Abstract
Background
Using a pilot system we have categorised this review as: Current question ‐ no update intended (topic covered in another review. Refer to: Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD007483. DOI: 10.1002/14651858.CD007483.pub2.) Please see "Published notes" section of the review for more details.
The World Health Organization recommends artemether‐lumefantrine for treating uncomplicated malaria. We sought evidence of superiority of the six‐dose regimen over existing treatment regimens as well as its effectiveness in clinical situations.
Objectives
To evaluate the six‐dose regimen of artemether‐lumefantrine for treating uncomplicated falciparum malaria.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register (April 2005), CENTRAL (The Cochrane Library 2005, Issue 1), MEDLINE (1966 to April 2005), EMBASE (1974 to April 2005), LILACS (1982 to April 2005), conference proceedings, and reference lists of articles. We also contacted experts in malaria research and the pharmaceutical company that manufactures artemether‐lumefantrine.
Selection criteria
Randomized controlled trials comparing six doses of artemether‐lumefantrine administered orally with standard treatment regimens (single drug or combination), or supervised with unsupervised treatment, for uncomplicated falciparum malaria.
Data collection and analysis
Two authors independently applied inclusion criteria to potentially relevant trials, assessed the risk of bias in the trials, and extracted data, including adverse events. Total failure by day 28 (day 42 for sulfadoxine‐pyrimethamine and day 63 for mefloquine) was the primary outcome.
Main results
Nine trials (4547 participants) tested the six‐dose regimen. Total failure at day 28 for artemether‐lumefantrine was lower when compared with amodiaquine (270 participants, 1 trial), amodiaquine plus sulfadoxine‐pyrimethamine (507 participants, 1 trial), but not with chloroquine plus sulfadoxine‐pyrimethamine (201 participants, 2 trials). In comparisons with artemisinin derivative combinations, artemether‐lumefantrine performed better than amodiaquine plus artesunate (668 participants, 2 trials), worse than mefloquine plus artesunate (270 participants, 4 trials), and no differently to dihydroartemisinin‐napthoquine‐trimethoprim (89 participants, 1 trial).
Authors' conclusions
The six‐dose regimen of artemether‐lumefantrine appears more effective than antimalarial regimens not containing artemisinin derivatives.
8 May 2019
No update planned
Other
No update planned. The six‐dose regimen is now used as first‐line treatment. It is included as a comparator in other Cochrane Reviews, for example Zani 2014 https://doi.org/10.1002/14651858.CD010927/full
Keywords: Humans, Antimalarials, Antimalarials/administration & dosage, , , /administration & dosage, Drug Combinations, Ethanolamines, Fluorenes, Fluorenes/administration & dosage, , /drug therapy, Randomized Controlled Trials as Topic, Sesquiterpenes, Sesquiterpenes/administration & dosage
Plain language summary
Artemether‐lumefantrine (six‐dose regimen) for treating uncomplicated malaria
Using a pilot system we have categorised this review as: Current question ‐ no update intended (topic covered in another review. Refer to: Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD007483. DOI: 10.1002/14651858.CD007483.pub2.) Please see "Published notes" section of the review for more details.
Malaria is a parasitic disease, spread by mosquitoes. It affects millions of people worldwide, and causes significant illness and mortality. Uncomplicated malaria presents with symptoms such as fever, headache, muscle pain, and vomiting. The parasite has become resistant to a number of previously effective drugs, and so combinations of drugs are used to try increase cure and to prevent further resistance. Artemether‐lumefantrine is one such drug combination. This review of trials showed that the six‐dose regimen of artemether‐lumefantrine was associated with high cure rates and was more effective that most other drug combinations used for uncomplicated malaria. Further research is needed to properly assess adverse outcomes.
Background
Malaria
Malaria is a major health problem with at least 300 to 500 million people diagnosed with the illness every year (WHO 2000a). The main cause is Plasmodium falciparum, one of the four species of malaria parasites found in humans. Uncomplicated malaria occurs in the majority of those affected, and is the form of the illness which presents with such symptoms as fever, headache, muscle pain (myalgia), vomiting, mild diarrhoea, anaemia, and enlarged spleen (splenomegaly). In addition, children commonly present with rapid breathing (tachypnoea), cough, and convulsions.
Antimalarial drug resistance
Resistance to antimalarial drugs emerged in South‐East Asia and South America (White 1999a), and then spread to Africa and western Oceania. Sulfadoxine‐pyrimethamine has replaced chloroquine as the first‐line treatment in some African countries (such as Malawi and Kenya), but resistance to this is now also emerging (WHO 2000a). Resistance to sulfadoxine‐pyrimethamine is relatively common in South‐East Asia (WHO 2001b), where resistance and declining sensitivity to mefloquine have also been reported (WHO 2000a). Mefloquine is contraindicated in areas of intensive malaria transmission, such as sub‐Saharan Africa, because its long half life may expose parasites to subcurative doses, which could result in the development of resistant strains (WHO 2000a).
Artemisinin drugs, including artemether and artesunate, are now used as first‐line treatment in some countries in South‐East Asia, but they are recommended only as combination treatment (WHO 2000a). Such combination therapy affords rapid clinical response and higher cure rates when compared with other antimalarial combinations (White 1999a). It is also thought combination therapy may slow the parasite developing resistance to the drug (White 1999b).
Artemether‐lumefantrine combination
The fixed‐dose combination of artemether‐lumefantrine, called co‐artemether, contains 20 mg of artemether and 120 mg of lumefantrine (previously called benflumetol). It was initially developed by scientists at the Academy of Military Medical Sciences in China before the pharmaceutical company Novartis (Switzerland) became a partner and was licensed to market it as Coartem® or Riamet®. This oral preparation has been designed for use against chloroquine‐resistant falciparum malaria. Artemether has a rapid onset of action and is rapidly eliminated from the plasma (half life of two to three hours; Lefèvre 1999). Lumefantrine is cleared more slowly and has a longer elimination half life (approximately 4.5 days; Ezzet 1998). The rationale behind this combination is that artemether initially provides rapid symptomatic relief by reducing the number of parasites present before lumefantrine eliminates any residual parasites. This is thought to minimize development of resistance because the malaria parasites are never exposed to artemether alone (due to its rapid elimination). Although they may be exposed to lumefantrine alone, the probability of resistance developing simultaneously to both drugs used in combination is thought to be low (Bloland 2000). Artemether‐lumefantrine also reduces gametocyte carriage and thus should have an impact on malaria transmission (Van Vugt 1998a).
There has been some concern about the possible risk of neurotoxicity with artemisinin derivatives that arose from animal studies using high doses of lipid‐soluble preparations given intramuscularly (WHO 1999). No serious adverse or persistent neurotoxic adverse events have been documented (Novartis 2005). There has been concern that the lumefantrine component could have adverse cardiac effects due to its similar structure to halofantrine (Bindschedler 2000). Artemether‐lumefantrine causes minimal QTc prolongation which was not associated with adverse clinical cardiac events (Novartis 2005). These potential adverse effects have to be considered when assessing the drug combination.
Artemether‐lumefantrine has been added to the WHO Model list of Essential Medicines and is being promoted in Africa as first‐line treatment for malaria by the World Health Organization. The World Health Organization has commended the company for providing the drug at discounted prices for developing countries in malaria endemic areas ( WHO 2001a).
Rationale for review
Since the first Cochrane Review on artemether‐lumefantrine was published (Omari 2002), the six‐dose regimen has become the standard, as researchers acknowledged the review findings that the four‐dose regimen was associated with treatment failures (Nosten 2003). Trials are generally using the six‐dose regimen, with the evidence for the four‐dose regimen maintained in a separate Cochrane Review (Omari 2006). This review aims to summarize the existing evidence of the six‐dose regimen of artemether‐lumefantrine and how it compares with other antimalarial drugs for treating uncomplicated falciparum malaria, including mefloquine, sulfadoxine‐pyrimethamine, and chloroquine.
For our endpoint, we use total failure by day 28 as the primary outcome measure, or day 42 for sulfadoxine‐pyrimethamine and day 63 for mefloquine because of their long half lives. In areas where malaria transmission is intense, recurrence of parasites by day 28 could also be due to reinfection, so we also examine the polymerase chain reaction (PCR) which is thought to distinguish between a new infection and recurrence of malaria (recrudescence) due to drug resistance.
Objectives
To evaluate the six‐dose regimen of artemether‐lumefantrine for treating uncomplicated falciparum malaria.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials.
Types of participants
Adults and children with acute uncomplicated malaria, as defined in WHO 2000b, with asexual P. falciparum parasitaemia confirmed using blood slides.
Types of interventions
Six doses of artemether‐lumefantrine administered orally versus standard treatment regimens (single drugs or combinations).
Supervised versus unsupervised treatment with the six doses of artemether‐lumefantrine.
Types of outcome measures
Primary
Total failure by day 28, day 42 (for sulfadoxine‐pyrimethamine), or day 63 (for mefloquine); defined as a recurrent malaria infection with or without clinical malaria.
Secondary
Total failure, defined as a recurrent malaria infection with or without clinical malaria, by day 7.
Total failure, defined as a recurrent malaria infection with or without clinical malaria, by day 14.
Total failure adjusted by PCR to exclude new infections by day 28 (recrudescent infections).
Parasite clearance time (PCT), defined as the time between commencing treatment and the first negative blood test when negativity persists for more than 48 hours; PCT 50, defined as the time taken for parasites to be reduced to 50% of first test value; and PCT 90, defined as the time taken for parasites to be reduced to 10% of first test value.
Fever clearance time, defined as the time between commencing treatment and the temperature returning to normal and remaining normal for more than 48 hours.
Gametocyte carriage on days 14 and 28.
Gametocyte clearance time, defined as the time taken for gametocytes to disappear (if present in the blood initially) after commencing treatment.
Adverse events
Adverse events requiring discontinuation of treatment, or are fatal, life‐threatening, or requiring hospitalization.
Other adverse events.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Databases
We searched the following databases using the search terms and strategy described in Appendix 1: Cochrane Infectious Diseases Group Specialized Register (April 2005); Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 1, 2005); MEDLINE (1966 to April 2005); EMBASE (1974 to April 2005); and LILACS (1982 to April 2005).
Conference proceedings
We searched the following conference proceedings for relevant abstracts: The Third Multilateral Initiative on Malaria Pan‐African Conference, 18 to 22 November 2002, Arusha, Tanzania; and the Second European Congress on Tropical Medicine, 14 to 18 September 1998, Liverpool, UK.
Researchers, organizations, and pharmaceutical companies
We contacted researchers working in the field, the World Health Organization, and the pharmaceutical company Novartis for unpublished and ongoing trials.
Data collection and analysis
Selection of studies
Aika Omari (AO) screened the results of the search strategy to identify potentially relevant trials. AO and Carrol Gamble (CG) independently assessed the eligibility of these trials for inclusion in the review using the stated inclusion criteria. Any differences in opinion between the authors were discussed with the third author Paul Garner.
Data extraction and management
AO and CG independently extracted data of trial characteristics including methods, participants, interventions, and outcomes, and recorded the data on standard forms. Where data from the published papers were insufficient or missing, we contacted the trial authors for additional information.
Where possible, we extracted data to allow an intention‐to‐treat analysis (the analysis should include all the participants in the groups to which they were originally randomly assigned). If the number randomized and the numbers analysed were inconsistent, we calculated the percentage loss to follow up. For dichotomous outcomes, we recorded the number of participants experiencing the event in each group of the trial. For continuous outcomes, we extracted arithmetic means and standard deviations and combined means using mean difference for each group where possible. If the data were reported using geometric means, we extracted standard deviations on the log scale, and extracted and reported the medians and ranges.
Assessment of risk of bias in included studies
We assessed of the generation of allocation sequence and concealment of allocation as adequate, inadequate, or unclear according to Jüni 2001. We described who was blinded to the interventions, such as the participants, care providers, or outcome assessors. We assessed the inclusion of all randomized participants in the main effectiveness analysis to be adequate if more than 90% were included in the analysis, inadequate if 90% or less, or unclear.
Data synthesis
We compared the drug with non‐artemisinin derivative regimens, other artemisinin regimens, and then other comparisons that examined delivery. Adverse events from all trials were reported together. We analysed data using Review Manager 5. We compared outcome measures for dichotomous data using risk ratio (RR), which is the risk of achieving an outcome in the artemether‐lumefantrine group relative to that in the control group. We used total failure (clinical or parasitological failure by day 28) as our main outcome, and we also conducted analysis excluding reinfection where PCR data were available. As the value of the risk ratio is constrained to lie between 0 and 1/CGER (control group event rate), large values of the risk ratio are impossible when events are common, so failure is preferred to treatment success. We would consider the DerSimonian Laird random‐effects model if there was significant heterogeneity.
We intend to explore the following potential sources of heterogeneity using subgroup analyses or meta‐regression: participant age (under five years versus five years or more); trial setting (high malaria transmission versus low transmission); and the presence of drug resistance to comparator drug, as new trials become available. Additional trials may allow sensitivity analyses according to blinding, allocation concealment, and whether the trials used an intention‐to‐treat analysis at a future date.
In determining the effectiveness of antimalarial treatment, we intended to extract the results of analyses conducted according to the intention‐to‐treat principle. This approach is considered to be more pragmatic as it attempts to estimate the effectiveness of the treatment in routine practice rather than in the context of a clinical trial. To allow the intention‐to‐treat principle to be applied, all participants should be followed for the duration of the trial irrespective of whether or not the treatment course was completed or other protocol deviations. Any reason for dropping out of the trial or being excluded from the trial should be documented (WHO 1996).
For total failure with trials that had conducted PCR analysis, we classified the infections into: recrudescent infection (matching genotypes on day 0 and day of recurrence); new infection (different genotypes on day 0 and day of recurrence); and missing values. We intended to conduct a sensitivity analysis around PCR examining the effect of missing data, but there were too few trials for us to do this.
Results
Description of studies
We identified 31 potentially relevant studies. Nine met the inclusion criteria (see 'Characteristics of included studies'); one trial was reported across two publications (Van Vugt 2000). We excluded 16 studies, including one reported in two separate publications (Hatz 1998), for the reasons given in the 'Characteristics of excluded studies'. We have requested data since 2003 on four studies from Novartis (cited in Novartis 1999), but have not yet received a response.
Trial location
Four trials were conducted in Africa, one in each of Burundi (Ndayiragije 2004), The Gambia (Sutherland 2005), Tanzania (Mutabingwa 2005), and Uganda (Piola 2005). The other five trials were conducted in South‐East Asia, in Lao Peoples Democratic Republic (PDR) (Mayxay 2004; Stohrer 2004) and in Thailand (Van Vugt 2000; Lefevre 2001; Krudsood 2003).
Trial funding
Two trials reported that they were sponsored by Novartis (Van Vugt 2000; Lefevre 2001). Other trials were funded by the Gates Malaria Partnership (Mutabingwa 2005; Sutherland 2005), the UNDP/World Bank/Special Programme for Research and Training in Tropical Diseases (Krudsood 2003), the Wellcome Trust (Mayxay 2004; Sutherland 2005), the World Health Organization (Ndayiragije 2004), USAID (Stohrer 2004), the Medical Research Council, UK (Sutherland 2005), and Médecins Sans Frontières (Piola 2005).
Participants
Four trials included 2933 children (Mayxay 2004; Ndayiragije 2004; Mutabingwa 2005; Sutherland 2005), three included 1265 adults and children (Van Vugt 2000; Stohrer 2004; Piola 2005), and two trials included 349 participants over 13 years of age (Lefevre 2001; Krudsood 2003).
Interventions
Two trials had more than two arms: chloroquine plus sulfadoxine‐pyrimethamine and mefloquine plus artesunate were the comparators in Mayxay 2004; and amodiaquine, amodiaquine plus sulfadoxine‐pyrimethamine, and amodiaquine plus artesunate were the comparators in Mutabingwa 2005.
Two trials each compared artemether‐lumefantrine with chloroquine plus sulfadoxine‐pyrimethamine (Mayxay 2004; Sutherland 2005) and amodiaquine plus artesunate (Ndayiragije 2004; Mutabingwa 2005). Other comparisons were with dihydroartemisinin‐napthoquine‐trimethoprim (Krudsood 2003), artesunate plus amodiaquine (Ndayiragije 2004), amodiaquine (Mutabingwa 2005), and amodiaquine plus sulfadoxine‐pyrimethamine (Mutabingwa 2005). Mefloquine plus artesunate was the comparator in four trials (Van Vugt 2000; Lefevre 2001; Mayxay 2004; Stohrer 2004).
One trial compared supervised and unsupervised treatment with artemether‐lumefantrine (Piola 2005).
Dose and regimen
All trials administered the six doses over 72 hours. Children received between 3.8 and 16 mg/kg of artemether and between 48 and 96 mg/kg of lumefantrine; adults received 480 mg of artemether and 2280 mg of lumefantrine.
Antimalarial drug resistance
Chloroquine resistance and sulfadoxine‐pyrimethamine resistance were reported in trials conducted in Tanzania, Burundi, The Gambia, Uganda, and Lao PDR. Multiple‐drug resistance was reported in Thailand.
Outcome measures
(See Appendix 2). Total failure (illness with parasitaemia or parasitaemia detected by day 28) was the most frequently reported outcome (six of the nine trials). Two trials reported failure by day 42 (Mayxay 2004; Stohrer 2004). Trials also reported the number of treatment failures at other time points (days one, two, three, seven, and 14). Fever clearance was reported in three trials, and time to parasite clearance was reported in three trials. Gametocyte carriage was reported in eight trials and gametocyte clearance in two trials. Polymerase chain reaction (PCR) analysis was reported in seven trials, and all trials reported adverse events.
Risk of bias in included studies
SeeTable 1for the assessment and the 'Characteristics of included studies' for details.
1. Risk of bias of each included studya.
| Study | Allocation sequence generation | Allocation concealment | Blinding | Inclusionb |
| Krudsood 2003 | Not described | Not described | No | Inadequate |
| Lefevre 2001 | Not described | Not described | No | Adequate |
| Mayxay 2004 | Not described | Adequate | No | Adequate |
| Mutabingwa 2005 | Adequate | Adequate | No | Adequate |
| Ndayiragije 2004 | Not described | Not described | No | Adequate |
| Piola 2005 | Adequate | Adequate | No | Adequate |
| Stohrer 2004 | Not described | Adequate | No | Adequate |
| Sutherland 2005 | Not described | Adequate | Single | Inadequate |
| Van Vugt 2000 | Not described | Adequate | No | Inadequate |
aInclusion of all randomized participants in the analysis; see the 'Methods of the review' for the assessment methods, and the 'Characteristics of included studies' for the methods used in each trial.
Generation of allocation sequence
All the trials were reported as randomized. Two trials reported using an adequate method to generate the allocation sequence. The remaining seven trials mentioned randomization, but they did not report how they generated the allocation sequence.
Concealment of allocation
Allocation concealment was adequate in the six trials that used central randomization, or numbered, sealed, opaque envelopes. The other three trials did not describe the method used to conceal allocation.
Blinding
One trial was single blind in which all staff apart from those in recruiting clinic and field assistants were not aware of the treatment group. The remaining eight trials were described as open.
Inclusion of randomized participants in the analysis
None of the nine trials had complete data for all participants randomized into the trial for the duration of follow up. This was partly because researchers stopped follow up after a participant withdrew. Therefore an intention‐to‐treat analysis was not possible for the trial investigators or for this review because data necessary for an intention‐to‐treat analysis were not collected. All trials gave results of analyses based on evaluable participants, that is, participants still on treatment at each time point. Three of the trials, however, also claimed to have reported cure rates as an 'intention‐to‐treat' analysis (Lefevre 2001; Mayxay 2004; Sutherland 2005) These are not the results of an intention‐to‐treat analysis, and differed from their evaluable participants analysis by assuming that all participants withdrawn from treatment or lost to follow up still had parasitaemia at all remaining time points. At the end of follow up, the number of participants evaluable for the primary outcome was greater than 90% in six trials and 85% to 90% in three trials .
Effects of interventions
1. Versus non‐artemisinin derivatives
1.1 Amodiaquine (789 participants, 1 trial)
Mutabingwa 2005, conducted in Tanzania, reported fewer total failures with artemether‐lumefantrine on day 28 (RR 0.29 95% CI 0.26 to 0.34; 724 participants, Analysis 1.1) and day 14 (RR 0.03, 95% CI 0.01 to 0.05; 750 participants, Analysis 1.2).
1.1. Analysis.
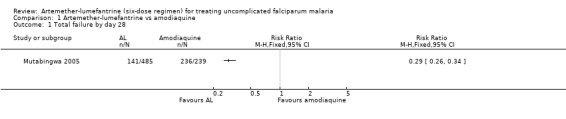
Comparison 1 Artemether‐lumefantrine vs amodiaquine, Outcome 1 Total failure by day 28.
1.2. Analysis.
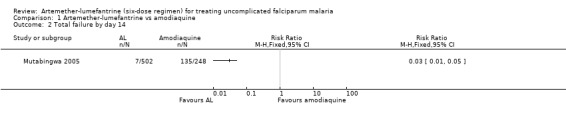
Comparison 1 Artemether‐lumefantrine vs amodiaquine, Outcome 2 Total failure by day 14.
Gametocyte carriage on day 14 was lower for artemether‐lumefantrine (RR 0.32, 95% CI 0.18 to 0.56; 461 participants, Analysis 1.3).
1.3. Analysis.
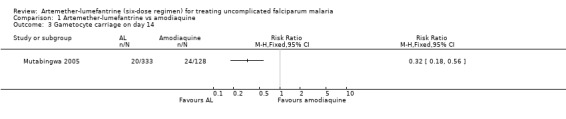
Comparison 1 Artemether‐lumefantrine vs amodiaquine, Outcome 3 Gametocyte carriage on day 14.
1.2 Chloroquine plus sulfadoxine‐pyrimethamine (717 participants, 2 trials)
Chloroquine plus sulfadoxine‐pyrimethamine was one of two comparators in the trial from Lao PDR (Mayxay 2004), and was the only comparator in the trial in Gambian children (Sutherland 2005). Sutherland 2005 reported on the outcome measures on days 28, 14, and 7, while Mayxay 2004 only reported on day 42.
Fewer total failures occurred in the artemether‐lumefantrine group, but the results were not statistically significant by day 42 (RR 0.95, 95% CI 0.48 to 1.87; 216 participants, Analysis 2.2), day 28 (RR 0.90, 95% CI 0.46 to 1.77; 427 participants, Analysis 2.1), day 14 (RR 0.44, 95% CI 0.11 to 1.74; 435 participants, Analysis 2.2), or day 7 (RR 0.22, 95% CI 0.01 to 3.48; 410 participants, Analysis 2.2).
2.2. Analysis.
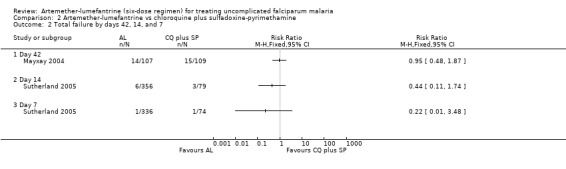
Comparison 2 Artemether‐lumefantrine vs chloroquine plus sulfadoxine‐pyrimethamine, Outcome 2 Total failure by days 42, 14, and 7.
2.1. Analysis.
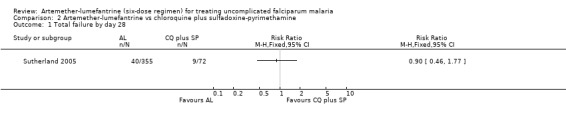
Comparison 2 Artemether‐lumefantrine vs chloroquine plus sulfadoxine‐pyrimethamine, Outcome 1 Total failure by day 28.
Mayxay 2004 reported that the parasite clearance time was significantly (P < 0.001) faster with artemether‐lumefantrine (2.08 days, 95% CI 2.0 to 2.1; 107 participants) than chloroquine plus sulfadoxine‐pyrimethamine (2.9 days, 95% CI 2.8 to 3.0; 102 participants); seeAppendix 3.
The mean fever clearance time was also statistically significantly (P < 0.001) faster with artemether‐lumefantrine (23.1 h, 95% CI 20.9 to 25.3; 107 participant) compared with chloroquine plus sulfadoxine‐pyrimethamine (40.2 h, 95% CI 35.9 to 44.4; 102 participants); seeAppendix 4.
Sutherland 2005 reported that gametocyte carriage was lower with artemether‐lumefantrine by day 28, day 14, and day 7 (Analysis 2.3). Mayxay 2004 reported that five of 100 participants in the artemether‐lumefantrine group, and 28 of 110 participants in the chloroquine plus sulfadoxine‐pyrimethamine were carrying gametocytes after treatment.
2.3. Analysis.
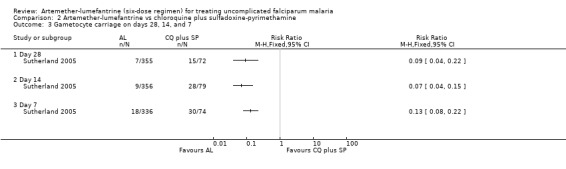
Comparison 2 Artemether‐lumefantrine vs chloroquine plus sulfadoxine‐pyrimethamine, Outcome 3 Gametocyte carriage on days 28, 14, and 7.
1.3 Amodiaquine plus sulfadoxine‐pyrimethamine (1026 participants, 1 trial)
Mutabingwa 2005 reported fewer total failures with artemether‐lumefantrine on day 28 (RR 0.36, 95% CI 0.32 to 0.42; 948 participants, Analysis 3.1) and day 14 (RR 0.05, 95% CI 0.02 to 0.11; 978 participants, Analysis 3.2). Gametocyte carriage on day 14 was lower for artemether‐lumefantrine (RR 0.23, 95% CI 0.15 to 0.37; 617 participants, Analysis 3.3).
3.1. Analysis.
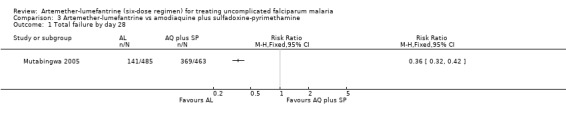
Comparison 3 Artemether‐lumefantrine vs amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 1 Total failure by day 28.
3.2. Analysis.
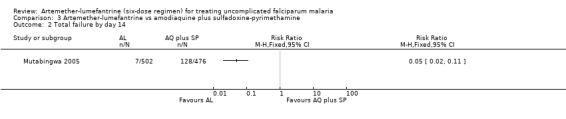
Comparison 3 Artemether‐lumefantrine vs amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 2 Total failure by day 14.
3.3. Analysis.
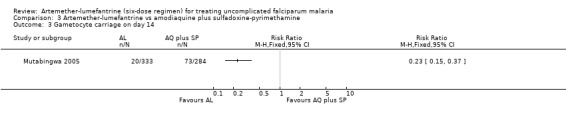
Comparison 3 Artemether‐lumefantrine vs amodiaquine plus sulfadoxine‐pyrimethamine, Outcome 3 Gametocyte carriage on day 14.
2. Versus other artemisinin derivatives
2.1 Amodiaquine plus artesunate (1329 participants, 2 trials)
The trials in Burundi and Tanzanian used this comparator (Ndayiragije 2004; Mutabingwa 2005).
On day 28, there were statistically significantly fewer total failures with artemether‐lumefantrine in Mutabingwa 2005 (RR 0.56, 95% CI 0.48 to 0.66; 957 participants, Analysis 4.1). On day 14, there were fewer parasitological failures in both trials (RR 0.11, 95% CI 0.05 to 0.23; 1283 participants, Analysis 4.2).
4.1. Analysis.
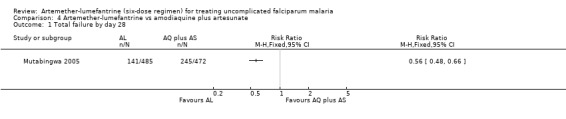
Comparison 4 Artemether‐lumefantrine vs amodiaquine plus artesunate, Outcome 1 Total failure by day 28.
4.2. Analysis.
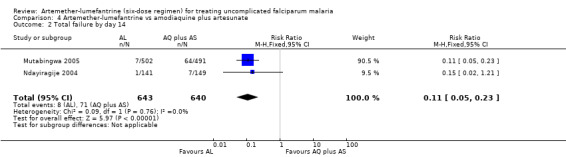
Comparison 4 Artemether‐lumefantrine vs amodiaquine plus artesunate, Outcome 2 Total failure by day 14.
On day 14, gametocyte carriage was significantly lower with artemether‐lumefantrine in Mutabingwa 2005, there was little difference in Ndayiragije 2004. The overall meta‐analysis showed an effect (RR 0.56, 95% CI 0.35 to 0.91; 941 participants, P = 0.27, Analysis 4.3). Ndayiragije 2004 also reported gametocyte carriage on day 7; it was lower with artemether‐lumefantrine (RR 0.68, 95% CI 0.33 to 1.41; 290 participants, Analysis 4.3).
4.3. Analysis.
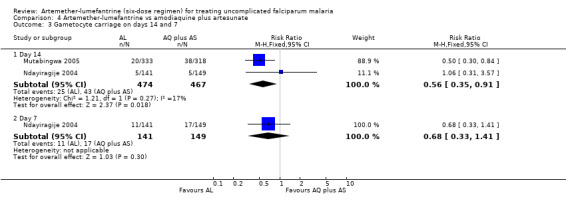
Comparison 4 Artemether‐lumefantrine vs amodiaquine plus artesunate, Outcome 3 Gametocyte carriage on days 14 and 7.
2.2 Mefloquine plus artesunate (419 participants, 4 trials)
Two of the four trials that used these antimalarials were conducted in Thailand (Van Vugt 2000; Lefevre 2001). The other two were conducted in Lao PDR (Mayxay 2004; Stohrer 2004); mefloquine plus artesunate was one of the three comparators in Mayxay 2004.
Total failure by day 28 was more common with artemether‐lumefantrine in the two trials that measured this (Van Vugt 2000; Lefevre 2001), but the results − individually and in a meta‐analysis − did not demonstrate a significant difference (RR 4.20, 95% CI 0.55 to 31.93; 389 participants, P = 0.81, Analysis 5.1). Of the 11 participants with parasitaemia on day 28, 10 had a PCR analysis to identify new infections from recrudescent infections; the analysis showed that only two were new infections (Analysis 5.2 and Appendix 5).
5.1. Analysis.
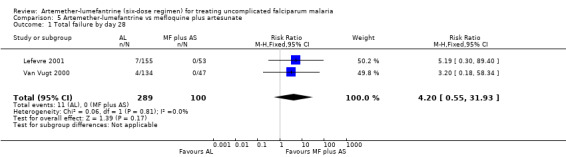
Comparison 5 Artemether‐lumefantrine vs mefloquine plus artesunate, Outcome 1 Total failure by day 28.
5.2. Analysis.
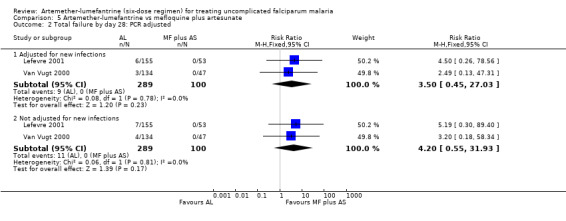
Comparison 5 Artemether‐lumefantrine vs mefloquine plus artesunate, Outcome 2 Total failure by day 28: PCR adjusted.
On day 42, more participants treated with artemether‐lumefantrine had treatment failures in Stohrer 2004 and Mayxay 2004, and the difference was significant with meta‐analysis (RR 2.93, 95% CI 1.48 to 5.80; 315 participants, P = 0.10, Analysis 5.3). All participants with parasitaemia on day 42 in Stohrer 2004 had a PCR analysis to identify new infections from recrudescent infections (Analysis 5.4). All eight failures in the mefloquine plus artesunate group were new infections, and of the 13 failures in the artemether‐lumefantrine group, 10 were new infections and three were recrudescent infections (Appendix 6). Mayxay 2004 reported PCR analysis on day seven but did not separate the treatment groups − of 25 failures, 20 were new and five were recrudescent infections.
5.3. Analysis.
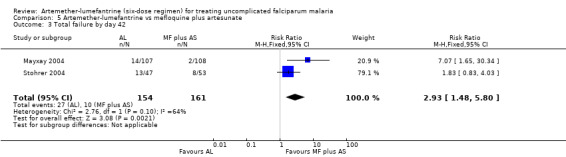
Comparison 5 Artemether‐lumefantrine vs mefloquine plus artesunate, Outcome 3 Total failure by day 42.
5.4. Analysis.
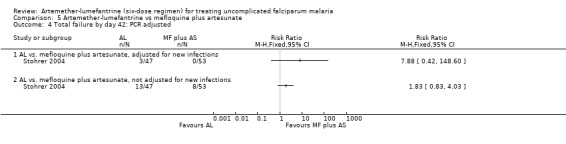
Comparison 5 Artemether‐lumefantrine vs mefloquine plus artesunate, Outcome 4 Total failure by day 42: PCR adjusted.
Lefevre 2001 reported no statistically significant difference in the parasite clearance time between artemether‐lumefantrine (median 29 h, 95% CI 29 to 32; 164 participants) and mefloquine plus artesunate (median 31 h, 95% CI 26 to 31; 55 participants), although there was no statistical test reported. In Mayxay 2004, parasite clearance times were similar between artemether‐lumefantrine (2.08 days, 95% CI 2.0 to 2.1; 107 participants) and mefloquine plus artesunate (2.07 days, 95% CI 2.0 to 2.1; 110 participants) (P value not reported); seeAppendix 3.
Lefevre 2001 reported a median fever clearance time of 29 hours (95% CI 23 to 37; 76 participants) for artemether‐lumefantrine compared with 23 hours (95% CI 15 to 30; 29 participants) for mefloquine plus artesunate, with no statistical test reported. Mayxay 2004 reported that the mean fever clearance times were similar for artemether‐lumefantrine (23.1 h, 95% CI 20.9 to 25.3; 107 participants) and mefloquine plus artesunate (24.6 h, 95% CI 21.8 to 27.3; 110 participants) (P value not reported); seeAppendix 4.
Lefevre 2001 and Stohrer 2004 reported gametocyte clearance times. In Lefevre 2001, the median time for artemether‐lumefantrine was 72 hours (95% CI 34 to 163; 26 participants) compared with 85 hours (95% CI 46 to 160; 10 participants) for mefloquine plus artesunate. As the confidence intervals overlap, it is unlikely the difference between the two groups is significant. In Stohrer 2004, the mean gametocyte clearance time for artemether‐lumefantrine was 10.5 days (95% CI 4.35 to 16.65; 47 participants) compared with 7.0 days (95% CI 7.0 to 7.0; 53 participants) for mefloquine plus artesunate; P = 0.6 with Mann‐Whitney U‐test; seeAppendix 7.
Stohrer 2004 and Mayxay 2004 also reported on gametocyte carriage on day 7. In Stohrer 2004, it was higher with artemether‐lumefantrine (RR 1.35, 95% CI 0.44 to 4.15; 100 participants, Analysis 5.5). Mayxay 2004 reported that the numbers of participants carrying gametocytes after treatment was 5/100 for artemether‐lumefantrine and 4/110 for mefloquine plus artesunate; no time point was given so it was not possible to include this in a meta‐analysis. Van Vugt 2000 and Lefevre 2001 reported gametocyte carriage within the first 72 hours; there was no significant difference in carriage between the groups (RR 1.09, 95% CI 0.58 to 2.06; 240 participants, P = 0.18, Analysis 5.5).
5.5. Analysis.
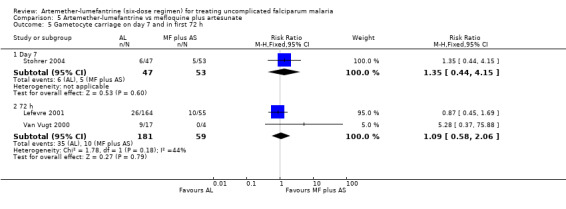
Comparison 5 Artemether‐lumefantrine vs mefloquine plus artesunate, Outcome 5 Gametocyte carriage on day 7 and in first 72 h.
2.3 Dihydroartemisinin‐napthoquine‐trimethoprim (DNP) (130 participants, 1 trial)
Krudsood 2003, which was conducted in Thailand, reported equal numbers of parasitological failures in both groups on day 28 (RR 2.35, 95% CI 0.15 to 36.54, Analysis 6.1). This result was not statistically significant, but it is imprecise due to the wide confidence interval.
6.1. Analysis.
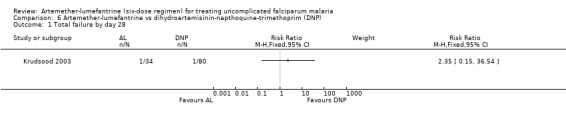
Comparison 6 Artemether‐lumefantrine vs dihydroartemisinin‐napthoquine‐trimethoprim (DNP), Outcome 1 Total failure by day 28.
The trial authors reported no statistically significant difference (P = 0.18) between the groups in the mean parasite clearance times for artemether‐lumefantrine (48.1 h; 34 participants) compared with DNP (43.0 h; 80 participants) (Appendix 3). This was also the case for the mean fever clearance times (P = 0.35): 41.2 hours (34 participants) for artemether‐lumefantrine compared with 32.8 hours (80 participants) for DNP (Appendix 4).
3. Supervised versus unsupervised treatment (957 participants, 1 trial)
Piola 2005, conducted in Uganda, compared supervised with unsupervised treatment with artemether‐lumefantrine. There was no statistically significant difference in the number of total failures by day 28 between the groups (RR 1.18, 95% CI 0.47 to 2.98; 918 participants, Analysis 7.1).
7.1. Analysis.
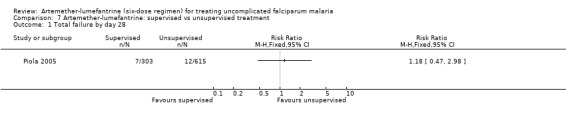
Comparison 7 Artemether‐lumefantrine: supervised vs unsupervised treatment, Outcome 1 Total failure by day 28.
4. Adverse events
All nine trials reported adverse events. The majority of adverse events reported were mild or moderate (Appendix 8), although some were severe (Appendix 9). One trial published adverse cardiac events separately and reported no clinically significant changes in the electrocardiographic intervals (Van Vugt 2000). One trial reported cardiac monitoring (Lefevre 2001), and one reported no difference in the QTc interval (difference between the longest and shortest measurable interval on the 12 lead electrocardiogram, corrected for heart rate) between treatment groups (Lefevre 2001).
Discussion
Trial methods
The risk of bias in several of the included trials was below average given current standards. Seven of the nine trials did not describe the method used to generate the allocation sequence and three did not describe how allocation was concealed. In seven trials, 90% or more of the participants were included in the final analysis for the reported primary outcome. The 'intention‐to‐treat' analysis for the primary outcome reported in five trials was actually a limited form of sensitivity analysis because they made the assumption that all participants lost to follow up were treatment failures. As results were not based on an intention‐to‐treat analysis, they are subject to attrition bias and the clinical effectiveness may be biased.
PCR analysis
Data for failure by day 14 and day 28 were corrected for new infections with missing samples or failed tests classified as treatment failures. This had a minimal effect for mefloquine plus artesunate and the result remained statistically insignificant in favour of mefloquine artesunate. Although PCR results were reported in seven trials, results from different groups were combined making it difficult to draw any valid conclusions and PCR data were not reported on all treatment failures.
Non‐artemisinin therapies
The results of a Cochrane Review of the four‐dose regimen showed that it was often less effective than other standard treatment regimens (Omari 2006). The review included a trial comparing four‐dose and six‐dose regimens, and the six‐dose regimen had fewer treatment failures, and this was statistically significant.
The six‐dose regimen of artemether‐lumefantrine performed better than amodiaquine and amodiaquine plus sulfadoxine‐pyrimethamine. Total failure was lower with artemether‐lumefantrine compared with chloroquine plus sulfadoxine‐pyrimethamine in two trials, but this was not statistically significant. Background resistance to chloroquine and sulfadoxine‐pyrimethamine in both trial areas could have affected the performance of the non‐artemisinin combination. One of the trials, Sutherland 2005, did not report outcomes on day 42, which would have been more informative due to the long half life of sulfadoxine‐pyrimethamine.
Parasite and fever clearance times were shorter for artemether‐lumefantrine when compared with chloroquine plus sulfadoxine‐pyrimethamine, which suggests that clinical symptoms may resolve faster.
Artemisinin combination therapies
In comparisons with other artemisinin‐combination therapies, fewer participants failed treatment with artemether‐lumefantrine compared with amodiaquine plus artesunate. However, the combination of mefloquine and artesunate was more effective at reducing parasitological failure on days 28 and 42. None of the trials reported outcomes on day 63 despite the long half life of mefloquine. There was no difference in parasitological failures between artemether‐lumefantrine and dihydroartemisinin‐napthoquine‐trimethoprim, but the trial may have been too small (130 participants) to detect any statistically significant difference.
There was no difference in the parasite, fever, and gametocyte clearance times in comparisons with mefloquine plus artesunate and dihydroartemisinin‐napthoquine‐trimethoprim. This is not surprising due to the artemisinin component in both therapies.
Supervised versus unsupervised treatment
Artemether‐lumefantrine given without supervision (which is normal clinical practice) showed no difference in the failure rate compared with supervised delivery.
Clearance times
Trials reported clearance times as medians, percentiles, and means. It would have been more informative reporting these as time‐to‐event analyses, as data on participants who did not reach the event would have been included in the analysis.
Adverse events
In some trials where adverse events were reported, no distinction was made between the treatment groups thereby making comparisons impossible. Although some trials reported adverse cardiac events, the evidence was insufficient to address concerns about the possible risk of cardiotoxicity. We, therefore, cannot justifiably comment on adverse events reported apart from reporting the details.
Authors' conclusions
Implications for practice.
The six‐dose regimen of artemether‐lumefantrine is associated with fewer failures and may be a suitable alternative to amodiaquine, amodiaquine plus sulfadoxine‐pyrimethamine, and amodiaquine plus artesunate. Available data suggest that mefloquine plus artesunate is as effective and possibly superior to artemether‐lumefantrine.
The comparative effectiveness of artemether‐lumefantrine was evaluated in a health service setting and the cure rates with unsupervised administration are acceptable.
Implications for research.
Trials should be of high quality, with careful attention to concealment of allocation. All participants should be followed up for the duration of the trial regardless of withdrawal from treatment or other protocol violations as this would permit an intention‐to‐treat analysis. Reasons for all treatment withdrawals should be documented.
Where possible, PCR analysis data should be reported on all treatment failures; if this is not possible, explanations should be given. Results from different groups should be reported separately.
What's new
| Date | Event | Description |
|---|---|---|
| 10 August 2011 | Amended | statement added to published notes section |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 5 August 2008 | Amended | Converted to new review format with minor editing. |
| 18 August 2006 | Amended | 2006, Issue 4: Amended the treatment failure outcomes for Mutabingwa 2005. Clinical failure has now been added to parasitological failure to give the 'total failure'. |
| 21 February 2006 | Amended | Added reference for artemether‐lumefantrine (four‐dose regimen) Cochrane Review (Omari 2006); editorial update. |
Notes
2011, Issue 9: The Cochrane Infectious Diseases Group are piloting a system to indicate whether the question is currently relevant, and the status of the review with regards to being up to date.
For relevance, we classify reviews into:
historical question, where an intervention or policy has been superseded by new medical developments (such as a new drug),
current question, which are still relevant to current policy or practice.
For status, we have three categories, with an explanation after each: “up to date”; “update pending”; “no update intended”.
For this review, we have categorised the review as: Current question ‐ no update intended (topic covered in another review. Refer to: Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin‐based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2009, Issue 3. Art. No.: CD007483. DOI: 10.1002/14651858.CD007483.pub2.)
2005, Issue 4 (first review version): We divided the original review on artemether‐lumefantrine (Omari 2002; Omari 2003) into two separate reviews, one of the six‐dose regimen (this review) and the other of the four‐dose regimen (Omari 2006), and added seven new trials.
Acknowledgements
This document is an output from a project funded by the UK Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID.
Appendices
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINEb | EMBASEb | LILACSb |
| 1 | artemether | artemether | artemether | artemether | artemether |
| 2 | lumefantrine | lumefantrine | lumefantrine | lumefantrine | lumefantrine |
| 3 | benflumetol | benflumetol | benflumetol | benflumetol | benflumetol |
| 4 | co‐artemether | co‐artemether | co‐artemether | co‐artemether | co‐artemether |
| 5 | coartem | coartem | coartem | coartem | coartem |
| 6 | coarteme | coarteme | coarteme | coarteme | 1 or 2 or 3 or 4 or 5 |
| 7 | riamet | riamet | riamet | riamet | malaria |
| 8 | CGP56697 | CGP56697 | CGP56697 | CGP56697 | 6 and 7 |
| 9 | — | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 | — |
| 10 | — | malaria | exp MALARIA | exp MALARIA | — |
| 11 | — | 9 and 10 | malaria | malaria | — |
| 12 | — | — | 10 or 11 | 10 or 11 | — |
| 13 | — | — | 9 and 12 | 9 and 12 | — |
| 14 | — | — | Limit 13 to human | Limit 13 to human | — |
aCochrane Infectious Diseases Group Specialized Register. bSearch terms used in combination with the search strategy for retrieving trials developed by The Cochrane Collaboration (Higgins 2005); upper case: MeSH or EMTREE heading; lower case: free text term.
Appendix 2. Outcomes reported in the included trials
| Trial | (Total) failurea | PCR analysisa | PCTb | FCTb | Gametocyte carriage | GCTb | Adverse events |
| Van Vugt 2000 | Day 28 | Yes | No | No | Yes | No | Yes |
| Lefevre 2001 | Day 28 | Yes | Yes | Yes | Yes | Yes | Yes |
| Krudsood 2003 | Day 28 | No | Yes | Yes | No | No | Yes |
| Mayxay 2004 | Day 42 | Yes | Yes | Yes | Yes | No | Yes |
| Ndayiragije 2004 | Day 14 | No | No | No | Yes | No | Yes |
| Piola 2005 | Day 28 | Yes | No | No | Yes | No | Yes |
| Stohrer 2004 | Day 42 | Yes | No | No | Yes | Yes | Yes |
| Mutabingwa 2005 | Days 14 and 28 | Yes | No | No | Yes | No | Yes |
| Sutherland 2005 | Days 7, 14, and 28 | Yes | No | No | Yes | No | Yes |
aFailure defined as a recurrent malaria infection with or without clinical malaria; polymerase chain reaction (PCR) analysis to exclude new infections. bPCT: parasite clearance time; FCT: fever clearance time; GCT: gametocyte clearance time.
Appendix 3. Parasite clearance time
| Trial | Intervention | No. participants | Median | 25‐75th percentile | 95% CI | P value |
| Lefevre 2001 | Artemether‐lumefantrine | 164 | 29 | 18 to 40 | 29 to 32 | — |
| Mefloquine‐artesunate | 55 | 31 | 24 to 35 | 26 to 31 | — | |
| Krudsood 2003 | Artemether‐lumefantrine | 34 | 48.1a | — | — | 0.18 |
| Dihydroartemisinin‐napthoquine‐trimethoprim | 80 | 43.0a | — | — | — | |
| Mayxay 2004 | Artemether‐lumefantrine | 107 | 2.08b | — | 2.0 to 2.1 | < 0.001c |
| Mefloquine‐artesunate | 110 | 2.07b | — | 2.0 to 2.1 | — | |
| Chloroquine plus sulfadoxine‐pyrimethamine | 102 | 2.9b | — | 2.8 to 3.0 | — |
aMean (h). bMean (d); CI: confidence interval. cArtemether‐lumefantrine versus chloroquine plus sulfadoxine‐pyrimethamine.
Appendix 4. Fever clearance time
| Trial | Intervention | No. participants | Median | 25‐75th percentile | 95%CI | P value |
| Krudsood 2003 | Artemether‐lumefantrine | 34 | 41.2a | — | — | 0.35 |
| Dihydroartemisinin‐napthoquine‐trimethoprim | 80 | 32.8a | — | — | — | |
| Lefevre 2001 | Artemether‐lumefantrine | 76 | 29 | 8 to 51 | 23 to 37 | — |
| Mefloquine‐artesunate | 29 | 23 | 15 to 31 | 15 to 30 | — | |
| Mayxay 2004 | Artemether‐lumefantrine | 107 | 23.1a | — | 20.9 to 25.3 | < 0.001b |
| Mefloquine‐artesunate | 110 | 24.6a | — | 21.8 to 27.3 | — | |
| Chloroquine plus sulfadoxine‐pyrimethamine | 102 | 40.2a | — | 35.9 to 44.4 | — |
aMean (h); CI: confidence interval. bArtemether‐lumefantrine versus chloroquine plus sulfadoxine‐pyrimethamine.
Appendix 5. Day 28 failures: polymerase chain reaction (PCR) results
| Comparator | Trial | Measure | Artemether‐lumefantrine | Comparator |
| Mefloquine‐artesunate | Van Vugt 2000 | Day 28 failures/follow up | 4/134 | 0/47 |
| PCR tested day 28/total failures day 28 | 3/4 | 0/0 | ||
| Missing sample or failed test | 1 | 0 | ||
| Recrudescent infections | 2 | 0 | ||
| New infections | 1 | 0 | ||
| Corrected day 28 failure rate | 3/134 | 0/47 | ||
| Lefevre 2001 | Day 28 failures/follow up | 7/155 | 0/53 | |
| PCR tested day 28/total failures day 28 | 7/7 | 0/0 | ||
| Missing sample or failed test | 0 | 0 | ||
| Recrudescent infections | 6 | 0 | ||
| New infections | 1 | 0 | ||
| Corrected day 28 failure rate | 6/155 | 0/53 |
Appendix 6. Day 42 failures: polymerase chain reaction (PCR) results
| Trial | Measure | Artemether‐lumefantrine | Mefloquine plus artesunate |
| Stohrer 2004 | Day 42 failures/follow up | 13/47 | 8/53 |
| PCR tested day 42/total failures day 42 | 13/13 | 8/8 | |
| Missing sample or failed test | 0 | 0 | |
| Recrudescence | 3 | 0 | |
| New infections | 10 | 8 | |
| Corrected day 42 failure rate | 3/47 | 0/53 |
Appendix 7. Gametocyte clearance time
| Trial | Intervention | No. participants | Median | 25‐75th percentile | 95% CI | P value |
| Lefevre 2001 | Artemether‐lumefantrine | 26 | 72 | 32 to 320 | 34 to 163 | — |
| Mefloquine‐artesunate | 10 | 85 | 46 to 328 | 46 to 160 | — | |
| Stohrer 2004 | Artemether‐lumefantrine | 47 | 10.5a | — | 4.35 to 16.65 | 0.6b |
| Mefloquine‐artesunate | 53 | 7.0a | — | 7.0 to 7.0 | — |
aMean in days. bMann‐Whitney U‐test; CI: confidence interval.
Appendix 8. Participants experiencing mild to moderate adverse events
| Comparator | Trial | Adverse event | n/Na (%) | |
| Artemether‐lumefantrine | Comparator | |||
| Chloroquine plus sulfadoxine‐pyrimethamine | Sutherland 2005 | Headache | 11/91 (12) | 45/406 (11) |
| Anorexia | 11/91 (12) | 65/406 (16) | ||
| Diarrhoea | 6/91 (7) | 16/406 (4) | ||
| Abdominal pain | 5/91 (5) | 20/406 (5) | ||
| Pruritis | 1/91 (1) | 4/406 (1) | ||
| Artesunate plus mefloquine | Stohrer 2004 | Gastrointestinal disorders | 6/47 (12.8) | 6/50 (12) |
| Central nervous system disorders | 14/47 (29.8) | 22/53 (41.5) | ||
| Versus dihydroartemisinin‐napthoquine‐trimethoprim | Krudsood 2003 | Nausea | 4/89 (4.5) | 2/41 (4.9) |
| Headache | 5/89 (5.6) | 2/41 (4.9) | ||
| Dizziness | 7/89 (7.9) | 4/41 (9.6) | ||
aNumber of participants with event calculated from percentage using the total number of participants randomized to each group originally.
Appendix 9. Severe adverse events
| Comparator | Trial | Adverse event | Artemether‐lumfantrine | Comparator |
| Amodiaquine | Mutabingwa 2005 | Died from severe malaria | 0 | 1 |
| Amodiaquine plus sulfadoxine‐pyrimethamine | Mutabingwa 2005 | Died from severe malaria | 0 | 1 |
| Mefloquine plus artesunate | Stohrer 2004 | Severe diarrhoea | 1 | 0 |
Data and analyses
Comparison 1. Artemether‐lumefantrine vs amodiaquine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Total failure by day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Gametocyte carriage on day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Artemether‐lumefantrine vs chloroquine plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Total failure by days 42, 14, and 7 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Day 42 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Gametocyte carriage on days 28, 14, and 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Day 7 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Artemether‐lumefantrine vs amodiaquine plus sulfadoxine‐pyrimethamine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Total failure by day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Gametocyte carriage on day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Artemether‐lumefantrine vs amodiaquine plus artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Total failure by day 14 | 2 | 1283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.23] |
| 3 Gametocyte carriage on days 14 and 7 | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Day 14 | 2 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.35, 0.91] |
| 3.2 Day 7 | 1 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.33, 1.41] |
Comparison 5. Artemether‐lumefantrine vs mefloquine plus artesunate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 2 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.20 [0.55, 31.93] |
| 2 Total failure by day 28: PCR adjusted | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Adjusted for new infections | 2 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.45, 27.03] |
| 2.2 Not adjusted for new infections | 2 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.20 [0.55, 31.93] |
| 3 Total failure by day 42 | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [1.48, 5.80] |
| 4 Total failure by day 42: PCR adjusted | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 AL vs. mefloquine plus artesunate, adjusted for new infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 AL vs. mefloquine plus artesunate, not adjusted for new infections | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Gametocyte carriage on day 7 and in first 72 h | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Day 7 | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.44, 4.15] |
| 5.2 72 h | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.58, 2.06] |
Comparison 6. Artemether‐lumefantrine vs dihydroartemisinin‐napthoquine‐trimethoprim (DNP).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 7. Artemether‐lumefantrine: supervised vs unsupervised treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total failure by day 28 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Krudsood 2003.
| Methods | Generation of allocation sequence: not described; randomization in ratio 2:1 Allocation concealment: not described Blinding: none Inclusion of all randomized participants in final analysis: 88% (114/130) |
|
| Participants | Number: 130 adults Inclusion criteria: acute uncomplicated falciparum malaria; positive blood slide; weight > 40 kg; age > 14 years; oral intake; agree to hospital admission Exclusion criteria: severe malaria; oral intake not possible; pregnancy or lactation; concomitant disease; taken other antimalarials within past 14 days; urine sulphonamides or 4‐aminoquinolones |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 72 h; artemether 80 mg/dose, lumefantrine 480 mg/dose 2. Dihydroartemisinin‐napthoquine‐trimethoprim (DNP): 2 tablets over 24 h | |
| Outcomes | 1. 28‐day cure 2. Parasite clearance time 3. Fever clearance time 4. Adverse events | |
| Notes | Location: Bangkok, Thailand 16 participants withdrew from trial (9 artemether‐lumefantrine, 7 DNP) Local antimalarial drug resistance: multiple‐drug resistance Malaria transmission: not specified |
|
Lefevre 2001.
| Methods | Generation of allocation sequence: not described; randomization 3:1 Allocation concealment: not described Blinding: none Inclusion of all randomized participants in the analysis: 95% (208/219) |
|
| Participants | Number: 219 participants aged 12 to 71 Inclusion criteria: microscopically confirmed Plasmodium falciparum Excluded: severe, complicated malaria |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 48 h; artemether 80 mg/dose, lumefantrine 480 mg/dose) 2. Mefloquine plus artesunate: mefloquine 2 doses over 48 h (day 2 = 15 mg/kg, day 3 = 10 mg/kg); artesunate 3 doses over 48 h (4 mg/kg/dose) | |
| Outcomes | 1. 28‐day cure 2. Parasite clearance time 3. Fever clearance time 4. Gametocyte carriage within first 72 h 5. Gametocyte clearance time 6. Parasite reduction at 24 h 7. Adverse effects 8. Polymerase chain reaction (PCR) analysis | |
| Notes | Location: Bangkok, Thailand Trial designed to compare artemether‐lumefantrine with historical controls in which artesunate‐mefloquine was used 11 not evaluated on day 28: 9 (artemether‐lumefantrine); 2 (artesunate‐mefloquine) Local antimalarial drug resistance: not specified Malaria transmission: low |
|
Mayxay 2004.
| Methods | Generation of allocation sequence: not described; block randomization Allocation concealment: sealed, opaque envelopes Blinding: none Inclusion of all randomized participants: 98% (324/330) |
|
| Participants | Number: 330 participants Inclusion criteria: Plasmodium falciparum of 5000 to 20,000/µL; age > 1 year; fever; no signs of severe malaria |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 72 h; artemether 1.3 to 2.6 mg/kg/dose, lumefantrine 8 to 16 mg/kg/dose 2. Chloroquine plus sulfadoxine‐pyrimethamine: chloroquine 25 mg base/kg; sulfadoxine 25 mg/kg, pyrimethamine 1.25 mg/kg 3. Mefloquine plus artesunate: mefloquine 12.5 mg/kg; artesunate 3 mg/kg/dose | |
| Outcomes | 1. 42‐day cure 2. Parasite clearance time 3. Fever clearance time 4. Gametocyte carriage 5. Polymerase chain reaction (PCR) analysis 6. Adverse events | |
| Notes | Location: Savannakhet Province, Lao People's Democratic Republic Local antimalarial drug resistance: chloroquine, sulfadoxine‐pyrimethamine Malaria transmission: not specified |
|
Mutabingwa 2005.
| Methods | Generation of allocation sequence: computer; block randomization Allocation concealment: sealed opaque numbered envelopes Blinding: none Inclusion of all randomized participants: 97% (1659/1811) |
|
| Participants | Number: 1811 children aged 4 to 59 months Inclusion criteria: microscopically confirmed Plasmodium falciparum parasitaemia > 2000/µL; oral intake; can attend clinic for follow up Exclusion criteria: severe malaria; mixed plasmodium infection; taken other antimalarial (apart from chloroquine) within past 7 days; known hypersensitivity to trial drugs; presence of disease masking assessment of response to antimalarial treatment |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 72 h; artemether 1 to 2 mg/kg/dose, lumefantrine 8 to 14 mg/kg/dose 2. Amodiaquine: 3 doses over 72 h; total dose 25 mg/kg 3. Amodiaquine plus sulfadoxine‐pyrimethamine: amodiaquine total dose 25 mg/kg (as 3 doses over 72 h); sulfadoxine 25 mg/kg, pyrimethamine 1.25 mg/kg (as single dose) 4. Amodiaquine plus artesunate: amodiaquine total dose 25 mg/kg as (3 doses over 72 h); artesunate 4 mg/kg over 72 h | |
| Outcomes | 1. 28‐day cure 2. 14‐day cure 3. Gametocyte carriage on day 14 4. Polymerase chain reaction (PCR) genotype 5. Haemoglobin 6. Adverse events | |
| Notes | Location: Muheza, Tanzania Local antimalarial drug resistance: chloroquine, sulfadoxine‐pyrimethamine Malaria transmission: perennial |
|
Ndayiragije 2004.
| Methods | Generation of allocation sequence: not described; block randomization Allocation concealment: not described Blinding: none Inclusion of all randomized participants: 98% (290/295) |
|
| Participants | Number: 295 children aged 6 to 59 months Inclusion criteria: weight > 7 kg; microscopically confirmed Plasmodium falciparum parasitaemia > 2000/µL; fever Excluded: severe malaria; severe malnutrition; other infectious febrile illness |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 60 h; artemether 1.3 to 2.6 mg/kg/dose, lumefantrine 8 to 16 mg/kg/dose 2. Artesunate: 3 doses over 48 h (4 mg/kg/dose); amodiaquine 3 doses over 48 h (10 mg/kg/dose) | |
| Outcomes | 1. 14‐day cure 2. Gametocyte carriage on days 0, 3, 7, and 14 3. Adverse effects | |
| Notes | Location: Buhiga and Kigobe, Burundi Local antimalarial drug resistance: chloroquine, sulfadoxine‐pyrimethamine Malaria transmission: not specified |
|
Piola 2005.
| Methods | Generation of allocation sequence: computer; block randomization Allocation concealment: sealed envelopes Blinding: none Inclusion of all randomized participants: 96% (918/957) |
|
| Participants | Number: 957 children and adults Inclusion criteria: fever; weight > 10 kg; monoinfection with Plasmodium falciparum; parasitaemia of 500 to 100,000 trophozoites/µL; no signs of severe malaria |
|
| Interventions | 1. Supervised artemether‐lumefantrine: 6 doses over 3 d (for each dose 1 tablet 10 to 14.9 kg; 2 tablets 15 to 24.9 kg; 3 tablets 25 to 34.9 kg; 4 tablets >35 kg) 2. Unsupervised artemether‐lumefantrine: 6 doses over 3 days | |
| Outcomes | 1. 28‐day cure 2. Proportion of afebrile patients on days 1, 2, and 3 3. Gametocyte carriage 4. Polymerase chain reaction (PCR) analysis 5. Haematological recovery 6. Adverse events | |
| Notes | Location: Mbarara, Uganda Local antimalarial drug resistance: chloroquine, sulfadoxine‐pyrimethamine Malaria transmission: perennial |
|
Stohrer 2004.
| Methods | Generation of allocation sequence: not described; block randomization Allocation concealment: sealed envelopes Blinding: none Inclusion of all randomized participants: 93% (101/108) |
|
| Participants | Number: 108 participants aged 2 to 66 years Inclusion criteria: fever; microscopically confirmed Plasmodium falciparum 1000 to 100,000 parasites/µL Exclusion criteria: severe or complicated malaria; severe malnutrition; weight < 10 kg |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 72 h; artemether 1.4 to 2 mg/kg/dose, lumefantrine 8.5 to 16 mg/kg/dose 2. Mefloquine plus artesunate: mefloquine total dose over 48 h (25 mg/kg); artesunate 3 doses over 72 h (4 mg/kg/dose) | |
| Outcomes | 1. 42‐day cure 2. Gametocyte carriage 3. Gametocyte clearance time 4. Polymerase chain reaction (PCR) analysis | |
| Notes | Location: Luang Namtha Province, Lao People's Democratic Republic Hospital‐ and community‐based study Local antimalarial drug resistance: chloroquine, sulfadoxine‐pyrimethamine Malaria transmission: perennial |
|
Sutherland 2005.
| Methods | Generation of allocation sequence: not described; block randomization Allocation concealment: numbered envelopes Blinding: single, all personnel apart from field assistants and recruiting clinic Inclusion of all randomized participants: 88% (368/419) |
|
| Participants | Number: 497 children Inclusion criteria: fever; microscopically confirmed Plasmodium falciparum > 500/µL Exclusion criteria: severe malaria; no oral intake; gametocyte carriage at presentation |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 72 h (for each dose, half‐tablet per 5 kg up to 2 tablets; children > 25 kg 3 tablets per dose) 2. Chloroquine plus sulfadoxine‐pyrimethamine: 30 mg/kg/base chloroquine; 250 mg sulfadoxine, 12.5 mg pyrimethamine; plus additional 12.5 mg sulfadoxine and 6.25 mg pyrimethamine for each 5 kg over 10 kg body weight | |
| Outcomes | 1. Infectiousness of patients to Anopheles mosquitoes from day 7 2. 7, 14, and 28‐day cure 3. Gametocyte carriage 4. Polymerase chain reaction (PCR) analysis 5. Adverse events | |
| Notes | Location: Farafenni, The Gambia Local antimalarial drug resistance: chloroquine and sulfadoxine‐pyrimethamine Malaria transmission: high seasonal |
|
Van Vugt 2000.
| Methods | Generation of allocation sequence: not described; block randomization (3) Allocation concealment: sealed envelopes Blinding: none Inclusion of all randomized participants: 90% (181/200) |
|
| Participants | Number: 200 participants aged 2 to 63 Inclusion criteria: parasitaemia > 500/µL Excluded: severe malaria |
|
| Interventions | 1. Artemether‐lumefantrine: 6 doses over 48 h; artemether 1.3 to 2.6 mg/kg/dose, lumefantrine 7.8 to 15 mg/kg/dose 2. Mefloquine plus artesunate: mefloquine 2 doses over 48 h (day 2, 15 mg/kg; day 3, 10 mg/kg); artesunate 3 doses over 48 h (4 mg/kg/dose) | |
| Outcomes | 1. 28‐day cure 2. Proportion of patients with fever on days 0 to 3 3. Proportion of patients with parasitaemia on days 0 to 3 4. Gametocyte carriage within first 72 h 5. Adverse events 6. Electrocardiogram (ECG) findings | |
| Notes | Location: Bangkok and Karen, Thailand 2 trial centres: Bangkok ‐ inpatients for 28 days; Karen ‐ outpatients 19 not evaluated on day 28: 16 (artemether‐lumefantrine), 3 (artemether plus mefloquine) Local antimalarial drug resistance: multiple‐drug resistance Malaria transmission: low |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Espino 2002 | Four‐dose regimen of artemether‐lumefantrine used |
| Falade 2005 | Not a randomized controlled trial |
| Hatz 1998 | Four‐dose regimen of artemether‐lumefantrine used |
| Jiao 1997 | Compared artemether‐lumefantrine with lumefantrine, which is not a recommended standard therapy for uncomplicated malaria |
| Karbwang 2002 | Artemether‐lumefantrine not compared with another antimalarial |
| Kshirsagar 2000 | Four‐dose regimen of artemether‐lumefantrine used |
| Lefevre 2002 | Parallel 3‐group trial where participants received sequential artemether‐lumefantrine and quinine |
| Looareesuwan 1999 | Four‐dose regimen of artemether‐lumefantrine used |
| Popov 2002 | Not a randomized controlled trial |
| Sun 2000 | Compared artemether‐lumefantrine with lumefantrine, which is not a recommended standard therapy for uncomplicated malaria |
| Van Agtmael 1999 | Four‐dose regimen of artemether‐lumefantrine used |
| Van Vugt 1998b | Four‐dose regimen of artemether‐lumefantrine used |
| Van Vugt 1999a | Compared four‐dose and six‐dose regimens of artemether‐lumefantrine |
| Von Seidlein 1997 | Not a randomized controlled trial (safety trial) |
| Von Seidlein 1998 | Four‐dose regimen of artemether‐lumefantrine used |
| Zhiwei 1999 | Compared artemether‐lumefantrine with lumefantrine, which is not recommended standard therapy for uncomplicated malaria |
Contributions of authors
Aika Omari and Carrol Gamble extracted and analysed data, and drafted the review. Paul Garner helped prepare the review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development, UK.
Declarations of interest
Paul Garner and Carrol Gamble (né Preston) were unpaid technical advisers to a World Health Organization meeting on 19 and 20 February, 2001 considering efficacy and effectiveness studies of co‐artemether‐lumefantrine. The World Health Organization paid for their travel and accommodation, and a representative of Novartis chaired the meeting. Aika Omari: none known
Unchanged
References
References to studies included in this review
Krudsood 2003 {published data only}
- Krudsood S, Chalermrut K, Pengruksa C, Srivilairit S, Silachamroon U, Treeprasertsuk S, et al. Comparative clinical trial of two‐fixed combinations dihydroartemisinin‐napthoquine‐trimethoprim (DNP) and artemether‐lumefantrine (Coartem/Riamet) in the treatment of acute uncomplicated falciparum malaria in Thailand. Asian Journal of Tropical Medicine and Public Health 2003;34(2):316‐21. [PMC free article] [PubMed] [Google Scholar]
Lefevre 2001 {published data only}
- Lefevre G, Looareesuwan S, Treeprasertsuk S, Krudsood S, Silachamroon U, Gathmann I, et al. A clinical and pharmacokinetic trial of six doses of artemether‐lumefantrine for multidrug‐resistant Plasmodium falciparum malaria in Thailand. American Journal of Tropical Medicine and Hygiene 2001;64(5‐6):247‐56. [DOI] [PubMed] [Google Scholar]
Mayxay 2004 {published data only}
- Mayxay M, Khanthavong M, Lindegardh N, Keola S, Barends M, Pongvongsa T, et al. Randomized comparison of chloroquine plus sulfadoxine‐pyrimethamine versus artesunate plus mefloquine versus artemether‐lumefantrine in the treatment of uncomplicated falciparum malaria in the Lao People's Democratic Republic. Clinical Infectious Diseases 2004;39(8):1139‐47. [DOI] [PubMed] [Google Scholar]
Mutabingwa 2005 {published data only}
- Mutabingwa TK, Anthony D, Heller A, Hallet R, Ahmed J, Drakeley C, et al. Amodiaquine alone, amodiaquine+sulfadoxine‐pyrimethamine, amodiaquine+artesunate, and artemether‐lumefantrine for outpatient treatment of malaria in Tanzanian children: a four‐arm randomised effectiveness trial. Lancet 2005;365(9469):1474‐80. [DOI] [PubMed] [Google Scholar]
Ndayiragije 2004 {published data only}
- Ndayiragije A, Niyungeko D, Karenzo J, Niyungeko E, Barutwanayo M, Ciza A, et al. Efficacy of therapeutic combinations with artemisinin derivatives in the treatment of non complicated malaria in Burundi [Efficacité de combinaisons thérapeutiques avec des dérivés de l'artémisinine dans le traitement de l'accès palustre non‐complique au Burundi]. Tropical Medicine & International Health 2004;9(6):673‐9. [DOI] [PubMed] [Google Scholar]
Piola 2005 {published data only}
- Piola P, Fogg C, Bajunirwe F, Biraro S, Grandesso F, Ruzagira E, et al. Supervised versus unsupervised intake of six‐dose artemether‐lumefantrine for treatment of acute, uncomplicated Plasmodium falciparum malaria in Mbarara, Uganda: a randomised trial. Lancet 2005;365(9469):1467‐73. [DOI] [PubMed] [Google Scholar]
Stohrer 2004 {published data only}
- Stohrer JM, Dittrich S, Thongpaseuth V, Vanisaveth V, Phetsouvanh R, Phompida S, et al. Therapeutic efficacy of artemether‐lumefantrine and artesunate‐mefloquine for treatment of uncomplicated Plasmodium falciparum malaria in Luang Namtha Province, Lao People's Democratic Republic. Tropical Medicine and International Health 2004;9(11):1175‐83. [DOI] [PubMed] [Google Scholar]
Sutherland 2005 {published data only}
- Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, et al. Reduction of malaria transmission to Anopheles mosquitoes with a six‐dose regimen of co‐artemether. PLoS Med 2005;2(4):338‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Vugt 2000 {published data only}
- Vugt M, Ezzet F, Nosten F, Gathmann I, Wilairatana P, Looareesuwan S, et al. No evidence of cardiotoxicity during antimalarial treatment with artemether‐lumefantrine. American Journal of Tropical Medicine and Hygiene 1999;61(6):964‐7. [DOI] [PubMed] [Google Scholar]
- Vugt M, Looareesuwan S, Wilairatana P, McGready R, Villegas L, Gathmann I, et al. Artemether‐lumefantrine for the treatment of multidrug‐resistant falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(5):545‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Espino 2002 {unpublished data only}
- Espino FE. Efficacy studies of chloroquine+sulfadoxine‐pyrimethamine, sulfadoxine‐pyrimethamine and artemether‐lumefantrine for uncomplicated malaria in Mindanao Island, Philippines. 2002.
Falade 2005 {published data only}
- Falade C, Makanga M, Premji Z, Ortmann CE, Stockmeyer M, Palacios PI. Efficacy and safety of artemether‐lumefantrine (Coartem) tablets (six‐dose regimen) in African infants and children with acute, uncomplicated falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2005;99(6):459‐67. [DOI] [PubMed] [Google Scholar]
Hatz 1998 {published data only}
- Hatz C, Abdulla S, Mull R, Schellenberg D, Gathmann I, Kibatala P, et al. Efficacy and safety of CGP 56697 (artemether and benflumetol) compared with chloroquine to treat acute falciparum malaria in Tanzanian children aged 1‐5 years. Tropical Medicine and International Health 1998;3(6):498‐504. [DOI] [PubMed] [Google Scholar]
- Irion A, Felger I, Abdulla S, Smith T, Mull R, Tanner M, et al. Distinction of recrudescences from new infections by PCR‐RFLP analysis in a comparative trial of CPG 56 697 and chloroquine in Tanzanian children. Tropical Medicine and International Health 1998;3(6):490‐7. [DOI] [PubMed] [Google Scholar]
Jiao 1997 {unpublished data only}
- Jiao XQ, Liu EY, Shan CQ, Dal P, Gathmann I, Mull R, et al. A double‐blind comparative trial of benflumetol, a novel antimalaria, and CGP 56697, a combination of benflumetol and artemether, in the treatment of acute P. falciparum malaria in adults in China. Proceedings of the 5th International Conference of Travel Medicine; 1997 Mar 24‐27; Geneva (Switzerland). 1997:Abstract 108.
Karbwang 2002 {unpublished data only}
- Karbwang J. Coartemether safety and efficacy evaluation of the six dose regimen in the treatment of uncomplicated malaria in children and infants. 2002.
Kshirsagar 2000 {published data only}
- Kshirsagar NA, Gogtay NJ, Moorthy NS, Garg MR, Dalvi SS, Chogle AR, et al. A randomized, double‐blind, parallel‐group, comparative safety, and efficacy trial of oral co‐artemether versus oral chloroquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in adults in India. American Journal of Tropical Medicine and Hygiene 2000;62(3):402‐8. [DOI] [PubMed] [Google Scholar]
Lefevre 2002 {published data only}
- Lefevre G, Carpenter P, Souppart C, Schmidli H, Martin JM, Lane A, et al. Interaction trial between artemether‐lumefantrine (Riamet) and quinine in healthy subjects. Journal of Clinical Pharmacology 2002;42(10):1147‐58. [DOI] [PubMed] [Google Scholar]
Looareesuwan 1999 {published data only}
- Looareesuwan S, Wilairatana P, Chokejindachai W, Chalermrut K, Wernsdorfer W, Gemperli B, et al. A randomized, double‐blind, comparative trial of a new oral combination of artemether and benflumetol (CGP 56697) with mefloquine in the treatment of acute plasmodium falciparum malaria in Thailand. American Journal of Tropical Medicine and Hygiene 1999;60(2):238‐43. [DOI] [PubMed] [Google Scholar]
Popov 2002 {published data only}
- Popov A F, Morokov VS, Chirkov VP, Popova NI, Lama N. Efficacy of mefloquine, halofantrine, and coarteme in the treatment of tropical malaria. Meditsinskaia parazitologiia i parazitarnye bolezni 2002;1:28‐9. [PubMed] [Google Scholar]
Sun 2000 {published data only}
- Sun ZW, Shan CQ, Li GF, Ding DB, Liu G, Wang JY, et al. Clinical comparative trial of co‐artemether and benflumetol (two formulations) in the treatment of falciparum malaria. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi [Chinese Journal of Parasitology and Parasitic Diseases] 2000;18(3):159‐61. [PubMed] [Google Scholar]
Van Agtmael 1999 {published data only}
- Agtmael M, Bouchaud O, Malvy D, Delmont J, Danis M, Barette S, et al. The comparative efficacy and tolerability of CGP 56697 (artemether + lumefantrine) versus halofantrine in the treatment of uncomplicated falciparum malaria in travellers returning from the Tropics to The Netherlands and France. International Journal of Antimicrobial Agents 1999;12(2):159‐69. [DOI] [PubMed] [Google Scholar]
Van Vugt 1998b {published data only}
- Vugt M, Brockman A, Gemperli B, Luxemburger C, Gathmann I, Royce C, et al. Randomized comparison of artemether‐benflumetol and artesunate‐mefloquine in treatment of multidrug‐resistant falciparum malaria. Antimicrobial Agents and Chemotherapy 1998;42(1):135‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Vugt 1999a {published data only}
- Vugt M, Wilairatana P, Gemperli B, Gathmann I, Phaipun L, Brockman A, et al. Efficacy of six doses of artemether‐lumefantrine (benflumetol) in multidrug‐resistant Plasmodium falciparum malaria. American Journal of Tropical Medicine and Hygiene 1999;60(6):936‐42. [DOI] [PubMed] [Google Scholar]
Von Seidlein 1997 {published data only}
- Seidlein L, Jaffer S, Pinder M, Haywood M, Snounou G, Gemperli B, et al. Treatment of African children with uncomplicated falciparum malaria with a new antimalarial drug, CGP 56697. Journal of Infectious Diseases 1997;176(4):1113‐6. [DOI] [PubMed] [Google Scholar]
Von Seidlein 1998 {published data only}
- Von Seidlein, Bojang K, Jones P, Jaffar S, Pinder M, Obaro S, et al. A randomized controlled trial of artemether/benflumetol, a new antimalarial and pyrimethamine/sulfadoxine in the treatment of uncomplicated falciparum malaria in African children. American Journal of Tropical Medicine and Hygiene 1998;58(5):638‐44. [DOI] [PubMed] [Google Scholar]
Zhiwei 1999 {published data only}
- Zhiwei S, Chengqi S, Guofu L, Jingyan W, Deben D, Guangyu L, et al. Treatment effects of Co‐artemether and Benflumetol capsule in naturally occuring falciparum malaria patients. Journal of Practical Parasitic Diseases 1999;7(2):49‐51. [Google Scholar]
Additional references
Bindschedler 2000
- Bindschedler M, Lefevre G, Ezzet F, Schaffer N, Meyer I, Thomsen MS. Cardiac effects of co‐artemether (artemether/lumefantrine) and mefloquine given alone or in combination to healthy volunteers. European Journal of Clinical Pharmacology 2000;56(5):375‐81. [DOI] [PubMed] [Google Scholar]
Bloland 2000
- Bloland PB, Ettling M, Meek S. Combination therapy for malaria in Africa: hype or hope?. Bulletin of the World Health Organization 2000;78(12):1378‐88. [PMC free article] [PubMed] [Google Scholar]
Ezzet 1998
- Ezzet F, Mull R, Karbwang J. Population pharmacokinetics and therapeutic response of CGP 56697 (artemether + benflumetol) in malaria patients. British Journal of Clinical Pharmacology 1998;46(6):553‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins J, Green S, editors. Highly sensitive search strategies for identifying reports of randomized controlled trials in MEDLINE. Cochrane Handbook for Systematic Reviews of Interventions 4.2.4 [updated March 2005]; Appendix 5b. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 1 April 2005).
Jüni 2001
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefèvre 1999
- Lefevre G, Thomsen MS. Clinical pharmacokinetics of artemether and lumefantrine (Riamet ®). Clinical Drug Investigation 1999;18(6):467‐80. [Google Scholar]
Nosten 2003
- Nosten F. Letters to the editor. The Newsletter of the East African Network for Monitoring Antimalarial Treatment 2003;6(1):10. [Google Scholar]
Novartis 2005
- Novartis. Coartem monograph. 4th Edition. Basel, Switzerland: Novartis Pharma AG, 2005. [Google Scholar]
Omari 2006
- Omari AAA, Gamble C, Garner P. Artemether‐lumefantrine (four‐dose regimen) for treating uncomplicated malaria. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Van Vugt 1998a
- Vugt M, Nosten F, Price RN, Looareesuwan S, White NJ. Antimalarial drug resistance: Treatment and prevention. Proceedings of the Second European Congress on Tropical Medicine. 1998 Sept 14‐18; Liverpool (UK). 1998:Abstract 463.
White 1999a
- White N. Antimalarial drug resistance and combination chemotherapy. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 1999;354(1384):739‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1999b
- White NJ, Nosten F, Looareesuwan S, Watkins WM, Marsh K, Snow RW, et al. Averting a malaria disaster. Lancet 1999;353(9168):1965‐7. [DOI] [PubMed] [Google Scholar]
WHO 1996
- World Health Organization. Division of Control of Tropical Diseases. Assessment of therapeutic efficacy of antimalarial drugs: for uncomplicated falciparum malaria in areas with intense transmission. Geneva: World Health Organization, 1996. [WHO/MAL/96.1077] [Google Scholar]
WHO 1999
- World Health Organization. Department of Communicable Disease Research and Development. WHO informal consultation on clinical neurological investigations required for patients treated with artemisinin compounds and derivatives. Report of an informal consultation convened by WHO, Geneva, 20 July 1998. Geneva: World Health Organization, 1998. [TDR/TDF/99.1] [Google Scholar]
WHO 2000a
- World Health Organization. WHO Expert Committee on Malaria (1998 : Geneva, Switzerland). WHO expert committee on malaria: twentieth report. World Health Organization Technical Report Series 2000; Vol. 892.
WHO 2000b
- World Health Organization. Communicable Diseases Cluster. Severe falciparum malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 2000;94(Suppl 1):1‐90. [PubMed] [Google Scholar]
WHO 2001a
- World Health Organization. Office of Press and Public Relations. WHO and Novartis join forces to combat drug resistant malaria. http://www.who.int/inf‐pr‐2001/en/pr2001‐26.html (accessed 23 May 2001).
WHO 2001b
- World Health Organization. Global Partnership to Roll Back Malaria. The use of antimalarial drugs. Report of a WHO informal consultation, 13‐17 November 2000. Geneva: World Health Organization, 2001. [WHO/CDS/RBM/2001.33] [Google Scholar]
References to other published versions of this review
Omari 2002
- Omari AAA, Gamble C, Garner P. Artemether‐lumefantrine for treating uncomplicated falciparum malaria. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omari 2003
- Omari AAA, Gamble C, Garner P. Artemether‐lumefantrine for treating uncomplicated falciparum malaria. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003125.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Omari 2004
- Omari AAA, Gamble C, Garner P. Artemether‐lumefantrine for uncomplicated malaria: a systematic review. Tropical Medicine & International Health 2004;9(2):192‐9. [DOI] [PubMed] [Google Scholar]


