over 5 years;18 males mean age 25 +/‐ 4 years, 37 females mean age 24 +/‐ 16 years) Inclusion criteria: clinical diagnosis of scabies Exclusion criteria: pregnancy; breastfeeding; impaired renal function; impaired liver function; treatment for scabies within previous 3 weeks |
|
| Interventions | 1. Oral ivermectin 200 µg/kg bodyweight single dose (29 participants) 2. Placebo (26 participants) | |
| Outcomes | 1. Number of participants clinically cured at 7 days (defined as absence of itching and no dermatologically active lesions) 2. Adverse events | |
| Notes | Location: Mexico Date: not stated Trial stopped at 7 days as ivermectin group significantly clinically better |
|
Madan 2001.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: outcomes assessor Inclusion of randomized participants in the analysis: 75% (50/200 lost to follow up) |
|
| Participants | Number: 200 enrolled (aged over 5 years; 132 males, 68 females) Inclusion criteria: clinical diagnosis of scabies (defined as nocturnal itching and/or family contact with similar complaint and/or typical lesions) Exclusion criteria: pregnancy; breastfeeding; severe cardiovascular, respiratory, or central nervous system disorders |
|
| Interventions | 1. Oral ivermectin 200 µg/kg bodyweight single dose (100 participants) 2. 1% lindane lotion applied neck to toe and left on overnight (100 participants) | |
| Outcomes | 1. Number of participants clinically cured at 4 weeks (defined as no signs or symptoms of scabies)
2. Adverse events Not included in this review: 3. Number of participants clinically cured at 2 weeks 4. Number of patients with good improvement at 4 weeks |
|
| Notes | Location: India Date: not stated Microscopic confirmation of diagnosis in 170/200 (85%) of participants Family contacts treated with 25% benzyl benzoate lotion for 3 days |
|
Maggi 1986.
| Methods | Design: randomized controlled trial Generation of allocation sequence: "randomly selected" Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 87 enrolled (children, age range not stated) Inclusion criteria: scabies, not further explained Exclusion criteria: pyodermatitis |
|
| Interventions | 1. 1% lindane suspension applied topically from chin to feet; 2 x 1‐h applications 7 days apart (45 participants) 2. 1% lindane suspension applied topically from chin to feet; 2 series of 4 daily applications, 7 days apart (42 participants) | |
| Outcomes | 1. Number of participants with absence of pruritus at 14 days Not included in this review: 2. Number of participants with absence of pruritus at 7 days 3. Numbers of participants with excoriations or burrows at days 7 and 14 |
|
| Notes | Location: Chile Date: March to November 1985 |
|
Nnoruka 2001.
| Methods | Design: randomized controlled trial Generation of allocation sequence: random‐number table Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 58 enrolled (aged 5 to 63 years, mean 27.9 years; 35 males, 33 females) Inclusion criteria: clinically diagnosed scabies (microbiologically confirmed in 43/58) Exclusion criteria: aged < 5 years | |
| Interventions | 1. Oral ivermectin 200 µg/kg bodyweight single dose (29 participants) 2. 25% benzyl benzoate emulsion applied neck to toe and left for 72 h (29 participants) | |
| Outcomes | 1. Number of participants clinically cured at 30 days (defined as complete disappearance of initial lesions and pruritus)
2. Adverse events Not included in this review: 3. Number of participants clinically cured at 7 days 4. Number of participants clinically cured at 14 days 5. Response of pruritus (graded on subjective scale) at 7, 14, and 30 days 6. Mean clinical score (based on number and activity of lesions) |
|
| Notes | Location: Nigeria Date: June 1998 All household contacts treated at same time as the participant (treatment not stated) |
|
Schenone 1986.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 127 enrolled (aged 4 to 19 years; 53 males, 74 females) Inclusion criteria: clinical diagnosis of scabies Exclusion criteria: none stated |
|
| Interventions | 1. 40 mL of 0.02% decamethrin lotion, applied everywhere except skull and face, daily for 2 days, and repeated on 2 more days 1 week later (53 participants) 2. 40 mL of 0.02% decamethrin lotion, applied everywhere except skull and face, daily for 4 days (74 participants) | |
| Outcomes | 1. Number of participants clinically cured at 21 days (defined as no active lesions) | |
| Notes | Location: Chile (18 boarding schools in Santiago) Date: 1985 Prevalence amongst boarding school children (aged 4 to 19): 127/868 (14.6%) Contacts treated with single dose of 0.02% decamethrin |
|
Schultz 1990.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: medication supplied to each trial centre in identical coded boxes Blinding: outcomes assessor Inclusion of randomized participants in the analysis: 87% (63/467 participants not analysed (for primary outcome)) |
|
| Participants | Number: 467 enrolled (aged 2 months to 75 years, mean age 22.1 years; 297 males, 170 females) Inclusion criteria: clinical diagnosis of scabies Exclusion criteria: pregnancy; breastfeeding; treatment with ectoparasiticide within previous 3 weeks; renal impairment; hepatic impairment; known allergy to permethrin or lindane |
|
| Interventions | 1. 5% permethrin cream applied to entire body below ears, single application (234 participants) 2. 1% lindane lotion applied from neck down, single application (233 participants) | |
| Outcomes | 1. Number of participants clinically cured at 28 +/‐ 7 days (defined as all original lesions healed and no new lesions)
2. Number of participants with persistence of itch
3. Adverse events Not included in this review: 4. Number of participants clinically cured at 14 +/‐ 3 days 5. Number of microbiologically confirmed cases clinically cured |
|
| Notes | Location: USA and Mexico (4 sexually transmitted diseases clinics, 2 dermatology clinics, and 2 family practice clinics, 1 of which was in Mexico and all others in USA) Date: not stated Personal contacts of 85% of participants provided with 1% lindane for their use Study supported in part by a grant from Burroughs Wellcome (manufacturers of permethrin) who also provided statistical assistance |
|
Singalavanija 2003.
| Methods | Design: randomized controlled trial Generation of allocation sequence: random‐number table Allocation concealment: unclear Blinding: unclear Inclusion of randomized participants in the analysis: 68% (32/100 participants lost to follow up) |
|
| Participants | Number: 100 enrolled (aged 6 months to 13 years; 60 males, 40 females) Inclusion criteria: clinically diagnosed and microbiologically confirmed scabies Exclusion criteria: resident in an orphanage; serious central nervous system illness; malnutrition; immunodeficiency |
|
| Interventions | 1. 10% sulfur ointment (50 participants)
2. 0.3% lindane gel (50 participants) Both medications applied neck to toe by parents for 7 consecutive nights |
|
| Outcomes | 1. Number of participants clinically cured (no new lesions and healing of all old lesions) at 4 weeks
2. Number of participants with decrease or absence of itching at 4 weeks
3. Adverse events Not included in this review: 4. Number of participants clinically cured at 2 weeks (defined as no new lesions and healing of all old lesions) 5. Number of participants with decrease or absence of itching at 2 weeks 6. Number of participants with absence of parasites on skin scraping at 2 and 4 weeks |
|
| Notes | Location: Thailand Date: December 1999 to May 2000 Contacts treated with either 25% benzyl benzoate (adults) or 10% sulfur (children) |
|
Taplin 1986.
| Methods | Design: randomized controlled trial Generation of allocation sequence: "randomized code" Allocation concealment: identical coded medication tubes; codes held by sponsor Blinding: investigators Inclusion of randomized participants in the analysis: 98% (1/52 participant lost to follow up) |
|
| Participants | Number: 52 enrolled (aged 2 to 40 years, mean age 9 years; 22 males, 29 females, 1 gender not stated) Inclusion criteria: clinically diagnosed scabies (confirmed microscopically in 46/52 cases) Exclusion criteria: unwell; febrile; taking any medication; treatment with pediculicides, scabicides, or other topical agent in previous 3 months |
|
| Interventions | 1. 5% permethrin cream (27 participants)
2. 1% lindane lotion (25 participants) Both medications applied as a single application head to toe |
|
| Outcomes | 1. Number of participants with no new lesions and healing of all original lesions at 1 month
2. Adverse events Not included in this review: 3. Number of participants with no new lesions and healing of all original lesions at 2 weeks |
|
| Notes | Location: Panama Date: not stated All family contacts treated with 1% lindane lotion Photographs taken before and after treatment and distribution of any lesions noted on diagrams Study supported in part by a grant from Burroughs Wellcome (manufacturers of permethrin) |
|
Taplin 1990.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: "medications supplied in identical ... tubes that were coded and randomized" Blinding: investigators Inclusion of randomized participants in the analysis: 98% (2/96 participants lost to follow up) |
|
| Participants | Number: 96 enrolled (aged 2 months to 5 years; 42 males, 54 females) Inclusion criteria: clinical diagnosis and the recovery of at least 1 live mite Exclusion criteria: none stated |
|
| Interventions | 1. 10% crotamiton cream (48 participants)
2. 5% permethrin cream (48 participants) Both medications applied as single application from head to toe and left for 8 to 10 h |
|
| Outcomes | 1. Number of participants with no new lesions and all original active lesions healed at 28 days
2. Number of participants with persistence of pruritus at 28 days
3. Adverse events Not included in this review: 4. Number of participants with no new lesions and all original active lesions healed at 14 days 5. Number of participants with persistence of pruritus at 14 days |
|
| Notes | Location: Panama Date: 1985 Household contacts were treated with 5% permethrin cream 65/96 (68%) participants had secondary cutaneous infection Study supported in part by a grant from Burroughs Wellcome (manufacturers of permethrin) |
|
Usha 2000.
| Methods | Design: randomized controlled trial Generation of allocation sequence: computer‐generated random‐number table Allocation concealment: investigators did not take part in allocation Blinding: none Inclusion of randomized participants in the analysis: 100% |
|
| Participants | Number: 88 enrolled (aged over 5 years with a mean age of 21.3 years (ivermectin) and 22.4 years (permethrin); 59 males, 26 females) Inclusion criteria: clinical diagnosis (3 out of burrow/lesions in classical sites/nocturnal itch/family history) or microscopic diagnosis Exclusion criteria: pregnancy; breastfeeding; treatment for scabies within previous 1 month; serious central nervous system, hepatic, cardiac, or renal disease |
|
| Interventions | 1. Oral ivermectin 200 µg/kg bodyweight single dose (43 participants)
2. 5% permethrin cream applied topically overnight (45 participants) Not included in this review 3. Second dose of oral ivermectin, 200 µg/kg for treatment failures in intervention group 1 (12 participants) 4. Second topical application 5% permethrin cream for treatment failures in intervention group 2 (1 participant) |
|
| Outcomes | 1. Number of participants clinically cured at 2 weeks (defined as symptom improvement)
2. Adverse events Not included is this review: 3. Number of participants clinically cured at 1, 4, and 8 weeks |
|
| Notes | Location: India Date: August 1996 to December 1997 Contacts treated with same drug as the index case, except contacts who were children under 5 or pregnant women; these were treated with 12.5% to 25% benzyl benzoate emulsion Author confirmed randomization method and blinding 3 participants in ivermectin group withdrawn due to using additional treatment |
|
Zargari 2006.
| Methods | Design: randomized controlled trial Generation of allocation sequence: unclear Allocation concealment: "drugs ... packaged in identical appearing tubes and randomized and coded by the manufacturer" Blinding: participants and investigators Inclusion of randomized participants in the analysis: 84.6% (18/117 lost to follow up) |
|
| Participants | Number: 117 enrolled (aged 6 to 64 years, mean age 30.2 years +/‐ 15.3; 55 males and 44 females followed up) Inclusion criteria: clinically diagnosed scabies (defined as burrow or typical lesions at classical sites plus nocturnal pruritus plus similar symptoms in contacts) and/or microscopically diagnosed scabies (demonstration of egg, larvae, mite, or faecal material) Exclusion criteria: < 5 years of age; treatment with antiscabietic medication or topical steroid in previous 4 weeks; pregnancy; breastfeeding; severe central nervous system, hepatic, or renal problems |
|
| Interventions | 1. 5% permethrin cream (59 participants)
2. 1% lindane cream (58 participants) Both medications applied as a single application head to toe, and repeated 1 week later |
|
| Outcomes | 1. Number of participants with no new lesions and improvement in itching at 14 days 2. Adverse events | |
| Notes | Location: Iran Date: December 2002 to October 2003 Treatment advised for all family members and close contacts Study supported by Gilaranco Company (manufacturers of permethrin and lindane) |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abedin 2007 | Non‐randomized study |
| Alebiosu 2003 | Allocation method inadequate; expressed preference of participants for different interventions taken into account |
| Amer 1981 | Non‐randomized study |
| Bockarie 2000 | Non‐controlled study |
| Burgess 1986 | Non‐randomized study |
| Cannon 1948 | Non‐controlled study |
| Chowdhury 1977 | Non‐controlled study |
| Cubela 1978 | Non‐randomized study |
| Curiati 1984 | Non‐randomized study |
| Damodaran 1979 | A trial of iron and folic acid supplementation |
| Daneshpajooh 2000 | Unclear if randomized |
| Dika 2006 | Non‐controlled study |
| Dourmishev 1998 | Non‐controlled study |
| Dunne 1991 | Study participants selected on basis of having onchocerciasis rather than scabies |
| Gallegos 1996 | Thesis unavailable |
| Gordon 1944 | Non‐randomized study |
| Grabner 1970 | Non‐randomized study |
| Hamm 2006 | Non‐controlled study |
| Hanna 1978 | Non‐controlled study |
| Haustein 1989 | Non‐randomized study |
| Henderson 1991 | Non‐randomized study |
| Henderson 1992 | Non‐randomized study |
| Kar 1994 | Case study |
| Kaur 1980 | Non‐randomized study |
| Kenawi 1993 | Non‐randomized study |
| Khan 2007 | Non‐randomized study |
| Konstantinov 1979 | Non‐randomized study |
| Landegren 1979 | Non‐randomized study |
| López 2003 | Non‐randomized study |
| Macotela‐Ruiz 1996 | Not truly randomized; unbalanced groups |
| Mapar 2008 | Non‐randomized study |
| Meinking 1995b | Non‐controlled study |
| Mellanby 1945 | Non‐randomized study |
| Mozgunov 1978 | Non‐controlled study |
| Nag 1995 | Non‐randomized study |
| Neto 1984 | Non‐randomized study |
| Oberoi 2007 | Non‐randomized study; cure not assessed |
| Oladimeji 2000 | Participants randomized to 1 of 3 treatments (lippia oil, benzyl benzoate, or liquid paraffin) but no clear randomization within these groups to 36 separate treatment schedule subgroups |
| Oladimeji 2005 | Trial design inadequate with control group consisting of participants excluded from intervention arms |
| Oyelami 2009 | Non‐controlled study |
| Paasch 2000 | Non‐randomized study |
| Paschoal 1985 | Not a trial of scabies treatment effectiveness |
| Pierce 1951 | Non‐randomized study |
| Regis 2003 | Outcome is reinfestation not treatment failure |
| Reid 1990 | Non‐controlled study |
| Sehgal 1972 | No assessment of any outcomes were reported |
| Srinivas 1996 | Randomization unclear; comparison of lindane applied by bath, paint brush, and spray |
| Srivastava 1980 | Allocation made on a "random basis and on availability of drugs" |
| Sule 2007 | Non‐randomized study |
| Suvanprakorn 1987 | Non‐controlled study |
| Taplin 1983a | Non‐randomized study |
| Taplin 1983b | Non‐controlled study |
| Taplin 1991 | Non‐controlled study |
| Tausch 1999 | Comparison between 2 different brands of the same drug (10% crotamiton lotion) |
| Thianprasit 1984 | Non‐controlled study |
| Woolridge 1948 | Non‐controlled study |
| Yonkonsky 1990 | Non‐controlled study |
Characteristics of ongoing studies [ordered by study ID]
Naeyaert ongoing.
| Trial name or title | "A randomised, double blind, double dummy study to compare the efficacy and safety of a single administration of ivermectin to a single administration of permethrin for the treatment of scabies" |
| Methods | — |
| Participants | Expected enrolment: 160
Minimum age: 5 years
Both genders Inclusion criteria: at least 1 of scabies tunnels or positive microscopic examination (acarids, faeces, or ova); at least two of non‐specific injuries with a typical distribution pattern, serious itching which increases during the night, or family or contacts with similar complaints Exclusion criteria: treatment for scabies < 4 weeks ago; treatment with corticoids < 1 week ago; pregnancy; breastfeeding; HIV; serious immunodepressive patients; sensitivity or allergy to 1 of the components of the study medication; damage of the central nerve system |
| Interventions | Administration of ivermectin or permethrin on day 0 |
| Outcomes | Primary: clinical healing of the skin injuries on day 28 Secondary: decrease of itching on day 28; amelioration of the life quality on day 28; number and gravity of adverse events |
| Starting date | July 2004 |
| Contact information | Jean‐Marie Naeyaert, Principal Investigator, University Hospital Ghent, Ghent 9000, Belgium |
| Notes | ClinicalTrials.gov identifier: NCT00262418 |
Contributions of authors
Mark Strong and Paul Johnstone jointly authored this review update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Medical Research Council, UK.
MS is funded by a Medical Research Council Health Services Research / Health of the Public research training fellowship [grant number G0601721].
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Amer 1992 {published data only}
- Amer M, el‐Gharib I. Permethrin versus crotamiton and lindane in the treatment of scabies. International Journal of Dermatology 1992;31(5):357‐8. [DOI] [PubMed] [Google Scholar]
Amerio 2003 {published data only}
- Amerio P, Capizzi R, Milani M. Efficacy and tolerability of natural synergised pyrethrins in a new thermo labile foam formulation in topical treatment of scabies: a prospective, randomised, investigator‐blinded, comparative trial vs. permethrin cream. European Journal of Dermatology 2003;13(1):69‐71. [PubMed] [Google Scholar]
Avila‐Romay 1991 {published data only}
- Avila‐Romay A, Alvarez‐Franco M, Ruiz‐Maldonado R. Therapeutic efficacy, secondary effects, and patient acceptability of 10% sulfur in either pork fat or cold cream for the treatment of scabies. Pediatric Dermatology 1991;8(1):64‐6. [DOI] [PubMed] [Google Scholar]
Bachewar 2009 {published data only}
- Bachewar N, Thawani V, Mali S, Gharpure K, Shingade V, Dakhale G. Comparison of safety, efficacy, and cost effectiveness of benzyl benzoate, permethrin, and ivermectin in patients of scabies. Indian Journal of Pharmacology 2009; Vol. 41, issue 1:9‐14. [DOI] [PMC free article] [PubMed]
Biele 2006 {published data only}
- Biele M, Campori G, Colombo R, Giorgio G, Frascione P, Sali R, et al. Efficacy and tolerability of a new synergized pyrethrins thermofobic foam in comparison with benzyl benzoate in the treatment of scabies in convicts: the ISAC study (Studio Della scabbia in ambiente carcerario). Journal of the European Academy of Dermatology and Venereology 2006;20(6):717‐20. [DOI] [PubMed] [Google Scholar]
Brooks 2002 {published data only}
- Brooks PA, Grace RF. Ivermectin is better than benzyl benzoate for childhood scabies in developing countries. Journal of Paediatrics and Child Health 2002;38(4):401‐4. [DOI] [PubMed] [Google Scholar]
Chouela 1999 {published data only}
- Chouela EN, Abeldano AM, Pellerano G, Forgia M, Papale RM, Garsd A, et al. Equivalent therapeutic efficacy and safety of ivermectin and lindane in the treatment of human scabies. Archives of Dermatology 1999;135(6):651‐5. [DOI] [PubMed] [Google Scholar]
Glaziou 1993 {published data only}
- Glaziou P, Cartel JL, Alzieu P, Briot C, Moulia‐Pelat JP, Martin PM. Comparison of ivermectin and benzyl benzoate for treatment of scabies. Tropical Medicine and Parasitology 1993;44(4):331‐2. [PubMed] [Google Scholar]
Gulati 1978 {published data only}
- Gulati PV, Singh KP. A family based study on the treatment of scabies with benzyl benzoate and sulphur ointment. Indian Journal of Dermatology, Venereology and Leprology 1978;44(5):269‐73. [Google Scholar]
Hansen 1986 {published data only}
- Hansen RC, Remmers E, Menter MA. A controlled comparative trial of permethrin 5 per cent cream and 1 per cent lindane lotion for the treatment of scabies. Clinical Research 1986;34:160. [Google Scholar]
Ly 2009 {published data only}
- Ly F, Caumes E, Ndaw CAT, Ndiaye B, Mahé A. Ivermectin versus benzyl benzoate applied once or twice to treat human scabies in Dakar, Senegal: a randomized controlled trial. Bulletin of the World Health Organization 2009;87:424‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macotela‐Ruiz 1993 {published data only}
- Macotela‐Ruiz E, Pena‐Gonzalez G. The treatment of scabies with oral ivermectin [Tratamiento de la escabiasis con ivermectina por via oral]. Gaceta Medica Mexico 1993;129(3):201‐5. [PubMed] [Google Scholar]
Madan 2001 {published data only}
- Madan V, Jaskiran K, Gupta U, Gupta DK. Oral ivermectin in scabies patients: a comparison with 1% topical lindane lotion. The Journal of Dermatology 2001;28(9):481‐4. [DOI] [PubMed] [Google Scholar]
Maggi 1986 {published data only}
- Maggi A, Subercaseaux B. Scabies treatment with lindane [Tratamiento de la sarna con lindano. Comparacion de esquemas terapeuticos]. Revista Medica de Valparaiso 1986;39(1):11‐14. [Google Scholar]
Nnoruka 2001 {published data only}
- Nnoruka EN, Agu CE. Successful treatment of scabies with oral ivermectin in Nigeria. Tropical Doctor 2001;31(1):15‐8. [DOI] [PubMed] [Google Scholar]
Schenone 1986 {published data only}
- Schenone H, Prieto R, Lobos M, Fabres P, Beresi R. Treatment of scabies with a dermatological lotion of decamethrin at 0.02%. Study in 127 patients by the use of 2 therapeutic regimens [Tratamiento de la sarna con loción dermatológica de decametrina al 0,02% estudio en 127 pacientes mediante la utilización de dos esquemas terapéuticos]. Boletin Chileno de Parasitologia 1986;41(1‐2):3‐7. [PubMed] [Google Scholar]
Schultz 1990 {published data only}
- Schultz MW, Gomez M, Hansen RC, Mills J, Menter A, Rodgers H, et al. Comparative study of 5% permethrin cream and 1% lindane lotion for the treatment of scabies. Archives of Dermatology 1990;126(2):167‐70. [PubMed] [Google Scholar]
Singalavanija 2003 {published data only}
- Singalavanija S, Limpongsanurak W, Soponsakunkul S. A comparative study between 10 per cent sulfur ointment and 0.3 per cent gamma benzene hexachloride gel in the treatment of scabies in children. Journal of the Medical Association of Thailand 2003;86 Suppl 3:531‐6. [PubMed] [Google Scholar]
Taplin 1986 {published data only}
- Taplin D, Meinking TL, Porcelain SL, Castilero PM, Chen JA. Permethrin 5% dermal cream: a new treatment for scabies. Journal of the American Academy of Dermatology 1986;15(5 Pt 1):995‐1001. [DOI] [PubMed] [Google Scholar]
Taplin 1990 {published data only}
- Taplin D, Meinking TL, Chen JA, Sanchez R. Comparison of crotamiton 10% cream (Eurax) and permethrin 5% cream (Elimite) for the treatment of scabies in children. Pediatric Dermatology 1990;7(1):67‐73. [DOI] [PubMed] [Google Scholar]
Usha 2000 {published and unpublished data}
- Usha V, Gopalakrishnan Nair TV. A comparative study of oral ivermectin and topical permethrin cream in the treatment of scabies. Journal of the American Academy of Dermatology 2000;42(2 Pt 1):236‐40. [DOI] [PubMed] [Google Scholar]
Zargari 2006 {published data only}
- Zargari O, Golchai J, Sobhani A, Dehpour AR, Sadr‐Ashkevari S, Alizadeh N, et al. Comparison of the efficacy of topical 1% lindane vs 5% permethrin in scabies: a randomized, double‐blind study. Indian Journal of Dermatology, Venereology and Leprology 2006;72(1):33‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abedin 2007 {published data only}
- Abedin S, Narang M, Gandhi V, Narang S. Efficacy of permethrin cream and oral ivermectin in treatment of scabies. Indian Journal of Pediatrics 2007;74(10):915‐6. [DOI] [PubMed] [Google Scholar]
Alebiosu 2003 {published data only}
- Alebiosu CO, Ogunledun A, Ogunleye DS. A report of clinical trial conducted on Toto ointment and soap products. Journal of the National Medical Association 2003;95(1):95‐105. [PMC free article] [PubMed] [Google Scholar]
Amer 1981 {published data only}
- Amer M, El‐Bayoumi M, Rizk MK. Treatment of scabies: preliminary report. International Journal of Dermatology 1981;20(4):289‐90. [DOI] [PubMed] [Google Scholar]
Bockarie 2000 {published data only}
- Bockarie MJ, Alexander ND, Kazura JW, Bockarie F, Griffin L, Alpers MP. Treatment with ivermectin reduces the high prevalence of scabies in a village in Papua New Guinea. Acta Tropica 2000;75(1):127‐30. [DOI] [PubMed] [Google Scholar]
Burgess 1986 {published data only}
- Burgess I, Robinson RJ, Robinson J, Maunder JW, Hassan Z. Aqueous malathion 0.5% as a scabicide: clinical trial. British Medical Journal 1986;292(6529):1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cannon 1948 {published data only}
- Cannon AB, McRae ME. Treatment of scabies. Report of one hundred patients treated with hexachlorocyclohexane in a vanishing cream base. Journal of the American Medical Association 1948;138:557‐560. [DOI] [PubMed] [Google Scholar]
Chowdhury 1977 {published data only}
- Chowdhury SP Roy. Nitofurazone in scabies. Lancet 1977;309(8003):152. [DOI] [PubMed] [Google Scholar]
Cubela 1978 {published data only}
- Cubela V, Yawalakar SJ. Clinical experience with Crotamiton cream and lotion in treatment of infants with scabies. British Journal of Clinical Practice 1978;32(8):229‐31. [PubMed] [Google Scholar]
Curiati 1984 {published data only}
- Curiati WJC. Double‐blind study with decamethrin in scabies and head louse [Estudo duplo‐cego com decametrina em escabiose e pediculose]. Revista Brasileira de Medicina 1984;41(2):81‐3. [Google Scholar]
Damodaran 1979 {published data only}
- Damodaran M, Naidu AN, Sarma KV. Anaemia and morbidity in rural preschool children. Indian Journal of Medical Research 1979;69:448‐56. [PubMed] [Google Scholar]
Daneshpajooh 2000 {published data only}
- Daneshpajooh M, Jafari F, Sadri MF, Valikhani M. Comparison of oral ivermectin and topical gamma benzene hexachloride 1% in the treatment of scabies. Iranian Journal of Dermatology 2000;3(10):3. [Google Scholar]
Dika 2006 {published data only}
- Dika E, Tosti A, Goldovsky M, Wester R, Maibach HI. Percutaneous absorption of crotamiton in man following single and multiple dosing. Cutaneous and Ocular Toxicology 2006;25(3):211‐6. [DOI] [PubMed] [Google Scholar]
Dourmishev 1998 {published data only}
- Dourmishev A, Serafimova D, Dourmishev L. Efficacy and tolerance of oral ivermectin in scabies. Journal of the European Academy of Dermatology and Venereology 1998;11(3):247‐51. [PubMed] [Google Scholar]
Dunne 1991 {published data only}
- Dunne CL, Malone CJ, Whiworth JA. A field study of the effects of ivermectin on ectoparasites of man. Transactions of the Royal Society of Tropical Medicine and Hygiene 1991;85(4):550‐1. [DOI] [PubMed] [Google Scholar]
Gallegos 1996 {published data only}
- Gallegos Polanco ERU. Tratamiento de escabiosis en el Hospital Militar de Arequipa, estudio comparativo de benzoato de bencilo, hexacloruro de benceno gama y permetrina en 130 pacientes [thesis]. Arequipa: Universidad Nacional de San Agustín de Arequipa, 1996. [Google Scholar]
Gordon 1944 {published data only}
- Gordon FM, Davey TH, Unsworth K, Hellier FF, Parry SC, Alexander JRH. Control of scabies by use of soap impregnated with tetra‐ethylthiuram monosulphide ("tetmosol"). British Medical Journal 1944;2:803‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grabner 1970 {published data only}
- Grabner K. Clinical and experimental studies with the antihistaminic Tavegyl in dermatologic patients [Klinische und experimentelle studien mit dem antihistaminikum Tavegyl an dermatologischem krankengut]. Wiener Klinische Wochenschrift 1970;82(36):625‐7. [PubMed] [Google Scholar]
Hamm 2006 {published data only}
- Hamm H, Beiteke U, Hoger PH, Seitz CS, Thaci D, Sunderkotter C. Treatment of scabies with 5% permethrin cream: results of a German multicenter study. Journal der Deutschen Dermatologischen Gesellschaft 2006;4(5):407‐13. [DOI] [PubMed] [Google Scholar]
Hanna 1978 {published data only}
- Hanna NF, Clay JC, Harris JR. Sarcoptes scabiei infestation treated with malathion liquid. British Journal of Venereal Diseases 1978;54(5):354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haustein 1989 {published data only}
- Haustein UF, Hlawa B. Treatment of scabies with permethrin versus lindane and benzyl benzoate. Acta Dermato‐Venereologica 1989;69(4):348‐51. [PubMed] [Google Scholar]
Henderson 1991 {published data only}
- Henderson C. Community control of scabies. Lancet 1991;337(8756):1548. [DOI] [PubMed] [Google Scholar]
Henderson 1992 {published data only}
- Henderson CA, Nykia M. Treatment of scabies in rural east Africa ‐ a comparative study of two regimens. Tropical Doctor 1992;22(4):165‐7. [DOI] [PubMed] [Google Scholar]
Kar 1994 {published data only}
- Kar SK, Mania J, Patnaik S. The use of ivermectin for scabies. National Medical Journal of India 1994;7:15‐6. [PubMed] [Google Scholar]
Kaur 1980 {published data only}
- Kaur GA, Nadeswary K. Field trials on the management of scabies in Jengka Triangle, Pahang. Medical Journal of Malaysia 1980;35:14‐21. [PubMed] [Google Scholar]
Kenawi 1993 {published data only}
- Kenawi MZ, Morsy TA, Abdalla KF, Hady H. Treatment of human scabies by sulfur and permethrin. Journal of the Egyptian Society of Parasitology 1993;23(3):691‐6. [PubMed] [Google Scholar]
Khan 2007 {published data only}
- Khan I, Yasmin R. Ivermectin in the treatment of scabies. Journal of Pakistan Association of Dermatologists 2007;17:78‐83. [Google Scholar]
Konstantinov 1979 {published data only}
- Konstantinov D, Stanoeva L. Crotamiton cream and lotion in the treatment of infants and young children with scabies. Journal of International Medical Research 1979;7(5):443‐8. [DOI] [PubMed] [Google Scholar]
Landegren 1979 {published data only}
- Landegren J, Borglund E, Storgards K. Treatment of scabies with disulfiram and benzyl benzoate emulsion: a controlled study. Acta Dermato‐venereologica 1979;59(3):274‐6. [PubMed] [Google Scholar]
López 2003 {published data only}
- López E, Alvarez M, Agorio C, Avendaño M, Bonasse J, Chavarría A, et al. Comparative treatment between oral ivermectina and topic permetrina in scabiosis [Tratamiento comparativo entre ivermectina vía oral y permetrina tópica en la escabiosis]. Revista Chilena de Dermatología 2003;19(1):27‐32. [Google Scholar]
Macotela‐Ruiz 1996 {published data only}
- Macotela‐Ruiz E, Islas CCM, Ramos QFBEN. Treatment of scabies with oral Ivermectin in an enclosed rural community [Tratamiento de escabiasis con Ivermectina por via oral en una comunidad rural cerrada. Implicaciones epidemiologicas]. Dermatologica Revista Mexicana 1996;40(3):179‐84. [Google Scholar]
Mapar 2008 {published data only}
- Mapar MA, Mali B. The comparison of oral ivermectin and topical lindane in the treatment of scabies. Iranian Journal of Dermatology 2008;11(4):147‐150. [Google Scholar]
Meinking 1995b {published data only}
- Meinking TL, Taplin D, Hermida JL, Pardo R, Kerdel FA. The treatment of scabies with ivermectin. New England Journal of Medicine 1995;333(1):26‐30. [DOI] [PubMed] [Google Scholar]
Mellanby 1945 {published data only}
- Mellanby K. Scabies prophylaxis using "tetmosol" soap. British Medical Journal 1945;1:38‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozgunov 1978 {published data only}
- Mozgunov VN, Klimenko AV. Effectiveness of preparations used in treating scabies. Voenno‐meditsinskii Zhurnal 1978, (12):74. [PubMed] [Google Scholar]
Nag 1995 {published data only}
- Nag SC, Barbhuiya JN, Datta PK, Banerjee PP. A comparative study of efficacy gama benzene hexachloride lotion and benzyl benzoate emulsion. Indian Journal of Dermatology 1995;40(2):86‐7. [Google Scholar]
Neto 1984 {published data only}
- Neto VS. Comparative study of monosulfiram and benzyl benzoate in the treatment of scabies [Estudo comparativo entre o monossulfiram e o benzoato de benzila no tratamentoda escabiose]. Anais Brasileiros de Dermatologia 1984;59:213‐4. [Google Scholar]
Oberoi 2007 {published data only}
- Oberoi S, Ahmed RS, Suke SG, Bhattacharya SN, Chakraborti A, Banerjee BD. Comparative effect of topical application of lindane and permethrin on oxidative stress parameters in adult scabies patients. Clinical biochemistry 2007;40(16‐17):1321‐4. [DOI] [PubMed] [Google Scholar]
Oladimeji 2000 {published data only}
- Oladimeji FA, Orafidiya OO, Ogunniyi TA, Adewunmi TA. Pediculocidal and scabicidal properties of Lippia multiflora essential oil. Journal of Ethnopharmacology 2000;72(1‐2):305‐11. [MEDLINE: ; CN‐00330021] [DOI] [PubMed] [Google Scholar]
Oladimeji 2005 {published data only}
- Oladimeji FA, Orafidiya LO, Ogunniyi TAB, Adewunmi TA, Onayemi O. A comparative study of the scabicidal activities of formulations of essential oil of Lippia multiflora Moldenke and benzyl benzoate emulsion BP. International Journal of Aromatherapy 2005;15(2):87‐93. [Google Scholar]
Oyelami 2009 {published data only}
- Oyelami OA, Onayemi A, Oyedeji OA, Adeyemi LA. Preliminary study of effectiveness of aloe vera in scabies treatment. Phytotherapy Research 2009;23(10):1482‐4. [DOI] [PubMed] [Google Scholar]
Paasch 2000 {published data only}
- Paasch U, Haustein UF. Management of endemic outbreaks of scabies with allethrin, permethrin, and ivermectin. International Journal of Dermatology 2000;39(6):463‐70. [DOI] [PubMed] [Google Scholar]
Paschoal 1985 {published data only}
- Paschoal LHC, Solowiejczyk D. Parallel double‐blind controlled clinical trial with dissulfiram suspension versus placebo in scabiotic patients: evaluation of side‐effects after alcohol ingestion [Estudo clínico controlado duplo‐cego paralelo com dissulfiram suspensäo versus placebo em pacientes escabióticos: avaliaçäo de efeitos colaterais após ingestäo de álcool ]. Revista Brasileira de Medicina 1985;42(7):65‐8. [Google Scholar]
Pierce 1951 {published data only}
- Pierce HE Jr. Scabies: epidemiology and management at a correctional institution. Journal of the National Medical Association 1951;43(2):107‐12. [PMC free article] [PubMed] [Google Scholar]
Regis 2003 {published data only}
- Regis Roggero A, Pancorbo Mendoza J, Lanchipa Yokota P, Regis Roggero RM, Aguero M. Tratamiento y reinfestación por escabiosis humana: estudio comparativo entre permetrina al 5 por ciento vs benzoato de bencilo al 25 por ciento [Treatment and reinfestation by human scabiosis: comparative study between 5 percentage permethrin and 25 percentage benzyl benzoate]. Dermatología Peruana 2003;13(1):30‐3. [Google Scholar]
Reid 1990 {published data only}
- Reid HF, Thorne CD. Scabies infestation: the effects of intervention by public health education. Epidemiology and Infection 1990;105(3):595‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sehgal 1972 {published data only}
- Sehgal VN, Rao TL, Rege VL, Vadiraj SN. Scabies: a study of incidence and a treatment method. International Journal of Dermatology 1972;11(2):106‐11. [DOI] [PubMed] [Google Scholar]
Srinivas 1996 {published data only}
- Srinivas CR, Pai S, Jana S. Treatment of scabies with 1 percent gamma benzene hexachloride : efficacy of drug delivery by bath, spray and paint brush. Indian Journal of Dermatology 1996;41(2):51‐2. [Google Scholar]
Srivastava 1980 {published data only}
- Srivastava BC, Chandra R, Srivastava VK, Saxena SC, Nandan D, Gupta RP, et al. Epidemiological studies of scabies and community control. Journal of Communicable Diseases 1980;12(3):134‐8. [PubMed] [Google Scholar]
Sule 2007 {published data only}
- Sule HM, Thacher TD. Comparison of ivermectin and benzyl benzoate lotion for scabies in Nigerian patients. American Journal of Tropical Medicine and Hygiene 2007;76(2):392‐5. [PubMed] [Google Scholar]
Suvanprakorn 1987 {published data only}
- Suvanprakorn P, Sukriket P, Bisalbutra P. Treatment of scabies with 1 per cent gamma benzene hexachloride. Journal of the Medical Association of Thailand 1987;70(5):252‐5. [PubMed] [Google Scholar]
Taplin 1983a {published data only}
- Taplin D, Riviera A, Walker JG, Roth WI, Reno D, Meinking T. A comparative trial of three treatment schedules for the eradication of scabies. Journal of the American Academy of Dermatology 1983;9(4):550‐4. [DOI] [PubMed] [Google Scholar]
Taplin 1983b {published data only}
- Taplin D, Arrue C, Walker JG, Roth WI, Rivera A. Eradication of scabies with a single treatment schedule. Journal of the American Academy of Dermatology 1983;9(4):546‐50. [DOI] [PubMed] [Google Scholar]
Taplin 1991 {published data only}
- Taplin D, Porcelain SL, Meinking TL, Athey RL, Chen JA, Castillero PM, et al. Community control of scabies: a model based on use of permethrin cream. Lancet 1991;337(8748):1016‐8. [DOI] [PubMed] [Google Scholar]
Tausch 1999 {published data only}
- Tausch I. Crotamiton ‐ An effective and safe drug for the treatment of scabies results of a controlled clinical trial. Zeitschrift fur Hautkrankheiten 1999;74:162‐6. [Google Scholar]
Thianprasit 1984 {published data only}
- Thianprasit M, Schuetzenberger R. Prioderm lotion in the treatment of scabies. Southeast Asian Journal of Tropical Public Health 1984;15(1):119‐20. [PubMed] [Google Scholar]
Woolridge 1948 {published data only}
- Woolridge WE. The gamma isomer of hexachlorcyclohexane in the treatment of scabies. Journal of Investigative Dermatology 1948;10:363‐6. [PubMed] [Google Scholar]
Yonkonsky 1990 {published data only}
- Yonkonsky D, Ladia L, Gackenheimer L, Schultz MW. Scabies in nursing homes: an eradication program with permethrin 5% cream. Journal of the American Academy of Dermatology 1990;23(6 Pt 1):1133‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Naeyaert ongoing {unpublished data only}
- Naeyaert J‐M. A randomised, double lind, double dummy study to compare the efficacy and safety of a single administration of ivermectin to a single administration of permethrin for the treatment of scabies. ClinicalTrials.gov identifier: NCT00262418.
Additional references
Alexander 1984
- Alexander, JO. Scabies. Arthropods and human skin. Berlin: Springer‐Verlag, 1984:227‐9. [Google Scholar]
Arlian 1989
- Arlian LG. Biology, host relations, and epidemiology of Sarcoptes scabiei. Annual Review of Entomology 1989;34:139‐61. [DOI] [PubMed] [Google Scholar]
Barkwell 1997
- Barkwell R, Shields S. Deaths associated with ivermectin treatment of scabies. Lancet 1997;349(9059):1144‐5. [DOI] [PubMed] [Google Scholar]
BNF 1997
- British National Formulary. Vol. 33, London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, 1997. [Google Scholar]
BNF 2010
- British National Formulary. Vol. 59, London: British Medical Association and the Royal Pharmaceutical Society of Great Britain, 2010. [Google Scholar]
Bredal 1997
- Reintjes R, Hoek C, Coyne PE, Addiss DG, Barkwell R, Shields S, et al. Deaths associated with ivermectin for scabies. Lancet 1997;350(9072):215‐6. [DOI] [PubMed] [Google Scholar]
Buffet 2003
- Buffet M, Dupin N. Current treatments for scabies. Fundamental & Clinical Pharmacology 2003;17(2):217‐25. [DOI] [PubMed] [Google Scholar]
Chosidow 2000
- Chosidow O. Scabies and pediculosis. Lancet 2000;355(9206):819‐26. [DOI] [PubMed] [Google Scholar]
Coleman 2005
- Coleman CI, Gillespie EL, White CM. Probable topical permethrin‐induced neck dystonia. Pharmacotherapy 2005;25(3):448‐50. [DOI] [PubMed] [Google Scholar]
Cox 2000
- Cox NH. Permethrin treatment in scabies infestation: importance of the correct formulation. BMJ 2000;320(7226):37‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coyne 1997
- Coyne PE, Addiss DG. Deaths associated with ivermectin for scabies. Lancet 1997;350(9072):215‐6. [DOI] [PubMed] [Google Scholar]
DeSole 1989
- Sole G, Remme J, Awadzi K, Accorsi S, Alley ES, Ba O, et al. Adverse reactions after large‐scale treatment of onchocerciasis with ivermectin: combined results from eight community trials. Bulletin of the World Health Organization 1989;67(6):707‐19. [PMC free article] [PubMed] [Google Scholar]
Diazgranados 1997
- Diazgranados JA, Costa JL. Deaths after ivermectin treatment. Lancet 1997;349(9066):1698. [DOI] [PubMed] [Google Scholar]
Downs 1999
- Downs AM, Harvey I, Kennedy CT. The epidemiology of head lice and scabies in the UK. Epidemiology and Infection 1999;122(3):471‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elgart 1996
- Elgart ML. A risk‐benefit assessment of agents used in the treatment of scabies. Drug Safety 1996;14(6):386‐93. [DOI] [PubMed] [Google Scholar]
Fain 1978
- Fain A. Epidemiological problems of scabies. International Journal of Dermatology 1978;17(1):20‐30. [DOI] [PubMed] [Google Scholar]
Green 1989
- Green M. Epidemiology of scabies. Epidemiologic Reviews 1989;11:126‐50. [DOI] [PubMed] [Google Scholar]
Hay 2004
- Hay RJ. Scabies ‐ learning from the animals. Journal of the European Academy of Dermatology and Venereology 2004;18:129‐30. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S. Intention to treat issues. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]; Section 8.4. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 September 2006).
Higgins 2006
- Higgins JPT, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]; Appendix 5b. www.cochrane.org/resources/handbook/hbook.htm (accessed 1 February 2007).
Juni 2001
- Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meinking 1995a
- Meinking TL, Taplin D. Infestations. In: Schachner LA, Hansen RC editor(s). Pediatric Dermatology. New York: Churchill Livingstone, 1995:1347‐92. [Google Scholar]
MHRA 2006
- MHRA 2006. Drug Analysis Prints. Data on suspected adverse drug reactions. www.mhra.gov.uk (accessed April 2006).
Mimouni 1998
- Mimouni D, Gdalevich M, Mimouni FB, Haviv J, Ashkenazi I. The epidemiologic trends of scabies among Israeli soldiers: a 28‐year follow‐up. International Journal of Dermatology 1998;37(8):586‐7. [DOI] [PubMed] [Google Scholar]
Mimouni 2003
- Mimouni D, Ankol OE, Davidovitch N, Gdalevich M, Zangvil E, Grotto I. Seasonality trends of scabies in a young adult population: a 20‐year follow‐up. British Journal of Dermatology 2003;149(1):157‐9. [DOI] [PubMed] [Google Scholar]
Orkin 1976
- Orkin M, Epstein E, Maibach HI. Treatment of today's scabies and pediculosis. Journal of the American Medical Association 1976;236(10):1136‐9. [PubMed] [Google Scholar]
Pacque 1990
- Pacque M, Munoz B, Greene BM, White AT, Dukuly Z, Taylor HR. Safety of and compliance with community‐based ivermectin therapy. Lancet 1990;335(8702):1377‐80. [DOI] [PubMed] [Google Scholar]
Petros 1990
- Petros S. Malathion poisoning. Tropical Doctor 1990;20(2):71. [PubMed] [Google Scholar]
Rauch 1990
- Rauch AE, Kowalsky SF, Lesar TS, Sauerbier GA, Burkart PT, Scharfman WB. Lindane (Kwell) ‐ induced aplastic anemia. Archives of Internal Medicine 1990;150(11):2393‐5. [PubMed] [Google Scholar]
Reintjes 1997
- Reintjes R, Hoek C, Coyne PE, Addiss DG, Barkwell R, Shields S, et al. Deaths associated with ivermectin for scabies. Lancet 1997;350(9072):215‐6. [DOI] [PubMed] [Google Scholar]
Review Manager 5.0 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Scheinfeld 2004
- Scheinfeld N. Controlling scabies in institutional settings: a review of medications, treatment models and implementation. American Journal of Clinical Dermatology 2004;5(1):31‐7. [DOI] [PubMed] [Google Scholar]
Victoria 2001
- Victoria J, Trujillo R. Topical ivermectin: a new successful treatment for scabies. Pediatric Dermatology 2001;18(1):63‐5. [DOI] [PubMed] [Google Scholar]
Wagner 2000
- Wagner SL. Fatal asthma in a child after use of an animal shampoo containing pyrethrin. Western Journal of Medicine 2000;173(2):86‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walton 2004
- Walton SF, Dougall A, Pizzutto S, Holt D, Taplin D, Arlian LG, et al. Genetic epidemiology of Sarcoptes scabiei (Acari: Sarcoptidae) in northern Australia. International Journal for Parasitology 2004;34(7):839‐49. [DOI] [PubMed] [Google Scholar]
WHO 1990
- International Programme on Chemical Safety. Deltamethrin (Environmental Health Criteria; 97). Geneva: World Health Organization, 1990. [Google Scholar]
WHO 2003
- World Health Organization. Topical use of lindane. WHO Drug Information 2003;17(1):26‐8. [Google Scholar]
Wong 2002
- Wong L‐C, Amega B, Barker R, Connors C, Dulla ME, Ninnal A, et al. Factors supporting sustainability of a community‐based scabies control program. Australasian Journal of Dermatology 2002;43(4):274‐7. [DOI] [PubMed] [Google Scholar]
Yeruham 1998
- Yeruham I, Hadani A. Control of human scabies by topical application of ivermectin. Annals of Tropical Medicine and Parasitology 1998;92(5):627‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Walker 1997
- Walker GJA, Johnstone PW. Drug treatment for scabies. Cochrane Database of Systematic Reviews 1997, Issue 4. [Google Scholar]
Walker 1999a
- Walker GJA, Johnstone PW. Interventions for treating scabies. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000320] [DOI] [PubMed] [Google Scholar]
Walker 1999b
- Walker GJA, Johnstone PW. Interventions for treating scabies. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD000320] [DOI] [PubMed] [Google Scholar]
Walker 2000
- Walker GJA, Johnstone PW. Interventions for treating scabies. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD000320] [DOI] [PubMed] [Google Scholar]


