Abstract
Background
Injected cholera vaccines are rarely used today, although they may have some benefit. It is valuable to summarize the evidence for effectiveness of injected cholera vaccines for comparison with newer oral vaccines (subject of a separate Cochrane Review).
Objectives
To evaluate killed whole cell (KWC) cholera vaccines and other inactive subunit vaccines (administered by injection) for preventing cholera and death, and to evaluate the adverse effects.
Search methods
In September 2008, we searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL (The Cochrane Library 2008, Issue 3), EMBASE, and LILACS. We also searched reference lists and handsearched the journal Vaccine up to 1997.
Selection criteria
Randomized and quasi‐randomized controlled trials comparing injected cholera vaccines (KWC or other inactive subunit) with placebo, control vaccines, or no intervention in adults and children irrespective of immune status or special risk category.
Data collection and analysis
Two authors extracted data and assessed trial methodological quality independently. Dichotomous data were reported using the risk ratio (RR) with 95% confidence intervals (CI). Vaccine efficacies were also calculated (% vaccine efficacy = (1‐RR) x 100%).
Main results
Sixteen trials, involving over one million adults, children and infants, fulfilled the inclusion criteria. Twenty‐four comparisons reported on vaccine efficacy (cholera cases and/or deaths) and 11 comparisons considered adverse effects (nine reported on both). Compared to placebo, vaccinees had a reduced risk of death from cholera (RR 0.49, 95% CI 0.25 to 0.93; 837,442 participants) and a reduced risk of contracting cholera at 12 months (RR 0.52, 95% CI 0.42 to 0.65, random‐effects model; 1,512,573 participants). This translates to an efficacy of 48%, 95% confidence interval 35% to 58%. Significant protection lasted for two years, even after only a single dose, and for three years with an annual booster. Children over five years and adults were protected for up to three years, while children under five years were protected for up to a year. Injected cholera vaccines were associated with more systemic and local adverse effects compared to placebo, but these were not severe or life‐threatening.
Authors' conclusions
Injected cholera vaccines appear to be safe and relatively more effective than usually realized. Protection against cholera persists for up to two years following a single dose of vaccine, and for three years with an annual booster. However, they have been superseded by oral vaccines.
23 April 2019
No update planned
Other
This is not a current question.
Plain language summary
Killed whole cell or other inactive subunit vaccines (injected) for preventing cholera
Cholera is an acute gastroenteritis caused by Vibrio cholerae. Infection causes profuse watery diarrhoea, and up to 40% of patients die if untreated. Cholera was a major cause of death in many countries in the past; epidemics are now less common, but cholera remains an important cause of death in developing countries, especially in Africa.
Vaccination against cholera was first tested in the nineteenth century and may play a role in controlling epidemics. Injected (parenteral) whole cell vaccines were used in the 1960s and 1970s, but they went out of favour as their efficacy was thought to be low and short‐lived, and associated with a high rate of adverse effects. This review summarizes the evidence for effectiveness of injected cholera vaccines. A separate Cochrane Review describes trials with oral cholera vaccines, which were introduced more recently and are used currently.
Sixteen trials, involving over one million adults, children, and infants, were included. Injected cholera vaccines reduced the risk of death from cholera and the risk of contracting cholera at 12 months. Significant protection lasted for two years. Injected cholera vaccines had more systemic and local adverse effects than placebo, but these adverse effects were relatively well tolerated and were not severe or life‐threatening.
The authors conclude that injected cholera vaccines appear to be relatively safe and more effective than usually realized. However, they are not currently available and therefore cannot be recommended for use. This review provides a solid background of evidence for the effects of cholera injected vaccines, against which to compare the effects of oral vaccines.
Background
Cholera is an acute infection that causes sudden onset of profuse watery diarrhoea, and up to 40% of patients die if untreated. Cholera was a major cause of death in many countries in the past, although epidemics are now less common. Nevertheless, cholera remains an important cause of death in developing countries. In 2005, there were a total of 131,943 reported cases of cholera throughout the world, including 2272 deaths (WHO 2006a), and it is known that there were many more cases that were not reported. Ninety‐five per cent of reported cases were in Africa. Cholera can lead to serious outbreaks: in 2005, the World Health Organization (WHO) confirmed 49 different outbreaks in 36 countries (WHO 2006a).
Cholera is caused by the Gram‐negative bacillus Vibrio cholerae. There are over a hundred serological groups of V. cholerae, each with varying potential to cause disease. Until recently only one of these (V. cholerae 01) caused epidemic cholera. In 1992 to 1993, an epidemic of cholera originating in the Indian subcontinent was found to be caused by V. cholerae 0139, also called 0139 Bengal. Cholera strains are also classified by their biotype (Classical or El Tor), and within the biotype, the serotype (Ogawa or Inaba). Serotype differences are based on differences in structure of the lipopolysaccharide membrane. The various serological groups are important as each vaccine component tends to be specific to particular groups of V. cholerae.
Transmission of V. cholerae occurs predominantly when people ingest faecal contaminated water or food. The disease spreads rapidly where there is poverty, poor hygiene, and lack of sanitation. Waterborne spread can be responsible for devastating epidemics such as that which occurred due to El Tor cholera in the refugee camps of Goma, Zaire in July 1994. This resulted in 70,000 cases and 12,000 deaths (Sánchez 1997).
V. cholerae colonize the gut using small hair like structures ("pili") that attach to the small bowel. High stomach pH and blood group O appear to make colonization more likely. The attached bacteria then release a soluble toxin, which results in the symptoms of the disease. This toxin is composed of two subunits, A and B. The A subunit stimulates cellular mechanisms in the bowel cells that disrupt sodium transport. The net result is a high sodium chloride (salt) concentration in the gut lumen, which holds on to water by osmotic forces, leading to profuse watery diarrhoea, severe dehydration, and eventually death. The B subunit of cholera toxin does not cause toxic effects but does stimulate an immune response from the host. Colonization can be inhibited by specific antibodies which are generated after infection with V. cholerae.
Intravenous rehydration therapy can be very effective in treatment of cholera. However, health services in cholera endemic or epidemic areas often do not have sufficient capabilities for such treatment. Improving hygienic practices in areas of poverty and limited water supply can also be problematic. This has led to attempts to prevent cholera by vaccination. The first vaccine effectively used against cholera was probably that of Ferran, who in 1884 apparently successfully controlled an epidemic in Spain. A vaccine was also produced by the Pasteur Institute in the 1920s.
Widespread use of cholera vaccines began in the 1960s when there was a series of large trials in what was then known as East Pakistan (now Bangladesh), India, and the Philippines. Most of the vaccines used in these trials were composed of whole V. cholerae serogroup 01 cells, usually a mixture of biotypes and serotypes, which were killed by either formalin, phenol, or heat. There were also trials of cholera toxoid vaccines in the 1970s. The killed whole cell vaccines, which were subsequently licensed, are injected and usually given in one or two doses.
Injected (parenteral) whole cell vaccines grew in popularity until the 1970s when they went out of favour (Bhadra 1994) on the grounds that efficacy was thought to be low and short‐lived, high titres of serum vibriocidal antibodies were thought not to provide sufficient intestinal immunity to prevent infection, and they were said to have a high rate of adverse effects. The advent of oral rehydration therapy, considered a highly effective treatment, was a major advance in reducing cholera morbidity, and led to a shift in interest away from injected vaccines. Even when injected cholera vaccines were in relatively widespread use in the early 1970s, it was never determined whether an individual's protection was likely to interrupt transmission to others in the community, or how important enteral or parenteral immunity is in bacterial shedding.
Recent cholera epidemics have shown that there still a requirement for an effective vaccine against this major disease (Sánchez 1997; Calain 2004; WHO 2006b). Oral vaccines have been under development since the 1980s, stimulated by the increasing recognition of the importance of stimulating local intestinal immunity in the prevention of the disease. Both killed and live oral vaccines are now licensed, but the injected vaccine is no longer used.
The original version of this review included both injected and oral cholera vaccines (Graves 2001), but this is now superseded and withdrawn. The current review assesses the results of trials with killed parenteral (injected) vaccines only. A separate Cochrane Review describes trials with oral cholera vaccines (Abba (in progress)).
Objectives
To evaluate killed whole cell cholera vaccines and other inactive subunit vaccines (administered by injection) for preventing cases of cholera and preventing death, and to evaluate the adverse effects associated with the vaccination.
Methods
Criteria for considering studies for this review
Types of studies
Randomized or quasi‐randomized controlled trials.
Exception: Phase 1 trials, reporting only adverse effects, for vaccines that never reached efficacy trials.
Types of participants
Well adults or children irrespective of immune status or special risk category.
Types of interventions
Intervention
Killed whole cell cholera vaccines or other inactive subunit vaccines administered by injection
Control
Placebo, control vaccines, or no intervention.
Types of outcome measures
Primary
Cholera cases, as defined by each trial (usually diarrhoea more than three times in 24 hours with bacteriological confirmation of V. cholerae).
All‐cause deaths.
Cholera deaths.
Adverse effects
-
Number and seriousness of adverse effects (classified as local and systemic).
Systemic adverse effects include cases of malaise, nausea, fever, arthralgias, rash, headache and more generalized and serious signs.
Local adverse effects include induration, soreness, and redness at the site of inoculation.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (1 September 2008); Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2008, Issue 3); MEDLINE (1966 to 1 September 2008); EMBASE (1974 to 1 September 2008); and LILACS (1982 to 1 September 2008).
We searched the bibliographies of included studies. Additionally, we handsearched the journal Vaccine from its first issue to the end of 1997 (Jefferson 1996; Jefferson 1998).
Data collection and analysis
Selection of studies
Four authors (VD, TJ, PG, and JD) read all trials retrieved in the search and applied the inclusion criteria to determine eligibility.
Data extraction and management
PG and JD independently extracted and double‐checked the following data: characteristics of participants (number, age, gender, ethnic group, risk category, and previous immunization status, if known); characteristics of interventions (vaccine type, placebo type, dose, immunization schedule, and length of follow up (in months); outcome measures; and trial date, location, sponsor, and publication status. All disagreements in the data extraction were resolved by discussion.
Adverse effect data were extracted individually for each adverse effect where possible. For trials where adverse effects were reported for more than one dose, the average of the number of people reporting each adverse effect for each dose was recorded. Where trials reported the occurrence of adverse effects over time following a single dose, the effects occurring in the first time period (typically 24 hours) were recorded if the total number of people reporting each effect in the complete follow‐up period was not given.
We extracted incidence of cholera cases and death over particular time periods of follow up (eg first year following vaccination, second year etc) to determine the duration of protection.
Assessment of risk of bias in included studies
PG and JD independently assessed each trial's method of treatment allocation (random, quasi‐random, sequential, not stated), blinding (double, single, or not blind), completeness (percentage of randomized participants completing the immunization schedule and the follow‐up period), and the surveillance procedure used to detect cases.
Data synthesis
The overall risk ratio (RR) was used to report the relative rates of cholera cases in vaccinated and placebo groups. This figure was converted to vaccine efficacy using the formula: % vaccine efficacy = (1‐RR) x 100%.
Overall risk ratio was also used for adverse effect rates and other outcomes.
We anticipated between‐trial variation in estimates of vaccine efficacy as there are several sources of heterogeneity which cannot be standardized. For example, the studies included in this review have been undertaken in a range of countries, each of which has a different pattern of exposure to the cholera pathogens. There are also major differences in the formulation of the vaccines.
To account for these differences in the analysis where significant heterogeneity (P < 0.1) was encountered between the study results, we have incorporated it into the analysis by reporting the results of the analysis using the random‐effects model, presented in the results section as a letter R following a result. Elsewhere we have reported the results of analyses using the fixed‐effect model.
It was defined a priori that subgroup analyses would be done for different age groups (under and over five years), and over time.
We split trials that included several active arms receiving separate vaccines into individual references (denoted as i, ii, iii, etc). As each active arm is compared to the same placebo group it is important that the analysis does not count the participants and cases in the placebo group more than once. This was prevented by dividing the placebo cases and participants as evenly as possible between the arms. The validity of this approach was confirmed in a second analysis in which the active arms within each trial were added together before the trials were pooled. This gave identical results in analyses using a fixed‐effect model, and very similar, but slightly less conservative, results when using a random‐effects model.
Results
Description of studies
Search results
Sixteen trials fulfilled the inclusion criteria, although 26 comparisons are included in the review since some trials had more than one arm and we reported on these separately ('Characteristics of included studies'). To avoid counting the control group more than once in these trials, the control cases and participants were divided as evenly as possible between the arms. Fifteen trials were excluded ('Characteristics of excluded studies').
Vaccines
All the included trials tested injected vaccines of which there were two kinds: killed whole cell (KWC) or purified antigen. Various serotypes and formulations of KWC vaccines or purified antigen fractions were tested. All compared the vaccine with placebo (active or inactive).
Trial sites
The trials were conducted starting in 1963 and continuing until the late 1970s. Most were large and required massive programs to undertake the logistics of vaccination and surveillance. Several series of large trials (a total of several hundred thousand people in each site) were conducted in four sites in endemic areas: the Matlab study area of East Pakistan (later Bangladesh); Calcutta, India; Negros Occidental province, Philippines; and Surabaya, Indonesia. One smaller trial (998 participants) was conducted in the former USSR (Burgasov 1976).
Outcome measures
Some of these trials (usually the first one in each series) investigated safety and immunogenicity. Most trials included clinical outcomes detected during massive population surveillance operations. All trials observed incidence of natural infection by cholera. The trial conducted in the former USSR investigated only safety and immunogenicity (Burgasov 1976). In terms of this review's outcome measures, 24 comparisons reported on vaccine efficacy (cholera cases and/or deaths) and 11 comparisons considered adverse effects. Nine reported on both types of outcome. Benenson 1968a and Burgasov 1976 provided data on adverse effects only.
Individual trial descriptions by location
East Pakistan (later Bangladesh)
Six quasi‐randomized controlled trials were conducted in this region, but nine comparisons were included since two trials had several arms (denoted as i, ii, and iii), and we reported on these separately; see Benenson 1968b‐i and Benenson 1968b‐ii; and Mosley 1970‐i, Mosley 1970‐ii, and Mosley 1970‐iii. One trial reported on adverse events only (Benenson 1968a). The comparisons differed in the participant age groups: four included all ages (Benenson 1968a; Oseasohn 1965; Benenson 1968b‐i; Benenson 1968b‐ii); four included children aged up to 14 years (McCormack 1969; Mosley 1970‐i; Mosley 1970‐ii; Mosley 1970‐iii); and one included females of all ages and males up to age 15 years (Curlin 1975).
The trials compared types of cholera vaccine with active placebos or various schedules of vaccine against active placebos (shown in order of date started):
Benenson 1968a: several types of injected KWC with one or two doses versus two active placebos (typhoid/paratyphoid A/paratyphoid B (TAB) and tetanus toxoid).
Oseasohn 1965: injected, single‐dose KWC versus active placebo (TAB).
Benenson 1968b‐i and Benenson 1968b‐ii: injected single dose vaccine (KWC vaccine in Benenson 1968b‐i and purified Ogawa antigen vaccine in Benenson 1968b‐ii) versus two types of active placebo (TAB and tetanus toxoid).
McCormack 1969: various schedules (one or two initial doses plus two annual boosters; two initial doses without boosters) of injected KWC versus two active placebos (tetanus and diphtheria toxoids).
Mosley 1970‐i, Mosley 1970‐ii, and Mosley 1970‐iii: three types of injected KWC vaccine (one initial dose, one booster dose at one year) versus two active placebos (tetanus and diphtheria toxoids). The three KWC vaccines were Classical Ogawa (Mosley 1970‐i), Classical Inaba (Mosley 1970‐ii), and El Tor (Mosley 1970‐iii).
Curlin 1975: two doses of lypohilized cholera toxoid (glutaraldehyde treated) versus active placebo (diptheria‐tetanus toxoid).
India (Calcutta)
Four randomized controlled trials were conducted in this region, but five comparisons are included. Two trials were reported in one publication (denoted as a and b), and one of these trials had two arms (denoted as i and ii): das Gupta 1965a; and das Gupta 1965b‐i and das Gupta 1965b‐ii. All age groups were included in the trials.
All four randomized controlled trials compared one dose of an injected KWC vaccine with an active placebo (shown in order of date started):
Taneja 1965: one‐dose injected KWC versus active placebo (TAB).
das Gupta 1965a: one‐dose injected KWC versus active placebo (TAB).
das Gupta 1965b‐i and das Gupta 1965b‐ii: one‐dose injected KWC vaccine (Classical KWC in das Gupta 1965b‐i and El Tor KWC in das Gupta 1965b‐ii) versus active placebo (TAB).
Pal 1980: one‐dose injected Classical KWC with alum adjuvant versus active placebo (tetanus toxoid).
Indonesia (Surabaya)
One randomized controlled trial was conducted in this region, although it had two arms (Saroso 1978i; Saroso 1978ii). The trial compared one‐dose injected KWC vaccine with an active placebo (tetanus toxoid) in all age groups. Saroso 1978i used a non‐aluminium‐hydroxide adsorbed KWC vaccine, while Saroso 1978ii used an aluminium hydroxide‐adsorbed KWC vaccine.
Philippines (Negros Occidental province)
Four randomized controlled trials were conducted in this region, but nine comparisons are included since two trials had several arms (denoted as i, ii, etc) and we reported on these separately. The trials were conducted in all age groups. All trials compared a KWC vaccine with active placebo (shown in order of date started):
Azurin 1965i, Azurin 1965ii, and Azurin 1965iii: three injected single‐dose Classical KWC vaccines versus active placebo (typhoid vaccine). The three KWC vaccines were Classical KWC (Azurin 1965i), El Tor KWC (Azurin 1965ii), and Classical KWC with oil adjuvant arm (Azurin 1965iii).
PCC 1968: one or two doses (at three‐week intervals) of El Tor KWC vaccines versus active placebo (typhoid vaccine).
PCC 1973a‐i, PCC 1973a‐ii, PCC 1973a‐iii, and PCC 1973a‐iv: four different types of injected single‐dose KWC vaccine versus active placebo (typhoid vaccine). The four KWC vaccines were El Tor Inaba (PCC 1973a‐i), El Tor Ogawa (PCC 1973a‐ii), Classical Ogawa (PCC 1973a‐iii), and Classical Inaba (PCC 1973a‐iv).
PCC 1973b: single‐dose Classical KWC injected subcutaneously or intradermally versus active placebo (typhoid vaccine).
Former USSR
One randomized controlled trial was conducted in this region (Burgasov 1976). This trial compared three types of one‐dose injected Classical KWC and a partially purified cholera toxoid with inert placebo (sterile physiological solution). Only adults (both sexes) were included.
Risk of bias in included studies
The details for each trial are given under 'Method' in the 'Characteristics of included studies'. We assessed the efficacy and adverse effect trials separately.
Efficacy trials: 14 trials with 24 comparisons
The methodological quality of the efficacy trials was relatively high, considering their age.
Method of treatment allocation
Nine trials with 16 comparisons stated that the allocation method was randomization although only one trial mentioned a particular method (Latin Square (Azurin 1965i; Azurin 1965ii; Azurin 1965iii)). The other five trials (eight comparisons) used a sequential method such as alternate census number (Curlin 1975, all East Pakistan trials). These trials have therefore been classified as quasi‐randomized controlled trials rather than randomized controlled trials, and allocation concealment is regarded as inadequate. All of the other trials mentioned some kind of coding system or identical preparation of placebo and are thus classified as adequate for allocation concealment.
Blinding
All efficacy trials were stated to be double blind with the exception of Curlin 1975 (single, possibly double).
Completeness
The major flaw in the reporting of the efficacy trials is the lack of information on the completeness (ie the percentage of randomized participants completing the immunization schedule and the follow‐up period). In many trials there was a large difference between the number randomized and the number who actually participated. Some trials reported on the number completing the vaccination schedule (71% for Curlin 1975). Most trial reports provided little information on the percentage of participants who completed even the initial period of follow up. The India, Indonesia, and Philippines trials gave no information on this aspect of the trials. The little information on follow up that can be gleaned from some of the other trials suggests that dropout was not a serious problem – for example, dropout appears to have been less than 5% by two years in McCormack 1969, about 5% by one year in Mosley 1970‐i, Mosley 1970‐ii, and Mosley 1970‐iii, and about 10% in Oseasohn 1965. It is easy to appreciate that keeping track of participants in trials with many thousands of people per arm would be a problem. However, since differential dropout is a serious potential source of bias in vaccine trials, and more information on this topic (perhaps by sampling a proportion of participants) would have been reassuring.
Surveillance procedure used to detect cases
Of the 14 efficacy trials, five used only active surveillance for cases, six used only passive, and three used both (details in Appendix 2). In all but one of the five East Pakistan/Bangladesh efficacy trials, it was claimed that daily or twice‐weekly surveillance was carried out at home. In India, all four trials used passive surveillance, whereby cases were not detected unless they presented for treatment or sent a postal or telephone message. The Indonesia trial also used passive surveillance. In the Philippines, one trial used active surveillance only and the other three used a combination of passive surveillance and house to house visits, although the frequency and duration of this activity is not stated.
Adverse effect trials: 7 trials with 11 comparisons
Method of treatment allocation
Allocation method in the adverse effect trials was stated to be randomization in all trials except Benenson 1968a (sequential by census), which was classified as a quasi‐randomized controlled trial. Allocation concealment was classed as inadequate in this trial; all other trials were classed as adequate for allocation concealment.
Blinding
Blinding was only single (possibly double, but not clear) in one of the adverse effect trials (Burgasov 1976). All other trials were stated to be double blind.
Completeness
In the adverse effect trials, follow up was usually short and dropouts minimal. Completion of follow up (ie the percentage of randomized participants completing the immunization schedule and the follow‐up period) was 100% in two of the trials (Benenson 1968a; Burgasov 1976) but not stated in the others.
Surveillance procedure used to detect cases
The seven trials reporting on adverse effects were very poor in reporting the methods of surveillance (details in Appendix 2). However, since most reported on adverse events within 24 hours of vaccination, it is likely that individuals were actively assessed during that time period. In Burgasov 1976, home follow up continued for 30 days, although the frequency was not stated. In one trial in the Philippines where adverse effects were assessed (Azurin 1965iii), passive surveillance occurred in addition to active follow up because numerous participants reported to health facilities with adverse events.
Effects of interventions
Outcomes considered were cholera cases assessed after different lengths of follow up (up to seven months, up to one year, during year two, three, four, and five after follow up) for which data were available. During the first year of follow up, data from trials may appear in either 'up to seven months follow up' or 'up to one year follow up' depending on the duration of surveillance. Trial results appear in both categories only if the trials reported additional data for the second half of the first year of follow up. It should be noted that only three trials continued follow up for more than two years.
Injected cholera vaccine vs placebo (no booster)
The vaccination schedule for the trials considered here was either a single dose (all trials except McCormack 1969 and PCC 1968) or a 'short schedule'. In McCormack 1969, half of the participants had one dose and the other half had a short schedule of two doses given up to 35 days apart. For this analysis, participants who had booster doses at one year in McCormack 1969 were only included before one year of follow up. In PCC 1968 the data were combined from groups given either one dose or two doses at three‐week intervals. Trials with booster doses at one year (Mosley 1970‐i; Mosley 1970‐ii) were excluded from this comparison after one year's follow up.
1. Cholera cases
Injected cholera vaccines were more effective than placebo at reducing risk of cholera cases for up to two years after immunization (Analysis 1.1): up to seven months (RR 0.44, 95% CI 0.37 to 0.53; 2,098,146 participants, random‐effects model); up to one year (RR 0.52, 95% CI 0.42 to 0.65, random‐effects model; 1,512,573 participants); and year two (RR 0.58, 95% CI 0.45 to 0.75; 718,579 participants). Considering all age groups together, the vaccines were not significantly efficacious in years three (33,028 participants), four (18,969 participants), and five (18,969 participants); see Analysis 1.1. The corresponding estimates for vaccine efficacy for all vaccines combined are 56% (95% CI 47% to 63%) for up to seven months, 48% (95% CI 35% to 58%) for up to one year, and 41% (95% CI 24% to 55%) for year two.
1.1. Analysis.
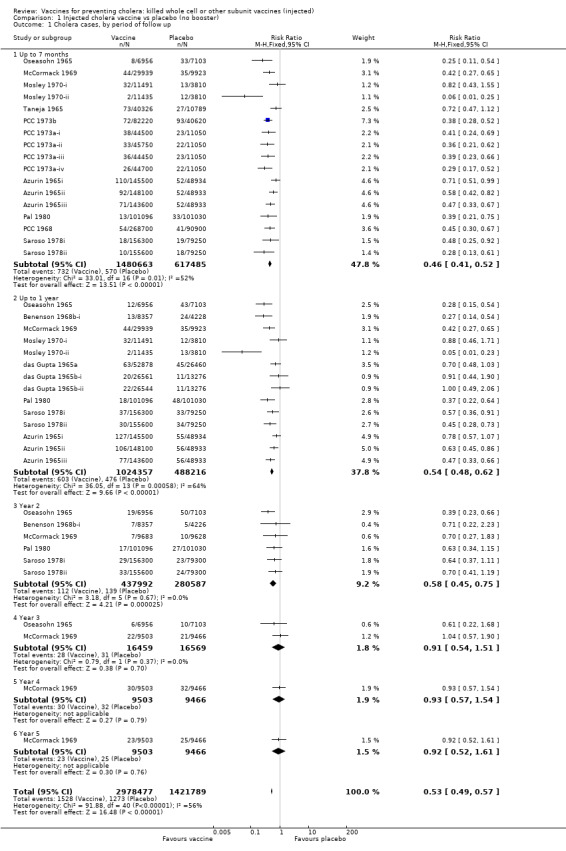
Comparison 1 Injected cholera vaccine vs placebo (no booster), Outcome 1 Cholera cases, by period of follow up.
2. Death
Analysis 1.2 examines deaths (all‐cause and cholera) in the first year of follow up. With the vaccine there was no reduction in all‐cause deaths (RR 0.99, 95% CI 0.72 to 1.34; 26,743 participants), but there was a significant reduction in cholera deaths (RR 0.49, 95% CI 0.25 to 0.93; 837,442 participants). Note that this comparison included the Philippines trial arm of Azurin 1965iii that tested the cholera oil adjuvant vaccine, which had serious adverse effects.
1.2. Analysis.
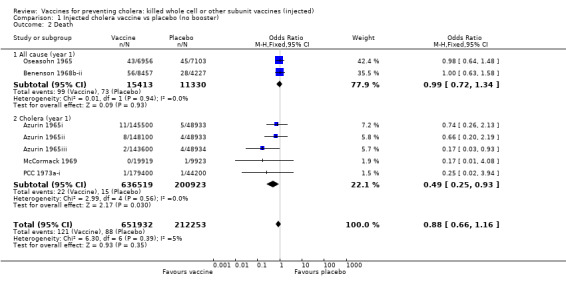
Comparison 1 Injected cholera vaccine vs placebo (no booster), Outcome 2 Death.
Injected cholera vaccine vs placebo (stratified analyses)
3.1. Cholera cases by age group
This comparison includes only those trials that reported age‐specific outcomes. A single dose or short schedule without booster was used by all trials except two trials that had one booster dose at one year after the first dose (Mosley 1970‐i; Mosley 1970‐ii). At each time point, we stratified the participants by those aged up to five years and those aged over five years: up to seven months' follow up (Analysis 2.1); up to one year follow up (Analysis 2.2); year two follow up (Analysis 2.3); year three follow up (Analysis 2.4); year four follow up (Analysis 2.5); and year five follow up (Analysis 2.6).
2.1. Analysis.
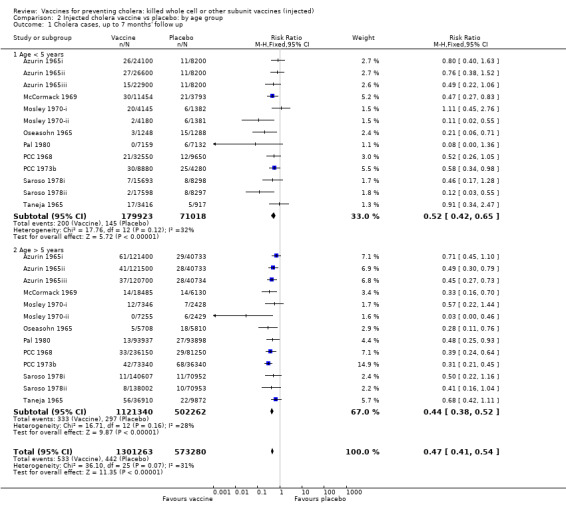
Comparison 2 Injected cholera vaccine vs placebo: by age group, Outcome 1 Cholera cases, up to 7 months' follow up.
2.2. Analysis.
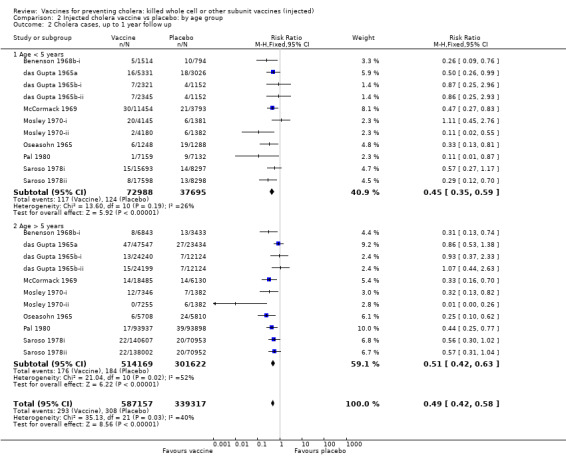
Comparison 2 Injected cholera vaccine vs placebo: by age group, Outcome 2 Cholera cases, up to 1 year follow up.
2.3. Analysis.
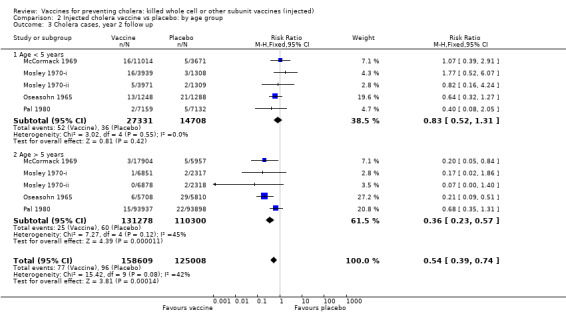
Comparison 2 Injected cholera vaccine vs placebo: by age group, Outcome 3 Cholera cases, year 2 follow up.
2.4. Analysis.
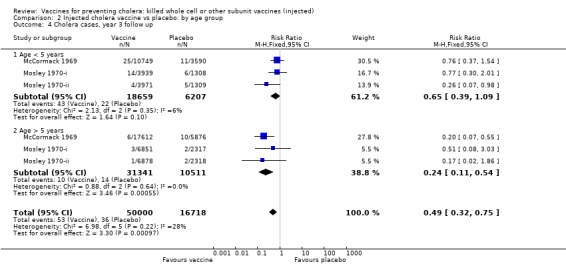
Comparison 2 Injected cholera vaccine vs placebo: by age group, Outcome 4 Cholera cases, year 3 follow up.
2.5. Analysis.
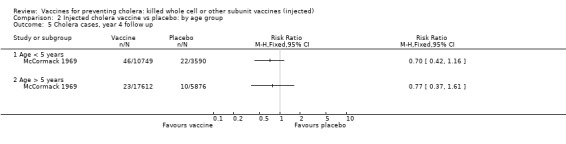
Comparison 2 Injected cholera vaccine vs placebo: by age group, Outcome 5 Cholera cases, year 4 follow up.
2.6. Analysis.
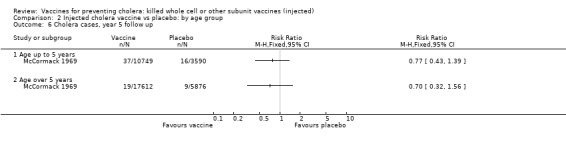
Comparison 2 Injected cholera vaccine vs placebo: by age group, Outcome 6 Cholera cases, year 5 follow up.
In the first year of follow up, there is little age‐related difference in the reduction in risk of cholera cases between the vaccine and placebo when stratified by age group (Analysis 2.2): up to five years (RR 0.45, 95% 0.35 to 0.59; 250,941 participants); and greater than five years (RR 0.51, 95% CI 0.42 to 0.63; 815,791 participants). These translate to efficacies of 55% (95% CI 41% to 65%) and 49% (95% CI 37% to 58%), respectively. There were two trials in which the efficacy at one year was notably better in the younger age group: Pal 1980 (89% versus 56%); and Saroso 1978ii (71% versus 43%). Both trials used alum‐absorbed vaccine, suggesting that this adjuvant may increase efficacy in young children. This effect was not observed in Saroso 1978i, which used the same vaccine as Saroso 1978ii without alum (efficacy 43% and 44% in participants aged up to five years and over five years, respectively).
In the second year of follow up, the vaccines were not significantly efficacious in children aged up to five years (RR 0.83, 95% CI 0.52 to 1.31; 42,039 participants) whereas they were in older participants (RR 0.36, 95% CI 0.23 to 0.57; 241,578 participants); see Analysis 2.3. These translate to efficacies of 17% (95% CI ‐31% to 48%) and 64% (95% CI 476% to 7686%), respectively. This difference was similar also at year three when the vaccines had little effect in children aged up to five years (RR 0.64, 95% CI 0.39 to 1.09; 24,866 participants), but they were still protective in the older participants (RR 0.24, 95% CI 0.11 to 0.54; 41,852 participants); see Analysis 2.4. The equivalent efficacies are 36% (95% CI ‐10% to 63%) and 76% (95% CI 45% to 90%), respectively.
3.2. Cholera cases by vaccine schedule
This comparison specifically examined the question of whether booster doses of injected vaccines improve the duration of protection. Two trials (one with two sub‐trials) included booster schedules: McCormack 1969 and Mosley 1970‐i and Mosley 1970‐ii. The McCormack 1969 trial included both non‐booster and booster arms: the non‐booster arm had two doses on a short schedule, while the booster arm had either one or two initial doses followed by additional doses after one and two years. Mosley 1970‐i and Mosley 1970‐ii tested Classical monovalent Ogawa (Mosley 1970‐i) or Inaba (Mosley 1970‐ii) vaccines with a single initial dose followed by one booster shot at one year. In both trials the non‐booster and/or placebo groups received placebo shots instead of booster doses to maintain blinding. For up to one year of follow up before the booster was given, no difference would be expected between short schedule and booster subgroups: RR 0.55 (95% CI 0.45 to 0.68, random‐effects model; 1,442,164 participants) for the single‐dose schedule; and RR 0.35 (95% CI 0.13 to 0.98, random‐effects model; 64,208 participants for the booster subgroups respectively; see Analysis 3.1. There was no difference between subgroups during year two either (Analysis 3.2). In the third year, significant protection was observed in the booster schedule group: RR 0.34 (95% CI 0.15 to 0.77, random‐effects model; 60,941 participants), but not in the other groups (Analysis 3.3). Similar trends were seen in year four (Analysis 3.4) and year five (Analysis 3.5).
3.1. Analysis.
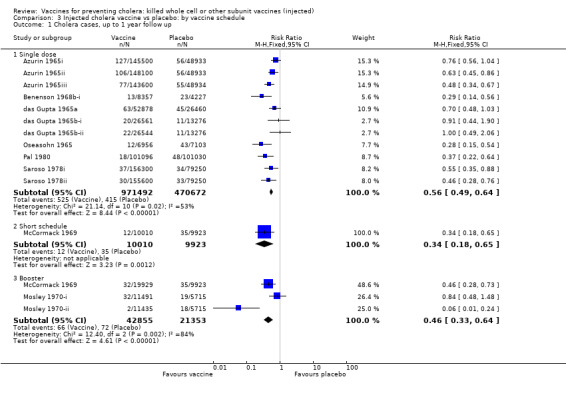
Comparison 3 Injected cholera vaccine vs placebo: by vaccine schedule, Outcome 1 Cholera cases, up to 1 year follow up.
3.2. Analysis.
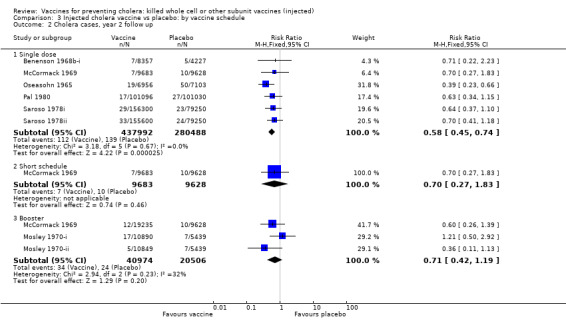
Comparison 3 Injected cholera vaccine vs placebo: by vaccine schedule, Outcome 2 Cholera cases, year 2 follow up.
3.3. Analysis.
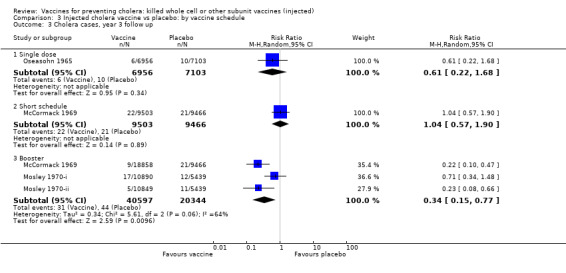
Comparison 3 Injected cholera vaccine vs placebo: by vaccine schedule, Outcome 3 Cholera cases, year 3 follow up.
3.4. Analysis.
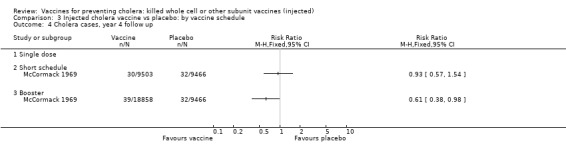
Comparison 3 Injected cholera vaccine vs placebo: by vaccine schedule, Outcome 4 Cholera cases, year 4 follow up.
3.5. Analysis.
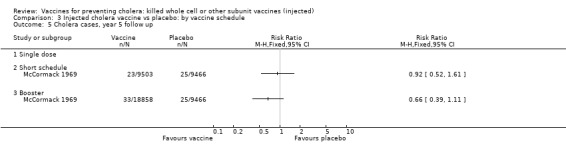
Comparison 3 Injected cholera vaccine vs placebo: by vaccine schedule, Outcome 5 Cholera cases, year 5 follow up.
3.3. Cholera cases by vaccine type
This comparison examines the efficacy of different biotypes and serotypes of cholera vaccine (including purified antigens) up to one year after immunization. Overall, all types of vaccine except a toxoid (Curlin 1975) demonstrated protective efficacy which ranged from 38% (10% to 57%) (R) for El Tor 01 Ogawa plus Inaba KWC injected, to 86% (25% to 97%) (R) for Classical 01 Inaba KWC injected (Analysis 4.1).
4.1. Analysis.
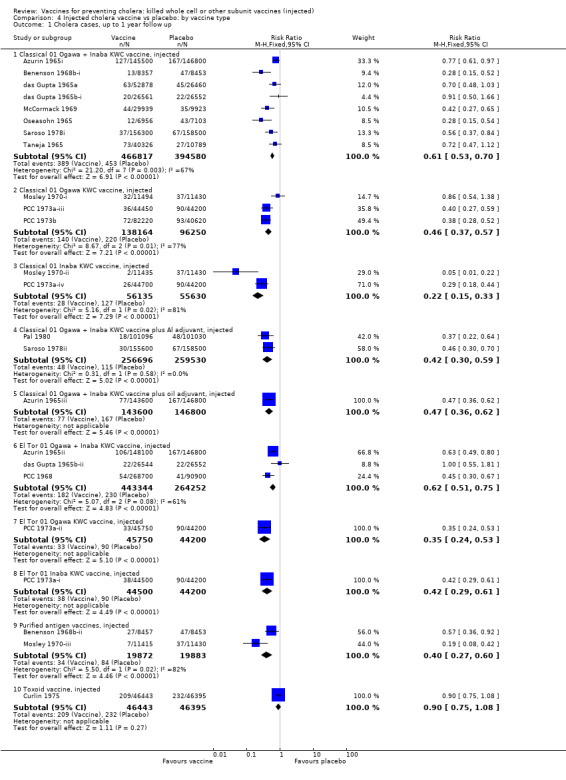
Comparison 4 Injected cholera vaccine vs placebo: by vaccine type, Outcome 1 Cholera cases, up to 1 year follow up.
4. Adverse effects
4.1. Versus inert placebo
Only one trial used an inert placebo (Burgasov 1976). As shown in Analysis 5.1, the vaccine used in this trial caused malaise in 11% of recipients (RR 4.36, 95% CI 1.79 to 10.60), tenderness in 38% of recipients (RR 9.56, 95% CI 4.82 to 18.95), erythema in 28% of recipients (RR 2.82, 95% CI 1.83 to 4.34), and local infiltration in 14% of recipients (RR 14.04, 95% CI 3.50 to 56.33). All other adverse effects had no higher frequency in the vaccinated versus placebo groups.
5.1. Analysis.
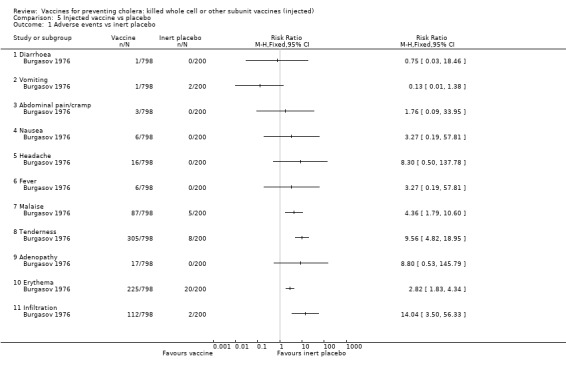
Comparison 5 Injected vaccine vs placebo, Outcome 1 Adverse events vs inert placebo.
4.2. Versus active placebo
When compared to active placebo (Analysis 5.2), the vaccines caused vomiting in 1.5% of recipients (RR 10.43, 95% CI 1.34 to 81.22) and tenderness in 26% of recipients (RR 1.26, 95% CI 1.04 to 1.53). There were between‐trial differences in the risk ratios for other adverse effects, with one trial (Pal 1980) showing large (RR > 1.5) increases in headache, fever, erythema, and swelling, although the averages across all trials were not statistically significant. In Benenson 1968a, the adverse effects were not classified beyond systemic or local;13% of participants had systemic effects (RR 2.30, 95% CI 1.10 to 4.80) and 40% had local effects (RR 3.48, 95% CI 2.14 to 5.63). One trial in the Philippines with three arms reported on trials with Classical vaccine (Azurin 1965i), El Tor vaccine (Azurin 1965ii), and Classical vaccine with oil adjuvant (Azurin 1965iii). There were serious adverse events observed in this trial when participants presented to health facilities (abscesses, ulcers, or hard masses at the site of vaccination), and it is reported that 96% of these occurred in the group who received the oil adjuvant vaccine (Azurin 1965iii). It was also reported that erythema, swelling, pain, induration, fever, and a feeling of weakness were experienced by participants. Overall the percentage of persons experiencing adverse events of any type was 0.8% in those receiving Classical vaccine (Azurin 1965i), 1.7% in those with El Tor vaccine (Azurin 1965ii), 96.1% in the Classical/oil adjuvant vaccine Azurin 1965iii, and 1.4% in the placebo group. However, no breakdown of specific symptom by vaccine group was given, except for the severe events mentioned above. Therefore we cannot include these results in the Analysis 5.2.
5.2. Analysis.
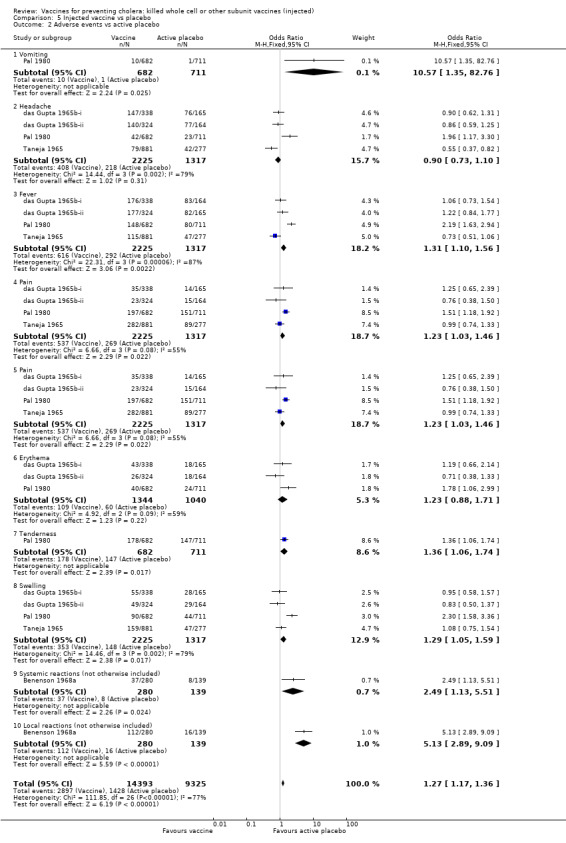
Comparison 5 Injected vaccine vs placebo, Outcome 2 Adverse events vs active placebo.
Discussion
We included trials of injected cholera vaccines that had clinical outcomes (cholera cases, deaths, and adverse effects). We excluded trials with only immunological outcomes because our main questions were the efficacy and safety of injected cholera killed or subunit vaccines. The number of cases of cholera at different time periods after vaccination was our major outcome measure. We also assessed deaths (both all‐cause and cholera specific) by year one of follow up, although this was investigated in few trials. Adverse effects were assessed by relatively few trials with a high variability in definition and measurement which made synthesis across different studies difficult.
The results of our review show that injected cholera vaccines are relatively efficacious in the first seven months. There was no evidence for a marked decline in efficacy in the second half of the first year or in year two, even without a booster dose.
Efficacy estimates stratified by age group (under or over five years) showed little difference in the first year. In year two, the vaccines were significantly less efficacious in children under five years than in older individuals. This difference persisted at year three when the vaccines had little effect in children aged less than five years (efficacy 20%, 95% CI ‐14% to 43%) but were still strongly protective in persons over five years (efficacy 57%, 95% CI 38 to 71%). By years four and five, neither age group was protected.
Both short vaccination schedules (single dose or two doses up to one month apart) and schedules with annual booster doses induced equivalent protection for two years in recipients. After year two, the booster schedule appeared to provide superior protection: efficacy was 9% for short schedules and 66% for booster schedules in year three and in year four the respective estimates were 7% and 39%. Our assessment of short versus booster schedules is based on a limited number of trials which included boosters (McCormack 1969; Mosley 1970‐i; Mosley 1970‐ii), only one of which continued beyond three years.
Injected cholera vaccines reduced cholera deaths by half, but they were of marginal efficacy in preventing all‐cause death.
Injected cholera vaccines appear to be reasonably safe and were relatively well tolerated. Injected cholera vaccines did not cause significant increase in most individual systemic adverse effects (fever, malaise, headache) compared to active placebo, although they did cause increased malaise compared to inert placebo, and increased vomiting and unspecified systemic reactions compared to active placebo.
Injected cholera vaccines caused an increased number of local adverse effects including erythema, tenderness, and infiltration compared to inert placebo, and unspecified local reactions when compared to active placebo.
Our decision not to include serological outcomes in this review is based partly on the uncertainty of the relationship between protection afforded by the vaccine and a rise in antibody titre following immunization. Previously, the mouse protection index was considered the best correlate measure (Joo 1974), but since then most studies have quantified immunogenicity in terms of serum anti‐toxin antibodies and/or vibriocidal antibodies, often in assays which are serotype specific (Inaba and Ogawa). Anti‐toxin antibodies may protect by neutralizing cholera toxin, while vibriocidal antibodies may protect against colonization. Currently the critical protective immunity is thought to be antibacterial (vibriocidal) rather than antitoxic (Davis 1995). However, elevated serum vibriocidal antibodies, which may exist in persons in endemic areas, are often not further boosted by either vaccination or exposure to cholera, so rates of seroconversion may not correlate well with vaccine efficacy in these areas. Moreover, studies with serological outcomes reported results in a variety of assays using different definitions of seroconversion, making it very difficult to sum‐up in a homogeneous way. Trials with clinical outcomes are the definitive method of assessing protection.
Historically six criticisms have been levelled at injected cholera vaccines. Firstly the protection from these vaccines is frequently stated to be below 30% at four to six months after vaccination (Sánchez 1997), or "modest and short lived" (Clemens 1994), or 50% to 70% with a short duration of three to six months (Feeley 1978), or not exceeding 50% to 60% (Joo 1974). We concur with the estimates of the latter two authors of overall protective efficacy, since our estimate is 57% (50% to 64%) after seven months. But we do not confirm the short duration of efficacy, since our estimate is 51% (4%1 to 59%) efficacy in the first year and 47% (36% to 56%) in the second year.
Secondly, injected cholera vaccines were stated to give "incidence of significant local reactions in up to 30% of vaccinees" (Sánchez 1997) and "immunization is generally accompanied by mild fever, malaise and headache" (Joo 1974). We found some basis for the former statement, but not for the latter since only up to 13% of vaccinees had systemic adverse effects. In general, injected cholera vaccines were well tolerated and the nature of the relatively minor adverse effects must be weighed against the possible severity and catastrophic impact of cholera.
Thirdly, protective efficacy in children aged less than five years was stated to be "below 30%" (Sánchez 1997) or "poor" in the same age group (Joo 1974). Again the letter of these statements is not borne out by the results of our meta‐analysis, as in the first year the level of protection is equivalent in under‐ and over‐five year olds (51% and 55% in the two groups). However, protection certainly persists longer in persons aged over five years, in whom efficacy was 57% in the third year after immunization.
Fourthly, it was suggested that injected cholera vaccines do not reduce carriage of V. cholerae 01 (Clemens 1994; Sánchez 1997). We are not able to comment on this statement as the trials reviewed to date have not addressed this issue specifically. Moreover Clemens 1994 noted that the role of asymptomatic excretion of V. cholerae in epidemics is unclear, and consequently the public health importance of interrupting 'carrier' status is not known. Cvjetanović 1978a and Cvjetanović 1978b suggested that lifelong healthy carriers epidemiologically play a negligible role.
Fifthly, Sommer 1973b thought that injected KWC vaccines have no role in controlling an epidemic, assuming an efficacy of around 50%. Although the included trials have not addressed this issue, it certainly appears likely that seeking out and vaccinating household contacts of cases (as considered by Sommer 1973b) would be too late to prevent infection of such secondary cases, and this is supported by one excluded trial (Sommer 1973a). However we believe that this does not discount the potential indirect effect that vaccinating a community would have on controlling or preventing an epidemic (Clemens 1996). Vaccine trials to date have involved individual, rather than community, randomization. The efficacy (or rather effectiveness) of a vaccine is likely to be much greater if given to a whole community rather than to dispersed individuals, if the vaccines reduced excretion of bacteria or if herd immunity was attained. This would have to be tested in trials of different design than the ones reviewed here.
Finally, it is asserted that injected cholera vaccines necessitate more than one inoculation to be effective. We did not find this to be the case for parenterally administered vaccines. Most of trials in our analysis used only one dose. For example, eight of the 10 trials (16 of 18 subtrials) of injected cholera vaccine analysed at seven months' follow up used a single dose, with a summary estimate of 54% efficacy. At two years, our summary estimate for these injected cholera vaccines was 39% (21% to 52%) protective efficacy in the second year of follow up; this was derived from six trials, in five of which only one dose was given. Booster doses do not provide enhanced protection until years three and four.
We conclude that injected cholera vaccines are generally safe and relatively effective, with a combined estimate of 57% efficacy at one year and 47% at two years. Injected cholera vaccines achieve this level of efficacy after one injection or a short schedule of two doses; extending this level of protective efficacy for up to four years requires an annual booster. Vaccines were of equivalent efficacy in children under five years as in older age groups in the first year, but protection persisted longer (up to three years) in older children and adults.
These data provide the background information against which to compare the efficacy of oral cholera vaccines, which are the subject of a separate Cochrane Review (Abba (in progress)).
Disaggregation of study results for this review caused a considerable conceptual and logistic burden. Study reports frequently discussed more than one separate trial in the same published report, and there were multiple publications from single trials. Repetition of study results is to a certain extent inevitable in such large studies of long duration, which lend themselves to multiple publications of progress reports or partial reports of different outcomes. However, we feel that unnecessarily complicated trials, combined with multiple reporting, may have contributed to the underestimation of the extent and longevity of protection induced by injected cholera vaccines. Nevertheless it is not clear how the myth of requirement for six‐monthly boosters for this type of vaccine originated, since no trial tested such a schedule, and the few trials comparing annual booster and non‐booster schedules showed no advantage of booster until the third year of follow up.
Over one million people, including infants and children, have taken part in large, good quality efficacy trials of injected cholera vaccines over the last 35 years. The overwhelming majority of these participants have been poor residents of cholera‐endemic areas. Also hundreds of researchers have devoted years of their careers to these trials. Their contributions deserve to be better recognized by thorough examination of the results of these trials. It appears that the adverse effects have been overestimated and relatively effective injected cholera vaccines have been underestimated.
Authors' conclusions
Implications for practice.
Injected cholera vaccines are not currently available and therefore not recommended for either residents of endemic areas or travellers. The accepted wisdom is that they provide weak, partial protection of very short duration and require multiple doses. However, this meta‐analysis demonstrated significant protection for populations living in endemic areas for up to two years following a single dose, and for three to four years with annual booster. Risk of death from cholera was also reduced by 50% in the first year after vaccination.
Implications for research.
All cholera vaccine trials to date have been individually rather than group randomized. Research is needed on whether cholera vaccines can control epidemics if given on a population basis.
Results of two trials showing that alum adjuvant KWC injected vaccines were more effective in young children than older persons suggest that modern adjuvants could possibly increase the efficacy of injected KWC vaccines in this age group.
This review provides a solid background of evidence for effects of cholera injected vaccines against which to compare the effects of oral vaccines.
What's new
| Date | Event | Description |
|---|---|---|
| 3 August 2010 | Review declared as stable | This review will no longer be updated because injected cholera vaccines are not available and no longer recommended for use. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 7 July 2010 | New search has been performed | This review is an update of the Cochrane Review of all cholera vaccines (Graves 2001). This update includes only injected vaccines. Oral vaccines are being covered in a new review (Abba (in progress)). |
| 6 July 2010 | New citation required but conclusions have not changed | A literature search (1 September 2008) did not identify any new trials that were not in Graves 2001. In this update, the authors have restructured the review. Mark Pratt stepped down as co‐author. |
Acknowledgements
The authors would like to thank Drs Daniela Rivetti, Franco Bottasso, Ron Behrens, and Alaistair MacMillan who applied quality criteria, and Professor Myron Levine and Mrs Carol Hobbs for assistance. We are very grateful to Mark Pratt who conducted literature searches and initial screening, and developed the trial register.
Appendices
Appendix 1. Detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | cholera | cholera | cholera | cholera | cholera |
| 2 | vaccin* | vaccin* | vaccin* | vaccin* | vaccin* |
| 3 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 | 1 or 2 |
| 4 | — | CHOLERA VACCINES | CHOLERA VACCINES | CHOLERA‐VACCINE | — |
| 5 | — | 3 or 4 | 3 or 4 | 3 or 4 | — |
| 6 | — | — | Limit 5 to human | Limit 5 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Lefebvre 2008); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Summary of trials, comparisons, outcomes, and surveillance methods
| Location | Trial name | Efficacy outcomes | Surveillance method (efficacy) | Adverse effects outcomes | Surveillance method (adverse effects) |
| East Pakistan (now Bangladesh) | Benenson 1968aa | — | — | Yes | Active (daily clinical assessments at home) |
| Oseasohn 1965b | Yes (cases, deaths) | Active (twice weekly at home) | — | — | |
| Benenson 1968b‐ib | Yes (cases, deaths) | Active (twice weekly at home) | — | — | |
| Benenson 1968b‐iib | — | — | |||
| McCormack 1969b | Yes (cases, deaths) | Active (daily at home) | — | — | |
| Mosley 1970‐ib | Yes (cases) | Active (daily at home) | — | — | |
| Mosley 1970‐iib | |||||
| Mosley 1970‐iiib | |||||
| Curlin 1975b | Yes (cases) | Passive (cases present to health facility) | — | — | |
| India | Taneja 1965c | Yes (cases) | Passive (cases make postal, telephone, or clinic notification) | Yes | Unclear; probably active (for 24 hours) |
| das Gupta 1965ab | Yes (cases) | Passive (cases make postal, telephone, or clinic notification) | — | — | |
| das Gupta 1965b‐ic | Yes (cases) | Passive (cases make postal, telephone, or clinic notification) | Yes | Unclear; probably active (for 24 hours) | |
| das Gupta 1965b‐iic | |||||
| Pal 1980c | Yes (cases) | Passive (attendance at hospital) | Yes | Unclear; probably active (for 24 hours) | |
| Indonesia | Saroso 1978ic | Yes (cases) | Passive (attendance at hospital or clinic) | Yes | Unclear |
| Saroso 1978iic | |||||
| Philippines | Azurin 1965ic | Yes (cases, deaths) | Active and passive (use of health facilities and house to house enquiry) | Yes | Active and passive |
| Azurin 1965iic | |||||
| Azurin 1965iiic | |||||
| PCC 1968b | Yes (cases) | Active (“kept under observation”) | — | — | |
| PCC 1973a‐ib | Yes (cases, deaths) | Active and passive (“kept under observation” and “house to house visits”; frequency not specified) | — | — | |
| PCC 1973a‐iib | |||||
| PCC 1973a‐iiib | |||||
| PCC 1973a‐ivb | |||||
| PCC 1973bb | Yes (cases) | Active and passive (“kept under observation” and “house to house visits”; frequency not specified) | — | — | |
| Former USSR | Burgasov 1976a | — | — | Yes | Active (medical surveillance for 30 days) |
Total trials: 16 (efficacy 14, adverse effects 7); comparisons: 26 (efficacy 24, adverse effects 11). aAdverse effects only 2 trials, 2 comparisons. bEfficacy only 9 trials, 15 comparisons. cBoth efficacy and adverse effects 5 trials, 9 comparisons.
Data and analyses
Comparison 1. Injected cholera vaccine vs placebo (no booster).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cholera cases, by period of follow up | 21 | 4.400266E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.49, 0.57] |
| 1.1 Up to 7 months | 17 | 2.098148E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.41, 0.52] |
| 1.2 Up to 1 year | 14 | 1.512573E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.48, 0.62] |
| 1.3 Year 2 | 6 | 718579 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.75] |
| 1.4 Year 3 | 2 | 33028 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.54, 1.51] |
| 1.5 Year 4 | 1 | 18969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.57, 1.54] |
| 1.6 Year 5 | 1 | 18969 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.52, 1.61] |
| 2 Death | 7 | 864185 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.16] |
| 2.1 All cause (year 1) | 2 | 26743 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.72, 1.34] |
| 2.2 Cholera (year 1) | 5 | 837442 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.25, 0.93] |
Comparison 2. Injected cholera vaccine vs placebo: by age group.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cholera cases, up to 7 months' follow up | 13 | 1.874543E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.41, 0.54] |
| 1.1 Age < 5 years | 13 | 250941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.42, 0.65] |
| 1.2 Age > 5 years | 13 | 1.623602E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.38, 0.52] |
| 2 Cholera cases, up to 1 year follow up | 11 | 926474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.42, 0.58] |
| 2.1 Age < 5 years | 11 | 110683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.35, 0.59] |
| 2.2 Age > 5 years | 11 | 815791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.42, 0.63] |
| 3 Cholera cases, year 2 follow up | 5 | 283617 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.39, 0.74] |
| 3.1 Age < 5 years | 5 | 42039 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.31] |
| 3.2 Age > 5 years | 5 | 241578 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.23, 0.57] |
| 4 Cholera cases, year 3 follow up | 3 | 66718 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.32, 0.75] |
| 4.1 Age < 5 years | 3 | 24866 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.39, 1.09] |
| 4.2 Age > 5 years | 3 | 41852 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.11, 0.54] |
| 5 Cholera cases, year 4 follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Age < 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Age > 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Cholera cases, year 5 follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Age up to 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Age over 5 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Injected cholera vaccine vs placebo: by vaccine schedule.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cholera cases, up to 1 year follow up | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Single dose | 11 | 1.442164E6 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.49, 0.64] |
| 1.2 Short schedule | 1 | 19933 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.18, 0.65] |
| 1.3 Booster | 3 | 64208 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.33, 0.64] |
| 2 Cholera cases, year 2 follow up | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Single dose | 6 | 718480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.45, 0.74] |
| 2.2 Short schedule | 1 | 19311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.27, 1.83] |
| 2.3 Booster | 3 | 61480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.19] |
| 3 Cholera cases, year 3 follow up | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Single dose | 1 | 14059 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.22, 1.68] |
| 3.2 Short schedule | 1 | 18969 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.57, 1.90] |
| 3.3 Booster | 3 | 60941 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.15, 0.77] |
| 4 Cholera cases, year 4 follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Single dose | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Short schedule | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Booster | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Cholera cases, year 5 follow up | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Single dose | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Short schedule | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Booster | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Injected cholera vaccine vs placebo: by vaccine type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cholera cases, up to 1 year follow up | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Classical 01 Ogawa + Inaba KWC vaccine, injected | 8 | 861397 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.53, 0.70] |
| 1.2 Classical 01 Ogawa KWC vaccine, injected | 3 | 234414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.37, 0.57] |
| 1.3 Classical 01 Inaba KWC vaccine, injected | 2 | 111765 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.15, 0.33] |
| 1.4 Classical 01 Ogawa + Inaba KWC vaccine plus Al adjuvant, injected | 2 | 516226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.30, 0.59] |
| 1.5 Classical 01 Ogawa + Inaba KWC vaccine plus oil adjuvant, injected | 1 | 290400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.36, 0.62] |
| 1.6 El Tor 01 Ogawa + Inaba KWC vaccine, injected | 3 | 707596 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.51, 0.75] |
| 1.7 El Tor 01 Ogawa KWC vaccine, injected | 1 | 89950 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.24, 0.53] |
| 1.8 El Tor 01 Inaba KWC vaccine, injected | 1 | 88700 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.29, 0.61] |
| 1.9 Purified antigen vaccines, injected | 2 | 39755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.27, 0.60] |
| 1.10 Toxoid vaccine, injected | 1 | 92838 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
Comparison 5. Injected vaccine vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events vs inert placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Diarrhoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Abdominal pain/cramp | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Headache | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 Fever | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 Malaise | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 Tenderness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 Adenopathy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 Erythema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 Infiltration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events vs active placebo | 5 | 23718 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.17, 1.36] |
| 2.1 Vomiting | 1 | 1393 | Odds Ratio (M‐H, Fixed, 95% CI) | 10.57 [1.35, 82.76] |
| 2.2 Headache | 4 | 3542 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.10] |
| 2.3 Fever | 4 | 3542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.10, 1.56] |
| 2.4 Pain | 4 | 3542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.03, 1.46] |
| 2.5 Pain | 4 | 3542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.03, 1.46] |
| 2.6 Erythema | 3 | 2384 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.88, 1.71] |
| 2.7 Tenderness | 1 | 1393 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.06, 1.74] |
| 2.8 Swelling | 4 | 3542 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.05, 1.59] |
| 2.9 Systemic reactions (not otherwise included) | 1 | 419 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.13, 5.51] |
| 2.10 Local reactions (not otherwise included) | 1 | 419 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.13 [2.89, 9.09] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Azurin 1965i.
| Methods | Randomized controlled trial Generation of allocation sequence: randomized according to a Latin Square Blinding: double blind Completeness: not assessable Surveillance: use of medical facilities and house‐to‐house enquiry |
|
| Participants |
Number: 584,026 (1000 for adverse events) Inhabitants (all ages) of Negros Occidental province, The Philippines, an area endemic for El Tor cholera (incidence estimated as 0.5/1000) 80% of the population (based on 1% sample) had been previously vaccinated against cholera |
|
| Interventions |
Injected cholera vaccines:
Placebo: active placebo (monovalent typhoid vaccine (Manila)) All vaccines contained 8 x 109 organisms/dose Route: injected subcutaneously Dose: 1 dose
|
|
| Outcomes |
|
|
| Location | Philippines | |
| Notes |
Length of follow up: 18 months Data for 'up to seven months' (210 days) and 'up to one year follow‐up' are reported from Azurin 1967 (see references listed under Azurin 1965i). Cases by age‐group are reported only for the first 6‐month period from Philippines Cholera Committee 1965 (see references listed under Azurin 1965i) The denominators in each group were calculated from a 1% sample of the total vaccinated population This trial Azurin 1965i reports the results of the Classical vaccine versus placebo (vaccine 1). Results for El Tor (vaccine 2) are reported in Azurin 1965ii. Results for the Classical oil adjuvant (vaccine 3) are reported in Azurin 1965iii Adverse effect data from these trials have not been entered due lack of information on outcomes by vaccine type, but gross reactions occurred in Azurin 1965iii (oil adjuvant vaccine). |
|
Azurin 1965ii.
| Methods | Randomized controlled trial | |
| Participants | Same as Azurin 1965i | |
| Interventions |
Injected cholera vaccine: El Tor bivalent (Ogawa 1418 + Inaba 6973) lyophilized killed whole cell, Manila Placebo: monovalent typhoid vaccine Route: injected Dose: 1 dose as Azurin 1965i |
|
| Outcomes | Same as Azurin 1965i | |
| Location | Philippines | |
| Notes | This trial reports the results of the El Tor vaccine (vaccine 2) in Azurin 1965i; see Azurin 1965i for additional information | |
Azurin 1965iii.
| Methods | Randomized controlled trial | |
| Participants | Same as Azurin 1965i | |
| Interventions |
Injected cholera vaccine: Classical bivalent (Ogawa 41 + Inaba 35A3) killed whole cell with oil adjuvant, Japan Placebo: monovalent typhoid vaccine Route: injected Dose: 1 dose as in Azurin 1965i |
|
| Outcomes | Same as Azurin 1965i | |
| Location | Philippines | |
| Notes | This trial reports the results for the Classical vaccine with oil adjuvant (vaccine 3 in the Azurin (Philippines) trials). See Azurin 1965i for further details This vaccine and adjuvant combination had serious adverse effects |
|
Benenson 1968a.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: sequential allocation Blinding: double blind Completeness: 100% with adverse effect data Surveillance: by daily clinical assessments at home |
|
| Participants |
Number: 2801 residents (all ages, both sexes) 87 staff of a military hospital (who had previously been vaccinated many times) and 419 residents of Kadamtali village, Matlab |
|
| Interventions |
Injected cholera vaccines:
Placebos:
Route: injected Dose: 1 or 2 doses |
|
| Outcomes |
|
|
| Location | East Pakistan: Bandar refugee colony, Mudafa (a rural area), and Kaliganj (a semi‐urban area) | |
| Notes | Only the military hospital and Kadamtali village have adverse effect data. Data from the military hospital is excluded as it is not possible to match the 164 vaccinations to the 87 subjects (each participant vaccinated twice, often with different vaccines). Data for Kadamtali (for vaccines 1 and 3 combined) have been estimated from a Figure. The placebo was tetanus toxoid | |
Benenson 1968b‐i.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: allocation according to odd and even census number Blinding: double blind Completeness: not ascertained Surveillance: twice weekly assessments at home |
|
| Participants |
Number: 25,267 All ages, comprising 78% of the residents of 35 villages in Matlab Thana, Comilla District, East Pakistan. These villages were adjacent to the 23 villages participating in the Oseasohn 1965. Previous immunization status or history of cholera not stated |
|
| Interventions |
Injected cholera vaccines: Classical bivalent (Ogawa 41, Inaba 35A3) phenol‐killed whole cell Placebo: Tetanus toxoid Route: injected Dose: 1 dose |
|
| Outcomes |
|
|
| Location | East Pakistan | |
| Notes |
Length of follow up: 2 years Vaccination occurred in September to November 1964 Follow up was divided into first cholera season (7 to 9 months after vaccination, up to June 1965) and second cholera season (July 1965 to June 1966) Cases from first season have been put in 'up to one year follow‐up' and cases from second season have been put in 'year 2 of follow‐up'. Age breakdown by cholera season is for 0 to 9 and ≥ 10 years. Breakdown by 0 to 4 and 5 to 9 years given only for first cholera season This trial reports results for Classical bivalent KWC vaccine. Benenson 1968b‐ii reports results of the purified Ogawa antigen vaccine |
|
Benenson 1968b‐ii.
| Methods | Quasi‐randomized controlled trial | |
| Participants | Same as Benenson 1968b‐i | |
| Interventions |
Injected cholera vaccine: purified freeze‐dried Ogawa antigen Placebo: tetanus toxoid |
|
| Outcomes | Same as Benenson 1968b‐i | |
| Location | East Pakistan | |
| Notes | This trial reports results results for the purified Ogawa antigen vaccine. Benenson 1968b‐i reports results for Classical bivalent KWC vaccine | |
Burgasov 1976.
| Methods | Randomized controlled trial Generation of allocation sequence: random allocation Blinding: single blind, possibly double blind Completeness: 100% Surveillance: medical surveillance for 30 days; frequency not stated |
|
| Participants |
Number: 998 Adults of both sexes aged over 17 years, not previously vaccinated against or contracted cholera |
|
| Interventions |
Injected cholera vaccines:
Placebo: sterile physiological solution Route: injected (syringe or injector) Dose: 1 dose of 8 x 109 organisms or 0.8 mg of toxoid |
|
| Outcomes |
|
|
| Location | USSR | |
| Notes | — | |
Curlin 1975.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation by census number Blinding: at least single blind Completeness: 71% received 2 doses Surveillance: cases presenting to health facility |
|
| Participants | Number: 92,838 participants (females all ages > 1 year; males aged 1 to 15 years) | |
| Interventions |
Injected cholera vaccine: Lyophilized cholera toxoid derived from Inaba 569B Placebo: Diphtheria‐tetanus toxoid vaccine Route: injected (jet injector) Dose: 2 doses of 0.5 mL containing 100 μg toxoid, 42 days apart |
|
| Outcomes | Cholera cases occurring > 2 weeks after first injection | |
| Location | Bangladesh | |
| Notes | Length of follow up: 1 year | |
das Gupta 1965a.
| Methods | Randomized controlled trial Generation of allocation sequence: not stated Blinding: double blind Completeness: not assessable Surveillance: through participant making a postal, telephone, or clinic notification |
|
| Participants |
Number: 79,340 Persons of all ages resident in 5 wards of north‐eastern Calcutta (Beliaghata, Maniktola and Ultadanga areas) Cholera incidence was > 4/1000 in previous years Previous vaccination status not stated explicitly, but it was alluded to in text as a possible factor leading to lower than expected cholera incidence during the trial |
|
| Interventions |
Injected cholera vaccines:
Both vaccines had 8 x 109 organisms/mL Placebo: TAB (CRI) Route: injected subcutaneously Dose: 1 dose; 2 to 4 years received 0.3 mL, 5 to 10 years received 0.5 mL, and ≥ 11 years received 1 mL; dose for infants not given, although from the tables it appears some were vaccinated |
|
| Outcomes | Cholera cases | |
| Location | India | |
| Notes |
Length of follow up (for both phases): was until 31 December 1965 (ie 6 to 10 months) This trial is part 1 of the 1965 trials in India reported in one publication by Das Gupta et al 1967 (see references listed under Taneja 1965 for details). In this first part, vaccination started on 2 February 1965 and finished on 22 March 1965. In the second part (see das Gupta 1965b‐i and das Gupta 1965b‐ii), 25,051 more people from the same Calcutta districts were vaccinated as were an additional 54,606 persons from an adjacent area of the city (total in part II: 79,657) during 2 April to 22 May 1965 The 2 vaccines used in das Gupta 1965a (both classical bivalent killed whole cell) were combined for this analysis |
|
das Gupta 1965b‐i.
| Methods | Randomized controlled trial Generation of allocation sequence: not stated Blinding: double blind Completeness: not assessable Surveillance: through participant making a postal, telephone, or clinic notification |
|
| Participants | Number: 79,657 persons of all ages resident in Calcutta; 25,051 from 5 wards in NE of city (same area as das Gupta 1965a; 54,606 from 5 wards in an adjacent area where the incidence of cholera was > 2/1000 in previous years | |
| Interventions |
Injected cholera vaccine:
All vaccines has 8 x 109 organisms/mL Placebo: TAB (CRI) Route: injected subcutaneously Dose: 1 dose; age 2 to 4 years received 0.3 mL, age 5 to 10 years received 0.5 mL, and age ≥ 11 years received 1 mL |
|
| Outcomes |
|
|
| Location | India | |
| Notes |
Length of follow up: 7 to 8 months This trial reports on 1 arm of part II of the 1965 India trials reported in das Gupta 1965b‐i, which reports the results of 1 of the Classical biotype killed whole cell vaccines (WRAIR) vs placebo |
|
das Gupta 1965b‐ii.
| Methods | Randomized controlled trial | |
| Participants | Same as das Gupta 1965b‐i | |
| Interventions |
Injected cholera vaccine: El Tor bivalent (Ogawa + Inaba) heat‐killed whole cell (Philippines) 8 x 109 organisms/mL Placebo: TAB (CRI) Route: injected Dose: 1 dose as in das Gupta 1965b‐i |
|
| Outcomes | Same as das Gupta 1965b‐i | |
| Location | India | |
| Notes |
Length of follow up: 7 to 8 months This trial reports 1 arm of part II of the 1965 India trials reported by das Gupta 1965b‐i, which reports the results of the El Tor biotype killed whole cell vaccine versus placebo |
|
McCormack 1969.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation according to census number Blinding: double blind Completeness: 97% at 1 year; 95% at 2 years Surveillance: daily clinical assessments at home |
|
| Participants |
Number: 39,862 children included from 53,868 considered Included: children aged 3 months to 14 years, resident in 132 villages in Matlab, Comilla Distirct, East Pakistan; about 50% of children > 5 years showed immunological evidence of previous exposure to cholera |
|
| Interventions |
Injected cholera vaccine: Classical bivalent (Ogawa NIH4, Inaba NIH 35A3) phenol killed whole cell, 4000 x106 organisms/dose Placebo: tetanus + diptheria toxoids Route: injected Dose: 0.5 mL/dose; 1 or 2 doses with 25 to 35 days between injections (September to November 1966) Initially there were 3 groups: OO (2 placebo doses); XO (1 vaccine, 1 placebo); and XX (2 vaccine). Later the trial was extended to 5 years, and third and fourth inoculations were given 1 and 2 years after the first, respectively (in September/October 1967 and September/October 1968). The third XX group was divided into 2. There were then 4 groups: 0000 (4 placebo); X0XX (1 vaccine, 1 placebo, 2 vaccine); XX00 (initially 2 vaccine then 2 placebo); and XXXX (4 vaccine) |
|
| Outcomes |
|
|
| Location | East Pakistan | |
| Notes |
Length of follow up: initially 7 months after first 2 doses; later extended to 5 years The XXXX, XXOO, XOXX groups are combined in the principal analyses. The XXXX and XOXX are combined as booster schedules, whilst the XXOO is analysed as a short schedule. Denominators for years 4 and 5 are assumed to be the same as year 3 (no others are given) |
|
Mosley 1970‐i.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation by census number Blinding: double blind Completeness: 95% at one year Surveillance: daily clinical assessments at home |
|
| Participants |
Number: 45,711 children Aged 0 to 14 years resident in 101 villages in Comilla District, adjacent to Matlab previous trials, East Pakistan; stratified by age (0 to 4, 5 to 9, and 10 to 14 years) |
|
| Interventions |
Injected cholera vaccines:
Placebo: Tetanus/diptheria toxoids Route: injected Dose: 2 doses of 0.5 mL; first dose in October/November 1968; second dose 1 year later in October 1969 (whole cell vaccines only) |
|
| Outcomes |
(Note: most cases (78/83) were due to Inaba serotypes. The paper restricts its analysis mainly to Inaba cases (Ogawa cases are given but not by age of participant)) |
|
| Location | East Pakistan | |
| Notes |
Length of follow up: 3 years from first dose This trial had a booster dose at 1 year. Therefore results for 1 year are after 1 dose, results for years 2 and 3 are after 2 doses This trial reports the results of the comparison Classical Ogawa versus placebo. Mosley 1970‐ii reports the results from the Classical Inaba vaccine versus placebo, while Mosley 1970‐iii reports results for the purified Inaba antigen |
|
Mosley 1970‐ii.
| Methods | Quasi‐randomized controlled trial | |
| Participants | Same as Mosley 1970‐i | |
| Interventions |
Injected cholera vaccine: Classical monovalent (Inaba NIH 35A3) formalin killed whole cell, 8 x 109/mL Placebo: Tetanus/diphtheria toxoids Route: injected Dose: 2 doses of 0.5 mL, 12 months apart |
|
| Outcomes | Same as Mosley 1970‐i | |
| Location | East Pakistan | |
| Notes | See trial Mosley 1970‐i. This trial reports the results of the Classical Inaba vaccine versus placebo | |
Mosley 1970‐iii.
| Methods | Quasi‐randomized controlled trial | |
| Participants | Same as Mosley 1970‐i | |
| Interventions |
Injected cholera vaccine: El Tor monovalent (Inaba V86) antigen 200 μg/mL Placebo: Tetanus/diphtheria toxoids Route: injected Dose: 1 dose |
|
| Outcomes | Same as Mosley 1970‐i | |
| Location | East Pakistan | |
| Notes | See Mosley 1970‐i. This trial reports the results of the Inaba purified antigen versus placebo. This group received 1 dose of the antigen followed by 1 dose of the placebo at 12 months | |
Oseasohn 1965.
| Methods | Quasi‐randomized controlled trial Generation of allocation sequence: alternate allocation according to census number Blinding: double blind Completeness: estimate 10% emigration in 2 years Surveillance: twice weekly assessments at home |
|
| Participants |
Number: 14,059 residents (all ages) 78% had history of prior cholera immunization; 6% had history of previous cholera |
|
| Interventions |
Injected cholera vaccine: classical bivalent (Inaba 35A3, Ogawa 41) phenol‐killed whole cell; 8 x 109 organisms/mL Placebo: TAB (typhoid ‐ paratyphoid A ‐ paratyphoid B) Route: injected Dose: 1, 0.5 mL (reduced to 0.4 mL about half way through trial) for ages > 12 years; 0.25 mL for ages 2 to 12 years; 0.1 mL for ages < 2 years |
|
| Outcomes |
|
|
| Location | East Pakistan: 23 villages in Matlab Thana | |
| Notes |
Length of follow up: 3 years; data reported separately for first year (Oseasohn et al 1965) and for each of 3 cholera 'seasons' (Benenson et al 1968), which were Season 1 (7 months after vaccination in November 1963 (December 1963 to June 1964), Season 2 (July 1964 to June 1965, and Season 3 (July 1965 to June 1966) Cases for season 1 have been entered in outcomes for 'up to 7 months' follow up', while cases for season 2 and 3 have been entered in outcomes for year 2 and 3 respectively (most cases in second and third cholera season fell in years 2 and 3 respectively) |
|
Pal 1980.
| Methods | Randomized controlled trial Generation of allocation sequence: not stated Blinding: double blind Completeness: not assessable Surveillance: through attendance at hospital with diarrhoea |
|
| Participants |
Number: 203,170 Persons aged > 1 year living in 17 municipal wards of Calcutta and 2 adjacent wards of South Dum Dum, India Average annual incidence of hospitalized cases 5/1000 |
|
| Interventions |
Injected cholera vaccine: Classical bivalent (Ogawa + Inaba) killed whole cell, adsorbed onto aluminium phosphate, (CRI Kasauli), 8 x 109 organisms/0.5 mL dose Placebo: tetanus toxoid (CRI Kasauli) Route: injected intramuscularly Dose: 1 dose; 1 to 5 years received 0.25 mL, and > 5 years received 0.5 mL |
|
| Outcomes |
|
|
| Location | India | |
| Notes | Length of follow up: 2 years | |
PCC 1968.
| Methods | Randomized controlled trial Generation of allocation sequence: randomized Blinding: double blind Completeness: not assessable Surveillance: vaccinees were kept under observation by the field team; method and frequency not stated |
|
| Participants |
Number: 359,600 residents (all ages) of Negros Occidental Province, The Philippines An estimated 80% had previously been immunized against cholera |
|
| Interventions |
Injected cholera vaccine: El Tor bivalent (Ogawa 1418, Inaba 6973) killed whole cell Placebo: active placebo (typhoid vaccine) Route: injected Dose: 2 doses at 3 week‐intervals, as follows:
|
|
| Outcomes | Cholera cases | |
| Location | Philippines | |
| Notes |
Length of follow up: 6 months Numbers in each group calculated from a 2% sample of the vaccinated population Results for vaccinated groups A, B, and C have been combined in this analysis |
|
PCC 1973a‐i.
| Methods | Randomized controlled trial Generation of allocation sequence: randomized Blinding: double blind Completeness: not stated Surveillance: vaccinees kept under observation by field team; method and frequency not stated |
|
| Participants |
Number: 223,566 Persons of all ages in coastal communities in Negros Occidental province, Philippines Trial in the same area as the Azurin trials and PCC 1968 |
|
| Interventions |
Injected cholera vaccines:
All vaccines contained 8 x 109 organisms/mL Placebo: active placebo (monovalent typhoid vaccine (BRL Manila)), 1 x 109 organisms/mL Route: injected subcutaneously Dose: 1 dose of 0.5 mL |
|
| Outcomes |
|
|
| Location | Philippines | |
| Notes |
Length of follow up: 7 months Denominators in each group estimated from a 2% sample Vaccines 3 and 4 are the same as those used in Mosley 1970‐i and Mosley 1970‐ii Cases not reported by age group, although it is stated in the text that protection was better in age groups > 5 years than for age 0 to 4 years All cases observed during follow up were El Tor Ogawa This trial reports the results of the El Tor Inaba vaccine (vaccine 1). See trials PCC 1973a‐ii, PCC 1973a‐iii, and PCC 1973a‐ivfor the other vaccines |
|
PCC 1973a‐ii.
| Methods | Randomized controlled trial | |
| Participants | Same as PCC 1973a‐i | |
| Interventions |
Injected cholera vaccine: El Tor monovalent (Ogawa 299 and 1418) whole cell Placebo: monovalent typhoid Route: injected Dose: 1 dose as PCC 1973a‐i |
|
| Outcomes | Same as PCC 1973a‐i | |
| Location | Philippines | |
| Notes | This trial reports the results of the El Tor Ogawa vaccine; see Philippines PCC 1973a‐i for more details | |
PCC 1973a‐iii.
| Methods | Randomized controlled trial | |
| Participants | Same as PCC 1973a‐i | |
| Interventions |
Injected cholera vaccine: Classical monovalent (Ogawa NIH 41) freeze‐dried formalin killed whole cell Placebo: monovalent typhoid Route: injected Dose: 1 dose, see PCC 1973a‐i |
|
| Outcomes | Same as PCC 1973a‐i | |
| Location | Philippines | |
| Notes | This trial reports results of the Classical Ogawa vaccine; see PCC 1973a‐i for more details | |
PCC 1973a‐iv.
| Methods | Randomized controlled trial | |
| Participants | Same as PCC 1973a‐i | |
| Interventions |
Injected cholera vaccine: Classical monovalent (Inaba NIH 42A3) freeze‐dried formalin killed whole cell Placebo: monovalent typhoid vaccine Route: injected Dose: 1 dose, see PCC 1973a‐i |
|
| Outcomes | Same as PCC 1973a‐i | |
| Location | Philippines | |
| Notes | This trial reports the results of the Classical Inaba vaccine; see PCC 1973a‐i for more details | |
PCC 1973b.
| Methods | Randomized controlled trial Generation of allocation sequence: random allocation Blinding: double blind Completeness: not assessable Surveillance: vaccinees kept under observation by field team; method and frequency not stated |
|
| Participants |
Number: 120,840 Persons of all ages resident in Negros Occidental province, Philippines |
|
| Interventions |
Injected cholera vaccines:
Both vaccines contained 8 x 109 organisms/mL Placebo: active placebo (typhoid vaccine (BRL Manila)), 1 x 109 organisms/mL Route and dose: injected subcutaneously (1 x 5 mL dose) or intradermally (1 x 0.2 mL dose) for all ages |
|
| Outcomes | Cholera cases | |
| Location | Philippines | |
| Notes |
Length of follow up: 6.5 months Numbers in each group estimated from a 5% sample The vaccine used (monovalent Ogawa) was chosen on the basis of its efficacy in the PCC 1973 trials against the predominant El Tor Ogawa endemic strains (however it does not appear to have been the most efficacious vaccine in that trial) This trial compared both intradermal and subcutaneous administration with placebo (subcutaneous). The 2 routes have been combined for this review The age groups given are 1 to 5 years and 5 to 14 years. It is unclear which group includes the children aged 5 years |
|
Saroso 1978i.
| Methods | Randomized controlled trial Generation of allocation sequence: allocation method not stated Blinding: double blind Completeness: not assessable Surveillance: through attendance at hospital or clinic with diarrhoea |
|
| Participants |
Number: 470,000 Persons of all ages resident in Surabaya, Indonesia Incidence in 1970 to 1972 was 23 to 74 per 100,000, with 15% of cases in the 1 to 4 years age group |
|
| Interventions |
Injected cholera vaccines:
Both contained 16 x 109 organisms/mL Placebo: Tetanus toxoid adsorbed to aluminium phosphate Route: injected subcutaneously Dose: 1 dose of 0.5 mL to all ages |
|
| Outcomes |
|
|
| Location | Indonesia | |
| Notes |
Length of follow up: 2 years, divided into 4 x 6‐month periods; cases reported by age group only up to 14 months No adverse effect data reported for the placebo group Numbers in each group calculated from a 1% sample of the vaccinated population This trial (Saroso 1978i) reports the results of the 'Plain' vaccine (vaccine 1). Saroso 1978ii reports the results of the classical Al(OH)3 adsorbed vaccine (vaccine 2) The vaccine biotype is not explicitly stated. The predominant cholera during follow‐up was El Tor, serotype Inaba |
|
Saroso 1978ii.
| Methods | Randomized controlled trial | |
| Participants | Same as Saroso 1978i | |
| Interventions |
Injected cholera vaccine: Bivalent killed whole cell aluminium hydroxide adsorbed vaccine Placebo: tetanus toxoid adsorbed to aluminium phosphate Route: injected Dose: 1 dose same as Saroso 1978i |
|
| Outcomes | Same as Saroso 1978i | |
| Location | Indonesia | |
| Notes | This trial reports the results of the Al(OH)3 adsorbed vaccine. Saroso 1978i reports the results of the 'plain vaccine' in the same trial. See Saroso 1978i for more details | |
Taneja 1965.
| Methods | Randomized controlled trial Generation of allocation sequence: not stated Blinding: double blind Completeness: not assessable Surveillance: through participant making a postal, telephone, or clinic notification |
|
| Participants |
Number: 51,135 persons of all ages and both sexes Previous vaccination status not explicitly given, but it is stated that "previous anti‐cholera vaccination, as usually practiced in an endemic zone" might have influenced the results |
|
| Interventions |
Injected cholera vaccine: Classical bivalent (Ogawa + Inaba)
Vaccines made in India contained 8 x 109/dose Placebo: TAB (CRI) Route: injected Dose: 1 dose; 0.2 mL for ages 2 to 4 years; 0.4 mL for ages 5 to 8 years; 0.6 mL for ages 9 to 12 years; 0.8 mL for ages 13 to 15 years; 1 mL for ages > 15 years |
|
| Outcomes |
|
|
| Location | India: Calcutta | |
| Notes |
Length of follow up: 6 months after the last vaccination (vaccination occurred during 12 March to 30 June 1964) WRAIR vaccine (No 3) supply ran short; this group had only 7975 participants compared to 10,784‐10,789 in the other groups Results from all 4 vaccine groups (all of which are Classical bivalent killed whole cell) have been combined at present Rates of adverse effects are very different in the 2 publications |
|
Al(OH)3: aluminium hydroxide (alum).
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Beran 1964 | Observational study |
| Beran 1965 | Nonrandomized study |
| Black 1979 | Nonrandomized study |
| Cabrera 2005 | Not a trial – preparation of vaccine. |
| Chandra Sekar 1947 | Observational study (note 'block' unit of observation) |
| Clasener 1968 | Immunological outcomes only |
| Ganguly 1975 | Immunological outcomes only |
| Gateff 1975 | Immunological outcomes only |
| Gupta 1998 | No placebo group; immunological and adverse outcomes only. Phase 1 trial comparing 2 polysaccharide cholera toxin conjugate vaccines and polysaccharide alone with licensed killed whole cell vaccine. None of the tested vaccines progressed to efficacy trials |
| McBean 1972 | Immunological outcomes only |
| Mosley 1968 | Seroprevalence study |
| Nimbkar 1975 | Immunological outcomes only |
| Peltola 1977 | Nonrandomized study |
| Russell 1927 | Nonrandomized study |
| Sommer 1973a | Randomized controlled trial, but vaccine given after exposure to cholera in family members |
Differences between protocol and review
The following phrase was added to 'Types of Interventions – Intervention': "Exception: Phase 1 trials, reporting only adverse effects, for vaccines that never reached efficacy trials."
The following phrase was deleted from "Types of Interventions – Control": "(trials) comparing types, doses or schedules of injected cholera vaccines". No such comparisons were made in the review.
Contributions of authors
Vittorio Demicheli, Tom Jefferson, Patricia Graves and Jon Deeks read all trials or trial abstracts and determined eligibility. Patricia Graves and Jon Deeks extracted trial data and assessed quality with the assistance of persons named in the Acknowledgments. Patricia Graves. Jon Deeks and Tom Jefferson conducted analyses with input from all authors on the results. All authors commented on the draft review.
Sources of support
Internal sources
Ministry of Defence, UK.
External sources
Department for International Development, UK.
European Commission (Directorate General XII), Belgium.
Declarations of interest
None known.
Unchanged
References
References to studies included in this review
Azurin 1965i {published data only}
- Azurin JC. A controlled field trial on the effectiveness of cholera and cholera el Tor vaccines in the Phillipines. Proceedings of the Cholera Research Symposium. 1965:369‐73. [Google Scholar]
- Azurin JC, Alvero M. Cholera incidence in a population offered cholera vaccination: comparison of cooperative and uncooperative groups. Bulletin of the World Health Organization 1971;44(6):815‐9. [PMC free article] [PubMed] [Google Scholar]
- Azurin JC, Cruz A, Pesigan TP, Alvero M, Camena T, Suplido R, Ledesma L, Gomez CZ. A controlled field trial of the effectiveness of cholera and cholera el Tor vaccines in the Phillipines. Bulletin of the World Health Organization 1967;37(5):703‐27. [PMC free article] [PubMed] [Google Scholar]
- [No authors listed]. A controlled field trial of the effectiveness of cholera and cholera el Tor vaccines in the Phillipines. Preliminary report; Phillipines Cholera Committee. Bulletin of the World Health Organization 1965;32(5):603‐25. [PMC free article] [PubMed] [Google Scholar]
Azurin 1965ii {published data only}
- See Azurin 1965i.
Azurin 1965iii {published data only}
- See Azurin 1965i.
Benenson 1968a {published data only}
- Benenson AS, Joseph PR, Oseasohn RO. Cholera vaccine field trials in East Pakistan. 1. Reaction and antigenicity studies. Bulletin of the World Health Organization 1968;38(3):347‐57. [PMC free article] [PubMed] [Google Scholar]
Benenson 1968b‐i {published data only}
- Benenson A, Mosley WH, Fahimuddin M, Oseasohn RO. Cholera vaccine field trials in East Pakistan. 2. Effectiveness in the field. Bulletin of the World Health Organization 1968;38(3):359‐72. [PMC free article] [PubMed] [Google Scholar]
- Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan 2. A comparison of antibody titres in the immunized and control populations of a cholera‐vaccine field trial area and the relation of antibody titre to cholera case rate. Bulletin of the World Health Organization 1968;38(3):335‐46. [PMC free article] [PubMed] [Google Scholar]
Benenson 1968b‐ii {published data only}
- See Benenson 1968‐i.
Burgasov 1976 {published data only}
- Burgasov PN, Sumarokov AA, Lelikov VL, Marcuk LM, Fedenev VG, Dzaparidze MN, et al. Comparative study of reactions and serological response to cholera vaccines in a controlled field trial conducted in the USSR. Bulletin of the World Health Organization 1976;54(2):163‐70. [PMC free article] [PubMed] [Google Scholar]
Curlin 1975 {unpublished data only}
- Curlin G, Levine M, Aziz KMA, Mizanur Rahman ASM, Verwey WF. Field trial of cholera toxoid. Proceedings of the eleventh joint conference on cholera. New Orleans: US‐Japan Cooperative Medical Science Program, 1975 Nov:314‐29.
- Curlin GT, Levine RJ, Ahmed A, Aziz KMA, Mizanur Rahman ASM, Verwey WF. Immunological aspects of a cholera toxoid field trial in Bangladesh. Dacca: Cholera Research Laboratory, 1978 March. Scientific Report No. 8. [Google Scholar]
das Gupta 1965a {published data only}
- das Gupta A, Sinha R, Shrivastava DL, De SP, Taneja BL, Rao MS, et al. Controlled field trial of the effectiveness of cholera and cholera el tor vaccines in Calcutta. Bulletin of the World Health Organization 1967;37(5):371‐85. [PMC free article] [PubMed] [Google Scholar]
das Gupta 1965b‐i {published data only}
- das Gupta A, Sinha R, Shrivastava DL, De SP, Taneja BL, Rao MS, et al. Controlled field trial of the effectiveness of cholera and cholera El Tor vaccines in Calcutta. Bulletin of the World Health Organization 1967;37(5):371‐85. [PMC free article] [PubMed] [Google Scholar]
das Gupta 1965b‐ii {published data only}
- See das Gupta A 1965b‐i.
McCormack 1969 {published data only}
- McCormack WM, Rahman ASMM, Chowdhury AKMA, Mosley WH, Phillips RA. Report of the 1966‐67 cholera vaccine trial in rural East Pakistan 3. The lack of effect of prior vaccination or circulating vibriocidal antibody on the severity of clinical cholera. Bulletin of the World Health Organization 1969;40(2):199‐204. [PMC free article] [PubMed] [Google Scholar]
- Mosley WH, Aziz KMA, Rahman ASMM, Chowdhury AKMA, Ahmed A, Fahimuddin M. Report of the 1966‐67 cholera vaccine trial in rural East Pakistan. 4. Five years of observation with a practical assessment of the role of a cholera vaccine in cholera control programmes. Bulletin of the World Health Organization 1972;47(2):229‐38. [PMC free article] [PubMed] [Google Scholar]
- Mosley WH, McCormack WM, Ahmed A, Chowdhury AKMA, Barui RK. Report of the 1966‐67 cholera vaccine field trial in rural East Pakistan. 2. Results of the serological surveys in the study population‐‐the relationship of case rate to antibody titre and an estimate of the inapparent infection rate with Vibrio cholerae. Bulletin of the World Health Organization 1969;40(2):187‐97. [PMC free article] [PubMed] [Google Scholar]
- Mosley WH, McCormack WM, Fahimuddin M, Aziz KM, Rahman AS, Chowdhury AK, et al. Report of the 1966‐67 cholera vaccine field trial in rural East Pakistan. I. Study design and results of the first year of observation. Bulletin of the World Health Organization 1969;40(2):177‐85. [PMC free article] [PubMed] [Google Scholar]
Mosley 1970‐i {published data only}
- Mosley WH, Aziz KM, Rahman AS, Chowdhury AK, Ahmed A. Field trials of monovalent Ogawa and Inaba cholera vaccines in rural Bangladesh‐‐three years of observation. Bulletin of the World Health Organization 1973;49(4):381‐7. [PMC free article] [PubMed] [Google Scholar]
- Mosley WH, Woodward WE, Aziz KM, Rahman AS, Chowdhury AK, Ahmed A, et al. The 1968‐1969 cholera‐vaccine field trial in rural East Pakistan. Effectiveness of monovalent Ogawa and Inaba vaccines and a purified Inaba antigen, with comparative results of serological and animal protection tests. Journal of Infectious Diseases 1970;121 Suppl:1‐9. [DOI] [PubMed] [Google Scholar]
Mosley 1970‐ii {published data only}
- See Mosley 1970‐i.
Mosley 1970‐iii {published data only}
- See Mosley 1970‐i.
Oseasohn 1965 {published data only}
- Benenson A, Mosley WH, Fahimuddin M, Oseasohn RO. Cholera vaccine field trials in East Pakistan. 2. Effectiveness in the field. Bulletin of the World Health Organization 1968;38(3):359‐72. [PMC free article] [PubMed] [Google Scholar]
- Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 2. A comparison of antibody titres in the immunized and control population of a cholera‐vaccine field‐trial area and the relation of antibody titre to cholera case rate. Bulletin of the World Health Organization 1968;38(3):335‐46. [PMC free article] [PubMed] [Google Scholar]
- Oseasohn RO, Benenson AS, Fahimuddin M. Cholera vaccine field trial in rural East Pakistan (first year of observation). Proceedings of the Cholera Research Symposium; 1965; Honolulu. 1965:362‐6. [Google Scholar]
Pal 1980 {published data only}
- Pal SC, Deb BC, Sen Gupta PG, De SP, Sircar BK, Sen D, et al. A controlled field trial of an aluminium phosphate‐adsorbed cholera vaccine in Calcutta. Bulletin of the World Health Organization 1980;58(5):741‐5. [PMC free article] [PubMed] [Google Scholar]
PCC 1968 {published data only}
- Philippines Cholera Committee. A controlled field trial of the effectiveness of various doses of cholera el Tor vaccine in the Philippines. Bulletin of the World Health Organization 1968;38(6):917‐23. [PMC free article] [PubMed] [Google Scholar]
PCC 1973a‐i {published data only}
- Philippines Cholera Committee. A controlled field trial of the effectiveness of monovalent classical and el tor cholera vaccines in the Philippines. Bulletin of the World Health Organization 1973;49(1):13‐9. [PMC free article] [PubMed] [Google Scholar]
PCC 1973a‐ii {published data only}
- See PCC 1973b‐i.
PCC 1973a‐iii {published data only}
- See PCC 1973b‐i.
PCC 1973a‐iv {published data only}
- See PCC 1973b‐i.
PCC 1973b {published data only}
- Philippines Cholera Committee. A controlled field trial on the effectiveness of the intradermal and subcutaneous administration of cholera vaccine in the Philippines. Bulletin of the World Health Organization 1973;49(4):389‐94. [PMC free article] [PubMed] [Google Scholar]
Saroso 1978i {published data only}
- Saroso JS, Bahrawi W, Witjaksono H, Budiarso RL, Brotowasisto, Bencić Z, et al. A controlled field trial of plain and aluminium hydroxide‐adsorbed cholera vaccines in Surabaya, Indonesia, during 1973‐‐75. Bulletin of the World Health Organization 1978;56(4):619‐27. [PMC free article] [PubMed] [Google Scholar]
Saroso 1978ii {published data only}
- See Saroso 1978i.
Taneja 1965 {published data only}
- Taneja BL. Controlled field trials of cholera vaccines in Calcutta. Proceedings of the Cholera Research Symposium. 1965:373‐379. [Google Scholar]
- das Gupta A, Sinha R, Shrivastava DL, De SP, Taneja BL, Rao MS, Abou‐Gareeb AH. Controlled field trial of the effectiveness of cholera and cholera el tor vaccines in Calcutta. Bulletin of the World Health Organization 1967;37(5):371‐385. [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Beran 1964 {published data only}
- Beran GW, Elvina O, Florendo FN Jr. Studies on the role of active immunization in the prevention of cholera El Tor. Journal of the Philippine Medical Association 1964;40(2):122‐35. [PubMed] [Google Scholar]
Beran 1965 {published data only}
- Beran GW, Elviña O, Florendo FN Jr. Human volunteer studies using el tor cholera vaccines. I. Post‐vaccinal reactions. Journal of the Philippine Medical Association 1965;41(3):183‐90. [PubMed] [Google Scholar]
Black 1979 {published data only}
- Black RE, Yunus MD, Eusof A, Ahmed A, Khan MR, Sack DA. Report on reactogenicity and immunogenicity of Wellcome cholera toxoids in Bangladeshi volunteers. Dacca, Bangladesh: International Centre for Diarrhoeal Disease Research, 1979 July. Scientific Report No. 29. [Google Scholar]
Cabrera 2005 {published data only}
- Cabrera O, Martínez ME, Cuello M, Soto CR, Valmaseda T, Cedré B, et al. Preparation and evaluation of vibrio cholerae O1 EL Tor Ogawa lipopolysaccharide‐tetanus toxoid conjugates. Vaccine 2006;2006(24 Suppl 2):74‐5. [DOI] [PubMed] [Google Scholar]
Chandra Sekar 1947 {published data only}
- Chandra Sekar C. Statistical assessment if the efficacy of anticholera inoculation from the data of 63 cheris in South Arcot district. Indian Journal of Medical Research 1947;35(3):153‐76. [Google Scholar]
Clasener 1968 {published data only}
- Clasener HA, Beunders BJ. Immunization of man with typhoid and cholera vaccine. Agglutinating antibodies after intracutaneous and subcutaneous injection. Acta Leidensia 1968;36:78‐85. [PubMed] [Google Scholar]
Ganguly 1975 {published data only}
- Ganguly R, Clem LW, Bencic Z, Sinha R, Sakazaki R, Waldman RH. Antibody response in the intestinal secretions of volunteers immunized with various cholera vaccines. Bulletin of the World Health Organization 1975;52(3):323‐30. [PMC free article] [PubMed] [Google Scholar]
Gateff 1975 {published data only}
- Gateff C, Dodin A, Wiart J. A comparison of the serological effects of classical cholera vaccine and of purified fraction vaccine, with or without simultaneous yellow fever vaccine (author's transl). Annales de Microbiologie 1975;126(2):231‐46. [PubMed] [Google Scholar]
Gupta 1998 {published data only}
- Gupta RK, Taylor DN, Bryla DA, Robbins JB, Szu SC. Phase 1 evaluation of Vibrio cholerae 01, serotype Inaba, polysaccharide‐cholera toxin conjugates in adult volunteers. Infection and Immunity 1998;66(7):3095‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
McBean 1972 {published data only}
- McBean AM, Agle AN, Compaore P, Foster SO, McCormack WM. Comparison of intradermal and subcutaneous routes of cholera vaccine administration. Lancet 1972;1(7749):527‐9. [DOI] [PubMed] [Google Scholar]
Mosley 1968 {published data only}
- Mosley WH, Benenson AS, Barui R. A serological survey for cholera antibodies in rural east Pakistan. 1. The distribution of antibody in the control population of a cholera‐vaccine field‐trial area and the relation of antibody titre to the pattern of endemic cholera. Bulletin of the World Health Organization 1968;38(3):327‐34. [PMC free article] [PubMed] [Google Scholar]
Nimbkar 1975 {published data only}
- Nimbkar YS, Karbhari RS, Cherian S, Chanderkar NG, Bhamaria RP, Ranadive PS, et al. Antibody response to two cholera vaccines in volunteers. Progress in Drug Research 1975;19:544‐62. [PubMed] [Google Scholar]
Peltola 1977 {published data only}
- Peltola H, Ruutu P, Palomäki H, Kaukinen K, Aho K. Intracutaneous vaccination technic with less side effects against typhoid and cholera. Duodecim 1977;93(7):431‐8. [PubMed] [Google Scholar]
Russell 1927 {published data only}
- Russell AJH. Bezredka's cholera bilivaccin versus anti‐cholera vaccine: a comparative field test. Transactions of the seventh congress of the Far East Association of Tropical Medicine 1927;1:523‐34. [Google Scholar]
Sommer 1973a {published data only}
- Sommer A, Khan M, Mosley WH. Efficacy of vaccination of family contacts of cholera cases. Lancet 1973;1(7814):1230‐2. [DOI] [PubMed] [Google Scholar]
Additional references
Abba (in progress)
- Abba K, Qadri F, Graves PM, Zaman K. Oral vaccines for preventing cholera. Cochrane Database of Systematic Reviews In progress. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhadra 1994
- Bhadra RK, Dasgupta U, Das J. Cholera vaccine: developmental strategies and problems. Indian Journal of Biochemistry and Biophysics 1994;31(6):441‐8. [PubMed] [Google Scholar]
Calain 2004
- Calain P, Chaine JP, Johnson E, Hawley ML, O'Leary MJ, Oshitani H, et al. Can oral cholera vaccination play a role in controlling a cholera outbreak?. Vaccine 2004;22(19):2444‐51. [DOI] [PubMed] [Google Scholar]
Clemens 1994
- Clemens J, Spriggs D, Sack D. Public health considerations for the use of cholera vaccines in cholera control programes. In: Wachsmuth K, Blake PA, Olvik O editor(s). Vibrio cholerae and cholera: molecular to global perspectives. Washington: American Society for Microbiology, 1994:25‐40. [Google Scholar]
Clemens 1996
- Clemens J, Brenner R, Rao M, Tafari N, Lowe C. Evaluating new vaccines for developing countries. Efficacy or effectiveness?. JAMA 1996;275(5):390‐7. [PubMed] [Google Scholar]
Cvjetanović 1978a
- Cvjetanović B, Grab B, Uemura K. Dynamics of acute bacterial diseases. Epidemiological models and their application in public health. Part I. Theory and practice of epidemiological models. Bulletin of the World Health Organization 1978;56 Suppl 1:9‐23. [PMC free article] [PubMed] [Google Scholar]
Cvjetanović 1978b
- Cvjetanović B, Grab B, Uemura K. Dynamics of acute bacterial diseases. Epidemiological models and their application in public health. Part II. Epidemiological models of acute bacterial diseases. Bulletin of the World Health Organization 1978;56 Suppl 1:25‐143. [PMC free article] [PubMed] [Google Scholar]
Davis 1995
- Davis R, Spencer CM. Live oral cholera vaccine: A preliminary review of its pharmacology and clinical potential in providing protective immunity against cholera. Clinical Immunotherapeutics 1995;4(3):235‐47. [Google Scholar]
Feeley 1978
- Feeley JC, Gangarosa EJ. Field trials of Cholera vaccine [Cholera and related diarrheas]. 43rd Nobel symposium; 1978; Stockholm, Sweden. Basel: Karger, 1980:204‐10. [Google Scholar]
Graves 2001
- Graves PM, Deeks JJ, Demicheli V, Pratt M, Jefferson T. Vaccines for preventing cholera. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000974] [DOI] [Google Scholar]
Jefferson 1996
- Jefferson T, Jefferson V. The quest for trials on the efficacy of human vaccines. Results of the handsearch of Vaccine. Vaccine 1996;14(6):461‐4. [DOI] [PubMed] [Google Scholar]
Jefferson 1998
- Jefferson T. Vaccine trial data systematically assembled, pooled and disseminated by the Cochrane Collaboration. Vaccine 1998;16(16):1487‐95. [DOI] [PubMed] [Google Scholar]
Joo 1974
- Joo I. Cholera vaccines. In: Barua D, Burrows W editor(s). Cholera. Philadelphia: Saunders, 1974:333‐5. [Google Scholar]
Lefebvre 2008
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 (updated February 2008). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org (accessed 1 September 2008).
Sommer 1973b
- Sommer A, Mosley WH. Ineffectiveness of cholera vaccination as an epidemic control measure. Lancet 1973;814:1232‐5. [DOI] [PubMed] [Google Scholar]
Sánchez 1997
- Sánchez JL, Taylor DN. Cholera. Lancet 1997;349(9068):1825‐30. [DOI] [PubMed] [Google Scholar]
WHO 2006a
- WHO. Cholera, 2005. Weekly Epidemiological Record 2006;81:297‐308. [Google Scholar]
WHO 2006b
- World Health Organization, Global Task Force on Cholera Control. Oral cholera vaccine use in complex emergencies: what next? Report of a WHO meeting, 14‐16 December 2005, Cairo, Egypt [WHO/CDS/NTD/IDM/2006.2]. Geneva: World Health Organization, 2006. [Google Scholar]


